chapter 86 Cutaneous Continent Urinary Diversion
General Considerations
Continent urinary diversion is widely accepted by both urologist and patient alike as an acceptable form of urinary reconstruction after cystectomy. Orthotopic urethral anastomotic procedures and continent catheterizable stomal reservoirs have stood the test of time, and both procedures should always be considered for all appropriate patients. Orthotopic continent diversion and the metabolic consequences of continent urinary diversion are covered in separate chapters. In this chapter the focus is on the continent cutaneous diversion surgeries associated with the highest success rates. Over the past 25 years the design of the reservoir has not substantially changed. However, an evolution has occurred in the techniques used to create antireflux and continence mechanisms in order to make them more effective and reliable. In addition, attention is given to the long-term quality of life outcomes of continent cutaneous reservoirs, as well as to the newer laparoscopic approaches used to create such reservoirs.
Despite the considerable enthusiasm for continent urinary diversion operations, those procedures requiring the use of external urinary collecting appliances remain more common. Although continent urinary diversion is certainly appropriate in selected patients, the procedures are technically more challenging and associated with higher short-term and long-term complication rates than those that use external collecting devices. However, the operating time associated with these more complex procedures has been significantly reduced by the widespread use of absorbable and metal staples in the construction of the reservoirs and limbs. These techniques are discussed in detail later. Also, as experience with continent urinary diversion has grown, complication rates have decreased dramatically. As a result, in some centers continent diversions are now more commonly employed than conduit diversions.
Patient Selection
Because the ability to self-catheterize is essential to the patient undergoing continent diversion, the patient must be assessed for the ability to care for himself or herself. Consultation between an enterostomal therapist and the patient is extremely helpful in this regard because the patient may be at greater ease with the therapist and more willing to express any concerns. Certain patients may not be able to comprehend the strict flushing and catheterization regimens that must be followed after continent urinary diversion or may lack the motor skills to independently perform self-care. Patients with multiple sclerosis, quadriplegic individuals, and frail or mentally impaired patients will at some point in their lives require family or visiting nurses for basic care and are therefore viewed as poor candidates for any form of continent diversion. Indeed, these patients may also require assistance with external appliances, but the degree of time and expertise required is much less burdensome on the care provider and the health care system. On the contrary, continent catheterizable diversion requires continuous attention and may limit patient and family options when determining long-term care needs.
Patient Preparation
All patients undergoing anticipated continent urinary diversion should be prepared for the possibility that a traditional ileal conduit might be performed. Although it is rare to abandon a continent diversion owing to unanticipated problems, this always remains a possibility. Therefore before starting the operation, the site for an external stoma should be selected with extreme care. In general, the location must be free from fat creases in both the standing and sitting position and it should not be close to prior abdominal scars that may interfere with proper adherence of an external appliance. Here, again, the aid of an enterostomal therapist is helpful. In general, the stoma should be brought through the right (or left) lower quadrant of the abdomen on a line extending from the umbilicus to the anterior superior iliac spine. The stoma should be as far lateral from the midline as possible, but the site selected should ensure that the bowel segment comprising the stoma traverses the rectus muscle. Failure to adhere to this rule increases the risk of parastomal hernias. The selected site for the stoma should be marked with an X scratched onto the anterior abdominal wall. Marking the stoma site with ink should be avoided because it may be washed away during the antiseptic preparation of the skin.
The surgeon undertaking continent urinary diversion should be familiar with more than one type of continent diversion technique. Although it is uncommon to have to abandon a given bowel segment for the reservoir, it is not uncommon to have to modify the antireflux or continence mechanism. In these circumstances, it is essential that the surgeon be able to select an alternate form of continent diversion from what was originally intended.
Renal and hepatic function must be reviewed carefully in the patient selected for continent diversion (Mills and Studer, 1999). The reabsorption and recirculation of urinary constituents and other metabolites require that liver function be normal and that serum creatinine levels be within normal range, or certainly below the level of 1.8 mg/dL. In cases in which renal function is borderline, creatinine clearance should be measured. A minimal level of 60 mL/min should be documented before deeming the patient an appropriate candidate for continent diversion. In patients with bilateral hydronephrosis in whom renal functional improvement might be anticipated on relief of the ureteral obstruction, the upper urinary tract should first be decompressed with either ureteral stenting or percutaneous nephrostomy(ies). Subsequent reevaluation of renal function should be performed before undertaking a continent diversion.
Procedures that will require use of the colon should always be preceded by a colonoscopic assessment of the entire large intestine. Performing only a sigmoidoscopy for a procedure that will use only this segment of the large bowel is insufficient because disease proximal to the resected segment may leave the patient with short colon syndrome. The preoperative assessment of the colon is not necessary if continent urinary diversion using small intestine is planned.
Healthy patients undergoing radical cystectomy can be admitted to the hospital on the day of surgery. A mechanical bowel preparation is administered after a liquid dinner on the night before surgery. The patient is instructed to drink copious amounts of water, and at 8 PM and 10 PM the patient is administered oral metronidazole (500 mg). In addition, the patient should receive cefoxitin (1 gm) intravenously 1 hour before the skin incision.
Cystectomy
All operations described require a midline incision, skirting the umbilicus to the side opposite the selected stoma site. The incision for a right colon pouch usually extends from the pubis to a point midway between the umbilicus and the xiphoid. The cranial extent of the incision is governed by the hepatic flexure, which must be divided to obtain sufficient colonic length and to allow for the right colon to easily fold on itself. On some occasions, the incision will be extended to the xiphoid. The incision for procedures using only the ileum should extend to just below the umbilicus. The cystectomy procedure is covered elsewhere in this text, and only those points germane to continent diversion are covered here.
After abdominal exploration, the ureters are isolated, transected, and transposed to an appropriate place for subsequent diversion. The right retroperitoneum is first opened over the iliac artery to expose the right ureter. In the typical circumstance of conduit diversion, the right ureter is transected below the common iliac artery. For all continent diversions, both ureters are transected as low as possible and shortened to the appropriate length once the final anatomy is determined. The sigmoid colon is freed from its lateral peritoneal attachments by incising along the line of Toldt. A wide tunnel is created by blunt finger dissection ventral to the aorta and common iliac arteries and caudal to the inferior mesenteric artery. This affords left ureteral access to the previously exposed right retroperitoneum. In cases of uroepithelial malignancy it is prudent to evaluate the margin status of the most distal portion of both ureters using frozen section analysis. In situations where substantial ureteral length is removed to obtain negative surgical margins, extension of the afferent limb mechanism may be necessary to allow tension-free ureteral intestinal anastomoses.
All sutures used in the urinary tract should be absorbable. The individual surgeon’s preference will dictate the caliber and type of suture material used. When carrying out bowel surgery for continent urinary diversions, stapling is the preferred method for division of the bowel segment, as well as for reconstruction of bowel continuity. This technique shortens operative times greatly and affords safe and reliable bowel anastomosis. Suturing is not necessary with the exception of placing two silk Lembert sutures at the apex of side-to-side stapled bowel anastomoses in order to prevent tension on the staple line. To avoid stone formation on the stapled proximal bowel segments, oversewing the stapled end of the conduit with absorbable material isolates the metal staple line from urinary contact within the lumen.
In constructing a nonappendiceal continent urinary diversion stoma, a skin button matching the diameter of the structure to be used in the diversion is resected. Cutaneous tissues are separated down to the level of the anterior rectus fascia, where a circle of similar diameter is excised from this fascia or, alternatively, the fascia is incised in a cruciate fashion. In carrying out this maneuver, it is essential that the fascia and skin are properly aligned in order to avoid angulation. Rectus muscle fibers are separated bluntly and an instrument passed through the posterior fascia and peritoneum. For appendiceal stomas, we prefer to perform a Y-shaped cutaneous incision that allows for a YV-type plasty incision between the appendiceal limb and the skin (Fig. 86–1). This will decrease the likelihood of subsequent stomal stenosis. Alternatively, the appendix lends itself to an umbilical stoma (Bissada, 1993; Gerharz et al, 1997). Favorable results with this appendiceal YV plasty technique to the umbilical site have been reported (Bissada, 1998).
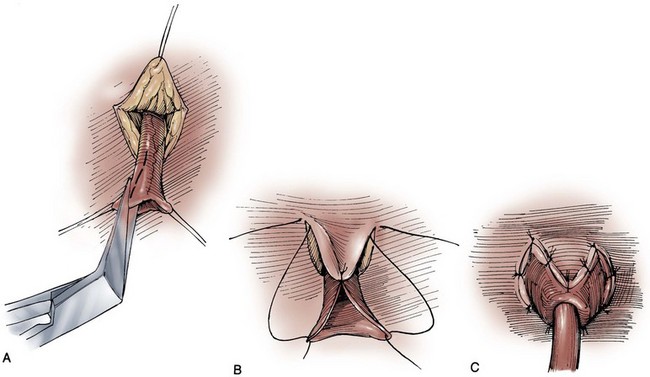
Figure 86–1 A to C, A V-flap is incised in the skin and a similar length incision made on the adjacent appendiceal surface. This is similar to the technique used to mature a cutaneous ureterostomy. For an appendiceal stoma, no eversion is required.
(From Hinman F Jr. Atlas of urologic surgery. Philadelphia: WB Saunders; 1989.)
It is standard procedure to use long, end-hole single J–type diverting stents in all continent cutaneous urinary diversions. These stents drain urine externally, ensuring that urine is safely diverted beyond any anastomotic site during the early healing period. They can also be safely manipulated or exchanged if necessary. The end hole allows for the passage of a straight wire through the stent, which decreases the likelihood of anastomotic trauma at the time of stent removal.
The authors advocate the use of closed suction drains in all cases of urinary diversion. Soft silicone Jackson-Pratt closed suction drains are preferred because they have less potential for tissue damage or migration into pouches.
Abdominal closure is performed according to the surgeon’s preference. In general a single-layer closure, using No. 2 nylon, Surgilene, or Prolene taken through all layers of fascia and muscle provides a rapid and secure abdominal closure in the majority of patients. In obese patients, those with tissues of poor quality, or nutritionally depleted patients, through-and-through stay sutures are also used. Ureteral stents are always brought through separate abdominal stab wounds, sutured to the anterior abdominal wall, and directed into separate drainage bags to monitor urine output. Even at this early stage it is important to ensure adequate drainage of the reservoir in order to prevent pouch rupture should the ureteral stents be displaced. In the case of limited pouch access such as with an appendiceal stoma, a Malecot tube should be placed directly into the reservoir and secured to the skin. The reservoir is sutured to the abdominal wall to prevent urine leakage into the peritoneal cavity when the tube is removed. This maneuver also helps to prevent migration and angulation of the reservoir, which could result in incontinence or catheterization difficulties.
Postoperative Care and Comments
Paralytic ileus is a common complication following urinary diversion procedures. Gastric decompression should be maintained until extubation. This can be achieved in the majority of patients by means of nasogastric intubation. However, certain patients may be managed best by formal gastrostomy decompression inserted intraoperatively. These individuals include those with multiple prior abdominal procedures in whom prolonged ileus is more likely. If the patient is nutritionally depleted preoperatively, hyperalimentation has been suggested to be of value if initiated during the preoperative interval (Hensle, 1983; Askanazi et al, 1985).
Ureteral stents are usually removed 1 week after surgery. Before any manipulation, a urine sample from each stent should be sent for culture and sensitivity testing. Before stent removal, radiographs of the pouch are obtained to ensure that the pouch is intact. Radiologic contrast studies are performed to ensure against ureteral anastomotic leakage. Each stent is injected with contrast agent in a search for extravasation; if none is seen, guidewires are advanced to each kidney and the stents removed. If there is any question of extravasation, stents can be advanced over the wires, positioned fluoroscopically, and left in situ for re-evaluation after additional healing has taken place.
Late malignancy has been reported in all bowel segments exposed to the urinary stream, whether or not there is a commingling with feces (Filmer and Spencer, 1990; Shokeir et al, 1995). A study by Gitlin and colleagues (1999) suggests that the malignancy may develop from the urothelial component and not as a result of urine affecting intestinal mucosa. As a result, urinary cytology should be performed in all patients undergoing a continent urinary diversion whether or not the diversion was performed secondary to a urothelial malignancy. When the ureters are directed into the fecal stream, routine colonoscopy should also be performed. Latency periods have been reported as short as 5 years, so all patients developing gross or microscopic hematuria should be fully evaluated (Golomb et al, 1989). If an anastomotic transitional cell cancer is discovered, the patient should be fully evaluated with upper tract imaging and ureteroscopy if possible. Antegrade ureteropyeloscopy can be employed if necessary. For an isolated anastomotic recurrence, distal ureterectomy and reimplantation may be appropriate. If nephroureterectomy is necessary, some patients may require removal of their continent diversion due to resulting renal insufficiency.
Continent Urinary Diversion
Continent, nonorthotopic urinary diversion can be divided into two major categories. First, the variations of ureterosigmoidostomy such as ileocecal sigmoidostomy, rectal bladder, and the sigmoid hemi-Kock operation with proximal colonic intussusception are discussed. These techniques allow for excretion of urine by means of evacuation. Second is the large category of continent diversions requiring clean intermittent catheterization of the constructed pouch for urine drainage at standard intervals.
The concept of refashioning bowel so that it serves as a urinary reservoir rather than a conduit has become universally accepted. This concept is based on original pioneering observations by Goodwin and colleagues in the development of the cystoplasty augmentation procedure (Goodwin et al, 1958). The destruction of peristaltic integrity and refashioning of bowel has led to the development of many innovative urinary reservoirs constructed from bowel. Several antireflux procedures have evolved to avoid upper tract urinary damage by sepsis or reflux, while other surgical techniques have been devised to achieve urinary continence.
Because there are numerous variants of continent urinary diversion used worldwide, a complete review of all operative techniques is beyond the scope of this or any chapter. However, many of these procedures are simple modifications of parent operations. In this chapter we describe in detail each parent operation, as well as major modifications. The fact that there are many continent urinary diversion procedures described reveals an obvious corresponding fact: the “best” continent diversion has yet to be devised. There is, to date, no consensus that would indicate one continent cutaneous diversion is superior to another, but it is becoming apparent that certain procedures are associated with lower early and late complication rates. Points of controversy include which bowel segment is most appropriate for fashioning into a urinary reservoir, the best techniques to use for achieving urinary continence, and the best technique for prevention of urine reflux into the upper tracts. There are now various continence mechanisms that appear reliable. In the authors’ experience, procedures using a right colon reservoir with some form of appendiceal continence mechanism are the fastest and easiest to perform.
It should be re-emphasized that all continent diversions will allow for substantial reabsorption of urinary constituents that will place an increased workload on the kidneys (Mills and Studer, 1999). No patient with substantial renal impairment should be considered for any of these procedures. The long-term sequelae of continent urinary diversion are well understood and, unfortunately, commonly involve significant renal damage. Although it has been suggested that the absence of reflux into the upper urinary tracts in catheterizable pouches may reduce the long-term impact of continent diversion procedures on renal function, it should be cautioned that long-term 15-year data are now available and, in some instances, antireflux procedures are associated with a higher risk of obstruction due to anastomotic stricture (Kristjansson et al, 1995). In addition to increased stricture rates, it is not clear whether antirefluxing mechanisms actually result in improved preservation of the upper tracts (Pantuck et al, 2000).
Multiple international studies have suggested an improved psychosocial adjustment of patients undergoing continent urinary and fecal diversion compared with those patients with diversions requiring collecting appliances (Gerber, 1980; McLeod and Fazio, 1984; Boyd et al, 1987; Salter, 1992a, 1992b; Bjerre et al, 1995; Filipas et al, 1997; Hart et al, 1999; McGuire et al, 2000). Although this is indeed true and is best exemplified by the individual with a conduit who desires conversion to a continent procedure, it is also true that many individuals seem to adjust well to wearing external appliances. The sense of body image is a remarkably personal and subjective parameter that varies greatly from patient to patient. In fact, the majority of patients are satisfied with their choice of urinary diversion, whether it is continent or not.
The process of patient counseling that we employ always refers to ileal conduit diversion as the gold standard against which the newer, more complex operations must be compared. The patient should be advised that continent diversion is, all other considerations being equal, associated with a longer hospital stay, higher complication rates, and greater potential for requiring reoperative surgery. However, it should be noted that an extensive review from our institution has demonstrated no statistically significant difference in reoperations, mortality, or hospital stay in patients undergoing continent diversion versus conduit diversion by the same three surgeons over a 3-year period (Benson et al, 1992). Analysis of the two patient groups, on the other hand, showed that, in general, those selected for continent diversion were 12 years younger and four times less likely to have significant concurrent illness. What this review suggests is that, with proper patient selection, continent diversion operations can be safely conducted with results similar to those for conduit diversion. To determine if continent diversion could be safely performed in selected elderly patients, Navon and colleagues (1995) compared the clinical course of 25 patients older than the age of 75 years undergoing a modified Indiana reservoir to a cohort of 25 randomly selected patients younger than 75. The mean age of the first group was 78.5 years, and the mean age of the second was 59.3 years. The complication rates between the two groups were acceptably low and surprisingly similar. Navon and colleagues concluded that age alone should not be a contraindication to continent diversion and that the Indiana reservoir can be successfully performed in elderly patients.
Rectal Bladder Urinary Diversion
Various innovative surgical techniques have been advocated for separating the fecal and urinary streams, while still employing the principles of ureterosigmoidostomy. These operations can generally be discussed together as rectal bladder urinary diversions. In each of these operations the ureters are transplanted into the rectal stump. The proximal sigmoid colon is managed by terminal sigmoid colostomy or, more commonly, by bringing the sigmoid to the perineum, thereby using the anal sphincter to achieve both bowel and urinary control. Although these operations continue to be commonly performed, they have never been well accepted in the United States. The principal reason is the potential for the calamitous complication of combined urinary and fecal incontinence, presumably occurring as a consequence of damage to the anal sphincter mechanism during the dissection processes (Culp, 1984).
If the urologist selects one of these procedures, the preoperative evaluation should include all of the caveats of ureterosigmoidostomy. Dilated ureters are not acceptable. Patients with extensive pelvic irradiation are not candidates, and neither are those with existing renal insufficiency. Anal sphincteric tone must be judged competent before electing these operations. Our preference has been to use a 400- to 500-mL thin mixture of oatmeal and water that the patient is asked to retain for 1 hour in the upright position (Spirnak and Caldamone, 1986). Finally, colonoscopy must be carried out before the procedure to rule out pre-existing colorectal disease, as well as after the procedure to guard against the potential development of colonic cancer. Procedures that separate the fecal and urinary streams but drain both through the rectal sphincter are not described here. Those wishing a detailed description of these procedures can find them in prior editions of this chapter. The following is a brief description of more modern surgical procedures that use the intact anal sphincter for urinary and fecal continence. However, the surgical techniques for these procedures will likewise not be discussed in this edition.
Folded Rectosigmoid Bladder
A modification of the ureterointestinal anastomosis was described by a group from Mansoura, Egypt (Hafez et al, 1995; El-Mekresh et al, 1997). This procedure creates a folded rectosigmoid bladder with anastomosis of the ureters via serosa-lined tunnels rather than into the taenia coli. This procedure has the advantage of a larger sigmoid reservoir, as well as the prevention of reflux by creating the above serous-lined tunnel for the anastomosis. This reimplantation technique was first described by Abol-Enein and Ghoneim (1993) and appears to have a lower complication rate than direct taenial implantation (Hafez et al, 1995).
Postoperative Care and Comments
Patients undergoing this procedure must be closely monitored for the development of hyperchloremic acidosis. This will occur in the majority of cases, and it is wise to initiate a bicarbonate replacement program following the operation. Because hypokalemia is also a feature of ureterosigmoidostomy, replacement of potassium along with bicarbonate may be achieved with oral potassium citrate. Routine nightly insertion of a rectal tube is advocated in the long-term care of the patient. However, many patients will reject this practice as uncomfortable and unappealing. Nighttime urinary drainage should be mandated in any patient who cannot maintain electrolyte homeostasis with oral medication. Bissada and colleagues (1995) reported that 30 of 61 patients were able to stay dry during the night without awakening. The other 31 required two or more awakenings to remain dry overnight. Hyperchloremic acidosis was reported in 4 of 61 noncompliant patients.
In 1997 El-Mekresh and colleagues (1997) reported on 64 patients (32 women, 20 men, and 12 children) who underwent their rectosigmoid bladder procedure between 1992 and 1995. Follow-up ranged from 6 to 36 months. Functional results were assessable in 57 patients: 1 died of a postoperative pulmonary embolism and 6 died from their disease. All patients were continent during the day with two to four emptyings, whereas all but four remained dry at night with zero to two emptyings. Four children experienced enuresis that responded to 25 mg of imipramine at bedtime. Importantly, upper urinary tract function was maintained or improved in 95% of patients. However, six renal units (5.3%) developed obstructive hydronephrosis secondary to ureterocolic anastomotic strictures. Two were remedied by antegrade dilation, one was repaired by open revision, and one nonfunctioning renal unit was removed. The fate of the remaining two units was not specified. No patient in this series developed a postoperative metabolic acidosis. However, all patients were maintained on prophylactic oral alkalinization.
Obviously, all patients undergoing these procedures have exposure of the urinary tract to fecal flora. Most authors would advocate chronic administration of an antibacterial agent in all patients (Duckett and Gazak, 1983; Spirnak and Caldamone, 1986). Ureteral strictures require reoperative surgery and are experienced in 26% to 35% of cases over time (Williams et al, 1969; Duckett and Gazak, 1983).
Because of the concern for development of rectal cancer anywhere between 5 and 50 years (average 21 years) after ureterosigmoidostomy (Ambrose, 1983), it is suggested that patients with long-term ureterosigmoidostomy undergo annual colonoscopy (Filmer and Spencer, 1990). Barium enemas are relatively contraindicated because reflux of this material into the kidneys (if the antireflux procedure fails) can result in dire consequences (Williams, 1984). Additional methods for colon carcinoma screening in this population are the evaluation of stool for blood, and the attempted cytologic examination of the mixed urine and feces specimen (Filmer and Spencer, 1990).
Augmented Valved Rectum
Kock developed this technique to be used in locales where stoma appliances were not readily available (Kock et al, 1988). This operation is similar to standard ureterosigmoidostomy except that a proximal intussusception of the sigmoid colon confines the urine to a smaller surface area, thus minimizing the problems of electrolyte imbalance. Additionally the rectum is patched with ileum to improve the urodynamic properties of the rectum as a urinary reservoir. Preoperative evaluation is similar to that used in ureterosigmoidostomy. The large bowel must be studied for pre-existing disease, and anal sphincteric integrity must be tested before surgery.
Hemi-Kock and T Pouch Procedures with Valved Rectum
In his description of the augmented valved rectum procedure, Kock described the use of a foreshortened hemi-Kock pouch to be used as a rectal patch when the ureters were too dilated to bring down between the leaves of the intussuscepted sigmoid (Kock et al, 1988). Skinner then modified this procedure by using an entire hemi-Kock segment to augment the rectum after sigmoid intussusception (Skinner et al, 1989).
After extensive experience with the Kock ileal reservoir, the group at the University of Southern California has attempted to improve on the intussuscepted Kock continence mechanism. The result has been the modification of the T pouch to serve as an ileal anal reservoir (Stein et al, 1999a). The technique consists of the construction of a hemi-Kock or T pouch employing doubly folded, marsupialized ileum and a proximal continence mechanism to prevent pouch-ureteral reflux. This pouch is then anastomosed to the rectum directly as a patch. Contact of urine with the proximal colon can be avoided by the intussusception of the sigmoid colon proximal to the anastomotic site (Fig. 86–2).
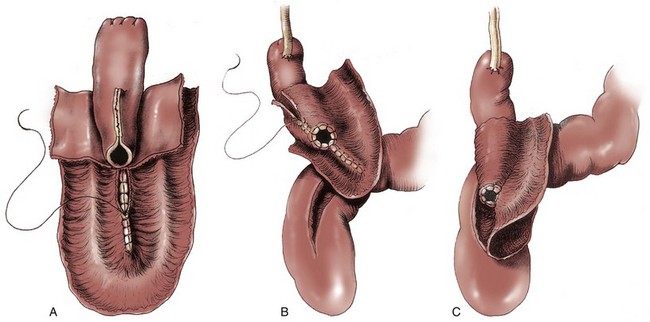
Figure 86–2 A, A 30-cm segment of ileum is selected, the first 10 cm for the T implant and the distal 20 cm for the patch. The 20-cm segment is folded into a U and opened as shown. The medial borders are closed with running absorbable sutures. B, The ostium of the T is secured to the walls of the ileum with interrupted absorbable sutures. The wall of the ileum is closed over the T mechanism with a running absorbable suture. C, The ureters are anastomosed to the top of the T in the usual end-to-side fashion. The T patch is then secured to the 15-cm proctotomy with a two-layer closure.
(From Stein JP, Buscarini M, DeFilippo RE, Skinner DG. Application of the T pouch as an ileo-anal reservoir. J Urol 1999;162:2052–3.)
Postoperative Care and Comments
Postoperative management and complications associated with this operation are similar to those that might be experienced after any procedure that directs the urinary stream into the rectum. Radiologic studies of the stents are carried out on the seventh postoperative day. Before conducting stent studies, a Gastrografin enema may be performed through the rectal tube to ensure that the region of ureterocolonic anastomosis is intact. Follow-up films are taken to ensure prompt drainage of the upper urinary tracts into the rectosigmoid region. The rectal tube may be removed at this point, but some believe that it is advisable to have it reinserted for evening drainage over the forthcoming week. The patient is instructed to empty the colon at intervals of no more than every 2 hours, particularly in the early postoperative period.
When the rectal tube is removed, as in other situations when the urinary tract is diverted to the rectum, the patient must be closely monitored for the development of hyperchloremic acidosis. Because hypokalemic metabolic acidosis often occurs after ureterosigmoidostomy, bicarbonate replacement with oral potassium citrate should begin in the immediate postoperative setting. Long-term care should include nightly insertion of a rectal tube despite the uncomfortable and unappealing nature of the process. In addition, nighttime urinary drainage is necessary in patients who cannot maintain electrolyte homeostasis with oral medication.
The hemi-T procedure with valved rectum has one theoretical advantage over the valved rectum operation itself: Because transitional ureteral epithelium is not in contact with colonic epithelium, there may be a reduced risk of developing colonic malignancy. As in the augmented valved rectum, the proximal colonic intussusception used in this procedure decreases the contact between urine and colonic epithelium, thereby potentially decreasing the risk of hyperchloremic acidosis. Nevertheless, attention should be paid to electrolyte levels after removal of stents and rectal tubes.
Skinner and colleagues reported on the results of the hemi-Kock procedure in 15 patients between 1987 and 1991 (Simoneau and Skinner, 1995). Four patients had prior bladder exstrophy and were converted to an ileoanal reservoir, and 11 patients underwent the procedure as a form of primary diversion after cystectomy. At the time of the report 10 patients were still alive and could be evaluated. Early postoperative complications occurred in three patients (20%): a colocutaneous fistula in two patients, urine leak in one, and deep venous thrombosis in another. Late complications included partial small bowel obstruction in four patients (with two requiring surgery), urinary retention requiring surgery in two patients, and metabolic acidosis in five patients. Two of the 11 patients undergoing primary construction never achieved continence; both were older than 68 years. The authors summarized their experience by concluding that the operation is best suited for the younger exstrophy patient and that it is essential to avoid colonic redundancy distal to the reservoir. The use of the T pouch as an ileoanal reservoir has been reported in one former exstrophy patient (Stein et al, 1999a), with no reported postoperative complications.
Sigma-Rectum Pouch, Mainz II
A variation of ureterosigmoidostomy was described by Fisch and Hohenfellner in 1991 and updated in 1996 (Fisch and Hohenfellner, 1991; Fisch et al, 1996). This operation, which they termed the sigma rectum or the Mainz II pouch, creates a low-pressure rectosigmoid reservoir of increased capacity. They viewed the simplicity and reproducibility of the operation as one of its major advantages.
Postoperative Care and Comments
The rectal tube is removed on the third to fifth postoperative day, and the ureteral stents are removed around the eighth day. On the 15th postoperative day the Mainz group performs an intravenous pyelogram to assess the upper tracts and the sigma rectum pouch construction. Radiography of the pouch is performed on the 17th postoperative day.
The results of the Mainz II pouch were reported by Fisch and colleagues in 1997. Between 1990 and 1993, 73 patients (59 adults and 14 children) underwent the Mainz II pouch procedure. Early complications were encountered in 5 of 73 patients (6.8%). These included single examples of a dislodged ureteral stent, pneumonia, pulmonary embolism, wound dehiscence, and ileus necessitating surgical intervention. There were eight (10.9%) late complications that required surgery: ureteral stenosis occurred in five patients (6.8%); one patient with nephrolithiasis was treated with extracorporeal shockwave lithotripsy; one patient with rupture of the anterior suture line required temporary colostomy; and one patient experienced perianal bleeding after chemotherapy that required endoscopic coagulation. Six patients presented with pyelonephritis (8.2%) and were treated with antibiotics. Daytime and nighttime continence were reported as 94.5% and 98.6%, respectively. Oral alkalinization to prevent metabolic acidosis was used in 49 of 73 patients (67.1%). Two patients who refused any oral medication developed metabolic acidosis. The Mainz group concluded that the overall complication rate was low and comparable with other techniques of continent urinary diversion.
Woodhouse and Christofides (1998) reported on their experience with the Mainz II pouch in 15 primary cystectomy patients and 4 patients with prior standard ureterosigmoidostomy who were incontinent. They reported excellent results: 14 of 15 (93.3%) of the primary patients achieved documented daytime and nighttime urinary control, while the remaining patient refused follow-up but reported continence. The four patients undergoing a salvage procedure fared less well. Only two patients became continent, while the remaining two were found to be in chronic retention. Their failed continence was believed to be secondary to inadequate pouch emptying. Similarly, excellent results have been achieved by Venn and Mundy (1999). They reported full daytime and nighttime urinary continence in 14 of 14 patients and no major postoperative complications.
Bastian and colleagues (2004) have reported on the health-related quality of life in 83 patients undergoing Mainz II urinary diversion. They found that quality of life was similar to that of age-matched controls except for diarrhea symptoms, with 100% daytime continence.
There appears to be no metabolic advantage to this procedure because the need for oral alkalinization is similar to standard ureterosigmoidostomy. In fact, the only difference between this operation and standard ureterosigmoidostomy is the partial reconfiguration of the rectosigmoid junction. It does appear that the reduced intracolonic pressures that result from the partial reconfiguration increases the sigmoid capacity and results in better daytime and nighttime continence. Whether the increased capacity and lower pressure of this pouch will decrease the incidence of upper tract complications remains to be determined by longer follow-up.
Continent Catheterizing Pouches
Numerous operative techniques have been developed for continent diversion wherein urine is emptied at intervals by clean intermittent self-catheterization. Many of these operations are described in this chapter, although certain pioneering procedures that used intact bowel (e.g., those of Gilchrist and colleagues [1950], Ashken [1987], Mansson and colleagues [1984, 1987], Benchekroun [1987]) are not. This is not to discredit the pioneers in the field but simply to allow this chapter to focus on those pouches that incorporate modern principles that attempt to achieve a spherical configuration and disruption of peristalsis.
In continent urinary diversion, the two favorite sites for stomal location are (1) at the umbilicus and (2) in the lower quadrant of the abdomen, through the rectus bulge and below the “bikini” line. This location is often preferred because it affords both men and women the opportunity to conceal the stoma. The umbilicus is a preferred location for the individual confined to a wheelchair and has been reported to have a lower incidence of stomal stenosis, especially when fashioning an appendiceal stoma. The umbilical location is also far easier for the paraplegic individual to catheterize without the need for chair transfer and disrobing. In individuals with a recessed umbilicus, the umbilical location of a stoma is barely perceptible from a normal umbilical dimple. Generally, the stoma site is covered with a gauze pad or square bandage to avoid mucous soiling of clothing. Patients undergoing continent urinary diversion to an umbilical location should be advised to wear a medical alert bracelet that informs the examiner of the umbilical stoma.
Before the extension of orthotopic neobladder construction to women, there was some enthusiasm for the orthotopic placement of a catheterizing portal. This procedure has been carried out in certain female patients with success. The construction of a neourethra to the introitus is attractive, provided there is no substantial difficulty in the catheterizing process. Because it can be difficult to direct a catheter through the “chimney” of an intussuscepted nipple valve, those continent diversions employing nipple valves are not particularly adaptable to orthotopic location, although they have been performed with success in a small number of patients (Olsson, 1987). In contrast, the imbricated and tapered ileal segment leading to an Indiana pouch is relatively easier to catheterize and can be used for orthotopic catheterizing diversion (Rowland et al, 1987). However, it may be difficult to obtain sufficient mesenteric length in some patients. The appendix can also be used as a neourethra, in which case mesenteric length should become less of a problem (Hubner and Pfluger, 1995).
Four general techniques have been employed to create a dependable, catheterizable continence zone. For right colon pouches, appendiceal techniques, pseudoappendiceal tubes fashioned from ileum or right colon, and the ileocecal valve plication are applicable. Appendiceal tunneling procedures are the simplest of all to perform because they use established surgical techniques already present in the urologic armamentarium. The in situ or transposed appendix is tunneled into the cecal taenia in a fashion similar to ureterocolonic anastomosis. Appendiceal continence mechanisms have been criticized for three general reasons. First, the appendix may be unavailable in some patients because of prior appendectomy. For those individuals, techniques have been developed that allow for the construction of a similar tube fashioned from ileum (Woodhouse and MacNeily, 1994) or from the wall of the right colon (Lampel et al, 1995a). Second, the appendiceal stump may be too short to reach the anterior abdominal wall or umbilicus while still maintaining sufficient length for tunneling. This criticism has been addressed by an operative variation described by Mitchell, in which the appendiceal stump can be lengthened by the inclusion of a tubular portion of proximal cecum (Burns and Mitchell, 1990) (Fig. 86–3). This lengthening procedure has the added advantage of allowing for a slightly larger stoma made of cecum that is less prone to stomal stenosis. Appendiceal continence mechanisms share the feature of allowing only small-diameter (14- to 16-Fr) catheters to be used for intermittent catheterization, whereas the large amount of mucus produced by an intestinal reservoir is more easily emptied or irrigated using a 20- to 22-Fr catheter. We believe that these criticisms are more theoretical and that the appendiceal or pseudoappendiceal continence mechanism remains an attractive and reliable continence mechanism.
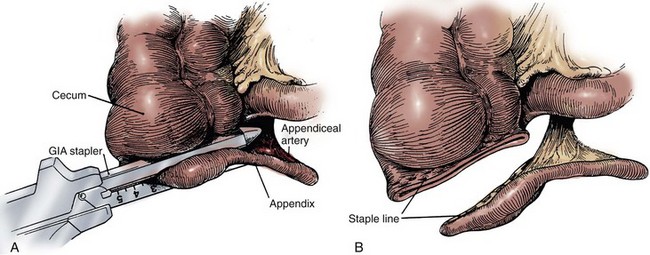
Figure 86–3 A, The appendiceal stump is lengthened by the inclusion of a tubular portion of proximal cecum by the application of the gastrointestinal anastomosis (GIA) stapler to the terminal cecum. A window is made in the mesoappendix, and the blade of the GIA stapler is advanced through the window. This maneuver ensures that the blood supply is not inadvertently damaged. B, The added length is demonstrated. The appendix is rotated and implanted into the taenia; the cecal tube serves as the stoma.
(From Burns MW, Mitchell ME. Tips on constructing the Mitrofanoff appendiceal stoma. Contemp Urol 1990;May:10–2.)
The second major type of continence mechanism used in right colon pouches is the tapered and/or imbricated terminal ileum and ileocecal valve. Here again the technology is rather simple, with imbrication or plication of the ileocecal valve region along with tapering of the more proximal ileum in the fashion of a neourethra (Rowland et al, 1985; Lockhart, 1987; Bejany and Politano, 1988). These techniques afford a reliable continence mechanism.
One feature of right colon pouches that has been criticized is the loss of the ileocecal valve. Although this does result in an increased frequency of bowel movements for some patients in the short term, the majority will experience bowel regularity either through intestinal adaptation or with the use of pharmacologic therapy. However, some patients have developed rather striking diarrhea/steatorrhea after the loss of the ileocecal valve. This may be particularly true in pediatric patients in whom there is neurogenic bowel dysfunction (e.g., myelomeningocele).
The third surgical principle used in constructing the continence mechanism is the use of the intussuscepted nipple valve or, more recently, the flap valve, which avoids the need for intussusception. The creation of nipple valves is by far the most technologically demanding of all the continence mechanisms, and it is associated with the highest complication and reoperation rates. There exists a significant learning curve before the surgeon achieves reproducible and dependable results. For this reason, nipple valve construction should probably not be chosen by the surgeon carrying out occasional construction of continent pouches. Furthermore, it should be noted that in the past 2 decades we have seen the introduction of numerous modifications of the original technique of Kock for construction of a stable nipple valve. The singular reason for all of these modifications is the rather disappointing long-term stability of the nipple valve in some patients. As a result, the group at the University of Southern California has developed the T pouch, which uses a flap valve (Stein et al, 1998). This procedure, which appears much simpler than the intussuscepted nipple valve, has been used to create both a continence and an antireflux mechanism. Nipple valve failure from slippage or valve effacement can be anticipated in 10% to 15% of cases even in the hands of the best and experienced surgeons. In addition to slippage, nipple valves are subject to ischemic atrophy. When this occurs, a new nipple valve must be fashioned from a new bowel segment.
A final feature of stapled nipple valves is the potential for stone formation on exposed staples. This was greatly lessened by the omission of staples at the tip of the intussuscepted nipple valve, as suggested by Skinner and colleagues (1984). However, more proximal staples occasionally erode into the pouch and serve as a nidus for stone formation. These stones are usually manageable endoscopically with forceps extraction or else with electrohydraulic or ultrasonic disintegration of the stone with subsequent forceps extraction of the staple. Although exposed staples may serve as a nidus for stone formation, continent urinary diversion in and of itself results in more urinary excretion of calcium, magnesium, and phosphate as compared with ileal conduit diversion (Terai et al, 1995). Thus all patients undergoing continent diversion are at an increased risk for the formation of reservoir stones.
The fourth major technique of continence mechanism construction is the provision of a hydraulic valve, as in the Benchekroun nipple (1987). In this procedure a small bowel segment is isolated, with subsequent reversed intussusception that effectively apposes the mucosal surfaces of the segment. Tacking sutures are placed on a portion of the circumference of the intussuscepted segment in order to stabilize the nipple valve while allowing urine to flow freely between the leaves of apposed ileal mucosa. As the pouch fills, hydraulic pressure closes the leaves, thereby ensuring continence. The premise of this technique is that as the reservoir fills, the pressure within the valve would also increase, resulting in continence. Concerns regarding stomal stenosis, especially in children, and nipple destabilization have resulted in this procedure being largely abandoned (Sanda et al, 1995). As a result, it is not discussed in this chapter.
General Procedural Methodology
During construction of the pouch, intraoperative testing for pouch integrity should always be performed. The continence mechanism is also tested for ease of catheterization, as well as continence after the pouch construction has been completed. The pouch is filled with saline, the continence mechanism catheter is removed, and the pouch is compressed lightly to look for points of leakage and to test the continence mechanism for its ability to contain urine. Thereafter, the continence mechanism is catheterized to ensure ease of catheter passage. This is an extremely important and crucial maneuver because the inability to catheterize is a serious complication that will often result in the need for reoperation. In general, all redundancy should be removed from the continence mechanism. It is often useful to secure the reservoir to the anterior abdominal wall in a manner that prevents the reservoir from migrating. This can prevent the development of a false passage or a kink, thereby facilitating catheterization.
Postoperatively, the larger-bore catheter used for drainage of the pouch should be irrigated at frequent intervals to prevent mucous obstruction. This can be performed at 4-hour intervals by simple irrigation with 45 to 50 mL of saline. Less frequent intervals of irrigation can be employed when the urine is totally diverted from the kidneys by means of long indwelling stents. It is essential that as soon as possible the patient be taught how to self-irrigate and what kind of regimen is required. This is performed to familiarize the patients with the catheterization process, to reduce the work burden on the nursing staff, and to allow for earlier discharge.
On the seventh postoperative day, a contrast study is performed to ensure pouch integrity. Thereafter, ureteral stents may be removed if no leaks are demonstrated by imaging studies. When it has been ascertained that the ureteral anastomoses and pouch are intact, the suction drain is removed. The suprapubic tube (if employed) can also be removed at this time, or it can be left in place until the patient is confident with self-catheterization. The patient is taught to irrigate the tube traversing the continence mechanism at 4-hour intervals and whenever any episode of intra-abdominal pressure or discomfort is experienced. Once these procedures are mastered and the patient is tolerating a regular diet, the patient can be discharged. This usually occurs between hospital days 6 and 8.
The following represents a summary of common patient questions and everyday solutions:
In the case of ileal pouches, pouch capacity will initially be low (150 mL). Therefore the frequency of catheterization will have to be significantly different in these individuals compared with those with right colon pouches in which initial comfortable capacities will be in excess of 300 mL. To ensure restful sleep, the smaller-capacity pouches may be managed best with indwelling catheterization during sleeping hours.
General Care
Because all patients with catheterized pouches will have chronic bacteriuria, the problem of antibiotic management should be discussed. Most authors would suggest that bacteriuria in the absence of symptomatology does not warrant antibiotic treatment (Skinner et al, 1987). The construction of an effective antireflux mechanism in these pouches may help protect against clinical episodes of pyelonephritis, in contrast to patients with freely refluxing conduits. Obviously, if clinical pyelonephritis does occur, antibiotic treatment should be instituted. Episodes of recurrent pyelonephritis should be evaluated with radiography of the pouch in order to diagnose failure of the antireflux mechanism or upper tract stone formation.
A condition termed “pouchitis” is manifested by pain in the region of the pouch along with increased pouch contractility. It should be mentioned that this condition, although infrequent, may result in temporary failure of the continence mechanism because of the hypercontractility of the bowel segment employed for construction of the pouch. The patient typically presents with a history of sudden explosive discharge of urine through the continence mechanism (rather than dribbling incontinence), along with discomfort in the region of the pouch. Appropriate antibiotic therapy will usually result in resolution of these symptoms. It has been our experience that short courses of antibiotics are not usually successful when treating pouch infections. This may be due to the larger amount of foreign material in the form of mucus and sediment within intestinal pouches as opposed to the bladder. Intestinal crypts may also serve as bacterial sanctuaries. Therefore whenever a pouch infection is diagnosed, antibiotic therapy should be continued for at least 10 days. Pyelonephritis will, of course, require longer courses of therapy.
Urinary retention is an infrequent but serious occurrence in catheterizable pouches. It is most commonly seen with pouches whose continence mechanism consists of a nipple valve. In these circumstances, when the chimney of the nipple valve is not near the abdominal surface, the catheter may be misdirected into folds of bowel rather than into the nipple valve proper, resulting in urinary retention. Pouch urinary retention represents a true emergency, and the patient must seek immediate attention so that catheterization and drainage by experienced personnel can be achieved promptly. The use of a coudé tipped catheter may be helpful. Rarely, a flexible cystoscope will be necessary. After the immediate problem has been resolved by emptying the pouch, a catheter should be left indwelling for 3 to 5 days to allow the edema and trauma to the catheterization portal to resolve. Before discharge, the patient should be observed to successfully self-catheterize on multiple occasions. The appropriate angle of entry should be taught to the patient until he or she is comfortable with the use of the new catheter. In fact, the authors prefer to routinely use coudé catheters with non–nipple valve pouches.
Intraperitoneal rupture of catheterizable pouches has been reported (Kristiansen et al, 1991; Thompson and Kursh, 1992; Watanabe et al, 1994). In general, these episodes are more common in the neurologic patient when sensation of pouch fullness may be less distinct (Hensle, personal communication; Mitchell, personal communication). Ruptures may also be associated with mild abdominal trauma, such as a fall. In general, these patients require immediate pouch decompression and radiographic pouch studies. For patients with large defects, surgical exploration and pouch repair are required. If the amount of urinary extravasation is small, and the patient does not have evidence of peritonitis, catheter drainage and antibiotic administration may suffice in treating an intraperitoneal rupture. Patients managed with this conservative approach require careful monitoring. If there is any sign of progressive peritonitis, surgical exploration and repair is imperative. The authors have successfully employed this nonoperative approach on patients with ruptured right colon pouches.
Continent Ileal Reservoir (Kock Pouch)
This operation was first reported for use in urinary diversion by Kock and colleagues in 1982. This report was singularly responsible for the renewed interest in continent diversion procedures at that time. An outgrowth of the Kock procedure for continent ileostomy (Kock, 1971), the Kock pouch combined reasonably dependable techniques for ensuring urinary continence and preventing reflux to the upper urinary tracts (nipple valves) with carefully refashioned bowel that provided a low-pressure urinary reservoir. This procedure and the similarly constructed T pouch are the only catheterizable continent diversions that preserve the ileocecal valve. Skinner and his colleagues (1989, 1992) have carefully studied and improved the technique over the years while amassing a prodigious experience with the operation and its variants. The high complication rate and the technical difficulties involved with constructing this reservoir have resulted in the procedure being abandoned by most individuals. As a result, this procedure is not discussed further in this edition. Those interested in a more detailed description should refer to previous editions of this text. However, the construction of a Kock limb remains an important procedure for use in repairing failed continence or reflux mechanisms and, as such, is described in more detail. It is Skinner’s operative description that will be followed closely in this chapter.
Procedure
A 15- to 20-cm length of ileum is selected for creating the intussuscepted nipple valve. The proximal 10 cm serve as the valve, and the distal 5 to 10 cm serve as the patch (Fig. 86–4A). The distal length is chosen on the basis of the volume lost after resection of the failed mechanism. Only 5 cm are necessary for the patch, but on some occasions the reservoir itself may require augmentation. The middle 6 to 8 cm of the 10-cm segment are denuded of mesentery by electrocoagulation. An Allis or Babcock clamp is advanced into the ileal terminus, grasping the full thickness of the intussusceptum and inverting the ileum into the pouch (see Fig. 86–4B). Using the TA-55 stapler, three rows of 4.8-mm staples are applied to the intussuscepted nipple valve (see Fig. 86–4C). The distal six staples from each cartridge are removed before staple application to ensure that the tip of the valve is free of staples. Most authors suggest that the pin of the stapling instrument should always be kept in place so that staple misalignment does not occur. This will result in a pinhole puncture site at the base of the nipple valve that should be oversewn with absorbable suture material to prevent fistula formation after staple application is complete. The nipple valve is then fixed to the back wall of the patch by one of two stapling techniques (Skinner et al, 1984). A small buttonhole may be made in the back wall of the ileal plate so that the anvil of the stapler can be passed through the buttonhole and advanced into the nipple valve before application of the fourth row of staples (see Fig. 86–4D). If this is carried out, the buttonhole is oversewn afterward with absorbable material. Alternatively, the anvil of the stapler can be directed between the two leaves of the intussuscipiens and the fourth row of staples used to fix the inner leaf of the nipple valve to the pouch wall (see Fig. 86–4E).
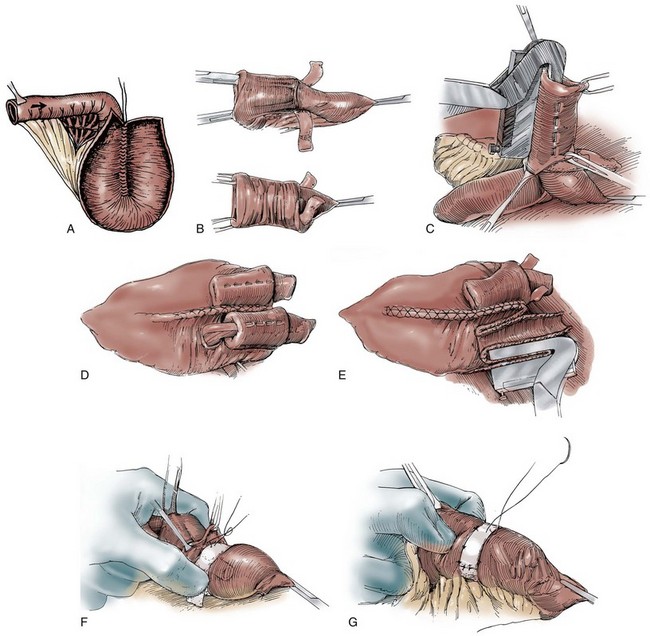
Figure 86–4 A, A 15-cm segment of terminal ileum is isolated and opened along its antimesenteric wall. The proximal 10 cm will serve as the continent intussusception and the distal 5 to 10 cm, the patch. The size of the patch will vary according to the size of the excised segment. B, An Allis or Babcock clamp is advanced into the ileal terminus, the full thickness of the intussuscipiens is grasped, and it is prolapsed into the pouch. C, Three rows of 4.8-mm staples are applied to the intussuscepted nipple valve using the TA-55 stapler. D, A small buttonhole is made in the back wall of the ileal plate to allow the anvil of the TA-55 stapler to be passed through and advanced into the nipple valve. A fourth row of staples is applied. The figure shows two valve mechanisms, but in this instance there would be only one. E, The anvil of the stapler can be directed between the two leaves of the intussuscipiens and the fourth row of staples applied in this manner. Two valve mechanisms are shown, but in this instance there would be only one. F, A 2.5-cm wide strip of absorbable mesh is placed through additional windows of Deaver at the base of each nipple valve. The mesh strips are fashioned into collars. G, The collars are sewn to the base of the pouch and the ileal terminus with seromuscular sutures.
(A, From Ghoneim MA, Lock NG, Lycke G, El-Din AB. An appliance-free, sphincter-controlled bladder substitute. J Urol 1987;138:1150–4; B to G, from Hinman F Jr. Atlas of urologic surgery. Philadelphia: WB Saunders; 1989.)
Some authors including Skinner and colleagues (1989) suggest the use of an absorbable mesh collar to anchor the base of the nipple valve. If a collar is used, a 2.5-cm wide strip of absorbable mesh is placed through an additional window of Deaver at the base of the nipple valve. The mesh strip is fashioned into a collar and sewn to the base of the patch with seromuscular sutures of absorbable material (see Fig. 86–4F and G). The patch is then sewn to the reservoir.
Double T Pouch
As indicated earlier, many surgeons have abandoned the Kock pouch largely due to the technical difficulties of creating the continence and antireflux mechanisms, as well as the high complication rates associated with them. This should not be viewed as a condemnation of the pioneering work of Kock and his colleagues. Without their initial efforts, many of the procedures described in this chapter would never have come into being. Rather, this represents the natural evolution of surgical techniques.
The group at the University of Southern California modified a technique described by Abol-Enein and Ghoneim (1993, 1994) to create a novel continence mechanism created entirely from ileum (Bochner et al, 1988). Abol-Einein and Ghoneim described a technique that created an extramural serosal tunnel into which the ureters were implanted. This extramural trough created a pseudotunnel that prevents reflux but in theory is associated with a lower risk of obstruction than either the Goodwin (1958), Leadbetter (1961), or LeDuc and colleagues’ (1987) techniques of direct transmural ureteral implantation. Stein and colleagues (1998) first reported on the use in a neobladder of a tapered ileal segment implanted into a serosal trough as the antireflux mechanism. In 1999 they reported on their adaptation of the technique to the ileal-anal reservoir, and in 1999 they presented their early experience with a double T pouch as a replacement for the Kock pouch at the meeting of the American Urological Association (Stein et al, 1999a). It was published elsewhere (Stein and Skinner, 2001) and is the technique presented in this section.
Procedure
A 70-cm segment of terminal ileum is isolated 15 to 20 cm from the ileocecal valve at the line of Treves. The proximal isoperistaltic 10- to 12-cm segment is isolated and will serve as the antireflux mechanism. The distal 12- to 15-cm segment is isolated and rotated in an antiperistaltic fashion and will create the cutaneous continence mechanism (Fig. 86–5A and B). A short 2- to 3-cm mesenteric incision is made to isolate the proximal limb, and a 4-cm incision is made for the distal limb, thereby preserving the major vascular arches. The proximal and distal segments can vary in length, depending on ureteral length and the thickness of the anterior abdominal wall. The middle 44 cm of ileum are folded in a W with each limb measuring 11 cm.
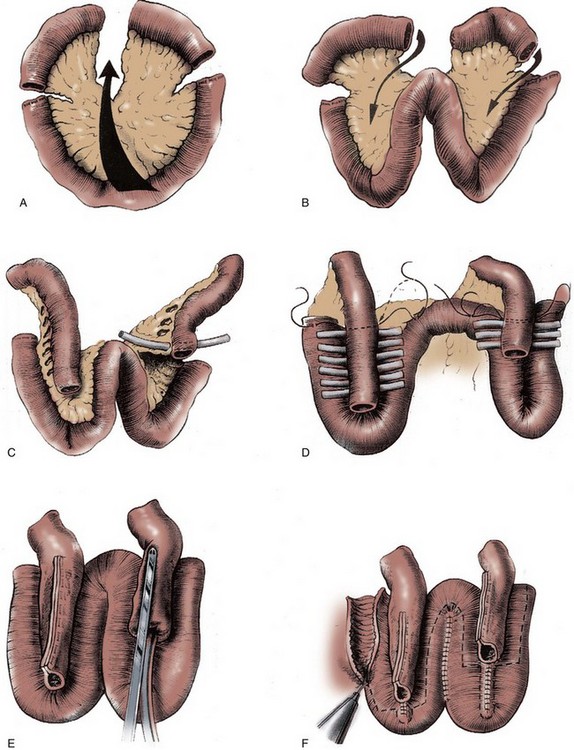
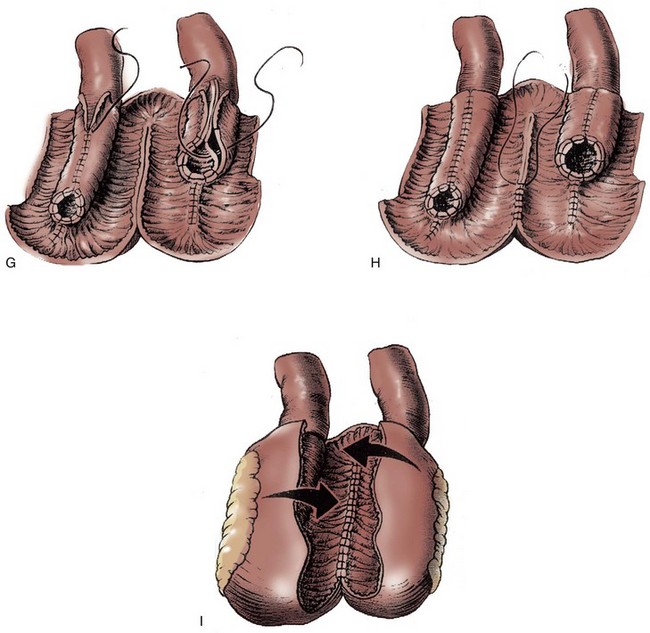
Figure 86–5 A, A 70-cm segment of terminal ileum is isolated 15 to 20 cm from the ileal cecal valve. B, A proximal 10-cm segment is isolated and rotated toward what will become the reservoir in an isoperistaltic direction. The distal 12 to 15 cm is rotated toward the reservoir in an antiperistaltic direction. C, The windows of Deaver are opened to allow the walls of the W reservoir to be apposed behind the valve mechanisms. Penrose drains are passed to guide suture passage. D, Horizontal mattress sutures of 3-0 silk are passed through each window. The distal continence mechanism is longer than the proximal antireflux mechanism. E, The proximal and distal mechanisms are tapered with a metal gastrointestinal anastomosis stapler. F, The bowel is incised along its antimesenteric border, where it will overlie the 2 Ts. Distal to the Ts, the bowel is incised close to the approximated limbs of the reservoir. G, The ostia of the valves are secured to the bowel wall with interrupted absorbable sutures. The two flaps of ileum are closed over the Ts with running absorbable sutures. H, The back wall of the reservoir is closed with running absorbable sutures. I, The lateral walls are folded medially, and the construction is completed with running absorbable sutures.
(From Stein JP, Buscarini M, DeFilippo RE, Skinner DG. Application of the T pouch as an ileo-anal reservoir. J Urol 1999;162:2052–3.)
The afferent antireflux mechanism is created by opening the windows of Deaver between the vascular arcades along the distal 3 to 4 cm. The efferent continence mechanism is created by opening the proximal 7 to 8 cm of vascular arcades (antiperistaltic) (see Fig. 86–5C). One-fourth-inch Penrose drains are then placed in each window of Deaver to facilitate passage of the 3-0 silk horizontal mattress sutures that are used to approximate the serosa of the corresponding 11-cm limbs of the W (see Fig. 86–5D). The 3- to 4-cm anchored portion of the proximal limb is then tapered over a 30-Fr catheter, and the 7- to 8-cm anchored portion of the efferent limb is tapered over a 16-Fr catheter. In both instances, tapering is performed with a gastrointestinal anastomosis (GIA) stapler (the staples will not be in contact with urine). Care must be taken in the efferent limb to create a gradual taper so that the catheter does not hit a false cul-de-sac (see Fig. 86–5E). The portions of the 11-cm W limbs not forming the troughs are then sutured together with a running 3-0 polyglycolic acid (PGA). The bowel is now incised along its antimesenteric border in the portion where the serosal trough exists and in close proximity to the medial PGA suture lines when beyond the two limbs (see Fig. 86–5F). The incised mucosa is then closed in two layers with a running suture of 3-0 PGA. The incised intestinal flaps (antimesenteric incision) are then sutured to each ostium with interrupted sutures of 3-0 PGA, and the two ileal flaps are sutured over each segment with a running suture of 3-0 PGA (see Fig. 86–5G). The reservoir is then closed side to side in two layers with 3-0 PGA, thereby completing its construction (see Fig. 86–5H and I). The ureters are anastomosed end to side over stents to the proximal limb, which has been closed with a running absorbable Parker-Kerr suture. The efferent limb is then brought to the abdominal wall stoma site, and redundant ileum is resected. The stoma is then matured with the reservoir lying immediately adjacent to the anterior abdominal wall.
Postoperative Care and Comments
Postoperative care is similar to that for any continent reservoir. Stein and colleagues (1999b) initially reported on nine patients, seven of whom could be evaluated for continence and long-term complications and two of whom died of disease during follow-up. All seven patients achieved immediate continence on catheter removal. However, two patients later became incontinent, with one requiring surgical revision. None of the nine patients experienced an early postoperative complication. One patient developed a reservoir stone 9 months after surgery that was removed endoscopically without sequelae. Pouch capacity was excellent: 400 to 700 mL (average 500 mL). There was no radiographic evidence of reflux in any patient, and there was no upper tract deterioration. This operative procedure appears to have many advantages over the Kock pouch, and good long-term continence results have been reported. Marino and colleagues (2002) reported on 18 patients with 1-year follow-up with 100% day and night continence and no delayed complications. Seifert and colleagues (2008) recently published their results on 19 patients who underwent ileal double T pouch construction between 1998 and 2006. Five patients (26%) had complications related to the diversion, some of which required surgical revision. Three patients (16%), all of whom had body mass indices of greater than 30, suffered necrosis of the efferent loop and subsequent cutaneous fistulas. Sixteen (84%) eventually developed both daytime and nighttime continence. Although a mild acidosis was common in this group, no urinary reflux was detected and no patients suffered significant upper tract deterioration or pyelonephritis.
Mainz Pouch I
The catheterizable Mainz pouch has undergone considerable modification over the years (Thuroff et al, 1985; Stein et al, 1995; Lampel et al, 1996; Gerharz et al, 1997). The main impetus for these changes has been the difficulty encountered with the nipple valve mechanism. The operative technique has now been modified to use the intact ileocecal valve as a means of further stabilizing the intussusception (Thuroff et al, 1988). This procedure is described here without further reference to earlier prototypes.
Procedure
The catheterizable Mainz pouch varies somewhat from the orthotopic, voiding Mainz pouch. First, a longer segment of bowel is used. A 10- to 15-cm portion of cecum and ascending colon is isolated along with two separate, equally sized limbs of distal ileum and an additional portion of ileum measuring 20 cm (Fig. 86–6A). The entire colon and distal segments of ileum are spatulated, taking care to preserve the ileocecal valve. These three bowel segments are folded in the form of an incomplete W and their posterior aspects sutured to one another to form a broad posterior plate (see Fig. 86–6B). A portion of the intact proximal ileal terminus is freed of its mesentery for a distance of 6 to 8 cm, and intussusception of the segment is achieved. Two rows of staples are applied on the intussusceptum itself (see Fig. 86–6C). Thereafter, the intussusceptum is led through the intact ileocecal valve and a third row of staples is applied to stabilize the nipple valve to the ileocecal valve (see Fig. 86–6D). Finally, a fourth row of staples is applied inferiorly, securing the inner leaf of the intussusception to the ileal wall (see Fig. 86–6E).
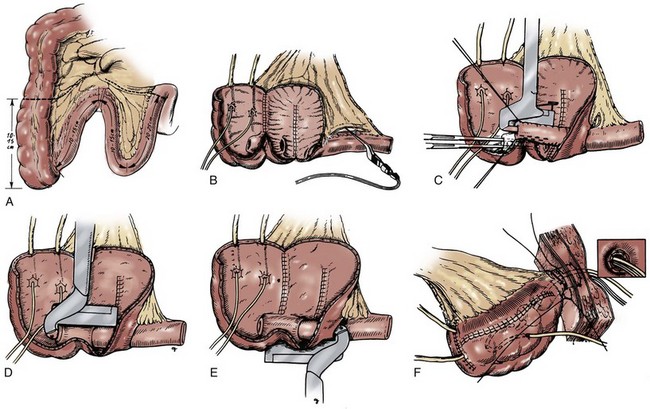
Figure 86–6 A, A 10- to 15-cm portion of cecum and ascending colon is isolated along with two separate equal-sized limbs of distal ileum and an additional portion of ileum measuring 20 cm. B, A portion of the intact proximal ileal terminus is freed of its mesentery for a distance of 6 to 8 cm. C, The intact ileum is intussuscepted, and two rows of staples are taken on the intussuscipiens itself. D, The intussuscipiens is led through the intact ileocecal valve, and a third row of staples is taken to stabilize the nipple valve to the ileocecal valve. E, A fourth row of staples is taken inferiorly, securing the inner leaf of the intussusception to the ileal wall. F, A button of skin is removed from the depth of the umbilical funnel, and the ileal terminus is directed through this buttonhole. Excess ileal length is resected, and the ileum is sutured at the depth of the umbilical funnel.
(A, From Thuroff JW, Alken P, Hohenfellner R. The MAINZ pouch [mixed augmentation with ileum ‘n’ cecum] for bladder augmentation and continent diversion. In: King LR, Stone AR, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book Medical Publishers; 1987. p. 252; B to F, from Thuroff JW, Alken P, Riedmiller H, et al. 100 cases of MAINZ pouch: continuing experience and evolution. J Urol 1988;140:283–8.)
Ureterocolonic anastomoses are created at the apex of the reservoir, which is then folded on itself in a side-to-side fashion to complete pouch construction. The entire pouch is rotated cephalad so as to bring the ileal terminus to the region of the umbilicus. A small button of skin is removed from the depth of the umbilical funnel, and the ileal terminus is directed through this buttonhole (see Fig. 86–6F). The pouch is secured to the posterior fascia with interrupted absorbable sutures, and the ileal terminus is sewn similarly to anterior fascia. Excess ileal length is resected, and the ileum is sutured at the depth of the umbilical funnel with interrupted absorbable sutures.
Postoperative Care and Comments
No specific differences in postoperative care or complications associated with the Mainz pouch need to be addressed. Initial pouch capacities are higher than in the Kock or T pouch. Final mean capacity averaging greater than 600 mL has been reported. Pouch pressures are 23 cm H2O at half capacity and 31 cm H2O when the pouch is full. Contraction waves beginning at 50% pouch fullness can be recorded at an amplitude of 12 cm H2O. Thus this pouch seems to produce a reasonably low-pressure urinary reservoir, although the pressures are not as low as those achieved with the use of small bowel alone.
Recently, the 10- and 12-year experiences with the Mainz pouch and the variations created by its developers were presented (Stein et al, 1995; Lampel et al, 1996). Between 1983 and 1994, 440 patients underwent a Mainz I operation in two urology departments, Mainz and Wuppertal. Continence mechanisms varied: in 146 cases the appendix was used as the continence mechanism; in 270 patients the intussuscepted nipple was used as the continent stoma; in 14 patients a submucosal, seromuscular bowel flap was employed; and in 10 patients a submucosal full-thickness bowel flap was used. The early complication rate was 12% and included mechanical ileus requiring open revision in 9 patients (1.6%), pouch leakage requiring revision in 5 patients (0.9%), wound dehiscence in 4 patients (0.7%), and fatal pulmonary emboli in 4 patients (0.7%).
The late complication rate was 37% and was predominantly attributable to the pouch. Stomal failure requiring open revision occurred in 45 patients (8%) and was directly related to the continence mechanism. Only 2 of 146 patients (1.4%) with an appendiceal continence mechanism were incontinent, but stomal stenosis occurred in 21%. The developers of this procedure were innovative in their attempts to bring the incontinence rate down to an acceptable level. To this end they tried multiple techniques, with variable success: an alloplastic stoma (4/4 incontinent); sutured intussusception (8/8 incontinent); stapled intussusception (5/22, 23% incontinent); and stapled ileocecal intussusception (10/204, 4.9% incontinent). The stapled ileocecal intussusception described previously is the current recommendation, and the long-term incontinence rate among the patients undergoing the stapled nipple valves was reduced to 10%. Other late complications included the need for ureteral reimplantation in 28 patients (4.9%) and stomal stenosis in 29 patients with an ileal nipple (11.7%) and in 17 patients with an appendiceal stoma (14.7%).
Calculus formation in the pouch occurred in 38 patients (6.8%), resulting in 36 percutaneous procedures. Despite the loss of the terminal ileum, no significant decrease in serum vitamin B12 levels has been reported and no patient has developed a macrocytic anemia or neurologic symptoms. However, 25% of patients are on oral alkalinization to avoid metabolic acidosis.
Since its inception, the overall complication rate for this procedure has been considered high (31%). However, as Stein and colleagues (1995) pointed out, 50% of the complications were manageable with percutaneous techniques. Additionally, since 1988 the incontinence rate has been only 3.2% and less than 2% in patients with an appendiceal mechanism.
Gerharz and colleagues (1997) from Marburg, Germany, reported their single-institution experience with the Mainz I ileocecal pouch. From 1990 to 1996, 202 consecutive patients underwent continent diversion, 96 with a submucosally embedded in-situ appendix and 106 with an intussuscepted ileal nipple. All patients had an umbilical stoma. In 172 of 200 patients (85%), no stomal complications occurred. In 17 of 96 patients (18%) with an appendiceal stoma, 23 revisions were performed for stomal stenosis. In contrast, only 13 of 106 patients (12%) with an intussuscepted ileal nipple developed problems with their stoma. However, these patients required more invasive, major procedures for correction, whereas those with an appendiceal stenosis could usually be repaired with a minor procedure. Three patients with an ileal nipple (3%) developed pouch calculi, whereas none of the patients with an appendiceal continence mechanism developed stones. As a result, the authors concluded that the appendix, when available, should be the intestinal continence mechanism of choice.
We share the enthusiasm for the use of the appendix as a continence mechanism. In our experience it has also been a reliable technique that is easy to perform. It has been our tendency in constructing right colon pouches employing an appendiceal continence mechanism to use the entire right colon inclusive of the hepatic flexure to form the reservoir, thereby preserving more terminal ileum. This has the theoretical advantage of fewer metabolic complications, but the Mainz group has not reported significant metabolic problems.
The introduction of the more reliable appendiceal continence mechanism has greatly increased the acceptance of the Mainz I procedure. The Mainz group has also developed two new techniques for construction of a Mitrofanoff, or appendiceal, type tube for use in patients whose appendix is either unsuitable or absent (Lampel et al, 1995a, 1995b; Lampel and Thurhoff, 1998). Both techniques use a small-caliber conduit fashioned from the large intestine in the region of the cecum. One technique uses a full-thickness tube lined by mucosa (Fig. 86–7) and the other a seromuscular tube lined by serosa (Fig. 86–8). Both techniques appear to be successful, although the full-thickness tube was associated with a lower complication rate and a higher success rate in their initial report (Lampel et al, 1995b). With longer follow-up, the authors have observed a similar success rates with both tubes; 93% of the patients (25 of 27) with a seromuscular tube and 92% of the patients (22 of 24) with a bowel wall tube were continent day and night (Lampel and Thurhoff, 1998). The authors believed that either tube was reliable and that each had its own unique advantages and disadvantages. In general, the full-thickness bowel wall tube was more adaptable, owing to the ability to create a longer tube. However, this came at the expense of a more tenuous blood supply. The decreased distal blood supply might be improved by creating a wider base on the tube. This could, however, make taenial implantation more difficult. The seromuscular tube was equally reliable but could only be anastomosed to the umbilicus, owing to the short adit tube. Either tube was believed to be indicated as a continence mechanism in the Mainz I pouch when the appendix was not available or as a continence mechanism for reservoirs created from other large intestinal segments. Either technique could be used as a salvage procedure when another primary continence mechanism had failed.
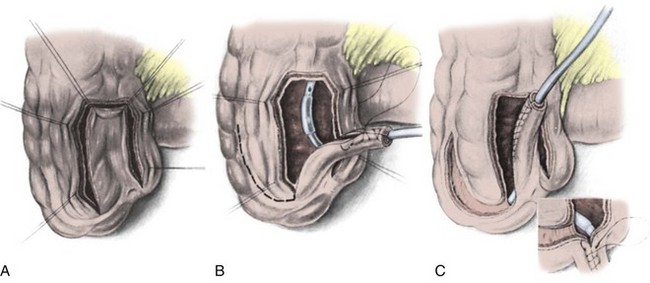
Figure 86–7 A to C, A full-thickness tube lined by mucosa is fashioned over an 18-Fr Foley catheter for tunneled reimplantation. The tube is closed with a running 3-0 absorbable suture. For longer tubes, the authors advise a wider base to prevent distal ischemia. The continence mechanism is created by placing the tube into the adjacent taenial trough.
(From Lampel A, Hohenfellner M, Schultz-Lampel D, Thuroff JW. In-situ tunneled bowel flap tubes: two new techniques of a continent outlet for Mainz pouch cutaneous diversion. J Urol 1995;153:308–15.)
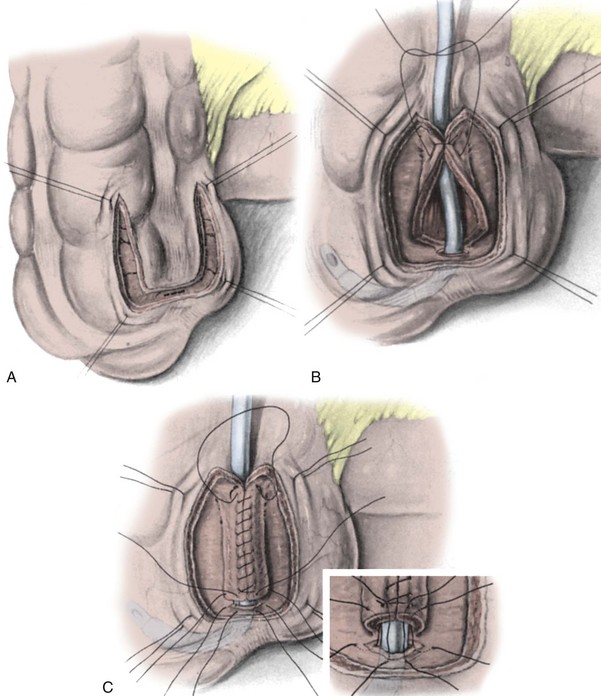
Figure 86–8 A to C, A 3- to 5-cm seromuscular tube denuded of mucosa and lined by serosa is fashioned for tunneled reimplantation. The tube is rolled over an 18-Fr Foley catheter. A mucosal window is opened at the base of the U, and the tube sutured to the mucosa with interrupted sutures.
(From Lampel A, Hohenfellner M, Schultz-Lampel D, Thuroff JW. In situ tunneled bowel flap tubes: two new techniques of a continent outlet for Mainz pouch cutaneous diversion. J Urol 1995;153:308–15.)
Another novel Mitrofanoff continence mechanism was described by Montie (1997), who conceived of a procedure in which a 2- to 3-cm segment of terminal ileum is isolated on its blood supply (Fig. 86–9A). The width of the segment was chosen to correspond to the circumference of the tube to be created. Once isolated, the segment is opened near one of its mesenteric junctions to create a longitudinal reconfiguration (see Fig. 86–9B and C). The tube is then closed with a running 3-0 absorbable suture (see Fig. 86–9D). It can now be used for a Mitrofanoff implant. When longer tubes are necessary, two adjacent segments can be isolated, reconfigured, and joined together (see Fig. 86–9E and F). Although originally described in dogs, the authors have used this technique in humans without complication. Montie (1997) reported on a high rate of stomal stenosis in dogs, but this may have been secondary to infrequent catheterizations. Stomal stenosis has not occurred in our limited series of patients. Other groups have used tapered ileum to create a tunneled access into the right colon (Fig. 86–10) (Woodhouse and MacNeily, 1994; Hampel et al, 1995). Using tapered ileum for this purpose has the advantage of a blood supply independent of the reservoir and no length restrictions while having the disadvantage of further limiting intestinal absorptive surface.
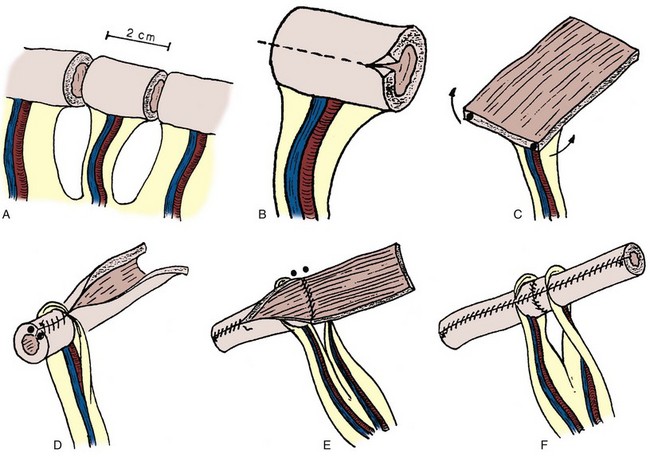
Figure 86–9 A, A 2- to 3-cm segment of terminal ileum is isolated on its blood supply. B and C, The tubular segment is opened approximately one fourth of the way up one side. This results in a well-vascularized rectangular plate. D, The rectangular tube is now closed over a catheter with a running absorbable suture. E and F, Two adjacent segments can be joined together to create one long tube.
(From Monti PR, Lara RC, Dutra MA, et al. New techniques for construction of efferent conduits based on the Mitrofanoff principle. Urology 1997;49:112–5.)
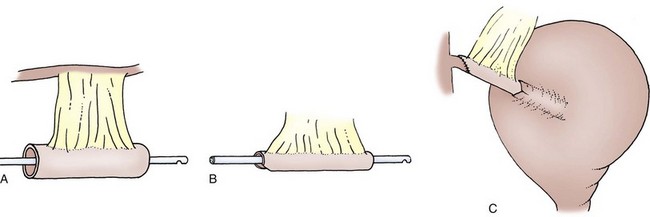
Figure 86–10 A to C, Woodhouse tapered ileum.
(From Woodhouse CR, MacNeily AE. The Mitrofanoff principle: expanding upon a versatile technique. Br J Urol 1994;74:447–53.)
Wiesner and colleagues (2007) recently compared their long-term results in 458 patients who underwent Mainz I pouch construction. The anastomosis was made using a submucosal tunnel in 809 renal-ureteric units and using a serosa-lined extramural tunnel in 74 units. At 17 months postoperatively they found a significantly higher occurrence of anastomotic obstruction in the submucosal tunnel group compared with the extramural group (7.3% vs. 4.1%, respectively). Importantly, they found a much higher rate of obstruction in patients with previously dilated upper tracts (14%) or with a history of neurogenic bladder (17%). No significant deterioration of the upper tracts was identified.
In another comparison of patients with a Mainz I pouch, Wiesner and colleagues (2006) reported on 800 patients with almost 8 years of follow-up. Overall continence was approximately 93%. Stomal stenosis occurred in 23.5% of patients with a submucosally embedded in-situ appendix, whereas stenosis was reported in 15.3% of patients with intussuscepted ileal nipple valves. Rates of calculi formation were the reverse: 10.8% incidence in patients with an ileal nipple valve and only in 5.6% of patients with an appendix stoma. Ischemic degeneration of the continence mechanism occurred almost three times more often in the appendiceal group.
Right Colon Pouches with Intussuscepted Terminal Ileum
Additional pouches using nipple valve technology for the continence mechanism include those right colon pouches in which intussusception of the terminal ileum and ileal cecal valve is employed. As such, they are variations on the continent cecal reservoir initially described by Mansson (1987) that employ an intact cecal segment. These three pouches are the Le Bag (Light and Scardino, 1986), Duke pouch (Webster and King, 1987), and UCLA pouch (Raz, personal communication, 1989). These surgeries differ from each other only by a few features mainly related to the technique employed for stabilizing the nipple valve. Unless the appendix is being used as a continence mechanism, appendectomy must be performed in all cases because an in-situ appendix would serve as a nidus for infection and abscess formation.
These operations were described in detail in the prior edition of this text. Since then, no new modifications to these procedures have been reported, and they are not further described here. The reader is referred to the prior edition of this text for an in-depth description of these operations.
Indiana Pouch
The concept of using the buttressed ileocecal valve as a dependable continence mechanism that can withstand the trauma of intermittent catheterization was first reported by Rowland and colleagues (1987) from Indiana University. This operation, which involved the partial spatulation of the cecal segment and attachment of an ileal patch, represented major contributions to the original ileocecal reservoir as described by Gilchrist and colleagues (1950), in which the intact bowel reservoir was employed and no attempt was made to strengthen the ileocecal valve. Originally, strengthening the ileocecal valve consisted of a double row of imbricating sutures taken to the entire ileal segment (Rowland et al, 1985, 1987). It soon became apparent that this was necessary only in the region of the ileocecal valve. “Neourethral” pressure profiles showed that the continence zone was confined to the region of the reconfigured ileocecal valve (Bejany and Politano, 1988). The remaining “neourethra” could be tapered and brought through an abdominal or perineal stoma. At Indiana University, as well as other institutions, it became clear that the concept of marsupializing only a portion of the ascending colon segment left enough peristaltic integrity in the cecal region to generate pressures sufficiently high to overcome the continence mechanism in some patients. A number of groups contributed to the concept of using the entire right colon or more, marsupializing the entire structure and refashioning it in a Heineke-Mikulicz configuration (Lockhart, 1987; Bejany and Politano, 1988; Benson et al, 1988; Rowland, personal communication, 1989). These variations have been entitled the Florida pouch (Lockhart, 1987) and the University of Miami pouch (Bejany and Politano, 1988). However, they represent relatively minor variations on the theme of the Indiana pouch.
Procedure
The Indiana pouch, in its present form, involves isolating a segment of terminal ileum approximately 10 cm in length along with the entire right colon to the junction of the right and middle colic artery blood supplies (Fig. 86–11A). After bowel continuity is re-established, appendectomy is performed and the appendiceal fat pad obscuring the inferior margin of the ileocecal junction is removed by cautery (see Fig. 86–11B). The entire right colon is opened along its antimesenteric border, and ureteral-taenial implants are fashioned (see Fig. 86–11C). The ileocecal junction is buttressed according to various reported techniques. Using nonabsorbable sutures, interrupted Lembert sutures are taken over a distance of 3 to 4 cm in two rows for the double imbrication of the ileocecal valve as described at Indiana University (see Fig. 86–11D). The second row of sutures should attempt to bring the opposite mesenteric edges of ileum together, usually over a 12- to 14-Fr catheter. These two rows of sutures should be placed approximately 8 mm from one another, and the initial suture in each row may be taken in a purse-string fashion around the cecal margin as well. Alternatively, the University of Miami group suggests placing purse-string sutures in the same ileal region (Bejany and Politano, 1988). Finally, the Tampa group suggests placement of apposing Lembert sutures on each side of the terminal ileum (see Fig. 86–11E). The remaining ileum can be tapered over the catheter and excess ileum removed with a stapling technique (see Fig. 86–11F).
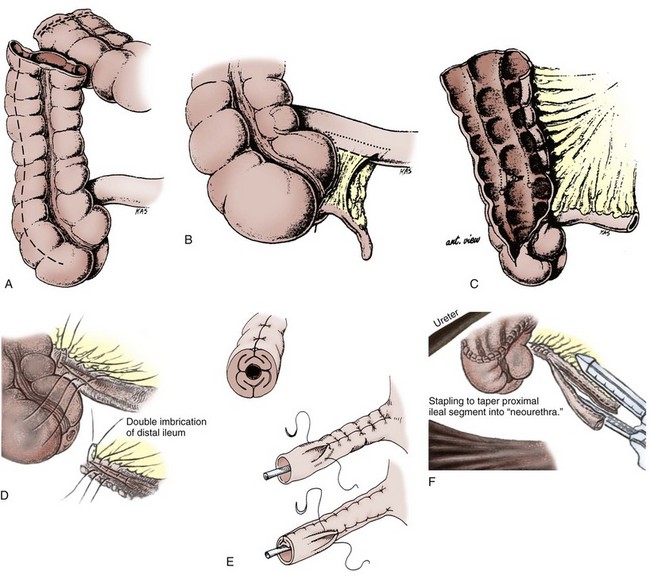
Figure 86–11 A, A segment of terminal ileum approximately 10 cm in length along with the entire right colon is isolated. B, An appendectomy is performed, and the appendiceal fat pad obscuring the inferior margin of the ileocecal junction is removed by cautery. C, The entire right colon is opened along its antimesenteric border. D, Interrupted Lembert sutures are taken over a short distance (3 to 4 cm) in two rows for the double imbrication of the ileocecal valve as described at Indiana. E, Application of opposing Lembert sutures on each side of the terminal ileum. F, Excess ileum can be tapered by stapling technique.
(A to C, From Benson MC, Sawczuk IS, Hensle TW, et al. Modified Indiana University continent diversion. Curr Surg Tech Urol 1988;1:1–8; D and F, from Olsson CA. Contemp Urol 1989;Sept:62–8; E, from Lockhart J. Remodeled right colon: an alternative urinary reservoir. J Urol 1987;138:730–4.)
It is important to carry out the imbrication while the cecal reservoir is still open (Rowland, 1996) so that the gradual closure of the ileocecal valve can be closely observed. The pouch is then closed in a Heineke-Mikulicz configuration with a running absorbable suture. Ureteral stents and a suprapubic tube are taken through a stab wound in the pouch and led through the right lower abdominal quadrant. The pouch is rotated so as to bring the ileal neourethra as close as possible to the selected stoma site. A fingerbreadth-width skin button is transected along with a similar button from the anterior and posterior fascia. The ileal neourethra is advanced between bundles of the rectus muscle through the stoma, and excess ileum is transected. The ileal edges are sewn to skin with interrupted sutures so as to create a flush stoma.
In addition to the differences in the technique of ileocecal valve imbrication, both the University of Miami and the Florida pouches differ in the amount of colon used. The entire ascending colon and the right third or half of the transverse colon is isolated along with 10 to 12 cm of ileum. The entire upper extremity of the large bowel is mobilized laterally in the fashion of an inverted U (Fig. 86–12A). The medial limbs of the U are sutured to one another after the bowel is spatulated (see Fig. 86–12B). The bowel plate is then closed side to side (see Fig. 86–12C). This inverted-U closure, however, is exactly the same as a Heineke-Mikulicz reconfiguration.
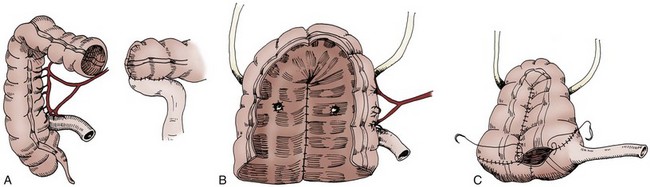
Figure 86–12 A, The entire ascending colon and the right third or half of the transverse colon is isolated along with 10 to 12 cm of ileum. B, The entire upper extremity of the large bowel is mobilized laterally in the fashion of an inverted U. The medial limbs of the U are sutured after the bowel is spatulated. C, The bowel plate is then closed side to side.
(From Lockhart J. Remodeled right colon: an alternative urinary reservoir. J Urol 1987;138:730–4.)
Recent modifications to the Indiana reservoir allow for more rapid construction and a lower complication rate (Rowland, 1996). The modifications incorporate the use of metal staples to create the efferent limb and absorbable staples to fashion the reservoir. The concept of using a metal GIA stapler to fashion the efferent limb was first introduced by Bejany and Politano (1988). Carroll and Presti (1992) reported on the urodynamic features of the stapled and plicated terminal ileum and found that the stapled limb performed equally well and was easier to construct. The use of absorbable staples to create this and other types of reservoirs is described later in the chapter.
Postoperative Care and Comments
The postoperative care of the patient with an Indiana pouch or its variants is not substantially different from that used in patients with other right colon catheterizable diversions. In early reports, Rowland recommended discharging the patient with the suprapubic tube in place until readmission to the hospital 3 weeks later for tube removal and instruction in self-catheterization. In the current medical climate, which places a premium on outpatient procedures, tube removal and catheterization instruction is now an ambulatory procedure at most institutions including Indiana University (Bihrle, 1997).
Average pouch capacities of 400 to 500 mL have been reported by the Indiana group (Rowland et al, 1987). Combining the partially and totally spatulated bowel procedures, this group reports a reoperation rate of 26%. Overall continence rates of 93% were achieved. Elegant urodynamic studies were conducted in Indiana pouch variants by Carroll and colleagues (1989). They found only 86% of patients totally continent in a small series. However, their pouch capacities exceeded 650 mL, and peak contractions of 47 cm H2O were recorded at capacity.
The last 81 patients operated on by Rowland underwent construction of a stapled efferent limb, and, in the last 20, the reservoir was created with absorbable staples (Rowland, 1996). The results in this group of patients were extremely favorable. Early pouch-related complications occurred in only three patients (3.7%). Two patients experienced a pouch leak that was managed conservatively, and one patient required open revision of the efferent limb owing to difficulty with catheterization. Early complications not directly attributable to the pouch occurred in seven patients (8.6%). Transient small bowel obstruction was the most common complication, occurring in four patients (4.9%). One patient developed a superficial wound infection, and one patient developed an abdominal abscess requiring surgery (1.2%). Late complications associated with the reservoir occurred in 23 patients (28.4%). Incontinence occurred in six patients (7.4%): In five patients it occurred secondary to high pouch pressures, whereas in the remaining patient it was due to failure of the efferent limb. One of the former and the latter patient underwent reoperation. Three patients (3.7%) developed stomal stenosis, and three had parastomal hernias; all six underwent surgery. Pouch stones occurred in three patients: One underwent open removal, and two had endoscopic extraction. Acute pyelonephritis was seen in four patients (4.9%). The most common late complication not related to the pouch was small bowel obstruction; this was seen in six patients and managed conservatively in five. In summary, the early reoperation rate was 2.5% and the late reoperation rate 14.8%. At 1 year, daytime and nighttime dry intervals of 4 hours or greater were achieved in 98% of patients. Eighty-four percent of patients stated they slept through the night without the need to awake for catheterization. Similarly excellent results in the last 150 patients, 50 with at least 2.5-year follow-up, were reported by Birhle (1997).
The Florida pouch has been performed in more than 190 patients (Helal et al, 1993). In 165 patients involving 326 ureters, no attempt was made to create a tunneled reimplantation. This approach was adopted owing to the high incidence of ureteral obstruction encountered in the first 30 ureters that were tunneled into a Florida pouch (4 patients, 13.3%). In the last 165 patients, 16/326 ureters (4.9%) developed primary obstruction and were treated by percutaneous balloon dilation, nephrectomy, or observation. Although no attempt is made to create an antirefluxing anastomosis, only 7.1% of the ureters implanted demonstrated reflux. All are being followed conservatively, and no renal deterioration has been demonstrated. In the initial 100 patients, a 7.2% reoperation rate was reported (Lockhart, 1987). Although hyperchloremia was noted in 70% of patients, only four patients (including those who had pre-existing renal disease) required treatment. Reservoir capacities ranged from 400 to 1200 mL, and maximal reservoir pressures at capacity ranged from 18 to 55 cm H2O (Lockhart, 1987). The reason why these authors experienced such a high incidence of ureteral obstruction with both nontunneled and tunneled ureteral colonic anastomoses is not clear. It is also surprising that only 23 of 326 ureters that were anastomosed end to side had reflux.
The University of Miami group has reported on its results in 75 patients. Early complications occurred in 19 patients (25%). Sixteen patients (21%) experienced late complications. The success rate of the ureterocolonic anastomosis was 90%, and total continence occurred in 98.6% of patients. Average pouch capacities were 750 mL or higher, and end filling pressures of 20 cm H2O were reported. No patient required alkali therapy.
The Indiana pouch remains one of the most reliable of all catheterizable reservoirs. It is among the easiest to construct and has low short-term and long-term complications.
Penn Pouch
The Penn pouch was the first continent diversion employing the Mitrofanoff (1980) principle in which the appendix served as the continence mechanism. As mentioned earlier, this operation enjoys the singular feature of affording a catheterizable continent diversion that can be performed using techniques already present in the urologic armamentarium.
Procedure
Two techniques of appendiceal continence mechanisms have been reported. Mitrofanoff reported excising the appendix with a button of cecum and reversing it on itself before tunneled reimplantation (Mitrofanoff, 1980; Duckett and Snyder, 1986). Alternatively, Riedmiller and colleagues (1990) left the appendix attached to the cecum and buried it into the adjacent taenia by rolling it back onto itself. A wide tunnel is created in the taenia extending 5 to 6 cm from the base of the appendix (Fig. 86–13). Windows are created in the mesoappendix between blood vessels. The appendix is folded cephalad into the tunnel, and seromuscular sutures are placed through the mesoappendix windows to complete the tunneling. The tip of the appendix is amputated and brought to the selected stoma site.
Figure 86–13 A to C, The appendix is left attached to the cecum and buried into the adjacent cecal taenia by rolling it back onto itself. A wide tunnel is created, extending 5 to 6 cm from the base of the appendix. Windows are created in the mesoappendix between blood vessels. The appendix is folded cephalad into the tunnel, and seromuscular sutures are placed through the mesoappendix.
As described by Duckett and Snyder (1986), an ileocecal pouch is created by isolating a segment of cecum up to the junction of the ileocolic and middle colic blood supplies along with a similar length of terminal ileum. These two structures are marsupialized on the antimesenteric borders and sutured to one another in the form of a neotubularized pouch. The superior margin of the pouch is sutured in a transverse fashion (all sutures being of absorbable material). A button of cecum surrounding the origin of the appendix is circumcised, and the resulting cecal aperture is closed with running absorbable suture. The mesentery of the appendix is dissected carefully from the base of the cecum, thereby preserving its blood supply. The appendix is then reversed on itself so that the cecal button can reach the anterior abdominal wall and the tail of the appendix can be directed to the taenia of the colon (Fig. 86–14). The appendiceal tip is obliquely transected and may be spatulated. Then a tunneled appendiceal-taenial implantation is carried out. If additional appendiceal length is required, the variation proposed by Burns and Mitchell (1990) of creating a tube from the base of the cecum can be employed (see Fig. 86–3). Instead of simply removing the appendix with a button of cecum before preparing it for tunneling, the entire base of the cecum leading to the appendix can be resected in continuity with the appendix by the application of the GIA stapler. The authors have found it helpful to spatulate the distal tip of the appendix until it accommodates a catheter at least 12- to 14-Fr in diameter.
Figure 86–14 A segment of cecum up to the junction of the ileocolic and middle colic blood supplies along with a similar length of terminal ileum is isolated and marsupialized on the antimesenteric borders. A button of cecum surrounding the origin of the appendix is circumcised. The mesentery of the appendix is dissected carefully from the base of the cecum, preserving its blood supply.
(From Duckett JW, Snyder HM III. The Mitrofanoff principle in continent urinary reservoirs. Semin Urol 1987;5:55–62.)
Postoperative Care and Comments
Although not shown in Duckett’s surgical drawings, the authors would suggest that a large-bore suprapubic tube be used to drain the pouch in the early postoperative interval. The size of the catheter admitted by the appendiceal stump is insufficient to allow for the passage of ureteral stents along with the 12- to 14-Fr catheter. In addition, safe irrigation of mucous debris is best managed by a larger-bore catheter.
Many groups have used the Mitrofanoff principle owing to the simplicity and reliability of the continence mechanism (Burger et al, 1992; Bissada, 1993; Sumfest et al, 1993; Woodhouse and MacNeily, 1994; Hampel et al, 1995). Woodhouse and MacNeily (1994) reported on a series of 100 patients who underwent surgery between 1985 and 1993. They employed seven different catheterizable conduits into six different types of reservoirs. Although they found the Mitrofanoff principle to be versatile and associated with a high success rate (91% continence), the reoperation rate for tube complications was 33%.
Sumfest and colleagues (1993) affirmed the use of the appendix as the Mitrofanoff segment of choice. They reported a continence rate of 96%. In their hands, late complications included difficulty with catheterization in 10.6% and stomal stenosis in 19.1%.
Urodynamic properties and pouch capacities will be a function of the reservoir constructed. Most often, the appendix is used in situ (Burger et al, 1992), and the right colon, either alone or with associated terminal ileum (Mainz), serves as the reservoir. We have used the in-situ appendix with a detubularized right colon reservoir and the native ileocecal valve as an antireflux mechanism (refluxing ureters implanted end to side into terminal ileum). In our hands this has resulted in an excellent success rate with no upper tract problems. The adequacy of the ileocecal valve as an antireflux mechanism was also reported by Alcini and colleagues (1994). In their series, however, the reservoir was not always detubularized and, as expected, upper tract complications ensued owing to high reservoir pressures. This procedure is uniquely capable of affording continent cutaneous diversion to the patient with short ureters because the terminal ileum can be left long enough to reach high into the retroperitoneum.
Gastric Pouches
Pioneering animal experimentation demonstrated the feasibility of employing stomach as a bladder patch or urinary reservoir (Sinaiko, 1956; Rudick et al, 1977; Leong, 1978). The use of the stomach to create a urinary reservoir has theoretical and real advantages (Adams et al, 1988). First, electrolyte reabsorption would be greatly diminished by using this bowel segment in the reservoir. This would potentially make the stomach the selected reservoir for individuals with pre-existing metabolic acidosis or renal insufficiency. Hyperchloremic acidosis would not be an anticipated problem; in fact, in addition to presenting a barrier against the absorption of chloride and ammonium, the gastric mucosa secretes chloride ions (Piser et al, 1987). Furthermore, in patients in whom shortening of the bowel may be expected to lead to some degree of malabsorption, the use of stomach is an attractive alternative. The acid pH of the urine may also reduce the risk of bacterial colonization. Finally, when the entire lower bowel has been irradiated, stomach tissue may provide healthy nonirradiated tissue for use in performing continent diversion. Given these theoretical advantages, a number of groups have initiated trials with gastric pouches and composite reservoirs in both pediatric (Adams et al, 1988) and adult populations (Lockhart et al, 1993; Austin et al, 1997).
Procedure
A wedge-shaped segment of stomach with maximal width of 7 to 10 cm is fashioned from the greater curvature. Care is taken not to extend the wedge through to the lesser curvature to preserve vagal innervation and normal gastric emptying. The left gastroepiploic artery is preferentially used as the blood supply for the isolated gastric wedge, dividing the short gastric vessels from the more proximal artery up to the gastric fundus. Alternatively, if there is a problem with the left artery, the right gastroepiploic vessel may be employed, dividing the short gastric vessels to the level of the pylorus (Fig. 86–15A and B). The stomach is then closed according to the surgeon’s preference. Neither gastroduodenostomy nor gastrojejunostomy is mandatory unless the antrum of the stomach has been used. The isolated wedge is refashioned into nearly a sphere by folding it back on itself and suturing the edges together with running absorbable material. Before pouch closure, one ureter is tunneled into the reservoir according to the surgeon’s preferred antireflux technique. Contralaterally, proximal transureteroureterostomy is performed. The contralateral distal ureter is used to create the continence mechanism. The distal ureter is tunneled into the reservoir in a fashion similar to an appendiceal implant. The free portion of the ureter can then be brought to the skin or to the introitus (or urethral stump in males) to serve as a catheterization portal (see Fig. 86–15C). Alternatively, the wedge of stomach can be incorporated into a reservoir composed of detubularized ileum (Lockhart et al, 1993). In this procedure, an 11-cm long segment of stomach is isolated on the right gastroepiploic blood supply (Fig. 86–16A). A 22-cm segment of ileum is then isolated, opened along its antimesenteric border, and refashioned in a U shape (see Fig. 86–16B). The edges of the stomach are then sutured to edges of the ileum with a running absorbable suture of 2-0 PGA. This completes the reservoir. The ureters are tunneled into the stomach, and a Mitrofanoff continence mechanism is created according to the preference of the surgeon. For example, the group from the University of South Florida employs a tapered segment of ileum (see Fig. 86–16C).
Figure 86–15 A and B, A wedge-shaped segment of stomach whose greatest width is 7 to 10 cm is fashioned from the greater curvature. The left gastroepiploic artery is preferentially used as the blood supply for the isolated gastric wedge, by dividing the short gastric vessels up to the gastric fundus. Alternatively, if there is a problem with the left artery, the right gastroepiploic vessel may be employed. C, The isolated wedge is refashioned into nearly a sphere by folding it back on itself and suturing the edges together with running absorbable material. One ureter is tunneled into the reservoir. A proximal transureteroureterostomy (TUU) is performed. The ipsilateral distal ureter is tunneled into the reservoir with its distal extent brought to the introitus to serve as a catheterization portal.
(From Adams MC, Mitchell ME, Rink RC. Gastrocystoplasty: an alternative solution to the problem of urological reconstruction in the severely compromised patient. J Urol 1988;140:1152–6.)
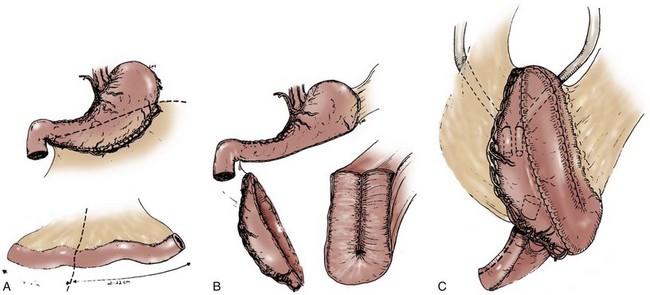
Figure 86–16 A, An 11-cm long segment of stomach is isolated on the right gastroepiploic blood supply using a gastrointestinal anastomosis-90 stapler. Usually, two staplers are required. B, A 22-cm segment of ileum is then isolated, opened along its antimesenteric border, and refashioned in a U shape. C, The edges of the stomach are then sutured to edges of the ileum with a running absorbable suture of 2-0 polyglycolic acid. This completes the reservoir. The ureters are tunneled into the stomach, and a Mitrofanoff continence mechanism is created with a tapered segment of ileum.
(From Lockhart JL, Davies R, Cox C, et al. The gastroileal pouch: an alternative continent urinary reservoir for patients with short bowel, acidosis and/or extensive pelvic radiation. J Urol 1993;150:46–51.)
Postoperative Care and Comments
Adams and colleagues (1988) report mean pouch capacities of 245 mL and end filling pressures averaging 35 cm H2O in a small patient sample. Combining their experience of gastric continent diversion and gastrocystoplasty, they report minimal mucus production: Only 3 of 13 patients required any irrigations, and the majority maintained sterile urine. Urine pHs have ranged from 4 to 7, but no introital ulceration from acid urine was reported. Three patients had minor elevations of serum gastrin, and none of the continent divisions required reoperation. Leong (1978) has used similar concepts in gastric pouch construction and has alluded to the creation of a voiding pouch created from stomach as well.
The construction of reservoirs entirely from stomach has not seen widespread acceptance. Rather, there has been greater use of stomach segments either for bladder augmentation or as a portion of a reservoir (composite) either alone or with an in-situ catheterizable tube fashioned from a portion of the stomach (Gosalbez et al, 1994; Carr and Mitchell, 1996).
Goslabez and colleagues (1994) reported on 15 patients who received a gastric tube as part of a composite gastric patch. Complications associated with the gastric patch and in-situ tube included one each of early traumatic perforation of the tube, distal tube stenosis, and mucosal redundancy. Two of these patients required reoperation. Peristomal skin irritation from acid secretion occurred in two patients but was not considered severe. This is a more frequent complication in other reports and has resulted in skin breakdown in some instances.
Over a 10-year period from January, 1985, to June, 1995, Carr and Mitchell (1996) reported on the use of stomach in 12 patients. Seven had urinary reservoirs totally constructed from stomach, whereas five had composite reservoirs. They report continence in all patients but that the continence mechanisms have often required revision. Average bladder capacity was 309 mL, and average compliance was 12.9 mL/cm H2O. When stomach is used as a bladder augment or as a portion of a neobladder, a dysuria and hematuria syndrome has been reported (Nguyen et al, 1993).
Austin and colleagues (1997) reported on nine adult patients with a mean follow-up of 54 months who underwent construction of a continent composite reservoir that was gastroileal in seven and gastrocolonic in two. All nine patients had either preexisting metabolic acidosis or a short bowel syndrome. All nine patients achieved electrolyte neutrality, and postoperative serum pH was significantly improved (P < .01). Three patients had a short-term serum gastrin elevation, which returned to normal during follow-up. One patient developed skin ulceration at the stoma site.
The use of stomach has particular appeal in the pediatric population in which the stomach’s unique acid-base properties can be used to not only reconstruct but also help correct the metabolic problems that are often associated with the need for pediatric urinary reconstruction (Carr and Mitchell, 1996). Although experience with use of the stomach remains small, its various unique intrinsic properties as a reservoir suggest that its use will continue in selected clinical situations.
Quality of Life Assessments
Extraordinarily few well-designed, prospectively conducted studies using validated instruments exist to assess quality of life after continent cutaneous urinary diversion. In fact, no randomized prospective trial has ever been conducted to compare the quality of life after continent cutaneous diversion with either orthotopic continent diversion or incontinent urostomy urinary diversion. Of those studies performed, there appear to be common flaws in the study design and methods used that make any direct comparisons between continent and incontinent diversions difficult (Gerharz et al, 2005).
In general, most quality of life studies show similar results between patients undergoing both ileal conduit and cutaneous continent diversion, with the latter being associated with improvements in stomal and urinary quality of life scores. In one of the few prospective studies to compare quality of life after continent cutaneous and ileal conduit diversion, Hardt and colleagues (2000) followed patients from the preoperative setting until 1 year after surgery. Using validated instruments tested for reliability, they found life satisfaction improved over time in patients with continent cutaneous diversion, whereas it worsened during the first year after ileal conduit construction. Using the Beck Depression Inventory and Profile of Mood States in adults, Boyd and colleagues (1987) found that patients choosing ileal conduit diversion had the lowest expectations of their quality of life. Interestingly, they found the highest overall satisfaction among patients undergoing conversion from ileal conduit to Kock cutaneous pouch diversion.
Mansson and colleagues (2002) found no difference in overall quality of life in men undergoing continent cutaneous diversion when compared with orthotopic neobladder using the FACT-BL and Hospital Anxiety and Depression Scale. In specific questions concerning intestinal, urinary, and sexual function, patients with cutaneous reservoirs experienced less difficulty with incontinence and emptied less frequently. Sexual function appeared better in patients undergoing orthotopic bladder substitution, likely due to urethral preservation.
Variations in Operative Technique
Minimally Invasive Continent Cutaneous Diversion
With recent improvements in both laparoscopic and robotic-assisted laparoscopic techniques, radical cystectomy can now be performed in selected centers using these minimally invasive techniques. The vast majority of centers performing minimally invasive cystectomy and continent cutaneous diversion perform the urinary diversion via standard open techniques. Turk has reported on an initial series of five patients who have undergone radical cystectomy with bilateral pelvic lymphadenectomy and continent urinary diversion using a rectosigmoid pouch performed with an intracorporeal laparoscopic technique. A bilateral stented antireflux ureteral reimplantation was used, and laparotomy was not performed. Operative time was 7.4 hours with minimal blood loss and a mean hospital stay of 10 days. No intraoperative or postoperative complications were encountered.
The complex nature of minimally invasive reconstructive surgery necessary in continent cutaneous diversion has limited these procedures to select centers. In addition, because of the prolonged time for return of postoperative bowel function, the benefits in hospital stay routinely encountered after laparoscopic nephrectomy do not seem to exist when urinary diversion is performed.
Conduit Conversion to a Continent Reservoir
The major indication for conversion of a functioning conduit to a continent urinary reservoir is the patient’s desire for improved quality of life. Pow-Sang and colleagues (1992) reported on conversion in 20 patients. Fifteen were converted from an ileal conduit, and one each from a cecal conduit, ureterosigmoidostomy, cutaneous ureterostomy, sigmoid conduit, and a suprapubic tube. In 14 of the 20 patients the conduit was discarded or used only as a patch to a colonic reservoir. Renal units that were obstructed preoperatively were associated with a 71% failure rate. Metabolic acidosis was seen in 15 (75%) but was believed to be mild. Pouch-related complications are, in general, a function of the reconstruction selected and should not necessarily be higher in this setting. However, patient selection is important in determining appropriate candidates for conversion.
The authors prefer to use the conduit in some form whenever possible. This strategy was supported in a report on two patients by Oesterling and Gearhart (1990). The use of an existing bowel segment has the potential to diminish metabolic sequelae and may result in a lower complication rate. The form of continent reconstruction chosen will have to depend on intraoperative findings, and no one procedure is more amenable than another. Before undertaking conversion, the patient should be fully evaluated for disease recurrence, renal functional status, urinary anatomy, hydronephrosis, intestinal length, and intestinal health.
Pahernik and colleagues (2004) have described the long-term outcomes of conversion of 39 patients from conduit diversion to Mainz I pouch diversion. With a mean follow-up of 102 months, the most common complications were stomal stenosis and pouch calculi. Long-term continence was achieved in 95% of patients.
Absorbable Stapling Techniques in Continent Urinary Diversion
The principle of bowel detubularization to increase reservoir capacity and diminish the effects of peristalsis is a fundamental principle of all contemporary continent urinary diversions. The process of detubularization and refashioning of the spatulated bowel segment consumes at least 1 hour of operating time and is by far the most time-consuming and tedious aspect of pouch construction. The use of absorbable staples has substantially reduced the time required to fashion bowel reservoirs and has demonstrated short-term and long-term reliability with respect to reservoir integrity and volume.
Bonney and Robinson (1990) first demonstrated the potential use of absorbable staplers to substitute for conventional suturing of bowel reservoirs. These authors used a bulky absorbable stapler (Polysorb staples in a TA Premium 55 stapler [US Surgical, Norwalk, CT]) to construct an S pouch configuration in a canine ileal urinary pouch model. Although the same stapler was used in humans by the authors in 1992 and by Cummings in 1995, its clinical use was never widely adopted because the bulky staple configuration destroyed a significant portion of the bowel diameter, particularly when applied to the small intestine. The fact that up to 20 costly staple cartridges were required to complete the closure of a bowel reservoir further reduced the potential benefits of absorbable pouch construction.
A 75-mm GIA instrument (PolyGIA [US Surgical, Norwalk, CT]) that incorporates substantially smaller absorbable staples was made available for clinical use in 1992. The stapler delivers four rows of absorbable polylactic acid and PGA blend copolymer staples, dividing the bowel between the second and third rows. Thus each staple line of the pouch has a double, staggered, stapled closure. This device has enabled both the refashioning and closure of bowel pouches to be performed with fewer staple applications and is strong and watertight. Finally, the width of bowel sacrificed with the new instrument is appreciably less than that with the older staple device. Several investigators have subsequently used the new “absorbable” GIA stapler to construct catheterizable pouches and neobladders (Olsson et al, 1993; Montie et al, 1994, 1995; Olsson and Kirsch, 1995). However, it is important to note that the absorbable PolyGIA staples must not overlap because this will result in the failure of the staples to lock together. This is in direct contrast to metal staples, which are meant to overlap to create anastomotic integrity. As a result of the need to prevent overlap of absorbable staples, the reservoir construction procedures must be varied when using such staples, as described next.
Surgical Techniques
Right Colon Pouch
In 1993 the authors described a technique using the absorbable GIA staplers to fully detubularize and refashion large bowel (Olsson et al, 1993). The technique of colon pouch construction described here incorporates the principles of bowel detubularization and refashioning using absorbable staplers in a simple “one-step” process.
The right colon and 10 cm of terminal ileum are mobilized by incising the peritoneum along the white line of Toldt and along the base of the mesentery and are isolated using metal GIA staplers (Fig. 86–17A). After bowel continuity is restored with standard metal GIA and TA staplers, the distal staple line of the right colon is excised and the bowel lumen is irrigated to remove residual enteric contents. Using electrocautery, a small opening (2 cm) is created on the antimesenteric border of the cecum to fit the absorbable stapler. The distal open end of the colon is aligned with the cecostomy by folding the right colon on itself, as depicted in Figure 86–17B. The limbs of the absorbable GIA stapler are inserted into the distal open end and into the cecostomy, and the stapler is fired along the antimesenteric line of the apposed folded bowel (see Fig. 86–17C). It is necessary to evert the bowel to continue subsequent staple applications. This may be achieved by placing Babcock clamps on each side of the distal staple line (see Fig. 86–17D). A small incision at the junction of each staple line is made to prevent overlap of the absorbable staple rows and to allow for the next staple application. Because of this incision, there is often a short unstapled area at the junction between each application of the stapler, requiring one or two simple figure-of-eight sutures of 2-0 absorbable material at each of these points. The last staple application traverses the apex of the fold of bowel. In adults, three to four applications of the stapling device have been required to construct the right colon pouch, whereas in children two to three staples suffice. The appearance of the nearly completed pouch is illustrated in Figure 86–17E.
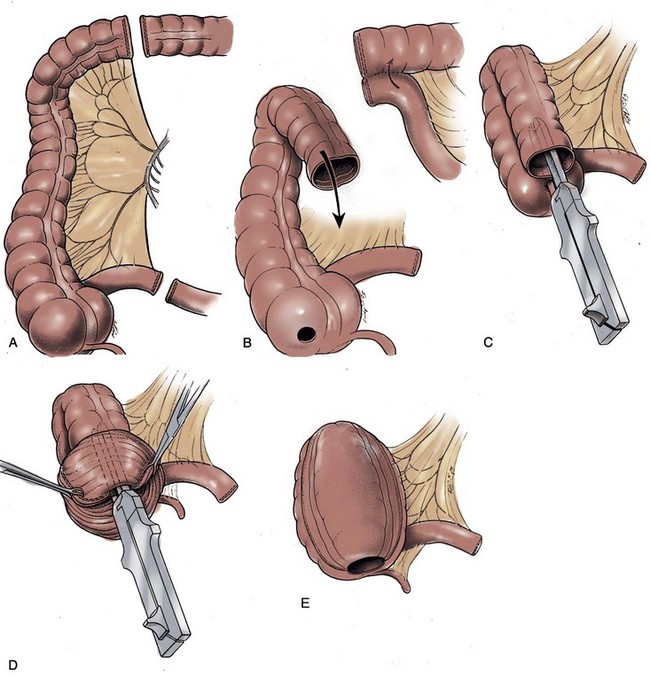
Figure 86–17 A, Isolation of colon and blood supply using metal gastrointestinal anastomosis staplers. B, Opening created in cecum and open end of colon to fit absorbable stapler. Bowel continuity restored between colon and ileum. C, Stapler activated along antimesenteric line and folded bowel. D, Inversion of bowel required to continue staple applications. E, Pouch inversion is complete.
(From Olsson CA, Kirsch AJ, Whang MI-S. Rapid construction of right colon pouch. Curr Surg Tech Urol 1993;6:1–8.)
Once the generic right pouch has been fashioned, several options exist for ureteral anastomosis and formation of a sphincter mechanism. These maneuvers may be approached through the coalesced distal colon opening and cecostomy, which accesses the interior of the pouch (see Fig. 86–17E). The opening permits appropriate stent placement or inspection of a buttressed ileocecal valve. Any of the Mitrofanoff techniques described in conjunction with the Mainz I procedure can be employed. Likewise, the terminal ileum can be either stapled or plicated to create a continence mechanism. Once construction of a continence mechanism and ureteral anastomoses has been performed, the opening can be closed with a running 2-0 absorbable suture or the application of an absorbable TA stapler of appropriate length.
Continent diversion procedures commonly employ the right colon or the cecum and terminal ileum. The array of right colon pouches that can be facilitated by this technique include all of the reservoirs described previously. Reservoirs using terminal ileum and cecum such as the Penn Pouch and the Mainz Pouch can also be fashioned in this manner.
Stapled Sigmoid Reservoir
The same stapling maneuvers can be applied to create a reservoir constructed from the sigmoid colon (Olsson and Kirsch, 1995). A portion of the sigmoid and descending colon measuring approximately 35 cm is mobilized by incising the peritoneum along the white line of Toldt. Once mesenteric windows have been created, the segment of colon is isolated using metal GIA staplers (Fig. 86–18A). Restoration of bowel continuity is achieved with either GIA, TA, or EEA staple devices.
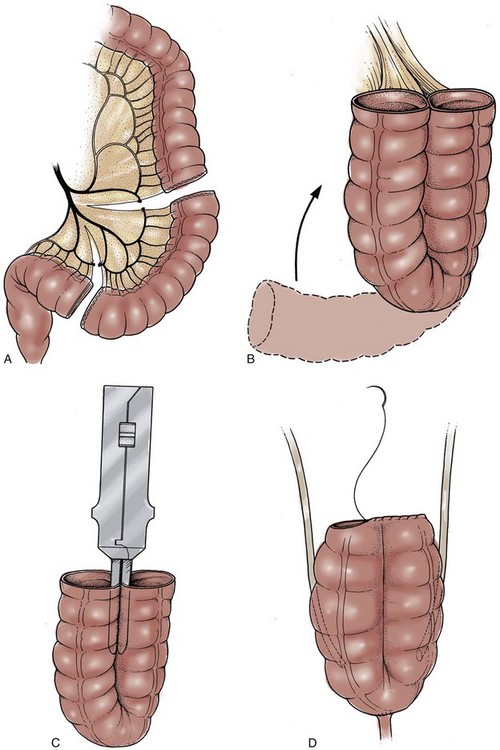
Figure 86–18 A, Isolation of colon and blood supply using metal gastrointestinal anastomosis staplers. B, Sigmoid bowel folded in a U shape. C, Stapler fired along antimesenteric border of folded sigmoid. D, Oversewing of superior open end completes pouch construction.
(From Olsson CA, Kirsch AJ, Whang MI-S. Rapid construction of right colon pouch. Curr Surg Tech Urol 1993;6:1–8.)
Each of the metal stapled ends of the isolated colon are excised, and the bowel lumen is irrigated. The isolated sigmoid is folded on itself in a U configuration, aligning both open ends (see Fig. 86–18B). The absorbable GIA stapler is inserted into the open bowel ends and fired along the antimesenteric line of the folded bowel (see Fig. 86–18C). Following the procedure for bowel eversion as described earlier completes the reservoir. Again, usually two or three applications of the stapler are required to complete the pouch, cutting each staple line tip to avoid staple overlap.
After bowel reinversion, ureteral implants into the taenia can be carried out, using the residual colon opening to assist stent passage. These stents and a suprapubic tube are led through a separate stab wound in the pouch and brought through a lower abdominal wall stab incision. A continence mechanism employing one of the Mitrofanoff variations is then performed.
W-Stapled Reservoir
Montie and colleagues (1994) used the absorbable GIA stapler to construct ileal neobladders in patients undergoing cystoprostatectomy. A segment of ileum measuring 50 cm is divided with a standard metal GIA stapler 20 cm from the ileocecal valve. The terminal ends of each limb of the isolated ileal segment are closed with an absorbable TA-55 stapler, and the metal staple line is resected. The bowel is aligned in a W configuration, and an enterotomy is made 10 cm from each end (Fig. 86–19A). To assist closure of the enterotomy with a TA instrument (see Fig. 86–19, inset), the enterotomy must be made midway between the mesentery and antimesenteric border. The absorbable GIA device is inserted through the enterotomy and is activated. This maneuver adjoins the two adjacent bowel segments. The enterotomy may be closed with the absorbable TA-55 instrument or running absorbable suture, completing the distal segment of the W. The middle and proximal segments are constructed similarly (see Fig. 86–19B). Montie stresses that the segments of the W must be offset to avoid staple lines that overlap each other. Exceeding a 3 to 6 cm overlap may result in bowel ischemia.
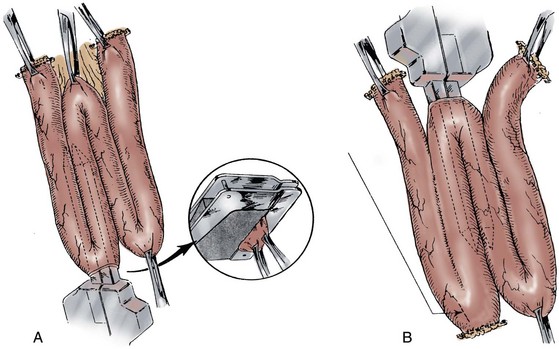
Figure 86–19 A, After closure of butt ends of reservoir with TA-55 stapler, enterotomy is made in dependent portion of first limb of the W. Absorbable stapler is inserted through enterotomy and fired, creating common lumen between two adjacent limbs. Arrow indicates close-up of closure of enterotomy with TA-55 stapler. B, In middle limb of W, stapler is placed through enterotomy at apex, and again common lumen is created. Staple lines are not directly opposing for their entire distance to avoid ischemia between adjacent staple lines. Arrow indicates site of enterotomy where urethro-ileal anastomosis will be performed.
(From Pontes JE. Genitourinary oncologic pelvic surgery. New York: John Wiley & Sons; 1993.)
Postoperative Care and Comments
In the first 50 adult patients to undergo our absorbable stapling technique in right colon pouch construction, with at least 7 years of follow-up, there have been no complications attributable to absorbable staples. Similar results have been reported by Rowland (1996). In the pediatric population, we have applied the absorbable stapler to continent urinary diversion, as well as to bladder augmentation (Hensle et al, 1995). In the first 18 children observed for up to 3 years, there have been no instances of pouch perforation or inadequate pouch capacity and, to date, only one of the patients has developed a reservoir calculus.
Montie and colleagues (1994) used absorbable staplers to create W-stapled ileal neobladders in 25 patients. Ileal pouch construction was performed in approximately 20 minutes, and functional aspects were comparable with bowel reservoirs constructed by conventional suturing. Urodynamic evaluation at 6 months, however, documented a small-capacity reservoir requiring augmentation enterocystoplasty in 3 of 25 patients (12%). Montie and colleagues attributed this complication to either the size of the staples or reservoir fibrosis secondary to foreign body reaction. It is conceivable that a similar situation would arise when constructing a W-stapled T pouch.
The authors have used the absorbable stapler to construct both large and small bowel reservoirs. In our experience, colonic pouches appear better suited for construction with the absorbable stapler because of their relatively larger lumen. The introduction of stapling devices delivering still smaller staples and automatic staple line sealing devices may prevent the problems presently seen when ileal pouches are constructed with current technology.
Summary
In summary, continent urinary diversion is now an accepted part of the urologic surgical armamentarium. A wide array of surgical techniques exists to accomplish the desired goal of creating a continent, stomal-free, nonrefluxing pouch. A paucity of long-term quality of life studies exists to compare outcomes between continent cutaneous urinary diversion and ileal conduit urinary diversion. Surgeons contemplating these forms of urinary diversion should familiarize themselves with several of the techniques and the management of the most common complications.
Key Points
Benson MC, Slawin KM, Wechsler MH, Olsson CA. Analysis of continent versus standard urinary diversion. Br J Urol. 1992;69:156-162.
Bonney WW, Robinson RA. Absorbable staples in continent ileal urinary pouch. Urology. 1990;3:57-62.
Boyd SD, Feinberg SM, Skinner DG, et al. Quality of life survey of urinary diversion patients: comparison of ileal conduits versus continent Kock ileal reservoirs. J Urol. 1987;138:1386-1389.
Gerharz EW, Kohl U, Weingartner K, et al. Complications related to different continence mechanisms in ileocecal reservoirs. J Urol. 1997;158:1709-1713.
Goodwin WE, Turner RD, Winter CC. Results of ileocystoplasty. J Urol. 1958;80:461-466.
Hardt J, Filipas D, Hohenfellner R, Egle UT. Quality of life in patients with bladder carcinoma after cystectomy: first results of a prospective study. Qual Life Res. 2000;9:1-12.
McGuire MS, Rimaldi G, Grotas J, Russo P. The type of urinary diversion after radical cystectomy significantly impacts on the patient’s quality of life. Ann Surg Oncol. 2000;7:4-8.
Olsson CA, Kirsch AJ, Whang M. Rapid construction of right colon pouch. Curr Surg Tech Urol. 1993;6:1-8.
Rowland RG, Mitchell ME, Bihrle R, et al. Indiana continent urinary reservoir. J Urol. 1987;137:1136-1139.
Stein JP, Lieskovsky G, Ginsberg DA, et al. The T pouch: an orthotopic ileal neobladder incorporating a serosal lined ileal antireflux technique. J Urol. 1998;159:1836-1842.
Stein R, Matani Y, Doi Y, et al. Continent urinary diversion using the Mainz Pouch I technique—ten years later. J Urol. 1995;153:251A.
Sumfest JM, Burns MW, Mitchell ME. The Mitrofanoff principle in urinary reconstruction. J Urol. 1993;150:1875-1878.
Thuroff JW, Alken P, Riedmiller H, et al. 100 cases of MAINZ pouch: continuing experience and evolution. J Urol. 1988;140:283-288.
Abol-Enein HC, Ghoneim MA. Optimization of uretero-intestinal anastomosis in urinary diversion: an experimental study in dogs: III. A new antireflux technique for ureteroileal anastomosis: a serous lined extramural tunnel. Urol Res. 1993;21:135-140.
Abol-Enein HC, Ghoneim MA. A novel uretero-ileal reimplantation technique: the serous lined extramural tunnel: a preliminary report. J Urol. 1994;151:1193-1197.
Adams MC, Mitchell ME, Rink RC. Gastrocystoplasty: an alternative solution to the problem of urological reconstruction in the severely compromised patient. J Urol. 1988;140:1152-1156.
Alcini E, Racioppi M, D’Addessi A, et al. Refluxes in orthotopic neobladders: can the ileocecal sphincter be considered an adequate antireflux mechanism? Urology. 1994;44:38-45.
Ambrose SS. Ureterosigmoidostomy. In: Glenn JF, editor. Urologic surgery. Philadelphia: JB Lippincott; 1983:511-520.
Ashken MH. Urinary cecal reservoir. In: King LR, Stone AR, Webster GD, editors. Bladder reconstruction and continent diversion. Chicago: Year Book Medical Publishers; 1987:238-251.
Askanazi J, Hensle TW, Starker P, et al. Effect of immediate postoperative nutritional support on length of hospitalization. J Urol. 1985;134:1032-1036.
Austin PF, DeLeary G, Homsy YL, et al. Long-term metabolic advantages of a gastrointestinal composite urinary reservoir. J Urol. 1997;158:1704-1708.
Bastian PJ, Albers P, Hanitzsch H, et al. Health-related quality-of-life following modified ureterosigmoidostomy (Mainz Pouch II) as continent urinary diversion. Eur Urol. 2004;46:591-597.
Bejany DE, Politano VA. Stapled and nonstapled tapered distal ileum for construction of a continent colonic urinary reservoir. J Urol. 1988;140:491-494.
Benchekroun A. The ileocecal continent bladder. In: King LR, Stone AR, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book Medical Publishers; 1987:224-237.
Benson MC, Sawczuk IS, Hensle TW, et al. Modified Indiana University continent diversion. Curr Surg Tech Urol. 1988;1:1-8.
Benson MC, Slawin KM, Wechsler MH, Olsson CA. Analysis of continent versus standard urinary diversion. Br J Urol. 1992;69:156-162.
Bihrle R. The Indiana pouch continent urinary reservoir. Urol Clin North Am. 1997;24:773-779.
Bissada NK. Characteristics of the in-situ appendix as a continent catheterization stoma for continent urinary diversion in adults. J Urol. 1993;150:151-152.
Bissada NK. Favorable experience with a simple technique to create a concealed umbilical stoma. J Urol. 1998;159:1174-1175.
Bissada NK, Morcos RR, Morgan WM, Hanash KA. Ureterosigmoidostomy: is it a viable procedure in the age of continent urinary diversion and bladder substitution? J Urol. 1995;153:1429-1431.
Bjerre BD, Johansen C, Steven K. Health-related quality of life after cystectomy: bladder substitution compared with ileal conduit diversion: a questionnaire survey. Br J Urol. 1995;75:200-205.
Bochner BH, Stein JP, Ginsberg DA, et al. A serous-lined antireflux valve: in vivo fluorourodynamic evaluation of antireflux continence mechanism. J Urol. 1988;160:112-115.
Bonney WW, Robinson RA. Absorbable staples in continent ileal urinary pouch. Urology. 1990;3:57-62.
Boyd SD, Feinberg SM, Skinner DG, et al. Quality of life survey of urinary diversion patients: comparison of ileal conduits versus continent Kock ileal reservoirs. J Urol. 1987;138:1386-1389.
Burger R, Wammack R, Fisch M, et al. The appendix as a continence mechanism. Eur Urol. 1992;22:255-262.
Burns MW, Mitchell ME. Tips on constructing the Mitrofanoff appendiceal stoma. Contemp Urol. May 1990:10-12.
Carr MC, Mitchell ME. Continent gastric pouch. World J Urol. 1996;14:112-116.
Carroll PR, Presti JCJr. Comparison of plicated and stapled continent ileocecal stoma. Urology. 1992;40:107-109.
Carroll PR, Presti JCJr, McAninch JW, Tanagho EA. Functional characteristics of the continent ileocecal urinary reservoir: mechanisms of urinary continence. J Urol. 1989;142(4):1032-1036.
Culp DA. Commentary: Heitz-Boyer-Havelacque procedure: not the procedure of choice. In: Whitehead ED, Leiter E, editors. Current operative urology. 2nd ed. Philadelphia: Harper and Row; 1984:782-783.
Cummings K. Personal communication. 1995.
Duckett JW, Gazak JM. Complications of ureterosigmoidostomy. Urol Clin North Am. 1983;10:473-481.
Duckett JW, Snyder HMIII. Use of the Mitrofanoff principle in urinary reconstruction. Urol Clin North Am. 1986;13:271-274.
El-Mekresh MM, Hafez AT, Abol-Enein H, Ghoneim MA. Double folded rectosigmoid bladder with a new ureterocolic antireflux technique. J Urol. 1997;157:2085-2089.
Filipas D, Egle UT, Budenbender C, et al. Quality of life and health in patients with urinary diversion: a comparison of incontinent versus continent urinary diversion. Eur Urol. 1997;32:23-29.
Filmer RB, Spencer JR. Malignancies in bladder augmentations and intestinal conduits. J Urol. 1990;143:671-678.
Fisch M, Hohenfellner R. Der sigma rectum pouch: eine modifikation der harnleiterdarn implantation. Akt Urol. 22, 1991. Operative Techniken
Fisch M, Wammack R, Hohenfellner R. The sigma-rectum pouch (Mainz pouch II). World J Urol. 1996;14:68-72.
Gerharz EW, Kohl U, Weingartner K, et al. Complications related to different continence mechanisms in ileocecal reservoirs. J Urol. 1997;158:1709-1713.
Gerharz EW, Mansson A, Hunt S, et al. Quality of life after cystectomy and urinary diversion: an evidence based analysis. J Urol. 2005;174:1729-1736.
Gerber A. Improved quality of life following a Kock continent ileostomy. West J Med. 1980;133:95.
Gilchrist RK, Merricks JW, Hamlin HH, Rieger IT. Construction of a substitute bladder and urethra. Surg Gynecol Obstet. 1950;90:752-760.
Gitlin JS, Wu X-R, Sun T-T, et al. New concepts of histological changes in experimental augmentation cystoplasty: insights into the development of neoplastic transformation at the enterovesical and gastrovesical anastomosis. J Urol. 1999;162:1096-1100.
Golomb J, Klutke CG, Lewin KJ, et al. Bladder neoplasms associated with augmentation cystoplasty: report of 2 cases and literature review. J Urol. 1989;142:377-380.
Goodwin WE, Turner RD, Winter CC. Results of ileocystoplasty. J Urol. 1958;80:461-466.
Gosalbez R, Padron OF, Singla AK, et al. The gastric augment single pedicle tube catheterizable stoma: a useful adjunct to reconstruction of the urinary tract. J Urol. 1994;152:2005-2007.
Hafez AT, El-Mekresh MM, Abol-Enein H, Ghoneim MA. Double folded rectosigmoid pouch with a novel ureterocolic reimplantation technique. In: Continent urinary reconstruction, Second International Meeting, Mainz, Germany, p 151, abstract 118, June 28–30, 1995.
Hampel N, Hasan ST, Marshall C, Neal DE. Continent urinary diversion using the Mitrofanoff principle. Br J Urol. 1995;74:454-459.
Hardt J, Filipas D, Hohenfellner R, Egle UT. Quality of life in patients with bladder carcinoma after cystectomy: first results of a prospective study. Qual Life Res. 2000;9:1-12.
Hart S, Skinner EC, Meyerowitz BE, et al. Quality of life after radical cystectomy for bladder cancer in patients with an ileal conduit, or cutaneous or urethral Kock pouch. J Urol. 1999;162:77-81.
Helal M, Poew-Sang J, Sanford E, et al. Direct (non-tunneled) ureterocolonic reimplantation in association with continent reservoirs. J Urol. 1993;150:835-837.
Hensle TW. Nutritional support of the surgical patient. Urol Clin North Am. 1983;10:109.
Hensle TW, Kirsch AJ, Olsson CA. Bowel reservoirs created with absorbable staples in a pediatric population. Presented at the annual meeting of the American Academy of Pediatrics, San Francisco, CA, October 1995.
Hubner WA, Pfluger H. Functional replacement of bladder and urethra after cystectomy for bladder cancer in a female patient. J Urol. 1995;153:1043-1046.
Kock NG. Ileostomy without external appliance: a survey of 25 patients provided with intestinal reservoir. Ann Surg. 1971;173:545.
Kock NG, Ghoneim MA, Lycke KG, Mahran MR. Urinary diversion to the augmented and valved rectum: preliminary results with a novel surgical procedure. J Urol. 1988;140:1375-1379.
Kock NG, Nilson AE, Nilsson LO, et al. Urinary diversion via a continent ileal reservoir: clinical results in 12 patients. J Urol. 1982;128:469.
Kristiansen P, Mansson W, Tyger J. Perforation of continent caecal reservoir for urine twice in one patient. Scand J Urol Nephrol. 1991;25:279-281.
Kristjansson A, Bajc M, Wallin L, et al. Renal function up to 16 years after conduit or continent urinary diversion: renal scarring and location of bacteriuria. Br J Urol. 1995;76:546-550.
Lampel A, Fisch M, Stein R, et al. Continent diversion with the Mainz pouch. World J Urol. 1996;14:85-91.
Lampel A, Hohenfellner M, Schultz-Lampel D, Thuroff JW. In situ tunneled bowel flap tubes: two new techniques of a continent outlet for Mainz pouch cutaneous diversion. J Urol. 1995;153:308-315.
Lampel A, Hohenfellner M, Schultz-Lampel D, Thuroff JW. Submucosal seromuscular tube and submucosal bowel flap tube: two new stoma techniques for Mainz pouch continent cutaneous urinary diversion. J Urol. 1995;153:305A.
Lampel A, Thuroff JW. Bowel-flap tubes for continent urinary diversion. World J Urol. 1998;16:235-241.
Leadbetter GWJr, Leadbetter WF. Ureteral re-implantation and bladder neck reconstruction: four and one-half years’ experience. JAMA. 1961;175:349.
LeDuc A, Camey M, Teillac P. An original antireflux ureteroileal implantation technique: long-term follow-up. J Urol. 1987;137:1156-1158.
Leong CH. Use of stomach for bladder replacement and urinary diversion. Ann R Coll Surg Engl. 1978;60:283-289.
Light JK, Scardino PT. Radical cystectomy with preservation of sexual and urinary function: use of the ileocolonic pouch (“Le Bag”). Urol Clin North Am. 1986;13:261-270.
Lockhart JL. Remodeled right colon: an alternative urinary reservoir. J Urol. 1987;138:730-734.
Lockhart JL, Davies R, Cox C, et al. The gastroileal pouch: an alternative continent urinary reservoir for patients with short bowel, acidosis and/or extensive pelvic radiation. J Urol. 1993;150:46-51.
Mansson W. The continent cecal urinary reservoir. In: King LR, Stone AR, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book Medical Publishers; 1987:209.
Mansson W, Colleen S, Forsberg L, et al. Renal function after urinary diversion: a study of continent caecal reservoir, ileal conduit, and colonic conduit. Scand J Urol Nephrol. 1984;18:307-315.
Mansson A, Davidsson T, Hunt S, Mansson W. The quality of life in men after radical cystectomy with a continent cutaneous diversion or orthotopic bladder substitution: is there a difference? BJU Int. 2002;90:386-390.
Marino G, Laudi M. Ileal T-pouch as a urinary continent cutaneous diversion: clinical and urodynamic evaluation. BJU Int. 2002;90:47-50.
McGuire MS, Rimaldi G, Grotas J, Russo P. The type of urinary diversion after radical cystectomy significantly impacts on the patient’s quality of life. Ann Surg Oncol. 2000;7:4-8.
McLeod RS, Fazio VW. Quality of life with the continent ileostomy. World J Surg. 1984;8:90.
Mills RD, Studer UE. Metabolic consequences of continent urinary diversion. J Urol. 1999;161:1057-1066.
Mitrofanoff P. Cystostomie continente trans-appendiculaire dans le traitement des vessies neurologiques. Chir Pediatr. 1980;21:297-305.
Montie JE. Ileal conduit diversion after radical cystectomy. Pro Urology. 1997;49:659-662.
Montie JE, Pontes JE, Parulkar BG, Selby T. W-stapled ileal neo-bladder formed entirely with absorbable staples. J Urol. 1994;151:1188-1192.
Montie JE, Pontes JE, Powell IJ. Orthotopic ileal neobladder after cystectomy: comparison of hand-sewn vs. absorbable staples for reservoir formation. J Urol. 1995;153:305A.
Navon JD, Wong AK, Weinberg AC, Ahlering TE. A comparative study of postoperative complications associated with the modified Indiana pouch in elderly versus younger patients. J Urol. 1995;154:1325-1328.
Nguyen DH, Bain MA, Salmonson KL, et al. The syndrome of dysuria and hematuria in pediatric urinary reconstruction with stomach. J Urol. 1993;150:707-709.
Oesterling JE, Gearhart JP. Utilization of ileal conduit in construction of continent urinary reservoir. Urology. 1990;36:15-19.
Olsson CA. Kock continent ileal reservoir for urinary diversion. In: King LR, Stone AR, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book Medical Publishers; 1987:291-310.
Olsson CA, Kirsch AJ. Stapled sigmoid neo-bladder. Curr Surg Tech Urol. 1995;8:1-8.
Olsson CA, Kirsch AJ, Whang M. Rapid construction of right colon pouch. Curr Surg Tech Urol. 1993;6:1-8.
Piser JA, Mitchell ME, Kulb TB, et al. Gastrocystoplasty and colocystoplasty in canines: the metabolic consequences of acute saline and acid loading. J Urol. 1987;138:1009.
Pahernik S, Stein R, Hohenfellner M, Thuroff JW. Conversion from colonic or ileal conduit to continent cutaneous urinary diversion. J Urol. 2004;171:2293-2297.
Pantuck AJ, Han KR, Perrotti M, Weiss RE, Cummings KB. Ureteroenteric anastomosis in continent urinary diversion: long-term results and complications of direct versus nonrefluxing techniques. J Urol. 2000;163:450-455.
Pow-Sang JM, Helal M, Figueroa E, et al. Conversion from external appliance wearing or internal urinary diversion to a continent urinary reservoir (Florida Pouch I and II): surgical technique, indications and complications. J Urol. 1992;147:356-360.
Riedmiller H, Steinbach F, Thuroff J, Hohenfellner R. Continent appendix stoma—a modification of the Mainz pouch technique. Presented E.A.U. Congress, Amsterdam, June 1990.
Rowland RG. Personal communication, 1989.
Rowland RG. Present experience with the Indiana pouch. World J Urol. 1996;14:92-98.
Rowland RG, Mitchell ME, Bihrle R. The cecoileal continent urinary reservoir. World J Urol. 1985;3:185-190.
Rowland RG, Mitchell ME, Bihrle R, et al. Indiana continent urinary reservoir. J Urol. 1987;137:1136-1139.
Rudick J, Schonholz S, Weber HN. The gastric bladder: a continent reservoir for urinary diversion. Surgery. 1977;82:1.
Salter MJ. What are the differences in body image between patients with a conventional stoma compared with those who have had a conventional stoma followed by a continent pouch? J Adv. Nursing. 1992;17:841-848.
Salter MJ. Aspects of sexuality for patients with stomas and continent pouches. J Adv Nursing. 1992;19:126-130.
Sanda MG, Jeffs RD, Gearhart JP. Evolution of outcomes with the ileal hydraulic valve continent diversion: re-evaluation of the Benchekroun catheterizable stoma. World J Urol. 1995;14:108-111.
Seifert HH, Obaje A, Müller-Mattheis V, et al. Clinical and functional results after continent cutaneous urinary diversion with the ileal double-T-pouch. Urol Int. 2008;80:8-12.
Shokeir AA, Shamaa M, El-Mekresh MM, et al. Late malignancy in bowel segments exposed to urine without fecal stream. Urology. 1995;46:657-661.
Simoneau AR, Skinner DG. Ileo-anal reservoir. J Urol. 1995;153:305A.
Sinaiko E. Artificial bladder segment of stomach and study of effect of urine on gastric secretion. Surg Gynecol Obstet. 1956;102:433.
Skinner DG. The Kock pouch for continent urinary reconstruction focusing on the afferent segment and the reservoir. Scand J Urol Nephrol. 1992;142:77-78.
Skinner DG, Boyd S, Lieskovsky G. Clinical experience with the Kock continent ileal reservoir for urinary diversion. J Urol. 1984;132:1101-1107.
Skinner DG, Lieskovsky G, Boyd S. Continent urinary diversion. J Urol. 1989;141:1323-1327.
Skinner DG, Lieskovsky G, Skinner E, Boyd S. Urinary diversion. Curr Prob Surg. 1987;24:401-471.
Spirnak JP, Caldamone AA. Ureterosigmoidostomy. Urol Clin North Am. 1986;13:285-294.
Stein JP, Buscarini M, DeFilippo RE, Skinner DG. Application of the T pouch as an ileo-anal reservoir. J Urol. 1999;162:2052-2053.
Stein JP, Lieskovsky G, Skinner DG. The Double T Pouch: a novel continent cutaneous ileal reservoir. J Urol. 1999;161(Suppl. 253A):67.
Stein JP, Lieskovsky G, Ginsberg DA, et al. The T pouch: an orthotopic ileal neobladder incorporating a serosal lined ileal antireflux technique. J Urol. 1998;159:1836-1842.
Stein JP, Skinner DG. T-mechanism applied to urinary diversion: the orthotopic T-pouch ileal neobladder and cutaneous double-T-pouch ileal reservoir. Tech Urol. 2001;7:209-222.
Stein R, Matani Y, Doi Y, et al. Continent urinary diversion using the Mainz Pouch I technique—ten years later. J Urol. 1995;153:251A.
Sumfest JM, Burns MW, Mitchell ME. The Mitrofanoff principle in urinary reconstruction. J Urol. 1993;150:1875-1878.
Terai A, Arai Y, Kawakita M, et al. Effect of urinary intestinal diversion urinary risk factors for urolithiasis. J Urol. 1995;153:37-41.
Thompson ST, Kursh ED. Delayed spontaneous rupture of an ileocolonic neobladder. J Urol. 1992;148:1890-1891.
Thuroff JW, Alken P, Riedmiller H, et al. The MAINZ pouch (mixed augmentation ileum ‘n zecum) for bladder augmentation and continent diversion. World J Urol. 1985;3:179-184.
Thuroff JW, Alken P, Riedmiller H, et al. 100 cases of MAINZ pouch: continuing experience and evolution. J Urol. 1988;140:283-288.
Venn SN, Mundy AR. Continent urinary diversion using the Mainz-type ureterosigmoidostomy—a valuable salvage procedure. Eur Urol. 1999;36:247-251.
Watanabe K, Kato H, Misawa K, Ogawa A. Spontaneous perforation of an ileal neobladder. Br J Urol. 1994;73:460-461.
Webster GD, King LR. Further commentary: cecal bladder. In: King LR, Stone AR, Webster GD, editors. Bladder reconstruction and continent urinary diversion. Chicago: Year Book Medical Publishers; 1987:206-208.
Wiesner C, Bonfig R, Stein R, et al. Continent cutaneous urinary diversion: long-term follow-up of more than 800 patients with ileocecal reservoirs. World J Urol. 2006;24:315-318.
Wiesner C, Pahernik S, Stein R, et al. Long-term follow-up of submucosal tunnel and serosa-lined extramural tunnel ureter implantation in ileocaecal continent cutaneous urinary diversion (Mainz pouch I). BJU Int. 2007;100:633-637.
Williams DF. Commentary: the value of ureterosigmoidostomy. In: Whithead ED, Leiter E, editors. Current operative urology. 2nd ed. Philadelphia: Harper & Row; 1984:742-744.
Williams DF, Burkholder GV, Goodwin WE. Ureterosigmoidostomy: a 15-year experience. J Urol. 1969;101:168-170.
Woodhouse CR, Christofides M. Modified ureterosigmoidostomy (Mainz II)—technique and early results. Br J Urol. 1998;81:247-252.
Woodhouse CR, MacNeily AE. The Mitrofanoff principle: expanding upon a versatile technique. Br J Urol. 1994;74:447-453.