chapter 23 Physiology of Penile Erection and Pathophysiology of Erectile Dysfunction
“The penis does not obey the order of its master, who tries to erect or shrink it at will. Instead, the penis erects freely while its master is asleep. The penis must be said to have its own mind, by any stretch of the imagination.”
Physiology of penile erection
Historical Aspects
The first description of erectile dysfunction (ED) dates from about 2000 BC and was set down on Egyptian papyrus. Two types were described: natural (“the man is incapable of accomplishing the sex act”) and supernatural (evil charms and spells). Later, Hippocrates reported many cases of male impotence among the rich inhabitants of Scythia and ascribed it to excessive horseback riding. Aristotle stated that three branches of nerves carry spirit and energy to the penis and that erection is produced by the influx of air (Brenot, 1994). His theory was well accepted until Leonardo da Vinci (1504) noted a large amount of blood in the erect penis of hanged men and cast doubt on the concept of the air-filled penis. His writings, however, were kept secret until the beginning of the 20th century (Brenot, 1994). Nevertheless, in 1585, in Ten Books on Surgery and the Book of Reproduction, Ambroise Paré gave an accurate account of penile anatomy and the concept of erection. He described the penis as being composed of concentric coats of nerves, veins, and arteries and of two ligaments (corpora cavernosa), a urinary tract, and four muscles. “When the man becomes inflamed with lust and desire, blood rushes into the male member and causes it to become erect,” Paré wrote. The importance of retaining blood in the penis was stressed by Dionis (1718; quoted by Brenot, 1994), who attributed this to the muscles cramping the veins at the proximal end, and by Hunter (1787), who thought that venous spasm prevented the exit of blood.
Modern investigations of penile hemodynamics began in the 1970s with xenon washout and cavernosography studies in human volunteers exposed to audiovisual sexual stimuli. These studies yielded conflicting results: Shirai and associates (1978) concluded that penile venous flow is increased during erection, but markedly increased arterial flow compensates for this; in contrast, Wagner (1981) also demonstrated increased arterial flow but concluded that venous drainage is decreased during erection.
Much of the current understanding of erectile physiology was gained in the 1980s and 1990s. In addition to the role of smooth muscle in regulating arterial and venous flow, the three-dimensional structure of the tunica albuginea and its role in venous occlusion were elucidated. An important breakthrough in the understanding of neural influences was the identification of nitric oxide (NO) as the major neurotransmitter for erection and of phosphodiesterases (PDEs) for detumescence. The role of endothelium in regulating smooth muscle tone and of the intercellular links affected by gap junctions has been uncovered. Furthermore, the importance of ion channels (potassium and calcium) and Rho/Rho kinase pathways in contraction and relaxation of smooth muscle has been shown. In pathophysiology, changes in smooth muscle, nerve endings, endothelium, and the fibroelastic framework associated with disease have been identified. These developments are discussed in detail in this chapter.
Functional Anatomy of the Penis
The penis is composed of three cylindrical structures: the paired corpora cavernosa and the corpus spongiosum (which houses the urethra), covered by a loose subcutaneous layer and skin. Its flaccid length is controlled by the contractile state of the erectile smooth muscle and varies considerably, depending on emotion and outside temperature. In one study, penile length, measured from the pubopenile junction to the meatus, was 8.8 cm flaccid, 12.4 cm stretched, and 12.9 cm erect, with neither age nor the size of the flaccid penis accurately predicting erectile length (Wessells et al, 1996). In another study, the author concluded that about 15% of men have a downward curve during erection; erect angle is below horizontal in one quarter; and shorter erect lengths (from 4.5 to 5.75 inches) occur in 40% of men (Sparling, 1997). Since then, more studies have been reported from several countries (Awwad et al, 2005) (Table 23–1). Regarding penile morphology and erection, one study showed that, during erection, the penile buckling forces are dependent not only on intracavernous pressures but also on penile geometry and erectile tissue properties. The authors concluded that, in patients with normal penile hemodynamics but without adequate rigidity, structural causes should be investigated (Udelson et al, 1998).
Tunica Albuginea
The tunica affords great flexibility, rigidity, and tissue strength to the penis (Hsu et al, 1992) (Fig. 23–1). The tunical covering of the corpora cavernosa is a bilayered structure with multiple sublayers. Inner-layer bundles support and contain the cavernous tissue and are oriented circularly. Radiating from this inner layer are intracavernous pillars that act as struts to augment the septum and provide essential support to the erectile tissue. Outer-layer bundles are oriented longitudinally, extending from the glans penis to the proximal crura; they insert into the inferior pubic rami but are absent between the 5 and the 7 o’clock positions. In contrast, the corpus spongiosum lacks an outer layer or intracorporeal struts, ensuring a low-pressure structure during erection.
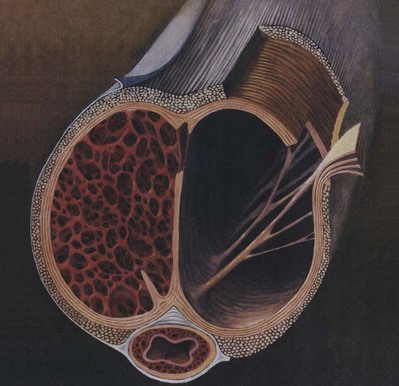
Figure 23–1 Artist’s cross-sectional drawing of the penis, depicting the inner circular and outer longitudinal layers of the tunica albuginea, as well as the intracavernous pillars. The longitudinal layer is absent in the ventral groove housing the corpus spongiosum.
(From Lue TF, Akkus E, Kour NW. Physiology of erectile function and dysfunction. Campbell’s Urology Update 1994;12:1–10.)
The tunica is composed of elastic fibers that form an irregular, latticed network on which the collagen fibers rest (Fig. 23–2). The detailed histologic composition of the tunica varies with anatomic location and function. Emissary veins run between the inner and outer layers for a short distance, often piercing the outer bundles obliquely. However, the cavernous artery and the branches of the dorsal artery that give additional blood supply to the corpus cavernosum take a more direct route and are surrounded by a periarterial soft-tissue sheath, which protects the arteries from occlusion by the tunica albuginea during erection.
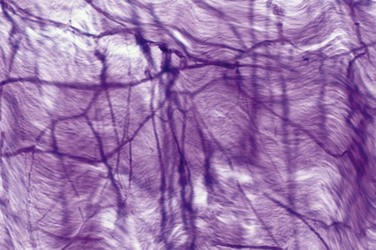
Figure 23–2 Micrograph of the human tunica albuginea, showing the interwoven elastic fibers and the finer collagen fibers (Hart stain ×100).
The outer tunical layer appears to play an additional role in compression of the emissary veins during erection. It also determines, to a large extent, the variability in tunical thickness and strength (Hsu et al, 1992). Between the 6 and 7 o’clock positions, the tunical thickness is 0.8 ± 0.1 mm; at the 9 o’clock position, 1.2 ± 0.2 mm; and at the 11 o’clock position, 2.2 ± 0.4 mm. At the 3, 5 to 6, and 1 o’clock positions, the measurements are nearly identical in mirror-image fashion. (Differences at specific locations have been found to be statistically significant.)
The stress on the tunica before penetration has been measured as 1.6 ± 0.2 × 107 N/m2 between the 6 and 7 o’clock positions, 3.0 ± 0.3 × 107 N/m2 at the 9 o’clock position, and 4.5 ± 0.5 × 107 N/m2 at the 11 o’clock position. The strength and thickness of the tunica correlate in a statistically significant fashion with location. The most vulnerable area is located on the ventral groove (between the 5 and 7 o’clock positions), where the longitudinal outer layer is absent; most prostheses tend to extrude here (Hsu et al, 1994).
The tunica albuginea is composed of fibrillar collagen (mostly type I, but also type III) in organized arrays interlaced with elastin fibers. Although collagen has a greater tensile strength than steel, it is unyielding. In contrast, elastin can be stretched up to 150% of its length. It is the elastin content that allows tunical expansion and helps to determine stretched penile length.
External penile support consists of two ligamentous structures: the fundiform and suspensory ligaments. The fundiform ligament arises from Colles’ fascia and is lateral, superficial, and not adherent to the tunica albuginea of the corpora cavernosa. The suspensory ligament arises from Buck fascia and consists of two lateral bundles and one median bundle, which circumscribe the dorsal vein of the penis. Its main function is to attach the tunica albuginea of the corpora cavernosa to the pubis, and thus it provides support for the mobile portion of the penis (Hoznek et al, 1998). In patients with congenital deficiency or in whom this ligament has been severed in “penile elongation” surgery, the erect penis may be unstable or droop.
Corpora Cavernosa, Corpus Spongiosum, and Glans Penis
The corpora cavernosa comprise two spongy, paired cylinders contained in the thick envelope of the tunica albuginea. Their proximal ends, the crura, originate at the undersurface of the puboischial rami as two separate structures but merge under the pubic arch and remain attached up to the glans. The septum between the two corpora cavernosa is incomplete in men but is complete in some species such as the dog.
The corpora cavernosa are supported by a fibrous skeleton that includes the tunica albuginea, the septum, the intracavernous pillars, the intracavernous fibrous framework, and the periarterial and perineural fibrous sheath (Goldstein and Padma-Nathan, 1990; Hsu et al, 1992). Within the tunica are the interconnected sinusoids separated by smooth muscle trabeculae surrounded by elastic fibers, collagen, and loose areolar tissue. The terminal cavernous nerves and helicine arteries are intimately associated with the smooth muscle. Each corpus cavernosum is a conglomeration of sinusoids, larger in the center and smaller in the periphery. In the flaccid state, the blood slowly diffuses from the central to the peripheral sinusoids and the blood gas levels are similar to those of venous blood. During erection, the rapid entry of arterial blood to both the central and the peripheral sinusoids changes the intracavernous blood gas levels to those of arterial blood (Sattar et al, 1995).
The structure of the corpus spongiosum and glans is similar to that of the corpora cavernosa, except that the sinusoids are larger; the tunica is thinner in the spongiosum (with only a circular layer [see earlier]) and is absent in the glans (Table 23–2).
Table 23–2 Penile Components and Their Function during Penile Erection
| Corpora cavernosa | Support corpus spongiosum and glans |
| Tunica albuginea (of corpora cavernosa) | Contains and protects erectile tissue |
| Provides rigidity of the corpora cavernosa | |
| Participates in veno-occlusive mechanism | |
| Smooth muscle | Regulates blood flow into and out of the sinusoids |
| Ischiocavernosus muscle | Pumps blood distally to hasten erection |
| Provides additional penile rigidity during rigid erection phase | |
| Bulbocavernosus muscle | Compresses the bulb to help expel semen |
| Corpus spongiosum | Pressurizes and constricts the urethral lumen to allow forceful expulsion of semen |
| Glans | Acts as a cushion to lessen the impact of penis on female organs |
| Provides sensory input to facilitate erection and enhance pleasure | |
| Facilitates intromission because of its cone shape |
Arteries
The source of penile blood is usually the internal pudendal artery, a branch of the internal iliac artery (Fig. 23–3A). In many instances, however, accessory arteries exist, arising from the external iliac, obturator, and vesical and femoral arteries, and they may in some men constitute the dominant or only arterial supply to the corpus cavernosum (Breza et al., 1989). In a study of 20 fresh human cadavers, Droupy and colleagues (1997) reported three patterns of penile arterial supply: type I arising exclusively from internal pudendal arteries (3/20); type II arising from both accessory and internal pudendal arteries (14/20); and type III arising exclusively from accessory pudendal arteries (3/20). Nehra and colleagues (2008) studied 79 consecutive patients with a history of erectile dysfunction (ED) and noted that 35% had an accessory pudendal artery, typically arising from the obturator artery. In these men, the accessory pudendal was the dominant blood supply in 54% and the only corporal blood supply in 11%. The importance of accessory pudendal artery preservation during radical prostatectomy was demonstrated by Mulhall and colleagues, who reported more rapid recovery of sexual function in men who underwent artery-sparing radical prostatectomy (Mulhall et al, 2008).
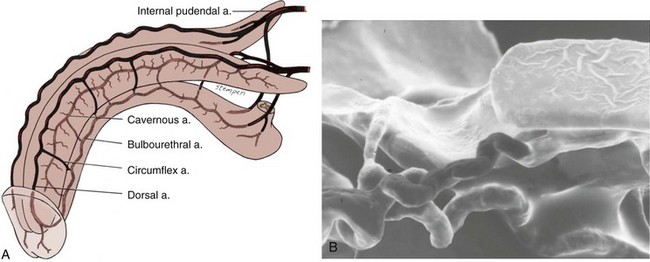
Figure 23–3 A, Penile arterial supply. B, Scanning electron micrograph of a human penile cast showing helicine arteries opening directly into the sinusoids without intervening capillaries.
The internal pudendal artery becomes the common penile artery after giving off a branch to the perineum. The three branches of the penile artery are the dorsal, bulbourethral, and cavernous. Distally, they join to form a vascular ring near the glans. The dorsal artery is responsible for engorgement of the glans during erection. The bulbourethral artery supplies the bulb and corpus spongiosum. The cavernous artery effects tumescence of the corpus cavernosum and enters it at the hilum of the penis, where the two crura merge. Along its course, it gives off many helicine arteries, which supply the trabecular erectile tissue and the sinusoids (Fig. 23–3B). These helicine arteries are contracted and tortuous in the flaccid state and become dilated and straight during erection.
Veins
The venous drainage from the three corpora originates in tiny venules leading from the peripheral sinusoids immediately beneath the tunica albuginea. These venules travel in the trabeculae between the tunica and the peripheral sinusoids to form the subtunical venous plexus before exiting as the emissary veins (Fig. 23–4A). Outside the tunica albuginea, venous drainage is as follows:
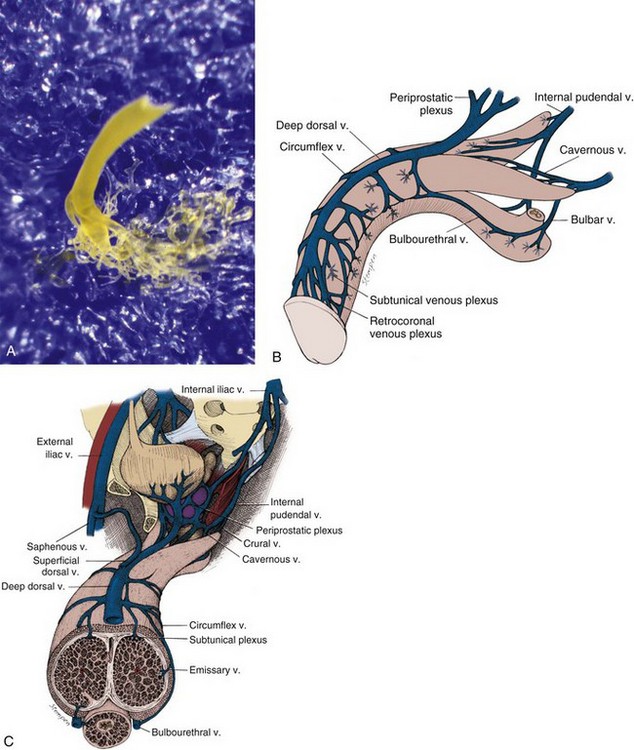
Figure 23–4 A, Photograph of an emissary vein with subtunical venous plexus of a human penile cast. The cast was made by injecting blue material into the corpus cavernosum and yellow material into the deep dorsal vein. The skin and tunica albuginea were then digested away with KOH solution. B and C, Penile venous drainage.
Skin and Subcutaneous Tissue
Multiple superficial veins run subcutaneously and unite near the root of the penis to form a single (or paired) superficial dorsal vein, which in turn drains into the saphenous veins. Occasionally, the superficial dorsal vein may also drain a portion of the corpora cavernosa.
Pendulous Penis
The emissary veins from the corpus cavernosum and spongiosum drain dorsally to the deep dorsal, laterally to the circumflex, and ventrally to the periurethral veins. Beginning at the coronal sulcus, multiple venous channels coalesce to form the deep dorsal vein, which is the main venous drainage of the glans penis and distal two thirds of the corpora cavernosa. Usually a single vein, but sometimes more than one deep dorsal vein, runs upwards behind the symphysis pubis to join the periprostatic venous plexus. There are also small venous channels accompanying the paired dorsal artery. In addition, many veins also travel longitudinally within the layers of the tunica albuginea to join the dorsal vein or Santorini plexus proximally (Hsu et al, 2003). These become enlarged after the deep dorsal vein is ligated and may be the cause of recurrent leakage in venogenic ED (Chen et al, 2005).
Infrapubic Penis
Emissary veins draining the proximal corpora cavernosa join to form cavernous and crural veins. These join the periurethral veins from the urethral bulb to form the internal pudendal veins.
The veins of the three systems communicate variably with each other. Indeed, variations in the number, distribution, and termination of these venous systems are common (Fig. 23–4B and C).
Hemodynamics and Mechanism of Erection And Detumescence
Corpora Cavernosa
The penile erectile tissue, specifically the cavernous smooth musculature and the smooth muscles of the arteriolar and arterial walls, plays a key role in the erectile process. In the flaccid state, these smooth muscles are tonically contracted, allowing only a small amount of arterial flow for nutritional purposes. The blood partial pressure of oxygen (pO2) is about 35 mm Hg (Sattar et al, 1995). The flaccid penis is in a moderate state of contraction, as evidenced by further shrinkage in cold weather and after phenylephrine injection.
Sexual stimulation triggers release of neurotransmitters from the cavernous nerve terminals. This results in relaxation of these smooth muscles and the following events (Fig. 23–5): (1) dilation of the arterioles and arteries by increased blood flow in both the diastolic and systolic phases; (2) trapping of the incoming blood by the expanding sinusoids; (3) compression of the subtunical venous plexuses between the tunica albuginea and the peripheral sinusoids, reducing venous outflow; (4) stretching of the tunica to its capacity, which occludes the emissary veins between the inner circular and outer longitudinal layers and further decreases venous outflow to a minimum; (5) an increase in PO2 (to about 90 mm Hg) and intracavernous pressure (around 100 mm Hg), which raises the penis from the dependent position to the erect state (the full-erection phase); and (6) a further pressure increase (to several hundred millimeters of mercury) with contraction of the ischiocavernosus muscles (rigid-erection phase).
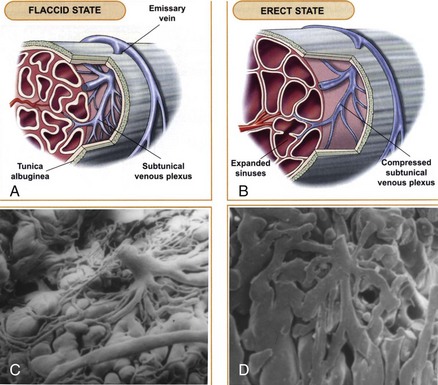
Figure 23–5 The mechanism of penile erection: A, In the flaccid state, the arteries, arterioles, and sinusoids are contracted. The intersinusoidal and subtunical venous plexuses are wide open, with free flow to the emissary veins. B, In the erect state, the muscles of the sinusoidal wall and the arterioles relax, allowing maximal flow to the compliant sinusoidal spaces. Most of the venules are compressed between the expanding sinusoids. The larger venules are sandwiched and flattened between the distended sinusoids and the tunica albuginea. This effectively reduces the venous capacity to a minimum. C and D, Scanning electron micrographs of casts of a canine subtunical venous plexus in the flaccid and erect states, respectively.
(A and B, From Lue TF, Giuliano F, Khoury S, Rosen R. Clinical manual of sexual medicine: sexual dysfunction in men. Paris: Health Publications; 2004.)
The angle of the erect penis is determined by its size and attachment to the puboischial rami (the crura) and the anterior surface of the pubic bone (the suspensory and funiform ligaments). In men with a long heavy penis or a loose suspensory ligament, the angle usually will not be greater than 90 degrees, even with full rigidity.
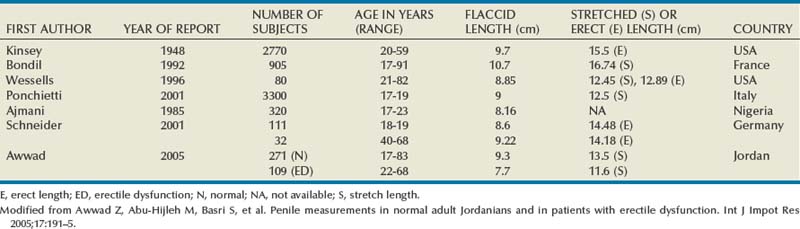
Three phases of detumescence have been reported in an animal study (Bosch et al, 1991). The first entails a transient intracorporeal pressure increase, indicating the beginning of smooth muscle contraction against a closed venous system. The second phase shows a slow pressure decrease, suggesting a slow reopening of the venous channels with resumption of the basal level of arterial flow. The third phase shows a fast pressure decrease with fully restored venous outflow capacity.
Erection thus involves sinusoidal relaxation, arterial dilation, and venous compression (Lue et al, 1983). The importance of smooth muscle relaxation has been demonstrated in animal and human studies (Saenz de Tejada et al, 1989a; Ignarro et al, 1990). To summarize the hemodynamic events of erection and detumescence, seven phases have been observed in animal experiments that reflect the changes in and the relationship between penile arterial flow and intracavernous pressure (Fig. 23–6).
Corpus Spongiosum and Glans Penis
The hemodynamics of the corpus spongiosum and glans penis are somewhat different from those of the corpora cavernosa. During erection, the arterial flow increases in a similar manner; however, the pressure in the corpus spongiosum and glans is only one-third to one-half that in the corpora cavernosa because the tunical covering, which is thin over the corpus spongiosum and virtually absent over the glans, ensures minimal venous occlusion. During the full-erection phase, partial compression of the deep dorsal and circumflex veins between Buck fascia and the engorged corpora cavernosa contributes to glanular tumescence, although the spongiosum and glans essentially function as a large arteriovenous shunt during this phase. In the rigid-erection phase, the ischiocavernosus and bulbocavernosus muscles forcefully compress the spongiosum and penile veins, resulting in further engorgement and increased pressure in the glans and spongiosum (Table 23–3).
Table 23–3 Comparison of Corpus Spongiosum and the Glans
| CORPUS SPONGIOSUM | GLANS PENIS | |
|---|---|---|
| Tunica albuginea | Thin (circular layer only) | Absent |
| Main blood supply | Bulbal and spongiosal arteries | Dorsal artery |
| Venous occlusion during erection | No | No |
| Compression by skeletal muscle | Yes (ischiocavernosus, bulbocavernosus) | No |
Neuroanatomy and Neurophysiology of Penile Erection
Spinal Centers and Peripheral Pathways
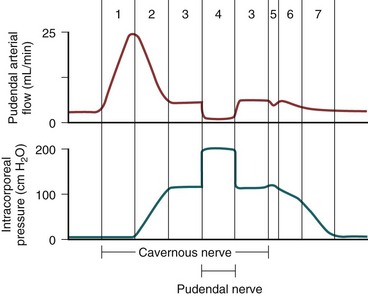
The innervation of the penis is both autonomic (sympathetic and parasympathetic) and somatic (sensory and motor) (Fig. 23–7). From the neurons in the spinal cord and peripheral ganglia, the sympathetic and parasympathetic nerves merge to form the cavernous nerves, which enter the corpora cavernosa and corpus spongiosum to modulate the neurovascular events during erection and detumescence. The somatic nerves are primarily responsible for sensation and the contraction of the bulbocavernosus and ischiocavernosus muscles.
Autonomic Pathways
The sympathetic pathway originates from the 11th thoracic to the 2nd lumbar spinal segments and passes through the white rami to the sympathetic chain ganglia. Some fibers then travel through the lumbar splanchnic nerves to the inferior mesenteric and superior hypogastric plexuses, from which fibers travel in the hypogastric nerves to the pelvic plexus. In humans the T10 to T12 segments are most often the origin of the sympathetic fibers, and the chain ganglia cells projecting to the penis are located in the sacral and caudal ganglia (de Groat and Booth, 1993).
The parasympathetic pathway arises from neurons in the intermediolateral cell columns of the second, third, and fourth sacral spinal cord segments. The preganglionic fibers pass in the pelvic nerves to the pelvic plexus, where they are joined by the sympathetic nerves from the superior hypogastric plexus. The cavernous nerves are branches of the pelvic plexus that innervate the penis. Other branches innervate the rectum, bladder, prostate, and sphincters. The cavernous nerves are easily damaged during radical excision of the rectum, bladder, and prostate. A clear understanding of the course of these nerves is essential to the prevention of iatrogenic ED (Walsh et al, 1990). Human cadaveric dissection has revealed medial and lateral branches of the cavernous nerves (the former accompanying the urethra and the latter piercing the urogenital diaphragm 4 to 7 mm lateral to the sphincter) and multiple communications between the cavernous and dorsal nerves (Fig. 23–8) (Paick et al, 1993). In addition to the cavernous nerve proper, pelvic ganglion cells also exist in and along the nerve components and pelvic viscera. These are seen at the bladder/prostate junction, the dorsal aspect of the seminal vesicles, and along the prostate. Takenaka and colleagues (2005) reported individual variations in distribution of these extramural ganglion cells in the male pelvis, which may complicate nerve-sparing efforts.
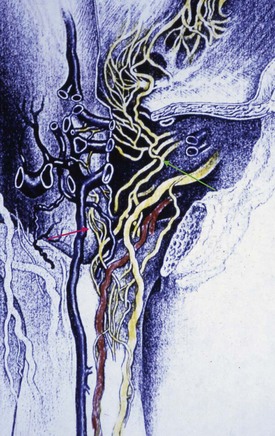
Figure 23–8 Drawing from a human cadaveric dissection shows the medial (red arrow) and lateral (green arrow) bundles of the cavernous nerve distal to the prostate.
(Reprinted with permission from Paick JS, Donatucci EF, Lue TF. Anatomy of cavernous nerves distal to prostate: microdissection study in adult male cadavers. Urology 1993;42:145–9, with permission from Exerpta Medica, Inc.)
Stimulation of the pelvic plexus and the cavernous nerves induces erection, whereas stimulation of the sympathetic trunk causes detumescence. This clearly implies that the sacral parasympathetic input is responsible for tumescence and the thoracolumbar sympathetic pathway is responsible for detumescence. In experiments with cats and rats, removal of the spinal cord below L4 or L5 reportedly eliminated the reflex erectile response, but placement with a female in heat or electrical stimulation of the medial preoptic area (MPOA) produced marked erection (Giuliano et al, 1996; Sato and Christ, 2000). Paick and Lee (1994) also reported that apomorphine-induced erection is similar to psychogenic erection in the rat and can be induced by means of the thoracolumbar sympathetic pathway in case of injury to the sacral parasympathetic centers. In man, many patients with sacral spinal cord injury retain psychogenic erectile ability even though reflexogenic erection is abolished. These cerebrally elicited erections are found more frequently in patients with lower motoneuron lesions below T12 (Courtois et al, 1999); no psychogenic erection occurs in patients with lesions above T9. The efferent sympathetic outflow is thus suggested to be at the levels T11 and T12 (Chapelle et al, 1980). These authors have also reported that, in patients with psychogenic erections, lengthening and swelling of the penis are observed but rigidity is insufficient.
It is therefore possible that, for rigid-erection production in normal men, cerebral impulses travel as follows: inhibiting the sympathetic pathway and thus decreasing norepinephrine release; through the parasympathetic, releasing NO and acetylcholine; and the somatic, releasing acetylcholine. In patients with a sacral cord lesion, the cerebral impulses can still travel by means of the sympathetic pathway to inhibit norepinephrine release, and NO and acetylcholine can still be released through synapse with postganglionic parasympathetic and somatic neurons. Because the number of these synapses is less than in men with an intact sacral spinal cord, the resulting erection will not be as strong.
Somatic Pathways
The somatosensory pathway originates at the sensory receptors in the penile skin, glans, and urethra and within the corpus cavernosum. In the human glans penis are numerous afferent terminations: free nerve endings and corpuscular receptors in a ratio of 10 : 1. The free nerve endings are derived from thin myelinated Ad and unmyelinated C fibers and are unlike any other cutaneous area in the body (Halata and Munger, 1986). The nerve fibers from the receptors converge to form bundles of the dorsal nerve of the penis, which joins other nerves to become the pudendal nerve. The latter enters the spinal cord via the S2-S4 roots to terminate on spinal neurons and interneurons in the central gray region of the lumbosacral segment (McKenna, 1998). Activation of these sensory neurons sends messages of pain, temperature, and touch by means of spinothalamic and spinoreticular pathways to the thalamus and sensory cortex for sensory perception.
The dorsal nerve of the penis used to be regarded as purely somatic; however, nerve bundles testing positive for NO synthase (NOS), which is autonomic in origin, have been demonstrated in the human by Burnett and colleagues (1993) and in the rat by Carrier and colleagues (1995). Giuliano and colleagues (1993) have also shown that stimulation of the sympathetic chain at the L4-L5 level elicits an evoked discharge on the dorsal nerve and that stimulation of the dorsal nerve evokes a reflex discharge in the lumbosacral sympathetic chain of rats. These findings clearly demonstrate that the dorsal nerve has both somatic and autonomic components that enable it to regulate both erectile and ejaculatory functions.
The Onuf nucleus in the second to fourth sacral spinal segments is the center of somatomotor penile innervation. These nerves travel in the sacral nerves to the pudendal nerve to innervate the ischiocavernosus and bulbocavernosus muscles. Contraction of the ischiocavernosus muscles produces the rigid-erection phase. Rhythmic contraction of the bulbocavernosus muscle is necessary for ejaculation. In animal studies, direct innervation of the sacral spinal motoneurons by brainstem sympathetic centers (A5-catecholaminergic cell group and locus ceruleus) has been identified (Marson and McKenna, 1996). This adrenergic innervation of pudendal motoneurons may be involved in rhythmic contractions of perineal muscles during ejaculation. In addition, oxytocinergic and serotonergic innervation of lumbosacral nuclei controlling penile erection and perineal muscles in the male rat has also been demonstrated (Tang et al, 1998).
Depending on the intensity and nature of genital stimulation, several spinal reflexes can be elicited (Table 23–4). The best known is the bulbocavernosus reflex, which is the basis of genital neurologic examination and electrophysiologic latency testing. Although impairment of bulbocavernosus and ischiocavernosus muscles may impair erection, the significance of obtaining a bulbocavernosus reflex in overall sexual dysfunction assessment is controversial.
Supraspinal Pathways and Centers
Integration and processing of afferent inputs (e.g., visual, olfactory, imaginative, genital stimulation) in the supraspinal centers are essential in the initiation and maintenance of penile erection. Many brain areas have been found to be associated with sexual function including the medial amygdala, the medial preoptic area (MPOA), the paraventricular nucleus (PVN), the periaqueductal gray, and ventral tegmentum (Table 23–5). Marson and colleagues (1993) injected pseudo-rabies virus into the rat corpus cavernosum and traced labeled neurons from major pelvic ganglia to neurons in the spinal cord, brainstem, and hypothalamus. Mallick and colleagues (1994) also showed that stimulation of the dorsal nerve in the rat influenced the firing rate of about 80% of the neurons in the MPOA but not in other areas of the hypothalamus. Efferent pathways from the MPOA enter the medial forebrain bundle and the midbrain tegmental region (near the substantia nigra). Pathologic processes in these regions such as Parkinson disease or cerebrovascular accidents are often associated with erectile dysfunction. Axonal tracing in monkeys, cats, and rats has shown direct projection from hypothalamic nuclei to the lumbosacral autonomic erection centers. The neurons in these hypothalamic nuclei contain peptidergic neurotransmitters including oxytocin and vasopressin, which may be involved in penile erection (Sachs and Meisel, 1988). Several brainstem and medullary centers are also involved in sexual function. The A5-catecholamine cell group and locus ceruleus have been shown to provide adrenergic innervation to the hypothalamus, thalamus, neocortex, and spinal cord. Projections from the nucleus paragigantocellularis, which provides inhibitory serotonergic innervation, have also been demonstrated in the hypothalamus, limbic system, neocortex, and spinal cord.
Table 23–5 Brain Centers Involved in Sexual Function
| LEVEL | REGION | FUNCTION |
|---|---|---|
| Forebrain | Medial amygdala Stria terminalis |
Control sexual motivation |
| Pyriform cortex | Inhibits sexual drive (hypersexuality when destroyed) | |
| Hippocampus | Involved in penile erection | |
| Right insula and inferior frontal cortex Left anterior cingulate cortex |
Increased activity during visually evoked sexual stimulation (sexual arousal) | |
| Hypothalamus | Medial preoptic area (MPOA) Lateral preoptic area (LPOA) |
Ability to recognize a sexual partner, integration of hormonal and sensory cues Control nocturnal penile tumescence in rats |
| Paraventricular nucleus (PVN) | Facilitates penile erection (via oxytocin neurons to lumbosacral spinal autonomic and somatic efferents) | |
| Brainstem | Nucleus paragigantocellularis | Inhibits penile erection (via serotonin neurons to lumbosacral spinal neurons and interneurons) |
| A5-catecholaminergic cell group Locus ceruleus |
Major noradrenergic center | |
| Midbrain | Periaqueductal gray | Relay center for sexually relevant stimuli |
Central Neural Activation during Arousal
Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have allowed a greater understanding of brain activation during human sexual arousal by demonstrating increases in regional cerebral blood flow or changes in regional cerebral activity during a particular moment in time. Generally, in young heterosexual men sexual arousal is triggered with sexually explicit pictures or videos. Scanned brain images taken during arousal are compared with images taken in response to sexually neutral media (e.g., documentaries or humorous video clips). Centers of activation and deactivation can be demonstrated. Although the simplicity of these study designs is elegant, multiple factors are involved in sexual arousal—especially when triggered by visual clues. The authors of these studies have placed many necessary conditions in an attempt to standardize the methods and participants; however, the complexity of human emotion and sexual response is extremely difficult to regulate.
In 1999 Stoléru and colleagues studied eight healthy right-handed heterosexual men with PET during visually evoked sexual arousal (Stoléru et al, 1999). Regions of brain activation were correlated with testosterone plasma levels and penile tumescence. Significant activation was seen in the bilateral inferior temporal cortices, right insula, right inferior frontal cortex, and left anterior cingulate cortex. From this landmark study a tentative model was introduced, suggesting that visually evoked sexual arousal has three components associated with neuroanatomic regions: (1) a perceptual-cognitive component that recognizes the visual stimuli as sexual and is performed in the bilateral inferior temporal cortices; (2) an emotional/motivational component that processes sensory information with motivational states and is performed in the right insula, right inferior frontal cortex, and left cingulate cortex (paralimbic areas); and (3) a physiologic component that coordinates the endocrine and autonomic functions and is performed in the left anterior cingulate cortex.
Bocher and colleagues (2001) demonstrated increased activation in the inferior lateral occipital cortex, bilateral posterior temporal cortices (right greater than left), right inferior lateral prefrontal cortex, left postcentral gyrus, bilateral inferior parietal lobules, left superior parietal lobules, frontal pole (Brodmann area 10), left prefrontal cortex, and midbrain regions. They also noted deactivation in the medial frontal and anterior cingulate, contrary to Stoleru’s report. Again, visual association centers were noted to be activated, in particular the posterior temporal cortices and postcentral gyrus. Interestingly, the midbrain activation seen in this study correlates with the location of the dopaminergic neurons. It was not demonstrated in other studies, but that may owe to these authors’ use of prolonged provocation: the visual sexual stimulus was a 30-minute continuous video clip, whereas other studies used brief visual stimuli (2 to 10 minutes).
Park and colleagues studied 12 healthy men with fMRI (Park et al, 2001) in response to erotic and nonerotic film clips. With the former, regional brain activation was generally seen in the inferior frontal lobe, cingulate gyrus, insular gyrus, corpus collosum, thalamus, caudate nucleus, globus pallidus, and inferior temporal lobes. Some activation regions were similar to those in other studies, in particular the inferior frontal lobes, inferior temporal lobes, and insular gyrus.
In a well-designed study with fMRI and visual stimuli, correlated with penile turgidity, Arnow and colleagues demonstrated a significant region of activation in the right subinsular/insular region, including the claustrum (Arnow et al, 2002), a response also seen in previous studies with PET (Stoléru et al, 1999; Redouté et al, 2000). This region has been associated with sensory processing, and this activation may represent somatosensory processing and recognition of erection. Other brain regions activated during visual sexual stimuli were the right middle gyrus, right temporal gyrus, left caudate and putamen, bilateral cingulate gyri, right sensimotor and premotor regions. Also, a lesser activation was seen in the right hypothalamus. (Dopamine is projected to the hypothalamus, and the evidence that dopamine assists male sexual behavior is substantial.) The activation of the right middle temporal gyrus is probably associated with visual processing.
In 2003 Mouras and colleagues studied eight men with fMRI. Video clips were not used; rather, still photographs (neutral and sexually arousing) were shown quickly. The authors believed that, by using shorter visual sexual stimuli, early neural responses would be generated instead of responses to the perception of penile tumescence. Again, activation of the middle and inferior occipital gyri was demonstrated, most likely linked to the visual stimuli, but not necessarily to the sexual component. In addition to multiple brain centers that showed activation with visual sexual stimuli (bilateral parietal lobules, left inferior parietal lobule, right postcentral gyrus, right parieto-occipital sulcus, left superior occipital gyrus, bilateral precentral gyrus), the cerebellum demonstrated activation in three subjects and deactivation in four. As many other reports have demonstrated activation of the cerebellum in response to erotic films and pictures of love partners (Garavan et al, 2000; Beauregard et al, 2001), visual sexual stimuli likely promote activation in regions within the cerebellum.
With the advances with fMRI, detailed comparisons of brain activation in response to visual sexual stimuli have been performed on varied groups. Stoléru and colleagues (2003) compared healthy men with men with hypoactive sexual desire disorder (HSDD) and reported that the left gyrus rectus, a portion of the medial orbitofrontal cortex, remained activated in the latter group in contrast to its deactivation in healthy men. This region is believed to mediate inhibition of motivated behavior, and its continued activation may help explain the pathophysiology of HSDD. Montorsi and colleagues (2003) compared men with psychogenic ED and potent controls after the administration of apomorphine. During visual sexual stimulation, the former group evidenced extended activation of the cingulated gyrus, frontal mesial, and frontal basal cortex, suggesting an underlying organic cause for psychogenic ED. However, their fMRI images after apomorphine were similar to those of the potent controls. Apomorphine caused additional activation of foci in the psychogenic ED patients (seen in the nucleus accumbens, hypothalamus, mesencephalon), and it was significantly greater in the right hemisphere than in the left. This greater right-sided activation is a common finding in sexually evoked brain activation studies.
Brain scanning with PET and fMRI has become a powerful tool in the study of central activation of sexual arousal, with many brain regions of activation demonstrated in these reports (Table 23–6). Psychogenic ED, premature ejaculation, sexual deviations, and orgasmic dysfunction are just a few conditions that may accompany alterations in higher brain function and perhaps now can be studied. As we begin to understand brain function with normal sexual response and arousal, the causes of dysfunction may be elucidated.
Table 23–6 Common Brain Activation Regions with Visual Sexual Stimuli*
| BRAIN ACTIVATION REGIONS | FUNCTIONAL ASSOCIATION |
|---|---|
| Bilateral inferior temporal cortex (right > left) | Visual association area |
| Right insula | Processes somatosensory information with motivational states |
| Right inferior frontal cortex | Processes sensory information |
| Left anterior cingulate cortex | Controls autonomic and neuroendocrine function |
| Right occipital gyrus | Visual processing |
| Right hypothalamus | Male copulatory behavior |
| Left caudate (the striatum) | Processes attention and guides responsiveness to new environmental stimuli |
* These regions demonstrate activation with visual sexual stimuli in multiple studies.
The structures discussed earlier are responsible for the three types of erection: psychogenic, reflexogenic, and nocturnal. Psychogenic erection is a result of audiovisual stimuli or fantasy. Impulses from the brain modulate the spinal erection centers (T11-L2 and S2-S4) to activate the erectile process. Reflexogenic erection is produced by tactile stimulation of the genital organs. The impulses reach the spinal erection centers; some then follow the ascending tract, resulting in sensory perception, while others activate the autonomic nuclei to send messages via the cavernous nerves to the penis to induce erection. This type of erection is preserved in patients with upper spinal cord injury. Nocturnal erection occurs mostly during rapid-eye-movement (REM) sleep. PET scanning of humans in REM sleep shows increased activity in the pontine area, the amygdalae, and the anterior cingulate gyrus but decreased activity in the prefrontal and parietal cortex. The mechanism that triggers REM sleep is located in the pontine reticular formation; the cholinergic neurons in the lateral pontine tegmentum are activated, while the adrenergic neurons in the locus ceruleus and the serotonergic neurons in the midbrain raphe are silent. This differential activation may be responsible for these nocturnal erections. In rats, the area of the brain that appears to control nocturnal penile tumescence is the lateral preoptic area (LPOA) (Schmidt et al, 2000).
The brain centers activated during orgasm and ejaculation have also been studied. Holstege and colleagues (2003) used positron emission tomography (PET) to measure increases in regional cerebral blood flow during ejaculation versus sexual stimulation without orgasm in heterosexual male volunteers. Manual penile stimulation was performed by the volunteer’s female partner. Primary brain activation was found in the mesodiencephalic transition zone (including the ventral tegmental area), an area frequently activated with “reward” behaviors and with injection of opioids such as heroin. Other activated mesodiencephalic structures included the midbrain lateral central tegmental field, the zona incerta, the subparafascicular nucleus, and the ventroposterior, midline, and intralaminar thalamic nuclei. Increased activation was also observed in the lateral putamen and adjoining parts of the claustrum. Neocortical activity was found in Brodmann areas 7/40, 18, 21, 23, and 47, exclusively on the right side. Conversely, in the amygdala and adjacent entorhinal cortex, a decrease in activation was observed. Remarkably strong blood flow increases were observed in the cerebellum. These findings corroborate the notion that the cerebellum plays an important role in emotional processing. Although activation of these particular areas is of great interest, it is apparent that further studies are necssary to truly understanding the neurobiologic basis of orgasm, ejaculation, and sexual satisfaction in men (Table 23–7).
Table 23–7 Brain Centers of Orgasm
| BRAIN AREAS | RELEVANCE | |
|---|---|---|
| Increased activity Primary area |
Mesodiencephalic transition zone (including the ventral tegmental area) | “Reward” center also activated by opioid |
| Increased activity Secondary areas | Midbrain lateral central tegmental field, the zona incerta, subparafascicular nucleus, ventroposterior, midline, and intralaminar thalamic nuclei Lateral putamen and adjoining parts of the claustrum Brodmann areas 7/40, 18, 21, 23, and 47, exclusively on the right side |
|
| Increased activity Other area |
Cerebellum | Emotional processing |
| Deceased activity | Amygdala and adjacent entorhinal cortex |
Neurotransmitters
Peripheral Neurotransmitters and Endothelium-Derived Factors
Flaccidity and Detumescence
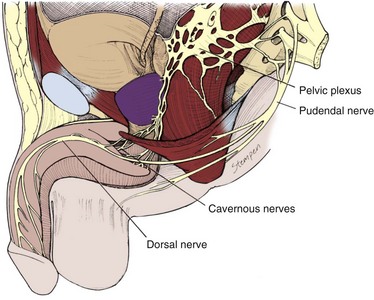
α-Adrenergic nerve fibers and receptors have been demonstrated in the cavernous trabeculae and surrounding the cavernous arteries, and norepinephrine has generally been accepted as the principal neurotransmitter to control penile flaccidity and detumescence (Hedlund and Andersson, 1985; Diederichs et al, 1990). Receptor-binding studies have shown the number of α adrenoceptors to be 10 times higher than the number of β adrenoceptors (Levin and Wein, 1980). Currently, it is suggested that sympathetic contraction is mediated by activation of postsynaptic α1a- and α1d-adrenergic receptors (Christ et al, 1990; Traish et al, 1995) and modulated by presynaptic α2-adrenergic receptors (Saenz de Tejada et al, 1989b). Contraction mediated by α2 receptors depends on the entry of calcium from the extracellular compartment, while the activation of α1 receptors provokes the release of intracellular calcium, initially, with subsequent extracellular calcium entry for the maintenance of the contractile tone.
Endothelin, a potent vasoconstrictor produced by the endothelial cells, has also been suggested to be a mediator for detumescence (Holmquist et al, 1990; Saenz de Tejada et al, 1991a). Endothelin-1 is a member of a family of three peptides and is a potent constrictor synthesized by the sinusoidal endothelium (Holmquist et al, 1990; Saenz de Tejada et al, 1991a). Its presence in human cavernous tissue suggests the participation of this peptide in the regulation of trabecular smooth muscle. Endothelin also potentiates the constrictor effects of catecholamines on trabecular smooth muscle (Christ et al, 1995b). Two receptors for endothelin, ETA and ETB, mediate the biologic effects of endothelin in vascular tissue: ETA receptors mediate contraction, whereas ETB receptors induce relaxation.
Several constrictor prostanoids including prostaglandin I2 (PGI2), PGF2α, and thromboxane A2 (TXA2) are synthesized by the human cavernous tissue. In-vitro studies have demonstrated that prostanoids are responsible for the tone and spontaneous activity of isolated trabecular muscle (Christ et al, 1990). Functional characterization of prostanoid receptors in human trabecular and arterial penile smooth muscle has revealed that only TP receptors mediate contractile effects of prostanoids in these tissues (Angulo et al, 2002). Also, it has been observed in vitro that constrictor prostanoids, simultaneously released with NO, attenuate the dilator effect of the latter (Azadzoi et al, 1992; Minhas et al, 2001).
The renin-angiotensin system may also play a significant role in the maintenance of penile smooth muscle tone. Angiotensin II has been detected in endothelial and smooth muscle cells of human corpus cavernosum (Kifor et al, 1997) and evokes contraction of human (Becker et al, 2001a) and rabbit (Park et al, 1997) corpus cavernosum in vitro. This contractile effect is mediated by interaction with AT-I subtype receptors (Park et al, 1997). Intracavernous injection of angiotensin II reverses spontaneous erections in dogs, while the AT-I receptor antagonist, losartan, increases the intracavernous pressure (Kifor et al, 1997). Finally, intracavernous blood levels of angiotensin II, which are higher than in systemic peripheral blood, increase in the detumescence phase (Becker et al, 2001b). Thus local production of angiotensin II may increase penile smooth muscle contractility by way of AT-I receptors, assisting penile detumescence. In addition, the endothelium has been shown to release potent vasoconstrictors including endoperoxides, thromboxane A2, and superoxide anions.
The current consensus holds that the maintenance of the intracorporeal smooth muscle in a semicontracted (flaccid) state likely results from three factors: intrinsic myogenic activity (Andersson and Wagner, 1995); adrenergic neurotransmission; and endothelium-derived contracting factors such as angiotensin II, PGF2α, and endothelins. On the other hand, detumescence after erection may be a result of cessation of NO release, the breakdown of cyclic guanosine monophosphate (cGMP) by phosphodiesterases, and/or sympathetic discharge during ejaculation.
Erection
Acetylcholine has been shown to be released with electrical field stimulation of human erectile tissue (Blanco et al, 1988). Traish and colleagues (1990) reported the density of muscarinic receptors in cavernous tissue to range from 35 to 65 fmol/mg protein and in endothelial cell membrane from 5 to 10 fmol/mg protein. However, intravenous or intracavernous injection of atropine has failed to abolish erection induced in animals by electrical neurostimulation (Stief et al, 1989a) and in men by erotic stimuli (Wagner and Uhrenholdt, 1980). Although acetylcholine is not the predominant neurotransmitter, it does contribute indirectly to penile erection by presynaptic inhibition of adrenergic neurons and stimulation of NO release from endothelial cells (Saenz de Tejada et al, 1989a).
Most researchers now agree that NO released from nonadrenergic/noncholinergic (NANC) neurotransmission and from the endothelium is the principal neurotransmitter mediating penile erection. NO increases the production of cGMP, which in turn relaxes the cavernous smooth muscle (Ignarro et al, 1990; Holmquist et al, 1991; Kim N et al, 1991; Pickard et al, 1991; Burnett et al, 1992; Knispel et al, 1992; Rajfer et al, 1992; Burnett et al, 1993; Trigo-Rocha et al, 1993a). The consensus is that NO derived from nNOS in the nitrergic nerves is responsible for the initiation and majority of the smooth muscle relaxation whereby NO from eNOS contributes to the maintenance of the erection (Hurt et al, 2002). (For a fuller discussion of NO, see specific “Nitric Oxide” sections later.)
Aside from its role in releasing vasoconstrictors, the endothelium can also release factors that induce smooth muscle relaxation including carbon monoxide, endothelium-derived hyperpolarizing factor (EDHF), prostacyclin (PGI2), and endothelin (which may induce relaxation via activation of ETB receptors).
Interactions among Nerves and Neurotransmitters
Acetylcholine, by acting on the presynaptic receptors on adrenergic neurons, has been shown to modulate the release of norepinephrine (Saenz de Tejada et al, 1989b), which also can be inhibited by PGE1 (Molderings et al, 1992). In the human corpus cavernosum, noradrenergic responses are under nitrergic control. Conversely, adrenergic neurons, through prejunctional α2 receptors, can also regulate the release of NO.
Several studies have demonstrated that the interaction between the two systems also occurs in the smooth muscle (Brave et al, 1993; Angulo et al, 2001). The NO-cGMP-PKGI pathway can lead to inhibition at several sites on the noradrenergic contractile pathway in the vascular smooth muscle, impairing IP3 production by phospholipase C (Hirata et al, 1990), IP3 receptor activity (Schlossmann et al, 2000), and the RhoA/Rho-kinase pathway (Sauzeau et al, 2000). However, interaction sites have not yet been identified in penile smooth muscle. A nitrergic-noradrenergic imbalance owing to defective nitrergic neurotransmission has been implicated in penile tissue from patients and in animal models with erectile dysfunction (Christ et al, 1995a; Cellek et al, 1999). Similar to the interaction between nitrergic and noradrenergic pathways, vasoconstrictive actions of endothelin have been shown to be inhibited by NO during erection (Mills et al, 2001).
A number of factors have been reported to increase both NOS activity and NO release. These include molecular oxygen, androgen, chronic administration of L-arginine, and repeated intracavernous injection of PGE1 (Kim N et al, 1993; Escrig et al, 1999; Marin et al, 1999). Decreased NOS activity has been associated with castration, denervation, hypercholesterolemia, and diabetes mellitus. Interaction of different types of NOS may also occur. For example, nNOS activity has been to shown to decrease and iNOS levels to increase after injection of transforming growth factor (TGF)-β1 into the penis (Bivalacqua et al, 2000), and eNOS levels are reportedly significantly higher in nNOS-knockout mice (Burnett et al, 1996).
In a study of neurotransmitters in human corpus cavernosum and spongiosum, Hedlund and colleagues (2000) reported that vesicular acetylcholine transporter (VAChT), vasoactive intestinal polypeptide (VIP), and neuronal nitric oxide synthase (nNOS) are found in the same nerve terminals. Tyrosine hydroxylase (TH) positive nerves do not contain VAChT, VIP, or NOS. Heme oxygenases (HO-1 and HO-2) and eNOS are localized to the endothelium. Interaction of these neurotrasmitters may modify the effect of parasympathetic and sympathetic activation on penile function.
Role of Caveolae
Caveolae are invaginated microdomains of plasma membrane that are rich in eNOS and caveolins, as well as cholesterol, sphingolipids, and glycosylphosphatidylinositol-linked proteins. In addition, caveolae contain numerous other signaling proteins such as receptors with seven-transmembrane domains, G-proteins, adenylyl cyclase, phospholipase C, protein kinase C, calcium pumps, and calcium channels. Decreased caveolin-1 expression has been reported in the cavernous smooth muscle of aged rat (Bakircioglu et al, 2001). Linder and colleagues (2006) demonstrated that penile erection requires association of soluble guanylyl cyclase with endothelial caveolin-1 in rat corpus cavernosum. Shakirova and colleagues (2009) reported that nerve-mediated relaxation of penile tissue from caveolin-1 deficient mice was impaired. Caveolon-1 in the cavernous smooth muscle and endothelium are both decreased after bilateral cavernous nerve injury (Becher et al, 2009). These reports strongly suggest that the caveolae and caveolin are involved in the regulation of penile function.
Central Neurotransmitters and Neural Hormones
A variety of neurotransmitters (dopamine, norepinephrine,5-hydroxytestosterone [5-HT], and oxytocin) and neural hormones (oxytocin, prolactin) have been implicated in regulation of sexual function. It is suggested that dopaminergic and adrenergic receptors may promote sexual function and 5-HT receptors inhibit it (Foreman and Wernicke, 1990).
Dopamine
There are many dopaminergic systems in the brain with ultrashort, intermediate, and long axons. The cell bodies are located in the ventral tegmentum, substantia nigra, and hypothalamus. One of these dopaminergic systems, the tuberoinfundibular system, secretes dopamine (DA) into the portal hypophysial vessels to inhibit prolactin secretion (Ganong, 1999a). Five different DA receptors have been cloned (D1 to D5), and several of these exist in multiple forms (Ganong, 1999b). In men, apomorphine, which stimulates both D1 and D2 receptors, induces erection that is unaccompanied by sexual arousal (Danjou et al, 1988). In male rats, Hull and colleagues (1992) have found that low levels of dopaminergic stimulation through the D1 receptor increase erection; higher levels or prolonged stimulation produces seminal emission through D2 receptors. The erectile response induced by injection of apomorphine into the paraventricular area can be suppressed by blockers of both DA and oxytocin receptors (Melis et al, 1989). Injection of oxytocin into the paraventricular area also induces erection, but this cannot be blocked by DA receptor blockers. These findings suggest that dopaminergic neurons activate oxytocinergic neurons in the paraventricular area and that the release of oxytocin produces erection (Melis et al, 1992).
In general terms, DA is supportive of copulation and 5-HT is inhibitory. DA is released in the MPOA at the time of ejaculation (5-HT is not), and changes in DA and 5-HT in different areas of the brain may promote copulation and sexual satiety, respectively (Hull et al, 1999). Testosterone enhances DA release in the MPOA at rest and with sexual challenge, possibly by upregulating NOS, which increases NO and thereby increases DA release. The same pattern of copulatory activity promoting DA release in the MPOA and the enhanced effect of the presence of sex hormones is seen in female rats. Longer-lasting changes may be seen through the postcopulatory effects of gene expression, and this expression increases with increased sexual experience, effectively changing the phenotype of certain cells in sexually experienced animals. Cells of the MPOA have high densities of α2-noradrenergic receptors, as well as DA receptors, and the effects of DA in the MPOA are most likely assisted by the activation of α2 (inhibition) and α1 (excitation) adrenoceptors owing to cross-talk within central nervous system (CNS) catecholamine systems (Cornil et al, 2002).
DA agonists (apomorphine and pergolide) and DA uptake inhibitors (nomifensine and bupropion) have been reported to enhance sexual drive (Stimmel and Gutierrez, 2006). Sublingual apomorphine is available for the treatment of ED in many countries but its utility is limited due to emetic side effects. A selective DA D4 receptor agonist, ABT-724, has been shown to assist penile erection in a dose-dependent fashion in conscious rats; interestingly, this drug lacked emetic effects in a ferret model (Brioni et al, 2004; Osinski et al, 2005). A similar compound, ABT-670, with superior oral bioavailability has also been identified for potential clinical use (Patel et al, 2006).
Serotonin
Neurons containing 5-HT have their cell bodies in the midline raphe nuclei of the brainstem and project to a portion of the hypothalamus, limbic system, neocortex, and spinal cord (Ganong, 1999a). Currently, 5-HT receptors 1 to 7 have been cloned and characterized. Within the 5-HT1 group are the 5-HT1 A, B, D, E, and F subtypes. Within the 5-HT2 group are the 5-HT2 A, B, and C subtypes. There are two 5-HT5 subtypes, 5-HT5 A and B (Ganong, 1999b). General pharmacologic data indicate that 5-HT pathways inhibit copulation, but 5-HT may have both facilitory and inhibitory effects on sexual function, depending on the receptor subtype, the receptor location, and the species investigated (de Groat and Booth, 1993). Andersson and Wagner (1995) have summarized the results of administration of selective agonists and antagonists as follows: 5-HT1A receptor agonists inhibit erectile activity but assist ejaculation; stimulation of 5-HT2C receptors causes erection; and 5-HT2 agonists inhibit erection but assist seminal emission and ejaculation. Steers and de Groat (1989) have also shown increased firing of the cavernous nerve and erection when m-chlorophenylpiperazine, a 5-HT2C receptor agonist, is given to rats. Applying a novel 5-HT2c receptor agonist (YM348) and antagonist SB242084, Kimura and colleagues (2006) confirmed the pro-erectile effect of the 5-HT2c receptor stimulation in rats. Stimulation of both 5-HT2 and 5-HT2C receptors has also been reported to increase oxytocin secretion (Bagdy et al, 1992). In addition, 5-HT may also affect the spinal reflex because Marson and McKenna (1992) have reported that endogenous 5-HT may act in the lumbar cord to inhibit sexual reflexes.
5-HT is believed to be an inhibitory transmitter in the control of sexual drive (Foreman et al, 1989). Suppressed libido has been reported in patients taking fenfluramine, a 5-HT-releasing agent, but elevated libido occurred in patients taking buspirone, a 5-HT neuron suppressor (Buffum, 1982).
Norepinephrine
The cell bodies of the norepinephrine-containing neurons are located in the locus ceruleus and the A5-catecholaminergic cell group in the pons and medulla. The axons of these noradrenergic neurons ascend to innervate the paraventricular, supraoptic, and periventricular nuclei of the hypothalamus, the thalamus, and neocortex. They also descend into the spinal cord and the cerebellum. Central norepinephrine transmission seems to have a positive effect on sexual function. In both humans and rats, inhibition of norepinephrine release by clonidine, an α2-adrenergic agonist, is associated with a decrease in sexual behavior, and yohimbine, an α2-receptor antagonist, has been shown to increase sexual activity (Clark et al, 1985). β Blockers have also been implicated in sexual dysfunction, probably because of their central side effects such as sedation, sleep disturbances, and depression.
γ-Aminobutyric Acid
γ-Aminobutyric acid (GABA) activity in the PVN provides a mechanism to balance (inhibit) proerectile signaling. Agonism for the type A receptor for GABA in the PVN can reduce both pharmacologically (apomorphine) and physiologically induced erections (Melis et al, 2001).
Opioids
Endogenous opioids are known to affect sexual function, but the mechanism of action is far from clear. Injection of small amounts of morphine into the MPOA assists sexual behavior in rats. Larger doses, however, inhibit both penile erection and yawning induced by oxytocin or apomorphine. It is suggested that endogenous opioids may exert an inhibitory control on central oxytocinergic transmission (Argiolas, 1992). Injection of morphine into the paraventricular nucleus of the hypothalamus prevents noncontact penile erections and impairs copulation in rats. It is speculated that intracellular nitric oxide may be involved in this process (Melis et al, 1999).
Cannabinoids
Cannabinoid CB1 receptor activation inhibits sexual function by modulating the paraventricular oxytocinergic neurons, which mediate erection. Antagonism of CB1 receptors in the paraventricular nucleus of male rats induces penile erection, which seems to involve glutamic acid and nitric oxide (Melis et al, 2004, 2006).
Oxytocin
Oxytocin is a neural hormone secreted by the neurons into the circulation. These are found in the posterior pituitary gland, but, because they are also found in the neurons projecting from the PVN to the brainstem and spinal cord, oxytocin can also function as a neurotransmitter. The blood level is increased during sexual activity in humans and animals and, when injected into the paraventricular area in rats, oxytocin produces yawning and penile erection. Calcium has been suggested as the second messenger mediating oxytocin-induced penile erection. Oxytocin release following stimulation of dompamine receptors in the paraventricular nucleus influences the appetitive and reinforcing effects of sexual activity (Succu et al, 2007). Because neurons in the paraventricular area have been shown to contain NOS, and NOS inhibitors prevent apomorphine- and oxytocin-induced erection, it is suggested that oxytocin acts on neurons whose activity is dependent on certain levels of NO (Vincent and Kimura, 1992; Melis and Argiolas, 1993).
Nitric Oxide
Nitric oxide (NO) is a gaseous molecule produced from various tissues, in particular the endothelium and nerve. It mediates penile erection at the level of the PVN (Melis et al, 1998) and at other levels of the neural pathway supporting sexual response. The presence of NO and the soluble guanylyl cyclase needed to generate cGMP is seen throughout the human brain. The NO/cGMP pathway (see later) is affected by aging in the brain and offers a potentially significant but unexplored site for mediating the deleterious effects of age on sexual function (Ibarra et al, 2001). Reduced nNOS protein within the PVN leading to blunting of the erectile response has been reported in streptoxotosin-induced diabetic rats (Zheng et al, 2007). Testosterone or its metabolite dihydrotestosterone (DHT) downregulates NOS activity and mRNA expression and the number of nNOS-containing neurons (Singh et al, 2000). Direct evidence of the importance of NO in central signaling related to erectile function resulted from a series of experiments designed to alter CNS NO activity (Sato et al, 2001): Manipulation of NO or cGMP levels altered MPOA-triggered intracavernous pressure response through CNS, not peripheral, mechanisms.
Melanocortins
Melanocortin-4 receptor (MC4R), implicated in the control of food intake and energy expenditure, also modulates erectile function and sexual behavior. Evidence supporting this notion is based on several findings: (1) a highly selective nonpeptide MC4R agonist augments erectile activity initiated by electrical stimulation of the cavernous nerve in wild-type, but not MC4R-null, mice; (2) copulatory behavior is enhanced by administration of a selective MC4R agonist and is diminished in mice lacking MC4R; (3) RT-PCR and non-PCR-based methods demonstrate MC4R expression in the rat and human penis and rat spinal cord, hypothalamus, brainstem, and pelvic ganglion (major autonomic relay center to the penis) but not in rat primary corpus cavernosum smooth muscle cells; and (4) in situ hybridization of glans tissue from the human and rat penis reveals MC4R expression in nerve fibers and mechanoreceptors in the glans. Collectively, these data implicate MC4R in the modulation of penile erectile function and provide evidence that MC4R-mediated proerectile responses may be activated through neuronal circuitry in spinal cord erectile centers and somatosensory afferent nerve terminals of the penis (Van der Ploeg et al, 2002).
Prolactin
Increased levels of prolactin suppress sexual function in men and experimental animals. In rats, high levels of prolactin decrease the genital reflex and disturb copulatory behavior (Rehman et al, 2000). It is suggested that the mechanism of prolactin’s action is through inhibition of dopaminergic activity in the MPOA and decreased testosterone. In addition, prolactin may have a direct effect on the penis through its contractile effect on the cavernous smooth muscle (Ra et al, 1996). In a study of sexual activity of married men with ED, men with sexual inactivity were noted to have a significanly higher mean prolactin level (Paick et al, 2006) (Table 23–8).
Table 23–8 Central Neurotransmitters and Their Function
| NEUROTRANSMITTER | RECEPTOR and FUNCTION |
|---|---|
| Dopamine | D1 and D4 receptor—enhances erection |
| D2 receptor—enhances seminal emission | |
| Serotonin (5HT) | 5HT—inhibits sex drive and spinal sexual reflex |
| 5HT1A—inhibits erection, facilitates ejaculation | |
| 5HT2C—enhances erection | |
| Norepinephrine | Enhances sexual function |
| Gamma-aminobutyric acid | Inhibits erectile signals |
| Opioids | Inhibit penile erection |
| Cannabinoids | Inhibit sexual function |
| Oxytocin | Enhances appetitive and reinforcing effects of sexual activity |
| Nitric oxide | Mediates erection at paraventricular nucleus |
| Melanocortins | McR4—enhances erection |
| Prolactin | Suppresses sexual function |
Smooth Muscle Physiology
Spontaneous contractile activity of cavernous smooth muscle has been recorded in vitro and in vivo. In isolated strips of rabbit corpus cavernosum, Mandrek (1994) demonstrated spontaneous mechanical activity with a frequency of 6 to 30 contractions per minute accompanied by fluctuations in membrane potential. In a human study, Yarnitsky and colleagues (1995) found two types of electrical activity recorded from the corpus cavernosum: spontaneous and activity induced. Levin and colleagues (1994) reported that in-vitro spontaneous contractile activity is correlated with a phasic increase in intracellular calcium and a biphasic change in the NADH/NAD ratio, suggesting an initial increase and then a decrease of intracellular energy. Italiano and colleagues (1998) suggested that phasic contraction of the penis is through the enzyme Na-K-ATPase and the resting tone is mediated by the endothelium through the release of PGF2a. Field stimulation results in a decrease in tension and intracellular calcium at low frequencies and an increase in tension and intracellular calcium at high frequencies. In general, the response to pharmacologic agents correlates with the change in intracellular calcium: for example, phenylephrine produces muscle contraction and an increase in intracellular calcium, whereas nitroprusside causes the opposite.
In a study of myosin isoforms in smooth muscle cells in the corpus cavernosum, DiSanto and colleagues (1998) reported their overall composition to be between that in aorta and bladder smooth muscles, which generally express tonic- and phasic-like characteristics, respectively. Further studies of isoform changes may elucidate the increased contractility or impaired relaxation of the cavernous smooth muscle in pathologic conditions.
Molecular Mechanism of Smooth Muscle Contraction
Smooth muscle contraction and relaxation are regulated by intracellular free calcium (Ca2+) acting through calmodulin. Calcium-bound calmodulin undergoes a conformational change, increasing its affinity for myosin light chain kinase (MLCK). MLCK is activated by binding of the calcium-calmodulin complex, leading to phosphorylation of the serine-19 residue of regulatory myosin light chain MLC20. In the presence of ATP, this phosphorylation enables actin to activate the myosin ATPase and initiates cross-bridge cycling. Hydrolysis of ATP by ATPase supplies the energy for the contractile process (Fig. 23–9).
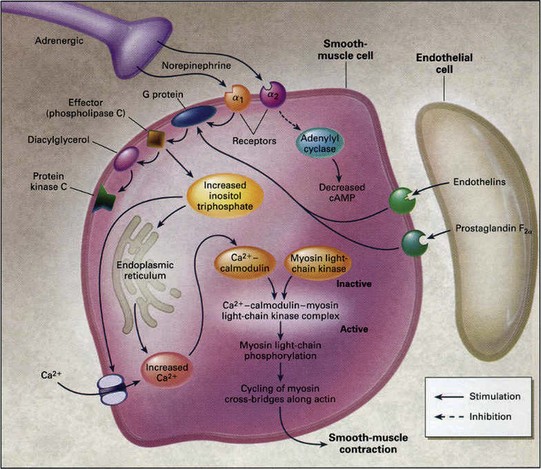
Figure 23–9 Molecular mechanism of penile smooth muscle contraction. Norepinephrine from sympathetic nerve endings and endothelins and prostaglandin F2α from the endothelium activate receptors on smooth muscle cells to initiate the cascade of reactions that eventually result in elevation of intracellular calcium concentrations and smooth muscle contraction. Protein kinase C is a regulatory component of the Ca2+-independent, sustained phase of agonist-induced contractile responses.
(From Lue TF. Erectile dysfunction. N Engl J Med 2000;342:1802–13.
Regulatory Molecules Affecting Smooth Muscle Contraction
The muscle contractile process ends when MLC20 is dephosphorylated (inactivated) by myosin light chain phosphatase (MLCP). MLCP is a holoenzyme consisting of a type 1 phosphatase (PP1c), a myosin-targeting subunit (MYPT1), and a 20 kD subunit of unknown function (Hersch et al, 2004; Ito et al, 2004). Theoretically, MLCP inhibition may lead to enhanced smooth muscle contraction. This is also termed “calcium sensitization” pathway. The activity of MLCP can be modulated by Rho/Rho-kinase pathway (Fig. 23–10). Rho-kinase can phosphorylate multiple substrates including MYPT1, the 17 kD protein kinase C-potentiated inhibitor protein (CPI-17), and MLC20. Phosphorylation of MYPT1 and CPI-17 results in the inhibition of PP1c phosphatase activity. RhoA and Rho-kinase have been shown to be expressed in penile smooth muscle (Rees et al, 2002; Wang et al, 2002). Interestingly, the amount of RhoA expressed in the cavernous smooth muscle is 17-fold that in vascular smooth muscle (Wang et al, 2002). A selective inhibitor of Rho-kinase has been shown to elicit relaxation of human corpus cavernosum in vitro and to induce erection in animal models (Chitaley et al, 2001; Rees et al, 2001). The emerging consensus is that phasic contraction of penile smooth muscle is regulated by an increase in cytosolic Ca2+ and that tonic contraction is governed by the calcium-sensitizing pathways (Cellek et al, 2002). The presence of CPI-17 protein has been detected in human and rabbit penile corpus cavernosum (Wang et al, 2002), but its functional significance remains to be determined.
Latch State—a Unique Characteristic of Smooth Muscle Contraction
Smooth muscle has the ability to maintain tension for prolonged periods with minimal energy expenditure. This efficiency has been termed the latch state and is critical for sustaining the “basal” tone of the smooth muscle. It has been proposed that dephosphorylated myosin remains bound to actin in the high-affinity state to help stabilize the latch state. Others have proposed that calponin participates in the latch state by simultaneously binding actin and myosin to stabilize cross-bridge interactions and slow the rate of detachment (Szymanski, 2004).
Pathways Involving IP3, DAG, and PKC
Vasoconstrictor agonists such as norepinephrine (α1-adrenergic receptors), endothelin-1 (ETA receptors), angiotensin II (AT1 receptors), prostaglandin F2α (FP receptors), and thromboxane A2 (TP receptors) bind their respective receptors to activate Gq, which in turn stimulates phospholipase C beta (PLC-β). This membrane-bound enzyme hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to liberate inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG). IP3 binds to specific receptors (IP3R) on the smooth endoplasmic reticulum (SER) to stimulate the release of Ca2+ from intracellular stores. Binding of IP3 to these receptors not only activates the channel but also increases the sensitivity of the IP3R to Ca2+ and assists calcium-induced calcium release (CICR).
Another mechanism of increased intracellular Ca2+ is by permitting entry of extracellular Ca2+ through receptor-operated channels without a change in membrane potential (Large, 2002). Norepinephrine, endothelin, vasopressin, and angiotensin II cause the opening of Ca2+-permeable, nonselective cation channels.
Molecular Mechanism of Smooth Muscle Relaxation
After contraction, relaxation of the muscle follows a decrease of free Ca2+ in the sarcoplasm. Calmodulin then dissociates from myosin light-chain kinase and inactivates it. Myosin is dephosphorylated by myosin light-chain phosphatase and detaches from the actin filament, and the muscle relaxes (Walsh, 1991) (Fig. 23–11).
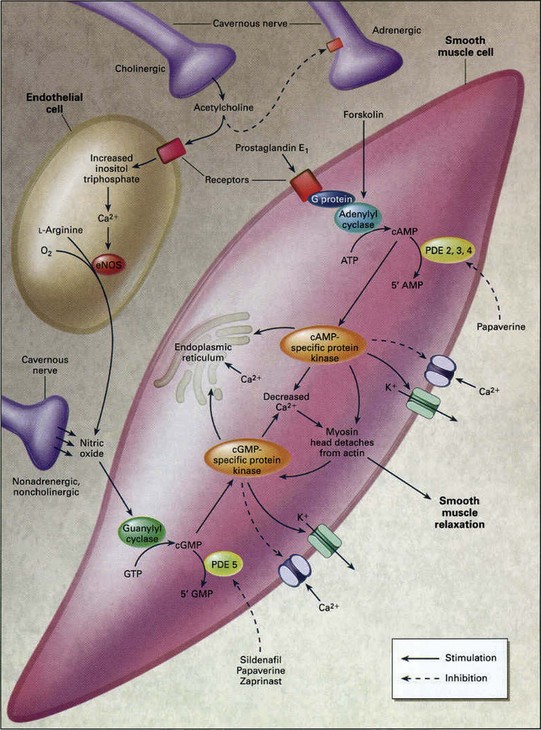
Figure 23–11 Molecular mechanism of penile smooth muscle relaxation. The intracellular second messengers mediating smooth muscle relaxation, cAMP and cGMP, activate their specific protein kinases, which phosphorylate certain proteins to cause opening of potassium channels, closing of calcium channels, and sequestration of intracellular calcium by the endoplasmic reticulum. The resultant fall in intracellular calcium leads to smooth muscle relaxation. Sildenafil inhibits the action of PDE5 and thus increases the intracellular concentration of cGMP. Papaverine is a nonspecific phosphodiesterase inhibitor. eNOS, endothelial nitric oxide synthase; GTP, guanosine triphosphate.
(From Lue TF. Erectile dysfunction. N Engl J Med 2000;342:1802–13.
Another mechanism of smooth muscle relaxation is through cyclic adenosine monophosphate (cAMP) and cGMP, which are the two major second messengers involved in smooth muscle relaxation. They activate cAMP- and cGMP-dependent protein kinases, which in turn phosphorylate certain proteins and ion channels, resulting in (1) opening of the potassium channels and hyperpolarization; (2) sequestration of intracellular calcium by the endoplasmic reticulum; and (3) inhibition of voltage-dependent calcium channels, blocking calcium influx. The consequence is a drop in cytosolic free calcium and smooth muscle relaxation.
Cyclic AMP-Signaling Pathway
Cyclic-AMP-signaling molecules include adenosine, calcitonin gene–related peptides (CGRPs), prostaglandins, and vasoactive intestinal peptide (VIP).
Adenosine
Adenosine is released from a variety of cells as a result of increased metabolic rates, and its actions on the vasculature are most prominent when oxygen demand is high (Tabrizchi and Bedi, 2001). However, the vascular response to the action of adenosine can be either relaxation or constriction, depending on which type of adenosine receptor is activated. Currently four AR subtypes (A1, A2A, A2B, and A3) belonging to the gene protein–coupled receptor (GPCR) superfamily have been recognized (Tabrizchi and Bedi, 2001). In general, the A1 receptor is believed to be coupled to Gi and Go proteins, and its activation results in inhibition of adenylyl cyclase (AC) and activation of phospholipase C, both of which lead to vasoconstriction. The A2 receptors are coupled to the Gs proteins, and their activation stimulates AC and thus vasorelaxation. The A3 receptor is coupled to Gi and Gq proteins, and its activation results in the activation of phospholipase C/D and the inhibition of AC, leading to vasoconstriction. The differential distribution of these adenosine receptor subtypes largely determines whether a particular vessel relaxes or contracts as a result of adenosine stimulation (Tabrizchi and Bedi, 2001). Whether adenosine plays a role in physiologic erection is unclear. Nevertheless, excessive adenosine accumulation in the penis, coupled with increased A(2B)R signaling, contributes to priapism in two independent lines of mutant mice. One is adenosine deaminase (ADA)-deficient mice (the only animal displaying spontaneously prolonged penile erection), and the other is sickle cell disease (SCD) transgenic mice, a well-accepted animal model for priapism (Bivalacqua et al, 2009; Dai et al, 2009).
Calcitonin Gene–Related Peptide Family
Calcitonin gene–related peptide, amylin, and adrenomedullin are members of the CGRP family. These short-chain peptides are potent vasodilators released from perivascular nerve fibers. They act through the calcitonin receptor–like receptor (CRLR), which belongs to the GPCR superfamily (Conner et al, 2002).
In rats the CGRP levels in the penis, bladder, kidney, testis, and adrenal gland were found to increase gradually up to maturity and then rapidly decline (Wimalawansa, 1992). In ED patients given CGRP intracavernously, a dose-related increase in penile arterial inflow (and erection) occurred (Stief et al, 1991). Adenovirus-mediated gene transfer of CGRP also enhanced erectile responses in aged rats, apparently through an increase of cAMP levels in the corpora cavernosa (Bivalacqua et al, 2001). Intracavernous administration of adrenomedullin also results in cavernous relaxation; however, the effect is through an NO-cGMP, rather than cAMP, pathway (Nishimatsu et al, 2001).
Prostaglandins
Prostaglandins (PGs) are a family of eicosanoids capable of initiating numerous biologic functions. The prime mode of PG action is through specific PG receptors that all belong to the GPCR family. There are at least nine known PG receptor subtypes in mouse and humans, as well as several additional splice variants with divergent carboxyl termini (Narumiya and FitzGerald, 2001). Four of the subtypes (EP1-EP4) bind PGE2, two (DP1 and DP2) bind PGD2, and the other three subtypes (FP, IP, and TP) bind PGF2α, PGI2, and TXA2, respectively. On the basis of signaling attributes, the PG receptors are classified into three types. The “relaxant” receptors IP, DP1, EP2, and EP4 are coupled to an αs-containing G-protein and therefore are capable of stimulating AC to increase intracellular cAMP. The “contractile” receptors EP1, FP, and TP are coupled to an αq-containing G-protein, which activates phospholipase C instead of AC. These contractile receptors therefore do not signal through the cAMP pathway, and their signaling outcome is an increase of intracellular calcium. The EP3 receptor is also a contractile receptor, but it is coupled to an αi-containing G-protein that inhibits AC to result in a decrease of cAMP formation.
Animal and human corpora cavernosa produce several PGs including PGF2α, PGE2, PGD2, PGI2, and TXA2 (Moreland et al, 2001). In studies in isolated human penile tissue, different PGs have been shown to elicit different effects in human corpus cavernosum, corpus spongiosum, and cavernous artery (Hedlund and Andersson, 1985). Although PGF2α, PGI2, and TXA2 contract the corpus cavernosum and corpus spongiosum, PGE1 and PGE2 (but not PGI2) relax the corpus cavernosum and spongiosum that have been precontracted with noradrenaline or PGF2α. Therefore although PGI2 is the predominant vasorelaxant in blood vessels, its action in the erectile tissue is either contractile or neutral. This disparity in the action of PGI2 between blood vessels and the erectile tissue and the difference between the effects of PGI2 and PGE1/2 in the erectile tissue are most likely due to differences in the distribution of PG receptors. Indeed, recent studies have shown that, in the corpus cavernosum, the relaxant effects of prostanoids are mediated by EP2 and/or EP4 receptors (for PGE1 and PGE2) but not IP receptor (for PGI2) (Angulo et al, 2002).
Although the production of PGs and the expression of PG receptors in the erectile tissue have been clearly demonstrated, their roles in physiologic erection are yet to be defined. On the other hand, the erectogenic effects of PGE1 as a pharmaceutic agent have been extensively documented. First described in 1998, intracavernous injection of PGE1 is one of the safest and most effective treatments for ED (Stackl et al, 1988). Transurethral application is an effective alternative.
Vasoactive Intestinal Peptide
The human or animal penis is richly supplied with nerves containing VIP (Andersson, 2001). Two subtypes of VIP receptors, VPAC1 and VPAC2, belonging to the GPCR family have been cloned from human and rat tissues. VPAC2, but not VPAC1 mRNA, has been identified in cultured rat cavernous smooth muscle cells (CSMCs) (Guidone et al, 2002). In the dog, intracavernous VIP injection has been found to induce penile erection (Juenemann et al, 1987); in men, it has not produced rigid erection but can improve success rates when combined with papaverine and phentolamine (Kiely et al, 1989). However, it has been shown that VIP release is not essential for neurogenic relaxation of human cavernous smooth muscle (Pickard et al, 1993), and VIP failed to stimulate cAMP production in cultured human CSMCs (Palmer et al, 1994).
Adenylyl Cyclase
Signaling molecules in the cAMP pathway bind to and activate specific cytoplasmic membrane receptors that, through their coupled G-proteins, activate ACs. To date, nine membrane-bound isoforms and one soluble form of mammalian AC have been cloned and characterized (Patel et al, 2001). Although different membrane-bound ACs are regulated differently, they are all stimulated by the GTP-bound form of the Gα subunit and are all (except AC9) stimulated by forskolin.
In rabbits with alloxan-induced diabetes, cAMP formation in the corpus cavernosum in response to forskolin has been shown to be reduced, suggesting impaired AC function in diabetes mellitus (Sullivan et al, 1998).
Protein Kinase A
PKA, also called cAMP-dependent kinase (cAK), is the principal receptor for cAMP, and it mediates the vast majority of the cellular effects of cAMP by phosphorylating a wide variety of downstream targets in both the cytoplasmic and nuclear compartments (Johnson et al, 2001). PKA is composed of two regulatory (R) and two catalytic (C) subunits that form a tetrameric holoenzyme R2C2. Binding of cAMP to the R subunits causes the holoenzyme to dissociate into an R2(cAMP)4 dimer and two free catalytically active C subunits. The presence of multiple C subunit genes further adds to the diversity and complexity of the various holoenzyme complexes, which differ in biochemical and functional properties, as well as patterns of expression and localization. These differences among the isozymes contribute to the broad specificity of PKA in a wide variety of physiologic processes in response to cAMP signaling.
More than 100 different cellular proteins have been identified as physiologic substrates of PKA, with more than 90% (135 of 145) being phosphorylated at serine and the remainder at threonine (Shabb, 2001). The predominant target sequence (>50%) is Arg-Arg-X-Ser, in which Ser is the phosphate acceptor. Three PKA substrate proteins have been identified in penile tissue: PDEs, cAMP-responsive element-binding protein (CREB), and ATP-sensitive potassium (KATP) channel.
Cyclic GMP-Signaling Pathway
Signaling molecules in the cGMP pathway include nitric oxide, carbon monoxide, and natriuretic peptides.
Nitric Oxide (NO)
Because of its small size, NO can diffuse inside its target cell, where it interacts with molecules that contain iron in either a heme or iron-sulfur complex. The most physiologically relevant receptor for NO is soluble guanylyl cyclase (sGC), and the NO-sGC-cGMP pathway is responsible for the vasorelaxing effect of many endothelium-dependent vasodilators including histamine, estrogens, insulin, corticotrophin-releasing hormone, nitrovasodilators, and acetylcholine. This pathway is also principally responsible for erection.
Synthesis of NO is catalyzed by NOS, which converts L-arginine and oxygen to L-citruline and NO. NOS exists as three isoforms in mammals: nNOS and eNOS are preferentially expressed in neurons and endothelial cells, respectively, and iNOS in virtually all cell types. All three NOS isoforms have been identified in the corpus cavernosum, with nNOS and eNOS being considered responsible for initiating and sustaining erection, respectively (Hurt et al, 2002). Downregulation of nNOS expression has been found in the corpus cavernosum of aging rats (Carrier et al, 1997), a model in which corpus cavernous smooth muscle relaxation is impaired (Cartledge et al, 2001). Despite its secondary role in erectile function, the endothelium-dependent cavernous smooth muscle relaxation is attenuated in the aging rabbit, and this age-related defect is paradoxically accompanied with upregulated eNOS expression in both the endothelium and cavernous smooth muscle (Haas et al, 1998; Bakircioglu et al, 2001). A contradictory observation, however, was made in another study, which showed downregulation of eNOS in the corpus cavernosum of aging rats (Rajasekaran et al, 2002).
Gene transfer of nNOS or eNOS to the penis has been shown to augment erectile responses in aging rats (Champion et al, 1999; Magee et al, 2002), and gene transfer of iNOS has enhanced intracavernous pressure (Chancellor et al, 2003). However, despite these encouraging results, it should be noted that mice with disrupted nNOS or eNOS gene have normal erectile function (Burnett et al, 1996; Burnett et al, 2002). Compensatory mechanisms, alternative splicing of the disrupted gene (Ferrini et al, 2003), and/or other unknown mechanisms are possibly involved in the preservation of erectile function in the NOS-knockout mice.
Carbon Monoxide (CO)
CO is a gaseous second messenger that occurs in biologic systems during the oxidative catabolism of heme by the heme oxygenase (HO) enzyme. HO exists as constitutive (HO-2, HO-3) and inducible isoforms (HO-1). The latter is upregulated in response to multiple stress stimuli. HO-1 confers protection in vitro and in vivo against oxidative cellular stress. CO regulates vascular processes such as vessel tone, smooth muscle proliferation, and platelet aggregation and may function as a neurotransmitter. The neurotransmitter effect of CO is dependent on the activation of guanylate cyclase by direct binding to the heme moiety of the enzyme, stimulating the production of cyclic AMP. Recent research indicates that CO exerts novel anti-inflammatory and anti-apoptotic effects dependent on the modulation of the p38 mitogen–activated protein kinase (MAPK)–signaling pathway. By virtue of these effects, CO confers protection in oxidative lung injury models and likely plays a role in HO-1–mediated tissue protection (Ryter et al, 2002).
Natriuretic Peptides
The natriuretic peptide family is involved in the regulation of cardiovascular homeostasis and consists of atrial (ANP), brain (BNP), and C-type (CNP) natriuretic peptides (Matsuo, 2001). Whereas ANP and BNP are ligands for the NPR-A receptor, CNP is a ligand for the NPR-B receptor. Both receptors are members of the guanylyl cyclase (GC) family and are therefore also called GC-A and GC-B.
The effects of ANP, BNP, and CNP on cGMP production and smooth muscle relaxation in isolated human and animal corpus cavernosum and in cultured CSMCs have been investigated (Kim et al, 1998; Guidone et al, 2002; Kuthe et al, 2003). The results indicate that CNP is the most potent natriuretic peptide and that it relaxes the isolated cavernous smooth muscle by binding to NPR-B. However, whether CNP and NPR-B play a role in physiologic erection remains to be seen.
Guanylyl Cyclase
In mammals, seven membrane-bound (particulate) GC isoforms (GC-A to GC-G) and one soluble isoform (sGC) have been identified (Andreopoulos and Papapetropoulos, 2000). On the basis of their ligand specificity, the particulate GCs (pGCs) have been classified as (1) natriuretic peptide receptors (GC-A and GC-B), which are activated by natriuretic peptides including ANP, BNP, and CNP; (2) intestinal peptide receptor (GC-C), which is activated by intestinal peptides including guanylin, uroguanylin, and lymphoguanylin; and (3) orphan receptors (GC-D, -E, -F, and -G). Although the membrane-bound GC system is not known to play a role in physiologic erection, expression of GC-B in human and rat corpus cavernosum and induction of cavernous smooth muscle relaxation by CNP (ligand for GC-B) have been demonstrated recently (Guidone et al, 2002; Kuthe et al, 2003).
The soluble isoform sGC plays a pivotal role in erectile function because it provides the link between NO and cGMP, which represent the extracellular and intracellular signaling molecules, respectively, in physiologic erection (Andersson, 2001). A heterodimeric protein, sGC consists of α and β subunits, each of which exists in two isoforms (α1, α2, and β1, β2) that are encoded by two separate genes (Andreopoulos and Papapetropoulos, 2000). Messenger RNAs of these four subunits have been detected in human corpus cavernosum (Behrends et al, 2000). In animal studies, sGC activator YC-1 has been shown to cause erectile responses (Mizusawa et al, 2002). However, mmunohistochemical examination has found sGC expression to be similar in the corpus cavernosum of potent and impotent patients (Klotz et al, 2000).
Protein Kinase G
Protein kinase G (PKG), also called cGMP-dependent kinase (cGK), is the principal receptor and mediator for cGMP signals. In mammals, PKG exists in two major forms, PKG-I and PKG-II, which are encoded by two separate genes. In smooth muscle, only PKGI is expressed and exists as two splice variants (PKG-Iα and PKG-Iβ). Immunoprecipitation studies in smooth muscle indicate that PKG-Iβ is associated with IP3R and a 125 kD protein known as IP3R-associated PKG substrate (IRAG), both of which act as substrates for the kinase (Schlossmann et al, 2000). Phosphorylation of IP3R and IRAG decreases agonist-induced Ca2+ release from the smooth endoplasmic reticulum (SER). Deletion mutation studies demonstrate that IRAG is a critical protein in mediating cGMP-dependent relaxation of smooth muscle (Geiselhöringer et al, 2004). In addition, PKG-I is known to phosphorylate phospholamban, a small membrane-associated protein (6 kD homopentamer) that constitutively inhibits SER. Phosphorylation of phospholamban inactivates its inhibitory control of SER Ca2+-ATPase pump (SERCA) and increases Ca2+ reuptake into the SER, where Ca2+ is bound by proteins such as calsequestrin and calreticulin. Interestingly, cAMP-mediated relaxation is unaffected by mutated, nonfunctional IRAG (Geiselhöringer et al, 2004), whereas PKA can phosphorylate phospholamban to increase Ca2+ reuptake (Raeymaekers et al, 1988) Thus the combined actions of PKG-I and PKA inhibit Ca2+ release from intracellular stores and stimulate Ca2+ reuptake.
Additionally, and perhaps of equal importance, cGMP and/or PKGI may induce relaxation via activation of the plasma membrane Ca2+-ATPase pump, inhibition of IP3 generation, inhibition of Rho-kinase, stimulation of MLCP, and phosphorylation of heat shock proteins (Carvajal et al, 2000; Lincoln et al, 2001). These mechanisms have been demonstrated in various cells, but their relevance to smooth muscle cells in genital tissues has not been explicitly shown. The PKG-I polypeptide contains three functional domains: the N-terminal, the regulatory, and the catalytic. The N-terminal domain has three functions: dimerization, autoinhibition, and localization. The regulatory domain contains two tandem cGMP-binding sites, and the catalytic domain catalyzes the transfer of the γ phosphate from ATP to a serine/threonine residue of the substrate protein.
Cavernous smooth muscle strips from PKG-I knockout mice cannot be relaxed by agents that raise cGMP levels, and these mice have a low ability to reproduce, presumably owing to ED (Hedlund et al, 2000). This observation further affirms the essential role of the cGMP/PKG-I pathway in physiologic erectile function.
Cross-Activation
Increased levels of intracellular cAMP and cGMP cause the activation of cAMP-dependent and cGMP-dependent protein kinases (PKA and PKG). Each cyclic nucleotide-dependent kinase can be activated by either cAMP or cGMP, although cross-activation requires an approximately 10-fold higher concentration of cyclic nucleotide (Walsh, 1994). Although PKA and PKG may phosphorylate a number of common substrates, several lines of evidence indicate that the activation of PKG by cGMP and cAMP is the predominant mechanism by which cyclic nucleotides decrease intracellular Ca2+ to cause vascular smooth muscle relaxation (Lincoln et al, 1990; Jiang et al, 1992; Komalavilas and Lincoln, 1996). On the other hand, PKA (but not PKG) can inhibit Ca2+-calmodulin activation of MLCK by phosphorylation and may play an important role in Ca2+ desensitization of the contractile apparatus (Hathaway et al, 1985).
Phosphodiesterase
In each episode of cyclic nucleotide signaling, the increase of intracellular cAMP or cGMP concentration is typically twofold to threefold baseline (Francis et al, 2001). Decline occurs rapidly and often during the continued presence of the signaling hormone (Francis et al, 2001). Termination of cyclic nucleotide signals is principally carried out by PDEs, which catalyze the hydrolysis of cAMP and cGMP to AMP and GMP, respectively. Feedback mechanisms that increase PDE activities and/or expression by the increased cyclic nucleotide level assist cyclic nucleotide degradation (Corbin et al, 2000; Lin et al, 2001a, 2001b).
The superfamily of mammalian PDEs consists of 11 families (PDE1 to PDE11) that are encoded from 21 distinct genes (Lin et al, 2003; Montorsi et al, 2004). Each PDE gene usually encodes more than one isoform through alternative splicing or from alternate gene promoters. Phosphodiesterase-1, PDE3, PDE4, PDE7, and PDE8 are multigene families, whereas PDE2, PDE5, PDE9, PDE10, and PDE11 are unigene families. PDE1, PDE2, PDE3, PDE10, and PDE11 hydrolyze both cAMP and cGMP; PDE4, PDE7, and PDE8 hydrolyze cAMP; and PDE5, PDE6, and PDE9 are specific for cGMP.
With the exception of PDE6, which is specifically expressed in photoreceptor cells, all PDEs have been identified in the corpus cavernosum (Küthe et al, 2001). However, there is ample evidence that PDE5 is by far the principal PDE for the termination of cavernous cGMP signaling (Fig. 23–12) (e.g., inhibition of the cGMP-catalytic activity of PDE5 by specific inhibitors has been shown to be highly effective in treating ED) (Eardley et al, 2002).
Figure 23–12 Immunohistochemistry of human penile tissue, showing positive PDE5 staining of cavernous smooth muscle fibers (small blue arrows), nerve (yellow arrow), and blood vessel wall (red arrow) (×100).
Phosphodiesterase-3 also appears to play a role in erection, as demonstrated by the erectogenic effect of a PDE3-specific inhibitor, milrinone (Kuthe et al, 2002). Furthermore, although direct inhibition of PDE5 is the main mechanism through which sildenafil exerts its erectogenic effect, it has been shown that sildenafil also significantly increases cAMP concentration in isolated human cavernous tissue strips (Stief et al, 2000). This effect is thought to involve PDE3 because cGMP, which is accumulated as a result of PDE5 inhibition by sildenafil, is capable of preventing cAMP degradation by competing for the same catalytic sites on the PDE3 molecules (Francis et al, 2001). This attenuating effect of cGMP on the cAMP-catalytic activity of PDE3 is also believed to explain why inhibition of PKG could suppress the relaxing effect of forskolin in isolated human cavernous smooth muscle (Uckert et al, 2004).
Ion Channels
In general, there are four major types of ion channels: (1) external ligand-gated, which open to a specific extracellular molecule (e.g., acetylcholine); (2) internal ligand-gated, which open or close in response to an intracellular molecule (e.g., ATP); (3) voltage-gated, which open in response to a change in membrane potential (e.g., sodium, potassium, and calcium channels); and (4) mechanically gated, which open in response to mechanical pressure.
Smooth muscle has neither a T-tubule system nor a well-developed sarcoplasmic reticulum. Therefore extracellular calcium plays an important role, and calcium must enter the cytoplasm through the plasma membrane during an action potential. Three transmembrane proteins are known to regulate calcium inflow and outflow: Calcium channels are the major inflow regulators, whereas the calcium-sodium exchanger and calcium-ATPase regulate calcium exit from muscle cells. The presence of voltage-dependent L-type calcium channels (long-duration current, slow calcium channel) in isolated cavernous smooth muscle and cultured muscle cells has been documented. Christ and colleagues (1993) have reported that both calcium influx through calcium channels and mobilization of intracellular calcium stores are involved during phenylephrine- and endothelin-induced contraction, but only calcium channel influx is apparent for potassium chloride-induced contraction.
Studies have shown at least four types of potassium channel subtypes in the cavernous smooth muscle: (1) the calcium-sensitive potassium channel (e.g., maxi-K); (2) the metabolically regulated potassium channels (KATP); (3) the delayed rectifier; and (4) the fast transient A current (IA) (Christ et al, 1993a; Fan et al, 1995). The calcium-sensitive potassium channels may be involved in cAMP-mediated smooth muscle relaxation. Decreased intracytosolic potassium and altered potassium conductance have been shown to occur in acetylcholine- and sodium nitroprusside-treated corpus cavernosum smooth muscle (Seftel et al, 1996). A stretch-sensitive chloride channel has also been demonstrated in CSMCs (Fan et al, 1999).
Hyperpolarization of the Smooth Muscle Cells
Hyperpolarization causes closure of voltage-dependent calcium channels, a decrease in the intracellular free calcium concentration, and relaxation of the smooth muscle. One of the hyperpolarization mechanisms is through the opening of potassium channels. The opening of ATP-sensitive K+ channels (KATP) and Ca2+-activated K+ channels (KCa) causes hyperpolarization and relaxation of vascular smooth muscle. These two types of channels are present in human corpus cavernosum smooth muscle (Christ et al, 1993b), and pharmacologic stimulation of KATP channels induces penile smooth muscle relaxation (Venkateswarlu et al, 2002). Furthermore, PNU-83757, an opener of KATP channels, has been shown to induce erection when given intracavernously to patients with ED (Vick et al, 2002). The opening of large-conductance KCa channels, also known as maxi-K, has been found to hyperpolarize and relax human corpus cavernosum (Spektor et al, 2002). The opening of K+ channels can be stimulated by PKA, PKG, or cGMP.
Hyperpolarization of penile smooth muscle is also important in endothelium-dependent relaxation of human penile arteries, in which significant relaxation remains despite blockade of NO and prostaglandin synthesis (Angulo et al, 2003). This activity has been attributed to the endothelium-derived hyperpolarizing factor (EDHF), which opens KCa channels and produces hyperpolarization and vasodilation. The nature of EDHF remains undetermined.
Molecular Oxygen as a Modulator of Penile Erection
The pO2 level of cavernous blood in the flaccid state is similar to that of venous blood (≈35 mm Hg). During erection, the large inflow of arterial blood raises pO2 to approximately 90 mm Hg (Sattar et al, 1995). Molecular oxygen is a substrate, together with L-arginine, for the synthesis of NO by NOS. In the flaccid state the low oxygen concentration inhibits NO synthesis; during erection, the higher level of substrate induces NO synthesis. It has been estimated that the minimal concentration of oxygen in the cavernous bodies necessary to reach full NOS activity is 50 to 60 mm Hg (Kim et al, 1993).
Similarly, prostaglandin H synthase is also an oxygenase (cyclooxygenase) and uses oxygen as substrate for the synthesis of prostanoids. Production of PGE1 has been shown to be inhibited in flaccidity and stimulated during erection. Endothelin synthesis is also modulated by oxygen: a low oxygen concentration promotes production, whereas a high concentration inhibits it.
Intercellular Communication
During erection and detumescence, communication should exist among cavernous smooth muscles to mediate synchronized relaxation and contraction (Christ et al, 1991). Several studies have demonstrated the presence of gap junctions in the membrane of adjacent muscle cells. These intercellular channels allow exchange of ions such as calcium and second-messenger molecules (Christ et al, 1993a). The major component of gap junctions is connexin-43, a membrane-sparing protein of less than 0.25 µm that has been identified between smooth muscle cells of human corpus cavernosum (Campos de Calvalho et al, 1993). Cell-to-cell communication through these gap junctions most likely explains the synchronized erectile response, although their pathophysiologic impact is still unclear.
Intracavernous Tissue Architecture
The trabeculae of the corpora cavernosa provide the structural support and regulatory mechanism for the endothelial-lined sinusoidal spaces, as well as the conduit for blood vessels and nerves. Relaxation of the trabeculae allows the expansion and filling of the sinuoids by the incoming blood, while “recoil” of the trabeculae expels blood to the emissary veins and returns the penis to a flaccid state. In 24 men undergoing penile prosthesis implantation for severe ED, Nehra and colleagues (1996) categorized the smooth muscle content of the corpus cavernosum into four groups: high (39% to 42%), intermediate (30% to 37%), low (13% to 29%), and normal (42% to 50%) and reported that increasing degree of venous leakage correlates with decreasing muscle content. Costa and colleagues (2006) showed that the major constituents of the trabeculae are collagen fibers (40.8%), smooth muscle (40.4%), and elastic fibers (13.2%) in specimens from six men who died of nongenital causes. Interestingly, in seven men undergoing penile prosthesis implantation, the content of the three components were collagen fibers (41.6%), smooth muscle (42%), and elastic fibers (9.1%); the only significant change in ED compared with normals was a reduction of elastic fibers. From these two reports, it seems likely that the histologic changes associated with ED consist primarily of decline in either smooth muscle or elastic fibers.
The complex architecture of the penis is maintained by the dynamic expression and interaction of numerous trophic factors. One of them is sonic hedgehog (SHH), which plays a key role in regulating vertebrate organogenesis such as the growth of digits on limbs and organization of the brain. SHH remains important in the adult. It controls cell division of adult stem cells and has been implicated in development of some cancers. SHH has been identified in the penis; inhibition of SHH in the adult rat leads to rapid atrophy and disorganization of the corpus cavernosum (Podlasek et al, 2003, 2005). In addition, SHH has been shown to stimulate the expression of vascular endothelial growth factor (VEGF) and nitric oxide synthase (NOS) in the penis (Podlasek et al, 2005) (Table 23–9).
Table 23–9 Key Molecules Involved in the Physiologic Regulation of Cavernous Smooth Muscle
| Contraction | |
| NAME | FUNCTION |
| High cytosolic calcium | Binds calmodulin to activate MLC kinase |
| MLC kinase | Converts MLC to active form, MLCp |
| Phosphorylated MLC (MCLp) | Cycling of myosin cross-bridges along actin results in muscle contraction |
| MLC phosphatase | Dephosphrylate MLCp to inactive form, MLC |
| Rho kinase | Inhibits MLC phosphatase to enhance contraction (calcium sensitization pathway) |
| Relaxation | |
| NAME | FUNCTION |
| Nitric oxide | Bind soluble guanylyl cyclase to produce cGMP |
| cGMP | Activate protein kinase G |
| Protein kinase G | Opens potassium channels and closes calcium channels |
| Low cytosolic calcium | Calcium dissociates from calmodulin, muscle relaxes |
Key Points
Smooth Muscle Physiology
Pathophysiology of Erectile Dysfunction
Incidence and Epidemiology
The increasing incidence of impotence with age was noted by Kinsey and colleagues in 1948: only 1 of 50 men at age 40 years, but 1 in 4 by age 65. In 1990 Diokno and colleagues reported that 35% of married men aged 60 years and older suffer from erectile impotence.
Modern probability sampling techniques were used by two surveys obtaining prevalence data of ED in the United States: the Massachusetts Male Aging Study (MMAS) and the National Health and Social Life Survey (NHSLS). The MMAS consisted of 1709 noninstitutionalized men between the ages of 40 and 70 years living in the greater Boston area first surveyed between 1987 and 1989 and resurveyed between 1995 and 1997 (Feldman et al, 1994; Johannes et al, 2000). Extensive physiologic measures, demographic information, and self-reported ED status (nine items related to potency on a questionnaire) were components of this report. The MMAS was the first cross-sectional, community-based, random-sample, multidisciplinary epidemiologic survey on ED and its physiologic and psychosocial correlates in men in the United States. From the prevalence rates reported in the MMAS study, between the ages of 40 and 70 years, the probability of complete ED increased from 5.1% to 15%, moderate dysfunction increased from 17% to 34%, and mild dysfunction remained constant at about 17%.
The NHSLS was a national probability survey of men (N = 1410) and women between the ages of 18 and 59 years living in households in the United States in 1992 (Laumann et al, 1999) and was principally a broad-ranging inquiry into sexual practices and beliefs within that age group. Therefore the survey collected only limited information on sexual function broadly defined. The following prevalence rates for ED were reported (responses to questions regarding obtaining and maintaining erection): 7% for ages 18 to 29 years, 9% for ages 30 to 39, 11% for ages 40 to 49, and 18% for ages 50 to 59.
Regarding worldwide prevalence of ED, 24 international studies were reported between 1993 and 2003 (Lewis et al, 2004). All that were stratified by age showed a rising prevalence of ED. Below age 40 the rate was 1% to 9%, from 40 to 59 it ranged from 2% to 9% to as high as 20% to 30%, with some studies showing marked differences between the 40 to 49 and the 50 to 59 groups. The 50- to 59-year groups showed the greatest range of reported prevalence rates. For the group from 60 to 69 years, most of the world showed a rather high rate (20% to 40%), with some increasing after age 65, except for the Scandinavian reports, in which the 70s and older were the time of major rate change. Almost all of the reports showed high prevalence rates for men in their 70s and 80s, ranging from 50% to 75%.
Incidence Studies
The MMAS (Johannes et al, 2000) is the only longitudinal study conducted in the United States (1987-1989 and 1995-1997). Analyses were performed on 847 of the 1297 men without ED at baseline (1987-1989) and with follow-up information from 1995 to 1997. The average age of these men at baseline was 52.2 years (range, 40 to 69 years). From this group of patients, the crude incidence rate of impotence in white men in the United States was 25.9 cases/1000 man-years (95% confidence interval [CI], 22.5 to 29.9). The annual incidence rates increased with each decade (per 1000 man-years): 12.4 cases for 40 to 49 years; 29.8 cases for 50 to 59 years; and 46.4 cases for 60 to 69 years. Age-adjusted risk (per 1000 man-years) of ED was higher for men with diabetes mellitus (50.7 cases), treated heart disease (58.3 cases), and treated hypertension (42.5 cases). By using these data and the known population of the United States, it was estimated that, for white men, the new cases in the 40- to 69-year age group would be 617,715 per year (Lewis et al, 2000). Rates reported from Europe and Brazil also suggest an incidence of 25 to 30/1000 man-years (Moreira et al, 2003; Schouten et al, 2005). A study using a validated questionnaire in a random sample of 2213 men conducted in Olmsted County, MN, from 1996 to 2004 revealed that the five sexual function domains change together over time in this community-based cohort. Erectile function, ejaculatory function, and sexual drive decrease over time with greater rates of decline for older men. However, older men are less likely to perceive these declines as a problem and are less likely to express dissatisfaction referable to them (Gades et al, 2009).
Risk Factors
Common risk factor categories associated with sexual dysfunction include the following: general health status, diabetes mellitus, cardiovascular disease, concurrence of other genitourinary disease, psychiatric/psychologic disorders, other chronic diseases, and socio-demographic conditions. In a study of race/ethnicity and socioeconomic status in 2301 men aged 30 to 79 years from the city of Boston, it is reported that men in the low socioeconomic status (SES) category had a greater than twofold increase in risk of ED (adjusted odds ratio of 2.26, 95% confidence interval 1.39, 3.66). The increased risk of ED in Black and Hispanic men in this study was thought to be associated with differences in SES rather than biologic factors (Kupelian et al, 2008).
For ED, smoking, medications, and hormonal factors also serve as well-defined risk-factor-associated conditions. In men, diabetes has been associated with a greater prevalence of decreased desire and orgasmic dysfunction, as well as ED. A higher odds ratio is seen with insulin-dependent diabetes mellitus; diabetes present for more than 10 years; fair or poor control based on glycosylated hemoglobin; management by means other than diet; a history of diabetes-related arterial, renal, or retinal disease and neuropathy; and concurrent cigarette smoking. Endothelial dysfunction is a condition present in many cases of ED, and thus there are common etiologic pathways for other vascular disease states (Lewis et al, 2004).
Classification
Many classifications have been proposed (Fig. 23–13). Some are based on the cause (diabetic, iatrogenic, traumatic) and some on the neurovascular mechanism (failure to initiate [neurogenic], failure to fill [arterial], and failure to store [venous] [I. Goldstein, personal communication, 1990]). A classification recommended by the International Society of Impotence Research is shown in Table 23–10 (Lizza and Rosen, 1999).
Figure 23–13 A functional classification of impotence. Note that it is unlikely for an individual patient’s impotence to derive solely from one source. Most cases have a psychogenic component of varying degree, and systemic diseases and pharmacologic effects can be concomitant and causative.
(Modified from Carrier S, Brock G, Kour NW, Lue TF. Pathophysiology of erectile dysfunction. Urology 1993;42:468–81, with permission of Exerpta Medica, Inc.)
Table 23–10 Classification of Male Erectile Dysfunction
| Organic |
| Psychogenic |
Psychogenic
Previously, psychogenic impotence was believed to be the most common, thought to affect 90% of impotent men (Masters and Johnson, 1965). This belief has given way to the realization that ED is usually a mixed condition that may be predominantly functional or physical.
Sexual behavior and penile erection are controlled by the hypothalamus, limbic system, and cerebral cortex. Therefore stimulatory or inhibitory messages can be relayed to the spinal erection centers to assist or inhibit erection. Two possible mechanisms have been proposed to explain the inhibition of erection in psychogenic dysfunction: direct inhibition of the spinal erection center by the brain as an exaggeration of the normal suprasacral inhibition (Steers, 2000); and excessive sympathetic outflow or elevated peripheral catecholamine levels, which may increase penile smooth muscle tone to prevent its necessary relaxation. Animal studies demonstrate that the stimulation of sympathetic nerves or systemic infusion of epinephrine causes detumescence of the erect penis (Diederichs et al, 1991a, 1991b). Clinically, higher levels of serum norepinephrine have been reported in patients with psychogenic ED than in normal controls or patients with vasculogenic ED (Kim and Oh, 1992).
Bancroft and Janssen theorized that male sexual response depends on the balance between excitatory and inhibitory impulses within the CNS (Bancroft and Junssen, 2000). One example is the high prevalence of sexual/erectile dysfunction in men with psychiatric disorders. Mosaku and Ukpong (2009) surveyed patients (mean age 39.6, standard deviation [SD] = 11.6 years) carrying the diagnosis of schizophrenia, bipolar affective disorder, recurrent depressive disorder, and/or substance use disorder with mean duration of illness of 10.24 years (SD 8.2 years) who were attending a psychiatry clinic. In this population the prevalence of ED was 83%; older age, unmarried status, use of medications, and the presence of comorbid medical conditions were significantly predictive of ED.
Neurogenic
It has been estimated that 10% to 19% of ED is neurogenic. If one includes iatrogenic causes and mixed ED, the prevalence is likely much higher. The presence of a neurologic disorder or neuropathy does not exclude other causes, and confirming that ED is neurogenic can be challenging. Because erection is a neurovascular event, any disease or dysfunction affecting the brain, spinal cord, and cavernous or pudendal nerves can induce dysfunction.
As discussed earlier, the MPOA, the PVN, and the hippocampus have been regarded as important integration centers for sexual drive and erection (Sachs and Meisel, 1988), and pathologic processes in these regions such as Parkinson disease, stroke, encephalitis, or temporal lobe epilepsy are often associated with ED. Parkinsonism’s effect may result from the imbalance of the dopaminergic pathways (Chaudhuri and Schapira, 2009). Other brain lesions associated with ED are tumors, dementias, Alzheimer disease, Shy-Drager syndrome, and trauma. In studies of sexual function in men after stroke, lack of sexual desire was found to be common (Jung et al, 2008). ED is more prevalent in patients who have cerebrovascular accident lesions in the thalamic area (Jeon et al, 2009).
In men with a spinal cord injury, its nature, location, and extent will largely determine erectile function. In addition to ED, they may also have impaired ejaculation and orgasm. Reflexogenic erection is preserved in 95% of patients with complete upper-cord lesions but in only about 25% of those with complete lower-cord lesions (Biering-Sørensen and Sønksen, 2001). Sacral parasympathetic neurons are important in the preservation of reflexogenic erection, although the thoracolumbar pathway may compensate for sacral loss through synaptic connections. In these patients, minimal tactile stimulation can trigger erection, albeit of short duration and requiring continuous stimulation. Other disorders at the spinal level (e.g., spina bifida, disc herniation, syringomyelia, tumor, transverse myelitis, and multiple sclerosis) may affect the afferent or efferent neural pathway in a similar manner.
Because of the close relationship between the cavernous nerves and the pelvic organs, the incidence of iatrogenic impotence from pelvic surgical procedures is reportedly high: radical prostatectomy, 43% to 100% (Walsh and Donker, 1982; Borchers et al, 2006), and abdominal perineal resection, 15% to 100% (Weinstein and Roberts, 1977).
An improved understanding of the neuroanatomy of the pelvic and cavernous nerves (Walsh and Donker, 1982) has resulted in modified surgery for cancer of the rectum, bladder, and prostate, producing a lower incidence of iatrogenic impotence. For example, the introduction of nerve sparing has reduced the incidence of impotence after radical prostatectomy to 30% to 50% (Catalona and Bigg, 1990; Quinlan et al, 1991) and less than 10% after radical rectal surgery (Liang et al, 2008).
In cases of pelvic fracture, ED can be a result of cavernous nerve injury or vascular insufficiency or both. In men with posterior urethral injury, early realignment has been associated with better potency preservation rate relative to delayed anastomosis (ED rate 34% vs. 42%) (Mouraviev et al, 2005). In diabetics, impairment of neurogenic and endothelium-dependent relaxation results in inadequate NO release (Saenz de Tejada, 1989a). Because autonomic penile innervation cannot be tested directly, clinicians should be cautious in diagnosing neurogenic ED. A corpus cavernosum electromyograph has been developed and refined for diagnosis of various conditions affecting the penis (including autonomic neuropathy), but the clinical utility of this device is still under investigation (Meuleman et al, 2007).
A decrease in penile tactile sensitivity with increasing age was also reported by Rowland and colleagues (1993). Sensory input from the genitalia is essential to achieve and maintain reflexogenic erection, and the input becomes even more important when older people gradually lose psychogenic erection. Therefore sensory evaluation should be an integral part of the evaluation for ED in all patients with or without an apparent neurologic disorder.
Endocrinologic
Hypogonadism is a frequent finding in the impotent population. Androgens influence the growth and development of the male reproductive tract and secondary sex characteristics; their effects on libido and sexual behavior are well established. In a review of published articles from 1975 to 1992, Mulligan and Schmitt (1993) concluded that testosterone (1) enhances sexual interest; (2) increases the frequency of sexual acts; and (3) increases the frequency of nocturnal erection, but has little or no effect on fantasy-induced or visually stimulated erections. Granata and colleagues (1997) have reported that the threshold level of testosterone for normal nocturnal erections is about 200 ng/dL. In a population-based, observational survey conducted in the Boston area, Araujo and colleagues (2007) reported a 5.6% prevalence of symptomatic androgen deficiency in men between the ages of 30 and 79 years, with older men at greater risk. Prevalence of symptoms were low libido (12%), erectile dysfunction (16%), osteoporosis/fracture (1%), and two or more nonspecific symptoms (20%); however, it is noteworthy that many men with low testosterone levels are asymptomatic. In a study of patients presented with ED, Köhler and colleagues (2008) reported androgen deficiency symptoms in 47%, 33%, 23%, and 7% in men with testosterone levels of less than 200, less than 300, less than 346, and less than 400 ng/dL, respectively. Age, the presence of uncontrolled diabetes, high total cholesterol, and anemia all correlated with significantly decreased testosterone levels in men with ED. In another report from the same group, waist circumference was noted to be the most important predictor of low testosterone and symptomatic androgen deficiency (Hall et al, 2008). In men with BMI of more than 30 kg/m2, total testosterone was subnormal in 57.5% and free testosterone subnormal in 35.6% of the subjects. Most of these men had isolated hypogonadotropic hypogonadism (Hofstra et al, 2008). In a comprehensive literature review, Traish and colleagues (2009) noted that low testosterone precedes elevated fasting insulin, glucose, and hemoglobin A1c (HbA1C) values in men who develop diabetes, suggesting that hypogonadism may be a sentinel event in the development of diabetes. The authors further suggest that androgen deficiency is associated with insulin resistance, type 2 diabetes, metabolic syndrome, and increased deposition of visceral fat. Visceral fat may serve as an endocrine organ, producing inflammatory cytokines and thus promoting endothelial dysfunction and vascular disease.
The mechanism of androgen’s effect has been examined by several investigators. Beyer and González-Mariscal (1994) have reported that testosterone and dihydrotestosterone (DHT) are responsible for male pelvic thrusting and estradiol or testosterone is responsible for female pelvic thrusting during copulation. Androgens have beneficial effects on endothelial cells and smooth-muscle cells: androgens promote endothelial cell survival, reduce endothelial expression of proinflammatory markers, and inhibit proliferation and intimal migration of vascular smooth-muscle cells. Low androgen levels are associated with apoptosis of endothelial cells and smooth-muscle cells. Moreover, low androgen levels impair proliferation, migration, and homing of endothelial progenitor cells, as well as myogenic differentiation of mesenchymal progenitor cells (Mirone et al, 2009). Testosterone and dihydrotestosterone may also relax penile artery and cavernous smooth muscle through their nongenomic effects (Waldkirch et al, 2008). In rats, castration has been reported to decrease arterial flow, induce venous leakage, and reduce the erectile response to stimulation of the cavernous nerve by about 50% (Mills et al, 1994; Penson et al, 1996). Castration also increases α-adrenergic responsiveness of penile smooth muscle (Traish et al, 1999). Clinically, many men on long-term androgen ablation therapy for prostate cancer have reported poor libido and ED.
Any dysfunction of the hypothalamic-pituitary axis can result in hypogonadism. Hypogonadotropic hypogonadism can be congenital or caused by a tumor or injury; hypergonadotropic hypogonadism may result from a tumor, injury, surgery, or mumps orchitis.
Hyperprolactinemia, whether from a pituitary adenoma or drugs, results in both reproductive and sexual dysfunction. Symptoms may include loss of libido, ED, galactorrhea, gynecomastia, and infertility. Hyperprolactinemia is associated with low circulating levels of testosterone, which appear to be secondary to inhibition of gonadotropin-releasing hormone secretion by the elevated prolactin levels (Leonard et al, 1989). Interestingly, in a study of subjects consulting for sexual dysfunction, prolactin in the lowest quartile levels was noted to be associated with metabolic syndrome and arteriogenic ED, as well as with premature ejaculation and anxiety symptoms (Corona et al, 2009).
ED may also be associated with both hyperthyroidism and hypothyroidism. The former is commonly associated with diminished libido (which may be caused by the increased circulating estrogen levels) and less often with ED. In hypothyroidism, low testosterone secretion and elevated prolactin levels contribute to ED.
Arteriogenic
Atherosclerotic or traumatic arterial occlusive disease of the hypogastric-cavernous-helicine arterial tree can decrease the perfusion pressure and arterial flow to the sinusoidal spaces, thus increasing the time to maximal erection and decreasing the rigidity of the erect penis. In the majority of patients with arteriogenic ED, the impaired penile perfusion is a component of the generalized atherosclerotic process. Common risk factors associated with arterial insufficiency include hypertension, hyperlipidemia, cigarette smoking, diabetes mellitus, blunt perineal or pelvic trauma, and pelvic irradiation (Feldman et al, 1994; Martin-Morales et al, 2001). Shabsigh and colleagues (1991) reported that abnormal penile vascular findings increased significantly as the number of risk factors for ED increased. On arteriography, bilateral diffuse disease of the internal pudendal, common penile, and cavernous arteries has been found in impotent patients with atherosclerosis. Focal stenosis of the common penile or cavernous artery is most often seen in young patients who have sustained blunt pelvic or perineal trauma (Levine et al, 1990). Long-distance cycling is also a risk factor for vasculogenic and neurogenic ED. The impact of cycling on penile circulation seems to depend on the rider position and the seat design (Cohen and Gross, 2005; Dettori and Norvell, 2006; Gemery et al, 2007; Schrader et al, 2008).
Erectile dysfunction and cardiovascular disease share the same risk factors such as hypertension, diabetes mellitus, hypercholesterolemia, and smoking (Feldman et al, 1994; Martin-Morales et al, 2001). Lesions in the pudendal arteries are much more common in impotent men than in the general population of similar age. Interestingly, natural remission and progression do occur in a substantial number of men with erectile dysfunction. The association of body mass index with remission and progression, as well as the association of smoking and health status with progression, offer potential avenues for facilitating remission and delaying progression using lifestyle intervention (Travison et al, 2007).
Atherosclerosis/Cardiovascular Diseases
High prevalence of ED has been reported in men with coronary, cerebral, and peripheral vascular diseases (Kloner et al, 2003; Montorsi et al, 2003; Bener et al, 2008; Chai et al, 2009). Among men with coronary arterial disease, the prevalence of ED increases as the severity of coronary arterial lesions increases (Montorsi et al, 2006). Similarly, cardiovascular diseases are also prevalent in men with ED, and ED tends to manifest before other signs of cardiovascular disease. In patients with chronic coronary disease who also had ED, onset of sexual dysfunction occurred before CAD onset in 93%, with a mean time interval of 24 (12 to 36) months (Montorsi et al, 2006). This has led some to advocate screening for ED as a means to identify men at risk for cardiovascular disease (Jackson, 2008).
Hyperlipidemia
Erectile dysfunction has been associated with a high prevalence of hyperlipidemia and coronary heart disease (Roumeguere et al, 2003). Hypercholesterolemia at baseline was also shown to be a predictor of subsequent ED over the course of 25 years in 570 male patients included in the Rancho Bernardo Study (Fung et al, 2004). On the other hand, a survey of 1899 men aged 30 to 79 in the Boston area did not show an association between untreated hyperlipidemia and ED (Hall et al, 2009). Although several reports suggest an association of ED and dyslipidemia, there is no consistency in the involvement of the different lipid fractions. Therefore a subcommittee of the Sexual Medicine Society of North America has suggested a more precise analysis of hyperlipidemia as an independent risk factor for ED (Mulhall et al, 2006).
The effect of hypercholesterolemia on erectile function has been studied in different experimental models. In hypercholesterolemic rabbits, examination of the corpus cavernosum ultrastructure revealed an early atherosclerotic process in the sinusoids (Kim et al, 1994). Although the endothelial NO/cGMP pathway is impaired in this model, neuronal vasodilation does not appear affected (Azadzoi et al, 1998). The NO/cGMP pathway effect likely owes to increased superoxide production (Kim et al, 1997) or endogenous NOS inhibitors such as NG-monomethyl-L-arginine monoacetate (L-NMMA) and asymmetric dimethylarginine (ADMA). L-arginine supplementation reverses endothelium-dependent relaxation impairment (Azadzoi et al, 1998). Vascular endothelial growth factor (VEGF) is an important angiogenic factor for maintenance of endothelial health. Ryu and colleagues (2006) reported that VEGF and VEGF receptor 2 (VEGFR-2) are downregulated in the corporal tissue of rats eating a 4% cholesterol diet for 3 months. In rabbits fed with 1% cholesterol diet, Xie and colleagues (2005) also noted that levels of VEGF mRNA are reduced with subsequent observation of impaired endothelium-dependent relaxation.
Studies have also been conducted in apolipoprotein E knock-out mice. After being fed a moderate- to high-cholesterol diet, these mice developed atherosclerosis and ED with reduced endothelium-dependent and endothelium-independent relaxation, as well as alteration of structure of the cavernosal tissue (Behr-Roussel et al, 2006; Xie et al, 2007).
In a more severe ischemic experimental model, rabbits underwent balloon de-endothelization of the iliac arteries followed by a high-cholesterol diet (Azadzoi et al, 1992). They developed both penile arterial insufficiency and veno-occlusive dysfunction owing to decreased expandability of the cavernous smooth muscle (Azadzoi et al, 1997; Nehra et al, 1998). Changes in iliac and penile vasculature were noted, associated with decreased NOS activity and reduced endothelium-dependent and neurogenic NO-mediated relaxation of the cavernous tissue (Azadzoi et al, 1999). As a result of the impaired NO activity, production of contractile thromboxane and prostaglandin increased, leading to potentiation of neurogenic contractions of the cavernous smooth muscle (Azadzoi et al, 1998, 1999).
In large arteries in rabbits, oxidized low-density lipoproteins (ox-LDLs) inhibited endothelium-dependent NO-mediated relaxation (Murohara et al, 1994). Enhanced corpus cavernosum muscle contractility by ox-LDL has also been reported by Ahn and colleagues (1999).
Hypertension
Hypertension is an independent risk factor for ED (Feldman et, 1994; Johannes et al, 2000), and its consequent cardiovascular complications such as ischemic heart disease and renal failure are associated with even higher ED prevalence (Feldman et, 1994; Kaufman et al, 1994; Johannes et al, 2000). However, in hypertension, the increased blood pressure itself does not impair erectile function; rather, the associated arterial biochemical and structural changes are thought to be the causes (Hsieh et al, 1989; Behr-Roussel et al, 2005).
In two analyses including more than 270,000 men with ED from a U.S. care claim database, the prevalence of hypertension in men with and without ED were 41.2% versus 19.2%, respectively (Seftel et al, 2004; Sun and Swindle, 2005).
The potential determinants for ED in hypertensive men include older age, longer duration of disease, greater severity of hypertension, and the use of antihypertensive medications (Doumas et al, 2006). Other researchers have studied arterial function and intima/media thickness in hypertensive men with ED and have suggested that high levels of the endogenous inhibitor of eNOS, asymmetric dimethylarginie (ADMA), are a potential marker of endothelial dysfunction (Vlachopoulos et al, 2008).
In animal models of spontaneously hypertensive rats (SHR) and DOCA-salt hypertensive rats, intracavernous pressure was decreased in two studies when expressed as a percentage of mean arterial pressure (Dorrance et al, 2002; Behr-Roussel et al, 2005). However, the absolute increases in intracavernous pressure did not appear different. Interestingly, in the longitudinal study of the SHR rats, the impairment of cavernosal endothelium-dependent and independent relaxation occurred before systemic vascular alteration, suggesting that corporal vascular tissue is affected early in the disease process (Behr-Roussel et al, 2005). In SHR rats, the impairment appears to involve both NO-mediated and carbon monoxide (CO)-mediated relaxation (Ushiyama et al, 2004). Treating these rats with an antioxidant, alpha-tocopherol, enhanced both NO- and CO-mediated relaxation, as well as endothelial function, suggesting that oxidative stress may play a significant role in this condition (Ushiyama et al, 2008). Increased activity of angiotensin II–mediated NADPH-oxidase in hypertensive rats is suggested to be the cause of increased superoxide anions (Jin et al, 2008).
Mechanism of Vascular Erectile Dysfunction
Structural Changes
In arteriogenic ED, oxygen tension in corpus cavernosum blood is less than that in psychogenic ED (Tarhan et al, 1997). Formation of PGE1 and PGE2 is oxygen dependent, and, in rabbit and human corpus cavernosum, increased oxygen tension was associated with elevation of PGE2 and suppression of TGF-β1-induced collagen synthesis (Moreland et al, 1995; Nehra et al, 1999). A decrease in oxygen tension may diminish cavernous trabecular smooth muscle content and lead to diffuse venous leakage (Saenz de Tejada et al, 1991; Nehra et al, 1998).
A narrowed lumen or increased wall-to-lumen ratio in the arteries contributes to increased peripheral vascular resistance in hypertension. Increased resistance has also been found in the penile vasculature of SHR—an alteration ascribed to structural changes of the arterial and erectile tissue (Gradin et al, 2006; Arribas et al, 2008). Mitochondrial damage (in both smooth muscle and endothelial cells) and nerve degeneration have been described in SHR rats (Jiang et al, 2005). Partial success in the prevention/reversal of the structural changes has been described when rats are treated with type 1 angiotensin II receptor (AT1) blocker, AT1 blocker with phosphodiesterase type 5 inhibitor, and a selective β1 blocker, nebivolol (Mazza et al, 2006; Toblli et al, 2006).
Enhanced Smooth Muscle Contraction and Vasoconstriction
Enhanced basal and myogenic tone has been observed in arteries from hypertensive rats (Schubert and Mulvany, 1999). The RhoA/Rho kinase calcium sensitization pathway is responsible for maintenance of muscle tone. Both hypercholesterolemia and hypertension enhance smooth muscle tone via RhoA/Rho kinase activity (Morikage et al, 2006; Fibbi et al, 2008). Interestingly, both statins and Rho kinase inhibitors enhance the erectile effect of PDE 5 inhibitors in hypertensive rats (Wilkes et al, 2004; Fibbi et al, 2008).
Endothelin-1 (ET-1) levels are elevated in plasma of men with atherosclerosis, hypertension, and hypercholesterolemia. Men with organic ED have higher venous and cavernous blood levels of ET-1 as well (Nohria et al, 2003; El Melegy et al, 2005). Despite this, a pilot study using an ETa receptor antagonist as a treatment for men with ED did not produce positive results (Kim et al, 2002). On the other hand, angiotensin type 1 receptor antagonist and angiotensin conversion enzyme inhibitor have shown promise in the treatment of men with ED hypertension and atherosclerosis, respectively (Speel et al, 2005; Baumhakel et al, 2008).
Impaired Endothelium-Dependent Smooth Muscle Relaxation
Endothelial dysfunction has been proposed as the common link between cardiovascular disease and ED (Brunner et al, 2005). Impairment of endothelium-dependent flow-mediated dilation (FMD) of the brachial artery has been reported in men with ED, and the degree of impairment correlates with the severity of ED (Kovacs et al, 2008). On the other hand, a plethysmography device designed to assess endothelium-dependent vasodilation of the penis did not find a correlation between brachial and penile arteries in ED men (Vardi et al, 2009).
Endothelial progenitor cells (EPCs) are regenerative cells produced in bone marrow that migrate to peripheral vessels to repair endothelial defects. The number of EPCs is reduced in men with ED and coronary heart disease, as well as in overweight men (Foresta et al, 2005; Baumhakel et al, 2006; Esposito et al, 2009). Importantly, both acute and chronic administration of PDE 5 inhibitors increases the number of circulating EPCs and improves both endothelial and erectile function (Foresta et al, 2005, 2009).
In SHR, the relaxing effect of acetylcholine is blunted in corporeal strips (Behr-Roussel et al, 2003). Impairment of endothelium-dependent relaxation in arteries from SHR could be ascribed to angiotensin II (Rajagopalan et al, 1996), thromboxane, and superoxide (Cosentino et al, 1998) or to high blood pressure per se (Paniagua et al, 2000) (Table 23–11).
Table 23–11 Vascular and Structural Changes Leading to Erectile Dysfunction (ED)
| PENILE STRUCTURE | CHANGES IN ED |
|---|---|
| Cavernous artery | Increased vascular resistance, narrow lumen |
| Smooth muscle | Increased tone (hypertonicity) |
| Decreases muscle content | |
| Alteration of K channels and GAP junctions | |
| Erectile tissue | Fibrosis |
| Impaired veno-occlusive mechanism | |
| Endothelium | Impaired endothelium dependent relaxation |
| Tunica albuginea | Alteration of elastic and collagen fibers |
| Neurotransmitters | Decreased nNOS, eNOS |
Cavernous (Venogenic)
Failure of adequate venous occlusion has been proposed as one of the most common causes of vasculogenic impotence (Rajfer et al, 1988). Veno-occlusive dysfunction may result from a variety of pathophysiologic processes: degenerative tunical changes; fibroelastic structural alterations; insufficient trabecular smooth muscle relaxation; and venous shunts.
Degenerative changes (Peyronie disease, old age, and diabetes) or traumatic injury to the tunica albuginea (penile fracture) can impair the compression of the subtunical and emissary veins (Gonzalez-Cadavid, 2009). In Peyronie disease, the inelastic tunica albuginea may prevent the emissary veins from closing (Metz et al, 1983). Chiang and colleagues (1992) have postulated that a decrease in the elastic fibers of the tunica albuginea and an alteration in its microarchitecture may contribute to impotence in some men. Changes in the subtunical areolar layer may impair the veno-occlusive mechanism, as is occasionally seen in patients after surgery for Peyronie disease (Dalkin and Carter, 1991).
Structural alterations in the fibroelastic components of the trabeculae, cavernous smooth muscle, and endothelium may result in venous leakage. Insufficient trabecular smooth muscle relaxation, causing inadequate sinusoidal expansion and insufficient compression of the subtunical venules, may occur in anxious individuals with excessive adrenergic tone or in patients with inadequate neurotransmitter release. It has been shown that alteration of an α adrenoceptor or a decrease in NO release may heighten smooth muscle tone and impair relaxation in response to endogenous muscle relaxant (Christ et al, 1990).
Acquired venous shunts—the result of operative correction of priapism—may cause persistent glans/cavernosum or cavernosum/spongiosum shunting.
Fibroelastic Component
Loss of compliance of the penile sinusoids associated with increased deposition of collagen and decreased elastic fiber may be seen in diabetes, hypercholesterolemia, vascular disease, penile injury, or old age. Sattar and colleagues (1994) have reported significant differences in the mean percentage of penile elastic fibers: 9% in normal men; 5.1% in patients with venous leakage; and 4.3% in patients with arterial disease. In an animal model of vasculogenic ED, Nehra and colleagues (1998) demonstrated that cavernous expandability correlates with smooth muscle content and may be used to predict trabecular histology. Moreland and colleagues (1995) have shown that PGE1 suppresses collagen synthesis by TGF-β1 in human cavernous smooth muscle, which implies that intracavernous injection of PGE1 may be beneficial in preventing intracavernous fibrosis.
Smooth Muscle
Because corporeal smooth muscle controls the vascular events leading to erection, a change in smooth muscle content and ultrastructure can be expected to affect erectile response. In a study of human penile tissue, Sattar and colleagues (1996) demonstrated a significant difference between the mean percentage of cavernous smooth muscle in normal potent men, stained with antidesmin (38.5%) or antiactin (45.2%), and that in a venogenic group (antidesmin, 27.4%; antiactin, 34.2%) or arteriogenic group (antidesmin, 23.7%, antiactin, 28.9%). An in-vitro biochemical study has shown impaired neurogenic and endothelium-related relaxation of penile smooth muscle in impotent diabetic men (Saenz de Tejada et al, 1989a). In vasculogenic and neurogenic ED, the damaged smooth muscle can be a key factor, aggravating the primary cause (Mersdorf et al, 1991). Pickard and colleagues (1994) have also shown impairment of nerve-evoked relaxation and α-adrenergic-stimulated contraction of cavernous muscle, as well as reduced muscle content in men with venous or mixed venous/arterial impotence.
Ion channels are intimately involved in the biochemical events of muscle function. Fan and colleagues (1995) reported an alteration of the maxi-K+ channel in cells from impotent patients and suggested that this might contribute to decreased hyperpolarizing ability, altered calcium homeostasis, and impaired smooth muscle relaxation. In animal studies, Junemann and colleagues (1991) showed significant smooth muscle degeneration with loss of cell-to-cell contact in rabbits fed a high-cholesterol diet for 3 months. In a rabbit model of vasculogenic impotence, Azadzoi and colleagues (1997) demonstrated that veno-occlusive dysfunction could be induced by cavernous ischemia. Cavernous nerve injury may also affect cavernous smooth muscle relaxation, as demonstrated in neurotomized dogs (Paick et al, 1991).
Gap Junctions
These intercellular communication channels are responsible for synchronization and coordination of the erectile response (Christ et al, 1991). In severe arterial disease, the presence of collagen fibers between cell membranes reduces or abolishes their contact (Persson et al, 1989). In aged and streptozotocin-induced diabetic rats, Suadicani and colleagues (2009) reported a significant decrease of gap junction protein connexin 43 in the corpus cavernosum.
Endothelium
By release of vasoactive agents, the endothelium of the corpus cavernosum can modify the tone of adjacent smooth muscle and affect the development or inhibition of erection. NO, prostaglandins, and the polypeptide endothelins have been identified in endothelial cells (Ignarro et al, 1990; Saenz de Tejada et al, 1991a, 1991b). Activation of cholinergic receptors on endothelial cells by acetylcholine or the cells’ expansion as a result of increased blood flow may elicit underlying smooth muscle relaxation through the release of NO (Saenz de Tejada et al, 1988). Diabetes and hypercholesterolemia have been shown to alter the function of endothelium-mediated relaxation of the cavernous muscle (Azadzoi et al, 1991) and impair erection. In a study of cell junction proteins in hypercholesterolemic mice, Ryu and colleagues (2009) reported downregulation of endothelium-specific cell-to-cell junction proteins including claudin-5, VE-cadherin, and PECAM-1, as well as decreased endothelial content, which may contribute to ED in these mice.
Maintenance of Structural Integrity
Sonic hedgehog homolog (SHH) is one of three proteins in the mammalian hedgehog family, the others being desert hedgehog (DHH) and Indian hedgehog (IHH). SHH is the best studied ligand of the hedgehog signaling pathway. It plays a key role in regulating vertebrate organogenesis, such as in the growth of digits on limbs and organization of the brain. SHH remains important in the adult. It controls cell division of adult stem cells and has been implicated in development of some cancers. SHH has been shown to regulate cavernous smooth muscle apoptosis in response to signals from cavernous nerve. In an animal model of neurogenic ED, SHH protein treatment of the penis prevents cavernous nerve (CN) injury–induced apoptosis and structural changes (Podlasek, 2009).
Markers of Erectile Function
Variable coding sequence protein A1 (Vcsa1) has been proposed as a marker of erectile function in rats. Vcsa1 is downregulated in animal models of neurogenic, diabetic, and aging-associated ED. In rat models of ED treated with human Ca2+-activated K+ channels (hslo) and tadalafil, the erectile function improved and the gene expression of Vcsa1 was also upregulated (Calenda et al, 2009). The Vcsa protein product sialorphin is an endogenous neutral endopeptidase inhibitor. Tong and colleagues (2008) proposed that downregulation of sialorphin could result in prolonged activation of G-protein activated signaling pathways by their peptide agonists and thus heighten the cavernous smooth muscle tone. In humans, there are at least three homologues to the Vcsa1 gene (hSMR3A, hSMR3B, and PROL1). Downregulation of hSMR3A has been reported in men with ED (Davies and Melman, 2008).
Drug-Induced
ED is common among older men and therefore will inevitably coexist with other conditions that are themselves risk factors for ED such as depression, diabetes, and cardiovascular disease (Feldman et al, 1994). In addition, sexual symptoms related to medication can involve a combination of complaints concerning desire, arousal, and orgasm rather than being limited to impaired function. Self-reported and questionnaire data concerning ED as a side effect of medication should be interpreted with caution.
Antihypertensive Agents
Almost all antihypertensive drugs have ED listed as a potential side effect, nevertheless, recent well-designed controlled clinical trials have clarified some myths.
Diuretics
Thiazide diuretics are carbonic anhydrase inhibitors that alkalinize cells and causes vasodilation. Nevertheless, the predominant activity of thiazide diuretics is to inhibit a directly coupled Na-Cl cotransporter (NCC) along the distal convoluted tubule of the kidney. Acutely, when ECF volume depletion occurs because of salt wasting, cardiac output tends to decline, resulting in reactive vasoconstriction. Chronically, however, cardiac output is regulated according to metabolic needs and vasodilation supervenes, returning cardiac output toward baseline; this transforms hypotension from hypovolemic to vasodilatory (Ellison et al, 2009).
This class of drug has been extensively studied. Data from a large U.K. trial showed that twice as many men taking thiazides for mild hypertension reported ED than did those treated with propranolol or placebo—the most common reason for withdrawal from the bendrofluazide arm of the study (Medical Research Council, 1981).
Similar findings were documented from the Treatment of Mild Hypertension Study (TOMHS), where the prevalence of ED at 2 years in men taking low-dose thiazide was twice that of those on placebo or alternative agents (Grimm et al, 1997). Interestingly, after 4 years of treatment, the prevalence of ED in the placebo group approached that of the thiazide group, a finding not fully explained by dropouts. It may be that thiazide therapy, rather than causing ED directly, unmasks it at an earlier stage. A study comparing sexual side effects of thiazide, placebo, or atenolol in hypertensive patients also found a higher rate of ED in the thiazide group, although this was ameliorated by weight loss (Wassertheil-Smoller et al, 1991). The mechanism of diuretic-induced ED remains to be elucidated.
β-Adrenergic Blockers
Receptor studies show that only 10% of adrenoceptors in the penile tissue are of the β type, and their stimulation is thought to mediate relaxation (Andersson and Wagner, 1995). This response is attenuated in vitro by nonselective drugs such as propranolol, possibly via a prejunctional β2-receptor effect (Srilatha et al, 1999), but not by cardiac-selective agents such as practolol. Beta antagonists also exert an inhibitory effect within the CNS, perhaps leading to lowered sex hormone levels (Suzuki et al, 1988).
The differential effects of β-adrenoceptor antagonists on erectile function may be explained by whether they are general antagonists, selective antagonists, or possess vasodilatatory properties. Nonselective drugs such as propanolol are associated with higher prevalence of ED compared with what is observed in patients treated with placebo or angiotensin-converting enzyme (ACE) inhibitors (Croog et al, 1986). Later trials using agents with higher selectivity for the β1 adrenoceptor such as acebutolol have shown a substantial reduction in ED with no difference between placebo and ACE inhibitor groups (Grimm et al, 1997). Carvedilol, a general β-adrenoceptor antagonist that also causes vasodilation by blocking α1 adrenoceptors, has been associated with worsening sexual function (Fogari et al, 2001). Some recently introduced β1-adrenoceptor antagonists, such as nebivolol, have vasodilatatory effects mediated by release of nitric oxide (Reidenbach et al, 2007). In cross-over studies using nebivolol versus the selective β1-adrenoceptor antagonists metoprolol and atenolol, nebivolol did not decrease sexual intercourse activity in hypertensive men and in some cases had positive effects on erectile function (Boydak et al, 2005; Brixius et al, 2007).
α-Adrenoceptor Blockers
Animal studies have demonstrated a positive effect on erection for α antagonists, particularly those acting on the α1 receptor, by increasing or prolonging the relaxant response of cavernous smooth muscle (Andersson and Wagner, 1995). In addition, prejunctional α2-receptor activation modulates the release of noradrenaline, suggesting a putative relaxant role for α2 blockers. In clinical observations, drugs such as doxazosin, used to treat hypertension (Grimm et al, 1997) or lower urinary tract symptoms (Flack, 2002), were not associated with complaints of ED and indeed had lower rates than in placebo groups. Not surprisingly, drugs stimulatory to the α2 receptor such as clonidine do result in diminished erectile function, both clinically and experimentally, by peripheral and central mechanisms (Srilatha et al, 1999). The centrally acting drug, methyldopa, has also been associated with ED in controlled trials comparing it with placebo and other antihypertensive agents (Croog et al, 1988,) and it may act by antagonizing hypothalamic α2 adrenoceptors.
Angiotensin-Converting Enzyme Inhibitors
These drugs lack any easily appreciated peripheral or central effect that would interfere with sexual function. An in-vivo experiment showed that the ACE inhibitor captopril did not cause any significant adverse effect on sexual function in awake normotensive rats (Srilatha et al, 1999). In three clinical studies of hypertension treatment comparing an ACE inhibitor with other agents and placebo, all found either no difference from placebo or improved sexual function over baseline when compared with other agents (Croog et al, 1988; Suzuki et al, 1988; Grimm et al, 1997).
Angiotension II Type 1 (AT1) Receptor Antagonist
In studies of hypertensive or aging normotensive animals, the AT1 receptor antagonists (e.g., losartan, valsartan, candesartan) reverse structural changes in the penile vasculature and appear to conserve erectile function (Hale et al, 2001, 2002; Park et al, 2005; Hannan et al, 2006). Moreover, in clinical cross-sectional studies, AT1 receptor antagonists, in contrast to other antihypertensive drugs, seem to improve erectile function (Doumas et al, 2006). In a cross-over study comparing valsartan with the β-adrenoceptor antagonist carvedilol, valsartan had a beneficial effect on preexisting sexual dysfunction and had no adverse sexual effects during 12 months of treatment (Fogari et al, 2001). Three-month treatment with losartan has also been reported to improve sexual function (Llisterri et al, 2001).
Calcium-Channel Blockers
Clinical studies have demonstrated no adverse effect on erection; ejaculatory complaints, which may owe to decreased force of bulbocavernous muscles, seem short-lived (Suzuki et al, 1988). In the TOMHS study there was no ED increase over placebo in the amlodipine group (Grimm et al, 1997). Another study also showed no increase in the prevalence of ED when hypertension was treated with diltiazem alone or in combination with an ACE inhibitor (Cushman et al, 1998).
Aldosterone Receptor Antagonist
Spironolacotone and eplerenone are mineralocorticoid-blocking agents used for their ability to block both the epithelial and nonepithelial actions of aldosterone. Spironolactone is a nonselective mineralocorticoid receptor antagonist with moderate affinity for both progesterone and androgen receptors. The latter property increases the likelihood of endocrine side effects including loss of libido, gynecomastia, and impotence. Eplerenone is a next-generation aldosterone receptor antagonist selective for aldosterone receptors alone. It has less affinity for progesterone and androgen receptors (Sica, 2005).
Summary
Treatment of mild to moderate hypertension requires agents with an acceptable side effect profile to minimize noncompliance. Thiazide diuretic is associated with higher rates of ED, although this may be reduced by combination therapy and weight loss. α1 Blockers and angiotensin II receptor blockers both tend to improve sexual functioning during treatment and may therefore be useful when commencing antihypertensive therapy in men with preexisting ED (Khan et al, 2002) (Table 23–12).
Table 23–12 Effect of Antihypertensive Agents on Sexual Function
| AGENT | EFFECT | MECHANISM |
|---|---|---|
| Diuretics | ED (twice as placebo) | Unknown |
| β blocker (nonselective) | ED | Prejunctional α2-receptor inhibition |
| β1 blocker (selective) | None | |
| α1 blocker | Decreases ED rate but may cause retrograde ejaculation | Failure of sympathetically induced closure of internal sphincter and proximal urethra during ejeculation |
| α2 blocker | ED | Inhibition of central α2 receptor |
| Angiotensin-converting enzyme inhibitor | None | |
| Angiotensin II receptor blocker | Decreases ED rate | |
| Calcium channel blocker | None |
Psychotrophic Medication
As with hypertension, the underlying disorder may be more relevant for ED than the medication. On the other hand, receptor complexity and interrelation of pathways within the CNS make it extremely likely that neurons and ganglia involved in sexual functioning will be affected by psychotropic drugs, leading to functional changes that may be positive or negative. One example is the loss of sexual desire among nonmedicated patients with schizophrenia, while those on antipsychotic drugs have shown greater desire but increased erectile and ejaculatory disturbance (Aizenberg et al, 1995).
Antipsychotics
Members of this class of drug have many effects on CNS receptors and may also act peripherally. Their therapeutic effect is thought to relate to doperminergic receptor blockade within the limbic and prefrontal areas of the brain. Their unwanted effects owe to β-adrenergic blockade and anticholinergic properties, as well as to antidopaminergic actions within the basal ganglia, causing extrapyramidal side effects that commonly produce sexual symptoms (Sullivan and Lukoff, 1990).
The occurrence of extrapyramidal effects differentiates the older “typical” antipsychotics, with which they are frequent, from the newer “atypical” antispychotics, with which they are less common. This difference probably relates to differential affinities for particular classes of receptor (Strange, 2001) or avidity for particular areas of the cerebral cortex (Westerink, 2002). An additional effect of DA blockade, hyperprolactinaemia, which also alters sexual function by reducing DA release in permissive cerebral centers, is more common with older “typical” agents (Smith and Talbert, 1986).
Animal experiments, chiefly in the rat, show that D1-receptor activation in the MPOA of the hypothalamus facilitates erection through intermediary oxytocinergic and spinal cholinergic pathways. It is also possible that activation of D2 receptors in this area has the opposite effect (Zarrindast et al, 1992). Older agents such as haloperidol and flupenthixol have been shown to reduce apomorphine-induced erections in experimental animals by means of D1-receptor antagonism (Andersson and Wagner, 1995). In addition, systemic administration of antipsychotic agents in the rabbit produced erection by a local nondoperminergic action, possibly involving anatagonism of α1 adrenoceptors (Naganuma et al, 1993). Therefore the clinical effect of antipsychotics on sexual function will vary according to their affinity for particular receptors.
In a nonrandomized comparative study of antipsychotic medications, the prevalence of sexual dysfunction ranged from 40% to 70% (Wirshing et al, 2002). Newer agents such as clozapine showed a lower reduction in sexual desire, and the group taking risperidone had the greatest decrease in erectile frequency.
Antidepressants
Sexual side effects in both men and women are varied but are important factors governing compliance because these drugs are commonly prescribed to younger and middle-aged adults. In a Cochrane review of 15 randomized trials, besides changing medications, addition of bupropion or a phosphodiesterase 5 inhibitor (PDE5I) to antidepressant seems to be an effective method to correct antidepressant-associated erectile dysfunction (Rudkin et al, 2004).
Tricyclics act by inhibiting the reuptake of catecholamines in the CNS. Their sexual side effect profile is thought to relate to peripheral anticholinergic and β-adrenergic effects. It is also possible that they antagonize 5-HT receptors. Controlled clinical studies suggest that orgasmic disorders in both sexes are frequent, explaining the use of these drugs as inhibitors of ejaculation (Harrison et al, 1986; Monteiro et al, 1987).
Monoamine oxidase inhibitors are associated with higher rates of orgasmic dysfunction in controlled trials (Harrison et al, 1986), but the nature of the central or peripheral mechanisms involved is uncertain.
Selective serotonin reuptake inhibitors (SSRIs) are the class of drug commonly used to treat depression at the present time. They inhibit the reuptake of 5-HT into CNS neurons and can therefore produce stimulatory effects on various 5-HT receptors. It is estimated that up to 50% of patients taking these drugs experience a change in sexual function (Rosen et al, 1999; Keltner et al, 2002). Possible mechanisms include stimulation of 5-HT2 and 5-HT3 receptors, which may inhibit erectogenic pathways within the spinal cord (Tang et al, 1998), decreased DA release in the MPOA (Maeda et al, 1994), inhibition of NOS, and lower serum levels of LH, FSH, and testosterone (Safarinejad, 2008). A controlled clinical study suggested that the improvement in sexual function resulting from SSRIs’ alleviation of clinical depression outweighed any negative effect (Michelson et al, 2001). However, other placebo-controlled randomized studies revealed increased sexual dysfunction, mainly anorgasmia, in the SSRI-treated group (Labbate et al, 1998; Croft et al, 1999). Further studies have suggested that these adverse effects can be modified by cotreatment with other drugs such as sildenafil (Fava et al, 2006) or mianserin (Aizenberg et al, 1997).
SSRIs differ in their ability to cause ED. A high incidence has been observed in patients treated with paroxetine (Kennedy et al, 2000), while a lesser impact has been reported with citalopram (Mendels et al, 1999). This suggests that mechanisms other than inhibition of serotonin reuptake may be involved, which is supported by a report that acute or chronic paroxetine, but not citalopram, caused ED in rats by inhibiting NO production (Angulo et al, 2001). Indeed, the inhibitory effects induced by acute paroxetine on erectile function in the rat can be prevented by inhibition of PDE5 with vardenafil (Angulo et al, 2003).
Other Antidepressants
Animal experiments suggest that stimulation of 5-HT-1 receptors within the CNS modulates sexual function, with the 5-HT-1A subtype increasing ejaculation and the 5-HT-1C subtype improving erection. Recently developed antidepressants such as mirtazapine and nefazodone tend to have beneficial effects on sexual function, possibly by activating the 5-HT-1C receptor, which augments sexual response (Stancampiano et al, 1994), although they may also antagonize the 5-HT-2C receptor (Millan et al, 2000). The isolated reports of priapism seen with a prototype agent, trazodone, may be related to the 5-HT-1C erectogenic effect seen with its primary metabolite, m-chlorophenylpiperazine, in experimental animals (Andersson and Wagner, 1995). In a clinical study, trazodone was shown to increase nocturnal erectile activity, despite reducing REM sleep (Ware et al, 1994).
Anxiolytics
Although not previously associated with ED, anxiolytics have been implicated in sexual problems by the MMAS study (Derby et al, 2001). Benzodiazepines are thought to potentiate the action of GABA in the reticular and limbic system, but they may also affect the serotonin and dopaminergic pathways. Experimental studies suggest that GABA-ergic drugs inhibit erection induced by apomorphine, a DA agonist (Zarrindast and Farahvash, 1994). A controlled clinical study demonstrated that a combination of lithium and benzodiazepine was associated with a significantly higher rate of sexual dysfunction than treatment with lithium alone (Ghadirian et al, 1992). More recent anxiolytic agents such as bupropion, acting mainly by inhibiting DA reuptake, and buspirone, acting on 5-HT-1A receptors, were not associated with sexual side effects in placebo-controlled trials (Coleman et al, 2001) and can be used to alleviate sexual symptoms caused by other antidepressant medication (Gitlin et al, 2002).
Anticonvulsants
Epileptic discharges may affect the function of the hypothalamic-pituitary axis and thus the level of hormones important for sexual function (Morris et al, 2005). Sexual function, bioavailable testosterone levels, and gonadal efficiency in men with epilepsy who take lamotrigine are comparable with control and untreated values and significantly greater than in men treated with carbamazepine or phenytoin (Herzog et al, 2004). Orgasmic dysfunction is common in patients who receive carbamazepine therapy, and loss of sexual desire is common in men treated with valproate (Kuba et al, 2006). There are reports of improved sexual function and hypersexuality in patients treated with lamotrigine (Gil-Nagel et al, 2006; Grabowska-Grzyb et al, 2006).
Antiandrogens
Androgens are believed to modify sexual behavior by modulating AR within the CNS. Antiandrogens cause partial or near-complete blockade of androgen’s action by inhibiting production of or antagonizing the androgen receptor (AR). The effects of androgen deficiency on sexual activity are variable, ranging from complete loss to normal function. Experimental studies in humans suggest that nocturnal erections during REM sleep are androgen dependent, while erections in response to visual sexual stimulation are independent (Andersson and Wagner, 1995). An additional peripheral effect has been suggested from animal experiments in which castration decreased nitric oxide synthase (NOS) activity within the rat corpus cavernosum, leading to reduced erectile activity. Testosterone restored NOS activity, but treatment with finasteride prevented this recovery, suggesting that dihydrotestosterone (DHT) may be the important androgen in penile tissue (Lugg et al, 1995).
Indeed, the 5-α-reductase inhibitor, finasteride, is the antiandrogen with the least effect on circulating testosterone. In randomized placebo-controlled studies of patients given finasteride (5 mg daily) for prostatic symptoms, approximately 5% complained of decreased desire and ED compared with 1% in the placebo group (Gormley et al, 1992). At the lower dose used to treat male-pattern alopecia (1 mg daily), no sexual dysfunction was seen (Tosti et al, 2001).
More complete androgen ablation is achieved by competitive antagonism at the AR, thus preventing signal transduction of testosterone and DHT. Nonsteroidal drugs such as flutamide and bicalutamide have relatively pure effects on the AR. The steroidal antiandrogen cyproterone acetate also has inhibitory effects on the hypothalamus. These drugs are used in the palliative treatment of locally advanced and metastatic prostate cancer, either alone or in combination with a luteinizing hormone-releasing hormone (LHRH) agonist. When used alone, nonsteroidal antiandrogens are associated with a rise in serum testosterone levels; when combined with an LHRH agonist, they reduce testosterone to the castrate range. The main effect is a reduction of sexual desire, which occurs in up to 70% (Iversen et al, 2001).
In a clinical trial with larger sample size and longer duration, treatment with bicalutamide alone resulted in a lesser decrease in sexual desire than did castration (Iversen et al, 2000). However, in another large controlled trial, treatment with either flutamide or cyproterone resulted in a gradual loss of sexual desire over 2 to 6 years in approximately 80% (Schroder et al, 2000). In a placebo-controlled study, half the patients on bicalutamide therapy suffered loss of erectile function, even at a low dose of 50 mg (Eri and Tveter, 1994).
The near-complete androgen deprivation achieved by medical castration with LHRH agonists results in a profound loss of sexual desire, which is usually accompanied by ED (Basaria et al, 2002). This was objectively confirmed by nocturnal penile tumescence (NPT) monitoring before and after initiation of therapy in a small study (Marumo et al, 1999).
Miscellaneous Drugs
Many other drugs are suggested as having sexual side effects, in particular that of ED in men, but these contentions are usually based on anecdotal case reports or postmarketing drug alerts rather than controlled trials.
Digoxin
In an experimental in-vitro study with isolated human corpus cavernosum tissue, digoxin attenuated the relaxant response to acetylcholine and intrinsic nerve stimulation; this was linked to findings of reduced penile rigidity not seen in men given a placebo after visual sexual stimulation (Gupta et al, 1998). A randomized clinical study confirmed a negative effect on general sexual functioning linked to a decrease in plasma testosterone (Neri et al, 1987). However, others did not find change in sex and adrenal hormone levels in men taking digitalis (Kley et al, 1984).
Statins
Statins are used to lower lipid levels and thus are commonly used in men likely to have established risk factors for sexual dysfunction, particularly ED. In a single placebo-controlled trial, the rate of ED was twice as high (12% vs. 6%) in men taking a statin, despite improvement in other parameters of hyperlipidemic endothelial dysfunction (Bruckert et al, 1996). In another study of 93 men attending cardiovascular risk clinics, after 6 months of statin therapy, the mean IIEF scores were reduced from 21 to 6.5 (range 0 to 25) (p < 0.001), and 22% experienced new-onset ED. The authors suggest that ED following statin therapy is more likely in patients with severe endothelial dysfunction due to established cardiovascular risk factors including age, smoking, and diabetes (Solomon et al, 2006). In contrast, in the large Scandinavian simvastatin survival study, 4444 patients with coronary arterial disease were randomized to treatment with simvastatin or placebo for up to 6 years. ED was found in 28 placebo-treated patients (8 resolved) and in 37 simvastatin-treated patients (14 resolved) (Pedersen and Faergeman, 1999). Therefore the underlying disease process appears to be the cause of ED in men treated with statins rather than the drug itself.
Regarding sexual side effects, the most studied statin is atorvastatin. In clinical studies, atorvastatin has been reported to have the following positive effects: (1) improvement in nocturnal penile activity and mean scores on the Sexual Health Inventory in Men (SHIM) questionnaire from 14.2 to 20.7 in hyperlipidemic patients treated for 4 months (Saltzman et al, 2004); (2) when combined with the ACE inhibitor quinapril, positive effects on ED in men with established penile disease and suboptimal response to phosphodiesterase inhibitors (Bank et al, 2006); (3) improvement in the response to sildenafil in men with ED not initially responsive to sildenafil (Herrmann et al, 2006); (4) when combined with sildenafil, improvement in erectile function recovery in men who had undergone bilateral nerve-sparing radical prostatectomy (Hong et al, 2007); and (5) positive effect on International Index of Erectile Function (IIEF) questionnaire scores in patients with hyperlipidemia followed for 12 months (Dogru et al, 2008).
Statins are classified as natural (lovastatin), semisynthetic (simvastatin), and synthetic (atorvastatin, cerivastatin) and are structurally heterogenous. Therefore the statins may have different effects on sexual function, which remain to be elucidated.
Histamine H2 Receptor Antagonists
Cimetidine and ranitidine were widely prescribed for prophylaxis and treatment of peptic ulcer disease. Case reports suggested that cimetidine was associated with ED. A single in-vitro animal study suggested that H2 receptor stimulation causes cavernous relaxation, possibly via endothelial release of NO (Andersson and Wagner, 1995).
Opiates
Long-term intrathecal administration of opiates results in hypogonadatropic hypogonadism and associated sexual dysfunction that can be restored with appropriate supplementation (Abs et al, 2000). However, administration of opioid antagonists to older men with ED was not found to improve erectile function measured objectively by NPT monitoring (Billington et al, 1990). Opioids do have a generalized depressant effect on sexual function when directly administered to the MPOA in rat brain, but treatment with the opioid receptor antagonist naloxone had no sexual effect on healthy male volunteers (Andersson and Wagner, 1995).
Antiretroviral Agents
Hypogonadism and erectile dysfunction (ED) appear to be more common among men infected with HIV compared with age-matched men within the general U.S. population (Crum et al, 2005). Sexual dysfunction seems to be a common event after the introduction of highly active antiretroviral therapy (HAART). The average prevalence is erectile dysfunction (46%), decreased libido (44%), ejaculatory disturbances (39%), and orgasmic disorders (27%) (Collazos, 2007). These disturbances seemed to be more common in patients treated with protease inhibitors. Because these patients may have diseases involving several organ systems and taking multiple drugs, the precise mechanism is difficult to determine.
Tobacco
Cigarette smoking may induce vasoconstriction and penile venous leakage because of its contractile effect on the cavernous smooth muscle (Juenemann et al, 1987). In an NPT study in cigarette smokers, Hirshkowitz and colleagues (1992) reported an inverse correlation between nocturnal erection (both rigidity and duration) and the number of cigarettes smoked per day: Men who smoked more than 40 cigarettes had the weakest and shortest nocturnal erections. The Boston Area Community Heath (BACH) survey used a multistage stratified random sample to recruit 2301 men, aged 30 to 79 years, from the city of Boston. The authors’ report indicates a dose-response association between smoking and ED with a statistically significant effect observed with 20 or more pack-years of exposure. Passive smoking is associated with a small, statistically insignificant increase in risk of ED comparable with approximately 10 to 19 pack-years of active smoking (Kupelian et al, 2007). In an experiment to elucidate the mechanisms of tobacco use–associated ED, nicotine- and tar-free cigarette smoke extract (CSE) was injected subcutaneously into adult male rabbits once a day for 5 weeks. The authors reported impaired NO production from blunted NOS activity, downregulation of nNOS protein, accumulation of endogenous NOS inhibitors, enhanced arginase activity, and upregulation of arginase I protein in cavernous tissue. CSE also caused accumulation of endogenous NOS inhibitors due to impaired dimethylarginine dimethylaminohydrolase (DDAH) activity and decreased expression of DDAH I protein. These alterations may be relevant to erectile dysfunction following CSE (Imamura et al, 2007).
Alcohol
Alcohol in small amounts improves erection and sexual drive because of its vasodilatory effect and suppression of anxiety; however, large amounts can cause central sedation, decreased libido, and transient ED. In the Western Australia Men’s Health Study, Chew and colleagues (2009) reported that, compared with never-drinkers, the age-adjusted odds of ED were lower among current, weekend, and binge drinkers and higher among exdrinkers.
Chronic alcoholism may result in liver dysfunction, decreased testosterone and increased estrogen levels, and alcoholic polyneuropathy, which may also affect penile nerves (Miller and Gold, 1988). In an in-vitro study of rabbits given 5% alcohol for 6 weeks, Saito and colleagues (1994) reported augmented smooth muscle contraction and relaxation to both electrical field stimulation and vasoconstrictors such as phenylephrine and potassium chloride, but not to sodium nitroprusside, suggesting changes in neurovascular function. In a study of subacute alcohol effect, mice were exposed to alcohol vapor for 7 or 14 days. The authors reported impaired endothelium-dependent relaxation of cavernous smooth muscle, as well as damage of endothelium in 14-day exposure group of mice but not the 7-day group (Aydinoglu et al, 2008) (Table 23–13).
Table 23–13 Drug-Induced Erectile Dysfunction (ED) and Suggested Alternatives
| CLASS | KNOWN TO CAUSE ED | SUGGESTED ALTERNATIVES |
|---|---|---|
| Antihypertensives | Thiazide diuretics General β blockers |
α blockers Calcium channel blockers Specific β blockers Angiotensin-converting enzyme inhibitors Angiotenin II receptor antagonists |
| Psychotropics | Antipsychotics Antidepressants Anxiolytics |
Newer anxiolytics (bupropion, buspirone) |
| Antiandrogen | Androgen receptor antagonists LHRH agonists 5-α reductase inhibitors |
|
| Opiates | ||
| Antiretroviral agents | ||
| Tobacco | Quit smoking | |
| Alcohol | Large amount | Small amount |
Aging, Systemic Disease, and Other Causes
A number of studies have indicated a progressive decline in sexual function in “healthy” aging men. Masters and Johnson (1977) noted a number of changes in older men including greater latency to erection, less turgidity, loss of forceful ejaculation and decreased volume, and a longer refractory period. Decreased frequency and duration of nocturnal erection with increasing age were reported in a group of men who had regular intercourse (Schiavi and Schreiner-Engel, 1988). Other research has also indicated a decrease in penile tactile sensitivity with age (Rowland et al, 1989). A heightened cavernous muscle tone may also contribute to the decreased erectile response in older men (Christ et al, 1990). In one study, a decrease in testosterone in aging impotent men in association with relatively normal gonadotropins was reported, suggestive of hypothalamic-pituitary dysfunction (Kaiser et al, 1988). Penile structural and functional changes have been documented in various animal studies. Costa and Vendeira (2008) reported progressive decline of smooth muscle content and increase in the caliber of vascular spaces in the corpus cavernosum with increasing age in Wister rats. This may be due to increases in smooth muscle cell apoptosis due to oxidative stress and/or increase in caspase 2-mediated intrinsic pathway signaling (Braga et al, 2008). Suadicani and colleagues (2009) showed a significant decrease in gap junction protein connexin 43 and purinoceptor subtypes P2X1R and an increase in P2X7R in the corpus cavernosum of aging Fischer-344 rats. Change of nitric oxide expression or activity has also been reported. Numao and colleagues (2007) have demonstrated attenuated neuronal nitric oxide synthase protein expression and accelerated arginase activity. Ferrini and colleagues (2001a and b) reported an increase of inducible nitric oxide, peroxynitrite formation, and elevation of apoptotic index in the corpus cavernosum and hypothalamic regions. The increased contractile property of the erectile tissue associated with aging may be due to elevated RhoA/Rho-kinase activity (Jin et al, 2006), enhanced rennin-agniotension system (Park et al, 2005) or impaired angiotensin-(1-7)-mediated relaxation (Yousif et al, 2007).
Diabetes Mellitus
Diabetes mellitus is a common chronic disease, affecting 0.5% to 2% of worldwide population. The prevalence of ED is three times higher in diabetic men (28% vs. 9.6%) (Feldman et al, 1994), occurs at an earlier age, and increases with disease duration, being approximately 15% at age 30 and rising to 55% at 60 years (McCullough et al, 1980, 1984). Erectile dysfunction among men with diabetes is more frequent in those with coexisting neuropathy. In a study of men presenting with ED, the authors found a twofold increase of hypogonadism in men with diabetes (24% vs. 12%) (Corona et al, 2006). The presence of ED is associated with more than 14 times higher risk for silent coronary artery disease, higher major cardiovascular morbidity, and mortality in diabetic men (Gazzaruso et al, 2004). This evidence indicates the presence of ED in diabetic patients could predict the future major cardiovascular events. Diabetes mellitus may cause ED by affecting one or a combination of the following: psychologic well-being, CNS function, androgen secretion, peripheral nerve activity, endothelial cell function, and smooth muscle contractility (Dunsmuir and Holmes, 1996).
In 12% of diabetic men, deterioration of sexual function can be the first symptom. Duplex ultrasound after intracavernous injection has revealed a high prevalence (>75%) of penile arterial insufficiency among diabetic men with ED (Wang et al, 1993). Pathologic changes in the cavernous arteries (Michal, 1980), ultrastructural changes in the cavernous smooth muscle (Mersdorf et al, 1991), and impaired endothelium-dependent relaxation of the corporeal smooth muscle (Saenz de Tejada et al, 1989a) have also been noted in penile specimens from diabetic men with ED. Hirshkowitz and colleagues (1990) have reported that impotent men with diabetes have fewer sleep-related erections, shorter tumescence time, diminished penile rigidity, decreased heart rate response to deep breathing, and lower penile blood pressure than do age-matched nondiabetic men.
A number of type 1 and type 2 diabetic animal models have been used to study the basic mechanisms of diabetes-induced ED. In these animals, diabetes causes endothelial cell dysfunction, resulting in an increased prevalence of vascular disease. Other effects include decreased nNOS, reduced activity of eNOS, oxidative stress, increased advanced glycation endproducts (AGEs), decreased elastin, reduced vascular endothelial growth factor (VEGF), hypercontractility of cavernous erectile tissue, and decreased smooth muscle/collagen ratio leading to impairment of the veno-occlusive mechanism. Summaries of mechanistic studies in humans and animal models, derived from the committee report of the Second International Consultation of Sexual Medicine (Saenz de Tejada et al, 2005), are shown in Tables 23-14 and 23-15.
Table 23–14 Summary Findings of Studies in Diabetic Patients
EDHF, endothelium-derived hyperpolarizing factor; NOS, nitric oxide synthase.
Table 23–15 Summary Findings of Studies in Diabetic Animal Models
Metabolic Syndrome
The metabolic syndrome (MetS) includes glucose intolerance, insulin resistance, obesity, dyslipidemia, and hypertension. Higher prevalence of ED (26.7%) in men with MetS relative to controls (13%) has been reported. Furthermore, the prevalence of ED increases as the number of MetS components increases (Esposito et al, 2005). In an analysis of the Baltimore Longitudinal Study of Aging, in which men were followed for a mean of 5.8 years, Rodriguez and colleagues (2007) confirmed that the prevalence of MetS increases with age and is associated with lower androgen levels. They also found that lower total T levels, along with lower sex hormone binding globulin (SHBG) levels, predicts a higher incidence of MetS. Men with MetS have an increased prevalence of ED, reduced endothelial function score, and higher circulating concentrations of C-reactive protein compared with men without metabolic disorders (Esposito et al, 2005). Low levels of androgens in men with erectile dysfunction and obesity were also reported by Corona and colleagues (2008).
Chronic Renal Failure
Sexual dysfunction is common in men with chronic renal failure. In a survey of 69 men on hemodialysis, only 55% were sexually active and the predominant sexual dysfunctions were loss of or diminished sexual needs (84.7%), erectile dysfunction (44.5%), and inhibited or lack of ejaculation (51.5%) (Lew-Starowicz and Gellert, 2009). Similarly, ED was reported in 52% of men undergoing peritoneal dialysis (Lai et al, 2007). The presence of depressive symptoms, highly prevalent in hemodialysis patients, is an independent factor of sexual dysfunction in male hemodialysis patients (Peng et al, 2007). Significant improvement of sexual function has been reported after kidney transplantation (Tavallaii et al, 2009). Nevertheless, in a report of 182 men who had undergone kidney transplantation, Espinoza and colleagues (2006) noted that 49% of men continued to have erectile dysfunction, whereas 33% of men had normal sexual function and 18% had no sexual activity. Many of the effects of uremia can potentially contribute to the development of ED including disturbance of the hypothalamic-pituitary-testis sex hormone axis, hyperprolactinemia, accelerated atheromatous disease, and psychologic factors (Ayub and Fletcher, 2000).
Bagcivan and colleagues (2003) suggest that ED may be due to either decreased production or reduced bioavailability of endogenous NO (Bagcivan et al, 2003). Evidence from animal models of chronic uremia therefore suggests that a decrease in functional NO may be responsible for vascular side effects including ED. Several putative mechanisms may lead to such a deficiency such as reduced bioavailability of the NO substrate L-arginine, reduced expression of NOS isoforms in the relevant organs, rapid quenching of NO by reactive oxygen species known to be increased in chronic renal failure, and the accumulation of uremic inhibitors of NOS (Vaziri, 2001).
Evidence of autonomic neuropathy as a factor contributing to ED in men with chronic renal failure comes from studies that found a high rate of abnormality in vascular and bulbocavernous reflexes, suggesting nerve dysfunction (Campese et al, 1982; Vita et al, 1999). Neuropathy is a common complication of end-stage kidney disease (ESKD), typically presenting as a distal symmetric process with insidious onset progressing over months. Neuropathy has been estimated to occur in 60% to 100% of patients on dialysis. Nerves of uremic patients have been shown to exist in a chronically depolarized state before initiation of dialysis, with subsequent improvement and normalization of resting membrane potential after initiation of dialysis. The degree of depolarization correlates with serum K(+), suggesting that chronic hyperkalemic depolarization plays an important role in the development of nerve dysfunction in ESKD (Krishnan and Kiernan, 2007). Investigation of cavernous vascular function in 20 men undergoing renal replacement therapy showed that 80% had both arterial insufficiency and veno-occlusive dysfunction (Kaufman et al, 1994). A link between impairment of the NO-cGMP pathway relating to failure of cavernous relaxation is provided by the finding of increased serum levels of asymmetric dimethylarginine (ADMA) in uremic patients (Kielstein and Zoccali, 2005).
Patients with severe pulmonary disease often fear aggravating dyspnea during sexual intercourse. Patients with angina, heart failure, or myocardial infarction can become impotent from anxiety, depression, or arterial insufficiency. Other systemic diseases such as cirrhosis of the liver, scleroderma, chronic debilitation, and cachexia are also known to cause ED.
Primary Erectile Dysfunction
Primary ED refers to a life-long inability to initiate and/or maintain erections beginning with the first sexual encounter. Although most cases owe to psychologic factors, a small number of afflicted men do have a physical cause resulting from maldevelopment of the penis or the blood and nerve supply. Primary psychologic dysfunction is usually related to anxiety about sexual performance stemming from adverse childhood events, traumatic early sexual experience, or misinformation. Endocrine abnormalities, particularly low testosterone levels, may also be implicated in primary ED, although lowered sex drive is likely to be the main symptom. Evidence to support these concepts is confined to observation studies with varying numbers of cases. The largest study described 67 patients, of whom 10 (15%) had a predominantly psychologic cause (Stief et al, 1989b). Those with physical abnormalities had a variety of neurologic, arterial, and veno-occlusive dysfunction.
Micropenis
Symmetric hypoplasia of the phallus, micropenis, is often related to urethral developmental abnormalities such as hypospadias and epispadias (Reilly and Woodhouse, 1989) or endocrine deficiency. The erectile tissue in such cases often functions normally; sexual dysfunction usually relates to lack of penile length or the degree of chordee, rather than to ED (Woodhouse, 1998).
Vascular Abnormalities
Primary ED in the presence of an externally normal phallus is unusual. Authors have described structural abnormalities of the cavernous tissue such as absence (Teloken et al, 1993) or replacement by fibrous tissue (Aboseif et al, 1992). Others have found vascular abnormalities including hypoplasia of the cavernous arteries (Montague et al, 1995) or veno-occlusive dysfunction owing to aberrant cavernous venous drainage (Lue, 1999). The underlying cause of these congenital abnormalities is unknown. Treatment in most cases has been vascular surgery or implantation of a penile prosthesis.
Key Points
Other Causes of Erectile Dysfunction