chapter 70 Electrical Stimulation and Neuromodulation in Storage and Emptying Failure
For the past century, the development of electrical neurostimulation and neuromodulation to alter physiologic processes responsible for lower urinary tract symptoms and dysfunctions has largely been the result of important landmark discoveries in the clinical application of electricity and in the understanding of neuromuscular physiology. In neurostimulation the use of electrical stimuli on nerves and muscles has mainly been developed to achieve immediate clinical responses in neurogenic conditions of pelvic organ dysfunction; in neuromodulation the application of electrical stimuli to nerves has been developed to alter neurotransmission processes in non-neurogenic and neurogenic conditions. In this chapter the focus is on reviewing the application (Table 70–1) and clinical outcomes of neurostimulation and neuromodulation therapies for pelvic organ dysfunction with respect to the development of our knowledge of pelvic neuromuscular physiology and its role in translational innovations.
Table 70–1 Potential Applications of Electrical Stimulation in the Treatment of Voiding Dysfunction
| PURPOSE | SITES OF STIMULATION | MECHANISM |
|---|---|---|
| Facilitate Filling-Storage | ||
| Inhibit detrusor contractility Increase bladder capacity Decrease urgency and frequency |
V, A, SP, PT CP, SR, IV |
Neuromodulation |
| Decrease nociception | V, A, SP, SR | Neuromodulation |
| Increase outlet resistance | V, A, SR | Direct stimulation (efferent nerves or roots) |
| Facilitate Emptying | ||
| Stimulate detrusor contraction (spinal cord–injured patient) | SAR | Direct stimulation (efferent nerves or roots) |
| Restore micturition reflex (idiopathic retention) | SR, IV | Neuromodulation |
A, anal; CP, common peroneal; IV, intravesical; PT, posterior tibial; SAR, sacral anterior (ventral) roots; SP, suprapubic; SR, sacral roots; V, vaginal.
History of Electrical Stimulation
Although the field of electrical stimulation of nerves to achieve muscle contractions was not truly realized until the early 1800s, its roots can be traced into the 1700s, when inadvertent electrical impulses were found to generate strong muscle convulsions (Galvani, 1786; Bell, 1811). Magendie (1822) was one of the first to conduct physiologic investigations of the spinal nerve roots, documenting in young dogs that transection of the posterior (dorsal) segments resulted in a lack of sensation but persistence of motor function, whereas anterior (ventral) root transection yielded preservation of sensation yet abolishment of motor function. These important findings created the foundation for our understanding of basic neurophysiology of micturition and led to further discoveries on bladder function in the setting of selective rhizotomy of both the pelvic and hypogastric nerves (Giannuzzi, 1863; Langley, 1895). Ultimately, Saxtorph in 1878 used these principles to directly stimulate the bladder in patients with urinary retention via a metal transurethral catheter (Madersbacher, 1999). His early findings allowed others to develop enthusiasm for direct stimulation of the bladder through both transurethral and direct detrusor routes (see section on direct stimulation).
Because Saxtorph had significant influence on the idea that direct bladder stimulation may lead to bladder contractility, McGuire used some of these same principles to perform direct bladder stimulation in dogs (Boyce et al, 1964). He concluded that multiple pairs of electrodes were required to achieve a more uniform pressure rise within the bladder during the stimulated contraction. Research continued throughout the late 1900s and moved toward development of new electrodes (Susset and Boctor, 1967) and differing wire configurations (Timm and Bradley, 1969; Tscholl et al, 1971).
Direct pelvic nerve stimulation and pelvic floor muscle stimulation were not well-known or well-studied concepts until the mid 1900s because emphasis was clearly on different areas of direct stimulation (i.e., bladder). Dees (1965) studied the bladder contraction state after stimulation to the pelvic nerve. He achieved contraction of both the detrusor muscle and urethral sphincter in a cat model and produced pain and hind leg contraction as well. Others demonstrated similar findings of nonspecific contraction of the bladder and pelvic floor and other areas with direct pelvic nerve stimulation and ultimately realized that direct pelvic nerve stimulation may not be suitable for treatment of bladder dysfunction (Burghele et al, 1962; Hald et al, 1966; Holmquist and Olin, 1968). It appears that direct pelvic nerve stimulation elicits pudendal nerve activity such that outlet resistance is increased, as is pain, through simultaneous hypogastric nerve stimulation. Thus, with the limited-potential clinical utility of the pelvic nerve for stimulation, efforts focused on the pelvic floor muscles, spinal cord, and sacral roots. Caldwell first reported experience with pelvic floor muscle stimulation with the goal of improving fecal continence and later urinary incontinence (Caldwell, 1963; Caldwell et al, 1965). Subsequently, interest increased and efforts toward external stimulation were described by anal, vaginal pessary, and direct vaginal stimulation (Hopkinson and Lightwood, 1967; Alexander and Rowan, 1968; Erlandson et al, 1977; Fall et al, 1977).
Spinal cord stimulation, by attempting to directly activate the micturition center, was thought to be a promising avenue of therapy (Nashold et al, 1971; Jonas et al, 1975; Jonas and Tanagho, 1975). Still, as in pelvic nerve stimulation, voiding was initiated; however, simultaneous sphincter activity precluded proper emptying. The subsequent series of developments pursued therapy for incomplete emptying by affecting not only bladder stimulation but, in some way, sphincter relaxation as well.
In 1972, Brindley began experimentation of sacral root stimulation that led to implantation of sacral anterior root stimulators in paraplegic patients with urinary incontinence (Brindley, 1972, 1974, 1977). Evolving data showed that, for optimal bladder emptying to be achieved, sacral anterior root stimulation with posterior rhizotomies of S2, S3, and S4 would be required (Sauerwein, 1990). The posterior rhizotomy would decrease the reflex activity of the detrusor and improve bladder compliance. Tanagho and coworkers then began further examining the sacral roots and their individual contributions to bladder and outlet function. In 1982, Tanagho and Schmidt presented initial experience in sacral root stimulation in paraplegic dogs. In this initial study they realized the design of a spiral electrode to minimize nerve damage and fixed the lead wire to the sacral lamina, thereby preventing tension on the electrode itself. They ultimately attained good bladder contraction in these dogs, with minimal sphincteric response. From these initial good results, Tanagho and coworkers then began human trials and characterized the sacral root stimulation patterns and the corresponding muscle responses (Table 70–2). In the course of the neurostimulation developments these researchers realized that sphincteric contraction abolished detrusor activity, and the role of the pudendal nerve and its modulation of bladder capacity began to evolve (Tanagho and Schmidt, 1988; Schmidt, 1989). Thus, neuromodulation was introduced as a concept by which activation of the sacral roots may, in fact, modulate external sphincter function and in turn inhibit detrusor activity as a normal reflex. These early discoveries created the platform for the current and future concepts and technologies that are used for neurostimulation and neuromodulation.
Table 70–2 Sacral Nerve Responses
| SACRAL NERVE | MOTOR AND SENSORY RESPONSE |
|---|---|
| S2 | Motor: Plantarflexion of the entire foot with lateral rotation and clamp movement of the anal sphincter |
| Sensory: Sensations in the leg and buttock | |
| S3 | Motor: Dorsiflexion of the great toe and bellows reflex (anal wink) |
| Sensory: Paresthesias or sensation of pulling in the rectum, scrotum, or vagina | |
| S4 | Motor: Bellows reflex only |
| Sensory: Sensation of pulling in the rectum only |
Neurophysiology of Electrical Stimulation for Storage and Emptying Disorders
The exact mechanisms of how neuromodulation works are not completely understood, but several plausible theories with testable hypotheses are under investigation. Most are founded on the basic neurophysiologic mechanisms that result in the normal storage and emptying functions of the bladder. Although there is an expanding amount of information on our knowledge of micturition neurophysiology as discussed in this chapter, in this section the specifics of the micturition pathway in relation to neuromodulation are addressed.
Normal detrusor function appears to be a sacral balance under suprasacral influences of the sympathetic and parasympathetic nervous systems in their respective abilities to maintain continence. The sympathetic tone, for the most part, is dominant for the majority of time and thus provides continence or storage of urine; the parasympathetic nervous system allows detrusor contractions for emptying of the bladder. Thus, the micturition reflex pathway is activated by initial bladder afferent excitation that then results in a bladder efferent excitation leading to a detrusor muscle contraction. The acquired and unique ability to void volitionally is due to either negative feedback (inhibition of voiding) or positive feedforward (induction of voiding) influences of supraspinal inputs from the pontine micturition center on this sacral micturition reflex pathway. Any loss of either central supraspinal inhibitory influences or increased sensitization of bladder afferent signaling can lead to unmasking of involuntary voiding. As such, this has been proven only in animals and, by deference, relates then to humans. There may be primitive reflexes that reside within the spinal cord that can be “awakened” by somatic and afferent nerve stimulation and may have something to do with the mechanism of action of neuromodulation (de Groat, 1975, 1976; de Groat and Ryall, 1968a, 1968b).
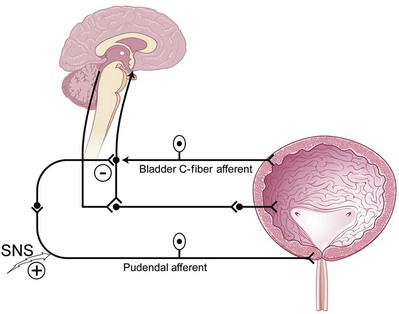
Bladder afferent nerve signaling sends information about pain and bladder fullness to the brain that will in turn initiate the micturition reflex. Bladder overactivity may be in part mediated by the loss of voluntary control of the voiding reflex and, furthermore, emergence of primitive voiding reflexes. In certain states of neurologic or inflammatory disease of the bladder, the previously silent C fibers may emerge and trigger the micturition reflex. Accordingly, blockade of this pathway by electrical neuromodulation, similar to pharmacologic blockade by capsaicin (a C-fiber blocker), may suppress detrusor overactivity (Maggi and Meli, 1988; Chen et al, 1993).
Reflexes That Promote Bladder Storage
Two important reflexes may play an important role in modulation of bladder function: the guarding reflex and the bladder afferent loop reflex. Both reflexes promote urine storage (guarding reflex under somatic influence and bladder loop reflex under sympathetic tone). The guarding reflex guards or prevents urine loss from times of cough or other physical stress that would normally trigger a micturition episode. Suprapontine input from the brain turns off the guarding reflex during micturition to allow efficient and complete emptying. The bladder afferent reflex works through sacral interneurons that then activate storage through pudendal nerve efferent pathways directed toward the urethral sphincter. As such, the activity has truly been realized only in cats but has been postulated to exist in humans and to function the same. Similar to the guarding reflex, the bladder afferent reflex promotes continence during periods of bladder filling and is quiet during micturition (Fig. 70–1).
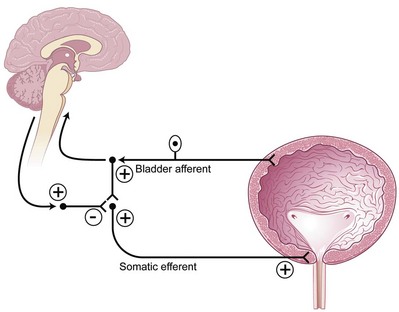
Figure 70–1 The guarding reflex promotes continence and allows the outlet to contract the urinary sphincter during periods of stress (e.g., cough). The brain can turn this reflex off during voiding.
(Modified from Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am 2005;32:11–8.)
Reflexes That Promote Bladder Emptying
Signals from the bladder that may modulate the need to void with fullness, pain, pressure, or stretch may elicit bladder afferent activity through the Aδ or even C fibers. These bladder afferent nerve fibers then synapse with both parasympathetic efferents (bladder-bladder reflex) and parasympathetic urethral efferents (bladder-urethral) reflex. The urge to void may then be translated as an initial activity (inhibitory) of the bladder-urethral reflex to allow the pressure in the urethral outlet to drop immediately before a bladder contraction ensues and simultaneously permit the bladder-bladder reflex to allow a smooth bladder contraction to occur as the reflex is maintained throughout the entire void (de Groat, 1978; de Groat et al, 1981, 1996).
Putative Mechanism of Action of Sacral Neuromodulation
Although our knowledge of how neuromodulation works is evolving, two main theories exist: (1) direct activation of efferent fibers to the striated urethral sphincter reflexively causes detrusor relaxation and (2) selective activation of afferent fibers causes inhibition at spinal and supraspinal levels. Accumulating evidence suggests that activation of somatic sacral afferent inflow at the sacral root level that in turn affects the storage and emptying reflexes in the bladder and central nervous system accounts for the positive effects of neuromodulation on both storage and emptying functions of the bladder (Yoshimura and de Groat, 1992, 1997; Leng and Chancellor, 2005). Malaguti and coworkers (2003), using detection of somatosensory evoked potentials during sacral neuromodulation, concluded that sacral neuromodulation therapy works by sacral afferent activity and concomitant activation of the somatosensory cortex. Because sacral neuromodulation has been clinically proven for both storage (urgency/frequency and urgency urinary incontinence) and emptying (nonobstructive urinary retention) dysfunctions of the bladder, isolating the mechanism of action to the micturition reflex pathway of sacral afferent and efferent pathways alone is challenging. However, by understanding the reflexes that influence the promotion of urine storage or emptying of the sacral micturition reflex pathway one begins to realize how neuromodulation may affect these reflexes and elicit symptomatic and functional improvement of voiding function (Fowler et al, 2000). Animal models of sacral neuromodulation for detrusor overactivity are now being developed and may shed more light on how this technology achieves its benefits (Riazimand and Mense, 2004; Vignes et al, 2009)
Putative Mechanism of Action of Sacral Neuromodulation in Overactive Bladder
The bladder storage and emptying reflexes are modulated by several centers in the brain and may be altered by neurologic injury that effectively unmasks involuntary bladder contractions. Thus, sacral neuromodulation of these primitive reflexes may restore normal micturition (de Groat, 1976). Animal data exist to support the fact that somatic afferent input to the spinal cord can affect the guarding and bladder-bladder reflexes (de Groat, 1978; Chen et al, 1993). It is believed that suppression of interneuronal transmission in the bladder reflex pathway may be how sacral neuromodulation affects detrusor overactivity (de Groat, 1976; Kruse and de Groat, 1993; Leng and Chancellor, 2005). The inhibition by electrical neuromodulation may, in part, modulate the sensory outflow from the bladder through the ascending pathways to the pontine micturition center, thereby preventing involuntary contractions by modulating the micturition reflex circuit but allowing voluntary voiding to occur (Fig. 70–2). The preservation of voluntary voiding may be due to selective avoidance of normal sensory ascending outflow pathways of the bladder from Aδ fibers to the pontine micturition center as well as initiation of the descending pathways from the pontine micturition center to sacral efferent outflow pathways. Therefore, as is seen in clinical practice, sacral neuromodulation may affect and improve the abnormal bladder sensations, involuntary voids, and detrusor contractions but still maintain normal bladder sensations and voluntary voiding patterns.
Putative Mechanism of Action of Sacral Neuromodulation in Urinary Retention
Sphincteric activity can be turned off by brain pathways to allow efficient and complete bladder emptying. If the suprasacral pathways are altered, the guarding and urethral reflexes still exist and cannot be turned off. This may cause retention, as in the spinal cord–injured patient who in turn has detrusor-sphincter dyssynergia resulting in urinary retention. Thus, inhibition of the guarding reflexes may allow urinary retention states to be improved (Fig. 70–3). Originally for urinary retention due to functional outlet obstruction, sacral neuromodulation was thought to directly turn off excitatory flow to the urethral outlet and facilitate bladder emptying. This may be an efferent effect of the primary afferent mechanism of sacral neuromodulation as reported by Dasgupta and colleagues (2005), who described novel neuroimaging evidence for the existence of abnormal interaction between the brainstem and cortical centers in women with urinary retention due to Fowler syndrome or overactive sphincteric activity. The direct therapeutic effect of sacral neuromodulation in patients with sphincter overactivity or functional outlet obstruction appears to be in the afferent restoration of a normal pattern of midbrain activity and decreased cortical activity leading to inhibition of the guarding reflexes.
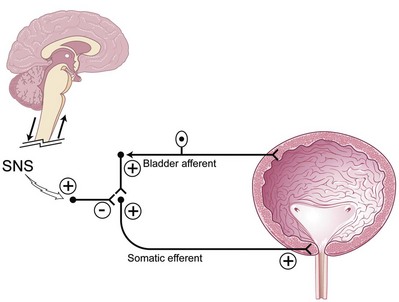
Figure 70–3 In neurologic disease the supraspinal circuitry is “disconnected” and therefore cannot turn off the spinal guarding reflex, and thus retention occurs. Sacral neuromodulation (SNS) can restore the normal voluntary pattern of micturition by inhibition of the spinal guarding reflex.
(Modified from Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am 2005;32:11–8.)
Electrical Stimulation for Storage Disorders
Criteria for Selection of Patients
Because many lower urinary tract symptoms and dysfunctions are secondary to a neuromuscular etiology, a thorough history and physical examination will often reveal the nature (acute vs. chronic) and help classify the cause (neurogenic, anatomic, postsurgical, functional, inflammatory, or idiopathic). In addition to the unique evaluation of pelvic floor muscle dysfunction (Siegel, 2005), a urinalysis is routinely performed; urine cytology should be considered in patients who present with refractory symptoms of dysuria, urgency, or frequency of urination, because carcinoma in situ and bladder tumors may present as irritative bladder symptoms without hematuria. Further assessment of the bladder function, urodynamic studies including cystometrography, pressure-flow studies, and electromyography of sphincters and pelvic floor muscles are performed on a selected basis, because most non-neurogenic assessments are completed routinely with the use of a voiding diary and a focused physical examination of the pelvis. Electromyography is recommended in suspected cases of neurogenic bladder dysfunction, detrusor-sphincter dyssynergia, or Fowler syndrome and may be considered for evaluation of inappropriate pelvic floor muscle behavior (Dasgupta and Fowler, 2003). As of now, urodynamics have not yielded adequate predictive factors that allow enhanced selection for patients undergoing sacral neuromodulation (Groenendijk et al, 2007). The characteristics of neurogenic bladders, as seen in patients with multiple sclerosis and spinal cord injury, can change with time and disease progression. Therefore, reevaluation with urodynamics and assessment of the upper urinary tracts may be needed when symptoms change despite active medical intervention.
Cystourethroscopy may yield information helpful in making a diagnosis. Anatomic lesions such as urethral stricture, bladder neck fibrosis, trabeculation, and bladder lesions have been found even in women with bladder outlet obstruction. Baseline upper tract imaging is performed in patients with neurologic disease or, if indicated, by physical or baseline studies or a patient’s history.
Sacral neuromodulation is frequently attempted in patients in whom traditional conservative measures (e.g., bladder retraining, pelvic floor biofeedback, and medications) have failed and before more invasive surgical procedures (e.g., enterocystoplasty and urinary diversion). Despite all the studies done to date there are no defined preclinical factors, such as urodynamic findings, that can predict which patients will or will not respond to sacral neuromodulation.
Although most patients are considered candidates for neurostimulation and neuromodulation therapies when more conservative treatment has failed there are some clinical considerations for excluding patients from this therapy. These include significant anatomic abnormalities in the spine or sacrum that may present challenges to gaining access; mental incapacitation of patients who cannot manage their device or judge the clinical outcome; physical limitations that prevent the patient from achieving normal pelvic organ function, such as functional urinary incontinence; and noncompliance of the patient.
Relative contraindications for patients who may be considering or who have an implantable electrical stimulation device are magnetic resonance imaging (MRI) and pregnancy. Magnetic fields produce currents in neuroelectrodes, and there is some concern that the magnetic field from MRI may damage the pulse generator, as discussed later. Many radiologists are reluctant to provide MRI services for patients with implantable electrical stimulation devices despite the anecdotal evidence that no adverse event has occurred when MRI has inadvertently or purposefully been done for emergent reasons or in small trials (Hassouna and Elkelini, 2005). For patients who have InterStim devices in place (see later), the authors advocate removal of the device in preparation for elective MRI based on current manufacturer recommendations. After the MRI procedure, a new neuroelectrode and generator may be placed. Anecdotal reports of patients safely undergoing the study (MRI) with the InterStim implant in place have occurred, but patients should have the devices turned off in anticipation. Because of the potential for teratogenicity or abortion from the effect of electrical stimulation, electrical stimulation has been considered contraindicated in pregnant women with various voiding dysfunctions. However, whether electrical stimulation can cause abortion or malformation is not known. Wang and Hassouna (1999) reported no adverse effects of electrical stimulation on pregnant rats and that termination of pregnancy is not advised for prospective mothers when electrical stimulation has been performed unknowingly in early pregnancy. Women with electrical stimulation devices for pelvic health conditions who become pregnant may simply turn off their devices during pregnancy.
Electrical Stimulation of the Bladder
Transurethral electrical bladder stimulation (TEBS) has been pursued not only for initiating sensory awareness of bladder filling and stimulating detrusor contractility (see later section) but also for increasing bladder capacity at low pressure in pediatric patients with myelomeningocele (Kaplan et al, 1989; Decter et al, 1994). The authors in these two cited references carried on an interesting point-counterpoint discussion in publications regarding the practical benefit of TEBS (Kaplan, 2000; Decter, 2000), which remains controversial.
Both authors seem to be saying the same thing with respect to results but attach a totally different significance to the practical implications of the results. Kaplan (2000) points out that when the procedure was initiated the goal was to provide children with neurogenic bladder dysfunction, mostly secondary to spina bifida, enough sensation to detect a filling or full bladder and to have them synergistically void or catheterize in a timely manner. As the results of treatment have been evaluated over the years, the real benefit of this program, to Kaplan at least, was the potential to increase bladder capacity while maintaining or decreasing end-filling bladder pressure (in essence, improving compliance). Decter (2000) gives what seems to be a reasonable summation of the largest multi-institutional report of this therapy involving 568 patients who underwent TEBS at 11 institutions, only 335 of whom had adequate pretreatment and post-treatment urodynamics for evaluation. Bladder capacity increased by 20% or more in 56% of the 335 patients, whereas pressure at bladder capacity decreased by 25% or more in 16% of those in whom the bladder capacity increased. Decter (2000) calculated that only 30 of 335 patients had both a 20% or more increase in bladder capacity and a 25% decrease in compliance. He reports his experience with 25 patients during a 4-year period as showing that bladder capacity increased more than 20% (values referred to a comparison to age-adjusted bladder capacity) and end-filling bladder pressures showed clinically significant decreases in 29% of patients. Putting this in perspective, he believes “the practical benefits our patients derive seem limited…the urodynamic improvements we achieved after stimulation did not materially alter daily voiding routine (i.e., clean intermittent catheterization) of these children.” Pugach and associates (2000) also reported the results of TEBS in a group of pediatric patients; only 7 of 44 (16%) had safe storage pressures with continence after treatment. There exist limited new data since 2000 on this treatment modality probably owing to other better potential nerve targets.
Sacral Rhizotomy
In most cases, bilateral anterior and posterior sacral rhizotomy or conusectomy converts a overactive detrusor to an areflexic one. This alone may be inappropriate therapy because it also adversely affects the rectum, anal and urethral sphincters, sexual function, and the lower extremities. In an attempt to leave sphincter and sexual function intact, selective motor nerve section was originally introduced as a treatment to increase bladder capacity by abolishing only the motor supply responsible for involuntary contractions. The initial use of this procedure followed the observation that the third anterior (ventral) sacral root provided the dominant motor innervation of the human bladder. To enhance the clinical response and to minimize side effects, differential sacral rhizotomy should always be preceded by stimulation and blockade of the individual sacral roots with cystometric and sphincterometric control.
Although technique refinements, such as percutaneous radiofrequency selective sacral rhizotomy and cryoneurolysis, have occurred, there is still controversy about the role of anterior rhizotomy procedures within a treatment plan for detrusor overactivity. Torrens (1985) summarized successful results in collected groups of patients that ranged from 48% for idiopathic overactivity to 81% for patients classified as having a “paraplegic bladder.” However, as he astutely pointed out, the definition of success varies from one series and from one patient to another. When these procedures are used, they should certainly be preceded by urodynamics and urologic evaluation of the effects of selective nerve blocks before performance, especially in patients without fixed neurologic disease or injury. Even then, unintended effects on pelvic and lower extremity sensory or motor functions may occur with disastrous medical and legal sequelae.
Both Tanagho and Schmidt (Tanagho and Schmidt, 1988; Tanagho et al, 1989) and Brindley (Brindley and Rushton, 1990) have popularized the concept of sensory deafferentation by dorsal or posterior rhizotomy to increase bladder capacity as part of their overall plan to simultaneously rehabilitate storage and emptying problems in patients with significant spinal cord injury or disease. These are patients in whom electrical stimulation also was used to alleviate emptying deficits (see section on emptying disorders). McGuire and Savastano (1984) also mentioned dorsal root ganglionectomy alone in such patients to increase bladder capacity.
Gasparini and colleagues (1992) reported durability of the deafferentation response to selective dorsal sacral rhizotomy up to 64 months after section. The technique involves selecting nerve roots whose intraoperative stimulation provokes an adequate detrusor response. The dorsal and ventral components of these roots are then separated and the dorsal root or roots severed. An increase in bladder capacity of 259 to 377 mL was noted in 16 of 17 patients studied (24 in the original series), with an increase in the volume to the first contraction of 99 to 270 mL. Fourteen patients were cured of incontinence, and 2 improved; the technique failed in 1 patient. Of seven potent men, two experienced a decrease in erectile frequency but were still able to achieve penetration. Bowel and sphincter function were unaffected. Koldewijn and associates (1994) reported on the effects of intradural bilateral posterior root rhizotomies from S2 to S5 with implantation of an anterior root stimulator in a group of patients with suprasacral spinal cord injury. All showed persistent detrusor areflexia afterward, although 2 required subsequent secondary rhizotomy at the level of the conus. A majority showed decreased bladder compliance up to 5 days postoperatively, followed by a rapid increase thereafter.
Brindley (1994) summarized the advantages of bilateral posterior sacral rhizotomy in treatment of voiding dysfunction after spinal cord injury as abolishing reflex incontinence, improving compliance, and abolishing striated sphincter dyssynergia without altering resting tone. Partial or selective procedures are considered only in such patients who retain some sensation or have excellent reflex erections. Madersbacher (2000) comments that posterior sacral rhizotomy for sacral deafferentation of the bladder is best achieved by the intradural approach, which has the advantage that, in this location, motor and sensory fibers can easily be separated, whereas distal to the spinal ganglion, motor and sensory fibers are intermingled and a clear separation is no longer possible. If an intradural procedure is not possible, he believes that a deafferentation at the level of the conus medullaris is preferable to an extradural sacral approach. He mentions that he has treated 65 tetraplegic or paraplegic patients with post–spinal cord injury reflex urinary incontinence who were resistant to all other means of conservative treatment. Incontinence was abolished in 90% of these patients.
Sacral Neuromodulation
Neuromodulation is an innovative treatment of lower urinary tract symptoms and dysfunctions of bladder storage secondary to neuromuscular causes. In addition to the application of evolving technologies for sacral neuromodulation therapy, expanding clinical indications such as neurogenic detrusor overactivity, post–sling-related voiding dysfunction, interstitial cystitis, pelvic pain, pediatric voiding dysfunction, and bowel disorders as well as novel forms of transcutaneous and implantable neuromodulation devices for different nerve roots are under investigation.
Technique
Sacral nerve stimulation (SNS) by the InterStim (Medtronic, Minneapolis, MN) procedure is performed in two stages: stage I, a clinical trial of a temporary or permanent lead for external stimulation; and stage II, implantation of a subcutaneous implantable pulse generator (IPG). Each stage can be performed with monitored anesthesia care supplemented by local anesthesia. During the initial introduction of sacral neuromodulation therapy, patients underwent a percutaneous nerve evaluation by the placement of a unilateral percutaneous lead in the S3 foramen with use of local injectable anesthesia. The lead was connected to an external pulse generator and worn by the patient for several days. A large number of false-negative results with therapy are attributed to improper lead placement and migration. Whereas some physicians still prefer to perform the first stage by a percutaneous nerve evaluation approach, most have adopted a permanent tined lead placement for the first stage in an attempt to avoid the issues related to high false-negative results with the first stage and high false-positive results with the second stage. Changes in lower urinary tract symptoms and postvoid residuals are recorded in a detailed bladder diary. If improvement is minimal or absent, revision or bilateral percutaneous lead placement may be attempted. If more than 50% improvement in symptoms of urgency/frequency or urgency urinary incontinence is attained, a permanent IPG is implanted. The length of the trial with the external pulse generator may vary slightly from patient to patient, by the indication, and by the surgeon’s practice preference. In patients with urgency/frequency and urgency urinary incontinence, a 1- to 2-week trial is generally adequate. For retention, a longer trial of 3 to 4 weeks or more may be necessary before a desired clinical response is obtained.
Previous lead placement required a more time-consuming surgical dissection of the layers above the sacral foramen and unreliable lead fixation with anchors. Recent technical advances have made implantation of the percutaneous lead easier and less prone to migration. Spinelli and colleagues (2003) were the first to present the advantages of the tined lead that used a percutaneous approach for placement and fixation (Fig. 70–4). Subsequent large-scale clinical experience worldwide confirms that the tined lead is less prone to migration and has decreased false-negative results with the screening trial. Furthermore, the false-positive rate of the screening trial is reduced when placement of a permanent lead with reliable fixation during the screening trial ensures that the same location of stimulation is achieved when the IPG is implanted. With a percutaneous nerve evaluation or similar temporary lead electrode during the screening trial, a different clinical outcome may occur when the permanent lead is placed at the time of the IPG implantation.
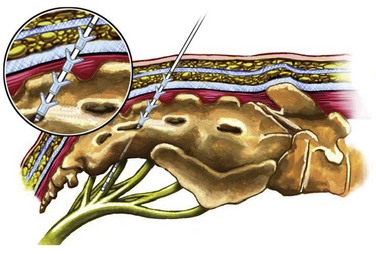
Figure 70–4 The tined lead is introduced typically into the S3 nerve foramen. The “tines” allow the lead to be fixed into the fascial layers above the sacrum. This lead has a quadripolar configuration (four contact points).
(Courtesy of Medtronic, Minneapolis, MN.)
For the first stage of the procedure, preoperative intravenous antibiotics are given before the procedure and aseptic techniques of foreign body implants are implemented. The patient is placed in the prone position, and the buttocks are held apart by wide tape retraction so that the anus is visible during test stimulation. The anus and tape are prepared in a sterile fashion and then covered with a separate plastic drape until visualization is needed during the procedure. The sterile drape covering the feet must be folded back such that the feet can be visualized during the procedure as well.
The location of the S3 foramen is approximated by measuring 9 cm cephalad to the dropoff of the sacrum and 1 to 2 cm lateral to the midline on either side. Alternatively, the site may also be localized by palpating the cephalad portions of the sciatic notches bilaterally and drawing a connecting line that intersects the midline of the sacrum; one fingerbreadth on either side of the midline of the sacrum at this intersection will define the location of the S3 foramen (Fig. 70–5). The foramen needle is then inserted into the S3 foramen. The pelvic plexus and pudendal nerve run alongside the pelvis, and therefore the needle should be placed just inside the ventral foramen. The position of the needle is confirmed by fluoroscopy. The nerve is tested for the appropriate motor response, which is dorsiflexion of the great toe and bellows contraction of the perineal area, which represents contraction of the levator muscles (bellows reflex). Simultaneous sensory responses determined at the time of lead placement help optimize positioning. Although this is controversial, if one can localize the stimulation at the time of lead positioning to the vagina-rectum juncture in females and perineoscrotal area in males this is proper localization of the device. The foramen needle stylet is then removed and replaced with the introducer sheath. The distal aspect of the lead consists of four electrodes numbered 0 through 3. The lead is placed into the introducer sheath as directed to expose the electrodes. Typically, electrodes are positioned such that electrodes 2 and 3 straddle the ventral surface of the sacrum (Fig. 70–6). Test stimulation is repeated on each electrode, and the responses are observed. An S3 response should be noted on at least two of the electrodes (see Table 70–2). Once the surgeon is satisfied with the position, the sheath is removed, releasing the tines that anchor the lead. A sensory response, sensation of stimulation in the perineum, is not needed to confirm proper placement if the correct S3 motor response is observed. However, when a motor response is absent, raising the conscious level of the patient during the procedure and detecting the correct sensory response will confirm proper localization, and a clinical response may still be obtained during the screening trial period despite the absence of the motor response.
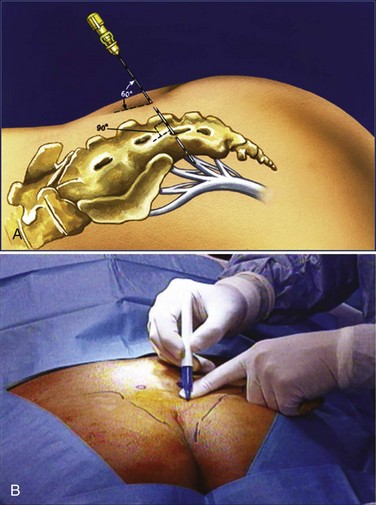
Figure 70–5 A, The percutaneous test stimulation is performed in an outpatient setting. A small lead wire is placed into S3 and connected to an external stimulator for 1 week to administer stimulation to the nerve roots. B, Measurement of the S3 nerve foramen is typically 9 cm to the coccyx and 11 cm to the anal verge. One measures 1 to 2 cm lateral to this mark to find the rough site of the S3 nerve foramen. The “cross hair” technique can be used to find the midline fluoroscopically and the lower aspect of the sacroiliac joints laterally to find the S3 nerve foramen as well.
(Courtesy of Medtronic, Minneapolis, MN.)
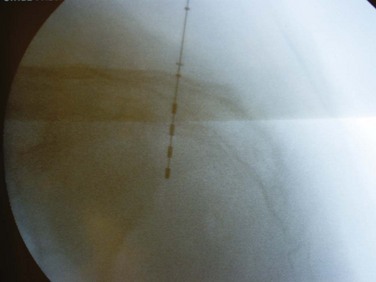
Figure 70–6 Fluoroscopy is used to confirm lead placement, typically with the lead configurations to obtain optimal muscle (bellows response and ipsilateral toe contractions) and sensory (vaginal, penile, or scrotal “pulsating” feeling) responses. The lead may then be deployed.
A 3- to 4-cm incision into the subcutaneous tissues in the upper lateral buttock is made below the beltline or below the level of the ischial wings for connecting the permanent lead to the percutaneous extension lead wire. If the screening trial is successful, this connection site will be the site of implantation for the IPG. With use of the tunneling device provided in the commercial kit, the permanent lead is transferred to the medial aspect of the lateral buttock incision. The lead is then connected to the extension wire, and the tunneling device is used again to transpose the extension wire from the medial aspect of the incision to an exit point on the contralateral side of the back. This transfer and long tunnel reduce the occurrence of infection from the percutaneous exit site of the wire. The extension wire is connected to the external pulse generator. Patients are able to resume their normal activities immediately but are advised to limit excessive movement-related activities, such as high-impact exercises, for the duration of the trial period.
The external generator can be flexibly programmed for the duration of the intended trial while the patient records symptoms and bladder function in a voiding diary. If there is more than 50% improvement in the symptoms or voiding function, a stage II procedure is performed.
A stage II procedure entails placement of the IPG. No fluoroscopy is required during stage II when a permanent neuroelectrode has been placed for the stage I procedure; however, if a percutaneous nerve evaluation was performed for stage I, fluoroscopic confirmation of the neuroelectrode placement is advised. The patient may be placed in the prone position or a lateral position with the site of the previous lateral incision for the lead connections placed upward (Fig. 70–7). The lateral position may improve ventilation during sedation. The previous buttock incision overlying the lead connections is opened, the percutaneous extension wire is removed, and the extension lead is secured to the permanent lead and subsequently to the IPG. A pocket is made in the subcutaneous tissue that is large enough to avoid tension on closure and at a depth to provide a covering layer of subcutaneous tissue anterior to the pulse generator to prevent erosion.
Outcomes
Urinary urgency, frequency, and urgency incontinence have a significant impact on medical health and quality of life. Neuromodulation offers an alternative to patients who may be considering irreversible surgical options when pelvic floor muscle reeducation and pharmacologic therapy have failed. Outcomes of SNS for the indications of idiopathic urgency/frequency and urgency urinary incontinence are derived from only two studies that have randomized patients to active or delayed therapy as well as reports from numerous prospective and retrospective reviews of case series and registry databases.
Schmidt and coworkers (1999) reported on SNS therapy in 76 patients with refractory urgency urinary incontinence from 16 centers worldwide randomized to active or delayed therapy (control group) during the study period of 6 months. Of the 34 patients receiving active SNS therapy compared with the delayed group, 16 (47%) were completely dry and an additional 10 (29%) demonstrated more than 50% reduction in incontinence episodes. Complications were IPG site pain in 16%, implant infections in 19%, and lead migration in 7%.
In a similar study design, Hassouna and colleagues (2000) reported the outcomes of SNS on refractory urgency/frequency conditions in 51 patients randomized from 12 centers during an initial 6-month period that was extended to 2 years. Outcomes at 6 months in the active SNS group showed improvement in the number of daily voids (16.9 ± 9.7 to 9.3 ± 5.1), volume voided (118 ± 74 mL to 226 ± 124 mL), degree of urgency (rank score of 2.2 ± 0.6 to 1.6 ± 0.9), and SF-36 quality of life measures. At 6 months after implantation, stimulators in the active group were turned off and urinary symptoms returned to baseline values. After reactivation of SNS, sustained efficacy was documented at 12 and 24 months.
Limited but conformational results of the earlier randomized trials have been obtained from prospective series (Shaker and Hassouna, 1998; Siegel et al, 2000; Janknegt et al, 2001) and registry studies (Spinelli et al, 2001; Hedlund et al, 2002) evaluating efficacy, safety, and quality of life measures. The results for the registration trial that led to approval by the U.S. Food and Drug Administration (FDA) for SNS (Pettit et al, 2002) reveal that 37 of 62 patients (60%) with refractory urgency/frequency or urgency urinary incontinence achieved an improvement of 50% or more in their condition. van Kerrebroeck and coworkers (2007) have reported on a 5-year prospective worldwide series for patients with the InterStim device for varying indications and for the urgency/frequency and urgency urinary incontinence populations, and good success was achieved in all groups. Of note is that the analysis was of patients including those in the initial registration trial who then “crossed over” to the prospective 5-year trial. This study, unfortunately, was influenced by several patients who did not attend all follow-up visits and, therefore, had missing data points, a problem reflective of this challenging subgroup. Outcomes data were based on “last observation carried forward,” which is of concern for not being statistically optimal, but nonetheless these are still the longest-term data this technology has available.
Special Populations
With the success of neuromodulatory therapies for refractory detrusor overactivity and urinary retention it should be no surprise that indications are expanding. Despite the fact that there is no true FDA-approved indication for neuromodulation in select populations, these groups all have some component of the indicated symptom-complex that includes urgency, frequency, urgency urinary incontinence, or urinary retention. The current expansion of indications for neuromodulation has developed into areas of neurogenic bladder (Parkinson disease, multiple sclerosis, spinal cord injury), interstitial cystitis (painful bladder syndrome), pelvic pain, fecal incontinence and bowel disorders, and pediatric voiding dysfunction.
Neurogenic Bladder
Patients with neurogenic bladder may have several urodynamic events that lead to their symptomatic voiding dysfunction. This may include neurogenic detrusor overactivity, detrusor-sphincter dyssynergia, and flaccid areflexia. Whereas neuromodulation has not been well examined in cases of obvious areflexia (possibly because of the need for some end-organ response for neuromodulation to have any benefit), it has been studied in small subgroups of patients with detrusor overactivity and possibly detrusor–external sphincter dyssynergia, although few published reports exist.
Because the spectrum of neurologic diseases with potential bladder manifestations is wide, one must be cognizant of a few important relative contraindications before contemplating current neuromodulatory therapies, as follows: significant bone abnormalities in the spine or sacrum that may present challenges to gaining access; mental incapacity of patients, who cannot manage the device; physical limitations that prevent voiding (functional incontinence); future need of MRI; and noncompliance.
Multiple Sclerosis
Multiple sclerosis (MS) is a chronic demyelinating disease of the central and peripheral nervous system that can cause a variety of voiding dysfunction scenarios including neurogenic detrusor overactivity, detrusor-sphincter dyssynergy, areflexia, and combinations of these. Because many patients have been refractory to standard therapies, neuromodulation or neurostimulation (to be addressed later in the section on neurostimulation for retention) may be considered a part of their treatment options. In an appropriately selected patient with MS, neuromodulation truly has some promise because it may balance the function of the bladder itself as well as that of the outlet (two of the main components of MS-related voiding dysfunction). This may be considered off-label use of neuromodulation because it is not approved specifically for MS-related voiding dysfunction, but approval may be based on symptoms such as urgency, frequency, or often urgency urinary incontinence and even nonobstructive urinary retention.
No prospective randomized trials exist on the use of neuromodulation in management of MS-related bladder dysfunction. Small series with encouraging results in patients with MS demonstrate that neuromodulation may have a role in treatment of MS-related voiding dysfunction (Bosch and Groen, 1996; Wallace et al, 2007). It appears that the best candidates are ones with mild, nonprogressive MS, with few functional issues, who also have detrusor overactivity or even retention but not areflexia. One of the major issues that arises in the patient with MS in particular is the potential change in the disease state that may be potentiated by neuromodulation. Although this may be theoretical, it was one of the factors cited by the FDA in the original sacral neuromodulation trials and precluded MS patients from being enrolled (Hassouna et al, 2000).
A secondary issue that may arise if a patient with MS is implanted with the lead and IPG system is the potential need for MRI in the future. One should maintain close contact with the patient’s neurologist as to the decision to place a neuromodulatory device to prevent any future need for MRI or selection of a patient in an active phase of disease who requires routine MRI. The main concern with MRI and implantable stimulator or pacemaker-type devices is that heating of the leads has been demonstrated in vivo and in vitro (Roguin et al, 2004; Martin, 2005). Whereas some question the clinical significance of the small temperature changes with the leads, the potential exists to elicit nerve damage with heating of the lead, and the magnetic field may change the generator itself (Gimbel and Kanal, 2004). At present, it is contraindicated to perform MRI of a patient with an implantable neurostimulator system.
Spinal Cord Injury
Many specialists who treat neurogenic disorders due to spinal cord injury realize that a patient may present with a variety of clinical and urodynamic findings. Many patients have neurogenic detrusor areflexic situations, but perhaps more often and sometimes more challenging is the subset who may be incontinent from neurogenic detrusor overactivity with or without concomitant sphincteric dyssynergy. Furthermore, the adverse sequelae of treated or untreated spinal cord injury may include infections, urolithiasis, reflux, or obstruction. The goal, then, is not only to prevent these adverse events but also to ensure a bladder that functions well, empties at a low pressure to protect the upper tracts, and maintains a good capacity and continence. Whereas it is implied that an intact reflex arc should be in place for neuromodulation to work, this has not been proved in clinical studies. Basic science data suggest that at least some communication should exist between sacral outflow and the pontine micturition center to allow processing for the reflexes that may be inhibited by the brain (de Groat et al, 1981). Thus, a patient with a complete spinal cord lesion may not have the same potential benefit from neuromodulation as does one with an incomplete lesion. Again, this fact has yet to be proved clinically in patients. Shaker and colleagues (2000) described their observations in a group of female rats that developed bladder overactivity 3 weeks after spinal cord transection, associated with an increase in neuropeptide content of the dorsal root ganglion of L6. The spinal cord transections were at level T10. Electrostimulation of S1 was carried out and abolished overactivity while still attaining the rise in neuropeptide content in the L6 dorsal root ganglion. This suggested that blockade of C-fiber afferent pathways may have been one of the mechanisms of action of neuromodulation of the sacral root. In addition, they believed the time course of the reduction in neuropeptide content may explain the long-term changes that occur with chronic neuromodulation and the time needed for detrusor overactivity to return toward baseline after neuromodulation is initiated.
From a clinical perspective, few studies exist in neurogenic patients alone for whom sacral neuromodulation was performed. Andrews and Reynard (2003) described a T8 paraplegic with residual urinary urgency and urgency urinary incontinence who underwent percutaneous tibial nerve stimulation (see later section in this chapter) for his problem. This patient experienced an almost twofold increase in bladder capacity; this was repeated, and again, increased cystometric capacity was demonstrated on follow-up. Patients with incomplete spinal cord injury may respond in a similar fashion to the remainder of the population, with fairly high success rates in motivated individuals (Lombardi and Del Popolo, 2010).
It is clear that this is an emerging area of interest in current techniques, and some have postulated a role for selective stimulation in neurogenic patients as a means to achieve better results. Future research will clearly need to be done in this challenging subset of patients.
Voiding Dysfunction after Prior Sling or Anti-Incontinence Surgery
It is well known that patients may develop urinary retention or some form of voiding dysfunction after vaginal sling surgery or other forms of surgical management for urinary incontinence. Whereas the mainstay of therapy is to surgically “undo” the operation, many patients who have been obstructed for some period of time may manifest long-term voiding difficulty. This problem may vary from urgency, frequency, to urgency urinary incontinence or to continued problems with retention and incomplete emptying despite adequate urethrolysis. This patient population (when refractory symptoms continue) may be ideally suited to the placement of a sacral neuromodulation device (Starkman et al, 2008). After implantation, patients had improved quality of life scores, improved urgency and frequency scores, with diminished urgency urinary incontinence in a small retrospective series.
Interstitial Cystitis (Painful Bladder Syndrome)
Chronic pelvic pain and interstitial cystitis are challenging and frustrating conditions for both the physician and the patient. Therapeutic options are limited and frequently ineffective. Whereas neuromodulation therapy in patients with interstitial cystitis has typically been reserved for patients considering major surgery (e.g., cystectomy and urinary diversion) when behavioral and pharmacologic therapies have failed, more innovative consideration has been given to earlier application of this therapy before chronic neuroplastic changes become irreversible.
Interstitial cystitis is not a true FDA-approved indication for neuromodulation; however, the symptom-complex of urinary urgency and frequency is well within the standard approved criteria. It may be realized that neuromodulation for interstitial cystitis may be best in combination with other therapies, because interstitial cystitis is thought to require a multimodal approach and neuromodulation should be considered only one part of the multimodal therapy.
Comiter (2003) performed a prospective evaluation of 25 patients with refractory interstitial cystitis. Seventeen of the 25 patients demonstrated more than 50% improvement in average pain score and voiding symptoms and therefore qualified for permanent IPG placement. At a mean of 14 months of follow-up, improvements were seen in frequency, nocturia, and mean voided volume. Average pain scores decreased from 5.8 to 1.6 on a 10-point scale, and Interstitial Cystitis Symptom Index (ICSI) and Problem Index (ICPI) scores decreased significantly. Furthermore, 94% of patients implanted had a sustained improvement in symptoms. Similar findings were noted by Whitmore and associates (2003) in 33 patients who had statistically improved parameters in frequency, pain, average voided volume, and maximum voided volume as well as ICSI and ICPI scores.
Peters and colleagues (2003) retrospectively evaluated 21 patients with refractory interstitial cystitis with pelvic pain who underwent permanent implantation of the IPG. The patients were contacted by mail and asked to respond to a questionnaire that addressed the use of narcotic pain medication. There was an average decrease in morphine dose-equivalents after implantation of 36%. In addition, subjects were asked to rate their pelvic pain complaints on a 7-point scale. Most patients reported a moderate to marked improvement in pain after sacral neuromodulation. Approximately one fourth of the patients were able to discontinue narcotics completely, and patients overall were satisfied with this form of therapy compared with previous ones.
Chai and coworkers (2000) have reported that perhaps some of the effects of neuromodulation in patients with interstitial cystitis may be due to changes in antiproliferative factor. His group has shown that levels of antiproliferative factor and epidermal growth factor were elevated in the urine of patients with interstitial cystitis and that these levels subsequently normalized after a short trial of sacral neuromodulation.
Chronic Pelvic Pain
Pelvic pain, much like interstitial cystitis, has been investigated with therapy like neuromodulation; again, this affects a challenging subset of patients who no other treatments seem to benefit. Bemelmans (1999) described the mechanism in pain inhibition as involving the gate control mechanism at the spinal segmental level. At this point, large somatic sensory fibers inhibit the activity in small Aδ or unmyelinated C fibers via sacral segmental interneurons or perhaps supraspinally by way of the spinobulbospinal reflex system. The hypothesis is that sacral root stimulation for the treatment of many disorders may result from decreasing pelvic floor spasticity.
Multimodal therapy is likely to be of benefit; however, results may not be optimal in all cases. Spinal cord stimulation at higher centers has been used by pain therapists and with moderate success; however, trial design and consistent entry criteria are debated (Feler, 2003; Mailis-Gagnon et al, 2004). Small series have looked at more selective stimulation, primarily at sacral roots. Aboseif and colleagues (2002) examined the effect of sacral neuromodulation on pelvic floor dysfunction in 41 of 64 patients thought to have chronic pelvic or perineal pain. Patients with chronic pelvic pain had decreased pain scores on average (5.8 to 3.7) after neuromodulation based on validated pain scales. Siegel and associates (2001) examined patients with intractable pelvic or genitourinary pain in the absence of neurologic or pelvic disease. Sacral neuromodulation did benefit patients, and they described a decrease in severity of pain and quality of life improvements. Bilateral caudal sacral stimulation may yield almost 40% improvement in pain scores in patients with mixed symptoms of voiding dysfunction and pelvic pain (Zabihi et al, 2008). Still, some form of placebo effect is likely to exist and is challenging to control in these small series.
Pediatric Voiding Dysfunction
Children often experience voiding dysfunction in fairly high rates and may in fact have refractory bladder problems that require advanced management schemes. Neuromodulation has been considered in this population because of the variety of lower tract dysfunctions that pediatric patients may have, including overactive bladder, urinary retention, and non-neurogenic neurogenic bladder (the Hinman bladder syndrome). Because neuromodulation truly represents a minimally invasive option for refractory management, it follows that this may be an approach to be used in pediatric lower urinary tract dysfunction. Still, sacral neuromodulation is not approved by the FDA for use in pediatric patients, perhaps owing to lack of data on what the sacral lead would do with concomitant growth of the spinal cord, nerve roots, and foramina. Accordingly, efforts in the past have been centered on alternative means of delivering electrical stimulation to the bladder and pelvic floor in these patients. Few studies exist of pediatric patients and sacral neuromodulation. Guys and coworkers (2004) prospectively examined 21 patients aged 5 to 21 years with sacral neuromodulation in the setting of neurologic disease consisting predominantly of spina bifida. The neuromodulation implant group had improved compliance and bladder capacity at 6 and 9 months but not at 12 months. Of the 21 patients, 9 improved their intestinal transit times and 1 patient had complete disappearance of urinary incontinence. No patients in the control group experience an improvement of their condition. McGee and associates (2009) described the use of an incisionless first- and second-stage sacral neuromodulation procedure with fairly high success rates in pediatric patients with dysfunctional elimination syndromes and had minimal complications.
Transcutaneous electrical nerve stimulation (TENS) has been used in pediatrics because it is noninvasive. One usually places a patch electrode on both sides of the S3 nerve foramen, and it is connected to a pulse generator and amplifier. Hoebeke and associates (2001) reported on this use in 41 children. In this case series, patients had urodynamically proven detrusor overactivity, and anticholinergic therapy had failed in all. Patients had daily therapy with the patch electrodes placed at S3, and stimulation was delivered at 2 Hz. A 76% response rate was observed, and this was due in part to increase in bladder capacity and reduction in urgency urinary incontinence and urgency symptoms. Of 41 patients, 21 (51%) were definitively cured; the remainder experienced relapse in the ensuing 1 year of follow-up. Bower and colleagues (1998) reported a similar fairly high success rate with 17 children treated with S3 transcutaneous stimulation and demonstrated dryness in 73.3% of patients and improved urgency and bladder capacity based on visual analog scales and voiding diaries. A more recent study by Malm-Buatsi and associates (2007) also showed continued benefit in patients when 8 of 12 (75%) subjects received statistically significant benefits when therapy was completed. Although this technology seems to have fairly good success there has been no trial in a randomized prospective controlled fashion that may increase its acceptance.
Posterior tibial nerve stimulation (PTNS), much like TENS, has been studied in pediatric patients because of its lack of invasiveness. DeGennaro and colleagues (2004) reported on PTNS in children in a subset of patients with refractory non-neurogenic voiding dysfunction; 80% of patients had symptom improvement and 44% were totally dry, and 62.5% had improvement in bladder capacity. Furthermore, 71% had improvement in urinary retention symptoms. No patients had significant problems from the therapy, and it was overall thought to be both safe and well tolerated. Hoebeke and associates (2002), in a pilot study of PTNS, demonstrated similar success with 32 children. Of the 28 children with urgency before therapy, the urgency disappeared after therapy in 7 and improved in 10. Of the 23 children with daytime incontinence before treatment, 4 became dry after stimulation, and in 12 patients the incontinence decreased. Of the 19 patients who reported abnormal voiding frequency of either less than 4 or more than 8 voids per day, 16 of 19 achieved a normal frequency of 4 to 6 voids daily.
Fecal Incontinence and Bowel Disorders
Sacral neuromodulation was investigated for bowel disorders on the basis of some of the early experience in bladder patients who exhibited treatment benefits with regard to the bowel symptoms (Pettit et al, 2002). The use of sacral neuromodulation in bowel disorders is being actively examined, but it is not yet approved for use in the United States. There exist two major areas of interest with regard to neuromodulation and bowel disorders: fecal incontinence and constipation.
Fecal Incontinence
Several studies have been done to examine the utility of sacral neuromodulation in fecal incontinence (Kenefick et al, 2002a, 2002b; Uludag et al, 2002; Melenhorst et al, 2007). It appears that patients with a variety of causes of the fecal incontinence seem to have benefited from sacral neuromodulation therapy in this setting. The Cleveland Clinic scoring system allows comparisons to be made with regard to outcome measures including incontinent episodes (solid, liquid, and flatus), pad use, and lifestyle changes. Several studies have used this scoring system, and all have shown improvements in these assessed parameters (Matzel et al, 2003; Jarrett et al, 2004). Sacral stimulation has been shown to be more effective than medical management based on randomized controlled trials for fecal incontinence (Tjandra et al, 2008). The mean incontinent (fecal) episodes decreased from 9.5 per week to 3.1 (P < .001), and perfect continence was gained in 47.2%. Similar improvements were demonstrated in quality of life parameters. The exact prognostic indicators for success have yet to be defined, particularly as they relate to etiology of the fecal incontinence (sphincter defect, neurologic, functional). The use of sacral neuromodulation is currently being investigated by the FDA for use in fecal incontinence.
Constipation
Constipation as such is a broad term, with the definition being in evolution. It is thought to be representative of difficult evacuation of feces and infrequent or inadequate defecation. Other refinements to the definition may include bowel frequency of fewer than three stools per week. The ROME II criteria categorize constipation into subtypes, including constipation-predominant irritable bowel syndrome, functional constipation, and pelvic floor dyssynergia (Drossman and Corazziari, 2000). Sacral neuromodulation has been examined in this regard and has had favorable results on the basis of some of the criteria listed for improvement. Ganio and associates (2001) described 16 patients who underwent permanent implantation of sacral leads for constipation whereby they had more than 50% decrease in difficulty emptying the rectum and more than 80% improvement in the Cleveland Clinic constipation score that persisted during the course of 1 year of follow-up. Other series (Kenefick et al, 2002a, 2002b) have shown similar improvements, although in smaller numbers. Still, with the small series available, it is difficult to make any meaningful analyses. The more important parameters, perhaps, in this setting relate to quality of life; these have been assessed with SF-36 questionnaires and have proved to be beneficial at least in Kenefick and associates’ study. All of these results suggest, at least, that there is some benefit of sacral neuromodulation in refractory constipation cases. Further study is warranted to assess prognostic factors to better decide on future candidates for this therapy.
Bilateral Stimulation and Neuromodulation
The current technique for sacral neuromodulation involves a unilateral lead at the S3 nerve foramen to achieve results in cases of urgency, frequency, urgency urinary incontinence, and idiopathic nonobstructive urinary retention. Bilateral stimulation has been suggested as an alternative, particularly in failed unilateral lead placements, for potential salvage or added benefit as the bladder receives bilateral innervation (van Kerrebroeck et al, 2005). The initial consideration of bilateral stimulation was based on animal studies demonstrating that bilateral stimulation yielded a more profound effect on bladder inhibition than did unilateral stimulation (Schultz-Lampel et al, 1998a, 1998b). An animal model of unilateral versus bilateral stimulation has suggested that bilateral stimulation may be more effective overall (based on reduction of detrusor overactive contractions) than unilateral stimulation (Kaufmann, 2008).
There has been only one prospective clinical study to demonstrate the differences in unilateral versus bilateral stimulation (Scheepens et al, 2002). This study was a prospective randomized crossover design in which all patients underwent unilateral as well as bilateral test stimulations to assess the benefits of bilateral stimulation. Both unilateral and bilateral test stimulation was continued for 72 hours, and the patients were randomly assigned to start with unilateral or bilateral stimulation. No significant difference was found in the unilateral versus bilateral group with regard to urgency urinary incontinence, frequency, or severity of leakage in the overactive bladder group, although overall results were impressive in both categories. The retention group had better parameters of emptying (volume per void) in bilateral compared with unilateral stimulation. Still, the numbers were too small in the retention group for adequate conclusions to be made. It appears that the data as presented, at least from a clinical perspective, do not suggest a large role for routine bilateral stimulation for most patients. Perhaps there will be subgroups that may benefit more than others (e.g., retention patients), but larger-scale studies with good methodology as shown in the Scheepens study will be required. Still, if one could increase the overall success rates of patients undergoing sacral neuromodulation, one could ultimately help more patients. Accordingly, Pham and colleagues (2008) examined 124 patients undergoing stage I sacral neuromodulation and stratified patients into unilateral and bilateral groups, retrospectively. Successful stage 1 trials were noted in 58% of unilateral patients and 76% of bilateral patients. An important component that still needs to be evaluated is whether it is cost effective to “routinely” place bilateral leads in the setting of most unilateral lead success rates approaching 70% and 80%.
Selective Nerve Stimulation
Pudendal Nerve
Because the bladder afferent reflex works through sacral interneurons that then activate storage through pudendal nerve efferent pathways directed toward the urethral sphincter, the pudendal nerve is a logical target for developing neuromodulation therapies. The earliest attempts to manipulate this reflex through electrical stimulation were based on direct pelvic floor muscle stimulation by Caldwell (1963, 1965) and others with the development of the first implantable and external pelvic floor stimulators, anal plug stimulator (Hopkinson and Lightwood, 1966, 1967), and intravaginal pessary stimulation (Alexander and Rowan, 1968; Erlandson et al, 1977; Fall et al, 1977; Fall, 1985). To deliver optimal stimulation to the nerve directly, selective pudendal nerve stimulation was introduced by Vodusek and coworkers (1986) and shown to have an inhibitory effect on the micturition reflex.
Neurophysiologic studies reveal that SNS works for bladder storage disorders by a similar inhibition of the micturition reflex as a result of electrical stimulation of sensory afferent fibers, in particular by depolarization of Aα and Aγ somatomotor fibers that affect the pelvic floor and external sphincter and thus inhibit detrusor activity (Hohenfellner et al, 1992). Because many of the sensory afferent nerve fibers contained in the sacral spinal nerves originate in the pudendal nerve, the pudendal nerve afferents are important targets for neuromodulating the inhibitory reflex on the micturition reflex (Peng et al, 2008; Woock et al, 2008; Yoo et al, 2008). Furthermore, high-frequency electrical stimulation of this nerve may achieve blockade of external sphincter contractions leading to sphincter relaxation (Gaunt and Prochazka, 2009). Direct pudendal nerve neuromodulation stimulates more pudendal afferents than SNS provides and may do so without the side effects of off-target stimulation of leg and buttock muscles. Thus, techniques for direct pudendal nerve stimulation at alternative locations to the sacral foramen are being developed. Spinelli and associates (2005) modified existing sacral neuromodulation technology and adapted it to pudendal nerve stimulation and realized the need for more sensitive neurophysiologic guidance to better guide stimulation to the pudendal nerve target. Trials using different techniques and devices are under way for selective pudendal nerve stimulation within the ischial rectal fossa and the pure sensory afferent branch of the pudendal nerve at the level of the symphysis bone referred to as the dorsal genital nerve.
The Bion device (Boston Scientific, Natick, MA) is a minimally invasive implantable mini-stimulator with an integrated electrode for nerve neuromodulation. Early feasibility trial results of the Bion device placed at the level of the pudendal nerve exiting the Alcock canal indicate that a considerable reduction in the degree of detrusor overactivity incontinence can be obtained in refractory cases, including those cases of failed SNS neuromodulation (Bosch, 2005). Clinical trials of the rechargeable Bion device were halted in the United States and Europe.
External Periurethral Nerve
A relatively new way to stimulate the bladder has been investigated and is now underway with clinical trials in the use of external periurethral neuromodulation (Nissenkorn et al, 2004, 2005). This device basically entailed placement of a lead and generator apparatus in the periurethral location while the generator was in the lower abdomen subcutaneous space. The lead then stimulated the sphincter apparatus and nerves associated with this structure presumably. Whereas their early results are fairly impressive, the device may help both urgency and stress urinary incontinence (16 patients with stress urinary incontinence were treated and 9 were dry during electrostimulation and the remainder had a 74% reduction in pad weights). The exact positioning of the electrodes seems to be in the area proximate to the external urethral sphincter, thereby allowing for direct access to afferent nerve fibers (Whiteside et al, 2009). How this therapy benefits patients will be interesting because it has many potential uses, including stress and urgency urinary incontinence, pain syndromes, and neuromuscular disorders of the pelvic outlet.
Dorsal Genital Nerve
The dorsal genital nerves (dorsal nerve of the penis in males, clitoral nerve in females) are the terminal and most superficial branches of the pudendal nerve found at the level of the symphysis pubis. The nerves are afferent nerves that carry sensory information from the glans of the penis or clitoris. Proximally, the dorsal genital nerves form a component of the pudendal nerve and then the sacral spinal roots. As a pure sensory afferent nerve branch of the pudendal nerve, the dorsal genital nerve contributes to the pudendal-pelvic nerve reflex that has been proposed as a mechanism of bladder inhibition. Whereas squeezing the glans penis or manipulation of the clitoris is clinically known to help suppress bladder contractions as observed in behaviors of voiding avoidance, direct electrical stimulation of these organs does not produce a significant effect on the micturition reflex as measured by urodynamics during the storage phase (Yalla et al, 1978; Kondo et al, 1982). However, direct dorsal genital nerve electrical stimulation in experimental and clinical studies appears promising in producing an inhibition of the micturition reflex.
Results in laboratory animals and in persons with spinal cord injury have demonstrated that electrical stimulation of the dorsal genital nerves inhibits bladder contractions (Craggs and McFarlane, 1999). In anesthetized cats (Sundin et al, 1974; Jiang and Lindstrom, 1999) and in unanesthetized chronic spinal cord–injured cats, reflex bladder contractions could be inhibited by stimulation of the genital nerves (Walter et al, 1993). Conditioning stimulation of afferents in the dorsal clitoral nerves has also been shown to suppress reflex bladder contractions in anesthetized cats (Jiang and Lindstrom, 1999). Similarly, recent work in anesthetized cats has shown that low-amplitude electrical stimulation of the S1 dorsal root (which in the cat carries the dorsal genital afferents) inhibits or abolishes ongoing reflex bladder contractions (Jezernik et al, 2001), resulting in significantly shorter bladder contractions. The micturition reflex can be activated and inhibited by stimulation of these dorsal penile afferent fibers in animal models (Woock et al, 2008).
Stimulation of the dorsal penile nerve has been tested in humans to control incontinence in individuals with spinal cord injury and increase bladder volume and reduce bladder overactivity (Wheeler et al, 1992, 1994). Penile nerve stimulation was painless with no side effects, was effective for inhibiting detrusor overactivity, and may be adaptable for chronic home use as an alternative to current therapy (Wheeler et al, 1992). Similar experiments have shown that stimulation of the dorsal nerve of the penis abolishes reflexive bladder contractions and increases bladder capacity in persons with spinal cord injury (Lee and Creasey, 2002). These results demonstrate that electrical stimulation of the dorsal genital nerves can abolish detrusor overactivity and increase bladder capacity in individuals with neurogenic detrusor overactivity due to spinal injury. Feasibility trials with this approach have been completed and demonstrate in a small series that 81% of patients experienced a 50% or greater improvement in urgency and 47% reported a 50% or greater reduction in incontinence episodes (Goldman et al, 2008). This approach seems to have further advantages in being minimally invasive, office based, and without the need for fluoroscopy or prone positioning.
Posterior Tibial Nerve
The posterior tibial nerve is a mixed sensory and motor nerve containing fibers originating from spinal roots L4 through S3 that modulate the somatic and autonomic nerves to the pelvic floor muscles, bladder, and urinary sphincter. On the basis of translational findings of the traditional Chinese practice of using acupuncture points over the common peroneal or posterior tibial nerve to inhibit bladder activity, McGuire and associates (1983) used transcutaneous stimulation of the common peroneal or posterior tibial nerve for inhibition of detrusor overactivity. PTNS (Urgent PC, CystoMedix, Anoka, MN) as approved by the FDA currently consists of weekly 30-minute stimulation treatments provided by insertion of a small-gauge stimulating needle approximately 5 cm cephalad from the medial malleolus and just posterior to the margin of the tibia with the grounding electrode pad placed on the medial surface of the calcaneus (Govier et al, 2001; Cooperberg and Stoller, 2005).
Clinical trials of PTNS have been performed in detrusor overactive conditions with and without pelvic pain (Klingler et al, 2000; van Balken et al, 2003; Vandoninck et al, 2003; Congregado Ruiz et al, 2004; Zhao et al, 2008) and urinary retention (van Balken et al, 2001; Vandoninck et al, 2003). Although clinical trials have produced variable results, PTNS is minimally invasive, demonstrates efficacy, and is easily applicable and well tolerated in all the lower urinary tract conditions studied. Recent urodynamic outcomes have been shown in an MS subpopulation and improvements during PTNS have been demonstrated, including increases in mean first involuntary detrusor contractions and mean cystometric capacity (Kabay et al, 2008). One major limitation of PTNS is that there does appear to be the need for chronic treatment that may be better derived from an implantable subcutaneous stimulation device (van Balken et al, 2003) and even continuous stimulation (Oliver et al, 2003). Perhaps the use of a sham control can help us better understand the potential clinical benefits of this therapy in the long run. A novel sham has been developed, and widespread study of PTNS with use of a sham control may help determine outcomes and eliminate placebo rates (Peters et al, 2009).
Transcutaneous Electrical Stimulation
Other methods of electrical stimulation have been used that seem to occupy a place midway between anal, vaginal, or perineal stimulation and sacral root stimulation. TENS devices have been used to limited degrees to achieve better tolerability to bladder filling and may have some efficacy in postponing voiding. Fall and associates (1980) described TENS use suprapubically in patients with interstitial cystitis, and subsequent studies have been done to gain wider use of this modality (Lindstrom et al, 1983; Fall and Lindstrom, 1991). The exact stimulation parameters are not agreed on because different frequencies have been used; 2 Hz may stimulate pudendal afferents, whereas 50 Hz may stimulate striated paraurethral musculature. Similarly, low-frequency TENS may have some use in abolishing detrusor contractility (Bower et al, 1998). As such, this technology is easy to do and apply, but it may be required for extended periods to gain treatment benefits. S2 or S3 TENS may make some sense, because direct stimulation transcutaneously of this area may yield better results than suprapubic stimulation. Positive results have been demonstrated on the basis of urodynamic data, with improved bladder capacity, delay in first urge to void, and reduced detrusor instability (Bower et al, 2001; Hoebeke et al, 2001). For adequate maintenance of the benefits of this therapy it must also be continued for longer durations. McGuire and associates (1983) described 16 patients with involuntary bladder contractions of varying etiology who were treated with common peroneal or posterior tibial nerve patch electrode stimulation: 12 patients initially were dry, 3 were improved, and 1 was “possibly improved.” Vereecker and colleagues (1984), however, were unable to suppress hyperactivity by this method in patients with suprasacral spinal cord injury or disease. Okada and associates (1998) reported a positive experience with transcutaneous stimulation of the thigh muscle in 19 patients with detrusor overactivity; the maximal cystometric capacity was increased by 57% in 11 of 19 patients.
Noninvasive magnetic stimulation of the sacral roots will also inhibit bladder contractions and cause effects that will persist for short times beyond the period of stimulation. This type of stimulation at present cannot be applied for prolonged periods and is currently unsuitable for long-term treatment, although it may be helpful for preliminary assessment of candidates for chronic sacral root neuromodulation. The extracorporeal magnetic stimulation provided by the “chair” has been used clinically for potential use in overactive bladder, stress incontinence, and pelvic pain (Galloway et al, 2000). No other prospective data on this technology are available for overactive bladder and, furthermore, the exact mechanism of action, if effective, remains to be explained, namely, magnetic, nerve root or peripheral nerve, or intramural nerve stimulation.
Complications and Troubleshooting of Sacral Neuromodulation
With the widespread adoption of sacral neuromodulation, an increasing need has developed to understand complications of this therapy and to learn how to troubleshoot the devices when responses change. It appears that the introduction of the tined lead concept has changed the frequency and profile of the complications that were once only technology related while keeping the patient-related complications at the same frequency.
Published Series
The SNS study group has published several reports on the efficacy and safety of the procedure for individual indications (Siegel et al, 2000). The complications were pooled from the different studies on the basis of the fact that the protocols, devices, efficacy results, and safety profiles were identical. The studies recruited 581 patients, 219 of whom underwent implantation of the InterStim system. The complications were divided into percutaneous test stimulation–related and postimplant-related problems. Of the 914 test stimulation procedures done on the 581 patients, 181 adverse events occurred in 166 of these procedures (18.2% of the 914 procedures). Most complications were related to lead migration (108 events, 11.8% of procedures). Technical problems and pain represented 2.6% and 2.1% of the adverse events, respectively. For the 219 patients who underwent implantation of the InterStim system (lead and generator), pain at the neurostimulator site was the most commonly observed adverse effect at 12 months (15.3%). Surgical revision of the implanted neurostimulator or lead system was performed in 33.3% of cases (73 of 219 patients) to resolve an adverse event. This included relocation of the neurostimulator because of pain at the subcutaneous pocket site and revision of the lead for suspected migration. Explant of the system was performed in 10.5% for lack of efficacy. One should consider the fact that, at the time, the generator was implanted in the lower abdomen.
Everaert and associates (2004) reported specifically on the complications with SNS. This was a retrospective study of 53 patients who had undergone implantation of the quadripolar electrode (Medtronic InterStim, model 3886 or 3080) and subcutaneous pulse generator in the abdominal site (Medtronic Itrel 2 IPG) between 1994 and 1998. Device-related pain was the most frequent problem and occurred equally in all implantation sites (sacral, flank, and abdominal). This occurred in 18 of the 53 patients (34%) and was more frequent in patients with dysuria and retention or perineal pain. Pain responded to physiotherapy in 8 patients, and no explantation was done for pain reasons. Current-related complications occurred in 11%. They performed 15 revisions in 12 patients. Revisions for prosthesis-related pain (n = 3) and for late failures (n = 6) were not successful. Similar series have been published by White and associates (2009) and show relatively low rates (30%) of adverse events and may have been predicted by trauma, body mass index and enrollment into a pain clinic, and history of adverse events.
A review (Hijaz and Vasavada, 2005) of the tined lead approach was performed at the Cleveland Clinic from June 2002 to June 2004 when 167 patients underwent sacral neuromodulation for indications of refractory overactive bladder, idiopathic and neurogenic urinary retention, and interstitial cystitis. In this cohort, 180 stage I operations used the tined lead approach. After 2 to 4 weeks of test stimulation, 130 (72.2%) patients proceeded to stage II implantation of the IPG.
Stage I complications can lead to explantation or revision of the tined lead. The reasons for either fall under response related, mechanical, or infection related. In this series, 50 tined leads were explanted (27.8%). The majority of lead explantations were performed for unsatisfactory or poor clinical response (46 of 50; 92%). The rest of the explantations were done for infection (4 of 50; 8%). Explantation for response reasons is not truly considered a complication as much as it is an integral part of the procedure. Stage I revisions totaled 22 of the 180 operations (12.2%). Revisions were done for marginal response (13 of 22), frayed subcutaneous extension wire (6 of 22), lead infection (3 of 22), and improper localization of stimulus (1 of 22). Eleven (50%) of the revisions proceeded to stage II generator implantation. When the revision was done for a marginal response (13 of 22), the response was ultimately clinically satisfactory in 5 of 13 (38.5%), and patients proceeded to generator implantation. Typically, when the patient reported a marginal or equivocal response during the test stimulation in the absence of infection or mechanical problems, a lead revision was offered with intraoperative sensory testing. Because 38.5% of these revisions eventually were successful and patients proceeded to stage II, this is an option to keep in mind in motivated patients with equivocal response.
As in stage I, stage II complications can be divided into explantation (generator and lead) or revision. Explantation was performed in 16 of 130 (12.3%). Explantations were done for infection and failure to maintain response in 56.3% and 43.7%, respectively. Revisions were done for infection, mechanical (generator related), and response causes. The revision rate of stage II in this series was 20% (26 of 130).
When infection at the generator site is diagnosed, the best management is explantation of the whole system.
Response-related complications necessitating revision are more common (18 of 26). The algorithm for management of a patient who presents with a decreased or absent response after a successful interval is outlined in Figure 70–8.
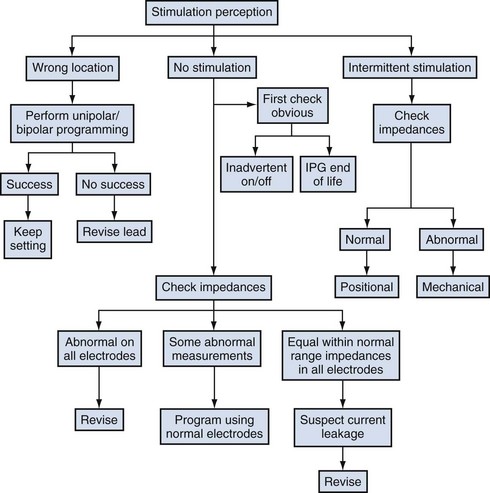
Figure 70–8 Diagnostic algorithm and troubleshooting for recurrent symptoms. IPG, implantable pulse generator.
The outlined algorithm includes testing of impedances (Fig. 70–9). Impedance describes the resistance to the flow of electrons through a circuit. Impedance or resistance is an integral part of any functioning circuit. However, if there is too much resistance, no current will flow (open). If there is too little resistance, excessive current flow results in diminished battery longevity (short). The electrical circuit that we are referring to starts at the neurostimulator’s circuitry and goes through the connectors to the extension wires, through the extension connector to the lead wires, through the lead’s electrodes to the patient’s tissue, and back either through another electrode and up the same path to the circuitry (bipolar) or to the neurostimulator case and into the circuitry (unipolar).
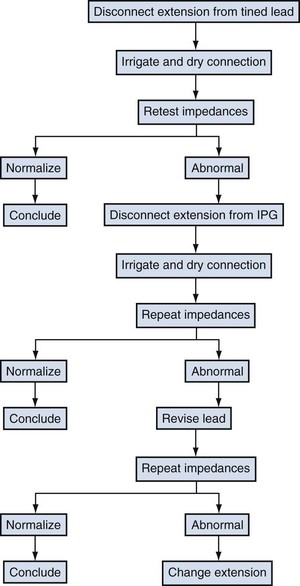
Figure 70–9 Intraoperative algorithm for impedance problem management. IPG, implantable pulse generator.
If the circuit is broken somehow, electrons cannot flow. This is called an open circuit, and impedance measurements are high. Open circuits can be caused by a fractured lead or extension wires and loose connections. Patients generally feel no stimulation if an open circuit is present. In measurement of impedances by the programmer, unipolar measurements are most useful for identifying open circuits because they take one lead wire measurement at a time, immediately identifying which connection or wire has the problem.
Short circuits, which are reflected in low impedance measurements, can be caused by body fluid intrusion into the connectors or crushed wires that are touching each other. The electrons will always follow the path of least resistance. Patients may or may not feel stimulation, or stimulation may not be present in the correct area (i.e., the generator site) or may vary in strength (i.e., a surging sensation). In measurement of impedances by the programmer, bipolar measurements are most useful for identifying shorts between two wires.
Therefore, impedance measurement is used as a troubleshooting tool to check the integrity of the system when a patient presents with a sudden or gradual disappearance of stimulation. Many measurements fall within the 400- to 1500-ohm range. High levels (>4000 ohms) identify open circuits, and low levels (<50 ohms) identify short circuits. Medtronic (manufacturer of the InterStim) recommends performing the impedance measurements at the time of closure of the incision, at the first programming session, to get a baseline measurement and at any time a problem is suspected. These measurements will identify which electrodes, if any, are intact and allow the programmer to proceed with programming of only those with acceptable impedance measurements. If all electrode measurements read above 4000 ohms, a revision may be necessary.
The intraoperative algorithm for management of impedance problems includes initial testing of impedances (see Fig. 70–9). First, the tined lead is disconnected from the extension to the IPG and then dried. The connection may be irrigated with sterile water, and then the connection is dried with the 3-Fr ENT suction device before it is reconnected again. At this stage the impedances are repeated. If they normalize, the revision can be concluded at this stage. If impedances continue to be abnormal, one can evaluate or change the 10-cm extension and retest impedances. If they continue to be abnormal, the lead is revised. It has been the authors’ experience that the connections to the IPG and the IPG itself seldom have anything to do with abnormal impedance values.
Troubleshooting Algorithm
After successful completion of stage II, a number of events can occur, and the treating physician should develop an algorithm to handle these events in a timely and efficient manner. These events with their probable causes and the troubleshooting algorithm are covered in this section.
Pocket (IPG Site) Discomfort
The probable causes of this symptom are pocket related or output related. Pocket-related causes of discomfort include infection, pocket location (waistline), pocket dimension (too tight, too loose), seroma, and erosion. Output-related causes include sensitivity to unipolar stimulation if this mode is used or current leak. To troubleshoot this problem the evaluating specialist is advised to do the following (Fig. 70–10):
Recurrent Symptoms
When the patient presents with recurrent symptoms, evaluation of the stimulation perception is necessary. The possibilities are that the patient perceives the stimulation in a wrong location compared with baseline, has no stimulation, or has intermittent stimulation.
Wrong Location
If the patient reports that the stimulation location or pattern has changed, it is best to go back to each unipolar setting and “map” out where the patient feels the stimulation. The device is set to 0−, case+ and the patient is asked where she or he feels the sensation; next it is set to 1−, case+ and the patient again is asked about the sensation; next it is set to 2−, case+ and finally to 3−, case+. If these combinations do not confirm the target area, the next step is to start programming bipolar combinations. When those are exhausted, sometimes increasing the pulse width widens the stimulation area. If the programming possibilities are exhausted, then revision for lead repositioning or relocation to the other side may be necessary.
No Stimulation
The obvious is checked first. The device parameters must be set high enough, an inadvertent on-off is checked (set magnet switch off to avoid inadvertent magnet activations), and whether the IPG is nearing the end of its life is checked. Next impedance readings are performed, paying close attention to unipolar impedances. These impedances measure one lead wire with the case, so it is easy to isolate a problem. Using unipolar impedances, it is possible to tell which lead wires are still intact and which ones are not, as mentioned previously. Programming of the electrodes is then continued with acceptable impedance measurements. Bipolar measurements are checked to rule out short circuits as well (very low impedance measurements). If programming around the malfunctioning lead does not restore the stimulation, the patient will often need to undergo revision.
Intermittent Stimulation
Again, inadvertent on-off is checked. Intermittent stimulation can be caused by either a loose connection or positional sensitivity. If a loose connection is suspected, palpating the connection site and re-creating the intermittency is a good clue as to where the problem lies. Taking impedances while the patient reports the stimulation intermittently determines whether the problem is positional (acceptable impedances are still present) or mechanical (when the patient feels stimulation go off, the impedances are high). With positional sensitivity, the lead position shifts when a patient moves in a certain direction (for instance, the patient reports that the stimulation goes away on standing). The lead position may have moved farther from the nerve during standing, and the amplitude may just need to be increased. Intermittent stimulation represents a challenging dilemma to troubleshoot.
Electrical Stimulation for Emptying Disorders
There exists strong evidence to suggest that neuromodulation works through supraspinal pathways (see the section on neurophysiology of electrical stimulation). The question of how it works for refractory detrusor overactivity as well as for urinary retention is based on changes in supraspinal pathways and perhaps the guarding reflex. It is important to understand that before neuromodulation, neurostimulation was applied in different forms to achieve bladder emptying. Still, neurostimulation has a role today in management of disorders of bladder emptying and has evolved through many different versions before its current techniques.
Electrical Stimulation Directly to the Bladder or Spinal Cord
Clinical trials of direct electrical stimulation of the bladder to facilitate emptying originated in 1940 but have met with only partial success and intermittent enthusiasm since then (Wein and Barrett, 1988). Direct electrical stimulation was most effective in patients with hypotonic and areflexic bladders. Initial success, defined as low postvoid residual urine volume with sterile urine, was achieved in only 50% to 60% of patients, and secondary failure often supervened, usually related to fibrosis, electrode malfunction, bladder erosion, or other equipment malfunction. The spread of current to other pelvic structures with a stimulus threshold lower than that of the bladder often resulted in abdominal, pelvic, and perineal pain; a desire to defecate or defecation; contraction of the pelvic and leg muscles; and erection and ejaculation in male patients. It was also noted that the increase in intravesical pressure was generally not coordinated with bladder neck opening or with pelvic floor relaxation and that other measures to accomplish these ends could be necessary. Direct electrical stimulation of the sacral spinal cord was also performed as an attempt to take advantage of the remaining motor pathways to initiate micturition. Although some short-term success was noted, many of the side effects seen with direct bladder stimulation occurred as well because the stimulus applied in this way was also unphysiologic. Enthusiasm for both of these approaches has waned considerably, and resurrection seems unlikely.
Electrical Stimulation to the Nerve Roots
For the past 25 to 30 years, Brindley (1993) and the Tanagho group (Tanagho and Schmidt, 1988; Tanagho et al, 1989) have pursued neurostimulation for the treatment of voiding dysfunction. The use of electrical stimulation for storage disorders and pelvic floor dysfunction has been covered. The focus of this section is on the use of anterior root electrical stimulation to facilitate emptying.
The Brindley device is the one most commonly used. Prerequisites for such use are described by Madersbacher and Fischer (Fischer et al, 1993) as (1) intact neural pathways between the sacral cord nuclei of the pelvic nerve and the bladder and (2) a bladder that is capable of contracting. The chief applications are in patients with inefficient or nonreflex micturition after spinal cord injury. Simultaneous bladder and striated sphincter stimulation is obviated by sacral posterior rhizotomy, usually complete, which eliminates reflex incontinence and improves low bladder compliance, if it is present (Brindley, 1994). The stimulation sequences and parameters themselves and their neurophysiologic consequences lead to less striated sphincter dyssynergia, even without posterior rhizotomy, than is seen in reflex micturition in a spinal cord–injured patient. Complete sacral deafferentation is usually performed, however, with the exception listed by Brindley as those patients who have genital sensation or useful reflex erections. Electrodes are applied intradurally to S2, S3, and S4 nerve roots, but the pairs can be activated independently (Fig. 70–11). The detrusor is usually innervated primarily by S3 and to a smaller extent by S2 or S4. Rectal stimulation is by means of all three roots equally. Erectile stimulation is chiefly by S2 with a small contribution from S3 and none from S4. Micturition, defecation, and erection programs are possible, with stimulus patterns set specifically for each patient (Brindley, 1994). The ventral sacral roots, however, carry both parasympathetic fibers to the bladder and somatic fibers to the striated sphincter. Ventral root stimulation therefore occasionally results in detrusor–striated sphincter dyssynergia.
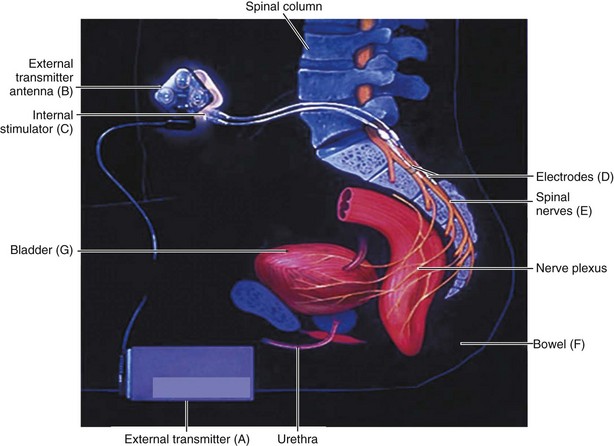
Figure 70–11 Brindley-Finetech system for sacral root stimulation.
(Courtesy of NDI Medical, Cleveland, OH.)
The current Brindley stimulator uses the principle of post-stimulus voiding, a term first introduced by Jonas and Tanagho (1975) to obviate this. Relaxation time of the striated sphincter after a stimulus train is shorter than the relaxation time of the detrusor smooth muscle. When interrupted pulse trains are used, voiding is achieved between the pulse trains because of the sustained high intravesical pressure. This concept and other methods that are available to overcome the stimulation-induced sphincter dyssynergia and allow low-pressure emptying are nicely reviewed by Rijkhoff and colleagues (1997). Post-stimulus voiding has a few shortcomings, described in this article as follows: voiding occurs in spurts at above-normal bladder pressures; when the stimulus parameters are not properly adjusted the detrusor pressures can become too high, putting the upper tracts at risk; and movement of the lower limbs occurs during stimulation because the nerve roots also contain fibers innervating leg musculature, and this movement can be cumbersome for the patient.
Brindley (1994) carefully reviewed the experience in the first 500 patients treated with his prosthesis with a total follow-up, at that time, of 2033.5 years. Of the total, 2 patients were lost to follow-up and 21 had died. Of the deaths, two were from septicemia (one definitely unrelated to the implant and the specifics of the other unmentioned) and one from related renal failure; the causes of five were unknown. Ninety-five reoperations were required for repair, six stimulators were removed (four infected), and two were awaiting repair. In 45 patients the stimulator was believed to be intact but not used for various reasons. In all others the stimulators were in use (411 for micturition and in most for defecation and in 13 for defecation alone) and the users were believed to be “pleased.” Upper tract deterioration was reported in only 2 of 365 patients with full deafferentation and in 10 of 135 with incomplete or no deafferentation. Two of these 10 had impaired renal function, and 1 died of this condition.
van Kerrebroeck and associates (1997) reported the results of use of the Finetech-Brindley stimulator in 52 patients. These patients were selected by screening 226 patients; complete posterior sacral root rhizotomies were performed in all. Thirty-seven of the patients had 6 months of follow-up; in these patients, complete daytime continence was achieved in 73% and night-time continence in 86%. There were significant increases in bladder capacity and bladder compliance, and residual urine was reduced significantly. Complications included cerebrospinal fluid leaks, which resolved spontaneously in 23 patients; nerve damage that resolved in 1 patient; and one implant failure caused by a cable fracture, which was successfully repaired. Sauerwein and colleagues (1999) reported in abstract form the results of sacral deafferentation and implantation of an anterior sacral root stimulator in 294 patients with spinal cord injury. Bladder spasticity was relieved in all. In 50%, micturition was achieved by stimulating S4 and S5 sacral ventral roots; in the remaining cases, it was achieved by stimulating the S2 and S3 roots. Variations in surgical approaches designed to achieve stimulation of only bladder contraction are described by Dahms and associates (2000), who also summarize overall success rates for sacral ventral root stimulation in spinal cord–injured patients at about 75%. Recent laparoscopic access techniques have minimized the invasiveness of this procedure and may have a role in the future in this select group of patients (Possover, 2009).
Extradural stimulation has been used by the Tanagho group (Tanagho and Schmidt, 1988; Schmidt, 1989; Tanagho et al, 1989) in the treatment of 19 patients with serious and refractory neuropathic voiding disorders. Extensive dorsal rhizotomy was performed, and a stimulator was implanted on the ventral component of S3 or S4 with selective peripheral neurotomy (Tanagho et al, 1989). In eight patients (42%), complete success was achieved with reservoir function, continence, and low-pressure/low residual voiding with electrical stimulation. Ten patients qualified as achieving partial success, regaining reservoir function and obtaining continence.
Electrical stimulation of the ventral sacral roots with some techniques to reduce detrusor hyperactivity and obviate striated sphincter dyssynergia has become an accepted treatment modality for lower urinary tract dysfunction in spinal cord–injured patients. Although more detailed follow-up is necessary, and further evolution will doubtless occur, these techniques have achieved remarkable improvements and success rates, which now seem to have stabilized at a high level.
Transurethral Electrical Bladder Stimulation
Intravesical electrotherapy is an old technique that has been resurrected with some interesting and promising results. The use of this technique to increase bladder capacity and to increase compliance has previously been discussed. This section deals with the use of TEBS to facilitate bladder emptying by establishing conscious control of the initiation and completion of a micturition reflex. Fischer and colleagues (1993) describe their concept of the basis for this use as follows. In patients with incomplete central or peripheral nerve lesions—and only these patients are suitable for this method—at least some nerve pathways between the bladder and the cerebral centers are preserved but are too weak to be efficient under normal circumstances. TEBS in this situation is hypothesized to activate specific mechanoreceptors in the bladder wall. With depolarization of these receptors, activation of the intramural motor system is said to occur, resulting in small local muscle contractions that further depolarize the receptor cells. As soon as this local motor reaction reaches a certain strength, “vegetative afferentation” begins, meaning that stimuli travel along afferent pathways to the corresponding cerebral structures with the occurrence of sensation. This, in time, reinforces efferent pathways, and their stimuli create centrally induced and more coordinated and stronger detrusor contractions. Ebner and coworkers (1992) simply conceptualize the mechanism as involving an artificial activation of the normal micturition reflex and further suggest that repeated activation of this pathway may “upgrade” its performance during voluntary micturition.
Children with congenital neurogenic bladder dysfunction who have never experienced the urge to void require a biofeedback system to realize the nature and meaning of this new sensation induced by TEBS. This exteroceptive stimulation is also important for other groups of patients because it signals detrusor contractions and whether, and to what degree, voluntary detrusor control is or has become possible and, by demonstrating progress, serves as positive feedback.
This technique involves direct intraluminal monopolar electrical stimulation with a special catheter equipped with a stimulation electrode. Saline solution is used as the current-leading fluid medium in the bladder. Exteroceptive reinforcement is achieved by visual recording of detrusor contractions on a water manometer connected to the stimulation catheter. An intensive bladder training program has to be combined with TEBS and must be highly individualized. Only patients with an incomplete spinal cord lesion and with receptors still capable of reactivity and with a detrusor still capable of contractility will benefit from this technique. The achievement of conscious control requires, in addition, an intact cortex.
Fischer and colleagues (1993) have used this technique in patients with incomplete spinal cord injury and other incomplete central or peripheral lesions of bladder innervation, in pediatric patients with congenital neurogenic lower urinary tract dysfunction, and in patients, especially children, with non-neurogenic dysfunction voiding. Only patients with preserved pain sensation in sacral dermatomes S2 through S4 improved with this technique. The technique is time consuming because stimulation must be performed on a daily basis for weeks and months, with an individual treatment time of about 90 minutes. Kaplan and Richards (1988) reported on such therapy in myelodysplastic children, performing the treatment for 60 minutes (during a 90-minute catheterization), 3 to 5 days a week for 15 to 30 daily sessions. Of 62 patients evaluated, 42 completed at least one series of treatment. “Success” was defined differently for infants than for older children. For infants, success implied a decrease in filling pressure, an increase in the quality of bladder contraction, and a decrease in residual urine. For older children, this type of result implied a heightened awareness of detrusor contractions before and during a contraction, maintenance of low-pressure filling, effectively emptying detrusor contractions with low residual urine, and either a conscious urinary control or timely enough sensory input to allow clean intermittent catheterization for continence. Of children who initially had some detrusor contraction on initial evaluation, 80% were said to have achieved some or all of the success parameters. Of those with no initial detrusor activity, 33% achieved some success.
Other reports have been less optimistic. Lyne and Bellinger (1993) reported the results of TEBS treatment of 17 patients with neurologic dysfunction, 10 with myelomeningocele, and 2 with lipomeningocele. Ultimately, all patients showed detrusor contraction during therapy (12 did so initially), but results related to increased bladder capacity and improved continence were disappointing. Five patients showed minor positive changes in continence. After completion of therapy in 12 patients who had serial cystometry, 5 experienced an increase in capacity (14% to 158%) and 4 a decrease (7% to 37%). Decter and associates (1994) used TEBS in 25 patients with neurogenic voiding dysfunction. TEBS was correlated with an increase from 18 to 24 in the number of patients who manifested contraction on stimulation and from 3 to 12 in the number who sensed contraction during stimulation. However, cystometry showed a more than 20% increase in the age-adjusted bladder capacity in only 6 of 18 patients with serial studies and clinically significant improvements in end-filling pressures in 5 of these. A telephone questionnaire revealed that 10 of 18 patients or parents perceived an improvement in bladder function, but the authors stated that “the limited urodynamic benefits our patients achieved have not materially altered the daily voiding regimen and, because of these factors, we are not enrolling any new patients in our…program.” This technique is certainly controversial. Some question the theoretical basis and the definitions of “success” applied to patients treated. Currently, even Kaplan (2000) does not seem enthusiastic about the use of this technique to attain the goal of volitional voiding, and Decter (2000), as previously pointed out, stated that, in his opinion, TEBS is a modality with limited clinical efficacy for facilitation of filling-storage and emptying.
Sacral Neuromodulation of Emptying Disorders
Sacral neuromodulation has been successful in patients with idiopathic nonobstructive retention, in patients with retention secondary to deafferentation of the bladder after hysterectomy, and in patients with Fowler syndrome (Dasgupta et al, 2004; De Ridder et al, 2007). Patient factors predictive of success have been sought, and increasing numbers of reports are differentiating results of sacral neurostimulation in urinary retention based on the functional disorders causing the condition, detrusor acontractility, and functional outlet obstruction. Bross and coworkers (2003) evaluated the predictive ability of the carbachol test and concomitant diseases in patients with an acontractile bladder. Whereas 33% of patients had a successful bilateral percutaneous nerve evaluation, a positive carbachol test result was not predictive of success. Goh and Diokno (2007) in a retrospective study of patients implanted for nonobstructive urinary retention found a statistically significant difference in predicting success of test stimulation for patients with a preimplantation ability to void (>50 mL) versus non-voiders. Further evidence of the predictive role of detrusor function was reported by Bertapelle and colleagues (2008) in developing a detrusor contractility test using urodynamics in conjunction with sacral neurostimulation in urinary retention as an exclusion criteria for sacral neurostimulation; this test appears to be a reliable tool to rule out detrusor acontractility due to irreversible bladder myopathy or complete neurogenic lesion and to predict success of permanent sacral neuromodulation.
A large, prospective, randomized multicenter trial to evaluate the efficacy of SNS for urinary retention was performed by Jonas and colleagues (2001). After a percutaneous nerve evaluation period of 3 to 7 days, 68 patients (38% of those evaluated) with chronic urinary retention qualified for permanent implantation. Patients were randomly assigned to the treatment or control group, in which treatment was delayed for 6 months. Successful results were initially achieved in 83% of patients who received the implant, with 69% able to discontinue intermittent catheterization completely. At 18 months, 71% of patients available for follow-up had sustained improvement. These results have been corroborated by others with longer follow-up (Datta et al, 2008; White et al, 2008).
Aboseif and coworkers (2002) evaluated the efficacy and change in quality of life in patients with idiopathic, chronic, nonobstructive functional urinary retention. Thirty-two patients with idiopathic retention requiring intermittent catheterization underwent percutaneous nerve evaluation. Permanent implants were placed in 20 patients (17 women) who showed more than 50% improvement in symptoms. Eighteen patients were subsequently able to void and no longer required intermittent catheterization; one patient required bilateral SNS implants. Average voided volumes increased from 48 to 198 mL, and postvoid residual volume decreased from 315 to 60 mL. Eighteen patients reported more than 50% improvement in quality of life, although the questionnaire used in the study was not described. Significant score improvements in the Beck Depression Inventory and SF-36 after sacral root neuromodulation for retention have been demonstrated by Shaker and Hassouna (1998). Overall success rates of the percutaneous nerve evaluation range from 33.3% to 100% (Koldewijn et al, 1994; Scheepens et al, 2002; Spinelli et al, 2003). Improvement in patients with retention may not be as rapid as in patients undergoing sacral root stimulation for other reasons. A percutaneous nerve evaluation period of at least 2 to 3 weeks and a permanent implant lead evaluation of 4 weeks or more have generally been recommended. Furthermore, bilateral or caudal SNS for urinary retention may be considered initially or for unilateral SNS trial failures, although this technique is performed infrequently and is less studied to date (van Kerrebroeck et al, 2005; Maher et al, 2007; Pham et al, 2008).
Future Research and Conclusions
During the past century, neurostimulation and neuromodulation that originated in the 19th century have been clinically adopted. Advances in electrical innovations and our understanding of neurophysiology have provided important discoveries for the care of people with neuromuscular causes of their pelvic organ dysfunctions. To date, influencing these dysfunctions at the level of the sacral roots appears to have stood the test of time and has become generalizable to physicians worldwide. At the start of our next century of using this expanding therapy, we will need to urgently focus on developing the supportive clinical research needed to drive further innovations and acceptance of wider clinical indications and applications. Long-term surveillance studies and randomized clinical trials to compare different techniques and nerve locations and to evaluate placebo effects are critically needed, as are more studies to elucidate modes of action to improve stimulation applications, selection of patients, and therapeutic results.
The introduction of new stimulation methods as well as application of these methods to different nerve locations will continue to provide improved treatment alternatives. In addition, these innovations will provide the ability to further develop testable hypotheses of more basic questions on electrical neurostimulation, neuromodulation, and neurophysiology of the autonomic, somatic, and central pathways that regulate pelvic organ function. Such questions need to address the observed lack of neural plasticity-induced changes with long-term neuromodulation and neurostimulation despite acute cellular changes identified in nerve signaling molecules secondary to magnetic and electrical field changes.
In their present form, neurostimulation and neuromodulation work acutely through continuous electrical activity without inducing long-term neuroplastic changes. Therefore, future technologies are likely to provide closed-loop conditional stimulation, much in the same manner as “on-demand” cardiac pacemakers and defibrillators do, as a means to obtain better efficacy without additional adverse electric field effects. The development of change from the present open-loop stimulation to closed-loop conditional stimulation will necessitate innovations in neurosensing of pathologic neuromuscular events of the pelvis that will lead to on-demand therapeutic electrical activity. These innovations will in turn have a profound effect on our diagnostic ability to predict clinical responses to neurostimulation and neuromodulation as we seek to maximize our benefit-risk and benefit-cost ratios in patient care.
Key Points: Electrical Stimulation
Brindley GS. Emptying the bladder by stimulating sacral ventral roots. J Physiol. 1974;237:15P-16P.
Chancellor MB, Chartier-Kastler E. Principles of sacral nerve stimulation (SNS) for the treatment of bladder and urethral sphincter dysfunctions. Neuromodulation. 2000;3:15-26.
Hassouna MM, Siegel SW, Nyeholt AA, et al. Sacral neuromodulation in the treatment of urgency-frequency symptoms: a multicenter study on efficacy and safety. J Urol. 2000;163:1849-1854.
Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005;32:11-18.
Siegel SW, Catanzaro F, Dijkema HE, et al. Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology. 2000;56(Suppl. 1):87-91.
Tanagho EA, Schmidt RA. Bladder pacemaker: scientific basis and clinical future. Urology. 1982;20:614-619.
Tanagho EA, Schmidt RA. Electrical stimulation in the clinical management of the neurogenic bladder. J Urol. 1988;140:1331.
van Kerrebroeck EV, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective worldwide clinical study. J Urol. 2007;178(5):2029-2034.
Wein AJ, Barrett DM. Voiding function and dysfunction—a logical and practical approach. Chicago: Year Book; 1988.
Yoshimura N, de Groat WS. Neural control of the lower urinary tract. Int J Urol. 1997;4:111.
Aboseif S, Tamaddon K, Chalfin S, et al. Sacral neuromodulation in functional urinary retention: an effective way to restore voiding. BJU Int. 2002;90:662-665.
Alexander S, Rowan D. An electric pessary for stress incontinence. Lancet. 1968;1:728.
Andrews BJ, Reynard JM. Transcutaneous posterior tibial nerve stimulation for treatment of detrusor hyperreflexia in spinal cord injury. J Urol. 2003;21:785-795.
Bell C. Idea of a new anatomy of the brain; submitted for the observation of his friends. Printed in 1811 by Strahan and Preston, London; not published, but privately circulated, pp 1–36. Reprinted in Cranefield PF, editor. The way in and the way out: François Magendie, Charles Bell and the roots of the spinal nerves. Mount Kisco (NY): Futura; 1974.
Bemelmans BHC, Mundy RM, Craggs MD. Neuromodulation by implant for treating lower urinary tract symptoms and dysfunction. Eur Urol. 1999;36(2):81-91.
Bertapelle P, Bodo G, Carone R. Detrusor acontractility in urinary retention: detrusor contractility test as exclusion criteria for sacral neuromodulation. J Urol. 2008;180(1):215-216.
Bosch JL. The Bion device: a minimally invasive implantable ministimulator for pudendal nerve neuromodulation in patients with detrusor overactivity incontinence. Urol Clin North Am. 2005;32:109-112.
Bosch JL, Groen J. Treatment of refractory urinary urge incontinence with sacral spinal nerve stimulation in multiple sclerosis patients. Lancet. 1996;348:717-719.
Bower WF, Moore KH, Adams RD. A pilot study of the home application of transcutaneous neuromodulation in children with urgency or urge incontinence. J Urol. 2001;166:2420-2422.
Bower WF, Moore KH, Adams RD, Shepherd R. A urodynamic study of surface neuromodulation versus sham in detrusor instability and sensory urgency. J Urol. 1998;160:2133.
Boyce WH, Lathem JE, Hunt LD. Research related to the development of an artificial electrical stimulator for the paralyzed human bladder: a review. J Urol. 1964;91:41-51.
Brindley GS. Electrode-arrays for making long-lasting electrical connexion to spinal roots. J Physiol. 1972;222:135P-136P.
Brindley GS. Emptying the bladder by stimulating sacral ventral roots. J Physiol. 1974;237:15P-16P.
Brindley GS. An implant to empty the bladder or close the urethra. J Neurol Neurosurg Psychiatry. 1977;40:358-369.
Brindley GS. Physiological considerations in the use of sacral anterior root stimulators. Neurourol Urodyn. 1993;12:485-486.
Brindley GS. The first 500 patients with sacral anterior root stimulator implants: general description. Paraplegia. 1994;32:795-805.
Brindley GS, Rushton DN. Long-term follow-up of patients with sacral anterior root stimulator implants. Paraplegia. 1990;28:469-475.
Bross S, Braun PM, Weiss J, et al. The role of the carbachol test and concomitant diseases in patients with nonobstructive urinary retention undergoing sacral neuromodulation. World J Urol. 2003;20:346-349.
Burghele T, Ichim V, Demetrescu M. Experimental study on emptying of the cord bladder. Transcutaneous stimulation of pelvic nerves by electromagnetic induction apparatus [paper 9]. Digest of the 15th Annual Conference on Engineering in Medicine and Biology, Chicago, 1962:58.
Caldwell KP. The electrical control of sphincter incompetence. Lancet. 1963;2:174-175.
Caldwell KP, Flack FC, Broad AF. Urinary incontinence following spinal injury treated by electronic implant. Lancet. 1965;1:846-847.
Chai TC, Zhang C, Warren JW, Keay S. Percutaneous sacral third nerve root neurostimulation improves symptoms and normalizes urinary HB-EGF levels and antiproliferative activity in patients with interstitial cystitis. Urology. 2000;55:643-646.
Chen C, Ma C, de Groat WC. Effects of capsaicin on micturition and associated reflexes in rats. Am J Physiol. 1993;265:R132-R138.
Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169:1369-1373.
Congregado Ruiz B, Pena Outeirino XM, Campoy Martinez P, et al. Peripheral afferent stimulation for treatment of lower urinary tract irritative symptoms. Eur Urol. 2004;45:65-69.
Cooperberg MR, Stoller ML. Percutaneous neuromodulation. Urol Clin North Am. 2005;32:71-78. vii
Craggs M, McFarlane J. Neuromodulation of the lower urinary tract. Exp Physiol. 1999;84:149-160.
Dahms SE, Hohenfellner M, Thuroff JW. Sacral neurostimulation and neuromodulation in urological practice. Curr Opin Urol. 2000;10:329-335.
Dasgupta R, Critchley HD, Dolan RJ, Fowler CJ. Changes in brain activity following sacral neuromodulation for urinary retention. J Urol. 2005;174(6):2268-2272.
Dasgupta R, Fowler CJ. The management of female voiding dysfunction: Fowler’s syndrome—a contemporary update. Curr Opin Urol. 2003;13:293-299.
Dasgupta R, Wiseman OJ, Kitchen N, Fowler CJ. Long-term results of sacral neuromodulation for women with urinary retention. BJU Int. 2004;94:335-337.
Datta SN, Chaliha C, Singh A, et al. Sacral neuromodulation for urinary retention: 10 year experience from one UK centre. BJU Int. 2008;101(2):192-196.
Decter RM. Intravesical electrical stimulation of the bladder: con. Urology. 2000;56:5-8.
Decter RM, Snyder P, Laudermilch C. Transurethral electrical bladder stimulation: a follow-up report. J Urol. 1994;152:812-817.
Dees JE. Contraction of the urinary bladder produced by electric stimulation: preliminary report. Invest Urol. 1965;2:539-547.
DeGennaro M, Capitanucci ML, Mastracci P, et al. Percutaneous tibial nerve neuromodulation is well tolerated in children and effective for treating refractory vesical dysfunction. J Urol. 2004;171:1911-1913.
de Groat WC. Nervous control of the urinary bladder of the cat. Brain Res. 1975;87:201.
de Groat WC. Mechanisms underlying recurrent inhibition in the sacral parasympathetic outflow to the urinary bladder. J Physiol. 1976;257:503.
de Groat WC. Inhibitory mechanisms in the sacral reflex pathways to the urinary bladder. In: Ryall RW, Kelly JS, editors. Iontophoresis and transmitter mechanisms in the mammalian central nervous system. Amsterdam: Elsevier; 1978:366-368.
de Groat WC, Nadelhaft I, Milne RJ, et al. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135-160.
de Groat WC, Ryall RW. The identification and antidromic responses of sacral preganglionic parasympathetic neurons. J Physiol (Lond). 1968;196:533.
de Groat WC, Ryall RW. Recurrent inhibition in sacral parasympathetic pathways to the bladder. J Physiol (Lond). 1968;196:579.
de Groat WC, Vizzard MA, Araki I, et al. Spinal interneurons and preganglionic neurons in sacral autonomic reflex pathways. Prog Brain Res. 1996;107:97.
De Ridder D, Dieter O, Bruyninckx F. The presence of Fowler’s syndrome predicts successful long-term outcome of sacral nerve stimulation in women with urinary retention. Eur Urol. 2007;51(1):229-233.
Drossman DA, Corazziari E. ROME II. The functional gastrointestinal disorders. diagnosis, pathophysiology, and treatment, 2nd ed. McLean, VA: Degnon Associates; 2000.
Ebner A, Jiang C, Lindstrom S. Intravesical electrical stimulation—an experimental analysis of the mechanism of action. J Urol. 1992;148:920-924.
Erlandson BE, Fall M, Carlsson CA. The effect of intravaginal electrical stimulation on the feline urethra and urinary bladder: electrical parameters. Scand J Urol Nephrol Suppl. 1977;44:5-18.
Everaert K, Kerckhaert W, Caluwaerts H, et al. A prospective randomized trial comparing the 1-stage with the 2-stage implantation of a pulse generator in patients with pelvic floor dysfunction selected for sacral nerve stimulation. Eur Urol. 2004;45:649-654.
Fall M. Conservative management of chronic interstitial cystitis: transcutaneous electrical nerve stimulation and transurethral resection. J Urol. 1985;133:774-778.
Fall M, Carlsson CA, Erlandson BE. Electrical stimulation in interstitial cystitis. J Urol. 1980;123:192.
Fall M, Erlandson BE, Carlsson CA, et al. The effect of intravaginal electrical stimulation on the feline urethra and urinary bladder: neuronal mechanisms. Scand J Urol Nephrol Suppl. 1977;44:19-30.
Fall M, Lindstrom S. Electrical stimulation: a physiologic approach to the treatment of urinary incontinence. Urol Clin North Am. 1991;18:393.
Feler CA. Sacral neuromodulation for chronic pain conditions. Anesthesiol Clin North Am. 2003;21:785-795.
Fischer J, Madersbacher H, Zechberger J, et al. Sacral anterior root stimulation to promote micturition in transverse spinal cord lesions. Zentralbl Neurochir. 1993;54:77-79.
Fowler CJ, Swinn MJ, Goodwin RJ, et al. Studies of the latency of pelvic floor contraction during peripheral nerve evaluation show that the muscle response is reflexly mediated. J Urol. 2000;163:881-883.
Galloway NT, El-Galley RE, Sand PK, et al. Update on extracorporeal magnetic innervation (EXMI) therapy for stress urinary incontinence. Urology. 2000;56(6 Suppl. 1):82-86.
Ganio E, Ratto C, Masin A, et al. Neuromodulation for fecal incontinence: outcome in 16 patients with definitive implant. The initial Italian Sacral Neurostimulation Group (GINS) experience. Dis Colon Rectum. 2001;44:965-970.
Gasparini ME, Schmidt RA, Tanagho EA. Selective sacral rhizotomy in the management of the reflex neuropathic bladder: a report on 17 patients with long-term follow-up. J Urol. 1992;148:1207-1210.
Gaunt RA, Prochazka A. Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehabil Neural Repair. 2009;23(6):615-626.
Giannuzzi J. Recherches physiologiques sur les nerfs moteurs de la vessie. J Physiol Homme Animaux. 1863;6:22-29.
Gimbel JR, Kanal E. Can patients with implantable pacemakers safely undergo magnetic resonance imaging? J Am Coll Cardiol. 2004;43:1325-1327.
Goh M, Diokno AC. Sacral neuromodulation for nonobstructive urinary retention—is success predictable? J Urol. 2007;178(1):197-199.
Goldman HB, Amundsen CL, Mangel J, et al. Dorsal genital nerve stimulation for the treatment of overactive bladder symptoms. Neurourol Urodyn. 2008;27(6):499-503.
Govier FE, Litwiller S, Nitti V, et al. Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. J Urol. 2001;165:1193-1198.
Groenendijk PM, Lycklama A, Nyeholt AA, et al. Urodynamic evaluation of sacral neuromodulation for urge urinary incontinence. BJU Int. 2007;101(3):325-329.
Guys JM, Haddad M, Planche D, et al. Sacral neuromodulation for neurogenic bladder dysfunction in children. J Urol. 2004;172(pt 2):1673-1676.
Hald T, Agrawal G, Kantrowitz A. Studies in stimulation of the bladder and its motor nerves. Surgery. 1966;60:848-856.
Hassouna MM, Elkelini M. Canadian experience in sacral neuromodulation. Urol Clin North Am. 2005;32(1):41-49.
Hassouna MM, Siegel SW, Nyeholt AA, et al. Sacral neuromodulation in the treatment of urgency-frequency symptoms: a multicenter study on efficacy and safety. J Urol. 2000;163:1849-1854.
Hedlund H, Schultz A, Talseth T, et al. Sacral neuromodulation in Norway: clinical experience of the first three years. Scand J Urol Nephrol Suppl. 2002;210:87-95.
Hijaz A, Vasavada S. Complications and troubleshooting of sacral neuromodulation therapy. Urol Clin North Am. 2005;32:65-69.
Hoebeke P, Renson C, Petillon L, et al. Percutaneous electrical nerve stimulation in children with therapy-resistant non-neuropathic bladder sphincter dysfunction: a pilot study. J Urol. 2002;168:2605-2607.
Hoebeke P, Van Laecke E, Everaert K, et al. Transcutaneous neuromodulation for the urge syndrome in children: a pilot study. J Urol. 2001;166:2416-2419.
Hohenfellner M, Thuroff JW, Schulz-Lampel D, et al. Sacral root stimulation to treat micturition disorders [Sakrale Neuromodulation zur Therapie von Miktionsstorungen.]. Aktuelle Urol. 1992;23:1-10.
Holmquist B, Olin T. Electromicturition in male dogs at pelvic nerve stimulation: an urethrocystographic study. Scand J Urol Nephrol. 1968;2:115-127.
Hopkinson BR, Lightwood R. Electrical treatment of anal incontinence. Lancet. 1966;1:297-298.
Hopkinson BR, Lightwood R. Electrical treatment of incontinence. Br J Surg. 1967;54:802-805.
Janknegt RA, Hassouna MM, Siegel SW, et al. Long-term effectiveness of sacral nerve stimulation for refractory urge incontinence. Eur Urol. 2001;39:101-106.
Jarrett ME, Varma JS, Duthie GS, et al. Sacral nerve stimulation for faecal incontinence in the UK. Br J Surg. 2004;91:755-761.
Jezernik S, Grill WM, Sinkjaer T. Detection and inhibition of hyperreflexia like bladder contractions in the cat by sacral nerve root recording and electrical stimulation. Neurourol Urodyn. 2001;20:215-220.
Jiang CH, Lindstrom S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol (Lond). 1999;517(pt 2):599-605.
Jonas U, Fowler CJ, Chancellor MB, et al. Efficacy of sacral nerve stimulation for urinary retention: results 18 months after implantation. J Urol. 2001;165:15-19.
Jonas U, Jones LW, Tanagho EA. Spinal cord stimulation versus detrusor stimulation: a comparative study in six “acute” dogs. Invest Urol. 1975;13:171-174.
Jonas U, Tanagho EA. Studies on the feasibility of urinary bladder evacuation by direct spinal cord stimulation. II. Poststimulus voiding: a way to overcome outflow resistance. Invest Urol. 1975;13:151-153.
Kabay SC, Yucel M, Kabay S. Acute effects of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with multiple sclerosis: a urodynamic study. Urology. 2008 Apr;71(4):641-645.
Kaplan WE. Intravesical electrical stimulation of the bladder: pro. Urology. 2000;56:2-4.
Kaplan WE, Richards I. Intravesical bladder stimulation in myelodysplasia. J Urol. 1988;140(pt 2):1282-1284.
Kaplan WE, Richards TW, Richards I. Intravesical transurethral bladder stimulation to increase bladder capacity. J Urol. 1989;142(pt 2):600-602.
Kaufmann S, Naumann CM, Hamann MF, et al. Unilateral vs bilateral sacral neuromodulation in pigs with formalin induced detrusor hyperactivity. BJU Int. 2008;103:260-263.
Kenefick NJ, Nicholls RJ, Cohen RG, Kamm MA. Permanent sacral nerve stimulation for treatment of idiopathic constipation. Br J Surg. 2002;89:882-888.
Kenefick NJ, Vaizey CJ, Cohen CR, et al. Double-blind placebo-controlled crossover study of sacral nerve stimulation for idiopathic constipation. Br J Surg. 2002;89:1570-1571.
Klingler HC, Pycha A, Schmidbauer Brindley J, Marberger M. Use of peripheral neuromodulation of the S3 region for treatment of detrusor overactivity: a urodynamic-based study. Urology. 2000;56:766-771.
Koldewijn E, Van Kerrebroeck PEV, Rosier PF, et al. Bladder compliance after posterior sacral root rhizotomies and anterior sacral root stimulation. J Urol. 1994;151(4):955-960.
Kondo A, Otani T, Takita T. Suppression of bladder instability by penile squeeze. Br J Urol. 1982;54:360-362.
Kruse MN, de Groat WC. Spinal pathways mediate coordinated bladder/urethral sphincter activity during reflex micturition in normal and spinal cord injured neonatal rats. Neurosci Lett. 1993;152:141.
Langley JN, Anderson HK. The innervations of the pelvic and adjoining viscera. II. The bladder. J Physiol. 1895;19:71-84.
Lee YH, Creasey GH. Self-controlled dorsal penile nerve stimulation to inhibit bladder hyperreflexia in incomplete spinal cord injury: a case report. Arch Phys Med Rehabil. 2002;83:273-277.
Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005;32:11-18.
Lindstrom S, Fall M, Carlsson CA, Erlandson BE. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J Urol. 1983;129:405.
Lombardi G, Del Popolo G. Clinical outcome of sacral neuromodulation in incomplete spinal cord injured patients suffering from neurogenic lower urinary tract symptoms. Spinal Cord. 2010;48(2):154-159.
Lyne CJ, Bellinger MF. Early experience with transurethral electrical bladder stimulation. J Urol. 1993;150(pt 2):697-699.
Madersbacher H. Konservative Therapie der neurogenen Blasendysfunktion. Urologe A. 1999;38:24-29.
Madersbacher H. Denervation techniques. BJU Int. 2000;85(Suppl 3):1-6.
Magendie F. Experiences sur les fonctions des raciness des nerfs rachidiens. J Physiol Exp Pathol. 1822;2:276-279. Alexander CA, transl. In. Flourens P, editor. Memoir of Magendie. Mount Kisco (NY) Futura, 1974
Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19:1.
Maher MG, Mourtzinos A, Zabihi N, et al. Bilateral caudal epidural neuromodulation for refractory urinary retention: a salvage procedure. J Urol. 2007;177(6):2237-2240.
Mailis-Gagnon A, Furlan AD, Sandoval JA, Taylor R. Spinal cord stimulation for chronic pain. Cochrane Database Syst Rev 2004;3:CD003783.
Malaguti S, Spinelli M, Giardiello G, et al. Neurophysiological evidence may predict the outcome of sacral neuromodulation. J Urol. 2003;170(pt 1):2323-2326.
Malm-Buatsi E, Nepple KG, Boyt MA, et al. Efficacy of transcutaneous electrical nerve stimulation in children with overactive bladder refractory to pharmacotherapy. Urology. 2007;70(5):980-983.
Martin ET. Can cardiac pacemakers and magnetic resonance systems coexist? Eur Heart J. 2005;26:325-327.
Matzel KE, Bittorf B, Stadelmaier U, et al. Sacral nerve stimulation in the treatment of faecal incontinence. Chirurg. 2003;74:26-32.
McGee SM, Granberg CF, Roth TJ, et al. Sacral neuromodulation in children with dysfunctional elimination syndrome: description of incisionless first stage and second stage without fluoroscopy. Urology. 2009;73(3):641-644.
McGuire EJ, Savastano JA. Urodynamic findings and clinical status following vesical denervation procedures for control of incontinence. J Urol. 1984;132:87-88.
McGuire EJ, Zhang SC, Horwinski ER, et al. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78-79.
Melenhorst J, Koch SM, Uludag O, et al. Sacral neuromodulation in patients with faecal incontinence: results of the first 100 permanent implants. Colorectal Dis. 2007;9(8):725-730.
Nashold BSJr, Friedman H, Boyarsky S. Electrical activation of micturition by spinal cord stimulation. J Surg Res. 1971;11:144-147.
Nissenkorn I, DeJong PR. A novel surgical technique for implanting a new electrostimulation system for treating female overactive bladder: a preliminary report. BJU Int. 2005;95(9):1253-1258.
Nissenkorn I, Shalev M, Radziszewski P, et al. Patient adjusted intermittent electrostimulation for treating stress and urge incontinence. BJU Int. 2004;94(1):105-109.
Okada N, Igawa Y, Ogawa A, Nishizawa O. Transcutaneous electrical stimulation of thigh muscles in the treatment of detrusor overactivity. Br J Urol. 1998;81:560-564.
Oliver S, Fowler C, Mundy A, Craggs M. Measuring the sensations of urge and bladder filling during cystometry in urge incontinence and the effects of neuromodulation. Neurourol Urodyn. 2003;22:7-16.
Peng CW, Chen JJ, Cheng CL, Grill WM. Improved bladder emptying in urinary retention by electrical stimulation of pudendal afferents. J Neural Eng. 2008;5(2):144-154.
Peters K, Carey JM, Konstandt DB. Sacral neuromodulation for the treatment of refractory interstitial cystitis: outcomes based on technique. Int Urogynecol J. 2003;14:223-228.
Peters K, Carrico D, Burks F. Validation of a sham for percutaneous tibial nerve stimulation (PTNS). Neurourol Urodyn. 2009;28(1):58-61.
Pettit P, Thompson JR, Chen AH. Sacral neuromodulation: new applications in the treatment of pelvic floor dysfunction. Curr Opin Obstet Gynecol. 2002;14:521-525.
Pham K, Guralnick ML, O’Connor RC. Unilateral versus bilateral stage I neuromodulatory lead placement for the treatment of refractory voiding dysfunction. Neurourol Urodyn. 2008;27(8):779-781.
Possover M. The sacral LION procedure for recovery of bladder/rectum/sexual functions in paraplegic patients after explantation of a previous Finetech-Brindley controller. J Minim Invasive Gynecol. 2009;16(1):98-101.
Pugach JL, Salvin L, Steinhardt GF. Intravesical electrostimulation in pediatric patients with spinal cord defects. J Urol. 2000;164(Pt. 2):965-968.
Riazimand S, Mense S. A rat model for studying effects of sacral neuromodulation on the contractile activity of a chronically inflamed bladder. BJU Int. 2004;94:158.
Rijkhoff NJ, Wijkstra H, van Kerrebroeck PE, Debruyne FM. Selective detrusor activation by electrical sacral nerve root stimulation in spinal cord injury. J Urol. 1997;157:1504-1508.
Roguin A, Zviman MM, Meininger GR, et al. Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation. 2004;110:475-482.
Sauerwein D. Die operative Behandlung der spastischen Blasenlahmung bei Querschnittlahmung. Sakrale Deafferentation (SDAF) mit der Implantation eines sakralen Voderwurzelstimulators (SARS). Urologe A. 1990;29:196-203.
Sauerwein DH, Domurath BKH, Kutzenberger JF, et al. Experience with sacral deafferentation and implantation of an anterior root stimulator in 294 spinal cord injury patients [abstract]. J Urol. 1999;161(Suppl. 4):275.
Scheepens WA, de Bie RA, Weil EH, van Kerrebroeck PE. Unilateral versus bilateral sacral neuromodulation in patients with chronic voiding dysfunction. J Urol. 2002;168:2046-2050.
Schmidt RA. Technique of pudendal nerve localization for block or stimulation. J Urol. 1989;142:1528-1531.
Schmidt RA, Jonas U, Oleson KA, et al. Sacral nerve stimulation for the treatment of refractory urinary urge incontinence. J Urol. 1999;162:352-357.
Schultz-Lampel D, Jiang C, Lindstrom S, Thuroff JW. Experimental results on mechanisms of action of electrical neuromodulation in chronic urinary retention. World J Urol. 1998;16:301-304.
Schultz-Lampel D, Jiang C, Lindstrom S, Thuroff JW. Summation effect of bilateral sacral root stimulation. Eur Urol. 1998;33(Suppl. 5):61.
Shaker HS, Hassouna MM. Sacral nerve root neuromodulation: an effective treatment for refractory urge incontinence. J Urol. 1998;159:1516-1519.
Shaker HS, Wang Y, Loung D, et al. Role of C-afferent fibers in the mechanism of action of sacral nerve root neuromodulation in chronic spinal cord injury. BJU Int. 2000;85:905-910.
Siegel SW. Selecting patients for sacral nerve stimulation. Urol Clin North Am. 2005;32:19-26.
Siegel SW, Catanzaro F, Dijkema HE, et al. Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology. 2000;56(Suppl. 1):87-91.
Siegel SW, Paszkiewicz E, Kirkpatrick C, et al. Sacral nerve stimulation in patients with chronic intractable pelvic pain. J Urol. 2001;166:1742-1745.
Spinelli M, Bertapelle P, Cappellano F, et al. Chronic sacral neuromodulation in patients with lower urinary tract symptoms. J Urol. 2001;166:541-545.
Spinelli M, Giardiello G, Arduini A, van den Hombergh U. New percutaneous technique of sacral nerve stimulation has high initial success rate: preliminary results. Eur Urol. 2003;43:70-74.
Spinelli M, Malaguti S, Giardiello G, et al. A new minimally invasive procedure for pudendal nerve stimulation to treat neurogenic bladder: description of the method and preliminary data. Neurourol Urodyn. 2005;24:305-309.
Starkman JS, Duffy JW, Wolter CE, et al. Refractory overactive bladder after urethrolysis for bladder outlet obstruction: management with sacral neuromodulation. Int J Urogynecol Pelvic Floor Dysfunct. 2008;19(2):277-282.
Sundin T, Carlsson CA, Kock NG. Detrusor inhibition induced from mechanical stimulation of the anal region and from electrical stimulation of pudendal nerve afferents. Invest Urol. 1974;5:374-378.
Susset JG, Boctor ZN. Implantable electrical vesical stimulator: clinical experience. J Urol. 1967;98:673-678.
Tanagho EA, Schmidt RA. Bladder pacemaker: scientific basis and clinical future. Urology. 1982;20:614-619.
Tanagho EA, Schmidt RA. Electrical stimulation in the clinical management of the neurogenic bladder. J Urol. 1988;140:1331.
Tanagho EA, Schmidt RA, Orvis BR. Neural stimulation for control of voiding dysfunction: a preliminary report in 22 patients with serious neuropathic voiding disorders. J Urol. 1989;142(2 Pt 1):340-346.
Timm GW, Bradley WE. Electrostimulation of the urinary detrusor to effect contraction and evacuation. Invest Urol. 1969;6:562-568.
Tjandra JJ, Chan MK, Yeh CH, Murray-Green C. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51:494-502.
Torrens M. The role of denervation in the treatment of detrusor instability. Neurourol Urodyn. 1985;4:353-358.
Tscholl R, Schreiter F, Jonas U. Direkte Stimulation des Detrusors mit einer simulierten Netzelektrode und transvasale Stimulation mit bipolaren Electroden am Hund. Urologe A. 1971;10:246-248.
Uludag O, Darby M, Dejong CH, et al. Sacral neuromodulation is effective in the treatment of fecal incontinence with intact sphincter muscles; a prospective study. Ned Tijdschr Geneeskd. 2002;146:989-993.
van Balken MR, Vandoninck V, Gisolf KW, et al. Posterior tibial nerve stimulation as neuromodulative treatment of lower urinary tract dysfunction. J Urol. 2001;166:914-918.
van Balken MR, Vandoninck V, Messelink BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43:158-163. discussion 163
Vandoninck V, van Balken MR, Finazzi Agrò E, et al. Percutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic data. Neurourol Urodyn. 2003;22:227-232.
van Kerrebroeck EV, Scheepens WA, de Bie RA, Weil EH. European experience with bilateral sacral neuromodulation in patients with chronic lower urinary tract dysfunction. Urol Clin North Am. 2005;31(1):51-57.
van Kerrebroeck EV, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective worldwide clinical study. J Urol. 2007;178(5):2029-2034.
van Kerrebroeck P, Scheepens WA, de Bie RA, Weil EH. European experience with bilateral sacral neuromodulation in patients with chronic lower urinary tract dysfunction. Urol Clin North Am. 2005;32:51-57.
van Kerrebroeck PE, van der Aa HE, Bosch JL, et al. Sacral rhizotomies and electrical bladder stimulation in spinal cord injury. I. Clinical and urodynamic analysis. Dutch Study Group on Sacral Anterior Root Stimulation. Eur Urol. 1997;31:263-271.
Vereecker RL, Das RJ, Grisar P. Electrical sphincter stimulation in the treatment of detrusor hyperreflexia of paraplegia. Neurourol Urodyn. 1984;3:145-149.
Vignes JR, Deloire M, Petry K. Animal models of sacral neuromodulation for detrusor overactivity. Neurourol Urodyn. 2009;28:8-12.
Vodusek DB, Light KJ, Libby JM. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol Urodyn. 1986;5:381-383.
Wallace PA, Lane FL, Noblett KL. Sacral nerve neuromodulation in patients with underlying neurologic disease. Am J Obstet Gynecol. 2007;197(1):96e1-5.
Walter JS, Wheeler JS, Robinson CJ, Wurster RD. Inhibiting the hyperreflexic bladder with electrical stimulation in a spinal animal model. Neurourol Urodyn. 1993;12:241-253.
Wang Y, Hassouna MM. Electrical stimulation has no adverse effect on pregnant rats and fetuses. J Urol. 1999;162:1785-1787.
Wein AJ, Barrett DM. Voiding function and dysfunction—a logical and practical approach. Chicago: Year Book; 1988.
Wheeler JSJr, Walter JS, Sibley P. Management of incontinent SCI patients with penile stimulation: preliminary results. J Am Paraplegia Soc. 1994;17:55-59.
Wheeler JSJr, Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol. 1992;147:100-103.
White WM, Dobmeyer-Dittrich C, Klein FA, Wallace LS. Sacral nerve stimulation for treatment of refractory urinary retention: long-term efficacy and durability. Urology. 2008;71(1):71-74.
White WM, Mobley JD3rd, Doggweiler R, et al. Incidence and predictors of complications with sacral neuromodulation. Urology. 2009;73(4):731-735.
Whiteside JL, Ensrud-Bowlin KM, Wang G, et al. Lead placement and associated nerve distribution of an implantable periurethral electrostimulator. Int Urogynecol J Pelvic Floor. 2009;20:325-329.
Whitmore KE, Payne CK, Diokno AC, Lukban JC. Sacral neuromodulation in patients with interstitial cystitis: a multicenter clinical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:305-309.
Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1880-R1889.
Yalla SV, Di Benedetto M, Blunt KJ, et al. Urethral striated sphincter responses to electro-bulbocavernosus stimulation. J Urol. 1978;119:406-409.
Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008;212(1):218-225.
Yoshimura N, de Groat WC. Patch clamp analysis of afferent and efferent neurons that innervated the urinary bladder of the rat. Soc Neurosci Abstr. 1992;18:127.
Yoshimura N, de Groat WS. Neural control of the lower urinary tract. Int J Urol. 1997;4:111.
Zabihi N, Mourtzinos A, Maher MG, et al. Short-term results of bilateral S2–4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):533-536.
Zhao J, Bai J, Zhou Y, et al. Posterior tibial nerve stimulation twice a week in patients with interstitial cystitis. Urology. 2008;71(6):1080-1084.