chapter 19 Regenerative Medicine in Urology
Stem Cells, Tissue Engineering, and Cloning
“So the Lord God caused the man to fall into a deep sleep, and while he was asleep, He took part of the man’s rib, and closed up the place with flesh. Then the Lord God made a woman from the part He had taken out of the man, and He brought her to the man.”
Four medical firsts are described in the passage cited above: the use of anesthesia, surgery, cloning, and tissue engineering. Even three decades ago, the performance of two of these, tissue engineering and cloning, was not possible. The use of one body part for another or the exchange of parts from one person to another was mentioned in the medical literature even in antiquity and captured the imagination of many over time. The maintenance of organs in culture was a major area of inquiry in the early 1900s. Charles Lindbergh, the first pilot to successfully fly across the Atlantic in the 1920s, joined forces with Alexis Carrel, a Nobel Prize winner in the field of medicine, to investigate the potential of keeping organs alive ex vivo long-term (Carrel et al, 1935).
The field of urology was the earliest to gain from the advent of transplantation, with the kidney being the first entire organ to be replaced in a human, in 1955 (Guild et al, 1955). In the early 1960s, Joseph Murray, who later received the Nobel Prize for his work, performed a nonrelated kidney transplantation from a nongenetically identical patient into another. This transplant, which overcame the immunologic barrier, marked a new era in medical therapy and opened the door for use of transplantation as a means of therapy for different organ systems. However, lack of good immune suppression and the ability to monitor and control rejection, as well as a severe organ donor shortage, opened the door for other alternatives.
As times evolved, synthetic materials were introduced to replace or rebuild diseased tissues or parts in the human body. The advent of new manmade materials, such as tetrafluoroethylene (Teflon) and silicone, opened a new field that included a wide array of devices that could be applied for human use. Although these devices could provide for structural replacement, the functional component of the original tissue was not achieved.
Simultaneous with this development was an increased body of knowledge of the biologic sciences that included new techniques for cell harvesting, culture, and expansion. The areas of cell biology, molecular biology, and biochemistry were advancing rapidly. Studies of the extracellular matrix and its interaction with cells, and with growth factors and their ligands, led the way to a further understanding of cell and tissue growth and differentiation. The concept of cell transplantation took hold in the research arena, and culminated with the first human bone marrow cell transplant in the 1970s.
In the 1970s, a natural evolution occurred wherein researchers started to combine the field of devices and materials sciences with cell biology, in effect starting a new field called tissue engineering. As more scientists from different fields came together with the common goal of tissue replacement, the field of tissue engineering became more formally established. Tissue engineering was defined as “an interdisciplinary field which applies the principles of engineering and life sciences towards the development of biological substitutes that aim to maintain, restore or improve tissue function” (Atala et al, 2001). The first use of the term “tissue engineering” in the literature can be traced to a reference dealing with corneal tissue in 1985 (Wolter et al, 1985).
The field of stem cells also received a large boost with the discovery of mouse embryonic stem cells in the early 1980s (Martin, 1981). However, the field remained relatively dormant until the description of human embryonic stem cells in 1998 (Thomson et al, 1998). The description of these cells led to one of the most contested ongoing ethical debates in the field of medicine. Just a year later, in 1999, the world awoke to the startling media announcement of the creation of the first cloned mammal, a sheep named Dolly (Wilmut et al, 1997). Although cloning, or nuclear transfer, had been done in amphibians and other animal models for years, this accomplishment in a mammal showed once again that concepts believed to be scientifically prohibitive were indeed possible.
The fields of cell transplantation, tissue engineering, and nuclear transfer all had one unifying concept—the regeneration of living tissues and organs. Thus in 1999, William Haseltine, then the scientific founder and Chief Executive Officer of Human Genome Sciences, coined the term “regenerative medicine,” in effect bringing all these areas under one defining field (Haseltine, 1999). Soon, the first online journal for the new field of tissue engineering, Regenerative Medicine, was formed, along with The Regenerative Medicine Society.
Organ transplantation remains a mainstay of treatment for patients with severely compromised organ function. Despite initiatives to increase the availability of transplant organs, however, the number of patients in need of treatment far exceeds the organ supply, and this shortfall is expected to worsen as the global population ages. One alternative treatment approach is regenerative medicine. In the last two decades, scientists have attempted to grow native and stem cells, engineer tissues, and design treatment modalities using regenerative medicine techniques for virtually every tissue of the human body. This chapter reviews some of the progress that has been achieved in the field of genitourinary regenerative medicine.
Regenerative Medicine: Strategies for Tissue and Organ Reconstitution
Regenerative medicine follows the principles of cell transplantation, materials science, and engineering toward the development of biologic strategies that can restore and maintain normal function. Regenerative medicine strategies usually fall into one of three categories: cell-based therapies, the use of biomaterials (scaffolds) alone, wherein the body’s natural ability to regenerate is used to orient or direct new tissue growth, or the use of scaffolds seeded with cells to create tissue substitutes.
Sources of Cells for Therapy
Stem Cells
The cells used for regenerative medicine can be autologous or heterologous, and from either native or stem cell sources. In general, there are three broad categories of stem cells obtained from living tissue that are used for cell therapies. Embryonic stem cells are obtained through the aspiration of the inner cell mass of a blastocyst or, more recently, a single cell from this mass. Fetal and neonatal amniotic fluid and placenta may contain multipotent cells that may be useful in cell therapy applications. Adult stem cells, on the other hand, are usually isolated from organ or bone marrow biopsies. Stem cells are defined as having three important properties: the ability to self-renew, the ability to differentiate into a number of different cell types, and the ability to easily form clonal populations (populations of cells derived from a single stem cell). Many techniques for generating stem cells have been studied over the past few decades. Some of these techniques have yielded promising results, but others require further research. The main techniques are discussed in detail below, and their advantages and limitations are summarized in Table 19–1.
Table 19–1 Summary of Alternate Methods for Generating Pluripotent Stem Cells
| METHOD | ADVANTAGES | LIMITATIONS |
|---|---|---|
| Somatic cell nuclear transfer | Customized stem cells Has been shown to work in nonhuman primates |
Requires oocytes Has not been shown to work in humans |
| Single cell embryo biopsy | Patient-specific to embryo Does not destroy or create embryos Has been done in humans |
Allogeneic cell types Is not known if single cells are totipotent Requires coculturing with a previously established human embryonic stem cell line |
| Arrested embryos | Cells obtained from discarded embryos Has been done in humans |
Allogeneic cell types Quality of cell lines might be questionable |
| Altered nuclear transfer | Customized stem cells | Ethical issues surround embryos with no potential Modified genome Has not been done with human cells |
| Reprogramming | Customized stem cells No embryos or oocytes needed Has been done with human cells |
Retroviral transduction Oncogenes |
Embryonic Stem Cells
In 1981 pluripotent cells were found in the inner cell mass of the human embryo, and the term “human embryonic stem cell” was coined (Martin, 1981). These cells are able to differentiate into all cells of the human body, excluding placental cells (only cells from the morula are totipotent; that is, able to develop into all cells of the human body). These cells have great therapeutic potential, but their use is limited by both biological and ethical factors.
The political controversy surrounding stem cells began in 1998 with the creation of human embryonic stem (hES) cells derived from discarded embryos. hES were isolated from the inner cell mass of a blastocyst (an embryo 5 days postfertilization) using an immunosurgical technique. Given that some cells cannot be expanded ex vivo, ES cells could be an ideal resource for regenerative medicine because of their fundamental properties: the ability to self-renew indefinitely and the ability to differentiate into cells from all three embryonic germ layers. Skin and neurons have been formed, indicating ectodermal differentiation (Reubinoff et al, 2001; Schuldiner et al, 2001; Zhang et al, 2001). Blood, cardiac cells, cartilage, endothelial cells, and muscle have been formed, indicating mesodermal differentiation (Kaufman et al, 2001; Kehat et al, 2001; Levenberg et al, 2002). Pancreatic cells have been formed, indicating endodermal differentiation (Assady et al, 2001). In addition, as further evidence of their pluripotency, embryonic stem cells can form embryoid bodies, which are cell aggregations that contain all three embryonic germ layers while in culture, and can form teratomas in vivo (Itskovitz-Eldor et al, 2000). These cells have demonstrated longevity in culture and can maintain their undifferentiated state for at least 80 passages when grown using current published protocols (Reubinoff et al, 2000; Thomson et al, 1998).
However, in addition to the ethical dilemma surrounding the use of embryonic stem cells, their clinical application is limited because they represent an allogenic resource and thus have the potential to evoke an immune response. New stem cell technologies (such as somatic cell nuclear transfer and reprogramming) promise to overcome this limitation.
Single Cell Embryo Biopsy
Because a major objection to hES cell research for some persons is that it results in the destruction of embryos, a method of isolating these cells without destroying the embryo would be advantageous. In 2006, Chung and colleagues were the first authors to report the generation of mouse embryonic stem cell lines in this manner. Their method was based on a technique used to obtain a single cell embryo biopsy for preimplantation genetic diagnosis. Cells were taken from eight-cell blastomeres rather than from blastocysts. The cells differentiated into derivatives of all three embryonic germ layers in vitro, and as well as into teratomas in vivo. In addition, the mouse embryos that resulted from the biopsied blastomeres developed to term without a reduction in their developmental potential.
Obtaining Cells from Arrested Embryos
hES cell lines can also be derived from arrested embryos (Zhang et al, 2006a). During in vitro fertilization, only a small proportion of zygotes produced will develop successfully to the morula and blastocyst stages. Over half the embryos stop dividing (Hardy, 1993; Geber et al, 1999) and are, therefore, considered dead embryos (Landry et al, 2004). Such embryos have unequal or fragmented cells and blastomeres and are usually discarded. Not all the cells within these arrested embryos, however, are abnormal (Martinez et al, 2002; Zhang et al, 2006a), and these embryos might be a source of hES cells. More studies are needed to characterize the full proliferation and differentiation potential of embryonic stem cells derived from arrested embryos.
Altered Nuclear Transfer
Altered nuclear transfer is a variation of somatic cell nuclear transfer (SCNT) in which a genetically modified nucleus from a somatic cell is transferred into a human oocyte. This embryo, which contains a deliberate genetic defect, is capable of developing into a blastocyst, but the induced defect prevents the blastocyst from implanting in the uterus. This process has the potential to generate customized hES cells from the blastocyst stage (Hurlbut, 2005). Human embryos with this genetic defect might lack the capacity to develop into viable fetuses, as a result of their inability to implant, thus providing a source of stem cells without destroying viable embryos. Proof of concept was obtained in mice by Meissner and Jaenisch (2006) by using embryos lacking the CDX2 homeobox gene.
The viability of human embryos lacking the CDX2 gene is unclear, as is whether this mutation restricts human developmental potential into certain lineages. Although much research must be done before therapeutic strategies based on this technique could ever enter the clinic, at this time, hES cells derived from altered nuclear transfer can provide opportunities to study pluripotency in hES cells without the need for destruction of viable embryos. The exact effects of CDX2 gene knockout on the development of human embryos are not well known. The effects of this gene however, have been thoroughly investigated in the gastric and intestinal epithelium (Benahmed et al, 2008; Vauhkonen et al, 2008).
Therapeutic Cloning (Somatic Cell Nuclear Transfer)
Somatic cell nuclear transfer, or therapeutic cloning, entails the removal of an oocyte nucleus in culture, followed by its replacement with a nucleus derived from a somatic cell obtained from a patient. Activation with chemicals or electricity stimulates cell division up to the blastocyst stage.
At this point, it is extremely important to differentiate between the two types of cloning that exist: reproductive cloning and therapeutic cloning. Both involve the insertion of donor deoxyribonucleic acid (DNA) into an enucleated oocyte to generate an embryo that has identical genetic material to its DNA source. However, the similarities end there. In reproductive cloning, the embryo is then implanted into the uterus of a pseudopregnant female to produce an infant that is a clone of the donor. A world-famous example of this type of cloning resulted in the birth of a sheep named Dolly in 1997 (Wilmut et al, 1997). However, there are many ethical concerns surrounding such practices, and as a result, reproductive cloning has been banned in most countries.
Although therapeutic cloning also produces an embryo that is genetically identical to the donor, this process is used to generate blastocysts that are explanted and grown in culture, rather than in utero. Embryonic stem cell lines can then be derived from these blastocysts, which are only allowed to grow to a 100-cell stage. At this time the inner cell mass is isolated and cultured, resulting in ES cells that are genetically identical to the patient. This process is detailed in Fig. 19–1. It has been shown that nuclear-transferred ES cells derived from fibroblasts, lymphocytes, and olfactory neurons are pluripotent and can generate live pups after injection into blastocysts. This shows that cells generated by SCNT have the same developmental potential as blastocysts that are fertilized and produced naturally (Hochedlinger et al, 2002; Eggan et al, 2004; Brambrink et al, 2006). In addition, the ES cells generated by SCNT are perfectly matched to the patient’s immune system, and no immunosuppressants would be required to prevent rejection should these cells be used in regenerative medicine applications.
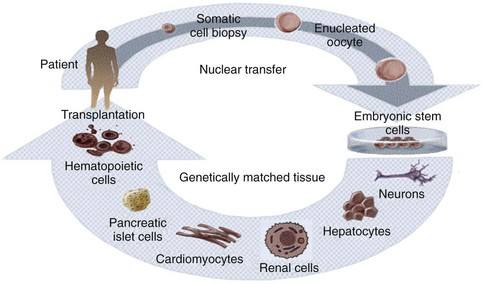
Figure 19–1 Therapeutic cloning strategy and its application to the engineering of tissues and organs.
Although ES cells derived from SCNT contain the nuclear genome of the donor cells, mitochondrial DNA (mtDNA) contained in the oocyte could lead to immunogenicity after transplantation. To assess the histocompatibility of tissue generated using SCNT, Lanza and colleagues (2002b) microinjected the nucleus of a bovine skin fibroblast into an enucleated oocyte. Although the blastocyst was implanted (reproductive cloning), the purpose was to generate renal, cardiac and skeletal muscle cells, which were then harvested, expanded in vitro, and seeded onto biodegradable scaffolds. These scaffolds were then implanted into the donor steer from which the cells were cloned to determine if cells were histocompatible. Analysis revealed that cloned renal cells showed no evidence of T-cell response, suggesting that rejection will not necessarily occur in the presence of oocyte-derived mtDNA. This finding represents a step forward in overcoming the histocompatibility problem of stem cell therapy.
Although promising, SCNT has certain limitations that require further improvement before its clinical application, in addition to the ethical considerations regarding the potential of the resulting embryos to develop into cloned embryos if implanted into a uterus. In addition, to date this technique has not been shown to work in humans. The initial failures and fraudulent reports of nuclear transfer in humans reduced enthusiasm for human applications (Simerly et al, 2003; Hwang et al, 2004; Hwang et al, 2005), although it was recently reported that nonhuman primate ES cell lines were generated by SCNT using nuclei from adult skin fibroblasts (Byrne et al, 2007; Mitalipov, 2007).
Before SCNT-derived ES cells can be used as clinical therapy, careful assessment of quality of the lines must be determined. For example, some cell lines generated by SCNT have contained chromosomal translocations, and it is not known whether these abnormalities originated from aneuploid embryos or if they occurred during ES cell isolation and culture. In addition, the low efficiency of SNCT (0.7%) and the inadequate supply of human oocytes further hinder the therapeutic potential of this technique. Still, these studies renew the hope that ES cell lines could one day be generated from human cells to produce patient-specific stem cells with the potential to cure many human diseases that are currently untreatable.
Reprogramming (Induced Pluripotent Stem Cells)
Recently, reports of the successful transformation of adult cells into pluripotent stem cells through a type of genetic “reprogramming” have been published. Reprogramming is a technique that involves de-differentiation of adult somatic cells to produce patient-specific pluripotent stem cells, eliminating the need to create embryos. Cells generated by reprogramming would be genetically identical to the somatic cells (and thus, the patient who donated these cells) and would not be rejected. Yamanaka’s group was the first to discover that mouse embryonic fibroblasts (MEFs) and adult mouse fibroblasts could be reprogrammed into an “induced pluripotent state” (iPS) (Takahashi and Yamanaka, 2006). These iPS cells possessed the immortal growth characteristics of self-renewing ES cells—expressed genes specific for ES cells, and generated embryoid bodies in vitro and teratomas in vivo. When iPS cells were injected into mouse blastocysts, they contributed to a variety of cell types. However, although iPS cells selected in this way were pluripotent, they were not identical to ES cells. Unlike ES cells, chimeras made from iPS cells did not result in full-term pregnancies. Gene expression profiles of the iPS cells showed that they possessed a distinct gene expression signature that was different from that of ES cells. In addition, the epigenetic state of the iPS cells was somewhere between that found in somatic cells and that found in ES cells, suggesting that the reprogramming was incomplete.
These results were improved significantly by Wernig and colleagues in July 2007 (Wernig et al, 2007). In this study, DNA methylation, gene expression profiles, and the chromatin state of the reprogrammed cells were similar to those of ES cells. Teratomas induced by these cells contained differentiated cell types representing all three embryonic germ layers. Most importantly, the reprogrammed cells from this experiment were able to form viable chimeras and contribute to the germ line like ES cells, suggesting that these iPS cells were completely reprogrammed.
It has recently been shown that reprogramming of human cells is possible (Yu et al, 2007). Yamanaka’s group generated human iPS cells that are similar to hES cells in terms of morphology, proliferation, gene expression, surface markers, and teratoma formation. These were generated via retroviral transduction of the stem cell markers OCT4, SOX2, NANOG, and LIN28 (Takahashi et al, 2007). However, in both studies, the human iPS cells were similar but not identical to hES cells. Although reprogramming is an exciting phenomenon, our limited understanding of the mechanism underlying it currently limits the clinical applicability of the technique, but the future potential of reprogramming is quite exciting.
Amniotic Fluid– and Placenta-Derived Stem Cells
The amniotic fluid and placental membrane contain a heterogeneous population of cell types derived from the developing fetus (Priest et al, 1978; Polgar et al, 1989). Cells found in this heterogeneous population include mesenchymal stem cells (in ’t Anker et al, 2003b; Tsai et al, 2004). In addition, the isolation of multipotent human and mouse amniotic fluid– and placenta-derived stem (AFPS) cells that are capable of extensive self-renewal and give rise to cells from all three germ layers was reported in 2007 (De Coppi et al, 2007a). AFPS cells represent approximately 1% of the cells found in the amniotic fluid and placenta. The undifferentiated stem cells expand extensively without a feeder cell layer and double every 36 hours. Unlike human embryonic stem cells, the AFPS cells do not form tumors in vivo. Lines maintained for over 250 population doublings retained long telomeres and a normal complement of chromosomes. AFPS cell lines can be induced to differentiate into cells representing each embryonic germ layer, including cells of adipogenic, osteogenic, myogenic, endothelial, neural-like and hepatic lineages. In addition to the differentiated AFPS cells expressing lineage-specific markers, such cells can have specialized functions. Cells of the hepatic lineage secreted urea and α-fetoprotein, while osteogenic cells produced mineralized calcium. In this respect, they meet a commonly accepted criterion for multipotent stem cells, without implying that they can generate every adult tissue.
AFS cells represent a new class of stem cells with properties somewhere between those of embryonic and adult stem cell types. They are probably more agile than adult stem cells, but less so than embryonic stem cells. Unlike embryonic and induced pluripotent stem cells, however, AFPS cells do not form teratomas, and if preserved for self-use, avoid the problems of rejection. The cells could be obtained either from amniocentesis or chorionic villous sampling in the developing fetus, or from the placenta at the time of birth. They could be preserved for self-use, and used without rejection, or they could be banked. A bank of 100,000 specimens could potentially supply 99% of the U.S. population with a perfect genetic match for transplantation. Such a bank may be easier to create than with other cell sources, because there are approximately 4.5 million births per year in the United States.
Since the discovery of the AFPS cells, other groups have published on the potential of the cells to differentiate to other lineages, such as cartilage (Kolambkar et al, 2007), kidney (Perin et al, 2007), and lung (Warburton et al, 2008). Muscle differentiated AFPS cells were also noted to prevent compensatory bladder hypertrophy in a cryoinjured rodent bladder model (De Coppi et al, 2007b).
Adult Stem Cells
Adult stem cells, especially hematopoietic stem cells, are the best understood cell type in stem cell biology (Ballas et al, 2002). However, adult stem cell research remains an area of intense study, because their potential for therapy may be applicable to a myriad of degenerative disorders. Within the past decade, adult stem cell populations have been found in many adult tissues other than the bone marrow and the gastrointestinal tract, including the brain (Taupin, 2006), skin (Jensen et al, 2008; Jiao et al, 2008), and muscle (Crisan et al, 2008). Many other types of adult stem cells have been identified in organs all over the body and are thought to serve as the primary repair entities for their corresponding organs (Weiner, 2008) (Fig. 19–2). The discovery of such tissue-specific progenitors has opened up new avenues for research.
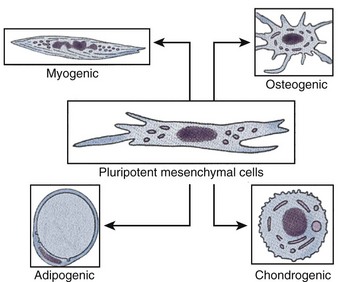
Figure 19–2 Schematic diagram of pluripotential stem cell lineages that can be derived from postnatal tissue.
A notable exception to the tissue specificity of adult stem cells is the mesenchymal stem cell, also known as the multipotent adult progenitor cell. This cell type is derived from bone marrow stroma (Devine, 2002; Jiang et al, 2002). Such cells can differentiate in vitro into numerous tissue types (Caplan, 2007; da Silva Meirelles et al, 2008) and can also differentiate developmentally if injected into a blastocyst. Multipotent adult progenitor cells can develop into a variety of tissues including neuronal (Duan et al, 2007), adipose (Crisan et al, 2008), muscle (Crisan et al, 2008; Luttun et al, 2006), liver (Ikeda et al, 2008; Mimeault et al, 2008), lungs (Nolen-Walston et al, 2008), spleen (in ’t Anker et al, 2003a), and gut tissue (Jiang et al, 2002), but notably not bone marrow or gonads.
Research into adult stem cells has, however, progressed slowly, mainly because investigators have had great difficulty in maintaining adult nonmesenchymal stem cells in culture. Some cells, such as those of the liver, pancreas, and nerve, have very low proliferative capacity in vitro, and the functionality of some cell types is reduced after the cells are cultivated. Isolation of cells has also been problematic, because stem cells are present in extremely low numbers in adult tissue (Hristov et al, 2008; Mimeault et al, 2008). Although the clinical utility of adult stem cells is currently limited, great potential exists for future use of such cells in tissue-specific regenerative therapies. The advantage of adult stem cells is that they can be used in autologous therapies, thus avoiding any complications associated with immune rejection.
Native Targeted Progenitor Cells
In the past, one of the limitations of applying cell-based regenerative medicine techniques to organ replacement was the inherent difficulty of growing certain human cell types in large quantities. Native targeted progenitor cells, or native cells, are tissue specific unipotent cells derived from most organs. The advantage of these cells is that they are already programmed to become the cell type needed, without any extralineage differentiation. By noting the location of the progenitor cells, as well as by exploring the conditions that promote differentiation and/or self-renewal, it has been possible to overcome some of the obstacles that limit cell expansion in vitro. One example is the urothelial cell. Urothelial cells could be grown in the laboratory setting in the past, but only with limited success. It was believed that urothelial cells had a natural senescence that was hard to overcome. Several protocols have been developed over the last two decades that have improved urothelial growth and expansion (Cilento et al, 1994; Liebert et al, 1997; Scriven et al, 1997; Puthenveettil et al, 1999). A system of urothelial cell harvesting was developed that does not use any enzymes or serum and has a large expansion potential. Using these methods of cell culture, it is possible to expand an urothelial strain from a single specimen that initially covers a surface area of 1 cm2 to one covering a surface area of 4202 m2 (the equivalent area of one football field) within 8 weeks (Cilento et al, 1994).
An additional advantage in using native cells is that they can be obtained from the specific organ to be regenerated, expanded, and used in the same patient without rejection, in an autologous manner (Atala et al, 1992b, 1993b, 1993c, 1994, 1995b, 1997, 1998; Cilento et al, 1994; Yoo et al, 1997; Fauza et al, 1998a, 1998b; Machluf et al, 1998; Yoo et al, 1998b; Amiel et al, 1999; Kershen et al, 1999; Oberpenning et al, 1999; Park et al, 1999).
Bladder, ureter, and renal pelvis cells can equally be harvested, cultured, and expanded in a similar fashion (Fig. 19–3). Normal human bladder epithelial and muscle cells can be efficiently harvested from surgical material, extensively expanded in culture, and their differentiation characteristics, growth requirements, and other biologic properties can be studied (Liebert et al, 1991; Cilento et al, 1994; Tobin et al, 1994; Harriss, 1995; Freeman et al, 1997; Liebert et al, 1997; Fauza et al, 1998a, 1998b; Lobban et al, 1998; Solomon et al, 1998; Nguyen et al, 1999; Puthenveettil et al, 1999; Rackley et al, 1999). Major advances in cell culture techniques have been made within the past decade, and these techniques make the use of autologous cells possible for clinical application. However, even now, not all human cells can be grown or expanded in vitro. Liver, nerve, and pancreas are examples of human tissues where the technology is not yet advanced to the point where these cells can be grown and expanded.
When cells are used for tissue reconstitution, donor tissue is dissociated into individual cells that are either implanted directly into the host or expanded in culture, then attached to a support matrix or reimplanted after expansion. The implanted tissue can be heterologous, allogeneic, or autologous. Ideally, this approach allows lost tissue function to be restored or replaced in toto, and with limited complications (Atala, 1997). Native cells and tissues are usually preferable for reconstruction. In most cases, the replacement of lost or deficient tissues with functionally equivalent cells and tissues would improve the outcome for these patients. This goal may be attainable with the use of regenerative medicine techniques.
Biomaterials for Genitourinary Regenerative Medicine
Synthetic materials have been used widely for urologic reconstruction. The most common type of synthetic prostheses for urologic applications is made of silicone. Silicone prostheses have been used for the treatment of urinary incontinence with the artificial urinary sphincter and detachable balloon system, for treatment of vesicoureteral reflux with silicone microparticles, and for impotence with penile prostheses (Atala et al, 1992a; Riehmann et al, 1993; Levesque et al, 1996; Buckley et al, 1997). There has also been a major effort directed toward the construction of artificial bladders made with silicone. In some disease states, such as urinary incontinence or vesicoureteral reflux, artificial agents (Teflon paste, glass microparticles) have been used as injectable bulking substances; however, these substances are not entirely biocompatible (Atala, 1994).
For regenerative medicine purposes, there are clear advantages in using degradable, biocompatible materials that can function as cell delivery vehicles, and/or provide the structural parameters needed for tissue replacement. Biomaterials in genitourinary regenerative medicine function as an artificial extracellular matrix (ECM) and elicit biologic and mechanical functions of native ECM found in tissues in the body. Native ECM brings cells together into tissue, controls the tissue structure, and regulates the cell phenotype (Alberts et al, 1994). Biomaterials facilitate the localization and delivery of cells and/or bioactive factors (e.g., cell adhesion peptides, growth factors) to desired sites in the body; define a three-dimensional space for the formation of new tissues with appropriate structure; and guide the development of new tissues with appropriate function (Kim et al, 1998). Direct injection of cell suspensions without biomaterial matrices has been used in some cases (Ponder et al, 1991; Brittberg et al, 1994), but it is difficult to control the localization of transplanted cells. In addition, the majority of mammalian cell types is anchorage-dependent and will die if not provided with a cell-adhesion substrate.
Design and Selection of Biomaterials
The design and selection of the biomaterial is critical in the development of engineered genitourinary tissues. The biomaterial must be capable of controlling the structure and function of the engineered tissue in a predesigned manner by interacting with transplanted cells and/or host cells. Generally, the ideal biomaterial should be biocompatible, promote cellular interaction and tissue development, and possess proper mechanical and physical properties.
The selected biomaterial should be biodegradable and bioresorbable to support the reconstruction of a completely normal tissue without inflammation. Such behavior of the biomaterials avoids the risk of inflammatory or foreign-body responses that may be associated with the permanent presence of a foreign material in the body. The degradation rate and the concentration of degradation products in the tissues surrounding the implant must be at a tolerable level (Bergsma et al, 1995).
The biomaterials should provide an appropriate regulation of cell behavior (e.g., adhesion, proliferation, migration, differentiation) to promote the development of functional new tissue. Cell behavior in engineered tissues is regulated by multiple interactions with the microenvironment, including interactions with cell-adhesion ligands (Hynes, 1992) and with soluble growth factors (Deuel, 1997). Cell adhesion–promoting factors (e.g., arg-gly-asp [RGD]) can be presented by the biomaterial itself or incorporated into the biomaterial to control cell behavior through ligand-induced cell receptor signaling processes (Barrera et al, 1993; Cook et al, 1997). The biomaterials provide temporary mechanical support sufficient to withstand in vivo forces exerted by the surrounding tissue and maintain a potential space for tissue development. The mechanical support of the biomaterials should be maintained until the engineered tissue has sufficient mechanical integrity to support itself (Atala, 2007). This potentially can be achieved by an appropriate choice of mechanical and degradative properties of the biomaterials (Kim et al, 1998).
The biomaterials need to be processed into specific configurations. A large ratio of surface area to volume is often desirable to allow the delivery of a high density of cells. A high-porosity, interconnected pore structure with specific pore sizes promotes tissue ingrowth from the surrounding host tissue. Several techniques, such as electrospinning, have been developed that readily control porosity, pore size, and pore structure (Lee et al, 2007; Yoo et al, 2007; Choi et al, 2008; Lee et al, 2008a, 2008b, 2008c).
Types of Biomaterials
Generally, three classes of biomaterials have been used for engineering of genitourinary tissues: naturally derived materials, such as collagen and alginate; acellular tissue matrices, such as bladder submucosa and small-intestinal submucosa; and synthetic polymers, such as polyglycolic acid (PGA), polylactic acid (PLA), and poly(lactic-co-glycolic acid) (PLGA). These classes of biomaterials have been tested in regard to their biocompatibility with primary human urothelial and bladder muscle cells (Pariente et al, 2001). Naturally derived materials and acellular tissue matrices have the potential advantage of biologic recognition. Synthetic polymers can be produced reproducibly on a large scale with controlled properties of strength, degradation rate, and microstructure.
Collagen is the most abundant and ubiquitous structural protein in the body, and it may be readily purified from both animal and human tissues with an enzyme treatment and salt/acid extraction (Li, 1995). Collagen has long been known to exhibit minimal inflammatory and antigenic responses (Furthmayr et al, 1976), and it has been approved by the U.S. Food and Drug Administration (FDA) for many types of medical applications, including wound dressings and artificial skin (Cen et al, 2008). Intermolecular cross-linking reduces the degradation rate by making the collagen molecules less susceptible to an enzymatic attack. Intermolecular cross-linking can be accomplished by various physical (e.g., ultraviolet radiation, dehydrothermal treatment) or chemical (e.g., glutaraldehyde, formaldehyde, carbodiimides) techniques (Li, 1995). Collagen contains cell-adhesion domain sequences (e.g., RGD) that exhibit specific cellular interactions. This may help to retain the phenotype and activity of many types of cells, including fibroblasts (Silver et al, 1992) and chondrocytes (Sams et al, 1995). This material can be processed into a wide variety of structures such as sponges (Fig. 19–4, top) fibers, and films (Yannas et al, 1980a, 1980b; Cavallaro et al, 1994).
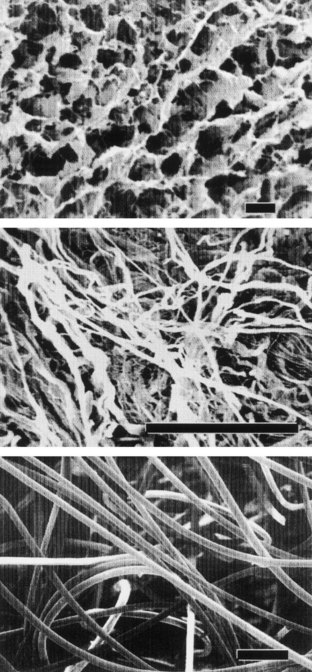
Figure 19–4 Scanning electron micrographs of biomaterials: collagen sponge (top), acellular matrix prepared from pig bladder submucosa (center), and polyglycolic acid fiber-based matrix (bottom). (Size bars = 100 µm.)
Alginate, a polysaccharide isolated from seaweed, has been used as an injectable cell delivery vehicle (Smidsrod et al, 1990) and a cell immobilization matrix (Lim and Sun, 1980) owing to its gentle gelling properties in the presence of divalent ions such as calcium. Alginate is a family of copolymers of D-mannuronate and L-guluronate. The physical and mechanical properties of alginate gel are strongly correlated with the proportion and length of the polyguluronate block in the alginate chains (Smidsrod et al, 1990). Efforts have been made to synthesize biodegradable alginate hydrogels with mechanical properties that are controllable in a wide range by intermolecular covalent cross-linking and with cell-adhesion peptides coupled to their backbones (Rowley et al, 1999).
Recently, natural materials such as alginate and collagen have been used as “bio-inks” in a newly developed bioprinting technique based on inkjet technology (Boland et al, 2006; Campbell et al, 2007). Using this technology, these scaffold materials can be “printed” into a desired scaffold shape using a modified inkjet printer. In addition, several groups have shown that living cells can also be printed using this technology (Laflamme et al, 2005; Nakamura et al, 2005). This exciting technique can be modified so that a three-dimensional construct containing a precise arrangement of cells, growth factors, and extracellular matrix material can be printed (Roth et al, 2004; Ilkhanizadeh et al, 2007; Xu et al, 2009). Such constructs may eventually be implanted into a host to serve as the backbone for a new tissue or organ.
Acellular tissue matrices are collagen-rich matrices prepared by removing cellular components from tissues (Fig. 19–4, center). The matrices are often prepared by mechanical and chemical manipulation of a segment of bladder tissue (Dahms et al, 1998; Piechota et al, 1998; Yoo et al, 1998b; Chen et al, 1999). The matrices slowly degrade after implantation and are replaced and remodeled by ECM proteins synthesized and secreted by transplanted or ingrowing cells. Acellular tissue matrices have been proved to support cell ingrowth and regeneration of genitourinary tissues, including urethra and bladder, with no evidence of immunogenic rejection (Probst et al, 1997; Chen et al, 1999). Because the structures of the proteins (e.g., collagen, elastin) in acellular matrices are well conserved and normally arranged, the mechanical properties of the acellular matrices are not significantly different from those of native bladder submucosa (Dahms et al, 1998).
Polyesters of naturally occurring α-hydroxy acids, including PGA, PLA, and PLGA, are widely used in regenerative medicine. These polymers have gained FDA approval for human use in a variety of applications, including sutures (Gilding, 1981). The degradation products of PGA, PLA, and PLGA are nontoxic, natural metabolites that are eventually eliminated from the body in the form of carbon dioxide and water (Gilding, 1981). Because these polymers are thermoplastics, they can easily be formed into a three-dimensional scaffold with a desired microstructure, gross shape, and dimension by various techniques, including molding, extrusion (Freed et al, 1994), solvent casting (Mikos et al, 1994), phase separation techniques, and gas foaming techniques (Harris et al, 1998). More recently, techniques such as electrospinning have been used to quickly create highly porous scaffolds in various conformations (Han et al, 2006; Choi et al, 2008; Lee et al, 2008a; Lee et al, 2008b).
Many applications in genitourinary regenerative medicine require a scaffold with high porosity and a high ratio of surface area to volume. This need has been addressed by processing biomaterials into configurations of fiber meshes (Fig. 19–4, bottom) and porous sponges using the techniques described previously. A drawback of the synthetic polymers is lack of biologic recognition. As an approach toward incorporating cell recognition domains into these materials, copolymers with amino acids have been synthesized (Barrera et al, 1993; Intveld et al, 1994; Cook et al, 1997). Other biodegradable synthetic polymers, including polyanhydrides and polyortho-esters), can also be used to fabricate scaffolds for genitourinary regenerative medicine with controlled properties (Peppas et al, 1994).
Nanotechnology, the ability to use small molecules that have distinct properties in a small scale, has been used to create “smart” biomaterials for regenerative medicine (Boccaccini et al, 2005; Harrison et al, 2007). Nanoscaffolds have been manufactured specifically for bladder applications (Harrington et al, 2006). The manufacturing of biomaterials can also lead to enhanced cell alignment and tissue formation (Choi et al, 2008).
Vascularization
The goals in regenerative medicine include the replacement of damaged, injured or missing body tissues with biological compatible substitutes. A limiting factor for the engineering of tissues is that cells cannot be implanted in volumes exceeding 3 mm3 (Folkman et al, 1973). Nutrition and gas exchange is limited by this maximal diffusion distance. If cells were implanted in volumes exceeding 3 mm3, only the cells on the surface would survive, and the central cell core would undergo necrosis resulting from a lack of vascularity. Therefore a critical obstacle in regenerative medicine is the ability to maintain large masses of cells alive upon transfer from the in vitro culture conditions into the host (in vivo) (Mooney et al, 1999). To achieve the goals of engineering large complex tissues, and possibly internal organs, vascularization of the regenerating cells is essential.
Formation of new blood vessels and capillaries comprises two different processes: vasculogenesis, the in-situ assembly of capillaries from undifferentiated endothelial cells (EC), and angiogenesis, the sprouting of capillaries from preexisting blood vessels. The formation of the first capillaries takes place mostly during the early stages of embryogenesis (Folkman et al, 1996; Yancopoulos et al, 1998).
Vasculogenesis can be divided into five consecutive steps: (1) EC are generated from precursor cells, called angioblasts, in the bone marrow; (2) EC form the vessel primordia and aggregates that establish cell-to-cell contact but have no lumen; (3) a nascent endothelial tube is formed, composed of polarized EC; (4) a primary vascular network is formed from an array of nascent endothelial tubes; and (5) pericytes and vascular smooth muscle cells are recruited (Drake et al, 1998).
Angiogenesis is a morphogenic process, which describes the formation of new blood capillaries from EC of preexisting vessels. There are six basic steps in angiogenesis: (1) vasodilatation of the parental vessel, reducing the contact between adjacent EC, (2) degradation of the basement membrane of a parental vessel by secretion and activation of a wide range of proteolytic enzymes, (3) EC migration and proliferation to form a leading edge of the new capillary, (4) generation of the capillary lumen and formation of a tubelike structure, (5) basement membrane synthesis, and (6) recruitment of pericytes and vascular smooth muscle cells (Folkman et al, 1992).
When converting to the angiogenic stage, EC will capture new properties that enable them to neovascularize the tissue (Hanahan et al, 1996). When the new vessels are in place and the vascular network matures, the neovascular EC resume their quiescent phenotype (Darland et al, 1999). Several growth factors serve as stimuli for EC conversion to the angiogenic phenotype (Battegay, 1995). Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) are two well-characterized and important angiogenic molecules that have a direct effect on EC.
The understanding of the angiogenic process and the isolation of potent and specific angiogenic growth factors has encouraged the use of these factors therapeutically (Loges et al, 2009; Phelps et al, 2009). Efforts have been aimed at incorporating the knowledge acquired in angiogenesis of ischemic tissues into practical approaches to vascularize bioengineered tissues (Stosich et al, 2009). Bioengineered tissues are usually supported by scaffolds of biocompatible matrices made from natural or artificial sources (Hubbell et al, 1991). Successful vascularization is dependent on the porosity of the supporting matrix. A positive correlation between the pore size of poly-L-lactic acid (PLLA) implants and the rate of vascularization has been observed (Mikos et al, 1993).
Three approaches have been used for vascularization of bioengineered tissue: (1) incorporation of angiogenic factors in the bioengineered tissue, (2) seeding EC with other cell types in the bioengineered tissue, and (3) prevascularization of the matrix prior to cell seeding. Angiogenic growth factors may be incorporated into the bioengineered tissue before implantation, to attract host capillaries and to enhance neovascularization of the implanted tissue. Angiogenic growth factors can be embedded in specific biomaterials, and can be controlled for slow release (Eiselt et al, 1998). Cells can also be genetically engineered to secrete high levels of angiogenic proteins (Springer et al, 1998).
Another approach for enhancing angiogenesis employed cultured EC, which are incorporated into the bioengineered tissue prior to implantation. Human penile corpus cavernosum–derived smooth muscle cells and EC were seeded on biodegradable polymer scaffolds, in order to reconstruct penile corporeal tissue in vitro and in vivo (Park et al, 1999). The use of EC improved the formation of the engineered tissue. In another study, the addition of both EC and angiogenic growth factors (VEGF) accelerated the formation of engineered muscle tissue (De Coppi et al, 2005). An alternative direction in vascularization of bioengineered tissue is the prevascularization of the supporting polymer prior to cell seeding. In this manner, the bioengineered tissue will be organized around the vascular network, providing sufficient tissue perfusion (Fontaine et al, 1995).
Despite the successes in bioengineering tissues consisting of thin layers of cells such as skin, a major challenge for regenerative medicine in the future is the production of larger organs with more complex structures like the kidney. Tissues with a large mass of cells will require a vascular network of arteries, veins, and capillaries to deliver nutrients to each cell. The development of efficient methods to vascularize bioengineered tissues is critical for a successful outcome. There are many obstacles to overcome before large entire tissue-engineered solid organs are produced. Recent developments in angiogenesis research may provide important knowledge and essential.
Regenerative Medicine of Urologic Structures
Urethra
Various strategies have been proposed over the years for the regeneration of urethral tissue. Woven meshes of PGA without cells (Bazeed et al, 1983; Olsen et al, 1992) or with cells (Atala et al, 1992b) were used to regenerate urethras in various animal models. Naturally derived collagen-based materials, such as bladder-derived acellular submucosa (Chen et al, 1999), and an acellular urethral submucosa (Sievert et al, 2000), have also been tried experimentally in various animal models for urethral reconstruction.
The bladder submucosa matrix (Chen et al, 1999), proved to be a suitable graft for repair of urethral defects in rabbits. The neourethras demonstrated a normal urothelial luminal lining and organized muscle bundles. These results were confirmed clinically in a series of patients with a history of failed hypospadias reconstruction wherein the urethral defects were repaired with human bladder acellular collagen matrices (Atala et al, 1999). The neourethras were created by anastomosing the matrix in an onlay fashion to the urethral plate. The size of the created neourethra ranged from 5 to 15 cm. After a 3-year follow-up, three of the four patients had a successful outcome in regard to cosmetic appearance and function (Fig. 19–5). One patient who had a 15-cm neourethra created developed a subglanular fistula. The acellular collagen-based matrix eliminated the necessity of performing additional surgical procedures for graft harvesting, and both operative time and the potential morbidity from the harvest procedure were decreased. Similar results were obtained in pediatric and adult patients with primary urethral stricture disease using the same collagen matrices (El-Kassaby et al, 2003). Another study in 30 patients with recurrent stricture disease showed that a healthy urethral bed (two or fewer prior urethral surgeries) was needed for successful urethral reconstruction using the acellular collage-based grafts (El Kassaby et al, 2008). More than 200 pediatric and adult patients with urethral disease have been successfully treated in an onlay manner with a bladder-derived collagen-based matrix. One of its advantages over nongenital tissue grafts used for urethroplasty is that the material is “off the shelf.” This eliminates the necessity of additional surgical procedures for graft harvesting, which may decrease operative time as well as the potential morbidity due to the harvest procedure.
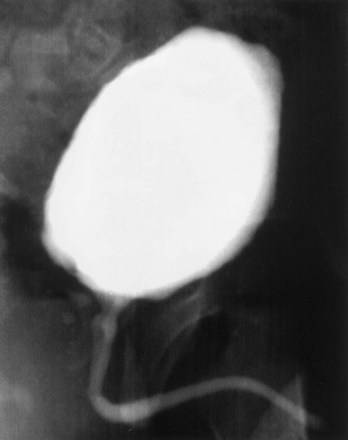
Figure 19–5 Urethrogram 6 months after surgery in a patient who had a portion of his urethra replaced with the use of regenerative medicine techniques.
The above techniques, using nonseeded acellular matrices, were applied experimentally and clinically in a successful manner for onlay urethral repairs. However, when tubularized urethral repairs were attempted experimentally, adequate urethral tissue regeneration was not achieved, and complications ensued, such as graft contracture and stricture formation (De Filippo et al, 2002). Autologous rabbit bladder epithelial and smooth muscle cells were grown and seeded onto preconfigured tubular matrices. Entire urethra segments were resected and urethroplasties were performed with tubularized collagen matrices, either seeded with cells or without cells. The tubularized collagen matrices seeded with autologous cells formed new tissue that was histologically similar to native urethra. The tubularized collagen matrices without cells led to poor tissue development, fibrosis, and stricture formation. These findings were confirmed clinically. A clinical trial using tubularized nonseeded small intestinal submucosa (SIS) for urethral stricture repair was performed with eight evaluable patients. Two patients with short inflammatory strictures maintained urethral patency. Stricture recurrence developed in the other six patients within 3 months of surgery (le Roux, 2005). Other cell types have also been tried experimentally in acellular bladder collagen matrices, including foreskin epidermal cells and oral keratinocytes (Fu et al, 2007; Li et al, 2008). Vascular endothelial growth factor gene–modified urothelial cells have also been used experimentally for urethral reconstruction (Guan et al, 2008).
The normal wound-healing response to injury has been studied extensively, and this knowledge has been helpful in maximizing success for the engineering of tissues. At the time of tissue injury, cell ingrowth is initiated from the wound edges to cover the tissue defect. The cells from the edges of the native tissue are able to traverse short distances without any detrimental effects. If the wound is large, more than a few millimeters in distance or depth, increased collagen deposition, fibrosis, and scar formation ensue. Matrices implanted in wound beds are able to lengthen the distances that cells can traverse without initiating an adverse fibrotic response. However, these distances are also limited. The maximum distance that adjacent cells from the wound edge have to travel to create normal tissue over a biologic matrix is approximately 1 cm (Dorin et al, 2008). Tissue defects greater than 1 cm, which are treated with a matrix alone (without cells), usually have increased collagen deposition, increased fibrosis, and scar formation. Cell-seeded matrices implanted in wound beds are able to further lengthen the distance for normal tissue formation without initiating an adverse fibrotic response. Studies in the field of regenerative medicine have shown that very large defects (>30 cm), can be successfully treated using cell-seeded scaffolds. This explains the described experimental and clinical results noted with urethral repair. Nonseeded matrices are able to replace urethral segments when used in an onlay fashion, because of the short distances required for tissue ingrowth. However, if a tubularized urethral repair is needed, the matrices need to be seeded with autologous cells in order to avoid the risk of stricture formation and poor tissue development.
Bladder
Currently, gastrointestinal segments are commonly used as tissues for bladder replacement or repair. However, gastrointestinal tissues are designed to absorb specific solutes, whereas bladder tissue is designed for the excretion of solutes. When gastrointestinal tissue is in contact with the urinary tract, multiple complications may ensue, such as infection, metabolic disturbances, urolithiasis, perforation, increased mucus production, and malignancy (McDougal, 1992; Atala et al, 1993a; Kaefer et al, 1997, 1998). Because of the problems encountered with the use of gastrointestinal segments, numerous investigators have attempted alternative reconstructive procedures for bladder replacement or repair. These include autoaugmentation (Cartwright et al, 1989a, 1989b) (Fig. 19–6) and ureterocystoplasty (Bellinger, 1993; Churchill et al, 1993; Adams et al, 1998). In addition, alternative methods for bladder reconstruction have been explored, such as the use of tissue expansion and regenerative medicine with cell transplantation.
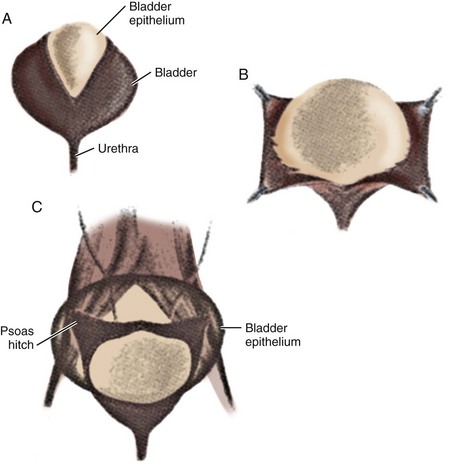
Figure 19–6 Autoaugmentation is performed by incising the detrusor (A) and stripping it from intact bladder epithelium (B), which allows the urothelial layer to bulge with bladder filling (C).
(From Cartwright PC, Snow BW. Bladder autoaugmentation: early clinical experience. J Urol 1989;142:505.)
Tissue Expansion for Bladder Augmentation
A system of progressive dilation for ureters and bladders has been proposed but has not been attempted clinically (Lailas et al, 1996; Satar et al, 1999). In an animal experiment, rabbits underwent unilateral ureteral ligation at the ureterovesical junction. A catheter was threaded into the proximal ipsilateral ureter and connected to an injection port that was secured subcutaneously. A saline-antibiotic solution was injected daily subcutaneously into the injection port. After 1 month of daily saline-antibiotic–solution injections, the ureteral units were dilated at least 10-fold, and the diameter exceeded that of adjacent colon in each instance (Fig. 19–7). Augmentation cystoplasty was performed with the reconfigured dilated ureteral segment, resulting in an increased bladder capacity ranging from 190% to 380% (Lailas et al, 1996). In a similar system, a dilating indwelling catheter was used to dilate ureteral tissue in pigs (Ikeguchi et al, 1998).
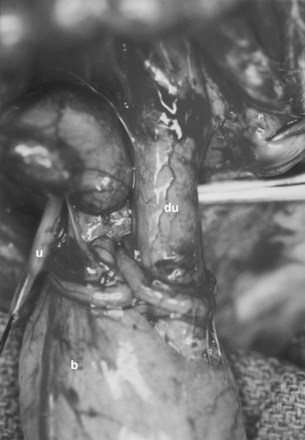
Figure 19–7 Progressive dilation can be performed in a normal-caliber ureter, which can be subsequently used for ureterocystoplasty. After placement of a ureteral dilation device, comparison is made of progressively dilated ureter (du) and native undilated ureter (u) coming off the bladder (b).
A system for the progressive expansion of native bladder tissue has also been used for augmenting bladder volumes (Satar et al, 1999). Beagle dog bladders were divided horizontally into two segments: a superior bladder neoreservoir, and an intact smaller bladder inferiorly with both ureters left intact and draining. A Silastic catheter was threaded into the newly formed, superiorly located neoreservoir and connected to an injection port, which was secured subcutaneously. Four weeks after surgery, a saline-antibiotic solution was injected daily into the palpable injection port, dilating the neoreservoir through the Silastic catheter. Within 30 days after progressive dilation, the neoreservoir volume was expanded at least 10-fold (Fig. 19–8). Urodynamic studies showed normal compliance in all animals, and microscopic examination of the expanded neoreservoir tissue showed a normal histology. A series of immunocytochemical studies demonstrated that the dilated bladder tissue maintained normal phenotypic characteristics (Satar et al, 1999).
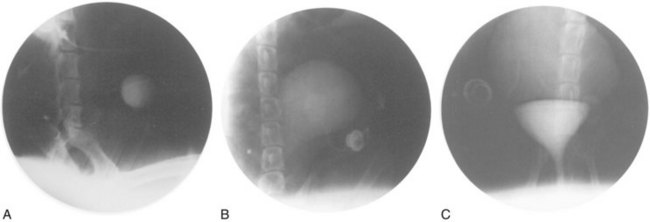
Figure 19–8 Progressive bladder dilation can be performed with adequate increases in capacity. Cystography of bladder neoreservoir before progressive dilation (A) is compared with cystography results after progressive dilation (B) and with cystogram showing dilated neoreservoir and intact bladder segment (C).
Ideally, bladder tissue expansion could be performed with an indwelling dilation catheter, similar to a Foley catheter with a large balloon. In the future, one could foresee placing a dilating catheter intravesically in a patient who requires augmentation, either in an intermittent fashion (e.g., four times daily) or left indwelling. An expanding balloon within the catheter could then be filled progressively, with either continuous or intermittent filling until the desired bladder volume is achieved.
Matrices for Bladder Regeneration
Over the last few decades, several bladder wall substitutes have been attempted with both synthetic and organic materials. Synthetic materials that have been tried in experimental and clinical settings include polyvinyl sponge, Teflon, collagen matrices, Vicryl (PGA) matrices, and silicone. Most of these attempts have failed because of mechanical, structural, functional, or biocompatibility problems. Usually, permanent synthetic materials used for bladder reconstruction succumb to mechanical failure and urinary stone formation, and use of degradable materials leads to fibroblast deposition, scarring, graft contracture, and a reduced reservoir volume over time (Atala, 1995a, 1998).
There has been a resurgence in the use of various collagen-based matrices for tissue regeneration. Nonseeded allogeneic acellular bladder matrices have served as scaffolds for the ingrowth of host bladder wall components. The matrices are prepared by mechanically and chemically removing all cellular components from bladder tissue (Sutherland et al, 1996; Probst et al, 1997; Piechota et al, 1998; Yoo et al, 1998b; Wefer et al, 2001). The matrices serve as vehicles for partial bladder regeneration, and relevant antigenicity is not evident.
Cell-seeded allogeneic acellular bladder matrices were used for bladder augmentation in dogs (Yoo et al, 1998b). The regenerated bladder tissues contained a normal cellular organization consisting of urothelium and smooth muscle and exhibited a normal compliance. Biomaterials preloaded with cells before their implantation showed better tissue regeneration compared with biomaterials implanted with no cells, in which tissue regeneration depended on ingrowth of the surrounding tissue. The bladders showed a significant increase (100%) in capacity when augmented with scaffolds seeded with cells, compared to scaffolds without cells (30%) (Fig. 19–9). The acellular collagen matrices can be enhanced with growth factors to improve bladder regeneration (Kikuno et al, 2008).
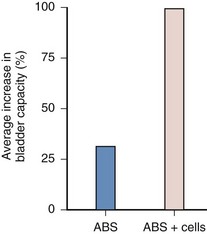
Figure 19–9 Bladders augmented with a collagen matrix derived from bladder submucosa seeded with urothelial and smooth muscle cells (allogenic bladder submucosa [ABS] + cells) showed a 100% increase in capacity compared with bladders augmented with the cell-free ABS, which showed only a 30% increase in capacity within 3 months after implantation.
SIS, a biodegradable, acellular, xenogeneic collagen-based tissue-matrix graft, was first described by Badylak and colleagues in the 1980s as an acellular matrix for tissue replacement in the vascular field (Badylak et al, 1989). It has been shown to promote regeneration of a variety of host tissues, including blood vessels and ligaments (Badylak, 2002). The matrix is derived from pig small intestine in which the mucosa is mechanically removed from the inner surface, and the serosa and muscular layer are removed from the outer surface. Animal studies have shown that the nonseeded SIS matrix used for bladder augmentation is able to regenerate in vivo (Kropp et al, 1996a, 1996b). Histologically, the transitional layer was the same as that of the native bladder tissue, but, as with other nonseeded collagen matrices used experimentally, the muscle layer was not fully developed. A large amount of collagen was interspersed among a smaller number of muscle bundles. A computer-assisted image analysis demonstrated a decreased muscle-to-collagen ratio with loss of the normal architecture in the SIS-regenerated bladders. In-vitro contractility studies performed on the SIS-regenerated dog bladders showed a decrease in maximal contractile response by 50% from those of normal bladder tissues. Expression of muscarinic, purinergic, and α-adrenergic receptors and functional cholinergic and purinergic innervation were demonstrated (Kropp et al, 1996b). Cholinergic and purinergic innervation also occurred in rats (Vaught et al, 1996).
Bladder augmentation using laparoscopic techniques was performed on mini-pigs with porcine bowel acellular tissue matrix, human placental membranes, or porcine SIS. At 12 weeks post-operatively, the grafts had contracted to 70%, 65%, and 60% of their original sizes, respectively, and histologically the grafts showed predominantly only mucosal regeneration (Portis et al, 2000). The same group evaluated the long-term results of laparoscopic hemicystectomy and bladder replacement with SIS with ureteral reimplantation into the SIS material in mini-pigs. Histopathology studies after 1 year showed muscle at the graft periphery and center, but it consisted of small fused bundles with significant fibrosis. Nerves were present at the graft periphery and center, but they were decreased in number. Compared with primary bladder closure after hemicystectomy, no advantage in bladder capacity or compliance was documented (Landman et al, 2004). More recently, bladder regeneration has been shown to be more reliable when the SIS was derived from the distal ileum (Kropp et al, 2004).
In multiple studies using various materials as nonseeded grafts for cystoplasty, the urothelial layer was able to regenerate normally, but the muscle layer, although present, was not fully developed (Kropp et al, 1996b; Probst et al, 1997; Sutherland et al, 1996; Jayo et al, 2008b; Yoo et al, 1998b; Zhang, 2008). Studies involving acellular matrices that may provide the necessary environment to promote cell migration, growth, and differentiation are being conducted (Chun et al, 2007). With continued bladder research in this area, these matrices may have a clinical role in bladder replacement in the future.
Regenerative Medicine for Bladder Using Cell Transplantation
Regenerative medicine with selective cell transplantation may provide a means to create functional new bladder segments (Atala, 1997). The success of cell transplantation strategies for bladder reconstruction depends on the ability to use donor tissue efficiently and to provide the right conditions for long-term survival, differentiation, and growth. Various cell sources have been explored for bladder regeneration. Native cells are currently preferable due to their autologous source, wherein they can be used without rejection (Cilento et al, 1994). It has been shown experimentally that the bladder neck and trigone area has a higher propensity of urothelial progenitor cells (Nguyen et al, 2007), and these cells are localized in the basal region (Kurzrock et al, 2008). Amniotic fluid and bone marrow–derived stem cells can also be used in an autologous manner and have the potential to differentiate into bladder muscle (De Coppi et al, 2007a; Shukla et al, 2008) and urothelium (Anumanthan et al, 2008). Embryonic stem cells also have the potential to differentiate into bladder tissue (Oottamasathien et al, 2007).
Formation of Bladder Tissue
Human urothelial and muscle cells can be expanded in vitro, seeded onto polymer scaffolds, and allowed to attach and form sheets of cells. The cell-polymer scaffold can then be implanted in vivo. Histologic analysis indicated that viable cells were able to self assemble back into their respective tissue types, and would retain their native phenotype (Atala et al, 1993c). These experiments demonstrated, for the first time, that composite layered tissue-engineered structures could be created de novo. Before this study, only nonlayered structures had been created in the field of regenerative medicine.
In order to determine the effects of implanting engineered tissues in continuity with the urinary tract, animal models of bladder augmentation were used (Yoo et al, 1998b). Partial cystectomies were performed in dogs. The animals were divided into two experimental groups. One group had their bladder augmented with a nonseeded bladder-derived collagen matrix, and the second group had their bladder augmented with a cell-seeded construct. The bladders augmented with matrices seeded with cells showed a 100% increase in capacity compared with bladders augmented with cell-free matrices, which showed only a 30% increase in capacity (see Fig. 19–9).
Most of the free grafts (without cells) used for bladder replacement in the past were able to show adequate histology in terms of a well-developed urothelial layer, but they were associated with an abnormal muscular layer that varied in terms of its full development (Atala, 1995a, 1998).
It has been well established for decades that the bladder is able to regenerate generously over free grafts. Urothelium is associated with a high reparative capacity (de Boer et al, 1994). Bladder muscle tissue is less likely to regenerate in a normal fashion. Both urothelial and muscle ingrowth are believed to be initiated from the edges of the normal bladder toward the region of the free graft (Baker et al, 1958; Gorham et al, 1989). Usually, however, contracture or resorption of the graft has been evident. The inflammatory response toward the matrix may contribute to the resorption of the free graft. It was hypothesized that building the three-dimensional structure constructs in vitro, before implantation, would facilitate the eventual terminal differentiation of the cells after implantation in vivo and would minimize the inflammatory response toward the matrix, thus avoiding graft contracture and shrinkage. The dog study demonstrated a major difference between matrices used with autologous cells (tissue-engineered matrices) and those used without cells (Yoo et al, 1998b). Matrices implanted with cells for bladder augmentation retained most of their implanted diameter, as opposed to matrices implanted without cells for bladder augmentation, in which graft contraction and shrinkage occurred. The histomorphology demonstrated a marked paucity of muscle cells and a more aggressive inflammatory reaction in the matrices implanted without cells. Epithelial-mesenchymal signaling is important for the differentiation of bladder smooth muscle (Master et al, 2003).
The results of initial studies showed that the creation of artificial bladders may be achieved in vivo; however, it could not be determined whether the functional parameters noted were caused by the augmented segment or by the intact native bladder tissue. To better address the functional parameters of tissue-engineered bladders, an animal model was designed that required a subtotal cystectomy with subsequent replacement with a tissue-engineered organ (Oberpenning et al, 1999).
Cystectomy-only and nonseeded controls maintained average capacities of 22% and 46% of preoperative values, respectively. An average bladder capacity of 95% of the original precystectomy volume was achieved in the cell-seeded tissue engineered bladder replacements. These findings were confirmed radiographically (Fig. 19–10). The subtotal cystectomy reservoirs that were not reconstructed and the polymer-only reconstructed bladders showed a marked decrease in bladder compliance (10% and 42% total compliance). The compliance of the cell-seeded tissue-engineered bladders showed almost no difference from preoperative values that were measured when the native bladder was present (106%). Histologically, the nonseeded scaffold bladders presented a pattern of normal urothelial cells with a thickened fibrotic submucosa and a thin layer of muscle fibers. The retrieved tissue-engineered bladders showed a normal cellular organization, consisting of a trilayer of urothelium, submucosa, and muscle (Fig. 19–11). Immunocytochemical analyses confirmed the muscle and urothelial phenotype. S-100 staining indicated the presence of neural structures (Oberpenning et al, 1999). These studies, performed with polyglycolic acid–based scaffolds, have been repeated by other investigators, showing similar results in large numbers of animals long-term (Jayo et al, 2008a, 2008b). The strategy of using biodegradable scaffolds with cells can be pursued without concerns for local or systemic toxicity (Kwon et al, 2008). However, not all scaffolds perform well if a large portion of the bladder needs replacement. In a study using SIS for subtotal bladder replacement in dogs, both the unseeded and cell-seeded experimental groups showed graft shrinkage and poor results (Zhang et al, 2006b). The type of scaffold used is critical for the success of these technologies. The use of bioreactors, wherein mechanical stimulation is started at the time of organ production, has also been proposed as an important parameter for success (Farhat et al, 2008).
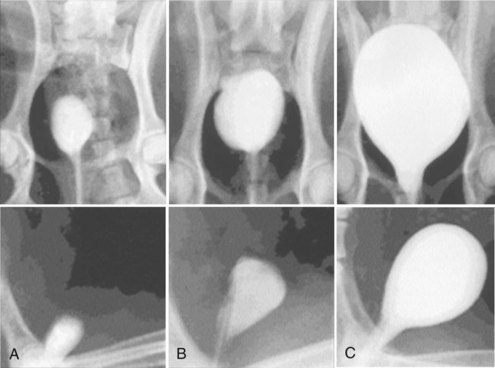
Figure 19–10 Radiographic cystograms in beagles 11 months after subtotal cystectomy without reconstruction (A); with reconstruction using a polymer without cells (B); and with reconstruction with a polymer and cell-seeded tissue-engineered organ (C). Organs after trigone-sparing cystectomy retained a small-sized reservoir. Tissue-engineered neobladders showed a normal configuration and a larger capacity than the trigones grafted with polymer only.
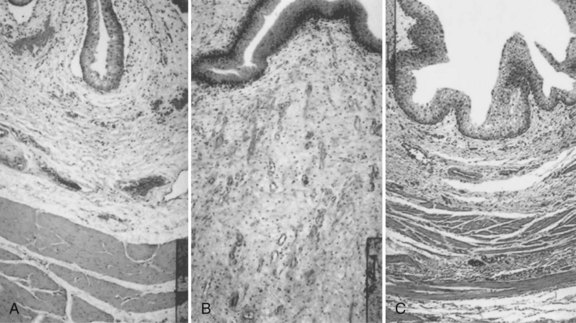
Figure 19–11 Hematoxylin and eosin staining shows histologic results 6 months after surgery (original magnification, ×250). A, Normal canine bladder. B, The bladder dome of the bladder reconstructed with cell-free polymer consists of normal urothelium over a thickened layer of collagen and fibrotic tissue; only scarce muscle fibers are apparent. C, The tissue-engineered neo-organ has a histomorphologically normal appearance. A trilayered architecture consisting of urothelium, submucosa, and smooth muscle is evident.
A clinical experience involving engineered bladder tissue for cystoplasty reconstruction was conducted starting in 1998. A small pilot study of seven patients was reported, using a collagen scaffold seeded with cells either with or without omentum coverage, or a combined PGA-collagen scaffold seeded with cells and omental coverage (Fig. 19–12). The patients reconstructed with the engineered bladder tissue created with the PGA-collagen cell-seeded scaffolds with omental coverage showed increased compliance, decreased end-filling pressures, increased capacities, and longer dry periods over time (Atala et al, 2006). It is clear from this experience that the engineered bladders continued their improvement with time, mirroring their continued development. Although the experience is promising in terms of showing that engineered tissues can be implanted safely, it is just a start in terms of accomplishing the goal of engineering fully functional bladders. This was a limited clinical experience, and the technology is not yet ready for wide dissemination, because further experimental and clinical studies are required. FDA Phase II studies have now been completed.
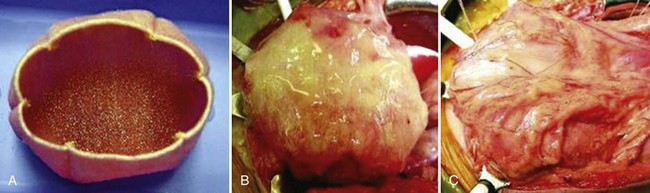
Figure 19–12 Construction of engineered bladder. A, Scaffold material seeded with cells for use in bladder repair. B, The seeded scaffold is anastomosed to native bladder with running 4-0 polyglycolic sutures. C, Implant covered with fibrin glue and omentum.
An area of concern in the field of tissue engineering in the past was the source of cells for regeneration. The concept of creating engineered constructs involves initially obtaining cells for expansion from the diseased organ. How can one be assured that the cell population that is obtained and being expanded for later autologous implantation is normal and will lead to normal tissue formation? For example, if one is dealing with a patient, will the cells obtained from a neuropathic bladder lead to the engineering of another neuropathic bladder? Cultured neuropathic bladder smooth muscle cells possess and maintain different characteristics than normal smooth muscle cells in vitro, as demonstrated by growth assays, contractility, adherence tests, and microarray analysis (Lin et al, 2004; Dozmorov et al, 2007; Hipp et al, 2008). However, when neuropathic smooth muscle cells were cultured in vitro, and seeded onto matrices and implanted in vivo, the tissue engineered constructs showed the same properties as the tissues engineered with normal cells (Lai et al, 2002). The progenitor cells, which reside within stem cell niches within each organ, are responsible for new cell differentiation and tissue formation during the normal process of tissue regeneration due to natural turnover, ageing, and tissue injury. It is known that genetically normal nonmalignant progenitor cells, which are the reservoirs for new cell formation, are programmed to give rise to normal tissue, regardless of whether the niche resides in either normal or diseased tissues (Faris et al, 2001; Lai et al, 2002; Haller et al, 2005). Therefore although the mechanisms for tissue self-assembly and regenerative medicine are not fully understood, it is known that the progenitor cells are able to “reset” their program for normal cell differentiation. The stem cell niche and its role in normal tissue regeneration remains a fertile area of ongoing investigation.
From the above studies, it is evident, as with the urethral studies, that the use of cell-seeded matrices is superior to the use of nonseeded matrices for the creation of engineered bladder tissues. Although advances have been made with the engineering of bladder tissues, many challenges remain. Current research in many centers is aimed at the development of biologically active and “smart” biomaterials that may improve tissue regeneration.
Ureters
Collagen tubular sponges have been used to transplant bladder cells for replacement of ureteral segments in dogs. The study showed severe stricture formation and papillary mucosal thickening at the anastomotic sites. In addition, muscle regeneration into the collagen grafts was not evident (Tachibana et al, 1985).
Ureteral decellularized matrices have been used as a scaffold for both the ingrowth of ureteral tissue (Dahms et al, 1997) and as a cell delivery vehicle using urothelial and bone marrow–derived cells (Matsunuma et al, 2006). Laparoscopic segmental ureteral replacement with an acellular matrix prepared from ureters and small-intestinal submucosa was performed in mini-pigs (Shalhav et al, 1999). At 12 weeks, all animals had complete obstruction at the level of the replacement. Ureteral replacement with polytetrafluoroethylene (Teflon) grafts was attempted in dogs, also with poor functional results (Baltaci et al, 1998). Nonseeded ureteral collagen acellular matrices were tubularized and used to replace 3 cm segments of canine ureters. At the time of sacrifice, there was moderate to marked hydroureteronephrosis above the level of the new tube in all dogs with significant narrowing of the ureteral lumen, up to complete occlusion (Osman et al, 2004).
Cell-seeded biodegradable polymer scaffolds have been used as cell transplantation vehicles to reconstruct ureteral tissues. In one study, urothelial and smooth muscle cells isolated from bladders and expanded in vitro were seeded onto PGA scaffolds with tubular configurations and implanted subcutaneously into athymic mice. After implantation, the urothelial cells proliferated to form a multilayered luminal lining of tubular structures, while the smooth muscle cells organized into multilayered structures surrounding the urothelial cells. Abundant angiogenesis was evident. The degradation of the polymer scaffolds resulted in the eventual formation of natural urothelial tissues (Atala et al, 1993c). This study suggested that it was possible to engineer urologic tissues containing multiple cell types. This approach was expanded to replacement of ureters in dogs by transplantation of smooth muscle cells and urothelial cells on tubular polymer scaffolds (Yoo et al, 1995). The limitations of transferring this technology to humans are the small number of patients requiring this type of tissue, and the large investment that would be required for regulatory approval.
Genital and Reproductive Tissues
Reconstructive surgery is required for a wide variety of pathologic penile conditions, including penile carcinoma, trauma, severe erectile dysfunction, and congenital conditions such as ambiguous genitalia, hypospadias, and epispadias. One of the major limitations of phallic reconstructive surgery is the availability of sufficient autologous tissue. Nongenital autologous tissue sources have been used for decades. Phallic reconstruction was initially attempted in the late 1930s, with rib cartilage used as a stiffener for patients with traumatic penile loss (Frumpkin, 1944; Goodwin et al, 1952). This method, involving multiple staged surgeries, was soon discouraged due to the unsatisfactory functional and cosmetic results. Silicone rigid prostheses were popularized in the 1970s and have been used widely (Small et al, 1975; Bretan, 1989). However, biocompatibility issues have been a problem in selected patients (Thomalla et al, 1987; Nukui et al, 1997). Tissue transfer techniques with flaps from various nongenital sources, such as the groin, dorsalis pedis, and forearm, have been used for genital reconstruction (Jordan, 1999). However, operative complications such as infection, graft failure, and donor site morbidity are not negligible. Phallic reconstruction with autologous tissue, derived from the patient’s own cells, may be preferable in selected cases.
Reconstruction of Corporeal Tissues
Reconstruction of Corporeal Smooth Muscle
One of the major components of the phallus is corporeal smooth muscle. The creation of autologous functional and structural corporeal tissue de novo would be beneficial. Initial experiments showed that cultured human corporeal smooth muscle cells may be used in conjunction with biodegradable polymers to create corpus cavernosum tissue de novo (Kershen et al, 2002). When grown on collagen, corporeal cavernosal endothelial cells formed capillary structures that created complex three-dimensional capillary networks (Fig. 19–13). In a subsequent study, human corporeal smooth muscle cells and endothelial cells seeded on biodegradable polymer scaffolds were able to form vascularized cavernosal muscle when implanted in vivo (Park et al, 1999). A naturally derived acellular corporal tissue matrix that possesses the same architecture as native corpora was developed (Fig. 19–14). Acellular collagen matrices were derived from processed donor rabbit corpora using cell lysis techniques. Human corpus cavernosal muscle and endothelial cells were derived from donor penile tissue, and the cells were expanded in vitro and seeded on the acellular matrices (Fig. 19–15). The matrices were covered with the appropriate cell architecture 4 weeks after implantation (Falke et al, 2003). The use of these tissue-derived matrices as cell-delivery scaffolds allowed the development of adequate structural and vascular corpora cavernosa constructs.
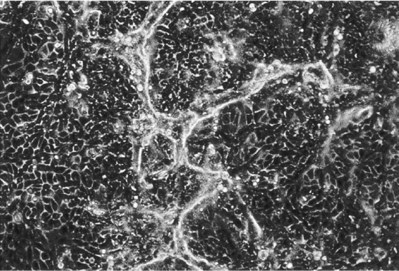
Figure 19–13 Human corporal cavernosal endothelial cells form capillary-like structures in vitro (original magnification, ×100).
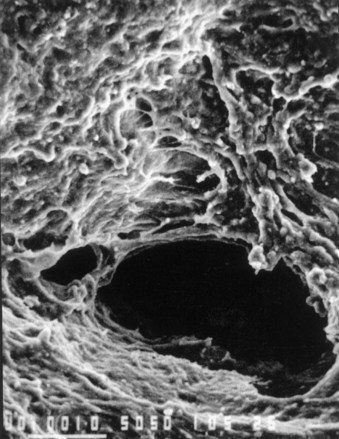
Figure 19–14 Scanning electron microscopy of human cavernosal smooth muscle and endothelial cells seeded on acellular matrices.
Figure 19–15 Cylindrical polymer scaffolds seeded with chondrocytes and implanted in vivo formed milky-white, rod-shaped, solid cartilaginous structures.
To look at the functional parameters of the engineered corpora, acellular corporeal collagen matrices were obtained from a donor rabbit penis, and autologous corpus cavernosal smooth muscle and endothelial cells were harvested, expanded, and seeded on the matrices. An entire cross-sectional segment of protruding rabbit phallus was excised, leaving the urethra intact and the cell-seeded matrices were interposed into the excised corporal space. Functional and structural parameters (cavernosography, cavernosometry, mating behavior, and sperm ejaculation) were followed, and histochemical, immunocytochemical and Western blot analyses were performed up to 6 months after implantation. The engineered corpora cavernosa achieved adequate structural and functional parameters (Kwon et al, 2002). This technology was further confirmed when the entire rabbit corpora was removed and replaced with the engineered scaffolds. The experimental corporeal bodies demonstrated intact structural integrity by cavernosography and showed similar pressure by cavernosometry when compared with the normal controls. The control rabbits without cells failed to show normal erectile function throughout the study period. Mating activity in the animals with the engineered corpora appeared normal by 1 month after implantation. The presence of sperm was confirmed during mating, and was present in all the rabbits with the engineered corpora. The female rabbits mated with the animals implanted with engineered corpora and also conceived and delivered healthy pups. Animals implanted with the matrix alone were unable to demonstrate normal mating activity and failed to ejaculate into the vagina. Grossly, the corporeal implants with cells showed continuous integration of the graft into native tissue. Histologically, sinusoidal spaces and walls, lined with endothelium and smooth muscle, were observed in the engineered grafts. Grafts without cells contained fibrotic tissue and calcifications with sparse corporal elements. Each cell type was identified immunocytochemically and by Western-blot analyses. The engineered corporeal tissues were able to contract and relax in response to electric field and pharmacologic stimulation, and the contractile response reached levels similar to normal corpora by 6 months after implantation (Chen et al, 2005). Dynamic cell seeding using bioreactor systems have been shown to enhance cell penetration in the acellular tissue-derived matrices (Eberli et al, 2008b).
The above series of studies demonstrate that penile corpora cavernosa tissue can be engineered. The engineered tissue is able to achieve adequate structural and functional parameters sufficient for erection, copulation, ejaculation, conception, and delivery in rabbits. Further studies will be needed to confirm the long-term functionality of these organs. In addition, further studies are needed to show that human structures can also be engineered.
Engineered Penile Prostheses
Although silicone is an accepted biomaterial for penile prostheses, biocompatibility is a concern (Thomalla et al, 1987; Nukui et al, 1997). The use of a natural prosthesis composed of autologous cells may be advantageous. A feasibility study for the creation of natural penile prostheses made of cartilage was performed initially (Yoo et al, 1998a).
Cartilage, harvested from the articular surface of calf shoulders, was isolated, grown, and expanded in culture. The cells were seeded onto preformed cylindrical polyglycolic acid polymer rods and implanted in mice. At retrieval, all polymer scaffolds seeded with cells formed milky-white, rod-shaped, solid cartilage structures, maintaining their preimplantation size and shape (see Fig. 19–15). In a subsequent study using an autologous system, the feasibility of applying the engineered cartilage rods in situ was investigated (Yoo et al, 1999). Autologous chondrocytes harvested from rabbit ear were grown and expanded in culture. The cells were seeded onto biodegradable poly-L-lactic acid coated polyglycolic acid polymer rods and implanted into the corporal spaces of rabbits. Examination at retrieval showed the presence of well-formed, milky-white cartilage structures within the corpora at 1 month. The animals were able to copulate and impregnate their female partners. In another study, human cartilage rods were engineered in vitro for potential use as penile prostheses. Chondrocytes isolated from human ear were seeded on rod-shaped biodegradable polymer scaffolds (1.2 cm in diameter, 6.0 cm long). The engineered human cartilaginous rods were flexible, elastic, and able to withstand high degrees of compressive forces. The mechanical properties were comparable to those of commercially available silicone prostheses (Kim et al, 2002).
Testis
Leydig cells are the major source of testosterone production in males. Patients with testicular dysfunction require androgen replacement for somatic development. Conventional treatment for testicular dysfunction consists of periodic intramuscular injections of chemically modified testosterone or, more recently, skin patch applications. However, long-term nonpulsatile testosterone therapy is not optimal and can cause multiple problems, including erythropoiesis and bone density changes.
A system was designed wherein Leydig cells were microencapsulated for controlled testosterone replacement. Microencapsulated Leydig cells offer several advantages, such as serving as a semipermeable barrier between the transplanted cells and the host’s immune system, as well as allowing for the long-term physiologic release of testosterone. Purified Leydig cells were isolated and encapsulated in an alginate-poly-L-lysine solution (Fig. 19–16). The encapsulated Leydig cells were injected into castrated animals, and serum testosterone was measured serially; the animals were able to maintain testosterone levels in the long term (Machluf et al, 1998). These studies suggest that microencapsulated Leydig cells may be able to replace or supplement testosterone in situations where anorchia or testicular failure is present. A novel technique to isolate Leydig stem cells and to study Leydig cell development has also been described (Lo et al, 2004). The successful transplantation of functional Leydig stem cells into a hypogonadal recipient showed that the de novo synthesis of testosterone is possible.
Figure 19–16 Left, Hydrogel microcapsules without cells. Right, Leydig cells encapsulated in hydrogel microcapsules secrete testosterone in vitro and in vivo.
Further studies showed that testicular prostheses created with chondrocytes in bioreactors, could be loaded with testosterone. The prostheses were implanted in athymic mice with bilateral anorchia, and testosterone was released long term, maintaining the androgen level at a physiologic range (Raya-Rivera et al, 2008). One could envision combining the Leydig cell technology described above with engineered prostheses for the long-term functional replacement of androgen levels. The ability to have spermatogenesis for infertility purposes has been a major area of interest in the last decade. Spermatogonial transplantation and reconstitution of donor cell spermatogenesis was shown in recipient mice (Nagano et al, 1998). The same group was able to show the restoration of fertility in mice with the transplantation of male germ-line stem cells (Ogawa et al, 2000). Spermatogonial stem cells can be isolated and enriched with specific markers (Lo et al, 2005). Sertoli cells, the main component of the testicular germ-cell niche, were recovered and dissociated from testes of donor male mice and microinjected into recipient testes, forming mature seminiferous tubule structures, and supporting spermatogenesis (Shinohara et al, 2003). The germ-line niche transplantation was also able to restore fertility in mice (Kanatsu-Shinohara et al, 2005).
Female Genital and Reproductive Tissues
Congenital malformations of the uterus may have profound implications clinically. Patients with cloacal exstrophy and intersex disorders may not have sufficient uterine tissue present for future reproduction. We investigated the possibility of engineering functional uterine tissue using autologous cells (Wang et al, 2003). Autologous rabbit uterine smooth muscle and epithelial cells were harvested, then grown and expanded in culture. These cells were seeded onto pre-configured uterine-shaped biodegradable polymer scaffolds, which were then used for subtotal uterine tissue replacement in the corresponding autologous animals. Upon retrieval 6 months after implantation, histological, immunocytochemical, and Western-blot analyses confirmed the presence of uterine tissue components. Biomechanical analyses and organ bath studies showed that the functional characteristics of these tissues were similar to those of normal uterine tissue. Breeding studies using these engineered uteri are currently being performed.
Similarly, several pathologic conditions, including congenital malformations and malignancy, can adversely affect normal vaginal development or anatomy. Vaginal reconstruction has traditionally been challenging due to the paucity of available native tissue. Acellular materials have been used experimentally for vaginal reconstruction in rats (Wefer et al, 2002). The feasibility of engineering vaginal tissue with cells in vivo was also investigated (De Filippo et al, 2003). Vaginal epithelial and smooth muscle cells of female rabbits were harvested, grown, and expanded in culture. These cells were seeded onto biodegradable polymer scaffolds, and the cell-seeded constructs were then implanted into mice. Functional studies in the tissue-engineered constructs showed similar properties to those of normal vaginal tissue. When these constructs were used for autologous total vaginal replacement in a rabbit model, patent functional vaginal structures were noted in the tissue-engineered specimens, while the non–cell-seeded structures were noted to be stenotic (De Filippo et al, 2008) (Fig. 19–17) These studies indicated that a regenerative medicine approach to clinical vaginal reconstruction would be a realistic possibility. Clinical trials are currently being conducted.
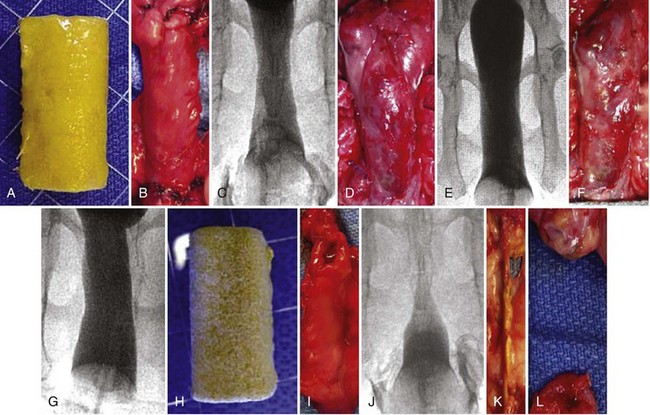
Figure 19–17 Appearance of tissue engineered neovaginas. A, Tubular polymer scaffold after cell seeding and 1-week in vitro culture, prior to implantation in vivo. B, D, and F indicate gross appearance and C, E, and G show vaginography of cell-seeded constructs 1, 3, and 6 months, postimplantation, respectively. H, Unseeded control scaffold prior to implantation. I, K, and L, Gross appearance of unseeded construct at 1, 3, and 6 months postimplantation. J, Vaginography of unseeded graft at 1 month.
Renal Structures
Although the kidney was the first organ to be substituted by an artificial device and the first successfully transplanted organ (Guild et al, 1955), current modalities of treatment are far from satisfactory. Renal tissue is arguably one of the most difficult tissues to replicate in the laboratory. The kidney is a complex organ and the unique structural and cellular heterogeneity present within this organ creates many challenges. The system of nephrons and collecting ducts within the kidney is composed of multiple functionally and morphologically distinct segments. For this reason, appropriate conditions must be provided to ensure the long-term survival, differentiation and growth of many types of cells. Efforts in the area of kidney tissue regeneration have focused on the development of a reliable cell source (Prockop, 1997; Kale et al, 2003; Lin et al, 2003; Ikarashi et al, 2005; Lin et al, 2005; Yokoo et al, 2005). Moreover, optimal growth conditions have been extensively investigated to provide adequate enrichment to achieve stable renal cell expansion systems (Milici et al, 1985; Carley et al, 1988; Humes et al, 1992; Schena, 1998).
Isolation of particular cell types that produce specific factors may be a good approach for selective cell therapies. For example, cells that produce erythropoietin have been isolated in culture, and these cells could eventually be used to treat anemia that results from end stage renal failure (Aboushwareb et al, 2008). Other more ambitious approaches involve working towards the goal of total renal function replacement. To create kidney tissue that would deliver full renal function, a culture containing all of the cell types comprising the functional nephron units should be used. Optimal culture conditions to nurture renal cells have been extensively studied and cells grown under these conditions have been reported to maintain their cellular characteristics (Lanza et al, 2002a). Cells obtained through the initial process of nuclear transfer were retrieved and expanded from cloned tissue. Moreover, renal cells placed in a three-dimensional culture environment are able to reconstitute into renal structures.
Recent investigative efforts in the search for a reliable cell source have been expanded to stem and progenitor cells. Use of these cells for tissue regeneration is attractive due to their ability to differentiate and mature into specific cell types needed. This is particularly useful in instances where primary renal cells are unavailable due to extensive tissue damage. Bone marrow–derived human mesenchymal stem cells have been shown to be a potential source due to their ability to differentiate into several cell lineages (Prockop, 1997; Kale et al, 2003; Ikarashi et al, 2005). These cells have been shown to participate in kidney development when they are placed in a rat embryonic niche that allows for continued exposure to a repertoire of nephrogenic signals (Yokoo et al, 2005). These cells, however, were found to contribute mainly to regeneration of damaged glomerular endothelial cells after injury. The major cell source of kidney regeneration was found to originate from intrarenal cells in an ischemic renal injury model (Lin et al, 2005). Systemic administration of bone marrow–derived mesenchymal stem cells in mice has led to prevention of nephropathy in a diabetic model, the acceleration of acute renal injury, and to angiogenesis and vasculogenesis in a renal injury model (Humphreys et al, 2008; Lin, 2008).
Another potential cell source for kidney regeneration is circulating stem cells, which have been shown to transform into tubular and glomerular epithelial cells, podocytes, mesangial cells, and interstitial cells after renal injury (Ito et al, 2001; Poulsom et al, 2001; Gupta et al, 2002; Iwano et al, 2002; Kale et al, 2003; Lin et al, 2003; Rookmaaker et al, 2003). Other stem cell types, such as human embryonic stem cells (Lin, 2006), and the more recently discovered human amniotic fluid and placental stem cells can also lead to renal differentiation (Perin et al, 2007). These observations suggest that controlling stem and progenitor cell differentiation may lead to successful regeneration of kidney tissues.
Although isolated renal cells are able to retain their phenotypic and functional characteristics in culture, transplantation of these cells in vivo may not result in structural remodeling. In addition, cell or tissue components cannot be implanted in large volumes due to limited diffusion of oxygen and nutrients (Folkman et al, 1973). Thus a cell-support matrix, preferably one that encourages angiogenesis, is necessary to allow diffusion across the entire implant. A variety of synthetic and naturally derived materials has been examined in order to determine the ideal support structures for regeneration (Tachibana et al, 1985; Atala, 1995b; Oberpenning et al, 1999; El-Kassaby et al, 2003; Atala et al, 2006). Biodegradable synthetic materials, such as poly-lactic and polyglycolic acid polymers, have been used to provide structural support for cells. Synthetic materials can be easily fabricated and configured in a controlled manner, which make them attractive options for regenerative medicine. However, naturally derived materials, such as collagen, laminin, and fibronectin, are much more biocompatible and provide a similar extracellular matrix environment to normal tissue. For this reason, collagen-based scaffolds have been used increasingly in many applications (De Filippo et al, 2002; El-Kassaby et al, 2003; Falke et al, 2003; Atala et al, 2006; Murray et al, 2006).
Developmental Approaches to Kidney Regeneration
Transplantation of a kidney precursor, such as the metanephros, into a diseased kidney has been proposed as a possible method for functional restoration. In an animal study, human embryonic metanephroi, transplanted into the kidneys of an immune-deficient mouse model, has developed into mature kidneys (Dekel et al, 2003). The transplanted metanephroi produced urine-like fluid, however, failed to develop ureters. This study suggests that development of an in-vitro system in which metanephroi could be grown may lead to transplant techniques that could produce a small replacement kidney within the host. In another study, the metanephros was divided into mesenchymal tissue and ureteral buds, and each of the tissue segments was cultured in vitro (Steer et al, 2002). After 8 days in culture, each portion of the mesenchymal tissues had grown to the original size. A similar method was used for ureteral buds, which also propagated. These studies were expanded further by staging renal structure reconstitution in vitro and later implanting these structures in vivo in a mouse model (Rosines et al, 2007). These studies indicate that if the mesenchyme and ureteral buds were placed together and cultured in vitro, a metanephros-like structure would develop and suggest that the metanephros could be propagated under an optimal condition.
In another study, transplantation of metanephroi into a nonimmunosuppressed rat omentum showed that the implanted metanephroi are able to undergo differentiation and growth that is not confined by a tight organ capsule (Rogers et al, 1998). When the metanephroi with an intact ureteric bud were implanted, the metanephroi are able to enlarge and become kidney shaped tissue within 3 weeks. The metanephroi transplanted into the omentum were able to develop into kidney tissue structure with a well-defined cortex and medulla. Mature nephrons and collecting system structures are shown to be indistinguishable from those of normal kidneys by light or electron microscopy (Hammerman, 2002a, 2002b). Moreover, these structures become vascularized by arteries that originate at the superior mesenteric artery of the host (Hammerman, 2002a, 2002b). It has been demonstrated that the metanephroi transplanted into the omentum survive for up to 32 weeks postimplantation (Rogers et al, 1999). These studies show that developmental approach may be a viable option for regenerating renal tissue for functional restoration.
Regenerative Medicine Approaches to Kidney Regeneration
The ability to grow and expand renal cells is one of the essential requirements in engineering tissues. The feasibility of achieving renal cell growth, expansion, and in-vivo reconstitution using regenerative medicine techniques was investigated (Atala, 1995b). Donor rabbit kidney cells, including distal tubules, glomeruli, and proximal tubules were plated separately in vitro and, after expansion, were seeded onto biodegradable polyglycolic acid scaffolds and implanted subcutaneously into host athymic mice. This included implants of proximal tubular cells, glomeruli, distal tubular cells, and a mixture of all three cell types. Histologic examination demonstrated progressive formation and organization of the nephron segments within the polymer fibers with time. BrdU incorporation into renal cell DNA was confirmed. These results demonstrated that renal specific cells can be successfully harvested and cultured, and can subsequently attach to artificial biodegradable polymers. However, it was unclear whether the tubular structures reconstituted de novo from dispersed renal elements, or if they merely represented fragments of donor tubules that survived the original dissociation and culture processes intact. Further investigation was conducted in order to examine the process (Fung, 1996). Mouse renal cells were harvested and expanded in culture. Subsequently, single isolated cells were seeded on biodegradable polymers and implanted into immune competent syngeneic hosts. Renal epithelial cells were observed to reconstitute into tubular structures in vivo. Sequential analyses of the retrieved implants over time demonstrated that renal epithelial cells first organized into a cordlike structure with a solid center. Subsequent canalization into a hollow tube could be seen by two weeks. Histologic examination with nephron segment-specific lactins showed successful reconstitution of proximal tubules, distal tubules, loop of Henle, collecting tubules, and collecting ducts. These results showed that single suspended cells are capable of reconstituting into tubular structures, with homogeneous cell types within each tubule.
In a subsequent study mouse renal cells were harvested, expanded in culture, and seeded onto a tubular device constructed from polycarbonate (Yoo et al, 1996). The tubular device was connected at one end to a Silastic catheter that terminated into a reservoir. The device was implanted subcutaneously in athymic mice. Histological examination of the implanted device demonstrated extensive vascularization, as well as formation of glomeruli (Fig. 19–18) and highly organized tubule-like structures. Immunocytochemical staining with antiosteopontin antibody, which is secreted by proximal and distal tubular cells and the cells of the thin ascending loop of Henle, stained the tubular sections. Immunohistochemical staining for alkaline phosphatase stained proximal tubule-like structures. Uniform staining for fibronectin in the extracellular matrix of newly formed tubes was observed. The fluid collected from the reservoir was yellow and contained 66 mg/dL uric acid (compared with 2 mg/dL in plasma), suggesting that these tubules are capable of unidirectional secretion and concentration of uric acid. The creatinine assay performed on the collected fluid showed an 8.2-fold increase in concentration when compared with serum. These results demonstrated that single cells form multicellular structures can become organized into functional renal units that are able to excrete high levels of solutes through a urine-like fluid (Yoo et al, 1996).
Figure 19–18 Retrieved tissue-engineered renal units demonstrate formation of glomeruli (hematoxylin and eosin stain; original magnification, ×400).
To determine whether renal tissue could be formed using an alternative cell source, nuclear transplantation (therapeutic cloning) was performed to generate histocompatible tissues, and the feasibility of engineering syngeneic renal tissues in vivo using these cloned cells was investigated (Lanza et al, 2002a) (Fig. 19–19). Nuclear material from bovine dermal fibroblasts was transferred into unfertilized enucleated donor bovine eggs. Renal cells from the cloned embryos were harvested, expanded in vitro, and seeded onto three-dimensional renal devices. The devices were implanted into the back of the same steer from which the cells were cloned, and were retrieved 12 weeks later. This process produced functioning renal units. Urine production and viability were demonstrated after transplantation back into the nuclear donor animal. Chemical analysis suggested unidirectional secretion and concentration of urea nitrogen and creatinine. Microscopic analysis revealed formation of organized glomeruli and tubular structures. Immunohistochemical and real-time polymerase chain reaction (RT-PCR) analysis confirmed the expression of renal mRNA and proteins. These studies demonstrated that cells derived from nuclear transfer can be successfully harvested, expanded in culture, and transplanted in vivo with the use of biodegradable scaffolds on which the single suspended cells can organize into tissue structures that are genetically identical to that of the host. These studies were the first demonstration of the use of therapeutic cloning for regeneration of tissues in vivo.
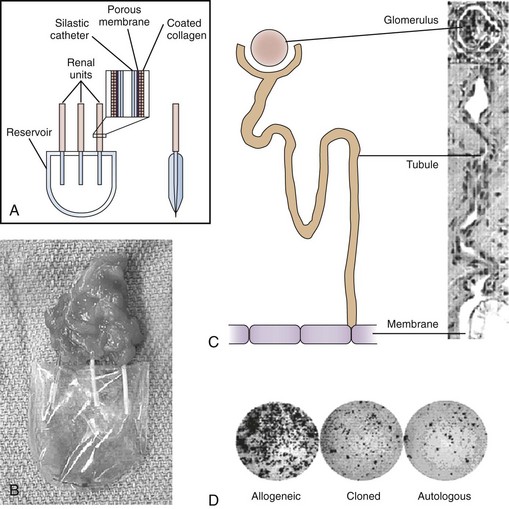
Figure 19–19 Production of kidney tissue by therapeutic cloning and regenerative medicine. A, Illustration of the tissue-engineered renal unit. B, Renal unit seeded with cloned cells, three months after implantation, showing the accumulation of urine-like fluid. C, There was a clear unidirectional continuity between the mature glomeruli, their tubules, and the polycarbonate membrane. D, ELISPOT analyses of the frequencies of T cells that secrete interferon-γ after primary and secondary stimulation with allogeneic renal cells, cloned renal cells, or nuclear donor fibroblasts.
A naturally derived tissue matrix with existing three-dimensional kidney architecture would be preferable to the artificial matrix used in the above experiments, because it would allow transplantation of a larger number of cells, resulting in greater renal tissue volumes. Thus an acellular collagen-based kidney matrix, which is similar to the native renal architecture, was developed. In a subsequent study, it was investigated whether these collagen-based matrices could accommodate large volumes of renal cells and form kidney structures in vivo (Amiel et al, 2000).
Acellular collagen matrices, derived from porcine kidneys, were obtained through a multistep decellularization process. During this process, serial evaluation of the matrix for cellular remnants was performed using histochemistry, scanning electron microscopy (SEM), and RT-PCR. Mouse renal cells were harvested, grown, and seeded on decellularized collagen matrices. Cell matrix constructs grown in vitro and implanted in the subcutaneous space of 20 athymic mice were analyzed over time. Renal cells seeded on the matrix adhered to the inner surface and proliferated to confluency by 7 days after seeding. Renal tubular and glomerulus-like structures were observed 8 weeks after implantation.
Other Applications of Genitourinary Regenerative Medicine
Please see the Expert Consult website![]() for this section, including Figure 19–20.
for this section, including Figure 19–20.
Fetal Regenerative Medicine
The prenatal diagnosis of fetal abnormalities is now more prevalent. Prenatal ultrasonography allows for a thorough survey of fetal anatomy. For example, the absence of bladder filling, a mass of echogenic tissue on the lower abdominal wall, or a low-set umbilicus on prenatal sonographic examination may suggest the diagnosis of bladder exstrophy. These findings and the presence of intraluminal intestinal calcifications suggest the presence of a cloacal malformation.
The natural consequence of the evolution in prenatal diagnosis was the use of intervention before birth to reverse potentially life-threatening processes. However, the concept of prenatal intervention itself is not limited to this narrow group of indications. A prenatal rather than a postnatal diagnosis of urologic conditions such as exstrophy may be beneficial under certain circumstances. There is now renewed interest in single-stage reconstruction surgery for some patients with bladder exstrophy. Limiting factors for choosing a single- or multiple-stage approach may include the finding of a small, fibrotic bladder patch without either elasticity or contractility and the finding of a hypoplastic bladder.
There are several strategies that may be pursued, using today’s technologic and scientific advances, to facilitate the future prenatal management of patients with urologic disease. Having a ready supply of urologic-associated tissue for surgical reconstruction at birth may be advantageous. Theoretically, once the diagnosis of the pathologic condition is confirmed prenatally, a small tissue biopsy could be obtained under ultrasound guidance. These biopsy materials could then be processed and the various cell types expanded in vitro. Using regenerative medicine techniques, reconstituted structures in vitro could then be readily available at the time of birth for reconstruction (Fig. 19–20).
Figure 19–20 In utero regenerative medicine strategy. Certain conditions, such as bladder exstrophy, can be diagnosed prenatally. Bladder, cartilage, skin, and other tissues can be retrieved by fetoscopy or percutaneously under ultrasound guidance. Cells can be harvested, expanded in vitro, and reconstituted into tissue structures. Tissue is then readily available at the time of birth for reconstruction.
Toward this end, a series of experiments was conducted on fetal lambs (Fauza et al, 1998a, 1998b). Bladder exstrophy was created in fetal lambs, and a bladder biopsy was processed and the cells expanded and seeded on biodegradable scaffolds. After delivery, the bladders were surgically closed using the tissue-engineered bladder tissue. Histologic analysis of the engineered tissue showed a normal histologic pattern, and good compliance (Fauza et al, 1998a). Similar prenatal studies were performed in lambs with engineering of skin for reconstruction at birth (Fauza et al, 1998b).
With regenerative medicine techniques, the bladder exstrophy complex could be managed not only in utero but also after birth in a similar manner, whenever a prenatal diagnosis is not certain. In these instances, bladder tissue biopsies could be obtained at the time of the initial surgery. Various tissues could be harvested and stored for future reconstruction, if necessary. A tissue bank for patients with exstrophy complex could preserve the different cell types.
Injectable Therapies
Both urinary incontinence and vesicoureteral reflux are common conditions affecting the genitourinary system for which injectable therapy may be useful. There are definite advantages to an endoscopic treatment, because the method is simple and can be completed in less than 15 minutes; it has a low morbidity; and it can be performed on an outpatient basis. The ideal substance for the endoscopic treatment of reflux and incontinence should be injectable, nonantigenic, nonmigratory, volume stable, and safe for human use (Kershen et al., 1999). Toward this goal, long-term studies were conducted to determine the effect of injectable chondrocytes in vivo (Atala et al, 1993b). The concept consisted of a biopsy of the ear, followed by chondrocyte processing and endoscopic injection of the autologous chondrocyte suspension for the treatment of incontinence. This system was adapted for the treatment of reflux in a porcine model (Atala et al, 1994). Chondrocytes were harvested, injected cystoscopically, and cartilage tissue formed, correcting reflux (Atala et al, 1994).
The first human application of cell-based regenerative medicine technology for urologic applications occurred with the injection of chondrocytes for the correction of vesicoureteral reflux in children and for urinary incontinence in adults. Two multicenter clinical trials were conducted using the above engineered chondrocyte technology. Patients with urinary incontinence secondary to intrinsic sphincter deficiency were treated endoscopically with injected chondrocytes at three different medical centers. Phase I trials showed an approximate success rate of 80% at both 3 and 12 months postoperatively (Bent et al, 2001). Patients with vesicoureteral reflux were treated at ten centers throughout the United States. The patients had a similar success rate as with other injectable substances in terms of cure. The overall success rate in 29 children (47 ureters) was 86%. At one-year follow-up, reflux correction was maintained in 70% of the ureters. Chondrocyte formation was not noted in patients who had treatment failure (Diamond et al, 1999). The success rate was higher with the treatment of incontinence than with reflux, probably related to the cells having time to “set” in the sphincter, as opposed to the bladder itself where immediate cycling occurs, and where the cell mound can get dissipated over a larger surface area. In fact, in the porcine model, the ureteral orifices exit in the urethral region, and the model does not necessarily replicate the human equivalent of reflux. There were several challenges that are unique to cell based therapies, such as cell processing, cell viability, and biological costs, underlying the importance of adequate planning with manufacturing for wider patient dissemination.
Using cell therapy techniques, the use of autologous smooth muscle cells was explored for both urinary incontinence and vesicoureteral reflux applications (Cilento et al, 1995). In-vivo experiments were conducted in mini-pigs, and reflux was successfully corrected. The potential use of injectable, cultured myoblasts for the treatment of stress urinary incontinence has also been investigated (Chancellor et al, 2000; Yokoyama et al, 2000). Labeled myoblasts were directly injected into the proximal urethra and lateral bladder walls of nude mice with a micro-syringe in an open surgical procedure. Tissue harvested up to 35 days postinjection contained the labeled myoblasts, as well as evidence of differentiation of the labeled myoblasts into regenerative myofibers. The authors reported that a significant portion of the injected myoblast population persisted in vivo. The authors subsequently demonstrated increased leak point
pressure and increased sphincter contractility after periurethral injection of muscle-derived stem cells in a rat model of stress urinary incontinence, and in a large-animal model (Cannon et al, 2003; Lee et al, 2003; Kwon et al, 2006). Similar techniques of sphincteric-derived muscle cells have been used for the treatment of urinary incontinence in a pig model (Strasser et al, 2004). The same group showed that urethral closing pressures and treatment efficacy in a porcine model was dose dependent (Mitterberger et al, 2007b).
The use of injectable muscle precursor cells has also been investigated for use in the treatment of urinary incontinence due to irreversible urethral sphincter injury or maldevelopment. Muscle precursor cells are the quiescent satellite cells found in each myofiber that proliferate to form myoblasts and eventually myotubes and new muscle tissue (Yiou et al, 2003b). Intrinsic muscle precursor cells have previously been shown to play an active role in the regeneration of injured striated urethral sphincter (Yiou et al, 2003a). In a subsequent study, autologous muscle precursor cells were injected into a rat model of urethral sphincter injury, and both replacement of mature myotubes, as well as restoration of functional motor units, were noted in the regenerating sphincteric muscle tissue (Yiou et al, 2003b). This is the first demonstration of the replacement of both sphincter muscle tissue and its innervation by the injection of muscle precursor cells. As a result, muscle precursor cells may be a minimally invasive solution for urinary incontinence in patients with irreversible urinary sphincter muscle insufficiency. A canine model of irreversible urethral sphincter injury was also created to test these technologies (Eberli et al, 2008a).
In addition, injectable muscle-based gene therapy and regenerative medicine were combined to improve detrusor function in a bladder injury model, and may potentially be a novel treatment option for urinary incontinence (Huard et al, 2002). Highly purified muscle-derived cells that display stem cell characteristics were genetically engineered to express the gene encoding β-galactosidase, then injected into the bladder walls of mice. The injectable cells were able to survive in the lower urinary tract and improve the contractility of the bladder following the induced injury, as well as become innervated into the bladder as early as 2 weeks after injection. Angiogenic gene modification of skeletal muscle cells has also been performed in order to compensate for ageing-induced decline in bioengineered functional muscle tissue (Delo et al, 2008). These findings may be useful in the setting of urinary incontinence where these muscle-derived cells may help to bulk up the urethral wall, enhance coaptation, and improve the urinary sphincter muscle.
The use of lipoaspirate cells has also been proposed for the treatment on urinary incontinence. The lipoaspirate cells were injected in mice bladders and urethras, and regenerated muscle tissue (Jack et al, 2005). The same cells were shown to differentiate into functional smooth muscle cells (Rodriguez et al, 2006).
Several clinical trials using myoblast injection have been conducted. A group of 42 patients, 29 women and 13 men with urinary stress incontinence, had myoblasts injected into the rhabdosphincter transurethrally and with ultrasound guidance. A cure was reported by 35 patients, and 7 had marked improvement (Strasser et al, 2007). The same group reported on an additional clinical trial in women with stress urinary incontinence (Mitterberger et al, 2007a). The authors compared the effectiveness and tolerability of ultrasonography-guided injections of autologous cells with those of endoscopic injections of collagen for stress incontinence in 63 patients, showing improved results for the patients receiving the myoblasts. However, controversy surrounding the trial ensued and the paper was retracted. In another trial, myoblasts isolated from the abdominal wall vasculature were injected in a series of bladder exstrophy patients with urinary incontinence. The authors reported that 88% of patients were socially dry, described as daytime dryness more than 3 hours. The patients were also on a pelvic floor electrical stimulation and pelvic floor exercise program (Kajbafzadeh et al, 2008). Another study described the use of autologous muscle-derived stem cell injection to treat stress urinary incontinence. After one year, 1 of 8 women achieved total continence, and 5 reported improvement (Carr et al, 2008). Activity has increased in the area of cell therapy for urinary incontinence in the last several years. Further trials and follow-up will be needed in order to determine efficacy long term.
Key Points
Summary
Regenerative medicine efforts are currently being undertaken for every type of tissue and organ within the urinary system. Most of the efforts expended in the genitourinary field have occurred within the last two decades. Regenerative medicine strategies involve the use of biomaterials alone, as has been accomplished clinically in patients with urethral disease; biomaterials with cells, as is currently being tested clinically with bladder and vaginal tissues; and cell therapies alone, as those applied clinically for urinary incontinence, initially with chondrocytes and now with muscle cells. Regenerative medicine is a multidisciplinary field that requires expertise in a wide variety of scientific disciplines, including cell and molecular biology, physiology, pharmacology, chemical engineering, biomaterials, nanotechnology, and clinical sciences. Although, to date, modest clinical success has been achieved in specific areas, the field is still in its infancy. Long-term studies are still essential to assure safety and efficacy before these technologies have widespread clinical application.
Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241-1246.
Bent AE, Tutrone RT, McLennan MT, et al. Treatment of intrinsic sphincter deficiency using autologous ear chondrocytes as a bulking agent. Neurourol Urodyn. 2001;20:157-165.
Carr LK, Steele D, Steele S, et al. 1-Year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:881-883.
De Coppi P, Bartsch JrG, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy, [see comment]. Nat Biotechnol, 2007;25, 100-106.
Diamond DA, Caldamone AA. Endoscopic correction of vesicoureteral reflux in children using autologous chondrocytes: preliminary results. J Urol. 1999;162:1185.
Kassaby EA, Yoo J, Retik A, Atala A. A novel inert collagen matrix for urethral stricture repair. J Urol. 2000;308S:70.
Lanza RP, Chung HY, Yoo JJ, et al. Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol. 2002;20:689-696.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
Wilmut I, Schnieke AE, McWhir J, et al. Viable offspring derived from fetal and adult mammalian cells, [see comment]; [erratum appears in Nature 1997;386(6621):200]. Nature, 1997;385, 810-813.
Zhang Y, Frimberger D, Cheng EY, et al. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006;98(5):1100-1105.
Aboushwareb T, Egydio F, Straker L, et al. Erythropoietin producing cells for potential cell therapy. World J Urol. 2008;26:295-300.
Adams MC, Brock JW, Pope JCT, et al. Ureterocystoplasty: is it necessary to detubularize the distal ureter? J Urol. 1998;160:851-853.
Alberts B, Bray D, Lewis JM. The extracellular matrix of animals. In: Alberts B, Bray D, Lewis JM, editors. Mollecular biology of the cell. New York: Garland Publishing; 1994:971-995.
Amiel GE, Atala A. Current and future modalities for functional renal replacement. Urol Clin North Am. 1999;26:235-246.
Amiel GE, Yoo J, Atala A. Renal tissue engineering using a collagen-based kidney matrix. Tissue Engineering Suppl. 2000;6:685.
Anumanthan G, Makari JH, Honea L, et al. Directed differentiation of bone marrow derived mesenchymal stem cells into bladder urothelium. J Urol. 2008;180:1778-1783.
Assady S, Maor G, Amit M, et al. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691-1697.
Atala A. Use of non-autologous substances in VUR and incontinence treatment. Dial Pediatr Urol. 1994;17:11-12.
Atala A. Commentary on the replacement of urologic associated mucosa. J Urol. 1995;156:338.
Atala A. Tissue engineering in the genitourinary system. In: Atala A, Mooney DJ, editors. Tissue engineering. Boston: Birkhauser Press; 1997:149.
Atala A. Autologous cell transplantation for urologic reconstruction. J Urol. 1998;159:2-3.
Atala A. Engineering tissues, organs and cells. J Tissue Eng Regen Med. 2007;1:83-96.
Atala A, Bauer SB, Hendren WH, et al. The effect of gastric augmentation on bladder function. J Urol. 1993;149:1099-1102.
Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty, [see comment]. Lancet, 2006;367, 1241-1246.
Atala A, Cima LG, Kim W, et al. Injectable alginate seeded with chondrocytes as a potential treatment for vesicoureteral reflux. J Urol. 1993;150:745-747.
Atala A, Freeman MR, Vacanti JP, et al. Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle. J Urol. 1993;150:608-612.
Atala A, Guzman L, Retik AB. A novel inert collagen matrix for hypospadias repair. J Urol. 1999;162:1148-1151.
Atala A, Kim W, Paige KT, et al. Endoscopic treatment of vesicoureteral reflux with a chondrocyte-alginate suspension. J Urol. 1994;152:641-643. discussion 644
Atala A, Lanza R. Preface. In: Atala A, Lanza R, editors. Methods of tissue engineering. San Diego: Academic Press, 2001.
Atala A, Peters CA, Retik AB, et al. Endoscopic treatment of vesicoureteral reflux with a self-detachable balloon system. J Urol. 1992;148:724-727.
Atala A, Schlussel RN, Retik AB. Renal cell growth in vivo after attachment to biodegradable polymer scaffolds. J Urol. 1995;153:4.
Atala A, Vacanti JP, Peters CA, et al. Formation of urothelial structures in vivo from dissociated cells attached to biodegradable polymer scaffolds in vitro. J Urol. 1992;148:658-662.
Badylak SF, Lantz GC, Coffey A, et al. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74-80.
Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377-383.
Baker R, Kelly T, Tehan T, et al. Subtotal cystectomy and total bladder regeneration in treatment of bladder cancer. JAMA. 1958;168:1178-1185.
Ballas CB, Zielske SP, Gerson SL. Adult bone marrow stem cells for cell and gene therapies: implications for greater use. J Cell Biochem Suppl. 2002;38:20-28.
Baltaci S, Ozer G, Ozer E, et al. Failure of ureteral replacement with Gore-Tex tube grafts, [see comment]. Urology, 1998;51, 400-403.
Barrera DA, Zylstra E, Lansbury PT, et al. Synthesis and RGD peptide modification of a new biodegradable copolymer poly (lactic acid-co-lysine). J Am Chem Soc. 1993;115:11010-11011.
Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med. 1995;73:333-346.
Bazeed MA, Thuroff JW, Schmidt RA, et al. New treatment for urethral strictures. Urology. 1983;21:53-57.
Bellinger MF. Ureterocystoplasty: a unique method for vesical augmentation in children. J Urol. 1993;149:811-813.
Benahmed F, Gross I, Gaunt SJ, et al. Multiple regulatory regions control the complex expression pattern of the mouse Cdx2 homeobox gene. Gastroenterology. 2008;135:1238-1247.
Bent AE, Tutrone RT, McLennan MT, et al. Treatment of intrinsic sphincter deficiency using autologous ear chondrocytes as a bulking agent. Neurourol Urodyn. 2001;20:157-165.
Bergsma JE, Rozema FR, Bos RR, et al. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polyactide particles, [see comment]. Biomaterials, 1995;16, 267-274.
Boccaccini AR, Blaker JJ. Bioactive composite materials for tissue engineering scaffolds. Expert Rev Med Devices. 2005;2:303-317.
Boland T, Xu T, Damon B, et al. Application of inkjet printing to tissue engineering. Biotechnol J. 2006;1:910-917.
Brambrink T, Hochedlinger K, Bell G, et al. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc Natl Acad Sci U S A. 2006;103:933-938.
Bretan PN. History of prosthetic treatment of impotence. In: Montague DK, editor. Genitourinary prostheses. Philadelphia: WB Saunders; 1989:1-5.
Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation, [see comment]. N Engl J Med, 1994;331, 889-895.
Buckley JF, Scott R, Aitchison M. Periurethral microparticulate silicone injection for stress incontinence and vesicoureteric reflux. Minim Invasive Ther. 1997;1:72.
Byrne J, Pedersen D, Clepper L, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer 1. Nature. 2007;450:497-502.
Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. Expert Opin Biol Ther. 2007;7:1123-1127.
Cannon TW, Lee JY, Somogyi G, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62:958-963.
Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341-347.
Carley WW, Milici AJ, Madri JA. Extracellular matrix specificity for the differentiation of capillary endothelial cells. Exp Cell Res. 1988;178:426-434.
Carr LK, Steele D, Steele S, et al. 1-Year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J. 2008;19:881-883.
Carrel A, Lindbergh CA. The culture of whole organs. Science. 1935;81:621-623.
Cartwright PC, Snow BW. Bladder autoaugmentation: early clinical experience. J Urol. 1989;142:505-508. discussion 520–1
Cartwright PC, Snow BW. Bladder autoaugmentation: partial detrusor excision to augment the bladder without use of bowel. J Urol. 1989;142:1050-1053.
Cavallaro JF, Kemp PD, Kraus KH. Collagen fabrics as biomaterials. Biotechnol Bioeng. 1994;43:781-791.
Cen L, Liu W, Cui L, et al. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res. 2008;63:492-496.
Chancellor MB, Yokoyama T, Tirney S, et al. Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn. 2000;19:279-287.
Chen F, Yoo JJ, Atala A. Acellular collagen matrix as a possible “off the shelf” biomaterial for urethral repair. Urology. 1999;54:407-410.
Chen KL, Yoo JJ, Atala A. Total penile corpora cavernosa replacement using tissue engineering techniques. In: Regenerate 2005 Proceedings. April 2005. Atlanta, GA.
Choi JS, Lee SJ, Christ GJ, et al. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29:2899-2906.
Chun SY, Lim GJ, Kwon TG, et al. Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials. 2007;28:4251-4256.
Chung Y, Klimanskaya I, Becker S, et al. Embryonic and extraembryonic stem cell lines derived from single mouse blastomeres. Nature. 2006;439:216-219.
Churchill BM, Aliabadi H, Landau EH, et al. Ureteral bladder augmentation. J Urol. 1993;150:716-720.
Cilento BG, Atala A. Treatment of reflux and incontinence with autologous chondrocytes and bladder muscle cells. Dial Pediatr Urol. 1995;18:11.
Cilento BG, Freeman MR, Schneck FX, et al. Phenotypic and cytogenetic characterization of human bladder urothelia expanded in vitro. J Urol. 1994;152:665-670.
Cook AD, Hrkach JS, Gao NN, et al. Characterization and development of RGD-peptide-modified poly(lactic acid-co-lysine) as an interactive, resorbable biomaterial. J Biomed Mater Res. 1997;35:513-523.
Crisan M, Casteilla L, Lehr L, et al. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells. 2008;26:2425-2433.
da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287-2299.
Dahms SE, Piechota HJ, Dahiya R, et al. Composition and biomechanical properties of the bladder acellular matrix graft: comparative analysis in rat, pig and human. Br J Urol. 1998;82:411-419.
Dahms SE, Piechota HJ, Nunes L, et al. Free ureteral replacement in rats: regeneration of ureteral wall components in the acellular matrix graft. Urology. 1997;50:818-825.
Darland DC, D’Amore PA. Blood vessel maturation: vascular development comes of age, [comment]. J Clin Invest, 1999;103, 157-158.
de Boer WI, Schuller AG, Vermey M, et al. Expression of growth factors and receptors during specific phases in regenerating urothelium after acute injury in vivo. Am J Pathol. 1994;145:1199-1207.
De Coppi P, Bartsch GJr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy, [see comment]. Nat Biotechnol, 2007;25, 100-106.
De Coppi P, Callegari A, Chiavegato A, et al. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol. 2007;177:369-376.
De Coppi P, Delo D, Farrugia L, et al. Angiogenic gene-modified muscle cells for enhancement of tissue formation. Tissue Eng. 2005;11:1034-1044.
De Filippo RE, Bishop CE, Filho LF, et al. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue, [erratum appears in Transplantation 2008;86(5):751; note: De Philippo, Roger E {corrected to De Filippo, Roger E}]. Transplantation, 2008;86, 208-214.
De Filippo RE, Yoo JJ, Atala A. Urethral replacement using cell seeded tubularized collagen matrices. J Urol. 2002;168:1789-1792. discussion 1792–1783
De Filippo RE, Yoo JJ, Atala A. Engineering of vaginal tissue in vivo. Tissue Eng. 2003;9:301-306.
Dekel B, Burakova T, Arditti FD, et al. Human and porcine early kidney precursors as a new source for transplantation. Nat Med. 2003;9:53-60.
Delo DM, Eberli D, Williams JK, et al. Angiogenic gene modification of skeletal muscle cells to compensate for ageing-induced decline in bioengineered functional muscle tissue. BJU Int. 2008;102:878-884.
Deuel TF. Growth factors. In: Lanza R, Langer R, Chick WL, editors. Principles of tissue engineering. New York: Academic Press; 1997:133-149.
Devine SM. Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem Suppl. 2002;38:73-79.
Diamond DA, Caldamone AA. Endoscopic correction of vesicoureteral reflux in children using autologous chondrocytes: preliminary results. J Urol. 1999;162:1185-1188.
Dorin RP, Pohl HG, De Filippo RE, et al. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration? World J Urol. 2008;26:323-326.
Dozmorov MG, Kropp BP, Hurst RE, et al. Differentially expressed gene networks in cultured smooth muscle cells from normal and neuropathic bladder. J Smooth Muscle Res. 2007;43:55-72.
Drake CJ, Hungerford JE, Little CD. Morphogenesis of the first blood vessels. Ann N Y Acad Sci. 1998;857:155-179.
Duan X, Chang JH, Ge S, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain, [see comment]. Cell, 2007;130, 1146-1158.
Eberli D, Andersson KE, Yoo J, et al. A canine model of irreversible urethral sphincter insufficiency. BJU Int. 2008;103:248-253.
Eberli D, Susaeta R, Yoo J, et al. A method to improve cellular content for corporal tissue engineering. Tissue Eng Part A. 2008;14:1581-1589.
Eggan K, Baldwin K, Tackett M, et al. Mice cloned from olfactory sensory neurons. Nature. 2004;428:44-49.
Eiselt P, Kim BS, Chacko B, et al. Development of technologies aiding large-tissue engineering. Biotechnol Prog. 1998;14:134-140.
El Kassaby A, Aboushwareb T, Atala A. Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol. 2008;179:1432-1436.
El-Kassaby AW, Retik AB, Yoo JJ, et al. Urethral stricture repair with an off-the-shelf collagen matrix. J Urol. 2003;169:170-173. discussion 173
Falke G, Yoo JJ, Kwon TG, et al. Formation of corporal tissue architecture in vivo using human cavernosal muscle and endothelial cells seeded on collagen matrices. Tissue Eng. 2003;9:871-879.
Farhat WA, Yeger H. Does mechanical stimulation have any role in urinary bladder tissue engineering? World J Urol. 2008;26:301-305.
Faris RA, Konkin T, Halpert G. Liver stem cells: a potential source of hepatocytes for the treatment of human liver disease, [see comment]. Artif Organs, 2001;25, 513-521.
Fauza DO, Fishman SJ, Mehegan K, et al. Videofetoscopically assisted fetal tissue engineering: bladder augmentation. J Pediatr Surg. 1998;33:7-12.
Fauza DO, Fishman SJ, Mehegan K, et al. Videofetoscopically assisted fetal tissue engineering: skin replacement. J Pediatr Surg. 1998;33:357-361.
Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138:745-753.
Folkman J, Brem H. Angiogenesis and inflammation. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: basic principles and clinical correlates. New York: Raven Press; 1992:821-839.
Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis?, [comment]. Cell, 1996;87, 1153-1155.
Fontaine M, Schloo B, Jenkins R, et al. Human hepatocyte isolation and transplantation into an athymic rat, using prevascularized cell polymer constructs. J Pediatr Surg. 1995;30:56-60.
Freed LE, Vunjak-Novakovic G, Biron RJ, et al. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (NY). 1994;12:689-693.
Freeman MR, Yoo JJ, Raab G, et al. Heparin-binding EGF-like growth factor is an autocrine growth factor for human urothelial cells and is synthesized by epithelial and smooth muscle cells in the human bladder. J Clin Invest. 1997;99:1028-1036.
Frumpkin AP. Reconstruction of male genitalia. Am Rev Sov Med. 1944;2:14.
Fu Q, Deng CL, Liu W, et al. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007;99:1162-1165.
Fung LCT, Elenius K, Freeman M, Donovan MJ, Atala A. Reconstitution of poor EGFr-poor renal epithelial cells into tubular structures on biodegradable polymer scaffold. Pediatrics. 1996;98(Suppl.):S631.
Furthmayr H, Timpl R. Immunochemistry of collagens and procollagens. Int Rev Connect Tissue Res. 1976;7:61-99.
Geber S, Sampaio M. Blastomere development after embryo biopsy: a new model to predict embryo development and to select for transfer. Hum Reprod. 1999;14:782-786.
Gilding D. Biodegradable polymers. In: Williams D, editor. Biocompatibility of clinical implant materials. Boca Raton, FL: CRC Press; 1981:209-232.
Goodwin WE, Scott WW. Phalloplasty. J Urol. 1952;68:903-908.
Gorham SD, French DA, Shivas AA, et al. Some observations on the regeneration of smooth muscle in the repaired urinary bladder of the rabbit. Eur Urol. 1989;16:440-443.
Guan Y, Ou L, Hu G, et al. Tissue engineering of urethra using human vascular endothelial growth factor gene-modified bladder urothelial cells. Artif Organs. 2008;32:91-99.
Guild WR, Harrison JH, Merrill JP, et al. Successful homotransplantation of the kidney in an identical twin. Trans Am Clin Climatol Assoc. 1955;67:167-173.
Gupta S, Verfaillie C, Chmielewski D, et al. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62:1285-1290.
Haller H, de Groot K, Bahlmann F, et al. Stem cells and progenitor cells in renal disease. Kidney Int. 2005;68:1932-1936.
Hammerman MR. Xenotransplantation of developing kidneys. Am J Physiol Renal Physiol. 2002;283:F601-F606.
Hammerman MR. Transplantation of embryonic kidneys. Clin Sci (Lond). 2002;103:599-612.
Han D, Gouma PI. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomedicine. 2006;2:37-41.
Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364.
Hardy K. Preimplantation embryo development. Bavister B. Springer, New York, 1993, 184-199.
Harrington DA, Cheng EY, Guler MO, et al. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J Biomed Mater Res A. 2006;78:157-167.
Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42:396-402.
Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28:344-353.
Harriss DR. Smooth muscle cell culture: a new approach to the study of human detrusor physiology and pathophysiology. Br J Urol. 1995;75(Suppl. 1):18-26.
Haseltine W. A brave new medicine. A conversation with William Haseltine. Interview by Joe Flower. Health Forum J. 1999;42:28-30.
Hipp J, Andersson KE, Kwon TG, et al. Microarray analysis of exstrophic human bladder smooth muscle. BJU Int. 2008;101:100-105.
Hochedlinger K, Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002;415:1035-1038.
Hristov M, Zernecke A, Schober A, et al. Adult progenitor cells in vascular remodeling during atherosclerosis. Biol Chem. 2008;389:837-844.
Huard J, Yokoyama T, Pruchnic R, et al. Muscle-derived cell-mediated ex vivo gene therapy for urological dysfunction. Gene Ther. 2002;9:1617-1626.
Hubbell JA, Massia SP, Desai NP, et al. Endothelial cell-selective materials for tissue engineering in the vascular graft via a new receptor. Biotechnology (NY). 1991;9:568-572.
Humes HD, Cieslinski DA. Interaction between growth factors and retinoic acid in the induction of kidney tubulogenesis in tissue culture. Exp Cell Res. 1992;201:8-15.
Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311-325.
Hurlbut WB. Altered nuclear transfer as a morally acceptable means for the procurement of human embryonic stem cells. Perspect Biol Med. 2005;48:211-228.
Hwang WS, Ryu YJ, Park JH, et al. Evidence of a pluripotent human embryonic stem cell line derived from a cloned blastocyst. Science. 2004;303:1669-1674.
Hwang WS, Roh SI, Lee BC, et al. Patient-specific embryonic stem cells derived from human SCNT blastocysts. Science. 2005;308:1777-1783.
Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11-25.
Ikarashi K, Li B, Suwa M, et al. Bone marrow cells contribute to regeneration of damaged glomerular endothelial cells. Kidney Int. 2005;67:1925-1933.
Ikeda E, Yagi K, Kojima M, et al. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495-505.
Ikeguchi EF, Stifelman MD, Hensle TW. Ureteral tissue expansion for bladder augmentation. J Urol. 1998;159:1665-1668.
Ilkhanizadeh S, Teixeira AI, Hermanson O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials. 2007;28:3936-3943.
in ’t Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845-852.
in ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548-1549.
Intveld PJA, Shen ZR, Takens GAJ. Glycine glycolic acid based copolymers. J Polym Sci Polym Chem. 1994;32:1063-1069.
Ito T, Suzuki A, Imai E, et al. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol. 2001;12:2625-2635.
Itskovitz-Eldor J, Schuldiner M, Karsenti D, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88-95.
Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341-350.
Jack GS, Almeida FG, Zhang R, et al. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol. 2005;174:2041-2045.
Jayo MJ, Jain D, Ludlow JW, et al. Long-term durability, tissue regeneration and neo-organ growth during skeletal maturation with a neo-bladder augmentation construct. Regen Med. 2008;3:671-682.
Jayo MJ, Jain D, Wagner BJ, et al. Early cellular and stromal responses in regeneration versus repair of a mammalian bladder using autologous cell and biodegradable scaffold technologies, [see comment]. J Urol, 2008;180, 392-397.
Jensen UB, Yan X, Triel C, et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci. 2008;121:609-617.
Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow, [see comment]; [erratum appears in Nature 2007;447(7146):879–880]. Nature, 2002;418, 41-49.
Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26:1221-1230.
Jordan GH. Penile reconstruction, phallic construction, and urethral reconstruction. Urol Clin North Am. 1999;26:1-13.
Kaefer M, Hendren WH, Bauer SB, et al. Reservoir calculi: a comparison of reservoirs constructed from stomach and other enteric segments, [see comment]. J Urol, 1998;160, 2187-2190.
Kaefer M, Tobin MS, Hendren WH, et al. Continent urinary diversion: the Children’s Hospital experience. J Urol. 1997;157:1394-1399.
Kajbafzadeh AM, Elmi A, Payabvash S, et al. Transurethral autologous myoblast injection for treatment of urinary incontinence in children with classic bladder exstrophy. J Urol. 2008;180:1098-1105.
Kale S, Karihaloo A, Clark PR, et al. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42-49.
Kanatsu-Shinohara M, Miki H, Inoue K, et al. Germline niche transplantation restores fertility in infertile mice. Hum Reprod. 2005;20:2376-2382.
Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716-10721.
Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes, [see comment]. J Clin Invest, 2001;108, 407-414.
Kershen RT, Atala A. New advances in injectable therapies for the treatment of incontinence and vesicoureteral reflux. Urol Clin North Am. 1999;26:81-94.
Kershen RT, Yoo JJ, Moreland RB, et al. Reconstitution of human corpus cavernosum smooth muscle in vitro and in vivo. Tissue Eng. 2002;8:515-524.
Kikuno N, Kawamoto K, Hirata H, et al. Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int. 2008;103:1424-1428.
Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16:224-230.
Kim BS, Yoo JJ, Atala A. Engineering of human cartilage rods: potential application for penile prostheses. J Urol. 2002;168:1794-1797.
Kolambkar YM, Peister A, Soker S, et al. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol. 2007;38:405-413.
Kropp BP, Rippy MK, Badylak SF, et al. Regenerative urinary bladder augmentation using small intestinal submucosa: urodynamic and histopathologic assessment in long-term canine bladder augmentations. J Urol. 1996;155:2098-2104.
Kropp BP, Sawyer BD, Shannon HE, et al. Characterization of small intestinal submucosa regenerated canine detrusor: assessment of reinnervation, in vitro compliance and contractility. J Urol. 1996;156:599-607.
Kropp BP, Cheng EY, Lin HK, et al. Reliable and reproducible bladder regeneration using unseeded distal small intestinal submucosa. J Urol. 2004;172:1710-1713.
Kurzrock EA, Lieu DK, Degraffenried LA, et al. Label-retaining cells of the bladder: candidate urothelial stem cells. Am J Physiol Renal Physiol. 2008;294:F1415-F1421.
Kwon D, Kim Y, Pruchnic R, et al. Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology. 2006;68:449-454.
Kwon TG, Yoo JJ, Atala A. Autologous penile corpora cavernosa replacement using tissue engineering techniques. J Urol. 2002;168:1754-1758.
Kwon TG, Yoo JJ, Atala A. Local and systemic effects of a tissue engineered neobladder in a canine cystoplasty model. J Urol. 2008;179:2035-2041.
Laflamme MA, Gold J, Xu C, et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663-671.
Lai JY, Yoon CY, Yoo JJ, et al. Phenotypic and functional characterization of in vivo tissue engineered smooth muscle from normal and pathological bladders. J Urol. 2002;168:1853-1857. discussion 1858
Lailas NG, Cilento B, Atala A. Progressive ureteral dilation for subsequent ureterocystoplasty. J Urol. 1996;156:1151-1153.
Landman J, Olweny E, Sundaram CP, et al. Laparoscopic mid sagittal hemicystectomy and bladder reconstruction with small intestinal submucosa and reimplantation of ureter into small intestinal submucosa: 1-year followup. J Urol. 2004;171:2450-2455.
Landry DW, Zucker HA. Embryonic death and the creation of human embryonic stem cells. J Clin Invest. 2004;114:1184-1186.
Lanza RP, Chung HY, Yoo JJ, et al. Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol. 2002;20:689-696.
Lanza RP, Chung HY, Yoo JJ, et al. Generation of histocompatible tissues using nuclear transplantation, [see comment]. Nat Biotechnol, 2002;20, 689-696.
le Roux PJ. Endoscopic urethroplasty with unseeded small intestinal submucosa collagen matrix grafts: a pilot study. J Urol. 2005;173:140-143.
Lee JY, Cannon TW, Pruchnic R, et al. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J. 2003;14:31-37. discussion 37
Lee SJ, Liu J, Oh SH, et al. Development of a composite vascular scaffolding system that withstands physiological vascular conditions. Biomaterials. 2008;29:2891-2898.
Lee SJ, Oh SH, Liu J, et al. The use of thermal treatments to enhance the mechanical properties of electrospun poly(epsilon-caprolactone) scaffolds. Biomaterials. 2008;29:1422-1430.
Lee SJ, Van Dyke M, Atala A, et al. Host cell mobilization for in situ tissue regeneration. Rejuvenation Res. 2008;11:747-756.
Lee SJ, Yoo JJ, Lim GJ, et al. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J Biomed Mater Res Part A. 2007;83:999-1008.
Levenberg S, Golub JS, Amit M, et al. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391-4396.
Levesque PE, Bauer SB, Atala A, et al. Ten-year experience with the artificial urinary sphincter in children. J Urol. 1996;156:625-628.
Li C, Xu Y, Song L, et al. Preliminary experimental study of tissue-engineered urethral reconstruction using oral keratinocytes seeded on BAMG. Urol Int. 2008;81:290-295.
Li ST. Biologic biomaterials: tissue derived biomaterials (collagen). In: Bronzino JD, editor. The biomedical engineering handbook. Boca Raton, FL: CRS Press; 1995:627-647.
Liebert M, Hubbel A, Chung M, et al. Expression of mal is associated with urothelial differentiation in vitro: identification by differential display reverse-transcriptase polymerase chain reaction. Differentiation. 1997;61:177-185.
Liebert M, Wedemeyer G, Abruzzo LV, et al. Stimulated urothelial cells produce cytokines and express an activated cell surface antigenic phenotype. Semin Urol. 1991;9:124-130.
Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908-910.
Lin F. Stem cells in kidney regeneration following acute renal injury. Pediatr Res. 2006;59:74R-78R.
Lin F. Renal repair: role of bone marrow stem cells. Pediatr Nephrol. 2008;23:851-861.
Lin F, Cordes K, Li L, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188-1199.
Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756-1764.
Lin HK, Cowan R, Moore P, et al. Characterization of neuropathic bladder smooth muscle cells in culture. J Urol. 2004;171:1348-1352.
Lo KC, Lei Z, Rao Ch V, et al. De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of Leydig stem cells. Endocrinology. 2004;145:4011-4015.
Lo KC, Brugh VM, Parker M, et al. Isolation and enrichment of murine spermatogonial stem cells using rhodamine 123 mitochondrial dye. Biol Reprod. 2005;72:767-771.
Lobban ED, Smith BA, Hall GD, et al. Uroplakin gene expression by normal and neoplastic human urothelium. Am J Pathol. 1998;153:1957-1967.
Loges S, Roncal C, Carmeliet P. Development of targeted angiogenic medicine. J Thromb Haemost. 2009;7:21-33.
Luttun A, Ross JJ, Verfaillie C, et al. Differentiation of multipotent adult progenitor cells into functional endothelial and smooth muscle cells. Curr Protoc Immunol. 2006. Chapter 22: Unit 22F.9
Machluf M, Atala A. Emerging concepts for tissue and organ transplantation. Graft. 1998;1:31-37.
Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634-7638.
Martinez F, Rienzi L, Iacobelli M, et al. Caspase activity in preimplantation human embryos is not associated with apoptosis. Hum Reprod. 2002;17:1584-1590.
Master VA, Wei G, Liu W, et al. Urothlelium facilitates the recruitment and trans-differentiation of fibroblasts into smooth muscle in acellular matrix. J Urol. 2003;170:1628-1632.
Matsunuma H, Kagami H, Narita Y, et al. Constructing a tissue-engineered ureter using a decellularized matrix with cultured uroepithelial cells and bone marrow-derived mononuclear cells. Tissue Eng. 2006;12:509-518.
McDougal WS. Metabolic complications of urinary intestinal diversion. J Urol. 1992;147:1199-1208.
Meissner A, Jaenisch R. Generation of nuclear transfer-derived pluripotent ES cells from cloned Cdx2-deficient blastocysts. Nature. 2006;439:212-215.
Mikos AG, Leite SM, Vacanti JP. Prevascularization of porous biodegradable polymers. Biotech Bioeng. 1993;42:716-723.
Mikos AG, Lyman MD, Freed LE, et al. Wetting of poly(L-lactic acid) and poly(DL-lactic-co-glycolic acid) foams for tissue culture. Biomaterials. 1994;15:55-58.
Milici AJ, Furie MB, Carley WW. The formation of fenestrations and channels by capillary endothelium in vitro. Proc Natl Acad Sci U S A. 1985;82:6181-6185.
Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4:27-49.
Mitalipov S. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum Reprod. 2007;22:2232-2242.
Mitterberger M, Marksteiner R, Margreiter E, et al. Autologous myoblasts and fibroblasts for female stress incontinence: a 1-year follow-up in 123 patients. BJU Int. 2007;100:1081-1085.
Mitterberger M, Pinggera GM, Marksteiner R, et al. Functional and histological changes after myoblast injections in the porcine rhabdosphincter, [see comment]; [erratum appears in Eur Urol 2008;54(5):1208; note: Bartsch, Georg {removed}]. Eur Urol, 2007;52, 1736-1743.
Mooney DJ, Mikos AG. Growing new organs. Sci Am. 1999;280:60-65.
Murray MM, Forsythe B, Chen F, et al. The effect of thrombin on ACL fibroblast interactions with collagen hydrogels. J Orthop Res. 2006;24:508-515.
Nagano M, Brinster RL. Spermatogonial transplantation and reconstitution of donor cell spermatogenesis in recipient mice. APMIS. 1998;106:47-55. discussion 56–47
Nakamura M, Kobayashi A, Takagi F, et al. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005;11:1658-1666.
Nguyen HT, Park JM, Peters CA, et al. Cell-specific activation of the HB-EGF and ErbB1 genes by stretch in primary human bladder cells. In Vitro Cell Dev Biol Anim. 1999;35:371-375.
Nguyen MM, Lieu DK, deGraffenried LA, et al. Urothelial progenitor cells: regional differences in the rat bladder. Cell Prolif. 2007;40:157-165.
Nolen-Walston RD, Kim CF, Mazan MR, et al. Cellular kinetics and modeling of bronchioalveolar stem cell response during lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1158-L1165.
Nukui F, Okamoto S, Nagata M, et al. Complications and reimplantation of penile implants. Int J Urol. 1997;4:52-54.
Oberpenning F, Meng J, Yoo JJ, et al. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering, [see comment]. Nat Biotechnol, 1999;17, 149-155.
Ogawa T, Dobrinski I, Avarbock MR, et al. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29-34.
Olsen L, Bowald S, Busch C, et al. Urethral reconstruction with a new synthetic absorbable device. An experimental study. Scand J Urol Nephrol. 1992;26:323-326.
Oottamasathien S, Wang Y, Williams K, et al. Directed differentiation of embryonic stem cells into bladder tissue. Dev Biol. 2007;304:556-566.
Osman Y, Shokeir A, Gabr M, et al. Canine ureteral replacement with long acellular matrix tube: is it clinically applicable? J Urol. 2004;172:1151-1154.
Pariente JL, Kim BS, Atala A. In vitro biocompatibility assessment of naturally derived and synthetic biomaterials using normal human urothelial cells. J Biomed Mater Res. 2001;55:33-39.
Park HJ, Yoo JJ, Kershen RT, et al. Reconstitution of human corporal smooth muscle and endothelial cells in vivo. J Urol. 1999;162:1106-1109.
Peppas NA, Langer R. New challenges in biomaterials, [see comment]. Science, 1994;263, 1715-1720.
Perin L, Giuliani S, Jin D, et al. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007;40:936-948.
Phelps EA, Garcia AJ. Update on therapeutic vascularization strategies. Regen Med. 2009;4:65-80.
Piechota HJ, Dahms SE, Nunes LS, et al. In vitro functional properties of the rat bladder regenerated by the bladder acellular matrix graft. J Urol. 1998;159:1717-1724.
Polgar K, Adany R, Abel G, et al. Characterization of rapidly adhering amniotic fluid cells by combined immunofluorescence and phagocytosis assays. Am J Hum Genet. 1989;45:786-792.
Ponder KP, Gupta S, Leland F, et al. Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proc Natl Acad Sci U S A. 1991;88:1217-1221.
Portis AJ, Elbahnasy AM, Shalhav AL, et al. Laparoscopic augmentation cystoplasty with different biodegradable grafts in an animal model. J Urol. 2000;164:1405-1411.
Poulsom R, Forbes SJ, Hodivala-Dilke K, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229-235.
Priest RE, Marimuthu KM, Priest JH. Origin of cells in human amniotic fluid cultures: ultrastructural features. Lab Invest. 1978;39:106-109.
Probst M, Dahiya R, Carrier S, et al. Reproduction of functional smooth muscle tissue and partial bladder replacement. Br J Urol. 1997;79:505-515.
Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74.
Puthenveettil JA, Burger MS, Reznikoff CA. Replicative senescence in human uroepithelial cells. Adv Exp Med Biol. 1999;462:83-91.
Rackley RR, Bandyopadhyay SK, Fazeli-Matin S, et al. Immunoregulatory potential of urothelium: characterization of NF-kappaB signal transduction. J Urol. 1999;162:1812-1816.
Raya-Rivera AM, Baez C, Atala A, et al. Tissue engineered testicular prostheses with prolonged testosterone release. World J Urol. 2008;26:307-314.
Reubinoff BE, Itsykson P, Turetsky T, et al. Neural progenitors from human embryonic stem cells, [see comment]. Nat Biotechnol, 2001;19, 1134-1140.
Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro, [see comment]; [erratum appears in Nat Biotechnol 2000;18(5):559]. Nat Biotechnol, 2000;18, 399-404.
Riehmann M, Gasser TC, Bruskewitz RC. The Hydroflex penile prosthesis: a test case for the introduction of new urological technology. J Urol. 1993;149:1304-1307.
Rodriguez LV, Alfonso Z, Zhang R, et al. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:12167-12172.
Rogers SA, Lowell JA, Hammerman NA, et al. Transplantation of developing metanephroi into adult rats. Kidney Int. 1998;54:27-37.
Rogers SA, Powell-Braxton L, Hammerman MR. Insulin-like growth factor I regulates renal development in rodents. Dev Genet. 1999;24:293-298.
Rookmaaker MB, Smits AM, Tolboom H, et al. Bone-marrow-derived cells contribute to glomerular endothelial repair in experimental glomerulonephritis. Am J Pathol. 2003;163:553-562.
Rosines E, Sampogna RV, Johkura K, et al. Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci U S A. 2007;104:20938-20943.
Roth EA, Xu T, Das M, et al. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707-3715.
Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45-53.
Sams AE, Nixon AJ. Chondrocyte-laden collagen scaffolds for resurfacing extensive articular cartilage defects. Osteoarthritis Cartilage. 1995;3:47-59.
Satar N, Yoo JJ, Atala A. Progressive dilation for bladder tissue expansion. J Urol. 1999;162:829-831.
Schena FP. Role of growth factors in acute renal failure. Kidney Int Suppl. 1998;66:S11-S15.
Schuldiner M, Eiges R, Eden A, et al. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001;913:201-205.
Scriven SD, Booth C, Thomas DF, et al. Reconstitution of human urothelium from monolayer cultures. J Urol. 1997;158:1147-1152.
Shalhav AL, Elbahnasy AM, Bercowsky E, et al. Laparoscopic replacement of urinary tract segments using biodegradable materials in a large-animal model. J Endourol. 1999;13:241-244.
Shinohara T, Orwig KE, Avarbock MR, et al. Restoration of spermatogenesis in infertile mice by Sertoli cell transplantation. Biol Reprod. 2003;68:1064-1071.
Shukla D, Box GN, Edwards RA, et al. Bone marrow stem cells for urologic tissue engineering. World J Urol. 2008;26:341-349.
Sievert KD, Bakircioglu ME, Nunes L, et al. Homologous acellular matrix graft for urethral reconstruction in the rabbit: histological and functional evaluation. J Urol. 2000;163:1958-1965.
Silver FH, Pins G. Cell growth on collagen: a review of tissue engineering using scaffolds containing extracellular matrix. J Long Term Eff Med Implants. 1992;2:67-80.
Simerly C, Dominko T, Navara C, et al. Molecular correlates of primate nuclear transfer failures. Science. 2003;300:297.
Small MP, Carrion HM, Gordon JA. Small-Carrion penile prosthesis. New implant for management of impotence. Urology. 1975;5:479-486.
Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71-78.
Solomon LZ, Jennings AM, Sharpe P, et al. Effects of short-chain fatty acids on primary urothelial cells in culture: implications for intravesical use in enterocystoplasties, [see comment]. J Lab Clin Med, 1998;132, 279-283.
Springer ML, Chen AS, Kraft PE, et al. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell. 1998;2:549-558.
Steer DL, Bush KT, Meyer TN, et al. A strategy for in vitro propagation of rat nephrons. Kidney Int. 2002;62:1958-1965.
Stosich MS, Moioli EK, Wu JK, et al. Bioengineering strategies to generate vascularized soft tissue grafts with sustained shape. Methods. 2009;47:116-121.
Strasser H, Berjukow S, Marksteiner R, et al. Stem cell therapy for urinary stress incontinence. Exp Gerontol. 2004;39:1259-1265.
Strasser H, Marksteiner R, Margreiter E, et al. Autologous myoblasts and fibroblasts versus collagen for treatment of stress urinary incontinence in women: a randomised controlled trial, [see comment]; [erratum appears in Lancet 2008;371(9611):474]; [retraction in Kleinert S, Horton R. Lancet 2008 6;372(9641):789–90; PMID: 18774408]. Lancet, 2007;369, 2179-2186.
Sutherland RS, Baskin LS, Hayward SW, et al. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J Urol. 1996;156:571-577.
Tachibana M, Nagamatsu GR, Addonizio JC. Ureteral replacement using collagen sponge tube grafts. J Urol. 1985;133:866-869.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
Taupin P. Therapeutic potential of adult neural stem cells. Recent Patents CNS Drug Discov. 2006;1:299-303.
Thomalla JV, Thompson ST, Rowland RG, et al. Infectious complications of penile prosthetic implants. J Urol. 1987;138:65-67.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts, [see comment]; [erratum appears in Science 1998;282(5395):1827]. Science, 1998;282, 1145-1147.
Tobin MS, Freeman MR, Atala A. Maturational response of normal human urothelial cells in culture is dependent on extracellular matrix and serum additives. Surg Forum. 1994;45:786.
Tsai MS, Lee JL, Chang YJ, et al. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450-1456.
Vaught JD, Kropp BP, Sawyer BD, et al. Detrusor regeneration in the rat using porcine small intestinal submucosal grafts: functional innervation and receptor expression. J Urol. 1996;155:374-378.
Vauhkonen M, Vauhkonen H, Sipponen P. Helicobacter pylori infection induces a reversible expression of the CDX2 transcription factor protein in human gastric epithelium, [see comment]. Scand J Gastroenterol, 2008;43, 915-921.
Wang T, Koh C, Yoo JJ. Creation of an engineered uterus for surgical reconstruction. In: Proceedings of the American Academy of Pediatrics section on urology. New Orleans; 2003.
Warburton D, Perin L, Defilippo R, et al. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc Am Thoracic Soc. 2008;5:703-706.
Wefer J, Sievert KD, Schlote N, et al. Time dependent smooth muscle regeneration and maturation in a bladder acellular matrix graft: histological studies and in vivo functional evaluation. J Urol. 2001;165:1755-1759.
Wefer J, Sekido N, Sievert KD, et al. Homologous acellular matrix graft for vaginal repair in rats: a pilot study for a new reconstructive approach. World J Urol. 20, 2002. 260–213
Weiner LP. Definitions and criteria for stem cells. Methods Mol Biol. 2008;438:3-8.
Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318-324.
Wilmut I, Schnieke AE, McWhir J, et al. Viable offspring derived from fetal and adult mammalian cells, [see comment]; [erratum appears in Nature 1997;386(6621):200]. Nature, 1997;385, 810-813.
Wolter JR, Meyer RF. Sessile macrophages forming clear endothelium-like membrane on the inside of successful keratoprosthesis. Graefes Arch Clin Exp Ophthalmol. 1985;222:109-117.
Xu T, Rohozinski J, Zhao W, et al. Inkjet-mediated gene transfection into living cells combined with targeted delivery. Tissue Eng Part A. 2009;15:95-101.
Yancopoulos GD, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border, [comment]. Cell, 1998;93, 661-664.
Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res. 1980;14:65-81.
Yannas IV, Burke JF, Gordon PL, et al. Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res. 1980;14:107-132.
Yiou R, Lefaucheur JP, Atala A. The regeneration process of the striated urethral sphincter involves activation of intrinsic satellite cells. Anat Embryol (Berl). 2003;206:429-435.
Yiou R, Yoo JJ, Atala A. Restoration of functional motor units in a rat model of sphincter injury by muscle precursor cell autografts. Transplantation. 2003;76:1053-1060.
Yokoo T, Ohashi T, Shen JS, et al. Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci U S A. 2005;102:3296-3300.
Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress urinary incontinence and bladder dysfunction. World J Urol. 2000;18:56-61.
Yoo J, Ashkar S, Atala A. Creation of functional kidney structures with excretion of kidney-like fluid in vivo. Pediatrics. 1996;98S:605.
Yoo J, Satar N, Retik AB, et al. Ureteral replacement using biodegradable polymer scaffolds seeded with urothelial and smooth muscle cells. J Urol. 1995;153:375A.
Yoo JJ, Atala A. A novel gene delivery system using urothelial tissue engineered neo-organs. J Urol. 1997;158:1066-1070.
Yoo JJ, Lee I, Atala A. Cartilage rods as a potential material for penile reconstruction. J Urol. 1998;160:1164-1168. discussion 1178
Yoo JJ, Lee JE, Kim HJ, et al. Comparative in vitro and in vivo studies using a bioactive poly(epsilon-caprolactone)-organosiloxane nanohybrid containing calcium salt. J Biomed Mater Res Part B Appl Biomater. 2007;83:189-198.
Yoo JJ, Meng J, Oberpenning F, et al. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. 1998;51:221-225.
Yoo JJ, Park HJ, Lee I, et al. Autologous engineered cartilage rods for penile reconstruction. J Urol. 1999;162:1119-1121.
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920.
Zhang SC, Wernig M, Duncan ID, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells, [see comment]. Nat Biotechnol, 2001;19, 1129-1133.
Zhang X, Stojkovic P, Przyborski S, et al. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669-2676.
Zhang Y, Frimberger D, Cheng EY, et al. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006;98:1100-1105.
Zhang Y. Bladder reconstruction by tissue engineering—with or without cells?, [comment]. J Urol, 2008;180, 10-11.