chapter 7 Fundamentals of Instrumentation and Urinary Tract Drainage
Endoscopy of the lower urinary tract and the establishment of urinary tract drainage are basic skills required by all urologists and uniquely define the specialty of urology. A basic understanding of the indications, instrumentation used, and the techniques involved in urethral catheterization, ureteral stent placement, and nephrostomy insertion are essential and are covered in this chapter.
Lower Urinary Tract
For Urethral Catheterization
Historical Background
Instruments to drain the urinary bladder are among the most ancient medical devices in the historical record. One of the earliest descriptions of a urinary catheter can be found in the Hippocratic text On Diseases (400 BC), in which bladder drainage was considered a basic skill in the armamentarium of Greek physicians (Moog et al, 2005a). The practice of bladder catheterization to permit urine drainage seems to have been widespread throughout the ancient world, with records of its application also found in India, Egypt, and the Byzantine and Roman empires. In Avicenna’s Canon of Medicine, mention is also made of urethral catheterization as a means to deliver intravesical therapy (Madineh, 2009). Surviving examples of early catheters include hollow tubes made of bronze, paper, animal hide, cloth soaked in wax, and lead (Moog et al, 2005b). In the 16th century, Ambroise Pare used catheters made of silver, brass, and copper. In the early 18th century, natural rubber was first used and the coudé tip was introduced by Mercier not long after. The self-retaining balloon catheter was introduced in the 20th century by Foley (Ellis, 2006), leading the way to permit development of catheters of synthetic materials with specialized coatings and drug-eluting properties.
Indications
The most common indications for the use of a bladder catheter can be broadly divided into two main categories: to obtain drainage or to allow the instillation of diagnostic or therapeutic agents.
The relief of acute or chronic urinary retention due to either bladder outlet obstruction or neurogenic bladder dysfunction is probably the most common indication for urethral catheterization (UC). Following in frequency is its use to monitor urinary output.
Urinary diversion by a catheter is used to allow healing after lower urinary tract surgery/trauma and to evacuate the bladder when the urine contains particulate matter, especially in combination with simultaneous irrigation (post transurethral resection, clot/purulent material evacuation). Other indications include the collection of microbiologic clean urine (uncooperative patients because of age or mental status or comorbidities that prevent voluntary voiding) and to measure postvoid residual urine volume samples for diagnostic purposes.
Urethral catheterization is also used to provide access to the bladder for urinary tract imaging studies such as cystography, which requires the instillation of radiographic contrast material.
UC with a pressure monitoring catheter is used during urodynamic testing for physiologic assessment of voiding function. It is also used to allow instillation of pharmacologic agents for local therapy of some bladder pathologies such as chemo/immunotherapy for transitional cell carcinoma (mitomycin, bacillus of Calmette Guerin), interstitial cystitis (dimethyl sulfoxide), and intractable hematuria (e.g., alum, formalin instillation).
Catheter Selection
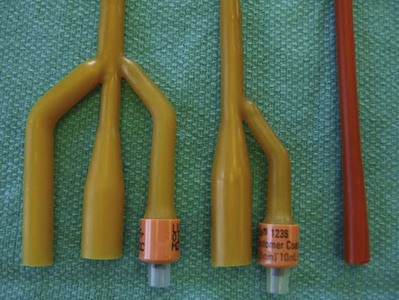
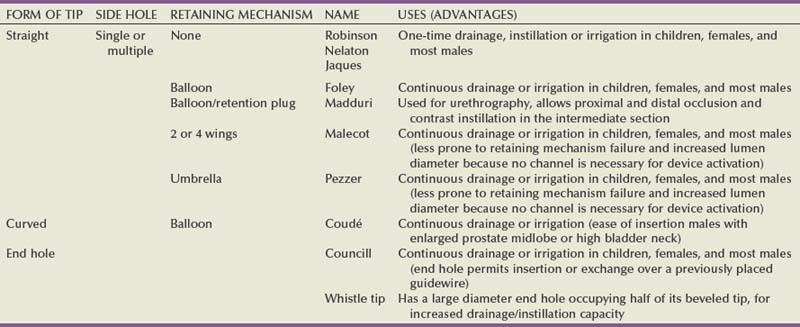
The size and type of urinary catheter used depends on the indication for catheter insertion, age of the patient, and type of fluid expected to be drained. Currently there is a wide variety of catheter types. Catheters can be classified on the basis of material, coating, number of channels (Fig. 7–1), and tip form (Fig. 7–2). Each of these characteristics is reviewed.
Catheter size is measured in the Charrière or French scale, whereby one Fr or Ch is equal to 0.33 mm. This measurement indicates the total circumference of the catheter and not the lumen size. As a general rule, catheter size should be the smallest size that can accomplish the desired drainage (i.e., 12 to 14 Fr for clear urine and 20 to 24 for thick pus or blood-filled urine). In children, choosing a catheter of appropriate size is essential to avoid unnecessary urethral trauma (Table 7–1). The use of feeding tubes as urethral catheters should be discouraged because their stiffness and length can be a source of complications (ischemic ulcers, urethral strictures, and knotting in the bladder) (Smith, 2003; Robson et al, 2006).
Table 7–1 Catheter Size Based on Age
| AGE IN YR | CATHETER SIZE (FR) |
|---|---|
| <5 | 5-8 |
| 5-10 | 8-10 |
| 10-14 | 10 |
| >14 | 10-14 |
Material
Modern urinary catheters are most frequently made of latex, rubber, silicone, and polyvinylchloride (PVC). Rubber and latex catheters are often chosen for short-term drainage. Silicone catheters are indicated when there is rubber/latex sensitivity or allergy and are particularly suited for patients requiring a longer period of indwelling time. Silicone is relatively inert, causing less tissue reaction, and is associated with less bacterial adherence than other catheter materials (Roberts et al, 1990). Evidence suggests that the use of silicone catheters is associated with a lower incidence of urinary tract infections compared with those made of latex (Crnich et al, 2007).
Coating
Various coatings on urethral catheters have been applied in an attempt to reduce urethral trauma and infection risks. The use of hydrophilic coatings has been evaluated among patients on chronic self-intermittent catheterization, with some studies suggesting less discomfort, a decrease in symptomatic urinary tract infection (UTI) rates, and urethral strictures (Wyndaele J, 2002; Vapnek et al, 2003; De Ridder et al, 2005; Bjerklund et al, 2007). Thus far, the evidence is contradictory for the use of antiseptic-coated catheters to prevent the incidence of UTIs, with most of the studies to date having been restricted to small pilot studies (Johnson et al, 2006).
The application of a viable bacterial coating onto catheter surfaces as a method of reducing catheter-associated urinary tract infection (CAUTI) by bacterial interference is a novel approach that has shown promise in a small pilot study involving the use of Escherichia coli–coated catheters. The rationale is based on natural competition by nonpathogenic bacteria overpowering any pathogenic bacteria that may enter the urinary tract (Trautner et al, 2007). Further study is necessary to confirm if this will be an effective strategy.
Number of Channels
The most basic catheters are constructed with a single lumen to permit urinary drainage or irrigation/instillation. Additional lumens are added to permit addition of a retention balloon (two-way catheter) and for simultaneous drainage and irrigation (three-way catheter). The ability to allow bidirectional flow with a three-way catheter is particularly useful when there is need to drain thick fluid such as pus or blood from the bladder because it allows thinning of the solute, preventing reaccumulation by rapid exchange of clean fluid in a washing effect. The insertion of a three-way catheter for continuous bladder irrigation is most often performed after transurethral resection procedures of the bladder or prostate, which additionally can be combined with a large-volume retention balloon (30 to 50 mL), which under slight traction can often achieve complete hemostasis of the resected prostatic fossa. It should be borne in mind, however, that the addition of a multichannel catheter is accomplished by decreasing the overall internal diameter or lumen of the main drainage channel; a 24-Fr three-way catheter has a smaller internal drainage diameter than a 24-Fr two-way, which has a narrower lumen than a 24-Fr one-way catheter.
Tip Shape
Most catheters are designed with a blunt straight tip that is blind ending. Catheters with curved tips or with an end hole have specific utility in certain clinical scenarios (e.g., a coudé tip for patients with a high bladder neck or prominent median prostate lobe, a Councill catheter when catheterization over a guidewire is required). The various catheters and their typical applications are presented in (Table 7–2).
Technique of Catheter Insertion
Once the need for urethral catheterization has been established, the procedure should be described to the patient and potential complications discussed. A focused clinical history including allergies, urologic pathology, previous urologic surgeries, and catheterization attempts may increase suspicion of the potential for difficulties with catheterization.
The patient should be in the supine position at a comfortable height for the physician performing catheterization. In female patients a “frog-leg” position is most suitable, and the use of stirrups can be considered, especially in the obese.
Catheterization should be carried out in a sterile fashion with antiseptic preparation and draping of the patient’s meatal and genital area.
When catheterization for more than just immediate urinary drainage is required, the catheter’s retention balloon should be tested and every catheter should be generously coated with sterile lubricant before insertion.
Anesthetic
The use of topical anesthetic gels before urethral catheterization is widely practiced; however; the evidence to support their use is conflicting. The agent used, temperature of the drug on administration, and indwell times before instrumentation are variable across the studies (McFarlane et al, 2001; Ho et al, 2003; Chung et al, 2007; Garbutt et al, 2008). There is some evidence that cooling to 4° C diminished the discomfort of lignocaine gel instillation, probably due to a cryo-analgesic effect (Thompson et al, 1999; Goel and Aron, 2003). Studies employing 2% lidocaine gel left indwelling at least 15 minutes have been reported to be associated with less pain (Siderias et al, 2004). If topical anesthesia is to be used, evidence suggests it requires a minimum of 10 minutes of exposure (depending on the agent), sufficient volume of the agent (20 to 30 mL), and slow instillation time (>3 to 10 seconds) (Schede and Thüroff, 2006; Tzortzis et al, 2009) to have the most effect.
The systemic absorption of topical urethral lidocaine through intact mucosa is minimal, resulting in low measurable concentrations at doses of up to 550 mg (Ouellette et al, 1985). Rare toxicity has been reported, however, after traumatic procedures that disrupt the mucosal barrier, leading to high and rapid peak concentrations. Seizures, confusion, and disorientation, which usually precede cardiovascular toxicity (Sundaram, 1987; Priya et al, 2005), have been described.
The main benefit of intraurethral anesthetic gel during bladder catheterization may be the additional lubrication providing less traumatic catheter passage and reduced patient discomfort on that basis.
The steps involved in urethral catheter placement obviously vary between males and females. Therefore the techniques for each are described separately. The techniques described pertain to the use of the standard Foley catheter, which is the most commonly used catheter for straightforward bladder drainage.
Male Patients
Anatomic Considerations
The male urethra follows a sigmoid curse, with a proximal curve at the junction of the membranous and bulbar urethra and another at the junction of the bulbar and penile urethra. The adult male urethra is approximately 18 to 20 cm in length, and its diameter is variable, from a mere slit to 6 mm during the passage of urine (Urinary system, 1995).
After sterile skin preparation and draping, grasp the shaft of the penis with the nondominant hand (which is now regarded as contaminated) and hold the penis at a 90-degree angle or perpendicular to the patient. This maneuver eliminates the distal or pendulous urethral curvature. Insert the lubricated tip of the catheter into the urethral meatus and gently but firmly continue to advance the catheter for 7 to 10 cm, while simultaneously bringing the shaft of the penis to the horizontal plane or parallel to the patient. Continue to advance the catheter, while expecting to feel a slight increase in resistance as the membranous urethra (external striated sphincter) is traversed.
Once the entire length of the catheter has been introduced (up to the juncture of the connector or to the two-way bifurcation), wait for spontaneous urine passage, confirming proper placement of the catheter. If spontaneous drainage of urine is not seen, gently press on the patient’s suprapubic area. If despite this maneuver no drainage occurs, slowly instill 20 mL of saline using a catheter-tipped syringe into the drainage port of the catheter and then slowly aspirate the fluid instilled. This maneuver should clear any obstruction of the catheter side hole by lubricant or other material. If the catheter is in the bladder, fluid should be aspirated without resistance. If the catheter is still within the urethra, the negative pressure produced during aspiration will cause collapse of the urethral wall and will not permit the return of the instilled fluid.
Only when the position of the catheter has been verified should the retaining balloon be inflated, with the amount of fluid indicated on the catheter. Most catheters do safely permit twice the indicated amount of fluid without risk of balloon rupture. Sterile water is the preferred solution for balloon inflation. Air is compressible and might leak, and electrolyte or glucose-based solutions can precipitate and occlude the tubing and valve mechanism.
The catheter should be attached to a sterile closed bag system as soon as urine is draining. The drainage bag should be placed below the level of the bladder to encourage one-way gravity flow with the tubing as straight as possible and avoiding kinks that might impair drainage. It has been shown that even the retention of 50 mL of urine in catheterized patients has been associated with an increase in UTIs in up to one third of the patients (Garcia et al, 2007).
The temporal exception to this is in patients with acute urinary retention with significant bladder distension in which rapid bladder drainage might precipitate decompression-induced hematuria or “ex vacuo hematuria.” In these patients the catheter should be intermittently clamped and released to permit gradual bladder decompression over 30 to 60 minutes.
If the patient is uncircumcised, at this point return the foreskin to its normal reduced position to avoid paraphimosis.
Secure the catheter to the patient, allowing for a normal range of motion and without tension, using adhesive tape or a commercial securing device.
Female Patients
Anatomic Considerations
The female urethra is approximately 3.5 to 4 cm long. The meatus is usually in an anterior location and the bladder neck in a posterior location in the horizontal plane, giving the urethra a slight posterior inclination.
After antiseptic preparation and sterile draping, use the nondominant hand to spread the patient’s labia (now considered contaminated) to reveal the urethral meatus. After lubrication, insert the tip of the catheter and gently advance using a slightly downward direction, until about half the length of the catheter has been inserted. Check for urine return and activate the anchoring mechanism if used.
Difficulties during female catheterization may be encountered for several reasons including the inability to locate the urethral meatus due to obesity and age-related changes and less frequently to strictures (postsurgery, radiotherapy, neoplastic causes).
In the obese patient, the use of one or more assistants to provide labial retraction or the use of stirrups can be helpful. In the case of postmenopausal vaginal atrophy or other conditions resulting in the urethral meatus receding into the introitus, we suggest the following alternatives. Holding the index and middle fingers of the nondominant hand together, slowly slide posterior along the introitus until the urethral meatus is palpated and then proceed to slide the fingers just distal to the inferior margin of the meatus. Using the dominant hand, pass the catheter along the groove made by the fingers (this serves a dual purpose—it creates a posterior border with the fingertips and provides a guide for the catheter). As the catheter tip crosses the meatus, it can be felt with the fingertips, thus ensuring proper placement. A second maneuver is to use a vaginal speculum to aid in the retraction and fixation of the introitus. Finally use a coudé tip catheter angled upward and gently slide the tip along the anterior vaginal wall in the midline, until it enters the meatus, and then advance into the bladder.
Special Considerations in Children
Whenever possible the procedure should be explained in clear and age-appropriate language to the child.
Catheterization in children is most commonly performed for drainage, performance of voiding cystourethrogram, or obtaining urine for culture. When attempting to obtain a urine sample for cultures, the use of a portable bladder ultrasound is recommended to ensure that an adequate amount of urine is present in the bladder, thus minimizing the risk of unproductive catheterization (Robson et al, 2006).
In female children the correct identification of the urethral meatus is essential to avoid unnecessary catheter contact with the sensitive introitus, leading to discomfort and possibly loss of cooperation by the child. The meatus is just above the superior margin of the introitus and frequently hidden by the superior portion of the hymen. Gentle downward pressure on the upper aspect of the hymen with a cotton ball may allow visualization of the meatus. Failing this maneuver, the catheter tip should be inserted just above the hymen in the midline.
In uncircumcised boys, retract the foreskin only until the meatus is visible. In infants and children younger than 3 years of age, when the normal foreskin adhesions have not yet involuted, simply align the preputial opening with the meatus to assist catheter insertion.
Difficult Catheterization
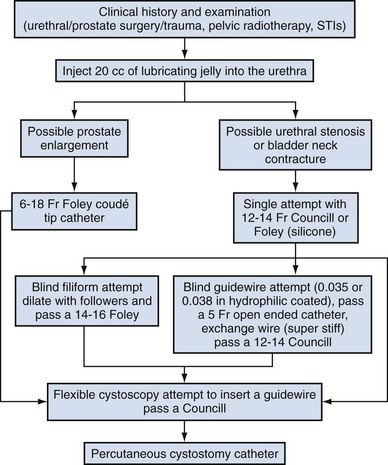
Difficulty inserting a catheter into the bladder is most commonly due to prostatic growth, urethral stricture(s), bladder neck contracture, or false passage from previous urethral instrumentation. Rarely it is the result of phimosis or urethral calculi. Although these difficulties occur mostly in men, the techniques described herein may be applied to place a catheter regardless of gender (Fig. 7–3).
If there is no clinical history of previous sexually transmitted infections (STIs), catheterization, trauma, urethral surgery, or radiotherapy in an adult male over 40 years of age, the most likely cause is prostatic enlargement. Using adequate urethral lubrication and a 16- or 18-Fr coudé tip silicone catheter is often successful in this scenario. If multiple previously unsuccessful attempts have been made and urethral trauma is suspected due to the appearance of a bloody urethral discharge, a false passage or a stricture is likely. A single atraumatic attempt can be made using a 12-Fr silicon/straight or coudé tip catheter. If this maneuver is unsuccessful, then depending on the availability of equipment and the level of experience of the clinician, several other options can be considered. The authors’ preference is to use a flexible cystoscope, allowing a direct visual approach that can be both diagnostic and therapeutic and minimizes the risk of further urethral injury. Under direct vision, the area where the false passage was created or the site of stricture formation is identified and an attempt is made to identify the true urethral lumen. Once identified, a 0.035 in Teflon-coated guidewire (e.g., Bentson type) is passed along the urethral lumen and into the bladder. Depending on the circumstances, urethral dilation may be required as in the situation of a tight urethral stricture or bladder neck contracture. Dilation can be accomplished over the guidewire using Amplatz dilators or purpose-built commercially available urethral dilators (Fig. 7–4A-C). Once the urethral lumen is deemed of adequate diameter to permit placement of the catheter size required, a Councill catheter, which can be advanced over the guidewire into the bladder, is selected.

Figure 7–4 A, Urethral dilators (12 and 18 Fr); B, dilator tip, showing gradual increase of diameter; C, Male urethral dilators.
If flexible instruments are unavailable and a urethral stricture is suspected, then a blind technique. although less desirable, can be used. This technique involves using filiforms and followers (Fig. 7–5).
The first step is to pass a small female filiform catheter. In many cases the initial filiform will frequently end up entrapped in the stricture membrane or in the false passage, rather than following the true lumen. Insertion of additional filiforms will then fill the false passage or stricture membrane until one is successfully directed into the true lumen. Once a filiform is successfully passed, male sections (followers) of increasing size are attached to the filiform to permit sequential dilation of the urethra. Once dilation to at least 14 to 16 Fr has been achieved, a 12- to 14-Fr catheter is then passed.
Alternatively, one can blindly pass a Councill catheter to the point of resistance and then attempt to advance a hydrophilic-coated guidewire. If guidewire advancement seems to go easily, a 5-Fr open-ended or angled ureteral catheter can be advanced over the wire to permit urine drainage and confirm bladder positioning. Once bladder positioning of the guidewire is assured, urethral dilation can be performed over the guidewire followed by Councill catheter placement.
Both blind techniques require a level of experience, especially a tactile sense of what is acceptable resistance during guidewire and filiform insertion. It is worth restating our belief that whenever possible the use of flexible cystoscopy and a direct visual approach are preferable. The use of fluoroscopy is another alternative, but the lack of immediate availability in most settings may logistically limit utility.
If none of these techniques are successful, a suprapubic catheter should then be inserted.
Complications of Urethral Catheterization
UTIs account for 40% of all nosocomial infections. The major risk factor is the use of urethral catheters, which are responsible for up to (80%) of UTIs in the hospital setting (Ha and Cho, 2006). Risk factors for CAUTIs include patients requiring more than 6 days of catheterization, female gender, active nonurinary infection sites, preexisting medical conditions, malnutrition, renal insufficiency, catheter insertion other than in the operating room, and having drainage tubing or a bag elevated above the level of the bladder (Maki and Tambyah, 2001).
Avoidance of unnecessary catheterization, atraumatic technique at insertion, and the use of a closed collection circuit have been shown to decrease the incidence of CAUTI. The use of aseptic technique, although universally practiced and recommended by expert opinion, is not evidence based (Pratt et al, 2007).
The use of antiseptic gels or irrigations of the bladder or collection bag, catheter clamping, or antireflux valves have not been shown to prevent CAUTIs (Phipps et al, 2006; Jahn et al, 2007; Tenke et al, 2008). Multiple trials to date have failed to show that hydrogel-coated catheters have a role in reducing the incidence of infections. The application of antibiotic coatings onto catheter surfaces and the concept of drug elution have shown some promise in preclinical investigations such as the application of nitrofurazone and minocycline/rifampin coatings, which have shown effectiveness in limited trials by reducing the incidence of asymptomatic bacteriuria (Lee et al, 2004; Stensballe et al, 2007). Evidence of the benefit of employing silver alloy coating to catheter surfaces has been conflicting (Davenport and Keeley, 2005; Srinivasan et al, 2006; Schumm et al, 2008). A recent Cochrane review reported a decrease in the rates of asymptomatic bacteriuria; however, whether this equates to a decrease in symptomatic infections is not clear (Jahn et al, 2007). The routine use of catheter coatings is currently not supported by the available literature.
A unique complication of urethral catheterization is the inability to remove the catheter from the bladder. This problem has been reported in the pediatric population when using feeding tubes that can become knotted or entangled. A more frequent occurrence, however, is the inability to remove a straight Foley catheter, either due to encrustation, entrapment by sutures, or inability to disengage/deflate the retaining balloon. The latter problem may be due to a faulty valve, inflation channel blockage, or rarely crystallization within the balloon.
If the catheter has been indwelling for a long period of time, encrustation should be considered and imaging studies (plain film or ultrasound) will be confirmatory. In many instances the encrustation is easily dislodged with gentle traction on the catheter. For more significant encrustations, one can consider using a semirigid ureteroscope and the holmium:YAG laser to remove the stone fragments. If the catheter is quite rigidly fixed and in the setting of recent bladder or prostate surgery, semirigid ureteroscopy along the catheter and using the holmium:YAG to release the suture have also been described (Bagley et al, 1998; Nagarajan et al, 2005). Because the suture materials used in bladder and prostate surgery are often absorbable, waiting for suture dissolution is another option.
An inability to deflate a Foley balloon can be managed using a stepwise approach. One should first attempt to place another 1 to 2 mL of fluid in the balloon to ensure normal balloon contour, which may be important with the large-volume balloons. Failing this maneuver, the next step is to cut the inflation port. If the valve is the source of the problem, balloon deflation should then occur.
If the balloon remains inflated, the problem is along the catheter’s inflation lumen or in the balloon itself. Although overinflation of the balloon has been described in an attempt to rupture the balloon, we do not recommend it because this maneuver may be painful to the patient and may cause bladder injury and fragmentation and retention of the balloon fragments (Gülmez et al, 1996). Similarly, the use of chemical instillations such as ether or toluene to induce balloon rupture should be discouraged because these agents can cause chemical cystitis (Patterson et al, 2006).
Our preferred approach is to insert a surgical steel wire (24 or 28 gauge; often included as an obturator for small-caliber ureteral catheters) or the stiff end of a 0.035-inch hydrophilic-coated guidewire through the valve inflation lumen. This usually bypasses the site of obstruction or creates a small perforation of the balloon.
Should none of these maneuvers be successful, the use of ultrasound-guided needle puncture can be conducted with a long spinal needle (22 gauge) using either a transrectal, transvaginal, or suprapubic surface probe (Daneshmand et al, 2002). In most instances, retained catheters can be removed without the need for open surgery.
Other complications of urethral catheterization include hematuria, urethral and meatal strictures, urethral perforation, and allergic reactions including anaphylaxis (Thomas et al, 2009; Wyndaele, 2002). Especially at risk are patients with long-term indwelling catheters, in whom other complications may also include malignant neoplasms (2.3% to 10%), stone formation (46% to 53%), bladder neck and urethral erosions (Igawa et al, 2008).
Suprapubic Catheterization
Indications
Suprapubic catheter (SC) insertion is most often selected for those patients in whom urethral access is not possible such as complete urethral stenosis, bladder neck contracture, and traumatic urethral disruption. Also, some patients who do not meet these criteria may be candidates for selective SC (Abrams et al, 2008), especially in the context of the need for a chronic indwelling catheter (i.e., neurogenic bladder, poor patient or cooperation/support for clean intermittent catheterization) because some evidence suggests that an SC may be associated with less discomfort than a urethral catheter (McPhail et al, 2006). The evidence that SC reduces the risk for symptomatic bacteriuria is relatively weak (Niël-Weise and van den Broek, 2005). Relative indications for SC placement also include patients who decline urethral catheterization for sexual or self-image purposes.
Indications for short-term SC as a method of postoperative urinary diversion to allow bladder or urethral healing include following surgical procedures (e.g., open prostatic adenectomy, intestinal bladder substitution) or in the pediatric patients following reflux surgery, and during trans urethral prostate resection to theoretically prevent fluid absorption in high risk surgical patients (Sanchez Zalabardo et al, 2003).
Technique (Percutaneous)
The distended bladder should be palpated or percussed to delineate its borders. Failure to palpate the bladder is a relative contraindication to blind percutaneous access techniques and should prompt the clinician to either wait until the bladder is more distended and palpable or use ultrasound imaging guidance for catheter placement (Aguilera et al, 2004). Because of the wide availability of portable ultrasound, ease of use, and added safety over the blind technique, we strongly advocate for its preferential use in percutaneous cystostomy placement to reduce possible complications.
The patient’s infraumbilical abdomen should be prepped and draped in a sterile field. Local anesthesia should be infiltrated into the skin and subcutaneous tissues. A 5- to 10-mm incision is then made 3 to 4 cm above the symphysis pubis in the midline of the abdomen.
Access can then be obtained using a trocar technique, in which a sharp stylet or trocar is used to penetrate the layers of the abdominal wall and bladder. Once entry into the bladder is confirmed by urine flashback or aspiration, the stylet is withdrawn while the catheter is advanced (coaxial system) or the catheter can be fed through the lumen (peel away sheath system). The anchoring mechanism is then activated, the catheter is sutured to the patient’s skin, and a sterile dressing is applied to the site.
An alternative approach employs the Seldinger technique. An 18-gauge hollow needle is advanced under continuous aspiration with a syringe until urine flashback appears, confirming the bladder has been entered. The needle is then advanced 1 cm more, the syringe is detached from the needle, and a floppy-tip guidewire (0.035 or 0.038 in Bentson) is advanced and coiled in the bladder. The needle is withdrawn, a small incision is made on the anterior abdominal fascia, and the tract is coaxially dilated either in one step with a balloon-dilating catheter or with sequential graduated dilators (Fig. 7–6). A 16- to 18-Fr Councill catheter can then be advanced over the guidewire and into the bladder.
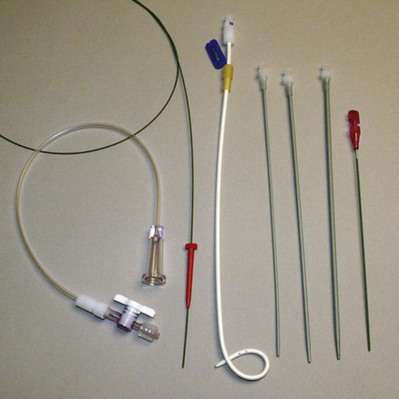
Figure 7–6 Percutaneous suprapubic catheter kit (left to right, guidewire, drainage bag connection tubing, Cope loop catheter, Amplaz dilators, and cystostomy needle).
(Courtesy of Cook Urological, Spencer, IN.)
Contraindications to the use of a blind percutaneous approach are uncorrected coagulopathy, previous lower abdominal surgery, or pelvic radiation (Lawrentschuk et al, 2003). In these situations, an open technique with exposure of the anterior surface of the bladder should be used. The use of a retrograde simultaneous cystoscopy to manually displace the bladder dome and serve as a visual and palpatory reference in both the closed and open technique has been described in an effort to diminish the chance of bowel injury (Alagiri and Seidmon, 1998; Lawrentschuk et al, 2003; Sawant et al, 2009).
Technique (Open)
The open technique entails prepping and draping the anterior abdomen of the patient. An incision is made approximately 2 to 3 cm above superior margin of the symphysis pubis in the midline. Both blunt and sharp dissections are then used to expose the anterior layer of rectus fascia, which is then incised. The Retzius space is developed with blunt dissection, taking care not to enter the peritoneal cavity until the anterior wall of the bladder is identified. Two 1-0 silk stay sutures are used to stabilize the bladder wall, and electrocautery is used to make a 1-cm incision in all layers of the bladder in between the sutures. Urine is drained, and a suprapubic catheter is placed through the abdominal wall by a stab incision, inserted into the bladder, and secured with a purse string or uninterrupted running suture (2-0 absorbable suture). The anchoring device is deployed at this point to avoid entrapment or puncture by the suture, and the anterior rectus fascia, sub-cutaneous tissue, and skin are closed per personal preferences.
Complications
Potential complications associated with suprapubic catheters include those related to initial insertion (hematuria, perivesical fluid collections, surrounding organ injury). With long-term use other adverse events can include catheter blockage/encrustation, dislodgement, skin site infections, symptomatic UTI, urothelial neoplasms, and stone formation (Shiøtz et al, 1989; Sheriff et al, 1998; Berman et al, 2003; Lawrentschuk et al, 2003; Kumar and Pati, 2005; Ahluwalia et al, 2006; Feifer and Corcos, 2008; Sugimura et al, 2008).
Upper Urinary Tract Ureteral Stents
Rudimentary versions of a ureteral stent were first conceived and used in the late 19th and early 20th centuries on the basis of Albarran’s pioneering work to aid in open surgery of the ureter. Zimskind in 1967 was the first to describe endoscopic stent placement to relieve ureteral obstruction. This early era was plagued with frequent stent migration and device expulsion. A novel design developed by Gibbons in the 1970s initially addressed this issue, but it was not until the revolutionary development of the Double J and pigtail stents independently by Finney and Hepperlen and colleagues (1978) that the use of ureteral stents became widespread (Watterson et al, 2002, Chew and Denstedt, 2004).
Ureteral stents have become a fundamental tool in today’s urologic armamentarium and are employed for a number of indications including obstruction relief as a prophylaxis against obstruction or ureteral injury and as use as a ureteral splint.
Indications for Ureteral Stents Placement
Stents are often inserted to relieve either extrinsic (tumor, retroperitoneal fibrosis) or intrinsic ureteral obstruction (stones, tumors, strictures) as a temporary measure while definitive treatment is instituted or as permanent measure when no corrective treatment is possible. In the context of bilateral obstruction, solitary kidney (anatomic or functional), refractory renal colic or obstruction associated with infection (fever, leukocytosis, pyuria) stent placement to secure drainage is often an emergent absolute indication.
Current percutaneous lithotripsy techniques do not require routine stenting. Exceptions to this practice include extensive perforation of the collecting system, significant stone burden remains with the need for subsequent shock wave lithotripsy (SWL), ureteral obstruction due to edema, concurrent ureteropelvic obstruction, stone fragment migration into the ureter, supracostal access, and persistent urinary fistula after nephrostomy tube removal.
The use of routine stenting before SWL is not associated with either a decrease of steinstrasse formation or increase in stone-free rates. However, stenting is clearly associated with increased morbidity. Kirkali and colleagues (1993) reported on 351 patients with renal stones less than 30 mm. The stone-free rate was not significantly different between groups (stented 31% vs. unstented 30%). Half of the patients in the stented group reported bladder discomfort and symptoms that were relieved on removal of the stent. In another report, stented subjects experienced significantly higher incidence and duration of urinary symptoms (urgency, hematuria, bladder discomfort, and frequency) compared with nonstented subjects (Preminger et al, 1989). Indications for pre-SWL stenting are stone burden greater than 2 cm (Libby et al, 1988; Sulaiman et al, 1999; Preminger et al, 2005), when bilateral SWL is to be undertaken (at least one renal unit), and when treating a solitary kidney.
In 2007 a cosponsored American Urological Association/European Association of Urology guideline panel recommended that routine ureteral stent insertion is not necessary after SWL for ureteral calculi because available evidence shows it does not improve patient outcomes (Preminger et al, 2007). Current evidence has also shown that routine stenting after uncomplicated ureteroscopy is unnecessary because unstented patients do not experience an increased risk of complications and have fewer postoperative symptoms (Hosking et al 1999; Rane et al, 2000; Denstedt et al, 2001). Stenting in association with ureteroscopy is recommended in patients with an impacted ureteral calculi, incomplete fragmentation, after formal ureteral dilation, and if there are procedural complications such as ureteral perforation.
Urinary calculi during pregnancy, although uncommon, presents a unique therapeutic challenge. Drainage, if indicated, can be achieved with an internal stent or a percutaneous nephrostomy tube; however, the indwelling time should be limited and exchange is recommended at a 6-week interval due to the increased risk of rapid encrustation triggered by gestational hyperuricosuria and hypercalciuria (Riddell and Denstedt, 2000).
Another indication for stenting (Double J or 5 open-ended ureteral catheter exteriorized and taped to the urethral catheter) is to serve as a surgical landmark for ureteral identification in order to avoid iatrogenic ureteral injury in abdominal or pelvic surgery. Light-emitting ureteral stents have been developed to serve as an additional optical aid, which may be especially helpful in the context of video-assisted surgery (i.e., laparoscopy), in which the tactile sense is diminished.
The use of a stent for ureteral splinting brings us back to the earliest uses of stents. In this setting the stent accomplishes two tasks—one is as a splint or scaffold to promote organized tissue healing and the other equally important role is to allow unhindered urinary flow across the healing segment of the ureter. The main areas where stents are used are following ureteral trauma such as perforation or transection and following ureteral repair (i.e., primary reanastomosis, urinary-intestinal anastomosis, ureterotomy, ureteral reimplantation). One particular indication is kidney transplants because stenting reduces the risk of major urologic complications (obstruction, urinary fistulas) (Wilson et al, 2005) without increasing the risk of infections (Eschwege et al, 1996).
Stents can be used to prevent the occurrence of urinary fistulas (i.e., after percutaneous nephrostolithotomy [PCNL], ureteral anastomosis) or treat them by creating a path of preferential flow away from the defect. Their use is indicated in cases of upper urinary tract fistulas (renal or ureteral) or retroperitoneal urinomas after blunt or open trauma (Haas et al, 1998).
Technique of Ureteral Stent Insertion
The insertion of a ureteral stent can be accomplished via one of several approaches (either open or endoscopic and by antegrade or retrograde techniques). The retrograde approach is most commonly used, often as an adjunct to ureteroscopy or SWL. Although retrograde insertion can be performed with either the aid of flexible or rigid cystoscopy, the need for fluoroscopy and a guidewire are common to both techniques. The safest way to place an internal ureteral stent is by advancing the stent over a guidewire. This can be accomplished by passing a stent that is open at both ends through the working channel of a rigid cystoscope through which a guidewire has been passed and advanced up the ureter to the renal pelvis. The stent is advanced with a pusher while visually observing the ureteral orifice through the cystoscope. Advancement of the stent up the ureter should be monitored fluoroscopically. When the pusher becomes visible at the level of the bladder neck, the wire is extracted, allowing the stent to curl in the bladder.
An alternative technique that relies more on fluoroscopic visualization for stent placement and less on cystoscopic visualization is often advantageous when dealing with more challenging stent placements such as impacted ureteral stones or malignant obstruction (Figs. 7-7 through 7-16).
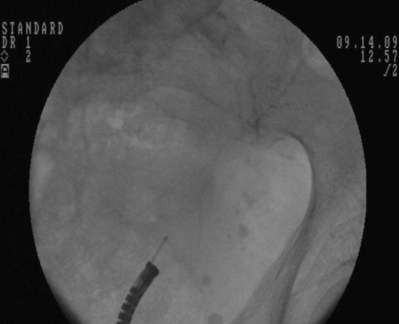
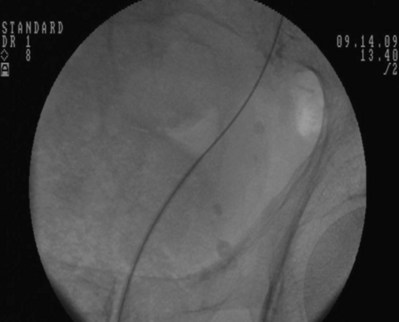
Figure 7–7 Flexible cystoscopy is used to locate and, under fluoroscopic guidance, pass a floppy tip guidewire through the ureteral meatus.
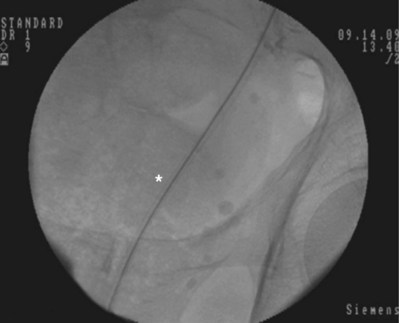
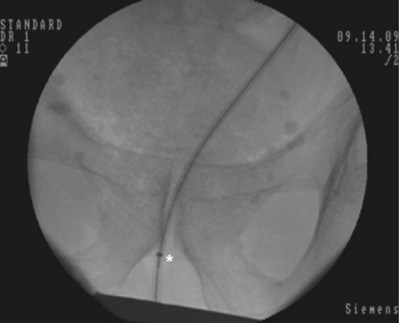
Figure 7–9 The 8 portion has been removed. Asterisk shows the faint outline of the 10 portion along the guidewire.
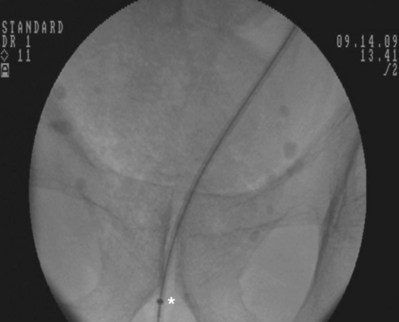
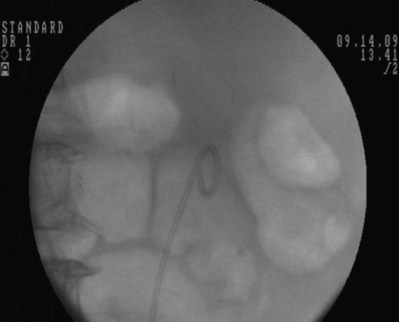
Figure 7–13 A metal tip pusher is used to advance the stent to the desired position. Asterisk shows the pushers radiopaque marker.
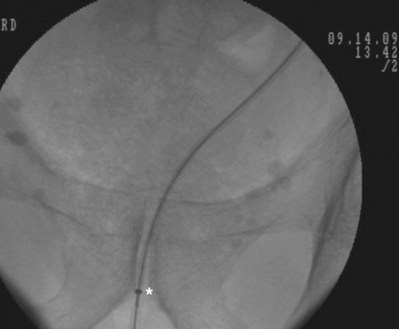
Figure 7–15 While maintaining the tip of the pusher fixed just distally to the inferior border of the pubic symphysis, the 10 portion of the dilator is removed. Asterisk shows the tip of the 10 portion.
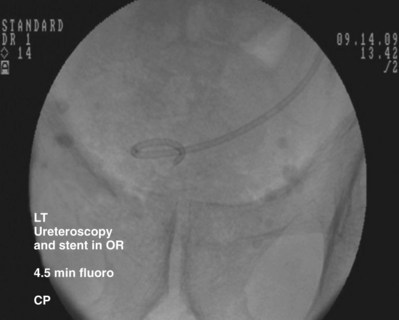
Figure 7–16 While maintaining the pusher’s position, the guidewire is removed to form the bladder coil. Then the pusher is removed.
After obtaining bladder access with either a flexible or rigid cystoscope, a floppy-tip guidewire is advanced into the ureteral meatus and coiled in the renal pelvis using fluoroscopy, the cystoscope is then removed and an 8- to 10-Fr coaxial dilator is advanced under fluoroscopy until the 10-Fr sheath is at the urethral meatus. The 8-Fr internal dilator is removed, leaving a conduit (10-Fr outer sheath) through which to advance the stent.
The ureteral stent is then advanced over the guidewire through the 10-Fr sheath by using a pusher with a small fluoroscopically visible metal band at its tip. The metal tip pusher is advanced under fluoroscopic control to the upper border of the pubic symphysis in male patients and lower border in female patients, while stabilizing the position of the lower end of the stent. The 10-Fr sheath and subsequently the guidewire are removed, allowing the stent to curl in the bladder.
Stent Technology
In the more than 30 years that have elapsed since the introduction of the first self-retaining ureteral stents, there have been ongoing efforts to design a stent with better patient tolerability and biocompatibility. In the following sections we address some of the characteristics of current stents, as well as future trends in stent technology.
The physical characteristics of a stent are dictated by the material employed and design used in its construction. Early stents were manufactured out of glass, tantalum, or vitalum tubes (Wignall and Denstedt, 2008). Polyethylene (PE) was the first plastic polymer used in stents; however, it proved to be fracture prone and has since fallen out of favor. Silicon is the most biocompatible material tested to date; however, due to its less than optimal handling characteristics (high friction coefficient and extreme flexibility) and low radial force, which makes it prone to occlusion (Chew and Denstedt, 2004), it is not widely used. Polyurethane shares characteristics common to both silicon and PE, making a good overall stent material. Various proprietary polymers are now available including C-flex, Silitek, Tecoflex, and Percuflex. These materials have been developed in an effort to increase biocompatibility and handling.
A further evolution in stent materials has been the design of biodegradable stents, which in theory could eliminate the need for cystoscopic stent removal. The main challenge of this technology has been the inability to reliably control the degradation rate. The first attempts used pH-triggered degradation of stent polymers (Shlick and Planz, 1997, 1998). In vitro studies demonstrated an ability to cause stent dissolution, but in vivo studies are lacking. Dissolution of a poly-L-lactic-co-glycolide (PLGA) stent has also been demonstrated in a porcine model for short-term drainage after endopyelotomy (Olweny et al, 2002). Although dissolution was achieved, tissue inflammation was a prominent finding. A poly-L-D-lactide copolymer stent tested in a canine model demonstrated 50% stent dissolution at 12 weeks, 100% dissolution, and no difference in histologic changes when compared with a nondegradable stent at 24 weeks (Lumiaho et al, 2000). Uriprene, a copolyester compound, has been tested in the porcine model showing complete degradation by 10 weeks without a difference in the inflammatory response when compared with conventional stents and without any retained fragments in the subepithelial tissues (Hadaschik et al, 2008). Only one trial to date involving human subjects has been reported using a proprietary polymer material stent (Lingeman et al, 2003). In 88 patients stented postureteroscopy, the stent was effective in providing ureteral drainage in 78.2% of patients with a 7.9% stent-related complication rate, mostly due to proximal/distal migration or fragment retention. Eighty-four percent of the patients were stent free at 30 days and 96.6% at 90 days. Although early in development, tissue-engineering techniques have been used to create stents made from chondrocytes over an absorbable scaffold (Amiel et al, 2001) and may overcome the limitations identified with prior dissolving stents.
The development of metallic mesh stents to combat stent occlusion by radial compression in the setting of chronic extrinsic ureteral obstruction is a relatively recent innovation. These devices can be self-expandable, balloon expandable, mesh, or continuous wire stents with or without thermo-expandable shape memory. Indications to date have primarily been for patients with malignant ureteral obstruction, in whom a conventional stent would not reliably relieve obstruction. The literature to date describing the experience with metallic stents is limited to small series, given the narrow indications for their use (Kulkarni and Bellamy, 2001). Complications reported include failure to relieve obstruction, erosion, encrustation, and migration (Pauer and Lugmayr, 1996; Diaz-Lucas et al, 1997; Arya et al, 2001; Agrawal et al, 2009; Liatsikos et al, 2010). The most recently released metallic stent is the titanium Resonance stent (Cook). Borin and colleagues (2006) reported follow-up of 14 months, with an overall 30% failure rate mostly due to epithelial ingrowth and 24% macroscopic encrustation rate. Garg and colleagues (2009) reported their experience with the use of the Resonance stent in benign conditions (ureteral and ureteroenteric strictures), finding an 89% rate of distal migration in their population. Nagele and colleagues (2008) reported that after a mean 8.6-month follow-up, 57% of the stents had to be removed (urinary symptoms, drainage failure, hematuria, infection, or encrustation).
Stent Design Modifications
Most ureteral stents on the market today are based on a common design consisting of a central lumen, multiple side holes, and a mechanism to limit stent movement (Fig. 7–17). The most commonly used stent is the double pigtail configuration in some variation such as the Double J stent, the dual durometer (softer distal curl and rigid proximal curl), or those with tail shape changes (e.g., Percuflex Tail Plus has a 3-Fr lumen-less soft tail, Polaris Loop has two thinner tail loops) (Dunn et al, 2000; Liatsikos et al, 2002).
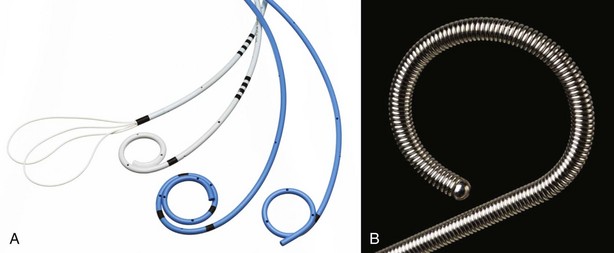
Figure 7–17 A, Types of ureteral stents. B, Metallic ureteral stent.
(A, Courtesy of Boston Scientific Corporation, Natick, MA. B, Courtesy of Cook Urological, Spencer, IN.)
Various design changes to the original standard double-coiled internal ureteral stent have appeared.
One such design modification aimed at improving stone clearance after lithotripsy is in the form of a self-expanding stent (OPEN Pass J) with multiple baskets in the stent body to increase ureteral lumen diameter (up to 6.5 mm) and trap stone fragments until stent removal (L’Esperance et al, 2007).
In an effort to improve urinary drainage in the setting of extrinsic ureteral compression, a double-lumen stent (SP-1 and SP-2 ACMI) demonstrated a lower flow versus two side-by-side 7-Fr stents but greater flow when compared with a single 7-Fr stent when tested in an ex-vivo model (Hafron et al, 2006). The Open-8 and Open-I are stents designed to provide drainage using a similar principle, on an I beam or open-sided omega configuration.
Other designs intended to improve drainage are the grooved stents (Towers Peripheral Ureteral Stent by Cook and Lithostent by ACMI), polyurethane metal-reinforced spiral stents (by Braun) designed to resist extrinsic compression and severe angulation (Tschada et al, 1994), and an externally ridged stent (Spirastent by Urosurge) theoretically aimed at increasing extra luminal flow and stone passage (Stoller et al, 2000).
Task-specific stents such as a stent incorporating a magnetic tip for noncystoscopic retrieval (Magnetip by ACMI) and variable-diameter tapered stents for splinting after endopyelotomy have also been developed.
Another exciting area in stent development involves application of coatings onto stents in an effort to reduce friction, encrustation, bacterial biofilm formation, or patient discomfort. Various coatings have been successfully applied, albeit with varying degrees of clinical effectiveness.
A hydrogel coating made of a hydrophilic polymer film attached to the stent surface allows water absorption, increasing the elasticity and decreasing friction in an attempt to ease insertion and enhance patient comfort.
Bacterial oxalate decarboxylase, monomethoxy poly(ethyleneglycol)-3,4-dihydroxyphenylalanine (mPEG-DOPA3), and diamond-like carbon coatings have all shown promising results in small trials to reduce encrustation or bacterial adherence both in vitro and in vivo (Watterson et al, 2003; Laube et al, 2007; Ko et al, 2008). Data on the use of heparin-coated stents have been conflicting. A recent in-vitro trial showed no difference in either bacterial adherence or biofilm formation (Lange et al, 2009), but small, uncontrolled clinical series (in total >20 patients) demonstrated a significant reduction in both encrustation and biofilm (Riedl et al, 2002; Tenke et al, 2004; Cauda et al, 2008). Additional clinical trials are necessary to fully validate these results. Stickler and colleagues (2002) have developed a phosphoryl-choline (PC)-coated stent in an attempt to mimic cell membrane lipids and to increase biocompatibility. In a 12-week trial involving 44 patients, the PC coating decreased encrustation by 14%, biofilm formation by 40%, and bacterial colonization by 17%.
Although the initial foray into stent coating development has failed to entirely eliminate the problems of stent discomfort and infections, the results have been encouraging enough to stimulate ongoing research in this area. Within the next decade it is likely we will see a number of products for clinical use using drug elution technology.
Stent Complications
Although ureteral stents play an essential role in various endourologic and open surgical procedures, the potential complications related to their use should not be underestimated. In one series, 32% of patients had their ureteral stents removed sooner than planned due to complications attributable to the stent (Ringel et al, 2000).
Stent Symptoms
From a patient perspective, stent morbidity can be significant. Lower urinary tract symptoms such as frequency, urgency, dysuria, and pain (flank or suprapubic) occur almost universally (Haleblian et al, 2008). More than 80% of patients experience stent-related pain affecting daily activities, 32% report sexual dysfunction, and 58% report reduced work capacity (Joshi et al, 2003).
The pathophysiology of these symptoms has not been completely elucidated, but it is most commonly believed that adverse stent symptoms are induced by bladder mucosal irritation due to contact by the distal curl of the stent, smooth muscle spasm, and reflux of urine resulting in flank pain.
The anatomic position of the stent curl has been shown to increase symptoms if it is located in a calyx or if it crosses the midline of the bladder (El Nahas et al, 2006). Rane and colleagues (2001) showed that an incomplete distal curl also increased discomfort. Stent composition does not seem to impact the incidence of symptoms with the currently available stent materials (Pryor et al, 1991).
Multiple pharmacologic approaches have been applied to stent symptoms such as oral anticholinergics, phenazopyridine, and standard analgesic medications (Norris et al, 2008). Intravesical instillation of ketorolac or oxybutynin decreased pain after stent insertion for up to 60 minutes when compared with placebo or to lidocaine (Beiko et al, 2004). The use of αl-adrenoreceptor blockers (tamsulosin and alfuzosin) has demonstrated beneficial effects reducing stent symptoms and pain (Deliveliotis et al, 2006; Damiano et al, 2008).
Urinary Tract Infection
As with any implanted foreign body, stents are a potential target for bacterial colonization and a source of urinary infection. The duration of stent insertion is a significant risk factor with 6.8% of patients becoming infected and 20.5% developing stent colonization at 30 days versus 28.6% developing a UTI and 64.9% stent colonization after 90 days. Predisposing factors include chronic renal failure, diabetes, and female gender (Kehinde et al, 2002).
Migration
Stent migration is reported in 2% to 8% of patients and is usually related to a stent that was incorrectly sized for the patient’s height (Breau and Norman, 2001). Jeon and colleagues (2007) have advocated for the determination of ureteral length measurement using a guidewire rather than patient height to more accurately allow stent length selection. Treatment for distal migration is reinsertion, and in the case of proximal migration the use of a ureteral dilation balloon (Chin and Denstedt, 1992) or ureteroscopy can be used to retrieve the stent.
Forgotten Stent
This is a source of considerable impact on patient well-being and a threat to renal function. Every effort should be made to counsel patients on the importance of returning for follow-up and stent removal. The establishment of a stent registry has also been described as a method of tracking patients in order to avoid the forgotten or neglected stent (Lynch et al, 2007).
Encrustation
Minor degrees of encrustation, particularly on the bladder curl, are not uncommon in many stented patients. More extensive encrustation often related to the forgotten/retained stent can be one of the most challenging tasks for the urologist requiring various endourologic interventions and on occasion open surgery. If left untreated, extreme degrees of encrustation may lead to compromise of the renal unit and even patient mortality (Walther et al, 1985; Singh et al, 2005).
The duration of indwelling time seems to affect the rate of stent encrustation with polyurethane stents (9.2% before 6 weeks, 47.5% in 6 to 12 weeks, and 76.3% after 12 weeks) (El-Faqih et al, 1991). An interesting observation, however, is that not all stents become encrusted, even after long indwell times. In a series of patients who had an indwelling stent for more than 1 year (mean 36 months), Park and colleagues (2004) found this occurred in only 33% of the stents. Reported risk factors are urinary tract infections (especially when involving urease-producing microorganisms) and pregnancy (Vanderbrink et al, 2008). Most manufacturers recommend that indwelling times should be limited to 4 months. Those at heightened risk of accelerated encrustation such as the pregnant patient should have their stents removed within 6 to 8 weeks (Aravantinos et al, 2006).
The discovery of the encrusted stent is often unsuspected until the withdrawal attempt is made and may be faced by the urologist in an office setting where fluoroscopy is not available. In this situation attempts to remove the stent are associated with resistance or even inability to pull the stent without causing severe pain to the patient. At this point no further traction should be attempted to avoid stent fragmentation, ureteral trauma, or even avulsion. Radiologic imaging, ideally with computed tomography (CT), must be arranged to assess the stone burden and proximal coil orientation. It has been our experience that often a kidney, ureter, bladder radiograph underestimates the degree of encrustation.
If no obvious encrustation is seen on CT, stent retrieval can be reattempted under fluoroscopic guidance. The distal end of the stent should be grasped and if possible exteriorized in an attempt to pass a guidewire through the stent lumen. This maneuver may allow straightening of the proximal coil, permitting stent removal.
If gross encrustation is present, additional treatment will be required. Depending on the sites of encrustation and the degree, single or multimodal approaches may be necessary. SWL has been successful for small-volume renal coil calcifications. PCNL for the larger proximal stone burdens is sometimes required. Cystolitholapaxy and ureteroscopy are used for mostly distally located encrustations. The holmium:YAG laser has been particularly useful in these settings. Renal function studies should be ordered in cases of severe gross encrustation. When renal function has been severely compromised, nephrectomy may be most appropriate.
Endourologic treatment either singly or in multiple stages ultimately succeeds in removing up to 85% to 97% of the stents (Bultitude et al, 2003; Gonzalez Ramirez et al, 2009).
Although chemolysis (Suby solution or hemiacidrin) has been reported as an alternative to invasive treatment of encrusted stents, Vanderbrink and colleagues (2008) suggest it should be reserved as a last resort measure due to its irritating effects on the urinary tract and potential electrolytic imbalances if systemic absorption occurs.
Nephrostomy Tube Drainage
The use of a percutaneously inserted nephrostomy tube for the direct drainage of a kidney was first described in 1955 by Goodwin and colleagues. The procedure remained relatively uncommon until 1976, when Fernström and Johansson purposely established a percutaneous tract for the removal of kidney calculi. With the popularity of PCNL, percutaneous nephrostomy access became a common technique in the endourologist’s armamentarium.
Clinical situations occur when a ureteral stent may not provide adequate urinary drainage of the upper urinary tract. Nephrostomy tube (NT) insertion may be a more effective option. As a method of urinary tract drainage, it has been shown in several studies that NTs provide better urine flow in comparison with internal stents. Stented subjects experience both low- and high-pressure vesico-ureteral reflux (Ramsay et al, 1985; Fine et al, 1989; Hübner et al, 1992, 1993) and may not drain at all, especially in the setting of extrinsic ureteral compression (Docimo and Dewolf, 1989). Stents can induce deleterious physiologic and histologic effects on the ureter not observed with NTs. Ryan and colleagues (1994) showed elevated intrapelvic pressures and reduced or absent and ureteral peristalsis, increasing the time it took an artificial stone to traverse the length of the ureter from 3.4 days to 16 days in stented versus unstented ureters, respectively. Microscopic analysis showed collagen deposition and muscular hypertrophy in ureters that were stented.
Nephrostomy Tube Design
Nephrostomy tubes must accomplish the same tasks as standard urethral catheters, mainly to provide unimpaired kidney drainage while being able to remain within the renal cavities resisting accidental dislodging; however, there is also the need to place the catheter blindly through a surgically created tract, so guidance capability is necessary. Councill catheters are commonly used as NTs because they have the previously mentioned qualities. Other catheters used for NT drainage are modified Foley (by cutting an end hole) and Pezzer and Malecot catheters. The latter two have the advantage of having a larger lumen due to their lack of retention balloon tubing.
Re-entry catheters are designed to permit nephrostomy drainage while ensuring access to the ureter should this be necessary (Paul et al, 2003) (Fig. 7–18).
Both the Smith and Hulbert percutaneous endopyelotomy stents (Cook Medical) have a variable diameter starting at 10 to 22 Fr and gradually tapering to 8.2 to 4.7 Fr, distally ending with a pigtail curl. This configuration allows for splinting of the upper urinary tract after endopyelotomy or ureterotomy, while allowing percutaneous drainage/access.
Circle or U-loop NT designs are available to provide continuous irrigation or drug instillation into the kidney while ensuring drainage.
Indications
Short Term
One of the most common indications for acute nephrostomy tube insertion is to establish urinary tract drainage following a failed attempt at retrograde stent insertion. An inability to insert a retrograde stent may be due to several reasons including an impacted ureteral stone or malignant obstruction. In patients with high-grade bilateral ureteric obstruction or obstruction of a solitary renal unit leading to obstructive nephropathy and metabolic derangements, the ability to place the nephrostomy tube in a radiology procedure room under local anesthesia may be the safest and most expeditious way to achieve upper tract drainage. Similarly, the patient presenting with urinary tract sepsis and hemodynamic instability may be a more appropriate candidate for urgent NT insertion rather than an attempt at ureteral stent placement in some circumstances and depending on the local logistics.
An NT is often placed as an adjunct to another procedure such as PCNL to aid hemostasis, drain purulent material or blood clots, and provide future renal access if necessary. Administration of therapeutic drugs (chemolysis, chemotherapy, or immunotherapy) in the context of renal calculi or upper urinary tract malignancy, respectively, is another well described use for an NT.
An NT can also serve a diagnostic role in the performance of the Whittaker test or contrast instillation to clarify complex anatomy (nephrostography).
The insertion of a nephrostomy tube at the completion of PCNL was long considered an essential step of the procedure until recently. Various authors have demonstrated NTs can be safely avoided in select patients (Karami et al, 2007; Shah et al, 2009).
Long Term
It is in the setting of palliative care and extrinsic obstruction where chronic NT tubes might have a significant role. Ku and colleagues (2004) compared NT versus ureteral stent in the setting of advanced extrinsic ureteral obstruction and found stents were exchanged more often than NTs and diversion failure was also more frequent with stents (11% vs. 1.3%). There was no difference in the rate of acute pyelonephritis; however, patient’s self-perception and quality of life were better with the use of chronic NTs.
Complications
NT-related complications can occur at the time of obtaining initial renal access, and additionally those that occur as a result of the nephrostomy tube itself. It is estimated that the overall complication rate associated with NTs is approximately 8.8% to 10%.
Complications related to the access procedure include hematuria; clot colic; and pulmonary injuries (pneumothorax, empyema, hydrothorax or hemothorax), which may be as high as 8% to 12% with upper calyceal approaches and sepsis (1.3% to 2.2%). Rarer complications include injuries to nearby organs (liver, spleen, and bowel) and arteriovenous fistulas. The need for blood transfusion related to the NT insertion alone is reported to be as high as 3.2% in some series (Lewis and Patel, 2004; Wah et al, 2004; Hausegger and Portugaller, 2006).
Biofilm Formation on Urinary Tract Biomaterials
Despite a wide array of novel materials available for the manufacture of urinary catheters, no biomaterial used to date is able to withstand the effects of the urinary environment (Wollin et al, 1998). Biofilms and their related complications are a significant cause of morbidity in the urologic patient requiring a urinary drainage device and remain the most common cause of stent failure (Chew et al, 2006).
A biofilm is the microorganism’s attempt to control its immediate environment by limiting exposure to harmful factors (waste products, antimicrobial agent, the host’s immune response) while enhancing the exposure to trophic factors. The organisms residing in the biofilm can also undergo other adaptative changes such as metabolic dormancy, which may lead to increased antibiotic resistance (Fujiwara et al, 1998; Donlan, 2003). The structure of a biofilm is complex, usually consisting of three layers: the innermost or linking film that is attached to the surface of the tissue or biomaterial, a base film that contains the attached microorganisms, and the outermost layer called the surface film that can be used as a point of access/egress for planktonic organisms (Beiko et al, 2004).
Biofilm structure and the bacterium residing within it behave more like a tissue of a higher organism (Costerton et al, 1995). It has been shown that bacteria within the biofilm can “communicate” with one another and initiate cell dispersal and detachment (Davies et al, 1998). The size of the biofilm can range from a few isolated monolayers to 400 cells deep, effectively covering the complete luminal area of a catheter and reaching a population of up to 5 × 109 colony-forming units (Ganderton et al, 1992).
The initial step in biofilm genesis is the deposition of a layer of urine constituents (proteins, electrolytes, and organic molecules) called a conditioning film. This step usually occurs within minutes after the device has been implanted (Tieszer et al, 1998; Wollin et al, 1998) and modifies the surface of the biomaterial by providing or blocking possible receptor sites for uropathogens. The next step in biofilm formation is microbial adhesion to the surface of the biomaterial, a process influenced by multiple physical properties such as electrostatic and hydrophobic interactions, ionic forces, osmolality and urinary pH (Gristina, 1987; Pratt-Terpstra et al, 1989; Uyen et al, 1989; Printzen, 1996; Reid et al, 1996, 1999). Following this event, the organism undergoes cell division, forming microcolonies that develop inside a matrix of bacterial exopolysaccharide and glycocalix. Once this point is reached, biofilm eradication is usually not possible with anything less than the removal of the involved biomaterial.
Biofilm formation in some cases is a preliminary step to biomaterial encrustation. Urease-producing bacteria cause an increase in urinary pH and, once attached to the biofilm, lead to the precipitation of magnesium and calcium, forming struvite encrustations across the surface (Sofer and Denstedt, 2000).
Thus the development of a biofilm-resistant stent has to achieve success over several factors: a large number of bacterial species able to cause stent-related infections, deposition of host urinary conditioning films that coat and negate the effects of a desirable surface or coating or both, and the microorganism’s ability to destroy and exclude host protection factors and antibiotics and to remain metabolically dormant (Denstedt and Cadieux, 2009).
Without question, the future of urinary catheter development requires a better understanding of biofilm formation and ways to avoid their occurrence. This knowledge along with improvements in biomaterials and in drug elution technology will pave the way for the next evolution in urologic device development.
Beiko DT, Knudsen BE, Watterson JD, et al. Urinary tract biomaterials. J Urol. 2004;171(6 Pt 1):2438-2444.
Cadieux P, Wignall G, Carriveau R. Introduction to biofilms in urology. In: Denstedt J, Atala A, editors. Biomaterials and tissue engineering in urology. Cambridge (UK): Woodhead Publishing Limited and CRC Press LLC, 2009.
Chew BH, Denstedt JD. Technology insight: novel ureteral stent materials and designs. Nat Clin Pract Urol. 2004;1(1):44-48.
Jahn P, Preuss M, Kernig A, et al. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev 2007;(3):CD004997.
Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7(2):342-347.
Tenke P, Kovacs B, Bjerklund Johansen TE, et al. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents. 2008;31(Suppl 1):S68-S78.
Tzortzis V, Gravas S, Melekos MM, de la Rosette JJ. Intraurethral lubricants: a critical literature review and recommendations. J Endourol. 2009;23(5):821-826.
Bagley DH, Schultz E, Conlin MJ. Laser division of intraluminal sutures. J Endourol. 1998;12(4):355-357.
Bjerklund Johansen T, Hultling C, Madersbacher H, et al. A novel product for intermittent catheterisation: its impact on compliance with daily life—international multicentre study. Eur Urol. 2007;52(1):213-220.
Chung C, Chu M, Paoloni R, et al. Comparison of lignocaine and water-based lubricating gels for female urethral catheterization: a randomized controlled trial. Emerg Med Australas. 2007;19(4):315-319.
Crnich CJ, Drinka PJ. Does the composition of urinary catheters influence clinical outcomes and the results of research studies? Infect Control Hosp Epidemiol. 2007;28(1):102-103.
Daneshmand S, Youssefzadeh D, Skinner EC. Review of techniques to remove a Foley catheter when the balloon does not deflate. Urology. 2002;59(1):127-129.
Davenport K, Keeley FX. Evidence for the use of silver-alloy-coated urethral catheters. J Hosp Infect. 2005;60(4):298-303.
De Ridder DJ, Everaert K, Fernández LG, et al. Intermittent catheterisation with hydrophilic-coated catheters (SpeediCath) reduces the risk of clinical urinary tract infection in spinal cord injured patients: a prospective randomised parallel comparative trial. Eur Urol. 2005;48(6):991-995.
Ellis H. The Foley catheter. J Perioper Pract. 2006;16(4):210-211.
Garbutt RB, McD Taylor D, Lee V, Augello MR. Delayed versus immediate urethral catheterization following instillation of local anaesthetic gel in men: a randomized, controlled clinical trial. Emerg Med Australas. 2008;20(4):328-332.
Garcia MM, Gulati S, Liepmann D, et al. Traditional Foley drainage systems—do they drain the bladder? J Urol. 2007;177(1):203-207.
Goel R, Aron M. Cooled lignocaine gel: does it reduce urethral discomfort during instillation? Int Urol Nephrol. 2003;35(3):375-377.
Gülmez I, Ekmekcioglu O, Karacagil M. A comparison of various methods to burst Foley catheter balloons and the risk of free-fragment formation. Br J Urol. 1996;77(5):716-718.
Ha US, Cho YH. Catheter-associated urinary tract infections: new aspects of novel urinary catheters. Int J Antimicrob Agents. 2006;28(6):485-490.
Ho KJ, Thompson TJ, O’Brien A, et al. Lignocaine gel: does it cause urethral pain rather than prevent it? Eur Urol. 2003;43(2):194-196.
Igawa Y, Wyndaele JJ, Nishizawa O. Catheterization: possible complications and their prevention and treatment. Int J Urol. 2008;15(6):481-485.
Jahn P, Preuss M, Kernig A, et al. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev 2007;(3):CD004997.
Johnson JR, Kuskowski MA, Wilt TJ. Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann Intern Med. 2006;144(2):116-126.
Lee SJ, Kim SW, Cho YH, et al. A comparative multicentre study on the incidence of catheter-associated urinary tract infection between nitrofurazone-coated and silicone catheters. Int J Antimicrob Agents. 2004;24(Suppl 1):S65-S69.
Madineh SM. Avicenna’s canon of medicine and modern urology. Part III: other bladder diseases. Urol J. 2009;6(2):138-144.
Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7(2):342-347.
McFarlane N, Denstedt J, Ganapathy S, Razvi H. Randomized trial of 10 mL and 20 mL of 2% intraurethral lidocaine gel and placebo in men undergoing flexible cystoscopy. J Endourol. 2001;15(5):541-544.
Moog FP, Karenberg A, Moll F. The catheter and its use in late antiquity and the early middle ages. J Urol. 2005;174(2):439-441.
Moog FP, Karenberg A, Moll F. The catheter and its use from Hippocrates to Galen. J Urol. 2005;174(4 Pt 1):1196-1198.
Nagarajan M, Weston PM, Biyani CS. Laser division of encircling sutures to remove retained urethral catheter after radical retropubic prostatectomy. J Endourol. 2005;19(1):83-85.
Ouellette RD, Blute RSr, Jaffee S, Bahde C. Plasma concentrations of lidocaine resulting from instillation of lidocaine jelly into genitourinary tract prior to cystoscopy. Urology. 1985;25(5):490-491.
Patterson R, Little B, Tolan J, et al. How to manage a urinary catheter balloon that will not deflate. Int Urol Nephrol. 2006;38(1):57-61.
Phipps S, Lim YN, McClinton S, et al. Short term urinary catheter policies following urogenital surgery in adults. Cochrane Database Syst Rev 2006;(2):CD004374.
Pratt RJ, Pellowe CM, Wilson JA, et al. Epic2: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2007;65(Suppl. 1):S1-64.
Priya V, Dalal K, Sareen R. Convulsions with intraurethral instillation of lignocaine. Acta Anaesthesiol Scand. 2005;49(1):124.
Roberts JA, Fussell EN, Kaack MB. Bacterial adherence to urethral catheters. J Urol. 1990;144(2 Pt 1):264-269.
Robson WL, Leung AK, Thomason MA. Catheterization of the bladder in infants and children. Clin Pediatr (Phila). 2006;45(9):795-800.
Schede J, Thüroff JW. Effects of intraurethral injection of anaesthetic gel for transurethral instrumentation. BJU Int. 2006;97(6):1165-1167.
Schumm K, Lam TB. Types of urethral catheters for management of short-term voiding problems in hospitalised adults. Cochrane Database Syst Rev 2008;(2):CD004013.
Siderias J, Guadio F, Singer AJ. Comparison of topical anesthetics and lubricants prior to urethral catheterization in males: a randomized controlled trial. Acad Emerg Med. 2004;11(6):703-706.
Smith L. Which catheter? Criteria for selection of urinary catheters for children. Paediatr Nurs. 2003;15(3):14-18.
Srinivasan A, Karchmer T, Richards A, et al. A prospective trial of a novel, silicone-based, silver-coated Foley catheter for the prevention of nosocomial urinary tract infections. Infect Control Hosp Epidemiol. 2006;27(1):38-43.
Stensballe J, Tvede M, Looms D, et al. Infection risk with nitrofurazone-impregnated urinary catheters in trauma patients: a randomized trial. Ann Intern Med. 2007;147(5):285-293.
Sundaram MB. Seizures after intraurethral instillation of lidocaine. CMAJ. 1987;137(3):219-220.
Tenke P, Kovacs B, Bjerklund Johansen TE, et al. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents. 2008;31(Suppl 1):S68-S78.
Thomas AZ, Giri SK, Meagher D, Creagh T. Avoidable iatrogenic complications of urethral catheterization and inadequate intern training in a tertiary-care teaching hospital. BJU Int. 2009;104(8):1109-1112.
Thompson TJ, Thompson N, O’Brien A, et al. To determine whether the temperature of 2% lignocaine gel affects the initial discomfort, which may be associated with its instillation into the male urethra. BJU Int. 1999;84(9):1035-1037.
Trautner BW, Hull RA, Thornby JI, Darouiche RO. Coating urinary catheters with an avirulent strain of Escherichia coli as a means to establish asymptomatic colonization. Infect Control Hosp Epidemiol. 2007;28(1):92-94.
Tzortzis V, Gravas S, Melekos MM, de la Rosette JJ. Intraurethral lubricants: a critical literature review and recommendations. J Endourol. 2009;23(5):821-826.
Urinary system. Gray’s anatomy. In Dyson M, editor: Gray’s anatomy, 38th ed, New York: Churchill Livingstone, 1995.
Vapnek JM, Maynard FM, Kim J. A prospective randomized trial of the LoFric hydrophilic coated catheter versus conventional plastic catheter for clean intermittent catheterization. J Urol. 2003;169(3):994-998.
Wyndaele JJ. Complications of intermittent catheterization: their prevention and treatment. Spinal Cord. 2002;40(10):536-541.
Abrams P, Agarwal M, Drake M, et al. A proposed guideline for the urological management of patients with spinal cord injury. BJU Int. 2008;101(8):989-994.
Aguilera PA, Choi T, Durham BA. Ultrasound-guided suprapubic cystostomy catheter placement in the emergency department. J Emerg Med. 2004;26(3):319-321.
Ahluwalia RS, Johal N, Kouriefs C, et al. The surgical risk of suprapubic catheter insertion and long-term sequelae. Ann R Coll Surg Engl. 2006;88(2):210-213.
Alagiri M, Seidmon EJ. Percutaneous endoscopic cystostomy for bladder localization and exact placement of a suprapubic tube. J Urol. 1998;159(3):963-964.
Berman ML, Truong TH, DiSaia PJ, Chan JK. Modified technique for suprapubic catheter insertion that avoids urinary leakage. Am J Obstet Gynecol. 2003;189(6):1670-1671.
Feifer A, Corcos J. Contemporary role of suprapubic cystostomy in treatment of neuropathic bladder dysfunction in spinal cord injured patients. Neurourol Urodyn. 2008;27(6):475-479.
Kumar P, Pati J. Suprapubic catheters: indications and complications. Br J Hosp Med (Lond). 2005;66(8):466-468.
Lawrentschuk N, Lee D, Marriott P, Russell JM. Suprapubic stab cystostomy: a safer technique. Urology. 2003;62(5):932-934.
McPhail MJ, Abu-Hilal M, Johnson CD. A meta-analysis comparing suprapubic and transurethral catheterization for bladder drainage after abdominal surgery. Br J Surg. 2006;93(9):1038-1044.
Niël-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev 2005;(3):CD004203.
Sánchez Zalabardo JM, Sánchez Elipe MA, Regojo Zapata O, et al. Modified technique for transurethral resection of the prostate with suprapubic drainage and local anesthesia. Actas Urol Esp. 2003;27(3):216-220.
Sawant AS, Patwardhan SK, Attar MI, et al. Suprapubic cystostomy using optical urethrotome in female patients. J Endourol. 2009;23(8):1325-1327.
Schiøtz HA, Malme PA, Tanbo TG. Urinary tract infections and asymptomatic bacteriuria after vaginal plastic surgery. A comparison of suprapubic and transurethral catheters. Acta Obstet Gynecol Scand. 1989;68(5):453-455.
Sheriff MK, Foley S, McFarlane J, et al. Long-term suprapubic catheterisation: clinical outcome and satisfaction survey. Spinal Cord. 1998;36(3):171-176.
Sugimura T, Arnold E, English S, Moore J. Chronic suprapubic catheterization in the management of patients with spinal cord injuries: analysis of upper and lower urinary tract complications. BJU Int. 2008;101(11):1396-1400.
Upper Urinary Tract Ureteral Stents
Agrawal S, Brown CT, Bellamy EA, Kulkarni R. The thermo-expandable metallic ureteric stent: an 11-year follow-up. BJU Int. 2009;103(3):372-376.
Amiel GE, Yoo JJ, Kim BS, Atala A. Tissue engineered stents created from chondrocytes. J Urol. 2001;165(6 Pt 1):2091-2095.
Aravantinos E, Gravas S, Karatzas AD, et al. Forgotten, encrusted ureteral stents: a challenging problem with an endourologic solution. J Endourol. 2006;20(12):1045-1049.
Arya M, Mostafid H, Patel HR, et al. The self-expanding metallic ureteric stent in the long-term management of benign ureteric strictures. BJU Int. 2001;88(4):339-342.
Beiko DT, Watterson JD, Knudsen BE, et al. Double-blind randomized controlled trial assessing the safety and efficacy of intravesical agents for ureteral stent symptoms after extracorporeal shockwave lithotripsy. J Endourol. 2004;18(8):723-730.
Borin JF, Melamud O, Clayman RV. Initial experience with full-length metal stent to relieve malignant ureteral obstruction. J Endourol. 2006;20(5):300-304.
Breau RH, Norman RW. Optimal prevention and management of proximal ureteral stent migration and remigration. J Urol. 2001;166(3):890-893.
Bultitude MF, Tiptaft RC, Glass JM, Dasgupta P. Management of encrusted ureteral stents impacted in upper tract. Urology. 2003;62(4):622-626.
Cauda F, Cauda V, Fiori C, et al. Heparin coating on ureteral Double J stents prevents encrustations: an in vivo case study. J Endourol. 2008;22(3):465-472.
Chew BH, Denstedt JD. Technology insight: novel ureteral stent materials and designs. Nat Clin Pract Urol. 2004;1(1):44-48.
Chin JL, Denstedt JD. Retrieval of proximally migrated ureteral stents. J Urol. 1992;148(4):1205-1206.
Damiano R, Autorino R, De Sio M, et al. Effect of tamsulosin in preventing ureteral stent-related morbidity: a prospective study. J Endourol. 2008;22(4):651-656.
Deliveliotis C, Chrisofos M, Gougousis E, et al. Is there a role for alpha1-blockers in treating Double-J stent-related symptoms? Urology. 2006;67(1):35-39.
Denstedt JD, Wollin TA, Sofer M, et al. A prospective randomized controlled trial comparing nonstented versus stented ureteroscopic lithotripsy. J Urol. 2001;165(5):1419-1422.
Díaz-Lucas EF, Martínez-Torres JL, Fernández Mena J, et al. Self-expanding wallstent endoprosthesis for malignant ureteral obstruction. J Endourol. 1997;11(6):441-447.
Dunn MD, Portis AJ, Kahn SA, et al. Clinical effectiveness of new stent design: randomized single-blind comparison of tail and double-pigtail stents. J Endourol. 2000;14(2):195-202.
El-Faqih SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146(6):1487-1491.
El-Nahas AR, El-Assmy AM, Shoma AM, et al. Self-retaining ureteral stents: analysis of factors responsible for patients’ discomfort. J Endourol. 2006;20(1):33-37.
Eschwège P, Blanchet P, Alexandre L, et al. Infectious complications after the use of Double J ureteral stents. Transplant Proc. 1996;28(5):2833.
Garg T, Guralnick ML, Langenstroer P, et al. Resonance metallic ureteral stents do not successfully treat ureteroenteric strictures. J Endourol. 2009;23(7):1199-1201.
Gonzalez Ramirez MA, Mendez Probst CE, Feria Bernal G. Factores de Riesgo y manejo en la calcificacion del cateter doble J. Rev Mex Urol. 2009;69(1):7-12.
Haas CA, Reigle MD, Selzman AA, et al. Use of ureteral stents in the management of major renal trauma with urinary extravasation: is there a role? J Endourol. 1998;12(6):545-549.
Hadaschik BA, Paterson RF, Fazli L, et al. Investigation of a novel degradable ureteral stent in a porcine model. J Urol. 2008;180(3):1161-1166.
Hafron J, Ost MC, Tan BJ, et al. Novel dual-lumen ureteral stents provide better ureteral flow than single ureteral stent in ex vivo porcine kidney model of extrinsic ureteral obstruction. Urology. 2006;68(4):911-915.
Haleblian G, Kijvikai K, de la Rosette J, Preminger G. Ureteral stenting and urinary stone management: a systematic review. J Urol. 2008;179(2):424-430.
Hosking DH, McColm SE, Smith WE. Is stenting following ureteroscopy for removal of distal ureteral calculi necessary? J Urol. 1999;161(1):48-50.
Jeon SS, Choi YS, Hong JH. Determination of ideal stent length for endourologic surgery. J Endourol. 2007;21(8):906-910.
Joshi HB, Stainthorpe A, MacDonagh RP, et al. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol. 2003;169(3):1065-1069.
Kehinde EO, Rotimi VO, Al-Awadi KA, et al. Factors predisposing to urinary tract infection after J ureteral stent insertion. J Urol. 2002;167(3):1334-1337.
Kirkali Z, Esen AA, Akan G. Place of double-J stents in extracorporeal shock wave lithotripsy. Eur Urol. 1993;23(4):460-462.
Ko R, Cadieux PA, Dalsin JL, et al. First prize: novel uropathogen-resistant coatings inspired by marine mussels. J Endourol. 2008;22(6):1153-1160.
Kulkarni R, Bellamy E. Nickel-titanium shape memory alloy Memokath 051 ureteral stent for managing long-term ureteral obstruction: 4-year experience. J Urol. 2001;166(5):1750-1754.
Lange D, Elwood CN, Choi K, et al. Uropathogen interaction with the surface of urological stents using different surface properties. J Urol. 2009;182(3):1194-1200.
Laube N, Kleinen L, Bradenahl J, Meissner A. Diamond-like carbon coatings on ureteral stents–a new strategy for decreasing the formation of crystalline bacterial biofilms? J Urol. 2007;177(5):1923-1927.
L’Esperance JO, Rickner T, Hoenig D, et al. Ureteral expanding stent: a new device for urolithiasis. J Endourol. 2007;21(5):533-537.
Liatsikos E, Kallidonis P, Kyriazis I, et al. Ureteral obstruction: is the full metallic double-pigtail stent the way to go? Eur Urol. 2010;57(3):480-486.
Liatsikos EN, Hom D, Dinlenc CZ, et al. Tail stent versus re-entry tube: a randomized comparison after percutaneous stone extraction. Urology. 2002;59(1):15-19.
Libby JM, Meacham RB, Griffith DP. The role of silicone ureteral stents in extracorporeal shock wave lithotripsy of large renal calculi. J Urol. 1988;139(1):15-17.
Lingeman JE, Preminger GM, Berger Y, et al. Use of a temporary ureteral drainage stent after uncomplicated ureteroscopy: results from a phase II clinical trial. J Urol. 2003;169(5):1682-1688.
Lumiaho J, Heino A, Pietiläinen T, et al. The morphological, in situ effects of a self-reinforced bioabsorbable polylactide (SR-PLA 96) ureteric stent; an experimental study. J Urol. 2000;164(4):1360-1363.
Lynch MF, Ghani KR, Frost I, Anson KM. Preventing the forgotten ureteral stent: implementation of a web-based stent registry with automatic recall application. Urology. 2007;70(3):423-426.
Nagele U, Kuczyk MA, Horstmann M, et al. Initial clinical experience with full-length metal ureteral stents for obstructive ureteral stenosis. World J Urol. 2008;26(3):257-262.
Norris RD, Sur RL, Springhart WP, et al. A prospective, randomized, double-blinded placebo-controlled comparison of extended release oxybutynin versus phenazopyridine for the management of postoperative ureteral stent discomfort. Urology. 2008;71(5):792-795.
Olweny EO, Landman J, Andreoni C, et al. Evaluation of the use of a biodegradable ureteral stent after retrograde endopyelotomy in a porcine model. J Urol. 2002;167(5):2198-2202.
Park K, Jeon SS, Park H, Kim HH. Clinical features determining the fate of a long-term, indwelling, forgotten double J stents. Urol Res. 2004;32(6):416-420.
Pauer W, Lugmayr H. Self-expanding permanent endoluminal stents in the ureter. 5 years results and critical evaluation. Urologe A. 1996;35(6):485-489.
Preminger GM, Assimos DG, Lingeman JE, et alAUA Nephrolithiasis Guideline Panel. Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol. 2005;173(6):1991-2000.
Preminger GM, Kettelhut MC, Elkins SL, et al. Ureteral stenting during extracorporeal shock wave lithotripsy: help or hindrance? J Urol. 1989;142(1):32-36.
Preminger GM, Tiselius HG, Assimos DG, et alEAU/AUA Nephrolithiasis Guideline Panel. 2007 guideline for the management of ureteral calculi. J Urol. 2007;178(6):2418-2434.
Pryor JL, Langley MJ, Jenkins AD. Comparison of symptom characteristics of indwelling ureteral catheters. J Urol. 1991;145(4):719-722.
Rane A, Cahill D, Larner T, et al. To stent or not to stent? That is still the question. J Endourol. 2000;14(6):479-481.
Rane A, Saleemi A, Cahill D, et al. Have stent-related symptoms anything to do with placement technique? J Endourol. 2001;15(7):741-745.
Riddell J, Denstedt JD. Urolithiasis in pregnancy. Cont Urol. 2000;12(3):12-26.
Riedl CR, Witkowski M, Plas E, Pflueger H. Heparin coating reduces encrustation of ureteral stents: a preliminary report. Int J Antimicrob Agents. 2002;19(6):507-510.
Ringel A, Richter S, Shalev M, Nissenkorn I. Late complications of ureteral stents. Eur Urol. 2000;38(1):41-44.
Schlick RW, Planz K. Potentially useful materials for biodegradable ureteric stents. Br J Urol. 1997;80(6):908-910.
Schlick RW, Planz K. In vitro results with special plastics for biodegradable endoureteral stents. J Endourol. 1998;12(5):451-455.
Singh V, Srinivastava A, Kapoor R, Kumar A. Can the complicated forgotten indwelling ureteric stents be lethal. Int Urol Nephrol. 2005;37(3):541-546.
Stickler DJ, Evans A, Morris N, Hughes G. Strategies for the control of catheter encrustation. Int J Antimicrob Agents. 2002;19(6):499-506.
Stoller ML, Schwartz BF, Frigstad JR, et al. An in vitro assessment of the flow characteristics of spiral-ridged and smooth-walled JJ ureteric stents. BJU Int. 2000;85(6):628-631.
Sulaiman MN, Buchholz NP, Clark PB. The role of ureteral stent placement in the prevention of Steinstrasse. J Endourol. 1999;13(3):151-155.
Tenke P, Riedl CR, Jones GL, et al. Bacterial biofilm formation on urologic devices and heparin coating as preventive strategy. Int J Antimicrob Agents. 2004;23(Suppl. 1):S67-S74.
Tschada RK, Henkel TO, Jünemann KP, et al. Spiral-reinforced ureteral stent: an alternative for internal urinary diversion. J Endourol. 1994;8(2):119-123.
Vanderbrink BA, Rastinehad AR, Ost MC, Smith AD. Encrusted urinary stents: evaluation and endourologic management. J Endourol. 2008;22(5):905-912.
Walther PJ, Robertson CN, Paulson DF. Lethal complications of standard self-retaining ureteral stents in patients with ileal conduit urinary diversion. J Urol. 1985;133(5):851-853.
Watterson JD, Cadieux PA, Beiko DT, et al. Oxalate-degrading enzymes from Oxalobacter formigenes: a novel device coating to reduce urinary tract biomaterial-related encrustation. J Endourol. 2003;17(5):269-274.
Watterson J, Cadieux P, Denstedt J. Ureteral stents: which, when, and why. AUA Update Ser. 2002;21(16):122-127.
Wignall GR, Denstedt JD. Ureteral stents. AUA Update Ser. 2008;27(12):102-107.
Wilson CH, Bhatti AA, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev 2005;(4):CD004925.
Nephrostomy Tube Drainage, Nephrostomy Tube Design
Docimo SG, Dewolf WC. High failure rate of indwelling ureteral stents in patients with extrinsic obstruction: experience at 2 institutions. J Urol. 1989;142(2 Pt 1):277-279.
Fernström I, Johansson B. Percutaneous pyelolithotomy. A new extraction technique. Scand J Urol Nephrol. 1976;10(3):257-259.
Fine H, Gordon RL, Lebensart PD. Extracorporeal shock wave lithotripsy and stents: fluoroscopic observations and a hypothesis on the mechanisms of stent function. Urol Radiol. 1989;11(1):37-41.
Goodwin WE, Casey WC, Woolf W. Percutaneous trocar (needle) nephrostomy in hydronephrosis. J Am Med Assoc. 1955;157(11):891-894.
Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006;16(9):2016-2030.
Hübner WA, Plas EG, Stoller ML. The double-J ureteral stent: in vivo and in vitro flow studies. J Urol. 1992;148(2 Pt 1):278-280.
Hübner WA, Plas EG, Trigo-Rocha F, Tanagho EA. Drainage and reflux characteristics of antireflux ureteral double-J stents. J Endourol. 1993;7(6):497-499.
Karami H, Jabbari M, Arbab AH. Tubeless percutaneous nephrolithotomy: 5 years of experience in 201 patients. J Endourol. 2007;21(12):1411-1413.
Ku JH, Lee SW, Jeon HG, et al. Percutaneous nephrostomy versus indwelling ureteral stents in the management of extrinsic ureteral obstruction in advanced malignancies: are there differences? Urology. 2004;64(5):895-899.
Lewis S, Patel U. Major complications after percutaneous nephrostomy—lessons from a department audit. Clin Radiol. 2004;59(2):171-179.
Paul EM, Marcovich R, Lee BR, Smith AD. Choosing the ideal nephrostomy tube. BJU Int. 2003;92(7):672-677.
Ramsay JW, Payne SR, Gosling PT, et al. The effects of double J stenting on unobstructed ureters. An experimental and clinical study. Br J Urol. 1985;57(6):630-634.
Ryan PC, Lennon GM, McLean PA, Fitzpatrick JM. The effects of acute and chronic JJ stent placement on upper urinary tract motility and calculus transit. Br J Urol. 1994;74(4):434-439.
Shah H, Khandkar A, Sodha H, et al. Tubeless percutaneous nephrolithotomy: 3 years of experience with 454 patients. BJU Int. 2009;104(6):840-846.
Wah TM, Weston MJ, Irving HC. Percutaneous nephrostomy insertion: outcome data from a prospective multi-operator study at a UK training centre. Clin Radiol. 2004;59(3):255-261.
Biofilm Formation on Urinary Tract Biomaterials
Beiko DT, Knudsen BE, Watterson JD, et al. Urinary tract biomaterials. J Urol. 2004;171(6 Pt. 1):2438-2444.
Chew BH, Duvdevani M, Denstedt JD. New developments in ureteral stent design, materials and coatings. Expert Rev Med Devices. 2006;3(3):395-403.
Costerton JW, Lewandowski Z, Caldwell DE, et al. Microbial biofilms. Annu Rev Microbiol. 1995;49:711-745.
Davies DG, Parsek MR, Pearson JP, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280(5361):295-298.
Denstedt JD, Cadieux PA. Eliminating biofilm from ureteral stents: the Holy Grail. Curr Opin Urol. 2009;19(2):205-210.
Donlan R. Problems of biofilms associated with medical devices and implants. In: Jass J, Surmann S, Walker J, editors. Medical biofilm: detection, prevention and control. Hoboken, NJ: John Wiley and Sons, 2003.
Fujiwara S, Miyake Y, Usui T, Suginaka H. Effect of adherence on antimicrobial susceptibility of Pseudomonas aeruginosa, Serratia marcescens, and Proteus mirabilis. Hiroshima J Med Sci. 1998;47(1):1-5.
Ganderton L, Chawla J, Winters C, et al. Scanning electron microscopy of bacterial biofilms on indwelling bladder catheters. Eur J Clin Microbiol Infect Dis. 1992;11(9):789-796.
Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588-1595.
Pratt-Terpstra I, Weerkamp A, Busscher H. Microbial factors in a thermodynamic approach of oral streptococcal adhesion to solid substrata. J Coll Interf Sci. 1989;129:568-574.
Printzen G. Relevance, pathogenicity and virulence of microorganisms in implant related infections. Injury.. 1996;27(Suppl. 3):SC9-S15.
Reid G, van der Mei HC, Tieszer C, Busscher HJ. Uropathogenic Escherichia coli adhere to urinary catheters without using fimbriae. FEMS Immunol Med Microbiol. 1996;16(3–4):159-162.
Reid G. Studying bacterial colonization of tubular medical devices. In: An YH, Friedman RJ, editors. Handbook of bacterial adhesion: principles, methods and applications. Humana Press Inc.; 1999:345-352.
Sofer M, Denstedt JD. Encrustation of biomaterials in the urinary tract. Curr Opin Urol. 2000;10(6):563-569.
Tieszer C, Reid G, Denstedt J. Conditioning film deposition on ureteral stents after implantation. J Urol. 1998;160(3 Pt. 1):876-881.
Uyen HM, van der Mei HC, Weerkamp AH, Busscher HJ. Zeta potential and the adhesion of oral streptococci to polymethylmethacrylate. Biomater Artif Cells Artif Organs. 1989;17(4):385-391.
Wollin TA, Tieszer C, Riddell JV, et al. Bacterial biofilm formation, encrustation, and antibiotic adsorption to ureteral stents indwelling in humans. J Endourol. 1998;12(2):101-111.