chapter 79 Surgical Procedures for Sphincteric Incontinence in the Male
The Artificial Genitourinary Sphincter and Perineal Sling Procedures
Urinary incontinence (UI), the complaint of any involuntary leakage of urine (Abrams et al, 2002), may be the result of congenital anomalies, injury, genitourinary surgery, and other conditions. The most common etiology of sphincteric UI in men is radical prostatectomy (RP), with the primary mechanism being failure to store urine secondary to inadequate resistance of the outlet sphincter. Despite improvements in surgical technique that have reduced the rate of post-prostatectomy UI, the burden of disease in the United States remains high and is expected to rise because of increasing numbers of RP performed annually (Stanford et al, 1999).
UI significantly compromises heath-related quality of life in men (Coyne et al, 2003) and can be improved by surgical treatment, including transurethral bulking agents, bulbar urethral slings, and the artificial urinary sphincter (AUS). These procedures prevent involuntary urinary loss by increasing outlet resistance. Although bulking agents have been used in the past as first-line treatment for male sphincteric UI, the severity of incontinence and postsurgical scarring in the vesicourethral region postprostatectomy have made surgical correction first-line treatment for the majority of cases.
After a number of generations, several bulbar urethral slings have emerged as viable treatment options for male UI, although the relative paucity of long-term follow-up and unsure reproducibility limits recommendations about the efficacy and broad applicability of sling procedures. The AUS remains the gold standard for the treatment of UI in males due to its long-term durability and effectiveness across the spectrum of moderate and severe degrees of urinary loss.
Classification, Pathophysiology, and Etiology
Urinary continence in the male depends on a compliant and contractile bladder body, functional posterior urethra, including the bladder neck and prostate (internal sphincter) and an intact rhabdosphincter (external sphincter). Thus stress urinary incontinence develops only in men with concomitant internal and external sphincter impairment. Internal sphincter incompetence results from pelvic surgery, bladder neck injury, specific sympathetic neuropathic dysfunction, or embryologic disruption. Incompetence of the external sphincter, known as intrinsic sphincter deficiency (ISD), occurs most frequently after radical prostatectomy, but also can result from prostatomembranous urethral distraction injuries, traumatic and acquired myelopathy, and congenital disorders, such as spinal dysraphism, sacral agenesis, and the exstrophy/epispadias complex. Table 79–1 shows conditions in which the bladder neck and rhabdosphincter may be dysfunctional or compromised. Associated bladder dysfunction, including decreased compliance or detrusor overactivity, may complicate or confound the diagnosis and management of male UI and requires careful evaluation (see below).
Male incontinence is rare in the general population, affecting only 5% of older men (Bortolotti, 2000). The incidence of UI in men parallels the rate of various surgical procedures and urologic conditions listed in Table 79–1. Incontinence after transurethral prostatectomy (TURP) may reflect persistent bladder overactivity but rarely results from damage to the external sphincter during transurethral resection. In a large Veterans Affairs Cooperative Study (Wasson et al, 1995), the rate of de novo UI after TURP was no different than in the watchful waiting group. The historical incidence of incontinence after radical prostatectomy varies from 2.5% to 87% (Foote et al, 1991). Progressive improvement in urinary control has been reported to occur for as long as 2 years after surgery; in one study the percentage of men who used one pad or less after RP was 71%, 87%, 92%, and 98.5% at 3, 6, 12, and 24 months, respectively (Lepor and Kaci, 2004). Nerve-sparing techniques pioneered by Walsh (Eggleston and Walsh, 1985) have been associated with a reduced incidence of postoperative UI (Wei et al, 2000a). Although the mechanism of this effect remains debatable, sensory and motor pudendal innervation of the rhabdosphincter is generally preserved post-RP; in contrast, autonomic afferent denervation and impaired membranous urethral sensitivity seems to be associated with post-RP UI (Catarin et al, 2008). Bladder neck contracture is also associated with a reduced continence rate after RP, probably due to secondary surgical intervention (Park et al, 2001).
Contemporary series demonstrate improved continence after prostatectomy when compared with historical rates, but wide variation in reported outcomes reflect patient- and surgeon-specific factors as well as methodology of data collection (Flynn and Webster, 2004). Validated disease-specific questionnaires, such as the University of California–Los Angeles (UCLA) Prostate Cancer Index (Litwin et al, 1998) or the Expanded Prostate Cancer Index Composite (Wei et al, 2000b), that capture symptoms of stress and urgency incontinence are recommended. Rates of incontinence are higher when calculated from such self-report instruments as compared with chart review or physician-recorded outcomes (Carlson and Nitti, 2001; Flynn and Webster, 2004). For example, Litwin and colleagues (1995) reported that up to 40% of patients in a large cohort study complained of persistent long-term urinary incontinence after RP. Although most patients reported mild UI, 4% complained of significant lifestyle-compromising leakage.
History and Development of Devices
Devices to control urinary incontinence in men have been described since antiquity (Schultheiss et al, 2000). One of the first modern prosthetic devices to treat incontinence in men was reported by Berry and associates in 1961 (Engel and Wade, 1969). This acrylic prosthesis was designed to kink and compress the bulbar urethra, but poor results, pain, and fistula formation led to its abandonment (Engel and Wade, 1969). In 1970, Kaufman reported the first of several procedures to provide continence by compressing the urethra with the Kaufman I procedure. This was modified by incorporating a tetrafluoroethylene mesh tape in the Kaufman II procedure and a silicone-gel–filled hemispherical prosthesis in the Kaufman III procedure, giving a success rate of 70%.
Slings to treat stress urinary incontinence (SUI) in men were introduced in 1975 by Kishev, who described the placement of a pliable prosthetic “wad” under the bulbar urethra. Schaeffer then described a bulbourethral sling that used bolsters suspended from the rectus fascia (Schaeffer et al, 1998; Clemens et al, 1999). Another report documented results with a composite graft of polypropylene and porcine skin collagen placed suburethrally (John, 2004). Male bulbourethral slings have continued to evolve with the development of bone screws for synthetic mesh fixation (InVance; American Medical Systems, Minnetonka, MN), transobturator fixation (AdVance; American Medical Systems), and a newly announced combined prepubic and transobturator sling (Virtue; Coloplast, Minneapolis, MN) (Comiter and Rhee, 2008).
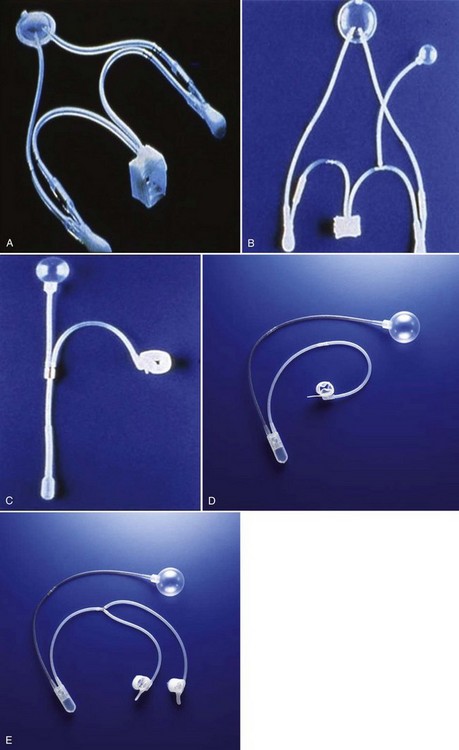
In 1976, Rosen designed the first model of an AUS, but hydraulic failure and fistula formation approached 100%, and this device was abandoned (Fowler and Auld, 1985). Scott presented his initial report with the AMS 721 (American Medical Systems, Minnetonka, MN), noting a 79% success rate (Timm et al, 1976; Scott, 1978). Over the next 10 years, further developments in design of the AUS were made. The AMS 742 allowed automatic cuff closure after cuff decompression. The AMS 791 and 792 used a silicone rubber cuff and a deactivation button (Scott, 1989). The AMS 800 included the deactivation button within the control pump; and, in 1987, the narrow-backed cuff was introduced (Fig. 79–1), which, by improving pressure transmission from the cuff pressure to the underlying tissue, has greatly decreased the incidence of urethral erosion and tissue atrophy (Light and Reynolds, 1992).
Evaluation and Diagnosis
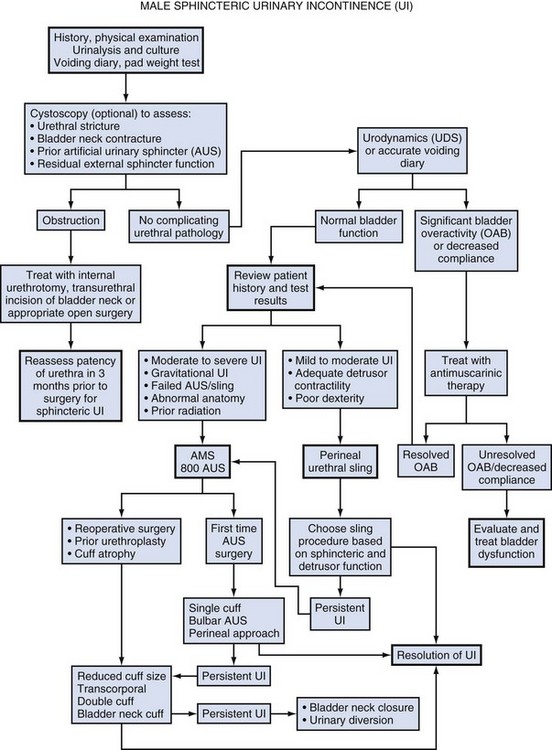
The initial evaluation of a man with UI requires a detailed history, physical examination, and urinalysis. Many cases of sphincteric UI will also require cystoscopy and pressure flow urodynamics to evaluate potential bladder neck contracture and bladder storage function respectively. Figure 79–2 shows the overall management algorithm.
History
The medical history should focus on the type, degree, and severity of UI; previous surgical procedures; and symptoms of neurologic disease. Specific evaluation of the failed AUS will be considered at the end of this section. Differentiation between stress and urge UI is important and can be aided by the voiding diary and pad test, simple inexpensive assessments of urinary incontinence that are recommended before proceeding with invasive testing (Flynn and Webster, 2004). The voiding diary reliably assesses the number of incontinent episodes and may uncover significant urgency and urge incontinence (Groutz et al, 2000). Self-reported daily pad usage varies considerably, with only moderate concordance with urinary incontinence volume (Dylewski et al, 2007). Thus the 24-hour pad weight test, which objectively measures the magnitude of the incontinence, may be helpful in directing appropriate therapy. However, because the effectiveness of current surgical therapies has not been stratified according to severity of UI, the most important current use for the pad test may be as an outcome measure postoperatively.
Physical Examination
A complete examination of the abdomen, back, genitalia, perineum, rectum, and neurologic system is essential to identify conditions associated with specific causes of UI. The skin should be inspected for signs of breakdown or secondary fungal or bacterial infection, which need to be treated prior to surgery. Previous surgical incisions should be noted when planning AUS pressure-regulating balloon (PRB) placement. Likewise, inguinal hernias should be identified and, if contralateral PRB placement is not possible, concomitant repair is advisable. Scrotal examination will detect pathology that may influence pump placement, such as hydrocele, hernias, and scrotal masses.
Laboratory
Urine analysis and culture are required prior to surgical correction of male UI, and all abnormalities need complete investigation. UTI must be appropriately evaluated and eradicated. Serum creatinine and prostate-specific antigen (PSA) assays should be obtained to assess renal function and cancer status post-RP. Renal insufficiency requires careful evaluation prior to proceeding with surgical intervention.
Cystoscopy
Detection of vesicourethral anastomotic stricture, bladder neck contracture, and bulbar urethral stricture prior to planned surgery may be achieved by history or uroflowmetry in most cases (Yurkanin et al, 2001). Because unrecognized urethral pathology can significantly complicate all surgical approaches, endoscopic evaluation is recommended prior to surgical correction of UI post-RP or -TURP even though bladder neck contracture is rare (Wasson et al, 1995; Erickson et al, 2009; Krambeck et al, 2009). Cystoscopic evaluation of the degree of residual function of the external sphincter may help identify appropriate candidates for transobturator sling procedures (see section on Transobturator Bulbourethral Sling and Fig. 79–3). Furthermore, in patients with recurrent incontinence after AUS implantation, cystoscopy may aid in differentiating mechanical failure from other causes such as urethral atrophy. Finally, when an AUS or sling (Harris et al, 2009) has been removed due to infection or erosion, repeat evaluation of the urethra prior to reimplantation is essential to identify stricture, diverticulum, and other urethral complications.
Urodynamics
Assessment of bladder capacity, compliance, and contractility is required prior to considering surgical correction of UI. A careful history and voiding diary may be sufficient to confirm the adequacy of bladder function; however, pressure-flow urodynamics permits an accurate assessment of bladder function, incontinence type, and severity. ISD will be identified in almost all cases. Although 40% to 45% of men with UI post-RP have bladder dysfunction, it is the sole cause of incontinence in a very small percentage (Leach et al, 1996; Ficazzola and Nitti, 1998). Filling cystometry can be difficult in men with severe incontinence, and occlusion of the bladder neck with a balloon catheter may be required to assess compliance and overactivity. Detrusor overactivity, although not a contraindication to surgery for UI if discovered, requires realistic counseling regarding the likelihood of a successful outcome. Reduced bladder compliance presents a more serious concern, because prolonged storage at high pressures may lead to deteriorating renal function. Notably, such urodynamic findings do not reliably predict worse post-AUS continence (Trigo-Rocha et al, 2008; Lai et al, 2009). This effectiveness, in spite of adverse urodynamic findings, could portend silent upper tract deterioration. Conversely, detrusor hypocontractility on pressure-flow urodynamics may indicate the need for AUS, if adequate detrusor function does not exist to overcome the fixed resistance of a compressive sling (Comiter, 2007).
Evaluation of Persistent Incontinence after AUS
The diagnostic evaluation of recurrent UI after AUS must differentiate between inadvertent deactivation, insufficient urethral compression (oversizing of cuff), mechanical failure with fluid loss, cuff erosion, bladder storage failure, urethral or bladder neck atrophy under the cuff, as well as rare causes such as a plugged delay-fill resistor or kinked tubing (Montague and Angermeier, 2001). The evaluation includes the same general approach described for de novo UI in Figure 79–2. A history of sudden loss of continence suggests deactivation or mechanical failure. Active cycling of the device excludes inadvertent deactivation. If the pump is deactivated with inadequate fluid to cycle, passive filling can be achieved by squeezing the pump on its lateral edges or by pushing on the pump with a cotton-tipped applicator opposite the deactivation button. Fluid loss from the device implies mechanical failure and leak. Plain radiography (for contrast-filled systems) or ultrasonography (for saline-filled systems) of the PRB during cycling can help differentiate fluid loss from cuff atrophy. If the PRB size changes with cycling and refills passively, mechanical failure is less likely, thus suggesting cuff atrophy (Taylor and Lebowitz, 1985; Lorentzen et al, 1987). Cystoscopy, in addition to excluding erosion, can be used to visualize the cuff during cycling and give insight into the likelihood of atrophy. Urodynamics should be performed when bladder storage failure is suspected.
Indications for Surgery
Surgical correction of UI is indicated in male patients with irreversible intrinsic sphincter deficiency and bothersome involuntary leakage of urine. After prostatectomy, all men should undergo a course of pelvic floor muscle exercise. Because progressive improvement in continence occurs after RP, some authors recommend a 1-year observation period (Peyromaure et al, 2002; Flynn and Webster, 2004). However, it is unnecessary to delay intervention for patients with severe or gravitational UI who show no improvement beyond 6 months, particularly if cystoscopy shows a significant external sphincter defect. Because of limited efficacy, submucosal bulking agents are no longer part of the treatment algorithm for post-RP UI (Kuznetsov et al, 2000; Montague and Angermeier, 2000; Abrams et al, 2009). Thus AUS and slings should be recommended as first-line surgical therapy for sphincteric incontinence in most men, although a trial of bulking agent may be appropriate in cases of neurogenic male stress UI.
Once the diagnostic evaluation has been completed (see Fig. 79–2), the best option for a given patient can be selected. Factors to consider include the severity of UI and associated bother; patient characteristics, including prior surgical procedures, bladder function, and cystoscopic findings; manual dexterity and cognitive function; efficacy of the various implants; long-term risk of complications and reoperation; and patient preference. Absolute contraindications to surgical correction of male UI are few, and include bladder disorders that jeopardize renal function, such as diminished vesical compliance and vesicoureteral reflux at low intravesical pressure. Inadequate tissue integrity at the bladder neck or urethra to accommodate a sling or AUS may require bladder neck closure or urinary diversion. Additionally, urinary tract abnormalities that require future transurethral management, such as bladder cancer or refractory vesicourethral anastomotic strictures, should be considered relative contraindications to surgery. In such cases, an AUS or sling procedure could impair transurethral access, and repeated instrumentation may put the devices at risk for infection or erosion. Although metastatic prostate cancer is not a contraindication to surgical correction of UI, improvements in quality of life must be balanced against performance status and life expectancy.
The AUS remains the established device for treatment of moderate to severe UI, supported by numerous publications documenting its benefits (Table 79–2). Advantages include reproducible and reliable implantation, ability of patients to empty the bladder without detrusor contraction, and proven efficacy after pelvic irradiation. A national study using validated questions from the University of California–Los Angeles Prostate Cancer Index (UCLA PCI) provides useful data on AUS results (Dalkin et al, 2003) (Table 79–3). Few men post-AUS have severe degrees of incontinence (9%), but many continue to use pads and report a moderately high urinary bother. There is accumulating data that men who are completely dry after radical prostatectomy have significantly better health-related quality of life compared with those who wear one pad daily (Cooperberg et al, 2003). Whether this is also the case after AUS remains unknown. Long-term durability of the AUS is well established, although a revision rate of 16% and 28% at 2 and 5 years, respectively highlights the limitations of the devices (Dalkin et al, 2003) and is consistent with a large single institution series in which 72% had the original sphincter in place and functioning at a mean follow-up of 69 months (Elliott and Barrett, 1998).
Table 79–3 National Questionnaire-Based Assessment of AUS Outcomes 2 and 5 Years after Implantation
| 2 YEARS POST-AUS | 5 YEARS POST-AUS | |
|---|---|---|
| Category | ||
| Urinary function* | 53 (25) | 40 (22) |
| Urinary bother* | 59 (24) | 48 (32) |
| Degree of Incontinence (%) | ||
| Mild | 59 | 38 |
| Moderate | 32 | 37 |
| Severe | 9 | 24 |
| Revision Rate (%) | 16 | 28 |
| N | 289 | 292 |
Age-adjusted urinary function and bother scores derived from the UCLA PCI.
AUS, artificial urinary sphincter; UCLA PCI, University of California–Los Angeles Prostate Cancer Index.
* Results are expressed as a numerical score (mean and SD) ranging from 0 to 100, with 100 being the highest possible response.
Adapted from Dalkin BL, Wessells H, Cui H: A national survey of urinary and health related quality of life outcomes in men with an artificial urinary sphincter for post-radical prostatectomy incontinence. J Urol 2003;169:237–9.
Table 79–2 Outcomes and Complication Rates of Surgical Therapy for Male Sphincteric Urinary Incontinence
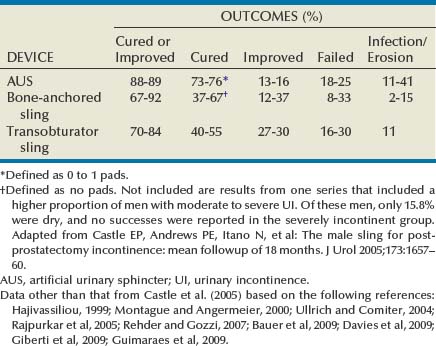
Male sling procedures represent a significant addition to the surgical armamentarium for UI by providing an alternative to AUS. They impart outlet resistance without occlusion of the urethra with a cuff, potentially reducing the risk of erosion and mechanical failure (Comiter, 2007). The bone-anchored sling is performed through a single perineal incision that results in short operative times and low erosion rates (Madjar, 2001a; Comiter, 2002). Titanium screws provide stable fixation of the sling to the inferior pubic ramus, avoiding the need for retropubic passage of suspension sutures. The mechanism of continence with the bone-anchored sling relies on compression of the urethra, as demonstrated by an increase in fixed resistance of the urethra (Ullrich and Comiter, 2004). However, no urodynamic evidence of increased detrusor pressure during voiding was seen. The results with this technique have been generally positive, although longer-term follow-up has revealed cases of persistent pain, infection and erosion (see Table 79–2) (Giberti et al, 2009).
A transobturator sling introduced by AMS (AdVance) has been shown to be effective in short-term follow up of well-characterized case series (see Table 79–2) (Rehder and Gozzi, 2007; Davies et al, 2009). In these highly selected populations, approximately 75% of patients are cured or significantly improved, with results persisting at 12 months. Unlike the bone-anchored sling, in which continence is improved by compression, it appears that the transobturator sling augments sphincteric function by repositioning and lengthening the membranous urethra (Firrozi and Vasavada, 2009). Although the absence of increased detrusor pressure during voiding was interpreted as a noncompressive mechanism (Davies et al, 2009), the significant tensioning required during sling placement suggests that compression may play a role in the function of the transobturator sling as well (Latini, 2009).
Bulbar urethral slings can be seen as alternatives for those who refuse AUS from fear of infection, erosion, or mechanical failure, as well as those with limited physical or cognitive capacity. Comiter (2007) notes that the techniques differ in their indications, relative success rates, and complication rates for patients with varying degrees of incontinence. The trade-off between risk and efficacy most be considered, with most authors recommending AUS for more severe UI. For mild UI, bulbar sling procedures become viable alternatives, whereas AUS may represent therapeutic overkill. Thus the bone-anchored and transobturator slings should be primarily used in cases with mild incontinence, which can be defined as a 24-hour pad weight of less than 150 grams (Flynn and Webster, 2004). A sling procedure should not be offered to those with prior radiation therapy or urethral erosion, because the degree of urine loss that exists in this group usually exceeds the limits of the procedure (Schaeffer et al, 1998; Dikranian et al, 2004; Castle et al, 2005; Giberti et al, 2009).
Key Points: Implant Selection
Techniques of Implantation
Operative Preparation
The entire operative site is shaved, and an antiseptic skin preparation is performed, including the lower abdomen, genitals, and perineum. Surgery is conducted under general or spinal anesthesia with the patient in the low lithotomy position. Intravenous antibiotics, including an aminoglycoside and a first- or second-generation cephalosporin or vancomycin, are administered within 60 minutes prior to skin incision according to American Urological Association (AUA) Guidelines on antimicrobial prophylaxis (Wolf et al, 2008). The patient is then carefully draped, and a urethral catheter is placed to drain the bladder and facilitate identification and dissection of the urethra.
Artificial Urinary Sphincter
The AUS (AMS 800) consists of a fluid-filled cuff placed around the bladder neck or bulbar urethra, a control pump placed in the scrotum, and a pressure-regulating balloon (PRB) placed in a preperitoneal or intraperitoneal location. Fluid transfer from the cuff to the PRB is accomplished by active “pumping” while refilling occurs passively by a pressure gradient from the PRB that traverses a resistor implanted in the pump. This valve prevents acute pressure transmission from the reservoir to the cuff.
The AUS cuff is most commonly placed around the bulbar urethra through a perineal incision, although a transscrotal approach popularized by Wilson and associates (Wilson et al, 2003) also may be selected (see below). The aim is to place the cuff as proximal on the bulbar urethra as possible—proximal to the fusion of the two corporeal bodies (Fig. 79–4A and B). This location allows safe circumferential dissection of the urethra, provides protection of the cuff from activation while sitting, and exposes the largest diameter of corpus spongiosum for placement of the cuff.
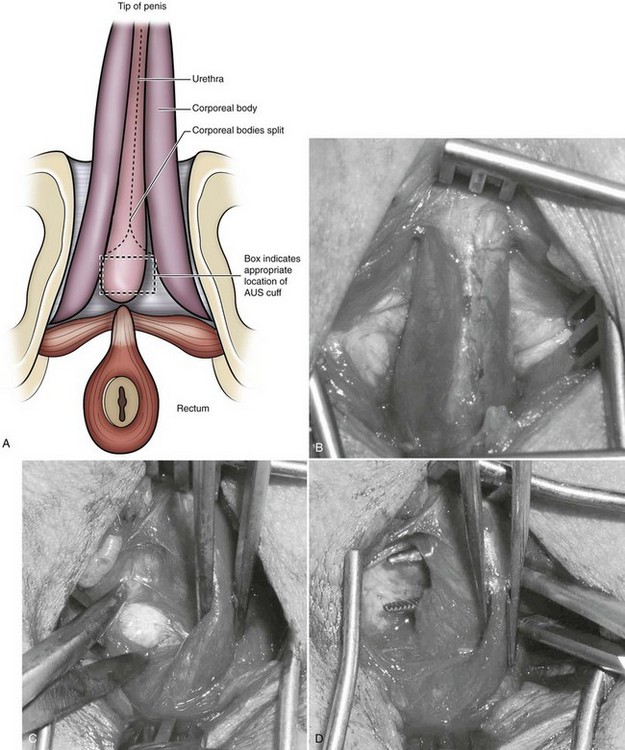
Figure 79–4 Perineal anatomy and bulbar urethral dissection. A, Preferred location for first-time bulbar cuff proximal to convergence of corpora cavernosa. Note box indicating ideal cuff placement. B, Exposure of the corpus spongiosum after division of the bulbospongiosus muscle. C, Dissection at interface between spongiosum and right crus of the cavernosum. D, Right-angle clamp behind corpus spongiosum after circumferential dissection. AUS, artificial urinary sphincter.
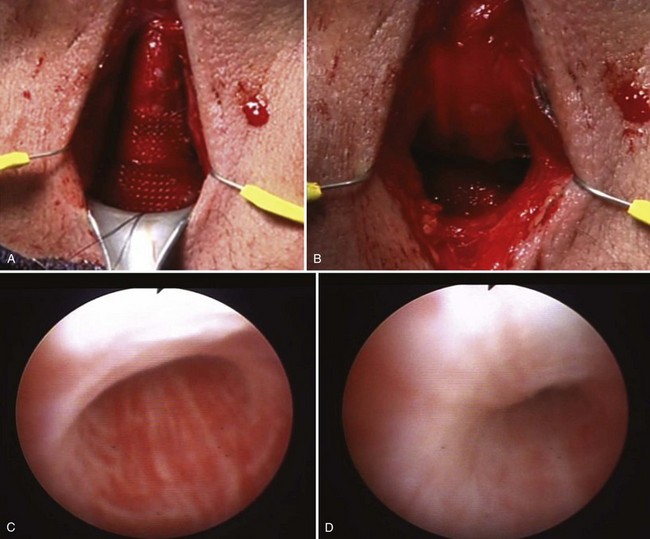
After incising the skin, Colles fascia, and bulbospongiosus muscle, Buck fascia is incised as it reflects off the bulbar urethra onto the diverging corporeal bodies (Fig. 79–4C). A 2-cm wide tunnel is created under direct vision using sharp dissection, dorsal to Buck fascia over the roof of the urethra. A right-angle clamp is then passed through this tunnel (Fig. 79–4D). Blunt spreading dissection is discouraged in this area, because it risks injury to the urethra, especially in reoperative cases. The circumference of the urethra is measured around the corpus spongiosum to guide selection of cuff size, most commonly 4.0 or 4.5 cm. The urethral catheter should be removed prior to measurement. Although some authors advocate selecting a cuff size 0.5 to 1 cm smaller than the measured circumference of the urethra to compensate for postoperative subcuff atrophy of the spongiosum, the best test of cuff fit is the visual and endoscopic appearance after it has been placed around the urethra (Fig. 79–5A and B). If the cuff size is obviously incorrect, the next appropriate size should be selected. The tubing from the AUS cuff is then passed through the overlying bulbospongiosus muscle into the deep perineal space beneath Colles fascia.
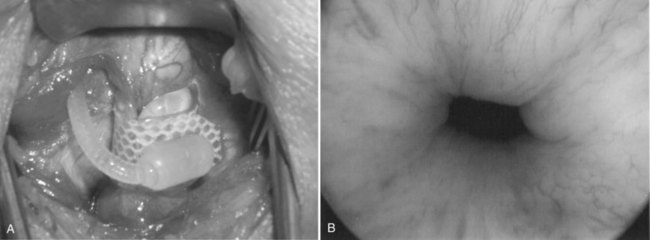
Figure 79–5 Appropriate appearance of cuff. A, Perineal placement encircling the bulbar urethra. B, Endoscopic appearance with cuff activated.
Pressure-Regulating Balloon (PRB)
The placement of the PRB may be achieved through a scrotal, perineal, or abdominal incision, depending on prior surgical incisions, body habitus, and surgeon preference. Implanters should be familiar with each approach. For abdominal placement, a horizontal lower-quadrant incision is made in the abdomen ipsilateral to pump placement. The rectus or external oblique fascia is incised, allowing the surgeon to split the underlying muscle and access the preperitoneal or intraperitoneal space. Digital dissection creates the space required for the PRB, which is small. We place a 61- to 70-cm H2O PRB in all bulbar AUS and reserve the 71- to 80-cm H2O PRB for bladder neck cuff placement. The abdominal wall fascia is closed around the tubing.
Alternatively, the PRB can be placed through the external inguinal ring, using techniques adapted from penile implant surgery. Using either a transverse scrotal or perineal incision, the external ring is identified and used to provide access to the retropubic space by penetrating through the floor of the inguinal canal with a fingertip or instrument. No sutures are required, because the transversalis fascia provides a natural barrier to PRB extrusion. Contraindications to this approach include mesh hernia repairs, radical cystectomy, and other extensive abdominal surgery. In such cases, the abdominal approach reduces the risk of bladder or intestinal injury.
The PRB is filled with 23 mL of sterile normal saline or contrast material, unless a bladder neck cuff is used, which requires additional fluid volume. Filling solutions include 0.9% normal saline, Cysto-Conray (Covidien Pharmaceuticals, Hazelwood, MO) 60 cc of contrast diluted with 15 cc of sterile water, or Hypaque (Amersham Health, Princeton, NJ) 25% 50 cc contrast diluted with 60 cc of sterile water. To provide an isotonic solution, contrast solutions should always be diluted with sterile water. Contrast media are contraindicated if the patient has an iodine or contrast allergy.
Control Pump Placement
The pump assembly is placed into the anterior scrotum from the inguinal, scrotal, or perineal incision. When the PRB has been placed abdominally, dissection proceeds inferiorly to the scrotum above the abdominal wall fascia, accessing the anterior scrotum deep to Scarpa fascia. A finger placed outside the dependent portion of the scrotum then invaginates this skin upward into the inguinal incision, allowing the fascial layers to be stripped off the dartos layer with an instrument, creating a small pocket for the pump (Fig. 79–6). The pump is then placed into that position with the tubing held loosely with a Babcock clamp. For the single-incision approaches, a small subdartos pouch is created directly from the scrotal or perineal incision, orienting the pump accordingly toward the foot (scrotal) or head (perineal).
Making the Connections
Connections are typically made in the abdominal incision, when used, thus protecting the connections from excess wear and allowing easy exploration at a later date. The quick connectors supplied by the manufacturer provide excellent, secure, and watertight connections for newly implanted devices. However, they cannot be used for revision surgeries, because a biofilm on the tubing interferes with watertight connection. Therefore one must use hand-tie connectors in revision cases where the entire device is not being replaced. After completing the connections, the device is cycled several times through the activation and deactivation states. We verify adequate coaptation of the urethra using urethroscopy. The closed cuff should cause slight blanching of the urethral tissue, indicating adequate urethral coaptation, filling, and connection of the device (see Fig. 79–5B).
Tandem-Cuff AUS
Up to 11% of men may have significant incontinence after single-cuff AUS placement, despite significant improvements in AMS 800 design and implantation techniques (Montague and Angermeier, 2000). In such patients, when the cause of persistent UI is incomplete urethral occlusion, the addition of a second cuff around the bulbar urethra (Fig. 79–7A and B) can yield satisfactory continence (Brito et al, 1993; Kabalin, 1996; DiMarco and Elliott, 2003; O’Connor et al, 2003). Indiscriminate use of the tandem-cuff approach as first-line surgical treatment should be tempered by higher rates of erosion associated with the distal cuff in several series (Kowalczyk et al, 1996b; Bell and Mulcahy, 2000).
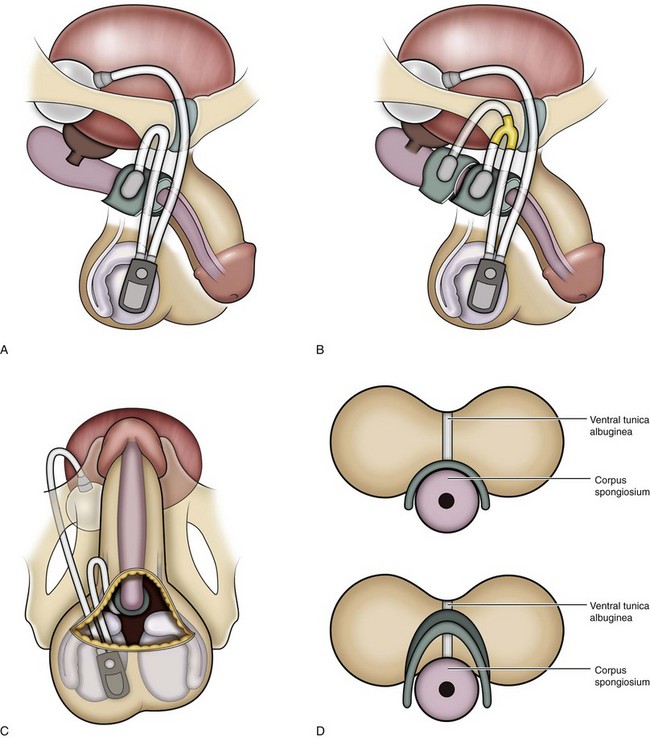
Figure 79–7 Diagram of the AUS including perineal (A), tandem (B), scrotal (C), and transcorporal (D) approaches. Note the incorporation of the ventral tunica albuginea with the corpus spongiosum in the transcorporal approach.
Placement of the second cuff can be accomplished through the perineal incision. After circumferentially dissection around the distal bulbar urethra, at least 1.5 to 2 cm distal to the primary cuff, a new cuff, usually 4.0 cm, is placed. Connection to the existing device requires division of the existing cuff tubing and use of a metal Y-connector. An additional 3 mL of fluid must be added to the system. When adding a new cuff to an existing system, the tubing must be connected with hand ties as described above. The entire device is left deactivated for 6 weeks postoperatively.
Transcorporal AUS Cuff
The transcorporal placement of the AUS provides another approach to difficult initial or revision surgery (Guralnick et al, 2002). Rather than dissecting between the corpus spongiosum and the ventral cavernosa, parallel longitudinal incisions in the tunica albuginea of the corpora cavernosa, near the interface with the corpus spongiosum, allow the plane of dissection to pass through the septum of the corpora from one side to the other. This technique leaves the ventral tunica albuginea attached to the dorsum of the spongiosum (Fig. 79–7D). The lateral edges of the tunica albuginea may need to be imbricated behind the cuff; preplacement of sutures to accomplish this is advisable. Advantages of the transcorporal approach include reduced risk of urethral injury during reoperation after prior erosion or urethra injury, better cuff fit because of the increased bulk of the urethra, and potential reduction in erosion risk. The technique is particularly useful when proximal cuff atrophy occurs under a 4.0-cm cuff. Transcorporal placement, 2 to 3 cm distally, leaves an intervening segment of normal urethra that may yield AUS continence results similar to those given in Table 79–2. Webster and associates reported a cure and improvement rate of 84% without intraoperative urethral injuries or postoperative urethral erosions at a mean follow-up of 17 months (Guralnick et al, 2002). An operative video in the AUA video series details the technique.
Transscrotal AUS
Wilson and associates (2003) described a technique in which all components of the AUS are placed through a transverse scrotal incision. Originally developed for reoperative surgery due to urethral atrophy, it allowed placement of a more distal cuff in tandem or with the transcorporal technique (Fig. 79–7C). Although the initial report was favorable in terms of continence outcomes, several reports suggested that the transscrotal technique yields inferior continence results (Stone et al, 2003; Henry et al, 2008). One possible explanation is that it does not allow equivalent placement of the cuff around the proximal, larger-diameter bulbar urethra when compared with the perineal approach. Corroborative evidence comes from data on implanted cuff size. In the Mayo clinic experience, 267 of the 272 perineal cuffs measured 4.5 cm while the remaining cuffs were 5.0 cm (Elliott and Barrett, 1998). Conversely, in the transscrotal series, 32 of the 37 cuffs measured 4.0 cm, and the remaining cuffs were 4.5 cm (Wilson et al, 2003). A modified version of the transscrotal approach, using enhanced exposure (personal communication: SK Wilson, August 7, 2009), has allowed the use of larger cuff sizes around the proximal bulbar urethra and may address the concerns raised in the literature.
Bladder Neck AUS
Bladder neck AUS remains an attractive, although more invasive, method of cuff placement in those men with sphincteric UI in whom the prostate is without external surgical or traumatic disruption. Thus for etiologies of exstrophy/epispadias, myelomeningocele, and other neuropathic disorders, it should be considered before bulbar AUS (Herndon et al, 2003). Its use is contraindicated after RP. Advantages include a lower likelihood of erosion and cuff atrophy. Placement of the cuff requires a lower abdominal incision, exposure of the bladder base, and circumferential dissection of the vesicoprostatic junction. Opening the endopelvic fascia helps to develop of the plane of dissection; a catheter placed in the urethra and another in the rectum helps in identifying these structures. If necessary, cystotomy with placement of a finger in the lumen of the bladder neck may also assist the dissection. The bladder neck in adults requires a much larger cuff implant (usually 8 cm or greater), higher PRB pressure (usually 71 to 80 cm H2O), and a larger fluid volume in the system. Manufacturer packaging includes details relevant to implantation. In children, cuff and PRB characteristics may need to be altered significantly (Ruiz et al, 2006).
Key Points: Implantation of AUS
Bone-Anchored Bulbourethral Sling
The bone-anchored perineal sling popularized using the AMS InVance implant is a synthetic mesh device placed outside the bulbospongiosus muscle and attached to the pubic rami. A vertical perineal incision centered over the bulbar urethra exposes the bulbospongiosus muscle and the medial aspect of each inferior pubic ramus (Fig. 79–8A). Three titanium bone screws preloaded with a pair of 1-0 polypropylene sutures are inserted in the anteromedial aspect of each descending pubic ramus using a bone drill (Fig. 79–8B). The proximal bone screws are placed just beneath the junction of the descending ramus and pubic symphysis, and the distal screws are inserted approximately 3 cm caudally at the level of the bulbar urethra. Comiter and colleagues (1997) described retrograde leak point pressure (RLPP) to tension the device (Comiter et al, 1997). The basic technique uses a 14-Fr catheter with the balloon inflated with 1 mL H2O in the distal penile urethra. Prior to tensioning the sling, perfusion sphincterometry is performed by connecting a bag of saline to the catheter through sterile tubing. The bag is elevated until fluid flows from the bag, through the drip chamber, and into the urethra (Comiter et al, 1997). The RLPP is recorded as the height of the fluid column above the symphysis at which fluid flow begins.
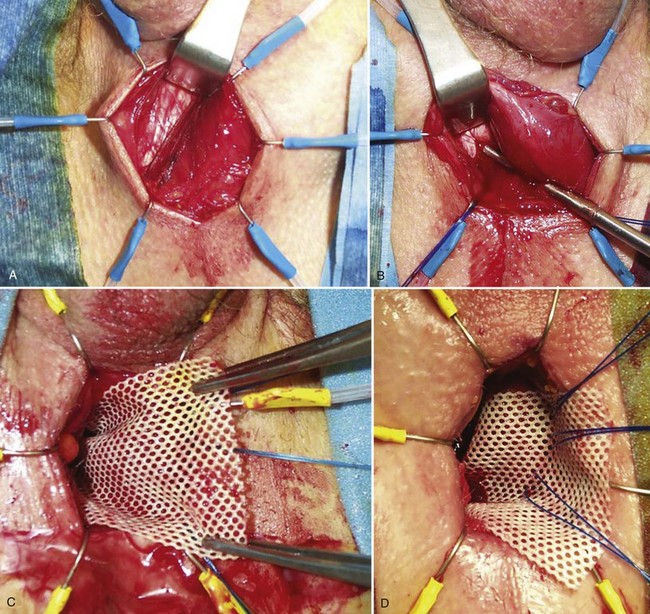
Figure 79–8 Bone-anchored sling insertion. A, Exposure of the right crus of the corpus cavernosum with bulbospongiosus muscle retracted medially. B, Screw insertion into inferior pubic ramus. C, Placement of the right side of the synthetic mesh device. D, Initial tensioning with sutures on the left side of the sling.
A 4- × 7-cm sling composed of synthetic or organic material is secured to the bone-anchored sutures by passing the sutures through the sling with the aid of an 18-gauge needle, 0.5 cm from the right edge, equally spaced along the width of the sling. The right-sided sutures are then tied down to the bone (Fig. 79-8C). With the sling positioned over the bulbospongiosus muscle, the contralateral sutures are passed through the left side of the sling and secured with a single throw of the suture over a silk safety tie. RLPP is repeated, allowing sling tension to be adjusted by moving the left-sided sutures more medially (tighter) or laterally (looser) until an RLPP of 60 cm H2O is achieved (Fig. 79-8D). The wound is irrigated and closed in two layers. The catheter is removed in the recovery room or the following morning for a trial of voiding.
Determining the appropriate tension of the sling is the most critical portion of the operation. Although the original choice of 60 cm H2O was based on the pressure range of the AUS PRB, subsequent studies have also demonstrated that patients who achieve a RLPP of 60 cm have better outcomes than those who demonstrate a lower compression pressure (Ullrich and Comiter, 2004). Alternative methods of sling tensioning have been described, such as an intraoperative cough test to determine sling tensioning (Madjar et al, 2001a; Rajpurkar et al, 2005).
Transobturator Bulbourethral Sling
Rehder and Gozzi (2007) described a male transobturator sling that provides an alternative surgical technique for suburethral placement. In contrast to the bone-anchored approach, the AdVance sling is designed to reposition and lengthen the membranous urethra. After exposing the corpus spongiosum in a manner identical to the AUS described above, the space between the corpus spongiosum and corpora cavernosa is developed on each side. The corpus spongiosum is dissected along its ventral surface proximally to the level of the perineal body, which is marked to facilitate later sling positioning. Using the inferior margin of the adductor longus muscle as a landmark, a small stab wound is made approximately one fingerbreadth below the muscle on each side. A spinal needle locates the obturator foramen immediately lateral to the pubic ramus. Then, using an outside-inside direction, the appropriate left- or right-sided helical passing device is inserted from the thigh through the obturator foramen and out of the perineal wound medial to the ipsilateral corporeal body. This needle should be brought out as close as possible to the superior aspect of the triangle formed by the corpus spongiosum and the pubic ramus. Once this has been completed bilaterally, the polypropylene mesh is loosely positioned in the perineum, attached to the passing devices, and pulled out of the obturator foramen to the medial thigh without tension. The midportion is placed on the corpus spongiosum, and the arms of the sling are pulled up to tension the device (Fig. 79–9). With tensioning, the perineal body and proximal bulbar urethra should move proximally and cephalad, 3 to 4 cm. The maneuver is repeated with cystoscopic visualization of the external sphincter region to confirm the repositioning of the urethra (Rehder and Gozzi, 2007). Once in position, the sling is flattened out again, attached with absorbable suture, and final tensioning is performed. The tail ends of the sling are trimmed and buried under the skin. The perineal incision is closed in layers, including the bulbospongiosus muscle, Colles fascia, and skin. Overnight catheter drainage is usually sufficient for decompression of the bladder.
Other Devices
The Prostate Adjustable Continence Therapy device (ProACT; Uromedica, Plymouth, MN) consists of two balloons placed on both sides of the bladder neck and is designed to increase resistance. Percutaneous adjustment of the pressure applied to the bladder neck is performed through titanium ports within the scrotum. Hübner and Schlarp showed significantly improved continence as they used this device over time, which stimulated the use of the ProACT device in centers in Europe (Hübner and Schlarp, 2005; Trigo-Rocha et al, 2006).
Another transobturator sling with limited clinical data has lateral strips for urethral transobturator elevation in addition to prepubic arms designed to provide concomitant compression. Early results have been published (Comiter, 2007), and the device in being commercialized.
Key Points: Sling Indications and Mechanisms
AUS Complications
Urinary Retention
Urinary retention in the immediate postoperative period should be managed by transurethral bladder drainage with a small (10-Fr or 12-Fr) catheter for 24 to 48 hours. Cuff deactivation must be confirmed before catheterization. If the patient fails a voiding trial at 48 hours, suprapubic drainage is recommended to reduce the risk of urethral erosion. Ultrasound or fluoroscopic guidance is recommended to prevent puncture or potential contamination of the PRB. Retention persisting beyond several weeks implies undersizing of the cuff; in such cases, reoperation and cuff replacement may be required. Correlation with preoperative urodynamic findings is advised in such cases. Late-onset urinary retention mandates endoscopic and urodynamic evaluation to rule out proximal urethral obstruction, erosion, or detrusor failure.
AUS Infection
Infection remains a serious and devastating complication of any implant surgery. The rate of infection with initial AUS surgery is 1% to 3% (Gundian et al, 1989; Marks and Light, 1989; Litwiller et al, 1996; Montague and Angermeier, 2000) but may be as high as 10% in patients after pelvic radiation and in reoperations (Montague, 1992). Skin pathogens are the most commonly cultured organisms, usually Staphylococcus epidermidis and S. aureus (Licht et al, 1995). The introduction of the InhibiZone Surface Treatment (American Medical Systems), combining rifampin and minocycline hydrochloride to the AUS cuff and pump, is likely to lead to a reduction in postoperative infection rates, although the results seen in the penile implant literature (Carson, 2004) have not yet been replicated in the AUS literature. Late infections (>4 months) may represent indolent organisms introduced at the time of infection or by hematogenous spread. Therefore in men with AUS, antibiotics should be considered before urinary tract manipulation in accordance with AUA Guidelines (Wolf et al, 2008).
The initial presentation of an early postoperative AUS infection is usually scrotal pain, although erythema, edema, and frank purulence will commonly accompany this symptom. Because implant infections are not amenable to antibiotic therapy, AUS infection will almost always require explantation. Traditional management includes device removal followed by a waiting period of several months with delayed reimplantation.
Immediate salvage of infected noneroded AUS can be accomplished with complete device removal, antiseptic irrigation, and immediate reimplantation (Kowalczyk et al, 1996a; Bell and Mulcahy, 2000; Bryan et al, 2002). This procedure includes an irrigation regimen used in penile prosthesis salvage protocols (Mulcahy et al, 1995). Mulcahy and associates used this approach to salvage seven of eight patients with infected noneroded AUS in a total of nine operations (Bryan et al, 2002). In all cases, the entire AUS was removed, and the wounds were copiously irrigated according to a seven-solution protocol prior to a new system being implanted. Contraindications to prosthesis salvage include sepsis, ketoacidosis, necrotizing infection, immunosuppression, and the finding of gross purulent material at the time of explantation.
Urethral Erosion
Urethral erosion is reported in up to 5% of AUS implantations (Gundian et al, 1989; Marks and Light, 1989; Litwiller et al, 1996; Singh and Thomas, 1996; Montague and Angermeier, 2000). Furlow and Barrett (1985) introduced the concept of postoperative deactivation during the healing process to decrease pressure-induced ischemia and necrosis. Delayed deactivation has lowered the risk of urethral erosion, especially in cases of reimplantation. Motley and Barrett (1990) saw a decrease in secondary urethral erosion from 18% to 1.3% with this technique. In an analysis of their 13-year experience in patients with urethral erosions, Webster and associates (Raj et al, 2006) determined that patients with hypertension, coronary artery disease, prior radiation therapy, and prior AUS revisions were more than twice as likely to suffer secondary urethral erosions.
Immediate removal of all the components of the AUS is imperative in cases of erosion, because they are assumed to be infected. The urethral injury is managed with urethral catheter drainage and or suprapubic cystostomy (Kowalczyk et al, 1996a; Flynn and Webster, 2004). Perineal wounds are considered infected and loosely approximated or allowed to close by secondary intention. Reimplantation is considered only after urethral healing is confirmed by urethrography and a delay of 3 to 6 months is observed.
Urethral patency must be confirmed by cystoscopy or retrograde urethrogram before attempted device replacement (Motley and Barrett, 1990; Kowalczyk et al, 1996a; Frank et al, 2000). A new cuff should be placed either proximal or distal to the previous site. Significant scarring, as well as a compromised vascular supply, makes replacement of the cuff at the erosion site difficult and risky (Motley and Barrett, 1990; Kowalczyk et al, 1996a). Frank and colleagues (2000) reported a successful outcome in 87% of de novo reimplantation after erosion or infection, with recurrent urethral erosion in 8.7%. The authors recommended nightly deactivation of the AUS in cases of reimplantation for prior erosion. More recently, in a series of 46 patients with prior AUS erosion who underwent reimplantation, 35% suffered another erosion within an average of 6.7 months (Raj et al, 2006). Use of the transcorporal approach in such cases may reduce the risk of urethral injury during the secondary surgery, as well as potentially provide greater protection against subsequent erosion.
Urethral Atrophy
Urethral atrophy results from the chronic compression of the spongy tissue under the occlusive cuff. This is the most common reason for revision of the AUS. Treatment options include cuff downsizing, movement of the cuff to a more proximal or distal location where the urethra may be thicker, or placement of a second cuff in tandem. Simple replacement of the PRB with a higher-pressure reservoir to overcome urethral atrophy is no longer recommended due to risk of erosion (Raj et al, 2005).
Our approach is to downsize the cuff in the same location when possible. If the existing cuff is already 4.0 cm, we place the cuff more proximally when feasible, or distally using the transcorporal technique if necessary. Saffarian and colleagues (2003) reported a significant improvement in daily pad usage from 3.9 to 0.5 in 17 patients with urethral atrophy. In a large series of secondary AUS surgery, the cuff was replaced in 142 cases, of which 33% of cuffs were placed distal to the original location, 11% were placed proximal, and 52% were placed at the original cuff location (Raj et al, 2005). The cuffs were downsized in 56% of cases, unchanged in 30%, and upsized in 13%, reflecting the new locations. In cases of urethral atrophy, several prominent centers have successfully added a second cuff in tandem to salvage continence (Brito et al, 1993; DiMarco and Elliott, 2003).
Mechanical Failure
The historical incidence of mechanical failure has diminished substantially after introduction of the narrow-backed cuff. Elliott and Barrett (1998) reviewed the long-term durability of the AMS 800 in 323 patients who were implanted at the Mayo clinic between 1983 and 1994. The change in design resulted in a decrease in nonmechanical failure from 17% to 9%, primarily due to a reduction in urethral atrophy. A decrease in mechanical failure from 21% to 7.6% was primarily due to a reduction in cuff leak along with improvements in the synthetic material, which lessened the risk of fracture or kinking of the device. Patients can generally expect a 7- to 10-year life expectancy for the AUS. In the absence of infection or erosion, replacement of an isolated malfunctioning component may be feasible if the revision occurs within 3 years of implantation. However, a slow leak from the PRB may be difficult to diagnose intraoperatively, and, if in doubt, total device replacement is prudent. Devices greater than 3 years old should be replaced in toto.
Special Circumstances
The management of urethral and vesicourethral anastomotic stricture encountered after AUS implantation proposes a unique challenge. Stricture at the site of the AUS cuff may result from compression or ischemia and may indicate impending urethral erosion. One such bulbar urethral stricture was successfully managed with periodic filiform and follower dilation (Debell and Wessells, 2001). Others have successfully treated strictures proximal to an AUS with balloon dilation (Westney et al, 1992) or holmium laser through a flexible ureteroscope (Anger et al, 2005). In the event significant endoscopic manipulation is required proximal to the cuff site, our practice is to surgically uncouple the cuff for the duration of the endoscopic procedure.
Sling Complications
The most commonly reported complications of male slings include perineal pain, urinary retention, infection, anchoring complications from bone anchors, and rare cases of erosion. Perineal pain may be reported in up to 74% of patients (Giberti et al, 2009) after bone-anchored sling procedures, but, for the majority, pain resolves within 3 months (Comiter, 2005). Retention is short lived and resolves within several weeks. Isolated cases have required release of the sling. The infection/erosion rate for both types of slings ranges from 2% to 15%, and the need for revision (secondary to bone-anchor dislodgement) has been reported for 2% to 4.2% of patients. Patients should be counseled about the possibility of persistent pain and osteitis pubis after a bone-anchored sling procedure.
Long-Term Results of AUS and Slings
Montague and Angermeier, in 2000, reviewed the results of the AUS in 286 patients from five centers. They noted that on average 76% of patients were dry (dry: 0 to 1 pads/day) and 13% were improved, for an overall success rate of 89% at a mean follow-up of 18 to 44 months. Revision of the device occurred due to urethral erosion (5%), AUS infection (3%), and mechanical failure (15%). Other studies have confirmed these results (Hajivassiliou, 1999; Venn et al, 2000). Although many patients still need minor protection with urinary pads, most are satisfied with the long-term outcome of the AUS (Haab et al, 1997; Montague et al, 2001; Dalkin et al, 2003). Success rates for revision surgery compare favorably with initial surgery, although infection and erosion rates are higher (Raj et al, 2005). Differences among centers may be related to surgical volume, inclusion of secondary implants, as well as the sensitivity and accuracy of outcome measures.
Initial results with the bone-anchored and transobturator sling appear similar to those with AUS (see Table 79–2). Results obtained by Ullrich and Comiter (2004) with the InVance sling showed a cured/improved rate of 92% at 25 months without significant morbidity. However, not all subsequent series have replicated these findings, with failure rates being as high as 33% (Giberti et al, 2009; Guimaraes et al, 2009). The place of the AdVance sling in the armamentarium remains unclear; early results are promising (Bauer et al, 2009), but patient selection criteria, particularly regarding assessment of external sphincter function and reproducibility of results requires further validation in other series.
Key Points: Complications
Summary
The expanding use of PSA testing and surgery for localized prostate cancer predict that male sphincteric UI will remain prevalent and require surgical treatment to improve symptoms and quality of life. The initial evaluation must include a focused history and physical examination, voiding diary, pad weight test, and, in many cases, cystoscopy and pressure-flow urodynamics. The AUS remains the gold standard treatment for moderate to severe male UI, especially in complex cases, with excellent overall success and acceptable complication rates. For mild to moderate UI in the absence of prior urethral surgery, radiation therapy, or detrusor acontractility, male slings provide efficacious alternatives. The outcomes after AUS and male sling procedures cannot be fully compared due to differences in endpoints, follow-up, and patient numbers. Nevertheless, regardless of incontinence severity, nearly 90% of men with sphincteric UI will have minimal leakage and an acceptable quality of life related to urinary symptoms after surgical treatment. Similar cured and improved rates have been reported with both the bone-anchored and transobturator slings. However, several caveats exist. Neither device has been compared to AUS in a controlled clinical trial. It is likely that selection bias regarding UI severity and other factors exists in choosing a sling versus AUS at most institutions. Furthermore, the bone-anchored and transobturator slings depend on subtle technical maneuvers relating to positioning and tensioning. Conversely, once the urethra has been circumferentially mobilized and measured, the effectiveness of AUS is largely independent of technique. Thus the occasional implanter will be more likely to achieve success with AUS versus either of the sling procedures, although the potential for erosion and mechanical failure will continue to drive the need for alternative solutions.
Brito CG, Mulcahy JJ, Mitchell ME, et al. Use of a double cuff AMS800 urinary sphincter for severe stress incontinence. J Urol. 1993;149:283-285.
Comiter CV. The male sling for stress urinary incontinence: a prospective study. J Urol. 2002;167:597-601.
Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159:1206-1208.
Guralnick ML, Miller E, Toh KL, et al. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol. 2002;167:2075-2078.
Rehder P, Gozzi C. Transobturator sling suspension for male urinary incontinence including post-radical prostatectomy. Eur Urol. 2007;52:860-866.
Abrams P, Andersson L, Birder L, et al. Evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. In: Abrams P, Cardozo L, Khoury K, Wein A, editors. Incontinence. 4th ed. Plymouth (UK): Health Publications; 2009:1767-1821.
Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report for the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178.
Anger JT, Raj GV, Delvecchio FC, et al. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol. 2005;173:1143-1146.
Bauer RM, Mayer ME, Gratzke C, et al. Prospective evaluation of the functional sling suspension for male postprostatectomy stress urinary incontinence: results after 1 year. Eur Urol. 2009;56:928-933.
Bell BB, Mulcahy JJ. Management of cuff erosion of the double cuff artificial urinary sphincter. J Urol. 2000;163:85-86.
Bortolotti A, Bernardini B, Colli E, et al. Prevalence and risk factors for urinary incontinence in Italy. Eur Urol. 2000;37:30-35.
Brito CG, Mulcahy JJ, Mitchell ME, et al. Use of a double cuff AMS800 urinary sphincter for severe stress incontinence. J Urol. 1993;149:283-285.
Bryan DE, Mulcahy JJ, Simmons GR. Salvage procedure for infected noneroded artificial urinary sphincters. J Urol. 2002;168:2464-2466.
Carlson KV, Nitti VW. Prevention and management of incontinence following radical prostatectomy. Urol Clin North Am. 2001;28:595-612.
Carson CC. Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol. 2004;171:1611-1614.
Castle EP, Andrews PE, Itano N, et al. The male sling for post-prostatectomy incontinence: mean followup of 18 months. J Urol. 2005;173:1657-1660.
Catarin MV, Manzano GM, Nobrega JA, et al. The role of membranous urethral afferent autonomic innervation in the continence mechanism after nerve sparing radical prostatectomy: a clinical and prospective study. J Urol. 2008;180:2527-2531.
Clemens JQ, Bushman W, Schaeffer AJ. Questionnaire based results of the bulbourethral sling procedure. J Urol. 1999;162:1972-1976.
Comiter CV. The male sling for stress urinary incontinence: a prospective study. J Urol. 2002;167:597-601.
Comiter CV. The male perineal sling: intermediate-term results. Neurourol Urodyn. 2005;24:648-653.
Comiter CV. Surgery insight: surgical management of postprostatectomy incontinence-the artificial urinary sphincter and male sling. Nat Clin Pract Urol. 2007;4:615-624.
Comiter CV, Rhee EY. The ‘ventral urethral elevation plus’ sling: a novel approach to treating stress urinary incontinence in men. BJU Int. 2008;101:187-191.
Comiter CV, Sullivan MP, Yalla SV. Retrograde leak point pressure for evaluating postradical prostatectomy incontinence. Urology. 1997;49:231-236.
Cooperberg MR, Master VA, Carroll PR. Health related quality of life significance of single pad urinary incontinence following radical prostatectomy. J Urol. 2003;170:512-515.
Coyne KS, Zhou Z, Thompson C, et al. The impact on health-related quality of life of stress, urge and mixed urinary incontinence. BJU Int. 2003;92:731-735.
Dalkin BL, Wessells H, Cui H. A national survey of urinary and health related quality of life outcomes in men with an artificial urinary sphincter for post-radical prostatectomy incontinence. J Urol. 2003;169:237-239.
Davies TO, Bepple JL, McCammon KA. Urodynamic changes and initial results of the AdVance male sling. Urology. 2009;74:354-357.
Debell M, Wessells H. Recurrent bulbar urethral stricture in the region of an artificial urinary sphincter. J Urol. 2001;166:1006-1007.
Dikranian AH, Chang JH, Rhee EY, et al. The male perineal sling: comparison of sling materials. J Urol. 2004;172:608-610.
DiMarco DS, Elliott DS. Tandem cuff artificial urinary sphincter as a salvage procedure following failed primary sphincter placement for the treatment of post-prostatectomy incontinence. J Urol. 2003;170:1252-1254.
Dylewski DA, Jamison MG, Borawski KM, et al. A statistical comparison of pad numbers versus pad weights in the quantification of urinary incontinence. Neurourol Urodyn. 2007;26:3-7.
Eggleston JC, Walsh PC. Radical prostatectomy with preservation of sexual function: pathological findings in the first 100 cases. J Urol. 1985;134:1146-1148.
Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159:1206-1208.
Engel RM, Wade JC. Experience with Berry prosthesis. J Urol. 1969;102:78-80.
Erickson BA, Meeks JJ, Roehl KA, et al. Bladder neck contracture after retropubic radical prostatectomy: incidence and risk factors from a large single-surgeon experience. BJU Int. 2009;104:1615-1619.
Ficazzola MA, Nitti VW. The etiology of post-radical prostatectomy incontinence and correlation of symptoms with urodynamic findings. J Urol. 1998;160:1317-1320.
Firrozi F, Vasavada S. Editorial comment. Urodynamic changes and initial results of the AdVance male sling. Urology. 2009;74:357-358.
Flynn BJ, Webster GD. Evaluation and surgical management of intrinsic sphincter deficiency after radical prostatectomy. Rev Urol. 2004;6:180-186.
Foote J, Yun S, Leach G. Postprostatectomy incontinence. Pathophysiology, evaluation, and management. Urol Clin North Am. 1991;18:229-241.
Fowler JW, Auld CD. The control of male stress incontinence by implantable prostheses. Br J Urol. 1985;57:175-180.
Frank I, Elliott DS, Barrett DM. Success of de novo reimplantation of the artificial genitourinary sphincter. J Urol. 2000;163:1702-1703.
Furlow WL, Barrett DM. The artificial urinary sphincter: experience with the AS 800 pump-control assembly for single-stage primary deactivation and activation—a preliminary report. Mayo Clin Proc. 1985;60:255-258.
Giberti C, Gallo F, Schenone M, et al. The bone anchor suburethral synthetic sling for iatrogenic male incontinence: critical evaluation at a mean 3-year followup. J Urol. 2009;181:2204-2208.
Groutz A, Blaivas J, Chaikin D, et al. Noninvasive outcome measures of urinary incontinence and lower urinary tract symptoms: a multicenter study of micturition diary and pad tests. J Urol. 2000;164:698-701.
Guimaraes M, Oliveira R, Pinto R, et al. Intermediate-term results, up to 4 years, of bone-anchored male perineal sling for treating male stress urinary incontinence after prostate surgery. BJU Int. 2009;103:500-504.
Gundian JC, Barrett DM, Parulkar BG. Mayo Clinic experience with use of the AMS800 artificial urinary sphincter for urinary incontinence following radical prostatectomy. J Urol. 1989;142:1459-1461.
Guralnick ML, Miller E, Toh KL, et al. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol. 2002;167:2075-2078.
Haab F, Trockman BA, Zimmern PE, et al. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of followup. J Urol. 1997;158:435-439.
Hajivassiliou CA. A review of the complications and results of implantation of the AMS artificial urinary sphincter. Eur Urol. 1999;35:36-44.
Harris SE, Guralnick ML, O’Connor RC. Urethral erosion of transobturator male sling. Urology. 2009;73:449-450.
Henry GD, Graham SM, Cleves MA, et al. Perineal approach for artificial urinary sphincter implantation appears to control male stress incontinence better than the transscrotal approach. J Urol. 2008;179:1475-1479.
Herndon CD, Rink RC, Shaw MB, et al. The Indiana experience with artificial urinary sphincters in children and young adults. J Urol. 2003;169:650-654.
Hübner WA, Schlarp OM. Treatment of incontinence after prostatectomy using a new minimally invasive device: adjustable continence therapy. BJU Int. 2005;96:587-594.
John H. Bulbourethral composite suspension: a new operative technique for post-prostatectomy incontinence. J Urol. 2004;171:1866-1870.
Kabalin JN. Addition of a second urethral cuff to enhance performance of the artificial urinary sphincter. J Urol. 1996;156:1302-1304.
Kowalczyk JJ, Nelson R, Mulcahy JJ. Successful reinsertion of the artificial urinary sphincter after removal for erosion or infection. Urology. 1996;48:906-908.
Kowalczyk JJ, Spicer DL, Mulcahy JJ. Long-term experience with the double-cuff AMS 800 artificial urinary sphincter. Urology. 1996;47:895-897.
Krambeck AE, DiMarco DS, Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448-453.
Kuznetsov DD, Kim HL, Patel RV, et al. Comparison of artificial urinary sphincter and collagen for the treatment of postprostatectomy incontinence. Urology. 2000;56:600-603.
Lai HH, Hsu EI, Boone TB. Urodynamic testing in evaluation of postradical prostatectomy incontinence before artificial urinary sphincter implantation. Urology. 2009;73:1264-1269.
Latini JM. Editorial comment. Urodynamic changes and initial results of the AdVance male sling. Urology. 2009;74:358.
Leach GE, Trockman B, Wong A, et al. Post-prostatectomy incontinence: urodynamic findings and treatment outcomes. J Urol. 1996;155:1256-1259.
Lepor H, Kaci L. The impact of open radical retropubic prostatectomy on continence and lower urinary tract symptoms: a prospective assessment using validated self-administered outcome instruments. J Urol. 2004;171:1216-1219.
Licht MR, Montague DK, Angermeier KW, et al. Cultures from genitourinary prostheses at reoperation: questioning the role of Staphylococcus epidermidis in periprosthetic infection. J Urol. 1995;154:387-390.
Light JK, Reynolds JC. Impact of the new cuff design on reliability of the AMS800 artificial urinary sphincter. J Urol. 1992;147:609-611.
Litwiller SE, Kim KB, Fone PD, et al. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol. 1996;156:1975-1980.
Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129-135.
Litwin MS, Hays RD, Fink A, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002-1012.
Lorentzen T, Dorph S, Hald T. Artificial urinary sphincters. Radiographic evaluation. Acta Radiol. 1987;28:63-66.
Madjar S, Jacoby K, Giberti C, et al. Bone anchored sling for the treatment of post-prostatectomy incontinence. J Urol. 2001;165:72-76.
Madjar S, Raz S, Gousse AE. Fixed and dynamic urethral compression for the treatment of post-prostatectomy urinary incontinence: is history repeating itself? J Urol. 2001;166:411-415.
Marks JL, Light JK. Management of urinary incontinence after prostatectomy with the artificial urinary sphincter. J Urol. 1989;142:302-304.
Montague DK. The artificial urinary sphincter (AS 800): experience in 166 consecutive patients. J Urol. 1992;147:380-382.
Montague DK, Angermeier KW. Postprostatectomy urinary incontinence: the case for artificial urinary sphincter implantation. Urology. 2000;55:2-4.
Montague DK, Angermeier KW. Artificial urinary sphincter troubleshooting. Urology. 2001;58:779-782.
Montague DK, Angermeier KW, Paolone DR. Long-term continence and patient satisfaction after artificial sphincter implantation for urinary incontinence after prostatectomy. J Urol. 2001;166:547-549.
Motley RC, Barrett DM. Artificial urinary sphincter cuff erosion. Experience with reimplantation in 38 patients. Urology. 1990;35:215-218.
Mulcahy JJ, Brant MD, Ludlow JK. Management of infected penile implants. Tech Urol. 1995;1:115-119.
O’Connor RC, Gerber GS, Avila D, et al. Comparison of outcomes after single or double-cuff artificial urinary sphincter insertion. Urology. 2003;62:723-726.
Park R, Martin S, Goldberg JD, et al. Anastomotic strictures following radical prostatectomy: insights into incidence, effectiveness of intervention, effect on continence, and factors predisposing to occurrence. Urology. 2001;57:742-746.
Peyromaure M, Ravery V, Boccon-Gibod L. The management of stress urinary incontinence after radical prostatectomy. BJU Int. 2002;90:155-161.
Raj GV, Peterson AC, Toh KL, et al. Outcomes following revisions and secondary implantation of the artificial urinary sphincter. J Urol. 2005;173:1242-1245.
Raj GV, Peterson AC, Webster GD. Outcomes following erosions of the artificial urinary sphincter. J Urol. 2006;175:2186-2190.
Rajpurkar AD, Onur R, Singla A. Patient satisfaction and clinical efficacy of the new perineal bone-anchored male sling. Eur Urol. 2005;47:237-242.
Rehder P, Gozzi C. Transobturator sling suspension for male urinary incontinence including post-radical prostatectomy. Eur Urol. 2007;52:860-866.
Ruiz E, Puigdevall J, Moldes J, et al. 14 years of experience with artificial urinary sphincter in children and adolescents without spina bifida. J Urol. 2006;176:1821-1825.
Saffarian A, Walsh K, Walsh IK, Stone AR. Urethral atrophy after artificial urinary sphincter placement: is cuff downsizing effective? J Urol. 2003;169:567-569.
Schaeffer AJ, Clemens JQ, Ferrari M, et al. The male bulbourethral sling procedure for post-radical prostatectomy incontinence. J Urol. 1998;159:1510-1515.
Schultheiss D, Hofner K, Oelke M, et al. Historical aspects of the treatment of urinary incontinence. Eur Urol. 2000;38:352-362.
Scott FB. The artificial sphincter in the management of incontinence in the male. Urol Clin North Am. 1978;5:375.
Scott FB. The artificial urinary sphincter. Experience in adults. Urol Clin North Am. 1989;16:105.
Singh G, Thomas DG. Artificial urinary sphincter for post-prostatectomy incontinence. Br J Urol. 1996;77:248-251.
Stanford JL, Stephenson RA, Coyle LM, et al. Prostate cancer trends 1973-1995. SEER Program, NIH Pub. No. 99-4543. Bethesda (MD): National Cancer Institute; 1999.
Stone AR, Nguyen M, Tse V. Re: new surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol. 2003;170:550-551.
Taylor GA, Lebowitz RL. Artificial urinary sphincters in children: radiographic evaluation. Radiology. 1985;155:91-97.
Timm GW, Bradley WE, Scott FB. [Development of an implantable artificial urinary sphincter (author’s transl)]. Urologe A. 1976;15:176-179.
Trigo-Rocha F, Gomes CM, Mitre AI, et al. A prospective study evaluating the efficacy of the artificial sphincter AMS 800 for the treatment of postradical prostatectomy urinary incontinence and the correlation between preoperative urodynamic and surgical outcomes. Urology. 2008;71:85-89.
Trigo-Rocha F, Gomes CM, Pompeo AC, et al. Prospective study evaluating efficacy and safety of Adjustable Continence Therapy (ProACT) for post radical prostatectomy urinary incontinence. Urology. 2006;67:965-969.
Ullrich NF, Comiter CV. The male sling for stress urinary incontinence: urodynamic and subjective assessment. J Urol. 2004;172:204-206.
Venn SN, Greenwell TJ, Mundy AR. The long-term outcome of artificial urinary sphincters. J Urol. 2000;164:702-706.
Wasson JH, Reda DJ, Bruskewitz RC, et al. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med. 1995;332:75-79.
Wei J, Dunn R, Marcovich R, et al. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol. 2000;164:744-748.
Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
Westney OL, Del Terzo MA, McGuire EJ. Balloon dilation of posterior urethral stricture secondary to radiation and cryotherapy in a patient with a functional artificial urethral sphincter. J Endourol. 1992;13:585-586.
Wilson SK, Delk JR, Henry GD, et al. New surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol. 2003;169:261-264.
Wolf JSJr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379-1390.
Yurkanin JP, Dalkin BL, Cui H. Evaluation of cold knife urethrotomy for the treatment of anastomotic stricture after radical retropubic prostatectomy. J Urol. 2001;165:1545-1548.