chapter 20 Male Reproductive Physiology
The male reproductive axis of hormones and organs is a remarkably efficient, well-orchestrated, and precisely managed biological system that has evolved over millions of years. It is responsible for reproductive tract formation and development, maturation of fertility potential at puberty, and the maintenance of maleness in the adult. This chapter explores our current understanding of this complex system by defining the anatomy and physiology of its component parts, including the hypothalamic-pituitary-gonadal hormonal axis, spermatogenesis and androgen production within the testicle, and maturation and transport of sperm in the excurrent ductal system. In addition, new concepts in genetic infertility, stem cell science, and the physiology of ejaculation are introduced and explained. Through such rigorous intellectual dissection, the true beauty and sophistication of the reproductive process is realized and appreciated.
Hypothalamic-Pituitary-Gonadal Axis
The hypothalamic-pituitary-gonadal (HPG) axis plays a critical role during development and adulthood in four physiological processes: 1) phenotypic gender development during embryogenesis, 2) sexual maturation at puberty, 3) testis endocrine function—testosterone production, and 4) testis exocrine function—sperm production.
Basic Endocrine Concepts
Two classes of hormones mediate communication in the reproductive axis: peptide and steroid. Peptide hormones are small, secretory proteins that act through cell surface receptors. Hormone signals are transduced by one of several second-messenger pathways (Fig. 20–1). Ultimately, most peptide hormones induce phosphorylation of proteins that alter cell function. Examples of peptide hormones are luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In contrast, steroid hormones are derived from cholesterol. They are not stored in secretory granules; consequently, steroid secretion rates directly reflect rates of production. In plasma, these hormones are usually bound to carrier proteins, and because they are lipophilic, steroid hormones are cell membrane–permeable. After binding to intracellular receptors, steroids are translocated to nuclear DNA recognition sites and regulate the transcription of target genes. Examples of reproductive axis steroid hormones are testosterone and estradiol.
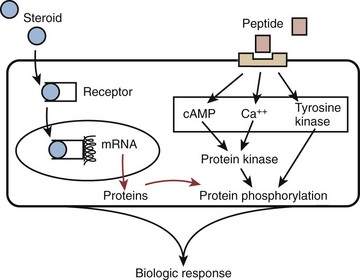
Figure 20–1 Two kinds of hormones classes mediate intercellular communication in the reproductive hormone axis: peptide and steroid.
(Adapted from Turek PJ. Male infertility. In: Tanagho EA, McAninch JC, editors. Smith’s urology. 16th ed. Stamford, CT: Appleton & Lange; 2008 [chapter 44–1].)
Hormonal signaling within the HPG axis is hierarchically governed by a free-running pulse generator within the hypothalamus. The specific amplitude and frequency with which hormone secretions occur within the reproductive axis determine downstream organ responsiveness. Feedback control is the principal mechanism through which hormonal regulation occurs in the HPG axis (Fig. 20–2). With feedback control, a hormone can regulate the synthesis and action of itself or of another hormone. In the HPG axis, negative feedback activity is primarily responsible for minimizing hormonal perturbations and maintaining homeostasis.
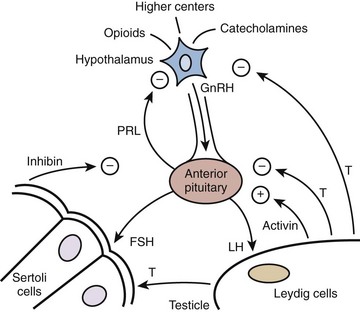
Figure 20–2 Diagram of the hypothalamic-pituitary-testis hormonal axis. +, positive feedback; −, negative feedback; FSH, follicle-stimulating hormone; GnRH, gonadoatrophin releasing hormone; LH, luteinizing hormone; PRL, prolactin; T, testosterone.
(Adapted from Turek PJ. Male infertility. In: Tanagho EA, McAninch JC, editors. Smith’s urology. 16th ed. Stamford, CT: Appleton & Lange; 2008 [chapter 44–2].)
Components of the Reproductive Axis
Hypothalamus
As the integrative center of the HPG axis, the hypothalamus receives neuronal input from the amygdala, thalamus, pons, retina, olfactory cortex, and many other areas (see Fig. 20–2). The pulse generator for the cyclical secretion of pituitary hormones, the hypothalalmus, is anatomically linked to the pituitary gland by both a portal vascular system and neuronal pathways. By avoiding the systemic circulation, the portal vascular system allows direct delivery of hypothalamic hormones to the anterior pituitary.
The most important hypothalamic hormone for reproduction is gonadotropin-releasing or LH-releasing hormone (GnRH or LHRH), a 10–amino acid peptide secreted from the neuronal cell bodies in the preoptic and arcuate nuclei. Currently, the only known function of GnRH is to stimulate the secretion of luteinizing hormone (LH) and follicle-stimulating hormones (FSH) from the anterior pituitary. GnRH has a plasma half-life of approximately 5 to 7 minutes, and is almost entirely removed on the first pass through the pituitary either by receptor internalization or enzymatic degradation. GnRH secretion results from integrated input from the effects of stress, exercise, and diet from higher brain centers, gonadotropins secreted from the pituitary, and circulating gonadal hormones. Substances known to regulate GnRH secretion are listed in Table 20–1. In Kallman syndrome, characterized by congenital hypogonadotropic hypogonadism, the GnRH precursor neurons fail to migrate normally with a subsequent absence of hypothalamic secretion of GnRH (Bick et al, 1992; Dode et al, 2003). Affected individuals present with delayed puberty or infertility due to lack of testosterone production.
Table 20–1 Substances That Modulate GnRH Secretion
| GnRH MODULATOR | TYPE OF FEEDBACK | EXAMPLES |
|---|---|---|
| Opioids | Negative | β-Endorphin |
| Catecholamines | Variable | Dopamine |
| Peptide hormones | Negative | FSH, LH |
| Sex steroids | Negative | Testosterone |
| Prostaglandins | Positive | PGE2 |
| Insulin | Positive | Insulin |
| Kisspeptins | Positive | Kisspeptin (puberty) |
| Leptins | Positive | Leptin |
FSH, follicle-stimulating hormone; LH, luteinizing hormone; PGE2, prostaglandin E2.
GnRH output exhibits several types of rhythmicity: seasonal, on a time scale of months and peaking in the spring; circadian, resulting in higher testosterone levels during the early morning hours; and pulsatile, with GnRH peaks occurring every 90 to 120 minutes on average. The importance of a pulsatile GnRH secretory pattern in normal HPG axis function is aptly demonstrated by the ability of exogenously given GnRH agonists (leuprolide acetate) to stop testicular testosterone production by changing pituitary exposure to GnRH from a cyclic to a constant pattern.
Anterior Pituitary
Located within the bony sella turcica of the cranium, the pituitary has two lobes: posterior and anterior. The posterior lobe, or neurohypophysis, secrets two hormones, oxytocin and vasopressin, and is driven by neural stimuli. In contrast, the anterior pituitary or adenohypophysis is regulated by blood-borne factors and is the site of action of GnRH (see Fig. 20–2). GnRH stimulates the production and release of FSH and LH by a calcium flux–dependent mechanism. The sensitivity of the pituitary gonadotrophs for GnRH varies with patient age and hormonal status. LH and FSH are the primary pituitary hormones that regulate testis function. They are glycoproteins composed of two polypeptide chain subunits, termed α and β, each coded by a separate gene. The α subunit of each hormone is identical and is similar to that of all other pituitary hormones; biologic and immunologic activities are conferred by the unique β subunit. Both subunits are required for endocrine activity. Sugars linked to these peptide subunits, consisting of oligosaccharides with sialic acid residues, differ in content between FSH and LH and likely account for differences in plasma clearance of these hormones. Secretory pulses of LH vary in frequency from 8 to 16 pulses in 24 hours and vary in amplitude by one- to threefold. These pulse patterns closely reflect GnRH release. Both androgens and estrogens regulate LH secretion through negative feedback. On average, FSH pulses occur approximately every 1.5 hours and vary in amplitude by 25%. The FSH response to GnRH is more difficult to measure than that of LH for two reasons: (1) FSH has a smaller amplitude response and a longer serum half-life, (2) the gonadal proteins inhibin and activin may affect FSH secretion and are thought to account for the relative secretory independence of FSH from GnRH secretion.
FSH and LH are only known to act in the gonads. They activate adenylate cyclase, which leads to increases in intracellular cAMP. In the testis, LH stimulates steroidogenesis within Leydig cells by inducing the mitochondrial conversion of cholesterol to pregnenolone and testosterone. FSH binds to Sertoli cells and spermatogonial membranes within the testis and is the major stimulator of seminiferous tubule growth during development. FSH is essential for the initiation of spermatogenesis at puberty. In the adult, the major physiologic role of FSH is to stimulate quantitatively normal levels of spermatogenesis.
A third anterior pituitary hormone, prolactin, can also affect the HPG axis and fertility. Prolactin is a large, globular protein of 199 amino acids (23 kD) that is responsible for milk synthesis during pregnancy and lactation in women. No human mutations have been found in either the human prolactin gene or its receptor (Goffin et al, 2002). The normal role of prolactin in men is less clear, but it may increase the concentration of LH receptors on Leydig cells and sustain normal, high intratesticular testosterone levels. It may also potentiate the effects of androgens on the growth and secretions of the male accessory sex glands (Wennbo et al, 1997; Steger et al, 1998). Normal prolactin levels may be important to maintain libido. Although low prolactin levels are not necessarily pathologic, hyperprolactinemia abolishes gonadotropin pulsatility by interfering with episodic GnRH release. In addition, the anterior pituitary contains cells that secrete other glycoprotein hormones: adrenocorticotropic hormone (ACTH), growth hormone (GH), and thyroid-stimulating hormone (TSH). These glycoprotein hormones can also have significant effects on male reproductive function.
Testis
Normal male virility and fertility requires the collaboration of the exocrine and endocrine testis (see Fig. 20–2). The interstitial compartment, composed mainly of Leydig cells, is responsible for steroidogenesis. The seminiferous tubules produce spermatozoa.
Normal testosterone production in men is approximately 5 g/day, and secretion occurs in a damped, irregular, pulsatile manner (nyctehemeral). Testosterone is metabolized into two major active metabolites in target tissue: (1) the major androgen dihydrotestosterone (DHT) from the action of 5α-reductase and (2) the estrogen estradiol through the action of aromatases. DHT is a much more potent androgen than is testosterone. In most peripheral tissues, testosterone reduction to DHT is required for androgen action, but in the testis and skeletal muscle, conversion to DHT is not essential for hormonal activity.
The primary site of FSH action is on Sertoli cells within seminiferous tubules. In response to FSH, Sertoli cells produce androgen-binding protein, transferrin, lactate, ceruloplasmin, clusterin, plasminogen activator, prostaglandins, and growth factors. Through these FSH-mediated factors, seminiferous tubule growth is stimulated during development, and sperm production is initiated during puberty. Interestingly, mice FSH knockout studies suggest that FSH is not essential for spermatogenesis, because affected mice can be fertile (Levallet et al, 1999). In humans, it is thought that FSH is required for normal spermatogenesis (Tapanainen et al, 1997).
The testis also produces the protein hormones inhibin and activin (Itman et al, 2006). Inhibin is a 32-kD protein made by Sertoli cells that inhibits FSH release from the pituitary. Within the testis, inhibin production is stimulated by FSH and acts by negative feedback at the pituitary or hypothalamus. Activin, a testis protein with close structural homology to transforming growth factor–β, exerts a stimulatory effect on FSH secretion. Activin receptors are found in a host of extragonadal tissues, suggesting that this hormone may have growth factor or regulatory roles in the body.
Negative feedback suppression of GnRH release by testosterone occurs through androgen receptors (AR) present in hypothalamic neurons and in the pituitary. In studies of genetic mutations, it is clear that both testosterone and estrogen participate in negative feedback (Shupnik and Schreihofer, 1997). Steroid negative feedback results mainly from AR binding to testosterone, with a smaller contribution from estradiol binding. Testosterone feedback occurs mainly at the hypothalamus, whereas estrogens feedback to the pituitary (Santen, 1975). It also appears that although testosterone is the primary regulator of LH secretion, estradiol (along with inhibin from Sertoli cells) is the predominant regulator of FSH secretion (Hayes et al, 2001).
Development of the HPG Axis
Sex determination is genetically determined in humans. A critical gene for sex determination is SRY (sex-determining region Y gene) on the short arm of the Y chromosome. The SRY gene product is a protein with a high mobility group box (HMG) sequence, a highly conserved DNA-binding motif that kinks DNA. This DNA bending effect alters gene expression, leading to testis formation and subsequently to the male phenotype. However, the SRY gene does not act in isolation to determine human sex. DAX1, a nuclear hormone receptor gene, can alter SRY activity during development by suppressing genes downstream to SRY that would normally induce testis differentiation. A second gene, WNT4, largely confined to the adult ovary, may also serve as an “antitestis” gene. The discovery of these genes has significantly altered theories of sex determination. In the past, the female genotype was considered the “default,” SRY-negative, developmental pathway. It is now clear that genes such as WNT4 and DAX1 can proactively induce female gonadal development, even in the presence of SRY (DiNapoli and Capel, 2008).
Once gonadal sex is determined, Leydig cells make testosterone that induces development of the internal genitalia (Fig. 20–3). Leydig cells also synthesize insulin-like-3 to promote transabdominal testis migration into the scrotum. DHT masculinizes the genital anlage to form the external genitalia (see Fig. 20–3). In addition, Sertoli cells within the developing testis synthesize müllerian-inhibiting substance (MIS, AMH) that prevents the müllerian duct from developing into uterus and fallopian tubes and keeps the early germ cells quiescent in the testis (Fig. 20–4). Deficiencies in these developmental pathways generally result in either birth defects or intersex disorders.
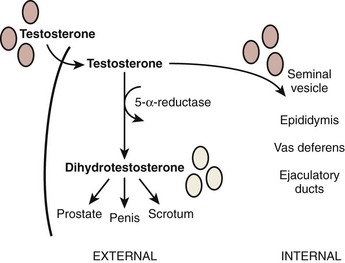
Figure 20–3 Diagram of internal and external genitalia development. Testosterone is the main androgenic steroid responsible for the developing male external genitalia, whereas dihydrotestosterone is the main androgen responsible for development of male internal genitalia.
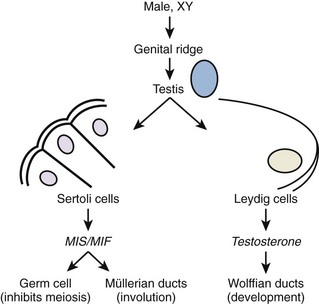
Figure 20–4 Early differentiation pathway of the male. MIF/MIS, müllerian inhibiting factor/substance.
(Adapted from Turek PJ: Male infertility. In: Tanagho EA, McAninch JC, editors. Smith’s urology. 16th ed. Stamford, CT: Appleton & Lange; 2008 [chapter 44–13].)
The hormonal feedback relationships within the HPG axis also become established during gestation. The expression of kisspeptin protein is in part responsible for activating GnRH neurons and triggering GnRH release. In addition, SF-1, an orphan nuclear receptor, is secreted by developing Sertoli cells and contributes to HPG axis development (Val et al, 2003). After the withdrawal of placental steroids at birth, there is a period of high gonadotropin secretion in the neonate. Subsequently, as axis sensitivity to gonadotropins increases, FSH and LH secretions fall to the low levels characteristic of childhood. Puberty begins with GnRH pulsing, leading gonadotropins to increase to adult levels and, subsequently, to increase sex hormones. The hypothalamic capacity to generate GnRH pulses arises at puberty, usually starting around the 12th year. Puberty begins at critical growth, weight, and nutritional rates for boys and girls, and is likely initiated by kisspeptin, melatonin, and leptin (Clement et al, 1998). The adipocyte hormone leptin is the body’s regulatory signal governing the size of the fat stores, and there is increasing evidence that leptin modulates hypothalamic and pituitary activity (Caprio et al, 1999; Kiess et al, 1999; Quinton et al, 1999).
Aging and the HPG Axis
A progressive decline in testosterone and sperm production occurs with age, such that men in the 7th decade have mean plasma testosterone levels 35% lower than young men (Vermeulen et al, 1995). The consequence of this is a phenomenon has been variously termed male menopause, male climacteric, andropause, or, more appropriately, partial androgen deficiency in the aging male (PADAM). The changes to the seminiferous epithelium with age include decreases in seminiferous tubule volume and length. An age-related decrease in sperm production in older testes appears to stem from a decrease in germ cell proliferation rather than cellular degeneration. Correspondingly, FSH levels also increase with age, with mean values threefold higher in older than younger men. The etiology of the age-related decline in HPG axis function is multifactorial. Testosterone production is reduced because of decreased numbers of Leydig cells and an increase in testosterone binding proteins. Diurnal variation of testosterone secretion is also lost in elderly men. There is also evidence for a blunted HPG feedback response to low testosterone (despite generally high levels of gonadotropins) and to GnRH stimulation. Finally, normal pulsatile GnRH release is replaced by irregular pulses that are less effective in stimulating gonadotropin release (Mulligan et al, 1997). A combination of these effects is likely responsible for diminished HPG axis function with age in men.
Key Points
HPG Axis
The Testis
Gross Architecture
The testis is a white, ovoid organ that is normally 15 to 25 mL in volume (Prader, 1966) and has a length of 4.5 to 5.1 cm (Tishler, 1971; Winter and Faiman, 1972). The tunica albuginea has smooth muscle cells that course through predominantly collagenous tissue (Langford and Heller, 1973). These smooth muscle cells may impart contractile capability to the capsule (Rikmaru and Shirai, 1972), may affect blood flow into the testis (Schweitzer, 1929), and promote the flow of seminiferous tubule fluid out of the testis (Davis and Horowitz, 1978).
The testis parenchyma is divided into compartments separated by septa. Each septum divides seminiferous tubules into lobes that each contain a centrifugal artery. Individual seminiferous tubules harbor developing germ cells and interstitial tissue. Interstitial tissue is composed of Leydig cells, mast cells, macrophages, nerves, and blood and lymph vessels. In humans, interstitial tissue comprises 20% to 30% of total testicular volume (Setchell and Brooks, 1988). The relationship between seminiferous tubules and interstitial tissue anatomy is demonstrated in Fig. 20–5. Seminiferous tubules are long, highly coiled, and looped. Both ends usually terminate in the rete testis. It is estimated that the combined length of the 600 to 1200 tubules in the human testis is approximately 250 meters (Lennox and Ahmad, 1970) (Fig. 20–6). The “hub” of the testis, also termed the rete testis, coalesces to form 6 to 12 ductuli efferentes that carry testicular fluid and spermatozoa into the caput epididymis (see Fig. 20–6).
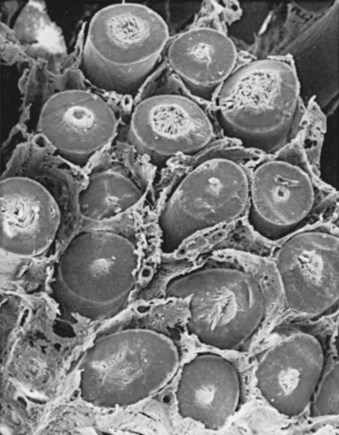
Figure 20–5 Scanning electron micrograph of the cut surface of the human testis. Note the relationship of interstitial tissue to seminiferous tubules.
(From Christensen AK. Leydig cells. In: Greep RO, Astwood EB, editors. Handbook of physiology. Washington, DC: American Physiology Society; 1975. p. 57–94.)
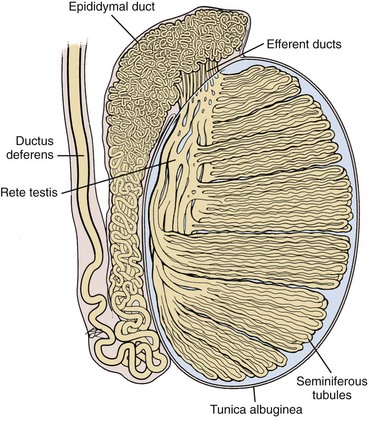
Figure 20–6 Drawing of the human testis showing the seminiferous tubules (250 meters length), epididymis (3 to 4 meters length), and vas deferens.
(Based on Hirsh AV. The anatomical preparations of the human testis and epididymis in the Glasgow Hunterian Collection. Hum Reprod Update 1995;1:515–21.)
The arterial supply to the testis and epididymis is derived from three sources: the internal spermatic artery, the deferential (vasal) artery, and the external spermatic (or cremasteric) artery (Harrison and Barclay, 1948). The internal spermatic artery arises from the abdominal aorta and is intimately associated with the pampiniform plexus of veins. The vascular arrangement within the pampiniform plexus, with the counterflowing artery and veins, facilitates the exchange of heat and small molecules. For example, testosterone passively diffuses from veins to the artery in a concentration-limited manner (Bayard et al, 1975). The countercurrent heat exchange supplies arterial blood to the testis that is 2° C to 4° C lower than the rectal temperature in normal men (Agger, 1971). A loss of the temperature differential is associated with testicular dysfunction in men with varicocele (Goldstein and Eid, 1989) and cryptorchidism (Marshall and Edler, 1982). As the spermatic cord is commonly dissected during varicocele repair, it is surgically relevant to know that a single artery is observed in 50% of spermatic cords, with two arteries in 30% and three arteries in 20% of cases (Beck et al, 1992).
Inferior to the scrotal pampiniform plexus and near the mediastinal testis, the spermatic artery is highly coiled and branches before entering the testis. Extensive interconnections, especially between the internal spermatic and deferential arteries, allow maintenance of testis viability even after division of the internal spermatic artery (Fig. 20–7). From angiographic studies, a single artery enters the testis in 56% of cases; two branches enter in 31% of cases and three or more branches in 13% of testes (Kormano and Suoranta, 1971). In men with a single testicular artery, its interruption can result in testicular atrophy (Silber, 1979). The testicular arteries penetrate the tunica albuginea and then travel inferiorly along the posterior surface of the testis within the parenchyma. Branching arteries pass anteriorly over the testicular parenchyma. Major testicular artery branches also travel over the inferior pole of the testis, pass anteriorly and branch out over the surface of the testis. The location of these vessels is clinically important, because they may be injured during orchiopexy or testis biopsy procedures (Jarow, 1991; Schlegel and Su, 1997). The midsection of the testis has relatively fewer vessels compared with superior or inferior areas of the testis. Individual arteries to the seminiferous tubules, termed centrifugal arteries, travel within the septa that contain tubules. Centrifugal artery branches give rise to arterioles that supply individual intertubular and peritubular capillaries (Muller, 1957). The intertubular capillaries are located within the columns of interstitial tissue, whereas the ladder-like capillaries running near the seminiferous tubule are called peritubular capillaries. Through this vascular complex, the human testis is provided with 9 mL of blood per 100 g of tissue per minute (Pettersson et al, 1973).
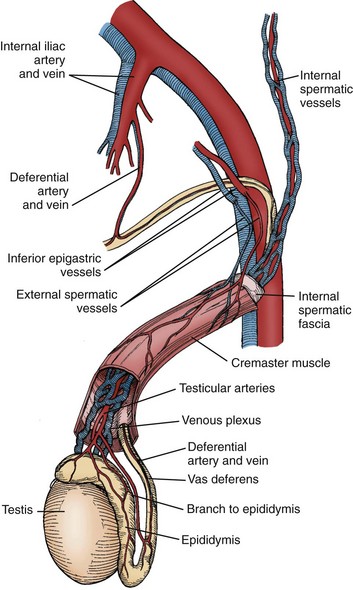
Figure 20–7 Schematic illustration of the interconnections between internal spermatic, external spermatic (cremasteric), and deferential vessels in the peritesticular region and spematic cord.
Veins within the testis are unusual in that they do not run with the corresponding intratesticular arteries. Small parenchymal veins empty into either the veins on the testis surface, or into a group of veins near the mediastinum testis that travels along the rete testis (Setchell and Brooks, 1988). These two sets of veins join together with deferential veins to form the pampiniform plexus as they ascend into the scrotum. Pampiniform plexus veins are thin walled, which likely contributes to the passive diffusion of testosterone and temperature exchange with the closely associated spermatic artery.
The testis has no known somatic innervation. It receives autonomic innervation primarily from the intermesenteric nerves and renal plexus (Mitchell, 1935). These nerves run along the testicular artery into the testis. It appears that testicular adrenergic innervation is restricted primarily to small blood vessels that supply Leydig cell clusters that may regulate Leydig cell steroidogenesis (Baumgarten et al, 1968; Turnbull and Rivier, 1997). It is thought that vascular tone in the testis may involve regulation at several levels (Linzell and Setchell, 1969), including autoregulation of capsular arteries (Davis et al, 1990), regional variation based on local metabolic need and governed by peptides such as atrial natriuretic peptide (Collin et al, 1997), and assisted transport of molecules such as LH across the vascular endothelium (Milgrom et al, 1997). Indeed, these observations suggest a highly specialized function for the microvasculature of the testis (see review by Desjardins [1989]).
Prominent lymphatics can be observed within the spermatic cord (Hundeiker, 1971). Obstruction of these ducts results in dilatation of the testis interstitium but not the seminiferous tubules, suggesting that the interstitial space is drained by lymphatics, but the seminiferous tubules are not. Lymphatic obstruction can also result in hydrocele formation, a known complication of certain varicocelectomy and herniorrhaphy procedures. The sperm containing intratubular fluid that baths Sertoli cells flows from the seminiferous tubules into the rete testis and, subsequently, into the caput epididymis. This fluid, isosmotic with plasma, is thought to be mainly of seminiferous tubule origin (Setchell and Brooks, 1988). Reabsorption of this fluid within the rete testis and efferent ductules is regulated by estrogens (Lee et al, 2000). Tubular fluid composition is markedly different from blood plasma or lymphatics, suggesting that substances are not freely diffusible into and out of the tubules (Setchell and Waites, 1975). This has led to the concept of a “blood-testis barrier” to be discussed later.
Testis Cytoarchitecture
Interstitium
Leydig Cells
The testis interstitium contains blood vessels, lymphatics, fibroblasts, macrophages, mast cells, and Leydig cells (Fig. 20–8). Leydig cells are responsible for the bulk of testicular steroid production. Leydig cells differentiate from mesenchymal precursor cells by the 7th week of gestation. The activation of Leydig cell steroidogenesis correlates with the onset of androgen-dependent differentiation of the male reproductive system. Although it is apparent that Leydig cells express steroidogenic enzymes before becoming responsive to LH (El-Gehani et al, 1998; Majdic et al, 1998), they also differentiate from precursor cells under the influence of LH, placental-derived human chorionic gonadotropin (HCG), and from the effect of local paracrine factors such as IGF-1 (Huhtaniemi and Pelliniemi, 1992; Teerds and Dorrington, 1993; Le Roy et al, 1999). At 2 to 3 months after birth, a second wave of Leydig cell differentiation occurs in response to gonadotropin production from the pituitary, briefly elevating testosterone levels in infants. Androgen produced during the the early male neonate’s life is thought to hormonally imprint the hypothalamus, liver, and prostate such that they respond appropriately to androgen stimulation later in life. After reactivation of the HPG axis at puberty, stereologic analysis has revealed that a single testis from a young adult contains approximately 700 million Leydig cells (Kaler and Neaves, 1978).
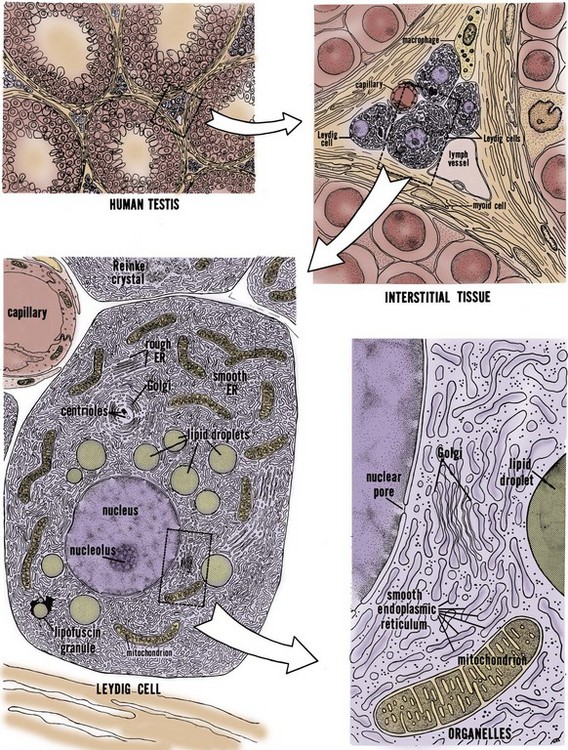
Figure 20–8 Fine structure of human Leydig cells. Leydig cells occur in clusters in the interstitium between seminiferous tubules (upper left). Interstitial tissue (upper right) contains macrophages and fibroblasts and capillaries and lymph vessels. The most abundant organelle within the Leydig cell cytoplasm is the smooth endoplasmic reticulum (lower left). Organelles seen in greater detail (lower right).
(From Christensen AK. Leydig cells. In: Greep RO, Astwood WB, editor. Handbook of physiology, section 7, Endocrinology. Baltimore: Williams & Wilkins; 1975.
Testosterone
Testosterone, synthesized from cholesterol, is the principal steroid produced by the testis (Lipsett, 1974). In addition, numerous C18, C19, and C21 steroids are also produced (Lipsett, 1974; Ewing and Brown, 1977). Cholesterol must be transported into Leydig cell mitochondria, where the cholesterol side-chain cleavage enzyme converts it to pregnenolone. The three main sources of cholesterol in the Leydig cell are (1) externally, from blood-borne lipoprotein and internalization of cholesterol-lipoprotein receptor complexes, (2) from de novo synthesis from acetate, and (3) from stored cholesterol esters in lipid droplets. Maintenance of cholesterol stores is part of the normal resting function of the Leydig cell; LH stimulation evokes cholesterol mobilization through cholesterol esterase activity. Pregnenolone is transported out of the mitochondrial membrane into the smooth endoplasmic reticulum, where it is converted into testosterone. Testosterone diffuses across the cell membrane and is trapped within the extracellular fluid and blood plasma by steroid-binding proteins.
Cholesterol transport to the inner membrane of the mitochondrion is regulated by two transport proteins: steroid acute regulatory protein (StAR) and peripheral benzodiazepine receptor (PBR). LH binding elicits protein synthesis in the Leydig cell, and the newly-synthesized StAR contains a signal sequence that enables it to be threaded through the outer mitochondrial membrane to faciliate cholesterol transport (Stocco, 2000). PBR forms a channel for cholesterol in the mitochondrial membrane (Culty et al, 1999), but it is not clear whether PBR functionally interacts with StAR (West et al, 2001).
The four major enzymes participating in testosterone biosynthesis from pregnenolone are cholesterol side-chain cleavage enzyme, 3β-hydroxysteroid dehydrogenase, cytochrome P450 17α-hydroxylase/C17-20-lyase, and 17β-hydroxysteroid dehydrogenase. The enzymology, chromosomal locations, and molecular genetics of these enzymes are well described (Payne and Hales, 2004). Gene mutations in the genes encoding these enzymes have been described and the resulting disorders of androgen biosynthesis are a relatively rare cause of sexual ambiguity in chromosomally normal males (Miller, 2002).
Control of Testosterone Synthesis
The control of Leydig cell steroidogenesis is complex and involves both pituitary and non-pituitary factors (Payne and Youngblood, 1995). The most important regulator of testosterone production is LH. LH binding, through the second messenger cyclic adenosine monophosphate (cAMP), initiates transport of cholesterol into mitochondria. Pituitary peptides other than LH (e.g., FSH and prolactin) modify the response to LH (Ewing, 1983). Other, nonpituitary factors capable of modifying steroid production by Leydig cells include GnRH (Sharpe, 1984), inhibin and activin (Bardin et al, 1989), epidermal growth factor (EGF), IGF-1, and transforming growth factor-β (TGF-β) (Ascoli and Segaloff, 1989; Saez et al, 1991), prostaglandins (Eik-Nes, 1975), and adrenergic stimulation (Eik-Nes, 1975). Moreover, direct inhibition of Leydig cell steroidogenesis may also occur through estrogens and androgens (Ewing, 1983; Darney et al, 1996).
Testosterone Cycles
Testosterone blood levels change dramatically during human fetal, neonatal, and adult life. Fig. 20–9 shows that a testosterone peak occurs in the human fetus between 12 and 18 weeks of gestation. Another testosterone peak occurs at approximately 2 months of age. A third testosterone peak occurs during the 2nd or 3rd decade of life. After this, there is a plateau, and then a slow decline with age. Superimposed on this, there are annual and daily rhythms of testosterone production (see Fig. 20–9, insets A and B) and irregular daily fluctuations in testosterone (see Fig. 20–9, inset C). These temporal changes in testosterone production during human life reflect a complex interaction between the pituitary gland and the testis. The testosterone peaks correspond temporally to the following developmental events: (1) the differentiation and development of the fetal reproductive tract, (2) the neonatal organization or “imprinting” of androgen-dependent target tissues, (3) the masculinization of the male at puberty, and (4) the maintenance of growth and function of androgen-dependent organs in the adult. This topic has been reviewed thoroughly by Swerdloff and Heber (1981).
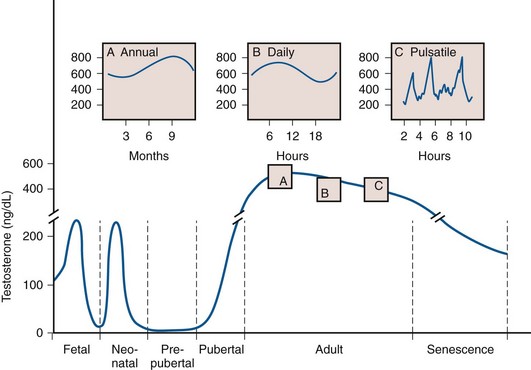
Figure 20–9 Peripheral blood testosterone levels in the human male during the life cycle. The fetal testosterone peak occurs between 12 and 18 weeks of gestation (lower left corner; gestational age not shown). The neonatal peak occurs at approximately 2 months of age. Testosterone declines to low levels during the prepubertal period. The pubertal increase in testosterone occurs between 12 and 17 years of age. Testosterone concentration in the adult reaches its maximum during the second or third decade of life and then declines slowly through the 5th decade. Testosterone declines dramatically during senescence. Inset A shows the annual rhythm in testosterone concentration in the human male. The peak and nadir occur in the fall and spring, respectively. Inset B shows the daily rhythm in testosterone concentration. The peak and nadir occur in the morning and evening, respectively. Inset C shows the frequent and irregular fluctuations in testosterone concentration.
(From Ewing LL, Davis JC, Zirkin BR. Regulation of testicular function: a spatial and temporal view. In: Greep RO, editor. International review of physiology. Baltimore: University Park Press; 1980. p. 41.)
Seminiferous Tubules
The seminiferous tubules consist of germ cells and supporting cells and are a unique environment for gamete production. Support cells include the Sertoli cells and fibrocyte and myoid cells of the basement membrane. The germ cells include a slowly dividing stem cell population, more rapidly proliferating spermatogonia and spermatocytes, and metamorphosing spermatids.
Sertoli Cells
The seminiferous tubules are lined with Sertoli cells that rest on the tubular basement membrane and extend cytoplasmic ramifications into its lumen (Fig. 20–10). The ultrastructural features of Sertoli cells are well described (Bardin et al, 1994). They have irregularly shaped nuclei, prominent nucleoli, low mitotic index, and exhibit unique tight junctional complexes between adjacent Sertoli cells. These tight junctions are the strongest intercellular barriers in the body. They divide the seminiferous tubule space into basal (basement membrane) and adluminal (lumen) compartments (see Fig. 20–10). This anatomic arrangement forms the basis for the “blood-testis barrier” and allows spermatogenesis to occur in an immunologically privileged site. Sertoli cells serve as “nurse” cells for spermatogenesis, nourishing developing germ cells that are arranged between Sertoli cell cytoplasmic projections. The undifferentiated spermatogonia are near the basement membrane of the tubule, whereas the more advanced spermatocytes and spermatids are located near the luminal surface. Thus the Sertoli cell is a polarized epithelium in which the base approximates the plasma environment, and its apex harbors an environment unique to the seminiferous tubule (Ewing et al, 1980).
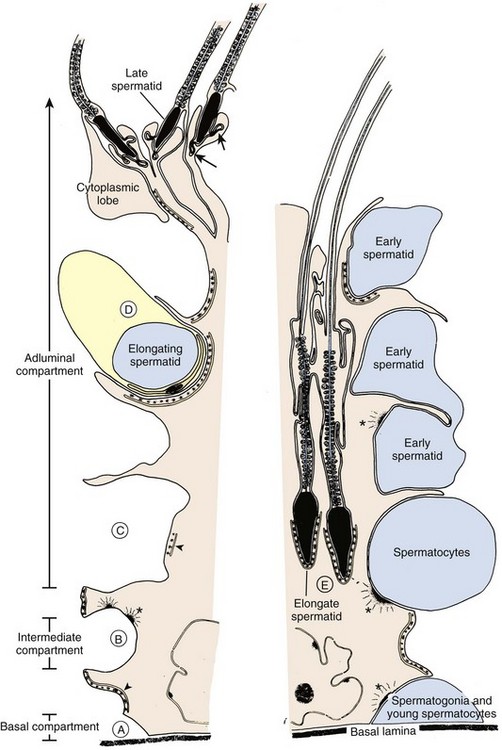
Figure 20–10 Representation of the tree-shaped Sertoli cell with a thickened central portion, or “trunk,” and more delicate processes, or “limbs.” The major configurational changes that occur during spermatogenesis are noted. Note the basal, intermediate, and adluminal compartments of the seminiferous epithelium. A, Spermatogonia and early spermatocytes share positions on the basal lamina and are enveloped by adjacent Sertoli cells that join to form tight junctional complexes (site of blood-testis barrier). B, Sertoli cells form junctional complexes both above and below leptotene-zygotene spermatocytes as they translocate from the basal to adluminal compartments. C, The spermatocytes enter the adluminal compartment when Sertoli tight junctions dissociate. D, The elongating spermatid is situated within a narrow recess of the Sertoli cell trunk. E, As the spermatid elongates further, the cell becomes lodged within the body of the Sertoli cell. The advanced spermatid moves toward the lumen of the epithelium in preparation for spermiation. Only the sperm head remains in intimate contact with the Sertoli cell. Specialized cell-to-cell contacts: asterisks, desmosome-gap junction complex; arrowheads, ectoplasmic specializations; isolated arrows, tubulobulbar complexes.
(From Russell L. Sertoli-germ cell interactions: a review. Gamete Res 1980;3:179.)
Sertoli cells nurture germ cell development by: (1) providing a specialized adluminal microenvironment, (2) supporting germ cells through gap junctions between Sertoli and germ cells, and (3) allowing migration of developing germ cells within the tubule (see Fig. 20–10). The tight junctions between Sertoli cells are constantly remodeled to allow “opening” and “closing” necessary for germ cell interaction and migration (Mruk and Cheng, 2004). Ligand-receptor complexes, such as c-kit and kit ligand, are likely involved in mediating communication between germ and Sertoli cells. Sertoli cells also participate in germ cell phagocytosis and produce and secrete fluid and important effector molecules. Androgen-binding protein (ABP) is one of earliest described Sertoli cell secretory products (Hansson and Djoseland, 1972). ABP is an intracellular carrier of androgen within the Sertoli cell. By binding testosterone, ABP maintains high levels of androgen (50-fold, as observed in serum) within the seminiferous tubules. Testosterone also plays an important role in the regulation of Sertoli cell function, including ABP production (Griswold, 1988). Inhibin is Sertoli cell–derived and plays an important regulatory role in the negative feedback loop of FSH secretion. Inhibin B is emerging as an important endocrine marker of Sertoli cell function in the male infertility evaluation.
As keepers of the immunological sanctuary in the testis, Sertoli cells maintain a germ cell microenvironment entirely distinct from that of plasma. As such, Sertoli cells secrete numerous other products including extracellular matrix components (lamin, collagen type IV, and collagen type I) and proteins such as ceruloplasmin, transferrin, glycoprotein 2, plasminogen activator, somatomedin-like substances, T proteins, H-Y antigen, clusterin, cyclic proteins, growth factors, and somatomedin (Mruk and Cheng, 2004). Steroids, such as DHT, testosterone, androstenediols, 17β-estradiol, and numerous other C21 steroids are also produced by Sertoli cells (Ewing et al, 1980; Mather et al, 1983). Although the function of many Sertoli cell– and peritubular-derived substances is unclear, further research will enlighten our understanding of how Sertoli cells orchestrate and support spermatogenesis.
Germ Cells
Within the human seminiferous tubule, germ cells give rise to approximately 123 × 106 (range: 21 to 374 × 106) spermatozoa daily (Amann and Howards, 1980). This equates to the production of about 1200 sperm per heartbeat. Within the seminiferous tubule, germ cells are arranged in a highly ordered sequence from the basement membrane to the lumen. Morphologic analysis of the various germ cells reveals at least 13 recognizable germ cell types in the human testis (Clermont, 1963; Heller and Clermont, 1964) (Fig. 20–11). Each cell type is thought to represent a different step in the spermatogenic process. Proceeding from the least to the most differentiated, based on morphologic appearance, they have been named dark type A spermatogonia (Ad); pale type A spermatogonia (Ap); type B spermatogonia (B); preleptotene (R), leptotene (L), zygotene (z), and pachytene primary spermatocytes (p); secondary spermatocytes (II); and Sa, Sb, Sc, Sd1, and Sd2 spermatids. The tight junctions maintain spermatogonia and early spermatocytes within the basal compartment and all subsequent germ cells in the adluminal compartment.
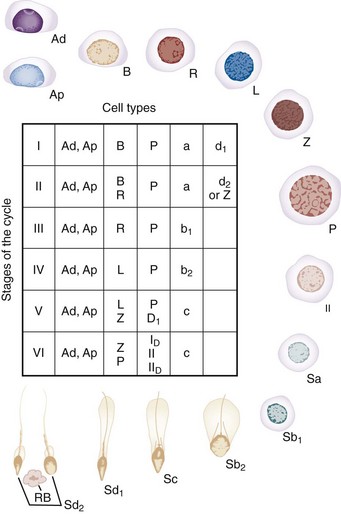
Figure 20–11 The steps of spermatogenesis in man. Ad, dark type A spermatogonium; Ap, pale type A spermatogonium; B, type B spermatogonium; II, secondary spermatocyte; L, leptotene spermatocyte; P, pachytene spermatocyte; R, resting or preleptotene primary spermatocyte; Rb, residual body; Sa(a), Sb1(b1), sb2(b2), Sc(c), sd1(d1), Sd2(d2), spermatids; Z, zygotene spermatocyte. The table shows cells that make up the six stages of the “cycle” of the seminiferous epithelium (I to VI): D1, diakenesis; ID and IID, first and second maturation divisions of spermatocytes.
(Modified from Clermont Y. Renewal of spermatogonia in man. Am J Anat 1966;118:509.)
Peritubular Structure
The human seminiferous tubule is surrounded by several layers of peritubular tissue (Hermo et al, 1977) (Fig. 20–12). The outer adventitial layer consists of fibrocytes. In the middle layer are myoid cells interspersed with connective tissue lamellae. The inner layer consists of a collagen matrix. In humans the peritubular myoid cells are thought to have contractile function (Toyama, 1977). Myoid cells actively secrete extracellular matrix components fibronectin and collagen type I, and produce the inner collagenous layer (Tung et al, 1984). Myoid cells may also affect Sertoli cell function and are known to associate with Sertoli cells in a precise mesenchymal-epithelial interaction. Skinner and coworkers (1988) isolated a paracrine factor produced by myoid cells, P-Mod-S (peritubular modifies Sertoli), that profoundly affects Sertoli cell synthetic and differentiation functions in vitro. Human peritubular cells have also been shown to secrete testosterone, and exert regulatory Sertoli cell secretory activity (Cigorraga et al, 1994).
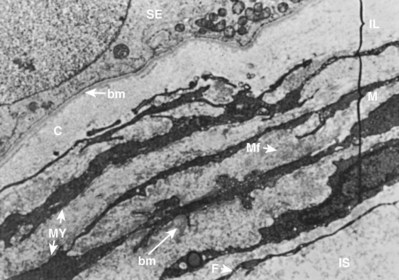
Figure 20–12 Low-power electron micrograph of human peritubular testis tissue. Peritubular tissue lies between the basement membrane (bm) of the seminiferous epithelium (SE) and the interstitial tissue (IS). Peritubular tissue has three zones: the inner lamella (IL); the myoid layer (M), containing myoid cells (MY) with abundant microfibrils (Mf); and an adventitial layer containing fibroblasts (F).
(From Hermo L, Lalli M, Clermont Y. Arrangement of connective tissue elements in the walls of seminiferous tubules of man and monkey. Am J Anat 1977;148:433–46.)
Blood Testis Barrier
Dyes and other substances, when injected into the bloodstream of animals, will rapidly appear throughout all body tissues but fail to penetrate regions of the brain and testis. From this observation, the concept of a “blood-testis barrier” was derived. More appropriately termed the “blood–seminiferous tubule barrier,” the barrier has two components: an anatomic or mechanical element and functional elements. The mechanical barrier is created, in part, by muscle-like myoid cells that surround seminiferous tubules (Dym and Fawcett, 1970; Fawcett et al, 1970). Regulation of molecular traffic also occurs at the level of capillary endothelial cells. However, the most important component of this barrier is the synaptic tight junctions between Sertoli cells that preclude the passage of large molecules and lymphocytes. These anatomical elements of the blood-testis barrier are necessary but not sufficient for maintaining the immunologic “sanctuary” status within the tubule, because they are not observed in other “protected” areas of the reproductive tract (Tung et al, 1971; Brown et al, 1972).
Thus, although the mechanical barrier contributes to the isolation of the testis, other “functional” components must also exist that suppress the normal immune response. Several mechanisms likely work in concert to protect sperm from destruction. First, lymphocytes are diverted by the vascular endothelium and are excluded from anatomically vulnerable regions in the germinal epithelium (Mahi-Brown et al, 1988). Second, these vulnerable regions harbor mainly T-suppressor cells (el-Demiry et al, 1985; Anderson and Hill, 1988). Due to deficiencies in antigen–HLA association, there may be a lack of sperm antigen presentation to lymphocytes, impairing the immune response (Jenkins et al, 1987; Anderson and Hill, 1988). There is also evidence to suggest that immunologic tolerance plays a role in the functional blood-testis barrier. Tung and colleagues have proposed that within the anatomically weaker areas (rete testis, efferent tubule, epididymis), there is a small, continuous leak of sperm antigens (Tung, 1980). This leak generates T-suppressor cells and immune tolerance, similar to desensitization protocols for common environmental allergens. However, with larger antigenic challenges, a true immune response results (Turek, 1997). Cytokines may contribute to immune tolerance, including interferon gamma, soluble Fc receptor, and transforming growth factor–β (Perussia et al, 1987; Ben Rafael, 1992; Turek, 1997). In addition, androgens have mild immunosuppressive activity and also appear to help regulate immunity (Diemer et al, 2003).
Why does the blood-testis barrier exist? Since it develops at spermarche, when spermatogenesis begins at puberty (Kormano, 1967), it is likely important for meiosis. In addition, it may immunologically isolate developing male gametes that are not recognized as “self” by the adult male immune system. In this sense, the value of a blood-testis barrier is fully realized after puberty, because foreign “antigens” on postmeiotic germ cells only exist after spermarche. A testicular insult such as biopsy, torsion, or trauma will not induce antisperm antibodies if it occurs before puberty. After puberty, however, immunologic infertility is a known risk (Turek, 1997). Clinically, the blood-testis barrier may also limit chemotherapy access to cancer cells sequestered behind it and result in isolated cancer recurrence within the testis.
Spermatogenesis
Spermatogenesis is a remarkably complex and specialized process of DNA reduction and germ cell metamorphosis. Older studies have estimated that the entire process in humans requires approximately 64 days (Clermont, 1972). However, a recent in-vivo kinetic study in healthy men revealed that the total time to produce an ejaculated sperm ranges from 42 to 76 days, suggesting that the duration of spermatogenesis can vary widely among individuals (Misell et al, 2006) (Fig. 20–13). Spermatogenesis involves (1) a proliferative phase as spermatogonia divide to replace their number (self-renewal) or differentiate into daughter cells that become mature gametes; (2) a meiotic phase when germ cells undergo a reduction division, resulting in haploid (half the normal DNA complement) spermatids; and (3) a spermiogenesis phase in which spermatids undergo a profound metamorphosis to become mature spermatozoa. (For excellent reviews, see Steinberger, 1976, and de Kretser and Kerr, 1988.)
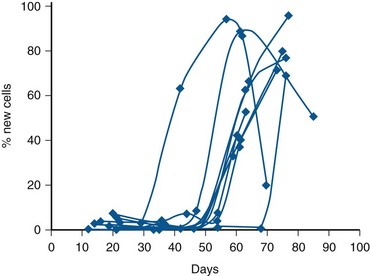
Figure 20–13 Time to make and ejaculate human sperm. Combined spermatocyte labeling curves for 11 individuals with normal semen quality who ingested 50 mL of 2H2O twice daily for 3 weeks. Note that new ejaculated sperm was found as soon as 42 days after ingesting the label, and that there is considerable interindividual variation in the time to make and ejaculate sperm among normal men.
(From Misell LM, Holochwost D, Boban D, et al. A stable isotope/mass spectrometric method for measuring the kinetics of human spermatogenesis in vivo. J Urol 2006;175:242–46.)
A cycle of spermatogenesis involves the division of primitive spermatogonial stem cells into subsequent germ cells. Several cycles of spermatogenesis coexist within the germinal epithelium at any one time, and they are described morphologically as stages. If spermatogenesis is viewed from a single fixed point within a seminiferous tubule, six recognizable cellular associations or stages exist in a predictable and constant fashion in humans (Heller and Clermont, 1964) (see Fig. 20–11). In addition, there is also a specific organization of spermatogenic cycles within the tubular space, termed spermatogenic waves. The best evidence suggests that human spermatogenesis exists in a spiral or helical cellular arrangement that ensures sperm production is a continuous and not a pulsatile process (Schulze, 1989) (Fig. 20–14).
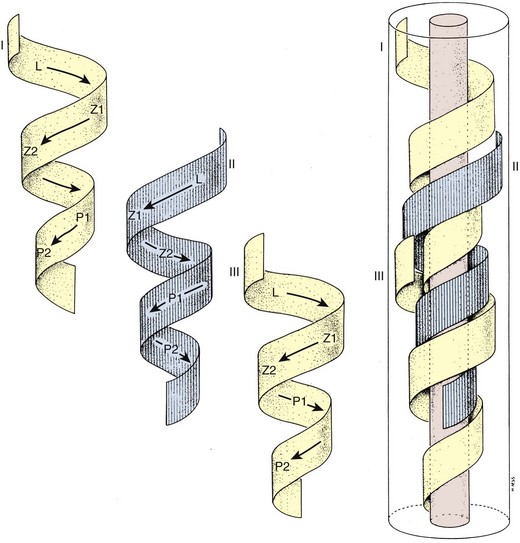
Figure 20–14 Helical configuration of seminiferous tubule epithelial cycles in man, forming overlapping “waves” of spermatogenesis that keep sperm production constant.
(From Schulze W, Rehder U. Organization and morphogenesis of the human seminiferous epithelium. Cell Tissue Res 1984;237:395–407.)
Testis Stem Cell Migration, Renewal, and Proliferation
Testis Stem Cell Migration
During early prenatal development, primordial germ cells migrate to the gonadal ridge and associate with Sertoli cells to form primitive testicular cords (Witschi, 1948). These primitive germline stem cells are termed gonocytes after the gonad differentiates into a testis by forming seminiferous cords. They are called spermatogonia after migration to the periphery of the tubule (Gondos and Hobel, 1971). Interestingly, these early migrating germ cells have properties very similar to embryonic stem cells and are likely the source of adult seminoma germ cell tumors (Ezeh et al, 2005).
Testis Stem Cell Renewal
Spermatogonia within the testis stem cell niche are replenished in a process termed stem cell renewal. The growth factor-receptor kit ligand/c-kit receptor system and the niche factor glial cell line-derived neurotrophic factor (GDNF) appear to be involved in this process (Oatley and Brinster, 2008). In fact, the c-kit receptor is a marker of spermatogonial stem cells in rats (Dym, 1994) and spermatogenesis in the rat is a c-kit–dependent process, whereas spermatogonial stem cell renewal may be c-kit–independent (Yoshinaga et al, 1991). Recent studies have also shown that human spermatogonial stem cells can be reprogrammed in vitro to become embryonic-like stem cells (Conrad et al, 2008; Kossack et al, 2009) (Fig. 20–15). Obtained from adult testis biopsies, the embryonic-like cells express distinct markers of pluripotency (OCT-4, SOX-2, STELLAR, GDF-3), can form all three germ layers, maintain a normal karyotype, form teratomas, and express appropriate levels of epigenetic markers and telomerase (Kossack et al, 2009). This finding suggests that, in the future, the testis may be a source of patient-specific, embryonic stem cells for cell-based therapy.
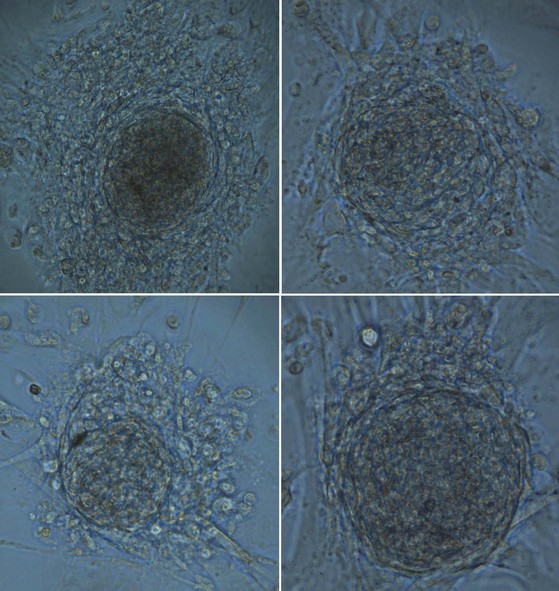
Figure 20–15 Microphotograph of four different colonies of adult testis spermatogonial–derived stem cells. These cell clusters are the result of reprogramming of adult spermatogonia in culture conditions used for human embryonic stem cells (HESCs). They exhibit the typical “cobblestone” appearance of HESCs and have also been shown to be functionally multipotent and even pluripotent.
Testis Stem Cell Proliferation
In the human, pale type A (Ap) spermatogonia in the basal, stem cell niche of the seminiferous tubule divide at 16-day intervals (Clermont, 1972) to form B spermatogonia. B spermatogonia are committed to become spermatocytes, but the cytoplasm between spermatogonial daughter cells remains conjoined after mitosis, forming cytoplasmic bridges between adjacent cells. These cytoplasmic bridges are observed between all classes of germ cells throughout spermatogenesis (Ewing et al, 1980). These bridges could be important for synchronized cellular proliferation and differentiation, and possibly for regulation of gene expression.
Meiosis
Somatic cells replicate by mitosis, in which genetically identical daughter cells are formed. Germ cells replicate by meiosis, in which the genetic material is halved to allow reproduction. Meiosis generates genetic diversity, providing a richer source of material on which natural selection can act. Cell replication by mitosis is a precise, well-orchestrated sequence of events involving duplication of the genetic material (chromosomes), breakdown of the nuclear envelope, and equal division of the chromosomes and cytoplasm into two daughter cells. The essential difference between mitotic and meiotic replication is that a single DNA duplication step is followed by only one cell division in mitosis, but two cell divisions in meiosis (four daughter cells). Consequently, daughter cells contain only half of the chromosome content of parent cells. Thus a diploid (2n) parent cell becomes a haploid (n) gamete. Other major differences between mitosis and meiosis are outlined in Table 20–2. Recent research has shown that small RNA molecules (small RNAs), including small interfering RNAs (siRNAs), microRNAs (miRNAs), and piwi-interacting RNAs (piRNAs), are important regulators of gene germ cell expression at the post-transcriptional or translation level (Tolia and Joshua-Tor, 2007; He et al, 2009).
Table 20–2 Essential Differences: Mitosis and Meiosis
| MITOSIS | MEIOSIS |
|---|---|
| Occurs in somatic cells | Occurs in sexual cells |
| 1 cell division, 2 daughter cells | 2 cell divisions, 4 daughter cells |
| Chromosome number maintained | Chromosome number halved |
| No pairing, chromosome homologs | Synapse of homologs, prophase I |
| No crossovers | >1 crossover per homolog pair |
| Centromeres divide, anaphase | Centromeres divide, anaphase II |
| Identical daughter genotype | Genetic variation in daughter cells |
Spermatogenesis begins with type B spermatogonia dividing mitotically to form primary spermatocytes within the adluminal compartment. Mature spermatocytes are the first germ cells to undergo meiosis (Kerr and de Kretser, 1981). In this process, a meiotic division is followed by a typical mitotic reduction division, resulting in daughter cells with a haploid chromosome complement. In addition, as a consequence of chromosomal recombination, each daughter cell contains different genetic information. The resultant cell is the Sa spermatid (see Fig. 20–11).
Chromosomal recombination, the defining feature of mammalian meiosis, ensures that haploid gametes differ genetically from their adult precursors and is the real engine of genetic diversity and evolution. During meiotic prophase, there is formation of a synaptonemal complex with pairing of homologous (maternal and paternal) chromosomes, and physical interaction and exchange of DNA through reciprocal sites of crossing over (chiasmata) between homologs. Recent research has shown that defects in the fidelity of recombination within human male germ cells can cause azoospermia and male infertility (Walsh et al, 2009). In one study, 10% of non-obstructive azoospermic men had significant defects in recombination compared to men with normal spermatogenesis (Gonsalves et al, 2004). In addition, among men with maturation arrest pattern on testis biopsy, faulty recombination was observed in about half of cases, providing evidence that faulty recombination is linked to poor sperm production (Gonsalves et al, 2004). Variations in recombination also have implications for sperm aneuploidy, because alterations in crossover position are risk factors for chromosomal nondisjunction. Indeed, molecular evidence suggests that the correlation of faulty recombination and sperm aneuploidy in azoospermic men is strong enough to explain the higher de novo rate of chromosomal abnormalities in offspring conceived with in-vitro fertilization–intracytoplasmic sperm injection (IVF-ICSI) (Sun et al, 2008).
Spermiogenesis
During spermiogenesis, round Sa spermatids mature into spermatozoa (see Fig. 20–11). During this maturation sequence, cell division does not occur, but there are extensive changes to the spermatid nucleus and cytoplasm. These include the loss of cytoplasm, migration of cytoplasmic organelles, formation of the acrosome from the Golgi apparatus, formation of the flagellum from the centriole, nuclear compaction to about 10% of former size, and reorganization of mitochondria around the sperm midpiece (Kerr and de Kretser, 1981). The nucleus of the round spermatid changes from spherical to asymmetrical as chromatin condenses. Many cellular elements contribute to the reshaping process, including chromosome structure, associated chromosomal proteins, the perinuclear cytoskeletal theca layer, the manchette of nuclear microtubules, subacrosomal actin, and Sertoli cell interactions. With completion of spermatid elongation, the Sertoli cell cytoplasm retracts around the developing sperm, stripping it of all unnecessary cytoplasm and extruding it into the tubule lumen. The mature sperm has remarkably little cytoplasm, and it is an elaborate, specialized cell produced in massive quantity—up to 300 per gram of testis per second.
Sertoli Cell–Germ Cell Interaction
A complex network of cell–cell interactions exists within the testis between Leydig cells and Sertoli cells, between Leydig cells and peritubular cells, between Sertoli and peritubular cells, and between Sertoli cells and germ cells. There are several kinds of Sertoli cell–germ cell associations in mammalian testes as illustrated in Figure 20–10 (Russell and Clermont, 1976; Romrell and Ross, 1979; Skinner, 1995). Physical contact between these cells plays a role in propelling the germ cell toward the tubule lumen and casting off of the residual cytoplasm from the spermatid. Lastly, there are factors that can reversibly disrupt the blood-testis barrier, including transforming growth factor–β3 (TGF-β3) and tumor necrosis factor–α (TNF-α). These substances act by reducing the levels of occludin and zonula occludens-1 (ZO-1) in the barrier through a p38 mitogen-activated protein (MAP) kinase signaling pathway (Xia et al, 2009). This represents only a piece of the remarkably complex and highly interactive process that characterizes spermatogenesis.
Genetics
Genetic causes of abnormal spermatogenesis have been identified as point mutations in single genes inherited in mendelian fashion (e.g., cystic fibrosis), and as chromosomal disorders, in which segments of (or entire) chromosomes have structural or numerical abnormalities. The reader is referred to Turek and Reijo Pera (2002) for a comprehensive review of such disorders. The postulation that deletions in the long arm of the Y chromosome cause azoospermia was made over thirty years ago (Tiepolo et al, 1976). Based on cytogenetic analysis, this theoretical region was termed the azoospermia factor (AZF). Currently, the positional patterns of deletions (termed “microdeletions”) in the AZF region are used to subdivide this region into AZFa, b, and c subregions (Vogt et al, 1996). Regional deletions of the Y chromosome, termed Yq microdeletions, occur in 6% to 8% of severely oligospermic men and in up to 15% of azoospermic men (Reijo et al, 1996). Taken together, such deletions are the most commonly defined molecular cause of male infertility (Kostiner et al, 1998).
There is emerging literature addressing the prognostic value of specific AZF deletions. In contrast to partial and complete AZFc-deletion patients, in which sperm is often found on semen analysis or testis biopsy, the chance of finding ejaculated or testis sperm in men with complete AZFa or AZFb deletions is highly unlikely (Hopps et al, 2003). Complete AZFa deletions are associated with germ cell aplasia or Sertoli cell–only histology. Complete AZFb deletions are generally associated with maturation arrest at the primary spermatocyte (early) or spermatid (late) stages. AZFc deletions are associated with hypospermatogenesis or a Sertoli cell–only pattern with foci of spermatogenesis. Sperm have been detected in ejaculates of men with presumed and confirmed partial AZFa and AZFb deletions (Foresta et al, 2001). Similarly, ejaculated sperm in men with AZFa + b, and AZFb + c deletions (presumably partial deletions) has also been reported, but the finding of AZFa−c deletions has been associated with azoospermia and no sperm on testis biopsy.
More recently, it has become clear that the X chromosome may also be important for spermatogenesis. Insight into the role of the X chromosome in male infertility has been garnered mainly from rodent studies. In 2001, Wang and colleagues reported on a systematic search for genes expressed exclusively in mouse spermatogonia (Wang et al, 2001). Twenty-five genes were identified by complementary DNA (cDNA) subtraction, of which 10 (9 novel) localized to the X chromosome, suggesting that the X chromosome may have a key role in premeiotic stages of spermatogenesis. Further, human homologues of six of these genes were mapped to chromosomal regions of conserved synteny between mouse and human genomes. Thus, similar to genes on the Y chromosome, it is possible that these X chromosome genes may also prove to be sites of mutation in cases of human spermatogenic failure. Studies have also examined mutations in X-linked genes in male infertility patients, including the SOX3 gene (sex determining region Y box 3) and the FATE gene (Olesen et al, 2003; Raverot et al, 2004). Although not commonly identified at this point, mutations on X chromosome genes will likely prove to contribute significantly to human male infertility in the future.
Genetics and Paternal Age
Age-Related Sperm Chromosomal Anomalies
The aneuploidy and polyploidy status of sperm were first investigated due to concern that advanced paternal age was associated with increased cases of trisomy, especially trisomy 21 or Down syndrome in offspring. With fluoresence in-situ hybridization technology (FISH), subtle paternal-age effects on sperm aneuploidy have been detected. The paternal age effect appears to increase the fraction of sperm with sex chromosomal aneuploidies (Wyrobek et al, 1996). However, there is little evidence to support a paternal age–related increase in aneuploid births, except for possibly trisomy 21 and disomy 1 (very rare). Examining sperm chromosome structural abnormalities, Martin and Rademaker (1987) found that a highly significant linear relationship exists between paternal age and the frequency of structural anomalies in sperm (r = 0.63). One explanation for this association may be that continued cell division during spermatogenesis places germ cells at risk for chromosomal injury, especially with advanced paternal age. Except for reciprocal translocations, however, there is little evidence to indicate that this association leads to an increased frequency of offspring with de novo structural chromosomal anomalies.
Age-Related Sperm Genetic Mutations
Single gene defects in sperm result from errors in DNA replication. To date, it has been difficult to assess the presence or absence of such defects in sperm. However, the effect of advanced paternal age on cases of conditions associated with single-gene deletions has been studied extensively. These disorders are listed in Table 20–3 and consist of autosomal dominant diseases that have known associations with advanced paternal age. They are termed “sentinel phenotypes” because they are disorders of significant frequency and low fitness, and occur sporadically due to highly penetrant mutations. One mechanism for the development of new single-gene mutations with age implicates the characteristic and continuous process of spermatogonial cell division in spermatogenesis. By puberty, 30 cell divisions of spermatogonia have occurred, resulting in a large pool of undifferentiated cells. After puberty, 23 divisions per year occur in these cells. The simple fact that the spermatogonia of older men have undergone numerous cell divisions may make them more likely to harbor errors in DNA transcription, the source of single-gene defects. Formal risk estimates exist for the contribution of advanced paternal age to autosomal dominant mutations: In men <29 years old, the risk of a mutation occurring in offspring is 0.22 per 1000. This risk doubles (0.45 per 1000) at paternal ages 40 to 44, and then climbs to 3.7 per 1000 at ages >45 (Friedman, 1981).
Table 20–3 Selected Genetic Disorders Associated with Advanced Paternal Age
The Epididymis
Gross Architecture
The epididymis is a comma-shaped organ located along the posterolateral surface of the testis. Passage through the epididymis induces many changes to newly formed sperm, including a gain in functional motility, and alterations in surface charge, membrane proteins, immunoreactivity, phospholipids, fatty acid content, and adenylate cyclase activity. These changes improve cell membrane structural integrity, increase fertilization ability, and improve motility. Spermatozoa within the testis have very poor or no motility. They become progressively motile and functional only after traversing the epididymis. The transit time of sperm through the epididymis is thought to take up to 12 days in humans (Johnson and Varner, 1988).
The epididymis is a tubule or duct that is 3 to 4 meters in length and is tightly coiled and encapsulated within the sheath of connective tissue of the tunica vaginalis (Lanz and Neuhauser, 1964; Turner et al, 1978). Extensions from the sheath enter interductal spaces and form septa that divide the duct into histologically characteristic regions (Kormano and Reijonen, 1976). Anatomically, these are classically divided into three regions: caput or head, corpus or body, and cauda (or tail) (Fig. 20–16). The caput epididymis consists of 8 to 12 ductuli efferentes from the testis. The lumen of the ductuli efferentes is large and somewhat irregular in shape near the testis, becoming narrow and oval near the junction with the ductus epididymis. Distal to this junction, the duct diameter increases slightly and, thereafter, remains constant in the corpus epididymis. In the bulky cauda epididymis, the tubule diameter enlarges substantially and acquires an irregular shape. Progressing distally, the tubule gradually assumes the characteristic appearance of the vas deferens.
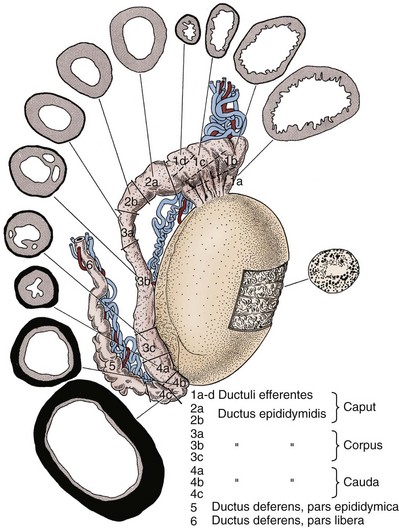
Figure 20–16 Drawing of the human epididymis showing regionalization of the ductal epithelium and muscle layer. Epididymal segment locations are shown in cross section and are identified by number.
(From Baumgarten HG, Holstein AF, Rosengren E. Arrangement, ultrastructure, and adrenergic innervation of smooth musculature of the ductal efferentes, ductus epididymidis, and ductus deferens in man. Z Zellforsch Mikrosk Anat 1971;120:37.)
Vascular and Lymph Supply
In humans, the caput and the corpus epididymis receive arterial blood from a branch of the testicular artery (see Fig. 20–7). It subsequently divides into superior and inferior epididymal branches (MacMillan, 1954). The epididymis also receives blood from branches of the deferential arteries (artery of the vas deferens), and collateral vessels connect the deferential artery to the testicular blood supply. The cauda epididymis is supplied by branches of the deferential artery. The deferential and cremasteric arteries serve as collateral sources to the epididymis, when the main testicular artery is obstructed or ligated. The arterial branches within the epididymis enter along septa formed from the connective tissue sheath. These vessels coil extensively before transforming into the straight vessels of the microvascular bed (Kormano and Reijonen, 1976). Microvascularization density varies significantly along the length of the epididymis, with the proximal caput containing the densest subepithelial capillary network, and the more distal segments harboring less dense vascularization. From animal studies, the epididymal capillary network is under hormonal control. For example, in rabbits, bilateral hormonal castration results in progressive deterioration and eventual disappearance of the epididymal capillary network (Clavert et al, 1981). It is not clear whether vascularization in the human epididymis is similarly controlled.
According to MacMillan (1954), venous drainage from the corpus and cauda epididymis joins to form the vena marginalis epididymis of Haberer. These veins drain into the pampiniform plexus through the vena marginalis testis, or through the cremasteric or deferential veins. Lymphatic drainage of the epididymis occurs through two routes (Wenzel and Kellermann, 1966). Lymph from the caput and corpus epididymis is removed through the same route as that described for the testis. These vessels course beside the internal spermatic vein and ultimately terminate in the preaortic nodes. Lymph vessels from the cauda epididymis join those draining the vas deferens and terminate in the external iliac nodes.
Innervation
The innervation of the human epididymis is derived primarily from the intermediate and inferior spermatic nerves that arise from the superior portion of the hypogastric plexus and pelvic plexus, respectively (Mitchell, 1935). The ductuli efferentes and the proximal segments of the epididymis are sparsely innervated by sympathetic fibers (Baumgarten and Holstein, 1967; Baumgarten et al, 1968). In these regions, the fibers are observed in a peritubular plexus and are principally associated with blood vessels. Many more fibers are observed in the midcorpus epididymis, and their density increases progressively with distal progression along the epididymis, coincident with the appearance and proliferation of smooth muscle cells in these areas (Baumgarten et al, 1971). The distribution of contractile cells and sympathetic nerves within the epididymis may explain the rhythmic peristaltic movements of the ductuli efferentes and initial epididymal segments, as well as the intermittent contractile activity of the cauda epididymis and the vas deferens during emission (Risely, 1963). These physiologic contractions are critical to the movement of sperm through the epididymis.
Cytoarchitecture
Epididymal Epithelium
The histology of the human epididymis has been reviewed by Holstein (1969) and Vendrely (1981). It consists of two main cells types: principal cells and basal cells (seen at low ultrastructural magnification in Fig. 20–17). Principal cells vary in height along the length of the epididymis due to the length of stereocilia (microvilli, not cilia). Tall stereocilia (120 µm) are generally found in the proximal epididymis, and smaller or shorter stereocilia (50 µm) are observed in more distal regions. The nuclei in principal cells are elongated and often possess large clefts and one or two nucleoli. Consistent with the idea that principal cells carry out both absorptive and secretive processes, their cellular apices have numerous coated pits, micropinocytotic vesicles, multivesicular bodies, irregularly shaped membranous vesicles, and an extensive Golgi apparatus. Because these cytologic features vary along the length of the epididymis, it suggests that there is varying absorptive and secretory capacity along the length of the duct (Vendrely and Dadoune, 1988).
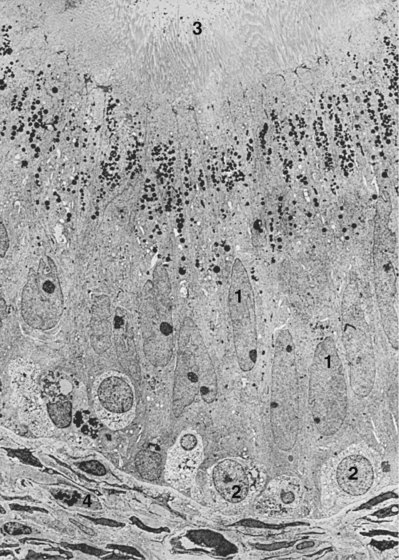
Figure 20–17 Electron micrograph of a human epididymis in cross section. Major components of the luminal epithelium are principal cells (1), basal cells (2), stereocilia (3), and myofilaments (4). Magnification approximately ×1800.
(From Holstein AF. In: Hafez ESE, editor. Human semen and fertility regulation in men. St Louis: Mosby; 1976.)
There are far fewer basal cells than principal cells lining the epididymal epithelium, and they are dispersed among the more numerous principal cells. Tear-shaped basal cells rest on the basal lamina and extend approximately 25 µm toward the lumen, their apices forming threads between adjacent principal cells. They are thought to be derived from macrophages. Unlike the principal cells, the morphology of basal cells remains relatively constant throughout the epididymal duct. They are thought to be the precursors of the principal cells.
Overall, the epithelium of the epididymis exhibits regional differences along the length of the duct. Within the epididymis proper, the epithelium is pseudostratified in nature and consists of principal and basal cells as described above. More proximally, at the junction of the rete testis and ductuli efferentes, there is a distinct transition from a low to a high cuboidal epithelium. The epithelium in the ductuli efferentes appears uneven and consists of ciliated and nonciliated cells (Holstein, 1969). The ciliated cells are dispersed throughout the epithelium and conduct sperm from the efferent ducts into the epididymis. The nonciliated cells with protruding apices are likely secretory in nature and predominate in the proximal ductuli efferentes (Vendrely, 1981). Other nonciliated cells have microvilli suggestive of resorptive activity and predominate in the distal ductuli efferentes. Nonciliated cells likely are involved in testis fluid reabsorption. Both nonciliated and ciliated cells are joined apically through junctional complexes. This suggests the existence of a blood-epididymis barrier analogous to the blood-testis barrier (Suzuki and Nagano, 1978; Hoffer and Hinton, 1984). Although not as dense as the blood-testis barrier, the blood-epididymis barrier extends from the caput to the cauda epididymis and may play an important role in influencing the composition of fluid within different segments of the epididymal lumen (Turner, 1979).
Epididymal Contractile Tissue
Peripheral to the basal lamina of the ductuli efferentes and the epididymal tubule, there are various contractile cells (Baumgarten et al, 1971) (see Fig. 20–17). In the ductuli efferentes (distal regions of the caput and the proximal corpus epididymis), the contractile cells form a loose layer, two to four cells deep, around the tubule. These cells contain myofilaments and are connected by numerous nexus-like junctions. In the distal corpus epididymis, there are larger contractile cells with fewer nexus-like intracellular junctions that resemble thin smooth muscle cells. In the cauda epididymis, the thin contractile cells are replaced by thick smooth muscle cells that form three layers—the outer two layers oriented longitudinally and the central layer circularly. This distal contractile layer increases in thickness as it forms the vas deferens. The contractile tissue throughout the epididymis is likely involved in sperm transport.
Epididymal Function
Described variations in the anatomy and histology of the epididymal tubule from the caput to cauda regions suggest that the epididymis is actually several different functional tissues (Vendrely, 1981). It is clear that sperm transport and storage, sperm-fertilizing ability, and motility maturation are several consequences of passage through this organ. This is addressed more fully in reviews by Robaire and Hermo (1988), and Moore and Smith (1988).
Sperm Transport
Sperm transport through the human epididymis has been calculated to take from 2 to 12 days (Johnson and Varner, 1988). Sperm transit time through the caput-corpus epididymis is roughly similar to the transit time through the cauda epididymis and is more likely related to daily testicular sperm production rather than a man’s age or the frequency of ejaculation (Amann, 1981; Johnson and Varner, 1988). In one study, sperm epididymal transit time averaged 2 days in men with a high daily rate of sperm production (137 million per testis), compared with 6 days in men with low daily sperm production (34 million per testis) (Johnson and Varner, 1988). Although the frequency of sexual activity does not affect sperm transit time through the caput and corpus epididymis, “recent emissions” can reduce transit time through the cauda epididymis by 68% (Amann, 1981).
Because normal human testicular sperm are immotile as they enter the epididymis, and remain relatively immotile within the caput, mechanisms other than sperm motility must exist to transport sperm through the epididymis. Animal studies have been very revealing in this regard (Bedford, 1975; Hamilton, 1977; Courot, 1981; Jaakkola and Talo, 1982; Jaakkola, 1983). Initially, sperm are carried into the ductuli efferentes by rete testis fluid, and fluid flow is facilitated by fluid resorption by ductal epithelial cells mediated by the estrogen receptor. Motile cilia and myoid cell contractions within the ductuli efferentes also assist with sperm movement. Within the epididymis proper, the principal mechanism responsible for sperm transport is likely the spontaneous, rhythmic contraction of the contractile cells surrounding the epididymal duct.
Sperm Storage
After migrating through the caput and corpus epididymis, sperm are retained in the cauda epididymis for varying lengths of time, depending on the frequency of sexual activity. In men 21 to 55 years of age, an average of 155 to 209 million sperm are present in each epididymis (Amann, 1981; Johnson and Varner, 1988), and approximately half are stored in the caudal region.
Spermatozoa stored in the cauda epididymis, unlike testicular sperm, are capable of progressive motility and are able to fertilize eggs. The exact amount of time that sperm can remain fertile within the epididymis is unclear, but animal studies have shown that sperm can remain viable for several weeks within the cauda epididymis after vas deferens ligation (Hammond and Asdell, 1926; Young, 1929). However, it is also clear that sperm fertility measured in vivo diminishes when sperm are maintained in the epididymis for prolonged periods of time (Cooper and Orgebin-Crist, 1977; Cuasnicu and Bedford, 1989). In humans, sperm aging as a result of extended epididymal transit time and prolonged storage may contribute to reduced fertility (Johnson and Varner, 1988).
The exact fate of unejaculated epididymal sperm is unknown. In animals, sperm are lost through spontaneous seminal discharge, oral self-cleaning (Martan, 1969), through urine (Lino et al, 1967), or by epididymal reabsorption (Amann and Almquist, 1961). Phagocytosis of spermatozoa by macrophages (spermiophages) within the epididymal lumen has been observed in humans after ligation of the vas deferens (Alexander, 1972). However, whether this mechanism can remove large numbers of spermatozoa from the epididymis of unvasectomized men is unclear.
Sperm Maturation
Sperm Motility
Sperm gain an increased capacity for motility with migration through the epididymis. This is observed as both a change in the pattern of motility, and as an increase in the proportion of sperm exhibiting “mature” motility patterns. Bedford and coworkers (1973) first observed that the majority of sperm from the ductuli efferentes, when placed in culture medium, are immotile or show only weak, twitching movement. Occasionally, they also observed sperm showing “immature” tail movements characterized by “thrashing” beats in wide arcs that result in little forward progression. The proportion of sperm with this immature motility pattern increased within the initial epididymal segment. However, in the corpus region, the proportion of sperm exhibiting this motility pattern decreased. Within the corpus region, there was an increase in the fraction of sperm with a “mature” motility pattern characterized by high-frequency, low-amplitude beats that result in progressive motility (Fig. 20–18). Within the cauda epididymis, >50% of sperm had a mature motility pattern, with the remainder either immotile or showing the immature motility patterns described earlier. Moore and colleagues (1983) also formally demonstrated the increased capacity of human sperm to show progressive forward motility with epididymal transit. When placed in buffer in vitro, 0%, 3%, 12%, 30%, and 60% of sperm were motile from the efferent ducts, caput, proximal corpus, distal corpus, and cauda epididymis, respectively (Fig. 20–19).
Figure 20–18 Patterns of tail movement of human epididymal sperm. A, The pattern shown by sperm taken from proximal epididymis is characterized by high-amplitude, low-frequency beats producing little forward movement. B, In contrast, tail movement in a large proportion of sperm from the cauda epididymis is characterized by low-amplitude, rapid beats with forward progression.
(From Bedford JM, Calvin HI, Cooper GW. The maturation of spermatozoa in the human epididymis. J Reprod Fertil 1973;18:199–213.)
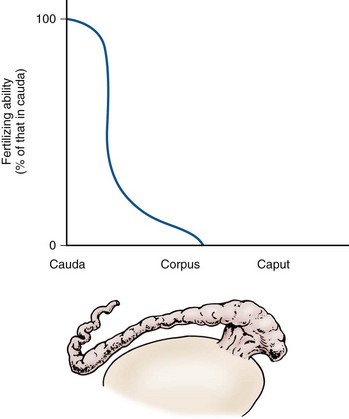
Figure 20–19 Sperm fertility maturation in the human epididymis. Sperm fertilizing ability was assessed using zona pellucida–free hamster eggs and by changes in motility.
(From Bedford JM. The bearing of epididymal function in strategies for in vitro fertilization and gamete intrafallopian transfer. Ann N Y Acad Sci 1988;541:284–91.)
The relative importance of overall epididymal contact time versus region-specific maturation to the gain in mature sperm motility patterns is unknown. Animal studies indicate that motility maturation may, in part, be an intrinsic sperm process that occurs independent of specific epididymal interactions. For example, although hamster and rabbit sperm are generally immotile within the caput epididymis, motile sperm are found in this region (albeit developing motility far more slowly and persisting for shorter periods than in the normal system) after epididymal duct ligation within the corpus region (Orgebin-Crist, 1969; Horan and Bedford, 1972). Human studies in obstructed patients with congenital absence of the vas deferens or epididymal obstruction also frequently report poor motility in spermatozoa aspirated from the distal epididymis, and better sperm motility in the proximal epididymis (Silber, 1989; Matthews et al, 1995). When combined, these observations suggest that spermatozoa are able to develop motility based on contact time with the proximal epididymal epithelium. However, this maturation process may not be the same as that which occurs through sperm interaction with the epididymis during migration through all ductal regions.
Sperm Fertility
Testicular sperm are incapable of fertilizing eggs unless injected into them with micromanipulation (Orgebin-Crist, 1969; Bedford, 1974; Yanagimachi, 2005). In most animals, the ability of sperm to fertilize eggs is acquired gradually as the sperm pass through the distal epididymis (see Fig. 20–19). Indeed, it has been shown in rabbits that sperm from the caput, corpus, and cauda epididymis can fertilize 1%, 63%, and 92% of rabbit eggs, respectively (Orgebin-Crist, 1969). Human in-vitro experiments using zona pellucida–free hamster eggs have corroborated these findings (Moore et al, 1983). In a study that assessed the fertilizing capacity of human epididymal sperm, Hinrichsen and Blaquier (1980) demonstrated that although sperm from the proximal epididymis are able to bind to zona-free eggs, only sperm from the cauda epididymis can both bind and penetrate eggs. Thus sperm fertility maturation is, for the most part, achieved at the level of the late corpus or early cauda epididymis.
Recent clinical observations, however, challenge the idea that fertility maturation requires sperm migration through the entire epididymis. Indeed, patients with epididymal obstruction or congenital absence of the vas deferens can achieve natural pregnancies after vasoepididymostomy at the level of the ductuli efferentes (Schoysman and Bedford, 1986; Silber, 1989). This suggests that obstruction induces proximal “skewing” of the maturation sequence along the epididymal duct, or that there may be a reduced flow of sperm through the epididymis after such bypass procedures, allowing more contact time and sperm maturation (Orgebin-Crist, 1969; Turner and Roddy, 1990). Despite this observation, is it generally believed that the likelihood of fertility is greater as the surgical anastomosis is performed more distally in the epididymis (Thomas, 1987).
Sperm Biochemical Changes
Sperm undergo many biochemical changes with passage through the epididymis (Brooks, 1983). Although human information is limited, epididymal sperm transit induces a net negative surface membrane charge (Bedford et al, 1973) and sperm membrane sulfhydryl groups oxidize to disulfide bonds, improving sperm structural rigidity necessary for progressive motility and egg penetration (Bedford et al, 1973; Reyes et al, 1976). Other post-testicular modifications of sperm membranes include changes in sperm lectin-binding properties (Courtens and Fournier-Delpech, 1979; Olson and Danzo, 1981), phospholipid and lipid content (Nikolopoulou et al, 1985), glycoprotein composition (Brown et al, 1983), immunoreactivity (Tezón et al, 1985), and iodination characteristics (Olson and Danzo, 1981). Overall, these membrane modifications during epididymal passage may enhance sperm adherence to the egg zona pellucida (Orgebin-Crist and Fournier-Delpech, 1982; Blobel et al, 1990). Sperm also undergo numerous metabolic changes during epididymal transit (Dacheux and Paquignon, 1980). These include gaining an increased capacity for glycolysis (Hoskins et al, 1975), changes in intracellular pH and calcium content, modification of adenylate cyclase activity (Casillas et al, 1980), and alterations in cellular phospholipid and phospholipid-like fatty acid content (Voglmayr, 1975).
Regulation of Epididymal Function
Sperm changes within the epididymis are likely influenced by fluids and secretions within the epididymal lumen (Robaire and Hermo, 1988; Blaquier et al, 1989). The biochemical composition of epididymal fluid not only differs from that of serum, but there are also regional differences in the osmolarity, electrolyte content, and protein composition of epididymal fluid (Robaire and Hermo 1988). These differences are likely the consequence of variations in vascularization, blood-epididymis barrier activity, and selective absorption and secretion of fluid constituents such as glycerylphosphorylcholine (GPC), carnitine, and sialic acids along the epididymal duct. Proteins within the epididymal fluid that are known to have physiologic effects on sperm in vitro include forward motility protein (Brandt et al, 1978), sperm survival factor (Morton et al, 1978), progressive motility sustaining factor (Sheth et al, 1981), sperm motility-inhibiting factor (Turner and Giles, 1982), acidic epididymal glycoprotein (Pholpramool et al, 1983) and the EP2-EP3 proteins that induce sperm binding to zona pellucida (Cuasnicu et al, 1984; Blaquier et al, 1988). Thus variations in epididymal tubule fluid characteristics play an important role in sperm maturation during epididymal transit. It is not surprising, then, that epididymis is a potentially important source of sperm dysfunction and male infertility.
Epididymal function is hormonally regulated. Testosterone and DHT are found in very high concentrations within the epididymis, and there are no regional gradients in androgen levels (Leinonen et al, 1980). This suggests the importance of androgens for epididymal function, a finding confirmed in animal studies (Brooks and Tiver, 1983). Bilateral castration results not only in the loss of androgen-dependent epididymal proteins but also losses in epididymal weight, changes in luminal histology, and alterations in the synthesis and secretion of epididymal fluid GPC, carnitine, and sialic acid. Ultimately, the castrated epididymis loses the ability to sustain sperm motility, fertility maturation, and sperm storage capacities, but most of these degenerative processes are reversed with androgen replacement.
Compared with other accessory sex glands, the epididymis requires relatively higher levels of androgen for maintenance of its structure and function (Prasad and Rajalakshmi, 1976). Androgen effects on the epididymis appear to be mediated mainly through dihydrotestosterone (DHT), the primary androgen in epididymal tissue extracts (Pujol et al, 1976) and/or 5α-androstane-3α, 17β-diol (3α-diol) (Orgebin-Crist et al, 1975). Indeed, this is corroborated by the fact that the enzymes Δ4-5α-reductase (catalyzes the DHT formation from testosterone), and 3α-hydroxysteroid dehydrogenase (converts DHT to 3α-diol) that produce these testosterone metabolites are also found in the human epididymis (Kinoshita et al, 1980; Larminat et al, 1980).
Epididymal function is also influenced by temperature (Foldesy and Bedford, 1982; Wong et al, 1982). Chronic exposure of the epididymis to elevated temperatures, by placing them within the abdomen, results in the loss of sperm storage and electrolyte transport functions. The influence of temperature on epididymal function in humans may be an important consideration for how varicocele and cryptorchidism induce male infertility. Abnormalities in epididymal myoid cell contractility may also influence epididymal function. In the rat, partial surgical denervation of the epididymis results in an abnormal accumulation of sperm within the cauda epididymis and a decrease in the swimming speed of retained sperm (Billups et al, 1990). These findings certainly have implications for infertility due to neuropathic causes such as spinal cord injury and diabetes mellitus.
Key Points
Epididymis
Ductus (Vas) Deferens
Gross Architecture
The vas deferens is a tubular organ derived from the mesonephric (wolffian) duct. In humans, the vas deferens is 30 to 35 cm long, beginning at the cauda epididymis and terminating in the ejaculatory duct, medial to the seminal vesicle and posterior to the prostate. It is classically divided into 5 regions: (1) the sheathless epididymal segment contained within the tunica vaginalis, (2) the scrotal segment, (3) the inguinal segment, (4) the retroperitoneal or pelvic portion, and (5) the ampulla (Lich et al, 1978). In cross section, the vas deferens consists of an outer adventitial connective tissue sheet containing blood vessels and small nerves, a muscular coat that consists of a middle circular layer surrounded by inner and outer longitudinal muscle layers, and an inner mucosal layer with an epithelial lining (Neaves, 1975). The outer diameter of the vas deferens varies from 1.5 to 3 μm, and the lumen of the unobstructed vas deferens varies from 200 to 700 mm in diameter (Middleton et al, 2009).
The vas deferens receives its blood supply from the deferential artery, a branch of the superior vesicle artery. Venous drainage corresponds to arterial supply. The vas deferens receives innervation from both the sympathetic and the parasympathetic nervous systems (Sjostrand, 1965). The cholinergic supply does not appear important for motor activity of the vas deferens (Baumgarten et al, 1975). There is a rich supply of sympathetic adrenergic nerves derived from hypogastric nerve coursing via the presacral nerve (Batra and Lardner, 1976; McConnell et al, 1982). Adrenergic nerve fibers have been observed in all three layers of the vas tunica muscularis, with the greatest concentration in the outer longitudinal layer (McConnell et al, 1982). The vas deferens also receives a short adrenergic nerve (Sjostrand, 1965) and has an abundance of ligand-gated, purinergic receptors in its smooth muscle membranes, suggesting sympathetic and purinergic cotransmission in sperm transport and ejaculation (Gur et al, 2007). Neurons containing other neurotransmitters, including neuropeptide Y, enkephalin, galanin, somatostatin, vasoactive intestinal polypeptide, and nitric oxide, have also been identified; however, their role in vas deferens function is unknown (Dixon et al, 1998). Interestingly, observations from human vas deferens specimens obtained at vasovasostomy from 1 to 15 years after vasectomy show a marked reduction in the density of muscular noradrenergic and subepithelial secretomotor nerves in testicular compared to abdominal segments. These changes after vasectomy may influence subsequent sperm maturation and transport, and hence procedural success after vasectomy reversal (Dixon et al, 1998).
Cytoarchitecture
The human vas deferens is lined by pseudostratified epithelium (Paniagua et al, 1981). The height of the epithelium decreases along the length of the vas deferens from the testis to the seminal vesicle. In addition, the longitudinal epithelial folds are simpler proximally near the testis and become more complex distally. The pseudostratified epithelium lining of the vas deferens is composed of basal cells and three types of tall, thin columnar cells (Hoffer 1976; Paniagua et al, 1981). The columnar cells, extending from the epithelial base to the lumen, include principal cells, but also pencil cells and mitochondria-rich cells. All columnar cells exhibit stereocilia and irregular convoluted nuclei. Principal cells are the most frequent columnar cell type in the proximal vas deferens, whereas both pencil cells and mitochondria-rich cells increase in density distally. The thickness of the total muscle layer gradually decreases along the length of the vas deferens. This complex cytoarchitecture strongly suggests that the vas deferens is more than simply a passive conduit for sperm transport.
Vas Deferens Function
Sperm Transport
Sperm transport through the vas deferens is influenced by several physiologic processes. First, the human vas deferens exhibits spontaneous motility (Ventura et al, 1973). It also has the capacity to respond when stretched (Bruschini et al, 1977). Finally, fluid within the vas deferens can be propelled into the urethra by strong peristaltic contractions elicited either by electrical stimulation of the hypogastric nerve (Bruschini et al, 1977) or by adrenergic neurotransmitters (Bruschini et al, 1977; Lipshultz et al, 1981). This suggests that immediately before emission, with sympathetic stimulation, rapid transport of sperm from the distal epididymis through the vas deferens to the ejaculatory duct occurs. This rapid transport ability is consistent with the vas deferens having the greatest muscle to lumen ratio (≈10 : 1) of any hollow viscus in the body.
Sperm reserves in the vas deferens have been estimated at approximately 130 million, suggesting that a significant proportion of human ejaculated sperm is stored in the vas deferens (Amann and Howards, 1980). In addition, vasal sperm quality, as assessed from fertile men at the time of vasectomy, is very similar to that of the ejaculate, with 71% motility and 91% viability (Bachtell et al, 1999). In the rabbit, it has been shown that during sexual rest, epididymal sperm are transported through the vas deferens and leak into the urethra in small amounts and at irregular intervals (Prins and Zaneveld, 1979, 1980a, 1980b). This suggests that the vas deferens is involved in ridding the epididymis of excess, stored sperm. Upon sexual stimulation, rabbit sperm are transported through the vas deferens similar to humans. After sexual stimulation, however, the vas deferens contents are propelled proximally toward the epididymis as the distal vas deferens contracts with greater amplitude, frequency, and duration than the proximal segment (Prins and Zaneveld, 1980a). Notably, with prolonged sexual rest, excess epididymal sperm are once again transported distally, supporting the idea that the vas deferens is important not only for sperm transport, but also for the maintenance of epididymal sperm reserves.
Absorption and Secretion
Based on its cytoarchitecture, it has been suggested that the human vas deferens has both absorptive and secretory functions (Hoffer 1976 and Paniagua et al, 1981). The principal cells are typical of cells that synthesize and secrete glycoproteins, as has been demonstrated in the rat model (Gupta et al, 1974; Bennett et al, 1974). The stereocilia, apical blebbing, and primary and secondary lysosomes within principal cells are also characteristic of cells involved in absorptive function (Murakami et al, 1988), which has also been confirmed in the rat (Friend and Farquhar, 1967). Lastly, spermiophagy by epithelial cells in the ampullary vas deferens has been observed with scanning electron microscopy in both men and monkeys (Murakami et al, 1988). Importantly, normal vas deferens function is likely to be androgen dependent because the vas deferens actively converts testosterone to DHT (Dupuy et al, 1979). Castration causes atrophy of—and testosterone treatment, restoration of—monkey vas cytoarchitecture (Dinakar et al, 1977), and spontaneous and α- and β-adrenergic–stimulated contractions of the rat vas deferens are altered by castration (Borda et al, 1981). Thus, although once thought to be a simple muscular conduit for sperm, the vas deferens is now viewed as a complex reproductive organ.
Seminal Vesicle and Ejaculatory Ducts
Gross and Cytoarchitecture
Seminal Vesicle
In the adult, the seminal vesicles are paired, elongated, hollow viscous organs located posterior to the prostate and bladder. Each seminal vesicle is 5 to 7 cm long and up to 1.5 cm wide. Each seminal vesicle actually consists of a tubule that is 15 cm long and highly coiled and convoluted. The tubule itself is composed of three layers: The inner lining is a moist and folded mucous membrane; the middle layer is largely collagenous; and the outer layer consists of circular and longitudinal muscles layers that constitute 80% of the wall thickness (Nguyen et al, 1996). The mucosa of the seminal vesicle, mainly nonciliated, pseudostratified columnar or cuboidal cells, is notable for many thin, complicated folds that produce numerous crypts. The excretory duct of the seminal vesicle opens into the ampullary vas deferens as it enters the prostate gland.
The blood supply to the seminal vesicle arises from the internal iliac artery and inferior vesicular artery through the prostatovesicular branch (Clegg, 1955). The prostatovesicular artery can also arise from the superior vesicular artery or from the pudendal artery. Most commonly, the prostatovesicular artery has anterior and posterior branches that supply the respective surfaces of the seminal vesicle. The lymphatic drainage of the seminal vesicle is through the internal iliac lymph nodes. The seminal vesicles are innervated through sympathetic nerves from the superior lumbar and hypogastric nerves. Parasympathetic innervation occurs through the pelvic plexus.
Ejaculatory Ducts
The ejaculatory ducts are paired, collagenous, tubular structures that commence at the junction of the vas deferens and seminal vesicle, course through the prostate, and empty into the prostatic urethra at the verumontanum. Histologically, the ejaculatory ducts are a continuation of the seminal vesicle, except that the outer circular muscle layer does not extend into the ducts (Nguyen et al, 1996). There are three distinct anatomic regions to the ejaculatory duct: the proximal, extraprostatic portion; the middle intraprostatic segment; and a short distal segment incorporating the lateral aspect of the verumontanum in the urethra (Nguyen et al, 1996) (Fig. 20–20). Although the ejaculatory duct contains an outer muscular layer in its extra- and intraprostatic segments, as the duct courses distally, the outer muscular layer dissipates, and there is no valvelike, muscular “sphincter” at the ejaculatory duct orifice, as was once thought (Nguyen et al, 1996) (Fig. 20–21). Instead, urinary reflux is prevented and ejaculatory continence is maintained by the acute angle of duct insertion into the urethra. The inner epithelial layer of the ejaculatory ducts is also complex and folded, and consists of simple and pseudostratified columnar cells. The ejaculatory ducts receive their blood supply from branches of the inferior vesicle artery and are innervated through the pelvic plexus.
Figure 20–20 Schematic anatomy of the human ejaculatory duct complex. A, Proximal, B, intraprostatic or middle, and C, distal ejaculatory duct regions. The inset shows how the muscle layer thins out in the middle segment.
(From Nguyen HT, Etzell J, Turek PJ, et al. Normal human ejaculatory duct anatomy: a study of cadaveric and surgical specimens. J Urol 1996;155:1639–42.)
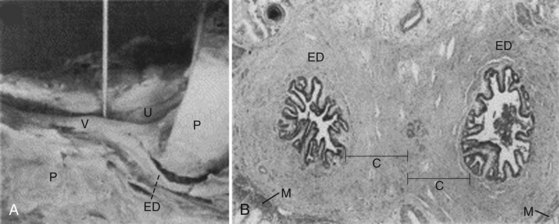
Figure 20–21 Human ejaculatory duct gross and microscopic anatomy from cadaver specimens. A, Shows sagital section through the midline with pin in ejaculatory duct orifice and ejaculatory duct (ED) and veru (V), urethra (U), and prostate (P) visible. B, Microphotograph of the paired ejaculatory ducts in the middle intraprostatic segment showing the thick collagenous layer (label C) surrounding the mucosa with a thin, outer muscular layer (label M).
(From Nguyen HT, Etzell J, Turek PJ, et al. Normal human ejaculatory duct anatomy: a study of cadaveric and surgical specimens. J Urol 1996;155:1639–42.)
Seminal Vesicle and Ejaculatory Duct–Unit Function
Animal studies suggest that the seminal vesicle and ejaculatory ducts are functionally similar to the bladder and urethra (Turek et al, 1998). The seminal vesicle is a contractile, compliant, smooth muscular organ with dynamic properties analogous to those of the bladder, and the ejaculatory ducts serve as a urethra-like conduit. This theory allows the classification of ejaculatory duct obstruction into two types of disorders, analogous to bladder outlet obstruction: (1) obstruction resulting from physical blockage of the ducts, similar to bladder outlet obstruction, and (2) “functional” obstruction of the seminal vesicle, similar to voiding dysfunction due to bladder myopathy. In addition, this has implications for the diagnosis of ejaculatory duct disorders because “static” anatomical imaging, such as transrectal ultrasonography, may not be sufficient to differentiate between these disorders, and medications and conditions (such as diabetes) might predispose the system to seminal vesicle dysfunction (Smith et al, 2008).
Seminal Vesicle Function
The seminal vesicles secrete a significant proportion (80%) of the seminal fluid, and these secretions are found in later fractions of the ejaculate, after the sperm-rich epididymal, and prostatic secretions. After ejaculation, sperm pass into and through the female cervical mucus and, subsequently, the uterus to enter the oviduct, where fertilization occurs. During residence in the female reproductive tract, sperm must undergo capacitation prior to oocyte fertilization. Capacitation occurs at different rates for individual sperm. During capacitation, the acrosome reaction and development of hyperactivated motility occurs (Yanagimachi, 1994). It is not clear if prostatic or seminal vesicle secretions contribute to capacitation.
In fact, the exact physiologic role of seminal vesicle fluid is not clear, although in rodents it functions as a plug or barrier that reduces the chances for sperm from a subsequent male to fertilize the oocyte. Before ejaculation, semen is a liquid, and after all components mix with the seminal vesicle secretions, it coagulates. The major component of the coagulum is semenogelin I, a 52-kD protein expressed exclusively in the seminal vesicles (Robert et al, 1999). Through coagulating semen, seminal vesicle secretions may promote sperm motility, increase stability of sperm chromatin, and suppress immune activity in the female reproductive tract. The best-elucidated function of human semen appears to be its ability to provide antioxidative protection to sperm. Semen is rich in antioxidant enzymes, including glutathione peroxidase, superoxide dismutase, and catalase (Yeung et al, 1998). In addition, the antioxidant molecules taurine, hypotaurine, and tyrosine are present in high concentrations (van Overveld et al, 2000). Lipofuscin granules from dead epithelial cells give seminal vesicle secretions a yellow-white color. In addition, seminal vesicle secretions are alkaline and contain fructose, mucus, vitamin C, flavins, phosphorylcholine, and prostaglandins. High fructose levels provide nutrient energy for the sperm when studied in vitro. The mixing of seminal vesicle with prostatic secretions results in human semen having a mildly alkaline pH. Acidic ejaculate (pH <7.2) is associated with blockage or absence of seminal vesicles (Turek, 2005).
Spermatozoa
Anatomy and Physiology
The human spermatozoon is approximately 60 µm in length and is divided into 3 morphological sections: head, neck, and tail (Fig. 20–22). The oval sperm head, about 4.5 µm long and 3 µm wide, contains a nucleus with highly compacted chromatin, and an acrosome, a membrane-bound organelle that harbors enzymes required for penetration of the outer vestments of the egg before fertilization (Yanagimachi, 1978). The sperm neck maintains the connection between the sperm head and tail. It consists of the connecting piece and proximal centriole. The axonemal complex extends from the proximal centriole through the sperm tail. The tail harbors the midpiece, principle piece, and end piece (Zamboni, 1992). The midpiece is 7 to 8 µm long and is the most proximal segment of the tail, terminating in the annulus. It contains the axoneme, which is the 9 + 2 microtubule arrangement, and surrounding outer dense fibers (Fig. 20–23). It also contains the mitochondrial sheath, which is helically arranged around the outer dense fibers. The outer dense fibers, rich in disulfide bonds, are not contractile proteins but are thought to provide the sperm tail with the elastic rigidity necessary for progressive motility (Oko and Clermont, 1990). Similar in structure to the midpiece, the principal piece has several columns of outer dense fibers that are replaced by the fibrous sheath. The fibrous sheath consists of longitudinal columns and transverse ribs. The sperm terminates in the endpiece, the most distal segment of the sperm tail, and contains axonemal structures and the fibrous sheath. Except for the end-piece region, the sperm is enveloped by a highly specialized plasma membrane that regulates the transmembrane movement of ions and other molecules (Friend, 1989).
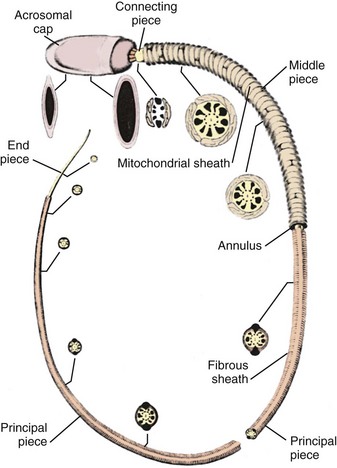
Figure 20–22 Diagram of a typical mammalian spermatozoon. The plasma membrane is omitted to illustrate the major cellular components. Cross-sectional insets show the orientation of the internal cell structures.
(From Fawcett DW. The mammalian spermatozoon. Dev Biol 1975;44:394–436.)
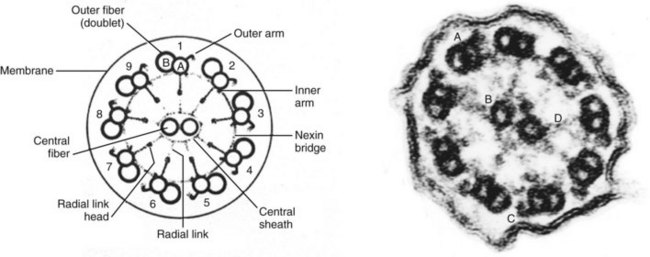
Figure 20–23 The “9 + 2” sperm axonemal structure. Left: schematic cross section of axoneme, demonstrating microtubule arrangement. Right: electron micrograph of axoneme. (A) outer doublet; (B) inner central doublet; (C) outer dynein arm; (D) radial link.
The spermatozoon is a remarkably complex metabolic and genetic machine. The 75 sperm mitochondria that surround the axoneme contain enzymes required for oxidative metabolism and produce adenosine triphosphate (ATP), the primary energy molecule for the cell. Mitochondria are semiautonomous organelles that produce cellular energy and can also cause apoptotic cell death through the release of cytochrome c. Mitochondria are composed of outer and inner membranes. As an organelle, the mitochrondrion has two compartments: the space between the outer and inner membranes, the intermembrane space, and the space surrounded by the inner membrane, termed the matrix. The inner membrane forms deep folds into the matrix, called the cristae, which make the surface area of the inner membrane larger than that of the outer membrane. Five distinct respiratory chain complexes span the width of the inner membrane and are necessary for oxidative phosphorylation: the nicotinamide adenosine diphosphate (NADPH) dehydrogenase, succinate dehydrogenase, cytochrome bc1, cytochrome c oxidase, and ATP synthase complexes. Contained within the matrix are citric acid cycle, fatty acid and amino acid oxidative enzymes, newly made ATP, mitochondrial DNA (mtDNA), and ribosomes. From animal studies, it is clear that the plasma membrane covering the sperm-head region harbors specialized proteins that participate in sperm–egg interaction (Saling, 1989). Indeed, carbohydrate-binding proteins on the sperm membrane interact with the species-specific ZP3 protein in the egg zona pellucida, resulting first in sperm binding to the zona and, subsequently, to induction of the acrosome reaction (Shabanowitz, 1990). Another sperm membrane protein, PH30, is present on testicular sperm and is modified during sperm migration through the epididymis and function as a fusion protein between the sperm and egg membranes at fertilization (Primakoff, 1987; Blobel et al, 1990).
Physiologically, the axoneme is the true motor assembly and requires 200 to 300 proteins for proper function. Among these, the microtubules are the best-understood components. Sperm microtubules are arranged in the classic “9 + 2” pattern of 9 outer doublets encircling an inner central doublet (Fig. 20–23). The complex protein dynein extends from one microtubule doublet to the adjacent doublet, and forms both the inner and outer “arms” of the axoneme. The sperm axoneme contains the enzymes and structural proteins necessary for the chemical transduction of ATP into mechanical movement and motility. Dynein is large (2000 kD), Mg+-stimulated, ATPase responsible for ATP-generated microtubule sliding that causes axonemal bending and, ultimately, sperm flagellar movement. The dynein structure has 2 to 3 globular, outer (heavy) chain heads (500 kD) joined to a common stem. The heads control movement along the microtubules. The inner (light) chain arms (14 to 120 kD) are the primary effectors of movement and are associated with the radial spokes of the dynein assembly. Sperm with outer arm mutants have reduced motility, and those with inner arm mutants have no motility. Radial links or spokes connect a microtubule of each doublet to the central inner doublet and consist of a complex of proteins. The central inner doublet is surrounded by a ringlike helical sheath to which the radial links from the outer doublets are attached. Tektins are proteins associated with the outer microtubular doublets, and nexin links are proteins that connect the outer doublets to each other and maintain the cylindrical axonemal shape.
The human mitochondrial DNA (mtDNA) is distinct from the sperm nuclear DNA. It consists of a circular, histone-free chromosome of 16,569 bp of DNA arranged in a single heavy and single light strand. MtDNA encodes 13 respiratory-chain-complex subunit proteins, 2 mitochondrial rRNAs, and 22 tRNAs used for protein synthesis. These genes have no introns. Expression of mtDNA genes are regulated by strand-specific, but not gene-specific, promoters in response to activation of a transcription factor (mtTFA). MtDNA is also far more susceptible to mutations than is nuclear DNA (estimated 40 to 100 times higher). Reasons for this may include the fact that mitochondria are near respiratory-chain complexes and may be easily attacked by reactive oxygen species. In addition, mtDNA is not coated with protective histones, and mitochondria have very limited DNA repair mechanisms (Hirata et al, 2002). The fact that mitochondria rapidly accumulate mutations suggests the necessity of degrading all paternal mtDNA in the fertilized egg. This degradation is likely mediated by the small proteolytic polypeptide ubiquitin that regulates proteolysis in many tissues (Sutovsky et al, 1999).
The phenotype of defective sperm structure has been recognized as ciliary dyskinesia. Although infertility is the rule with ciliary dyskinesias, ejaculated sperm can be motile and sperm concentrations can be normal. With ICSI, clinical pregnancies and live births have been reported using affected sperm (Cayan et al, 2001). Because the inheritance is usually recessive, normal offspring are likely. Patients suspected of harboring sperm structural defects generally exhibit severely compromised sperm motility (<10%). Sperm electron microscopy can reveal ultrastructural or functional abnormalities of the sperm. Sperm structural abnormalities are currently categorized by Chemes (2000) as follows:
Summary
Spermatogenesis is a remarkably intricate and complex process that is driven by precisely regulated and pulsatile secretions of GnRH, LH, and FSH from the hypothalamic-pituitary-gonadal axis. Pertubations in this hormonal milieu are common causes of male infertility. Sperm production occurs in the testis, a specialized structure that functions optimally at 2° C to 4° C below body temperature and generates a mature human sperm in 64 days. Well-integrated cycles and waves of spermatogenesis ensure that human sperm production is constant at about 1200 sperm per second. Spermatogenesis is an androgen-dependent process that occurs with very high intratesticular levels of testosterone. The product of spermatogenesis, the spermatozoa, leave the testis as immotile cells with limited capacity to fertilize an oocyte. After epididymal transit, sperm are typically motile and capable of fertilization. During ejaculation, sperm are rapidly transported through the ejaculatory ducts into the urethra from the distal epididymis. The ejaculate itself supports sperm metabolism, motility, serves as an antioxidant, and serves as a barrier to exclude subsequent gamete deposits from gaining access to the egg.
Akre O, Richiardi L. Does a testicular dysgenesis syndrome exist? Hum Reprod. 2009;24:2053-2060. [An excellent and critical review of the TDS concept]
Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213-227. [Up-to-date review of epididymal biology]
De Jonge CJ, Barratt CLR, editors. The sperm cell: production, maturation, fertilization, regeneration. New York: Cambridge University Press, 2006. [An up-to-date review of mammalian and human sperm biology, genetics, and function]
DiNapoli L, Capel B. SRY and the standoff in sex determination. Mol Endocrinol. 2008;22:1-9. [A review of the new theory of sex determination in which “testis genes” coexist with “antitestis genes”]
Itman C, Mendis S, Barakat B, Loveland KL. All in the family: TGF-beta family action in testis development. Reproduction. 2006;132:233-246. [An excellent review of HPG axis factors inhibin and activin]
Masters V, Turek PJ. Ejaculatory physiology and dysfunction. Urol Clin North Am. 2001;28:363. [Review of the fundamentals of the physiology and pathology of ejaculation]
Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947-970. [Review of the complex biology of steroid hormone production]
Robaire B, Hinton BT, editors. The epididymis: from molecules to clinical practice: a comprehensive survey of the efferent ducts, the epididymis and vas deferens. New York: Kluwer Academic and Plenum, 2002. [Easily the most comprehensive basic science text on the biology of the epididymis and vas deferens]
Skinner MK, Griswold MD, editors. Sertoli cell biology. San Diego: Elsevier Academic Press, 2005. [State-of-the-art update on Sertoli cells and male reproduction]
Smith JF, Turek PJ. Ejaculatory duct obstruction. Urol Clin North Am. 2008;35:221-227. [Comprehensive review of the biology, physiology, and pathology of the seminal vesicle and ejaculatory duct complex]
Turek PJ, Reijo Pera RA. Current and future genetic screening for male infertility. Urol Clin North Am. 2002;29:767-792. [Comprehensive review of genetic associations with male infertility]
Turek PJ. Male infertility. In Tanagho EA, McAninch JC, editors: Smith’s urology, 15th ed, Stamford (CT): Appleton & Lange, 2003. [chapter 46]. [Basic review of human meiosis]
Walker WH. Molecular mechanisms of testosterone action in spermatogenesis. Steroids. 2009;74:602. [Up-to-date review of the hormone biology of spermatogenesis]
Agger P. Scrotal and testicular temperature: its relation to sperm count before and after operation for varicocele. Fertil Steril. 1971;22:286-297.
Alexander NJ. Prenatal development of the ductus epididymidis in the rhesus monkey: the effects of fetal castration. Am J Anat. 1972;135:119-134.
Amann RP. A critical review of methods for evaluation of spermatogenesis from seminal characteristics. J Androl. 1981;2:37-58.
Amann RP, Almquist JO. Reproductive capacity of dairy bulls: VI. Effect of unilateral vasectomy and ejaculation frequency on sperm reserves: aspects of epididymal physiology. J Reprod Fertil. 1961;3:260-268.
Amann RP, Howards SS. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J Urol. 1980;124:211-215.
Anderson DJ, Hill JA. Cell-mediated immunity in infertility. Am J Reprod Immunol Microbiol. 1988;17:22.
Ascoli M, Segaloff DL. Regulation of the differentiated functions of Leydig tumor cells by epidermal growth factor. Ann NY Acad Sci. 1989;564:99-115.
Bachtell N, Conaghan J, Turek PJ. The relative viability of human spermatozoa from the testis, epididymis and vas deferens before and after cryopreservation. Hum Reprod. 1999;14:101-104.
Bardin CW, Cheng CY, Musto N, Gunsalus G. The Sertoli cell. New York: Raven Press; 1994.
Bardin CW, Morris PL, Shaha C, et al. Inhibin structure and function in the testis. Ann N Y Acad Sci. 1989;564:10-23.
Batra SK, Lardner TJ. Sperm transport in the vas deferens. St Louis: Mosby; 1976.
Baumgarten HG, Falck B, Holstein AF, et al. Adrenergic innervation of the human testis, epididymis, ductus deferens and prostate: a fluorescence microscopic and fluorimetric study. Z Zellforsch Mikrosk Anat. 1968;90:81-95.
Baumgarten HG, Holstein AF. Catecholaminehaltige nervenfasern im hoden des menschen. Z Zellforsch Mirosk Anat. 1967;79:389-395.
Baumgarten HG, Holstein AF, Rosengren E. Arrangement, ultrastructure, and adrenergic innervation of smooth musculature of the ductuli efferentes, ductus epididymidis and ductus deferens of man. Z Zellforsch Mikrosk Anat. 1971;120:37-79.
Baumgarten HG, Owan C, Sjoberg NO. Neural mechanisms in male fertility. New York: Harper & Row; 1975.
Bayard F, Boulard PY, Huc A, Pontonnier F. Arteriovenous transfer of testosterone in the spermatic cord of man. J Clin Endocrinol Metab. 1975;40:345-346.
Beck EM, Schlegel PN, Goldstein M. Intraoperative varicocele anatomy: a macroscopic and microscopic study. J Urol. 1992;148:1190-1194.
Bedford JM. Maturation, transport and fate of spermatozoa in the epididymis. In: Greep RO, Astman EB, editors. Handbook of physiology. Baltimore: Williams & Wilkins; 1975:303-317.
Bedford JM. Report of a workshop: maturation of the fertilizing ability of mammalian spermatozoa in the male and female reproductive tract. Biol Reprod. 1974;11:346-362.
Bedford JM, Calvin HI, Cooper GW. The maturation of spermatozoa in the human epididymis. J Reprod Fertil. 1973;18:199-213.
Bennett G, Leblond CP, Haddal A. Migration of glycoprotein from the Golgi apparatus to the surface of various cell types as shown by autoradiography after labelled fucose injection into rats. J Cell Biol. 1974;60:258-284.
Ben-Rafael Z, Orvieto R. Cytokines–involvement in reproduction. Fertil Steril. 1992;58:1093.
Bick DP, Franco B, Sherins RJ, et al. Brief report: intragenic deletion of the Kalig-1 gene in Kallmann’s syndrome. N Engl J Med. 1992;326:1752.
Billups KL, Tillman S, Chang TSK. Ablation of the inferior mesenteric plexus in the rat: alteration of sperm storage in the epididymis and vas deferens. J Urol. 1990;143:625-629.
Blaquier JA, Cameo MS, Cuasnicu PS, et al. The role of epididymal factors in human sperm fertilizing ability. Ann N Y Acad Sci. 1988;541:292-296.
Blaquier J, Cameo MS, Dawidowski A, et al. On the role of epididymal factors in sperm maturation in man. Serono Symp Publ. 1989;53:37-44.
Blobel CP, Myles DG, Primakoff P, White JM. Proteolytic processing of a protein involved in sperm-egg fusion correlates with acquisition of fertilization competence. J Cell Biol. 1990;111:69-78.
Borda E, Agostini M, Gimeno MF, Gimeno AL. Alpha and beta sympathetic responses to isoproterenol by the isolated rat vas deferens. Pharmacol Res Comm. 1981;13:487-499.
Brandt H, Acott TS, Johnson DJ, Hoskins DD. Evidence for an epididymal origin of bovine sperm forward motility protein. Biol Reprod. 1978;19:830-835.
Brooks DE. Epididymal functions and their hormonal regulation. Aust J Biol Sci. 1983;36:205-221.
Brooks DE, Tiver K. Analysis of surface proteins of rat spermatozoa during epididymal transit and identification of antigens common to spermatozoa, rete testis fluid and cauda epididymal plasma. J Reprod Fertil. 1983;71:249-257.
Brown CR, von Glos KI, Jones R. Changes in plasma membrane glycoproteins of rat spermatozoa during maturation in the epididymis. J Cell Biol. 1983;96:256-264.
Brown PC, Dorling J, Glynn LE. Ultrastructural changes in experimental allergic orchitis in guinea-pigs. J Pathol. 1972;106:229.
Bruschini H, Schmidt RA, Tanagho EA. Studies on the neurophysiology of the vas deferens. Invest Urol. 1977;15:112-116.
Caprio M, Isidori AM, Carta AR, et al. Expression of functional leptin receptors in rodent Leydig cells. Endocrinology. 1999;140:4939-4947.
Casillas ER, Elder CM, Hoskins DD. Adenylate cyclase activity of bovine spermatozoa during maturation in the epididymis and the activation of sperm particulate adenylate cyclase by GTP and polyamines. J Reprod Fertil. 1980;59:297-302.
Cayan S, Schriock E, Conaghan J, Turek PJ. Birth after intracytoplasmic sperm injection using testicular sperm from men with Kartagener/immotile cilia syndrome. Fertil Steril. 2001;76:1.
Chemes HE. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl. 2000;21:799.
Cigorraga SB, Chemes H, Pellizzari E. Steroidogenic and morphogenic characteristics of human peritubular cells in culture. Biol Reprod. 1994;51:1193-1205.
Clavert A, Cranz C, Brun B. Epididymal vascularization and microvascularization. In: Bollack C, Clavert A, editors. Progress in reproductive biology. Basel: Karger; 1981:48-57.
Clegg EJ. The arterial supply of the human prostate and seminal vesicle. J Anat. 1955;89:205-216.
Clement K, Vaisse C, Lahlou S, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398-401.
Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat. 1963;112:35-51.
Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198-236.
Collin O, Lissbrant E, Bergh A. Atrial natriuretic peptide, brain natriuretic peptide and c-type natriuretic peptide: effects on testicular microcirculation and immunohistochemical localization. Int J Androl. 1997;20:55-60.
Conrad S, Renninger M, Hennenlotter J, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344-349.
Cooper TG, Orgebin-Crist MC. Effect of aging on the fertilizing capacity of testicular spermatozoa from the rabbit. Biol Reprod. 1977;16:258-266.
Courot M. Transport and maturation of spermatozoa in the epididymis of mammals. In: Bollack C, Clavert A, editors. Progress in reproductive biology. Basel: Karger; 1981:67.
Courtens JL, Fournier-Delpech S. Modifications in the plasma membranes of epididymal ram spermatozoa during maturation and incubation in utero. J Ultrastruct Res. 1979;68:136-148.
Cuasnicu PS, Bedford JM. The effect of moderate epididymal aging on the kinetics of the acrosome reaction and fertilizing ability of hamster spermatozoa. Biol Reprod. 1989;40:1067-1073.
Cuasnicu PS, Gonzales-Echeverria F, Piazza A, et al. Epididymal proteins mimic the androgenic effect of zona pellucida recognition by immature hamster spermatozoa. J Reprod Fertil. 1984;71:427-431.
Culty M, Li H, Boujrad N, et al. In vitro studies on the role of the peripheral-type benzodiazepine receptor in steroidogenesis. J Steroid Biochem Mol Biol. 1999;69:123-130.
Dacheux JL, Paquignon M. Relations between the fertilizing ability, motility and metabolism of epididymal spermatozoa. Reprod Nutr Dev. 1980;20:1085-1089.
Darney KJJr, Zirkin BR, Ewing LL. Testosterone autoregulation of its biosynthesis in the rat testis: inhibition of 17 alpha-hydroxylase activity. J Androl. 1996;17:137-142.
Davis AG, Horowitz AM. Age-related differences in the response of the isolated testicular capsule of the rat to norepinephrine, acetylcholine and prostaglandins. J Reprod Fertil. 1978;54:269-274.
Davis CM, Papadopoulos V, Sommers CL, et al. Differential expression of extracellular matrix components in rat Sertoli cells. Biol Reprod. 1990;43:860-869.
de Kretser DM, Kerr JB. The cytology of the testis. New York: Raven Press; 1988.
Desjardins C. The microcirculation of the testis. Ann NY Acad Sci. 1989;564:243-249.
Diemer T, Hales DB, Weidner W. Immune-endocrine interactions and Leydig cell function: the role of cytokines. Andrologia. 2003;35:55.
Dinakar RA, Dinaker N, Prasad MRN. Response of the epididymis, ductus deferens and accessory glands of the castrated prepubertal rhesus monkey to exogenous administration of testosterone of 5a-dihydrotestosterone. Indian J Exp Biol. 1977;15:829-834.
DiNapoli L, Capel B. SRY and the standoff in sex determination. Mol Endocrinol. 2008;22:1-9.
Dixon JS, Jen PY, Gosling JA. Structure and autonomic innervation of the human vas deferens: a review. Microsc Res Tech. 1998;42:423-432.
Dode C, Levilliers J, Dupont JM, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463-465.
Dupuy GM, Boulanger KD, Roberts KD, et al. Metabolism of sex steroids in the human and canine vas deferens. Endocrinology. 1979;104:1553-1558.
Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308.
Dym M. Spermatogonial stem cells of the testis. Proc Natl Acad Sci USA. 1994;91:11287-11289.
el-Demiry MI, Hargreave TB, Busuttil A, et al. Lymphocyte sub-populations in the male genital tract. Br J Urol. 1985;57:769.
Eik-Nes KB. Biosynthesis and secretion of testicular steroids. In: Greep RO, Astwood EB, editors. Handbook of physiology. Baltimore: Williams & Wilkins; 1975:95.
El-Gehani F, Zhang FP, Pakarinen P, et al. Gonadotropin-independent regulation of steroidogenesis in the fetal rat testis. Biol Reprod. 1998;58:116-123.
Ewing LL, Brown B. Testicular steroidogenesis. In: Johnson AD, Gomes WR, editors. The testis. New York: Academic Press; 1977:239.
Ewing LL. Leydig cell. In: Lipshultz LI, Howards S, editors. Infertility in the male. New York: Churchill Livingstone; 1983:43.
Ewing LL, Davis JC, Zirkin BR. Regulation of testicular function: a spatial and temporal view. In: Greep RO, editor. International review of physiology. Baltimore: University Park Press; 1980:41.
Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104:2255-2265.
Fawcett DW, Leak LV, Heidger PMJr. Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl. 1970;10:105.
Foldesy RG, Bedford JM. Biology of the scrotum: I. Temperature and androgen as determinants of the sperm storage capacity of the rat cauda epididymis. Biol Reprod. 1982;26:673-682.
Foresta C, Moro E, Ferlin A. Prognostic value of Y deletion analysis. The role of current methods. Hum Reprod. 2001;16:1543-1547.
Friedman JM. Genetic disease in the offspring of older fathers. Obstet Gynecol. 1981;57:745.
Friend DS. Sperm maturation: membrane domain boundaries. Ann N Y Acad Sci. 1989;567:208-221.
Friend DS, Farquhar MG. Functions of coated vesicles during protein absorption in the rat vas deferens. J Cell Biol. 1967;35:357.
Goffin V, Binart N, Touraine P, Kelly P. Prolactin: the new biology of an old hormone. Annu Rev Physiol. 2002;64:47-67.
Goldstein M, Eid JF. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol. 1989;142:743-745.
Gondos B, Hobel CJ. Ultrastructure of germ cell development in the human fetal testis. Z Zellforsch Mikrosk Anat. 1971;119:1-20.
Gonsalves J, Sun F, Schlegel PN, et al. Defective recombination in infertile men. Hum Mol Genet. 2004;13:2875-2883.
Griswold MD, Morales C, Sylvester SR. Molecular biology of the Sertoli cell. Oxf Rev Reprod Biol. 1988;10:53-123.
Gupta G, Rajalakshmi N, Prasad MRN, Moudgal NR. Alteration of epididymal function and its relation to maturation of spermatozoa. Andrologia. 1974;6:35-44.
Gur S, Kadowitz PJ, Hellstrom WJ. Purinergic (P2) receptor control of lower genitourinary tract function and new avenues for drug action: an overview. Curr Pharm Des. 2007;13:3236-3244.
Hamilton DW. The epididymis. In: Greep RO, Koblinsky MA, editors. Frontiers in reproduction and fertility control: II. Cambridge, MA: MIT Press; 1977:411.
Hammond J, Asdell SA. The vitality of the spermatozoa in the male and female reproductive tract. J Exp Biol. 1926;4:155.
Hansson V, Djoseland O. Preliminary characterization of the 5a-dihydrotestosterone binding protein in the epididymal cytosol fraction: in vivo studies. Acta Endocrinol. 1972;71:614-624.
Harrison RG, Barclay AE. The distribution of the testicular artery (internal spermatic artery) to the human testis. Br J Urol. 1948;20:5.
Hayes FJ, Pitteloud N, DeCruz S, et al. Importance of inhibin B in the regulation of FSH secretion in the human male. J Clin Endocrinol Metab. 2001;86:5541-5546.
He Z, Kokkinaki M, Pant D, et al. Small RNA molecules in the regulation of spermatogenesis. Reproduction. 2009;137:901-911.
Heller CG, Clermont Y. Kinetics of the germinal epithelium in man. Rec Prog Horm Res. 1964;20:545-575.
Hermo L, Lalli M, Clermont Y. Arrangement of connective tissue elements in the walls of seminiferous tubules of man and monkey. Am J Anat. 1977;148:433-446.
Hinrichsen MJ, Blaquier JA. Evidence supporting the existence of sperm maturation in the human epididymis. J Reprod Fertil. 1980;60:291-294.
Hirata S, Hoshi K, Shoda T, Mabuchi T. Spermatozoon and mitochondrial DNA. Reprod Med and Biol. 2002;1:41.
Hoffer AP. The ultrastructure of the ductus deferens in man. Biol Reprod. 1976;14:425-443.
Hoffer AP, Hinton BT. Morphological evidence for a blood-epididymis barrier and the effects of gossypol on its integrity. Biol Reprod. 1984;30:991-1004.
Holstein AF. Morphologische studien am nebenhoden des menschen. Zwanglose Abhaudl Gebeit Norm Pathol Anat. 1969;20:1.
Hopps CV, Mielnik A, Goldstein M, et al. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18:1660-1665.
Horan AH, Bedford JM. Development of the fertilizing ability of spermatozoa in the epididymis of the Syrian hamster. J Reprod Fertil. 1972;30:417-423.
Hoskins DD, Munsterman D, Hall ML. The control of bovine sperm glycolysis during epididymal transit. Biol Reprod. 1975;12:566-572.
Huhtaniemi I, Pelliniemi LJ. Fetal Leydig cells: cellular origin, morphology, life span, and special functional features. Proc Soc Exp Biol Med. 1992;201:125-140.
Hundeiker M. Lymphgefasse in parenchym des menschlichen hoden. Arch Klin Exp Dermatol. 1971;235:271.
Itman C, Mendis S, Barakat B, Loveland KL. All in the family: TGF-beta family action in testis development. Reproduction. 2006;132:233-246.
Jaakkola UM. Regional variations in transport of the luminal contents of the rat epididymis in vivo. J Reprod Fertil. 1983;68:465-470.
Jaakkola UM, Talo A. Relation of electrical activity to luminal transport in the cauda epididymis of the rat in vitro. J Reprod Fertil. 1982;64:121-126.
Jarow JP. Clinical significance of intratesticular arterial anatomy. J Urol. 1991;145:777-779.
Jenkins MK, Pardoll DM, Mizuguchi J, et al. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci U S A. 1987;84:5409.
Johnson L, Varner DD. Effect of daily spermatozoan production but not age on transit time of spermatozoa through the human epididymis. Biol Reprod. 1988;39:812-817.
Kaler LW, Neaves WB. Attrition of human Leydig cell population with advancing age. Anat Rec. 1978;192:513-521.
Kerr JB, de Kretser DM. The cytology of the human testis. In: Burger H, de Kretser D, editors. The testis. New York: Raven Press; 1981:141-169.
Kiess W, Reich A, Meyer K, et al. A role for leptin in sexual maturation and puberty? Horm Res. 1999;51:55-63.
Kinoshita Y, Hosaka M, Nishimura R, Takai S. Partial characterization of 5a-reductase in the human epididymis. Endocrinol Jpn. 1980;27:277-284.
Kormano M. Dye permeability and alkaline phosphatase activity of testicular capillaries in the postnatal rat. Histochemie. 1967;9:327-338.
Kormano M, Reijonen K. Microvascular structure of the human epididymis. Am J Anat. 1976;145:23-27.
Kormano M, Suoranta H. An angiographic study of the arterial pattern of the human testis. Anat Anz. 1971;128:69-76.
Kossack N, Meneses J, Shefi S, et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138-149.
Kostiner DR, Turek PJ, Reijo RA. Male infertility: analysis of the markers and genes on the human Y chromosome. Hum Reprod. 1998;13:3032-3038.
Langford GA, Heller CG. Fine structure of muscle cells of the human testicular capsule: basis of testicular contractions. Science. 1973;179:573.
Lanz TV, Neuhauser G. Morphometrische Analyse des menschlichen Nebenhodens. Z Anat Entwicklungsgesch. 1964;124:126.
Larminat MA, Hinrichsen MJ, Scroticati C, et al. Uptake and metabolism of androgen by the human epididymis in vitro. J Reprod Fertil. 1980;59:397-402.
Leinonen P, Hammond GL, Vihko R. Testosterone and some of its precursors and metabolites in the human epididymis. J Clin Endocrinol Metab. 1980;51:423-428.
Le Roy C, Lejeune H, Chuzel F, et al. Autocrine regulation of Leydig cell differentiated functions by insulin-like growth factor I and transforming growth factor beta. J Steroid Biochem Mol Biol. 1999;69:379-384.
Lee KH, Hess RA, Bahr JM, et al. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod. 2000;63:1873-1880.
Lennox B, Ahmad KN. The total length of tubules in the human testis. J Anat. 1970;107:191.
Levallet J, Pakarinen P, Huhtaniemi IT. Follicle-stimulating hormone ligand and receptor mutations, and gonadal dysfunction. Arch Med Res. 1999;30:486-494.
Lich RJr, Howerton LW, Amin M. Anatomy and surgical approach to the urogenital tract in the male. In: Harrison JH, Gittes RF, Perlmutter AD, et al, editors. Campbell’s urology, vol. 1. Philadelphia: WB Saunders; 1978:3-33.
Lino BF, Braden AWH, Turnbull KE. Fate of unejaculated spermatozoa. Nature. 1967;213:594-595.
Linzell JL, Setchell BP. Metabolism, sperm and fluid production of the isolated perfused testis of the sheep and goat. J Physiol. 1969;201:129-143.
Lipsett MB. Steroid secretion by the testis in man. In: James VH, Serio M, Martini L, et al, editors. The endocrine function of the human testis. New York: Academic Press, 1974.
Lipshultz LI, McConnell J, Benson GS. Current concepts of the mechanism of ejaculation. J Reprod Med. 1981;26:499-507.
MacMillan EW. The blood supply of the epididymis in man. Br J Urol. 1954;26:60-71.
Mahi-Brown CA, Yule TD, Tung KS. Evidence for active immunological regulation in prevention of testicular autoimmune disease independent of the blood-testis barrier. Am J Reprod Immunol Microbiol. 1988;16:165.
Majdic G, Saunders PT, Teerds KJ. Immunoexpression of the steroidogenic enzymes 3b hydroxysteroid dehydrogenase and 17a-hydroxylase, C17,20 lyase and the receptor for luteinizing hormone (LH) in the fetal rat testis suggests that the onset of Leydig cell steroid production is independent of LH action. Biol Reprod. 1998;58:520-525.
Marshall FF, Edler JS. Cryptorchidism and related anomalies. New York: Praeger; 1982.
Martan J. Epididymal histochemistry and physiology. Biol Reprod. 1969;1:34-54.
Martin RH, Rademaker AW. The effect of age on the frequency of sperm chromosomal abnormalities in normal men. Am J Hum Genet. 1987;41:484.
Mather JP, Gunsalus GL, Musto NA, et al. The hormonal and cellular control of Sertoli cell secretion. J Steroid Biochem. 1983;19:41-51.
Matthews GJ, Schlegel PN, Goldstein M. Patency following microsurgical vasoepididymostomy and vasovasostomy: temporal considerations. J Urol. 1995;154:2070-2073.
McConnell J, Benson GS, Wood JG. Autonomic innervation of the urogenital system: adrenergic and cholinergic elements. Brain Res Bull. 1982;9:679-694.
Middleton WD, Dahiya N, Naughton CK, et al. High-resolution sonography of the normal extrapelvic vas deferens. J Ultrasound Med. 2009;28:839-846.
Milgrom E, de Roux N, Ghinea N, et al. Gonadotrophin and thyrotrophin receptors. Horm Res. 1997;48:33-37.
Miller WL. Disorders of androgen biosynthesis. Semin Reprod Med. 2002;20:205-216.
Misell LM, Holochwost D, Boban D, et al. A stable isotope/mass spectrometric method for measuring the kinetics of human spermatogenesis in vivo. J Urol. 2006;175:242-246.
Mitchell GAG. The innervation of the kidney, ureter, testicle and epididymis. J Anat. 1935;70:10.
Moore HD, Hartman TD, Pryor JP. Development of the oocyte-penetrating capacity of spermatozoa in the human epididymis. Int J Androl. 1983;6:310-318.
Moore HD, Smith CA. The role of the epididymis during maturation of mammalian spermatozoa in vivo and in vitro. Reprod Nutr Dev. 1988;28:1217-1224.
Morton BE, Sagadraca R, Fraser CF. Sperm motility within the mammalian epididymis: species variation and correlation with free calcium levels in epididymal plasma. Fertil Steril. 1978;29:695-698.
Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747-806.
Muller I. Kanalchen und Capillararchitektonik des ratten Hodens. Z Zellforsch. 1957;45:522-537.
Mulligan T, Iranmanesh A, Johnson ML, et al. Aging alters feed-forward and feedback linkages between LH and testosterone in healthy men. Am J Physiol. 1997;273(4 Pt. 2):1407-1413.
Murakami M, Yokoyama R, Nishida T, et al. Scanning and transmission electron microscope observations of the terminal segment of the cat seminiferous tubule: epithelial phagocytosis of spermatozoa and latex beads. Arch Histol Cytol. 1988;51:185-192.
Neaves WB. Biological aspects of vasectomy. In: Greep RO, Astwood EB, editors. Handbook of physiology. Baltimore: Williams & Wilkins; 1975:383-404.
Nguyen HT, Etzell J, Turek PJ. Normal human ejaculatory duct anatomy: a study of cadaveric and surgical specimens. J Urol. 1996;155:1639-1642.
Nikolopoulou M, Soucek DA, Vary JC. Changes in the lipid content of boar sperm plasma membranes during epididymal maturation. Biochim Biophys Acta. 1985;815:486-498.
Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263-286.
Oko R, Clermont Y. Mammalian spermatozoa: structure and assembly of the tail. In: Gagnon C, editor. Controls of sperm motility: biological and clinical aspects. Boca Raton, FL: CRC Press; 1990:3-27.
Olesen C, Silber J, Eiberg H, et al. Mutational analysis of the human FATE gene in 144 infertile men. Hum Genet. 2003;113:195-201.
Olson GE, Danzo BJ. Surface changes in rate spermatozoa during epididymal transit. Biol Reprod. 1981;24:431-443.
Orgebin-Crist MC. Studies on the function of the epididymis. Biol Reprod. 1969;1:155-175.
Orgebin-Crist MC, Danzo BJ, Davies J. Endocrine control of the development and maintenance of sperm fertilizing ability in the epididymis. In: Greep RO, Astwood EB, editors. Handbook of physiology, section 7, Endocrinology. Baltimore: Williams & Wilkins; 1975:319-338.
Orgebin-Crist MC, Fournier-Delpech S. Sperm-egg interaction: evidence for maturational changes during epididymal transit. J Androl. 1982;3:429-433.
Paniagua R, Regader J, Nistal M, Abaurrea MA. Histological, histochemical an ultrastructural variations along the length of the human vas deferens before and after puberty. Acta Anat. 1981;111:190.
Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947-970.
Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol Reprod. 1995;52:217-225.
Perussia B, Kobayashi M, Rossi ME, et al. Immune interferon enhances functional properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987;138:765.
Pettersson S, Soderholm B, Persson JE, et al. Testicular blood flow in man measured with venous occlusion plethysmography and xenon-133. Scand J Urol Nephrol. 1973;7:115-119.
Pholpramool C, Lea OA, Burrow PV, et al. The effects of acidic epididymal glycoprotein (AEG) and some other proteins on the motility of rat epididymal spermatozoa. Int J Androl. 1983;6:240-248.
Prader A. Testicular size: assessment and clinical importance. Triangle. 1966;7:240-243.
Prasad MR, Rajalakshmi M. Target sites for suppressing fertility in the male. Adv Sex Horm Res. 1976;2:263-287.
Primakoff P, Hyatt H, Tredick-Kline J. Identification and purification of a sperm surface protein with a potential role in sperm-egg membrane fusion. J Cell Biol. 1987;104:141-149.
Prins GS, Zaneveld LJ. Distribution of spermatozoa in the rabbit vas deferens. Biol Reprod. 1979;21:181-185.
Prins GS, Zaneveld LJD. Contractions of the rabbit vas deferens following sexual activity: a mechanism for proximal transport of spermatozoa. Biol Reprod. 1980;23:904.
Prins GS, Zaneveld LJD. Radiographic study of fluid transport in the rabbit vas deferens during sexual rest and after sexual activity. J Reprod Fertil. 1980;58:311-319.
Pujol A, Bayard F, Louvet JP, Boulard C. Testosterone and dihydrotestosterone concentrations in plasma, epididymal tissues and seminal fluid of adult rats. Endocrinology. 1976;98:111-113.
Quinton ND, Smith RF, Clayton PE, et al. Leptin binding activity changes with age: the link between leptin and puberty. J Clin Endocrinol Metab. 1999;84:2336-2341.
Raverot G, Lejeune H, Kotlar T, et al. X-linked sex-determining region Y box 3 (SOX3) gene mutations are uncommon in men with idiopathic oligoazoospermic infertility. J Clin Endocrinol Metabol. 2004;89:4146-4148.
Reijo R, Alagappan RK, Patrizio P, et al. Severe oligozoospermia resulting from deletions of azoospermia factor gene on Y chromosome. Lancet. 1996;347:1290-1293.
Reyes FI, Winter JS, Faiman C. Gonadotropin-gonadal interrelationships in the fetus. Prog Clin Biol Res. 1976;10:83-106.
Rikmaru A, Shirai M. Response of the human testicular capsule to electrical stimulation and to autonomic drugs. Tohoku J Exp Med. 1972;108:303-304.
Risely PL. Physiology of the male accessory organs. New York: Pergamon Press; 1963.
Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: structure, function, and their regulation. In: Knobil E, Neil J, editors. The physiology of reproduction. New York: Raven Press; 1988:999-1080.
Robert M, Gagnon C, Semenogelin I. A coagulum forming, multifunctional seminal vesicle protein. Cell Mol Life Sci. 1999;55:944-960.
Romrell LJ, Ross MH. Characterization of Sertoli cell-germ junctional specializations in disassociated testicular cells. Anat Rec. 1979;193:23-41.
Russell L, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976;185:259-278.
Saez JM, Avallet O, Lejeune H, Chatelain PG. Cell-cell communication in the testis. Horm Res. 1991;36:104-115.
Saling PM. Mammalian sperm interaction with extracellular matrices of the egg. Oxf Rev Reprod Biol. 1989;11:339-388.
Santen RJ. Is aromatization of testosterone to estradiol required for inhibition of luteinizing hormone secretion in men? J Clin Invest. 1975;56:1555-1563.
Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod. 1997;12:1688-1692.
Schoysman RJ, Bedford JM. The role of the human epididymis in sperm maturation and sperm storage as reflected in the consequences of epididymovasostomy. Fertil Steril. 1986;46:293-299.
Schulze W. Structural principles underlying the spermatogenic process in man and a non-human primate (Macaca cynomolgus). In: Reproductive biology and medicine. Berlin: Diesbach Verlag; 1989:58-65.
Schweitzer R. Uber die Bedeutung der Vascularisation, der Binnendruckes und der Zwischenzellen fur die Biologie des Hodens. Anat Entwickl. 1929;89:775-796.
Setchell BP, Brooks DE. Anatomy, vasculature, innervation and fluids of the male reproductive tract. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1988:753-836.
Setchell BP, Waites GMH. The blood-testis barrier. In: Greep RO, Astwood EB, editors. Handbook of physiology. Baltimore: Williams & Wilkins; 1975:143-172.
Shabanowitz RB. Mouse antibodies to human zona pellucida: evidence that human ZP3 is strongly immunogenic and contains two distinct isomer chains. Biol Reprod. 1990;43:260-270.
Sharpe RM. Intratesticular factors controlling testicular function. Biol Reprod. 1984;30:29-49.
Sheth AR, Gunjikar AN, Shah GV. The presence of progressive motility sustaining factor (PMSF) in human epididymis. Andrologia. 1981;13:142-146.
Shupnik MA, Schreihofer DA. Molecular aspects of steroid hormone action in the male reproductive axis. J Androl. 1997;18:341-344.
Silber SJ. Microsurgical aspects of varicocele. Fertil Steril. 1979;31:230-232.
Silber SJ. Apparent fertility of human spermatozoa from the caput epididymidis. J Androl. 1989;10:263-269.
Sjostrand NO. The adrenergic innervation of the vas deferens and the accessory male genital glands. Acta Physiol Scand. 1965;65:7-82.
Skinner MK. Interactions between germ cells and Sertoli cells in the testis. Biol Reprod. 1995;52:211-216.
Skinner MK, Fetterhoff PM, Anthony CT. Purification of a paracrine factor, P-Mod-S, produced by testicular peritubular cells that modulates Sertoli cell function. J Biol Chem. 1988;263:2884-2890.
Smith JF, Walsh TM, Turek PJ. Ejaculatory duct obstruction. Urol Clin North Am. 2008;35:221-227.
Steger RW, Chandrashekar V, Zhao W, et al. Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology. 1998;139:3691-3695.
Steinberger E. Molecular mechanisms concerned with hormonal effects on the seminiferous tubule and endocrine relationships at puberty in the male. In: Spilman CH, Lobl TJ, Kirton KT, et al, editors. Regulatory mechanisms of male reproductive physiology. Amsterdam: Excerpta Medica; 1976:29-34.
Stocco DM. Intramitochondrial cholesterol transfer. Biochim Biophys Acta. 2000;1486:184-197.
Sun F, Mikhaail-Philips M, Oliver-Bonet M, et al. Reduced meiotic recombination on the XY bivalent is correlated with an increased incidence of sex chromosome aneuploidy in men with nonobstructive azoospermia. Mol Hum Reprod. 2008;14:399-404.
Sutovsky P, Moreno R, Ramalho-Santos J, et al. Ubiquitin tag for sperm mitochondria. Nature. 1999;402:371-372.
Suzuki F, Nagano T. Development of tight junctions in caput epididymal epithelium of mouse. Dev Biol. 1978;63:321.
Swerdloff RS, Heber D. Endocrine control of testicular function from birth to puberty. In: Burger H, de Kretser D, editors. The testis. New York: Raven Press; 1981:107-126.
Tapanainen JS, Aittomaki K, Min J, et al. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15:205-206.
Teerds KJ, Dorrington JH. Localization of transforming growth factor beta 1 and beta 2 during testicular development in the rat. Biol Reprod. 1993;48:40-45.
Tezón JG, Ramella E, Cameo MS, et al. Immunochemical localization of secretory antigens in the human epididymis and their association with spermatozoa. Biol Reprod. 1985;32:591-597.
Thomas AJJr. Vasoepididymostomy. Urol Clin N Am. 1987;14:527-538.
Tiepolo L, Zuffardi O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet. 1976;34:119-124.
Tishler PV. Diameter of testicles. N Engl J Med. 1971;285:1489.
Tolia NH, Joshua-Tor L. Slicer and the argonautes. Nat Chem Biol. 2007;3:36-44.
Toyama Y. Actin-like filaments in the myoid cell of the testis. Cell Tissue Res. 1977;177:221-226.
Tung KSK. Autoimmunity of the testis. In: Dhindsa DS, Schumacher GFB, editors. Immunological aspects of infertility and fertility regulation. New York: Elsevier-North Holland; 1980:33.
Tung KS, Skinner MK, Fritz IB. Fibronectin synthesis is a marker for peritubular cell contaminants in Sertoli cell-enriched cultures. Biol Reprod. 1984;30:199-211.
Tung KS, Unanue ER, Dixon FJ. Pathogenesis of experimental allergic orchitis. II. The role of antibody. J Immunol. 1971;106:1463.
Turek PJ. Immunopathology and infertility. In Lipshultz LI, Howards SS, editors: Infertility in the male, 3rd ed, Philadelphia: Mosby Year Book, 1997.
Turek PJ. Practical approach to the diagnosis and management of male infertility. Nat Clin Pract Urol. 2005;2:1-13.
Turek PJ, Aslam KH, Younes AK, Nguyen HT. Observations on seminal vesicle dynamics in an in vivo rat model. J Urol. 1998;159:1731-1734.
Turek PJ, Reijo Pera RA. Current and future genetic screening for male infertility. Urol Clin North Am. 2002;29:767-792.
Turnbull AV, Rivier C. Inhibition of gonadotropin-induced testosterone secretion by the intracerebroventricular injection of interleukin-1 beta in the male rat. Endocrinology. 1997;138:1008-1013.
Turner TT. On the epididymis and its function. Invest Urol. 1979;16:311-321.
Turner TT, D’Addario D, Howards SS. Further observations on the initiation of sperm motility. Biol Reprod. 1978;19:1095-1101.
Turner TT, Giles RD. Sperm motility-inhibiting factor in rat epididymis. Am J Physiol. 1982;242:R199-R203.
Turner TT, Roddy MS. Intraluminal androgen binding protein alters 3H-androgen uptake by rat epididymal tubules in vitro. Biol Reprod. 1990;43:414-419.
Val P, Lefrançois-Martinez A-M, Veyssière G, Martinez A. SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept. 2003;1:8.
van Overveld FW, Haenen GR, Rhemrev J, et al. Tyrosine as important contributor to the antioxidant capacity of seminal plasma. Chem Biol Interact. 2000;127:151-161.
Vendrely E. Histology of the epididymis in the human adult. In: Bollack C, Clavert A, editors. Progress in reproductive biology, vol. 8. Epididymis and fertility: biology and pathology. Basel: Karger; 1981:21-33.
Vendrely E, Dadoune JP. Quantitative ultrastructural analysis of the principal cells in the human epididymis. Reprod Nutr Dev. 1988;28:1225-1235.
Ventura WP, Freund M, Davis J, Pannuti MS. Influence of norepinephrine on the motility of the human vas deferens: a new hypothesis of sperm transport by the vas deferens. Fertil Steril. 1973;24:68-77.
Vermeulen A, Kaufman JM. Ageing of the hypothalamo-pituitary-testicular axis in men. Horm Res. 1995;43:25-28.
Vogt PH, Edelmann A, Kirsch S, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5:933-943.
Voglmayr JK. Metabolic changes in spermatozoa during epididymal transit. In: Greep RO, Astwood EB, editors. Handbook of physiology, section 7, Endocrinology. Baltimore: William & Wilkins; 1975:437-451.
Walsh TJ, Reijo Pera RA, Turek PJ. Genetic male infertility. Semin Reprod Med. 2009;2:124-136.
Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27:422-426.
Wennbo H, Kinblom J, Isaksson OGP, Törnell J. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement in the prostate gland. Endocrinology. 1997;138:4410-4415.
Wenzel J, Kellermann P. Vergleichende untersuchungen uber das Lymphgefasssytem des Nebenhodens und Hodesn von Mensch, hund unk Kaninchwen. Z Mikrosk Anat Forsch. 1966;75:368-387.
West LA, Horvat RD, Roess DA, et al. Steroidogenic acute regulatory protein and peripheral-type benzodiazepine receptor associate at the mitochondrial membrane. Endocrinology. 2001;142:502-505.
Winter JSD, Faiman C. Pituitary-gonadal relations in male children and adolescents. Pediatr Res. 1972;6:126-131.
Witschi E. Migration of the germ cells of human embryos from the yolk sac to the primitive gonadal fold. Carnegie Institute Wash Contrib Embryol. 1948;209:67-80.
Wong PY, Au CL, Bedford JM. Biology of the scrotum: II. Suppression by abdominal temperature of transepithelial ion and water transport in the cauda epididymidis. Biol Reprod. 1982;26:683-689.
Wyrobek AJ, Aardema M, Eichenlaub-Ritter U, et al. Mechanisms and targets involved in maternal and paternal age effects on numerical aneuploidy. Environ Mol Mutag. 1996;28:254-264.
Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48-61.
Yanagimachi R. Fertilization and developmental initiation of oocytes by injection of spermatozoa and pre-spermatozoal cells. Ital J Anat Embryol. 2005;110:145-150.
Yanagimachi R. Fertilization. In: Knobil E, Neill JD, Greenwald GS, et al, editors. The physiology of reproduction. New York: Raven; 1994:189-317.
Yanagimachi R. Sperm-egg association in mammals. In: Moscona AA, Monroy A, editors. Current topics in developmental biology. New York: Academic Press; 1978:83-105.
Yoshinaga K, Nishikawa S, Ogawa M, et al. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689-699.
Yeung CH, Sonnenberg-Riethmacher E, Cooper TG. Receptor tyrosine kinase c-ros knockout mice as a model for the study of epididymal regulation of sperm function. J Reprod Fertil. 1998;53(Suppl.):137-147.
Young WC. A study of the function of the epididymis: II. The importance of an ageing process in sperm for the length of the period during which fertilizing capacity is retained by sperm isolated in the epididymis of the guinea pig. J Morphol. 1929;48:475-491.
Zamboni L. Sperm structure and its relevance to infertility. An electron microscopic study. Arch Pathol Lab Med. 1992;116:325.