chapter 25 Priapism
Priapism describes a persistent erection arising from dysfunction of the mechanisms regulating penile tumescence, rigidity, and flaccidity. A correct diagnosis of priapism is a matter of urgency requiring identification of the underlying hemodynamics.
Scientific organizations have recommended guidelines for the management of priapism including the American Urological Association in 2003 (www.auanet.org) and the International Society for Sexual Medicine in 2006 (www.issm.info). Both groups have noted that the literature on priapism is composed mainly of small case series and individual case reports and includes inconsistent definitions and methodologies with little long-term erectile function outcome data. Recent case series have included detailed methodology including duration of priapism, etiology of priapism, and erectile function outcomes. The basic science on the pathogenesis of priapism and clinical research supporting the most effective treatment strategies are summarized in this chapter. Recommendations for best clinical practice and suggestions for research are made.
Defining Priapism
Priapism is a full or partial erection that continues more than 4 hours beyond sexual stimulation and orgasm or is unrelated to sexual stimulation.
Ischemic Priapism (Veno-Occlusive, Low-Flow)
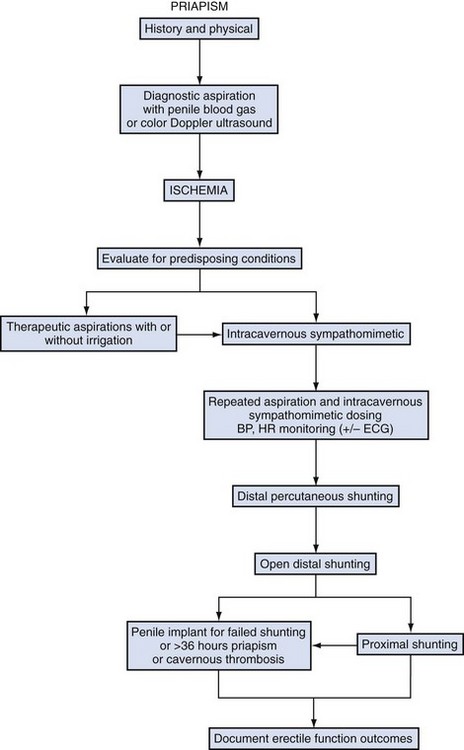
Ischemic priapism is a persistent erection marked by rigidity of the corpora cavernosa and little or no cavernous arterial inflow. In ischemic priapism there are time-dependent changes in the corporal metabolic environment with progressive hypoxia, hypercarbia, and acidosis. The patient typically complains of penile pain after 6 to 8 hours, and the examination reveals a rigid erection. The condition is analogous to a muscle compartment syndrome, with initial occlusion of venous outflow and subsequent cessation of arterial inflows. Well-documented histologic changes occur within the corporal smooth muscle as a consequence of prolonged ischemia. Interventions beyond 48 to 72 hours of onset may help relieve erection and pain but have little benefit in preserving potency. Histologically by 12 hours corporal specimens show interstial edema, progressing to destruction of sinusoidal endothelium, exposure of the basement membrane, and thrombocyte adherence at 24 hours. After 48 hours thrombus can be found in the sinusoidal spaces and smooth muscle necrosis with fibroblast-like cell transformation is evident (Spycher, 1986). Ischemic priapism is an emergency. When left untreated, resolution may take days and erectile dysfunction (ED) invariably results (Fig. 25–1A and B).
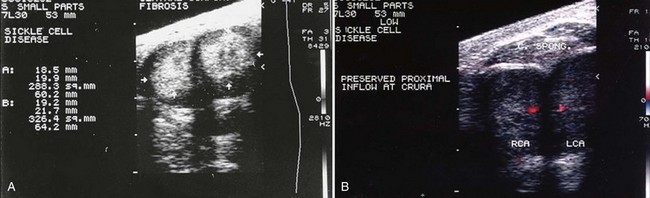
Figure 25–1 A, Transverse gray-scale ultrasound image of pendulous penis in a 30-year-old sickle cell disease patient, with history of multiple episodes of priapism, shows hyperechoic corporal fibrosis replacing normal tissues (arrows). B, Color duplex Doppler in same patient following intracavernous injection of alprostadil. The right and left (RCA, LCA) cavernous arteries show preservation of blood flow only at the base of the penis.
Stuttering Priapism (Intermittent)
Stuttering priapism characterizes a pattern of recurrence. The term has historically described recurrent unwanted and painful erections in men with sickle cell disease (SCD) (Serjeant, 1985). Patients typically awaken with an erection that persists for several hours. Unfortunately, males with SCD may experience stuttering priapism from childhood; in these patients the pattern of stuttering may increase in frequency and duration, leading up to a full episode of unrelenting ischemic priapism. Unfortunately, any patient who has experienced an episode of ischemic priapism is also at risk for stuttering priapism.
Nonischemic Priapism (Arterial, High-Flow)
Nonischemic priapism is a persistent erection caused by unregulated cavernous arterial inflow. Typically, the corpora are tumescent but not rigid and the penis is not painful. A history of blunt trauma to the penis or an iatrogenic needle injury is common. Whatever the mechanism of injury, the result is a disruption of the cavernous arterial anatomy creating an arteriolar-sinusoidal fistula. The cavernous environment does not become ischemic and cavernous blood gases do not show hypoxia, hypercarbia, or acidosis. This type of priapism, once properly diagnosed, does not require emergent intervention. Beyond the acute trauma, patients do not complain of pain. Normal erectile function has been reported after recovery from the initial event, despite persistence of nonsexual partial erection.
Priapism: Historical Perspectives
The term priapism has its origin in reference to the Greek god Priapus, who was worshipped as a god of fertility and protector of horticulture. Priapus is memorialized in sculptures for his giant phallus. The first recorded account of priapism in English medical literature appears in the Lancet and is attributed to Tripe (1845). Historically the most commonly cited observation on this condition in North American literature is Frank Hinman Senior’s landmark article describing the natural history of priapism (Hinman, 1914). Subsequently in 1960 his son Frank Hinman Jr. proposed that venous stasis, increased blood viscosity, and ischemia were responsible for priapism and emphasized that failure to correct these abnormalities in the penile environment was essentially responsible for treatment nonresponse (Hinman, 1960). Advances in our understanding of the physiology of erection and the pathophysiology of ED substantiated early hypotheses that prolonged veno-occlusion within the corporal bodies is analogous to a compartment syndrome. Hauri demonstrated the radiologic differences between veno-occlusive and arterial priapism (1983).
Frank Hinman (1914) first described “acute transitory attacks of priapism” as opposed to persistence or rapid recurrence of a single episode. The actual term of stuttering priapism is attributed to Emond and colleagues (1980) in observations of patients with SCD in a Jamaican clinic. Stuttering priapism episodes were seen to increase in frequency and length, leading to major, unrelenting occurrence of ischemic priapism. Attempts to manage SCD patients with stuttering ischemic priapism resulted in the early recommendation for hormonal suppression of nocturnal erections and stuttering with estrogen (Serjeant, 1985).
Nonischemic priapism is described far less commonly than ischemic priapism in the urologic literature. Nonischemic priapism is invariably associated with antecedent perineal or penile trauma. It was first described in the English literature by Burt (1960).
Key Points
Priapism Definitions
Epidemiology and Pathophysiology of Priapism
Etiology of Ischemic Priapism (Veno-Occlusive, Low-Flow)
Ischemic priapism accounts for the majority of cases described in the literature. The erection of ischemic priapism may begin with sexual stimulation or the administration of pharmacologic agents. Once an erection persists beyond 4 hours and is not relieved by cessation of sexual stimulation or orgasm, the physiologic phenomena of ischemic priapism have begun. Erections lasting up to 4 hours are by consensus defined as “prolonged”; manufacturers of erection-facilitating pharmacotherapies (oral, injectable, and intraurethral) recommend that the patient seek emergent medical consultation for prolonged erection.
Population-based studies estimate cases per 100,000 person-years (the number of patients with a first episode of priapism divided by the accumulated amount of person-time in the study population). Cases per 100,000 person-years have been calculated in several countries; these data depend on recording of presentations to clinics and hospitals where cases are registered. Kulmala and colleagues (1995) calculated the cases per 100,000 person-years to be 0.34 to 0.52 from 1975-1990 in Finland; Eland and colleagues (2001) calculated the cases in the Netherlands to be 1.5/100,000 person-years; Earle and colleagues (2003) calculated 0.84/100,000 person-years in Australia from 1985-2000. These reported incidences were statistically significantly impacted by the introduction and proliferation of intracavernous vasoactive injections for the management of erectile dysfunction; in Finland during the last 3 years of the study the incidence of priapism doubled to 1.1 cases/100,000 person-years. These and other reports on the epidemiology and etiology of priapism are also greatly influenced by the prevalence of SCD in the populations described. The life-time probability of a man with SCD developing ischemic priapism ranges from 29% to 42% (Emond, 1980).
In 1986 Pohl and colleagues reported on 230 cases. The etiology of priapism was identified as idiopathic in the majority, and 21% of cases were associated with alcohol or drug use/abuse, 12% with perineal trauma, and 11% with SCD (Pohl, 1986). Although SCD is a predominant etiology of veno-occlusive priapism cases in the literature, there is a wide variety of reported associations from urinary retention to insect bites (Hoover, 2004). Priapism has even been reported following spider bites and envenomation from the “Brazilian banana spider,” Phoneutria nigriventer (Andrade, 2008; Villanova, 2009). The genus Phoneutria (Greek for murderess) has eight species. P. nigriventer is known to hide in dark and moist places, wander the jungle floor, and stowaway within banana shipments. P. nigriventer is blamed for most cases of envenomation in Brazil; the venom contains a neurotoxin that has calcium channel blocking properties, inhibits glutamate release, and inhibits calcium reuptake and glutamate reuptake. Bites can cause intense pain, loss of muscle control—paralysis, breathing problems—asphyxiation, and priapism (www.wikipedia.org). Two peptides isolated from the venom of P. nigriventer have been directly linked with the induction of persistent and painful erections in mammals (Tx2-5 and TX2-6). The protein has been named eretina and has been shown to have highly specific interference at the molecular level with nitric oxide pathway (NO). Penile erection has been induced in vivo with eretina by direct intraperitoneal injection with a minimum effective dosage of 0.006 mcg/kg (Andrade, 2008).
Hematologic dyscrasias are a major risk factor for ischemic priapism. Priapism has been described as a complication of SCD, thalasssemia, hemoglobin Olmsted, and thrombophilia (Burnett, 2005). Thrombotic disease states have also been cited as precipitants of ischemic priapism: asplenism, erythropoietin use, hemodialysis with heparin use, and cessation of coumadin therapy. Intracavernous heparin given as a therapy for priapism due to rebound hypercoagulable states has actually worsened the condition (Fassbinder et al, 1976; Bschieipfer, 2001). Priapism may occur in patients with excessive white blood cell counts. The incidence of priapism in adult male patients with leukemia is 1% to 5% (Chang et al, 2003). Hyperleukocystosis causes priapism in these patients; it is believed that mechanical pressure on abdominal veins secondary to splenomegaly causes congestion of cavernous outflow and sludging of leukemic cells within the corpora cavernosa. When priapism presents in the oncology setting, evaluation and management of the predisposing condition must accompany interventions directed at the penis. In hematologic malignancies, leukaphresis and cytotoxic therapy (hydroxurea, ctosine arabinoside) may reduce the numbers of circulating white blood cells (Ponniah et al, 2004; Manuel et al, 2007). Priapism secondary to metastatic infiltrating solid lesions rather than leukemoid reaction is extremely rare. In most case reports of metastatic priapism, the primary malignancy is genitourinary (prostate and bladder). Metastatic infiltration of the penis may proceed with solid replacement or focal deposits within the corpora cavernosa, glans, and corpus spongiosum. Theoretically, metastatic deposits within the corpora could obstruct venous outflow resulting in ischemic priapism. Depending on the status of the patient, metastatic lesions may be managed expectantly, with partial or total penectomy, chemotherapy, or irradiation. These cases are too rarely and poorly described to define best practice recommendations (Robey, 1984; Chan, 1998; Guvel, 2003; Celma-Domenech, 2008) (Table 25–1).
Table 25–1 Etiologies of Priapism
| α-Adrenergic Receptor Antagonists |
| Prazosin, terazosin, doxazosin, tamsulosin |
| Antianxiety Agents |
| Hydroxyzine |
| Anticoagulants |
| Heparin, warfarin |
| Antidepressants and Antipsychotics |
| Trazodone, bupropion, fluoxetine, sertraline, lithium, clozapine, resperidone, olazapine, chlorpromazine, thoridazine, phenothaizines |
| Antihypertensives |
| Hydralazine, guanethidine, propanolol |
| Drugs (Recreational) |
| Alcohol, cocaine (intranasal and topical), crack cocaine, marijuana |
| Genitourinary |
| Straddle injury, coital injury, pelvic trauma, kick to penis/perineum, arteriovenous or arteriocavernous bypass surgery, urinary retention |
| Hematologic Dyscrasias |
| Sickle cell disease, thalassemia, granulocytic leukemia, myeloid leukemia, lymphocytic leukemia, multiple myeloma, haemoglobin Olmsted variant, fat emboli associated with hyperalimentation, hemodialysis, glucose 6-phosphate dehydrogenase deficiency |
| Hormones |
| Gonadotropin-releasing hormone, testosterone |
| Infectious (Toxin Mediated) |
| Scorpion sting, spider bite, rabies, malaria |
| Metabolic |
| Amyloidosis, Fabry disease, gout |
| Neoplastic (Metastatic or Regional Infiltration) |
| Prostate, urethra, testis, bladder, rectal, lung, kidney |
| Neurogenic |
| Syphilis, spinal cord injury, cauda equina compression, autonomic neuropathy, lumbar disk herniation, spinal stenosis, cerebral vascular accident, brain tumor, spinal anesthesia, cauda equina syndrome |
| Vasoactive Erectile Agents |
| Papaverine, phentolamine, prostaglandin E1, oral phosphodiesterase type 5 inhibitors, combination intracavernous therapy |
Modified from Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction and priapism. In: Walsh PC, Retik AB, Vaughan ED, et al, editors. Campbell’s urology. Philadelphia: WB Saunders; 2002. p. 1610–96.
Sickle Cell Disease
Blood dyscrasias are a risk factor for ischemic priapism. SCD priapism has traditionally been ascribed to stagnation of blood within the sinusoids of the corpora cavernosa during physiologic erection, secondary to obstruction of venous outflow by sickled erythrocytes (Lue, 2002). Nelson and Winters described a series of cases in which SCD was the primary etiology of ischemic priapism in 23% of adults and 63% of children (Nelson, 1977). Sickle cell hemoglobinopathy accounts for at least a third of all cases of priapism, and indeed prevalence of ischemic priapism will vary significantly with the population of males in a community with SCD. From Emond and colleagues’ 1980 observational study comes the most commonly quoted incidence: Among 104 men attending an outpatient sickle cell clinic in Kingston, Jamaica the incidence of priapism in men with homozygous sickle cell (SS) disease was 42% (Emond, 1980). In a U.S. clinical series, Tarry and Duckett (1987) found that 6.4% of male children in an outpatient sickle cell clinic had history of priapism (Tarry, 1987). Adeyoju and colleagues (2002), in an international multicenter observational study of SCD, mailed or interviewed 130 patients attending SCD clinics in the United Kingdom and Nigeria. Respondents ranged in age from 4 to 66 years old with the mean age of 25. The authors cited mean age of onset of priapism as 15 years, with 75% of patients having their first episode before age 20 and rare first-time presentations by the third decade of life. In the questionnaires a clear distinction was made between acute severe prolonged priapism lasting longer than 24 hours requiring emergency attention and stuttering recurrent priapism of shorter and self-limiting duration. In this population the incidence of acute priapism was 35%; of these patients 72% gave a history of stuttering. The median frequency of occurrence of stuttering priapism was three times per month; the median duration of each espisode was 1.2 hours, with the longest being 8 hours. Precipitating events reported from greatest to least were sexual arousal/intercourse, fever, sleep, cold weather, and dehydration. Self-administered regimens were analgesics, drinking water, and exercise. Twenty-one percent of patients reporting history of priapism also reported erectile dysfunction. Only 7% of young men who had not experienced priapism were even aware that priapism was a potential complication of their SCD. On the basis of the World Health Organization global prevalence map of SCD, Aliyu (2008) estimates that 20 to 25 million individuals worldwide have homozygous SCD: 12 to 15 million in sub-Saharan Africa, 5 to 10 million in India, and 3 million in other world regions. Aliyu (2008) also found that 70,000 patients with SCD live in the United States.
The sickle cell genetic mutation is the result of a single amino acid substitution in the beta-globin subunit of haemoglobin. The clinical features are seen in homozygous SCD patients: chronic hemolysis, vascular occlusion, tissue ischemia, and end-organ damage. HbS polymerizes when deoxygenated, injuring the sickle erythrocyte, activating a cascade of hemolysis and vaso-occlusion. Membrane damage results in dense sickling of red cells, causing adhesive interactions among sickle cells, endothelial cells, and leukocytes. Hemolysis releases hemoglobin into the plasma. Free Hbg reacts with nitric oxide (NO) to produce methemoglobin and nitrate. This is a scavenging reaction; the vasodilator NO is oxidized to inert nitrate. Sickled erythrocytes release arginase-I into blood plasma, which converts L-arginine into ornithine, effectively removing substrate for NO synthesis. Oxidant radicals further reduce NO bioavailability. The combined effects of NO scavenging and arginine catabolism result in a state of NO resistance and insufficiency termed hemolysis-associated endothelial dysfunction (Morris, 2005; Rother, 2005; Kato, 2007; Aliyu, 2008).
Contemporary science implicates hemolysis and reduced nitric oxide in the pathogenesis of pulmonary hypertension, leg ulcers, priapism, and stroke in SCD patients. Increased blood viscosity is believed to be responsible for painful crises, osteonecrosis, and acute chest syndrome. SCD patients with priapism have a fivefold greater risk of developing pulmonary hypertension. SCD priapism is also associated with reduced hemoglobin levels and increased hemolytic markers: reticulocyte count, bilirubin, lactate dehydrogenase, and aspartate aminotransferase (Kato, 2006). Cerebral vascular accidents are more frequent, close to episodes of full-blown priapism; the ASPEN syndrome (priapism, exchange transfusion, and neurologic events) describes cerebral vascular accidents in SCD patients who have received exchange transfusions (Siegel, 1993; Merritt, 2006). Sickle cell trait is considered a benign condition; a few complications have been associated with extreme physical exertions. There have been case reports of sickle cell trait as the predisposing factor to ischemic priapism (Larocque, 1974; Birnbaum, 2008).
Iatrogenic Priapism: Intracavernous Injections
Prolonged erection is more commonly reported than is priapism, following therapeutic or diagnostic injection of intracavernous vasoactive medications (Broderick, 2002). Despite the introduction of effective oral medications for ED in 1998, ICI remains an important therapeutic option for men with severe ED failing a phosphodiesterase type-5 inhibitor or for men who cannot take PDE-5 inhibitors because they require or carry nitrates. In many communities patients receiving intracavernous medications for ED will outnumber patients with SCD. Priapism following intracavernous injection (ICI) is a problem all urologists will encounter and must be prepared to manage. In a review of worldwide reports on intracavernous injection (ICI) programs, Junemann and colleagues (1990) noted that diagnostic injection resulted in 5.3% of men getting ischemic priapism and 0.4% of men reported priapism after injecting at home (Junemann, 1990). In papaverine-based ICI programs, reports of prolonged erections and priapism are poorly distinguished and range from 0% to 35% (Broderick, 2002). In worldwide clinical trials of the Alprostadil Study Group, prolonged erection (defined as 4 to 6 hours) was 5%, and priapism (>6 hours) was described in 1% of subjects (Porst, 1996). In papaverine/phentolamine/alprostadil ICI programs prolonged erections have been reported in 5% to 35% of patients (Broderick, 2002).
Iatrogentic Priapism: Oral Phosphodiesterase Type 5 Inhibitors
All PDE5 inhibitors have similar side effects related directly to their mode of action, tissue content of substrate, and pharmacologic selectivity for type 5 inhibition versus other phosphodiesterase enzymes. Those side effects include headache, flushing, dyspepsia, rhinitis, light sensitivity, and myalgia. Morales and colleagues (1998) analyzed data from 4274 men who received double-blind treatment with sildenafil or placebo for up to 6 months and 2199 who received long-term open-label sildenafil for up to 1 year (Morales, 1998). No cases of priapism (erection lasting >4 hours) were reported. No cases of priapism were reported by Montorsi and colleagues (2004) in a multicenter, open-label, 24-month extension of 8- or 12-week double-blind, placebo-controlled studies assessing the long-term efficacy, safety, and tolerability of tadalafil in 1173 men with ED (Montorsi, 2004). Nonetheless, the INDICATION and USAGE section of the U.S. Food and Drug Administration (FDA)-approved product labeling for PDE type 5 inhibitors (USPI) does contain this warning: There have been rare reports of prolonged erection greater than 4 hours and priapism (painful erections >6 hours duration) for this class of compounds. Both the USPI and European Summary of Product Characteristics label information contain warning or precautionary language about the use of these agents in men who have conditions predisposing them to priapism. Tadalafil has recently been approved in many regions of the world for daily dosing in the management of ED and is currently being studied for use in the management of symptoms due to benign prostatic hypertrophy. Tadalafil 5-mg daily dosing caused no priapism in a phase II clinical study of 281 men with history of lower urinary tract symptoms secondary to benign prostatic hyperplasia for 6 weeks, followed by dosage escalation to 20 mg once daily for 6 weeks (McVary, 2007).
From 1999-2007 there have been at least nine case-based reports of oral phosphodiesterase type 5 inhibitor dosing and adult priapism and at least one pediatric case: Aoyagi (1999), Sur (2000), Kassim (2000), Goldmeier (2002), McMahon (2003), Wilt (2004), Gallati (2005), King (2005), Kumar (2005), and Wills (2007). The majority of cases reports detailing priapism following a PDE type 5 inhibitor reveal histories of men with increased risk for priapism: SCD, spinal cord injury, men who used PDE5 inhibitor recreationally, men who used PDE5 inhibitor in combination with intracavernous injection, men with history of penile trauma, men on psychotropic medications, and men abusing narcotics. Wills (2007) described a 19-month-old male child weighing 10 kg who accidentally ingested up to 6 tablets of sildenafil 50 mg. The child presented with persistent sinus tachycardia and partial erection for 24 hours; the authors presume this was a high-flow priapism (HFP) because the shaft was neither completely rigid nor painful. Erection in the child subsided spontaneously after overnight intravenous hydration and observation.
Key Points
Ischemic Priapism as a Complication of Erectile Dysfunction Therapy
Etiology of Stuttering (Intermittent) Priapism
Stuttering (intermittent) priapism describes a pattern of recurrent priapism. The term has traditionally been used to describe recurrent unwanted and painful erections in men with SCD. Patients typically awaken with an erection that persists up to 4 hours and becomes progressively painful secondary to ischemia. SCD patients may experience stuttering priapism from childhood. Any patient who has experienced ischemic priapism is at risk for stuttering priapism. Patients with stuttering priapism will experience repeated painful intermittent attacks up to several hours before remission. Affected young men suffer embarrassment, sleep deprivation, and performance anxiety with sexual partners (Chow, 2008). In a study of 130 patients with SCD, Adeyoju and colleagues (2002) reported that 46 (35%) had a history of priapism and, of these, 33 (72%) had a history of stuttering priapism. In 75% of patients the first episode of stuttering occurred before the age of 20. Two thirds of males presenting with SCD ischemic priapism will describe prior stuttering attacks (De Jesus, 2009). Commonly reported precipitants of full-blown SCD priapism are stuttering nocturnal/early morning erections, dehydration, fever, and exposure to cold.
Etiology and Pathophysiology of Nonischemic (Arterial, High-Flow) Priapism
HFP is a persistent erection caused by unregulated cavernous arterial inflow. The epidemiologic data on nonischemic priapism is almost exclusively derived from small case series or individual case reports. Nonischemic priapism is much rarer than ischemic priapism, and the etiology is largely attributed to trauma. Forces may be blunt or penetrating, resulting in laceration of the cavernous artery or one of its branches within the corpora. The etiology most commonly reported is a straddle injury to the crura. Other mechanisms include coital trauma, kicks to the penis or perineum, pelvic fractures, birth canal trauma to the newborn male, needle lacerations, complications of penile diagnostics, and vascular erosions complicating metastatic infiltration of the corpora (Witt, 1990; Brock, 1993; Dubocq, 1998; Burgu, 2007; De Jesus, 2009). Although accidential blunt trauma is the most common etiology, HFP has been described following iatrogenic injury: cold-knife urethrotomy, Nesbitt corporoplasty, and deep dorsal vein arterialization (Wolf, 1992; Liguori, 2005). Any mechanism that lacerates a cavernous artery or arteriole can produce unregulated pooling of blood in sinusoidal space with consequent erection. Nonischemic priapism is typically delayed in onset compared with the episode of blunt trauma (Ricciardi, 1993). Sustained partial erection may develop 24 hours following perineal or penile blunt trauma. It is believed that the hemodynamics of a nocturnal erection disrupts the clot and the damaged artery/arteriole ruptures; the unregulated arterial inflow creates a sinsusoidal fistula. As healing progresses with clearing of clot and necrotic smooth muscle tissue, the fistula forms a pseudocapsule. Formation of a pseudocapsule at the site of fistula may take several weeks to months.
Contemporary reports suggest that HFP may have a unique subvariety. Several authors have noted that following either aggressive medical management of ischemic priapism or surgical shunting, priapism may rapidly recur with conversion from ischemia to high flow. HFP has been reported after aspiration and injection of α-adrenergics in the management of ischemic priapism (McMahon, 2002; Rodriguez, 2006; Bertolotto, 2009). Color Doppler ultrasound has shown formation of an arteriolar-sinusoidal fistula at the site of intervention (needle laceration or shunt site) (Fig. 25–2A to C). On rare occasion following reversal of ischemic priapism, a new high-flow hemodynamic state of the cavernous arteries occurs with no evidence of fistula. This presentation of HFP should be suspected in cases where rapid recurrence, persistence of erection with partial penile rigidity, or stuttering priapism not associated with pain is evident. Nonfistula type of arterial priapism is the result of dysregulation of cavernous inflows. Nonfistula arterial priapism is a rare complication following management ischemic priapism (Seftel, 1998; Cruz-Guerra, 2004; Wallace, 2009). Penile tenderness to palpation is easily confused with the ongoing ache of persistent ischemia. Soft tissue edema and ecchymosis render the physical examination equivocal after medical and surgical maneuvers to alleviate priapism. Dysregulated arterial inflows with or without a fistula can best be distinguished from persistent ischemic priapism by color Doppler ultrasound.
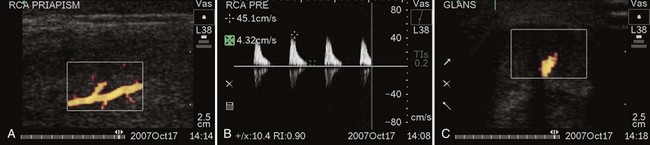
Figure 25–2 A, Color duplex Doppler ultrasound, showing right cavernous arterial inflow (RCA). The patient had a 30-hour ischemic priapism reversed with a Winter shunt. He developed a persistent partial erection, converting from ischemia to high-flow priapism. B, Peak systolic velocity in right cavernous artery is 45 cm/sec. C, Arterial sinusoidal fistula has formed at the site of the previous Winter shunt, as shown in this transverse color Doppler image of the glans penis.
Priapism in Children
Priapism in children and adolescents is most commonly related to SCD. The literature suggests that the incidence of priapism in pediatric sickle cell clinics is 2% to 6% (Tarry and Duckett, 1987; De Jesus, 2009). The majority of SCD priapism is ischemic. In the newborn period, fetal hemoglobin predominates, not hemoglobin S (Burgu, 2007). SCD phenotypes related to ischemic or occlusive crises are unlikely to be evident while fetal hemoglobin persists. Newborn priapism is an extremely rare phenomenon with only limited case reports and rare application of contemporary diagnostic modalities. Erection is frequently elicited in males during the newborn period. In newborn males simple tactile stimulation such as diaper changing, bathing, and urethral catheterization may result in erection; the erection quickly subsides following cessation of stimuli. Fewer than 20 cases of newborn priapism have been reported in the literature; rarely has etiology been defined: polycythemia, blood transfusion, and birth canal trauma (Amlie, 1977; Leal, 1978; Shapiro, 1979; Walker, 1997). The majority of cases have been conservatively managed with spontaneous resolution reported from hours to days. Minimally invasive diagnostics (color Doppler ultrasound) should be performed (Pietras, 1979; Meijer, 2003). In children who develop priapism following straddle trauma, every effort should be made to localize the arteriolar-sinusoidal fistula. Hatzichristou and colleagues (2002) reported that identification of the fistula by Doppler ultrasound coupled with direct manual compression softens the high-flow erection and may speed spontaneous resolution. They suggested that this noninvasive therapy likely works in children and not adults because the perineum has considerably less subcutaneous fat and crural bodies are more easily compressed (Hatzichristou, 2002).
Molecular Basis of Ischemic and Stuttering Priapism
Advances in our understanding of the molecular basis of priapism have drawn significantly from both in vitro and in vivo experimental studies using animal models. Data on the true inciting mechanisms involved in ischemic priapism are emerging. Ischemic priapism consists of an imbalance of vasoconstrictive and vasorelaxatory mechanisms predisposing the penis to hypoxia and acidosis. In-vitro studies have demonstrated that when corporal smooth muscle strips and cultured corporal smooth muscle cells are exposed to hypoxic conditions, α-adrenergic stimulation fails to induce corporal smooth muscle contraction (Broderick, 1994; Saenz de Tejada, 1997; Muneer, 2005). Extended periods of severe anoxia significantly impair corporal smooth muscle contractility and cause significant apoptosis of smooth muscle cells and, ultimately, fibrosis of the corpora cavernosa.
In experimental animal models of ischemic priapism, lipid peroxidation, an indicator of injury induced by reactive oxygen species (ROS), and increased hemo-oxygenase expression occurs in the penis during and after ischemic priapism (Munarriz, 2003; Jin, 2008). Additional pathophysiologic mechanisms involved in the progression of ischemia-induced fibrosis are the upregulation of hypoxia-induced growth factors. TGF-β is a cytokine that is vital to tissue repair. However, excess amounts may induce tissue damage and scarring. Upregulation of TGF-β occurs during hypoxia and in response to oxidative stress (Moreland, 1995; Jin, 2008). It is hypothesized that TGF-β may be involved in the progression of the corporal smooth muscle to fibrosis (Bivalacqua, 2000; Jeong, 2004).
Transgenic mouse models of SCD manifest priapism (Beuzard, 1996; Bivalacqua, 2009). There have been two major discoveries in elucidation of the molecular mechanism of ischemic priapism. Mi and colleagues (2008) have shown that transgenic sickle cell mice corpora cavernosa have enhanced smooth muscle relaxation to electrical field stimulation. Transgenic sickle cell mice and mice lacking endothelial nitric oxide synthase (eNOS) gene expression display supraphysiologic erections and spontaneously phasic priapic activity in vivo (Bivalacqua, 2006, 2007).
Endothelial cells actively regulate basal vascular tone and vascular reactivity, by responding to mechanical forces and neuro-humoral mediators with the release of a variety of relaxing and contracting factors. In the penis the vascular endothelium is a source of vaso-relaxing factors such as NO and adenosine, as well as vaso-constrictor factors such as RhoA/Rho-kinase. Recent evidence suggests that in states of priapism there may be aberrant NO and adenosine signaling, thus identifying a potential role for NO/cGMP, as well as adenosine and RhoA/Rho-kinase signaling in the pathophysiology of ischemic priapism (Champion, 2005; Mi, 2008; Bivalacqua, 2009).
eNOS−/− mutant mice have an exaggerated erectile response to cavernous nerve stimulation and have phenotypic changes in erectile function consistent with priapism (Champion, 2005; Bivalacqua, 2006). Mice lacking the eNOS gene manifest a priapism phenotype through mechanisms involving defective phosphodiesterase type 5 (PDE5) regulatory function in the penis, resulting from altered endothelial nitric oxide/cGMP signaling in the organ (Lin, 2003; Bivalacqua, 2006). Supporting this hypothesis, PDE5 expression is significantly reduced in corpora cavernosa smooth muscle cells (CCSMC) grown under anoxic and hypoxic cell culture conditions (Lin, 2003). In the context of molecular dysregulation, the cyclic nucleotide cGMP is produced in low steady-state amounts under the influence of priapism-related destruction of the vascular endothelium and thus reduced endothelial nitric oxide activity; this situation thereby downregulates the set point of PDE type 5 function, secondary to altered cGMP-dependent feedback control mechanisms (Champion, 2005; Bivalacqua, 2006; Burnett, 2007). Under these conditions, when NO is neuronally produced in response to an erectogenic stimulus or with nocturnal erections, cGMP production surges in a manner that leads to excessive erectile tissue relaxation because of basally insufficient PDE type 5 enzyme to degrade the cyclic nucleotide. Additionally, reduced Rho-kinase activity (contractile mediator) may contribute to the susceptibility of corporal tissue to excessive relaxation via two distinct molecular mechanisms. These two distinct molecular mechanisms may act in concert to promote stuttering ischemic priapism: enhanced vaso-relaxation by uninhibited cGMP and diminished contractile effects of Rho-kinase. Transgenic sickle cell mice also have significant reductions in penile NO/cGMP signaling leading to deficient PDE5 expression/activity, as well as reduced RhoA/Rho-kinase expression from which they manifest enhanced erectile responses and recurrent priapism (Champion, 2005). Another potential cause of enhanced corporal smooth muscle relaxation in SCD-associated priapism is elevated penile adenosine levels, thus causing the corpora cavernosa to be in a chronically vasodilated state (Mi, 2008). Taken together, these data suggest that ischemic priapism and most importantly stuttering priapism is a direct result of NO imbalance resulting in aberrant molecular signaling, PDE5 dysregulation, adenosine overproduction, and reductions in Rho-kinase activity, translating into enhanced corporal smooth muscle relaxation and inhibition of vasoconstriction in the penis.
Key Points
Sickle Cell Disease and Priapism
Evaluation and Diagnosis of Priapism
History
In order to initiate appropriate management, the physician must determine whether the underlying priapism hemodynamics are ischemic or nonischemic. Emergency management of ischemic priapism is recommended. Ischemia should be suspected when the patient has progressive penile pain, associated with the duration of erection; has used a known drug associated with priapism; has SCD or another blood dyscrasia; or has a known neurologic condition, especially those affecting the spinal cord. Stuttering priapism history is one of recurrent episodes of prolonged erections, usually nonresolving morning erections. Nonischemic priapism should be suspected when there is no pain and the erection duration has not been accompanied by progressive discomfort. There is a history of straddle injury, coital trauma, blunt trauma to the penis or perineum, penile injection, penile surgery or a diagnostic procedure of the pelvic and penile vessels. The onset of post-traumatic HFP in adults and children may be delayed by hours to several days following the initial injury (Table 25–2).
Table 25–2 Elements in Taking the History of Priapism
Physical Examination
Inspection and palpation of the penis is recommended to determine the extent and degree of tumescence and rigidity; the involvement of the cavernous bodies; the presence of pain; and the evidence of trauma to the perineum. In ischemic priapism the corporal bodies will be completely rigid; the glans penis and corpus spongiosum are not. Although malignancies rarely cause priapism, examination of the abdomen, testicles, perineum, rectum, and prostate may help identify a cancer primary. Malignant infiltration of the penis causes indurated nodules within or replacing corporal tissue. The subtle differences in the penile examination may be apparent to the experienced urologist but can be overlooked by emergency personnel on initial evaluation (Fig. 25–3A and B). If physical examination reveals the penis is nontender, tumesced, or partially erect, nonischemic priapism is suspected. In nonischemic priapism the corpora will be tumescent but not completely rigid. In children and adults with high-flow priapism, depending on the location of trauma and time since the traumatic event, there may be residual bruising at the perineum from straddle injury (Table 25–3).
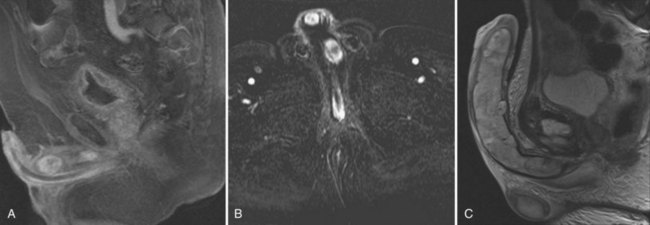
Figure 25–3 A, Saggital MRI of the penis showing metastatic deposits of prostate cancer to the corpus cavernosum. B, Coronal MRI of the same patient. Note the proximal and distal metastic deposits of prostate cancer. C, T2-weighted MRI showing chondrosarcoma replacing corpus cavernosum.
(Courtesy of David Ralph.)
Table 25–3 Key Findings in Priapism
| FINDINGS | ISCHEMIC PRIAPISM | NONISCHEMIC PRIAPISM |
|---|---|---|
| Perineal trauma | Seldom | Usually |
| Hematologic abnormalities | Usually | Seldom |
| Recent intracorporal injection | Sometimes | Sometimes |
| Corpora cavernosa fully rigid | Usually | Seldom |
| Penile pain | Usually | Seldom |
| Abnormal penile blood gas | Usually | Seldom |
| Cavernous inflow (by Doppler) | Seldom | Usually |
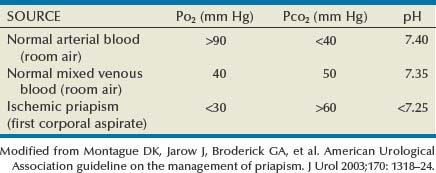
Modified from Montague DK, Jarow J, Broderick GA, et al. American Urological Association guideline on the management of priapism. J Urol 2003;170:1318–24.
Laboratory Testing
Evaluation should include a CBC, WBC with blood cell differential, platelet count, and coagulation profile to assess anemia, rule out infection, detect hematologic abnormalities, and ensure that the patient can safely tolerate surgical interventions should initial medical management fail. In African-Americans a sickle cell prep and hemoglobin electrophoresis should be requested. Other hematologic abnormalities may cause priapism and should be sought if etiology is not evident: leukemia, platelet abnormalities, and thalassemia. An elevated reticulocyte count is nonspecific and may be elevated in both priapism due to SCD and thalassemia. Urine and serum toxicology panels should be done if recreational narcotic or prescription psychoactive drugs are suspected from the history. A corporal blood gas by aspiration is recommended in the emergency evaluation of priapism. The corporal blood aspirate differentiates ischemic from nonischemic priapism. Aspiration may be both diagnostic and therapeutic. Visual inspection of the color and consistency of an initial penile aspirate will reveal dark deoxygenated blood, with a “crankcase oil” appearance in ischemic priapism. The initial corporal apirate may be sent for blood gas testing to document pH, PO2, and PCO2 (Table 25–4). Color duplex Doppler ultrasonography should be initiated if the history suggests penile/perineal trauma or if the corporal aspirate reveals well-oxygenated blood (Fig. 25–4A and B).
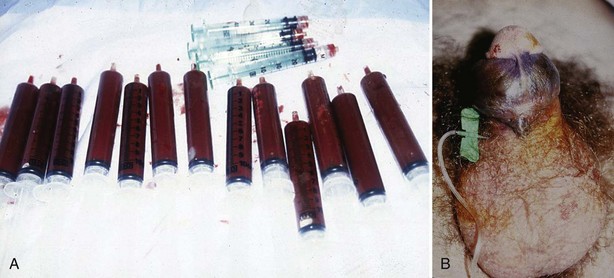
Figure 25–4 A, Initial corporal aspirate in ischemic priapism show dark, deoxygenated blood. Subsequent aspirations will show brighter blood as corpus cavernosum is reoxygenated by inflow. Empty syringes are from successive injections of phenylephrine. B, A butterfly needle for aspiration and injection should be placed at the penoscrotal junction. Initial failed efforts in the emergency room were due to distal placement of butterfly needle and failure to repeat aspirations.
Penile Imaging
Color duplex Doppler ultrasonography (CDU) of the penis and perineum is recommended in the evaluation of priapism. CDU is an adjunct to the corporal aspirate in differentiating ischemic from nonischemic priapism. Patients with prolonged ischemic priapism will have no blood flow in the cavernous arteries; the return of the cavernous artery waveform will accompany successful detumescence. Patients with nonischemic priapism have normal to high blood flow velocities detectable in the cavernous arteries; an effort should be made to localize the characteristic blush of color emanating from the disrupted cavernous artery/arteriole (Broderick, 2002). Examination of the entire penile shaft and perineum is recommended; this can be done with the patient supine but frog legged (Fig. 25–5). Penile arteriography should be reserved for the management of high-flow priapism, when embolization is planned; arteriography is too invasive as a diagnostic procedure to differentiate ischemic from nonischemic priapism (Burnett, 2004). The data from penile blood gas assessments become confusing following interventions. Color Doppler ultrasound should always be considered in the evaluation of a full or partial erection after treatments for ischemic priapism. The differential diagnosis includes resolved ischemia with penile edema, persistent ischemia, and conversion to high-flow state. Chiou and colleagues (2009) have recommended that to accurately categorize presentations as nonischemic or ischemic, careful interpretation of color Doppler ultrasound hemodynamics must be done in conjunction with the clinical assessment. They describe eight patients with priapism following intracavernous injection (duration ≤7 hours), all of whom showed presence of cavernous arterial inflows with varied peak systolic velocities and end-diastolic velocities. They concluded that most patients with priapism following ICI (and duration <7 hours) have a hemodynamic picture of mixed arteriogenic and veno-occlusive priapism. In their series, men with idiopathic ischemic priapism longer than 20 hours showed no detectible cavernous arterial inflows.
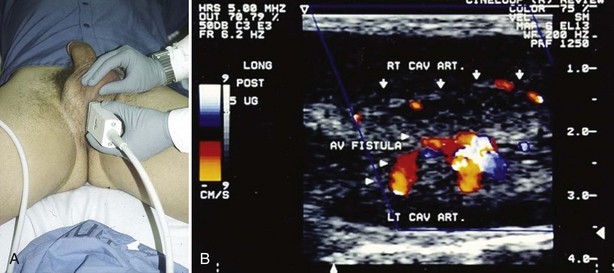
Figure 25–5 A, Examination of the crural bodies is required when searching for arterial sinusoidal fistula following straddle injury. B, Color Doppler image of arterial sinusoidal fistula of left cavernous artery.
There have recently been reports on the use of magnetic resonance imaging in priapism. Kirkam and colleagues (2008) noted that there are three possible roles for magnetic resonance imaging (MRI) to help in the assessment of priapism; the primary role would be in the imaging of a well-established arteriolar-sinusoidal fistula (Kirkham, 2008). The authors acknowledge a limitation of MRI is resolution; MRI cannot demonstrate small vessels as clearly as high-frequency Doppler sonography or angiography. The second would be in ischemic priapism to demonstrate the presence and extent of tissue thrombus and corporal smooth muscle infarction. Ralph (2009) used MRI in the prospective management of 50 patients with refractory ischemic priapism failing medical/surgical management from 24 to 72 hours. Patients underwent MRI to characterize the extent of smooth muscle necrosis before placement of penile prosthesis (Fig. 25–6A and B). The third role for MRI would be in the imaging of corporal metastasis mimicking priapism, or causing true ischemic priapism by obstruction of venous outflow.
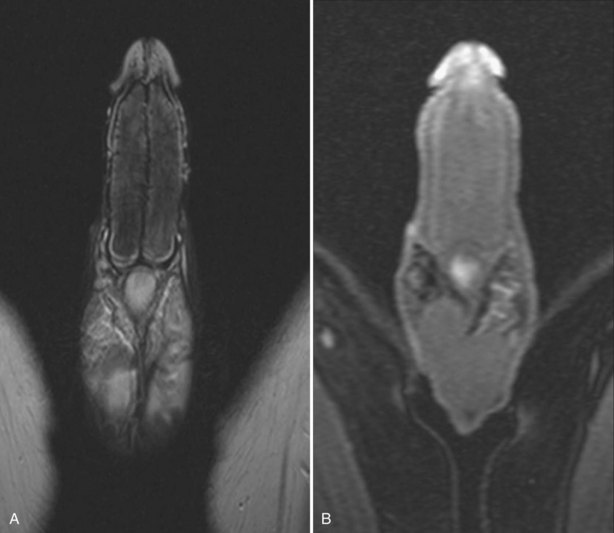
Figure 25–6 A, T2-weighted MRI showing cavernous body thrombosis. B, Same patient. There is no enhancement following gadolinium infusion. At operation, extensive smooth muscle necrosis and thrombus was found. Patient had untreated ischemic priapism lasting several days.
(Courtesy of David Ralph.)
Key Points
Priapism Imaging
Medical Treatments
Ischemic Priapism
Historically, first aid was applied by the patient or recommended by a health practitioner unfamiliar with the hemodynamics of priapism; these interventions included ejaculation, ice packs, cold baths, and cold water enemas. Each of these remedies was thought to end erection by inducing vasoconstriction. Some historical reports advised voiding and exercise. Oral sympathomimetic drugs (etilefrine, pseudoephedrine, phenylpropanolamine, and terbutaline) have been reported to effectively reverse prolonged erection (<4 hours) initiated by intracavernous injection therapies with efficacies of 28% to 36% (Lowe, 1993). Lowe and Jarow (1993) compared oral terbutaline with pseudoephedrine or placebo in 75 patients with prolonged erection induced by intracavernous injection of Alprostadil; they reported detumescence in 38% of cases following terbutaline, 28% following pseudoephedrine, and 12% following placebo. In a follow-up study Priyadarshi (2004) specifically investigated the efficacy of oral terbutaline in the management of prolonged erection following intracavernous injections (papaverine/chlorproamazine); he administered oral terbutaline 5 mg or placebo to men with persisting erection for more than 2.5 hours. Detumescence was acheieved in 42% and 15% of cases, respectively, treated with terbutaline/placebo. Terbutaline treatment was unsuccessful in 58% of cases; all of those patients responded to intracavernous injection of an α-adrenergic. In this author’s experience, when diagnostic injections result in prolonged erections with duration from 2 to less than 4 hours, aspiration may not be necessary. An ultrafine needle and 1-mL syringe (phenylephrine 200 µg) may be used for single injection of α-adrenergic.
Oral agents are not recommended in the management of acute ischemic priapism (>4 hours). The recommended initial treatment of ischemic priapism is the decompression of the corpora cavernosa by aspiration. Aspiration will immediately soften the erection and relieve pain. Aspiration alone may relieve priapism in 36% of cases. The AUA Guidelines Panel (2003) advised that there were not sufficient data to conclude that aspiration followed by saline intracorporal irrigation was any more effective than aspiration alone (Montague, 2003). Subsequently, Ateyah (2005) reported that a combination of corporal blood aspiration and cold saline irrigation effectively terminated priapism in 66% of cases compared with aspiration alone (24%). Data to support the efficacy of cold saline are limited. Aspiration should be repeated until no more dark blood can be seen coming out from the corpora and fresh bright red blood is obtained. This process leads to a marked decrease in the intracavernous pressure, relieves pain, and resuscitates the corporal environment removing anoxic, acidotic, and hypercarbic blood. A single, large-bore, 19-gauge needle should be inserted at the peno-scrotal junction at 3 or 9 o’clock, to avoid piercing the dorsal neurovascular bundle. The surgeon should compress the penile shaft between the thumb and first digit, just below the 19-gauge needle, aspirating the shaft until it is soft. Leaving the needle in place, the shaft is permitted to refill. Compression is reapplied and aspiration repeated. These maneuvers may need to be serially repeated. Several small, empty syringes should be available (3-mL to 12-mL syringes).
Corporal aspiration, if unsuccessful, should be followed by α-adrenergic injection or irrigation. Aspiration followed by the intracavernous injection of sympathomimetic drugs is recommended by the AUA Guidelines Panel, 2003 (Montague, 2003). Sympathomimetic drugs (phenylephrine, etilephrine, ephedrine, epinephrine, norepinephrine, metaraminol) cause cavernous smooth muscle (CSM) contraction. In the laboratory, normal CSM preparations from humans, rabbits, and rodents show concentration-dependent contractions on exposure to phenylephrine, if the corporal environment is well oxygenated and has a normal pH (Broderick, 1994; Muneer, 2008). Broderick and Harkaway (1994) described time-dependent changes in the corporal environment beginning within 6 hours of persistent erection (Broderick, 1994) in humans. Animal models of ischemic priapism have demonstrated impairment in smooth muscle contraction with progressive acidosis, hypoxia, and glucopenia (Broderick, 1994; Saenz de Tejada, 1997; Munnarriz, 2006; Muneer, 2008). Corpus cavernosum specimens from patients presenting with prolonged priapism show no contractions to high-dose phenylephrine in vitro.
Phenylephrine is a selective α1-adrenergic receptor agonist without β-mediated ionotropic and chronotropic cardiac effects; it is the agent of choice by AUA consensus recommendation (Montague, 2003; Pryor, 2004). There are no comparative trials of sympathomimetics in the management of priapism, nor are there dosage-tolerating studies to report. Case reports vary in efficacy from 43% to 81%, with time-dependent efficacies for each agent reported. Phenylephrine is usually diluted in normal saline with a concentration of 100 to 500 µg/mL and given in 1-mL dosages every 3 to 5 minutes. Dosing should be intermittent over the course of an hour. In this author’s experience phenylephrine can be concentrated as 200 µg/mL in saline and administered intermittently as 0.5 mL to 1.0 mL, every 5 to 10 minutes to a maximum dosage of 1 mg. This will permit up to 10 separate injections of 0.5 mL (100 µg each) or 5 separate injections of 1 mL (200 µg each). Aspirate the penis between successive injections by tightly pinching the shaft at the peno-scrotal junction, just below the site of needle insertion. Aspirate until distal shaft is empty. Then inject phenylephrine, releasing the compression at the peno-scrotal junction, allowing the shaft to refill with fresh blood. Extremes of age and preexisting cardiovascular diseases should be taken into consideration before intracavernous sympathomimetic dosing. Serial monitoring of blood pressure and pulse should be performed during and immediately following intracavernous injection of sympathomimetic drugs. Potential side effects of intracavernous sympathomimetics include headache, dizziness, hypertension, reflex bradycardia, tachycardia, and irregular cardiac rhythms. Davila (2008) reported subarachnoid hemorrhage in a case of SCD ischemic priapism. The patient was a 24-year-old African-American male who reported sudden and severe headache immediately following phenylephrine intracorporal dosing of 500 µg/mL repeated every 3 minutes for a total 4 mL (2000 µg = 2 mg). In 2005 in its Patient Safety News: Caution on Fatal Dosing Errors with Epinephrine (#35), the FDA reported a case of a 16-year-old boy who received 4 mL of undiluted 1:1000 epinephrine solution intracavernously to treat priapism. A 1:1,000,000 solution should have been used, so the boy received a massive overdosage; he arrested and could not be resuscitated (www.fda.gov/psn). Whichever intracavernous sympathomimetic agent is chosen for the management of ischemic priapism, urologists are well advised to consult their pharmacies and develop clear mixing and dosing protocols for safe administration (Fig. 25–7).
SCD and hematologic malignancies are rare but important causes of ischemic priapism. Classically, treatment of SCD-induced ischemic priapism involved analgesics, hydration, oxygen, bicarbonate, and blood transfusion. However, systemic therapy alone is not effective management of SCD priapism (Rogers, 2005). A recent report suggested that blood transfusion may have no effective role in the treatment of sickle cell-induced priapism (Merritt, 2006). Reports from hematology centers suggest high success rates using penile aspiration/injection/irrigation of intracavernous sympathomimetics for SCD priapism (Mantadakis, 2000). Mantadakis and colleagues (2000) conducted a prospective trial for the management of children with SCD presenting with prolonged erection, ages 3 to 18 years (no placebo group). For erections lasting longer than 4 hours and less than 12 hours, emergency department interventions were local anesthetic, cavernous aspiration, and irrigation with 10 mL of a 1:1,000,000 solution of epinephrine. If detumescence lasted for 30 minutes, patients were discharged to home. They described 15 patients receiving 39 interventions, of which 37 were successful; 67% required only one aspiration and irrigation treatment. In the management of SCD pediatric patients with stuttering priapism, several levels of escalating intervention are necessary with parental and emergency department staff education comprising the first level. Gbadoe and colleagues (2001) described the treatment of 11 SCD patients (ages 30 months to 15 years) who presented with acute ischemic priapism or stuttering priapism. In their series of cases if the patient presented with priapism lasting less than 6 hours, aspiration and injection of 5 mg of etilefrine was given in the emergency department; for stuttering priapism, patients were given oral etilefrine 0.5 mg/kg nightly for 1 month, or 0.25 mg/kg twice daily. Patients (parents) also administered injections at home to reverse painful erection lasting longer than 1 hour. The authors reported no significant hypertension and only one case of “agitation” attributed to daily dosing.
Key Points
Medical Management of Ischemic Priapism
Stuttering Priapism
Various factors need to be considered when treating stuttering priapism. Although an episode may last less than 4 hours, increasing frequency or duration of stuttering episodes may herald a major ischemic priapism. Multiple frequent visits to the emergency department to resolve the priapism are disruptive to the patient’s life and embarrassing. If attacks follow sexual activity, patients may become sexually avoidant (Adeyoju, 2002; Chow, 2008). Safety and efficacy of various treatments are poorly characterized in the literature. The side effects of recommended medications should be understood by the patient. Patients on chronic medical therapy to decrease the frequency of stuttering episodes may significantly benefit from performing a single sympathomimetic intracorporal injection at home as part of a personal treatment algorithm (Virag, 1996; Teloken, 2005). Multiple treatment options have been described: oral and injectable α-adrenergic agonists, terbutaline, digoxin, antisickling agent hydroxacabamide, estrogens, GnRH analogues, antiandrogens, baclofen, gabapentin, and recently phophodiesterase type 5 inhibitors (Chow, 2008).
Etilefrine is an α agonist available as an oral or injectable treatment in some European countries. Maximum oral dosing is 100 mg in 24 hours (Okpala, 2002). Okpala (2002) followed 18 adults (17 SCD patients and one with sickle trait), all with history of stuttering priapism. Patients were given oral etilefrine in escalating dosages from 25 mg at bedtime to a maximum of 100 mg each day. Stuttering episodes were reduced in frequency and duration in 72%. A small series of six SCD children were followed with dosing twice daily with 0.25 mg/kg of etilefrine (Gbadoe, 2002). The experience of multiple investigators using oral α-adrenergic dosing in the management of SCD stuttering ischemic priapism suggests that oral α-adrenergics at limited daily dosing should be considered in the management of stuttering priapism; drug therapy is typically initiated at bedtime.
Hormonal Therapies
The primary action of systemic hormonal therapy in stuttering priapism is the suppression of the androgenic effects on penile erection. Attempts to treat stuttering priapism with hormones have exploited known regulators of male sexual function by targeting the pituitary gland (GnRH agonists), suppressing pituitary function through feedback inhibition (diethylstilbestrol), blocking androgen receptors (antiandrogens), and reducing testicular and adrenal synthesis (ketoconazole). In the only randomized placebo-controlled trial, a synthetic estrogen, diethylstilbestrol (DES), caused termination of the stuttering episodes in all patients subjected to treatment (Chinegwundoh, 2004). However, in more than 50% of patients (five of nine) priapism recurred after treatment cessation. Similar results are described by others in case reports (Gbadoe, 2002; Shamloul, 2005). Long-term estrogen therapy is not recommended due to the potential cardiovascular side effects. GnRH analogues, goserelin acetate and leuprolide acetate, have been described in case reports (Levine, 1993; Shamloul, 2005). Chronic dosing with GnRH analogues in combination with penile injection of alpha adrenergics as needed have been reported in the management of ischemic stuttering priapism (Steinberg, 1995). Discontinuation of GnRH analogues typically leads to stuttering resumption. Antiandrogens carry their actions by direct suppression of the penile androgen receptors. These agents including flutamide, bicalutamide, and chlormadinone have been able to cause considerable relief of stuttering priapism in a number of case reports; antiandrogens may have benefit to the patients over the GnRH analogues—orally administered patients have reported continued ability to have sexually stimulated erections (Costabile, 1998; Dahm, 2002; Yamashita, 2004). Rachid-Filho and colleagues (2009) have recently described the efficacy of oral finasteride in the management of sickle cell stuttering priapism. They administered finasteride to 35 patients over 120 days in dosages that decreased monthly from 5 mg/day to 3 mg/day and then 1 mg/day in the final month. This was not a controlled trial, but careful observation of stuttering episodes was made; they found at the beginning of treatment the mean episode of stuttering priapism per patient was 22.7, and at the end of 4 months the mean episodes per patient were 2.1. The optimal effects were found at 5- and 3-mg daily dosing. Finasteride is a 5-α-reductase inhibitor approved in the United States for management of symptomatic benign prostatic hyperplasia and male pattern alopecia; it is not approved for use in stuttering ischemic priapism, and this was not a placebo-controlled trial. Six of 35 patients in this study developed painless gynecomastia. Abern and Levine (2009) used nightly dosing with oral ketoconazole and prednisone to suppress nocturnal erections as a preventive strategy for recurrent ischemic priapism; their protocol required titrating dosages and monitoring of nocturnal erections and serum testosterone levels.
Baclofen
Studies in both rats and humans suggest that baclofen inhibits penile erection and ejaculation, through GABA receptor activity. In rats, stimulation of GABAB receptors in the lumbosacral spinal cord inhibits erection (Bitran, 1988; Paredes, 1995; Vaidyanathan, 2004). Denys and colleagues (1998) reported on nine men with multiple sclerosis or spinal cord injuries who were treated for 44 months with intrathecal baclofen for muscle spasticity; eight of nine reported decreased erectile function, which reversed on cessation of baclofen. Rourke and colleagues (2002) first reported on the use of oral nightly baclofen 40 mg in the management of recurrent priapism in patients with neurologic lesions. D’Aleo and colleagues (2009) were the first to report on the use of an intrathecal pump dosing baclofen 180 µg daily for the management of skeletal muscle spasm and recurrent priapism in a patient with spinal cord injury; the patient was refractory to treatment with oral dosing of 75 mg/day but responded to a test dosage of 25 µg intrathecally. The neurologic literature generally fails to categorize these erectile events as ischemic or nonischemic. Triggering events may be tactile nonsexual stimulation causing repeated reflexogenic erections. Better characterization of these unwanted reflexogenic erections is necessary to appreciate hemodynamics, inciting events, duration, and impact on erectile function. In non-neurogenic patients baclofen daily dosing is associated with drowsiness, nausea, complaints of fatigue, and ED. Recurrent reflexogenic erections are clearly an unwanted condition associated with muscle spasticity in men with spinal cord lesions and neurologic disease, but it remains to be demonstrated that the duration and hemodynamics of such erectile events are similar to ischemic stuttering priapism in SCD.
Phophodiesterase Type 5 Inhibitors in the Management of Stuttering Priapism: a Counter-Intuitive Treatment Strategy
Bialecki and Bridges (2002) first reported on sildenafil having a paradoxical effect in controlling stuttering priapism in three patients with SCD (Bialecki, 2002). Although this proposal would immediately seem illogical on the basis of the understanding that PDE5 inhibitors exert erectogenic effects, there is a scientific basis for using these agents to treat priapism.
In a small case series, Burnett and colleagues have shown that daily sildenafil or tadalafil therapy reduces ischemic priapism episodes in men with stuttering priapism (Burnett, 2006). When used in long-term dosing regimens unassociated with erection stimulatory conditions, PDE5 inhibitor therapy alleviates recurrent priapism episodes in men with SCD-associated priapism without affecting normal erectile capacity (Burnett, 2006; Bivalacqua, 2009). The working theory is that surges of cGMP go unchecked because of down-regulated levels of PDE type 5; this results in stimuli like nocturnal erection, which causes unchecked corporal smooth muscle relaxation. In initial series, the short-acting PDE5 inhibitor sildenafil citrate was given in a dose of 25 mg oral daily, with escalation to 50 mg daily. Subsequently these investigators have reported on tadalafil at a dose of 5 or 10 mg oral dosage taken three times weekly. Multicenter, randomized, double-blind, placebo-controlled clinical trials are under way. PDE5 Inhibitors should be started under conditions of complete penile flaccidity, not during a stuttering episode. Efficacy is seen after a week or more of dosing.
Key Points
Medical Management of Stuttering Priapism
Surgical Management of Ischemic Priapism
Surgical management of ischemic priapism is indicated after repeated penile aspirations and injections of sympathomimetics have failed or if such an attempt has resulted in a significant cardiovascular side effect. At present there is a paucity of data regarding the timing of surgical intervention following initiation of medical treatment, although the 2004 International Consultation on Sexual Medicine in Paris recommended corporal aspiration and α-adrenergic agonists for at least 1 hour before consideration of surgery (Pryor, 2004). Early surgical intervention may be preferable in patients with malignant or poorly controlled hypertension or for men who are using monoamine oxidase inhibitor medications contraindicating α-adrenergic therapies. A comprehensive discussion and documentation that includes baseline erectile function, duration of priapism, risks and benefits of the surgery, and ED should be held with the patient/guardian and an informed consent form signed by the patient/guardian.
Shunting
It is generally accepted that the longer an episode of ischemic priapism lasts, the greater the likelihood of compromised erectile function will be in the future. Previous reviews have concluded that priapism lasting longer than 24 hours was associated with a 90% ED rate (Pryor, 1982). Kulmala and colleagues (1995) reported 92% erectile function preservation among patients with ischemic priapism reversed in less than 24 hours, but only 22% preservation of erectile function among men with priapism lasting longer than 7 days. Recommendations based on well-documented erectile function outcomes are few. One recent study does document erectile function outcomes by contemporary standards (International Index of Erectile Function). Bennett and Mulhall (2008) carefully documented 39 cases of SCD priapism presenting to their emergency department over 8 years; men were routinely interviewed for erectile function status within 4 weeks of priapism/interventions. Of the 39 African-American men followed, 73% acknowledged prior episodes of stuttering; 85% had previously been diagnosed with SCD; and only 5% had been counseled in SCD clinics or were aware that priapism was a complication of SCD. A standard protocol of aspiration and phenylephrine injection was performed; shunting for failure of medical management was performed in 28%. In those patients where priapism was reversed, spontaneous erections (with or without use of sildenafil) were reported in 100% of men when priapism was reversed by 12 hours; 78% when reversed by 12 to 24 hours; and 44% when reversed by 24 to 36 hours. No patient reported spontaneous erections after priapism duration of longer than 36 hours. The International Society for Sexual Medicine Standards Committee (expert opinion) stated that shunting is to be considered for ischemic priapism events lasting 72 hours or less. Consideration should be given to foregoing a shunt in priapism events lasting longer, in particular where cavernous thrombosis is evident and no blood can be aspirated from the corporal bodies (Pryor, 2004; Mulhall, 2006).
The objective of shunt surgery is reoxygenation of the cavernous smooth muscle. The shared principle of shunt procedures is to reestablish corporal inflow by relieving venous outflow obstruction; this requires creation of a fistula between the corpora cavernosa (CC) and glans penis, CC and corpus sponsigosum, or CC and dorsal/saphenous veins. Shunt procedures are subdivided on the basis of anatomic location on the penis (Lue, 2006) (Figs. 25-8 and 25-9).
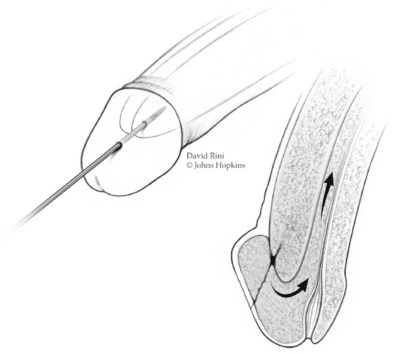
Figure 25–8 Winter shunt. A distal cavernoglanular shunt procedure is depicted by the transglanular placement of a large-bore needle or angiocatheter in the distal glans and corpus cavernosum.
(© Brady Urological Institute.)
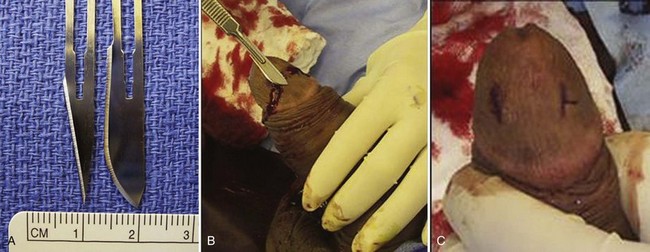
Figure 25–9 A, A No. 11 blade is used for an Ebbehoj percutaneous cavernoglanular shunt, and a No. 10 blade is used for a T shunt. B and C, Note the differences between the Ebbehoj and T shunts. In the Ebbehoj technique the No. 11 blade leaves a straight incision into the glans and corporus cavernosum. In the creation of a T shunt the No. 10 blade is rotated 90 degrees after insertion and withdrawn. In both the percutaneous techniques deoxygenated blood is milked out of the open wounds; once bright red blood is seen, the skin is closed, leaving the deeper incision as the open fistula. In either procedure the maneuver may be repeated on the opposite corpora.
(courtesy of Tom Lue.)
A distal cavernoglanular shunt should be the first choice of shunting procedures because it is technically easier to perform than proximal shunting. Percutaneous distal shunting is less invasive than open distal shunting and can be performed with local anesthetic in the emergency department. The most recently described distal shunt (Brant, 2009) creates a T-shaped shunt between the CC and glans penis. Brant (2009) describes 13 men with priapism durations longer than 24 hours (6 of 13 had failed other distal or proximal shunt procedures). All T shunts were performed following penile anesthetic block; 12 of 13 patients were successfully reversed by initial intervention. In T shunting a No. 10 blade is placed vertically through the glans 4 mm away from the meatus; the blade pierces through the glans to CC and is rotated 90 degrees away from the urethra and removed. Deoxygenated blood is milked out of the wound. The glans is then sutured with absorbable suture. The authors recommend discharge home if the penis remains flaccid for 15 minutes. If erection returns or persists, a second T shunt is recommended on the opposite side of the meatus. In those cases where ischemic priapism has been present for more than 36 hours, the authors recommend immediate bilateral T shunts with passage of 20-Fr dilators into the fistula tract and well into the CC down to the crus. This technique is more traumatic and will require anesthetic. Burnett and Perorazio (2009) have described a similar technique to resolve ischemic priapism refractory to first-line interventions. Their procedure known as the “corporal snake” is a modification of the Al-Ghorab corporoglanular shunt (Fig. 25–10). Under general anesthetic, a 2-cm transverse incision is made on the glans; the distal tips of the rigid CC are incised and grasped with 2-0 stay sutures or Kocher clamps. Deoxygenated blood is milked out of the CC, but rather than excising a wedge of tunica and underlying CC muscle, a 7/8 Hegar dilator is advanced through each of the tunica windows proximally several centimeters to release blood and thrombus. The penis is made flaccid by repeated manual compression and release; the glans skin is then approximated with 4-0 chromic sutures; urethral catheter is placed and lightly compressive dressing is applied to the genitalia.
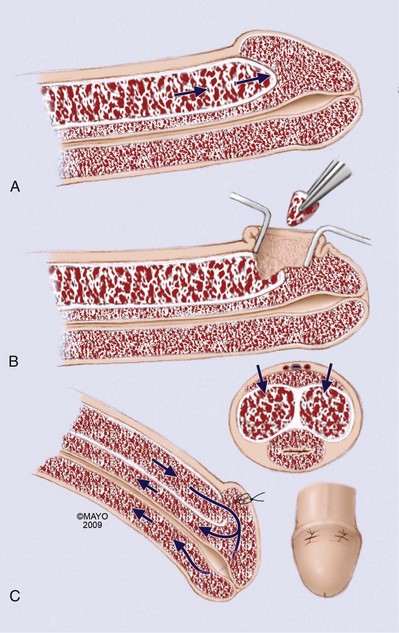
Figure 25–10 A-C, An open corporoglanular shunt is indicated if percutaneous shunting fails to reestablish cavernous blood inflow. The Al-Ghorab shunt requires the excision of circular cone segments of the distal tunica albuginea (5 × 5 mm).
(By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
The key factors determining successful surgical reversal of ischemic priapism are evacuation of thrombus, reestablishing cavernous inflow, and patency of shunt. Theoretically, larger open shunt procedures are likely to result in higher shunt patency rates; there are no data comparing percutaneous and open distal shunts. The surgeon must be guided by familiarity with various techniques: percutaneous shunting, open distal shunting, proximal shunting, and vein shunting. Although distal shunting can be performed with penile block and sedation in the emergency department, open shunting, especially that requiring passage of dilators into the CC, will likely require general anesthesia and an operating room suite. At the completion of the shunt, patency can be verified in the operating room and subsequently recovery room in a number of ways: bright oxygenated blood should be seen emanating from the corporal bodies; intracavernous pressures should fall; the penis should detumesce and refill with sequential compression and release; and color Doppler ultrasound will show resumption of cavernous artery inflow (Lue, 2002; Nixon, 2003; Chiou, 2009) (Table 25–5). Complications of shunting include penile edema, hematoma, infection, urethral fistula, penile necrosis, and pulmonary embolism.
Table 25–5 Assessing Corpora Cavernosa Shunt Patency
The most commonly described proximal shunt is the unilateral shunt, described by Quackles in 1964 (Fig. 25–11). Proximal corpus cavernosum to spongiosum (CC-CS) shunt procedures require a transscrotal or transperineal approach (Quackles, 1964). There are no data comparing bilateral (Sacher, 1972) and unilateral CC-CS shunts (Quackles, 1964). Typically, bilateral shunts are staggered; the right side and left side are separated by a distance of at least 1 cm in an effort to minimize the risk of urethral stricture at the point of CC-CS communication (Fig. 25–12). In cases where proximal shunt fails, some have advocated saphenous vein bypass or deep dorsal vein shunt (Fig. 25–13A and B). A wedge of tunica albuginea is removed and the vein is anastamosed end to side of CC. There are no comparative trials of vein shunting for ischemic priapism. Authors have described a significant risk of sapheno-femoral vein thrombus and pulmonary embolism with vein shunting (Kandel, 1968).
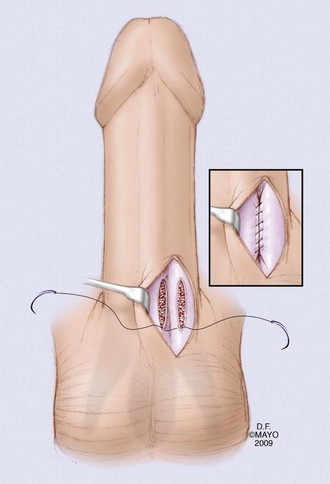
Figure 25–11 The proximal open shunt technique to establish communication between the corpus spongiosum and corpus cavernosum was first described by Quackles in 1964.
(By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
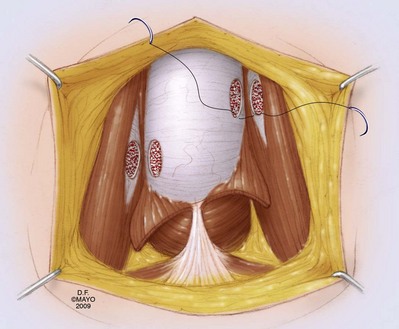
Figure 25–12 Bilateral shunts are staggered. The right and left sides are separated by a distance of at least 1 cm in an effort to minimize the risk of urethral stricture at the point of corpus cavernosum to spongiosum communication (Sacher, 1972).
(By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
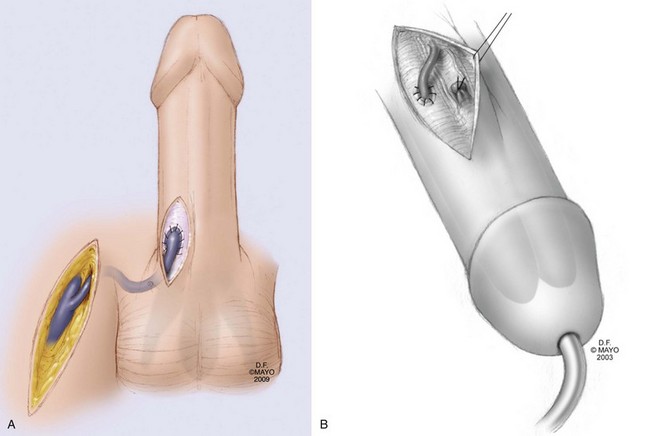
Figure 25–13 A, Venous bypass to control ischemic priapism was first described by Grayhack in 1964. The Grayhack shunt mobilizes the saphenous vein below the junction of the femoral vein and anastamoses the vein end to side into the corpus cavernosum. B, Deep dorsal vein (DDV) shunt with distal ligation of DDV and anastamosis of proximal DDV to corpus cavernosum. A wedge of tunica albuginea is removed.
(By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
Key Points
Surgical Management of Ischemic Priapism
Immediate Implantation of Penile Prosthesis
Unfortunately the natural history of untreated ischemic priapism or priapism refractory to interventions is severe fibrosis, penile length loss, and complete ED (see Fig. 25–1). Kelami (1985) described the implantaion of the Small-Carrion penile prosthesis through an infrapubic incision in the management of postpriapic erectile dysfunction. Bertram and colleagues (1985) described six postpriapic cases of penile prosthesis; five of six men had successful implantation of semirigid prostheses. Both groups described extensive corporal fibrosis and suggested that semirigid implants were preferable because inflatable implants would not overcome the corporal fibrosis sufficiently to erect the penis. Douglas and colleagues (1990) reported on penile prosthesis in five SCD postpriapic men; they described a surgical technique of tunneling and corporal excavation. Inadvertent damage to the tunica albuginea was common, as was subsequent migration of hardware; 11 additional procedures were required following the initial implants. The average time from priapism to implant in Douglas’s series was 4 years. Monga and colleagues (1996) described implants in young SCD patients (six patients, average age 26); inflatable implants were placed to both treat ED and circumvent ongoing episodes of stuttering priapism. They suggested that both potency and recurrent episodes of ischemic priapism could be managed by “early” implantation. Some have suggested performing immediate penile prosthesis in the acute management of ischemic priapism, when the patient has failed sympathomimetic intracavenous therapies and shunting (Rees et al, 2002). There are two distinct advantages to immediate implantation: corporal fibrosis is not yet established, and penile length may be preserved. The exact time point at which prosthetic insertion becomes a reasonable option for managing ischemic priapism is unclear. Should medical management of ischemic priapism be followed by distal percutaneous shunt, by open distal shunt, and subsequently by proximal shunting before penile implant? Should men with delayed presentation of ischemic priapism and evident corporal thrombus be triaged to immediate penile implant? What is clear is that any discussion pertaining to early prosthesis insertion should be documented and include a comprehensive review of the theoretic advantages and actual risks. Compared with prosthesis insertion in a typical patient with erectile dysfunction, there are significantly higher rates of complications noted in priapism cases: infection, urethral injury, device migration, device erosion, and revision surgeries. The surgeon must be familiar with the additional technical concerns posed by weaknesses in the tunica albuginea in the region of prior shunts.
The advantages of early penile implantation in the acute management of ischemic priapism are preservation of penile length and technically easier implant insertion. Delayed placement of penile prosthesis is technically challenging due to corporal fibrosis. Ralph (2009) reported on 50 patients with ischemic priapism. All patients failed conservative management with the instillation of α-adrenergic agents (200 µg phenylephrine repeated to a maximum dosage of 1500 µg). Unsuccessful shunts were performed in 13 of 50 cases (Ralph, 2009). Mean duration of priapism was 209 hours (range 24 to 72 hours). All patients had evidence of cavernous thrombus and smooth muscle necrosis on MRI and all 50 underwent insertion of penile prosthesis in the acute setting of refractory ischemic priapism. Revision rates were significantly high 24% (12 of 50 patients). The infection rate of 6% was also notably high and likely related to multiple factors including ischemic tissues and preceding penile interventions.
Key Points
Surgical Management of Ischemic Priapism with Immediate Penile Implant
Interventional Angiography in the Management of Arterial (Nonischemic, High-Flow) Priapism
Arterial priapism is not an emergency. Spontaneous resolution or response to conservative therapy has been reported in up to 62% of published series (Montague et al, 2003; Pryor et al, 2004). Persistent partial erection from HFP may be evident for months to years, without adverse impact on erectile function (Bastuba et al, 1995). Kumar et al (2006) described a case of HFP in a 24-year-old patient 10 days after straddle injury on a bicycle. The patient had no erection for the first 4 days following injury. Examination revealed a tumescent penis that was compressible. Penile aspiration and blood gas analysis revealed oxygenated corporal blood. Color Doppler ultrasound of the cavernous artery revealed arterio-sinusoidal fistula. Partial erection spontaneously resolved 4 days after diagnostic evaluation, with the patient reporting normal erections 2 weeks later. The authors hypothesized that in cases of blunt penile and perineal trauma an arterio-lacunar fistula forms; these fistulae, unlike arteriovenous communications, may spontaneously resolve as the less rigid walls of the lacunae are prone to spontaneous thrombosis.
There are no comparative outcome studies of intervention versus conservative management in high-flow priapism; there are sufficient case descriptions, especially in children, to recommend initial watchful waiting (Nehra, 2006). Initial observation is recommended for this type of priapism. Conservative measures include ice applied to the perineum and site-specific compression. Cavernous aspiration has only a diagnostic role in high-flow priapism. Repeated aspirations, injection, and irrigation with intracavernous sympathomimetics have no role in the treatment of nonischemic priapism.
Patients demanding immediate relief can be offered selective arterial embolization. The pathognomonic arteriographic finding is an arterial-lacunar fistula; a characteristic intracavernosal cone-shaped blush of contrast is seen at the site of the cavernous artery or arteriole laceration (Fig. 25–14). Selective internal pudendal catheterization and subsequent embolization have been reported with various agents: microcoils, polyvinyl alcohol, N-butylcyanoacrylate, gel-foam, and autologous blood clot (Kuefer, 2005). Permanent materials pose a greater theoretic risk of erectile dysfunction; many authors recommend use of autologous blood clot and absorbable gels (Pryor, 2004; Kim, 2007). Autologous blood clot has a low risk of foreign body reaction, or antigenicity; it is a temporary occlusive agent and should permit recanalization of the cavernous artery (Park, 2001). The success rates with selective pudendal artery catherterization followed by embolization are quite high (89% to 100%), regardless of the embolization material used (Kuefer, 2005; Numan, 2008). Similar results have been reported by others (Savoca, 2004; Alexander, 2006). Normal postembolization erectile function has been reported in 75% to 86% of patients (Cakan, 2006; Numan, 2008). It should be noted that a single treatment of embolization carries a recurrence rate of 30% to 40% (Ciampalani, 2002; Gandini, 2004; Ozturk, 2009). Although ultimately successful, embolization of HFP may require retreatment. The most notable side effect of arterial embolization is erectile dysfunction. Although it was previously reported that nonpermanent embolization materials cause less ED than permanent ones (5% vs. 39%), recent reports employing International Index of Erectile Function in evaluation of postembolization erectile function describe similar rates of erectile dysfunction, 15% and 20% (Savoca, 2004; Alexander, 2006). Other reported adverse effects include penile gangrene, gluteal ischemia, purulent cavernositis, and abscess of the perineum (Hakim, 1996; Sandock, 1996) (Fig. 25–15).
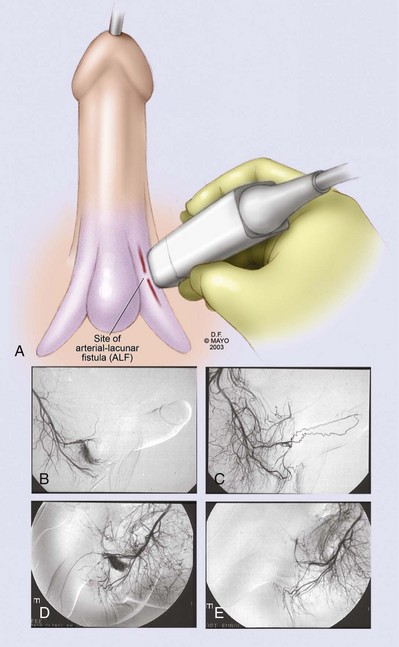
Figure 25–14 Color Doppler ultrasonography of the penis and perineum is recommended in the evaluation of priapism, when the history or examination suggests penile trauma (A). Doppler sonography for localization of a fistula correlates well with selective pudendal angiography (B, C, D, E); a characteristic fistula blush is shown (B, D), along with normal arteriograms (C, E).
(A, By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
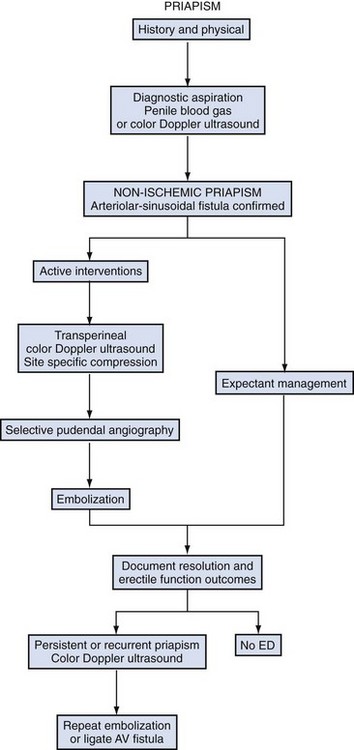
Figure 25–15 Algorithm for managing high-flow priapism. AV, arteriovenous; ED, erectile dysfunction.
Puppo (1985) compared perineal duplex ultrasound and selective internal pudendal arteriography showing excellent sensitivity in detecting the arterial-lacunar fistula on ultrasound that is seen angiographically (12 of 12 cases). Several reports describe combined ultrasound-guided compression with selective arterial embolization to increase success rates in the treatment of nonischemic priapism (Hatzichristou, 2002; Bartsch, 2004; Cakan, 2006). If the follow-up clinical examination is equivocal for recurrence of high-flow priapism, a perineal duplex Doppler ultrasound can determine the need for repeat arteriography and embolization (Kim, 2007).
Surgical Management of Arterial (Nonischemic, High-Flow) Priapism
Arterial priapism is not a urologic emergency. HFP is painless and there have been reports of partial erection persisting for years (Nehra, 2006). Any intervention must follow a comprehensive discussion with the patient regarding risks and benefits of any of the procedures advocated by the clinician. In cases of long-standing arterial priapism where a pseudocapsule around the fistula has developed, surgical ligation has been reported to be successful. Formation of a pseudocapsule may take weeks to months following trauma. Corporal exploration before the formation of a pseudocapsule may result in ligation of cavernous artery rather than selective ligation of the fistula. Currently this intervention is reserved for patients who do not wish to pursue expectant management or who are poor candidates for angio-embolization. It is also reserved for patients who refuse the procedure; for patients in places where technology is not available; and for patients whose angio-embolization failed (Ji, 1994; Berger, 2001; Mulhall, 2006). The surgical approach is transcorporal. Intraoperative Doppler ultrasound guidance is recommended.
Key Points
Evaluation and Management of High-Flow Priapism
Conclusions and Recommendations for Research in Priapism
Prompt diagnosis and appropriate management of priapism are necessary to spare patients ineffective interventions and optimize erectile function outcomes. Future research is necessary to understand each type of priapism: (a) corporal smooth muscle pathology associated with genetic and acquired conditions that result in ischemic priapism; (b) the molecular mechanisms involved in the pathogenesis of stuttering ischemic priapism; (c) documentation of onset of HFP in relation to time of injury and response to conservative management versus angiographic or surgical interventions. Future research should include animal models to study the physiology of ischemic priapism. These models should examine the clinical observation that interventions for ischemic priapism may result in a phenomenon of conversion to high flow. Specific questions need to be addressed: Is cavernous arterial dysregulation a normal postischemic response? When does postischemic high flow resolve? Sickle cell patients should receive education on priapism and its management. Recent studies suggest that only 5% of SCD patients recalled learning that priapism was a complication of SCD. Urologists preparing case reports should document prepriapism erectile function, duration of priapism with respect to initiation of each intervention, history of stuttering priapism, and recovery of erectile function using standardized questionnaires. Documenting erectile function outcomes on the basis of duration of ischemic priapism, time to interventions, and types of interventions will establish evidence-based guidance on how and when to apply those interventions.
Aliyu ZY, Kato GJ, Taylor J, et al. Sickle cell disease and pulmonary hypertension in Africa: a global perspective and review of epidemiology, pathophysiology, and management. Am J Hematol. 2008;83:63-70.
Bastuba MD, de Tejada IS, Dinlenc CZ, Sarazen A, Krane RJ, Goldstein I. Arterial priapism: diagnosis, treatment and long-term follow up. J Urol. 1995;151:1231-1237.
Bennett N, Mulhall J. Sickle cell disease status and outcomes of African American men presenting with priapism. J Sex Med. 2008;5(5):1244-1250.
Bivalacqua TJ, Champion HC, Mason W, et al. Long-term phosphodiesterase type 5 inhibitor therapy reduces priapic activity in transgenic sickle cell mice. J Urol. 2006;175:387.
Brant WO, Garcia MM, Bella AJ, et al. T-shaped shunt and intracavernous tunneling for prolonged ischemic priapism. J Urol. 2009;181:1699-1705.
Broderick GA, Gordon D, Hypolite J, Levin RM. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
Broderick GA, Harkaway R. Pharmacologic erection: time-dependent changes in the corporal environment. Int J Impot Res. 1994;6:9-16.
Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Feasibility of the use of phosphodiesterase type 5 inhibitors in a pharmacologic prevention program for recurrent priapism. J Sex Med. 2006;3:1077-1084.
Chan PTK, Begin LR, Arnold D, et al. Priapism secondary to penile metastasis: a report of two cases and a review of the literature. J Surg Oncol. 1998;68(1):51-59.
Chiou RK, Aggarwal H, Chiou C, et al. Colour Doppler ultrasound hemodynamic characteristics of patient with priapism before and after therapeutic interventions. Can Urol Assoc J. 2009;3(4):304-311.
Montague DK, Jarow J, Broderick GA, et al. American Urological Association guideline on the management of priapism. J Urol. 2003;170:1318-1324.
Ozturk MH, Gumus M, Donme H, et al. Materials in embolotherapy of high-flow priapism: results and long-term follow-up. Diagn Interv Radiol. 2009;15(3):215-220.
Ralph DJ, Garaffa G, Muneer A, et al. The immediate insertion of a penile prosthesis for acute ischaemic priapism. Eur Urol. 2009;56:1033-1038.
Seftel A, Haas CA, Brown SL, et al. High flow priapism complicating veno-occlusive priapism: pathophysiology of recurrent idiopathic priapism?. J Urol, 159, 1998, 1300-1301.
Abern MR, Levine LA. Ketoconazole and prednisone to prevent recurrent ischemic priapism. J Urol. 2009;182(4):1401-1406.
Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int. 2002;90(9):898-902.
Alexander Tønseth K, Egge T, et al. Evaluation of patients after treatment of arterial priapism with selective micro-embolization. Scand J Urol Nephrol. 2006;40:49-52.
Aliyu ZY, Kato GJ, Taylor J, et al. Sickle cell disease and pulmonary hypertension in Africa: a global perspective and review of epidemiology, pathophysiology, and management. Am J Hematol. 2008;83:63-70.
Andrade E, Villanova F, Borra P, et al. Penile erection induced in vivo by a purifed toxin from the Brazilian spider Phoneutria nigriventer. BJU Int. 2008;120(7):835-837.
Amlie RN, Borgeois B, Huxtable R. Priapism in preterm infant. Urol. 1977;9:558-559.
Aoyagi T, Hayakawa K, Miyaji K, et al. Sildenafil induced priapism. Bull Tokyo Dent Coll. 1999;40(4):215-217.
Ateyah A, Rahman El-Nashar A, Zohdy W, et al. Intracavernosal irrigation by cold saline as a simple method of treating iatrogenic prolonged erection. J Sex Med. 2005;2:248-253.
Barry JM. Priapism. Treatment with corpus cavernosum to dorsal vein of penis shunts. J Urol. 1976;116:754-756.
Bartsch GJr, Kuefer R, Engel O, Volkmer BG. High-flow priapism: colour-Doppler ultrasound-guided supraselective embolization therapy. World. J Urol. 2004;22:368-370.
Bastuba MD, de Tejada IS, Dinlenc CZ, et al. Arterial priapism: diagnosis, treatment and long-term follow up. J Urol. 1995;151:1231-1237.
Bennett N, Mulhall J. Sickle cell disease status and outcomes of African American men presenting with priapism. J Sex Med. 2008;5(5):1244-1250.
Berger R, Billups K, Brock G, et al. AFUD Thought Leader Panel on Evaluation and Treatment of Priapism. Report of the American Foundation for Urologic Disease (AFUD) Thought Leader Panel for evaluation and treatment of priapism. Int J Impot Res. 2001 De;13(Suppl 5):S39-S43.
Bertolotto M, Ciampalini S, Martingano P, Mucelli FP. High flow priapism complicating ischemic priapism following iatrogenic laceration of the dorsal artery during a Winter procedure. J Clin Ultrasound. 2009;37(1):61-64.
Bertram RA, Carson CC, Webster GD. Implantation of penile prostheses in patient impotent after priapism. Urology. 1985;26(4):325-327.
Beuzard Y. Transgenic mouse models of sickle cell disease. Curr Opin Hematol. 1996;3:150-155.
Bialecki ES, Bridges KR. Sildenafil relieves priapism in patients with sickle cell disease. Am J Med. 2002;113(3):252.
Birnbaum BF, Pinzone JJ. Sickle cell trait and priapism: a case report and review of the literature. Cases J. 2008;1:429.
Bitran D, Miller SA, McQuade DB, et al. Inhibition of sexual reflexes by lumbosacral injection of a GABA-B agonist in the male rat. Pharmacol Biochem Beha. 1988;31:657-666.
Bivalacqua TJ, Champion HC, Mason W, et al. Long-term phosphodiesterase type 5 inhibitor therapy reduces priapic activity in transgenic sickle cell mice. J Urol. 2006;175:387.
Bivalacqua TJ, Diner EK, Novak TE, et al. A rat model of Peyronie’s disease associated with a decrease in erectile activity and an increase in inducible nitric oxide synthase protein expression. J Urol. 2000;163:1992-1998.
Bivalacqua TJ, Liu T, Musicki B, et al. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51:1732-1740.
Bivalacqua TJ, Musicki B, Champion HC, Burnett AL. Phosphodiesterase type 5 inhibitor therapy for priapism. In: Carson CC, Kirby RS, Goldstein I, Wyllie MG, editors. Textbook of erectile dysfunction. 2nd ed. London: Informa Healthcare; 2009:428-433.
Bivalacqua TJ, Musicki B, Hsu LL, et al. Establishment of a transgenic sickle cell mouse model to study the pathophysiology of priapism. J Sex Med. 2009;6:2494-2504.
Borrelli M, Mitre AI, Alfer Júnior W, et al. [Surgical treatment of priapism using Al-Ghorab’s technic.]. Rev Paul Med. 1983;101:27. [in Portugese]
Brant WO, Garcia MM, Bella AJ, et al. T-shaped shunt and intracavernous tunneling for prolonged ischemic priapism. J Urol. 2009;181:1699-1705.
Brock G, Breza J, Lue TF, Tanagho EA. High flow priapism: a spectrum of disease. J Urol. 1993;150(160):969-971.
Broderick GA, Gordon D, Hypolite J, Levin RM. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
Broderick GA, Harkaway R. Pharmacologic erection: time-dependent changes in the corporal environment. Int J Impot Res. 1994;6:9-16.
Broderick GA, Lue TF. Evaluation and nonsurgical management of erectile dysfunction and priapism. In: Walsh PC, Retik AB, Vaughan ED, et al, editors. Campbell’s urology. Philadelphia: WB Saunders; 2002:1619-1671.
Bschieipfer TH, Hauck EW, Diemer TH, et al. Heparin-induced priapism. Int J Impot Res. 2001;13:357-359.
Burgu B, Talas H, Erdeve O, et al. Approach to newborn priapism: a rare entity. J Ped Urol. 2007;3:509-511.
Burnett AL. Therapy insight: priapism associated with hematologic dyscrasias. Nat Clin Pract Urol. 2005;2(9):449-456.
Burnett AL, Pierorazio P. Corporal “snake” maneuver: corporoglanular shunt surgical modification for ischemic priapism. J Sex Med. 2009;6:1171-1176.
Burnett AL. Priapism. In: Walsh PC, Retik AB, Vaughan ED, et al, editors. Campbell’s urology. Philadelphia: WB Saunders; 2004:839-849.
Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34:631-642.
Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Feasibility of the use of phosphodiesterase type 5 inhibitors in a pharmacologic prevention program for recurrent priapism. J Sex Med. 2006;3:1077-1084.
Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Long-term oral phosphodiesterase 5 inhibitor therapy alleviates recurrent priapism. Urology. 2006;67:1043-1048.
Burt FB, Schimer HK, Scott WW. A new concept in the management of priapism. J Urol. 1960;83:60-61.
Cakan M, Altu Gcaron U, Ademir M. Is the combination of superselective transcather autologous clot embolization and duplex sonography-guided compression therapy useful treatment option for the patients with high-flow priapism? Int J Impot Res. 2006;18:141-145.
Celma-Domenech A, Planas-Morin J, de Tores-Ramirez I, et al. Priapism secondary to contiguity bladder carcinoma infiltration of the wpenis. Actas Urol Esp. 2008;32(7):749-751.
Champion HC, Bivalacqua TF, Takimoto E, et al. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661-1666.
Chan PTK, Begin LR, Arnold D, et al. Priapism secondary to penile metastasis: a report of two cases and a review of the literature. J Surg Oncol. 1998;68(1):51-59.
Chang MW, Tang CC, Chang SS. Priapism: a rare presentation in chronic myeloid leukemia. Chang Gun Med J. 2003;26:288-292.
Chinegwundoh FI, Anie KA. Treatments for priapism in boys and men with sickle cell disease. Cochrane Database Syst Rev 2004;(4):CD004198.
Chiou RK, Aggarwal H, Chiou C, et al. Colour Doppler ultrasound hemodynamic characteristics of patient with priapism before and after therapeutic interventions. Can Urol Assoc J. 2009;3(4):304-311.
Chiou RK, Aggarawal H, Mues AC, et al. Clinical experience and sexual function outcome of patients with priapism treated with penile cavernosal-dorsal vein shunt using saphenous vein graft. Urology. 2009;73:556-561.
Chow K, Payne S. The pharmacological management of intermittent priapismic states. BJU Int. 2008;102:1515-1521.
Ciampalani S, Savoca G, Buttazi L, et al. High-flow priapism treatment and follow up. Urology. 2002;59:110-113.
Costabile RA. Successful treatment of stutter priapism with an antiandrogen. Tech Urol. 1998;4:167-168.
Cruz Guerra NA, Pozo Mengual B, Perales Cespedes MP, et al. Metachronous recurrence of idiopathic high flow priapism. Arch Esp Urol. 2004;57(1):82-84.
Dahm P, Rao DS, Donatucci CF. Antiandrogens in the treatment of priapism. Urology. 2002;59:138.
D’Aleo G, Rifici C, Kofler M, et al. Favorable response to intrathecal, but not oral, baclofen of priapism in a patient with spinal cord injury. Spine. 2009;34(3):E127-E129.
Davila HH, Parker J, Webster JC, et al. Subarachnoid hemorrhage as complication of phenylephrine injection for the treatment of ischemic priapism in sickle cell disease patient. J Sex Med. 2008;5:1025-1028.
De Jesus LE, Dekermacher S. Priapism in children: review of pathophysiology and treatment. J Pediatr (Rio J). 2009;85(3):194-200.
Denys P, Mane M, Azouvi P, et al. Side effects of chronic intrathecal baclofen on erection and ejaculation in patients with spinal cord lesions. Arch Phys Med Rehab. 1998;79(5):494-496.
Douglas L, Fletcher H, Serjeant GR. Penile prostheses in the management of impotence in sickle cell disease. Br J Urol. 1990;65(5):533-535.
Dubocq FM, Tefilli MV, Grignon DJ, et al. High flow malignant priapism with isolated metastasis to the corpora cavernosa. Urology. 1998;51:324-326.
Earle CM, Stuckey BG, Ching HL, Wisnewski ZS. The incidence and management of priapism in western Australia. Int J Imp Res. 2003;15(4):272-276.
Ebbehoj J. A new operation for priapism. Scand J Plast Reconstr Surg. 1974;8:241-242.
Eland IA, van der Lei J, Stricker BH, Sturkenboom MJ. Incidence of priapism in the general population. Urol. 2001;57(5):970-972.
Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Inter Med. 1980;140:1434-1437.
Fassbinder W, Frei U, Issantier R, et al. Factors predisposing to priapism in heamodialysis patient. Proc Eur Dial Transplant Assoc. 1976;12:380-386.
Galatti L, Fioraavanti A, Salvo F, et al. Interaction between tadalafil and itraconazole. Ann Pharmacother. 2005;39:200.
Gandini A, Spinelli A, Konda D, et al. Superselective emboliation in posttraumatic priapism with glubran 2 acrylic glue. Cardiovasc Intervent Radiol. 2004;27:544-548.
Gbadoe AD, Assimadi JK, Segbena YA. Short period of administration of diethylstilbestrol in stuttering priapism in sickle cell anemia. Am J Hematol. 2002;69:297-298.
Gbadoe AD, Atakouma Y, Kusiaku K, et al. Management of sickle cell priapism with etilefrine. Arch Dis Chil. 2001;85:52-53.
Goldmeier D. Prolonged erection produced by dihydrocodeine and sildenafil. BMJ. 2002;524:1555.
Grayhack JT, McCullough W, O’Connor VJ, Trippel O. Venous bypass to control priapism. Investig Urol. 1964;1:509-513.
Guvel S, Kilinc F, Torun D, et al. Malignant priapism secondary to bladder cancer. J Androl. 2003;24(4):499-500.
Hakim LS, Kulaksuzoglu H, Mulligan S, et al. Evolving concepts in the diagnosis and treatment of arterial high-flow priapism. J Urol. 1996;155:541-545.
Hanafy HM, Saad SM, El-Rifaie M, Al-Ghorab MM. Early Arabian medicine: contribution to urology. Urology. 1976;8(1):63-67.
Hatzichristou D, Salpiggidis G, Hatzimouratidis K, et al. Management strategy for arterial priapism: therapeutic dilemmas. J Urol. 2002;168:2074-2077.
Hauri D, Spycher M, Brühlmann W. Erection and priapism: a new physiopathological concept. Urol Int. 1983;38:138-141.
Hinman F. Priapism: report of case in a clinical study of the literature with reference to its pathogenesis and surgical treatments. Ann Surg. 1914;60:689-716.
Hinman FJr. Priapism: reasons for failure of therapy. J Urol. 1960;83:420-428.
Hoover NG, Fortenberry JD. Use of antivenin to treat priapism after a black widow spider bite. Pediatrics. 2004;114:128-129.
Jeong WI, Do SH, Yun HS, et al. Hypoxia potentiates transforming growth factor-beta expression of hepatocyte during the cirrhotic condition in rat liver. Liver Int. 2004;24:658-668.
Ji MX, He NS, Wang P, Chen G. Use of selective embolization of the bilateral cavernous arteries for post-traumatic arterial priapism. J Urol. 1994;151:1641.
Jin YC, Gam SC, Jung JH, et al. Expression and activity of heme oxygenase-1 in artificially induced low-flow priapism in rat penile tissues. J Sex Med. 2008;5:1876-1882.
Junemann KP, Persson-Junemann C, Alken P. Pathophysiology of erectile dysfunction. Semin Urol. 1990;8(2):80-93.
Kandel GL, Bender LI, Grove JS. Pulmonary embolism a complication of corpus saphenous shunt for priapism. J Urol. 1968;99:196-197.
Kassim AA, Fabry ME, Nagel RL. Acute priapism associated with the use of sildenafil in a patient with sickle cell trait. Letter to the editor. Blood. 2000;95(5):1878-1879.
Kato GJ, Gladwin MT, Steinber MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37-47.
Kato GJ, McGowan VR, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patents with sickle cell disease. Blood. 2006;107:2279-2285.
Kelami A. Implantaton of small-carrion posthesis in the treatment of erectile impotence after priapism: difficulties and effects. Urol Int. 1985;40(6):343-346.
Kim KR, Shin JH, Song HY, et al. Treatment of high-flow priapism with superselective transcatheter embolization in 27 patients: a multicenter study. J Vasc Interv Radiol. 2007;18:1222-1226.
King SH, Hallock M, Strote J, Wessells H. Tadalafil associated priapism. Urology. 2005;66(2):432.
Kirkham A, Illing RO, Minahas S, Allen C. MR imaging of nonmalignant penile lesions. RadioGraphics. 2008;28:837-853.
Kuefer R, Bartsch GJr, Herkommer K, et al. Changing diagnostic and therapeutic concepts in high-flow priapism. Int J Impot Res. 2005;17:109-113.
Kulmala RV, Lehtonen TA, Tammela TL. Priapism its incidence and seasonal distribution in. Finland. Scan J Urol Nephrol. 1995;29(1):93-96.
Kulmala RV, Lehtonen TA, Tammela TL. Preservation of potency after treatment for priapism. Scan J Urol Nephrol. 1995;29(1):93-96.
Kumar R, Jindal L, Seth A. Priapism following oral sildenafil abuse. Natl Med J India. 2005;18(1):49.
Kumar R, Shrivastava DN, Seth A. Spontaneous resolution of delayed onset, posttraumatic high-flow priapism. J Postgrad Med. 2006;52(4):298-299.
Larocque MA, Cosgrove MD. Priapism: a review of 46 cases. J Urol. 1974;112(6):770-772.
Leal J, Walker D, Egan EA. Idiopathic priapism in the newborn. J Urol. 1978;120:376.
Levine LA, Guss SP. Gonadotropin releasing hormone analogues in the treatment of sickle cell anemia-associated priapism. J Urol. 1993;150:475-477.
Liguori G, et al. High flow priapism secondary to Nesbit operation: management by percutaneous embolization and colour Doppler guided compression. Int J Impot Res. 2005;17:304-306.
Lin G, Xin ZC, Lue TF, Lin CS. Up and down-regulation of phosphodiesterase-5 as related to tachyphylaxis and priapism. J Urol. 2003;170:S15-S19.
Lowe FC, Jarow JP. Placebo-controlled study of oral terbutaline in management of prostaglandin E1 induced prolonged erections. J Urol. 1993;42(1):51-53.
Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction and priapism. In: Walsh PC, Retik AB, Vaughan ED, et al, editors. Campbell’s urology. Philadelphia: WB Saunders; 2002:1610-1696.
Lue TF, Pescatori ES. Distal cavernosum-glans shunts for ischemic priapism. J Sex Med. 2006;3:749-752.
Mantadakis E, Ewalt DH, Cavender JD, et al. Outpatient penile aspiration and epinephrine irrigation for young patients with sickle cell anemia and prolonged priapism. Blood. 2000;95:78-82.
Manuel MB, Leak A, Carroll SA. Priapism in the oncology setting. Clin J Onc Nursing. 2007;11(1):23-25.
McMahon CG. High flow priapism due to an arterial-sinusoidal fistula complicating initial veno-occlusive priapism. Int J Impot Res, 14, 2002, 195-196.
McMahon CG. Priapism associated with concurrent use of phosphodiesterase inhibitor drugs and intracavernous injection therapy. Int J Impot Res. 2003;15:383-384.
McVary KT, Roehrborn CG, Kaminetsky JC, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007;177(4):1401-1407.
Meijer B, Bakker HHR. Management of priapism in the newborn. Urol. 2003;61(1):224.
Merritt AL, Haiman C, Henderson SD. Myth: blood transfusion is effective for sickle cell anemia associated priapism. CJEM. 2006;8:119-122.
Mi T, Abbasi S, Zhang H, et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118:1491-1501.
Monga M, Broderick GA, Hellstrom WJ. Priapism in sickle cell disease: the case for early implataion of the penile prosthesis. Eur Urol. 1996;30(1):54-59.
Montague DK, Jarow J, Broderick GA, et al. American Urological Association guideline on the management of priapism. J Urol. 2003;170:1318-1324.
Montorsi F, Verheyden B, Meuleman E, et al. Long-term safety and tolerability of tadalafil in the treatment of erectile dysfunction. Eur Urol. 2004;45:339-345.
Morales A, Gingell C, Collins M, et al. Clinical safety of oral sildenafil citrate (VIAGRA) in the treatment of erectile dysfunction. Int J Impot Res. 1998;10(2):69-73.
Moreland RB, Traish A, McMillin MA, et al. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle. J Urol. 1995;153:826-834.
Morris CR, Kato GJ, Pojakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension and mortality in sickle cell disease. JAMA. 2005;294:81-90.
Mulhall J. Priapism—guidelines for surgical management of priapism. In: Porst H, Buvat J, editors. Standard practice of sexual medicine. Oxford (UK): Blackwell Science; 2006:180-190.
Munarriz R, Park K, Huang YH, et al. Reperfusion of ischemic corporal tissue: physiologic and biochemical changes in an animal model of ischemic priapism. Urology. 2003;62:760-764.
Muneer A, Cellek S, Dogan A, et al. Investigation of cavernosal smooth muscle dysfunction in low flow priapism using an in vitro model. Int J Impot Res. 2005;17:10-18.
Muneer A, Minhas S, Freeman A, et al. Investigating the effects of high-dose phenylephrine in the management of prolonged ischaemic priapism. J Sex Med. 2008;5:2152-2159.
Munnarriz R, Wen CC, McAuley I, et al. Management of ischemic priapism with high-dose intracavernosal phenylephrine: from bench to bedside. J Sex Med. 2006;3:918-922.
Nehra A. Priapism: pathophysiology and non-surgical management in standard practice. In: Porst H, Buvat J, editors. Standard practice of sexual medicine. Oxford (UK): Blackwell; 2006:174-179.
Nelson JH, Winter CC. Priapism: evolution of management in 48 patients in a 22 year series. J Urol. 1977;117:455-458.
Nixon RG, O’Connor JL, Milam DF. Efficacy of shunt surgery for refractory low flow priapism: a report on the incidence of failed detumescence and erectile dysfunction. J Urol. 2003;170:883.
Numan F, Cantasdemir M, Ozbayrak M, et al. Postraumatic nonischemic priapism treated with autologous blood clot embolization. J Sex Med. 2008;5:173-179.
Okpala I, Westerdale N, Jegede T, Cheung B. Etilefrine for the prevention of priapism in adult sickle cell disease. Br J Haematol. 2002;118:918-921.
Ozturk MH, Gumus M, Donme H, et al. Materials in embolotherapy of high-flow priapism: results and long-term follow-up. Diagn Interv Radiol. 2009;15(3):215-220.
Paredes RG, Agmo A. The GABA-B antagonist CGP 35348 inhibits the effects of baclofen on sexual behavior and motor coordination. Brain Res Bull. 1995;36:495-497.
Park JK, Jeong YB, Han YM. Recanalisation of embolised cavernosal artery: restoring potency in a patient with high-flow priapism. J Urol. 2001;165:2002-2003.
Pietras JR, Cromie WJ, Duckett JW. Ketamine as a detumescence agent during hypospadius repair. J Urol. 1979;121:654.
Pohl J, Pott B, Kleinhans G. Priapism: a three-phase concept of management according to etiology and prognosis. Br J Urol. 1986;58:113.
Ponniah A, Brown CT, Taylor P. Priapism secondary to leukemia; effective management with prompt leukapheresis. Int J Urol. 2004;11:809-810.
Porst H. The rational for prostaglandin E1 in erectile failure: a survey of worldwide experience. J Urol. 1996;155:802-815.
Priyadarshi S. Oral terbutaline in the management of pharmacologically induced prolonged erection. Int J Imp Res. 2004;16:424-426.
Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:16-20.
Pryor JP, Hehir M. The management of priapism. Br J Urol. 1982;54:751.
Puppo P, Belgrano E, Germinale F, et al. Angiographic treatment of high-flow priapism. Eur Urol. 1985;11:397.
Quackles R. Treatment of a case of priapism by cavernospongious anastamosis. Acta Urol Belg. 1964;32:5-13.
Rachid-Filho D, Cavalcanti AG, Favorito LA, et al. Treatment of recurrent priapism in sickle cell anemia with finasteride: a new approach. Urology. 2009;74(5):1054-1057.
Ralph DJ, Garaffa G, Muneer A, et al. The immediate insertion of a penile prosthesis for acute ischaemic priapism. Eur Urol. 2009;56:1033-1038.
Rees RW, Kalsi J, Minhas S, et al. The mangement of low-flow priapism with the immediate insertion of a penile prosthesis. BJU Int. 2002;90(9):893-897.
Ricciardi R, Bhatt GM, Cynamon J, et al. Delayed high-flow priapism: pathophysiology and management. J Urol. 1993;149:119-121.
Robey EL, Schellhammer PF. Four cases of metastases to the penis and a review of the literature. J Urol. 1984;132(5):992-994.
Rodriguez J, Cuadrado JM, Frances A, Franco E. High flow priapism as a complication of veno-occlusive priapism. Two case reports. Int J Impot Res, 18, 2006, 215-217.
Rogers ZR. Priapism in sickle cell disease. Hematol Oncol Clin North Am. 2005;19(5):917-928.
Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequela of intravscular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1652-1662.
Rourke KF, Fischler AH, Jordan GH. Treatment of recurrent idiopathic priapism with oral baclofen. J Urol. 2002;168:2552-2553.
Sacher EC, Sayegh E, Frensilli F, et al. Cavernospogiosum shunt in treatment of priapism. J Urol. 1972;108:97-100.
Saenz de Tejada I, Kim NN, Daley JT, et al. Acidosis impairs rabbit trabecular smooth muscle contractility. J Urol. 1997;157:722-726.
Sandock DS, Seftel AD, Herbener TE, et al. Perineal abcess after embolization for high-flow priapism. Urology. 1996;308:48.
Savoca G, Pietropaolo F, Scieri F, et al. Sexual function after highly selective embolisation of cavernous artery in patients with high-flow priapism: long-term follow-up. J Urol. 2004;172:644-647.
Seftel A, Haas CA, Brown SL, et al. High flow priapism complicating veno-occlusive priapism: pathophysiology of recurrent idiopathic priapism?. J Urol, 159, 1998, 1300-1301.
Serjeant GR, de Ceulaer K, Maude GH. Stilboestrol and stuttering priapism in homozygous sickle-cell disease. Lancet. 1985;2:1274-1276.
Shamloul R, el Nashaar A. Idiopathic stuttering priapism treated successfully with low-dose ethinyl estradiol: a single case report. J Sex Med. 2005;2:732-734.
Shapiro SR. Idiopathic priapism in the newborn. J Urol. 1979;121:838.
Siegel JF, Rich MA, Brock WA. Association of sickle cell disease, priapism, exchange transfusion and neurological events: ASPEN syndrome. J Urol. 1993;150:1480-1482.
Spycher MA, Hauri D. The ultastructure of the erectile tissue in priapism. J Urol. 1986;135:142-147.
Steinberg J, Eyre RC. Management of recurrent priapism with epinephrine self-injection and gonadotropin-releasing hormone analogue. J Urol. 1995;153:152-153.
Sur RL, Kane CJ. Sildenafil citrate-associated priapism. Urology. 2000;55(6):950.
Tarry WF, Duckett JW, Snyder H. Urological complications of sickle cell disease in a pediatric population. J Urol. 1987;138:592.
Teloken C, Ribeiro EP, Chammas M, et al. Intracavernosal etilefrine self-injection therapy for recurrent priapism. Uro. 2005;65(5):1002.
Tripe JW. Case of continued priapism. Lancet. 1845;2:8.
Vaidyanathan S, Watt JW, Singh G, et al. Management of recurrent priapism in a cervical spinal cord injury patient with oral baclofen therapy. Spinal Cord. 2004;42(2):134-135.
Villanova FE, Andrade E, Leal É, et al. Erection induced by Tx2–6 toxin of Phoneutria nigriventer spider: expression profile of genes in the nitric oxide pathway of penile tissue of mice. Toxicon. 2009;54(6):793-801.
Virag R, Buchi D, Lee K, Galacteros E. Preventive treatment of sickle cell disease with oral and self-administered intracavernous injection of etilefrine. Urology. 1996;47(5):777-781.
Walker JR, Casale AJ. Prolonged penile erection in the newborn. Urol. 1997;50:796-799.
Wallace CJ, Hoag N, Pommerville PJ, Huk ME. Recurrent idiopathic high-flow priapism treated with selective arterial embolization after repeated initial treatments for low-flow priapism. Can Urol Assoc J. 2009;3(1):60-63.
Wills B, Albinson C, Wahl M, Clifton J. Sildenafil citrate ingestion and prolonged priapism and tachycardia in a pediatric patient. Clin Tox. 2007;45:798-800.
Wilt TJ, Fink HA. Is antidepressant plus sildenafil a recipe for priapism? Postgrad Med. 2004;116(6):11-12.
Winter CC. Cure of idiopathic priapism: new procedure for creating fistula between glans penis and corpora cavernosa. Urology. 1976;8:389-391.
Witt MA, Goldstein I, Saenz de Tejada I, et al. Traumatic laceration of intracavernosal arteries: the pathophysiology of nonischemic, high flow areterial priapism. J Urol. 1990;143:129-132.
Wolf JS, Lue TF. High flow priapism and glans hypervascularization following deep dorsal vein arterialization for vasculogienc impotence. Urol Int. 1992;49:227-229.
Yamashita N, Hisasue S, Kato R, et al. Idiopathic stuttering priapism: recovery of detumescence mechanism with temporal use of antiandrogen. Urology. 2004;63:1182-1184.