chapter 78 Bladder and Female Urethral Diverticula
Bladder Diverticula
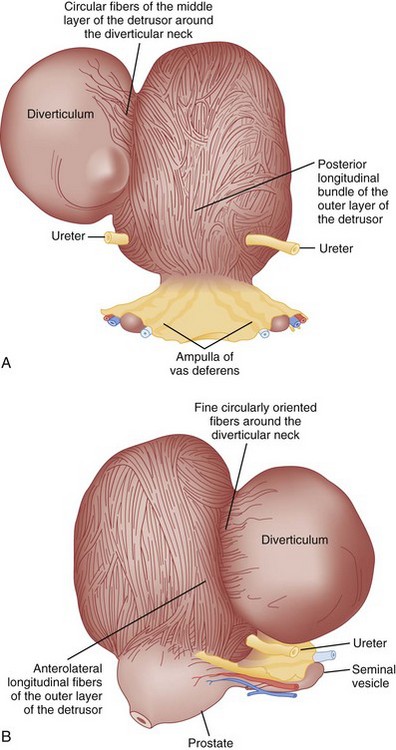
Bladder diverticula represent a herniation of the bladder urothelium through the muscularis propria of the bladder wall. This results in the typical finding of a variably sized, thin-walled, urine-filled structure adjacent to and connecting with the bladder lumen through a narrow neck, or ostium. Histologically, the diverticulum wall is composed of mucosa, subepithelial connective tissue or lamina propria, scattered thin muscle fibers, and an adventitial layer (Fig. 78–1) (Peterson et al, 1973; Gil-Vernet, 1998). A fibrous capsule or pseudocapsule outer shell is often present and may be a useful surgical plane for excision (see later discussion). The outside wall of the bladder diverticulum may contain some residual scattered strands or bundles of smooth muscle; however, these are disorganized and nonfunctional. Therefore bladder diverticula generally empty poorly during micturition, leaving a large postvoid residual urine volume that results in the characteristic findings on presentation and imaging.
Classification, Pathophysiology, and Etiology
Bladder diverticula may be classified as either congenital or acquired. Pathophysiology, presentation, and imaging may differentiate these two types. Congenital diverticula usually present during childhood, with a peak incidence in those less than 10 years old (Boechat and Lebowitz, 1978). These are usually solitary, occur most commonly in males (Stage and Tank, 1992; Sarihan and Abes, 1998; Evangelidis et al, 2005; Garat et al, 2007), and are located lateral and posterior to the ureteral orifice, often in association with vesicoureteral reflux (Evangelidis et al, 2005; Garat et al, 2007). The primary causation in those without coexisting lower urinary tract conditions appears to be a congenital weakness at the level of the ureterovesical junction and not bladder outlet obstruction (Johnston, 1960; Hutch, 1961; Hutch et al, 1961; Stephens, 1979). Blane and colleagues (1994) reported the incidence of bladder diverticula in children to be approximately 1.7% in a pediatric genitourinary database of more than 5000 cases. Congenital bladder diverticula are usually solitary but larger in comparison with those associated with obstruction or neurogenic bladder dysfunction (Gearhart, 2002). The most common presentation of congenital bladder diverticula is urinary tract infection (Bauer and Retik, 1974; Pieretti and Pieretti-Vanmarcke, 1999; Evangelidis et al, 2005; Garat et al, 2007). Congenital bladder diverticula may occur in the presence of normal voiding dynamics in the absence of bladder outlet obstruction (Cendron and Alain, 1972; Barrett et al, 1976), but secondary bladder outlet obstruction may occur when the diverticulum extends distally toward the bladder neck (Taylor et al, 1979; Epstein et al, 1982; Verghese and Belman, 1984; Oge et al, 2002). Typically, congenital bladder diverticula are found in smooth-walled bladders and are not associated with significant trabeculation on cystoscopic examination (Hutch, 1961). However, in patients with prune-belly syndrome or posterior urethral valves, bladder diverticula may be located at the dome and be associated with aberrant voiding dynamics and/or anatomy. These are to be distinguished from the urachal diverticula seen in some pediatric urologic conditions. Congenital bladder diverticula have been noted in association with a number of congenital syndromes, including Menkes syndrome (kinky hair or copper deficiency syndrome) (Harcke et al, 1977; Daly and Rabinovitch, 1981), Williams syndrome (Babbitt et al, 1979; Blane et al, 1994; Schulman et al, 1996), Ehlers-Danlos syndrome (Breivik et al, 1985; Levard et al, 1989; Schippers and Dittler, 1989; Rabin et al, 1991; Bade et al, 1994; Cuckow et al, 1994; Burrows et al, 1998), and fetal alcohol syndrome (Lewis and Woods, 1994). Whether there is a genetic predisposition to the formation of bladder diverticula in individuals without congenital syndromes is unclear, although congenital bladder diverticula have been reported in twins (Beall and Berger, 1978) and possibly as an autosomal-dominant trait in a family (Hofmann et al, 1984).
Acquired (also termed “secondary”) diverticula occur most commonly in the setting of bladder outlet obstruction or neurogenic vesicourethral dysfunction. Similar to the congenital type, these diverticula are also located most commonly at the ureterovesical hiatus (Van Arsdalen and Wein, 1992) but also occur elsewhere in the bladder. Acquired diverticula in males usually occur after age 60, which corresponds to the age of the development of prostatic enlargement (Fig. 78–2). Bladder outlet obstructions, including those due to benign and malignant disease of the prostate or urethral stricture, are commonly associated factors in adults, although obstruction is not considered to be present in all cases (Blacklock et al, 1983). Acquired diverticula are often multiple, typically found in association with significant bladder trabeculation (Wesselhoeft et al, 1963), and much more common in males than females (Senger et al, 1952; Pool and Hacker, 1966). Bladder diverticula in females are uncommon (Gillon et al, 1988) and quite rare in the absence of obstruction (Safir et al, 1998) (Fig. 78–3). Historically, the reported prevalence of moderate- to large-sized bladder diverticula in association with “prostatism” is approximately 1% to 6% (Burns, 1944). It is important to note that an acquired bladder diverticulum may also be found in children and young adults secondary to a number of conditions, including bladder neck dysfunction, posterior urethral valves, and neurogenic vesicourethral dysfunction. If the diverticulum encompasses the ureteral orifice in the setting of a neurogenic bladder and vesicoureteral reflux, it is termed a “Hutch” diverticulum (Hutch, 1952). These diverticula may also occur in the setting of dysfunctional voiding.
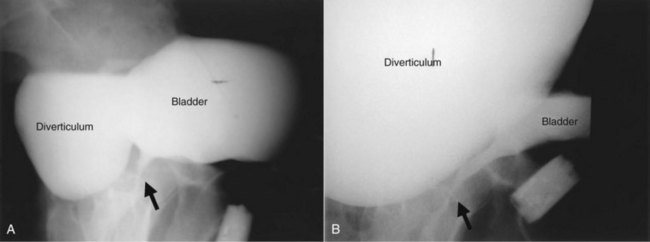
Figure 78–2 Voiding cystourethrogram (VCUG) demonstrating a bladder diverticulum in a male. A, The diverticulum is seen posterior to the bladder on this lateral voiding image (arrow points to urethra). There is a fluid-fluid level within the diverticulum representing the relatively denser contrast media settling below the urine. B, Later image from the VCUG demonstrates near emptying of the bladder with enlargement of the diverticulum.
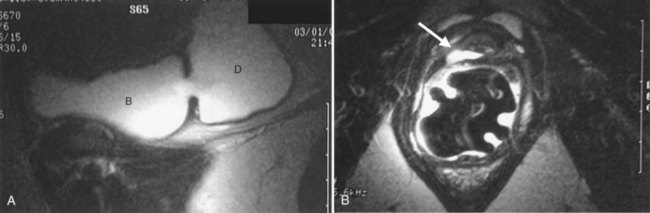
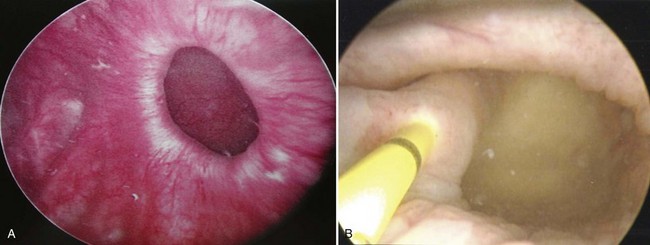
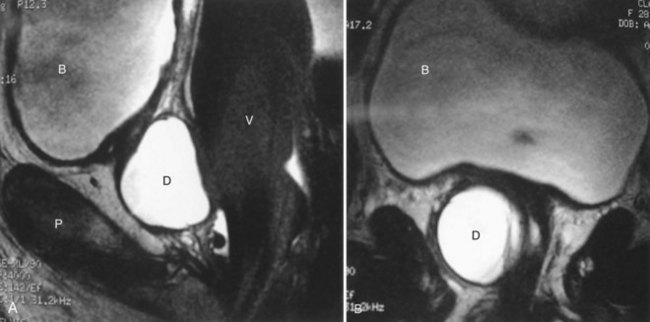
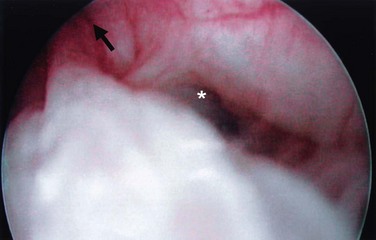
Figure 78–3 Bladder and urethral diverticula in a female. A, MRI of a bladder diverticulum. B, Endoluminal MRI demonstrating urethral diverticulum (arrow points to urethral diverticulum). B, bladder; D, bladder diverticulum.
Bladder diverticula may also be iatrogenic (Hernandez et al, 1997; Suzuki et al, 2002; Chertin and Prat, 2008). Inadequate closure of the muscular layers of the bladder wall following a cystotomy for any indication may result in formation of a bladder diverticulum at a weak point of the suture line. Bladder diverticula may also occur following ureteral reimplantation surgery at the ureteroneocystostomy site (Ahmed and Tan, 1982; Sheu et al, 1998).
Diagnosis and Evaluation
Presentation
Acquired bladder diverticula do not typically produce specific symptoms. Because large bladder diverticula empty poorly or incompletely during voiding, symptoms and signs, if present, are usually attributed to urinary stasis within the diverticulum or, alternatively, to its mass effect in the lower abdomen and pelvis. Retrospectively, when queried, patient symptoms such as incomplete bladder emptying, lower abdominal fullness, and double voiding may be attributed to some large bladder diverticula. These symptoms, however, are nonspecific and can be due to prostatic enlargement, obstruction, or a number of other lower urinary tract conditions. Most bladder diverticula are found during the investigation of nonspecific lower urinary symptoms, hematuria, or infection—or, alternatively, noted incidentally during radiographic or endoscopic investigation of these conditions. As noted previously, congenital bladder diverticula most often present with urinary tract infection (Bauer and Retik, 1974; Pieretti and Pieretti-Vanmarcke, 1999; Evangelidis et al, 2005; Garat et al, 2007), but hematuria, abdominal pain, or an abdominal mass may be present as well. Inguinal hernias containing bladder diverticula have also been reported (Scardino and Upson, 1953; Bolton and Joyce, 1994; Buchholz et al, 1998; Schewe et al, 2000).
Urinary tract infection (UTI) in the male has been associated with bladder diverticula, because it is often an infection in a male that provides the impetus for further clinical investigation uncovering a diverticulum. A bladder diverticulum and subsequent urinary stasis may result in a predisposition for UTI and may also confer an increased difficulty in eradicating an existing infection (Shah, 1979; Taylor et al, 1979). Nevertheless, urine analysis and urine culture are important in the evaluation of bladder diverticula. Abnormalities of the urine sediment are common in patients with bladder diverticula. Pyuria and hematuria are often present. In fact, relapsing or persistent pyuria unresponsive to antibiotic therapy may be an indication for bladder diverticulectomy. Urine cytology should be considered in most patients with bladder diverticula, especially when nonoperative management is being considered.
Imaging
The diagnosis of bladder diverticula relies on radiographic and endoscopic findings. Bladder diverticula are part of the radiologic continuum that includes cellules and saccules. Cellules, saccules, and bladder diverticula are felt to represent increasingly larger, therefore more severe manifestations of the same pathologic process involving elevated intravesical voiding pressure (Talner et al, 2000). Cellules and saccules represent small outpouchings between hypertrophied bands of bladder muscle, with saccules generally being larger than cellules. However, there are no universally agreed upon minimum size requirements between cellules, saccules, and bladder diverticula, and the differentiation between these three entities is often subjective and arbitrary (Talner et al, 2000).
As noted previously, bladder diverticula are often found incidentally in the radiographic investigation of recurrent urinary tract infections or other nonspecific lower urinary tract symptoms or signs. Fluoroscopically monitored voiding cystourethrography is an excellent study to detect bladder diverticula; however, false negatives are possible (Hernanz-Schulman and Lebowitz, 1985). A voiding urethrocystogram (VCUG) with anterior-posterior, oblique, and lateral images provides information regarding anatomy, location, and size and also associated vesicoureteric reflux and, importantly, emptying of the bladder diverticulum with voiding. Anomalous voiding into the diverticulum during a detrusor contraction may result in paradoxical enlargement of the bladder diverticulum during micturition (Wesselhoeft et al, 1963) (see Fig. 78–2). Presumably, this occurs as the contrast flows from an area of relatively high pressure in the bladder, during the detrusor contraction, to the diverticulum, which represents an area of low pressure. In some instances, the bladder may empty partly into the diverticulum and partly through the urethra. The amount of postvoid residual urine in the bladder diverticulum and bladder should be noted. Incomplete bladder emptying suggests that a urodynamic abnormality may be present, requiring further study.
If the neck of the diverticulum is obstructed from a tumor or otherwise not patent, cross-sectional imaging may be required for diagnosis (Dondalski et al, 1993). Cross-sectional imaging, including computed tomography (CT) and magnetic resonance imaging (MRI) may also be useful for the evaluation of masses within the diverticulum (see under Associated Conditions). Review of the radiographic films should accurately characterize the number, anatomy, and location of the diverticula. Filling defects within the diverticula or other bladder abnormalities should prompt further investigation (Fig. 78–4).
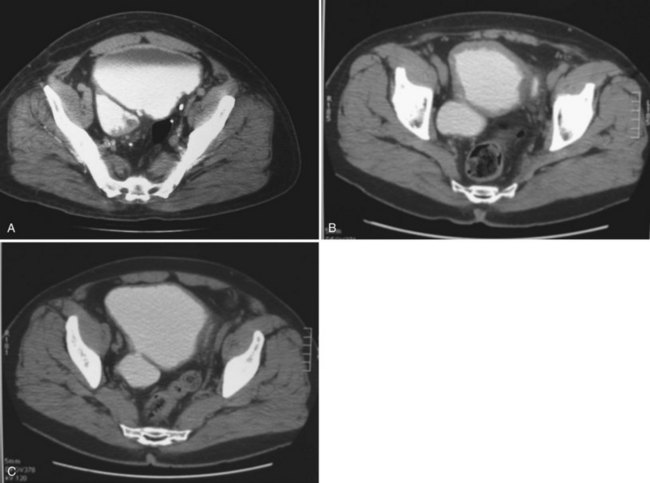
Figure 78–4 CT scans demonstrating several abnormalities in the setting of a bladder diverticulum. A, Diverticulum with multiple filling defects. Cystoscopy and biopsy revealed transitional cell carcinoma. B, Diffusely thickened and irregular bladder wall due to chronic bladder outlet obstruction in association with two diverticula. Cystoscopy confirmed trabeculation without mucosal abnormalities. C, Asymmetric bladder wall thickening in association with a bladder diverticulum. The ostium of the diverticulum is seen connecting to the bladder.
Imaging of the upper urinary tract may include intravenous pyelography (IVP), ultrasonography, CT, or MRI. In the absence of hematuria or a known or suspected urinary tract malignancy, the goal of upper tract imaging is to evaluate for asymptomatic or silent hydroureteronephrosis related to the diverticulum (Lebowitz et al, 1979; Sharma et al, 1997). Hydronephrosis may be related to obstruction of the ureter (Kwan and Lowe, 1992; Livne and Gonzales, 1985), an underlying urodynamic abnormality that resulted in the formation of the diverticulum, vesicoureteral reflux in association with the diverticulum, inflammation (Bellinger et al, 1985), or may be completely unrelated to the bladder diverticulum. A multichannel urodynamic study in combination with a voiding cystourethrogram (VCUG) or, alternatively, a videourodynamic study is useful in this setting to differentiate upper from lower urinary tract causes of the hydronephrosis.
A bladder diverticulum may cause deviation with or without compression of the ipsilateral ureter. On intravenous urography, medial deviation of the pelvic ureter is most commonly seen; however, lateral deviation may also occur (Talner et al, 2000) (Fig. 78–5). Furthermore, a bladder diverticulum that encompasses the ureteral orifice may create a functionally shortened intramural ureteral segment and result in vesicoureteric reflux (see later discussion). In these patients, excision of the bladder diverticulum with ureteroneocystotomy may be necessary.
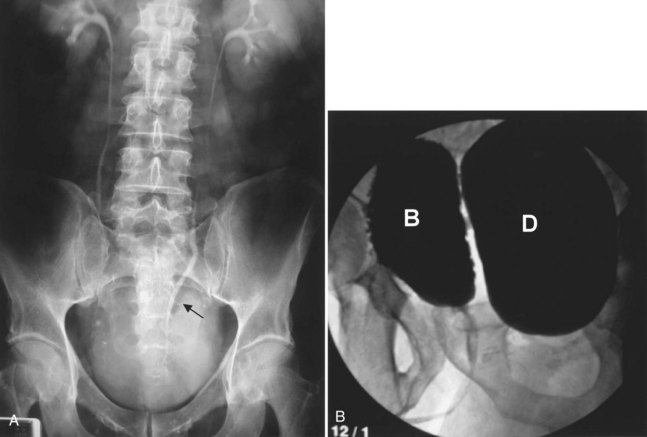
Figure 78–5 Bladder diverticulum with deviation of the ureter. This patient had a long history of recurrent urinary tract infections due to presumed prostatitis. A, 10-min film from the intravenous urogram demonstrating medial deviation of the pelvic ureter (arrow). B, Voiding image from the voiding cystourethrogram revealed a large, smooth-walled bladder diverticulum (D) emanating from the trabeculated bladder (B).
Urodynamics
Bladder outlet obstruction, impaired compliance, and/or neurogenic voiding dysfunction may result in the formation of bladder diverticula. Therefore a urodynamic study can be very helpful in the investigation of these patients. Failure to identify and treat an existing underlying urodynamic abnormality prior to or, concomitantly, with definitive surgical therapy for bladder diverticula may result in a high risk of recurrent diverticula or other problems following surgery. Successful treatment of the urodynamic abnormality may improve bladder emptying and may potentially result in resolution of the symptoms and/or complications. Importantly, bladder contractility may appear to be diminished on urodynamics due to the “pressure sink” effect of the bladder diverticulum. This artifact occurs as the detrusor contracts and the intravesical contents are decompressed through the path of least resistance into the bladder diverticulum as opposed to the urethra (Wilson and Klufio, 1985). Nevertheless, when carefully measured, bladder contractility is not significantly different from those individuals with benign prostatic hyperplasia (BPH) without bladder diverticula (Adot Zurbano et al, 2005).
Endoscopic Examination
Endoscopically, the entire interior of each bladder diverticulum should be thoroughly inspected for stones or abnormal-appearing epithelium. Flexible cystoscopy is often required to examine the entire interior of some diverticula. The location of the diverticula relative to the ureters and bladder outlet is noted, and the size of the diverticulum is carefully recorded (Fig. 78–6). Ideally, urine cytology should be obtained from the diverticulum during endoscopic examination. Any abnormal-appearing epithelium or lesions within the diverticulum are carefully biopsied. Extreme care must be taken during the biopsy to prevent perforation, because the wall of the diverticulum is very thin due to the lack of a muscularis propria layer. Patients who elect nonoperative management of bladder diverticula are usually followed closely with periodic endoscopic examinations and cytology. The natural history of untreated bladder diverticula is unknown, but the possibility of malignant transformation over time due to urinary stasis within the diverticulum should be considered.
Associated Conditions
Acquired bladder diverticula are commonly found to exist in the setting of bladder outlet obstruction. Common causes of bladder outlet obstruction in the male include benign and malignant disease of the prostate, and urethral stricture disease. Less common causes include bladder neck hypertrophy (primary bladder neck obstruction), vesicourethral anastomotic stricture following prostatectomy, and functional obstruction as a result of neurogenic vesicourethral dysfunction (bladder neck and/or striated sphincter dyssynergia). Strong consideration should be given toward management of bladder outlet obstruction prior to or concomitant with treatment of the bladder diverticulum. In some patients, treatment of bladder outlet obstruction alone may subsequently allow low pressure and complete emptying of the bladder diverticulum with micturition, thus reducing the potential for complications associated with these lesions and possibly avoiding the need for future operative intervention in the form of diverticulectomy.
The finding of a neoplasm within a bladder diverticulum has particularly important diagnostic and therapeutic considerations. The overall prevalence of malignant tumors within a bladder diverticulum has been reported as ranging from 0.8% to 10% (Montague and Boltuch, 1976; Micic and Ilic, 1983; Melekos et al, 1987; Baniel and Vishna, 1997; Golijanin et al, 2003). However, it is likely that the prevalence of bladder diverticula overall is under-reported and, as such, the prevalence of tumors within diverticula is probably considerably smaller than that reported above. Nevertheless, the most common histologic type of malignancy seen within bladder diverticula is transitional cell carcinoma in approximately 70% to 80% of cases, followed by squamous cell carcinoma in 20% to 25% of cases (Montague and Boltuch, 1976; Redman et al, 1976; Micic and Ilic, 1983; Van Arsdalen and Wein, 1992; Yu et al, 1993; Baniel and Vishna, 1997; Golijanin et al, 2003). Tumors in bladder diverticula occur almost exclusively in adults with a peak occurrence between ages 65 and 75 (Ostroff et al, 1973). The prognosis for patients with these tumors has been historically reported as poor (Faysal and Freiha, 1981; Micic and Ilic, 1983; Das and Amar, 1986; Melekos et al, 1987), although some reports may suggest otherwise in selected patients (Montague and Boltuch, 1976; Baniel and Vishna, 1997). The reason for the poor prognosis has been attributed to delayed diagnosis and advanced stage at presentation (Ostroff et al, 1973; Fellows, 1978; Faysal and Freiha, 1981; Micic and Ilic, 1983) in some patients. The lack of a well-defined muscularis layer of bladder diverticula is likely to be a very important factor. Because bladder diverticula lack a muscular layer beyond the urothelium, there is a theoretically greater risk for early transmural involvement with local extension to the perivesical fat through the comparatively thinner diverticular wall as compared with the normal bladder wall. In addition, the lack of a defined muscular wall risks early dissemination of tumor cells into an extravesical location during transurethral resection of these lesions and makes precise pathologic staging difficult. Accurate identification of tumors within bladder diverticula has been demonstrated using a variety of radiographic techniques, including CT, MRI, and ultrasonography (Dragsted and Nilsson, 1985; Saez et al, 1985; Williams and Gooding; 1985; Lowe et al, 1989; Dondalski et al, 1993; Durfee et al, 1997; Mallampati and Siegelman, 2004) (see Fig. 78–4). Whether these modalities can reliably predict extravesical invasion and therefore affect staging and prognosis is not clear. It has been suggested that clinical stage at presentation is the most important prognostic factor for patients with tumors in bladder diverticula, with 5-year actuarial survival ranging from 83 ± 9% in patients with superficial disease to 45 ± 14% in those presenting with extradiverticular disease (Golijanin et al, 2003). One small series suggests that aggressive individualized multimodal therapy in these patients, including surgery, chemotherapy, and radiation therapy may improve prognosis (Garzotto et al, 1996). Because pathologic staging following transurethral resection is difficult and often inaccurate, some authors have suggested a very aggressive approach to these tumors. This surgical staging approach involves an open exploration and partial or radical cystectomy without a prior transurethral resection (Redman et al, 1976). Others have advocated a selective individualized approach, taking into account the clinical stage and pathologic grade of the tumor (Golijanin et al, 2003). Low-grade, low-stage tumors may be successfully treated with diverticulectomy alone (Baniel and Vishna, 1997; Sulaiman et al, 1998); however, it is important to recognize that the ability to reliably predict stage and grade preoperatively is limited, and therefore this approach should be undertaken only with caution in select cases, with adequate counseling and follow-up. In any case, close surveillance of these patients is warranted whether or not aggressive therapy is undertaken.
The location of many bladder diverticula at the level of the ureterovesical junction may explain the high incidence of associated ipsilateral ureteral abnormalities. For a ureter found within a diverticulum, the lack of a muscular backing to the diverticulum results in a functionally shortened intramural tunnel (see Fig. 78–6B). A very high prevalence of ipsilateral vesicoureteral reflux has been noted in association with congenital bladder diverticula (Amar, 1972). One study noted reflux in 83 of 89 diverticulum associated ureteric units (Barrett et al, 1976). Other considerations in the evaluation and management of bladder diverticula include the potential development of stones within the diverticulum, ureteral obstruction (Lebowitz et al, 1979; Bellinger et al, 1985; Kwan and Lowe, 1992; Khan et al, 1994; Sharma et al, 1997), and even the potential for the rare but life-threatening complication of perforation and/or rupture of the bladder diverticulum (Mitchell and Hamilton, 1971; Keeler and Sant, 1990; Itoh and Kounami, 1994; Jorion and Michel, 1999).
Key Points: Bladder Diverticula—Diagnosis
Management
In general, if the etiology of the bladder diverticulum is felt to be consistent with bladder outlet obstruction, definitive treatment of the bladder outlet is indicated prior to formal diverticulectomy. This approach allows reassessment of the diverticulum following relief of outlet obstruction and, if bladder emptying is satisfactory, then the diverticulum may not require excision. However, simultaneous open bladder diverticulectomy/open prostatectomy, as well as simultaneous laparoscopic bladder diverticulectomy/transurethral prostatectomy (TURP) can be performed (Porpiglia et al, 2004). If treatment of the obstruction is pursued initially and results in satisfactory emptying of the diverticulum postoperatively, and complicating factors such as reflux, infection, malignancy, or stones are absent, then ongoing surveillance of the diverticulum may be all that is required. Emptying of the diverticulum following relief of outlet obstruction may be assessed on ultrasonography, VCUG, or by videourodynamics.
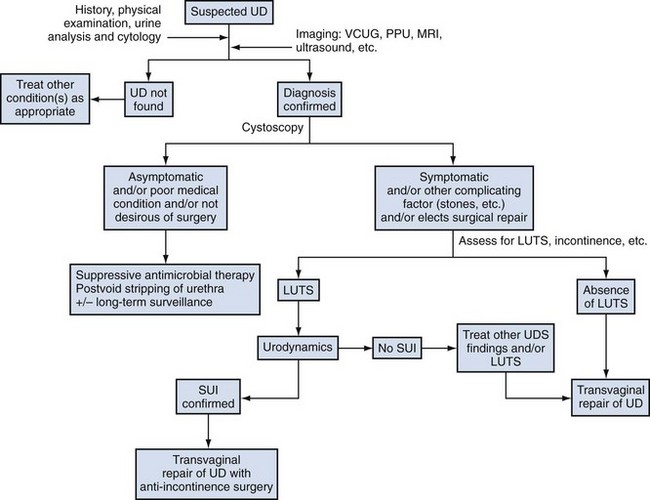
Management options in the treatment of bladder diverticula include observation, endoscopic management, and surgical excision; however, there are many factors to consider during the evaluation of these lesions prior to deciding on appropriate therapy (Fig. 78–7).
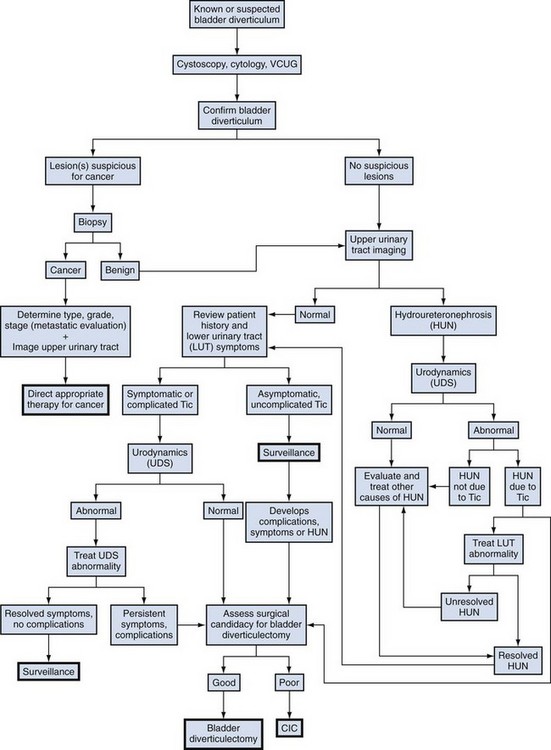
Figure 78–7 Algorithm for the evaluation and therapy of bladder diverticula. CIC, clean intermittent catheterization; VCUG, voiding cystourethrogram.
(Adapted from Rovner ES, Wein AJ. Bladder diverticula in adults. In: Resnick M, Elder JA, Spirnak JP, editors. Decision making in urology. 3rd ed. Hamilton [Ontario]: B.C. Decker; 2004. p. 260–3.)
Observation/Expectant Management
Patients who have poor bladder emptying following relief of obstruction, or who are unable or unwilling to undergo surgical excision of the bladder diverticulum, may be effectively treated with clean intermittent catheterization (CIC) or an indwelling catheter. In this setting, and in the absence of future complicating factors, surveillance of the bladder diverticulum may be all that is required. Patients who demonstrate significantly impaired bladder contractility on preoperative urodynamics, or who fail to empty their bladder (independent of the emptying of the diverticulum) on preoperative VCUG or videourodynamics following relief of obstruction, may have persistent failure to empty their bladder even following successful diverticulectomy. Therefore long-term CIC may be a viable option in these patients.
Indications for Intervention
Incidentally found congenital or acquired bladder diverticula may require no further therapy unless associated with persistent symptoms, recurrent infections, obstruction, stones, malignancy, or other complicating factors such as ipsilateral vesicoureteral reflux. Symptoms or complications related to bladder diverticula are most often due to poor emptying of the diverticulum and urinary stasis. Therefore excision of the diverticula would be expected to improve emptying of the lower urinary tract, provided that the primary problem that resulted in the formation of the bladder diverticulum (i.e., obstruction) has been adequately addressed. Therefore bladder diverticulectomy is indicated for the treatment of symptoms related to the diverticulum, or for the major complications directly related to it, including chronic relapsing urinary tract infection, stones within the diverticulum, carcinoma or premalignant change, and, finally, upper urinary tract deterioration as a result of obstruction or reflux.
Bladder diverticula can vary tremendously in size and, in some instances, are larger than the bladder itself. The size of the diverticulum does not appear to correlate with symptoms or complications and therefore cannot be used as an absolute indication to proceed with surgery. Asymptomatic patients without complicating factors may be followed closely with endoscopic examination, urine cytology, and cultures. Controversy exists as to whether prophylactic excision of asymptomatic bladder diverticula should be carried out to prevent malignant transformation or whether close observation is sufficient. Cytologic techniques and endoscopic equipment allow excellent follow-up, but patients should be counseled that the treatment and prognosis of malignant lesions, should they develop in follow-up, remain variable and unpredictable.
Excision of bladder diverticula is most commonly elective. The relative merits of surgical excision versus surveillance should be carefully considered and discussed with each patient individually. The patient should be in relatively good health and be a reasonable surgical risk prior to considering the procedure. Preoperative medical status should be assessed and reversible risk factors corrected and/or optimized (nutritional, renal, cardiac, pulmonary, etc.). Preoperative urinary tract infection should be treated. Patients who are prohibitive surgical risks due to concurrent medical illness or other factors should not undergo surgical excision but may be candidates for CIC or endoscopic treatment.
Endoscopic Management
Endoscopic management of bladder diverticulum may be considered in patients who are aged or somewhat debilitated, those who are not good candidates for an open operative approach, or for those undergoing transurethral resection of prostate in whom there exists an associated poorly draining diverticulum (Orandi, 1977; Vitale and Woodside, 1979). Transurethral resection of the diverticular neck has been reported to be successful in select cases (Vitale and Woodside, 1979). This technique is performed with a standard resectoscope. The neck of the diverticulum is incised using the resectoscope loop or Collins knife. Incisions are carried down to the muscular fibers of the bladder at the level of the ostium of the diverticulum. Circumferential resection of the entire neck may be performed. When successful, this procedure enlarges the neck of the diverticulum, disrupting the narrow sphincteric-like properties of its connection to the bladder lumen, thereby permitting improved emptying of the diverticulum during micturition. Although generally safe and well tolerated, this technique has resulted in urinary retention due to a reversal of flow during micturition and “venting” of the bladder contents into the diverticulum postoperatively (Schulze and Hald, 1983). Transurethral resection of the diverticular neck may be combined with fulguration of the entire urothelial lining of the diverticulum (Clayman et al, 1984; Adachi et al, 1991a, 1991b; Yamaguchi et al, 1992). Fulguration of the lining of the diverticulum should result in obliteration of the diverticulum or a considerable reduction in its size.
Open Surgical Management
Open excision is usually performed through a transvesical approach, although extravesical and combined approaches have been described. In cases of marked prostatic enlargement and obstruction, open suprapubic prostatectomy and transvesical bladder diverticulectomy may be performed concomitantly. Adequate preoperative imaging and endoscopic evaluation should provide information regarding the size, number, and location of all bladder diverticula. Furthermore, the relationship of the adjacent anatomic structures, including the major pelvic vessels, ureters, and bowel, should be noted. Surgically, the bladder is most commonly approached extraperitoneally through a low midline or transverse incision. The retropubic space is entered and developed. Regardless of the technique used, careful dissection is required during excision of the diverticulum to avoid ureteral injury, because many bladder diverticula are located adjacent to the ureter or may be adherent to it. Ureteral stents are often placed preoperatively or intraoperatively to facilitate dissection and avoid ureteric injury. Often, several bladder diverticula are noted preoperatively. Simultaneous resection of all existing bladder diverticula should be performed to optimize postoperative bladder emptying (Gil-Vernet, 1998).
Transvesical Bladder Diverticulectomy
The transvesical approach to bladder diverticulectomy was first reported by Hugh Hampton Young in 1906 (Gil-Vernet, 1998). The bladder is opened along the anterior wall and suitable retraction is placed in order to visualize the neck of the diverticulum. For the excision of small diverticula, saccules or cellules, a locking instrument such as an Allis-type clamp is passed through the neck of the diverticulum, and the base or bottom of the diverticulum is grasped, pulled, and everted back into the bladder. In the absence of extravesical adhesions or inflammation, a small diverticulum can be completely everted into the bladder and exposed in this manner. The urothelium of the diverticulum at the level of the neck is incised circumferentially, and the diverticulum is removed. The defect in the bladder wall is closed with absorbable suture in two layers. Care must be taken when performing this technique to avoid injuring structures adherent or adjacent to external surface of the diverticulum, because they may be blindly pulled into the bladder with the diverticulum wall and inadvertently injured during resection.
If eversion of the diverticulum is not possible due to adhesions or inflammation, or the diverticulum is too large to permit complete exposure during eversion, submucosal excision of the diverticulum may be performed. An anterior cystotomy is performed, the bladder is opened and suitable retraction is placed to visualize the neck of the diverticulum within the bladder. The neck of diverticulum is then identified and mobilized initially, similar to the technique used for the first part of the dissection of the ureter during ureteroneocystostomy. The diverticular neck is circumscribed sharply with scissors or electrocautery, and the plane between the wall of the diverticulum and the surrounding fibrous capsule is defined (Fig. 78–8A to D). Traction is placed on the edges of the neck of the diverticulum with Allis clamps circumferentially, and the neck and then the exterior walls of the diverticulum are carefully mobilized from the surrounding tissues and delivered into the bladder lumen. Sharp and blunt dissection on the exterior wall of the diverticulum is performed in a well-defined periadventitial plane between the diverticulum wall and the fibrous pseudocapsule. Packing of the diverticulum with gauze may facilitate dissection and provide some countertraction (Fig. 78–9A to D). The diverticulum is completely freed from its fibrous pseudocapsule and removed. The bladder wall defect is then repaired in two layers with absorbable sutures. Drainage of the potential space left by the pseudocapsule is usually not necessary (Firstater and Farkas, 1977).
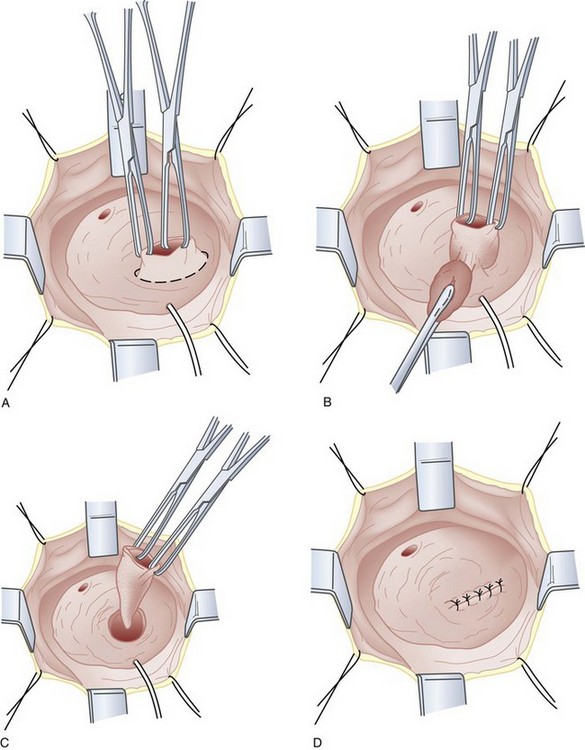
Figure 78–8 Intravesical submucosal bladder diverticulectomy. A, The neck of the diverticulum is grasped and circumscribed sharply. B, The adventitial tissue is dissected free from the diverticulum wall. C, The diverticulum is completely removed. D, The bladder is repaired.
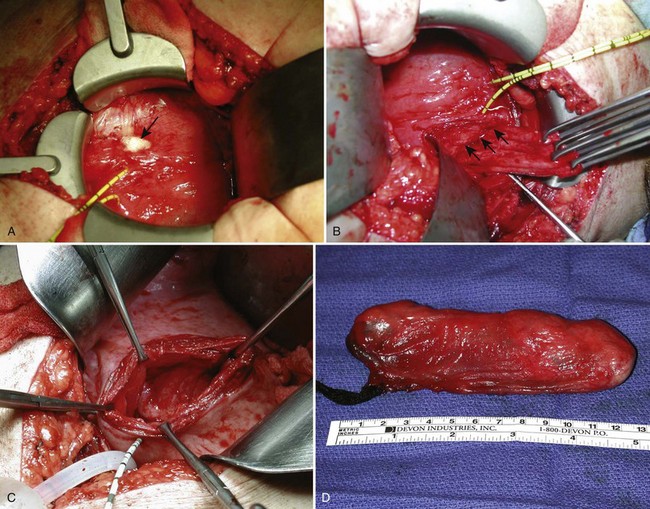
Figure 78–9 Transvesical submucosal bladder diverticulectomy. A, Intraoperative packing of the diverticulum (arrow) with gauze may facilitate dissection. Ureteric catheters demonstrate the proximity of the diverticulum to the right ureter. B, Allis clamps are seen on the edge of the neck of the diverticulum providing traction. The arrows show the plane of dissection along the pseudocapsule of the diverticulum as it is dissected free from the retroperitoneal tissues. C, View into the retroperitoneal space through the bladder wall defect created by removal of the diverticulum. D, Intact diverticulum specimen packed with gauze.
Combined Intravesical/Extravesical Approach
For individuals with large diverticula and/or considerable peridiverticular inflammation, a purely transvesical approach may not be feasible. In addition, involvement of the ureter within the diverticulum, or severe peridiverticular inflammation encompassing the ureter may have altered the usual course of the ureter and may incur a prohibitive risk of injuring the ureter with a transvesical approach. Therefore a combined intravesical and extravesical approach may be warranted. Ureteral catheters are usually placed to prevent ureteral injury. The bladder is opened as if for a transvesical diverticulectomy. The diverticular neck is incised circumferentially. The surgeon’s finger is then inserted into the diverticulum to identify the location of the rest of the diverticulum (Fig. 78–10). The diverticulum may be packed with gauze as well. If the neck of the diverticulum is sufficiently mobile, the anterior aspect of the neck is brought up into the operative field outside the bladder using the surgeon’s finger. The overlying tissue is dissected free, and the anterior portion of the neck of the diverticulum is exposed and incised extravesically. The neck is circumferentially mobilized, and then transected. The diverticular wall is then mobilized and dissected free of its attachments as described previously. In some cases, anterior reflection of the surgeon’s finger allows opening of the diverticulum itself, and dissection can be carried out in the plane between the pseudocapsule and the urothelium in a completely extravesical fashion. In some cases, it may be necessary to divide the ipsilateral superior vesical pedicle to facilitate exposure and delivery of the diverticulum. If additional difficulty is encountered in exposing the diverticulum, the original cystotomy incision can be extended or enlarged in a “T” fashion over to the neck of the diverticulum. This procedure generally removes the entire diverticulum, including the mucosa and fibrous pseudocapsule. In cases where considerable inflammation is encountered and the diverticulum is closely adhering to adjacent vital structures, it may be necessary to leave portions of the fibrous pseudocapsule in situ. The bladder is closed as described previously.
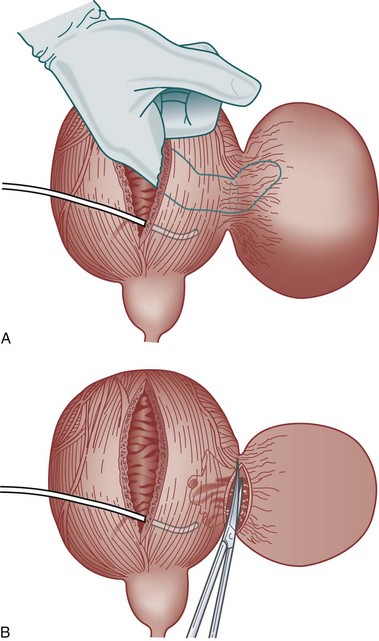
Figure 78–10 Combined intravesical-extravesical bladder diverticulectomy. A, The surgeon’s finger is inserted into the diverticulum through a cystotomy allowing identification of the diverticular neck. B, The diverticular neck is divided and the remaining portion of the diverticulum is dissected free from the surrounding tissues.
Laparoscopic and Robotic Diverticulectomy
Minimally invasive techniques, such as laparoscopy and robotics (Myer and Wagner, 2007), have been applied to surgical diverticulectomy using a similar surgical approach (Das, 1992; Parra et al, 1992; Jarrett et al, 1995; Faramarzi-Roques et al, 2004; Abdel-Hakim et al, 2007; Macejko et al, 2008). Intra- and extraperitoneal approaches have been reported. Approaching the diverticulum extravesically using such a minimally invasive approach can be challenging; however, simultaneous intraoperative cystoscopic transillumination may aid in locating the diverticulum within the retroperitoneal tissues (Macejko et al, 2008). Small, non-randomized case series suggest that outcomes are similar to established open techniques (Porpiglia et al, 2004; Myer and Wagner, 2007).
Complications
The most devastating complication during bladder diverticulectomy is ureteral injury. Fortunately, this is rare and can be avoided by careful attention to technique. Placement of a ureteral catheter can be very helpful in identifying the ureter during surgery. If identified intraoperatively, a partial transaction may be repaired primarily and stented. Complete transection usually mandates ureteral reimplantation into the bladder with or without a psoas hitch. Other complications include urinary tract infection, bleeding, prolonged urinary extravasation postoperatively, urinary fistula, and bowel injury.
Key Points: Bladder Diverticula—Management
Female Urethral Diverticula (UD)
A urethral diverticulum in the female can be generally described as a periurethral cystic structure adjacent to the urethra. Diverticula of the female urethra present some of the most challenging diagnostic and reconstructive problems in female urology. UD are notable for a bewildering variety of clinical presentations ranging from completely asymptomatic, incidentally noted lesions on physical examination or radiographs, to very debilitating, painful vaginal masses associated with incontinence, stones, and/or tumors. Anatomical variations between patients and in the location, size, and complexity of these lesions, ensure that each case is unique.
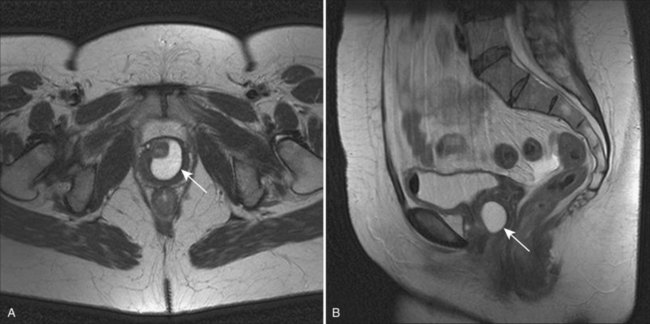
Although described as early as the 19th century (Hey, 1805), the modern era of female urethral diverticula (UD) began in 1956 with the advent of positive-pressure urethrography by Davis and Cian (1952). Over the next several years, there was a dramatic increase in number of cases of UD reported in the literature. A published series of 121 cases by Davis and Telinde (1958) approximately doubled the number of cases reported during the previous 60 years. Development of adjuvant imaging techniques, such as ultrasonography and surface coil/endoluminal MRI, in the past 2 to 3 decades have contributed greatly to further understanding of UD (Fig. 78–11).
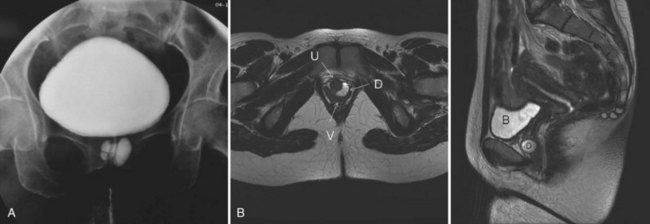
Figure 78–11 Imaging of urethral diverticula. A, Anterior-posterior view from a voiding cystourethrogram demonstrating a collection of contrast below the bladder, suggesting a urethral diverticulum. B, Surface coil MRI with both sagittal and axial T2 images demonstrating the relevant anatomy of a patient with a urethral diverticulum. B, bladder; D, urethral diverticulum; U, urethra; V, vagina.
Anatomy of the Female Urethra
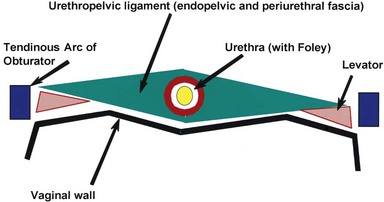
The normal female urethra is a musculofascial tube approximately 3 to 4 cm in length, extending from the bladder neck to the external urethral meatus, suspended to the pelvic sidewall and pelvic fascia (tendinous arc of the obturator muscle) by a sheet of connective tissue termed the urethropelvic ligament. The urethropelvic ligament is composed of two layers of fused pelvic fascia that extend toward the pelvic sidewall bilaterally (Fig. 78–12). This structure can be considered to have an abdominal side (the endopelvic fascia) and a vaginal side (periurethral fascia). Within and between these two leaves of fascia lies the urethra and the location of most urethral diverticula.
The urethral lumen is lined by an epithelial layer that is a transitional cell type proximally and nonkeratinized stratified squamous cell type distally. The urethra may be conceptualized as a rich, vascular, spongy cylinder surrounded by an envelope consisting of smooth and skeletal muscle and fibroelastic tissue (Young et al, 1996). Within the thick, vascular lamina propria/submucosal layer are the periurethral glands (Fig. 78–13). These tubuloalveolar glands exist over the entire length of the urethra posterolaterally; however, they are most prominent over the distal two thirds, with the majority of the glands draining into the distal one third of the urethra. Skene glands are the largest and most distal of these glands. These glands drain outside the urethral lumen, lateral to the urethral meatus. It is from pathologic processes involving the periurethral glands that most acquired female urethral diverticula are thought to originate.
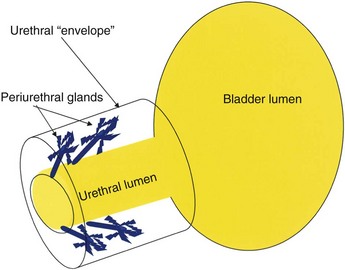
Figure 78–13 Periurethral glands are located within submucosa of urethra deep to the muscular envelope draining distally but arborizing proximally.
The urethra has several muscular layers: an internal longitudinal smooth muscle layer, an outer circular smooth muscle layer, and a skeletal muscle layer. The skeletal muscle component spans much of the length of the urethra but is more prominent in the middle third. It has a U-shaped configuration, being deficient dorsally. Ventral to the urethra, but separated from it by the periurethral fascia, lies the anterior vaginal wall (see Fig. 78–12). The location and competence of the urethral sphincters have important implications when considering surgical repair of urethral diverticula due to the anatomic overlap of these two entities.
Arterial inflow to the urethra derives from two sources. The proximal urethra has a blood supply similar to the adjacent bladder, whereas the distal urethra derives its blood supply from the terminal branches of the inferior vesical artery through the vaginal artery that runs along the superior lateral aspect of the vagina (Hinman, 1993). Lymphatic drainage of the female urethra is to the external and internal iliac nodes from the proximal urethra, and to the superficial and deep inguinal lymph nodes from the distal urethra. Innervation to the female urethra is from the pudendal nerve (S2-S4), and afferents from the urethra travel through the pelvic splanchnic nerves.
Urethral Diverticula
Pathophysiology and Etiology
As conceptualized by Young and colleagues (1996), urethral diverticula represent a cavity dissecting within the confines of the fascia of the urethropelvic ligament. This defect is often an isolated cystlike appendage with a single discreet connection to the urethral lumen termed the neck, or ostium (Fig. 78–14). However, complicated anatomic patterns may exist, and in certain cases the UD may extend partially (“saddlebag” UD) around the urethra, anterior to the urethra (Vakili et al, 2003), or circumferentially around the entire urethra (Rovner and Wein, 2003) (Fig. 78–15).
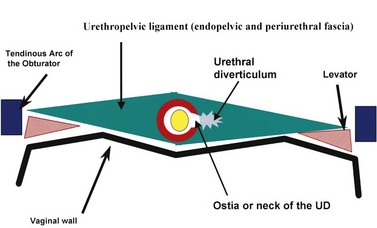
Figure 78–14 Diagram of urethral diverticulum. The urethral diverticulum forms within and between the layers of the urethropelvic ligament.
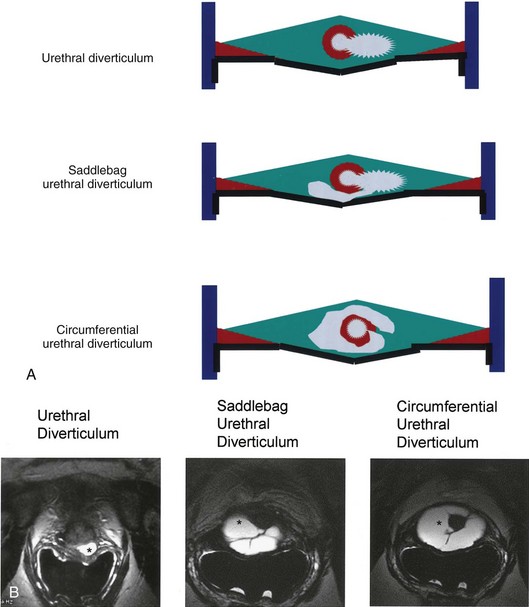
Figure 78–15 Multiple different morphologies of urethral diverticulum (UD) may exist. A, Schematic diagrams representing three different morphologies of UD. B, Endoluminal MR images corresponding to each of the configurations in three different patients (asterisk indicates urethral diverticulum).
The exact origin of UD is still unproven. A major debate in the earlier part of the 20th century focused on whether UD were congenital or acquired lesions (Johnson, 1938; Gilbert and Rivera-Cintron, 1954; Pinkerton, 1963). Although this condition exists in children, the diagnosis may represent a different clinical entity from adult female UD. Scattered reports of congenital UD in female infants have been described (Glassman et al, 1975). Marshall (1981) reported five cases of UD in young females, of whom three underwent spontaneous regression. Congenital anterior urethral diverticulum is a well-described entity in boys (Kirks and Grossman, 1981; Lau and Ong, 1981; Kaneti et al, 1984), but this is considered to be an entirely different clinical entity from UD in the female. Congenital Skene gland cysts have been reported (Kimbrough and Vaughan, 1977; Lee and Kim, 1992) but are considered extremely rare. Diverticula in the pediatric population have been attributed to a number of congenital anomalies, including an ectopic ureter draining into a Gartner duct cyst and a form fruste of urethral duplication (Silk and Lebowitz, 1969; Vanhoutte, 1970; Boyd and Raz, 1993). The vast majority of UD, however, are likely to be acquired and are diagnosed in adult females. In two large series of UD, there were no patients reported who were younger than 10 years of age (Davis and Robinson, 1970; Davis and TeLinde, 1958), arguing against a congenital etiology for these lesions. Although it is possible that there exists a congenital defect in patients that results in or represents a precursor to UD, which then only becomes symptomatic later in life, it is still unproven.
There are multiple theories regarding the formation of acquired UD. For many years, acquired UD were felt to be most likely due to trauma from vaginal childbirth (McNally, 1935). It was postulated that mechanical trauma during vaginal delivery resulted in herniation of the urethral mucosa through the muscular layers of the urethra with the subsequent development of a UD. However, up to 20% to 30% of patients in some UD series are nulliparous (Lee, 1984; Ganabathi et al, 1994), which may significantly discount parity as a risk factor. Trauma with forceps delivery, however, has been reported to cause UD (Klyszejko et al, 1985), and the radiographic appearance of periurethral injectable agents, including collagen (Clemens and Bushman, 2001; Bridges et al, 2005) and other injectable agents (Castillo-Vico et al, 2007), can simulate a urethral diverticulum.
The periurethral glands are felt to be the probable site of origin of acquired UD (Young et al, 1996). Huffman’s anatomic work with wax models of the female urethra was critical to the early theories regarding the pathophysiology of UD and the involvement of the periurethral glands (Huffman, 1948). By reviewing 10-µm transverse sections, he refuted earlier anatomic descriptions of the glandular anatomy of the female. He characterized the periurethral glands as located primarily dorsolateral to the urethra, arborizing proximally along the urethra, and yet draining into ducts located in the distal one third of the urethra (see Fig. 78–13). Furthermore, he noted that periductal and interductal inflammation was found commonly. In support of these observations and an infectious (acquired) etiology of UD, in over 90% of UD cases, the ostium is located posterolaterally in the mid- or distal urethra, which corresponds to the anatomic location of the periurethral glands (Lang and Davis, 1959; MacKinnon et al, 1959).
Although there are probably other unknown factors that may facilitate the initiation, formation or propagation of UD, infection of the periurethral glands seems to the most generally accepted common etiologic factor in most cases. Peters and Vaughan (1976) found a strong association with concurrent or previous infection with Neisseria gonorrhea and UD. However, the initial infection and, especially, subsequent reinfections may originate from a variety of sources, including E. coli and other coliform bacteria as well as vaginal flora. Nevertheless, UD have been attributed historically to recurrent infection of the periurethral glands, with obstruction, suburethral abscess formation, and subsequent rupture of these infected glands into the urethral lumen. Continual filling and pooling of urine in the resultant cavity may lead to stasis, recurrent infection, and eventual epithelialization of the cavity, forming a permanent urethral diverticulum. This concept was first popularized by Routh (1890) over a century ago and has now become the most widely accepted theory regarding the formation of female UD. Reinfection, inflammation, and recurrent obstruction of the neck of the cavity are theorized to result in patient symptoms and enlargement of the diverticulum. This proposed pathophysiology appears to adequately explain the anatomic location and configuration of most UD and is supported by the work of Huffman noted previously. However, it should be noted that Daneshgari and colleagues (1999) have reported noncommunicating urethral diverticula diagnosed by MRI. Whether this lesion represents a forme fruste of UD or simply a UD with an obstructed ostium is unclear.
Young and colleagues (1996) have formulated a modern hypothesis regarding the pathogenesis of UD through extensive clinical experience with this entity, including the diagnosis, imaging, and surgical repair of UD. These authors have proposed that acquired UD results from infection and obstruction of the periurethral glands. These glands are normally found in the submucosal layer of the spongy tissue of the distal two thirds of the urethra. Repeated infection and abscess formation in these obstructed glands eventually result in enlargement and expansion. Initially the expanding mass displaces the spongy tissue of the urethral wall and then enlarges to disrupt the muscular envelope of the urethra. This results in herniation into the periurethral fascia. The enlarging cavity can then expand and dissect within the leaves of the periurethral fascia and urethropelvic ligament. This expansion occurs most commonly ventrally, resulting in the classic anterior vaginal wall mass palpated on physical examination in some patients with UD. However, it is important to note that these may also expand laterally, or even dorsally, about the urethra. Eventually, the abscess cavity ruptures into the urethral lumen, resulting in the communication between the UD and the urethral lumen.
Prevalence
Moore (1952) stated that UD as an entity is “…found in direct proportion to the avidity with which it is sought.” Although it is not considered a rare lesion today, less than 100 cases of UD had been reported in the literature prior to 1950. With the development of sophisticated imaging techniques, including positive-pressure urethrography in the 1950s, the diagnosis of UD became increasingly common.
The true prevalence of female UD, however, is not known; it is reported to occur in up to 1% to 6% of adult females in some series. Determining the true prevalence of UD would require appropriate screening and imaging of a large number of symptomatic and asymptomatic adult female subjects in a primary care setting, which, to date, has not been done. Bruning (1959) found UD in 3 of 500 female autopsy specimens. In 1967, Andersen reported the results of positive-pressure urethrography done for 300 women with cervical cancer but without lower urinary tract symptoms (LUTS) and found UD in 3%. Aldridge (1978) reported a prevalence of UD in 1.4% of women presenting with incontinence and related symptoms. Stewart (1981) found UD in 16 of 40 highly symptomatic females investigated with positive-pressure urethrography. Endorectal coil MRI was performed on 140 consecutive female patients with LUTS, and the incidence of UD was approximately 10% (Lorenzo et al, 2003). However, this represented a series of symptomatic females at a tertiary referral center and therefore probably is not reflective of general population. More recently, Burrows and colleagues (2004) reviewed U.S. hospital and ambulatory surgery databases and concluded that the rate of urethral diverticulectomy surgery from 1994 to 1996 was approximately 6.7 per 1 million females. However, this is well below the historical prevalence figures cited previously.
Some series have suggested a definite racial predilection, with African-Americans being as much as six times as likely to develop UD as their white counterparts (Davis and Robinson, 1970). The reasons for this racial distribution are not well understood. Recent data suggests that inpatient surgery for urethral diverticulum occurs threefold more commonly in African-Americans than in whites (Burrows et al, 2004).
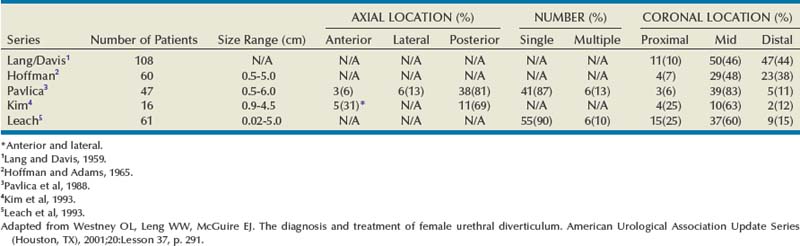
Diverticular Anatomy
Most commonly, UD represent an epithelialized cavity with a single connection to the urethral lumen. The size of the lesion may vary from only a few millimeters to several centimeters. In addition, the size may vary over time due to inflammation, intermittent obstruction of the ostium, and subsequent drainage into the urethral lumen.
The epithelium of UD may be columnar, cubiodal, stratified squamous, or transitional. In some cases, the epithelium is absent, and the wall of the UD consists of only fibrous tissue. UD are found within the periurethral fascia bordered by the anterior vaginal wall ventrally. In the sagittal plane, UD are most often located and centered at the level of the middle third of the urethra, with the luminal connection, or ostium, located posterolaterally. They may extend distally along the vaginal wall, almost to the urethral meatus or proximally up to and beyond the bladder neck underneath the trigone of the bladder (Fig. 78–16). A bewildering array of configurations can be noted on imaging and at surgical exploration (Table 78–1). In the axial plane, the UD cavity may extend laterally along the urethral wall and, in some cases, around to the dorsal side of the urethra or wrap circumferentially around the entire urethra. UD may be bilobed (dumbbell shaped), extending across the midline in a so-called “saddle bag” configuration (see Fig. 78–15). Multiple loculations are not uncommon, and at least 10% of patients have multiple UD at presentation. Varying degrees of sphincteric compromise may exist due to the location of diverticulum relative to the proximal and distal urinary sphincter mechanisms, or sphincteric compromise may coexist with UD due to other factors. This is important to note when considering surgical repair as discussed later.
Presentation
The majority of patients with UD present between the third and seventh decade of life (Johnson, 1938; Moore, 1952; Pathak and House, 1970; Ginsburg and Genadry, 1983; Ganabathi et al, 1994). The presenting symptoms and signs in patients with UD are protean (Table 78–2). The classic presentation of UD has been described historically as the “three Ds”: dysuria, dyspareunia, and dribbling (postvoid). However, individually or collectively, these symptoms are neither sensitive nor specific for UD. Although presentation is highly variable, the most common symptoms are irritative (frequency, urgency, etc.) lower urinary tract symptoms (LUTS), pain, and infection (Davis and TeLinde, 1958; Davis and Robinson, 1970; Peters and Vaughan, 1976; Leach et al, 1986). Dyspareunia will be noted by 12% to 24% of patients (Davis and TeLinde, 1958; Davis and Robinson, 1970). Approximately 5% to 32% of patients will complain of postvoid dribbling (Davis and Robinson, 1970; Ganabathi et al, 1994). Recurrent cystitis or urinary tract infection is also a frequent presentation in one third of subjects (Davis and Robinson, 1970; Ganabathi et al, 1994), probably due to urinary stasis in the UD. Multiple bouts of recurrent cystitis should alert the clinician to the possibility of a UD. Other complaints include pain, a vaginal mass, hematuria, vaginal discharge, obstructive symptoms or urinary retention, and incontinence (stress or urge). Patients may present with complaints of a tender or nontender anterior vaginal wall mass, which upon gentle compression may reveal retained urine or purulent discharge per the urethral meatus. Although spontaneous rupture of these lesions is extremely rare, urethrovaginal fistula may result under these circumstances (Nielsen et al, 1987). Notably, up to 20% of patients diagnosed with UD may be completely asymptomatic, having the lesions diagnosed incidentally on imaging or physical examination.
Table 78–2 Signs and Symptoms of Urethral Diverticula
| Symptoms |
| Signs |
It is important to note that the size of the UD does not correlate with symptoms. In some cases, very large UD may result in minimal symptoms, and, conversely, some UD that are non-palpable may result in considerable patient discomfort and distress. Finally, symptoms may wax and wane and even resolve for long periods of time. The reasons for these exacerbations and remissions are poorly understood but may be related to periodic and repeated episodes of infection and inflammation.
Because many of the symptoms associated with UD are nonspecific, patients may often be misdiagnosed and treated for years for a number of unrelated conditions before the diagnosis of UD is made. This may include therapies for interstitial cystitis, recurrent cystitis, vulvodynia, endometriosis, vulvovestibulitis, and other conditions. In one series of 46 consecutively examined women eventually diagnosed with UD, the mean interval from onset of symptoms to diagnosis was 5.2 years (Romanzi et al, 2000). In this series, women consulted with an average of nine physicians prior to the definitive diagnosis being made, despite the fact that 52% of women had a palpable mass on exam. This underscores the importance of a baseline level of suspicion and a thorough pelvic exam in female patients complaining of LUTS or other symptoms that may be associated with UD.
Evaluation and Diagnosis
The diagnosis and complete evaluation of UD can be made with a combination of a thorough history, physical examination, appropriate urine studies (including urine culture and analysis), endoscopic examination of the bladder and urethra, and selected radiologic imaging. A urodynamic study may also be helpful in selected cases.
Physical Examination
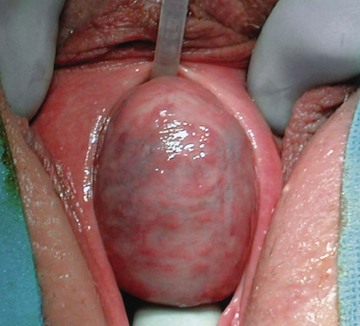
During physical examination, the anterior vaginal wall should be carefully palpated for masses and tenderness. The location, size, and consistency of any suspected UD should be recorded. Most UD are located ventrally over the middle and proximal portions of the urethra, corresponding to the area of the anterior vaginal wall 1 to 3 cm inside the introitus (Fig.78–17). However, UD may also be located anterior to the urethra or extend partially or completely around the urethral lumen. These particular configurations may have significant implications when undertaking surgical excision and reconstruction. UD may also extend proximally toward the bladder neck. These UD may produce distortion of the bladder outlet and trigone of the bladder on cystoscopy or on radiographic imaging. Special care should be taken during surgical excision and reconstruction due to concerns for intraoperative bladder and ureteral injury, as well as the potential development of postoperative voiding dysfunction and urinary incontinence. Distal vaginal masses or perimeatal masses may represent other lesions, including abnormalities of Skene glands (see later discussion). The differentiation between these lesions sometimes cannot be made on the basis of a physical examination alone and may require additional radiologic imaging. A particularly hard anterior vaginal wall mass may indicate a calculus or malignancy within the UD and mandates further investigation. During physical examination, the urethra may be gently “stripped” or “milked” distally in an attempt to express purulent material or urine from within the UD cavity. Although often described for the evaluation of UD, this maneuver is not successful in producing the characteristic discharge per urethral meatus in the majority of patients (Leach and Bavendam, 1987).
Figure 78–17 Large anterior vaginal wall mass. The urethral catheter is seen superiorly, and a weighted vaginal speculum is seen inferiorly. Scott retractor hooks are seen exposing the anterior vaginal wall in this intraoperative photograph.
The vaginal walls are assessed for atrophy, rugation, and elasticity. Poorly estrogenized, atrophic tissues are important to note if surgery is being considered for definitive treatment. These tissues are mobilized intraoperatively and may be used for flaps during excision and reconstruction. The distal vagina and vaginal introitus are also assessed for capacity. These factors may impact on surgical planning, because a narrow introitus can make surgical exposure difficult and may mandate an episiotomy or other measures. Finally, during physical examination, a provocative maneuver to elicit stress incontinence should be performed, as well as an assessment of the presence or absence of any vaginal prolapse.
Urine Studies
Urine analysis and culture should be performed. The most common organisms isolated in patients with UD is E. coli. However, other gram-negative enteric flora, as well as N. gonorrhea, Chlamydia, streptococci, and staphylococci, are often present (Davis and TeLinde, 1958; Hoffman and Adams, 1965). A sterile urine culture does not exclude infection, because these patients are often on antibiotic therapy at presentation. In patients with irritative symptoms or where a malignancy is suspected, urine cytology can be performed.
Cystourethroscopy
Cystourethroscopy is performed both in an attempt to visualize the UD ostium as well as to rule out other causes for the patient’s LUTS. A flexible fiberoptic cystoscope or specially designed rigid female cystoscope can be extremely helpful in evaluating the female urethra. The short beak of the female cystoscope and the lack of a beak on a flexible cystoscope maintains the discharge of the irrigation solution immediately adjacent to the lens and thus aids in distention of the relatively short (as compared to the male) urethra, permitting improved visualization. It may also be advantageous to compress the bladder neck while simultaneously applying pressure to the diverticular sac with an assistant’s finger. Luminal discharge of purulent material can often be seen with this maneuver or with simple digital compression of the UD during urethroscopy. The UD ostium is most often located posterolaterally at the level of the midurethra but can be very difficult to identify in some patients (Fig. 78–18). The success in identifying a diverticular ostium on cystourethroscopy is highly variable and is reported to be 15% to 89% (Davis and Robinson, 1970; Leach and Bavendam, 1987; Ganabathi et al, 1994). As a note of caution, patients with UD are often highly symptomatic, and endoscopic examination can be very difficult to initiate or complete without anesthesia. Notably, a positive exam may help in surgical planning; however, the failure to locate an ostium on cystourethroscopy should not influence the decision to proceed with further investigations or surgical repair.
Urodynamics
For patients with UD and urinary incontinence or significant voiding dysfunction, a urodynamic study may be helpful in accurately characterizing these symptoms (Bhatia et al, 1981; Reid et al, 1986; Summitt and Stovall, 1992). Urodynamics may document the presence or absence of stress urinary incontinence (SUI) prior to repair. Approximately 50% of women with UD will demonstrate SUI on urodynamic evaluation (Bass and Leach 1991; Ganabathi et al, 1994). A videourodynamic study combines both a voiding cystourethrogram and a urodynamic study, thus consolidating the diagnostic evaluation and decreasing the number of required urethral catheterizations during the patient’s clinical workup. For patients undergoing surgery for UD—with coexistent symptomatic SUI demonstrated on physical examination, urodynamically demonstrable SUI, or in those found to have an open bladder neck on preoperative evaluation—a concomitant anti-incontinence surgery can be offered. Multiple authors have described successful repair of urethral diverticula and stress incontinence in the same operative setting (Bass and Leach, 1991; Swierzewski and McGuire, 1993; Ganabathi et al, 1994; Faerber, 1998). Alternatively, a small number of patients may have evidence of bladder outlet obstruction on urodynamic evaluation due to the obstructive or mass effects of the UD on the urethra. It should be noted that SUI may coexist with obstruction (Bradley and Rovner, 2004) but, nevertheless, both conditions can be treated successfully with a carefully planned and executed operation. Urethral pressure profilometry has also been used by some authors to assess and/or diagnose patients with UD, noting a biphasic pattern or pressure drop at the level of the lesion during the study (Bhatia et al, 1981; Wagner et al, 1986; Summitt and Stovall, 1992).
Imaging
High-quality preoperative imaging is important in the diagnosis and therapy of female urethral diverticula. Aside from its utility as a diagnostic entity, radiologic imaging should also provide an accurate reflection of the relevant anatomy of the UD, including its relationship to the proximal urethra and bladder neck.
Diagnostic Contrast Radiography: PPU, VCUG, and IVU
A number of imaging techniques have been applied to the study of female UD, and no single study can be considered the gold standard or optimal imaging study for the evaluation of UD. Each technique has relative advantages and disadvantages, and the ultimate choice of diagnostic study in many centers often depends on several factors, including local availability, cost, and the experience and expertise of the radiologist. Currently available techniques for the evaluation of UD include double-balloon positive-pressure urethrography (PPU), voiding cystourethrography (VCUG), intravenous urography (IVU), ultrasonography (US), and magnetic resonance imaging (MRI) with or without an endoluminal coil (eMRI).
Historically, double-balloon PPU had been considered to be the optimal study for the diagnosis and assessment of female UD (Davis and Cian, 1952; Davis and TeLinde, 1958; Greenberg et al, 1981; Ganabathi et al, 1994). In this technique, a highly specialized catheter with two balloons separated by several centimeters is inserted into the female urethra (Fig. 78–19). This catheter contains a channel within the catheter that exits through a side hole between the two balloons. One balloon is positioned adjacent to the external urethral meatus, and the other balloon is situated at the bladder neck. Both balloons are inflated, creating a seal about the urethral lumen. Contrast is then infused through the channel under slight pressure, distending the urethral lumen between the two balloons and forcing contrast into the UD, thereby opacifying the cavity. This highly specialized study provides outstanding images of the urethra and UD and, importantly unlike VCUG, is not dependent on the patient successfully voiding during the study. However, PPU is not widely performed clinically.
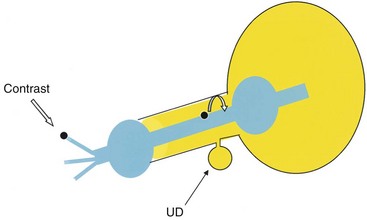
Figure 78–19 Schematic diagram of a double-balloon catheter. The curved white arrow represents flow of the contrast as it enters the urethral lumen. UD, urethral diverticulum.
As an alternative to PPU, VCUG may provide excellent imaging of UD (Fig. 78–20). It is widely available and is a familiar diagnostic technique to most radiologists. Sensitivity for UD with this technique varies from 44% to 95% (Ganabathi et al, 1994; Jacoby and Rowbotham, 1999). Patients often will have difficulty in initiating micturition in the radiology suite due to the pain associated with urethral catheterization, psychogenic inhibition due to voiding in the presence of others, or other factors. In the absence of voiding, the urethral diverticulum will not be seen. Therefore a VCUG which does not demonstrate a urethral diverticulum but did not contain voiding images or postvoid images is nondiagnostic. If the patient is unable to void under fluoroscopy during the VCUG, an attempt should be made to void in the privacy of an adjacent bathroom. If voiding in private was successful, a postvoid film taken under these circumstances will likely show a collection of contrast inferior to the bladder demonstrating the urethral diverticulum. Unfortunately, an inability to generate an adequate flow rate during the VCUG will result in suboptimal filling of the UD and an underestimation of its size and complexity (Fig. 78–21). Three-dimensional CT VCUG with reconstructions is being investigated as a novel imaging technique for UD but is not yet widely available clinically (Kim et al, 2005).
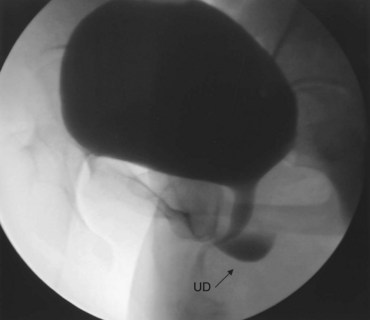
Figure 78–20 Voiding image from a voiding cystourethrogram demonstrating a urethral diverticulum (UD).
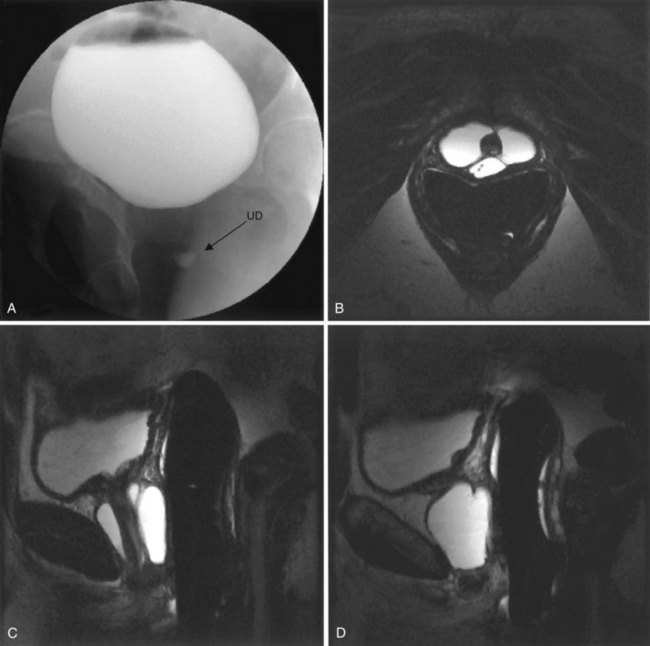
Figure 78–21 Voiding cystourethrogram (VCUG) and MRI from a patient with a large circumferential urethral diverticulum (UD). The voiding image from the VCUG (A) shows poor opacification of the proximal urethra with suboptimal distention of the UD due to a poor voiding effort. The endoluminal MRI demonstrates the full extent and complexity of the lesion on the T2 axial (B), midline sagittal (C), and parasagittal (D) images.
IVU (intravenous urography) may be considered in patients in whom it is necessary to delineate the upper urinary tract or to evaluate for the possibility of a congenital ectopic ureteral anomaly as the cause of an anterior vaginal wall mass (Blacklock and Shaw, 1982). The post-void film of the urogram can be helpful for the diagnosis of UD in some patients (Stern and Patel, 1976; Goldfarb et al, 1981).
Ultrasonography
This study has been advocated for the preoperative assessment of vaginal masses and UD by multiple authors (Lee and Keller, 1977; Wexler and McGovern, 1980; Baert et al, 1992; Martensson and Duchek, 1994; Chancellor et al, 1995; Vargas-Serrano et al, 1997; Dmochowski, 2001; Fortunato et al, 2001; Lee et al, 2001; Gerrard et al, 2003). Abdominal, transvaginal, translabial, and transurethral techniques have been described. Transvaginal imaging often provides information regarding the size and location of UD. On ultrasonographic imaging, the UD appears as an anechoic or hypoechoic area with enhanced through transmission. Ultrasonography (US) is relatively noninvasive and does not expose the patient to radiation. Another significant advantage of US is that successful imaging of UD does not require voiding. However, US may not produce detailed high-resolution images that demonstrate precise surgical anatomy.
MRI
As an alternative to the radiologic investigations noted previously, MRI permits relatively noninvasive, high-resolution, multiplanar imaging of UD. UD appear as areas of decreased signal intensity on T1 compared with the surrounding soft tissues, and have high signal intensity on T2 images. A distinct advantage of MRI compared with VCUG is that successful imaging of UD is wholly independent of voiding and that it is free from ionizing radiation. Surface coil (Hricak et al, 1991; Kim et al, 1993) (Fig. 78–22) and endoluminal techniques (Siegelman et al, 1997; Wang and Wang, 2000; Blander et al, 2001; Lorenzo et al, 2003) have been described. Endoluminal imaging (eMRI) places the magnetic coil into a body cavity adjacent to the area of interest. This location produces an improved signal-to-noise ratio and high-resolution imaging of these areas (Siegelman et al, 1997; Blander et al, 2001). For the evaluation of UD, the coil is placed intravaginally or intrarectally. Both surface coil MRI and eMRI appear to be superior to VCUG and/or PPU in the evaluation of UD (Kim et al, 1993; Neitlich et al, 1998; Blander et al, 2001), but the technology is expensive and not widely available. Contraindications to MRI for UD are few; these include metallic foreign body fragments, claustrophobia, and an inability to tolerate the endoluminal probe.
Differential Diagnosis of UD: Periurethral Masses Other Than Urethral Diverticula
Periurethral masses other than UD comprise a wide spectrum of conditions that must be differentiated from each other and UD. It may often be possible to make a definitive diagnosis based on history and physical exam alone; in other cases, judicious use of radiographic and cystoscopic studies will be necessary to exclude UD.
Vaginal Leiomyoma
Vaginal leiomyomata are benign mesenchymal tumors of the vaginal wall that arise from smooth muscle elements. They commonly present as a smooth, firm, round mass on the anterior vaginal wall (Fig. 78–23). It is an uncommon lesion, with approximately 300 cases reported in the literature (Young et al, 1991). In a recent series of 79 patients with periurethral masses, 4 (5%) were found to have vaginal leiomyoma (Blaivas et al, 2004). These masses were all apparent on physical examination as freely mobile, firm, nontender masses on the anterior vaginal wall. They may be misdiagnosed as UD (Shirvani and Winters, 2000). Symptoms, if they exist, are usually related to the size of the lesion and include a mass effect, obstruction, pain, and dyspareunia. They commonly present in the fourth to fifth decade. Similar to uterine leiomyoma, these lesions are usually estrogen dependent and have been demonstrated to regress during menopause (Liu, 1988). Excision or enucleation (Young et al, 1991) through a vaginal approach is often curative and is recommended to confirm the diagnosis, exclude malignant histology, and also to alleviate symptoms.
Skene Gland Abnormalities
Skene gland cysts and abscesses are similar lesions that are differentiated based on clinical findings (Fig. 78–24). Both lesions generally present as small, cystic masses just lateral or inferolateral to the urethral meatus. They may be lined with transitional or stratified squamous epithelium. Abscesses may be extremely tender and inflamed, and, in some cases, purulent fluid can be expressed from the ductular orifice. Classically, in contrast to UD, these lesions do not communicate with the urethral lumen. Skene gland cysts are not uncommonly noted in neonatal girls and young to middle-aged female patients (Lee and Kim, 1992). Symptoms may include dysuria, dyspareunia, obstruction, and pain. Differentiation from UD can often be made on physical examination, because these lesions are located relatively distally on the urethra, often distorting the urethral meatus as compared with UD, which most commonly occur over the mid- and proximal urethra. Various treatments for abnormalities of Skene gland abnormalities have been described, including aspiration, marsupialization, incision and drainage, and simple excision.
Figure 78–24 Skene gland cyst in a 19-year-old female. Note the large periurethral mass with displacement of the urethral meatus.
Adenocarcinoma arising in Skene glands has been reported. Because of homology with the prostate, these patients may demonstrate elevated PSA levels that normalize with treatment (Dodson et al, 1994).
Gartner Duct Abnormalities
Gartner duct cysts represent mesonephric remnants and are found on the anterolateral vaginal wall from the cervix to the introitus. Because these are mesonephric remnants, they may drain ectopic ureters from poorly functioning or nonfunctioning upper pole moieties in duplicated systems (Fig. 78–25). They have also been reported with single-system ectopia, although this is much less common in females (Gadbois and Duckett, 1974; Currarino, 1982). It is not clear what proportion of patients with Gartner duct cysts will have ipsilateral renal abnormalities, but upper tract evaluation is recommended. In contrast, approximately 6% of subjects with unilateral renal agenesis will have a Gartner duct cyst (Eilber and Raz, 2003). Up to 50% of patients with Gartner duct cysts and renal dysplasia may also have ipsilateral müllerian duct obstruction (Sheih et al, 1998).
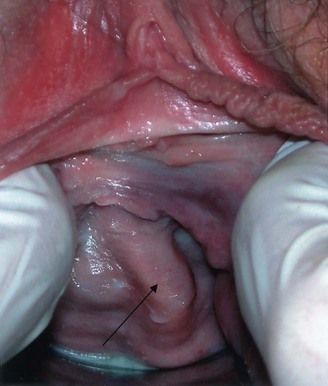
Figure 78–25 Upper pole ectopic ureter in a 39-year-old female being evaluated for lifelong urinary incontinence and recurrent pyelonephritis. A tubelike structure (arrow) representing the turgid, debris-filled ectopic ureter is seen on the anterior vaginal wall.
Treatment depends on symptoms and association with ectopic ureters. If the lesions are asymptomatic and are associated with a nonfunctioning renal moiety, they can be observed. Aspiration and sclerotherapy has been successful (Abd-Rabbo and Atta, 1991). Simple excision or marsupialization has also been recommended for symptomatic lesions. If the cyst is associated with a functioning renal moiety, treatment must be individualized.
Vaginal Wall Cysts
Vaginal wall cysts usually present as small asymptomatic masses on the anterior vaginal wall (Deppisch, 1975) but may enlarge to cause lower urinary tract symptoms or dyspareunia (Fig. 78–26). They may arise from multiple cell types: mesonephric (Gartner duct cysts), paramesonephric (müllerian), endometriotic, urothelial, or epidermoid (inclusion cyst). A specific diagnosis cannot be reliably made until the specimen is removed and examined by a pathologist. The histologic subtype is usually of little consequence, although epidermoid cysts are usually associated with previous trauma or vaginal surgery. Pradhan and Tobon (1986) described the pathologic characteristics of 43 vaginal cysts removed over a 10-year period from 41 women. The derivation of the cyst was müllerian in 44%, epidermoid in 23%, and mesonephric in 11%. The remainder were a Bartholin gland type, endometriotic and indeterminate. As with other periurethral masses, they must be differentiated from UD. Treatment is usually by simple excision in symptomatic patients.
Urethral Mucosal Prolapse
Urethral prolapse presents as a circumferential herniation or eversion of the urethral mucosa at the urethral meatus. The prolapsed mucosa commonly appears as a beefy red “doughnut”-shaped lesion that completely surrounds the urethral meatus. It may be asymptomatic or present with bleeding, spotting, pain, or urinary symptoms. It is commonly noted in two separate populations: postmenopausal women and prepubertal girls. Although thought to be more common in young African-American girls, more recent series do not confirm this predilection (Fernandes et al, 1993; Rudin et al, 1997). In children, it is often causally related to Valsalva or constipation. Eversion of the mucosa may then occur due to a pathologically loose attachment between smooth muscle layers of the urethra (Lowe et al, 1986). Etiology is much less clear for postmenopausal women, although it has been epidemiologically linked to estrogen deficiency. Treatment may be medical or surgical. Medical treatment involves topical creams (estrogen, anti-inflammatory) and/or sitz baths. Various surgical techniques have been described, including cauterization, ligation around a Foley catheter, and complete circumferential excision. Circumferential excision with suture reapproximation of the remaining urethral mucosa to the vaginal wall can be performed with few complications. Rudin and colleagues (1997) reported outcomes in 58 girls with urethral prolapse. Medical treatment was initially successful in 20 patients among whom there were five recurrences. The remaining 38 patients failed initial conservative management and underwent surgical excision with four complications, including urethral stenosis in 2 patients. Jerkins and colleagues (1984) found superior results in surgically treated patients when compared with medical management or catheter ligation.
Urethral Caruncle
Urethral caruncle is an inflammatory lesion of the distal urethra that is most commonly diagnosed in postmenopausal women. It usually appears as a reddish exophytic mass at the urethral meatus, which is covered with mucosa. These lesions are often symptomatic and noted incidentally on gynecologic examination. When irritated, they may cause underwear spotting or become painful. Less commonly they may cause voiding symptoms. Rarely, these lesions may thrombose, resulting in a discolored periurethral mass (Fig. 78–27). Etiologically, they are related to mucosal prolapse. Chronic irritation contributes to hemorrhage, necrosis, and inflammatory growth of the tissue that corresponds to the histology of excised lesions. If the lesion is atypical in appearance or behavior, excision may be warranted to exclude other entities. Intestinal metaplasia, tuberculosis, melanoma, and lymphoma have all been reported to either coexist with or mimic urethral caruncles (Willett and Lack, 1990; Indudhara et al, 1992; Khatib et al, 1993; Lopez et al, 1993; Atalay et al, 1998).
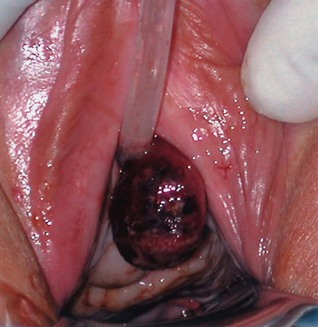
Figure 78–27 Thrombosed urethral caruncle in a postmenopausal female. The Foley catheter is seen in the urethra. Simple excision was curative.
There is a paucity of literature regarding optimal treatment of urethral caruncle. Most authors recommend initial conservative management with topical estrogen or anti-inflammatory creams and sitz baths. Large or refractory lesions may be managed with simple excision. The tip of the lesions should be grasped and traction employed to fully expose the base of the caruncle. The lesion can then easily be excised. If a large defect remains, the mucosa may be reapproximated with absorbable suture. In most instances, the urethral mucosa will heal around a Foley catheter, which may be left in place for several days.
Periurethral Bulking Agents
The transurethral or periurethral injection of bulking agents for the treatment of stress incontinence may result in an anterior vaginal wall mass that appears cystic on imaging, consistent with a urethral diverticulum (Bridges et al, 2005). A careful history will elicit this possibility. Typically, these lesions do not communicate with the urethra and are largely asymptomatic. In the absence of symptoms or complications, many bulking agents will reabsorb with time, and thus observation is all that is required.
Classification of Urethral Diverticula
Although not yet widely adopted, a classification system for UD has been proposed by Leach and colleagues (1993). This staging system for UD, termed the L/N/S/C3 classification system, is similar to that used for cancer staging and is based on several characteristics of UD, including location, number, size, anatomic configuration, site of communication to the urethral lumen, and continence status of the patient. This system attempts to standardize description of UD, but currently it has not been prospectively applied or validated by other authors. Another proposed classification scheme uses the location of the UD as the primary determinant of surgical approach, with distal lesions undergoing marsupialization and more proximal lesions undergoing excision and reconstruction (Ginsburg and Genadry, 1983).
Finally, a classification system proposed by Leng and McGuire (1998) divides UD into two categories based on the presence or absence of a preserved periurethral fascial layer. In some patients with UD who have undergone prior vaginal or urethral surgery, the periurethral fascial layer may be deficient, resulting in a pseudodiverticulum. These authors suggest that the recognition of this anatomic configuration has important implications for surgical reconstruction. These patients may require additional reconstruction or interposition of a tissue flap or graft for reconstruction.
Key Points: Female Urethral Diverticula—Diagnosis
Surgical Repair of Female Urethral Diverticula
Indications for Repair
Although often highly symptomatic, not all urethral diverticula mandate surgical excision. Some patients may be asymptomatic at presentation, with the lesion incidentally diagnosed on imaging for another condition or, perhaps, incidentally noted on routine physical examination. Other patients may be unwilling or medically unable to undergo surgical removal. Very little is known regarding the natural history of untreated urethral diverticula. Whether these lesions will progress in size, symptomatology, or complexity with time is unknown. For these reasons, and due to the lack of symptoms in selected cases, some patients may not desire surgical therapy. However, it should be noted that there are multiple reports in the literature of carcinomas arising in UD (Marshall and Hirsch 1977; Prudente et al, 1978; Tesluk, 1981; Tines et al, 1982; Patanaphan et al, 1983; Gonzalez et al, 1985; Thomas and Maguire, 1991; Rajan et al, 1993; Seballos and Rich, 1995; Hickey et al, 2000), and it is possible that certain carcinomas arising in UD are asymptomatic and may not be prospectively identified on radiologic imaging. Thus patient counseling is necessary in patients who elect primary nonoperative management. Patients electing nonoperative management can be treated with low-dose antibacterial suppressants and digital stripping of the anterior vaginal wall following micturition to prevent postvoid dribbling and reduce the risk of UTI due to stasis in the UD. Whether long-term surveillance is required in these patients, with periodic physical examinations, radiographic imaging, or endoscopic examination, is unknown.
Symptomatic patients may be offered surgical excision, including those with dysuria, refractory bothersome postvoid dribbling, recurrent UTIs, dyspareunia, and pelvic pain, in whom the symptoms can be attributed to the UD. Those with UD and symptomatic stress urinary incontinence can be considered for a concomitant anti-incontinence procedure at the time of UD excision (see later discussion) (Fig. 78–28).
Techniques for Repair
Alternative Techniques
A variety of surgical interventions for urethral diverticula have been reported since 1805 when Hey described transvaginal incision of the UD and packing the resulting cavity with lint. Approaches have included transurethral (Davis and Robinson, 1970) and open (Spence and Duckett, 1970; Roehrborn, 1988) marsupialization, endoscopic unroofing (Lapides, 1978; Spencer and Streem, 1987), fulguration (Saito, 2000), incision and obliteration with oxidized cellulose (Ellick, 1957) or polytetrafluoroethylene (Mizrahi and Bitterman, 1988), coagulation (Mizrahi and Bitterman, 1988), and excision with reconstruction. Most commonly, a complete excision and reconstruction is performed as described below. However, for distal lesions, a transvaginal marsupialization as described by Spence and Duckett may reduce operative time, blood loss, and recurrence rate (Spence and Duckett, 1969, 1970; Roehrborn, 1988). During this procedure, care must be taken to avoid aggressively extending the incision proximally, which could result in vaginal voiding or potentially damage the proximal and distal sphincteric mechanism, resulting in stress urinary incontinence. Another potentially significant complication with the Spence-Duckett procedure is that in sexually active women, a pseudoseptum may be created by the marsupialization of the urethra with respect to the anterior vaginal wall, which may result in dyspareunia. Therefore this approach is probably only applicable to UD in very select cases involving the distal one third of the urethra. It is not commonly performed.
Excision and Reconstruction
Excision and reconstruction is probably the most common surgical approach to UD in the modern era. The principles of the urethral diverticulectomy operation have been well described (Table 78–3). There are only a few minor issues about which some surgeons may disagree, including the type of vaginal incision (inverted “U” vs. inverted “T”), whether it is necessary to remove the entire mucosalized portion of the lesion, and, finally, the optimal type of postoperative catheter drainage (urethra only vs. urethra and suprapubic).
Table 78–3 Principles of Transvaginal Urethral Diverticulectomy (UD)
Complex urethral reconstructive techniques for the repair of UD have been described. Fall (1995) described the use of a bipedicled vaginal wall flap for urethral reconstruction in patients with UD and urethrovaginal fistula. Laterally based vaginal flaps have also been used as an initial approach to UD (Woodhouse et al, 1980; Appell and Suarez, 1982). Complex anatomic configurations may exist, and many novel approaches have been described for complicated anteriorly located or circumferential lesions (Clyne and Flood, 2002; Rovner and Wein, 2003; Vakili et al, 2003).
The technique described herein is similar to that described by Leach and Raz (Leach et al, 1986), based on earlier work by Benjamin and colleagues (1974) and Busch and Carter (1973).
Preoperative Preparation
Prophylactic antibiotics are often used preoperatively to ensure sterile urine at the time of surgery. Patients can also be encouraged to strip the anterior vaginal wall following voiding, thereby consistently emptying the UD and preventing urinary stasis and recurrent UTIs. This may not be possible in those with noncommunicating UD or in those who have significant pain related to the UD. In some patients with postmenopausal atrophic vaginitis, application of topical estrogen creams for several weeks prior to surgery may be beneficial in improving the overall quality of the tissues with respect to planned operative dissection and mobilization. Preoperative parenteral antibiotics are often administered especially for those with recurrent or persistent UTIs.
Urethral diverticulectomy surgery is complex and sometimes quite technically challenging. Further complicating these cases are the associated preoperative symptoms, such as pain, dyspareunia, voiding dysfunction, urinary tract infections, and urinary incontinence. These associated symptoms are often, but not always, improved or eliminated with surgical repair despite even a technically successful surgery. Therefore the importance of appropriate preoperative patient counseling regarding surgery and postoperative expectations of cure cannot be overemphasized.
Patients with symptomatic stress urinary incontinence can be offered simultaneous anti-incontinence surgery. Preoperative videourodynamics may be helpful in evaluating the anatomy of the UD, assessing the competence of the bladder neck, and confirming the diagnosis of stress urinary incontinence. In patients with SUI and UD, Ganabathi and others have described excellent results with concomitant needle bladder neck suspension in these complex patients (Lockhart et al, 1990; Ganabathi et al, 1994). More recently, pubovaginal fascial slings have been used in patients with UD and stress urinary incontinence with satisfactory outcomes (Swierzewski and McGuire, 1993; Faerber, 1998; Romanzi et al, 2000). The role of synthetic midurethral slings, however, has not been well defined in this population. Placement of synthetic material adjacent to a fresh suture line following diverticulectomy in the setting of potentially infected urine may increase the risk of complications, such as urethral erosion and vaginal extrusion of the sling material, as well as urethrovaginal fistula formation.
Procedure
The patient is placed in the lithotomy position with all pressure points well padded. The use of padded adjustable stirrups for the lower extremities greatly enhances operative access to the female perineum. A standard vaginal antiseptic preparation is applied. A weighted vaginal speculum and Scott retractor with hooks aid in exposure. A posterolateral episiotomy may be beneficial in some patients for additional exposure, although the midurethral (and therefore somewhat distal in the vaginal canal) location of most UD usually precludes the need for this type of adjunctive procedure. A Foley catheter is placed per urethra. If desired, a suprapubic tube is placed at the start of the procedure, either using the Lowsley retractor or proceeding percutaneously under direct transurethral cystoscopic visual guidance. Placement of the suprapubic tube at the end of the case is not advisable because this will require traversing the fresh urethral suture line and risk disruption of the repair.
An inverted U is marked along the anterior vaginal wall with the base of the U at the level of the distal urethra and the limbs extending to the bladder neck or beyond (Fig. 78–29A). Care is taken to ensure that the limbs of the U are progressively wider proximally (toward the bladder neck) to ensure adequate vascularity at the distal lateral margins of the anterior vaginal wall flap. Compared with the inverted T incision, the inverted U incision provides excellent exposure laterally at the level of the midvagina and can be extended proximally as needed for lesions that extend beyond the bladder neck. Injectable saline can be infused along the lines of the incision to facilitate dissection. An anterior vaginal wall flap is created by careful dissection in the potential space between the vaginal wall and the periurethral fascia. The use of sufficient counter-traction during this portion of the procedure is important in maintaining the proper plane of dissection. Care is taken to preserve the periurethral fascia and avoid inadvertent entry into the UD.
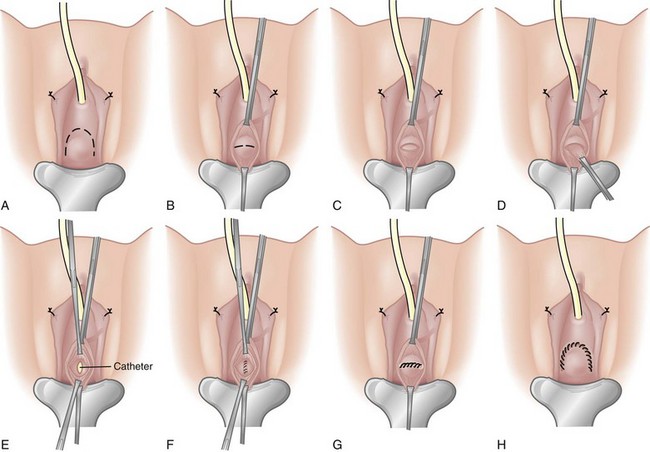
Figure 78–29 A, An inverted U incision is marked on the anterior vaginal wall. Retraction is aided by the use of Allis clamps and a ring retractor with hooks. B, After reflection of the anterior vaginal wall, a transverse incision is made in the periurethral fascia. The dotted line represents the intended incision line. C, The periurethral fascia is incised and dissected from the underlying urethral diverticulum. D, The diverticular sac is freed from the periurethral fascia. E, The urethral catheter is seen after complete excision of the diverticular sac. F, The urethra is closed with absorbable suture. G, The periurethral fascia is closed with care to obliterate any dead space. H, The anterior vaginal wall flap is advanced over the periurethral suture line and secured with running interlocking absorbable suture.
A distinct layer of periurethral fascia is usually interposed between the vaginal wall and the UD. Preservation and later reconstruction of this layer is of paramount importance to prevent recurrence, close dead space, and avoid urethrovaginal fistula formation postoperatively. Pseudodiverticula have been described where this layer of tissue is considerably attenuated or even absent (Leng and McGuire, 1998). In these patients, an interpositional flap or graft, such as a pubovaginal sling, may be used for reconstruction.
The periurethral fascia is incised transversely (Fig. 78–29B). Proximal and distal layers of periurethral fascia are carefully developed, avoiding entrance into the UD. The UD is then grasped and dissected back to its origin on the urethra within the leaves of the periurethral fascia (Fig. 78–29C). In many cases, it is necessary to open the UD to facilitate dissection from the surrounding tissues. The ostium or connection to the urethra is identified and the walls of UD are completely removed. Every effort should be made to remove the entire mucosalized surface of the UD to prevent recurrence (Ganabathi et al, 1994; Fortunato et al, 2001). This may involve removing small adherent or inflamed portions of the urethral wall, especially in the area of the ostium (Fig. 78–29D). All abnormal tissue in the area of the ostium should be removed, if possible, to ensure that no mucosal elements of the UD wall remain, which could result in postoperative urine leakage and recurrence. Elaborate methods of identifying the full extent of the UD cavity have been described, including catheterization of the UD with urinary (Moore, 1952; Kohorn and Glickman, 1992) and Fogarty (Wear, 1976) catheters, packing the UD with gauze (Hyams and Hyams, 1939), infusing and staining the UD with methylene blue (Gilbert and Rivera Cintron, 1954), and the use of silicone (Hirschhorn, 1964) or cryoprecipitate (Feldstein, 1981) to create a solid mass and ease dissection. However, these measures are mostly of historical interest only and are usually not necessary in modern UD surgery (Leach and Bavendam, 1987; Ganabathi et al, 1994).
The Foley catheter is usually seen following complete excision of UD (Fig. 78–29E). The urethra can be reconstructed over as small as a 12-Fr Foley catheter without long-term risk of urethral stricture (Young et al, 1996) and should be closed in a watertight fashion with absorbable suture (Fig. 78–29F). The closure should be tension-free. Uncommonly, a UD may extend circumferentially around the urethra and require segmental resection of the involved portion of the urethra and complex reconstruction (Tamada et al, 2000; Rovner and Wein, 2003).
The periurethral fascial flaps are reapproximated with absorbable suture in a perpendicular orientation to the urethral closure line to minimize overlap and the risk of postoperative urethrovaginal fistula formation (Fig. 78–29G). Care is taken to secure the periurethral fascial flaps so that all dead space is closed.
If desired, a fibrofatty labial (Martius) flap can be harvested at this point and placed over the periurethral fascia as an additional layer of closure (Dmochowski, 2001). Indications for such a flap are not universally agreed upon. However, in those patients with poor-quality tissues, attenuated periurethral fascia, or in whom significant inflammation is encountered intraoperatively, a well-vascularized adjuvant flap, such as a Martius flap, may reduce the risk of wound breakdown and subsequent complications, such as urethrovaginal fistula.
The anterior vaginal wall flap is then repositioned and reapproximated with absorbable suture (Fig. 78–29H). This completes a three-layer closure (four layers if a Martius flap is used). An antibiotic-impregnated vaginal pack is placed.
Postoperative Care
Antibiotics are continued for 24 hours postoperatively. The vaginal packing is removed and the patient discharged home with closed urinary drainage. Antispasmodics are used liberally to reduce bladder spasms. A pericatheter VCUG is obtained at 14 to 21 days postoperatively. If there is no extravasation, the catheters are removed. If extravasation is seen, then repeat pericatheter VCUGs are performed weekly until resolution is noted. In the vast majority of cases, extravasation will resolve in several weeks with this type of conservative management (Schwab and Rovner, 2003).
Complications
Careful adherence to the principles of transvaginal urethral diverticulectomy should minimize postoperative complications. Nevertheless, complications may arise (Table 78–4). One small series suggested that large diverticula (>4 cm) or those associated with a lateral or horseshoe configuration may be associated with a greater likelihood of postoperative complications (Porpiglia et al, 2002). Common complications include recurrent UTIs, urinary incontinence, or recurrent UD. Urethrovaginal fistula is a devastating complication of urethral diverticulectomy and deserves special mention. A urethrovaginal fistula located beyond the sphincteric mechanism should not be associated with symptoms other than perhaps a split urinary stream and/or vaginal voiding. As such, an asymptomatic distal urethrovaginal fistula may not require repair, although some patients may request repair. Conversely, a proximal fistula located at the bladder neck or at the midurethra in patients with an incompetent bladder neck will likely result in considerable symptomatic urinary leakage. These patients should undergo repair with consideration for the use of an adjuvant tissue flap, such as a Martius flap, to provide a well-vascularized additional tissue layer. The actual timing of the repair relative to the initial procedure is controversial. Meticulous attention to surgical technique, good hemostasis, avoidance of infection, preservation of the periurethral fascia (Fig. 78–30A and B) and a well-vascularized anterior vaginal wall flap, and a multilayered closure with nonoverlapping suture lines should minimize the potential for postoperative urethrovaginal fistula formation.
Table 78–4 Complications of Transvaginal Urethral Diverticulectomy (UD)
| Complication (% Range of Reported Incidence) |
Adapted from Dmochowski R. Surgery for vesicovaginal fistula, urethrovaginal fistula, and urethral diverticulum. In: Walsh PC, Retik AB, Vaughan ED Jr, et al, editors. Campbell’s urology. 8th ed. Philadelphia: WB Saunders; 2002.
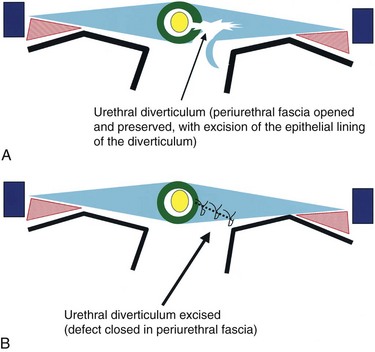
Figure 78–30 Diagrams demonstrating the importance of preserving and reconstructing the periurethral fascia. A, The periurethral fascia has been opened and the urethral diverticulum has been excised. B, The periurethral fascia has been closed.
Significant postoperative de novo stress urinary incontinence may occur in 7% to 16% of individuals undergoing urethral diverticulectomy surgery without a concomitant anti-incontinence surgery (Han et al, 2007; Ljungqvist and Fall, 2007; Stav et al, 2008). However, Lee and colleagues (2008) noted at least minor de novo SUI in 49% of patients following urethral diverticulectomy, the majority of which was minor and did not require additional therapy. Only 10% of these individuals underwent a subsequent SUI operation. Risk factors for de novo SUI may include the size of the diverticulum (>30 mm) and more proximal location (Stav et al, 2008).
Persistence of Symptoms Following Urethral Diverticulectomy
Some patients will have persistence or recurrence of their preoperative symptoms postoperatively. The finding of a UD following a presumably successful urethral diverticulectomy may occur as a result of a new medical problem (e.g., UTI, etc.), a new UD, or, alternatively, as a result of recurrence of the original lesion. Recurrence of UD may be due to incomplete removal of the UD, inadequate closure of the urethra or residual dead space, or other technical factors. Lee (1983) noted recurrent urethral diverticulum in 8 of 85 patients at follow-up of 2 to 15 years from the initial UD resection, while Ljungqvist and colleagues (2007) reported recurrence in 11 of 68 patients during a 26-year follow-up (Ljungqvist and Fall, 2007). The risk of recurrence of UD following transvaginal excision may be related to the complexity of the anatomic configuration. Han and colleagues (2007) reported no recurrent UD in 17 patients with simple UD, but of the 10 patients with circumferential UD, recurrence was noted in 6 (60%). Notably in this series, secondary procedures were not as successful in completely removing the UD. Ockrim and colleagues (2009) similarly cured all 19 patients presenting with simple urethral diverticula on the first attempt, but the 11 patients with complex anatomic configurations required a total of 17 procedures for success. Repeat urethral diverticulectomy surgery can be challenging due to altered anatomy, scarring, and the difficulty in identifying the proper anatomic planes. Complications such as fistula and recurrence of the UD are more common in reoperative cases (Ljungqvist and Fall, 2007).
Female Urethral Diverticula and Associated Conditions
Malignant and benign tumors may be found in urethral diverticula. Approximately 10% of urethral diverticulectomy specimens may demonstrate significant histopathologic abnormalities, including metaplasia, dysplasia, or frank carcinoma that require long-term follow-up or additional therapy (Thomas et al, 2008). Less than 100 cases of carcinoma within UD have been reported in the English-language literature (Rajan et al, 1993). The most common malignant pathology in UD is adenocarcinoma, followed by transitional cell and squamous cell carcinomas (Rajan et al, 1993; Thomas et al, 2008). This is in direct contrast to primary urethral carcinoma, in which the primary histologic type is squamous cell carcinoma. Some authors have suggested that UD is associated with the development of urethral adenocarcinoma in the female (Oliva and Young, 1996). If this is true, then nonexcisional therapy of UD, such as marsupialization or endoscopic incision, should always be combined with a biopsy to rule out malignancy (McLoughlin, 1975). There is no consensus on proper treatment in these cases, and recurrence rates are high with local treatment alone (Rajan et al, 1993). The incidental finding of malignancy in these cases can be particularly troubling when found intraoperatively, or even more disturbing on the postoperative pathology report. Although it is interesting to speculate, it has not been conclusively demonstrated that any particular preoperative imaging modality, such as ultrasonography or MRI can reliably and prospectively diagnose a small malignancy arising in a UD. When considering curative therapy, it is unclear whether extensive surgery, including cystourethrectomy with or without adjuvant external beam radiotherapy, is superior to local excision followed by radiotherapy (Patanaphan et al, 1983).
Multiple benign lesions, including both nephrogenic adenoma and endometriosis, have been described within urethral diverticula (Palagiri, 1978; Peterson and Matsumoto, 1978; Piazza et al, 1987; Paik and Lee, 1997). Pathologically, nephrogenic adenoma can be difficult to differentiate from adenocarcinoma.
Calculi within UD are not uncommon and may be diagnosed in 4% to 10% of cases (Ward et al, 1967; Ginesin et al, 1988; Romanzi et al, 2000) and is most likely due to urinary stasis and/or infection (Fig. 78–31). This may be suspected by physical exam findings or noted incidentally on imaging evaluation. The presence of a stone will not significantly alter the evaluation or surgical approach and can be considered an incidental finding. The stone is removed with the UD specimen at the time of surgery.
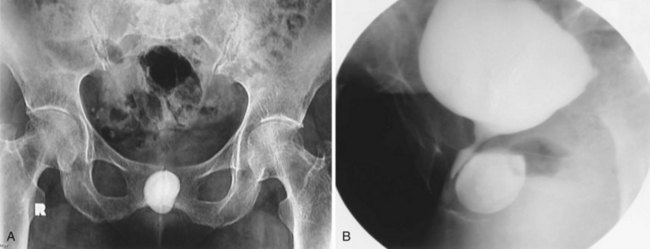
Figure 78–31 Calculi within a urethral diverticulum. A, Scout film shows a calcified density overlying the symphysis pubis. B, Voiding images from a voiding cystourethrogram demonstrates that the density seen on the scout film represents a stone within a urethral diverticulum.
UD has also been reported to present during pregnancy. Moran reported four cases of UD diagnosed during pregnancy (Moran et al, 1998). Conservative treatment included antibiotics and aspiration or incision and drainage. Two women delivered vaginally and the other two delivered by cesarean section for unrelated reasons. In one patient, drainage was performed during labor to facilitate delivery. Three of the four women had definitive repair performed after delivery. It is not known if pregnancy is associated with formation of UD, although patients may be more likely to become symptomatic during this period. Usually, conservative management with antibiotics may be desirable until after delivery to avoid precipitating premature labor, although successful surgical treatment during pregnancy has been reported (Wittich, 1997).
Bauer SB, Retik AB. Bladder diverticula in infants and children. Urology. 1974;3(6):712-715.
Garzotto MG, Tewari A, Wajsman Z. Multimodal therapy for neoplasms arising from a vesical diverticulum. J Surg Oncol. 1996;62(1):46-48.
Gil-Vernet JM. Bladder diverticulectomy. In: Graham SD, editor. Glenn’s urologic surgery. 5th ed. Philadelphia: Lippincott-Raven; 1998:205-209.
Davis HJ, TeLinde RW. Urethral diverticula: an assay of 121 cases. J Urol. 1958;80:34-39.
Eilber KS, Raz S. Benign cystic lesions of the vagina: a literature review. J Urol. 2003;170(3):717-722.
Ganabathi K, Leach GE, Zimmern PE, Dmochowski R. Experience with the management of urethral diverticulum in 63 women. J Urol. 1994;152(5 Pt. 1):1445-1452.
Lee RA. Diverticulum of the urethra: clinical presentation, diagnosis, and management. Clin Obstet Gynecol. 1984;27(2):490-498.
Abd-Rabbo MS, Atta MA. Aspiration and tetracycline sclerotherapy: a novel method for management of vaginal and vulval Gartner cysts. Int J Gynaecol Obstet. 1991;35(3):235-237.
Abdel-Hakim AM, El Feel A, Abouel-Fettouh H, Saad I. Laparoscopic vesical diverticulectomy. J Endourol. 2007;21(1):85-89.
Adachi M, Nakada T, Suzuki H, et al. Successful repair of huge bladder diverticulum with a transurethral fulguration. Report of a case. Urol Int. 1991;46(1):87-89.
Adachi M, Nakada T, Yamaguchi T, et al. Transurethral treatment of bladder diverticula. Eur Urol. 1991;19(2):104-108.
Adot Zurbano JM, Salinas CJ, Dambros M, et al. [Urodynamics of the bladder diverticulum in the adult male]. [Spanish]. Arch Esp Urol. 2005;58(7):641-649.
Ahmed S, Tan H. Complications of transverse advancement ureteral reimplantation: diverticulum formation. J Urol. 1982;127(5):970-973.
Aldridge CWJr, Beaton JH, Nanzig RP. A review of office urethroscopy and cystometry. Am J Obstet Gynecol. 1978;131(4):432-437.
Amar AD. Vesicoureteral reflux associated with congenital bladder diverticulum in boys and young men. J Urol. 1972;107(6):966-968.
Andersen MJ. The incidence of diverticula in the female urethra. J Urol. 1967;98(1):96-98.
Appell RA, Suarez BC. Experience with a laterally based vaginal flap approach for urethral diverticulum. J Urol. 1982;127(4):677-678.
Atalay AC, Karaman MI, Basak T, et al. Non-Hodgkin’s lymphoma of the female urethra presenting as a caruncle. Int Urol Nephrol. 1998;30(5):609-610.
Babbitt DP, Dobbs J, Boedecker RA. Multiple bladder diverticula in Williams Elfin-Facies syndrome. Pediatr Radiol. 1979;8(1):29-31.
Bade JJ, Ypma AF, van Elk P, Mensink HJ. A pelvic mass: bladder diverticulum with haemorrhage in Ehlers-Danlos patient [Review]. Scand J Urol Nephrol. 1994;28(3):319-321.
Baert L, Willemen P, Oyen R. Endovaginal sonography: new diagnostic approach for urethral diverticula. J Urol. 1992;147(2):464-466.
Baniel J, Vishna T. Primary transitional cell carcinoma in vesical diverticula. Urology. 1997;50(5):697-699.
Barrett DM, Malek RS, Kelalis PP. Observations on vesical diverticulum in childhood. J Urol. 1976;116(2):234-236.
Bass JS, Leach GE. Surgical Treatment of Concomitant urethral diverticulum and stress urinary incontinence. Urol Clin NA. 1991;18:365-373.
Bauer SB, Retik AB. Bladder diverticula in infants and children. Urology. 1974;3(6):712-715.
Beall ME, Berger MP. Congenital bladder diverticula in adult twins. Urology. 1978;11(5):498-499.
Bellinger MF, Gross GW, Boal DK. Bladder diverticulum associated with ureteral obstruction. Pediatr Radiol. 1985;15(3):207-228.
Benjamin J, Elliott L, Cooper JF, Bjornson L. Urethral diverticulum in adult female. Clinical aspects, operative procedure, and pathology. Urology. 1974;3(1):1-7.
Bhatia NN, McCarthy TA, Ostergard DR. Urethral pressure profiles of women with urethral diverticula. Obstet Gynecol. 1981;58(3):375-378.
Blacklock AR, Geddes JR, Shaw RE. The treatment of large bladder diverticula. Br J Urol. 1983;55(1):17-20.
Blacklock ARE, Shaw RE, Geddes JR. Late presentation of ectopic ureter. Br J Urol. 1982;54:106-110.
Blaivas JG, Flisser AJ, Bleustein CB, Panagopoulos G. Periurethral masses: etiology and diagnosis in a large series of women. Obstet Gynecol. 2004;103(5 Pt. 1):842-847.
Blander DS, Rovner ES, Schnall MD, et al. Endoluminal magnetic resonance imaging in the evaluation of urethral diverticula in women. Urology. 2001;57(4):660-665.
Blane CE, Zerin JM, Bloom DA. Bladder diverticula in children. Radiology. 1994;190(3):695-697.
Boechat MI, Lebowitz RL. Diverticula of the bladder in children. Pediatr Radiol. 1978;7(1):22-28.
Bolton DM, Joyce G. Vesical diverticulum extending into an inguinal hernia. Br J Urol. 1994;73(3):323-324.
Boyd S, Raz S. Ectopic ureter presenting in midline urethral diverticulum. Urology. 1993;41(6):571-574.
Bradley CS, Rovner ES. Urodynamically defined stress urinary incontinence and bladder outlet obstruction can coexist in women. J Urol. 2004;171:757-761.
Breivik N, Refsum SJr, Oppedal BR, Vesterhus P. Ehlers-Danlos syndrome and diverticula of the bladder. Z Kinderchir. 1985;40(4):243-246.
Bridges MD, Petrou SP, Lightner DJ. Urethral bulking agents: imaging review. AJR Am J Roentgenol. 2005;185(1):257-264.
Bruning EJ. Die Pathologie der weiblichen Urethra und des Parurethrium. Stuttgart: Enke; 1959.
Buchholz NP, Biyabani R, Talati J. Bladder diverticulum as an unusual content of a femoral hernia. Br J Urol. 1998;82(3):457-458.
Burns E. Diverticula of the urinary bladder. Ann Surg. 1944;119:656.
Burrows LJ, Howden NL, Meyn L, Weber AM. Surgical procedures for urethral diverticula in women in the United States, 1979-1997. Int Urogynecol J Pelvic Floor Dysfunct. 2004;16(2):158-161.
Burrows NP, Monk BE, Harrison JB, Pope FM. Giant bladder diverticulum in Ehlers-Danlos syndrome type I causing outflow obstruction. Clin Exp Dermatol. 1998;23(3):109-112.
Busch FM, Carter FH. Vaginal flap incision for urethral diverticula. [Abstract]. Western Section Meeting, American Urological Association; June 29, 1973; Honolulu.
Castillo-Vico MT, Checa-Vizcaino MA, Paya-Panades A, et al. Periurethral granuloma following injection with dextranomer/hyaluronic acid copolymer for stress urinary incontinence. Int Urogynecol J. 2007;18(1):95-97.
Cendron J, Alain JL. [Bladder diverticulum in children without obstruction of the lower urinary tract. Apropos of 43 cases]. [French]. J Urol Nephrol (Paris). 1972;78(10):793-805.
Chancellor MB, Liu JB, Rivas DA, et al. Intraoperative endo-luminal ultrasound evaluation of urethral diverticula. J Urol. 1995;153(1):72-75.
Chertin B, Prat O. Iatrogenic bladder diverticula following caesarean section. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1707-1709.
Clayman RV, Shahin S, Reddy P, Fraley EE. Transurethral treatment of bladder diverticula. Alternative to open diverticulectomy. Urology. 1984;23(6):573-577.
Clemens JQ, Bushman W. Urethral diverticulum following transurethral collagen injection. J Urol. 2001;166(2):626.
Clyne OJ, Flood HD. Giant urethral diverticulum: a novel approach to repair. J Urol. 2002;167(4):1796.
Cuckow PM, Blackhall RJ, Mouriquand PD. Huge bladder diverticula associated with Ehlers-Danlos syndrome. J R Soc Med. 1994;87(5):290-291.
Currarino G. Single vaginal ectopic ureter and Gartner’s duct cyst with ipsilateral renal hypoplasia and dysplasia (or agenesis). J Urol. 1982;128(5):988-993.
Daly WJ, Rabinovitch HH. Urologic abnormalities in Menkes’ syndrome. J Urol. 1981;126(2):262-264.
Daneshgari F, Zimmern PE, Jacomides L. Magnetic resonance imaging detection of symptomatic noncommunicating intraurethral wall diverticula in women. J Urol. 1999;161(4):1259-1261.
Das S. Laparoscopic removal of bladder diverticulum. J Urol. 1992;148(6):1837-1839.
Das S, Amar AD. Vesical diverticulum associated with bladder carcinoma: therapeutic implications. J Urol. 1986;136(5):1013-1014.
Davis BL, Robinson DG. Diverticula of the female urethra: assay of 120 cases. J Urol. 1970;104(6):850-853.
Davis HJ, Cian LG. Positive pressure urethrography: a new diagnostic method. J Urol. 1952;68:611-616.
Davis HJ, TeLinde RW. Urethral diverticula: an assay of 121 cases. J Urol. 1958;80:34-39.
Deppisch LM. Cysts of the vagina: classification and clinical correlations. Obstet Gynecol. 1975;45(6):632-637.
Dmochowski R. Urethral diverticula: evolving diagnostics and improved surgical management. Curr Urol Rep. 2001;2(5):373-378.
Dodson MK, Cliby WA, Keeney GL, et al. Skene’s gland adenocarcinoma with increased serum level of prostate-specific antigen. Gynecol Oncol. 1994;55(2):304-307.
Dondalski M, White EM, Ghahremani GG, Patel SK. Carcinoma arising in urinary bladder diverticula: imaging findings in six patients. AJR Am J Roentgenol. 1993;161(4):817-820.
Dragsted J, Nilsson T. Urothelial carcinoma in a bladder diverticulum evaluated by transurethral ultrasonography. Scand J Urol Nephrol. 1985;19(2):153-154.
Durfee SM, Schwartz LH, Panicek DM, Russo P. MR imaging of carcinoma within urinary bladder diverticulum. Clin Imaging. 1997;21(4):290-292.
Eilber KS, Raz S. Benign cystic lesions of the vagina: a literature review. J Urol. 2003;170(3):717-722.
Ellick M. Diverticulum of the female urethra: a new method of ablation. J Urol. 1957;77:243-246.
Epstein ES, Siegel MJ, Manley CB. Acute urinary retention caused by vesical diverticula. Urol Radiol. 1982;4(4):263-265.
Evangelidis A, Castle EP, Ostlie DJ, et al. Surgical management of primary bladder diverticula in children. J Pediatr Surg. 2005;40(4):701-703.
Faerber GJ. Urethral diverticulectomy and pubovaginal sling for simultaneous treatment of urethral diverticulum and intrinsic sphincter deficiency. Tech Urol. 1998;4(4):192-197.
Fall M. Vaginal wall bipedicled flap and other techniques in complicated urethral diverticlum and urethrovaginal fistula. J Amer Coll Surg. 1995;180:150-156.
Faramarzi-Roques R, Calvet C, Gateau T, Ballanger PH. Surgical treatment of bladder diverticula: laparoscopic approach. J Endourol. 2004;18(1):69-72.
Faysal MH, Freiha FS. Primary neoplasm in vesical diverticula. A report of 12 cases. Br J Urol. 1981;53(2):141-143.
Feldstein MS. Cryoprecipitate coagulum as an adjunct to surgery for diverticula of the female urethra. J Urol. 1981;126:698-699.
Fellows FJ. The association between vesical carcinoma and diverticulum of the bladder. Eur Urol. 1978;4(3):185-186.
Fernandes ET, Dekermacher S, Sabadin MA, Vaz F. Urethral prolapse in children. Urology. 1993;41(3):240-242.
Firstater M, Farkas A. Transvesical submucosal diverticulectomy. Experience with 48 cases. Urology. 1977;10(5):436-438.
Fortunato P, Schettini M, Gallucci M. Diagnosis and therapy of the female urethral diverticula. Int Urogynecol J. 2001;12(1):51-57.
Gadbois WF, Duckett JWJr. Gartner’s duct cyst and ipsilateral renal agenesis. Urology. 1974;4(6):720-721.
Ganabathi K, Leach GE, Zimmern PE, Dmochowski R. Experience with the management of urethral diverticulum in 63 women. J Urol. 1994;152(5 Pt. 1):1445-1452.
Garat JM, Angerri O, Caffaratti J, et al. Primary congenital bladder diverticula in children. Urology. 2007;70(5):984-988.
Garzotto MG, Tewari A, Wajsman Z. Multimodal therapy for neoplasms arising from a vesical diverticulum. J Surg Oncol. 1996;62(1):46-48.
Gearhart JP. Exstrophy, epispadias, and other bladder anomalies. In: Walsh PC, Retik AB, Vaughan EDJr, et al, editors. Campbell’s urology. 8th ed. Philadelphia: WB Saunders; 2002:2136-2196.
Gerrard ERJr, Lloyd LK, Kubricht WS, Kolettis PN. Transvaginal ultrasound for the diagnosis of urethral diverticulum. J Urol. 2003;169(4):1395-1397.
Gil-Vernet JM. Bladder diverticulectomy. In: Graham SDJr, editor. Glenn’s urologic surgery. 5th ed. Philadelphia: Lippincott-Raven; 1998:205-209.
Gilbert CR, Rivera-Cintron FJ. Urethral diverticula in the female; review of the subject and introduction of a different surgical approach. Am J Obstet Gynecol. 1954;67(3):616-627.
Gillon G, Nissenkorn I, Servadio C. Bladder diverticula in elderly females with urgency, dysuria and incontinence. Eur Urol. 1988;14(1):34-36.
Ginesin Y, Bolkier M, Nachmias J, Levin DR. Primary giant calculus in urethral diverticulum. Urol Int. 1988;43(1):47-48.
Ginsburg D, Genadry R. Suburethral diverticulum: classification and therapeutic considerations. Obstet Gynecol. 1983;61(6):685-688.
Glassman TA, Weinerth JL, Glenn JF. Neonatal female urethral diverticulum. Urology. 1975;5(2):249-251.
Goldfarb S, Mieza M, Leiter E. Postvoid film of intravenous pyelogram in diagnosis of urethral diverticulum. Urology. 1981;17(4):390-392.
Golijanin D, Yossepowitch O, Beck SD, et al. Carcinoma in a bladder diverticulum: presentation and treatment outcome. J Urol. 2003;170(5):1761-1764.
Gonzalez MO, Harrison ML, Boileau MA. Carcinoma in diverticulum of female urethra. Urology. 1985;26(4):328-332.
Greenberg M, Stone D, Cochran ST, et al. Female urethral diverticula: double-balloon catheter study. AJR Am J Roentgenol. 1981;136(2):259-264.
Han DH, Jeong YS, Choo MS, Lee KS. Outcomes of surgery of female urethral diverticula classified using magnetic resonance imaging. Eur Urol. 2007;51(6):1664-1670.
Harcke HTJr, Capitanio MA, Grover WD, Valdes-Dapena M. Bladder diverticula and Menkes’ syndrome. Radiology. 1977;124(2):459-461.
Hernandez J, Waguespack RL, Horton M, Rozanski TA. Acute urinary retention due to an iatrogenic bladder diverticulum. J Urol. 1997;158(5):1907.
Hernanz-Schulman M, Lebowitz RL. The elusiveness and importance of bladder diverticula in children. Pediatr Radiol. 1985;15(6):399-402.
Hey W. Practical observations in surgery. Philadelphia: James Humphries; 1805.
Hickey N, Murphy J, Herschorn S. Carcinoma in a urethral diverticulum: magnetic resonance imaging and sonographic appearance. Urology. 2000;55(4):588-589.
Hinman F. Female genital tract and urethra. In: Hinman FJr, editor. Atlas of urosurgical anatomy. Philadelphia: WB Saunders; 1993:389-416.
Hirschhorn RC. A new surgical technique for removal of urethral diverticula in the female patient. J Urol. 1964;92:206-209.
Hoffman MJ, Adams WE. Recognition and repair of urethral diverticula: a report of 60 cases. Am J Obstet Gynecol. 1965;92:106-111.
Hofmann R, Hegemann M, Mauermayer W, Endres M. Hereditary autosomal dominant form of bladder diverticula in male patients. J Urol. 1984;131(2):338-339.
Hricak H, Secaf E, Buckley DW, et al. Female urethra: MR imaging. Radiology. 1991;178(2):527-535.
Huffman JW. The detailed anatomy of the paraurethral ducts in the adult human female. Am J Obstet Gynecol. 1948;55:86.
Hutch JA. Vesico-ureteral reflux in the paraplegic. Cause and correction. J Urol. 1952;68:457-467.
Hutch JA. Saccule formation at the ureterovesical junction in smooth walled bladders. J Urol. 1961;86:390-399.
Hutch JA, Ayers RD, Loquvan GS. The bladder musculature with special reference to the ureterovesical junction. J Urol. 1961;85:531-539.
Hyams JA, Hyams MN. A new operative procedure for the treatment of diverticulum of the female urethra. J Urol. 1939;43:573-577.
Indudhara R, Vaidyanathan S, Radotra BD. Urethral tuberculosis. Urol Int. 1992;48(4):436-438.
Itoh N, Kounami T. Spontaneous rupture of a bladder diverticulum: ultrasonographic diagnosis. J Urol. 1994;152(4):1206-1207.
Jacoby K, Rowbotham RK. Double balloon positive pressure urethrography is a more sensitive test than voiding cystourethrography for diagnosing urethral diverticulum in women. J Urol. 1999;162(6):2066-2069.
Jarrett TW, Pardalidis NP, Sweetser P, et al. Laparoscopic transperitoneal bladder diverticulectomy: surgical technique. J Laparoendosc Surg. 1995;5(2):105-111.
Jerkins GR, Verheeck K, Noe HN. Treatment of girls with urethral prolapse. J Urol. 1984;132(4):732-733.
Johnson CM. Diverticula and cyst of the female urethra. J Urol. 1938;39:506-516.
Johnston JH. Vesical diverticula without urinary obstruction in childhood. J Urol. 1960;84:535.
Jorion JL, Michel M. Spontaneous rupture of bladder diverticula in a girl with Ehlers-Danlos syndrome. J Pediatr Surg. 1999;34(3):483-484.
Kaneti J, Sober I, Bar-Ziv J, Barki Y. Congenital anterior urethral diverticulum. Eur Urol. 1984;10(1):48-52.
Keeler LL, Sant GR. Spontaneous rupture of a bladder diverticulum. J Urol. 1990;143(2):349-351.
Khan MS, Uwechue EJ, O’Brien A. Acute renal failure due to bladder diverticula. Br J Urol. 1994;73(4):466-467.
Khatib RA, Khalil AM, Tawil AN, et al. Non-Hodgkin’s lymphoma presenting as a urethral caruncle. Gynecol Oncol. 1993;50(3):389-393.
Kim B, Hricak H, Tanagho EA. Diagnosis of urethral divert icula in women: value of MR imaging. AJR Am J Roentgenol. 1993;161(4):809-815.
Kim SH, Kim SH, Park BK, et al. CT voiding cystourethrography using 16-MDCT for the evaluation of female urethral diverticula: initial experience. AJR Am J Roentgenol. 2005;184(5):1594-1596.
Kimbrough HMJr, Vaughan EDJr. Skene’s duct cyst in a newborn: case report and review of the literature. J Urol. 1977;117(3):387-388.
Kirks DR, Grossman H. Congenital saccular anterior urethral diverticulum. Radiology. 1981;140(2):367-372.
Klyszejko C, Ilnicki W, Klyszejko D, et al. Development of a urethral diverticulum after forceps delivery. Ginekol Pol. 1985;56:766-769.
Kohorn EI, Glickman MG. Technical aids in investigation and management of urethral diverticula in the female. Urology. 1992;40(4):322-325.
Kwan DJ, Lowe FC. Congenital bladder diverticulum: an unusual presentation with abdominal mass, urinary retention, and renal failure in a young adult. Urol Radiol. 1992;14(3):194-196.
Lang ED, Davis HJ. Positive pressure urethrography: a roentgenographic diagnostic method for urethral diverticula in the female. Radiology. 1959;72:410.
Lapides J. Transurethral treatment of urethral diverticula in women. Trans Am Assoc Genitourin Surg. 1978;70:135-137.
Lau JT, Ong GB. Congenital diverticulum of the anterior urethra. ANZ J Surg. 1981;51(3):305-306.
Leach GE, Bavendam TG. Female urethral diverticula. Urology. 1987;30(5):407-415.
Leach GE, Schmidbauer H, Hadley HR, et al. Surgical treatment of female urethral diverticulum. Semin Urol. 1986;4:33.
Leach GE, Sirls LT, Ganabathi K, Zimmern PE. L N S C3: a proposed classification system for female urethral diverticula. Neurourol Urodyn. 1993;12(6):523-531.
Lebowitz RL, Colodny AH, Crissey M. Neonatal hydronephrosis caused by vesical diverticula. Urology. 1979;13(3):335-341.
Lee DI, Bagley DH, Liu JB. Experience with endoluminal ultrasonography in the urinary tract. J Endourol. 2001;15(1):67-74.
Lee NH, Kim SY. Skene’s duct cysts in female newborns. J Pediatr Surg. 1992;27(1):15-17.
Lee RA. Diverticulum of the female urethra: postoperative complications and results. Obstet Gynecol. 1983;61(1):52-58.
Lee RA. Diverticulum of the urethra: clinical presentation, diagnosis, and management. Clin Obstet Gynecol. 1984;27(2):490-498.
Lee TG, Keller FS. Urethral diverticulum: diagnosis by ultrasound. AJR Am J Roentgenol. 1977;128(4):690-691.
Lee UJ, Goldman H, Moore C, et al. Rate of de novo stress urinary incontinence after urethal diverticulum repair. Urology. 2008;71(5):849-853.
Leng WW, McGuire EJ. Management of female urethral diverticula: a new classification. J Urol. 1998;160(4):1297-1300.
Levard G, Aigrain Y, Ferkadji L, et al. Urinary bladder diverticula and the Ehlers-Danlos syndrome in children. J Pediatr Surg. 1989;24(11):1184-1186.
Lewis DD, Woods SE. Fetal alcohol syndrome. Am Fam Physician. 1994;50(5):1025-1032. 1035–6
Liu MM. Fibromyoma of the vagina. Eur J Obstetr Gynecol Reprod Biol. 1988;29(4):321-328.
Livne PM, Gonzales ETJr. Congenital bladder diverticula causing ureteral obstruction. Urology. 1985;25(3):273-276.
Ljungqvist LPR, Fall M. Female urethral diverticulum: 26-year followup of a large series. J Urol. 2007;177:219-224.
Lockhart JL, Ellis GF, Helal M, Pow-Sang JM. Combined cystourethropexy for the treatment of type 3 and complicated female urinary incontinence. J Urol. 1990;143(4):722-725.
Lopez JI, Angulo JC, Ibanez T. Primary malignant melanoma mimicking urethral caruncle. Case report. Scand J Urol Nephrol. 1993;27(1):125-126.
Lorenzo AJ, Zimmern P, Lemack GE, Nurenberg P. Endorectal coil magnetic resonance imaging for diagnosis of urethral and periurethral pathologic findings in women. Urology. 2003;61(6):1129-1133.
Lowe FC, Goldman SM, Oesterling JE. Computerized tomography in evaluation of transitional cell carcinoma in bladder diverticula. Urology. 1989;34(6):390-395.
Lowe FC, Hill GS, Jeffs RD, Brendler CB. Urethral prolapse in children: insights into etiology and management. J Urol. 1986;135(1):100-103.
Macejko AM, Viprakasit DP, Nadler RB. Cystoscope- and robot-assisted bladder diverticulectomy. J Endourol. 2008;22(10):2389-2391.
MacKinnon M, Pratt JH, Pool T. Diverticulum of the female urethra. Surg Clin North Am. 1959;39:953-962.
Mallampati GK, Siegelman ES. MR imaging of the bladder, Magn Reson Imaging. Clin North Am. 2004;12(3):545-555.
Marshall S. Urethral diverticula in young girls. Urology. 1981;17(3):243-245.
Marshall S, Hirsch K. Carcinoma within urethral diverticula. Urology. 1977;10(2):161-163.
Martensson O, Duchek M. Translabial ultrasonography with pulsed colour-Doppler in the diagnosis of female urethral diverticula. Scand J Urol Nephrology. 1994;28(1):101-104.
McLoughlin MG. Carcinoma in situ in urethral diverticulum: pitfalls of marsupialization alone. Urology. 1975;6(3):343.
McNally A. Diverticula of the female urethra. Am J Surg. 1935;28:177.
Melekos MD, Asbach HW, Barbalias GA. Vesical diverticula: etiology, diagnosis, tumorigenesis, and treatment. Analysis of 74 cases. Urology. 1987;30(5):453-457.
Micic S, Ilic V. Incidence of neoplasm in vesical diverticula. J Urol. 1983;129(4):734-735.
Mitchell RJ, Hamilton SG. Spontaneous perforation of bladder diverticula. Br J Surg. 1971;58(9):712.
Mizrahi S, Bitterman W. Transvaginal, periurethral injection of polytetrafluoroethylene (polytef) in the treatment of urethral diverticula. Br J Urol. 1988;62(3):280.
Montague DK, Boltuch RL. Primary neoplasms in vesical diverticula: report of 10 cases. J Urol. 1976;116(1):41-42.
Moore TD. Diverticulum of the female urethra. An improved technique of surgical excision. J Urol. 1952;68:611-616.
Moran PA, Carey MP, Dwyer PL. Urethral diverticula in pregnancy. ANZ J Obstet Gynaecol. 1998;38(1):102-106.
Myer EG, Wagner JR. Robotic assisted laparoscopic bladder diverticulectomy. J Urol. 2007;178(6):2406-2410.
Neitlich JD, Foster HEJr, Glickman MG, Smith RC. Detection of urethral diverticula in women: comparison of a high resolution fast spin echo technique with double balloon urethrography. J Urol. 1998;159(2):408-410.
Nielsen VM, Nielsen KK, Vedel P. Spontaneous rupture of a diverticulum of the female urethra presenting with a fistula to the vagina. Acta Obstet Gynecol Scand. 1987;66(1):87-88.
Ockrim JL, Allen DJ, Shah J, Greenwell TJ. A tertiary experience of urethral diverticulectomy: diagnosis, imaging and surgical outcomes. BJU Int. 2009;103(11):1550-1554.
Oge O, Gemalmaz H, Ozeren B. Acute urinary retention in a child caused by a congenital bladder diverticulum. J Pediatr Surg. 2002;37(6):926-927.
Oliva E, Young RH. Clear cell adenocarcinoma of the urethra: a clinicopathologic analysis of 19 cases. Mod Pathol. 1996;9(5):513-520.
Orandi A. Transurethral fulguration of bladder diverticulum: new procedure. Urology. 1977;10(1):30-32.
Ostroff EB, Alperstein JB, Young JDJr. Neoplasm in vesical diverticula: report of 4 patients, including a 21-year-old. J Urol. 1973;110(1):65-69.
Paik SS, Lee JD. Nephrogenic adenoma arising in an urethral diverticulum. Br J Urol. 1997;80:150.
Palagiri A. Urethral Diverticulum with endometriosis. Urology. 1978;11:271-272.
Parra RO, Jones JP, Andrus CH, Hagood PG. Laparoscopic diverticulectomy: preliminary report of a new approach for the treatment of bladder diverticulum. J Urol. 1992;148(3):869-871.
Patanaphan V, Prempree T, Sewchand W, et al. Adenocarcinoma arising in female urethral diverticulum. Urology. 1983;22(3):259-264.
Pathak UN, House MJ. Diverticulum of the female urethra. Obstet Gynecol. 1970;36(5):789-794.
Pavlica P, Viglietta G, Losinno F, et al. I diverticoli dell’uretra femminile: studio radiologico ed ecografico. Radiol Med. 1988;75:521-527.
Peters W3rd, Vaughan EDJr. Urethral diverticulum in the female. Etiologic factors and postoperative results. Obstet Gynecol. 1976;47(5):549-552.
Peterson LJ, Matsumoto LM. Nephrogenic adenoma in urethral diverticulum. Urology. 1978;11(2):193-195.
Peterson LJ, Paulson DF, Glenn JF. The histopathology of vesical diverticula. J Urol. 1973;110(1):62-64.
Piazza R, Aragona F, Pizzarella M, et al. Nephrogenic adenoma in urethral diverticulum: an unusual finding. Urol Int. 1987;42(1):69-70.
Pieretti RV, Pieretti-Vanmarcke RV. Congenital bladder diverticula in children. J Pediatr Surg. 1999;34(3):468-473.
Pinkerton JH. Urethral diverticula in women. Br Med J. 1963;5366:1204-1205.
Pool TL, Hacker PK. Vesical diverticulum of the urinary bladder in the female. Arch Surg. 1966;92(2):266-268.
Porpiglia F, Destefanis P, Fiori C, Fontana D. Preoperative risk factors for surgery female urethral diverticula. Our experience. Urol Int. 2002;69(1):7-11.
Porpiglia F, Tarabuzzi R, Cossu M, et al. Is laparoscopic bladder diverticulectomy after transurethral resection of the prostate safe and effective? Comparison with open surgery. J Endourol. 2004;18(1):73-76.
Pradhan S, Tobon H. Vaginal cysts: a clinicopathological study of 41 cases. Int J Gynecol Pathol. 1986;5(1):35-46.
Prudente DT, Dias Montellato NI, Arap S, et al. Carcinoma in diverticulum of female urethra. Urol Int. 1978;33(6):393-398.
Rabin JM, Hirschfield L, Badlani GH. Type IX Ehlers-Danlos syndrome: bladder diverticula with transitional cell carcinoma. Urology. 1991;38(6):563-566.
Rajan N, Tucci P, Mallouh C, Choudhury M. Carcinoma in female urethral diverticulum: case reports and review of management. J Urol. 1993;150:1911-1914.
Redman JF, McGinnis TB, Bissada NK. Management of neoplasms in vesical diverticula. Urology. 1976;7(5):492-494.
Reid RE, Gill B, Laor E, et al. Role of urodynamics in management of urethral diverticulum in females. Urology. 1986;28(4):342-346.
Roehrborn CG. Long-term follow-up study of the marsupialization technique for urethral diverticula in women. Surg Gynecol Obstet. 1988;167(3):191-196.
Romanzi LJ, Groutz A, Blaivas JG. Urethral diverticulum in women: diverse presentations resulting in diagnostic delay and mismanagement. J Urol. 2000;164(2):428-433.
Routh A. Urethral diverticula. BMJ. 1890;1:361-362.
Rovner ES, Wein AJ. Diagnosis and reconstruction of the dorsal or circumferential urethral diverticulum. J Urol. 2003;170(1):82-86.
Rudin JE, Geldt VG, Alecseev EB. Prolapse of urethral mucosa in white female children: experience with 58 cases. J Pediatr Surg. 1997;32(3):423-425.
Saez F, Pena JM, Martinez A, et al. Carcinomas in vesical diverticula: the role of ultrasound. J Clin Ultrasound. 1985;13(1):45-48.
Safir MH, Gousse AE, Raz S. Bladder diverticula causing urinary retention in a woman without bladder outlet obstruction. J Urol. 1998;160(6 Pt. 1):2146-2147.
Saito S. Usefulness of diagnosis by the urethroscopy under anesthesia and effect of transurethral electrocoagulation in symptomatic female urethral diverticula. J Endourol. 2000;14(5):455-457.
Sarihan H, Abes M. Congenital bladder diverticula in infants. Eur Urol. 1998;33(1):101-103.
Scardino PL, Upson TE. Inguinal hernia and bladder diverticulum. J Urol. 1953;69(2):282-283.
Schewe J, Brands EH, Pannek J. The inguinal bladder diverticulum: a rare differential diagnosis of hernias. Int Urol Nephrol. 2000;32(2):255-256.
Schippers E, Dittler HJ. Multiple hollow organ dysplasia in Ehlers-Danlos syndrome. J Pediatr Surg. 1989;24(11):1181-1183.
Schulman SL, Zderic S, Kaplan P. Increased prevalence of urinary symptoms and voiding dysfunction in Williams syndrome. J Pediatr. 1996;129(3):466-469.
Schulze S, Hald T. Voiding inability after transurethral resection of a bladder diverticulum. Scand J Urol Nephrol. 1983;17(3):377-378.
Schwab CW, Rovner ES. Utility of radiologic imaging and prolonged catheterization following complex lower urinary tract reconstruction [Abstract]. Mid Atlantic Section Meeting, American Urological Association; October 26, 2003; Boca Raton, FL.
Seballos RM, Rich RR. Clear cell adenocarcinoma arising from a urethral diverticulum. J Urol. 1995;153(6):1914-1915.
Senger FL, Bottone JJ, Rothfeld SH. Bladder diverticulum in the female. J Urol. 1952;68(4):699-702.
Shah KJ. Bladder diverticulum: an uncommon cause of acute retention of urine in a male child. Br J Radiol. 1979;52(618):504-506.
Sharma R, Mondal A, Sherigar R, et al. Giant diverticulum of urinary bladder causing bilateral hydronephrosis in an adult. Diagnostic features on radionuclide scintigraphy. Clin Nucl Med. 1997;22(6):385-387.
Sheih CP, Li YW, Liao YJ, et al. Diagnosing the combination of renal dysgenesis, Gartner’s duct cyst and ipsilateral mullerian duct obstruction. J Urol. 1998;159(1):217-221.
Sheu JC, Huang YH, Chang PY, et al. Results of surgery for vesicoureteral reflux in children: 6 years’ experience in an Asian country. Pediatr Surg Int. 1998;13(2-3):138-140.
Shirvani AR, Winters JC. Vaginal leiomyoma presenting as a urethral diverticulum. J Urol. 2000;163(6):1869.
Siegelman ES, Banner MP, Ramchandani P, Schnall MD. Multicoil MR imaging of symptomatic female urethral and periurethral disease. Radiographics. 1997;17(2):349-365.
Silk MR, Lebowitz JM. Anterior urethral diverticulum. J Urol. 1969;101:66-67.
Spence HM, Duckett JWJr. Motion picture: simple operation for cure of diverticula of female urethra. Trans Am Assoc Genitourin Surg. 1969;61:78-79.
Spence HM, Duckett JWJr. Diverticulum of the female urethra: clinical aspects and presentation of a simple operative technique for cure. J Urol. 1970;104(3):432-437.
Spencer WF, Streem SB. Diverticulum of the female urethral roof managed endoscopically. J Urol. 1987;138(1):147-148.
Stage KH, Tank ES. Primary congenital bladder diverticula in boys. Urology. 1992;40(6):536-538.
Stav K, Dwyer PL, Rosamilia A, Chao F. Urinary symptoms before and after female urethral diverticulectomy—can we predict de novo stress urinary incontinence? J Urol. 2008;180:2088-2090.
Stephens FD. The vesicoureteral hiatus and paraureteral diverticula. J Urol. 1979;121(6):786-791.
Stern AJ, Patel SK. Diverticulum of the female urethra. The value of the post-void bladder film during excretory urography. Radiology. 1976;121(1):222.
Stewart M, Bretland PM, Stidolph NE. Urethral diverticula in the adult female. Br J Urol. 1981;53(4):353-359.
Sulaiman MN, Buchholz NP, Khan MA. Carcinoma in a bladder diverticulum: the place of diverticulectomy. Arch Ital Urol Androl. 1998;70(4):195-197.
Summitt RLJr, Stovall TG. Urethral diverticula: evaluation by urethral pressure profilometry, cystourethroscopy, and the voiding cystourethrogram. Obstet Gynecol. 1992;80(4):695-699.
Suzuki K, Tanaka O, Saito T, Tokue A. Giant bladder diverticulum due to previous bullet injury: findings of gadolinium-enhanced magnetic resonance imaging. Int J Urol. 2002;9(9):517-519.
Swierzewski SJ3rd, McGuire EJ. Pubovaginal sling for treatment of female stress urinary incontinence complicated by urethral diverticulum. J Urol. 1993;149(5):1012-1014.
Talner LB, O’Reilly PH, Roy C. Specific causes of obstruction. In: Pollack HM, McClennan BL, Dyer RB, Kenney PJ, editors. Clinical urography. 2nd ed. Philadelphia: WB Saunders; 2000:1967-2136.
Tamada S, Iwai Y, Tanimoto Y, et al. [Urethral diverticula surrounding the urethra in women: report of 2 cases]. [Japanese]. Hinyokika Kiyo. 2000;46(9):639-642.
Taylor WN, Alton D, Toguri A, et al. Bladder diverticula causing posterior urethral obstruction in children. J Urol. 1979;122(3):415.
Tesluk H. Primary adenocarcinoma of female urethra associated with diverticula. Urology. 1981;17(2):197-199.
Thomas AA, Rackley RR, Lee U, et al. Urethral diverticula in 90 female patients: a study with emphasis on neoplastic alterations. J Urol. 2008;180(6):2463-2467.
Thomas RB, Maguire B. Adenocarcinoma in a female urethral diverticulum. ANZ J Surgery. 1991;61(11):869-871.
Tines SC, Bigongiari R, Weigel JW. Carcinoma in diverticulum of the female urethra. AJR Am J Roentgenol. 1982;138(3):582-585.
Vakili B, Wai C, Nihira M. Anterior urethral diverticulum in the female: diagnosis and surgical approach. Obstet Gynecol. 2003;102(5 Pt. 2):1179-1183.
Van Arsdalen K, Wein AJ. Bladder diverticulectomy. In: Droller MJ, editor. Surgical management of urologic disease: an anatomic approach. St Louis: Mosby; 1992:629-639.
Vanhoutte JJ. Ureteral ectopia into a Wolffian duct remnant presenting as a urethral diverticulum in two girls. Am J Roentgenol Radium Ther Nucl Med. 1970;110(3):540-545.
Vargas-Serrano B, Cortina-Moreno B, Rodriguez-Romero R, Ferreiro-Arguelles I. Transrectal ultrasonography in the diagnosis of urethral diverticula in women. J Clin Ultrasound. 1997;25(1):21-28.
Verghese M, Belman AB. Urinary retention secondary to congenital bladder diverticula in infants. J Urol. 1984;132(6):1186-1188.
Vitale PJ, Woodside JR. Management of bladder diverticula by transurethral resection: re-evaluation of an old technique. J Urol. 1979;122(6):744-745.
Wagner U, Debus-Thiede G, Christ F. [Significance of the urethral pressure profile in the diagnosis of urethral diverticulum]. Geburtshilfe Frauenheilkd. 1986;46(7):456-458.
Wang AC, Wang CR. Radiologic diagnosis and surgical treatment of urethral diverticulum in women. A reappraisal of voiding cystourethrography and positive pressure urethrography. J Reprod Med. 2000;45(5):377-382.
Ward JN, Draper JW, Tovell HM. Diagnosis and treatment of urethral diverticula in the female. Surg Gynecol Obstet. 1967;125(6):1293-1300.
Wear JB. Urethral diverticulectomy in females. Urol Times. 1976;4:2-3.
Wesselhoeft CWJr, Perlmutter AD, Berg S, et al. Pathogenesis and surgical treatment of diverticulum of the urinary bladder. Surg Gynecol Obstet. 1963;116:719-725.
Wexler JS, McGovern TP. Ultrasonography of female urethral diverticula. AJR Am J Roentgenol. 1980;134(4):737-740.
Willett GD, Lack EE. Periurethral colonic-type polyp simulating urethral caruncle. A case report. J Reprod Med. 1990;35(11):1017-1018.
Williams MJ, Gooding GA. Sonographic diagnosis of a neoplasm in a bladder diverticulum. J Ultrasound Med. 1985;4(4):203-204.
Wilson B, Klufio G. The radiological and urodynamic significance of large bladder diverticula. Clin Radiol. 1985;36(5):521-524.
Wittich AC. Excision of urethral diverticulum calculi in a pregnant patient on an outpatient basis. J Am Osteopath Assoc. 1997;97(8):461-462.
Woodhouse CRJ, Flynn JT, Molland EA, Blandy JP. Urethral diverticulum in females. Br J Urol. 1980;52:305-310.
Yamaguchi K, Kotake T, Nishikawa Y, et al. Transurethral treatment of bladder diverticulum. Urol Int. 1992;48(2):210-212.
Young GPH, Wahle GR, Raz S. Female urethral diverticulum. In: Raz S, editor. Female urology. Philadelphia: WB Saunders; 1996:477-489.
Young SB, Rose PG, Reuter KL. Vaginal fibromyomata: two cases with preoperative assessment, resection, and reconstruction. Obstet Gynecol. 1991;78(5 Pt. 2):972-974.
Yu CC, Huang JK, Lee YH, et al. Intradiverticular tumors of the bladder: surgical implications—an eleven-year review. Eur Urol. 1993;24(2):190-196.