chapter 11 Prostatitis and Related Conditions, Orchitis, and Epididymitis
Prostatitis and Related Conditions
Historical Aspects
Modern descriptions of the clinical presentation, pathology, and microscopic evaluation of prostate-specific specimens of prostatitis patients were firmly established by Young, Gereghty, and Stevens (1906) by the turn of the 20th century. Bacterial and cytologic localization studies of the lower urinary tract were described shortly thereafter (Hitchens and Brown, 1913) and standardized by 1930 (Von Lackum, 1927, 1928; Nickel, 1930, 1999a). The primary form of therapy for prostatitis during most of the 20th century was repetitive prostate massage (Farman, 1930; O’Conor, 1936; Henline, 1943; Campbell, 1957). With the introduction of sulfanilamide in the 1930s, antimicrobial therapy became the main therapeutic approach (Ritter and Lippow, 1938). However, even in the 1950s and 1960s, the significance of inflammatory cells and bacteria in the expressed prostatic secretion (EPS) was questioned (O’Shaughnessy et al, 1956; Bowers and Thomas, 1958; Bourne and Frishette, 1967), and it was even recognized that, in many cases, antibiotics were performing little better than placebo in the treatment of prostatitis (Gonder, 1963).
The next era of prostatitis management began in the 1960s with Meares and Stamey’s (1968) description of the four-glass lower urinary tract segmented localization study. Prostatic massage as the mainstay of prostatitis therapy was abandoned, and antimicrobial therapy was rationalized for the very small percentage of patients with bacteria localized to prostate-specific specimens. Unfortunately the vast majority of patients who were diagnosed with a nonbacterial cause continued to suffer the indignities of dismal urologic management (Nickel, 1998a). The establishment of new definitions and a classification system, better understanding of the etiopathogenesis, completion of randomized placebo-controlled trials with validated outcome indices, and the evolving insight that patients with prostatitis have variable clinical phenotypes has radically changed the way this condition is managed.
Epidemiology
Prostatitis is the most common urologic diagnosis in men younger than age 50 years and the third most common urologic diagnosis in men older than age 50 years after benign prostatic hyperplasia (BPH) and prostate cancer (McNaughton Collins et al, 1998). There were almost 2 million U.S. physician visits annually from 1990 to 1994 with prostatitis listed as the diagnosis (McNaughton Collins et al, 1998), Approximately 5% of visits to U.S. urologists were reported to be for inflammatory diseases of the prostate (Schappert, 1994). In the mid 1990s urologists in Wisconsin in the United States saw an average of 173 patients with prostatitis per year (Moon, 1997) whereas Canadian urologists reported an average of 264 patients with prostatitis per year, with 38% of whom newly diagnosed (Nickel et al, 1998a). Based on both a physician survey study in Dane County, Wisconsin (Moon, 1997), and a survey of younger men from a Wisconsin National Guard unit (Moon et al, 1997), it was estimated that 5% of men (aged 20 to 50 years) have a history of prostatitis. In the Netherlands, de la Rosette and colleagues (1992a) noted that 4% of respondents reported a history of prostatitis, whereas researchers in Finland reported a 14% overall lifetime prevalence in that country (Mehik et al, 2000). McNaughton Collins and colleagues (2002) noted that 16% of health care professionals in the United States report either a previous or current diagnosis of prostatitis. Roberts and associates (1998) reviewed the medical records of 2113 men from July 1992 through February 1996 (a median of 50 months’ follow-up) in the Olmsted County community-based cohort in Minnesota and found the overall prevalence of a physician’s diagnosis of prostatitis was 9%.
Many of these epidemiologic studies are limited by physicians’ or patients’ long-term recollection and the unreliability of a physician’s coding or diagnosis of prostatitis. Population-based studies employing the validated National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) (Litwin et al, 1999) to determine the prevalence of prostatitis-like symptoms in the general population of men showed widely variant results (12.2% in Nigeria [Ejike and Ezeanyika, 2008], 8.0% in Malaysia [Cheah et al, 2003], 6.6% in Canada [Nickel et al, 2001b], 2.7% in Singapore [Tan et al, 2002], and approximately 2.2% in older men in Olmsted County [Roberts et al, 2002]). Employing NIH-CPSI definitions of prostatitis-like symptoms in a managed care population, Clemens and colleagues (2006) estimated that between 5.9% and 11.2% have prostatitis-like symptoms depending on the specific definition. Whereas retrospective billing data studies have indicated that up to 8% of U.S. male patients seen in urologic outpatient practice have a diagnosis of prostatitis (McNaughton Collins et al, 1998), prospective practice audit studies have shown that as many as 12% of men presenting to Italian urologists are diagnosed with prostatitis (Rizzo et al, 2003), whereas only 2.8% of men had a clinical diagnosis of prostatitis in a similar study carried out in Canada (Nickel et al, 2005c). Symptoms of prostatitis wax and wane, with approximately one third to one half of patients experiencing relief of symptoms over a 1-year period (Nickel et al, 2002b; Turner et al, 2004b, Propert et al, 2006b).
It has traditionally been believed that prostatitis is a disease of younger men (e.g., aged 35 to 50 years). Epidemiologic studies described in this section confirm that prostatitis affects men of all ages, unlike BPH and prostate cancer, which are predominantly diseases of older men. Compared with men aged 66 years and older, a diagnosis of prostatitis is 1.6-, 2.6-, and 2.1-fold greater in men aged 18 to 35, 36 to 50, and 51 to 65 years, respectively, in the Olmsted County study (Roberts et al, 1998). The age-specific prevalence of a physician-assigned diagnosis of prostatitis was highest in patients between the ages of 20 and 49 years and increased again in those older than 70 years. The cumulative probability of having a diagnosis of prostatitis (acute or chronic) by 85 years of age was 26%. In the Canadian prevalence study (Nickel et al, 2001b) there was no significant difference in the prevalence of prostatitis-like symptoms in men younger than 50 years (11.5% reported at least mild symptoms over the previous week) compared with men older than 50 years (8.5% reported at least mild symptoms over the previous week). As many as 20% of men diagnosed with BPH also report prostatitis-like symptoms (Nickel 2005a). Interestingly, although prostatitis symptoms appear to be as prevalent in older versus younger males they are reported to be less distressing with age (Hedelin and Jonsson, 2007).
Chronic prostatitis is associated with substantial costs and significant predicted resource consumption (Calhoun et al, 2004; Turner et al, 2004a; Duloy et al, 2007). Overall spending in the United States for the diagnosis and management of prostatitis, exclusive of pharmaceutical spending, totaled 84 million dollars in 2000 and appears to be increasing (Pontari et al, 2007). This economic factor needs increased attention when evaluating the incidence and treatment of this prevalent condition.
Histopathology
For the pathologist, prostatitis is defined as an increased number of inflammatory cells within the prostatic parenchyma (Cotran et al, 1999). Prostatic inflammation may or may not be noted in patients with a diagnosis of prostatitis (True et al, 1999), BPH (Nickel et al, 1999c), or prostate cancer (Zhang et al, 2000) and is noted in autopsy series in as many as 44% of prostate tissue samples from men without any definitive prostate disease (McNeal, 1968).
Key Points
Epidemiology
A consistent description of fairly distinct, although often coexisting, patterns of chronic inflammation can be found in the prostate gland of patients with or without prostate disease. The most common pattern of inflammation is a lymphocytic infiltrate in the stroma immediately adjacent to the prostatic acini (Kohnen and Drach, 1979; Nickel et al, 1999b). The intensity of the inflammatory process varies considerably from only scattered lymphocytes to dense lymphoid nodules. Stromal lymphocytic infiltrates frequently coexist with periglandular inflammation. Sheets, clusters, and occasional nodules of lymphocytes and scattered plasma cells are seen within the fibromuscular stroma with no apparent relationship to the ducts and acini. Infiltrates of inflammatory cells restricted to the glandular epithelium and lumen are found in association with prostatitis and BPH but can be found in asymptomatic patients. The intraepithelial inflammatory cells may be neutrophils, lymphocytes, or macrophages, or all of these, whereas neutrophils and macrophages are typically found in the lumen. Figure 11–1 illustrates the various inflammatory patterns seen in a prostate specimen of a patient with chronic prostatitis.
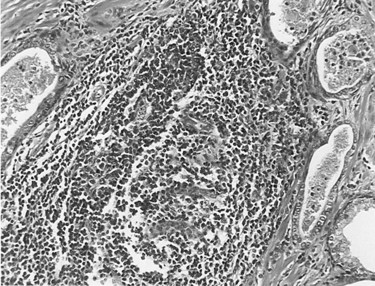
Figure 11–1 Histologic preparation of a prostate specimen demonstrating areas of glandular, periglandular, and stromal inflammation (×400).
(Courtesy of Dr. Alexander Boag.)
Corpora amylacea, which may develop from the deposition of prostatic secretions around a sloughed epithelial cell or other irritant, are not usually associated with inflammation unless they become large enough to distend or obstruct the prostatic gland (Attah, 1975). Prostatic calculi may contribute to prostatic inflammation by obstructing central prostate ducts and thus preventing drainage or providing a nidus in which bacteria can survive host defenses and antibiotics (Meares, 1974; Roberts et al, 1997).
Granulomatous prostatitis presents a nonspecific and variable histologic pattern typified by heavy lobular, mixed, inflammatory infiltrates that include abundant histiocytes, lymphocytes, and plasma cells. Small, discrete granulomas may be present, or the pattern may be typified by well-defined granulomas. Granulomatous prostatic inflammation is a common consequence of surgery (Eyre et al, 1986) or therapy with bacillus Calmette-Guérin (BCG) (Lafontaine et al, 1997) and a rare event in patients with systemic tuberculosis (Saw et al, 1993).
A consensus group of urologists and pathologists have developed a classification system to describe histologic inflammatory patterns in the prostate (Nickel et al, 2001d), but it is only useful for comparative research purposes.
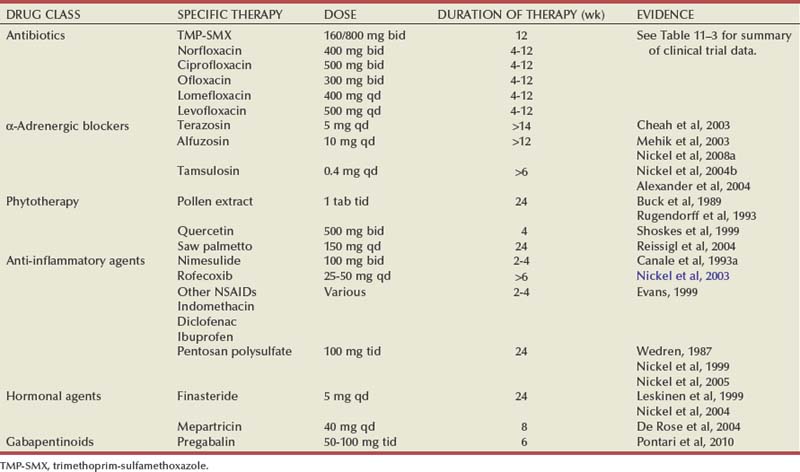
Etiology
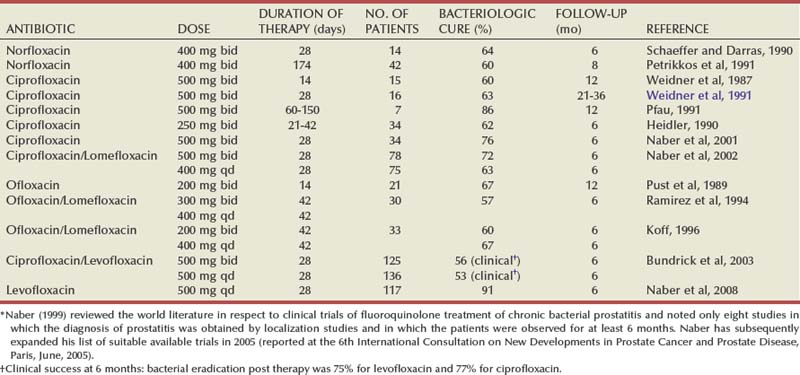
Microbiologic
Gram-Negative Uropathogens
Acute bacterial prostatitis is a generalized infection of the prostate gland and is associated with both lower urinary tract infection (UTI) and generalized sepsis. Chronic bacterial prostatitis is associated with recurrent lower UTIs (i.e., cystitis) secondary to areas of focal uropathogenic bacteria residing in the prostate gland. The most common cause of bacterial prostatitis is the Enterobacteriaceae family of gram-negative bacteria, which originate in the gastrointestinal flora. The most common organisms are strains of Escherichia coli, which are identified in 65% to 80% of infections (Stamey, 1980; Lopez-Plaza and Bostwick, 1990; Weidner et al, 1991b; Schneider et al, 2003). Pseudomonas aeruginosa, Serratia species, Klebsiella species, and Enterobacter aerogenes are identified in a further 10% to 15% (Meares, 1987; Weidner et al, 1991b). However, in acute bacterial prostatitis, the organisms that result from previous manipulation of the lower urinary tract show different patterns of virulence and resistance (e.g., to ciprofloxacin and cephalosporins) compared with the organisms associated with spontaneous acute prostatitis (Millan-Rodriquez et al, 2006; Ha et al, 2008).
Urovirulence factors play a significant role in the pathogenesis of bacterial prostatitis (Ruiz et al, 2002; Johnson et al, 2005). For instance, bacterial P-fimbria (or pili) bind to the urothelial receptors, and this subsequently facilitates ascent into the urinary tract as well as establishing deep infections in the prostate gland itself (Dilworth et al, 1990; Neal et al, 1990; Andreu et al, 1997). Colonization of the lower urinary tract by E. coli is also facilitated by the presence of the type 1 fimbria, also known as mannose-sensitive fimbria. The receptor is a common moiety of the uroepithelial uromucoid; this association has been shown to be important in the development of cystitis in humans, and its presence in prostatitis has also been documented (Correll et al, 1996). Phase variation of type 1 pili during the establishment of acute bacterial prostatitis may occur in the setting of prostatitis (Schaeffer, 1991). Multiple virulence factors appear to be necessary to produce prostatitis (Mitsumori et al, 1999; Ruiz et al, 2002). Bacteria reside deep in the ducts of the prostate gland and when threatened, with host defense and antimicrobial therapy, tend to form aggregates (also called biofilms), which appears to be a protective mechanism allowing bacteria to persist in the prostate gland even when the cystitis is treated with antibiotics (Nickel and Costerton, 1993; Nickel et al, 1994). Hemolysin appears to be a virulence factor associated with E. coli acute prostatitis, but hemolysin may also be associated with increased ability of certain strains of E. coli to persist in the prostate as biofilms in patients with chronic bacterial prostatitis (Soto et al, 2007).
Gram-Positive Bacteria
Enterococci are believed to account for 5% to 10% of documented prostate infections (Drach, 1974a; Meares, 1987; Bergman, 1994). The role of other gram-positive organisms, which are also commensal organisms in the anterior urethra, is controversial (Jimenez-Cruz et al, 1984; Fowler and Mariano, 1984b, Krieger et al, 2002). An etiologic role for gram-positive organisms such as Staphylococcus saprophyticus, hemolytic streptococci, Staphylococcus aureus, and other coagulase-negative staphylococci has been suggested by a number of authors (Drach, 1974a, 1986; Bergman, 1994). Nickel and Costerton (1992) have shown coagulase-negative Staphylococcus to be present in the EPS as well as transperineal prostate biopsy tissue of men with chronic prostatitis (microscopy and culture). Although this and other studies (Carson et al, 1982; Pfau, 1983; Bergman et al, 1989; Wedren, 1989) suggested that coagulase-negative staphylococci are involved in the pathogenesis of chronic prostatitis, these studies did not conclusively demonstrate that these bacteria were actually causing the inflammation and symptom-complex rather than simply colonizing the prostate (Krieger et al, 2002). However, eradication of gram-positive bacteria in the prostate of men experiencing recent onset of prostatitis symptoms resulted in similar clinical results compared with men with gram-negative uropathogens localizing to the prostate (Magri et al, 2007a; Nickel and Xiang, 2008). In both cases, eradication of the bacteria localized to the prostate was strongly correlated with a good clinical outcome. However, the inconsistent localization of gram-positive bacteria in prostate-specific specimens from patients with chronic prostatitis suggests that this relationship may not be as strong as suggested (e.g., Krieger et al, 2005).
Anaerobic Bacteria
In studies in which the prostate-specific specimens were cultured anaerobically, anaerobic bacteria could be identified in a small number of patients (Nielsen and Justesen, 1974; Mardh and Colleen, 1975; Szoke et al, 1998). This has not been a consistent finding, and the role of anaerobic bacteria is essentially unknown.
Corynebacterium Infection
Corynebacterium species have usually been acknowledged as prostate nonpathogens but have been suggested as potential etiologic agents in this disease (Riegel et al, 1995; Domingue, 1998). Domingue and colleagues (1997) suggested that these difficult-to-culture coryneforms could be missed by routine culturing of EPS. Direct Gram staining of the EPS showed gram-variable pleomorphic coccobacillary rods that do not usually grow on routine media. The presence of these pleomorphic swollen rods was also shown by fluorescent acridine orange staining. Tanner and associates (1999), using polymerase chain reaction (PCR) techniques, were able to identify a bacterial signal (phylogenetically gram-positive organisms with predominance of Corynebacterium species) in 65% of 17 patients with chronic prostatitis. Approximately half of these patients tended to respond to antimicrobial therapy, whereas patients in whom molecular signals for these bacteria could not be identified did not.
Chlamydial Infection
The evidence supporting the role of Chlamydia trachomatis as an etiologic agent in chronic prostatic inflammation is both confusing and conflicting. Mardh and Colleen (1972) found that one third of men with chronic prostatitis had antibodies to C. trachomatis compared with 3% of controls. Shortliffe and coworkers (1992) found that 20% of patients with nonbacterial prostatitis had antichlamydial antibody titers in the prostatic fluid. Koroku and associates (1995) detected C. trachomatis–specific immunoglobulin A (IgA) in 29% of men with chronic nonbacterial prostatitis. Bruce and colleagues (1981) found that 56% of patients with “subacute or chronic prostatitis” were infected with C. trachomatis (after examining early morning urine, prostatic fluid, or semen specimens). In a follow-up study, Bruce and Reid (1989) found that 6 of 55 men with abacterial prostatitis, including 31 believed to have chlamydial prostatitis, met strict criteria for positive diagnosis for chlamydial prostatitis based on identification of the organisms by culturing or immunofluorescence. Kuroda and colleagues (1989) identified C. trachomatis in the urethras of 20% of men with prostatitis. Other investigators have come to similar conclusions (Nilsson et al, 1981; Weidner et al, 1983). Chlamydia has also been isolated in prostate tissue specimens. Poletti and coworkers (1985) isolated C. trachomatis from prostate samples obtained by transrectal aspiration biopsy of men with “nonacute abacterial prostatitis.” Abdelatif and colleagues (1991) identified intracellular Chlamydia employing “in-situ hybridization techniques” in transurethral prostate chips from 30% of men with histologic evidence of “chronic abacterial prostatitis.” Shurbaji and associates (1998) identified C. trachomatis in paraffin-embedded secretions in 31% of men with histologic evidence of prostatitis compared with none in patients with BPH without inflammation.
Although Mardh and Colleen (1972) suggested that C. trachomatis may be implicated in as many as one third of men with chronic prostatitis, their follow-up studies employing culturing and serologic tests could not confirm C. trachomatis as an etiologic agent in idiopathic prostatitis (Mardh and Colleen, 1975; Mardh et al, 1978). Shortliffe and Wehner (1986) came to a similar conclusion when her group evaluated antichlamydial antibody titers in prostatic fluid. Twelve percent of controls (compared with 20% of patients with nonbacterial prostatitis) had detectable antibodies. Berger and coworkers (1989) could not culture C. trachomatis from the urethras in men with chronic prostatitis nor did they find a serologic or local immune response to C. trachomatis in such patients. Doble and associates (1989b) were not able to culture or detect by immunofluorescence Chlamydia in transperineal biopsy specimens of abnormal areas of the prostate in men with chronic abacterial prostatitis. Krieger and colleagues (1996b) were only able to find Chlamydia in 1% of prostate tissue biopsy specimens in men with chronic prostatitis. A further localization and culture series by Krieger and associates (2000) also failed to culture Chlamydia from either urethral or prostate specimens. Further elucidation of the role of chlamydial etiology of prostate infection is required to make any definitive statement on the association between isolation of this organism and its prostatic origin and effect (Weidner et al, 2002).
Ureaplasma Infection
Ureaplasma urealyticum is a common organism isolated from the urethra of both asymptomatic men and men with nonspecific urethritis. Weidner and colleagues (1980) found high U. urealyticum concentrations in prostate-specific specimens in patients with signs and symptoms of abacterial prostatitis. Isaacs (1993) cultured U. urealyticum from prostate secretion in 8% of patients with chronic nonbacterial prostatitis. Fish and Danziger (1993) found significant U. urealyticum concentrations in 13% of patients with prostatitis. Treatment with specific antimicrobial therapy cleared the organisms in all cases. Ohkawa and associates (1993a) isolated U. urealyticum cells from the prostates of 18 of 143 patients with chronic prostatitis. Antibiotics eradicated the organism in all, improved the symptoms in 10, and cleared the leukocytes in the EPS in 4 (Ohkawa et al, 1993b).
Other investigators (Mardh and Colleen, 1975), employing similar techniques, were unable to implicate U. urealyticum in patients with nonbacterial prostatitis. The problems encountered in all these studies include the absence of controls and the fact that it was difficult to account for possible urethral contamination in collecting specific prostate specimens.
Other Microorganisms
Candida (Golz and Mendling, 1991; Indudhara et al, 1992) and other mycotic infections such as aspergillosis and coccidioidomycosis (Schwarz, 1982; Chen and Schijj, 1985; Campbell et al, 1992; Truett and Crum, 2004) have been implicated in prostatic inflammation. However, in most cases it was usually an isolated finding in immunosuppressed patients or those with systemic fungal infection. Viruses (Doble et al, 1991; Benson and Smith, 1992) have also been implicated in prostatic inflammation, but no systematic evaluation of the role of these agents in prostatitis has been undertaken. Trichomonas has been described in the prostate glands of patients complaining of prostatitis-like symptoms (Kuberski, 1980; Gardner et al, 1996; Skerk et al, 2002a).
Nonculturable Microorganisms
There are significant limitations to the culture techniques employed to attempt to identify etiologic microorganisms associated with prostatitis (Lowentritt et al, 1995; Domingue et al, 1997; Domingue, 1998). Bacteria may exist in aggregated biofilms adherent to the prostatic ductal walls or within the obstructed ducts in the prostate (Nickel and MacLean, 1998). Nickel and Costerton (1993) observed that 60% of patients with previously diagnosed chronic bacterial prostatitis who progressed to sterile EPS cultures but continued to have symptoms despite antimicrobial therapy had positive cultures in prostate biopsy specimens showing an organism similar to the initial organism. As discussed earlier, such organisms appear to persist in small aggregates or biofilms in the ducts and acini of the prostate gland.
Berger and associates (1997) cultured urine specimens and transperineal prostate biopsy specimens specifically for commensal and fastidious organisms. These investigators demonstrated that, in prostate biopsy cultures, men with evidence of inflammation in EPS are more likely to have bacteria isolated, positive cultures for anaerobic bacteria, higher total bacterial counts, and more bacterial species isolated than men without EPS inflammation. Krieger and colleagues (1996b), Riley and coworkers (1998), and Tanner and associates (1999) used a combination of clinical, culture, and molecular biologic methods (i.e., PCR) and found a strong correlation between inflammation and EPS and the detection of bacteria-specific 16S rRNA (gram-negative and gram-positive organisms) in the prostate tissue. But other researchers did not show any association between culture and PCR findings in men with nonbacterial prostatitis compared with men with prostatitis symptoms (Keay et al, 1999; Lee et al, 2003; Leskinen et al, 2003b). Nanobacteria are intriguing organisms that are difficult to isolate and culture but may be implicated in some chronic urologic conditions, including chronic prostatitis (Wood and Shoskes, 2006). A number of investigators (Shoskes et al, 2005; Short et al, 2008) have demonstrated the possibility that nanobacteria associated with and without prostatic calculi may be implicated in some cases of chronic prostatitis.
It has been estimated that less than 10% of all environmental bacteria have been identified (Domingue, 1998), so it is possible that fastidious and nonculturable microorganisms might be present in the prostate gland and that such organisms might be involved in the inflammatory process and subsequent development of symptoms.
Altered Prostatic Host Defense
Risk factors that allow bacterial colonization or infection of the prostate with potentially pathogenic bacteria include intraprostatic ductal reflux (Kirby et al, 1982); phimosis (VanHowe, 1998); specific blood groups (Lomberg et al, 1986); unprotected penetrative anal rectal intercourse; UTI; acute epididymitis (Berger et al, 1987); indwelling urethral catheters and condom catheter drainage (Meares, 1998); and transurethral surgery, especially in men who have untreated, infected urine (Meares, 1989). Secretory dysfunction of the prostate characterized by an alteration in the composition of prostatic secretions can be diagnostic of patients with prostatitis: there is a decrease in the levels of fructose; citric acid; acid phosphatase; the cations zinc, magnesium, and calcium; and the zinc-containing prostatic antibacterial factor; but pH, the ratio of isoenzymes lactate dehydrogenase-5 to lactate dehydrogenase-1, levels of inflammatory proteins such as ceruloplasmin, and complement C3 are increased (Meares, 1989). These defined alterations in the prostate secretory function have also been blamed for adversely affecting the normal antibacterial nature of prostatic secretions. A decrease in prostatic antibacterial factor may reduce the intrinsic antibacterial activity of the prostatic fluid (Fair et al, 1976), whereas the alkaline pH may hamper diffusion of certain basic antimicrobial drugs into the prostatic tissue and fluid (Fair and Cordonnier, 1978). However, caution is warranted because it is not known whether these compositional changes are a cause or a consequence of inflammation.
Dysfunctional Voiding
Anatomic or neurophysiologic obstruction resulting in high-pressure dysfunctional flow patterns has been implicated in the pathogenesis of the prostatitis syndrome. Blacklock (1974, 1991) demonstrated that bladder neck, prostatic, and urethral anatomic abnormalities predisposed some men to developing prostatitis. Urodynamic studies confirm that many patients, particularly those with prostatodynia, have decreased maximal urinary flow rates and obstructive-appearing flow patterns (Barbalias et al, 1983; Ghobish, 2002). On video-urodynamic studies, many patients with prostatitis syndromes show incomplete funneling of the bladder neck as well as vesicourethral dyssynergic patterns (Kaplan et al, 1994, 1997; Hruz et al, 2003). Investigators (Dellabella et al, 2006) have described ultrasound alterations of the preprostatic sphincter in men with chronic prostatitis. In a study of 48 treatment refractory chronic prostatitis patients with no associated infection, Hruz and associates (2003) determined that 29 (60%) had bladder neck hypertrophy diagnosed by endoscopic and urodynamic criteria. This dyssynergic voiding may lead to an autonomic overstimulation of the perineal-pelvic neural system with subsequent development of a chronic neuropathic pain state. Alternatively, this high-pressure, dysfunctional voiding may result in intraprostatic ductal reflux in susceptible individuals (see the next section).
Intraprostatic Ductal Reflux
Reflux of urine and possibly bacteria into the prostatic ducts has been postulated as one of the most important etiologic mechanisms involved in the pathogenesis of chronic bacterial and nonbacterial prostatic inflammation. Anatomically, the ductal drainage of the peripheral zone is more susceptible than other prostatic zones to intraprostatic ductal reflux (Blacklock, 1974, 1991). Kirby and associates (1982) instilled a carbon particle solution into the bladders of men diagnosed with nonbacterial prostatitis. Carbon particles were found in the EPS macrophages and prostatic acini and ductal system after surgery in men with nonbacterial prostatitis. Persson and Ronquist (1996) noted high levels of urate and creatinine in EPS, which they postulated was caused by urine reflux into the prostatic ducts. Terai and coworkers (2000) provided molecular epidemiologic evidence for ascending infection in acute bacterial prostatitis.
Prostatic calculi are composed of substances found only in urine, not in prostatic secretions (Sutor and Wooley, 1974; Ramiraz et al, 1980), further evidence that urinary intraprostatic reflux occurs and likely contributes to the formation of prostatic calculi. If pathogenic bacteria reflux into the prostate gland, they may exist in protected aggregates within prostatic calculi themselves. High culture counts of pathogens encrusted in prostatic calculi have been demonstrated by Eykyn and colleagues (1974). This type of bacterial colonization in protective bacterial aggregates or biofilms associated with prostatic calculi may lead to recalcitrant chronic prostatitis and subsequent recurrent UTIs despite what seems to be adequate antibiotic therapy. Ludwig and coworkers (1994), employing transrectal ultrasonography, showed that men with chronic inflammatory prostatitis had a significantly increased frequency of prostatic calculi compared with men without prostate inflammation (prostatodynia). It appears that prostatic calcification is common in patients with nonbacterial chronic prostatitis and is associated with greater inflammation, bacterial colonization, pelvic floor spasm, and symptom duration (Shoskes et al, 2007). The inflammation resulting from chemical, bacterial, or immunologic stimulation has been shown to possibly cause an increase in intraprostatic pressures, measurable with transperineally inserted pressure transducers (Mehik et al, 2002).
Immunologic Alterations
The local prostatic immune system is activated by infection in bacterial prostatitis. In acute bacterial prostatitis, serum and prostatic fluid antigen-specific (i.e., bacterial antigen) IgG and IgA can be detected immediately after the onset of infection, and after successful antibiotic therapy they decline to normal levels over the next 6 to 12 months (Fowler and Mariano, 1984a; Meares, 1977, 1998; Kumon, 1992). Prostate-specific antigen (PSA) levels can be markedly elevated during an acute episode of bacterial prostatitis (Dalton, 1989; Moon et al, 1992; Neal et al, 1992) and slowly resolve to normal levels over the course of 6 weeks, provided there is no recrudescence of the infection. In chronic bacterial prostatitis, no serum immunoglobulin elevation is detected, whereas prostatic fluid IgA and IgG levels are both increased (Shortliffe and Wehner, 1986; Kumon, 1992). After successful antibiotic therapy, IgG levels return to normal after several months but the IgA (particularly secretory IgA) levels remain elevated for almost 2 years (Shortliffe et al, 1981a, 1981b; Fowler and Mariano, 1984a). Antibody-coated bacteria detected in urine, EPS, and semen is another prominent feature of chronic bacterial prostatitis (Riedasch et al, 1984, 1991).
Noninfectious inflammation (nonbacterial prostatitis) might also be secondary to immunologically mediated inflammation due to some unknown antigen or perhaps even related to an autoimmune process. IgA and IgM antibody levels (not microorganism-specific) are elevated (Shortliffe and Wehner, 1986; Shortliffe et al, 1989, 1992), and similar antibodies as well as fibrinogen and complement C3 (Vinje et al, 1983; Doble et al, 1990) have been identified in prostatic biopsy specimens from patients with chronic prostatitis. Both animal model studies (Donadio et al, 1998; Ceri et al, 1999; Lang et al, 2000) and human studies (Alexander et al, 1997; Batstone et al, 2002; Maake et al, 2003; Motrich et al, 2007) have suggested that prostatitis may be an autoimmune process. A number of candidates have been suggested for the self-antigen, including PSA (Ponniah et al, 2000). Other specific immunologic and neuroendocrine alterations such as cytokine production (Alexander et al, 1998; Jang et al, 2003) and nerve growth factor (Miller et al, 2002) have a subsequent role to play in the process of inflammation. Specifically, interleukin-10 has been implicated in the etiology and clinical manifestations in chronic prostatitis (Miller et al, 2002; Shoskes et al, 2002) but other cytokines such as interleukin (IL)-1β and tumor necrosis factor-α have also been implicated (Nadler et al, 2000). IL-8 is the most common cytokine localized to the semen in men with chronic prostatitis (Khadra et al, 2006; Penna et al, 2007). There may be a genetic phenotype that promotes specific immunologic parameters that predispose to immunologically induced prostatic inflammation (Riley et al, 2002; Shoskes et al, 2002). These immunophenotypic patterns have even been observed in noninflammatory category IIIB chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) (Barghorn et al, 2001). Whatever the initiating event, the immunologic cascade appears to have an important role in the development of prostatitis in those patients who develop prostatic inflammation (Moon, 1998; Kumon, 1999).
Chemically Induced Inflammation
Investigators have demonstrated that urine and its metabolites (e.g., urate) are present in the prostatic secretion of patients with chronic prostatitis (Persson and Ronquist, 1996). These investigators have hypothesized that the prostatic inflammation and subsequent symptoms may be simply due to a chemically induced inflammation secondary to the noxious substances in the urine that have refluxed into the prostatic duct.
Pelvic Floor Muscle Abnormalities
Some investigators (Zermann et al, 1999) propose that the sensory or motor disturbances or both consistent with neural dysregulation of the lower urinary tract may be a consequence of acquired abnormalities in the central nervous system. Certainly, extraprostatic tenderness is identified in many patients with chronic prostatitis (Berger et al, 2007; Shoskes et al, 2008). Zermann and Schmidt (1999) describe 103 patients with chronic pelvic pain whom they evaluated at a specialized neurourologic unit. They showed that a majority of the men had insufficient conscious control of their somatically innervated striated pelvic floor muscles. The patients showed various levels of identity with their pelvic floor muscles, but none was able to demonstrate the full range of pelvic floor contraction and relaxation repetitively and effortlessly. This was true whether or not there was evidence of inflammation. They conclude that their findings reflect a functional disassociation between the central nervous system and the peripheral target, the pelvic floor muscles.
Other clinicians (Anderson, 1999; Potts, 2003) believe that the source of the pain is specifically at the pelvic musculature attachment area at the sacrum, coccyx, ischial tuberosity, pubic rami, and endopelvic fascia. These areas are immediately adjacent to the prostate and bladder and can be recognized by the demonstration of a hyperirritable spot or myofascial trigger point that is painful on compression. It is hypothesized that the formation of myofascial trigger points in this area results from mechanical abnormalities in the hip and lower extremities, chronic holding patterns such as those that occur during toilet training, sexual abuse, repetitive minor trauma and constipation, sports that create chronic pelvic stimulation, trauma or unusual sexual activity, recurrent infections, and surgery (Anderson, 1999). More recently, it has been hypothesized that the pain experienced in some men with CPPS may be explained by pudendal nerve entrapment, which causes subsequent neuropathic pain (Antolak et al, 2002). Unfortunately, all these studies examining pelvic floor muscle abnormalities did not compare findings to a control group (a criticism that can apply to many of the studies cited in this etiology section).
Neuroendocrine Mechanisms
The pain associated with the chronic prostatitis syndromes is similar in many respects to neuropathic pain. Objective autonomic nervous system changes can be observed in men with a chronic prostatitis, suggesting that altered autonomic nervous system responses may be responsible for the pain associated with this chronic pelvic pain syndrome (Yilmaz et al, 2007). Pain that may have originated in the prostate or pelvic floor muscles, through mechanisms of cross-sensitization, may have spread to adjacent organs and/or structures. We are only recently beginning to understand the complexity of overlapping neuropathways and possible mechanisms underlying pelvic organ crosstalk (Malykhina, 2007).
It has been shown that men with chronic prostatitis showed evidence of dysfunctional hypothalamic-pituitary-adrenal axis function reflected in augmented awakening cortisol responses (Anderson et al, 2008). Another study evaluating the adrenocortical hormone abnormalities in men with chronic prostatitis suggested that some men with this condition may even meet the diagnostic criteria for nonclassic congenital hyperplasia (Dimitrakov et al, 2008).
Psychological Factors
Psychological factors have always been considered to play an important role in the development or exacerbation of the chronic prostatitis syndromes. Some researchers who have investigated the psychopathology in these patients concluded that this syndrome should be viewed as a psychosomatic disorder (Mendlewich et al, 1971; Mellan et al, 1973; Keltikangas-Jarvinen et al, 1982). De la Rosette and associates (1993b) compared a group of 50 patients with chronic prostatitis with a group of 50 patients seen for vasectomy and showed that, although there were significant statistical differences between the groups (with patients with chronic prostatitis scoring consistently higher personality disorder scores), these differences in scores were quite small compared with those between patients with prostatitis and psychiatric patients. Berghuis and coworkers (1996) compared 51 prostatitis patients with a group of 34 men without any chronic pain condition and concluded that depression and psychological disturbances are common among prostatitis patients. Egan and Krieger (1994) compared prostatitis patients with those seeking treatment for chronic low back pain. Major depression was more common in prostatitis patients, but back pain caused more somatically focused depression and anxiety. Ku and coworkers (2002) suggested that depression and weak masculine identity may be associated with an early stage of chronic prostatitis. A large case-control study confirmed that depression and panic disorders are significantly more common in men with chronic pelvic pain conditions than in control subjects (Clemens et al, 2008). These more recent studies demonstrate that psychological factors are involved in the disease, but it seems unjustified to label this group of patients as neurotic or as having a psychopathologic condition. However, recent analyses of the large prostatitis cohorts showed that psychological variables, such as depression, maladaptive coping techniques (e.g., pain catastrophizing and pain contingent resting), poor social support, and stress are important in chronic prostatitis outcomes (Tripp et al, 2004, 2005, 2006; Ulrich et al, 2005; Nickel et al, 2008b). Factors such as catastrophizing are particularly important because they have been found to be stronger predictors of patient pain reports than depression (Tripp et al, 2006), indicating that negative cognitive appraisals of pain experience may be a primary target for psychosocial interventions. This may be especially important given the strong association that pain catastrophizing has shown with elevations in depression, disability, and lower quality of life manifest in patients with chronic prostatitis (Tripp et al, 2005, 2006; Nickel et al, 2008b).
Association with Interstitial Cystitis (Painful Bladder Syndrome)
Interstitial cystitis, now referred to many as painful bladder syndrome or bladder pain syndrome, is an ill-defined CPPS occurring primarily in females, and a number of investigators have hypothesized that chronic nonbacterial prostatitis may have a similar cause (Pontari, 2006; Forrest et al, 2007). Unfortunately the cause of interstitial cystitis remains unknown, but the pathogenic mechanisms are theorized to be very similar to those that cause chronic prostatitis in men (Sant and Nickel, 1999; Eisenberg et al, 2003; Parsons 2003). Some researchers have proposed that in some patients diagnosed with prostatitis, a bladder-orientated interstitial cystitis mechanism actually accounts for the symptoms and that the prostate is only indirectly involved (Sant and Kominski, 1997). Certainly, the pain and voiding symptoms of interstitial cystitis and chronic prostatitis overlap to some extent (Miller et al, 1995; Novicki et al, 1998; Sant and Nickel, 1999; Forrest and Schmidt, 2004), and men with prostatitis diagnoses have findings on cystoscopic (Berger et al, 1998), urodynamic (Siroky et al, 1981), and potassium sensitivity testing (Parsons et al, 2002, 2005) very similar to those of patients with interstitial cystitis. However, Yilmaz and associates (2004) did not confirm positive potassium sensitivity testing in prostatitis patients and Keay and colleagues (2004) have shown that men diagnosed with chronic prostatitis (pain only) have normal antiproliferative factor activity whereas men diagnosed with interstitial cystitis (pain and irritative voiding symptoms) have detectable levels of urine antiproliferative factor.
An Interrelated, Pluricausal, Multifactorial Etiology
It is likely that the nonbacterial prostatitis syndromes have a multifactorial etiology, either a spectrum of etiologic mechanisms or, more likely, a progression or cascade of events after some initiating factor. In an important review on mechanisms involved in the pathogenesis of chronic prostatitis, Pontari and Ruggieri (2004) concluded that, “the symptoms of chronic prostatitis/chronic pelvic pain syndrome appear to result from an interplay between psychological factors and dysfunction in the immune, neurological and endocrine systems.” Figure 11–2 describes a hypothetical scenario that could potentially involve most of the proposed and interrelated causes described in this section.
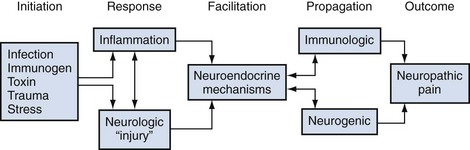
Figure 11–2 It is very likely that the etiology and pathogenesis of chronic prostatitis/chronic pelvic pain syndrome (category III CPPS) involves a pluricausal, multifactorial mechanism. An initiating stimulus such as infection, reflux of some “toxic” or “immunogenic” urine substance, perineal/pelvic “trauma,” and/or psychological stress starts a cascade of events in an anatomically or genetically susceptible man, resulting in a local response of either inflammation or neurogenic injury (or both). Further immunologic or neuropathic (possibly interrelated) mechanisms mediated by neuroendocrine pathways propagate or sustain the chronicity of the initial (or ongoing) event. The final outcome is the clinical manifestation of chronic perineal/pelvic pain–associated symptoms associated with local and central neuropathic mechanisms.
Definition and Classification
Traditional Classification
The traditional classification system is based on the landmark paper by Meares and Stamey (1968) describing the differential diagnosis of the prostatitis syndromes. This classic paper described in great details the serial cultures (and treatment) in four patients with chronic prostatitis and introduced the so-called Meares-Stamey four-glass test. This localization test, which segmentally assesses inflammation and cultures of the male lower urinary tract, is described later in this chapter (see Lower Urinary Tract Evaluation). Based on 10 years of clinical experience with this test, a classification system describing four categories of prostatitis was described by Drach and colleagues in 1978. Differentiation of the four categories depended on an analysis of prostatic fluid, which included microscopy (examination for white blood cells [WBCs], inflammatory cell clumps, mucus debris, oval fat bodies, and macrophages) and culturing (identifying traditional uropathogens).
Acute bacterial prostatitis was diagnosed when prostatic fluid was clinically purulent, systemic signs of infectious disease were present, and bacteria were cultured from prostatic fluid. Chronic bacterial prostatitis was diagnosed when pathogenic bacteria were recovered in significant numbers from a purulent prostatic fluid in the absence of a concomitant UTI or significant systemic signs. Nonbacterial prostatitis was diagnosed when significant numbers of bacteria could not be cultured from prostatic fluid but the fluid consistently revealed microscopic purulence. Prostatodynia was diagnosed in the remaining patients who had persistent pain and voiding complaints as in the previous two categories but who had no significant bacteria or purulence in the prostatic fluid. This clinical differentiation of the prostatitis syndromes is now referred to as the “traditional” classification system (Table 11–1).
Table 11–1 Classification System for the Prostatitis Syndromes
| TRADITIONAL | NATIONAL INSTITUTES OF HEALTH | DESCRIPTION |
|---|---|---|
| Acute bacterial prostatitis | Category I | Acute infection of the prostate gland |
| Chronic bacterial prostatitis | Category II | Chronic infection of the prostate gland |
| N/A | Category III chronic pelvic pain syndrome (CPPS) | Chronic genitourinary pain in the absence of uropathogenic bacteria localized to the prostate gland employing standard methodology |
| Nonbacterial prostatitis | Category IIIA (inflammatory CPPS) | Significant number of white blood cells in expressed prostatic secretions, post–prostatic massage urine sediment (VB3) or semen |
| Prostatodynia | Category IIIB (noninflammatory CPPS) | Insignificant number of white blood cells in expressed prostatic secretions, post–prostatic massage urine sediment (VB3) or semen |
| N/A | Asymptomatic inflammatory prostatitis (AIP) | White blood cells (and/or bacteria) in expressed prostatic secretions, post–prostatic massage urine sediment (VB3), semen or histologic specimens of prostate gland |
The significant limitations of this classification system included the abandonment by most physicians of the rigorous Meares-Stamey four-glass test (Moon, 1997; Nickel et al, 1998a; McNaughton Collins et al, 1999, 2000a), the realization that only the very rare patient would be subsequently diagnosed with chronic bacterial prostatitis, the perception that patients responded to specific therapy (e.g., antibiotics) independent of this classification system, and the dawning realization that, in many cases, patients presenting with prostatitis syndromes were not easily classified into one of these categories (e.g., patients in whom specific cultures were negative because of previous antibiotic therapy or those thought to have a cause other than the prostate gland) (Nickel, 1998a).
National Institutes of Health Classification
The limitations of the traditional diagnostic algorithm and traditional classification system led to the development of the National Institutes of Health (NIH) classification system (see Table 11–1) (Krieger et al, 1999). The new definition recognized that pain is the main symptom in “abacterial chronic prostatitis” (with variable voiding and sexual dysfunction), and it was the optimal criterion to differentiate chronic prostatitis patients from control patients or patients experiencing other genitourinary problems (e.g., BPH). This classification differed from the traditional system in two main areas: the descriptions of category III CP/CPPS and category IV asymptomatic inflammatory prostatitis.
Category I is identical to the acute bacterial prostatitis category of the traditional classification system. Category II is identical to the traditional chronic bacterial prostatitis classification, except that it now usually refers to patients with recurrent lower urinary tract infections (with a prostate nidus of infection) (Schaeffer, 2006). Category III is defined as the “presence of genitourinary pain in the absence of uropathogenic bacteria detected by standard microbiologic methodology.” This syndrome is further categorized into category IIIA, or inflammatory CP/CPPS (based on the presence of excessive leukocytes in EPSs or post–prostatic massage urine or semen), and category IIIB, or noninflammatory CP/CPPS (no significant leukocytes in similar specimens). Category IV, or asymptomatic inflammatory prostatitis, addressed one of the major problems and omissions of the traditional classification system. Patients are classified as having category IV prostatitis by the presence of significant leukocytes (or bacteria or both) in prostate-specific specimens (EPS, semen, and tissue biopsies) in the absence of typical chronic pelvic pain.
The NIH International Prostatitis Collaborative Network met in Washington in 1998 and confirmed the value of this classification system, not only in clinical research studies but also in clinical practice (Nickel et al, 1999c).
Clinical Presentation
Category I: Acute Bacterial Prostatitis
Acute bacterial prostatitis, category I, is a rare but important lower urinary tract infectious disease. It is characterized by an acute onset of pain combined with irritative and obstructive voiding symptoms in a patient with manifestations of a systemic febrile illness. The patient typically complains of urinary frequency, urgency, and dysuria. Obstructive voiding complaints including hesitancy, poor interrupted stream, strangury, and even acute urinary retention are common. The patient complains of perineal and suprapubic pain and may have associated pain or discomfort of the external genitalia. In addition, there are usually significant systemic symptoms including fever, chills, malaise, nausea and vomiting, and even frank septicemia with hypotension. The combination and severity of symptoms in category I, acute bacterial prostatitis, vary from patient to patient. Approximately 5% of patients with acute bacterial prostatitis may progress to chronic bacterial prostatitis (Cho et al, 2005).
Category II: Chronic Bacterial Prostatitis
The most important clue in the diagnosis of category II, chronic bacterial prostatitis, is a history of documented recurrent UTIs. From 25% to 43% of patients diagnosed with chronic bacterial prostatitis employing a four-glass test had a history of recurrent UTIs (Weidner and Ludwig, 1994; Wright et al, 1994). Patients may be relatively asymptomatic between acute episodes, or they may present with a long history of a CPPS, which is described extensively in the next section. The prevalence of bacterial prostatitis ranges from 5% to 15% of prostatitis cases (Schaeffer et al, 1981; Krieger and Egan, 1991; Weidner and Ludwig, 1994). In one of the largest and most comprehensive clinical series, Weidner and associates (1991b) found significant bacteriuria (with uropathogenic organisms) in 4.4% of patients with symptoms of chronic prostatitis.
Category III: CP/CPPS
The presenting symptoms of inflammatory category IIIA CP/CPPS (similar to the previous category of chronic nonbacterial prostatitis) are indistinguishable from those of patients with noninflammatory category IIIB disease (previously referred to as prostatodynia). The symptoms experienced by patients with CP/CPPS have been studied extensively by Krieger and colleagues (1996a). They evaluated 50 patients with CP/CPPS seen in a prostatitis clinic (compared with 75 control patients). Alexander and Trissel (1996) surveyed a cohort of 163 prostatitis patients on the Internet. These symptoms were best defined in the development of prostatitis symptom scores by Neal and Moon (1994), Krieger and colleagues (1996a), Nickel and Sorensen (1996b), and Brahler and coworkers (1997). The predominant symptom in all these studies was pain, which was most commonly localized to the perineum, suprapubic area, and penis but can also occur in the testes, groin, or low back. Pain during or after ejaculation is one of the most prominent, important, and bothersome features in many patients (Shoskes et al, 2004). Irritative and obstructive voiding symptoms including urgency, frequency, hesitancy, and poor interrupted flow are associated with this syndrome in many patients. Erectile dysfunction and sexual disturbances have been reported in patients with CPPS (Mehik et al, 2001; Liang et al, 2004; Zaslau et al, 2005; Muller and Mulhall, 2006; Marzalek et al, 2007; Smith et al, 2007a, 2007b; Lee et al, 2008a) but are not pathognomonic features of this syndrome. The best description of the CP/CPPS patient was provided by the NIH Chronic Prostatitis Cohort Study (Schaeffer et al, 2002). A detailed description of 488 men with CP/CPPS noted that the most frequently reported pain/discomfort was in the perineum, followed by pain/discomfort in the suprapubic area. Over half of the men had pain/discomfort during or after sexual climax (ejaculatory pain may be the most discriminatory symptom).
By definition, the syndrome becomes chronic after 3 months’ duration. The symptoms tend to wax and wane over time; approximately one third of patients improve over 1 year (usually patients with shorter duration and fewer symptoms) (Nickel et al, 2002; Turner et al, 2004; Propert et al, 2006). An age-matched case-control study of risk factors in men with CP/CPPS (Pontari et al, 2005) showed that compared with asymptomatic controls, men with CP/CPPS reported a significantly greater life time prevalence of nonspecific urethritis (12% vs. 4%), cardiovascular disease (11% vs. 2%), neurologic disease (41% vs. 14%), psychiatric conditions (29% vs. 11%), and blood or infectious disease (41% vs. 20%).
The impact of this condition on health status is significant. The quality of life of many patients diagnosed with CP/CPPS is greatly diminished. Wenninger and associates (1996), employing a generic health status measure, the Sickness Impact Profile, showed that the mean scores were within the range of scores reported in the literature for patients with a history of myocardial infarction, angina, or Crohn disease. McNaughton Collins and coworkers (2001b) employed similar quality of life assessment instruments in the NIH Chronic Prostatitis Cohort Study of almost 300 patients and confirmed this finding. These investigators noted that the mental health component was affected more than the physical component of the quality of life assessment. CP/CPPS patients’ quality of life was lower than those observed in the most severe subgroups of men with congestive heart failure and diabetes mellitus. This significant impact on quality of life has also been reported in a cohort of CP/CPPS men evaluated in a primary care setting (Turner et al, 2002). Patients with a diagnosis of CP/CPPS may present with depression (Tripp et al, 2005, 2006), stress (Ulrich et al, 2005), or a history of abuse (sexual, physical, or emotional) (Hu et al, 2007). Depression, maladaptive coping techniques (e.g., catastrophizing and pain contingent resting) and poor social support are associated with a poorer quality of life (Nickel et al, 2008b).
Category IV: Asymptomatic Inflammatory Prostatitis
Category IV, asymptomatic inflammatory prostatitis, by definition, does not cause symptoms. The patients present with BPH, an elevated PSA level, prostate cancer, or infertility. Subsequent microscopy of EPS or semen and/or histologic examination of BPH chips, prostate cancer specimens, or prostate biopsy specimens disclose evidence of prostatic inflammation.
Symptom Assessment
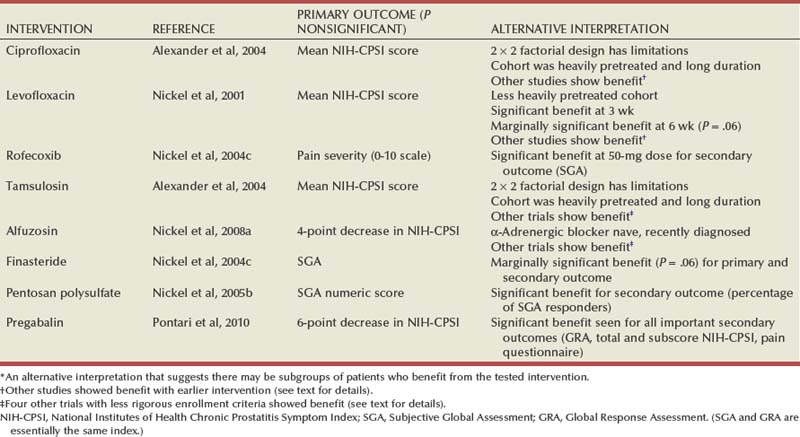
In a syndrome such as CP/CPPS, in which the objective parameters of the disease are unknown, controversial, or not validated, evaluation outcomes become of critical importance. In a syndrome defined primarily by its symptom-complex, analysis of specific prostatitis-like symptoms, the quality of life, the patient’s functional status, and the patient’s satisfaction with medical care will result in not only better evaluation of the prostatitis patient but also improved therapeutic follow-up. Scientifically validated symptom indices not only improve the care of patients but also optimize clinical decision making in terms of comparing clinical trial outcomes. Since the early 1990s, several different symptom indices have been described in clinical research (Neal and Moon, 1994; Krieger et al, 1996a; Nickel and Sorensen, 1996b; Brahler et al, 1997; Chiang et al, 1997) and have been sporadically employed in clinical practice (McNaughton Collins and O’Leary, 1999). Although each of these symptom indices was successful at the time it was developed for the specific purpose or study, none was believed to be ideal for use in general research or clinical practice because they were not validated according to the rigorous standards that now must be met for an accepted urologic disease-specific index (O’Leary et al, 1992).
The NIH Chronic Prostatitis Collaborative Research Network (CPCRN) developed a reproducible and valid instrument to measure the symptoms and quality of life of patients with chronic prostatitis for use in research protocols as well as clinical practice (Litwin et al, 1999). The steps followed in the development of the NIH-CPSI included a systematic literature review, focus groups, cognitive testing, an expert panel review, a validation test, and psychometric analyses. The final index consists of nine questions that address the three most important domains of the chronic prostatitis experience. Pain (which is the primary symptom of CP/CPPS) was captured in four questions that focused on its location, severity, and frequency. Urinary function, the second most important component of patients’ symptoms, was captured in two questions, one concerning irritative, and the other obstructive, function. The quality of life or impact was captured in three additional questions that asked about the effect of symptoms on daily activities. The NIH-CPSI (Fig. 11–3) has now been accepted by the international prostatitis research community as an accepted outcome measure (Nickel et al, 1999c) and has shown validity and responsiveness in primary care samples (Turner et al, 2003) and clinical trials (Propert et al, 2006). It has been translated and validated in many languages other than English (Collins et al, 2001; Kunishima et al, 2002; Leskinen et al, 2003a; Schneider et al, 2004; Karakiewicz et al, 2005). The symptom index has also proved its usefulness in the evaluation and follow-up of patients in general clinical urologic practice (Nickel, 1999b, 2001c).

Figure 11–3 The National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) captures the three most important domains of the prostatitis experience: pain (location, frequency, and severity), voiding (irritative and obstructive symptoms), and quality of life (including impact). This index is useful in research studies and clinical practice.
(Reprinted with permission from Litwin MS, McNaughton Collins M, Fowler FJ, et al. The NIH Chronic Prostatitis Symptom Index [NIH-CPSI]: development and validation of a new outcome measure. J Urol 1999;162:369–375.)
Lower Urinary Tract Evaluation
Physical Examination
Physical examination is an important part of the evaluation of a patient with prostatitis, but it is usually not helpful in making a definitive diagnosis or further classifying the disorder. It assists in ruling out other perineal, anal, neurologic, pelvic, or prostate abnormalities and is an integral part of the lower urinary tract evaluation by providing prostate-specific specimens.
In category I, acute bacterial prostatitis, the patient may be systemically toxic, that is, flushed, febrile, tachycardic, tachypneic, and even hypotensive. The patient usually has suprapubic discomfort and perhaps has clinically detectable acute urinary retention. Perineal pain and anal sphincter spasm may complicate the digital rectal examination. The prostate itself is usually described as hot, boggy, and exquisitely tender. The expression of prostatic fluid is believed to be totally unnecessary and perhaps even harmful.
The physical examination of a patient with category II, chronic bacterial prostatitis and category III CPPS is usually unremarkable (except for pain). Careful examination and palpation of external genitalia, groin, perineum, coccyx, external anal sphincter (tone), and internal pelvic floor and side walls may pinpoint prominent areas of pain or discomfort. The digital rectal examination should be performed after the patient has produced preprostatic massage urine specimens (see later). The prostate may be normal in size and consistency, and it has also been described as enlarged and boggy (loosely defined by the author as softer than normal). The degree of elicited pain during prostatic palpation is variable and is unhelpful in differentiating a prostatitis syndrome. The prostate should be carefully checked for prostatic nodules before a vigorous prostatic massage is performed to produce prostate-specific specimens (EPS and post–prostatic massage urine sample).
Lower Urinary Tract Cytologic Examination and Culture Techniques
In patients presenting with category I, acute bacterial prostatitis, a urine culture is the only laboratory evaluation of the lower urinary tract required. It has been suggested that the vigorous prostatic massage necessary to produce EPS can exacerbate the clinical situation, although such fears have never been substantiated in the literature. A midstream urine specimen will show significant leukocytosis and bacteriuria microscopically, and culturing usually discloses typical uropathogens. Blood cultures may show the same organism.
In 1968, Meares and Stamey described the classic four-glass urine collection technique to distinguish urethral, bladder, and prostate infections in men with chronic prostatitis, and for 3 decades this has remained the “gold standard” for the evaluation of this lower urinary tract syndrome. The voided bladder-1 (VB1) specimen includes the first 10 mL of urine and represents the urethral specimen. The voided bladder-2 (VB2) specimen is similar to a midstream urine collection and represents the bladder urine. EPS should be collected directly into a sterile container during prostatic massage. The voided bladder-3 (VB3) specimen, the first 10 mL of urine voided after prostatic massage, includes any EPS trapped in the prostatic urethra. The three urine specimens are centrifuged for 5 minutes and the sediment examined under high power for leukocytes (including aggregates of leukocytes), macrophages, oval fat bodies, erythrocytes, bacteria, and fungal hyphae. A wet mount of a drop of EPS can be examined under a coverslip in a similar manner. Some researchers (Muller et al, 2001; Krieger et al, 2003) point out that quantitative determination of the EPS WBC concentration by a counting chamber method is superior to the standard wet mount method but probably only indicated in research studies. In fact, the NIH Chronic Prostatitis Cohort Study (Schaeffer et al, 2002; Nickel et al, 2003a) suggested that leukocyte determination did not appear to add significant clinical information to the assessment of a patient with CP/CPPS. All four specimens are sent to the laboratory for quantitative culturing. Figure 11–4 illustrates the technique and interpretation of the four-glass test.
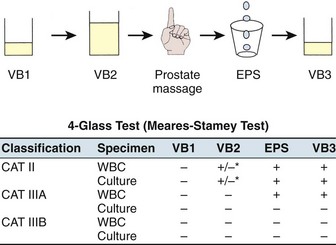
Figure 11–4 Technique and interpretation of the Meares-Stamey four-glass lower urinary tract localization test for CP/CPPS.
Category II, chronic bacterial prostatitis, is diagnosed if there is a 10-fold increase in bacteria in the EPS or VB3 specimen compared with the VB1 and VB2 specimens. In a patient who has acute cystitis this localization is impossible, and in this case the patient can be treated with a short course (1 to 3 days) of therapy with an antibiotic such as nitrofurantoin, which penetrates the prostate poorly but eradicates the bladder bacteriuria. Subsequent localization of bacteria in the post–prostatic massage urine or EPS is then diagnostic of category II prostatitis. Category IIIA CP/CPPS is diagnosed when no uropathogenic bacteria are cultured, but excessive leukocytosis (usually defined as more than 5 to 10 WBCs/high-power field [hpf]) is noted in the prostate-specific specimens (EPS or VB3 or both). Category IIIB CP/CPPS is diagnosed when no uropathogenic bacteria are cultured and there is no significant leukocytosis noted on microscopic examination of EPS or the sediment of VB3.
Although the four-glass test remains the gold standard diagnostic evaluation of prostatitis patients, numerous surveys (Moon, 1997; Nickel et al, 1998a; McNaughton Collins et al, 1999, 2000a) have confirmed that clinicians have more or less abandoned this time-consuming and expensive rigorous evaluation. The pre-massage and post-massage test (or two-glass test), originally suggested by Weidner and Ebner (1985) and popularized by Nickel (1995, 1996, 1997a), is a simple, cost-effective screen to categorize patients with chronic prostatitis. The patient provides a midstream pre-massage urine specimen and a urine specimen (initial 10 mL) after prostatic massage. Microscopy (sediment) and culturing of these two screening urine specimens allows categorization of the majority of patients presenting with a chronic prostatitis syndrome. Figure 11–5 illustrates the technique and interpretation of the two-glass pre-massage and post-massage test.
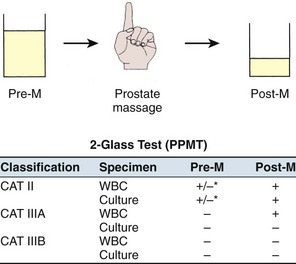
Figure 11–5 Technique and interpretation of the pre- and post-massage two-glass lower urinary tract localization test for CP/CPPS.
In a retrospective personal series and a review of series in the literature, Nickel (1997a) noted that this test had 91% sensitivity and specificity compared with the gold standard Meares-Stamey test. Its limitations were thought to be due to the exclusion of the urethral and EPS specimen. However, in patients without clinical urethritis, Krieger and associates (2000) demonstrated that urethral swabs are more efficient in picking up urethral inflammation than the VB1 specimen. But in this series of 235 patients only 3% had more than 1 WBC/hpf. Therefore the urethral specimens rarely detected significant urethral inflammation, and in this series rarely did cultured organisms change the direction of clinical therapy in patients with prostatitis (without clinical urethritis). In the same study (Krieger et al, 2000) comparing EPS with post–prostatic massage urine, the investigators demonstrated that EPS examination detected 76%, whereas post-massage urine examination detected 82% of the patients who had inflammation on one or both tests. Ludwig and associates (2000), in a series of 328 patients in whom both EPS and a VB3 specimen were obtained, demonstrated that VB3 is almost as accurate as EPS (92% sensitivity; 99% specificity) in detecting prostate-specific inflammation. Seiler and associates (2003) came to the same conclusion in their study of 143 chronic prostatitis patients. Nickel and colleagues from the NIH CPCRN (2006) examined a cohort of 353 CP/CPPS men with complete four-glass data and noted that the two-glass test predicted a positive four-glass result with clinically acceptable accuracy (over 95% of men would have had the same diagnosis if the four-glass test was performed). This test, however, is only a screening test, and in patients in whom it is important to localize bacteria to the prostate versus the urethra (e.g., patients with recurrent UTIs, suspicion of urethral abnormality), a follow-up VB1 specimen or urethral swab may be very helpful. If typical urethral organisms are localized to the prostate when the pre-massage and post-massage test is used and the clinician is inclined to consider them pathogenic and subsequently treat the patients, urethral and EPS specimens to definitively localize the specific bacteria to the prostate are appropriate. As a general rule, it is always best to examine the EPS (if obtainable) microscopically.
Key Point
Lower Urinary Tract Culture Technique
Microbiologic Considerations
Both the traditional and the NIH classification systems depend on culturing for standard uropathogens. The Enterobacteriaceae (e.g., E. coli, Serratia, Klebsiella, Proteus, Pseudomonas) represent the most common uropathogens, followed by gram-positive enterococci. However, as discussed earlier in the section on etiology, other gram-positive organisms that typically colonize the urethra (Staphylococcus epidermidis, Staphylococcus saprophyticus, Streptococcus species, Corynebacterium, and Bacteroides) can be localized to the prostate specimens, including semen (>10-fold colony-forming unit count in prostate-specific specimens compared with pre–prostatic massage specimens), and their association with the prostatic inflammation symptom-complex remains unclear. At this time, these patients are still considered as having category III CP/CPPS, but as more research results become available this may change as our understanding of bacterial pathogenicity in the prostate gland evolves (Nickel and Moon, 2005; Nickel and Xiang, 2008).
Cytologic Considerations
The differentiation of the two subtypes of category III CP/CPPS depend on cytologic examination of the urine or EPS or both. The urine specimens are centrifuged for 5 minutes, and the sediment is resuspended under a coverslip and examined at high power (×300 to ×400) while the wet mount of a drop of EPS is examined under a coverslip at the same power. WBCs have traditionally been reported as numbers of leukocytes per high-power field (Fig. 11–6). There is no validated cutoff point for the level of WBCs per high-power field that is required to differentiate an inflammatory from a noninflammatory CP/CPPS. Although the suggested limits have ranged from as low as 2 (Anderson and Weller, 1979) to as high as 20 (Blacklock and Beavis, 1978) the consensus appears to favor 5 to 10 WBCs/hpf in EPS as the upper level of normal (Meares and Stamey, 1968; Pfau et al, 1978; Schaeffer et al, 1981). But inflammatory cells in the EPS fluctuate over time (Anderson and Weller, 1979; Schaeffer et al, 1981) and with the frequency of ejaculation (Jameson, 1967; Yavascaoglu et al, 1999). A disadvantage of looking at a drop of prostatic fluid or urine sediment is that the cells may clump or aggregate, which renders quantifying them virtually impossible. Also, an unstained specimen does not allow differentiation of the type of WBC present (e.g., polymorphonuclear leukocytes, lymphocytes, monocytes, macrophages). If accuracy is required (i.e., research), then the WBCs can be counted in a glass hemacytometer (so they may be quantified as cells per square millimeter) and subsequently stained to differentiate the inflammatory cell subtype (Anderson and Weller, 1979).
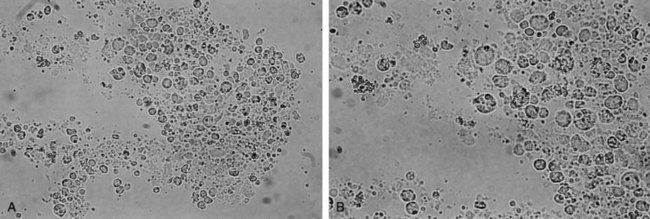
Figure 11–6 A and B, Unstained photomicrographs showing individual white blood cells, clumps of white blood cells, and lipid-laden macrophages in the expressed prostatic secretion (EPS) of a patient with category IIIA CPPS (A, ×250; B, ×400).
The clinical relevance of adding cytologic examination of semen specimens (which is difficult without special staining techniques) is unknown. Certainly, semen examination increases the percentage of patients identified as inflammatory category IIIA CP/CPPS (Krieger et al, 2000).
Nickel and colleagues (2003a) compared the number of WBCs in the EPS in patients with CP/CPPS to EPS specimens from normal asymptomatic control men and noted that while there was a statistically significant difference in WBC counts in the CP/CPPS men the clinical significance was not apparent (e.g., 50% of CPPS men had >5 WBCs/hpf compared with 40% of control men). The relevance of examining urine and EPS for white cells in routine clinical practice has been challenged (Nickel et al, 2003a). In fact, we have not been able to confirm the association between histologically proven prostate inflammation and prostatitis symptoms (Nickel et al, 2007), further confusing the issue of whether we really need to determine prostate-specific specimen inflammation, which is really just a surrogate for prostate inflammation. However, some investigators (Nickel, 2002d) have recommended that urine cytology (for malignant cells) become a standard diagnostic test for men presenting with prostatitis-like symptoms, particularly if the symptom-complex includes irritative voiding symptoms, dysuria, and/or suprapubic/bladder pain.
Urodynamics
Pain is the dominant symptom in patients presenting with CP/CPPS, but a wide constellation of irritative and obstructive voiding symptoms are associated with this syndrome. Proposed causes to account for the persistent irritative and obstructive voiding symptoms include detrusor vesical neck or external sphincter dyssynergia, proximal or distal urethral obstruction, and fibrosis or hypertrophy of the vesical neck (Blacklock, 1974, 1986; Bates et al, 1975; Orland et al, 1985; Theodorou et al, 1999). These abnormalities can often be clarified and diagnosed by urodynamics, particularly video-urodynamics. Others have suggested that men with defined primary voiding dysfunction have been misdiagnosed with chronic prostatitis (Webster et al, 1980; Siroky et al, 1981; Murnaghan and Millard, 1984). Siroky and associates (1981) noted that urodynamics revealed that 50% of 47 men with recurrent voiding symptoms, perigenital pain, or both previously diagnosed as chronic prostatitis had bladder acontractility during a study with nonrelaxing perineal floor (striated muscle spasm) and that another 36% had detrusor overactivity with appropriate striated sphincter relaxation. Barbalias (1990) and Barbalias and colleagues (1983) noted decreased peak and mean urinary flow rates, a significantly elevated maximal urethral closing pressure, and incomplete funneling of the bladder neck accompanied by urethral narrowing at the level of the external urinary sphincter during voiding with urodynamic evaluation of men diagnosed with chronic prostatitis. Hellstrom and colleagues (1987) also noted elevated urethral pressures, “hyperreflexia” of the external urethral sphincter, and intraprostatic reflux in three patients with unremitting symptoms of chronic nonbacterial prostatitis.
Kaplan and associates (1994, 1996, 1997) postulated that chronic lower urinary tract symptoms in young men are often misdiagnosed as chronic nonbacterial prostatitis when in fact they indicate a cohort of men with undiagnosed chronic voiding dysfunction. This conclusion is based on the video-urodynamic studies of 137 consecutive men 50 years of age or younger diagnosed with chronic prostatitis (Kaplan et al, 1996). These researchers demonstrated a variety of urodynamic abnormalities, including 54% of patients with primary vesical neck obstruction, 24% with functional obstruction localized to the membranous urethra (pseudodyssynergia), 17% with impaired bladder contractility, and 5% with an acontractile bladder. They noted detrusor overactivity in 49% of the men. Simple documentation of uroflowmetry and residual urine bladder scan abnormalities may suggest proceeding to more sophisticated urodynamics (Ghobish, 2000). Other groups dispute the benefits of urodynamics and have noted very few urodynamic abnormalities in patients presenting with classic chronic prostatitis symptoms (Mayo et al, 1998).
Endoscopy
Clinical experience (rather than controlled clinical studies) suggests that lower urinary tract endoscopy (i.e., cystoscopy) is not indicated in the majority of men presenting with CP/CPPS. However, cystoscopy is indicated in patients in whom the history (e.g., hematuria), lower urinary tract evaluation (e.g., VB1 urinalysis), or ancillary studies (e.g., urodynamics) indicate the possibility of a diagnosis other than CP/CPPS. In these patients, occasionally lower urinary tract malignancy, stones, urethral strictures, bladder neck abnormalities, and so forth that can be surgically corrected are discovered. Cystoscopy can probably be justified in men refractory to standard therapy.
Transrectal Ultrasonography
Transrectal ultrasonography has become one of the best radiologic methods to evaluate prostate disease and has become an especially helpful clinical tool for the assessment of prostate volume and ultrasound guidance of biopsy needles. The diagnostic value of ultrasonography in differentiating benign from malignant prostate disease is controversial, and the further differentiation of the various benign conditions of the prostate is even more so. Di Trapani and colleagues (1988) described inhomogeneous echo structures, constant dilatation of periprostatic venous plexus, elongated seminal vesicles, and thickening of the inner septa in patients with prostatitis. Doble and Carter (1989) described seven ultrasound signs associated with the presence of symptoms of chronic prostatitis compared with controls, and although the sensitivity increased with higher leukocyte counts the signs were not sufficiently specific to differentiate clinical groups.
Peeling and Griffiths (1984) describe the heterogeneity of the echo pattern and prostatic calculi as ultrasound features related to prostatitis. Ludwig and coworkers (1994) described the ultrasound features such as prostatic calcifications and seminal vesicle abnormalities that appear to be indicative of signs of inflammation but not proof of the presence of chronic prostatitis. Harada and associates (1980) concluded that the presence of stones is not related to a specific prostate disease process. De la Rosette and colleagues (1992b) performed ultrasonography in 22 patients with nonbacterial prostatitis and compared the results with those of a control group of 22 patients without lower urinary tract symptoms. This study indicated that there were no significant differences in ultrasound patterns of patients with nonbacterial prostatitis and the control group. Others have employed color Doppler ultrasonography (Veneziano et al, 1995) and automated computer analysis (de la Rosette et al, 1995) in an attempt to improve the value of transrectal ultrasonography in the evaluation of prostatitis patients; however, the results are not conclusive enough to indicate that this is a clinically useful tool.
Transrectal ultrasonography can be valuable in diagnosing medial prostatic cysts in patients with prostatitis-like symptoms (Dik et al, 1996), diagnosing and draining prostatic abscesses (Granados et al, 1992), or diagnosing and draining obstructed seminal vesicles (Littrup et al, 1988). It is not required in all cases of acute bacterial prostatitis but rather only in those patients who are failing appropriate antimicrobial therapy (Horcajada et al, 2003).
Prostate Biopsy
Occasionally, because of an elevated PSA level or abnormal digital rectal examination, prostate biopsy is indicated (Kawakami et al, 2004). Some clinicians will consider starting patients with elevated screening PSA levels and a history of prostatitis or symptoms of CPPS on antibiotics, but this practice is really only rational in patients with acute or chronic bacterial prostatitis (Nickel, 2002c), conditions that invariably lead to elevated PSA levels. The diagnosis of CP/CPPS should be used only as a reason against a prostate biopsy if the clinician is looking for an excuse not to biopsy (Nickel, 2002c). A comprehensive review on PSA and prostatitis is available (Kawakami et al, 2004).
Out of desperation, urologists sometimes resort to prostate biopsy in an attempt either to demonstrate histologic evidence of prostatic inflammation or to culture an organism that cannot be cultured employing the standard approach. The importance and interpretation of prostate biopsies in prostatitis performed for reasons other than prostate cancer screening is unclear. Doble and associates (1990) demonstrated immune complexes in the prostate of patients with prostatitis but found culture of the prostatic tissue unhelpful (Doble et al, 1989a). Nickel and Costerton (1993) were able to confirm the presence of potentially uropathogenic bacteria in patients with a documented history of chronic bacterial prostatitis in whom EPS cultures became sterile after antibiotic therapy. Berger and associates (1997) also confirmed the presence of potential uropathogenic bacteria in prostate biopsy specimens (which correlated to some extent with prostatic inflammation in EPS) in patients in whom the same bacteria did not grow standard prostatic specimens (e.g., EPS). Krieger and colleagues (1996b) demonstrated the possible presence of microorganisms in the prostate gland of a majority of men with chronic prostatitis syndrome using the molecular biologic technique of polymerase chain reaction. At this time, histologic, culture, and molecular biologic evaluations of prostate biopsy specimens in patients with CP/CPPS remain research tools only.
Seminal Vesiculitis
Occasionally, seminal vesiculitis can occur as a consequence of local bacterial infection in acute and chronic bacterial prostatitis (Zeitlin, 1999) and patients can present with seminal vesicle abscesses (Stearns, 1963; Kennelly and Oesterling, 1989). Seminal vesicle abscesses were traditionally diagnosed clinically by a positive ejaculate culture and seminal vesiculography (Dunnick et al, 1982; Baert et al, 1986) but is now imaged with computed tomography (Patel and Wilbur, 1987), transrectal ultrasonography (Littrup et al, 1988), magnetic resonance imaging (Sue et al, 1989) or recently with technetium-99m ciprofloxacin radioisotope scan (Choe et al, 2003).
Other Possible Markers
Wishnow and associates (1982) found that control patients (10 patients) and men with chronic abacterial prostatitis (4 patients) had no antibodies to gram-negative bacterial antigens, in contrast to men with bacterial prostatitis (6 patients). They hypothesized that immunologic analysis may provide a better diagnostic tool than culturing and microscopy. Shortliffe and coworkers (1981a, 1981b, 1986, 1989, 1992) found that the total IgA and IgG levels in the prostatic fluid in men with chronic abacterial prostatitis were higher than those of controls. They also discovered that prostatic fluid from control or abacterial prostatitis patients did not contain specific antibodies to gram-negative urinary pathogens (in contrast to men with bacterial prostatitis). Nickel and colleagues (2001a) used a similar antibody screening procedure in evaluation of 102 men with CP/CPPS who were subsequently treated with quinolone antibiotics. However, “antibody-positive” patients did not have a better response to antibiotic therapy than “antibody-negative” patients after 12 weeks of therapy. Li and associates (2001) demonstrated increased endotoxin concentrations in EPS and VB3 of men with bacterial prostatitis and inflammatory category IIIA CPPS and suggested that endotoxin levels might be used to identify these categories of patients with chronic prostatitis.
Alexander and colleagues (1998) discovered that men with chronic abacterial prostatitis had higher mean levels of the proinflammatory cytokines IL-1α and TNF-α in seminal plasma compared with controls. Ruggieri and coworkers (2000) noted that levels of both IL-1α and IL-8 were significantly higher in semen in category IIIA patients (leukocytes) than in category IIIB patients, but there was no statistically significant difference in levels of TNF-α, IL-1α, or IL-6. This group found no correlation between cytokine levels and the number of leukocytes in EPS. The increased IL-8 levels in the semen of patients with prostatitis symptoms has recently been confirmed by Khadra and coworkers (2006) and Penna and associates (2007), suggesting that this could be a surrogate marker for CP/CPPS. Nadler and colleagues (2000) found that mean levels of IL-1α in EPS were higher in men with inflammatory chronic abacterial prostatitis and noninflammatory chronic bacterial prostatitis compared with controls. Hochreiter and associates (2000a) did find a direct significant correlation between the number of leukocytes in EPS and IL-1α levels in EPS. One of the most intriguing possible biomarkers includes monocyte chemoattractant protein-1 and macrophage inflammatory protein-1α detected in EPS. Both of these chemokines are elevated in category IIIA and IIIB CP/CPPS, whereas macrophage inflammatory protein-1α may be a further marker for clinical pain in these patients (Desireddi et al, 2008). The sensitivity, specificity, and, more importantly, the clinical applicability of all these immunologic tests is really unknown and none of them is yet indicated in clinical practice.
Marmar and associates (1980) hypothesized that zinc levels in EPS would be a useful marker for prostatitis and found that, indeed, zinc levels in men with chronic abacterial prostatitis and bacterial prostatitis were significantly lower than zinc levels in control patients and men with prostatodynia. However, Zaichick and colleagues (1996) found no differences in zinc levels between patients with chronic abacterial prostatitis, BPH, and controls. At this time the measurement of zinc levels in prostatic or semen specimens is clinically unhelpful.
Tanner and associates (1999) detected positive signals (rRNA-based molecular technique with prostatic fluid) in 65% of patients with chronic prostatitis. Seven of 11 patients with bacterial signals but none of 6 patients without bacterial signals was improved on antibiotic therapy. The same group (Shoskes and Shahed, 2000) subsequently confirmed this finding with a larger cohort of patients. These results are intriguing, and controlled studies evaluating the potential clinical significance of differentiating patients based on molecular biologic techniques are required.
An Approach to Diagnosis and Classification
A diagnostic algorithm that provides a practical approach to the workup of the majority of men presenting with CP/CPPS is shown in Figure 11–7. Table 11–2 shows the tests recommended by the NIH Third International Prostatitis Collaborative Network (Nickel, 2003).
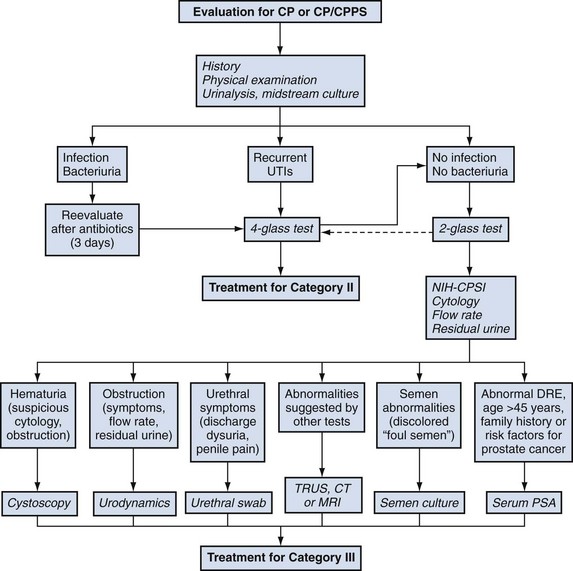
Figure 11–7 A suggested diagnostic algorithm for the evaluation of patients presenting with CP/CPPS. UTIs, urinary tract infections; TRUS, transrectal ultrasonography; CT, computed tomography; MRI, magnetic resonance imaging; DRE, digital rectal examination; PSA, prostate-specific antigen.
Table 11–2 Suggested Evaluation of a Man with Chronic Pelvic Pain Syndrome*
| Mandatory |
| Recommended |
| Optional |
* See text for explanation, rationale, and description of each test.
Treatment
In this section the rationale for each of the various treatments advocated for the prostatitis syndromes is presented followed by a review of the clinical trial data that support (or not) the use of those specific therapeutic modalities in clinical practice. Recent rigorous prospective studies in chronic bacterial prostatitis and randomized placebo-controlled trials employing standardized definitions and validated outcomes in CP/CPPS have allowed us to develop a best-evidence-based treatment strategies in a therapeutic field that used to be based on poor clinical data, dogma, and anecdotal experience (McNaughton Collins et al, 2000b, 2001a; Nickel, 2002a, 2002b, 2004; Schaeffer, 2006; Nickel, 2008b).
Antimicrobial Agents
Rationale
It is generally accepted that acute and chronic bacterial prostatitis are etiologically secondary to bacterial infection of the prostate gland. Many urologists further believe that, although bacteria are cultured in only 5% to 10% of cases of prostatitis, bacteria may be the cause of prostatitis in a significant percentage of patients presenting with this syndrome. Antimicrobial therapy is the most commonly prescribed treatment for the chronic prostatitis syndromes (Moon, 1997; Nickel et al, 1998a; McNaughton Collins et al, 2000b, 2001a), independent of culture status.
Pharmacology and Pharmacokinetics
Most antimicrobial pharmacokinetic studies were performed in animal models (dogs and rats) (Madsen et al, 1978; Nickel, 1997b). Stamey (1980) and Stamey and associates (1970) found that acid antibiotic drugs can be detected in prostatic secretions only in very low concentrations, even when plasma concentrations of the drug are very high. Alkaline antibiotic drugs are found in concentrations greater than the simultaneous plasma levels. In dogs, this was explained by the presence of a pH gradient across the prostate epithelium. This phenomenon of ion trapping, and the fact that drug penetration was believed to be a passive transport mechanism based on diffusion and concentration, suggested that drug penetration is dependent on the lipid solubility, degree of ionization, degree of protein binding, and size and shape of the antimicrobial molecule. In dogs, the pH of plasma was found to be 7.4, whereas that of prostatic secretion is 6.4. Therefore, in this model, weak acids (low pKa) concentrate on the plasma side whereas antibiotics with a higher pKa (weak bases) concentrate in the prostatic secretion.
Because infection may alter the local prostatic environment, thus changing the pharmacokinetic parameters, animal models were developed that introduced infection into the process (Baumueller and Madsen, 1977; Madsen et al, 1994; Nickel et al, 1995). All these animal studies (with and without infection) showed that trimethoprim concentrates in prostatic secretion and prostatic interstitial fluid (exceeding plasma levels), whereas sulfamethoxazole and ampicillin do not. The fluoroquinolones, which are neither pure acids nor bases but have characteristics of both, being zwitterionic drugs (e.g., those that have two pKa values) (Gasser et al, 1986), should allow drug concentration in the prostate at various pH ranges. In the dog model, ciprofloxacin and norfloxacin did not concentrate in prostatic secretion as predicted, and this may be accounted for by their lipid solubility and protein-binding characteristics. Carbenicillin, which for years was the only antibiotic approved by the U.S. Food and Drug Administration for the treatment of bacterial prostatitis, could not be detected in prostatic secretion at all. Aminoglycosides did not concentrate in prostatic secretion and for unknown reasons may not follow the rules of nonionic diffusion of drugs across biologic membranes.
It is difficult to extrapolate the animal pharmacokinetic studies to humans (Sharer and Fair, 1982). Fair and Cordonnier (1978) found that the prostatic secretion of normal men is slightly alkaline (pH ~ 7.3) but also that the pH of prostatic secretion in men with prostatic infection is markedly increased (pH ~ 8.3). This has been confirmed in other studies (Anderson and Fair, 1976; Blacklock and Beavis, 1978; Pfau et al, 1978), and because the pH gradation is crucial to ion trapping we should not apply the results from animal studies directly to humans. Unfortunately, drug diffusion studies are difficult to perform in humans and most studies determine antibiotic concentrations in transurethrally resected BPH adenomas. These studies are further complicated because the high drug concentrations in urine can substantially alter the results. Employing a method to reduce urine contamination, Naber and Madsen (1999) demonstrated that for most fluoroquinolones the ratio of concentrations in prostatic fluid to concentrations in plasma is less than 1 (norfloxacin ratio 0.12, ciprofloxacin ratio 0.18 to 0.26, lomefloxacin ratio 0.48). Concentrations in seminal fluid usually exceed corresponding plasma concentrations of ciprofloxacin and ofloxacin, with ciprofloxacin demonstrating the highest ratio of seminal fluid to plasma (Naber, 1999). The numerous studies evaluating fluoroquinolone concentrations in prostatic tissue demonstrated that the fluoroquinolone concentration in the adenoma tissue is usually higher than that in plasma.
Clinical Trial Data
Unless the patient has a significant anatomic abnormality of the lower urinary tract or develops a prostate abscess, antimicrobial therapy is universally successful in eradicating the bacteria and curing the patient presenting with acute bacterial prostatitis (Nickel and Moon, 2005). In the acutely inflamed prostate gland the pharmacokinetic considerations described in the previous section probably do not play a significant role in antibiotic penetration, and it is believed that most antibiotics achieve reasonable intraprostatic concentrations in the acute phase of the disease. Although prospective clinical trial data are unavailable, most experts suggest therapy initially with parenteral antibiotics (depending on the seriousness of the infection) followed by oral antibiotics with wide-spectrum antimicrobial activity (Becopoulos et al, 1990). The most common drugs suggested for initial therapy (Neal, 1999; Ludwig, 2008; Benway and Moon, 2008) are a combination of penicillin (i.e., ampicillin) and an aminoglycoside (i.e., gentamicin), second- or third-generation cephalosporins, or one of the fluoroquinolones. Once the acute infection has settled down, therapy should be continued with one of the oral antimicrobial agents appropriate for the treatment of chronic bacterial prostatitis (e.g., trimethoprim or fluoroquinolones). The duration of optimal therapy is unknown; between 2 and 4 weeks has been suggested (Nickel, 1998b; Bjerklund-Johansen et al, 1998; Wagenlehner et al, 2007; Ludwig, 2008).
In the 1970s to 1990s the most commonly used antimicrobial agent in the treatment of chronic prostatitis was trimethoprim-sulfamethoxazole (co-trimoxazole) (Moon, 1997; Nickel et al, 1998a) and, to a lesser extent, trimethoprim alone. In patients with chronic bacterial prostatitis, eradication of pathogens (the only objective measurement in most chronic prostatitis studies) with trimethoprim-sulfamethoxazole or trimethoprim alone ranged from a low of 0% (Smith et al, 1979) to a high of 67% (Paulson and White, 1978), with most studies demonstrating an efficacy rate of between 30% and 50% (Meares, 1973, 1975, 1978; Drach, 1974b; McGuire and Lytton, 1976). It appears that longer-duration therapy (90 days) provides the best clinical results. Trimethoprim-sulfamethoxazole is less effective both in bacterial eradication and cost-effectiveness when compared with the newer fluoroquinolones (Kurzer and Kaplan, 2002).
Except for the well-studied fluoroquinolones, most antibiotics (including minocycline, cephalexin, and carbenicillin) do not demonstrate significant clinical efficacy in clinical studies in which patients were observed for sufficient time (Paulson and White, 1978; Oliveri et al, 1979; Mobley, 1981). One notable exception has been the macrolides, erythromycin (Mobley, 1974), azithromycin (Skerk et al, 2003), and clarithromycin (Skerk et al, 2002b), particularly when C. trachomatis is implicated.
The fluoroquinolones have demonstrated improved therapeutic results, especially in prostatitis caused by E. coli and other members of the Enterobacteriaceae but not necessarily in prostatitis due to P. aeruginosa or enterococci. Naber (1999) analyzed the many studies available in the literature evaluating fluoroquinolones in the treatment of chronic prostatitis and found eight comparable studies in which the diagnosis was obtained by localization studies and in which the patients were observed for a sufficient time after completion of therapy (Weidner et al, 1987; Pust et al, 1989; Schaeffer and Darras, 1990; Heidler, 1990; Weidner et al, 1991a; Pfau, 1991; Ramirez et al, 1994; Koff, 1996); in these studies the researchers evaluated norfloxacin, ciprofloxacin, ofloxacin, and lomefloxacin. In 2005 Naber (reported at the Sixth International Consultation on New Developments in Prostate Cancer and Prostate Disease, Paris, June, 2005) added three more recent studies that met these strict criteria (Naber et al, 2000, 2002; Bundrick et al, 2003). These studies are outlined in Table 11–3. A subsequent study has suggested that levofloxacin may also be effective in treating chronic bacterial prostatitis (Naber et al, 2008). For chronic prostatitis caused by E. coli, treatment duration of 1 month for the fluoroquinolones seems to be superior to the usual 3-month treatment with trimethoprim-sulfamethoxazole. It has been suggested that antibiotics should be continued only for 4 to 6 weeks if pretreatment cultures are positive and/or the patient has reported positive effects from treatment (Wagenlehner et al, 2007). Some clinicians have observed that as many as 20% of patients who fail an initial treatment period could be rescued with a second cycle of treatment with another antibiotic (Magri et al, 2007). In microbiologically diagnosed chronic bacterial prostatitis, eradication of bacteria is associated with both short-term and long-term clinical success (Nickel and Xiang, 2008). This appears to be true in men with recent onset of prostatitis associated with bacterial localization with the traditional uropathogens (gram-negative uropathogens and enterococci species) as well as nontraditional bacteria (gram-positive such as coagulase-negative staphylococcal and streptococcal species) (Magri et al, 2007; Nickel and Xiang, 2008). A number of investigators (Baert and Leonard, 1988; Jimenez-Cruz et al, 1988; Yamamoto et al, 1996; Guercini et al, 2005b) have advocated direct injection of antibiotics into the prostate gland, but this method has never been rigorously evaluated or become popular among urologists.
Many studies evaluating physicians’ practice patterns in prostatitis syndromes (de la Rosette et al, 1992a; Moon, 1997; Nickel et al, 1998a; McNaughton Collins et al, 1998, 2000a) have confirmed that most patients diagnosed with chronic prostatitis, irrespective of culture results, are treated with antimicrobial therapy. Older studies have generally indicated that approximately 40% of patients with nonbacterial chronic prostatitis have some symptomatic improvement with antimicrobial therapy (Berger et al, 1989; Weidner, 1992; de la Rosette et al, 1993a; Ohkawa et al, 1993b; Bergman, 1994; Bjerklund-Johansen et al, 1998; Tanner et al, 1999; Nickel et al, 2001a). Antibiotic therapy may benefit CP/CPPS patients by three different mechanisms: a strong placebo effect, the eradication or suppression of noncultured microorganisms (Nickel et al, 2001a), or the independent anti-inflammatory effect of some antibiotics (Yoshimura et al, 1996; Galley et al, 1997). It has been suggested by a European consensus group evaluating the role of antibiotics in the treatment of chronic prostatitis (Bjerklund-Johansen et al, 1998) that antibiotics should be considered empirical treatment for category IIIA CP/CPPS but the benefits should be appraised after a minimum of 2 to 4 weeks of therapy. The antibiotics could be continued for 4 to 6 weeks if the patient has reported positive effects from treatment (Wagenlehner et al, 2007). These recommendations remain controversial, particularly because new data appear to provide conflicting interpretations. Two multicenter randomized placebo controlled studies have assessed the efficacy of 6 weeks of levofloxacin (Nickel et al, 2003b) and ciprofloxacin (Alexander et al, 2004) in men with CP/CPPS. In these trials the participants had chronic symptoms for a long duration (many years) and had been heavily treated (including treatment with antibiotics). In the study by Nickel and associates (2003b), 80 patients were randomized to levofloxacin or placebo whereas in the NIH-sponsored study reported by Alexander and colleagues (2004) 196 men with CP/CPPS were randomized in a 2 × 2 factorial design to ciprofloxacin, tamsulosin, the combination of ciprofloxacin and tamsulosin, or placebo. In both of these prospective-designed controlled multicenter trials, no significant difference was reported between the fluoroquinolone and placebo in terms of symptom amelioration. Antibiotics should not be prescribed for previously treated men with CP/CPPS of long duration. However, two recent prospective trials comparing the effect of 4 to 6 weeks of antibiotics (Magri et al, 2007; Nickel and Xiang, 2008) in men with localization of both traditional uropathogens and organisms not usually believed to be uropathogenic (and therefore classified as category III CP/CPPS) showed similar eradication and clinical success rates (75% to 80%). Furthermore, in the study by Nickel and Xiang (2008), the eradication of those organisms, whether or not they were considered to be uropathogens, correlated with both short- and long-term clinical success. Because the majority of patients in Nickel and Xiang’s study (2008) had a short history of prostatitis and were antibiotic naive for that episode, it was concluded that antibiotic treatment may be considered for antibiotic-naive patients with recent diagnosis of prostatitis, regardless of culture status.
α-Adrenergic Blocker Therapy
Rationale
Patients with CP/CPPS have significant lower urinary tract symptoms, which appear to be related to poor relaxation of the bladder neck during voiding (Barbalias et al, 1983; Murnaghan and Millard, 1984; Blacklock, 1986; Hellstrom et al, 1987; Barbalias, 1990; Kaplan et al, 1997). The subsequent turbulent “dysfunctional” voiding may predispose the patient to reflux of urine into the prostatic ducts, causing intraprostatic inflammation and subsequently pain (Kirby et al, 1982). The bladder neck and prostate are rich in a receptors, and it is hypothesized that α-adrenergic blockade may improve outflow obstruction, improving urinary flow and perhaps diminishing intraprostatic ductal reflux.
Clinical Trial Data
A number of older clinical trials suggested that the α-adrenergic blockers diphenoxybenzamine (Dunzendorfer et al, 1981), phenoxybenzamine (Osborn et al, 1981), alfuzosin (de la Rosette et al, 1992c; Barbalias et al, 1998), terazosin (Neal and Moon, 1994; Barbalias et al, 1998; Lacquaniti et al, 1999; Gül et al, 2001), doxazosin (Evliyaoglu and Burgut, 2002) and tamsulosin (Lacquaniti et al, 1999) resulted in significant symptomatic improvement of prostatitis-related symptoms; however, these trials were small, most were uncontrolled, and outcome measures were not validated. The study by Barbalias and associates (1998) further seemed to indicate that the combination of antibiotics and α-adrenergic blockers improved the clinical result in patients with chronic bacterial prostatitis.
Four randomized placebo controlled trials with clearly defined CP/CPPS patients (NIH classification) and employing the NIH-CPSI as the outcome parameter appear to have confirmed the efficacy of α-adrenergic blockers but only in men who have recent onset disease not those heavily pretreated and who are on therapy for longer than 6 weeks. Cheah and colleagues (2003) randomized 86 patients with chronic prostatitis to either terazosin or placebo for 14 weeks. Patients on terazosin had a 50% reduction in mean symptom score compared with 37% in the placebo-treated group. Terazosin resulted in modest but significant improvement in all domains of the NIH-CPSI. Mehik and colleagues (2003) followed 19 patients randomized to 6 months of alfuzosin treatment and 20 patients on 6 months of placebo therapy and both groups were followed for a further 6 months after discontinuing the active or placebo medication. Patients in the alfuzosin group had a significant amelioration of symptoms compared with the placebo therapy group that was evident at 4 months and became even more clinically significant by 6 months. At 6 months 65% of alfuzosin patients were rated as responders compared with 24% of placebo group. The beneficial effect appeared to wear off over the next 6 months after the alfuzosin was discontinued. Nickel and colleagues (2004b) randomized 57 men with CP/CPPS to tamsulosin, 0.4 mg, or placebo after a 2-week placebo run-in and observed the two groups for 6 weeks. Patients treated with tamsulosin had a statistically significant (but only modest clinically significant) treatment effect compared with patients taking a placebo. A significant treatment effect was not observed in patients who had mild symptoms, but patients with severe symptoms (75th percentile) had a statistically and clinically significant response compared with placebo. It appears that the response to α-adrenergic blockers is durable, for at least up to 24 to 38 weeks (Mehik et al, 2003; Cheah et al, 2004). Another study (Tugcu et al, 2007) included 90 treatment-naive patients with CP/CPPS randomized to receive doxazosin, 4 mg/day, alone or a triple therapy (doxazosin, 4 mg/day plus an anti-inflammatory agent—ibuprofen, 400 mg/day, and a myorelaxant-thiocolchicoside, 12 mg/day) or placebo. Over 6 months, the total NIH-CPSI score significantly improved in the doxazosin group (from 23.1 to 10.5 points) and triple-therapy groups (from 21.9 to 9.2) while it remained stable in the placebo group (from 22.9 to 21.9).
In contrast, the results from the NIH CPCRN randomized controlled trial (Alexander et al, 2004) comparing 6 weeks of ciprofloxacin, tamsulosin, and the combination of ciprofloxacin and tamsulosin to placebo in very chronic and heavily pretreated patients failed to show any improvement in patients treated with tamsulosin (± ciprofloxacin) compared with patients treated with placebo. A number of meta-analyses and comprehensive reviews of these data recommended that α-adrenergic blockers provided significant symptom amelioration only after more than 6 weeks of therapy in less heavily treated patients with recent onset of moderate to severe symptoms (Mishra et al, 2007; Yang et al, 2006; Nickel, 2008a). To test this hypothesis, an NIH multicenter, randomized, double-blind, placebo-controlled trial was conducted to evaluate the efficacy of 12 weeks of alfuzosin or placebo to reduce symptoms in 272 randomized men with CP/CPPS diagnosed within 2 years previously and who had not been previously treated with an α-adrenergic blocker (Nickel et al, 2008a). The rate of the primary outcome (reduction of at least 4 points in NIH-CPSI total score from baseline) was 49% in both treatment groups. The response rates at 12 weeks measured with a global response assessment were also similar, 34% and 35%, for the placebo and alfuzosin groups, respectively (P = .90). These important findings did not support the use of α-adrenergic blockers in recently diagnosed α-adrenergic blocker–naive men with CP/CPPS.
Anti-Inflammatory Agents and Immune Modulators
Rationale
Prostatic inflammation is associated with category IIIA CP/CPPS, and elevated cytokine levels are noted in the semen (Alexander et al, 1998; Ruggieri et al, 2000) and EPS (Nadler et al, 2000; Hochreiter et al, 2000b) of patients with inflammatory CPPS. Nonsteroidal anti-inflammatory drugs, corticosteroids, and immunosuppressive drugs theoretically should improve the inflammatory parameters within the prostate and possibly result in a reduction of symptoms (Pontari, 2002).
Clinical Trial Data
Canale and associates (1993a) found that nimesulide (a nonsteroidal anti-inflammatory drug) quickly reduced inflammatory-type symptoms such as dysuria, strangury, and painful ejaculation. A second study by Canale’s group (1993b) found that, by the rectal route, ketoprofen was inferior to nimesulide (both drugs were used as suppositories). Prednisolone has been suggested as a potent anti-inflammatory for chronic prostatitis (Bates and Talbot, 2000) and a randomized study presented by Dimitrakov and associates (2004) indicates that high-dose methylprednisolone (followed by rapid tapering of dose) may have more efficacy than placebo, even after 12 months, but the side effect profile makes this type of therapy less attractive. A small randomized trial evaluating oral corticosteroids did not show superiority of the active therapy over placebo (Bates et al, 2007).
The new class of cyclooxygenase-2 inhibitors has proved successful for long-term treatment of other chronic inflammatory conditions such as rheumatoid arthritis and chronic osteoarthritis, and many urologists employed these medications for prostatitis patients with some anecdotal successes reported. The results of a North American randomized control trial comparing the cyclooxygenase-2 inhibitor rofecoxib to placebo indicated that many men with CPPS benefited (in terms of pain and quality of life) from rofecoxib therapy compared with placebo. In this study in which 161 patients were randomized to rofecoxib 25 mg, rofecoxib 50 mg, or placebo, only patients on the high dose showed any clinical improvement compared with the placebo. Very few patients, however, had complete resolution of their symptoms (Nickel et al, 2003c). Another study from China (Zeng et al, 2004) assessing the effectiveness of two doses of the cyclooxygenase-2 inhibitor celecoxib also demonstrated a dose-dependent response (200 mg twice a day for 6 weeks was more effective than 200 mg once a day). At this time, high-dose, long-duration therapy with cyclooxygenase-2 inhibitors is not recommended.
Because the clinical and pathologic characteristics are similar to those of interstitial cystitis, Wedren (1987) compared the efficacy of pentosan polysulfate, a glycosaminoglycan drug that has been used in the treatment of interstitial cystitis, with placebo. In this small study the treated group was noted to have a statistically significant improvement in symptoms but the major symptom that improved was nonspecific myalgias and arthralgias. An uncontrolled pilot study evaluating oral pentosan polysulfate in 32 men with CPPS demonstrated amelioration of symptoms and improvement in the quality of life in over 40% after treatment for 6 months (Nickel et al, 2000). The results of a multicenter, randomized, placebo-controlled trial that randomized 100 men to pentosan polysulfate, 900 mg/day (three times the usual dose), or placebo indicated this medication provided modest benefit for some men with CPPS (Nickel et al, 2005b).
Thalidomide, a cytokine modulating drug, was assessed in 30 men with chronic abacterial prostatitis and abnormal semen cytokine levels (IL-2, IL-6, IL-8, IL-10, and TNF-α) in a randomized placebo-controlled trial (Guercini et al, 2005). Despite a significant reduction in cytokine levels in semen, no difference in symptom relief was noted. A similar lack of efficacy was noted in a small placebo-controlled trial evaluating the leukotriene antagonist zafirlukast (Goldmeier et al, 2005).
The potential of various anti-inflammatory agents, immune modulators, and cytokine inhibitors makes these classes of drugs potentially useful as adjunctive therapy for the chronic prostatitis syndromes, but clinical trials suggest that they are not a useful monotherapy.
Muscle Relaxants
Rationale
Many investigators believe that CPPS is the ultimate reflection of a smooth and skeletal neuromuscular dysregulatory phenomenon in the perineum or pelvic floor (Osborn et al, 1981; Egan and Krieger, 1997; Anderson, 1999; Zermann and Schmidt, 1999). The use of α-blockers to relax smooth muscle (see earlier discussion of α-adrenergic blockers) and skeletal muscle relaxants combined with adjuvant medical and physical therapies has been advocated and promoted (Anderson, 1999; Zerman and Schmidt, 1999).
Clinical Trial Data
In one of the few studies to compare muscle relaxants to placebo, Osborn and associates (1981) conducted a prospective double-blind study comparing phenoxybenzamine, baclofen (a striated muscle relaxant), and placebo in 27 patients with prostatodynia (category IIIB). Patients were treated with each agent for 1 month in a crossover trial. Symptomatic improvement was seen in 37% of the patients treated with baclofen compared with 8% treated with placebo. Simmons and Thin (1985) compared diazepam with an antibiotic in patients with chronic abacterial prostatitis and found no difference in symptom improvement between the diazepam group (8 of 11 men improved) and the antibiotic group (7 of 12 men improved). Unfortunately these studies were hindered by a lack of controlled and defined entry criteria and no quantified measurement of patients’ responses and therefore the role of muscle relaxants has yet to be determined.
Hormone Therapy
Rationale
Prostate growth and function are influenced by the local hormonal milieu, especially by androgens. Theoretically, antiandrogens (including 5α-reductase inhibitors) could result in regression of prostatic glandular tissue (inflammation is believed to begin at the level of the ductal epithelium), improved voiding parameters (especially in older patients with BPH and prostatitis), and reduced intraprostatic ductal reflux (Nickel, 1999c).
Clinical Trial Data
Holm and Meyhoff (1996) were the first to note that the 5α-reductase inhibitor finasteride had potential in alleviating symptoms by observing the effect of finasteride therapy in 4 patients with chronic prostatitis or prostatodynia. Leskinen and colleagues (1999) randomized 41 patients with chronic idiopathic prostatitis (i.e., nonbacterial prostatitis and prostatodynia) to treatment with placebo (25%, or 10 patients) or finasteride (75%, or 31 patients) for 1 year. Compared with placebo, finasteride reduced prostatitis and BPH symptom scores; however, there was no statistically significant difference in pain between the two groups. The baseline characteristics of the two groups were not comparable, and the enrolled patients consisted of an unknown mixed population with inflammatory and non-inflammatory prostatitis syndromes. A randomized open label comparative trial in CP/CPPS men showed significantly more improvement in men treated for a year with finasteride compared with saw palmetto, an herbal therapy (Kaplan et al, 2004). A randomized controlled trial compared the reduction of NIH-CPSI in 64 men with CP/CPPS randomized to finasteride or placebo (Nickel et al, 2004c). Six months of finasteride resulted in a numerical but not statistically significant reduction in symptoms compared with the symptom reduction noted in the placebo group. Finasteride cannot be recommended as a monotherapy except perhaps in men with associated BPH.
Testosterone and dihydrotestosterone are not the only hormones with a possible effect on prostate inflammation; estrogens may also play a role. A number of small, poorly controlled studies (Cavallini, 2001; Saita et al, 2001) suggested that mepartricin (a drug that lowers estrogen levels in the prostate) may be useful in the treatment of CP/CPPS. A small prospectively designed trial randomized 26 men with CP/CPPS to 60 days therapy with mepartricin or placebo (De Rose et al, 2004). The study showed a statistical and perhaps clinically significant benefit (60% vs. 20% improvement, respectively) that should stimulate further research in the role of hormonal manipulation (in this case estrogens) in the treatment of CP/CPPS.
Phytotherapeutic Agents
Rationale
A number of plant extracts have been shown in many in-vitro experiments to have 5α-reductase activity, α-adrenergic blockade activity, effects on bladder contractility, and anti-inflammatory properties (Lowe and Fagelman, 1999; Shoskes, 2002).
Clinical Trial Data
Three specific phytotherapeutic agents have been tested in clinical trials, Cernilton, a pollen extract (Buck et al, 1989; Rugendorff et al, 1993), Quercetin, a natural bioflavonoid (Shoskes et al, 1999) and Serenoa repens (saw palmetto berry) extract (Kaplan et al, 2004; Reissigl et al, 2004). Rugendorff and coworkers (1993) noted that over half of 72 patients with chronic prostatitis without other lower urinary tract abnormalities had favorable improvements in pain and irritative voiding symptoms when treated with Cernilton, but no control group was included in this study. A randomized study of pollen extract (Cernilton) in 122 men with category IIIA showed that men on the active treatment had statistically significant improvements in the pain and quality-of-life components of the CPSI (Wagenlehner et al, 2009). A controlled, randomized study of a similar preparation, Prostat/Poltit (grass pollen extract, including rye pollen), in 60 patients showed greater improvement in patients on active therapy compared with placebo but no validated outcome index incorporated into the study design (Elist, 2006). Shoskes and associates (1999) randomized 15 patients to the bioflavonoid Quercetin and 13 patients to placebo for 1 month. Sixty-seven percent of the patients in the treatment group were considered responders compared with only 20% of the patients in the placebo arm. Kaplan and associates (2004) did not note any appreciable long-term improvement in any CP/CPPS parameters when compared with 12 months of finasteride in a randomized open-label comparative study. However, Reissigl and colleagues (2004) reported that there was a moderate/marked improvement in over 60% of 72 CP/CPPS patients after 12 months of therapy with Serenoa repens extract compared with less than 25% in the 70 men in the placebo-treated group. However, further follow-up did not support the durability of this therapy (Reissigl et al, 2005). Phytotherapy for CP/CPPS may look promising but further multicenter randomized controlled trials with well-characterized, standardized, and stable herbal components should be considered to assess their role in therapy.
Neuromodulator Therapy
Rationale
One proposed mechanism is that CP/CPPS, particularly in chronic cases of long standing, represents a neurogenic pain syndrome and that the subsequent pain is actually a neuropathic pain (Pontari and Ruggieri, 2004). Patients with CP/CPPS have a history of neurologic disease that is almost five times more likely among cases than control subjects (Pontari et al, 2005), and men with CP/CPPS have been found to have abnormalities of both the afferent and efferent autonomic nervous systems (Yang et al, 2003; Yilmaz et al, 2007). This type of neuropathic pain related to central nervous system sensitization responds to gabapentinoids in other chronic pain conditions (Rosenstock et al, 2004; Crofford et al, 2005).
Clinical Trial Data
A recent NIH CPCRN randomized placebo-controlled trial evaluated effect of the gabapentinoid pregabalin on symptoms of men with long-standing, treatment-refractory CP/CPPS (Pontari et al, 2010). Among the 103 men assigned to pregabalin, 47% reported at least a 6-point decrease in total NIH-CPSI score at 6 weeks (primary end point) compared with 35.8% of 106 men assigned to placebo (P = .072). The NIH-CPSI total score decreased by a mean of 6.6 and 4.2 points (of 43) in the pregabalin and placebo groups respectively (P = .008), whereas significantly more men in the pregabalin arm reported they were markedly or moderately improved compared with placebo (31% and 19%, respectively; P = .023). Although 6 weeks of pregabalin therapy was not superior to placebo for treating symptoms of CP/CPPS based on the primary end point, the impressive differences in secondary end points suggest that pregabalin may prove effective in some men with long-standing CP/CPPS.
Allopurinol
Rationale
Persson and Ronquist (1996) theorized that the intraprostatic ductal reflux of urine increases the concentration of metabolites containing purine and pyrimidine bases in the prostatic ducts, causing inflammation.
Clinical Trial Data
Persson and associates (1996) compared allopurinol therapy with placebo in a double-blind controlled study in 54 men. The allopurinol groups had lower levels of serum urate, urine urate, and EPS urate and xanthine. With variations in accepted statistical methodology, the investigators were able to show a difference in the mean patient-reported discomfort score between the study and the control groups at certain times in this trial with 330 days of follow-up. However, a re-examination of the data employing more standardized statistical analyses did not convince other groups that changes in the urine and prostatic secretion of purine and pyrimidine bases resulted in significant amelioration of symptoms in this particular trial (Nickel et al, 1996a). A follow-up randomized clinical trial further showed no advantage of allopurinol compared with placebo (Ziaee et al, 2006).
Prostatic Massage
Prostatic massage has been the principal therapy for prostatitis since the turn of the 20th century (O’Conor, 1936; Campbell, 1957). With the introduction of the scientific approach advocated by Meares and Stamey in 1968, prostatic massage became important only as a diagnostic tool, but as a therapy it was abandoned by urologists. Currently it is regaining some popularity, primarily because of the failure of standard medical therapy in patients with refractory symptoms of chronic prostatitis. Its benefits are believed to arise from draining theoretically occluded prostatic ducts and improving circulation and antibiotic penetration (Hennenfent and Feliciano, 1998). Independent but uncontrolled studies (Nickel et al, 1999a; Shoskes and Zeitlin, 1999) describe clinical benefits in one third to two thirds of patients treated with repetitive prostatic massage (two to three times per week) for 4 to 6 weeks along with antibiotic therapy. However, another trial indicated that prostatic massage does not significantly improve the response of men with CP/CPPS treated with antibiotics (Ateya et al, 2006). It appears that some patients may improve with prostatic massage, but a panel of North American “prostatitis experts” (Nickel et al, 1999) could not come to a consensus on the potential overall benefit or even the mechanism of achieving that benefit if it does occur. A subsequent systematic review of the literature concluded that evidence for a role of repetitive prostatic massage as an adjunct in the management of chronic prostatitis is at most “soft” but could be considered as part of multimodal therapy in selected patients (Mishra et al, 2008). Frequent ejaculation may achieve the same function as prostatic massage (Yavascaoglu et al, 1999).
Perineal or Pelvic Floor Massage and Myofascial Trigger Point Release
Most clinicians recognize that men with prostatitis syndromes, especially the noninflammatory category or prostatodynia, have specific anatomic areas that cause discomfort. Anderson (1999) believes that prolonged or chronic tension, distention, or distortion in the muscle bands (e.g., in the perineum) leads to a painful trigger point that is responsible for the pain. Predisposing factors leading to the formation of myofascial trigger points in the perineum or pelvis may include mechanical abnormalities in the hip and lower extremities, chronic urinary holding patterns (dysfunctional toilet training), sexual abuse, repetitive minor trauma, constipation, trauma, unusual sexual activity, recurrent infections or surgery, and perhaps stress and anxiety. Treatment of these trigger points includes heat therapy, physiotherapy massage, ischemic compression, stretching, anesthetic injections, acupuncture, electroneural modulation, and mind-body interactions such as progressive relaxation exercises, yoga, and hypnosis (Potts, 2003). Anderson and associates (2005) report that employing these techniques with a team consisting of a urologist, physiotherapist, and psychologist results in more than half of patients having or demonstrating a clinically detectable improvement. A case study analysis indicates that this may be an effective therapeutic approach in some patients (Anderson et al, 2005) and may result in improvement not just in pain but also in sexual function (Anderson et al, 2006). A recently reported NIH pilot study of men and women with chronic pelvic pain treated with either relaxation massage or specific pelvic massage therapy demonstrated improvement in women but could not corroborate these findings in men (Peters K, presented at AUA annual meeting, May 19, 2008).
Pudendal Nerve Entrapment Therapy
It has been hypothesized that the symptoms of CPPS could be caused by entrapment of the pudendal nerve, perhaps between the sacrotuberous and sacrospinous ligaments, in the canal of Alcock or by the falciform process of the sacrotuberous ligament (Robert et al, 1998). Pudendal nerve blocks (Thoumas et al, 1999; McDonald and Spigos, 2000; Peng and Tumber, 2008) and neurolysis surgery (Robert et al, 1993; Mauillon et al, 1999) have been suggested for treatment. The role of the pudendal nerve in chronic perineal pain deserves more scientific scrutiny.
Biofeedback
It is possible that the voiding and pain symptoms associated with CP/CPPS may be secondary to some form of pseudodyssynergia during voiding or repetitive perineal muscle spasm; biofeedback has the potential to improve this process. Kaplan and associates (1997), Nadler (2002), Ye and colleagues (2003), and Cornel and coworkers (2005) have demonstrated in small uncontrolled studies that biofeedback does ameliorate specific prostatitis-like symptoms in some men. Controlled clinical trials will be necessary to evaluate this mode of therapy.
Acupuncture
Acupuncture is an accepted traditional Chinese therapy for chronic pain, including prostatitis (Ge et al, 1988; Katai, 1992; Ikeuchi and Iguchi, 1994). Chen and Nickel (2003) determined in a pilot study of 12 treatment-refractory men that acupuncture was safe and provided effective and durable symptom improvement. A subsequent study comparing 10 weeks of acupuncture versus sham acupuncture treatment indicated that the active acupuncture proved to be almost twice as likely as sham treatment to improve CP/CPPS symptoms (Lee et al, 2008).
Psychological Support
Data from the NIH Prostatitis Cohort (Tripp et al, 2005, 2006; Nickel et al, 2008b) support a biopsychosocial model that associates the chronic pain and poor quality of life of CP/CPPS with depression and suggests that physicians may be able to advise patients to avoid certain pain coping strategies that can be associated with greater depression. Nickel and colleagues (2008) have developed an evidence-based cognitive behavioral treatment program for men with CP/CPPS. This program specifically targets empirically supported biopsychosocial variables (e.g., pain catastrophizing, depressive thinking, social support) and encourages patients to critically evaluate their patterns of thinking and to entertain novel thinking and behavioral responses to their troublesome symptoms, with an end objective to improve overall quality of life.
Studies also show that the maladaptive pain coping technique of employing “pain contingent resting” (using rest rather than more active behaviors to control pain) is reported by CP/CPPS patients in response to their pain (Tripp et al, 2006; Nickel et al, 2008b). It was suggested by Tripp and coworkers (2006) that such sedentary behaviors in the presence of pain may be associated with elevated disability in men with CP/CPPS. A double-blind randomized study showed that men participating in aerobic exercise were significantly better than those who were randomized to stretching and motion exercise, suggesting that increased physical activity is a valid option in men with CP/CPPS (Giubilei et al, 2007). The results of a study examining perceived helpfulness of medical and self-management strategies suggested that clinicians may find it useful to support patients’ use of safe, inexpensive, self-management approaches, such as warm baths, increased water intake, exercise, and avoidance of prolonged sitting (Turner et al, 2006).
Minimally Invasive Therapies
Balloon Dilatation
Lapatin and coworkers (1990) employed balloon dilatation in an uncontrolled trial of seven patients with nonbacterial prostatitis and prostatodynia and showed improvement in voiding symptoms during a 1- to 5-month follow-up. Pain and discomfort were not assessed. This treatment effect has never been substantiated, and balloon dilatation has not been routinely employed in clinical practice. Suzuki and coworkers (1995) combined the potential beneficial effects of balloon dilatation with prostatic hyperthermia in five men with CP/CPPS and demonstrated significant improvement in symptoms in one patient and partial improvement in three. Nickel and associates (1998b) were not able to duplicate this beneficial effect in a small pilot trial evaluating the “hot balloon” (heating by radiofrequency energy rather than laser energy).
Transurethral Needle Ablation
Chiang and associates (1997) employed transurethral needle ablation (TUNA) of the prostate in 7 patients with chronic nonbacterial prostatitis, assessed the patients before and after therapy (6 months of follow-up) employing a modification of the Symptom Severity Index (Nickel and Sorensen, 1996b), and reported favorable results in 4. A follow-up study by Chiang and Chiang (2004) showed significant improvement in symptoms in the majority of 32 patients treated with TUNA. But Leskinen and colleagues (2002) investigated the effectiveness and durability of TUNA in 25 patients randomized to TUNA and 8 patients randomized to sham treatment and they reported that the efficacy of TUNA in CP/CPPS is comparable to sham treatment and so could not recommend TUNA as therapy for CP/CPPS.
Other Minimally Invasive Surgeries
Serel and colleagues (1997) reported significantly meaningful beneficial effects in 30 patients with chronic abacterial prostatitis and prostatodynia employing the neodymium:yttrium-aluminum-garnet laser. Ruedi and associates (2003) suggested that high-frequency electrostimulation may be harnessed to treat chronic prostatitis. A number of other minimally invasive treatments have been examined in small pilot studies. These include pelvic and sacral electromagnetic therapy (Leippold et al, 2005; Rowe et al, 2005), extracorporeal shockwave therapy (Zimmerman et al, 2008), and injection of botulinum toxin directly into the prostate (Chuang and Chancellor, 2006).
Sham control trials will be required before minimally invasive surgery can be recommended as a treatment for CP/CPPS.
Microwave Hyperthermia and Thermotherapy
It is believed that the heat applied to the prostate gland by the microwave process could shorten the natural resolution of the inflammatory process, perhaps by accelerating the process of fibrosis or scar formation in the area of chronic inflammation. In addition, heat therapy, particularly with the higher temperatures achieved with transurethral microwave thermotherapy, could alter the afferent nerve fibers that convey the objective symptom of pain from the inflamed prostate gland (intraprostatic sympathectomy) (Perachino et al, 1993). It may even be possible that the microwave energy kills nonculturable or cryptic bacteria within the prostate gland (Sahin et al, 1998).
Although many uncontrolled trials employing heat therapy have shown benefit (Nickel, 1999d; Zeitlin, 2002), only three published studies have used sham controls. Vassily and associates (1999) noted symptom improvement in 75% of men in a transrectal microwave hyperthermia-treated group compared with 52% of men in the sham-treated group. Shaw and colleagues (1993) documented treatment success (defined as a greater than 50% improvement in symptoms) in 55% of the men in a transrectal microwave hyperthermia group (15 patients) compared with 10% of patients treated with sham therapy (13 patients) at 3 months. Nickel and Sorensen (1996b) examined the safety and efficacy of transurethral microwave thermotherapy in 20 men randomized to therapy or sham. At 3 months’ follow-up, the transurethral microwave thermotherapy–treated patients had significantly improved symptom scores compared with sham-treated patients (7 of 10 men treated with transurethral microwave thermotherapy had a favorable result compared with 1 of 10 men treated with a sham therapy). A recently reported study in men with CP/CPPS treated with cooled transurethral microwave thermotherapy using the NIH-CPSI as an outcome (Kastner et al, 2004) again suggested that thermotherapy remains a promising treatment for intractable chronic prostatitis, particularly when it is associated with concomitant BPH. Although this prospective study showed a significant reduction in NIH-CPSI score compared with baseline in 35 men followed for 12 months, it was not a randomized sham controlled trial. Heat therapy appears to be a promising therapeutic approach but, until larger-scale studies are performed, should be restricted to patients with refractory or end-stage symptoms.
Surgery
In acute bacterial prostatitis (category I), urinary obstruction is a very common symptom. Traditionally, it has been suggested that the insertion of a suprapubic cystotomy tube is the optimal therapy because an indwelling Foley catheter may further obstruct urethral ducts, resulting in the potential to develop prostate abscesses (Dajani and O’Flynn, 1968; Pai and Baht, 1972; Weinberger et al, 1988). In most patients, however, an in-and-out catheterization to relieve the initial obstruction or short-term (12 hours) indwelling catheterization with a small-caliber Foley catheter is appropriate. A developing prostate abscess, best detected with transrectal ultrasonography or computed tomography (Rovik and Doehlin, 1989), in patients who fail to respond quickly to antibiotics is optimally drained by the transurethral incision route (Pai and Baht, 1972). However, transperineal incision and drainage (Granados et al, 1992) must be considered when the abscess has penetrated beyond the prostatic capsule or penetrated through the levator ani muscle. More recently it has been suggested that percutaneous drainage of the abscess is the most effective and less morbid procedure (Varkarakis et al, 2004).
Surgery does not have an important role in the treatment of most chronic prostatitis syndromes unless a specific indication is discovered during the evaluation of the patient (Kirby, 1999). These indications are usually noted during specific and ancillary investigations such as cystoscopy, transrectal ultrasonography, urodynamics, or computed tomography/magnetic resonance imaging. Certainly, patients with urethral strictures benefit from surgical correction. Kaplan and associates (1994) have suggested that men with chronic nonbacterial prostatitis-like symptoms and urodynamic evidence of vesical neck obstruction benefit from endoscopic incision of the bladder neck.
Seminal vesicle abscesses can be managed with antibiotic therapy, transrectal aspiration, and, if necessary, an operation to remove the seminal vesicles. Traditionally seminal vesiculectomy was performed as a difficult open procedure, but recently laparoscopic excision of the seminal vesicles was reported to be the least morbid procedure (Nadler and Rubenstein, 2001).
Radical transurethral resection of the prostate (Barnes et al, 1982; Sant et al, 1984) has been advocated in patients who have either relapsing or refractory chronic bacterial prostatitis (category II) secondary to bacterial persistence within the prostate gland. Although prostatic calculi are not pathognomonic of prostatitis (Harada et al, 1980), it has been clearly shown that bacteria can persist in protective biofilms or aggregates within the interstices or on the surface of the calculus material (Meares, 1974; Nickel et al, 1994). Theoretically, removal of all the infected material, including potentially infected calculi, can be achieved (with appropriate intraoperative radiographs or ultrasound studies), but except for small anecdotal case series (Barnes et al, 1982; Sant et al, 1984) there is no substantial proof in the literature as to the efficacy of major prostate surgery in category II chronic prostatitis. Radical transurethral resection of the prostate has not been advocated for category III CP/CPPS, but open radical prostatectomy has been shown anecdotally to benefit a few patients with symptoms of nonbacterial prostatitis or prostatodynia or both (Davis and Weigel, 1990; Frazier et al, 1992). No definitive clinical series or long-term follow-up has ever been presented, and this type of surgery should not be encouraged or recommended at this time.
Treatment Summary
Acute bacterial prostatitis is relatively simple to treat; eradicate the bacteria with appropriate antibiotic therapy. The objective for chronic bacterial prostatitis is similar—eradication of bacteria, but long-term symptom amelioration sometimes eludes us. Our standard therapies for CP/CPPS, when used as monotherapy, offer only modest improvement in symptoms (Nickel et al, 2004a; Nickel, 2008b). Multimodal therapy employing multiple concurrent treatment strategies may offer the best results at this time (Shoskes et al, 2003; Shoskes and Katz, 2005). However, a number of well-controlled prospective studies did not demonstrate increased efficacy by combining α-adrenergic blockers and antibiotics (Alexander et al, 2004) or α-adrenergic blockers and anti-inflammatory agents (Batstone et al, 2005). The explanation for this difficulty in treating chronic prostatitis may be that the patients become peripherally and centrally sensitized and that treatment targeted to the initiators of the process may not work as well when the condition becomes chronic (Yang et al, 2003; Pontari and Ruggieri, 2004; Pontari, 2007). Table 11–4 is a list of the various standard medical therapies that are currently recommended. Further investigations should focus not only on the mechanisms inducing the symptoms, including the pain, but also on the mechanisms maintaining the pain.
To evaluate and compare the many clinical trials assessing the various therapies advocated for CP/CPPS it is important to clearly define and classify the patient population (NIH classification system), determine results by employing a standardized outcome index (NIH-CPSI), prospectively compare a treated group to a similar group randomized to placebo, and fulfill the requirements of peer review for publication in a reputable journal (Nickel et al, 1999c; Propert et al, 2002). In the past several years the results of a significant number of such trials have been published (Nickel, 2004, 2008a; Schaeffer, 2006), allowing the reader to assess and compare the efficacy of antibiotics, α-adrenergic blockers, anti-inflammatory agents, phytotherapies, and hormonal agents in CP/CPPS (Table 11–5). A suggested treatment algorithm based on these assessments is presented in Figure 11–8.
Table 11–5 Clinical Trials Evaluating Therapy for CP/CPPS That Met the Evidence-Based Criteria Established by Nickel (see text)
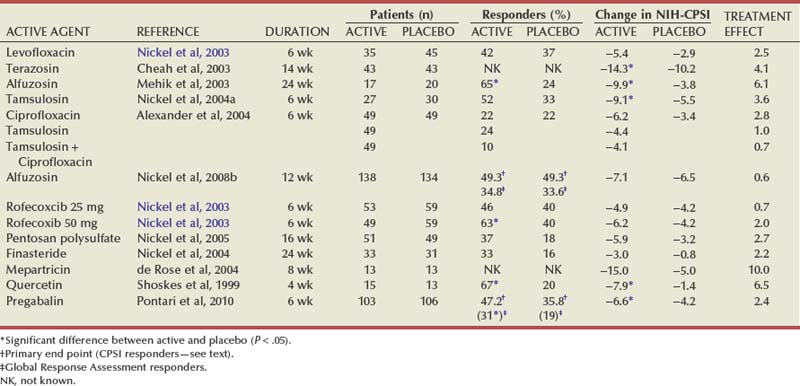
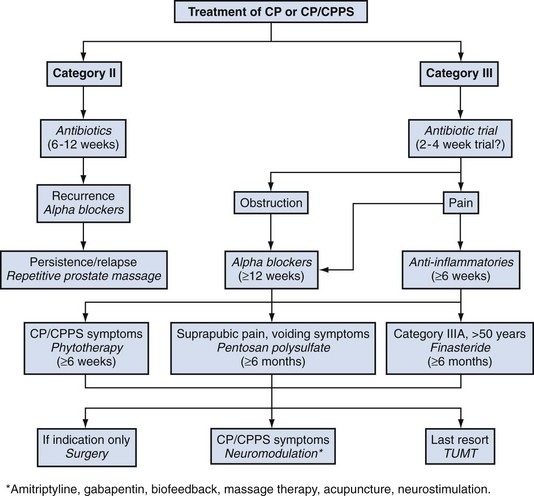
Figure 11–8 A suggested therapeutic algorithm for the treatment of patients presenting with CP/CPPS. TUMT, transurethral microwave thermotherapy.
Phenotypic Approach to Pelvic Pain (PAPP): UPOINT Management of CP/CPPS
Many clinicians dedicated to practicing an evidence-based approach to managing patients with CP/CPPS have become frustrated. Many of the traditional and new promising therapies that appear to work in real-life clinical practice fail to show significant efficacy when subjected to large randomized placebo-controlled trials (Schaeffer, 2006; Nickel, 2008b). Only recently have we become aware that patients with urologic chronic pelvic pain syndromes, such as CP/CPPS, are not a homogeneous group of patients with identical etiologic mechanisms, genitourinary pain, voiding symptoms, and/or psychosexual problems but rather a heterogeneous group of individual patients with widely differing clinical phenotypes. This realization has led the NIH to fund the Multidisciplinary Approach to Pelvic Pain (MAPP) study group to explore basic science (particularly biomarker and etiologic studies) and epidemiology to better understand the differences in this very heterogeneous group of patients. It is hoped that “phenotyping” patients may explain our very inconsistent therapeutic results and that the concept eventually may be applicable to direct better management strategies.
There are a number of reasons why the majority of randomized placebo-controlled trials reported in the literature and this chapter have been “negative,” making it difficult to develop evidence-based management guidelines. The first reason is because treatments based on a single etiologic mechanism are doomed to fail when tested in the whole CP/CPPS population. As discussed earlier under Etiology, most of the mechanisms examined are based on sound scientific theory and all are associated with at least some confirmatory clinical data. We must accept that there is no one all encompassing etiologic mechanism responsible for all cases of CP/CPPS. In addition, we cannot be sure that the patients we routinely manage in clinical practice are the same patients who have been enrolled in clinical trials. In fact, the most rigorously designed NIH-sponsored randomized controlled trials (Alexander et al, 2004; Nickel et al, 2008a; Pontari et al, 2010) did not enroll over 90% of the CP/CPPS patients who were screened. Finally, were the negative trials reported in the literature and this chapter really negative? A reappraisal of the study results would suggest otherwise. Table 11–6 outlines such a reappraisal of results in “negative” studies in CPPS. Antibiotics tended to work better in less chronic heavily pretreated patients (marginally significant improvement in levofloxacin trial) (Nickel et al, 2003b) compared with ciprofloxacin trial (Alexander et al, 2004), further substantiated by the 75% improvement seen using ciprofloxacin or levofloxacin treatment in patients with very early (4 to 8 weeks of symptoms associated with that particular episode) presentation (Nickel and Xiang, 2008). Whereas large NIH-sponsored multicenter studies failed to confirm the benefits of α-adrenergic blockers in both chronic heavily pretreated (Alexander et al, 2004) and recently diagnosed α-adrenergic blocker–naive (Nickel et al, 2008a) CP/CPPS patients, at least four other randomized controlled trials (Cheah et al, 2003; Mehik et al, 2003; Nickel et al, 2004b; Tugcu et al, 2007) with less rigorous selection criteria did show significant efficacy with α-adrenergic blockers. Although trials examining anti-inflammatory agents (Nickel et al, 2004c), pentosan polysulfate (Nickel et al, 2005b), finasteride (Nickel et al, 2004c), and pregabalin (Pontari et al, 2010) were considered negative trials based on the primary end point analysis, these trials showed efficacy for many of the validated outcomes (including responder analyses employing the validated subjective global or global response assessment scale) of statistical (Nickel et al, 2003c; Nickel et al, 2005b; Pontari et al, 2010) or marginal significance (Nickel et al, 2004c) (see Table 11–6 for details). So what do all these seemingly negative trials mean to physicians treating CP/CPPS? It is very likely that we will never discover a single overall cure for all patients diagnosed with this condition. This reevaluation of trial results, however, strongly suggests that some patients do, in fact, respond to these various therapies. We must be able to identify patients who may respond to specific therapies.
A clinically practical phenotyping classification system for patients diagnosed with urologic chronic pelvic pain syndromes (CP/CPPS and Interstitial Cystitis) has been proposed (Shoskes et al, 2009a, 2009b). UPOINT is a 6-point clinical classification system that categorizes the phenotype of patients with UCPPS into one or more of six clinically identifiable domains: Urinary, Psychosocial, Organ-Specific, Infection, Neurologic/Systemic, and Tenderness (muscle) (Fig. 11–9). It has been suggested that UPOINT will be a new clinical tool for urologists to use to direct individually based therapy. UPOINT has been evaluated and validated in female interstitial cystitis (Nickel et al, 2009) and male CP/CPPS (Shoskes et al, 2009). For both of these urologic chronic pelvic pain conditions each domain has been clinically defined using standard clinical assessment, linked to specific mechanisms of symptom production or propagation, and associated with specific therapy (criteria for inclusion in the specific CP/CPPS domains are listed in Table 11–7).
Figure 11–9 The UPOINT phenotypic classification system has six clinically defined domains (Urinary, Psychosocial, Organ Specific, Infection, Neurologic/Systemic, and Tenderness). Because each individual patient presents with a unique phenotype, the six-point UPOINT system has been called the “snowflake hypothesis.”
Table 11–7 Phenotypic Approach to Pelvic Pain (PAPP): UPOINT Classification and Directed Therapy for CP/CPPS
| UPOINT DOMAIN | CLINICAL CRITERIA | POSSIBLE THERAPY* |
|---|---|---|
| Urinary | Bothersome urgency, frequency, and/or nocturia Increased postvoid residual urine Dysuria |
Diet modification α-Adrenergic blockers Pyridium Anticholinergic agents |
| Psychosocial | Depression Maladaptive coping Social dysfunction Stress Anxiety |
Cognitive behavioral therapy Counseling Antidepressants Antianxiolytics |
| Organ specific | Specific prostate tenderness Leukocytosis in prostate specimens Hematospermia Extensive prostate calcification Lower urinary tract obstruction |
Quercetin α-Adrenergic blockers Prostate massage Surgery |
| Infection | Exclude patients with clinical category I or II prostatitis† Gram-negative bacilli or enterococci in prostate-specific specimens History of previous resolution with antibiotics |
Antibiotics |
| Neurologic/Systemic | Pain beyond abdomen and pelvis Associated medical conditions such as irritable bowel syndrome, fibromyalgia, etc. |
Gabapentinoids Amitriptyline Neuromodulation |
| Tenderness (of skeletal muscles) | Palpable tenderness, painful spasm or trigger points in pelvis or abdomen | Physiotherapy Muscle relaxants Exercises |
* Therapies listed are not necessarily evidence based but suggested on best-available evidence, interpretation of clinical trial data, and clinical experience. Therapies should be targeted against specific symptom or clinical assessment within a particular phenotype. Some treatments will be effective only in a subcategory of a specific phenotype and not effective in others.
† This category does not include category II chronic bacterial prostatitis patients, defined as men with recurrent urinary tract infections (usually same organism) with identical organism identified in prostate-specific specimens between episodes of infection (see text). These patients have the requirements for inclusion in the category III CP/CPPS: genitourinary and/or pelvic pain with no history of recurrent urinary tract infections.
In one study researchers determined the phenotype of a cohort of men with documented CP/CPPS using the UPOINT system and assessed the frequency of individual domains and their effect on symptom severity (Shoskes et al, 2009). The percentage of patients positive for each domain was 52%, 34%, 61%, 16%, 37%, and 53% for the urinary, psychosocial, organ-specific, infection, neurologic/systemic, and tenderness domains, respectively. Only 22% were positive for only one domain, and a significant stepwise increase was found in the total CPSI score as the number of positive domains increased (in other words, symptom severity was associated with the number of identified domains). As symptom duration increased, so did the number of positive domains (suggesting a phenotype progression). The domains with the most significant effect on symptoms included the urinary, psychosocial, organ-specific, and neurologic/systemic domains. For pain, the psychosocial, neurologic/systemic, and tenderness domains had significantly greater scores, whereas only the psychosocial and neurologic/systemic domains influenced the patients’ quality of life. This suggests that domains active outside the pelvis may have the most profound effect on symptoms and quality of life. It is postulated that identifying and managing these phenotypic domains may result in more effective amelioration of CP/CPPS symptoms and greater improvement in quality of life. Based on best available evidence to date, suggested treatment regimens based on this new clinical phenotyping strategy are presented in Table 11–7. The author and his colleagues are currently developing and testing a specific questionnaire that will provide urologists with a clinical instrument to identify the six major phenotypes but also the subclassifications that will likely be relevant within each specific domain. A better understanding of etiology, mechanisms of disease, and disease progression and the discovery of specific biomarkers that will allow better phenotype identification will further improve management of CP/CPPS.