chapter 126 Posterior Urethral Valves
Posterior Urethral Valves
Description, Classification, and Embryology
Congenital obstruction of the urethra is one of the most devastating anomalies that occur in the urinary tract and is one of the few that are life threatening in the neonatal period. These lesions usually result in lifelong disabilities with incontinence and decreased renal function despite optimal medical management. The condition of posterior urethral valves is still relatively common and makes up the vast majority of congenital urethral obstructions.
Langenbeck is credited with first reporting congenital obstruction of the prostatic urethra in 1802 (Dewan et al, 1999). Despite this observation it was left for Hugh Hampton Young, over a century later, to define and name the condition as posterior urethral valves (Young et al, 1919). His remarkably accurate observations were made only with gross pathologic dissection and with the primitive cystoscopic instruments of his day. Young observed the anatomic variability of the anomaly and developed a classification that is still used almost 100 years later.
The term valves is unfortunate because it implies function. This obstructive membrane has no active function and is not a developmental stage in the embryology of the urethra. It is simply a passive barrier to urine flow. Young adopted this term when he found that a urethral sound would pass easily from the urethral meatus into the bladder in a retrograde manner while the same sound (or urine for that matter) would not pass antegrade out of the bladder and down the urethra. This unidirectional access through the urethra suggested to Young the function of a one-way valve, and he described the condition as such.
Posterior urethral valves occur in 1 in 8000 to 25,000 live births and make up 10% of urinary obstructions diagnosed in utero (Atwell, 1983; Thomas and Gordon, 1989; Casale, 1990). The diagnosis has been made on average in 1 in 1250 fetal ultrasound screenings (Gunn et al, 1995). There are reported cases of congenital urethral obstruction in females, but the classic posterior urethral valves occur only in males (Nesbit et al, 1964). The incidence of posterior urethral valves is dropping in some populations due to the effects of prenatal diagnosis and subsequent termination of affected fetuses. In one report, fetuses diagnosed with valves were electively terminated in 46% of cases (Cromie et al, 2001).
Despite several alternative proposals, Young’s classification is still most commonly used (Dewan, 1993) (Fig. 126–1). He described three types of posterior urethral valves:
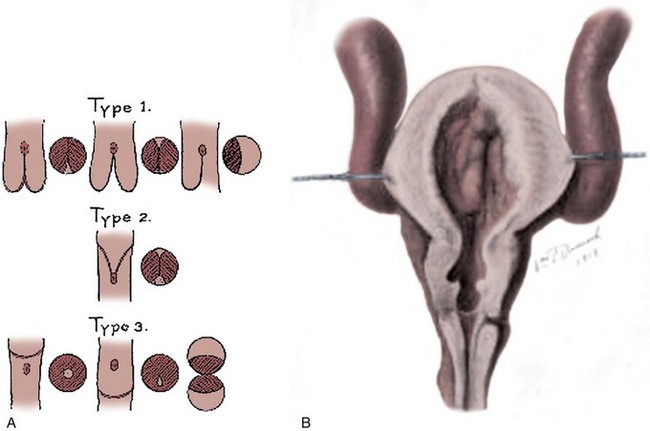
Figure 126–1 A, Young’s original figures from his 1919 article. He described three types of posterior urethral valves and diagrammed them. B, William P. Didusch drew this pathologic dissection that shows the features of posterior urethral valves, the thickened bladder wall with an elevated bladder neck, and enlarged posterior urethra. The two valve leaflets of type I valves are seen. The ureters are massively dilated.
(From Young HH, Frontz WA, Baldwin JC. Congenital obstruction of the posterior urethra. J Urol 1919;3:289.)
Type I. In the most common type there is a ridge lying on the floor of the urethra, continuous with the verumontanum, which takes an anterior course and divides into two forklike processes in the region of the bulbomembranous junction. These processes are continued as thin membranous sheets, directed upward and forward, which may be attached to the urethra throughout its entire circumference. In the majority of cases of this general type the fusion of the valves anteriorly is not complete, there existing at this point a slight separation of the folds. However, in a few of the cases…the anterior fusion is complete while a cleft exists between the folds posteriorly.
Recently it has been proposed that all boys with valves originally have complete fusion along the anterior urethra, leaving only an open channel along the posterior urethral wall. The frequently observed cleft in the membrane may be iatrogenic, created by instrumentation or catheterization that erodes the valve tissue in the anterior midline and splits the valve in two leaflets (Robertson and Hayes, 1969; Dewan et al, 1992).
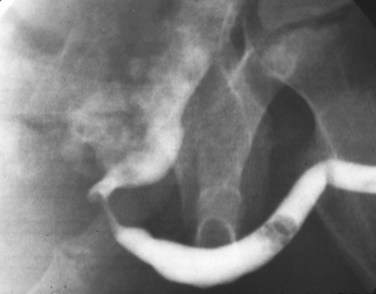
Young’s type I valves make up 95% of all posterior urethral obstructions (Fig. 126–2).
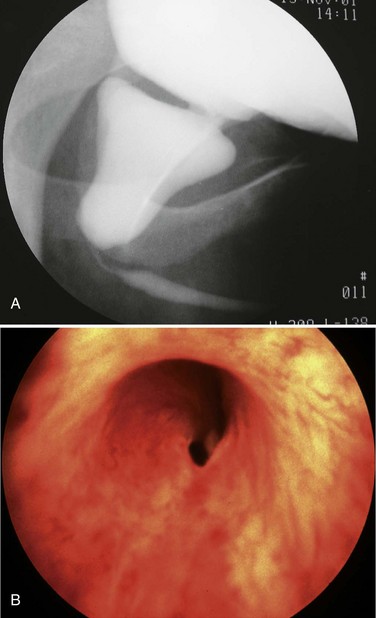
Figure 126–2 A, Voiding cystourethrogram demonstrates a typical type I posterior urethral valve with a dilated posterior urethra, elevated bladder neck, and valve leaflets obstructing the flow of contrast material from the bladder. B, This cystoscopic image from the distal urethra shows the valve leaflets forced into the lumen with the bladder full, illustrating their obstructive effect.
The thickness of the valves themselves varies from rigid, thick tissue to thin, almost transparent membranes. The valve itself is made of a fibrous stroma covered on each side by transitional epithelium. There is no muscle within the valve. The degree of obstruction also varies widely and, in turn, the amount of damage to the urinary tract.
The embryology of posterior urethral valves is speculative but may be related to an abnormal insertion of the mesonephric ducts into the fetal cloaca. Evidence supporting this theory lies in the fact that type I valve patients lack plicae colliculi, mucosal folds found in the normal male urethra that are believed to represent a remnant of the normal pathway of migration of the mesonephric ducts toward the verumontanum (Stephens, 1983).
Young described a type II valve as arising from the verumontanum and extending along the posterior urethral wall toward the bladder neck. This description was based on the similar appearance of these folds to the membranes found in type I. These type II folds are not obstructive and probably result from hypertrophy of muscles of the superficial trigone and prostatic urethra in response to high voiding pressure from distal obstruction. They can be seen in response to many obstructive conditions such as urethral strictures, posterior urethral valves, anterior urethral valves, and detrusor-sphincter dyssynergia. These type II folds are no longer referred to as valves.
Young described type III valves as a membrane lying transversely across the urethra with a small perforation near its center (Fig. 126–3). The membrane is distal to the verumontanum and sometimes is elongated like a wind sock reaching the bulbous urethra (Field and Stephens, 1974). Type III valves make up only 5% of the total. Young described them as follows:
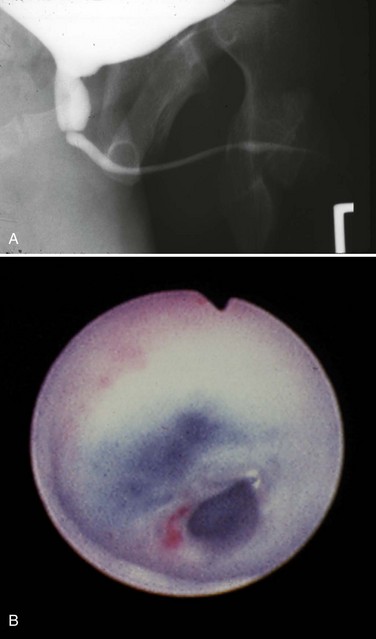
Figure 126–3 A, Voiding cystourethrogram demonstrates a typical type III posterior urethral valve with a transverse septum crossing the urethra at a right angle distal to the verumontanum. B, A cystoscopic image from the distal urethra shows the thin membrane and a small off-center opening.
There is a third type which has been found at different levels of the posterior urethra and which apparently bears no such relation to the verumontanum as the types just considered.…This obstruction was attached to the entire circumference of the urethra, there being a small opening in the center. Incomplete varieties of this type have been described, the most common being a more or less crescentic or semicircular fold crossing the urethra and being attached either to the roof or floor.
The embryologic origin of type III valves has been attributed to incomplete dissolution of the urogenital portion of the cloacal membrane. Type III valves present in the same manner and are managed in the same way as the more common type I, although there is some evidence that type III valves have a worse prognosis (Rosenfeld et al, 1994).
The inheritance of posterior urethral valves is poorly understood and may involve several genes and inheritance patterns (Livne et al, 1983; Weber et al, 2005). Valves have occurred in siblings, twins, and in successive generations (Farkas and Skinner, 1976; Hanlon-Lundberg et al, 1994; Morini et al, 2002; Maruotti et al, 2006). The timing of valve development is also speculative, but it would appear that they become obstructive during or after the eighth week of life by which time the prostatic urethra has developed from the urogenital sinus (Mitchell and Close, 1996). In a recent review of the literature, Baskin and colleagues concluded that the precise origins regarding the anatomy and embryology of posterior urethral valves remain undefined. Although theories of etiology abound, there are a limited number of modern methodical anatomic and histiologic studies to support them (Krishnan et al, 2006).
Pathophysiology
The effects of congenital obstruction of the urethra are reflected in the entire urinary tract above the level of obstruction (Table 126–1). Proximal urethra, prostate, bladder neck, bladder, ureters, and kidneys are all affected and suffer various forms of damage.
Table 126–1 Damage Due to Posterior Urethral Valves
| ORGAN | EFFECT | NATURAL HISTORY |
|---|---|---|
| Lung | Pulmonary hypoplasia | May be fatal in newborns; if infant survives, there are few long-term problems |
| Kidney | ||
| Glomerular injury | ||
| Obstructive uropathy | Reversible renal insufficiency | Usually improves with initial treatment but can recur with bladder dysfunction |
| Dysplasia | Irreversible renal insufficiency | Permanent level of renal damage that limits growth; leads to progressive renal failure and hypertension |
| Tubular injury | Inability to limit sodium and water loss | Progressive with age; nephrogenic diabetes insipidus |
| Bladder | Poor sensation, hypercontractility, low compliance, and eventual myogenic failure all may contribute to incontinence and poor emptying | Bladder problems are lifelong and change with age |
| Ureters | Poor contractility and inability to coapt and transport urine | Most will improve initially, but most have chronic hydronephrosis |
Lower Urinary Tract
The injury to the lower urinary tract appears to be caused by high-pressure urine storage and voiding (McConnell, 1989; Keating, 1994). Bladder histology of fetuses with posterior urethral valves shows hypertrophy and hyperplasia of the detrusor muscle along with increased connective tissue. The ratio of muscle to connective tissue is the same as in normal bladders, but there is conflicting evidence that the type of collagen within the bladder is altered (Kim et al, 1991; Ewalt et al, 1992). Some bladder findings in neonates such as wall thickness and poor compliance will improve after relief of obstruction, but the valve bladders seldom if ever achieve normal function.
High voiding pressures distend and thin the prostatic urethra. The storage capacity of the prostatic urethra sometimes exceeds that of the bladder because of the relative lack of muscle there. The verumontanum is distorted, and the ejaculatory ducts may be dilated from refluxing urine. The bladder neck is rigid and hypertrophied (Fig. 126–4). This high bladder neck was once mistaken as another cause of obstruction and was surgically incised to facilitate bladder emptying. Unfortunately, this practice often resulted in total incontinence. Today it is understood that the appearance of the bladder neck is a result of distal obstruction and not obstructive lesion itself. Bladder neck appearance and function usually improves after the obstructive valves are destroyed.
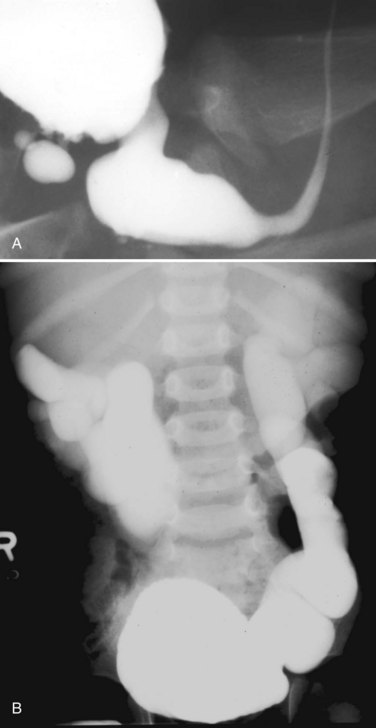
Figure 126–4 A, Voiding cystourethrogram shows a posterior bladder diverticulum near the base of the bladder and an extremely high bladder neck. A catheter passed blindly can fail to pass over the bladder neck and coil in the dilated posterior urethra. B, Massive vesicoureteral reflux in a boy with valves.
Upper Urinary Tract
Ureteral damage is usually severe. The appearance and function of the ureters are markedly abnormal. The ureteral wall is thickened and the lumen massively dilated. The ureter is at the mercy of the ureterovesical junction (UVJ). If this junction functions to prevent reflux, the ureter and kidney are somewhat protected from the complete force of the bladder contractions. If the junction allows reflux, the entire pressure of the thickened and hypercontractile bladder is transmitted directly to the upper tract with severe consequences. Virtually all valve patients have hydroureteronephrosis, and this can be the result of obstruction from the valves, the UVJ, or both. Although reflux is variable, hydroureteronephrosis is a constant in these patients. Severely dilated ureters coapt poorly and contract weakly, if at all (Gearhart et al, 1995). The propulsion of urine down the ureter is diminished in the infant, and this ureteral dysfunction may persist throughout life despite relief of distal obstruction.
The nature of the renal injury in boys with posterior urethral valves is complex and appears to have two distinct components. Some damage, described as obstructive uropathy, is caused by persistent high pressure. It is ongoing and can be progressive, but it is potentially reversible with relief of high pressures. Other damage, termed renal dysplasia, may be due to either increased pressure during the development of the kidney or abnormal embryologic development. Renal dysplasia is not reversible, and therefore the degree of dysplasia is critical in determining eventual renal function in valve patients.
Dysplasia is, in fact, a histologic diagnosis and depends on identifying a fundamental disorganization of the renal parenchyma on a cellular and tissue level. The elements of dysplasia include embryonic tubules, cartilage, cysts, and mesenchymal connective tissue. The particular type of dysplasia in valve patients is termed microcystic and can be observed primarily in the peripheral cortical zone of the kidney.
The exact cause of dysplasia is a matter of continued debate. Beck and Glick demonstrated that dysplasia could be produced by early urinary obstruction in lambs (Beck, 1971; Glick et al, 1984). Maizels and colleagues (1983) also produced a form of dysplasia in chicken embryos by obstructing the fetal urinary tract. Ample evidence indicates that a form of dysplasia can be induced by early fetal urinary obstruction and resultant high intrarenal urinary pressure.
On the other hand, Stephens and Henneberry (1980) have demonstrated that dysplasia may be found in patients who do not seem to have a history of obstruction but instead have abnormal ureteral development. In patients with severe vesicoureteral reflux, the ureteral orifice is usually in an abnormal position. These patients frequently demonstrate renal dysplasia, and the degree of dysplasia is proportional to how much the position of the ureter deviates from normal. Stephens postulated that this dysplasia was the result of a faulty ureteral bud arising in an abnormal position along the mesonephric duct. Because the ureteral bud induces the development of the kidney, it follows that an abnormal bud may induce an abnormal kidney.
Dysplasia therefore occurs in association with obstruction and with reflux, seemingly unrelated conditions. Both theories are plausible and not exclusive. Whatever the cause, dysplasia is the defining factor in long-term renal function in most patients with valves. Because this type of renal damage is not reversible, the extent of dysplasia will ultimately determine the growth and functional potential of the infant’s kidneys. A number of other related factors may contribute to declining renal function in valve patients including infection, persistent obstruction, hypertension, hyperfiltration of damaged parenchyma, and perhaps high-protein diet, but none are as important as the degree of dysplasia (Brenner et al, 1982; Klahr, 1989; Farnham et al, 2005).
All posterior urethral valve patients have a degree of associated obstructive uropathy. Obstructive uropathy involves both glomerular injury and tubular injury. Glomerular injury occurs when high pressures result in decreased renal perfusion and filtration. These changes are partially reversible when pressures are dropped and obstruction is relieved. The potential for recovery is felt to be time sensitive, and early relief of obstruction should favor recovery. Some degree of scarring and fibrosis within the parenchyma persists, but unlike dysplasia, obstructive uropathy usually improves with treatment.
Tubular damage from increased urinary pressure results in failure to concentrate and acidify the urine. This occurs in up to 59% of valve patients and sometimes occurs in patients without evidence of glomerular injury (Parkhouse and Woodhouse, 1990; Dinneen et al, 1995). Concentrating defects in the form of nephrogenic diabetes insipidus lead to increased urine output independent of the patient’s state of hydration. Patients are at risk for electrolyte imbalances and dehydration due to inability to conserve sodium and water even in the face of dehydration (Gonzales, 1978). Pathologically large urine output also stresses the ureters and bladder and contributes to persistent hydroureteronephrosis and bladder dysfunction. Tubular damage worsens with age despite early relief of obstruction in many patients, and resultant high urine volumes contribute to the deterioration of renal and bladder function in late childhood.
Key Points: Pathophysiology
Vesicourethral Reflux and Dysplasia and Pop-Off Mechanisms
Hoover and Duckett identified the relationship between valves, reflux, and dysplasia (Hoover and Duckett, 1982). They recognized that valve infants with unilateral reflux had markedly different renal function when comparing refluxing and nonrefluxing kidneys. Reflux was consistently associated with dsyplastic and damaged kidneys. They postulated that the reflux allowed the high bladder pressures to focus on the refluxing kidney, sparing and protecting the nonrefluxing kidney. The vesicoureteral reflux and dysplasia (VURD) relationship is found in 13% of valve patients, and interestingly the refluxing kidney is on the left side in 92%.
The proposed mechanism of this protection is that the refluxing collecting system acts as a pressure pop-off valve. The belief was that the protected kidney would ultimately lead to a better long-term prognosis compared with boys without unilateral reflux. The long-term protective effect of VURD has not been as helpful as originally thought. Longitudinal studies in VURD patients have shown that by midchildhood both serum creatinine and glomerular filtration rate (GFR) were significantly decreased compared with normal children (Cuckow et al, 1997). In addition, scarring has been observed with dimercaptosuccinic acid scanning in half of the contralateral, nonrefluxing kidneys with possible negative long-term consequences (Narasimhan et al, 2005). Other types of pop-off pressure systems include bladder diverticuli, urinomas, and urinary ascites (Rittenberg et al, 1988). The protective effect of these clinical situations is highly variable.
Clinical Presentation
In the past boys with posterior urethral valves presented with a variety of symptoms and at various ages (Hendren, 1971). They ranged from newborns with life-threatening renal and pulmonary conditions to older children with minor voiding dysfunction. In general, the symptoms are age dependent with the more severely affected boys presenting earlier in life (Dinneen and Duffy, 1996).
Neonates
Today, most patients with posterior urethral valves are diagnosed with prenatal ultrasound. Obstruction leads to decreased fetal urine output and results in oligohydramnios. The observation of marked hydroureteronephrosis, a distended bladder, and a thickened bladder wall in utero strongly support the diagnosis of valves (Fig. 126–5). Often the urologist has been consulted long before delivery, and in some cases intervention has been undertaken in utero.
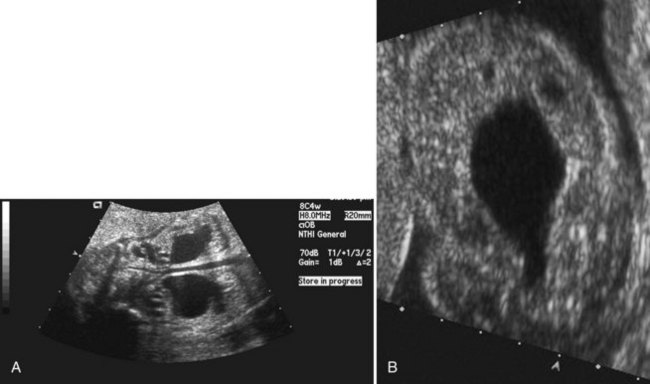
Figure 126–5 A, Ultrasound examination shows severe hydroureteronephrosis in a fetus with posterior urethral valves. B, Fetal ultrasound examination demonstrates a severely thickened bladder wall with a distended bladder and posterior urethra. These two fluid collections, a larger one above the smaller one, produce the “keyhole” sign indicative of posterior urethral valves.
Pulmonary Hypoplasia
The most severe clinical problem facing the neonate with posterior urethral valves is pulmonary hypoplasia. The infants are often cyanotic and require respiratory support at delivery. Pulmonary hypoplasia is a direct result of oligohydramnios and accounts for most of the mortality today (Churchill et al, 1990). The exact etiology of the pulmonary hypoplasia is unclear, but contributing factors include physical restriction of fetal breathing motion (Landers and Hanson, 1990). The lack of amniotic fluid to surround and buffer the fetus from intra-abdominal pressure leads to a small chest cavity and prevents chest wall breathing motion and normal musculoskeletal development. Amniotic fluid may be important in development of the fetal pulmonary tree by providing needed intraluminal pressure, volume, and flow. In addition, the amniotic fluid may provide some cellular or molecular stimulus to the developing lung. The results of oligohydramnios are often life threatening, and some infants succumb despite heroic measures. The mortality of valves has decreased significantly over the past two decades with development of better methods to manage pulmonary hypoplasia by neonatology. It is rare to have an infant die today with the quality of pulmonary support available in the newborn intensive care unit (Dejter and Gibbons, 1989; Gibbons et al, 1993).
Renal Insufficiency
In addition to pulmonary distress, the newborn with posterior urethral valves usually presents with signs of severe systemic illness such as intrauterine growth retardation, failure to thrive, lethargy, and poor feeding. The infants may be pale and have poor muscle tone. They may demonstrate the classic signs of oligohydramnios such as Potter facies and bowed and deformed limbs with pressure dimples over knees and elbows from intrauterine pressure. Examination of the abdomen may reveal masses due to hydroureteronephrosis and a distended bladder. The infant may suffer from extensive edema and urinary ascites. The caregivers may notice a diminished force of urinary stream, but this sign is not reliable to predict valves (Smith et al, 1996). If not diagnosed in utero, the most severely affected valve patients present as newborns. Often urinary tract infection or urosepsis precipitates evaluation and diagnosis.
Ascites
Forty percent of neonatal ascites is caused by urinary conditions (Fig. 126–6) (Adzick et al, 1985). Urinary ascites occurs when high intraluminal pressure forces urine to extravasate from the kidney, usually across a renal fornix. Urine then enters the retroperitoneum and travels across the peritoneum as a transudate. If aspirated from the peritoneal cavity, the ascites or extravasated urine contains electrolyte and creatinine levels similar to serum. The urine within the peritoneum is subject to the large absorptive mesothelial surface that quickly normalizes these values, masking the identity of ascitic fluid as urine. The diagnosis of urinary ascites may be difficult and may require definitive upper tract drainage in the form of nephrostomy tubes in order to establish the etiology of the ascites and allow its resolution. Urinary ascites in the case of distal obstruction may serve to lower urinary pressures and offer some protection to the developing kidneys (Conner et al, 1988).
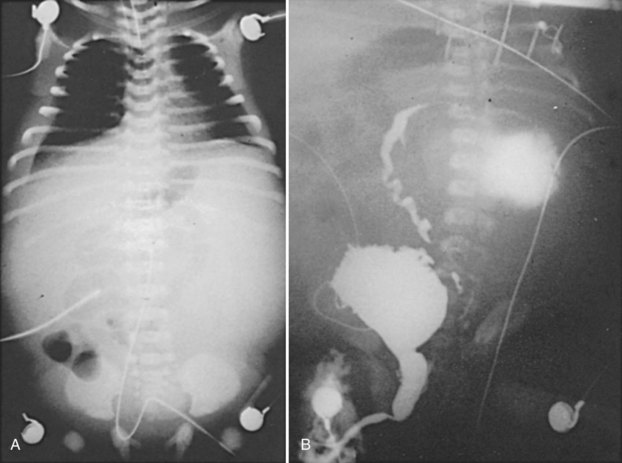
Figure 126–6 A, Abdominal radiograph shows the ground-glass appearance of an infant with ascites. B, Voiding cystourethrogram demonstrates reflux of contrast material, which is then seen extravasating into a urinoma and later flowing into the abdominal cavity as urinary ascites.
(From Gonzales ED. Posterior urethral valves and other ureteral anomalies. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell’s urology. 8th ed. Philadelphia: WB Saunders; 2002.)
Patel and colleagues (2003) reviewed a large group of 615 valve patients and analyzed the protective effect of ascites compared with unilateral and bilateral urinomas. The authors found that the kidney associated with a unilateral urinoma is often impaired. Bilateral urinomas are associated with good renal function, but urinary ascites alone has a poorer prognosis. The GFR of patients with urinary ascites alone was 29 mL/min per 1.73 m3 compared with 36 mL for boys with unilateral urinoma alone, 74 mL for boys with urinoma plus ascites, and 104 mL for bilateral urinomas.
Heikkila and colleagues (2008a) studied 200 patients with valves and found that the incidence of urinoma approached 15% after the introduction of ultrasound. This group included nine with perirenal urine collections, six with urinary ascites, and two with urothorax. They found initial and post-treatment serum creatinine levels, incidence of reflux, median split renal function, and risk for end-stage renal disease (ESRD) in the 17 patients with urinoma to be statistically similar to a control group of valve patients without urinoma. Their conclusion was that renal function is similar in patients with or without urinoma. Other authors have also found no association between renal function and urinoma formation in valve patients (Kleppe et al, 2006).
Older Children
The majority of boys who present later in life do so with urinary tract infection and/or voiding dysfunction. With a better understanding of functional voiding disorders, valves are now an uncommon cause of minor voiding dysfunction (Pieretti, 1993). Due to widespread prenatal ultrasound, it is now rare to see a new patient with posterior urethral valves past the neonatal period. Although boys who present later in life are generally believed to have more normal urinary tracts, this is not a uniform finding. One series reported that patients who presented at school age suffered renal insufficiency in 35% of cases (Bomalaski et al, 1999).
Initial Diagnostic Evaluation
Ultrasound
The widespread use of maternal ultrasound in the past 25 years in the United States has resulted in at least 80% of women undergoing ultrasound screening study during pregnancy. Posterior urethral valves is the third most common antenatal genitourinary diagnosis made today and accounts for 10% of all fetal uropathy (Thomas and Gordon, 1989). Although it was reported that two thirds of patients with valves presented on fetal ultrasound during the 1990s, this percentage has continued to rise (Greenfield, 1997). The quality and availability of ultrasound technology continues to improve, and with it the ability to image the fetus.
Ultrasound is sensitive in detecting fetal hydronephrosis, but the specific diagnosis of posterior urethral valves is more difficult. The differential diagnosis of bilateral hydronephrosis includes posterior urethral valves, prune-belly syndrome, bilateral ureteropelvic junction obstruction, bilateral high-grade vesicoureteral reflux, bilateral ureterovesical junction obstruction, congenital urethral atresia, and anterior urethral obstruction. Valves were correctly diagnosed in only 66% of 18 fetuses who were identified with hydronephrosis (Skolder et al, 1988). In a review of 42 patients with valves, only 45% were detected on antenatal ultrasound (Dinneen et al, 1993).
The timing of screening for fetal uropathy is an important factor in accuracy. There is compelling evidence that valves may be missed if the screening ultrasound is done before 24 weeks’ gestation (Jee et al, 1993; Hutton et al, 1994). In Dinneen’s series, 92% of the 36 patients scanned before 24 weeks’ gestation were not detected. The classic ultrasound findings in patients with valves include bilateral hydroureteronephrosis, distended bladder, dilated posterior urethra, and a thickened bladder wall. The “keyhole” sign of a dilated bladder above a dilated prostatic urethra is also helpful. Bladder wall thickness is an important diagnostic sign. The findings of an excessively thick bladder wall and a distended bladder indicate distal obstruction (Kaefer et al, 1997a).
Renal echogenicity is another parameter that may aid in the diagnosis of valves. Increased echogenicity predicted an obstructive process in a study of fetuses with bilateral hydroureteronephrosis and distended bladders (Kaefer et al, 1997b). Patients with a nonobstructive process such as megacystis-megaureter association failed to demonstrate increased echogenicity, while 87.5% of valve patients had increased echogenicity at a mean gestational age of 26 weeks. The same predictive value was found with oligohydramnios. Any male fetus with bilateral hydroureteronephrosis, decreased amniotic fluid, and a distended bladder should be considered to have posterior urethral valves until proved otherwise. The ultrasound findings of a neonate with posterior urethral valves are identical to those of a fetus.
Voiding Cystourethrography
The voiding cystourethrogram (VCUG) remains the most important study in diagnosing posterior urethral valves because it defines the anatomy and gross function of the bladder, bladder neck, and urethra (Fig. 126–7). When the diagnosis of valves is in question, a VCUG should be obtained as soon as possible. On imaging the bladder and upper tracts of children with neuropathic bladder, urethral stricture, anterior urethral obstruction, and posterior urethral valves may all appear identical. Images of the urethra during voiding are necessary to make the correct diagnosis.
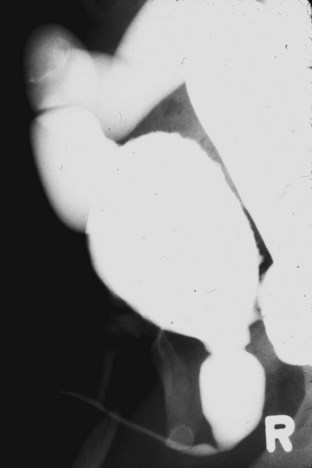
Figure 126–7 Voiding cystourethrogram reveals massive vesicoureteral reflux in an infant with posterior urethral valves.
In posterior urethral valves the bladder is thickened and trabeculated. The VCUG often demonstrates bladder diverticuli and severe vesicoureteral reflux. From the lateral projection the bladder neck is elevated. The proximal urethra is quite dilated, and the actual valve structure is often visible. Vesicoureteral reflux is present in at least 50% of valve patients at the time of diagnosis (Churchill et al, 1990). The incidence of reflux has been found to be higher in neonates than in older children. Because most valve patients are now discovered in utero, the incidence of reflux may continue to increase compared with historical studies. There is an 80% incidence of reflux on the left side in patients with unilateral reflux for no apparent reason (Hoover and Duckett, 1982).
Ultrasound imaging of the posterior urethra from the perineum has been suggested as a noninvasive way to diagnose valves (Good et al, 1996). Valve leaflets can be visualized on ultrasound while bladder wall thickness and posterior urethral diameter before and during voiding is increased in valve patients. Although this application has little to add to the newborn evaluation, it can be used to evaluate older children without catheterization.
Radionuclide Renal Scan
In addition to the VCUG and renal ultrasound, the radionuclide renal scan has become an important part of the evaluation. Mercaptoacetyltriglycine (MAG-3) is the most useful agent for this imaging because it provides functional data and adequate imaging of both the parenchyma and collecting system. Scans provide differential renal function and some insight about drainage of the upper urinary tracts. In patients with reflux the bladder must be emptied during the entire study with a catheter in order to avoid artificial enhancement of renal function by reflux of the isotope from the bladder. If the catheter cannot keep the bladder empty through gravity, it may be necessary to aspirate it with a syringe during the study.
Laboratory Evaluation
Initial laboratory evaluation of the newborn with valves is usually misleading due to the effects of maternal renal function mediated through the placenta. Initial creatinine and blood urea nitrogen (BUN) levels may be artificially low, and it will take approximately 48 hours for the serum levels to accurately represent the child’s intrinsic renal function. Creatinine, BUN, and electrolytes should be followed twice daily for the first few days of life until they plateau. Serum bicarbonate, sodium, and potassium are all critical factors to monitor.
Initial Management of Posterior Urethral Valves
Bladder Drainage
Initial management of all patients with posterior urethral valves requires the immediate establishment of urinary catheter drainage from the bladder. This should be performed even if the diagnosis has not been confirmed by VCUG. Neonates can be catheterized with a 3.5- or 5-Fr pediatric feeding tube. Foley catheters have been used with success, but there have also been reports that the balloon causes irritation and resultant bladder spasms. The bladder may be extremely irritable, and the presence of any catheter can cause spasms so severe as to obstruct the flow of urine into the bladder (Noe and Jerkins, 1983; Jordan and Hoover, 1985). Accurate catheter placement in the bladder must be documented either by irrigation of the catheter or by bladder imaging. Because it is difficult to pass the catheter over the elevated bladder neck and to avoid placing the catheter in the dilated prostatic urethra instead of the bladder, a one-shot cystogram is often advisable.
The ill newborn with posterior urethral valves is usually in the hands of the neonatal service for the management of the most threatening issues such as pulmonary hypoplasia and renal insufficiency. It is in these areas where improvement of medical management has decreased early mortality in valve babies (Nakayama et al, 1986). These infants may require maximal ventilatory support, extracorporeal membrane oxygenation, dialysis, parenteral nutrition, control of hypertension, and a host of other services provided by neonatology and pediatric nephrology (Gibbons et al, 1993).
Valve Ablation
After successful initial bladder drainage and when the patient’s medical condition has stabilized, the next step is to permanently destroy the valves. In the past, destruction of valves was done by open surgery or patients were managed with long-term suprapubic tube drainage (Gonzalez et al, 1990). Both of these early therapies can lead to unacceptable complications such as incontinence, stones, and infection (Churchill et al, 1983).
Today a number of successful surgical techniques are available to disrupt or destroy posterior urethral valves including hooks, balloon catheters, and valvulotomes (Deane et al, 1988; Kolicinski, 1988; Cromie et al, 1994; Chandna et al, 1996; Chertin et al, 2002). One of the more common methods uses the Whitaker hook. This instrument looks like a crochet hook and has been used successfully to cut valves either blindly or with fluoroscopic control (Whitaker and Sherwood, 1986). New, smaller pediatric cystoscopes with improved optics are favored today because of the ability to perform the procedure under direct vision. A bugbee electrode or a pediatric resectoscope with a hook or cold knife can be used to incise the valves (Fig. 126–8). A number of authors report using a cystoscope and laser to disrupt valves (Ehrlich and Shanberg, 1988; Biewald and Schier, 1992; Gholdoian et al, 1998). Although most surgeons incise the valves from a retrograde position viewed through the urethra, others have found that an antegrade approach through a vesicostomy or suprapubic puncture of the bladder is helpful (Zaontz and Firlit, 1985).
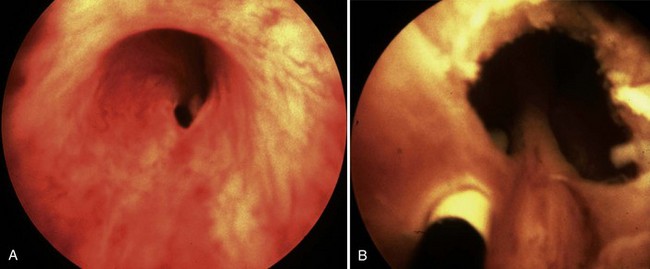
Figure 126–8 Cystoscopic photographs made from the distal urethra show the posterior urethral valves before ablation (A) and after ablation (B). The ureteral catheter has been passed through a perforation in the valve leaflet.
Originally surgeons attempted to completely resect the valves. This practice produced frequent complications from urethral stricture resulting from electrosurgical and instrument damage to the urethra. Modern appropriately sized cystoscopic equipment and limited use of electrosurgery have reduced the incidence of urethral injury to 5% (Nijman and Scholtmeyer, 1991). Today the goal is not to remove the valves but to incise them so that they are not suspended across the urethra obstructing urine flow. Well-placed incisions can disrupt their integrity and allow the valves to lie freely along the walls of the urethra when the child voids. The exact point of incision can vary; some surgeons prefer the 12 o’clock position, others prefer incisions at 4 and 8 o’clock, and others all three. Although most valves are quite thin and do not bleed at surgery, it is sometimes preferable to leave a catheter in place for 24 hours after incision. The valve remnants involute after incision, and there is often no evidence of them on later cystoscopic examination.
Documenting that the valve remnants are no longer obstructive after valve ablation is imperative. The timing of a postoperative VCUG is debatable, but one should be done to not only view the urethra but also evaluate bladder size and reflux. Decreased trabeculation, resolution of reflux, and a more uniform urethral diameter are all signs of successful relief of obstruction. One can measure the ratio of the diameter of the posterior urethra to that of the anterior urethra as an indicator of obstruction. A postablation urethral ratio of 2.5 to 3.0 is considered an acceptable postoperative result at 12 weeks (Gupta et al, 2009). Evidence indicates that early valve ablation allows the bladder to cycle, thus improving compliance and bladder stability in infancy and resulting in less bladder dysfunction in older children (Youssif et al, 2009).
Transurethral incision of the bladder neck at the time of valve incision has not been part of standard management; however, there is a report that this procedure has produced improved bladder performance with fewer inhibited contractions, lower mean maximum voiding pressures, and less need for anticholinergic medications than an age-matched control group after 4 years (Kajbafzadeh et al, 2008). Additional study of the modern use of this procedure is necessary.
Cutaneous Vesicostomy
If the infant is too small to instrument safely for valve ablation, then a cutaneous vesicostomy can be performed as a temporary measure (Fig. 126–9). Temporary vesicostomy drainage allows the urologist to incise the valves later when the patient is older and healthier. The vesicostomy has proven to be a safe and efficient treatment with long-term results in preserving renal function and somatic growth equal to primary valve ablation (Walker and Padron, 1990; Narasimhan et al, 2004). The vesicostomy provides adequate drainage of the upper tracts in more than 90% of cases (Krahn and Johnston, 1993). Vesicostomy itself is not without complication, however, and one study reported an 8.6% reoperation rate (Noe and Jerkins, 1985). Some people have questioned whether vesicostomy would cause permanent loss of bladder volume, but this has not proven to be true and vesicostomy does not significantly affect bladder capacity (Khoury et al, 1990). Some authors report that compliance may be decreased in vesicostomy bladders compared with those treated with primary ablation (Kim et al, 1996; Podesta et al, 2000). In general, primary ablation is the preferred surgical procedure to treat posterior urethral valves and vesicostomy is reserved for very small or very ill infants. Vesicostomy remains an excellent alternative treatment in these difficult situations.
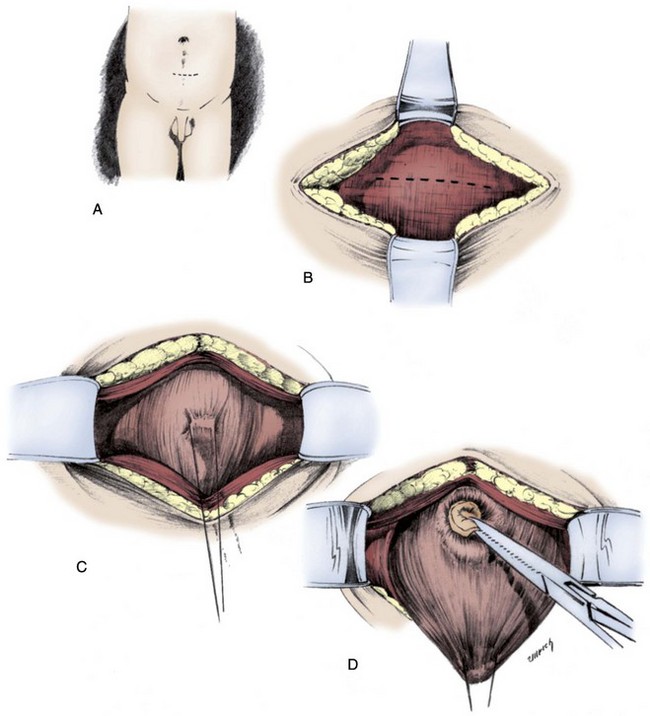
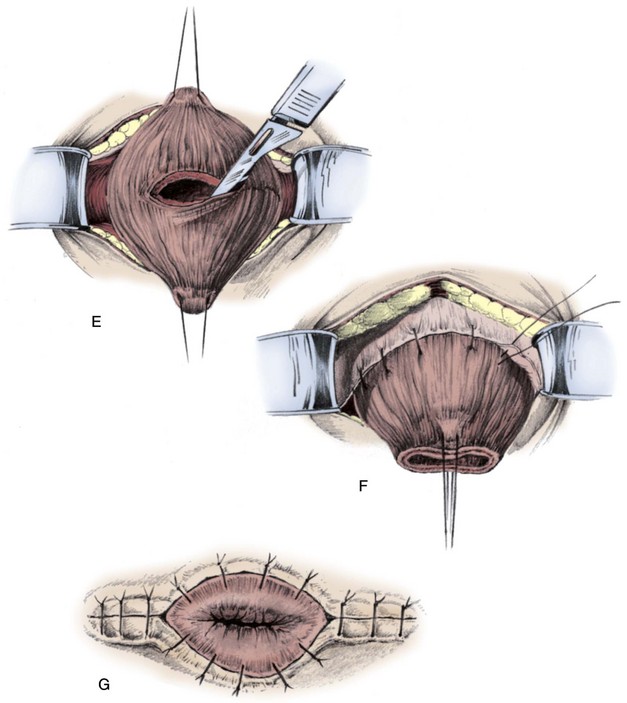
Figure 126–9 Blocksom technique for performance of cutaneous vesicostomy. A, After the bladder is filled, a transverse incision is made at the upper limit of the filled bladder and near the midpoint between the pubis and umbilicus. B, A transverse incision is made in the rectus fascia. C and D, The bladder is mobilized with a stay stitch and blunt dissection to free the peritoneum away from the bladder dome. E, A transverse incision is made into the bladder. F, The bladder is sewn to the rectus fascia, placing these sutures 1 cm away from the edge of the bladder incision. G, The bladder wall is sewn flush to the skin.
(From Gonzales ED. Posterior urethral valves and other ureteral anomalies. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell’s urology. 8th ed. Philadelphia: WB Saunders; 2002.)
Upper Tract Diversion
In the past, initial management of posterior urethral valves relied on upper tract diversion with ureterostomy or pyelostomy (Johnston, 1963). Diversion was effective in decompressing the upper tracts and controlling infection. Infants with high urinary diversion, however, often faced difficult urinary reconstructive problems later in life. In addition, high diversion produces stomas that present chronic management problems with incontinence. Even with these difficulties urinary diversion provided the best management available for valve patients for many years.
With the development of new cystoscopic equipment, Johnston demonstrated that endoscopic ablation could be performed safely and with good initial results (Johnston, 1966). Once the option of primary valve ablation was available, a long and thorough debate over the relative merits of initial upper tract diversion versus primary valve ablation followed.
Because both approaches yield good initial control of infection and adequate decompression of the upper urinary tract in most cases, the controversy focused on long-term results and measured renal function, bladder function, and somatic growth in each group. Conflicting data have been found regarding ultimate renal function and somatic growth, with Krueger and colleagues (1980) presenting data supporting ureterostomy from the large Toronto experience. He found that patients initially diverted had better eventual renal function and somatic growth. On the other hand, Reinberg, Duckett, and Hendren presented data supporting the conclusion that there was no significant difference in long-term outcomes between the two initial treatments (Hendren, 1978; Duckett and Norris, 1989; Reinberg et al, 1992a). Although more study may help clarify the situation, the current consensus is that neither initial treatment is superior in promoting renal function and somatic growth.
Despite early concerns that a bladder that is left without urine flow by proximal diversion would deteriorate from disuse, more recent data have shown that most of these bladders do not lose significant function. After upper tract diversion, urodynamics have shown that bladder capacity is preserved in 80% and compliance in 69% (Lome et al, 1972; Tanagho, 1974; Ghanem and Nijman, 2005). Primary valve ablation may give the best chance for optimal bladder rehabilitation because of the ability of the bladder to cycle, but temporary supravesical diversion remains a reasonable alternative in the complex valve patient and usually leads to a marginal, if any, decrease in long-term bladder function (Close et al, 1997; Podesta et al, 2002).
The current consensus is that both approaches eventually yield similar results and that infants who undergo initial upper tract diversion are at the disadvantage of needing more surgical procedures. Today upper tract diversion is usually limited to those patients who fail to respond to bladder level drainage. The specific clinical situation for upper tract diversion in posterior urethral valves today develops when bladder level drainage is insufficient to prevent infection or adequately drain the upper tracts. The decision to perform upper tract diversion relies on renal function, infection, and to a lesser extent, degree of hydronephrosis. Kidney development and growth in early life is critical, and neonatal kidneys may potentially recover function and continue to produce new cells during this period (Mayor et al, 1975; Hayslett, 1983). It is compelling to place these damaged kidneys in the best possible physiologic situation to recover, but this must be balanced with the knowledge that although upper tract diversion does initially improve renal function and drainage, there is no evidence that it offers any long-term advantage over primary ablation.
After the bladder has been adequately decompressed, the decision of whether to perform upper tract diversion is one of clinical judgment. Because hydronephrosis usually takes months to improve significantly, renal function is a better early parameter to follow. If the serum creatinine level drops below 2.0 it is safe to rely on improved bladder drainage for additional kidney improvement. If the creatinine level remains above 2.0 after 10 days of adequate bladder decompression and if hydronephrosis is unimproved, then upper tract diversion may be considered. The type of diversion remains the surgeon’s choice, and there are proponents of high loop ureterostomy, ring ureterostomy, pyelostomy, and end ureterostomy (Sober, 1972; Williams and Cromie, 1975; Kitchens et al, 2007). The type of upper tract diversion the authors prefer is a low loop ureterostomy because it provides adequate drainage, places the stoma under the diaper, and offers the most logical and simple reconstruction later.
If upper tract diversion is performed, reconstructive surgery to internalize the urinary tract should be delayed until the bladder and upper tracts have improved as much as can be expected. Practically this usually occurs between 3 and 5 years of age. If the patient is destined to reach ESRD, then reconstruction should be delayed until transplantation can be performed (Sheldon et al, 1992). It is imperative to establish an efficient method to empty the bladder, either by voiding or intermittent catheterization, before undiversion (Fig. 126–10).
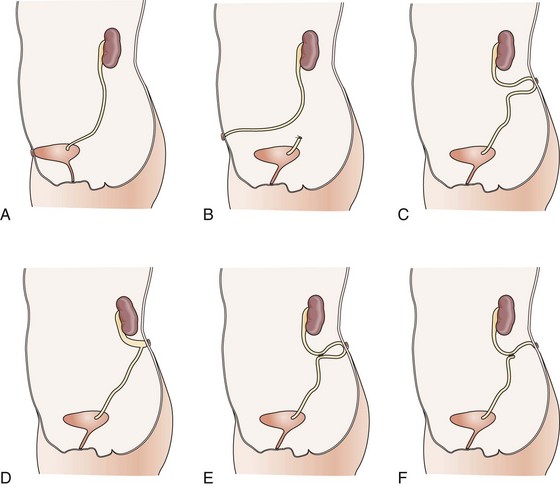
Figure 126–10 The variety of urinary tract diversions useful in posterior urethral valves. A, Vesicostomy. B, Distal ureterostomy. C, Proximal loop ureterostomy. D, Cutaneous pyelostomy. E, Ring ureterostomy. F, Sober Y ureterostomy.
(From Glassberg KI, Horowitz M. Urethral valves and other anomalies of the male urethra. In: Belman AB, King LR, Kramer SA, editors. Clinical pediatric urology. 4th ed. London: Martin Dunitz; 2002. p. 899–945.)
Management of Vesicoureteral Reflux
Between 50% and 70% of patients with posterior urethral valves will have vesicoureteral reflux at the time of diagnosis (Fig. 126–11) (Hulbert and Duckett, 1992). Scott (1985) studied 46 affected boys with valves and found reflux in 72% of patients and in 53% of ureters. Bilateral reflux occurred in 32% of this study group.
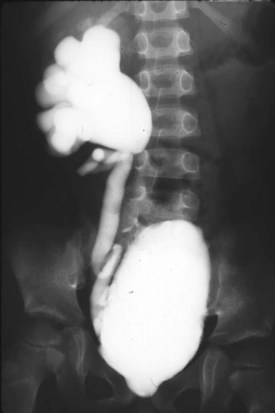
Figure 126–11 Voiding cystourethrogram shows persistent high-grade vesicoureteral reflux in a boy with posterior urethral valves. Note the large-capacity, mildly trabeculated bladder.
Reflux in posterior urethral valves is considered secondary to bladder outlet obstruction so that the initial management of reflux is relief of obstruction. Reflux resolves after valve ablation in between 20% and 32% of refluxing ureters (Scott, 1985). Most reflux resolves within several months, but some can take as long as 3 years. Reflux is more likely to resolve when associated with a better functioning kidney. Hassan found that reflux resolved in only 10% of kidneys with less than 20% split renal function in a series of 73 boys with valves (Hassan et al, 2003). Children with initial bilateral reflux are more likely to have reflux resolve than those with unilateral reflux. As with all children with vesicoureteral reflux, they must be maintained on prophylactic antibiotics to prevent infection.
If persistent high-grade reflux is a clinical problem because of urinary tract infections or incontinence, then bladder function and drainage must be reviewed. Bladder dysfunction in the form of inadequate emptying and high storage pressures are the usual causes of persistent reflux. Surgery to repair reflux in valve patients must be undertaken with great care, and reimplanting ureters into a valve bladder that has not been adequately rehabilitated will lead to a high failure and complication rate (Warshaw et al, 1985; Hulbert et al, 1992; El-Sherbiny, 2002a).
The first step in evaluation of persistent reflux is to repeat the VCUG to look for persistent valve remnants. If no remnants are found, then urodynamics are necessary to evaluate the bladder. If the bladder function is adequate and persistent reflux remains a problem, surgery to correct reflux may be necessary. Ureteroneocystostomy is usually limited to the situation where infections cannot be controlled and bladder function is adequate.
High-grade reflux is often associated with severe dysplasia and nonfunction in the associated kidney in valve patients (Heikkila and Rintala, 2009). In the past these nonfunctional kidneys and dilated collecting systems were removed early in life. It has become evident that there is no urgency to remove these units as long as they do not lead to recurrent infection. They are best retained until the bladder has rehabilitated as much as possible. The dilated collecting system could possibly be used as a ureteral augmentation if bladder volume or pressure warrants (Bellinger, 1993). Ureter is the best material available for augmentation cystoplasty because it is lined with urothelium and the ureteral muscle will not contribute to high pressures from detrusor contractions (Landau et al, 1994). It is also possible to leave these nonfunctional units in place and successfully repair the reflux (Kim et al, 1997a). This is a reasonable alternative when faced with the need for reimplant surgery on the contralateral side.
Management of Hydronephrosis
Almost all patients with posterior urethral valves have severe hydroureteronephrosis at diagnosis. Scott found hydronephrosis in 96.5% of valve patients, and in 78% it was bilateral (Scott, 1985). Hydronephrosis may or may not be associated with reflux. Like reflux, hydronephrosis is a secondary phenomenon in valve patients and like reflux, it often resolves once the primary pathology, obstruction, is relieved. Nonrefluxing hydronephrosis resolves in 49% of patients and may do so quite rapidly after valve ablation. This leaves a significant population of valve patients with persistent hydronephrosis for years despite adequate bladder emptying (Johnston and Kulatilake, 1971). Hulbert and Duckett (1988) found that 25% of valve patients have dilated upper tracts 5 to 15 years after successful valve ablation.
The majority of patients with persistent hydronephrosis do not have obstruction either at the bladder outlet or the ureterovesical junction. Whitaker developed his antegrade perfusion test specifically to examine chronic hydronephrosis in valve patients and document pressures within the upper urinary tract and across the UVJ (Whitaker, 1973). Tietjen also used the Whitaker test to evaluate 25 valve patients with persistent hydronephrosis and found only 8% of patients to have an element of obstruction at the ureterovesical junction (Tietjen et al, 1997). The majority of patients with hydronephrosis had a measured mean perfusion pressure of only 3 cm of H2O (normal is <15 cm of H2O). The ureterovesical junction may be functionally obstructed before ablation of the valves, but this usually resolves with bladder rehabilitation (Glassberg et al, 1982).
Valve Bladder Syndrome
The valve bladder or full valve bladder are terms coined by Mitchell to describe a chronic condition in valve patients where despite successful valve ablation, intrinsic bladder dysfunction leads to deterioration of the upper urinary tracts and incontinence (Mitchell, 1982). The combination of poor sensation, high bladder volumes, and poor compliance produce storage pressures high enough to prevent adequate drainage of the upper tracts. These boys are comfortable with large bladder volumes at high pressures. They frequently have overflow incontinence and void infrequently and incompletely. Videourodyanmics are necessary to diagnose this condition, and there is a direct correlation of abnormal urodynamics indicating poor compliance and detrusor overactivity with poor renal function (Ghanem et al, 2004). Initial management is usually timed voiding, but if this does not successfully lower pressures and empty the bladder, α blockers or clean intermittent catheterization (CIC) can be used (Austin et al, 1999). α Blockers can be effective in reducing postvoid residual urine and improving the urinary stream. Abraham and colleagues (2009) studied 42 children with valves, and residual urine volume was significantly reduced in 95%. The mean reduction of residual volume was 85%.
Glassberg (2001) has suggested that proactive urodynamic-directed bladder management might improve long-term bladder outcome. Koff and colleagues (2002) also proposed that valve bladder syndrome is not a permanent state due to prenatal bladder damage but is an induced condition due to a combination of polyuria, impaired bladder sensation, and residual urine volume. Bladder decompensation in response to this situation results in increasing residual urine, upper tract dilation, and renal injury.
Koff proposed intermittent catheterization with continuous nocturnal bladder emptying. He found a significant improvement of hydronephrosis in 10 boys with valves treated in this manner. Nguyen also found significant improvement in frequency of urinary tract infection, upper tract dilatation, and continence in valve patients who underwent overnight bladder drainage (Nguyen et al, 2005). Holmdahl and colleagues (2003) studied 35 boys with valve bladder and found that intermittent catheterization not only improved GFR but also improved bladder compliance and capacity more than expected for age and when compared with valve patients who did not catheterize.
Glassberg and colleagues (1982) categorized the hydronephrotic upper tracts of valve patients into three types: those that drain independent of bladder volume, those that drain efficiently only with the bladder empty, and those that are obstructed independent of bladder volume. Duckett applied this classification to 100 valve patients and found only four cases of true obstruction of the upper tracts independent of bladder dysfunction, and all had undergone ureteral reimplantation (Smith and Duckett, 1996).
Evaluation of persistent hydronephrosis in valve patients is complex and may require measurement of voided volumes to evaluate the relationship between renal tubular competence and bladder function, videourodynamics to evaluate the ability of the bladder to hold and empty adequate volumes at acceptable pressures, ultrasound to evaluate degree of hydronephrosis, lasix renography to evaluate relative renal function and induced drainage, and Whitaker perfusion test to measure intrarenal pressures at a given drainage rate. Access to all of these modalities is necessary to choose proper management of the valve patient with persistent hydronephrosis. Luckily, most children with persistent hydronephrosis do not need surgical therapy outside of valve ablation and attention to bladder function.
Management of Bladder Dysfunction
Posterior urethral valves affect no organ more consistently and profoundly than the bladder. The degree of damage varies considerably, and it is the rare patient who does not suffer significant lifelong bladder dysfunction. The bladder causes two types of long-term problems for the valve patient: interference with upper tract drainage and incontinence. The bladder subjects the upper tracts to a form of obstruction at two points of life, as newborns and late in childhood. At birth the bladder is thick and trabeculated with saccules and diverticuli. It contracts with great force, and compliance is low. Although urodynamics are difficult to perform in infants, particularly in the presence of reflux and diverticuli, the bladder can be seen on imaging to undergo strong contractions. This stage of bladder dysfunction can persist for months despite successful valve ablation. Voiding pressures are high in male infants with valves but no higher than their normal peers. Furthermore, high voiding pressures are not associated with reduced kidney function (Taskinen et al, 2009). Storage pressures, compliance, and ability to empty are other key elements of bladder function in these children.
Late in childhood the bladder is less contractile but displays poor compliance, and this along with poor sensation leads to the valve bladder syndrome discussed earlier. Both the hypercontractile bladder of infancy and the rigid bladder of late childhood inhibit upper tract drainage.
Incontinence is a major problem for valve patients. A review of 100 patients revealed 81% had delayed day and night continence at age 5 years (Smith et al, 1996). Another large series discovered that only 53% were dry by age 12 (Churchill et al, 1990). This problem improves in most by age 20, but the delay in attaining continence creates painful difficulties during adolescence (Parkhouse et al, 1988). In the past, incontinence was felt to be due to sphincter injuries and bladder neck dysfunction, but today this problem is thought to be multifactorial and due to a combination of poor bladder sensation, poor bladder compliance, detrusor instability, and polyuria. Renal tubular damage, which progresses during childhood, leads to inability to conserve sodium and free water and can lead to nephrogenic diabetes insipidus and urine volumes up of several liters per day. These exaggerated volumes can overcome any bladder’s functional ability, especially one with limited potential.
Peters identified three urodynamics patterns in valve boys: myogenic failure, detrusor hyperreflexia, and decreased compliance/small capacity (Peters et al, 1990). Myogenic failure usually occurs in older children and leads to overflow incontinence and incomplete bladder emptying. Management includes timed voiding, double voiding, α blockers, and intermittent catheterization. The urinary frequency and urge incontinence due to detrusor hyperreflexia is managed with anticholinergic medications. Anticholinergics may also help with those patients with small poorly compliant bladders but may not be sufficient to prevent the need for augmentation cystoplasty to provide adequate urine storage necessary for continence and upper tract drainage. Inappropriate use of anticholinergic medications may induce iatrogenic myogenic failure and therefore they should only be used with urodynamic monitoring (Misseri et al, 2002).
Holmdahl and colleagues (1995) studied valve patients with urodynamics over many years to assess changes in bladder function. They found that the functional patterns outlined by Peters overlap in the majority of patients and that the dominant pattern changed with age. They concluded that these patterns are stages of development. In infants, poor compliance dominates; in older children instability from hypercontractility is the dominant pattern; in postpubertal boys myogenic failure becomes the norm. The valve bladder is surely one in constant transition during childhood. Urodynamics are necessary to track changes and to alter management in these patients throughout the first two decades of life (Kim et al, 1997b).
Boys with valves who present later in childhood frequently have only voiding complaints and a more functional urinary tract. In a study of 70 children who presented at average age of 7.5 years, Schober and Dulabon (2004) found that ablation of valves will dramatically improve symptoms of urinary frequency and diurnal and nocturnal enuresis. Despite this improvement, most boys will continue to have a degree of hydronephrosis and some voiding difficulties.
In some boys with valves, bladder performance is not adequate to protect the upper tracts from the deleterious effects of high storage pressures and recurrent infection despite maximal medical management. Treatment of these children depends on their age and their ability to undergo CIC in most cases. If the boy can be catheterized, then anticholinergics and CIC will often improve the bladder environment enough to allow the bladder to rehabilitate and develop a better compliance and pressure profile. If this approach fails, then a vesicostomy is usually an excellent alternative.
After the age of toilet training the expectations change, and if anticholinergics and CIC fail to provide either a safe environment for the kidneys or the boy remains incontinent, then augmentation cystoplasty may be indicated (Kajbafzadeh et al, 1995; Bhatti et al, 2007). Although ileal augmentation is the historical norm and works well, it carries the disadvantages of mucous production, risk of rupture, stone production, and the potential for bladder cancers later in life. Because many valve patients have massive hydronephrosis and some have a unilateral nonfunctional kidney, ureteral augmentation is a somewhat popular alternative (Fisang et al, 2010). Ureteral augmentation has the advantage of leaving the bladder lined only with native urothelium and avoiding mucus, stones, and cancer. Bladder performance in 13 of 17 patients (10 with valves) after ureteral augmentation was significantly improved with a mean follow-up of 4.5 years when measured by capacity, mean end filling pressure, and compliance (Johal et al, 2008).
Augmentation in valve patients was more popular in the past before the national history of valve bladder development, and the pathophysiology of tubular damage in dysplastic kidneys was as well defined. Today it is somewhat rare and many patients are managed medically with CIC, medicine, and nighttime catheter drainage until their bladder pressures decrease and/or their renal failure is treated definitively with transplantation.
Catheterizable urinary channels are useful in many boys because the urethra is sensate and the high bladder neck often makes catheterization technically difficult (Cain et al, 1999). Appendicovesicostomy is slightly more difficult in valve patients because of the thickness and relative fibrotic bladder wall, but once accomplished it works well. This gives the boys easy access for CIC and nighttime catheter drainage. The authors perform appendicovesicostomy routinely in all older valve boys who require CIC.
In summary, management of bladder dysfunction in boys with posterior urethral valves is an intensively individualized process with close monitoring of urodynamics, urine volumes, renal function, infections, and hydronephrosis. Patients may need anticholinergics, α blockers, intermittent catheterization, continuous nocturnal bladder drainage, timed voiding, or any combination of management options. In addition, because the bladder is in transition throughout most of childhood, their management needs will change as they mature.
Management of Glomerular Injury and Renal Function
The primary goal of management in posterior urethral valves is to preserve renal function and to maximize renal growth and development. Renal injury in posterior urethral valves includes two initial glomerular insults: dysplasia and obstructive uropathy. Dysplasia is by far the most important because it is irreversible and not only produces a level of renal insufficiency at diagnosis but also limits future growth and development of the kidney. Obstructive uropathy may produce ongoing injury and is potentially reversible. It is mandatory to provide low-pressure storage and drainage of urine in order to prevent further renal damage.
Other threats to glomerular function come from persistent or progressive obstruction and infection. Hyperfiltration has been recognized in animal models as a cause of additional injury, but its importance in humans remains undocumented (Brenner et al, 1982). As somatic growth makes additional demands on the kidneys, valve patients can experience proteinuria, hypertension, and progressive loss of renal function (Tejani et al, 1986; Burbige and Hensle, 1987; Parkhouse et al, 1988; Reinberg et al, 1992a; Farnham et al, 2005). Poorly controlled hypertension also can add to renal deterioration. Consistent and regular urologic care with monitoring of bladder function and infection will place the kidneys in the best position to grow and develop to their full potential.
Antenatal Diagnosis and Management
The incidence of urologic anomalies is 1 in 500 fetuses detected on ultrasounds (Colodny, 1987). Posterior urethral valves are the most common cause of bilateral hydronephrosis in male fetuses. Most valve patients are diagnosed by prenatal ultrasound, and the ability to image the fetus continues to improve with the technology of ultrasound. The accuracy of ultrasound diagnosis is proportional to fetal age, and valves may be missed in scans done before 24 weeks’ gestation (Skolder et al, 1988; Abbott et al, 1998). The differential diagnosis of a male fetus with bilateral hydroureteronephrosis and a distended bladder includes prune-belly syndrome and severe reflux. Bladder wall thickness is impressively increased only with valves. Fetal urine parameters may also be useful in predicting renal damage in utero. Aspirated fetal bladder urine with high sodium content and isosthenuria compared with serum values suggests poor renal function and that fetal intervention would not be helpful from a renal standpoint (Glick et al, 1985).
Valves have emerged as the only urologic anomaly to be considered for antenatal intervention. Fetal intervention, although championed in the 1980s, has become quite rare due to a lack of objective evidence that it alters renal development (Reinberg et al, 1992b; Freedman et al, 1999). Although antenatal decompression of the urinary tract and the subsequent restoration of amniotic fluid are usually accomplished by placing a vesicoamniotic shunt, there have been reports of fetal valve ablation (Quintero et al, 1995). Restoring the volume of amniotic fluid can be a life-saving maneuver because of its effect on pulmonary function (Harrison et al, 1982). For optimal results this intervention must be performed during the appropriate stage of pulmonary development, older than 20 weeks and younger than 32 weeks. Oligohydramnios earlier than 20 weeks is usually incompatible with life due to pulmonary hypoplasia, and if diagnosed later than 32 weeks early delivery is preferable to fetal intervention. The decision to undertake fetal intervention must weigh the potential benefit for the fetus versus the risk of complications to both fetus and mother that occurred in 44% of those cases reviewed by the International Fetal Surgery Registry (Elder et al, 1987) (Fig. 126–12).
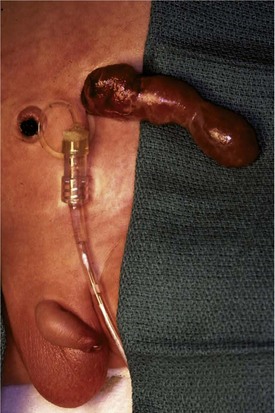
Figure 126–12 This boy had a vesicoamniotic shunt placed at 18 weeks of gestational age. The shunt has been attached to a drainage bag in the intensive care unit. The structure extending from the shunt site is herniated omentum. There is a significant risk of abdominal wall hernia at the shunt site.
Key Points: Management of Posterior Urethral Valves
Prognostic Indicators of Renal Function
Four basic predictors of renal function exist in patients with posterior urethral valves: ultrasound appearance, serum chemistries, age at diagnosis, and presence of reflux. Ultrasound, age at diagnosis, and presence of reflux can be determined at diagnosis, but serum chemistries require some time to develop.
Ultrasound appearance is a method of estimating the amount of dysplasia present in the kidneys. Dysplasia, strictly speaking, is a histologic diagnosis of tissue disorganization and includes fibrosis and cysts. This disorganization appears more echogenic on ultrasound and may be obvious by comparing the kidney to the liver and spleen as controls (Sanders et al, 1988). Kidneys that are considerably brighter than the liver usually contain significant dysplasia. Hyperechogenic kidneys diagnosed on ultrasound before 25 weeks’ gestational age were predictive of renal dysplasia on postmortem examination of 24 male fetuses with urethral obstruction (Robyr et al, 2005). Loss of the normal corticomedullary junction normally visible on ultrasound has also been correlated with poor long-term renal function (Hulbert et al, 1992).
Age at diagnosis may be helpful in predicting prognosis. In the past, infants who presented younger than 1 year of age had a worse prognosis than those who presented later in life. Older boys usually present with voiding difficulty. They were long considered to represent the least affected of valve patients, but recent evidence has shown that some boys who present later in life suffer significant renal damage (Pieretti, 1993; El-Sherbiny et al, 2002b). One study of boys who presented older than 5 years revealed that 35% had renal insufficiency and 10% reached ESRD (Bomalaski et al, 1999).
In a review of 70 boys who presented at the mean age of 7.5 years, Schober found that all had a normal serum creatinine (Schrober et al, 2004). Ansari and colleagues (2008) compared 95 patients with valves who presented before 2 years of age to 99 patients who presented after 2 years. They found that the incidence of azotemia at presentation, mean serum creatinine level, and risk for ESRD were worse in the boys who presented after age 2 years. The conflicting data in numerous studies seem to indicate that postnatal age at diagnosis alone is not a reliable factor in predicting outcome in valve patients, but it may be helpful in understanding the risks for an individual when considered with other clinical factors.
Today with most infants diagnosed antenatally and improved prenatal care, mortality of newborns with valves has decreased from almost 50% to between 3% and 10% (Williams, 1954; Churchill et al, 1990). Despite this improvement in mortality, positive effects of prenatal diagnosis on long-term renal function have been difficult to determine. Multiple authors have found that early gestational age at the time of diagnosis (before 24 weeks) is a significant negative factor in predicting long-term renal failure (Hutton et al, 1994; Sarhan et al, 2008).
Reflux in valve patients carries a poor prognosis. Johnston reviewed a series of valve patients in which the mortality was 57% in patients with bilateral reflux, 17% with unilateral reflux, and 9% with no reflux (Johnston, 1979). Many other authors have noted the grave effect of bilateral reflux on patients with posterior urethral valves (Parkhouse et al, 1988; Churchill et al, 1989). The commonly accepted explanation is that the incompetent ureterovesical junction that allows free reflux into the kidney also transmits the high pressures generated by the bladder. On the other hand, Stevens and others believe that severe reflux is associated with dysplasia primarily because the ureteric bud is abnormal and, in turn, induces an abnormal kidney.
The final prognostic factor is based on serum chemistries and relies on serial measurements of renal performance over time. Warshaw reported that a nadir creatinine of less than or equal to 0.8 mg/dL during the first year of life predicts likely good long-term renal function (Warshaw et al, 1985). Other authors found the same relationship with a nadir creatinine level of 1 mg/dL during the first year of life (Conner and Burbige, 1990; Sarhan et al, 2009; Ansari et al, 2010). Early renal function may be a helpful predictor as well. Denes reported on 32 patients with posterior urethral valves treated with immediate drainage. Serum creatinine was measured at diagnosis, after 4 days of drainage, and the nadir in the first year of life. After a mean of 10 years the authors found that patients fell into two groups, one with a GFR of greater than 70 mL/1.73 m2 and the other with a GFR less than 70. The group with worse long-term function had initial creatinine of 3.6 mg/dL, a postdrainage creatinine of 2.4, and a nadir of 1.7. The group with better long-term renal function had initial creatinine of 1.3 mg/dL, postdrainage creatinine 0.6, and a 1-year nadir of 0.4 (Denes et al, 1997).
In a multivariate analysis of 119 valve patients seen over 30 years with approximately half presenting as newborns, DeFoor identified two independent risk factors that were statistically significant in predicting ESRD: increased nadir serum creatinine and severe bladder dysfunction. Reflux was associated with an increased risk of ESRD, but this did not reach statistical significance (DeFoor et al, 2008). Ansari studied 260 valve patients and found that nadir serum creatinine level, severe bladder dysfunction, and bilateral reflux were significant predictors of eventual ESRD (Ansari et al, 2010). The most powerful tools yet proposed in predicting outcomes in posterior urethral valves may lie at the genetic or molecular level (Woolf and Winyard, 2000).
Transplantation in Valve Patients
Obstructive uropathy is the most common cause of ESRD in children and accounts for 16.3% of children presenting for transplantation (Seikaly et al, 2001). The most common condition producing this degree of dysfunction is posterior urethral valves. Up to 50% of boys with posterior urethral valves will reach ESRD in their lifetime. Most of these will need dialysis or transplantation during the first two decades of life. Because renal transplantation allows a better quality of life and optimal somatic growth, it is the preferred method of managing ESRD in children and adolescents. The damaged bladder of valve patients presents a technical challenge for renal transplantation, and abnormal bladder function has been considered a threat to the success of transplantation.
Early transplant series in children with valves demonstrated significantly decreased success in transplant graft survival and in graft function compared with those who suffered ESRD due to reflux nephropathy (Churchill et al, 1988; Reinberg et al, 1988). The principal cause of graft failure was chronic rejection, and the relationship between bladder function and rejection was not apparent. Valve patients are known to have decreased bladder compliance and increased storage pressures, but in the absence of obstructive hydronephrosis or infection these factors can be managed successfully to protect the kidney.
More recent data have shown improved results with some series demonstrating good graft survival but a statistically significant increase in serum creatinine levels compared with controls (Bryant et al, 1991; Salomon et al, 2000). Still others have found no significant difference between valve patients and others in either graft survival or renal function (Groenewegen et al, 1993; Ross et al, 1994; DeFoor et al, 2003; Mendizabal et al, 2005; Otukesh et al, 2008). Because the lower urinary tract is markedly abnormal in valve patients, it is not surprising that urologic complications are higher than in patients with normal bladders. Urethral strictures, stones, and urinary retention were among the urologic complications that occurred in 19% of valve patients undergoing transplantation (Ross et al, 1994). Mochon found an increased risk of urinary tract infection in valve patients compared with others after transplantation, but this relationship may have been present before transplant and did not affect graft function (Mochon et al, 1992).
It is safe to say that renal transplantation is an important part of management of some valve patients and that success rates are high. It must be undertaken with the knowledge that urinary tract management may be more complex and complications more common than with other children undergoing transplantation. Careful urodynamic evaluation of bladder function before transplantation is vital, and the patient may need intermittent catheterization and/or augmentation cystoplasty in order to function safely with the new kidney (Zaragoza et al, 1993; Indudhara et al, 1998; Koo et al, 1999; Salomon et al, 2000; Mendizabal et al, 2005; Ozcan et al, 2006).
Quality of Life with Posterior Urethral Valves
Although study in this area is somewhat limited, with modern management of renal insufficiency and bladder dysfunction most boys with posterior urethral valves feel they have a good quality of daily life (Hellström et al, 2006). Intermittent catheterization is seen as an obstacle in the company of friends and has required boys to develop strategies to perform this discreetly. Overall, boys with valves saw their treatment as a small part of their lives and valued friends, being able to participate in health care decisions, and having their own doctor and nurse.
The generally positive attitude toward life by valve patients can lead to their being lost from the health care system, and almost half of a group of 31- to 44-year-olds with valves had not been checked since adolescence. Among this group, a third was uremic and 40% had signs of bladder dysfunction despite being continent. The ability to father children was dependent on the presence of uremia. This study highlights the importance of lifelong follow-up care to monitor the often silent complications of valves, which ultimately affect quality of life (Holmdahl and Sillen, 2005). Boys with posterior urethral valves have a higher incidence of cryptorchidism (16%) and inguinal hernia (11%) (Heikkila et al, 2008b).
Anterior Urethral Valves
Anterior urethral obstruction is rare compared with posterior urethral valves. Although these obstructive structures may be called valves, they often occur in the form of a diverticulum of the urethra with one wall acting as an obstructive “valve” (Fig. 126–13) (Tank, 1987). There are also some reported cases of urethral flaps or valves in the anterior urethra (DeCastro et al, 1987; Scherz et al, 1987). The first case was reported in 1906, and the total number in the literature is small (Watts, 1906; Williams and Retik, 1969; Firlit et al, 1978).
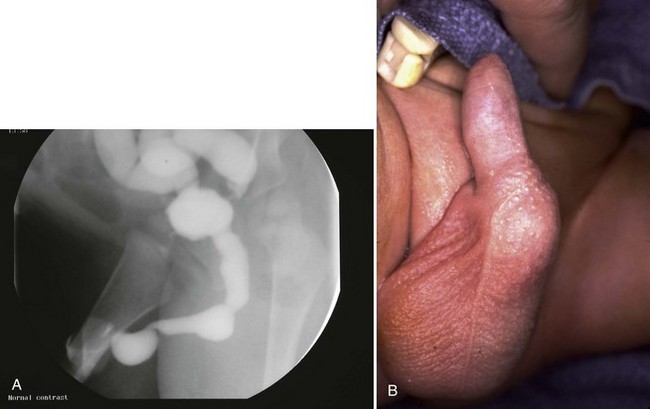
Figure 126–13 A, Voiding cystourethrogram shows a severe case of anterior urethral valves. The bladder is almost empty, and there is massive reflux. The valve is in the form of a diverticulum. B, The filled diverticulum can sometimes be seen as a mass on the penis that resolves between voids. Pressing on the mass may express urine for some time after voiding.
Anterior urethral diverticuli typically occur where there is a defect of the corpus spongiosum, leaving a thin-walled urethra (Fig. 126–14). This segment of urethra balloons during voiding and causes a mass that is sometimes visible along the ventral wall of the penis. The mass resolves as urine drains from it between voids, and when full, urine can be expressed from the diverticulum by applying pressure. The distal edge of the diverticulum forms a flap that obstructs the flow of urine, and the effects of anterior urethral valves can be as damaging to the urinary tract as those of the posterior urethra. The embryology of these structures remains unclear but seems to be unrelated to the development of posterior urethral valves.
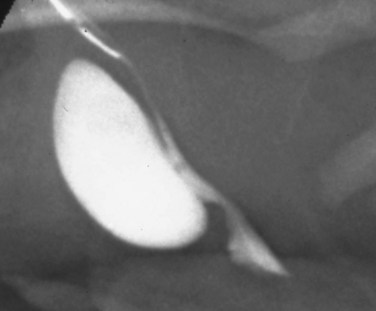
Figure 126–14 Voiding cystourethrogram demonstrates an anterior urethral diverticulum trapping urine during voiding.
Boys with anterior urethral valves often present later in childhood with infection, straining to void, or incontinence. One third present with voiding symptoms, a third with antenatal hydronephrosis, and the remainder with a visible diverticulum (Van Savage et al, 1997). The degree of obstruction can be severe and produce pressures as high as with the posterior urethral valve. Spontaneous rupture of the fetal bladder in anterior urethral valves has been reported (Merrot et al, 2003).
Diagnosis depends on VCUG or cystoscopy. It may be difficult to pass a catheter into the bladder because the catheter often easily enters the diverticulum and becomes trapped. Treatment is similar to that of posterior urethral valves in that relief of obstruction is the most important goal. In severely ill children or young infants a vesicostomy is a good option for temporary drainage, allowing the urinary tract to decompress and delaying urethral repair until conditions are optimal (Rushton et al, 1987).
Treatment of anterior urethral valves may include transurethral incision to allow free flow of urine or open urethral reconstruction. If the diverticulum is small or there is a urethral flap, transurethral incision is preferred. If the diverticulum is large, it will usually cause pooling of urine and repeated infection. The best option is an open excision of diverticulum and repair of the urethra. This can be difficult surgery and requires catheter drainage to facilitate urethral healing.
The long-term outcome of anterior urethral obstruction is similar to that of the posterior urethra. In general the severity of obstruction, degree of hydronephrosis, and incidence of renal failure is usually better in anterior urethral valves (Rawat et al, 2009). Less than 5% of patients with anterior urethral valves have reached ESRD compared with more than 30% of those with posterior urethral valves (Kaplan and Scherz, 1992).
Congenital Urethral Stricture
Congenital urethral strictures are rare anomalies that produce the same pathologic and clinical problems as posterior urethral valves (Fig. 126–15) (Currarino and Stephens, 1981). They occur in the posterior urethra and lead to oligohydramnios, bilateral hydroureteronephrosis, and a distended bladder (Narborough et al, 1990). Initial treatment in the newborn is almost always vesicostomy. Because these strictures are usually too long to incise and too tight to dilate with sounds, they may be dilated gradually with increasingly larger catheters with good results (Passerini-Glazel et al, 1988).
Urethral Polyps
Urethral polyps are rare anomalies of the male urethra (Fig. 126–16). Patients with urethral polyps usually present with intermittent voiding complaints such as hematuria, stranguria, and dysuria (Raviv et al, 1993; Walsh et al, 1993). Unlike urethral valves, polyps do not produce extensive damage to the urinary tract. They usually occur in the prostatic urethra near the bladder neck and may be quite mobile on a long stalk. Anterior urethral polyps have been reported (Coleman and Nense, 1991).
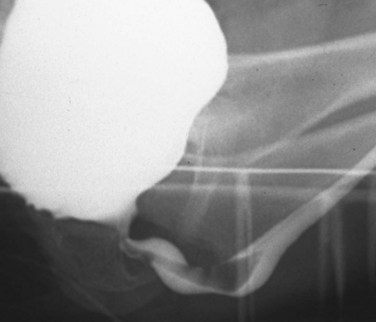
Figure 126–16 This urethral polyp is on a long stalk and caused intermittent dysuria and straining to void.
The polyps are usually diagnosed on VCUG, and the diagnosis is confirmed by cystoscopy. They are benign but may be confused with rhabdomyosarcoma of the prostate. Benign urethral polyps are usually solitary, whereas the polypoid form of rhabdomyosarcoma usually presents with multiple polyps and a mass extending beyond the bladder or prostate. Transurethral resection is curative in urethral polyps (Gleason et al, 1994).
Urethral Duplication
Duplication of the urethra is a rare anomaly. Various types and several classification systems exist (Fig. 126–17) (Effmann et al, 1976; Woodhouse and Williams, 1979). In practice, urethral duplication is so rare that it is usually described by anatomic landmarks. Most urethral duplications occur in the same sagittal plane and can be divided into either dorsal or ventral. Some more rare duplications occur in the same horizontal plane, and the urethras lie next to one another, one left and one right. This horizontal type may be associated with duplicated phallus or complete bladder duplication (Woodhouse and Williams, 1979). Some patients present with incontinence or infection, but most are discovered in infancy on examination by the mother or pediatrician.
Figure 126–17 A to J, Variations of urethral duplications in the sagittal plane. Each of these drawings shows the ventral urethra as the functional one as it passes through the prostate and sphincter.
(From Colodny A. Urethral lesions in infants and children. In: Gillenwater JT, Howards SS, Duckett JW, editors. Adult and pediatric urology. 2nd ed. St Louis: Mosby-Year Book; 1991. p. 2013.)
Dorsal urethral duplication occurs when there is a normal urinary meatus, dorsal chordee of the penis, and a second epispadiac meatus along the dorsal aspect of the penis. The foreskin may be incomplete dorsally, and the epispadiac meatus can occur anywhere along the midline of the penis (Sharma et al, 1987). The normal urethra is the ventral one that ends on the glans. The epispadiac duplicate urethra may be blind ending or extend to the bladder. If it communicates with the bladder, the patient may be incontinent. These patients may have a widened symphysis pubis similar to epispadias or exstrophy.
Ventral urethral duplications are extremely rare and may also be complete or incomplete with a blind-ending urethra. There may be a normally placed urethra on the glans and a second along the ventral aspect of the penis or even along the perineum. The dorsal urethra may be of normal caliber or narrow. The ventral urethra is considered the more normal because it usually passes out of the bladder neck and sphincter (Salle et al, 2000). The “Y” duplication occurs when the prostatic urethra splits into two channels with one extending to the glans and the more functional ventral one coursing to the perineum near the rectum.
Diagnosis of urethral duplication depends on the type of anomaly. If the duplication is complete, a VCUG will demonstrate both urethras during voiding (Fig. 126–18) (Hoekstra et al, 1985). If the duplication is blind ending, a retrograde injection of contrast and cystoscopy may be required to delineate the anatomy (Fig. 126–19) (Podesta et al, 1998).
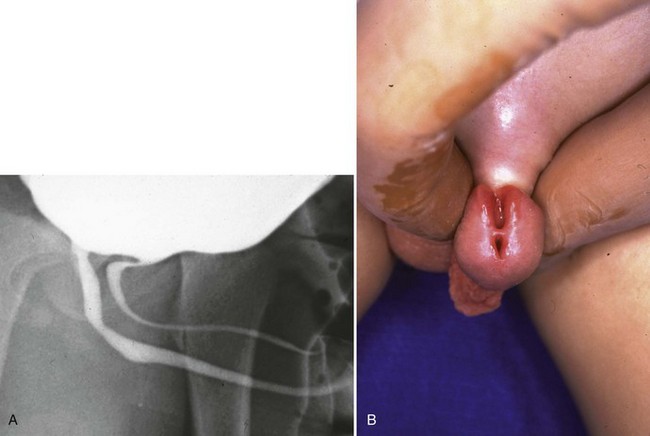
Figure 126–18 A, Voiding cystourethrogram shows a complete duplication of the urethra with an epispadiac dorsal segment. B, The ventral urethra is in the center of the glans, and the dorsal urethra ends in the dorsal glans cleft in an epispadiac location.
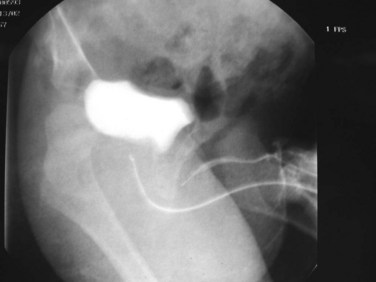
Figure 126–19 This urethral duplication has a blind-ending dorsal segment that ends under the pubis.
Management of urethral duplication varies with symptoms and the severity of the anomaly. Some patients do not require treatment if they are free from infection and incontinence. Simple accessory duplicate urethras may be fulgurated with a bugbee and allowed to scar and close (Holst and Peterson, 1988). Others need to be excised to make the patient dry and prevent infection. If both urethras are functional and end adjacent to one another on the glans, the septum between the two can be excised at the meatus, giving the patient a single urinary meatus. The most complex “Y” fistulae may require extensive urethroplasties requiring tissue transfer such as buccal mucosa grafts (Ortolano and Nasaralloh, 1986).
Urinary Fistula in Boys with Anorectal Anomalies
More than 80% of boys born with anorectal malformations have a fistula between the rectum and the urinary tract (Hong et al, 2002) (Fig. 126–20). The vast majority have imperforate anus, but occasionally a congenital fistula occurs between the urinary tract and the rectum in a boy with an external anal opening (H-type fistula). Fistulae are usually discovered by the pediatric general surgery service in their evaluation of the newborn with an anorectal anomaly and may be demonstrated with a VCUG. Because the initial treatment of most patients with anorectal anomalies is a diverting colostomy, many fistulae are diagnosed at 6 months of age with a distal colostogram. The distal colostogram is done by injecting contrast into the distal limb of the diverting colostomy before surgical repair of the anus.
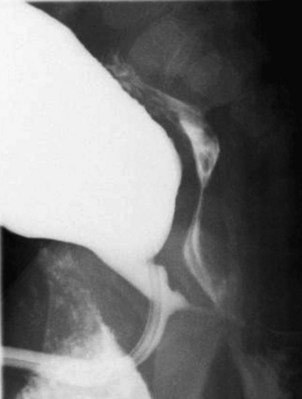
Figure 126–20 This voiding cystourethrogram demonstrates a posterior urethral fistula to the rectum discovered after an anoplasty. The patient presented with recurrent urinary tract infection and diarrhea due to urine passing into the rectum.
Pediatric urologists are involved early in the child’s life when recurrent urinary infections are a problem due to distal colostomy limb bacterial overgrowth, which leads to contamination of the bladder or urethra. This may be treated with antibiotic prophylaxis, enemas to empty the distal bowel, surgical separation of the proximal and distal limbs of the colostomy to prevent spillage, or in some cases early repair of the anus with a pull-through procedure. Upper urinary tract anomalies have been reported in up to 60% of boys with anorectal anomalies and may include vesicoureteral reflux, renal anomalies including solitary kidney, and hydronephrosis (Misra et al, 1996). Because 40% of these patients have sacral anomalies and 55% of those have severe lower urinary tract dysfunction comparable with patients with myelodysplasia, the threat of continued contamination of the urinary tract is serious (Boemers et al, 1996).
Definitive treatment of congenital recto-urinary fistulae is done at the time of anoplasty by the pediatric general surgery service. If this procedure is successful, the fistula is taken down at the time of rectal repair. Urethral injuries occur in 3% to 25% and may present immediately or in a delayed fashion (see Fig. 126–20). Fistulae may be either persistent or recurrent after rectal surgery or acquired by injuring the urethra at the time of anoplasty. In addition, a section of the rectum may be left attached to the fistula, creating a urethral diverticulum that may present with incontinence or UTI later in childhood. Rarely, the urethra may be divided or injured so severely that it causes stricturing.
The urologist should be involved with injuries to the urethra or bladder. Cystoscopy with catheterization of the fistula and imaging will often be helpful to the surgeon, and if the urinary injury is more than a simple persistent fistula the pediatric urologist should repair the urinary tract. Repair of fistula in infants and children follows the same set of operative rules that apply to adults. Any obstructive element of the urethra must be eliminated, the rectum and urethra (or bladder) must be adequately separated, the fistula must be ligated, and healthy tissue must be interposed between the urinary tract and gut. Often, pediatric surgeons pull down fresh bowel at the time of revision and uninjured rectal wall opposes the urinary fistula, allowing healing. If this is not possible, it may be necessary to interpose omentum or a Dartos fascial flap as a barrier to repeat fistula (Youssef et al, 1998). The importance of having a catheter in the urethra during any rectal surgery cannot be overstated. In addition, adequate preoperative imaging with VCUG, colostogram, and magnetic resonance imaging will give the operating surgeon the best opportunity to have a successful outcome to this difficult surgical problem. Symptomatic urethral diverticuli must be excised using many of the same surgical techniques.
After any type of fistula or diverticulum repair, a urinary catheter should be left in place for at least 7 days. These children will need lifelong monitoring due to the risk of stricture, recurrent fistulae, or neurogenic bladder dysfunction.
Churchill BM, McLorie GA, Khoury AE, et al. Emergency treatment and long-term follow-up of posterior urethral valves. Urol Clin North Am. 1990;17:343.
Dinneen MD, Duffy PG. Posterior urethral valves. Br J Urol. 1996;78:275.
Ghanem MA, Wolffenbuttel KP, De Vylder A, et al. Long-term bladder dysfunction and renal function in boys with posterior urethral valves based on urodynamic findings. J Urol. 2004;171:2409.
Glassberg KL, Schneider M, Haller JO, et al. Observations on persistently dilated ureter after posterior urethral valve ablation. Urology. 1982;20:20.
Hassan JM, Pope JC, Brock JW, et al. Vesicoureteral reflux in patients with posterior urethral valves. J Urol. 2003;170:1677.
Holmdahl G, Sillen U, Hanson E, et al. Bladder dysfunction in boys with posterior urethral valves before and after puberty. J Urol. 1996;155:694.
Indudhara R, Joseph DB, Perez LM, Diethelm AG. Renal transplantation in children with posterior urethral valve revisited: a 10-year follow-up. J Urol. 1998;160:1201.
Koff SA, Mutabagani KH, Jananthi VR. The valve bladder syndrome: pathophysiology and treatment with nocturnal bladder emptying. J Urol. 2002;167:297.
Narasimhan KL, Kaur B, Chowdhary SK, et al. Does mode of treatment affect the outcome of neonatal posterior urethral valves? J Urol. 2004;171:2423.
Young HH, Frontz WA, Baldwin JC. Congenital obstruction of the posterior urethra. J Urol. 1919;3:289.
Abbott JF, Levine D, Wapner R. Posterior urethral valves: Inaccuracy of prenatal diagnosis. Fetal Diagn Ther. 1998;13:179.
Abraham MK, Nasir AR, Sudarsanan B, et al. Role of alpha adrenergic blocker in the management of posterior urethral valves. Pediatr Surg Int. 2009.
Adzick NS, Harrison MR, Flake AW, de Lorimier AA. Urinary extravasation in the fetus with obstructive uropathy. J Pediatr Surg. 1985;20:608.
Ansari MS, Gulia A, Srivastava A, Kapoor R. Risk factors for progression to end-stage renal disease in children with posterior urethral valves. J Pediatr Urol. 2010;6:261-264.
Ansari MS, Singh P, Mandhani A, et al. Delayed presentation in posterior urethral valve: long-term implications and outcome. Urology. 2008;71:230.
Atwell JC. Posterior urethral valves in the British Isles: a multicenter BAPS review. J Pediatr Surg. 1983;18:70.
Austin PF, Homsy YL, Masel JL, et al. Alpha-adrenergic blockade in children with neuropathic and nonneuropathic voiding dysfunction. J Urol. 1999;162:1064.
Beck AD. The effect of intra-uterine urinary obstruction upon the development of the fetal kidney. J Urol. 1971;105:784.
Bellinger MF. Ureterocystoplasty: a unique method for vesical augmentation in children. J Urol. 1993;149:811.
Bhatti W, Sen S, Chacko J, et al. Does bladder augmentation stabilize serum creatinine in urethral valve disease? A series of 19 cases. J Pediatr Urol. 2007;3(2):122-126.
Biewald W, Schier F. Laser treatment of posterior urethral valves in neonates. Br J Urol. 1992;69:425.
Boemers TML, Beek FJA, van Gool JD, et al. Urologic problems in anorectal malformations. Part 1: urodynamic findings and significance of sacral anomalies. J Ped Surg. 1996;31:407.
Bomalaski MD, Arema JG, Coplen DE, et al. Delayed presentation of posterior urethral valves: a not so benign condition. J Urol. 1999;162:2130.
Brenner BM, Meyer TW, Hosteller TH. Dietary protein intake and the progressive nature of kidney disease. N Engl J Med. 1982;307:652.
Bryant JE, Joseph DB, Kohaut EC, Diethelm AG. Renal transplantation in children with posterior urethral valves. J Urol. 1991;146:1585.
Burbige KA, Hensle TW. Posterior urethral valves in a newborn: treatment and functional results. J Pediatr Surg. 1987;22:165.
Cain MP, Casale AJ, King SJ, et al. Appendicovesicostomy and newer alternatives for the Mitrofanoff procedure: results in the last 100 patients at Riley Children’s Hospital. J Urol. 1999;162:1749.
Casale AJ. Early ureteral surgery for posterior urethral valves. Urol Clin North Am. 1990;17:361.
Chandna JB, Dickson BA, Gough D. The Whitaker hook in the treatment of posterior urethral valves. Br J Urol. 1996;78:783.
Chertin B, Cozzi D, Puri P. Long-term results of primary avulsion of posterior urethral valves using a Fogarty balloon catheter. J Urol. 2002;168:1841.
Churchill BM, Khoury AE, McLorie GA. Posterior urethral valves. Acta Urol Belg. 1989;57:435.
Churchill BM, Krueger RP, Fleisher MH, et al. Complications of posterior urethral valve surgery. Urol Clin North Am. 1983;10:519.
Churchill BM, McLorie GA, Khoury AE, et al. Emergency treatment and long-term follow-up of posterior urethral valves. Urol Clin North Am. 1990;17:343.
Churchill BM, Sheldon CA, McLorie GA, et al. Factors influencing patients and graft survival in 300 cadaveric pediatric renal transplants. J Urol. 1988;140:1129.
Close CE, Carr MC, Burns MW, Mitchell ME. Lower urinary tract changes after early valve ablation in neonates and infants: is early diversion warranted? J Urol. 1997;157:984.
Coleman NH, Nensle TW. Anterior urethral polyp associated with hematuria in six year old child. Urology. 1991;38:143.
Colodny A. Antenatal diagnosis and management of urinary abnormalities. Pediatr Clin North Am. 1987;34:1365.
Conner JP, Burbige KA. Long-term urinary continence and renal function in neonates with posterior urethral valves. J Urol. 1990;144:1209.
Conner JP, Hensle TW, Berdon W, Burbige KA. Contained neonatal urinoma: management and functional results. J Urol. 1988;140:1319.
Cromie WJ, Cain MP, Bellinger MF, et al. Urethral valve incision using a modified venous valvulotome. J Urol. 1994;151:1053.
Cromie WJ, Kwang L, Kara H, et al. Implications of prenatal ultrasound screening in the incidence of major genitourinary malformations. J Urol. 2001;165:1677.
Cuckow PM, Dinneen MD, Risdon RA, et al. Long-term renal function in the posterior urethral valves, unilateral reflux and renal dysplasia syndrome. J Urol. 1997;158:1004.
Currarino G, Stephens FD. An uncommon type of bulbar urethral stricture, sometimes familial, of unknown cause: congenital versus acquired. J Urol. 1981;126:658.
Deane AM, Whitaker RH, Sherwood T. Diathermy hook ablation of posterior urethral valves in neonates and infants. Br J Urol. 1988;62:593.
DeCastro R, Battaglino F, Casolari E, et al. Valves of the anterior urethra without diverticulum: description of three cases. Pediatr Med Chir. 1987;9:211.
DeFoor W, Tackett L, Minevich E, et al. Successful renal transplantation in children with posterior urethral valves. J Urol. 2003;170:2402.
DeFoor W, Clark C, Jackson E, et al. Risk factors for end stage renal disease in children with posterior urethral valves. J Urol. 2008;180:1705.
Dejter SWJr, Gibbons MD. The fate of infant kidneys with fetal hydronephrosis but initially normal postnatal sonography. J Urol. 1989;142(2 Pt. 2):661-662.
Denes ED, Barthold JS, Gonzalez R. Early prognostic value of serum creatinine levels in children with posterior urethral valves. J Urol. 1997;157:1441.
Dewan PA. Congenital obstructing posterior urethral membrane (COPUM): further evidence for a common morphological diagnosis. Pediatr Surg Int. 1993;8:45.
Dewan PA, Goh DG, Crameri J. Urethral valves or COPUM? Changing the nomenclature. Contemp Urol. 1999;11:15.
Dewan PA, Zappala PG, Ransley PG, et al. Endoscopic reappraisal of the morphology of congenital obstruction of the prostatic urethra. Br J Urol. 1992;70:439.
Dinneen MD, Dhillon HK, Ward HC, et al. Antenatal diagnosis of posterior urethral valves. Br J Urol. 1993;72:364.
Dinneen MD, Duffy PG. Posterior urethral valves. Br J Urol. 1996;78:275.
Dinneen MD, Duffy PG, Barratt TM, Ransley PG. Persistent polyuria after posterior urethral valves. Br J Urol. 1995;75:236.
Duckett JW, Norris M. The management of neonatal valves with advanced hydronephrosis and azotemia. In: Carlton CE, editor. Controversies in urology. Chicago: Year Book; 1989:2.
Effmann EL, Lebowitz RL, Colodny AH. Duplication of the urethra. Radiology. 1976;119:179.
Ehrlich RM, Shanberg A. Neodymium-YAG laser ablation of posterior urethral valves. Dialogues Pediatr Urol. 1988;11:29.
Elder JS, Duckett JW, Snyder HM. Intervention for fetal obstructive uropathy: specific outcomes diagnosis. Lancet. 1987;2:1007.
El-Sherbiny M, Hafez AT, Ghoneim MA. Ureteroneocystostomy in children with posterior urethral valves: indications and outcome. J Urol. 2002;168:1836.
El-Sherbiny M, Hafez AT, Shokeir AA. Posterior urethral valves: does young age at diagnosis correlate with poor renal function? Urology. 2002;60:335.
Ewalt DH, Howard PS, Blyth B, et al. Is lamina propria matrix responsible for normal bladder compliance? J Urol. 1992;148:544.
Farkas A, Skinner DG. Posterior urethral valves in siblings. Br J Urol. 1976;48:76.
Farnham SB, Adams MC, Brock JW, et al. Pediatric urological causes of hypertension. J Urol. 2005;173:697.
Field PL, Stephens FD. Congenital urethral membranes causing urethral obstruction. J Urol. 1974;111:250.
Firlit RS, Firlit CF, King LR. Obstructing anterior urethral valves in children. J Urol. 1978;119:819.
Fisang C, Hauser S, Muller SC. Ureterocystoplasty: an ideal method for vesical augmentation in children. Aktuelle Urologie. 2010;41(Suppl. 1):S50-S52.
Freedman AL, Johnson MP, Smith CA, et al. Long-term outcome in children after antenatal intervention for obstructive uropathies. Lancet. 1999;354:374.
Gearhart JP, Lee BR, Partin AW, et al. A quantitative histological evaluation of the dilated ureter of childhood: II. Ectopia, posterior urethral valves, and the prune belly syndrome. J Urol. 1995;153:172.
Ghanem MA, Nijman RJM. Long-term followup of bilateral high (Sober) urinary diversion in patients with posterior urethral valves and its effect on bladder function. J Urol. 2005;173:1721.
Ghanem MA, Wolffenbuttel KP, De Vylder A, et al. Long-term bladder dysfunction and renal function in boys with posterior urethral valves based on urodynamic findings. J Urol. 2004;171:2409.
Gholdoian CG, Thayer K, Hald D, et al. Applications of the KTP laser in the treatment of posterior urethral valves, ureteroceles, and urethral strictures in the pediatric patient. J Clin Laser Med Surg. 1998;16:39.
Gibbons MD, Horan JJ, Dejter SW, Keszler M. Extracorporeal membrane oxygenation: an adjunct in the management of the neonate with severe respiratory distress and congenital urinary tract anomalies. J Urol. 1993;150:434.
Glassberg KI. The valve bladder syndrome: 20 years later. J Urol. 2001;166:1406.
Glassberg KL, Schneider M, Haller JO, et al. Observations on persistently dilated ureter after posterior urethral valve ablation. Urology. 1982;20:20.
Gleason PE, Kramer SA. Genitourinary polyps in children. Urology. 1994;44:106.
Glick PL, Harrison MR, Adzick NS, et al. Correction of congenital hydronephrosis in utero IV: in utero decompression prevents renal dysplasia. J Pediatr Surg. 1984;19:649.
Glick PL, Harrison MR, Golbus MS, et al. Management of the fetus with congenital hydronephrosis: II. Prognostic criteria and selection for treatment. J Pediatr Surg. 1985;20:376.
Gonzales ET. Posterior urethral valves and bladder neck obstruction. Urol Clin North Am. 1978;3:57.
Gonzalez R, Reinberg Y, Burke B, et al. Early bladder outlet obstruction in fetal lambs induces renal dysplasia and the prune-belly syndrome. J Pediatr Surg. 1990;25:342.
Good CD, Vinnicombe SJ, Minty Il, et al. Posterior urethral valves in male infants and newborns: detection with US of the urethra before and during voiding. Radiology. 1996;198:387.
Greenfield SP. Posterior urethral valves: new concepts [Editorial]. J Urol. 1997;157:996.
Groenewegen AA, Sukhai RN, Nanta J, et al. Results of renal transplantation in boys treated for posterior urethral valves. J Urol. 1993;149:1517.
Gunn TR, Mora JD, Pease P. Antenatal diagnosis of urinary tract abnormalities by ultrasonography after 28 weeks gestation: incidence and outcome. IS J Obstet Gynecol. 1995;172:479.
Gupta RK, Shah HS, Jadhav V, et al. Urethral ratio on voiding cystourethrogram: a comparative method to assess success of posterior urethral valve ablation. J Pediatr Urol. 2009.
Hanlon-Lundberg KM, Verp MS, Loy G. Posterior urethral valves in successive generations. Am J Perinatol. 1994;11:37.
Harrison MR, Nakayamo DK, Rooll R. Correction of congenital hydronephrosis in utero: II. Decompression versus the effects of obstruction on the fetal lung and urinary tract. J Pediatr Surg. 1982;17:965.
Hassan JM, Pope JC, Brock JW, et al. Vesicoureteral reflux in patients with posterior urethral valves. J Urol. 2003;170:1677.
Hayslett JP. Effect of age on compensatory renal growth. Kidney Int. 1983;23:599.
Heikkila J, Rintala R, Taskinen S, et al. Vesicoureteral reflux in conjunction with posterior urethral valves. J Urol. 2009;182(4):1555-1560.
Heikkila J, Taskinen S, Rintala R. Urinomas associated with posterior urethral valves. J Urol. 2008;180:1476.
Heikkila J, Taskinen S, Topari J, Rintala R. Posterior urethral valves are often associated with cryptorchidism and inguinal hernias. J Urol. 2008;180(2):715-717.
Hellström AL, Berg M, Solsnes E. Feeling good in daily life: from the point of view of boys with posterior urethral valves. J Urol. 2006;176:1742.
Hendren WH. Posterior urethral valves in boys. A broad clinical spectrum. J Urol. 1971;106:298.
Hendren WH. Complications of ureterostomy. J Urol. 1978;120:269.
Henneberry MO, Stephens FA. Renal hypoplasia and dysplasia in infants with posterior urethral valves. J Urol. 1980;123:912.
Hoekstra I, Jones B. Duplication of the male urethra: report of three cases. Clin Radiol. 1985;36:529.
Holmdahl G, Sillen U, Hanson E, et al. Bladder dysfunction in boys with posterior urethral valves before and after puberty. J Urol. 1996;155:694.
Holmdahl G, Sillen U, Hellstrom L, et al. Does treatment with clean intermittent catheterization in boys with posterior urethral valves affect bladder and renal function? J Urol. 2003;170:1681.
Holmdahl G, Sillen U. Boys with posterior urethral valves: outcome concerning renal function, bladder function and paternity at ages 31 to 44 years. J Urol. 2005;174:1031.
Holst S, Peterson NE. Fulguration-ablation of atypical accessory urethra. J Urol. 1988;140:347.
Hong AR, Acuna MF, Pena A, et al. Urologic injuries associated with repair of anorectal malformations in male patients. J Surg. 2002;37:339.
Hoover DL, Duckett JW. Posterior urethral valves, unilateral reflux, and renal dysplasia: a syndrome. J Urol. 1982;128:994.
Hulbert WC, Duckett JW. Current views on posterior urethral valves. Pediatr Ann. 1988;17:31.
Hulbert WC, Duckett JW. Posterior urethral valve obstruction [AUA Update Series]. II: Lesson 26. American Urology Association. 1992.
Hulbert WC, Rosenberg HK, Cartwright PC, et al. The predictive value of ultrasonography in evaluation of infants with posterior urethral valves. J Urol. 1992;148:122.
Hutton KAR, Thomas DFM, Arthur RF, et al. Prenatally detected posterior urethral valves: is gestational age at detection a predictor of outcome? J Urol. 1994;152:698.
Indudhara R, Joseph DB, Perez LM, Diethelm AG. Renal transplantation in children with posterior urethral valve revisited: a 10-year follow-up. J Urol. 1998;160:1201.
Jee LD, Rickwood AM, Turnock RR. Posterior urethral valves: does prenatal diagnosis influence prognosis. Br J Urol. 1993;72:830.
Johal NS, Hamid R, Aslam Z, et al. Ureterocystoplasty: long-term results. J Urol. 2008;179:2373.
Johnston JH. Temporary cutaneous ureterostomy in the management of advanced congenital urinary obstruction. Arch Dis Child. 1963;38:161.
Johnston JH. Posterior urethral valves: an operative technique using an electric auriscope. J Pediatr Surg. 1966;1:583.
Johnston JH. Vesicoureteric reflux with urethral valves. Br J Urol. 1979;51:100.
Johnston JH, Kulatilake AE. The sequelae of posterior urethral valves. Br J Urol. 1971;43:743.
Jordan GH, Hoover DL. Inadequate decompression of the upper tracts using a Foley catheter in the valve bladder. J Urol. 1985;134:137.
Kaefer M, Barnewolt C, Retik AB. The sonographic diagnosis of infravesical obstruction in children: evaluation of bladder wall thickness indexed to bladder filling. J Urol. 1997;157:989.
Kaefer M, Peters CA, Retik AB. Increased renal echogenicity: a sonographic sign in differentiating between obstructive and nonobstructive etiologies of in utero bladder distension. J Urol. 1997;158:1026.
Kajbafzadeh AM, Payabvash S, Karimian G. The effects of bladder neck incision on urodynamics abnormalities of children with posterior urethral valves. J Urol. 2008;178:2142.
Kajbafzadeh AM, Quinn FM, Duffy PG, et al. Augmentation cystoplasty in boys with posterior urethral valves. J Urol. 1995;154:874.
Kaplan GW, Scherz HC. Infravesical obstruction. In: Kelalis PP, King LR, Belman AB, editors. Clinical pediatric urology. 3rd ed. Philadelphia: Saunders; 1992:821-864.
Keating MA. The noncompliant bladder: principles in pathogenesis and pathophysiology. Prob Urol. 1994;8:348.
Khoury AE, Houle AM, McLorie GA, et al. Cutaneous vesicostomy effect on bladder’s eventual function. Dial Pediatr Urol. 1990;13:4.
Kim KM, Kogan BA, Massad CA, et al. Collagen and elastin in the obstructed fetal bladder. J Urol. 1991;146:528.
Kim YH, Horwitz M, Combs A. Comparative urodynamic findings after primary valve ablation, vesicostomy, or proximal diversion. J Urol. 1996;156:673.
Kim YH, Horwitz M, Combs AJ, et al. The management of unilateral poorly function kidneys in patients with posterior urethral valves. J Urol. 1997;158:1001.
Kim YH, Horwitz M, Combs AJ, et al. Management of posterior urethral valves on the basis of urodynamic findings. J Urol. 1997;158:101.
Kitchens DM, DeFoor W, Minevich E, et al. End cutaneous ureterostomy for the management of severe hydronephrosis. J Urol. 2007;177:1501-1504.
Klahr S. The modification of diet in renal disease study. N Engl J Med. 1989;320:864.
Kleppe S, Schmitt J, Geipel A, et al. Impact of prenatal urinomas in patients with posterior urethral valves and postnatal renal function. J Perinatal Med. 2006;34:425.
Koff SA, Mutabagani KH, Jananthi VR. The valve bladder syndrome: Pathophysiology and treatment with nocturnal bladder emptying. J Urol. 2002;167:297.
Kolicinski ZH. Foley balloon procedure in posterior urethral valves. Dialogues Pediatr Urol. 1988;11:7.
Koo HP, Bunchman TE, Flynn JT, et al. Renal transplantation in children with severe lower urinary tract dysfunction. J Urol. 1999;161:240.
Krahn CG, Johnston HW. Cutaneous vesicostomy in the young child: indications and results. Urology. 1993;41:558.
Krishnan A, de Souza A, Konijeti R, et al. The anatomy and embryology of posterior urethral valves. J Urol. 2006;175:1214.
Krueger RP, Hardy BE, Churchill BM. Growth in boys with posterior urethral valves. Urol Clin North Am. 1980;7:265.
Landau EH, Jayanthi VR, Khoury AE, et al. Bladder augmentation: ureterocystoplasty versus ileocystoplasty. J Urol. 1994;152:716.
Landers S, Hanson TN. Pulmonary problems associated with congenital renal malformations. In: Gonzales ET, Roth DR, editors. Common problems in pediatric urology. Houston: Mosby-Year Book; 1990:85.
Livne PM, Delaune J, Gonzales ET. Genetic etiology of posterior urethral valves. J Urol. 1983;130:781.
Lome LG, Howat JM, Williams DI. The temporarily defunctionalized bladder in children. J Urol. 1972;107:469.
Maizels M, Stephens FD, King LR, Firlit CF. Cowper’s syringocele: a classification of dilatations of Cowper’s duct based upon clinical characteristics of 8 boys. J Urol. 1983;129:111.
Maruotti GM, Agangi A, Martinelli P, et al. Early prenatal diagnosis of concordant posterior urethral valves in male monochorionic twins. Prenatal Diagnosis. 2006;26:67.
Mayor G, Genton R, Torrado A, Geirgnard J. Renal function in obstructive nephropathy: long-term effects of reconstructive surgery. Pediatrics. 1975;58:740.
McConnell JD. Detrusor smooth muscle development. Dialogues Pediatr Urol. 12, 1989.
Mendizabal S, Estornell F, Zamora I, et al. Renal transplantation in children with severe bladder dysfunction. J Urol. 2005;173:226.
Merrot T, Chaumoitre K, Shojai R, et al. Fetal bladder rupture due to anterior urethral valves. Urology. 2003;61:1259.
Misra D, Chana J, Drake DP, et al. Operative trauma to the genitourinary tract in the treatment of anorectal malformations: 15 years experience. Urology. 1996;47:559.
Misseri R, Combs AJ, Horowitz M, et al. Myogenic failure in posterior urethral valve disease: real or imagined. J Urol. 2002;168:1844.
Mitchell ME. Persistent ureteral dilation following valve resection. Dial Pediatr Urol. 1982;5:8.
Mitchell ME, Close CE. Early primary valve ablation for posterior urethral valves. Semin Pediatr Surg. 1996;5:66.
Mochon M, Kaiser BA, Dunn S, et al. Urinary tract infection in children with posterior urethral valves after kidney transplantation. J Urol. 1992;148:1874.
Morini F, Ilari M, Casati A, et al. Posterior urethral valves and mirror image anomalies in monozygotic twins. Am J Med Genet. 2002;111:210.
Nakayama DK, Harrison MR, de Lorimier AA. Prognosis of posterior urethral valves presenting at birth. J Pediatr Surg. 1986;21:43.
Narasimhan KL, Kaur B, Chowdhary SK, et al. Does mode of treatment affect the outcome of neonatal posterior urethral valves? J Urol. 2004;171:2423.
Narasimhan KL, Mahajan JK, Kaur B, et al. The vesicoureteral reflux dysplasia syndrome in patients with posterior urethral valves. J Urol. 2005;174:1433.
Narborough GC, Elliott S, Minford JE. Congenital stricture of the urethra. Clin Radiol. 1990;42:402.
Nesbit RM, McDonald HPJr, Busby S. Obstructing valves in the female urethra. J Urol. 1964;91:79-84.
Nguyen MT, Pavlock CL, Zderic S, et al. Overnight catheter drainage in children with poorly compliant bladders improves post-obstructive diuresis and urinary incontinence. J Urol. 2005;174:1633.
Nijman RJ, Scholtmeyer RJ. Complications of transurethral electro-incision of posterior urethral valves. Br J Urol. 1991;67:324.
Noe HN, Jerkins GR. Oliguria and renal failure following decompression of the bladder in children with posterior urethral valves. J Urol. 1983;129:595.
Noe HN, Jerkins GR. Cutaneous vesicostomy experience in infants and children. J Urol. 1985;134:301.
Ortolano V, Nasaralloh PF. Urethral duplication. J Urol. 1986;136:909.
Otukesh H, Basiri A, Simfroosh N, et al. Kidney transplantation in children with posterior urethral valves. Pediatr Transplant. 2008;12(5):516-519.
Ozcan O, Tekgul S, Duzova A, et al. How does the presence of urological problems change the outcome of kidney transplantation in the pediatric age group. Transplantation Proc. 2006;38:552.
Parkhouse HF, Barratt TM, Dillon MJ, et al. Long-term outcome of boys with posterior urethral valves. Br J Urol. 1988;62:59.
Parkhouse HF, Woodhouse CR. Long-term status of patients with posterior urethral valves. Urol Clin North Am. 1990;17:373.
Passerini-Glazel G, Araguna F, Chiozza L, et al. The P.A.D.U.A. (Progressive Augmentation by Dilating the Urethra Anterior) procedure for the treatment of severe urethral hypoplasia. J Urol. 1988;140:1247.
Patel KK, Wilcox DT, Samuel M, et al. Management of urinary extravasation in 18 boys with posterior urethral valves. J Urol. 2003;169:1508.
Peters CA, Bolkier M, Bauer SB, et al. The urodynamic consequences of posterior urethral valves. J Urol. 1990;144:122.
Pieretti RV. The mild end of the clinical spectrum of posterior urethral valves. J Pediatr Surg. 1993;28:701.
Podesta ML, Medil R, Castera R, et al. Urethral duplication in children: surgical treatment and results. J Urol. 1998;160:1830.
Podesta M, Ruarte AC, Gargiulo C. Bladder function associated with posterior urethral valves after primary valve ablation or proximal urinary diversion in children and adolescents. J Urol. 2002;168:1830.
Podesta M, Ruarte AC, Gargiulo C, et al. Urodynamic findings in boys with posterior urethral valves after treatment with primary valve ablation or vesicostomy and delayed ablation. J Urol. 2000;164:139.
Quintero RA, Hume R, Smith C, et al. Percutaneous fetal cystoscopy and endoscopic fulguration of posterior urethral valves. Am J Obstet Gynecol. 1995;172:206.
Raviv G, Leibovitch I, Hanani J, et al. Hematuria and voiding disorders in children caused by congenital urethral polyps: principles of diagnosis and management. Eur Urol. 1993;23:382.
Rawat J, Khan TR, Singh S, et al. Congenital anterior urethral valves and diverticula: diagnosis and management in six cases. Afr J Paediatr Surg. 2009;6(2):102-105.
Reinberg Y, deCastano I, Gonzalez R. Influence of initial therapy on progression of renal failure and body growth in children with posterior urethral valves. J Urol. 1992;148:532.
Reinberg Y, deCastano I, Gonzalez R. Prognosis for patients with prenatally diagnosed posterior urethral valves. J Urol. 1992;148:125.
Reinberg Y, Gonzalez R, Fryd D, et al. The outcome of renal transplantation in children with posterior urethral valves. J Urol. 1988;140:1491.
Rittenberg MH, Hulbert WC, Snyder HM, Duckett JW. Protective factors in posterior urethral valves. J Urol. 1988;140:993.
Robertson WB, Hayes JA. Congenital diaphragmatic obstruction of the male posterior urethra. Br J Urol. 1969;41:592.
Robyr R, Benachi A, Daikha-Dahmane F, et al. Correlation between ultrasound and anatomic findings in fetuses with lower urinary tract obstruction in the first half of pregnancy. Ultrasound Ob Gyn. 2005;25:478.
Rosenfeld B, Greenfield SP, Springate JE, Feld LG. Type III posterior urethral valves: presentation and management. J Pediatr Surg. 1994;29:81.
Ross JH, Kay R, Novick AC, et al. Long-term results of renal transplantation into the valve bladder. J Urol. 1994;151:1500.
Rushton HG, Parrott TS, Woodard JR, Walther M. The role of vesicostomy in the management of anterior urethral valves in neonates and infants. J Urol. 1987;138:107.
Salle JL, Sibai H, Rosenstein D, et al. Urethral duplication in the male: review of 16 cases. J Urol. 2000;163:1936.
Salomon L, Fontaine E, Guest G, et al. Role of the bladder in delayed failure of kidney transplants in boys with posterior urethral valves. J Urol. 2000;163:1282.
Sanders RC, Nussbaum AR, Solez R. Renal dysplasia: sonographic findings. Radiology. 1988;167:623.
Sarhan O, El-Dahshan K, Sarhan M. Prognostic value of serum creatinine levels in children with posterior urethral valves treated by primary valve ablation. J Pediatr Urol. 2009.
Sarhan O, Zaccaria I, Macher MA. Long-term outcome of prenatally detected posterior urethral valves: single center study of 65 cases managed by primary valve ablation. J Urol. 2008;179:307.
Schrober JM, Dulabon LM, Woodhouse CR. Outcome of valve ablation in late-presenting posterior urethral valves. Br J Urol. 2004;94:616.
Scherz HC, Kaplan GW, Packer MG. Anterior urethral valves in the fossa navicularis in children. J Urol. 1987;138:1211.
Scott JES. Management of congenital posterior urethral valves. Br J Urol. 1985;57:71.
Seikaly M, Ho PL, Emmett L, et al. The 12th annual report of the North American Pediatric Renal Transplant Cooperative Study: renal transplantation from 1987 through 1998. Pediatr Transplant. 2001;5:215.
Sharma SK, Kapoor R, Kumar A, Mandal AK. Incomplete epispadiac urethral duplication with dorsal penile curvature. J Urol. 1987;138:585.
Sheldon CA, Churchill BM, McLorie GA, et al. Evaluation of factors contributing to mortality in pediatric renal transplant recipients. J Pediatr Surg. 1992;27:629.
Skolder AJ, Maizels M, Depp R, et al. Caution in antenatal intervention. J Urol. 1988;139:1026.
Smith GHH, Canning DA, Schulmon SL, et al. The long-term outcome of posterior urethral valves treated with primary valve ablation and observation. J Urol. 1996;155:1730.
Smith GHH, Duckett JW. Urethral lesions in infants and children. In: Gillenwater JY, Grayhack JT, Howards SS, Duckett JW, editors. Adult and pediatric urology. 3rd ed. St. Louis: Mosby-Year Book; 1996:2411.
Sober I. Pelvioureterostomy-en-Y. Urology. 1972;107:473.
Stephens FD. Congenital malformations of the urinary tract. New York: Praeger; 1983. 96, 103
Tanagho EA. Congenitally obstructed bladders: fate after prolonged defunctionalization. J Urol. 1974;111:102.
Tank ES. Anterior urethral valves resulting from congenital urethral diverticula. Urology. 1987;30:467.
Taskinen S, Heikkila J, Rintala R. Posterior urethral valves: primary voiding pressures and kidney function in infants. J Urol. 2009;182(2):699-702. discussion 702–3
Tejani A, Butt K, Glassberg K, et al. Predictors of eventual end stage renal disease in children with posterior urethral valves. J Urol. 1986;136:857.
Thomas DFM, Gordon AC. Management of prenatally diagnosed uropathies. Arch Dis Child. 1989;64:58.
Tietjen DN, Gloor JM, Husmann DA. Proximal urinary diversion in the management of posterior urethral valves: is it necessary? J Urol. 1997;158:1008.
Van Savage JG, Khoury AE, McLorie GA. An algorithm for the management of anterior urethral valves. J Urol. 1997;158:1030.
Walker RD, Padron M. Management of posterior urethral valves by initial vesicostomy and delayed valve ablation. J Urol. 1990;144:1212.
Walsh IK, Keane PF, Herron B. Benign urethral polyps. Br J Urol. 1993;72:937.
Warshaw BL, Hymes LC, Trulock TS, et al. Prognostic features in infants with obstructive uropathy due to posterior urethral valves. J Urol. 1985;133:240.
Watts SH. Urethral diverticula in the male. Johns Hopkins Hosp Rep. 1906;13:49.
Weber S, Mir S, Schlingmann KP, et al. Gene locus ambiguity in posterior urethral valves/prune-belly syndrome. Pediatr Nephrol. 2005;20:1036.
Whitaker RH. The ureter in posterior urethral valves. Br J Urol. 1973;45:395.
Whitaker RH, Sherwood T. An improved hook for destroying posterior urethral valves. J Urol. 1986;135:531.
Williams DI. Congenital valves in the posterior urethra. Br Med J. 1954;1:623.
Williams DI, Cromie WJ. Ring ureterostomy. Br J Urol. 1975;47:789.
Williams DI, Retik AB. Congenital valves and diverticula of the anterior urethra. Br J Urol. 1969;41:228.
Woolf AS, Winyard PJ. Gene expression and cell turnover in human renal dysplasia. Histol Histopathol. 2000;15:159.
Woodhouse CR, Williams DI. Duplications of the lower urinary tract in children. Br J Urol. 1979;51:481.
Young HH, Frontz WA, Baldwin JC. Congenital obstruction of the posterior urethra. J Urol. 1919;3:289.
Youssef AH, Fath-Alla M, El-Kassaby AW. Perineal subcutaneous dartos pedicled flap as a new technique for repairing urethrorectal fistula. J Urol. 1998;161:1498.
Youssif M, Dawood W, Shabaan S, et al. Early valve ablation can decrease the incidence of bladder dysfunction in boys with posterior urethral valves. J Urol. 2009;182(4 Suppl.):1765-1768.
Zaontz MR, Firlit CF. Percutaneous antegrade ablation of posterior urethral valves in premature or underweight term neonates: an alternative to primary vesicostomy. J Urol. 1985;134:139.
Zaragoza MR, Ritchey ML, Bloom DA, McGuire EJ. Enterocystoplasty in renal transplantation candidates: urodynamic evaluation and outcome. J Urol. 1993;150:1463.