chapter 72 Vaginal and Abdominal Reconstructive Surgery for Pelvic Organ Prolapse
Surgical treatment of pelvic organ prolapse (POP) is one of the most challenging problems faced by surgeons who perform procedures to correct disorders of the pelvic floor. The cause is usually multifactorial, and the clinical presentation and the impact on a patient’s quality of life may vary considerably. Surgical correction is aimed at addressing defects in the supporting structures, which requires a comprehensive understanding of pelvic floor anatomy and function. Pelvic floor defects have been traditionally named for the prolapsing visceral organs; however, it is important to conceptualize that these disorders have less to do with the prolapsing organs and more to do with compartmental defects in the supportive tissues. Just as any hernia represents a defect in the fascia, POP represents herniations or descensus of the bladder, rectum, bowel, uterus, and urethra through defects in the pelvic floor connective tissues. Procedures must be tailored to the patient’s unique presenting factors, such as comorbidities and concomitant pathology of the pelvic floor, as well as patient goals and expectations in surgical restoration of pelvic organ support. The aim of this chapter is to provide a practical overview of the surgical anatomy and discuss the vaginal and open abdominal techniques for the treatment of POP.
Pelvic organ defects occur as compartmental defects of vaginal support. Patients may present with any combination of compartmental defects, and multiple defects are very common. The aims of surgical management include restoration of normal vaginal anatomy while maintaining visceral and sexual function. It is critical to understand the patient’s goals and expectations of treatment to ensure that the prescribed treatment will meet these goals. This is the art of tailoring the appropriate treatment for each individual surgical patient.
Surgical Anatomy of the Pelvic Floor
Anatomy
Supporting Structures
Bony Scaffolding
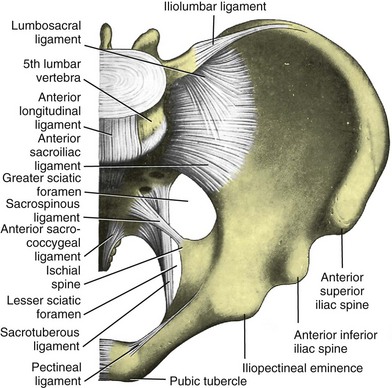
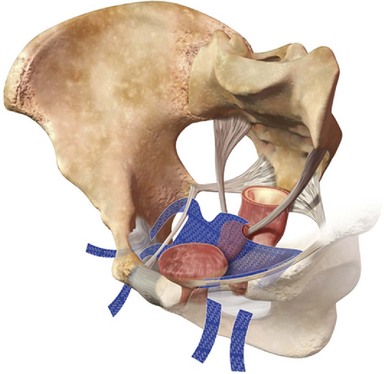
The bones of the pelvis provide the scaffold on which the soft tissue supports (muscles, ligaments, and fascia) are anchored. They include paired innominate or hip bones on either side of the sacrum, which comprise the pelvic girdle. The innominate bones are further divided into the ileum, the ischium, and the pubis. The ischial spine provides attachment for the arcus tendineus fasciae pelvis (ATFP), the sacrospinous ligament, and the coccygeus muscle (Fig. 72–1). The obturator foramen is formed by the superior pubic ramus above, the pubic body and inferior ramus medially, and the anterior border of the ischial body below (Newell, 2005) (Fig. 72–2).
Muscular Supports of the Pelvic Floor
The pelvic floor comprises all of the soft tissues that essentially hold the pelvic viscera in place at the base of the bony pelvis and the levator ani muscle complex. The pelvic floor is a three-dimensional structure that functions as a unit (Hurt, 2000). The pelvic diaphragm is composed of the striated pubococcygeus, iliococcygeus, and coccygeus (Fig. 72–3). The levator ani muscles are composed of the pubococcygeus and iliococcygeus. The coccygeus is also termed the ischiococcygeus and is attached to the lateral margins of the coccyx and fifth sacral segment (Mundy, 2005). It is attached laterally to the ischial spine. The iliococcygeus is attached to the ischial spine, the arcus tendineus levator ani laterally and posteriorly, the tip of the sacrum, and the coccyx. The pubococcygeus is attached to the back of the pubis, and it courses lateral to the urethra (in males it is the pubourethralis, and in females, because it forms a sling around the vagina, it is the pubovaginalis). In both men and women, fibers of the pubococcygeus attach to the perineal body (Mundy, 2005). The pubococcygeus compresses the visceral canals, which cross the pelvic floor. The puborectalis portion of the pubococcygeus helps to create the anorectal angle. Contraction of the puborectalis causes the rectoanal junction to move toward the pubic symphysis, which is critical in maintaining fecal continence (Rogers, 2003). Although the muscles are referred to separately, like other structures of the pelvic floor the boundaries are often difficult to delineate and they perform similar physiologic functions (Mundy, 2005). The posterior levator ani group (iliococcygeus and coccygeus) fuses in the midline and attaches to the coccyx. The complex formed by this fusion is the levator plate, which serves as a supporting structure for the upper vagina and cervix, This serves not only to stabilize the upper vagina in a horizontal plane but also to provide a protective mechanism preventing downward forces onto the perineal body (Wall and Menafee, 2002).
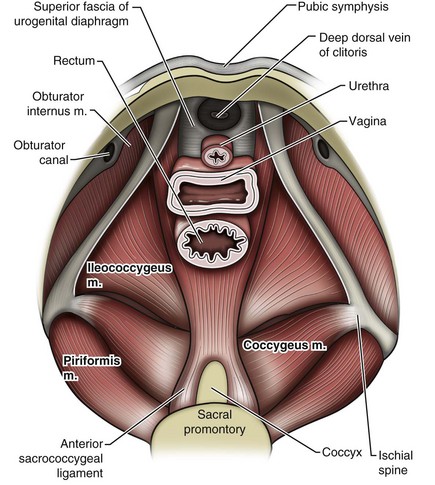
Figure 72–3 Muscles of the female pelvic diaphragm.
(From Anderson J, Genadry R. Anatomy and embryology. In: Novak’s text of gynecology. 13th ed. Philadelphia: Lippincott Williams & Wilkins; 2002, with permission.)
Beneath the pelvic diaphragm is the diamond-shaped urogenital diaphragm (Fig. 72–4). The boundaries are the pubic symphysis anteriorly, the tip of the coccyx posteriorly and laterally, and the ischiopubic rami on either side. It can be further divided anteriorly and posteriorly by imagining a line between each ischial tuberosity. This results in the urogenital triangle anteriorly and the anorectal triangle posteriorly. The deep transversus perinei muscle, also referred to as the perineal membrane, is contained by the urogenital triangle, in which the urethra and vagina traverse. In contrast to the perineal membrane in males, which is a sheetlike structure, the perineal membrane in females is a three-dimensional structure that has two regions, dorsal and ventral (DeLancey, 1999; Stein and DeLancey, 2008) (Fig. 72–5). The dorsal region is attached to the perineal body and the lateral wall of the vagina via the ischiopubic rami. The ventral region is part of a solid three-dimensional mass that is contiguous with the paraurethral and paravaginal connective tissues. This portion contains the compressor urethrae and urethrovaginal sphincter muscles of the distal urethra (Stein and DeLancey, 2008).
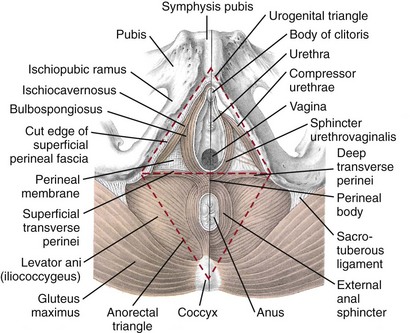
Figure 72–4 Muscles of the female perineum. The cut edge on the right side is the cut edge of the superficial fascia that has been removed. The left side depicts the deep perineal muscles (seen without the superficial perineal muscles and overlying fascia.
(From Mundy A. True pelvis, pelvic floor and perineum. In: Standrinak W, editor. Gray’s anatomy: the anatomical basis of clinical practice. 39th ed. New York: Elsevier; 2005.)
Figure 72–5 A and B, Perineal membrane. The two halves of this triangular sheet of fibromuscular connective tissue form a central tendon referred to as the perineal body.
(From DeLancey JO. Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am J Obstet Gynecol 1999;180:815–23.)
The anorectal triangle contains the external anal sphincter, which attaches to the anococcygeal ligament and fuses to the superficial transverse perinei muscle. External to the urogenital triangle are the external genital muscles, bulbospongiosus, ischiospongiosus, and superficial transversus perinei. The hymen is located just inside the labia minora and is the fixed point of reference recommended by the International Continence Society Committee on Standardisation of Terminology, Subcommittee on Pelvic Organ Prolapse and Pelvic Floor Dysfunction to grade prolapse (Bump et al, 1996). This is in contrast to the term introitus, which is ill defined.
The urogenital hiatus is the opening within the levator ani muscle through which the urethra and vagina pass. Because the rectum is attached directly to the muscles at this level it is not within the urogenital hiatus. The hiatus is supported anteriorly by the pubic bones and levator ani muscles and posteriorly by the perineal body and external anal sphincter (Ashton-Miller and DeLancey, 2007). The urogenital hiatus elongates and descends with POP.
Endopelvic Fascia and Connective Tissue Supports
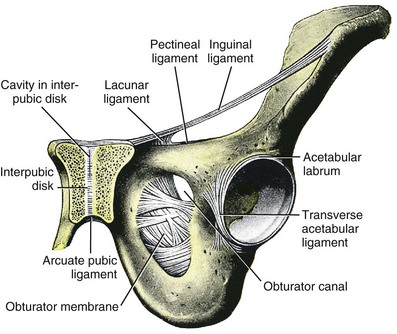
The endopelvic fascia is a network of fibromuscular tissue located between the peritoneum and the levator muscles. It surrounds and attaches the bladder, uterus, vagina, and rectum to the pelvic walls, thereby stabilizing the pelvic viscera. The endopelvic fascia is one continuous unit; however, distinct areas are named separately. The parametrium (broad, cardinal, and uterosacral ligaments) attaches the uterus and upper vagina to the pelvic sidewall. The paracolpium (the arcus tendineum levator ani and the ATFP) attaches the vagina to the pelvic sidewalls. In his landmark article, DeLancey (1992) described the supports of the vagina and conceptually divided them into three parts according to the region of vaginal support (Fig. 72–6). The structures supporting the uterus and the cephalad 2 to 3 cm of the vagina comprise level I support, and these fibers originate from the greater sciatic foramen, the sacroiliac region, and the lateral sacrum. The fibers are primarily vertical in their orientation and are the longest fibers of the endopelvic fascia, thereby suspending the uterus and upper vagina, and comprise the cardinal/uterosacral ligament complex. Level II support is at the mid vagina. These fibers are shorter than level I support but longer than those at level III. The orientation of the attaching fibers is lateral, and they are more dense than the cardinal/uterosacral ligament complex. The endopelvic fascia splits at this level to encompass the bladder and urethra such that the abdominal leaf is still named the endopelvic fascia and the vaginal leaf is the pubocervical and periurethral fascia. Posteriorly, the endopelvic fascia, which attaches laterally to the superior fascia of the levator ani muscles, is the rectovaginal fascia. Level III support of the vagina starts at the introitus and extends 2 to 3 cm above the hymenal ring. In this most distal location there is no intervening paracolpium and the vagina is fused directly to the urethra and is embedded in the connective tissue of the perineal membrane (urogenital diaphragm). Laterally it blends into the medial margins of the levator ani muscles, and posteriorly it blends into the perineal body.
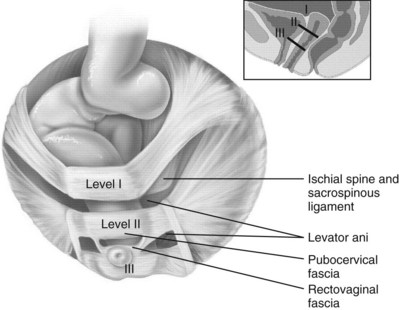
Figure 72–6 Vaginal and visceral supportive structures as defined by DeLancey. The fibers of level I support are oriented vertically and suspend the uterus and upper vagina. Level II support is more horizontal in its orientation and attached to the mid vagina. Distally, level III support fuses directly into the support structures.
(From DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 1992;166:1717–28.)
The endopelvic fascia is a composite of tissues of fibers embedded in a matrix. The variability of this tissue matrix may lead to its inherent weakness and inconsistency in reconstructive surgery. In fact, fresh strips of pubocervical fascia have been shown to shorten 15% to 20% of its length (Richardson, and DeLancey, 2000). Thus, these tissues are supportive and contractile. Histologically, this tissue is unlike abdominal wall fascia or the fascial coverings of other muscles such that they lack the organization of the fascial coverings of the skeletal muscle.
Support by Compartment
Anterior Compartment Supports
Because the urethra is fused to the anterior vaginal wall for much of its length, the supporting structures of the urethra and distal anterior vagina are one in the same. The major components are the endopelvic fascia (periurethral and pubocervical fascia), ATFP, and the levator ani muscles (Ashton-Miller and DeLancey, 2007). At this level the endopelvic fascia surrounds the vagina and attaches to the ATFP bilaterally. Each ATFP is attached to the pubic bone anteriorly and to the ischial spine dorsally. This structure appears as a band closer to the pubic bone, and as it courses toward the ischial spine it fans out into a broad aponeurotic structure. Laterally it merges with the levator ani muscles at the confluence of the iliococcygeus and obturator internus.
Apical (Middle) Compartment Supports
This support is provided by the cardinal/uterosacral ligament complex, which are level I supports as described by DeLancey (1992). They originate from fibers of the pelvic sidewall and extend to the area around the greater sciatic foramen and extend to the second, third, and fourth sacral segments. From there they fan out as they attach to the cervix and the upper vagina. The medial edge of this complex is the area of the uterosacral ligaments. The uterosacral ligaments are visible and palpable with traction of the cervix or the cuff of the vagina after hysterectomy. The fibers encircle the cervix and in aggregate are called the pericervical ring.
Posterior Compartment Supports
The rectovaginal fascia is also sometimes referred to as Denonvilliers fascia in the female (Richardson and DeLancey, 2000). It has more dense strands of elastin and less smooth muscle compared with the pubocervical fascia. It is stabilized in the upper vagina by the cardinal/uterosacral ligament complex, because it is contiguous with this structure. The uterosacral ligament inserts into the rectovaginal fascia just below the attachment to the posterior cervix. Because the rectovaginal fascia is connected to the sacrum and the perineal body it provides continuous posterior support.
The perineal body is a condensation of fibromuscular tissue and collagen that is located in the midline between the vagina and the anus. It serves as a point of fixation for the rectovaginal fascia, the levator ani muscles, the transverse perinei muscles, and the external anal sphincter (Rosenblum et al, 2005).
Pathophysiology of Pelvic Organ Prolapse: Surgical Correlation
The levator ani muscles comprise both type I and type II fibers. Type I muscle fibers are slow twitch and provide sustained tonicity of the pelvic floor. This function serves a crucial role in providing dynamic pelvic floor support, thus taking the mechanical stress off of the endopelvic connective tissue attachments. Type II fibers consists of fast-twitch fibers that are mainly responsible for the reflex contractions of the pelvic floor associated with sudden increases in intra-abdominal pressure. Thus, the levator ani muscle complex fulfills multiple functions. First, the tonic contraction of the pubococcygeus muscle narrows the genital hiatus. Second, contraction of the pelvic floor leads to the elongation and elevation of the pelvic organs facilitating urinary and fecal continence. Additionally, posterior levator ani tonicity elevates the upper vagina and stabilizes it into a horizontal plane near the hollow of the sacrum (Fig. 72–7) (Wall and Menafee, 2002). Direct muscular damage, neuromuscular dysfunction, and inherent tissue defects may predispose to a dysfunction of the levator ani musculature. As a result, the burden of support shifts mostly to the endopelvic connective tissues. As these structures weaken, various “breaks” occur that lead to vaginal support defects.
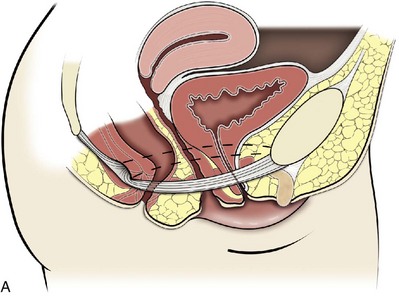
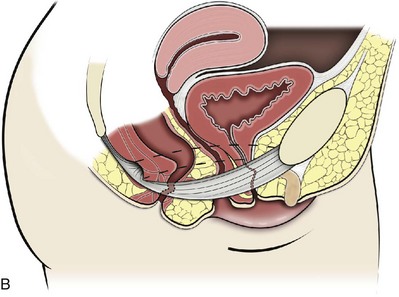
Figure 72–7 Function of the levator ani. The tonic contraction of the pubococcygeus muscle narrows the genital hiatus and leads to elongation and elevation of the pelvic organs, facilitating continence (A at rest; B contracted). The posterior levator ani elevate the upper vagina and stabilize it on the levator plate, which provides a supportive role to the perineal body.
(From Wall LL, Menefee S. Incontinence, prolapse and disorders of the pelvic floor. In: Berek JS, editor. Novak’s textbook of gynecology. 13th ed. Philadelphia: Lippincott Williams & Wilkins; 2002, with permission.)
It is most useful to define defects by compartment while keeping in mind that many patients present with multicompartmental defects. Additionally, some patients will present with several defects within the same compartment.
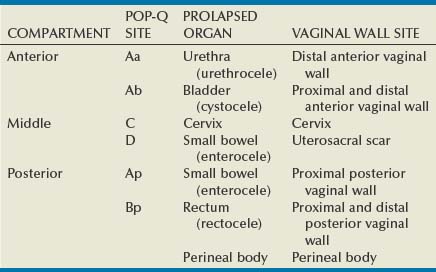
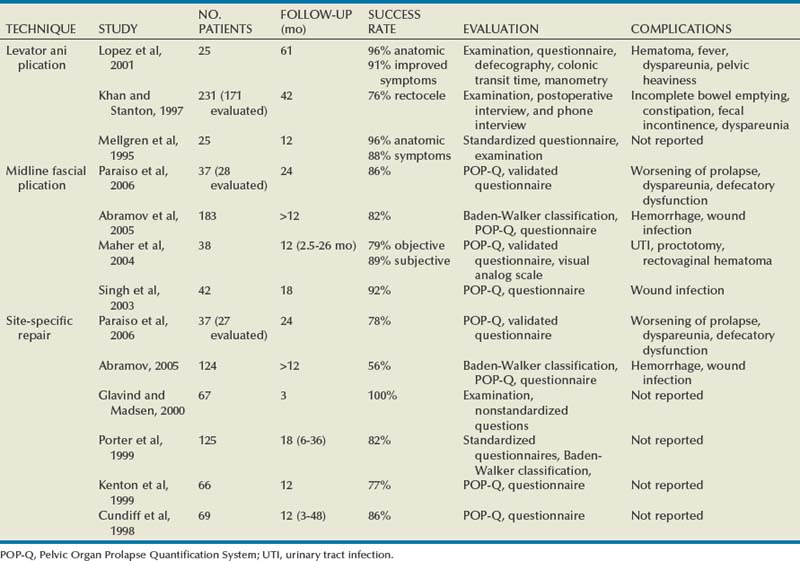
Site-Specific Defects by Compartment (Identification) (Table 72–1)
Anterior Compartment
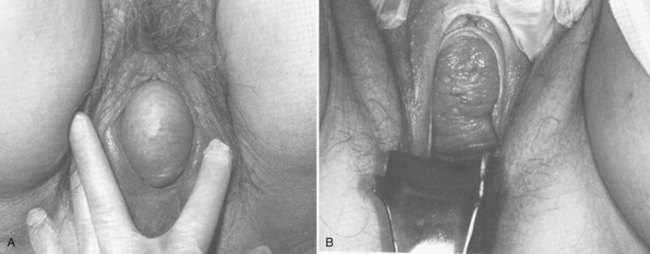
Within the anterior compartment two types of defects can lead to cystoceles. The central cystocele results from attenuation or separation of the pubocervical fascia, resulting in a protrusion of anterior compartment structures through this defect. This protrusion results in the classic appearance of a loss of the rugae or vaginal folds of the anterior vaginal wall. Detachment of the pubocervical fascia and the pubourethral ligaments to the ATFP results in a lateral cystocele and can also involve the urethra. This usually results in a preservation of the rugal folds of the vaginal wall and a “rotational” prolapse of the anterior wall (Fig. 72–8). Urethroceles, which are distal anterior compartment defects, usually result in urethral hypermobility (Weber and Walter, 1997).
Apical Compartment
Apical compartment defects involve a disruption in the uterosacral/cardinal ligament complex. This defect may lead to prolapse of the uterus, the vaginal cuff after hysterectomy, and the peritoneum cul-de-sac with or without bowel (enterocele). Enteroceles are most commonly associated with apical or high posterior compartment defects; however, enteroceles may rarely occur anteriorly. Enteroceles can occur with or without vaginal vault prolapse. Complete vaginal vault prolapse contains enteroceles in 75% of patients. Waters (1956) described four types of enteroceles by etiology: congenital, pulsion, traction, and iatrogenic. Congenital enteroceles occur either from the failure of the peritoneum to fuse with the perineal body or from reopening of previously fused structures. Pulsion defects occur with increased intra-abdominal pressures, and traction enteroceles occur by a pulling of the vaginal epithelium from other prolapsing organs. Iatrogenic enteroceles are created when a surgical procedure is performed that alters the normal vaginal axis or when the pubocervical fascia and the rectovaginal septum are not reapproximated after hysterectomy (Wiskind et al, 1992).
Uterine prolapse occurs with loss of support of the cardinal and uterosacral ligaments. The broad ligaments also provide uterine support and are located above insertion of the cardinal uterosacral ligaments. They are located within the leaves of the anterior and posterior peritoneum. Within this fused structure are the fallopian tubes and the round and ovarian ligaments along with their blood supply (Rosenblum et al, 2005). It is difficult to differentiate other prolapsing organs with loss of apical support high in the vagina. As such, careful dissection is often needed to identify other prolapsing organs with uterine prolapse. This reinforces the concept that the endopelvic fascia is best considered as a contiguous unit that can fail together. Consequently, the bladder, small bowel, and rectum are often found prolapsing with the uterus.
Vaginal vault prolapse can occur after hysterectomy if support of the vault is not reconstituted by suspending it to the cardinal/uterosacral ligament complex. Incidence of vaginal vault prolapse after hysterectomy has been reported to be as high as 18.2%, and it can contribute to prolapse in other compartments (Richter, 1982). Because of the redundancy of the support by the endopelvic fascia, failure to reattach the suspensory component does not lead to immediate vaginal eversion after hysterectomy (DeLancey, 1992). Complex vaginal eversion is vaginal eversion associated with cystocele, rectocele, or both (DeLancey, 1992). Complex vaginal eversion has been reported as high as 67% of vault prolapse (Morley and DeLancey, 1988). In this group of patients, 7% had apical prolapse and cystocele, 30% had apical prolapse with rectocele, and 30% had prolapse with both cystocele and rectocele.
Posterior Compartment
The posterior vaginal compartment comprises the peritoneum of the cul-de-sac, the rectum, and the perineum. Defects in the rectovaginal fascia either in the form of attenuated fascia or site-specific tears will result in herniation of the rectum and sometimes the small bowel into the vagina. Rectoceles may be divided into low, midvaginal, or high depending on the location of loss of support, and it may present as a combination of defects. Richardson was the first to describe site-specific defects of the rectovaginal fascia in 1993 (Fig. 72–9). Defects in the cardinal/uterosacral ligament complex can result in high rectoceles and can involve enteroceles. Enteroceles are estimated to be present in 0.1% to 16% of women undergoing surgery for POP (Chou et al, 2000). Loss of support in the mid vagina from the lateral attachments to the arcus tendineus fascia rectovaginalis will result in a bulging in the midportion of the posterior compartment. The perineal body is suspended from the sacrum by the contiguous support of the uterosacral ligaments and rectovaginal septum. Any break in this support system will lead to a hypermobile perineal body that descends with increases in intra-abdominal pressures (Richardson, 1993). Separation of the perineal body at the level of the rectovaginal fascia results in perineal descent or a low rectocele. The rectocele will be visible just inside the hymen posteriorly (Fig. 72–10). If perineal body detachment is left untreated at the time of rectocele repair, a perineal rectocele can occur in which the rectum bulges into the perineal body, termed a perineal rectocele (Richardson, 1995).
Preparing the Patient for Prolapse Surgery
Because pelvic organ prolapse is, for the most part, a quality of life issue, consideration must be given to the grade of prolapse, patient’s symptoms, and the degree to which the patient’s quality of life is impaired (Novara and Artibani, 2005). In selecting surgical procedure(s), one must individualize management based on the unique clinical presentation of each patient, taking into account physiologic age, comorbidities, previous surgeries, and level of physical and sexual activity (Flynn and Webster, 2002). Once the decision has been made to proceed with surgery, it is imperative to recall that the best chance at restoring normal support and function is to reapproximate normal anatomy during the first surgery (Rogers, 2003). After the first procedure, the normal anatomic planes will no longer be present, which may add to the difficulty and complexity of subsequent surgeries. Recurrence rates increase with each attempt to surgically correct the defect(s) (Birch, 2005; Maher et al, 2006). In addition, the surgeon who operates in the region of the pelvic floor must understand the anatomic alterations that result from each technique and how that surgery will affect the continuity and function of the endopelvic fascia and its related viscera (Rogers, 2003).
Repairs can be defined as restorative, compensatory, and obliterative (Van Rooyen and Cundiff, 2005). In patients whose comorbidities preclude prolonged surgery, obliterative procedures provide symptom relief with minimal morbidity. These procedures are contraindicated in patients who wish to remain sexually active. For those patients who have discrete defects in the fibromuscular layer of the endopelvic fascia, restorative repairs are an appropriate option. These repairs correct the defined defects in the native tissues by utilizing endogenous support structures. For patients whose native tissues are particularly weak or in those who have had failed surgeries, compensatory procedures have been used. These procedures involve placing a graft to reinforce the repair. Grafts are made of synthetic materials and biologic and autologous tissues.
In assessing tension of the repair at the time of surgery, the pelvic floor surgeon should recall that the muscles of the pelvic floor are relaxed under anesthesia (general or spinal). Postoperatively, these muscles will contract along with the endopelvic fascia due to its own contractile fibers. Furthermore, scarring and postmenopausal atrophy may also cause further narrowing (Segal and Karram, 2002). Although the etiology of postsurgical dyspareunia is not fully understood, these factors should be kept in mind when repairing the pelvic floor.
Pelvic floor reconstruction may be performed through a vaginal, open abdominal, laparoscopic approach or any combination of these techniques. Because POP is often mixed, surgery commonly involves a combination of repairs addressing any affected compartments. In addition, an anti-incontinence procedure may be performed at the same time. In comparing the vaginal approach with the abdominal approach there is a benefit with the vaginal approach in that complications and recovery time are less with the vaginal approach (Weber and Richter, 2005). The operative time is generally less with the vaginal approach, as is the hospital stay and recovery time (Morley and DeLancey, 1988; Shull, 1999). However, there is evidence that the abdominal approach is more durable compared with a vaginal technique (Benson et al, 1996). This fact should be considered in younger and more active patients.
Preoperative Counseling of Patient for Vaginal POP Surgery
Over the past decade the definition of success has been expanded to include patient satisfaction and symptom improvement in addition to objective measures. Patients’ expectations and readiness to undergo surgery for POP have been shown to impact their satisfaction and how they perceive their improvement (Kenton et al, 2007). In the first article to examine patient-selected goals and surgical outcomes, Elkadry and coworkers (2003) found that patient satisfaction after surgery for POP correlated highly with achievement of self-described goals. In contrast, patient satisfaction correlated poorly with objective measures of surgical success. Dissatisfaction was highly associated with feeling unprepared for surgery. Furthermore, patients perceived the experience of routine postoperative events such as pain, hospital discharge with a catheter, constipation, urgency incontinence, and minor effects of anesthesia as surgical complications. These perceived complications were also associated with dissatisfaction. Additionally, they also demonstrated dissatisfaction despite high cure rates. Thus, patient perception of perioperative events and bother from recurrent symptoms equate largely to postoperative dissatisfaction even in the presence of high objective cure rates. It is vitally important to recognize that the absence of symptoms influences patient satisfaction greater than the elimination of anatomic prolapse. Kenton and associates (2007) evaluated patients with standardized counseling using a three-page handout on what to expect after surgery. Women who perceived they were fully prepared for surgery were more likely to be improved on the Patient Global Impression of Improvement (PGI-I) scale (Yalcin and Bump, 2003) and had lower postoperative scores on the Pelvic Organ Prolapse Distress Inventory (POPDI) (Barber et al, 2001) and Urogenital Distress Inventory (UDI) (Shumaker et al, 1994). Also, they found that objective measures of cure did not differ by preparedness. In conclusion, the preoperative surgeon-patient interaction is a key time that can be used to improve postoperative patient satisfaction. Therefore it is recommended to complete a thorough consent about the surgery, alternatives to surgery, the purpose of planned surgery (what it can and cannot accomplish), the benefits of surgery (what symptoms can be improved), the risks, and complications. In addition, it is useful to review what to expect while the patient is in the hospital, what to expect at home, and strategies on coping with a catheter both in the hospital and at home if needed (Kenton et al, 2007).
In 2001 the Agency for Healthcare Research and Quality (AHRQ) identified perioperative interventions to improve patient safety (Shojania et al, 2001). These include asking the patient to recall and restate what she was told during the informed consent process, administering appropriate antibiotic prophylaxis to prevent postoperative infections, and implementing prophylaxis to prevent venous thromboembolism in patients at risk.
The second most common source of nosocomial infections is infection at the surgical site (Bratzler and Houck, 2004). Current guidelines by the Centers for Medicare and Medicaid Services recommend that prophylactic antibiotics be administered within 60 minutes of incision time and discontinued within 24 hours of surgery (Centers for Medicare and Medicaid Services, 2008). The antibiotic chosen should be active against the likely infectious organisms to be encountered during the surgery performed. Although the American College of Obstetricians and Gynecologists has provided recommendations for antibiotic prophylaxis in patients undergoing hysterectomy there are no current guidelines addressing prolapse operations without hysterectomy (ACOG, 2009). Most favor the use of prophylactic antibiotics in reconstructive vaginal surgery, especially in surgery involving synthetic and biologic grafts.
The risk of deep venous thrombosis (DVT) after major gynecologic surgery in patients not receiving prophylaxis is between 15% and 40% (Gutt et al, 2005). Risk factors include increasing age, previous venous thromboembolism, gynecologic malignancy surgery, and major pelvic reconstructive surgery. Waetjen and associates (2003) found that 42% of patients having surgery for POP were older than 60. The Centers for Medicare and Medicaid Services has endorsed a risk stratification presented by Geerts and colleagues (2004) that states that patients older than age 60 undergoing major surgery are at high risk for DVT. Without prophylaxis, high-risk patients have a 20% to 40% risk for calf DVT, a 2% to 4% risk for pulmonary embolism, and a 1% risk of fatal pulmonary embolism. They have recommended both anticoagulant-based and mechanical prophylaxis in patients at high risk for DVT. Specifically, heparin 5000 units three times a day and low-molecular-weight heparin with or without sequential compression devices are acceptable choices.
Patients with advanced POP and incomplete bladder emptying may be taught to perform clean intermittent catheterization (CIC) preoperatively in the event that complete bladder emptying becomes a problem postoperatively. In postmenopausal women, local estrogen therapy with vaginal cream to treat atrophic vaginitis is started 6 weeks preoperatively. The administration of unopposed low-dose vaginally administered estrogen has been shown to be both safe and effective (Robinson and Cardozo, 2003). Also, local estrogens may increase vascularity and facilitate healing (Cervigni, 1996). The use of yellowfin (Allen, Acton, MA) or comparable stirrups, careful positioning of the patient in a neutral anatomic position, and avoiding leaning on or into the patient will minimize the risk of neuropathy, specifically to the lateral peroneal nerves.
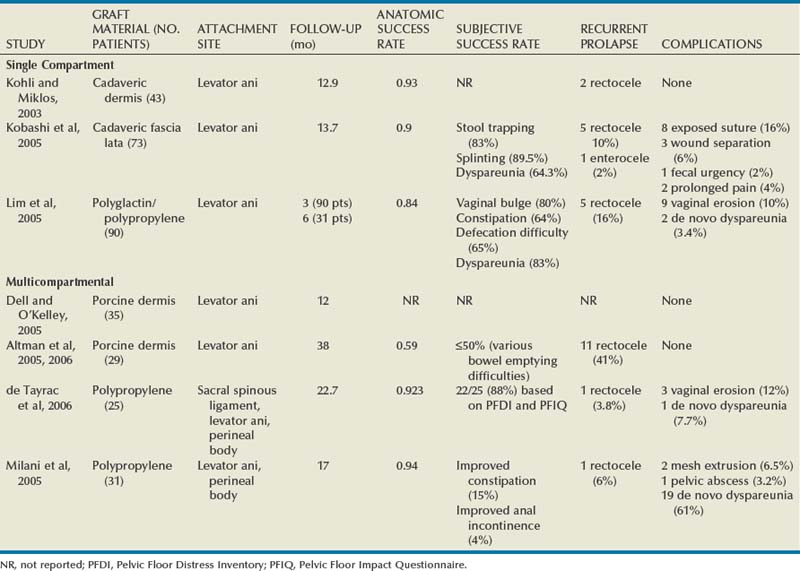
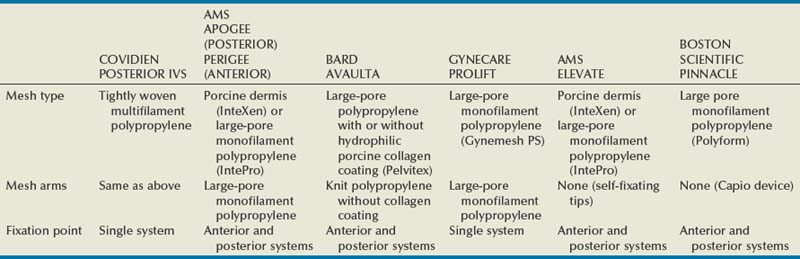
Biologic and Synthetic Materials in Prolapse Surgery
Although there has been an increased use of interposition graft materials in the area of pelvic floor reconstruction there are little controlled data to recommend their use. Graft materials have been used in an effort to improve outcomes of transvaginal prolapse repairs. The risk factors for failure of prolapse surgery include age, vaginal parity, smoking, deficient tissue quality, conditions that impair wound healing (diabetes mellitus and corticosteroid use), and conditions that stress the repair (chronic constipation, chronic obstructive pulmonary disease, and obesity). When examining the issue of failed prolapse surgery there are limitations. The definition of failure is not standardized, and many studies are short term, lacking controls. However, in spite of the limitations it is clear that failure of prolapse surgery is not uncommon. Some patients with POP have specific deficiencies in their tissues, which may predispose them to failure with traditional restorative procedures. Abnormal type I to type III collagen ratios have been identified, leading to a less organized collagen matrix (Falconer et al, 1998). Normal collagen protects fibroblasts from apoptosis, whereas abnormal fibroblasts are not protective (He et al, 2002). Decreased numbers and impaired function of fibroblasts have also been cited (Poncet et al, 2005). Overexpression of matrix metalloproteinases that break down extracellular matrix proteins have been demonstrated in women with prolapse (Jackson et al, 1996). These factors imply that these affected tissues may be less likely to respond to the dynamic forces placed on the female pelvic floor (Chen et al, 2004). As a result of these potential tissue disorders and inherent tissue weakness, graft interposition has been utilized with increased frequency. Although there is level I evidence supporting the use of synthetic graft materials for abdominal sacrocolpopexy (Davila et al, 2005) there is little evidence regarding the most appropriate usage of transvaginal mesh in prolapse repairs.
Classification of Graft Materials
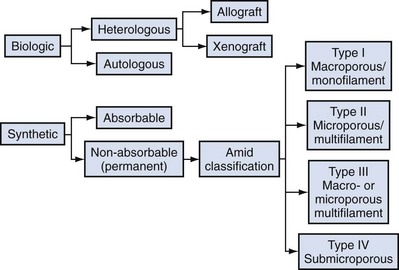
The ideal prosthetic implant would be biocompatible, chemically inert, noncarcinogenic, mechanically strong, and sterile, have minimal allergic or inflammatory reaction, and avoid shrinkage and mechanical stress (Birch, 2005). In addition, the ideal implant should be readily available and affordable. Grafts may be categorized as either synthetic or biologic, and both graft types are used in pelvic floor reconstruction. Synthetic graft materials are usually classified as absorbable or nonabsorbable (permanent). Permanent graft materials are usually classified by pore size (macroporous, microporous, submicroporous, and combined) and by material structure (monofilament or multifilament). Biologic grafts are classified by source—autologous or heterologous—and further categorized as allografts or xenografts (Fig. 72–11).
Basic Science: Graft-versus-Host Interaction
Graft incorporation of the tissues involves a foreign body response. Although many materials used for synthetic grafts are reported to be chemically and physically inert and nonimmunogenic (Davila et al, 2005; Deprest et al, 2006), none is biologically inert. Regardless of the material type, wound healing with a graft follows a stepwise cascade. Initially a biofilm is produced in response to tissue injury. Low-molecular-weight protein adsorption occurs quickly at the interface and does not require a cellular response. Complex proteins such as fibrinogen, immunoglobulins, and extracellular matrix proteins follow. If bacteria are incorporated into the biofilm at this point later wound healing can be affected (Deprest et al, 2006). These complex proteins undergo a conformational change to become more immunogenic, which leads to an inflammatory cascade by activating complement, binding antibodies, leukocytes, blood clotting, and fibrinolysis (Tang and Eaton, 1995). At this point the acute inflammatory response becomes chronic and granulation tissue forms. This involves fibroblasts, macrophages, giant cells, and, later, neovascularization and fibrosis. At this point the implant is mechanically stable. The amount of foreign body reaction is proportional to the surface area of the material exposed to the host. The degree of response and amount of tissue ingrowth is determined by the nature of the material, its structure, and the amount implanted for biologic grafts (Deprest et al, 2006). Ideally, host-tissue integration into the graft takes place, leading to long-term graft function after this biologic transformation. This illustrates an important concept in that the graft, biologic or synthetic, acts as a scaffold to provide a substrate to facilitate tissue ingrowth, rather than it functioning as a permanent mechanical support (Birch, 2005).
Once a graft has been implanted into the human host, one of four processes may occur: (1) rejection in which there is severe and chronic inflammatory reaction around the implant, (2) degeneration (the result is necrosis from in-situ exothermic polymerization by the host), (3) encapsulation (minimal foreign body response in which there is fibrosis around the graft), or (4) incorporation into the human host tissue (a response in which there are inflammatory cells and giant cells leading to ingrowth of host tissues into the graft) (Williams, 1973). For long-term graft survival it appears that incorporation by the host through a process known as graft remodeling is necessary (Fig. 72–12).
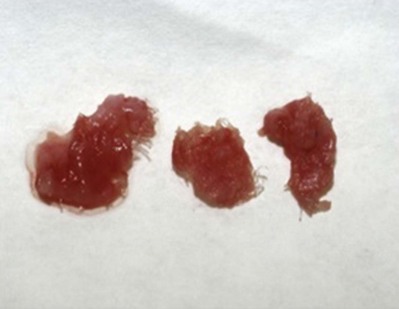
Figure 72–12 Host tissue ingrowth (process of graft remodeling) into polypropylene mesh.
(From Woodruff A, Cole E, Scarpero H, et al. A histologic analysis of sling graft materials: a comparative study. Urology 2008;72:85–9, with permission.)
Pore size affects host fibroblast infiltration, flexibility, and mechanical integration of the graft (Klinge et al, 2002). Large pore size has been shown to facilitate this process by enhancing host tissue infiltration and reducing the amount of inflammatory reaction with increased fibroblast infiltration. The large pores become integrated in a loose network of perifilament granulomas filled with fat, which maintains elasticity. After ingrowth of host tissue, a process of transformation occurs to complete the process of remodeling. Solid products or products with small pores (< 50 µm) induce a foreign body reaction that fills the entire pore. This promotes the bridging of the filaments to each other, thereby predisposing to encapsulation (Beets et al, 1996).
Kaupp and colleagues (1979) described four histologic stages of the host to biologic graft implantation:
Once the entire graft is infiltrated with host tissue the transformation process is complete. If this remodeling process characterized by ingrowth and transformation occurs before the graft substrate dissolves, long-term viability of the implant may be ensured. Although synthetic mesh material is a permanent substrate, the principles of tissue incorporation are necessary to prevent infection, extrusion, or erosion. Extrusion is thought to be secondary to exposed graft, which is attributable to localized infection, inadequate closure of the wound, or poor tissue quality (decreased vascularity or thickness) (Birch, 2005). Erosion is thought to be a chronic process related to host inflammatory response at the tissue-graft interface.
Clinical Application of Graft Materials
Biologic Grafts
Because of concerns regarding potential complications associated with synthetic grafts, some authors have turned to biologic grafts to circumvent these complications (Togami et al, 2008). Biologic grafts are categorized as autologous (patient serves as the donor), heterologous (same species, different individual [cadaveric]), and xenogenic (obtained from other species, typically porcine). The advantages of biologic grafts include in-vivo tissue remodeling, histologic similarity, and decreased propensity to elicit local complications (Silva and Karram, 2005).
The donor sites for autologous grafts include rectus fascia, fascia lata, and vaginal epithelium, with the most common being rectus fascia and fascia lata. Autologous grafts are ideal in that they are well incorporated, pose no threat of disease transmission, and have minimal risk of encapsulation or rejection. The major drawback is the harvesting process, with increased morbidity, time, and potential for complications at the donor site. These factors are eliminated by using allografts and xenografts (Jarvis et al, 1985). Allografts are obtained from cadaveric donors and include dura mater, fascia lata, and dermis. Cadaveric fascia lata and dermis are used most commonly. A major concern when utilizing biologic materials is the potential for disease transmission. Choe and Bell (2001) demonstrated intact DNA in freeze-dried/irradiated cadaveric fascia lata and freeze-dried cadaveric dermal allograft. Fitzgerald and associates (2000) also examined irradiated freeze-dried or solvent dehydrated cadaveric fascia lata and found that it retained the donor human leukocyte antigen (HLA) class I and II antigens. Although extensive steps are taken to prevent infection exposure, risk of prion and human immunodeficiency virus transmission is estimated to be 1 in 1.7 million (Buck et al, 1989). To date there have not been any reports of disease transmission from grafts used for POP.
The harvesting techniques of nonautologous biologic graft materials are standardized. In their review, Chen and colleagues (2007) described the process of allograft acquisition. Before allografts are harvested the donor undergoes serologic testing for hepatitis B and C, human immunodeficiency virus, and human T-lymphotropic virus type 1. Aseptic technique is used during harvest, and cultures are obtained at the time. The source animals for xenografts are specifically raised for medical purposes with production being strictly controlled by U.S. Food and Drug Administration (FDA) guidelines (Deprest et al, 2006). Materials available are derived from porcine subintestinal mucosa, porcine dermis, bovine dermis, and bovine pericardium, although the most commonly used are from porcine sources. These function as acellular collagen–based scaffolds to serve as a platform for host infiltration.
Allografts and xenografts must undergo tissue processing before implantation, and, unlike harvesting, there is a variance in the processing techniques of these materials. This variance may affect the biologic and biomechanical properties of the grafts. There is no consensus as to which method should be used to optimize tissue properties. In some cases, processing is carried out to affect the long-term effects of the tissue to decrease graft breakdown and increase host tissue ingrowth. Sterilization is achieved by freeze-drying, solvent dehydration, and/or gamma irradiation. Lemer and associates (1999) demonstrated that freeze-dried cadaveric fascia demonstrated the least desirable characteristics when compared with autologous rectus fascia, cadaveric dermis, and solvent dehydrated cadaveric fascia lata. The freeze-dried cadaveric fascia demonstrated a reduced maximum load to failure and stiffness. In addition there was greater variability in the tissue’s strength and stiffness throughout its entire length.
Cross-linking is another processing technique that can affect graft performance. This is done to delay reabsorption by collagenases (Badylak et al, 2002). Several methods are used, including aldehyde cross-linking and hexamethylene di-isocyanate (HMDI). Aldehydes are cytotoxic in high concentrations and may increase concentrations of gelatinases, which may actually increase the rate of degradation (Jorge-Herrero et al, 2001). In addition, aldehydes may cause calcification of the grafts, adversely affecting their function. Studies performed in rats have shown no graft mineralization with HMDI cross-linked dermal implantation at 2 years in contrast to aldehydes (Oliver, 1987).
Local complications such as encapsulation may occur after the use of porcine dermis grafts (Cole et al, 2003). Graft fenestrations have been reported to enhance ingrowth and angiogenesis (Taylor et al, 2008). They may also decrease seroma formation and local complications. Porcine small intestine submucosa (Surgisis, Cook Medical, Bloomington, IN) is a non–cross-linked graft processed so that the complex extracellular matrix and natural growth factors are left intact (Cook Medical, 2007). There is histologic evidence that by 1 month the strength and histology of the graft is identical to native material and at 2 years it exceeds the strength of native tissue (Konstantinovic et al, 2005).
The lack of in-vivo data limits the study of many biologic and synthetic grafts. Naturally it would be of benefit to demonstrate how the biomechanical properties of these materials are altered or remodeled by the host. Several animal models have been used to study grafts and meshes. In the rabbit model a free or pedicle flap of autologous rectus fascia decreased 37% in length, 63% in width, and 53% in tensile strength after implantation for 12 weeks. Neovascularization, minimal inflammation, and fibrosis was noted only along the permanent suture used to secure the graft (Fokaefs et al, 1997). In a rabbit model, freeze-dried, irradiated cadaveric fascia lata had a 90% decrease in tensile strength 12 weeks after implantation (Walter et al, 2003). There was variability in tensile strength from lot to lot and from grafts taken from different areas in the same lot. In an extensive rabbit study examining six different graft materials, tensile strength and stiffness of human cadaveric fascia and porcine xenografts decreased by 60% to 89%. Polypropylene (Prolene) mesh and anterior rectus fascia had no change in tensile strength from baseline (Dora et al, 2004). When comparing a tension-free vaginal tape (TVT) (Ethicon, Somerville, NJ) polypropylene sling to a cadaveric fascia lata sling in the rat model there was an advantage for the polypropylene sling in the breakload and maximum average load compared with cadaveric fascia lata (Spiess et al, 2004). There is a wide variation in the types of grafts available and tissue processing they undergo. It is unclear how this affects the performance of the grafts because there are little data comparing them. Before implantation, dermal allografts, solvent dehydrated fascia lata, and synthetic mesh have equal or higher tensile strength compared with autologous fascia. In some studies, freeze-dried grafts have a decreased tensile strength compared with similar grafts that have been solvent dehydrated. After implantation, autologous fascia and synthetic mesh seem to retain more of their tensile strength compared with allografts or xenografts (Chen et al, 2007).
Synthetic Grafts
Synthetic mesh can be permanent or absorbable. Absorbable mesh has several desirable characteristics. It promotes postoperative fibroblast activity, has less infectious disease risk, is not rejected, and has not been reported to be harmful to the viscera. One disadvantage is that the resultant scar tissue may not be as strong as the native tissue it is reinforcing (Klinge et al, 2001). Classification of the synthetic meshes occurs by type of mesh (absorbable or nonabsorbable), pore size (macroporous or microporous), and filament type (monofilament or multifilament). Two types of absorbable meshes are available: polyglycolic acid (Dexon, Davis and Geck, American Cyanamid, Danbury, CT) and polyglactin 910 (Vicryl, Ethicon, Somerville, NJ). They differ in the duration in the tissues. Polyglactin 910 starts to hydrolyze by 21 days and loses its mechanical support by 30 days. Polyglycolic acid takes 90 days for absorption.
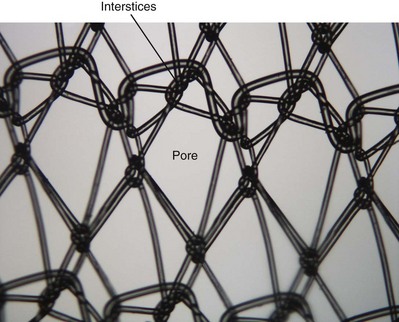
The most important characteristic of a synthetic mesh is its pore size. Meshes are divided into macroporous (>75 µm) or microporous (<10 µm). Pore size is important from an infectious standpoint because it allows cellular elements such as macrophages and granulocytes to enter the mesh construct. In addition, it is important for tissue ingrowth with fibroblasts, blood vessels, and collagen fibrils (Amid, 1997; Deprest et al, 2006; Dwyer, 2006) (Fig. 72–13). Most bacteria are less than 1 µm; granulocytes and macrophages are greater than 10 µm in diameter, but 75 µm is the key size that allows the tissue ingrowth. Pore size also determines the flexural rigidity. The larger the pore size, the more flexible the mesh (Dietz et al, 2003). This property may impact local tissue trauma and erosive risk (Birch, 2005).
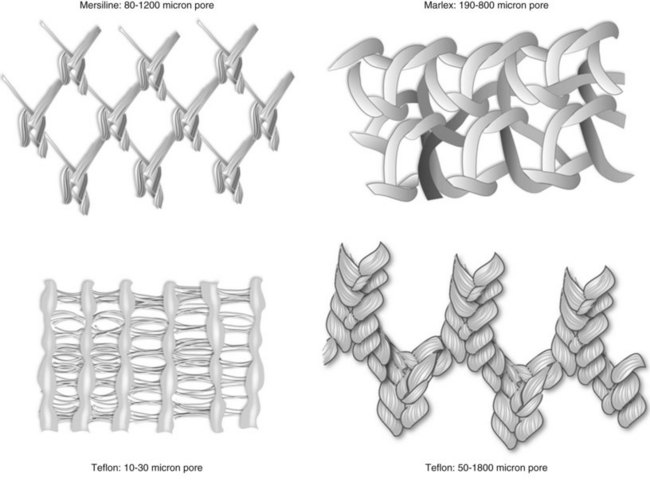
Figure 72–13 Synthetic graft pore and interstices: Note the multifilamentous nature of Mersilene, which may promote bacterial adherence.
(From Togami J, Krlin R, et al. Graft materials in prolapse surgery. AUA Update Series 2008;27(31):294–303.)
Synthetic mesh can be monofilament or multifilament. Multifilament synthetics may have pore sizes that allow them to be classified as macroporous; however, between the fibers the size is less than 10 µm, owing to the way they are either woven or knitted. The spaces are small enough to allow bacteria into confines less than 10 µm, which carries a greater theoretical risk than a monofilament mesh. The construct of the mesh affects pore size, and the interstitial distance is measured between the synthetic fibers (Fig. 72–14). Woven mesh has small pore size and interstices, whereas knitted materials are able to assume a macroporous configuration and are flexible with high tissue conformity.
Although there is no classification system specifically used for mesh in pelvic floor reconstruction, the Amid classification, which was originally used to describe mesh used in the treatment of abdominal wall hernias, is currently the standard (Amid, 1997). This classification emphasizes the importance of pore size and filament type (see Fig. 72–11).
Type I promotes host defenses and tissue infiltration. Type II and type III meshes result in a greater foreign body response and are associated with greater rates of erosion (Julian, 1996; Debodinance et al, 1999). The most desirable synthetic for pelvic floor reconstruction are monofilament, macroporous mesh, most commonly polypropylene mesh.
Surgical Management of Pelvic Organ Prolapse (Table 72–2)
Anterior Compartment
Anterior Colporrhaphy
Kelly described his method of cystocele repair as a treatment for urinary incontinence in 1913. He emphasized the importance of repairing the pubocervical fascia with plication sutures to repair the central defect. This procedure eventually became known as the anterior colporrhaphy, and it corrects the central defect in the anterior compartment. However, anterior compartment defects are commonly combined central and lateral defects. Thus, an isolated anterior colporrhaphy is not recommended except in rare circumstances. In addition, the American Urological Association Female Stress Urinary Incontinence Clinical Guidelines Panel, in 1997, performed a meta-analysis and found that the failure rate of anterior colporrhaphy as a treatment for stress urinary incontinence (SUI) was close to 40% after 4 years of follow-up (Leach et al, 1997). As such, anterior colporrhaphy is not recommended as treatment for SUI.
Table 72–2 Surgical Approach to Pelvic Organ Prolapse
| POP-Q SITE | VAGINAL | ABDOMINAL |
|---|---|---|
| Aa | Retropubic urethropexy | |
| Urethra | ||
| Ba | ||
| Bladder | ||
| C | ||
| Cervix/cuff | ||
| D | McCall culdoplasty | |
| Cul-de-sac | ||
| Ap | Colpoperineopexy |
Technique (Fig. 72–15)
After placement of the patient in the dorsal lithotomy position an indwelling urethral catheter is placed and the bladder is kept drained throughout the procedure. Hydrodissection may be utilized to facilitate the dissection. A midline incision is made in the anterior vaginal wall, extending from the vaginal apex to the bladder neck. The incision should not extend to the urethra when a simultaneous midurethral sling is performed. Optimally the sling should be placed through a separate, suburethral incision. However, when performing a pubovaginal sling, it is desirable to create one incision that extends to the distal urethra and facilitates more precise sling location. The vaginal epithelium is dissected off the pubocervical fascia, and the dissection should be carried out sufficiently to delineate the defect. Usually, the incision should extend as far laterally as possible. Allis clamps or a self-retaining ring retractor may be utilized to provide optimal exposure. The bladder is then reduced with a finger or instrument, and this facilitates exposure and reapproximation of the pubocervical tissues. Once these tissues are identified, interrupted 2-0 delayed absorbable plication sutures are then placed from the bladder neck to the apex. The plication sutures are then tied. Care must be taken to avoid excessively deep suture placement to avoid ureteral injury. If the pubocervical fascia is too attenuated, a piece of allograft or mesh can be placed under the vaginal wall on one side to strengthen the repair and prevent suture pull-through (Chesson et al, 1999). After completion of the colporrhaphy, cystoscopy is performed after the administration of indigo carmine or methylene blue to inspect the bladder for any iatrogenic injury and to visualize urine effluxing from each ureteral orifice. Finally, the vaginal wall is trimmed and closed with a 2-0 absorbable suture.
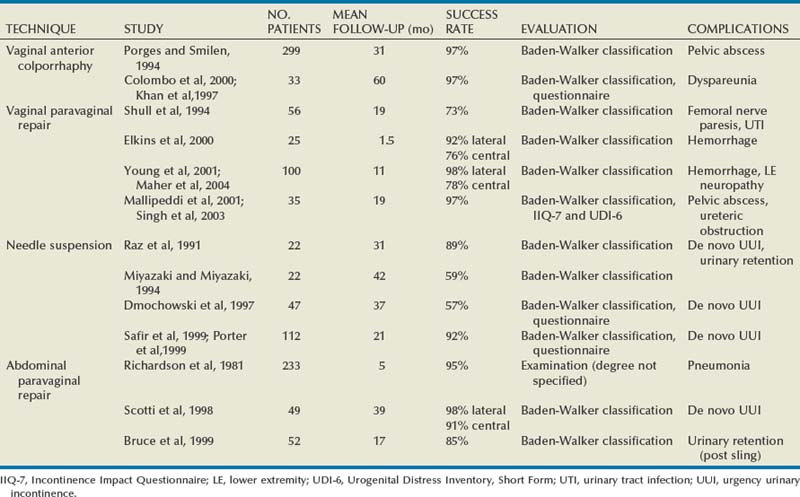
Results
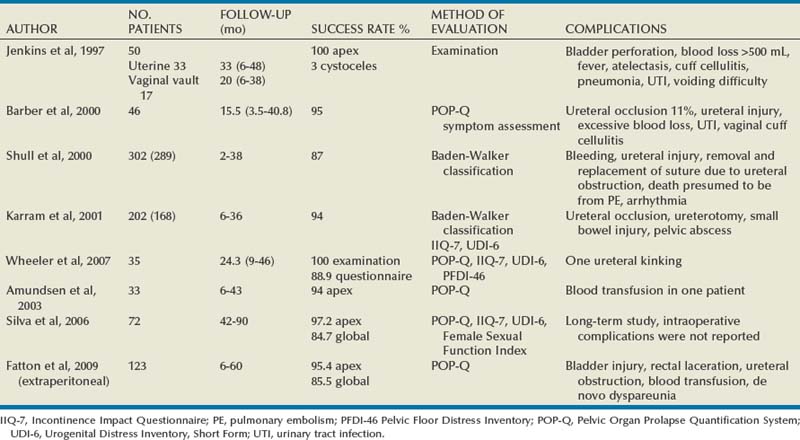
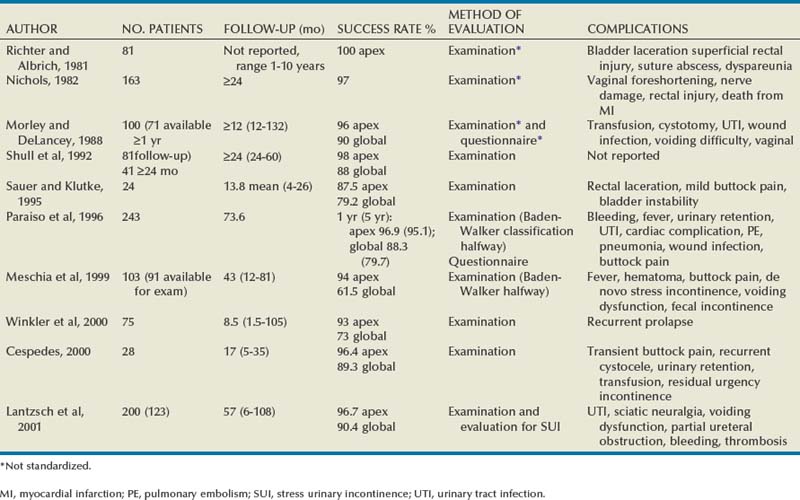
In earlier studies, the reported cure rate of cystocele approached 95% (Table 72–3). In a large, retrospective study that included 299 patients with anterior vaginal wall prolapse, Porges and Smilen (1994) reported a recurrence rate of only 3%, with a mean follow-up of 31 months. Recurrence was defined as only symptomatic descent for which further surgery was performed or recommended. Also, in a prospective, randomized trial between Burch colposuspension versus anterior colporrhaphy, Colombo and colleagues (2000) reported a recurrence rate again of only 3% in the 33 patients who underwent anterior colporrhaphy. The mean follow-up was 5 years. All of the patients in both groups underwent concomitant hysterectomy and uterosacral ligament fixation. Recent series have reported recurrence rates for standard anterior colporrhaphy that approach 40% (Table 72–4). Recurrence in these series included asymptomatic grade 2 or stage 2 anterior compartment prolapse. Sand and associates (2001) reported on 161 women randomized to either anterior colporrhaphy with polyglactin suture or anterior colporrhaphy with a free polyglactin mesh inlay, which was placed underneath the trigone after plicating the pubocervical fascia. After 1 year of follow-up, the women randomized to polyglactin mesh had a failure rate of 25% compared with the 43% failure rate in those women who underwent the plication with suture alone (P = .02). Weber and colleagues (2001) compared anterior colporrhaphy reinforced with polyglactin mesh to traditional plication without tension using polydioxanone suture and “ultralateral” plication using both polydioxanone suture and tension. In this study, 114 women with symptomatic cystoceles (mostly stages 2 and 3 on the pelvic organ prolapse quantification [POP-Q] system) were randomized between the three groups. With a mean follow-up of 23 months there was no significant difference in failure rate (defined as stage 2 or greater on the POP-Q) between the three groups. The failure rate was 30% in those who underwent traditional plication, 46% in those who underwent ultralateral plication, and 42% in those who underwent traditional plication augmented with polyglactin mesh. Unfortunately, the study did not recruit enough women to achieve statistical power to detect differences between the groups. This is in contrast to an earlier paper by the same author that stated that anterior vaginal wall prolapse recurs after standard anterior colporrhaphy in 20% of patients (Weber and Walter, 1997). The Sand and Weber trials are not similar enough to do a meta-analysis. Yet, the high recurrence rates observed with anterior colporrhaphy illustrates the main concern regarding the durability of this procedure in most women with moderate- or high-grade anterior compartment defects. The most likely contributing factor is the concomitant presence of other compartmental defects.
Complications
Reported complications from anterior colporrhaphy include de novo SUI (if the patient had SUI masked by a high-grade cystocele), de novo detrusor overactivity, postoperative urinary retention, incomplete bladder emptying, significant bleeding of more than 350 mL, wound infection, bladder or ureteral injuries, vaginal shortening, and dyspareunia. De novo detrusor overactivity can occur in 5% to 7% of patients after standard colporrhaphy (Raz et al, 1991). Yet, preexisting detrusor overactivity has been reported to resolve in up to 63% of patients after surgical repair of pelvic organ prolapsed (Nguyen and Bhattia, 2001). Urinary retention usually happens in cases during which an anti-incontinence procedure is performed. In most instances, the retention resolves after a brief period of CIC. If the retention persists and the patient had a concomitant anti-incontinence procedure, urethrolysis is often required. When impaired bladder emptying is due to preexisting hypocontractility, the patient may require chronic CIC or neuromodulation.
Bleeding during anterior colporrhaphy is usually due to both the high vascularity of the vagina and to the venous complex beneath the inferior pubic bone. During transvaginal anterior colporrhaphy, problematic bleeding can occur if the dissection is carried out in the wrong plane. During anterior colporrhaphy, the vaginal wall should be dissected off of the pubocervical fascia directly on its white shiny surface. In patients undergoing repeat anterior colporrhaphy, scarring will often obscure the correct dissection plane. As such, these patients are at higher risk for bleeding.
Bladder or ureteral injuries are rare during standard anterior colporrhaphy. Bladder injuries can occur when perforation into the retropubic space occurs. Ensuring bladder drainage before perforating the endopelvic fascia may reduce this. Should an inadvertent bladder injury occur, a two-layer closure should be performed with absorbable suture. If the patient has a history of pelvic irradiation or if the repair quality seems tenuous, a labial fat pad interposition should be considered to prevent vesicovaginal fistula formation (Kreder, 1993). Cystoscopy after the administration of indigo carmine should be routinely performed with every anterior colporrhaphy. If blue-stained urine is not seen effluxing from either ureteral orifice, ureteral catheterization should be considered before taking down the plication sutures. In some cases the ureter is patent and an alternative reason will explain the lack of efflux. Appropriate steps must be taken to ensure ureteral patency if the ureter cannot be catheterized. This may include taking down the repair.
Procedures for Lateral and Combined Defects
Vaginal Paravaginal Repair
In his first description of a vaginal paravaginal repair, George White (1909) described a cystocele repair that involved suturing the lateral sulci of the vagina to the white line of the pelvic fascia. White believed that the cause of a cystocele was weakness of the lateral vaginal attachments to the ATFP. As Kelly’s anterior colporrhaphy became the primary technique of cystocele repair for the next 70 years, White’s concept fell into disfavor. In 1976, Richardson and coworkers (1976) reintroduced the paravaginal defect repair, but they described the technique using an abdominal approach. Regardless of the approach the goal of a paravaginal repair is to repair a lateral compartment defect by reattaching the pubocervical fascia to the ATFP. A vaginal paravaginal repair may be combined with anterior colporrhaphy in patients with combined lateral and central defects.
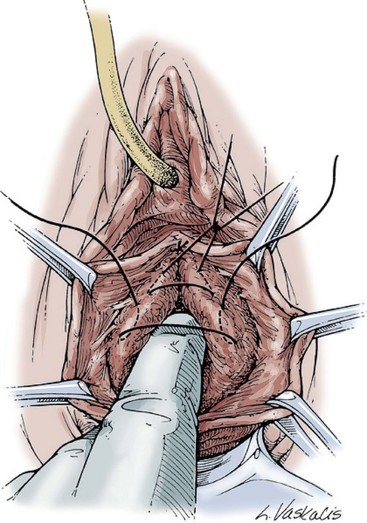
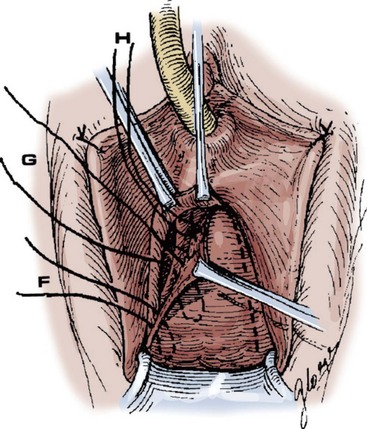
Technique (Fig. 72-16)
A midline anterior vertical incision is made through the vaginal epithelium from the bladder neck to the vaginal apex. After the vaginal epithelium is sharply dissected off the attenuated pubocervical fascia and bladder the dissection is carried laterally to the pelvic sidewall. Blunt dissection can be used to facilitate this. Access to the pelvic sidewall is achieved by either continued lateral dissection or perforation of the endopelvic fascia. On entry into the retropubic space, palpation is utilized to identify the ATFP as it courses from the ischial spine to the inferior aspect of the pubic ramus. Deep, lighted retractors may be useful to facilitate exposure. With the bladder retracted medially, five to seven interrupted nonabsorbable sutures are placed at 1-cm intervals through the ATFP. A Capio Suture Capturing Device (Boston Scientific, Natick, MA) may expedite placement of these sutures. These sutures are then passed through the lateral edge of the detached pubocervical fascia. No sutures should be tied until all are placed on either one side or both sides (if bilateral defects). At this point, if indicated, central defect plication sutures may be placed to complete a combined repair. As with anterior colporrhaphy, cystoscopy must be performed to confirm ureteral patency and the absence of intravesical sutures. The vaginal wall is trimmed and closed with a 2-0 absorbable suture. Vaginal packing is placed.
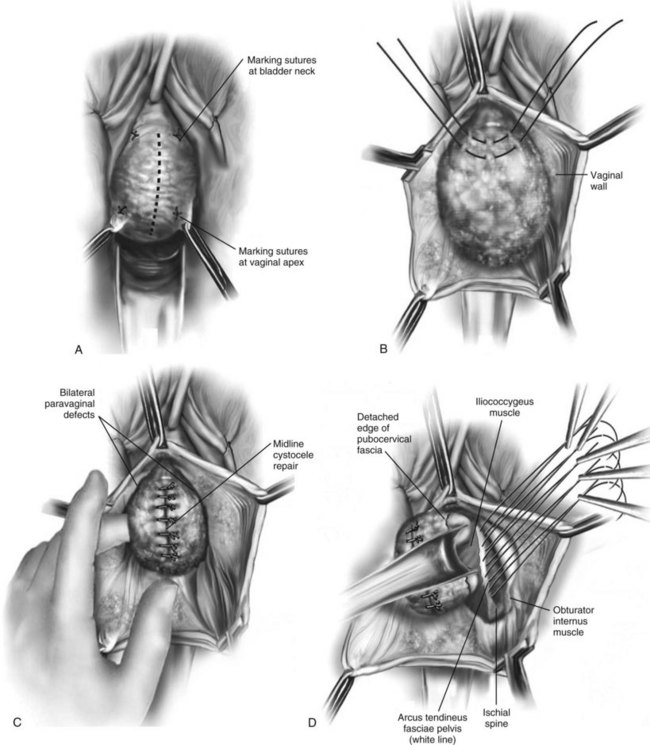
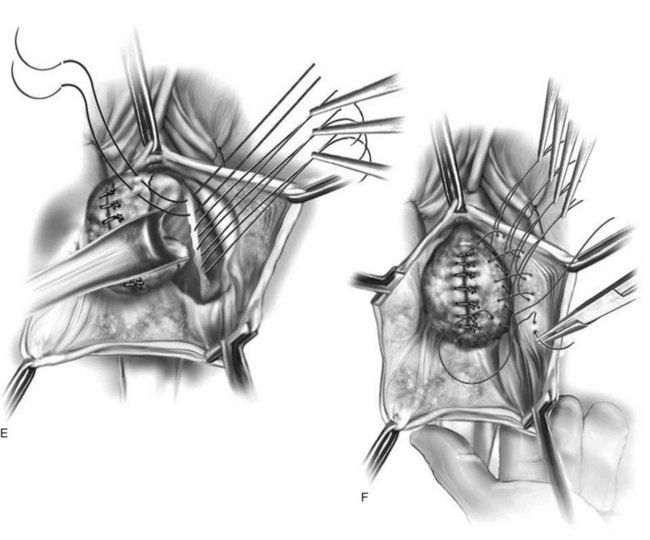
Figure 72–16 Vaginal paravaginal repair. A, Unopened anterior vaginal wall with marking sutures placed at anatomic level of bladder neck and vaginal apex. B, Anterior vaginal wall opened via a midline incision. Sutures placed for midline cystocele repair. C, Midline cystocele repair completed. Bilateral paravaginal defects identified. D, Bladder retracted medially to expose lateral pelvic sidewall. Permanent sutures have been passed through the white line. E, Top two sutures have been passed through detached edge of pubocervical fascia. F, Three-point closure is completed with all sutures passed through the pubocervical fascia and inside wall of the vagina.
(From Mallipeddi PK, Steele AC, Kohli N, et al. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior vaginal wall prolapse. Int Urogynecol J 2001;12: 83–88, with permission.)
Results (see Table 72–4)
Shull and colleagues (1994) reported that 41 patients (73%) had no cystocele recurrence with vaginal paravaginal repair after a mean follow-up of 1.6 years. Of the 15 recurrences, only 4 developed to or through the hymen, and in these the prolapse was less than what was noted preoperatively. Elkins and associates (2000) reported an 8% lateral cystocele recurrence rate and a 22% central cystocele recurrence rate 0.5 to 3 years after vaginal paravaginal repair in 25 patients with grade 3 or greater anterior wall vaginal prolapse. Midline central defects were common, leading surgeons to perform joint repairs with an anterior colporrhaphy. Young and coworkers (2001) retrospectively evaluated 100 patients with symptomatic grade 2 to 4 combined central and lateral cystoceles. All 100 patients underwent both vaginal paravaginal repair and concomitant anterior colporrhaphy. With a mean follow-up of 11 months, the lateral cystocele recurrence rate was only 2% and the central cystocele recurrence rate was 22%. Mallipeddi and colleagues (2001) reported a 3% central cystocele recurrence 20 months after vaginal paravaginal repair in 35 patients with grades 2 to 4 anterior vaginal wall prolapse. Of the 21 patients with concomitant SUI, 12 (57%) had persistent SUI after the paravaginal repair. The paravaginal repair, like standard anterior colporrhaphy, is ineffective in the treatment of SUI. Finally, there are no studies comparing paravaginal defect repair with or without midline anterior repair to standard anterior colporrhaphy alone.
Although complications after vaginal paravaginal repair are infrequent, they are significant. Young and associates (2001) reported 21 major complications, including three major intraoperative hemorrhagic complications. Two patients developed lower extremity neuropathy, one of whom was in the lithotomy position for a prolonged period of time. Elkins and associates (2000) reported a transfusion rate of 12%. Mallipeddi and coworkers (2001) reported 2 patients with vaginal abscesses that required drainage, 1 patient with retropubic hematoma who required reexploration, and 1 patient with bilateral ureteric obstruction that resolved after takedown of the repair and suture replacement.
The vaginal paravaginal repair as described is technically more challenging than the standard anterior colporrhaphy. Complication rates are higher and usually more serious. In addition, the conventional vaginal paravaginal repair relies on suture placement through weakened pubocervical fascia. To compensate for these factors, most surgeons describe transvaginal correction of lateral defects using placement of grafts, which are attached to the pelvic sidewalls bilaterally.
Anterior Compartment Repairs Utilizing Grafts
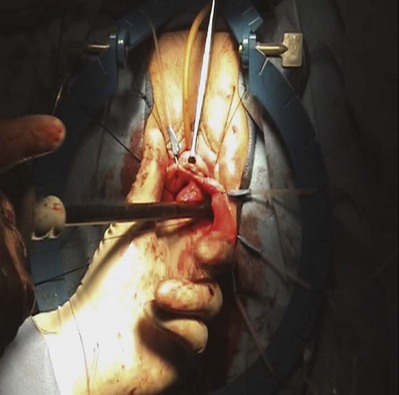
Technique
The incision and dissection into the retropubic space are performed in a similar manner as for the vaginal paravaginal repair. Once access to the pelvic sidewall is achieved, the bladder is retracted medially, and 2 to 3 sutures (permanent or delayed absorbable) are placed into the ATFP and/or obturator internus fascia, which will serve as adequate lateral fixation for the graft. A Capio Suture Capturing Device (Boston Scientific, Natick, MA) may expedite placement of these sutures (Fig. 72–17). Before fashioning the graft it is useful to estimate the distance between the proximal and distal ipsilateral sutures and the distance between the sutures on each side. These measurements serve to determine the dimensions of the graft material, which is trimmed to size. At this point, central plication sutures may be placed. The previously placed pelvic sidewall sutures are then brought out to the corresponding locations on the graft. The sutures are then tied, securing the graft to the pelvic sidewall. It is useful to leave the sutures long until after cystoscopy confirms patency of the ureters. The vaginal wall is trimmed and closed with a 2-0 absorbable suture. Vaginal packing is placed at the end of the case.
Results
Polypropylene Mesh (see Table 72–4)
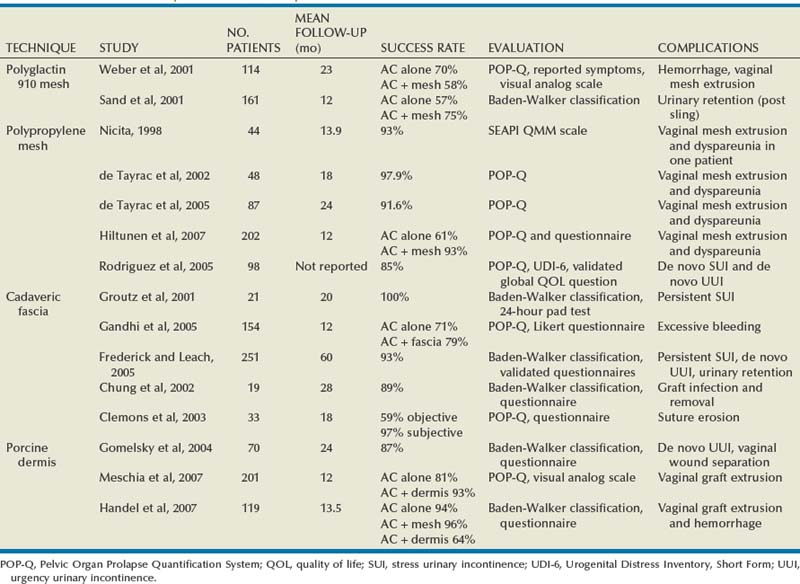
Nicita (1998) described the use of polypropylene mesh for the treatment of anterior vaginal wall prolapsed. He sutured the mesh to the anterior aspect of the ATFP on each side using 3-0 polypropylene sutures. With 2 years of follow-up, only 3 of the 44 patients (7%) had prolapse recurrence. The only patient who developed dyspareunia had vaginal mesh extrusion (exposure), and this was managed with mesh trimming and vaginal closure.
de Tayrac and associates, in 2002, described placement of polypropylene mesh (Gynemesh; Ethicon, Somerville, NJ) into the retropubic space in a tension-free fashion without suture fixation. The lateral extensions of the mesh were placed in contact with the ATFP and anchored with a transobturator approach. A total of 48 women with grade 3 and 4 cystoceles underwent this procedure. After a mean follow-up of 18 months the authors reported an anatomic success rate of 97.9%. Vaginal mesh extrusion was noted in 8.3%. In a subsequent series with 87 women and with 24 months of follow-up, de Tayrac and associates again (2005) used GyneMesh and found that 77 women (91.6%) were cured of their symptomatic anterior vaginal wall prolapse, defined as POP-Q stage 0 or 1. Of the 7 patients with recurrent cystoceles, 5 were asymptomatic. Vaginal mesh extrusion was again noted in 8.3%, and the de novo dyspareunia rate was 16.7%.
In a randomized controlled trial of 202 women with anterior vaginal wall prolapse, Hiltunen and coworkers (2007) compared anterior colporrhaphy (n = 97) to the same procedure reinforced with low-weight polypropylene mesh (n = 105). After 1 year of follow-up the addition of the polypropylene mesh to the anterior repair did statistically decrease the risk of recurrent stage 2 or greater anterior prolapse. Recurrence was noted in 7 of 104 women in the mesh group versus 37 of 96 women in the group with no graft (P < .001). However, there was no statistically significant difference in symptomatic outcomes (pelvic pressure, vaginal bulging, or difficulty with bladder emptying) between the groups. Importantly, 18 patients in the mesh group (17.3%) had vaginal extrusion of the mesh, although only 4 of the 18 patients were symptomatic and none required total removal of the mesh.
In a prospective study of 98 patients with stage 3 and 4 cystoceles using the POP-Q, Rodriguez and associates (2005) attached a 5 × 5-cm piece of polypropylene mesh to the obturator internus fascia using 2-0 polyglactin suture to repair the paravaginal defect (Fig. 72–18). This mesh was placed after repair of the central defect with horizontal mattress sutures of 3-0 polyglactin. All patients underwent a distal urethral polypropylene sling regardless of any SUI symptoms. They reported an 85% anatomic success rate, defined as stage 0 or 1. No patient experienced urinary retention. Three patients who did not report SUI before the procedure developed mild de novo SUI after the procedure. Overall quality of life due to genitourinary symptoms improved from 4.7 (unhappy) to 1 (pleased) (P < .005).
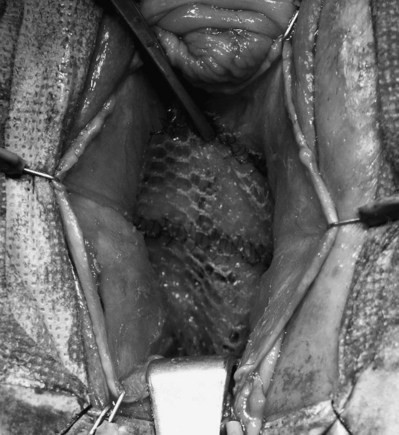
Figure 72–18 Anterior compartment repair with polypropylene mesh graft. Polypropylene mesh is anchored to the uterosacral ligament at the bladder base, laterally to the obturator fascia and distally to the bladder neck using 2-0 polyglactin suture.
(From Rogriguez LR, Bukkapatnam R, Shah SM, et al. Transvaginal paravaginal repair of high-grade cystocele central and lateral defects with concomitant suburethral sling: report of early results, outcomes, and patient satisfaction with a new technique. Urology 2005;66[Suppl. 5A]:57–65.)
Cadaveric Fascia (see Table 72–4)
Groutz and colleagues (2001) prospectively enrolled 21 women with grade 3 or 4 anterior vaginal wall prolapse to cystocele repair using solvent dehydrated cadaveric fascia lata (Fig. 72–19). The graft was anchored transversally between the bilateral arcus tendineus and the cardinal and uterosacral ligaments. Also, 19 of the 21 women underwent concomitant pubovaginal sling for either symptomatic or occult SUI. After a mean follow-up 20 months, none of the patients developed a recurrent cystocele. In addition, no patient developed new-onset dyspareunia, pelvic pain, or postoperative vaginal stenosis.
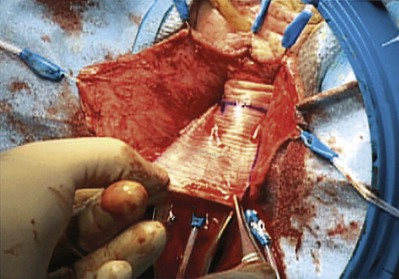
Figure 72–19 Anterior compartment repair with cadaveric fascia graft. Cadaveric fascia patch was tailored appropriately. Typically a 5- × 7-cm patch is more than adequate.
(From Groutz A, Chaikin DC, Theusen E, et al. Use of cadaveric solvent-dehydrated fascia lata for cystocele repair—preliminary results. Urology 2001:58:179–83.)
Gandhi and colleagues (2005) published a prospective randomized controlled trial comparing ultralateral anterior colporrhaphy with 0 polyglactin suture (n = 78) to the same colporrhaphy reinforced with cadaveric fascia lata (n = 76) in women with grade 2 or greater anterior vaginal wall prolapse. Previous surgery did not preclude participation. Although there was a trend toward improved anatomic outcome with graft use, the addition of the fascia lata patch to the anterior repair did not statistically decrease the risk of recurrent grade 2 or greater anterior vaginal wall prolapse (P = .23). Of the 39 total patients with recurrent prolapse, 26 (67%) were asymptomatic. Interestingly, concomitant transvaginal Cooper ligament sling procedures were associated with a decrease in recurrent grade 2 anterior vaginal wall prolapse (P < .0001).
Kobashi and coworkers (2000) first described the cadaveric prolapse repair and sling procedure (CaPS) as combined treatment of both symptomatic cystocele and SUI. The procedure is performed transvaginally using a 6 × 8-cm piece of cadaveric fascia lata and bone anchors. In a noncomparative study of 251 patients with a mean follow-up of 2 years after CaPS, Frederick and Leach (2005) noted a symptomatic cystocele recurrence rate of 7%. Because the cured/dry rate for SUI was 56%, with most of the failures occurring beyond 1 year, the authors questioned the long-term efficacy of the sling portion of the CaPS.
Cadaveric dermis was evaluated by Chung and colleagues (2002) in 19 patients with combined SUI and symptomatic grade 3 cystocele. The proximal portion of the 3 × 7-cm graft was sutured to the uterosacral/cardinal ligament complex to repair the central cystocele defect, and the distal portion of the allograft was used as the sling. Patients with lateral and paravaginal defects were excluded. The mean follow-up was 28 months. One patient developed a graft infection and underwent graft removal. Of the remaining 18 patients, 16 patients had no cystocele recurrence. Seventeen of the 18 patients (94%) were cured of their SUI, including 10 patients who had prior incontinence surgery. Clemons and coworkers (2003) described a vaginal paravaginal repair using cadaveric dermis in 33 women with recurrent stage 2 or with primary or recurrent POP-Q stage 3 or 4 cystoceles. The 3 × 7-cm graft was positioned over the bladder base and secured to the ATFP with four sutures of 2-0 braided permanent polyester bilaterally. The mean follow-up was 18 months. Objectively, 13 patients (41%) had recurrent stage 2 cystoceles; however, only 1 patient (3%) had a symptomatic recurrence. All of the objective failures were failures of point Aa on the POP-Q, implying that the anterior vaginal wall was well supported proximally but not distally. Eight women underwent a concurrent TVT sling, and the TVT sling did not prevent objective failure.
Porcine Dermis (see Table 72–4)
The majority of clinical experience in the use of xenografts for pelvic organ prolapse has been with porcine dermis. Pelvicol (CR Bard, Murray Hill, NJ) is acellular cross-linked porcine dermis (Chen et al, 2007). Gomelsky and associates (2004) reported favorable, durable outcomes using a 6 × 8-cm piece of porcine dermis sutured laterally to the ATFP to correct high-grade cystoceles (Fig. 72–20). In a retrospective review of 70 patients, 61 patients (87%) had no cystocele recurrence after a mean follow-up of 24 months. Of the 9 patients (13%) with recurrent cystoceles, none was symptomatic. One patient had superficial vaginal wound separation, which was treated with conservative measures.
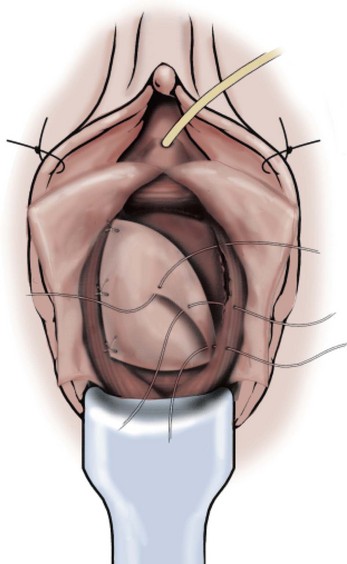
Figure 72–20 Anterior compartment repair with porcine dermis graft. Three pairs of delayed absorbable sutures are inserted into the arcus tendineus fasciae pelvis and into the porcine dermis patch.
(From Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ procedures. J Urol 2004;171:1581–4.)
Meschia and colleagues (2007) published a prospective randomized controlled trial comparing anterior colporrhaphy with 0 polyglactin suture (n = 103) to the same colporrhaphy reinforced with a 4 × 7-cm piece of Pelvicol (n = 98) in women with stage 2 or greater anterior vaginal wall prolapse as defined on the POP-Q. After 1 year of follow-up, the women randomized to receive the Pelvicol graft experience graft failure 7% of the time, compared with the 19% failure rate in those women who underwent the anterior colporrhaphy with suture plication alone (P = .019). One patient who received the Pelvicol graft had vaginal extrusion of the mesh 1 month after surgery. Finally, there were no differences between the two groups in the incidence of postoperative dyspareunia.
In a recent retrospective study, Handel and associates (2007) compared two different grafts (polypropylene mesh and porcine dermis) to traditional anterior colporrhaphy with suture alone. In a nonrandomized comparison, 119 patients underwent cystocele repair using one of the three techniques. Traditional anterior colporrhaphy was performed in 18 patients, colporrhaphy reinforced with a 3 × 7-cm piece of polypropylene mesh was performed in 25 patients, and colporrhaphy reinforced with a 3 × 7-cm piece of porcine dermis was performed in 56 patients. For patients undergoing repair with a graft, it was anchored to the levator fascia bilaterally and to the uterosacral/cardinal ligament complex. With a mean follow-up of 13.5 months there was no significant difference in failure rate (defined as grade 2 or greater on the Baden-Walker classification) between the three groups. Unfortunately, the study was not powered adequately because there was no sample size justification among the groups.
Complications
Vaginal exposure (extrusion) has been reported in anterior compartment prolapse repair with most materials. Rates from 0% to 13% have been reported in recent series with synthetic grafts (Sand et al, 2001; Weber et al, 2001; Dwyer and O’Reilly, 2004; de Tayrac et al, 2005; Milani et al, 2005; Rodriguez et al, 2005) and are less common with biologic grafts. Dyspareunia has been reported by 3.1% to 19% of patients with anterior colporrhaphy (Dwyer and O’Reilly, 2004; Yan et al, 2004; Milani et al, 2005).
Other Procedures to Correct Anterior Compartment Defects
Six-Corner Needle Suspension
Raz and colleagues (1989) originally described the four-corner needle suspension in 1989 for women with SUI due to urethral hypermobility and mild to moderate lateral cystocele defect. With a mean follow-up of 24 months, they reported a 94% subjective correction of SUI and a 98% correction of cystocele on physical examination. The procedure did not include anterior colporrhaphy. The technique was modified to a six-corner suspension in 1996 by Albo and associates to treat larger-grade cystoceles.
Technique (Fig. 72–21)
The technique begins by making two parallel, oblique incisions from the bladder neck to the proximal vagina. Alternatively, this can also be accomplished through a midline incision. The dissection is then carried lateral to expose the pubocervical fascia. The endopelvic fascia is perforated, and the retropubic space is entered. Three sets of size 1 polypropylene suture are placed on each side. Each suture incorporates multiple passes through the tissue. The proximal sutures are placed through the cardinal ligament and vaginal wall to support the bladder base. The middle and distal sutures are placed to support the bladder neck and proximal urethra, respectively. Next, a small suprapubic incision is made, and the double-pronged ligature carrier is passed under direct fingertip control through the retropubic space, exiting in the vaginal incision. All six sutures are transferred individually to the suprapubic incision. After the administration of intravenous indigo carmine, cystoscopy is performed to confirm both ureteral patency and the absence of suture in either the bladder or urethra. The polypropylene sutures are then lifted to ensure anatomic reduction of the cystocele. The vaginal incision is closed with a running 2-0 polyglactin suture. Finally, the polypropylene suspension sutures are tied sequentially to themselves and to the corresponding one from the opposite side. It is important to tie the polypropylene suspension sutures without tension to avoid postoperative urinary retention. The suprapubic site is irrigated with povidone-iodine solution, and the wound is closed with 4-0 polyglactin.
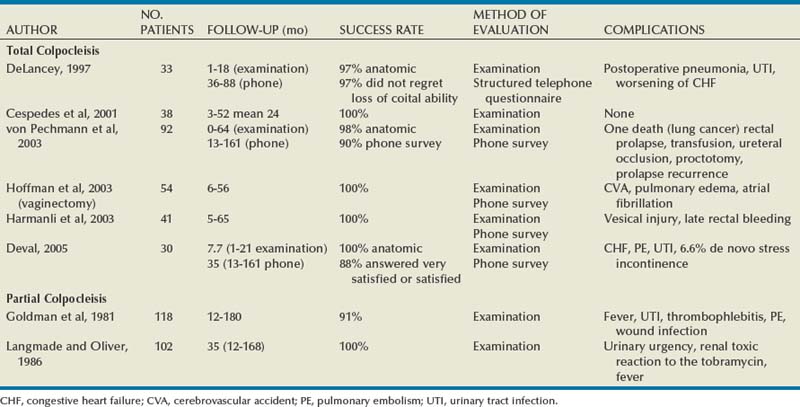
Results (see Table 72–4)
For larger cystoceles with a central defect, Raz and colleagues (1991) combined anterior colporrhaphy with his four-corner needle suspension. Of the 46 patients who underwent the repair, 5 (11%) had prolapse recurrence with a mean follow-up of 2.8 years. Miyazaki and colleagues (1994) noted a prolapse recurrence rate of 59% in the 22 patients they followed for 3.5 to 4 years after four-corner needle suspension. In a prospective study of 47 patients, Dmochowski and associates (1997) noted that 27 patients (57%) had grade 1 or 2 cystoceles after a four-corner needle suspension with a mean follow-up of 37 months. Recurrent prolapse symptoms were noted in 12 of these 27 patients. Safir and associates (1999) described the four-defect repair of grade 4 cystoceles, whereby a vaginal wall sling and anterior colporrhaphy reinforced with polyglactin mesh are added to the traditional Raz four-corner needle suspension. With a mean follow-up of 21 months, 92% of the 112 patients had excellent objective and subjective results for cystocele repair. Of the patients with preoperative SUI, 90% had excellent or good subjective results.
Complications
Complications of four- and six-corner suspensions include bladder or urethral injury with suture passage, ureteral obstruction, and injury or entrapment of the genital branch of the genitofemoral or ilioinguinal nerve.
A number of needle suspension reports have demonstrated high failure rates utilizing the six-corner repair in the treatment of anterior compartment defects. Perhaps, suture displacement through weakened tissue contributes to these failures. Although not commonly utilized at present, these procedures were influential in exposing urologists to the technique of endopelvic fascia perforation and access into the retropubic space, which is a useful adjunct to many commonly performed procedures utilized today in the correction of POP.
Abdominal Paravaginal Repair
Technique (Fig. 72–22)
Positioning and preparation of the patient to allow abdominal and vaginal access initiate the repair. The bladder is drained. The retropubic space is entered through either a low midline or transverse incision. Additionally, this may be accomplished laparoscopically. The bladder neck, symphysis pubis, endopelvic fascia, ATFP, and obturator fascia should all be clearly identified. The normal site of normal vaginal attachment on the pelvic side wall from the interior aspect of the superior pubic ramus to the ischial spine is then identified. The surgeon’s nondominant hand is placed into the vagina and used to elevate the lateral superior vaginal sulcus to its site of normal attachment along the course of the ATFP. With the bladder retracted medially, four to six interrupted nonabsorbable sutures are placed at 1-cm intervals through the ATFP extending from the ischial spine to the pubic bone. These sutures are then placed in the appropriate location in the lateral wall of the vagina. Care is taken to avoid paravaginal veins, which commonly course through this area. Elevating the vagina to its normal anatomic position to localize suture placement site may facilitate vaginal suture placement. After the sutures are tied, cystoscopy must be performed to confirm ureteral patency and the absence of intravesical sutures. The incision is closed and the catheter is left indwelling.
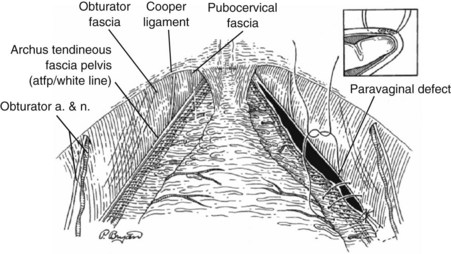
Figure 72–22 Abdominal paravaginal repair. Paravaginal defect repair as viewed from the retropubic space: approximation of the pubocervical fascia medially to the arcus tendineus fasciae pelvis laterally with 2-0 braided nonabsorbable suture. Note the vertical orientation of the vaginal vessels in relation to the transverse orientation of the bladder vessels. Inset shows suture being passed beneath the vaginal vessels to ensure generous purchase of pubocervical fascia and control of hemostasis.
(From Bruce RG, El-Galley R, Galloway NT. Paravaginal defect repair in the treatment of female stress urinary incontinence and cystocele. Urology 1999;54:647–51.)
Results (see Table 72–4)
Richardson and coworkers (1976) popularized the abdominal paravaginal defect repair. In a retrospective study of 233 patients with follow-up spanning 2 to 8 years, they reported an anatomic cure of 95%. Of these patients, 53 (23%) had previously undergone one or more successful anterior vaginal wall prolapse repairs. No patient required transfusion. Eighty-eight percent of the women with preoperative SUI were cured after abdominal paravaginal repair (Richardson et al, 1981).
Other authors published the results of case series that demonstrated similar encouraging results with SUI cure rates of 85% to 97% after abdominal paravaginal repair (Baden and Walker, 1987; Shull and Baden, 1989). However, recent evidence over the past 15 years suggests that abdominal paravaginal defect repair is not as effective as either the Burch colposuspension or suburethral sling for the treatment of SUI. In 1996, Colombo and coworkers (2000) published the results of a randomized trial comparing paravaginal defect repair to Burch colposuspension for the treatment of SUI in 18 patients. With a mean follow-up of 2.2 years, the objective (urodynamic) cure rates were 100% for Burch colposuspension and 61% for abdominal paravaginal defect repair. Colombo and coworkers found that the Burch colposuspension increased the functional urethral length and pressure transmission ratio in the proximal urethra whereas the abdominal paravaginal repair did not. Bruce and associates (1999) examined the abdominal paravaginal defect repair (PVdR) in the treatment of women with both lateral cystocele and symptomatic SUI. In this retrospective cohort of 52 patients, half underwent abdominal PVdR and half underwent abdominal PVdR with pubovaginal sling using rectus muscle. All the patients who underwent concomitant sling had intrinsic sphincter deficiency. With a mean follow-up of 17 months, 4 patients (8%) had recurrent cystocele, 3 patients developed vault prolapse, and 1 patient developed an enterocele. Thus, the overall prolapse cure rate was 85%. Finally, the overall cure rate for SUI was 72% in the group that underwent PVdR alone and 85% in the group that underwent PVdR and pubovaginal sling.
Anterior Compartment Repair with Sling
In patients with high-grade anterior compartment prolapse the descent may create urethral kinking and urethral compression (Gallentine and Cespades, 2001) (Fig. 72–23). This may predispose a patient to develop SUI after prolapse correction. Documenting the presence of SUI in patients with the prolapse reduced is helpful in the preoperative management of women with high-grade anterior compartment prolapse. SUI occurring in presumably continent women after prolapse correction is known as occult SUI. It is essential for women with high-grade prolapse to undergo an evaluation with the prolapse reduced to identify if they are at risk for occult SUI. In two prospective studies POP repair in clinically continent women was shown to result in postoperative SUI in 11% to 22% (Stanton et al, 1982; Borstad and Rud, 1989).
Figure 72–23 Urethral kinking caused by large cystocele. Fluorourodynamic study shows a large cystocele before (A) and after (B) Valsalva maneuver was performed. The urethra (arrow) clearly can be seen to “kink off” during the Valsalva maneuver. The patient did demonstrate severe SUI after prolapse reduction.
(From Gallentine ML, Cespedes RD. Occult stress urinary incontinence and the effect of vaginal vault prolapsed on abdominal leak point pressures. Urology 2001;57:40–4.)
To diagnose occult SUI, the prolapse may be reduced with vaginal packing, rectal swabs, a speculum blade, or pessary. Ghoniem and coworkers (1994) used a vaginal pack (formed from two rolled 4 × 4-inch gauze pads) to reduce large cystoceles and found occult SUI in 69% of the women. They found that the vaginal pack provided superior visualization of the vesicourethral angle during fluoroscopic urodynamics. Gallentine and Cespades (2001) used gauze packing and a speculum blade to reduce high-stage POP and found occult SUI in at least 50% of the women with high-grade anterior compartment prolapse. Using a fitted vaginal pessary, Chaikin and coworkers (2000) detected occult SUI in 58% of the women. They also noted that none of the women had urethral obstruction after pessary placement.
There are multiple techniques used to reduce prolapse, and these methods are not standardized. This has led to variance in the reported incidence of occult SUI (Haessler et al, 2005). Veronikis and associates (1997) compared prolapse reduction with rectal swabs, Gellhorn pessary, and a Graves speculum blade. Because prolapse reduction with rectal swabs revealed a significantly lower midurethral closure pressure, these authors concluded that the rectal swabs were superior. Sensitivity and specificity data are lacking with all of the methods used to reduce POP. As such, no method for prolapse reduction has been proven to be superior.
Performing a concomitant sling at the time of anterior vaginal wall prolapse repair in women without symptoms of SUI or occult SUI remains controversial. Twiss and colleagues (2007) recommended a simultaneous anti-incontinence procedure at the time of high-grade POP repair because they contend there is no correlation between the preoperative position of the urethra utilizing any method of cystocele reduction and the ultimate position of the urethra after surgery. Thus they believe that the diagnosis of occult SUI is inconsistent and not reproducible. Consequently, occult SUI could be missed. It has also been reported that a concomitant suburethral sling may contribute to the long-term success of anterior compartment repairs. Goldberg and associates (2001) demonstrated a 55% reduction in postoperative cystocele recurrence in patients who underwent suburethral sling at the time of prolapse repair. Also, Cross and coworkers (1997) reported that the support created by the simultaneous placement of a pubovaginal sling in grade 3 and 4 cystoceles was improved. It remains an important distinction that the protection from recurrent anterior compartment defects has only been described for bladder neck slings, which are not the most commonly performed midurethral sling procedures.
Liang and associates (2004) prospectively evaluated 49 patients with severe POP and occult urinary incontinence. POP repair alone was performed in 17 patients with occult SUI, and POP repair with simultaneous TVT was performed in 32 patients. They found that the addition of a prophylactic TVT to the prolapse repair significantly decreased postoperative objective and subjective SUI rates. Yet, in this series some patients with occult SUI did not undergo sling. Of the 30 patients without occult SUI preoperatively, none had SUI postoperatively. Also, complication rates were higher in the group undergoing concomitant sling.
The Colpopexy and Urinary Reduction Efforts trial is the only large, randomized, controlled trial evaluating a simultaneous anti-incontinence procedure with POP repair (Brubaker et al, 2006). The investigators evaluated 322 patients with stage 2 to 4 POP who underwent abdominal sacrocolpopexy, of whom 157 were randomized to concomitant Burch colposuspension and 165 were randomized to abdominal sacrocolpopexy alone. The addition of a Burch colposuspension to abdominal sacrocolpopexy decreased postoperative SUI from 44.1% to 23.6% at 3 months postoperatively. In a subsequent study examining 2-year outcomes in the same group of patients, the authors found that 32% of patients who underwent concomitant Burch colposuspension were incontinent versus 45% of patients who underwent abdominal sacrocolpopexy alone (Brubaker et al, 2008). Approximately a third of the women after a Burch colposuspension still had SUI. Thus the major implication of this work is that women undergoing colpopexy should have an anti-incontinence procedure. However, these data cannot be generalized to women who undergo transvaginal POP repair or anti-incontinence procedures other than Burch colposuspension.
Most authors who have examined this issue have reported that the majority of women without occult SUI or symptoms of SUI do not leak after prolapse surgery. Chaikin and colleagues (2000) reported that all (100%) of their patients without occult SUI did not leak after prolapse. They concluded that the decision to perform a concomitant prophylactic anti-incontinence procedure should be tailored to individual urodynamic findings. Ballert and associates (2009) reported that 22 of 24 women (91.7%) without symptomatic or occult SUI did not leak after repair of stage 2 to 4 POP. They also found that the risk of intervention due to obstruction after receiving a midurethral sling was 8.5%. Therefore, the risk of intervention due to sling obstruction was equal to the risk of intervention due to SUI when no clinical, urodynamic, or occult SUI was present and no miduretheral sling was placed. Because the need to intervene for complications resulting from a midurethral sling equaled the risk of having to perform a secondary sling, the necessity for secondary surgery in women without SUI after repair of anterior vaginal wall prolapse is not decreased.
In summary, there is no standardized terminology or accurate testing for occult SUI. In addition, there are no controlled studies proving that continent women (no clinical, urodynamic, or occult SUI) develop postoperative SUI. However, it is clear that all women with advanced stage anterior compartment prolapse should be screened for the presence of SUI with the prolapse reduced and consideration should be given to a concomitant anti-incontinence procedure. All women with preoperative symptoms of SUI, occult SUI, or urodynamically proven SUI should undergo a simultaneous anti-incontinence procedure. The risks and benefits of a prophylactic anti-incontinence procedure on continent women should be reviewed with each patient because the current literature supports selective use of an anti-incontinence procedure at the time of POP repair.
Apical Vaginal Compartment
Middle compartment defects involve the vaginal apex. The organs involved in defects of the middle compartment include the uterus, bowel or omentum, and, depending on the size, bladder and rectum. The apex remains the cornerstone of vaginal support, and failure to ensure apical support at the time of prolapse correction will undoubtedly increase the risk of recurrence exponentially.
McCall (1957) highlighted this importance when he described his technique of posterior culdoplasty. This technique closed the peritoneal cul-de-sac posteriorly, thereby preventing enterocele formation, and emphasized the importance of apical fixation. In this landmark paper, he described the following technique:
The posterior culdoplasty is a simple procedure which obliterates the redundant cul-de-sac of Douglas by a series of continuous sutures so as to suspend it by the uterosacral ligaments which then are brought together in the midline…The first suture picks up the left uterosacral ligament about 2 cm above its cut edge. Several bites of redundant sac are then taken at 1-2 cm intervals until the right uterosacral ligament is reached and picked up…Three external through-and-through sutures of this type are usually inserted, each one higher than the last, so as to be placed through the uterosacral ligaments at intervals between the internal sutures. The highest of these is placed just at the top of the newly supported vagina. It is this suture, which brings the new vaginal vault to the highest possible level thus insuring that the vagina will be as long as possible in each instance. The internal sutures are now tied and the previously herniated cul-de-sac obliterated into a firm, shelflike structure. The external sutures are then tied and the vaginal mucosa snugged against this shelf.
Apical Vaginal Prolapse Repairs
The uterosacral vault suspension preserves the orientation of the vaginal axis in its natural position, thus potentially preventing recurrence of prolapse in other segments (Silva et al, 2006). Several authors have reported that the uterosacral ligaments break at specific points rather than an attenuation of the uterosacral/cardinal ligament complex, thus allowing for a more site-specific repair (Jenkins, 1997; Barber et al, 2000; Shull et al, 2000). These findings are in contrast to a histologic assessment of the uterosacral ligaments, which calls into question the integrity of this structure for long-term apical support (Cole et al, 2006).
Surgical Anatomy of the Uterosacral Ligaments
The ligament is attached broadly to S1 to S3 and variably to S4 (Buller et al, 2001) (Figs. 72-24 and 72-25). It proceeds in a fanlike manner anterolaterally to the cervical os and also onto the proximal portion of the posterior vagina. The ligament can be divided into sacral, intermediate, and cervical portions. Beneath the sacral portion of the ligament runs the superior gluteal vein. Although there is great variability in the location of the ureter relative to the spine (Karram et al, 2001), the ureter has been reported to be near the anterior margin of the uterosacral ligament near the cervix. As the ureter courses distally, the distance between the uterosacral ligament and ureter decreases from 4 cm near the sacrum to 0.9 cm near the cervix (Buller et al, 2001). Because of the fibrous tissues of the ureteral sheath, the ureter is subject to kinking from traction caused by sutures placed in the adjacent tissue (Dwyer and Fatton, 2008). If sutures are placed close to the sacrum there is a risk of entrapping fibers of the sacral plexus trunk of S1 to S4 (Siddique et al, 2006; Wieslander et al, 2007). This can result in buttock pain, which radiates to the posterior thigh and popliteal fossa. Placing sutures in the intermediate portion of the uterosacral ligament appears to be the optimal site because there are fewer structures to be potentially affected and it provides a stable point of fixation. There is a decreased chance of traction affecting the ureter at this point, because it is further away from the ureter and its sheath. Placing the sutures from lateral to medial will also minimize accidentally catching either the ureter or its attachments in the stitch (Shull et al, 2000). The intermediate segment can be located 1 cm posterior along the palpable uterosacral ligament at the level of the ischial spine with the uterosacral ligament placed on tension (Buller et al, 2001). An advantage of suspension to the uterosacral ligament is that it minimizes injury to the pudendal and gluteal vessels relative to sacrospinous ligament fixation (Barber et al, 2000).
Figure 72–24 Right hemipelvis: sagittal view. The uterosacral ligament (USL) has been reflected to show subjacent anatomy.
(From Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol 2001;97:873–9.)
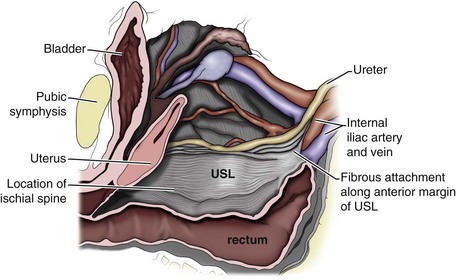
Figure 72–25 Right hemipelvis sagittal view. The peritoneum has been removed and the uterosacral ligament (USL) was left intact to show the proximity of the ureter in this specimen.
(From Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol 2001;97:873–9, with permission.)
Technique
High Uterosacral Vaginal Vault Suspension
This procedure starts intraperitoneally (Karram et al, 2001). After hysterectomy the apex is identified on the vaginal cuff at the 3- and 9-o’clock positions, which represent the attachments of the cardinal and uterosacral ligaments (Shull et al, 1993). It is useful to place stay sutures at these dimples before incision. The vaginal epithelium is dissected away from the peritoneum of the hernia sac. Once the dissection has mobilized the hernia sac from the epithelium to the neck of the sac, it is entered and the sac excised (Fig. 72–26). Laparotomy pads are placed to pack the bowel away. Ischial spines can then be palpated transperitoneally. The uterosacral ligaments will be located posteriorly and medially to the ischial spines. By placing traction to the stay sutures or by placing an Allis clamp at the 5- and 7-o’clock positions of the apex the uterosacral ligament will tent and two to four nonabsorbable sutures can be placed through the uterosacral/cardinal ligament complex (Fig. 72–27). The ureters should be located lateral to the uterosacral ligaments; however, it should be noted that depending on the degree of the prolapse there can be significant variability in their location relative to the edge of the uterosacral ligament (Silva and Karram, 2006). If there is any question as to their location, temporary stents may be placed to facilitate their palpation. In placing the needles through the uterosacral ligaments it is important to pass them lateral to medial, exiting away from the ureter to avoid inadvertent inclusion into the tie (Shull et al, 2000). To ensure the proper depth, the suture should be passed at the level of the ischial spines. Subsequent sutures are placed proximal to the last. If an anterior colporrhaphy or sling is planned it may be done at this point. Suspensory sutures in the uterosacral ligaments are then placed in the most apical portions of the pubocervical and rectovaginal fascia. The highest sutures are then sewn to the most medial portions of the pubocervical and rectovaginal fascia. The more distal sutures are placed most laterally in the pubocervical and rectovaginal fascia and passed out of the vaginal epithelium on each side (Fig. 72–28). Cystoscopy with indigo carmine is an essential step after this procedure to confirm ureteral patency and should be performed before trimming the suture because this will facilitate identification of the sutures if they need to be removed due to obstruction. If no efflux of urine is seen the sutures should be removed starting with the most lateral suture of the ipsilateral side. In addition, it is important to perform the cystoscopy after the suture is tied, not just placed (Yazdany and Bhatia, 2008). After ureteral patency is ensured, it is useful to close the vaginal cuff at least one third of the length of the incision before tying down the uterosacral sutures to avoid the difficulty of placing the closing sutures high in the vagina once it is suspended. As one ties these suspending sutures, the rectovaginal fascia and pubocervical fascia are brought together at the uterosacral/cardinal ligament complex, thus resuspending the apex of the vagina and closing the cul-de-sac (Fig. 72–29).
Figure 72–26 Vaginal enterocele repair. Enterocele sac is identified and opened to reduce its contents. It is then tied in a purse-string suture and the excess is excised. Before closure of the enterocele sac the peritoneal cavity is entered to access the uterosacral ligament.
(From Cespedes RD, Winters CW. Colpocleisis for the treatment of severe vaginal vault prolapse. In: Vasavada SP, Appell RA, Sand PK, Raz S, editors. Female urology, urogynecology, and voiding dysfunction. Boca Raton [FL]: Taylor & Francis; 2005.)
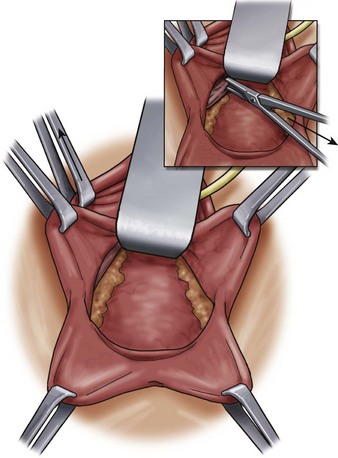
Figure 72–27 To identify the uterosacral ligament, an Allis clamp is placed on the vaginal epithelium at the right apex and pulled straight upward. Placing the right uterosacral ligament on tension aids in visualizing this structure within the pelvis. Inset, A long Allis clamp is used to grasp the right uterosacral ligament.
(From Walters M, Muir T. Surgical treatment of vaginal apex prolapse. In: Vasavada SP, Appell RA, Sand PK, Raz S, editors. Female urology, urogynecology, and voiding dysfunction. Boca Raton [FL]: Taylor & Francis; 2005.)
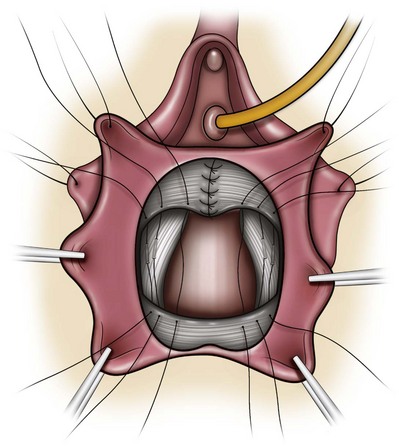
Figure 72–28 High uterosacral ligament vaginal vault suspension. Three sutures are placed from lateral to medial in each uterosacral ligament. The sutures are brought through the vaginal muscularis anteriorly (pubocervical fascia) and posteriorly (rectovaginal fascia).
(From Walters M, Muir T. Surgical treatment of vaginal apex prolapse. In: Vasavada SP, Appell RA, Sand PK, Raz S, editors. Female urology, urogynecology, and voiding dysfunction. Boca Raton [FL]: Taylor & Francis; 2005, with permission.)
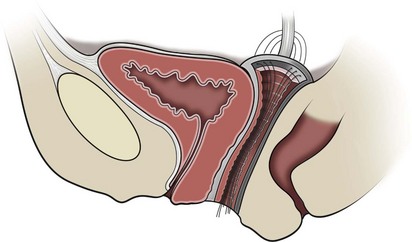
Figure 72–29 Completed uterosacral vaginal vault suspension with anterior colporrhaphy.
(From Walters M, Muir T. Surgical treatment of vaginal apex prolapse. In: Vasavada SP, Appell RA, Sand PK, Raz S, editors. Female urology, urogynecology, and voiding dysfunction. Boca Raton [FL]: Taylor & Francis; 2005, with permission.)
An extraperitoneal approach has also been described (Dwyer and Fatton, 2008). A midline incision is made, depending on concomitant prolapse. The vaginal epithelium is then dissected away from the endopelvic fascia, and the enterocele sac is identified and dissected free of the vault. Dissection is continued laterally until the uterosacral/cardinal ligament complex is identified posterior and medial to the ischial spine. Palpation to tent the uterosacral/cardinal ligament complex is facilitated by placing tension on the vagina. Use of the Breisky-Navratil retractor to retract the bladder and ureters anteriorly has prevented ureteral obstruction through traction or injury (Fatton et al, 2009). Once the ligaments are identified, the sutures are placed in a similar fashion to the intraperitoneal approach. Cystoscopy and vaginal closure are performed as described previously.
Abdominal Approach to the Uterosacral Ligaments
The uterosacral ligaments may be accessed transabdominally (Lowenstein et al, 2009). This technique begins by elevating the uterus at the time of abdominal hysterectomy. Three permanent sutures are placed in each of the uterosacral ligaments proximal and medial to the ischial spine. They are numbered in the manner described by Shull and colleagues (2000) (Fig. 72–30). Once the hysterectomy has been performed and the cuff has been closed, the sutures are placed sequentially through the anterior and posterior leaves of the endopelvic fascia. By reapproximating the anterior and posterior muscularis of the vagina, any potential enterocele defects are closed and the cuff is elevated toward the sacrum, re-creating the normal vaginal axis. Cystoscopy is performed after tying the sutures, which are left uncut until efflux of urine is demonstrated. If no urine is seen, the most lateral ipsilateral suture is removed and subsequent sutures are taken down in sequence.
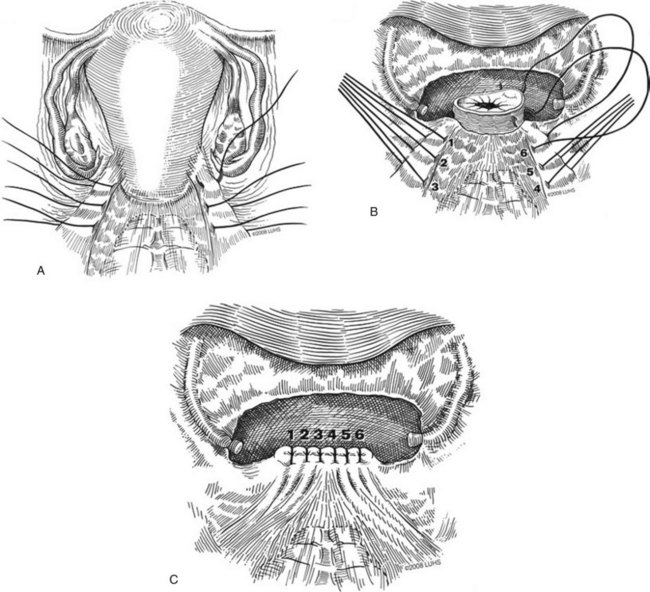
Figure 72–30 A, Three permanent sutures are placed in each of the uterosacral ligaments medial to the ischial spine. B, One end of each of the six sutures is placed serially across vaginal apex through the anterior endopelvic fascia and the other end is placed through the posterior endopelvic fascia. C, All sutures are tied to reapproximate the anterior and posterior vaginal muscularis, to close any potential enterocele defect, and to elevate the vaginal apex toward the sacrum.
(© 2008 Loyola University Health System. Used with permission from Mary Pat Fitzgerald, MD.)
Results (Table 72–5)
Jenkins (1997) reported his experience in 50 patients who underwent vaginal vault suspension to the uterosacral ligaments, 33 of whom underwent concomitant vaginal hysterectomy. There was no recurrence of vault prolapse; however, 2 patients had asymptomatic cystoceles. Three had erosion of the suture (monofilament) into the vagina. Although not sexually active, 1 patient who had vaginal narrowing complained of tightness of the bladder and vagina. Three had de novo dyspareunia, with resolution in 2 by 6 months. Barber and associates (2000) reported on 46 women who underwent site-specific repair of the endopelvic fascia defects with suspension of the vaginal cuff to the proximal uterosacral ligaments. At a median follow-up of 15.5 months, 67% had POP-Q stage 1 or less prolapse of any compartment, and 95% had correction of apical prolapse. Voiding dysfunction was noted to improve from 59% to 7%. Eleven percent of patients were noted to have entrapment of the ureter, which was recognized intraoperatively. Two had to undergo ureteral reimplantation, which was performed at the time of the original surgery. This emphasizes the need for intraoperative cystoscopy to identify ureteral injury. Shull and colleagues (2000) reported a large series of patients who underwent uterosacral ligament suspension. Of 289 patients who were observed over time, a majority (87%) had no recurrence of support defect at any site on any postoperative examination. Only 5% of the patients had grade 2 prolapse or greater. The most common site was the anterior compartment in which 10 patients had grade 2 or 3 defects. One patient required ureteroneocystostomy. Two patients required removal of suspensory sutures. Karram and coworkers (2001) reported on 202 patients who underwent vaginal vault suspension. Of interest, uterosacral ligament suspension could not be performed in six patients because the enterocele sac could not be identified or exposure of the cul-de-sac could not be carried out. These patients underwent sacrospinous ligament fixation. Ninety-nine percent had a successful vaginal vault suspension. Ten women had recurrent grade 2 prolapse at other sites. Wheeler and associates (2007) reported patient satisfaction of 89% in 35 patients. No ureteral injuries were noted, and none of the patients required operation for recurrent prolapse, urinary incontinence, or urinary tract injury. Amundsen and associates (2003) reported on a retrospective analysis of 33 patients who underwent bilateral uterosacral ligament suspension with concomitant pubovaginal sling and site-specific endopelvic fascial repair. Vaginal prolapse symptoms resolved in 82%, and SUI improved in 82%. No dyspareunia was reported postoperatively. Eighty-three percent of women who needed to perform vaginal or perineal splinting ceased to do so postoperatively. POP-Q measurements improved significantly (P < .05) for all sites. Although these investigators noted a decrease in total vaginal length of 0.9 cm there was no corresponding loss of sexual function. Silva and Karram (2006) evaluated 5-year anatomic and functional outcomes of high uterosacral vaginal vault suspension. Apical recurrence occurred in 2 of 78 patients. Seven patients had de novo postoperative dyspareunia. Short-form Incontinence Impact Questionnaire/Urinary Distress Inventory scores indicated improvement in all three domains of irritative, obstructive, stress, and overall symptoms compared with preoperative scores. Rare complications such as blood transfusion (1% to 3%), bowel injury (0% to 0.6%), and pelvic infection (0.6%) have all been reported after the uterosacral ligament suspension (Shull et al, 2000; Karram et al, 2001; Amundsen et al, 2003).
Fatton and colleagues (2009) examined their results performing an extraperitoneal bilateral uterosacral vaginal vault suspension over 2 years. Some of the patients in this series also had mesh reinforcement both anteriorly (47.1%) and posteriorly (53.7%). Recurrence at the apex was noted in 4.6% of patients. Mesh exposure occurred and was diagnosed within 6 months in the majority of the patients. Their results are similar to that reported for an intraperitoneal approach.
Sacrospinous Ligament Fixation
The sacrospinous ligament fixation was first described by Richter in 1942 but was not widely utilized until Nichols reported on the technique forty years later (Nichols, 1982). The sacrospinous ligament fixation has been shown to be an effective method to correct apical prolapsed (Cruikshank and Muniz, 2003). This technique is appealing because of its retroperitoneal approach and consistency as a strong structure on which to anchor the apex. Access to the spine was originally achieved from the posterior approach, although dissection from the anterior approach may also be performed via the paravaginal space (Cespedes, 2000; Winkler et al, 2000). The advantages of sacrospinous ligament suspension are success rates comparable to abdominal procedures (Brown et al, 1989; Imparato et al, 1992), ability to repair concomitant pelvic floor defects, absence of a laparotomy (Morley and DeLancey, 1988), shorter hospital stay (Brown et al, 1989), preservation of vaginal length and function (Morley and DeLancey, 1988; Brown et al, 1989), and cost effectiveness (Brown et al, 1989). The structures at risk with this technique include the pudendal or inferior gluteal vessels and the sciatic or pudendal nerves. Pudendal nerve entrapment results in posterior buttocks pain, which may radiate down the back of the thigh. A disadvantage of this approach is the alteration in vaginal axis, which results in apical displacement posteriorly and to the right side when unilateral fixation is used. This posterior displacement can result in anterior compartment recurrence even when an anterior repair is performed (Morley and DeLancey, 1988; Shull et al, 1992; Sauer and Klutke, 1995).
Sacrospinous fixation may be performed unilaterally or bilaterally (Pohl and Frattarelli, 1997). With the unilateral suspension, right-sided placement is generally preferred. Technically, it is easier to place the sutures on the right ligament for a right-handed surgeon (Sauer and Klutke, 1995). Cespedes (2000) reported success using the bilateral anterior support and noted the advantage of more midline location of the vaginal apex. Although bilateral placement has been advocated (Nichols, 1996), there is little to support the advantage of bilateral fixation over unilateral fixation when examining outcomes (Morley and DeLancey, 1988).
Surgical Anatomy of the Sacrospinous Ligament Fixation
The sacrospinous ligament is 7 to 8 cm in length (Morley and DeLancey, 1988). It extends from the ischial spine laterally, courses medially under the coccygeus muscle, and inserts into the sacrum. The pudendal nerves and vessels are in close proximity to the ligament just proximal to their course around the ischial spine (Fig. 72–31). Additionally, the hypogastric plexus of veins are located superiorly and the hemorrhoidal vessels are located medially to the sacrospinous ligament (Morley and DeLancey, 1988); thus retraction should be carried out carefully in these respective areas. To avoid the gluteal vessels, the suture should be placed into the ligament and not behind it (Kettel and Herbertson, 1989). Pudendal nerve entrapment may result in pain, which localizes to the buttocks or perineum. This may be avoided by placing sutures more medially about 1.5 cm from the ischial spine (Sauer and Klutke, 1995; Alevizon and Finan, 1996). Thus, the optimal position of the suture placement is 1.5 to 2.0 cm medial to the ischial spine, directly into the sacrospinous ligament. Placing it too far medially may risk failure of the repair because the sacrospinous ligament fans and thins as it inserts into the sacrum (Sauer and Klutke, 1995). In addition, there is a higher concentration of sacral nerves in this medial location. Barksdale and associates (1997) performed a cadaveric study to assess the histology of the sacrospinous ligament. Segments were taken near the ischial spine, including middle and sacral portions of the ligament. The highest concentration of nerves was found in the sacral portion of the ligament, with the lowest being near the spine. Although it was postulated that the nerves embedded in the sacrospinous ligament were a potential source of neuropathic pain, no clinical correlation could be made in this cadaveric study. Lantzsch and coworkers (2001) concluded that localization and depth of the suture can also influence the occurrence and intensity of sciatic neuralgia.
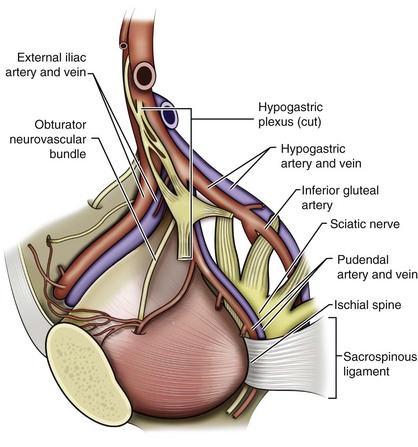
Figure 72–31 Anatomy of the sacral spinous ligament and surrounding structures. Note the close proximity of the pudendal vessels, hypogastric plexus, inferior gluteal vessels, and sciatic nerve.
(From Walters M, Muir T. Surgical treatment of vaginal apex prolapse. In: Vasavada SP, Appell RA, Sand PK, Raz S, editors. Female urology, urogynecology, and voiding dysfunction. Boca Raton [FL]: Taylor & Francis; 2005, with permission.)
Technique
The procedure starts by marking the apex of the vagina. The vaginal apex is then reduced to the sacrospinous ligament(s) intended to be used. This site of attachment is then tagged for later fixation. A midline vaginal incision is fashioned, the location of which is largely dependent on the need for other compartmental repairs. The vaginal epithelium is separated from the rectovaginal septum posteriorly and/or pubocervical fascia anteriorly. If an anterior compartment repair will be performed, the sacrospinous ligament may be approached anteriorly, exposing the paravesical space. The tissue attachments overlying the spine and ligament are swept medially. Alternatively, if the posterior approach is used, one must enter the perirectal space by bluntly mobilizing the rectum medially. On entering the perirectal space the ischial spine is identified. With further dorsal and medial blunt dissection, the sacrospinous ligament is palpated. Blunt dissection is continued to ensure that the rectum is retracted medially, and the ligament is adequately exposed. At this point Heaney or Breisky-Navratil retractors are very useful to visualize the ligament and facilitate suture placement. Additionally, long lighted retractors can provide added visualization. The sacrospinous ligament is then palpated. The suture is placed approximately one to two fingerbreadths medial from the spine, to avoid damage to the structures in the Alcock canal. Several different methods have been used to fix the suture to the sacrospinous ligament. The Capio Suture Capturing Device (Boston Scientific, Natick, MA) enables the suture to be placed by palpation. Alternatively, the Miya Hook (Cooper Surgical, Trumbull, CT) (Miyazaki, 1987), the Autosuture Endo Stitch Suturing Device (Covidien, Norwalk, CT) (Schlesinger, 1997), and Deschamps Ligature Carrier may be utilized to place the sutures under direct vision. Two sutures are most commonly placed. In cases of bilateral sacrospinous ligament fixation, one suture may be placed on each ligament. In the case of unilateral sacrospinous ligament fixation the placement of the sutures on the ligament 2 cm apart will prevent constriction of the vaginal apex. Additional repairs may be performed at this point. Following this, the previously placed sacrospinous ligament sutures are brought out of the vagina to the previously marked vaginal apex (Fig. 72–32). If a permanent suture is utilized for sacrospinous ligament fixation, a (pulley stitch) configuration, which ensures that the knot of the suture will remain internal, is needed. To create a pulley stitch, tying a knot onto the fibromuscular portion on the visceral side of the vagina creates a fixed point. The free end of the suture is then pulled, thus suspending the apex to the sacrospinous ligament, and this is then secured by tying a secure knot. Importantly, this resultant knot remains internal and is not within the vaginal canal. Closure of the vaginal wall halfway before tying down the sutures is useful because the suspended apex may be quite difficult to reach. The sutures should be tied such that the vaginal apex is firmly attached to the coccygeal/sacrospinous ligament complex with no intervening suture material bridging a gap (Morley and DeLancey, 1988; Sze and Karram, 1997). Palpation of the suture behind the vaginal cuff will ensure that the suture is tied securely. It is useful to leave the suture untrimmed until the cystoscopy is performed in case the suture needs to be removed. Once efflux has been ensured, the suture is trimmed and the remainder of the vaginal wall is closed.
Figure 72–32 Sacral spinous ligament fixation. With a unilateral suspension, the vagina is deflected to the right side and caudally.
(From Richter K, Albright W. Long-term results following fixation of the vagina on the sacrospinous ligament by the vaginal route [vaginaefixatio sacrospinalis vaginalis]. Am J Obstet Gynecol 1981;141:811–6, with permission.)
Results (Table 72–6)
Nichols (1982) reported outcomes of sacrospinous ligament fixation in 163 patients, some with “massive” vaginal eversion and procidentia, and had no recurrences of vaginal vault prolapse. Nine patients reported that the vagina was too narrow postoperatively. Richter and Albrich (1981) reported excellent patient satisfaction, although eight patients were reluctant to resume sexual relations because of a perceived narrowed vagina. Morley and DeLancey (1988) reported on 100 patients who underwent sacrospinous ligament fixation, 33 of whom underwent isolated apical treatment. Sixty-seven had concomitant prolapse repairs. Apical success was noted in 95.7%, 90.1% globally, with four patients having prolapse in the anterior compartment. They noted that the long-term results were acceptable with minimal adverse effects.
Bilateral sacrospinous ligament suspension was compared with abdominal sacrocolpopexy in a prospective randomized study (Benson et al, 1996). Time to failure was shorter in the sacrospinous ligament suspension group compared with the abdominal sacrocolpopexy group (11 vs. 22 months) with the vaginal group having 12 cystoceles requiring reoperation versus 4 cystoceles in the abdominal group. Sauer and Klutke (1995) reported on 24 patients with a mean follow-up of 13.8 months. They had very little blood loss, and no transfusions were needed. Mild buttock pain was noted that resolved by 3 months. In 21 of 24 patients there was no recurrence of prolapse at the apex. One patient underwent repeat operation for a significant cystocele. Meschia and associates (1999) reported on 103 patients who underwent sacrospinous ligament fixation and found excellent results for the posterior and apical compartments but disappointing results for the anterior compartment, with 16% having grade 2 or greater prolapse.
The anterior compartment has been reported as a particularly vulnerable site for developing a defect after sacrospinous vaginal vault suspension. Recurrent cystoceles have been reported in 7.6% to 92% of patients postoperatively (Richter and Albrich, 1981; Morley and DeLancey, 1988; Pasley, 1995). This has been attributed to vaginal retroversion. This is noted not only postoperatively but intraoperatively, although many who develop anterior defects remain asymptomatic and do not undergo subsequent surgery to correct the anterior compartment defect (Paraiso et al, 1996).
Several authors have demonstrated the feasibility and efficacy of an anterior approach to the sacrospinous ligament vaginal vault suspension (Cespedes, 2000; Winkler et al, 2000). Winkler and coworkers (2000) reported that the anterior compartment recurrence rate after an anterior sacrospinous ligament fixation and anterior repair was 9%, in contrast to a 24% after sacrospinous ligament fixation with posterior repair. Cespedes (2000) reported on the bilateral anterior approach and noted no recurrences. Some of the theoretical advantages of the anterior approach include improved ability of the vagina to withstand increased intra-abdominal pressures (Pohl and Frattarelli, 1997) and less likelihood of rectal injury (Sauer and Klutke, 1995). Again, a low incidence of anterior compartment recurrence was noted (7%) (Cespedes, 2000).
Successful healing in the short term may predict long-term success of the sacrospinous ligament suspension. Shull and colleagues (1992) reported on 81 women undergoing sacrospinous ligament suspension, 72% of whom had undergone previous pelvic surgery. Those who showed absence of any compartment defect at the 6-week follow-up visit had a 3% likelihood of requiring additional reconstructive surgery within 2 to 5 years.
Complications
Cruikshank and Muniz (2003) reported 15% of patients experienced gluteal pain after sacrospinous ligament fixation on the ipsilateral side. Generally this pain resolves spontaneously within 2 to 3 months when delayed absorbable sutures are used (Maher et al, 2001). Postoperative neuropathic pain can be managed with observation, and patients should be counseled that duration may be as long as 3 months (Sauer and Klutke, 1995). Injection of the nerve with local anesthetic has also been reported (Lantzsch et al, 2001). Rectal perforation has been reported, recognized at the time of surgery, and repaired primarily with no sequelae (Richter and Albrich, 1981; Sauer and Klutke, 1995). In addition, injury to the pudendal nerve and internal pudendal vessels may occur with medially placed sutures near the ischial spine.
Iliococcygeus Suspension
The iliococcygeus suspension has the advantage of maintaining the vagina in the normal axis and is useful in cases in which the uterosacral ligaments are insufficient for attachment or cannot be identified. In this technique the vaginal vault is attached to the fascia of the iliococcygeus muscle as the anchoring site in contrast to the uterosacral or sacrospinous ligaments.
Technique and Results
The approach is similar to the uterosacral and sacrospinous ligament fixation and is usually approached extraperitoneally. The fascia of the iliococcygeus muscle is identified lateral to the rectum and anterior to the ischial spine (Shull et al, 1993). The point of suture fixation is placed 1 cm distal to the ischial spine, near the insertion of the ATFP. This suture is then passed through the condensation of connective tissue at the apex of the vagina anteriorly through the pubocervical fascia and posteriorly through the rectovaginal fascia (Fig. 72–33). Another suture is placed on the contralateral side in a similar manner. It is recommended that bilateral suture fixation be performed to achieve optimal results. Once the sutures are tied securely the vagina will be suspended bilaterally. As in sacrospinous ligament fixation the sutures may be placed through an anterior or posterior approach. Special needle drivers and lighted retraction are useful to facilitate suture placement. In cases utilizing permanent suture a pulley stitch technique is applied tying the knot internally.
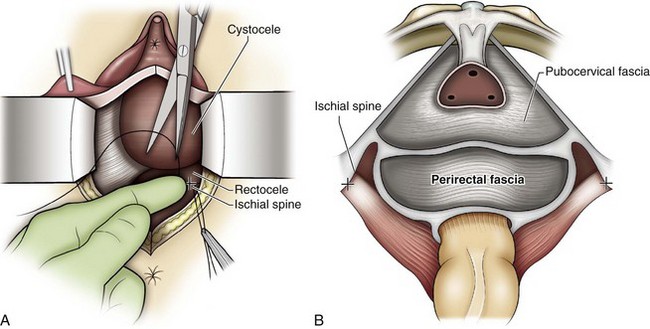
Figure 72–33 Iliococcygeus fascia suspension. A, With the surgeon’s finger deflecting the rectum downward, the right iliococcygeus fascia sutures are placed. Inset, View of the dissected vagina. B, Abdominal view of the endopelvic fascia. Approximate location of the sutures is delineated by the + sign.
(From Walters M, Muir T. Surgical treatment of vaginal apex prolapse. In: Vasavada SP, Appell RA, Sand PK, Raz S, editors. Female urology, urogynecology, and voiding dysfunction. Boca Raton [FL]: Taylor & Francis; 2005, with permission.)
Shull and colleagues (1993) reported on bilateral attachment of the vaginal cuff to the iliococcygeus fascia in 42 patients. Eight patients experienced pelvic support loss postoperatively, 3 in the middle compartment, 2 in the posterior compartment, and 4 in the anterior compartment. Six patients had additional surgery. Meeks and associates (1994) reported an anatomic cure of 96% in 110 patients. Intraoperative complications included rectal and bladder laceration and hemorrhage requiring transfusion. Postoperative complications included vaginal cuff abscess, fever, and transient femoral neuropathy. Maher and coworkers (2001) reported a 53% cure rate in 50 patients, and 19% of the patients experienced buttock pain.
Abdominal Sacrocolpopexy
The abdominal sacrocolpopexy should be considered a treatment option in the following clinical scenarios: in a patient with a failed previous vaginal repair, in a patient with isolated apical prolapse and/or enterocele, and in a younger woman with an active lifestyle who desires to continue sexual intercourse. In these patients, abdominal sacrocolpopexy is an excellent choice because it maximizes functional vaginal length and a near-normal vaginal axis. As discussed earlier, other pelvic floor defects and occult SUI can be addressed at the same time. In addition to open surgery, robotic-assisted laparoscopic and pure laparoscopic approaches have all been described (Sundaram et al, 2004; Rozet et al, 2005; Daneshgari et al, 2006; Elliott et al, 2006). The principles of the operation remain the same regardless of the approach.
Technique
The critical elements of the operation include the use of permanent mesh (type I macroporous, monofilament) as graft material, secure fixation of the graft to the sacral promontory and vaginal cuff, and complete enterocele reduction and culdoplasty.
The patient is positioned in the low lithotomy position, providing both transvaginal and transabdominal access. A Pfannenstiel or low midline abdominal incision may be used. On entry into the peritoneal cavity it is important to achieve exposure of true pelvis by careful packing of the small intestine and sigmoid colon. This is accomplished by releasing all adhesions in the pelvis and packing the bowel above the level of the sacral promontory. An incision is made in the posterior peritoneum over the sacral promontory, extending inferiorly along the right lateral aspect of the rectum. Electrocautery is used when dividing the fatty tissue over the promontory. Careful dissection in this area is essential to avoid shearing of presacral veins because severe bleeding may occur. The middle sacral vein traverses over the promontory and should also be avoided. As the fatty tissue is dissected free, the anterior surface of the sacral promontory is visualized, usually by identification of the anterior longitudinal ligament. Two or three permanent sutures are placed into the sacral promontory. These sutures are safely tagged for later placement into the graft. Alternatively, commercially available tacking devices may be used for securing the graft to the sacral promontory (Fig. 72–34).
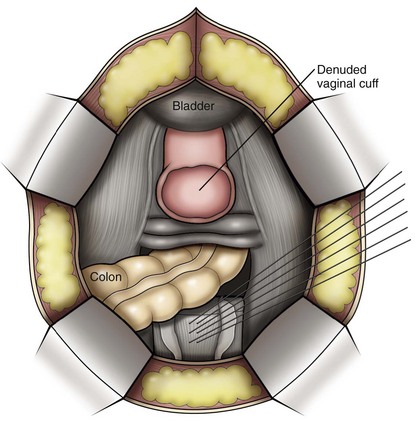
Figure 72–34 Abdominal sacral colpopexy. A, Incision in peritoneal cuff overlying the vagina, exposing the vaginal cuff. B, Two permanent sutures are placed in the sacral promontory to secure the graft material.
(From Delacroix S, Winters J. Abdominal sacral colpopexy. In: Graham SD, Keane TE, editors. Glenn’s atlas of urologic surgery. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2009, with permission.)
After placing an obturator in the vagina (large end-to-end anastomosis sizers are useful) the enterocele sac is identified and secured with an Allis clamp. If the enterocele is large, a Halban culdoplasty is performed by placing linear permanent (or delayed absorbable) sutures through the posterior peritoneum and on the outer surface of rectum up to the vaginal cuff (Geomini et al, 2001). Upward retraction of the sizer will assist in exposing the cul-de-sac. Usually four to six sutures are used to complete adequate cul-de-sac closure. This technique of closure (Fig. 72–35) is in contrast to that of the Moschowitz procedure, which involves purse-string closure and may predispose to ureteral angulation and obstruction.
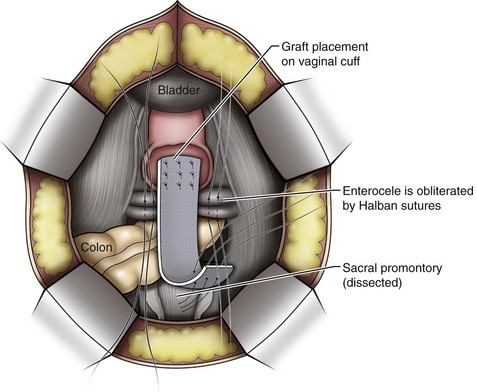
Figure 72–35 Abdominal sacral colpopexy: Synthetic graft material is sutured securely to vaginal cuff using multiple interrupted permanent sutures. The peritoneal cul-de-sac is closed using linearly placed sutures to obliterate this potential space.
(From Winters J, Cespedes R, Vanlangendonck R. Abdominal sacrocolpopexy and abdominal enterocele repair in the treatment of vaginal vault prolapse. Urology 2000;56:55–63, with permission.)
The peritoneum over the vaginal cuff is incised, and the peritoneum is dissected off of the cuff of the vagina (see Fig. 72–35). A 2.0-cm wide segment of graft is sutured to the exposed vaginal cuff using six to eight sutures of permanent suture material (Fig. 72–36). Care is taken to identify the border of the bladder to avoid suturing the graft to the bladder. An obturator in the vagina is useful to facilitate suture placement and secure the mesh to the vagina. A polypropylene, macroporous, monofilament, nonabsorbable mesh is recommended. The graft is secured to the vagina by folding over the cuff of the vagina and allowing the long end of the graft to exit posteriorly and extend to the sacrum. (Alternatively, a T-shaped configuration of the graft may be created. The short arm of the T is placed on the top of the vagina, and the long arm of the T is secured to the lower end of the vagina.) Additionally, a long segment of posterior mesh may be attached to the perineal body, a procedure termed colpoperineopexy (Cundiff et al, 1997). At this step the central sutures from the culdoplasty are placed through the long arm of the mesh if desired.
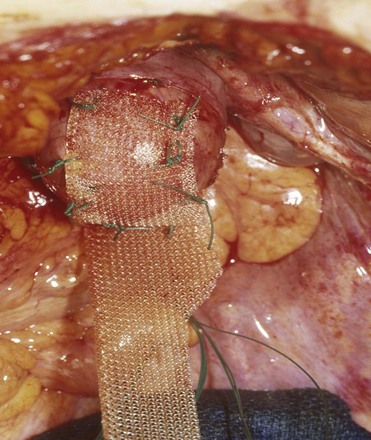
Figure 72–36 Abdominal sacral colpopexy: intraoperative view. Permanent mesh material sutured to vaginal cuff. End-to end anastomosis sizer was utilized to provide exposure of vaginal cuff.
(From Scarpero H, Cespedes R, Winters J. Abdominal approach to the repair of vaginal vault prolapse. Tech Urol 2001;7:139–45, with permission.)
The graft is then secured to the promontory. Care must be taken to avoid excessive tension. Placing the obturator all the way into the vagina but not pushing the vagina upward establishes the proper length of the graft. The graft is placed around the right lateral aspect of the rectum. A space of 2 fingerbreadths between the graft and the rectum prevents compression of the rectum over the graft (Fig. 72–37). The excessive length of the graft is trimmed after fixation to the sacrum. Lastly, the graft is positioned in the retroperitoneal space by closing the presacral peritoneum over the graft and covering the graft on the vagina with the superior edge of the peritoneum over the vagina. Procedures for SUI (if indicated) are then performed. The patient is then examined to determine if any ancillary prolapse repairs are needed.
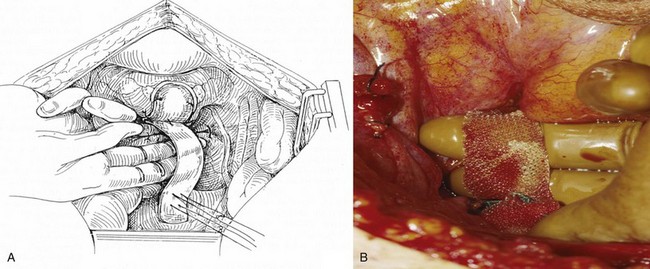
Figure 72–37 Abdominal sacral colpopexy. A, Graft is secured without tension to sacral promontory. B, Intraoperative view.
(A, From Winters J, Cespedes R, Vanlangendonck R. Abdominal sacrocolpopexy and abdominal enterocele repair in the treatment of vaginal vault prolapse. Urology 2000;56:55–63, with permission; B, from Scarpero H, Cespedes R, Winters J. Abdominal approach to the repair of vaginal vault prolapse. Tech Urol 2001;7[2]:139–45.)
Abdominal sacrocolpopexy maintains a functional vagina and restores maximum vaginal length and support. In a comparative study, vaginal length was longer after abdominal sacrocolpopexy compared with sacrospinous fixation (Given et al, 1993). A nonabsorbable monofilament synthetic mesh should be used during abdominal sacrocolpopexy. In a study by Culligan and coworkers (2005), patients undergoing abdominal sacrocolpopexy using either absorbable cadaveric fascia lata graft (Tutoplast) or nonabsorbable monofilament polypropylene were randomized. The objective failure rate for recurrence at any other vaginal site was 14 of 44 in the fascial group and 4 of 45 in the mesh group.
There are numerous reports by multiple authors that confirm the success of abdominal sacrocolpopexy. Multiple authors have reported success rates greater than 90% (Snyder and Krantz, 1991; Addison and Timmons, 1993; Webb et al, 1998; Menefee et al, 1999). There are several randomized, prospective trials comparing sacrospinous ligament fixation with abdominal sacrocolpopexy. In a comparative study between abdominal sacrocolpopexy versus vaginal repair, Benson and colleagues (1996) reported a lower success rate with vaginal repair, and an equivalent hospital stay between the two groups. Lo and Wang (1998) reported on their results of abdominal sacrocolpopexy versus sacrospinous ligament fixation at a duration of 2.1 years: 94.2% success in abdominal sacrocolpopexy compared with 80% in sacrospinous ligament fixation. Maher and coworkers (2001) reported on a comparative study between abdominal sacrocolpopexy and sacrospinous ligament fixation. At 2 years follow-up, symptoms and anatomic success were equivalent but asymptomatic failures to introitus and recurrent cystoceles were less after abdominal sacrocolpopexy. Overall, vaginal (sacrospinous ligament fixation) procedures have a higher rate of recurrent anterior defects even when performed in the setting of a concomitant anterior repair (colporrhaphy) (Holley et al, 1995). After an isolated abdominal repair of vaginal vault prolapse without addressing concomitant pelvic floor defects, recurrent distal defects such as cystocele and rectocele may occur up to a third of the time. This may predispose to decreased patient satisfaction and the possibility of secondary vaginal repair (Blanchard et al, 2006). Although most patients remain asymptomatic, secondary repair of these defects may be required (Blanchard et al, 2006). It is important to counsel patients preoperatively about the possibility of distal anterior or posterior defects after abdominal sacrocolpopexy and the need for secondary vaginal repair, which can occur up to 20% of the time (Blanchard et al, 2006).
In a comprehensive review by Nygaard and coworkers (2004), intraoperative complications of sacrocolpopexy included hemorrhage or transfusion in 4.4%, cystotomy in 3.1%, enterotomy in 1.6%, and ureteral injury in 1.0%. Postoperative complications included urinary tract infection in 10.9%, wound infection in 4.6%, ileus in 3.6%, deep venous thrombosis or pulmonary embolism in 3.3%, and small bowel obstruction in 1.1%. The risk of significant bleeding has been reported from 1.6% to 4.4% and may be controlled with the use of stainless steel thumbtacks (Timmons et al, 1991). Significant hemorrhage may occur from disruption of the presacral vessels, and the occurrence of this complication may be reduced when fixation of the graft is performed high on the sacral promontory. The rate of reoperation for gastrointestinal complications is 1.2% (Whitehead et al, 2007). Vaginal exposure of mesh is heralded by persistent pain, discharge, or infections, and clinicians must be vigilant in follow-up (Karlovsky et al, 2005). In a recently reported meta-analysis, mesh erosion into the vagina is reported at 3.4% to 5.4% and may vary on the type of mesh used (Brizzolara and Pillai-Allen, 2003; Nygaard et al, 2004). Erosions are more prevalent with Teflon or Gore-Tex materials and rare with macroporous, monofilament meshes. Several authors have suggested that hysterectomy at the time of colpopexy increases the rate of mesh extrusion, although these findings are not uniform (Nygaard et al, 2004; Marinkovic, 2008) and no randomized trials address this issue (Nygaard et al, 2004). In 60 patients undergoing concomitant hysterectomy using a two-layer closure of the vaginal cuff followed by abdominal sacrocolpopexy with synthetic nonabsorbable monofilament mesh, the erosion rate was 0.8% compared with 0% in the 64 patients with a prior hysterectomy (Wu et al, 2006). In patients undergoing a combined operation, supracervical hysterectomy or a meticulous two-layer closure of the cuff should be performed to decrease the incidence of mesh erosion. Surgeons must be aware that the concerns of mesh erosion after abdominal sacrocolpopexy are higher after hysterectomy. Options in the management of eroded mesh after colpopexy may also include mesh excision with partial colpocleisis (Quiroz et al, 2008) and abdominal mesh excision.
Colpocleisis
Colpocleisis is an obliterative procedure used in the treatment of posthysterectomy vaginal vault prolapse or significant uterovaginal prolapse. In properly counseled, older patients who do not desire functional vaginal anatomy for sexual activity, colpocleisis may be an appropriate procedure. The total colpocleisis refers to removal of the vaginal epithelium to 0.5 to 2 cm from the urethral meatus with complete closure. A partial colpocleisis is used in cases of uterovaginal prolapse, and this procedure involves creating lateral channels to allow uterine drainage. Advantages of the colpocleisis procedures are shorter operative time, ability to use regional anesthesia or local anesthesia with sedation, minimal complications, and decreased recuperative time (Cespedes et al, 2001; FitzGerald et al, 2008). In those whose uterus is to be left in situ, preoperative evaluation should include a Papanicolaou smear, pelvic ultrasonography, and endometrial biopsy if indicated. Performance of hysterectomy at the time of colpocleisis eliminates the risk of developing cervical or endometrial cancer and eliminates the risk of developing pyometra, which is a serious complication of partial colpocleisis when the channels become obstructed (Shayya et al, 2009). However, most patients selected for colpocleisis are older with significant comorbidities that make a concomitant hysterectomy undesirable.
Technique
Partial Colpocleisis
The patient is placed in the standard lithotomy position. A Foley catheter is placed into the bladder. If an anti-incontinence procedure is needed, it may be performed first. The cervix is grasped with a tenaculum, and a rectangular segment of vaginal epithelium is marked anteriorly and posteriorly. The excision of the vaginal epithelium will extend 3 cm from the urethral meatus to 3 cm from the cervix (Cespedes et al, 2001). It is important to leave sufficient vaginal epithelium laterally to allow creation of the lateral channels to facilitate drainage. The epithelium is then excised. It is optional to place a 14-Fr red Robinson catheter along the vaginal sidewalls to assist in forming the channels. Then, starting at the leading edge of the prolapse, one places successive layers of 2-0 delayed absorbable sutures, which plicate and reduce the prolapse until the prolapsed tissues are above the levator plate (Fig. 72-38A). The sutures are placed in transverse rows consisting of linear sutures through the pubocervical fascia anteriorly and rectovaginal fascia posteriorly. As these sutures are tied, the apical prolapse is reduced proximally (see Fig. 72-38B). Successive rows of sutures are placed until the apical prolapse is completely reduced. A high perineorrhaphy is performed by removing a triangular segment of the vaginal epithelium. The fibromuscular tissues of the perineal body are approximated in the midline with absorbable sutures to narrow the introitus. The completed procedures are shown in Figure 72–39. Indigo carmine is administered and a cystoscopy is carried out to confirm ureteral patency.
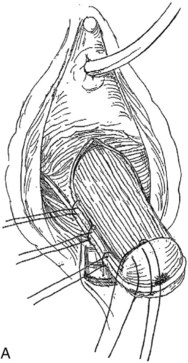
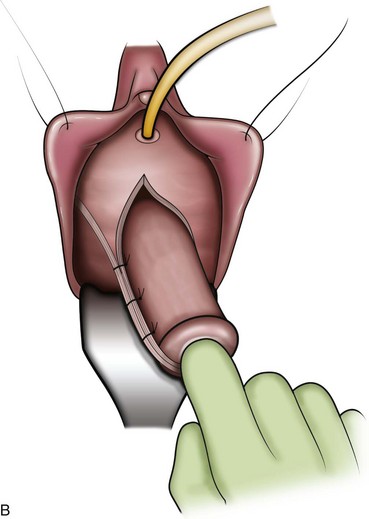
Figure 72–38 A, Partial colpocleisis is started by excising rectangular portions of the vaginal epithelium, leaving lateral channels. The cervix is then everted over a red Robinson catheter. B, The cervix is everted as the prolapse is reduced.
(From Cespedes RD, Winters CW. Colpocleisis for the treatment of severe vaginal vault prolapse. In: Vasavada SP, Appell RA, Sand PK, Raz S, editors. Female urology, urogynecology, and voiding dysfunction. Boca Raton [FL]: Taylor & Francis; 2005, with permission.)
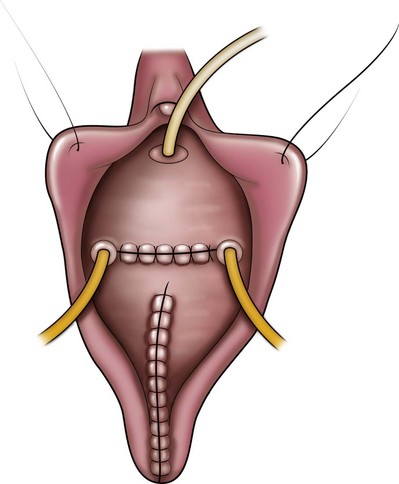
Figure 72–39 The partial colpocleisis is completed along with perineorrhaphy.
(From Cespedes RD, Winters CW. Colpocleisis for the treatment of severe vaginal vault prolapse. In: Vasavada SP, Appell RA, Sand PK, Raz S, editors. Female urology, urogynecology, and voiding dysfunction. Boca Raton [FL]: Taylor & Francis; 2005, with permission.)
Total Colpocleisis (Fig. 72–40)
The technique differs from partial colpocleisis in that the lateral channels are not left in place, but, rather, the entire prolapsed vagina is denuded of its epithelium from the apex to 3 cm proximal to the urethral meatus. The dissection is carried laterally and continued to the posterior lateral sulcus. The prolapse sac is dissected away from the vaginal epithelium. Usually the enterocele reduces easily and no further treatment is necessary (Cespedes et al, 2001). At this point the colpocleisis is carried out starting at the leading edge of the prolapse. Purse-string sutures of 2-0 absorbable sutures are placed circumferentially around the prolapse (see Fig. 72–37A). As each progressive suture is tied, the prolapse is reduced proximally (see Fig. 72-37B). These progressive purse-string sutures are tied until the prolapse is adequately reduced. Alternatively, the prolapse may be reduced as described for the partial colpocleisis by placing transverse rows of interrupted sutures. As these rows are tied the prolapse is reduced proximally. Successive rows of sutures are placed until the prolapse is fully reduced. The remainder of the procedure is performed as described for the partial colpocleisis. After the prolapse is reduced the posterior vaginal wall and perineum are repaired. A high perineorrhaphy is important to narrow the introitus. Cystoscopy to ensure patency of the ureters is recommended. For both techniques, success depends on the amount of vaginal tissue sutured together. This creates a septum of support, which is enhanced by bringing the levator muscles together along with the perineorrhaphy.
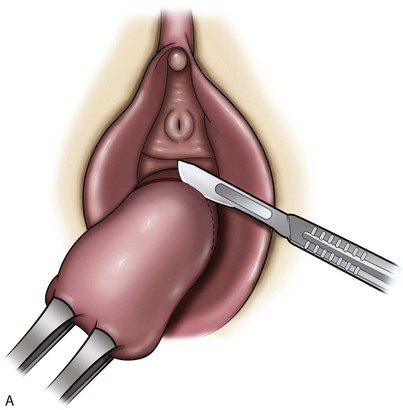
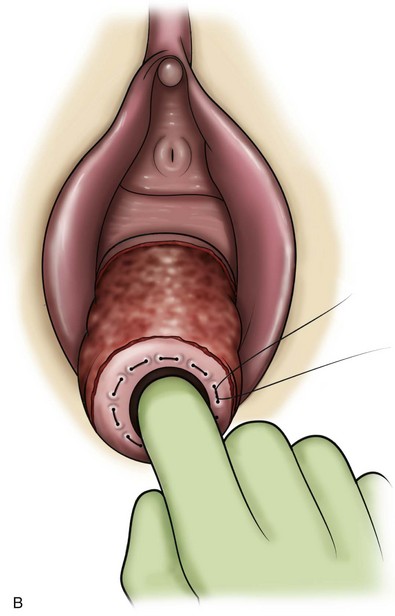
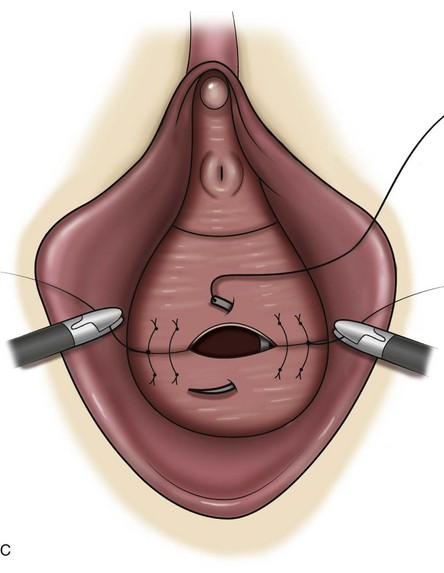
Figure 72–40 Total colpocleisis. A, A circumferential incision is created at the base of the prolapse. B, Purse-string sutures are placed in the prolapsed tissues to reduce it. C, The vaginal epithelium is closed transversely.
(From Delancey JO, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol 1997;176:1228–35, with permission.)
Several authors have reported that it is unnecessary to open the enterocele sac if one is present, because the repair will close the potential space in which the bowel might protrude (DeLancey, 1997; Glavind and Kempf, 2005). If the enterocele sac cannot be reduced, then purse-string absorbable sutures incorporating the sac and the uterosacral remnants can be used to reduce the sac (Cespedes et al, 2001).
Results (Table 72–7)
Total Colpocleisis
DeLancey (1997) reported on 33 women who underwent colpocleisis over a 20-year period. In this study, opening of the enterocele sac was avoided. One patient had recurrence of a vaginal eversion and was treated with repeat colpocleisis and had a good response 1 year after the second colpocleisis. All but one patient expressly denied regret over the decision to have an operation that precludes the ability to have intercourse; however, one “accepted” her sexual inactivity. Many indicated relief of the discomfort associated with the prolapse and were positive about the outcome of the operation. Cespedes and coworkers (2001) reported on 38 patients who underwent a mean of 24 months follow-up. There was no recurrent prolapse. Three patients who had a Kelly plication for incontinence had mild SUI, and two were treated successfully with collagen. All were satisfied with the procedure, and no complications were noted. von Pechmann and colleagues (2003) reported on a large study (92 patients) who underwent colpocleisis. Patients were followed by examination and telephone survey, which appraised satisfaction and regret. Regret over the loss of coital ability was expressed in 12.9% (eight patients). In a multicenter, prospective study designed to study the effect of colpocleisis on pelvic support, symptoms, quality of life, morbidity, and postoperative satisfaction, FitzGerald and associates (2006) reported on 152 patients with 1-year follow-up. Eighty-four percent stated they were either very satisfied or satisfied about the decision for vaginal closure. One patient was dissatisfied, and one was very dissatisfied. One patient underwent repeat colpocleisis for recurrence. Most patients did not experience worsening of their body image. Hoffman and coworkers (2003) reported favorably on vaginectomy or hysterovaginectomy in which the denuded tissues are brought together with serial purse-string sutures. They preferred this approach because of optimal exposure to the cul-de-sac and the ability to perform multicompartmental repair.
Partial Colpocleisis (see Table 72–7)
Goldman and coworkers (1981) reported on 118 patients who underwent Le Fort colpocleisis. Good anatomic results were noted in 107 patients, with 101 reporting relief of symptoms. There were three recurrences but no patient underwent further operative therapy. Immediate postoperative complications were one pulmonary embolism, fever, and urinary tract infection. Langmade and Oliver (1986) reported on 102 patients. There were no recurrences and no de novo stress incontinence. Sixteen patients had transient postoperative urinary retention. No patient changed her mind regarding the ability to engage in sexual intercourse.
Urinary incontinence after colpectomy may occur and has been attributed to several mechanisms. The first, occult stress incontinence, is when correction of the urethral angulation will unmask preexisting stress incontinence. The other is from traction of the urethra when it is approximated with the posterior vaginal muscularis (FitzGerald et al, 2006). Thus it is recommended to modify the procedure so that the distal edge of the colpocleisis is at least 1.5 cm from the urethral meatus (FitzGerald et al, 2006). As with other prolapse cases, the recommendation of a concomitant anti-incontinence procedure in an asymptomatic patient is controversial and remains to be substantiated by prospective studies. However, it is clear that these women may develop SUI postoperatively. Thus all women before undergoing colpocleisis should be screened for the presence of occult SUI, and anti-incontinence procedures should be done concomitantly in women with symptoms of SUI or occult SUI. Additionally, in these frail, elderly patients there is a concern of urinary retention, which frequently is the result of impaired contractility and other perioperative factors. This may be more difficult to manage because these patients are more likely to be unable to perform intermittent catheterization. Thus these possibilities must be discussed with the patients in advance of surgery. Patients considered at significant risk for retention should be offered a suprapubic tube to assist in bladder management postoperatively.
Uterine Prolapse
Pelvic organ prolapse was the indication for 16.3% of all hysterectomies performed between 1988 and 1990. Although the majority of hysterectomies are performed transabdominally, the vaginal approach offers the advantages of less invasive surgery and the ability to repair concomitant pelvic floor defects (ACOG, 2009; Eilber et al, 2004).
The uterus is suspended in the pelvis by the cardinal/uterosacral ligament complex. The body of the uterus is enveloped between the two leaves of the broad ligament, which also surrounds the fallopian tube, round ligament, and ovarian ligament along with the uterine and ovarian vessels. The broad ligament does not provide significant support (Eilber et al, 2004). Therefore the defect prompting uterine prolapse is in the form of attenuated ligaments or specific breaks in the continuity of the cardinal/uterosacral ligament complex. Contraindications for the vaginal approach are endometriosis of unknown extent, obliteration of the cul-de-sac, large fibroids (size disproportion to the introitus), pelvic tumor, adnexal tumor, and malignancy of the uterus or ovaries (Eilber et al, 2005).
Transvaginal Hysterectomy
Technique
The patient is placed in the dorsal lithotomy position. A catheter is placed to drain the bladder. Retraction of the labia with a ring retractor and stays is useful. Two Lahey clamps are placed on the cervix. Care must be taken to place it on the most distal portion because the bladder may descend fairly low on the anterior vaginal wall. The incision is made full thickness through the vaginal epithelium around the cervix approximately 1 cm from the cervical os (Fig. 72–41). Because the posterior cul-de-sac is entered first, a U-shaped incision is made initially from the 3-o’clock position to the 6-o’clock position to the 9-o’clock position. The posterior peritoneal fold is exposed (Fig. 72–42). Applying traction to the tenaculum and using a right angle retractor to apply countertraction to the vagina facilitate this exposure. This maneuver will help to identify the correct plane, because the vagina will retract when the correct depth is reached. Once the posterior peritoneal fold is identified, it is opened sharply to enter the cul-de-sac. Visualization of bowel and omentum will confirm correct entry into the peritoneal cavity. By entering the peritoneal cavity posteriorly, control of the uterosacral ligaments can be attained before the anterior dissection (Fig. 72–43). This prevents potential anterior bleeding from obscuring the posterior portion of the dissection and facilitates mobilization of the uterus when the uterosacral ligaments are divided. Countertraction and use of gauze will aid with separation of the peritoneum off of the posterior aspect of the uterus. Once the cul-de-sac is entered, the uterus and adnexa may be palpated to check for further pathology. A long, weighted vaginal speculum is placed with the beak into the abdominal cavity. The anterior dissection is now started. The circumferential incision is completed anteriorly. It is imperative to keep the dissection close to the uterus by placing the tips of the curved Metzenbaum scissors toward the cervix. The glistening white surface of the uterus confirms the correct plane of dissection. Traction on the cervix with countertraction applied with a right angle (Heaney) retractor will facilitate with the dissection of the vesicouterine space. The peritoneum is identified anteriorly and entered. Visualization of the bowel or omentum will confirm the location in the peritoneal cavity. Once the peritoneum has been entered anteriorly and posteriorly the ureters can be palpated to verify the location. The uterosacral ligament can then be shortened to facilitate suspension into the pelvis to restore the vaginal cuff once the uterus has been removed.
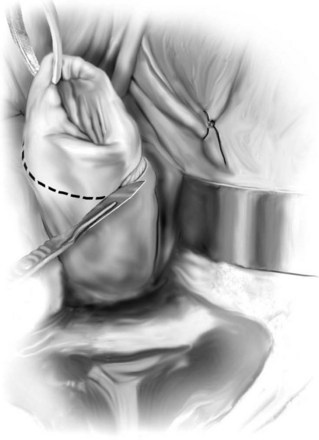
Figure 72–41 Circumferential incision through the vaginal epithelium is made at the level of the cervix.
(From Rooney CM, Karram MM. Vaginal hysterectomy in the treatment of vaginal prolapse. In Raz S, Rodriguez LV, editors. Female urology. 3rd ed. Philadelphia: Elsevier Saunders; 2008.)
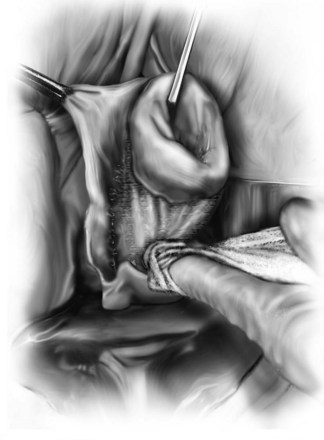
Figure 72–42 The vaginal epithelium is sharply dissected from the cervix revealing the posterior peritoneal fold.
(From Rooney CM, Karram MM. Vaginal hysterectomy in the treatment of vaginal prolapse. In: Raz S, Rodriguez LV, editors. Female urology. 3rd ed. Philadelphia: Elsevier Saunders; 2008.)
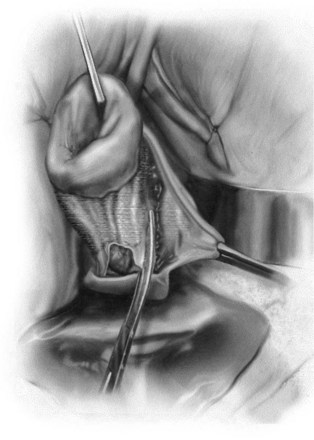
Figure 72–43 Clamping of the uterosacral ligaments.
(From Rooney CM, Karram MM. Vaginal hysterectomy in the treatment of vaginal prolapse. In: Raz S, Rodriguez LV. Female urology. 3rd ed. Philadelphia: Elsevier Saunders; 2008.)
The cardinal and uterosacral ligaments are isolated with a large right-angle clamp and Phaneuf clamp, divided, and then suture ligated with delayed absorbable suture. First, the uterosacral ligament is identified. A small stump of tissue should be left rather than cutting flush with the clamp to facilitate later identification. This is done bilaterally. The pedicles should be tagged with a curved hemostat. The uterus will then descend. The cardinal ligament is then grasped by swinging the clamp laterally, which shortens the pedicle. It is not unusual to have an elongated cervix with prolapse, which will make visualization of the fundus of the uterus challenging. The uterine arteries are cross clamped, suture ligated, and then divided, being careful to leave enough of a stump so that the suture does not slip (Fig. 72–44). Including the peritoneum anteriorly and posteriorly will ensure the complete inclusion of the artery in the clamp. Next comes the broad ligament. If the ovaries and adnexa are to be left, the utero-ovarian ligament, fallopian tube, and round ligaments are visualized from anterior to posterior and are divided and ligated. The broad ligament is then clamped and divided bilaterally. All the pedicles are examined for hemostasis.

Figure 72–44 The uterine vessels are clamped and the vascular pedicle suture ligated. Care should be taken to leave enough of a stump to prevent the suture from slipping off the pedicle.
(From Rooney CM, Karram MM. Vaginal hysterectomy in the treatment of vaginal prolapse. In Raz S, Rodriguez LV. Female urology. 3rd ed. Philadelphia: Elsevier Saunders; 2008.)
Vaginal vault suspension is crucial because the hysterectomy alone will not restore the anatomy of the vaginal vault. Two maneuvers are important to perform. The pubocervical fascia and the rectovaginal fascia are approximated to close the cul-de-sac and a culdoplasty is done. The culdoplasty should be performed first. This is started by placing a suture into the uterosacral ligaments and a suture at the corners of the vaginal cuff. These are not tied down until after the cuff is closed, because doing so will suspend the vaginal vault. Two purse-string sutures of 0 polyglactin are placed to close the cul-de-sac. These sutures will incorporate the pubocervical fascia, rectovaginal septum, cardinal/uterosacral ligament complex, broad ligaments, and perivesical fascia. The sutures are not placed too laterally on the pubocervical fascia because this can cause ureteral injury. The purse-string sutures are tagged and not tied down at this point. The vaginal cuff can then be closed in a continuous locking or interrupted fashion. The sutures from the uterosacral ligaments are brought to the midline, and then the purse-string sutures are tied. The suspension sutures are then tied, thus suspending the vaginal vault and restoring vaginal depth. At this point any other repair is performed, but extending the anterior or posterior incisions to the previously incised vaginal cuff (T colpotomy) should be avoided because this inherently weakens the repair (Fine and DeLancey, 2005). A cystoscopy is then performed before cutting the excess suture material.
Posterior Compartment Repair
Symptoms attributable to posterior compartment prolapse can be divided conceptually as herniation symptoms, defecatory dysfunction, and sexual dysfunction (Cundiff and Fenner, 2004). Herniation symptoms include vaginal bulging and bleeding of the epithelium from excoriation. Defecatory dysfunction includes stool trapping requiring vaginal splinting or manual digitations, defecatory urgency, and constipation. It is important to differentiate between outlet obstruction (including defects in the support of the posterior compartment, perineum, and rectum), motility disorders, and anismus (Cundiff and Fenner, 2004). Anismus is the failure of the puborectalis to relax during defecation. Motility disorders, which usually involve impaired transit of the rectum and anus, are treated with dietary modifications and medication. Anismus responds to biofeedback, and pelvic floor support defects are treated surgically. In combined disorders it is recommended that nonsurgical treatment for anismus or slow transit constipation (the most common disorder of motility) be treated before embarking on surgical intervention. Sexual dysfunction is thought to be secondary to dyspareunia, although decreased desire and anorgasmia may also be contributing factors (Handa et al, 2004). There is conflicting evidence between the correlation both in sexual dysfunction and defecatory dysfunction and the degree of POP (Weber et al, 1998; Ellerkman et al, 2001; Fialkow et al, 2002; Jelovsek et al, 2005; Bradley et al, 2006).
Several authors have sought to identify patient factors that would predict who might benefit most from rectocele repair (Murthy et al, 1996; Watson et al, 1996). These include sensation of vaginal mass or bulge, need for digitalization to complete rectal evacuation, nonemptying or partial emptying on defecography, and presence of a large rectocele. Sensation of incomplete emptying and constipation are not specific to rectoceles and may be associated with other disorders, including irritable rectum and slow transit constipation. Patients should be counseled that surgery may reduce the vaginal protrusion and decrease or eliminate the need for vaginal splinting; however, some patients may have persistence of constipation because motility disorders and anismus can coexist with prolapse.
Rectoceles can be approached transanally or transvaginally. Nieminen and coworkers (2004) randomized 30 patients to rectovaginal fascia plication or transanal repair. Both approaches resulted in a high rate of symptom resolution (93% for the vaginal approach vs. 73% for the transanal approach). However, the vaginal approach had better objective findings and a lower rate of prolapse recurrence (7% vs. 66%). The traditional posterior colporrhaphy was devised in the 19th century to treat perineal tears, which occurred during vaginal delivery. The original description involved plicating the pubococcygeus muscles and the posterior vaginal wall and reconstruction of the perineal body, which was termed posterior colpoperineorrhaphy (Cundiff and Fenner, 2004). This creates a rigid inferior shelf, which reduced the herniation of the posterior wall and prevented descensus of the vaginal vault or uterus. In 1961, Francis and Jeffcoate (1961) reported a high incidence of dyspareunia after colporrhaphy with levator plication. In their series of 243 women, 50% developed postoperative dyspareunia. In addition, there is evidence to suggest that the traditional posterior colporrhaphy with levator plication may worsen defecatory symptoms. Khan and Stanton (1997) reported increased symptoms of fecal incontinence, constipation, incomplete evacuation, and dyspareunia postoperatively. Because of the increase in dyspareunia postoperatively, plication of the levator ani muscles has largely been abandoned. Site-specific repairs and midline fascial plication without levator ani plication have emerged as the predominant surgical treatments of rectocele.
Posterior Colporrhaphy
Technique (Fig. 72–45)
The patient is placed in the dorsal lithotomy position. If a patient requires an anterior or middle compartment repair, it is performed first (Rovner and Ginsberg, 2001). The anterior wall can be retracted with a Heaney retractor to improve visualization. Hydrodissection may be accomplished by injecting saline or a local anesthetic. A midline incision is made on the posterior vaginal epithelium. For a high rectocele the incision may be as high as the vaginal vault; for smaller rectoceles the incision is started at the most caudal position. The rectovaginal fascia is separated from the vaginal epithelium with Metzenbaum scissors. The tips of the scissors should be pointed toward the vagina to avoid rectal injury. The dissection proceeds laterally until the pararectal attachments to the pelvic sidewall are visualized. In cases of large posterior defects, a purse-string suture of 2-0 or 3-0 absorbable suture may be placed at the base of the rectal herniation to reduce it. Additionally, this acts to bring the attenuated rectovaginal tissue together to aid in its reapproximation. The rectovaginal tissue is then plicated in the midline with either interrupted or continuous 2-0 absorbable suture. Care is taken to avoid excessively lateral placement of these sutures, because this may result in a painful “ridge” along the posterior vaginal wall. This is extended distally and incorporated into a perineal body reapproximation. The excess vaginal epithelium is trimmed, and the vaginal epithelium is closed with an absorbable suture. A perineorrhaphy may also be performed before the vaginal epithelium is closed.
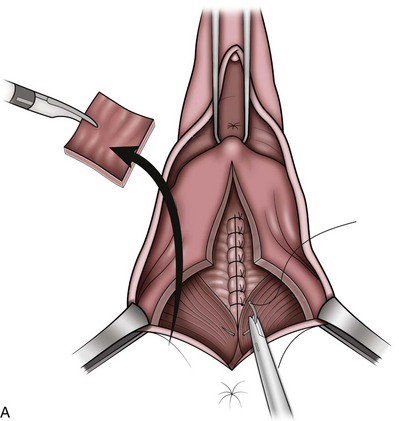
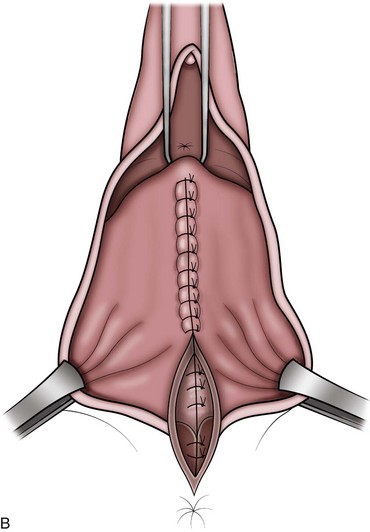
Figure 72–45 A and B, Technique of posterior colporrhaphy with rectovaginal tissue plication.
From Ginsberg D. Treatment of vaginal wall prolapse. In: Goldman H, Vasabada S, editors. Female urology: a practical clinical guide. Totowa [NJ]: Humana Press; 2007. p. 281–96, with permission.)
Site-Specific Repair
To minimize complications associated with posterior colporrhaphy, a site-specific defect repair was described. Richardson (1993) described discrete defects in the rectovaginal fascia found in both patients undergoing posterior colporrhaphy and cadaveric dissections. He found that the most common defect was the transverse configuration, separating the vaginal septum from the perineal body (Fig. 72–46). This essentially results in a separation of the rectovaginal tissues from the perineal body. The goal of the site-specific repair is to restore the anatomy by closing these discrete defects.
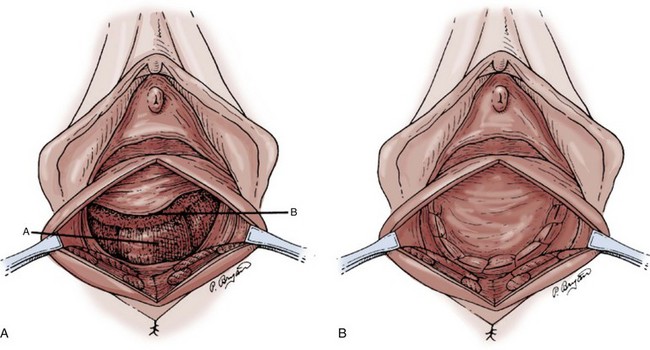
Figure 72–46 Site-specific rectocele repair. A low transverse defect is identified and repaired primarily.
(From Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol 1993;36:976–83.)
A midline incision is made on the posterior vaginal epithelium. For a high rectocele the incision may be as high as the vaginal vault; for smaller rectoceles the incision is started at the most caudal position. The rectovaginal fascia is separated through a virtually bloodless plane from the vaginal epithelium. The index finger of the nondominant hand is then inserted into the rectum to facilitate identification of the fascial defect. The defect is closed with absorbable interrupted sutures. The excess vaginal epithelium is trimmed, and the vaginal epithelium is closed with interrupted sutures.
Perineorrhaphy
A triangular incision at the mucocutaneous junction is removed (Rovner and Ginsberg, 2001). The triangular island of posterior vaginal wall is sharply removed from the prerectal levator fascia. Horizontal mattress sutures are used to approximate the attenuated perineal body fibromuscular tissue. Once these are brought together, the muscles of the urogenital diaphragm are reconstituted and support is restored to the central tendon. It is important that a smooth contour be created along the suture line, because ridges may cause dyspareunia. A vaginal packing soaked with the antibiotic solution or metronidazole gel is then placed.
Results (Table 72–8)
Khan and Stanton (1997) reported on the anatomic and functional results of posterior repair utilizing levator plication. Both reported anatomic success; however, many patients complained of bowel symptoms and dyspareunia postoperatively. Because of these adverse events associated with levator plication, this technique has been largely abandoned.
Paraiso and associates (2006) randomized patients prospectively to posterior colporrhaphy (rectovaginal fascial plication), site-specific repair, or site-specific repair with porcine small intestinal submucosa graft. They found that posterior colporrhaphy and site-specific rectocele repair had similar functional and anatomic outcomes. The addition of the porcine-derived graft did not improve anatomic outcomes. Rates of dyspareunia did not differ when comparing all three groups. In a follow-up study of these same patients specifically looking at bowel symptoms, Gustilo-Ashby and coworkers (2007) found that anatomic cure was associated with a reduced risk of postoperative straining and sensation of incomplete bowel evacuation but not with other bowel symptoms. They found that bowel symptoms, including feeling of incomplete emptying, straining to defecate, splinting to defecate, and fecal incontinence improved significantly after rectocele repair. Abramov and associates (2005) retrospectively studied patients with advanced posterior vaginal prolapse and compared patients who underwent posterior repair to those who had site-specific repair. They found that the recurrence of posterior defects was higher in the site-specific group compared with the midline plication of the rectovaginal fascia (33% vs. 14% for second-degree repair and 11% vs. 4% for third-degree repair). In addition, recurrence of symptomatic rectocele was greater in the site-specific group (11% vs. 4%). Rates of de novo dyspareunia and postoperative bowel symptoms were the same in both groups. Maher and colleagues (2004) prospectively studied the efficacy of posterior repair with plication of the rectovaginal fascia and found that improved anatomic outcome correlated with improved functional outcomes. Eighty-seven percent of patients no longer experienced obstructed defecation postoperatively. Significant improvements were seen in awareness of prolapse, obstructed defecation, straining to defecate, hard stools, dyspareunia, and digitations. Dyspareunia decreased from 37% to 5%. Singh and associates (2003) reported a prospective study of 42 women evaluated for bowel, sexual, urinary, and prolapse symptoms as well as anatomic outcomes after fascial plication technique. At 6-week follow-up, 87% noted an improvement of bowel symptoms. At 18 months they estimated the relief of vaginal symptoms to be between 73% and 92%. Sixty-five percent had improvement of the defecatory symptoms and 38% had improvement of sexual discomfort. None of the patients reported de novo dyspareunia or bowel symptoms.
In contrast to the generalized repair of midline plication of the rectovaginal fascia, several authors have reported their experience with site-specific repair. Glavind and Madsen (2000) prospectively studied 67 patients who underwent a discrete repair. Of the 67 patients, 64 were found to have a discrete defect, which was repaired; and in 3 there was an attenuation of the tissue. At 3-month follow-up, 85% of those who reported bowel symptoms preoperatively reported resolution of symptoms. Two patients (3%) had de novo dyspareunia. Porter and colleagues (1999) examined anatomic, functional, and quality of life aspects of posterior colporrhaphy in a retrospective study. Improvement or cure was noted for pain or pressure, vaginal mass, splinting, and difficulty with defecation. Constipation did not significantly change. Preoperative dyspareunia improved in 73% patients, worsened in 19%, and occurred de novo in three patients. Emotional health improved, specifically thoughts of embarrassment and frustration. Kenton and coworkers (2007) reported on 66 patients who underwent site-specific repair; all patients had reattachment of the rectovaginal septum to the perineal body. Statistically significant symptom relief was noted in protrusion, manual evacuation, difficult defecation, and dyspareunia. Constipation was unchanged. Three patients developed de novo dyspareunia, and one had de novo constipation. At 1 year, 7 of 11 patients who used manual evacuation preoperatively returned to doing so. The authors commented that this may be due to a functional decompensation of the rectum that is not corrected surgically. Finally, Cundiff and colleagues (1998) reported on the anatomic and functional aspects of discrete fascial defect repair of the posterior compartment. At 12-month follow-up, 86% were noted to have stage 1 or less. Splinting was eliminated in 63% of patients who reported this symptom preoperatively. The mean satisfaction score was 8.6 (10 highest). The mean improvement did not correlate to the anatomic correction but did correlate with alleviation of defecatory symptoms, stressing the importance of symptom relief being of priority to the patient over anatomic correction.
Interposition Graft Repairs of the Posterior Compartment (Table 72–9)
Both synthetic mesh and biologic grafts have been used in posterior repairs, although data are lacking regarding routine use. Also, several authors caution against the use of synthetic materials in the posterior compartment, owing to the potential for dyspareunia and visceral erosion, and favor using biologic grafts (Chen et al, 2007). Kohli and Miklos (2003) reported anatomic outcomes on 43 patients who underwent a site-specific repair with cadaveric dermis: 30 were available for evaluation at an average of 12.9 months. The graft was fixed proximally to the vaginal apex, laterally to the levator ani muscles, and distally to the perineal body. They reported a 93% success rate using the POP-Q system. Kobashi and associates (2005) reported using cadaveric fascia lata in 73 patients. With a mean follow-up of 13.7 months, there was an 83% to 89% improvement in symptoms. Ninety percent of those examined had a grade 0 rectocele by the Baden-Walker classification. Dell and O’Kelley (2005) used Pelvisoft, a fenestrated, porcine dermal acellular matrix, and reported no wound complications. Despite these encouraging results, Altman and associates (2005) reported on 29 patients using collagen mesh for symptomatic rectocele. Twenty-three were available at 3-year follow up: 41% had recurrence of stage 2 or greater, and 12 of 23 patients reported incomplete rectal evacuation. They advocated further study before recommending the routine use of graft-augmented tissue repair.
A number of authors are utilizing permanent mesh materials via a posterior approach. By using permanent materials, multicompartmental repairs are more commonly performed extending from the sacrospinous ligament to the perineal body. de Tayrac and associates (2006) reported on 26 patients with 2-year follow up. Objective and subjective cure rates of 92.3% and 88%, respectively, were observed. Dwyer and O’Reilly (2004) used a technique attaching Atrium mesh to the sacrospinous ligament bilaterally to the perineal body. Fifty patients underwent posterior repair, 17 of whom had both anterior and posterior repair. No rectocele recurrence was seen at 13 months. Lim and associates (2005) reported using a composite polyglactin/polypropylene mesh (Vypro II, Ethicon, Somerville, NJ). In a descriptive study, 90 patients underwent interposition graft placement with no primary repair. Mesh was placed loosely. Success was noted in 27 of 31 (84%) at minimum 6-month follow-up. The 5 patients with recurrence of the rectocele did not have prolapse in other compartments. Mercer-Jones and coworkers (2004) reported 22 patients who underwent transperineal placement of either polyglactin/polypropylene or just polypropylene mesh: 77% reported moderate, good, or excellent results after mesh repair.
In the posterior compartment, graft extrusion rates of 1.3% to 12% have been reported (Kobashi et al, 2005; Lim et al, 2005; de Tayrac et al, 2006, 2007). Dyspareunia has also been reported with rates as high as 60% when using mesh posteriorly (Milani et al, 2005).
Vaginal Kits
There has been a rapid expansion of the use of vaginal kits in the treatment of pelvic organ prolapse. With the popularity of the transobturator approach of the midurethral sling it was not long before techniques involving the blind passage of trocars to the level of the sacrospinous ligament via the paravaginal space were developed. Although some have been marketed as single-compartment repairs, in actuality the presence of the mesh at the level of the vaginal apex with fixation turns a single-compartment repair into a multicompartmental repair, thus making comparisons to traditional prolapse repairs challenging. Lo and Wang (1998) reported on their results of abdominal sacrocolpopexy versus sacrospinous ligament fixation at a duration of 2.1 years. Success was noted in 94.2% of patients after abdominal sacrocolpopexy compared with 80% success after the sacrospinous ligament fixation.
Transvaginal Kits
Tension-free vaginal kits have been introduced to provide a graft augmented durable repair. The proposed advantages of kits are “less invasive,” standardization of technique, standardization of mesh, and the ability to repair multiple compartments through a vaginal approach. Currently, there are a number of vaginal prolapse repair kits on the market (Table 72–10), with Prolift (Ethicon, Johnson and Johnson, Somerville, NJ) and Apogee/Perigee (American Medical Systems, Minnetonka, MN) being most commonly utilized. Short-term cure rates of approximately 90% have been reported in several series (de Tayrac et al, 2007; Fatton et al, 2007; Gauruder-Burmester et al, 2007). These transvaginal kit procedures have gained widespread acceptance in light of little controlled, quality data that strongly support their use. Implanting surgeons must realize that these procedures are utilizing a significantly higher volume of mesh than conventional midurethral slings and that the trocars are passed deeply into the pelvic musculature. The potential for organ injury and hemorrhage are greater with these procedures. In addition, functional implications regarding these mesh procedures remain largely unknown. Thus, pelvic surgeons must make an informed decision with the patient, which weighs the apparent advantage in anatomic efficacy, decreased invasiveness, and potential durability against the potential morbidity associated with mesh erosion and the possibility of more significant complications unique to these procedures.
Technique
The anterior prolapse repair follows many of the same basic steps for all of the kit procedures. A midline incision is created in the anterior vaginal wall. Hydrodissection may facilitate a deep, full-thickness vaginal dissection. The full-thickness dissection is important to minimize the occurrence of vaginal exposure of mesh. The dissection progresses laterally to the level of the endopelvic fascia, which is entered by sharp or blunt dissection. The pelvic sidewalls are developed until the ischial spines and the ATFP are palpated. Stab incisions are made overlying the obturator foramen. The distal incisions are placed in the anteromedial edge of the obturator foramen at the level of the clitoris, and the proximal incisions are placed 2 cm below and 1 cm lateral to the distal incision. The trocars are utilized to position the arms of the mesh patch along the ATFP. The distal trocars are placed approximately 2 cm from the distal aspect of the pubic bone, and the proximal trocars are advanced along the ATFP, exiting 1 cm distal to the ischial spine. The proximal trocars in the Perigee system are different than the distal needles, which facilitate a deep passage of the proximal devices to the ischial spine. The mesh arms are utilized to position the mesh appropriately without tension. Sutures are used to fix the mesh both proximally at the level of the vaginal cuff (preferably with delayed absorbable or permanent sutures) and distally proximal to the bladder neck with absorbable sutures. Prior to mesh placement, anterior colporrhaphy may be performed to correct a central defect if present. Cystoscopy is performed to confirm lower urinary tract integrity. Care is taken to confirm the absence of excessive tension on each of the mesh arms (Fig. 72–47). The vagina is then closed without trimming the vaginal wall to minimize the occurrence of vaginal mesh extrusion. A loosely interrupted vertical mattress closure may be used to create a virtual two-layer closure of the vaginal epithelium.
The posterior and apical approach to kit placement does vary between kit devices. The Prolift device utilizes proximal attachment through the sacrospinous ligament, whereas the Apogee device is fixed proximally 1 cm distal to the ischial spine. For all procedures, the dissection begins with a posterior vaginal incision, which is full thickness. The dissection proceeds laterally and proximally to expose the ischial spine (Apogee) and the sacrospinous ligament (Prolift). Two stab incisions are made 3 cm lateral and below the anus. With the surgeon’s hand in the vagina retracting the rectum, the trocars are advanced through the ischiorectal fossa. The Prolift is brought through the sacrospinous ligament 3 cm medial to the ischial spine. The Apogee trocars exit 1 cm distal to the ischial spine. The mesh is trimmed so that it does not extend to within 1 cm of the perineal body. This avoids mesh complications in the perineum. The mesh is fixed laterally with absorbable sutures. The mesh is fixed with permanent or delayed absorbable suture proximally to the vaginal apex or posterior lip of the cervix. As with the anterior compartment, no trimming of the epithelium is recommended and closure is completed with absorbable suture.
There are new kits, which do not utilize trocars for mesh placement. The Pinnacle device (Boston Scientific, Nantucket, MA) uses a Capio Needle Driver System to pass the arms of the mesh through the sacrospinous ligament proximally and the ATFP and obturator internus fascia distally to provide anterior support. The mesh may also be deployed posteriorly using the Capio device as well. The Elevate (American Medical Systems, Minnetonka, MN) has a unique “tacking” device, which anchors the graft in the sacrospinous ligament as well as ATFP. There is a special tool that facilitates adjustment of mesh tension after the tacking mechanism is deployed. Both of these devices have not been in use very long and there are little meaningful data at this time.
Results
When evaluating the results of the vaginal kit procedures in the treatment of prolapse there are a paucity of peer review data to guide most appropriate usage. Much of the literature regarding kits include preliminary data in abstract form. The multicenter preliminary results of Prolift have been published by Fatton and associates (2007): 110 patients were observed for 3 months, and a 94.3% success rate was reported. Gauruder-Burmester and colleagues (2007) reported on a retrospective analysis of 121 women utilizing Apogee/Perigee. Success rates of 93% were achieved. Sanses and colleagues (2009) compared the outcomes of Prolift, uterosacral ligament suspension and abdominal sacrocolpopexy for the treatment of apical prolapse. Prolift, uterosacral ligament suspension, and abdominal sacrocolpopexy were performed for 206, 231, and 305 subjects, respectively. There was no difference in apical success after Prolift (98.8%) compared with uterosacral ligament suspension (99.1%) or abdominal sacrocolpopexy (99.3%) (both P = 1.00) 3 to 6 months after surgery. The average elevation of the vaginal apex was lower after Prolift (−6.9 cm) than uterosacral ligament suspension (−8.05 cm) and abdominal sacrocolpopexy (−8.5 cm) (both P < .001) (Sanses et al, 2009). Feiner and associates (2009) performed a meta-analysis of the peer-reviewed literature and select peer-reviewed gynecologic abstracts to evaluate outcomes and complications after transvaginal synthetic apical suspension procedures. After the Apogee procedure, 525 women had a mean follow-up of 26 weeks (range 10 to 56 weeks). Mean objective success was 95% (range 81 to100). In the same meta-analysis, a posterior or total Prolift procedure was performed in 1295 women with mean follow-up time of 30 weeks (range 12 to 52 weeks), and the mean objective success rate was 87% (range 75% to 94%). Although these early data demonstrate successful outcomes, it is important to note that serious complications, some unique to these procedures, are being reported. These concerns have prompted comments regarding new procedures and materials for the treatment of incontinence and prolapse, warning that there are insufficient data supporting the routine use of these devices (Ostergaard, 2007). In light of this, it is incumbent on physicians to openly discuss these issues with patients.
Complications
Mesh complications are most commonly in the form of mesh exposure or “extrusion.” Extrusion rates of approximately 10% can be expected (Gauruder-Burmester et al, 2007; Feiner et al, 2009). The exposures seem to occur more frequently on the anterior wall, and a concomitant hysterectomy significantly increases the risk (de Tayrac et al, 2007; Gauruder-Burmester et al, 2007). Measures to minimize the occurrence of vaginal mesh exposure are minimizing excessive vaginal wall trimming and closing without tension. Some advocate closing with a vertical mattress technique to separate the graft from the wound (de Tayrac et al, 2006). Symptoms of mesh extrusion include vaginal discharge, persistent bleeding, pain, dyspareunia, partner pain, dysuria, and recurrent urinary tract infections. Examination by palpation as well as visualization is important to detect this complication. While some patients can be managed with either observation or local treatment, many will require excision with primary vaginal closure (de Tayrac et al, 2006). Infected vaginal mesh can lead to sinus formation, abscess, and enterovaginal fistula formation. One case of necrotizing fasciitis with Staphylococcus aureus requiring extensive perineal debridement and colostomy has been reported after a kit procedure (Abdel-Fattah et al, 2008). Persistent groin pain has been reported after transobturator grafts and may signify an erosion or abscess of a transobturator graft (Mahajan et al, 2005; Rafii et al, 2006).
Unfortunately, significant lower urinary tract erosion into the bladder or urethra have also been reported, with significant consequences (Yamada et al, 2006). Altman and Falconer (2007) found the overall risk of serious complications of the Prolift procedure is 4.4%. The majority of serious complications were visceral perforations. Including transvaginal kits systems, the incidences of visceral (including urethra) injuries have been reported from 2.7% to 4.4% (Altman and Falconer, 2007; de Tayrac et al, 2007). The rates of bladder perforation vary between 0.9% and 2.8% (Altman and Falconer, 2007; de Tayrac et al, 2007; Fatton et al, 2007). Bladder perforation after retropubic midurethral slings is not uncommon and appears to be a benign event in the presence of adequate bladder drainage (Kuuva and Nilsson, 2002). The fate of bladder injury at the time of mesh prolapse repairs is much less certain. Two studies have reported on placement of mesh with single, small, uncomplicated bladder injuries (Fatton et al, 2007; Popovic et al, 2007). If the bladder is injured, it is the opinion of the authors that the procedure should be completed without a mesh interposition of the anterior compartment, because placing mesh in the setting of a bladder laceration would prohibitively increase the risk of erosion. Extensive erosions into the bladder requiring partial cystectomy have been reported (Abdel-Fattah et al, 2008). With urethral injury, the defect should be repaired primarily, and, again, mesh/graft placement should not be done.
Rectal perforation is reported in 0.7% to 2.8% of cases (Altman and Falconer, 2007; de Tayrac et al, 2007). If rectal injury is recognized intraoperatively, synthetic mesh should not be placed (Mercer-Jones et al, 2004; de Tayrac et al, 2006). Rectal erosion of synthetic mesh may require both rectal and vaginal excision (Hurtado et al, 2007). From these outcomes it is clear that if the rectum is perforated a multilayer closure should be performed, povidone-iodine distention of the rectum should follow to ensure the integrity of the repair, and the procedure should be abandoned.
The trocars are passed through the pelvic muscular complex both for anterior and posterior kit repairs. The trocars pass near the ischial spine, and significant intraoperative bleeding may occur. This would usually emanate from the pudendal neurovascular bundle, and embolization of the source of bleeding has been reported (Mokrzycki and Hampton, 2007). Also multiple authors have reported pelvic hematomas after transvaginal kit procedures (Ignjatovic and Stosic, 2007; LaSala and Schimpf, 2007; Abdel-Fattah et al, 2008). Abdel-Fattah and colleagues described vascular complications after Prolift and Apogee/Perigee procedures, including arterial injury requiring embolization. Perhaps blind passage of the trocars through the levator musculature poses a risk for these hemorrhagic complications. Hematomas have also been reported after these procedures (Ignjatovic and Stosic, 2007). Commonly, patients will present with unusually more pelvic pain than typically encountered. Computed tomography is an excellent method to detect hematomas. Thus, if excessive postoperative pain occurs after the procedure, one should consider an evaluation with computed tomography to detect a hematoma. If a hematoma is present, observation with serial hematocrit levels is the appropriate initial evaluation; however, surgical drainage may be required for expanding hematomas, decreasing hematocrits, refractory pain, or infection.
Local complications such as pelvic pain, defecatory pain, and dyspareunia have been reported after kit procedures (Altman and Falconer, 2007; de Tayrac et al, 2007). When examining these patients, one must carefully look for areas of impaired healing, banding or tenting of the mesh, “trigger points” that elicit pain, and signs of focal inflammation. If these abnormal areas are identified, release of the arms or site of mesh tension may alleviate these areas of discomfort. When performing the mesh release, one should excise as much offending mesh material as possible before vaginal closure. In cases of infection, granulomas, or persistent draining sinuses, all mesh involved in the infected areas must be removed. If no local incriminating factors are found, a period of conservative therapy consisting of physical therapy, trigger point injections, and other adjunctive techniques should be attempted first.
U.S. Food and Drug Administration Public Health Notification
A public health notification is an important message from the FDA for devices and radiologic health to the health care community (Schultz, 2008). This message describes a risk associated with the use of a medical device and provides recommendations to avoid or reduce the risk. In October 2008 the FDA released a public health statement that “Serious Complications are Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse and Stress Urinary Incontinence.” This report was designed to alert health care practitioners of complications associated with transvaginal placement of surgical mesh to treat POP and SUI. This report cited that over 3 years the FDA received more than 1000 reports from nine surgical mesh manufacturers of complications that were associated with surgical mesh devices used to repair POP and SUI. These potential complications are outlined previously and in some cases lead to a significant decrease in quality of life due to discomfort and pain, including dyspareunia. As a result, a number of recommendations were made to physicians that include obtaining specialized training for each mesh placement technique and to inform patients that implantation of surgical mesh is permanent. As a result, some complications associated with the implanted mesh may require additional surgery that may or may not correct the complication. In addition it is recommended to inform patients about the potential for serious complications and their adverse effect on quality of life. Also, it is recommended that the physicians provide patients with a written copy of the patient labeling from the surgical mesh manufacturer, if available.
The impact of these recommendations or the evidence that these recommendations will improve outcomes is unknown. However, it is important for pelvic surgeons to be aware of the growing concerns regarding the potential for serious complications associated with the use of these materials. Patients must be informed of the indications for and benefits and risks of mesh placement before any surgical intervention, and surgeons must remain vigilant in assessing their own outcomes. It is only through well-designed and sufficiently powered randomized controlled trials that these questions will be answered.
Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1422.
Blanchard K, Vanlangendonck R, Winters JC. Recurrent pelvic floor defects after abdominal sacral colpopexy. J Urol. 2006;175(3 Pt. 1):1010-1013. discussion 1013
Culligan PJ, Blackwell L, Goldsmith LJ, et al. A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol. 2005;106(1):29-37.
DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
DeLancey JO, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol. 1997;176:1228-1235.
Feiner B, Jelovsek JE, Maher C. Efficacy and safety of transvaginal mesh kits in the treatment of prolapse of the vaginal apex: a systematic review. Br J Obstet Gynaecol. 2009;116(1):15-24.
Kenton K, Pham T, Mueller E, Brubaker L. Patient preparedness: an important predictor of surgical outcome. Am J Obstet Gynecol. 2007;197:654.e1-654.e6.
Maher CF, Murray CJ, Carey MP, et al. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001;98:40-44.
Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805-823.
Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;4:214-221.
Richardson AC, DeLancey J. Anatomy of genital support. In: Hurt WG, editor. Urogynecologic surgery. Philadelphia: Lippincott Williams & Wilkins; 2000:22.
Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
Togami J, Krlin R, et al. Graft materials in prolapse surgery. AUA Update Series. 2008;27(31):294-303.
Winters JC, Cespedes R, Vanlangendonck R. Abdominal sacrocolpopexy and abdominal enterocele repair in the treatment of vaginal vault prolapse. Urology. 2000;56:55-63.
Abdel-Fattah M, et al. Retrospective multicentre study of the new minimally invasive mesh repair devices for pelvic organ prolapse. Br J Obstet Gynaecol. 2008;115:22-30.
Abramov Y, et al. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005;105:314-318.
2009 ACOG practice bulletin No. 104: Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113(5):1180-1189.
Addison WA, Timmons MC. Abdominal approach to vaginal eversion. Clin Obstet Gynecol. 1993;36(4):995-1004.
Albo M, Dupont MC, et al. Transvaginal correction of pelvic prolapse. J Endourol. 1996;10(3):231-239.
Alevizon SJ, Finan MA. Sacrospinous colpopexy: management of postoperative pudendal nerve entrapment. Obstet Gynecol. 1996;88:713-715.
Altman D, et al. Functional and anatomic outcome after transvaginal rectocele repair using collagen mesh: a prospective study. Dis Colon Rectum. 2005;48:1233-1242.
Altman D, Falconer C. Perioperative morbidity using transvaginal mesh in pelvic organ prolapse repair. Obstet Gynecol. 2007;109(2 Pt. 1):303-308.
Amid P. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia. 1997;1:15-21.
Amundsen CL, Flynn BJ, Webster GD. Anatomical correction of vaginal vault prolapse by uterosacral ligament fixation in women who also require a pubovaginal sling. J Urol. 2003;169:1770-1774.
Ashton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Ann NY Acad Sci. 2007;1101:266-296.
Baden WF, Walker T. Urinary stress incontinence: evolution of paravaginal repair. Female Patient. 1987;12:89-105.
Badylak S, et al. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103:190-202.
Ballert KN, et al. Managing the urethra at transvaginal pelvic organ prolapse repair: a urodynamic approach. J Urol. 2009;181:679-684.
Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185:1388-1395.
Barber MD, Visco AG, Weidner AC, et al. Bilateral uterosacral ligament vaginal vault suspension with site specific endopelvic fascia defect repair. Am J Obstet Gynecol. 2000;183:1402-1411.
Barksdale PA, et al. Intraligamentous nerves as a potential source of pain after sacrospinous ligament fixation of the vaginal apex. Int Urogynecol J. 1997;8:121-125.
Beets GL, Go PM, van Mameren H. Foreign body reactions to monofilament and braided polypropylene mesh used as preperitoneal implants in pigs. Eur J Surg. 1996;162:823-825.
Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1422.
Birch C. The use of prosthetics in pelvic reconstructive surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:979-991.
Blanchard K, Vanlangendonck R, Winters JC. Recurrent pelvic floor defects after abdominal sacral colpopexy. J Urol. 2006;175(3 Pt. 1):1010-1013. discussion 1013
Borstad E, Rud T. The risk of developing urinary stress incontinence after vaginal repair in continent women: a clinical and urodynamic followup study. Acta Obstet Gynecol Scand. 1989;68:545-549.
Bradley C, Brown M, Cundiff G, et al. Bowel symptoms in women planning surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;195:1814-1819.
Bratzler D, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38:1706-1715.
Brizzolara S, Pillai-Allen A. Risk of mesh erosion with sacral colpopexy and concurrent hysterectomy. Obstet Gynecol. 2003;102(2):306-310.
Brown W, Hoffman M, et al. Management of vaginal vault prolapse: retrospective comparison of abdominal versus vaginal approach. J Fla Med Assoc. 1989;76:249-252.
Brubaker L, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354:1557-1566.
Brubaker L, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
Bruce RG, et al. Paravaginal defect repair in the treatment of female stress urinary incontinence and cystocele. Urology. 1999;54:647-651.
Buck B, et al. Bone transplantation and human immunodeficiency virus: an estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop Relat Res. 1989;240:129-136.
Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
Bump RC, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
Centers for Medicare and Medicaid Services (CMS). Surgical care improvement project. Specifications manual, version 2.3b, section 2.4. Baltimore: CMS; 2008.
Cervigni M. Hormonal influences in the lower urinary tract. In: Raz S, editor. Female urology. Philadelphia: WB Saunders; 1996:539.
Cespedes RD. Anterior approach bilateral sacrospinous ligament fixation for vaginal vault prolapse. Urology. 2000;56(Suppl. 6A):70-75.
Cespedes RD, et al. Colpocleisis for the treatment of vaginal vault prolapse. Tech Urol. 2001;7:152-160.
Chaikin DC, Groutz A, Blaivas J. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
Chen B, et al. Elastolytic activity in women with stress urinary incontinence and pelvic organ prolapse. Neurourol Urodynam. 2004;23:119-126.
Chen C, et al. Biologic grafts and synthetic meshes in pelvic reconstructive surgery. Clin Obstet Gynecol. 2007;50:383-411.
Chesson RR, et al. The use of fascia lata graft for correction of severe or recurrent anterior vaginal wall defects. J Pelvic Surg. 1999;5:96-103.
Choe JM, Bell T. Genetic material is present in cadaveric dermis and cadaveric fascia lata. J Urol. 2001;166:122-124.
Chou Q, et al. Clinical presentation of enterocele. Obstet Gynecol. 2000;96:599-603.
Chung SY, et al. Technique of combined pubovaginal sling and cystocele repair using a single piece of cadaveric dermal graft. Urology. 2002;59:538-541.
Clemons JL, et al. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618.
Cole E, Gomelsky A, Dmochowski RR. Encapsulation of a porcine dermis pubovaginal sling. J Urol. 2003;170:1950.
Cole E, Leu PB, Gomelsky A, et al. Histopathological evaluation of the uterosacral ligament: Is this a dependable structure for pelvic reconstruction. BJU Int. 2006;97:345-348.
Colombo M, et al. Randomised comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. Br J Obstet Gynaecol. 2000;107:544-551.
Cook Medical. Surgisis Biodesign advanced tissue repair products. http://www.cookmedical.com/feature.do?id=Biodesign, 2007. [accessed 13.10.09]
Cross CA, Cespedes RD, McGuire EJ. Treatment results using pubovaginal slings in patients with large cystoceles and stress incontinence. J Urol. 1997;158:431-434.
Cruikshank SH, Muniz M. Outcomes study: a comparison of cure rates in 695 patients undergoing sacrospinous ligament fixation alone and with other site-specific procedures—a 16-year study. Am J Obstet Gynecol. 2003;188:1509-1515.
Culligan PJ, Blackwell L, Goldsmith LJ, et al. A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol. 2005;106(1):29-37.
Cundiff GW, Fenner D. Evaluation and treatment of women with rectocele: focus on associated defecatory and sexual dysfunction. Obstet Gynecol. 2004;104:1403-1421.
Cundiff GW, Harris RL, et al. Abdominal sacral colpoperineopexy: a new approach for correction of posterior compartment defects and perineal descent associated with vaginal vault prolapse. Am J Obstet Gynecol. 1997;177(6):1345-1353. discussion 1353–5
Cundiff GW, Weidner AC, Visco AG, et al. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1451-1457.
Daneshgari F, Paraiso MF, et al. Robotic and laparoscopic female pelvic floor reconstruction. BJU Int. 2006;98(Suppl. 1):62-68. discussion 69
Davila G, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int J Gynecol Obstet. 2005;17:S51-S55.
de Tayrac R, Devoldere G, et al. Prolapse repair by vaginal route using a new protected low-weight polypropylene mesh: 1-year functional and anatomical outcome in a prospective multicentre study. Int J Gynecol Obstet. 2007;18:251-256.
de Tayrac R, Gervaise A, Chauveaud A, et al. Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med. 2005;50:75-80.
de Tayrac R, Gervaise A, Fernandez H. Cystocele repair by the vaginal route with a tension-free sub-bladder prosthesis. J Gynecol Obstet Biol Reprod. 2002;31:597-599.
de Tayrac R, Picone O, Chauveaud-Lambling A, Fernandez H. A 2-year anatomical and functional assessment of transvaginal rectocele repair using a polypropylene mesh. Int J Gynecol Obstet. 2006;17:100-105.
Debodinance P, Cossen M, Burlet G. Tolerance of synthetic tissues in touch with vaginal scars: review to the point of 287 cases. Eur J Obstet Gynecol Reprod Biol. 1999;87:23-30.
DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
DeLancey JO. Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am J Obstet Gynecol. 1999;180:815-823.
DeLancey JO, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol. 1997;176:1228-1235.
Dell JR, O’Kelley KR. PelviSoft BioMesh augmentation of rectocele repair: the initial clinical experience in 35 patients. Int Urogynecol J. 2005;16:44-47.
Deprest J, et al. The biology behind fascial defects and the use of implants in pelvic organ prolapse repair. Int Urogynecol J. 2006;17:S16-S25.
Deval B. Hysterocolpectomy with colpocleisis for massive genital prolapse in women aged over 70 years. Eur J Obstet Gynecol Reprod Biol. 2005;123:249-253.
Dietz HP, et al. Mechanical properties of urogynecologic implant materials. Int Urogynecol J. 2003;14:239-243.
Dmochowski RR, Zimmern PE, et al. Role of the four-corner bladder neck suspension to correct stress incontinence with a mild to moderate cystocele. Urology. 1997;49(1):35-40.
Dora CD, et al. Time dependent variations in biomechanical properties of cadaveric fascia, porcine dermis, porcine small intestine submucosa, polypropylene mesh and autologous fascia in the rabbit model: implications for sling surgery. J Urol. 2004;171:1970-1973.
Dwyer P. Evolution of biological and synthetic grafts in reconstructive pelvic surgery. Int Urogynecol J. 2006;17:S10-S15.
Dwyer P, Fatton B. Bilateral extraperitoneal uterosacral suspension: a new approach to correct posthysterectomy vaginal vault prolapse. Int Urogynecol J. 2008;19:283-292.
Dwyer P, O’Reilly B. Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. Br J Obstet Gynaecol. 2004;111:831-836.
Eilber KS, et al. Vaginal hysterectomy. In: Graham SJ, editor. Glenn’s urologic surgery. Philadelphia: Lippincott Williams & Wilkins; 2004:363-368.
Eilber KS, et al. Surgical therapy for uterine prolapse. In: Vasavada AR, Sand PK, Raz S, editors. Female urology, urogynecology and voiding dysfunction. Boca Raton (FL): Taylor & Francis, 2005.
Elkadry EA, et al. Patient-selected goals: a new perspective on surgical outcome. Am J Obstet Gynecol. 2003;189:1551-1558.
Elkins TE, et al. Transvaginal paravaginal repair: a useful adjunctive procedure in pelvic relaxation surgery. J Pelvic Surg. 2000;6:11-15.
Ellerkmann R, Cundiff G, Melick C, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
Elliott DS, Krambeck AE, et al. Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. J Urol. 2006;176(2):655-659.
Falconer C, et al. Different organization of collagen fibrils in stress-incontinent women of fertile age. Acta Obstet Gynecol Scand. 1998;77:87-94.
Fatton B, Amblard J, Debodinance P, et al. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
Fatton B, Dwyer PL, Achtari C, Tan PK. Bilateral extraperitoneal uterosacral vaginal vault suspension: a 2-year follow-up longitudinal case series of 123 patients. Int Urogynecol J. 2009;20:427-434.
Feiner B, Jelovsek JE, Maher C. Efficacy and safety of transvaginal mesh kits in the treatment of prolapse of the vaginal apex: a systematic review. Br J Obstet Gynaecol. 2009;116(1):15-24.
Fialkow M, Gardella C, Melville J, et al. Posterior vaginal wall defects and their relation to measures of pelvic floor neuromuscular function and posterior compartment symptoms. Am J Obstet Gynecol. 2002;187:1443-1448.
Fine PM, DeLancey JO. Vaginal hysterectomy and other operations for uterine prolapse. In: Vasavada AR, Sand PK, Raz S, editors. Female urology, urogynecology and voiding dysfunction. Boca Raton (FL): Taylor & Francis; 2005:545-559.
FitzGerald MP, et al. The antigenicity of fascia lata allografts. BJU Int. 2000;86:826-828.
FitzGerald MP, et al. Colpocleisis: a review. Int Urogynecol J. 2006;17:261-271.
FitzGerald MP, et al. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J. 2008;19:1603-1609.
Flynn BJ, Webster GD. Surgical management of the apical vaginal defect. Curr Opin Urol. 2002;12:353-358.
Fokaefs ED, et al. Experimental evaluation of free versus pedicled fascial flaps for sling surgery of urinary stress incontinence. J Urol. 1997;157:1039-1043.
Francis WJA, Jeffcoate TN. Dyspareunia following vaginal operations. J Obstet Gynaecol Br Comm. 1961;68:1-10.
Frederick RW, Leach GE. Cadaveric prolapse with sling: intermediate outcomes with 6 month to 5 years of followup. J Urol. 2005;173:1229-1233.
Gallentine ML, Cespades RD. Occult stress urinary incontinence and the effect of vaginal vault prolapse on abdominal leak point pressures. Urology. 2001;57:40-44.
Gandhi S, et al. A prospective randomized trial using solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005;192:1649-1654.
Gauruder-Burmester A, Koutouzidou P, et al. Follow-up after polypropylene mesh repair of anterior and posterior compartments in patients with recurrent prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(9):1059-1064.
Geerts WH, Pineo GF, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl.):338S-400S.
Geomini PM, Brolmann HA, et al. Vaginal vault suspension by abdominal sacral colpopexy for prolapse: a follow up study of 40 patients. Eur J Obstet Gynecol Reprod Biol. 2001;94(2):234-238.
Ghoniem GM, et al. The value of the vagina pack test in large cystoceles. J Urol. 1994;152:931-934.
Given FTJr, Muhlendorf IK, et al. Vaginal length and sexual function after colpopexy for complete uterovaginal eversion. Am J Obstet Gynecol. 1993;169(2 Pt. 1):284-287. discussion 287–8
Glavind K, Kempf L. Colpectomy or Le Fort colpocleisis—a good option in selected elderly patients. Int J Gynecol Obstet. 2005;16:48-51.
Glavind K, Madsen H. A prospective study of the discrete fascial defect rectocele repair. Acta Obstet Gynecol Scand. 2000;79:145-147.
Goldberg RP, et al. Protective effect of suburethral slings on postoperative cystocele recurrence after reconstructive pelvic operation. Am J Obstet Gynecol. 2001;185:1307-1312.
Goldman J, Ovadia J, Feldberg D. The Neugebauer-Le Fort operation: a review of 118 partial colpocleisis. Eur J Obstet Gynecol Reprod Biol. 1981;12:31-35.
Gomelsky A, Rudy DC, Dmchowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol. 2004;171:1581-1584.
Groutz A, Chaikin DC, Theusen E, Blaivas JG. Use of cadaveric solvent-dehydrated fascia lata for cystocele repair—preliminary results. Urology. 2001;58:179-183.
Gustilo-Ashby AM, et al. Bowel symptoms 1 year after surgery for prolapse: further analysis of a randomized trial of rectocele repair. Am J Obstet Gynecol. 2007;197:76.e1-76.e5.
Gutt CN, Oniu T, et al. Prophylaxis and treatment of deep vein thrombosis in general surgery. Am J Surg. 2005;189(1):14-22.
Haessler AL, et al. Reevaluating occult incontinence. Curr Opin Obstet Gynecol. 2005;17:535-540.
Handa VL, et al. Sexual function among women with urinary incontinence and pelvic organ prolapse. Am J Obstet Gynecol. 2004;191:751-756.
Handel LN, et al. Results of cystocele repair: a comparison of traditional anterior colporrhaphy, polypropylene mesh, and porcine dermis. J Urol. 2007;178:153-156.
Harmanli O, Dandolu V, Chatwani A, Grody M. Total colpocleisis for severe pelvic organ prolapse. J Reprod Med. 2003;48:703-706.
He Y, et al. Type I collagen inhibits hydroxyl radical-induced apoptosis. J Biochem. 2002;132:373-379.
Hiltunen R, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110:455-462.
Hoffman MS, et al. Vaginectomy with pelvic herniorrhaphy for prolapse. Am J Obstet Gynecol. 2003;189:364-471.
Holley RL, Varner RE, et al. Recurrent pelvic support defects after sacrospinous ligament fixation for vaginal vault prolapse. J Am Coll Surg. 1995;180(4):444-448.
Hurt WG, editor. Urogynecologic surgery. Philadelphia: Lippincott Williams & Wilkins, 2000.
Hurtado E, Bailey H, et al. Rectal erosion of synthetic mesh used in posterior colporrhaphy requiring surgical removal. Int J Gynecol Obstet. 2007;18:1499-1501.
Ignjatovic I, Stosic D. Retrovesical haematoma after anterior Prolift procedure for cystocele correction. Int Urogynecol J. 2007;18:1495-1497.
Imparato E, et al. Surgical management and prevention of vaginal vault prolapse. Surg Gynecol Obstet. 1992;175:233-237.
Jackson SR, et al. Changes in metabolism of collagen in genitourinary prolapse. Lancet. 1996;347:1658-1661.
Jarvis G, Fowlie A. Clinical and urodynamic assessment of the porcine dermis bladder sling in the treatment of genuine stress incontinence. Br J Obstet Gynaecol. 1985;92:1189-1191.
Jelovsek J, Barber M, Paraiso M, Walters MD. Functional bowel and anorectal disorders in patients with pelvic organ prolapse and incontinence. Am J Obstet Gynecol. 2005;193:2105-2111.
Jenkins V. Uterosacral ligament fixation for vaginal vault suspension in uterine and vaginal vault prolapse. Am J Obstet Gynecol. 1997;177:1337-1344.
Jorge-Herrero E, et al. Calcification and identification of metalloproteinases in bovine pericardium after subcutaneous implantation in rats. J Mater Sci Mater Med. 2001;12:1013-1017.
Julian T. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
Karlovsky ME, Thakre AA, et al. Biomaterials for pelvic floor reconstruction. Urology. 2005;66(3):469-475.
Karram MM, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1343.
Kaupp LA, et al. Graft infection or graft rejection? Arch Surg. 1979;114:1419-1422.
Kelly HA. Incontinence of urine in women. Urol Cutaneous Rev. 1913;17:291-293.
Kenton K, Pham T, Mueller E, Brubaker L. Patient preparedness: an important predictor of surgical outcome. Am J Obstet Gynecol. 2007;197:654.e1-654.e6.
Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999;181:1360-1363.
Kettel LM, Herbertson RM. An anatomic evaluation of the sacrospinous ligament colpopexy. Surg Gynecol Obstet. 1989;168:318-322.
Khan MA, Stanton S. Posterior colporrhaphy: its effects on bowel and sexual function. Br J Obstet Gynaecol. 1997;104:882-886.
Klinge U, et al. Functional assessment and tissue response of short- and long-term absorbable surgical meshes. Biomaterials. 2001;22:1415-1424.
Klinge U, et al. Impact of polymer pore size on the interface scar formation in a rat model. J Surg Res. 2002;103:208-214.
Kobashi KC, Leach G, Frederick R, et al. Initial experience with rectocele repair using nonfrozen cadaveric fascia lata interposition. Urology. 2005;66:1203-1208.
Kobashi KC, Mee SL, Leach GE. A new technique for cystocele repair and transvaginal sling: the cadaveric prolapsed repair and sling (CAPS). Urology. 2000;56:9-14.
Kohli N, Miklos JR. Dermal graft-augmented rectocele repair. Int Urogynecol J. 2003;14:146-149.
Konstantinovic MJ, et al. Comparison of host response to polypropylene and non–cross-linked porcine small intestine serosal-derived collagen implants in a rat model. Br J Obstet Gynaecol. 2005;112:1554-1560.
Kreder KJ. Female urethral diverticulum and urethral fistulae. In: Kirby R, Webster GD, Goldwasser B, editors. Reconstructive urology. Oxford: Blackwell, 1993.
Kuuva N, Nilsson C. A nationwide analysis of complications associated with the tension-free vaginal tape procedure. Acta Obstet Gynecol Scand. 2002;81:72-77.
Langmade CF, Oliver JAJr. Partial colpocleisis. Am J Obstet Gynecol. 1986;154:1200-1205.
Lantzsch T, et al. Sacrospinous ligament fixation for vaginal vault prolapse. Arch Gynecol Obstet. 2001;265:21-25.
LaSala C, Schimpf M. Occurrence of postoperative hematomas after prolapse repair using a mesh augmentation system. Obstet Gynecol. 2007;109:569-572.
Leach GE, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. The American Urological Association. J Urol. 1997;158:875-880.
Lemer M, Chaikin DC, Blaivas J. Tissue strength analysis of autologous and cadaveric allografts for pubovaginal sling. Neurourol Urodynam. 1999;18:497-503.
Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
Lim Y, Rane A, Muller R. An ambispective observational study in the safety and efficacy of posterior colporrhaphy with composite Vicryl-Prolene mesh. Int J Gynecol Obstet. 2005;16:126-131.
Lo TS, Wang AC. Abdominal colposacropexy and sacrospinous ligament suspension for severe uterovaginal prolapse: a comparison. J Gynecol Surg. 1998;14:59-64.
Lowenstein L, Fitz A, Kenton K, et al. Transabdominal uterosacral suspension: outcomes and complications. Am J Obstet Gynecol. 2009;200(6):656.e1-656.e5.
Mahajan S, Kenton K, et al. Transobturator tape erosion associated with leg pain. Int Urogynecol J. 2005;17:66-68.
Maher CF, Baessler K. Surgical management of anterior vaginal wall prolapse: an evidence based literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:195-201.
Maher CF, Murray CJ, Carey MP, et al. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001;98:40-44.
Maher CF, Qatawneh AM, Baessler K, et al. Midline rectovaginal fascial plication for repair of rectocele and obstructed defecation. Obstet Gynecol. 2004;104:685-689.
Mallipeddi PK, Kohli SA, et al. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior vaginal wall prolapse. Int Urogynecol J. 2001;12:83-88.
Marinkovic S. Will hysterectomy at the time of sacrocolpopexy increase the rate of polypropylene mesh erosion? Int J Gynecol Obstet. 2008;19:199-203.
McCall ML. Posterior culdoplasty. Obstet Gynecol. 1957;10:595-602.
Meeks GR, et al. Repair of vaginal vault prolapse by suspension of the vagina to the iliococcygeus (prespinous) fascia. Am J Obstet Gynecol. 1994;171:1444-1454.
Mellgren A, Anzen B, Nilsson B, et al. Results of rectocele repair. Dis Colon Rectum. 1995;38:7-13.
Menefee SA, et al. Results of abdominal sacral colpopexy using polyester mesh in the treatment of posthysterectomy vaginal vault prolapse and enterocele. Obstet Gynecol. 1999;l54:563-565.
Mercer-Jones M, Sprowson A, Varma JS. Outcome after transperineal mesh repair of rectocele: a case series. Dis Colon Rectum. 2004;47:864-868.
Meschia M, Bernasconi F, Amicarelli F, et al. The sacrospinous vaginal vault suspension: critical analysis of outcomes. Int Urogynecol J. 1999;10:155-159.
Meschia M, Pifarotti P, Bernasconi F, et al. Porcine skin collagen implants to prevent anterior vaginal wall prolapsed recurrence: a multicenter, randomized study. J Urol. 2007;177:192-195.
Milani R, Salvatore S, et al. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with Prolene mesh. Br J Obstet Gynecol. 2005;112:107-111.
Miyazaki F, Miyazaki D. Raz four-corner suspension for severe cystocele: poor results. Int Urogynecol J. 1994;5:94-97.
Miyazaki F. Miya Hook ligature carrier for sacrospinous ligament suspension. Obstet Gynecol. 1987;70:286-288.
Mokrzycki ML, Hampton BS. Pelvic arterial embolization in the setting of acute hemorrhage as a result of the anterior Prolift procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):813-815.
Morley GW, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-881.
Mundy AR. True pelvis, pelvic floor and perineum. In: Williams A, editor. Gray’s anatomy. Philadelphia: Elsevier, 2005.
Murthy VK, et al. Excellent outcome using selective criteria for rectocele repair. Dis Colon Rectum. 1996;39:374-378.
Newell R. Pelvic girdle, gluteal region and hip joint. In: Williams A, editor. Gray’s anatomy. Philadelphia: Elsevier, 2005.
Nguyen JK, Bhattia NN. Resolution of motor urge incontinence after surgical repair of pelvic organ prolapse. J Urol. 2001;166:2263-2266.
Nichols DH. Sacro-spinous fixation for massive eversion of the vagina. Am J Obstet Gynecol. 1982;142:901-904.
Nichols DH. Transvaginal sacrospinous colpopexy. J Pelvic Surg. 1996;2:87-91.
Nicita G. A new operation for genitourinary prolapse. J Urol. 1998;160:741-745.
Nieminen K, et al. Transanal or vaginal approach to rectocele repair: a prospective, randomized pilot study. Dis Colon Rectum. 2004;47:1636-1642.
Novara G, Artibani W. Surgery for pelvic organ prolapse: current status and future perspectives. Curr Opin Urol. 2005;15:256-262.
Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805-823.
Oliver RF. Scars and collagen implantation. Burns Incl Therm Inj. 1987;13(Suppl.):S49-S55.
Ostergaard D. Lessons from the past: directions for the future. Int J Gynecol Obstet. 2007;18:591-598.
Paraiso MF, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1431.
Paraiso MF, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
Pasley WW. Sacrospinous suspension: a local practitioner’s experience. Am J Obstet Gynecol. 1995;173:440-448.
Pohl JF, Frattarelli JL. Bilateral transvaginal sacrospinous colpopexy: preliminary experience. Am J Obstet Gynecol. 1997;177:1356-1362.
Poncet S, et al. The expression and function of the endothelin system in contractile properties in vaginal myofibroblasts of women with uterovaginal prolapse. Am J Obstet Gynecol. 2005;192:426-432.
Popovic I, Debodinance P, et al. Prosthetic reinforcements: how to manage bladder injuries? Int J Gynecol Obstet. 2007;18:1215-1217.
Porges RF, Smilen SS. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1526.
Porter WE, et al. The anatomic and functional outcomes of defect-specific rectocele repairs. Am J Obstet Gynecol. 1999;181:1353-1359.
Quiroz L, Guman R, et al. Partial colpocleisis for the treatment of sacrocolpopexy mesh erosions. Int J Gynecol Obstet. 2008;19:261-266.
Rafii A, Jacob D, et al. Obturator abscess after transobturator tape for stress urinary incontinence. Obstet Gynecol. 2006;108:720-723.
Raz S, Klutke CG, et al. Four-corner bladder and urethral suspension for moderate cystocele. J Urol. 1989;142(3):712-715.
Raz S, Little NA, Juma S, Sussman EM. Repair of severe anterior vaginal wall prolapse (grade IV cystourethrocele). J Urol. 1991;146(4):988-992.
Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36:976-983.
Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;4:214-221.
Richardson AC, DeLancey J. Anatomy of genital support. In: Hurt WG, editor. Urogynecologic surgery. Philadelphia: Lippincott Williams & Wilkins; 2000:22.
Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to fascial defect. Obstet Gynecol. 1981;57:357-362.
Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-571.
Richter K. Massive eversion of the vagina: pathogenesis, diagnosis, and therapy of the “true” prolapse of the vaginal stump. Clin Obstet Gynecol. 1982;25:897-912.
Richter K, Albrich W. Long-term results following fixation of the vagina on the sacrospinal ligament by the vaginal route (vaginaefixatio sacrospinalis vaginalis). Am J Obstet Gynecol. 1981;141:811-816.
Robinson D, Cardozo LD. The role of estrogens in female lower urinary tract dysfunction. Urology. 2003;62(4 Suppl. 1):45-51.
Rodriguez LR, Bukkapatnam R, Shah SM, et al. Transvaginal paravaginal repair of high grade cystocele central and lateral defects with concomitant suburethral sling: report of early results, outcomes and patient satisfaction with a new technique. Urology. 2005;66(Suppl. 5A):57-65.
Rogers RJ. Anatomy of pelvic support. In: Cundiff GW, Bent AE, Ostergaard DR, Swift SE, editors. Ostergaard’s urogynecology and pelvic floor dysfunction. Philadelphia: Lippincott Williams & Wilkins, 2003.
Rosenblum N, et al. Anatomy of pelvic support. In: Vasavada AR, Sand PK, Raz S, editors. Female urology, urogynecology and voiding dysfunction. Boca Raton (FL): Taylor & Francis, 2005.
Rovner ES, Ginsberg DA. Posterior vaginal wall prolapse: transvaginal repair of pelvic floor relaxation, rectocele and perineal laxity. Tech Urol. 2001;7:161-168.
Rozet F, Mandron E, et al. Laparoscopic sacral colpopexy approach for genito-urinary prolapse: experience with 363 cases. Eur Urol. 2005;47(2):230-236.
Safir MH, et al. 4-Defect repair of grade 4 cystocele. J Urol. 1999;161(2):587-594.
Sand P, Koduri S, Lobel RW, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001;184:1357-1362.
Sanses TV, Shahryarinejad A, Molden S, et al. Anatomic outcomes of vaginal mesh procedure (Prolift) compared with uterosacral ligament suspension and abdominal sacrocolpopexy for pelvic organ prolapse: a Fellows’ Pelvic Research Network study. Am J Obstet Gynecol. 2009 Nov;201(5):519.e1-519.e8.
Sauer HA, Klutke CG. Transvaginal sacrospinous ligament fixation for the treatment of vaginal prolapse. J Urol. 1995;154:1008-1012.
Schlesinger RE. Vaginal sacrospinous ligament fixation with the Autosuture Endostitch device. Am J Obstet Gynecol. 1997;176:1358-1362.
Schultz DG. Serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. http://www.fda.gov/cdrh/safety/102008-surgicalmesh.html, 2008. [accessed 15.10.09]
Scotti R, Garely A, Gretson W, et al. Paravaginal repair of lateral vaginal wall defects by fixation to the ischial periosteum and obturator membrane. Am J Obstet Gynecol. 1998;179:1436-1445.
Segal JL, Karram MM. Evaluation and management of rectoceles. Curr Opin Urol. 2002;12:345-352.
Shayya RF, Weinstein MM, Lukacz ES. Pyometra after Le Fort colpocleisis resolved with interventional radiology drainage. Obstet Gynecol. 2009;113:566-568.
Shojania K, Duncan B, et al. Making health care safer: a critical analysis of patient safety practices. Rockville (MD): Agency for Healthcare Research and Quality; 2001.
Shull BL. Pelvic organ prolapse: anterior, superior, and posterior vaginal segment defects. Am J Obstet Gynecol. 1999;181:6-11.
Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
Shull BL, Baden WF. A six-year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160:1432-1439.
Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcomes. Am J Obstet Gynecol. 1994;171:1429-1439.
Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
Shull BL, Capen CV, Riggs MW, et al. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 1993;168:1669-1677.
Shumaker S, et al. Health-related quality of life measures for women with urinary incontinence: the incontinence impact questionnaire and the urogenital distress inventory. Quality Life Res. 1994;3:291.
Siddique SA, et al. Relationship of the uterosacral ligament to the sacral plexus and to the pudendal nerve. Int Urogynecol J. 2006;17:642-645.
Silva WA, Karram MM. Scientific basis for the use of grafts during vaginal reconstructive procedures. Curr Opin Obstet Gynecol. 2005;17:519-529.
Silva WA, et al. Uterosacral ligament vault suspension. Obstet Gynecol. 2006;108:255-263.
Singh K, et al. Evaluation of the fascial technique for surgical repair of isolated posterior vaginal wall prolapse. Obstet Gynecol. 2003;101:320-324.
Snyder TE, Krantz KE. Abdominal-retroperitoneal sacral colpopexy for the correction of vaginal prolapse. Obstet Gynecol. 1991;77:944-949.
Spiess PE, et al. The tensile properties of tension-free vaginal tape and cadaveric fascia lata in an in vivo rat model. BJU Int. 2004;93:171-173.
Stanton SL, et al. Clinical and urodynamic effects of anterior colporrhaphy and vaginal hysterectomy for prolapsed with and without incontinence. Br J Obstet Gynaecol. 1982;89:459-463.
Stein TA, DeLancey JO. Structure of the perineal membrane in females. Obstet Gynecol. 2008;111:686-693.
Sundaram CP, Venkatesh R, et al. Laparoscopic sacrocolpopexy for the correction of vaginal vault prolapse. J Endourol. 2004;18(7):620-623. discussion 623–4
Sze EH, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89:466-475.
Tang L, Eaton JW. Inflammatory responses to biomaterials. Am J Clin Pathol. 1995;103:466-471.
Taylor GB, et al. Posterior repair with perforated porcine dermal graft. Int Braz J Urol. 2008;34:84-90.
Timmons MC, Kohler MF, et al. Thumbtack use for control of presacral bleeding, with description of an instrument for thumbtack application. Obstet Gynecol. 1991;78(2):313-315.
Togami J, Krlin R, et al. Graft materials in prolapse surgery. AUA Update Series. 2008;27(31):294-303.
Twiss C, Triaca V, et al. Re: Reevaluating occult stress incontinence. Eur Urol. 2007;51:850-851.
Van Rooyen JB, Cundiff GW. Surgical management of pelvic organ prolapse. In: Cundiff GW, Bent AE, Ostergaard DR, Swift SE, editors. Ostergaard’s urogynecology and pelvic floor dysfunction. Philadelphia: Lippincott Williams & Wilkins, 2005.
Veronikis DK, Nichols DH, Wakamatsu MM. The incidence of low-pressure urethra as a function of prolapsed-reducing technique in patients with massive pelvic organ prolapsed (maximum descent at all vaginal sites). Am J Obstet Gynecol. 1997;177:1305-1313.
von Pechmann WS, Mutone M, Fyffe J, Hale DS. Total colpocleisis with high levator plication for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol. 2003;189:121-126.
Waetjen LE, Subak LL, et al. Stress urinary incontinence surgery in the United States. Obstet Gynecol. 2003;101(4):671-676.
Wall L, Menafee S. Novak’s textbook of gynecology. Philadelphia: Lippincott Williams & Wilkins; 2002.
Walter AJ, et al. Changes in tensile strength of cadaveric human fascia lata after implantation in a rabbit vagina model. J Urol. 2003;169:1907-1910.
Waters EG. Vaginal prolapse technic for correction and prevention at hysterectomy. Obstet Gynecol. 1956;8:432-436.
Watson SJ, Loder PB, Halligan S, et al. Transperineal repair of symptomatic rectocele with Marlex mesh: a clinical, physiological and radiologic assessment of treatment. J Am Coll Surg. 1996;183:257-261.
Webb MJ, Aronson MP, Ferguson LK, Lee RA. Posthysterectomy vaginal vault prolapse: primary repair in 693 patients. Obstet Gynecol. 1998;92:281-285.
Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106:615-634.
Weber AM, Walters M. Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311-318.
Weber A, Walters M, Ballard L, et al. Posterior vaginal prolapse and bowel function. Am J Obstet Gynecol. 1998;179:1446-1449.
Weber AM, Walters M, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1306.
Wheeler TL, et al. Outcomes of vaginal vault prolapse repair with a high uterosacral suspension procedure utilizing bilateral single sutures. Int Urogynecol J. 2007;18:1207-1213.
White GR. Cystocele. JAMA. 1909;21:1701-1710.
Whitehead WE, Bradley CS, et al. Gastrointestinal complications following abdominal sacrocolpopexy for advanced pelvic organ prolapse. AM J Obstet Gynecol. 2007;197(1):78. e1–7
Wieslander CK, et al. Uterosacral ligament suspension sutures: anatomic relationships in unembalmed female cadavers. Am J Obstet Gynecol. 2007;197:672. e1–672.e6
Williams WD. The response of the body environment to implants. In: Williams RR, Williams DF, editors. Implants in surgery. Philadelphia: WB Saunders; 1973:203-297.
Winkler HA, Tomeszko JE, Sand PK. Anterior sacrospinous vaginal vault suspension for prolapse. Obstet Gynecol. 2000;95:612-615.
Wiskind AK, Creighton SM, Stanton SL. The incidence of genital prolapse after the Burch colposuspension. Am J Obstet Gynecol. 1992;167:399-405.
Wu JM, Wells EC, et al. Mesh erosion in abdominal sacral colpopexy with and without concomitant hysterectomy. Am J Obstet Gynecol. 2006;194(5):1418-1422.
Yalcin I, Bump R. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003;189(1):98-101.
Yamada BS, Govier FE, et al. Vesicovaginal fistula and mesh erosion after Perigee (transobturator polypropylene mesh anterior repair). Urology. 2006;68(5):1121. e5–7
Yan A, Anne M, Karine A, et al. Cystocele repair by a synthetic vaginal mesh secured anteriorly through the obturator foramen. Eur J Obstet Gynecol Reprod Biol. 2004;115:90-94.
Yazdany T, Bhatia N. Uterosacral ligament vaginal vault suspension: anatomy, outcome and surgical considerations. Curr Opin Obstet Gynecol. 2008;20:484-488.
Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1367.