chapter 27 Prosthetic Surgery for Erectile Dysfunction
Three sentinel events define the history of the treatment of erectile dysfunction (ED): the introduction of the inflatable penile prosthesis (Scott et al, 1973); intracavernous injection therapy (Virag, 1982); and effective systemic therapy with sildenafil citrate (Goldstein et al, 1998). Today the treatment of ED can be compared with the treatment of osteoarthritis, another common disorder that also becomes more prevalent with age. In both, a progressive treatment model may be employed (Table 27–1). Most men with ED will initially be offered systemic therapy with a PDE5 inhibitor. If that fails and the men want to continue treatment, second- and third-line therapies should be discussed. If these fail or are rejected, penile prosthesis implantation is usually appropriate.
Table 27–1 Progressive Treatment Model
| OSTEOARTHRITIS | ERECTILE DYSFUNCTION | |
|---|---|---|
| First-line therapy | NSAIDs | PDE5 inhibitors |
| Second-line therapy | Joint injections | VCDs, IUD |
| Third-line therapy | Arthroscopic surgery | Intracavernous injections |
| Fourth-line therapy | Prosthetic joint replacement | Penile prosthesis implantation |
IUD, intraurethral drugs; NSAIDs, nonsteroidal antiinflammatory agents; PDE5, type 5 phosphodiesterase; VCDs, vacuum constriction devices.
Types of Prostheses
Currently available penile prostheses in the United States are shown in Table 27–2. Malleable prostheses are semirigid devices with a central core that allows the penis to be bent down for dressing and bent upward for coitus. However, for most men this malleable core does not maintain these positions well. Malleable devices have the advantage of low mechanical failure rates and ease of use. Disadvantages include constant penile rigidity and an increased risk of erosion (Steidle and Mulcahy, 1989; Zermann et al, 2006).
Table 27–2 Penile Prosthesis Types
| PROSTHESIS TYPE | AMERICAN MEDICAL SYSTEMS* | COLOPLAST CORPORATION† |
|---|---|---|
| Semirigid rod | AMS Malleable 600 | Genesis |
| AMS Malleable 650 | ||
| Positionable | Dura II | |
| Two-piece inflatable | AMS Ambicor | Excel‡ |
| Three-piece inflatable | AMS 700 CX | Titan |
| AMS 700 CXR | Titan Narrow Base | |
| AMS 700 LGX |
* American Medical Systems (AMS), Minnetonka, MN.
† Coloplast Corporation, Minneapolis, MN.
‡ Not available in the United States.
The positionable penile prosthesis (Dura II) is a semirigid device with a central series of articulating segments held together with a spring on each end. This device, compared with malleable prostheses, is better able to maintain its upward and downward positions.
The two-piece inflatable penile prosthesis (AMS Ambicor) consists of two cylinders connected to a small scrotal pump (Levine et al, 2001; Lux et al, 2007). Squeezing this pump transfers a small volume of fluid from the rear tip reservoirs of the cylinders into a nondistensible central chamber producing rigidity comparable with that of a malleable device. When the device is deflated, the central chamber partially collapses, providing better flaccidity than a malleable implant. The two-piece prosthesis has as its primary advantage ease of implantation because there is no third piece (abdominal fluid reservoir). A disadvantage compared with malleable devices is the increased risk of mechanical failure. Excel, a two-piece inflatable prosthesis (Coloplast), is not available in the United States.
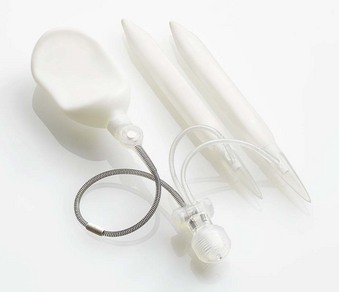
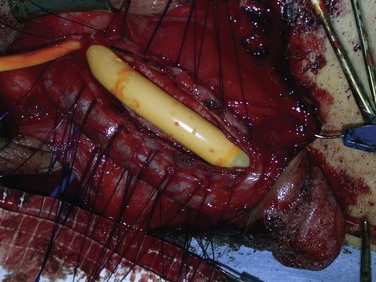
The ideal prosthesis would provide its recipient with a penis that resembles as closely as possible normal penile flaccidity and erection. Only three-piece inflatable devices that transfer a large volume of fluid into the penile cylinders for erection and out of the cylinders for flaccidity approach this ideal. Three-piece prostheses have paired corporeal cylinders, a scrotal pump, and an abdominal fluid reservoir. An example of a three-piece inflatable prosthesis is the Coloplast Titan Inflatable Penile Prosthesis (Fig. 27–1). All three-piece devices provide penile girth expansion and rigidity similar to that of a normal erection. One device, the AMS 700 LGX Inflatable Penile Prosthesis, also provides length expansion (Fig. 27–2).
Preoperative Patient-Partner Counseling
Ideally, discussions regarding the treatment of ED should include the partner; however, this is not always possible. Once a decision has been made that penile prosthesis implantation should be considered, it is important to inform the patient about alternative treatments and the advantages and disadvantages of each. By this time the patient has either failed or is not a candidate for systemic treatment with PDE5 inhibitors. He should be aware that in addition to penile prosthesis implantation, therapies with vacuum constriction devices, intraurethral prostaglandin, and intracavernous injection are available. An option such as penile prosthesis implantation should not be considered to treat a man with erectile dysfunction that is situational, the result of a relationship conflict, or one that is potentially reversible. For these men and their partners, psychologic consultation and sex therapy are more appropriate.
The various types of penile prostheses along with their advantages and disadvantages (see above) should be explored. Most penile prosthesis implantation is done under spinal or general anesthesia on an outpatient basis with discharge on the same or next day. The intensity and duration of postoperative pain is variable. Most men require oral narcotics for about 1 week. After that pain can often be managed with a nonsteroidal antiinflammatory agent. When an abdominal fluid reservoir has been placed, strenuous activity and heavy lifting are proscribed for 4 weeks. Patients can drive and return to nonstrenuous work once they are no longer taking narcotics. Coitus is usually possible in 4 to 6 weeks.
The patient should understand that penile prostheses produce an erection-like state. The following considerations refer to the three-piece inflatable devices. With the prosthesis deflated most men feel comfortable in a locker room situation. With inflation the erection-like state produces girth expansion and rigidity that for most men approaches that of a normal erection. The glans, however, is not included in the erection, and for most men the erection will be shorter than their normal erection. We demonstrate to men their stretched penile length and tell them that this will be the approximate length of their prosthetic erection. One device, the AMS 700 LGX (formerly the AMS 700 Ultrex), produces both girth and length expansion with length expansion ranging from 1 to 4 cm (mean 1.9 cm) (Montague and Lakin, 1992).
Key Points
Patient Expectations
Many men with ED have normal libido, and most have normal penile sensation and orgasm with ejaculation. If present, penile prosthesis implantation will preserve orgasm and ejaculation but not restore them if absent.
The patient should be informed that infection will probably require complete removal of the implant with resulting scarring of the corporeal smooth muscle. With prosthesis reimplantation after infection, the penis is frequently smaller and cylinder implantation is usually difficult. On rare occasions reimplantation may not be possible. Erosion of the prosthesis through the skin or into the urethra also requires device removal. The potential implant recipient should understand that mechanical failure is possible and correcting this requires device revision or replacement.
Surgical Approaches
Surgical approaches for penile prosthesis implantation include subcoronal (used only for implantation of malleable or positionable devices), infrapubic, and penoscrotal. We prefer the penoscrotal approach for the reasons listed in Table 27–3. When corporeal fibrosis is present, the penoscrotal incision can be extended to expose almost the entire extent of both corpora cavernosa.
Table 27–3 Comparison of Infrapubic and Penoscrotal Implant Approaches
| INFRAPUBIC APPROACH | PENOSCROTAL APPROACH | |
|---|---|---|
| Advantages | Reservoir placement under direct vision | Better corporeal exposure No dorsal nerve injury Pump fixation possible |
| Disadvantages | Limited corporeal exposure Possible dorsal nerve injury Inability to anchor pump |
Blind reservoir placement |
A potential disadvantage of the penoscrotal incision not listed in Table 27–3 is the presence of excess tubing in the scrotum or at the base of the penis. We avoid this by implanting the preconnected cylinders and pump first using a penoscrotal incision with a transverse flap through the deep layers of dartos fascia. A second incision is made through dartos fascia in the midline of the scrotum to develop a pouch for the pump. A stab incision is then made deep through the back wall of the pouch (the transverse dartos flap), and the pump is brought through this to place it into the pouch. This helps anchor the pump in the pouch and buries the tubing in the upper scrotum. This is advantageous not only from a cosmetic standpoint but also because it reduces the risk of device infection. If a superficial penoscrotal wound infection occurs and the fascia is intact, a deep infection in the space around the prosthesis, which would require device removal, is not likely to occur.
Increasingly, men who have had radical prostatectomy require penile prosthesis implantation. Some question the feasibility of three-piece inflatable prosthesis implantation in this group because of scarring of the lower abdominal fascia and concern regarding scarring in the retropubic (prevesical) space. Lane and colleagues (2007) reported 115 consecutive implants of three-piece inflatable penile prostheses done through a single transverse penoscrotal incision with no reservoir-related complications. The use of LGX cylinders in men who have had radical prostatectomy may be of particular benefit because radical prostatectomy has been shown to result in a loss of penile length in many individuals (Gontero et al, 2007; Mulhall, 2005; Savoie et al, 2003).
Ams 700 Lgx Inflatable Penile Prosthesis Implantation By A Combined Longitudinal-Transverse Penoscrotal Approach
The authors perform this procedure with the patient in the supine position under either spinal or general anesthesia. Shaving of the skin is done in the operating room to avoid bacterial colonization of small skin breaks that might occur if shaving were done before the surgery. A 10-minute skin prep is performed. Paper drapes are used because when cloth drapes become wet, they do not provide a good bacterial barrier. We do not place a urethral catheter at this point because it is easier to judge the quality of the erection when a catheter is not present.
A longitudinal penoscrotal incision is made, and the superficial layers of dartos fascia are divided longitudinally. At the penoscrotal junction the deep layers of dartos fascia are divided transversely. This transverse incision through the fascia should be directed toward the urethra and corporeal bodies rather than toward the scrotum. Allis clamps are placed on the lower margin of the fascia, and the underside of dartos fascia is dissected off the urethra and the proximal corpora (crura). This combined longitudinal-transverse penoscrotal incision provides a transverse dartos flap to bury tubing in the upper scrotum with the cosmetic advantages of a skin incision along the midline penoscrotal raphe. The authors use a ring retractor they have modified by placing slits in it so that exposure may be maintained by a combination of hook stays and retractor blades.
Two-centimeter corporotomies are made, and two horizontal mattress sutures of 2-0 PDS II (polydioxanone, Ethicon, Inc., Somerville, NJ) are placed on each side of the corporotomy. These sutures are used as guide sutures during corporeal dilation and measurement, and they are also used to close the corporotomy after the cylinders are placed (Montague, 1993). Dilation starts with an 8-mm Hegar dilator and proceeds to 16 mm proximally and 14 mm distally. The greater proximal dilation is necessary to accommodate both the cylinder and cylinder tubing. If dilation to these diameters is not possible, the smaller-diameter AMS 700 CXR or the Mentor Titan Narrow Base prosthesis should be used.
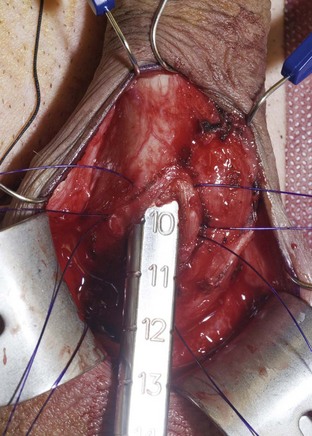
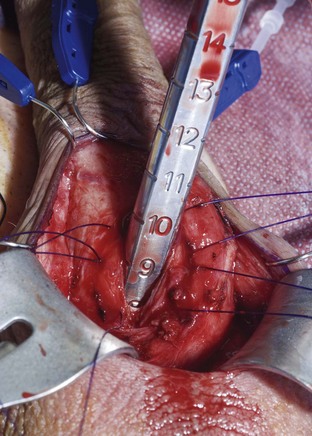
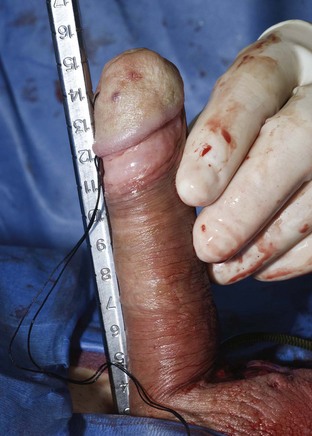
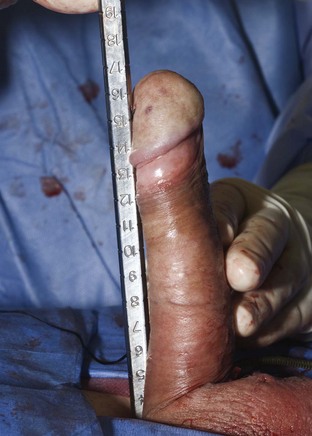
Using a sizing instrument, the distal measurement taken from the distal end of the corporotomy is 10 cm (Fig. 27–3), and the proximal measurement taken from the proximal end of the corporotomy is 8 cm (Fig. 27–4). It is important to size cylinders exactly to fill the entire corpora. Cylinders should be neither too short, producing the supersonic transporter (SST) deformity (Ball, 1980), nor too long, producing bucking within the corpora. We do not include the 2-cm corporotomy in the measurement because we believe that surface sizing with a rigid measuring tool introduces a 2-cm measuring error (Montague et al, 2003). With standard measuring techniques from a midcorporotomy reference point, the cylinder placed is about 2 cm too long. When the length-expanding LGX cylinder is used, this leads to the so-called S-shaped cylinder deformity (Wilson et al, 1996). Although no data support this, the authors believe that with non–length-expanding cylinders, a too-long cylinder may cause premature failure (cylinder failures are most common in the area where the buckling with a too-long cylinder occurs).
After corporeal measurements are taken, the appropriate cylinder size is selected. LGX and CX cylinders come in the following lengths: 12, 15, 18, and 21 cm. Adjustments between these sizes are made by the addition of rear tip extenders. The cylinders are filled with normal saline, and the authors inject saline until the cylinders are full (rounded) but not inflated under pressure. Having the cylinders full rather than collapsed makes it easier to judge whether the cylinder fit following insertion is correct. The pump is filled by cycling the pump while the pump tubes are submersed in a basin of normal saline. The reservoir is filled with normal saline to displace air; the saline is then removed because the reservoir will be filled after it is implanted. In this case the total corporeal length was 18 cm (10 cm + 8 cm), and the authors elected to use 15-cm LGX cylinders with 3-cm rear tip extenders. Figure 27–5 shows the filled prosthesis components ready for implantation. For illustrative purposes one cylinder is inflated to show both its girth and length expansion. It will be deflated before implantation.
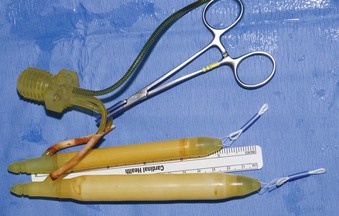
Figure 27–5 The prosthesis components prepared for implantation. One cylinder is inflated to demonstrate its girth- and length-expanding properties. It is deflated before insertion.
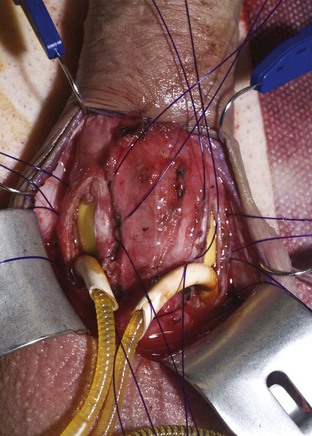
Distal cylinder insertion is aided by the use of the Furlow cylinder inserter (American Medical Systems, Minnetonka, MN). The guide sutures in the tip of the cylinder are threaded through the needle provided in the accessory package, and this needle with its sutures is placed into the distal end of the inserter. The inserter is then placed to the distal end of the corpus cavernosum. The plunger of the cylinder inserter is pushed in delivering the needle and guide sutures through the tip of the corpus cavernosum and out through the glans. The guide sutures are used to pull the distal cylinder into the distal corpus cavernosum. If rear tip extenders are necessary, they are added now and the proximal portion of the cylinder is manually inserted. If the cylinder fit is proper, the proximal portion will be all the way down to the attachment of the crus to the pelvic bone, and the distal portion will be in the distal corpus cavernosum under the mid glans penis. When these conditions have been met, the rounded visible portion of the cylinder will lie flatly in the open corporotomy (Fig. 27–6). The penis should then be released from the retractor, and the distal position of the cylinders and glans support should be evaluated (Fig. 27–7). If the cylinder is too long or too short, the proximal portion can be removed and length adjustment can be made by adding or removing rear tip extenders. The corporotomies are closed by tying the proximal ends and then the distal ends of the preplaced horizontal mattress sutures (Montague, 1993).
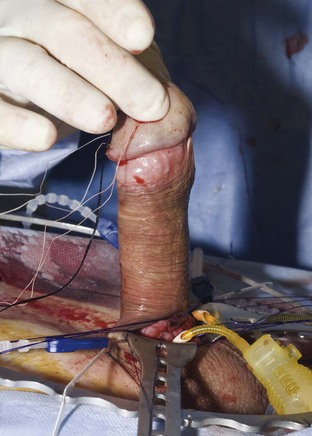
Figure 27–7 The penis has been released from the retractor. Both cylinder tips reach the ends of the corpora and provide excellent glans support.
In preparation for pump placement, a second incision through dartos fascia is made in the scrotal septum. A deep septal pouch is developed with ring forceps. A passage way is made with a right angle clamp through the back wall of this pouch deep in the scrotum, and the pump is brought through this passage way into the pouch (Fig. 27–8). After irrigation with antibiotic solution, the second dartos incision is closed with running 3-0 Dexon II (polyglycolic acid suture, United States Surgical, Tyco Healthcare Group, Norwalk, CT).
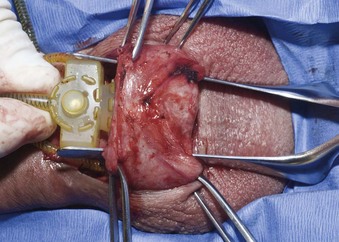
Figure 27–8 The pump is brought through a stab incision deep through the back wall of the subdartos pouch.
A 50-mL syringe filled with normal saline, which serves as a temporary reservoir, is then connected to the reservoir tube from the pump. The pubis to glans tip penile length measurement is 15 cm with the cylinders deflated (Fig. 27–9) and 17 cm with the cylinders inflated (Fig. 27–10). The use of a device with LGX cylinders in this patient has given him a prosthetic erection that is 2 cm longer than that obtainable with any other inflatable device.
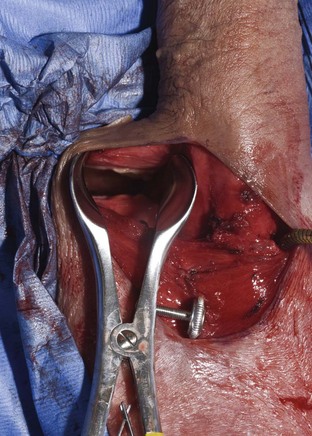
Safe reservoir insertion into the retropubic space through the primary penoscrotal incision is possible only if the bladder is completely empty. The authors insert an 18-Fr urethral catheter attached to closed gravity drainage and apply suprapubic pressure over the bladder until it is empty. The surgeon introduces his or her finger into the incision and moves this up to the external inguinal ring on either side. If the external ring is not palpable, a point just above the pubic tubercle is chosen. Metzenbaum scissors or a Kelly clamp is used to perforate the transversalis fascia. If all layers of the fascia are perforated, the surgeon’s index finger enters the retropubic space. Correct entry is confirmed if the back of the symphysis pubis and the balloon in the empty bladder are palpated. A nasal speculum with long blades is substituted for the finger; this maintains the fascial opening (Fig. 27–11). The empty reservoir is introduced into the retropubic space; the hublike junction between the reservoir and the tube should be located in the fascial defect.
The AMS reservoirs are available in two sizes: 65 mL and 100 mL. The former is recommended for all cylinder sizes except for the 18- and 21-cm LGX cylinders, which require the 100-mL reservoir size. The reservoir is then filled with the requisite amount of normal saline, and then the position of the reservoir is confirmed by palpation. The reservoir itself, which is below the fascia, should not be palpable. The tubing hub should be palpable as it protrudes through the fascia. A back pressure test is done; if fluid exits under pressure, as little as 85 mL can be left in a 100-mL reservoir and as little as 50 mL can be left in a 65-mL reservoir. The reservoir tubing is brought down into the incision in the upper scrotum, where a connection to the pump-reservoir tubing is made over a sutureless straight connector.
A long right-angle clamp is introduced through the incision to the inguinal area on the side of the reservoir. A stab incision is made and through this a closed-suction type of drain is introduced. The drain is brought down to the base of the penis, where it will lie on top of the tubes and connectors. Dartos fascia is then closed transversely with running 3-0 Dexon. The skin is closed with running subcuticular 4-0 Vicryl.
Postoperative Care
The urethral catheter and the closed suction drain are removed the next morning after an overnight stay in the ambulatory surgery center. Most men require an oral narcotic for about 1 week. After that a nonsteroidal antiinflammatory agent will usually suffice. If an abdominal fluid reservoir has been placed, lifting and other activities that might result in reservoir displacement from the retropubic space into the inguinal area are proscribed for 1 month. The patient is instructed to keep his penis up on the lower abdomen pointing to the umbilicus. If the penis is worn in a downward position as healing takes place, ventral curvature of the penis may result.
One month after surgery most men are ready for instructions on cycling the device. We instruct the patient to fully inflate and deflate his device twice daily for 1 month. This serves a dual purpose: practice on cycling, as well as activity that stretches the pseudo capsule that by now has formed around the cylinders. In the beginning pumping requires considerable force, but with time it becomes much easier.
The patient is given permission to begin coitus whenever inflation can be accomplished without discomfort. Because anxiety on the part of the partner may inhibit vaginal lubrication, the couple is advised to use a water-soluble lubricant at least initially. Retarded ejaculation (failure to reach orgasm) may occur when men first use their prosthesis for coitus. To avoid this, the couple is counseled to have adequate foreplay so that both partners are sexually aroused before intromission.
Complications
Infection
Infection in the periprosthetic space usually does not cause significant illness; however, to eradicate the infection, removal of all components of the prosthesis is almost always required. Therefore infection is considered by many to be the most significant complication of genitourinary prosthetic surgery.
Penile prosthesis implantation should be delayed in patients with urinary tract infections or cutaneous infections in the operative area. Shaving of the operative area is done just before surgery to avoid bacterial colonization of small skin breaks that might occur if shaving was done before the day of surgery. The operative area is prepared with a 10-minute skin prep. Broad-spectrum prophylactic antibiotics are given 1 hour before the case begins so that adequate tissue levels are present when the incision is made. We use gentamicin and vancomycin for this purpose. Paper rather than cloth drapes are used because the latter are permeable to bacteria when wet. The prosthesis is kept in its sterile package until it is ready to be filled and implanted. It is then submersed in an antibiotic solution unless it has an antibiotic coating, in which case it is placed in a sterile basin and covered with a paper towel. Silicone has a positive charge and attracts air borne particles; therefore as it is placed in the body, it is irrigated with antibiotic solution and irrigated again as tissues are closed over it. For our antibiotic solution we use 50,000 units of bacitracin in a liter of normal saline.
Infections occurring after penile prosthesis are either early (in the first few weeks following implant) or late (6 months to 1 or 2 years after implant). The former are often associated with gram-negative bacteria. whereas the latter are usually associated with gram-positive bacteria such as Staphylococcus epidermidis (Kabalin and Kessler, 1988; Licht et al, 1995). Infections with other organisms such as fungi (Peppas et al, 1988) and gonococci (Nelson and Gregory, 1988) have also been rarely reported. It was originally believed that all infections originated from the time of surgery. Carson showed that late prosthetic infections can occur by hematogenous spread from a distant source (Carson and Robertson, 1988). The authors have shown that late infection can also arise after a mechanical malfunction (fluid leak) from a prosthetic device (Milbank et al, 2004). Fluid leak likely disrupts the balance with a biofilm, favoring bacterial growth (Silverstein et al, 2003). Alternatively, the fluid itself could be a bacterial reservoir.
Evidence is divided as to whether men with diabetes mellitus are at increased risk for infection following penile prosthesis implantation and whether the degree of diabetes control at the time of surgery is related to the risk of infection. In one study four infections occurred in 13 diabetics with a glycosylated hemoglobin above 11.5%, whereas in 19 diabetics with a glycosylated hemoglobin under 11.5%, only one infection occurred (Bishop et al, 1992). In another study of penile prosthesis recipients, infections occurred in 10 of 114 diabetics (8.7%) and in 11 of 275 nondiabetics (4%). However, there was no statistically significant increased infection rate with increased levels of glycosylated hemoglobin (Wilson et al, 1998). In a study of 556 prosthesis recipients, there was no significant difference in the incidence of infection in diabetic versus nondiabetic patients (Montague et al, 2001).
In a study looking at the surgical approach for penile prosthesis implantation, 4 of 139 devices implanted via the infrapubic approach became infected (2.9%), whereas 2 of 221 implanted via the penoscrotal approach became infected. This difference was not statistically significant (Garber and Marcus, 1998).
Early infections are likely to be evident by swelling, erythema, tenderness, possible purulent drainage, and occasionally fever. Late infections may only be manifested by persistent or recurrent long-term pain. With long-term infections the scrotal skin may be adherent to the pump. Infections are sometimes evident by erosion, particularly of the pump through the scrotum (Fig. 27–12). This should never be considered infection of the pump alone. All components of the device are connected by tubing, and the entire device is considered to be infected.
Treatment of a prosthetic infection with appropriate antibiotics usually results in clinical improvement; however, antibiotic treatment will rarely permanently eradicate this type of infection. This is thought to be due to the harboring of microorganisms within a biofilm that is adherent to the device (Abouassaly et al, 2004). For this reason, when a prosthetic infection is present, all components of the prosthesis should be removed.
Penile prosthesis reimplantation following infected device removal presents certain difficulties. Pump and reservoir reimplantation is seldom a problem. However, after infected device removal, the corporeal smooth muscle becomes replaced in varying degrees with scar. In the past reimplantation was often delayed as long as a year. During this time the scar within the corpora would mature, resulting in contraction, a smaller penis size, and more difficulty with dilation before cylinder reimplantation. To minimize loss of penile size and to facilitate corporeal dilation, the authors now perform prosthesis reimplantation as soon as possible after device removal for infection. Once all incisions have healed and postoperative edema has resolved (usually 6 to 12 weeks after device removal), reimplantation is advised because early fibrosis is easier to dilate and the scar contraction that leads to shortening has not yet occurred.
Mulcahy introduced the concept of prosthesis salvage for infection in 1996 (Brant et al, 1996), and he updated his experience with this in 2000 (Mulcahy, 2000). His protocol involves removal of all prosthetic components and foreign bodies followed by irrigation with seven antibacterial solutions. A new device is implanted, and the patient is placed on antibiotics. In 55 patients available for follow-up, 45 (82%) were free of infection with follow-up ranging from 6 to 93 months. When salvage procedures are successful, they maintain penile size and correct the problem with only one operation.
Recent advances in prosthetic design have been made in attempts to reduce infection. A surface treatment combining rifampin and minocycline (InhibiZone) was introduced in 2001 by American Medical Systems for its three-piece inflatable penile prosthesis product line. In a report comparing 2261 rifampin-minocycline–coated devices with 1944 controls, the infection rate at 180 days was 0.68% in the treated group compared with 1.61% in the control group (Carson, 2004).
Mentor (now Coloplast) introduced a hydrophilic surface coating for their three-piece inflatable prosthesis in 2002 (Coloplast Titan). This coating, polyvinylpyrrolidone (PVP), reduces bacterial adhesion and absorbs the antibiotics in which the prosthesis is submerged by the surgeon just before implantation. In a report comparing 2357 hydrophilic-coated devices with 482 controls, the infection rate was 2.07% in the treated group compared with 1.06% in the control group (Wolter and Hellstrom, 2004).
The incidence of infection during first-time penile prosthesis implantation ranges from 1% to 3%, whereas with revision surgery it is considerably higher (7% to 18%) (Kabalin and Kessler, 1988; Wilson and Delk, 1995; Jarow, 1996; Henry et al, 2005). In the early days of penile prosthesis surgery, mechanical failures were common and frequently occurred within the first few years following device implantation. The reports of increased infection rates following prosthesis revision occurred when only the failed component of the prosthesis was replaced. If the entire device is explanted and all prosthesis compartments are thoroughly irrigated before a new prosthesis is implanted, the infection rate is not significantly different from the rates seen with first-time prosthesis implantation (Montague et al, 2001; Henry et al, 2005).
Mechanical failures today are less common and typically occur more than 5 years after implantation.
Key Points
Prosthesis Infections
Perforation and Erosion
Perforation is an event that occurs intraoperatively; whereas erosion is an event that occurs or is only recognized postoperatively. When the surgeon is dilating the proximal corpora (crura), a sudden give of the dilator suggests the crus has been perforated on its medial aspect near its attachment to the pelvic bone. The dilator, almost always a smaller size, travels out into the soft tissues of the perineum. Although Mulcahy (1987) suggested the “wind sock” correction for this, it is rarely necessary if the perforation is recognized and larger-diameter dilators are used to dilate the correct tract. When the proximal portion of the cylinder is inserted, it will stay within the crus and the small area of perforation will heal over it.
With distal dilation, crossover to the opposite side may occur or the urethra may be perforated. If urethral perforation occurs, the implant procedure should be abandoned and a urethral catheter should be left in for 7 to 10 days. Prosthesis reimplantation may be done at a later date. To avoid urethral perforation, the surgeon should keep the tip of the dilator under the dorsolateral surface of the corpus cavernosum. This maneuver also helps to prevent crossover to the opposite side. After the first cylinder is implanted, the surgeon should resound the other side both proximally and distally to see if crossover in either direction has occurred.
Erosion of the distal cylinder lateral to the distal corpus cavernosum is usually most effectively corrected by a technique described by Mulcahy (1999). With this approach the distal cylinder is removed (folded back) and an incision through the back wall of the capsule of the cylinder compartment is made in an area proximal to the site where the erosion has occurred. This allows distal dilation within the true corpus cavernosum. Using the Furlow cylinder inserter, the distal cylinder is then reimplanted within the distal corpus cavernosum.
Erosion of the distal end of the prosthesis may occur into the urethra, in which case it is visible through the meatus. This occurs more commonly after semirigid rod implantation, presumably due to constant internal pressure from the rod device (Steidle and Mulcahy, 1989). It also occurs more commonly in men with spinal cord injury because of their lack of sensation (Zermann et al, 2006). In the case of a semirigid rod device, only the eroded side needs to be removed and this can usually be done in the office by grasping the distal end of the rod with a towel clip and then pulling it out. In the case of urethral erosion, a urethral catheter is placed for 10 days to allow urethral healing. Many patients can have adequate coitus with only one rod in place; hence a procedure to reimplant the second rod is usually unnecessary.
In the case of erosion of one cylinder of an inflatable device into the meatus or through the glans, this cylinder should be removed as soon as possible to avoid spread of bacteria along the cylinder to its tubing and thus to the rest of the prosthesis. After the cylinder is removed, an implantable plug (supplied by the device manufacturer) is placed in the pump tubing that led to that cylinder. Again, many men can have coitus with only one inflatable cylinder; thus a later procedure to reimplant the second cylinder is not always necessary.
Poor Glans Support
Poor support of the glans penis by cylinder or rod tips leads to a drooping appearance of the glans, which is commonly referred to as the “SST deformity” after the supersonic transport (Concorde) nose appearance on takeoff and landing (Ball, 1980). This may result from inadequate distal dilation; too short cylinders; or in the case of minor deformity, variations in anatomy.
Correction of this deformity can be done in one of two ways. The definitive correction involves removing both cylinders, perforating the distal capsule with Metzenbaum scissors, redilating the distal corpora, resizing, and then inserting longer cylinders or the same cylinders with longer rear tip extenders. Alternatively, dorsal plication of the glans back onto the shaft of the penis can be performed (Fig. 27–13) (Ball, 1980; Mulhall and Kim, 2001). This latter procedure is preferable when there are minor but otherwise bothersome degrees of SST deformity.
Oversized Cylinder or Rod
When a semirigid rod prosthesis that is too long is implanted, the patient may complain of pain that does not subside as healing takes place following device implantation. Alternatively, this may lead to erosion of the rod either into the meatus or through the glans. Reoperation with placement of smaller rods will usually relieve the pain and avoid impending erosion.
As already discussed, cylinders that are too long may result in a sigmoid (S) penile deformity in the case of Ultrex or LGX cylinders. In the case of non–length-expanding cylinders, usually there is no problem, although theoretically it might lead to premature cylinder failure.
Pump Complications
The technique for pump implantation discussed earlier helps to avoid upward pump migration, which tends to take place during healing because of the action of the cremasteric muscles. If upward pump migration occurs, the pump may impinge on the base of the penis, making use of the pump difficult and also interfering with intromission. Revision is sometimes necessary, at which time the pump is relocated to its correct position.
The pump may also be difficult to use if a hematoma or seroma forms around it. These may reabsorb with time; if they do not, pump revision may be necessary.
Autoinflation
Autoinflation occurs when the inflatable penile prosthesis partially inflates with physical activity. This can be minimized by placing the reservoir in the prevesical (retropubic) space and by performing the back pressure test as described earlier. The cylinders should also be kept deflated during healing after surgery and when the prosthesis is not being used.
The Coloplast Titan prosthesis has a reservoir with a lock-out valve in the stem. Initial experience with this device suggests that it reduces the incidence of this complication (Hollenbeck et al, 2002; Wilson et al, 2002). The MS pump of the AMS 700 series three-piece inflatable penile prostheses has a lock-out valve in the top, deflation portion of the pump.
Penile Prosthesis Implantation in Special Cases
Peyronie Disease
Men who have acquired erectile deformity secondary to Peyronie disease along with significant ED may be candidates for penile prosthesis implantation. When prosthesis implantation by itself does not produce satisfactory straightening of the penis, a corporoplasty may also be necessary (Mulcahy and Wilson, 2002). Corporoplasty was frequently necessary when girth- plus length-expanding Ultrex cylinders were used and not necessary when girth-only expanding CX cylinders were used (Montague et al, 1996). Wilson showed that modeling or forcefully bending the penis with the cylinders inflated usually obviated the need for corporoplasty (Wilson et al, 2001).
Multiple relaxing incisions to correct curvature and increase penile length have been used in men with Peyronie disease undergoing penile prosthesis implantation (Montorsi et al, 2001; Rajpurkar et al, 1999). When a relaxing incision leaves a gap greater than 1 cm, covering the defect to prevent herniation of the cylinder is recommended. Materials used to cover the defect include human cadaveric dura mater (Fallon, 1990), polytetrafluoroethylene (Fig. 27–14) (Herschorn and Ordorica, 1995), human cadaveric pericardium (Palese and Burnett, 2001), and porcine small intestinal submucosa (Knoll and Center for Urological Treatment, 2002).
Cavernosal Fibrosis
In contrast to Peyronie disease, in which scarring is present in the tunica albuginea, scarring may occur in the cavernosal smooth muscle that forms much of the contents of the corporeal bodies. Cavernosal fibrosis is most often seen after removal of an infected penile prosthesis; it also occurs in men with ED as a result of ischemic priapism. In both cases, the penis is often significantly smaller owing to decreased volume of the corpora cavernosa. Dilation of the corpora and placement of semirigid rods or cylinders is also frequently quite difficult.
In preparing the corpora for cylinder implantation, the authors start with a 2-cm standard corporotomy. If the corpora cannot be dilated without force, they abandon the use of Hegar dilators and instead use Metzenbaum scissors with rounded tips. Gently spreading the tips as the scissors are advanced is often effective. After the scissors tips reach the distal and proximal ends of the corpora, dilation with special cutting dilators is helpful (Mooreville et al, 1999).
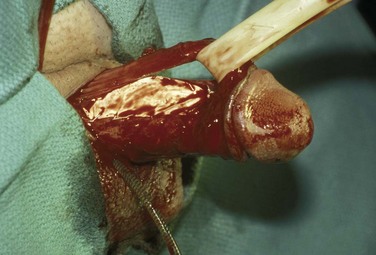
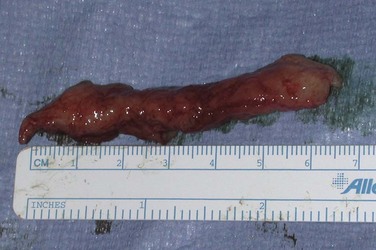
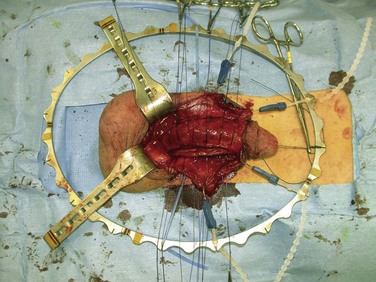
If these maneuvers are not successful, the authors extend the penoscrotal incision to the frenulum. Extended corporotomies are made permitting either dilation with more control or resection of scar tissue by establishing a plane between the fibrotic core and the inner surface of the tunica albuginea (Fig. 27–15), and this is facilitated by placing a Penrose drain around the core (Fig. 27–16) (Montague and Angermeier, 2006). The result is removal of the fibrotic contents of the corpus cavernosum (Fig. 27–17). Multiple horizontal mattress sutures of 2-0 PDS are then placed along the edges of the tunica albuginea (Fig. 27–18). The prosthetic cylinder can then be laid into the empty corporeal shell (Fig. 27–19), with primary closure of the tunica albuginea being possible (Fig. 27–20).
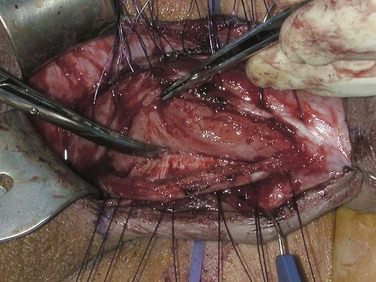
Figure 27–15 A plane of dissection is established between the fibrotic core and the inner surface of the tunica albuginea.
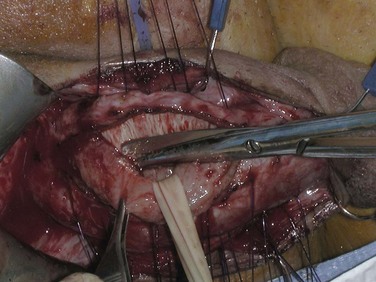
Figure 27–16 A Penrose drain is used to place traction of the fibrotic core, facilitating further dissection.
Results
Mechanical Failure Rates
Much of the early reporting of penile prosthesis results in terms of survival free of mechanical failure was limited because of data that usually gave only the range and mean follow-up for the entire series. It was not possible with this type of reporting to meaningfully compare different devices or the same device in different series because of failures to take into account differences in follow-up. Contemporary reporting standards should require reporting device survival free of mechanical failure by using Kaplan-Meier projections (Kaplan and Meier, 1958; Montague et al, 2005).
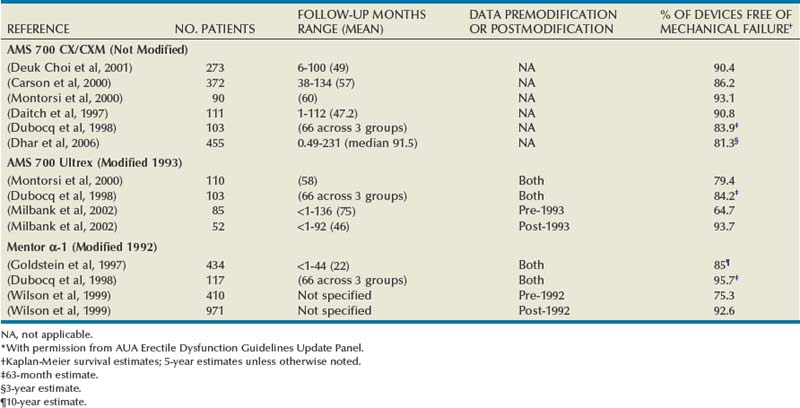
The results reported herein are limited to the three-piece inflatable penile prostheses available today (the AMS 700 CX/CXM, the AMS 700 Ultrex, and the Mentor Alpha-1) and include only those studies that report Kaplan-Meier projections. Eight such studies were found (Table 27–4). The AMS 700 CX/CXM prosthesis was introduced in 1987; no significant design changes have occurred with this device since its introduction. Five studies with a total of 949 implant recipients reveal that freedom from mechanical failure ranges from 83.9% (63 months) to 93.1% (5 years).
The AMS 700 Ultrex prosthesis was introduced in 1990, and the cylinders were modified in 1993. Results from two studies (Dubocq et al, 1998; Montorsi et al, 2000) with a total of 213 device recipients included both pre- and post-1993 Ultrex modified devices. Survival free of mechanical failure ranges were 79.4% (5 years) and 84.2% (63 months). One study compared 5-year device survival before the 1993 cylinder modification (64.7%, N = 85) to after the 1993 cylinder modification (93.7%, N = 52) (Milbank et al, 2002).
The Mentor Alpha-1 prosthesis introduced in 1989 underwent pump modification in November 1992. Two studies with a total of 55l implant recipients included both prepump and postpump modification devices. Survival free of mechanical failure was 85% at 3 years (Goldstein et al, 1997) and 95.7% at 63 months (Dubocq et al, 1998). One study compared 5-year device survival before pump modification (75.3%, N = 410) and after pump modification (92.6%, N = 971) (Wilson et al., 1999).
Dhar and colleagues reported on 455 men implanted with CX/CXM devices. Median follow-up was 91.5 months. Ten-year Kaplan-Meier estimates of freedom from mechanical failure were 81.3% (95% CI, 75.7 to 87.3) (Dhar et al, 2006).
Patient (Partner) Satisfaction
Early satisfaction studies were retrospective and used nonstandardized questionnaires. In one such study of the AMS 700 inflatable (pre-CX and pre-Ultrex) prosthesis, 387 recipients were mailed questionnaires and 272 were returned. This retrospective review showed that 83% of the men and 70% of their partners were satisfied with the use of this device (McLaren and Barrett, 1992). In another retrospective study of 145 AMS 700 Ultrex inflatable penile prosthesis recipients using a nonstandardized questionnaire, 85% of the men and 76% of their partners were satisfied (Holloway and Farah, 1997).
Prospective studies using structured questionnaires can provide more useful information regarding patient and partner satisfaction following penile prosthesis implantation. In one such study a psychosexual questionnaire containing 13 questions scored on a scale of 1 to 5 was administered to 35 inflatable penile prosthesis recipients preoperatively and again at 3, 6, and 12 months after surgery. This study demonstrated significant improvements in the psychosexual well-being of these prosthesis recipients with attainment of a high level of patient satisfaction up to 1 year after surgery. This study permitted these results to be broken down into erectile ability, libido, frequency of sexual activity, satisfaction with sexual activity, and a decrease in feelings of sadness, depression, anxiety, anger, frustration, and embarrassment related to sexual activity (Tefilli et al, 1998).
In another prospective study of 96 inflatable penile prosthesis recipients, the International Index of Erectile Function (IIEF) (Rosen et al, 1997) and the Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) (Althof et al, 1999) were administered preoperatively and 3, 6, and 12 months postoperatively (IIEF) and 3, 6, and 12 months postoperatively (EDITS). All 12 month scores were significantly higher than baseline scores, and the 12-month scores were significantly higher than the 6-month scores for the IIEF satisfaction domain and for EDITS (Mulhall et al, 2003).
One study compared penile prosthesis implantation to penile injection therapy. In this retrospective study a structured telephone questionnaire was administered to 115 intracavernosal injection patients and 65 penile prosthesis recipients. Mean follow-up of all patients was 5.4 years. At the time of contact 70% of the patients with prostheses were still sexually active, whereas only 41% of the penile injection patients were sexually active (Sexton et al., 1998).
Another retrospective study compared erectile function status and satisfaction in patients receiving treatment with sildenafil citrate, intracavernous injection therapy, and inflatable penile prosthesis implantation. The patients were administered EDITS and the erectile function domain of the IIEF. There were 31 patients in the sildenafil citrate group, 22 in the penile injection therapy group, and 32 who had received a penile prosthesis. At a mean follow-up of 19.54 months, penile prosthesis recipients had significantly better erectile function and treatment satisfaction than did patients in the other two groups (Rajpurkar et al, 2003).
In a study of 114 penile prosthesis recipients, IIEF was administered preoperatively and IIEF, EDIT, and a Global Satisfaction Questionnaire were given at least 6 months postoperatively (Akin-Olugbade et al, 2006). Subgroups evaluated included recipients with Peyronie disease, body mass index (BMI) greater than 30 kg·m2, radical prostatectomy, and patient age older than 70 years. All groups showed significant improvement in IIEF and EDITS scores. Men with Peyronie disease, radical prostatectomy, and BMI greater than 30 had lower satisfaction rates than the general penile implant group.
Abouassaly R, Montague DK, Angermeier KW. Antibiotic-coated medical devices: with an emphasis on inflatable penile prosthesis. Asian J Androl. 2004;6:249-257.
Milbank AJ, Montague DK, Angermeier KW, et al. Mechanical failure of the American Medical Systems Ultrex inflatable penile prosthesis: before and after 1993 structural modification. Urology. 2002;167(6):2502-2506.
Mulcahy JJ. Long-term experience with salvage of infected penile implants. J Urol. 2000;163:481-482.
Mulhall JP, Ahmed A, Branch J, et al. Serial assessment of efficacy and satisfaction profiles following penile prosthesis surgery. J Urol. 2003;169(4):1429-1433.
Tefilli MV, Dubocq F, Rajpurkar A, et al. Assessment of psychosexual adjustment after insertion of inflatable penile prosthesis. Urology. 1998;52:1106-1112.
Wilson SK, Cleves MA, Delk JR2nd. Comparison of mechanical reliability of original and enhanced Mentor Alpha I penile prosthesis. J Urol. 1999;162:715-718.
Abouassaly R, Montague DK, Angermeier KW. Antibiotic-coated medical devices: with an emphasis on inflatable penile prosthesis. Asian J Androl. 2004;6(3):249-257.
Akin-Olugbade O, Parker M, Guhring P, Mulhall J. Determinants of patient satisfaction following penile prosthesis surgery. J Sex Med. 2006;3(4):743-748.
Althof SE, Corty EW, Levine SB, et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology. 1999;53(4):793-799.
Ball TPJ. Surgical repair of penile “SST” deformity. Urology. 1980;15:603.
Bishop JR, Moul JW, Sihelnik SA, et al. Use of glycosylated hemoglobin to identify diabetics at high risk for penile periprosthetic infections. J Urol. 1992;147(2):386-388.
Brant MD, Ludlow JK, Mulcahy JJ. The prosthesis salvage operation: immediate replacement of the infected penile prosthesis [see comments]. J Urol. 1996;155(1):155-157.
Carson CC3rd. Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol. 2004;171(4):1611-1614.
Carson CC, Mulcahy JJ, Govier FE. Efficacy, safety and patient satisfaction outcomes of the AMS 700CX inflatable penile prosthesis: results of a long-term multicenter study. AMS 700CX Study Group. J Urol. 2000;164(2):376-380.
Carson CC, Robertson CN. Late hematogenous infection of penile prostheses. J Urol. 1988;139(1):50-52.
Daitch JA, Angermeier KW, Lakin MM, et al. Long-term mechanical reliability of AMS 700 series inflatable penile prostheses: comparison of CX/CXM and Ultrex cylinders. J Urol. 1997;158(4):1400-1402.
Deuk Choi Y, Jin Choi Y, Hwan Kim J, Ki Choi H. Mechanical reliability of the AMS 700CXM inflatable penile prosthesis for the treatment of male erectile dysfunction. J Urol. 2001;165(3):822-824.
Dhar NB, Angermeier KW, Montague DK. Long-term mechanical reliability of AMS 700CX/CXM inflatable penile prosthesis. J Urol. 2006;176(6 Pt. 1):2599-2601. discussion 2601
Dubocq F, Tefilli MV, Gheiler EL, et al. Long-term mechanical reliability of multicomponent inflatable penile prosthesis: comparison of device survival. Urology. 1998;52(2):277-281.
Fallon B. Cadaveric dura mater graft for correction of penile curvature in Peyronie disease. Urology. 1990;35(2):127-129.
Garber BB, Marcus SM. Does surgical approach affect the incidence of inflatable penile prosthesis infection? Urology. 1998;52(2):291-293.
Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338(20):1397-1404.
Goldstein I, Newman L, Baum N, et al. Safety and efficacy outcome of mentor alpha-1 inflatable penile prosthesis implantation for impotence treatment. J Urol. 1997;157(3):833-839.
Gontero P, Galzerano M, Bartoletti R, et al. New insights into the pathogenesis of penile shortening after radical prostatectomy and the role of postoperative sexual function. J Urol. 2007;178(2):602-607.
Henry GD, Wilson SK, Delk JR2nd, et al. Revision washout decreases penile prosthesis infection in revision surgery: a multicenter study. J Urol. 2005;173(1):89-92.
Herschorn S, Ordorica RC. Penile prosthesis insertion with corporeal reconstruction with synthetic vascular graft material. J Urol. 1995;154(1):80-84.
Hollenbeck BK, Miller DC, Ohl DA. The utility of lockout valve reservoirs in preventing autoinflation in penile prostheses. Int Urol Nephrol. 2002;34(3):379-383.
Holloway FB, Farah RN. Intermediate term assessment of the reliability, function and patient satisfaction with the AMS700 Ultrex penile prosthesis. J Urol. 1997;157(5):1687-1691.
Jarow JP. Risk factors for penile prosthetic infection. J Urol. 1996;156(2 Pt. 1):402-404.
Kabalin JN, Kessler R. Infectious complications of penile prosthesis surgery. J Urol. 1988;139(5):953-955.
Kaplan E, Meier P. Nonparametric estimations from incomplete observations. J Am Statist Assoc. 1958;53:457-481.
Knoll LD, Center for Urological Treatment NTUSA. Use of porcine small intestinal submucosal graft in the surgical management of tunical deficiencies with penile prosthetic surgery. Urology. 2002;59(5):758-761.
Lane BR, Abouassaly R, Angermeier KW, Montague DK. Three-piece inflatable penile prostheses can be safely implanted after radical prostatectomy through a transverse scrotal incision. Urology. 2007;70(3):539-542.
Levine LA, Estrada CR, Morgentaler A. Mechanical reliability and safety of, and patient satisfaction with the Ambicor inflatable penile prosthesis: results of a 2 center study. J Urol. 2001;166(3):932-937.
Licht MR, Montague DK, Angermeier KW, Lakin MM. Cultures from genitourinary prostheses at reoperation: questioning the role of Staphylococcus epidermidis in periprosthetic infection. J Urol. 1995;154(2):387-390.
Lux M, Reyes-Vallejo L, Morgentaler A, Levine LA. Outcomes and satisfaction rates for the redesigned 2-piece penile prosthesis. J Urol. 2007;177(1):262-266.
McLaren RH, Barrett DM. Patient and partner satisfaction with the AMS 700 penile prosthesis. J Urol. 1992;147(1):62-65.
Milbank AJ, Montague DK, Angermeier KW. A new mechanism for delayed genitourinary prosthesis infections. J Sex Med. 2004;1(Suppl. 1):83.
Milbank AJ, Montague DK, Angermeier KW, et al. Mechanical failure of the American Medical Systems Ultrex inflatable penile prosthesis: before and after 1993 structural modification. J Urol. 2002;167(6):2502-2506.
Montague DK. Penile prosthesis corporotomy closure: a new technique. J Urol. 1993;150:924-925.
Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology. 2006;67(5):1072-1075.
Montague DK, Angermeier KW, Lakin MM. Penile prosthesis infections. Int J Impot Res. 2001;13(6):326-328.
Montague DK, Angermeier KW, Lakin MM, Ingleright BJ. AMS 3-piece inflatable penile prosthesis implantation in men with Peyronie’s disease: comparison of CX and Ultrex cylinders. J Urol. 1996;156(5):1633-1635.
Montague DK, Angermeier KW. Cylinder sizing: less is more. Int J Impot Res. 2003;15(Suppl. 5)):S132-S133.
Montague DK, Jarow JP, Broderick GA, et al. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol. 2005;174(1):230-239.
Montague DK, Lakin MM. Early experience with the controlled girth and length expanding cylinder of the American Medical Systems Ultrex penile prosthesis. J Urol. 1992;148(5):1444-1446.
Montorsi F, Rigatti P, Carmignani G, et al. AMS three-piece inflatable implants for erectile dysfunction: a long-term multi-institutional study in 200 consecutive patients. Eur Urol. 2000;37(1):50-55.
Montorsi F, Salonia A, Maga T, et al. Reconfiguration of the severely fibrotic penis with a penile implant. J Urol. 2001;166(5):1782-1786.
Mooreville M, Adrian S, Delk JR2nd, Wilson SK. Implantation of inflatable penile prosthesis in patients with severe corporeal fibrosis: introduction of a new penile cavernotome. J Urol. 1999;162(6):2054-2057.
Mulcahy JJ. A technique of maintaining penile prosthesis position to prevent proximal migration. J Urol. 1987;137:294-296.
Mulcahy JJ. Distal corporoplasty for lateral extrusion of penile prosthesis cylinders. J Urol. 1999;161(1):193-195.
Mulcahy JJ. Long-term experience with salvage of infected penile implants. J Urol. 2000;163(2):481-482.
Mulcahy JJ, Wilson SK. Management of Peyronie’s disease with penile prostheses. Int J Impot Res. 2002;14(5):384-388.
Mulhall JP. Penile length changes after radical prostatectomy. BJU Int. 2005;96(4):472-474.
Mulhall JP, Ahmed A, Branch J, Parker M. Serial assessment of efficacy and satisfaction profiles following penile prosthesis surgery. J Urol. 2003;169(4):1429-1433.
Mulhall JP, Kim FJ. Reconstructing penile supersonic transporter (SST) deformity using glanulopexy (glans fixation). Urology. 2001;57(6):1160-1162.
Nelson RP, Gregory JC. Gonococcal infections of penile prostheses. Urology. 1988;31:391-394.
Palese MA, Burnett AL. Corporoplasty using pericardium allograft (tutoplast) with complex penile prosthesis surgery. Urology. 2001;58(6):1049-1052.
Peppas DS, Moul JW, McLeod DG. Candida albicans corpora abscess following penile prosthesis placement. J Urol. 1988;140:1541-1542.
Rajpurkar A, Dhabuwala CB. Comparison of satisfaction rates and erectile function in patients treated with sildenafil, intracavernous prostaglandin E1 and penile implant surgery for erectile dysfunction in urology practice. J Urol. 2003;170(1):159-163.
Rajpurkar A, Li H, Dhabuwala CB. Penile implant success in patients with corporal fibrosis using multiple incisions and minimal scar tissue excision. Urology. 1999;54(1):145-147.
Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
Savoie M, Kim SS, Soloway MS. A prospective study measuring penile length in men treated with radical prostatectomy for prostate cancer. J Urol. 2003;169(4):1462-1464.
Scott FB, Bradley WE, Timm GW. Management of erectile impotence: use of implantable inflatable prosthesis. Urology. 1973;2:80-82.
Sexton WJ, Benedict JF, Jarow JP. Comparison of long-term outcomes of penile prostheses and intracavernosal injection therapy [see comments]. J Urol. 1998;159(3):811-815.
Silverstein A, Donatucci CF. Bacterial biofilms and implantable prosthetic devices. Int J Impot Res. 2003;15(Suppl. 5)):S150-S154.
Steidle CP, Mulcahy JJ. Erosion of penile prostheses: a complication of urethral catheterization. J Urol. 1989;142(3):736-739.
Tefilli MV, Dubocq F, Rajpurkar A, et al. Assessment of psychosexual adjustment after insertion of inflatable penile prosthesis. Urology. 1998;52(6):1106-1112.
Virag R. Intracavernous injection of papaverine for erectile failure, Letter to the editor. Lancet, 1982;2, 938.
Wilson SK, Carson CC, Cleves MA, Delk JR2nd. Quantifying risk of penile prosthesis infection with elevated glycosylated hemoglobin. J Urol. 1998;159(5):1537-1539.
Wilson SK, Cleves MA, Delk JR2nd. Ultrex cylinders: problems with uncontrolled lengthening (the S-shaped deformity). J Urol. 1996;155(1):135-137.
Wilson SK, Cleves MA, Delk JR2nd. Comparison of mechanical reliability of original and enhanced Mentor Alpha I penile prosthesis. J Urol. 1999;162(3 Pt. 1):715-718.
Wilson SK, Cleves MA, Delk JR2nd. Long-term followup of treatment for Peyronie’s disease: modeling the penis over an inflatable penile prosthesis. J Urol. 2001;165(3):825-829.
Wilson SK, Delk JR2nd. Inflatable penile implant infection: predisposing factors and treatment suggestions. J Urol. 1995;153(3 Pt. 1):659-661.
Wilson SK, Henry GD, Delk JrJR, Cleves MA. The mentor Alpha 1 penile prosthesis with reservoir lock-out valve: effective prevention of auto-inflation with improved capability for ectopic reservoir placement. J Urol. 2002;168(4 Pt. 1):1475-1478.
Wolter CE, Hellstrom WJ. The hydrophilic-coated inflatable penile prosthesis: 1-year experience. J Sex Med. 2004;1(2):221-224.
Zermann DH, Kutzenberger J, Sauerwein D, et al. Penile prosthetic surgery in neurologically impaired patients: long-term followup. J Urol. 2006;175(3 Pt. 1):1041-1044. discussion 1044