chapter 88 Genital and Lower Urinary Tract Trauma
Injuries of the External Genitalia
Penis
Traumatic injuries to the genitalia are uncommon, in part because of the mobility of the penis and scrotum. Blunt phallic traumatic injury is usually of concern only with an erect penis, when fracture of the tunica albuginea may result. In general, prompt surgical reconstruction of most penile injuries usually leads to adequate and acceptable cosmetic and functional results.
Fracture
Etiology
Penile fracture is the disruption of the tunica albuginea with rupture of the corpus cavernosum. Fracture typically occurs during vigorous sexual intercourse, when the rigid penis slips out of the vagina and strikes the perineum or pubic bone, producing a buckling injury.
The tunica albuginea is a bilaminar structure (inner circular, outer longitudinal) composed of collagen and elastin. The outer layer determines the strength and thickness of the tunica, which varies in different locations along the shaft and is thinnest ventrolaterally (Hsu et al, 1994; Brock et al, 1997). The tensile strength of the tunica albuginea is remarkable, resisting rupture until intracavernous pressures rise to more than 1500 mm Hg (Bitsch et al, 1990). When the erect penis bends abnormally, the abrupt increase in intracavernosal pressure exceeds the tensile strength of the tunica albuginea, and a transverse laceration of the proximal shaft usually results.
Whereas penile fracture has been reported most commonly with sexual intercourse, it has also been described with masturbation, rolling over or falling onto the erect penis, and myriad other scenarios. In the Middle East, self-inflicted fractures predominate; the erect penis is forcibly bent during masturbation or as a means to achieve rapid detumescence, the practice of taghaandan (Zargooshi, 2000).
Mydlo (2001) reported that 94% of fractures in Philadelphia, Pennsylvania, were a result of sexual intercourse; Zargooshi (2000) described 69% of fractures in Kermanshah, Iran, as being due to self-manipulation. The tunical tear is usually transverse and 1 to 2 cm in length (Asgari et al, 1996; Mydlo, 2001). The injury is usually unilateral, although tears in both corporeal bodies have been reported (Mydlo, 2001; El-Taher et al, 2004). Although the site of rupture can occur anywhere along the penile shaft, most fractures are distal to the suspensory ligament. Injuries associated with coitus are usually ventral or lateral (Mydlo, 2001; Lee et al, 2007), where the tunica albuginea is the thinnest (Hsu et al, 1994).
Diagnosis and Imaging
The diagnosis of penile fracture is often straightforward and can be made reliably by history and physical examination. Patients usually describe a cracking or popping sound as the tunica tears, followed by pain, rapid detumescence, and discoloration and swelling of the penile shaft. If the Buck fascia remains intact, the penile hematoma remains contained between the skin and tunica, resulting in a typical eggplant deformity. If the Buck fascia is disrupted, hematoma can extend to the scrotum, perineum, and suprapubic regions (Fig. 88–1 on the Expert Consult website).![]() The swollen, ecchymotic phallus often deviates to the side opposite the tunical tear because of hematoma and mass effect. The fracture line in the tunica albuginea may be palpable. Because fear and embarrassment are commonly associated, the patient’s presentation to the emergency department or clinic is sometimes significantly delayed.
The swollen, ecchymotic phallus often deviates to the side opposite the tunical tear because of hematoma and mass effect. The fracture line in the tunica albuginea may be palpable. Because fear and embarrassment are commonly associated, the patient’s presentation to the emergency department or clinic is sometimes significantly delayed.
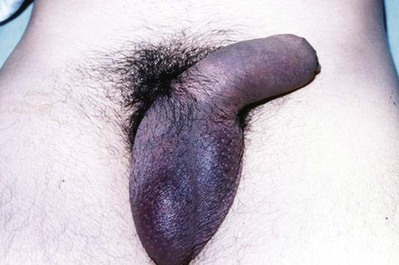
Figure 88–1 “Eggplant deformity,” the classic appearance of penile fracture sustained during intercourse, with hematoma of the penile shaft and ecchymosis extending into the scrotum.
The incidence of urethral injury is significantly higher in the United States and Europe (20%) than in Asia, the Middle East, and the Mediterranean region (3%), probably owing to the different etiology—intercourse trauma versus self-inflicted injury (Eke, 2002; Zargooshi, 2002; Jack et al, 2004; Derouiche et al, 2008). Most urethral injuries are associated with gross hematuria, blood at the meatus (Fig. 88–2), or inability to void, although the absence of these findings does not definitively rule out urethral injury (Tsang and Demby, 1992; Mydlo, 2001; Jack et al, 2004). Given that urethral injury occurs not infrequently, preoperative urethrography should be considered when urethral injury is suspected. However, because urethrography can be time consuming and inaccurate (Kamdar et al, 2008), intraoperative flexible cystoscopy is now performed routinely just before catheter placement at the time of penile exploration when urethral injury is suspected.
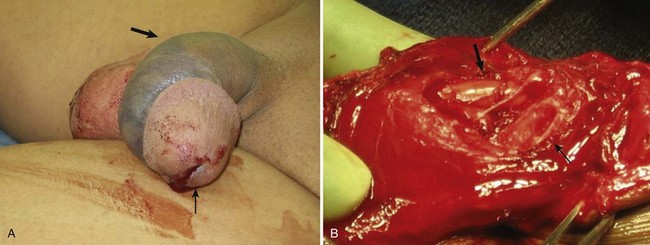
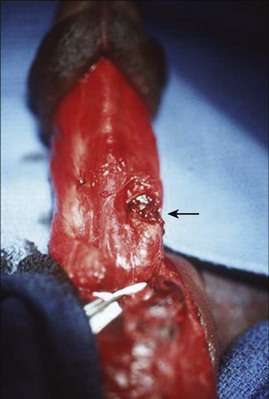
Figure 88–2 A, Large arrow indicates pronounced ecchymosis and swelling in this patient with a penile fracture sustained during intercourse. Small arrow indicates blood at urethral meatus. B, During surgical exploration and repair, urethral laceration with exposed Foley catheter is noted (large arrow). Small arrow indicates laceration of corpus cavernosum.
The typical history and clinical presentation of fractured penis usually make adjunctive imaging studies unnecessary. Although cavernosography has been advocated to assist in diagnosis, false-negative studies have been reported (Mydlo, 2001); false-positive studies can result from inadequate filling of one corporeal body and misinterpretation of complex venous drainage (Pliskow and Ohme, 1979; Beysel et al, 2002). Cavernosography is discouraged in the evaluation of a suspected penile fracture because it is time consuming and unfamiliar to most urologists and radiologists (Morey et al, 2004). Ultrasonography, although noninvasive and easy to perform, has also been associated with significant false-negative studies (Koga et al, 1993; Fedel et al, 1996).
Magnetic resonance imaging (MRI) is a noninvasive and accurate means of demonstrating disruption of the tunica albuginea (Fedel et al, 1996; Uder et al, 2002). Arguments against the routine use of MRI are the expense, limited availability, and time requirements involved with the study. MRI is reasonable in the evaluation of patients without the typical presentation and physical findings of penile fracture.
False fracture has been reported in patients who present with penile swelling and ecchymosis, and some even describe the classic “snap-pop” or rapid detumescence typically associated with fracture (Feki et al, 2007). Physical examination may not be adequate for definitive diagnosis of a corporeal tear in these circumstances (Shah et al, 2003). Surgical exploration (Fig. 88–3 on the Expert Consult website)![]() or evaluation with MRI should be considered. Another condition that may mimic penile fracture is rupture of the dorsal penile artery or vein during sexual intercourse (Armenakas et al, 2001; Bar-Yosef et al, 2007).
or evaluation with MRI should be considered. Another condition that may mimic penile fracture is rupture of the dorsal penile artery or vein during sexual intercourse (Armenakas et al, 2001; Bar-Yosef et al, 2007).
Management
Multiple contemporary publications indicate that suspected penile fractures should be promptly explored and surgically repaired. Although small lateral incisions may be used for localized hematomas or palpable tunical defects (El Bahnasawy and Gomha, 2000; Nasser and Mostafa, 2008), a distal circumcising incision is appropriate in most cases, thus providing exposure to all three penile compartments. Closure of the tunical defect with interrupted 2-0 or 3-0 absorbable sutures is recommended; deep corporeal vascular ligation or excessive debridement of the delicate underlying erectile tissue must be avoided. Induction of an artificial erection with saline or colored dye may aid in locating the corporeal laceration (Shaeer, 2006). Partial urethral injuries should be oversewn with fine absorbable suture over a urethral catheter. Complete urethral injuries should be debrided, mobilized, and repaired in a tension-free fashion over a catheter. Therapy with broad-spectrum antibiotics and 1 month of sexual abstinence are recommended. In uncircumcised patients, strong consideration should be given to performing limited circumcision at the conclusion of the repair because wide mobilization of the foreskin may place the distal prepuce at risk for ischemia.
Outcome and Complications
Immediate surgical reconstruction results in faster recovery, decreased morbidity, lower complication rates, and lower incidence of long-term penile curvature (Nicolaisen et al, 1983; Orvis and McAninch, 1989; Hinev, 2002; El-Taher et al, 2004; Muentener et al, 2004). Although immediate repair results in penile curvature in less than 5% of patients (El Atat et al, 2008), conservative management of penile fracture has been associated with penile curvature in more than 10% of patients, abscess or debilitating plaques in 25% to 30%, and significantly longer hospitalization times and recovery (Meares, 1971; Nicolaisen et al, 1983; Kalash and Young, 1984; Orvis and McAninch, 1989). Zargooshi (2002) reported in a personal surgical series of 170 patients that surgical management of penile fractures resulted in erectile function comparable to that of a control population. Timing of surgery may also influence long-term success—those undergoing repair within 8 hours of injury had significantly better long-term results than did those having surgery delayed 36 hours after the fracture occurred (Asgari et al, 1996; Karadeniz et al, 1996).
Gunshot and Penetrating Injuries
Gunshot Wounds
The majority of penetrating wounds to the genitalia are due to gunshots (Mohr et al, 2003), and most require surgical exploration. Treatment principles include immediate exploration, copious irrigation, excision of foreign matter, antibiotic prophylaxis, and surgical closure. Gunshot injuries to the phallus are rarely isolated wounds—nearly all victims have significant associated injuries, including abdominal, pelvic, lower extremity, vascular, or additional genitourinary injuries (Bandi and Santucci, 2004; Kunkle et al, 2008). Excellent cosmetic and functional outcomes can be expected with immediate reconstruction (Gomez et al, 1993; Cavalcanti et al, 2006). An artificial erection may be induced to ensure penile straightness, and plication techniques may be used to correct any curvature resulting from closure of a large corporeal injury (Kunkle et al, 2008).
Urethral injuries have been reported in 15% to 50% of penile gunshot wounds (Miles et al, 1990; Goldman et al, 1996; Mohr et al, 2003). Retrograde urethrography should be strongly considered in any patient with penetrating injury to the penis, especially with high-velocity missile injuries, blood at the meatus, or difficulty voiding and when the trajectory of the bullet was near the urethra (Goldman et al, 1996; Mohr et al, 2003; Bandi and Santucci, 2004, Phonsombat et al, 2008). Alternatively, intraoperative retrograde urethral injection of methylene blue or indigo carmine may identify the site of injury and the adequacy of closure. If a catheter has already been placed, pericatheter injection may help to ascertain urethral integrity.
Urethral injuries due to penetrating trauma should be closed primarily by use of standard urethroplasty principles whenever possible—excellent results have been reported (Miles et al, 1990; Bandi and Santucci, 2004). Patients with urethral injury and extensive tissue damage from high-velocity weapons or close-range shotgun blasts may require staged repair and suprapubic urinary diversion (Bandi and Santucci, 2004), especially those located in the penile urethra (Cavalcanti et al, 2006).
Animal and Human Bites
The morbidity of animal bites is directly related to the severity of the initial wound. Most victims are boys, and dog bites are the most common injury (Gomes et al, 2001; Van der Horst et al, 2004). Infectious complications are unusual because treatment is sought early. Initial management of dog bites includes copious irrigation, debridement, and immediate primary closure along with prophylactic use of broad-spectrum antibiotic (Cummings and Boullier, 2000). Tetanus and rabies immunizations should be used as appropriate. Because of the risk of polymicrobial infection and the antimicrobial susceptibilities of typical organisms, recommended empiric antimicrobial therapy choices include beta lactam antibiotic with a beta lactamase inhibitor (i.e., amoxicillin-clavulinic acid); a second-generation cephalosporin with anaerobic efficacy (i.e., cefoxitin, cefotetan); or clindamycin with a fluoroquinolone (Talan et al, 1999).
Human bites produce contaminated wounds that often should not be closed primarily. Most human bite victims seek medical attention after a substantial delay and thus are more likely to present with gross infection. Empirical antibiotic administration is warranted with amoxicillin/clavulanic acid or moxifloxacin (Talan et al, 2003).
Amputation
Traumatic amputation of the penis, although rare, is usually the result of genital self-mutilation. Sixty-five to 87 percent of patients performing genital self-mutilation are psychotic (Greilsheimer and Groves, 1979; Aboseif et al, 1993; Romilly and Isaac, 1996). Psychiatric consultation should be sought in all such cases.
Reconstruction of the urethra and reanastomosis of the corporeal bodies with microsurgical repair of dorsal penile vessels and nerves achieves remarkably good results. Patients should be transferred to a facility with microsurgical capabilities; however, if this is unavailable, macroscopic anastomosis of the urethra and corporeal bodies can be performed with good erectile results, albeit with potential compromise of sensation and skin loss. Every attempt should be made to locate, clean, and preserve the severed portion in a “double bag” technique. The distal penis should be rinsed in saline solution, wrapped in saline-soaked gauze, and sealed in a sterile plastic bag. The bag should then be placed into an outer bag with ice or slush (Jezior et al, 2001). Hypothermic injury to the amputated segment can occur if it is in direct contact with ice for a prolonged period. Successful reimplantation is possible after 16 hours of cold ischemia time or 6 hours of warm ischemia (Lowe et al, 1991). If the severed part is not available, the penile stump should be formalized by closing the corpora and spatulating the urethral neomeatus, similar to a partial penectomy procedure for malignant disease.
Microvascular reconstruction of the dorsal arteries, vein, and nerves is the preferred method of repair for the amputated penis. Adequate erectile function is possible with both microvascular reanastomosis and macroscopic replantation, with more than 50% of men able to achieve erection with either technique (Bhanganada et al, 1983; Lowe et al, 1991; Aboseif et al, 1993). However, complications such as urethral strictures, skin loss, and sensory abnormalities are all less common with microvascular repair (Jezior et al, 2001).
Normal penile sensation returns in 0% to 10% of patients after macroscopic replantation (Bhanganada et al, 1983; Lowe et al, 1991), whereas sensation is present in more than 80% of microscopic replantations (Jordan and Gilbert, 1989; Lowe et al, 1991; Jezior et al, 2001). Penile skin necrosis, sometimes complete, is often a troublesome problem, although less common with microsurgical repair. This is because the blood supply of the skin is independent of the corporeal bodies and because without repair of the superficial vascular structures the penile skin is essentially a free graft (Jezior et al, 2001) Split-thickness skin grafts are applied when the native skin becomes necrotic (Ozturk et al, 2009). An alternative strategy is to denude the phallus of all skin and bury it in the scrotum, leaving the glans exposed, followed by separation of the structures after 2 months (Bhanganada et al, 1983; Jordan and Gilbert, 1989). Adjuvant techniques after penile replant include the use of hyperbaric oxygen to promote healing (Landström et al, 2004; Zhong et al, 2007) or medical leeches on the penis after macroreplantation to augment venous outflow and decrease edema (Mineo et al, 2004).
Key Points: Step-by-Step Approach to Penile Reattachment
Zipper Injuries
Zipper injuries to the penis usually happen more often to impatient boys or intoxicated adults. Multiple maneuvers are available to free the entrapped skin and to remove the mechanism. After a penile block the zipper slider and adjacent skin can be lubricated with mineral oil, followed by a single attempt to unzip and untangle the skin (Kanegaye and Schonfeld, 1993; Mydlo, 2000). The cloth material connected to the zipper can be incised with perpendicular cuts in between each zipper tooth to release the lateral support of the zipper, allowing the device to fall apart and release the trapped skin (Oosterlinck, 1981). A bone cutter or similar tool can be used to cut the median bar (diamond-shaped connection) of the slider. This maneuver allows separation of the upper and lower shields of the slider, and the entire zipper falls apart (Flowerdew et al, 1977; Saraf and Rabinowitz, 1982). Some children may require more than local anesthesia or sedation; circumcision or an elliptical skin excision can be performed in the operating room under anesthesia (Yip et al, 1989; Mydlo, 2000).
Strangulation Injuries
Accidental injuries with thread, hair, or rubber bands occur in children, but child abuse must be considered in such cases. Any child with unexplained penile swelling, erythema, or difficulty voiding should be examined closely for a hidden strangulating hair or string. Adults may place objects around the shaft as a means of sexual pleasure or to prolong an erection. The constricting device can reduce blood flow, cause edema, and induce ischemia; gangrene and urethral injury may develop in delayed presentations. Emergent treatment requires decompression of the constricted penis to allow blood flow and micturition. Depending on the constricting device, significant resourcefulness may be required of the physician.
String, hair, and rubber bands can be incised. Initial attempts to remove a solid constricting device causing penile strangulation involve lubrication of the shaft and foreign body and attempted direct removal. Edema distal to the strangulation often makes removal difficult. A string or latex tourniquet can be wrapped around the distal shaft to decrease swelling and to improve the odds of removing the device with lubrication. If the constricting object cannot be severed or removed, a string technique should be considered (Browning and Reed, 1969; Vahasarja et al, 1993; Noh et al, 2004). A thick silk suture or umbilical tape is passed proximally under the strangulation object and wound tightly around the penis distally toward the glans. The tag of suture or tape proximal to the ring is grasped; unwinding from the proximal end will push the object distally. Glanular puncture with a needle or blade will allow escape of dark trapped blood and improve the odds of removing the object with the string method (Browning and Reed, 1969; Noh et al, 2004).
Plastic constricting devices can be incised with a scalpel or an oscillating cast saw (Pannek and Martin, 2003), but metal objects present a more difficult challenge. Readily available hospital equipment (ring cutters, bolt cutters, dental drills, commercially available rotary tools, orthopedic and neurosurgical operative drills) may not be adequate to cut through heavy iron or steel items. The use of industrial drills, steel saws, hacksaws, saber saws, and high-speed electric drills has been reported (Perabo et al, 2002; Santucci et al, 2004). On occasion, fire department and emergency medical services equipment may be required to cut through iron and steel rings. The phallus should be protected from thermal injury, sparks, and the cutting blade by use of tongue depressors, sponges, or malleable retractors; continuous saline irrigation may be used for cooling. Such elaborate undertakings are best accomplished in the operating room under anesthesia. If decompression is delayed and the patient is distended and unable to void, a suprapubic bladder catheter should be placed. Outcomes are generally good with device removal alone, although the surgeon should be prepared to consider reconstructive techniques such as skin grafting if the strangulation injury causes skin necrosis (Ivanovski et al, 2007).
Testis
Etiology
Although the testis is relatively protected by the mobility of the scrotum, reflexive cremasteric muscle contraction, and its tough fibrous tunica albuginea, blunt injury (usually the result of assault, sports-related events, and motor vehicle accidents) can result in rupture of the tunica albuginea, contusion, hematoma, dislocation, or torsion of the testis. Testicular injury results from blunt trauma in about 75% of cases (McAninch et al, 1984; Cass and Luxenberg, 1991), whereas penetrating injuries due to firearms, explosions, or impalement make up the remainder.
Whereas only 1.5% of blunt testis injury involves both gonads, about 30% of penetrating scrotal trauma results in bilateral injury (Cass and Luxenberg, 1988, 1991). Like penetrating urethral injuries, penetrating scrotal trauma (roughly 80%) usually involves neighboring structures, including the thigh, penis, perineum, bladder, urethra, and/or femoral vessels (Gomez et al, 1993; Cline et al, 1998). In contemporary military conflicts, genital wounds account for a larger percentage of urologic injuries because of the powerful explosive weapons involved and absence of protective body armor over the genitalia (Thompson et al, 1998). Blast injuries are typically associated with extensive scrotal skin loss, multiple projectile injuries of both testes, and concomitant extensive destruction of the lower extremities and abdomen.
Diagnosis
Rupture of the testis must be considered in all cases of blunt scrotal trauma. Most patients complain of exquisite scrotal pain and nausea. Swelling and ecchymosis are variable, and the degree of hematoma may not correlate with the severity of testicular injury; absence does not entirely rule out testicular rupture, and contusion without fracture can present as significant bleeding. Scrotal hemorrhage and hematocele along with tenderness to palpation often limit a complete physical examination. Concomitant urethral injury should be suspected and evaluated when examination reveals blood at the meatus or if the mechanism of injury or hematuria suggests this possibility. Penetrating injuries mandate careful examination of surrounding structures, especially the femoral vessels.
Ultrasonography can be helpful to assess the integrity and vascularity of the testis in equivocal cases. Ultrasonography is rapid, readily available, and noninvasive. Because it may be operator dependent, false-positive and false-negative studies range from 56% to 94% (Fournier et al, 1989; Corrales et al, 1993; Herbener, 1996; Dreitlein et al, 2001). Ultrasound findings suggestive of testicular fracture include a heterogeneous echo pattern of the testicular parenchyma and disruption of the tunica albuginea (Micallef et al, 2001; Buckley and McAninch, 2006) (Fig. 88–4). Although ultrasonography may assist in detection of testicular fracture or hematoma (Guichard et al, 2008), a normal or equivocal ultrasound study should not delay surgical exploration when physical examination findings suggest testicular damage; definitive diagnosis is often made in the operating room. Although MRI may effectively demonstrate testicular integrity, its widespread use is not the norm because of expense, limited availability, and potential delay in definitive surgical care of the patient (Serra et al, 1998; Muglia et al, 2002; Kim et al, 2009).
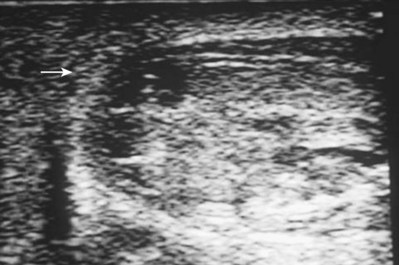
Figure 88–4 Ultrasound examination demonstrates hypoechoic intratesticular areas (arrow) consistent with testicular rupture sustained by blunt trauma. Scrotal exploration revealed large hematocele and exposed seminiferous tubules.
Differential diagnosis of testicular fracture includes hematocele without rupture, torsion of the testis or an appendage, reactive hydrocele, hematoma of the epididymis or spermatic cord, and intratesticular hematoma. A nonpalpable testis in a trauma patient should raise the possibility of dislocation outside the scrotum. This entity usually occurs after motorcycle crashes when extreme forces on the scrotum expel the testis into surrounding tissues such as the superficial inguinal pouch (50%) or to a pubic, penile, pelvic, abdominal, or perineal location (Schwartz and Faerber, 1994; Bromberg, 2003). Bilateral dislocation after trauma has been reported (Bromberg et al, 2003; O’Brien et al, 2004). Manual or surgical reduction of the displaced testis is indicated. Finally, approximately 5% of spermatic cord torsions are believed to be precipitated by trauma; torsion should be considered in all cases of significant scrotal pain without signs or symptoms of major scrotal trauma (Elsaharty et al, 1984; Manson, 1989; Lrhorfi et al, 2002).
Management
Early exploration and repair of testicular injury is associated with increased testicular salvage, reduced convalescence and disability, faster return to normal activities, and preservation of fertility and hormonal function (Kukadia et al, 1996). Minor scrotal injuries without testicular damage can be managed with ice, elevation, analgesics, and irrigation and closure in some circumstances.
The objectives of surgical exploration and repair are testicular salvage, prevention of infection, control of bleeding, and reduced convalescence. Transverse scrotal incision is preferable in most cases. The tunica albuginea should be closed with small absorbable sutures after removal of necrotic and extruded seminiferous tubules. Even small defects in the tunica albuginea should be closed, because progressive swelling and intratesticular pressure can continue to extrude seminiferous tubules. Every attempt to salvage the testis should be performed; loss of capsule tissue may require removal of additional parenchyma to allow closure of the remaining tunica albuginea. A flap or graft of tunica vaginalis may be used to cover a large defect in the tunica albuginea in an otherwise salvageable testis (Fig. 88–5); synthetic grafts are not recommended for this purpose (Ferguson and Brandes, 2007). Significant intratesticular hematomas should be explored and drained even in the absence of testicular rupture to prevent progressive pressure necrosis and atrophy, delayed exploration (40%), and orchiectomy (15%) (Cass and Luxenberg, 1988). Significant hematoceles should also be explored, regardless of imaging studies, because up to 80% are caused by testicular rupture (Vaccaro et al, 1986).
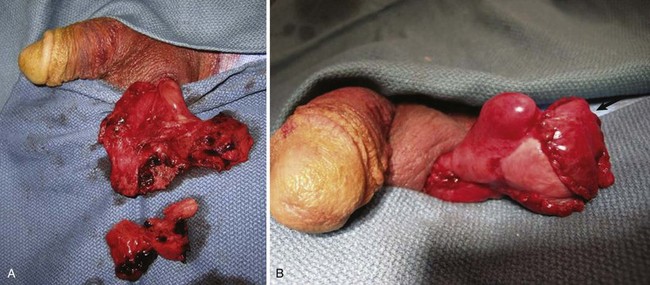
Figure 88–5 A, Testicular rupture after blunt trauma. B, Reconstructed testis after debridement and closure. Arrow indicates placement of tunica vaginalis graft.
Penetrating scrotal injuries should be surgically explored to inspect for vascular and vasal injury; as in blunt trauma the same principles of salvage, hemostasis, and reconstruction apply. The vas deferens is injured in 7% to 9% of scrotal gunshot wounds (Gomez et al, 1993; Brandes et al, 1995). The injured vas should be ligated with nonabsorbable suture and delayed reconstruction performed if necessary. Approximately 30% of gunshot wounds injure both testes—exploration of the contralateral testis should be considered, depending on the findings of physical examination and the path of the projectile.
Outcome and Complications
Nonoperative management of testicular rupture is frequently complicated by infection, atrophy, necrosis, chronic unrelenting pain, and delayed orchiectomy. Testicular salvage rates exceed 90% with exploration and repair within 3 days of injury (Del Villar et al, 1973; Schuster, 1982; Fournier et al, 1989; Cass and Luxenberg, 1991) versus orchiectomy rates threefold to eightfold higher with conservative management and delayed surgery (Cass and Luxenberg, 1991). Testicular salvage rates with conservative management are as low as 33%, with delayed orchiectomy rates between 21% and 55% (Schuster, 1982; Cass and Luxenberg, 1991; McAleer and Kaplan, 1995). Approximately 45% of patients initially managed conservatively will ultimately undergo surgical exploration for pain, infection, and persistent hematoma (Del Villar et al, 1973; Cass and Luxenberg, 1991). Convalescence and time of return to normal activities are significantly reduced after early surgical repair.
Unlike blunt testis rupture, for which salvage rates are very high, penetrating testicular trauma has historically been associated with gonad salvage in only 32% to 65% of cases (Bickel et al, 1990; Gomez et al, 1993; Brandes et al, 1995; Cline et al, 1998). Improved salvage rates as high as 75% have been reported both in recent civilian (Phonsombat et al, 2008) and combat series (Waxman et al, 2009). The overwhelming majority of surgical patients have adequate preservation of hormonal and fertility function (Kukadia et al, 1996). Sperm production has been documented in men with appropriately repaired bilateral testis rupture and bilateral penetrating injuries (Pohl et al, 1968; Brandes et al, 1995).
Urologists may be consulted for opinion and guidance with regard to boys with a solitary testis who play a contact sport. Fortunately, testicular injuries are exceedingly rare in boys involved in individual or team contact sports and recreational activities (McAleer et al, 2002; Wan 2003a, 2003b). Parents should be appropriately counseled and a protective cup device recommended. The American Academy of Pediatrics Committee on Sports Medicine and Fitness (2001) recommended that many factors be considered regarding whether to allow a child with a solitary testis to play sports; their recommendation was an unqualified yes in this circumstance.
Genital Skin Loss
Etiology
Necrotizing gangrene due to polymicrobial infection in the genital area, or Fournier gangrene, is the most common cause of extensive genital skin loss (McAninch et al, 1984). Skin loss is iatrogenic, caused by the necessity for acute debridement of necrotic genital skin when the patient is seen initially.
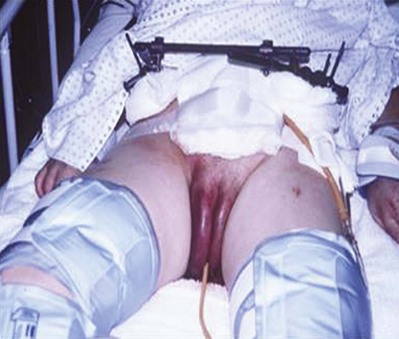
Penile skin loss can result from traction by mechanical devices, such as farm or industrial machinery, or by suction devices, such as vacuum cleaners. Because the superficial penile tissue is loose areolar tissue, it is often torn free without damage to the underlying structures. Significant scrotal skin loss resulting from penetrating trauma is uncommon, but it has been seen more recently in battlefield explosive injuries (Fig. 88–6).
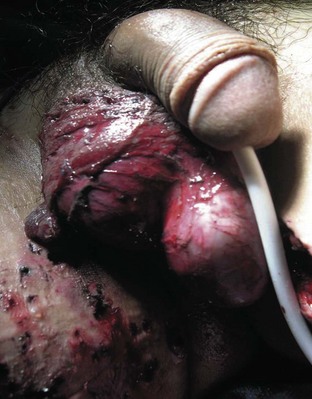
Figure 88–6 Extensive loss of scrotal skin from explosive blast injury. Note numerous concomitant shrapnel injuries to thigh.
Penile burns, although rare, are often full thickness because the penile skin is so thin (Horton, 1990). Constricting bands placed on the penis can result in significant skin loss, although a more common injury involves direct pressure necrosis under the band, which usually heals well with device removal alone.
Diagnosis and Initial Management
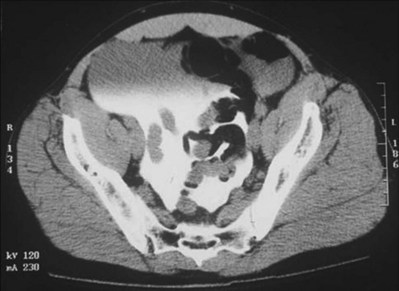
Although both cellulitis and Fournier gangrene are commonly associated with significant genital edema and erythema, skin ischemia is the hallmark of Fournier gangrene. The finding of loss of scrotal rugae is highly suggestive of tissue necrosis. Scrotal ultrasonography (Kane et al, 1996) and computed tomography (CT) may reveal subcutaneous air, a helpful indicator of necrotizing infection (Fig. 88–7).
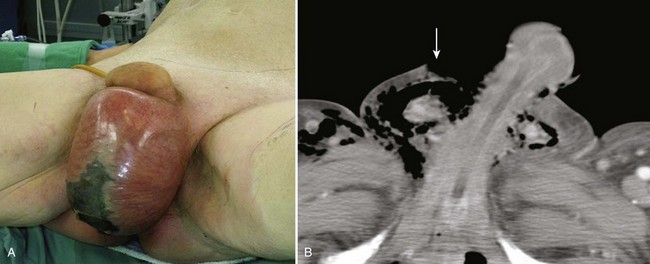
Figure 88–7 A, Large erythematous scrotum with central necrosis suggestive of necrotizing infection. B, CT reveals subcutaneous air in scrotum (arrow) secondary to Fournier gangrene.
In most cases of Fournier gangrene, multiple debridements of ischemic or frankly necrotic skin are required over a period of several days until active infection is controlled. Wounds are treated with frequent wet-to-dry dressing changes or with vacuum-assisted closure therapy (Cyzmek et al, 2009) until primary coverage is planned. Inspection at least daily by the surgical team is mandatory. Suprapubic urinary diversion should be strongly considered for extensive injuries to simplify wound care and to prevent urethral complications related to prolonged catheterization. Hyperbaric oxygen treatment has been advocated as an adjunctive measure to promote wound healing, although the authors do not recommend this owing to the considerable increase in expense and logistical complexity in the absence of proven benefit (Mindrup et al, 2005).
Genital burns are largely treated like other burns, with early resection of burn eschar and coverage with split-thickness skin grafts when possible. Partial-thickness skin loss or genital burns may be treated with silver sulfadiazine cream. For deep penile electrical burns, a conservative approach is warranted because the ultimate outcome will usually be autopenectomy and/or death due to extensive concurrent injuries.
Penile Reconstruction
In selected uncircumcised patients, mobilization of redundant foreskin may allow primary closure of middle to distal penile skin loss (Horton, 1990). Scrotal rotation flaps can be used for more proximal defects if skin loss is limited, but the hair-bearing nature of scrotal skin risks an unacceptable cosmetic result. Local flaps, such as from the abdomen and thigh, also can be used but are cosmetically inferior to split-thickness skin grafts. Skin coverage with avulsed skin should be avoided because it often becomes necrotic.
Thick (0.012- to 0.015-inch), nonmeshed, split-thickness skin grafts (McAninch et al, 1984) are preferred for penile reconstruction. Meshed grafts can be used but have a tendency to contract and are cosmetically inferior to unmeshed grafts. Grafts are usually harvested from the anterior thigh with a pneumatic dermatome. If grafts are to be used, care must be taken to remove any subcoronal skin remaining after debridement. Lymphatic obstruction of this distal foreskin, if it is not excised, will result in circumferential lymphedema (McDougal, 2003). Graft stabilization in the immediate postoperative period may be achieved by either tie-over-bolster technique or with a circumferential vacuum dressing (Weinfeld et al, 2005; Senchenkov et al, 2006). Skin grafts placed on the penile shaft never regain normal sensation (Horton, 1990), although sexual function is often preserved because of intact sensation in the glans.
Scrotal Reconstruction
Scrotal skin loss defects of up to 50% can often be closed directly. For extensive injuries, the testes may be placed in thigh pouches or treated with wet dressings for up to several weeks until reconstruction (Cummings and Boullier, 2000; Gomes et al, 2001). Thigh pouches are not recommended initially, until the infection is stabilized, because transmission of the infectious process into uninvolved tissues may occur. During scrotal reconstruction, local skin flaps should be mobilized initially to cover as much of the tissue defect as possible (Fig. 88–8 on the Expert Consult website).![]() Meshed split-thickness skin grafts can then be employed for reconstruction of the remainder. In addition to providing an excellent cosmetic result, meshing allows exudate to escape from the interstices, thus improving graft take. The spermatic cords and testes are sewn together in multiple areas before grafting to prevent a bifid neoscrotum. The neoscrotum may appear unnaturally tight initially, but after 6 to 12 months the testes eventually occupy a more natural dependent position. Thigh flaps can be used to reconstruct the scrotum when the testes have been buried in the thighs after traumatic or surgical scrotal removal (Morey and McAninch, 1999). Fibrin sealant has proved useful as a tissue glue to promote healing and to reduce drainage during complex genital reconstruction cases (Morris et al, 2006).
Meshed split-thickness skin grafts can then be employed for reconstruction of the remainder. In addition to providing an excellent cosmetic result, meshing allows exudate to escape from the interstices, thus improving graft take. The spermatic cords and testes are sewn together in multiple areas before grafting to prevent a bifid neoscrotum. The neoscrotum may appear unnaturally tight initially, but after 6 to 12 months the testes eventually occupy a more natural dependent position. Thigh flaps can be used to reconstruct the scrotum when the testes have been buried in the thighs after traumatic or surgical scrotal removal (Morey and McAninch, 1999). Fibrin sealant has proved useful as a tissue glue to promote healing and to reduce drainage during complex genital reconstruction cases (Morris et al, 2006).
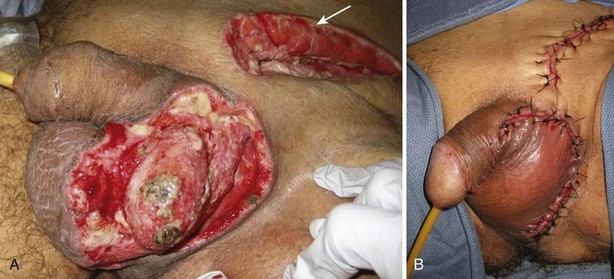
Figure 88–8 A, Scrotal defect before final debridement and closure of left hemiscrotum for Fournier gangrene. Arrow indicates inguinal counterincision made owing to extension of infection to inguinal canal. B, Delayed primary closure. The right testis and tunica vaginalis were mobilized off the overlying scrotal tissue, which was then used to close the scrotal defect.
Bladder Injuries
Etiology
The urinary bladder is generally protected from external trauma because of its deep location in the bony pelvis. Most blunt bladder injuries are the result of rapid-deceleration motor vehicle collisions, but many also occur with falls, crush injuries, assault, and blows to the lower abdomen. Disruption of the bony pelvis tends to tear the bladder at its fascial attachments, but bone fragments can also directly lacerate the organ. Other important causes of bladder rupture include penetrating trauma, iatrogenic surgical complications, and spontaneous rupture in patients with a history of neuropathic disease, pre-existing bladder disease, or prior urologic surgery.
Bladder injures that occur with blunt external trauma are rarely isolated injuries—80% to 94% of patients have significant associated nonurologic injuries (Cass, 1984; Volpe et al, 1999; Hsieh et al, 2002; Parry et al, 2003). Mortality in these patients with multiple injuries is usually related to nonurologic injuries and ranges from 8% to 44% (Carroll and McAninch, 1984; Cass and Luxenberg, 1987; Corriere and Sandler, 1989; Volpe et al, 1999; Alli et al, 2003; Parry et al, 2003). The most common associated injury is pelvic fracture, which is associated with 83% to 95% of bladder injuries (Cass, 1989; Corriere and Sandler, 1989; Morey et al, 2001; Parry et al, 2003). Conversely, bladder injury has been reported to occur in only 5% to 10% of pelvic fractures (Cass, 1989; Peters, 1989; Aihara et al, 2002). Sudden force applied to a full bladder may result in a rapid rise in intravesical pressures and lead to rupture without pelvic fracture.
Penetrating bladder trauma is also associated with significant nonurologic injuries and mortality rate. Nearly half of all bladder injuries are iatrogenic (Dobrowolski et al, 2002)—obstetric and gynecologic complications are the most common causes of bladder injuries during open surgery (Dobrowolski et al, 2002; Gomez et al, 2004).
Diagnosis
Extraperitoneal bladder injury is usually associated with pelvic fracture. Intraperitoneal injuries can be associated with pelvic fracture but are more commonly due to penetrating injuries or burst injuries at the dome by direct blow to a full bladder. Appropriate diagnostic imaging is important because of the marked influence on management.
Clinical Signs and Symptoms
Bladder rupture does not occur as an isolated, asymptomatic event in normal individuals. Conscious patients present with pronounced nonspecific symptoms such as suprapubic pain combined with inability to void. Physical signs include suprapubic tenderness, lower abdominal bruising, muscle guarding and rigidity, and diminished bowel sounds. Associated abdominal and pelvic injuries may mask bladder symptoms, and those who are unresponsive due to intoxication or altered sensorium similarly warrant higher suspicion for bladder injury.
Immediate catheterization should be performed when blunt bladder rupture is suspected because the most reliable indicator is gross hematuria, which is present in nearly all cases (Iverson and Morey, 2001; Hsieh et al, 2002; Parry et al, 2003; Gomez et al, 2004). If blood is noted at the meatus or the catheter does not pass easily, retrograde urethrography should be performed first because urethral injuries occur concomitantly in 10% to 29% of patients with bladder rupture (Cass, 1989; Dobrowolski et al, 2002).
Radiographic Imaging
Imaging of the bladder is performed on the basis of clinical suspicion. After blunt external trauma the absolute indication for immediate cystography is gross hematuria associated with pelvic fracture—approximately 29% of patients presenting with this combination of findings have bladder rupture (Morey et al, 2001). Relative indications for cystography after blunt trauma include gross hematuria without pelvic fracture and microhematuria with pelvic fracture. The diagnosis of bladder rupture is extremely low in these atypical groups (e.g., 0.6% in patients with pelvic fracture and microhematuria), but the index of suspicion should be raised by associated clinical indicators of bladder injury. Conversely, penetrating injuries of the buttock, pelvis, or lower abdomen with any degree of hematuria warrant cystography.
Key Points: Clinical Indicators of Bladder Injury
Retrograde or stress cystography is nearly 100% accurate for bladder injury if performed appropriately. The bladder should be filled in cooperative and conscious patients to a sense of discomfort, otherwise to 350 mL. For a plain film technique, three images are obtained: one before administration of a contrast agent, one full-bladder anteroposterior film, and one drainage film. Posterior extravasation of the contrast medium can be missed without a drainage film. Significant bladder distention is required to visualize small tears. False-negative studies have been reported with retrograde instillation of only 250 mL (Peters, 1989; Morey and Carroll, 1997). Although hematuria and mechanism of injury mandate consideration of upper tract imaging studies, upper and lower urinary tract injuries are almost never coincident (0.4%) (Cass and Luxenberg, 1990).
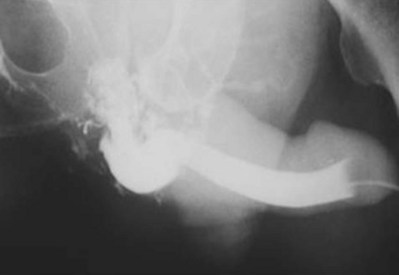
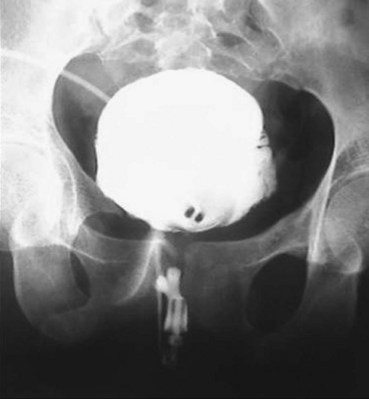
A dense, flame-shaped collection of contrast material in the pelvis is characteristic of extraperitoneal extravasation (Fig. 88–9). Depending on fascial integrity, contrast material may extend beyond the confines of the pelvis and be visualized in the retroperitoneum, scrotum, phallus, thigh, and/or abdominal wall. The amount of extravasation is not always proportional to the extent of bladder injury. Intraperitoneal extravasation is identified when contrast material outlines loops of bowel and/or the lower lateral portion of the peritoneal cavity.
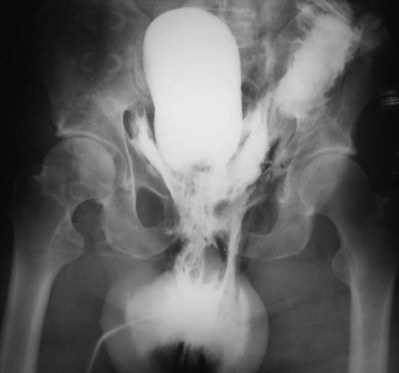
Figure 88–9 Plain film cystogram reveals extraperitoneal bladder rupture with extravasation into scrotum. Surgical exploration revealed anterior bladder neck and prostatic urethral laceration.
Because CT is now routinely used to assess trauma patients, concomitant CT cystography is now frequently selected as a more efficient means to assess the bladder. CT cystography is as accurate and reliable as plain film cystography to evaluate suspected bladder injury (Fig. 88–10), as long as the bladder is filled in retrograde fashion with contrast material diluted to 2% to 4% (6 : 1 with saline) to a volume of 350 mL (Peng et al, 1999; Hsieh et al, 2002). Drainage films are not required after CT cystography because the retrovesical space can be well visualized with axial images (Morey and Carroll, 1997). Dilution of the contrast material is mandatory because undiluted contrast material is so dense that the CT quality is compromised by scatter artifact. Clamping the urethral catheter in an attempt to allow antegrade distention of the bladder by intravenous contrast medium is inadequate for diagnosis of bladder rupture—retrograde filling is required. Conventional abdominal CT of the trauma patient may show findings suggestive of bladder injury but is not considered to be adequate for bladder evaluation without retrograde contrast distention (Mee et al, 1987; Udekwu et al, 1996; Hsieh et al, 2002).
Management
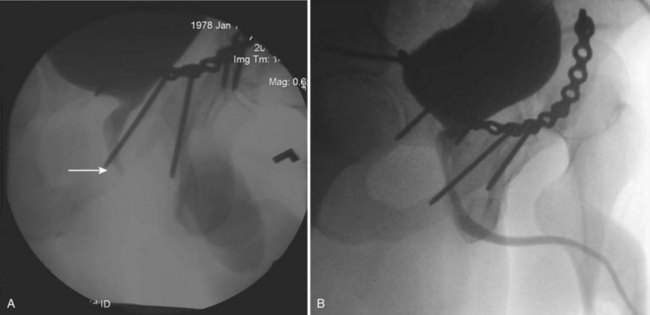
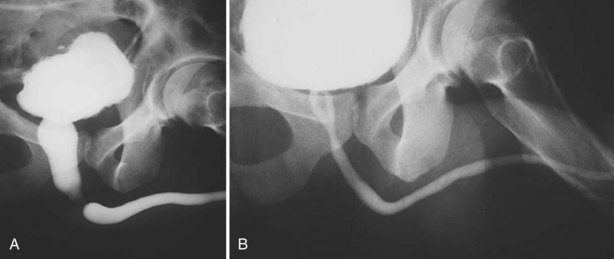
The usual treatment of uncomplicated extraperitoneal bladder ruptures, when conditions are ideal, is conservative management with urethral catheter drainage alone (Fig. 88–11). A large-bore (22 Fr) Foley catheter should be used to promote adequate drainage—if output is poor, fluoroscopic cystography should be considered to ensure proper catheter placement. Cystography is necessary to verify complete healing before catheter removal 14 days after injury; occasionally, extravasation may persist for several additional weeks but will resolve with continuation of urethral catheter drainage, after which radiographic confirmation of healing is essential. Bone spicules within the bladder wall (Fig. 88–12 on the Expert Consult website)![]() may compromise healing. Antimicrobial agents are instituted on the day of injury and continued for at least 1 week to prevent infection of the pelvic hematoma.
may compromise healing. Antimicrobial agents are instituted on the day of injury and continued for at least 1 week to prevent infection of the pelvic hematoma.
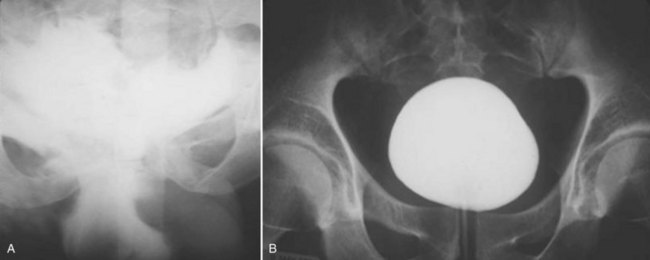
Figure 88–11 A, Dense flame-shaped pattern of contrast extravasation in pelvis due to extraperitoneal bladder rupture. B, Repeated cystogram in same patient after 2 weeks of catheter drainage shows completely healed bladder.
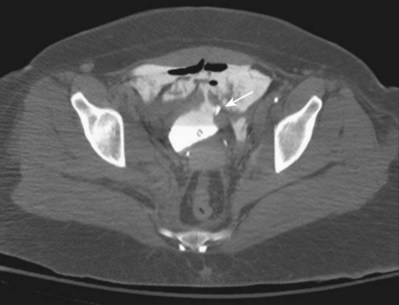
Figure 88–12 CT cystogram of patient with extraperitoneal bladder rupture after motor vehicle/pedestrian collision and extensive pelvic fracture. Arrow indicates fragment of bone in the bladder, removed at time of laparotomy and repair of bladder.
Several authors (Cass, 1989; Kotkin and Koch, 1995) have reported fewer complications, such as fistula, failure to heal, clot retention, and sepsis, with open repair (5% overall) versus conservative management (12% overall). For this reason, blunt extraperitoneal injuries warrant immediate open repair to prevent complications such as fistula, abscess, and prolonged leak in the presence of any complicating features. If a patient whose condition is stable is undergoing exploratory laparotomy for other associated injuries or internal fixation of pelvic fracture, it is prudent to surgically repair the extraperitoneal rupture at the same setting. The anterior bladder wall is entered, and the tear is closed intravesically with absorbable suture. The perivesical pelvic hematoma should not be disturbed. When internal fixation of pelvic fractures is performed, concomitant bladder repair is recommended because urine leakage from the injured bladder onto the orthopedic fixative hardware is prevented, thereby reducing the risk of hardware infection. Drainage of the repaired bladder can be safely accomplished with a large-bore Foley catheter alone, and cystography performed 1 week after repair should verify bladder healing.
Key Points: Indications for Immediate Repair of Bladder Injury
All penetrating or intraperitoneal injuries resulting from external trauma should be managed by immediate operative repair. These injuries are often larger than suggested on cystography and are unlikely to heal spontaneously, and continued leak of urine causes a chemical peritonitis. Although most injuries are repaired with open surgery, select patients may undergo laparoscopic repair (Kim et al, 2008)—primarily those whose bladder may have been injured during laparoscopic surgical procedures. When bladder injuries are explored after penetrating trauma without preliminary imaging, the ureteral orifices should be inspected for clear efflux; ureteral integrity may also be ensured by intravenous administration of indigo carmine or methylene blue or retrograde passage of a ureteral catheter. Any penetrating injury involving the ureteral orifice or intramural ureter warrants primary closure with stented reimplantation of the ureter. A perivesical drain should be employed. In patients with intraperitoneal rupture, antimicrobial agents are administered for 3 days, in the perioperative period only. If the bladder has been repaired, a cystogram is obtained 7 to 10 days after surgery (Corriere and Sandler, 1989). Several studies have now shown that suprapubic tube drainage provides no benefit over urethral catheter drainage alone (Volpe et al, 1999; Alli et al, 2003; Parry et al, 2003), although maximal urinary drainage using both is recommended when complex injuries are encountered. When concurrent rectal or vaginal injuries exist, the organ walls should be separated, overlapping suture lines avoided, and every attempt made to interpose viable tissue in between the repaired structures. Fibrin sealant injected over the bladder wall closure may help reduce complications when intervening tissue is unavailable (Evans et al, 2003).
Outcomes and Complications
Prompt diagnosis and appropriate management of bladder injuries promotes excellent results and minimal morbidity. Serious complications are usually associated with delayed diagnosis or treatment due to misdiagnosis, delayed presentation, or complex injuries resulting from devastating pelvic trauma. Unrecognized bladder injuries may manifest as acidosis, azotemia, fever and sepsis, low urine output, peritonitis, ileus, urinary ascites, or respiratory difficulties. Unrecognized bladder neck, vaginal, and rectal injury associated with the bladder rupture can result in incontinence, fistula, stricture, and difficult delayed major reconstruction. Severe pelvic fractures may cause a transient or permanent neurologic injury and result in voiding difficulties despite an adequate bladder repair.
Urethral Injuries
Posterior Urethral Injuries
Etiology
Urethral disruption injuries typically occur in conjunction with multisystem trauma from vehicular accidents, falls, or industrial accidents. Fracture of the anterior pelvic ring or pubic diastasis are almost always present when urethral disruption is encountered, and a greater degree of displacement has been correlated to a higher risk of urethral injury (Basta et al, 2007). “Straddle fractures” (Fig. 88–13 on the Expert Consult website)![]() involving all four pubic rami and fractures resulting in both vertical and rotational pelvic instability are associated with the highest risk of urologic injury (Mundy, 1996; Koraitim, 1999; Brandes and Borelli, 2001). Urethral injury has been reported to occur in approximately 10% of males and up to 6% of females sustaining pelvic fractures (Koraitim et al, 1999; Black et al, 2006). Girls younger than age 17 years have a higher risk of urethral injury compared with women, perhaps owing to greater compressibility of the pelvic bones (Hemal, 1999).
involving all four pubic rami and fractures resulting in both vertical and rotational pelvic instability are associated with the highest risk of urologic injury (Mundy, 1996; Koraitim, 1999; Brandes and Borelli, 2001). Urethral injury has been reported to occur in approximately 10% of males and up to 6% of females sustaining pelvic fractures (Koraitim et al, 1999; Black et al, 2006). Girls younger than age 17 years have a higher risk of urethral injury compared with women, perhaps owing to greater compressibility of the pelvic bones (Hemal, 1999).
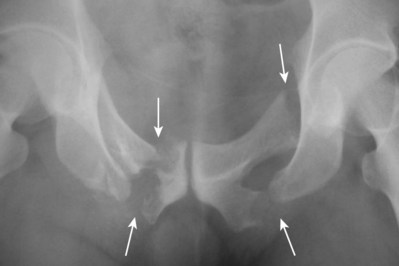
Figure 88–13 Anteroposterior film of the pubis shows a straddle fracture involving all four pubic rami (arrows) in a patient with a posterior urethral disruption in whom initial perineal posterior urethroplasty failed because of severe bone distortion. Reconstruction was successfully performed by an abdominoperineal technique.
Because the posterior urethra is densely adherent to the pubis via both the urogenital diaphragm and the puboprostatic ligaments, the bulbomembranous junction is more vulnerable to injury during pelvic fracture than is the prostatomembranous junction (Colapinto and McCallum, 1977; Brandes and Borelli, 2001). Endoscopic and urodynamic evaluation has confirmed that the membranous urethral sphincter complex tends to remain functionally intact while being avulsed vertically, posteriorly, and/or laterally from the underlying bulb (Mundy, 1997; Andrich and Mundy, 2001). In children, injuries are more likely to extend proximally to the bladder neck because of the rudimentary nature of the prostate (Devine et al, 1989; Al-Rifaei et al, 1991; Boone et al, 1992).
Diagnosis
Examination
Urethral disruption is heralded by the triad of blood at the meatus, inability to urinate, and palpably full bladder. Because these and other classic findings such as a “high-riding” prostate or a “butterfly” perineal hematoma may frequently be absent (Sandler and Corriere, 1989), urethral disruption is often first detected when a urethral catheter cannot be placed by the emergency department trauma team or when it is misplaced into a pelvic hematoma. Pelvic hematoma often obscures the prostatic contour, resulting in a misdiagnosis of impalpable prostate (Koraitim et al, 1996). Females may also develop proximal urethral avulsion injuries, although much more rarely than males; they present with vulvar edema (Fig. 88–14) and blood at the vaginal introitus, thus indicating the need for careful vaginal examination in all female patients with pelvic fracture (Perry and Husmann, 1992).
Urethrography
When blood at the urethral meatus is discovered, an immediate retrograde urethrogram should be performed to rule out urethral injury (Fig. 88–15). A small-bore urethral catheter (16 Fr) is placed unlubricated 1 cm into the fossa navicularis, and the balloon is filled with 1 cm of water to achieve a snug fit (Sandler and Corriere, 1989). Alternatively, a Brodney clamp or rolled gauze bandage can be used to provide penile traction. Patients should be placed in an oblique or lateral decubitus position, and it is preferable to perform the study under fluorography when it is available; 25 mL of contrast medium is injected gently by a 60-mL catheter-tip syringe, and the film is taken during injection. Direct inspection by urethroscopy is suggested in lieu of urethrography in females with suspected urethral injury (Perry and Husmann, 1992; Koraitim, 1999).
Initial Management
Immediate Open Reconstruction
Immediate anastomotic reconstruction of posterior urethral disruption injuries in men has been abandoned because of its association with unsatisfactory outcomes, such as impotence and incontinence, stricture formation, and operative blood loss (Webster et al, 1983; Koraitim, 1996). In cases of female urethral disruption related to pelvic fracture, most authorities suggest immediate primary repair, or at least urethral realignment over a catheter, to avoid subsequent urethrovaginal fistulas or urethral obliteration (Fig. 88–16) (Koraitim et al, 1996; Dorairajan et al, 2004, Black et al, 2006). Concomitant vaginal lacerations also must be closed acutely to prevent vaginal stenosis. Delayed reconstruction is problematic because the female urethra is too short (about 4 cm) to be amenable for mobilization during an anastomotic repair when it becomes embedded in scar (Podesta, 2001); however, the authors have found that a suprapubic approach with partial pubectomy provides excellent exposure enabling female bladder neck reconstruction.
Suprapubic Cystostomy
Immediate suprapubic tube placement remains the standard of care in men with posterior urethral injuries. This may be accomplished through a small infraumbilical incision, which allows inspection and repair of the bladder and proper placement of a large-bore catheter at the bladder dome. Trocar suprapubic tube placement is both safe and expedient when the bladder is obviously distended and no other indications for surgery exist; over the long term, however, smaller “punch” suprapubic tubes are less robust, tending to fracture or become obstructed with debris and often requiring urgent replacement.
Increasingly, patients with pelvic ring fracture undergo early surgical fixation by orthopedists to decrease bleeding, improve healing, and speed ambulation (Connor et al, 2003). Although orthopedists frequently suggest that a suprapubic tube not be placed if anterior pubic hardware is used in pelvic fracture repair because of concern that the suprapubic tube will lead to hardware infection (Patterson, 1995), the authors and others (Borrelli and Brandes, 2004; Bepple et al, 2007) have repeatedly found that suprapubic cystostomy can safely be used without complications throughout the course of care (Fig. 88–17 on the Expert Consult website).![]() The authors use a large-bore (24 Fr) Foley catheter placed high in the bladder and tunneled through the skin as high as possible on the lower abdominal midline to keep the tube away from the plated symphysis.
The authors use a large-bore (24 Fr) Foley catheter placed high in the bladder and tunneled through the skin as high as possible on the lower abdominal midline to keep the tube away from the plated symphysis.
Primary Realignment
An attempt at primary realignment of the distraction with a urethral catheter is reasonable in patients whose condition is stable (Elliott and Barrett, 1997), either acutely or within several days of injury. The authors prefer a simple technique consisting of passage of a coudé catheter antegrade from an anterior cystotomy to the urethral meatus, then tying it to another catheter that is drawn back into the bladder. A variety of more elaborate approaches have been described, frequently with retrograde and anterograde flexible cystoscopes (Follis et al, 1992; Routt et al, 1996; Elliott and Barrett, 1997; Porter et al, 1997; Asci et al, 1999; Mouraviev et al, 2005, Hadjizacharia et al, 2008), although the authors have observed that prolonged endoscopic realignment attempts risk infection of the pelvic hematoma (Morey et al, 1999) and thus cannot be recommended.
When the urethral catheter is removed after 4 to 6 weeks, it is imperative to retain a suprapubic catheter because many patients will, despite realignment, develop posterior urethral stenosis. If the patient voids satisfactorily through the urethra, the suprapubic catheter can be removed 7 to 14 days later. Primary realignment may sometimes allow healing without stricture (Elliott and Barrett, 1997), but mild stenosis 1 to 2 cm in length develops in many patients (Kotkin and Koch, 1996; Routt et al, 1996; Asci et al, 1999). Patients managed with suprapubic tubes alone virtually always develop complete stenosis requiring posterior urethroplasty (Kotkin and Koch, 1996; Mouraviev et al, 2005). Although realignment may not always prevent symptomatic stenosis, it may allow resultant strictures to be managed endoscopically or ease the difficulty of open posterior urethroplasty by bringing the prostate and urethra into alignment (Mouraviev et al, 2005; Hadjizacharia et al, 2008).
Incomplete urethral tears are best treated by stenting with a urethral catheter. The authors and others (Al-Ali and Husain, 1983; Mundy, 1991; Kotkin and Koch, 1996) have not seen any evidence that a gentle attempt to place a urethral catheter can convert an incomplete into a complete transection. Caution is warranted because misplacement outside the bladder is distinctly possible; radiographic confirmation of adequate positioning is imperative. In no case is traction used after urethral catheter placement; it is unnecessary and may cause incontinence (Asci et al, 1999).
Complex Injuries
Some authors advocate open exploration with realignment in cases of high-riding or “pie-in-the-sky” bladder or associated bladder neck tear in males (Webster et al, 1983). Associated rectal injuries require open exploration, repair, irrigation, and placement of drains.
Delayed Reconstruction
In posterior urethral disruption the rupture defect between the two severed ends fills with scar tissue, resulting in a complete lack of urethral continuity. This separation is not a stricture; it is a true urethral rupture defect filled with fibrosis. At 3 months, scar tissue at the urethral disruption site is stable enough to allow posterior urethroplasty to be undertaken safely, provided that associated injuries are stabilized and the patient is ambulatory. Suprapubic cystostomy drainage should be maintained until the associated injuries have healed and the patient can be appropriately positioned for the reconstructive procedure.
Preoperative Evaluation
Before the reconstructive procedure is planned, imaging studies are necessary to delineate the characteristics of the urethral rupture defect. A cystogram and retrograde urethrogram should be obtained simultaneously (“up-and-down-o-gram,” Fig. 88–18). The patient is asked to attempt to void with the bladder filled. Ideally, the prostatic urethra should be visualized as the bladder neck opens, enabling measurement of the distance between the severed urethral ends. Should the bladder neck not open, flexible endoscopy should be used to supplement radiographic imaging (Mundy, 1997). The appearance of the bladder neck on preoperative imaging, either open or closed, does not correlate well with bladder neck behavior postoperatively (Mundy, 1997), thus making it difficult to predict bladder neck incompetence or obstruction. MRI has been used successfully to define defect length and to determine the extent and direction of urethral dislocation (Dixon et al, 1992) and the extent of prostatic displacement, and it may help in planning the surgical approach (Koraitim and Reda, 2007).
Endoscopic Treatments
Endoscopic treatments such as direct-vision internal urethrotomy are best reserved for selected short urethral stenoses, such as partial distraction injuries for which early catheterization achieved urethral continuity. In most cases, when preoperative evaluation indicates defects 1 cm or longer, endoscopic procedures such as cutting through the pelvic scar to provide a channel between the two ends of the avulsed urethra (“cut-to-the-light” procedure) are ineffective and have no advantage other than reduced operative time (Levine and Wessels, 2001); moreover, aggressive endoscopic treatments have been associated with complications such as coring of a false passage that inadvertently bypasses the bladder neck (Turner-Warwick, 1989). Cut-to-the-light or similar core-through procedures typically require multiple urethrotomies or long-term dilation by the patient or urologist to keep the channel open. Inevitably, the fibrosis will contract, leading to difficult self-catheterization, false passage, and/or acute urinary retention. In such cases, a 3-month period of “urethral rest” via suprapubic urinary diversion is advised before open reconstruction.
Surgical Reconstruction
Open posterior urethroplasty through a perineal anastomotic approach is the treatment of choice for most urethral distraction injuries because it definitively cures the patient without the need for multiple procedures. Once preoperative studies have determined that the prostatic urethral apex can be reached by a perineal approach, the patient is placed in the high lithotomy position and a midline or lambda-shaped incision is made. The bulbar urethra is freed and mobilized from the site of urethral rupture to the midscrotum. The scar tissue of the urethral rupture defect is excised, and the prostatic urethra is identified at the apex of the prostate. Care must be taken to carefully and meticulously excise all fibrotic tissue from the proximal urethral margin until at least a 28-Fr bougie passes without resistance (Fig. 88–19; Turner-Warwick, 1989; Morey and McAninch, 1997). The bulbar urethra is then anastomosed in a tension-free manner to the prostatic urethra.
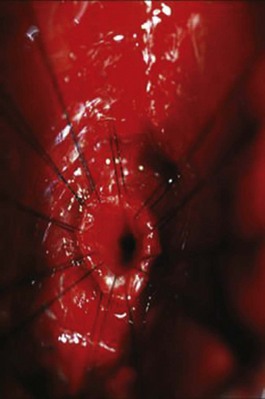
Figure 88–19 Intraoperative view of normal membranous urethra after fibrotic tissue was excised during perineal bulbomembranous urethroplasty.
In 95% of patients, posterior urethral anastomosis will be successfully achieved through a perineal approach alone (Carr and Webster, 1997). Adjunctive maneuvers such as corporeal separation, inferior pubectomy, and corporeal rerouting have been routinely implemented if direct anastomosis proves difficult (Mundy, 1997; Flynn et al, 2003). Others have found these maneuvers, especially supracrural rerouting, to be unnecessary and/or unhelpful (Morey and McAninch, 1997; Rosenstein and Jordan, 2003; Cooperberg et al, 2007; Kizer et al, 2007).
Total removal of the symphysis pubis, first reported by Pierce in 1962, has been recommended when severe injuries result in complicating features such as fistula or marked displacement or retropubic fixation of the prostate (Netto, 1985; McAninch, 1989; Asci et al, 1999). Alternatively, a combined abdominoperineal approach (with or without partial pubectomy) has proved helpful in cases of severe fibrosis, fistula, previous failed anastomotic urethroplasty, and associated bladder neck injury and in pediatric cases (Waterhouse, 1976; Al-Rifaei et al, 1991; Koraitim, 2003, 2005; Pratap et al, 2006). It is important to limit the lithotomy time to 5 hours or less to prevent lower extremity complications (Anema et al, 2000) when any complex urethral reconstruction is undertaken.
Complications
Erectile Dysfunction
Some degree of impotence is noted in up to 82% of patients with pelvic fracture and urethral distraction injury (Flynn et al, 2003), although the average reported rate is approximately 50% (Corriere et al, 1994; Routt et al, 1996; Elliott and Barrett, 1997; Asci et al, 1999; Koraitim, 2005). The etiology is multifactorial and variably attributed to cavernous nerve injury, arterial insufficiency, venous leak, and direct corporeal injury (Narumi et al, 1993; Munarriz et al, 1995; Shenfeld et al, 2003).
Some surgical series have shown a small number of patients with new-onset or worsened erectile dysfunction after reconstruction (Tunc et al, 2000); however, the complications of the original pelvic injury are difficult to differentiate from the complications of attempts to repair urethral and bladder injuries. Accounts of greater risk of erectile dysfunction and incontinence with primary realignment are prior to the advent of flexible endoscopes (Koraitim, 1996); Several studies have shown that patients treated with modern techniques of primary endoscopic realignment have rates of impotence and incontinence similar to those of patients who have had either no treatment or delayed open reconstruction (Kotkin and Koch, 1996; Asci et al, 1999; Koraitim, 2005; Mouraviev et al, 2005). These studies support the conclusion that these complications are usually the result of the injury itself and not of the treatment (Follis et al, 1992; Elliott and Barrett, 1997; Porter et al, 1997; Corriere, 2001). Some patients who become impotent after injury spontaneously recover erectile function 1 or 2 years later (Turner-Warwick, 1989; Morey and McAninch, 1997; Koraitim, 2005).
Many patients who become impotent as a result of pelvic fracture have some degree of arterial insufficiency (Armenakas, 1993; Matthews, 1995). Because impotent patients may be more vulnerable to restenosis after posterior urethroplasty as a result of bulbar urethral ischemia, some experts have suggested that “at-risk” patients undergo preoperative penile arterial duplex Doppler studies to identify candidates suitable for initial penile revascularization (Matthews et al, 1995; Rosenstein and Jordan, 2003). Indications for penile revascularization as a treatment for post-traumatic erectile dysfunction are extremely limited, however (Kawanishi et al, 2004). Overall rates of incontinence, anejaculation, and areflexic bladder are low (2% to 4%) (Corriere et al, 1994; Elliott and Barrett, 1997; Asci et al, 1999; Anger et al, 2008) and also tend to be secondary to the original injury.
Recurrent Stenosis
After posterior urethroplasty, 5% to 15% of patients have recurrent stenosis at the anastomosis (Mundy, 1996; Flynn et al, 2003; Koraitim 2005; Cooperberg et al, 2007). Fortunately, endoscopic treatment (e.g., with direct-vision internal urethrotomy) is often successful in this setting because the majority of fibrotic tissue has been eliminated (Netto et al, 1989).
Incontinence
Urinary continence after urethral distraction is the rule rather than the exception, despite destruction of the external sphincter from either the injury itself or the subsequent repair. Incontinence rates after reconstruction are surprisingly low, less than 4% (Koraitim, 2005). The mechanism of continence is thought to be largely attributable to bladder neck function (Iselin and Webster, 1999). Recent urodynamic data show that a significant proportion of patients also have identifiable distal rhabdosphincteric function (Whitson et al, 2008).
Anterior Urethral Injuries
In contrast to posterior urethral distraction, anterior injuries are most often isolated (Kiracofe et al, 1975). The majority occur after straddle injury and involve the bulbar urethra, which is susceptible to compressive injury because of its fixed location beneath the pubis. A smaller percentage of injuries to the anterior urethra are the result of direct penetrating injury to the penis.
As with posterior urethral injury, a high index of suspicion must be maintained in all patients with blunt or penetrating trauma in the urogenital region, and urethrography should be performed in any case of suspected urethral injury (Husmann et al, 1993). Clinical signs of anterior urethral injuries include blood at the meatus, perineal hematoma, gross hematuria, and urinary retention. In severe trauma the Buck fascia may be disrupted, resulting in blood and urinary extravasation into the scrotum (Fig. 88–20 on the Expert Consult website).![]() The primary morbidity of straddle injury is urethral stricture, which may become symptomatic even years later (Park and McAninch, 2004).
The primary morbidity of straddle injury is urethral stricture, which may become symptomatic even years later (Park and McAninch, 2004).
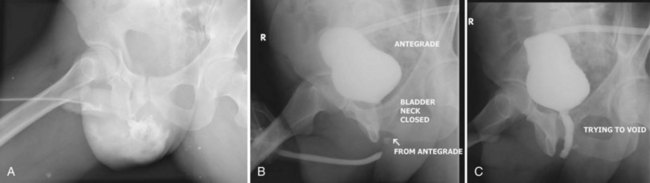
Figure 88–20 A, Extravasation of contrast medium seen in scrotum on retrograde urethrogram in a patient with a straddle injury. B, Retrograde and antegrade urethrogram shows distal limit of urethral patency, but bladder neck does not open. C, Repeat antegrade urogram study showing proximal limit of urethral patency.
Initial Management
Armenakas and McAninch (1996) proposed a simple, practical classification scheme dividing anterior urethral injuries on the basis of radiographic findings into contusion, incomplete disruption, and complete disruption. Contusions and incomplete injuries can be treated with urethral catheter diversion alone. Initial suprapubic cystostomy is the standard of care for major straddle injuries involving the urethra (Park and McAninch, 2004); however, primary anterior urethral realignment has shown promising results with respect to stricture rate and erectile dysfunction in patients with straddle injuries of lesser magnitude (Ying-Hao et al, 2000; Yu et al, 2007).
Primary surgical repair is recommended for low-velocity urethral gunshot injuries (Kunkle et al, 2008)—catheter alignment alone is associated with a far worse stricture rate (Husmann et al, 1993). Debridement of the corpus spongiosum after trauma should be limited because corporeal blood supply is usually robust, enabling spontaneous healing of most contused areas (Kiracofe et al, 1975; Husmann et al, 1993). Initial suprapubic urinary diversion is recommended after high-velocity gunshot wounds to the urethra, followed by delayed reconstruction.
Delayed Reconstruction
Before any planned procedure, a retrograde urethrogram and voiding cystourethrogram should be obtained to define the site and length of the obliterated urethra clearly. Urethral ultrasound examination may help delineate the length and severity of stricture. Retrograde injection of saline combined with antegrade bladder filling will fill the urethra proximally and distally, and a 10-MHz sonogram will clearly define the nondistensible segment to be excised. Dense fibrous tissue from trauma often demonstrates a fixed, nondistensible appearance sonographically with significant shadowing (Morey and McAninch, 2000). Ultrasound imaging of urethral strictures may be more accurate than retrograde urethrography (Mitterberger et al, 2007), although it is highly operator dependent and currently not widely used.
Anastomotic urethroplasty is the procedure of choice in the totally obliterated bulbar urethra after a straddle injury. The typical scar is 1.5 to 2 cm long and can readily be completely excised. The proximal and distal urethra can be mobilized for a tension-free, end-to-end anastomosis. This is a highly successful procedure in more than 95% of cases (Santucci et al, 2002).
Endoscopic incision through the scar tissue of an obliterated urethra is a hopeless procedure doomed to failure. Partial urethral narrowing can initially be treated by endoscopic incision or dilation with higher success. It is now recognized that repeated endoscopic manipulation is neither clinically effective nor cost effective for the treatment of urethral strictures (Greenwell et al, 2004). Patients who undergo repeated endoscopic procedures are also more likely to require complex reconstructive procedures such as grafts (Park and McAninch, 2004). Open repair should be delayed for several weeks after instrumentation to allow the urethra to stabilize, and a 2-month period of suprapubic urinary diversion may be prudent preoperatively to optimize conditions for repair of complex or recurrent strictures that have been catheter dependent. Finally, UroLume stents are contraindicated in the setting of traumatic urethral strictures (Wilson et al, 2002).
Andrich DE, Mundy AR. The nature of urethral injury in cases of pelvic fracture urethral trauma. J Urol. 2001;165:1492-1495.
Black PC, Miller EA, Porter JR, Wessells H. Urethral and bladder neck injury associated with pelvic fracture in 25 female patients. J Urol. 2006;175:2140-2145.
Buckley JC, McAninch JW. Use of ultrasonography for the diagnosis of testicular injuries in blunt scrotal trauma. J Urol. 2006;175:175-178.
Cooperberg MR, McAninch JW, Alsikafi NF, Elliot SP. Urethral reconstruction for traumatic posterior urethral disruption: outcomes of a 25-year experience. J Urol. 2007;178:2006-2010.
Morey AF, Iverson AJ, Swan A, et al. Bladder rupture after blunt trauma: guidelines for diagnostic imaging. J Trauma. 2001;51:683-686.
Morey AF, Metro MJ, Carney KJ, et al. Consensus on genitourinary trauma. BJU Int. 2004;94:507-515.
Penis, Testis, and Genital Skin Loss
Aboseif S, Gomez R, McAninch JW. Genital self-mutilation. J Urol. 1993;150:1143-1146.
American Academy of Pediatrics. Medical conditions affecting sports participation. Pediatrics. 2001;107:1205-1209.
Armenakas NA, Hochberg DA, Fracchia JA. Traumatic avulsion of the dorsal penile artery mimicking a penile fracture. J Urol. 2001;166:619.
Asgari MA, Hosseini SY, Safarinejad MR, et al. Penile fractures: evaluation, therapeutic approaches and long-term results. J Urol. 1996;155:148-149.
Bandi G, Santucci RA. Controversies in the management of male external genitourinary trauma. J Trauma. 2004;56:1362-1370.
Bar-Yosef Y, Greenstein A, Beri A, et al. Dorsal vein injuries observed during penile exploration for suspected penile fracture. J Sex Med. 2007;4:1142-1146.
Beysel M, Tekin A, Gürdal M, et al. Evaluation and treatment of penile fractures: accuracy and clinical diagnosis and the value of corpus cavernosography. Urology. 2002;60:492-496.
Bhanganada K, Chayavatana T, Pongnumkul C, et al. Surgical management of an epidemic of penile amputations in Siam. Am J Surg. 1983;146:376-382.
Bickel A, Mata J, Hochstein LM, et al. Bowel injury as a result of penetrating scrotal trauma: review of associated injuries. J Urol. 1990;143:1017-1018.
Bitsch M, Kromann-Andersen B, Schou J, Sjontoft E. The elasticity and tensile strength of tunica albuginea of the corpora cavernosa. J Urol. 1990;143:642-645.
Brandes SB, Buckman RF, Chelsky MJ, Hanno PM. External genitalia gunshot wounds: a ten-year experience with fifty-six cases. J Trauma. 1995;39:266-271.
Brock G, Hsu G, Nunes L, et al. The anatomy of the tunica albuginea in the normal penis and Peyronie’s disease. J Urol. 1997;157:276-281.
Bromberg W, Wong C, Kurek S, Salim A. Traumatic bilateral testicular dislocation. J Trauma. 2003;54:1009-1011.
Browning WH, Reed DC. A method of treatment for incarceration of the penis. J Urol. 1969;101:189-190.
Buckley JC, McAninch JW. Use of ultrasonography for the diagnosis of testicular injuries in blunt scrotal trauma. J Urol. 2006;175:175-178.
Cass AS, Luxenberg M. Value of early operation in blunt testicular contusion with hematocele. J Urol. 1988;139:746-747.
Cass AS, Luxenberg M. Testicular injuries. Urology. 1991;37:528-530.
Cavalcanti AG, Krambeck R, Araujo A, et al. Penile lesion from gunshot wound: a 43-case experience. Int Braz J Urol. 2006;32:56-63.
Cline KJ, Mata JA, Venable DB, Eastham JA. Penetrating trauma to the male external genitalia. J Trauma. 1998;44:492-494.
Corrales JG, Corbel L, Cipolla B, et al. Accuracy of ultrasound diagnosis after blunt testicular trauma. J Urol. 1993;150:1834-1836.
Cummings JM, Boullier JA. Scrotal dog bites. J Urol. 2000;164:57-58.
Czymek R, Schmidt A, Eckmann C, et al. Fournier’s gangrene: vacuum-assisted closure versus conventional dressings. Am J Surg. 2009;197:168-176.
Del Villar RG, Ireland GW, Cass AS. Early exploration following trauma to the testicle. J Trauma. 1973;13:600-601.
Derouiche A, Belhaj K, Hentati H, et al. Management of penile fractures complicated by urethral rupture. Int J Impot Res. 2008;20:111-114.
Dreitlein DA, Suner S, Basler J. Genitourinary trauma. Emerg Med Clin North Am. 2001;19:569-590.
Eke N. Fracture of the penis. Br J Surg. 2002;89:555-565.
Elsaharty S, Pranikoff K, Magoss IV, Sufrin G. Traumatic torsion of the testis. J Urol. 1984;132:1155-1156.
El Atat R, Sfaxi M, Renslama MR, et al. Fracture of the penis: management and long-term results of surgical treatment. Experience in 300 cases. J Trauma. 2008;64:121-125.
El-Bahnasawy MS, Gomha MA. Penile fractures: the successful outcome of immediate surgical management. Int J Impot Res. 2000;12:273-277.
El-Taher AM, Aboul-Ella HA, Sayed MA, Gaafar AA. Management of penile fracture. J Trauma. 2004;56:1138-1140.
Feki W, Derouiche A, Belhaj K, et al. False penile fracture: report of 16 cases. Int J Impot Res. 2007;19:471-473.
Fedel M, Venz S, Andreessen R, et al. The value of magnetic resonance imaging in the diagnosis of suspected penile fracture with atypical clinical findings. J Urol. 1996;155:1924-1927.
Ferguson GG, Brandes S. Gunshot wound injury of the testis: the use of tunica vaginalis and polytetrafluoroethylene grafts for reconstruction. J Urol. 2007;178:2462-2465.
Flowerdew R, Fishman IJ, Churchill BM. Management of penile zipper injury. J Urol. 1977;117:671.
Fournier GR, Laing FC, McAninch JW. Scrotal ultrasonography and the management of testicular trauma. Urol Clin North Am. 1989;16:377-385.
Goldman HB, Dmochowski RR, Cox CE. Penetrating trauma to the penis. J Urol. 1996;155:551-553.
Gomes CM, Ribeiro-Filho L, Giron AM, et al. Genital trauma due to animal bites. J Urol. 2001;165:80-83.
Gomez RG, Castanheira AC, McAninch JW. Gunshot wounds to the male external genitalia. J Urol. 1993;150:1147-1149.
Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36:441-446.
Guichard G, El Ammari J, Del Coro C, et al. Accuracy of ultrasonography in diagnosis of testicular rupture after blunt scrotal trauma. Urology. 2008;71:52-56.
Herbener TE. Ultrasound in the assessment of the acute scrotum. J Clin Ultrasound. 1996;24:405-421.
Hinev AI. Re: Penile injury [letter]. J Urol. 2002;167:1802-1803.
Horton CE, Dean JA. Reconstruction of traumatizing acquired defects of the phallus. World J Surg. 1990;14:757-762.
Hsu GL, Brock G, Martinez-Pineiro L, et al. Anatomy and strength of the tunica albuginea: its relevance to penile prosthesis extrusion. J Urol. 1994;151:1205-1208.
Ivanovski O, Stankov O, Kuzmanoski M, et al. Penile strangulation: two case reports and review of the literature. J Sex Med. 2007;4:1775-1780.
Jack GS, Garraway I, Reznichek R, Rajfer J. Current treatment options for penile fractures. Rev Urol. 2004;6:114-120.
Jezior JR, Brady JD, Schlossberg SM. Management of penile amputation injuries. World J Surg. 2001;25:1602-1609.
Jordan GH, Gilbert DA. Management of amputation injuries of the male genitalia. Urol Clin North Am. 1989;16:359-367.
Kalash SS, Young JD. Fracture of penis: controversy of surgical versus conservative treatment. Urology. 1984;24:21-24.
Kamdar C, Mooppan UM, Kim H, et al. Penile fracture: preoperative evaluation and surgical technique for optimal patient outcome. BJU Int. 2008;102:1640-1644.
Kane CJ, Nash P, McAninch JW. Ultrasonographic appearance of necrotizing gangrene: aid in early diagnosis. Urology. 1996;48:142-144.
Kanegaye JT, Schonfeld N. Penile zipper entrapment: a simple and less threatening approach using mineral oil. Pediatr Emerg Care. 1993;9:90-91.
Karadeniz T, Topsakal M, Ariman A, et al. Penile fracture: differential diagnosis, management and outcome. Br J Urol. 1996;77:279-281.
Kim SH, Park S, Choi SH, et al. The efficacy of magnetic resonance imaging for the diagnosis of testicular rupture: a prospective preliminary study. J Trauma. 2009;66:239-242.
Koga S, Saito Y, Arakaki Y, et al. Sonography in penile fracture. Br J Urol. 1993;72:228-229.
Kukadia AN, Ercole CJ, Gleich P, et al. Testicular trauma: potential impact on reproductive function. J Urol. 1996;156:1643-1646.
Kunkle DA, Lebed BD, Mydlo JH, Pontari MA. Evaluation and management of gunshot wounds of the penis: 20-year experience at an urban trauma center. J Trauma. 2008;64:1038-1042.
Landström JT, Schuyler RW, Macris GP. Microsurgical penile replantation facilitated by postoperative HBO treatment. Microsurgery. 2004;24:49-55.
Lee SH, Bak CW, Choi MH, et al. Trauma to male genital organs: a 10-year review of 156 patients, including 118 treated by surgery. BJU Int. 2007;101:211-215.
Lowe MA, Chapman W, Berger RE. Repair of a traumatically amputated penis with return of erectile function. J Urol. 1991;145:1267-1270.
Lrhorfi H, Manunta A, Rodriguez A, Lobel B. Trauma induced testicular torsion. J Urol. 2002;168:2548.
Manson AL. Traumatic testicular torsion: case report. J Trauma. 1989;29:407-408.
McAleer IM, Kaplan GW. Pediatric genitourinary trauma. Urol Clin North Am. 1995;22:177-188.
McAleer IM, Kaplan GW, LoSasso BE. Renal and testis injuries in team sports. J Urol. 2002;168:1805-1807.
McAninch JW, Kahn RI, Jeffrey RB, et al. Major traumatic and septic genital injuries. J Trauma. 1984;24:291-298.
McDougal WS. Lymphedema of the external genitalia. J Urol. 2003;170:711-716.
Meares EM. Traumatic rupture of the corpus cavernosum. J Urol. 1971;105:407-408.
Micallef M, Ahmad I, Ramesh N, et al. Ultrasound features of blunt testicular injury. Injury. 2001;32:23-26.
Miles BJ, Poffenberger RJ, Farah RN, Moore S. Management of penile gunshot wounds. Urology. 1990;36:318-321.
Mindrup SR, Kealey GP, Fallon B. Hyperbaric oxygen for the treatment of Fournier’s gangrene. J Urol. 2005;173:1975-1977.
Mineo M, Jolley T, Rodriguez G. Leech therapy in penile replantation: a case of recurrent penile self amputation. Urology. 2004;63:981-983.
Mitterberger M, Christian G, Pinggera GM, et al. Gray scale and color Doppler sonography with extended field of view technique for the diagnostic evaluation of anterior urethral strictures. J Urol. 2007;177:992-996.
Mohr AM, Pham AM, Lavery RF, et al. Management of trauma to the male external genitalia: the usefulness of American Association for the Surgery of Trauma organ injury scales. J Urol. 2003;170:2311-2315.
Morey AF, McAninch JW. Genital skin loss and scrotal reconstruction. In: Alter G, Ehrlich R, editors. Reconstructive and plastic surgery of the external genitalia. Philadelphia: WB Saunders; 1999:414-422.
Morey AF, Metro MJ, Carney KJ, et al. Consensus on genitourinary trauma. BJU Int. 2004;94:507-515.
Morris MS, Morey AF, Stackhouse DA, Santucci RA. Fibrin sealant as tissue glue: preliminary experience in complex genital reconstructive surgery. Urology. 2006;67:688-691.
Muentener M, Suter S, Hauri D, Sulser T. Long-term experience with surgical and conservative treatment of penile fracture. J Urol. 2004;172:576-579.
Muglia V, Tucci SJr, Elias JJr, et al. Magnetic resonance imaging of scrotal diseases: when it makes the difference. Urology. 2002;59:419-423.
Mydlo JH. Treatment of a delayed zipper injury. Urol Int. 2000;64:45-46.
Mydlo JH. Surgeon experience with penile fracture. J Urol. 2001;166:526-529.
Nasser TA, Mostafa T. Delayed surgical repair of penile fracture under local anesthesia. J Sex Med. 2008;5:2464-2469.
Nicolaisen GS, Melamud A, Williams RD, McAninch JW. Rupture of the corpus cavernosum: surgical management. J Urol. 1983;130:917-919.
Noh J, Kang TW, Heo T, et al. Penile strangulation treated with the modified string method. Urology. 2004;64:591.
O’Brien MF, Collins DA, McElwain JP, et al. Traumatic retrovesical testicular dislocation. J Urol. 2004;171:798.
Oosterlinck W. Unbloody management of penile zipper injury. Eur Urol. 1981;7:365-366.
Orvis BR, McAninch JW. Penile rupture. Urol Clin North Am. 1989;16:369-375.
Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum assisted closure therapy in the management of Fournier’s gangrene. Am J Surg. 2009;197(5):660-665. discussion 665
Pannek J, Martin W. Penile entrapment in a plastic bottle. J Urol. 2003;170:2385.
Perabo FG, Steiner G, Albers P, Muller SC. Treatment of penile strangulation caused by constricting devices. Urology. 2002;59:137.
Phonsombat S, Master VA, McAninch JW. Penetrating external genital trauma: a 30-year single institution experience. J Urol. 2008;180:192-196.
Pliskow RJ, Ohme RK. Corpus cavernosography in acute fracture of the penis. Am J Radiol. 1979;133:331-332.
Pohl DR, Johnson DE, Robison JR. Bilateral testicular rupture. J Urol. 1968;99:772-773.
Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55:427-431.
Santucci RA, Deng D, Carney J. Removal of metal penile foreign body with a widely available emergency-medical-services-provided air-driven grinder. Urology. 2004;63:1183-1184.
Saraf P, Rabinowitz R. Zipper injury of the foreskin. Am J Dis Child. 1982;136:557-558.
Schuster G. Traumatic rupture of the testicle and a review of the literature. J Urol. 1982;127:1194-1196.
Schwartz SL, Faerber GJ. Dislocation of the testis as a delayed presentation of scrotal trauma. Urology. 1994;43:743-745.
Senchenkov A, Knoetgen J, Chrouser KL, Nehra A. Application of vacuum-assisted closure dressing in penile skin graft reconstruction. Urology. 2006;67:416-419.
Serra AD, Hricak H, Coakley FV, et al. Inconclusive clinical and ultrasound evaluation of the scrotum: impact of magnetic resonance imaging on patient management and cost. Urology. 1998;51:1018-1021.
Shaeer O. Methylene blue-guided repair of fractured penis. J Sex Med. 2006;3:349-354.
Shah DK, Paul EM, Meyersfield SA, Schoor RA. False fracture of the penis. Urology. 2003;61:1259.
Talan DA, Citron DM, Abrahamian FM, et al. Bacteriologic analysis of infected dog and cat bites. N Engl J Med. 1999;340:85-92.
Talan DA, Talan DA, Abrahamian FM, et al. Clinical presentation and bacteriologic analysis of infected human bites in patients presenting to emergency departments. Clin Infect Dis. 2003;37:1481-1489.
Thompson I, Flaherty S, Morey AF. Battlefield urologic injuries: the Gulf War experience. J Am Coll Surg. 1998;187:139-141.
Tsang T, Demby AM. Penile fracture with urethral injury. J Urol. 1992;147:466-468.
Uder M, Gohl D, Takahashi M, et al. MRI of penile fracture: diagnosis and therapeutic follow-up. Eur Radiol. 2002;12:113-120.
Vaccaro JA, Davis R, Belville WD, Kiesling VJ. Traumatic hematocele: association with rupture of the testicle. J Urol. 1986;136:1217-1218.
Vahasarja VJ, Hellstrom PA, Serlo W, Kontturi MJ. Treatment of penile incarceration by the string method: 2 case reports. J Urol. 1993;149:372-373.
Van der Horst C, Martinez Portillo FJ, Seif C, et al. Male genital injury: diagnostics and treatment. BJU Int. 2004;93:927-930.
Wan J, Corvino TF, Greenfield SP, DiScala C. The incidence of recreational genitourinary and abdominal injuries in the western New York pediatric population. J Urol. 2003;170:1525-1527.
Wan J, Corvino TF, Greenfield SP, DiScala C. Kidney and testicle injuries in team and individual sports: data from the national pediatric trauma registry. J Urol. 2003;170:1528-1532.
Waxman S, Beekley A, Morey A, Soderdahl D. Penetrating trauma to the external genitalia in Operation Iraqi Freedom. Int J Impot Res. 2009;21:145-148.
Weinfeld AB, Kelley P, Yuksel E, et al. Circumferential negative-pressure dressing (VAC) to bolster skin grafts in the reconstruction of the penile shaft and scrotum. Ann Plast Surg. 2005;54:178-183.
Wolf JS, Turzan C, Cattolica EV, McAninch JW. Dog bites to the male genitalia: characteristics, management and comparison with human bites. J Urol. 1993;149:286-289.
Yip A, Ng SK, Wong WC, et al. Injury to the prepuce. Br J Urol. 1989;63:535-538.
Zargooshi J. Penile fracture in Kermanshah, Iran: report of 172 cases. J Urol. 2000;164:364-366.
Zargooshi J. Penile fracture in Kermanshah, Iran: the long-term results of surgical treatment. BJU Int. 2002;89:890-894.
Zhong Z, Dong Z, Lu Q, et al. Successful penile replantation with adjuvant hyperbaric oxygen treatment. Urology. 2007;69:983.e3-983.e5.
Aihara R, Blansfield JS, Millham FH, et al. Fracture locations influence the likelihood of rectal and lower urinary tract injuries in patients sustaining pelvic fractures. J Trauma. 2002;52:205-209.
Al-Ali IH, Husain I. Disrupting injuries of the membranous urethra—the case for early surgery and catheter splinting. Br J Urol. 1983;55:716-720.
Alli MO, Singh B, Moodley J, Shaik AS. Prospective evaluation of combined suprapubic and urethral catheterization to urethral drainage alone for intraperitoneal bladder injuries. J Trauma. 2003;55:1152-1154.
Al-Rifaei MA, Gaafar S, Abdel-Rahman M. Management of posterior urethral strictures secondary to pelvic fractures in children. J Urol. 1991;145:353-356.
Andrich DE, Mundy AR. The nature of urethral injury in cases of pelvic fracture urethral trauma. J Urol. 2001;165:1492-1495.
Anema JG, Morey AF, McAninch JW, et al. Complications related to the high lithotomy position during urethral reconstruction. J Urol. 2000;164:360-363.
Anger JT, Sherman ND, Webster GD. Ejaculatory profiles and fertility in men after posterior urethroplasty for pelvic fracture-urethral distraction defect injuries. BJU Int. 2008;102:351-353.
Armenakas NA, McAninch JW. Acute anterior urethral injuries: diagnosis and initial management. In: McAninch JW, editor. Traumatic and reconstructive urology. Philadelphia: WB Saunders; 1996:543-550.
Armenakas NA, McAninch JW, Lue TF, et al. Posttraumatic impotence: magnetic resonance imaging and duplex ultrasound in diagnosis and management. J Urol. 1993;149:1272-1275.
Asci R, Sarikaya S, Buyukalpelli R, et al. Voiding and sexual dysfunctions after pelvic fracture urethral injuries treated with either initial cystostomy and delayed urethroplasty or immediate primary urethral realignment. Scand J Urol Nephrol. 1999;33:228-233.
Basta AM, Blackmore CC, Wessells H. Predicting urethral injury from pelvic fracture patterns in male patients with blunt trauma. J Urol. 2007;177:571-575.
Bepple JL, Virasoro R, Williams MB, et al. Incidence of infection in patients with orthopedic interventions for a pelvic fracture and a suprapubic catheter placed secondary to a posterior urethral distraction injury. J Urol. 77, 2007. [abstract 115]
Black PC, Miller EA, Porter JR, Wessells H. Urethral and bladder neck injury associated with pelvic fracture in 25 female patients. J Urol. 2006;175:2140-2145.
Boone TB, Wilson WT, Husmann DA. Postpubertal genitourinary function following posterior urethral disruptions in children. J Urol. 1992;148:1232-1234.
Borrelli J, Brandes SB. Pelvic fractures: assessment and management for the urologist. AUA Update Series. 2004;23:82-86.
Brandes SB, Borelli J. Pelvic fracture and associated urologic injuries. World J Surg. 2001;25:1578-1587.
Carr LK, Webster GD. Posterior urethral reconstruction. Atlas Urol Clin North Am. 1997;5:125-137.
Carroll PR, McAninch JW. Major bladder trauma: mechanisms of injury and a unified method of diagnosis and repair. J Urol. 1984;132:254-257.
Cass AS. The multiple injured patient with bladder trauma. J Trauma. 1984;24:731-734.
Cass AS. Diagnostic studies in bladder rupture. Urol Clin North Am. 1989;16:267-273.
Cass AS, Luxenberg M. Features of 164 bladder ruptures. J Urol. 1987;138:743-745.
Cass AS, Luxenberg M. Simultaneous upper and lower urinary tract injury from external trauma. Urology. 1990;36:226-227.
Colapinto V, McCallum RW. Injury to the male posterior urethra in fractured pelvis: a new classification. J Urol. 1977;118:575-580.
Cooperberg MR, McAninch JW, Alsikafi NF, Elliot SP. Urethral reconstruction for traumatic posterior urethral disruption: outcomes of a 25-year experience. J Urol. 2007;178:2006-2010.
Connor GS, McGwin G, MacLennan PA, et al. Early versus delayed fixation of pelvic ring fractures. Am Surg. 2003;69:1019-1024.
Corriere JN. 1-Stage delayed bulboprostatic anastomotic repair of posterior urethral rupture: 60 patients with 1-year followup. J Urol. 2001;165:404-407.
Corriere JN, Rudy DC, Benson GS. Voiding and erectile function after delayed one-stage repair of posterior urethral disruptions in 50 men with a fractured pelvis. J Trauma. 1994;37:587-589.
Corriere JN, Sandler CM. Management of extraperitoneal bladder rupture. Urol Clin North Am. 1989;16:275-277.
Devine CJJr, Jordan GH, Devine PC. Primary realignment of the disrupted prostatomembranous urethra. Urol Clin North Am. 1989;16:291-295.
Dixon CM, Hricak H, McAninch JW. Magnetic resonance imaging of traumatic posterior urethral defects and pelvic crush injuries. J Urol. 1992;148:1162-1165.
Dobrowolski ZF, Lipczynski W, Drewniak T, et al. External and iatrogenic trauma of the urinary bladder: A survey in Poland. BJU Int. 2002;89:755-756.
Dorairajan LN, Gupta H, Kumar S. Pelvic fracture–associated urethral injuries in girls: experience with primary repair. BJU Int. 2004;94:134-136.
Elliott DS, Barrett DM. Long-term followup and evaluation of primary realignment of posterior urethral disruptions. J Urol. 1997;157:814-816.
Evans LA, Ferguson KH, Foley JP, et al. Fibrin sealant for the management of genitourinary injuries, fistulas, and surgical complications. J Urol. 2003;169:1360-1362.
Flynn BJ, Delvecchio FC, Webster GD. Perineal repair of pelvic fracture urethral distraction defects: experience in 120 patients during the last 10 years. J Urol. 2003;170:1877-1880.
Follis HW, Koch MO, McDougal WS. Immediate management of prostatomembranous urethral disruptions. J Urol. 1992;147:1259-1262.
Gomez RG, Ceballos L, Coburn M, et al. Consensus statement on bladder injuries. BJU Int. 2004;94:27-32.
Greenwell TJ, Castle C, Andrich DE, et al. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective. J Urol. 2004;172:275-277.
Hadjizacharia P, Inaba K, Teixeira PGR, et al. Evaluation of immediate endoscopic realignment as a treatment for traumatic urethral injuries. J Trauma. 2008;64:1443-1450.
Hemal AK, Singh I, Chahal R, Gupta NP. Core through internal urethrotomy in the management of post-traumatic isolated bladder neck and prostatic urethral strictures in adults: a report of 4 cases. Int Urol Nephrol. 1999;31:703-708.
Hsieh C, Chen R, Fang J, et al. Diagnosis and management of bladder injury by trauma surgeons. Am J Surg. 2002;184:143-147.
Husmann DA, Boone TB, Wilson WT. Management of low velocity gunshot wounds to the anterior urethra: the role of primary repair versus urinary diversion alone. J Urol. 1993;150:70-72.
Iselin CE, Webster GD. The significance of the open bladder neck associated with pelvic fracture urethral distraction defects. J Urol. 1999;162:347-351.
Iverson AJ, Morey AF. Radiographic evaluation of suspected bladder rupture following blunt trauma: critical review. World J Surg. 2001;25:1588-1591.
Kawanishi Y, Kimura K, Nakanishi R, et al. Penile revascularization surgery for arteriogenic erectile dysfunction: the long-term efficacy rate calculated by survival analysis. BJU Int. 2004;94:361-368.
Kim FJ, Chammas MFJr, Gewehr EV, et al. Laparoscopic management of intraperitoneal bladder rupture secondary to blunt abdominal trauma using intracorporeal single layer suturing technique. J Trauma. 2008;65:234-236.
Kiracofe HL, Pfister RR, Peterson NE. Management of non-penetrating distal urethral trauma. J Urol. 1975;114:57-62.
Kizer WS, Armenakas NA, Brandes SB, et al. Simplified reconstruction of posterior urethral disruption defects: limited role of supracrural rerouting. J Urol. 2007;177:1378-1382.
Koraitim MM. Pelvic fracture urethral injuries: the unresolved controversy. J Urol. 1999;161:1433-1441.
Koraitim MM. Failed posterior urethroplasty: lessons learned. Urology. 2003;62:719-722.
Koraitim MM. On the art of anastomotic posterior urethroplasty: a 27-year experience. J Urol. 2005;173:135-139.
Koraitim MM, Marzouk ME, Atta MA, Orabi SS. Risk factors and mechanism of urethral injury in pelvic fractures. Br J Urol. 1996;77:876-880.
Koraitim MM, Reda IS. Role of magnetic resonance imaging in assessment of posterior urethral distraction defects. Urology. 2007;70:403-406.
Kotkin L, Koch MO. Morbidity associated with nonoperative management of extraperitoneal bladder injuries. J Trauma. 1995;38:895-898.
Kotkin L, Koch MO. Impotence and incontinence after immediate realignment of posterior urethral trauma: result of injury or management? J Urol. 1996;155:1600-1603.
Levine L, Wessels H. Comparison of open and endoscopic treatment of posttraumatic posterior urethral strictures. World J Surg. 2001;25:1597-1601.
Matthews LA, Herbener TE, Seftel AD. Impotence associated with blunt pelvic and perineal trauma: penile revascularization as a treatment option. Semin Urol. 1995;13:66-72.
McAninch JW. Pubectomy in repair of membranous urethral stricture. Urol Clin North Am. 1989;16:297-302.
Mee SL, McAninch JW, Federle MP. Computerized tomography in bladder rupture: diagnostic limitations. J Urol. 1987;137:207-209.
Morey AF, Carroll PR. Evaluation and management of adult bladder trauma. Contemp Urol. 1997;9:13-22.
Morey AF, Hernandez J, McAninch JW. Reconstructive surgery for lower urinary tract trauma. Urol Clin North Am. 1999;26:49-60.
Morey AF, Iverson AJ, Swan A, et al. Bladder rupture after blunt trauma: guidelines for diagnostic imaging. J Trauma. 2001;51:683-686.
Morey AF, McAninch JW. Reconstruction of posterior urethral disruption injuries: outcome analysis in 82 patients. J Urol. 1997;157:506-510.
Morey AF, McAninch JW. Sonographic staging of anterior urethral strictures. J Urol. 2000;163:1070-1075.
Mouraviev VB, Coburn M, Santucci RA. The treatment of posterior urethral disruption associated with pelvic fractures: comparative experience of early realignment versus delayed urethroplasty. J Urol. 2005;173:873-876.
Munarriz RM, Yan QR, ZNehra A, et al. Blunt trauma: the pathophysiology of hemodynamic injury leading to erectile dysfunction. J Urol. 1995;153:1831-1840.
Mundy AR. The role of delayed primary repair in the acute management of pelvic fracture injuries of the urethra. Br J Urol. 1991;68:273-276.
Mundy AR. Urethroplasty for posterior urethral strictures. Br J Urol. 1996;78:243-247.
Mundy AR. Reconstruction of posterior urethral distraction defects. Atlas Urol Clin North Am. 1997;5:139-174.
Narumi Y, Hricak H, Armenakas NA, et al. MR imaging of traumatic posterior urethral injury. Radiology. 1993;188:439-443.
Netto NRJr. The surgical repair of posterior urethral strictures by the transpubic urethroplasty or pull-through technique. J Urol. 1985;133:411-412.
Netto NRJr, Lemos GC, Claro JF. Internal urethrotomy as a complementary method after urethroplasties for posterior urethral stenosis. J Urol. 1989;141:50-51.
Park S, McAninch JW. Straddle injuries to the bulbar urethra: management and outcomes in 78 patients. J Urol. 2004;171:722-725.
Parry NG, Rozycki GS, Feliciano DV, et al. Traumatic rupture of the urinary bladder: is the suprapubic tube necessary? J Trauma. 2003;54:431-436.
Patterson BM. Pelvic ring injury and associated urologic trauma: an orthopaedic perspective. Semin Urol. 1995;13:25-33.
Peng MY, Parisky YR, Cornwell EEIII, et al. CT cystography versus conventional cystography in evaluation of bladder injury. AJR Am J Roentgenol. 1999;173:1269-1272.
Perry MO, Husmann DA. Urethral injuries in female subjects following pelvic fractures. J Urol. 1992;147:139-143.
Peters PC. Intraperitoneal rupture of the bladder. Urol Clin North Am. 1989;16:279-282.
Pierce JMJr. Exposure of the membranous and posterior urethra by total pubectomy. J Urol. 1962;88:256-257.
Podesta ML, Jordan GH. Pelvic fracture urethral injuries in girls. J Urol. 2001;165:1660-1665.
Porter JR, Takayama TK, Defalco AJ. Traumatic posterior urethral injury and early realignment using magnetic urethral catheters. J Urol. 1997;158:425-430.
Pratap A, Agrawal CS, Tiwari A, et al. Complex posterior urethral disruptions: management by combined abdominal transpubic urethroplasty. J Urol. 2006;175:1751-1754.
Rosenstein D, Jordan GH. The perineal approach to membranous urethral distraction injuries. Atlas Urol Clin North Am. 2003;11:51-63.
Routt ML, Simonian PT, Defalco AJ, et al. Internal fixation in pelvic fractures and primary repairs of associated genitourinary disruptions: a team approach. J Trauma. 1996;40:784-790.
Sandler CM, Corriere JNJr. Urethrography in the diagnosis of acute urethral injuries. Urol Clin North Am. 1989;16:283-289.
Santucci RA, Mario LA, McAninch JW. Anastomotic urethroplasty for bulbar urethral stricture: analysis of 168 patients. J Urol. 2002;167:1715-1719.
Shenfeld OZ, Kiselgorf D, Gofrit ON, et al. The incidence and causes of erectile dysfunction after pelvic fractures associated with posterior urethral disruption. J Urol. 2003;169:2173-2176.
Tunc HM, Tefekli AH, Kaplancan T, Esen T. Delayed repair of post-traumatic posterior urethral distraction injuries: long-term results. Urology. 2000;55:837-841.
Turner-Warwick R. Prevention of complications resulting from pelvic fracture urethral injuries—and from their surgical management. Urol Clin North Am. 1989;16:335-358.
Udekwu PO, Gurkin B, Oller DW. The use of computed tomography in blunt abdominal injuries. Am Surg. 1996;62:56-59.
Waterhouse K. The surgical repair of membranous urethral strictures in children. J Urol. 1976;116:363-365.
Webster GD, Mathes GL, Selli C. Prostatomembranous urethral injuries: a review of the literature and a rational approach to their management. J Urol. 1983;130:898-902.
Weinfeld AB, Kelley P, Yuksel E, et al. Circumferential negative-pressure dressing (VAC) to bolster skin grafts in reconstruction of the penile shaft and scrotum. Ann Plast Surg. 2005;54:178-183.
Whitson JM, Tanagho EA, Metro MJ, Rahman NU. Mechanism of continence after repair of posterior urethral disruption: evidence of rhabdosphincter activity. J Urol. 2008;179:1035-1039.
Wilson TS, Lemack GE, Dmochowski RR. UroLume stents: lessons learned. J Urol. 2002;167:2477-2480.
Volpe MA, Pachter EM, Scalea TM, et al. Is there a difference in outcome when treating traumatic intraperitoneal bladder rupture with or without a suprapubic tube? J Urol. 1999;161:1103-1105.
Ying-Hao S, Chuan-Liang X, Xu G, et al. Urethroscopic realignment of ruptured bulbar urethra. J Urol. 2000;164:1543-1545.
Yu JJ, Xu YM, Qiao Y, Gu BJ. Urethral cystoscopic realignment and early end-to-end anastomosis develop different influence on erectile function in patients with ruptured bulbous urethra. Arch Androl. 2007;53:59-62.