chapter 71 Retropubic Suspension Surgery for Incontinence in Women
Continence in the woman results from a complex interplay of anatomic and physiologic properties of the lower urinary tract (bladder, urethra and sphincter, and pelvic floor) acting under the coordinating control of an intact central and peripheral nervous system. The role of the pelvic floor is to provide support to both the bladder and urethra and to facilitate normal abdominal pressure transmission to the proximal urethra, thereby maintaining continence.
Stress incontinence is a symptom, a sign, and a clinical diagnosis; the clinical symptom is that of involuntary loss of urine associated with increased intra-abdominal pressure, such as occurs during coughing and sneezing. The International Continence Society (ICS) defines urodynamic stress incontinence as the involuntary loss of urine during increased intra-abdominal pressure during filling cystometry, in the absence of detrusor (bladder wall muscle) contraction (Abrams et al, 2002). Thus, urodynamic evaluation is a prerequisite for the diagnosis of urodynamic stress incontinence. Therefore, in discussing stress incontinence, this review refers to women with stress urinary incontinence (SUI) diagnosed on the basis of symptoms alone or urodynamically proven, so-called urodynamic stress incontinence.
Treatment options for SUI include conservative techniques and both pharmacologic and surgical interventions. Surgical procedures to treat SUI generally aim to improve the support to the urethrovesical junction and to correct deficient urethral closure. There is a contemporary lack of consensus, however, regarding the precise mechanism by which continence is achieved in the “normal asymptomatic female” and therefore not surprisingly in how “normality” is restored by surgical manipulation. Anti-incontinence surgery is generally used to address the failure of normal anatomic support of the bladder neck and proximal urethra and intrinsic sphincter deficiency. Anti-incontinence surgery does not necessarily work by restoring the same mechanism of continence that was present before the onset of incontinence. Rather, it works by a compensatory approach, creating a new mechanism of continence (Jarvis, 1994a).
Therapeutic Options
The surgeon’s preference, coexisting problems, and anatomic features of the patient and her general health condition influence the choice of procedure. Numerous surgical methods have been described, but they essentially fall into seven categories (Table 71–1).
This wide variety of treatment options for stress incontinence indicates the lack of a clear consensus as to which procedure is the most effective. Several groups have reviewed the literature, often using systematic and methodical analyses of well-designed randomized controlled trials (Jarvis, 1994b; Black and Downs, 1996; Fantl et al, 1996; Leach et al, 1997; Moehrer et al, 2002, 2003; Lapitan et al, 2003). Most of these reviews, however, are hampered by the quality of the existing evidence base, and these reviews are based on studies of mixed quality with little standardization of the points in Table 71-2.
Table 71–2 Standardization Needed for Studies
A review of existing literature on confounding variables affecting outcome of therapy (Smith et al, 2005) concluded the following:
Choice of Surgical Technique
Two types of stress incontinence have been suggested: one associated with a hypermobile but otherwise healthy urethra, a manifestation of weakened support of the proximal urethra, and one arising from a deficiency of the urethral sphincter mechanism itself, thereby compromising the ability of the urethra to act as a watertight outlet. Hypermobility of the bladder neck and proximal urethra results from a weakening or loss of their supporting elements (ligaments, fasciae, and muscles), which in turn may be a consequence of aging, hormonal changes, childbirth, and prior surgery. It seems likely that the majority of women with SUI will also have an element of intrinsic sphincteric weakness with a variable degree of loss of the normal anatomic support of the bladder neck and proximal urethra, resulting in hypermobility. The compelling observation that one can cite for this is that a normal asymptomatic individual will not leak however much she strains.
Differentiating Relative Contributions of Hypermobility and Intrinsic Sphincter Deficiency
The influence of urethral function defined by leak point pressure or maximum urethral closure pressure (MUCP) is difficult to define on account of the large variation in outcome measures employed. Furthermore, other variables such as urethral mobility are often not controlled. Intrinsic sphincteric deficiency (ISD) is usually defined as a leak point pressure of less than 60 or MUCP of less than 20. A standardized test is not available to differentiate the relative contributions of intrinsic sphincter deficiency and hypermobility; therefore few studies have been able to accurately separate their individual contributions to the development of incontinence (Chapple et al, 2005). Retropubic procedures act to restore the bladder neck and proximal urethra to a fixed, retropubic position and are used when hypermobility is believed to be an important factor in the development of that woman’s stress incontinence. This may facilitate the function of a marginally compromised intrinsic urethral sphincter mechanism, but if significant intrinsic sphincter deficiency is present it is likely that SUI will persist despite efficient surgical repositioning of the bladder neck and proximal urethra; at present this hypothesis remains unproven. In such circumstances then a sling procedure (particularly a “snug” fascial sling) or an artificial sphincter is most likely to be the therapy of choice.
In the normal continent woman, the bladder neck and proximal urethra are supported in a retropubic position, with the bladder base being dependent. Increases in intra-abdominal pressure are transmitted to both the bladder and the proximal urethra such that the pressure difference between the two is unchanged, promoting continence (Einhorning, 1961). A valvular effect at the bladder neck created by the transmission of abdominal pressure to the dependent bladder base may also be operative here (Penson and Raz, 1996). Furthermore, with proper bladder neck support, reflex contraction of the pelvic floor muscles during Valsalva maneuvers and coughing acts as a backboard for urethral compression (Staskin et al, 1985).
Surgical Procedures
Retropubic surgical procedures, usually chosen as surgical therapy for patients with SUI in which there is a significant component of hypermobility, are discussed here.
Open retropubic colposuspension is the surgical approach of lifting the tissues near the bladder neck and proximal urethra into the area of the pelvis behind the anterior pubic bones. When it is an open procedure the approach is through an incision over the lower abdomen. There are four variations of open retropubic colposuspension: Marshall-Marchetti-Krantz (MMK), Burch, vagino-obturator shelf (VOS), and paravaginal.
The term colposuspension was originally used to denote suspension of the urethra by the vaginal wall; however, by common usage, it now generally includes the paraurethral fascia and sometimes only this without the vagina. Retropubic colposuspension urethral repositioning can be achieved by three distinctly different procedures; these are all based on a similar underlying principle but in a spectrum in relation to the degree of the support/elevation they achieve, and their outcomes differ somewhat in the longer term.
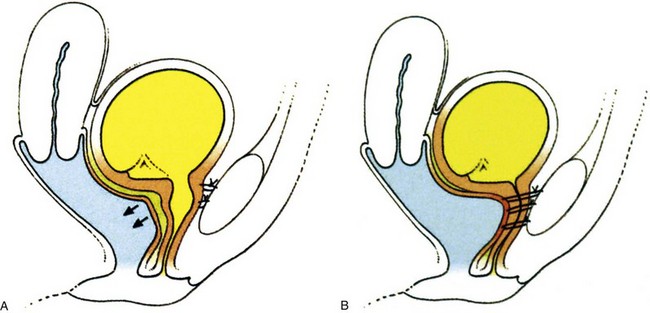
The Burch colposuspension (Fig. 71–1A) is the elevation of the anterior vaginal wall and paravesical tissues toward the iliopectineal line of the pelvic sidewall with use of two to four sutures on either side (Burch, 1961). The VOS repair (see Fig. 71–1B) aims to anchor the vagina to the internal obturator fascia and is a modification of a combination of the Burch and paravaginal defect repair, with placement of the sutures laterally anchored to the internal obturator fascia rather than hitching the vagina up to the iliopectineal line (Turner-Warwick, 1986), although a more recent modification does, where appropriate, insert sutures into both the internal obturator and iliopectineal line (see Fig. 71–1C). The paravaginal defect repair (see Fig. 71–1D) aims to close a presumed fascial weakness laterally at the site of attachment of the pelvic fascia to internal obturator fascia (Richardson et al, 1976). The MMK procedure (see Fig. 71–1E) is the suspension of the vesicourethral junction (bladder neck) toward the periosteum of the symphysis pubis (Marshall et al, 1949) and was thought to act by buttressing the paraurethral area and bringing the vesicourethral junction into a more elevated “intra-abdominal” position.
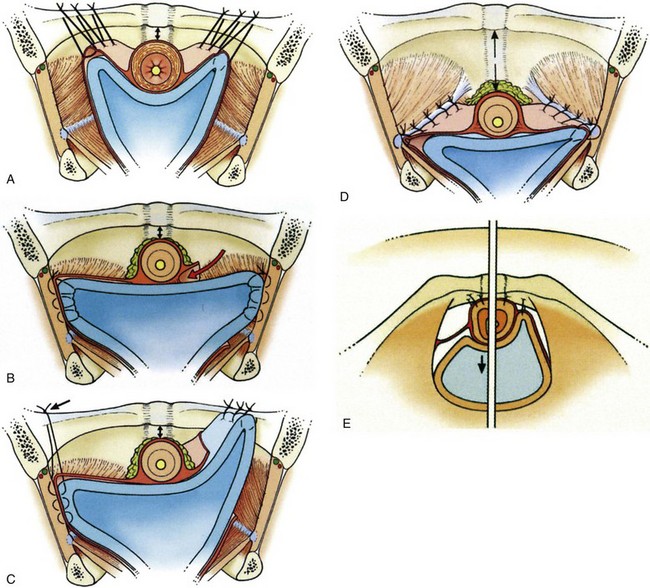
Figure 71–1 A, Coronal view, diagram of a Burch colposuspension. B, Coronal view, diagram of a vagino-obturator shelf procedure. C, Coronal view, diagram of a vagino-obturator shelf procedure on the left, augmented by suturing to the iliopectineal line, and a Burch colposuspension on the right. D, Coronal view, diagram of a paravaginal repair. E, Diagram showing the sutures in a Marshall-Marchetti-Krantz procedure and their proximity to the urethra.
The Degree of Urethral Elevation
The extent of the urethral elevation achieved by both the Burch (see Fig. 71–1A) and the VOS suspensions (see Fig. 71–1B and C) is higher than the arcus tendineus anchorage of the paravaginal defect repair (see Fig. 71–1D).
The Configuration of the Suspensions
A particular advantage of the horizontal urethral elevation achieved by both the VOS and the paravaginal repair suspensions is the significantly less susceptibility to tension on the urethra and to obstructive problems than with the V-shaped configuration of the Burch suspension (see Fig. 71–1A).
Tissue Approximation
Both the VOS and the paravaginal repair suspensions are anchored by tissue-approximating sutures; thus, unlike the Burch colposuspension, neither the VOS nor the paravaginal repair suspension is suture dependent in the longer term once the initial healing is complete because there is direct tissue adhesion. The VOS anchorage and suspension is significantly more robust than that of the paravaginal defect repair; the elevation it achieves can be further augmented by additionally including the iliopectineal ligament in the upper sutures (see Fig. 71–1C).
Laparoscopic colposuspension is the most popular of the laparoscopic incontinence procedures that were first introduced in the early 1990s (Vancaillie and Schuessler, 1991) with the premise that as minimally invasive procedures they would benefit patients by avoiding the major incision of conventional open surgery and shorten the time for a return to normal activity. As in open colposuspension, sutures are inserted into the paravaginal tissues on either side of the bladder neck and then attached to the iliopectineal ligaments on the same side. There are, however, technical variations in surgery with respect to the laparoscopic approach (transperitoneal into the abdominal cavity or extraperitoneal) and in the number and types of sutures, the site of anchor, and the use of mesh and staples (Jarvis et al, 1999).
Assessing Outcomes of Therapy
Before the best procedure can be determined, several issues regarding outcome reporting need to be addressed.
Duration of Follow-up
It is recognized that prolonged follow-up is required to assess the true benefit of an incontinence procedure. Short-term follow-up should be considered to have begun in all studies after participants have reached 1 year of follow-up (Abrams et al, 2005). In the short term (2 years), most procedures are successful and success rates between procedures are similar (Leach et al, 1997). However, with longer follow-up (>5 years), failures become manifest and the true benefit of the better procedures is realized. Most studies report outcomes after short-term follow-up, and thus results must be interpreted with caution.
The Issue of Intrinsic Sphincter Deficiency
There is no consistency in the existing literature database to support the likelihood that intrinsic sphincter deficiency can influence either the outcomes or the type of surgical treatment. The main problem is that there is no consensus on the meaning of intrinsic sphincter deficiency and how to diagnose it (Smith et al, 2005, 2009). Nevertheless it is likely in the author’s view (unsubstantiated by any unequivocal evidence) that mild degrees of intrinsic sphincter deficiency coexist with hypermobility in most cases. In a situation in which intrinsic sphincter deficiency is the predominant problem, then a repositioning procedure such as a colposuspension is less likely to be successful than a tight fascial sling or artificial sphincter.
The Definition of Cure
The definition of cure varies between studies. Some authors report cure of SUI only, whereas others define cure as complete continence postoperatively, implying the absence of urgency incontinence as well. The assessment of cure may vary. Some authors report subjective cure based on patient history, questionnaire, bladder diary, or medical chart review; others use more objective measures, such as pad tests, stress tests, and urodynamics.
Finally, one must question whether the goal of complete continence is reasonable, given that the condition of SUI is generally a degenerative one and corrective surgery does not replace the defective components. As well, even in normal healthy women, urinary continence is a spectrum of dryness; approximately 40% of nulliparous 30- to 49-year-olds experience some degree of incontinence with exercise (Nygaard et al, 1990). It seems unreasonable to expect surgery for a degenerative condition to achieve results that are better than the nondegenerative state.
The Patient’s versus the Physician’s Perspective
A patient’s satisfaction with treatment is often based on the difference between her expectations and her experiences (Sofaer and Firminger, 2005). Thus, fulfillment of positive expectations is a key element of a patient’s satisfaction (Sitzia and Wood, 1997). Because expectations vary widely, satisfaction is not a standard concept. Consequently, treatment plans must be tailored to meet a nonstandard goal. An integral step in achieving this goal is the development of a patient-physician partnership that promotes the negotiation of realistic expectations.
Logically, agreement of patient and physician with respect to treatment plan and goals should improve outcomes. When a diagnosis has been made, asking patients what they already know about the condition may give clues to expectations for treatment. The “ask-tell-ask” method may be employed to mend the gaps between the physician’s and the patient’s expectations. The physician explains the proposed treatment plan and expectations for the outcome, then encourages the patient to ask questions. The physician provides the information requested and invites questions again, continuing the process until a mutual understanding of treatments and expectations is reached (Barrier et al, 2003). This approach may prevent “surprises” such as unexpected pain of treatment, adverse events of medication, and prolonged recovery time. Elkadry and associates (2003) emphasized this point by demonstrating a significant association between feeling unprepared for surgery and the patient’s dissatisfaction after pelvic reconstruction. The same investigators also reported that achievement of patient-defined goals was more predictive of a patient’s satisfaction than were objective measures of surgical success.
Clearly, one or more high-quality validated symptom and quality of life instruments should be chosen at the outset of a clinical trial representing the patient’s viewpoint, accurately defining baseline symptoms as well as any other areas in which treatment may be beneficial and assessing the objective severity and subjective impact of both.
Whereas many, including the author, think that urodynamic studies are helpful in defining the underlying pathophysiologic process in cases with incontinence, they have not been proved to have adequate sensitivity, specificity, or predictive value (Chapple et al, 2005). The ICI meeting concluded that although urodynamic studies, such as frequency-volume charts and pad tests, are useful there is inadequate evidence to justify pressure-flow studies for routine testing as either entry criteria or outcome measures in clinical trials. It was recommended that most large-scale clinical trials enroll subjects by using carefully defined symptom-driven criteria when the treatment will be given on an empirical basis (Abrams et al, 2005).
Indications for Retropubic Repair
The treatment of SUI in women must be tailored to the individual patient. Once evaluation has identified contributing factors, a trial of conservative therapy should be pursued and surgery considered for patients who fail to respond to these conservative measures. Careful assessment of the patient is essential in making an accurate diagnosis (Fig. 71–2).
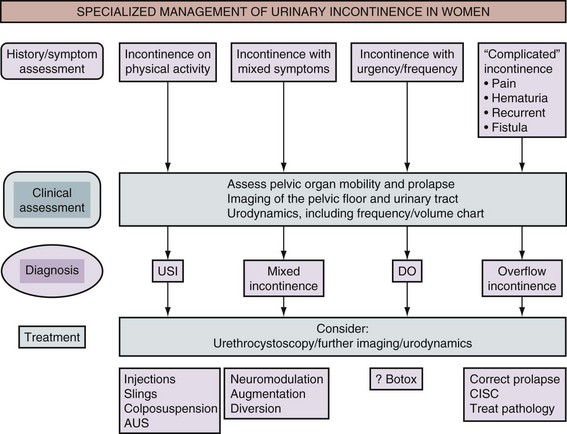
Figure 71–2 Algorithm for the specialized management of stress urinary incontinence (after the Third International Consultation on Incontinence, Monaco [Abrams et al, 2005]). USI, urodynamic stress incontinence; DO, detrusor overactivity; AUS, artificial urinary sphincter; CISC, clean intermittent self-catheterization.
The selection of technique is largely based on the surgeon’s preference and prior experience; bladder base and urethral hypermobility may be surgically corrected by either a vaginal or a retropubic approach. Although it has been suggested that a retropubic colposuspension should be considered in patients who frequently generate high intra-abdominal pressure (e.g., those with chronic cough from obstructive pulmonary disease and women in strenuous occupations) (Appell, 1993), it could also be argued that these patients may be better served by a pubovaginal sling as well.
Specific Indications
A retropubic approach for the correction of anatomic SUI is indicated (1) for a patient undergoing a laparotomy for concomitant abdominal surgery that cannot be performed vaginally and (2) where there is limited vaginal access.
Potential Contraindications
If there is a history of prior failed incontinence procedures the existence of significant sphincteric deficiency must be suspected, even if hypermobility exists, and consideration given to performing a pubovaginal sling, although retropubic colposuspensions may be successful in this scenario as well (Cardozo et al, 1999; Maher et al, 1999; Nitahara et al, 1999).
In the author’s view, when SUI exists solely due to intrinsic sphincter deficiency (i.e., a fixed, nonfunctional proximal urethra with intrinsic urethral sphincter dysfunction), a retropubic suspension procedure is less likely to be successful because there is no hypermobility to correct and the patient is better served by a pubovaginal sling, collagen injections, or an artificial sphincter (Bergman et al, 1989b). This represents a personal view that is at variance with the ICI’s conclusion statement on the role of urethral occlusive forces, which reads as follows: “It would appear that a low leak point pressure is less predictive of outcome on this data when compared to the presence or absence of urethral hypermobility” (Smith et al, 2009).
In cases with a pan–pelvic floor weakness, a colposuspension should not be used in isolation but should be part of a comprehensive approach to the pelvic floor and combined as appropriate with other alternative pelvic floor repair procedures. A retropubic colposuspension does not always adequately correct the associated vaginal prolapse that frequently coexists with bladder neck hypermobility. Although lateral defect cystocele and enterocele lend themselves to retropubic repair, a central defect cystocele, rectocele, and introital deficiency do not.
A retropubic colposuspension is contraindicated when there is an inadequate vaginal length or mobility of the vaginal tissues, for example, after prior vaginal surgery or radiotherapy or such as that after a prior vaginal incontinence procedure (Appell, 1993). The lysis of retropubic adhesions can be performed adequately and safely by a vaginal approach in conjunction with a needle suspension procedure or pubovaginal sling.
Vaginal versus Retropubic Surgery
From a review of the literature there is clearly a difference in the success rate of vaginal versus retropubic surgery alone with respect to correction of stress incontinence. An anterior colporrhaphy can certainly be efficacious for the correction of prolapse, with a reported efficacy in randomized controlled studies of 42% and 57% in the management of cystoceles (Sand et al, 2001; Weber et al, 2001). For the treatment of both a cystocele and SUI, an anterior colporrhaphy should be combined with a sling procedure. Goldberg and colleagues (2001), in a case-control series, demonstrated that in women with a cystocele and SUI the addition of a pubovaginal sling to an anterior colporrhaphy significantly decreased the recurrence rate from 42% in the control group to 19% in the anterior colporrhaphy group.
Glazener and Cooper (2001) reviewed the literature on randomized or quasi-randomized trials that included anterior vaginal repair for the treatment of urinary incontinence. Nine trials were identified that included 333 women having an anterior vaginal repair and 599 who received comparison interventions. They concluded that anterior vaginal repair was less effective than open abdominal retropubic suspension on the basis of patient-reported cure rates in eight trials both in the medium term (failure rate within 1 to 5 years after anterior repair, 97 of 259 [38%] versus 57 of 327 [17%]; relative risk [RR], 2.29; 95% confidence interval [CI], 1.7 to 3.08) and in the long term (after 5 years, 49 of 128 [38%] versus 31 of 145 [21%]; RR, 2.02; 95% CI, 1.36 to 3.01). There was evidence from three of these trials that this was reflected in a need for more repeated operations for incontinence (25 of 107 [23%] vs. 4 of 164 [2%]; RR, 8.87; 95% CI, 3.28 to 23.94). These findings held irrespective of the coexistence of prolapse (pelvic relaxation), although fewer women had a prolapse after anterior repair (RR, 0.24; 95% CI, 0.12 to 0.47) and later prolapse operation appeared to be equally common after either a vaginal (3%) or an abdominal (4%) operation.
Long-term follow-up beyond the first year is available in only three randomized controlled trials (Bergman et al, 1989a; Liapis et al, 1996; Colombo et al, 2000). There is a low morbidity with vaginal repair, but long-term success rates decrease with time to the extent that a 63% cure rate at 1 year fell to 37% at 5 years of follow-up (Bergman and Elia, 1995).
It can be concluded that with short-term follow-up vaginal and open retropubic suspension procedures have similar success rates in the treatment of stress incontinence. However, with longer follow-up (and with the exception of the pubovaginal sling and loose midurethral tapes (see later) patients who have retropubic procedures fare better than those undergoing vaginal repairs.
The recommendation from the ICI committee (Smith et al, 2009) is that anterior colporrhaphy should not be used in the management of SUI alone (grade A).
General Technical Issues
Retropubic Dissection
In open retropubic suspension procedures good access to the retropubic space is crucial. This is best performed with the patient in the supine position with the legs abducted, in either a low or a modified dorsal lithotomy position with the use of stirrups, allowing access to the vagina during the procedure and a perineal-abdominal progression. A urethral Foley catheter is inserted; the catheter balloon is used for subsequent identification of the urethra and bladder neck, and, indeed, it is invaluable in allowing palpation of the edges of the bladder by appropriate manipulation. A Pfannenstiel or lower midline abdominal incision is made, separating the rectus muscles in the midline and sweeping the anterior peritoneal reflection off the bladder. It is essential to optimize the access to the retropubic space, and if a Pfannenstiel skin incision is made it is advisable to use the suprapubic V-shaped modification described by Turner-Warwick and colleagues (1974). Likewise, whatever incision is made, extra valuable access to the retropubic space is obtained by extending the division of the rectus muscles down to the pubic bone and elevating the aponeurotic insertion of the rectus muscle off the upper border of the pubic bone.
The retropubic space is then developed by teasing away the retropubic fat and underlying retropubic veins from the back of the pubic bone. The bladder neck, anterior vaginal wall, and urethra are then easy to identify, often facilitated by the presence of the Foley balloon. In patients who have had previous retropubic surgery, the dissection is performed sharply and it is important to take down all old retropubic adhesions, particularly in the presence of a prior failed repair. If difficulty is encountered in the identification of the bladder neck, the bladder may be partially filled or even opened to identify its limits and an examining finger in the vagina is invaluable in aiding the dissection (Symmonds, 1972; Gleason et al, 1976).
It is important to identify the lateral limits of the bladder as it reflects off the vaginal wall because only in this manner can one avoid inadvertent suturing of the bladder itself. Dissection over the bladder neck and urethra in the midline is to be avoided so as not to damage the intrinsic musculature. The lateral bladder wall may be “rolled off” medially and cephalad from the vaginal wall with a mounted swab and by use of countertraction with a finger in the vagina. In the author’s experience it is necessary to incise the endopelvic fascia. Occasional venous bleeding from the large vaginal veins is controlled by suture ligature, although it often resolves with tying of elevating sutures. To aid in the identification of the lateral margin of the bladder it is helpful to displace the balloon of the Foley catheter into the lateral recess, where it easily can be palpated through the bladder wall.
Suture Material
Absorbable sutures were used in the original descriptions of the MMK procedure (chromic catgut), Burch colposuspension (chromic catgut), and VOS procedure (polyglycolic acid or polydioxanone), whereas the original paravaginal repair used nonabsorbable sutures (silicon-coated Dacron). Fibrosis during subsequent healing is likely to be the most important factor in providing continued fixation of the perivaginal fascia to the suspension sites (Tanagho, 1996); nevertheless, some surgeons believe that a nonabsorbable suture material is better because of the risk of suture dissolution before the development of adequate fibrosis (Penson and Raz, 1996). Clearly, the choice of suspension suture material is a personal choice, but erosion of nonabsorbent sutures into the lumen of the bladder is a not uncommon complication and a not uncommon source of medical litigation (Woo et al, 1995).
Bladder Drainage
Some degree of immediate postoperative voiding difficulty can be expected after retropubic suspensions (Lose et al, 1987; Colombo et al, 1996a). Immediately postoperatively, bladder drainage may take the form of a urethral or a suprapubic catheter, generally based on the surgeon’s preference. A voiding trial is usually performed around the fifth day postoperatively. However, there is some evidence that a suprapubic catheter may be advantageous with respect to a lower incidence of asymptomatic and febrile urinary tract infection and earlier resumption of normal bladder function (Andersen et al, 1985; Bergman et al, 1987). In addition, the use of a suprapubic tube is generally more comfortable, allows the patient to participate in catheter management, and avoids the need for clean intermittent self-catheterization. Catheterization can be discontinued when efficient voiding has resumed, which is usually indicated by a postvoid residual urine volume either less than 100 mL or less than 30% of the functional bladder volume.
Drains
A tube drain may be placed in the retropubic space when there is concern about ongoing bleeding from perivaginal veins that may prove difficult to control with suture and electrocautery. Often, tying the suspension sutures is sufficient to stop this bleeding; but when the bleeding persists, drainage of the retropubic space is indicated. The drain is generally removed on the first to third day, when minimal output is noted.
Marshall-Marchetti-Krantz Procedure
Technique
Marshall, Marchetti, and Krantz in 1949 described a retropubic approach for the elevation and fixation of the anterolateral aspect of the urethra to the posterior aspect of the pubic symphysis and the adjacent periosteum. Technically, the original description of the MMK procedure reported a double suture bite of the paraurethral tissue included with the vaginal wall; this may be generically entitled a cystourethropexy. In 1949, Marshall and coworkers described their retropubic vesicourethral suspension in 50 patients; 38 of the patients had symptoms of SUI, 25 of whom had failed prior gynecologic operations for urinary incontinence. A simple suprapubic procedure was described by which the vesical outlet was suspended to the pubis (Marshall et al, 1949). In the original description, three pairs of sutures (taking double bites of tissue) were placed on each side of the urethra, incorporating full-thickness vaginal wall (excluding mucosa) and lateral urethral wall (excluding mucosa) (Marshall et al, 1949). Marchetti (1949) then modified the procedure to omit the tissue bite through the urethral wall because of concern about urethral injury. Apart from modifications in suture number and material over the years, the procedure remains the same today.
Cystourethropexy was often used as a secondary procedure for the resolution of persistent leaking after an anterior colporrhaphy. A cystourethropexy does not support the posterior wall of the urethra unless the sutures include the paraurethral vaginal wall, nor does it positively reduce an anterior vaginal wall prolapse in the same way that the true retropubic colposuspension procedures do. If there is a significant urinary residual after colporrhaphy with associated laxity of the anterior vagina wall, then with the descent, this applies traction to the posterior aspect of the bladder neck and tends to “tent” it open because the anterior aspect is tethered by sutures to the back of the pubis (see Fig. 71–1E). Sutures are placed on either side of the urethra (avoiding the urethral wall), taking bites through the paraurethral fascia and anterior vaginal wall (excluding mucosa). The most proximal sutures are placed at the level of the bladder neck. Each suture is then passed into an appropriate site in the cartilaginous portion of the symphysis (Fig. 71–3). However, the main technical problem relating to the MMK procedure is the difficulty of obtaining an adequately robust anchorage of the anterior wall of the urethra and the paraurethral fascia to the symphysis and the periosteum of the pubis where the suture bites are relatively insecure. As shown in Figure 71–3 these sutures can potentially either distort the bladder neck and impair sphincter function (see Fig. 71–3A) or obstruct the bladder neck (see Fig. 71–3B). All sutures are inserted; and while an assistant elevates the anterior vaginal wall, each suture is individually tied, starting with the more distal pair. The proximal, or bladder neck, suture frequently needs to be passed through the insertion of the rectus abdominis. Additional sutures may or may not be placed between the anterior bladder wall and the rectus muscles to pull the bladder farther anteriorly.
Results
Krantz described a personal series of 3861 cases with a follow-up of up to 31 years and a 96% subjective cure rate (Smith et al, 2005). Short- and medium-term results with the MMK procedure have been good. Mainprize and Drutz (1988) reviewed 58 articles (predominantly retrospective) published between 1951 and 1988 for treatment outcomes in 3238 cases. The cure rate, mostly based on subjective criteria, was 88%, with an improvement rate of 91%. Jarvis’s meta-analysis of studies in the literature (1994b) noted subjective continence in 88.2% (range, 72% to 100%) of 2460 patients with 1- to 72-month follow-up and objective continence in 89.6% (range, 71% to 100%) of 384 patients with 3- to 12-month follow-up. Whether the procedure was being done primarily or secondarily affected the outcome, with subjective continence in 92% if it was done primarily versus 84.5% if it was done secondarily. Longer-term data are limited in amount. McDuffie (1981) reported 75% success at 15 years. More recently, Clemens and coworkers (1998) noted subjective cure or improvement (SUI and urgency urinary incontinence) in only 41% of patients with a mean follow-up of 17 years, and Czaplicki and colleagues (1998) noted decreasing continence rates from 77% at 1 year to 57% at 5 years to 28% at 10 years, with a mean duration of continence of 78.5 months. There are significant limitations to the data since most series are retrospective, with preoperative assessment based mainly on the history and physical examination and few studies using objective data as outcome measures.
Complications occur in up to 21% of cases (Mainprize and Drutz, 1988), and the placement of sutures through the pubic symphysis incurs the risk of osteitis pubis, a potentially devastating complication of the MMK procedure that has been reported in 0.9% to 3.2% of patients (Lee at al, 1979; Mainprize and Drutz, 1988; Zorzos and Paterson, 1996). Patients usually present 1 to 8 weeks postoperatively with acute pubic pain radiating to the inner thighs, aggravated by moving. Physical examination reveals tenderness over the pubic symphysis, and radiography demonstrates haziness to the borders of the pubic symphysis and possibly lytic changes. Treatment is with bed rest, analgesics, and possibly corticosteroids (Lee at al, 1979). Other specific complications of the MMK procedure have included the occasional erosion of nonabsorbable cystourethropexy sutures into the bladder lumen with stone formation. Also, the positioning of sutures in the endopelvic fascia close to the bladder neck can result in a significant outlet obstruction.
Whereas the MMK procedure produces a cure rate similar to that of colposuspension, the complication of osteitis pubis means that there is little to support its use as an alternative to other colposuspension procedures. Indeed the ICI committee (Smith et al, 2009) concluded that although short-term results indicate comparable cure rates to colposuspension there is limited evidence that the longer-term outcome is poorer after MMK (level 1) and declines further over time (level 3). There is no evidence to support the continued use of MMK over colposuspension.
The recommendation from the ICI committee (Smith et al, 2009) is that the MMK procedure is not advised for the treatment of SUI (grade A).
Burch Colposuspension
Technique
Burch’s original description of the colposuspension in 1961 followed his original procedure, which was essentially a paravaginal repair attaching the paravaginal fascia to the white line of the pelvis, the arcus tendineus. The Burch colposuspension was a novel approach to restore the urethrovesical junction to a retropubic location by approximating the periurethral fascia to the tough bands of fibrous tissue running along the superior aspect of the pubic bone (Cooper [iliopectineal] ligament) with three pairs of sutures. The original Burch retropubic colposuspension is appropriate only if the patient has adequate vaginal mobility and capacity to allow the lateral vaginal fornices to be elevated toward and approximated to the Cooper ligament on either side. This technique has been modified. Tanagho’s modification (1978) approximated the vaginal wall to the lateral pelvic wall, with the sutures holding the anterior vaginal wall to the Cooper ligament, being tied loosely so that two fingers could be placed between the symphysis and urethra. This achieved broad support for the urethra and bladder neck and potentially minimized the risk of postoperative voiding dysfunction. A more recent modification (Shull and Baden, 1989; Turner-Warwick and Chapple, 2002) involves a hybrid approach whereby the vaginal tissues are approximated to the internal obturator fascia with an anchoring bite to the iliopectineal ligament (see VOS repair later).
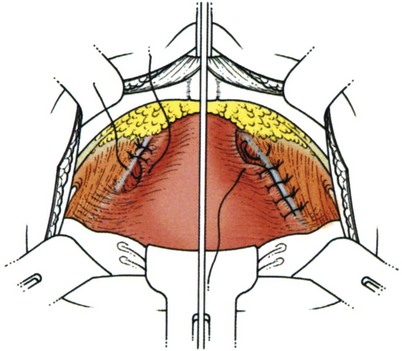
Suture placement is facilitated by the elevation of the dissected anterolateral vaginal wall into the field by the surgeon’s fingers inserted in the vagina (Fig. 71–4). The bladder is retracted to the opposite side with a mounted swab. Two to four sutures are placed on each side, each suture taking a good bite of fascia and vaginal wall, with care taken not to pass through the vaginal mucosa. Some recommend taking double bites of tissue to lessen the risk of suture pull-through (Jarvis, 1994a). The most distal suture is at the level of the bladder neck and placed no closer than 2 cm lateral to it, although some place distal sutures at the midurethral level (Tanagho, 1978). The suspension suture bites of the paraurethral fascia should not be positioned too close to the bladder neck and the urethra, as they are in the cystourethropexy procedures (MMK), because the unwanted effect of lateral traction-tension created by their anchorage to the iliopectineal ligaments may tether the sphincteric occlusive effect on the urethra or create a degree of obstructed voiding. Subsequent sutures are placed proximal to the level of the bladder neck, at about 1-cm intervals. The sutures are then placed into corresponding sites in the Cooper ligament, the emphasis being on a mediolateral direction for the sutures. The exact mechanism of continence of the Burch colposuspension is still unknown. Burch (1968) thought it to be secondary to elevation and stabilization of the bladder neck and urethra. In support of the suggestion regarding suture placement, Digesu and colleagues (2004) reviewed magnetic resonance imaging findings before and 1 year after open Burch colposuspension in 28 women to see if this would explain the mechanism. In the 86% who were cured the distance between the levator ani muscle and bladder neck was significantly shorter than in those whose treatment failed. Their suggestion is that insertion of sutures in a medial-lateral direction as opposed to an anteroposterior direction may better appose the levator ani muscle and bladder neck. The highly vascular vaginal wall may bleed profusely during suture placement, and large vaginal veins often need to be oversewn, but most bleeding ceases once the sutures are tied and the vagina is suspended. To facilitate tying of the sutures, the assistant elevates the appropriate portion of the vaginal wall as each suture is tied, beginning with the more distal pair.
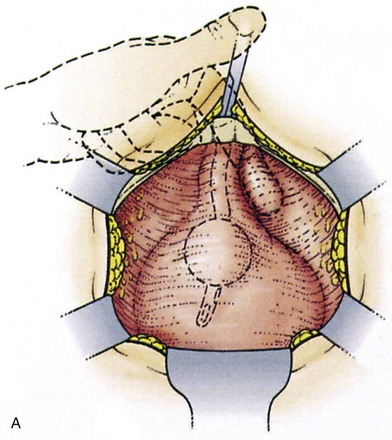
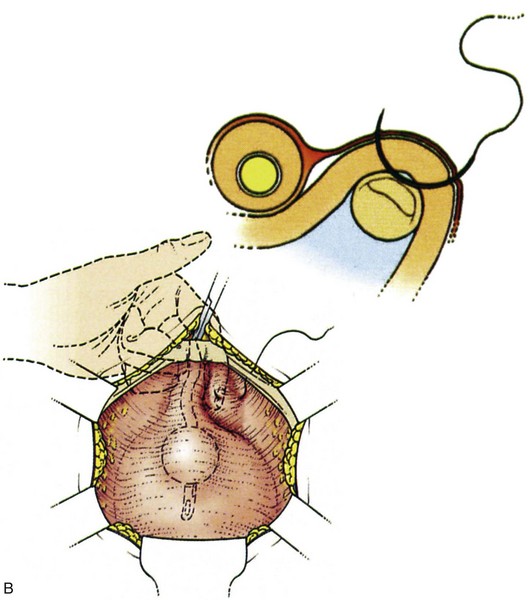
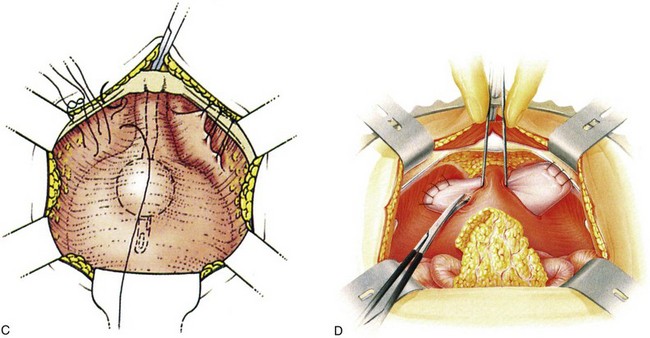
Figure 71–4 A, The use of a finger in the vagina when the pelvic tissues are dissected superomedially off of the vagina. B, Medial suture placement; note the role of the finger in steering the suture (inset). C, Note the mediolateral orientation of the sutures. D, Completion of a retropubic colposuspension with a vagino-obturator hitch on the left and a Burch colposuspension on the right.
No attempt should be made to tie the sutures tightly. Often, the vaginal wall does not approximate to the Cooper ligaments, and free suture material is seen between the vagina and the ligaments. The principle is to approximate the vaginal wall to the lateral pelvic wall, where it will heal and promote adhesion formation (Tanagho, 1978; Shull and Baden, 1989; Turner-Warwick and Chapple, 2002), thereby creating a broad support for the urethra and bladder neck.
Results
As in the MMK procedure, short- and medium-term outcomes with the Burch colposuspension have been good. In Jarvis’s meta-analysis (1994b), subjective continence was achieved in 91% (range, 63% to 97%) of more than 1300 patients with 3 to 72 months of follow-up and objective continence in 84% of more than 1700 patients with 1 to 60 months of follow-up. Lapitan and associates (2003) reviewed 33 trials involving a total of 2403 women who underwent open colposuspension and found an overall cure rate between 68.9% to 88.0%, with a 1-year cure rate of 85% to 90%. This decreased to 70% at 5 years. Although there may be a decline in the cure rate of only 15% to 20% beyond 5 years, Alcalay and colleagues (1995) noted a subjective and objective SUI cure rate of 69% with a mean follow-up of 13.8 years. Baessler and Stanton (2004) examined the impact of surgery on coital incontinence. Of the 30 women available for postoperative evaluation, 73% preoperatively had incontinence with penetration, 10% with orgasm only, and 17% with both. Postoperatively, 70% were cured of their coital incontinence. Moreover, in those who were subjectively cured of their stress incontinence, 87% were also cured of their coital incontinence.
Unlike with the MMK procedure, the good results with the Burch colposuspension appear to be durable with longer follow-up. Lapitan and associates (2003) reached the conclusion from a review of two trials comparing the Burch colposuspension and the MMK procedure that the Burch technique results in higher cure rates. Thus, it should be regarded as the standard open retropubic colposuspension procedure.
Open colposuspension is as effective as any other procedure in primary or secondary surgery at curing SUI with proven long-term success (level 1 evidence, grade A recommendation) (Smith et al, 2005).
Prophylactic Colposuspension
In 2006, the Colpopexy and Urinary Reduction Efforts trial demonstrated that the postoperative risk of stress incontinence in stress-continent women undergoing open abdominal sacrocolpopexy could be substantially reduced by the addition of a Burch colposuspension (Brubaker et al, 2006). Initial results at 3 months after the procedure demonstrated reduction of de novo stress incontinence from 44% in the untreated group to 24% in the Burch group, without increased rates of voiding dysfunction or urgency symptoms. Subsequent 1- and 2-year outcomes from this trial showed continued benefit in patients who received the concomitant Burch colposuspension (Burgio et al, 2007; Brubaker et al, 2008).
Reoperative Surgery
Poorer results are likely to occur when the procedure is performed secondarily. Scarring and fibrosis from previous surgery can prevent adequate suspension in some cases, and suture cut-through is more likely. Furthermore, after failed surgery, patients may often have coexisting sphincteric weakness that places them at greater risk of recurrence after colposuspension (Bowen et al, 1989; Koonings et al, 1990).
Nevertheless, Maher and colleagues (1999) and Cardozo and associates (1999) have both shown good objective (72% and 79%) and subjective (89% and 80%) success with repeated colposuspension at a mean follow-up of 9 months. Nitahara and coworkers (1999) reported a 69% subjective success at a mean follow-up of 6.9 years. Urgency incontinence and sphincteric weakness are the main causes of failure and dissatisfaction. Urgency incontinence accounted for 63% (12 of 19) of failures; the remaining 7 with persistent stress incontinence in Nitahara’s series demonstrated sphincteric deficiency with mean Valsalva leak point pressures of 65 cm H2O. The low-pressure urethra has often been quoted as an adverse risk factor for colposuspension (Haab et al, 1996; Bowen et al, 1989; Koonings et al, 1990), but this topic also remains controversial. Several authors have studied the urethral pressure profilometry changes after colposuspension and have noted a statistically significant increase in the postoperative pressure transmission ratio but minimal changes in the postoperative MUCP, functional urethral length, and continence area (Faysal et al, 1981; Weil et al, 1984; Feyersiel et al, 1994). Although a low-pressure urethra (MUCP ≤ 20 cm H2O) is considered a contraindication to the Burch procedure, a modification of the standard Burch operation has had some success in managing SUI associated with the low-pressure urethra. Bergman and colleagues (1989c) combined a standard Burch procedure with the Ball procedure (Ball, 1963) whereby, before the Cooper ligament suspension is performed, two or three sutures are used to plicate the anterior urethral wall at the level of the proximal and middle urethra. They retrospectively noted greater success with this technique than with a standard Burch procedure for the low-pressure urethra, and this was comparable to the success obtained with a standard Burch procedure in patients with a normal-pressure urethra (MUCP > 20 cm H2O). With longer follow-up, the Ball-Burch procedure continued to yield better results than the standard Burch procedure in patients with a low-pressure urethra, with a documented 5-year cure rate of SUI of 84% (Bergman et al, 1991; Elia and Bergman, 1995). However, there are no randomized studies addressing the issue, and whether these results can be extrapolated for the use of this technique in patients with intrinsic sphincter deficiency is debatable.
As with any major abdominal or pelvic surgical procedure, intraoperative and perioperative complications that may occur after a retropubic suspension include bleeding, injury to genitourinary organs (bladder, urethra, ureter), pulmonary atelectasis and infection, wound infection or dehiscence, abscess formation, and venous thrombosis or embolism. Other complications more specific to retropubic suspension procedures include postoperative voiding difficulty, detrusor overactivity, and vaginal prolapse. These are discussed in more detail along with other reported complications in a later section in this chapter.
The recommendations from the ICI committee (Smith et al, 2009) are as follows: Open retropubic colposuspension can be recommended as an effective treatment for primary SUI, which has longevity (grade A). Although open colposuspension has to a large extent been superseded by the less invasive midurethral tapes, it should still be considered for those women in whom an open abdominal procedure is required concurrently with surgery for SUI (grade D).
Paravaginal Repair
Technique
The origins of the paravaginal repair date to White (1912), who described the importance of the “white line” of the pelvis (arcus tendineus) as an integral structure supporting the proximal urethra and bladder base to the pelvic wall and the development of paravaginal fascial tears predisposing to cystocele formation. He performed the paravaginal repair by a vaginal approach but envisioned that it would be easier if it was performed abdominally (White, 1912). Later, in his original description, Burch (1961) attached the vaginal wall to the arcus tendineus in seven patients, only to realize that the attachment may not be secure, prompting him to use the Cooper ligament as an attachment site. In the 1970s, Richardson and coworkers (1976) reintroduced the concept of a lateral defect cystourethrocele as an etiologic factor in the genesis of SUI and popularized the paravaginal repair as a technique for management.
The patient is placed in a low lithotomy position, just as for the MMK procedure and Burch colposuspension. If there are retropubic adhesions resulting from prior surgery they are sharply incised; the dissection is facilitated by placement of two fingers of the surgeon’s left hand in the vagina. The bladder and urethra are not mobilized from the vaginal attachments. Richardson and colleagues (1981) describe an extensive reattachment of the lateral vaginal sulcus with its overlying fascia to the arcus tendineus fasciae pelvis from the back of the lower edge of the symphysis pubis to the ischial spine, using six to eight sutures placed at 1-cm intervals. The vaginal wall in the region of the bladder neck is identified, and these interrupted sutures are placed at approximately 1-cm intervals through the paravaginal fascia and vaginal wall (excluding vaginal mucosa) beginning at the urethrovesical junction. The sutures are then passed through the adjacent obturator fascia and underlying muscle at the site of the arcus tendineus fascia (Fig. 71–5). If the arcus is not visible, the obturator foramen may be used as a landmark. It is situated 1.5 to 2 cm above the white line.
The end point that should be achieved is the reestablishment of the urethral axis in an anatomic position, easily allowing three fingerbreadths between the pubic symphysis and the proximal urethra but providing secure fixation and preventing rotational descent. Consequently, it has been reported that postoperative voiding difficulties are uncommon (Richardson et al, 1981).
Results
Few reports on the use of this technique have been published. With variable follow-up, cure rates greater than 90% have been reported for the paravaginal repair (Richardson et al, 1981; Shull and Baden, 1989). There is only a single randomized comparison of colposuspension with paravaginal repair including 36 patients who were randomly allocated to treatment by either colposuspension or paravaginal repair with nonabsorbable suture material. At 6 months of follow-up there was an objective cure rate of 100% for those undergoing colposuspension and 72% for those undergoing paravaginal repair (Colombo et al, 1996a).
Small series have reported on vaginal approach paravaginal repairs (Scotti et al, 1998; Mallipeddi et al, 2001). In particular, Mallipeddi and colleagues observed 45 patients (21 with SUI) after this approach for a mean of 1.6 years, and 57% had persistent stress incontinence; their conclusion was that this technique had limited applicability for SUI. It can be concluded that there is level 1/2 evidence that abdominal paravaginal repair is less effective than colposuspension. There are limited data (level 3/4) on laparoscopic and vaginal paravaginal repairs, but interpretation of these data is hampered by the small numbers of patients, the short follow-up, and a combination of this procedure with other types of incontinence procedures (Smith et al, 2005).
There is limited evidence (level 2) that abdominal paravaginal defect repair is less effective than open colposuspension (Smith et al, 2009).
The recommendation from the ICI committee (Smith et al, 2009) is that paravaginal defect repair is not recommended for the treatment of SUI alone (grade A).
Vagino-Obturator Shelf Repair
Technique
Turner-Warwick (1986; Turner-Warwick and Chapple, 2002) reported his variant of the paravaginal repair, which he called the vagino-obturator shelf (VOS) repair. The premise for this is that there should be no restriction to the intrinsic sphincteric function of the urethra by fixation or paraurethral tethering, and there should be no urethral compression (Turner-Warwick and Kirby, 1993).
Just as for other retropubic suspension procedures (Fig. 71–6), the anterior wall of the lower segment of the bladder wall is exposed and the position of the bladder neck is identified by gentle traction on a balloon catheter in the urethra. The surgeon’s forefinger in the vagina elevates its anterior wall and the overlying endopelvic fascia on either side of the urethra. Lateral displacement of the catheter balloon with the finger in the vagina facilitates the identification of the inferolateral margin of the bladder, lateral to the bladder neck; and its separation from the paraurethral endopelvic fascia is achieved by simple blunt dissection with a sponge or scissor-tip retraction. This naturally exposes the surface of the obturator muscle and the arcus tendineus origin of the levator muscles deep in the sulcus below this, the site of suture placement in the paravaginal repair (which is well below the vaginal anchorage point in the VOS procedure). The obturator nerves lie superolaterally; their canals run high up in a groove under the superior pubic ramus so that once they are identified, injury to them can be avoided. The full thickness of the vagina and its overlying layer of endopelvic fascia are elevated by the surgeon’s finger in the vagina and are approximated to the internal obturator muscle and anchored to the bulk of this with absorbable 0 or 1 sutures mounted on robust 35- to 40-mm half-circle needles. Slight flexion of the fingertip tents the vaginal wall and facilitates the full-thickness insertion of these suture bites through it, thus avoiding inclusion of the surgical glove. Three or four successive sutures are inserted. For knot security these are best tied continuously, head to tail, as they are inserted, rather than separating them individually. Each tied suture bite facilitates the insertion of the next (unlike in the Burch procedure, in which the sutures are not tied until they have all been inserted). A similar obturator suspension of the elevated protrusion of the vagina and its overlying endopelvic fascia is achieved on the opposite side. Some additional elevation of the lateral anchorage of the VOS by the inclusion of a bite of the iliopectineal ligament within the suture bite approximating the vagina to the obturator muscle (like a Burch procedure) may be used to reinforce the repair (Shull and Baden, 1989). This modification is one the author favors and represents a hybrid with the Burch procedure, facilitating reattachment of the pubocervical fascia to the arcus tendineus fasciae pelvis, tissue apposition to the lateral pelvic wall, and nonobstructive elevation of the urethra and urethrovesical junction. In addition, obliteration of the pouch of Douglas (culdoplasty) may be needed to prevent enterocele (Shull and Baden, 1989; Turner-Warwick and Kirby, 1993).
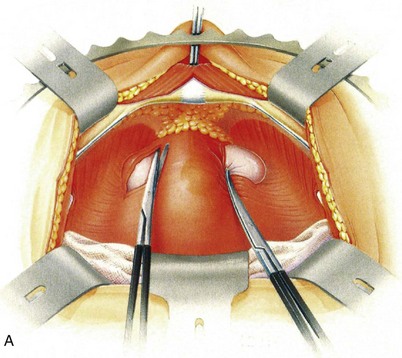
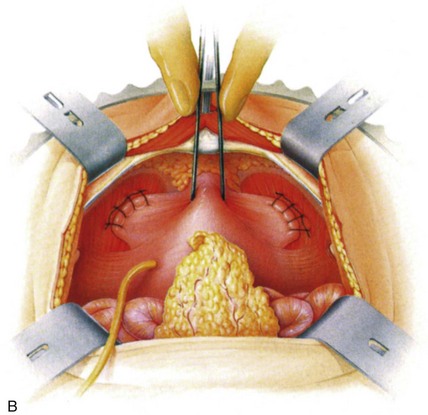
Figure 71–6 A, Mobilizing the tissues. The tissues should be stripped by blunt dissection with a mounted swab or the edge of a pair of scissors; only occasionally is sharp dissection necessary, provided the stripping action starts far laterally adjacent to the pubic bone. B, Completed vagino-obturator shelf procedure.
Results
There are limited data available for the VOS repair, which has had reported cure rates of 60% to 86%, depending on whether the procedure was performed primarily or secondarily (Turner-Warwick, 1986; German et al, 1994). German and colleagues (1994) reported that the VOS procedure is less likely to be successful in those patients who had undergone previous surgery.
Ultimately, as with all such reconstructive surgery, the surgeon should select the correct procedure for the individual patient. Although the VOS, which is a synthesis of the principles of the paravaginal repair and the Burch colposuspension, is of interest, further clinical results are necessary before definitive conclusions can be drawn.
Laparoscopic Retropubic Suspension
Laparoscopic techniques for retropubic suspension were introduced by Vancaillie and Schuessler in 1991. They essentially performed an MMK urethropexy laparoscopically and, since that time, laparoscopic techniques have been applied to both the Burch procedure and the paravaginal repair. Subsequent modifications to the suspension suturing techniques have been introduced, including the use of mesh (Ou et al, 1993), staples (Lyons, 1994), and fibrin sealant (Kiilholma et al, 1995), but all adhere to the same principles of their open counterparts. Proposed advantages to the laparoscopic approach include improved intraoperative visualization, less postoperative pain, shorter hospitalization, and quicker recovery times (Liu, 1993). Disadvantages include greater technical difficulty with resultant longer operating times and higher operating costs (Paraiso et al, 1999).
The procedure may be performed extraperitoneally or transperitoneally, and each approach has its proponents. Although the extraperitoneal technique may be associated with shorter operating times, easier dissection, and fewer bladder injuries (Frankel and Kantipong, 1993; Raboy et al, 1995), the transperitoneal approach provides a larger operating space and the ability to perform concomitant intraperitoneal procedures and apical prolapse repair (Paraiso et al, 1999). The specifics of the different procedures are beyond the scope of this chapter.
Short- and medium-term outcomes with the laparoscopic retropubic suspensions have become available. In their review of 13 studies of laparoscopic retropubic suspensions, Paraiso and colleagues (1999) found cure rates to range from 69% to 100% with follow-up of 1 to 36 months. This is comparable to outcomes of the open procedures as already noted. Both retrospective (Polascik et al, 1995) and randomized, prospective comparisons (Summitt et al, 2000) between open and laparoscopic techniques have demonstrated similar short-term success. However, with longer follow-up, laparoscopic retropubic suspensions appear to fail more frequently. McDougall’s group (1999) retrospectively noted only 30% cure of SUI and 50% cure or improvement by a laparoscopic Burch procedure with 45 months of follow-up, and this was no different from the results with a Raz procedure.
Five trials summarized in a Cochrane review have compared laparoscopic with open colposuspension (Burton, 1997, 1999; Su et al, 1997; Carey et al, 2000; Summitt et al, 2000; Fatthy et al, 2001; Moehrer et al, 2002). All had different lengths of follow-up: 6 months (Carey et al, 2000); 1 year (Su et al, 1997; Summitt et al, 2000); 6 and 18 months (Fatthy et al, 2001); and 6 months, 1 year, 3 years, and 5 years (Burton, 1997, 1999). Outcome data for 6 months to 18 months were therefore available for all studies. Longer-term data are currently available only for Burton’s study. The ability to synthesize data was also limited by the variable tests and definitions used to measure subjective and objective outcomes across the trials (Moehrer et al, 2003). Moehrer and colleagues, in their meta-analysis, noted that a total of 233 women received a laparoscopic and 254 women an open colposuspension, and the confidence intervals are generally all wide as a consequence. Four trials comparing laparoscopic with open colposuspension were otherwise of good quality (Burton, 1997, 1999; Carey et al, 2000; Summitt et al, 2000; Fatthy et al, 2001). Burton’s study had the potentially confounding factors of use of absorbable sutures and of the surgeons having performed only a relatively small number of laparoscopic colposuspensions (<20) before beginning the trial. These factors may have influenced his results, in particular since there is believed to be a definite albeit relatively steep learning curve associated with laparoscopic colposuspensions. The fifth trial had methodologic problems with corrupted randomization and confounding factors of performance of additional surgery in some patients and the use of a different number of sutures for laparoscopic (one suture) and open (three sutures) colposuspension (Su et al, 1997). The number of sutures used appears to have a significant influence on the cure rate, with more sutures resulting in a significantly higher success rate. Persson and Wolner-Hanssen (2000) compared different numbers of paravaginal sutures and found a significantly higher objective 1-year cure rate (dry on “ultrashort” pad test) for women randomized to two sutures compared with one suture, with a cure rate of 83% for two sutures and 58% for one suture. Only one trial currently has data beyond 18 months of follow-up (Burton, 1997, 1999). This suggested poorer long-term results after laparoscopic surgery. This finding should be interpreted cautiously, however, because there are concerns that the surgeon’s laparoscopic performance may have been suboptimal because he had performed few laparoscopic colposuspensions when the trial started. Data from other larger trials with multiple operators are now needed to assess whether this is a real effect. All the other trials had data up to a maximum of 18 months. These show some inconsistencies. Outcome assessed by the women participating (arguably the most important outcome) appeared equally good in the two groups. Urodynamic investigations were used to assess cure objectively in all five studies. Overall, there was a significantly higher success rate after open colposuspension (RR, 0.89; 95% CI, 0.82 to 0.98) equivalent to an absolute difference of an additional 9% risk of failure after laparoscopic surgery. No significant differences between the two groups were observed for postoperative urgency, voiding dysfunction, or de novo detrusor overactivity. A trend was shown toward a higher complication rate, less postoperative pain, shorter hospital stay, and more rapid return to normal function for laparoscopic colposuspension. The operating time tended to be longer, the intraoperative blood loss less, and the duration of catheterization shorter for laparoscopic compared with open colposuspension (Moehrer et al, 2003).
In a previous review of laparoscopic colposuspension, Paraiso and colleagues (1999) noted major intraoperative and short-term complications in up to 25% of cases, with bladder injury being the most common complication and declining with experience; ureteric injury has also been reported (Aslan and Woo, 1997). The use of mesh and tacks or staples may be complicated by foreign body erosion (Arunkalaivanan and Arunkalaivanan, 2002; Kenton et al, 2002), and in a randomized study (Ankardal et al, 2004) it was reported that the use of sutures was superior to the laparoscopic mesh and staple technique.
A previous Cochrane review (Moehrer et al, 2002) was dependent on small trials, recruiting a total of 487 women between them, with mostly only medium-term follow-up data (18 months) and limited evidence from other small studies and concluded that laparoscopic colposuspension had the benefit of a more rapid recovery but might have a higher complication rate and be more expensive and possibly less effective in the long term than open colposuspension. This highlighted the need for further well-designed clinical studies.
Carey and colleagues (2006) have reported a randomized trial that recruited 200 women during 1997 and 1998, with 2-year follow-up available for 83% of participants and longer-term subjective data from a similar proportion. The primary outcome measure was objective cure (absence of urodynamic stress incontinence) at 6 months, powered to detect a difference of 20% between the two study arms. Secondary measures included patient satisfaction, quality of life and general well-being, and complications. In the same year Kitchener and coworkers (2006) reported the results of the COLPO trial, which recruited 291 women between 1999 and 2001, with a 2-year follow-up rate of more than 80%. The primary outcomes were objective (dry pad test) and subjective (symptom report) cure at 24 months. Secondary outcomes included recovery time, complications, and a formal cost-benefit analysis. The trial was powered to show noninferiority of laparoscopic colposuspension to open colposuspension, assuming a cure rate of 80% for the open procedure. Both studies were well designed, well conducted, and clearly reported.
Carey and coworkers found no difference in urodynamic cure rate, incidence of detrusor overactivity, or patient satisfaction at 6 months, with an overall objective cure rate of 75%. Detrusor overactivity occurred in 12% of women; 66% of women were urodynamically “normal.” If the raw data are converted to percentage scores, there was 89% satisfaction with the treatment outcomes and 88% satisfaction with the care received. The overall satisfaction was 87%. At 24 months after surgery there were no significant differences between the two treatment groups with respect to reporting SUI, urgency, and urgency incontinence and a satisfaction score of greater than or equal to 80. Across both treatment groups, by 24 months after surgery, 66% of women reported no SUI, 38% reported no urgency, 47% reported no urgency incontinence, and 64% reported a satisfaction score of greater than or equal to 80. Although follow-up information was only available on approximately 80% of all subjects by 24 months, a sensitivity analysis was performed assuming that all women who did not complete the 24-month follow-up had either occasional or frequent SUI. With these assumptions, cure rates decreased to 61% for open colposuspension and 50% for laparoscopic colposuspension. There was no significant difference between the two groups treatment groups, even when adjusted for surgeon experience (P = .08). The study population was contacted by telephone for further follow-up at a mean of 3.7 years (range 3 to 5 years) after surgery. This follow-up was undertaken at a single point in time. A total of 164 women were contacted, 88 of whom underwent open colposuspension and 76 of whom underwent laparoscopic colposuspension. There were no significant differences between the two treatment groups at 3 to 5 years after surgery and the findings were similar to the 24-month data. Mean operating time was approximately twice as long for laparoscopic colposuspension, but surgeon’s estimates of blood loss and patient’s estimates of immediate postoperative pain at rest were significantly less after the laparoscopic procedure with a return to normal activities, on average, 5 days earlier (P = .01).
Kitchener and colleagues also found no difference in the objective cure rate (79% for laparoscopic vs. 70% for open) or subjective cure rate (55% vs. 54%) between the study arms, again showing the now well-recognized mismatch between objective and subjective outcomes. The intention-to-treat analysis indicated no significant difference in cure rates between open and laparoscopic surgery. The study was slightly underpowered based on the sample size calculations, but the analyses clearly demonstrated that laparoscopic colposuspension is not inferior to open colposuspension. The complication rate was low in both arms, with more bowel and bladder injuries in the laparoscopic arm and more wound infections in the open arm. Each demonstrated comparable improvements in generic quality-of-life scores. Other points of note include the careful selection of surgeons who had experience of both open and laparoscopic surgery and interestingly as contrasted to the Carey study contrary to the previously held beliefs of laparoscopic surgery is that it would be associated with longer operating times, less postoperative pain, and shorter hospital stay. For hospital stay there was only a small advantage for laparoscopic surgery, with a median stay of 5 days compared with 6 days for open surgery. The operating times were fairly similar in terms of “knife to skin” to completion of surgery, there being a median time of 65 and 51 minutes for laparoscopic and open surgery, respectively, but postoperative pain was significantly less in the laparoscopic surgery group. But there were no differences in time of return to work identified in this study. Complications of surgery were low generally, with a higher bladder injury rates in the laparoscopic procedure and a higher wound infection with open surgery as would be expected. The impact of these differences was analyzed in a cost-benefit analysis accompanying this study that suggested that a greater quality-adjusted life year total is achieved in the laparoscopic arm at both 6 and 24 months, suggesting that laparoscopic surgery may confer an additional benefit of well-being (Manca et al, 2006). But the additional costs of laparoscopic surgery were only recouped after 24 months of follow-up, thus highlighting the need to address long-term outcomes in any surgical study.
The last major publication in this field was a meta-analysis of all of the comparative studies published between 1995 and 2006 of laparoscopic versus open colposuspension (Tan et al, 2007). End points evaluated were operative outcomes and subjective/objective cure. A random-effect model was used and sensitivity analysis performed to account for bias in patient selection. Sixteen studies matched the selection criteria, reporting on 1807 patients, of whom 861 (47.6%) underwent laparoscopic and 946 (52.4%) underwent open colposuspension. Length of hospital stay and return to normal life were significantly reduced after laparoscopic surgery. These findings remained consistent on sensitivity analysis. Bladder injuries occurred more often in the laparoscopic group, but only with marginal statistical significance. Comparable bladder injury rates were found when studies were matched for quality, year, and randomized trials. Cure rates were similar between the two procedures at 2 years follow-up.
The current evidence would suggest that in adequately experienced hands there is no difference in overall safety and efficacy between laparoscopic and open colposuspension. Clearly another concern is how generalizable the data are on laparoscopic colposuspension because the majority of reported studies are from expert laparoscopists or surgeons working in specialized units. The evidence base on both laparoscopic and open colposuspension is limited by relatively short-term follow-up (robust data are needed out to 5 years) and the tendency toward small numbers, and poor methodology limits the interpretation of most studies with the exception of those reported by Carey and coworkers (2006) and Kitchener and colleagues (2006). Laparoscopic colposuspension shows comparable subjective outcome, but poorer objective outcome than both open colposuspension and tension-free vaginal tape procedure in the short to medium term; longer term outcomes are unknown (level 2). It may not be good value for money when compared with open colposuspension in the short term (i.e., first 6 months after surgery), but it could be a cost-effective alternative over 24 months (level 1); other comparisons, however, suggest that minimally invasive midurethral tape procedures may be dominant in health economic terms.
Recommendations from the ICI committee (Smith et al, 2009) include the following:
Complications of Retropubic Repairs
As with any major abdominal or pelvic surgical procedure, intraoperative and perioperative complications that may occur after a retropubic suspension include bleeding, injury to genitourinary organs (bladder, urethra, ureter), pulmonary atelectasis and infection, wound infection or dehiscence, abscess formation, and venous thrombosis or embolism. Other common complications more specific to retropubic suspension procedures include postoperative voiding difficulty, detrusor overactivity, and vaginal prolapse. Potentially, it is overcorrection of the urethrovesical angle that may be a major contributory factor in the development of the long-term complications of de novo urgency, voiding dysfunction, and enterocele formation.
Nevertheless, the reported incidence of these problems is relatively low. In their meta-analysis, Leach and associates (1997) noted a 3% to 8% transfusion rate for retropubic suspensions and no significant difference in the overall medical and surgical complication rates between retropubic suspensions, needle suspensions, anterior colporrhaphy, and pubovaginal slings. Mainprize and Drutz (1988), in their review of the MMK procedure literature (2712 patients), noted an overall complication rate of 21%, with wound complications and urinary infections making up the majority (5.5% and 3.9%, respectively). Direct surgical injury to the urinary tract occurred in only 1.6%, and genitourinary tract fistulas occurred in 0.3%. Ureteral obstruction has been reported rarely after Burch colposuspension, and it usually results from ureteral kinking after elevation of the vagina and bladder base, although direct suture ligation of the ureter can occur (Applegate et al, 1987). If it is identified intraoperatively, it is best remedied by removal of the offending ligature and temporary placement of a ureteral stent. The so-called post-colposuspension syndrome, which has been described as pain in one or both groins at the site of suspension, has been noted in up to 12% of patients after a Burch procedure (Galloway et al, 1987). More recently, Demirci and colleagues (2001, 2002) reported the occurrence of groin or suprapubic pain in 15 of 220 women (6.8%) after Burch colposuspension with a follow-up of 4.5 years.
Postoperative Voiding Difficulty
Postoperative voiding difficulty after any type of retropubic suspension is not uncommon, and undoubtedly its occurrence is more likely if there is preexisting detrusor dysfunction or denervation resulting from extensive perivesical dissection. In most cases, however, it is the result of overcorrection of the urethral axis from inappropriately placed or excessively tightened sutures. If the sutures are placed too medially, they may also transfix the urethra or distort it.
Preoperatively, at-risk patients may be identified by their history of prior voiding dysfunction or episodes of urinary retention. These women should be carefully counseled preoperatively about the potential for postoperative voiding difficulty and the possible need for self-catheterization intermittently. Their incontinence should be of sufficient magnitude that its correction offsets the risk of the need for self-catheterization.
Women with postcystourethropexy voiding problems who have obstruction often do not exhibit the classic urodynamic features of obstruction. However, the history of postoperative voiding symptoms and associated new-onset bladder storage symptoms and a finding of a retropubically angulated and fixed urethra generally indicate that obstruction does exist (Carr and Webster, 1997). In such cases, revision of the retropubic suspension by releasing the urethra into a more anatomic position resolves voiding symptoms in up to 90% of cases (Webster and Kreder, 1990; Nitti and Raz, 1994; Carr and Webster, 1997).
The meta-analysis by Leach and coworkers (1997) noted that the risk of temporary urinary retention lasting more than 4 weeks postoperatively is 5% for all retropubic suspensions, and the risk for permanent retention is estimated to be less than 5%. These risks are not significantly different from those for needle suspensions or pubovaginal slings. Mainprize and Drutz’s review of the literature (1988) yielded a 3.6% incidence of postoperative voiding problems after an MMK procedure, whereas the Burch procedure literature reports an incidence of postoperative voiding disorders ranging from 3% to 32% (Hilton and Stanton, 1983; Galloway et al, 1987; Eriksen et al, 1990; Alcalay et al, 1995; Colombo et al, 1996a). In more recent literature, voiding dysfunction may be persistent, as noted in 3.5% of a series of 310 women with a mean follow-up of 36 months (Viereck et al, 2004). Nonpersistent voiding dysfunction has been reported in 12.5% (6% to 37.2%) after primary surgery (Smith et al, 2005). After colposuspension conducted as a secondary procedure, Bidmead and associates (2001) reported voiding difficulties requiring intermittent self-catheterization in 6% of cases.
Because the paravaginal repair aims to restore normal anatomy there is theoretically little chance of overcorrection of the urethral axis, which should translate into a lower risk of postoperative obstruction. In Richardson and coworkers’ study (1981), 80% of patients were able to void immediately after paravaginal repair, and “all patients had satisfactory bladder function at the time of discharge.” However, temporary voiding difficulty has been noted in up to 17% of patients after a VOS procedure (German et al, 1994), and chronic (>2 years) voiding difficulty has been noted in up to 11% of patients after the paravaginal repair (Colombo et al, 1996a).
All patients should be counseled before surgery about the potential need for intermittent self-catheterization.
Bladder Overactivity
Bladder overactivity commonly accompanies anatomic SUI, and its incidence preoperatively has been reported to be as high as 30% in patients undergoing either first correction or repeated operations (McGuire, 1981). Provided it is considered as a diagnosis, urodynamic evaluation is performed to show whether detrusor overactivity is present, an attempt at treatment of the related overactive bladder symptoms has been made (with or without success), and the patient has been advised that the presence of detrusor overactivity will increase the risk of continuing storage symptoms postoperatively, then preoperative bladder overactivity does not contraindicate a retropubic suspension procedure, provided that anatomic SUI has also been demonstrated. In the majority of cases, the bladder overactivity symptoms resolve after surgical repair (McGuire, 1988). Leach and coworkers’ meta-analysis (1997) found the risk of urgency after a retropubic suspension to be 66% if urgency and detrusor overactivity were present preoperatively, 36% if there was urgency but no documented overactivity preoperatively, and only 11% if there was neither urgency nor overactivity preoperatively. There was no significant difference in the incidence of postoperative urgency between retropubic suspensions, needle suspensions, and pubovaginal slings. Postoperative urgency was noted in only 0.9% of MMK procedures in Mainprize and Drutz’s meta-analysis of 15 series (1988), although Parnell and associates (1982) reported that 28.5% of their patients developed postoperative storage symptoms. Jarvis’s meta-analysis (1994b) of Burch procedures found the incidence of de novo bladder overactivity to be 3.4% to 18%. More recently, Smith and colleagues (2005) quoted a figure for postoperative detrusor overactivity of 6.6% for colposuspension (range, 1.0% to 16.6%), whereas the incidence of postoperative urgency or of urgency incontinence after the paravaginal/VOS repair has been reported to be 0% to 6% (Shull and Baden, 1989; German et al, 1994; Colombo et al, 1996a).
For those patients in whom postoperative storage symptoms persist, proven to be associated with detrusor overactivity and intractable to management with anticholinergic therapy and behavioral modification, surgical techniques including intravesical botulinum toxin therapy, neuromodulation, augmentation cystoplasty, or detrusor myectomy may be indicated.
Bladder storage symptoms arising de novo after retropubic suspension may be associated with bladder outlet obstruction. This premise is supported by the frequent coexistence of these symptoms with impaired voiding after suspension procedures and confirmed by the finding that urethrolysis, by freeing the urethra from an obstructed position, often resolves both storage and voiding symptoms (Raz, 1981; Webster and Kreder, 1990).
Vaginal Prolapse
Retropubic suspensions alter vaginal and bladder base anatomy, and thus postoperative vaginal prolapse is a potential complication. Genitourinary prolapse has been reported as a sequel to Burch colposuspension in 22.1% of women (range, 9.5% to 38.2%) by Smith and colleagues (2005) in their review of the literature. The Burch colposuspension, because of lateral vaginal elevation, may aggravate posterior vaginal wall weakness, predisposing to enterocele. The incidence varies between 3% and 17% (Burch, 1961, 1968; Galloway et al, 1987; Wiskind et al, 1992); because of this, prophylactic obliteration of the cul-de-sac of Douglas is sometimes considered in performing retropubic suspensions (Shull and Baden, 1989; Turner-Warwick and Kirby, 1993). However, simultaneous hysterectomy is not recommended prophylactically because it does not enhance the outcome of a retropubic suspension and should be performed only if there is concomitant uterine disease (Milani et al, 1985; Langer et al, 1988). Although the Burch colposuspension and paravaginal/VOS repair both correct lateral defect cystourethroceles, recurrent cystourethroceles were noted in 11% and 39% of Burch colposuspensions and paravaginal repairs, respectively (Colombo et al, 1996a). In Mainprize and Drutz’s review (1988), postoperative cystocele was noted in only 0.4% of patients after an MMK procedure.
Wiskind and coworkers (1992) noted that 27% of patients who had undergone a Burch colposuspension developed prolapse requiring surgery: rectocele in 22%, enterocele in 11%, uterine prolapse in 13%, and cystocele in 2%. More recently, it has been suggested that most women are asymptomatic, and less than 5% have been reported to request further surgery (Smith et al, 2005). Ward and Hilton (2004) reported that 4.8% of women needed a posterior repair, whereas Kwon and associates (2003) reported that 4.7% required subsequent pelvic reconstruction.
Auwad and colleagues (2006) have reported the findings of the first prospective study to determine the prevalence of pelvic organ prolapse (POP) after colposuspension and to investigate possible preoperative and operative risk factors. Seventy-seven women who underwent colposuspension between 1996 and 1997 were investigated. POP was assessed before colposuspension using the pelvic organ prolapse quantification (POP-Q) system (Bump et al, 1988). Women were reassessed at 1 and at 7 to 8 years (or when referred with symptomatic POP). By 7 to 8 years, of the 77 women, 29 (38%) had developed symptomatic prolapse, 29 (38%) had asymptomatic prolapse, 7 (9%) had no symptoms and no prolapse, and 12 (15%) could not be assessed. POP at 1 year was significantly associated with the presence of posterior vaginal descent before colposuspension (odds ratio 3.07, 95% CI 1.10 to 8.60, P = .03). No potentially predisposing variable reached statistical significance by 8 years after colposuspension. The results add support to the view that there is an association between colposuspension and the development of symptomatic POP (requiring surgery).
Because retropubic suspensions are unable to correct central defect cystoceles, patients must be carefully examined preoperatively to exclude their presence and must be counseled if undergoing a colposuspension of the high risk of needing further surgery in the long term. Particularly those in whom a weakness of the posterior compartment is identified preoperatively and with a past history of hysterectomy might be at increased risk.
Comparisons between Incontinence Procedures
Retropubic Repair versus Needle Suspension and Anterior Repair
Three articles that reviewed the literature on incontinence procedures all found retropubic suspensions to be more effective than either needle suspensions or anterior colporrhaphies (Jarvis, 1994b; Black and Downs, 1996; Leach et al, 1997). Cure rates were approximately 85% for the retropubic suspensions compared with 50% to 70% for the needle suspensions and anterior colporrhaphies. Results were more durable for the retropubic suspensions and better if the procedure was primary.
Overall therefore there is now high-level evidence that indicates that needle suspension procedures are as effective as anterior colporrhaphy but less effective than colposuspension even in the short-term (level 1). Long-term studies indicate lack of longevity even of the initial modest results; long-term complications remain a concern (level 3) (Smith et al, 2009).
The recommendation from the ICI committee (Smith et al, 2009) is that endoscopic and nonendoscopic bladder neck needle suspension procedures, with and without bone anchors, are not recommended for the treatment of SUI (grade A).
Retropubic Repair versus Pubovaginal Sling
Most studies in the historical literature have not demonstrated a significant difference in cure rates between retropubic suspensions (generally a Burch colposuspension) and pubovaginal slings (Jarvis, 1994b; Black and Downs, 1996; Leach et al, 1997). However, often, selection bias exists in that the pubovaginal sling is generally reserved for patients with multiple prior failed incontinence procedures and with less prolapse, and the presence of presumed intrinsic sphincter deficiency (a fixed urethra with periurethral fibrosis) is often used in clinical practice as a contraindication to a retropubic suspension. In an interesting randomized study in patients with a prior failed incontinence procedure (anterior repair) but without a low-pressure urethra (i.e., MUCP > 20 cm H2O), Enzelsberger’s group (1996) found no significant difference in cure (subjective or objective) at 32 to 48 months between the Burch colposuspension and the Lyodura sling. However, they noted significantly more postoperative voiding difficulty with the pubovaginal sling (13% vs. 3%) and more vaginal prolapse (enterocele or rectocele) with the Burch colposuspension (13% vs. 3%).
A recent high-quality multicenter randomized clinical trial in women with stress incontinence compared the Burch colposuspension with a pubovaginal sling using autologous rectus fascia (Aldo et al, 2007). Women were eligible for the study if they had predominant symptoms associated with the condition, a positive stress test, and urethral hypermobility. The primary outcomes were success in terms of overall urinary incontinence measures, which required a negative pad test, no urinary incontinence (as recorded in a 3-day diary), a negative cough and Valsalva stress test, no self-reported symptoms, no re-treatment for the condition, and success in terms of specific measures of stress incontinence as well as an assessment of postoperative urgency incontinence, voiding dysfunction, and adverse events. A noteworthy aspect of the study was the careful approach to standardization (using the recommendations from the standardization committees of the ICI with regard to clinical terms, urodynamic nomenclature, and methods of evaluation of patients across all sites. Key elements of the two surgical procedures were standardized among all participating surgeons and included the use of preoperative antibiotics, skin-incision length, number and type of Burch sutures, fascial-sling length and width, and cystoscopic evaluation of the bladder. A criticism that can be leveled at the study is the choice of technique for the Burch colposuspension with very medial paraurethral sutures. The techniques utilized are demonstrated in Figure 71–7.
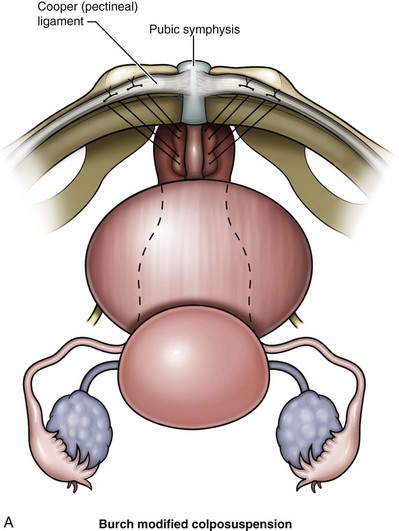
Figure 71–7 A, In the Burch colposuspension, sutures are placed in the anterior vaginal wall at the level of the bladder neck and proximal urethra and sutured to the iliopectineal ligament. B, In the autologous sling procedure a strip of rectus fascia is harvested and permanent sutures placed at both ends. The sling is positioned beneath the urethra via a vaginal incision. The two ends of the sling are then secured to the anterior abdominal wall, either together or to the rectus fascia.
(Modified from Aldo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med 2007;356:2143–55.)
Because these procedures are frequently performed in conjunction with surgery for pelvic prolapse, abdominal and vaginal approaches for both pelvic prolapse repair and hysterectomy were permitted; however, surgeons were required to declare before randomization which concomitant procedures would be performed.
A total of 655 women were randomly assigned to study groups: 326 to undergo the sling procedure and 329 to undergo the Burch procedure; 520 women (79%) completed the outcome assessment. At 24 months, success rates were higher for women who underwent the sling procedure than for those who underwent the Burch procedure, for both the overall category of success (47% vs. 38%, P = .01) and the category specific to stress incontinence (66% vs. 49%, P < .001). There was no significant difference between the sling and Burch groups in the percentage of patients who had serious adverse events (13% and 10%, respectively; P = .20). However, more women who underwent the sling procedure had adverse events than in the Burch group, with 415 events among 206 women in the sling group as compared with 305 events among 156 women in the Burch group. This difference was due primarily to urinary tract infections; 157 women in the sling group (48%) had 305 events and 105 women in the Burch group (32%) had 203 events. When urinary tract infections were excluded, the rates of adverse events were similar in the two groups.
The distribution of time to return to normal voiding differed significantly between the two groups (P < .001). Voiding dysfunction was more common in the sling group than in the Burch group (14% vs. 2%, P < .001). Consequently, surgical procedures to reduce voiding symptoms or improve urinary retention were performed exclusively in the sling group, in which 19 patients underwent 20 such procedures (63% vs. 47%, P < .001). Treatment satisfaction rates for the 480 subjects who answered the satisfaction question at 24 months were significantly higher in the sling group than in the Burch group (86% vs. 78%, P = .02).
A further analysis of this study focused on sexual activity as assessed by the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) among those sexually active at baseline and 2 years after surgery (Brubaker et al, 2009). This report demonstrated that sexual function improves after successful surgery and does not differ between the Burch and sling operations.
It therefore can be reliably concluded that in specialist centers working in a standardized fashion, the autologous fascial sling results in a higher rate of successful treatment of stress incontinence but also greater morbidity than the Burch colposuspension.
Burch Colposuspension versus Marshall-Marchetti-Krantz Procedure versus Paravaginal Repair
Comparisons between the MMK and the Burch procedures have generally yielded similar results. Jarvis, in his meta-analysis of the literature (1994b), noted that overall continence rates were 89.6% and 83.9% for the MMK and the Burch procedures, respectively. When he looked at the effect of prior incontinence surgery, the continence rates were 92.1% and 94% when there was no prior surgery and 84.5% and 84% when there was a history of prior incontinence surgery for the MMK and Burch procedures, respectively. Similarly, Black and Downs (1996), in reviewing five studies (one randomized) that directly compared the MMK procedure with the Burch procedure, found that there was no significant difference in cure rates between the two procedures, although the Burch procedure generally yielded better results. However, they pointed out that overall the studies were of poor quality with small sample sizes.
Two randomized studies that assessed urethral sphincteric function by urethral pressure profilometry had conflicting results. Quadri’s group (1999) compared the MMK procedure with the Burch procedure in women with a hypermobile, low-pressure urethra (MUCP ≤ 20 cm H2O) and noted significantly higher subjective and objective 1-year cure rates with the MMK procedure. They used urethroscopy to facilitate MMK suture placement. On the other hand, Colombo’s group (1994) excluded women with a low-pressure urethra (MUCP < 30 cm H2O) and performed a cystotomy to facilitate MMK suture placement. With 2- to 7-year follow-up (mean, 3 years), they noted higher subjective and objective cure rates with the Burch procedure, although these were not statistically significant. In addition, significantly more MMK patients had persistent postoperative voiding difficulties (28% vs. 8%).
The literature on the paravaginal repair is sparse. The only randomized study that compared the Burch procedure with a paravaginal repair found significantly greater subjective and objective cure with the Burch procedure (Colombo et al, 1996a). Until large, randomized studies with prolonged follow-up are available, the issue of which is the best procedure will remain unresolved.
Tension-Free Vaginal Tape Procedure versus Colposuspension
Since its introduction in 1996 by Ulmsten and colleagues, the tension-free vaginal tape (TVT) procedure has gained widespread acceptance for treatment of SUI, given its low morbidity, short-term success rates, and ability to be performed as an outpatient procedure under local or regional anesthesia. The 7-year objective and subjective success rate for TVT is 81% (Nilsson et al, 2004). More recently in reviewing this series, 77% of the initial cohort of 90 women and 89% of those alive and capable of cooperating were assessed 11.5 years after the TVT operation. Ninety percent of the women had both a negative stress test and a negative pad test and were considered to be objectively cured. Subjective cure by patients’ global impression was found in 77%, 20% being improved and only 3% regarding the operation as a failure. No late-onset adverse effects of the operation were found, and no case of tape erosion was seen (Nilsson et al, 2008).
There have been a number of well-designed, prospective, randomized trials comparing the Burch colposuspension (two open and two laparoscopic) with TVT.
Liapis and coworkers (2002) reported a prospective randomized comparative study of Burch colposuspension (n = 35) and TVT (n = 36). They concluded that at 2-year follow-up the TVT and Burch procedures were equally effective with an objective pad test cure rate of 84% and 86%, respectively. TVT had a shorter operative time and entailed less postoperative pain with a faster return to normal activity.
Ward and Hilton (2004) published a 2-year follow-up of their landmark prospective randomized trial comparing TVT and open Burch colposuspension. At 2 years, 74% of the initial TVT and 69% of the open colposuspension patients completed the evaluation. The objective cure rates for the TVT (81%) and colposuspension (80%) were not significantly different. However, if the missing patients were evaluated by the last observation carried forward analysis, the cure rates would favor TVT (78% vs. 68%). It is interesting that the subjective cure rates differ from the objective rates dramatically, with only 43% and 37% reporting subjective cure of stress incontinence after TVT and colposuspension, respectively. At 2 years the colposuspension group still had significantly lower scores on mental and emotional health. The incidences of enterocele and vault prolapse were higher in the colposuspension group, requiring significantly more prolapse surgery. Likewise, the number of patients still requiring intermittent catheterization was higher in the colposuspension group. The intraoperative complications were higher in the TVT group, whereas the postoperative complications were higher in the colposuspension group. The authors’ conclusion at 2 years, because of patients lost to follow-up, is unchanged from their conclusion at 6 months, namely, that TVT may be better than, worse than, or the same as colposuspension.
A Cochrane review of open colposuspension examined a total of seven trials comparing TVT with open colposuspension (Lapitan et al, 2005), although the trial by Ward and Hilton noted earlier dominated the analysis. The review concluded that TVT and open colposuspension were equally effective but that TVT carried an increased risk of complications, particularly bladder perforation. A further cost-benefit analysis based on the Ward and Hilton study showed that TVT was a cost-effective alternative to colposuspension, largely based on shorter hospital stay and rapid return to work (Manca et al, 2003).
In a further report from the Ward and Hilton study, 98 of those who had TVT and 79 of those having colposuspension returned for 5-year follow up; 72 in the TVT group and 49 in the colposuspension group had full subjective and objective data (Ward et al, 2008). The primary outcome in this analysis was a negative 1-hour pad test and this was not significantly different between the two groups, being recorded in 58/72 (81%) women in the TVT group and 44/49 (90%) in the colposuspension group (P = .21, Fisher’s exact test) at 5 years. Significantly, more women in the colposuspension group (11 [7.5%]) underwent surgery for prolapse during the follow-up period than those in the TVT group (3 [1.8%]). Tape-related complications were seen in six women. In the first year, one tape was divided for obstructed voiding; there was one suprapubic extrusion and one vaginal erosion. Two further vaginal erosions were detected at 5-year follow-up; and, in addition, one woman was found to have tape within the bladder at cystoscopy after complaining of symptoms of overactive bladder. There were no reported suture-related complications in the colposuspension group.
The effect of both procedures on cure of incontinence and improvement in quality of life appears from this follow-up of a proportion of the originally treated patients to be maintained in the long term out to 5 years (Ward et al, 2008), with 81% of the women who had undergone TVT and 90% who had undergone colposuspension as satisfied or very satisfied with the results of their surgery at 5 years. The key messages in terms of adverse events are that vault and posterior vaginal wall prolapse are seen more commonly after colposuspension and that late tape erosion may occur several years after surgery.
Persson and coworkers (2002) were one of the first groups to compare TVT and laparoscopic Burch colposuspension. At 1-year follow-up there was no difference between objective and subjective cure rates. Valpas and associates (2004) conducted a prospective randomized trial comparing TVT and laparoscopic mesh colposuspension at 1-year follow-up. The objective cure rate (negative stress test, 86% vs. 57%), satisfaction, and quality of life were statistically better for the TVT group.
Paraiso and colleagues (2004) prospectively randomized 72 patients into laparoscopic Burch colposuspension and TVT at a mean follow-up of 20 months, but only 17 and 16 patients were available in the laparoscopic and TVT groups, respectively. Whereas objective and subjective cure rates were significantly higher in the TVT group, satisfaction of patients was equal in each group and no difference was reported in voiding dysfunction, urgency, or symptomatic pelvic prolapse. All these laparoscopic studies require longer follow-up with greater power to demonstrate a persistent difference between TVT and Burch colposuspension.
A meta-analysis of the literature has been reported by Novara and colleagues (2007) in which they combined the results of open and laparoscopic colposuspension. To date, nine RCTs compared TVT to Burch colposuspension as primary treatment for SUI (Liapis et al, 2002; Persson et al, 2002; Ward et al, 2002, 2004; Ustun et al, 2003; Paraiso et al, 2004; Valpas et al, 2004; Bai et al, 2005; El-Barky et al, 2005). A further study compared the suprapubic arc sling to laparoscopic Burch colposuspension (Foote et al, 2006). TVT was followed by significantly higher continence rates compared with Burch colposuspension, considering success rates evaluated according to any definition of continence (OR = 0.58; 95% CI = 0.42 to 0.79; P = .0007), presence of negative stress test (OR = 0.38; 95% CI = 0.25 to 0.57; P < .0001), and negative pad test (OR = 0.59; 95% CI = 0.41 to 0.85; P = .005).
A recent study has contrasted direct health care costs of treatment of SUI in Sweden to four different procedures: (1) open Burch colposuspension (OBC), (2) laparoscopic colposuspension with sutures (LCS), (3) laparoscopic colposuspension with mesh and staples (LCM), and (4) tension-free vaginal tape (TVT) (Ankardal et al, 2007). A model was constructed representing a hospital with standardized surgical equipment, staff, and average unit costs in 2003 euros. The time used for anesthesia and surgery was calculated. Clinical data were collected from three different sources: a multicenter, randomized, prospective study comparing OBC with LCM with 1-year follow-up; a three-armed, prospective study in which women were randomized to either OBC, LCM, or LCS with 1-year follow-up; and a descriptive study reporting results of TVT with 5-year follow-up. Data collected from the studies and hospital cost data were put into the model to create the different cost elements. The total cost per individual, showed a lower cost for TVT compared with the other alternatives. The direct costs for a TVT were only 56% of the costs for an OBC (P < .001) and 59% of the costs for an LCS (P < .001). Thus, when using a model and comparing health care costs for surgical treatment of female SUI in Sweden, the TVT procedure generated a lower direct cost than both open and laparoscopic colposuspension.
The shift in practice pattern away from Burch colposuspension for the treatment of SUI to midurethral slings as the primary treatment of SUI shows one of the difficulties in conducting surgical randomized controlled trials, especially when the field has changed as quickly as it has in recent years in the treatment of urinary incontinence. A recent survey of urogynecology fellows found that the average third-year fellow had performed 257 midurethral sling procedures and only 13 Burch procedures (LeBrun et al, 2008). It would be interesting to determine if a surgeon’s experience with Burch colposuspension is correlated with outcomes. Indeed, the outcomes reported in the existing literature base are likely to be very different to those seen in many clinicians’ clinical practices in which this has become a rare operation and where many have had very limited experience of performing the procedure during their training over the past decade.
At this time the TVT appears to be an equivalent operation to the open or laparoscopic Burch colposuspension. A recent meta-analysis (Novara et al, 2007) suggested superiority for the TVT, but this combined open and laparoscopic procedures and studies of variable quality. It is likely that greater reliability should be placed on single high-quality comparative studies (Ward et al, 2004, 2008). The TVT has now largely supplanted the colposuspension in contemporary practice. An open colposuspension should still be considered as a surgical option in patients undergoing open surgery and still has a role when there is associated prolapse. The laparoscopic colposuspension has been shown in the short term to have some advantages over open colposuspension in experienced hands but as a procedure has largely been supplanted by the TVT in contemporary practice.
Key Points
Acknowledgment
I would like to thank Richard Turner-Warwick for allowing the use of figures from our book in this chapter.
Abrams P, Cardozo L, Fall M, et al. Standardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167-178.
Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 4th International Consultation on Incontinence. Paris: Health Publications, 2009.
DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713-1720.
DeLancey JO. The pathophysiology of stress urinary incontinence in women and its implications for surgical treatment. World J Urol. 1997;15:268-274.
International Continence Society, http://www.icsoffice.org/.
Petros PP, Ulmsten U. An integral theory of female urinary incontinence: experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990;153:7-31.
Abrams P, Andersson KE, Brubaker L, et al. Third International Consultation on Incontinence. Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and faecal incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 3rd International Consultation on Incontinence, Vol. 2. Plymouth (UK): Plymbridge Distributors; 2005:1589-1626. Management
Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178.
Alcalay M, Monga A, Stanton SL. Burch colposuspension: a 10-20 year follow up. Br J Obstet Gynaecol. 1995;102:740-745.
Aldo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
Andersen JT, Heisterberg L, Hebjorn S, et al. Suprapubic versus transurethral bladder drainage after colposuspension/vaginal repair. Acta Obstet Gynecol Scand. 1985;64:139-143.
Ankardal M, Ekerydh A, Crafoord K, et al. A randomised trial comparing open Burch colposuspension using sutures with laparoscopic colposuspension using mesh and staples in women with stress urinary incontinence. Br J Obstet Gynecol. 2004;111:974-981.
Ankardal M, Jarbrink K, Milsom I, et al. Comparison of health care costs for open Burch colposuspension, laparoscopic colposuspension and tension-free vaginal tape in the treatment of female urinary incontinence. Neurourol Urodyn. 2007;26:761-766.
Appell RA. Retropubic procedures for female stress incontinence. In: Webster GD, Kirby R, King L, Goldwasser B, editors. Reconstructive urology. Oxford (England): Blackwell Scientific; 1993:887-894.
Applegate GB, Bass KM, Kubik CJ. Ureteral obstruction as a complication of the Burch colposuspension procedure: case report. Am J Obstet Gynecol. 1987;156:445.
Arunkalaivanan AS, Smith AR. Bladder calculus after laparoscopic colposuspension. J Obstet Gynaecol. 2002;22:101.
Aslan P, Woo HH. Ureteric injury following laparoscopic colposuspension. Br J Obstet Gynaecol. 1997;104:266-268.
Auwad W, Bombieri L, Adekanmi O, et al. The development of pelvic organ prolapse after colposuspension: a prospective, long-term follow-up study on the prevalence and predisposing factors. Int Urogynecol J. 2006;17:389-394.
Baessler K, Stanton SL. Does Burch colposuspension cure coital incontinence? Am J Obstet Gynecol. 2004;190:1030-1033.
Bai SW, Sohn WH, Chung DJ, et al. Comparison of the efficacy of Burch colposuspension, pubovaginal sling, and tension-free vaginal tape for stress urinary incontinence. Int J Gynaecol Obstet. 2005;91:246-251.
Ball TL. Gynecologic surgery and urology, 2nd ed. St. Louis: Mosby; 1963. p. 165–209
Barrier PA, Li JT, Jensen NM. Two words to improve physician-patient communication: what else? Mayo Clin Proc. 2003;78:211-214.
Berglund AL, Eisemann M, Lalos A, Lalos O. Social adjustment and spouse relationships among women with stress incontinence before and after surgical treatment. Soc Sci Med. 1996;42(11):1537-1544.
Bergman A, Ballard CA, Koonings PP. Comparison of three different surgical procedures for genuine stress incontinence: prospective randomized study. Am J Obstet Gynecol. 1989;160(5 Pt. 1):1102-1106.
Bergman A, Elia G. Three surgical procedures for genuine stress incontinence: five-year follow-up of a prospective randomized study. Am J Obstet Gynecol. 1995;173(1):66-71.
Bergman A, Koonings PP, Ballard CA. Negative Q-tip test as risk factor for failed incontinence surgery in women. J Reprod Med. 1989;34:193.
Bergman A, Koonings PP, Ballard CA. Proposed management of low urethral pressure type of genuine stress incontinence. Gynecol Obstet Invest. 1989;27:155-159.
Bergman A, Koonings PP, Ballard CA. The Ball-Burch procedure for stress incontinence with low urethral pressure. J Reprod Med. 1991;36:137-140.
Bergman A, Matthews L, Ballard CA, Roy S. Suprapubic versus transurethral bladder drainage after surgery for stress urinary incontinence. Obstet Gynecol. 1987;69:546-549.
Bidmead J, Cardozo L, McLellan A, et al. A comparison of the objective and subjective outcomes of colposuspension for stress incontinence in women. Br J Obstet Gynecol. 2001;108:408-413.
Black NA, Downs SH. The effectiveness of surgery for stress incontinence in women: a systematic review. Br J Urol. 1996;78(4):497-510.
Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case controlled urodynamic study. Am J Obstet Gynaecol. 1989;160:452-458.
Brieger G, Korda A. The effect of obesity on the outcome of successful surgery for genuine stress incontinence. Aust N Z J Obstet Gynaecol. 1992;32(1):71-72.
Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200:562.
Brubaker L, Cundiff GW, Fine P, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354:1557-1566.
Brubaker L, Nygaard I, Richter HE, et al. Pelvic Floor Disorders Network. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
Bump RC, Fant JA, Jurt WG. The mechanism of urinary continence in women with severe uterovaginal prolapse: results of barrier studies. Obstet Gynecol. 1988;72:291.
Burch JC. Urethrovaginal fixation to Cooper’s ligament for correction of stress incontinence, cystocoele and prolapse. Am J Obstet Gynecol. 1961;81(2):281-290.
Burch JC. Cooper’s ligament urethrovesical suspension for stress incontinence. Am Obstet Gynecol. 1968;100:764-774.
Burgio KL, Nygaard IE, Richter HE, et al. Bladder symptoms one year after abdominal sacrocolpopexy with and without Burch colposuspension in women without preoperative stress incontinence symptoms. Am J Obstet Gynecol. 2007;197:647.
Burton G. A three-year prospective randomised urodynamic study comparing open and laparoscopic colposuspension. Neurourol Urodyn. 1997;16(5):353-354.
Burton G. A five-year prospective randomised urodynamic study comparing open laparoscopic colposuspension [abstract 42]. Neurourol Urodyn. 1999;18:295-296.
Cardozo L, Hextall A, Bailey J, Boos K. Colposuspension after previous failed incontinence surgery: a prospective observational study. Br J Obstet Gynaecol. 1999;106:340-344.
Carey M, Goh JT, Rosamilia A, et al. Laparoscopic versus open Burch colposuspension: a randomized controlled trial. Br J Obstet Gynaecol. 2006;113:999-1006.
Carey M, Rosamilia A, Dwyer P, et al. Laparoscopic versus open colposuspension: a prospective multicentre randomised single-blind comparison. Neurourol Urodyn. 2000;19(4):389-391.
Carr LK, Webster GD. Voiding dysfunction following incontinence surgery: diagnosis and treatment with retropubic or vaginal urethrolysis. J Urol. 1997;157:821-823.
Chapple CR, Wein AJ, Artibani W, et al. A critical review of diagnostic criteria for evaluating patients with symptomatic stress urinary incontinence. BJU Int. 2005;95(3):327-334.
Chilaka VN, Amu O, Mayne CJ. Factors affecting outcome of colposuspension. J Obstet Gynaecol. 2002;22(1):72-74.
Clarke B. The role of urodynamic assessment in the diagnosis of lower urinary tract disorders. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(4):196-199.
Clemens JQ, Stern JA, Bushman WA, Schaeffer AJ. Long-term results of the Stamey bladder neck suspension: direct comparison with the Marshall-Marchetti-Krantz procedure. J Urol. 1998;160:372-376.
Colombo M, Milani R, Vitobello D, Maggioni A. A randomized comparison of Burch colposuspension and abdominal paravaginal defect repair for female stress urinary incontinence. Am J Obstet Gynecol. 1996;175:78-84.
Colombo M, Scalambrino S, Maggioni A, Milani R. Burch coloposuspension versus modified Marshall-Marchetti-Krantz urethropexy for primary genuine stress incontinence: a prospective, randomized clinical trial. Am J Obstet Gynecol. 1994;171:1573-1579.
Colombo M, Vitobello D, Proietti F, Milani R. Randomised comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. Br J Obstet Gynaecol. 2000;107(4):544-551.
Colombo M, Zanetta G, Vitobello D, Milani R. The Burch colposuspension for women with and without detrusor overactivity. Br J Obstet Gynaecol. 1996;103:255-260.
Czaplicki M, Dobronski P, Torz C, Borkowski A. Long-term subjective results of Marshall-Marchetti-Krantz procedure. Eur Urol. 1998;34:118-123.
Demirci F, Yildirim U, Demirci E, et al. Ten-year results of Marshall-Marchetti-Krantz and anterior colporraphy procedures. Aust N Z J Obstet Gynaecol. 2002;42(5):513-514.
Demirci F, Yucel O, Eren S, et al. Long-term results of Burch colposuspension. Gynecol Obstet Invest. 2001;51(4):243-247.
Digesu GA, Bombieri L, Hutchings A, et al. Effects of Burch colposuspension on the relative positions of the bladder neck to the levator ani muscle: an observational study that used magnetic resonance imaging. Am J Obstet Gynecol. 2004;190:614-619.
Einhorning G. Simultaneous recording of intravesical and intraurethral pressure. Acta Chir Scand. 1961;276:1-6.
El-Barky E, El-Shazly A, El-Wahab OA, et al. Tension-free vaginal tape versus Burch colposuspension for treatment of female stress urinary incontinence. Int Urol Nephrol. 2005;37:277-281.
Elia G, Bergman A. Genuine stress urinary incontinence with low pressure urethra: five-year follow-up after the Ball-Burch procedure. J Reprod Med. 1995;40:503-506.
Elkadry EA, Kenton KS, FitzGerald MP, et al. Patient-selected goals: a new perspective on surgical outcome. Am J Obstet Gynecol. 2003;189:1551-1557. discussion 1557–8
Enzelsberger H, Helmer H, Schatten C. Comparison of Burch and lyodura sling procedures for repair of unsuccessful incontinence surgery. Obstet Gynecol. 1996;88:251-256.
Eriksen BC, Hagen B, Eik-Nes SH, et al. Long-term effectiveness of the Burch colposuspension in female urinary stress incontinence. Acta Obstet Gynecol Scand. 1990;69:45-50.
Fantl JA, Newman DK, Colling J, et al. Urinary incontinence in adults: acute and chronic management. Clinical practice guideline. No. 2, 1996 update. AHCPR Publication No.: 96-0682. Rockville (MD): US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1996.
Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparoscopists. 2001;8(1):99-106.
Faysal M, Constantinou CE, Rother L, Govan DE. The impact of bladder neck suspension on the resting and stress urethral profile: a prospective study comparing controls with incontinent patients preoperatively and postoperatively. J Urol. 1981;125:55.
Feyersiel JF, Dreher E, Haenggi W, et al. Long-term results after Burch colposuspension. Am J Obstet Gynaecol. 1994;171:647-652.
Foote AJ, Maughan V, Carne C. Laparoscopic colposuspension versus vaginal suburethral slingplasty: a randomised prospective trial. Aust N Z J Obstet Gynaecol. 2006;46:517-520.
Frankel G, Kantipong M. Sixteen-month experience with video-assisted extraperitoneal laparoscopic bladder neck suspension. J Endourol. 1993;9:259-264.
Galloway NTM, Davies N, Stephenson TP. The complications of colposuspension. Br J Urol. 1987;60:122-124.
German KA, Kynaston H, Weight S, Stephenson TP. A prospective randomized trial comparing a modified needle suspension procedure with the vagina/obturator shelf procedure for genuine stress incontinence. Br J Urol. 1994;74:188-190.
Gillon G, Stanton SL. Long-term follow-up of surgery for urinary incontinence in elderly women. Br J Urol. 1984;56:478-481.
Glazener CMA, Cooper K. Anterior vaginal repair for urinary incontinence in women. Cochrane Database Syst Rev 2001;(1):CD001755.
Gleason DM, Reilly RJ, Pierce JA. Vesical neck suspension under vision with cystotomy enhances treatment of female incontinence. J Urol. 1976;115:555-557.
Goldberg RP, Koduri S, Lobel RW, et al. Protective effect of suburethral slings on postoperative cystocele recurrence after reconstructive pelvic operation. Am J Obstet Gynecol. 2001;185(6):1307-1312. discussion 1312–13
Haab F, Zimmern PE, Leach GE. Female stress urinary incontinence due to intrinsic sphincteric deficiency: recognition and management. J Urol. 1996;156:3-17.
Hilton P, Stanton SL. A clinical and urodynamic assessment of the Burch colposuspension for genuine stress incontinence. Br J Obstet Gynaecol. 1983;90:934-939.
International Continence Society, http://www.icsoffice.org/.
Jarvis GJ. Stress incontinence. In: Mundy AR, Stephenson TP, Wein AJ, editors. Urodynamics: principles, practice and application. 2nd ed. New York: Churchill Livingstone; 1994:299-326.
Jarvis GJ. Surgery for genuine stress incontinence. Br J Obstet Gynaecol. 1994;101(5):371-374.
Jarvis GJ, Bent A, Cortesse A, et al. Surgical treatment for incontinence in adult women surgery of female lower genitourinary fistulae. In: Abrams P, Khoury S, Wein A, editors. Incontinence: 1st International Consultation on Incontinence. Recommendations of the International Scientific Committee: the evaluation and treatment of urinary incontinence, Vol. 15. Plymouth (UK): Health Publication; 1999:637-668.
Kenton K, FitzGerald MP, Brubaker L. Multiple foreign body erosions after laparoscopic colposuspension with mesh. Am J Obstet Gynecol. 2002;187:252-253.
Kiilholma P, Haarala M, Polvi H, et al. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;2:441-444.
Kitchener HC, Dunn G, Lawton V, et al. Laparoscopic versus open colposuspension—results of a prospective randomised controlled trial. Br J Obstet Gynaecol. 2006;113:1007-1013.
Koonings PP, Bergman A, Ballard CA. Low urethral pressure and stress urinary incontinence in women: risk factor for failed retropubic surgical procedure. Urology. 1990;35:245-248.
Kwon CH, Culligan PJ, Koduri S, et al. The development of pelvic organ prolapse following isolated Burch retropubic urethropexy. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:321-325. discussion 325
Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:323-326. discussion 326–7
Langer R, Ron-El R, Neuman M, et al. The value of simultaneous hysterectomy during Burch colposuspension for stress urinary incontinence. Obstet Gynecol. 1988;72:866-869.
Lapitan MC, Cody DJ, Grant AM. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev 2003;(1):CD002912.
Lapitan MC, Cody DJ, Grant AM. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev 2005;(3):CD002912.
Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. The American Urological Association. J Urol. 1997;158(3 Pt. 1):875-880.
LeBrun EE, Sears CL, Young SB. An anonymous survey of urogynecology fellowship surgical experience: a pilot study. J Pelvic Med Surg. 2008;14:115.
Lee RA, Symmonds RE, Goldstein RA. Surgical complications and results of modified Marshall-Marchetti-Krantz procedure for urinary incontinence. Obstet Gynecol. 1979;53:447-450.
Liapis A, Asimiadis V, Loghis CD, Pyrgiotis E, Zourlas PA. A randomized prospective study of three operative methods for genuine stress incontinence. J Gynecol Surg. 1996;12(1):7-14.
Liapis A, Bakas P, Creatsas G. Burch colposuspension and tension-free vaginal tape in the management of stress urinary incontinence in women. Eur Urol. 2002;41(4):469-473.
Liu CY. Laparoscopic retropubic colposuspension (Burch procedure)—a review of 58 cases. J Reprod Med. 1993;38:526-530.
Lose G, Jørgensen L, Johnsen A. Predictive value of detrusor instability index in surgery for female urinary incontinence. Neurourol Urodyn. 1988;7:141-148.
Lose G, Jørgensen L, Mortensen SO, et al. Voiding difficulties after colposuspension. Obstet Gynecol. 1987;69:33-37.
Lyons TL. Minimally invasive retropubic urethropexy: the Nolan-Lyons modification to the Burch procedure. Gynecol Endosc. 1994;3:40-42.
Maher C, Dwyer P, Carey M, Gilmour D. The Burch colposuspension for recurrent urinary stress incontinence following retropubic continence surgery. Br J Obstet Gynaecol. 1999;106:719-724.
Mainprize TC, Drutz HP. The Marshall-Marchetti-Krantz procedure: a critical review. Obstet Gynecol Surv. 1988;43:724-729.
Mallipeddi PK, Steele AC, Kohli N, Karram MM. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior vaginal wall prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(2):83-88.
Manca A, Dumville J, Kitchener H, et al. Cost-effectiveness analysis of open colposuspension versus laparoscopic colposuspension in the treatment of urodynamic stress incontinence. Br J Obstet Gynaecol. 2006;113:1014-1022.
Manca A, Sculpher MJ, Ward K, Hilton P. A cost-utility analysis of tension-free vaginal tape versus colposuspension for primary urodynamic stress incontinence. Br J Obstet Gynaecol. 2003;110:255-262.
Marchetti AA. The female bladder after correction for stress incontinence. Am J Obstet Gynecol. 1949;58:1145.
Marshall FV, Marchetti AA, Krantz KE. The correction of stress incontinence by simple vesicourethral suspension. Surg Gynecol Obstet. 1949;88:509-518.
McDougall EM, Heidorn CA, Portis AJ, Klutke CG. Laparoscopic bladder neck suspension fails the test of time. J Urol. 1999;162:2078-2081.
McDuffie RWJr, Litin RB, Blundon KE. Urethrovesical suspension (Marshall-Marchetti-Krantz): experience with 204 cases. Am J Surg. 1981;141(2):297-298.
McGuire EJ. Urodynamic findings in patients after failure of stress incontinence operations. Prog Clin Biol Res. 1981;78:351-360.
McGuire EJ. Bladder instability in stress urinary incontinence. Neurourol Urodyn. 1988;7:563.
Milani R, Scalambrino S, Quadri G, et al. Marshall-Marchetti-Krantz procedure and Burch colposuspension in the surgical treatment of female urinary incontinence. Br J Obstet Gynaecol. 1985;92:1050-1053.
Moehrer B, Carey M, Wilson D. Laparoscopic colposuspension: a systematic review. B J Obstet Gynaecol. 2003;110:230-235.
Moehrer B, Ellis G, Carey M, Wilson PD. Laparoscopic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev 2002;(1):CD002239.
Nilsson CG, Falconer C, Rezapour M. Seven- year follow-up of tension-free vaginal tape procedure for treatment of urinary incontinence. Obstet Gynecol. 2004;104:1259-1262.
Nilsson CG, Palva K, Rezapour M, Falconer C. Eleven years prospective follow-up of the tension-free vaginal tape procedure for treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(8):1043-1047.
Nitahara KS, Aboseif S, Tanagho EA. Long-term results of colpocystourethropexy for persistent or recurrent stress urinary incontinence. J Urol. 1999;162:138-141.
Nitti VW, Raz S. Obstruction following anti-incontinence procedures: diagnosis and treatment with transvaginal urethrolysis. J Urol. 1994;152:93-98.
Novara G, Ficarra V, Boscolo-Berto R, et al. Tension-free midurethral slings in the treatment of female stress urinary incontinence: a systematic review and meta-analysis of randomized controlled trials of effectiveness. Eur Urol. 2007;52:663-678.
Nygaard I, DeLancey JO, Arnsdorf L, Murphy E. Exercise and incontinence. Obstet Gynecol. 1990;75:848-851.
Ou SC, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
Paraiso MFR, Falcone T, Walters MD. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
Paraiso MFR, Walters MD, Karram MM, Barber MD. Laparoscopic Burch colposuspension versus tension-free vaginal tape: a randomized trial. Obstet Gynecol. 2004;104:1249-1258.
Parnell JP, Marshall VF, Vaughn ED. Primary management of urinary stress incontinence by the Marshall-Marchetti-Krantz vesicourethropexy. J Urol. 1982;127:679-682.
Penson DF, Raz S. Why anti-incontinence surgery succeeds or fails. In: Raz S, editor. Female urology. 2nd ed. Philadelphia: WB Saunders; 1996:435-442.
Persson J, Teleman P, Eten-Berquist C, Wolner-Hanssen P. Cost analyses based on a prospective randomized study comparing laparoscopic colposuspension with a tension-free vaginal tape procedure. Acta Obstet Gynecol Scand. 2002;81:1066-1073.
Persson J, Wolner-Hanssen P. Laparoscopic Burch colposuspension for stress urinary incontinence: a randomized comparison of one or two sutures on each side of the urethra. Obstet Gynaecol. 2000;95(1):151-155.
Petrou SP, Frank I. Complications and initial continence rates after a repeat pubovaginal sling procedure for recurrent stress urinary incontinence. J Urol. 2001;165(6 Pt. 1):1979-1981.
Polascik TJ, Moore RG, Rosenberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic urethropexy for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
Quadri G, Magatti F, Belloni C, et al. Marshall-Marchetti-Krantz urethropexy and Burch colposuspension for stress urinary incontinence in women with low pressure and hypermobility of the urethra: early results of a prospective randomized clinical trial. Am J Obstet Gynecol. 1999;181:12-18.
Raboy A, Hakim LS, Ferzli G, et al. Extraperitoneal endoscopic vesicourethral suspension. J Laparoendosc Surg. 1995;3:505-508.
Raz S. Modified bladder neck suspension for female stress incontinence. Urology. 1981;17:82-85.
Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357-362.
Richardson AC, Lyon FB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-573.
Sand PK, Koduri S, Lobel RW, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001;184(7):1357-1362. discussion 1362–4
Scotti RJ, Garely AD, Greston WM, et al. Paravaginal repair of lateral vaginal wall defects by fixation to the ischial periosteum and obturator membrane. Am J Obstet Gynecol. 1998;179(6 Pt. 1):1436-1445.
Shull BL, Baden WF. A six year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160:1432-1440.
Sitzia J, Wood N. Patient satisfaction: a review of issues and concepts. Soc Sci Med. 1997;45(12):1829-1843.
Smith ARB, Chang D, Dmochowski R, et al. Surgery for urinary incontinence in women. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 4th International Consultation on Incontinence. Paris: Health Publications; 2009:1191-1272.
Smith ARB, Daneshgari F, Dmochowski R, et al. Surgery for urinary incontinence in women. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 3rd International Consultation on Incontinence, Vol. 2. Plymouth (UK): Plymbridge Distributors; 2005:1297-1370. Management
Sofaer S, Firminger K. Patient perceptions of the quality of health services. Annu Rev Public Health. 2005;26:513-559.
Stanton SL, Cardozo L, Williams JE, et al. Clinical and urodynamic features of failed incontinence surgery in the female. Obstet Gynecol. 1978;51(5):515-520.
Staskin DR, Zimmern PE, Hadley HR, Raz S. The pathophysiology of stress incontinence. Urol Clin North Am. 1985;12:271-278.
Su TH, Wang KG, Hsu CY, et al. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress incontinence. Acta Obstet Gynecol Scand. 1997;6(76):576-582.
Summitt RL, Lucente V, Karram MM, et al. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95(4 Suppl. 1):S2.
Symmonds RE. The suprapubic approach to anterior vaginal relaxation and urinary stress incontinence. Clin Obstet Gynecol. 1972;15:1107-1121.
Tamussino KF, Zivkovic F, Pieber D, et al. Five-year results after anti-incontinence operations. Am J Obstet Gynecol. 1999;181(6):1347-1352.
Tan E, Tekkis PP, Cornish J, et al. Laparoscopic versus open colposuspension for urodynamic stress incontinence. Neurourol Urodyn. 2007;26:158-169.
Tanagho EA. Colpocystourethropexy: the way we do it. J Urol. 1978;116:751-753.
Tanagho EA. Colpocystourethropexy. In: Raz S, editor. Female urology. 2nd ed. Philadelphia: WB Saunders; 1996:319-324.
Turner-Warwick R. The Turner-Warwick vagino-obturator shelf urethral-repositioning procedure. In: Debruyne FMJ, Van Kerrebroeck PEVA, editors. Practical aspects of urinary incontinence. Dordrecht, The Netherlands: Martinus Nijhoff; 1986:100-104.
Turner-Warwick R, Chapple CR. Functional reconstruction of the urinary tract and gyneco-urology: an exposition of functional principles and surgical procedures. Oxford (England): Blackwell Science; 2002.
Turner-Warwick R, Kirby RS. Principles of sphincteroplasty. In: Webster GD, Kirby R, King L, Goldwasser B, editors. Reconstructive urology. Oxford (England): Blackwell Scientific; 1993:657-686.
Turner-Warwick R, Worth PHL, Milroy EJG, Duckett J. The suprapubic V incision. Br J Urol. 1974;46:39-45.
Ulmsten U, Henrikson L, Johnson P, Varhos G. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:81-86.
Ustun Y, Engin-Ustun Y, Gungor M, Tezcan S. Tension-free vaginal tape compared with laparoscopic Burch urethropexy. J Am Assoc Gynecol Laparosc. 2003;10:386-389.
Valpas A, Kivela A, Penttinen J, et al. Tension-free vaginal tape and laparoscopic mesh colposuspension for stress urinary incontinence. Obstet Gynecol. 2004;104:42-49.
Vancaillie TG, Schuessler W. Laparoscopic bladderneck suspension. J Laparoendosc Surg. 1991;1(3):169-173.
Viereck V, Pauer HU, Bader W, et al. Introital ultrasound of the lower genital tract before and after colposuspension: a 4-year objective follow-up. Ultrasound Obstet Gynecol. 2004;23:277-283.
Ward K, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.
Ward K, Hilton P. A prospective multicenter randomized trial of tension-free vaginal tape and colposuspension for primary urodynamic stress incontinence: two-year follow-up. Am J Obstet Gynecol. 2004;190:324-331.
Ward K, Hilton P, et al. Tension-free vaginal tape versus colposuspension for primary urodynamic stress incontinence: 5-year follow-up. Br J Obstet Gynaecol. 2008;115:226-233.
Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185(6):1299-1304. discussion 1304–6
Webster GD, Kreder KJ. Voiding dysfunction following cystourethropexy: its evaluation and management. J Urol. 1990;144:670-673.
Weil A, Reyes H, Bischoff P, et al. Modifications of the urethral rest and stress profiles after different types of surgery for stress incontinence. Br J Obstet Gynaecol. 1984;91:46.
White GR. An anatomic operation for the cure of cystocele. Am J Obstet Dis Women Child. 1912;65:286.
Wiskind AK, Creighton SM, Stanton SL. The incidence of genital prolapse after the Burch colposuspension. Am J Obstet Gynecol. 1992;167:399-405.
Woo HH, Rosario DJ, Chapple CR. Stone formation on permanent suture material used previously in colposuspension. Br J Urol. 1995;76:139-140.
Zivkovic F, Tamussino K, Pieber D, Haas J. Body mass index and outcome of incontinence surgery. Obstet Gynecol.. 1999;93(5 Pt. 1):753-756.
Zorzos I, Paterson PJ. Quality of life after a Marshall-Marchetti-Krantz procedure for stress urinary incontinence. J Urol. 1996;155:259-262.