chapter 96 Pathology of Prostatic Neoplasia
This chapter covers the pathology of adenocarcinoma of the prostate from its precursor lesions to invasive carcinomas, from needle biopsies to radical prostatectomies. Other tumors involving the prostate are also discussed. In particular, practical points of pathology that are critical for urologists to know for the management of their patients are emphasized.
Prostatic Intraepithelial Neoplasia
Prostatic intraepithelial neoplasia (PIN) consists of architecturally benign prostatic acini or ducts lined by cytologically atypical cells and is classified as low-grade and high-grade neoplasias (McNeal and Bostwick, 1986) (Fig. 96–1). Diagnostic reports should not comment on low-grade PIN. First, pathologists cannot reproducibly distinguish between low-grade PIN and benign prostate tissue (Epstein et al, 1995). Second, when low-grade PIN is diagnosed on needle biopsy, these patients are at no greater risk of having carcinoma on repeated biopsy than are men with a benign biopsy finding (Epstein and Herawi, 2006). Evidence that high-grade PIN (HGPIN) is a precursor to some prostate carcinomas includes the following: There is an increase in the size and number of high-grade PIN foci in prostates with cancer compared with prostates without carcinoma; with increasing amounts of high-grade PIN, there are a greater number of multifocal carcinomas; both high-grade PIN and carcinoma preferentially involve the peripheral zone; and biomarkers and molecular changes show similarity between high-grade PIN and carcinoma (Bostwick et al, 1996; Haggman et al, 1997). About 20% of high-grade PIN lesions harbor a TMPRSS2-ERG fusion gene, which is a common molecular abnormality detectable in about 50% of prostate cancers (Cerveira et al, 2006; Perner et al, 2007).
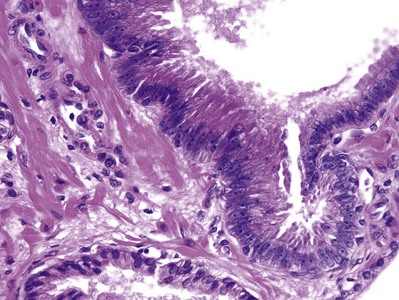
Figure 96–1 High-grade prostatic intraepithelial neoplasia. Note cytologically atypical cells with prominent nucleoli in an architecturally benign gland (top), contrasted to a benign gland (bottom).
The incidence of high-grade PIN on biopsy averages 7.6% among 38 studies, with a median of 5.2%. There is a tremendous variation in the percentages reported, ranging from 0% to 25%, with no apparent correlation to number of cores sampled or whether the study was from an academic or community hospital setting (Epstein and Herawi, 2006). The most likely explanation accounting for this variation is interobserver threshold. The distinction between low-grade and high-grade PIN is based on the prominence of the nucleoli. This is a subjective exercise, and those pathologists with a lower threshold as to what defines prominent nucleoli will have a higher incidence of high-grade PIN. The largest studies reported a 16% to 44.6% risk of cancer on subsequent biopsy. Of the 11 studies with at least 50 cases of high-grade PIN on needle biopsy with follow-up, the mean risk of cancer is 26.4% (Epstein and Herawi, 2006). In the majority of studies, serum prostate-specific antigen (PSA) levels, results of digital rectal examination, and transrectal ultrasonography findings do not enhance the prediction of who is more likely to have carcinoma on repeat biopsy. PIN by itself does not give rise to elevated serum PSA values (Ronnett, et al, 1993). Of 8 studies in which the risk of cancer after a needle biopsy diagnosis of high-grade PIN was compared with the risk of cancer after a benign biopsy finding, 6 showed no difference between the groups (Epstein and Herawi, 2006). For patients diagnosed with unifocal high-grade PIN on extended initial core sampling, a repeat biopsy within the first year is unnecessary in the absence of other clinical indicators of cancer and in the absence of extensive high-grade PIN. High-grade PIN on greater than or equal to two cores is associated with a sufficiently high risk of subsequent cancer that rebiopsy within a year of the initial PIN diagnosis is warranted (Abdel-Khalek et al, 2004; Netto and Epstein, 2006; Merrimen et al, 2009). Lefkowitz and colleagues (2002) described 31 men who had an initial diagnosis of high-grade PIN on 12-core biopsy with an interval to follow-up prostate biopsy of 3 years. The rate of cancer on repeat 12-core biopsy was 25.8% compared with their earlier study in which rebiopsy within 1 year of a diagnosis of high-grade PIN yielded cancer in only 2.3%. They hypothesized that the 3-year interval allowed unsampled small cancers that were associated with the high-grade PIN at the time of initial biopsy to grow to a size that was detectable on repeat biopsy, or alternatively, some of the high-grade PIN lesions progressed to cancer during the 3-year interval. If a repeat prostate needle biopsy is performed, it should sample the entire prostate and not just the initial sextant site where the high-grade PIN was found (Epstein and Herawi, 2006).
The significance of HGPIN on transurethral resection (TUR) is not clear, with conflicting data as to the risk for subsequent discovery of cancer (Gaudin et al, 1997; Pacelli and Bostwick, 1997). In an elderly patient with HGPIN on TUR, often no further workup is instituted. In a younger man, a more aggressive workup to rule out a clinically significant tumor is warranted. The one piece of evidence we have for premalignant lesions in other organs that is lacking in the prostate is the natural history of high-grade PIN. With the prostate, there is no capability to monitor a PIN focus to determine whether there is already infiltrating carcinoma at that site or whether infiltrating carcinoma has evolved in the immediate vicinity of the PIN focus. Because we do not know what percentage of patients develop infiltrating carcinoma during a given follow-up interval, when high-grade PIN is found on biopsy material, most authorities do not use the term carcinoma in situ of the prostate. It appears that high-grade PIN is a precursor lesion to many peripheral intermediate- to high-grade adenocarcinomas of the prostate. However, PIN need not be present for carcinoma to arise. Low-grade carcinomas, especially those present within the transition zone, are not closely related to high-grade PIN.
Intraductal carcinoma of the prostate (IDC-P) has, in several studies, been described in radical prostatectomy specimens (McNeal et al, 1986; McNeal and Yemoto, 1996; Rubin et al, 1998; Wilcox et al, 1998; Cohen et al, 2007; Robinson and Epstein, 2010). Rarely, IDC-P may be identified on biopsy material in the absence of infiltrating carcinoma (Guo and Epstein, 2006). IDC-P on prostate biopsies is frequently associated with high-grade cancer and poor prognostic parameters at radical prostatectomy, as well as potentially advanced disease following other therapies (Guo and Epstein, 2006). These findings support the idea that in most cases IDC-P represents intraductal spread of carcinoma within pre-existing ducts and acini and that IDC-P in the vast majority of cases should not be categorized as a preinvasive neoplastic condition. Consideration should be given to aggressively treating patients with IDC-P on biopsy, even in the absence of documented infiltrating cancer.
Adenocarcinoma
Location
In clinical stage T2 carcinomas and in 85% of nonpalpable tumors diagnosed on needle biopsy (stage T1c), the major tumor mass is peripheral in location (McNeal, 1969; Byar and Mostofi, 1972; Epstein et al, 1994b). In the remaining cases, tumors are predominantly located in the transition zone (i.e., periurethrally or anteriorly). Tumors that appear to be unilateral on rectal examination are bilateral in approximately 70% of cases when they are examined for pathology. Adenocarcinoma of the prostate is multifocal in more than 85% of cases (Byar and Mostofi, 1972). In many of these cases of bilateral or multifocal tumor, the other tumors are small, low grade, and clinically insignificant. Consequently, the distinction between pathologic stages T2a and T2b is meaningless (Freedland et al, 2004). In a highly selected population with limited unilateral biopsy cancer, the mean number of separate tumor nodules per radical prostatectomy is 2.9. A contralateral tumor to the positive biopsy side at radical prostatectomy is typically small. However, 20% have some contralateral adverse pathology in terms of size, extraprostatic extension, grade, or margins (Yoon et al, 2008).
Spread of Tumor
Because the prostate lacks a discrete histologic capsule, extraprostatic extension is preferable to “capsular penetration” as the term to describe a tumor that has extended out of the prostate into periprostatic soft tissue (Ayala et al, 1989). Some authors use the term capsular invasion when they believe that the “capsule” is infiltrated by a tumor but the tumor does not extend out of the prostate. Because there is no such entity as the prostatic capsule, “capsular invasion” makes no sense. Peripherally located adenocarcinomas of the prostate tend to extend out of the prostate through perineural space invasion (Villers et al, 1989). Perineural invasion by itself in radical prostatectomy specimens does not worsen prognosis, because perineural invasion merely represents extension of a tumor along a plane of decreased resistance and not invasion into lymphatics (Hassan and Maksem, 1980). In contrast, vascular invasion increases the risk of recurrence after radical prostatectomy (Baydar et al, 2008). Extraprostatic extension preferentially occurs posteriorly and posterolaterally, paralleling the location of most adenocarcinomas.
Further local spread of tumor may lead to seminal vesicle invasion, which is diagnosed when a tumor extends into the muscle wall of the seminal vesicle. The most common route of seminal vesicle invasion is by tumor penetration out of the prostate at the base of the gland, with growth and extension into the periseminal vesicle soft tissue and eventually into the seminal vesicles. Less commonly, there may be direct extension through the ejaculatory ducts into the seminal vesicles or direct extension from the base of the prostate into the wall of the seminal vesicles. Least commonly, there may be discrete metastases to the seminal vesicle (Fry et al, 1979; Ohori et al, 1993). Local spread of prostate cancer may also rarely involve the rectum, where it may be difficult to distinguish from a rectal primary tumor (Fry et al, 1979; Lane et al, 2008).
The most frequent sites of metastatic prostate carcinoma are lymph nodes and bone. Prostate cancer may present with metastases to the left supradiaphragmatic, typically the supraclavicular, lymph nodes (Cho and Epstein, 1987). Lung metastases from prostate carcinoma are extremely common at autopsy, and almost all cases have bone involvement as well (Varkarakis et al, 1974). Metastatic lesions usually take the form of multiple small nodules or diffuse lymphatic spread rather than large metastatic deposits. Clinically, prostate carcinoma metastatic to the lung is usually asymptomatic. In addition to lymph nodes, bones, and lung, the next most common regions of spread of prostate cancer at autopsy are bladder, liver, and adrenal gland (Hess et al, 2006).
Tumor Volume
In general, the size of a prostate cancer correlates with its stage. Extraprostatic extension is uncommon in tumors of less than 0.5 cm3, and tumors that are less than 4 cm3 uncommonly reveal lymph node metastases or seminal vesicle invasion (McNeal et al, 1990). Tumor volume is also proportional to grade (see following discussion). The location and grade of the tumor also modulate the effect of tumor volume (Christensen et al, 1990; McNeal et al, 1990; Greene et al, 1991). For example, transition zone tumors extend out of the prostate at larger volumes than do peripheral zone tumors, because of their lower grade and greater distance from the edge of the gland.
Grade
Although numerous grading systems exist for the evaluation of prostatic adenocarcinoma, the Gleason grading system is the most widely accepted (Gleason and Mellinger, 1974). The Gleason system is based on the glandular pattern of the tumor as identified at relatively low magnification (Fig. 96–2). Cytologic features play no role in the grade of the tumor. Both the primary (predominant) and the secondary (second most prevalent) architectural patterns are identified and assigned a grade from 1 to 5, with 1 being the most differentiated and 5 being the least differentiated (Table 96–1). Because both the primary and the secondary patterns are influential in predicting prognosis, there is a Gleason sum or score obtained by the addition of the primary and secondary grades. If a tumor has only one histologic pattern, then for uniformity, the primary and secondary patterns are given the same grade. Gleason scores range from 2 (1 + 1 = 2), which represents tumors uniformly composed of Gleason pattern 1 tumor, to 10 (5 + 5 = 10), which represents totally undifferentiated tumors. This author prefers to assign both a primary and a secondary pattern even when presented with limited cancer so as to not to create any confusion. For example, cases that the pathologist signs out as only “Gleason grade 4” could be interpreted to mean Gleason pattern 4 (high-grade cancer) or Gleason score 4 (low-grade cancer). In radical prostatectomy specimens, it has been demonstrated that tertiary (third most common pattern) high-grade components adversely affect biologic behavior, yet are not always equivalent to the sum of the primary pattern and highest-grade pattern. It is recommended that in radical prostatectomy specimens, the routine Gleason score, consisting of the most prevalent and the second most prevalent architectural patterns, be recorded along with a note stating that there is a tertiary high-grade pattern (Pan et al, 2000; Trock et al, 2009). There are a few exceptions to the Gleason system, as described above. For needle biopsy specimens in which the typical scenario includes tumors with patterns 3, 4, and 5 in various proportions, both the primary pattern and the highest grade should be added to derive the Gleason score. Any amount of high-grade tumor sampled on needle biopsy most likely indicates a more significant amount of high-grade tumor within the prostate because of the correlation of grade and volume and the problems inherent with needle biopsy sampling. In the setting of high-grade cancer, one should ignore lower-grade patterns if they occupy less than 5% of the area of the tumor. For example, a tumor composed of 98% Gleason pattern 4 and 2% Gleason pattern 3 should be diagnosed as Gleason score 4 + 4 = 8 (Epstein et al, 2005).
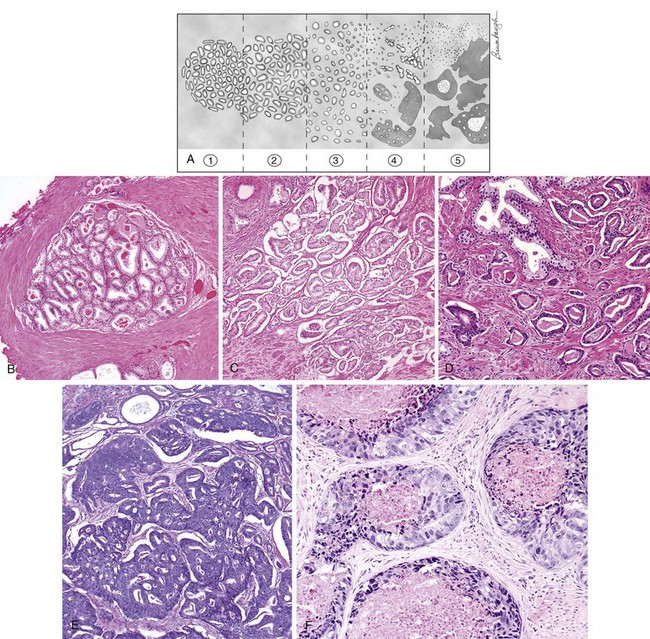
Figure 96–2 The Gleason grading system. A, Schematic diagram of the Gleason grading system. B, Gleason pattern 1: Well-circumscribed nodule of closely packed glands. C, Gleason pattern 2: nodule with more loosely arranged glands. D, Gleason pattern 3: small glands with an infiltrative pattern between benign glands. E, Gleason pattern 4: large irregular cribriform glands. F, Gleason pattern 5: solid nests of tumor with central comedonecrosis.
Table 96–1 2005 International Society of Urological Pathology Modified Gleason System
| Pattern 1 |
| Pattern 2 |
| Pattern 3 |
| Pattern 4 |
| Pattern 5 |
It is important to recognize Gleason pattern 4 tumors because tumors with this pattern have a significantly worse prognosis than those with pure Gleason pattern 3 (McNeal et al, 1990; Epstein et al, 1993b). It has also been demonstrated in radical prostatectomy specimens that tumors with Gleason score 4 + 3 = 7 have a worse prognosis than those with Gleason score 3 + 4 = 7 (Chan et al, 2000). There is fairly good interobserver reproducibility of the Gleason system among uropathology experts and poorer reproducibility among practicing pathologists (Allsbrook et al, 2001a, 2001b). It has been demonstrated that although current use of the Gleason grading system is not optimal, significant improvements can be made after participation in relatively brief educational programs, such as those available on websites (e.g., www.isuporg.org [International Society of Urological Pathology]).
The Gleason grade on biopsy material has also been shown to correlate fairly well with that of the subsequent prostatectomy specimen (Fine and Epstein, 2008). Several studies have demonstrated that there is better correlation between the biopsy and prostatectomy grade with extended as opposed to sextant needle biopsy sampling. In general, a Gleason score less than or equal to 6 or greater than or equal to 7 on biopsy corresponds to a Gleason score less than or equal to 6 or greater than or equal to 7 in the radical prostatectomy, respectively, in 80% of cases. An unavoidable cause of discordant grading between the biopsy and subsequent prostatectomy specimen(s) concerns sampling errors with the needle biopsy. One of the most frequent causes of discordant grading is grading of tumors that straddle two grades. One way the practice of Gleason scoring can be improved is by virtually never assigning Gleason score 2 to 4 for adenocarcinoma of the prostate on needle biopsy. The reasons for this approach are as follows: (1) most tumors graded as Gleason score 2 to 4 on needle biopsy are graded as Gleason score 5 to 6 or higher when reviewed by uropathology experts (Steinberg et al, 2005); (2) there is poor reproducibility in the diagnosis of Gleason score 2 to 4 on needle biopsy even among uropathology experts (Allsbrook et al, 2001b); and (3) most important, assigning Gleason score 2 to 4 to an adenocarcinoma on needle biopsy can adversely affect the patient’s care, because clinicians may incorrectly assume that all low-grade cancers on needle biopsy do not need definitive therapy. Although low volume, Gleason score 2 to 4 adenocarcinoma of the prostate, on transurethral resection of the prostate, has a relatively indolent course; low-grade cancer on needle biopsy does not. Pathologists, in general, are less frequently overdiagnosing Gleason scores 2 to 4 on biopsy in recent years. In one study, 24% of pathologists rendered a diagnosis of Gleason score 2 to 4 on biopsy in 1991, which decreased to 2.4% in 2001 (Ghani et al, 2005).
The ultimate value of any grading system is its prognostic ability. Both Gleason’s data with 2911 patients and subsequent studies with long-term follow-up have demonstrated a good correlation between the Gleason sum and prognosis (Mellinger, 1977; Sogani et al, 1985). When stage of disease is factored in with grade, prognostication is enhanced. Some men with low-grade cancers develop high-grade tumors after several years (Brawn, 1983). It is not clear whether the residual low-grade cancer progressed or whether there was a subsequent development of a multifocal, more aggressive tumor. Although, in general, larger tumors are high grade and small tumors are low grade, exceptions occur (Epstein et al, 1994a). There is a tendency to hypothesize that tumors begin as low-grade tumors and, on reaching a certain size, dedifferentiate into higher-grade lesions, accounting for the relationship between size and grade. Alternatively, high-grade tumors may be high grade at their inception but are detected at an advanced size because of their rapid growth. Similarly, low-grade tumors may evolve so slowly that they tend to be detected at lower volumes. During a 2- to 3-year period after biopsy, there is no evidence that prostate cancer grades worsen significantly (Sheridan et al, 2008).
Assessment of Needle Biopsy Specimens
Processing
When biopsy specimens are taken from different areas of the prostate, they should be submitted to pathology in separate containers (Table 96–2). As long as cores are submitted in separate containers or the cores are in the same container yet specified by the urologist as to their location (i.e., by different color inks), pathologists should assign individual Gleason scores to separate cores (Epstein et al, 2005). If cores are combined in a container, one can try to give separate scores for each core or can give an overall Gleason score. For example, in a case with Gleason score 4 + 4 = 8 on one core and with pattern 3 (3 + 3 = 6, 3 + 4 = 7, 4 + 3 = 7) on other cores in the same container, the overall score for that container, averaging all involved needle biopsy specimens together as if they were one long positive core, would be Gleason score 4 + 3 = 7 or 3 + 4 = 7, depending on whether pattern 4 or 3 predominated.
Table 96–2 Reasons to Submit Needle Cores in Separate Jars for Each Sextant Site
Differential Diagnosis
The underdiagnosis of limited adenocarcinoma of the prostate on needle biopsy is one of the most frequent problems in prostate pathology (Epstein, 2004). There are also numerous benign mimickers of adenocarcinoma of the prostate (Srigley, 2004). In some of these cases, the use of antibodies to high-molecular-weight cytokeratin and p63 may resolve the diagnosis (Wojno and Epstein, 1995). Benign glands contain basal cells and are labeled with these antibodies, whereas prostate cancer shows no staining. Immunohistochemistry with antibodies to α-methylacyl-CoA racemase, which preferentially labels prostatic carcinoma and high-grade PIN, can also be used as an adjunct in the diagnosis of limited cancer, yet pathologists must be careful because false-positive and false-negative staining with α-methylacyl-CoA racemase has been reported (Jiang et al, 2004).
In certain cases, there are findings suggestive of but not diagnostic of carcinoma. The incidence of atypical needle biopsy specimens reported from the 39 studies in the literature averages 7.6% with a median of 5.2% (Epstein and Herawi, 2006). Pathologists should sign out atypical cases descriptively as “a focus of atypical glands” rather than use ambiguous terminology such as “atypical hyperplasia” or “atypical small acinar proliferation.” A comment should be added in the report describing why the focus is suggestive of cancer yet not diagnostic, with a recommendation for repeat biopsy. In this way, there is no confusion in the urologist’s mind that he or she is dealing with a lesion likely to be infiltrating cancer but for which the pathologist is not comfortable establishing the diagnosis. The likelihood of cancer after an atypical diagnosis is 42% or 49%, according to the two largest studies from the Mayo Clinic and Johns Hopkins Hospital, respectively (Iczkowski et al, 1997; Chan and Epstein, 1999). Surprisingly, in men with a prior atypical biopsy result, the level of serum PSA elevation or the results of digital rectal examination do not correlate with the risk of a subsequent biopsy specimen showing carcinoma. Regardless of the serum PSA level, all patients with an initial atypical diagnosis on needle biopsy should undergo a repeat biopsy. In a series of second-opinion prostate needle biopsy cases that were patient- and urologist-driven, on expert review, only 36.8% of outside atypical diagnoses were confirmed; on review, 92 of the 204 (45.1%) outside atypical cases in one study were diagnosed as cancer and 33 (16.2%) as benign (Chan and Epstein, 2005). Cases diagnosed as atypical have the highest likelihood of being changed on expert review, and urologists should consider sending such cases for consultation to attempt to resolve the diagnosis as either definitively benign or malignant before subjecting the patient to repeat biopsy.
Prognosis
Adverse findings on needle biopsy, in terms of Gleason grade and tumor extent, in general accurately predict adverse findings in the radical prostatectomy specimen. However, favorable findings on needle biopsy do not necessarily predict favorable findings in the radical prostatectomy specimen, as a result of sampling error. The ways in which cancer may be measured on needle biopsy include number of positive cores, total millimeters of cancer among all cores, percentage of cancer per core, and total percentage of cancer in the entire specimen. An equal number of studies claim superiority of one technique over the other, with no one technique adopted uniformly (Epstein, 2004). It is proposed that pathologists report the number of positive cores along with one other measurement of tumor extent. At the Johns Hopkins Hospital, the percentages of each involved core are reported, for example, as “adenocarcinoma of the prostate, Gleason grade 3 + 3 = 6, involving two cores (70%, 30%).” By combining needle biopsy grade with clinical stage and serum PSA values, the extent of cancer within the prostate can be more accurately predicted (Makarov et al, 2007).
Whether perineural invasion that is extensive enough to be sampled on needle biopsy signals an increased risk of extraprostatic extension of cancer is controversial. Harnden (2007), in a systematic review of the importance of perineural invasion on needle biopsy to predict recurrence following radiotherapy or surgery, concluded that the weight of the evidence in 21 studies showed it was a significant prognostic indicator, particularly in specific patient subgroups defined by serum PSA and Gleason scores. Over two thirds of the studies using external beam radiotherapy but none using brachytherapy showed prognostic significance for perineural invasion. Given that perineural invasion is readily identifiable in most cases, that it is prognostic in some studies, although the data is conflicting, and that it is uncertain what factors an individual clinician may consider in treatment decisions, it is the opinion of these authors that perineural invasion should be noted on the biopsy pathology report.
One can also use information from needle biopsy pathology reports to help determine whether to sacrifice the neurovascular bundle on a given side in cases with a higher likelihood of extraprostatic extension (Shah et al, 2003; Ohori et al, 2004; Tsuzuki et al, 2005).
There is emerging data that atrophy and associated inflammation are linked with prostate carcinogenesis (DeMarzo et al, 2003). However, the hypothesis is that these factors are involved in the initiation of prostate cancer and are not proximately related to cancer by the time atrophy is identified on needle biopsy. Atrophy of all morphologic types are very common on needle biopsy and are not associated with an increased risk of cancer or PIN on subsequent biopsy (Postma et al, 2005).
Assessment of Transurethral Resection Specimens
Processing
The recommended system is based on the percentage of the specimen involved by tumor, with 5% being the cutoff between stage T1a and T1b (Cantrell et al, 1981). All stage T1b tumors are detected by processing of between 6 and 8 cassettes of a transurethral resection (TUR) specimen. By processing 8 to 10 cassettes, more than 90% of stage T1a lesions are identified (Newman et al, 1982; Murphy et al, 1986; Vollmer, 1986; Rohr, 1987). Depending on the institution, all TUR tissues may be examined in men younger than 65 years in whom aggressive therapy for stage T1a disease might be pursued. There are conflicting opinions as to whether the grade of the cancer should be factored into the staging system for limited cancer detected on transurethral prostatectomy. On the basis of data from the author’s institution, cases with less than 5% of tumor detected on TUR and with Gleason scores below 7 are staged T1a; if the Gleason scores are above 7, they should be staged T1b (Larsen et al, 1991).
Differential Diagnosis
One of the most common lesions to be confused with low-grade adenocarcinoma is adenosis (atypical adenomatous hyperplasia) (Gaudin and Epstein, 1994). In the author’s institution, 1.6% of benign TUR specimens and 0.8% of all needle biopsy specimens contain adenosis. It is characteristically found in the transition zone of the prostate, is frequently multifocal, and most often is an incidental finding in TURs performed for urinary obstruction. Although adenosis mimics carcinoma, there is no conclusive evidence suggesting that patients with adenosis have an increased risk of harboring or developing adenocarcinoma of the prostate.
Assessment of Radical Prostatectomy Specimens
Assessment
Within institutions that do not totally embed radical prostatectomy specimens, there are sampling techniques that provide accurate pathologic staging (Hall et al, 1992; Sehdev et al, 2001). Whole-mount sectioning of the prostate provides more aesthetically pleasing sections for teaching and publication, yet the information obtained by routine sections is identical. In a minority of cases, routinely processed thinly sliced sections allow identification of positive margins not identified with use of thicker slices of tissue, which are necessary for whole-mount processing.
It is recommended that pathologists assign a separate grade to each dominant tumor nodule. Most often, the dominant nodule is the largest tumor, which is also the tumor associated with the highest stage and highest grade. In the unusual occurrence of a nondominant nodule (i.e., smaller nodule) that is of higher stage, one should also assign a grade to that nodule. If one of the smaller nodules is the highest-grade focus within the prostate, the grade of this smaller nodule should also be recorded. In general, this will be the exception; in most cases, separate grades will be assigned to only one or at most two dominant nodules.
If the urologist plans on aborting radical prostatectomy when lymph nodes are positive, the pathologist should freeze representative nodes, because approximately two thirds of microscopic metastases can be identified with random frozen sections. For urologists who perform radical prostatectomy in the face of microscopic metastases for local control, it has been demonstrated that men with a Gleason score below 8 on needle biopsy have a relatively prolonged interval before distant metastases appear; the pathologist need not examine the nodes at frozen section in these cases, because the urologist will proceed with surgery even if nodal metastases are found (Sgrignoli et al, 1994). Preoperatively, the physician and patient should decide and agree on what to do if there is a positive lymph node when the biopsy grade is Gleason score 8 to 10. In patients with Gleason scores of 8 to 10 on needle biopsy, it has been shown that if the lymph nodes are involved, these men will not benefit from radical surgery, whereas if the nodes are free of tumor, cure after radical prostatectomy is possible (Sgrignoli et al, 1994). Current data support that in men with Gleason scores of 8 to 10 on biopsy, all the nodes should be examined at the time of surgery, and the urologist should abort the prostatectomy if the nodes are positive. Other features that have correlated with prognosis are extent, grade, and ploidy of nodal metastases (Boorjian et al, 2007).
Prognosis
Only 25% of men with seminal vesicle invasion and none with lymph node metastases are biochemically progression-free at 10 years after radical prostatectomy, with grade and, in some studies, margins of resection influencing prognosis (Epstein et al, 1993b; Salomon et al, 2003; Bloom et al, 2004; Johnson et al, 2004). There is some controversy as to the definition of seminal vesicle invasion. Some investigators recognize both an intraprostatic and an extraprostatic portion of the seminal vesicle, whereby one can have organ-confined disease and seminal vesicle invasion (Ohori et al, 1993; Wheeler et al, 1998). However, these studies have demonstrated that the finding of intraprostatic seminal vesicle invasion does not adversely affect prognosis beyond that of finding extraprostatic extension without seminal vesicle invasion. Consequently, the author diagnoses seminal vesicle invasion only when the tumor extends out of the prostate into the muscle coat of the seminal vesicle; with use of this definition, seminal vesicle invasion is associated with a markedly adverse prognosis (Epstein et al, 1993b). The presence of extraprostatic extension and its extent also influence progression. Pathologists frequently underdiagnose extraprostatic extension. When tumor extends out of the prostatic gland, it induces a dense desmoplastic response in the periprostatic adipose tissue, where it can be difficult to judge whether the tumor has extended out of the gland or is within the fibrous tissue of the prostate. Posterior, posterolateral, and lateral regions account for 18%, 17%, and 4% of positive margins, respectively, in the author’s material. These sites parallel the location of most stage T2 carcinomas. In particular, these sites are disproportionately involved toward the apex (Stamey et al, 1990; Epstein et al, 1993b). Only approximately 50% of men with positive margins progress after radical prostatectomy (Epstein et al, 1993b). A major source of this discrepancy is that even in cases in which margins histologically appear to be positive, additional tissue removed from the site does not always show tumor (Epstein, 1990). It has also been demonstrated that tumor close to the margins does not result in a higher risk of recurrence (Epstein, 1990; Epstein and Sauvageot, 1997). Artifactually positive margins relate to the scant tissue surrounding the prostate, which may easily be disrupted during surgery or pathologic evaluation of the gland. Positive margins also may arise as a result of the surgical transection of intraprostatic tumor (intraprostatic incision). The reported incidence of intraprostatic incision ranges from 1.3% to as high as 71%. In the author’s opinion, much of this variation relates to the difficulties described in recognizing extraprostatic extension. If an extraprostatic tumor associated with a desmoplastic stromal response at a margin is misdiagnosed as organ-confined, it will be misclassified as a positive margin due to intraprostatic incision. At the apex of the prostate, the boundaries of the prostate are especially vague such that it can be difficult to determine whether a cancer with a positive margin is intraprostatic. The other relatively frequent site of intraprostatic incision is in the region of the neurovascular bundles, where the urologist tries to preserve the bundle(s) for potency, yet cuts into the prostate. This author diagnoses a positive margin as a result of intraprostatic incision only if both tumor and benign glands are transected in the same area and are present at the inked margin. Intraprostatic incision is associated with an increased risk of postoperative progression equivalent to that associated with focal extraprostatic extension and a positive margin (Chuang et al, 2007). Preliminary studies have not shown a difference in sites of positive margins between open surgery and robotic radical prostatectomy, with the apex being the most commonly affected region (Smith et al, 2007). In a multivariate analysis, Gleason grade, extraprostatic extension, and margins of resection are strong independent predictors of progression (i.e., elevated postoperative serum PSA level). A more refined prognostication is not needed for men with Gleason scores of 2 to 4, because these men are almost all invariably cured by surgery. Men with Gleason scores of 8 to 10 have a poor prognosis after prostatectomy, with nodal metastases as the major prognostic determinant. Of cases with negative seminal vesicles and lymph nodes, Gleason scores of 5 to 7 account for 88% of tumors removed by radical prostatectomy and have a prognosis that can be stratified by various clinical and pathologic parameters (Epstein, in press; Epstein et al, 1993b; Diblasio and Kattan, 2003; Stephenson et al, 2005).
Tumor volume correlates well with pathologic stage and Gleason grade in clinical stage T2 cancers (McNeal et al, 1990). However, most studies have found that tumor volume does not independently predict postradical prostatectomy progression once grade, pathologic stage, and margins are taken into account (Epstein et al, 1993a; Kikuchi et al, 2004). Consequently, it is not currently recommended that tumor volume be calculated for clinical purposes in radical prostatectomy specimens. Rather, there should be some overall subjective indication of tumor volume to identify those cases with a minute amount of tumor and an excellent prognosis versus those cases with extensive tumor and a worse prognosis.
The evaluation of ploidy on radical prostatectomy specimens is controversial (Shankey et al, 1993; Adolfsson, 1994). The strongest data to support the prognostic importance of ploidy are in patients undergoing radical prostatectomy with pelvic node metastases (Boorjian et al, 2007). This author does not recommend that ploidy be routinely evaluated on radical prostatectomy specimens.
Adenocarcinoma with Treatment Effect
The major effect of combination endocrine therapy on benign prostate tissue is the presence of atrophic changes with immature squamous metaplasia (Vailancourt et al, 1996). This therapy may result in three different histologic patterns in prostate cancer: (1) atrophic prostate cancer, (2) cells with pyknotic nuclei with abundant xanthomatous cytoplasm resembling histiocytes, and (3) individual tumor cells resembling lymphocytes (Vailancourt et al, 1996). One of the problems with evaluating carcinomas that have been treated with hormone therapy is that the grade often appears artifactually higher (Smith and Murphy, 1994). Pathologists should not assign a Gleason score to carcinomas with treatment effect. However, if other areas of the tumor do not show a pronounced hormone effect, these areas can be Gleason graded. It has been demonstrated that finasteride does not alter the histology of either benign or malignant tissue (Yang et al, 1999; Rubin et al, 2005).
Radiation-related changes in the prostate are usually seen in patients who have been irradiated for adenocarcinoma of the prostate. When carcinoma is present in a biopsy specimen obtained 12 to 18 months after radiotherapy, it is a powerful predictor of either local or distant postirradiation failure (Scardino et al, 1986). Some studies have demonstrated that the morphologic appearance of the cancer (whether or not the cancer appears altered by the radiation) correlates with prognosis (Crook et al, 1997). Radiation alters the histologic features of benign prostate tissue so that it may mimic prostate cancer (Bostwick et al, 1982). Radiation atypia in benign prostate glands may persist for a long time (up to 72 months) after the initial treatment, resulting in a significant pitfall in evaluating prostate biopsy specimens (Magi-Galluzzi et al, 2003). If clinicians are aware of such treatment, this information should be provided to the pathologist.
Subtypes of Prostate Adenocarcinoma
Mucinous adenocarcinoma of the prostate gland is one of the least common morphologic variants of prostatic carcinoma (Epstein and Lieberman, 1985; Ro et al, 1990). They behave like nonmucinous prostate carcinomas, having a propensity to develop bone metastases with advanced disease. Mucinous adenocarcinoma of the prostate treated by radical prostatectomy is not more aggressive than nonmucinous prostate cancer (Osunkoya et al, 2008). Even in ordinary adenocarcinomas of the prostate without light microscopic evidence of neuroendocrine differentiation, almost half show neuroendocrine differentiation on evaluation with immunohistochemistry for multiple neuroendocrine markers (di Sant’Agnese, 1992). Most studies do not demonstrate a convincing relation between the extent of neuroendocrine differentiation in ordinary prostate cancer and prognosis. Small cell carcinomas of the prostate are identical to small cell carcinomas of the lung (Tetu et al, 1987, 1989). In approximately 50% of the cases, the tumors are mixed small cell carcinoma and adenocarcinoma of the prostate. Although most small cell tumors of the prostate lack clinically evident hormone production, they account for the majority of prostatic tumors with clinically evident adrenocorticotropic hormone or antidiuretic hormone production. The average survival of patients with small cell carcinoma of the prostate is less than a year. There is no difference in prognosis between patients with pure small cell carcinoma and those with mixed glandular and small cell carcinomas. Small cell carcinomas are not assigned a Gleason grade.
Between 0.4% and 0.8% of prostatic adenocarcinomas arise from prostatic ducts (Epstein and Woodruff, 1986; Christensen et al, 1991). When prostatic duct adenocarcinomas arise in the large primary periurethral prostatic ducts, they may grow as an exophytic lesion into the urethra, most commonly in and around the verumontanum, and give rise to either obstructive symptoms or hematuria. Tumors arising in the more peripheral prostatic ducts may present like ordinary (acinar) adenocarcinoma of the prostate and may be diagnosed on needle biopsy (Brinker et al, 1999). Tumors are often underestimated clinically because rectal examination findings and serum PSA levels may be normal. Most prostatic duct adenocarcinomas are advanced stage at presentation and have an aggressive course; they should be regarded as Gleason score 4 + 4 = 8, because of their shared cribriform morphologic features with acinar adenocarcinoma Gleason score 8 and similar prognosis (Brinker et al, 1999). Pure primary squamous carcinoma of the prostate is rare and is associated with poor survival (Parwani et al, 2004). These tumors develop osteolytic metastases, do not respond to estrogen therapy, and do not develop elevated serum acid phosphatase levels with metastatic disease. More commonly, squamous differentiation occurs in the primary and metastatic deposits of adenocarcinomas that have been treated with estrogen therapy. Sarcomatoid carcinomas (carcinosarcomas) have also been reported within the prostate and have a dismal prognosis (Lauwers et al, 1993).
Mesenchymal Tumors
Sarcomas of the prostate account for 0.1% to 0.2% of all malignant prostatic tumors (Sexton et al, 2001). Rhabdomyosarcomas are the most frequent mesenchymal tumor within the prostate and are seen almost exclusively in childhood. Leiomyosarcomas are the most common sarcomas involving the prostate in adults (Cheville et al, 1995). A spindle cell lesion that can occur at any age can closely simulate a leiomyosarcoma. Inflammatory myofibroblastic tumors may occur soon after TUR or without a history of TUR (Montgomery et al, 2006). There are also mesenchymal tumors of the prostate arising from the unique prostatic specialized stroma. These lesions range from prostatic stromal tumors of uncertain malignant potential to prostatic sarcomas. On histologic examination, these lesions are variable; one subtype resembles a tumor seen in the breast and is termed phyllodes tumor of the prostate (Herawi and Epstein, 2006).
Urothelial Carcinoma
Primary urothelial carcinoma of the prostate without bladder involvement accounts for 1% to 4% of all prostate carcinomas (Sawczuk et al, 1985). Primary urothelial carcinomas of the prostate show a propensity to infiltrate the bladder neck and the surrounding soft tissue such that more than 50% of the patients present with stage T3 or T4 tumors (Greene et al, 1976). Twenty percent of the patients present with distant metastases, with bone (predominantly osteolytic), lung, and liver being the most common sites. Treatment of stage T3 disease with radiation results in a 5-year survival of approximately 34%. In the minority of cases with tumor localized to the prostate (T2), radical surgery has resulted in long-term disease-free survival in several patients.
More commonly, urothelial carcinoma involves prostatic ducts and acini in patients with a history of carcinoma in situ (CIS) of the bladder who have been treated for a period of months to years with intravesical topical chemotherapy (Schellhammer et al, 1977; Mahadevia et al, 1986; Wood et al, 1989; Njinou Ngninkeu et al, 2003). Between 35% and 45% of cystoprostatectomies performed for urothelial carcinoma contain prostatic involvement. However, this number is dependent on the amount of histologic sampling of the prostate tissue and may be much higher in completely mapped specimens. If cystoprostatectomy is performed and only intraductal urothelial carcinoma is present, the prostatic involvement does not worsen the prognosis, which is determined by the stage of the bladder tumor (Esrig et al, 1996). Intraductal urothelial carcinoma of the prostate appears to involve the prostate by direct extension from the overlying urethra, which is usually involved by CIS. Intraductal and infiltrating urothelial carcinoma involving the prostate tends to be seen in higher-stage bladder tumors, in which the patients have a poor prognosis attributable to either advanced bladder or prostatic disease. A minority of these cases will have low-stage bladder tumor and a poorer prognosis, demonstrating the adverse effect of prostatic stromal infiltration (Esrig et al, 1996). Extensive sampling of the periurethral area in cystoprostatectomy specimens performed for urothelial carcinoma is necessary to identify and to evaluate the prostate for urothelial carcinoma.
Finally, one may find direct invasion from bladder urothelial carcinoma into the stroma of the prostate. The distinction between poorly differentiated urothelial carcinoma and poorly differentiated adenocarcinoma of the prostate can be difficult. Approximately 95% of poorly differentiated prostatic adenocarcinomas show PSA staining, although it may be focal (Chuang et al, 2007). In some cases of poorly differentiated prostatic adenocarcinomas, there may be weak or negative PSA staining where the tumor reacts to a greater degree with newer prostate specific markers, including prostate specific membrane antigen (PSMA), p501S (Prostein), and NKX 3.1 (Chuang et al, 2007). However, the lack of immunoreactivity to prostate specific markers in a poorly differentiated tumor within the prostate, especially if present in limited amount, does not exclude the diagnosis of a poorly differentiated prostatic adenocarcinoma. Although CK7 and CK20 are more frequently seen in urothelial carcinoma as compared to adenocarcinoma of the prostate, they may also be positive in adenocarcinoma of the prostate. Markers that label urothelial carcinoma and not prostate carcinoma include uroplakin and thrombomodulin (49% to 69% sensitivity) and TP63 and high molecular weight cytokeratin (83% to 90% sensitivity) (Chuang et al, 2007).
Miscellaneous Malignant Tumors
Primary prostatic lymphoma without lymph node involvement appears to be much less common than secondary infiltration of the prostate (Bostwick and Mann, 1985). The most common form of leukemic involvement of the prostate is that of chronic lymphocytic leukemia, although monocytic, granulocytic, and lymphoblastic leukemias have also been found in the prostate (Dajani and Burke, 1976).
Key Points
Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics: tumours of the urinary system and male genital organs. Lyon (France): IARC Press; 2004.
Epstein JI, Cubilla AL, Humphrey PA. AFIP atlas of tumor pathology, series 4. Tumors of the prostate gland, seminal vesicles, penis, and scrotum. Washington (DC): ARP Press; 2011.
Epstein JI, Netto GJ. Prostate biopsy interpretation. Philadelphia: Lippincott Williams & Wilkins; 2008.
Humphrey PA. Prostate pathology. Chicago: ASCP Press; 2003.
Abdel-Khalek M, El-Baz M, Ibrahiem el-H. Predictors of prostate cancer on extended biopsy in patients with high-grade prostatic intraepithelial neoplasia: a multivariate analysis model. BJU Int. 2004;94:528-533.
Adolfsson J. Prognostic value of deoxyribonucleic acid content in prostate cancer: a review of current results. Int J Cancer. 1994;58:211-216.
Allsbrook WCJr, Mangold KA, Johnson MH, et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum Pathol. 2001;32:81-88.
Allsbrook WCJr, Mangold KA, Johnson MH, et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: urologic pathologists. Hum Pathol. 2001;32:74-80.
Ayala AG, Ro JY, Babaian R, et al. The prostatic capsule: does it exist? Its importance in the staging and treatment of prostatic carcinoma. Am J Surg Pathol. 1989;13:21-27.
Baydar DE, Baseskioglu B, Ozen H, Geyik PO. Prognostic significance of lymphovascular invasion in clinically localized prostate cancer after radical prostatectomy. Sci World J. 2008;8:303-312.
Bloom KD, Richie JP, Schultz D, et al. Invasion of seminal vesicles by adenocarcinoma of the prostate: PSA outcome determined by preoperative and postoperative factors. Urology. 2004;63:333-336.
Boorjian SA, Thompson RH, Siddiqui S, et al. Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol. 2007;178:864-870. discussion 870–1
Bostwick DG, Egbert BM, Fajardo LF. Radiation injury of the normal and neoplastic prostate. Am J Surg Pathol. 1982;6:541-551.
Bostwick DG, Mann RB. Malignant lymphomas involving the prostate. A study of 13 cases. Cancer. 1985;56:2932-2938.
Bostwick DG, Pacelli A, Lopez-Beltran A. Molecular biology of prostatic intraepithelial neoplasia. Prostate. 1996;29:117-134.
Brawn PN. The dedifferentiation of prostate carcinoma. Cancer. 1983;52:246-251.
Brinker DA, Potter SR, Epstein JI. Ductal adenocarcinoma of the prostate diagnosed on needle biopsy: correlation with clinical and radical prostatectomy findings and progression. Am J Surg Pathol. 1999;23:1471-1479.
Byar DP, Mostofi FK. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Examined by the step-section technique. Cancer. 1972;30:5-13.
Cantrell BB, DeKlerk DP, Eggleston JC, et al. Pathological factors that influence prognosis in stage A prostatic cancer: the influence of extent versus grade. J Urol. 1981;125:516-520.
Cerveira N, Ribeiro FR, Peixoto A, et al. TMPRSS2-ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia. 2006;8:826-832.
Chan TY, Epstein JI. Follow-up of atypical prostate needle biopsies suspicious for cancer. Urology. 1999;53:351-355.
Chan TY, Epstein JI. Patient and urologist driven second opinion of prostate needle biopsies. J Urol. 2005;174:1390-1394. discussion 1394; author reply 1394
Chan TY, Partin AW, Walsh PC, Epstein JI. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823-827.
Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76:1422-1427.
Cho KR, Epstein JI. Metastatic prostatic carcinoma to supradiaphragmatic lymph nodes. A clinicopathologic and immunohistochemical study. Am J Surg Pathol. 1987;11:457-463.
Christensen WN, Partin AW, Walsh PC, Epstein JI. Pathologic findings in clinical stage A2 prostate cancer: relation of tumor volume, grade, and location to pathologic stage. Cancer. 1990;65:1021-1027.
Christensen WN, Steinberg G, Walsh PC, Epstein JI. Prostatic duct adenocarcinoma. Findings at radical prostatectomy. Cancer. 1991;67:2118-2124.
Chuang AY, Nielsen ME, Hernandez DJ, et al. The significance of positive surgical margin in areas of capsular incision in otherwise organ confined disease at radical prostatectomy. J Urol. 2007;178:1306-1310.
Cohen RJ, Wheeler TM, Bonkhoff H, Rubin MA. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch Pathol Lab Med. 2007;131:1103-1109.
Crook JM, Bahadur YA, Robertson SJ, et al. Evaluation of radiation effect, tumor differentiation, and prostate specific antigen staining in sequential prostate biopsies after external beam radiotherapy for patients with prostate carcinoma. Cancer. 1997;79:81-89.
Dajani YF, Burke M. Leukemic infiltration of the prostate: a case study and clinicopathological review. Cancer. 1976;38:2442-2446.
DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955-964.
Diblasio CJ, Kattan MW. Use of nomograms to predict the risk of disease recurrence after definitive local therapy for prostate cancer. Urology. 2003;62(Suppl. 1):9-18.
di Sant’Agnese PA. Neuroendocrine differentiation in human prostatic carcinoma. Hum Pathol. 1992;23:287-296.
Epstein JI. Evaluation of radical prostatectomy capsular margins of resection. The significance of margins designated as negative, closely approaching, and positive. Am J Surg Pathol. 1990;14:626-632.
Epstein JI. Diagnosis and reporting of limited adenocarcinoma of the prostate on needle biopsy. Mod Pathol. 2004;17:307-315.
Epstein JI. Prognostic significance of tumor volume in radical prostatectomy and needle biopsy specimens. J Urol (in press).
Epstein JI, Allsbrook WCJr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228-1242.
Epstein JI, Carmichael M, Partin AW, Walsh PC. Is tumor volume an independent predictor of progression following radical prostatectomy? A multivariate analysis of 185 clinical stage B adenocarcinomas of the prostate with 5 years of follow-up. J Urol. 1993;149:1478-1481.
Epstein JI, Carmichael MJ, Partin AW, Walsh PC. Small high grade adenocarcinoma of the prostate in radical prostatectomy specimens performed for nonpalpable disease: pathogenetic and clinical implications. J Urol. 1994;151:1587-1592.
Epstein JI, Grignon DJ, Humphrey PA, et al. Interobserver reproducibility in the diagnosis of prostatic intraepithelial neoplasia. Am J Surg Pathol. 1995;19:873-886.
Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006;175:820-834.
Epstein JI, Lieberman PH. Mucinous adenocarcinoma of the prostate gland. Am J Surg Pathol. 1985;9:299-308.
Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993;71:3582-3593.
Epstein JI, Sauvageot J. Do close but negative margins in radical prostatectomy specimens increase the risk of postoperative progression? J Urol. 1997;157:241-243.
Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368-374.
Epstein JI, Woodruff JM. Adenocarcinoma of the prostate with endometrioid features. A light microscopic and immunohistochemical study of ten cases. Cancer. 1986;57:111-119.
Esrig D, Freeman JA, Elmajian DA, et al. Transitional cell carcinoma involving the prostate with a proposed staging classification for stromal invasion. J Urol. 1996;156:1071-1076.
Fine SW, Epstein JI. A contemporary study correlating prostate needle biopsy and radical prostatectomy gleason score. J Urol. 2008;179:1335-1338. discussion 1338–9
Freedland SJ, Partin AW, Epstein JI, Walsh PC. Biochemical failure after radical prostatectomy in men with pathologic organ-confined disease: PT2a versus pT2b. Cancer. 2004;100:1646-1649.
Fry DE, Amin M, Harbrecht PJ. Rectal obstruction secondary to carcinoma of the prostate. Ann Surg. 1979;189:488-492.
Gaudin PB, Epstein JI. Adenosis of the prostate. Histologic features in transurethral resection specimens. Am J Surg Pathol. 1994;18:863-870.
Gaudin PB, Sesterhenn IA, Wojno KJ, et al. Incidence and clinical significance of high-grade prostatic intraepithelial neoplasia in TURP specimens. Urology. 1997;49:558-563.
Ghani KR, Grigor K, Tulloch DN, et al. Trends in reporting Gleason score 1991 to 2001: changes in the pathologist’s practice. Eur Urol. 2005;47:196-201.
Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58-64.
Greene DR, Wheeler TM, Egawa S, et al. A comparison of the morphological features of cancer arising in the transition zone and in the peripheral zone of the prostate. J Urol. 1991;146:1069-1076.
Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: histologic features and clinical significance. Mod Pathol. 2006;19:1528-1535.
Haggman MJ, Macoska JA, Wojno KJ, Oesterling JE. The relationship between prostatic intraepithelial neoplasia and prostate cancer: critical issues. J Urol. 1997;158:12-22.
Hall GS, Kramer CE, Epstein JI. Evaluation of radical prostatectomy specimens. A comparative analysis of sampling methods. Am J Surg Pathol. 1992;16:315-324.
Harnden P, Shelley MD, Clements H, et al. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer. 2007;109:13-24.
Hassan MO, Maksem J. The prostatic perineural space and its relation to tumor spread: an ultrastructural study. Am J Surg Pathol. 1980;4:143-148.
Herawi M, Epstein JI. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2006;30:694-704.
Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624-1633.
Iczkowski KA, MacLennan GT, Bostwick DG. Atypical small acinar proliferation suspicious for malignancy in prostate needle biopsies: clinical significance in 33 cases. Am J Surg Pathol. 1997;21:1489-1495.
Jiang Z, Woda BA, Wu CL, Yang XJ. Discovery and clinical application of a novel prostate cancer marker: alpha-methylacyl CoA racemase (P504S). Am J Clin Pathol. 2004;122:275-289.
Johnson CW, Anastasiadis AG, McKiernan JM, et al. Prognostic indicators for long term outcome following radical retropubic prostatectomy for prostate cancer involving the seminal vesicles. Urol Oncol. 2004;22:107-111.
Kikuchi E, Scardino PT, Wheeler TM, et al. Is tumor volume an independent prognostic factor in clinically localized prostate cancer? J Urol. 2004;172:508-511.
Lane Z, Epstein JI, Ayub S, Netto GJ. Prostatic adenocarcinoma in colorectal biopsy: clinical and pathologic features. Hum Pathol. 2008;39:543-549.
Larsen MP, Carter HB, Epstein JI. Can stage A1 tumor extent be predicted by transurethral resection tumor volume, per cent or grade? A study of 64 stage A1 radical prostatectomies with comparison to prostates removed for stages A2 and B disease. J Urol. 1991;146:1059-1063.
Lauwers GY, Schevchuk M, Armenakas N, Reuter VE. Carcinosarcoma of the prostate. Am J Surg Pathol. 1993;17:342-349.
Lefkowitz GK, Taneja SS, Brown J, et al. Followup interval prostate biopsy 3 years after diagnosis of high grade prostatic intraepithelial neoplasia is associated with high likelihood of prostate cancer, independent of change in prostate specific antigen levels. J Urol. 2002;168:1415-1418.
Magi-Galluzzi C, Sanderson H, Epstein JI. Atypia in nonneoplastic prostate glands after radiotherapy for prostate cancer: duration of atypia and relation to type of radiotherapy. Am J Surg Pathol. 2003;27:206-212.
Mahadevia PS, Koss LG, Tar IJ. Prostatic involvement in bladder cancer. prostate mapping in 20 cystoprostatectomy specimens. Cancer. 1986;58:2096-2102.
Makarov DV, Trock BJ, Humphreys EB, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095-1101.
McNeal JE. Origin and development of carcinoma in the prostate. Cancer. 1969;23:24-34.
McNeal JE, Bostwick DG. Intraductal dysplasia: a premalignant lesion of the prostate. Hum Pathol. 1986;17:64-71.
McNeal JE, Reese JH, Redwine EA, et al. Cribriform adenocarcinoma of the prostate. Cancer. 1986;58:1714-1719.
McNeal JE, Villers AA, Redwine EA, et al. Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer. 1990;66:1225-1233.
McNeal JE, Yemoto CE. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol. 1996;20:802-814.
Mellinger GT. Prognosis of prostatic carcinoma. Recent Results Cancer Res. 1977;60:61-72.
Merrimen JL, Jones G, Walker D, et al. Multifocal high grade prostatic intraepithelial neoplasia is a significant risk factor for prostatic adenocarcinoma. J Urol. 2009;182:485-490.
Montgomery EA, Shuster DD, Burkart AL, et al. Inflammatory myofibroblastic tumors of the urinary tract: a clinicopathologic study of 46 cases, including a malignant example inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol. 2006;30:1502-1512.
Murphy WM, Dean PJ, Brasfield JA, Tatum L. Incidental carcinoma of the prostate. How much sampling is adequate? Am J Surg Pathol. 1986;10:170-174.
Netto GJ, Epstein JI. Widespread high-grade prostatic intraepithelial neoplasia on prostatic needle biopsy: a significant likelihood of subsequently diagnosed adenocarcinoma. Am J Surg Pathol. 2006;30:1184-1188.
Newman AJJr, Graham MA, Carlton CEJr, Lieman S. Incidental carcinoma of the prostate at the time of transurethral resection: importance of evaluating every chip. J Urol. 1982;128:948-950.
Njinou Ngninkeu B, Lorge F, Moulin P, et al. Transitional cell carcinoma involving the prostate: a clinicopathological retrospective study of 76 cases. J Urol. 2003;169:149-152.
Ohori M, Kattan MW, Koh H, et al. Predicting the presence and side of extracapsular extension: a nomogram for staging prostate cancer. J Urol. 2004;171:1844-1849. discussion 1849
Ohori M, Scardino PT, Lapin SL, et al. The mechanisms and prognostic significance of seminal vesicle involvement by prostate cancer. Am J Surg Pathol. 1993;17:1252-1261.
Osunkoya AO, Nielsen ME, Epstein JI. Prognosis of mucinous adenocarcinoma of the prostate treated by radical prostatectomy: a study of 47 cases. Am J Surg Pathol. 2008;32:468-472.
Pacelli A, Bostwick DG. Clinical significance of high-grade prostatic intraepithelial neoplasia in transurethral resection specimens. Urology. 1997;50:355-359.
Pan CC, Potter SR, Partin AW, Epstein JI. The prognostic significance of tertiary Gleason patterns of higher grade in radical prostatectomy specimens: a proposal to modify the Gleason grading system. Am J Surg Pathol. 2000;24:563-569.
Parwani AV, Kronz JD, Genega EM, et al. Prostate carcinoma with squamous differentiation: an analysis of 33 cases. Am J Surg Pathol. 2004;28:651-657.
Perner S, Mosquera JM, Demichelis Fet al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882-888.
Postma R, Schroder FH, van der Kwast TH. Atrophy in prostate needle biopsy cores and its relationship to prostate cancer incidence in screened men. Urology. 2005;65:745-749.
Ro JY, Grignon DJ, Ayala AG, et al. Mucinous adenocarcinoma of the prostate: histochemical and immunohistochemical studies. Hum Pathol. 1990;21:593-600.
Robinson BD, Epstein JI. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings. J Urol. 2010;184:1328-1333.
Rohr LR. Incidental adenocarcinoma in transurethral resections of the prostate. Partial versus complete microscopic examination. Am J Surg Pathol. 1987;11:53-58.
Ronnett BM, Carmichael MJ, Carter HB, Epstein JI. Does high grade prostatic intraepithelial neoplasia result in elevated serum prostate specific antigen levels? J Urol. 1993;150:386-389.
Rubin MA, Allory Y, Molinié V, et al. Effects of long-term finasteride treatment on prostate cancer morphology and clinical outcome. Urology. 2005;66:930-934.
Rubin MA, de La Taille A, Bagiella E, et al. Cribriform carcinoma of the prostate and cribriform prostatic intraepithelial neoplasia: incidence and clinical implications. Am J Surg Pathol. 1998;22:840-848.
Salomon L, Porcher R, Anastasiadis AG, et al. Introducing a prognostic score for pretherapeutic assessment of seminal vesicle invasion in patients with clinically localized prostate cancer. Radiother Oncol. 2003;67:313-319.
Sawczuk I, Tannenbaum M, Olsson CA, deVere White R. Primary transitional cell carcinoma of prostatic periurethral ducts. Urology. 1985;25:339-343.
Scardino PT, Frankel JM, Wheeler TM, et al. The prognostic significance of post-irradiation biopsy results in patients with prostatic cancer. J Urol. 1986;135:510-516.
Schellhammer PF, Bean MA, Whitmore WFJr. Prostatic involvement by transitional cell carcinoma: pathogenesis, patterns and prognosis. J Urol. 1977;118:399-403.
Sehdev AE, Pan CC, Epstein JI. Comparative analysis of sampling methods for grossing radical prostatectomy specimens performed for nonpalpable (stage T1c) prostatic adenocarcinoma. Hum Pathol. 2001;32:494-499.
Sexton WJ, Lance RE, Reyes AO, et al. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166:521-525.
Sgrignoli AR, Walsh PC, Steinberg GD, et al. Prognostic factors in men with stage D1 prostate cancer: identification of patients less likely to have prolonged survival after radical prostatectomy. J Urol. 1994;152:1077-1081.
Shah O, Robbins DA, Melamed J, Lepor H. The New York University nerve sparing algorithm decreases the rate of positive surgical margins following radical retropubic prostatectomy. J Urol. 2003;169:2147-2152.
Shankey TV, Kallioniemi OP, Koslowski JM, et al. Consensus review of the clinical utility of DNA content cytometry in prostate cancer. Cytometry. 1993;14:497-500.
Sheridan TB, Carter HB, Wang W, et al. Change in prostate cancer grade over time in men followed expectantly for stage T1c disease. J Urol. 2008;179:901-904. discussion 904–5
Smith DM, Murphy WM. Histologic changes in prostate carcinomas treated with leuprolide (luteinizing hormone-releasing hormone effect). Distinction from poor tumor differentiation. Cancer. 1994;73:1472-1477.
Smith JAJr, Chan RC, Chang SS, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385-2389. discussion 2389–90
Sogani PC, Israel A, Lieberman PH, et al. Gleason grading of prostate cancer: a predictor of survival. Urology. 1985;25:223-227.
Srigley JR. Benign mimickers of prostatic adenocarcinoma. Mod Pathol. 2004;17:328-348.
Stamey TA, Villers AA, McNeal JE, et al. Positive surgical margins at radical prostatectomy: importance of the apical dissection. J Urol. 1990;143:1166-1172. discussion 1172–3
Steinberg DM, Sauvageot J, Piantadosi S, Epstein JI. Correlation of prostate needle biopsy and radical prostatectomy Gleason grade in academic and community settings. Eur Urol. 2005;47:196-201.
Stephenson AJ, Scardino PT, Eastham JA, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005-7012.
Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59:1803-1809.
Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of prostate associated with myasthenic (Eaton-Lambert) syndrome. Urology. 1989;33:148-152.
Trock BJ, Guo CC, Gonzalgo ML, et al. Impact of tertiary Gleason patterns on biochemical recurrence following radical prostatectomy: proposal for a modified Gleason scoring system. J Urol. 2009;182:1364-1370.
Tsuzuki T, Hernandez DJ, Aydin H, et al. Prediction of extraprostatic extension in the neurovascular bundle based on prostate needle biopsy pathology, serum prostate specific antigen and digital rectal examination. J Urol. 2005;173:450-453.
Vailancourt L, Ttu B, Fradet Y, et al. Effect of neoadjuvant endocrine therapy (combined androgen blockade) on normal prostate and prostatic carcinoma. A randomized study. Am J Surg Pathol. 1996;20:86-93.
Varkarakis MJ, Winterberger AR, Gaeta J, et al. Lung metastases in prostatic carcinoma. Clinical significance. Urology. 1974;3:447-452.
Villers A, McNeal JE, Redwine EA, et al. The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J Urol. 1989;142:763-768.
Vollmer RT. Prostate cancer and chip specimens: complete versus partial sampling. Hum Pathol. 1986;17:285-290.
Wheeler TM, Dillioglugil O, Kattan MW, et al. Clinical and pathological significance of the level and extent of capsular invasion in clinical stage T1-2 prostate cancer. Hum Pathol. 1998;29:856-862.
Wilcox G, Soh S, Chakraborty S, et al. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol. 1998;29:1119-1123.
Wojno KJ, Epstein JI. The utility of basal cell-specific anti-cytokeratin antibody (34 beta E12) in the diagnosis of prostate cancer. A review of 228 cases. Am J Surg Pathol. 1995;19:251-260.
Wood DPJr, Montie JE, Pontes JE, et al. Transitional cell carcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. J Urol. 1989;141:346-349.
Yang XJ, Lecksell K, Short K, et al. Does long-term finasteride therapy affect the histologic features of benign prostatic tissue and prostate cancer on needle biopsy? PLESS Study Group. Proscar Long-Term Efficacy and Safety Study. Urology. 1999;53:696-700.
Yoon GS, Wang W, Osunkoya AO, et al. Residual tumor potentially left behind after local ablation therapy in prostate adenocarcinoma. J Urol. 2008;179:2203-2206. discussion 2206