chapter 62 Urodynamic and Video-Urodynamic Evaluation of the Lower Urinary Tract
Urodynamics (UDS) is the term used to describe testing and measurements of the function of the lower urinary tract. The lower urinary tract has two essential functions: the storage of urine at low pressure and the voluntary evacuation of urine. Low-pressure storage is essential to protect kidneys and ensure continence, whereas voluntary evacuation allows for the elimination of urine in socially acceptable situations without fear of leakage or overdistention. It is clear that a number of conditions and diseases affect the lower urinary tract and disrupt the storage and/or evacuation of urine. This can lead to bothersome symptoms (e.g., urinary incontinence or pain from failure to empty) or, in some cases, potentially harmful sequela. In many cases, a precise assessment of storage and emptying is necessary to optimally treat patients. UDS is the dynamic study of the transport, storage, and evacuation of urine. It is composed of a number of tests that individually or collectively can be used to gain information about urine storage and evacuation. UDS involves the assessment of the function and dysfunction of the urinary tract and includes the actual tests that are performed (UDS studies) and the observations during the testing (UDS observations) (Abrams et al, 1988, 2002). In this chapter we discuss the use of urodynamics to help in the assessment and treatment of voiding dysfunction. We keep that discussion in practical terms and supplement it with evidence for the technical performance and clinical utility of UDS.
Role of Urodynamic Testing in Clinical Practice
UDS has been used for decades, yet clear-cut, level 1, evidenced-based “indications” for its use are surprisingly lacking. There are a number of reasons for this. It is difficult to conduct proper randomized controlled trials on UDS for conditions in which lesser levels of evidence and expert opinion strongly suggest clinical utility and in which “empiric treatment” is potentially harmful or even life threatening (e.g., neurogenic voiding dysfunction). Additionally, symptoms can be caused by a number of different conditions and it is difficult to study pure or homogeneous patient populations. Given the current state of evidence for UDS studies, what is most important is that the clinician has clear-cut reasons for performing the study and that the information obtained will be used to guide treatment of the patient. Therefore it is probably more useful to describe the role of UDS in clinical practice rather than precise “indications” for its use.
In practical terms, UDS is most useful when history, physical examination, and simple tests are not sufficient to make an accurate diagnosis and/or institute treatment. This has clinical applicability in two general scenarios:
Rather than refer to a list of “indications for UDS” that often are not evidence based at all, it is more useful for clinicians to think of how UDS should be used in a broader clinical perspective. In keeping with that theme, the role of UDS in clinical practice has been nicely summarized by Hosker and colleagues (2009) for the following situations:
In order to use UDS in a practical and effective way, it is important that the clinician has the proper expertise to know when and why to perform a UDS study. Despite many technical advances in the recording, processing, and printing of UDS studies, careful attention to technical details to ensure accurate collection of data remains the cornerstone of a good study. Because not all patients undergo UDS for the same reasons, the clinician should “customize” UDS to the patients’ symptoms and condition. That means deciding on the questions to be answered before starting each study and designing that study to obtain the answers to those questions. It is important to remember that UDS is performed in an “unnatural setting” and therefore does not always duplicate real life. A UDS study that does not duplicate complaints or symptoms when an abnormality is recorded is not necessarily diagnostic. In addition, failure to record an abnormality does not always rule out its existence (e.g., failure to demonstrate detrusor overactivity in a patient with urge incontinence). Finally, not all UDS observations are clinically significant. Therefore it is important to interpret UDS studies in the context of the patient’s history including symptoms and concomitant diseases/conditions, as well as other information like postvoid residual volumes and frequency volume charts (voiding and intake diaries) when clinically applicable.
Functional Classification of Voiding Dysfunction: Applicability to Urodynamic Testing
In order to formulate a set of questions to be answered by a urodynamic test, an understanding of the possible causes of symptoms and the possible urodynamic manifestations of a preexisting condition is necessary. In order to accomplish this, a practical classification of voiding dysfunction is invaluable. The system proposed and popularized by Wein (1981) is simple and allows one to classify voiding dysfunction according to urodynamic findings. Functional abnormalities of the lower urinary tract can be divided into the following:
In addition, functional abnormalities can be subclassified to the anatomic region of the lower urinary tract that is affected and how it is affected. Thus storage and emptying abnormalities can be caused by the following:
The beauty of a functional classification system is that it helps to clarify treatment options for a given patient. Thus in practical terms, the UDS evaluation should help to determine if there is bladder or bladder outlet dysfunction (or both) and whether there is a storage and/or emptying problem. By providing answers to these simple questions, the urodynamic test can lead to a correct diagnosis and, equally as important, institution of appropriate treatment. Obviously, an understanding of the physiology of urine storage and voiding and the pathophysiology of voiding dysfunction (see Chapter 61) is required to formulate appropriate questions to be answered by a urodynamic study. However, all too often clinicians get caught up in the intricate neurophysiologic aspects of voiding and storage dysfunction and fail to think in practical terms. One should always focus on the possible urodynamic findings in a given case and how each of the findings may ultimately affect the patient and his or her treatment. Obviously, these potential findings will be determined by symptoms and/or underlying conditions or diseases.
Conducting A Urodynamic Study: Patient and Technical Factors
Preparing for Urodynamic Study: Clinician, Patient, and Facility
Once the decision has been made to perform UDS on a particular patient, it is important to consider what information is expected from the test. The simple fact that a patient has symptoms or a disorder that may affect the lower urinary tract is not sufficient to start the UDS evaluation. A list of problems or questions that should be solved or answered by UDS should be made before any testing is performed. All patients are not alike, so each urodynamic evaluation may be different depending on the information needed to answer the questions relevant to a particular patient. We follow three important rules before starting the UDS evaluation (Nitti and Combs, 1998):
By following these simple rules, one can maximize the chance of obtaining useful information from a study. If a particular question is not answered, the study can be repeated in the same session. Most people who do urodynamics regularly would concur that a urodynamic test is not always perfect in answering all important questions, but by defining the information needed before starting the study, “unanswered questions” can be kept to a minimum. As mentioned earlier, one of the most important parts of UDS is its proper performance with careful attention to technical details so that accurate interpretation is possible. It is beyond the scope of this chapter to describe the proper performance of UDS in detail; however, the reader is referred to Schafer and colleagues (2002) for good urodynamic practices and Abrams and colleagues (2002) for terminology. The International Continence Society (ICS) has now defined the term “urodynamic observations” to denote those observations that occur during and are measured by the UDS test itself. In order to be consistent, it is recommended that all clinicians performing and interpreting UDS use the current ICS terminology (Abrams et al, 2002). A list of common UDS terms is provided in Table 62–1.
Table 62–1 Terminology for Common Urodynamic Terms and Observations According to the International Continence Society Standardization Subcommittee
|
The ICS has now defined the term urodynamic observations to denote observations that occur during and are measured by the urodynamics (UDS) test itself. In order to be consistent, it is recommended that all clinicians performing and interpreting UDS use the current ICS terminology (Abrams et al, 2002).
Two principal methods of urodynamic investigation exist:
The following are required of both types of studies:
Abdominal pressure: the pressure surrounding the bladder; currently it is estimated from rectal, vaginal, or extraperitoneal pressure or a bowel stoma.
Detrusor pressure: the component of intravesical pressure created by forces on the bladder wall that are both passive and active.
Filling cystometry: the method by which the pressure and volume relationship of the bladder is measured during bladder filling.
Bladder sensation during filling cystometry:
Normal bladder sensation, defined by three points noted during filling cystometry and evaluated in relation to the bladder volume at that moment and in relation to the patient’s symptomatic complaints.
First sensation of bladder filling: the volume at which the patient first becomes aware of the bladder filling.
First desire to void: the feeling during filling cystometry that would lead the patient to pass urine at the next convenient moment.
Nonspecific bladder sensation: the individual is aware of bladder filling because of other sensations such as abdominal fullness or vegetative symptoms.
Normal detrusor function: allows bladder filling with little or no change in pressure, no involuntary contractions despite provocative maneuvers.
Detrusor overactivity: involuntary detrusor contractions during the filling phase, spontaneous or provoked.
Phasic detrusor overactivity: a characteristic waveform that may or may not lead to urinary incontinence.
Terminal detrusor overactivity: a single involuntary detrusor contraction occurring at cystometric capacity that cannot be suppressed, resulting in incontinence with bladder emptying.
Detrusor overactivity incontinence: incontinence related to involuntary detrusor contractions. This may be qualified according to cause.
|
|
Cystometric capacity: the bladder volume at the end of the filling cystogram when permission to void is given.
Maximum cystometric capacity: the volume at which the patient feels he or she can no longer delay micturition and has a strong desire to void.
Maximum anesthetic bladder capacity: the volume to which the bladder can be filled under deep general or spinal anesthesia. This should be qualified as to what type of anesthesia is used, the rate of filling, the length of time of filling, and the pressure to which the bladder is filled.
Normal urethral closure mechanism: this maintains a positive urethral closure pressure during bladder filling even in the presence of increased abdominal pressure.
Incompetent urethral closer mechanism: this is defined as one allowing leakage of urine in the absence of detrusor contraction.
Urethral relaxation incontinence: leakage related to urethral relaxation in the absence of raised abdominal pressure or detrusor overactivity.
Urodynamic stress incontinence: noted during filling cystometry and defined as the involuntary leakage of urine during increased abdominal pressure in the absence of a detrusor contraction. This currently replaces genuine stress incontinence.
Urethral pressure measurements:
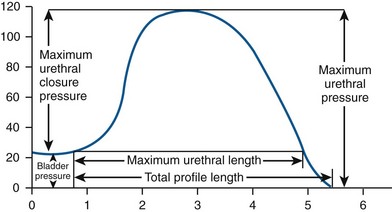
Urethral pressure profile: a graph indicating the intraluminal pressure along the length of the urethra.
Maximum urethral closure pressure (MUCP): the maximum difference between the urethral pressure and the intravesical pressure.
Abdominal leak point pressure: the intravesical pressure at which urine leakage occurs because of increased abdominal pressure in the absence of a detrusor contraction.
Detrusor leak point pressure: the lowest detrusor pressure at which urine leakage occurs in the absence of either a detrusor contraction or increased abdominal pressure.
Pressure-flow studies: the method by which the relationship between pressure in the bladder and urine flow rate is measured during bladder emptying.
|
From Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn 2002;21:167–78; and Schafer W, Abrams P, Liao L, et al. Good urodynamic practices: uroflowmetry, filling, cystometry, and pressure-flow studies. Neurourol Urodyn 2002;21:261–74.
Ideally a room of suitable size should be dedicated to urodynamics (Nitti and Combs, 1998). This area does not have to be exclusively for urodynamics, but when a study is being performed, there should not be distractions from people walking into and out of the area for other reasons. A quiet, private area is the best. It is difficult enough to recreate a natural environment during testing without outside distractions. The room should be large enough to allow for the patient to lie down to have catheters placed and also to be able to stand and sit on a commode as needed. Many patients undergoing urodynamic testing will have neurologic problems that limit mobility and will require assistance with positioning. This includes patients in wheelchairs. This must be considered when determining the size of the room. Centers that perform video-urodynamics (VUDS) will require a larger area to allow for x-ray equipment.
The importance of a well-trained, attentive, and supportive staff involved with the UDS study cannot be overemphasized. With that said, in general, UDS is well tolerated. However, patients should be properly prepared and told why the test is being done, how the results may affect treatment, and what to expect during the actual UDS test. Scarpero and colleagues (2005) used a questionnaire-based study to assess patient expectations of anxiety, pain, embarrassment, and apprehension before UDS and compared it with the patient’s actual experience. They found that UDS was associated with minimal to moderate degrees of anxiety, discomfort, and embarrassment. After testing, most respondents (>90% per question) thought that the test was the same or better than expected and had an expected or less than expected level of pain and embarrassment. This did not vary between the sexes, but a higher number of younger individuals found that the test experience was worse than expected, whereas a higher number of older individuals found that it was better than expected. Therefore younger patients may require more reassurance and attention in preparation for the procedure.
Many patients undergoing urodynamic testing will have been placed on medications that affect bladder function (e.g., antimuscarinics). For such patients the clinician should decide in advance what information is desired and whether or not the study should be done on or off medication. For example, if the goal of the study is to determine the therapeutic effect of a medication, obviously the UDS should be done with the patient on a regular dosing schedule for that medication. On the other hand, if medication was started empirically to treat symptoms and the goal of the urodynamic test is to uncover the etiology of those symptoms, then consideration can be given to discontinuing the medication before testing because this may give the highest yield.
Components of the Urodynamic Study
Before discussing the details of the UDS test itself, it is useful to be familiar with its different components. These tests within the test can be used individually or in combination depending on the information desired. For the purposes of this chapter we discuss each component as part of the entire multichannel UDS or video UDS study. Uroflow and postvoid residual (PVR) determination are two simple, noninvasive tests that can be used to evaluate voiding function and perhaps prompt further testing. In addition, both are part of a multichannel UDS study.
PVR is an excellent assessment of bladder emptying. It can be performed by ultrasound (bladder scan) or catheterization. Elevation of PVR indicates a problem with emptying but does not tell why. An elevated PVR may prompt further testing.
Uroflowmetry is measurement of the rate of urine flow over time. It is also an assessment of bladder emptying. Normal uroflow is a bell-shaped curve. When the flow rate is reduced or the pattern is altered, this could indicate bladder (underactive) or bladder outlet (obstruction) dysfunction (Fig. 62–1).
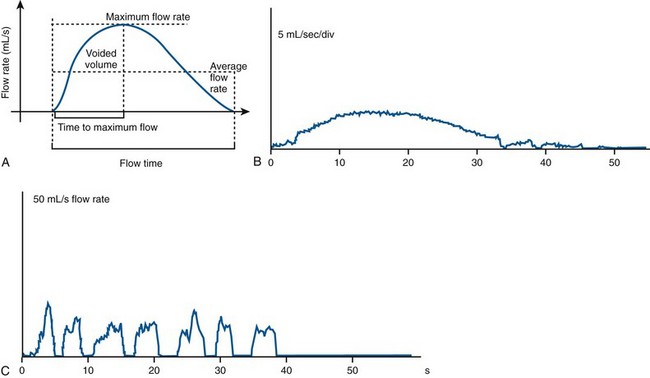
Figure 62–1 Examples of uroflow curves. A, Normal bell-shaped flow curve of flow rate versus time. B, A flattened pattern is usually indicative of obstruction. C, An interrupted or straining pattern, which can be seen with impaired bladder contractility, obstruction, or voiding with or by abdominal straining.
(A from Wein AJ, English WS, Whitemore KE. Office urodynamics. Urol Clin North Am 1988;15:609; B and C from Boone TB, Kim YH. Uroflowmetry. In: Nitti VW, editor. Practical urodynamics. Philadelphia: WB Saunders; 1998. p. 28–51.)
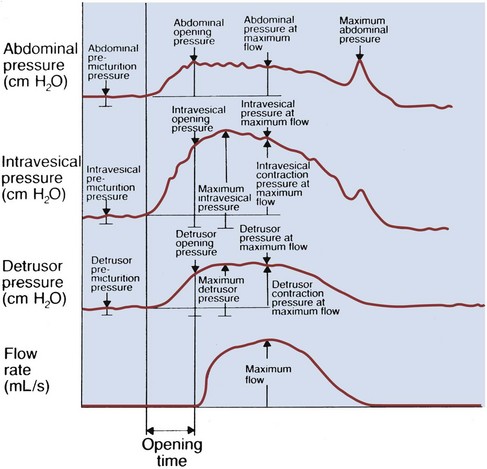
Cystometrogram (CMG): Cystometry or, more appropriately, “filling cystometry” is the method by which the pressure/volume relationship of the bladder is measured during bladder filling. The filling phase starts when filling commences and ends when the patient and urodynamicist decide that “permission to void” has been given. CMG can be performed by the single measurement of bladder pressure via a bladder catheter (urethral or suprapubic); however, changes in bladder pressure can represent a change in detrusor pressure (Pdet) or a change in abdominal pressure (Pabd) (see later). Therefore it is recommended that CMG be performed by measuring both the total vesical pressure (Pves) and Pabd (measured by a catheter placed in the rectum or vagina). To calculate Pdet, the following equation is used: Pdet = Pves − Pabd (Fig. 62–2).
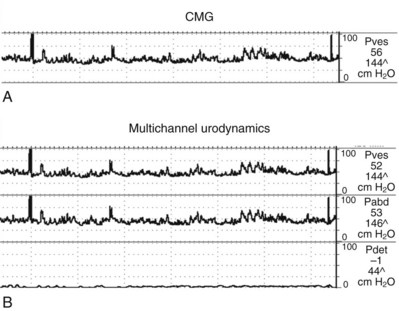
Figure 62–2 Cystometrogram (CMG) measures the pressure in the bladder as the bladder fills over time. Standard fill rates in adults are 30 to 100 cm/H2O per minute. Adding intra-abdominal pressure monitoring gives a better representation of the true detrusor pressure. A, Single-channel CMG where only the total vesical pressure (Pves) is measured. Note the multiple spikes and rises in pressure. Without having simultaneous monitoring of intra-abdominal pressure, it is impossible if these pressure spikes are due to a rise in detrusor or abdominal pressure. B, This same tracing with intra-abdominal pressure (Pabd) monitoring added (Multichannel UDS). This allows for the determination of (subtracted) detrusor pressure (Pdet). Now it can be clearly seen that changes in Pves were due to the changes in Pabd (e.g., movement, coughing). The Pdet curve is noted to be flat and without any rises in pressure.
(From Nitti VW. Cystometry and abdominal pressure monitoring. In Nitti VW, editor. Practical urodynamics. Philadelphia: WB Saunders; 1998. p. 38–51.)
Electromyography (EMG) is the study of the electronic potentials produced by the depolarization of muscle membranes. In the case of UDS, EMG measurement of the striated sphincteric muscles of the perineum is done to evaluate possible abnormalities of perineal muscle function, which are often associated with lower urinary tract symptoms and dysfunction. EMG activity is measured during both filling and emptying. EMG is obtained via electrodes placed in or near the muscle to be measured.
Urethral pressure profile (UPP) is a graph indicating the intraluminal pressure along the length of the urethra. Urethral pressure is defined as the fluid pressure needed to just open a closed urethra. UPP is obtained by the withdrawal of a pressure sensor (catheter) along the length of the urethra.
Pressure-flow studies (PFS) of voiding are the method by which the relationship between pressure in the bladder and urine flow rate is measured during bladder emptying. Detrusor pressure is measured as explained earlier with the simultaneous measurement of flow rate by a uroflowmeter. The voiding phase starts when “permission to void” is given, or when uncontrollable voiding begins, and ends when the patient considers voiding has finished.
Urodynamic Equipment
Urodynamic Systems
Various urodynamic systems are available today. They range in price from several thousand dollars to more than 100,000 dollars depending on their complexity and the features they contain. Most high-end systems are computer-based digital systems that allow for easy data storage and postprocessing of the study. In addition, they allow for hardware and software upgrades as needed. It is beyond the scope of this chapter to describe in detail the options available for UDS systems. However, it is recommended that, when choosing a system, one must consider the patient population and spectrum of diseases frequently encountered, space, convenience of operation (if a factor), and the need for data storage and processing. In addition, it is recommended that a multichannel system in which channels are available to measure vesical pressure, abdominal pressure (and subtracted detrusor pressure), and flow rate is used. Some clinicians may also desire channels for electromyography and urethral pressure measurement. The UDS system and software market is constantly changing, so what is state-of-the-art today may seem “outdated” tomorrow. However, despite all the advances, the clinician performing the study remains the most important constant in data collection and interpretation. For the most realistic assessment, the infusant should be a liquid (e.g., normal saline or radiographic contrast) that will most approximate urine. The use of gas such as carbon dioxide is no longer recommended. Many advanced urodynamic centers now perform VUDS. Adding this capability is expensive but allows one to perform the most comprehensive study possible. There are certain clinical settings in which VUDS is the test of choice (see later). In addition to the necessary urodynamic hardware and software, a fluoroscopy unit and room of adequate size are required. Obviously, this is not practical or necessary in every setting. Video-urodynamic studies also require a greater time commitment on the part of the clinician to ensure accurate data collection.
Signal Transmission and Transducers
Transducers are the hardware that allows pressure in the patient to be measured and transferred to the UDS system. External strain-gauge transducers located “between” the patient and the urodynamic machine have been popular for years. Pressurized tubing (to avoid dampening or dissipation of the pressure) extends from the pressure transducer to the catheters placed in the patient. An electronic cable or “wireless transmission” brings the signal from the transducer to the urodynamic machine. Traditionally a water-filled system in which the entire system from transducer to patient is filled with water has been used. Since this system depends on the transmission of pressure through fluid (water), it is crucial that there are no air bubbles in the transducer or tubing. The pressurized tubing transmission lines should be lucent to allow for easy recognition of air in the line. The transducers are usually set at the level of the patient’s bladder (symphysis pubis) at the start of the study. This is important because if the patient changes position during the test (e.g., standing to sitting), the height of the transducer can be adjusted so that it remains at the level of the bladder.
More recently, air-charged catheters have become popular for pressure measurement (T-Doc, Wenonah, NJ). This catheter uses a miniature, air-filled balloon placed circumferentially around a polyethylene catheter. External forces on the balloon of the catheter are transmitted to the air-filled catheter lumen and communicated to an external semiconductor transducer. The technology of the balloon system allows circumferential measurement readings. The catheters are disposable and single use. Air-charged catheters have several practical advantages over fluid-filled pressure lines because there is no fluid connection between the patient and the urodynamic equipment—just air. This means there is no hydrostatic pressure effect to account for so that there is no need to position anything at the level of the symphysis pubis and there is no need to flush the system through to exclude air (essential when using a fluid-filled system). Also, there are no artifactual fluctuations in pressure produced when the patients move. It must also be remembered that many UDS nomograms and other “standards of measurement” were determined using fluid-filled systems. Although there is comparative evidence for the use of air-charged catheters to measure urethral pressure (Pollak et al, 2004), no studies have shown definitively that air-charged catheters provide an acceptable alternative to fluid-filled lines for measuring intravesical and intra-abdominal pressure in UDS. Thus it is recommended that investigators planning to use air-charged catheters for intravesical and intra-abdominal pressure monitoring check for themselves that they have an equivalent performance to their current system for measuring pressure (Hosker et al, 2009).
Finally a microtip or fiberoptic system can be used to process pressure transmission. Here the transducer is contained within the catheter. This in turn is connected directly into the urodynamic machine via a cable. These catheters are quite expensive but reusable. They must be sterilized before each use.
Uroflowmeters
Urine flow rate or uroflow can be determined by a number of different types of devices or uroflowmeters. Modern uroflowmeters use weight, electrical capacitance, or a rotating disc to determine urinary flow rates. The two most common techniques used today are the weight transducer or load cell method and the rotating disc method. With the load cell the voided “weight” is measured and is then differentiated with respect to time to determine the flow rate. In the rotating disc method the urine stream is directed onto a rotating disc, and the power necessary to keep a disc rotating at a constant rate is measured. This power is proportional to the flow rate. The electronic dipstick flowmeter measures the electrical capacitance of a dipstick mounted in a collecting chamber. The output of the signal is proportional to the accumulated volume, and the volumetric flow rate is determined by differentiation. Each of these methods has advantages and disadvantages. The weight transducer method is simple, reliable, and accurate regardless of the site of stream impact but requires that the density of urine must be set. The rotating disc method is also reliable and accurate, and it provides a direct measurement without need for differentiation of volume with respect to time. Electronic flowmeters provide a range of electronically read flow parameters with graphical depiction of the uroflow and have sufficient precision for clinical use with error rates of 1% to 8% in voided volume and 4% to 15% in flow rate (Susset, 1983). Variations in specific gravity of the fluid voided (infusant when doing UDS studies) can affect the calculated flow rate. Most systems allow for calibrations for various fluids such as radiographic contract.
Electromyography
Muscle depolarization must be detected by an electrode placed in or near the muscle. Several different methods are available to do this including surface electrodes and needle electrodes (the two most common methods), as well as anal plug or urethral catheter–mounted electrodes (O’Donnell, 1998). Surface electrodes are self-adhesive skin patch electrodes that are applied over the skin of the anal sphincter. Except in some neurologic diseases, the external anal sphincter EMG will be the same as the external urethral sphincter EMG. Surface electrodes have a significant advantage compared with the needle electrode regarding patient convenience and comfort. However, the surface electrodes provide an inferior signal source and must be precisely placed to provide an adequate signal source. Most clinicians feel that the concentric needle electrode is the superior technique for obtaining a signal source of EMG activity of the striated external sphincter muscles. Compared with the surface electrode, placement of the needle electrode has the disadvantage of being uncomfortable for the patient, especially if more than one attempt at placement of the electrode is required to obtain an adequate signal. Also, the needle electrode is easily dislodged and may require replacement during the study. Patients typically have a low tolerance for replacement of the needle electrode during urodynamic studies. The performance of an EMG and the selection of the type of electrode to be used depend on the UDS question to be answered.
Urodynamic Study: Analysis and Interpetation
The author has found it useful to divide the UDS test into filling/storage and voiding phases. This allows for ease of classification of voiding dysfunction according to the functional classification system mentioned previously. The filling/storage phase consists primarily of the CMG, provocative testing (e.g., measurement of abdominal leak point pressure), and urethral pressure measurement during storage. The voiding phase evaluates bladder contractility, bladder outlet resistance, and sphincter coordination by pressure-flow analysis and EMG.
Filling and Storage Phase
The CMG assesses the bladder’s response to filling. The CMG can measure filling pressure, sensation, involuntary contractions, compliance, and capacity. Sensation is the part of cystometry that is truly subjective and therefore requires an alert and attentive patient and clinician. Several subjective parameters that can be recorded during filling are recognized by the ICS (see Table 62–1).
Normal Filling and Storage
Normally, the bladder should store urine at low pressure and not contract involuntarily. Once capacity is reached or voluntary voiding is desired, intravesical pressure will increase (voluntary detrusor contraction). In actuality this is preceded by a relaxation of the external sphincter. A normalized adult CMG is shown in Figure 62–3. Normally detrusor pressure should remain near zero during the entire filling cycle until voluntary voiding is initiated. That means baseline pressure stays constant (and low) and there are no involuntary contractions.
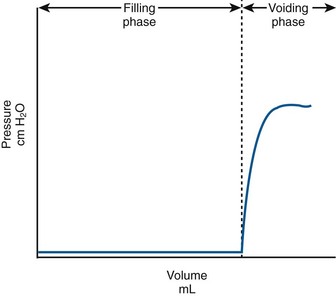
Figure 62–3 Normal, idealized adult cystometrogram with low pressure storage until the patient is given the command to void and the voiding phase starts. Note that the baseline bladder pressure is near zero (compliant) and there are no involuntary contractions.
As mentioned previously, the simultaneous measurement of Pabd, usually by a rectal or vaginal catheter, and Pves during urodynamics provides a means of calculating the true Pdet. The ability to calculate subtracted Pdet allows one to distinguish between a true rise in detrusor pressure (either via a contraction or loss of compliance) and the effect of increased abdominal pressure (e.g., straining, Valsalva). This is especially important when rises in detrusor pressure are small or when they are accompanied by changes in abdominal pressure.
Abnormalities of Bladder Filling—Detrusor Overactivity and Impaired Compliance
During filling, involuntary detrusor contractions (IDCs) can occur. These are often associated with urgency and even urgency incontinence. Detrusor overactivity (DO) is a urodynamic observation characterized by IDCs during the filling phase, which may be spontaneous or provoked (Fig. 62–4). DO may be further characterized as neurogenic DO, which means it is associated with a relevant neurologic condition (e.g., spinal cord injury, multiple sclerosis) or idiopathic DO, which means there is “no defined cause” (non-neurogenic) (Abrams et al, 2002). The term idiopathic is a bit of a misnomer in that the cause of DO in a non-neurogenic patient may be readily apparent (e.g., bladder outlet obstruction, inflammatory process) or may be truly “unknown.” Thus from a practical standpoint, the terms neurogenic and non-neurogenic DO make sense but do not fit the ICS definitions. (It should be noted that the term neurogenic DO replaced the term detrusor hyperreflexia, and the term idiopathic DO replaced the term detrusor instability in previous ICS terminology). Neurogenic and idiopathic DO may look identical on CMG. These terms are strictly defined by the patient’s neurologic status and not the CMG appearance of the IDCs.
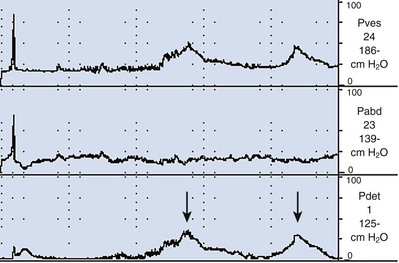
Figure 62–4 Detrusor overactivity. In this tracing there are two involuntary detrusor contractions (arrows). There is a rise in Pves with no associated rise in Pabd, and therefore the subtracted Pdet looks identical to the Pves.
The presence of DO during UDS must be interpreted in the context of the patient’s symptoms and condition. Ideally, a patient’s symptoms should be reproduced during UDS, so we would expect DO to be accompanied by urgency or urgency incontinence, although it can occur and be significant without being symptomatic, particularly in neurogenic DO. However, DO can also be “test induced” or clinically insignificant. It has been reported in 14% to 18% of healthy asymptomatic volunteers undergoing UDS (van Waalwijk van Doorn et al, 1992; Robertson, 1999; Wyndaele et al, 2002). This is even more dramatic in ambulatory UDS studies where DO has been found in up to 69% of asymptomatic females (van Waalwijk van Doorn, et al, 1996). Conversely, failure to demonstrate DO on UDS does not rule out its existence. It is well known that up to 50% of women with urgency incontinence do not demonstrate DO on UDS. However, the ability to suppress DO during UDS test may in and of itself be significant. For example, Osman (2003) showed that patients with mixed incontinence and a normal CMG (no DO) not only had excellent cure rates for stress incontinence but also had an 87% cure rate for urgency incontinence (compared with only 43% cure for urgency incontinence for women randomized to receive antimuscarinic medication instead of stress incontinence surgery).
It is important that the person performing the UDS study be absolutely sure that the contraction is indeed involuntary. Sometimes a patient may become confused during the study and actually void as soon as the desire is felt. The volume at which contractions occur and the pressure of the contractions should be recorded. It is often worthwhile to repeat the CMG at a slower filling rate if the patient experiences uncharacteristic symptoms associated with DO. If the patient experiences incontinence during an involuntary contraction (DO incontinence), this should be noted.
In addition to the presence of DO, its characteristics can be noted. DO can be observed as a single event or as multiple IDCs. It can be phasic (continuous), sporadic, or terminal (occurring at the end of filling near capacity). It can also be suppressed or nonabortable and may lead to leakage or precipitant micturition. Classifying DO in such a way can be valuable in certain circumstances. For example, overactivity bladder symptoms associated with obstruction have been shown to have a higher likelihood of resolving with intervention (e.g., transurethral resection of the prostate [TURP]) when DO occurs as a single terminal IDC rather than continuous or sporadic IDCs (Kageyama et al, 2000).
The viscoelastic properties of the bladder, on the basis of its composition of smooth muscle, collagen, and elastin, normally produce a highly compliant structure. Therefore as the bladder fills there is little change in pressure (see normalized CMG Fig. 62–3). Compliance is the relationship between change in bladder volume and change in detrusor pressure (Δ volume/Δ pressure) and is measured in mL/cm H2O. The ICS recommends two standard points, the Pdet at the start of bladder filling (usually zero) and the Pdet at cystometric capacity or before the start of any detrusor contraction that results in significant leakage (Abrams et al, 2002). Both points are measured excluding any detrusor contractions. It is difficult to define what “normal compliance” is in terms of mL/cm H2O. Several authors have shown that mean values for compliance in healthy subjects range from 46 to 124 mL/cm H2O (Sorensen et al, 1988; van Waalwijk van Doorn et al, 1992; Hosker, 2004). However, there is great variation. For example, van Waalwijk van Doorn and colleagues (1992) showed a variation of compliance from 11 to 150 mL/cm H2O (mean 46) in 17 healthy subjects. Some of the variation in “normal” is likely due to the fact that compliance per se is dependent on bladder capacity. Furthermore, various definitions of impaired compliance have been used (e.g., between 10 and 20 mL/cm H2O); however, there is no consistent definition based on mL/cm H2O. Stohrer and colleagues (1999) suggest that a value of less than 20 mL/cm H2O is consistent with impaired compliance and implies a poorly accommodating bladder. However, examples in which this may not be the case can be cited (e.g., small cystometric capacity). Therefore in practical terms, absolute pressure is probably more useful than a “compliance number” or value. For example, it has been shown that storage greater than 40 cm H2O is associated with harmful effects on the upper tracts (McGuire et al, 1981) (Fig. 62–5). Also, depending on the clinical scenario, a particular compliance in terms of mL/cm H2O can mean different things (Fig. 62–6). As a general rule, prolonged storage at high pressures can lead to upper tract deterioration. Elevated storage pressures and impaired compliance should be interpreted in the context of the clinical scenario. It appears that conventional cystometry may provoke filling pressures higher than natural filling in some cases. Robertson (1999) showed that for six patients with neuropathic bladder and severely impaired compliance on conventional cystometry, compliance was actually normal on ambulatory monitoring with natural filling.
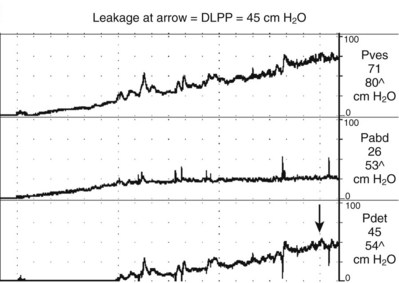
Figure 62–5 Impaired compliance. Note the rise in Pves (and Pdet) with bladder filling. The Pdet at the end of filling is approximately 45 cm H2O, which is a potentially dangerous situation. In this case the bladder was filled to a volume of 300 mL, so the compliance is 6.67 mL/cm H2O. The arrow is the point at which incontinence was demonstrated, which is the detrusor leak point pressure (DLPP).
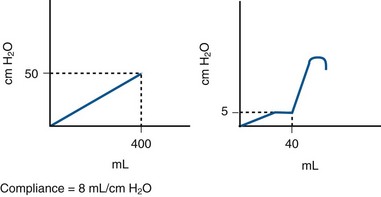
Figure 62–6 A problem with measuring compliance. Shown are two theoretical cystometrograms. The one on the left demonstrates impaired compliance with a constant rise in Pdet throughout filling. At 400 mL the Pdet is 50 cm H2O (8 mL/cm H2O), a dangerous situation. There is significant storage time where the det is greater than 40 cm H2O. The CMG on the right could represent a small-capacity bladder with DO and precipitant micturition. At a volume of only 40 mL, an IDC occurs. The Pdet just before this was only 5 cm H2O. The calculated compliance would be 6 mL/cm H2O, the same as the one on the left. Yet the CMG on the right does not demonstrate a dangerous situation, just a highly symptomatic (incontinent) patient.
Impaired compliance is seen in a variety of neurologic conditions (spinal cord injury/lesion, spina bifida) and usually results from increased outlet resistance (e.g., detrusor-external sphincter dyssynergia [DESD]) or decentralization in the case of lower motor neuron lesions. It can also result from long-term bladder outlet obstruction (e.g., BPH) (Leng and McGuire, 2003) or structural changes like radiation cystitis or tuberculosis.
The measurement of compliance can be affected by a number of factors. Sometimes an increase in Pdet during cystometry is seen as a result of rapid filling (filling during cystometry is almost always faster than physiologic filling). This is more of an accommodation problem rather than a true decrease in compliance. When Pdet is seen to be rising, filling may be stopped or reduced to see if the effect is real. An IDC, particularly if it is of sustained and low amplitude, can be confused with impaired compliance. If filling is stopped and the pressure returns to baseline, then the compliance is not impaired. Finally, a number of “pop-off” mechanisms can make compliance seem better than it actually is. Vesicoureteral reflux and bladder diverticulum are two examples. With reflux, pressure is actually transferred to the refluxing renal unit and may be harmful. The author has seen instances where the upper tract holds more urine than the bladder. VUDS (see later) is useful in these cases. Because a bladder diverticulum is actually part of the bladder, it may provide a protective effect for the upper tracts. Finally, an incompetent outlet may be a pop-off mechanism. This may only become apparent when outlet resistance is increased, which can be done during cystometry by occluding the outlet but may not be seen until the outlet resistance is surgically increased (e.g., with an artificial urinary sphincter or sling procedure).
Leak Point Pressures
Two distinct types of leak point pressures can be measured in the incontinent patient: abdominal leak point pressure (ALPP) and detrusor leak point pressure (DLPP). The two are independent of each other and conceptually measure completely different things.
ALPP is a measure of sphincteric strength or the ability of the sphincter to resist changes in abdominal pressure (McGuire et al, 1993). ALPP is defined as the intravesical pressure at which urine leakage occurs due to increased abdominal pressure in the absence of a detrusor contraction (Abrams et al, 2002). This measure of intrinsic urethral function is applicable to patients with stress incontinence. An ALPP can only be demonstrated in a patient with stress urinary incontinence (SUI). Conceptually, the lower the ALPP, the weaker the sphincter. There is no normal ALPP because patients without stress incontinence will not leak any physiologic abdominal pressure. ALPP should be measured as the total abdominal pressure required to cause leakage, not the change in pressure (McGuire et al, 1993). Therefore if ALPP is measured in the standing position, it should include the baseline Pabd (or Pves), which is usually about 20 to 40 cm H2O. Classically, the reading is taken from the Pves channel as long as there is no involuntary contraction (Fig. 62–7). In cases where patients do not leak with a urethral catheter in place, the ALPP can be measured from the abdominal pressure channel either rectally or vaginally (Stohrer et al, 1999). The original description of ALPP was done at an arbitrary bladder volume of 150 milliliters; however, in some cases it is necessary to fill the bladder more. The volume at which ALPP is determined should be noted as some investigators have found that it decreases at higher volumes (Faerber and Vashi, 1998). As a general rule, one should start testing at 150 mL and then every 50 mL thereafter until SUI is demonstrated. If there is no SUI demonstrated at capacity, the urethral catheter is removed and ALPP is measured via the rectal catheter (provided there is no increase in Pdet from DO or impaired compliance).
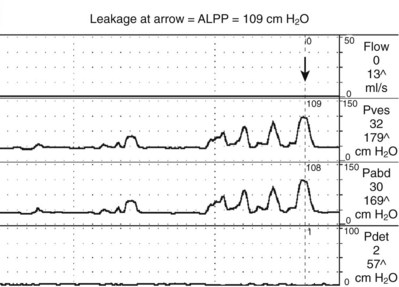
Figure 62–7 Abdominal leak point pressure measurement. After progressive Valsalva maneuvers, leakage is demonstrated on the last one at 109 cm H2O (arrow). There is no rise in Pdet.
Attempts have been made to quantify intrinsic sphincter deficiency (ISD) in women using ALPP. In 1993 McGuire and colleagues measured ALPP in 125 women with SUI. When the ALPP was less than 60 cm H2O, all patients had high-grade incontinence with 81% having continuous leakage and 75% having a fixed urethra (no urethral hypermobility). When ALPP was between 61 and 89 cm H2O, 80% had pronounced urethral hypermobility and moderate to high-grade incontinence. When ALPP was greater than 90 cm H2O, patients had lesser grades of incontinence and minimal to gross urethral hypermobility. The inferences are that:
Current technology does not permit a method to distinguish between ISD in the face of urethral hypermobility in women. Therefore although these ALPP values are often used as guidelines, they should be interpreted with caution. For example, if there is no urethral hypermobility, SUI must be caused by ISD, regardless of the ALPP. Furthermore, Fleischmann and colleagues (2003) found that urethral hypermobility was equally common in women with lower versus higher ALPP. ISD and urethral hypermobility may coexist, and they do not define discrete classes of patients with stress urinary incontinence. Thus an isolated measure of ALPP without considering other factors such as cystometry and urethral mobility is of limited utility in predicting success for commonly performed female SUI procedures (Hosker et al, 2009).
The term ALPP has been used interchangeably with “Valsalva” leak point pressure (VLPP); however, this is not entirely correct. An ALPP can be measured during UDS testing by a voluntary Valsalva maneuver (VLPP) or by a cough (cough leak point pressure or CLPP). In the same person, VLPP tends to be significantly lower than CLPP. Therefore exact terminology and methods should be used when describing an ALPP. ALPP can also be influenced by the presence or the size of a urethral catheter (Bump et al, 1995; Huckabay et al, 2005; Türker et al, 2010). It has been shown in women with SUI that the larger the catheter, the lower the ALPP. ALPP can also be measured without a urethral catheter by assessing the abdominal pressure via a rectal (or vaginal) catheter. It has been shown that 15% of women with SUI (Türker et al, 2010) and 35% of men with postprostatectomy SUI (Huckabay et al, 2005) will demonstrate an ALPP only with the urethral catheter removed.
The second type of leak point pressure is the detrusor leak point pressure (DLPP), which is a measure of detrusor pressure in patients with decreased bladder compliance. It is defined as the lowest detrusor pressure at which urine leakage occurs in the absence of either a detrusor contraction or increased abdominal pressure (Abrams et al, 2002) (see Fig. 62–5). The higher the urethral resistance, the higher the DLPP. One can imagine that in a poorly compliant bladder, if outlet resistance is low, incontinence will occur at a relatively low or “safe” pressure. However, if outlet resistance is high, the pressure in the bladder will continue to increase as the bladder fills. There is potentially less incontinence, but eventually the pressure is transmitted to the upper tracts (Fig. 62–8).
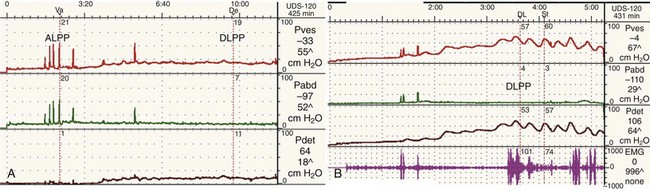
Figure 62–8 Outlet resistance causes impaired compliance. Urodynamic (UDS) studies of two children with the same neurologic problem and symptoms but dramatically different findings. A, UDS tracing of a young boy with spina bifida who is incontinent in between catheterizations. The study shows a low-pressure system with poor outlet resistance and stress incontinence (ALPP demonstrated) and a low DLPP. His upper tracts are protected. B, UDS tracing of a young girl with spina bifida who is incontinent in between catheterizations. The study shows a high-pressure system with strong outlet resistance and a high DLPP. There was no stress incontinence. Her upper tracts are at risk. The difference in these two cases is difference in storage pressures caused by the difference in outlet resistance.
From a clinical perspective, DLPP is most useful in patients with lower motor neuron disease causing “decentralization” and in non-neurogenic patients with low bladder compliance (after multiple bladder surgeries, radiation, and tuberculous cystitis). The higher the DLPP, the more likely is upper tract damage because intravesical pressure is transferred to the kidneys. McGuire (1981) documented the deleterious effects that a detrusor high-leak point pressure has on the upper urinary tracts; detrusor leak point pressures greater than 40 cm H2O result in hydronephrosis or vesicoureteral reflux in 85% of myelodysplastic patients.
The significance of an elevated DLPP is that bladder pressures are getting too high before the “pop-off” mechanism of urethral leakage occurs. In most cases treatment is aimed at lowering bladder pressures, so the DLPP is never reached. In some cases DLPP can be lowered by decreasing outlet resistance, for example, with a sphincterotomy in a patient with DESD.
Key Point: Leak Point Pressures
Stress-Induced Detrusor Overactivity
Sometimes detrusor overactivity can be triggered by a rise in abdominal pressure (Fig. 62–9). Thus the symptom may appear to be stress incontinence, but the condition causing the symptom is actually an involuntary contraction, not sphincteric weakness. In a patient with stress-induced detrusor overactivity (SIDO), it is important to note if there is also urodynamic SUI and/or DO independent of the SIDO.
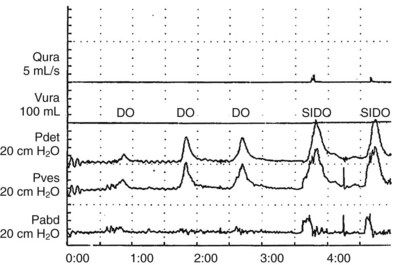
Figure 62–9 Stress-induced detrusor overactivity. In this case, there are three episodes of detrusor overreactivity (DO) preceding two episodes of stress-induced DO (SIDO). In the case of SIDO, note that as Pabd increases, so does Pves. Shortly after this, Pdet rises and continues long after Pabd returns to baseline. With both episodes of stress-induced instability, incontinence occurred, as can be seen on the flow (Qura) curve.
(From Nitti VW: Cystometry and abdominal pressure monitoring. In Nitti VW, editor. Practical urodynamics. Philadelphia: WB Saunders; 1998. p. 38–51.)
Occult Stress Incontinence
Stress incontinence that is demonstrated in a clinically continent woman with pelvic prolapse, only when the prolapse is reduced, is referred to as occult incontinence (Ballert et al, 2009). Prolapse reduction can be done with a pessary, a packing, a forceps, or manually. Technically, if this is demonstrated during urodynamic testing, it may be referred to as urodynamic occult SUI.
Urethral Pressure Profilometry
The method of urethral pressure profilometry (UPP) was popularized by Brown and Wickman in 1969 using a small catheter with lateral apertures. Fluid is continuously infused through this type of catheter. Simultaneous bladder and urethral pressure is measured as the catheter is slowly withdrawn along the course of the urethra. The urethral pressure transducer measures the fluid pressure required to lift the urethral wall off the catheter side holes and thus elevates the circumferential and radial stresses induced by the presence of the catheter in the urethra and the slow urethral profusion. Thus urethral pressure is defined as the fluid pressure needed to just open a closed urethra (Abrams et al, 2002). Accurate measurements are recorded only in those cases in which the urethra is distensible and therefore able to create a perfect seal.
Despite an abundant literature on urethral profilometry, its clinical relevance is controversial. Many urologists do not routinely perform urethral profilometry. The urethral pressure profile (UPP) represents the intraluminal pressure along the length of the urethra in graphic form (Fig. 62–10). Several parameters can be obtained from the UPP:
In most continent women, the functional urethral length is approximately 3 cm and the MUCP is 40 to 60 cm H2O, but normal values vary widely. MUCP has also been used to define ISD. McGuire (1981) performed a retrospective evaluation of women who failed SUI surgery and found that a preoperative MUCP less than 20 cm H2O resulted in higher surgical failure rates. These patients represented a specific subtype of SUI caused by a fixed, open urethra (type III SUI). In 1992 the term was redefined as ISD. Many authors have used the definition of MUCP less than 20 to define ISD; however, this definition has many of the same problems as ISD definitions for ALPP. Another caveat of UPP is that its measurement does not diagnose stress incontinence and SUI is not required to measure it (contrary to ALPP). MUCP in incontinent women has been shown to be lower than in continent women, but there is certainly overlap (Schick et al, 2004). In addition, MUCP is not always indicative of the severity of incontinence. For example, there is a difference between the urethra of an incontinent patient whose MUCP equals 38 cm H2O and that of a continent woman with the same MUCP.
In 2002 the ICS standardization subcommittee concluded that the clinical utility of urethral pressure measurement is unclear (Lose et al, 2002). Furthermore, there are no urethral pressure measurements that (1) discriminate urethral incompetence from other disorders; (2) provide a measure of the severity of the condition; and (3) provide a reliable indicator to surgical success and return to normal after surgical intervention (Lose et al, 2002).
Voiding and Emptying Phase
Normal Voiding and Emptying
Evaluation of the voiding phase provides an assessment of both detrusor contractility and bladder outlet resistance, the two parameters that are critical for normal bladder emptying. In simple terms, abnormalities of bladder emptying are caused by too much outlet resistance, inadequate (strength or duration) bladder contractility, or a combination of both. The simultaneous measurement of detrusor pressure and urinary flow rate during voluntary voiding, known as a PFS, is the most accurate way to access these two critical parameters (Fig. 62–11).
In order to understand the relationship between bladder contractility and outlet resistance, one must start with an understanding of the normal micturition process. Normal voiding is accomplished by activation of the micturition reflex, which involves the following (Fig. 62–12):
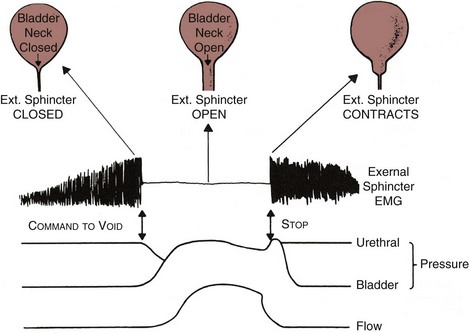
Figure 62–12 Physiology of micturition. See text for details.
(From Blaivas JG. Pathophysiology of lower urinary tract dysfunction. Clin Obstet Gynecol 1885;12:295–309.)
This occurs as a result of coordination between pontine and sacral micturition centers with suprapontine input, which allows for voluntary control of the micturition reflex.
UDS can evaluate the critical parameters during the voiding phase: detrusor contractility, relaxation of the bladder outlet, and coordination of sphincters (Fig. 62–13).
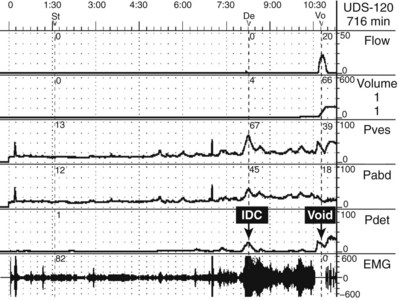
Figure 62–13 Multichannel urodynamics study showing filling and voiding phases with pressure and electromyography (EMG) readings. In this case, the patient experienced an involuntary detrusor contraction (IDC) that led to increased external sphincter contraction and an increase in EMG activity (guarding reflex). However, shortly after that the patient is given permission to void. First there is quieting of the EMG (sphincter relaxation) followed by an increase in Pdet and volitional voiding with a normal-appearing uroflow curve.
According to the ICS, normal detrusor function is characterized by a voluntarily initiated continuous contraction that leads to complete bladder emptying within a normal time span, and in the absence of obstruction. Detrusor underactivity is defined as a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying within a normal time span. Finally, an acontractile detrusor is when there is no demonstrable contraction during UDS (Abrams et al, 2002). Normal detrusor function and detrusor underactivity are “nebulously defined” in terms of absolute pressure because that will depend on outlet resistance. When evaluating detrusor function urodynamically, UDS must be correlated with clinical findings. For example, if a patient who normally voids is unable to void during a UDS study, one cannot make a definitive diagnosis of “acontractile detrusor.”
Urinary flow rate in combination with PVR is a useful clinical tool to assess emptying. When flow rate is reduced or PVR is elevated, it tells us that emptying is not complete but does not tell us why (e.g., obstruction vs. impaired contractility). Flow rate also depends on voided volume because there is a linear relationship between Qmax and voided volume with a voided volume above and a hyperbolic relationship below a voided volume of 150 mL (Drach et al, 1979). Therefore many authors have recommended a minimum voided volume of 150 mL to accurately assess uroflow. However, establishing a minimum voided volume puts major limitations on uroflowmetry because many patients with voiding dysfunction do not routinely void large enough volumes to be evaluable. The corrected form of Qmax, Qmax divided by the square root of voided volume, may provide useful information in such patients (Boone and Kim, 1998). Over the years several nomograms have been developed to define normal flow rates for a specified population and correct for voided volume. These include the Siroky nomogram (Siroky et al, 1979, 1980) for men and the Liverpool nomogram (Haylen et al, 1989) for men and women.
The bethanechol supersensitivity test has been used to help distinguish the cause of detrusor underactivity as neurogenic or myogenic. It is based on the Cannon theory of denervation, which states that denervated structures develop increased sensitivity to chemical stimulation. This concept was applied to the bladder by Lapides and colleagues (1962). The original bethanechol supersensitivity test described by these authors was performed by infusing liquid at a rate of 1 mL/sec to a volume of 100 mL, where the pressure is measured. This can be done up to three times, and the pressure values averaged. The patient is then given 2.5 mg of bethanechol chloride (later revised to 0.035 mg/kg) subcutaneously, and the study is repeated at 10, 20, and 30 minutes. A normal bladder (or myogenically impaired bladder) should show an increase of less than 15 cm H2O above control value at 100 mL at 30 minutes. This is considered a negative study. A positive study, indicating a sensory or motor paralytic bladder, is a response of at least 15 cm H2O above the control value. More recent studies have indicated that the bethanechol supersensitivity test is rather unreliable in predicting “neurogenic bladder.” Blaivas and colleagues (1980) reported only 76% sensitivity and 50% specificity in doing this. Another problem is that even if the test is able to differentiate between neurogenic and myogenic dysfunction, treatment is often the same (e.g., clean intermittent catheterization). Bethanechol chloride, whether administered subcutaneously or orally, has not proved to be a consistently effective treatment for the underactive detrusor (Wein et al, 1978, 1980). In addition, a positive test does not predict improved voiding when it is used therapeutically. Therefore we feel that there is a limited role for the use of the bethanechol supersensitivity test.
Pressure-Flow Relation
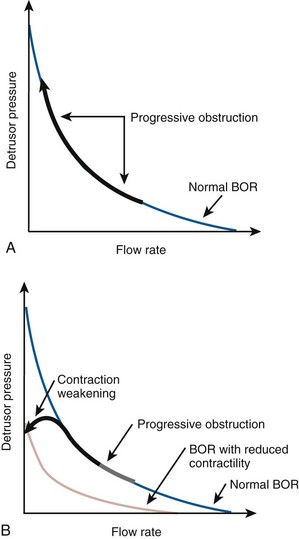
As mentioned previously, detrusor pressure during voiding is a function of outlet resistance. For a normal detrusor, the greater the outlet resistance is, the higher the detrusor pressure during voiding will be. This is accompanied by a reduced flow rate. A healthy bladder is able to overcome obstruction by contracting more forcefully. Although the flow may be slower, the bladder can empty itself. Over time, the detrusor may decompensate and no longer be able to generate the necessary pressure to overcome obstruction. When this occurs, the result will be incomplete bladder emptying or retention of urine. Thus the urodynamic manifestation of bladder outlet obstruction (BOO) is high-pressure and low-flow voiding (or more practically speaking, increased pressure and reduced flow). Over time, if bladder decompensation results, detrusor underactivity or impaired contractility can result.
In order to use the common measures of obstruction and impaired contractility that are used today, it is important to understand basic bladder output and urethral resistance relations. Attempts to mathematically define urethral resistance date back to 1962 (Gleason and Lattimer). Early equations calculating urethral resistance followed standard hydrodynamic formulas calculating outlet resistance. These concepts failed to consider that the urethra is not a rigid tube but rather has an active and a distensible nature. They also failed to consider the importance of bladder volume. Rigid tube hydrodynamics were abandoned in favor of more dynamic ways to analyze micturition. In 1972 Griffiths introduced Bladder Output Relation (BOR), which depicts the interrelations between bladder pressure and uroflow at a given volume and essentially measures the function of the bladder independent of the function of the urethra. Griffiths further defined a method to evaluate urethral resistance independent of bladder function, the urethral resistance relation (URR). According to this relation, as bladder pressure rises the flow rate will be zero until the intrinsic bladder pressure equals the intrinsic urethral pressure. At this point flow will start and the flow rate will rise rapidly with further increases in the intrinsic bladder pressure. If pairs of simultaneously measured values of detrusor pressure and flow rate are plotted against one another throughout the course of a micturition event, a curve is obtained. The curve shows the resistance to flow independent of detrusor function, representing the urethral resistance relation. If the urethra were relaxed or tightened during voiding, the URR would move toward the left or right, respectively. Because the BOR represents the function of the bladder independent of the urethra and the URR depicts urethral function independent of bladder function, the actual detrusor pressure and flow rate are determined by the intersection of the bladder output relation and the urethral resistance relation, which is the point at which intrinsic bladder pressure equals urethral pressure (Fig. 62–14). A change in one of these relations during micturition would not affect the curve representing the other relation but would result in the point of intersection to moving along that curve.
Bladder Outlet Obstruction in Men
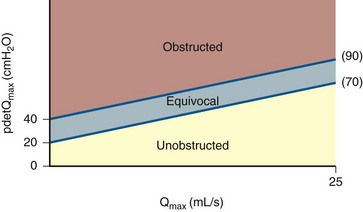
The value of making a precise diagnosis of obstruction in men comes from the assumption that the outcomes of surgery to treat benign prostatic hyperplasia (BPH) and its consequent lower urinary tract symptoms (LUTS) are improved when obstruction can be documented. Because BPH and benign prostatic obstruction (BPO) are highly prevalent conditions, it was intuitive to use them as a model for defining obstruction. Most of the analytic work has focused on defining obstruction-based PFS. Three well-known nomograms based on PFS have been described to diagnose men as obstructed, equivocal, or unobstructed. These are the Abrams Griffiths nomogram (Abrams and Griffiths, 1979); the Urethra Resistance Factor (URF) (Griffiths et al, 1989); and the Linear Passive Urethral Resistance Relation or Schafer nomogram (Schafer, 1990). The categories of obstruction described in these nomograms are based on observations of men who underwent surgery for LUTS (mainly transurethral resection of the prostate, TURP). After surgery, the detrusor pressure at maximum flow (PdetQmax) was reduced in the obstructed group, reduced unpredictably in the equivocal group, and unchanged in the unobstructed group. Subsequently, Lim and Abrams (1995) showed that patients were similarly classified by all three methods. They described a number, the AG number (now known as the bladder outlet obstruction index [BOOI]), derived from the equation for the slope of the line dividing obstructed from equivocal in the Abrams Griffiths nomogram, which is the same line dividing obstructed from slightly obstructed in the Schafer nomogram: BOOI = PdetQmax − 2(Qmax). Subsequently, Griffiths and colleagues (1997) (Fig. 62–15) described the ICS provisional nomogram, which is now suggested for use in diagnosing obstruction in men with LUTS suggestive of BPH (Abrams, 1999). Men are considered obstructed if BOOI is greater than 40, unobstructed if BOO is less than 20, and equivocal if BOOI is 20 to 40.
Voiding PFS show consistent reproducibility in the diagnosis of BOO in men. In a review of the topic, Abrams and colleagues (2001) concluded that random variations of about 9 to 14 cm H2O in pressure measurement and about 0.4 to 2 mL/sec in maximum flow rate occur. In repeated studies during the same session there is usually a systematic decrease of up to 4 cm H2O in detrusor pressure and 0.4 mL/sec in maximum flow rate. These variations have little clinical importance because they cause only 10% to 16% of patients to change classification on the ICS nomogram, and in all but about 1% that change is only by one class (e.g., from equivocal to unobstructed or from obstructed to equivocal).
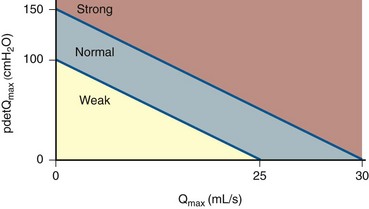
Although much effort has focused on defining outlet resistance (i.e., obstruction), an index for bladder contractility can also be derived from the contractility groups Schafer (1995) described (strong, normal, weak, very weak). The slope of Schafer’s lines, now known as the bladder contractility index (BCI), is given by the formula: PdetQmax + 5(Qmax) (Abrams, 1999). Strong contractility is a BCI greater than 150, normal contractility a BCI of 100 to 150, and weak contractility a BCI of less than 100 (Fig. 62–16).
Although contractility and obstruction can be independently measured, it is sometimes difficult to diagnosis obstruction in the face of impaired contractility using the ICS or other nomograms. For example, in order to make a diagnosis of unequivocal obstruction, BOOI must be at least 40 cm H2O. That means that a Pdet of at least 40 cm H2O must be generated and that assumes flow is zero. If the Qmax is 5 mL/sec, then PdetQmax must be at least 50 cm H2O. Thus the ICS nomogram can exclude obstructed patients whose impaired contractility is the result of long term obstruction. In such cases, clinical judgment becomes important.
BOO is associated with abnormalities of storage as well. This is presumably due to changes in ultrastructure that occur with obstruction. Detrusor overactivity and impaired compliance occur in conjunction with obstruction. For example, about two thirds of men with symptomatic BPO have DO that resolves 50% to 67% of the time with treatment of obstruction (Abrams et al, 1979). Reduced compliance is also associated with obstruction and has been shown to improve with treatment of obstruction (TURP) (Leng and McGuire, 2003).
An alternative to voiding PFS as a way of measuring outlet resistance is the micturitional urethral pressure profile (MUPP), or voiding profilometry. This technique, popularized by Yalla, can both diagnose and localize obstruction (Yalla et al, 1980, 1981). The MUPP is performed with a triple-lumen catheter under fluoroscopic guidance similar to the static UPP described earlier. During voiding the catheter is slowly withdrawn and the pressure is measured from the bladder neck through the anterior urethra (Steele et al, 1998). Normally during voiding, the pressure in the bladder is isobaric with the prostatic urethra and then pressure decreases across the membranous urethra and gradually decays along the rest of the anterior urethra. The membranous urethra is the narrowest segment of the bladder outlet during voiding, which accounts for the expected pressure drop of 20 to 30 cm H2O. In patients with obstruction secondary to BPH, the MUPP is quite different. One will typically see a pressure disparity somewhere along the prostatic urethra. When this pressure disparity is greater than 5 cm H2O, obstruction at the point of pressure drop is present. MUPP has been shown to be as effective in diagnosing bladder outlet obstruction as standard pressure-flow studies (DuBeau et al, 1995). An analysis of symptomatic BPH patients has shown that successful treatment outcomes have been achieved with treatment based on MUPP results (Lecanwasam et al, 1994).
Bladder Outlet Obstruction in Women
Bladder outlet obstruction in women can present more of a diagnostic dilemma than in men. Because there is no highly prevalent condition (like BPH) that causes female obstruction, it is difficult to establish nomograms. Furthermore, nomograms derived for men cannot be applied to women because voiding dynamics differ. In addition, anatomic differences allow many women to empty their bladders by simply relaxing the pelvic floor, and some will augment voiding by abdominal straining. Minor elevations in detrusor pressure or decreases in flow rate, which might be considered insignificant in the male population, might signify BOO in women. Accordingly, clinicians must have a high index of suspicion on the basis of the presence of lower urinary tract symptoms, incomplete emptying, persistent urinary tract infections, and a history of anti-incontinence surgery, prolapse, or other conditions.
In an effort to develop cut-off values for pressure and flow for the diagnosis of obstruction in women, Chassagne and colleagues (1998) studied a group of “clinically obstructed women” (after incontinence surgery; secondary to cystocele; or “other etiologies”) and compared them with a group of controls (women with stress incontinence). Using receiver operator (ROC) curve analysis, they found the optimum sensitivity and specificity for predicting obstruction was obtained with a Qmax less than 15 mL/sec and a PdetQmax of greater than 20 cm H2O (74.3% sensitivity and 91.1% specificity). In 2000 with an expanded population, the authors (Lemack and Zimmern, 2000) revised this to Qmax = 11 mL/sec and PdetQmax = 21 cm H2O as optimal for the selection of patients with BOO. In the most recent publication (Defreitas et al, 2004), these authors used normal asymptomatic women as the control group and found the highest sensitivity and specificity for predicting obstruction was obtained with Qmax less than 12 mL/sec and PdetQmax greater than 25 cm H2O. These cut point studies have some limitations, namely that obstruction was predefined clinically and only patients with anatomic obstruction were included. Women with functional obstruction (e.g., from primary bladder neck obstruction or dysfunctional voiding) were not included in any of the cut point analyses. This would be a difficult group of women to define “clinically” without any testing.
In 1999 Nitti and colleagues showed that the addition of fluoroscopic imaging to urodynamics was helpful in diagnosing female BOO (see section on VUDS). In this study, patients were classified as obstructed if there was radiographic evidence of obstruction between the bladder neck and distal urethral in the presence of a sustained detrusor contraction of any magnitude. In addition to diagnosing BOO, it also localizes the site of obstruction and allows for the diagnosis of obstruction in the face of impaired contractility if indeed the site can be localized. With both the video-urodynamic and cut point criteria there is a significant difference in mean Qmax and PdetQmax in the group of obstructed versus the group of unobstructed women, but there is a large overlap of values between obstructed and unobstructed patients. This demonstrates that absolute pressure and flow values are imprecise and that another parameter (e.g., radiographic or clinical evidence of obstruction) is necessary for diagnosis.
Blaivas and Groutz (2000) presented a nomogram for defining female BOO. Citing the fact that in their series there was a significantly higher flow rate in the same woman without a catheter, they chose to use noninvasive flow rate in their nomogram. Also, because they found no statistical difference between PdetQmax and Pdetmax in obstructed or unobstructed patients, they chose Pdetmax as the pressure parameter. Using cluster analysis to classify patients with low- and moderate-grade obstruction, they formulated the nomogram. The nomogram places women into four zones: no, mild, moderate, and severe obstruction. An obvious criticism of the nomogram is that it is based on two separate voids (invasive and noninvasive), and one must assume that the pressure characteristics of the void are the same. Akikwala and colleagues (2006) compared the three methods of diagnosing BOO in women and found good concordance between the video-urodynamic and cut point criteria. They also noted that the Blaivas Groutz nomogram over diagnosed obstruction compared with the other two methods.
Obstruction in women cannot be defined by the ICS nomogram or the BOOI because these will grossly underestimate female BOO. This is because normally women void at much lower pressures than men, and therefore the obstructed female bladder outlet may not respond as dramatically (or at least with the same pressures) as in the male. Unfortunately there is no condition in women that causes BOO as commonly as BPO in men, and therefore creating a consistent standard is difficult. Thus concepts are the same (higher pressure and lower flow), but the values are different and less well defined. Those who are interested are referred to suggested readings (Nitti et al, 1999; Blaivas and Groutz, 2000; Defreitas et al, 2004; Akikwala et al, 2006).
Sphincter Coordination
External Sphincter
Normal voiding requires external sphincter relaxation followed by contraction of the detrusor. The external sphincter (and internal sphincter) should remain relaxed until voiding is complete. In normal voluntary voiding, a rise in detrusor pressure is preceded by a fall in urethral pressure and relaxation of the external sphincter as measured by electromyography (EMG). The sphincter and urethral pressure remain low during voiding and then increase when voiding is completed (Fig. 62–17). Failure of the sphincter to relax or stay completely relaxed during micturition is abnormal (Abrams et al, 2002). Thus normally EMG activity decreases before a voluntary bladder contraction; however, it is not abnormal for EMG activity to increase with an involuntary contraction as part of a guarding reflex to inhibit the IDC (see Fig. 62–11).
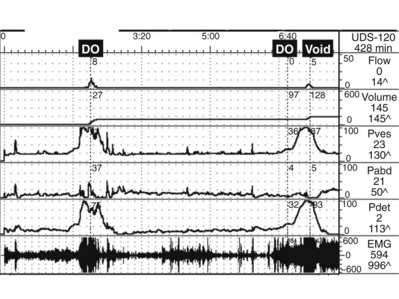
Figure 62–17 Urodynamic tracing of a patient with myelodysplasia and neurogenic detrusor overactivity (DO) and detrusor-external sphincter dyssynergia (DESD). Note the initial involuntary detrusor contraction associated with DESD and incontinence (measured on the flow channel). With refilling there is again DO with DESD. Then the patient is told to voluntarily void, and there is persistent increased electromyographic activity. As a result there is high-pressure, low-flow voiding (obstruction from the dyssynergic sphincter).
Several abnormalities are related to external sphincter relaxation (or lack thereof). DESD occurs when there is an involuntary increase external sphincter associated with DO and also with voiding (see Fig. 62–17). It is caused by a neurologic lesion in the suprasacral spinal cord. DESD can produce profound changes as the detrusor involuntarily contracts against a relatively closed sphincter. This will result in high pressures and can even cause impaired bladder compliance over time. Because long periods of elevated detrusor pressure during bladder filling or (abnormally prolonged) voiding put the upper urinary tract at risk (McGuire et al, 1996; Kurzrock and Polse, 1998; Tanaka et al, 1999). DESD may be considered a urodynamic risk factor for upper tract deterioration. True DESD only occurs when there is a known neurologic lesion above the sacral micturition center. The higher the lesion, the more likely DESD will occur (Blaivas, 1982). If there is no neurologic lesion, then the “dyssynergia” is considered to be a learned behavior and is known as dysfunctional voiding. The term dysfunctional describes malfunction (failure to relax or involuntarily contraction of the external sphincter) during the voiding phase only and says nothing about the storage phase (Nevéus et al, 2006). However, it is entirely possible and quite common for a patient to experience storage symptoms (and UDS abnormalities) associated with dysfunctional voiding. Although the condition has been extensively described in children, it has also been described in adult men (Kaplan et al, 1997; Nitti et al, 2001; He et al, 2009) and women (Carlson et al, 2001) and can be a major cause of LUTS. It is recommended that when dysfunctional voiding is diagnosed by UDS, the flow pattern (reduced and/or intermittent) is confirmed by noninvasive uroflowmetry to rule out a test-induced phenomenon (Barrett and Wein, 1981; Carlson et al, 2001). He and colleagues (2010) used the following diagnostic criteria in men: nothing abnormal detected in the history and no symptoms on an examination for neurologic diseases; transient and intermittent closure of the external sphincter during voiding detected by EMG and fluoroscopic cystourethrography; a higher external sphincter EMG activity with no abdominal pressure increase in the voiding phase. Uroflowmetry was assessed individually to show any discontinuity in a diagram of urinary flow, in conditions with as little external interference as possible.
Internal Sphincter
Just as there can be a lack of coordination of the detrusor and external sphincter, so too can there be dyscoordination of the internal sphincter or bladder neck. In the case of neurologic disease, if a suprasacral spinal cord lesion is above the level of the sympathetic ganglia (T10-L1), detrusor-internal sphincter-dyssynergia may occur in conjunction with external sphincter dyssynergia (Pan et al, 2009). In non-neuropathic men, women, and children the phenomenon of bladder neck dyssynergia or primary bladder neck obstruction is a well-known cause of LUTS, although its exact etiology is not known (Diokno et al, 1984; Norlen and Blaivas, 1986; Combs et al, 2005). Conditions of internal sphincter dysfunction require VUDS for an exact diagnosis and are described later in the next section.
Video-Urodynamics
VUDS consists of the simultaneous measurement of UDS parameters and imaging of the lower urinary tract. It provides the most precise evaluation of voiding function and dysfunction and is particularly useful when anatomic structure and function are important (Keane at al, 1993; McGuire et al, 1996). Examples of where VUDS are useful include the localization of obstruction, detecting incontinence not seen on physical examination, and evaluating vesicoureteral reflux during storage and/or voiding. It can be particularly useful in cases of neuropathic voiding dysfunction. VUDS is the only way to evaluate bladder neck dysfunction and can confirm sphincteric dysfunction diagnosed by EMG. Also, there are instances where a known anatomic abnormality exists and simultaneous imaging can determine if that abnormality is playing a role in voiding dysfunction (e.g., bladder or urethral diverticulum, vesicoureteral reflux).
VUDS can be performed using various different methods. Most commonly fluoroscopy is employed using a C-arm. This gives the most flexibility in allowing patient positioning. However, a fixed unit with a fluoroscopy table that can move from 90 to 180 degrees may also be used. It is important that the patient can be positioned properly to evaluate the desired function and anatomy. For example, SUI in men and women is best evaluated in the standing position. Voiding is best evaluated in the position that the patient characteristically voids (usually sitting for women and standing for men). It is always recommended that fluoroscopy time be limited and focus on situations of high yield such as during provocative maneuvers to demonstrate SUI, during rises in pressure associated with impaired compliance or involuntary contractions, and during voiding.
VUDS can be extremely useful for the diagnosis of bladder outlet obstruction in women (Nitti el al, 1999; Blaivas and Groutz, 2000). In fact, Nitti and colleagues (1999) described the VUDS criteria for the diagnosis obstruction in which radiographic evidence of obstruction between the bladder neck and urethral meatus during voluntary voiding defines and localizes obstruction. Primary bladder neck obstruction can only be diagnosed on VUDS. Figures 62-18 and 62-19 distinguish between the two most common causes of functional obstruction in women, primary bladder neck obstruction, and dysfunctional voiding.
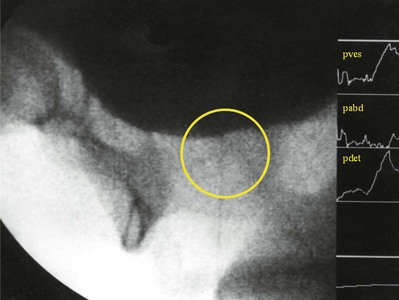
Figure 62–18 Primary bladder neck obstruction in a 35-year-old woman with obstructive voiding symptoms and intermittent urinary retention. Note the failure of the bladder neck to open at all, despite a detrusor contraction of greater than 60 cm H2O.
(From Nitti VW. Primary bladder neck obstruction in men and women. Rev Urol 2005;7[Suppl 8]:S12–7.)
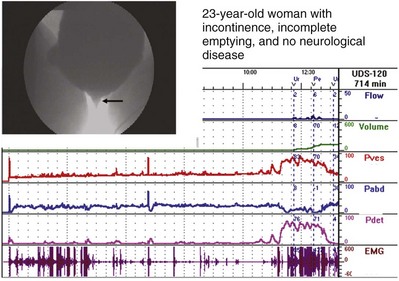
Figure 62–19 Dysfunctional voiding—a urodynamics study of a 23-year-old woman with urgency incontinence, incomplete emptying, and no neurologic disease. Just before voiding there is an involuntary detrusor contraction. With voiding there is increased EMG activity. The fluoroscopic picture taken during voiding shows a characteristic “spinning top urethra” with the level of obstruction at the external sphincter. The high-pressure–low-flow voiding is also characteristic of obstruction.
Similarly, VUDS can be used to evaluate LUTS in young men and in particular make a diagnosis of primary bladder neck obstruction (Norlen and Blaivas, 1986; Kaplan et al, 1996). Although obstruction can be diagnosed by PFS alone, many surgeons would not feel comfortable performing surgical intervention on a young man without localizing that obstruction. In addition, sometime bladder neck obstruction can present without classical findings of high pressure and low flow. Three distinct types have been described (Nitti et al, 2001): classic high pressure—low flow (type I); normal pressure—low flow with narrowing at the bladder neck (type II); and delayed opening of the bladder neck (type III). Figure 62–20 shows types I and II male primary bladder neck obstruction. Fluoroscopy is critical to the diagnosis, especially in types II and III.
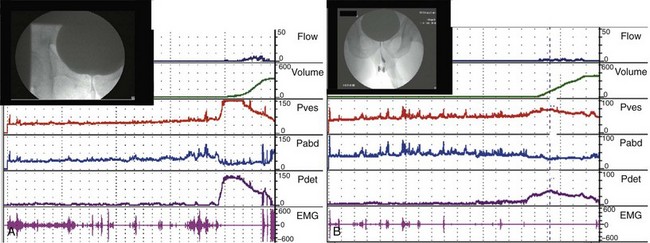
Figure 62–20 Primary bladder neck obstruction (dysfunction). A, Type 1—high-pressure, low-flow voiding in a 45-year-old male with severe lower urinary tract symptoms (LUTS) including frequency, urgency, and decreased force of stream. Image is taken during voiding—note the incompletely opened bladder neck. B, Type 2—normal-pressure, low-flow voiding in a 35-year-old male with similar LUTS as A. There is also an incompletely open bladder neck during voiding. The much lower voiding pressures compared with A should still be enough to empty normally, though there may be a component of impaired contractility because the bladder is unable to compensate for the increased resistance at the bladder neck.
(From Nitti VW. Primary bladder neck obstruction in men and women. Rev Urol 2005;7[Suppl 8]:S12–7.)
VUDS is an important aid in diagnosing neuropathic voiding dysfunction, as well as other conditions that may cause elevated storage pressures. In cases where vesicoureteral reflux occurs, the volume and pressure at which it starts can be documented. In fact, in cases of impaired compliance, in which there is compensation by the “pop-off” mechanism of vesicoureteral reflux, the impaired compliance might not be identified unless the reflux is also recognized by fluoroscopy. In addition, an accurate DLPP can be obtained in cases where it would otherwise be impossible to position a patient to observe leakage (e.g., some tetraplegics). Furthermore, in cases of possible internal sphincter dyssynergia (often found in conjunction with external sphincter dyssynergia), VUDS is the only way to make the diagnosis and can dramatically change treatment (Fig. 62–21). The latest ICI recommendations (Hosker et al, 2009) include that VUDS be used, if possible, to evaluate patients with suspected neuropathic lower urinary tract dysfunction to “establish the state and function of the lower urinary tract.” Although VUDS can be helpful in many cases, it is not readily available to all physicians.
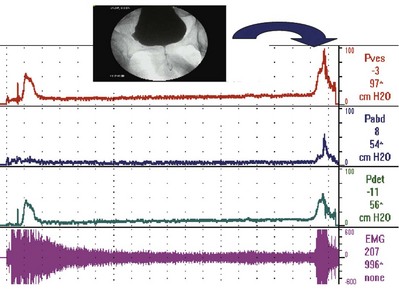
Figure 62–21 Video-urodynamics evaluation of a cervical spine–injured man with neuropathic voiding dysfunction with incontinence and incomplete emptying. The urodynamic tracing clearly shows detrusor overactivity with detrusor-external sphincter dyssynergia (increased electromyographic activity with involuntary contraction). However, it is only with the fluoroscopic view of the bladder outlet during an involuntary contraction that detrusor-internal sphincter dyssynergia is diagnosed (the bladder neck remains relatively closed).
Key Points: Video-Urodynamics
Ambulatory Urodynamics
Ambulatory urodynamic studies are defined as a functional test of the lower urinary tract, using natural filling and reproducing the subject’s everyday activities (Abrams et al, 2002). The development of natural and slow-filling urodynamic studies was initially undertaken in the investigation of neuropathic patients by Comarr (1957) using diuresis-induced natural filling. He demonstrated an increase in bladder capacity and decreases in bladder pressures during filling when compared with CMG. Similar investigations of spinal injury patients by Tsiju and colleagues (1960) demonstrated increased phasic activity associated with incontinence during natural filling. Today it is a well-established method for investigating lower urinary tract function under the conditions of normal daily activities. Ambulatory UDS has its greatest value in patients in whom conventional UDS is not suitable or is unable to reproduce symptoms in question.
In 2000 the ICS published guidelines for the performance of ambulatory UDS (van Waalwijk van Doorn et al, 2000). Before the investigation, patients receive detailed information describing the test and the necessary preparation. Patients are instructed on how to accurately record symptoms and how to identify catheter displacement and hardware failure. A sample diary is given to record all relevant events so that UDS findings can be correlated with symptoms. Most systems employ microtip transducer catheters, which allow the most mobility. These are placed transurethrally to record bladder pressure and transrectally to record abdominal pressure. These catheters are firmly secured to the patient and are connected to a portable recording device. Some systems contain a third channel, which can be used for measuring urinary leakage objectively using an absorbent electronic (capacitance change) nappy pad (Robertson and Neil, 1998). This allows accurate data on the relationship of urinary leakage to detrusor activity to be obtained. Home uroflowmetry units are also available. After the completion of testing, ambulatory UDS tracings are analyzed, and this can be a time-consuming process depending on the length of the study. This must be done with great care and frequent quality checks to make sure that urethral and abdominal catheters are properly transducing pressure (e.g., using cough tests). In addition, the reader must be able to identify physiologic artifacts (after contractions and aberrant rectal pressures) and technical artifacts (movement or variation in pressure and lack of balance in the transducer lines), which could affect the interpretation of the study. The ambulatory study should be designed to reproduce symptoms. For example, if the patient complains of stress incontinence, a standard protocol of exercises can be performed and recorded (e.g., jumping up and down, squatting, coughing).
Clinical Utility of Ambulatory Urodynamics
Ambulatory UDS is performed in an effort to capture more realistic or more physiologic observations, especially of incontinence episodes (Hosker et al, 2009). It attempts to increase sensitivity by providing a longer time for DO (and other abnormalities) to manifest itself. Practically speaking, ambulatory UDS is most useful when standard UDS is inconclusive and diagnosis and, more importantly, treatment are uncertain. Ambulatory UDS has been most commonly used to diagnose the cause of urinary incontinence, but it has also been applied to the diagnosis of male bladder outlet obstruction and neuropathic voiding dysfunction.
It has been shown that ambulatory monitoring detects more actual incontinence than conventional cystometry (Cassidenti and Ostergard, 1999). Several studies have shown ambulatory UDS to be more sensitive than conventional CMG for the diagnosis of DO (Robertson et al, 1994; Heslington and Hilton, 1996). Radley and colleagues (2001) found that ambulatory monitoring revealed DO in 70 of 106 women with symptoms suggestive of DO (twice as many as conventional cystometry with provocation by hand washing) and it detected DO incontinence (DOI) in 40 of the 70. The observation of DOI was correlated with symptom severity, but it is not clear how many women complaining of urgency incontinence showed DOI. Thus the sensitivity is unknown. The finding of higher rates (and sensitivity) of DO found on ambulatory monitoring must be weighed against the fact that ambulatory UDS has found rates of DO asymptomatic volunteers. DO has been found in asymptomatic females as often as 69% versus 18% for conventional CMG (vanWaalwijk van Doorn et al, 1996) and in 38% versus 17% in asymptomatic men and women (Robertson et al, 1994). Thus some degree of detrusor overactivity may be normal in the setting of a urethral catheter for a prolonged period of time, making some sort of standardization important.
In a review of 422 female ambulatory UDS studies over a 12-year period, Patravali (2007) argued for the value of the study. It was seen that 85% of patients complaining of urinary incontinence showed detectable leakage with a diagnosable mechanism on ambulatory study, and in 77% of 74 women with a normal CMG ambulatory UDS diagnosed the cause of incontinence and provided “clear added value.” DO was a component in 42 of these 57 patients (76%). What is unknown is exactly what was meant by “clear added value” and how treatment was affected. In a smaller study of 25 patients, Pannek and Pieper (2008) had similar findings but a more useful interpretation of those findings. They found that ambulatory UDS was helpful (versus standard UDS) in diagnosing lower urinary tract dysfunction in 72% of the evaluable examinations. However, 24% of the studies done were not evaluable owing to technical problems or catheter dislocation. Thus ambulatory UDS was clinically useful in only 48% of the patients who underwent this examination. When a diagnosis was made on ambulatory UDS, successful treatment was established in 42% of the patients. However, when ambulatory UDS was not helpful and patients were treated on the basis of clinical symptoms, 33.3% were treated successfully. Gorton and Stanton (2000) also looked at the effect of ambulatory UDS on clinical management. In a retrospective review of 71 women there were technical difficulties in 42% of the studies with 2 being uninterpretable. DO was found in 45% of the women, and nearly all were treated with medication. Among the remainder without DO, fewer received medication. However, less than half of those who received medication improved. The authors concluded that ambulatory UDS was not helpful in deciding on management.
Ambulatory UDS has been used for the diagnosis of obstruction in men with LUTS and inconclusive (borderline or nondiagnostic) conventional PFS. Rosario and colleagues (1999) reclassified 24% of such patients as either obstructed or nonobstructed. However, Robertson and colleagues (1996) found no difference in the classification of patients with ambulatory versus conventional PFS. It has been demonstrated that storage pressures in patients with chronic obstruction and neuropathic voiding dysfunction are lower on ambulatory, natural-fill UDS than on conventional CMG (Webb et al, 1989, 1992). Patients with poor compliance (high filling pressures) and hydronephrosis on conventional CMG were found to have normal compliance but significant phasic DO on ambulatory monitoring. It has been suggested that phasic DO and not impaired compliance may lead to upper tract deterioration in these patients. Although this may be true, it needs to be proven in a series of patients followed over time, and for now, on the basis of the available evidence, one must still conclude that significantly impaired compliance on conventional CMG is a risk factor for upper tract damage.
Clinical Applications of Urodynamic Studies: Evidence-Based Review
Thus far this chapter has described the technical aspects of UDS and general indications for its use. This final section provides an evidence-based review of UDS for different common clinical applications. In many situations there is not enough evidence in the literature to conclude how useful UDS is for a particular case, and as such, its use should be based on a clinical impression. Nevertheless, there are some instances and indications where the evidence is strong enough to provide guidance as to how useful UDS will be. In cases where recommendations are made, these are based on the Oxford system: Grade A recommendation usually depends on consistent level 1 evidence and often means that the recommendation is effectively mandatory and placed within a clinical care pathway. Grade B recommendation usually depends on consistent level 2 and/or 3 evidence studies or “majority evidence” from randomized controlled trials. Grade C recommendation usually depends on level 4 evidence studies or “majority evidence” from level 2/3 studies or from “expert opinion.” Four areas in which UDS is commonly used for evaluation are addressed. Enough evidence exists to make valid conclusions or recommendations for women with SUI, men with LUTS, neuropathic voiding dysfunction, and pediatric urologic conditions.
Evaluation of Women with Stress Incontinence
Several studies have shown that for women with pure SUI without urge symptoms who empty normally and demonstrated SUI on physical examination, UDS will not provide much useful information. For example, in a randomized trial comparing the efficacy of Burch procedure versus pubovaginal sling in 655 selected women with pure or predominate SUI who had fewer than 12 micturitions/day, a positive standardized stress test (volume < 300 mL), a maximum cystometric capacity of at least 200 mL, a PVR of less than 150 mL and unobstructed voiding (Qmax > 12 mL/sec, PdetQmax < 50 cm H2O), UDS including ALPP, and presence of DO added little to help determine surgical outcomes with respect to efficacy (Nager et al, 2008) or postoperative voiding dysfunction (Lemack et al, 2008). In 2006, in the United Kingdom, the National Institute for Health and Clinical Excellence issued guidelines that UDS was recommended before surgery for urinary incontinence only if (1) there is a clinical suspicion of DO, (2) there has been previous surgery for stress incontinence or anterior compartment prolapse, or (3) there are symptoms suggestive of voiding dysfunction. In other words, urodynamics is not routinely recommended for women before surgery for a “clearly defined clinical diagnosis of stress urinary incontinence.”
However, many women with SUI who are considering surgical correction have mixed symptoms or emptying difficulties and it is here that UDS probably has its most significant role for female SUI. In addition, previous studies have found significant variation of the predictive value of symptoms in identifying the three UDS observations of urodynamic SUI, DOI, and mixed urinary incontinence (Harvey and Versi, 2001; Homma, 2002; Agur et al, 2009). This variation is probably explained by the nonhomogeneous patient populations and an inconsistency in the clinical and the UDS diagnosis between the studies.
UDS does seem to have a valuable role in the preoperative evaluation of patients with mixed symptoms. In fact, several studies have shown excellent cure rates for both stress and urge symptoms in women with urodynamic SUI and a normal CMG (no DO) for pubovaginal sling (Chou, 2003; Osman, 2003); Burch procedure (Osman, 2003); and tension-free vaginal tape (Rezapour and Ulmstem, 2001).
In view of these discrepancies between symptoms and urodynamic findings, the soundest basis for the clinical diagnosis and evaluation of stress urinary incontinence and urgency urinary incontinence remains to be established, as does the significance of the corresponding urodynamic observations such as USI, DOI, and DO (Hosker et al, 2009). In cases of straightforward SUI with no significant urgency symptoms and with normal bladder emptying, it seems reasonable to forego UDS evaluation if the clinician and patient are comfortable with the limitations of symptoms as a predictor of all possible lower urinary tract abnormalities. These considerations are best reflected in the following grade A recommendation from the Fourth ICI Committee on Dynamic Testing (Hosker, 2009):
“the cost effectiveness of urodynamic testing is kept in mind when discussing ‘cost and gain’ of the various methods of diagnosis for urinary incontinence, in relation to the method of treatment.”
Many attempts to measure and quantify the severity of SUI urodynamically have been made using urethral pressure profilometry and leak point pressure measurements. In a review of static urethral pressure profilometry parameters such as MUCP or cough profile parameters such as pressure transmission ratio, Weber (2001a) concluded that these measures cannot be used to characterize the severity of incontinence. Another review by the same author (Weber, 2001b) suggested that ALPP might be a useful tool to quantify urethral dysfunction associated with SUI. However, other studies have found no correlation between leak point pressures and the severity of urinary incontinence as measured by bladder diaries and quality of life instruments (Theofrastous et al, 1995; Nager et al; 2001; Fleischmann et al, 2003; Albo et al, 2007; Chen et al, 2008). Leak point pressures have also been criticized as a diagnostic tool because of a lack of standardization of the technique (Rodrigues et al, 2006). Furthermore, leak point pressure and urethral pressure profilometry have not been shown to consistently predict outcomes for surgical treatment of SUI. In a review of this topic, Lemack (2004) found little evidence to suggest that women with “more severe forms” of SUI as measured urodynamically fare more poorly after commonly performed surgical procedures than those with “less severe forms.” Recently it has been suggested that lower urethral pressure cut points are predictive of failure for transobturator versus retropubic midurethral slings (Miller et al, 2006; O’Connor et al, 2006; Guerette et al, 2008). However, this has not been confirmed by randomized studies to date. Other investigators have shown that MUCP is not a predictor or surgical failure (Homma, 2002; Bai et al, 2007). Finally, in the prospective, randomized Stress Incontinence Surgical Treatment Efficacy (SISTEr) trial comparing Burch colposuspension and pubovaginal sling, leak point pressures were not found to be predictive of surgical outcome for either procedure (Nager et al, 2008).
In 2009 the Fourth ICI Committee on Dynamic Testing made the following grade B/C recommendations on the basis of available evidence-based literature (Hosker et al, 2009):
Evaluation of Men With Lower Urinary Tract Symptoms
LUTS are common among men of 50 years and older. The cause of male LUTS is multifactorial, comprising at least four conditions: (1) BOO, (2) impaired contractility, (3) detrusor overactivity, and (4) sensory urgency (Blaivas, 1988). Often storage symptoms of frequency and urgency accompany voiding symptoms of decreased force of stream and hesitancy. Urgency incontinence can also occur as a result of DO with or without BOO. It has been established that coexistence of BOO and DO increases with age and with the degree of BOO (Vesely et al, 2003; Oelke et al, 2008). In such cases UDS can be helpful to establish the underlying bladder and/or bladder outlet abnormality.
The question of how much help UDS is in the evaluation and treatment of male LUTS has been debated for years. How necessary is a diagnosis of BOO before transurethral prostate resection, for example? The answer depends on how comfortable the clinician is making a diagnosis and treating with less invasive and less definitive testing. Few would argue that in order to institute an α blocker for the treatment of LUTS in a man with relatively normal bladder emptying, UDS is not required. However, as the scenario becomes more complex and the treatment more invasive and potentially morbid, a precise diagnosis will be helpful in many cases. Often it is ultimately up to the clinician to decide how much information is useful or necessary to make a treatment decision and properly counsel patients.
It is well documented that in men with BOO, surgery such as transurethral prostate resection (by any means) reduces obstruction and relieves symptoms. However, storage symptoms like urgency frequency and urgency incontinence will persist in about one third of cases. It has been shown that when storage symptoms are associated with DO and BOO, UDS can help predict resolution of those symptoms. In such a scenario, storage symptoms have a higher likelihood of resolving with intervention (e.g., TURP) when DO occurs as a single terminal IDC rather than continuous or sporadic IDCs (Kageyama et al, 2000). Such information can be quite valuable when counseling patients. For example, given the same UDS presentation of BOO plus continuous IDCs, a patient primarily concerned with his inability to empty may opt for surgery, whereas a patient who is primarily concerned about OAB symptoms may not.
As for the utility of UDS before surgical treatment or no treatment of LUTS thought to be secondary to BPH, the literature is mixed. Several studies support its necessity (Javle et al, 1998; Rodrigues et al, 2001; Porru et al, 2002; Thomas et al, 2004), whereas others have concluded that UDS is not necessary (Pannek et al, 1998; Kanik et al, 2004) or necessary only in inconclusive cases (Ignjatovic, 1997). Three reviews (Abrams et al, 2001; Homma, 2001; Clemens, 2003) have suggested that UDS has some but not strong predictive value for the outcome of treatment. Another review by Bhargava and colleagues (2004) concluded that conventional urodynamic studies are useful in providing preoperative information about detrusor function and to exclude patients less likely to benefit from prostate surgery. Despite this, some regard the need for performing urodynamic evaluation routinely, before transurethral resection of the prostate, as still controversial. This led the experts at the Sixth International Consultation on New Developments in Prostate cancer and Diseases to conclude that although almost all evidence for the advantages of UDS before invasive therapy for BPO is level 3 (good-quality retrospective “case-control studies” or “case series”), the quantity of evidence allows a grade B recommendation (Abrams et al, 2006).
Most of the time when BOO is treated, it is done so because of symptoms. However, “symptomatic” BOO, if not relieved in time, may lead to progression of the disease and affect organ function. For many years attention was paid only to the backpressure effects of obstruction on the kidneys (from high voiding pressures and/or impaired compliance). However, it is now known that BOO affects bladder function, leading to structural changes. Because the changes may eventually become irreversible, some would argue that management should be directed toward early relief of significant obstruction (Flanigan et al, 1998; Lu et al, 2000; Brierly et al, 2003). However, at this time, a critical level of obstruction (e.g., based on the BOOI) has not been defined. No evidence-based studies suggest when surgical relief is indicated to prevent bladder decompensation. However, many papers have shown that if there is no evidence of obstruction on PFS, the results of surgical relief are not as good (Porru et al, 2002; Thomas, 2004). Significantly impaired compliance remains the only “absolute urodynamic indication” for treating BOO.
Evaluation of Neuropathic Lower Urinary Tract Dysfunction
Neuropathic lower urinary tract dysfunction usually presents clinically as incontinence and/or inability to empty the bladder. Incontinence may be of bladder origin (detrusor overactivity or impaired compliance) or sphincter origin. Poor emptying can also be of bladder origin (impaired detrusor contractility) of sphincter origin (dyssynergia). In addition to symptomatic presentation, neuropathic lower urinary tract dysfunction can present as upper urinary tract decompensation with hydroureteronephrosis and renal insufficiency without bothersome symptoms. The goal of management in these patients is to prevent upper tract decompensation and relieve symptoms. A specific understanding of the pathophysiology of the condition in each individual is essential for the correct choice of therapy (Stohrer, 1990; Stohrer et al, 1994; Rivas and Chancellor, 1995). UDS probably has its most important role in the diagnosis and management of patients with neuropathic voiding dysfunction.
The aims of therapy for neuropathic lower urinary tract dysfunction are to achieve physiologic filling (and if possible voiding) conditions, as well as to control symptoms and create a management situation acceptable to the patient in daily life. Much of the evidence base for management of the lower urinary tract in the neuropathic patient consists of level 3 evidence. This is primarily because of the potential negative consequences of untreated neuropathic dysfunction. As such, randomized, controlled trials are thought by many to be dangerous and unethical. Because prolonged periods of elevated detrusor pressure during bladder filling or abnormally prolonged elevated pressures during voiding have been found to put the upper urinary tract at risk (McGuire et al, 1996; Kurzrock and Polse, 1998; Tanaka et al, 1999), primary aim of therapy in patients with such problems is conversion to a low pressure bladder during filling even if this leads to incomplete emptying and the need to supplement emptying with catheterization. Adequate therapy depends on whether the detrusor is overactive or has reduced compliance, and only urodynamics can answer those questions unequivocally. Timely and adequate diagnosis of DO and impaired compliance is thought to be of paramount importance for the patient’s quality of life (Stohrer, 1990; Stohrer et al, 1994; Bomalaski et al, 1995; Cardenas et al, 1995). UDS is also essential for assessing the response to treatment and following any sequelae of the disease and its management (Hosker et al, 2009).
In 2009 the Fourth Consultation on Incontinence committee on dynamic testing made the following grade B/C recommendation on the basis of the available evidence-based literature (Hosker et al, 2009): Patients with suspected neurogenic dysfunction of the LUT should receive comprehensive UDS evaluation including VUDS, if possible, to establish the state and function of the lower tract. In addition, UDS testing in this group of patients should be done in a specialized environment with trained personnel.
Evaluation of Children
Many of the conditions for which UDS is used in children involve anatomic and neurologic abnormalities in which lower urinary tract function is variable and unpredictable. UDS is used to establish as clearly as possible the baseline situation so that changes as a result of treatment and/or growth can be assessed, and some guidance is obtained in the choice of treatment even if the result of UDS testing is not necessarily the deciding factor (Hosker et al, 2009). In the pediatric population, the aim of UDS is not only to provide precise knowledge of lower urinary tract function but also to provide an understanding of the current and future condition to the caregiver and to the patient (and his or her parents). Of course, it is still imperative that the testing should be relevant, reliable, and reproducible. We briefly describe the evidence base for several commonly studied pediatric conditions. The reader is referred to the relevant chapters on pediatric urology for a more detailed discussion.
Pediatric Neurologic Conditions: Myelodysplasia, Occult Spinal Dysraphism, and Sacral Agenesis
When evaluating children with myelodysplasia, a number of effects on the lower urinary tract can be seen. Detrusor overactivity (on filling) or underactivity (on voiding), DESD, impaired compliance, and elevated DLPP are the key parameters evaluated on UDS testing in this population (McGuire et al, 1981; Bauer et al, 1984). Retrospective and prospective studies have shown that the urodynamic diagnosis of DO and/or reduced detrusor compliance in patients with myelodysplasia or (occult) spinal dysraphism is not predictable on the basis of clinical signs, symptoms, or level of lesion (Dator et al, 1992; Landau et al, 1994; Pontari et al, 1995; Kaefer et al, 1997; Palmer et al, 1997), making UDS an important tool in assessing the lower urinary tract. Furthermore, these studies reveal clinically relevant results with regard to detrusor storage function. In a retrospective study of 64 children, Rendeli and colleagues (2007) showed that UDS evaluation and the presence of neurologic impairment have crucial roles in determining the optimal timing of surgery in patients with lipomeningocele and in diagnosing the onset of tethered cord. Finally, it has been demonstrated that lower urinary tract function may change over time (McGuire et al, 1981; Bauer et al, 1984; Sidi et al, 1986; Wang et al, 2006) and repeat evaluation may be necessary. However, no studies have helped to determine the optimum timing and frequency of UDS follow-up.
Sacral agenesis is often missed in infancy because of its subtle clinical manifestations and no obvious loss of lower extremity motor and sensory function. Similar to myelodysplasia, patients can present with a variety of lower urinary tract dysfunctions. Several studies have shown that 30% to 40% of patients present with DO and an intact but dyssynergic sphincter (upper motor neuron lesion), 25% to 50% will have an acontractile detrusor with a denervated sphincter (lower motor neuron lesion), and 15% to 20% have normal lower urinary tract function (Koff et al, 1977; Guzman et al, 1983, Jabokson et al, 1985). In eight reports that provide adequate data, UDS has been 90% accurate in delineating the neurologic deficit and in managing incontinence and upper urinary tract abnormalities (Koff et al, 1977, 1983; Jabokson et al, 1985; Gotoh et al, 1991; Saito et al, 1991; Boemers et al, 1994, 1996; Wilmshurst et al, 1999; Torre et al, 2002).
In 2009 the Fourth Consultation on Incontinence committee on dynamic testing made the following recommendations for children with myelodysplasia or occult spinal dysraphism and sacral agenesis on the basis of the available evidence-based literature (Hosker et al, 2009):
Pediatric Anatomic Abnormalities: Posterior Urethral Valves, Bladder Extrophy, Vesicoureteral Reflux
A number of congenital and acquired anatomic abnormalities can affect lower urinary tract function. Because of a lack of prospective data, it is recommended that for each of the abnormalities presented, clinicians should consider UDS, depending on the clinical scenario (Hosker et al, 2009). Studies on posterior urethral valves have shown that some boys will have significant lower urinary tract dysfunction, namely DO and impaired compliance (Kim et al, 1997; Kajbafzadeh et al, 2007). When storage pressures are considered to be dangerously elevated, improvements in DO and compliance after valve ablation have been documented (De Gennaro et al, 1996, 2000; Holmdahl et al, 1996; Kim et al, 1997; Donohoe et al, 2004). Myogenic failure and poor emptying are late sequela of posterior urethral valves, and persistence of upper tract changes is related to high storage pressure and/or incomplete emptying. Several studies have shown the predictability of developing upper tract decompensation on the basis of UDS parameters of persistent poor compliance, high detrusor pressures, and impaired contractility during voiding (Bauer et al, 1979; Peters et al, 1990; Holmdahl et al, 1995, 1996; Lopez Pereira et al, 2002).
Bladder extrophy is another condition in which UDS may be helpful. A few studies have shown that after bladder neck reconstruction, the prevalence of DO increases and compliance worsens in up to 50% of cases (Hollowell et al 1992, 1993; Diamond et al, 1999; Caione et al, 2005; Burki, 2006). Although there is no prospective information regarding long-term outcomes based on UDS finding, this information may be helpful in management.
There is increasing evidence that a significant number of vesicoureteral reflux cases are due to bladder dysfunction (DO) and not necessarily an anatomic abnormality at the ureterovesical junction (Koff et al, 1979; Griffiths and Scholtmeijer, 1987; Sillen et al, 1996; Capitanucci, 2000; Greenfield and Wan, 2000; Podesta, 2004). DO may be a result of high voiding pressures, particularly in boys, or dysfunctional voiding. Four separate studies have shown that treating DO and/or voiding with antimuscarinics leads to a rapid rate of reflux resolution (63% to 92%) within 1 year (Homsy et al, 1985; Scholtmeijer and Nijmanl, 1994; Batista Miranda et al, 1997). This is higher than the 25% to 54% rate of reflux resolution seen with treatment using antibiotics alone to prevent recurrent infection (Seruca, 1989). Treating dysfunctional voiding with biofeedback has also been shown to reduce the grade of reflux (Kuo and Liu, 2006). Because of these findings, many clinicians advocate UDS for children with high-grade vesicoureteral reflux, especially those with incontinence, renal damage, or pending surgery to correct reflux (Scholtmeijer and Nijman, 1994; Moran Penco et al, 2004; Musquera et al, 2004; Fotter and Riccabona, 2005). On the basis of the available literature, the Fourth ICI Committee had the following grade C recommendation regarding UDS testing of children with anatomic abnormalities discussed earlier: Clinicians should consider complete urodynamic testing, at least once, in children with posterior urethral valves, urethral stricture, ectopic ureterocele, vesicoureteral reflux, and bladder extrophy. Clinicians should consider regular uroflowmetry and PVR in the management and follow-up of children with posterior urethral valves, urethral stricture, ectopic ureterocele, vesicoureteral reflux, and bladder extrophy.
Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU Int. 1999;84:14.
Abrams P, Cardoza L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178.
Abrams P, D’Ancona C, Griffiths D, van Kerrebroeck P, et al. Lower tract symptom: etiology, patient assessment and predicting outcome from therapy. In: McConnell J, Abrams P, Denis L, et al, editors. Male lower urinary tract dysfunction evaluation and management. Plymouth (UK): Health Publication; 2006:69-142.
Abrams PH, Farrar DJ, Turner-Warwick RT, et al. The results of prostatectomy: a symptomatic and urodynamic analysis of 192 patients. J Urol. 1979;121:640.
Blaivas JG. The neurophysiology of micturition: a clinical study of 550 patients. J Urol. 1982;127:958-963.
Blaivas JG, Groutz A. Bladder outlet obstruction nomogram for women with lower urinary tract symptomatology. Neurourol Urodyn. 2000;19:553-564.
Defreitas GA, Zimmern PE, Lemack GE, Shariat SF. Refining diagnosis of anatomic female bladder outlet obstruction: comparison of pressure-flow study parameters in clinically obstructed women with those of normal controls. Urology. 2004;64:675-679.
Fleischmann N, Flisser AJ, Blaivas JG, Panagopoulos G. Sphincteric urinary incontinence: relationship of vesical leak point pressure, urethral mobility and severity of incontinence. J Urol. 2003;69:999-1002.
Griffiths D, Hofner K, van Mastrigt R, et al. Standardization of terminology of lower urinary tract function: pressure-flow studies of voiding, urethral resistance, and urethral obstruction. International Continence Society Subcommittee on standardization of terminology of pressure-flow studies. Neurourol Urodyn. 1997;16:1.
Griffiths DJ. The mechanics of the urethra and of micturition. Br J Urol. 1973;45:497.
Hosker G, Rosier P, Gajewski J, et al. Dynamic Testing. In: Abrams P, Cardoza L, Khoury S, Wein A, editors. Incontinence: 4th International Consultation on Incontinence. United Kingdom: Health Publications; 2009:413-552.
Lemack GE, Krauss S, Litman H, et al. Normal preoperative urodynamic testing does not predict voiding dysfunction after Burch colposuspension versus pubovaginal sling. J Urol. 2008 Nov;180:2076-2080.
Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol. 1995;13:34-39.
Lose G, Griffiths D, Hosker G, et al. Standardisation of urethral pressure measurement: report from the Standardisation Sub-Committee of the International Continence Society. Neurourol Urodyn. 2002;21:258-260.
McGuire EJ, Fitzpatrick CC, Wan J, et al. Clinical assessment of urethral sphincter function. J Urol. 1993;150:1452.
McGuire EJ, Woodside JR, Borden TA. Prognostic value of urodynamic testing in myeloplastic children. J Urol. 1981;126:205.
Nager CW, FitzGerald M, Kraus SR, et al. Urodynamic measures do not predict stress continence outcomes after surgery for stress urinary incontinence in selected women. J Urol. 2008;179:1470-1474.
Nitti VW, Tu LM, Gitlin J. Diagnosing bladder outlet obstruction in women. J Urol. 1999;161:1535.
Scarpero HM, Kaufman MR, Koski M, Dmochowski RR. Urodynamics best practices. American Urological Association Update Series. 2009. Lesson 9
Schafer W. Principles and clinical application of advanced urodynamic analysis of voiding function. Urol Clin North Am. 1990;17:553.
Schafer W, Sterling AM, Liao L, et al. Good urodynamic practice: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodynam. 2002;21:261.
van Waalwijk van Doorn E, Anders K, Khullar V, et al. Standardisation of ambulatory urodynamic monitoring: report of the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2000;19:113-125.
Wein AJ. Classification of neurogenic voiding dysfunction. J Urol. 1981;125:605.
Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU Int. 1999;84:14-15.
Abrams P, Blaivas JG, Stanton SL, Andersen JT. The standardisation of terminology of lower urinary tract function. The International Continence Society committee on standardisation of terminology. Scand J Urol Nephrol Suppl. 1988;114:5-19.
Abrams P, Cardoza L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178.
Abrams P, D’Ancona C, Griffiths D, et al. Lower tract symptom: etiology, patient assessment and predicting outcome from therapy. In: McConnell J, Abrams P, Denis L, et al, editors. Male lower urinary tract dysfunction evaluation and management. Plymouth (UK): Health Publication, Ltd.; 2006:69-142.
Abrams P, Griffiths D, Hoefner K, et al. The urodynamic assessment of lower urinary tract symptoms. In: Chatelain C, Denis L, Foo K, et al, editors. Benign prostatic hyperplasia. Plymouth (UK): Health Publication, Ltd.; 2001:227-281.
Abrams P, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol. 1979;51:129-134.
Abrams PH, Farrar DJ, Turner-Warwick RT, et al. The results of prostatectomy: a symptomatic and urodynamic analysis of 192 patients. J Urol. 1979;121:640.
Agur W, Housami F, Drake M, Abrams P. Could the National Institute for Health and Clinical Excellence guidelines on urodynamics in urinary incontinence put some women at risk of a bad outcome from stress incontinence surgery? BJU Int. 2009;103:635-639.
Akikwala TV, Fleischman N, Nitti VW. Comparison of diagnostic criteria for female bladder outlet obstruction. J Urol. 2006;176:2093-2097.
Albo M, Wruck L, Baker J. The relationships among measures of incontinence severity in women undergoing surgery for stress urinary incontinence. J Urol. 2007;177:1810-1814.
Bai SW, Jung YH, Jeon MJ, et al. Treatment outcome of tension-free vaginal tape in stress urinary incontinence: comparison of intrinsic sphincter deficiency and nonintrinsic sphincter deficiency patients. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1431-1434.
Ballert KN, Biggs GY, Isenalumhe A, et al. Managing the urethra at transvaginal pelvic organ prolapse repair: a urodynamic approach. J Urol. 2009;181:679-684.
Barrett DM, Wein AJ. Flow evaluation and simultaneous external sphincter electromyography in clinical urodynamics. J Urol. 1981;125:538-541.
Batista Miranda J, Arano Bertran P, Caffaratti J, et al. Efficacy of oxybutynin chloride in children with vesicoureteral reflux and detrusor instability. Anales Espanoles de Pediatria. 1997;47:251-257.
Bauer SB, Dieppa RA, Labib KK, Retik AB. The bladder in boys with posterior urethral valves: a urodynamic assessment. J Urol. 1979;121:769-773.
Bauer SB, Hallett M, Khoshbin S, et al. Predictive value of urodynamic evaluation in newborns with myelodysplasia. JAMA. 1984;252:650-652.
Bhargava S, Canda AE, Chapple CR. A rational approach to benign prostatic hyperplasia evaluation: recent advances. Curr Opin Urol. 2004;14:1-6.
Blaivas JG. The neurophysiology of micturition: a clinical study of 550 patients. J Urol. 1982;127:958-963.
Blaivas JG, Groutz A. Bladder outlet obstruction nomogram for women with lower urinary tract symptomatology. Neurourol Urodyn. 2000;19:553-564.
Blaivas JG, Labib KB, Michalik SJ, Zayed AA. Failure of bethanechol denervation supersensitivity as a diagnostic aid. J Urol. 1980;123:199-201.
Boemers TM, Beek FJ, van Gool JD, et al. Urologic problems in anorectal malformations. Part 1: urodynamic findings and significance of sacral anomalies. J Pediatr Surg. 1996;31(3):407-410.
Boemers TM, van Gool JD, de Jong TP, Bax KM. Urodynamic evaluation of children with the caudal regression syndrome (caudal dysplasia sequence). J Urol. 1994;151:1038-1040.
Bomalaski MD, Teague JL, Brooks B. The longterm impact of urological management on the quality of life of children with spina bifida. J Urol. 1995;154:778-781.
Boone TB, Kim YH. Uroflowmetry. In: Nitti VW, editor. Practical urodynamics. Philadelphia: WB Saunders; 1998:28-51.
Brierly RD, Hindley RG, McLarty E, et al. A prospective evaluation of detrusor intrastructural changes in bladder outlet obstruction. BJU Int. 2003;91:360-364.
Brown M, Wickham JEA. The urethral pressure profile. Br J Urol. 1969;41:211-217.
Bump RC, Elser DM, Theofrastous JP, McClish DK. Valsalva leak point pressures in women with genuine stress incontinence: reproducibility, effect of catheter caliber, and correlations with other measures of urethral resistance. Continence Program for Women Research Group. Am J Obstet Gynecol. 1995;173:551-557.
Burki T, Hamid R, Duffy P, et al. Long-term followup of patients after redo bladder neck reconstruction for bladder exstrophy complex. J Urol. 2006;176:1138-1142.
Caione P, Capozza N, Zavaglia D, De Dominicis M. Anterior perineal reconstruction in exstrophy-epispadias complex. Eur Urol. 2005;47:872-878.
Capitanucci ML, Silveri M, Mosiello G, et al. Prevalence of hypercontractility in male and female infants with vesico-ureteral reflux. Eur J Pediatr Surg. 2000;10:172-176.
Cardenas DD, Mayo ME, Turner LR. Lower urinary changes over time in suprasacral spinal cord injury. Paraplegia. 1995;33:326-329.
Carlson KV, Rome S, Nitti VW. Dysfunctional voiding in women. J Urol. 2001;165:143-147.
Cassidenti AP, Ostergard DR. Multichannel urodynamics: ambulatory versus standard urodynamics. Curr Opin Obstet Gynecol. 1999;11:485-487.
Chassagne S, Bernier PA, Haab F, et al. Proposed cutoff values to define bladder outlet obstruction in women. Urology. 1998;51:408-411.
Chen CC, Rooney CM, Pariaso MF, et al. Leak point pressure does not correlate with incontinence severity or bother in women undergoing surgery for urodynamic stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1193-1198.
Chou ECL, Flisser AJ, Panagopoulis G, Blaivas JG. Effective treatment for mixed urinary incontinence with a pubovaginal sling. J Urol. 2003;170:494-497.
Clemens JQ. The role of urodynamics in the diagnosis and treatment of benign prostatic hyperplasia. Curr Urol Rep. 2003;4:269-275.
Comarr EA. Excretory cystometry: a more physiological method. J Urol. 1957;77:622-633.
Combs AJ, Grafstein N, Horowitz M, Glassberg KI. Primary bladder neck dysfunction in children and adolescents I: pelvic floor electromyography lag time—a new noninvasive method to screen for and monitor therapeutic response. J Urol. 2005;173:207-210.
Dator DP, Hatchett L, Dyro FM, et al. Urodynamic dysfunction in walking myelodysplastic children. J Urol. 1992;148:362-365.
Defreitas GA, Zimmern PE, Lemack GE, Shariat SF. Refining diagnosis of anatomic female bladder outlet obstruction: comparison of pressure-flow study parameters in clinically obstructed women with those of normal controls. Urology. 2004;64:675-679.
De Gennaro M, Capitanucci ML, et al. The changing urodynamic pattern from infancy to adolescence in boys with posterior urethral valves. BJU Int. 2000;85:1104-1108.
De Gennaro M, Mosiello G, Capitanucci ML, et al. Early detection of bladder dysfunction following posterior urethral valves ablation. Eur J Pediatr Surg. 1996;6:163-165.
Diamond DA, Bauer SB, Dinlenc C, et al. Normal urodynamics in patients with bladder exstrophy: are they achievable? J Urol. 1999;162:841-845.
Diokno AC, Hollander JB, Bennett CJ. Bladder neck obstruction in women: a real entity. J Urol. 1984;132:294-298.
Donohoe JM, Weinstein RP, Combs AJ, et al. When can persistent hydroureteronephrosis in posterior urethral valve disease be considered residual stretching? J Urol. 2004;172:706-711.
Drach GW, Layton TN, Binard WJ. Male peak urinary flow rate: relationships to volume voided and age. J Urol. 1979;122:210-214.
DuBeau CE, Sullivan MP, Cravalho EG, et al. Correlation between micturitional pressure profile and pressure-flow criteria in bladder outlet obstruction. J Urol. 1995;154:489-503.
Faerber GJ, Vashi AR. Variations in Valsalva leak point pressure with increasing vesical volume. J Urol. 1998;159:1909-1911.
Flanigan RC, Reda DJ, Wasson JH, et al. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans study. J Urol. 1998;160:12-17.
Fleischmann N, Flisser AJ, Blaivas JG, Panagopoulos G. Sphincteric urinary incontinence: relationship of vesical leak point pressure, urethral mobility and severity of incontinence. J Urol. 2003;169:999-1002.
Fotter R, Riccabona M. Functional disorders of the lower urinary tract in children. Radiologe. 2005;45:1085-1091.
Gorton E, Stanton S. Ambulatory urodynamics: do they help clinical management? BJOG. 2000;107:316-319.
Gotoh T, Shinno Y, Kobayashi S, et al. Diagnosis and management of sacral agenesis. Eur Urol. 1991;20:287-292.
Greenfield SP, Wan J. The relationship between dysfunctional voiding and congenital vesicoureteral reflux. Curr Opin Urol. 2000;10:607-610.
Griffiths D, Hofner K, van Mastrigt R, et al. Standardization of terminology of lower urinary tract function: pressure-flow studies of voiding, urethral resistance, and urethral obstruction. International Continence Society Subcommittee on Standardization of Terminology of Pressure-Flow Studies. Neurourol Urodyn. 1997;16:1-18.
Griffiths DJ, Scholtmeijer RJ. Vesicoureteral reflux and lower urinary tract dysfunction: evidence for two different reflux/dysfunction complexes. J Urol. 1987;137:240-244.
Griffiths DJ, van Mastrigt R, Bosch R. Quantification of urethral resistance and bladder function during voiding, with special reference to the effects of prostate size reduction in urethral obstruction due to benign prostatic hyperplasia. Neurourol Urodyn. 1989;8:29-52.
Guerette NL, Bena JF, Davila W. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:97-102.
Guzman L, Bauer SB, Hallett M, et al. Evaluation and management of children with sacral agenesis. Urology. 1983;22:506-510.
Harvey MA, Versi E. Predictive value of clinical evaluation of stress urinary incontinence: a summary of the published literature. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:31-37.
Haylen BT, Ashby D, Sutherst JR, et al. Maximum and average urine flow rates in normal male and female populations—the Liverpool nomograms. Br J Urol. 1989;64:30-38.
He W, Chen M, Zu X, et al. Chronic prostatitis presenting with dysfunctional voiding and effects of pelvic floor biofeedback treatment. BJU Int. 2010;105:975-977.
Heslington K, Hilton P. Ambulatory monitoring and conventional cystometry in asymptomatic female volunteers. Br J Obstet Gynaecol. 1996;103:434-441.
Hollowell JG, Hill PD, Duffy PG, Ransley PG. Lower urinary tract function after exstrophy closure. Pediatr Nephrol. 1992;6:428-432.
Hollowell JG, Hill PD, Duffy PG, Ransley PG. Evaluation and treatment of incontinence after bladder neck reconstruction in exstrophy and epispadias. Br J Urol. 1993;71:743-749.
Holmdahl G, Sillén U, Bachelard M, et al. The changing urodynamic pattern in valve bladders during infancy. J Urol. 1995;153:463-467.
Holmdahl G, Sillén U, Hanson E, et al. Bladder dysfunction in boys with posterior urethral valves before and after puberty. J Urol. 1996;155:694-698.
Homma Y. Pressure-flow studies in benign prostatic hyperplasia: to do or not to do for the patient? BJU Int. 2001;87:19-23.
Homma Y. The clinical significance of the urodynamic investigation in incontinence. BJU Int. 2002;90:489-497.
Homsy YL, Nsouli I, Hamburger B, et al. Effects of oxybutynin on vesicoureteral reflux in children. J Urol. 1985;134:1168-1171.
Hosker G. Urodynamics. In: Hillard T, Purdie D, editors. The yearbook of obstetrics and gynecology. London: RCOG Press; 2004:233-254.
Hosker G, Rosier P, Gajewski J, et al. Dynamic testing. In: Abrams P, Cardoza L, Khoury S, Wein A, editors. 4th International Consultation on Incontinence. United Kingdom: Health Publications; 2009:413-552.
Huckabay C, Twiss C, Berger A, Nitti VW. A urodynamics protocol to optimally assess men with post-prostatectomy incontinence. Neurourol Urodynam. 2005;24:622-626.
Ignjatovic I. Prediction of unfavourable symptomatic outcome of transurethral prostatectomy in patients with the relative indication for operation. Int Urol Nephrol. 1997;29:653-660.
Jabokson H, Holm-Bentzen M, Hald T. The evaluation and management of children with sacral agenesis and dysgenesis. Neurourol Urodynam. 1985;4:99.
Javle P, Jenkins SA, Machin DG, Parsons KF. Grading of benign prostatic obstruction can predict the outcome of transurethral prostatectomy. J Urol. 1998;160:1713-1717.
Kaefer M, Rosen A, Darbey M, et al. Pressure at residual volume: a useful adjunct to standard fill cystometry. J Urol. 1997;158:1268-1271.
Kageyama S, Watanabe T, Kurita Y, et al. Can persisting detrusor hyperreflexia be predicted after transurethral prostatectomy for benign prostatic hypertrophy? Neurourol Urodyn. 2000;19:233-240.
Kajbafzadeh AM, Payabvash S, Karimian G. The effects of bladder neck incision on urodynamic abnormalities of children with posterior urethral valves. J Urol. 2007;178:2142-2149.
Kanik EA, Erdem E, Abidinoglu D, et al. Can the outcome of transurethral resection of the prostate be predicted preoperatively? Urology. 2004;64:302-305.
Kaplan SA, Ikeguchi EF, Santarosa RP, et al. Etiology of voiding dysfunction in men less than 50 years of age. Urology. 1996;47:836-839.
Kaplan SA, Santarosa RP, D’Alisera PM, et al. Pseudodyssynergia (contraction of the external sphincter during voiding) misdiagnosed as chronic nonbacterial prostatitis and the role of biofeedback as a therapeutic option. J Urol. 1997;157:2234-2237.
Kim YH, Horowitz M, Combs AJ, et al. Management of posterior urethral valves on the basis of urodynamic findings. J Urol. 1997;158:1011-1016.
Koff SA. Estimating bladder capacity in children. Urology. 1983;21:248.
Koff SA, Deridder PA. Patterns of neurogenic bladder dysfunction in sacral agenesis. J Urol. 1977;118:87-89.
Koff SA, Lapides J, Piazza DH. Association of urinary tract infection and reflux with uninhibited bladder contractions and voluntary sphincteric obstruction. J Urol. 1979;122:373-376.
Kuo HC, Liu HT. Investigation of dysfunctional voiding in children with urgency frequency syndrome and urinary incontinence. Urol Int. 2006;76:72-76.
Kurzrock EA, Polse S. Renal deterioration in myelodysplastic children: urodynamic evaluation and clinical correlates. J Urol. 1998;159:1657-1661.
Landau EH, Churchill BM, Jayanthi VR, et al. The sensitivity of pressure specific bladder volume versus total bladder capacity as a measure of bladder storage dysfunction. J Urol. 1994;152:1578-1581.
Lapides J, Friend CR, Ajemian EP, et al. Denervation supersensitivity as a test for neurogenic bladder. Surg Gynecol Obstet. 1962;114:241.
Lecanwasam HS, Sullivan MP, Desirreddi N, et al. Voiding profilometry as a diagnostic aid in patients with prostatism: assessment with post-therapeutic symptom evaluation. Neurourol Urodyn. 1994;13:391.
Lemack GE. Urodynamic assessment of patients with stress incontinence: how effective are urethral pressure profilometry and abdominal leak point pressures at case selection and predicting outcome? Curr Opin Urol. 2004;14:307-311.
Lemack GE, Krauss S, Litman H, et al. Normal preoperative urodynamic testing does not predict voiding dysfunction after Burch colposuspension versus pubovaginal sling. J Urol. 2008;180:2076-2080.
Lemack GE, Zimmern PE. Pressure flow analysis may aid in identifying women with outflow obstruction. J Urol. 2000;163:1823-1828.
Leng WW, McGuire EJ. Obstructive uropathy induced bladder dysfunction can be reversible: bladder compliance measures before and after treatment. J Urol. 2003;169:563-566.
Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol. 1995;13:34-39.
Lopez Pereira P, Martinez Urrutia MJ, Espinosa L, et al. Bladder dysfunction as a prognostic factor in patients with posterior urethral valves. BJU Int. 2002;90:308-311.
Lose G, Griffiths D, Hosker G, et al. Standardisation of urethral pressure measurement: report from the Standardisation Sub-Committee of the International Continence Society. Neurourol Urodyn. 2002;21:258-260.
Lu SH, Wei YH, Chang LS, et al. Morphological and morphometric analysis of human detrusor mitochondria with urodynamic correlation after partial bladder outlet obstruction. J Urol. 2000;163:225-229.
McGuire EJ. Urodynamic findings in patients after failure of stress incontinence operations. Prog Clin Biol Res. 1981;78:351-354.
McGuire EJ, Cespedes RD, O’Connell HE. Leakpoint pressures. Urol Clin North Am. 1996;23:253-262.
McGuire EJ, Fitzpatrick CC, Wan J, et al. Clinical assessment of urethral sphincter function. J Urol. 1993;150:1452-1454.
McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myeloplastic children. J Urol. 1981;126:205-209.
Miller JJ, Botros SM, Akl MN, et al. Is transobturator tape as effective as tension-free vaginal tape in patients with borderline maximum urethral closure pressure? Am J Obstet Gynecol. 2006;195:1799-1804.
Moran Penco JM, Gomez Fraile A, Rodríguez Alarcon J, et al. Evolution of the treatment of vesicoureteral reflux in Spain. J Urol. 2004;171:834-837.
Musquera F, Errando SC, Prados SM, et al. False postvoid residual volume diagnosed by videourodynamics. Actas Urol Esp. 2004;28:792-795.
Nager CW, FitzGerald M, Kraus SR, et al. Urodynamic measures do not predict stress continence outcomes after surgery for stress urinary incontinence in selected women. J Urol. 2008;179:1470-1474.
Nager CW, Schulz JA, Stanton SL, Monga A. Correlation of urethral closure pressure, leak-point pressure and incontinence severity measures. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:395-400.
National Institute for Health and Clinical Excellence. Urinary incontinence—the management of urinary incontinence in women. London: National Institute for Health and Clinical Excellence; 2006.
Nevéus T, von Gontard A, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol. 2006;176:314-324.
Nitti VW, Blaivas JG. Urinary incontinence, epidemiology, pathophysiology, evaluation, and management overview. In: Wein AJ, Kavoussi LR, Novick AC, et al, editors. Campbell-Walsh urology. Philadelphia: Elsevier Saunders; 2007:2045-2078.
Nitti VW, Lefkowitz G, Ficazzola M, Dixon CM. Lower urinary tract symptoms in young men: videourodynamic findings and correlation with noninvasive measures. J Urol. 2001;168:135-138.
Nitti VW, Tu LM, Gitlin J. Diagnosing bladder outlet obstruction in women. J Urol. 1999;161:1535-1540.
Norlen LJ, Blaivas JG. Unsuspected proximal urethral obstruction in young and middle-aged men. J Urol. 1986;135:972-976.
O’Connor RC, Nanigian DK, Lyon MB, et al. Early outcomes of mid-urethral slings for female stress urinary incontinence stratified by Valsalva leak point pressure. Neurourol Urodyn. 2006;25:685-688.
O’Donnell PD. Electromyography. In: Nitti VW, editor. Practical urodynamics. Philadelphia: WB Saunders; 1998:65-71.
Oelke M, Baard J, Wijkstra H, et al. Age and bladder outlet obstruction are independently associated with detrusor overactivity in patients with benign prostatic hyperplasia. Eur Urol. 2008;54:419-426.
Osman T. Stress incontinence surgery for patients presenting with mixed incontinence and a normal cystometrogram. BJU Int. 2003;92:964-968.
Palmer LS, Richards I, Kaplan WE. Age related bladder capacity and bladder capacity growth in children with myelomeningocele. J Urol. 1997;158:1261-1264.
Pan D, Troy A, Rogerson A, et al. Long-term outcomes of external sphincterotomy in a spinal injured population. J Urol. 2009;181:705-709.
Pannek J, Berges RR, Haupt G, Senge T. Value of the Danish prostate symptom score compared to the AUA symptom score and pressure/flow studies in the preoperative evaluation of men with symptomatic benign prostatic hyperplasia. Neurourol Urodynam. 1998;17:9-18.
Pannek J, Pieper P. Clinical usefulness of ambulatory urodynamics in the diagnosis and treatment of lower urinary tract dysfunction. Scand J Urol Nephol. 2008;42:428-432.
Patravali N. Ambulatory urodynamic monitoring: are we wasting our time? J Obstet Gynaecol. 2007;27:413-415.
Peters CA, Bolkier M, Bauer SB, et al. The urodynamic consequences of posterior urethral valves. J Urol. 1990;144:122-126.
Podesta ML, Castera R, Ruarte AC. Videourodynamic findings in young infants with severe primary reflux. J Urol. 2004;171:829-833.
Pollak JT, Neimark M, Connor JT, Davila GW. Air-charged and microtransducer urodynamic catheters in the evaluation of urethral function. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:124-128.
Pontari M, Keating M, Kelly M, et al. Retained sacral function in children with high level myelodysplasia. J Urol. 1995;154:775-777.
Porru D, Jallous H, Cavalli V, et al. Prognostic value of a combination of IPSS, flow rate and residual urine volume compared to pressure-flow studies in the preoperative evaluation of symptomatic BPH. Eur Urol. 2002;41:246-249.
Radley SC, Rosario DJ, Chapple CR, Farkas AG. Conventional and ambulatory urodynamic findings in women with symptoms suggestive of bladder overactivity. J Urol. 2001;166:2253-2258.
Rendeli C, Ausili E, Tabacco F. Urodynamic evaluation in children with lipomeningocele: timing for neurosurgery, spinal cord tethering and followup. J Urol. 2007;177:2319-2324.
Rezapour M, Ulmsten U. Tension-Free vaginal tape (TVT) in women with recurrent stress urinary incontinence—a long-term follow up. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S9-11.
Rivas DA, Chancellor MB. Neurogenic vesical dysfunction. Urol Clin North Am. 1995;22:579-591.
Robertson A, Neal DE. Ambulatory urodynamics. In: Nitti VW, editor. Practical urodynamics. Philadelphia: WB Saunders; 1998:273-286.
Robertson AS. Behaviour of the human bladder during natural filling: the Newcastle experience of ambulatory monitoring and conventional artificial filling cystometry. Scand J Urol Nephrol Suppl. 1999;201:19-24.
Robertson AS, Griffiths C, Neal DE. Conventional urodynamics and ambulatory monitoring in the definition and management of bladder outflow obstruction. J Urol. 1996;155:506-511.
Robertson AS, Griffiths CJ, Ramsden PD, et al. Bladder function in healthy volunteers: ambulatory monitoring and conventional urodynamic studies. Br J Urol. 1994;73:242-249.
Rodrigues P, Alfonzo Y, Hering FO, et al. Valsalva leak point pressure to determine internal sphincter deficiency in stress urinary incontinence. Urol Int. 2006;76:154-158.
Rodrigues P, Lucon AM, Freire GC, Arap S. Urodynamic pressure flow studies can predict the clinical outcome after transurethral prostatic resection. J Urol. 2001;165:499-502.
Rosario DJ, MacDiarmid SA, Radley SC, Chapple CR. A comparison of ambulatory and conventional urodynamic studies in men with borderline outlet obstruction. BJU Int. 1999;83:400-409.
Saito M, Kondo A, Kato K. Diagnosis and treatment of neurogenic bladder due to partial sacral agenesis. Br J Urol. 1991;67:472-476.
Scarpero HM, Kaufman MR, Koski M, Dmochowski RR. Urodynamics best practices. American Urological Association Update Series. 2009. Lesson 9
Scarpero HM, Padmanabhan P, Xue X, Nitti VW. Patient perception of videourodynamic testing: a questionnaire based study. J Urol. 2005;173:555-559.
Schafer W. Principles and clinical application of advanced urodynamic analysis of voiding function. Urol Clin North Am. 1990;17:553-566.
Schafer W. Analysis of bladder outlet function with the linearized passive urethral resistance relation, linPURR, and a disease-specific approach for grading obstruction from complex to simple. World J Urol. 1995;13:47-58.
Schafer W, Sterling AM, Liao L, et al. Good urodynamic practice: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodynam. 2002;21:261-274.
Schick E, Dupont C, Bertrand PE, et al. Predictive value of maximum urethral closure pressure, urethral hypermobility and urethral incompetence in the diagnosis of clinically significant female genuine stress incontinence. J Urol. 2004;171:1871-1875.
Scholtmeijer RJ, Nijman RJ. Vesicoureteric reflux and videourodynamic studies: results of a prospective study after three years of follow-up. Urology. 1994;43:714-718.
Seruca H. Vesicoureteral reflux and voiding dysfunction: a prospective study. J Urol. 1989;42:494-501.
Sidi AA, Dykstra DD, Gonzalez R. The value of urodynamic testing in the management of neonates with myelodysplasia: a prospective study. J Urol. 1986;135:90-93.
Sillen U, Bachelard M, Hansson S. Video cystometric recording of dilating reflux in infancy. J Urol. 1996;155:1711-1715.
Siroky MB, Olsson CA, Krane RJ. The flow rate nomogram: I. Development. J Urol. 1979;122:665-668.
Siroky MB, Olsson CA, Krane RJ. The flow rate nomogram: II. Clinical correlation. J Urol. 1980;123:208-210.
Sorensen S, Gregersen H, Sorensen SM. Long term reproducibility of urodynamic investigations in healthy fertile females. Scand J Urol Nephrol Suppl. 1988;114:35-41.
Steele GS, Sullivam MP, Yalla SV. Urethral pressure profilometry: vesicourethral pressure measurements under resting and voiding conditions. In: Nitti VW, editor. Practical urodynamics. Philadelphia: WB Saunders; 1998:108-130.
Stohrer M. Alterations in the lower urinary tract after spinal cord injury—diagnosis, prevention and therapy of late sequelae. World J Urol. 1990;7:205.
Stohrer M, Goepel M, Kondo A, et al. The standardization of terminology in neurogenic lower urinary tract dysfunction with suggestions for diagnostic procedures. Neurourol Urodyn. 1999;18:139-158.
Stohrer M, Kramer G, Lochner-Ernst D, et al. Diagnosis and treatment of bladder dysfunction in spinal cord injury patients. Eur Urol Update series. 1994.
Susset JG. Development of nomograms for application of uroflowmetry. In: Hinman F, Boyarski S, editors. Benign prostatic hypertrophy. New York: Springer-Verlag; 1983:528-538.
Tanaka H, Kakizaki H, Kobayashi S, et al. The relevance of urethral resistance in children with myelodysplasia: its impact on upper urinary tract deterioration and the outcome of conservative management. J Urol. 1999;161:929-932.
Theofrastous JP, Bump RC, Elser DM, et al. Correlation of urodynamic measures of urethral resistance with clinical measures of incontinence severity in women with pure genuine stress incontinence. The Continence Program for Women Research Group. Am J Obstet Gynecol. 1995;173:407-414.
Thomas AW, Cannon A, Bartlett E, et al. The natural history of lower urinary tract dysfunction in men: the influence of detrusor underactivity on the outcome after transurethral resection of the prostate with a minimum 10-year urodynamic follow-up. BJU Int. 2004;93:745-750.
Torre M, Planche D, Louis-Borrione C, et al. Value of electrophysiological assessment after surgical treatment of spinal dysraphism. J Urol. 2002;168:1759-1763.
Tsiju I, Kuroda K, Nakajima F. Excretory cystography in paraplegic patients. J Urol. 1960;83:839-844.
Türker P, Kilic G, Tarcan T. The presence of transurethral cystometry catheter and type of stress test affect the measurement of abdominal leak point pressure (ALPP) in women with stress urinary incontinence (SUI). Neurourol Urodyn. 2010;29:536-539.
van Waalwijk van Doorn E, Anders K, Khullar V, et al. Standardisation of ambulatory urodynamic monitoring: report of the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2000;19:113-125.
van Waalwijk van Doorn ES, Meier AH, et al. Ambulatory urodynamics: extramural testing of the lower and upper urinary tract by Holter monitoring of cystometrogram, uroflowmetry, and renal pelvic pressures. Urol Clin North Am. 1996;23:345-371.
van Waalwijk van Doorn ES, Remmers A, Janknegt RA. Conventional and extramural ambulatory urodynamic testing of the lower urinary tract in female volunteers. J Urol. 1992;147:1319-1326.
Vesely S, Knutson T, Fall M, et al. Clinical diagnosis of bladder outlet obstruction in men with lower urinary tract symptoms: reliability of commonly measured parameters and the role of idiopathic detrusor overactivity. Neurourol Urodyn. 2003;22:301-305.
Wang QW, Wen JG, Song DK, et al. Is it possible to use urodynamic variables to predict upper urinary tract dilatation in children with neurogenic bladder-sphincter dysfunction? BJU Int. 2006;98:1295-1300.
Webb RJ, Griffiths CJ, Ramsden PD, Neal DE. Ambulatory monitoring of bladder pressure in low compliance neurogenic bladder dysfunction. J Urol. 1992;148:1477-1481.
Webb RJ, Styles RA, Griffiths CJ, et al. Ambulatory monitoring of bladder pressures in patients with low compliance as a result of neurogenic bladder dysfunction. Br J Urol. 1989;64:150-154.
Weber AM. Is urethral pressure profilometry a useful diagnostic test for stress urinary incontinence? Obstet Gynecol Surv. 2001;56:720-735.
Weber AM. Leak point pressure measurement and stress urinary incontinence. Curr Womens Health Rep. 2001;1:45-52.
Wein AJ. Classification of neurogenic voiding dysfunction. J Urol. 1981;125:605-609.
Wein AJ, Hanno PM, Dixon DO, et al. The effect of oral bethanechol chloride on the cystometrogram of the normal adult male. J Urol. 1978;120:330-331.
Wein AJ, Raezer DM, Malloy TR. Failure of the bethanechol supersensitivity test to predict voiding after subcutaneous bethanechol administration. J Urol. 1980;123:202-203.
Wilmshurst JM, Kelly R, Borzyskowski M. Presentation and outcome of sacral agenesis: 20 years’ experience. Dev Med Child Neurol. 1999;41:806-812.
Wyndaele JJ, De Wachter S. Cystometrical sensory data from a normal population: comparison of two groups of young healthy volunteers examined with 5 years interval. Eur Urol. 2002;42:34-38.
Yalla SV, Blute R, Waters W, et al. Urodynamic evaluation of prostatic enlargements with vesicourethral static pressure profiles. J Urol. 1981;125:685-689.
Yalla SV, Sharma GV, Barsamian EM. Micturitional urethral pressure profile during voiding and the implications. J Urol. 1980;124:649-656.