chapter 6 Core Principles of Perioperative Care
Preoperative Evaluation
The perioperative management of patients undergoing urologic surgery continues to evolve. Over the past 2 decades, the economics of health care has added increasing pressure for more outpatient surgery and decreased hospital stays. Furthermore, the acuity of surgical patients is increasing in that patients are older with more significant comorbidities. It has become standard for patients undergoing even the most sophisticated and complex urologic procedures in the hospital to be admitted on the same day of the surgery. Therefore the urologic surgeon is responsible for assuring that the patient has been thoroughly evaluated by the other physicians in the health care team and presents to the operating room in the most optimized medical condition. The preoperative use of appropriate medical specialist consults will result in improved patient safety and obviate the need for unnecessary cancelled surgeries due to the inadequacy of medical optimization.
Presurgical Testing
The goal of presurgical testing is to identify undiagnosed comorbidity or significant exacerbation of existing comorbid illnesses that may affect the operative outcome (Townsend et al, 2008). Although the preoperative evaluation should be individualized on the basis of age, history, and physical examination, each hospital or surgery center generally has specific guidelines for the necessary presurgical tests. Interestingly, routine testing has never been shown to be cost effective. In fact, it is less predictive of perioperative morbidity than the American Society of Anesthesiologists (ASA) status or the American Heart Association (AHA)/American College of Cardiology (ACC) guidelines for surgical risk. Generally, presurgical testing includes complete blood count (CBC), basic metabolic panel (BMP), prothrombin time/partial prothrombin time/international normalized ratio (PT/PTT/INR; controversial), electrocardiogram (ECG), and chest radiograph. The routine use of a PT/PTT in a patient not using warfarin or in a patient who gives no prior history of increased bleeding with other surgical procedures is controversial. Often overlooked but extremely important is the requirement for a urine pregnancy test on the morning of surgery in any woman of childbearing age unless the ovaries or uterus have been previously surgically removed (Halaszynski et al, 2004). The value of a preoperative ECG in identification of underlying acute cardiac disease and predicting perioperative cardiac morbidity is also controversial. Some studies show that ECG abnormalities have no significant predictive value (Goldman et al, 1978), whereas others have shown that an abnormal ECG was the best diagnostic predictor of an adverse cardiac event (Carliner et al, 1985). Nonetheless, current recommendations generally suggest that a preoperative ECG be done in patients older than 40 years of age or those with a history of any cardiac disease. Similarly, the routine preoperative use of a chest radiograph, in the absence of preexisting cardiopulmonary disease, is not indicated. Overall, even an ASA Task Force on preanesthesia evaluation could not make firm recommendations other than “preoperative tests may be ordered, required, or performed on a selective basis for purposes of guiding or optimizing perioperative management” (Practice advisory, 2002).
Surgical Risk Evaluation
American Society of Anesthesiologists Classification and Risk Stratification
Approximately 27 million patients undergo surgery each year in the United States and 8 million or 30% have significant coronary artery disease or other cardiac comorbidities. Appropriately, the cardiovascular system is targeted during the preoperative assessment of patients. The ASA classification was first developed in 1961 and has been revised to categorize risk into six stratifications.
The goal of the classification system is to assess the overall physical status of the patient before surgery (not to assess surgical risk), and although quite subjective, it remains as a significant independent predictor of mortality (Davenport et al, 2006). Other tools to assess the preoperative risks were developed by multivariate statistical analysis of patient-related factors correlated with surgical outcomes. One such scoring system, Goldman’s criteria (Table 6–1), assigns points to easily reproducible characteristics. The points are then added to compute the perioperative risk of cardiac-related complication. Another system, the Cardiac Risk Index, simplified this concept employing only six predictors to estimate cardiac complication risk in noncardiac surgical patients (Table 6–2) (Akhtar and Silverman, 2004).
Table 6–1 Goldman’s Cardiac Risk Index
| PATIENT RISK FACTORS | POINTS |
|---|---|
| Third heart sound or jugular venous distention | 11 |
| Recent myocardial infarction | 10 |
| Nonsinus rhythm or premature atrial contraction on electrocardiogram | 7 |
| >5 premature ventricular contractions | 7 |
| Age >70 yr | 5 |
| Emergency operations | 4 |
| Poor general medical condition | 3 |
| Intrathoracic, intraperitoneal, or aortic surgery | 3 |
| Important valvular aortic stenosis | 3 |
| For noncardiac surgery, the risk of cardiac complications are: |
|
Adapted from Akhtar S, Silverman DG. Assessment and management of patients with ischemic heart disease. Crit Care Med 2004;32:S126–36.
Table 6–2 Modified Cardiac Risk Index
| PATIENT RISK FACTORS | POINTS |
|---|---|
| Ischemic heart disease | 1 |
| Congestive heart failure | 1 |
| Cerebral vascular disease | 1 |
| High-risk surgery | 1 |
| Preoperative insulin treatment for diabetes | 1 |
| Preoperative creatinine of ≥2 mg/dL | 1 |
| Each increment in point increases risk of perioperative cardiovascular morbidity | |
Adapted from Akhtar S, Silverman DG. Assessment and management of patients with ischemic heart disease. Crit Care Med 2004;32:S126–36.
Cardiac Evaluation
The preoperative cardiac evaluation, which consists of an initial history and physical examination and ECG, attempts to identify potential serious cardiac disorders such as coronary artery disease, heart failure, symptomatic arrhythmias, the presence of a pacemaker or implantable defibrillator, or a history of orthostatic hypotension (Eagle et al, 1996). Furthermore, it is essential to define the severity and stability of existing cardiac disease before surgery. Cardiac-specific risk is also altered by the patient’s functional capacity, age, and other comorbid conditions such as diabetes, peripheral vascular disease, renal dysfunction, and chronic obstructive pulmonary disease. The American College of Cardiology and American Heart Association recently collaborated to develop guidelines regarding perioperative cardiac evaluation before surgery (Fleisher et al, 2007a). The guidelines generally use three categories of clinical risk predictors: clinical markers, functional capacity, and type of surgical procedure (Eagle et al, 2002).
Clinical Markers
The major clinical predictors of increased perioperative cardiovascular risk are a documented acute myocardial infarction less than 7 days previously, a recent myocardial infarction (defined as >7 days but <1 month before surgery), unstable angina, evidence of any ischemic burden by clinical symptoms or noninvasive testing, decompensated heart failure, significant arrhythmias, and severe valvular disease. Intermediate predictors include mild angina, previous myocardial infarction by history or pathologic Q waves, compensated heart failure, diabetes, or renal insufficiency (creatinine >2 mg/dL). Minor predictors of risk are advanced age, abnormal electrocardiogram, rhythms other than sinus (i.e., atrial fibrillation), history of stroke, or uncontrolled systemic hypertension. The historical dictum of the timing of elective surgery after a myocardial infarction into a 3-month and 6-month interval is now currently avoided (Tarhan et al, 1972). The ACC cardiovascular database committee stratifies risk on the basis of the severity of the myocardial infarction and the likelihood of reinfarction based on a recent exercise stress test. However, in the absence of adequate clinical trials on which to base firm recommendations, it is reasonable to wait 4 to 6 weeks after myocardial infarction to perform elective surgery.
Functional Capacity
Functional capacity, or one’s ability to meet aerobic demands for a specific activity, is quantified as metabolic equivalents or METs. For example, a 4-MET demand is comparable with a patient’s ability to climb two flights of stairs. This simple measurement continues to be an easy and inexpensive method to determine a patient’s cardiopulmonary functional capacity (Biccard, 2005). The Duke Activity Status Index (Table 6–3) allows the physician to easily determine a patient’s functional capacity (Hlatky et al, 1989). Generally, a capacity of 4 METs indicates no further need for invasive cardiac evaluation.
Table 6–3 Duke Activity Status Index*
| ACTIVITY | YES | NO |
|---|---|---|
| Can you take care of yourself (eating, dressing, bathing, or using the toilet)? | 2.75 | 0 |
| Can you walk indoors such as around your house? | 1.75 | 0 |
| Can you walk a block or two on level ground? | 2.75 | 0 |
| Can you climb a flight of stairs or walk up a hill? | 5.50 | 0 |
| Can you run a short distance? | 8.00 | 0 |
| Can you do light work around the house like dusting or washing dishes? | 2.70 | 0 |
| Can you do moderate work around the house like vacuuming, sweeping floors, or carrying in groceries? | 3.50 | 0 |
| Can you do heavy work around the house like scrubbing floors or lifting and moving heavy furniture? | 8.00 | 0 |
| Can you do yardwork like raking leaves weeding or pushing a power mower? | 4.50 | 0 |
| Can you have sexual relations? | 5.25 | 0 |
| Can you participate in moderate recreational activities like golf, bowling, dancing, doubles tennis, or throwing a baseball or football? | 6.00 | 0 |
| Can you participate in strenuous sports like swimming, singles tennis, football, basketball, or skiing? | 7.50 | 0 |
Duke activity status index (DASI) = SUM (values for all 12 questions).
Estimated Peak Oxygen Uptake (VO2peak) in mL/min = (0.43 × [DASI]) + 9.6.
VO2peak mL/kg/min × 0.286 (mL/kg/min)−1 = METS.
* The most widely recognized measure of cardiorespiratory fitness is maximal oxygen consumption (VO2peak) measured in mL/kg/min. The Index score correlates directly with VO2peak and therefore is an indirect measure of maximal METS.
Adapted from Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989;64:651–4.
Surgery-Specific Cardiac Risk
Two important factors determine the surgery-specific cardiac risk: the type of surgery and the degree of hemodynamic stress. Surgery-specific risk is stratified into high-, intermediate-, and low-risk procedures. High-risk procedures include both major emergent surgery, particularly in the elderly, and surgery associated with increased operative time resulting in large fluid shifts or blood loss. Intermediate risk procedures include intraperitoneal surgery, laparoscopic procedures, and robotic-assisted laparoscopic surgeries. Low-risk procedures include endoscopic procedures or superficial surgeries (i.e., not involving entrance into a body cavity) (Eagle et al, 2002).
Pulmonary
Preoperative pulmonary evaluation is important in all urologic procedures but critical in those surgeries involving the thoracic or abdominal cavities. These later procedures, which include intra-abdominal, laparoscopic, or robotic surgeries, can decrease pulmonary function and predispose to pulmonary complications. Accordingly, it is wise to consider pulmonary functional assessment in patients who have significant underlying medical disease, significant smoking history, or overt pulmonary symptoms. Pulmonary function tests that include a forced expiratory volume in 1 second (FEV1), forced vital capacity, and the diffusing capacity of carbon monoxide are quite easily obtainable and provide a preoperative baseline. Patients with an FEV1 of less than 0.8 L/sec or 30% of predicted are at high risk for complications (Arozullah et al, 2003). Specific pulmonary risk factors include chronic obstructive pulmonary disease, smoking, preoperative sputum production, pneumonia, dyspnea, and obstructive sleep apnea. It has been shown that smokers have a fourfold increased risk for postoperative pulmonary morbidity and as high as a 10-fold higher mortality rate (Fowkes et al, 1982). In general, it is interesting to note that patients with restrictive pulmonary disease fare better than those with obstructive pulmonary disease because the former group maintains an adequate maximal expiratory flow rate, which allows for a more effective cough with less sputum production (Pearce and Jones, 1984). In addition to the specific pulmonary risk factors, general factors contribute to increased pulmonary complications such as increased age, lower serum albumin levels, obesity, impaired sensorium, previous stroke, immobility, acute renal failure, and chronic steroid use.
Specific preoperative interventions can decrease pulmonary complications. Smoking must be discontinued at least 8 weeks before surgery to achieve a risk reduction. Patients who discontinue smoking less than 8 weeks before surgery may actually have a higher risk of complication because the acute absence of the noxious effect of cigarette smoke decreases postoperative coughing and pulmonary toilet. However, patients who have stopped smoking at least 8 weeks preoperatively will significantly lower their complication rate, and patients who have ceased smoking for more than 6 months have a pulmonary morbidity comparable with nonsmokers (Warner et al, 1989). The use of preoperative bronchodilators in COPD patients can dramatically reduce postoperative pulmonary complications. Aggressive treatment of preexisting pulmonary infections with antibiotics, as well as the pretreatment of asthmatic patients with steroids, is essential in optimizing pulmonary performance. Likewise, the use of epidural and regional anesthetics, vigorous pulmonary toilet, rehabilitation, and continued bronchodilation therapy are all beneficial (Arozullah et al, 2003).
Hepatobiliary
Because the survival of patients with advanced liver disease has improved over the past decade, surgery is being performed more frequently in these patients. Furthermore, patients with mild to moderate hepatic disease are often asymptomatic. These patients need to be identified and evaluated before surgery. Patients are usually aware of a prior diagnosis of hepatitis, and they should be questioned regarding the timing of diagnosis and the precipitating factors. This history is particularly important in a situation when a member of the health care team is inadvertently stuck with a needle or scalpel during the surgical procedure. A review of systems should include questions regarding pruritus, excessive bleeding, abnormal abdominal distention, and weight gain. On physical examination, jaundice and scleral icterus may be evident with serum bilirubin levels higher than 3 mg/dL. Skin changes such as caput medusae, palmar erythema, spider angiomas, and clubbing all indicate hepatic dysfunction. Severe manifestations include abdominal distention, encephalopathy, asterixis, or cachexia. Again, identification of underlying hepatic illness is important in the preoperative risk assessment of the patient. Although the estimation of perioperative mortality is limited by the lack of high-quality clinical studies, the use of the Child classification and Model for End-Stage Liver Disease (MELD) score offers a reasonable estimation.
The Child classification assesses perioperative morbidity and mortality in patients with cirrhosis and is based on the patient’s serum markers (bilirubin, albumin, prothrombin time) and severity of clinical manifestations (i.e., encephalopathy and ascites). Mortality risk for patients undergoing surgery stratified by Child class is as follows: Child Class A—10%, Child Class B—30%, and Child Class C—76% to 82%. The Child classification also correlates with the frequency of complications such as liver failure, encephalopathy, bleeding, infection, renal failure, hypoxia, and intractable ascites. Independent risk factors other than the Child class that can increase the mortality rate in patients with liver disease include emergency surgery and chronic obstructive pulmonary disease (O’Leary et al, 2009; Pearce and Jones, 1984).
The MELD score is perhaps a more accurate assessment of perioperative mortality in patients with hepatic dysfunction. The score is derived from a linear regression model based on serum bilirubin, creatinine levels, and the international normalized ratio (INR). It is more accurate than the Child classification in that it is objective, gives weights to each variable, and does not rely on arbitrary cut-off values (Teh et al, 2007). Clinicians can use a website (http://mayoclinic.org/meld/mayomodel9.html) to calculate the 7-day, 30-day, 90-day, 1-year, and 5-year surgical mortality risk on the basis of the patient’s age, ASA class, INR, serum bilirubin, and creatinine levels. Taken together, the Child classification and the MELD score complement each other and provide an accurate assessment of the risk of surgery in cirrhotic patients (O’Leary and Friedman, 2007; O’Leary et al, 2009).
Optimization of Comorbid Illness
Just as adequate preoperative evaluation is important, optimization of comorbid illness is critical in reducing perioperative morbidity and mortality. With regards to cardiac disease, many studies have evaluated the prophylactic use of nitrates, calcium-channel blockers, and β-blockers for patients who are at risk for perioperative myocardial ischemia. Only β-blockade has shown to improve outcomes (Pearse et al, 2004). In a landmark study, Mangano and colleagues reported in the New England Journal of Medicine that there was an improvement in outcomes with the prophylactic use of atenolol in patients undergoing vascular surgery (Mangano et al, 1996). Similarly, a retrospective, cooperative group study of more than half a million patients showed that perioperative beta blockade is associated with a reduced risk of death among high-risk patients undergoing major noncardiac surgery (Lindenauer et al, 2005). In addition to β-blockade, the concept of goal-directed therapy, employing the judicious use of fluids, inotropes, and oxygen therapy to achieve therapeutic goals, may further reduce perioperative risk (Pearse et al, 2004). This concept was validated by Shoemaker, who reported an impressive reduction in mortality from 28% to 4% (P < .02) when goal-directed therapy was used (Shoemaker et al, 1988).
As with cardiac comorbidity, the preoperative management of the diabetic patients is quite important. Perioperative hyperglycemia can lead to impaired wound healing and a higher incidence of infection (Golden et al, 1999). Hypoglycemia in an anesthetized or sedated diabetic patient may be unrecognized and carries its own significant risks. Noninsulin diabetics may need to discontinue long-acting hypoglycemics because of this risk of intraoperative hypoglycemia. Shorter-acting agents or sliding scale insulin regimens are generally preferable. It is recommended that blood glucose levels be controlled between 80 and 250 mg/dL. Frequent fingerstick glucose checks and the use of a sliding scale short-acting insulin regimen are used in the postoperative period. Once the patient is eating, the usual insulin regimen can be resumed. Patients who monitor their diabetes with the use of insulin pumps should continue their basal insulin infusions on the day of surgery. The pump is then used to correct the glucose level as it is measured. It is important to know the sensitivity factor that corrects the glucose so that the patient’s sugars can be managed in the operating room (Townsend et al, 2008).
Patients with either hyperthyroidism or hypothyroidism should be evaluated by an endocrinologist and surgery should be deferred until a euthyroid state is achieved. The greatest risk in the hypothyroid patient is thyrotoxicosis or thyroid storm, which can present with fevers, tachycardia, confusion, and cardiovascular collapse. Atrial fibrillation may also be present in 20% of hyperthyroid patients (Klein and Ojamaa, 2001). With regards to hyperthyroidism, careful attention should be given to the airway because the trachea can be compressed or deviated by a large goiter. Generally, antithyroid medications such as propylthiouracil or methimazole, as well as β-blockers, are continued on the day of surgery. In the event of thyroid storm, iodine and steroids may be necessary (Schiff and Welsh, 2003). Hypothyroidism is generally associated with an increased sensitivity to medications such as anesthetic agents and narcotics. Severe hypothyroidism can be associated with myocardial dysfunction, coagulopathy, electrolyte imbalance, and a decreased gastrointestinal motility. Symptoms include lethargy, cold intolerance, hoarseness, constipation, dry skin, and apathy. The decrease in metabolic rate produces periorbital edema, thinning of the eyebrows, brittle hair, dry skin, hyperthermia, bradycardia, and a prolonged relaxation of the deep tendon reflexes (Murkin, 1982). Once the diagnosis is confirmed by a low thyroxine level and an elevated thyroid stimulating hormone level, thyroid replacement with levothyroxine can be initiated (Schiff and Welsh, 2003).
The evaluation of the patient either taking corticosteroids or suspected of having an abnormal response of the hypothalamic-pituitary-adrenal axis (HPA) is also important. There is a wide variability in the HPA suppression in patients on exogenous steroids. Nonetheless, it seems clear that oral steroids equivalent to less than 5 mg of prednisone for any duration of time does not cause clinically significant suppression of the HPA axis. By contrast, any patient taking more than 20 mg of prednisone or its equivalent per day for more than 3 weeks or who is clinically Cushingoid has probable HPA axis suppression (LaRochelle et al, 1993). HPA suppression can even occur in patients using potent topical steroids at doses of 2 g per day, as well as in patients using inhaled corticosteroids at doses of 0.8 mg per day. Although the duration of a functional HPA axis suppression after glucocorticoids have been stopped is debatable, perioperative supplemental steroids are recommended for patients who had HPA axis suppressive doses within 1 year of surgery. A low-dose ACTH stimulation test can be used to assess the HPA axis and the need for stress steroids. For patients who take 5 mg of prednisone or the equivalent each day, no supplemental steroids are necessary and the usual daily glucocorticoid dose may be given in the perioperative period. For those in whom the HPA axis is presumed to be suppressed or is documented to be suppressed, then 50 to 100 mg of intravenous hydrocortisone is given before the induction of anesthesia and 25 to 50 mg of hydrocortisone is given every 8 hours thereafter for 24 to 48 hours until the usual steroid dose can be resumed. Minor procedures under local anesthesia do not require stress-dose steroids (Schiff and Welsh, 2003).
Special Populations
Elderly
In the next 20 years, the percentage of patients older than 85 years of age may reach 5% or 15 million people. This represents a rapidly growing segment of our aging population (Monson et al, 2003). Accordingly, octogenarians and nonagenarians are undergoing an increasing number of surgeries annually. Because of the elderly patients’ special physiologic, pharmacologic, and psychologic needs, a unique set of health care challenges are encountered. It is still unclear whether advanced age independently predicts surgical risk or whether it is coexisting medical conditions that adversely affect surgical outcomes. However, in a large study published by Turrentine, it was shown that increased age independently predicted morbidity and mortality (Turrentine et al, 2006). This confirmed the study by Vemuri, who also found increased age as an independent risk factor for morbidity and mortality in patients undergoing aneurysm surgery (Vemuri et al, 2004). The studies suggest that perhaps the elderly patient cannot meet the increased functional demand required during perioperative and postoperative period. Hypertension and dyspnea were the most frequently seen comorbid risk factors in patients older than 80 years, and preoperative transfusion history, emergency operation, and weight loss best predicted postoperative morbidity. Each 30-minute increment of operative time increased the odds of mortality by 17% in octogenarians (Turrentine et al, 2006). A unique and important factor in the perioperative care of the elderly is in the identification and prevention of delirium. Often overlooked as “sundowning,” delirium can be the first clinical sign of metabolic and infectious complications (Townsend et al, 2008).
Morbid Obesity
With the rising incidence of obesity, as well as the vast experience gathered from bariatric surgery, the care of the morbidly obese patient has been extensively studied. One must carefully weigh the risk of any surgical procedure with the natural history of the disease when deciding the optimal time of the surgery in the morbidly obese. It is estimated that patients with a body mass index (BMI) of greater than or equal to 45 kg/m2 may lose anywhere from 8 to 13 years of life (Fontaine et al, 2003). The careful selection of the morbidly obese patient for elective surgery is of paramount importance. Cardiac symptoms such as exertional dyspnea and lower extremity edema are nonspecific in the morbidly obese patients, and many of these patients have poor functional capacity. The physical examination often underestimates cardiac dysfunction in the severely obese patient. Severely obese patients with greater than three coronary heart disease risk factors may require noninvasive cardiac evaluation (Poirier et al, 2009). Obesity is associated with a vast array of comorbidities. Morbidly obese patients often have atherosclerotic cardiovascular disease, heart failure, systemic hypertension, pulmonary hypertension related to sleep apnea and obesity, hypoventilation, cardiac arrhythmias, deep vein thrombosis, history of pulmonary embolism, and poor exercise capacity. There are also numerous pulmonary abnormalities that result in a ventilation perfusion mismatch and alveolar hypoventilation. Obesity is a risk factor for postoperative wound infections, and, when appropriate, laparoscopic surgery should be considered.
Pregnancy
Urologic surgery in the pregnant woman is generally related to the management of renal colic and urinary tract stones. In the asymptomatic woman, the stones can be discovered during the sonographic evaluation of the fetus or during the evaluation of the pregnant woman who is experiencing renal colic. The fetus is at the highest risk from radiation exposure from the preimplantation period to approximately 15 weeks’ gestation. Because the radiation dose that is associated with congenital malformations is 10 cGy, the evaluation of renal colic in a pregnant patient is performed usually with sonography (radiation dose with abdominal CT: 1 cGy, intravenous pyelogram: 0.3 cGy). The indications for operative intervention in the pregnant patient are discussed elsewhere in this book. Anesthetic risks during pregnancy concern both the mother and the fetus. During the first trimester, the fetus may be directly exposed to the teratogenic effects of certain anesthetic agents. Later in pregnancy, anesthesia places the mother at risk for preterm labor and the fetus at risk for hypoxemia secondary to changes in uterine blood flow and maternal acid base balance. These risks seem to be greatest during the first and third trimesters. For semielective procedures, an attempt should be made to delay surgery until after the first trimester. However, one must consider the continued exposure of the underlying condition in relation to the operative risks to both the mother and the fetus. The second trimester is the safest time to perform surgery because organ system differentiation has occurred and there is almost no risk for anesthetic-induced malformation or spontaneous abortion. When contemplating surgery on the pregnant female, consultation with the obstetrician, perinatologist, and anesthesiologist is essential. These specialists will help determine the optimum technique to monitor the status of the fetus in the pregnant mother. Fetal heart rate monitors and tocometer monitoring for uterine activity are used before and after the procedure. Postoperative pain is best managed with narcotic analgesics because they have not been shown to cause birth defects in humans when used in normal dosages. Nonsteroidal anti-inflammatory medication should be avoided because of the risk for premature closure of the ductus arteriosus. Chronic use of narcotics during pregnancy may cause fetal dependency, and it is recommended that the pregnant postsurgical patient be weaned off narcotic use as soon as possible (Mikami et al, 2008).
Nutritional Status
Malnutrition compromises host defenses and increases the risk of perioperative morbidity and mortality. Adequate nutritional status is essential for proper wound healing, management of infections, return of gastrointestinal activity, and maintenance of vital organ function (McDougal, 1983). The preoperative evaluation of the patient’s nutritional status consists of the assessment of any recent weight loss and the measurement of the lymphocyte count and serum albumin. A 20-pound weight loss in the preceding 3 months before surgery is considered to be a reflection of severe malnutrition. The lymphocyte count and serum albumin level reflect visceral protein status with lower levels indicating malnutrition (Reinhardt et al, 1980). There are two methods for nutritional support. Total parenteral nutrition (TPN) is used for patients who are severely malnourished and who have a nonfunctioning gastrointestinal tract. Several studies have shown that 7 to 10 days of preoperative parenteral nutrition improves postoperative outcome in undernourished patients (Von Meyenfeldt et al, 1992). On the contrary, its use in well-nourished or mildly undernourished patients is either of no benefit or even with increased risk of sepsis (Perioperative total parenteral nutrition in surgical patients, 1991). On the other hand, enteral nutrition has a fewer complications than TPN and can provide a more balanced physiologic diet. Elemental nutrition is accomplished via a feeding tube, a gastrostomy, or feeding jejunostomy. Enteral nutrition maintains the gut-associated lymphoid tissue, enhances mucosal blood flow, and maintains the mucosal barrier. There are hundreds of enteral products on the market, and most have a caloric density of 1 to 2 kcal/mL. These formulas are also lactose free and provide the recommended daily allowances of vitamins and minerals in less than 2 L per day. The patients on enteral feeds must be monitored for improvement in nutritional status, gastrointestinal intolerance, and fluid and electrolyte imbalance. Preoperative enteral feedings can decrease postoperative complication rates by 10% to 15% when used for 5 to 20 days before surgery (Guidelines, 2002). The guidelines recommend postoperative parenteral nutrition in patients who are unable to meet their caloric requirements within 7 to 10 days. Just as in the perioperative state, enteral feedings are preferred over parenteral nutrition when feasible. Moreover, the routine use of postoperative TPN has not proven useful in well-nourished patients or in those with adequate oral intake within 1 week after surgery (Byers and Hameed, 2008). Complications can occur with either enteral nutrition or parenteral nutrition. Dislodgement of nasoenteral tubes and percutaneous enteral catheters can result in pulmonary and peritoneal complications. Adynamic ileus may also occur because of decreased splanchnic perfusion, sympathetic tone, or opiate use. With regards to TPN, establishing central access is associated with a significant risk of complications. These include pneumothorax/hemothorax secondary to poor line placement and chylothorax secondary to thoracic duct injury. Line sepsis is the most common complication of indwelling central catheters and necessitates catheter removal. Venous thrombosis with associated thrombophlebitis and extremity edema has been reported. Catheter thrombosis has also been reported and can be treated with thrombolytic agents (Guidelines, 2002).
Preparation for Surgery
Antibiotic Prophylaxis
In 1999 the Centers for Disease Control (CDC) issued its third report on the prevention of surgical site infections (SSIs) highlighting the importance of standardization of prophylaxis treatment to prevent this universal surgical complication (Mangram et al, 1999). The report indicated that SSIs account for approximately 40% of nosocomial infections in surgical patients and potentially prolong hospital stay by 7 to 10 days. A study of national SSIs from the 2005 Healthcare Cost and Utilization Project National Inpatient Sample (HCUP NIS) calculated an increase in hospital stay of 9.7 days and in per-patient cost of $20,892 (de Lissovoy et al, 2009). This translated nationally into an additional 1 million inpatient hospital days and additional health care cost of $1.6 billion. Bowater and colleagues recently published a systematic review of meta-analyses (level 1 evidence) and concluded that there was substantial evidence that antibiotic prophylaxis was an effective prevention for SSI over a wide variety of surgical procedures (Bowater et al, 2009). Given both the ethical responsibility of the surgeon to decrease surgical morbidity and the recent policy shift by the Centers of Medicare and Medicaid Services to withhold reimbursement for hospital admissions secondary to specific SSI, it is mandatory for urologists to understand the principles behind and practice SSI prevention.
Along with antibiotic prophylaxis, proper hand washing/scrubbing and sterile preparation of the operative field have always been central to the prevention of SSI. For procedures involving the gastrointestinal tract, mechanical and oral antibiotic bowel preparation had been standard practice until more recent literature calling into question its usefulness (discussed later). Preoperative hair removal has not been associated with a decrease in SSI, but if performed, use of mechanical clippers or depilatory creams as opposed to razors are associated with a decrease risk of SSI (Wolf et al, 2008).
The risk of SSI and therefore the recommendation for antibiotic prophylaxis is composed of three risk factors: the patient’s susceptibility to and the ability to respond to localized and systemic infection, procedural risk of infection, and the potential morbidity of infection. Patient-related factors, listed in Table 6–4, increase risk by decreasing natural defenses, increasing the local bacterial concentration, and/or altering the spectrum of bacterial flora. Secondly, surgical procedure–specific factors can affect the route of entry, site of infection, and pathogen involved. This idea was first described in the landmark study from the National Research Council and later formalized by the CDC; specifically, surgical wounds are now classified by degree of contamination (i.e., the inoculum of potential pathogen) (Table 6–5; Hart et al, 1968). To predict the risk of SSI, several scoring systems have been developed incorporating patient-related factors with wound classification. Finally, the risk to the patient from SSI is an important consideration in determining the need for prophylaxis. For example, routine cystoscopy in the evaluation of microhematuria in an otherwise young, healthy patient may not warrant prophylaxis; however, the same procedure in an elderly, insulin-dependent diabetic (immunocompromised) does warrant prophylaxis given the high likelihood that a postprocedural urinary tract infection would result in a significant deterioration in the patient’s overall health. Understanding the three factors together then allows the urologist to make a rational decision as to the risk/benefit of antibiotic prophylaxis.
Table 6–4 Patient Factors That Increase the Risk of Infection
Data from Cruse PJ. Surgical wound infection. In: Wonsiewicz MJ, editor. Infectious disease. Philadelphia: WB Saunders; 1992. p. 758–764; and Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999;20:250–78; quiz 279–80.
Table 6–5 Surgical Wound Classification
| Clean | |
| Clean contaminated | |
| Contaminated | |
| Dirty infected |
Data from Garner JS. CDC guideline for prevention of surgical wound infections, 1985. Supersedes guideline for prevention of surgical wound infections published in 1982. (Originally published in 1995.) Revised. Infect Control 1986;7(3):193–200; and Simmons BP. Guideline for prevention of surgical wound infections. Infect Control 1982;2:185–96.
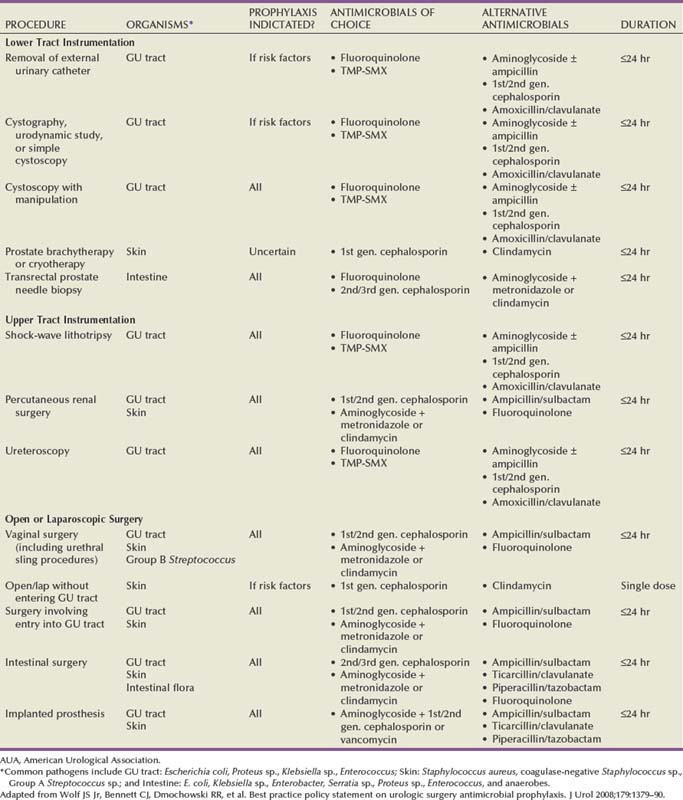
Once the decision for antibiotic prophylaxis is made, the keys to successful prevention are proper timing and administration of the antibiotic and the proper choice of antibiotic for the particular procedure. Since the pivotal study by Classen and colleagues, particular emphasis has been placed on the timing of prophylaxis to be given within 2 hours of incision (Classen et al, 1992). This emphasis is exemplified by the Joint Commission’s Surgical Care Improvement Project (SCIP) guideline for administration of antibiotic prophylaxis 60 minutes before incision in a broader effort to decrease surgical complications by 25% by 2010. A recent multi-institutional trial involving more than 4400 patients at 29 institutions reported results of their analysis on the optimal timing of antibiotic prophylaxis (Steinberg et al, 2009). Their results suggested an improvement in prevention of SSI when antibiotics were administered within 30 minutes of incision as compared with 31 to 60 minutes (adjusted odds ratio [OR] 1.48, P = .06). More importantly, this larger study confirmed the significantly increased risk of SSI when antibiotics were administered at the time of or following incision with adjusted OR of 2.20, P = .02. The duration of antibiotic prophylaxis is more controversial; however, most recommendations advocate no more than 24 hours in a patient without an established infection. Routine antibiotic use beyond 24 hours increases the risk of Clostridium difficile colitis, increases the development of antibiotic resistance, and increases costs. Along with timing and duration, proper administration of antibiotics implies proper dosing. Antibiotic dose is dependent on the patient’s body weight, renal function/hepatic function, and duration of procedure (requiring re-dosing if >4 hours). The second key to successful prevention is the proper choice of antibiotic for the procedure in question. As mentioned earlier, surgery-specific factors affect the type of pathogen, route of entry, and likelihood of systemic infection. For example, the choice of antibiotic is different before a transurethral resection of the prostate (need coverage for common urinary tract pathogens) as compared with a cystectomy with planned sigmoid colon urinary diversion (need coverage for anaerobic bacteria). Another important consideration is the rate of antibiotic resistance in the community. Although there is level 1 evidence for the use of fluoroquinolones as prophylaxis for urologic endoscopic procedures, the emerging Escherichia coli resistance in the community is changing practice patterns in many practices and high-resistance hospitals. One resource that is particularly useful is the hospital antibiogram. These reports are published monthly at most major hospitals and quantify the susceptibility/resistance of common organisms to a wide variety of antibiotics. A summary of the recent American Urological Association (AUA) best practice statement on antibiotic prophylaxis is shown in Table 6–6.
Bowel Preparation
Since antibiotics were first shown to reduce infectious complications in gastrointestinal surgery, mechanical and antibiotic bowel preparation has been a mainstay of urologic surgery employing intestinal segments. The rationale for bowel preparation before intestinal surgery is to decrease intraluminal feces and decrease bacterial colony counts in order to decrease the rate of anastomotic leak, intra-abdominal abscesses, and wound infections. The bacterial flora in the bowel consists of aerobic organisms, the most common of which are Escherichia coli and Enterococcus faecalis, and anaerobic organisms, the most common of which are Bacteroides species and Clostridium species. The bacterial concentration ranges from 10 to 105 organisms per gram of fecal content in the jejunum, 105 to 107 in the distal ileum, 106 to 108 in the ascending colon, and 1010 to 1012 in the descending colon. The preparation itself consists of two components: antibiotic preparation and mechanical preparation. Because there are only a few small series in the urologic literature, the rationale for each must be inferred from the general surgery literature, specifically, from colorectal surgery literature.
Although preoperative parenteral antibiotic prophylaxis before intestinal surgery is well established and widely used, oral antibiotic preparation is still somewhat controversial. Several oral antibiotic regimens are used today. The most commonly used regimen, oral neomycin and erythromycin, first became established with the landmark study by Drs. Nichols and Condon in 1977 (Clarke et al, 1977). In a double-blind, placebo-controlled study, 167 patients undergoing elective colonic surgery were randomized to receive mechanical bowel preparation with or without oral neomycin and erythromycin. The overall septic complications were 43% in the mechanical-only prep and 9% in the antibiotic plus mechanical prep group, P = .001. With the widespread use of preoperative parenteral antibiotics, the benefit of oral preparation has been debated. Several older studies reported decreased infectious complications; however, these studies were small and there have been no randomized controlled trials to document the benefit. The disadvantage of oral antibiotic preparation is primarily related to increased incidence of pseudomembranous colitis secondary to C. difficile infection. In a retrospective analysis of 304 patients, Wren and colleagues reported a significantly decreased incidence of C. difficile colitis in patients who did not receive oral antibiotics before elective colorectal surgery (2.6% vs. 7.2%, P = .03) (Wren et al, 2005). Despite the lack of evidence in the literature, a recent survey of colorectal surgeons revealed that up to 87% of surgeons continue to administer oral antibiotic bowel preparation before elective surgery (Zmora et al, 2003).
Mechanical bowel preparation predates the use of antibiotics in intestinal surgery and was thought to decrease the rate of anastomotic complications. Before the development of nonabsorbable liquids, patients underwent several days of oral laxatives, bowel irrigations via nasogastric tubes, and repeat enemas. These regimens were associated with significant patient discomfort and clinical morbidity due to electrolyte imbalances. The development of polyethylene glycol solution (GoLytely) and sodium phosphate solution (Fleet’s Phospho-soda) reduced much of the electrolyte disturbances and allowed for mechanical bowel preparation to be done in the outpatient setting. Both regimens are suitable for most patients; however, polyethylene glycol is preferred in the elderly and in patients with renal insufficiency, congestive heart failure, existing electrolyte disturbances, and cirrhosis because it is completely nonabsorbable.
The benefit of mechanical bowel preparation has been assumed for decades as evidenced by 99% positive response by colorectal surgeons when asked if mechanical preparation is routinely used (Zmora et al, 2003). However, recent randomized controlled trials (RCT) have called into doubt the true benefit. Slim and colleagues recently published a meta-analysis of RCTs including a total of 4859 patients (Slim et al, 2009). The analysis included 14 trials including 2 recently completed large trials from the Netherlands and Sweden (Contant et al, 2007; Jung et al, 2007). Overall, the analysis revealed that mechanical bowel preparation provided no benefit for anastomotic leak (OR 1.12, 95%; CI 0.82 to 1.53; P = .46), abdominal/pelvic abscess (OR 0.90, CI 0.47 to 1.72; P = .75), or mortality (OR 0.91, CI 0.57 to 1.45; P = .70). In fact, when considering overall SSI, mechanical bowel preparation was associated with a significantly increased risk (OR 1.40, CI 1.05 to 1.87; P = .02). These results were reiterated in a recent Cochrane review, which found no significant differences in anastomotic leak rate, wound infection, need for reoperation, and mortality rates (Guenaga et al, 2009). The authors concluded that there was no evidence that mechanical bowel preparation improves patient outcome following elective colorectal surgery. Although similar studies have not been done in patients undergoing elective urologic surgery, urologists can infer from the colorectal literature and should re-evaluate the common practice of mechanical bowel preparation before urologic intestinal surgery. Two specific exceptions are transrectal ultrasound-guided prostate needle biopsy (PNBx) and laparoscopic urologic surgery. Given the portal of entry and subsequent risk of bacteremia, most urologists advocated for mechanical rectal cleansing with an enema before transrectal PNBx. With regards to laparoscopy, minimally invasive surgeons have long believed that preoperative bowel preparation improves operative exposure due to bowel decompression and decreases the incidence of postoperative ileus. However, to date there have been no trials to support this assertion.
Venous Thromboembolic Prophylaxis
Venous thromboembolic (VTE) complications are a major cause of potentially preventable morbidity and mortality among surgical patients in the United States. A recent study from the Center for Quality Improvement and Patient Safety and the Agency for Healthcare Research and Quality found postoperative VTE as the second most common cause of excess length of stay, charges, and mortality among surgical patients discharged from acute care hospitals (Zhan and Miller, 2003). Urology patients in particular have an increased incidence estimated to be 10% to 40% in patients without any prophylaxis (Geerts et al, 2008). Although these estimates are based on historical studies before the routine use of mechanical prophylaxis and the recognition of the benefits of early ambulation, the increased risk continues in more recent studies with reported incidence of 1% to 5%. Urologic patients followed prospectively in the European RISTOS study developed VTE in 1.9% undergoing open surgery despite a high rate of prophylaxis (Scarpa et al, 2007). Overall, VTE represents the most important cause of nonsurgical mortality among urology patients (Forrest et al, 2009).
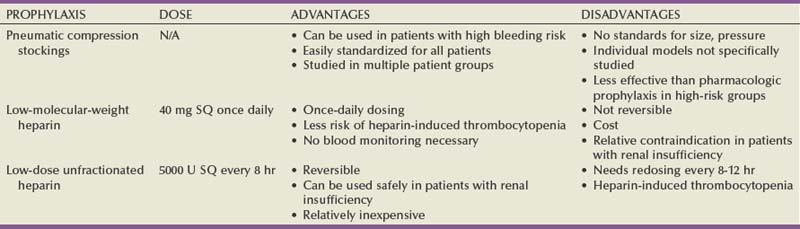
Although the use of perioperative mechanical prophylaxis (pneumatic compression stockings) is fairly universal, pharmacologic prophylaxis is only administered after weighing the risk of VTE versus risk of perioperative bleeding complications (Table 6–7). Leonardi and colleagues reviewed and analyzed 33 randomized controlled trials to assess the incidence of bleeding complications in general surgery patients receiving pharmacologic prophylaxis (Leonardi et al, 2006). Although there was a significantly higher rate of minor complications (injection site bruising and wound hematoma), there was no significant difference in major complications (i.e., GI tract [0.2%] or retroperitoneal bleeding [<0.1%]). Although these results are generally applicable to urology patients, certain urologic procedures have a specifically higher rate of bleeding complications such as transurethral resection of the prostate and partial nephrectomy. Regarding an individual’s risk of VTE, both surgery-related risk factors and patient-related risk factors must be considered. Surgical factors specific to urologic surgery to be weighed include general versus neuraxial anesthesia, supine versus dorsal lithotomy position, abdominal versus pelvic surgery with/without lymphadenectomy, and open versus laparoscopic approach. Patient-related risk factors are listed in Table 6–8 with increasing age, malignancy, history of cancer therapy, and others being fairly common among urology patients. In fact, both the RISTOS study and a recent report on minimally invasive radical prostatectomy confirmed several of these factors as increased risk of VTE in urologic patients (Scarpa et al, 2007; Secin et al, 2008). In 2008 the American College of Chest Physicians (ACCP) issued guidelines on the prevention of VTE with a strong recommendation that hospitals develop a formal, active strategy to address VTE prevention. Although prior recommendations from the ACCP advocated individualized risk assessment models to guide therapy, the current recommendations advocate implementation of group-specific thromboprophylaxis routinely for all patients who belong to each of the major surgical groups (i.e., urologic surgery) (Geerts et al, 2008). The AUA recently published a best practice statement on the use of VTE prophylaxis in urologic patients (Forrest et al, 2009). Their recommendations combine an individualized risk assessment model with each type of urologic surgery. For example, a high-risk patient (multiple patient risk factors) and low-risk surgery may require pharmacologic prophylaxis just as a low-risk patient undergoing high-risk surgery. The recommendations are summarized in Table 6–9.
Table 6–8 Patient-Related Factors Increasing Risk for Venous Thromboembolism (VTE)
Adapted from Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest 2008;133:381S–453S.
Table 6–9 Patient Risk Assessment Model (A) and American Urological Association Best Practice Recommendations (B)
| A Patient Risk Stratification |
|
| Low risk | |
| Moderate risk | |
| High risk | |
| Highest risk | |
| B Level of Risk |
Recommendations |
| Low risk | |
| Moderate risk | |
| High risk | |
| Highest risk | |
Adapted from Forrest JB, Clemens JQ, Finamore P, et al. AUA Best Practice Statement for the prevention of deep vein thrombosis in patients undergoing urologic surgery. J Urol 2009;181:1170–7.
Antithrombotic Therapy
With most urologic patients suffering from medical comorbidities, urologists frequently encounter patients on chronic vitamin K antagonists (e.g., warfarin) or anti-platelet therapy for the management of atrial fibrillation, mechanical heart valves, or coronary artery disease. The perioperative management of interruption of this antithrombotic therapy can be a challenging problem. Unlike VTE pharmacologic prophylaxis, warfarin and antiplatelet therapy have been shown to be associated with significant bleeding complications after surgery. Therefore urologists must carefully consider the risk of interruption of chronic anticoagulation to determine the best course of perioperative management of these medications.
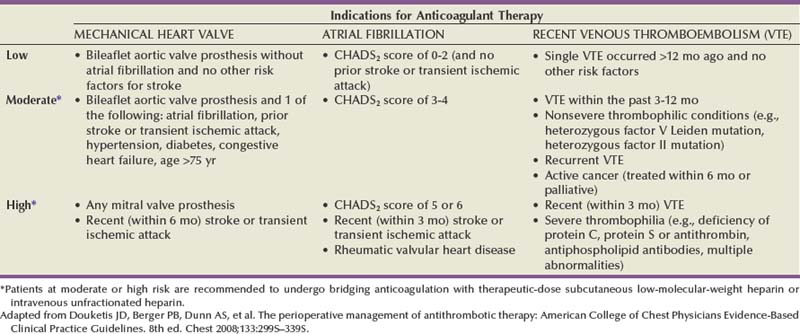
Chronic anticoagulation with warfarin is most frequently encountered in patients with atrial fibrillation, mechanical heart valves, or prior VTE. The pharmacologic half-life of warfarin is 36 to 42 hours, and therefore most guidelines recommend cessation of therapy 5 days before surgery to ensure an INR less than 1.5. The larger issue is whether patients require bridge with short-term anticoagulation between the time of subtherapeutic INR and surgery. The decision is based on risk of thrombotic event. Regarding atrial fibrillation, clinical scoring systems such as congestive heart failure-hypertension-age-diabetes-stroke (CHADS2) stratify patients into risk groups that predict risk of stroke off anticoagulation. Patients with mechanical heart valves can also be stratified into risk groups according to the location (mitral versus aortic) and type of valve used. Similarly, patients with a prior history of VTE are stratified according to duration since last VTE and the patient’s risk of recurrent VTE (Table 6–10). In general, the ACCP, which released its guidelines in 2008, recommends that patients in the moderate- and high-risk groups undergo bridging anticoagulation with therapeutic dose subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (Douketis et al, 2008).
Table 6–10 Risk Stratification for Arterial/Venous Thromboembolism Events during Perioperative Period in Patients on Chronic Anticoagulant Therapy
An increasing number of patients are receiving chronic antiplatelet therapy in the prevention of cardiovascular events and, more importantly, in the prevention of coronary stent thrombosis. Although the former indication poses little controversy for the urologist, the latter indication presents a significant and complex clinical question in which the urologist must weigh the risk of bleeding with the potentially devastating risk of perioperative stent thrombosis. Aspirin and clopidogrel are the two most commonly used antiplatelet drugs and are frequently used together. Both are irreversible inhibitors of platelet function and therefore need to be stopped 7 to 10 days before surgery in order to minimize bleeding risk. Current recommendations require dual antiplatelet therapy for 6 weeks after bare metal coronary stents and 12 months for drug-eluting stents. Premature interruption of antiplatelet therapy has been associated with a 25% to 50% risk of significant myocardial infarction with resultant increased perioperative mortality (O’Riordan et al, 2009). In most cases, urologists should defer elective surgery until after antiplatelet therapy can be safely interrupted. Even then, because acute stent thrombosis has been described with drug-eluting stents after 12 months, urologists should strongly consider at least single-agent antiplatelet therapy in these patients. Given the current lack of clinically useful alternatives to antiplatelet therapy, in cases in which surgery cannot be delayed (i.e., malignancy), the ACCP strongly recommends continuing aspirin and clopidogrel during the perioperative period (Douketis et al, 2008). Obviously, communication between the urologist and the cardiologist throughout the perioperative period is essential to minimize complications.
Anesthetic Considerations
The basic tenet of anesthesia is to deliver hypnosis, amnesia, and analgesia while maintaining satisfactory operating conditions. An understanding of the basic pharmacologic principles, anesthetic equipment and monitoring, and patient analgesia is important to any surgeon including the urologist for successful operative outcomes and avoidance of surgical complications. Although urologists are performing increasingly more procedures in the office, the bulk of urologic surgery occurs in the operating room under monitored anesthesia care (MAC), regional anesthesia, or general anesthesia. Current practice in operative anesthesia employs a combination of inhalational agents and intravenous medications along with analgesics (for pain control) and benzodiazepines (for anxiolysis and amnesia). Of course, improved presurgical evaluation, pharmacologic drugs, and perioperative monitoring have dramatically decreased the risks of anesthesia. A recent study of New York hospital-based and free-standing ambulatory surgical centers reported the risk of all-cause mortality of 1 in 49,012 and immediate rate of admission to an inpatient facility of 0.6% (Fleisher et al, 2007b).
Selection of Mode of Anesthesia
An important role of the urologist in the anesthetic evaluation is to determine what mode of anesthesia is best for the particular patient and surgical procedure. The choice depends on patient-related factors including comorbidities, airway, and patient preference and procedural factors including complexity, duration, anatomic location, and expected fluid/blood loss. A basic understanding of each method of anesthesia and the pharmacologic principles will aid the urologist in making recommendations to the anesthesiologist.
Monitored Anesthesia Care
Although monitored anesthesia care (MAC) is defined as conscious sedation under the care of an anesthesiologist in a monitored situation, MAC encompasses a wide range of levels of anesthesia from minimal sedation to brief intervals of unconscious general anesthesia. Most commonly, anesthesiologists combine intravenous opioid analgesics and benzodiazepines to maintain a sufficient level of patient comfort and anxiolysis. MAC is widely used in urology in the ambulatory setting and is suitable for short-duration endoscopic procedures, transrectal ultrasound-based procedures, and, when combined with a local anesthetic, superficial procedures of the external genitalia. Conscious sedation can be administered in the office setting but only with proper monitoring of the patient during and after the procedure. The Joint Commission has strict guidelines to ensure that the patients receive the same level of monitoring as if under the care of an anesthesiologist including a requirement for a trained monitoring assistant, immediate access to airway and resuscitation equipment, and specific preprocedure/postprocedure evaluations.
Regional Anesthesia
Regional anesthesia incorporates different levels of anesthesia directed to the surgical site including local anesthesia, spinal anesthesia, and epidural anesthesia. The use of local anesthetics is typically combined with MAC for superficial procedures in an isolated anatomic location. The keys to proper local anesthetic administration are avoidance of intravascular injection and knowledge of pharmacology. The two most commonly used drugs are lidocaine and bupivacaine with the primary difference being the onset and duration of action.
Spinal and epidural anesthesia involves injection of anesthetic (most commonly lidocaine or bupivacaine) into the subarachnoid space or epidural space with direct effect on the spinal cord resulting in sensory, motor, and sympathetic blockade. In urologic procedures, epidural anesthesia is most useful for postoperative pain management for major abdominal procedures, thereby avoiding the adverse effects of high doses of intravenous opioids (i.e., respiratory depression, gastrointestinal dysfunction). Spinal anesthesia is suitable for most urologic endoscopic procedures and lower abdominal surgical procedures and is limited only by the duration of anesthesia required. Spinal anesthesia avoids the cardiopulmonary effects and complications of general anesthesia. Several factors affect the spinal level and efficacy of administration. Generally, larger volume and increased doses result in longer duration and increased cephalad migration. The addition of low-dose opioids and/or vasoconstrictors prolongs the duration of analgesia while reducing the dose of anesthetic. The anesthetic-related adverse effect is hypotension as a result of sympathetic blockade and occurs in 10% to 40% of patients (Di Cianni et al, 2008). The primary technique-related complication is postdural puncture headache (results from cerebrospinal fluid leak) with an incidence of less than 2% with currently used 29-gauge pencil-tipped needles (Turnbull and Shepherd, 2003). Overall, spinal anesthesia has become safe with the incidence of serious neurologic deficits being 0.05%.
General Anesthesia
Inhalational General Anesthesia
Inhalational drug development has emphasized inhalational agents that facilitate rapid induction and emergence and are nontoxic. Two of the most important characteristics of inhalational anesthetics are the blood/gas (B/G) solubility coefficient and the minimum alveolar concentration (MAC). The B/G refers to the serum uptake of the inhaled agent, and the MAC is a measure of the potency of a volatile anesthetic (i.e., the serum level required to prevent movement in response to a skin incision in 50% of patients). The various inhalational agents differ not only in the B/G and MAC but also in the cardiopulmonary effects. Obviously, a basic understanding of these properties is important for the urologic surgeon, especially during instances of surgical complication.
Nitrous oxide (NO) is one of the most commonly used agents due to its propensity of rapid induction and emergence; however, due to its low potency, it is often combined with other agents. Because of NO’s high B/G and tendency to increase the volume and pressure of closed spaces, its use is contraindicated in certain clinical situations such as small bowel obstruction and pneumothorax. During laparoscopic abdominal procedures, surgeons often prefer to avoid the use of NO due to resultant bowel distention and subsequent interference in the operative field. Although this effect is debated in the surgical literature, El-Galley and colleagues reported significantly increased bowel distention and surgical interference with NO use in patients undergoing laparoscopic donor nephrectomy (El-Galley et al, 2007).
Once introduced in the 1950s, halothane rapidly became one of the most commonly used anesthetic agents due to its high potency. However, halothane has several important risks that have since limited its use. It has significant cardiac effects and can precipitate failure in patients with left ventricular dysfunction. Furthermore, it sensitizes the myocardium to the effects of catecholamines (relevant for local anesthetics injected into the surgical site). Finally, there is a 1 in 35,000 incidence of fulminant hepatitis, which can be lethal due to overaccumulation of toxic metabolites. More recent advancements in inhalational agents have focused on reduction in toxicity while maintaining the potency and rapidity of halothane. Three of the most commonly used current agents are isoflurane, sevoflurane, and desflurane. Isoflurane, less expensive than the other agents due to availability of generic equivalents, is widely used due to its low cardiac depression, less myocardial sensitization to catecholamines, and minimal metabolism. The primary unique toxicity is variable response tachycardia, which can lead to significantly increased myocardial oxygen consumption. Unlike isoflurane which has a putrid odor, sevoflurane is often used for inhalation induction (odorless) due to its rapid induction and emergence, decreased incidence of postoperative nausea (important in outpatient surgery), and minimal cardiac toxicity. It is the generally preferred agent for difficult airways requiring mask induction and in patients with severe bronchospastic disease. Desflurane, like isoflurane, has a pungent odor and is not used for inhalational induction. Its primary advantage over isoflurane is a more rapid recovery in patients requiring anesthesia over 3 hours.
Intravenous General Anesthesia
Intravenous anesthesia consists of a combination of induction agent, opioid, and neuromuscular relaxant. Anesthesiologists often prefer intravenous induction with a combination of inhalational and intravenous agents for maintenance of anesthesia. Intravenous induction offers several advantages in that it is rapid, minimizes patient discomfort, and is preferred by children and most adults. Thiopental, the oldest and least expensive agent, is a suitable choice for uncomplicated patients but is limited in more complex cases due to its significant vasodilation, cardiac depression, and risk of bronchospasm, especially in patients with reactive airway disease. Ketamine is a preferred choice for procedures that are brief and superficial because of its profound amnesia and somatic analgesia. It is associated with increased arteriolar and bronchomotor tone and is advantageous during induction for hypovolemic and asthmatic patients. Propofol is among the most commonly used anesthetic agents, especially in outpatient surgery. It has a rapid onset, produces excellent bronchodilation in patients with reactive airway disease, and perhaps most importantly, is associated with smooth, nausea-free emergence from anesthesia. Its primary adverse effect is significant blood pressure reduction. Midazolam, never used as a single agent, produces profound amnesia and anxiolysis while having rapid onset/short duration and producing minimal cardiac side effects.
Although these agents induce unconsciousness and amnesia, opioids have become an integral component to all forms of anesthesia. Opioids result in significant analgesia without an increase in cardiac side effects. Several studies have documented the decreased requirements of other agents when used in combination with opioids, thus reducing the overall cardiopulmonary side effects of anesthesia (Fukuda, 2009). Opioids themselves are differentiated in their potency, onset of action, duration of action, and metabolism/excretion. Fentanyl (synthetic opioid) is probably the most widely used because of its potency (100 to 150 times that of morphine), rapid onset, and short duration of action. Newer synthetics are geared to shorter duration and more rapid metabolism.
For major operative cases, complete neuromuscular relaxation is required for sufficient exposure and successful outcome. Although full relaxation can be achieved with intravenous and inhalational agents, the dose required is extremely high. The use of intravenous neuromuscular blockers allows for neuromuscular relaxation and minimization of inhalational/intravenous drugs. There are two types of neuromuscular blockers: depolarizing drugs, which depolarize the plasma membrane of skeletal muscle fibers making the fibers resistant to further stimulation by acetylcholine, and nondepolarizing drugs, which block the binding of acetylcholine to cholinergic receptors on the presynaptic and postsynaptic membrane. Succinylcholine, the only depolarizing drug on the market, is chosen for its rapid onset (used in rapid induction sequences), relatively short duration (around 5 minutes), and rapid metabolism. Its use is limited due to the risk of malignant hyperthermia (when used in combination with volatile inhalational agent), hyperkalemia, and bradycardia in children. In cases where succinylcholine is contraindicated, nondepolarizing agents are used. Several nondepolarizing drugs are available and differ in routes of metabolism and adverse effects. Furthermore, multiple medications including desflurane can alter the metabolism of these drugs and potentiate their actions. The most important consideration in the use of neuromuscular blockers is the assessment of adequate return of neuromuscular function following withdrawal of the drug. The most common complication of neuromuscular-blocking drugs is inadequate reversal resulting in respiratory failure and reintubation. Numerous reports in the literature correlate residual neuromuscular blockade with increased postoperative pulmonary complications in the postanesthetic care unit (PACU) and in the postoperative period. The concept of train of four fade ratio (TOF) was developed to devise an objective measure of adequate neuromuscular function. This concept refers to the magnitude of the fourth of four twitches in response to maximal stimuli to the ulnar nerve delivered at 0.5-sec intervals. Historically, a TOF of 0.7 (meaning that the fourth twitch was 70% the magnitude of the first twitch) correlated with adequate return of neuromuscular function; however, more recent standards have raised the threshold to 0.9 as an indicator of complete return of neuromuscular function (Kopman et al, 1997). Currently, anesthesiologists use several clinical assessments including head lift, tongue depressor test, and hand grip to estimate a TOF of 0.9. A recent study revealed that using clinical assessments alone, 16% and 45% of patients 2 hours after single intubating dose of neuromuscular blocker had TOF of less than 0.7 and less than 0.9, respectively, in the PACU (Debaene et al, 2003). As such, current recommendations are that quantitative TOF measurement (acceleromyography) be combined with clinical assessments before extubation in the operating room (Viby-Mogensen, 2009).
Blood Products
Given the vascular nature of urologic organs, the urologist often confronts the issue of indication and necessity of transfusion in the perioperative period. Therefore it is important that the urologist understand the indications, implications, and risks associated with blood product transfusion. Before the AIDS epidemic, blood transfusion was liberally administered often for any patient with hematocrit less than 30%. However, fear and concern about the infectious risk led to the convening of a National Institutes of Health (NIH) panel to develop consensus recommendations for the indication of blood product transfusion (NIH Consensus Statement, 1988). The principles in these guidelines largely hold true today as reflected by the ASA practice guidelines issued in 2006 (Practice guidelines, 2006). To summarize, the guidelines indicate that transfusion is rarely indicated with hematocrit greater than 30% and often indicated for hematocrit less than 21%. For levels between 21% and 30%, clinical factors such as risk of complications from inadequate oxygenation should guide the need for transfusion balancing the risks and benefits. In general, patients with relatively minor comorbidities can tolerate hematocrit of greater than 21% before transfusion is indicated. Patients with moderate to severe comorbidity (i.e., significant pulmonary compromise, coronary artery disease, or vascular insufficiency, or with signs/symptoms of hypovolemic, hemorrhagic shock) warrant transfusion to achieve hematocrit greater than 30%. Ultimately, until technology is derived to directly measure inadequate oxygen-carrying capacity, the urologist should individualize the decision to transfuse for each patient and clinical situation.
A major advance in blood banking and product transfusion has been the development of component therapy allowing for administration for specific fractions of whole blood. Packed red blood cells (PRBCs) are equivalent to whole blood minus the plasma component. Whereas the hematocrit in whole blood is 40%, it is 70% in PRBC units. These units are reconstituted and administered with crystalloid. Given the lack of the remaining components, in instances of massive PRBC transfusions and associated bleeding, platelets and occasionally fresh frozen plasma should be given to avoid dilutional coagulopathy. Platelet transfusion is rarely indicated empirically except in patients with significant thrombocytopenia (<50,000/mm3) and planned surgical procedure or with moderate thrombocytopenia (50,000-100,000/mm3) and either high-risk procedure or evidence of platelet dysfunction. Similarly, empiric transfusion with fresh frozen plasma (FFP) for massive transfusion is not indicated. With the development of component therapy, the use of FFP increased dramatically, leading to consensus statements from the NIH and the ASA to guide practitioners (Consensus conference, 1985; Practice guidelines, 2006). The current indications for FFP transfusion are immediate reverse of warfarin-induced coagulopathy, replacement in patients with specific clotting factor deficiencies, and evidence of bleeding and INR greater than 1.5. According the ASA guidelines, in patients with massive transfusion and no INR readily available, FFP should be given after replacement of 1 blood volume.
There are well-documented risks of transfusion, and these risks should always be discussed with the patient before administration. Hemolytic transfusion reactions occur due to incompatibility between donor and recipient (either ABO or non-ABO incompatibility). According to the U.S. Food and Drug Administration (FDA) Annual Summary, from 2005-2007 transfusion reactions accounted for 22% of transfusion-related fatality in the United States (FDA Annual Summary for Fiscal Year 2007, 2007). Transfusion reactions occur relatively frequently and, if identified early, can be treated with rare catastrophic events. The early signs/symptoms include fever, chills, chest pain, hypotension, and bleeding diathesis occurring during or immediately after transfusion. Reactions may also occur in a delayed fashion, which is characterized by significant intravascular hemolysis secondary to recipient antibodies. The treatment of transfusion reaction is centered on fluid resuscitation, cessation of the transfusion, and alkalinization of the urine to prevent renal failure. The most common cause of transfusion-related fatality is transfusion-related acute lung injury (TRALI). This entity accounted for 55% of transfusion mortality from 2005-2007. The injury is characterized by noncardiogenic pulmonary edema injury and manifests itself 1 to 2 hours after transfusion. Although no specific treatment other than supportive measures are indicated, most patients recover without significant sequelae. Finally, one of the most feared complications (at least in the public eye) is the transmission of bacterial/viral infection. Although the risk of hepatitis virus and human immunodeficiency virus (HIV) transmission were unacceptably high in the 1970s and 1980s, the initiation of more stringent screening procedures for high-risk populations and the development of nucleic acid amplification technology (PCR and transcription-mediated amplification) have resulted in dramatically reduced risk and incidence of viral transmission. Currently, the risk of HIV and hepatitis C transmission is approximately 1 in 2 million cases, whereas the risk of hepatitis B is 1 in 200,000. The highest risk of infectivity occurs with platelet transfusion in which bacterial contamination develops at a rate of 1 in 5000 units (Eder et al, 2007).
Patient Environment
Patient Temperature
Although hypothermia can be therapeutic in certain situations of trauma and brain injury, for elective surgical procedures, hypothermia is associated with significantly increased morbidity to the patient. There are two primary reasons for hypothermia to develop in the operating room. Anesthetic agents induce peripheral vasodilation redistributing heat from the core (trunk, head) with resultant drop in immediate core temperature after induction. Throughout the rest of the procedure, radiation and conductive heat loss account for most of the heat loss during a surgical procedure. Normothermia is defined as core temperature between 36° C and 38° C, and even hypothermia of 1° C to 2° C results in adverse effect. Rajagopalan and colleagues performed a meta-analysis of randomized controlled trials and reported that mild hypothermia (decrease of 1°C) resulted in a 16% increase in estimated blood loss and 22% increase in transfusion requirements (Rajagopalan et al, 2008). The increased bleeding risk is thought to result from a hypothermia-associated decrease in clotting cascade enzymatic function and platelet aggregation. Even more significant is the increase in the risk of surgical site infections (SSI) associated with mild hypothermia (34° C to 36° C). Hypothermia increases the risk of SSI by impairing immune mechanisms and vasoconstriction, resulting in regional tissue hypoxia. In a landmark study, Kurz and colleagues with The Study of Wound Infection and Temperature Group tested the hypothesis that hypothermia increases the rate of wound infection and hospital stay in 200 patients undergoing elective colorectal surgery (Kurz et al, 1996). Hypothermia was associated with a three times increased risk of wound infection and a 2.6-day increase in hospitalization. More recent studies have generally confirmed these findings in other series of surgical patients (Mauermann and Nemergut, 2006). In its overall goal of reducing SSI, the SCIP has also included perioperative normothermia as one of its guidelines. Strategies to improvement maintenance of normothermia including regular use of warming blankets, warmed intravenous fluids, warmed irrigation fluids (especially during TURP and other prolonged endoscopic procedures), warmed/humidified CO2 gas during laparoscopy, and increase in ambient operating room temperature. Although there have been few studies in the urologic literature, the findings can be generalized to all surgical patients.
Skin Preparation
Sterile skin preparation is fundamental in the prevention of SSI for any procedure. Currently, the most commonly used skin antiseptics are alcohol, povidone-iodine, or chlorhexidine based. Whichever antiseptic is chosen, the solution should be applied in concentric circles from the center of the surgical site and be allowed to dry before incision. A recent review from the Cochrane database did not find sufficient evidence to recommend one skin preparation over another (Edwards et al, 2004). Furthermore, although the CDC clearly recommends preoperative showering/bathing to reduce SSI, there is no evidence that bathing with an antiseptic solution reduces the rate of infection (Webster and Osborne, 2007). Regarding hair removal, the CDC recommends that if hair removal is performed, it should be performed immediately before the surgical procedure and performed with clippers (rather than shaving) (Mangram et al, 1999).
Patient Safety
In 1991 Brennan and colleagues published their seminal work describing adverse events, defined as injuries caused by medical management in hospitalized patients, revealing that 48% of the events accompanied a surgical operation (Brennan et al, 1991; Leape et al, 1991). This important study inspired the publication of “To Err Is Human, Building a Safer Health System,” a comprehensive study by the Institute of Medicine on medical errors. Regarding surgical patients, the most frequent venue of preventable injuries is the operating room. Although the surgeon is the “captain of the ship” and ultimately responsible, it takes cognizance and attention to detail from each member of the operating room team to prevent iatrogenic injuries to the patient. Three causes of immediately preventable injuries are retractor-associated injuries, thermal injuries, and patient position–related injuries. There are several reports in the literature documenting increased rate of neuropathy (especially femoral nerve) following laparotomy with self-retaining retractors versus without self-retaining retractors (Irvin et al, 2004). Careful attention to be certain that the lateral blades do not directly compress the psoas muscle and only cradle the rectus abdominal muscles will ensure avoidance of femoral neuropathy. Furthermore, periodic reinspection of the retractor blades is also warranted. Many devices used in urologic surgery employ thermal energy for desired effect and therefore can result in thermal injury to the patient. These include Bovie cautery, argon beam coagulator, bipolar devices, and laser. In both endoscopic and laparoscopic surgery, high-wattage light sources are used to illuminate the operative field. While illuminated, the ends of the light cords can result in burns when in direct contact with the patient (even through draping). These light sources should be turned off at all times when not in use. Special mention is deserved for the morbidly obese patient. The operating room should be equipped with a hydraulic table, extra-long instruments, additional padding, wide venous compression devices, and side extensions to ensure a safe operating room environment for the patient.
Patient Positioning
Although often given only a cursory evaluation, proper patient positioning in the operating room can prevent potentially devastating complications. Ultimately, proper positioning is the shared responsibility of each member of the operating room team. Much of the knowledge and guidelines for avoidance of position-related injury are drawn from the anesthesia literature. In fact, in response to a 1999 study of the ASA Closed Claims Database, which found neuropathy as second-leading cause of liability, the ASA published a practice advisory for the prevention of perioperative peripheral neuropathies (Practice advisory, 2000). The recommendations are listed in Table 6–11. Although the exact mechanisms of peripheral neuropathy are not always known, the etiology of position-related neuropathy is generally secondary to excessive stretch, prolonged compression, or ischemia. Given the variety of different patient positions used in urologic surgery, it is critical for the urologist to be an active participant in the positioning of the patient and to understand the potential patient compromise that accompanies each position.
Table 6–11 American Society of Anesthesiologists Task Force Recommendations on the Prevention of Perioperative Peripheral Neuropathies
| Preoperative assessment | When judged appropriate, it is helpful to ascertain that patients can comfortably tolerate the anticipated operative position |
| Upper extremity positioning | Arm abduction should be limited to 90 degrees in supine patients; patients who are positioned prone may comfortably tolerate arm abduction greater than 90 degrees Arms should be positioned to decrease pressure on the postcondylar groove of the humerus (ulnar groove). When arms are tucked at the side, a neutral forearm position is recommended. When arms are abducted on armboards, either supination or a neutral forearm position is acceptable Prolonged pressure on the radial nerve in the spiral groove of the humerus should be avoided Extension of the elbow beyond a comfortable range may stretch the median nerve |
| Lower extremity positioning | Lithotomy positions that stretch the hamstring muscle group beyond a comfortable range may stretch the sciatic nerve Prolonged pressure on the peroneal nerve at the fibular head should be avoided Neither extension nor flexion of the hip increases the risk of femoral neuropathy |
| Protective padding | Padded armboards may decrease the risk of upper extremity neuropathy The use of chest rolls in laterally positioned patients may decrease the risk of upper extremity neuropathies Padding at the elbow and at the fibular head may decrease the risk of upper and lower extremity neuropathies, respectively |
| Equipment | Properly functioning automated blood pressure cuffs on the upper arms do not affect the risk of upper-extremity neuropathies Shoulder braces in steep head-down positions may increase the risk of brachial plexus neuropathies |
| Postoperative assessment | A simple postoperative assessment of extremity nerve function may lead to early recognition of peripheral neuropathies Documentation Charting specific positioning actions during the care of patients may result in improvements of care by (1) helping practitioners focus attention on relevant aspects of patient positioning and (2) providing information that continuous improvement processes can use to effect refinements in patient care |
Adapted from Practice advisory for the prevention of perioperative peripheral neuropathies: a report by the American Society of Anesthesiologists Task Force on Prevention of Perioperative Peripheral Neuropathies. Anesthesiology 2000;92:1168–82.
The supine position, used in abdominal, pelvic, and penile procedures, is generally considered the safest patient position. However, several specific issues should be considered. Excessive upper extremity abduction (>90 degrees) can lead to tension on the brachial plexus, leading to upper extremity neuropathy. The arm board should be padded to avoid excessive pressure on the ulnar groove and spiral groove of the humerus (radial nerve injury). In cases in which the arms are tucked at the patient’s side, care must be taken to avoid excessive pressure on hand and forearm. Moreover, peripheral intravenous catheter infiltration must be identified quickly because forearm compartment syndrome may develop.
One of the most frequent positions used in urology is the lithotomy position. Improper positioning can lead to transient and occasionally prolonged lower extremity neuropathy. In a retrospective evaluation of more than 190,000 cases from 1957 to 1991 involving the lithotomy position, persistent neuropathy was found in 0.03%; however, the same group in a prospective study of 991 patients reported an incidence of 1.5% (15 patients) with resolution of symptoms by 6 months in all but one patient (Warner et al, 1994, 2000). The basic principle of position involves manipulation of both lower extremities simultaneously with flexion of the hips at 80 to 100 degrees with 30- to 45-degree abduction. The legs should be padded to avoid excessive compression against the stirrup. Particular caution should be given to the patient’s hands to avoid entrapment within the moving parts of the stirrups.
For most open and laparoscopic upper urothelial tract and renal procedures, the patient is placed in some degree of lateral decubitus position. Proper padding of the patient is important with appropriate anterior and posterior support to maintain the decubitus position. The most frequent focus of compromise involves positioning of the arms and potential for brachial plexus injury. The ipsilateral arm should be placed on an elevated arm rest or gel pad avoiding abduction more than 90 degrees and excessive stretch on the shoulder. The contralateral arm should be placed on an arm board with ulnar padding. Furthermore, in patients in full flank position, an axillary roll should be placed just caudal to the axilla (not in the axilla) to avoid compression of the contralateral brachial plexus. Finally, after the patient is positioned and before sterile draping, the operating table should be fully rotated to ensure that the patient is adequately secured in all positions.
Two patient positions used in specific urologic cases deserve attention: the prone position for percutaneous nephroscopy and the full Trendelenburg position for robotic-assisted laparoscopic procedures in the pelvis. In the prone position, special care should be taken to pad the torso, elbows, hips, and legs. The anesthesiologist should ensure that the endotracheal tube and all vascular access are properly secured. Coordination of the entire team is required during transfer from the supine position on the stretcher to the prone position on the operating room table. A stretcher should always be available immediately in case of airway compromise and the need for rapid transfer to the supine position. Regarding the full Trendelenburg position for minimally invasive pelvic procedures, the primary issues involve the physiologic changes in respiratory function, cardiovascular function, and increases in central venous and intracranial pressures. Patient positioning should focus on properly securing and padding the patient to the operating table to prevent cephalad sliding. Although fixed shoulder braces will undoubtedly prevent patient movement, these braces should be avoided due to the risk of brachial plexus compression and resultant neuropathy.
Abdominal Incisions and Wound Closure
Abdominal Incisions
Urologic surgery can encompass a large area of the trunk, and therefore the urologist should be familiar with all type of incisions of the abdomen. The most commonly used incision in surgery including urology is the midline abdominal incision. This incision can provide access to the entire peritoneum and retroperitoneum. For procedures focused on particular areas of the abdomen, alternative incisions provide more focused exposure with certain benefits. A Pfannenstiel incision (transverse lower abdominal incision) can be used for virtually all pelvic procedures and results in improved cosmesis and possibly decreased pain. For access to the lower third of the ureter, a Gibson incision (i.e., an oblique incision in the lower quadrant) can be used. Using a Gibson incision, entry into the retroperitoneum is gained by splitting the external and internal oblique muscles in the direction of its fibers. Access to the upper abdomen and retroperitoneum for renal and adrenal surgery can be gained using various kinds of incisions. An extraperitoneal approach is best performed via a flank incision over the 11th or 12th rib, with or without partial rib removal. An extraperitoneal approach avoids the complications of transperitoneal surgery such as bowel injury, postoperative ileus, and adhesion formation. Transperitoneal access can be obtained via an anterior subcostal incision (two finger breadths below the costal margin). This incision provides better access to the midline vascular structures by allowing for complete medial mobilization of the posterior peritoneum. For large or locally advanced (vena cava thrombus) tumors, a thoracoabdominal or chevron incision generally provides the best exposure. A thoracoabdominal approach is preferred for large upper retroperitoneal tumors or tumors with extension into the thoracic cavity (supradiaphragmatic vena cava tumor thrombus). On the other hand, a chevron incision is preferred for access to both the right and left abdomen (e.g., bilateral renal tumors). In summary, proper choice of incision is often critical to successful surgical outcome, especially for complex operative cases.
Wound Healing
Knowledge of the basic principles of wound healing is important in order to properly assess incision closure and its associated complications. All cutaneous wounds progress stepwise through a series of events toward complete wound repair, understanding that in a particular wound, different phases of events may occur simultaneously. The series of steps can be divided broadly into three stages: reactive phase, proliferative phase, and maturational phase. The reactive phase occurs immediately with the two primary responses of hemostasis and inflammation. Disruption of vascular membranes results in platelet activation and aggregation, which in turn initiate the inflammatory response. During this stage of wound healing, inflammatory cells including polymorphonuclear cells, macrophages, and lymphocytes migrate into the wound and become activated, leading to cytokine activation and secretion of various growth factors. The second stage, proliferative, results in the formation of granulation tissue. The stage is characterized by proliferation of endothelial cells and fibroblasts leading angiogenesis and epithelialization, which eventually results in growth of immature blood vessels and deposition of extracellular matrix and early collagen scaffolding. Finally, the maturation stage occurs with further deposition of collagen and wound contraction. The maturational phase begins approximately 1 week after injury and progresses rapidly over 6 weeks with increasing wound strength over the next 12 months. The scar regains approximately 3% strength after 1 week, 20% after 3 weeks, and 80% after 3 months (Witte and Barbul, 1997).
Wound Closure
Along with choice of incision, proper closure is necessary to avoid certain surgical complications including wound infection and fascial dehiscence. In general there are three types of wound closure: primary, secondary, and tertiary (or delayed primary closure). In the vast majority of elective cases, the urologist should attempt a permanent closure following the operation (primary closure). Secondary closure is reserved for heavily contaminated wounds for which the fascia is closed primarily, but the skin/subcutaneous tissues are allowed to heal re-epithelialization and contraction. Tertiary closures are reserved for patients with abdominal compartment syndrome or in patients requiring re-explorations in which temporary closure is initially performed with intention of future permanent closure. Unless the case is heavily contaminated, incision closure involves reapproximation of the fascia (in one or multiple layers) and the skin. Choice of suture type by the surgeon depends on preferences of braided versus nonbraided, monofilament versus multifilament, and absorbable versus nonabsorbable. A full description of different suture types and their properties is listed in Table 6–12 (Hochberg et al, 2009). Although the method of fascial closure has been studied extensively, a definitive, superior method is not universally agreed on. van’t Reit and colleagues performed the most recent meta-analysis of available randomized controlled trials (van ‘t Riet et al, 2002). In all, 6566 patients from 15 studies were included with the primary outcome measure being incidence of incisional hernia. Their analysis indicated that between slowly absorbable or nonabsorbable sutures, there was no difference in risk of incisional hernia in continuous versus interrupted fascial closures although nonabsorbable closure was associated with increased wound pain and sinus formation. For rapidly absorbable suture types, continuous fascial closure was significantly associated with increased rate of incisional hernias. Due to the limited number of patients, a definitive conclusion could not be made for interrupted rapidly absorbable suture closure versus continuous slowly nonabsorbable suture closure. The authors, however, concluded that mass closure with slowly absorbable suture in a continuous fashion is the optimal method. To address the limitation found in the recent meta-analysis, Seiler and colleagues completed a randomized control trial of 625 patients (Seiler et al, 2009). Patients were randomized to one of three arms: interrupted closure with rapidly absorbable suture or continuous closure with one of two different slowly absorbable sutures. They found no significant difference in incisional hernia (15.9% vs. 8.4% vs. 12.2%, P = .09), fascial dehiscence, wound infection, or serious adverse events. In conclusion, although incisions may be closed either with interrupted, rapidly absorbable suture closure or with continuous slowly absorbable suture closure, careful attention should be given to the technique given the relatively high incidence of incisional hernia.
Perhaps the most frequently encountered complication of abdominal wounds is the surgical site infection. Because of different reporting methods and descriptions in the literature, the National Health Care Safety Network of the CDC published definitions of surgical site infections with clearly defined, objective criteria (Kirby and Mazuski, 2009). The infections are classified as follows:
Specific risk factors predisposing for surgical site infections were previously listed in Table 6–4. The mainstay of treatment centers on adequate drainage of the infected area. Superficial infections and some deep incisional infections can generally be managed with opening of the skin incision and packing of the wound. Care should be taken to open the incision widely to ensure complete drainage of underlying purulent fluid. The use of oral/intravenous antibiotics is not necessary unless it is associated with significant skin cellulitis (erythema extending >2 cm from incision edge or systemic signs of toxicity, e.g., fever, sepsis) (Barie and Eachempati, 2005). Once opened, the wound should be allowed to heal by secondary intention. Many deeper incisional infections are too extensive for bedside incision and require operative debridement under anesthesia. It is critical for careful examination of any infected wound for signs of necrotizing infection most commonly secondary to Clostridia perfringens. Signs include drainage of grayish, dishwater-colored fluid, frank necrosis of the fascial layer, and wound crepitus. A necrotizing infection requires immediate return to the operating room for wide debridement and washout. In contrast to incisional infections, deeper organ/space infections may present without superficial signs at the level of the incision. Rather, patients often show systemic signs of infection, pain, and or sepsis leading to cross-sectional imaging revealing the putative source. Again, the principle of adequate drainage applies, and management involves percutaneous or operative drainage of the abscess fluid. A controversial issue in the prevention of organ/space infections is the routine placement of drainage systems at the time of the initial operative procedure. A wide variety of surgical drains is available. Drains are broadly categorized as open/nonsuction, closed/nonsuction, and closed/suction drains. Open/nonsuction drains are employed when accurate quantification of drainage amount is not necessary. These drains are associated with less patient discomfort and are the easiest to remove. Closed drains are chosen if quantification of drainage amounts or characterization of drainage fluid is necessary. The use of suction in closed drain systems is generally preferred if immediate recognition of small amounts of drainage is important (e.g., a drain around an uretero-intestinal anastomosis). Although drains continue to be widely used in various urologic procedures, several prospective studies in the general surgical literature have failed to show significant benefit; therefore most experts caution against their routine use (unless strong indication exists) (Barie, 2002).
Perhaps the most dreaded complication of surgical incisions is acute wound failure (or fascial dehiscence). Overall, the incidence of fascial dehiscence is 1% to 2% and usually occurs 1 week after surgery, although it may occur up to 30 days postoperatively. Risk factors for dehiscence include patient-related factors (advanced age, malnutrition, corticosteroid use, obesity, and prior history of radiotherapy); surgical site infection; and technical errors at the time of wound closure. The most common technical errors associated with wound failure are placing the suture too close to the fascial edge, knot slippage, and excessive suture tension. Multiple studies have investigated the type of closure and there is no difference in dehiscence risk between interrupted and continuous closures as long as rapidly absorbable sutures are not used with the latter. Historically, in high-risk patients retention sutures were strongly advocated and widely used as a preventive measure for wound dehiscence. Literature evidence, however, was primarily retrospective and subsequent prospective trials have failed to show evidence of benefit (Carlson, 1997). One recent trial by Rink and colleagues randomized 95 high-risk patients to retention sutures versus standard closure reporting that although no difference in wound failure rates was seen, retention sutures were associated with significantly increased rate of patient morbidity, primarily pain (Rink et al, 2000). As such, recent surgical trends suggest a movement away from the use of retention sutures and, alternatively, toward the increased use of grafts and synthetic mesh in wounds thought to be at increased risk of fascial dehiscence. When acute wound failure does occur, it is usually immediately preceded by a sudden gush of serosanguineous fluid from the wound. Some small fascial disruptions can be managed conservatively with wound packing and close observation, but the majority of disruptions, particularly in the setting of bowel evisceration, mandate urgent return to the operating room. At the bedside, sterile, saline-moistened towel should be placed over the eviscerated components while the patient is being prepared for the operating room. At the time of reoperation, the fascial edges are inspected for the etiology of the disruption. In cases of technical errors or fascial tearing in which the fascial edges are healthy and can be brought together without tension, a primary closure is appropriate. In all other cases, the fascial edges are debrided and the wound is closed with absorbable mesh or biologic prosthetic grafts. A full discussion of the different materials is beyond the scope of this chapter, but needless to say, strong consideration should be given for an intraoperative general surgical consult.
Key Points
Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370-376.
Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:299S-339S.
Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol. 2002;39:542-553.
Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:381S-453S.
2006 Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198-208.
van ‘t Riet M, Steyerberg EW, Nellensteyn J, et al. Meta-analysis of techniques for closure of midline abdominal incisions. Br J Surg. 2002;89:1350-1356.
Wolf JSJr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
Akhtar S, Silverman DG. Assessment and management of patients with ischemic heart disease. Crit Care Med. 2004;32:S126-S136.
Arozullah AM, Conde MV, Lawrence VA. Preoperative evaluation for posoperative pulmonary complications. Med Clin North Am. 2003;87:153-173.
Barie PS. Are we draining the life from our patients? Surg Infect. 2002;3:159-160.
Barie PS, Eachempati SR. Surgical site infections. Surg Clin North Am. 2005;85:1115-1135.
Biccard BM. Relationship between the inability to climb two flights of stairs and outcome after major non-cardiac surgery: implications for the pre-operative assessment of functional capacity. Anaesthesia. 2005;60:588-593.
Bowater RJ, Stirling SA, Lilford RJ. Is antibiotic prophylaxis in surgery a generally effective intervention? Testing a generic hypothesis over a set of meta-analyses. Ann Surg. 2009;249:551-556.
Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370-376.
Byers PM, Hameed SM. Preoperative and postoperative nutritional support: strategies for enteral and parenteral therapies. In: Asensio J, Trunkey D, editors. Current therapy of trauma and surgical critical care. Elsevier; 2008:710-717.
Carliner NH, Fisher ML, Plotnick GD, et al. Routine preoperative exercise testing in patients undergoing major noncardiac surgery. Am J Cardiol. 1985;56:51-58.
Carlson MA. Acute wound failure. Surg Clin North Am. 1997;77:607-636.
Clarke JS, Condon RE, Bartlett JG, et al. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg. 1977;186:251-259.
Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281-286.
Consensus conference. Fresh-frozen plasma. Indications and risks. JAMA. 1985;253:551-553.
Contant CM, Hop WC, van’t Sant HP, et al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet. 2007;370:2112-2117.
Davenport DL, Bowe EA, Henderson WG, et al. National Surgical Quality Improvement Program (NSQIP) risk factors can be used to validate American Society of Anesthesiologists Physical Status Classification (ASA PS) levels. Ann Surg. 2006;243:636-641. discussion 641–4
de Lissovoy G, Fraeman K, Hutchins V, et al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387-397.
Debaene B, Plaud B, Dilly MP, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology. 2003;98:1042-1048.
Di Cianni S, Rossi M, Casati A, et al. Spinal anesthesia: an evergreen technique. Acta Biomed. 2008;79:9-17.
Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133:299S-339S.
Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol. 2002;39:542-553.
Eagle KA, Brundage BH, Chaitman BR, et al. Guidelines for perioperative cardiovascular evaluation for noncardiac surgery. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. Circulation. 1996;93:1278-1317.
Eder AF, Kennedy JM, Dy BA, et al. Bacterial screening of apheresis platelets and the residual risk of septic transfusion reactions: the American Red Cross experience (2004–2006). Transfusion. 2007;47:1134-1142.
Edwards PS, Lipp A, Holmes A. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev 2004: CD003949.
El-Galley R, Hammontree L, Urban D, et al. Anesthesia for laparoscopic donor nephrectomy: is nitrous oxide contraindicated? J Urol. 2007;178:225-227. discussion 227
Fatalities reported to FDA following blood collection and transfusion: annual summary for fiscal year 2007: Food and Drug Administration, 2007.
Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2007;50:e159-e242.
Fleisher LA, Pasternak LR, Lyles A. A novel index of elevated risk of inpatient hospital admission immediately following outpatient surgery. Arch Surg. 2007;142:263-268.
Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA. 2003;289:187-193.
Forrest JB, Clemens JQ, Finamore P, et al. AUA Best Practice Statement for the prevention of deep vein thrombosis in patients undergoing urologic surgery. J Urol. 2009;181:1170-1177.
Fowkes FG, Lunn JN, Farrow SC, et al. Epidemiology in anaesthesia. III: mortality risk in patients with coexisting physical disease. Br J Anaesth. 1982;54:819-825.
Fukuda K. Opioids. In: Miller RD, Eriksson LI, Fleisher LA, et al, editors. Miller’s anesthesia, vol 2. Churchhill Livingstone; 2009:769-824.
Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133:381S-453S.
Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408-1414.
Goldman L, Caldera DL, Southwick FS, et al. Cardiac risk factors and complications in non-cardiac surgery. Medicine (Baltimore). 1978;57:357-370.
Guenaga KK, Matos D, Wille-Jorgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2009: CD001544.
2002 Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26:1SA-138SA.
Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32:S76-S86.
Hart D, Postlethwait RW, Brown IWJr, et al. Postoperative wound infections: a further report on ultraviolet irradiation with comments on the recent (1964) national research council cooperative study report. Ann Surg. 1968;167:728-743.
Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651-654.
Hochberg J, Meyer KM, Marion MD. Suture choice and other methods of skin closure. Surg Clin North Am. 2009;89:627-641.
Irvin W, Andersen W, Taylor P, Rice L. Minimizing the risk of neurologic injury in gynecologic surgery. Obstet Gynecol. 2004;103:374-382.
Jung B, Pahlman L, Nystrom PO, Nilsson E. Multicentre randomized clinical trial of mechanical bowel preparation in elective colonic resection. Br J Surg. 2007;94:689-695.
Kirby JP, Mazuski JE. Prevention of surgical site infection. Surg Clin North Am. 2009;89:365-389.
Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509.
Kopman AF, Yee PS, Neuman GG. Relationship of the train-of-four fade ratio to clinical signs and symptoms of residual paralysis in awake volunteers. Anesthesiology. 1997;86:765-771.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209-1215.
LaRochelle GEJr, LaRochelle AG, Ratner RE, Borenstein DG. Recovery of the hypothalamic-pituitary-adrenal (HPA) axis in patients with rheumatic diseases receiving low-dose prednisone. Am J Med. 1993;95:258-264.
Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377-384.
Leonardi MJ, McGory ML, Ko CY. The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis: a systematic review of 33 randomized controlled trials. Arch Surg. 2006;141:790-797. discussion 797–9
Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349-361.
Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335:1713-1720.
Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250-278. quiz 279–80
Mauermann WJ, Nemergut EC. The anesthesiologist’s role in the prevention of surgical site infections. Anesthesiology. 2006;105:413-421. quiz 439–40
McDougal WS. Surgical nutrition. American Urological Association Update Series. 1983:16.
Mikami DJ, Beery PR, Ellison EC. Surgery in the pregnant patient. In: Townsend CMJ, Beauchamp RD, Evers BM, Mattox KL, editors. Sabiston’s textbook of surgery. Philadelphia: Saunders Elsevier; 2008:2236-2248.
Monson K, Litvak DA, Bold RJ. Surgery in the aged population: surgical oncology. Arch Surg. 2003;138:1061-1067.
Murkin JM. Anesthesia and hypothyroidism: a review of thyroxine physiology, pharmacology, and anesthetic implications. Anesth Analg. 1982;61:371-383.
1988 NIH Consensus Statement available on perioperative red cell transfusion. Am J Public Health. 1988;78:1588.
O’Leary JG, Friedman LS. Predicting surgical risk in patients with cirrhosis: from art to science. Gastroenterology. 2007;132:1609-1611.
O’Leary JG, Yachimski PS, Friedman LS. Surgery in the patient with liver disease. Clin Liver Dis. 2009;13:211-231.
O’Riordan JM, Margey RJ, Blake G, O’Connell PR. Antiplatelet agents in the perioperative period. Arch Surg. 2009;144:69-76. discussion 76
Pearce AC, Jones RM. Smoking and anesthesia: preoperative abstinence and perioperative morbidity. Anesthesiology. 1984;61:576-584.
Pearse RM, Rhodes A, Grounds RM. Clinical review: how to optimize management of high-risk surgical patients. Crit Care. 2004;8:503-507.
1991 Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325:525-532.
Poirier P, Alpert MA, Fleisher LA, Thompson PD, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation. 2009;120:86-95.
2006 Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198-208.
2002 Practice advisory for preanesthesia evaluation: a report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2002;96:485-496.
2000 Practice advisory for the prevention of perioperative peripheral neuropathies: a report by the American Society of Anesthesiologists Task Force on Prevention of Perioperative Peripheral Neuropathies. Anesthesiology. 2000;92:1168-1182.
Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71-77.
Reinhardt GF, Myscofski JW, Wilkens DB, et al. Incidence and mortality of hypoalbuminemic patients in hospitalized veterans. JPEN J Parenter Enteral Nutr. 1980;4:357-359.
Rink AD, Goldschmidt D, Dietrich J, et al. Negative side-effects of retention sutures for abdominal wound closure: a prospective randomised study. Eur J Surg. 2000;166:932-937.
Scarpa RM, Carrieri G, Gussoni G, et al. Clinically overt venous thromboembolism after urologic cancer surgery: results from the @RISTOS Study. Eur Urol. 2007;51:130-135. discussion 136
Schiff RL, Welsh GA. Perioperative evaluation and management of the patient with endocrine dysfunction. Med Clin North Am. 2003;87:175-192.
Secin FP, Jiborn T, Bjartell AS, et al. Multi-institutional study of symptomatic deep venous thrombosis and pulmonary embolism in prostate cancer patients undergoing laparoscopic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;53:134-145.
Seiler CM, Bruckner T, Diener MK, et al. Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541). Ann Surg. 2009;249:576-582.
Shoemaker WC, Appel PL, Kram HB, et al. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176-1186.
Slim K, Vicaut E, Launay-Savary MV, et al. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg. 2009;249:203-209.
Steinberg JP, Braun BI, Hellinger WC, et al. Timing of antimicrobial prophylaxis and the risk of surgical site infections: results from the Trial to Reduce Antimicrobial Prophylaxis Errors. Ann Surg. 2009;250:10-16.
Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220:1451-1454.
Teh SH, Nagorney DM, Stevens SR, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132:1261-1269.
Townsend CMJ, Beauchamp RD, Evers BM, Mattox KL. Sabiston textbook of surgery. Philadelphia: Saunders Elsevier; 2008.
Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718-729.
Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865-877.
van ‘t Riet M, Steyerberg EW, Nellensteyn J, et al. Meta-analysis of techniques for closure of midline abdominal incisions. Br J Surg. 2002;89:1350-1356.
Vemuri C, Wainess RM, Dimick JB, et al. Effect of increasing patient age on complication rates following intact abdominal aortic aneurysm repair in the United States. J Surg Res. 2004;118:26-31.
Viby-Mogensen J. Neuromuscular monitoring. In: Miller RD, Eriksson LI, Fleisher LA, et al, editors. Miller’s anesthesia, Vol 1. Churchill Livingstone; 2009:1515-1531.
Von Meyenfeldt MF, Meijerink WJ, Rouflart MM, et al. Perioperative nutritional support: a randomised clinical trial. Clin Nutr. 1992;11:180-186.
Warner MA, Martin JT, Schroeder DR, et al. Lower-extremity motor neuropathy associated with surgery performed on patients in a lithotomy position. Anesthesiology. 1994;81:6-12.
Warner MA, Offord KP, Warner ME, et al. Role of preoperative cessation of smoking and other factors in postoperative pulmonary complications: a blinded prospective study of coronary artery bypass patients. Mayo Clin Proc. 1989;64:609-616.
Warner MA, Warner DO, Harper CM, et al. Lower extremity neuropathies associated with lithotomy positions. Anesthesiology. 2000;93:938-942.
Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev 2007: CD004985.
Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509-528.
Wolf JSJr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
Wren SM, Ahmed N, Jamal A, Safadi BY. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg. 2005;140:752-756.
Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868-1874.
Zmora O, Wexner SD, Hajjar L, et al. Trends in preparation for colorectal surgery: survey of the members of the American Society of Colon and Rectal Surgeons. Am Surg. 2003;69:150-154.