chapter 103 Laparoscopic and Robotic-Assisted Laparoscopic Radical Prostatectomy and Pelvic Lymphadenectomy
Evolution of Minimally Invasive Laparoscopic Prostatectomy
Over a century has passed since Hugh Hampton Young (1905) performed the first open prostatectomy for carcinoma through a perineal approach. In 1947, Millin was the first to describe the retropubic approach to radical prostatectomy. In the late 1970s and early 1980s several detailed anatomic studies performed in fetal and adult cadavers provided important insights into the periprostatic anatomy, especially that of the dorsal vein complex (DVC; Reiner and Walsh, 1979), the neurovascular bundle (NVB; Walsh and Donker, 1982), and the striated urethral sphincter (Oelrich, 1980). These observations provided a more anatomic approach to radical prostatectomy with a consequent reduction in operative morbidity. Subsequently, anatomic, nerve-sparing radical prostatectomy has maintained a cardinal role in the management of localized prostate cancer for more than 2 decades.
Schuessler and colleagues (1997) performed the first successful laparoscopic radical prostatectomy (LRP) in 1997. In their series of nine patients, operative duration was lengthy (8 to 11 hours) and the length of stay was 7.3 days on average. Although the authors concluded that cure rates with LRP may be comparable with open surgery, they could not define any significant advantages. As a result, LRP was not immediately widely adopted in the field of urology.
Advances in task-specific surgical instrumentation, optics, digital video equipment, and computer and robotic technology opened a new frontier for minimally invasive laparoscopic prostatectomy. These advances led urologists to revisit LRP, spearheaded by two centers in France that reported on their techniques and early results (Abbou et al, 2000; Guillonneau and Vallancien, 2000). Their stepwise approach to LRP proved to be both reproducible and teachable, although the learning curve remained challenging. Operative times were in a more acceptable 4- to 5-hour range with reported overall positive margin rates of 15% to 28%. Both groups had good continence and potency rates. This work rekindled worldwide interest in LRP, and in the ensuing years surgeons at a number of centers throughout the world acquired the skills and experience to perform LRP. However, advanced laparoscopic skills are necessary to perform a proficient LRP, especially for suturing of the vesicourethral anastomosis.
Computer-assisted surgical devices using mechanical robotic arms were adopted for use with radical prostatectomy in part due to their ability to aid the surgeon in performing the challenging task of laparoscopic suturing. One such device, the da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) has become the dominant robotic surgical device in the field of urology. By incorporating sophisticated wristed technology at the terminal ends of the robotic instruments, a surgeon can operate, dissect, and suture with the facility of a human wrist. In addition, a 10× magnified, three-dimensional image is displayed as a result of a specialized stereo endoscope lens and camera, providing the surgeon with an unprecedented view of the operative field and periprostatic anatomy. The first-generation robotic platform, originally launched in the United States in 2000, allows for the surgeon to control three robotic arms simultaneously, two arms for robotic instrumentation and a third arm that controls the stereo endoscope and camera. The newer da Vinci S system, made available in 2006, incorporates high-definition image capability with an additional fourth robotic arm for grasping and retraction. Finally the latest-generation robot, the da Vinci Si HD, which was launched in 2009, offers two separate surgeon consoles allowing two surgeons to operate simultaneously, providing an opportunity for improved operative efficiency and teaching.
There has been an almost revolutionary introduction of robotic surgery into the surgical management of carcinoma of the prostate. Since its introduction into the United States in 2000, robotic-assisted laparoscopic prostatectomy (RALP) has rapidly grown in popularity with surgeons and patients alike. With rapid dissemination of this robotic platform into large tertiary referral centers and community hospitals throughout the country, it is now estimated that RALP is the dominant surgical approach for radical prostatectomy in the United States. In addition, robotic technology is rapidly expanding throughout centers worldwide.
There have been considerable and ongoing debates about the merits of RALP versus an open surgical approach by either the retropubic or perineal route. Issues with equipment expense, the learning curve for the surgeon and surgical team, and patient-related outcomes remain. Nonetheless, RALP has virtually replaced LRP in the United States, and the overwhelming majority of new surgeons have adopted RALP as their preferred surgical approach for prostate cancer. Thus it seems virtually certain that the use of RALP will continue to proliferate.
This chapter highlights some of the surgical advances for both LRP and RALP. Further, technical details for the surgical dissection and currently available data on oncologic, as well as functional outcomes, are presented. Finally, the authors review the role of pelvic lymph node dissection (PLND) in conjunction with minimally invasive approaches to prostatectomy along with technical considerations and potential complications.
Patient Selection
Indications and Contraindications
The indications for LRP and RALP are identical to that for open surgery (i.e., patients with ≤ clinical stage T2 with no evidence of metastasis either clinically or radiographically [computed tomography (CT) and bone scan]). Absolute contraindications to minimally invasive laparoscopic prostatectomy include uncorrectable bleeding diatheses and the inability to undergo general anesthesia because of severe cardiopulmonary compromise. Patients who have received neoadjuvant hormonal therapy or who have a history of prior complex lower abdominal and pelvic surgery such as partial colectomy, inguinal mesh herniorrhaphy, or prior transurethral resection of the prostate (TURP) pose a greater technical challenge due to distortion of normal anatomy and adhesions but are not an absolute contraindication to LRP and RALP. In those patients with a history of prior laparoscopic extraperitoneal mesh herniorrhaphy, a transperitoneal approach may be preferred over the extraperitoneal approach because dense adhesions in the retropubic space often make attempts at initial access to the space of Retzius challenging.
Morbidly obese patients pose additional challenges due to the potential respiratory compromise encountered when placing these patients in a steep Trendelenburg position, as well as the relatively limited working space and limitations of trocar size and instrumentation length, especially with LRP. Patients with large prostate volumes (e.g., >70 g) are often met with longer operative times, blood loss, and hospital stay than those with smaller glands; however, long-term urinary outcomes appear comparable (Levinson et al, 2008, 2009; Link et al, 2008). Salvage surgery after failure of primary treatment (e.g., radiation, brachytherapy, cryotherapy, high-intensity focused ultrasound) has been successfully reported in properly selected patients but should be approached with caution due to the attendant risks and complications (Kaouk, 2008; Boris et al, 2009). Due to the effects of prior local radiotherapy or ablation, the tissue planes surrounding the prostate and especially between the posterior prostate and anterior rectum are often fibrotic and obliterated, increasing the risk of inadvertent entry into the rectum during salvage surgery. As a result, patients undergoing salvage prostatectomy need to be counseled on the potential risk of rectal injury in addition to the higher incidence of impotence and incontinence as compared with primary surgery. It is strongly advised that these more complex patient scenarios be avoided in a surgeon’s early experience with LRP and RALP; however, these patient features are not by themselves absolute contraindications for a minimally invasive approach to prostatectomy (Brown et al, 2005a; Erdogru et al, 2005; Singh et al, 2005; Stolzenburg et al, 2005).
Instrumentation
Instrumentation required for LRP and RALP is dependent on the chosen approach and model of da Vinci system being used (i.e., three- vs. four-arm robot) in the case of RALP. A list of suggested instrumentation for LRP and RALP are listed in Table 103–1. For LRP, the AESOP 3000 robotic arm (Intuitive Surgical, Inc., Sunnyvale, CA) may be used to stabilize and control the laparoscopic lens and camera by hand-held remote control, voice activation, or foot pedal control. Alternatively, a surgical assistant can be used for this purpose. During RALP, the use of the da Vinci S or Si HD system allows the surgeon to control a total of four robotic arms with one being the endoscope as compared with only three robotic arms with the first-generation standard robotic platform. The surgeon generally begins the operation by using a 0-degree stereo endoscope and controlling a grasping forceps in the left robotic arm (such as the Maryland curved bipolar forceps or plasma kinetic dissector) and the curved monopolar scissors in the right robotic arm. The fourth robotic arm controls the ProGrasp forceps (Intuitive Surgical, Inc., Sunnyvale, CA), a large atraumatic blunt grasper for retraction and exposure of tissues. The surgeons then toggle between control of any two of the three working robotic arms at any given time to allow for greater autonomy and to achieve optimal exposure and dissection.
Table 103–1 Suggested Instrumentation for LRP and RALP
| Laparoscopic Radical Prostatectomy |
| Bipolar forceps |
| Robotic-Assisted Laparoscopic Prostatectomy |
LRP, laparoscopic radical prostatectomy; RALP, robotic-assisted laparoscopic prostatectomy.
Preoperative Preparation
Bowel Preparation
As with open surgery, a preoperative mechanical bowel preparation may be used. Most commonly, one bottle of citrate of magnesium is taken the day before surgery and the patient’s diet is limited to clear liquids. An enema administered the morning of surgery is recommended by some surgeons. A broad-spectrum antibiotic such as cefazolin is administered intravenously 30 minutes before surgery.
Informed Consent
In addition to bleeding, transfusion, and infection, patients undergoing LRP and RALP must be aware of the potential for conversion to open surgery. As with open surgery, patients must be counseled on the risk of impotence, incontinence, incisional hernia, and adjacent organ injury (e.g., ureter, rectum, bladder, small bowel). The risks of general anesthesia must also be presented to the patient because LRP and RALP cannot be performed under regional anesthesia. In addition, it may be appropriate for the surgeon to discuss his or her overall operative experience with radical prostatectomy and provide a realistic forecast of cancer control, as well as return to normal urinary and sexual function on the basis of each patient’s unique characteristics.
Operating Room Personnel
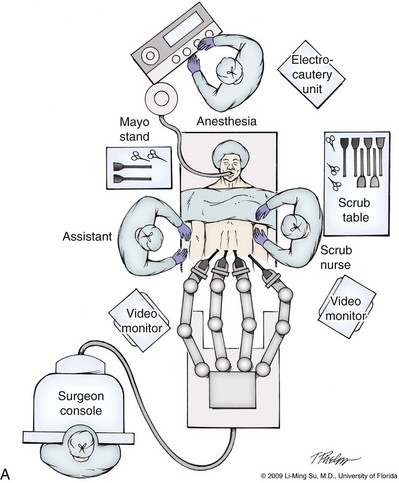
LRP and RALP require that the surgical team including the scrub technician, circulating nurse, and surgical assistant(s) be fully trained and skilled in the instrumentation, operative setup, and technical steps of these minimally invasive techniques. Only one skilled assistant is generally required for these procedures, but a second assistant may be used if available to provide retraction of tissues. The scrub technician is an integral part of the operative team and must be versed in the wide array of laparoscopic and robotic instruments that may be used to accomplish this procedure. For RALP, it is important for the tableside assistant to have adequate training in basic laparoscopy and the mechanics, setup, and troubleshooting of the robotic system. Typical operating room equipment and setup for RALP and LRP are shown in Figure 103–1.
Patient Positioning
After induction of general endotracheal anesthesia, the patient is placed in a supine position in steep Trendelenburg with arms tucked and padded at the sides. Sequential compression stocking devices are placed on both legs and activated. The patient’s legs are spread apart and supported by spreader bars to allow for access to the rectum and perineum. Alternatively, the patient’s legs may be placed in stirrups in the low lithotomy position. The patient is then secured to the table using tape and egg-crate padding. An orogastric tube and urethral catheter are placed to decompress the stomach and bladder, respectively.
Anesthesia Considerations
Both LRP and RALP require general anesthesia. Because the patient’s arms are tucked at the side and difficult to access, establishing accurate pulse oximetry, blood pressure cuff placement, and intravenous access is critical before patient positioning. The anesthesiologist must be aware of the potential consequences of CO2 insufflation and pneumoperitoneum including oliguria and hypercarbia. Prompt adjustments in minute and tidal volumes may be required by the anesthesiologist in the event of rising end-tidal CO2 levels and hypercarbia (Meininger et al, 2004). Adjustments in CO2 insufflation pressures may also be required by the surgeon to reduce the risk of continued hypercarbia. Taken together, maintaining good communication between the surgeon and the anesthesia team is important during these procedures.
Surgical Technique
Robotic-Assisted versus Pure Laparoscopic Approach
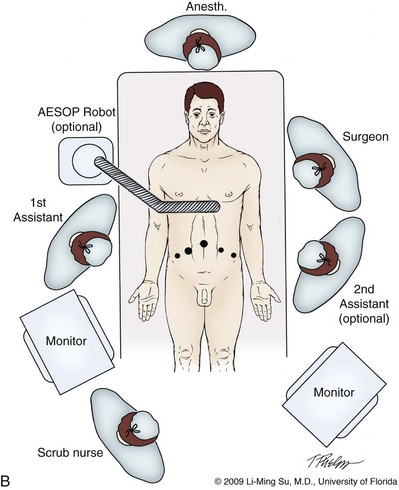
Most of the principles and considerations for the surgical dissection are similar regardless of whether a pure laparoscopic or robotic-assisted approach is used. For RALP, the da Vinci Surgical System is a master/slave system with three components: surgical robot (also called the patient side cart), surgeon console, and video cart (Fig. 103–2). For the purpose of this chapter and for simplicity, the technique using the four-arm da Vinci S system is described. The robot is docked at the foot of the operating table between the patient’s legs. The tableside assistant is responsible for docking/undocking the robot, suction-irrigation, retraction of tissues, passing sutures into the operative field, and robotic instrument changes. The surgeon is seated at the surgeon console, which provides a superb three-dimensional, 10× magnified, high-definition operative view and allows for the surgeon to have complete control of all camera movements and three additional robotic arms. The surgeon’s thumb and index fingers are inserted into master controls that allow natural hand and wrist movements to be precisely replicated by wristed instruments at the terminal ends of the robotic arms in real time.
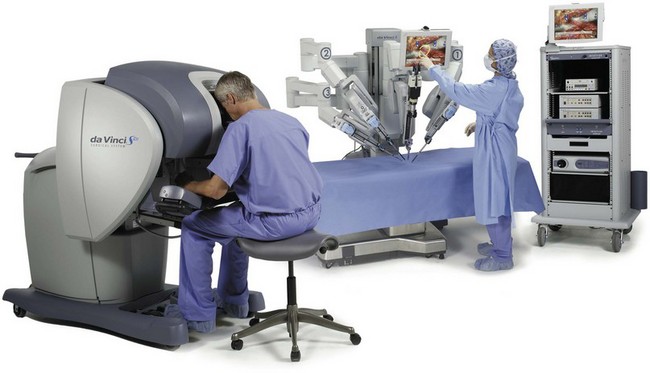
Figure 103–2 Three components of the da Vinci S Surgical System (Copyright 2009, Intuitive Surgical, Inc.)—the surgeon console, patient side cart, and video cart.
Highly skilled laparoscopic surgeons may find the robotic technology unnecessary and discover that they are equally as facile with pure laparoscopic suturing and dissection as with the robot (Guillonneau, 2005). Most surgeons, however, feel that the robotic technology significantly facilitates suturing of the vesicourethral anastomosis and aids in other aspects of the surgical dissection such as achieving the critical angles of dissection required to optimize cavernous nerve preservation.
Other than setup of the operating room and surgical fields, there is little difference in the surgical technique between LRP and RALP. In general, the following discussion of technique and the pros and cons of various maneuvers and approaches apply to either surgical approach.
Transperitoneal Approach
The most common approach to LRP and RALP is the transperitoneal anterior approach in which following transperitoneal access and insufflation, the space of Retzius is immediately entered and the prostate gland, seminal vesicle, and vasa are approached and dissected from an anterior approach. This is in contrast to the transperitoneal retrovesical approach in which the seminal vesicles and vasa are initially approached and completely dissected behind the bladder near the cul-de-sac. The transperitoneal access and approaches are favored by most surgeons over the extraperitoneal approach due to the greater working space and familiar landmarks of the pelvis and its contents. For the purposes of this chapter, the transperitoneal anterior approach will be primarily described with brief mention about the extraperitoneal approach.
Abdominal Access, Insufflation, and Trocar Placement
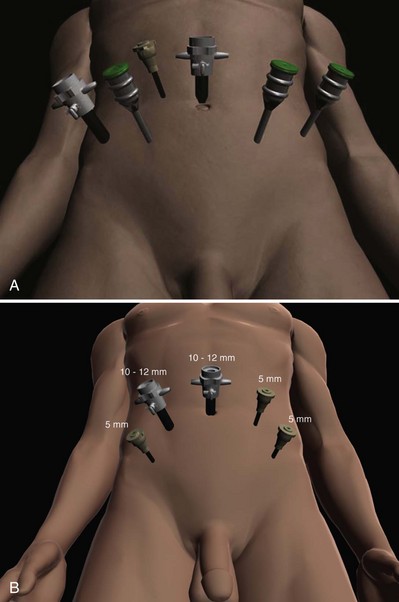
For a transperitoneal approach, pneumoperitoneum is established using either a Veress needle inserted at the base of the umbilicus or an open Hasson technique. Following initial trocar placement, CO2 insufflation pressure in general is maintained between 12 and 15 mm Hg. Secondary trocars are then placed under laparoscopic view. For RALP, an example of a trocar configuration is shown in Figure 103–3A. A 12-mm trocar is initially placed at or slightly above the umbilicus for insertion of the stereo endoscope. In a morbidly obese or very tall patient, infraumbilical camera placement may be preferable. Three 8-mm metal robotic trocars are used by the working robotic arms of the surgeon while the assistant provides retraction, suction, and irrigation and passes clips and sutures via the 12-mm and 5-mm trocars placed along the patient’s right side. The surgeon controls camera movement by depressing a foot pedal and using brief, simultaneous arm movements to affect camera positioning and rotation. Endoscopes with either angled (30-degree) or straight-ahead (0-degree) viewing are available and interchangeable at various portions of the procedure. In general, most surgeons use the 0-degree endoscope lens throughout the operation; however, some surgeons prefer to switch to the 30-degree down lens when approaching the bladder neck, NVBs, and apical dissection. Figure 103–3B depicts the trocar configuration for LRP. The surgeon stands at the patient’s left side and operates through the two pararectus trocars while one or two assistants use the lateralmost trocars. The endoscope is held and controlled by an AESOP robotic arm or surgical assistant through the umbilical trocar.
Extraperitoneal Approach
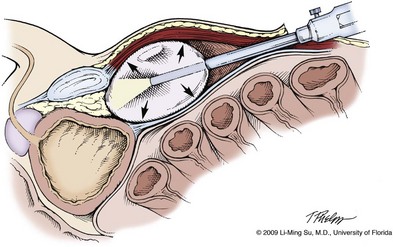
For an extraperitoneal approach, a 1.5-cm incision is made at the level of the umbilicus and dissection is carried out down through the anterior rectus sheath. Using blunt finger dissection, a space is created immediately anterior to the posterior rectus sheath and underlying peritoneum. A trocar-mounted balloon dilator device (PDB Balloon, Covidien Autosuture, Mansfield, MA) is inserted into the preperitoneal space anterior to the posterior rectus sheath and advanced down to the pubis along the midline. Using a 0-degree, 10-mm endoscope inserted through the balloon trocar, approximately 500 mL of air is inflated to develop the space of Retzius under laparoscopic view (Fig. 103–4). Secondary trocars are then inserted as described previously under laparoscopic view. The operation then proceeds in the exact manner to that of the transperitoneal anterior approach.
Pros and Cons of Extraperitoneal versus Transperitoneal Approach
In a retrospective comparison between extraperitoneal versus transperitoneal LRP, Hoznek and colleagues (2003) found that the mean operative time was shorter with the extraperitoneal approach (169.6 vs. 224.2 minutes, P < .001) with the greatest time saved during access to the space of Retzius. They suggested that time to full diet was less with the extraperitoneal versus the transperitoneal LRP approach (1.6 vs. 2.6 days, P = .002) because the peritoneum had not been violated and postoperative ileus was minimized. Eden and colleagues (2004) found a statistical significant advantage in operative time, hospital stay, and return of early continence favoring patients undergoing extraperitoneal versus transperitoneal LRP, postulating that earlier return to urinary control may be secondary to less bladder dissection and, perhaps, less bladder dysfunction as compared with transperitoneal LRP. Most studies, however, have found little or no difference in operative time and perioperative outcomes between transperitoneal and extraperitoneal approaches (Cathelineau et al, 2004a; Erdogru et al, 2004; Brown et al, 2005b; Atug et al, 2006).
With an extraperitoneal approach, the simultaneous laparoscopic management of concurrent inguinal hernias using prosthetic mesh is feasible (Stolzenburg et al, 2003). Simultaneous inguinal herniorrhaphy has also been reported during transperitoneal LRP (Allaf et al, 2003); however, proper coverage of the mesh prosthesis is necessary using peritoneal flaps, omentum, or a second absorbable mesh to reduce the risk of direct contact between the mesh and bowel with subsequent fistula. The extraperitoneal technique may be preferable in patients with previous extensive abdominal surgery or morbid obesity. With the extraperitoneal approach, the peritoneum acts as a natural barrier, minimizing the potential for bowel injury and preventing the bowels from falling into the operative field and obscuring the surgeon’s view. Furthermore, this approach helps to confine any urine leak that may occur from the vesicourethral anastomosis within the extraperitoneal space. One limitation with the extraperitoneal approach is the reduced working space as compared with the relatively larger working space of the peritoneal cavity gained with transperitoneal access. This is especially relevant when a well-meaning assistant attempts to clear the operative field of blood or smoke. Suctioning can evacuate CO2 gas and rapidly collapse the already limited extraperitoneal working space, thus significantly compromising visualization. A second limitation to the extraperitoneal approach is in patients with a prior history of laparoscopic extraperitoneal mesh herniorrhaphy because the retropubic space is often obliterated, making attempts at extraperitoneal access challenging. Lastly, higher CO2 absorption has been reported with extraperitoneal versus transperitoneal insufflation, requiring a higher minute volume to compensate for hypercarbia and associated acidosis (Meininger et al, 2004). Overall, whether to use an extraperitoneal or transperitoneal approach for LRP or RALP is largely a matter of surgeon preference and experience and there is no consistently demonstrated advantage for either approach.
Developing the Space of Retzius
Following abdominal access and trocar placement for the transperitoneal anterior approach, the pelvic contents are inspected (Fig. 103–5) and adhesions are lysed if present. The initial step is entry and development of the space of Retzius. The bladder is dissected from the anterior abdominal wall by dividing the urachus high above the bladder and incising the peritoneum bilaterally immediately lateral to the medial umbilical ligaments using monopolar electrocautery. The presence of prevesical fatty alveolar tissue confirms the proper plane of dissection. Applying posterior and cephalad traction on the urachus, the retropubic space is rapidly developed with a combination of blunt and sharp dissection along the relatively avascular plane within the space of Retzius (Fig. 103–6). Lateral dissection of the bladder is carried out down toward the crossing of the median umbilical ligament and vas deferens in order to ensure optimal mobility of the bladder and to minimize future tension at the vesicourethral anastomosis. The fat overlying the prostate is removed using sharp dissection and electrocautery as needed, and the superficial branches of the DVC coagulated using bipolar electrocautery.
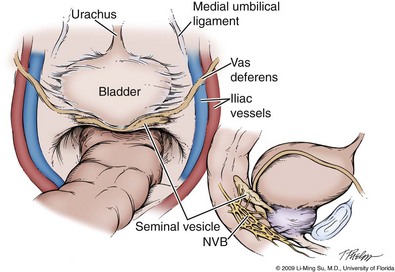
Figure 103–5 Initial transperitoneal view detailing the relevant landmarks within the male pelvis. NVB, neurovascular bundle.
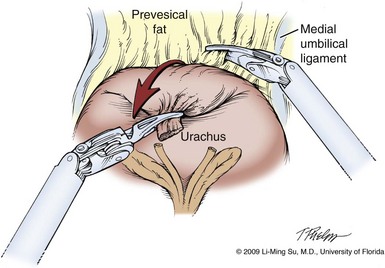
Figure 103–6 Division of urachus and entry into the space of Retzius. Cephalad traction on the urachus with the left hand helps to identify the fatty alveolar tissue immediately anterior to the bladder, which marks the proper plane of dissection.
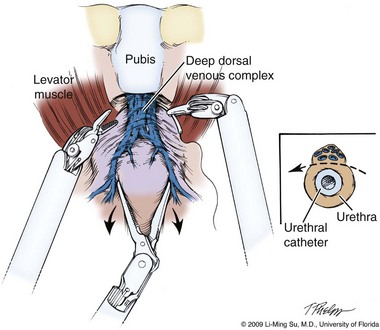
At this point, visible landmarks include the anterior aspect of the bladder and prostate, puboprostatic ligaments, endopelvic fascia, and pubis (Fig. 103–7). The endopelvic fascia and puboprostatic ligaments are sharply divided, exposing levator muscle fibers attached to the lateral and apical portions of the prostate. These fibers are meticulously and bluntly dissected from the surface of the prostate exposing the deep DVC and urethra at their confluence with the apex of the prostate. Electrocautery is avoided if possible to minimize thermal damage to the external sphincter and nearby NVBs.
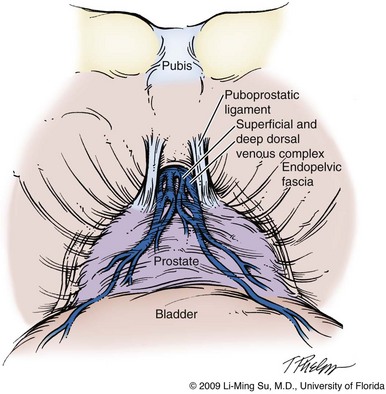
Figure 103–7 Retropubic view of the bladder and prostate following entry into the space of Retzius. The fatty tissue overlying the anterior aspect of the prostate has been removed, exposing the puboprostatic ligaments, superficial and deep dorsal venous complex, and endopelvic fascia.
Accessory pudendal arteries traveling longitudinally along the anteromedial aspect of the prostate are easily recognized during LRP and RALP. Attempt at preservation of these arteries is important for erectile function because in some men these arteries may be the dominant source of arterial blood supply to the corpora cavernosa (Nehra et al, 2008). These accessory arteries can usually be preserved, although separation of the artery from the prostatic apex can be somewhat challenging. Most commonly, though, careful and meticulous dissection allows enough mobilization of the artery away from the deep DVC and prostatic apex that both good anatomic dissection of the prostatic apex and preservation of the accessory vessel are feasible.
Ligation of the Deep Dorsal Venous Complex
As with open surgery, different methods have been described for control of the DVC. A common observation, though, is that the profuse bleeding, which is sometimes encountered during open surgery, is not usually problematic because of the tamponade effect on venous bleeding offered by the pneumoperitoneum. In fact, some techniques involve simple cutting of the DVC with or without the use of electrocautery and placement of a suture only if necessary. Typically, there are one or two small arteries in the DVC. Most described techniques use placement of an absorbable suture for hemostasis before initiation of the surgical dissection of the prostate. During RALP, the ProGrasp forceps can be used for fixed cephalad retraction of the prostate and bladder to achieve optimal exposure of the DVC and prostatic apex before DVC ligation. Similar retraction can be applied by the surgical assistant during LRP. The deep DVC is suture ligated using a 0-polydioxanone suture or polyglactin suture as close to the pubis and as far from the prostatic apex as possible (Fig. 103–8). Securing the DVC as far away from the prostatic apex as possible can help minimize iatrogenic entry into the prostatic apex during later division of the DVC. The needle is passed beneath the DVC and anterior to the urethra. An alternative method to DVC ligation is the use a laparoscopic linear stapling device, which ligates and divides the DVC all in one step (Ahlering et al, 2004b; Nguyen et al, 2008). Regardless of the method used, it is important to avoid damage to the anterior urethral sphincter muscle from placing the sutures or staples too deep. With most techniques, the DVC is not divided until later in the operation and immediately before prostatic apical dissection and division of the urethra. A back-bleeding suture may be placed along the anterior base of the prostate to help identify the contour of the prostate and to aid in subsequent bladder neck identification and transection.
Bladder Neck Identification and Transection
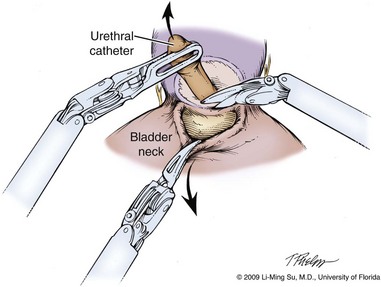
Proper identification of the bladder neck during RALP and LRP can be initially challenging due to the lack of tactile feedback in delineating the precise margin between the prostate and bladder. Several maneuvers are helpful in identifying the proper plane of dissection and in minimizing inadvertent entry into the prostate. First, visual identification of the point of transition of the prevesical fat to the anterior prostate can serve as a guide. Second, intermittent and repetitive caudal retraction of the urethral catheter balloon can help identify and confirm the transition between bladder neck and prostate. Third, using a forceps to grasp and retract the dome of the bladder in a cephalad direction results in “tenting” of the bladder neck at its attachment to the prostate. Lastly, further confirmation of this margin between the bladder and prostate is made by a bimanual “palpation” or “pinch” of the bladder neck using the tips of two robotic or laparoscopic instruments.
The anterior bladder is divided horizontally using monopolar electrocautery along the midline until the urethral catheter is identified. The anterior bladder neck incision should not be carried too laterally because branches of the bladder pedicle are often encountered, resulting in unwanted bleeding. The balloon is decompressed, and the tip of the urethral catheter is brought through the anterior bladder neck opening and lifted anteriorly with the assistant applying counter traction externally at the penile meatus to suspend the prostate.
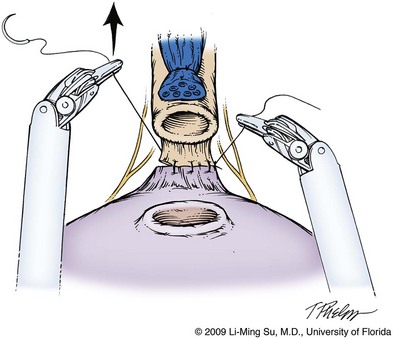
The posterior bladder neck is inspected for the presence of a median lobe and to locate the ureteral orifices. If a vertical drop-off of the posterior bladder neck mucosa is noted, this would suggest the absence of a median lobe. Alternatively, if a mass effect from a large median lobe is identified, further exposure may be required to visualize beneath the protruding median lobe and identify the posterior bladder neck. The median lobe is lifted anteriorly using either the ProGrasp forceps or the surgical assistant. The posterior bladder neck is horizontally divided with monopolar electrocautery, staying along the midline to avoid bleeding from the lateral pedicles (Fig. 103–9). Dissection is carried out in a 45-degree downward angle to avoid entry into the base of the prostate, as well as creating a buttonhole in the posterior wall of the bladder. In case of a prior TURP, the bladder neck margin is less evident and often distorted as a result of prior resection and scarring. Careful inspection is made of the posterior bladder neck paying specific attention to the location of the ureteral orifices because they are often found close to the posterior bladder neck margin. Attempt at bladder neck sparing should be avoided in post-TURP and median lobe cases. When in doubt, the posterior bladder neck should be divided slightly more proximally in these particular cases so as to avoid inadvertent entry into the prostate gland.
Dissection of the Seminal Vesicles and Vasa Deferentia
Following bladder neck transection, the seminal vesicles and vasa deferentia are individually identified, dissected, and divided, avoiding electrocautery if possible to prevent damage to the nearby NVB (Fig. 103–10). One unique distinction between the transperitoneal anterior and retrovesical approach is in dissection of the seminal vesicles and vasa deferentia. During a transperitoneal retrovesical approach, the initial step of the operation is complete dissection of the vasa deferentia and seminal vesicles deep within the cul-de-sac. Following abdominal access, the vasa are dissected from lateral to medial toward their confluence at the ejaculatory ducts. The seminal vesicles are found immediately lateral to the vasa and are dissected free from the nearby NVB using hemoclips while avoiding the use of thermal energy (Fig. 103–11). With the dissection of the seminal vesicles and vasa now completed, once the bladder neck is divided from the prostate, these structures are simply grasped and brought through the opening. This retrovesical approach to the seminal vesicles and vasa deferentia is particularly useful in cases of a median lobe where identification and dissection of these structures by the anterior approach may be more challenging due to the protruding median lobe.
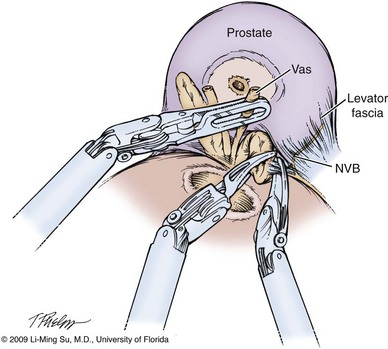
Figure 103–10 Dissection of seminal vesicles and vasa deferentia via the transperitoneal anterior approach. The seminal vesicles and vasa are identified and dissected within the opening created between the posterior bladder neck and prostate following division of the bladder neck. Hemoclips are used in lieu of electrocautery to avoid thermal injury to the nearby neurovascular bundles (NVB).
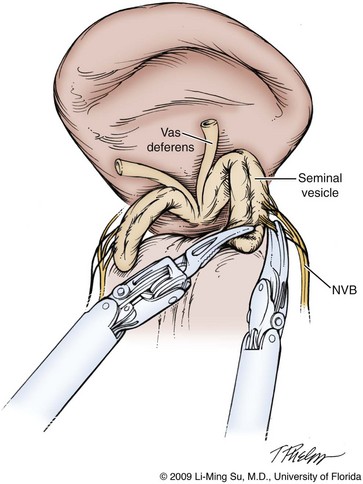
Figure 103–11 Dissection of the seminal vesicles and vasa deferentia via the transperitoneal retrovesical approach. The vasa and seminal vesicles are identified as the initial step in this approach deep within the retrovesical space. The neurovascular bundles (NVB) are dissected off of the seminal vesicles in an antegrade direction from the tip toward the base using hemoclips.
Development of the Plane between the Prostate and Rectum
Separation of the posterior prostate from the anterior rectal wall is a key surgical maneuver to avoid rectal injury but also to permit adequate identification of the prostatic pedicles and establish the medial border of the NVB. Development of this plane in an antegrade fashion is a maneuver often unfamiliar to surgeons experienced with open surgery but one that is rapidly adaptable to laparoscopic and robotic approaches. Anterior retraction of the vasa deferentia and seminal vesicles by either a surgical assistant or the robotic ProGrasp forceps helps with identification of the proper plane for the initial dissection (Fig. 103–12).
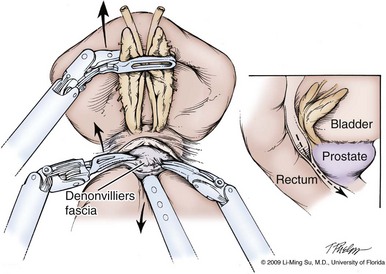
Figure 103–12 Development of the plane between the prostate and rectum. As the assistant or ProGrasp forceps is used to apply anterior traction on the seminal vesicles and vasa deferentia and downward traction on the rectum, a transverse incision is made in Denonvilliers fascia below the seminal vesicles, and blunt dissection is used to develop a plane between the posterior prostate and the rectum. Inset demonstrates the direction of dissection toward the prostatic apex.
Denonvilliers fascia is an inferior extension of the peritoneal cul-de-sac that lies between the prostate and rectum. With an intrafascial or interfascial dissection, Denonvilliers fascia can be separated from the posterior prostate by careful blunt and sharp dissection. The separation can be carried all the way to the prostatic apex and laterally to the prostatic pedicle. The proper surgical plane is relatively avascular. When a wider margin of tissue is desired on the posterior prostate such as in cases of palpable disease, Denonvilliers fascia should be sharply incised just posterior to the junction of the seminal vesicle and the prostate. This allows immediate entry into the anterior perirectal fat plane of dissection. Good visualization can be achieved as the dissection proceeds distally toward the apex staying between Denonvilliers fascia anteriorly and the anterior propria fascia of the rectum posteriorly.
Substantial bleeding typically suggests that the dissection may be too close to the prostate. If difficulty is encountered in establishing the proper plane of dissection, a new attempt can be directed to one side or the other of the initial entry point. Once the proper plane is entered, the dissection characteristically progresses rather smoothly. The rectal wall should be mobilized far enough laterally that there is sufficient separation for dissection of the NVB and prostatic apex.
Prostatic Pedicle Control
Various methods have been described for control of the prostatic pedicle. Some techniques use pure electrocautery, either monopolar or bipolar. Because of the propagation of thermal energy through tissue, which may result in damage to the nearby NVB, limitation of the use of electrocautery is advised even in the prostatic pedicle. Locking polymer Hem-o-Lok clips (Teleflex Medical, Research Triangle Park, NC) are commonly employed, but engagement of the clipping mechanism does require good delineation and thinning of the pedicle tissue so that the clipping mechanism will engage. This is best facilitated by adequate mobilization of the rectum and lateral prostate for identification of the prostatic pedicle. Application of a bulldog clamp to the pedicle with subsequent suturing after the specimen has been removed has also been described (Ahlering et al, 2005; Gill et al, 2005). Regardless of the method used, though, successful division of the prostatic pedicle in the correct anatomic location is an important step in avoiding positive margins and damage to the nearby NVB.
Preservation of the Neurovascular Bundle
Preservation of the periprostatic parasympathetic nerve fibers important for erectile function is one of the key and most difficult maneuvers during radical prostatectomy, regardless of surgical approach. Increasingly, it is recognized that the periprostatic nerves of significance have a more diffuse and highly variable course than previously believed (Costello et al, 2004; Takenaka et al, 2004; Lunacek et al, 2005), but there is a confluence of nerves along the posterolateral prostate commonly termed the NVB. Nerve tissue extends posteriorly around the prostate, forming a virtual hammock of nerves. In addition, nerve fibers can be identified in the periprostatic tissue along the more anterior portion of the prostate gland, although there is still debate about their relative contribution to erectile function. A well-performed, nerve-sparing radical prostatectomy takes all of these considerations into account and preserves as much periprostatic nerve tissue as possible.
The superb visualization of the periprostatic tissues with laparoscopic surgery has led to a greater appreciation of the periprostatic fascial layers. Although there is some confusion in the literature regarding the terminology used for the various fascial layers, an extra fascial dissection typically implies dissection laterally between the lateral prostatic fascia and the levator fascia and posteriorly between Denonvilliers fascia and the anterior propria rectal fascia. An interfascial dissection is carried out laterally between the prostatic fascia and the levator fascia and posteriorly between Denonvilliers fascia and the posterior prostate (Fig. 103–13). This is the preferred approach in patients with presumed organ-confined disease because it permits safe preservation of the cavernous nerves without violating the anatomic limits of the prostate gland and capsule. An intrafascial dissection is between the prostatic capsule and the prostatic fascia and leaves virtually no periprostatic tissue overlying the prostate. Although technically feasible, this approach may lead to a higher incidence of positive margins due to the relatively closer dissection to the prostate gland. Even though these fascial layers as described do have some true integrity for intraoperative identification, it is also well recognized that they can be multilayered.
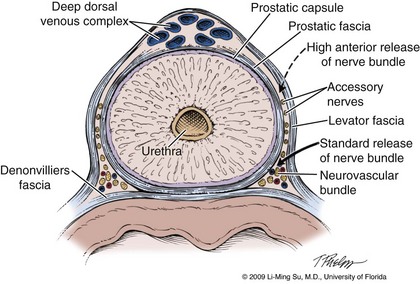
Figure 103–13 Cross section of the prostate demonstrating the periprostatic fascial planes with respect to the location of the neurovascular bundles. The dashed line indicates the direction of interfascial dissection (i.e., between the levator and prostatic fascia) to accomplish a high anterior release of the neurovascular bundle from the prostate and establish the lateral neurovascular bundle groove. The solid line indicates the incision made for a standard release of the neurovascular bundle.
Damage to the NVB can occur because of direct incision, entrapment in a suture or clip, thermal injury, or traction. Some surgeons advocate release of the NVB from the prostate before mobilization of the specimen to help avoid traction injury. With the antegrade approach typically used for LRP and RALP, identification and at least partial release of the NVB along the lateral aspect of the prostate before controlling the prostatic pedicle can help drop the nerve tissue away from the gland and permit a more precise placement of sutures, hemoclips, or clamps on the pedicle while preventing inadvertent entrapment of the nerve bundles. To accomplish this, the levator fascia is first incised sharply along the anteromedial aspect of the midprostate entering into the interfascial plane of dissection (Fig. 103–14). Blunt dissection is carried out along the prostatic fascia in a posterolateral direction, thus partially releasing the lateral NVB and developing a visible NVB groove, which aids in precise hemoclip placement and division of the prostatic pedicle (Fig. 103–15). The ergonomics and scaled motion of the wristed robotic instruments are helpful in achieving this delicate dissection. During LRP, specially designed fine-tipped (0.8-mm) dissectors have been described to aid in NVB dissection and preservation (Su et al, 2004). Some discussion and debate has centered around how far anteriorly on the prostate the interfascial dissection of the nerve bundles should be performed. Whether high release (vs. standard release) preserves important nerves or simply allows for a physical handle to permit more precise, meticulous dissection and preservation of the true cavernous nerves located at the 5- and 7-o’clock positions without direct manipulation of these main nerve fibers is uncertain (see Fig. 103–13). However, there is a general consensus that thermal energy should be minimized and, ideally, completely avoided during dissection of the NVB. These microscopic postganglionic parasympathetic nerve fibers are highly susceptible to thermal injury as shown in both animal and human studies (Ong et al, 2004; Ahlering et al, 2005). Bleeding along the NVB is usually relatively minimal and may require no specific hemostatic measures. There may be small arteries or larger veins that require suturing with small tissue bites to avoid entrapment of adjacent nerves. Overdissection of the NVB at the prostatic apex should be avoided.
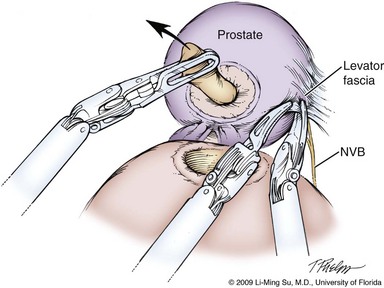
Figure 103–14 Entering into the interfascial plane of dissection for neurovascular bundle (NVB) preservation. The levator fascia is first incised along the anteromedial aspect of the midprostate, allowing entry into the interfascial plane of dissection.
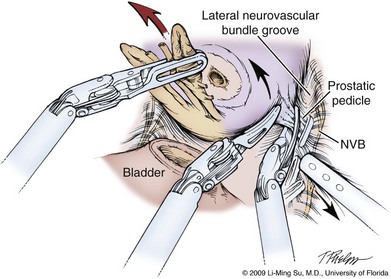
Figure 103–15 Developing the interfascial plane of dissection. Using blunt dissection, the nerve bundle is partially released from the prostate in a posterolateral direction, forming a visible lateral neurovascular bundle groove. This step serves to delineate the prostatic pedicle and course of the nerve bundle and allow for precise placement of hemoclips, while avoiding nerve entrapment. NVB, neurovascular bundle.
Patients with extremely large prostate glands (especially those > 100 g) offer a unique challenge during preservation of the NVB. Maneuvering a large prostate gland in the tight confines of the bony pelvis can be challenging, especially during exposure of the prostatic pedicles and NVB. Effective exposure of tissues by the surgical assistant, as well as the fourth robotic arm, is critical and can optimize visualization during prostatic pedicle ligation and NVB preservation.
Apical Dissection
The prostatic apex is a common location for tumor involvement and the most common site of positive margins with radical prostatectomy. Further, the steps required for apical dissection are crucial to preservation of erectile function and avoidance of urinary incontinence. The visualization of the operative field and the ability to limit bleeding from the DVC facilitate apical prostatic dissection during LRP and RALP.
Up to this point in the operation, antegrade dissection has permitted complete mobilization of the lateral, base, and posterior prostate, leaving division of the DVC and urethra from the prostatic apex for last. It is critical to avoid entry into the anterior prostate during division of the DVC because this may result in an iatrogenic positive margin. Although the previously placed DVC stitch may become dislodged or divided during this step, further sutures to secure the DVC can be easily placed. Also, bleeding from the DVC during attempts at resuturing can be kept to a minimum by transiently increasing the CO2 insufflation pressure to 20 mm Hg to improve the tamponade effect on venous bleeding. Once the DVC is divided, there should be good visualization of the prostatic apex and its junction with the urethra (Fig. 103–16). The anatomy of the prostatic apex is variable and should be carefully inspected before division of the urethra. As much urethral length as possible should be maintained, but an overlying anterior lip of prostate must be recognized, as well as posterior extension of prostatic tissue beneath the urethra. Nevertheless, leaving a small rim of urethra along the prostatic apex may be advisable to reduce the incidence of apical positive margins because this does not appear to have an adverse effect on the return of urinary continence (Borin et al, 2007). Sharp dissection with limited use of electrocautery is preferred during the prostatic apical dissection and division of the urethra so as to prevent thermal injury to the external striated sphincter and nearby NVBs.
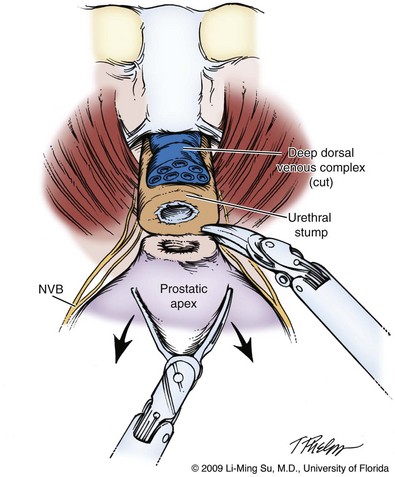
Figure 103–16 Division of urethra. Following division of the deep dorsal venous complex, the anterior and posterior urethra is divided sharply without electrocautery. A small rim of urethra may be safely left on the prostate apex to avoid an iatrogenic positive apical margin. Great care must be taken to avoid damage to the nearby nerve bundles. NVB, neurovascular bundle.
Intraoperative Inspection of Prostate
Upon completely freeing the prostate gland and before entrapment of the specimen, the entire surface of the gland can be inspected laparoscopically to assess the adequacy of resection and integrity of the tissues covering the prostate specimen. If concern exists regarding a close surgical margin, additional tissue may be excised specific to the location of concern; however, with experience this should rarely be necessary.
Pelvic Lymphadenectomy
It is generally at this time that pelvic lymphadenectomy takes place because prior mobilization of the bladder allows for excellent exposure of the obturator lymph node region. The extent of pelvic lymphadenectomy remains controversial but can be tailored on the basis of patient-specific risk factors including prostate-specific antigen (PSA), clinical stage, and Gleason score. Technical description of laparoscopic pelvic lymphadenectomy follows later in this chapter.
Entrapment of Specimens
The prostate and pelvic lymph nodes are entrapped within a laparoscopic entrapment sack introduced into the abdomen by the surgical assistant through the 12-mm assistant trocar and stored in the abdomen until completion of the vesicourethral anastomosis.
Bladder Neck Reconstruction
The bladder opening is often slightly larger than the urethral lumen, but a parachuting effect can be achieved during the vesicourethral anastomosis that allows direct approximation of the bladder neck to the urethra. If the bladder neck opening is considerably larger than the urethra, a tennis racquet handle closure can be performed using absorbable suture either posteriorly or anteriorly to allow a better size match with the urethra. In the event of a large prostate gland, median lobe, or prior TURP, the bladder neck may often be disproportionately larger than the urethra and thus may require extensive reconstruction before performing the vesicourethral anastomosis. Often, in these circumstances, the ureteral orifices are located at or near the posterior margin of the bladder neck where they are at risk of injury or obstruction during suturing of the anastomosis. In such cases the ureteral orifices can be imbricated by placing a few interrupted sutures at the 5- and 7-o’clock position along the posterior bladder neck using 3-0 polyglactin or polydioxanone suture. This maneuver can help avoid inadvertent sutures passing through or near the ureteral orifices during the anastomosis while at the same time reduce the size of the bladder neck opening.
Posterior Support of the Vesicourethral Anastomosis
As a result of prostatectomy the posterior supportive layers of the bladder and prostate are divided, including the Denonvilliers fascia and its confluence with the posterior rhabdosphincter. Recent reports of attempts at reconstruction of these posterior supportive structures have suggested improvement in earlier postoperative return of urinary control (Rocco et al, 2007), whereas others have found no significant benefit (Menon et al, 2008). Reconstituting the posterior support to the anastomosis is accomplished by reapproximating the remnant Denonvilliers fascia and posterior bladder neck to the posterior rhabdosphincter beneath the urethra using a running continuous 2-0 poliglecaprone (Monocryl) suture before completion of the vesicourethral anastomosis (Fig. 103–17). Although the exact mechanism remains unclear, suggested mechanisms include re-establishment of the posterior anatomic support to the bladder and urethra improving urethral coaptation during voiding, reduced tension at the vesicourethral anastomosis, and increase in the functional length of the striated urethral sphincteric complex. Despite the ongoing debate on its effectiveness, many believe that this step at the very least reduces the distance between the bladder neck and urethra, thus facilitating completion of a tension-free vesicourethral anastomosis. Resuspension of the anastomosis and distal bladder neck to the arcus tendineus is used by some to restore anterior urethral support and preserve the vesicourethral angle (Tewari et al, 2007).
Vesicourethral Anastomosis
With LRP, the vesicourethral anastomosis is one of the most difficult aspects of the procedure due to the challenges of performing laparoscopic suturing. The da Vinci surgical robot greatly facilitates suturing of the anastomosis due to the ergonomics of the wristed robotic instrumentation. Although interrupted sutures may be used for the anastomosis, van Velthoven and colleagues (2003) described a running suture technique, which distributes tension broadly across multiple points along the bladder and urethra. Typically, two separate sutures are tied together at their ends, each 6 to 8 inches in length. The anastomosis between the bladder and urethra begins posteriorly, leaving two needles to run progressively in an anterior direction on either side, finally ending at a single anterior tie. Dedicated mucosal eversion sutures at the bladder neck, commonly used during radical retropubic prostatectomy (RRP), are seemingly unnecessary with the excellent running mucosa-to-mucosa anastomosis achieved with LRP and RALP. Multiple sutures are first placed through the urethra and bladder before progressive cinching of the anastomosis by lifting each arm of the suture in an anterior direction (Fig. 103–18). Either an assistant or the robotic ProGrasp forceps can be used to grasp one arm of the suture to maintain tension and approximation of the posterior anastomosis while the surgeon reapproximates the contralateral side of the anastomosis using the second suture. The final urethral catheter is passed under direct vision immediately before completion of the anastomosis and the bladder is irrigated to make certain that there are no anastomotic leaks. Further sutures may be required if a leak is identified.
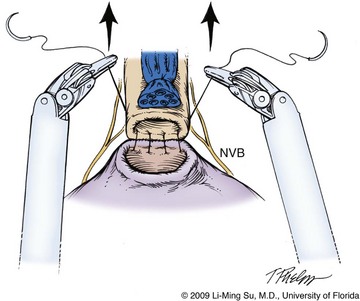
Figure 103–18 Running vesicourethral anastomosis. The posterior anastomosis is reapproximated after preplacing two to three sutures on either side and cinching the sutures by lifting anteriorly. Great care must be taken to avoid incorporating the neurovascular bundles (NVB) when placing sutures within the urethra.
Delivery of the Specimens and Exiting the Abdomen
Prior to undocking the robot and removal of the specimens, the pelvis and operative field should be carefully reinspected for bleeding under low insufflation pressure (<10 mm Hg). The bowel should be examined closely to make certain that there is no injury resulting from instrument exchanges. The string for the laparoscopic entrapment sack is transferred to the camera port site at the umbilicus and the abdomen is completely desufflated. The specimens within the laparoscopic entrapment sack are extracted intact through extension of the periumbilical trocar site. The fascial defect is then closed by open suture placement, and the skin defects are closed with a subcuticular absorbable suture or a skin adhesive. Closure of the fascial defect for the 5-mm trocar sites is generally not necessary. Due to the potential risk of a trocar site hernia, it is advisable to close the 12-mm trocar sites with a Carter Thomason fascial closure device, especially if a bladed (vs. dilating) trocar is used.
Postoperative Management
With the secure and watertight running anastomosis typically achieved, a pelvic drain may not always be necessary. However, a drain may be placed through one of the 8-mm robotic trocar sites and does not require a separate stab wound. The drain may evacuate an unanticipated urine leak or lymphatic fluid collection. Often, though, drainage is minimal and the drain typically can be removed on the first postoperative day. Parenteral narcotic medications may be required for the first 24 hours following surgery but should be used sparingly. Instead, ketorolac may be used in select patients for postoperative pain control.
The duration of time required for the urethral catheter depends to a great extent on the preferred approach of the surgeon, as well as the extent of bladder neck reconstruction. The 2-week period commonly used with open surgery is in general unnecessary if a good running anastomosis is achieved. With 1 week or more of an indwelling urethral catheter, the vast majority of patients are able to void adequately with minimal risk of urinary retention and need for catheter replacement. Performing a cystogram before removal of the catheter is based on surgeon preference. If catheter removal before a week is planned, it may be advisable to obtain a cystogram to ensure that there is no extravasation from the anastomotic site. In cases of a leak, longer duration of the urethral catheter is required to allow for spontaneous healing. Although this may prolong the time to achieve urinary continence, long-term urinary outcomes do not appear to be affected (Patil et al, 2009). Some surgeons are exploring an approach that uses suprapubic catheter drainage of the bladder with a specially designed anastomotic splint rather than a urethral catheter with good initial results (Tewari et al, 2008a).
Even with LRP and RALP, some degree of postoperative ileus occurs. Most patients can tolerate a regular diet within 24 hours of surgery, and hospitalization beyond the first postoperative day is not typically necessary. Patients can characteristically return to their preoperative activities shortly after catheter removal but must avoid strenuous activity.
Key Points: Technique of Laparoscopic and Robotic-Assisted Laparoscopic Radical Prostatectomy
Results
Published results continue to emerge with regards to midterm outcomes of LRP and RALP both in the United States and abroad, suggesting comparable outcomes with RRP. Most of these reports, however, are case series. No randomized trials have been conducted evaluating laparoscopic and robotic versus open techniques, and retrospective comparisons are limited by disparities in surgeon experience, the influence of patient selection, and nonstandardized methods of outcome assessment.
Perioperative Outcomes
Operative Time
The duration of surgery is typically longer with LRP or RALP compared with open surgery, especially early in the surgeon’s experience. In fact, operating times are often used as a surrogate for assessing the “learning curve” with laparoscopic prostatectomy (Herrell and Smith, 2005). As both surgeon and operating team experience is gained, virtually all reported series have documented a substantial decrease in operative times that approach and in some series surpass those for open surgical techniques. At experienced centers of excellence with LRP, operative times less than 3 to 4 hours are common (Turk et al, 2001; Salomon et al, 2004; Stolzenburg, 2008). Similar findings have been observed with RALP. Inexperience of both the console surgeon and the tableside operating team can lead to lengthy procedures initially. Once experience is gained, operative times of less than 3 hours have been reported for the total procedure (Smith, 2004; Badani et al, 2007; Patel et al, 2008).
Postoperative Pain
One of the distinct advantages of laparoscopy for many surgical procedures (e.g., laparoscopic nephrectomy) is its minimally invasive nature resulting in less postoperative pain than comparative open approaches. For radical prostatectomy, however, this advantage seems to be less dramatic because RRP is performed through an infraumbilical muscle-splitting incision. In addition, relatively little pain occurs after radical perineal prostatectomy. Some series have shown decreased pain in patients undergoing either LRP or RALP compared with RRP (Menon et al, 2002; Bhayani et al, 2003). Other reports have shown no substantial difference in postoperative narcotic use or in patient-reported pain (Webster et al, 2005). The lack of a significant advantage for laparoscopic prostatectomy is attributable primarily to low pain scores even in the open surgical group.
Intraoperative Blood Loss
Because most of the blood loss that occurs during radical prostatectomy is from venous sinuses, the tamponade effect from the pneumoperitoneum helps diminish ongoing blood loss during LRP and RALP. Further, the antegrade approach used during LRP and RALP entails earlier control of the prostatic pedicles and late division of the DVC as compared with RRP, in which the DVC is divided early and the arterial supply to the prostate managed late in the operation. Thus the potential for ongoing bleeding is reduced during LRP and RALP as compared with open surgery. Both of these factors, as well as the excellent visualization with laparoscopy, account for the minimal blood loss reported in most series. Blood loss of less than a few hundred milliliters is routinely reported (Ficarra et al, 2009).
Perhaps the most meaningful parameter clinically is the proportion of patients requiring transfusion of homologous blood products. Most studies have shown a significant decrease in transfusion requirement for patients undergoing LRP or RALP compared with RRP (Tewari et al, 2003; Ahlering et al, 2004a). Others have shown no statistically significant difference if the transfusion requirement with RRP can be limited to only a few percent of patients (Farnham et al, 2006).
Hospital Stay
Over the past decade, hospital stay after radical prostatectomy has diminished remarkably regardless of the surgical approach. Some centers have reported a length of stay as short as 1 or 2 days after RRP (Holzbeierlein and Smith, 2000). With LRP and RALP, a hospital stay of only 1 day has become routine in many centers. Ileus and inability to tolerate a regular diet are the most common factors limiting early discharge. Pain control does not typically contribute to prolonged length of stay because parenteral narcotics are rarely required. With early discharge programs for radical perineal and RRP being commonly used at many centers within the United States, no distinct advantage exists for LRP or RALP, although discharge on the first postoperative day may be more easily accomplished routinely with the minimally invasive approaches.
Functional Outcomes
The complications of radical prostatectomy with the greatest potential for an adverse effect on quality of life are urinary incontinence and erectile dysfunction. Greater surgical experience with radical prostatectomy and refinements in surgical technique have reduced the frequency with which these problems are observed in most radical prostatectomy series from centers of excellence. However, most large population-based studies show substantial rates of erectile dysfunction and incontinence after both RRP and radical perineal prostatectomy (Fowler et al, 1993). Whether laparoscopic or robotic approaches offer improved functional outcomes is still a matter of debate, and comparison of published series is difficult because of differences in patient populations and methods of outcome assessment.
Urinary Incontinence
Urinary incontinence after radical prostatectomy is usually manifested as stress incontinence secondary to intrinsic sphincter deficiency. Although more than 90% of patients ultimately regain good urinary control and do not require pads for incontinence in reports from high-volume centers (Walsh, 1998; Catalona et al, 1999), other studies have shown that a substantial proportion of patients may be bothered by some degree of stress incontinence (Fowler et al, 1993). The exact physiologic mechanisms that contribute to urinary control after radical prostatectomy are not entirely understood and are likely multifactorial. However, surgical technique is undoubtedly a contributing factor (Smith, 2002).
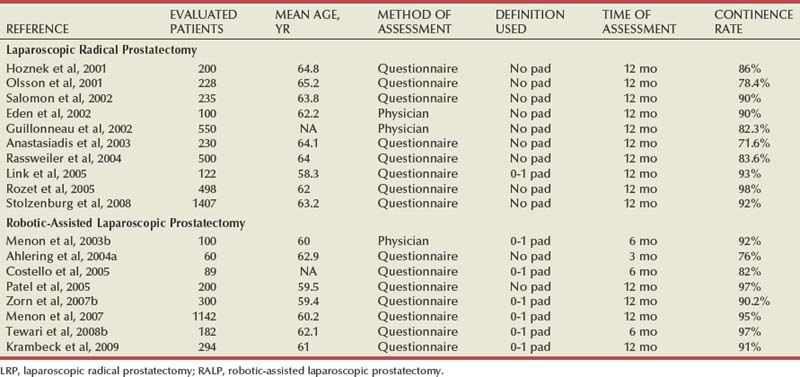
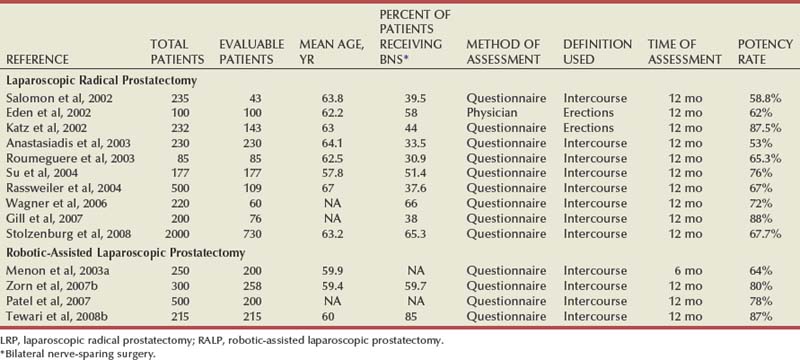
With LRP and RALP, visualization of the prostatic apex is typically superb. The minimal bleeding and magnification of the operative field allow precise dissection of the prostatic apex with limited trauma to the periurethral striated sphincter and genitourinary diaphragm. As mentioned previously, the ability to more reliably accomplish a tension-free, watertight anastomosis under the superior and direct visualization offered by laparoscopic approaches in theory favors LRP and RALP over open surgery. A common observation after radical prostatectomy, regardless of surgical approach, is that urinary incontinence improves substantially within the first 3 to 6 months after surgery and to some extent for another year or more. Therefore the time points at which data on incontinence are collected are highly influential. Differences exist whether the information is gathered by questionnaire, the physician, or an independent third party. Further, even though validated instruments for assessment of incontinence exist, the manner and location in which the data are collected can affect results. Although the method used to evaluate continence in reported series varies, the recovery of urinary continence is excellent at 1 year following LRP and RALP (Table 103–2). More recent reports of techniques that provide both posterior and anterior support to the vesicourethral anastomosis report even further improvements in urinary continence, especially at earlier time points (Tewari et al, 2007).
Erectile Dysfunction
Preservation of erectile function after radical prostatectomy depends on precise and meticulous separation of the cavernous nerves within the NVB from the prostate gland (Walsh and Donker, 1982). The anatomic course of these nerves has been described but can be variable (Costello et al, 2004; Takenaka et al, 2004; Lunacek et al, 2005). Intraoperative localization using nerve stimulation has not been sufficiently accurate for clinical utility (Holzbeierlein et al, 2001). The principles and anatomic dissection for nerve preservation are the same regardless of surgical approach. It is still uncertain whether the magnified image of the operative field afforded by laparoscopy and the precision of the surgical instruments allows more anatomically accurate and less traumatic dissection of the NVBs, resulting in improved postoperative erectile function. As with incontinence, comparison of the published literature is difficult (Salomon et al, 2004). Differences in the method of assessment, definition of potency (e.g., spontaneous erections vs. intercourse), and patient selection complicate comparisons. In addition, the use of adjunctive therapies such as phospho-diesterase-5 inhibitors or vasoactive injections can substantially influence results. Also, in concordance with other nerve injuries, improvement in erectile function is a prolonged process that is ongoing for years after radical prostatectomy. Good results with erectile function after both LRP and RALP have been reported (Table 103–3). However, there are no published data that allow definitive conclusions about the relative merits of either LRP or RALP in avoiding erectile dysfunction compared with open surgical approaches. Nevertheless, the avoidance of hemostatic energy sources during NVB preservation and the performance of meticulous interfascial dissection and preservation of the cavernous nerves appear to be critical to optimizing postoperative recovery of potency.
Anatomic studies indicate that the cavernous nerves in the NVB course posterolateral to the prostate and urethra. A technique during RALP for preservation of more anteromedial periprostatic fascia in addition to the conventional NVB regions has been reported to significantly improve potency outcomes (Menon et al, 2005; Savera et al, 2006). Although some neural tissue can be shown histologically to travel within the anterior and medial periprostatic fascia, the purpose and significance of these nerves and their relative contribution to erectile function remains uncertain. Nevertheless, the concept of optimizing both qualitative and quantitative preservation of nerve fibers traveling within the periprostatic fascial planes irrespective of whether they affect penile erections or urinary continence seems reasonable. In cases where wide excision of the nerve bundles is required, cavernous nerve grafting and nerve advancement have been described; however, the true merits of these techniques remain unclear (Martinez-Salamanca et al, 2007; Zorn et al, 2008).
Oncologic Outcomes
Surgical margin status and biochemical recurrence have generally been used as surrogates for oncologic efficacy following radical prostatectomy.
Surgical Margins
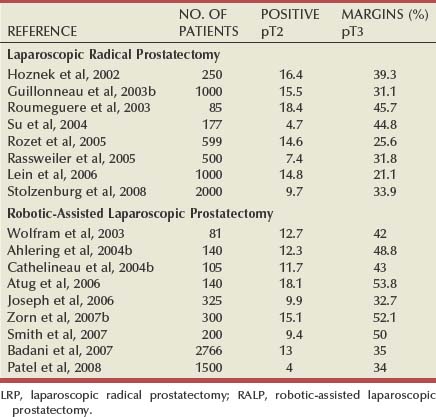
The goal of radical prostatectomy is complete surgical removal of the entire prostate and its investing fascia, as well as the seminal vesicles. Because most adenocarcinomas of the prostate occur in the peripheral zone and approach the capsular margin, surgical technique can influence oncologic outcomes. Proper surgical dissection should allow negative margins with pathologic stage T2 tumors while also permitting complete excision and negative margins for some extracapsular lesions. Efforts to avoid urinary incontinence or erectile dysfunction by dissecting too closely to the prostatic apex or the posterolateral aspect of the prostate can compromise margins, regardless of the surgical approach. Once again, comparison of positive margin rates in reported series is difficult because of patient selection. Importantly, the method and detail of pathologic analysis of the surgical specimen can be highly influential in assessing surgical margin status. Some reports have used only biopsies of remaining tissue after removal of the surgical specimen to assess margin status, whereas others rely on step-sectioned routine or whole mount histology. According to the World Health Organization International Consultation Consensus Committee guidelines established for the pathologic analysis of prostatectomy specimens, whole mount histologic sectioning may miss areas of extraprostatic extension in 7% to 15% of cases and positive margins in up to 12% as compared with specimens analyzed by routine sectioning (WHO International Consultation on Prediction of Patient Outcome in Prostate Cancer, 2004). This finding is thought to be due to the relatively thicker slices required during sectioning of the prostate for whole mount technique as compared with the serial 3- to 5-mm slices used during routine sectioning.
In most series of LRP and RALP, positive margin percentages decrease with experience (Ahlering et al, 2004b; Salomon et al, 2004; Rassweiler et al, 2005). This implies that inexperience with the surgery accounts for positive margins in some cases. Sometimes, this may be from difficulty in identifying the proper anatomic plane of dissection between the bladder neck and the base of the prostate. The most common site of a positive margin, whether the operation is performed via open or laparoscopic approaches, is the prostatic apex (Touijer et al, 2005). Insufficient removal of prostatic tissue at the apex in an effort to optimize urethral length and avoid incontinence can result in positive margins even with tumors that do not pathologically violate the capsule (i.e., stage pT2). As mentioned previously in the surgical techniques section of this chapter, adhering to specific surgical principles can help reduce site-specific positive margins at the apex, bladder neck, and posterolateral regions of the prostate. Published rates of pathologic stage-specific positive margins for LRP or RALP are shown in Table 103–4.
The primary factor that determines the positive margin rate in a given series is patient selection. As discussed earlier, the method and detail of pathologic analysis is also influential. Evaluating positive margin rates from one series to another, then, is not necessarily a comparison of surgical technique. A more accurate technical comparison is analysis of the pathologic results in stage T2 tumors wherein a positive margin implies surgical violation of the prostatic capsule. Even in this circumstance, though, the methodology for pathologic sampling is important. Intrainstitutional comparisons from some studies have shown a reduced rate of positive margins with laparoscopic approaches compared with RRP. However, comparison of margin status between high-volume centers with operations performed by experienced surgeons has shown no definitive advantage for one surgical approach over the other in achieving negative surgical margins (Brown et al, 2003; Khan et al, 2005).
Biochemical Recurrence
Biochemical recurrence following prostatectomy may perhaps provide a more accurate assessment of oncologic control than margin status. Guillonneau and colleagues reported on their oncologic outcomes with 1000 consecutive LRPs performed over a 4-year period with a median follow-up period of 12 months (Guillonneau et al, 2003b). Their overall actuarial biochemical progression-free survival rate was 90.5% at 3 years. By pathologic stage, the rates were 92% for pT2a, 88% for pT2b, 77% for pT3a, and 44% for pT3b. Pavlovich and colleagues (2008) reported on 528 consecutive LRP patients with a mean follow-up of 13 months. The 3-year, actuarial, biochemical-free survival was 94.5% overall, 98.2% for pT2, and 78.7% for pT3 disease.
With regard to RALP, Badani and colleagues in 2007 reported on their large series of 2766 consecutive RALP patients with a mean follow-up period of 22 months. Their overall 5-year, actuarial, biochemical-free survival was 84% overall, 84% for pT2, and 66% for pT3 patients. It should be noted that their patient population included higher-risk patients than most reported series with a Gleason score of 7 or higher in 64% and a pathologic stage of pT3 or higher in 22% of their patients. In a retrospective comparison of biochemical recurrence between RALP and a contemporary series of RRP performed at a single center, no difference was found between the two groups after controlling for clinical and pathologic features (Schroeck et al, 2008). In the end, longer-term follow-up is required to compare the results of LRP and RALP with results of RRP from established and experienced centers of excellence.
Economic Considerations
Both the duration of surgery and equipment expenses contribute to operating room costs for LRP and RALP, which typically are higher than those for open approaches (Link et al, 2004; Lotan et al, 2004; Scales et al, 2005). This is particularly true with robotic-assisted surgery. Current purchase cost of a da Vinci S system is approximately $1.65 million with an average cost of $2400 for each multiple-use (10 lives) robotic instrument. For robotic instrumentation, this would translate to approximately $1200 per case for the use of five separate robotic instruments with an additional $325 per case for disposables (sterile robotic drapes and trocar seals).
In the study by Link and colleagues (2004), the factors that most influenced overall LRP cost in order of importance included operative time, length of hospital stay, and consumable items (e.g., disposable laparoscopic equipment and trocars). They found that the calculated cost equivalence between RRP and LRP could be met if disposable equipment was eliminated by using reusable items and operative times for LRP were reduced to 3.4 hours. Lotan and colleagues (2004) found that RALP costs were approximately $1155 per case more than RRP if the initial purchase cost of the robot were excluded. In a local cost analysis performed by Scales and colleagues (2005), they found that RALP would be less expensive than RRP in some practice settings in which RALP hospital stay was less than 1.5 days if case volumes increased to 14 cases per week. This study suggested that RALP may be more economically viable in high-volume centers with multispecialty robotic use.
These increased costs may be partially mitigated by shorter hospital stays compared with open surgery. The decrease in expense for hospital stay depends partly on the discharge day for the laparoscopic procedure but also the customary length of stay at a given hospital for open radical prostatectomy. Published reports detailing a length of stay of a week or more for RRP are not in concordance with other contemporary reports wherein patients are discharged on the second or even first postoperative day after radical perineal or RRP (Holzbeierlein and Smith, 2000).
Complications
Complications Related to Patient Positioning
LRP and RALP are typically performed with the patient in a steep exaggerated Trendelenburg position and with the legs in lithotomy position. Careful padding of vulnerable body parts such as the hips, shoulders, knees, and calves is important to prevent pressure injury and neuromuscular complications. Increased intraocular pressure can occur in patients in an exaggerated Trendelenburg position, but in patients undergoing RALP this does not appear to have any apparent clinical long-term consequence. However, there may be an increased risk of corneal abrasion, making it even more important for the anesthesiologist to maintain good eye lubrication and protection.
Vascular and Bowel Injury
Vascular or bowel perforations are rare but can occur during placement of abdominal trocars. In addition, once safe trocar placement is established and the robot is docked, care must be taken to avoid injury along the path of the multiple instruments, which must be interchanged and directed toward the pelvis. The key to avoiding major postoperative complications is prompt recognition and immediate management of injury to bowel or blood vessels. Often, relatively minor injuries can be repaired laparoscopically, although there should be no hesitation to convert to an open approach in the face of a difficult problem.
Open Conversion
Open conversion is rare (<2%) and has been cited in the literature, usually during a surgeon’s early experience with LRP or RALP, mainly as a result of failure to progress or uncertainty of dissection planes (Bhayani et al, 2004). With experience, the need for open conversion is rare; however, patients must be properly counseled on this possibility.
Rectal Injury
Rectal injuries, although uncommon during LRP and RALP (0.7% to 2.4%), have been reported and repaired successfully by laparoscopic means (Guillonneau et al, 2003a; Katz et al, 2003; Gonzalgo et al, 2005). Intraoperative recognition and repair of the injury is crucial. Multilayered primary closure and interposition of omentum between the rectum and anastomosis can usually avoid long-term problems, as well as the need for open conversion and intestinal diversion. Inadequate closure or lack of recognition can result in a fistula between the vesicourethral anastomosis and the rectum. If a small rectal injury is suspected but not readily identified, insufflation of air into the rectum using a catheter inserted into the rectum with fluid within the pelvis can often demonstrate small bubbles at the site of the injury.
Thromboembolic Complications
The incidence of thromboembolic complications is low after LRP and RALP (0.5%) likely, in part, due to faster postoperative patient mobilization and Trendelenburg positioning, used for decreasing the venous stasis in the lower extremities as compared with open surgery (Secin et al, 2008). The American Urological Association guidelines do not recommend routine use of prophylactic anticoagulants for laparoscopic procedures unless a patient has known risk factors such as obesity or a prior history of deep venous thrombosis. Nonetheless, because of the known venous stasis and hypercoagulable state that can occur during pelvic surgery in patients with known malignancy, these patients are still at risk for thromboembolic problems. Secin and colleagues (2008) propose the use of heparin only in patients at high risk for venous thromboembolism. Sequential compression stockings are advised during the operative and perioperative period. The presentation of deep venous thrombosis in the lower extremities should prompt a pelvic CT scan or ultrasound to exclude a lymphocele, hematoma, or urinoma that could be compressing the external iliac vein.
Anastomotic Complications
Failure to achieve a watertight closure of the anastomosis can result in urinary extravasation and accumulation of urine even if a pelvic drain is placed. This is even more problematic with a transperitoneal approach because the entire abdominal cavity becomes accessible for urine egress. In such cases a cystogram should be performed to make certain that there is some degree of integrity of the anastomosis. Fluid accumulations may require percutaneous drainage. If complete disruption of the anastomosis has occurred, surgical revision, either laparoscopic or open, is indicated if the problem is recognized in the first few days after surgery. Anastomotic stricture resulting in bladder neck contracture seemingly occurs at a lower rate after LRP and RALP compared with open surgical approaches, especially in the hands of experienced surgeons. Rates of less than 2% have been reported (Msezane et al, 2008; Webb et al, 2009). This implies that achievement of a watertight anastomosis with good mucosal approximation is the key measure in preventing postoperative bladder neck contracture.
Bleeding and Transfusion
Virtually all published reports have documented a distinct advantage for laparoscopic surgery in diminishing the amount of bleeding that occurs with radical prostatectomy. Transfusion requirements of 2% or less are commonly reported (Ficarra et al, 2009). The tamponade effect of the pneumoperitoneum compresses venous bleeding, and the superb visualization allows prospective identification of bleeding vessels that require hemostasis. However, in addition to the risk of major vascular injury from the surgical dissection or trocar placement, there is the possibility of postoperative bleeding once the pneumoperitoneum is relieved. As mentioned previously, the pelvis and surgical field should be carefully inspected for the presence of bleeding at the end of the operation under low insufflation pressure. Because of the low incidence of postoperative bleeding, though, routine use of topical hemostatic agents along the prostate bed is not generally required.
Equipment Malfunction
The surgeon is highly dependent on sophisticated technology and equipment for performance of LRP and, in particular, RALP. Equipment malfunction, especially with RALP, can create problems that make it difficult to progress with surgery and may result in case cancellation or conversion to pure laparoscopic or even open surgery. Zorn and colleagues (2007a) identified recoverable errors in 0.4% of RALP cases performed at their institution. Lavery and colleagues (2008) found a 0.4% nonrecoverable malfunction rate in their multi-institutional study of high-volume RALP centers. Although extremely rare, patients need to be properly counseled about the possibility of conversion to a pure laparoscopic or open surgical approach in the event of an unrecoverable equipment malfunction.
Key Points: Results and Complications of Laparoscopic and Robotic-Assisted Laparoscopic Radical Prostatectomy
Laparoscopic Pelvic Lymph Node Dissection
Indications
Currently, PLND is rarely indicated as an independent staging procedure. In some patients with a significant risk for nodal metastasis, such as those with a high Gleason grade tumor, a large tumor volume, or a markedly elevated PSA level, PLND may be useful for staging and selection of therapy before external beam irradiation. Also, staging PLND may have a role in some patients in whom radical perineal prostatectomy is planned. With RRP, LRP, or RALP, it is the usual practice that PLND be performed simultaneously with the radical prostatectomy.
Expert opinion about the role of PLND in patients undergoing surgery for carcinoma of the prostate is evolving. Current debate centers around the anatomic boundaries for the procedure, the merits of an extended lymph node dissection, and whether there is any meaningful clinical (i.e., therapeutic) benefit to surgical removal of involved nodes. Most studies show histologic evidence of nodal metastasis in less than 5% of patients with low-risk features in the primary tumor. Consequently, PLND may not be required in these patients.
PLND is generally recommended in patients with intermediate or high-risk parameters of the primary tumor, generally implying a PSA of greater than 10, a large palpable nodule, or a Gleason sum of 7 or greater.
Surgical Technique
In laparoscopic staging PLND, trocar configuration is similar to that of LRP and RALP but only one assistant trocar is generally necessary, if at all. Abdominal access is established, and an incision is made just lateral to the medial umbilical ligament back toward its confluence with the hypogastric artery and down to the pubis (Fig. 103–19). Great care must be taken so as to avoid injury to the nearby ureter. If PLND is performed during LRP or RALP, the dissection is simplified because previous mobilization of the bladder provides excellent exposure of the obturator space. As with an open approach, a key initial step to standard laparoscopic PLND is separation of the nodal packet from the external iliac vein. The lymph node packet is grasped and retracted medially. A relatively avascular plane between the lymph node packet and lateral pelvic sidewall is identified and dissected bluntly using a suction-irrigation device. Dissection is carried out proximally to the iliac bifurcation and distally to the pubis, thus defining the lateral extent of the lymph node packet. By retracting the lymph node packet medially, the precise course of the obturator nerve and vessels can be identified and protected (Fig. 103–20). After securing the distal extent of the lymph node packet, the packet is divided, retracted cranially, and bluntly separated from the obturator vessels and nerve. Hemoclips are used to control lymphatic vessels at the most proximal and distal extent of the dissection (Fig. 103–21). The lymph nodes can usually be removed as a single packet and extracted either through the 12-mm trocar or by placing them in the entrapment sack along with the prostate specimen.
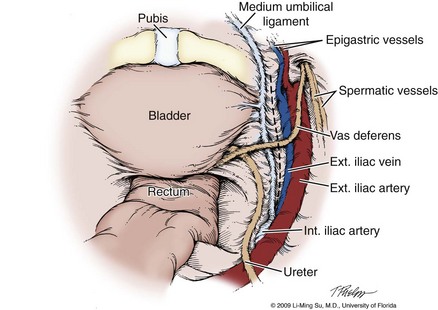
Figure 103–19 Laparoscopic pelvic lymph node dissection. Initial transperitoneal view of the obturator fossa and relevant anatomy. The dashed line indicates the longitudinal incision that is made in the peritoneum lateral and parallel to the median umbilical ligament back toward the bifurcation of the iliac vessels in efforts to provide exposure to the obturator fossa and lymph nodes.
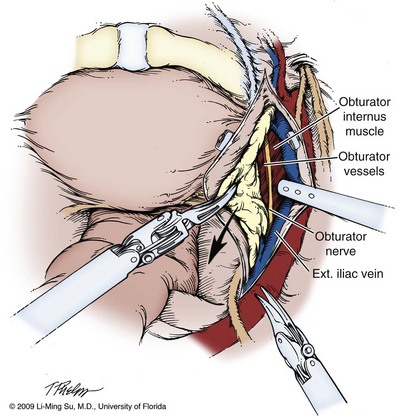
Figure 103–20 Initial dissection of a standard pelvic lymph node template. The vas deferens has been clipped and divided. With medial traction on the lymph node packet, the lateral extent of the dissection is defined using mainly blunt dissection.
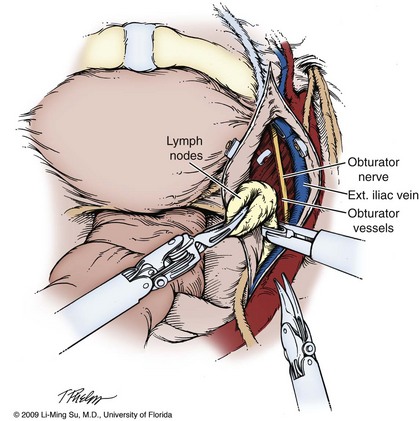
Figure 103–21 Final dissection of a standard pelvic lymph node template. The proximal and distal extent of the lymph node packet are clipped and divided, taking great care to avoid injury to the obturator nerve and vessels, as well as the accessory obturator vein.
An extended lymph node dissection typically includes nodes categorized as external iliac, obturator, and hypogastric and has a boundary of the genitofemoral nerve laterally, the bladder wall medially, the node of Cloquet distally, and a point 2 cm above the bifurcation of the common iliac artery proximally. Importantly, the posterior dissection involves complete skeletonization of the hypogastric artery and its medial branches, as well as the area posterior to the obturator. Studies show that an extended dissection has a higher diagnostic yield than standard or limited PLND because it recognizes and removes positive lymph nodes more commonly.
Extended lymph node dissection can be performed laparoscopically and robotically. The da Vinci S system allows more proximal angulation of the instruments, and this can significantly facilitate dissection around the bifurcation of the common iliac artery. A thorough dissection adds time to the surgical procedure, but this is true regardless of whether laparoscopic or open surgery is performed. No valid comparative studies exist to support the superiority of open versus laparoscopic PLND. Using lymph node count as a surrogate for adequacy of the dissection is problematic because the method and thoroughness of pathologic evaluation is, perhaps, even more influential than the anatomic extent of the surgical dissection. With careful attention to meticulous dissection, though, all of the fibrous, fatty, and lymphatic tissue within the commonly accepted anatomic boundaries for an extended lymph node dissection can be removed laparoscopically or robotically. Use of clips on identifiable lymphatic channels can minimize the occurrence of a postoperative lymphocele.
Complications of Pelvic Lymph Node Dissection
Because, by definition, PLND requires skeletonization of portions of the common iliac, external iliac, and hypogastric arteries and veins, the possibility of major vascular injury exists. A small venotomy or arteriotomy can be closed laparoscopically with a fine polypropylene (Prolene) suture. A major injury may require rapid conversion to an open approach. However, the incidence of major vascular injury resulting in enough bleeding to require transfusion is well under 1% with PLND.
Transection of the obturator nerve may occur. Direct repair with suturing of the ends of the obturator nerve can restore partial function. Ureteral injury is uncommon, but caution must be observed during the proximal portion of the dissection as the ureter crosses the anterior portion of the common iliac artery.
A transperitoneal approach is not protective against the formation of a lymphocele. Theoretically, the communication with the entire peritoneum would allow distribution and absorption of any lymphatic fluid throughout the peritoneal lining of the abdomen and decrease the risk of lymphocele. Despite this, however, a loculation of lymphatic fluid can occur. Asymptomatic lymphoceles do not necessarily require drainage or treatment. A larger collection can compress the bladder and cause voiding symptoms. Compression of the external iliac vein can predispose the patient to deep vein thrombosis in the lower extremity. Secondary infection of lymphoceles may also occur. In the presence of symptoms or complications from a lymphocele, percutaneous drainage is typically successful. However, lymphatic fluid can reaccumulate, requiring repeat drainage with injection of a sclerosing agent or surgical opening of a window with marsupialization of the lymphocele wall by laparoscopic means.
Key Points: Laparoscopic Pelvic Lymph Node Dissection
Summary
In the past decade, LRP and RALP have become accepted surgical approaches for the management of patients with localized carcinoma of the prostate both in the United States and abroad. As expertise with these procedures is achieved, operative times have diminished. In experienced centers operative times are similar to that of RRP. The robotic-assisted technique provides ergonomic advantages for the surgeon and facilitates suturing and other technical aspects of the operation for surgeons who do not have highly advanced laparoscopic skills.
Comparison of outcomes between reported series is imprecise because of differences in patient selection, methods of collecting and reporting data, and the technique of pathologic sectioning and analysis. However, intraoperative blood loss with LRP and RALP has consistently been reported as minimal and transfusion is required in only a small percentage of patients. Postoperative morbidity and return to activity are both improved compared with open surgery in most reports. Good results with postoperative urinary continence and erectile function are reported with mature LRP and RALP series, although there is still debate about whether these outcomes are superior to open RRP performed by experienced surgeons. Pathologic tumor margins status seems to be comparable between laparoscopic, robotic, and open series overall (Parsons and Bennett, 2008; Ficarra et al, 2009).
Improvements in the available instruments are highly likely to advance even further the technologic capabilities of surgeons performing LRP and RALP. Equipment expense remains a significant issue for some hospitals, and not all may be able to offer this state-of-the-art technology. Nonetheless, there seems to be little doubt that minimally invasive approaches for radical prostatectomy, especially RALP, have become the dominant surgical treatment for localized prostate cancer in the United States and are having continued growth and acceptance worldwide.
Badani KK, Kaul S, Menon M. Evolution of robotic radical prostatectomy: assessment after 2766 procedures. Cancer. 2007;110:1951-1958.
Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004;94:1071-1976.
Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037-1063.
Link RE, Su L-M, Sullivan W, et al. Health related quality of life before and after laparoscopic radical prostatectomy. J Urol. 2005;173:175-179.
Lotan Y, Cadeddu JA, Gettman MT. The new economics of radical prostatectomy: cost comparison of open, laparoscopic and robot assisted techniques. J Urol. 2004;172:1431-1435.
Menon M, Kaul S, Bhandari A, et al. Potency following robotic radical prostatectomy: a questionnaire based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J Urol. 2005;174:2291-2296.
Ong AM, Su LM, Varkarakis I, et al. Nerve sparing radical prostatectomy: effects of hemostatic energy sources on the recovery of cavernous nerve function in a canine model. J Urol. 2004;172(4 Pt. 1):1318-1322.
Patel VR, Palmer KJ, Coughlin G, Samavedi S. Robot-assisted laparoscopic radical prostatectomy: perioperative outcomes of 1500 cases. J Endourol. 2008;22:2299-2305.
Pavlovich CP, Trock BJ, Sulman A, et al. 3-year actuarial biochemical recurrence-free survival following laparoscopic radical prostatectomy: experience from a tertiary referral center in the United States. J Urol. 2008;179:917-921.
Stolzenburg JU, Rabenalt R, Do M, et al. Endoscopic extraperitoneal radical prostatectomy: the University of Leipzig experience of 2000 cases. J Endourol. 2008;22(10):2319-2325.
Abbou CC, Salomon L, Hoznek A, et al. Laparoscopic radical prostatectomy: preliminary results. Urology. 2000;55:630-633.
Ahlering TE, Eichel L, Chou D, Skarecky DW. Feasibility study for robotic radical prostectomy cautery-free neurovascular bundle preservation. Urology. 2005;65:994-997.
Ahlering TE, Eichel L, Edwards RA, et al. Robotic radical prostatectomy: a technique to reduce pT2 positive margins. Urology. 2004;64:1224-1228.
Ahlering TE, Wood D, Eichel L, et al. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon’s outcomes. Urology. 2004;63:819-822.
Allaf ME, Hsu TH, Sullivan W, Su LM. Simultaneous laparoscopic prosthetic mesh inguinal herniorrhaphy during transperitoneal laparoscopic radical prostatectomy. Urology. 2003;62:1121.
Anastasiadis AG, Salomon L, Katz R, et al. Radical retropubic versus laparoscopic prostatectomy: a prospective comparison of functional outcome. Urology. 2003;62:292-297.
Atug F, Castle EP, Woods M, et al. Transperitoneal versus extraperitoneal robotic-assisted radical prostatectomy: is one better than the other? Urology. 2006;68:1077-1081.
Badani KK, Kaul S, Menon M. Evolution of robotic radical prostatectomy: assessment after 2766 procedures. Cancer. 2007;110:1951-1958.
Bhayani SB, Pavlovich CP, Hsu TS, et al. Prospective comparison of short-term convalescence: laparoscopic radical prostatectomy versus open radical retropubic prostatectomy. Urology. 2003;61:612-616.
Bhayani SB, Pavlovich CP, Strup SE, et al. Laparoscopic radical prostatectomy: a multi-institutional study of conversion to open surgery. Urology. 2004;63:99-102.
Borin JF, Skarecky DW, Narula N, Ahlering TE. Impact of urethral stump length on continence and positive surgical margins in robot-assisted laparoscopic prostatectomy. Urology. 2007;70:173-177.
Boris RS, Bhandari A, Krane LS, et al. Salvage robotic-assisted radical prostatectomy: initial results and early report of outcomes. BJU Int. 2009;103:952-956.
Brown JA, Garlitz C, Gomella LG, et al. Pathologic comparison of laparoscopic versus open radical retropubic prostatectomy specimens. Urology. 2003;62:481-486.
Brown JA, Rodin DM, Lee B, Dahl DM. Laparoscopic radical prostatectomy and body mass index: an assessment of 151 sequential cases. J Urol. 2005;173:442-445.
Brown JA, Rodin D, Lee B, Dahl DM. Transperitoneal versus extraperitoneal approach to laparoscopic radical prostatectomy: an assessment of 156 cases. Urol. 2005;65:320-324.
Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433-438.
Cathelineau X, Cahill D, Widmer H, et al. Transperitoneal or extraperitoneal approach for laparoscopic radical prostatectomy: a false debate over a real challenge. J Urol. 2004;171(2 Pt. 1):714-716.
Cathelineau X, Rozet F, Vallancien G. Robotic radical prostatectomy: the European experience. Urol Clin North Am. 2004;31:693-699.
Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004;94:1071-1976.
Costello AJ, Haxhimolla H, Crowe H, Peters JS. Installation of telerobotic surgery and initial experience with telerobotic radical prostatectomy. BJU Int. 2005;96:34-38.
Eden CG, Cahill D, Vass JA, et al. Laparoscopic radical prostatectomy: the initial UK series. BJU Int. 2002;90:876-882.
Eden CG, King D, Kooiman GG, et al. Transperitoneal or extraperitoneal laparoscopic radical prostatectomy: does the approach matter? J Urol. 2004;172(6 Pt. 1):2218-2223.
Erdogru T, Teber D, Frede T, et al. Comparison of transperitoneal and extraperitoneal laparoscopic radical prostatectomy using match-pair analysis. Eur Urol. 2004;46:312-319. discussion 320
Erdogru T, Teber D, Frede T, et al. The effect of previous transperitoneal laparoscopic inguinal herniorrhaphy on transperitoneal laparoscopic radical prostatectomy. J Urol. 2005;173:769-772.
Farnham SB, Webster TM, Herrell SD, Smith JAJr. Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology. 2006;67:360-363.
Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037-1063.
Fowler FJJr, Barry MJ, Lu-Yao G, et al. Patient-reported complications and follow-up treatment after radical prostatectomy. The national Medicare experience: 1988-1990 (updated June 1993). Urology. 1993;42:622-629.
Gill IS, Ukimura O. Thermal energy-free laparoscopic nerve-sparing radical prostatectomy: one-year potency outcomes. Urology. 2007;70:309-314.
Gill IS, Ukimura O, Rubinstein M, et al. Lateral pedicle control during laparoscopic radical prostatectomy: refined technique. Urology. 2005;65:23-27.
Gonzalgo ML, Pavlovich CP, Trock BJ, et al. Classification and trends of perioperative morbidities following laparoscopic radical prostatectomy. J Urol. 2005;174:135-139.
Guillonneau B, Cathelineau X, Doublet JD, et al. Laparoscopic radical prostatectomy: assessment after 550 procedures. Crit Rev Oncol Hematol. 2002;43:123-133.
Guillonneau B, el-Fettouh H, Baumert H, et al. Laparoscopic radical prostatectomy: oncological evaluation after 1,000 cases at Montsouris Institute. J Urol. 2003;169:1261-1266.
Guillonneau B, Gupta R, El Fettouh H, et al. Laparoscopic management of rectal injury during laparoscopic radical prostatectomy. J Urol. 2003;169:1694-1696.
Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: the Montsouris technique. J Urol. 2000;163:1643-1649.
Guillonneau BD. Laparoscopic versus robotic radical prostatectomy. Nat Clin Pract Urol. 2005;2:60-61.
Herrell SD, Smith JA.Jr. Robotic-assisted laparoscopic prostatectomy: what is the learning curve? Urology. 2005;66:105-107.
Holzbeierlein JM, Peterson M, Smith JAJr. Variability of results of cavernous nerve stimulation during radical prostatectomy. J Urol. 2001;165:108-110.
Holzbeierlein JM, Smith JAJr. Radical prostatectomy and collaborative care pathways. Semin Urol Onc. 2000;8:60-65.
Hoznek A, Antiphon P, Borkowski T, et al. Assessment of surgical technique and perioperative morbidity associated with extraperitoneal versus transperitoneal laparoscopic radical prostatectomy. Urol. 2003;61(3):617-622.
Hoznek A, Salomon L, Olsson LE, et al. Laparoscopic radical prostatectomy. The Creteil experience. Eur Urol. 2001;40:38-45.
Hoznek A, Sammadi DB, Salomon L, et al. Laparoscopic radical prostatectomy. Curr Urol Report. 2002;3:141-147.
Joseph JV, Rosenbaum R, Madeb R, et al. Robotic extraperitoneal radical prostatectomy: an alternative approach. J Urol. 2006;175:945-951.
Kaouk JH, Hafron J, Goel R, et al. Robotic salvage retropubic prostatectomy after radiation/brachytherapy: initial results. BJU Int. 2008;102:93-96.
Katz R, Borkowski T, Hoznek A, et al. Operative management of rectal injuries during laparoscopic radical prostatectomy. Urology. 2003;62:310-313.
Katz R, Salomon L, Hoznek A, et al. Patient reported sexual function following laparoscopic radical prostatectomy. J Urol. 2002;168:2078-2082.
Khan MA, Partin AW. Surgical margin status after radical retropubic prostatectomy. BJU Int. 2005;95:281-284.
Krambeck AE, DiMarco DS, Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448-452.
Lavery HJ, Thaly R, Albala D, et al. Robotic equipment malfunction during robotic prostatectomy: a multi-institutional study. J Endourol. 2008;22:2165-2168.
Lein M, Stibane I, Mansour R, et al. Complications, urinary continence, and oncologic outcomes of 1000 laparoscopic transperitoneal radical prostatectomies-experience at the Charité Hospital Berlin, Campus Mitte. Eur Urol. 2006;50:1278-1284.
Levinson AW, Bagga HS, Pavlovich CP, et al. The impact of prostate size on urinary quality of life indexes following laparoscopic radical prostatectomy. J Urol. 2008;179:1818-1822.
Levinson AW, Ward NT, Sulman A, et al. The impact of prostate size on perioperative outcomes in a large laparoscopic radical prostatectomy series. J Endourol. 2009;23:147-152.
Link BA, Nelson R, Josephson DY, et al. The impact of prostate gland weight in robot assisted laparoscopic radical prostatectomy. J Urol. 2008;180:928-932.
Link RE, Su LM, Bhayani SB, Pavlovich CP. Making ends meet: a cost comparison of laparoscopic and open radical retropubic prostatectomy. J Urol. 2004;172:269-274.
Link RE, Su L-M, Sullivan W, et al. Health related quality of life before and after laparoscopic radical prostatectomy. J Urol. 2005;173:175-179.
Lotan Y, Cadeddu JA, Gettman MT. The new economics of radical prostatectomy: cost comparison of open, laparoscopic and robot assisted techniques. J Urol. 2004;172:1431-1435.
Lunacek A, Schwentner C, Fritsch H, et al. Anatomical radical retropubic prostatectomy: ‘curtain dissection’ of the neurovascular bundle. BJU Int. 2005;95:1226-1231.
Martinez-Salamanca JI, Rao S, Ramanathan R, et al. Nerve advancement with end-to-end reconstruction after partial neurovascular bundle resection: a feasibility study. J Endourol. 2007;21:830-835.
Meininger D, Byhahn C, Wolfram M, et al. Prolonged intraperitoneal versus extraperitoneal insufflation of carbon dioxide in patients undergoing totally endoscopic robot-assisted radical prostatectomy. Surg Endosc. 2004;18(5):829-833.
Menon M, Kaul S, Bhandari A, et al. Potency following robotic radical prostatectomy: a questionnaire based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J Urol. 2005;174:2291-2296.
Menon M, Muhletaler F, Campos M, Peabody JO. Assessment of early continence after reconstruction of the periprostatic tissues in patients undergoing computer assisted (robotic) prostatectomy: results of a 2 group parallel randomized controlled trial. J Urol. 2008;180:1018-1023.
Menon M, Shrivastava A, Kaul S, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007;51:648-658.
Menon M, Shrivastava A, Sarle R, et al. Vattikuti Institute Prostatectomy: a single-team experience of 100 cases. J Endourol. 2003;17:785-790.
Menon M, Tewari A, Baize B, et al. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti urology institute experience. Urology. 2002;60:864-868.
Menon M. Tewari A, members of the Vattikuti Institute Prostatectomy Team. Robotic radical prostatectomy and the Vattikuti Urology Institute technique: an interim analysis of results and technical points. Urology. 2003;61:15-20.
Millin T. Retropubic urinary surgery. London: Livingstone; 1947.
Msezane LP, Reynolds WS, Gofrit ON, et al. Bladder neck contracture after robot-assisted laparoscopic radical prostatectomy: evaluation of incidence and risk factors and impact on urinary function. J Endourol. 2008;22:377-383.
Nehra A, Kumar R, Ramakumar S, et al. Pharmacoangiographic evidence of the presence and anatomical dominance of accessory pudendal artery(s). J Urol. 2008;179:2317-2320.
Nguyen MM, Turna B, Santos BR, et al. The use of an endoscopic stapler vs suture ligature for dorsal vein control in laparoscopic prostatectomy: operative outcomes. BJU Int. 2008;101:463-466.
Oelrich TM. The urethral sphincter muscle in the male. Am J Anat. 1980;158:229-296.
Olsson LE, Salomon L, Nadu A, et al. Prospective patient-reported continence after laparoscopic radical prostatectomy. Urology. 2001;58:570-572.
Ong AM, Su LM, Varkarakis I, et al. Nerve sparing radical prostatectomy: effects of hemostatic energy sources on the recovery of cavernous nerve function in a canine model. J Urol. 2004;172(4 Pt. 1):1318-1322.
Parsons JK, Bennett JL. Outcomes of retropubic, laparoscopic, and robotic-assisted prostatectomy. Urology. 2008;72:412-416.
Patel VR, Tully AS, Holmes R, Lindsay J. Robotic radical prostatectomy in the community setting—the learning curve and beyond: initial 200 cases. J Urol. 2005;174:269-272.
Patel VR, Thaly R, Shah K. Robotic radical prostatectomy: outcomes of 500 cases. BJU Int. 2007;99:1109-1112.
Patel VR, Palmer KJ, Coughlin G, Samavedi S. Robot-assisted laparoscopic radical prostatectomy: perioperative outcomes of 1500 cases. J Endourol. 2008;22:2299-2305.
Patil N, Krane L, Javed K, et al. Evaluating and grading cytographic leakage: correlation with clinical outcomes in patients undergoing robotic prostatectomy. BJU Int. 2009;103:1108-1110.
Pavlovich CP, Trock BJ, Sulman A, et al. 3-year actuarial biochemical recurrence-free survival following laparoscopic radical prostatectomy: experience from a tertiary referral center in the United States. J Urol. 2008;179:917-921.
Rassweiler J, Schulze M, Teber D, et al. Laparoscopic radical prostatectomy: functional and oncological outcomes. Curr Opin Urol. 2004;14:75-82.
Rassweiler J, Schulze M, Teber D, et al. Laparoscopic radical prostatectomy with the Heilbronn technique: oncological results in the first 500 patients. J Urol. 2005;173:761-764.
Reiner WB, Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorini’s plexus during radical retropubic surgery. J Urol. 1979;121:198-200.
Rocco F, Carmignani L, Acquati P, et al. Early continence recovery after open radical prostatectomy with restoration of the posterior aspect of the rhabdosphincter. Eur Urol. 2007;52:376-383.
Roumeguere T, Bollens R, Vanden Bossche M. Radical prostatectomy: a prospective comparison of oncologic and functional results between open and laparoscopic approaches. World J Urol. 2003;20:360-363.
Rozet F, Galiano M, Cathelineau X, et al. Extraperitoneal laparoscopic radical prostatectomy: a prospective evaluation of 600 cases. J Urol. 174, 2005. 980-911
Salomon L, Anastasiadis AG, Katz R, et al. Urinary continence and erectile function: a prospective evaluation of functional results after radical laparoscopic prostatectomy. Eur Urol. 2002;42:338-343.
Salomon L, Sebe P, De la Taille A, et al. Open versus laparoscopic radical prostatectomy: part I and II. BJU Int. 2004;94:238-250.
Savera AT, Kaul S, Badani K, et al. Robotic radical prostatectomy with the “Veil of Aphrodite” technique: histologic evidence of enhanced nerve sparing. Eur Urol. 2006;49:1065-1073.
Scales CDJr, Jones PJ, Eisenstein EL, et al. Local cost structures and the economics of robot assisted radical prostatectomy. J Urol. 2005;174:2323-2329.
Schroeck FR, Sun L, Freedland SJ, et al. Comparison of prostate-specific antigen recurrence-free survival in a contemporary cohort of patients undergoing either radical retropubic or robot-assisted laparoscopic radical prostatectomy. BJU Int. 2008;102:28-32.
Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854-857.
Secin FP, Jiborn T, Bjartell AS, et al. Multi-institutional study of symptomatic deep venous thrombosis and pulmonary embolism in prostate cancer patients undergoing laparoscopic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;53:134-145.
Singh A, Fagin R, Shah G, Shekarriz B. Impact of prostate size and body mass index on perioperative morbidity after laparoscopic radical prostatectomy. J Urol. 2005;173:552-554.
Smith JAJr. Outcome after radical prostatectomy depends on surgical technique but not approach. Curr Urol Rep. 2002;3:179-181.
Smith JAJr. Robotically assisted laparoscopic prostatectomy: an assessment of its contemporary role in the surgical management of localized prostate cancer. Am J Surg. 2004;188:63S-67S.
Smith JAJr, Chan RC, Chang SS, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385-2390.
Stolzenburg JU, Ho KM, Do M, et al. Impact of previous surgery on endoscopic extraperitoneal radical prostatectomy. Urology. 2005;65:325-331.
Stolzenburg JU, Rabenalt R, Dietel A, et al. Hernia repair during endoscopic (laparoscopic) radical prostatectomy. J Laparoendosc Adv Surg Tech A. 2003;13:27-31.
Stolzenburg JU, Rabenalt R, Do M, et al. Endoscopic extraperitoneal radical prostatectomy: the University of Leipzig experience of 2000 cases. J Endourol. 2008;22(10):2319-2325.
Su LM, Link RE, Bhayani SB, et al. Nerve-sparing laparoscopic radical prostatectomy: replicating the open surgical technique. Urology. 2004;64:123-127.
Takenaka A, Murakami G, Soga H, et al. Anatomical analysis of the neurovascular bundle supplying penile cavernous tissue to ensure a reliable nerve graft after radical prostatectomy. J Urol. 2004;172:1032-1035.
Tewari A, Jhaveri J, Rao S, et al. Total reconstruction of the vesico-urethral junction. BJU Int. 2008;101:871-877.
Tewari A, Rao S, Mandhani A. Catheter-less robotic radical prostatectomy using a custom-made synchronous anastomotic splint and vesical urinary diversion device: report of the initial series and perioperative outcomes. BJU Int. 2008;102:1000-1004.
Tewari A, Srivasatava A, Menon M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92:205-210.
Tewari AK, Bigelow K, Rao S, et al. Anatomic restoration technique of continence mechanism and preservation of puboprostatic collar: a novel modification to achieve early urinary continence in men undergoing robotic prostatectomy. Urology. 2007;69:726-731.
Touijer K, Kuroiwa K, Saranchuk JW, et al. Quality improvement in laparoscopic radical prostatectomy for pT2 prostate cancer: impact of video documentation review on positive surgical margin. J Urol. 2005;173:765-768.
Turk I, Deger S, Winkelmann B, et al. Laparoscopic radical prostatectomy. Technical aspects and experience with 125 cases. Eur Urol. 2001;40:46-53.
van Velthoven RF, Ahlering TE, Peltier A, et al. Technique for laparoscopic running urethrovesical anastomosis: the single knot method. Urology. 2003;61:699-702.
Wagner A, Link R, Pavlovich C, et al. Use of a validated quality of life questionnaire to assess sexual function following laparoscopic radical prostatectomy. Int J Impot Res. 2006;18:69-76.
Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160:2418-2424.
Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492-497.
Webb DR, Sethi K, Gee K. An analysis of the causes of bladder neck contracture after open and robot-assisted laparoscopic radical prostatectomy. BJU Int. 2009;103:957-963.
Webster TM, Herrell SD, Chang SS, et al. Robotic assisted laparoscopic radical prostatectomy versus retropubic radical prostatectomy: a prospective assessment of postoperative pain. J Urol. 2005;174:912-914.
WHO International Consultation on Prediction of Patient Outcome in Prostate Cancer, Prognostic Factors in Radical Prostatectomy Specimens, Stockholm, 2004.
Wolfram M, Brautigam R, Engl T, et al. Robotic-assisted laparoscopic radical prostatectomy: the Frankfurt technique. World J Urol. 2003;21:128-132.
Young HH. The early diagnosis and radical cure of carcinoma of the prostate: being a study of 40 cases and presentations of a radical operation which was carried out in 4 cases. Johns Hopkins Hosp Bull. 1905;16:315.
Zorn KC, Bernstein AJ, Gofrit ON, et al. Long-term functional and oncological outcomes of patients undergoing sural nerve interposition grafting during robot-assisted laparoscopic radical prostatectomy. J Endourol. 2008;22:1005-1012.
Zorn KC, Gofrit ON, Orvieto MA, et al. Robotic-assisted laparoscopic prostatectomy: functional and pathologic outcomes with interfascial nerve preservation. Eur Urol. 2007;51:755-763.
Zorn KC, Gofrit ON, Orvieto MA, et al. Da Vinci robot error and failure rates: single institution experience on a single three-arm robot unit of more than 700 consecutive robot-assisted laparoscopic radical prostatectomies. J Endourol. 2007;21:1341-1344.