chapter 87 Orthotopic Urinary Diversion
Evolution of Orthotopic Urinary Diversion
Since the early 1900s, innovative surgeons have investigated how best to replace the original bladder when it must be removed for either benign or malignant disease. Contemporary objectives of lower urinary tract reconstruction have had an ultimate goal of finding a system that allows volitional voiding through the native urethra while eliminating the need for a cutaneous urinary stoma or intermittent catheterization. If continued progress is to be made in the field of urinary diversion, a thorough understanding and appreciation of previous techniques and accomplishments must be achieved.
Ureterosigmoidostomy is the oldest form of clinically applied urinary diversion. The first reported urinary diversion into a segment of bowel was by Simon in 1852. He attempted a ureterosigmoidostomy in an exstrophy patient by drawing the ureters into the rectum with the use of needles and suture to create a fistula. Although the patient died of sepsis 12 months later, this marked the first reported attempt at some form of urinary diversion (Simon, 1852). Over the following 100 years the evolution of urinary diversion was marked by a continued search for better methods and techniques to reconstruct the lower urinary tract. A number of technical modifications of the ureterosigmoidostomy ensued, particularly related to the ureteral implantation technique (Hinman, 1936). The rates of obstruction and ascending pyelonephritis in patients with ureterosigmoidostomy were significantly reduced after a direct anastomosis of the ureter into the sigmoid colon, incorporating an antireflux submucosal tunnel (Leadbetter, 1951; Goodwin et al, 1953).
Ureterosigmoidostomy remained the diversion of choice until the late 1950s, but long-term electrolyte imbalance, upper tract obstruction and infection, and secondary malignant neoplasms arising at the ureteral implantation site were observed (Leadbetter and Clarke, 1954; Clarke and Leadbetter, 1955; Ridlon, 1963; Wear and Barquin, 1973). These significant complications inspired surgeons to develop better forms of urinary diversion. In 1950 Bricker refined and popularized the ileal conduit form of urinary diversion, building on an original description by Zaayer in 1911 (Zaayer, 1911; Bricker, 1950). The ileal conduit is a technically simple urinary diversion. This form of reconstruction was a reliable technique for urinary diversion and became widely accepted. It has remained the “gold standard” to which other types of urinary diversion are compared. It continues to be the most common form of urinary diversion performed throughout the world today.
Since the 1950s, the further evolution of lower urinary tract reconstruction has developed along three distinct paths: (1) variations of cutaneous conduit forms of urinary diversion (ileal or colon conduit); (2) continent cutaneous forms of urinary diversion to the skin (such as the cutaneous Kock pouch and Indiana pouch); and (3) most recently, the orthotopic form of diversion to the native, intact urethra (neobladder).
Long-term complications with the Bricker ileal conduit started to come to light in the 1970s. Although problems with hyperchloremic metabolic acidosis and pyelonephritis were substantially less common than in patients with ureterosigmoidostomy, late complications with the ileal conduit such as stomal stenosis, pyelonephritis, calculus formation, ureteral obstruction, and renal deterioration became more apparent with longer follow-up (Butcher et al, 1962; Shapiro et al, 1975; Middleton, 1976; Johnson and Lamy, 1977; Pitts and Muecke, 1979; Sullivan et al, 1980). These clinical sequelae were thought to be related to the reflux of infected urinary or obstruction of the upper urinary tract. It was postulated that the addition of an antireflux technique to a conduit form of diversion could help diminish the problems of reflux and renal deterioration in these patients. In fact, well-performed laboratory experiments provided evidence to support the advantage of nonrefluxing colonic conduits over ileal conduits (Richie et al, 1974; Claesson et al, 1985). Unfortunately, with longer follow-up, similar complications with colon conduits including upper urinary tract damage developed, again dampening enthusiasm for this form of urinary diversion (Morales and Golimbu, 1975; Althausen et al, 1978; Elder et al, 1979).
The first continent diversion was described by Gilchrist and colleagues. This form of urinary reconstruction incorporated a cecal reservoir with the ileocecal valve as the continence mechanism and the distal ileum as a catheterizable stomam (Gilchrist et al, 1950). However, this innovation attracted little attention at the time. The concept of a continent cutaneous diversion was subsequently reintroduced by Kock and colleagues (1982). This diversion was originally designed for a continent terminal ileostomy and used an intussuscepted nipple valve to maintain continence and avoid reflux. In animal experiments and then in humans, Kock demonstrated the importance of complete detubularization of the bowel segment and the double-folding technique that creates the most spherical shape possible (Eckman et al, 1964; Kock et al, 1982). These concepts are the cornerstone of current cutaneous and orthotopic reservoirs. After Kock described his results in his initial 12 patients, Donald Skinner began performing this diversion in adults undergoing cystectomy for bladder cancer. Although this form of urinary diversion required catheterization of an abdominal stoma, it eliminated the need for and associated problems with an external urostomy appliance. It was a popular concept for patients and referring physicians alike, and Skinner quickly amassed a large clinical experience with this type of diversion (Skinner et al, 1984, 1987).
The “Achilles’ heel” of continent cutaneous reservoirs is the design of a reliable, durable, efferent continence mechanism that is easily catheterizable. A number of techniques have been described using large and small bowel and even stomach, with many ingenious continence mechanisms. However, stones, difficulty catheterizing, peristomal hernias, and the development of leakage are potential problems with all of them, often requiring open surgical revision to resolve (Lieskovsky et al, 1987; Rowland, 1995).
The concept of orthotopic diversion began even before Gilchrist’s continent cutaneous diversion. Tizzoni and Poggi (1988) were the first to experiment in a dog transplanting the ureters into an isolated loop of ileum interposed between the ureters and the urethra. The dog was reportedly continent and subsequently underwent three successful pregnancies before expiring 30 months postoperatively (Tizzoni and Poggi, 1988). Lemoine is credited with performing the first orthotopic reconstruction in a human subject. This patient initially underwent a cystectomy with ureterosigmoidostomy. Complications related to recurrent pyelonephritis led to undiversion in this patient; the rectal segment was isolated, transected, and anastomosed to the urethra, and the sigmoid colon was brought down and anastomosed to the anus (Lemoine, 1913).
In 1979 Camey and Le Duc reported their pioneering and extensive clinical experience with orthotopic substitution to the native urethra in male bladder cancer patients (Camey and Le Duc, 1979). This was a substantial accomplishment that demonstrated the feasibility of lower urinary tract reconstruction to the native urethra. The initial Camey diversion used an intact segment of ileum, resulting in a high-pressure reservoir. Subsequently the Camey II detubularized reservoir (Camey, 1990); the Hautmann W-neobladder (Hautmann et al, 1988); the Studer pouch (Studer et al, 1989); the T pouch (Stein et al, 1998b); stomach neobladder (Hauri, 1998); cecal and ileocecal neobladders (Light and Englemann, 1986; Mansson and Colleen, 1990); and sigmoid reservoir (Reddy and Lange, 1987) have been described. Many of these now have a large clinical experience with long-term follow-up demonstrating good renal preservation and continence results (discussed further later). Initially these techniques were only applied to male patients because continence in the female was believed to be dependent on an intact bladder neck. In the mid-1990s it was discovered through anatomic dissections and initial clinical experience that women could remain continent with a low-pressure reservoir and preservation of only the urethra itself (Borirakchanyavat et al, 1997; Colleselli, 1998). In addition there were several careful pathologic studies of female cystectomy specimens showing that preservation of the urethra was safe in the majority of women with bladder cancer without compromising the oncologic efficacy of the operation (Stein et al, 1995, 1998a; Stenzl, 1995b; Maralani, 1997).
Although the ideal bladder substitute remains to be developed, the orthotopic neobladder most closely resembles the original bladder in both location and function. This form of lower urinary tract reconstruction relies on the intact external rhabdosphincter continence mechanism, usually does not require intermittent catheterization, and avoids the difficulties associated with the efferent continence mechanism of continent cutaneous reservoirs. Voiding is accomplished by concomitantly increasing intra-abdominal pressure (Valsalva maneuver) with relaxation of the pelvic floor musculature. The majority of patients undergoing orthotopic reconstruction are continent and void to completion without the need for intermittent catheterization. The pioneering work of Camey and Le Duc with orthotopic reconstruction in carefully selected male patients has subsequently evolved into a common form of lower urinary tract reconstruction potentially applicable to most patients requiring urinary diversion (Camey and Le Duc, 1979).
In many centers worldwide orthotopic reconstruction has replaced the ileal conduit as the standard form of reconstruction. The experience of urinary diversion at the Keck University of Southern California School of Medicine demonstrates this evolution (Fig. 87–1). Beginning in 1986, the number of orthotopic bladder substitutes performed dramatically increased while the number of conduits dramatically declined. Currently the authors perform continent orthotopic diversion in approximately 90% of male and 75% of female patients undergoing radical cystectomy. It is, however, incumbent on the surgeon who is actively involved in lower urinary tract reconstruction to understand the indications for and contraindications to orthotopic diversion, as well as to be familiar with the various reconstructive options. This will help ensure optimal clinical outcomes and improved satisfaction of patients.
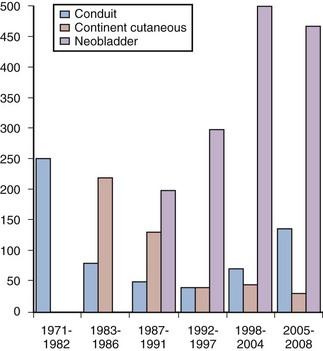
Figure 87–1 Evolution of urinary diversion at the University of Southern California from 1971 to 2008. Orthotopic reconstruction was first applied to men in 1986 and to women in 1990, with a steady increase in the number of bladder substitutes since that time and a decline in the number of conduit forms of diversion during the same period. Recent increases in the percentage of older patients undergoing this surgery have resulted in a slight increase in ileal conduit diversion in the past few years.
Key Points: Historic Evolution of Orthotopic Urinary Diversion
Basic Principles of Continent Orthotopic Urinary Diversion
Many methods for construction of an orthotopic neobladder using intestinal segments exist, but three basic principles must be satisfied for a successful outcome. First, the patient must have an adequate external sphincter mechanism and nonobstructed urethra. This aspect is discussed in more detail later in the sections on patient selection and description of the anatomy of the external sphincter and surgical techniques for continence preservation.
Secondly, the reservoir must be sufficiently compliant to maintain a low pressure throughout the filling phase. This is best achieved by splitting the bowel segment open longitudinally to completely detubularize it and folding it to create a spherical shape. This concept was described by Goodwin and colleagues (1959) and further developed by Kock in elegant animal experiments (Eckman et al, 1964). The sphere has the greatest internal volume for a given surface area and thus the greatest capacity. By Laplace’s law (T = PR) for a given wall tension (T), the internal pressure (P) decreases as the radius (R) of the sphere increases. The compliance of the wall relates to the physical characteristics of the bowel wall itself and is greater in small bowel compared with large bowel. The double-folded technique of Kock, or S- or W-shaped reservoirs all effectively ablate the coordinated high-pressure contractions of the bowel wall, allowing the reservoir to maintain low internal pressure throughout the filling phase (Kock et al, 1982; Hinman, 1988). Early techniques for bladder augmentation using an intact cecal segment and the early Camey orthotopic ileal neobladder failed to incorporate the important step of detubularization and thus resulted in unacceptable incontinence and upper tract deteriorization (Camey and Le Duc, 1979; Lilien and Camey, 1984). All current techniques use detubularized bowel to construct the reservoir portion of the orthotopic diversion.
Thirdly, the reservoir must have adequate volume to allow for reasonable voiding intervals. This generally should be at least 300 to 500 mL. All bowel segments effectively stretch over time if there is adequate outflow resistance. The standard 44-cm length of ileum formed into a double-folding reservoir by the Kock technique (also used for both the Studer and T pouch neobladders) holds less than 200 mL initially. When used for a cutaneous pouch with high outflow resistance, this segment routinely stretches up over time to hold more than 1000 mL at low pressure. Such high volumes are not ideal for orthotopic diversion because of the difficulty emptying such large reservoirs, so patients are encouraged to empty more frequently. Nevertheless, this emphasizes that larger initial volumes are not necessary to ultimately achieve an adequate voiding volume. However, colonic segments do not stretch up as easily and a larger initial volume may be necessary for pouches constructed out of colon. In general small bowel, when available, has advantages over colon in terms of wall compliance and ability to stretch, as well as reduced mucous formation (Khafagy, 2006).
Key Points: Three Basic Principles of Orthotopic Neobladder Construction
Patient Selection
Nearly all patients who undergo radical cystectomy should be considered at least potential candidates for orthotopic urinary diversion. There are few absolute contraindications. However, a number of factors should be considered in counseling the individual patient as to the best diversion for him or her, and these can be divided into cancer-related factors and patient factors.
Cancer-Related Factors
Risk of Urethral Recurrence in Men
The primary cancer-related contraindication for orthotopic diversion is presence of urothelial carcinoma in the urethral stump to which the neobladder is to be connected. In the male patient involvement of the prostatic urethra is associated with a higher risk of subsequent urethral recurrence. Ashworth first reported that patients with prostatic urethral involvement are at increased risk for urethral tumor involvement after cystectomy and ileal conduit. Prostatic urethral tumor involvement was found in five of seven patients (71%) who later developed anterior urethral tumors (Ashworth, 1956). Other series have subsequently confirmed this finding (Raz et al, 1978; Faysal, 1980; Hardeman, 1990; Levinson et al, 1990; Tobisu et al, 1991; Nieder et al, 2004). Freeman and colleagues (1994) reviewed six studies, in which 31 of 122 patients (25%) with some form of prostatic urethral involvement developed an anterior urethral tumor after radical cystectomy for bladder cancer. These findings have subsequently been confirmed in our cystectomy series in which the overall probability of urethral recurrence was estimated to be approximately 7% at 5 years and 9% at 10 years. Recurrences were observed at a median of 2 years after cystectomy (range 0.2 to 13 years). The risk was only 5% at 5 years in the 639 patients without any prostate tumor involvement compared with 11% for the 129 men with any prostate involvement (Stein et al, 2005). In a large, comprehensive, pooled analysis of 25 series, Stenzl and colleagues (1995b) reported a total of 256 anterior urethral tumor recurrences in 3165 patients (8.1%) undergoing cystectomy for bladder cancer.
Specific characteristics of the primary bladder cancer have been analyzed to determine if any particular histopathologic parameters can identify patients at increased risk for urethral recurrence after cystectomy. Various pathologic risk factors have been implicated including the presence of papillary tumors, tumor multifocality, trigone or bladder neck tumor involvement, associated carcinoma in situ in the bladder or upper tracts, and various degrees of involvement of the prostate (Freeman et al, 1996). Several investigators have evaluated the presence of carcinoma in situ in the bladder as a risk factor for urethral recurrence with variable results (Hardeman and Soloway, 1990; Levinson et al, 1990; Tobisu et al, 1991; Stein et al, 2005). Tobisu and colleagues (1991) combined these pathologic risk factors and showed in a multivariate analysis that the risk of urethral recurrence more than doubled each time the number of risk factors was increased by one.
Of all the risk factors, the degree of prostatic tumor involvement is most useful in predicting the risk of subsequent urethral recurrence. In general, prostatic involvement can be divided into urethral mucosa only (including carcinoma in situ), ductal involvement with carcinoma in situ, and prostatic stromal invasion. Hardeman and Soloway (1990) evaluated 30 patients with some involvement of the prostate and found anterior urethral recurrence in 11 (37%). No patient with only mucosal involvement developed a recurrence, whereas 25% of men with prostatic ductal involvement and 64% of men with prostatic stromal involvement developed urethral recurrence. In the same series, only 2 of 56 patients (4%) without prostatic urethral tumor involvement in the primary tumor suffered subsequent urethral recurrence (Hardeman and Soloway, 1990). In another study, Levinson and colleagues (1990) found similar results, with urethral recurrences rates of 0% for mucosa only, 10% for ductal involvement, and 30% with stromal invasion. Overall, 67% of their patients with urethral recurrences had a history of prostatic urethral involvement in their primary tumor (Levinson et al, 1990). Hassan and colleagues (2004) reported an even lower risk of urethral recurrence, with only one recurrence observed in more than 400 patients, though with relatively short follow-up.
These observations were confirmed in our series of 768 men undergoing cystectomy without urethrectomy with long-term follow-up (median 13 years) (Stein et al, 2005). The extent of prostatic tumor involvement was the most significant predictor for urethral tumor recurrence. Of the 129 patients with pathologic transitional cell carcinoma involving the prostate at cystectomy, 14 (11%) developed urethral recurrence. The 5-year estimated probability of urethral recurrence for superficial (mucosa and ductal, without stroma) involvement was 12%, compared with 18% with prostatic stroma invasion. In a multivariate analysis, any prostate tumor involvement (superficial or invasive) remained an independent and significant predictor of a urethral tumor recurrence. Carcinoma in situ and tumor multifocality were not individually associated with a significant risk for anterior urethral recurrence (Stein et al, 2005). Collectively, these studies confirm that prostatic stromal invasion is the single strongest pathologic predictor of subsequent recurrence in the anterior urethra after cystectomy for bladder cancer. It does not appear that the presence of carcinoma in situ in the bladder alone is a clear risk factor for subsequent urethral recurrence.
Some evidence indicates that orthotopic diversion itself may provide some protection against urethral recurrence. In the University of Southern California experience, the type of diversion was an independent variable predicting urethral recurrence. In patients without prostatic involvement, the risk of urethral recurrence at 5 years was 3% versus 8% in patients with orthotopic versus cutaneous diversion, respectively. Interestingly, there was no benefit seen if patients with any prostatic involvement were taken as a whole. However, in patients with the highest-risk disease (prostatic stromal invasion) there did seem to be some protection for those undergoing orthotopic diversion, with a 5-year risk of recurrence of 11% versus 24% for those with cutaneous diversion. The reason for this observation is unclear, though there has been speculation that continued flow of urine, perhaps with changes in urinary characteristics due to the interposed bowel, might be responsible (Stein et al, 2005).
There is some controversy about the importance of attempting to identify prostatic urethral involvement preoperatively, as well as what to recommend for those patients in whom prostatic involvement is identified. At the time of transurethral resection of the primary bladder tumor, the surgeon may take deep transurethral resection biopsies of the prostate, preferably at the 5- and 7-o’clock positions lateral to the verumontanum. This should certainly be done if the mucosa of the prostate looks suspicious or in cases with obvious tumor at the bladder neck. Some authors have recommended that this should be done routinely and have advocated repeat transurethral resection (TUR) before cystectomy in cases where it was omitted (Wood et al, 1989; Sakamoto et al, 1993). However, the reliability of preoperative transurethral prostatic biopsies has been challenged by others (Lebret et al, 1998; Donat et al, 2001). In a prospective series of 118 patients, Lebret and colleagues examined the utility of preoperative prostatic biopsies compared with intraoperative frozen-section analysis of the prostatic urethral margin at the time of cystectomy in predicting urethral recurrence. They found that intraoperative frozen-section analysis was more accurate than any preoperative parameter including preoperative prostate biopsies in predicting urethral recurrence (Lebret et al, 1998). In another series of 246 men who underwent preoperative transurethral loop biopsy of the prostate, Donat and colleagues (2001) reported that this preoperative pathologic evaluation did not accurately determine prostatic tumor involvement—both false-negative and false-positive results were observed. Forty-three percent of patients with prostatic involvement on final pathology were missed on the TUR biopsy, and 12 of 36 patients with prostatic stromal invasion identified on TUR specimen had no residual prostatic involvement on the final cystectomy specimen (Donat et al, 2001). In light of this it is questionable whether the risk of the additional anesthetic and the potential delay to definitive surgery warrants the additional information garnered by a repeat TUR with prostatic urethral biopsy. The authors have not routinely recommended repeat TUR before cystectomy in the majority of patients. Other centers have adopted a similar philosophy with comparable clinical outcomes (Iselin et al, 1997; Hautmann, 2003).
It has been the authors’ practice to counsel patients with documented prostatic mucosal, ductal, or stromal invasion about the increased risk of urethral recurrence if the urethra is left in situ and to help them weigh that risk against any perceived advantage of an orthotopic diversion. If an orthotopic diversion is still preferred, the authors will depend on the intraoperative frozen section of the urethral margin to make the final decision. Patients with extensive prostatic stroma invasion, similar to those with clinical extravesical disease, should also be strongly considered for neoadjuvant chemotherapy before cystectomy (Grossman et al, 2003). If the patient opts for a cutaneous diversion, the authors will perform an en bloc urethrectomy at the time of cystectomy.
Risk of Urethral Recurrence in Women
In the past, urethrectomy was routinely performed at the time of radical cystectomy in women. With the acceptance of continent neobladder in men a number of investigators began evaluating the feasibility of preservation of the urethra in women. This was initially attempted in women with nontransitional cell carcinoma in whom there was little concern about possible urethral recurrence with excellent functional results (Stein et al, 1996). At the time it was generally believed that the bladder neck was the primary continence mechanism in women, so it was somewhat surprising that one could achieve excellent continence dividing distal to the bladder neck (Tanagho et al, 1966).
Two important pathologic studies were critical to the ultimate decision to expand urethral-preserving cystectomy to women with urothelial carcinoma. Stein and colleagues (1995) retrospectively evaluated a series of archival cystectomy specimens from female patients undergoing cystectomy for bladder cancer. Sixty-seven consecutive female cystectomy specimens removed for transitional cell carcinoma of the bladder were pathologically re-evaluated. Histologic evidence of tumor involving the urethra was found in 9 women (13%), whereas 17 patients (25%) demonstrated bladder neck tumors. All female patients with an uninvolved bladder neck also had an uninvolved urethra (no skip lesions), whereas approximately 50% of patients with a bladder neck tumor had concomitant urethral tumor involvement. Risk factors for urethral involvement in this study included increased grade, stage, and lymph node involvement, but the presence of carcinoma in situ did not predict urethral involvement. Vaginal wall involvement was also a major risk factor for urethral involvement. Although vaginal wall invasion was a relatively rare event (1%), all of these patients also had bladder neck involvement and 50% had urethral extention (Stein et al, 1995).
Stenzl and colleagues (1995b) also studied the risk of synchronous or secondary urethral tumors with long-term follow-up in women with bladder cancer. The charts of women treated for various stages of bladder cancer during a 19-year period were reviewed. They evaluated 356 women with a mean follow-up of 5.5 years. Overall, 7 of 356 patients (2%) had a urethral tumor at presentation (Stenzl et al, 1995b). This is a similar incidence as had been observed by Ashworth in his earlier endoscopic analysis. Of the women with clinical stages T2 to T3b, N0, M0 (potential candidates for radical cystectomy with preservation of the urethra), only 1% had urethral tumor (Ashworth, 1956). The authors’ evaluation of the impact of tumor localization revealed that bladder neck involvement was most significantly associated with secondary urethral tumor in these women (Stenzl et al, 1995b). Furthermore, no patient in this analysis had an isolated urethral tumor without concomitant bladder neck tumor involvement. The authors emphasized that the only consistent risk factor for urethral tumor involvement was concurrent tumor at the bladder neck. No correlation between urethral tumors and other pathologic factors such as carcinoma in situ or tumor multifocality was found. They concluded that the urethra can be safely preserved in selected female cystectomy patients provided that neither preoperative biopsy specimens of the bladder neck nor intraoperative frozen-section specimens of the proximal urethra demonstrate any tumor or atypia (Stenzl, 1995b). It has been suggested that the apparent lower incidence of urethral tumors in women (compared with men) may be related to the fact that transitional cell mucosa in women covers a smaller urethral segment, with the remainder being normal or metaplastic squamous cell mucosa. The area at risk in the female urethra is therefore smaller and probably diminishes with increasing age as the demarcation line between squamous and transitional cell mucosa migrates cranially during menopause. In the sixth and seventh decades of life, when most bladder tumors occur, metaplastic squamous cell mucosa may cover the entire urethra, the bladder neck, and even a portion of the trigone (Peckhman, 1971).
This pathologic finding was confirmed by Maralani and colleagues (1997), who retrospectively evaluated 43 female cystectomy specimens removed for bladder cancer. They reported a 16% incidence of urethral tumor involvement, and vaginal involvement in this study was the most significant risk factor for urethral tumor involvement (Maralani et al, 1997). Similarly, Chen and colleagues also retrospectively reviewed the risk of secondary urethral, vaginal, and cervical involvement by transitional cell carcinoma in women undergoing radical cystectomy. They found an overall 8% incidence of tumor of the urethra, and approximately 50% of patients with vaginal or cervical invasion also demonstrated urethral tumor involvement. They similarly confirmed previous reports that the most significant risk factor for urethral tumor involvement is tumor at the bladder neck (Chen et al, 1997). Similar conclusions had been previously reached by Coloby and da Paepe (De Paepe et al, 1990; Coloby et al, 1994).
Stein and colleagues (1998a) embarked on a prospective study to evaluate and confirm the previously established pathologic risk factors in women undergoing cystectomy for bladder cancer to determine if these criteria safely identify appropriate female candidates for orthotopic diversion. Final pathologic analysis of the bladder neck and proximal urethra was performed and compared with the intraoperative frozen-section analysis of the distal surgical margin (proximal urethra). Tumor involvement at the bladder neck and proximal urethra was found in 14 (19%) and 5 (7%) cystectomy specimens, respectively. All patients with urethral tumors also demonstrated concomitant bladder neck tumors. Bladder neck tumor involvement was again found to be the most significant risk factor for tumor involving the urethra, confirming the findings from retrospective series (Stein et al, 1995). However, approximately half of patients with bladder neck tumors had a normal (tumor-free) proximal urethra. Furthermore, no patient with a normal bladder neck demonstrated tumor involvement of the urethra. In all cases, intraoperative frozen-section analysis of the proximal urethra correlated with and was correctly confirmed by final permanent section (Stein et al, 1998a). These results were virtually identical to the results from previous retrospective study (Stein et al, 1995) and suggest that preoperative biopsy of the bladder neck or urethra is not necessary, but rather one may depend on the intraoperative frozen section to determine the feasibility of orthotopic diversion. Clinical follow-up data of the first 88 women undergoing orthotopic diversion at that institution showed that no urethral recurrences were observed with a median follow-up of 30 months in this group (Stein et al, 2002). More recently Al el Dein and colleagues (2004) prospectively evaluated 145 women undergoing cystectomy and orthotopic diversion. Two patients developed urethral recurrence. One had a primary squamous cell cancer of the bladder, and the other had urothelial carcinoma with carcinoma in situ of the trigone (Al el Dein et al, 2004).
Under no circumstance should the adequacy of the cancer operation be compromised by the type of reconstruction. The authors continue to recommend the intraoperative frozen-section analysis of the proximal urethral margin in both female and male patients as an appropriate mechanism to determine who is an appropriate candidate for orthotopic diversion from a cancer perspective.
Locally Advanced Tumor Stage
Many urologists are hesitant to perform continent orthotopic diversion in patients with locally extensive disease. This is based on two factors: (1) concern about the possible impact of local recurrence on the neobladder itself and (2) a belief that these patients are doomed to suffer distant recurrence and have a shortened life expectancy and will not benefit from the neobladder.
Radical cystectomy with bilateral pelvic iliac lymphadenectomy provides excellent local (pelvic) control for the treatment of invasive bladder cancer. Stein and colleagues (2001a) reported the clinical outcomes for 1054 patients who underwent radical cystectomy for bladder cancer with a median follow-up of more than 10 years. In this series, an overall local pelvic recurrence rate of 7% was observed for the entire group of patients. The risk of recurrence ranged from 6% with organ-confined, node-negative disease to 13% for patients with extravesical or node-positive disease (Stein et al, 2001a). These results suggest that local recurrence even for patients demonstrating locally advanced or lymph node–positive disease is relatively infrequent and should not necessarily preclude an orthotopic form of urinary diversion. In addition, the results of this study show that a significant percentage of patients with locally advanced disease will be long-term survivors and may benefit from a continent form of urinary diversion. Nearly 50% of patients with extravesical tumor extension and 30% of patients with lymph node–positive disease were still alive without evidence of disease 5 years following cystectomy (Stein et al, 2001a).
If local tumor recurrence does develop in patients with an orthotopic diversion, only a minority will develop problems related to the urinary diversion itself. Hautmann and colleagues evaluated this question in 43 of 357 men who underwent radical cystectomy and ileal neobladder and developed local recurrence. Most of them (84 %) had advanced disease (≥pT3a) on final pathology at the time of cystectomy. A total of 17 patients (43%) had concomitant distant metastasis at the time of diagnosis of the local recurrence. Local recurrence interfered with the upper urinary tract in 24 cases, the neobladder in 10 (23%), and the intestinal tract in 7; only 1 patient required removal of the neobladder because of an intestinal fistula. The authors concluded that most patients can anticipate normal neobladder function even in the presence of locally recurrent disease (Hautmann and Simon, 1999).
Similar results have been reported by others. Tefilli and colleagues (1999) found that 1 of 11 patients with orthotopic diversion required conversion to an ileal conduit after local recurrence. To evaluate the impact of orthotopic diversion on the quality of the cystectomy and ensuring appropriate cancer control, Yossepowitch and colleagues retrospectively evaluated 214 patients who underwent radical cystectomy and orthotopic reconstruction and compared them with 269 patients similarly treated with an ileal conduit diversion. Adjusting for pathologic stage, there was no cancer-specific survival difference between the two diversion groups. Patterns of relapse in 62 of the 214 patients (29%) with an orthotopic neobladder included local recurrence in 11%, distant recurrence in 9%, and combined local and distant recurrence in 18%. Only one patient in this series required the neobladder to be converted to an ileal conduit secondary to a relapse at the ureteroenteric anastomosis and expanding into the pouch. The authors concluded that the low risk of local recurrence showed that in this cohort of patients the oncologic efficacy of the operation was not compromised (Yossepowitch et al, 2003).
Key Points: Cancer-Related Factors in Patient Selection
Patient-Related Factors
The type of urinary diversion to be performed depends on a combination of the cancer-related factors and patient-related factors. The latter includes the patient’s general health and social circumstances, baseline renal function, presence of a functional urethra, manual dexterity, and previous treatments including pelvic radiation, prostate surgery, or bowel resection. Perhaps equally important is the patient’s personal preference and attitudes about the risk of incontinence, need to self-catheterize, and management of an external appliance. The relative importance of each of these factors in determining how to counsel an individual patient must be decided on a case-by-case basis. The patient and his or her family must have a realistic understanding of the pros and cons of each type of diversion before making a decision. There is a natural inclination for most patients to opt for an orthotopic reconstruction because it is the most “natural.” They must gain the understanding that problems can occur with each type of diversion, although the specific types of problems differ. An honest, informed discussion, carefully explaining the various options along with the short- and long-term risks and benefits of each form of urinary diversion, must be performed. It may also be helpful to have the patient talk with other patients who have undergone the various forms of urinary reconstruction.
Patients with poor general health, difficult social circumstances, or poor cognitive function are probably best managed with an ileal conduit. Severe urethral stricture disease in men is a contraindication for orthotopic diversion. Poor external sphincter function in a man highly motivated to undergo orthotopic diversion may be managed with a concomitant artificial urinary sphincter device. Similarly a female with significant stress incontinence might undergo a concomitant pubourethral sling procedure, though she would most likely be dependent on intermittent catheterization to drain properly.
Age
Many authors have evaluated the success of continent diversion in elderly patients (Lance et al, 2001; Clark et al, 2005; Sogni et al, 2008). Although elderly patients undergoing orthotopic diversion may regain continence more slowly and have a higher rate of mild stress incontinence, ultimately older patients achieve daytime and nighttime continence rates similar to those for younger patients (Elmajian et al, 1996). The clear consensus is that age alone is not a contraindication for continent diversion and options should be considered for each patient on the basis of other factors. Medical comorbidities; renal, cardiac, pulmonary, and cognitive function; and manual dexterity are all important factors that should be considered, along with the patient’s social support situation. A frail, sedentary, elderly person is probably best served with an expeditious conduit. Similarly, a conduit may be easier for a caregiver to manage than an orthotopic diversion with the risk of incontinence. However, an active, generally healthy, elderly patient may certainly be considered a reasonable candidate for orthotopic diversion depending on his or her wishes.
Renal Function
One of the most important contraindications for continent neobladder reconstruction is compromised renal function. Urinary electrolytes including urea, potassium, and bicarbonate are reabsorbed from the small bowel mucosa, resulting in an increased acid load that must be handled by the kidneys. In patients with compromised renal function, hyperchloremic metabolic acidosis can develop along with worsening dehydration, uremia, nausea, and bone loss. The exact level of acceptable renal function for consideration for continent diversion is somewhat controversial. As a general rule, a serum creatinine level of less than 1.7 to 2.2 mg/dL (150 to 200 µmol/L) or an estimated creatinine clearance of greater than 35 to 40 mL/min is recommended (Hautmann, 2003; Hautmann et al, 2007). However, decision making in individual patients can at times be less clear-cut. Acute upper tract obstruction caused by the tumor often results in a transient rise in creatinine, which would be expected to improve after cystectomy.
Body Habitus
Obesity is not a contraindication for orthotopic diversion, although working with the thickened bowel mesentery may be challenging. In fact, obese patients may be better served with orthotopic diversion because of the difficulty constructing functional conduit stomas on their abdomens.
Manual Dexterity and Willingness to Do Self-Catheterization
The need to do occasional or routine self-catheterization is reported in 10% to 50% of men and in up to 30% to 50% of women (Hautmann et al, 2007). It is generally impossible to predict which patients will require catheterization to empty, and retention can occur many years after the initial surgery. Thus all patients considered for continent diversion should be willing and able to do self-catheterization. The authors have each patient meet with a specially trained enterostomal therapist before surgery to go over this technique in addition to other perioperative issues. It has been rare for a patient to decide against a continent orthotopic diversion because of this requirement.
Prior Pelvic Radiation
It is not uncommon for patients to present for surgical management of invasive bladder cancer after previous failed radiation therapy or after radiation treatment of a previous pelvic malignancy. Stein and colleagues (2001a) found that 8.5% of 1471 patients had some prior pelvic radiation. This exposure can significantly increase the difficulty of the surgery and can affect wound healing and postoperative complications. Many surgeons are hesitant to offer orthotopic diversion in this setting.
Ahlering and colleagues (1988) retrospectively evaluated patients undergoing cystectomy and cutaneous continent diversion comparing 44 patients who had a history of prior radiation with 42 matched patients who did not. They found no significant difference between irradiated and nonirradiated patients in operative time, blood loss, transfusion requirements, and wound or ureteral complications (Ahlering et al, 1988). Kim and Steinberg found an increased risk of surgical complications, especially those that required percutaneous or surgical intervention, in 23 patients undergoing cystectomy and conduit following radiation compared with 23 matched controls (Kim and Steinberg, 2001). Chang and colleagues (2004) evaluated outcomes of ileal conduits in 36 patients with prior radiation. They found ureteroileal complications occurred in 9% of patients by 5 years and concluded that it was appropriate to use ileum for diversion rather than a colon conduit.
The complications and ultimate functional outcome of orthotopic neobladders in patients with prior pelvic radiation have been evaluated by a number of authors. Gschwend and colleagues reported on 11 such patients. The postoperative course in this group including duration of hospital stay, perioperative complications, and early functional results did not differ from that of a control group of nonirradiated patients. They concluded that high-dose pelvic irradiation should not be a primary contraindication for orthotopic urinary diversion with segments of small intestine (Gschwend et al, 1996).
Gheiler and colleagues (1997) evaluated their clinical outcomes in three patients undergoing orthotopic substitution after cystoprostatectomy for radiorecurrent prostate cancer. Postoperative complications included pyelonephritis in one patient and prolonged ileus in another. All patients with an orthotopic neobladder were continent during the day; one patient required a single pad at night. This group concluded from their small series that orthotopic urinary diversion is a valid option for well-selected patients with radiorecurrent prostate cancer who require salvage cystoprostatectomy and that it can be performed with minimal complications, resulting in good continence and quality of life (Gheiler et al, 1997).
Bochner and colleagues (1998) described their experience with salvage surgery and orthotopic bladder substitution after failed radical radiation therapy. A total of 18 patients who had failed definitive radiation therapy (total minimum dose, ≥60 Gy) for bladder or prostate cancer and had undergone a salvage procedure with construction of an orthotopic neobladder were evaluated. Operative characteristics, postoperative outcomes, and complications (related or unrelated to the urinary diversion) were found to be similar in irradiated and nonirradiated patients. Good daytime and nighttime continence after surgery was reported by 67% and 56% of irradiated patients, respectively. Patients with poor postoperative continence (22%) were successfully treated with the placement of an artificial urinary sphincter. These authors concluded that salvage surgery and orthotopic urinary reconstruction appear to be safe, effective procedures that provide a functional lower urinary tract in patients in whom definitive pelvic radiation therapy has failed (Bochner et al, 1998). Nieuwenhuijzen and colleagues (2004) reported on 27 patients who underwent salvage cystectomy, 9 of whom had an orthotopic diversion. Eight of them achieved complete daytime continence, similar to results expected in nonirradiated patients (Nieuwenhuijzen et al, 2004).
It is clear that in carefully selected patients, orthotopic lower urinary tract reconstruction can be performed after definitive, full-dose pelvic irradiation. Even selected women with a history of pelvic irradiation may be appropriate candidates for orthotopic reconstruction with good clinical outcomes (Stein et al, 2002; Lee et al, 2004). However, these are challenging procedures that clearly require technical expertise and keen intraoperative judgment. Previous high-dose prostate radiation (external beam or brachytherapy) or a vaginal implant for cervical cancer cause more scarring around the rhabdosphincter area than does radiation for either bladder cancer or previous colon cancer. Similarly, interstitial seed implants for prostate cancer may result in severe scarring around the area of the external sphincter, depending on the placement of the seeds. Preoperative evaluation including cystoscopy is mandatory to evaluate the integrity of the mucosa around the area of the sphincter. However, it may not be possible to accurately predict the degree of radiation damage found at surgery, so careful intraoperative tissue assessment and determination of the condition of the urethra, ureters, and bowel must be performed to make a final decision about the feasibility of orthotopic diversion (Abbas et al, 2001). These patients should always be counseled preoperatively that the orthotopic diversion may not be possible.
Prior Prostate Surgery or Bowel Resection
Prior abdominal or pelvic surgery may also present challenges for the surgeon performing orthotopic diversion. Patients who have had a prior radical prostatectomy may have a particularly difficult dissection around the proximal urethra at the prior vesicourethral anastomosis. Nevertheless, this is often feasible with careful dissection. The health of the proximal urethra can be assessed at surgery and acceptable continence can be obtained in selected patients (Schuster et al, 2003). In general, with careful dissection a patient who was continent following the first surgery can be expected to have an acceptable result with a neobladder.
A patient with multiple prior bowel resections may be at risk of developing chronic diarrhea or even short bowel syndrome after an additional 45 to 60 cm of small bowel is resected. In these patients alternatives for orthotopic diversion such as a sigmoid neobladder might be entertained. Generally prior bowel resections can be managed by carefully dissecting out all of the small bowel, taking down any adhesions before performing the diversion. It is critical to identify the old bowel anastomosis and, whenever possible, take that down and use that site as one end of the continent reservoir. This avoids potential devascularization of the bowel segment between the old and new bowel anastomoses.
Key Points: Previous Pelvic Radiation or Pelvic Surgery
Continence Mechanism in Patients Undergoing Orthotopic Diversion
An understanding of the anatomy of the urethral continence mechanism in men and women is crucial to preserving this function at the time of radical cystectomy. The surgical dissection at the prostatic apex in men and bladder neck in women must be carefully and precisely performed to achieve optimum continence while taking care not to compromise the oncologic effectiveness of the surgery.
Much of what has been learned of the rhabdosphincter complex comes from elegant neuroanatomic studies of the female urethra. Colleselli and colleagues (1998) performed extensive microneuroanatomic dissections, histologic examination, and three-dimensional reconstructive imaging to better define the urethral sphincteric and rhabdosphincteric anatomy in women. The female urethral sphincter system consists of smooth muscle innervated by the autonomic nervous system and striated muscle supplied by somatic nerves. There is general agreement that the autonomic nerves that serve the smooth muscle sphincter originate in the pelvic plexus. These autonomic fibers emerge from the the pelvic plexus and course along the lateral aspect of the rectum and vagina toward the bladder neck and very proximal urethra. Some of these fibers branch off from a thick fiber at the lower margin of the lateral vaginal wall and enter the bladder neck and cranial portion of the urethra from the dorsolateral aspect. These autonomic nerves do not appear to play a significant role in the innervation of the rhabdosphincter or the continence mechanism and are essentially sacrificed during the exenterative portion of the operation without compromising continence (Colleselli et al, 1998).
Innervation of the voluntary urinary sphincter system, however, is a matter of some controversy. Most interested investigators agree that the rhabdosphincter is probably supplied primarily by the branches of the pudendal nerve (Borirakchanyavat et al, 1997; Stenzl et al, 1997; Colleselli et al, 1998). Although these dissections were performed on female cadavers, the observations and findings have been similarly described in men (Strasser, 2000). Collectively, these findings have allowed a more precise and anatomic approach to maintain the continence mechanism in all patients undergoing cystectomy and orthotopic substitution.
In the same study, Colleselli and colleagues (1998) found that the major portion of the striated muscle that corresponds to the striated rhabdosphincter is located on the ventral and lateral aspects (omega shaped) of the urethra. No clear, defined line could be identified between the transverse smooth muscle cranially and the striated muscle caudally. Rather, a gradual transition was noted in the middle third of the urethra, with intermingling fibers of both types of muscle (Colleselli et al, 1998). This area has been found to correspond to the area of continence region on fluorourodynamic studies performed on women who had undergone orthotopic reconstruction after cystectomy (Grossfeld, 1996). Branches off the pudendal nerve coursing beneath the levator muscle can be traced to the rhabdosphincter. Delicate fibers from the perineal portion of the pudendal nerve course underneath the urogenital diaphragm, entering the caudal portion of the urethra laterally.
In a neuroanatomic study performed in male human cadaveric pelves, similar anatomic findings and innervation were described. Strasser and Bartsch (2000) described the male rhabdosphincter as an independent muscle unit that is not in direct contact with the fibers of the levator ani muscle. These dissections demonstrated that the male sphincter does not form a horizontal muscular ring around the membranous urethra. Rather, the male rhabdosphincter is a muscular coat situated ventral and lateral to the membranous urethra and prostate, the core of which is an omega-shaped loop that surrounds the membranous urethra. The innervation of the male rhabdosphincter was also found to originate from fine branches that arise off the pudendal nerve. These authors suggested that injury to either the rhabdosphincter or the pudendal innervation may impair the sphincter mechanism in men (Strasser and Bartsch, 2000).
Surgical Techniques for Continence Preservation during Radical Cystectomy
Attention to anatomic and surgical detail is important to optimize functional and clinical outcomes in patients undergoing orthotopic diversion after radical pelvic surgery. Minimal manipulation of the muscle fibers of the rhabdosphincter, fascial attachments, and corresponding innervation is essential in providing optimal urinary continence (Stein et al, 1997, 2001b; Colleselli et al, 1998; Stenzl et al, 1998; Strasser, 2000).
Anterior Apical Dissection in the Male Patient
The technique of an extended bilateral pelvic lymphadenectomy with radical cystoprostatectomy is well established (Stein and Skinner, 2004). Urethral preparation with preservation of the continence mechanism is critical when orthotopic diversion is anticipated. The continence mechanism in men may be maximized if dissection in the region of the pelvic floor and anterior urethra is minimized. Attention to surgical technique is important and is described in detail elsewhere (Stein et al, 2001b). Several fundamental key surgical issues in the preparation of the urethra in patients undergoing orthotopic diversion deserve special mention.
In a standard cystectomy the bladder and prostate are completely freed off of the rectum and mobile posteriorly before the urethral dissection. Posterior dissection should not extend distal to the apex of the prostate. If a nerve-sparing approach is planned, the urethra may be divided after taking down the lateral pedicles to the bladder (anterior branches of the internal iliac vessels) before performing the posterior dissection. The prostate is then dissected in a retrograde fashion off of the rectum and bilateral neurovascular bundles, and the posterior pedicles are divided last.
In either approach, all fibroareolar connections along the anterior bladder wall, prostate, and undersurface of the pubic symphysis are divided. The endopelvic fascia is incised adjacent to the prostate, and the levator muscles are carefully swept off the lateral and apical portions of the prostate. The superficial branch of the deep dorsal vein is identified, ligated, and divided. With tension placed posteriorly on the prostate, the puboprostatic ligaments are identified and slightly divided just beneath the pubis and lateral to the deep dorsal venous complex, which courses between these ligaments. Care should be taken to avoid any extensive dissection in this region. The puboprostatic ligaments need to be incised only enough to allow proper apical dissection of the prostate.
Several methods can be performed to properly control the dorsal venous complex (DVC) (Stein et al, 2001b; Stein and Skinner, 2006). One may carefully pass an angled clamp beneath the dorsal venous complex, anterior to the urethra to pass a suture to ligate the complex. Alternatively, a suture on a long curved needle may be directly passed just anterior to the urethra, posterior to the venous complex, and tied as a simple or figure-of-eight stitch. A third alternative is to gather the complex in a long Allis clamp before passing the suture. The suture is best placed with the surgeon facing the head of the table and with the needle holder perpendicular to the patient. These latter maneuvers avoid passage of any instruments between the dorsal venous complex and the rhabdosphincter, which could potentially injure these structures and compromise the continence mechanism. Alternatively, especially in a patient with a broad, short DVC, the complex can simply be sharply divided and oversewn as needed to obtain adequate hemostasis. Absorbable suture should be used to avoid any risk of subsequent suture erosion into the urethral anastomosis.
Once the venous complex is ligated, it may be divided close to the apex of the prostate. Any bleeding from the transected venous complex can be controlled with an absorbable 2-0 polyglycolic acid suture. However, only suturing that is absolutely necessary should be performed and care should be taken to avoid deep suture bites into the complex or levator muscles, which could injure the continence mechanism. The DVC suture can then be used to suspend the venous complex anteriorly to the periosteum of the symphysis pubis to re-establish anterior fixation of the dorsal venous complex and puboprostatic ligaments, which may enhance continence recovery.
The anterior urethra is now exposed. Before division of the urethra, the authors prefer to place a heavy suture around the apex of the prostate encircling the catheter to decrease the risk of urine spill when the urethra is divided. The anterior urethra is then incised just beyond the apex of the prostate. Six 2-0 absorbable monofilament or woven polyglycolic acid sutures are placed in the urethra circumferentially, carefully incorporating only the mucosa and submucosa of the urethra without incorporating the levator muscles. The urethral catheter is clamped and divided distally. Two additional sutures are then placed, incorporating the rectourethral muscle posteriorly and the caudal extent of Denonvillier fascia. After this, the posterior urethra is divided and the specimen removed. The urethral sutures are appropriately tagged to identify their location and are placed under a towel until the urethroenteric anastomosis is performed.
Regardless of the technique, frozen-section analysis of the distal urethral margin (prostatic apex) on the cystectomy specimen is performed to exclude tumor involvement. The decision to perform an orthotopic bladder substitute is ultimately made at this time. If there is no evidence of tumor, orthotopic reconstruction may be performed.
Urethral Dissection in the Female Patient
When orthotopic diversion is considered in female patients, several technical issues should be noted to optimize the continence mechanism (Stein et al, 2001b; Stein and Skinner, 2004). A standard female cystectomy includes removal of the uterus, cervix, and ovaries (anterior exenteration). However, in selected females with clinically lower-stage disease some authors have advocated preservation of the uterus and ovaries (Chang et al, 2002; Zippe et al, 2005). In either case, whenever possible the bladder is dissected completely off of the vagina, but a tumor on the posterior bladder wall may necessitate excision of a portion of the anterior vaginal wall. This does increase the risk of subsequent pouch-vagina fistula but is not an absolute contraindication to orthotopic reconstruction. However, a patient with a significant tumor at the bladder neck or with palpable extention into the vaginal wall is a poor candidate for neobladder and should undergo en bloc urethrectomy and cutaneous diversion.
In developing the posterior pedicles, the posterior vagina is incised at the posterior fornix posterior to the cervix. If the patient has undergone prior hysterectomy, a sponge stick in the vagina facilitates identification of the vaginal apex. In that case the vagina is generally not entered. Before dissecting off the bladder, the posterior vascular pedicles are developed and divided. The vaginal apex may be grasped with a curved Kocher clamp to provide countertraction, and scissors are used to dissect right along the lateral vaginal wall. This develops the posterior pedicle coming around the rectum and vagina toward the bladder, which can be controlled with hemoclips, vascular staples, or a LigaSure (Covidien Ltd., Mansfield, MA) or similar device. Care is taken to dissect along the midlateral vaginal border, rather than dissecting back along the rectum or anterior into the bladder.
The issue of nerve-sparing cystectomy in women is controversial. Some authors have suggested that a sympathetic nerve-sparing cystectomy may be important in maintaining continence in women undergoing orthotopic diversion (Stenzl et al, 1995a; Hautmann, 1997; Turner et al, 1997; Bhatta et al, 2007). Others have routinely sacrificed the autonomic nerves coursing along the lateral aspect of the uterus and vagina and have successfully relied on the pudendal innervation of the rhabdosphincter complex for continence (Stein et al, 1997, 2004; Ali-El-Dein et al, 2002). In fact, the authors have observed good continence results in women undergoing complete bowel and urinary tract reconstruction after total pelvic exenteration with removal of all pelvic autonomic innervation. This, again, supports the concept that autonomic innervation is not critical to the continence mechanism.
Once the posterior pedicles are divided, careful dissection of the bladder off of the anterior vaginal wall is performed sharply. Care must be taken to dissect in the proper plane to prevent entry into the posterior bladder and reduce the amount of bleeding in this vessel-rich area. Dissection is carried just distal to the vesicourethral junction. Palpation of the Foley catheter balloon assists in identifying this region.
Once the posterior dissection is completed, the fatty tissue overlying the anterior urethra is swept off and the vesico-urethral junction is carefully identified using the Foley catheter balloon. The endopelvic fascia and periurethral tissue anteriorly should not be disturbed. A pedicle clamp is placed across the bladder neck, which prevents any tumor spill from the bladder when the urethra is transected. With gentle traction, the proximal urethra and Foley catheter are sharply divided just distal to the bladder neck. It is not necessary to place the urethral sutures as the urethra is divided because it does not retract away as it does in the male. Frozen-section analysis is performed on the distal surgical margin of the cystectomy specimen to exclude any tumor. Once the decision is made to perform the orthotopic diversion, 8 to 10 sutures of 2-0 absorbable suture are placed circumferentially through the urethra, tagged, and set aside.
In the case of a deeply invasive posterior bladder, the anterior vaginal wall may be removed en bloc with the cystectomy specimen by incision along the long axis of the lateral vaginal wall. This leaves the anterior vaginal wall attached to the posterior bladder specimen. Again, the Foley catheter balloon facilitates identification of the vesicourethral junction. A rim of anterior vaginal wall must be preserved proximal to the urethra to close the vagina. The vagina may be closed in a clamshell or horizontal manner. Other reconstructions to increase vaginal volume are feasible, but it may be difficult to perform a complex vaginal reconstruction along with a neobladder simply because of a lack of adequate room in the pelvis.
If the anterior vaginal wall has been preserved, the vagina is closed at the apex with absorbable suture and then suspended. One may suspend the vagina to the Cooper ligament or to preserved cut ends of the round ligaments to prevent postoperative vaginal prolapse. However, the authors routinely perform a colposacroplexy incorporating a short strip of permanent mesh to fix the vagina to the sacral promontory at a more natural angle without any tension. This may be important to support the neobladder and avoid a possible cause of late voiding difficulty. Regardless of the form of vaginal reconstruction, a well-vascularized omental pedicle graft must be placed between the reconstructed vagina and the neobladder and properly secured to the levator ani muscles to separate the suture lines and prevent fistula formation between the vaginal and urethral anastomosis to the neobladder.
Key Points: Continence Mechanism and Surgical Techniques for Continence Preservation
Techniques for Orthotopic Bladder Substitution
Choice of Bowel Segment
A number of different procedures for orthotopic reconstruction incorporating different segments of bowel have been described. There are clearly some physiologic indications to use various segments of intestine, but the surgeon’s preference may be even more influential. It has been suggested that excellent functional and clinical outcomes with voiding can be achieved regardless of the segment of bowel chosen as long as the principles of preservation of the rhabdosphincter as a continence mechanism and construction of an adequate capacity, low-pressure reservoir are maintained (Parekh et al, 2000b; Lee et al, 2003). Ideally the surgeon performing orthotopic urinary diversion should be comfortable using a variety of the techniques described later so that he or she can adapt the technique to the needs of the individual patient.
Reservoirs made of detubularized ileum or ileum and colon together appear to have the greatest compliance and lowest likelihood of generating intermittent high pressure contractions. Hohenfellner and colleagues elegantly evaluated the properties of gut smooth muscle layers (circular and longitudinal) of ileal and cecal segments in a canine model. The circular muscle layer of ileum was found to be most distensible, followed by the colonic circular and longitudinal ileal layers. The longitudinal layer of the colonic segment was relatively nondistensible (Hohenfellner et al, 1993).
Several clinical studies have demonstrated that the urodynamic characteristics of the ileum appear to be superior to those of the colon (Berglund et al, 1987; Lytton and Green, 1989; Davidsson et al, 1992; Schrier et al, 2005). Stomach and sigmoid colon have been found to have particularly poor compliance and high pressures (Santucci et al, 1999). Davidsson and colleagues (1992) evaluated the urodynamic profiles of neobladders constructed from the ileum and the right colon and found that although the volume capacity was similar, the pressure at maximum capacity was much lower with the ileal reservoir. Schrier and colleagues (2005) found that neobladders made of ileum had larger capacity, lower filling pressures, lower maximum capacity pressures, and better compliance. The distal small bowel mesentery also tends to have the greatest mobility and generally reaches to the urethra without much difficulty. In patients with short ureteral length due to malignancy or other pathology of the ureters, an ileal pouch with a “tail” (such as the Studer) can be extended to reach all the way to the renal pelvis. A final advantage of ileum is the atrophy of the intestinal mucosa as it is exposed to urine over time. This results in decreased mucous production and decreased reabsorption of urinary electrolytes in the mature reservoir. Mucosal atrophy appears to be more reliable in small bowel than in large bowel reservoirs (Norlen and Trasti, 1978; Mills and Studer, 1999).
The primary disadvantage of using distal ileum lies in the potential loss of absorption of vitamin B12. This segment may also be unacceptably damaged by prior pelvic radiation or may be unavailable due to multiple prior bowel resections or to inflammatory conditions like Crohn disease. Ideally the surgeon performing orthotopic urinary diversion should be comfortable using a variety of the techniques described later so that he or she can adapt the technique to the needs of the individual patient. Whenever available, the authors preferentially use ileum for orthotopic diversion.
Bladder replacement using stomach has also been described, although it has not been a popular technique (Adams et al, 1988; Nguyen, 1991; Hauri, 1998; Lin, 2000). The primary advantage of gastric segments is that the gastric mucosa excretes chloride and hydrogen ions, effectively reversing the acidosis of renal insufficiency. The latter is worsened with a pouch made of small or large bowel. The excreted acid from a stomach segment can also reduce the risk of bacterial colonization, and there is less mucous production. However, the disadvantage of using stomach is that some patients will suffer from dysuria or hematuria from the excreted acid (Nguyen, 1991). Lin and colleagues (2000) reported their clinical outcomes in eight patients undergoing gastric neobladder construction and found that pouch capacity and compliance were worse compared with ileal or ileocecal neobladders. Combining gastric and small or large intestinal segments can counteract some of these side effects and may be ideal from a purely metabolic standpoint (Lockhart et al, 1993). This construction can theoretically be performed in a patient with compromised renal function when continent diversion is generally contraindicated. It may also provide another option in a patient with prior high-dose pelvic radiation or multiple previous bowel resections. Few urologists have been trained in the surgical anatomy of the stomach, and most who perform a significant number of cystectomies currently rarely, if ever, using this type of diversion.
Isolation of the segment of bowel to be used for the diversion must be performed carefully in order to preserve blood supply to the pouch, as well as to the bowel anastomosis. If using ileum one should avoid deep incision into the mesentery at the proximal bowel division to avoid compromising the blood supply to the reservoir. In addition, in patients who have had previous bowel resection it is important to take down the old anastomosis rather than choosing another site nearby because that may result in a poorly vascularized segment between the new and old bowel anastomoses. Gastrointestinal complications are a common consequence of cystectomy and diversion, and care must be taken in ensuring a secure bowel anastomosis.
Need to Prevent Reflux
The deleterious effect of vesicoureteral reflux in children is well accepted, especially in the face of infection. In the context of urinary diversion the importance of preventing reflux was first identified in patients undergoing ureterosigmoidostomy (Clarke and Leadbetter, 1955; Wear and Barquin, 1973) and, subsequently, ileal conduit urinary diversion (Middleton and Hendren, 1976; Shapiro et al, 1975). It has been believed that the frequent long-term deterioration of renal function and upper tract changes seen in up to 50% of patients with ileal conduit urinary diversions by 15 years are due to high-pressure reflux of infected urine (Clark et al, 1999; Madersbacher et al, 2003). Mean time to development of kidney problems in conduit patients is 5 years, and the incidence of such complications seems to increase steadily with time (Clark et al, 1999).
However, studies that have compared refluxing versus nonrefluxing urinary diversion have generally been limited by short follow-up, patient selection bias, retrospective design, or relatively small patient numbers. Richie and colleagues (1974) showed a protective effect of nonrefluxing anastomoses in a dog model. Kristjansson and colleagues (1995a, 1995b) found no difference in colon conduits with nonrefluxing anastomoses versus ileal conduits with refluxing anastomoses. However, continent diversions with antireflux techniques appeared to have less upper tract scarring and bacteruria. Studies by both Elder and Hill showed upper tract deterioration was common in both colonic and ileal conduits, although each had high rates of stomal stenosis and ureteroenteric stenosis (Elder et al, 1979; Hill and Ransley, 1983). Althausen showed better results using nonrefluxing colon conduits, but the follow-up in this study was only 3 years (Althausen, 1978). Song and Hautmann each found no differences in renal function or hydronephrosis in refluxing or nonrefluxing orthotopic neobladders in nonrandomized retrospective studies (Song et al, 2006; Hautmann et al, 2006). All of these studies were retrospective and nonrandomized and generally included relatively small numbers of patients.
With orthotopic diversion, most patients void by Valsalva maneuver with relaxation of the external sphincter. The resulting increase in pressure in the reservoir should be transmitted equally to the rest of the system, limiting any resulting back pressure even without an antireflux mechanism (Thoeny et al, 2002; Studer et al, 2006). However, free reflux into the upper tracts of the Studer pouch is often visible on cystogram with as little as 300 mL of filling, well within the typical voided volume of these patients. It is likely that there is some retrograde flow of urine in these patients at some times. In patients who require intermittent catheterization to empty, such reflux could potentially occur at relatively high pressures when the reservoir is full. Finally, although symptomatic infections are uncommon, it is not unusual for these patients to have asymptomatic bacteruria, especially if they are on self-catheterization. Steven reported 34% 3-year and 24% 5-year prevalence of bacteriuria in 166 men undergoing orthotopic reconstruction (Steven and Poulsen, 2000). Whether any of this is physiologically important is still unclear (Suriano et al, 2008). Wood and colleagues (2003) looked at 66 patients undergoing orthotopic refluxing ileal neobladder. Of these patients, 17% required clean intermittent catheterization, 67% had positive urinalyses, and 39% had a documented positive urine culture. For those who voided without the need for catheterization, they reported an estimated 5-year probability of urinary tract infection of 58% and urosepsis of 18% (Wood et al, 2003).
Medium to long-term results in ileal neobladders without antireflux valves have generally been good. Normal renal function and preserved upper tract anatomy are possible in the majority of patients (Thoeny et al, 2002; Perimenis et al, 2004; Minervini, 2005). Thoeny and colleagues examined long-term results in 76 patients with 5 or more years follow-up with a Studer pouch and found preserved renal function and anatomy in 95% of the patients (Thoeny et al, 2002).
It is clear as well that any mechanism introduced to prevent reflux may also potentially cause upper tract obstruction. Initial experience with the intussuscepted Kock nipple valve showed that it provided reliable protection against reflux and seemed to have few drawbacks. However, as surviving patients were followed out to 10 years and beyond it became clear that approximately 5% of patients experienced obstruction from stenosis of the afferent nipple valve, and an additional 5% developed stone on or extussussception of the afferent nipple valve (Stein et al, 1996). Afferent valve problems were often clinically silent: Patients who were not followed carefully occasionally presented with acute renal failure and bilateral hydroureteronephrosis with significant renal damage. Similarly, ureteroileal obstruction with the antireflux Camey–Le Duc ureteral implantation technique has been reported to occur in 7% to 29%, compared with less than 3% for a direct ureteroileal anastomosis (Le Duc et al, 1987; Shaaban et al, 1992; Pitts and Muecke, 1979; Roth, 1997; Pantuck et al, 2000).
Shaaban and colleagues (2006) performed one of the few prospective randomized clinical trials evaluating this question. They studied 60 patients who all had normal renal function preoperatively and no hydronephrosis. In each patient one randomly chosen ureter was implanted in an antireflux manner (using an extraserosal tunnel), and the other was implanted directly into a small tabularized chimney on a standard W ileal neobladder. At 2 years median follow-up they found more deterioration in the renal units drained with the antireflux technique, which resulted in five cases of documented obstruction than in those with a direct refluxing anastomosis (Shaaban et al, 2006). However, longer-term follow-up may be necessary to see any cumulative damage from the reflux itself.
Abol-Enein and Ghoneim (2001) developed a serous-lined ureteral implantation technique with an ileal neobladder and reported their intermediate experience (mean follow-up of 38 months) in 450 patients. In this large series, 96% of upper tracts remained unchanged or improved; reflux was observed in only 3% of patients. They reported that in their series an anastomotic ureteral stricture occurred in 3.8% of patients, an incidence similar to that of most refluxing ureteroileal anastomoses (Pantuck et al, 2000). The T pouch was designed building on this technique to provide protection from reflux while avoiding some of these complications of the Kock nipple valve. This is accomplished by preserving optimum blood supply to the tunneled ileal afferent limb segment while allowing the ureters to be directly implanted into the ileum, allowing for ureters that are dilated or must be divided high (Stein et al, 1998b; Stein and Skinner, 2006). Stein and colleagues (2004) reported results with this type of diversion with 33 months median follow-up showing that renal function and anatomy were preserved in 90% of patients, with only 2% developing stenosis related to the afferent T limb during this time. In recent years the technique has been modified to avoid tapering the afferent limb to attempt to limit further the development of late stenosis (see later).
As the overall therapy (medical and surgical) for pelvic malignant neoplasms improves and patients live longer after cystectomy and urinary diversion (placing them at further long-term risk for renal deterioration), reflux prevention may become a more important issue. The basic question remains whether any benefit in upper tract protection afforded by the antireflux technique outweighs the potential risk of upper tract obstruction due to the antireflux technique itself in patients with bladder cancer. Further clarification of this question awaits the results of prospective randomized studies currently under way (Skinner and Skinner, 2010).
Key Points: Choice of Bowel Segment and the Need to Prevent Reflux
General Perioperative Management
Perioperative management of patients undergoing cystectomy and orthotopic diversion is not materially different than for patients undergoing other types of diversion. A careful history and physical examination should focus on prior abdominal or pelvic surgery or radiation, current voiding patterns and continence, and general medical problems. If colon may be used for the diversion, a colonoscopy is recommended to rule out polyps or tumors. A contrast study demonstrating the patient’s upper tract anatomy is helpful in planning management of the ureters.
There is no consensus on the ideal management of ureteral stents or catheters in patients undergoing orthotopic diversion. Most authors do recommend the use of ureteral stents in the early postoperative period (Mattei et al, 2008). The authors routinely use 8-Fr plastic feeding tubes that are tied to the urethral catheter so that they can be removed at the same time and the patient has fewer external bags to manage. They also use a 24-Fr stiff hematuria catheter, which facilitates routine irrigation of the pouch. The disadvantage of this system is the need to irrigate the catheter several times daily to ensure that the catheter does not plug up with mucus. Many surgeons use externalized ureteral stents brought through the skin and drained separately. The latter allows for less urine to pass through the pouch while it is healing and may decrease dependence on attentive nursing. A pelvic drain should be placed in every patient. The authors routinely use a soft plastic Penrose drain. This type of drain promotes passive drainage without drawing urine out of the pouch by the suction used and can be safely left in for long periods of time.
Although no strict guidelines are available regarding optimal timing of reservoir catheter removal, it has been the authors’ practice for patients undergoing orthotopic and continent cutaneous forms of diversion to wait until 3 weeks postoperatively to remove the Foley catheter. When patients return at the 3-week postoperative mark, if there is minimal drainage from the Penrose drain (<100 mL during 24 hours), the catheter is removed, followed by the drain. Routine pouchograms or radiographic studies of the neobladder are not routinely performed unless a significant output from the drain is observed (Ankem et al, 2004). Patients receive education throughout the perioperative period regarding catheter management and proper voiding technique.
Surgical Techniques
A large number of modifications of the techniques presented next have been described in a small number of patients, and this is by no means an exhaustive list.
Ileal Reservoirs
Most ileal reservoirs use from 60 to 75 cm of terminal ileum, which is detubularized and folded in a variety of ways to attempt to create a spherical shape. Modifications primarily include variations in the exact folding technique and variations in management of the ureters, with or without an antireflux mechanism. All reservoirs are generally closed with continuous absorbable suture. The use of nonabsorbable suture and metal staples should be avoided because of the potential for stone formation (Stein et al, 1994b). Absorbable staples have been developed, and some authors have found them useful, though they tend to be somewhat bulky (Bonney and Robinson, 1990; Olsson et al, 1995; Kirsch et al, 1996, Montie et al, 1996).
Camey II
The Camey II orthotopic substitute (Camey, 1990) is a modification of the original Camey bladder substitute (Camey I) (Lilien and Camey, 1984), which was a simple segment of ileum anastomosed to the ureters and urethra. The modification includes detubularization and folding to eliminate peristaltic activity.
A total of 65 cm of ileum is isolated, with an area of the ileum identified to reach to the region of the urethra in a tension-free manner. After the integrity of the bowel is restored, the mesenteric trap is closed and the isolated portion of ileum is opened along the antimesenteric border for the entire length, except the area previously identified for the urethral anastomosis. In this region, the ileal incision is directed toward the mesenteric border. The ileum is then placed in a transverse U orientation. The medial borders of the U are sutured together with a running absorbable suture. A fingertip opening is made in the preselected area for the ileourethral anastomosis, the entire ileal plate is brought down to the pelvis, and the urethral anastomosis is performed. The ureteroileal anastomosis is then performed by a Le Duc technique (Le Duc et al, 1987). The reservoir is completed by folding the ileal plate and suturing with a running absorbable suture. The ends of the U are anchored to the pelvic floor to reduce tension (Fig. 87–2). A modification of the Camey II has been described by Barre and colleagues (1996). This places the ileum in a Z configuration and reportedly has the advantages of shorter length requirements, improved reservoir capacity, and potentially improved functional (continence) results (Barre et al, 1996).
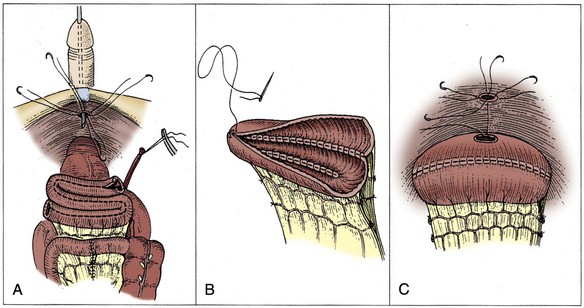
Figure 87–2 Construction of the modified Camey II. A, The ileal loop is folded three times (Z shaped) and incised on the antimesenteric border. B, The reservoir is closed with a running suture to approximate the incised ileum. C, The urethral anastomosis is performed, and the ureters are implanted using a Le Duc antireflux technique.
Ileal Neobladder (Hautmann Pouch)
This ileal neobladder was developed by Hautmann and colleagues (1988). This neobladder is an intentionally large-capacity, spherical (W configuration) ileal reservoir that is constructed in an attempt to optimize initial volume and potentially reduce nighttime incontinence.
A segment of terminal ileum of approximately 70 cm is selected. The bowel is reconstituted, and the mesenteric trap is closed. The ileal section that reaches the urethra most easily is identified and marked with a traction suture along the antimesenteric border. The isolated bowel segment is then arranged in either an M or W shape and is opened along the antimesenteric border except for a 5-cm section along the traction suture where the incision is curved to make a U-shaped flap.
The four limbs of the M or W are then sutured to one another with a running absorbable suture. A small full-thickness segment of bowel is excised in the site for the urethral anastomosis, which is then performed with the sutures tied from inside the neobladder. Once the ileal neobladder is situated in the pelvis and the urethral sutures are tied, the ureters are implanted from inside the neobladder through a small incision in the ileum at a convenient site. The remaining portion of the anterior wall is then closed with a running absorbable suture (Fig. 87–3).
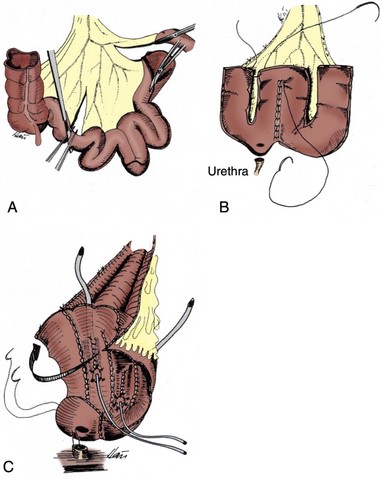
Figure 87–3 Construction of the Hautmann ileal neobladder. A, A 70-cm portion of terminal ileum is selected. The isolated segment of ileum is incised on the antimesenteric border. B, The ileum is arranged into an M or W configuration with the four limbs sutured to one another. C, After a buttonhole of ileum is removed on an antimesenteric portion of the ileum, the urethral anastomosis is performed. The ureteral anastomoses are performed using a Le Duc technique or direct implantation, are stented, and the reservoir is then closed in a side-to-side manner. As an alternative, the two ends of the W may be left slightly longer as a short chimney on either side for implantation of the ureters.
Initially Hautmann and colleagues (1988) used an antireflux anastomosis similar to that described by Le Duc and colleagues (1987). However, ureteroileal strictures were fairly common and beginning in 1997, the author modified this technique and now employs a freely refluxing, open end-to-side anastomosis implanted into short tabularized segments at each end of the W. This resulted in a decrease in the risk of ureteroileal stenosis from 9.5% to 1% (Hautmann, 2001; Hautmann et al, 1999, 2006; Volkmer et al, 2009).
The potential downside of this reservoir is the large initial capacity, which may result in an increased incidence of late urinary retention and increased electrolyte reabsorption from the pouch. Sevin has reported a modified Hautmann ileal neobladder using only 40 cm of ileum to reduce these potential issues with acceptable clinical outcomes (Sevin et al, 2004). The classic configuration of the pouch also could not accommodate short ureters, but in a popular revised technique one or both ends of the W could be left long to anastomoses to one or both shortened ureters (Hollowell et al, 2000).
Orthotopic Kock Ileal Reservoir (“Hemi-Kock”)
The Kock ileal reservoir was first employed as a continent cutaneous ileal reservoir incorporating intussuscepted nipple valves for both the afferent (antireflux) and efferent (continence) limbs (Kock et al, 1982; Skinner et al, 1984). This subsequently evolved into an orthotopic form of diversion in which the afferent intussuscepted limb was maintained to prevent urinary reflux (Fig. 87–4) (Ghoneim et al, 1987; Skinner et al, 1991). Skinner and colleagues (1998) performed more than 500 of these procedures from 1982 to 1995, with excellent continence and a low ureteroileal stricture rate of less than 3% (Elmajian et al, 1996). However, the technique required the use of metal surgical staples to fix the intussuscepted nipple valve, which was a source of potential stone formation. In addition, many surgeons found the technique difficult to master. Schreiter and Deliveliotis each described ileal S bladder modifications of the of the orthotopic Kock ileal reservoir (Schreiter and Noll, 1989; Deliveliotis et al, 2001). Both involve forming the detubularized reservoir into an S shape instead of the double-folded U configuration.
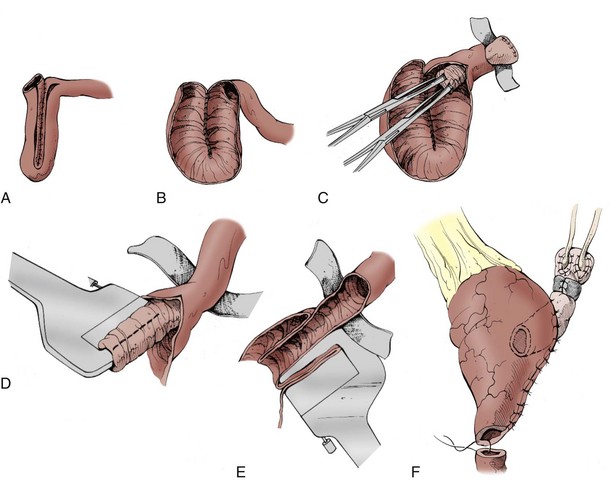
Figure 87–4 Construction of the Kock ileal reservoir. A, A total of 61 cm of terminal ileum is isolated. Two 22-cm segments are placed in a U configuration and opened adjacent to the mesentery. The more proximal 17-cm segment of ileum will be used to make the afferent intussuscepted nipple valve. B, The posterior wall of the reservoir is then formed by joining the medial portions of the U with a continuous running suture. C, A 5- to 7-cm antireflux valve is made by removing the mesentery under that segment and then intussuscepting the afferent limb with the use of Allis forceps clamps. D, The afferent limb is fixed with two rows of staples placed within the leaves of the valve. E, The valve is then fixed to the back wall from outside the reservoir with additional surgical staples. F, After completion of the nipple valve, the reservoir is completed by folding the ileum on itself and closing it, leaving the most dependent end of the suture line open for the urethral anastomosis.
With long-term follow-up, late complications associated with the afferent intussuscepted antireflux nipple developed in a small but significant number of patients (Stein et al, 1994, 1996). The primary late complications include stone formation on exposed staples (5%), stenosis of the afferent nipple ostium thought to be secondary to a compromised blood supply (4%), and extussusception or prolapse of the afferent limb (1%). These complications occasionally presented more than 10 years after construction of the reservoir and were clinically silent until the patient developed bilateral hydronephrosis or even renal failure. The technical difficulty of the intussuscepted nipple valve and the associated complications, along with the development of effective alternative antireflux techniques, has made the Kock afferent intussuscepted nipple valve a technique primarily of historic interest today.
Studer Ileal Bladder
This ileal bladder substitute initially described by Studer and colleagues (1989) uses a long, afferent, isoperistaltic, tubular ileal segment. It is believed that the long segment functionally prevents vesicoureteral reflux when the patient voids by Valsalva maneuver (Studer et al, 1989). It is straightforward to construct and has become the most popular form of orthotopic diversion in the United States. The advantages of this bladder substitute include the simplicity of construction, no requirement for surgical staples, and the ability to accommodate short ureters. The reservoir portion uses the double-folded U configuration originally described by Kock (1989). Studer’s group reported on 480 of these procedures performed from 1985 through 2005 with excellent long-term results in terms of continence, preservation of renal function, and less than 3% ureteroileal stricture rate (Studer et al, 2006). The original description used a 20-cm afferent segment, with 40 cm used for the reservoir. In more recent years Studer has advocated using a somewhat shorter afferent ileal segment, with similar results (Studer et al, 2006).
A portion of terminal ileum 54 to 56 cm long is isolated approximately 25 cm proximal to the ileocecal valve. Bowel continuity is restored, and the ends of the isolated segment are closed with a running absorbable suture. The distal 40- to 44-cm segment of ileum is placed in a U shape and opened along the antimesenteric border. The ureters are spatulated and anastomosed in an end-to-side fashion to the proximal 14- to 16-cm afferent tubular portion of ileum. The two medial borders of the U-shaped ileum are then oversewn with a running absorbable suture. The bottom of the U is folded over between the two ends of the U. After the lower half of the anterior wall and part of the upper half are closed, a finger is introduced through the remaining reservoir opening to determine the most caudal part of the neobladder. A hole is cut out in this dependent portion of ileum, away from the suture line, which allows urethral anastomosis. The urethral anastomosis is performed, and the remaining portion of the reservoir is then closed (Fig. 87–5).
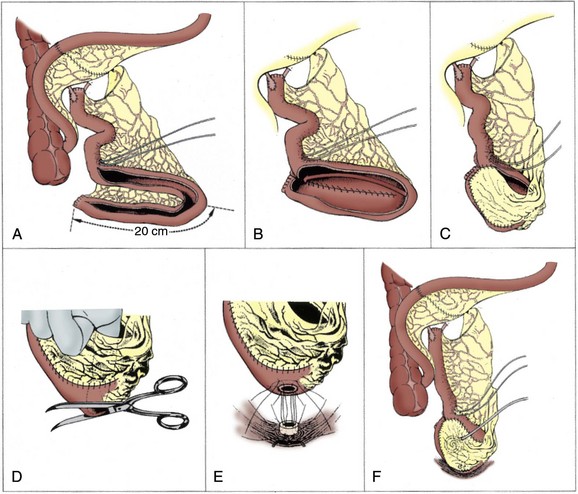
Figure 87–5 Construction of Studer pouch ileal neobladder with an isoperistaltic afferent ileal limb. A, A 60- to 65-cm distal ileal segment is isolated, and the distal 40 to 45 cm are folded into a U configuration. It is opened on the antimesenteric border while the more proximal 20 to 25 cm of ileum remain intact (afferent limb). B, The posterior wall of the reservoir is closed with a continuous running suture. The ureteroileal anastomoses are performed in a standard end-to-side technique to the proximal portion (afferent limb) of the ileum. Ureteral stents are used and brought out anteriorly through separate stab wounds. C, The reservoir is folded and oversewn (anterior wall). D, Before complete closure, a buttonhole opening is made in the most dependent (caudal) portion of the reservoir. E, The urethral anastomosis is performed. F, A cystostomy tube is placed, and the reservoir is closed completely.
Serous-Lined Extramural Tunnel
On the basis of experimental studies, Abol-Enein and Ghoneim demonstrated that an effective reflux mechanism could be made by bringing the ureters into a reservoir through extramural serous-lined tunnels. The authors believed that the construction of an extramural serous-lined tunnel provides several advantages. Metallic staples or synthetic materials are not required. The serous-lined tunnel protects the implanted portion of the ureter from exposure to urine so that sound healing without scarring is ensured. Moreover, a relatively short segment of bowel is used, and the procedure is versatile and not technically difficult (Abol-Enein and Ghoneim, 1993, 1994). The authors updated their excellent results in 450 patients with this technique and demonstrated that the serous-lined extramural tunnel is an effective and durable antireflux technique, with more than 93% of patients with unidirectional, unobstructed urinary flow (Abol-Enein and Ghoneim, 2001).
A 40-cm ileal segment is isolated from the distal ileum and arranged in a W configuration. The antimesenteric border of the isolated intestine is opened, and the edges of the medial flaps are joined with a running absorbable suture. On either side, the two lateral flaps are joined by a seromuscular continuous suture of silk (3-0). This forms two serous-lined intestinal troughs. Each ureter is laid down in its corresponding trough. A mucosa-to-mucosa anastomosis is performed with the spatulated ureter and the intestinal mucosa at the distal end of the trough. The mucosal edges on each side are then approximated over the reimplanted ureter. The anterior wall of the pouch is then closed in a side-to-side fashion. The suture line of the most dependent portion of the pouch close to the urethral stump is reopened to make a hole that will be anastomosed to the urethra (Fig. 87–6).
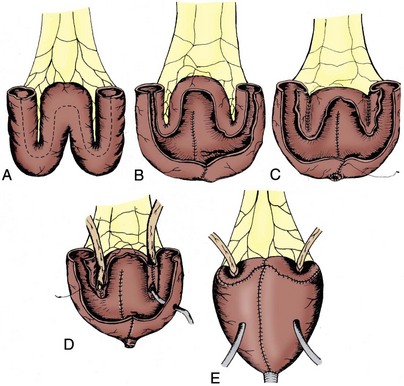
Figure 87–6 Construction of the serous-lined, extramural ileal neobladder (Ghoneim). A, A 40-cm segment of ileum is isolated and fashioned into a W configuration. B, The ileum is opened along the antimesenteric border for the entire length. C, The incised mucosa is joined in the middle with a running suture. The two lateral ileal flaps are joined by a seromuscular continuous suture to make the backing for the serous-lined ureteral tunnels. D, The spatulated ureters are laid into each trough, anastomosed to the intestinal mucosa, and stented. The tunnel is then closed over the implanted ureter. E, The anterior wall of the reservoir is formed by folding the ileum side to side. The reservoir is then anastomosed to the urethra.
Good results with this technique have been reported by others (Papadopoulos and Jacobsen, 2001). A modification of this orthotopic substitute with a serous-lined extramural ureteral reimplantation technique has also been reported by Kato and colleagues (2001) with similar results. The primary disadvantage of this technique is the requirement for long ureteral length, inability to accommodate dilated ureters, and a possibly increased risk of ureteral strictures.
T Pouch Ileal Neobladder
In an effort to preserve an antireflux mechanism but avoid the potential long-term complications seen with the Kock nipple valve, as well as allow for more flexibility in managing the ureters, Stein and Skinner developed the T pouch as a modification of Ghoneim and Abol-Enein’s ureteral serous-lined tunnel and updated their results with intermediate follow-up of 209 patients (Abol-Enein and Ghoneim, 1994; Stein et al, 2004). The evolution of this technique is detailed elsewhere (Stein et al, 2005).
The orthotopic T pouch uses the Kock double-folded U configuration. The afferent antireflux mechanism uses a short segment of ileum, which is fixed in an extraserosal tunnel that functions as a flap valve. If necessary, the afferent limb may be made longer to accommodate short ureters. It has the advantage of a classic Leadbetter ureteroileal anastomosis with its low ureteral stricture rate, and the antireflux mechanism functions regardless of the degree of dilation of the ureters. Maintenance of the vascular arcades of the distal afferent limb preserves the blood supply and should reduce the risk of late stenosis.
The T pouch is constructed from 44 cm of distal ileum placed in a U configuration; a proximal 8- to 10-cm segment of ileum is used to form the afferent antireflux mechanism. If ureteral length is short or compromised, a longer afferent ileal segment may be used. The ileum is divided between the proximal afferent ileal segment and the reservoir segment. The proximal end of the isolated afferent ileal segment is closed with a running absorbable suture. Both ends of the reservoir portion are also closed with absorbable suture.
Windows in the mesentery adjacent to the bowel wall at the distal end of the afferent segment are made (“windows of Deaver”), and the places held with small pieces of Penrose drain. A series of 3-0 silk sutures are then used to approximate the serosa of the two adjacent 22-cm ileal segments at the base of the U, with the sutures passed through the previously opened windows of Deaver. Two or three individual stitches are placed in each window to secure the tunnel. This process is repeated through each individual window until the distal 3 to 4 cm of the afferent segment are permanently fixed in the serous-lined ileal trough (Fig. 87–7).
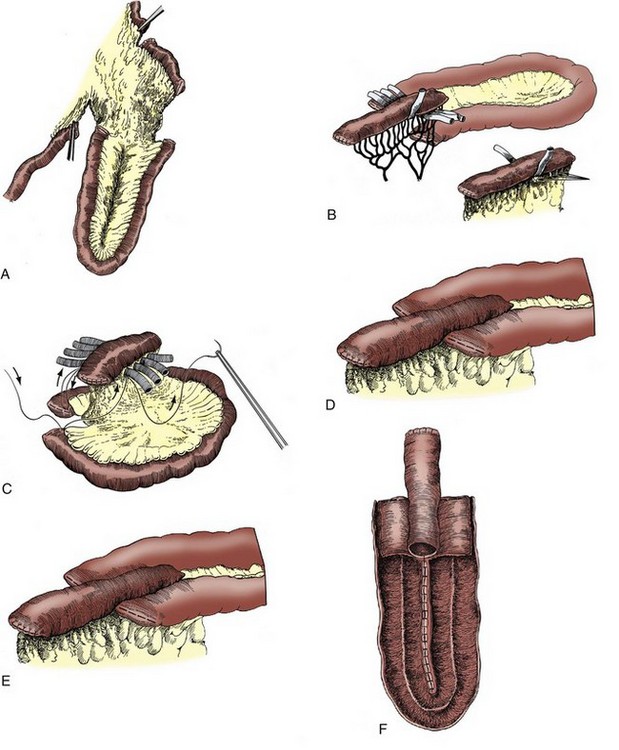
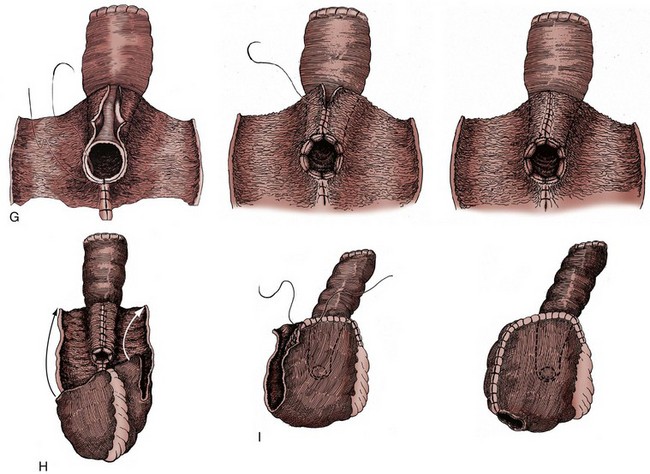
Figure 87–7 Construction of the T pouch orthotopic ileal neobladder. A, A total of 62 to 64 cm of distal ileum is isolated. The proximal 8- to 10-cm section is separated from the rest, and the two ends of the 44-cm segment are closed. The long segment is folded in a U shape. B, The antireflux mechanism is constructed. Windows in the mesentery of the distal 3 to 4 cm of the short segment are opened just outside of the bowel wall (“windows of Deaver”) and held in place with short pieces of small Penrose drains in each window. Note that the blood supply remains intact to this afferent ileal segment. C, A series of interrupted silk sutures are used to bring together the serosa of the adjacent 22-cm limbs (cephalad portion) through these windows. Then three to four silk sutures are placed through each window to make a secure backing for the extraserosal tunnel. D, the anchored afferent limb in place. E, The ileal segments are opened along the medial mesentery. Once the incision reaches the level of the ostium of the afferent limb, it swings wide to create two flaps to close over the top of the anchored afferent limb. F, The incised ileal mucosa is oversewn with absorbable running suture. G, The ostium of the afferent segment is sutured to the ileal flaps. The ileal flaps are then brought over and oversewn to cover the tapered afferent ileal segment. This completes the posterior wall of the reservoir and forms the antireflux flap-valve mechanism. H, The reservoir is folded in the opposite direction from which it was opened. The ureteral anastomoses are performed in an end-to-side Leadbetter fashion to the afferent limb. I, The suture line is left open to accommodate one fingerbreadth on the far right corner for the urethral anastomosis. This becomes the most dependent portion of the reservoir when it is positioned in the pelvis. The urethral anastomosis is the last step of the pouch.
Initial descriptions of the T pouch included tapering the distal portion of the afferent segment after it had been fixed into the tunnel to decrease its diameter and decrease the risk of reflux. However, these efforts appeared to be associated with occasional late stenosis of the end of the afferent valve. In 2004 the authors stopped tapering the distal afferent limb and have not observed any increase in reflux, but they have seen a decrease in stenosis. Therefore the authors no longer recommend tapering the afferent limb.
The reservoir portions are then opened along the medial mesentery, swinging wide on either side of the fixed afferent segment to construct flaps to close over the top of that segment. The suture lines are all closed with running absorbable suture. The flaps are brought over the top of the afferent limb, and it is matured into the reservoir. Finally, the pouch is folded in the opposite direction and that suture line closed, leaving a fingerbreadth opening at the far right corner for the urethral anastomosis. The ureters are anastomosed to the afferent limb in a direct end-to-side fashion, and the urethral anstomosis is performed last.
Colon and Iliocolonic Pouches
Orthotopic neobladders constructed completely of colon are a good option for patients with multiple previous small bowel resections or who have diseased ileum. Colon segments are less distensible than ileal segments and may be more likely to produce higher pressure waves causing incontinence (Khafagy et al, 2006). As a consequence initial volume should be larger than for an ileal pouch. Combined colon and ileal segments can mitigate this risk.
Orthotopic Mainz Pouch (Mainz III)
The Mainz (“mixed augmented ileum and zecum”) pouch was initially described as a continent catheterizable reservoir that was subsequently modified into an orthotopic neobladder (Thuroff et al, 1986; Eisenberger et al, 1999). A 10- to 15-cm segment of cecum, in continuity with a 20- to 30-cm segment of ileum, is isolated. An ascending ileocolostomy is performed. The entire segment of bowel is opened along the antimesenteric border, sacrificing the ileocecal valve. The bowel is placed in a W configuration, with the first limb of the W represented by cecum and the middle two limbs represented by ileum. The adjacent three limbs are sutured together with an absorbable suture, forming the posterior plate of the reservoir.
At the cephalad portion of the cecum, tunneled ureterocolonic anastomosis is performed. A buttonhole incision is made in the cecum at the base of the reservoir, and a ureterocolonic anastomosis is performed. After this, the reservoir is closed side to side with absorbable suture (Fig. 87–8).
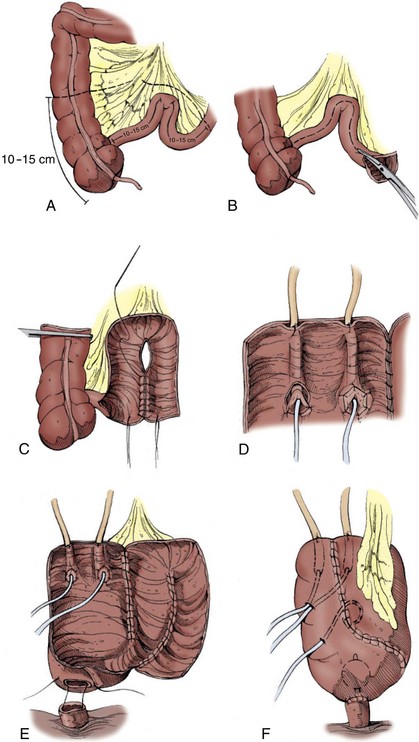
Figure 87–8 Construction of the Mainz ileocolonic orthotopic reservoir. A, An isolated 10 to 15 cm of cecum in continuity with 20 to 30 cm of ileum are isolated. B, The entire bowel segment is opened along the antimesenteric border. An appendectomy is performed. C, The posterior reservoir is closed by joining the opposing three limbs together with a continuous running suture. D, An antireflux implantation of the ureters through a submucosal tunnel is performed and stented. E, A buttonhole incision in the dependent portion of the cecum is made to provide for the urethral anastomosis. F, The reservoir is closed side to side with a cystostomy tube and the stents exiting.
Ileocolonic (Le Bag) Pouch
The Le Bag ileocolonic bladder substitute was initially described by Light and Engelmann (1986). A 20-cm segment of ascending colon and a corresponding length of terminal ileum are isolated, and bowel continuity is restored. The antimesenteric border is incised for the entire length of the colon and ileum to make two flat sheets (one small and one large bowel). These sheets are then sewn to one another to form the posterior plate. Initially, in the early experience with this neobladder, the incision in the small bowel commenced 2 inches from the cut end so that this intact tube of ileum could be anastomosed end to end to the urethra. This technique was subsequently modified because the small peristaltic ileal segment (intact) was thought to promote urinary incontinence. After this, the entire length of small bowel was incised (Light and Marks, 1990; Kolettis et al, 1996), and the urethra was anastomosed, end to side, to the cecum. The ureters are then brought into the colonic segment and implanted within the reservoir, and the neobladder is closed anteriorly (Fig. 87–9).
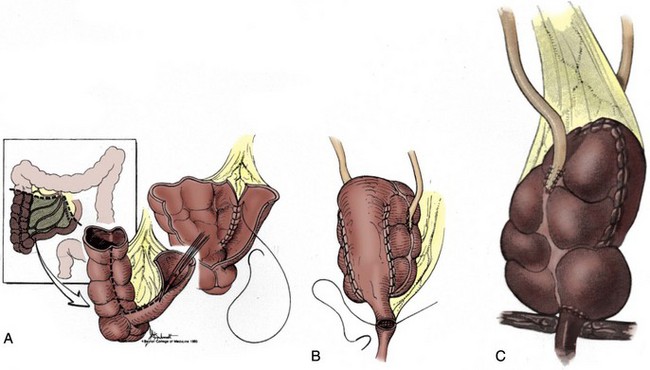
Figure 87–9 Construction of Le Bag (ileocolonic) orthotopic reservoir. A, A total of 20 cm of ascending cecum and colon, with a corresponding length of adjacent terminal ileum, is isolated. The bowel is opened along the entire antimesenteric border, and the two incised segments are then sewn to one another. This forms the posterior plate of the reservoir. B, This reservoir is folded and rotated 180 degrees into the pelvis with the most proximal portion of the ileum (2 cm nondetubularized) anastomosed to the urethra. C, Modification is performed with complete detubularization of the bowel segment, which is then anastomosed to the urethra.
(© Baylor College of Medicine.)
A modified version of the Le Bag ileocolonic neobladder has been reported by Baniel and Tal in which a Studer-like ileal chimney is incorporated as the afferent limb (Baniel and Tal, 2004).
Right Colon Pouch
An orthotopic substitute employing the right colon without ileum has been reported (Goldwasser et al, 1986; Mansson and Colleen, 1990; Goldwasser, 1995; Mansson et al, 2003). The entire right colon and cecum are isolated, and a transverse ileocolonic anastomosis is performed to provide bowel continuity. The ileal stump at the ileocecal valve is closed with a running absorbable suture. The colonic segment is then opened along the anterior tenia, leaving the proximal 2 to 3 inches of cecum intact. An appendectomy is performed, and the ureters are implanted in an antireflux fashion within the reservoir. The colon is then folded in a Heineke-Mikulicz manner and closed with a running absorbable suture. The ureterocolonic anastomosis is then performed.
Sigmoid Pouch
Patients who are candidates for radical cystectomy often have a redundant sigmoid colon, which is readily available for use. The only concern is the potential compromise of the vasculature of the distal colon segment due to interruption of branches of the internal iliac artery during the cystectomy. It is important therefore to maintain as much of the vascular supply to both ends of the bowel anastomosis as possible. Some patients will complain of frequent stools or rectal urgency for a period of time after sigmoid colectomy.
The use of the sigmoid in construction of an orthotopic substitute was initially described by Reddy and Lange (1987) (Fig. 87–10). A 35-cm portion of descending colon and sigmoid is isolated and arranged in a U configuration on the basis of the inferior mesenteric artery. The medial taenia of the U is incised down to an area just short of the urethral anastomosis. The incised medial limbs of the U are then brought together with an absorbable suture. Ureteral implantation is performed in a tunnel antireflux fashion. A small button of colon is removed from the most dependent portion of the reservoir, and the urethroenteric anastomosis is performed. The reservoir is then closed side to side.
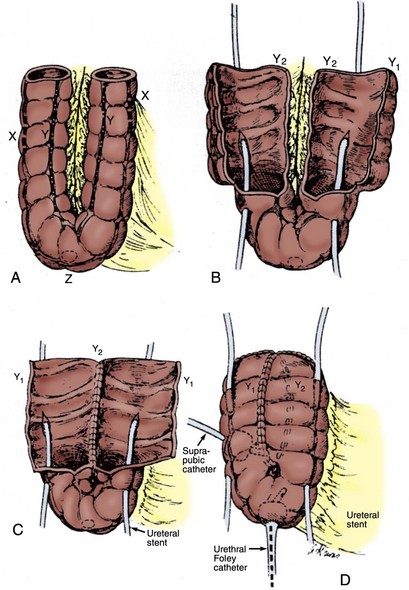
Figure 87–10 Construction of the sigmoid (Reddy) neobladder. A, A 35-cm segment of descending and sigmoid colon is isolated and folded into a U shape. B, The colon is incised along the medial tenia down to a point a few centimeters short of the entire colon. A buttonhole is made in the caudal portion of the colon that is later anastomosed to the urethra. C, The medial portions of the U are sewn together, and a tunneled ureterocolonic anastomosis is performed. D, The colonic pouch is closed by folding the reservoir side to side, and it is anastomosed to the urethra.
A modification of this technique was reported by DaPozzo and colleagues in which the entire bowel is detubularized and then folded in a Heineke-Mikulicz fashion to provide a more spherical reservoir (DaPozzo et al, 1994).
Adaptation of Orthotopic Diversion to Minimally Invasive Techniques
Over the past 10 years a number of innovators have begun to perform cystectomy using laparoscopic or robotic-assisted techniques (Gill et al, 2002; Stephenson and Gill, 2008; Huang et al, 2008; Pruthi and Wallen, 2008; Wang et al, 2008; Yuh et al, 2008). Initial attempts to perform the diversion completely intracorporeally were fraught with difficulty and were gradually abandoned by most groups. Currently most reports describe completing the cystectomy and lymph node dissection with the laparoscopic or robotic techniques, then delivering the bowel through a small incision to construct the diversion extracorporeally (Haber et al, 2008). This has allowed construction of continent diversions, both cutaneous and orthotopic. Ileal neobladders using the Studer technique have been the most popular because of ease of mobilization of the segment and simpler bowel anastomosis with a low abdominal incision. In the case of orthotopic neobladders, some authors then reestablish the pneumoperitoneum and use robotic assistance to complete the urethral anastomosis. Others perform the anastomosis in an open fashion (Pruthi and Wallen, 2008).
Although most reports to date contain relatively few patients and relatively short follow-up, there are a few groups with extensive experience and oncologic results to date appear to be comparable to open surgical approaches (Haber et al, 2008, Guillotreau et al, 2009). Postoperative complications and length of stay in most series have only been modestly decreased, with longer operative times but decreased blood loss and in some cases reduced ileus and shorter hospital stay. These techniques continue to evolve and may ultimately be applied more widely. As with other cancer surgeries, it is mandatory that the oncologic efficacy of the operation not be compromised in the use of minimally invasive techniques. Most importantly in treating invasive bladder cancer, the surgeon must attempt to achieve negative surgical margins and there are clear oncologic advantages to a more extensive lymphadenectomy (Skinner et al, 2007).
Results and Complications of Orthotopic Neobladders
Continence
In evaluating the continence results and clinical outcomes of various series of cystectomy and orthotopic neobladders, several considerations must be kept in mind. The prevalence and severity of urinary incontinence may be confounded by many variables including age and gender of the patients, surgical indications, prior treatments (such as radiation or prior transurethral resection of the prostate), and technique of urinary diversion. In addition, the definitions of incontinence, the techniques used to collect the information (i.e., chart review, telephone interview, or anonymous validated questionnaire), and the length and diligence of follow-up are all crucial when trying to compare results from different reported series (Thuroff et al, 1996) (Table 87–1).
Common observations from nearly all series of patients undergoing orthotopic diversion include a gradual period of improvement in daytime continence over the first 6 to 12 months and a higher incidence of persistent nighttime incontinence seen in 20% to 50% of patients. In a pooled analysis of 2238 patients with various forms of orthotopic neobladder, daytime incontinence was reported in an average of 13% of patients. Risk factors in this study for daytime incontinence included advanced age of the patient (older than 65 years), use of colonic segments, and in some series lack of nerve-sparing techniques (Steers, 2000).
Kessler and colleagues (2004) assessed various factors influencing urinary continence after radical cystectomy and ileal orthotopic bladder substitution in 331 male patients. In a multivariate analysis, the time to achieve daytime continence and the ultimate rate of success were significantly higher in patients younger than 65 and in those with attempted nerve-sparing techniques (Kessler et al, 2004). These findings strengthen the relationship of nerve-sparing techniques and continence in these cystectomy patients with an orthotopic substitute. It is the authors’ conviction that in patients with bulkier tumors (>T2), especially on the posterior bladder, trigone, or bladder neck, and in most patients older than age 70, that the advantage of nerve-sparing techniques is outweighed by a potential increased risk of incomplete tumor excision. Positive surgical margins in this disease are almost always ultimately fatal, so appropriate selection of patients for nerve-sparing is crucial.
Nighttime incontinence remains one of the most bothersome sequelae of neobladders, seen in 7% to 70% of patients with an average of 28% (Steers, 2000). Nocturnal incontinence after orthotopic reconstruction results in part from the absence of neurologic feedback and sphincter detrusor reflex, as well as decreased sphincter tonus at night. Similar to daytime incontinence, nighttime incontinence generally improves as functional capacity increases and as the absorptive capacity of the mucosa decreases over time (El Bahnasawy et al, 2000). The latter results in a reversal of the normal antiduiretic effect of nighttime dehydration. In elderly patients there is also a physiologic diuresis associated with increasing age. Instructing the patient to completely empty immediately before bedtime and to awaken two or three times with the assistance of an alarm clock may help reduce some of the issues of nighttime incontinence. The authors and others have observed that nighttime incontinence may improve as late as 24 months after surgery (Elmajian et al, 1996; Granberg et al, 2008).
Decreased urethral sensitivity has been proposed as a potential factor contributing to urinary incontinence after radical cystectomy and orthotopic diversion. It has been suggested that conscious or unconscious sensation of urine leakage in the membranous urethra may normally produce either a reflex or voluntary contraction with increased tone of the external urethra (Kessler et al, 2007). This may be impaired in some patients with an orthotopic diversion. This reflex may also diminish with age and contribute to gradually decreasing continence in some individuals after orthotopic reconstruction (Hugonnet et al, 1999; Madersbacher et al, 2002). To some degree this worsening continence with age might be offset by the lack of problems related to prostatic enlargement, detrusor hypertrophy, and overactive bladder, all of which are common in elderly patients.
The evaluation and management of urinary incontinence after orthotopic diversion should be delayed until the neobladder has had time to enlarge. This may take 6 months to a year after surgery (Grossfeld, 1996; Granberg et al, 2008). Physical therapy with biofeedback focused on the pelvic floor muscles may help some patients attain initial continence (Parekh et al, 2003). Urodynamic investigation may be indicated to ensure adequate capacity, without pressure waves. If reduction in maximal urethral closure or low Valsalva leak pressure is demonstrated, transurethral injection of bulking agents may be considered but it has had only marginal results in the authors’ experience. Alternatively, in men an artificial urinary sphincter can be considered and may provide a more definitive treatment. In women, although injection of urethral bulking agents provides a minimally invasive approach with some positive results (Tchetgen et al, 2000), it is best employed for women with minimal symptoms of incontinence, with overall success rates below 50% and with less than optimal durable responses (Wilson et al, 2004). Pubovaginal sling procedures for incontinence may be more effective in women with incontinence after orthotopic reconstruction (Quek et al, 2004). Female patients undergoing a sling, however, must be informed of the high likelihood of urinary retention and the need to perform intermittent catheterization. Importantly, one should avoid blind passage of needles or sling material into the pelvis in these patients because of a high risk of injury to the pouch or a loop of bowel stuck to the pubic symphysis. Use of infrapubic bone anchors or a prepubic approach may provide the safest surgical options (Quek et al, 2004).
Urinary Retention
Failure to empty or urinary retention has been reported in 4% to 25% of patients undergoing orthotopic reconstruction and is more common in women than in men (Steers, 2000; Nagele et al, 2006). Stein and colleagues (2004) reported that 25% of 209 patients with an orthotopic T pouch required at least occasional intermittent catheterization (20% of men and 43% of women).
Patients with incomplete emptying may present with acute retention but more often present with recurrent infections or the new onset of overflow incontinence. They may also be discovered on routine follow-up with a palpable suprapubic mass or distended reservoir on imaging. The development of retention is often a late event, so the reported rates are heavily influenced by the length of follow-up of the series. Risk factors for urinary retention in patients with an orthotopic reservoir include the use of excessive intestinal length for the reservoir (>60 cm of ileum) and the use of prostate-sparing or nerve-sparing surgical procedures (Steers, 2000; Steven and Poulsen, 2000; Stein et al, 2004). Some authors have suggested that the location of the urethral anastomosis in the neobladder may contribute to retention in men (Thurairaja and Studer, 2008), but the chapter authors’ experience using the end of the suture line for the anastomosis has resulted in a low incidence of retention (Elmajian et al, 1996; Stein et al, 2004).
Rectal or vaginal examination and cystoscopy should be performed in patients who develop retention to rule out a urethral anastomotic stricture or tumor recurrence. Urinary retention is best managed by intermittent self-catheterization (Steers, 2000). Pharmacologic intervention for patients with urinary retention does not appear to be an effective measure to improve this voiding dysfunction. Biofeedback training in pelvic floor relaxation may be helpful, and some authors have suggested that many of these patients have a flap of mucosa causing the obstruction that may be incised endoscopically with good effect (Thurairaja and Studer, 2008). Others have not found this to be a common cause of retention (Simon et al, 2006). Patients should be advised during the first few years of follow-up of the importance of adequate emptying and the need to consciously relax the external sphincter along with using Valsalva pressure during voiding.
A significant number of patients with orthotopic reservoirs will develop abdominal wall or incisional hernias postoperatively. These fascial defects will reduce the efficiency in completely evacuating the neobladder by reducing the ability to effectively increase intra-abdominal pressure. These hernias should be identified and surgically repaired.
In women it appears that posterior prolapse of the pouch may contribute to late retention, and posterior support by means of omental flaps and sacroculpopexy has been advocated (Ali-el-Dein et al, 2002; Stenzl and Höltl, 2003; Lee et al, 2004; Stein and Skinner, 2004). Ali-el-Dein and colleagues elegantly studied the possible causes of chronic retention after radical cystectomy and orthotopic bladder substitution in women. A total of 136 women underwent a standard radical cystectomy and orthotopic substitution, with 100 patients evaluable at a mean follow-up of 36 months. Overall, 95% of the women were continent in the day, 86% were continent at night, 2% were completely incontinent, and 16% were in chronic retention. Video-urodynamics showed that retention appeared to be mechanical in nature due to the pouch’s falling back in the wide pelvic cavity, resulting in acute angulation of the posterior pouch-urethral junction. In addition, herniation of the pouch wall through the prolapsed vaginal stump was observed in most cases. Pelvic floor electromyography demonstrated complete pelvic floor silence during voiding, suggesting that urinary retention was not related to a neurogenic cause. The authors suggested that omental packing behind the reservoir, suturing of the peritoneum on the rectal wall to the vaginal stump, suspension of the vaginal stump by the preserved round ligaments, and suspension of the pouch near the dome to the back of the rectus muscle at cystectomy will help reduce the incidence of chronic retention (Ali-El-Dein et al, 2002). The proposed technical modifications to increase back support of the pouch with ventral suspension near its dome and support of the vaginal stump should help reduce the incidence of urinary retention in these patients.
Key Points: Continence and Urinary Retention
Complications of Orthotopic Diversion
Complications of radical cystectomy and diversion are common, and many are severe and potentially life threatening (Konety et al, 2006; Quek et al, 2006; Ali El Dein et al, 2008). Most early complications include bleeding, thrombotic events, infection, and cardiovascular and pulmonary complications are not directly related to the urinary diversion and do not appear to be different in patients undergoing different types of diversion. Gastrointestinal complications are also common after cystectomy and are at least partially related to the need for a bowel anastomosis, which is common to all types of diversion. Similarly, urine leak and ureteral complications are possible with any type of diversion (Jensen et al, 2006). The overall rates of diversion-related early and late complications reported in some recent published series of patients undergoing orthotopic diversion are listed in Table 87–2. A number of authors have shown that the overall rate of complications, hospital stay, and reoperation rates are not increased by using continent diversion compared with ileal conduit diversion (Benson et al, 1992; Gburek et al, 1998; Parekh et al, 2000a).
Table 87–2 Suggested Follow-up Protocol for Bladder Cancer Patients following Cystectomy and Orthotopic Diversion
| Every 4 Months First Year, Then Every 6 Months to 3 Years, Then Annually |
| Annual Visits Only |
| Imaging Depending on Cystectomy Pathology |
Urine leaks may be more common in continent diversion than in conduits because of the long suture lines, but with properly managed stents and drains these generally resolve with observation alone. If a patient has an undrained leak, an attempt at percutaneous drainage and/or bilateral nephrostomy tube placement is preferable to open surgical repair. The latter is extremely difficult during the first few weeks after the initial surgery and is likely to be complicated by enterotomies and a risk of fistula formation. In the authors’ experience approximately 5% of patients require some sort of percutaneous drain or nephrostomy tube placement during the early postoperative period to manage these problems.
Late complications, however, are often influenced by the type of urinary diversion. Complications may also be influenced by other factors including tumor recurrence and the use of adjuvant or salvage systemic chemotherapy or radiation, and these causes may be difficult to separate out from causes related to the surgery. Complications not directly related to the diversion include late bowel obstruction, ventral hernia, late thrombotic events, and cardiovascular problems common to patients in this age group. The primary late complications of orthotopic diversion that may be related to the diversion itself include urinary tract infection, ureteroileal or afferent limb obstruction, urethral stricture, upper tract and pouch stones, and incontinence. Most of these complications can be managed by endoscopic procedures and rarely require open surgical revision (Jensen et al, 2006). This is in contradistinction to cutaneous continent diversion, in which open revision is often required for stomal problems such as difficulty catheterizing or leakage (Rowland, 1995).
Pouch perforation is rare in continent diversion in general, especially in orthotopic diversion because outlet resistance is usually low. The risk may be increased in patients who have had previous radiation therapy. It is a potentially life-threatening complication when it occurs. The authors have seen at least two deaths due to pouch perforation of an orthotopic diversion that was unrecognized when our patients were admitted to an outside hospital. Patients generally present with acute abdominal pain and distention, often with signs of sepsis. Computed tomography (CT) cystogram is usually diagnostic. These patients should be generally managed with exploration and repair, though conservative management with percutaneous drains has been described (Mansson et al, 1997; Singh and Choon, 2004).
Obstruction from an antireflux valve has been seen in both hemi-Kock pouches and in the extraserosal tunneled afferent limb of the T pouch (Stein et al, 1996, 2004). These may be clinically silent until the patient presents with bilateral hydronephrosis or even renal failure. Diagnosis may be suspected on CT or ultrasound and can be confirmed on retrograde pyelography. In both types of diversion these may be managed by endoscopic incision of the valve mechanism. It can be technically challenging to find the afferent limb in some cases, and special techniques may be necessary to catheterize and incise it (Dunn et al, 2007).
Pouch-vaginal fistula is a unique complication of orthotopic neobladder in women that can be quite difficult to resolve. The reported incidence in larger series is 5% to 10%, and the risk is increased if a portion of the anterior vaginal wall must be excised along with the cystectomy specimen and in irradiated patients (Al el Dein et al, 2008; Granberg et al, 2008). Prevention methods are crucial including careful water-tight closure of the vaginal cuff and placement of an omental flap between the vagina and neobladder, tacked to the perivaginal tissue on either side of the urethral anastomosis (Stein and Skinner, 2006). In the early postoperative period any concern about vaginal urinary leakage should be evaluated with a lateral cystogram before removal of the catheter. Fistula should also be ruled out in any woman with persistent incontinence after the first few months of recovery.
Beyond the initial few weeks a pouch-vaginal fistula is unlikely to heal spontaneously or with urinary diversion with a catheter or percutaneous nephrostomy tubes alone. Repair may be attempted transvaginally, though reported success varies (Rapp et al, 2004; Smith, 2005; Al el Dein et al, 2008). Repair may ultimately require transabdominal exploration or even conversion to a cutanous form of diversion.
Follow-up for Patients with Orthotopic Diversion
There is no consensus on the ideal follow-up regimen for patients with orthotopic diversion. Imaging techniques include intravenous urography, CT, magnetic resonance imaging, and ultrasound. Physicians in the United States tend to depend more on radiographic imaging, whereas those in Europe often rely more on ultrasound evaluation. Regardless of the type of imaging, the follow-up regimen can be divided into three time segments:
Our current follow-up scheme is shown in Table 87–2, but others have been described (Thurairaja and Studer, 2008). Positron emission tomography–CT imaging is a promising new tool for follow-up of higher-risk patients (Bouchelouche and Oehr, 2008). The authors routinely perform cytology on both voided urine and urethral wash in male patients, especially those with carcinoma in situ in their primary pathology. It does require a cytologist with experience looking at urine specimens from neobladder patients to be adept at evaluating them. Women can be evaluated with bimanual examination and voided cytology or a urethral swab processed as a thin prep smear.
Monitoring the retained urethra for all patients after radical cystectomy for bladder cancer is particularly important in patients undergoing an orthotopic form of urinary diversion. At a minimum, this should include annual voided cytology and full evaluation including urethroscopy in patients who develop symptoms related to the urethra or a change in voiding pattern or hematuria. Symptomatic patients with a urethral tumor recurrence usually present with bloody urethral discharge or gross hematuria but may also present with new-onset urinary retention or change in voiding pattern.
Clark and colleagues (2004) evaluated 47 men who developed urethral recurrence after cystectomy. Although this study was not specific for orthotopic diversion, the majority of patients were symptomatic on presentation for a urethral tumor recurrence. Among the 37 patients in whom urethral tumor recurrence was detected during the long-term follow-up period, cytologic screening alone diagnosed 13 of 37 patients (35%). Among the patients with cytology data available in this study, 94% had a positive cytology result. However, 4 of the 14 patients with an orthotopic neobladder who developed a urethral recurrence presented with a change in voiding habits, which in one case was the patient’s only complaint (Clark et al, 2004). It is therefore important to consider a urethral recurrence in the differential diagnosis whenever a patient who has undergone lower urinary tract reconstruction with an orthotopic neobladder presents with any change in voiding habits.
Key Points: Complications from Orthotopic Neobladders and Recommended Follow-Up
Quality of Life after Urinary Diversion
During the past decade, there has been an increasing focus on quality of life issues and outcomes in various urologic diseases. This has been aided by the development of new, health-related quality of life instruments for use specifically in urology. Health-related quality of life can be defined as a patient’s evaluation of the impact of a health condition and its treatment on relevant aspects of his or her life. Quality of life issues are becoming increasingly important in selection of the type of urinary diversion and are likely to play a larger role in future management of patients undergoing lower urinary tract reconstruction after cystectomy. One of the perceived advantages of the various forms of continent urinary diversion (particularly orthotopic diversion) is a presumed improvement in quality of life compared with a conduit form of diversion. However, these more “complicated” forms of urinary diversion may have some disadvantages as well. Continent diversions are technically more challenging and time consuming. Postoperatively, patients leave the hospital with indwelling catheters and may have a longer convalescence. Once the catheters are removed, patients generally require a period of education in the techniques required to properly care for the reservoir or neobladder. In addition, patients with continent diversions may be at slightly higher risk for bowel dysfunction (diarrhea and vitamin B12 malabsorption).
A number of studies have been undertaken to try to measure the impact of cystectomy and diversion on quality of life and to try to measure differences between the various types of diversion. These studies have been limited by the fact that there has never been a randomized study of conduits compared with continent diversion. In addition, there are often major differences in the baseline characteristics of patients undergoing the different types of diversion, making it difficult to compare them. Patients with different diversion also experience different types of difficulties, and it is difficult to write a questionnaire that captures them all equally.
Initial studies by Boyd, Mansson, and their colleagues in the 1980s pioneered comparative evaluation regarding quality of life issues in patients with different forms of urinary diversion after cystectomy. Boyd and colleagues (1987) compared quality of life in patients with an ileal conduit and in patients with a continent cutaneous Kock ileal reservoir. They found that all patients surveyed in the study were generally satisfied with their diversion and adapted reasonably well socially, physically, and psychologically. The key to adaptation seemed to be a detailed and realistic preoperative education or discussion about the type of diversion. Patients with ileal conduit diversions, however, had the lowest expectations of the form of diversion, as defined by preoperative awareness of the need to wear an external ostomy appliance along with the associated inconveniences and changes in external body image. Postoperatively, ileal conduit patients also had the poorest self-images as defined by a decrease in sexual desire and in all forms of physical contact (sexual and nonsexual). Interestingly, a subset of patients who underwent conversion from conduit diversions to continent cutaneous pouches were statistically the most satisfied and the most physically and sexually active. Although not directly related to orthotopic diversion, this early study suggested that from a quality of life perspective, a continent diversion may provide an important alternative to noncontinent forms of urinary diversion (Boyd et al, 1987). Mansson and colleagues (1988) also evaluated quality of life issues in patients undergoing cystectomy and urinary diversion. They demonstrated that urinary diversion affects most aspects of life in all patients. Although problems related to the diversion procedure tend to be fewer in patients with continent cutaneous reservoirs than in patients with conduits, both forms of diversion could be associated with serious social, sexual, mental, and emotional problems (Mansson et al, 1988).
Hobisch and colleagues (2000) compared 69 patients with orthotopic diversion to 33 patients who underwent an ileal conduit using both standardized and institutionally developed questionnaires. Patients with the continent diversion were more likely to recommend the procedure to friends, more likely to describe themselves as feeling “completely safe” with their diversion (74.6% vs. 33.3%), and more likely to feel “not handicapped at all” (92.8% vs. 66.7%). However, the groups were not totally comparable. The ileal conduit patients were significantly older, more likely to be female and unmarried, and less likely to be working (Hobisch et al, 2000). All of these factors can affect quality of life.
These initial quality of life studies in patients with urinary diversion paved the way to evaluate and compare patients undergoing an orthotopic form of diversion. Bjerre and colleagues (1995) compared health-related quality of life in patients undergoing an orthotopic neobladder (38 patients) or an ileal conduit (29 patients) form of diversion. Despite higher daytime and nighttime urine leakage in the bladder substitute group, the urine leakage affected conduit patients more severely, and they scored higher on a leakage distress scale. The ileal conduit group was also found not to retain healthy body image as well as patients with a bladder substitute (Bjerre et al, 1995).
In one of the first studies to compare long-term quality of life outcomes, Hart and colleagues (1999) reported on a total of 224 participating patients who completed four self-reporting questionnaires including a profile of mood states and adapted versions of the sexual history form, body image dissatisfaction scale, and quality of life questionnaire. This study compared self-reports of emotional distress; global quality of life; sexuality; body image dissatisfaction; urinary diversion problems; and problems with social, physical, and functional activities. The diversions included an ileal conduit in 25, a cutaneous Kock pouch in 93, and an orthotopic neobladder in 103 patients. Men who had or had not received an inflatable penile prosthesis after cystectomy were also compared with regard to quality of life variables. Regardless of the form of urinary diversion, the majority of patients reported good overall quality of life, little emotional distress, and few problems with social, physical, or functional activities. Problems with urinary diversion and sexual function were identified as most common. After controlling for age, no significant differences in any quality of life area were found among the urinary diversion subgroups. However, controlling for age in men indicated that penile prosthesis placement was significantly associated with better sexual function and satisfaction. Quality of life also appeared good in those patients who were long-term survivors of bladder cancer. The type of urinary diversion, at least in this study, did not appear to be associated with significant differences in quality of life. Furthermore, the authors suggested that the option of erectile aids in men after cystectomy should be considered (Hart et al, 1999).
Weijerman and colleagues (1998) compared quality of life issues in patients undergoing either an orthotopic or a cutaneous continent urinary diversion. Quality of life assessment in this study revealed only a minor advantage for an orthotopic placement. Importantly, quality of life assessment was found to be favorable for both types of urinary diversion (Weijerman et al, 1998). Similar results have been reported by others (Sullivan et al, 1998).
Most of the studies that evaluated and compared quality of life issues in patients undergoing various forms of urinary diversion have been criticized for methodologic problems that limit their conclusions (Gerharz et al, 2005; Gerharz, 2007). Most of the early studies were retrospective and failed to employ a well-validated measure of assessing quality of life that compared different groups. In one of the better studies, McGuire and colleagues (2000) used a well-validated survey to assess the impact of different forms of urinary diversion on overall quality of life in patients with locoregional bladder cancer after cystectomy. This study evaluated a total of 92 patients without evidence of disease recurrence who had undergone one of three different forms of urinary diversion. Patients completed a validated quality of life survey by mail. A total of 38 men with an orthotopic substitute had a mean physical score of 48.4 and a mean mental score of 51; 16 men and women with an Indiana pouch had a mean physical score of 48.4 and a mean mental score of 55.7. These results were not statistically different from published age-based and sex-based population norms. Thirty-eight men with an ileal conduit diversion had a mean physical score of 41.4 and a mean mental score of 48.2. The physical score was not statistically different from the population-based norm; however, the mental score was significantly decreased from the published norm. These authors concluded that patients with ileal conduits have significantly decreased mental health quality of life, whereas patients with continent urinary diversions do not. Furthermore, they suggested that when it is not medically contraindicated, patients should be offered a continent form of diversion after cystectomy. Although not specific for an orthotopic substitute, these data again provide further support for a continent form of urinary diversion (McGuire et al, 2000).
Hautmann and Paiss (1998) attempted to determine if the option of a neobladder substitute stimulates the decisions of patient and physician toward an earlier cystectomy in patients with bladder cancer. They reported on a total of 213 men undergoing cystectomy for bladder malignant neoplasm, 135 patients with an ileal neobladder and 78 with an ileal conduit diversion. The interval from the primary diagnosis was 11.8 months in the neobladder and 16.7 months in the conduit group. Five-year survival rate was significantly higher for all disease stages in the neobladder group than in the conduit group. The authors concluded that the availability of ileal neobladder may decrease the physician’s reluctance to perform cystectomy early in the disease process, thus increasing the survival rate. They also implied that orthotopic urinary diversion positively influenced patients and physicians to choose radical surgery earlier in the course of disease (Hautmann and Paiss, 1998).
Critical analyses of the current quality of life literature suggest that there is not enough evidence to conclude that there is a clear quality of life advantage for patients undergoing continent diversion. Porter and Penson (2005) performed a systematic review to determine if any differences exist in health-related quality of life outcomes among different types of urinary diversion after radical cystectomy. They performed a detailed MEDLINE search of appropriate studies inclusive of the dates 1966 to January 2004. Inclusion criteria included adult patients, patients with bladder cancer, comparative studies, original research, primary study outcome related to quality of life, and use of a quality of life instrument to measure outcomes. Only studies comparing neobladder, continent reservoir, or conduit diversion were included. Of 378 initial articles, only 15 studies met all the inclusion criteria. None of the studies were randomized trials, and only one was prospective. Common limitations included unvalidated health-related quality of life outcomes instruments, use of general health-related quality of life outcomes instruments only, lack of baseline data, cross-sectional analysis, and retrospective study design. The authors found that the current body of published literature is insufficient for it to be concluded that any form of urinary diversion is superior to another on the basis of health-related quality of life outcomes (Porter and Penson, 2005). Gerharz came to similar conclusions (Gerharz et al, 2005; Gerharz, 2007).
Clearly, to better understand and evaluate these quality of life issues in patients undergoing various forms of urinary diversion, future studies in this area must incorporate prospective data collection, provide longer-term follow-up, and incorporate validated disease-specific health-related quality of life outcomes instruments.
In summary, although there are not clear data showing an overall quality of life advantage for patients undergoing neobladder reconstruction, in fact many patients will chose this option simply because it seems the most natural. Patients must, however, have realistic expectations about the risk of incontinence and the possible need for self-catheterization. However, the majority of patients who undergo continent orthotopic diversion are happy with the results and would recommend it to a friend who was having a cystectomy.
Abol-Enein H, Ghoneim MA. Functional results of orthotopic ileal neobladder with serous-lined extramural ureteral reimplantation: experience with 450 patients. J Urol. 2001;165:1427.
Ali-El-Dein B, Gomha M, Ghoneim MA. Critical evaluation of the problem of chronic urinary retention after orthotopic bladder substitution in women. J Urol. 2002;168:587.
Bochner BH, Figueroa AJ, Skinner EC, et al. Salvage radical cystoprostatectomy and orthotopic urinary diversion following radiation failure. J Urol. 1998;160:29.
Colleselli K, Stenzl A, Eder R, et al. The female urethral sphincter: a morphological and topographical study. J Urol. 1998;160:49.
Gerharz EW, Mansson A, Hunt S, Skinner EC. Quality of life after cystectomy and urinary diversion: an evidence based analysis. J Urol. 2005;174:1729-1736.
Gilchrist RK, Merricks JW, Hamlin HH, Rieger IT. Construction of a substitute bladder and urethra. Surg Gynecol Obstet. 1950;9:752.
Guillotreau J, Gamé X, Mouzin M, et al. Radical cystectomy for bladder cancer: morbidity of laparoscopic versus open surgery. J Urol. 2009;181:554-559.
Hautmann RE, Abol-Enein H, Hafez K, et al. World Health Organization (WHO) Consensus Conference on Bladder Cancer: urinary diversion. Urology. 2007;69(1 Suppl.):17-49.
Hautmann RE, DePetriconi R, Gottfried HW, et al. The ileal neobladder: complications and functional results in 363 patients after 11 years of followup. J Urol. 1999;161:422.
Jensen JB, Lundbeck F, Jensen KM-E. Complications and neobladder function of the Hautmann orthotopic ileal neobladder. BJU Int. 2006;98:1289-1294.
Nieuwenhuijzen JA, Horenblas S, Meinhardt W, et al. Salvage cystectomy after failure of interstitial radiotherapy and external beam radiotherapy for bladder cancer. BJU Int. 2004;94:793-797.
Simon J, Bartsch GJr, Kufer R, et al. Neobladder emptying failure in males: incidence, etiology and therapeutic options. J Urol. 2006;176:1468-1472.
Skinner DG, Lieskovsky G, Boyd SD. Continuing experience with the continent ileal reservoir (Kock pouch) as an alternative to cutaneous urinary diversion: an update after 250 cases. J Urol. 1987;137:1140-1145.
Stein JP, Esrig D, Freeman JA, et al. Prospective pathologic analysis of female cystectomy specimens: risk factors for orthotopic diversion in women. Urology. 1998;51:951.
Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666.
Stein JP, Skinner DG. Surgical atlas: the orthotopic T-pouch ileal neobladder. BJU Int. 2006;98:469-482.
Stenzl A, Draxl H, Posch B, et al. The risk of urethral tumors in female bladder cancer: can the urethra be used for orthotopic reconstruction of the lower urinary tract? J Urol. 1995;153:950.
Studer UE, Burckard FC, Schumacher M, et al. Twenty years experience with an ileal orthotopic low pressure bladder substitute—lessons to be learned. J Urol. 2006;176:161-166.
Abbas F, Biyabani SR, Talati J. Orthotopic bladder replacement to the urethra following salvage radical cystoprostatectomy in men with failed radiation therapy. Tech Urol. 2001;7:20-26.
Abol-Enein H, Ghoneim MA. Optimization of uretero-intestinal anastomosis in urinary diversion: an experimental study in dogs. III. A new antireflux technique for uretero-ileal anastomosis: a serous-lined extramural tunnel. Urol Res. 1993;21:135.
Abol-Enein H, Ghoneim MA. A novel uretero-ileal reimplantation technique: the serous lined extramural tunnel. A preliminary report. J Urol. 1994;151:1193.
Abol-Enein H, Ghoneim MA. Functional results of orthotopic ileal neobladder with serous-lined extramural ureteral reimplantation: experience with 450 patients. J Urol. 2001;165:1427.
Adams MC, Mitchell ME, Rink RC. Gastrocystoplasty: an alternative solution to the problem of urological reconstruction in the severely compromised patient. J Urol. 1988;140:1152.
Ahlering TE, Kanellos A, Boyd SD, et al. A comparative study of perioperative complications with Kock pouch urinary diversion in highly irradiated versus nonirradiated patients. J Urol. 1988;139:1202.
Ali-El-Dein B, Abdel-Latif M, Ashamallah A, et al. Local urethral recurrence after radical cystectomy and orthotopic bladder substitution in women: a prospective study. J Urol. 2004;171:275.
Ali-El-Dein B, Gomha M, Ghoneim MA. Critical evaluation of the problem of chronic urinary retention after orthotopic bladder substitution in women. J Urol. 2002;168:587.
Ali-el-Dein B, Shaaban AA, Abu-Eideh RH, et al. Surgical complications following radical cystectomy and orthotopic neobladder in women. J Urol. 2008;180:206-210.
Althausen AF, Hagen-Cook K, Hendren WHD. Non-refluxing colon conduit: experience with 70 cases. J Urol. 1978;120:35.
Ankem MK, Han K-R, Hartanto V, et al. Routine pouchograms are not necessary after continent urinary diversion. Urology. 2004;63:435.
Ashworth A. Papillomatosis of the urethra. Br J Urol. 1956;28:3.
Baniel J, Tal R. The “B-bladder”—an ileocolonic neobladder with a chimney: surgical technique and long-term results. Eur Urol. 2004;45:794.
Barre PH, Herve JM, Botto H, Camey M. Update on the Camey II procedure. World J Urol. 1996;14:27.
Benson MC, Slawin KM, Wechsler MH, Olsson CA. Analysis of continent versus standard urinary diversion. Br J Urol. 1992;69:156.
Berglund B, Kock NG, Norlen L, Philipson BM. Volume capacity and pressure characteristics of the continent ileal reservoir used for urinary diversion. J Urol. 1987;137:29.
Bhatta DN, Kessler TM, Mills RD, et al. Nerve-sparing radical cystectomy and orthotopic bladder replacement in female patients. Eur Urol. 2007;52:1006-1014.
Bjerre BD, Johansen C, Steven K. Health-related quality of life after cystectomy: bladder substitution compared with ileal conduit diversion. A questionnaire survey. Br J Urol. 1995;75:200.
Bochner BH, Figueroa AJ, Skinner EC, et al. Salvage radical cystoprostatectomy and orthotopic urinary diversion following radiation failure. J Urol. 1998;160:29.
Bonney WW, Robinson RA. Absorbable staples in continent ileal urinary pouch. Urology. 1990;35:57.
Borirakchanyavat S, Aboseif SR, Carroll PR, et al. Continence mechanism of the isolated female urethra: an anatomical study of the intrapelvic somatic nerves. J Urol. 1997;158:822.
Bouchelouche K, Oehr P. Positron emission tomography and positron emission tomography/computerized tomography of urological malignancies: an update review. J Urol. 2008;179:34-45.
Boyd SD, Feinberg SM, Skinner DG, et al. Quality of life survey of urinary diversion patients: comparison of ileal conduits versus continent Kock ileal reservoirs. J Urol. 1987;138:1386.
Bricker EM. Bladder substitution after pelvic evisceration. Surg Clin North Am. 1950;30:1511.
Butcher HRJr, Sugg WL, McAffee CA, Bricker EM. Ileal conduit method of ureteral urinary diversion. Ann Surg. 1962;156:682.
Camey M. Detubularized U-shaped cystoplasty (Camey II). Curr Surg Tech Urol. 1990;3:1.
Camey M, Le Duc A. L’enterocystoplastie avec cystoprostatectomie totale pour cancer de la vessie. Ann Urol. 1979;13:114.
Cancrini A, DeCarli P, Pompeo V, et al. Lower urinary tract reconstruction following cystectomy: experience and results in 96 patients using the orthotopic ileal bladder substitution of Studer et al. Eur Urol. 1996;29:204.
Chang SS, Alberts GL, Smith JAJr, Cookson MS. Ileal conduit urinary diversion in patients with previous history of abdominal/pelvic irradiation. World J Urol. 2004;22:272-276.
Chang SS, Cole E, Smith JAJr, Cookson MS. Pathological findings of gynecologic organs obtained at female radical cystectomy. J Urol. 2002;168:147-149.
Chen ME, Pisters LL, Malpica A, et al. Risk of urethral, vaginal and cervical involvement in patients undergoing radical cystectomy for bladder cancer: results of a contemporary cystectomy series from M.D. Anderson Cancer Center. J Urol. 1997;157:2120.
Claesson K, Frodin L, Lorelius LE. Ureteral reflux and ileal conduit pressure following diversion with a reflux-preventing technique. Ups J Med Sci. 1985;90:119.
Clark PE, Montie JE, Klein EA. Long-term follow up of renal function and upper tract status after ileal conduit urinary diversion. J Urol. 1999;161:247A.
Clark PE, Stein JP, Groshen S, et al. The management of urethral transitional cell carcinoma after radical cystectomy for bladder cancer. J Urol. 2004;172:1342.
Clark PE, Stein JP, Groshen SG, et al. Radical cystectomy in the elderly: comparison of clinical outcomes between younger and older patients. Cancer. 2005;104:36-43.
Clarke BG, Leadbetter WF. Ureterosigmoidostomy: collective review of results in 2897 cases. J Urol. 1955;73:999.
Colleselli K, Stenzl A, Eder R, et al. The female urethral sphincter: a morphological and topographical study. J Urol. 1998;160:49.
Coloby PJ, Kakizoe T, Tobisu K, Sakamoto M. Urethral involvement in female bladder cancer patients: mapping of 47 consecutive cysto-urethrectomy specimens. J Urol. 1994;152:1438.
DaPozzo LF, Colombo R, Pompa P, et al. Detubularized sigmoid colon for bladder replacement after radical cystectomy. J Urol. 1994;152:1409.
Davidsson TP, Poulsen AL, Hedlund H, et al. A comparative urodynamic study of the ileal and the colonic neobladder. Scand J Urol Nephrol. 1992;142:143.
De Paepe ME, Andre R, Mahadevia P. Urethral involvement in female patients with bladder cancer. A study of 22 cystectomy specimens. Cancer. 1990;65:1237.
Deliveliotis C, Alargoff E, Skolarikos A, et al. Modified ileal neobladder for continent urinary diversion: experience and results. Urology. 2001;58:712.
Donat SM, Wei DC, McGuire MS, Herr HW. The efficacy of transurethral biopsy for predicting the long-term clinical impact of prostatic invasive bladder cancer. J Urol. 2001;165:1580.
Dunn MD, Kalisvaart M, Stein JP, et al. Endoscopic incision of stenotic afferent antireflux valves in continent urinary diversions: rate of recurrent stenosis and comparison of treatment modalities. J Urol. 2007;177:187A.
Eckman H, Jacobsson B, Kock NG, Sundin T. The functional behavior of different types of intestinal bladder substitutes. Contrib Soc Int Urol. 1964;11:213-219.
Eisenberger CF, Schoenberg M, Fitter D, Marshall FF. Orthotopic ileocolic neobladder reconstruction following radical cystectomy: history, technique and results of the Johns Hopkins experience, 1986-1998. Urol Clin North Am. 1999;26:149-156.
El Bahnasawy MS, Osman Y, Gomha MA, et al. Nocturnal enuresis in men with an orthotopic ileal reservoir: urodynamic evaluation. J Urol. 2000;164:10.
Elder DD, Moisey CU, Rees RW. A long-term follow-up of the colonic conduit operation in children. Br J Urol. 1979;51:462-465.
Elmajian DA, Stein JP, Esrig D, et al. The Kock ileal neobladder: updated experience in 295 male patients. J Urol. 1996;156:920.
Faysal MH. Urethrectomy in men with transitional cell carcinoma of bladder. Urology. 1980;16:23.
Freeman JA, Esrig D, Stein JP, Skinner DG. Management of the patient with bladder cancer. Urethral recurrence. Urol Clin North Am. 1994;21:645.
Freeman JA, Tarter TA, Esrig D, et al. Urethral recurrence in patients with orthotopic ileal neobladders. J Urol. 1996;156:1615.
Gburek BM, Lieber MM, Blute ML. Comparison of Studer ileal neobladder and ileal conduit urinary diversion with respect to perioperative outcome and late complications. J Urol. 1998;160:721.
Gerharz EW. Is there any evidence that one continent diversion is any better than any other or than ileal conduit? Curr Opin Urol. 2007;17:402-407.
Gerharz EW, Mansson A, Hunt S, Skinner EC. Quality of life after cystectomy and urinary diversion: an evidence based analysis. J Urol. 2005;174:1729-1736.
Gheiler EL, Wood DPJr, Montie JE, Pontes JE. Orthotopic urinary diversion is a viable option in patients undergoing salvage cystoprostatectomy for recurrent prostate cancer after definitive radiation therapy. Urology. 1997;50:580.
Ghoneim MA, Kock NG, Lycke G, el-Din AB. An appliance-free, sphincter-controlled bladder substitute: the urethral Kock pouch. J Urol. 1987;138:1150.
Gilchrist RK, Merricks JW, Hamlin HH, Rieger IT. Construction of a substitute bladder and urethra. Surg Gynecol Obstet. 1950;9:752.
Gill IS, Kaouk JH, Meraney AM, et al. Laparoscopic radical cystectomy and continent orthotopic ileal neobladder performed completely intracorporeally: the initial experience. J Urol. 2002;168:13-18.
Goldwasser B. The colonic orthotopic bladder. Urology. 1995;45:190.
Goldwasser B, Barrett DM, Benson RCJr. Bladder replacement with use of a detubularized right colonic segment: preliminary report of a new technique. Mayo Clin Proc. 1986;61:615.
Goodwin WE, Harris AP, Kaufman JJ, Beal JM. Open, transcolonic ureterointestinal anastomosis: a new approach. Surg Gynecol Obstet. 1953;97:295.
Goodwin WE, Winter CC, Baker WF. “Cup-patch” technique of ileocystoplasty for bladder enlargement or partial substitution. Surg Gynecol Obstet. 1959;108:240.
Granberg CF, Boorjian SA, Crispen PL, et al. Functional and oncological outcomes after orthotopic neobladder reconstruction in women. BJU Int. 2008;102:1551-1555.
Grossfeld GD, Stein JP, Bennett J, et al. Lower urinary tract reconstruction in the female using the Kock ileal reservoir with bilateral ureteroileal urethrostomy: update of continence results and fluorourodynamic findings. Urology. 1996;48:383-388.
Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859-866.
Gschwend JE, May F, Paiss T, et al. High-dose pelvic irradiation followed by ileal neobladder urinary diversion: complications and long-term results. Br J Urol. 1996;77:680.
Guillotreau J, Gamé X, Mouzin M, et al. Radical cystectomy for bladder cancer: morbidity of laparoscopic versus open surgery. J Urol. 2009;181:554-559.
Haber GP, Crouzet S, Gill IS. Laparoscopic and robotic assisted radical cystectomy for bladder cancer: a critical analysis. Eur Urol. 2008;54:54-62.
Hardeman SW, Soloway MS. Urethral recurrence following radical cystectomy. J Urol. 1990;144:666.
Hart S, Skinner EC, Meyerowitz BE, et al. Quality of life after radical cystectomy for bladder cancer in patients with an ileal conduit, cutaneous or urethral Kock pouch. J Urol. 1999;162:77.
Hassan JM, Cookson MS, Smith JAJr, Chang SS. Urethral recurrence in patients following orthotopic urinary diversion. J Urol. 2004;172:1338-1341.
Hauri D. Can gastric pouch as orthotopic bladder replacement be used in adults? J Urol. 1998;156:931.
Hautmann RE. The ileal neobladder to the female urethra. Urol Clin North Am. 1997;24:827.
Hautmann RE. The ileal neobladder. Atlas Urol Clin North Am. 2001;9:85.
Hautmann RE. Urinary diversion: ileal conduit to neobladder. J Urol. 2003;169:834.
Hautmann RE, Abol-Enein H, Hafez K, et al. World Health Organization (WHO) Consensus Conference on Bladder Cancer: urinary diversion. Urology. 2007;69(1 Suppl.):17-49.
Hautmann RE, DePetriconi R, Gottfried HW, et al. The ileal neobladder: complications and functional results in 363 patients after 11 years of followup. J Urol. 1999;161:422.
Hautmann RE, Egghart G, Frohneberg D, Miller K. The ileal neobladder. J Urol. 1988;139:39.
Hautmann RE, Paiss T. Does the option of the ileal neobladder stimulate patient and physician decision toward earlier cystectomy? J Urol. 1998;159:1845.
Hautmann RE, Simon J. Ileal neobladder and local recurrence of bladder cancer: patterns of failure and impact on function in men. J Urol. 1999;162:1963.
Hautmann S, Chun KHF, Currlin E, et al. Refluxing chimney versus nonrefluxing Le Duc ureteroileal anastomosis for orthotopic ileal neobladder: a comparative analysis for patients with bladder cancer. J Urol. 2006;175:1389-1394.
Hill JT, Ransley PG. The colon conduit: a better method of urinary diversion? Br J Urol. 1983;55:629-631.
Hinman F. Selection of intestinal segments for bladder substitution: physical and physiological characteristics. J Urol. 1988;139:519.
Hinman F, Weyrauch HMJr. A critical study of the different principles of surgery which have been used in ureterointestinal implantation. Trans Am Assoc Genitourin Surg. 1936;29:15.
Hobisch A, Tosun K, Kinzl J, et al. Quality of life after cystectomy and orthotopic neobladder versus ileal conduit urinary diversion. World J Urol. 2000;18:338.
Hohenfellner M, Burger R, Schad H, et al. Reservoir characteristics of Mainz pouch studied in animal model. Osmolality of filling solution and effect of oxybutynin. Urology. 1993;42:741.
Hollowell CM, Christiano AP, Steinberg GD. Technique of Hautmann ileal neobladder with chimney modification: interim results in 50 patients. J Urol. 2000;163:47.
Huang J, Lin T, Xu K, et al. Laparoscopic radical cystectomy with orthotopic ileal neobladder: a report of 85 cases. J Endourology. 2008;22:939-946.
Hugonnet CL, Danuser H, Springer JP, et al. Decreased sensitivity in the membranous urethra after orthotopic ileal bladder substitution. J Urol. 1999;161:418.
Iselin CE, Robertson CN, Webster GD, et al. Does prostate transitional cell carcinoma preclude orthotopic bladder reconstruction after radical cystoprostatectomy for bladder cancer? J Urol. 1997;158:2123.
Jensen JB, Lundbeck F, Jensen KM-E. Complications and neobladder function of the Hautmann orthotopic ileal neobladder. BJU Int. 2006;98:1289-1294.
Johnson DE, Lamy SM. Complications of a single stage radical cystectomy and ileal conduit diversion: review of 214 cases. J Urol. 1977;117:171.
Kato H, Kiyokawa H, Igawa Y, Nishizawa O. The serous-lined tunnel principle for urinary reconstruction: a more rational method. BJU Int. 2001;87:783.
Kessler TM, Burkhard FC, Perimenis P, et al. Attempted nerve sparing surgery and age have a significant effect on urinary continence and erectile function after radical cystoprostatectomy and ileal orthotopic bladder substitution. J Urol. 2004;172:1323-1327.
Kessler TM, Studer UE, Burkhard FC. Increased proximal urethral sensory threshold after radical pelvic surgery in women. Neurourol Urodyn. 2007;26:208-212.
Khafagy M, Shaheed FA, Moneim TA. Ileocaecal vs ileal neobladder after radical cystectomy in patients with bladder cancer: a comparative study. BJU Int. 2006;97:799-804.
Kim HL, Steinberg GD. Complications of cystectomy in patients with a history of pelvic radiation. Urology. 2001;58:557-560.
Kirsch AJ, Hensle TW, Olsson CA. Absorbable stapling techniques in continent urinary diversion. World J Urol. 1996;14:117.
Kock NG, Ghoneim MA, Lycke KG, Mahran MR. Replacement of the bladder by the urethral Kock pouch: functional results, urodynamics and radiological features. J Urol. 1989;141:1111.
Kock NG, Nilson AE, Nilsson LO, et al. Urinary diversion via a continent ileal reservoir: clinical results in 12 patients. J Urol. 1982;128:469.
Kolettis PN, Klein EA, Novick AC, et al. The Le Bag orthotopic urinary diversion. J Urol. 1996;156:926.
Konety BR, Allareddy V, Herr H. Complications after radical cystectomy: analysis of population-based data. Urology. 2006;68:58-64.
Kristjansson A, Bajc M, Wallin L, Mansson W. Renal function up to 16 years after conduit (refluxing or anti-reflux anastomosis) or continent urinary diversion. 2. Renal scarring and location of bacteriuria. Br J Urol. 1995;76:546-550.
Kristjansson A, Wallin L, Mansson W. Renal function up to 16 years after conduit (refluxing or anti-reflux anastomosis) or continent urinary diversion. 1. Glomerular filtration rate and patency of uretero-intestinal anastomosis. Br J Urol. 1995;76:539-545.
Laguna MP, Brenninkmeier M, Belon JA, et al. Long-term functional and urodynamic results of 50 patients receiving a modified sigmoid neobladder created with a short distal segment. J Urol. 2005;174:963-967.
Lance RS, Dinney CP, Swanson D, et al. Radical cystectomy for invasive bladder cancer in the octogenarian. Oncol Rep. 2001;8:723-726.
Le Duc A, Camey M, Teillac P. An original antireflux ureteroileal implantation technique: long-term followup. J Urol. 1987;137:1156.
Leadbetter WF. Consideration of problems incident to performance of ureteroenterostomy: report of technique. J Urol. 1951;65:818.
Leadbetter WF, Clarke BG. Five years’ experience with ureteroenterostomy by the “combined technique.”. J Urol. 1954;73:67.
Lebret T, Herve JM, Barre P, et al. Urethral recurrence of transitional cell carcinoma of the bladder. Predictive value of preoperative latero-montanal biopsies and urethral frozen sections during prostatocystectomy. Eur Urol. 1998;33:170.
Lee CT, Hafez KS, Sheffield JH, et al. Orthotopic bladder substitution in women: nontraditional applications. J Urol. 2004;171:1585.
Lee KS, Montie JE, Dunn RL, Lee CT. Hautmann and Studer orthotopic neobladders: a contemporary experience. J Urol. 2003;169:2188.
Lemoine G. Creation d’une vessie nouvelle par un procede personnel apres cystectomie totale pour cancer. J Urol Med Chir. 1913;4:367.
Levinson AK, Johnson DE, Wishnow KI. Indications for urethrectomy in an era of continent urinary diversion. J Urol. 1990;144:73.
Lieskovsky G, Boyd SD, Skinner DG. Management of late complications of the Kock pouch form of urinary diversion. J Urol. 1987;137:1146-1150.
Light JK, Engelmann UH. Le Bag: Total replacement of the bladder using an ileocolonic pouch. J Urol. 1986;136:27.
Light JK, Marks JL. Total bladder replacement in the male and female using the ileocolonic segment (Le Bag). Br J Urol. 1990;65:467.
Lilien OM, Camey M. 25-year experience with replacement of the human bladder (Camey procedure). J Urol. 1984;132:886.
Lin DW, Santucci RA, Mayo ME, et al. Urodynamic evaluation and long-term results of the orthotopic gastric neobladder in men. J Urol. 2000;164:356.
Lockhart JL, Davies R, Cox C, et al. The gastroileoileal pouch: an alternative continent urinary reservoir for patients with short bowel, acidosis and/or extensive pelvic radiation. J Urol. 1993;150:46.
Lytton B, Green DF. Urodynamic studies in patients undergoing bladder replacement surgery. J Urol. 1989;141:1394.
Madersbacher S, Mohrle K, Burkhard F, Studer UE. Long-term voiding pattern of patients with ileal orthotopic bladder substitutes. J Urol. 2002;167:2052.
Madersbacher S, Schmidt J, Eberle JM, et al. Long-term outcome of ileal conduit urinary diversion. J Urol. 2003;169:985-990.
Mansson W, Bakke A, Bergman B, et al. Perforation of continent urinary reservoirs. Scandinavian experience. Scand J Urol Nephrol. 1997;31:529.
Mansson W, Colleen S. Experience with a detubularized right colonic segment for bladder replacement. Scand J Urol Nephrol. 1990;24:53.
Mansson W, Davidsson T, Konyves J, et al. Continent urinary tract reconstruction—the Lund experience. BJU Int. 2003;92:271.
Mansson A, Johnson G, Mansson W. Quality of life after cystectomy. Comparison between patients with conduit and those with continent caecal reservoir urinary diversion. Br J Urol. 1988;62:240.
Maralani S, Wood DPJr, Grignon D, et al. Incidence of urethral involvement in female bladder cancer: an anatomic pathologic study. Urology. 1997;50:537.
Mattei A, Birkhaeuser FD, Baermann C, Warncke SH. Studer UE. To stent or not to stent perioperatively the ureteroileal anastomosis of ileal orthotopic bladder substitutes and ileal conduits? Results of a prospective randomized trial. J Urol. 2008;179:582-586.
McGuire MS, Grimaldi G, Grotas J, Russo P. The type of urinary diversion after radical cystectomy significantly impacts on the patient’s quality of life. Ann Surg Oncol. 2000;7:4.
Middleton ANJr, Hendren WH. Ileal conduit in children at the Massachusetts General Hospital from 1955 to 1970. J Urol. 1976;115:591.
Mills RD, Studer UE. Metabolic consequences of continent urinary diversion. J Urol. 1999;161:1057.
Minervini A, Boni G, Salinitri G, et al. Evaluation of renal function and upper urinary tract morphology in the ileal orthotopic neobladder with no antireflux mechanism. J Urol. 2005;173:144-147.
Montie JE, Pontes JE, Powell IJ. A comparison of the W-stapled ileal reservoir with hand-sewn reservoirs for orthotopic bladder replacement. Urology. 1996;47:476.
Morales P, Golimbu M. Colonic urinary diversion: 10 years of experience. J Urol. 1975;113:302.
Nagele U, Kuczyk M, Anastasiadis G, et al. Radical cystectomy and orthotopic bladder replacement in females. Eur Urol. 2006;50:249-257.
Nguyen DH. Gastric bladder reconstruction. Urol Clin North Am. 1991;18:649.
Nieder AM, Sved PD, Gomez P, et al. Urethral recurrence after cystoprostatectomy: implications for urinary diversion and monitoring. Urology. 2004;64:950.
Nieuwenhuijzen JA, Horenblas S, Meinhardt W, et al. Salvage cystectomy after failure of interstitial radiotherapy and external beam radiotherapy for bladder cancer. BJU Int. 2004;94:793-797.
Norlen L, Trasti H. Functional behavior of the continent ileum reservoir for urinary diversion: an experimental and clinical study. Scand J Urol Nephrol. 1978;49:33.
Olsson CA, Hensle TW, Kirsch AJ. Absorbable stapling techniques in bowel reservoirs. Atlas Urol Clin North Am. 1995;3:69.
Pantuck AJ, Han KR, Perrotti M, et al. Ureteroenteric anastomosis in continent urinary diversion: long-term results and complications of direct versus nonrefluxing techniques. J Urol. 2000;163:450.
Papadopoulos I, Jacobsen KW. Experiences with the entero-ureteral anastomosis via the extramural serous-lined tunnel: procedure of Abol-Enein. Urology. 2001;57:234.
Parekh AR, Feng MI, Kirages D, et al. The role of pelvic floor exercises on post-prostatectomy incontinence. J Urol. 2003;170:130-133.
Parekh DJ, Gilbert WB, Kock MO, Smith JJJr. Continent urinary reconstruction versus ileal conduit: a contemporary single-institution comparison of perioperative morbidity and mortality. Urology. 2000;55:852-855.
Parekh DJ, Gilbert WB, Smith JAJr. Functional lower urinary tract voiding outcomes after cystectomy and orthotopic neobladder. J Urol. 2000;163:56.
Peckhman DA. The epithelial lining of the female trigone and urethra. Br J Urol. 1971;43:201.
Perimenis P, Burkhard F, Kessler TM, et al. Ileal orthotopic bladder substitute combined with an afferent tubular segment: long-term upper urinary tract changes and voiding pattern. Eur Urol. 2004;46:604-609.
Pitts WRJr, Muecke EC. A 20-year experience with ileal conduits: the fate of the kidneys. J Urol. 1979;122:154.
Porter MP, Penson DF. Health related quality of life after radical cystectomy and urinary diversion for bladder cancer: a systematic review and critical analysis of the literature. J Urol. 2005;173:1318.
Pruthi RS, Wallen EM. Is robotic radical cystectomy an appropriate treatment for bladder cancer? Short-term oncologic and clinical follow-up in 50 consecutive patients. Urology. 2008;72:617-620.
Quek ML, Ginsberg DA, Wilson S, et al. Pubovaginal slings for stress urinary incontinence following radical cystectomy and orthotopic neobladder reconstruction in women. J Urol. 2004;172:219.
Quek ML, Stein JP, Daneshmand S, et al. A critical analysis of perioperative mortality from radical cystectomy. J Urol. 2006;175:886-890.
Rapp DE, O’Connor RC, Katz EE, Steinberg GD. Neobladder-vaginal fistula after cystectomy and orthotopic neobladder construction. BJU Int. 2004;94:1092.
Raz S, McLorie G, Johnson S, Skinner DG. Management of the urethra in patients undergoing radical cystectomy for bladder carcinoma. J Urol. 1978;120:298.
Reddy PK, Lange PH. Bladder replacement with sigmoid colon after radical cystoprostatectomy. Urology. 1987;29:368.
Richie JP, Skinner DG, Waisman J. The effect of reflux on the development of pyelonephritis in urinary diversion: an experimental study. J Surg Res. 1974;16:256.
Ridlon HC. Ureterosigmoidostomy: a comparison of two techniques. J Urol. 1963;89:167.
Roth S, van Ahlen H, Semjonow A. Does the success of ureterointestinal implantation in orthotopic bladder substitution depend more on surgeon level of experience or choice of technique? J Urol. 1997;157:56.
Rowland RG. Complications of continent cutaneous reservoirs and neobladders—series using contemporary techniques. AUA Update Series. 1995;14:201.
Sakamoto N, Tsuneyoshi M, Naito S, Kumazawa J. An adequate sampling of the prostate to identify prostatic involvement by urothelial carcinoma in bladder cancer patients. J Urol. 1993;149:318.
Santucci RA, Park CH, Mayo ME, et al. Continence and urodynamic parameters of continent urinary reservoirs: comparison of gastric, ileal, ileocolic, right colon and sigmoid segments. Urology. 1999;54:252.
Schreiter F, Noll F. Kock pouch and S bladder: two different ways of lower urinary tract reconstruction. J Urol. 1989;142:1197.
Schrier BP, Laguna MP, van der Pal F, et al. Comparison of orthotopic ileal neobladder: continence and urodynamic parameters. Eur Urol. 2005;47:679.
Schuster TG, Marcovich R, Sheffield J, et al. Radical cystectomy for bladder cancer after definitive prostate cancer treatment. Urology. 2003;61:342-347.
Sevin G, Soyupek S, Armagan A, et al. Ileal orthotopic neobladder (modified Hautmann) via a shorter detubularized ileal segment: experience and results. BJU Int. 2004;94:355.
Shaaban AA, Abdel-Latif M, Mosbahh A, et al. A randomized study comparing an antireflux system with a direct ureteric anastomosis in patients with orthotopic neobladders. BJU Int. 2006;97:1057-1062.
Shaaban AA, Gaballah MA, el-Diasty TA, Ghoneim MA. Urethral controlled bladder substitution: a comparison between the intussuscepted nipple valve and the technique of Le Duc as antireflux procedures. J Urol. 1992;148:1156.
Shapiro SR, Lebowitz R, Colodny AH. Fate of 90 children with ileal conduit urinary diversion a decade later; analysis of complications, pyelography, renal function and bacteriology. J Urol. 1975;114:289.
Simon J. Extrophia vesicae (absence of the anterior walls of the bladder and pubic abdominal parietes); operation for directing the orifices of the ureters into the rectum; temporary success; subsequent death; autopsy. Lancet. 1852;2:568.
Simon J, Bartsch GJr, Kufer R, et al. Neobladder emptying failure in males: incidence, etiology and therapeutic options. J Urol. 2006;176:1468-1472.
Singh S, Choon S. Rupture and perforation of urinary reservoirs made from bowel. World J Urol. 2004;22:222-226.
Skinner DG, Boyd SD, Lieskovsky G. Clinical experience with the Kock continent ileal reservoir for urinary diversion. J Urol. 1984;132:1101.
Skinner DG, Boyd SD, Lieskovsky G, et al. Lower urinary tract reconstruction following cystectomy: Experience and results in 126 patients using the Kock ileal reservoir with bilateral ureteroileal urethrostomy. J Urol. 1991;146:756.
Skinner DG, Lieskovsky G, Boyd SD. Continuing experience with the continent ileal reservoir (Kock pouch) as an alternative to cutaneous urinary diversion: an update after 250 cases. J Urol. 1987;137:1140-1145.
Skinner DG, Lieskovsky G, Boyd SD. Continent urinary diversion—a  year experience. Ann Surg. 1998;208:337-344.
year experience. Ann Surg. 1998;208:337-344.
Skinner EC, Skinner DG. Does reflux in orthotopic diversion matter? A randomized prospective comparison of the Studer and T-pouch ileal neobladders. World J Urol. 2010;57:174.
Skinner EC, Stein JP, Skinner DG. Surgical benchmarks for the treatment of invasive bladder cancer. Urol Oncol. 2007;25:66-71.
Smith JAJr. Neobladder-vaginal fistula after cystectomy and orthotopic neobladder construction. J Urol. 2005;174:970-971.
Sogni F, Brausi M, Frea B, et al. Morbidity and quality of life in elderly patients receiving ileal conduit or orthotopic neobladder after radical cystectomy for invasive bladder cancer. Urology. 2008;71:919-923.
Song C, Kang T, Hong J-H, et al. Changes in the upper urinary tract after radical cystectomy and urinary diversion: a comparison of antirefluxing and refluxing orthotopic bladder substitutes and ileal conduit. J Urol. 2006;175:185-189.
Steers WD. Voiding dysfunction in the orthotopic neobladder. World J Urol. 2000;18:330.
Stein JP, Clark P, Miranda G, et al. Urethral tumor recurrence following cystectomy and urinary diversion: clinical and pathological characteristics in 768 male patients. J Urol. 2005;173:1163.
Stein JP, Cote RJ, Freeman JA, et al. Indications for lower urinary tract reconstruction in women after cystectomy for bladder cancer: a pathological review of female cystectomy specimens. J Urol. 1995;154:1329.
Stein JP, Dunn MD, Quek ML, et al. The orthotopic T pouch ileal neobladder: experience with 209 patients. J Urol. 2004;172:584.
Stein JP, Esrig D, Freeman JA, et al. Prospective pathologic analysis of female cystectomy specimens: risk factors for orthotopic diversion in women. Urology. 1998;51:951.
Stein JP, Freeman JA, Esrig D, et al. Complications of the afferent antireflux valve mechanism in the Kock ileal reservoir. J Urol. 1996;155:1579-1584.
Stein JP, Ginsberg DA, Skinner DG. Indications and technique of the orthotopic T-pouch ileal neobladder. Urol Clin North Am. 2002;29:725.
Stein JP, Grossfeld GD, Freeman JA, et al. Orthotopic lower urinary tract reconstruction in women using the Kock ileal neobladder: updated experience in 34 patients. J Urol. 1997;158:400.
Stein JP, Huffman JL, Freeman JA, et al. Stenosis of the afferent antireflux valve in the Kock pouch continent urinary diversion: diagnosis and management. J Urol. 1994;151:338.
Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666.
Stein JP, Lieskovsky G, Ginsberg DA, et al. The T pouch: an orthotopic ileal neobladder incorporating a serosal lined ileal antireflux technique. J Urol. 1998;159:1836.
Stein JP, Quek ML, Skinner DG. Contemporary surgical techniques for continent urinary diversion. Atlas Urol Clin North Am. 2001;9:147.
Stein JP, Skinner DG. Surgical atlas, radical cystectomy. BJU Int. 2004;84:197.
Stein JP, Skinner DG. Surgical atlas: the orthotopic T-pouch ileal neobladder. BJU Int. 2006;98:469-482.
Stein JP, Stenzl A, Esrig D, et al. Lower urinary tract reconstruction following cystectomy in women using the Kock ileal reservoir with bilateral ureteroileal urethrostomy: initial clinical experience. J Urol. 1994;152:1404.
Stenzl A, Colleselli K, Bartsch G. Update of urethra-sparing approaches in cystectomy in women. World J Urol. 1997;15:134.
Stenzl A, Colleselli K, Poisel S, et al. Rationale and technique of nerve sparing radical cystectomy before an orthotopic neobladder procedure in women. J Urol. 1995;154:2044.
Stenzl A, Colleselli K, Poisel S, et al. Anterior exenteration with subsequent ureteroileal urethrostomy in females. Anatomy, risk of urethral recurrence, surgical technique, and results. Eur Urol. 1998;33:18.
Stenzl A, Draxl H, Posch B, et al. The risk of urethral tumors in female bladder cancer: can the urethra be used for orthotopic reconstruction of the lower urinary tract? J Urol. 1995;153:950.
Stenzl A, Höltl L. Orthotopic bladder reconstruction in women—what we have learned over the past decade. Crit Rev Oncol Hematol. 2003;47:147.
Stephenson AJ, Gill IS. Laparoscopic radical cystectomy for muscle-invasive bladder cancer: pathological and oncological outcomes. BJU Int. 2008;102:1296-1301.
Steven K, Poulsen AL. The orthotopic Kock ileal neobladder: functional results, urodynamic features, complications and survival in 166 men. J Urol. 2000;164:288.
Strasser H, Bartsch G. Anatomy and innervation of the rhabdosphincter of the male urethra. Semin Urol Oncol. 2000;18:2.
Studer UE, Ackermann D, Casanova GA, Zingg EJ. Three years’ experience with an ileal low pressure bladder substitute. Br J Urol. 1989;63:43.
Studer UE, Burckard FC, Schumacher M, et al. Twenty years experience with an ileal orthotopic low pressure bladder substitute—lessons to be learned. J Urol. 2006;176:161-166.
Studer UE, Danuser H, Hochreiter W, et al. Summary of 10 years’ experience with an ileal low-pressure bladder substitute combined with an afferent tubular isoperistaltic segment. World J Urol. 1996;14:29.
Sullivan JW, Grabstald H, Whitmore WF. Complications of ureteroileal conduit with radical cystectomy: review of 336 cases. J Urol. 1980;124:797.
Sullivan LD, Chow VD, Ko DS, et al. An evaluation of quality of life in patients with continent urinary diversions after cystectomy. Br J Urol. 1998;81:699.
Suriano F, Gallucci M, Flammia GP, et al. Bacteriuria in patients with an orthotopic ileal neobladder: urinary tract infection or asymptomatic bacteriuruia? BJU Int. 2008;101:1576-1579.
Tanagho EA, Miller ER, Meyers FH, Corbett RK. Observations on the dynamics of the bladder neck. Br J Urol. 1966;38:72.
Tchetgen MB, Sanda MG, Montie JE, Faerber GJ. Collagen injection for the treatment of incontinence after cystectomy and orthotopic neobladder reconstruction in women. J Urol. 2000;163:212.
Tefilli MV, Gheiler EL, Tiguert R, et al. Urinary diversion-related outcome in patients with pelvic recurrence after radical cystectomy for bladder cancer. Urology. 1999;53:999.
Thoeny HC, Sonnenschein MJ, Madersbacher S, et al. Is ileal orthotopic bladder substitution with an afferent tubular segment detrimental to the upper urinary tract in the long term? J Urol. 2002;168:2030.
Thurairaja R, Studer UE. How to avoid intermittent catheterization in men with ileal bladder substitution. J Urol. 2008;180:2301-2302.
Thuroff JW, Alken P, Riedmiller H, et al. The Mainz pouch (mixed augmentation ileum and cecum) for bladder augmentation and continent diversion. J Urol. 1986;136:17.
Thuroff JW, Mattiasson A, Andersen JT, et al. The standardization of terminology and assessment of functional characteristics of intestinal urinary reservoirs. Scand J Urol Nephrol. 1996;30:349.
Tizzoni G, Poggi A. Die Wiederherstellung der Harnblase: Experimentelle Untersuchungen. Centrlbl Chir. 1888;15:921.
Tobisu K, Tanaka Y, Mizutani T, Kakizoe T. Transitional cell carcinoma of the urethra in men following cystectomy for bladder cancer: multivariate analysis for risk factors. J Urol. 1991;146:1551.
Turner WH, Danuser H, Moehrle K, Studer UE. The effect of nerve sparing cystectomy technique on postoperative continence after orthotopic bladder substitution. J Urol. 1997;158:2118.
Volkmer BG, de Petricone RC, Hautmann RE. Lessons learned from 1000 ileal neobladders: the early complication rate. J Urol. 2009;181(Suppl. 1):265A.
Wang GJ, Barocas DA., Raman JD., Scherr DS. Robotic vs open radical cystectomy: prospective comparison of perioperative outcomes and pathological measures of early oncological efficacy. BJU Int. 2008;101:89-93.
Wear JB, Barquin OP. Ureterosigmoidostomy: long-term results. Urology. 1973;1:192.
Weijerman PC, Schurmans JR, Hop WC, et al. Morbidity and quality of life in patients with orthotopic and heterotopic continent urinary diversion. Urology. 1998;51:51.
Wilson S, Quek ML, Ginsberg DA. Transurethral injection of bulking agents for stress urinary incontinence following orthotopic neobladder reconstruction in women. J Urol. 2004;172:244.
Wood DPJr, Bianco FJJr, Pontes JE, et al. Incidence and significance of positive urine cultures in patients with an orthotopic neobladder. J Urol. 2003;169:2196.
Wood DPJr, Montie JE, Pontes JE, Levin HS. Identification of transitional cell carcinoma of the prostate in bladder cancer patients: a prospective study. J Urol. 1989;142:83.
Yossepowitch O, Dalbagni G, Golijanin D, et al. Orthotopic urinary diversion after cystectomy for bladder cancer control and patterns of disease recurrence. J Urol. 2003;169:177.
Yuh B, Padalino J, Butt ZM, et al. Impact of tumour volume on surgical and pathological outcomes after robot-assisted radical cystectomy. BJU Int. 2008;102:840-843.
Zaayer EJ. Discussion. Intra-abdominale plastieken. Ned Tijdschr Genesk. 1911;65:836.
Zippe C, Nandipati KC, Agarwal A, Raina R. Female sexual dysfunction after pelvic surgery: the impact of surgical modifications. BJU Int. 2005;96:959-963.