chapter 16 Tuberculosis and Other Opportunistic Infections of the Genitourinary System
Genitourinary Tuberculosis
Tuberculosis has been one of the great imitators of all time, and only a few years ago was thought to be a disease of the past. Tuberculosis (TB) is a deadly infectious disease with a rising incidence worldwide. The urologist’s awareness of the clinical features of genitourinary (GU) TB is necessary to effectively treat patients with this disease. A review of the literature will reveal that the clinical features and pathology of genitourinary tuberculosis described in the earlier part of the 20th century has remained essentially valid to date. Current research focuses on epidemiology of the new resurgence of the disease, as well as rapid diagnostic modalities and new treatment regimens to combat multidrug-resistant TB.
History
Akhenaton, a Pharaoh of the eighteenth dynasty of Egypt, and his wife Nefertiti both are believed to have died from tuberculosis, and evidence indicates that hospitals for tuberculosis existed in Egypt as early as 1500 BCE (Madkour, 2003). Signs of the disease have also been found in the spines of Egyptian mummies dating between 3000 and 2400 BCE (Zink et al, 2003).
Tuberculosis became known as “the consumption” during the 1700s in Europe when infections reached epidemic proportions, causing one fourth of the deaths in England (Daniel, 2000). The bacillus causing tuberculosis, Mycobacterium tuberculosis, was identified and described on March 24, 1882 by Robert Koch. He demonstrated that Mycobacterium was the single cause of tuberculosis in all of its forms. His conclusion was based on the observations that Mycobacterium was found in all cases of the disease, could be prepared as a pure culture, and the original infection could be reproduced in an inoculated animal, from which it could be cultured again (Koch, 1882). These observations lead to postulates that have become known as the “Koch postulates,” and they form the basis for the study of all infectious diseases. March 24th has become “World Tuberculosis Day.”
Epidemiology
Incidence
The World Health Organization (WHO) estimates that 9.27 million new cases of TB occurred in 2007, compared with 9.24 million new cases (140 per 100,000) in 2006. An estimated 1.37 million (14.8%) of the cases in 2007 were HIV-positive (World Health Organization and Global Tuberculosis Programme, 2008).
In the United States, the incidence of TB showed a resurgent peak in 1992 after having continually declined until 1985, coinciding with the emergence of HIV-AIDS. In 2008, 12,904 cases were reported (4.2 cases per 100,000), representing a decline of 2.9% compared with 2007.
The trend of the declining annual case rate has slowed, from an annual average decline of 5.6% for 1993 through 2002 to an annual average decline of 2.6% for 2003 through 2008.
Genitourinary TB
Extrapulmonary sites account for 10% of tuberculosis cases. Genitourinary TB accounts for 30% to 40% of all extrapulmonary TB, second only to lymphonodal affection (Eastwood et al, 2001). In developed countries, urogenital tuberculosis occurs in 2% to 10% of cases of pulmonary tuberculosis, while in developing countries it occurs in as many as 15% to 20% of cases.
Transmission and Development of Disease
Controversy existed as early as the latter part of the 19th century as to whether pulmonary tuberculosis spread to the genitourinary tract by bacterial retrograde ascent or hematogenous dissemination. In 1885, injection of the renal artery with TB bacillus was shown to produce renal tuberculosis in the kidney of an experimental animal. Ekehorn, in 1908, postulated that TB bacilli lodged in renal glomeruli flourished into renal infection. These suspicions were confirmed in 1949 by Medlar and associates, proving that renal cortical TB was a “metastatic” infection spread by the hematogenous route (Medlar et al, 1949).
The kidney, epididymis in men, and fallopian tubes in women are the primary landing sites for hematogenous spread of TB. The prostate is also considered one of the sites for hematogenous spread, though its involvement with TB bacilli in urine is more common. Other genitourinary organs are involved by direct, endoluminal, or lymphatic spread from these sites.
Immunology and Pathogenesis
In the lung, inhaled tubercle bacilli implant in the respiratory bronchioles and alveoli. The interaction between bacterial virulence and host immunity determines whether an infection is established or aborted (Dannenberg, 1993). If infection occurs, the mycobacteria slowly divide within alveolar macrophages. Two to 12 weeks often ensue before mycobacterial numbers are sufficient to mount a clinically detectable cellular immune response (Dannenberg, 1994).
The interval before the development of cellular immunity is when tubercle bacilli spread through the lymphatics to the hilar lymph nodes and ultimately through the bloodstream to seed distant organs. Mycobacteria deposited in the upper lung zones, kidneys, bones, and brain find such environments favorable, and bacterial multiplication may occur before specific cellular immunity can limit bacterial activity.
In the presence of intact cell-mediated immunity, macrophages, T lymphocytes, B lymphocytes, and fibroblasts aggregate to form a granuloma, with lymphocytes surrounding the infected macrophages and organisms localized in the center of the granuloma. This pathognomonic lesion prevents dissemination of the mycobacteria. Immune cells communicate through cytokines within the milieu of the granuloma. T lymphocytes secrete interferon gamma, which induces intracellular killing of mycobacteria within infected macrophages (Kaufmann, 2002). An antibody response against M. tuberculosis has been demonstrated but does not appear to be protective (Abebe and Bjune, 2009). Tubercle bacilli often remain viable within the tubercle, become dormant, and finally result in a latent infection.
The risk of reactivation of dormant TB foci increases with diabetes mellitus and diseases associated with immunosuppression, e.g., HIV infection and malignancies, as well as by use of corticosteroids, chemotherapy, and other immunosuppressive drugs.
Pathologic Features
Kidney
The kidneys are the primary site of hematogenous spread of TB. Mycobacteria lodge in the renal capillaries causing microscopic foci near the glomeruli bilaterally, the cortex being favored due to its greater blood supply and higher oxygen tension (Pasternak, 2001). An initial acute inflammatory response ensues, resulting in polymorphonuclear leukocytes infiltration. Over the following 3 to 6 weeks cell-mediated immunity developing in macrophages may inhibit the M. tuberculosis by containing the bacterial replication and halting the disease in the renal cortex, leading to the formation of dormant TB foci.
Upon activation of the disease, a chronic inflammatory process arises with the subsequent development of characteristic granulomata, tubercles consisting of multinucleated Langhans giant cells, lymphocytes, and fibroblasts. Central caseous necrosis builds up within the tubercles, and neighboring tuberculous foci coalesce to form confluent areas of caseation. With progression of the disease, inflammatory changes extend into the renal tubules and medulla with further tubercle formation and caseous necrosis. Renal papilla involvement results in sloughing and caseous material gaining access to the collecting system by calyceal ulceration (Medlar, 1926). Renal tuberculosis often becomes clinically evident at this stage. Extensive fibrosis accompanying healing tubercles results in cicatricial complications, such as calyceal infundibular narrowing, ureteropelvic junction scarring, and disfiguration leading to segmental or generalized hydronephrosis respectively, and adds an obstructive element to the ongoing renal damage (Medlar, 1926).
Adrenal
Adrenal tuberculosis is seen in less than 6% of active TB cases. The lesion may be unilateral, but is usually bilateral. Tuberculosis causes necrosis of the adrenal gland, which manifests as Addison disease. Primary caseous tuberculosis of the adrenals is probably the most common lesion seen. The glands are enlarged, surrounded by a thickened capsule, and have irregular nodular surfaces with infrequent calcifications. Caseous cavitary destruction is found upon section. As a result, up to 56% of patients with adrenal TB will have a subnormal cortisol response to corticotrophin stimulation (Hawken et al, 1996).
Ureter
Tuberculosis of the ureter is almost always a direct extension of TB of the kidney. The passage of caseous material rich in mycobacteria leads to tubercle formation within the ureteric mucosa. This usually affects the lower ureter, commonly the ureterovesical junction, less commonly the middle and upper ureter (Shin et al, 2002). Tubercle formation is soon followed by ulceration of the mucosa and subsequent fibrosis and scarring, leading to ureteric stricture disease and obstruction. Lesions extending into the ureteric wall will also initiate a dense fibrosis on the ureteric serosa, leading to encasement of the ureter and angulation by contracted cicatricial bands (Johnson, 1911).
Bladder
Tuberculosis of the bladder occurs secondary to TB of the kidney. The bladder urothelium is very resistant to infection by TB bacilli. Urine of renal tuberculosis patients may contain mycobacteria for years prior to the involvement of the bladder. The most common sites affected by TB are the areas surrounding the ureteric orifices and the trigone. The urothelium is initially swollen and inflamed, following the formation of tubercles within the bladder mucosa. The ureteric orifice may be completely obscured by the swollen mucosa. In modern times the progression to the stage of mucosal ulceration is rare. However, if it does occur, the coalescence of tubercles will lead to larger areas of caseated mucosa, the top of which ulcerates leading to the formation of a classical undermined tuberculous ulcer with “worm-eaten” ragged edges (Johnson, 1911).
Epididymis, Vas, and Testis
Tubercle bacilli reach the epididymis by hematogenous spread. The disease initially affects the more vascular globus minor. Tubercles form within the epididymal epithelium eliciting a chronic inflammatory reaction that subsequently leads to fibrous narrowing and possible obliteration of the lumen. With disease progression, large caseous foci may form leading to a nodular epididymis. These lesions may become adherent to the overlying skin and ulcerate through giving the classic picture of a tuberculous sinus that is typically located on the posterior surface of the scrotum. Spread of the infection along the lumen, with the flow of secretion, will lead to affection of the vas deferens in a similar pattern. The classical finding is that of a beaded vas as a result of tubercles eventually inducing dense fibrosis.
Tuberculosis of the testis is secondary to that of the epididymis and is due to direct extension of the disease. Tubercles form within the seminiferous tubular epithelium as well as in the connective tissue septa of the testis. As a result the affected testicular tissue is eventually replaced by caseous material and fibrosis. Such lesions may be clinically difficult to distinguish from a testicular tumor.
Prostate and Seminal Vesicles
The prostate is rarely affected, it is however one of the sites of hematogenous spread of TB. The lesions are often incidentally found on TUR specimens. In cases where progression occurs, caseous destruction of prostatic tissue ensues that may be significant enough to cause a noticeable reduction in semen volume (Marconi et al, 2009). Densely fibrotic nodules may form and are indistinguishable from cancer.
Tuberculosis of the seminal vesicle is rarely seen in modern times. The bacilli can reach the seminal vesicles through the vas deferens in cases of tuberculosis of the testis or epididymis, or through the urethra and ejaculatory duct because of tuberculosis of the kidneys, bladder, or prostate. Caseous masses develop in the walls, and the lumen may be filled caseation.
Penis and Urethra
Tuberculosis of the penis in adults is very rare and is secondary to infection of the kidney and bladder. It starts as a chronic process, by the formation of infected granulation tissue, which gradually infiltrates the glanular and cavernous tissues and may invade the whole thickness of the penis. As the infection progresses, isolated pea-sized masses can be felt in the cavernous bodies and urethra, and in some cases directly under the skin. On pathologic section, these lesions are found to be masses of caseous tubercles.
One rare presentation is an ulcerative lesion of the glans. This primary infection can be acquired by sexual relations with partners having genital or perineal tuberculous lesions (Angus et al, 2001). Several cases of primary TB of the penis were reported in young Jewish boys following circumcision. Hemorrhage was stopped by sucking the penis with the mouth. Rabbis with open pulmonary TB transmitted the infection through infected sputum. Urethral tuberculosis is rare, often only seen at the meatus. Small miliary tubercles are seen over the surface and throughout the urethra. Late cases may have advanced fibrotic strictures.
Clinical Manifestations
In the words of Chang: “the kidney is an inarticulate organ; its vocal cords are the bladder” (Chang, 1976). Tuberculosis can often mimic a wide range of nonspecific urologic symptoms. It is thus, no wonder that many cases of genitourinary TB are easily overlooked. A high index of clinical suspicion of TB is required to further investigate cases of unexplained symptoms in the urinary tract. This is especially important when there is a failure to respond to initial treatments given for lower urinary tract symptoms or when urinalysis and routine culture reveal “sterile pyuria.” The fact that 18 out of 25 physicians with renal TB presented only after cavitary lesions developed is a measure of how silently the destructive process occurs (Lattimer, 1965).
Genitourinary TB is more commonly seen in men (male : female ratio of 2 : 1), usually presenting in the fourth decade of life. Lower urinary tract symptoms are the most common presentation, with over 50% of patients presenting with storage symptoms. Hematuria and loin pain are the presentation in one third of cases (Figueiredo et al, 2008). Passage of caseous material, necrotic renal papillary tissue, clots, or stones account for renal colic in 10% of patients (Simon et al, 1977). Less than 20% of cases will present with constitutional symptoms of fever, anorexia, weight loss, and night sweats. The presence of these symptoms, however, can alert to the presence of active TB elsewhere in the body (Simon et al, 1977).
Physical examination is often of limited value in the diagnostic process, because physical signs develop late in the disease. The most common physical finding is an abnormal scrotal exam in about half the patients (Figueiredo et al, 2008). Epididymal hardening, modularity, or scrotal fistulae are among the signs seen. A chronic renal fistula tract, often with a history of prior renal surgery is another late physical sign. Enlarged, firm seminal vesicles, or prostatic nodules on rectal examination, though nonspecific, should arouse suspicion in clinically suggestive cases. It remains a fact that up to 25% of patients will present only with sterile pyuria and 13% might have gross or microscopic hematuria as their only presentation (Wise and Shteynshlyuger, 2008). Functional loss of the affected kidney can be present in up to 25% of cases, and renal failure is present in 7.4% of cases (Figueiredo et al, 2008). Genitourinary TB may be diagnosed during a workup for infertility as a cause of epididymal and vasal obstruction (Paick et al, 2000). TB should be considered in all cases of recurrent hemospermia. Adrenal tuberculosis may present with an addisonian type of clinical picture.
Diagnosis
Urinalysis and Culture
Historically, the diagnosis of genitourinary TB has relied on the identification of Mycobacterium tuberculosis in the urine. Unlike sputum examination, Ziehl-Neelsen staining of concentrated urine samples for acid-fast bacilli is often negative. Of note, a large majority of patients with genitourinary TB have “sterile pyuria,” often accompanied by hematuria and proteinuria, whereas up to 20% may have superimposed bacterial infection (Gow and Barbosa, 1984).
Urine cultures are carried out on standard solid media optimized for mycobacterial growth, namely egg-based (Löwenstein-Jensen) or agar-based (e.g., Middlebrook 7H10) media. Optimizing factors include aniline dyes, such as malachite green, that inhibit growth of bacterial contaminants. Agar-based media are transparent and facilitate earlier visualization of micro-colonies by approximately 1 week. Intermittent release of the organism in urine makes multiple sampling necessary. Three to five early-morning urine samples should be cultured soon after collection rather than 24-hour samples, because exposure to urine acidity for prolonged periods retards mycobacterial growth (American Thoracic Society [ATS] and Centers for Disease Control and Prevention [CDC], 2000; Sommers, 1979). One other caveat is that chronic renal lesions may no longer discharge tuberculous material in urine due to dense fibrosis that is a barrier to the collecting system. In such cases diagnostic methods other than urine testing must be applied. Notably, cultures optimized for mycobacterial growth will favor the growth of mycobacterial contaminants as well as TB. Species identification is done using growing colonies tested with DNA strip assays. These provide rapid confirmation of pathogenic Mycobacterium TB in culture (Piersimoni et al, 2002).
Urine cultures are sensitive in 80% to 90% of cases and have a specificity of nearly 100% (Sorlozano et al, 2009). However, they may take up to 6 weeks to yield clinically reliable results. Radiometric detection of mycobacterial activity in liquid culture media allows a more rapid diagnosis. Inoculation of specimens in broth with radiolabelled 14C-palmitate results in liberation of 14CO2 by mycobacterial metabolism, which is then detected by a radiometric analyzer. Mycobacterial detection and drug sensitivity testing using this method is possible as early as 7 to 14 days after inoculation (Watterson and Drobniewski, 2000; Sorlozano et al, 2009). One of the most popular systems using this principle is the BACTEC 460TB (Becton, Dickinson, Franklin Lakes, NJ). Nonradiometric methods using advanced fluorometric technology to detect O2 consumption also yield rapid detection of mycobacteria without the use of radiation. One example of this detection method is the mycobacterial growth indicator tube (MGIT; Becton, Dickinson; Watterson and Drobniewski, 2000).
Antibiotic sensitivity testing (AST) is also done using traditional and rapid culture methods.
Purified Protein Derivative–Tuberculin Test—Mantoux Test
Charles Mantoux, a French physician who developed on the work of Koch, described this test in 1907. Purified protein derivative (PPD) tuberculin is a precipitate of non–species-specific molecules obtained from glycerol extracted filtrates of sterilized, concentrated cultures of tubercle bacilli. In the United States, a standard dose of 5 tuberculin units (0.1 mL) is injected intradermal (between the layers of dermis) into the volar or dorsal surface of the forearm and read 48 to 72 hours later. T-cell–mediated delayed-type hypersensitivity reaction to this intradermal antigen is the principle of the test. Antigen stimulation of memory cells leads to cytokine release that induces induration via local vasodilatation, fibrin deposition, and recruitment of other inflammatory cells into the area. These begin to accumulate within 24 hours and reach their peak after 48 to 72 hours, hence the timing of test interpretation. Patients who have been exposed to TB are expected to mount an immune response to PPD (Daniel, 1980).
Tuberculin test is nondiagnostic, and is of value only if positive. Three distinct cut points for positivity have been defined to optimize sensitivity and specificity for the test in different patient populations. The test must be interpreted in light of the recommendations in Table 16–1.
Table 16–1 Interpretation of Tuberculin Test: ATS and CDC Guidelines
From American Thoracic Society and Centers for Disease Control and Prevention. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161(4 Pt. 1):1376–95.
It is important to note that prior vaccination with bacillus Calmette-Guérin (BCG) may lead to a false-positive tuberculin test. Interestingly, a PPD skin test done before initiation of BCG therapy for superficial bladder cancer converted from negative to positive in 68% of patients (Bilen et al, 2003).
Nucleic Acid Amplification (NAA) Testing—PCR
Nucleic acid amplification tests (NAAT), such as the polymerase chain reaction (PCR) and other methods for amplifying DNA and RNA, facilitate rapid detection of microorganisms, particularly those difficult to culture. The high sensitivity of PCR is particularly useful in non-pulmonary tuberculosis where discharge of the organism is sporadic and present in small amounts (Manjunath et al, 1991). Multiple sampling is also necessary for this method. The PCR test has been extensively studied and has shown reliably high sensitivity, specificity, and rapid results. In various studies, data show sensitivity ranging from 87% to 95% (usually >90%) and specificity from 92% to 99.8% (usually >95%) as compared to culture. Staining for acid-fast bacilli, bladder biopsies, and intravenous pyelography (IVP) examinations all yielded inferior results (Hemal et al, 2000b; Moussa et al, 2000). Numerous commercial tests and kits are available with near-equivalent quality.
Some caveats exist in the interpretation of NAAT results, because they are best used in conjunction with clinical judgment and TB cultures. Urine is known to have naturally occurring enzyme inhibitors in up to 10% of cases, which suppress the enzymatic reactions of DNA/RNA amplification (van Vollenhoven et al, 1996). This may result in false-negative tests (Moussa et al, 2000). It is thus important to corroborate with the laboratory that appropriate measures have been done to confirm a PCR result as negative rather than an inhibited reaction, especially in clinically suspicious cases. Moreover, NAA tests can amplify nucleic acids from dead organisms, thus yielding positive results even after effective chemotherapy. Thus they should be used for diagnosis only and not as a follow-up to treatment (ATS and CDC, 2000).
Rapid molecular testing for drug resistance is available by detecting resistance mutations in three Mycobacterium TB genes. Results are available in 1 to 2 days (Barnard et al, 2008).
Radiography and Endoscopy
A wide spectrum of imaging findings has been described. Though each finding is nonspecific, multiple findings are common and are collectively suggestive of tuberculosis.
Plain Radiograph
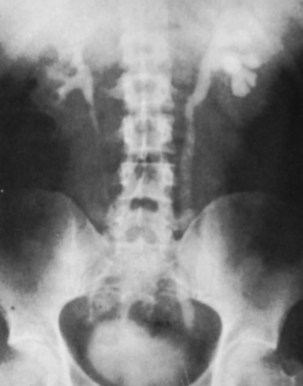
Plain radiographic findings in genitourinary tuberculosis may be seen in the GU tract, surrounding tissues, and up to 50% of patients may show positive findings on chest radiograph. Disparity in renal size on plain films may indicate early increase in size of the affected kidney due to caseous lesions or a shrunken fibrotic kidney of autonephrectomy. Calcifications are seen in 30% to 50% of cases (Roylance et al, 1970). Focal calcifications occur within the caseating lesions (Fig. 16–1). A characteristic diffuse, uniform, extensive parenchymal, putty-like calcification, forming a lobar cast of the kidney is seen with autonephrectomy (Muttarak et al, 2005). Calculi may also be seen in the collecting system or ureter secondary to stricture formation. Ureteral calcifications are rare and are characteristically intraluminal as opposed to the mural calcifications of schistosomiasis. Bladder wall calcifications are not very common except in late cases of bladder contraction. Calcifications of the prostate and seminal vesicles are seen in 10% of cases (Burrill et al, 2007).
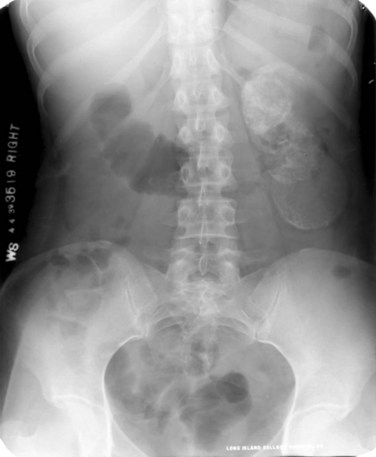
Figure 16–1 Kidney-ureter-bladder radiographic view in a patient with left renal tuberculosis with associated calcifications.
Plain film findings suggestive of tuberculosis may be seen in surrounding tissues such as erosions of the vertebral bodies or calcifications in a cold abscess of the psoas muscle (Burrill et al, 2007).
Intravenous Urography (IVU)
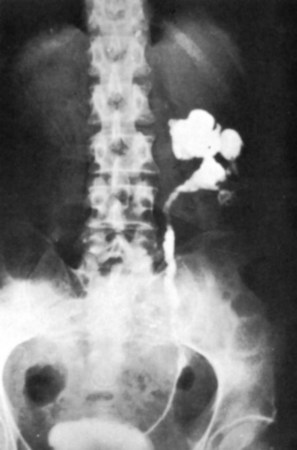
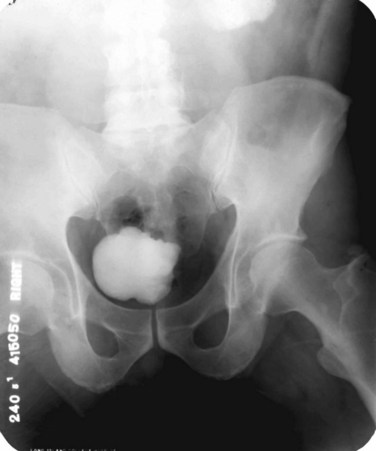
The majority of cases will show positive findings on excretory urography, the most common findings being hydrocalycosis, hydronephrosis, or hydroureter due to stricture formation (Wang et al, 2003). Early signs include the moth-eaten appearance of calyceal erosion and papillary irregularity. These signs are best seen on early excretory films, because they are often masked by increasing density of the contrast on later films of the IVU. Cavitary lesions communicating with the collecting system are characteristic of TB. These lesions eventually enlarge as parenchymal destruction ensues, and a picture similar to chronic pyelonephritis may be seen. Fibrotic distortion of the collecting system and ureter is also seen. Calyceal obliteration and amputation, hydrocalycosis, segmental or total hydronephrosis, and a shriveled reduced-capacity renal pelvis may all be signs of renal tuberculosis (Figs. 16-2 and 16-3). Scarring and angulation of the ureteropelvic junction (UPJ) may also occur, the so-called “Kerr’s kink” (Matos et al, 2005). Ultimately diminished or absent function and extensive calcification may be seen with autonephrectomy. If nonvisualized on IVU, the kidney is best evaluated by computed tomography (CT) or ultrasonography.
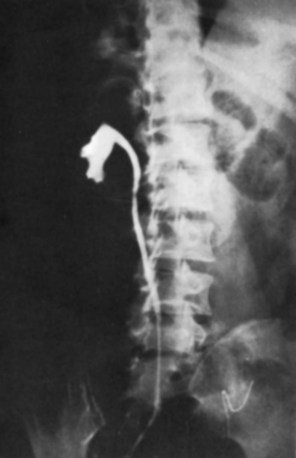
Tuberculosis of the ureter is commonly seen as a rigid, straightened “pipe-stem” ureter. A beaded, corkscrew appearance is sometimes also seen. Ureterovesical junction obstruction is caused by tuberculous cystitis or strictures of the distal third of the ureter (Fig. 16–4). Secondary stone formation on top of this stricture is an occasional finding. The cystogram films may show a small contracted bladder due to excessive fibrosis (Fig. 16–5). Of note, although IVU is being phased out by CT-urography in many developed countries (Stacul et al, 2008), IVU continues to be a reliable imaging modality for genitourinary TB in most parts of the world.
Computed Tomography
The most common findings on contrast-enhanced CT include renal parenchymal masses and scarring, thick urinary tract walls (ureter and bladder) and extraurinary tubercular manifestations particularly in miliary TB (Wang et al, 2003). Coalescence of caseating granulomata may lead to a renal mass (tuberculoma), which must be differentiated from renal cell carcinoma. CT allows for evaluation of renal function, grading of hydronephrosis and parenchymal scarring (Fig. 16–6). CT is most sensitive in detecting renal calcifications (Premkumar, et al, 1987). Most CT findings are in themselves nonspecific, and the collective interpretation of multiple findings in conjunction with the clinical picture is the best option in decision making (Wang et al, 2003).
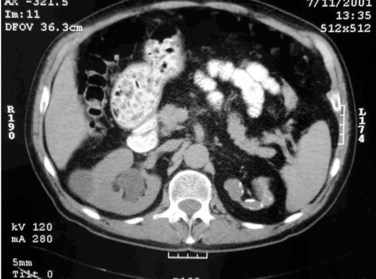
Figure 16–6 CT after oral contrast medium in a patient with bilateral tuberculosis. The right kidney is hydronephrotic secondary to infundibular stenosis but has retained good function. The left kidney is an end-stage nonfunctioning atrophic kidney with calcification.
CT is also helpful in the diagnosis of adrenal tuberculosis, which may appear as bilaterally enlarged glands with areas of necrosis (caseation) early in the disease. Dotlike calcifications and atrophy of the adrenal gland are common late findings (Wang, et al, 1998).
Tuberculosis of the prostate or seminal vesicles may lead to calcification, caseation, and necrosis causing hypoattenuation or cavity formation that can be visualized on contrast-enhanced CT scan of the pelvis. However, in the absence of calcification, tuberculous prostatic lesions may mimic a pyogenic abscess or carcinoma, especially that prostate-specific antigen (PSA) may be elevated in one third of cases (Lee et al, 2001). Needle biopsy may be needed in these cases.
Ultrasonography
Ultrasonography has a limited role in diagnosis because it provides nonspecific findings. However, it may be used to follow up cases presenting with hydronephrosis during the course of treatment; fibrotic scarring during healing may lead to worsening of hydronephrosis, thus requiring surgical attention. Ultrasonography is useful in the demonstration of epididymal and testicular lesions. Transrectal ultrasonography may also show seminal vesicular and prostatic lesions. All findings are nonspecific and should be interpreted in light of the clinical picture.
Retrograde Pyelogram and Antegrade Pyelogram
Diagnosis using contrast studies by directly injecting a contrast medium, whether using endoscopy or percutaneous puncture, have largely been superseded by noninvasive imaging techniques such as CT urography and magnetic resonance imaging (MRI). The value of these modalities lies in the ability to obtain a urine sample from the upper tract, particularly where it is important to identify the affected side (as in surgical planning). Percutaneous puncture is particularly useful in cases where fibrosis has sealed the affected side, such that organisms are no longer discharged in urine. A ureteric stent or nephrostomy tube may be placed, at that time, to bypass a stricture or drain infected material in a septic patient.
Cystoscopy and Ureteroscopy
Endoscopy plays a limited role in the diagnosis of TB. Despite direct visualization of lesions, there are no pathognomonic findings that are specific for tuberculosis. Ulcerative lesions may mimic malignancy. A “golf-hole” ureteric orifice is suggestive of tuberculosis, and, when found, upper tract imaging or endoscopy should be obtained (Fig. 16–7). A positive urine culture or stain for acid-fast bacilli obviates the need for a biopsy, especially because it is diagnostic in only 18% to 45% of cases (Wong et al, 1984; Hemal et al, 2000b). However, a biopsy should be done when in doubt of malignancy.
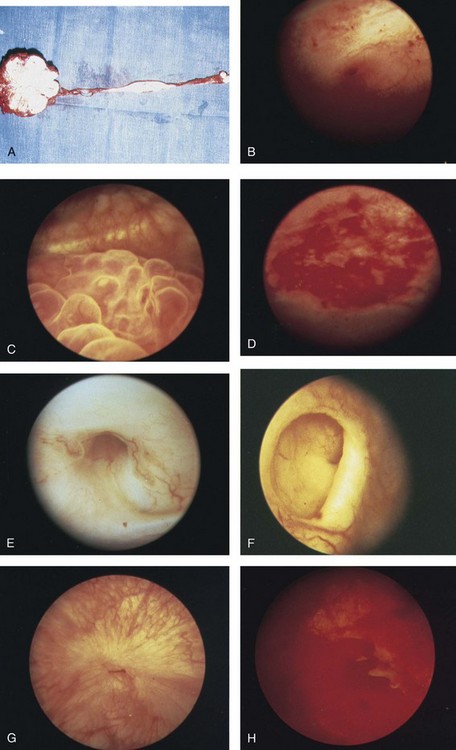
Figure 16–7 A, Extensive tuberculosis of the kidney and ureter with calcification and stricture formation. B, Acutely inflamed ureteric orifice. C, Tuberculous bullous granulations. D, Acute tuberculous ulcer. E, Tuberculous golf-hole ureter. F, Tuberculous golf-hole ureter, severely withdrawn. G, Healed tuberculous lesion. H, Acute tuberculous cystitis with ulceration.
Treatment
Successful treatment of genitourinary tuberculosis relies on the early diagnosis and the prompt initiation of an adequate drug regimen. Prior to successful antituberculous chemotherapy extirpative surgery was the initial form of treatment. Currently surgical treatment is reserved for advanced cases, often to correct the obstructive effects of fibrosis and scarring rather than the removal of infected tissues. Thus the balanced medical-surgical approach is ideally aimed at the preservation of renal (organ) function and eradication of mycobacteria. Despite standardized regimens and surgical indications, treatment must also be individualized; “No disease process is as atypical as a typical tuberculous process” (Cooper and Robinson, 1972).
Medical Therapy
On November 20, 1944, streptomycin was administered for the first time to a critically ill TB patient. His advanced disease was clearly arrested, and mycobacteria disappeared from his sputum. A succession of antimycobacterial agents rapidly appeared in the years that followed.
The principle underlying medical treatment is the effective eradication of the slowly dividing mycobacteria from tissues and urine. To understand the basis for modern-day multidrug regimens for tuberculosis, it is important to contemplate the following dilemma: Mycobacteria exist in several different environments in genitourinary tract TB. The largest population is the more actively dividing extracellular mycobacteria that exist within cavitary lesions, often at a neutral or alkaline pH. Another population exists in the intracellular acidic environment within macrophages. A smaller population of slowly dividing organisms can be found enclosed within caseous material at a neutral pH or freely in the acidic pH of urine (Dutt and Stead, 1982). The differential ability of various antimycobacterial drugs to penetrate tissues, inherent effects on the tubercle bacillus (bactericidal vs. bacteriostatic), and their levels of activity in the wide range of pH required to manage genitourinary TB (as discussed under the individual drugs below), dictates the use of a multidrug regimen. This is in addition to the standard principle of lowering the emergence of drug-resistant strains.
Genitourinary TB can safely be managed with short-course chemotherapy (Dutt et al, 1986; Small and Fujiwara, 2001). This treatment is effective because there are fewer organisms in genitourinary lesions; isonicotinic acid hydrazide (isoniazid, INH) and rifampicin have good penetration of lesions at lethal concentrations; and isoniazid, rifampicin, and pyrazinamide attain high concentrations in urine (Gow and Barbosa, 1984).
Classically, antimycobacterial regimens rely on a first line of drugs, namely, rifampicin, INH, pyrazinamide, and ethambutol. A second line of drugs is reserved for cases that fail to respond to first-line therapy or in drug-resistant cases. Finally, it is important to acknowledge the rising incidence of multidrug resistant (MDR-TB) and extensively drug resistant TB (XDRTB) infections, and the resultant implications for the drug regimens used (Johnston et al, 2009).
A standard treatment regimen for tuberculosis requires 6 months of therapy. The first 2 months involve three to four drugs: Rifampicin, isoniazid, and pyrazinamide are administered daily; ethambutol is added if drug resistance to isoniazid is suspected. An additional 4 months of rifampicin and isoniazid daily, twice per week, or three times per week are used (Small and Fujiwara, 2001). The intensive 2-month, three-drug regimen targets rapid multipliers, and the prolonged 4-month, two-drug regimen eradicates slow, sporadic multipliers and persistent bacteria. Directly observed therapy is often best to ensure patient compliance and completion of treatment.
It is important to obtain adequate specimens for culture and susceptibility testing before treatment is initiated. Ethambutol can be stopped if lack of resistance is demonstrated. Baseline measurements of hepatic enzymes, bilirubin, and creatinine, and a complete blood count with a platelet count, should be performed. During treatment, all patients should be monitored for adverse effects on a monthly basis. Routine follow-up of liver enzymes is important to detect hepatotoxicity of rifampicin. If pyrazinamide is used, uric acid levels should be measured, and if ethambutol is given, visual acuity and red-green color perception should be monitored (Small and Fujiwara, 2001) (Tables 16-2 and 16-3).
Table 16–3 Some Second-Line Antituberculous Drugs
| DRUG/FORMULATION | ADULT DOSAGE (DAILY) |
MAIN ADVERSE EFFECTS |
|---|---|---|
| Streptomycin* | 15 mg/kg IM, IV (max 1 g) | Vestibular and auditory toxicity, renal damage |
| Capreomycin (Capastat) | 15 mg/kg IM, IV (max 1 g) | Auditory and vestibular toxicity, renal damage, electrolyte imbalance |
| Kanamycin (Kantrex and others) | 15 mg/kg IM, IV (max 1 g) | Auditory toxicity, renal damage |
| Amikacin (Amikin) | 15 mg/kg IM, IV (max 1 g) | Auditory toxicity, renal damage |
| Cycloserine† (Seromycin and others) | 10-15 mg/kg in two doses (max 500 mg bid) PO | Psychiatric symptoms, seizures |
| Ethionamide (Trecator-SC) | 15-20 mg/kg in two doses (max 500 mg bid) PO | Gastrointestinal and hepatic toxicity, hypothyroidism |
| Levofloxacin (Levaquin) | 500-1000 mg PO, IV | Nausea, abdominal pain, restlessness, confusion, rash dysglycemia |
| Moxifloxacin (Avelox) | 400 mg PO, IV | Nausea, abdominal pain, restlessness, confusion, rash dysglycemia |
| Aminosalicylic acid (PAS; Paser) | 8-12 g in 2-3 doses PO | Gastrointestinal disturbance |
* Streptomycin is generally given 5 to 7 times per week (15 mg/kg, or a maximum of 1 g per dose) for an initial 2- to 12-week period and then, if needed, two to three times per week (20 to 30 mg/kg, or a maximum of 1.5 g per dose). For patients >59 years old, dosage is reduced to 10 mg/kg/day (max 750 mg/day). Dosage should be decreased if renal function is diminished.
† Some authorities recommend pyridoxine, 50 mg, for every 250 mg of cycloserine to decrease the incidence of adverse neurologic effects.
From The Medical Letter. Drugs for tuberculosis. Treat Guidel Med Lett 2009;7(86):75–82; quiz 2 (after p. 82).
Antituberculous Drugs
Isoniazid
Isoniazid (INH) acts by inhibition of cell wall lipid synthesis, depletion of nucleic acid pools, and metabolic depression in mycobacteria (Timmins and Deretic, 2006). It penetrates caseous material and is active within the macrophages. INH is rapidly and completely absorbed orally; this may be slowed by food. It is widely distributed in the body, and tissue levels are similar to serum levels. Seventy percent of all administered INH is excreted by the kidneys, most in an inactive form. Dose modification in renal failure is usually not necessary but is recommended in hepatic failure, especially in persons who are slow hepatic acetylators.
INH is associated with hepatic toxicity in 10% to 20% of patients, usually in the form of asymptomatic elevations of transaminase levels. This occurs after 6 to 8 weeks of therapy and may normalize with continued INH treatment. Baseline liver function tests should be obtained, and INH should be stopped if the aminotransferase level triples or if symptoms suggestive of hepatitis develop (fatigue, nausea, anorexia), because severe hepatic necrosis has been reported. Peripheral neuropathy due to INH can be prevented by supplementation with pyridoxine (vitamin B6) 20 to 50 mg/day. INH is an inhibitor of the hepatic cytochrome P-450 system; thus it increases serum levels of anticonvulsants, warfarin, benzodiazepines, haloperidol, theophylline, tacrolimus, and ketoconazole.
Rifampicin
Rifampicin acts by inhibiting the β-subunit–dependent DNA-directed RNA polymerase of mycobacteria, leading to suppression of DNA synthesis (Sousa et al, 2008). The drug is lipid soluble, enters macrophages, and is excreted in the urine.
Hepatotoxicity is the major adverse reaction to rifampicin. Liver failure requires a moderate reduction in dosage, but full doses can be given with renal insufficiency. Rifampicin decreases serum levels of digoxin, verapamil, diltiazem, warfarin, sulfonylureas, progestins, theophylline, diazepam, corticosteroids, and several other drugs.
Streptomycin
Streptomycin is an aminoglycoside and binds to the S12 protein of the 30S unit of the bacterial ribosome, interfering with bacterial protein synthesis. It penetrates the walls of tuberculous abscesses at lethal concentrations, even in caseous material, and is active mainly against extracellular tubercle bacilli because its penetration into cells is poor. High concentrations are obtained in the urine. Isolates resistant to streptomycin are not resistant to other aminoglycosides. It must be given intramuscularly because it is very poorly absorbed orally. Streptomycin is ototoxic, causing vertigo and hearing loss. It is also nephrotoxic. Toxicity is dose related, and the risk is increased in the elderly; dose adjustment according to renal function is necessary.
Pyrazinamide
Pyrazinamide is a derivative of nicotinamide. Its target and mechanism of action are not fully known. Being highly active at pH 5.5 makes it suitable for genitourinary tuberculosis. It is readily taken up by macrophages and is active in the acidic intracellular milieu against mycobacteria within lysosomes. Gastrointestinal disturbances, hepatotoxicity (in 1% to 5% of patients), arthralgias, and hyperuricemia are some of its side effects.
Ethambutol
Ethambutol halts mycobacterial cell wall synthesis by inhibition of arabinosyl transferases. It is active against M. tuberculosis strains resistant to INH and other commonly used antituberculous drugs. It is well absorbed after oral administration. Up to 80% is excreted in the urine as active unchanged drug; dosage should be reduced in renal failure. Ethambutol rarely causes retrobulbar neuritis and should be discontinued if ocular changes occur. Changes in visual acuity and red-green color perception are early findings, and these parameters should be tested at baseline and monthly therafter.
Corticosteroids
Steroids have a limited role in management of genitourinary TB. The general rational behind their use is lowering the host immune response that is responsible for the tissue destruction and subsequent scarring. In cases of severe acute tuberculous cystitis, steroids may reduce mucosal inflammation and improve symptoms. Of note, higher doses of oral steroids are needed because rifampicin will increase their hepatic metabolism.
Multidrug-Resistant TB
Drug-resistant tuberculosis is defined as M. tuberculosis that is resistant to one of the first-line antituberculosis drugs (isoniazid, rifampin, pyrazinamide, or ethambutol).
Multidrug-resistant tuberculosis (MDR-TB) refers to M. tuberculosis that is resistant to at least isoniazid and rifampin, and possibly additional chemotherapeutic agents.
Extensively drug-resistant tuberculosis (XDR-TB) refers to M. tuberculosis that is resistant to at least isoniazid and rifampin, and is additionally resistant to fluoroquinolones and either aminoglycosides (amikacin, kanamycin) or capreomycin, or both (Centers for Disease Control and Prevention [CDC], 2007). Typical MDR-tuberculosis regimens can consist of up to five drugs, used for a minimum of 18 months after culture conversion to negative. XDR tuberculosis requires individualized treatment based on drug-susceptibility testing of the particular organism (Rich and World Health Organization, 2006). Drug regimens combining linezolid with other companion drugs selected according to individual drug history, and tailored to the susceptibility results of each isolate, are currently being evaluated (Schecter et al, 2010).
Surgical Therapy
About 55% of patients with genitourinary TB will require surgical intervention. This rate is lower in areas where the disease is diagnosed early, while still asymptomatic. In developing countries, where the disease is diagnosed in late stages, often after examination of nephrectomy specimens, this rate maybe as high as 95% (Figueiredo et al, 2008). The role of surgery has changed in the era of effective antitubercular treatment. Surgical intervention compliments medical treatment in preservation and restoration of organ function. Ablative surgery is performed less commonly and should be considered carefully. The earlier diagnosis and chemotherapy have allowed reconstructive procedures to be performed more commonly today, even in advanced cases. Currently, more than half of surgeries performed for TB are reconstructive (Gupta et al, 2006). Moreover, surgical treatment is best carried out after an initial 3 to 6 weeks of medical treatment. This interval allows intense inflammatory changes to resolve and lesions to stabilize, permitting a better assessment of the extent of destruction, and hence doing the appropriate procedure. In the setting of obstruction and deteriorated kidney function, initiation of medical treatment can temporize definitive surgical intervention until renal function recovers, provided that measures are taken to relieve the obstruction (e.g., ureteric stenting or percutaneous nephrostomy).
Indications for surgical management follow broad lines: procedures to relieve obstruction and drain infected material, definitive local treatment, upper urinary tract reconstruction, lower urinary tract reconstruction, and surgery for genital TB.
Procedures to Relieve Obstruction
The prompt relief of obstruction is emergently required in cases of uremia or sepsis. Bilateral hydronephrosis or unilateral hydronephrosis of a solitary or functionally solitary kidney is often the cause of renal failure. Early ureteral stenting or percutaneous nephrostomy (PCN) for tuberculous ureteral strictures have been demonstrated to decrease the loss of renal function and increase the opportunity for later reconstructive surgery (Shin et al, 2002). In such cases, temporary and immediate drainage of hydronephrosis, preferably by retrograde ureteric stenting if possible, is required. An indwelling double J stent can be left until the patient’s condition is optimized. Retrograde placement is successful in 41% of cases (Ramanathan et al, 1998). When this is not technically feasible, percutaneous puncture of the hydronephrotic kidney is done to pass an antegrade stent. If that also fails, a percutaneous tube is left until definitive management is done. In cases of segmental hydronephrosis, more than one PCN may be required to achieve adequate drainage (Carl and Stark, 1997). It is important to understand that PCN placement must be followed by correcting the cause of obstruction. A tuberculous cutaneous fistula invariably develops if the PCN is simply removed. If the renal unit is deemed unsalvageable or shows no function, a nephrectomy is inevitable to prevent this complication. One pitfall to avoid during stent placement is the use of high-contrast injection pressures, because this may lead to possible dissemination of infection (Salem, 2008).
Nephrectomy
Upfront (primary) nephrectomy to remove an infected kidney or debride caseous tissue is no longer the preferred line of treatment of genitourinary TB in the era of modern antitubercular chemotherapy. Today, the decision for a nephrectomy is based upon the extent of renal parenchymal destruction and, more importantly, the function of the kidney (Ramanathan et al, 1998). The indications for nephrectomy have thus been reduced to the excision of a renal unit that is nonfunctioning despite adequate drainage and medical treatment (Gupta et al, 2006). Other indications include extensive parenchymal destruction involving the whole kidney associated with hypertension (Flechner and Gow, 1980). Coexisting renal carcinoma also mandates nephrectomy. The current paradigm implies that chemotherapy is sufficient to render lesions free of mycobacteria even in nonfunctioning kidneys. However, removal of a calcified nonfunctioning kidney may be curative in patients with persistent symptoms (predominantly those of cystitis), because up to 50% of cases may still discharge mycobacteria in urine despite adequate chemotherapy (Fischer and Flamm, 1990).
A standard lumbar incision provides adequate exposure sufficient to carry out the dissection and control of the kidney. Every effort should be taken to avoid inadvertent entry into the peritoneal or pleural cavities. It is often possible to preserve the adrenal gland. In rare cases, the perinephric fat may appear to have tubercles or caseous cavities. These should not be dissected from the kidney and should be removed with the specimen. Dense fibrosis, due to healing of such lesions after chemotherapy, is more commonly encountered in the perirenal tissues or surrounding the renal pedicle. Control of the renal artery and vein, separately, may be difficult in these cases, and the pedicle may be controlled in toto by suture ligation. Routine removal of the ureter is not necessary. Retroperitoneoscopic nephrectomy has been attempted successfully for tuberculous kidneys (Hemal et al, 2000a).
Partial Nephrectomy
Partial nephrectomy is permissible only where parenchymal destruction is clearly localized, such as a calcified polar cavitary lesion or localized lesions that progress to calcification despite 6 weeks of adequate chemotherapy. Early localized lesions are adequately managed by medical treatment. It is prudent to ensure that no obstruction is present at the level of the renal pelvis or ureter to avoid tuberculous fistula formation.
Ureteropelvic and Ureteral Surgery
Strictures of the UPJ and ureter may be temporarily stented, as mentioned above, to allow improvement of renal function, after which a definitive decision on the appropriate management of the stricture is made. Upper and midureteric strictures are rare and may be amenable to endourologic treatment. Lower ureteric strictures are the commonest form and will often require surgical intervention. Additionally, the length of a stricture, whether it is passable or not, and renal function are important factors to be considered.
Endoscopic Management
Several options are available for the endourologic management of ureteric strictures. Tuberculous ureteric strictures are characterized by mucosal ischemia and dense fibrosis. Hence, success rates of endoscopic management of strictures due to other etiologies may not necessarily apply to TB strictures. Generally, short, passable strictures, with good renal function yield the best endoscopic outcome.
Strictures forming during medical treatment and managed by early stenting (double J placement) have been shown to stabilize and require no further treatment (Shin et al, 2002). Balloon dilatation by retrograde or antegrade access has been described for TB strictures of the ureter, UPJ, and calyceal infundibula (Murphy et al, 1982; Kim et al, 1993). A stent is left after dilatation. Due to high failure rates, repeated procedures are needed.
Follow-up of all cases of ureteric strictures by imaging (ultrasonography or IVU), especially those managed endoscopically, is needed because some strictures will worsen during the healing process due to fibrosis and cicatrization. Corticosteroids may be added if deterioration is detected. Consequently, up to 72% of cases will show relief of obstruction (Horne and Tulloch, 1975), and stent placement, if still needed, is often possible at this time. Failure to respond or progression after 6 weeks is an indication for definitive management.
Surgical Options
Long, complex strictures require surgical repair. It must be kept in mind that due to loss of elasticity, fibrosis, and reduced vascularity, mobilization of structures may be difficult, and thus the surgeon should be open to alternative options.
Repair of the ureteropelvic junction scarring is more challenging in TB cases than for congenital stenosis. The choice of procedure largely depends on the degree of scarring and contraction of the pelvis. Dismembered pyeloplasty is feasible for extrarenal pelvis with short segment scarring. Nondismembered (flap) pyeloplasty is preferred for longer strictures, but may not be feasible due to excessive scarring of the pelvis. When anatomic reconstruction is not possible, ureterocalicostomy (ureter to the lower pole calyx) is an option. It is important to cover the exposed lower pole parenchyma (using preserved capsule or omentum) to avoid fibrosis and constriction around the anastomosis (Carl and Stark, 1997).
Upper and middle ureteric strictures can be managed by excision of the diseased segment and, with adequate mobilization, a primary tension-free ureteroureterostomy can be performed if endourologic management has failed. Alternatively, lysis of adhesions and intubation (Davis intubated ureterotomy) may be done.
Lower ureter strictures requiring surgery are best managed by complete excision of the entire affected ureteric segment back to healthy ureteric mucosa having good blood supply. Affection of the bladder around the ureteric orifice is also common, and the area of anastomosis must also be healthy. The main focus then becomes to bridge the resultant gap with a tension-free, well-vascularized anastomotic technique. An array of ureteroneocystostomy procedures exist to bring the bladder closer to the ureteric end. Salient points are discussed briefly here; surgical details can be found under ureteroneocystostomy. Simple mobilization of the lateral attachments of the bladder on the contralateral side, accompanied by dividing the superior vesical artery, may provide 2 to 3 cm of length to bridge a small gap. In patients with good bladder capacity, a psoas hitch may also be performed. The genitofemoral and femoral nerves are close in the vicinity, and care must be taken to avoid their injury when placing these sutures. A well-performed psoas hitch can bridge a gap of up to 5 cm. Constructing a Boari flap is another method of bridging a longer gap of up to 10 cm, and may also be performed in combination with a psoas hitch. It is important to note that a poorly executed Boari flap can compromise bladder capacity. In addition, small or contracted fibrotic bladders of tuberculous cystitis may not have sufficient wall area to allow flap creation. Finally, ileal interposition (ileal ureteric replacement) can be done in cases of multiple or recurrent strictures, where the native ureter is no longer an adequate conduit.
Bladder Surgery
Augmentation cystoplasty and bladder substitution are options in the management of the tuberculous contracted bladder. A capacity of less than 100 mL is commonly the indication to augment. Extremely contracted bladders (thimble bladders of 20 mL capacity) are best managed by orthotopic bladder substitution (Hemal and Aron, 1999). Various bowel segments have been used, and the general rules of incorporating the bowel into the urinary tract apply, such as thoroughly evaluating renal functions, reconfiguring a low pressure reservoir (de Figueiredo et al, 2006), patient education, and long-term follow-up.
Prostate and Urethra
Bladder neck contracture or severe granulomatous prostatitis is best managed endoscopically by transurethral incision of the contracture or resection of the prostate. A tuberculous prostatic abscess can be managed by transurethral drainage or aspiration with ultrasound guidance.
Urethral strictures may also be managed endoscopically and will often require repeated procedures. Tuberculous urethral fistulae are best managed by initiation of medical therapy and suprapubic bladder drainage. Delayed reconstruction is preferred.
Drainage of a seminal vesicle TB cavity into the bladder by cold knife incision has been reported (Dewani et al, 2006).
Genital TB
Surgery is considered only for established male genital tuberculosis when symptoms have failed to respond to chemotherapy and for nonresolving caseating abscesses. Surgical options involve excision of the diseased tissue. Involvement of the epididymis without clear affection of the testis is often the case, and every effort should be made to perform an epididymectomy rather than excision of the testis. Preserving testicular blood supply is important during the tedious dissection of the epididymis. Initiating dissection at the globus minor after ligation of the vas facilitates excision. If testicular involvement is evident, a scrotal orchiectomy is done. Involvement of the vas deferens by TB is usually distal to the external ring and ligation of at the level of the ring is possible and sufficient.
Management of Genitourinary TB in Special Situations
Pregnancy and Lactation
Females of the childbearing age should be advised to avoid pregnancy while on antituberculous treatment. If the diagnosis is discovered during pregnancy, prompt treatment is initiated because risks to the fetus from TB outweigh the risk of adverse drug effects. Pregnancy is allowed to continue, and a drug regimen consisting of isoniazid, ethambutol, and rifampicin, in addition to pyridoxine supplements, is started and should continue for 9 months.
During lactation, pyridoxine should be given while on isoniazid because this drug is significantly excreted in breast milk. Ethambutol is sparsely excreted in milk, and risk of ocular toxicity is rare.
HIV Infection
Patients diagnosed with TB should be tested for HIV infection. HIV-positive individuals with recent contact with a TB case should receive empiric treatment of latent TB, regardless of screening test results. Patients on maintenance treatment for active TB should be placed on daily or three times a week regimens rather than weekly regimens to avoid the emergence of drug-resistant strains, particularly in patients with CD4 counts <100 cells/mm3 (Nettles et al, 2004). Treatment should continue for a minimum of 9 months. Drug interactions with components of a highly active antiretroviral (HAART) regimen should be taken into consideration. Rifampicin may decrease the serum levels of HAART drugs to possibly ineffective levels. Dose increases may be needed to compensate for this effect.
Renal Transplant Recipients
Tuberculosis should be considered in the differential evaluation of a recipient with unexplained fever and constitutional manifestations. The risk is highest in recipients born in or who live in endemic areas, and the peak incidence occurs during the first year after transplantation (el-Agroudy et al, 2003). Urinary tract involvement occurs in up to 50% of cases. PPD skin testing may be falsely negative in 70% of cases due to anergy. A repeat test after one week may increase the diagnostic yield by 10%. Thus PCR analysis, or DNA probing, of urine is the recommended method of detection. Rejection may also complicate the presentation of allograft tuberculosis.
PPD-positive patients in pretransplantation evaluation should receive isoniazid prophylaxis for at least 6 months. Four drug regimens are used for treatment, which typically lasts for 18 months. Rifampicin induces the hepatic P-450 microsomal enzyme and may substantially decrease serum levels of cyclosporine, tacrolimus, and prednisolone below therapeutic levels, necessitating dose increases and frequent monitoring of serum drug levels. Rifampicin-free regimens have also been used (Khaira et al, 2009).
Renal Failure
Renal failure patients, particularly those on chronic dialysis, have a markedly increased risk for TB. Early diagnosis prevents a high mortality associated with this presentation (Hussein et al, 2003). Most antitubercular drugs need dose adjustments in the presence of various degrees of renal failure and in patients on hemodialysis and chronic ambulatory peritoneal dialysis. Reference should be made to dosing methods (interval increase or dose reduction) and clearance on dialysis of each drug.
Parasitic Diseases of the Genitourinary System
Parasitic diseases remain a significant health problem, especially in resource-poor countries. The human urogenital tract is typically only affected by a few species of parasites (Richens, 2004; Kehinde, 2008). This chapter will discuss in detail Schistosoma haematobium, a blood trematode that causes severe genitourinary tract disease, and the filarial helminth infections of the generas Wuchereria, Brugia, and Onchocerca, which may result in hydrocele, chyluria, funiculoepididymitis, and genital elephentiatis. Involvement of the genitourinary tract by other parasites (except sexually transmitted protozoa) is either subordinate to systemic manifestations or rare.
Billions of people harbor parasites, yet symptomatic infections are substantially less common. Parasitic infection does not always result in parasitic disease, depending on the intensity and duration of infection, as well as the contributing sequelae of inflammatory responses to the presence of parasites and/or their biologic products.
Increasing international travel, globalization of the economy, and jet travel increasingly results in uncommon and tropical diseases presenting in the offices of physicians far from endemic areas. The most important requirement for recognition of parasitic infections is awareness, based on knowledge of common clinical presentations, pathogenesis, and geographic distribution of parasitic diseases. The importance of careful history-taking should include questions of recent and remote travel. Additional probing can assist in establishing the likelihood of specific parasitic infections. Helpful cogent information on parasitic infections is available from the Centers for Disease Control and Prevention at www.dpd.cdc.gov/dpdx/. One of the best available resources for current knowledge on available drug therapies for parasitic infections is available from The Medical Letter (2010) (www.themedicalletter.com).
Urinary Schistosomiasis
History
Schistosomiasis is a chronic infection caused by the digentic parasitic trematodes of the genus Schistosoma. Human infection begins with free-swimming cercariae penetrating the skin and eventual development of the adult male and female worms. The threadlike female schistosome lies within a cleft in the body of the male worm, remaining in a state of permanent copulo for many years. The paired adult worms reside in the venous plexuses of the abdominal viscera. S. mansoni and S. japonicum reside in the mesenteric veins, leading to gastrointestinal and/or hepatic disease, while S. haematobium dwells principally in the perivesical venous plexus, resulting in urinary schistosomiasis.
Urinary schistosomiasis has been a scourge of the Middle East and Africa for millennia and remains a major cause of morbidity and mortality in these areas. Egyptian physicians of the twelfth dynasty (1900 BC) recognized hematuria as the cardinal sign and symptom of this disease. Theodore Bilharz, a German pathologist working in Cairo in 1852, first described worm pairs in mesenteric veins at autopsy and linked these to eggs found in human excreta.
Biology and Life Cycle
The life cycle of S. haematobium is outlined in Fig. 16–8 (King, 2006). The male and female worm pair and attach to the blood vessel endothelia, producing and depositing 200 to 500 eggs per day. The estimated common life span of 3 to 6 years (Butterworth et al, 1988) means a single worm pair can produce 250,000 to 600,000 eggs. Rare cases of long-lived worm pairs, of up to 30 years, are capable of inducing greater pathology (De Gentile et al, 1988).
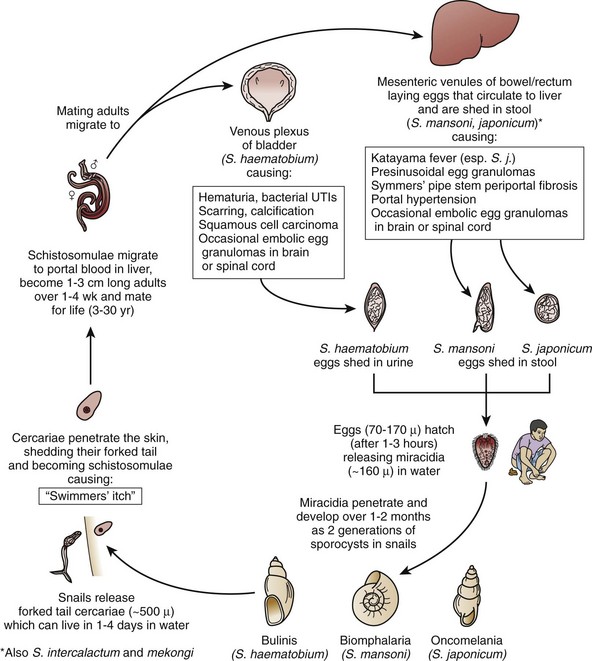
Figure 16–8 Life cycle of a schistosome. UTIs, urinary tract infections.
(From King CH. Schistosomiasis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases, principals, pathogens, and practice. 2nd ed. Philadelphia: Elsevier Churchill Livingstone; 2006. p. 1341–8.)
According to experimental data, the majority of eggs microembolize in the microvasculature of the lungs, liver, and other sites (Cheever and Anderson, 1971), while 20% of the eggs cross into the lumina of the hollow viscera near the adult pair. Hence, for S. haematobium the eggs are predominantly excreted in the urine with some finding their way into the feces. The eggs entrapped in tissue become calcified and accumulate in the viscera at a rate of 90 to 100 per worm pair per day (Cheever et al, 1977). These eggs are destroyed by the host gramulomitis immune response which may result in clinical morbidity and possible mortality.
S. haematobium eggs measure 80 to 150 µm, are ovoid, and have a small spine at their terminal end. This differentiates them from the laterally spined S. mansoni eggs. S. chyponecum has a tiny lateral spine. The only other schistosome that is pathogenic for humans and has a terminally spined egg, S. interculotum, is rarely seen outside limited foci in the Republic of Congo, Gabon, and Cameroon (WHO, 1993). Diagnostic terminally spined eggs can be found in the urine, feces, or human tissue especially intestinal or bladder wall biopsy. Due to the ongoing nature of infection and egg production, all egg stages are seen during active infection. Once the infection is treated, only degenerated or calcified eggs can be found.
If viable eggs are placed in fresh water, the miracidia emerge as short-lived ciliated larvae that swim to seek intermediate snail hosts. For S. haematobium snails of the Bulinus species (Ross et al, 2002) can perpetuate the life cycle. The miracidia migrate through tissues and then transform into successive generations of sporocysts. An amplification cascade finds each miracidium developing into a sporocyst, which produces 20 to 40 daughter sporocysts, which each in turn produce 200 to 400 cercariae. The cercariae escape the daughter sporocyst, migrate to the snail surface, and emerge into the surrounding fresh water. This enormous asexual multiplication from a single miracidium to 105 cercariae compensates for the attrition during aquatic parts of the life cycle. The cercariae must penetrate unbroken skin of appropriate hosts within a few hours or they die. For S. haematobium, the developmental process from cercarial body to schistosomulum to adult pair may require 80 to 110 days.
Epidemiology
Of the 200 million persons afflicted with schistosomiasis, 80 to 90 million are infected with S. haematobium (Mahmoud and Abdel Wahab, 1990; WHO, 1998, 2002; Engels et al, 2002), and as many as 10 to 40 million have obstructive uropathy or other complications secondary to this parasitic disease. Transmission of S. haematobium occurs in many countries in the Middle East and in most of the African continent. In Southwest Asia, the disease is found in Southern Yemen, Yemen, Saudi Arabia, Lebanon, Syria, Turkey, Iraq, and Iran (WHO, 1998).
Clinical and autopsy studies have demonstrated a correlation between the prevalence of infection and its intensity with levels of egg excretion or tissue egg burden (Smith et al, 1974b). The tissue egg burden is related to the severity of the disease and to the frequency of complications. Autopsy studies have shown that severe urinary pathology is uncommon when the frequency of infection in a population is less than 30%, but increases linearly after this threshold is exceeded (Cheever et al, 1978).
Pathogenesis and Pathology
Schistosomal disease results directly from the granulomatous host response to schistosome eggs (Phillips and Colley, 1978; Cheever et al, 1985; Waine and McManus, 1997). During ongoing human infections all stages of granulomas are simultaneously present; whereas, in treated or burned-out infection the granulomas are uniform (Cheever et al, 1985). Four factors contribute to this spectrum of significant disease related to S. haematobium: intensity, duration, activity, and focality. These factors also determine the morbidity, mortality, and treatment of urinary schistosomiasis.
Though S. haematobium adult worm pairs are widely distributed in the pelvic venous plexuses, egg-laying occurs mainly in the lower urinary tract, the site of pathologic manifestations (Cheever et al, 1977). It is typical to see composite granulomas in tissue rather than a small single granuloma, because S. haematobium eggs are deposited in groups more often than singly. Even though S. mansoni may lay eggs in the lower urinary tract, no lesions have been ascribed solely to S. mansoni (Cheever et al, 1978).
The immune response to S. haematobium is complex and multifaceted. The embolized eggs induce a vigorous eosinophilic and granulomatous immune response. Significant cellular and humoral host responses develop (Phillips and Colley, 1978; Butterworth, 1993; Mwatha et al, 1998; De Jesus et al, 2002; Pearce and MacDonald, 2002; Leutscher et al, 2005). These immune responses partially abrogate (but do not eliminate) subsequent reinfection in human hosts (Hagan et al, 1985, 1987, 1991; Woolhouse et al, 1991). Areas of the world endemic for schistosomiasis often have very high rates of HIV coinfection. Egg excretion in HIV-seronegative persons is significantly higher than HIV-seropositive individuals, though circulating schistosomal antigen levels are similar (Karanja et al, 1997). These observations are compatible with the hypothesis that the transit of schistosome eggs through the tissue is facilitated by a competent immune system.
Microscopic examination of tissue shows polypoid patches consisting of scattered and massed composite granulomas separated by edematous granulation tissue diffusely infiltrated by eosinophils, lymphocytes, and plasma cells. On gross examination, the areas of granulomatous inflammation result in large, bulky, hyperemic, and polypoid masses projecting into the lumen (Fig. 16–9). In the active stage of disease, schistosomiasis is characterized by multiple large inflammatory polyps related to the heavy localized egg burden (Smith et al, 1977c). In the endemic areas, these polypoid patches occur mainly in children through their early teens. However, up to 60% of urinary bladder polypoid lesions in patients with schistosomiasis result from conditions other than schistosomiasis, such as polypoid cystitis (Smith et al, 1974a).
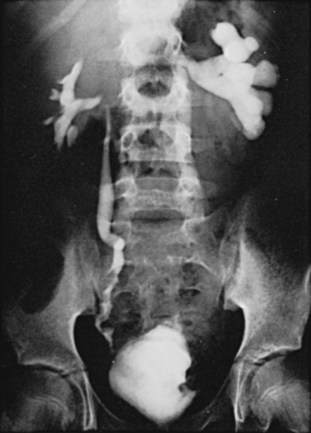
Figure 16–9 Intravenous urogram in an Egyptian boy shows scalloping of the bladder and right lower ureter by schistosomal polypoid lesions.
Inactive urinary schistosomiasis, occurs after adult worms have died, and is characterized by the absence of viable eggs in tissue or urine and the presence of “sandy patches”—relatively flat, tan mucosal lesions of various depth, with somewhat ill-defined borders (Fig. 16–10). These patches are formed as the inflammation associated with active egg-laying wanes. The remaining entrapped eggs are destroyed or calcified and the tissues undergo fibrotic reaction, which is the histologic characterization of the sandy-patch lesions. Urinary excretion of dead or calcified eggs is rare. However, the bladder may contain a sufficient number of calcified eggs to result in a thin outline of the bladder on plain radiographs.
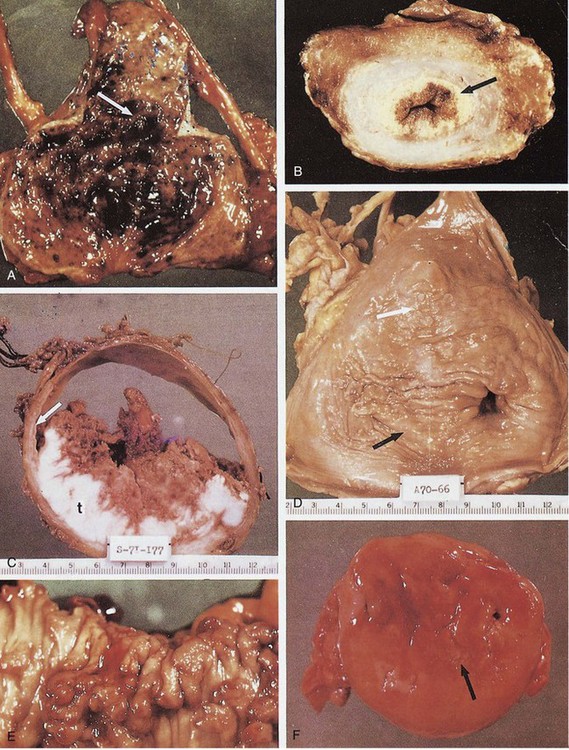
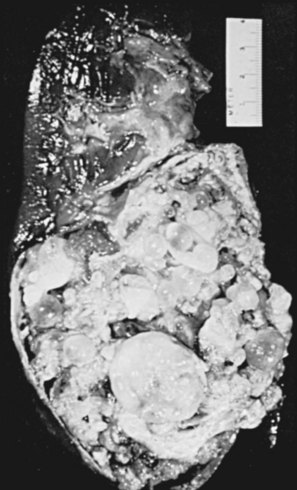
Figure 16–10 Macroscopic appearance of human urinary schistosomiasis. A, Urinary bladder opened with an anterior Y incision. The posterior and apical walls have many erythematous, granular, sessile, and pedunculated polyps (arrow), characteristic of the early active stage of urinary schistosomiasis. B, Coronal section through the apex of a formalin-fixed urinary bladder. The lamina propria has been expanded and is replaced by a yellow-tan, finely granular, sandy patch (arrow), which is characteristic of chronic inactive foci. Small sandy patches are sprinkled through the fibrotic, atrophic detrusor muscle, even in perivesical fat. The more superficial erythematous portion of the lamina propria contains some viable eggs with granulomatous response (chronic active stage of urinary schistosomiasis). C, Coronal section through the middle of a urinary bladder after formalin inflation and fixation. The lamina propria (arrow) has been replaced by a concentric sandy patch, most prominent at the margin of the exophytic, moderately differentiated squamous cell carcinoma. The bladder wall is attenuated except for the tumor (t). No evidence of recent oviposition was found in the lower urinary tract (chronic inactive stage of urinary schistosomiasis, usually found with the bilharzial bladder cancer syndrome). D, Urinary bladder opened with anterior Y incision shows several features of severe chronic inactive urinary schistosomiasis. The entire lamina propria has been replaced by a sandy patch. Foci of epidermization are seen at or near the white arrow. The left ureteral orifice (right) is markedly dilated (the so-called golf-hole ureter of schistosomal uropathy). The right ureteral orifice (point of black arrow) is markedly stenotic. E, Rectosigmoid colon with polyposis. Numerous sessile and pedunculated polyps are seen. Many are erythematous, indicative of active oviposition with granuloma formation. Some have necrotic hemorrhagic tips. F, Mucosal surface of partial cystectomy specimen (4- to 5-cm ellipse) from a patient with the chronic inactive stage of the disease. There is a stellate chronic schistosomal ulcer. Despite the inactivity of the disease, these ulcers may bleed profusely. Pale mucoid flecks at the margin of the ulcer (arrow) are areas of adenoid (goblet cell) metaplasia.
The clinical pathology of chronic schistosomiasis is often the development of obstructive uropathy. There are two components to schistosomal obstructive uropathy: obstruction and its effect on the proximal ureter. Schistosomal obstructive uropathy is usually asymmetric (Smith et al, 1974a). The location of the obstruction varies from the urethral meatus (1%), interstitial ureter (10% to 30%), juxtavesical ureter (20% to 60%), lower third of the ureter (15% to 50%), or a contiguous combination of these areas (30% to 60%) (Gelfand, 1948; Smith et al, 1977b; Al-Shukri and Alwan, 1983). Three types of hydroureter are associated with obstructive schistosomiasis: segmental (i.e., cylindrical or fusiform), tonic, and atonic (Smith et al, 1977b). Approximately 25% of obstructive uropathy cases involve segmental ureteral dilation, with 80% of those cases occurring in the lower ureter. The dilations occur above areas of concentric ureteral muscular obliteration associated with fibrosis and sandy patches. It is rare for segmental lesions to cause significant hydronephrosis. Up to 30% of obstructive uropathy is due to tonic hydroureter. This is characterized by dilated, tortuous, thick-walled, and trabeculated ureters with marked ureteral muscular hypertrophy and decreased peristaltic action. Typically, the entire ureter proximal to an obstructive lesion is involved, creating a functional stenosis. This is usually accompanied by significant hydronephrosis, which is reversible if the obstruction is relieved (Smith et al, 1977b). Atonic hydroureters are found in the remaining patients with schistosomal obstructive uropathy. This form is characterized by markedly dilated, very tortuous, thin-walled ureters, without peristalsis and associated with atrophic fibrotic ureteral muscle.
Hydroureter usually precedes hydronephrosis (Lehman et al, 1973; Smith et al, 1974a, 1977b; Cheever et al, 1978). Left untreated, schistosomal hydronephrosis advances from progressive renal pelvic dilation to medullary atrophy to near total medullary effacement before cortical atrophy ensues (Smith et al, 1974a, 1977b). This specific pathophysiology explains the abrogation of tubular function (especially maximal urine concentration) before compromise of glomerular function (Lehman et al, 1971, 1973).
Bladder cancer is the final pathologic sequela of schistosomiasis. Linkage between urinary schistosomiasis and the development of bladder cancer has been postulated since the end of the nineteenth century. Bladder cancer in the setting of S. haematobium has an early onset (40 to 50 years) and a high frequency of squamous cell carcinomas (60% to 90%), with 5% to 15% adenocarcinomas (Cheever, 1978; Lucas, 1982; Al-Shukri et al, 1987; Thomas et al, 1990; Bedwani et al, 1998). More than 40% of schistosomiasis-associated squamous cell carcinomas of the bladder are well-differentiated or verrucous carcinomas that are exophytic and carry an overall good prognosis. Tumors are found on the posterior wall about 50% of the time and on the lateral wall roughly 30% of the time. Exophytic tumors constitute about two thirds of schistosomal bladder cancers, while one third are ulcerative endophytic tumors. Mass schistosomiasis treatment campaigns in Egypt are associated with an overall reduction of all bladder cancers (27.6% to 11.7%) and a shift to more transitional cell carcinoma from squamous cell carcinoma (Gouda et al, 2007). Although less frequent transitional cell carcinomas of the bladder are associated with S. haematobium infection (Michaud, 2007), some epidemiologists feel the relatively high rate of smoking in this region may increase the risk of bladder cancer. Notably, however, some unselected autopsy series from the same regions have shown similar frequencies of bladder cancers in patients without schistosomiasis (Smith et al, 1977a; Cheever et al, 1978).
Clinical Manifestations
Acute Schistosomiasis
Acute schistosomiasis is also referred to as Katayama fever, rarely found among endemic populations. First coined about S. japonicum infection, it refers to a first, and presumably heavy, exposure of a noninfected individual that leads to fever, lymphadenopathy, splenomegaly, eosinophilia, urticaria, and other manifestations of a serum sickness–like disease (Doherty et al, 1996; De Jesus et al, 2002). This presentation is relatively rare in S. haematobium and more common in S. japonicum, which is probably the result of the significantly greater egg fecundity of S. japonicum. Acute schistosomiasis generally occurs 3 to 9 weeks after infection, coinciding with the onset of egg-laying, but may be delayed for up to 4 months (Young et al, 1986). Of note, this symptomatic phase often precedes the occurrence of eggs in the urine. Hematuria is the first sign of established S. haematobium infection, often appearing 10 to 12 weeks after infection (Kehinde et al, 2008).
Acute schistosomiasis is rarely associated with atopic egg-laying because worms meander toward the pelvic venous plexuses and become delayed or lost. These ectopic eggs incite an intense granulomatous inflammatory reaction in aberrant sites such as the skin (Edington et al, 1975), epididymis (Elem et al, 1989), or spinal cord or nerve roots or both (Pitella, 1997).
Chronic Schistosomiasis
Chronic schistosomiasis is far more common than acute disease and has several different clinical manifestations. The prepatent period, between penetration of the cercariae and onset of egg deposition in the tissues, is usually 2 to 3 months but may last more than 7 months (Young et al, 1986). The most typical presentation of active schistosomiasis is hematuria with terminal dysuria. This hematuria can be sufficient to induce anemia (Wilkins et al, 1985). Some patients develop early polypoid lesions of the bladder that result in urethral or ureteral obstruction or develop heavy bleeding, enough to produce clot retention. Because water contact is essentially universal, active schistosomiasis usually begins in childhood. In some cultures, hematuria in males may be seen as a sign of puberty. In this active infection stage, eggs are deposited in the tissues, traverse the bladder or rectosigmoid mucosa, and are excreted in the urine (or less commonly in the feces). This provides an opportunity for diagnosis.
S. haematobium egg burdens of the seminal vesicles and the ejaculatory ducts are high, and either blood and schistosome eggs or both may appear in the ejaculate before they appear in the urine. Patients with involvement of these genitourinary structures often present with scrotal pain or a testicular mass. Egg burdens of the uterus, vagina, and testes are lower than those of the epididymis, ovaries, and fallopian tubes (Cheever et al, 1977, 1978; Helling-Giese et al, 1996).
S. haematobium causes genital disease in 30% of infected women (Poggensee and Feldmeier, 2001; Kjetland et al, 2005). Schistosomal cervicitis and vaginitis may be asymptomatic or have clinical manifestations similar to cervicitis and vaginitis due to other causes (Williams, 1967). A routine Papanicolaou smear may reveal diagnostic eggs of S. haematobium infection. Similar to other ulcerating sexually transmitted infections, schistosomal lesions of the vagina and cervix may predispose patients to the acquisition of HIV infection (Feldmeier et al, 1994). Histologic evaluation of the placenta and amniotic fluid at term has shown schistosome eggs in these structures, but there are no documented reports of fetal schistosomiasis.
Over time, a late, chronic, active stage develops when egg burdens in the tissue are at their highest. Clinically constant, deep lower abdominal and pelvic pain with associated urinary urgency, frequency, and incontinence are noted with the “schistosomal contracted bladder” (Duvie, 1986). Anatomically, the trigone appears normal or minimally hyperemic and edematous, while the detrusor muscle is indurated and thickened, along with the entire bladder wall. The bladder lumen is reduced to as little as 50 mL of functional capacity.
After some years, the active infection enters a more quiescent period, in which egg deposition and excretion continue at a lower rate and symptoms are diminished. Over 30% of light infections “resolve” spontaneously in some endemic areas (Rutasitara and Chimbe, 1985). The painful presentations of acute schistosomiasis are typically absent at this point. However, silent obstructive uropathy may develop throughout this phase as fibrosis replaces polypoid lesions and the bladder and ureters undergo irreversible damage. A slow, insidious evolution may result in enormous hydroureters and hydronephrosis with few symptoms.
Patients finally enter a chronic inactive phase, in which viable eggs are no longer detected in urine or tissues. Signs and symptoms at this stage are caused by sequelae and complications of the immune reaction to the eggs rather than the schistosomal infection itself. Unfortunately, among patients with schistosomal obstructive uropathy, 40% to 60% present to urologists at this stage of their disease (Smith and Christie, 1986). In heavily endemic areas, it is common to find nonfunctioning kidneys in patients who are otherwise apparently well. About 50% of patients will develop chronic or acute bacterial urinary tract infection complications superimposed on their schistosomal obstructive uropathy. The bacterial organisms associated with schistosomiasis are the same organisms that cause infections in patients without schistosomiasis. Some series have noted an association of chronic or recurring urinary tract infections caused by Salmonella species, often associated with intermittent bacteremia in some patients with urinary schistosomiasis (King, 2001). Salmonella organisms reside in the apical invaginations of the schistosome tegument, where they are protected from host defenses and antibiotics. Awareness of this association can lead to treatment of both conditions with good response. Antibiotics alone do not fully resolve this process.
Another manifestation of schistosomal disease is the development of bladder ulcers, which are usually of two types (Smith et al, 1977a). Acute schistosomal ulcers will rarely present in the active stage, when a necrotic polyp sloughs into the urine. The more common chronic schistosomal ulcer is a late sequela of heavy infection. This lesion is associated with a constant “burning” micturition and intense pelvic and suprapubic pain. Over 90% of these patients have a history of previous urinary schistosomiasis, 20% have histories of previous sequelae and complications, and 10% have had previous surgical intervention for urinary schistosomiasis. Gross hematuria and gross pyuria are found in more than half of these patients.
Diagnosis
The presence of terminally spined eggs in the urinary sediment is diagnostic of active S. haematobium infection. The eggs of S. mansoni and S. japonicum have a lateral spine. Eggs may also be seen in the rectal stool samples examined for ova and parasites. If eggs are not found in the urine or stool, a rectal biopsy should be attempted before a bladder biopsy, because eggs are nearly as common in the rectum mucosa and the risk of a urinary tract infection is avoided. A squash preparation of the biopsy specimen between glass slides is better than histopathologic analysis, because it is more sensitive and allows determination of egg viability. Ova are excreted in the urine in proportion to the viable worm burden in the lower urinary tract tissues (Smith et al, 1974b; Smith and Christie, 1986). The intensity of the current infection in its early stages can be estimated by the examination of urine and quantitative urinary egg count. This also has some prognostic significance. Moderate to heavy infections will reveal eggs in almost all routine examinations of the urinary sediment, although lighter infections may require filtration or concentration of the urine (Mott, 1983). S. haematobium egg excretion peaks between 10 AM and 2 PM. Interestingly, this circadian phenomenon reverses in night-shift workers without the variation induced by athletic activity or water loading (McMahon, 1976).
Serology tests of the blood that combine a FAST-ELISA followed by Western blot analysis are available at the CDC (Wilson et al, 1995; Al-Sherbiny et al, 1999). In cases where the diagnosis is highly suspected but eggs are not present, the serologic tests can be useful, but do not distinguish between acute and chronic disease. Antibody titers can remain positive even after curative treatment. Although serologic testing is a valuable diagnostic and epidemiologic tool, finding eggs is the gold standard for diagnosis of active infection. Chronic inactive urinary schistosomiasis is characterized by the lack of egg secretion even in patients with severe clinical sequelae. When tissue biopsies are taken, up to 106 eggs/g of tissue have been found (Smith et al, 1974b). Real-time PCR of the urine is in developmental testing, and may hold some promise in the future. Specificity of nearly 100% is better than sensitivity of 89% in studies, consistent with other nucleic acid–based diagnostic assays (Obeng et al, 2008). Radiography is an important diagnostic tool in the evaluation of sequelae and complications of urinary schistosomiasis. A plain radiograph of the abdomen may reveal calcifications in the urinary tract. The classic presentation of a calcified bladder, which may even look like a fetal head in the pelvis, is pathognomonic of chronic urinary schistosomiasis (Fig. 16–11). The seminal vesicles, prostate, posterior urethra, distal ureters, and, in rare instances, the colon may also show calcified lesions. The earliest radiographic changes appear to be striations in the ureters and renal pelvis (Hugosson, 1987). Ureteral calcification is typically mural, and the ureter is dilated. This differs from the calcification seen in tuberculosis, which forms a cast of a nondilated ureter.
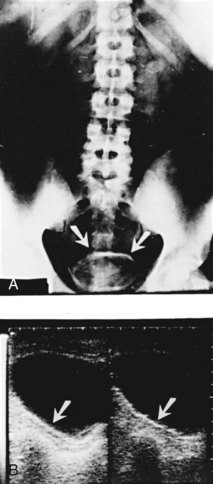
Figure 16–11 Bladder calcification in a 30-year-old Egyptian farmer. A, Plain x-ray film of the abdomen shows a rim of calcification surrounding the urinary bladder (arrows). B, Abdominal ultrasound study shows a bright line surrounding the bladder with a definite dark rim behind it (arrows).
(A and B, Courtesy of G. Thomas Strickland, MD. From Abdel-Wahab MF, Ramzy I, Esmat G, et al. Ultrasonography for detecting Schistosoma haematobium urinary tract complications: comparison with radiographic procedures. J Urol 1992;148:346.)
Hydroureter, hydronephrosis, nonfunctioning kidney, ureteral stenosis, and bladder and ureteral filling defects caused by polypoid lesions are readily observed on a standard intravenous urogram. Similar lesions can also be seen with ultrasonography. In the presence of severe obstructive uropathy, delayed films are often needed to discern distended ureters and kidneys. Postvoid films may indicate bladder neck obstruction with retention. Fluoroscopy can differentiate tonic and atonic ureters (Abdel-Halim et al, 1985) and identify nonstenotic, immotile ureters. Computer tomography (CT) scans have the advantage of being able to detect both obstructive uropathy and calcified lesions in the urinary tract and colon (Jorulf and Linstedt, 1985), giving them advantage over the intravenous urogram. MRI scanning has been tried but so far has not shown significant enhanced diagnostic ability to warrant the additional expense (Kohono et al, 2008).
Another useful imaging modality is vesicocystourethrography. It indicates the presence of vesicoureteral reflux, which occurs in 25% of infected ureters. Abdominal ultrasound is a useful method to detect focal thickening of the bladder wall and polypoid lesions of the urinary tract. It also detects hydroureter, hydronephrosis, and heavily calcified patches (Medhat et al, 1997).
Treatment
Medical Management
The development of safe and effective antischistosomal drugs has dramatically changed the management of schistosome infections (WHO, 1993; Richter, 2003; Berhe et al, 2008). All patients with schistosomiasis should be treated regardless of the intensity or apparent activity of their infection. Praziquantel is the drug of choice for all schistosoma species (The Medical Letter, 2010). It is active against all clinical forms and stages of the disease after the first 3 to 4 weeks of infection, because it works better in the presence of antibodies against schistosomal organisms. Praziquantel interferes with the ion transport in the schistosome tegument, resulting in calcium and sodium fluxes, metabolic alterations, and sudden contraction of the parasite’s musculature. Cure rates for S. haematobium infections are 73% to 100% (Mott et al, 1985; Leutscher et al, 2008). The drug also has activity against most other flukes and tapeworms, which is a great advantage in the developing world. The current recommendation is 2 oral doses of 20 mg/kg (or a single 40 mg/kg dose) in 1 day for S. haematobium (The Medical Letter, 2010). Praziquantel is extremely well tolerated, with occasional gastrointestinal complaints noted as the more common side effects. Headache, dizziness, and fever have occasionally been reported. The lack of serious side effects has made it an excellent agent of choice in mass chemotherapy campaigns (King et al, 1990).
Surgical Management
The efficacy and ease of current drug therapy for schistosomiasis, together with the possible reversibility of disease (Berhe et al, 2008) provides rationale for medical therapy trials prior to elective surgical approaches for genital urinary disease associated with schistosomal infection (Cioli et al, 1995). In general, surgery is reserved for complications that have not responded to adequate medical treatment within a reasonable follow-up time, or for those settings where immediate surgical intervention is necessary. Obstructive neuropathy is the most common chronic condition requiring surgery with severe bladder hemorrhage, the most common cause for urgent surgical intervention.
Significant prostatitis and hypertrophy are uncommon in schistosomiasis. There is no evidence of bladder outlet obstruction in S. haematobium infections in multiple autopsy studies (Smith et al, 1974a; Cheever et al, 1977, 1978). However, clinical studies consistently report cystoscopic (Fam, 1964), urodynamic (Sabha and Nilsson, 1988), and postvoid residual urine, which are evidence of functional bladder outlet obstruction that occasionally requires surgical intervention in patients with severe inactive urinary schistosomiasis (Abdel-Halim, 1984). Due to scrotal pain, induration, and enlargement associated with schistosomal epididymitis, surgery is often performed for the suspicion of a testicular tumor.
As previously discussed, schistosomal disease of the urinary bladder leads to a spectrum of clinical outcomes, including schistosomal polyposis, sandy patches, contracted bladder, schistosomal ulceration, urothelial hyperplasia, metaplasia, dysplasia, and bladder cancer. In particular instances, such as the chronically contracted bladder, surgical intervention is indicated and takes the form of vesical denervation, urinary diversion, ileocystoplasty, or hydrodistention. Any treatment, however, must be done in conjunction with medical chemotherapy. Chronic deep bladder ulcers may necessitate a partial cystectomy, because fulguration rarely produces either symptomatic relief or healing of the ulcer. Urothelial hyperplasia is strongly associated with severe urinary schistosomiasis, whereas urothelial metaplasia and dysplasia commonly accompany schistosomal bladder cancer (Khafagy et al, 1972). Treatment of bladder cancer secondary to schistosomiasis is usually surgical and discussed elsewhere in this textbook.
The most common sequelae of urinary schistosomiasis result from ureteral involvement causing obstructive uropathy (Lehman et al, 1973; Smith et al, 1974a; Cheever et al, 1978; Smith and Christie, 1986). Hydroureter and hydronephrosis are related to the intensity of S. haematobium infection. Because ureteral obstruction seen during active schistosomiasis is most often caused by concentric or hemiconcentric polypoid lesions that “girdle” the ureteral muscle in the intramural and adjacent extravesical ureter, it responds well to medical management alone. Complete resolution of deteriorated renal function occurs within 1 to 2 months of antischistosomal chemotherapy when obstruction is caused by active disease (Lehman et al, 1973). One study reported that chemotherapy not only reverses schistosomal obstructive uropathy but also prevents it, even in persons who continue to become reinfected (Subramanian et al, 1999). In late chronic active and inactive urinary schistosomiasis, on the other hand, anatomic obstruction is more prominent.
Anatomic ureteral stenosis, with or without calculi, has been identified in up to 80% of obstructions (Lehman et al, 1973; Smith et al, 1977b; Al-Shukri and Alwan, 1983; El-Nahas et al, 2003). Therefore when there is residual ureteral stenosis after successful chemotherapy it is usually amenable to surgical intervention. Depending on the extent and location of the stricture, procedures involving excision or dilatation have been used. Balloon dilatation has reportedly proved effective with anatomic stenosis (Jacobsson et al, 1987), but mechanical dilatation is frequently followed by repeat stenosis (Wishahi, 1987). When the ureteral meatus, intermural ureter, ureterovesical junction, or lower ureter is involved, a variety of plastic operations to reconstruct a functional valve are available. Most of these procedures are variants of the Leadbetter-Politano operation (Politano and Leadbetter, 1958; Leadbetter and Leadbetter, 1961). Although highly effective for some patients (Smith et al, 1977b; Al-Shukri and Alwan, 1983), other authors have noted restenosis (Umerah, 1981).
In long or multisegmental lesions, excision of the affected portion leaves an inadequate residual ureter for reimplantation; in these cases, surgeons have successfully employed the Boari-Ockerblad bladder flap, Boari-Küss flap, ileal conduit, suprapubic intravesical ureterostomy, and ureteroileocystostomy, taking care to have an isoperistaltic direction of the ileal segment (Abdel-Halim, 1980, 1984; Al-Shukri and Alwan, 1983; Abu-Aisha et al, 1985). Isolated meatal stenosis may be amenable to simple meatoplasty (Al-Shukri and Alwan, 1983). When a ureter is hopelessly obstructed, long-term nephrostomy drainage or creation of an ileal ureter can provide relief.
Prognosis
Tens of millions of people are infected with S. haematobium, but most have mild infections, and good prognosis. However, the morbidity and mortality of schistosomiasis are determined by the overall intensity of infection. No mortality was observed in areas in which the prevalence of schistosomiasis and frequency and severity of schistosomal obstructive uropathy were low (Nigeria). Yet when Egypt had a prevalence of 50%, attributable mortality approached 10% (Smith et al, 1974a; Cheever et al, 1978). Among patients with severe disease, mortality reached 50% in 2 to 5 years (Lehman et al, 1970).
Patients who die of schistosomal obstructive uropathy (bilateral end-stage hydronephrosis) are usually in their 20s, have early-stage disease, and have heavy total egg burdens. Patients who develop the complications of pyelonephritis and urothelial cancer are usually older than age 40, consistent with time- and intensity-linked pathology (Christie et al, 1986; Smith and Christie, 1986).
The prognosis for persons with demonstrable urinary tract lesions has dramatically improved with praziquantel therapy. In children with obstructive polyps, the uropathy usually completely resolves within 2 to 6 weeks of treatment. For patients with chronic obstructive uropathy due to sandy patches and fibrosis, the prognosis is less clear. Some individuals tolerate advanced obstructive uropathy with little, if any, deterioration in renal function. Schistosomal obstructive uropathy, urolithiasis, bladder outlet obstruction, and bacterial cystitis all predispose to pyelonephritis. Bacterial superinfection is a serious prognostic event and should be treated as vigorously and as soon as possible. Furthermore, for those who develop a bladder malignancy, their prognosis is dependent on the aggressiveness of their tumor.
Prevention and Control
Travelers in endemic areas should be advised of the hazard of infected fresh water streams, rivers, ponds, and lakes and should avoid contact with such water. Boiling water kills the cercariae. Measures of control in endemic areas have utilized several approaches: destruction of the snail host, elimination of urine and fecal contamination of water, and reduction of contact with infected water (WHO, 1993). In many endemic areas, such measures are expensive, unfeasible, or poorly tolerated by the local population. Therefore mass therapy by drugs has been emphasized, and all major control campaigns today include drug treatment as a major component, often age-directed or annual or both (King et al, 1991; Engels et al, 2002; WHO, 2002). Vaccines to prevent disease or to reduce infection are not currently available (Pearce, 2003).
Genital Filariasis
In broad terms, filarial diseases are classified as lymphatic and nonlymphatic. Wuchereria bancrofti accounts for 90% of the cases of human lymphatic filariasis, notably widespread throughout tropical regions of the world. This human parasite has no animal reservoir. Brugia malayi and B. timori cause human lymphatic filariasis in the Far East, but also have primate and feline reservoirs. Nonlymphatic filariasis is caused by Onchocerca volvulus, Loa loa or Mansonella perstans. O. volvulus, the agent of river blindness, is found predominantly in Africa with small scattered foci in South and Central America. Although dermatitis, keratitis, and chorioretinitis are the common features of O. volvulus infection (Freedman, 2006); subcutaneous nodules and massive inguinal lymphadenopathy may result in “hanging groin” and scrotal elephantiasis.
Very rarely, Loa loa or M. perstans have been isolated from ovarian follicular fluid and associated with infertility (Chang et al, 2008; Bazi et al, 2006).
Lymphatic Filariasis
The symptoms of bancroftian and brugian filariasis range from acute lymphatic inflammation characterized by fever, localized lymphangitis, and transitory lymphadenopathy to chronic lymphatic dilation with hydrocele, elephantiasis of the limbs, and chyluria (Ottesen, 1989; Dreyer et al, 2000). Lymphatic filariasis affects 120 million people living in 73 countries, with India accounting for 40% of the global infections (Ramaiah et al, 2000).
Biology and Life Cycle
The lymphatic filariae are elongated (100 × 0.3 mm for female and 40 × 0.1 mm for male W. bancrofti) nematodes. Their cycle proceeds from human to mosquito and back. The most common urban vectors of W. bancrofti are the ubiquitous Culex pipiens complex, but filariae have adapted to a wide variety of mosquitoes in different areas. Only small portions of mosquito bites (1%) are infective, even in hyperendemic areas. Typically, obstructive lymphatic disease occurs in people repeatedly infected over many years.
Female mosquitoes ingest microfilariae with their blood meals. These rapidly become infective larvae and go to the mosquitoes’ salivary glands. The infective larvae are then deposited on the skin during the next mosquito feeding. Although unable to traverse unbroken skin, the infective larvae can enter the bite site or can cross the normal conjunctiva or buccal mucosa (Ah et al, 1974; Sullivan and Chernin, 1976). The adult filaria then migrates to and lives in the larger lymphatic vessels. W. bancrofti adults have a predilection for periaortic, iliac, inguinal, and intrascrotal lymph vessels, whereas B. malayi prefers inguinal and more distal lymphangioles.
Most of the mature Wuchereria female is composed of the uterus, which contains all stages, from eggs to mature microfilariae. Microfilaria discharge into the blood is regulated by a feedback system that maintains a constant level of microfilaremia. There is, however, no constant relationship between the number of worms and the level of microfilaremia. Indeed, microfilaremia is found in only 30% to 40% of all infections. The timing of peak microfilaremia in each endemic focus corresponds to the peak feeding period of local mosquito vectors.
Pathology and Pathogenesis
Epidemiologic findings and animal experiments clearly indicate that host reactions to microfilariae differ from those to adult worms. Although pathologic and clinical features differ between patients from endemic and from nonendemic areas, significant humoral and cellular immune responses develop in both groups (Steel et al, 1996). Filaria-specific IgE titers rise, and IgE-eosinophil–mediated killing of microfilariae has been noted in vitro. However, patients from endemic regions manifest IgG4-blocking antibodies and antigen-specific suppressor T cells. The extent of immune down regulation correlates with an absence of pathologic sequelae and the presence of microfilaremia (Piessens, 1982; Davis, 1989; Ottesen, 1989).
Early Infection
Lesions of established infection do not remit, but either persist actively or result in significant scarring and lymphatic obstruction. Lesions may appear at the worms’ nesting areas (funiculoepididymitis, orchitis, filarial lymphangitis, filarial abscess), in their lymphatic distribution (e.g., hydrocele, lymphadenitis, genital edema), or both. Dying or dead adult filariae are often detected in surgically excised tissue, which suggests that worm death provokes the inflammatory response.
The lesions vary from nodular inflammation, simulating neoplasm, to suppuration, simulating acute bacterial disease. Histologically, nodular lesions show massive granulomas around cuticular fragments or necrotic filaria. Tissue eosinophilia is a useful diagnostic hint but may be absent. The most acute subcutaneous filarial lesions simulate fluctuant abscesses with bacteriologically sterile but purulent exudate that surrounds a dying worm (O’Connor and Hulse, 1935).
Episodic filarial inflammation eventually abates, leaving obliterated lymphatics surrounded by poorly organized scar tissue. A palpable mass or indurated cord may develop. The consequences of lymphatic obstruction vary with the location of the obstruction, but the common feature is the accumulation of lymphedema or hydrocele fluid associated with lymphoid infiltrates.
Late Infection
The complications of late infection include huge hydroceles and scrotal and penile elephantiasis (Fig. 16–12). Lymphangiographic studies reveal that the initial obliteration of lymphatic vessels is bypassed by collateral formation. As these collaterals become progressively obstructed, lymph dilatation follows. Lymphangiograms in late filariasis show poorly draining networks of communicating lymph vessels extending through lymphedematous tissues into superficial dermal layers (Kanetkar et al, 1966).
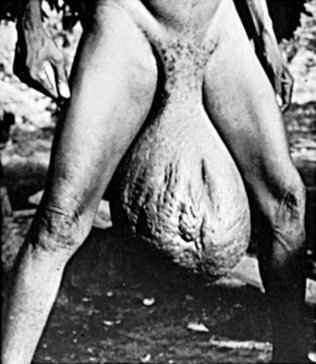
Figure 16–12 Huge hydrocele and scrotal elephantiasis.
(Courtesy of Dr. B.H. Kean. From Zaiman H. A pictorial presentation of parasites, Valley City, ND.)
Patients demonstrating chyluria and filarial hydrocele rarely experience bacterial super-infection. In contrast, elephantiasis and lymphedematous scrotum are often superinfected (Olszewski et al, 1997). Further supporting the role of infection in classic elephantiasis is noted by the ability to prevent this manifestation when patients with lymphedema are instructed to use antiseptic soap on a regular basis and treated with antibiotics (Maher and Ottesen, 2000). Bacterial superinfections are thus considered an additional contributing factor to the development of sequelae from filarial infection (Dreyer et al, 2000).
Serial autopsy studies of men show that the tail of the epididymis and the lower spermatic cord are the most constant locations of worms (Galindo et al, 1962). Correspondingly, funiculoepididymitis and hydrocele are the most common consequences of bancroftian filariasis. Assuming that heavy infections saturate the preferred habitat and compel adult filariae to spread centrifugally to increasingly distant sites, saturation of inguinal lymphatic trunks (yielding scrotal edema) initiates encroachment into femoral lymphatics (causing elephantiasis of lower extremities) and proximally to renal lymphangioles (producing lymph varices whose intrapelvic rupture causes chyluria).
Clinical Manifestations
Clinical lymphatic filariasis manifests as one of four forms:
Asymptomatic Infection
Persons in endemic areas may have positive serology or skin test reactivity to filarial antigens but no microfilaremia and no clinical evidence of filariasis. Asymptomatic microfilaremia may occur in patients with downregulated effector immune responses (Ottesen, 1989). Such individuals probably developed tolerance to filarial antigens in utero (Weil et al, 1983). In addition, generation of elevated levels of interleukin-12 has been noted in individuals with chronic microfilaremia (King et al, 1995).
Filarial Fevers
Patients with “filarial fever” sustain episodic fevers, lymphangitis-lymphadenitis, funiculoepididymitis, transient edema, and small acute hydroceles. They are typically amicrofilaremic. Patients with endemic filarial fever usually inexorably progress due to ongoing reinfection and persistent inflammatory reaction. Expatriates who may experience parasite- related fevers will abate after repatriation and treatment.
Chronic Filariasis
Early established filariasis progresses to the late chronic stage with a series of progressive lesions: funiculoepididymitis, hydrocele, orchitis, scrotal and penile elephantiasis, lymph scrotum, and chyluria. These do not resolve with treatment, reflective of the chronic tissue changes induced by earlier inflammatory reaction. This pathology alters local lymph fluid drainage (Fan et al, 1995).
Funiculoepididymitis
Most symptomatic cases of filarial funiculoepididymitis appear before patients’ fourth decade. The attack may be isolated, with remission, or may be repetitive and progressive. Pain radiating to the testis and simulating ureteral colic may accompany the systemic symptoms.
Palpable cordlike swelling may mimic an intrascrotal tumor or torsion of the cord and be accompanied by hydrocele or soft tissue edema. Eosinophilia is common because of the persistent tissue migratory nature of this parasitic infection. Varicocele, or thrombosis of the pampiniform plexus, may complicate inflammation, increasing pain and swelling. Bacterial superinfection of acute filarial corditis, is a rare but often lethal complication, with exquisite pain and septic thrombophlebitis as a contributing cause.
Mild cases may involute spontaneously, but, in an endemic setting, they more often point to future recurrences or the development of chronic lymphedema or both (Kazura et al, 1995). The disease frequently simulates malignancy, and many patients ultimately undergo operations including orchiectomy. Even in severe filarial funiculitis, the spermatic cord is usually intact and patent. Sterility and orchitis due to filariasis are rare.
Hydrocele
In endemic areas, differentiation of filarial from idiopathic hydrocele is difficult on either clinical or laboratory grounds. Microfilariae or adult worms are rarely detected in the hydrocele fluid, but a milky or sediment-rich hydrocele fluid suggests a filarial origin. Hydrocele accompanied by nodules in the cord or epididymis and a history of travel to or residence in an endemic area suggests filariasis. Discovery of a thick, fibrous tunica, especially with cholesterol or calcium deposits, should also suggest a diagnosis of filariasis. Tunical calcification is very rare in idiopathic hydrocele.
Scrotal and Penile Elephantiasis
Mild scrotal edema is not unusual during early infection or with established hydrocele. Penile edema, on the other hand, is unusual, and the monstrous elephantine enlargements of the scrotum or penis depicted in textbooks occur largely in populations without access to medical care. Elephantiasis of the limbs poses the differential diagnosis between filarial and other causes, but genital elephantiasis is rarely due to other causes, such as malignancy.
Chyluria
Chyluria occurs with or without microfilaremia, usually in young adults. It occurs earlier in the natural history of filariasis than does genital elephantiasis. Dying worms provoke lymphatic obstruction with proximal lymphangiolar dilatation. Rupture of a lymphatic varix into the urinary collecting system has been demonstrated by lymphangiography. Chyluria may initially alarm patients but often is disregarded and may result in severe protein loss, leading to hypoalbuminemia and anasarca. Chyluria is usually intermittent and may spontaneously remit with bed rest.
Tropical Pulmonary Eosinophilia
Tropical pulmonary eosinophilia occurs in patients with an allergic response to microfilarial antigens; such patients are amicrofilaremic (Ong and Doyle, 1998). This syndrome is characterized by marked, sustained peripheral eosinophilia, only temporarily affected by corticosteroids but responsive to antifilarial drugs. There is an absence of classic filarial lesions, such as lymphedema or funiculoepididymitis. However, the presence of lymphadenopathy that can be marked enough to mimic lymphoma and symptomatic pulmonary infiltrates suggests asthma (Danaraj et al, 1966). Patients develop very high levels of filaria-specific IgE and pulmonary reticulonodular densities on chest radiographs that reveal an eosinophilic interstitial pneumonitis on biopsy. The frequency of this syndrome varies markedly between endemic foci and is most common in southern India and Singapore.
Diagnosis
Careful history-taking and physical examination are paramount in suggesting a filarial lymphedema. TB, S. haematobium, and gonorrhea may also produce funiculoepididymitis. Idiopathic hydrocele with or without varicocele or hernia is common in tropical and nontropical areas. However, hydrocele occurs at an earlier age and in greater frequency in areas of endemic filariasis (Jachowski et al, 1962).
The histologic finding of adult worms is a diagnostically definitive but insensitive technique. The presence of microfilariae in peripheral blood, chylous urine, or hydrocele fluid is also diagnostic. Blood samples must be taken when peak microfilaremia occurs (e.g., midnight in the case of nocturnal periodic W. bancrofti). Peripheral blood is best examined by the thick-drop technique with Giemsa stain. It is important to remember that filaremia may be absent in both early and late filariasis, after treatment with a microfilaricide, and in tropical pulmonary eosinophilia. Immunoassays for measuring antibody and circulating antigens, as well as molecular biologic assays for detecting parasite DNA, have been developed (McCarthy, 1996; Harnett et al, 1998; Mishra, 2005).
Direct ultrasonographic observation of adult filariae has been reported in lymph vessels of microfilaremic and otherwise asymptomatic patients, made possible by energetic movements of the worms (“filarial dance sign”) (Amaral et al, 1994; Chaubal et al, 2003). Lymphangiography may distinguish filariasis from other causes of lymphatic obstruction, especially in conditions with reduced numbers and competence of lymph vessels. Plain radiographs may reveal calcified worms, which are diagnostic.
Treatment and Prevention
There is continual development of new control strategies and treatment programs for filariasis. The new methodology of control leverages the benefits of mass treatment programs and individual patient treatment to dramatically reduce transmission (Ottesen et al, 1997). Four drugs are now used singly or in various combinations and doses: diethylcarbamazine (DEC), ivermectin, albendazole, and doxycycline (Shenoy et al, 1999; Taylor et al, 1999; Mand et al, 2009). DEC has been used for over 50 years and is predominantly effective against adult worms. Ivermectin has efficacy against microfilaremia, but is relatively ineffective against adult parasites. The objective of therapy is to kill adult worms and abolish microfilaremia. Unfortunately, patients may have prominent immune reactions to the enhanced antigenic exposure associated with dying filariae, the severity directly proportional to the magnitude of the microfilaremia. Patients with high microfilarial counts should start with lower doses when DEC is used for the first several days to minimize these reactions. Some treatment protocols include premedication with corticosteroids or antihistamines to reduce the inflammatory symptoms. Additional treatment-related effects, including fever, headache, nausea, vomiting, and arthralgias, may occur in the first few days of treatment.
Ivermectin is used in a single dose of 200 to 400 µg/kg. The microfilaricidal effect is comparable to that of DEC. There is no effect on adult filariae. In contrast, albendazole kills both adults and microfilariae in lymphatic disease (Ottesen et al, 1999; Bookaire et al, 2002; Gardon et al, 2002). These medications are the cornerstone of mass treatment programs; they successfully interrupt transmission by reducing the microfilariae available for the arthropod vectors in endemic areas.
The recognition that all human pathogenic filariae harbor the bacterial endosymbiont (Wolbachia spp.) has led to trials of doxycycline, which kills the filarial by killing the Wolbachia. Subsequent use of the three classic antifilarial agents may still have a role in full eradication (Coulibaly et al, 2009; Mand et al, 2009). Three-week doxycycline therapy for W. bancrofti cases followed by a single dose of DEC has been shown to reduce or resolve lymphatic pathology in GU filariasis (Mand et al, 2009).
Use of elastic stockings and elevation of extremities are important adjunctive measures in reducing lymphedema. Meticulous skin care and aggressive treatment of secondary infections may halt or even reverse the lymphedema and prevent the development of elephantiasis (Ottesen et al, 1997). Abdominal binders have been used to increase intra-abdominal pressure in an effort to stop chyluria but are controversial because they may increase chyluria in some patients (Ahrens, 1970). Finally, diagnostic retrograde lymphangiography curatively scleroses lymphatic fistulas in 48% of patients (Gandhi, 1976). Thus surgical correction is unnecessary in many of these cases, and operative intervention is challenging because the varices are hard to identify and eliminate.
Genital elephantiasis is rarely amenable to surgery. Additionally, procedures such as lymphadenectomy may further compromise lymph drainage and exacerbate complications. There are, however, a few instances when surgical intervention is necessary to treat the complication of filariasis. For example, patients with filarial funiculoepididymitis should first undergo a thorough workup for malignant, filarial, and bacterial causes of their symptoms. Then, appropriate antibiotics should be prescribed for bacterial disease, and treatment of the filariasis may require surgical decompression or excision of filarial nodules, preserving the testis and cord. When funiculoepididymitis is recurrent, painful, and deforming or complicated by blood vessel involvement, more radical surgery is warranted. Next, for the treatment of large or symptomatic hydroceles, hydrocelectomy is indicated. Excision of the intact hydrocele sac is the treatment of choice; an alternative method is inversion with partial excision (Jachowski et al, 1962). Small hydroceles that do not enlarge can be ignored. Finally, a variety of procedures have been devised to remove redundant tissue and to reconstruct the scrotum or vulva.
For prevention of disease, DEC has been used as a prophylactic drug, given as an annual dose of 6 mg/kg. Trials with prophylactic ivermectin have had similar beneficial effects. Major control methods, however, depend on the use of residual insecticides, domestic mosquito netting, and the reduction of mosquito breeding sites.
Onchocerciasis
Onchocerca volvulus is the agent of river blindness and of severe, debilitating, chronic dermatitis. Onchocerciasis is common throughout tropical Africa, and endemic foci also exist in Central and South America. This filaria differs sharply from Brugia and Wuchereria: (1) It is transmitted by black flies of the Simulium species; (2) adult worms inhabit subcutaneous tissue and cause palpable fibrous nodules in which they are encapsulated; and (3) microfilariae travel through the dermis (and the eye) but are not in the peripheral blood. Diagnosis and estimation of infection intensity are made by microscopic examination of skin snips immersed in normal saline solution for 20 to 30 minutes under a coverslip on a slide, or with Giemsa stain. Serologic and antigen detection tests have also been developed (Vincent et al, 2000; Pischke et al, 2002).
In late stages, onchocerca infection may produce “hanging groin” or scrotal elephantiasis. Histology demonstrates atrophy and fibrosis of the inguinal lymph nodes with subcutaneous edema and fibrosis superimposed on the typical onchocercal dermatitis (Connor et al, 1970). In addition, onchocerciasis is occasionally accompanied by giant inguinal lymphadenopathy, which can also produce “hanging groin.”
A single oral dose of ivermectin (150 µg/kg) repeated every 2 to 6 months until the patient is asymptomatic (resolution of pruritis and rash) has proved to be a successful onchocercal microfilaricide with few side effects (Greene et al, 1985; The Medical Letter, 2010). DEC should not be used for treatment of this disease, because of the severe allergic immune responses to microfilariae dying in the skin and other sites (the Mazzotti reaction). Ivermectin distribution has been successfully used for curbing O. volvulus transmission in an international campaign against African river blindness under the auspices of the WHO (Dull and Meredith, 1998). An advance with potential therapeutic implications is the demonstration that a 6-week course of doxycycline renders the female worm sterile by reducing the symbiotic Wolbachia endobacteria (Hoerauf, 2003).
Other Parasitic Diseases of the Genitourinary Tract
This section describes parasitic infections that are rare, or in which urogenital manifestations are overshadowed by diseases and other organs. They are, nonetheless, occasionally clinically important for individual patients. The organisms involved include the intestinal helminth Enterobius; the larval tapeworm of hydatid disease, Echinococcus; Entamoeba histolytica; Gnathostomiasis; Trichomonas vaginalis; Plasmodium falciparum; Strongyloides stercoralis; and, very rarely, locally inoculated fly larvae.
Enterobiasis
The common intestinal pinworm Enterobius vermicularis, which is ubiquitous worldwide, occasionally migrates from its nocturnal swarming site, the anus, upward into the vagina, reaching the peritoneal cavity through the uterus and fallopian tubes. In that unnatural habitat, worm movement and egg-laying may continue, producing inflammation of the pelvic peritoneum with pain, fever, simulated acute appendicitis, or other lesions. Dead worms and eggs incite granulomas and adhesions. Careful searching for enterobiasis eggs in vaginal secretions may properly diagnose a persistent vaginal discharge that was originally thought to be caused by more typical organisms. Treatment of pelvic enterobiasis is with pyrantel pamoate, mebendazole, or albendazole (The Medical Letter, 2010).
Hydatid Disease
No part of the human anatomy is invulnerable to hydatid cysts, but renal hydatids occur in only about 2% of cases (Musacchio and Mitchell, 1966; Kehinde et al, 2008). The hydatid is the larval form of Echinococcus granulosus, whose definitive host is the dog, and whose principal intermediate host is the sheep. The major endemic loci of hydatid disease are sheep-herding areas, such as Australia, Argentina, Greece, Spain, and the Middle East. In addition, feral life cycles lead to sporadic human cases in sites without sheep. Echinococcal cysts of other species are found in Alaska, Siberia, parts of Europe (Echinococcus multilocularis) (Rausch, 1967), and Central America (Echinococcus vogeli) (D’Alessandro, 1979). In each instance, humans acquire the cysts by accidentally eating eggs excreted in the feces of dogs or alternative feral hosts.
In the kidney and other sites, hydatid cysts evolve by slow, asymptomatic, concentric growth over years and may invoke pressure symptoms or flank pain, depending on their location and size. Cysts can reach over 20 cm in diameter (Fig. 16–13). A host fibrous shell with scant inflammatory reaction envelops the cyst. They often enlarge 1 to 2 cm per year.
The most common urologic presentation is of chronic dull flank or lower back discomfort from cystic pressure (Gogus et al, 2003). The cysts seldom affect renal function. Diagnosis can be made by plain film radiography, ultrasonography, or CT, which shows a thick-walled, fluid-filled spherical cyst, often with a calcific cyst wall (Horchani et al, 2001; Turgut et al, 2009). Serologic testing (available through state laboratories or the CDC) has proved useful but has a sensitivity of only 60% to 90%. Albendazole, 400 mg twice daily for 1 to 6 months, is the recommended medical therapy. Some patients may require surgical excision of their cysts because of the size or location of the lesions (Handa and Harjai, 2005). Praziquantel and albendazole have been recommended preoperatively for 7 to 10 days to minimize or prevent secondary seeding by daughter cysts if they accidentally contaminate the operative field (The Medical Letter, 2010). Spillage of cystic products into the peritoneum or the bloodstream may result in metastatic infection. Rupture of cysts may result in systemic anaphylaxis.
Amebiasis
E. histolytica is a rare cause of renal abscess (Brandt and Perez Tamayo, 1970). Liver abscesses invariably accompany the abscess. The right kidney is more frequently involved. Hematuria may be a prominent manifestation, especially if the abscess induces renal vein thrombosis. Medical therapy (metronidazole or tinidazole) must be promptly instituted (The Medical Letter, 2010). Surgery, if necessary, should be delayed until drug therapy has been initiated; otherwise, disastrous spread of amebic infection is likely (Grigsby, 1969). Good reviews on the pathology (Brandt and Perez Tamayo, 1970), surgery (Grigsby, 1969), and medical management of amoebiasis (Ravdin and Stauffer, 2005) are available.
Gnathostomiasis
Gnathostomiasis is a food-borne zoonotic nematode. Gnathostoma spinigerum is the most common species to infect humans. Disease is acquired by consumption of infected fish when eaten raw or undercooked. Humans represent an accidental host, which means the parasite is unable to reach full maturity. It wanders through tissues resulting in local symptoms, depending on location, and typically a very predominant eosinophilia (often greater than 50%) (McCarthy and Moore, 2000). While the disease typically has superficial subcutaneous manifestations, deeper visceral tissues may be involved. Very rare genitourinary cases have been reported (Rusnak and Lucey, 1993).
Serologic assays have been developed with sensitivity and specificity of 93% and 98%, respectively, but are not readily available. The definitive treatment is surgical excision when possible. Albendazole is a safe and well-tolerated medical treatment that is often successful (Kraivichian et al, 1992).
Trichomoniasis
Trichomonas vaginalis is a flagellate protozoal organism, commonly found in the secretions of inflamed genital surfaces, such as the vagina or the preputial skin or the urethral mucosa (Kehinde, et al, 2008). Infection from this parasite is most commonly transmitted through intimate sexual contact and may involve the Cowper or Skeen glands. In women, the prevalence of trichomoniasis is estimated to be 3% to 48% in various studies (Sena et al, 2007). Vaginitis is the most frequent manifestation; however, it has been associated with preterm delivery in pregnant women (Cotch et al, 1997). The majority of male partners of women with trichomoniasis are infected even when the men are asymptomatic. Men may experience urethritis, epididymitis, prostatitis, and infertility, probably through infection of the seminal vesicles.
Diagnosis can be relatively straightforward if secretions are examined under a microscope and the highly active motile flagellum is visualized. However, the sensitivity of this diagnostic step is limited. Newer nucleic acid amplification tests have been developed with sensitivities ranging from 85% to 100% (Kaydos et al, 2002).
Malaria
Plasmodium falciparum is a protozoal parasite that causes severe malaria. The urogenital manifestation is through the phenomenon of black water fever due to hemoglobinuria (Kehinde et al, 2008). This is a rare manifestation involving a complement-mediated immune reaction found in patients who have resided 2 to 3 months in endemic areas, and who have also been treated with inadequate doses of antimalarial medication. The treatment includes appropriate full-course doses of effective antimalarials together with supportive medical care if the hemoglobinuria has resulted in acute renal tubular necrosis.
Strongyloidiasis
Strongyloides stercoralis is the fourth most important intestinal nematode infection in the world, with distribution throughout the tropical and temperate world. The parasite has the capacity for autoreinfection, which perpetuates infection for decades after exposure (Siddiqui et al, 2006). Typically, S. stercoralis infection produces an asymptomatic chronic upper gastrointestinal infection. However, in immunocompromised individuals, especially those receiving corticosteroids, strongyloides hyperinfection can develop with dissemination of the larvae to extraintestinal organs with mortality rates as high as 85%. Genitourinary infection is extremely rare and is usually an indication of disseminated disease. Motile larvae are rarely visible on urinalysis (Rifaat et al, 1973). The migrating worms carry gram-negative bacteria on their coat, which may result in bacteremia or infection of the genitourinary system if the parasites accidentally migrate to the genitourinary organs.
Diagnosis is usually best accomplished with blood serology, although the high sensitivity decreases in the face of immunosuppression. The organism exists as worms and larvae in typically low numbers, making direct observation challenging. The high numbers seen in disseminated infection can increase this sensitivity. Ivermectin represents the treatment of choice at a dose of 200 µg/kg daily for 2 days in normal hosts. Longer courses of treatment may be necessary in patients with disseminated disease. Of note, thiabendazole, the historical drug of choice, should no longer be used due to its prominent gastrointestinal and neuropsychologic side effects. Albendazole has some activity but a lower cure rate than ivermectin (The Medical Letter, 2010).
Key Points
Parasitic Diseases of the Genitourinary System
Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689-1694.
Dreyer G, Noroes J, Figueredo-Silva J, Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol Today. 2000;16:544.
Engin G, Acunas B, Acunas G, Tunaci M. Imaging of extrapulmonary tuberculosis. Radiographics. 2000;20:471. quiz 529, 532
Fan-Harvard P, O’Donovan C, Smith SM, et al. Oral fluconazole versus amphotericin B bladder irrigations for treatment of candidal funguria. Clin Infect Dis. 1995;21:960-965.
Jorulf H, Linstedt E. Urogenital schistosomiasis: CT evaluation. Radiology. 1985;157:745.
Kauffman CA. Candiduria. Clin Infect Dis. 2005;41:S371-S376.
Kauffman CA, Vazquez JA, Sobel JD, et alThe National Institute of Allergy and Infectious Diseases (NIAID) Mycoses Study Group. A prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14-18.
Kehinde EO, Anin JT, Hira PR. Parasites of urological importance. Urol Int. 2008;81:1-13.
Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161-189.
Ross A, Bartley P, Sleigh A, et al. Schistosomiasis. N Engl J Med. 2002;346:1212.
The Medical Letter. Drugs for tuberculosis. Treat Guidel Med Lett. 2004;28:83-88.
The Medical Letter. Antifungal drugs. Treat Guidel Med Lett. 2005;30:7-14.
The Medical Letter. Drugs for parasitic infections. Med Lett Treat Guide. 2010;8:e1-13.
Turgut AT, Ödev K, Kabaalioglu A, et al. Multitechnique evaluation of renal hydatid disease. Am J Roentgenol. 2009;192:462-467.
Wise GJ, Marella VK. Genitourinary manifestations of tuberculosis. Urol Clin North Am. 2003;30:111-121.
Abebe F, Bjune G. The protective role of antibody responses during mycobacterium tuberculosis infection. Clin Exp Immunol. 2009;157(2):235-243.
Angus BJ, Yates M, Conlon C, Byren I. Cutaneous tuberculosis of the penis and sexual transmission of tuberculosis confirmed by molecular typing. Clin Infect Dis. 2001;33(11):E132-E134.
American Thoracic Society (ATS), Centers for Disease Control and Prevention (CDC). Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;61(4 Pt. 1):1376-1395.
Barnard M, Albert H, Coetzee G, et al. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177(7):787-792.
Bilen CY, Inci K, Erkan I, Oze H. The predictive value of purified protein derivative results on complications and prognosis in patients with bladder cancer treated with Bacillus Calmette-Guerin. J Urol. 2003;169(5):1702-1705.
Burrill J, Williams CJ, Bain G, et al. Tuberculosis: a radiologic review. Radiographics. 2007;27(5):1255-1273.
Carl P, Stark L. Indications for surgical management of genitourinary tuberculosis. World J Surg. 1997;21(5):505-510.
Centers for Disease Control and Prevention (CDC). Extensively drug-resistant tuberculosis—United States, 1993-2006. MMWR Morb Mortal Wkly Rep. 2007;56(11):250-253.
Chang CY. Genitourinary tuberculous infection. In: Lapides J, Nesbit RM, editors. Fundamentals of urology. Philadelphia: Saunders; 1976:295.
Cooper HG, Robinson EG. Treatment of genitourinary tuberculosis: report after 24 years. J Urol. 1972;108(1):136-142.
Daniel TM. The immunology of tuberculosis. Clin Chest Med. 1980;1(2):189-201.
Daniel TM. Pioneers of medicine and their impact on tuberculosis. Rochester, NY: University of Rochester Press; 2000.
Dannenberg AM. Pathogenesis of pulmonary tuberculosis: host-parasite interactions, cell-mediated immunity, and delayed-type hypersensitivity. In: Schlossberg D, editor. Tuberculosis. 3rd ed. New York: Springer-Verlag; 1994:323.
Dannenberg AMJr. Immunopathogenesis of pulmonary tuberculosis. Hosp Pract. 1993;28(1):51-58.
de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440.
Dewani P, Dewani N, Bhatia D. Case report: tubercular cold abscess of seminal vesicle: minimally invasive endoscopic management. J Endourol. 2006;20(6):436-442.
Dutt AK, Moers D, Stead WW. Short-course chemotherapy for extrapulmonary tuberculosis. nine years’ experience. Ann Intern Med. 1986;104(1):7-12.
Dutt AK, Stead WW. Present chemotherapy for tuberculosis. J Infect Dis. 1982;146(5):698-704.
Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. JASN. 2001;12(6):1307-1314.
el-Agroudy AE, Refaie AF, Moussa OM, Ghoneim MA. Tuberculosis in Egyptian kidney transplant recipients: study of clinical course and outcome. J Nephrol. 2003;16(3):404-411.
Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832.
Fischer M, Flamm J. [The value of surgical therapy in the treatment of urogenital tuberculosis. Urologe A. 1990;29(5):261-264.
Flechner SM, Gow JG. Role of nephrectomy in the treatment of non-functioning or very poorly functioning unilateral tuberculous kidney. J Urol. 1980;123(6):822-825.
Gow JG, Barbosa S. Genitourinary tuberculosis. A study of 1117 cases over a period of 34 years. Br J Urol. 1984;56(5):449-455.
Gupta NP, Kumar R, Mundada OP, et al. Reconstructive surgery for the management of genitourinary tuberculosis: a single center experience. J Urol. 2006;175(6):2150-2154. discussion 2154
Hawken MP, Ojoo JC, Morris JS, et al. No increased prevalence of adrenocortical insufficiency in human immunodeficiency virus-associated tuberculosis. Tubercle Lung Dis. 1996;77(5):444-448.
Hemal AK, Aron M. Orthotopic neobladder in management of tubercular thimble bladders: initial experience and long-term results. Urology. 1999;53(2):298-301.
Hemal AK, Gupta NP, Kumar R. Comparison of retroperitoneoscopic nephrectomy with open surgery for tuberculous nonfunctioning kidneys. J Urol. 2000;164(1):32-35.
Hemal AK, Gupta NP, Rajeev TP, et al. Polymerase chain reaction in clinically suspected genitourinary tuberculosis: comparison with intravenous urography, bladder biopsy, and urine acid fast bacilli culture. Urology. 2000;56(4):570-574.
Horne NW, Tulloch WS. Conservative management of renal tuberculosis. Br J Urol. 1975;47(5):481-487.
Hussein MM, Mooij JM, Roujouleh H. Tuberculosis and chronic renal disease. Semin Dial. 2003;16(1):38-44.
Johnson AB. Tuberculosis of the kidney. Surgical diagnosis, 2nd ed. New York, London: D Appleton; 1911.
Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PloS One. 2009;4(9):e6914.
Kaufmann SH. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann the Rheum Dis. 2002;61(Suppl. 2):ii54-ii58.
Khaira A, Bagchi S, Sharma A, et al. Renal allograft tuberculosis: report of three cases and review of literature. Clin Exp Nephrol. 2009;13(4):392-396.
Kim SH, Yoon HK, Park JH, et al. Tuberculous stricture of the urinary tract: antegrade balloon dilation and ureteral stenting. Abdom Imag. 1993;18(2):186-190.
Koch R. Die atiologie der tuberculose. Facsimile of the original contribution by Robert Koch in “berliner klinische wochenschrift” 10 april 1882. [I. Die Aetiologie der Tuberculose. (Nach einem in der physiologischen Gesellschaft zu Berlin am 24. Marz er, gehaltenen Vortrage).]. Fortschr Med. 1982;100(12):539.
Lattimer JK. Renal tuberculosis. N Engl J Med. 1965;273:208-211.
Lee Y, Huang W, Huang J, et al. Efficacy of chemotherapy for prostatic tuberculosis—a clinical and histologic follow-up study. Urology. 2001;57(5):872-877.
Madkour MM. Textbook of tuberculosis. New York: Springer; 2003.
Manjunath N, Shankar P, Rajan L, et al. Evaluation of a polymerase chain reaction for the diagnosis of tuberculosis. Tubercle. 1991;72(1):21-27.
Marconi M, Pilatz A, Wagenlehner F, et al. Impact of infection on the secretory capacity of the male accessory glands. Int Braz J Urol. 2009;35(3):299-308. discussion 308–9
Matos MJ, Bacelar MT, Pinto P, Ramos I. Genitourinary tuberculosis. E J Radiol. 2005;55(2):181-187.
The Medical Letter. Drugs for parasitic infections. Med Lett Treat Guide. 2010;8:e1-13.
Medlar EM. Cases of renal infection in pulmonary tuberculosis: evidence of healed tuberculous lesions. Am J Pathol. 1926;2(5):401-14.15.
Medlar EM, Spain DM, Holliday RW. Post-mortem compared with clinical diagnosis of genito-urinary tuberculosis in adult males. J Urol. 1949;61(6):1078-1088.
Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588.
Murphy DM, Fallon B, Lane V, O’Flynn JD. Tuberculous stricture of ureter. Urology. 1982;20(4):382-384.
Muttarak M, ChiangMai WN, Lojanapiwat B. Tuberculosis of the genitourinary tract: imaging features with pathological correlation. Singapore Med J. 2005;46(10):568-574. quiz 575
Nettles RE, Mazo D, Alwood K, et al. Risk factors for relapse and acquired rifamycin resistance after directly observed tuberculosis treatment: a comparison by HIV serostatus and rifamycin use. Clin Infect Dis. 2004;38(5):731-736.
Paick J, Kim SH, Kim SW. Ejaculatory duct obstruction in infertile men. BJU Int. 2000;85(6):720-724.
Pasternak MS, Rubin RH. Urinary tract tuberculosis. In Schrier RW, editor: Diseases of the kidney and urinary tract, 7th ed, Philadelphia: Lippincott Williams & Wilkins, 2001.
Piersimoni C, Scarparo C, Piccoli P, et al. Performance assessment of two commercial amplification assays for direct detection of mycobacterium tuberculosis complex from respiratory and extrapulmonary specimens. J Clin Microbiol. 2002;40(11):4138-4142.
Premkumar A, Lattimer J, Newhouse JH. CT and sonography of advanced urinary tract tuberculosis. AJR. 1987;148(1):65-69.
Ramanathan R, Kumar A, Kapoor R, Bhandari M. Relief of urinary tract obstruction in tuberculosis to improve renal function. analysis of predictive factors. Br J Urol. 1998;81(2):199-205.
Rich M, World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization; 2006.
Roylance J, Penry JB, Davies ER, Roberts M. The radiology of tuberculosis of the urinary tract. Clin Radiol. 1970;21(2):163-170.
Salem B. Disseminated tuberculosis following the placement of ureteral stents: a case report. Cases J. 2008;1(1):383.
Schecter GF, Scott C, True L, et al. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010;50(1):49-55.
Shin KY, Park HJ, Lee JJ, et al. Role of early endourologic management of tuberculous ureteral strictures. J Endourol. 2002;16(10):755-758.
Simon HB, Weinstein AJ, Pasternak MS, et al. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420.
Small PM, Fujiwara PI. Management of tuberculosis in the United States. N Engl J Med. 2001;345(3):189-200.
Sommers HM. The laboratory diagnosis of mycobacterial disease. In: Youmans GP, editor. Tuberculosis. Philadelphia: Saunders; 1979:511.
Sorlozano A, Soria I, Roman J, et al. Comparative evaluation of three culture methods for the isolation of mycobacteria from clinical samples. J Microbiol Biotechnol. 2009;19(10):1259-1264.
Sousa M, Pozniak A, Boffito M. Pharmacokinetics and pharmacodynamics of drug interactions involving rifampicin, rifabutin and antimalarial drugs. J Antimicrob Chemother. 2008;62(5):872-878.
Stacul F, Rossi A, Cova MA. CT urography: the end of IVU? La Radiologia Medica. 2008;113(5):658-669.
Timmins GS, Deretic V. Mechanisms of action of isoniazid. Molec Microbiol. 2006;62(5):1220-1227.
van Vollenhoven P, Heyns CF, de Beer PM, et al. Polymerase chain reaction in the diagnosis of urinary tract tuberculosis. Urol Res. 1996;24(2):107-111.
Wang LJ, Wu CF, Wong YC, et al. Imaging findings of urinary tuberculosis on excretory urography and computerized tomography. J Urol. 2003;169(2):524-528.
Wang YX, Chen CR, He GX, Tang AR. CT findings of adrenal glands in patients with tuberculous Addison’s disease. J Belge Radiol. 1998;81(5):226-228.
Watterson SA, Drobniewski FA. Modern laboratory diagnosis of mycobacterial infections. J Clin Pathol. 2000;53(10):727-732.
Wise GJ, Shteynshlyuger A. An update on lower urinary tract tuberculosis. Curr Urol Rep. 2008;9(4):305-313.
Wong SH, Lau WY, Poon GP, et al. The treatment of urinary tuberculosis. J Urol. 1984;131(2):297-301.
World Health Organization, Global Tuberculosis Programme. Global tuberculosis control. Geneva: World Health Organization; 2008.
Zink AR, Sola C, Reischl U, et al. Characterization of mycobacterium tuberculosis complex DNAs from Egyptian mummies by spoligotyping. J Clin Microbiol. 2003;41(1):359-367.
Parasitic Diseases of the Genitourinary System
Abdel-Halim RE. Ileal loop replacement and restoration of kidney function in extensive bilharziasis of the ureter. Br J Urol. 1980;52:280.
Abdel-Halim RE. Bilharzial uropathies and a scheme for primary medical care. Br J Urol. 1984;56:13.
Abdel-Halim RE, Al-Mashad S, Al-Dabbagh A. Fluoroscopic assessment of bilharzial ureteropathy. Clin Radiol. 1985;36:89.
Abu-Aisha H, Reddy JJ, Hussain S, Balbeesi A. Long-term ureterostomy with suprapubic intravesical drainage used to bypass severe schistosomal obstructive uropathy—preliminary report. Urol Res. 1985;13:263.
Al-Sherbiny MM, Osman AM, Hancock K, et al. Application of immunodiagnostic assays: detection of antibodies and circulating antigens in human schistosomiasis and correlation with clinical findings. Am J Trop Med Hyg. 1999;60:960.
Al-Shukri S, Alwan MH. Bilharzial strictures of the lower third of the ureter: a critical review of 560 strictures. Br J Urol. 1983;55:477.
Al-Shukri S, Alwan MH, Nayef M. Bilharziasis in malignant tumors of the urinary bladder. Br J Urol. 1987;59:59.
Bedwani R, Renegenathan E, El Kwasky F, et al. Schistosomiasis and the risk of bladder cancer in Alexandria, Egypt. Br J Cancer. 1998;77:1186.
Berhe N, Myrvang B, Gundersen SG. Reversibility of schistosomal periportal thickening/fibrosis after praziquantel therapy: a twenty-six month follow-up study in Ethiopia. Am J Trop Med Hyg. 2008;78:228-234.
Butterworth AE. Immunology of schistosomiasis. In: Jordan P, Webbe G, Sturrock RE, editors. Human schistosomiasis. London: Baillière-Tindall; 1993:331.
Butterworth AE, Fulford AJ, Dunne DW, et al. Longitudinal studies on human schistosomiasis. Philos Trans R Soc Lond B Biol Sci. 1988;321:495.
Cheever AW. Schistosomiasis and neoplasia [editorial]. J Natl Cancer Inst. 1978;61:13.
Cheever AW, Anderson LA. Rate of destruction of Schistosoma mansoni eggs in the tissues of mice. Am J Trop Med Hyg. 1971;20:62.
Cheever AW, Byram JE, Hieny S, et al. Schistosomiasis in B cell–depleted mice. Parasite Immunol. 1985;7:399.
Cheever AW, Kamel IA, Elwi AM, et al. Schistosoma mansoni and Schistosoma haematobium infections in Egypt: II. Quantitative parasitological findings at necropsy. Am J Trop Med Hyg. 1977;26:702.
Cheever AW, Kamel IA, Elwi AM, et al. Schistosoma mansoni and Schistosoma haematobium infections in Egypt: III. Extrahepatic pathology. Am J Trop Med Hyg. 1978;27:55.
Christie JD, Crouse D, Smith JH, et al. Patterns of Schistosoma haematobium egg distribution in the human lower urinary tract: II. Obstructive uropathy. Am J Trop Med Hyg. 1986;35:752.
Cioli D, Pica-Mattoccia L, Archer S. Antischistosomal drugs: past, present … and future? Pharmacol Ther. 1995;68:35.
De Gentile L, Fayad M, Denis P, Lecastre MJ. Erratic localization and uncommon longevity of Schistosoma haematobium: apropos of a case. J Urol. 1988;94:163.
De Jesus AR, Silva A, Santana LB, et al. Clinical and immunological evaluation of 31 patients with acute Schistosomiasis mansoni. J Infect Dis. 2002;185:98.
Doherty JF, Moody AH, Wright SG. Katayama fever: an acute manifestation of schistosomiasis. BMJ. 1996;313:1071.
Duvie SOA. Cup-patch ileocystoplasty in treatment of bilharzial contracted bladder. J R Coll Surg Edinb. 1986;31:56.
Edington GM, Nwabuebo I, Junaid TA. The pathology of schistosomiasis in Ibadan, Nigeria, with special reference to the appendix, brain, pancreas and genital organs. Trans R Soc Trop Med Hyg. 1975;69:153.
Elem B, Patil PS, Lambert TK. Giant fibrous pseudotumor of the testicular tunics in association with Schistosoma haematobium infection. J Urol. 1989;141:376.
El-Nahas AR, Shoma AM, El-Baz M, et al. Bilharzial pyelitis: a rare cause of secondary ureteropelvic junction obstruction. Urology. 2003;170:1946.
Engels D, Chitsulo L, Montresor A, et al. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139.
Fam A. The problem of the bilharzial ureter. Br J Urol. 1964;36:211.
Feldmeier H, Krantz I, Poggensee G. Female genital schistosomiasis as a risk factor for the transmission of HIV. Int J STD AIDS. 1994;5:368.
Freedman DO. Onchocerciasis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases, principals, pathogens, and practice. 2nd ed. Philadelphia: Elsevier Churchill Livingstone; 2006:1176-1188.
Gelfand M. Bilharzial affection of the ureter: study of 110 consecutive necropsies showing vesicle bilharziasis. BMJ. 1948;1:1228.
Gouda I, Mokhtar N, Bilal D. Bilharziasis and bladder cancer: a time trend analysis of 9843 patients. J Egypt Natl Cancer Inst. 2007;19:158-162.
Hagan P, Blumenthal UJ, Chaudri M, et al. Resistance to reinfection with Schistosoma haematobium in Gambian children: analysis of their immune responses. Trans R Soc Trop Med Hyg. 1987;81:938.
Hagan P, Blumenthal UJ, Dunn D, et al. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243.
Hagan P, Wilkins A, Blumenthal UJ, et al. Eosinophilia and resistance to Schistosoma haematobium in man. Parasite Immunol. 1985;7:625.
Helling-Giese G, Kjetland EF, Gundersen SG, et al. Schistosomiasis in women: manifestations in the upper reproductive tract. Acta Trop. 1996;62:225.
Hugosson CO. Striation of the renal pelvis and ureter in bilharziasis. Clin Radiol. 1987;38:407.
Jacobsson B, Lindstedt E, Narasimham DL, et al. Balloon dilatation of bilharzial ureteral strictures. Br J Urol. 1987;60:28.
Jorulf H, Linstedt E. Urogenital schistosomiasis: CT evaluation. Radiology. 1985;157:745.
Karanja DM, Colley DG, Nahlen BL, et al. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg. 1997;56:515.
Kehinde EO, Anin JT, Hira PR. Parasites of urological importance. Urol Int. 2008;81:1-13.
Khafagy MM, El-Bolkainy MN, Mansour MA. Carcinoma of the bilharzial bladder: a study of associated mucosal lesions in 86 cases. Cancer. 1972;30:150.
King CH. Disease in schistosomiasis haematobia. In: Mahmoud AAF, editor. Schistosomiasis. London: Imperial College Press; 2001:265.
King CH. Schistosomiasis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases, principals, pathogens, and practice. 2nd ed. Philadelphia: Elsevier Churchill Livingstone; 2006:1341-1348.
King CH, Lombardi G, Lombardi C, et al. Chemotherapy-based control of schistosomiasis haematobia: II. Metrifonate vs. praziquantel in control of infection-associated morbidity. Am J Trop Med Hyg. 1990;42:587.
King CH, Muchiri E, Ouma JH, Koech D. Chemotherapy-based control of schistosomiasis haematobium: IV. Impact of repeated annual chemotherapy on prevalence and intensity of Schistosoma haematobium infection in an endemic area of Kenya. Am J Trop Med Hyg. 1991;45:498.
Kjetland EF, Ndhlovu PD, Mduluza T, et al. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2005;72:311.
Kohono M, Kuwatsuru R, Suzuki K, et al. Imaging findings from a case report of bilharziasis in a patient with gross hematuria of several years’ duration. Radiat Med. 2008;26:553-556.
Leadbetter GWJr, Leadbetter WF. Ureteral re-implantation and bladder neck reconstruction. JAMA. 1961;175:349.
Lehman JSJr, Farid Z, Bassily S. Mortality in urinary schistosomiasis. Lancet. 1970;2:822.
Lehman JSJr, Farid Z, Bassily S, Kent DC. Hydronephrosis, bacteriuria, and maximal urine concentration in urinary schistosomiasis. Ann Intern Med. 1971;75:49.
Lehman JSJr, Farid Z, Smith JH, et al. Urinary schistosomiasis in Egypt: clinical, radiological, bacteriological and parasitological correlations. Trans R Soc Trop Med Hyg. 1973;67:384.
Leutscher PD, Pedersen M, Raharisolo C, et al. Increased prevalence of leukocytes and elevated cytokine levels in semen from Schistosoma haematobium–infected individuals. J Infect Dis. 2005;191:1639.
Leutscher PD, van Dam GT, Reimert CM, et al. Eosinophil cationic protein, soluble egg antigen, circulating anodic antigen, and egg excretion in male urogenital schistosomiasis. Am J Trop Med Hyg. 2008;79(3):422-426.
Lucas S. Squamous cell carcinoma of the bladder and schistosomiasis. East Afr Med J. 1982;59:345.
Mahmoud AAF, Abdel Wahab MF. Schistosomiasis. In: Warren KS, Mahmoud AAF, editors. Tropical and geographical medicine. 2nd ed. New York: McGraw-Hill; 1990:458.
McMahon JE. Circadian rhythm in Schistosoma haematobium egg excretion. Int J Parasitol. 1976;6:373.
Medhat A, Zarzoura A, Nafeh M, et al. Evaluation of an ultrasonographic score for urinary bladder morbidity in Schistosoma hematobium infection. Am J Trop Med Hyg. 1997;57:16.
The Medical Letter. Drugs for parasitic infections. Med Lett Treat Guide. 2010;8:e1-13.
Michaud DS. Chronic inflammation and bladder cancer. Urol Oncol. 2007;25:260-268.
Mott KE. A reusable polyamide filter for the diagnosis of Schistosoma haematobium infection by urine filtration. Bull Soc Pathol Exot. 1983;76:101.
Mott KE, Dixon H, Osei-Tutu E, et al. Effect of praziquantel on hematuria and proteinuria in urinary schistosomiasis. Am J Trop Med Hyg. 1985;34:1119.
Mwatha JK, Kiamani G, Kamau T. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-gamma but low levels of IL-5 are associated with hepatosplenic disease in human schistosomiasis mansoni. J Immunol. 1998;160:1992.
Obeng BB, Aryeetey YA, de Dood CJ, et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. 2008;102:625-633.
Pearce EJ. Progress towards a vaccine for schistosomiasis. Acta Trop. 2003;86:309.
Pearce EJ, MacDonald A. The immmunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499.
Phillips SM, Colley DG. Immunologic aspects of host responses to schistosomiasis: resistance, immunopathology, and eosinophil involvement. Prog Allergy. 1978;24:49.
Pitella JE. Neuroschistosomiasis. Brain Pathol. 1997;7:649.
Poggensee G, Feldmeier H. Female genital schistosomiasis: facts and hypotheses. Acta Trop. 2001;79:193-210.
Politano V, Leadbetter NF. An operative technique for correction of vesicoureteral reflux. J Urol. 1958;79:932.
Richens J. Genital manifestations of tropical diseases. Sex Transm Infect. 2004;80:12.
Richter J. The impact of chemotherapy on morbidity due to schistosomiasis. Act Trop. 2003;86:161.
Ross A, Bartley P, Sleigh A, et al. Schistosomiasis. N Engl J Med. 2002;346:1212.
Rutasitara WK, Chimbe A. Spontaneous cure in schistosomiasis. East Afr Med J. 1985;62:408.
Sabha M, Nilsson T. Urodynamic evaluation of calcified bilharzial bladders. APMIS Suppl. 1988;3:50.
Smith JH, Christie JD. The pathobiology of Schistosoma haematobium infection in humans. Hum Pathol. 1986;17:333.
Smith JH, Elwi A, Kamel IA, von Lichtenberg F. A quantitative postmortem analysis of urinary schistosomiasis in Egypt: I. Pathology and pathogenesis. Am J Trop Med Hyg. 1974;23:1054.
Smith JH, Kelada AS, Khalil A. Schistosomal ulceration of the urinary bladder. Am J Trop Med Hyg. 1977;26:89.
Smith JH, Kelada AS, Khalil A, Torky AH. Surgical pathology of schistosomal obstructive uropathy: a clinicopathologic study. Am J Trop Med Hyg. 1977;26:96.
Smith JH, Torky H, Kelada AS, Farid Z. Schistosomal polyposis of the urinary bladder. Am J Trop Med Hyg. 1977;26:85.
Smith JH, Torky H, Mansour N, Cheever AW. Studies on egg excretion and tissue egg burden in urinary schistosomiasis. Am J Trop Med Hyg. 1974;23:163.
Subramanian AK, Mungai P, Ouma JH, et al. Long-term suppression of adult bladder morbidity and severe hydronephrosis following selective population chemotherapy for Schistosoma haematobium. Am J Trop Med Hyg. 1999;61:476.
Thomas JE, Bassett MT, Sigola LB, Taylor P. Relationship between bladder cancer incidence, Schistosoma haematobium infection, and geographical region in Zimbabwe. Trans R Soc Trop Med Hyg. 1990;84:551.
Umerah BC. Bilharzial hydronephrosis: a clinicoradiologic study. J Urol. 1981;126:164.
Waine GJ, McManus DP. Schistosomiasis vaccine development. Curr Picture Bioassays. 1997;19:435.
Wilkins HA, Goll PH, Moore PJ. Schistosoma haematobium infections and hemoglobin concentrations in a Gambian community. Ann Trop Med Parasitol. 1985;79:159.
Williams AO. Pathology of schistosomiasis of the uterine cervix due to S. haematobium. Am J Obstet Gynecol. 1967;98:784.
Wilson M, Schantz P, Pieniazek N. Diagnosis of parasitic infections: immunologic and molecular methods. In Murray PR, editor: The manual of clinical microbiology, 6th ed, Washington DC: American Society for Microbiology Press, 1995.
Wishahi M. The role of dilatation in bilharzial ureters. Br J Urol. 1987;59:405.
Woolhouse ME, Taylor P, Matanhire D, Chandiwana SK. Acquired immunity and epidemiology of Schistosoma haematobium. Nature. 1991;351:757.
World Health Organization. The control of schistosomiasis (Technical Report 728:1). Geneva: World Health Organization; 1993.
World Health Organization. Report of the WHO Informal Consultation on Schistosomiasis Control. Geneva: World Health Organization; 1998.
World Health Organization Expert Committee. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. WHO Tech Rep Ser. 2002;912:1.
Young D, Beland JE, Kloos H, et al. Schistosomiasis in an American medical investigator. J Clin Gastroenterol. 1986;8:589.
Ah HS, Klei TR, McCall JW, Thompson PE. Brugia pahangi infections in Mongolian jirds and dogs following the ocular inoculation of infective larvae. J Parasitol. 1974;60:643.
Ahrens EHJr. Clinical research on patients with chyluria. Jpn J Trop Med. 1970;11:53.
Amaral F, Dreyer G, Figuereido-Silva J, et al. Live adult worms detected by ultrasonography in human bancroftian filariasis. Am J Trop Med Hyg. 1994;50:753.
Bazi T, Finan R, Zourob D, et al. Filariasis infection is a probable cause of implantation failure in in-vitro fertilization cycles. Fertil Steril. 2006;85:1822. e13–15
Bookaire MJ, Tisch DJ, Kastens W, Alexander WD. Mass treatment to eliminate filariasis in Papua New Guinea. N Engl J Med. 2002;347:1841.
Chang LW, Reller ME, Bishop JA, et al. A 41-year-old woman from Cameroon with infertility. Clin Infect Dis. 2008;47:141-143.
Chaubal NG, Pradhan GM, Chaubal JN, Ramani SK. Dance of live adult filarial worms is a reliable sign of scrotal filarial infection. J Ultrasound Med. 2003;22:765.
Connor DH, Morrison NE, Kerdel-Vegas F, et al. Onchocerciasis: onchocerciasis dermatitis, lymphadenitis, and elephantiasis in the Ubangi territory. Hum Pathol. 1970;1:553.
Coulibaly YI, Nutman TB, Klion AD, et al. A randomized trial of doxycycline for Mansonella perstans infection. N Engl J Med. 2009;361:1448-1458.
Danaraj TJ, Pacheco W, Shanmugaratnam K, Beaver PC. The etiology and pathology of eosinophilic lung (tropical eosinophilia). Am J Trop Med Hyg. 1966;19:181.
Davis BR. Filariasis. Dermatol Clin. 1989;7:313.
Dreyer G, Noroes J, Figueredo-Silva J, Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol Today. 2000;16:544.
Dull HB, Meredith SE. The Mectizan Donation Progamme—a 10-year report. Ann Trop Med Parasitol. 1998;92(Suppl. 1):S69.
Fan PC, Peng HW, Chen CC. Follow-up investigations on clinical manifestations after filariasis eradication by diethylcarbamazine medicated common salt on Kinmen (Quemoy) Islands, Republic of China. J Trop Med Hyg. 1995;98:461.
Galindo L, von Lichtenberg F, Baldizon C. Bancroftian filariasis in Puerto Rico: infection pattern and tissue lesions. Am J Trop Med Hyg. 1962;11:739.
Gandhi GM. Role of lymphangiography in management of filarial chyluria. Lymphology. 1976;9:11.
Gardon J, Boussinesq M, Kamgno J, et al. Effects of standard and high doses of ivermectin on adult worms of Onchocerca volvulus: a randomized controlled trial. Lancet. 2002;360:203.
Greene BM, Taylor HR, Cupp EW, et al. Comparison of ivermectin and diethylcarbamazine in the treatment of onchocerciasis. N Engl J Med. 1985;313:133-138.
Harnett W, Bradley JE, Garate T. Molecular and immunodiagnosis of human filarial nematode infections. Parasitology. 1998;117(Suppl.):S59.
Hoerauf A, Mand S, Volkmann L, et al. Doxycycline in the treatment of human onchocerciasis: kinetics of Wolbachia endobacteria reduction and of inhibition of embryogenesis in female Onchocerca worms. Microbes Infect. 2003;5:261.
Jachowski LA, Gonzalez-Flores B, von Lichtenberg F. Filarial etiology of tropical hydroceles in Puerto Rico. Am J Trop Med Hyg. 1962;11:220.
Kanetkar AV, Deshmukh SM, Pradham RS, et al. Lymphangiographic patterns in filarial edema of lower limbs. Clin Radiol. 1966;17:258.
Kazura J, Bockarie M, Alexander N, et al. Risk factors for acute morbidity in bancroftian filariasis. Am J Trop Med Hyg. 1995;53:100.
Kehinde EO, Anin JT, Hira PR. Parasites of urological importance. Urol Int. 2008;81:1-13.
King CL, Hakimi J, Shata MT, Medhat A. IL-12 regulation of parasite antigen-driven IgE production in human helminth infections. J Immunol. 1995;155:454.
Maher D, Ottesen EA. The global lymphatic filariasis initiative. Trop Doct. 2000;30(3):178.
Mand S, Pfarr K, Sahoo PK, et al. Macrofilaricidal activity and amelioration of llymphatic pathology in bancroftian filariasis after 3 weeks of doxycycline followed by single-dose diethylcarbamazine. Am J Trop Med Hyg. 2009;81:702-711.
McCarthy JS, Zhong M, Gopinath R, et al. Evaluation of a polymerase chain reaction-based assay for diagnosis of Wuchereria bancrofti infection. J Infect Dis. 1996;173:1510-1514.
The Medical Letter. Drugs for parasitic infections. Med Lett Treat Guide. 2010;8:e1-13.
Mishra K, Raj DK, Dash AP, Hazra RK. Acta Tropica. 2005;93:233-237.
O’Connor FW, Hulse CR. Studies in filariasis: I. In Puerto Rico. Puerto Rico J Public Health Trop Med. 1935;11:167.
Olszewski WL, Jamal S, Manokaran G, et al. Bacteriologic studies of skin, tissue fluid, lymph and lymph nodes in patients with filarial lymphedema. Am J Trop Med Hyg. 1997;57:7.
Ong RK, Doyle RL. Tropical pulmonary eosinophilia. Chest. 1998;113:1673.
Ottesen EA. Filariasis now. Am J Trop Med Hyg. 1989;41(Suppl. 3):9.
Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491.
Ottesen EA, Ismail MM, Horton J. The role of albendazole in programmes to eliminate lymphatic filariasis. Parasitol Today. 1999;15:382.
Piessens WF. Immunology of lymphatic filariasis and onchocerciasis. In: Cohen S, Warren KS, editors. Immunology of parasitic disease. 2nd ed. Oxford: Blackwell Scientific Publications; 1982:622-653.
Pischke S, Buttner DW, Liebau E, Fischer P. An internal control for the detection of Onchocerca volvulus DNA by PCR-ELISA and rapid detection of specific PCR products by DNA detection test strips. Trop Med Int Health. 2002;7:526.
Ramaiah KD, Das PK, Michael E, Guyatt H. The economic burden of lymphatic filariasis in India. Parasitol Today. 2000;16:251.
Shenoy RK, Dalia S, John A, et al. Treatment of the microfilaraemia of asymptomatic brugian filariasis with single doses of ivermectin, diethylcarbamazine or albendazole, in various combinations. Ann Trop Med Parasitol. 1999;93:643.
Steel C, Guinea A, Ottesen EA. Evidence for protective immunity to bancroftian filariasis in the Cook Islands. J Infect Dis. 1996;174:598.
Sullivan JJ, Chernin E. Oral transmission of Brugia pahangi and Dipetalonema viteae to adult and neonatal jirds. Int J Parasitol. 1976;6:75.
Taylor MJ, Hoerauf A. Wolbachia bacteria of filarial nematodes. Parasitol Today. 1999;15:437-442.
Vincent JA, Lustigman S, Zhang S, Weil GJ. A comparison of newer tests for the diagnosis of onchocerciasis. Ann Trop Med Parasitol. 2000;94:253.
Weil GJ, Hussain R, Kumaraswami V, et al. Prenatal allergic sensitization to helminth antigens in offspring of parasite-infected mothers. J Clin Invest. 1983;71:1124.
Other Parasitic Diseases of the Genitourinary Tract
Brandt H, Perez Tamayo R. Pathology of human amoebiasis. Hum Pathol. 1970;1:351.
Cotch MF, Pastorek JG, Nugent RP, et al. Trichomonas vaginalis with low birth weight and preterm delivery. Sex Transm Dis. 1997;24:353-360.
D’Alessandro RL. Echinococcus vogeli in man with a review of polycystic disease in Columbia and neighboring countries. Am J Trop Med Hyg. 1979;28:303.
Gogus C, Safak M, Baltaci S, Turkolmez K. Isolated renal hydatidosis: experience with 20 cases. J Urol. 2003;169:186.
Grigsby WP. Surgical treatment of amoebiasis. Surg Gynecol Obstet. 1969;128:609.
Handa R, Harjai MM. Hydatid cyst of the renal pelvis. Pediatr Surg Int. 2005;21:410.
Horchani A, Nouira Y, Chtourou M, et al. Retrovesical hydatid disease: a clinical study of 27 cases. Eur Urol. 2001;40:655.
Kaydos SC, Swygard H, Wise SL, et al. Development and validation of a PCR-based enzyme-linked immunosorbent assay with urine for use in clinical research settings to detect Trichomonas vaginalis in women. J Clin Microbiol. 2002;40:89-95.
Kehinde EO, Anin JT, Hira PR. Parasites of urological importance. Urol Int. 2008;81:1-13.
Kraivichian P, Kulkumthorn M, Yingyourd P, et al. Albendazole for the treatment of human gnathostomiasis. Trans R Soc Trop Med Hyg. 1992;86:418-421.
McCarthy J, Moore TA, et al. Emerging helminth zoonoses. Intl J Parasitol. 2000;30:1351-1360.
The Medical Letter. Drugs for parasitic infections. Med Lett Treat Guide. 2010;8:e1-13.
Musacchio F, Mitchell N. Primary renal echinococcosis: a case report. Am J Trop Med Hyg. 1966;15:168.
Rausch RL. On the occurrence and distribution of Echinococcus sp. (Cestoda, Taeniidae) and characteristics of their development in the intermediate host. Am J Parasitol. 1967;42:19.
Ravdin JI, Stauffer WM. Entamoeba histolytica (amebiasis). In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:3097-3111.
Rifaat MA, Ghanam NS, el-Kaneshy MH, et al. Letter: a case of genito-urinary strongyloidiasis. Trans R Soc Trop Med Hyg. 1973;67:722-723.
Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
Sena AC, Miller WC, Hobbs MM, et al. Trichomonas vaginalis infection in male sexual partners: implications for diagnosis, treatment, and prevention. Clin Infect Dis. 2007;44:13-22.
Siddiqui AA, Genta RM, Berk SL. Strongyloidiasis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases, principals, pathogens, and practice. 2nd ed. Philadelphia: Elsevier Churchill Livingstone; 2006:1274-1285.
Turgut AT, Ödev K, Kabaalioglu A, et al. Multitechnique evaluation of renal hydatid disease. Am J Roentgenol. 2009;192:462-467.