chapter 24 Evaluation and Management of Erectile Dysfunction
Historical Perspective
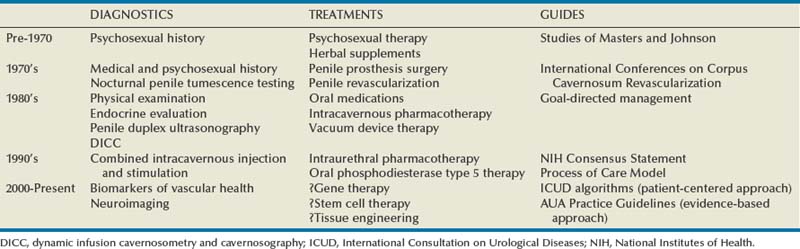
Over just the past quarter century, the clinical management of erectile dysfunction (ED) has changed remarkably (Table 24–1). This comment relates to the success of greatly improved therapeutic options in the field, well demonstrated by oral phosphodiesterase type 5 (PDE5) inhibitor pharmacotherapy. Previously, ED management was mostly empirical and frequently used options were psychoanalysis, sex therapy, and endocrine treatments. Other notional prescriptions ranged from aphrodisiacs and erection enhancement pills to penile rigidity-inducing devices and surgically implanted penile prosthetics.
The modern management of ED marks a surge in the science of penile erection and a trend toward applying an evidence-based methodology (guided by outcomes from controlled and systematic scientific research) in the evaluation and institution of clinical therapies. Movement in this direction was witnessed in the early 1970s with the development of penile revascularization as a plausibly curative intervention for ED, which hinged on new insights regarding the vascular etiopathology of the problem. Although the place of the surgery is today relegated to select ED clinical presentations, it nonetheless ushered in a new era for therapies having an evidentiary basis. This era espoused therapeutic applications based on real scientific advances, from deciphering the pathophysiologic grounds for ED to elucidating the essential biochemical and molecular factors governing the erectile response. Additionally, efficacies of treatments were demonstrated by way of proper clinical investigation. Innovative pharmacologic delivery routes including intracavernosal, intraurethral, and oral treatments were similarly devised and introduced successively in the 1980s, mid-1990s, and late 1990s.
Besides advances in its therapeutic dimension, the field emerged overall as a recognized clinical discipline. Additional highlights in this regard included reaffirmations of its nosological foundations and its principles of practice for the patient with ED. Physiologic penile erection and its impairment were described within the context of the male sexual response cycle, and the historically pejorative and ambiguous term “impotence” was replaced with the more euphemistic and well-defined term “erectile dysfunction.” Along the way, the importance of the patient’s subjective claim of the existence of the problem was fully recognized, with emphasis given to the roles of both the patient and partner in its evaluation and management. Lue originally proposed the concept of the “goal-directed approach” for the management of ED, recognizing that lesser-invasive, reversible therapeutic methods had become available by the 1980s that did not require extensive and costly diagnostic testing before their implementation (Lue, 1990). The approach pronounced the imperative role of the clinician in performing a standard evaluation on the basis of a thorough and adequate sexual, medical, and psychosocial history in combination with a focused physical examination and select laboratory testing. However, it also acknowledged that diagnostic and therapeutic decisions should rely on the goals and preferences of the patient (and partner). This manner emphasizes the “patient-centered” intent brought to ED management (Rosen et al, 2004c).
The clinician’s responsibility in recognizing and managing ED, as for all sexual dysfunctions, has gained the support of many thought leaders in the field of sexual medicine. Guidelines for the management of sexual dysfunctions with worldwide acceptance have resulted from numerous consensus meetings. Prominent among these were the series of International Consultations on Sexual Medicine (ICSM), cosponsored variously by the World Health Organization, International Consultation on Urological Diseases, American Urological Association, Société Internationale d’Urologie, and the International Society for Sexual Medicine, the first held in July 1999 with subsequent conferences convened in July 2004 and in July 2009 (Jardin et al, 2000; Lue et al, 2004; Montorsi et al, 2010). Published proceedings from these congresses established diagnostic and therapeutic algorithms, which were founded on the principles of the goal-directed clinical management approach and obliged the trained clinician/physician to assess the patient and couple completely and prescribe therapy appropriately. They have also proclaimed that sexual medicine should be practiced in accordance with the highest standards of ethics, quality, safety and cost-effectiveness.
Public Health Significance
ED is a medical condition of major health significance, with implications that extend beyond treating the occasionally presenting patient who possesses a problem of seemingly non-life-threatening magnitude. The value of properly assessing and managing ED relates not only to affected individuals and their partners but also to society as a whole, and its scope encompasses physical and mental wellness aspects related to addressing (or failing to address) the sexual dysfunction, concurrent disease management issues, and socioeconomic burden.
Epidemiology
Epidemiologic investigation, which specifies that study results are readily generalized to the overall male population, has provided powerful information regarding the nature, etiology, and prognostic ramifications of ED. The most thoroughly studied sexual dysfunction in the context of epidemiologic research, ED is estimated to carry an overall adult male (older than 20 years of age) prevalence rate of 10% to 20% worldwide, with the majority of studies reporting a rate closer to 20% (Derogatis and Burnett, 2008). It was estimated that there were more than 152 million men worldwide who experienced ED in 1995, with a projection of the prevalence reaching approximately 322 million men having ED by 2025 (Aytac et al, 1999). This trend is maintained irrespective of racial/ethnic background or geographic region. Current data have also confirmed that the prevalence of ED mounts with increasing age and the presence of comorbid medical conditions, which include type 2 diabetes mellitus, obesity, cardiovascular disease, hypertension, dyslipidemia, depression, and prostate disease/benign prostatic hypertrophy (BPH) (Braun et al, 2000; Martin-Morales et al, 2001; Nicolosi et al, 2004; Rosen et al, 2004b; Saigal et al, 2006; Laumann et al, 2007; Selvin et al, 2007). This correlation has supported the premise that ED and comorbid medical conditions share pathophysiologic mechanisms such as endothelial dysfunction, arterial occlusion, and systemic inflammation (Solomon et al, 2003; Montorsi et al, 2004; Billups, 2005; Ganz, 2005; Kloner, 2005; Guay, 2007).
Although they are few in number, prospectively conducted longitudinal studies have documented the true incidence and disease risk relationships for ED. In one study, a crude ED incidence rate was 25.9 cases/1000 man-years among men aged 40 to 69 years (Johannes et al, 2000). According to another study, incident ED statistics were 57% at 5 years and 65% at 7 years in men 55 years or older (Thompson et al, 2005). Such studies have uniquely affirmed predictors for the development of ED, which include age, lower education, diabetes, cardiovascular disease, hypertension, cigarette smoking, cigar smoking, passive exposure to cigarette smoke, and overweight condition (Feldman et al, 2000; Johannes et al, 2000; Inman et al, 2009).
However, the strength of the risk association is also gauged from the opposite analytic direction, and incident ED may indeed inform the risk of subsequent disease morbidity and mortality. This relationship has been best demonstrated so far with respect to cardiovascular disease. The placebo arm of the Prostate Cancer Prevention Trial found that ED is a sentinel for future risk of cardiovascular events, comparable with that of current cigarette smoking or a family history of myocardial infarction (Thompson et al, 2005). This study established that men with ED were 45% more likely than men without ED to experience a cardiac event after 5 years of follow-up (Thompson et al, 2005). In another population-based study of community-dwelling men followed longitudinally, ED was associated with an approximately 80% higher risk of subsequent coronary artery disease at 10 years (Inman et al, 2009). In a long-term follow-up (15 years) of the Massachusetts Male Aging Study (Feldman et al, 1994), ED was found to be positively associated with subsequent all-cause and cardiovascular disease mortality and constituted a risk in this regard similar to that of conventional risk factors such as increased body mass index, diabetes, and hypertension (Araujo et al, 2009). These compelling data, considered alongside results generated from multiple smaller clinical cohort studies in the field, have contributed profoundly toward the understanding that the diagnosis of ED represents a clinical barometer of overall male health status and further serves to catalyze efforts to prevent disease, promote health and, moreover, improve survival.
Health Policy
Sexual dysfunctions and ED specifically have taken on increasing importance with respect to their socioeconomic impact. Besides its medical comorbidity associations, ED is recognized to adversely affect quality of life, decrease occupational productivity, and increase health care resource utilization (Krane et al, 1989; Litwin et al, 1998). Because of the heightened ease of use and availability of effective first-line treatments combined with a growing societal awareness of ED and acceptance of its treatment, it is understandable that a trend toward increased health care services utilization surrounding ED has been observed (Wessells et al, 2007).
ED can be included among a host of urologic diseases having a substantial burden on the public financially. Total expenditures for outpatient clinical management of ED (exclusive of pharmaceutical costs) in the United States in 2000 approximated $330 million, ranking ninth most costly among most frequent urologic diagnoses (Litwin et al, 2005). By contrast, this cost was approximately $185 million in 1994 (Wessells et al, 2007). Individual-level expenditures on an annual basis associated with an ED diagnosis (inclusive of pharmaceutical costs) among affected 18- to 64-year-old males in the United States in 2002 were calculated to be $1107 (Wessells et al, 2007). These data have enormous implications for governmental, as well as nongovernmental agencies in the United States and worldwide, whose work must consider the practical distribution and fiscal allocation of health care services for ED.
Key Points
Epidemiology and Health Policy
Management Principles
The approach to the evaluation and treatment of ED is most assuredly different from that of many other urologic diseases in several basic respects. The diagnosis of ED customarily involves an acknowledgment of the subjective complaint of erectile inability by the patient (or patient and partner), and extensive diagnostic procedures are generally not required to proffer the diagnosis. Additionally, current first-line intervention in the form of effective oral pharmacotherapy is easily prescribed and administered and is frequently successful for the majority of patients. However, notwithstanding the semblance that the management of ED is fairly uncomplicated, it is a structured process that critically incorporates several clinical practice concepts for bringing the best therapeutic outcomes to patients.
Early Detection
Epidemiologic and clinical investigation has suggested that many patients with ED retain adverse clinical conditions and also lifestyle factors (e.g., diabetes, cardiovascular disease, prostate disease, overweight condition, current cigarette smoking, physical inactivity) that potentially compromise erectile function (Saigal et al, 2006; Laumann et al, 2007; Selvin et al, 2007). It is estimated that as much as 75% of men with diabetes have ED to some degree (Hakim and Goldstein, 1996). Similar rates of ED diagnoses are described for men presenting clinically with other chronic diseases, which constitute risk factors for ED (Jackson, 1999; Burchardt et al, 2000; Montorsi et al, 2003b; Solomon et al, 2003).
In addition to adverse health conditions having risk associations with ED, medication use has also been associated with ED in up to 25% of presentations (Keene and Davies, 1999; Francis et al, 2007). The most commonly implicated classes of drug include antihypertensive drugs such as thiazide diuretics and β-adrenoceptor antagonists and psychotherapeutic drugs, particularly selective serotonin reuptake inhibitor (SSRI) antidepressants. Table 24–2 lists several drug classes commonly associated with ED. It is importantly recognized that medications may affect other components of the male sexual response cycle including sexual desire, arousal, and orgasm, which secondarily hampers erectile function. Of additional importance, the assignment of causation of ED for any particular medication is conditional, requiring that an increased prevalence exists in the target population compared with the placebo group after stratification for known risk factors or compared with another drug with an equivalent therapeutic effect, and further, a credible physiologic mechanism should be established experimentally (Sáenz de Tejada et al, 2005).
Table 24–2 Drugs Associated with Erectile Dysfunction
| CLASS | SPECIFIC AGENTS |
|---|---|
| Antihypertensives | Thiazide diuretics, nonselective β-blockers |
| Antidepressants | Tricyclics; selective serotonin reuptake inhibitors |
| Antipsychotics | Phenothiazines |
| Antiandrogens | Nonsteroidal (flutamide); steroidal (cyproterone acetate); luteinizing hormone-releasing hormone analogues |
| Antiulcer drugs | Histamine H2 receptor antagonists (cimetidine) |
| Cytotoxic agents | Cyclophosphamide, methotrexate |
| Opiates | Morphine |
Calculated odds ratios underscore the extent to which various ED risk factors correlate with ED (Table 24–3). These data support the contention that patients with identifiable ED risk factors likely experience the sexual dysfunction currently or will eventually develop it at some time. Clinical screening of such patients based on these indications may allow advantageous opportunities to diagnose and treat ED.
Table 24–3 Major Erectile Dysfunction Risk Factors
| CONDITION | MULTIVARIATE ADJUSTED ODDS RATIO |
|---|---|
| Diabetes mellitus | 2.9 |
| Hypertension | 1.6 |
| Cardiovascular disease | 1.1 |
| Hypercholesterolemia | 1.0 |
| Benign prostate enlargement | 1.6 |
| Obstructive urinary symptoms | 2.2 |
| Increased body mass index (>30 kg/m2) | 1.5 |
| Physical inactivity | 1.5 |
| Current cigarette smoking | 1.6 |
| Antidepressant use | 9.1 |
| Antihypertensive use | 4.0 |
From Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007;120:151–57; and Francis ME, Kusek JW, Nyberg LM, Eggers PW. The contribution of common medical conditions and drug exposures to erectile dysfunction in adult males. J Urol 2007;178:591–6.
Goal-Directed Management
A goal-directed approach to the management of patients with ED has largely been practiced in the field over the past two decades since Lue’s original description (Lue, 1990). The approach dictates that the diagnostic evaluation and therapeutic plan relates to the individual patient’s presentation and manner of deriving satisfaction, in accordance with a patient-centered framework (Hatzichristou et al, 2010). The basic aim of goal-directed management is to allow the patient or couple to make an informed selection of the preferred therapy for sexual fulfillment on the basis of a sound understanding of all treatment options after completing a thorough discussion with the treating clinician. The approach recognizes that patients vary in their acceptance of their sexual disorders and in their interest to pursue management. Their decisions accordingly follow individual preferences, needs, and expectations regarding management options. Evaluations of this approach have affirmed its utility and demonstrated that patient therapeutic preferences accord with the least invasive forms of therapy (Jarow et al, 1996; Hanash, 1997).
Role of Partner Interview
The partner interview is a critical component in initiating management of ED. Partner interviews have been shown to impact diagnosis and treatment in up to 58% of cases (Tiefer and Schuetz-Mueller, 1995; Chun and Carson, 2001). The partner may be the source of important information that guides optimal intervention and response to therapy. The partner may share a new and different perspective on sexual issues affecting the couple, provide insight into the quality of the couple’s relationship, and relate his/her role in the sexual dysfunction (Speckens et al, 1995; Fisher et al, 2009). The partner’s involvement and attitude may also affect the patient’s initiation of and adherence to therapy (Jackson and Lue, 1998; Fisher et al, 2005).
An important additional consideration is that partners’ well-beings may be affected by the patients’ ED conditions. Studies have shown that women partners of men with ED are themselves more likely to have sexual dysfunction or to cease sexual activity entirely (Ichikawa et al, 2004; Montorsi and Althof, 2004; Fisher et al, 2005; Sand and Fisher, 2007). This observation further prompts the facilitatory role of the partner in ED management, which maximizes the success of therapy and inherently satisfaction of the couple.
In practice, additional office visits as needed, in which the partner accompanies the patient, and the communication of educational information to the partner via the patient are recommended techniques for involving partners in ED management (Dean et al, 2008).
Cardiac Risk Assessment
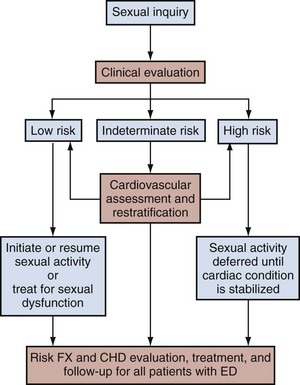
The frequent coexistence of ED and cardiovascular disease, as established by clinical epidemiologic study and by basic science research, has steered ED management to include procedures that account for the ED patient’s cardiovascular health risks. The second Princeton Consensus Guidelines Panel reinforced the linkage between sexual activity and cardiac risk, which was acknowledged with the first conference (DeBusk et al, 2000), and pronounced that all men with ED, even in the absence of manifesting cardiac symptoms, should be regarded as having potential risks for cardiovascular disease (Kostis et al, 2005; Jackson et al, 2006).
ED patients are recommended to undergo a full medical assessment with stratification of cardiovascular risk as high, medium, or low (Fig. 24–1). Patients classified as having high risk would be those with unstable or refractory angina, a recent history of myocardial infarction, certain arrhythmias, or uncontrolled hypertension. For these patients, sexual activity with any particular ED therapy should be deferred until the cardiac condition is stabilized. Such patients should ideally undergo cardiologic referral for cardiovascular stress testing and subsequent risk reduction therapy. Importantly, even patients at low risk for cardiovascular events should receive the minimum recommendations of cardiovascular disease management. Basic intervention includes counseling for lifestyle modifications such as increased physical activity and improved weight control combined with regular health monitoring by the patient’s general practitioner (Kostis et al, 2005).
Step-Care Approach
Practitioners of ED management have always sought a rational approach for implementing diagnostic and therapeutic options. The “Process of Care Model for Erectile Dysfunction” was proposed as a stepwise methodology, combining processes, actions, and outcomes in the management of the ED patient (Process of Care Consensus Panel, 1999). It specified an algorithm for therapeutic decision making that takes into account patient needs and preferences (goal-directed management), although it was also based on specific criteria such as ease of administration, reversibility, relative invasiveness, and cost of therapies. This algorithm presented a strategy of staged therapy (i.e., first-, second-, and third-line interventions), which ranged from lifestyle modification to surgery. In concept, the scheme has been borrowed and endorsed by other consensus panels, which acknowledged the purpose of patient education and counseling along with medical therapies as initial forms of ED management in common practice (Montague et al, 2005; Hatzichristou et al, 2010).
Shared Decision Making and Treatment Planning
The therapeutic plan may vary for each patient and couple and ultimately depends on a host of factors including patient considerations and clinical indications and contraindications. An informed decision-making process should dictate the best therapeutic option. It follows a balanced and thorough discussion led by the clinician of all treatment options, both medical and nonmedical, and their expected advantages and disadvantages. Perceived risks and benefits, which may be influenced by the individual clinical situation, should be weighed. It is understood that the patient may appropriately select a preferred treatment option without necessarily adhering to a strictly prescribed succession of attempted therapies. Indeed, the patient may elect to defer treatment altogether. Whatever the patient (or couple) chooses, this option can then be pursued within the boundaries of safety, under the supportive partnership of his clinician.
Specialist Referral
The advent of effective oral pharmacotherapy for ED has recently enabled many primary practitioners to feel comfortable with managing the majority of clinical presentations of ED. At the same time, it is understood that situations arise in which the patient or primary practitioner may request the assistance of a consultant/specialist (e.g., cardiologist, endocrinologist, psychologist, urologist) for further diagnostic evaluation and treatment beyond the boundaries of initial management (Process of Care Consensus Panel, 1999). Such referrals may be required for individuals with complicated or atypical presentations of ED, representing diagnostic challenges that exceed common clinical practices of nonspecialists. Specialized evaluation and management potentially offer improved therapeutic outcomes for these presentations.
Generally recommended indications for specialized evaluations and associated consultants are failure of initial treatment, referred to a urologist; younger patients with a history of pelvic or perineal trauma, referred to a urologist; patients with significant penile deformity (e.g., Peyronie disease, congenital chordee), referred to a urologist; complicated endocrinopathies (e.g., secondary hypogonadism, pituitary adenoma), referred to an endocrinologist; complicated psychiatric or psychosexual disorders (e.g., refractory depression, hypoactive sexual desire), referred to a psychiatrist; presentations requiring vascular or neurosurgical intervention (e.g., aortic aneurysm, lumbosacral disc disease), referred to a vascular surgeon or neurosurgeon, respectively; medicolegal reasons (e.g., workman’s compensation claims), referred to a urologist.
A caveat is that effort should be taken at the time of referral to be sure that patients are fully informed about the rationale, costs, potential risks, and potential outcomes of the referral and possible additional procedures. This recommendation is made in accordance with the principles of patient-centered medicine, by which patients (and partners where possible) should be included in the decision-making process.
Follow-up Care
Follow-up care is an essential part of ED management and should not be overlooked. The objectives of this action are manifold. A primary basis is to ensure continual success with the therapeutic outcome. It has been shown that treatment discontinuation occurs at high rates among patients who are not reassessed regularly (Albaugh et al, 2002). Additional purposes are to reassess medical and psychosocial conditions adversely impacting ED and success of therapy, evaluate the need for dosage titration or treatment substitution, and monitor adverse drug interactions or drug interaction effects. As always, follow-up attention offers educational opportunities for patient and partner with regard to addressing sexual health concerns, as well as lending guidance for related health care matters.
Diagnostic Evaluation
The cornerstone in the evaluation of ED involves a detailed case history, preferably taken from patient and partner, physical examination, and proper laboratory tests (Fig. 24–2). The diagnosis can be submitted on the basis of an individual’s report of consistent inability to attain and maintain an erection of the penis sufficient to permit satisfactory sexual intercourse (NIH Consensus Statement, 1992; Lewis et al, 2004). It is noteworthy that the original National Institutes of Health definition did not specify a parameter for the duration of symptoms to accept the diagnosis. Subsequent organizational statements did apply a 3-month interval as a minimal requirement diagnostically, except for cases of trauma or surgically induced ED (Lewis et al, 2004).
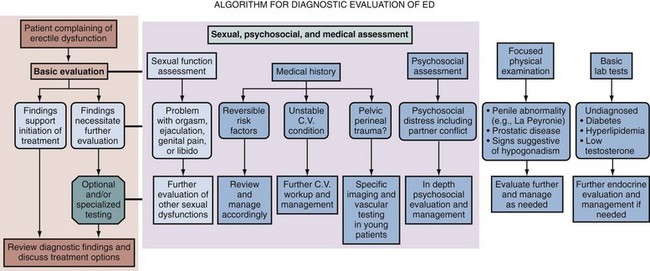
Figure 24–2 Diagnostic algorithm for erectile dysfunction (ED) recommended by the International Consultations on Sexual Medicine.
Sexual, Medical, and Psychosocial History
The comprehensive assessment of any sexual problem begins with the performance of a detailed case history including sexual, medical, and psychosocial components. The clinician may employ brief checklists or questionnaires for the purpose of recognizing the problem and initiating its evaluation, although he or she should standardly perform a detailed interview to understand the nature of the sexual complaint. The sexual history component in particular should be elicited with utmost sensitivity, given the intrapersonal and interpersonal aspects of sexual dysfunction (Rosen et al, 2004c). Additional emphasis has recently been given to providing cultural competence when interacting with patients (Hatzichristou et al, 2010). All discussion of sexual matters is done privately and confidentially, and the clinician is required to express trust and concern, as well as a nonjudgmental manner that epitomizes the doctor-patient relationship. The clinician should not assume that every patient is involved in a monogamous, heterosexual relationship. However, the situation may be presented whereby the partner can be interviewed, and this opportunity may be used, with the approval of the patient, to corroborate aspects of the clinical history and confirm mutual therapeutic goals.
Sexual History
The sexual history is the central component of the clinical history and serves to confirm the patient’s sexual dysfunction complaint of ED. Objectives of the interview are also to delineate the problem according to such features as its onset, duration, conditions, severity, and etiology. The conditions of the problem are often determined by reviewing circumstances that facilitate or hinder erectile function. Circumstances for achievable erections include stimuli used during sexual encounters, erections on awakening, and the role of self-stimulation. Circumstances associated with erectile difficulty include performance anxiety, inability to perform with a designated partner, and motivational factors affecting lovemaking. Other pertinent issues include availability, interest and health of the partner, changes in medical status or other events relating to the onset of ED, and prior attempts to manage the problem by the patient or another caregiver.
The severity of ED can be defined as mild, moderate, or severe/complete, according to increasing degrees of loss of penile rigidity and the associated interference with sexual activity. For instance, mild ED may refer to minimally decreased ability to attain and/or maintain an erection with intermittent satisfactory sexual performance, moderate ED may refer to minimally decreased ability to attain and/or maintain an erection with infrequent satisfactory sexual performance, and severe ED may refer to substantially decreased ability to attain and/or maintain an erection with rare or absent satisfactory performance.
The potential etiology of ED is commonly probed and may be categorized as psychogenic, organic, or mixed according to whether there is a presumed psychologic or interpersonal determinant (psychogenic); a specific endocrinologic, neurologic, or cardiovascular cause (organic); or coexistence of psychologic or relationship factors and organic causes (mixed) (Table 24–4) (Ralph and McNicholas, 2000). It is accepted that ED many times cannot be fully dichotomized into psychogenic and organic categories. However, its characterization by a predominant etiologic basis may nonetheless assist therapeutic objectives. The interview should also assess whether ED is the primary source of the presenting complaint or secondary to some other aspect of the sexual response cycle (e.g., desire, ejaculation, orgasm) that may also relate to the clinical presentation (Rosen et al, 2004c). The association of decreased arousal, if present, may be explored as well and evaluated as to whether it preceded or was incidental to the development of ED.
Table 24–4 Classification of Erectile Dysfunction
| PSYCHOGENIC | ORGANIC |
|---|---|
| Sudden onset | Gradual onset |
| Complete immediate loss | Incremental progression |
| Situational dysfunction | Global dysfunction |
| Waking erections present | Waking erections poor/absent |
Adapted from Ralph D, McNicholas T. UK management guidelines for erectile dysfunction. BMJ 2000;321:499–503.
Medical History
The medical history primarily serves to identify and evaluate predictors and risk factors associated with ED. The main objective is to explore the role of possibly related or underlying medical conditions and to ascertain the existence of comorbidities. Recognition of the association between medical conditions and ED not only may lend insight into the possible basis for the ED, which may guide choice of therapy, but it may also specify reversible or treatable factors associated with ED that may be corrected with an expectation of improving the level of erectile function.
Medical conditions associated with ED include disease states (e.g., type 2 diabetes mellitus, cardiovascular disease, hypertension, dyslipidemia, neurologic disease, hypogonadism, thyroid disorders); consequences of trauma involving aspects of the body, pelvis, or genitalia (e.g., spinal cord injury, pelvic surgery or radiation, sexual injury); and side effects of medications or recreational substances that disturb biochemical processes of penile erection. Age is recorded, in accordance with the well-known association between aging and ED. Comorbidities (e.g., depression, anxiety, anger) are importantly registered because of their bidirectional relationship with ED.
Psychosocial History
The intake of psychosocial history is a necessary part of the clinical history. The very best sexual performance most assuredly implies wellness of mind and body acting together, and unstable psychosocial circumstances of both intrapersonal and interpersonal contexts may adversely affect sexual function. Accordingly, the presence and interaction of mental health problems, emotional stressors, and interpersonal relationship difficulties, both past and present, should be ascertained. Additional questions may be asked relating to occupational status, financial security, family life, and social support, which may also influence sexual function.
Physical Examination
The physical examination is a necessary component of the comprehensive assessment of sexual dysfunctions and complements the clinical case history. It may reveal possible etiologies for ED.
This evaluation consists of basic anthropometrics (i.e., height, weight, waist circumference); assessment of body habitus (appearance of secondary sexual characteristics); and examination of relevant body parts pertaining to cardiovascular, neurologic, and genital systems, with a particular focus on the external genitalia. The observation of a classically distinctive body habitus consistent with Kallman or Klinefelter syndrome or obvious physical signs of hypogonadism such as gynecomastia and general poor masculine development may suggest an endocrinologic basis for ED.
Findings of obesity, elevated blood pressure, or abnormal femoral or pedal pulses—all signs representative of cardiovascular disease—convey a potential vascular causation. Findings of abnormal genital and perineal sensation or bulbocavernosus reflex, indicative of a peripheral neuropathy, suggest the involvement and effects of a neurologic disorder or diabetes.
Detection of a penile deformity such as micropenis, congenital chordee, or Peyronie disease–related fibrous plaques in the corpora cavernosa supports the possibility that a physical impediment accounts for ED. Genital examination findings of abnormal position, size, and consistency of testes may also suggest hypogonadism and suggests that ED exists on endocrinologic grounds.
Questionnaires and Sexual Function Symptom Scores
Self-administered ED questionnaires are extremely useful adjuncts to the case history, and they concur with the patient’s self-report in establishing the diagnosis. Early supplied questionnaires in the field such as the Derogatis Sexual Function Inventory (245 items) (Derogatis and Melisaratos, 1979) and the Golombok Rust Inventory of Sexual Satisfaction (GRISS) (28 items) (Rust and Golombok, 1986) were detailed, and they commonly aimed to differentiate psychogenic and nonpsychogenic ED or evaluate sexual functioning in the context of the couple. More recently developed instruments were implemented primarily in clinical trials associated with new drug development, and they captured particular efficacy end points including sexual interest, performance, and satisfaction. However, as part of practice pattern shifts that have occurred in ED management in recent years, there has been a growing emphasis on and application of patient self-reported instruments for clinical practice. These self-report measures have been meant to be brief and practical and to serve in documenting the presence, severity, and responsiveness to treatment of ED.
The most widely referenced instruments include the International Index of Erectile Function (IIEF) by Rosen and colleagues (1997), the Brief Male Sexual Function Inventory (BMSFI) by O’Leary and colleagues (1995), the Center for Marital and Sexual Health Sexual Functioning Questionnaire by Glick and colleagues (1997), the Changes in Sexual Functioning Questionnaire by Clayton and colleagues (1997), and the Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) by Althof and colleagues (1999). The IIEF, which contains 15 items that address and quantify five domains—erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction—is the most widely used questionnaire (Fig. 24–3). An abridged five-item version of this instrument, the IIEF-5, has been useful to clinicians in routine clinical practice specifically for the evaluation of ED (Rosen et al, 1999). The instrument classifies ED severity into five categories: severe (5 to 7), moderate (8 to 11), mild to moderate (12 to 16), mild (17 to 21), and no ED (22 to 25). The Male Sexual Health Questionnaire offers another instrument that assesses core components of male sexual function (i.e., desire, erection, ejaculation, satisfaction) and has utility in both clinical and research settings (Rosen et al, 2004a). The Sexual Experience Questionnaire has also recently been developed as a tool for evaluating health-related quality of life concepts and comprises erection, individual satisfaction, and couple satisfaction domains (Mulhall et al, 2008).
A known limitation of self-administered questionnaires is that they do not distinguish an etiologic basis for ED, that is, they do not differentiate among the various causes of ED (Blander et al, 1999; Kassouf and Carrier, 2003). Further, they may not sufficiently indicate the severity of ED that is evidenced on objective grounds (Tokatli et al, 2006). Although the exact nature of the ED diagnosis arguably is not absolutely necessary to initiate ED treatment today with current management options, it is understood that further clinical evaluation with diagnostic tests may be required to discern the basis and extent of the ED by system (e.g., vascular, neurologic, endocrinologic) and take action that may be most effective and possibly corrective.
Laboratory Tests
Appropriate laboratory testing can be considered part of a systematic clinical evaluation for individuals presenting with ED. Such evaluation may confirm or define etiologic medical conditions associated with the sexual dysfunction. At times, it may identify treatable conditions or previously undetected disease states that may contribute to ED. A standardized panel of tests can be offered for the man presenting routinely with sexual dysfunction including ED. Further laboratory testing can be tailored to the clinical situation. Similarly, specialized endocrinologic assessment can be performed when indicated for select clinical presentations.
Recommended laboratory tests for men with sexual problems typically include serum chemistries, fasting glucose, complete blood count, lipid profile, and serum total testosterone. Total testosterone, measured from a morningtime blood draw, serves to screen androgenic status and, if abnormally low, serum-free (or bioavailable) testosterone and leutinizing hormone should be measured. Prolactin measurement may also be done for hormonal assessment. Thyroid function tests may be performed at the clinician’s discretion. Serum PSA testing is performed as needed if prostate pathology is suspected that might be promoted by exogenously administered testosterone. Dipstick analysis of urine may reveal glucosuria, which suggests the diagnosis of diabetes.
Specialized Evaluation and Testing
The implicit goal of specialized evaluations in medicine in general is to improve diagnostic accuracy and direct successful therapy on the basis of the specific diagnosis. A similar principle applies to sexual medicine. However, at the present time, despite the availability of various technologies that may specify and define the causation for ED (i.e., vasculogenic, neurogenic, endocrinogenic, psychogenic), the treatment plan for this sexual dysfunction can often be formulated without carrying out extensive diagnostic testing. Nonetheless, such testing is frequently applied for diagnostic precision, typically by specialists, particularly in settings of complex clinical presentations. Table 24–5 summarizes the most frequently used evidence-based test procedures for diagnostic evaluations of ED (Rosen et al, 2004c).
Table 24–5 Evidence-Based Tests for Organic Erectile Dysfunction and Recommendations
| TEST | RECOMMENDATION* |
|---|---|
| Vascular | |
| Dynamic infusion cavernosometry and cavernosography (DICC) | B |
| Intracavernous injection pharmacotesting (ICI) | B |
| ICI and color duplex ultrasound | B |
| Arteriography | C |
| Computed tomography angiography | D |
| Magnetic resonance imaging (MRI) | D |
| Infrared spectrophotometry | D |
| Radioisotope penography | D |
| Audiovisual Sexual Stimulation (AVSS) | |
| Independent or jointly with vascular testing | C |
| With or without: pharmacologic stimulation (oral, ICI) | C |
| Neurophysiologic | |
| Nocturnal penile tumescence and rigidity (NPTR) | B |
| Erectiometer/rigidometer | D |
| Biothesiometry (vibratory thresholds) | C |
| Dorsal nerve conduction velocity | C |
| Bulbocavernosus reflex latency | B |
| Plethysmography/electrobioimpedance | D |
| Corpus cavernosum electromyography (CC-EMG) | C |
| MRI or positron emission tomography scanning of brain (during AVSS) | D |
Modified from Rosen RC, Hatzichristou D, Broderick G, et al. Clinical evaluation and symptom scales: sexual dysfunction assessment in men. In: Lue TF, Basson R, Rosen F, et al, editors. Sexual medicine: sexual dysfunctions in men and women. Paris: Health Publications; 2004. p. 173–220; and Harbour R, Miller J. A new system for grading recommendations in evidence-based guidelines. BMJ 2001;323:334–6.
Vascular Evaluation
The vascular evaluation for ED conceptually connotes surveying the vascular requirements of the sexual organ for the erectile response: arterial blood inflow, blood engorgement, and blood retention within the corporal structures. From a diagnostic standpoint, the studies aim to assist in deriving the classical diagnoses of arterial impairment and veno-occlusive dysfunction. As for all diagnostic testing, hemodynamic tests of the penis require that the patient is counseled regarding the purpose, alternatives, risks, and benefits of any procedure before its implementation.
Combined Intracavernous Injection and Stimulation
The CIS test serves as a first-line evaluation of penile blood flow because of its basic manner of administration and assessment. The test involves the intracavernous injection of a vasodilatory drug or drugs as a direct pharmacologic stimulus, combined with genital or audiovisual sexual stimulation, and the erectile response is observed and rated by an independent assessor (Donatucci and Lue, 1992; Katlowitz et al, 1993). The test is designed to bypass neurologic and hormonal influences involved in the erectile response and allows the clinician to evaluate the vascular status of the penis directly and objectively.
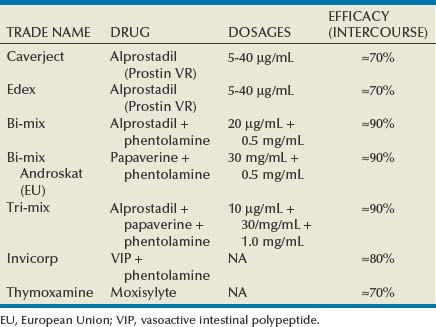
The clinician may decide the protocol for using vasodilator drugs. Alternative regimens include alprostadil alone (Caverject or Edex, 10 to 20 µg), a combination of papaverine and phentolamine (Bimix, 0.3 mL), or a mixture of all three of these agents (Trimix, 0.3 mL). The procedure requires a syringe with a 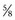 -inch needle (27 to 29 gauge), which is inserted at the lateral base of the penis directly into the corpus cavernosum for medication delivery. After needle withdrawal, manual compression is applied to the injection site for 5 minutes to prevent local hematoma formation. The assessment is done periodically afterwards to rate both rigidity and duration of response. Repeated dosing may be performed if the initial erectile response is poor. Return to penile flaccidity is required before allowing the patient to leave the office, and if detumescence does not occur spontaneously in approximately an hour after dosing, intracavernous injection of a diluted phenylephrine solution (500 µg/mL) can be done every 3 to 5 minutes until flaccidity returns.
-inch needle (27 to 29 gauge), which is inserted at the lateral base of the penis directly into the corpus cavernosum for medication delivery. After needle withdrawal, manual compression is applied to the injection site for 5 minutes to prevent local hematoma formation. The assessment is done periodically afterwards to rate both rigidity and duration of response. Repeated dosing may be performed if the initial erectile response is poor. Return to penile flaccidity is required before allowing the patient to leave the office, and if detumescence does not occur spontaneously in approximately an hour after dosing, intracavernous injection of a diluted phenylephrine solution (500 µg/mL) can be done every 3 to 5 minutes until flaccidity returns.
A normal CIS test, based on the assessment of a sustainably rigid erection, is understood to signify normal erectile hemodynamics. Alternative diagnoses of psychogenic, neurogenic, or endocrinogenic ED may then be considered. However, it is known that false-positive results may occur in up to 20% of patients with borderline arterial inflow (as defined by the measurement of 25 to 35 cm/sec peak cavernous artery systolic flow on duplex ultrasound) (Pescatori et al, 1994). False-negative results are also possible and occur most commonly because of patient anxiety, needle phobia, or inadequate dosage.
Duplex Ultrasonography (Gray Scale or Color Coded)
Duplex ultrasound of the penis following pharmacostimulation or CIS represents second-line evaluation of penile blood flow. However, it is the most reliable and least invasive diagnostic modality for assessing ED. The test adds an imaging dimension and a quantification component to the evaluation of blood flow in the penis distinct from first-line evaluation, which relies on the assessor’s judgment alone.
The technique consists of high-resolution (7 to 10 MHz) real-time ultrasonography and color pulsed Doppler, which serves to visualize the dorsal and cavernous arteries selectively and to perform hemodynamic blood flow analysis (Lue et al, 1989). Scanning is applied to the surface of the penis and may include the entire penis from the crura in the perineum to the tip. Color-coded duplex ultrasound indicates the direction of blood flow within vessels, with red designating direction toward the probe and blue designating direction away from the probe (Broderick and Arger, 1993; Herbener et al, 1994). Flow velocities are measured at baseline before injection and commonly every 5 minutes afterwards up to 20 minutes. Cavernous arterial diameters may also be measured. Vascular anatomic communications between the paired cavernous arteries or between the dorsal and cavernous arteries should be noted (Fig. 24–4). Erection quality should also be simultaneously assessed and rated. An observed poor erection, possibly associated with patient anxiety, should prompt vasodilator redosing as recommended for the CIS test.
Figure 24–4 Collateral circulation connecting the right dorsal artery (RDA) to the right cavernous artery (RCA) and the left cavernous artery (LCA) is shown by color duplex ultrasound in a longitudinal view.
A standard pattern of Doppler waveforms occurs with hemodynamic changes in corporeal pressure during progression to normal full erection (Fig. 24–5) (Schwartz et al, 1991). In the filling phase when sinusoidal resistance is low (within 5 minutes after vasodilator injection), the waveform increases in size consistent with high forward flow during both systole and diastole. As intracavernous pressure increases, diastolic velocities decrease. With full erection, the systolic waveforms sharply peak and may be slightly less than during full tumescence. At maximal rigidity, when intracavernous pressure exceeds systemic diastolic blood pressure, diastolic flow may be zero. The sonographic color pattern of the cavernous artery may demonstrate an impressive shift from red to blue in association with the reversal of diastolic flow.
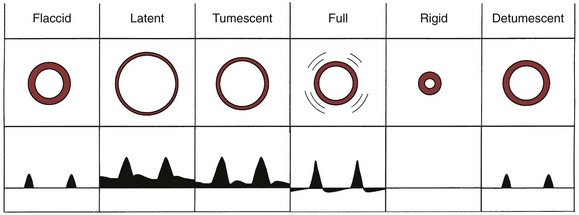
Figure 24–5 Artist’s conception of the changes in diameter and flow waveform in the cavernous arteries induced by intracavernous injection of prostaglandin E1 in a potent young man as demonstrated by duplex ultrasound. Forceful concentric pulsations are particularly noticeable during full erection.
Normative values have been described for peak systolic velocity (PSV) and diameter of the cavernous arteries during increases in arterial inflow to the penis. Early studies documented that the PSV of the cavernous arteries consistently exceeded 25 cm/sec within 5 minutes of vasodilator injection in patients with nonarteriogenic causes of ED (i.e., psychogenic, neurogenic) (Lue et al, 1985; Mueller and Lue, 1988). Investigators subsequently confirmed mean PSV of cavernous arteries after pharmacostimulation to range from 35 cm/sec to 47 cm/sec in normal subjects (Benson and Vickers, 1989; Shabsigh et al, 1990). A cut point at 25 cm/sec had a sensitivity of 100% and a specificity of 95% in patients with abnormal pudendal arteriography (Quam et al, 1989). Diameter changes of the cavernous artery after vasodilator injection were found to increase less than 75% and rarely exceed 0.7 mm in patients with severe vascular ED (Lue and Tanagho, 1987; Mueller and Lue, 1988). Importantly, unlike PSV changes, percentage of cavernous arterial vasodilation was not found to correlate well with findings on pudendal arteriography (Jarow et al, 1993).
Vascular arterial anatomic variants may confound the interpretation of duplex ultrasonography (Breza et al, 1989; Jarow et al, 1993). Early cavernous arterial branching or the presence of multiple such branches may affect blood flow velocity determinations of the main cavernous artery. The presence of distal arterial perforators extending from the dorsal or spongiosal arteries also may alter the measurement of cavernous arterial blood flow velocity. Accordingly, the clinician must recognize these variants to avoid making the incorrect diagnosis of arteriogenic ED. On the other hand, asymmetric blood flow of the cavernous arteries may have diagnostic significance. The findings of dissimilar cavernous artery velocity measurements, which are greater than 10 cm/sec between sides, or reversal of flow across a collateral may suggest a significant atherosclerotic lesion (Benson et al, 1993).
Duplex ultrasound measurements are informative for diagnosing vasculogenic ED (Rosen et al, 2004c). Cavernous arterial insufficiency is suggested when PSV is less than 25 cm/sec; a PSV consistently greater than 35 cm/sec defines normal cavernous arterial inflow. Cavernous artery acceleration time (i.e., PSV divided by systolic rise time) greater than 122 msec may also indicate this diagnosis. Cavernous veno-occlusive dysfunction, which refers to failure of erection maintenance despite adequate cavernous arterial inflow, is suggested by assorted sonographic parameters. Generally meaningful at 15 to 20 minutes after stimulatory onset, these parameters include persistent high systolic flow velocities (i.e., PSV > 25 cm/sec) and high end-diastolic flow velocities (EDV > 5 cm/sec), accompanied by rapid detumescence, following stimulatory onset. In addition, vascular resistive index (RI), based on the formula: RI equals PSV minus EDV that is then divided by PSV, has had tremendous diagnostic utility in this regard. The parameter is based on the concept that, as penile intracavernous pressure during erection achievement equals or exceeds diastolic pressure, diastolic flow in the corporal bodies will approach zero and the value for RI will approach one. An RI greater than 0.9 has been associated with normal penile vascular function, and that less than 0.75 is consistent with veno-occlusive dysfunction (Naroda et al, 1996).
Several technical modifications of sonographic evaluation of the penis have been described. A portable Midus pulsed Doppler unit connected to a laptop computer for in-office testing reliably records the Doppler waveform of the cavernous arteries despite not providing a real-time ultrasound image (Metro and Broderick, 1999). Power Doppler offers an even more specialized technique to visualize distal ramifications of the main cavernous artery down to the level of arterioles (Sarteschi et al, 1998; Golubinski and Sikorski, 2002). A somewhat more invasive approach that evaluates the integrity of cavernosal arterial flow involves the measurement of the cavernous artery systolic occlusion pressure (CASOP) by a Doppler transducer during saline intracavernous infusion (Rhee et al, 1995). As a variation on the stimulatory component of penile sonographic testing, a combination of an oral PDE5 inhibitor in association with visual erotic stimulation has been shown to be an effective, noninvasive method (Bacar et al, 2001; Speel et al, 2001). Sonographically measured postocclusive vasodilation of the cavernous arteries, which is believed to relate to the level of intact endothelial function in the penis, has been found to be diagnostic for organic ED (Virag et al, 2004). Cavernous artery intima media thickness as demonstrated by high-resolution echo color Doppler ultrasound has been suggested to be more accurate than PSV in predicting vasculogenic ED (Caretta et al, 2009).
Dynamic Infusion Cavernosometry and Cavernosography
Cavernosometry and cavernosography, precisely referring to functional hemodynamic and radiographic assessments of the corpora cavernosa, represents third-line evaluation of the vascular integrity of the penis. The testing is indicated for select patients who are suspected to have a site-specific vasculogenic leak resulting from perineal or pelvic trauma or who have had life-long ED (primary ED). When used, it generally precedes consideration for corrective penile vascular surgery.
The technique involves two needles inserted into the penis for simultaneous saline infusion and intracavernous pressure monitoring following intracavernous pharmacologic injection. The testing requires complete trabecular smooth muscle relaxation to avoid erroneous results, and repeated and maximal pharmacologic dosing protocols are recommended (Hatzichristou et al, 1995). Measurements of maintenance flow rate, pressure drop, and CASOP are done to verify complete smooth muscle relaxation (Fig. 24–6).
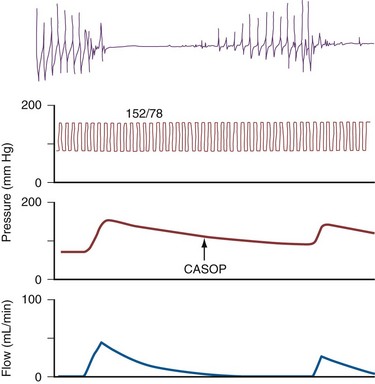
Figure 24–6 This tracing depicts four simultaneous variables obtained during the third phase of dynamic infusion cavernosometry and cavernosography. Top to bottom, Cavernosal artery flow recorded by using a continuous-wave Doppler ultrasound probe; systemic brachial systolic and diastolic arterial blood pressure (150/87 mm Hg); intracavernosal pressure, which varied from 70 to 160 mm Hg in this tracing; and intracavernosal heparinized saline inflow. The intracavernosal pressure at which the cavernosal artery pulsations returned, the effective cavernosal artery systolic occlusion pressure (CASOP), was 108 mm Hg. The gradient between the brachial and the cavernosal artery systolic occlusion pressures was 150 to 108, or 42 mm Hg, which is abnormal.
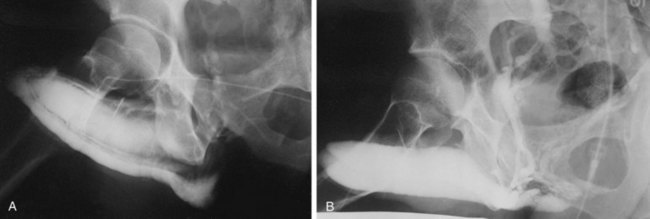
Dynamic infusion cavernosometry and cavernosography evaluate the penile venous outflow system. The existence of veno-occlusive dysfunction is indicated by the failure to increase intracavernous pressure to the level of the mean systolic blood pressure with saline infusion or the demonstration of a rapid drop of intracavernous pressure after cessation of saline infusion (Puyau and Lewis, 1983; Rudnick et al, 1991; Shabsigh et al, 1991; Motiwala, 1993). The flow rate required to maintain erection at an intracavernous pressure of more than 100 mm Hg is normally less than 3 to 5 mL/min, and the pressure decrease in 30 seconds from 150 mm Hg is normally less than 45 mm Hg. Cavernosography follows cavernosometric evaluation and is intended to reveal the site of venous leakage (Fig. 24–7). With normal veno-occlusive function, there should be opacification of the corpora cavernosa with minimal or no visualization of venous structures or corpus spongiosum. With impaired veno-occlusive function, leakage may be identified into such sites as the glans, corpus spongiosum, superficial dorsal veins, and cavernous and crural veins. More than one site is visualized in the majority of patients (Lue et al, 1986; Rajfer et al, 1988; Shabsigh et al, 1991).
Penile Angiography
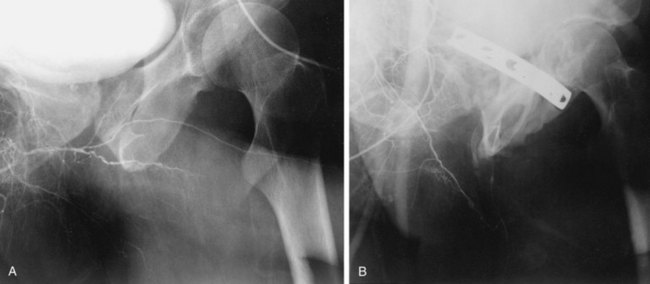
Penile angiography essentially refers to an anatomic study of the arterial vasculature of the penis and also represents third-line evaluation of the penile vascular system. It is commonly reserved for the young patient with ED secondary to a traumatic arterial disruption or the patient with a history of penile compression injury, who is being considered for penile revascularization surgery.
The procedure involves selective cannulation of the internal pudendal artery and injection of radiographic contrast. The intracavernous injection of a vasodilating agent is optimally used to induce maximal vasodilation of the penile arterial supply. The anatomy and radiographic appearance of the iliac, internal pudendal, and penile arteries are then evaluated and documented (Fig. 24–8). The inferior epigastric arteries are frequently studied as well to determine their suitability for use in surgical revascularization. It should be recognized that significant variation of the intrapenile arterial anatomy exists, challenging the angiographer to differentiate congenital variations from acquired abnormalities and establish their clinicopathologic relevance (Bähren et al, 1988; Benson et al, 1993).
Historical and Investigational Studies of Penile Blood Flow
Penile Brachial Pressure Index
This test refers to the penile systolic blood pressure divided by the brachial systolic blood pressure. The technique involves applying a small pediatric blood pressure cuff to the base of the flaccid penis and measuring the systolic blood pressure with a continuous-wave Doppler probe. A PBI of 0.7 or less has been used to indicate arteriogenic ED (Metz and Bengtsson, 1981). The technique has not been found to be valid, basically because it does not assess the hemodynamic properties of a functionally relevant, induced erection, and thus it is not recommended for use (Aitchison et al, 1990; Mueller et al, 1990).
Penile Plethysmography (Penile Pulse Volume Recording)
This test evaluates arterial pressure waveforms in the penis with an aggregate of the contributions of all penile vessels (Kedia, 1983). It requires the application of a 2.5- or 3-cm cuff connected to an air plethysmograph applied to the base of the penis, inflating the cuff to a pressure above brachial systolic pressure, and then decreasing the pressure by 10–mm Hg increments while recording pressure waveform tracings. Abnormal pressure waveforms by diagnostic criteria have been used to indicate vasculogenic ED (Doyle and Yu, 1986). Because this study is done in the flaccid penis like the PBI, its clinical relevance has been questioned. Despite this concern, a technical modification that measures postischemic flow-mediated dilation was introduced as being informative regarding penile vascular endothelial function (Dayan et al, 2005; Vardi et al, 2009).
Radioisotopic Penography
This test quantifies changes in penile blood volume after intracavernous injection of a vasoactive agent using 99mTc-labeled red blood cells (Shirai et al, 1976). Extremely low flow is understood to mean arteriogenic ED (Smith et al, 1998). An evaluation comparing color Duplex ultrasound and radionuclide penography showed poor correlation (Glass et al, 1996).
Penile Magnetic Resonance Imaging
This test has significant potential applications for the assessment of anatomic details of the penis and penile microcirculation. Angiographic techniques may be combined with it to evaluate the anatomic condition of the internal iliac and penile vasculature. Magnetic resonance angiography has been shown to have good correlation with color duplex ultrasound testing (Stehling et al, 1997; John et al, 1999).
Penile Near-Infrared Spectrophotometry
This test provides continuous, quantitative measurements of penile blood flow using a specialized near-infrared spectrophotometry instrument (Burnett et al, 2000). It may be applied with an erectile stimulus and documents the hemodynamic phenomena of erection. Penile spectrophotometry has been further investigated in combination with intraurethral pharmacotherapy documenting blood flow increase to the penis with this erectogenic modality (Padmanabhan and McCullough, 2007). Further investigation of this technique is necessary to establish its clinical utility.
Cavernous Smooth Muscle Content
This test evaluates the smooth muscle composition of the corporeal tissue by light microscopic and computed morphometric assessment of biopsies of the penis and may serve adjunctively in the diagnosis of vasculogenic ED (Wespes et al, 1992). A reduced proportion of corporeal smooth muscle (and correspondingly increased collagen) has been observed in older men with veno-occlusive dysfunction (19% to 36% smooth muscle) and arteriogenic ED (10% to 25%), compared with that of young, healthy men with normal erections and penile curvature (40% to 52%) (Wespes et al, 1991). In part because of its invasiveness, the test is controversial and thus it remains investigational at present.
Psychophysiologic Evaluation
The psychophysiologic evaluation of ED seeks to evaluate the erectile response applying techniques that directly measure penile tumescence and rigidity. From the historical perspective of ED diagnostics, testing was applied primarily to differentiate psychogenic from organic ED. In general, the documentation of a full erection indicates functional integrity of the neurovascular axis regulating penile erection and thereby raises suspicion of a psychogenic etiology. There are several approaches to perform this evaluation. Importantly, the psychophysiologic evaluation does not currently represent first-line evaluation for ED, largely because of technical and cost limitations associated with current techniques. When considered to undergo any of these tests as part of a diagnostic plan, patients are counseled with regard to their expected utility and risks and benefits.
Penile Tumescence and Rigidity Monitoring
Nocturnal penile tumescence (NPT) monitoring, which describes the study of erections that occur with nighttime sleep, was classically described as a technique offering assessment of physiologic erectile ability (Wasserman et al, 1980). Standardly, sleep laboratory nocturnal penile tumescence and rigidity (NPTR) testing applies nocturnal monitoring devices that measure the number of episodes, tumescence (circumference change by strain gauges), maximal penile rigidity, and duration of nocturnal erections (Kessler, 1988). The conventional approach is to perform monitoring in conjunction with electroencephalography, electro-oculography, and electromyography (EMG), with nasal airflow and with oxygen saturation to document rapid eye movement (REM) sleep and the presence or absence of hypoxia (sleep apnea). Importantly, documentation of REM sleep is done because of the observation that true erectile phenomena occurring during sleep are associated with the REM sleep phase (Fisher et al, 1965). Sleep movement patterns are also monitored because periodic limb movement disorders are associated with abnormal NPT. Axial rigidity is measured along with photography of the erect penis on awakening the patient at maximal tumescence; a buckling device is applied to the tip of the penis to measure resistance (500 g minimum for vaginal penetration, 1.5 kg suggestive of complete rigidity) (Karacan et al, 1977). NPT has traditionally been performed over two to three nights to overcome the so-called first-night effect when REM sleep is inconsistent. Formal testing, which involves a specially equipped sleep laboratory staffed with trained observers, is costly. The monitoring of diurnal penile tumescence, in reference to monitoring performed during daytime napping, has served alternatively as an in-office evaluation (Morales et al, 1994).
Rigiscan (Timm Medical Technologies, Inc. Minneapolis, MN) is an automated, portable device used for NPTR, which combines the monitoring of radial rigidity, tumescence, number, and duration of erectile events (Bradley et al, 1985). The device employs two loops, one placed at the base of the penis and the other placed at the coronal sulcus (respectively, base and tip recording sites), which record penile tumescence (circumference) and radial rigidity with timed, standardized constrictions of the loops. A baseline initialization is done with the patient in the office, and then it is calibrated for home use. At home, registrations of penile rigidity are done every 3 minutes and increased to every 30 seconds when the base loop detects a circumference increase of greater than 10 mm (Fig. 24–9). Recommended criteria for normal NPTR include four to five erectile episodes per night, mean duration longer than 30 minutes, an increase in circumference of more than 3 cm at the base and more than 2 cm at the tip, and maximal rigidity above 70% at both base and tip (Cilurzo et al, 1992). A computerized program has yielded standardized data measurements according to cumulative distribution of time-intensity measures, defined as tumescence activity units (TAU) and radial rigidity activity units (RAU) (Burris et al, 1989; Levine and Carroll, 1994). Potential limitations of Rigiscan include that radial rigidity does not accurately predict axial rigidity (Allen et al, 1993; Licht et al, 1995) and considerable variability apparently exists even in normal subjects (Levine and Carroll, 1994). Further, the manner of testing does not allow verification of the presence of REM sleep.
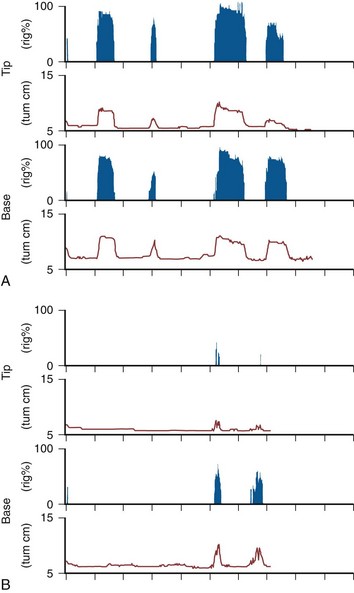
Figure 24–9 The RigiScan device has been designed to measure penile rigidity during home nocturnal monitoring. A, A study in a patient with at least two episodes of well-sustained, completely rigid nocturnal erections. B, A study with two episodes of poorly sustained, poorly rigid nocturnal erections. Such home studies fail to document sleep quality.
NPT electrobioimpedance (NEVA, American Medical Systems, Inc., Minnetonka, MN) is a more recently introduced device that assesses volumetric changes in the penis during nocturnal erections (Knoll and Abrams, 1999). The device consists of three small electrode pads applied to the hip and the penile base and glans and a small recording device attached to the patient’s thigh. In operation, an undetectable alternating current is transmitted from the glans electrode to the hip ground, and the penile base electrode measures impedance and changes in penile length. Impedance measures decrease in concert with increases in cross-sectional area of the penis during nocturnal tumescence. Further investigation is necessary to establish the relationship of volumetric changes and rigidity of the penis. Similar to Rigiscan, the technique does not include REM sleep monitoring and correlations.
In summary, NPTR monitoring is an attractive approach for objectively evaluating the somatic basis of erectile ability, theoretically devoid of psychologic interference. However, it has several apparent shortcomings, which limit its routine use for diagnostic purposes (Jannini et al, 2009). Central issues are that the testing does not indicate the cause and severity of ED and that it may be poorly reproducible. A fundamental issue, too, is whether the testing appropriately evaluates wakeful, sexually relevant erections. Indeed, erections observed during NPTR monitoring do not unequivocally equate with erections sufficient for sexual performance, and false-positive results are possible for various clinical situations (e.g., multiple sclerosis). False-negative results may occur in aging patients and in patients with depression or anxiety, which may conditionally affect the physiology of sleep-related erectile phenomena. Nonetheless, NPTR monitoring may be considered in special circumstances such as when the cause of ED is obscure and noninvasive testing is desirable.
Audiovisual and Vibratory Stimulation
Alternative erectogenic methods can be used in conjunction with diagnostic testing of erectile function. Erotic stimulation by explicit videotape material with monitoring has been used as a reliable and time- and cost-effective alternative to NPTR for differentiating between organic and psychogenic ED presentations (Sakheim et al, 1987; Bancroft et al, 1991). It is also considered more physiologic, consistent with erectile behavior when awake. The testing has potential limitations, with possible false-negative responses occurring in the presence of endocrine abnormalities (Carani et al, 1992; Greenstein et al, 1995) and false-positive responses occurring in psychologic situations such as erotic excitement inhibition (Chung and Choi, 1990). As one may infer, these methods can be applied in conjunction with other stimulatory conditions (e.g., pharmacologic erection testing), as well as erectile function assessment approaches (e.g., Rigiscan monitoring) (Katlowitz et al, 1993; Martins and Reis, 1997).
Neuroimaging
Diagnostic techniques to evaluate central mechanisms of male sexual arousal have contributed to the psychophysiologic investigation of ED. Positron emission tomography (Miyagawa et al, 2007) and functional magnetic resonance imaging (Park et al, 2001; Montorsi et al, 2003; Mouras et al, 2003; Ferretti et al, 2005) have been used in association with video sexual stimulation or an erectogenic pharmacologic stimulus (e.g., oral apomorphine). Studies have documented key brain regions associated with sexual arousal that induce penile erection (i.e., anterior cingulate, insula, amygdala, hypothalamus, and secondary somatosensory cortices). Interestingly, functional abnormalities in the brain have been shown in patients with psychogenic ED, suggesting that this diagnosis may be attributable to an actual biologic basis. More investigation in this area is necessary before determining its clinical role.
Psychologic Evaluation
The psychologic evaluation of ED addresses psychogenic contributions to clinical presentations, essentially psychologic and interpersonal factors interfering with erectile function. These aspects should not be underestimated, and it is well documented in population studies that ED is associated with anxiety, depression, low degrees of self-esteem, negative outlook on life, self-reported emotional stress, and a history of sexual coercion (Feldman et al, 1994; Laumann et al, 1999). The urologist’s role in initiating a psychologic evaluation is not necessarily complicated, and a basic attempt employing queries about a patient’s psychologic health is useful in assessing sexual health (Rowland et al, 2005).
The diagnostic interview is central to the psychologic evaluation, and this process should be done straightforwardly. Immediately discernible causes of sexual dysfunction may be elicited such as fear of failure; performance anxiety (for widowers, this may include complex interactions of dating, new partners, and unresolved mourning/guilt); insufficient sexual stimulation; loss of attraction for the partner; adjustment to a chronic illness or surgery; and relationship conflicts. Less immediately discernible causes may be identified as well to include unresolved parental attachments, sexual identity issues, history of sexual trauma, occurrence of extramarital affairs, and cultural-religious taboos (Leach and Bethune, 1996; Laumann et al, 1999).
The interviewer should be mindful of the possibility of a primary psychogenic ED presentation (Turnbull and Weinberg, 1983). In the absence of organic risk factors, a primary psychogenic ED causation may be suspected. Further support for the diagnosis may follow the confirmation of noncoital erections (i.e., masturbatory, nocturnal or on awakening). Clinical subtypes of psychogenic ED may be further identified: (1) generalized versus situational and (2) lifelong (primary) versus acquired (secondary including substance abuse or major psychiatric illness).
The interviewer should also inquire about relationship factors (Rosen, 2001). Relationship conflicts may be the source of psychogenic ED or otherwise may exacerbate organic ED. Couple’s issues include intimacy and trust, status and dominance, loss of sexual attraction, ability to achieve sexual satisfaction without erection, and communication problems. Important information may derive not just from interviewing the patient alone, and interviews both with the couple together and of partners separately may prove insightful.
Complex intrapsychic causes of sexual dysfunction are often relevant for the ED presentation and may become evident during the diagnostic interview. The clinical history may reveal a significant traumatic life experience, cultural or religious strife, compulsive sexual behavior, or neurotic process. It may suggest the presence of serious psychiatric comorbidities such as substance abuse, depressive symptoms, anxiety disorder, or personality disorder. It is recognized that the urologist may not have the professional background, comfort, or time to address these issues definitively, and a referral to a psychologic expert for further attention would certainly be appropriate.
Neurologic Evaluation
The neurologic evaluation of ED is concerned with neurogenic associations with ED presentations. The importance of testing for deficits in the neurologic system relates to the principal regulatory role of this system for governing erectile function. Target sites for evaluation include peripheral, spinal, and supraspinal centers, as well as both somatic and autonomic pathways involved in this biologic response. In line with this purpose, several diagnostic tests have been introduced. However, thus far they have had limited impact on routine clinical management decisions, and much of the available testing in this realm is reserved for research protocols and medicolegal investigations. Additionally, fundamental problems surround the lack of sensitivity, reproducibility, reliability, and validity for many of these tests. This concern is particularly so for autonomic function tests, distinct from somatic function testing, which has been shown to be reproducible and valid. Otherwise, tests that could be most useful for evaluating penile erection (e.g., neurotransmitter release) await development altogether.
Somatic Nervous System
Biothesiometry
This test represents a technique to assess afferent sensory function of the penis (Padma-Nathan, 1988). Testing involves a hand-held electromagnetic device placed on the pulp of the index fingers, both sides of the penile shaft, and the glans penis. Measurements of sensory perception threshold are obtained in response to various amplitudes of vibratory stimulation. Investigators have questioned the utility of penile glans biothesiometry, which does not accurately portray neurophysiologic function of the dorsal penile nerve because of limitations in recording responses to vibratory stimuli of glanular skin (Bemelmans et al, 1995).
Sacral Evoked Response—Bulbocavernosus Reflex Latency
This test is used to assess the somatosensory reflexogenic mechanism of penile erection. Testing consists of a direct-current stimulator, which delivers square-wave impulses via two stimulating ring electrodes placed around the penis, one secured near the corona and the other secured 3 cm more proximally, and a recorder, which gauges responses via concentric needle electrodes placed in the right and left bulbocavernous muscles. Latency period is measured as the interval from the beginning of each stimulus to the beginning of each response. An abnormal latency time, defined as a value more than three standard deviations above the mean (30 to 40 msec), indicates a high probability of neuropathology (Padma-Nathan, 1988). However, the utility of this test has been questioned, and it has been shown that a full battery of electrophysiologic tests evaluating limb nerve function is more sensitive in diagnosing neuropathy than such tests specific to pudendal nerve function alone (Vodusek et al, 1993; Ho et al, 1996).
Dorsal Nerve Conduction Velocity
This test in concept extends from pudendal nerve function reflex testing and involves electrophysiologic stimulation with two stimulating electrodes placed at the glans and the base of the penis for obtaining two bulbocavernosus reflex latency measurements. Conduction velocity of the dorsal nerve is represented by dividing the distance between the two stimulating electrodes by the difference in latency times recorded from both sites. An average conduction velocity of 23.5 m/sec with a range of 21.4 to 29.1 m/sec is found in normal subjects (Gerstenberg and Bradley, 1983). Abnormal nerve conduction velocities were found to be diagnostic for neurogenic ED in patients with diabetes (Kaneko and Bradley, 1987).
Genitocerebral Evoked Potential
This test is designed to assess afferent sensory mechanisms and stimulus processing at spinal and supraspinal nervous system levels. The testing requires complex electronic equipment for recording the evoked potential waveforms overlying the sacral spinal cord and cerebral cortex in response to dorsal penile nerve electrical stimulation (Spudis et al, 1989). Central conduction time is recorded as the difference between the latency times after stimulation of the first replicated spinal response and the first replicated cerebral response (Padma-Nathan, 1988). The test has been questioned as having poor discriminatory value of response latencies (Pickard et al, 1994). However, it may still serve as an objective tool to define characteristics of afferent penile sensory dysfunction in patients with subtle abnormalities on neurologic examination.
Autonomic Nervous System
Heart Rate Variability and Sympathetic Skin Response
The test of heart rate control (mainly parasympathetic) consists of measuring heart rate variations during quiet breathing, deep breathing, and in response to raising the feet. Normative parameters have been documented. The test of sympathetic skin response involves producing an electrical shock stimulus at a certain location (e.g., median or tibial nerve) and recording the evoked potential elsewhere (e.g., contralateral hand or foot or penis). Recording from the penis is considered to be a potentially useful method of testing penile autonomic innervation (Daffertshofer et al, 1994).
Penile Thermal Sensory Testing
This test serves to assess the conductance of small sensory nerve fibers, which are affected by autonomic disturbances consistent with neuropathy. The testing measures thermal threshold. In studies of the penis, it seems to correlate very well with the clinical determination of neurogenic ED (Lefaucheur et al, 2001; Bleustein et al, 2003).
Corpus Cavernosum Electromyography (CC-EMG) and Single Potential Analysis of Cavernous Electrical Activity
This test offers a direct recording of cavernous electrical activity, which varies between penile flaccidity and tumescence (Wagner et al, 1989). In the normally flaccid penis, electrical activity is described to have a rhythmic slow wave with intermittent bursts of activity. As penile tumescence occurs (such as in response to visual sexual stimulation or after intracavernous injection of a smooth muscle relaxant), this activity ceases. During detumescence, the baseline electrical activity returns. Patients with suspected autonomic neuropathy were demonstrated to display a discordant pattern, having continued electrical activity during erectogenic stimuli (Wagner et al, 1989). Recording techniques have been standardized, and normative values have been defined to include maximum peak-to-peak amplitudes between 120 and 500 mV and mean potential durations of 12 seconds (Stief et al, 1994). However, its clinical utility remains in question (Kellner et al, 2000; Jiang et al, 2003).
Hormonal Evaluation
The hormonal evaluation for ED explores an endocrinologic basis for the sexual dysfunction and recognizes accumulating evidence that endocrinopathies potentially affect the physiology of penile erection (Traish and Guay, 2006; Mirone et al, 2009). Several endocrine conditions are particularly relevant in this regard: hypogonadism (decrease or absence of hormonal secretion from the gonads), hyperthyroidism (excessive thyroid hormone release), and diabetes (altered modulation of androgen function). The diagnostic evaluation may be undertaken in view of their possible influences on erectile function. The clinical history may raise suspicion of the diagnosis, although the clinical presentation of an endocrinopathy may be quite variable. Several questionnaires have been proposed for use in screening, particularly with respect to hypogonadism (Morley et al, 2000; Daig et al, 2003; Heinemann, 2005). However, their general lack of specificity for most presentations and lack of sensitivity for some others has limited their widespread applications (Morales et al, 2007). The central feature of this evaluation involves biochemical testing for serum hormonal levels.
Serum Testosterone Measurements
Much focus in assessing the impact of endocrinopathies on male sexual function has centered on the role of androgens. Androgen deficiency or low testosterone levels are observed in as low as 2% and as high as one-third of men presenting clinically with ED (Korenman et al, 1990; Citron et al, 1996; Soran and Wu, 2005). Differences in patient populations under study likely account for the variation in statistics. In acknowledgment that aging may represent the primary cause of declining androgens, thought leaders have variously applied such terms as androgen deficiency of the aging male (ADAM), partial androgen deficiency of the aging male (PADAM), hypoandrogenism, symptomatic late-onset hypogonadism (SLOH), and andropause to designate this association.
It is important to understand the biology of testosterone production and function in order to proceed with its laboratory evaluation. Testosterone circulates in three fractions: free (0.5% to 3%), tightly bound to sex hormone binding globulin (SHBG) (≈30%), and loosely bound to albumin and other serum proteins (≈67%) (Basaria and Dobs, 2001; Freeman et al, 2001). Free testosterone and albumin-bound portions comprise the bioavailable testosterone fraction. The relative concentrations of the carrier proteins (SHBG and albumin) serve to modulate androgen function. Numerous conditions can alter the SHBG fraction and accordingly affect bioavailable testosterone to some extent even if the total testosterone measurement is unchanged (Bhasin et al, 2006). Decreased SHBG is associated with moderate obesity, nephrotic syndrome, and hypothyroidism, as well as use of glucocorticoids, progestins, and androgenic steroids, and it produces an elevation in bioavailable testosterone. Increased SHBG is associated with aging, hepatic cirrhosis, hyperthyroidism, human immunodeficiency virus infection, and use of anticonvulsants and estrogens, and it produces a lowering in bioavailable testosterone. Despite the observation that lower levels of SHBG are associated with insulin resistance (Stellato et al, 2000), variable SHBG levels have been documented in diabetic men, possibly because of confounding obesity and aging factors, and the diagnosis of hypogonadism in this population should rely on the measurement of a low bioavailable testosterone level (see later) (Kapoor et al, 2007).
Theoretically, the unbound or free fraction measurement of testosterone offers the most relevant determination of testosterone bioavailability. However, commercial assays for free testosterone are known to be inconsistent and have been considered to be invalid by some investigators (Vermeulen et al, 1999). The best indicator of androgen status is the calculated bioavailable testosterone (free testosterone and albumin-bound testosterone). A formula for this calculation is found on the website of the International Society for the Study for the Aging Male at http://www.issam.ch/freetesto.htm and requires entries for the values of total testosterone and SHBG. In men with serious liver disease or hypoalbuminemia, entry of the serum albumin value may be useful for obtaining the best calculation.
For screening purposes, measurement of total serum testosterone level is generally sufficient. It is recommended that the blood draw be done between 7 and 11 AM, when there is a peak serum testosterone level, accounting for the fact that diurnal variation occurs in younger and middle-aged men (Wang et al, 2009). The typical reference range for the total testosterone measurement is 280 to 1000 ng/dL. It is recognized that because of individual variability the normal range for testosterone beyond which replacement therapy should be initiated remains unresolved. If the testosterone level is below or at the low limit of normal, it should be repeated for confirmation. On the other hand, a mildly abnormal testosterone may be found to be normal in 30% of patients on repeat testing (Bhasin et al, 2006). The clinical scenario, such as the presence of conditions that alter testosterone carrier proteins, may prompt further testing and assessment decisions.
Serum Gonadotropin Measurements
When proceeding with a second total testosterone determination, assessment of leutinizing hormone (LH) and prolactin should also be included. Measurement of serum gonadotropins will help to localize the source of the hypogonadism. It is understood that testosterone release involves the integrative activity of the hypothalamic-pituitary-gonadal axis and its regulatory feedback mechanisms, and disruption at any level of this axis may account for hypogonadism (Bhasin et al, 2006). Low testosterone results in decreased negative feedback to the hypothalamus and pituitary, causing increased secretion of LH and follicle-stimulating hormone (FSH). Elevated serum LH and FSH releases are appropriate pituitary responses to low serum testosterone levels, which is consistent with testicular failure (primary hypogonadism). In contrast, normal or low serum LH and FSH releases in the setting of low serum testosterone levels indicate an inappropriate response and suggest a central disorder (secondary hypogonadism).
Serum Prolactin Measurement
Hyperprolactinemia causes hypogonadism by suppression of gonadotropin-releasing hormone from the hypothalamus, which impairs pulsatile LH secretion required for serum testosterone production by the gonads (Morales et al, 2004). An additional possible mechanism for sexual dysfunction, specifically loss of sexual libido, in patients with hyperprolactinemia independent of the circulating level of testosterone relates to an interference of the peripheral conversion of testosterone to dihydrotestosterone (Lobo and Kletzky, 1983). Suspicion of hyperprolactinemia is raised in the patient with low serum testosterone and low or inappropriately normal LH. However, controversy surrounds the consideration of routine determinations of prolactin in men with ED, with some indicating the low yield in doing so (Johnson and Jarow, 1992; Govier et al, 1996) and others finding that low serum testosterone or low sexual desire does not always coincide with the diagnosis (Buvat and Lemaire, 1997; Johri et al, 2001). Causes of the condition include various medications such as antipsychotic agents, tricyclic depressants and opiates, prolactin-secreting tumors, hypothyroidism, hypothalamic lesions, renal insufficiency, cirrhosis, and chest wall lesions (Zeitlin and Rajfer, 2000; Molitch, 2005).
Magnetic Resonance Imaging Scans
Cases of central (hypogonadotropic) hypogonadism and unexplained hyperprolactinemia prompt central imaging of the pituitary. This evaluation commonly involves magnetic resonance imaging, which can identify structural abnormalities (Citron et al, 1996; Petak et al, 2002; Rhoden et al, 2003). Generally accepted guidelines provide indications for pituitary imaging: cases of severe central hypogonadism (testosterone <150ng/dL) and suspicion of pituitary disease (i.e., panhypopituitarism, persistent hyperprolactinemia, or symptoms of tumor mass effect).
Serum Thyroid Function Tests
Hyperthyroidism is associated with ED, possibly by increasing aromatization of testosterone into estrogen (which raises levels of SHBG) (Morales et al, 2004) or by increasing adrenergic tone (which causes smooth muscle contractile effects or exerts psycho-behavioral effects) (Carani et al, 2005). Symptoms of hyperthyroidism such as hyperactivity, irritability, heat intolerance, palpitations, fatigue, and weight loss are often reported, and physical signs such as tachycardia, tremor, goiter, and eyelid retraction are often identified. The diagnosis is made biochemically by measurement of high levels of thyroid hormone (total or free T4 or T3) with a low serum thyroid-stimulating hormone level.
Key Points
Diagnostic Evaluation
Treatment Considerations
The treatment of ED axiomatically follows an appropriate diagnostic workup. Although current interventions are both etiologically specific and nonspecific, an intervention that is specific for the cause of ED ideally offers the opportunity to treat ED with a corrective purpose in mind. Current recommendations adhere to a patient goal-directed focus to therapy and specify that therapeutic options are presented according to a step-care clinical management approach (Fig. 24–10) (Montague et al, 2005; Hatzichristou et al, 2010).
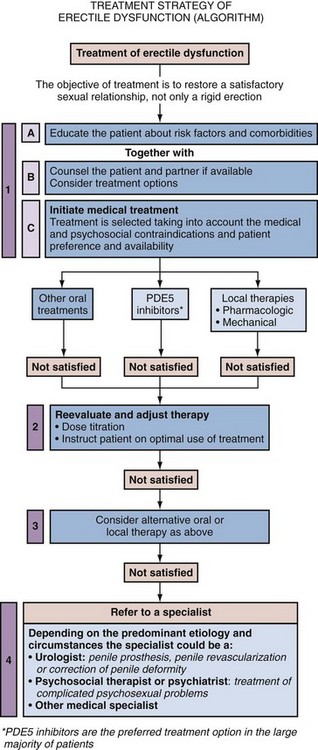
Figure 24–10 Treatment algorithm for erectile dysfunction recommended by the International Consultations on Sexual Medicine.
Lifestyle Modification
The risk of developing ED is highly associated with the presence of comorbid health conditions such as diabetes, cardiovascular disease, and metabolic syndrome that are either preventable or to a minimal extent treatable in the endeavor to optimize health status (Kostis et al, 2005). It stands to reason that optimization of these diseases offers opportunities to prevent the development of ED or ameliorate its extent.
Epidemiologic studies have examined potentially modifiable risk factors and in some instances provided support that risk modification may indeed improve erectile function. For instance, several reports have suggested that discontinuation of cigarette smoking results in a recovery of functional erection status (Mannino et al, 1994; Feldman et al, 2000; Bacon et al, 2006). A beneficial role of increasing exercise for those with sedentary lifestyle in men with ED was also shown (Feldman et al, 1994; Derby et al, 2000). In a recent prospective study, obese men with moderate ED and no overt symptoms of cardiovascular disease showed significant improvements in IIEF scores after exercise and weight control compared with a control group, which followed an educational program alone (Esposito et al, 2004). Significant changes in body mass index, C-reactive protein and physical activity scores were observed in the intervention group compared with the control group. Mediterranean-style diets and a reduction in caloric intake have been found to improve erectile function in men with metabolic syndrome (Esposito et al, 2006). A change to a no-nose saddle from a conventional saddle was shown to recover erectile function, presumably by alleviating perineal trauma, in a short-term interventional study of men with ED associated with occupational bicycle riding (Schrader et al, 2008).
Reports indicating that ED is potentially ameliorated by lifestyle modifications of risk factors that predispose this sexual dysfunction are most illuminative. More definitive clinical and basic science evidence is certainly necessary to evaluate biologic mechanisms associated with these risk factors and affirm the benefits of risk modification. However, current knowledge about these risks still prompts recommendations for a healthy and fit lifestyle for maximal erectile function preservation.
Medication Change
It is quite possible that a certain medication is an offending factor resulting in the clinical presentation of ED. After this inference is made, an appropriate next step would be to change to a different dose or type of medication entirely considering that this action may reverse ED in some patients (Ralph and McNicholas, 2000). For instance, switching antihypertensive therapies from thiazide diuretics and β-blockers to calcium channel blockers and renin-angiotensin system inhibitors (i.e., angiotensin-converting enzyme inhibitors and angiotensin receptor blockers) may recover erectile function in men developing ED in this clinical setting. Similarly, in patients suffering adverse sexual dysfunction effects from use of selective serotonin reuptake inhibitors (SSRI) (e.g., ED, retarded ejaculation), such treatment strategies as drug substitution (e.g., bupropion, nefazodone, buspirone, mirtazapine), drug holidays, SSRI dosage reduction, watchful waiting, and administration of PDE5 inhibitors have enabled sexual function recovery (Rosen et al, 1999; Nurnberg et al, 2001).
Psychosexual Therapy
Because of the frequent interplay of psychologic and interpersonal factors in clinical presentations of ED, it is hardly surprising to consider that psychosexual therapy should be included in the therapeutic armamentarium for this sexual dysfunction. A strong limitation of this area is that evidence-based investigation and well-controlled, large-scale outcome research documenting the efficacy of interventions are generally lacking. In practice, psychosexual therapy does represent an ill-defined combination of interventions and interpretations on the basis of behavioral, relational, psychoanalytic, and cognitive psychology concepts. A variety of interventions are used: systematic anxiety reduction/desensitization, sensate focus, interpersonal therapy, cognitive-behavior therapy, sex education, couples’ communication and sexual skills training, and masturbation exercises (Althof et al, 2005). Integrated treatments, which combine psychosexual interventions with medical therapies such as oral therapy, intracavernosal injection, or vacuum device therapy have been successful, too, in managing ED presentations, particularly those associated with motivational obstacles (Hawton, 1998; Althof et al, 2005). For urologists, collaborative efforts with other specialists who have expertise in psychosexual therapy may be advantageous to provide effective, comprehensive treatment.
Hormonal Therapy
A prescription of hormonal therapy is considered for the patient in whom a hormonal disturbance is identified to affect the clinical presentation of ED. The urologist’s role is fitting for the treatment of primary hypogonadism and hyperprolactinemia, whereas endocrinologists would be considered foremost consultants for other endocrinopathies.
Testosterone Replacement
Androgen replacement straightforwardly addresses the clinical complaint that is associated with hypogonadism. As a general principle of sex steroid replacement therapy, serum hormone levels to be achieved over the 24 hours of the day should ideally achieve normal reference values and resemble the normal diurnal pattern. Evaluating serum testosterone levels both before and during treatment is imperative, although the efficacy of testosterone supplementation is best judged by clinical response rather than a precise testosterone determination. Current recommendations suggest that a short (e.g., 3-month) therapeutic trial is justified, and in the absence of a response testosterone administration should be discontinued (Wang et al, 2009). Potential adverse effects of androgen therapy (i.e., erythrocytosis, sleep apnea, urinary symptoms, prostate cancer progression risk, gynecomastia, acne) should be recognized (Morales et al, 2004; Wald et al, 2006). Monitoring of patients on therapy consists of a baseline assessment that includes digital rectal examination and serum PSA testing along with laboratory evaluation (i.e., hemoglobin/hematocrit levels, liver function tests, cholesterol level, and lipid profile) followed by assessment of treatment efficacy after 1 to 3 months and annually thereafter to assess symptom response and any adverse events (Morales et al, 2004; Bhasin et al, 2006). Short-acting preparations may be preferred over long-acting depot preparations in the initial treatment of patients so that therapy can be discontinued on the occasion of an adverse event (Wang et al, 2009).
For the treatment of hypogonadism, several testosterone preparations are offered and can be delivered by various routes: injectable, subcutaneous, transdermal (patch and gel), buccal, and oral (Edelstein et al, 2006; Morgentaler et al, 2008; Wang et al, 2009). A brief description of available therapies follows (see also Table 24–6).
Injectable
Testosterone enanthate, an injectable depot preparation of testosterone, is delivered by deep intramuscular injection 150 to 300 mg every 2 to 4 weeks). The schedule of therapy results in supraphysiologic levels of testosterone for 72 hours with a steady exponential decline to subphysiologic levels by 10 to 12 days. Alternative dosing of 100 mg every 7 to 10 days can be considered in situations when patients experience symptomatic mood or sexual fluctuations associated with documented early hypogonadal troughs. Another parenteral preparation, testosterone cypionate, is also delivered intramuscularly at a dosage 200 mg every 2 to 3 days because of its shorter half-life, and it may also display serum testosterone fluctuations. Testosterone undecanoate (TU), as a depot formulation consisting of 750 mg or 1000 mg dosages administered at 10-week dosing intervals, has been used in Europe since 2003 but is not yet available in the United States.
Subcutaneous
Pellets offer a subcutaneous, long-acting depot formulation of testosterone. Testopel is a pellet containing 75 mg of testosterone. Dosing usually requires two to six pellets (150 to 450 mg testosterone) implanted subcutaneously every 3 to 6 months.
Transdermal
Transdermal delivery options comprise patches and gels, with a delivery approach that intendedly simulates normal circadian levels of testosterone. When patients apply medication in the morning, higher initial absorption will mimic normal diurnal variation.
Testoderm was approved initially as a daily administered scrotal patch without adhesive (4 to 6 mg), but it came under disuse because of difficulties with its application and the requirement for scrotal shaving, as well as finding that it significantly produced high levels of dihydrotestosterone (DHT) by conversion by 5α-reductase activity that is plentiful on scrotal skin. Testoderm TTS represented an alternative formulation avoiding the inconveniences of the scrotal application. Its application is to the arm, back, or buttock as a 5-mg patch. Androderm, an alternative product, delivers 2.5 or 5 mg testosterone daily. Both patches have been associated with itching, chronic skin irritation, and allergic contact dermatitis. The skin irritation is alleviated by local application of cortisone cream. Patients are advised to alternate application sites and avoid sun-exposed areas.
AndroGel (testosterone gel) 1% gel pack is a topical gel, which contains 50, 75, or 100 mg of testosterone, with only 10% of the drug being absorbed during a 24-hour period. Testim, also providing 1% testosterone, is an alternative product packaged as a 5-g tube containing 50 mg of testosterone. Both are similarly applied once daily in the morning to clean, dry skin over the shoulders, upper arms, or abdomen and allowed to dry before dressing.
Buccal
Striant refers to a tablet-like, mucoadhesive treatment system (30 mg of testosterone) that continuously delivers medication. It is applied twice daily to the gum tissue above the incisors, allowing testosterone to be absorbed through the buccal mucosa.
Oral
Oral testosterone preparations are limited. Concern is associated with the liver toxicity of testosterone (i.e., hepatitis, cholestatic jaundice, hepatomas, hemorrhagic liver cysts, and hepatocellular carcinoma) related to large doses necessary to achieve normal serum levels (Bagatell and Bremner, 1996). Large doses (>200 mg/day) are required orally because much of the administration is rendered metabolically inactive during the “first pass” circulation through the liver. Chemical modifications of oral testosterone have been explored to overcome adverse reactions. Both 17α-methyltestosterone and fluoxymesterone have been formulated, but because of their patient variability of effect with potential liver toxicity risk, they should not be prescribed (Wang et al, 2009). TU, as an oral formulation in oleic acid, is safe by partly escaping hepatic inactivation (Köhn and Schill, 2003). However, it has shown a large individual variability for the time of maximal responses, as well as when maximal serum testosterone is attained. Oral TU remains unapproved in the United States.
Alternative Hormone Treatments
Alternative hormonal replacement therapies have been suggested, and they have posed certain desirable features and caveats. DHT as a direct mode of therapy is attractive because of its action as a pure androgen that is not aromatizable to estradiol, and accordingly it has been demonstrated that the hormone does not exert adverse estrogenic effects on prostate growth or lipid profile measurements (Kunelius et al, 2002; Sakhri and Gooren, 2007). A therapeutic DHT gel is available at a dose of 125 to 250 mg/day, yielding plasma DHT levels comparable with physiologic testosterone levels (Kunelius et al, 2002). Dehydroepiandrosterone (DHEA), a hormone supplement with androgen-like and estrogen-like effects, has been used although limited evidence exists showing it improves sexual function (Baulieu et al, 2000; Morales et al, 2004). Importantly, the treatment cannot be considered harmless, and the potential exists for DHEA and other non-testosterone androgen precursor preparations (e.g., DHEA-S, androstenediol, androstenedione) to stimulate hormone-sensitive diseases such as breast or prostate cancer. Human chorionic gonadotropin (HCG) has been found to increase total and free testosterone and estradiol 50% above baseline and conceivably would benefit hypogonadal men similar to effects of androgen administration. Clinical investigation of HCG has shown some anthropometric effects (i.e., decrease in fat mass, increase in lean body mass) and improvements in serum testosterone concentrations in androgen deficient men without documented benefits for sexual function (Liu et al, 2002; Tsujimura et al, 2005). Anti-estrogens and aromatase inhibitors have been shown to increase endogenous testosterone levels, and selective androgen receptor modulators are under development. Because of insufficient evidence about the therapeutic benefits and adverse effects of alternative replacement therapies in older men with hypogonadism, they are not currently recommended for use (Wang et al, 2009).
Hyperprolactinemia Treatments
The treatment of hyperprolactinemia is undertaken acknowledging that testosterone replacement therapy is neither corrective nor sufficient to improve sexual function. The therapeutic objective, rather, is to identify and address the underlying cause, which may then ameliorate ED. Offending drugs such as estrogens, morphine, sedatives, and neuroleptics should be discontinued (Molitch, 2008). A prolactin-secreting adenoma should be treated medically and, if necessary, surgically. Bromocriptine, a dopamine agonist that lowers prolactin level and restores testosterone production to normal, serves to reduce the size of the tumor. Neurosurgical ablation becomes necessary if the therapeutic response to medication does not occur or visual effects are noted in association with optic nerve compression (Gillam et al, 2006). Erection recovery is most evident after treatment of men with significant serum elevations of prolactin (>40 ng/mL) (Netto Júnior and Claro, 1993).
Pharmacologic Therapies
The premise of pharmacologic therapies is that they simulate the biochemical and molecular mechanisms of action naturally governing the erectile response. Conceptually, erectogenic therapies serve strategically either to promote proerectile mechanisms or oppose antierectile mechanisms, at both peripheral and central levels of the neurovascular axis responsible for penile erection (Rowland and Burnett, 2000). At a peripheral level, these mechanisms influence corporal smooth muscle tone. Promotion of proerectile mechanisms is achieved by either inducing corporal smooth muscle activation through cell-receptor agonists or effectors of tissue relaxant pathways (e.g., stimulating second messenger cyclic nucleotide [cyclic guanosine monophosphate, cGMP or cyclic adenosine monophosphate, cAMP] synthesis) or inhibiting the deactivation of smooth muscle relaxation pathways (e.g., inhibiting phosphodiesterases), whereas opposition of antierectile mechanisms are achieved by decreasing smooth muscle contraction through receptor antagonists of tissue contractile pathways (e.g., α1-adrenergic inhibitors). At a central nervous system level (i.e., brain or spinal cord), neuronal pathways are affected and potential opportunities exist to promote proerectile pathways (e.g., agonists of dopaminergic D2 receptors in the medial hypothalamus) or oppose antierectile pathways (e.g., antagonists of 5-HT1A/2 [serotonergic] receptors in the spinal cord).
Diverse therapies have been touted over time, although their efficacy and safety characteristics have not always been clearly defined. Current standards of regulatory agency approval have helped to clarify the qualifications of commercially developed and marketed therapies (Hirsch et al, 2004).
Oral Therapy
Orally administered medication for ED meets many of the attributes of “ideal therapy,” which include convenience, simplicity, and noninvasiveness (Morales et al, 1995). Oral therapies are increasingly demanded to meet the therapeutic objective of clinical efficacy as well.
Phosphodiesterase Type 5 Inhibitors
This class of medication was famously inaugurated as an effective ED treatment in the United States following U.S. Food and Drug Administration (FDA) approval of sildenafil citrate (Viagra, Pfizer, Inc, New York) in 1998 and vardenafil hydrochloride (Levitra, Bayer Schering Pharma AG, Berlin) and tadalafil (Cialis, Lilly LLC, Indianapolis, IN) in 2003 (Burnett, 2005). PDE5 inhibitors similarly work to block the catalytic action of the enzyme that degrades cGMP, the downstream effector of the erection mediator nitric oxide, which then facilitates the signal transductional mechanisms of corpus cavernosal smooth muscle relaxation required for penile erection. It is important to recognize that the medications augment but do not induce the erectile response and the induction of penile erection requires the release of nitric oxide from penile nerve endings and vascular endothelium under the influence of sexual stimulation. The high concentration of PDE5 in the smooth muscle of the penile corpora cavernosa accounts for the selectivity of their effect.
Despite their similar modes of actions, PDE5 inhibitors differ somewhat in their biochemical properties, pharmacokinetic profiles, and clinical performances (Table 24–7). The chemical structures of sildenafil and vardenafil are similar, unlike that for tadalafil, and this difference does explain some phenomenological differences observed between the agents (Corbin and Francis, 1999). Distinct from the action of tadalafil, sildenafil and vardenafil cross-react slightly with phosphodiesterase (PDE) type 6, which is expressed in the retina, and this difference may explain the complaint of visual disturbances observed with sildenafil and vardenafil use. Tadalafil minimally cross-reacts with PDE type 11, unlike the other two PDE5 inhibitors, although the significance of this effect is unclear. The remaining side effects commonly observed with PDE5 inhibitor treatment are associated with inhibition of PDE5 localized in other target tissues such as vascular and gastrointestinal smooth muscle. Tadalafil possesses a longer half-life of elimination than the other two PDE5 inhibitors. This feature suggests a longer therapeutic window uniquely afforded for tadalafil, which may translate into increased convenience for couples to have sexual intercourse with this agent.
All three PDE5 inhibitors have demonstrated equivalent efficacy and tolerability in clinical trials for the treatment of ED of varying severity and cause (Carson and Lue, 2005; Hellstrom, 2007; Giuliano et al, 2009). Trial designs for the three PDE5 inhibitors have differed, limiting useful comparisons between them, and in the absence of directly comparative studies superiority cannot be claimed for any particular agent (Carson and Lue, 2005). In general, the agents effectively achieve successful sexual intercourse rates of approximately 70% (Carson and Lue, 2005). A somewhat reduced intercourse success rate of 40% to 50% has been reported in diabetic patients with ED (Fonseca et al, 2004; Safarinejad, 2004) and in patients with ED associated with radical prostatectomy in general (Hatzimouratidis et al, 2009). However, the intercourse success rate for patients specifically after bilateral nerve-sparing radical prostatectomy is somewhat better than for the entire group, and reports have commonly documented rates of functional erections with therapy that approach 60% to 70%.
According to standard dosing recommendations, patients are instructed to take the medications on demand approximately an hour before intended sexual activity. This lead-time interval is specified to take advantage of the duration by which the medications achieve peak serum concentrations (i.e., approximately 1 hour for sildenafil and vardenafil and 2 hours for tadalafil). Although the onset of activity has been documented to occur possibly within 20 minutes for each agent, this characteristic is less important to patients than erection hardness and maintenance of erections with therapy (Claes et al, 2008). A daily dosing regimen has been approved for tadalafil as an alternative treatment schedule to afford patients greater convenience in having sexual intercourse while using this agent (Porst et al, 2006; Shabsigh et al, 2010). Optimization of effect for all PDE5 inhibitors is also achieved by properly applying sexual stimulation as a prerequisite for nitric oxide release; reducing food intake, which may delay drug absorption; escalating drug dosing as needed; and repeating attempts with the medications several times (up to 9 or 10 attempts affords maximal probability of success) (McCullough et al, 2002; Barada, 2003; Shindel, 2009). Correcting or improving adverse health conditions (e.g., glycemic control, hyperlipidemic control, androgen replacement), which affect drug efficacy, has also been demonstrated to be potentially beneficial (Guay, 2003; Sadovsky et al, 2009). As evidence of therapeutic efficacy, patient and partner satisfaction with therapy (as shown for sildenafil) has been well demonstrated (Montorsi and Althof, 2004). Suboptimal acceptance or lack of long-term adherence to therapy (up to 47% of patients) has been reported, which may indicate the influence of psychosocial factors or challenges of a treatment that requires repeated dosing (Seftel, 2002; Al-Shaiji and Brock, 2009).
Patients using PDE5 inhibitors should be thoroughly counseled regarding precautions (Table 24–8). Cardiovascular safety using the medication has been well demonstrated, although it should be emphasized that given the cardiovascular risks of sexual activity and potential for adverse drug interactions with this therapy, cardiovascular risk assessment and stabilization should be considered for all men before institution of PDE5 inhibitor therapy. Controlled and postmarketing studies involving these agents have shown that they do not cause an increase in myocardial infarction or death rates when compared with expected rates in study control populations (Jackson et al, 2006; Hellstrom, 2007; Nehra, 2009). In addition, patients with known coronary artery disease or heart failure receiving PDE5 inhibitors did not exhibit worsening ischemia, coronary vasoconstriction, or worsening hemodynamics on exercise testing or cardiac catheterization. Caution is advised for the use of PDE5 inhibitors in patients with certain conditions: aortic stenosis, left ventricular outflow obstruction, hypotension, and hypovolemia. The agents have a minimal effect on QTc interval (Morganroth et al, 2004). Vardenafil among PDE5 inhibitors is not recommended in patients who take type-1A antiarrhythmics (e.g., quinidine or procainamide) or type-3 antiarrhytmics (e.g., sotalol, amiodarone) or in patients with congenital prolonged QT syndrome.
Table 24–8 Warnings and Drug Interactions
Certain drugs such as ketoconazole and itraconazole and protease inhibitors such as ritonavir can impair the metabolic breakdown of PDE5 inhibitors by blocking the CYP3A4 pathway. Such agents may increase blood levels of inhibitors, requiring a PDE5 dose reduction. On the other hand, agents such as rifampin may induce CYP3A4, enhancing the breakdown of inhibitors and requiring higher PDE5 doses. Kidney or hepatic dysfunction may require dose adjustments or warnings.
Nitrate use in any form (e.g., sublingual nitroglycerin, isosorbide dinitrate, other nitrate preparations used to treat angina, amyl nitrite, and amyl nitrate “poppers”) represents an absolute contraindication. Past use of nitrates (more than 2 weeks before the use of PDE5 inhibitors) is not considered a contraindication. If angina occurs during sexual activity having used a PDE5 inhibitor, patients should cease this activity and seek emergency care immediately. They should inform medical personnel that a PDE5 inhibitor was taken and avoid nitroglycerin use for a period of 24 hours for sildenafil and vardenafil and 48 hours for tadalafil (Cheitlin et al, 1999). If acute myocardial infarction occurs with the use of PDE5 inhibitors, usual therapies with the exception of organic nitrates may be administered. If hypotension results from PDE5 inhibitor use, patients should be placed in the Tredelenburg position and given intravenous fluids along with administration of α-adrenergic agonists (e.g., phenylephrine) as needed. Refractory hypotension warrants intra-aortic balloon counterpulsation, as specified by the American College of Cardiology/American Heart Association guidelines. No pharmacologic antidote to the PDE5 inhibitor/nitrate interaction exists. Caution is advised when PDE5 inhibitors are coadministered with α-adrenergic blockers because both agents are vasodilators with blood pressure–lowering effects.
Side effects observed with PDE5 inhibitor therapy include headache (15% to 16%), dyspepsia (4% to 10%), flushing (4% to 10%), myalgia/back pain (0% to 3%), nasal congestion (3% to 4%), and visual disturbances (e.g., photophobia, blue vision) (0% to 3%). Randomized, controlled trials have documented that flushing and visual side effects are more common in patients receiving sildenafil or vardenafil, whereas back pain/myalgia is more common in patients receiving tadalafil. These events have been found to be mild, abate with time, and prompt discontinuation in few patients (Hellstrom, 2007). The concern of the development of nonarteritic anterior optic neuropathy (NAION), which can cause blindness, with PDE5 inhibitor therapy has been posed, although a careful review of pooled data from clinical trials for all three agents did not demonstrate an increased risk of NAION or other adverse ocular events associated with their use (Laties, 2009). Affected patients in postmarketing reports possibly carried risk factors for blindness to include hypertension, diabetes, and hyperlipidemia. At this time, despite the absence of a proven link between PDE5 inhibitor use and serious ocular disorders, physicians should continue to advise patients to stop use of PDE5 inhibitors and seek immediate medical attention as a safety measure in the event of a sudden loss of vision (Laties, 2009).
The interest to extend the utility of PDE5 inhibitors beyond an on-demand erectogenic role and rather apply them to recover or maintain the natural vitality of the penis in the face of an ED-associated disease state or condition has been investigated. This proposal has been considered particularly in the clinical context of radical prostatectomy and introduced as a therapeutic strategy à la “penile rehabilitation,” by which the medications are taken in some regularly scheduled fashion to promote the recovery of spontaneous erectile function. Presently, this role remains unclear. Opposing outcomes were found in two controversial albeit well-designed and conducted (i.e., randomized, controlled) clinical trials of PDE5 inhibitor use after bilateral nerve-sparing radical prostatectomy. In one trial involving sildenafil treatment of 36 weeks starting 4 weeks after the surgery, 27% of patients using the agent recovered erections defined as “good enough for sexual activity” compared with 4% of patients on placebo at about 1 year after surgery (Padma-Nathan et al, 2008). In another trial involving vardenafil treatment of 9 months either on demand or daily starting 14 days after surgery, erection recovery was no different in patients administered vardenafil by either form of administration or placebo at about 1 year after surgery (Montorsi et al, 2008). Randomized, controlled trials in other ED contexts have also failed to show sustained natural erectile function improvement after discontinuing continuous regimens of PDE5 inhibitor therapy (Zumbé et al, 2008; Burnett et al, 2009).
The notion to combine PDE5 inhibitors with other ED therapies such as vasoactive penile pharmacotherapies has been proposed (Lau et al, 2006; McMahon et al, 2006). This strategy is to be considered “off label,” and clinical precautions are advised.
α-Adrenoceptor Antagonists
Phentolamine mesylate is a nonspecific α-adrenergic receptor antagonist with equal affinity for blocking both α1- and α2-adrenoreceptors. Its mode of action presumably is to produce corporal smooth muscle relaxation by blocking the (antierectile) postsynaptic α1-adrenergic receptor (Juenemann et al, 1986). Clinical trials suggested an efficacy rate in men with minimal ED of approximately 40% (Goldstein, 2000). The drug was considered to be relatively safe, with less than 10% of patients using the 40-mg dosage experiencing headaches, facial flushing, or nasal congestion. However, further investigation is required before determining whether it will produce erectile responses of sufficient quality for reliable sexual intercourse, particularly in men with more severe ED.
Yohimbine hydrochloride (Yocon), an indolalkylamine alkaloid derived from the bark of the yohimbe tree, reportedly exerts central effects on the mediation of penile erection operating as an α2-adrenoreceptor antagonist (Clark, 1991; Giuliano and Rampin, 2000). Originally proposed to be an erectogenic and aphrodisiac agent, the drug has been investigated recently as an authentic ED treatment. It is conventionally prescribed orally at a dosage of 5.4 mg three times daily with observation for improvement over at least a month. A meta-analysis of all randomized, placebo-controlled trials involving yohimbine suggested a superior effect for the medication compared with placebo (Ernst and Pittler, 1998). However, the drug does not appear to enable successful sexual intercourse any better than placebo in men with confirmed organic ED (Montague et al, 1996; Teloken et al, 1998). Adverse effects appear to be relatively infrequent but include hypertension, anxiety, tachycardia, and headache. Although yohimbine may be well tolerated, its modest results suggest that it may be best limited to men with psychogenic ED.
Dopaminergic Agonists
Apomorphine (Uprima, TAP Pharmaceutical Products Inc, Lake Forest, IL) is a dopaminergic agent activating D1 and D2 receptors at a central level within the paraventricular nucleus of the brain, indicating its particular relevance in the treatment of men with psychogenic ED (Lal et al, 1987). The medication is taken in sublingual form, having a dosage range of 2, 4, and 6 mg, and it has no erectile efficacy if it is swallowed (Heaton, 2000). It has a rapid onset of action, with a mean time to erection of 12 minutes. Apomorphine achieves a maximal plasma concentration in 50 minutes, although its window of opportunity extends for approximately 2 hours from administration. In clinical trials involving men with ED of varying severities and etiologies, the drug achieved a successful sexual intercourse rate of 50.6% at the 4-mg dosage compared with the 33.8% placebo rate (Heaton, 2000). Side effects include nausea (16.9%), dizziness (8.3%), yawning (7.9%), somnolence (5.8%), sweating (5%), and emesis (3.7%). Syncope occurred in 0.6% of patients using the medication at the highest recommended dosage and was accompanied by a prodrome consisting of nausea, vomiting, sweating, dizziness, and light-headedness but no cardiac sequelae. Side effects were minimized when patients were titrated from higher to lower dosages. The drug achieved regulatory approval for commercialization by European authorities in early 2001, but it has not been so approved in the United States.
Melanocortin-Receptor Agonists
Melanocortin analogues (e.g., melanotan II, PT-141) have been studied showing efficacy in inducing erectile responses in early clinical trials (Wessells et al, 2000; Diamond et al, 2004). These drugs operate centrally at melanocortin-4 receptors, which have been implicated in controlling food intake and energy expenditure, as well as modulating erectile function and sexual behavior. Flushing and nausea have been reported as side effects. The drugs have not achieved regulatory approval for the treatment of ED.
Serotonin-Receptor Effectors
Trazodone (Desyrel) is an antidepressant that has been associated with priapism, prompting its “off-label” investigation as a possible treatment for ED (Lal et al, 1990). It is purported to work through mechanisms at the spinal cord level with multiple serotonergic effects (Allard and Giuliano, 2001). The active metabolite of trazodone acts as an agonist of the proerectile 5-HT2C receptor through reuptake inhibition, with some affinity for the 5-HT2A receptor, although it may also operate as an antagonist of antierectile 5-HT1A receptors (Andersson and Wagner, 1995). Rigorous recent evaluations have not shown clinical efficacy that exceeds placebo responses in eliciting penile erection (Costabile and Spevak, 1999). Given its potential side effects (i.e., drowsiness, nausea, emesis, blood pressure changes, urinary retention, and priapism) and general lack of effect, this medication would appear to have a limited role for ED treatment.
Other Oral Therapies
Additional possibilities for the oral treatment of ED including L-arginine (the amino acid precursor of nitric oxide), L-dopa (dopamine precursor), limaprost (prostaglandin E1), and naltrexone (opioid antagonist) have been proposed (Burnett, 1999). Each of these agents has a plausible mechanism of action to induce erections. However, they remain insufficiently studied and their clinical roles remain unclear.
Intracavernous Injection
The discovery in 1982 that vasoactive agents, delivered by injection into the penis, induced erections is credited with launching the movement toward medical therapies for the treatment of ED (Virag, 1982; Zorgniotti, 1985). Since that time, there has been an explosion of basic scientific and clinical research leading to the development and use of various locally administered vasoactive medications having mechanisms of action that result in corporal smooth muscle relaxation. Although a host of medications have been explored for this purpose, three medications are used regularly in clinical practice: alprostadil, papaverine, and phentolamine (Table 24–9). These have been administered clinically as a single agent (i.e., monotherapy) or in various combinations (e.g., bi-mix, tri-mix). Combination therapy offers a synergistic mechanism of the vasoactive agents to elicit maximal erectile responses, particularly among patients who have failed monotherapy (Zorgniotti and Lefleur, 1985; Bennett et al, 1991; Floth and Schramek, 1991). This alternative may also be used to circumvent side effects of a certain agent (e.g., penile pain associated with alprostadil).
A general rule of thumb is to start with a small dose of medication, especially in patients with nonvasculogenic forms of ED. In-office self-injection training and education are recommended before home injection, and this opportunity may also be used to titrate medication toward a dosage that safely yields an erection of sufficient rigidity for sexual intercourse yet lasts no more than an hour (Benard and Lue, 1990; Fallon, 1995). The therapy is contraindicated for men with psychologic instability, a history or risk for priapism, histories of severe coagulopathy or unstable cardiovascular disease, reduced manual dexterity (although the partner can be trained in the injection technique), and use of monoamine oxidase inhibitors (because of the risk of precipitating a life-threatening hypertensive crisis in the event that an intracavernosal α-adrenergic agonist is used to reverse a priapic episode) (Sharlip, 1998).
Alprostadil
Alprostadil (Prostin VR) is a synthetic form of a naturally occurring fatty acid, prostaglandin E1, which binds with specific receptors on smooth muscle cells and activates intracellular adenylate cyclase to produce cAMP, which in turn induces tissue relaxation through a second messenger system (Palmer et al, 1994). Currently it is the only FDA-approved injectable medication for ED, and it is marketed for this purpose by the trade names Caverject (Pfizer Inc, New York) and Edex (Schwarz Pharma, Milwaukee, WI) (Linet and Ogrinc, 1996; Porst, 1996; Buvat et al, 1998). After intracavernous injection, the medication is locally metabolized by 96% within 60 minutes and does not appreciably enter the peripheral circulation (van Ahlen et al, 1994). At dosages of 10 to 20 µg, alprostadil produces full erections in 70% to 80% of patients with ED (Linet and Neff, 1994). Side effects of treatment are most commonly pain at the injection site or during erection (in 11% of patients), hematoma/ecchymosis (1.5%), prolonged erection/priapism (1% to 5%), and penile fibrotic lesions (2%) (Linet and Ogrinc, 1996). Perceived advantages of alprostadil for intracavernous pharmacotherapy relative to other agents are lower incidences of prolonged erection, systemic side effects, and penile fibrosis. Disadvantages include a higher incidence of painful erection and higher cost, and once reconstituted into liquid from powder it has a shortened half-life if not refrigerated.
Papaverine
Papaverine, an alkaloid isolated from the opium poppy, is a nonspecific PDE inhibitor, which prevents the degradation of cAMP and cGMP so that these cyclic nucleotides accumulate in smooth muscle cells and thereby increasingly promote tissue relaxation (Kukovetz et al, 1975). The compound also blocks voltage-dependent calcium channels along the membrane wall, thus impeding calcium influx to the cell, a process known to trigger smooth muscle contraction (Brading et al, 1983; Sunagane et al, 1985). Papaverine is metabolized in the liver, and the plasma half-life is 1 to 2 hours. Its general efficacy in promoting penile erection after intracavernous administration is less than 55%. The drug is inexpensive and stable at room temperature. However, disadvantages of commonly observed liver enzyme elevations, priapism risk (up to 35%), and penile fibrosis risk (1% to 33%) have led to its abandonment as monotherapy (Lakin et al, 1990; Fallon, 1995; Porst, 1996; Moemen et al, 2004).
α-Adrenoceptor Antagonists
Besides its purported oral role for ED therapy, phentolamine mesylate is more familiarly applied as an intracavernous agent (Regitine). Although its erectogenic effect is mediated by blocking the (antierectile) postsynaptic α1-adrenergic receptor (Sironi et al, 2000), because of its potential inhibition of the prejunctional α2-adrenergic receptor, which interferes with norepinephrine reuptake, the drug’s tissue relaxant effect for penile erection is believed to be antagonized (Juenemann et al, 1986). This dual effect of the drug probably accounts for its limited success when administered intracavernously as a sole agent (Blum et al, 1985). It has a short plasma half-life (30 minutes). Common side effects associated with the drug include systemic hypotension, reflex tachycardia, nasal congestion, and gastrointestinal upset. Moxisylyte (Thymoxamine) is a competitive blocker of α1-adrenoceptors with impressive erectogenic effects (Buvat et al, 1989). Its availability is restricted in the United States.
Vasoactive Intestinal Polypeptide
Vasoactive intestinal polypeptide (VIP), a hormone having 28 amino acids originally isolated from the small intestine, was proposed early on to be the elusive nonadrenergic noncholinergic (NANC) mediator of penile erection because of its potent vasodilatory effects in various tissues (Adaikan et al, 1986). Its mechanism of action in smooth muscle is achieved through specific protein receptor binding and activation of adenylate cyclase, therebypromoting synthesis of cAMP and subsequent tissue relaxation (Andersson and Wagner, 1995). The drug has had disappointing effects when administered alone, although in separate combination with other drugs such as papaverine and phentolamine, erection responses were elicited (Kiely et al, 1989; Dinsmore and Wyllie, 2008). VIP in combination with phentolamine (Invicorp) is currently being sought for regulatory approval in the United States.
Intraurethral Suppositories
The administration of vasoactive drugs via the urethral channel of the penis was introduced with the hope of affording a less invasive procedure than intracavernous needle injections to induce penile erection. This technique relies on the absorption of medication through the mucosal lining into the surrounding corpus spongiosum, with passage via small vascular channels into the main erectile bodies, the corpora cavernosa. The transfer of drug from the urethra to the cavernous tissue varies across men according to anatomic variability. Following an initial trial that demonstrated prostaglandin E2 was effective in inducing full tumescence in 30% of patients and partial tumescence in 40% of patients (Wolfson et al, 1993), a synthetic formulation of prostaglandin E1 was developed and FDA approved in November 1996 as MUSE (Medicated Urethral System for Erection, Vivus, Menlo Park, CA) (Hellstrom et al, 1996; Padma-Nathan et al, 1997). MUSE employs a suppository inserted into the urethral opening that dispenses a semisolid pellet (1 × 3 mm) of alprostadil (125, 250, 500, and 1000 µg dosages) into the distal urethra (3 cm from the external urethral meatus). Several technical procedures optimize the success of the treatment including properly depositing and manually distributing the medication into the penis and the patient’s remaining in the upright position for several minutes after its application. In-office training and monitoring of initial response may afford advantages for optimizing technique and making dosage adjustments before performing treatment at home.
A calculated final responder rate to MUSE is approximately 50%, and among responders approximately 70% of administrations result in sexual intercourse (Hellstrom et al, 1996; Padma-Nathan et al, 1997; Guay et al, 2000). The combined use of an adjustable penile constriction band (ACTIS) was designed and FDA approved to enhance the local retention and effect of the medication (Lewis, 2000). A transurethral bi-mix consisting of alprostadil and α1-adrenergic antagonist prazosin (ALIBRA) was introduced and in a multicenter trial of nearly 400 patients was shown to increase the at-home responder rate for successful sexual intercourse from 47% with alprostadil alone to 70% with ALIBRA (Qureshi, 2001).
The most common side effects of MUSE include local urogenital pain (approximately one third of patients) and minor urethral bleeding (5%) (Padma-Nathan et al, 1997; Guay et al, 2000). Other complications such as hypotension (3%), dizziness (4%), and priapism (0.1%) have been observed as well. MUSE is contraindicated in patients with known hypersensitivity to alprostadil, abnormal penile anatomy, and conditions that increase the risk of priapism. MUSE seems safe for female partners, producing only a 5.8% incidence of vaginal burning or itching, although it should not be used for intercourse with a pregnant woman without a condom.
Transdermal/Topical Pharmacotherapy
The notion to apply vasoactive drugs directly to the surface of the penis is consistent with the general appeal of many transdermal therapies (e.g., gels, creams) in medicine on the basis of their delivery route: convenience, simplicity, and putatively limited systemic adverse effects. Several topical therapies have been explored for ED treatments, although certain obstacles have limited their widespread use. Nitroglycerine, a nitric oxide donor formulated as a 2% paste, was found to produce tumescence but rarely penile rigidity sufficient for sexual intercourse (Owen et al, 1989). This relative inefficacy combined with its headache side effects for both patient and partner following absorption and action of the drug as a potent systemic vasodilator have precluded its use in clinical practice. Papaverine, formulated as a gel, was investigated but then abandoned as a topical ED treatment on finding that its large molecular size (molecular weight 376 Da) interfered with its transdermal absorption (Kim et al, 1995).
Alprostadil has been a more promising prospect, subjected to commercial development by two companies for penile glans administration in combination with transdermal delivery enhancers: alprostadil 1% + SEPA (soft enhancer of percutaneous absorption), referred to as Topiglan (Macrochem, Lexington, MA), and alprostadil + NexACT, referred to as Alprox-TD (NexMed, Inc., Robbinsville, NJ). Applied intrameatally, both agents in clinical trials have shown efficacy with rates of vaginal penetration and intercourse success that were small but significantly greater than placebo rates and caused minor side effects of site-specific burning or warmth that were comparable with placebo rates (McVary et al, 1999; Goldstein et al, 2001; McMahon, 2002; Padma-Nathan and Yeager, 2006; Rooney et al, 2009). Prostaglandin E1 ethyl ester, which is a prodrug of prostaglandin E1, is believed to possess an improved transdermal permeation and less skin irritation than enhancing agents because of its esterification (Schanz et al, 2009). Applied to the shaft of the penis in early clinical trials, this drug achieved significantly higher rigidity scores than placebo. In general, transdermal therapy with alprostadil is likely to meet similar clinical roles as that assigned to transurethral pharmacotherapy. Further clinical trials will be useful to define and establish their place in the treatment of ED.
Medical Devices
In patients who do not respond to or decline oral or local vasoactive pharmacotherapeutic options, vacuum erection device therapy may be alternatively explored. The principle of vacuum erection device therapy is to mechanically create negative pressure surrounding the penis in order to engorge it with blood and then restrain blood egress from the organ to maintain the erection-like effect (Broderick et al, 1992). Although the treatment does not produce a truly physiologic erection and the engorged blood predominantly consists of venous blood (Bosshardt et al, 1995), the effect resembles a normal erection and is sufficient for coitus. A particular feature is that the glans penis, and not solely the corpora cavernosa, is engorged with blood by the treatment, such that the treatment is further advantageous for patients experiencing glanular insufficiency.
The vacuum erection device standardly consists of a usually clear plastic suction cylinder and vacuum-generating source (manual or battery-operated pump) in one piece. It is placed directly over the flaccid penis and operated, and after the penis is erected an elastic constriction ring or band is positioned at the base of the penis; then the vacuum is released and the device is removed (Montague et al, 1996; McMahon, 1997). The cylinder has a pressure release valve designed to prevent penile injury from excessive negative pressure. Sexual intercourse may then ensue, although it is recommended that the ring should not be left in place for longer than 30 minutes. Prescription devices are advised, and metal or other inelastic rings are contraindicated.
Efficacy rates in achieving satisfactory erections of 67% to 90% have been reported for ED associated with various severities and etiologies, but satisfaction rates with the device are lower, ranging from 34% to 68% (Hellstrom et al, 2010). Attrition is reported to occur and may relate to lack of efficacy with more severe forms of ED, although long-term continuation rates have ranged from 19% to 42%. Success is limited in patients with severe vascular abnormalities such as proximal venous leakage or arterial insufficiency or fibrosis secondary to priapism or prosthesis infection (Marmar et al, 1988). Patient preferences also dictate long-term success. The device is more acceptable to older men in a steady relationship compared with young, single men.
Among basic expectations of the treatment, patients should be informed of possible local discomfort or pain associated with the constriction band, a pivoting effect of the penis because turgidity exists only distal to the band’s location, a cyanotic discoloration and coolness of the penis resulting from extracorporeal congestion, and trapping of the ejaculation caused by urethral constriction (Witherington, 1989; Sidi et al, 1990; Cookson and Nadig, 1993). Common complications are minor and include penile pain and numbness, difficult ejaculation, ecchymosis, and petechiae, and major complications (e.g., penile skin necrosis, urethral varicosities, Fournier gangrene) are infrequent. Patients receiving anticoagulant therapy (e.g., aspirin, warfarin) and patients with bleeding disorders should use the device with caution (Limoge et al, 1996).
Special uses for this therapy have been sought. It has been successfully combined with oral, intracavernous, and intraurethral pharmacotherapies to produce erectile responses (Marmar et al, 1988; Chen et al, 1995; John et al, 1996; Chen et al, 2004; Canguven et al, 2009). The device has enhanced erectile effects in the presence of a malfunctioning penile prosthesis (Sidi et al, 1990; Korenman and Viosca, 1992). Further, it may offer a means to preserve the elasticity of penile tissues after priapism or penile prosthesis explantation (Moul and McLeod, 1989; Soderdahl et al, 1997) or after surgical correction of Peyronie disease (Yurkanin et al, 2001), and it has been suggested to facilitate erection recovery after treatments for prostate cancer (Raina et al, 2006; Kohler et al, 2007).
Surgery
Surgical interventions have always served an important role in the armamentarium of ED treatments. Often, they are applied in the face of penile injury resulting from genital or pelvic trauma, penile structural deformity occurring in association with Peyronie disease, or possibly cavernosal fibrosis secondary to prolonged ischemic priapism or infection. They are also considered when medical therapy for ED is contraindicated, unsuccessful, or undesirable.
Penile Prosthesis Surgery
Penile prosthesis or implant surgery is a mechanism for creating penile rigidity that differs from a physiologic or pharmacologically induced erection. Both malleable (semi-rigid) and inflatable (hydraulic) devices are currently available for this purpose. Details of this treatment option are presented elsewhere.
Penile Revascularization Surgery
On the basis of the requirements of inflow of blood and its retention in the penis for penile erection to occur, it is hardly a wonder to think that vascular surgeries have aggressively been pursued to facilitate or restore these biologic processes.
Arterial Revascularization
In concept, arterial revascularization surgery was designed to create arterial inflow to the corpora cavernosa, in turn addressing the presentation of arteriogenic ED. Several procedures have been described to meet this objective, similarly creating an anastomosis of the inferior epigastric artery either to the corpus cavenosum directly or to vascular conduits of the penis such as the dorsal artery (i.e., revascularization), the deep dorsal vein (i.e., arterialization), or the deep dorsal vein with venous ligation (i.e., arterialization with venous reconstruction) (Hellstrom et al, 2010). Success of these surgeries has been variable and depends on careful patient selection. Penile arteriography is required to establish a penile arterial anatomic defect, and other organic causes of ED (e.g., venous incompetence) that would limit surgical success should be excluded. According to the current literature, the following inclusion criteria should be met to select patients for arterial surgery: age younger than 55 years, nonsmoker, nondiabetic, absence of venous leakage, and radiographic confirmation of stenosis of the internal pudendal artery (Hellstrom et al, 2010). The highest success rates are reported in young men (younger than 30 years of age) with isolated arterial stenosis following perineal or pelvic trauma (Babaei et al, 2009). Complications of arterial revascularization surgery include glans hyperemia (13%), shunt thrombosis (8%), and inguinal hernias (6.5%) (Manning et al, 1998; Kawanishi et al, 2004).
Venous Reconstruction
Venous reconstruction was proposed to prevent the pathologic blood egress from the penis, understandably serving to correct veno-occlusive ED. Most surgical procedures have centered on ligating or embolizing penile veins (e.g., superficial dorsal vein, deep dorsal vein, crural vein) or surgically compressing the penile crura (e.g., crural plication/ligation, pericavernoplasty) (Hellstrom et al, 2010). Success with these surgeries has not been affirmed, owing primarily to inaccurate or deficient methods for diagnosing and correcting the relevant anatomic defect. The optimal surgical approach remains to be defined, and thus venous reconstructive surgery is presently considered investigational (Montague et al, 2005; Hellstrom et al, 2010). Reported complications of this surgery include glanular hypoesthesia/anesthesia, skin necrosis, wound infections, penile curvature/shortening, and glans hyperemia.
Combination Therapies
It is well recognized that many patients with ED will not respond acceptably to monotherapy. Some patients indeed may achieve optimal therapeutic responses by combining treatment options. In addition, it is possible that a dose-limiting adverse effect is associated with ED monotherapy such that combined treatments may then seem advantageous. Multiple combinations may certainly be proposed for ED treatment. The extant literature describes several successful combinations: oral PDE5 inhibitors with psychosocial counseling (Althof et al, 2005), oral PDE5 inhibitors with testosterone replacement therapy (Shabsigh et al, 2004), oral PDE5 inhibitors with transurethral alprostadil (Mydlo et al, 2000; Nehra et al, 2002), oral PDE5 inhibitors and intracavernous pharmacotherapy (McMahon et al, 1999), oral PDE5 inhibitors and vacuum erection device (Chen et al, 2004; Canguven et al, 2009), intracavernous pharmacotherapy and vacuum erection device (Chen et al, 1995), transurethral pharmacotherapy and vacuum erection device (John et al, 1996), and transurethral pharmacotherapy and penile prosthesis surgery (Benevides and Carson, 2000). Caution is advised when initiating combination therapy to observe for potential complications that may be compounded by combined treatments, and in-office evaluations before continuing treatments at home may be considered to offer an additional measure of safety.
Alternative Therapies
Alternative therapies have long been considered for the treatment of ED, from herbs, ointments, and concoctions of antiquity to vitamins, nutraceuticals, and dietary supplements in commercial supply today. The movement toward alternative medicines in this field actually gained momentum over the past decade with the emergence of effective oral therapy in the form of PDE5 inhibitors, which created avenues for producing PDE5 inhibitor-like counterfeit and imitation substances and promoting regulatory agency unapproved products in general. Indeed, the true efficacies of proposed alternative therapies (e.g., ginkgo biloba, L-arginine, Korean red ginseng) remain uncertain in the absence of evidenced benefit in randomized, controlled clinical trials (Moyad et al, 2004). The success of these products is ascribed in some measure to the known placebo effect of agents to treat ED, which has been observed to amount to as much as 25% to 50% in properly conducted clinical trials. Before the use of alternative therapies can be advocated, further research that demonstrates their mechanisms of action and meaningful efficacies must be done.
Key Points
Treatment Considerations
Future Directions
The National Urology Research Agenda Summit Meeting, which was convened in the fall of 2009 and sponsored by the American Urological Association Foundation, included sexual dysfunctions among its priority research areas for advancing urologic health. Among the assembly of scientific experts and leaders in urologic research, the working group on sexual medicine enumerated several broad thematic categories of research for advancing ED management. These categories encompassed all subject areas of epidemiology, basic scientific research, and clinical investigation. Five top recommendations were submitted.
First, the epidemiologic relationships and pathogenic mechanisms linking ED and its comorbidity risk factors should be further clarified. It was considered that the definition and implementation of health prevention efforts for ED and for general health disorders have potentially mutual gains and should predictably improve overall health and longevity outcomes for patients.
Second, further research emphasis should be given to understanding and addressing iatrogenic ED. This objective recognizes that ED is a complication associated with numerous medical and surgical treatments that are performed in urology and in other disciplines. The scope in urology includes treatments for prostate cancer, benign prostate conditions, voiding dysfunctions, and possibly even pelvic pain disorders.
Third, basic scientific research in such areas as endothelial mechanisms, smooth muscle biology, and neurobiology referable to the sexual organ system must be supported with the understanding that research discoveries are the foundation for continued advances in the clinical practice of sexual medicine. Interdisciplinary research collaborations and interactions with researchers in other urologic research fields in particular are likely to facilitate the translational purpose of this objective.
Fourth, research focus should be given to the development of innovative therapeutics for ED. It is recognized that today’s advances in oral pharmacotherapy, despite their noted advantages, do not fully achieve goals of ideal therapy. Ideal therapy for ED management should go further, such as to restore function or improve it effectively for the long term. This prospect underscores the purpose of molecular and cellular therapeutic targeting, which is specific for etiopathogenic factors underlying ED. Exciting directions, from up-and-coming pharmacotherapeutics and technologies to futuristic approaches such as gene therapy, stem cell therapies, and tissue engineering, are all worth investigating.
Fifth, outcomes research is highly relevant for this field. This objective pertains to the rigorous evaluation of diagnostic assessments and treatments associated with ED management to ascertain their roles. It also applies to clinical contexts in which accurate erectile function assessments such as outcome reporting for pelvic surgery are necessary. This research area will profit from the ongoing development, validation and proper use of questionnaires, biomarkers, and other specialized clinical tools.
Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257-280.
Hellstrom WJ. Current safety and tolerability issues in men with erectile dysfunction receiving PDE5 inhibitors. Int J Clin Pract. 2007;61:1547-1554.
Kostis JB, Jackson G, Rosen R, et al. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference). Am J Cardiol. 2005;96:85M-93M.
Montague DK, Jarow JP, Broderick GA, et al. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol. 2005;174:230-239.
Montorsi F, Adaikan G, Becher E, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2010;7:3572-3588.
Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30:1-9.
Adaikan PG, Kottegoda SR, Ratnam SS. Is vasoactive intestinal polypeptide the principal transmitter involved in human penile erection? J Urol. 1986;135:638-640.
Aitchison M, Aitchison J, Carter R. Is the penile brachial index a reproducible and useful measurement? Br J Urol. 1990;66:202-204.
Albaugh J, Amargo I, Capelson R, et al. Health care clinicians in sexual health medicine: focus on erectile dysfunction. Urol Nurs. 2002;22:217-231.
Allard J, Giuliano F. Central nervous system agents in the treatment of erectile dysfunction: how do they work? Curr Urol Rep. 2001;2:488-494.
Allen RP, Smolev JK, Engel RM, Brendler CB. Comparison of RigiScan and formal nocturnal penile tumescence testing in the evaluation of erectile rigidity. J Urol. 1993;149:1265-1268.
Al-Shaiji TF, Brock GB. Phosphodiesterase type 5 inhibitors for the management of erectile dysfunction: preference and adherence to treatment. Curr Pharm Des. 2009;15:3486-3495.
Althof SE, Corty EW, Levine SB, et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology. 1999;53:793-799.
Althof SE, Leiblum SR, Chevret-Measson M, et al. Psychological and interpersonal dimensions of sexual function and dysfunction. J Sex Med. 2005;2:793-800.
Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191-236.
Araujo AB, Travison TG, Ganz P, et al. Erectile dysfunction and mortality. J Sex Med. 2009;6:2445-2454.
Aytac IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50-56.
Babaei AR, Safarinejad MR, Kolahi AA. Penile revascularization for erectile dysfunction: a systematic review and meta-analysis of effectiveness and complications. Urol J. 2009;6:1-7.
Baçar MM, Batislam E, Altinok D, et al. Sildenafil citrate for penile hemodynamic determination: an alternative to intracavernosal agents in Doppler ultrasound evaluation of erectile dysfunction. Urology. 2001;57:623-626.
Bacon CG, Mittleman MA, Kawachi I, et al. A prospective study of risk factors for erectile dysfunction. J Urol. 2006;176:217-221.
Bagatell CJ, Bremner WJ. Androgens in men—uses and abuses. N Engl J Med. 1996;334:707-714.
Bähren W, Gall H, Scherb W, et al. Arterial anatomy and arteriographic diagnosis of arteriogenic impotence. Cardiovasc Intervent Radiol. 1988;11:195-210.
Bancroft J, Smith G, Munoz M, Ronald P. Erectile response to visual erotic stimuli before and after intracavernosal papaverine, and its relationship to nocturnal penile tumescence and psychometric assessment. Br J Urol. 1991;68:629-638.
Barada JH. Optimizing outcomes of oral therapy for patients with erectile dysfunction. Rev Urol. 2003;5(Suppl. 7):S28-S34.
Basaria S, Dobs AS. Hypogonadism and androgen replacement therapy in elderly men. Am J Med.. 2001;110:563-572.
Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000;97:4279-4284.
Bemelmans BL, Hendrikx LB, Koldewijn EL, et al. Comparison of biothesiometry and neuro-urophysiological investigations for the clinical evaluation of patients with erectile dysfunction. J Urol. 1995;153:1483-1486.
Bénard F, Lue TF. Self-administration in the pharmacological treatment of impotence. Drugs. 1990;39:394-398.
Benevides MD, Carson CC. Intraurethral application of alprostadil in patients with failed inflatable penile prosthesis. J Urol. 2000;163:785-787.
Bennett AH, Carpenter AJ, Barada JH. An improved vasoactive drug combination for a pharmacological erection program. J Urol. 1991;146:1564-1565.
Benson CB, Aruny JE, Vickers MAJr. Correlation of duplex sonography with arteriography in patients with erectile dysfunction. AJR Am J Roentgenol. 1993;160:71-73.
Benson CB, Vickers MA. Sexual impotence caused by vascular disease: diagnosis with duplex sonography. AJR Am J Roentgenol. 1989;153:1149-1153.
Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
Billups KL. Erectile dysfunction as a marker for vascular disease. Curr Urol Rep. 2005;6:439-444.
Blander DS, Sánchez-Ortiz RF, Broderick GA. Sex inventories: can questionnaires replace erectile dysfunction testing? Urology. 1999;54:719-723.
Bleustein CB, Eckholdt H, Arezzo JC, Melman A. Quantitative somatosensory testing of the penis: optimizing the clinical neurological examination. J Urol. 2003;169:2266-2269.
Blum MD, Bahnson RR, Porter TN, Carter MF. Effect of local alpha-adrenergic blockade on human penile erection. J Urol. 1985;134:479-481.
Bosshardt RJ, Farwerk R, Sikora R, et al. Objective measurement of the effectiveness, therapeutic success and dynamic mechanisms of the vacuum device. Br J Urol. 1995;75:786-791.
Brading AF, Burdyga TV, Scripnyuk ZD. The effects of papaverine on the electrical and mechanical activity of the guinea-pig ureter. J Physiol. 1983;334:79-89.
Bradley WE, Timm GW, Gallagher JM, Johnson BK. New method for continuous measurement of nocturnal penile tumescence and rigidity. Urology. 1985;26:4-9.
Braun M, Wassmer G, Klotz T, et al. Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey’. Int J Impot Res. 2000;12:305-311.
Breza J, Aboseif SR, Orvis BR, et al. Detailed anatomy of penile neurovascular structures: surgical significance. J Urol. 1989;141:437-443.
Broderick GA, Arger P. Duplex Doppler ultrasonography: noninvasive assessment of penile anatomy and function. Semin Roentgenol. 1993;28:43-56.
Broderick GA, McGahan JP, Stone AR, White RD. The hemodynamics of vacuum constriction erections: assessment by color Doppler ultrasound. J Urol. 1992;147:57-61.
Burchardt M, Burchardt T, Baer L, et al. Hypertension is associated with severe erectile dysfunction. J Urol. 2000;164:1188-1191.
Burnett AL. Oral pharmacotherapy for erectile dysfunction: current perspectives. Urology. 1999;54:392-400.
Burnett AL. Phosphodiesterase 5 mechanisms and therapeutic applications. Am J Cardiol. 2005;96:29M-31M.
Burnett AL, Allen RP, Davis DM, et al. Near infrared spectrophotometry for the diagnosis of vasculogenic erectile dysfunction. Int J Impot Res. 2000;12:247-254.
Burnett AL, Strong T, Trock BJ, et al. Serum biomarker measurements of endothelial function and oxidative stress after daily dosing of sildenafil in type 2 diabetic men with erectile dysfunction. J Urol. 2009;181:245-251.
Burris AS, Banks SM, Sherins RJ. Quantitative assessment of nocturnal penile tumescence and rigidity in normal men using a home monitor. J Androl. 1989;10:492-497.
Buvat J, Costa P, Morlier D, et al. Double-blind multicenter study comparing alprostadil alpha-cyclodextrin with moxisylyte chlorhydrate in patients with chronic erectile dysfunction. Urol. 1998;159:116-119.
Buvat J, Lemaire A. Endocrine screening in 1,022 men with erectile dysfunction: clinical significance and cost-effective strategy. J Urol. 1997;158:1764-1767.
Buvat J, Lemaire A, Buvat-Herbaut M, Marcolin G. Safety of intracavernous injections using an alpha-blocking agent. J Urol. 1989;141:1364-1367.
Canguven O, Bailen J, Fredriksson W, et al. Combination of vacuum erection device and PDE5 inhibitors as salvage therapy in PDE5 inhibitor nonresponders with erectile dysfunction. J Sex Med. 2009;6:2561-2567.
Carani C, Bancroft J, Granata A, et al. Testosterone and erectile function, nocturnal penile tumescence and rigidity, and erectile response to visual erotic stimuli in hypogonadal and eugonadal men. Psychoneuroendocrinology. 1992;17:647-654.
Carani C, Isidori AM, Granata A, et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab. 2005;90:6472-6479.
Caretta N, Palego P, Schipilliti M, et al. Cavernous artery intima-media thickness: a new parameter in the diagnosis of vascular erectile dysfunction. J Sex Med. 2009;6:1117-1126.
Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257-280.
Cheitlin MD, Hutter AMJr, Brindis RG, et al. ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;33:273-282.
Chen J, Godschalk MF, Katz PG, Mulligan T. Combining intracavernous injection and external vacuum as treatment for erectile dysfunction. J Urol. 1995;153:1476-1477.
Chen J, Sofer M, Kaver I, et al. Concomitant use of sildenafil and a vacuum entrapment device for the treatment of erectile dysfunction. J Urol. 2004;171:292-295.
Chun J, Carson CC3rd. Physician-patient dialogue and clinical evaluation of erectile dysfunction. Urol Clin North Am. 2001;28:249-258.
Chung WS, Choi HK. Erotic erection versus nocturnal erection. J Urol. 1990;143:294-297.
Cilurzo P, Canale D, Turchi P, et al. The Rigiscan system in the diagnosis of male sexual impotence. Arch Ital Urol Nefrol Androl. 1992;64(Suppl. 2):81-85.
Citron JT, Ettinger B, Rubinoff H, et al. Prevalence of hypothalamic-pituitary imaging abnormalities in impotent men with secondary hypogonadism. J Urol. 1996;155:529-533.
Claes H, Opsomer RJ, Andrianne R, et al. Characteristics and expectations of patients with erectile dysfunction: results of the SCORED study. Int J Impot Res. 2008;20:418-424.
Clark JT. Suppression of copulatory behavior in male rats following central administration of clonidine. Neuropharmacology. 1991;30:373-382.
Clayton AH, McGarvey EL, Clavet GJ. The Changes in Sexual Functioning Questionnaire (CSFQ): development, reliability, and validity. Psychopharmacol Bull. 1997;33:731-745.
Cookson MS, Nadig PW. Long-term results with vacuum constriction device. J Urol. 1993;149:290-294.
Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem. 1999;274:13729-13732.
Costabile RA, Spevak M. Oral trazodone is not effective therapy for erectile dysfunction: a double-blind, placebo controlled trial. J Urol. 1999;161:1819-1822.
Daffertshofer M, Linden D, Syren M, et al. Assessment of local sympathetic function in patients with erectile dysfunction. Int J Impot Res. 1994;6:213-225.
Daig I, Heinemann LA, Kim S, et al. The Aging Males’ Symptoms (AMS) scale: review of its methodological characteristics. Health Qual Life Outcomes. 2003;15(1):77.
Dayan L, Greunwald I, Vardi Y, Jacob G. A new clinical method for the assessment of penile endothelial function using the flow mediated dilation with plethysmography technique. J Urol. 2005;173:1268-1272.
Dean J, Rubio-Aurioles E, McCabe M, et al. Integrating partners into erectile dysfunction treatment: improving the sexual experience for the couple. Int J Clin Pract. 2008;62:127-133.
DeBusk R, Drory Y, Goldstein I, et al. Management of sexual dysfunction in patients with cardiovascular disease: recommendations of the Princeton Consensus Panel. Am J Cardiol. 2000;86:62F-68F.
Derby CA, Mohr BA, Goldstein I, et al. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology. 2000;56:302-306.
Derogatis LR, Burnett AL. The epidemiology of sexual dysfunctions. J Sex Med. 2008;5:289-300.
Derogatis LR, Melisaratos N. The DSFI: a multidimensional measure of sexual functioning. J Sex Marital Ther. 1979;5:244-281.
Diamond LE, Earle DC, Rosen RC, et al. Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int J Impot Res. 2004;16:51-59.
Dinsmore WW, Wyllie MG. Vasoactive intestinal polypeptide/phentolamine for intracavernosal injection in erectile dysfunction. BJU Int. 2008;102:933-937.
Donatucci CF, Lue TF. The combined intracavernous injection and stimulation test: diagnostic accuracy. J Urol. 1992;148:61-62.
Doyle DL, Yu J. Comparison of Doppler and strain-gauge plethysmography to detect vasculogenic impotence. Can J Surg. 1986;29:338-339.
Edelstein D, Dobs A, Basaria S. Emerging drugs for hypogonadism. Expert Opin Emerg Drugs. 2006;11:685-707.
Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol. 1998;159:433-436.
Esposito K, Ciotola M, Giugliano F, et al. Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int J Impot Res. 2006;18:405-410.
Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978-2984.
Fallon B. Intracavernous injection therapy for male erectile dysfunction. Urol Clin North Am. 1995;22:833-845.
Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54-61.
Feldman HA, Johannes CB, Derby CA, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30:328-338.
Ferretti A, Caulo M, Del Gratta C, et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage. 2005;26:1086-1096.
Fisher C, Gorss J, Zuch J. Cycle of penile erection synchronous with dreaming (REM) sleep. Preliminary report. Arch Gen Psych. 1965;12:29-45.
Fisher WA, Eardley I, McCabe M, Sand M. Erectile dysfunction (ED) is a shared sexual concern of couples I: couple conceptions of ED. J Sex Med. 2009;6:2746-2760.
Fisher WA, Rosen RC, Eardley I, et al. Sexual experience of female partners of men with erectile dysfunction: the female experience of men’s attitudes to life events and sexuality (FEMALES) study. J Sex Med. 2005;2:675-684.
Floth A, Schramek P. Intracavernous injection of prostaglandin E1 in combination with papaverine: enhanced effectiveness in comparison with papaverine plus phentolamine and prostaglandin E1 alone. J Urol. 1991;145:56-59.
Fonseca V, Seftel A, Denne J, Fredlund P. Impact of diabetes mellitus on the severity of erectile dysfunction and response to treatment: analysis of data from tadalafil clinical trials. Diabetologia. 2004;47:1914-1923.
Francis ME, Kusek JW, Nyberg LM, Eggers PW. The contribution of common medical conditions and drug exposures to erectile dysfunction in adult males. J Urol. 2007;178:591-596.
Freeman ER, Bloom DA, McGuire EJ. A brief history of testosterone. J Urol. 2001;165:371-373.
Ganz P. Erectile dysfunction: pathophysiologic mechanisms pointing to underlying cardiovascular disease. Am J Cardiol. 2005;96:8M-12M.
Gerstenberg TC, Bradley WE. Nerve conduction velocity measurement of dorsal nerve of penis in normal and impotent males. Urology. 1983;21:90-92.
Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27:485-534.
Giuliano F, Jackson G, Montorsi F, et al. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2009 Nov 9. [Epub ahead of print]
Giuliano F, Rampin O. Central noradrenergic control of penile erection. Int J Impot Res. 2000;12(Suppl. 1):S13-S19.
Glass JM, Vale JA, Belcaro G, et al. A comparison of isotope penile blood flow and colour Doppler ultrasonography in the assessment of erectile dysfunction. Br J Urol. 1996;77:566-570.
Glick HA, McCarron TJ, Althof SE, et al. Construction of scales for the Center for Marital and Sexual Health (CMASH) Sexual Functioning Questionnaire. J Sex Marital Ther. 1997;23:103-117.
Goldstein I. Oral phentolamine: an alpha-1, alpha-2 adrenergic antagonist for the treatment of erectile dysfunction. Int J Impot Res. 2000;12(Suppl. 1):S75-S80.
Goldstein I, Payton TR, Schechter PJ. A double-blind, placebo-controlled, efficacy and safety study of topical gel formulation of 1% alprostadil (Topiglan) for the in-office treatment of erectile dysfunction. Urology. 2001;57:301-305.
Golubinski AJ, Sikorski A. Usefulness of power Doppler ultrasonography in evaluating erectile dysfunction. BJU Int. 2002;89:779-782.
Govier FE, McClure RD, Kramer-Levien D. Endocrine screening for sexual dysfunction using free testosterone determinations. J Urol. 1996;156:405-408.
Greenstein A, Plymate SR, Katz PG. Visually stimulated erection in castrated men. J Urol. 1995;153:650-652.
Guay AT. Optimizing response to phosphodiesterase therapy: impact of risk-factor management. J Androl. 2003;24(6 Suppl.):S59-S62.
Guay AT. ED2: erectile dysfunction = endothelial dysfunction. Endocrinol Metab Clin North Am. 2007;36:453-463.
Guay AT, Perez JB, Velásquez E, et al. Clinical experience with intraurethral alprostadil (MUSE) in the treatment of men with erectile dysfunction. A retrospective study. Medicated urethral system for erection. Eur Urol. 2000;38:671-676.
Hakim LS, Goldstein I. Diabetic sexual dysfunction. Endocrinol Metab Clin North Am. 1996;25:379-400.
Hanash KA. Comparative results of goal oriented therapy for erectile dysfunction. J Urol. 1997;157:2135-2138.
Hatzichristou DG, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt. 2):337-348.
Hatzichristou DG, Saenz de Tejada I, Kupferman S, et al. In vivo assessment of trabecular smooth muscle tone, its application in pharmaco-cavernosometry and analysis of intracavernous pressure determinants. J Urol. 1995;153:1126-1135.
Hatzimouratidis K, Burnett AL, Hatzichristou D, et al. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334-347.
Hawton K. Integration of treatments for male erectile dysfunction. Lancet. 1998;351:7-8.
Heaton JP. Apomorphine: an update of clinical trial results. Int J Impot Res. 2000;12(Suppl. 4):S67-S73.
Heinemann LA. Aging Males’ Symptoms scale: a standardized instrument for the practice. J Endocrinol Invest. 2005;28:34-38.
Hellstrom WJ. Current safety and tolerability issues in men with erectile dysfunction receiving PDE5 inhibitors. Int J Clin Pract. 2007;61:1547-1554.
Hellstrom WJ, Bennett AH, Gesundheit N, et al. A double-blind, placebo-controlled evaluation of the erectile response to transurethral alprostadil. Urology. 1996;48:851-856.
Hellstrom WJ, Montague DK, Moncada I, et al. Implants, mechanical devices, and vascular surgery for erectile dysfunction. J Sex Med. 2010;7(1 Pt. 2):501-523.
Herbener TE, Seftel AD, Nehra A, Goldstein I. Penile ultrasound. Semin Urol. 1994;12:320-332.
Hirsch M, Donatucci C, Glina S. Standards for clinical trials in male sexual dysfunction: erectile dysfunction and rapid ejaculation. J Sex Med. 2004;1:87-91.
Ho KH, Ong BK, Chong PN, Teo WL. The bulbocavernosus reflex in the assessment of neurogenic impotence in diabetic and non-diabetic men. Ann Acad Med Singapore. 1996;25:558-561.
Ichikawa T, Takao A, Manabe D, et al. The female partner’s satisfaction with sildenafil citrate treatment of erectile dysfunction. Int J Urol. 2004;11:755-762.
Inman BA, Sauver JL, Jacobson DJ, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. 2009;84:108-113.
Jackson G. Erectile dysfunction and cardiovascular disease. Int J Clin Pract. 1999;53:363-368.
Jackson G, Montorsi P, Cheitlin MD. Cardiovascular safety of sildenafil citrate (Viagra): an updated perspective. Urology. 2006;68(3 Suppl.):47-60.
Jackson G, Rosen RC, Kloner RA, Kostis JB. The second Princeton consensus on sexual dysfunction and cardiac risk: new guidelines for sexual medicine. J Sex Med. 2006;3:28-36.
Jackson SE, Lue TF. Erectile dysfunction: therapy health outcomes. Urology. 1998;51:874-882.
Jannini EA, Granata AM, Hatzimouratidis K, Goldstein I. Use and abuse of Rigiscan in the diagnosis of erectile dysfunction. J Sex Med. 2009;7:1820-1829.
Jardin A, Wagner G, Khoury S, Giuliano F, Padma-Nathan H, Rosen R. Erectile dysfunction. United Kingdom: Health Publication Ltd; 2000.
Jarow JP, Nana-Sinkam P, Sabbagh M, Eskew A. Outcome analysis of goal directed therapy for impotence. J Urol. 1996;155:1609-1612.
Jarow JP, Pugh VW, Routh WD, Dyer RB. Comparison of penile duplex ultrasonography to pudendal arteriography. Variant penile arterial anatomy affects interpretation of duplex ultrasonography. Invest Radiol. 1993;28:806-810.
Jiang XG, Speel TG, Wagner G, et al. The value of corpus cavernosum electromyography in erectile dysfunction: current status and future prospect. Eur Urol. 2003;43:211-218.
Johannes CB, Araujo AB, Feldman HA, et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460-463.
John H, Kacl GM, Lehmann K. Clinical value of pelvic and penile magnetic resonance angiography in preoperative evaluation of penile revascularization. Int J Impot Res. 1999;11:83-86.
John H, Lehmann K, Hauri D. Intraurethral prostaglandin improves quality of vacuum erection therapy. Eur Urol. 1996;29:224-226.
Johnson AR3rd, Jarow J. Is routine endocrine testing of impotent men necessary? J Urol. 1992;147:1542-1543.
Johri AM, Heaton JP, Morales A. Severe erectile dysfunction is a marker for hyperprolactinemia. Int J Impot Res. 2001;13:176-182.
Juenemann KP, Lue TF, Fournier GRJr, Tanagho EA. Hemodynamics of papaverine- and phentolamine-induced penile erection. J Urol. 1986;136:158-161.
Kaneko S, Bradley WE. Penile electrodiagnosis. Value of bulbocavernosus reflex latency versus nerve conduction velocity of the dorsal nerve of the penis in diagnosis of diabetic impotence. J Urol. 1987;137:933-935.
Kapoor D, Aldred H, Clark S, et al. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30:911-917.
Karacan I, Scott FB, Salis PJ, et al. Nocturnal erections, differential diagnosis of impotence, and diabetes. Biol Psychiatry. 1977;12:373-380.
Kassouf W, Carrier S. A comparison of the International Index of Erectile Function and erectile dysfunction studies. BJU Int. 2003;91:667-669.
Katlowitz NM, Albano GJ, Morales P, Golimbu M. Potentiation of drug-induced erection with audiovisual sexual stimulation. Urology. 1993;41:431-434.
Kawanishi Y, Kimura K, Nakanishi R, et al. Penile revascularization surgery for arteriogenic erectile dysfunction: the long-term efficacy rate calculated by survival analysis. BJU Int. 2004;94:361-368.
Kedia KR. Penile plethysmography useful in diagnosis of vasculogenic impotence. Urology. 1983;22:235-239.
Keene LC, Davies PH. Drug-related erectile dysfunction. Adverse Drug React Toxicol Rev. 1999;18:5-24.
Kellner B, Stief CG, Hinrichs H, Hartung C. Computerized classification of corpus cavernosum electromyogram signals by the use of discriminate analysis and artificial neural networks to support diagnosis of erectile dysfunction. Urol Res. 2000;28:6-13.
Kessler WO. Nocturnal penile tumescence. Urol Clin North Am. 1988;15:81-86.
Kiely EA, Bloom SR, Williams G. Penile response to intracavernosal vasoactive intestinal polypeptide alone and in combination with other vasoactive agents. Br J Urol. 1989;64:191-194.
Kim ED, el-Rashidy R, McVary KT. Papaverine topical gel for treatment of erectile dysfunction. J Urol. 1995;153:361-365.
Kloner RA. Erectile dysfunction and cardiovascular risk factors. Urol Clin North Am. 2005;32:397-402.
Knoll LD, Abrams JH. Application of nocturnal electrobioimpedance volumetric assessment: a feasibility study in men without erectile dysfunction. J Urol. 1999;161:1137-1140.
Köhler TS, Pedro R, Hendlin K, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int. 2007;100:858-862.
Köhn FM, Schill WB. A new oral testosterone undecanoate formulation. World J Urol. 2003;21:311-315.
Korenman SG, Morley JE, Mooradian AD, et al. Secondary hypogonadism in older men: its relation to impotence. J Clin Endocrinol Metab. 1990;71:963-969.
Korenman SG, Viosca SP. Use of a vacuum tumescence device in the management of impotence in men with a history of penile implant or severe pelvic disease. J Am Geriatr Soc. 1992;40:61-64.
Kostis JB, Jackson G, Rosen R, et al. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference). Am J Cardiol. 2005;96:85M-93M.
Krane RJ, Goldstein I, Saenz de Tejada I. Impotence. N Engl J Med. 1989;321:1648-1659.
Kukovetz WR, Pöch G, Wurm A. Quantitative relations between cyclic AMP and contraction as affected by stimulators of adenylate cyclase and inhibitors of phosphodiesterase. Adv Cyclic Nucleotide Res. 1975;5:395-414.
Kunelius P, Lukkarinen O, Hannuksela ML, et al. The effects of transdermal dihydrotestosterone in the aging male: a prospective, randomized, double blind study. J Clin Endocrinol Metab. 2002;87:1467-1472.
Lakin MM, Montague DK, VanderBrug Medendorp S, et al. Intracavernous injection therapy: analysis of results and complications. J Urol. 1990;143:1138-1141.
Lal S, Laryea E, Thavundayil JX, et al. Apomorphine-induced penile tumescence in impotent patients—preliminary findings. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:235-242.
Lal S, Rios O, Thavundayil JX. Treatment of impotence with trazodone: a case report. J Urol. 1990;143:819-820.
Laties AM. Vision disorders and phosphodiesterase type 5 inhibitors: a review of the evidence to date. Drug Saf. 2009;32:1-18.
Lau DH, Kommu S, Mumtaz FH, et al. The management of phosphodiesterase-5 (PDE5) inhibitor failure. Curr Vasc Pharmacol. 2006;4:89-93.
Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
Laumann EO, West S, Glasser D, et al. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the male attitudes regarding sexual health survey. J Sex Med. 2007;4:57-65.
Leach MM, Bethune C. Assisting sexually abused adults. Practical guide to interviewing patients. Can Fam Physician. 1996;42:82-86.
Lefaucheur JP, Yiou R, Colombel M, et al. Relationship between penile thermal sensory threshold measurement and electrophysiologic tests to assess neurogenic impotence. Urology. 2001;57:306-309.
Levine LA, Carroll RA. Nocturnal penile tumescence and rigidity in men without complaints of erectile dysfunction using a new quantitative analysis software. J Urol. 1994;152:1103-1107.
Lewis RW. Intraurethral and topical agents. Drugs Today (Barc). 2000;36:113-119.
Lewis RW, Fugl-Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
Licht MR, Lewis RW, Wollan PC, Harris CD. Comparison of RigiScan and sleep laboratory nocturnal penile tumescence in the diagnosis of organic impotence. J Urol. 1995;154:1740-1743.
Limoge JP, Olins E, Henderson D, Donatucci DF. Minimally invasive therapies in the treatment of erectile dysfunction in anticoagulated cases: a study of satisfaction and safety. J Urol. 1996;155:1276-1279.
Linet OI, Neff LL. Intracavernous prostaglandin E1 in erectile dysfunction. Clin Invest. 1994;72:139-149.
Linet OI, Ogrinc FG. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction. The Alprostadil Study Group. N Engl J Med. 1996;334:873-877.
Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159-166.
Litwin MS, Saigal CS, Yano EM, et al. Urologic diseases in America Project: analytical methods and principal findings. J Urol. 2005;173:933-937.
Liu PY, Wishart SM, Handelsman DJ. A double-blind, placebo-controlled, randomized clinical trial of recombinant human chorionic gonadotropin on muscle strength and physical function and activity in older men with partial age-related androgen deficiency. J Clin Endocrinol Metab. 2002;87:3125-3135.
Lobo RA, Kletzky OA. Normalization of androgen and sex hormone-binding globulin levels after treatment of hyperprolactinemia. J Clin Endocrinol Metab. 1983;56:562-566.
Lue TF. Impotence: a patient’s goal-directed approach to treatment. World J Urol. 1990;8:67-74.
Lue TF, Giuliano F, Montorsi F, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2004;1:6-23.
Lue TF, Hricak H, Marich KW, Tanagho EA. Evaluation of arteriogenic impotence with intracorporeal injection of papaverine and the duplex ultrasound scanner. Semin Urol. 1985;3:43-48.
Lue TF, Hricak H, Schmidt RA, Tanagho EA. Functional evaluation of penile veins by cavernosography in papaverine-induced erection. J Urol. 1986;135:479-482.
Lue TF, Mueller SC, Jow YR, Hwang TI. Functional evaluation of penile arteries with duplex ultrasound in vasodilator-induced erection. Urol Clin North Am. 1989;16:799-807.
Lue TF, Tanagho EA. Physiology of erection and pharmacological management of impotence. J Urol. 1987;137:829-836.
Manning M, Jünemann KP, Scheepe JR, et al. Long-term followup and selection criteria for penile revascularization in erectile failure. J Urol. 1998;160:1680-1684.
Mannino DM, Klevens RM, Flanders WD. Cigarette smoking: an independent risk factor for impotence? Am J Epidemiol. 1994;140:1003-1008.
Marmar JL, DeBenedictis TJ, Praiss DE. The use of a vacuum constrictor device to augment a partial erection following an intracavernous injection. J Urol. 1988;140:975-979.
Martin-Morales A, Sanchez-Cruz JJ, Saenz de Tejada I, et al. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J Urol. 2001;166:569-574.
Martins FE, Reis JP. Visual erotic stimulation test for initial screening of psychogenic erectile dysfunction: a reliable noninvasive alternative? J Urol. 1997;157:134-139.
McCullough AR, Barada JH, Fawzy A, et al. Achieving treatment optimization with sildenafil citrate (Viagra) in patients with erectile dysfunction. Urology. 2002;60(2 Suppl. 2):28-38.
McMahon CG. Nonsurgical treatment of cavernosal venous leakage. Urology. 1997;49:97-100.
McMahon CG. Topiglan MacroChem. Curr Opin Investig Drugs. 2002;3:602-606.
McMahon CG, Samali R, Johnson H. Treatment of intracorporeal injection nonresponse with sildenafil alone or in combination with triple agent intracorporeal injection therapy. J Urol. 1999;162:1992-1997.
McMahon CN, Smith CJ, Shabsigh R. Treating erectile dysfunction when PDE5 inhibitors fail. BMJ. 2006;332:589-592.
McVary KT, Polepalle S, Riggi S, Pelham RW. Topical prostaglandin E1 SEPA gel for the treatment of erectile dysfunction. J Urol. 1999;162:726-730.
Metro MJ, Broderick GA. Diabetes and vascular impotence: does insulin dependence increase the relative severity? Int J Impot Res. 1999;11:87-89.
Metz P, Bengtsson J. Penile blood pressure. Scand J Urol Nephrol. 1981;15:161-164.
Mirone V, Imbimbo C, Fusco F, et al. Androgens and morphologic remodeling at penile and cardiovascular levels: a common piece in complicated puzzles? Eur Urol. 2009;56:309-316.
Miyagawa Y, Tsujimura A, Fujita K, et al. Differential brain processing of audiovisual sexual stimuli in men: comparative positron emission tomography study of the initiation and maintenance of penile erection during sexual arousal. Neuroimage. 2007;36:830-842.
Moemen MN, Hamed HA, Kamel II, et al. Clinical and sonographic assessment of the side effects of intracavernous injection of vasoactive substances. Int J Impot Res. 2004;16:143-145.
Molitch ME. Medication-induced hyperprolactinemia. Mayo Clin Proc. 2005;80:1050-1057.
Molitch ME. Drugs and prolactin. Pituitary. 2008;11:209-218.
Montague DK, Barada HJ, Belker AM, et al. Clinical guidelines panel on erectile dysfunction: summary report of the treatment of organic erectile dysfunction. The American Urological Association. J Urol. 1996;156:2007-2011.
Montague DK, Jarow JP, Broderick GA, et al. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol. 2005;174:230-239.
Montorsi F, Adaikan G, Becher E, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2010;7:3572-3588.
Montorsi F, Althof SE. Partner responses to sildenafil citrate (Viagra) treatment of erectile dysfunction. Urology. 2004;63:762-767.
Montorsi F, Briganti A, Salonia A, et al. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44:360-364.
Montorsi F, Brock G, Lee J, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924-931.
Montorsi F, Perani D, Anchisi D, et al. Apomorphine-induced brain modulation during sexual stimulation: a new look at central phenomena related to erectile dysfunction. Int J Impot Res. 2003;15:203-209.
Montorsi P, Briganti A, Salonia A, et al. Impaired vascular reactivity in patients with erectile dysfunction. J Am Coll Cardiol. 2004;44:1339-1340.
Morales A, Buvat J, Gooren LJ, et al. Endocrine aspects of sexual dysfunction in men. J Sex Med. 2004;1:69-81.
Morales A, Condra M, Heaton JP, et al. Diurnal penile tumescence recording in the etiological diagnosis of erectile dysfunction. J Urol. 1994;152:1111-1114.
Morales A, Heaton JP, Johnston B, Adams M. Oral and topical treatment of erectile dysfunction. Present and future. Urol Clin North Am. 1995;22:879-886.
Morales A, Spevack M, Emerson L, et al. Adding to the controversy: pitfalls in the diagnosis of testosterone deficiency syndromes with questionnaires and biochemistry. Aging Male. 2007;10:57-65.
Morganroth J, Ilson BE, Shaddinger BC, et al. Evaluation of vardenafil and sildenafil on cardiac repolarization. Am J Cardiol. 2004;93:1378-1383. A6
Morgentaler A, Dobs AS, Kaufman JM. Long acting testosterone undecanoate therapy in men with hypogonadism: results of a pharmacokinetic clinical study. J Urol. 2008;180:2307-2313.
Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239-1242.
Motiwala HG. Dynamic pharmacocavernosometry: a search for an ideal approach. Urol Int. 1993;51:1-8.
Moul JW, McLeod DG. Negative pressure devices in the explanted penile prosthesis population. J Urol. 1989;142:729-731.
Mouras H, Stoléru S, Bittoun J, et al. Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. Neuroimage. 2003;20:855-869.
Moyad MA, Barada JH, Lue TF, et alSexual Medicine Society Nutraceutical Committee. Prevention and treatment of erectile dysfunction using lifestyle changes and dietary supplements: what works and what is worthless, part II. Urol Clin North Am. 2004;31:259-273.
Mueller SC, Lue TF. Evaluation of vasculogenic impotence. Urol Clin North Am. 1988;15:65-76.
Mueller SC, von Wallenberg-Pachaly H, Voges GE, Schild HH. Comparison of selective internal iliac pharmaco-angiography, penile brachial index and duplex sonography with pulsed Doppler analysis for the evaluation of vasculogenic (arteriogenic) impotence. J Urol. 1990;143:928-932.
Mulhall JP, King R, Kirby M, et al. Evaluating the sexual experience in men: validation of the sexual experience questionnaire. J Sex Med. 2008;5:365-376.
Mydlo JH, Volpe MA, Macchia RJ. Initial results utilizing combination therapy for patients with a suboptimal response to either alprostadil or sildenafil monotherapy. Eur Urol. 2000;38:30-34.
Naroda T, Yamanaka M, Matsushita K, et al. Clinical studies for venogenic impotence with color Doppler ultrasonography—evaluation of resistance index of the cavernous artery. Nippon Hinyokika Gakkai Zasshi. 1996;87:1231-1235.
Nehra A. Erectile dysfunction and cardiovascular disease: efficacy and safety of phosphodiesterase type 5 inhibitors in men with both conditions. Mayo Clin Proc. 2009;84:139-148.
Nehra A, Blute ML, Barrett DM, Moreland RB. Rationale for combination therapy of intraurethral prostaglandin E(1) and sildenafil in the salvage of erectile dysfunction patients desiring noninvasive therapy. Int J Impot Res. 2002;14(Suppl 1):S38-S42.
Netto Júnior NR, Claro Jde A. The importance of hyperprolactinemia in impotence. Rev Paul Med. 1993;111:454-455.
Nicolosi A, Laumann EO, Glasser DB, et alGlobal Study of Sexual Attitudes and Behaviors Investigators’ Group. Sexual behavior and sexual dysfunctions after age 40: the global study of sexual attitudes and behaviors. Urology. 2004;64:991-997.
NIH Consensus Statement: Impotence 1992;10:1–33.
Nurnberg HG, Gelenberg A, Hargreave TB, et al. Efficacy of sildenafil citrate for the treatment of erectile dysfunction in men taking serotonin reuptake inhibitors. Am J Psychiatry. 2001;158:1926-1928.
O’Leary MP, Fowler FJ, Lenderking WR, et al. A brief male sexual function inventory for urology. Urology. 1995;46:697-706.
Owen JA, Saunders F, Harris C, et al. Topical nitroglycerin: a potential treatment for impotence. J Urol. 1989;141:546-548.
Padma-Nathan H. Neurologic evaluation of erectile dysfunction. Urol Clin North Am. 1988;15:77-80.
Padma-Nathan H, Hellstrom WJ, Kaiser FE, et al. Treatment of men with erectile dysfunction with transurethral alprostadil. Medicated Urethral System for Erection (MUSE) Study Group. N Engl J Med. 1997;336:1-7.
Padma-Nathan H, McCullough AR, Levine LA, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20:479-486.
Padma-Nathan H, Yeager JL. An integrated analysis of alprostadil topical cream for the treatment of erectile dysfunction in 1732 patients. Urology. 2006;68:386-391.
Padmanabhan P, McCullough AR. Penile oxygen saturation in the flaccid and erect penis in men with and without erectile dysfunction. J Androl. 2007;28:223-228.
Palmer LS, Valcic M, Melman A, et al. Characterization of cyclic AMP accumulation in cultured human corpus cavernosum smooth muscle cells. J Urol. 1994;152:1308-1314.
Park K, Seo JJ, Kang HK, et al. A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res. 2001;13:73-81.
Pescatori ES, Hatzichristou DG, Namburi S, Goldstein I. Positive intracavernous injection test implies normal veno-occlusive but not necessarily normal arterial function: a hemodynamic study. J Urol. 1994;151:1209-1216.
Petak SM, Nankin HR, Spark RF, et alAmerican Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr Pract. 2002;8:440-456.
Pickard RS, Powell PH, Schofield IS. The clinical application of dorsal penile nerve cerebral-evoked response recording in the investigation of impotence. Br J Urol. 1994;74:231-235.
Porst H. The rationale for prostaglandin E1 in erectile failure: a survey of worldwide experience. J Urol. 1996;155:802-815.
Porst H, Giuliano F, Glina S, et al. Evaluation of the efficacy and safety of once-a-day dosing of tadalafil 5 mg and 10 mg in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2006;50:351-359.
Process of Care Consensus Panel. The process of care model for evaluation and treatment of erectile dysfunction. Int J Impot Res. 1999;11:59-70.
Puyau FA, Lewis RW. Corpus cavernosography. Pressure flow and radiography. Invest Radiol. 1983;18:517-522.
Quam JP, King BF, James EM, et al. Duplex and color Doppler sonographic evaluation of vasculogenic impotence. AJR Am J Roentgenol. 1989;153:1141-1147.
Qureshi AA. Alibra (VIVUS). Curr Opin Investig Drugs. 2001;2:550-554.
Raina R, Agarwal A, Ausmundson S, et al. Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. Int J Impot Res. 2006;18:77-81.
Rajfer J, Rosciszewski A, Mehringer M. Prevalence of corporeal venous leakage in impotent men. J Urol. 1988;140:69-71.
Ralph D, McNicholas T. UK management guidelines for erectile dysfunction. BMJ. 2000;321:499-503.
Rhee E, Osborn A, Witt M. The correlation of cavernous systolic occlusion pressure with peak velocity flow using color duplex Doppler ultrasound. J Urol. 1995;153:358-360.
Rhoden EL, Estrada C, Levine L, Morgentaler A. The value of pituitary magnetic resonance imaging in men with hypogonadism. J Urol. 2003;170:795-798.
Rooney M, Pfister W, Mahoney M, et al. Long-term, multicenter study of the safety and efficacy of topical alprostadil cream in male patients with erectile dysfunction. J Sex Med. 2009;6:520-534.
Rosen RC. Psychogenic erectile dysfunction. Classification and management. Urol Clin North Am. 2001;28:269-278.
Rosen RC. Evaluation of the patient with erectile dysfunction: history, questionnaires, and physical examination. Endocrine. 2004;23:107-111.
Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-326.
Rosen RC, Catania J, Pollack L, et al. Male Sexual Health Questionnaire (MSHQ): scale development and psychometric validation. Urology. 2004;64:777-782.
Rosen RC, Fisher WA, Eardley I, et al. Men’s Attitudes to Life Events and Sexuality (MALES) Study: The multinational Men’s Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20:607-617.
Rosen RC, Hatzichristou D, Broderick G, et al. Clinical Evaluation and Symptom Scales: Sexual Dysfunction Assessment in Men, 2004 edition. Lue TF, Basson R, Rosen R, et al, editors. Sexual medicine: sexual dysfunctions in men and women. Health Publications, France, 2004, 173-220.
Rosen RC, Lane RM, Menza M. Effects of SSRIs on sexual function: a critical review. J Clin Psychopharmacol. 1999;19:67-85.
Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822-830.
Rowland DL, Burnett AL. Pharmacotherapy in the treatment of male sexual dysfunction. J Sex Res. 2000;37:226-243.
Rowland DL, Thornton JA, Burnett AL. Recognizing the risk of erectile dysfunction in a urology clinic practice. BJU Int. 2005;95:1034-1038.
Rudnick J, Bödecker R, Weidner W. Significance of the intracavernosal pharmacological injection test, pharmacocavernosography, artificial erection and cavernosometry in the diagnosis of venous leakage. Urol Int. 1991;46:338-343.
Rust J, Golombok S. The GRISS: a psychometric instrument for the assessment of sexual dysfunction. Arch Sex Behav. 1986;15:157-165.
Sadovsky R, Brock GB, Gutkin SW, Sorsaburu S. Toward a new “EPOCH”: optimising treatment outcomes with phosphodiesterase type 5 inhibitors for erectile dysfunction. Int J Clin Pract. 2009;63:1214-1230.
Sáenz de Tejada I, Angulo J, Cellek S, et al. Pathophysiology of erectile dysfunction. J Sex Med. 2005;2:26-39.
Safarinejad MR. Oral sildenafil in the treatment of erectile dysfunction in diabetic men: a randomized double-blind and placebo-controlled study. J Diabetes Complications. 2004;18:205-210.
Saigal CS, Wessells H, Pace J, et alUrologic Diseases in America Project. predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166:207-212.
Sakheim DK, Barlow DH, Abrahamson DJ, Beck JG. Distinguishing between organogenic and psychogenic erectile dysfunction. Behav Res Ther. 1987;25:379-390.
Sakhri S, Gooren LJ. Safety aspects of androgen treatment with 5alpha-dihydrotestosterone. Andrologia. 2007;39:216-222.
Sand M, Fisher WA. Women’s endorsement of models of female sexual response: the nurses’ sexuality study. J Sex Med. 2007;4:708-719.
Sarteschi LM, Montorsi F, Menchini Fabris F, et al. Cavernous arterial and arteriolar circulation in patients with erectile dysfunction: a power Doppler study. J Urol. 1998;159:428-432.
Schanz S, Hauck EW, Schmelz HU, et al. Topical treatment of erectile dysfunction with prostaglandin E ethyl ester. J Dtsch Dermatol Ges. 2009 May 14. [Epub ahead of print.
Schrader SM, Breitenstein MJ, Lowe BD. Cutting off the nose to save the penis. J Sex Med. 2008;5:1932-1940.
Schwartz AN, Lowe M, Berger RE, et al. Assessment of normal and abnormal erectile function: color Doppler flow sonography versus conventional techniques. Radiology. 1991;180:105-109.
Seftel AD. J Challenges in oral therapy for erectile dysfunction. J Androl. 2002;23:729-736.
Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151-157.
Shabsigh R, Donatucci C, Costabile R, et al. Reliability of efficacy in men with erectile dysfunction treated with tadalafil once daily after initial success. Int J Impot Res. 2010;22:1-8.
Shabsigh R, Fishman IJ, Shotland Y, et al. Comparison of penile duplex ultrasonography with nocturnal penile tumescence monitoring for the evaluation of erectile impotence. J Urol. 1990;143:924-927.
Shabsigh R, Fishman IJ, Toombs BD, Skolkin M. Venous leaks: anatomical and physiological observations. J Urol. 1991;146:1260-1265.
Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol. 2004;172:658-663.
Sharlip ID. Evaluation and nonsurgical management of erectile dysfunction. Urol Clin North Am. 1998;25:647-659.
Shindel AW. 2009 update on phosphodiesterase type 5 inhibitor therapy part 2: updates on optimal utilization for sexual concerns and rare toxicities in this class. J Sex Med. 2009;6:2352-2364. quiz 2365–6
Shirai M, Nakamura M, Ishii N, et al. Determination of intrapenial blood volume using 99mTc-labeled autologous red blood cells. Tohoku J Exp Med. 1976;120:377-383.
Sidi AA, Becher EF, Zhang G, Lewis JH. Patient acceptance of and satisfaction with an external negative pressure device for impotence. J Urol. 1990;144:1154-1156.
Sironi G, Colombo D, Poggesi E, et al. Effects of intracavernous administration of selective antagonists of alpha(1)-adrenoceptor subtypes on erection in anesthetized rats and dogs. J Pharmacol Exp Ther. 2000;292:974-981.
Smith EM, Netto IC, Gladden KH, et al. Role of radionuclide phallogram in therapeutic decision-making for erectile dysfunction. Urology. 1998;51(5A Suppl.):175-178.
Soderdahl DW, Petroski RA, Mode D, et al. The use of an external vacuum device to augment a penile prosthesis. Tech Urol. 1997;3:100-102.
Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart. 2003;89:251-253.
Soran H, Wu FC. Endocrine causes of erectile dysfunction. Int J Androl. 2005;28(Suppl. 2):28-34.
Speckens AE, Hengeveld MW, Lycklama à Nijeholt G, et al. Psychosexual functioning of partners of men with presumed non-organic erectile dysfunction: cause or consequence of the disorder? Arch Sex Behav. 1995;24:157-172.
Speel TG, Bleumer I, Diemont WL. The value of sildenafil as mode of stimulation in pharmaco-penile duplex ultrasonography. Int J Impot Res. 2001;13:189-191.
Spudis EV, Stubbs AJ, Skowronski T. Cerebral-evoked response from stimulation of dorsal nerve in impotent men. Urology. 1989;34:370-375.
Stehling MK, Liu L, Laub G, et al. Gadolinium-enhanced magnetic resonance angiography of the pelvis in patients with erectile impotence. MAGMA. 1997;5:247-254.
Stellato RK, Feldman HA, Hamdy O, et al. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490-494.
Stief CG, Jünemann KP, Kellner B, et al. Consensus and progress in corpus cavernosum-EMG (CC-EMG). Int J Impot Res. 1994;6:177-182.
Sunagane N, Ogawa T, Uruno T, Kubota K. Mechanism of relaxant action of papaverine. VI. Sodium ion dependence of its effect on 45Ca-efflux in guinea-pig taenia coli. Jpn J Pharmacol. 1985;38:133-139.
Telöken C, Rhoden EL, Sogari P, et al. Therapeutic effects of high dose yohimbine hydrochloride on organic erectile dysfunction. J Urol. 1998;159:122-124.
Thompson IM, Tangen CM, Goodman PJ, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996-3002.
Tiefer L, Schuetz-Mueller D. Psychological issues in diagnosis and treatment of erectile disorders. Urol Clin North Am. 1995;22:767-773.
Tokatli Z, Akand M, Yaman O, et al. Comparison of international index of erectile function with nocturnal penile tumescence and rigidity testing in evaluation of erectile dysfunction. Int J Impot Res. 2006;18:186-189.
Traish AM, Guay AT. Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med. 2006;3:382-404.
Tsujimura A, Matsumiya K, Takao T, et al. Treatment with human chorionic gonadotropin for PADAM: a preliminary report. Aging Male. 2005;8:175-179.
Turnbull JM, Weinberg PC. Psychological factors involved in impotence. A review of the literature. J Androl. 1983;4:59-66.
Van Ahlen H, Peskar BA, Sticht G, Hertfelder HJ. Pharmacokinetics of vasoactive substances administered into the human corpus cavernosum. J Urol. 1994;151:1227-1230.
Vardi Y, Dayan L, Apple B, et al. Penile and systemic endothelial function in men with and without erectile dysfunction. Eur Urol. 2009;55:979-985.
Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666-3672.
Virag R. Intracavernous injection of papaverine for erectile failure. Lancet. 1982;2:938.
Virag R, Floresco J, Richard C. Impairment of shear-stress-mediated vasodilation of cavernous arteries in erectile dysfunction. Int J Impot Res. 2004;16:39-42.
Vodusek DB, Ravnik-Oblak M, Oblak C. Pudendal versus limb nerve electrophysiological abnormalities in diabetics with erectile dysfunction. Int J Impot Res. 1993;5:37-42.
Wagner G, Gerstenberg T, Levin RJ. Electrical activity of corpus cavernosum during flaccidity and erection of the human penis: a new diagnostic method? J Urol. 1989;142:723-725.
Wald M, Meacham RB, Ross LS, Niederberger CS. Testosterone replacement therapy for older men. J Androl. 2006;27:126-132.
Wang C, Nieschlag E, Swerdloff R, et alInternational Society of Andrology (ISA); International Society for the Study of Aging Male (ISSAM); European Association of Urology (EAU); European Academy of Andrology (EAA); American Society of Andrology (ASA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30:1-9.
Wasserman MD, Pollak CP, Spielman AJ, Weitzman ED. The differential diagnosis of impotence. The measurement of nocturnal penile tumescence. JAMA. 1980;243:2038-2042.
Wespes E, Goes PM, Schiffmann S. Computerized analysis of smooth muscle fibers in potent and impotent patients. J Urol. 1991;146:1015-1017.
Wespes E, Moreira de Goes P, Schulman C. Vascular impotence: focal or diffuse penile disease. J Urol. 1992;148:1435-1436.
Wessells H, Joyce GF, Wise M, Wilt TJ. Erectile dysfunction. J Urol. 2007;177:1675-1681.
Wessells H, Levine N, Hadley ME, et al. Melanocortin receptor agonists, penile erection, and sexual motivation: human studies with melanotan II. Int J Impot Res. 2000;12(Suppl. 4):S74-S79.
Witherington R. Vacuum constriction device for management of erectile impotence. J Urol. 1989;141:320-322.
Wolfson B, Pickett S, Scott NE, et al. Intraurethral prostaglandin E-2 cream: a possible alternative treatment for erectile dysfunction. Urology. 1993;42:73-75.
Yurkanin JP, Dean R, Wessells H. Effect of incision and saphenous vein grafting for Peyronie’s disease on penile length and sexual satisfaction. J Urol. 2001;166:1769-1772.
Zeitlin SI, Rajfer J. Hyperprolactinemia and erectile dysfunction. Rev Urol. 2000;2:39-42.
Zorgniotti AW. Intracavernous injections of vasoactive substances for the treatment of impotence. Nippon Hinyokika Gakkai Zasshi. 1985;76:1620.
Zorgniotti AW, Lefleur RS. Auto-injection of the corpus cavernosum with a vasoactive drug combination for vasculogenic impotence. J Urol. 1985;133:39-41.
Zumbé J, Porst H, Sommer F, et al. Comparable efficacy of once-daily versus on-demand vardenafil in men with mild-to-moderate erectile dysfunction: findings of the RESTORE study. Eur Urol. 2008;54:204-210.