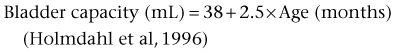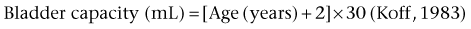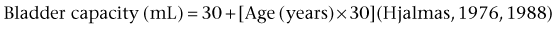chapter 127 Non-Neuropathic Dysfunction of the Lower Urinary Tract in Children
Our increasing understanding and recognition of non-neuropathic lower urinary tract dysfunction as a cause of various urologic disorders in childhood has had a profound influence on our management strategies over the past decade. What was known as primary or idiopathic, such as primary vesicoureteral reflux (VUR) or primary nocturnal enuresis, is often associated with underlying bladder dysfunctions evident on urodynamics and resolves with successful correction of the bladder physiology (Koff and Murtagh, 1983; Homsy et al, 1985; Watanabe et al, 1994; Yeung et al, 1998, 1999). Conversely, failure of recognition and treatment of the associated problem can result in persistence or even further deterioration.
The understanding of the spectrum of dysfunction of the bladder and even of normal bladder physiology in infants and young children is complex. Not only are the dynamics and functional disturbances of the lower urinary tract different from those in adults, but the evolution in normal bladder-sphincteric function during growth and maturation in children poses continuous changes (Yeung, 1995; Yeung et al, 1995; Sillen et al, 1996; Holmdahl, 1997). More confusingly, one type of bladder dysfunction may often progress with time and evolve imperceptibly into another, without a sharp distinction between the different stages. This is further confounded by a lack of age- and sex-specific normal reference values for various urodynamic parameters, especially for the very young age groups (Yeung et al, 1995).
Recent advances and development in the use of urodynamic techniques specially designed for infants and young children have allowed more accurate assessment of bladder function and provided much better understanding of bladder pathophysiology. This has allowed for better recognition of functional disorders, as well as to provide a scientific basis for their therapy (Hjalmas, 1988; Perez et al, 1992; Bauer, 1997; Nevéus et al, 2007). In view of the important association of bladder dysfunctions with various common urologic disorders, urologists should be acquainted with the spectrum of dysfunction and the techniques to facilitate proper diagnosis and treatment.
Normal Bladder Function in Infants and Children
Anatomy of Bladder
The bladder is a unique organ of the human body in that it not only carries a dual function of both storage and emptying of urine but also has a complex innervation of voluntary and involuntary control of function. Understanding of the functional anatomy of the lower urinary tract stems from extensive postmortem studies carried out over decades (Gosling et al, 1981; Gosling, 1985; DeLancey, 1988; de Groat, 1993; Zvara et al, 1994).
The bladder is an abdominal organ, and when full it can be readily palpable in infants and young children due to a shallow pelvis (Wiegel, 1990). The bladder wall consists of three layers: mucosa, detrusor, and adventitia. The detrusor consists of a meshwork of smooth muscle fibers arranged into a single functioning unit with an ability to elicit nearly maximum active tension over a wide range of length. This allows the bladder to fill with urine from the upper tract at low pressures (compliance) (Mattiasson, 1994). The ability of the bladder to store urine (reservoir function) is determined by the concomitant activity of the detrusor muscle and the bladder outlet (consisting of the bladder neck, proximal urethra, and striated muscle of the pelvic floor) (de Groat, 1993).
The bladder sphincter (external and internal) plays a major role in urinary continence by closure of the bladder neck and proximal urethra. The anatomy of the external urinary sphincter consists of a cylindrical structure, which is accentuated anteriorly and thinned out or actually absent posteriorly, thus giving a characteristic horseshoe or omega shape on cross section. It has an inner layer of smooth muscle and an outer layer of striated muscle, extending from the apex of the prostate to invest the length of the membranous urethra in males. In the females this is less well developed and extends from the bladder neck to the midurethra. The internal sphincter, however, has not been well delineated anatomically. It has generally been accepted that it consists of smooth muscle fibers continuing from the bladder base and trigone, which traverse inferiorly through the bladder neck to extend toward the proximal urethra. Its existence has been better delineated on radiologic and urethral pressure measurement studies. During micturition, the bladder base, bladder neck, and proximal urethra can be shown to contract simultaneously as a unit, producing a funneling effect that opens up the bladder outlet with initiation of voiding.
Little, too, is known about the natural course of development or maturation of the structure and function of the sphincter mechanism. Literature suggests that immature detrusor-sphincter coordination, manifested as detrusor hypercontractility and interrupted voiding, commonly occurs in the first 1 to 2 years of life, causing some degree of functional bladder outflow obstruction (Sillen et al, 1992; Yeung et al, 1998). In a postmortem study of the ontogeny of the external urinary sphincter in human fetuses, infants, and young children, Kokoua and Homsy found significant age-related differences in the histologic structure of the sphincter as compared with that in adults. Striated muscle fibers of the sphincter first appeared at around 20 weeks of gestation and then became arranged in a concentric pattern as a closed ring, fused posteriorly to form a tail-like structure that was directed to the perineal body. Posterior splitting of the striated sphincter, starting first caudally and progressively in a cephalad manner, then occurred during the first year of life, coinciding in parallel with gradual resorption of the “tail,” to eventually giving way to a mature omega-shaped structure (Kokoua et al, 1992). As a complete closed ring of striated sphincteric muscle was present up to 1 year of age in more than 40% of cases, it may well be conjectured that this could be related to the high intravesical pressures and interrupted voiding that are commonly observed during urodynamic studies in infants (Sillen et al, 1992; Yeung et al, 1995, 1998).
Innervation of Bladder
Activation, coordination, and integration of various parts of the bladder-sphincteric complex involve both the central somatic and autonomic nervous systems through three sets of peripheral nerves: sacral parasympathetic (pelvic nerve), thoracolumbar sympathetic (hypogastric nerves and sympathetic chain), and sacral somatic nerves (primarily the pudendal nerve) (Fig. 127–1) (deGroat, 1993; Mattiasson, 1994).
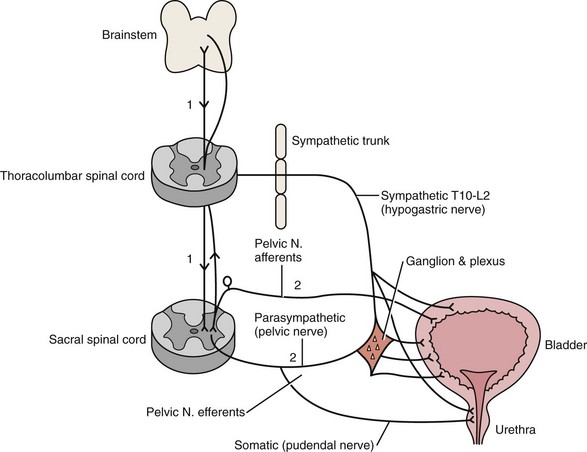
Figure 127–1 Diagram to illustrate the innervation of the bladder-sphincter complex.
(From Yeung CK, Barker GM, Läckgren G. Pathophysiology of bladder dysfunction. In: Gearhart JP, Rink RC, Mouriquand PDE, editors. Pediatric urology. 2nd ed. Philadelphia: Elsevier Saunders; 2010. p. 353–65.)
Parasympathetic nerve fibers run in the pelvic nerve (S2-S4) to supply the pelvic and vesical plexuses before entering the bladder. Parasympathetic ganglia are found within these plexuses and in the bladder wall. Sympathetic nerves arise from segments T10-L2 of the spinal cord and go to the inferior mesenteric ganglion through the sympathetic trunk. From the inferior mesenteric ganglion the nerve fibers pass to the pelvic plexus and bladder through the hypogastric nerves. There is also sympathetic innervation originating from T10-L2 supplying the detrusor and urethral sphincter (Bradley et al, 1974). The somatic nervous system (pudendal nerve) supplies the periurethral pelvic floor musculature (Mattiasson, 1994). The sensory and motor nerves carried by all three nerves innervate both the bladder and urethral sphincter. They originate from parasympathetic ganglia located in the second, third, and fourth segments of the sacral spinal cord (Bradley et al, 1974). Within the spinal cord, information from bladder afferents is integrated with that from other viscera and somatic sources and projected to the brainstem centers that coordinate the micturition cycle (Harrison and Abrams, 1994).
Development of Normal Bladder Function and Micturition Control
Urodynamic studies on normal infant bladders have shown that bladder function in young children is different from that in adults. During the first 2 to 3 years of life there is progressive development from an initially indiscriminate infantile voiding pattern to a more socially conscious and voluntary or adult type of micturition. This is achieved through an active learning process whereby the child acquires the ability to voluntarily inhibit or initiate voiding at socially convenient times. This natural evolution of bladder control entails an intact nervous system and depends on at least three main events occurring in parallel: (1) a progressive increase in bladder functional storage capacity; (2) maturation of voluntary control over the urethral striated muscle sphincter; and, perhaps most importantly, (3) development of direct volitional control over the bladder-sphincteric unit so that the child can voluntarily initiate or inhibit the micturition reflex. This process can also be influenced by an awareness of the accepted social norms in families during toilet training (Yeung et al, 2010).
Change in Bladder Functional Parameters
Voiding Frequency
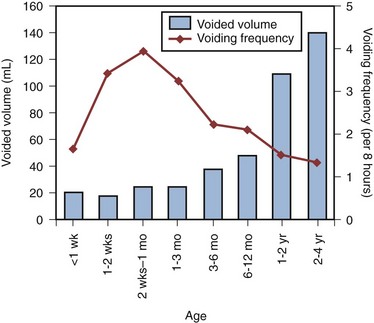
During the third trimester of pregnancy, the fetus is voiding at the rate of approximately 30 times every 24 hours (Goellner et al, 1981). However, immediately after birth, this drops dramatically for the first few days of life, only to increase again after the first week to reach a peak by weeks 2 to 4 to an average of once per hour. Subsequently this rate declines again to about 10 to 15 times per day between 6 and 12 months and to about 8 to 10 times per day by 2 to 3 years (Goellner et al, 1981; Yeung, 1995; Holmdahl et al, 1996). This reduction in voiding frequency observed during the first few years of life appears to be related mainly to an increase in bladder capacity in parallel to body growth, which is proportionately greater than simultaneous increase in urine volume production (Yeates, 1973; Koff, 1997). By the age of 12, the voiding pattern is similar to that in an adult and usually comprises 4 to 6 voids per day (Fig. 127–2).
Bladder Capacity, Voided Volume, and Emptying Efficiency
The increase in bladder capacity with the growth of the child is a crucial step in the development of bladder function and urinary continence. An adequate reservoir function for urine storage is necessary to meet the increased rate of urine production and decreased voiding frequency in the growing child.
Several studies have shown that functional bladder capacity at a certain age can be accurately estimated and expressed as a function of age with no difference in sex. For young infants it can be expressed as:
For older children, one of the most widely accepted formulas is the Koff formula:
Or similarly, the Hjalmas formula follows:
In parallel to the increase in bladder capacity, the mean voided volume of each micturition increases with age. Of note, urodynamic studies have shown that a significant proportion of infants with incomplete maturation of detrusor-sphincter coordination before the age of 1 are still able to achieve satisfactory bladder emptying (>80% efficacy) (Yeung, 1995; Yeung et al, 1995, 1998; Holmdahl et al, 1996; Bachelard et al, 1999; Sillen et al, 2000).
Detrusor Pressure at Voiding
Studies on detrusor pressures at voiding in normal infants are limited due to (1) the technical difficulties involved in performing urodynamic studies in young infants and (2) an ethical consideration in justifying doing so. From the data in a natural filling cystometric study of infants with normal lower urinary tracts (as indicated by a normal micturating cystourethrogram) and who had undergone either dismembered pyeloplasty for pelviureteric junction obstruction or nephrectomy for dysplastic kidney, the authors have documented significantly higher maximum detrusor pressures with micturition (Pdetmax) than in normal adults. It was also noted that male infants voided with significantly higher pressures than females (mean Pdetmax: 118 versus 75 cm H20 respectively, P < .03) (Yeung et al, 1995a, 1995b, 1998). Similar findings were reported in healthy, asymptomatic infant siblings of children with VUR (Bachelard et al, 1999).
Studies have also shown that these high detrusor pressures noted during micturition were mainly observed only during the first year of life and decreased progressively with age. Furthermore, an interrupted or “staccato” type of urinary stream was noted in more than half of the patients (Yeung et al, 1995a, 1995b, 1998). This was demonstrated by fluctuations of the detrusor pressure when it reached maximum during voiding and resumption of urinary stream in conjunction with a sharp fall in the detrusor pressure. The high detrusor pressures during voiding are thought to represent variations among individual infants in the maturation process of detrusor and sphincter coordination during the first 1 to 2 years of life (Yeung et al, 1995, 1998; Holmdahl et al, 1996; Bachelard et al, 1999; Sillen et al, 2000).
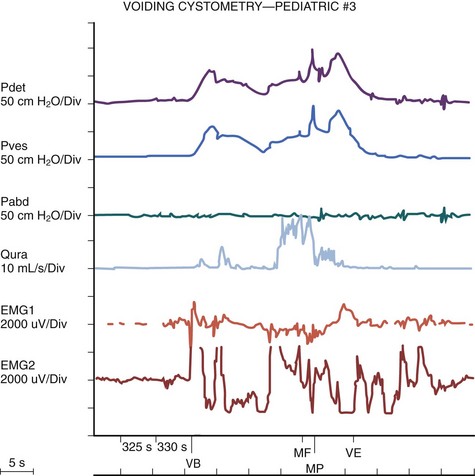
The authors have further confirmed this finding using video cystometry under fluoroscopy combined with natural fill urodynamics and perineal electromyography (EMG) in infants with a history of urinary tract infections (UTIs). Periods of increase in perineal or sphincteric EMG activities were noted during voiding and associated with a sudden cessation of urinary flow with a simultaneous isometric rise or high peak of detrusor pressure. In contrast, resumption of urinary flow was associated with relaxation of the external urinary sphincter and a paradoxical drop in detrusor pressure. Also, the detrusor pressure associated with the initiation of urinary flow was usually significantly lower than the maximal detrusor pressure during micturition (Pdetmax) and that the Pdetmax was significantly higher than those recorded in normal adults (Fig. 127–3).
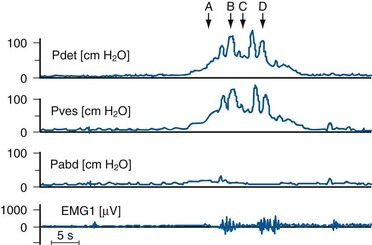
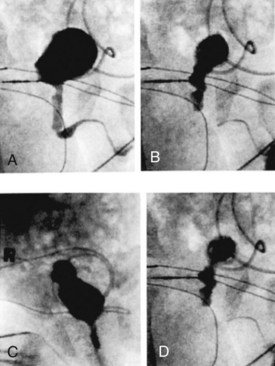
Figure 127–3 Interrupted voiding pattern due to detrusor-sphincter discoordination as revealed by urodynamic study with simultaneous perineal electromyographic (EMG) monitoring and videocystourethrography. Arrows with letters in top graph correspond to urethrograms at bottom. A, Urinary flow started. B, Premature sphincteric contraction and urethral closure as evidenced by the urethrogram and increase in sphincteric EMG activities, leading to a sharp spike of detrusor pressure (isometric increase) associated with a paradoxical abrupt cessation of urinary flow. C, Urinary flow resumed in parallel with sphincter relaxation. D, Repeated sphincteric contraction leading to another sharp increase in detrusor pressure and paradoxical interruption of urinary flow. P abd, abdominal pressure; Pdet, detrusor pressure; Pves, intravesical pressure.
(From Yeung CK, Barker GM, Läckgren G. Pathophysiology of bladder dysfunction. In: Gearhart JP, Rink RC, Mouriquand PDE, editors. Pediatric urology. 2nd ed. Philadelphia: Elsevier Saunders; 2010. p. 353–65.)
Evolution of Normal Micturition Control
Traditionally, it has been assumed that micturition in newborns and young infants occurs automatically with a full bladder by a simple spinal cord reflex, with little or no mediation by the higher neural centers, and that with progressive maturation, voluntary inhibition of the bladder emptying reflex is achieved by adulthood. A delay in the normal maturation of bladder control was attributed to certain conditions such as primary nocturnal enuresis and hence the traditional belief that all enuretics would get better with age (Nash, 1949). However, more recent studies have indicated that this is an oversimplification of what actually occurs. Even in full-term fetuses and newborns, it has been shown that micturition is modulated by higher centers. Ohel and colleagues (1995) showed that intrauterine micturition almost exclusively occurs while the fetus is awake rather than randomly distributed over various behavioral (sleep/arousal) states. Furthermore, it has been observed that micturition in a full-term fetus can be elicited by vibroacoustic stimulation, all of which indicates that the micturition reflex is probably under higher neural control even at near gestational term (Zimmer et al, 1993). Further extensive modulation occurs during the postnatal period.
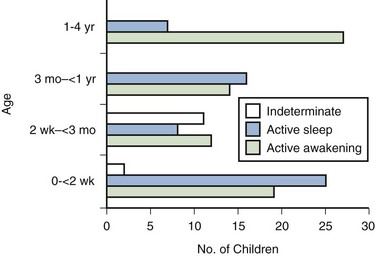
Studies on normal neonates using ambulatory bladder monitoring techniques in conjunction with polysomnographic recordings have shown that even in newborns, micturition does not occur during sleep (Yeung et al, 1995). During sleep the bladder is normally quiescent and stable with lack of facilitation of detrusor contractions, whereas during wakefulness marked detrusor overactivity is observed. Clear electroencephalographic (EEG) evidence of cortical arousal or actual awakening occurs in response to bladder distension, and sleeping infants are noted to wake up before bladder activity returns and voiding occurs. However, this arousal period may often be transient with the infant crying or moving for a brief period, micturating, and then going back to sleep without being noticed to have awakened. This wakening response to bladder distension probably involves more complicated neural pathways and higher centers than has been appreciated up until now (Fig. 127–4).
These results also correlate with recent animal studies showing a sophisticated integration of preexisting central and peripheral neural pathway in micturition control at birth with remodulation occurring in the early postnatal period (Maggi et al, 1986; Thor et al, 1989). Extensive studies using experimental animals by de Groat and colleagues (1993, 1998) have indicated that early postnatal maturation of bladder function probably occurs at different levels: (1) changes in the properties of detrusor muscle; (2) developmental modifications in the peripheral innervation of the bladder; and (3) alterations in central synaptic circuitry and neuroplasticity in the parasympathetic reflex pathways to the bladder (Sugaya and de Groat, 1994; Araki and de Groat, 1997; Sugaya et al, 1997). Recordings of spontaneous activity in bladder smooth muscle in neonatal rats showed much larger amplitude and more synchronous rhythmic contractions compared with those observed in adult rats (Sugaya and de Groat, 1994). This suggests that there is a progressive reduction in intercellular communication between detrusor smooth muscle cells, resulting in less spontaneous activities and hence more efficient urine storage, during early postnatal development. In addition, peripheral and central neural mechanisms also change extensively during this period. In cats (and some other species) micturition during the newborn period is dependent on an exteroreceptive somatovesical reflex triggered when the mother licks the perineum of the kittens (de Groat et al, 1993, 1998; Araki and de Groat, 1997). This somatovesical reflex, processed in the sacral spinal cord, disappears in older animals but may reappear after spinal cord injury. Further neuroanatomic studies have indicated that spinal bladder reflexes are mediated via interneurons located immediately adjacent to, and synapsing with, the sacral preganglionic neurons (Sugaya et al, 1997). This interneuron–preganglionic neuron synaptic transmission is efficient immediately after birth but is abruptly downregulated during the third postnatal week when the mature supraspinal micturition reflexes start to appear (Araki and de Groat, 1997). Transection of the spinal cord prevents this downregulation, indicating that the higher neural centers play an important role in this synaptic remodeling, which contributes to postnatal development of micturition reflexes.
During the second or third year of life there is progressive development toward a socially conscious continence and a more voluntary or adult type of micturition control. The child becomes more aware of the sensation of bladder distension and the urge to urinate, as well as the social norm and embarrassment associated with urinary incontinence. Through an active learning process, the child acquires the ability to voluntarily inhibit and delay voiding until a socially convenient time and then actively initiate urination even when the bladder is not very full and allow it to proceed to completion. This natural evolution of micturition control mechanisms depends on an intact neural pathway and awareness of social norms, as well as multiple factors including the gradual increase in functional bladder capacity, maturation of detrusor-sphincter coordination, and progressive development of voluntary control over the whole bladder-sphincteric-perineal complex. The final steps are usually achieved at around the age of 3 to 4 years, when most children have developed the adult pattern of urinary control and will be dry both day and night. The child has learned to inhibit a micturition reflex, postpone voiding, and voluntarily initiate micturition at socially acceptable and convenient times and places. This development of continence and voluntary micturition is also dependent on behavioral learning and can be influenced by toilet training, which in turns depends on the cognitive perception of the maturing urinary tract. It is understandable therefore that this series of complex events is highly susceptible to the development of various types of dysfunctions.
Neurologic control of normal micturition occurs at different levels of the central nervous system (CNS), from the spinal cord with the “sacral micturition center,” to the brainstem with the “pontine micturition center,” the cerebellum, basal ganglia, limbic system, thalamus and hypothalamus, and cerebral cortex (Blaivas, 1982; McLorie and Husman, 1987; de Groat, 1993; Fernandes et al, 1994). It should be noted that the bladder is unique among other visceral organs in that its function is under the control of both the somatic and the autonomic nervous system. Besides acetylcholine and norepinephrine, various other neurotransmitters including prostaglandin substance P, opioid peptides, vasoactive intestinal peptide, and neuropeptide Y are involved during bladder stimulation (Fernandes et al, 1994). Simple manipulation of adrenergic and cholinergic receptors may only partially abolish the effect of neural stimulation, which explains why pharmacologic blockage of the classic neurotransmitters (acetylcholine and norepinephrine) alone may fail to elicit the expected full clinical response.
Transitory Detrusor-Sphincter Discoordination in Infancy
Studies have shown that in making the transition from an infantile to an adult pattern of micturition control, all children may transiently display some degree of abnormal bladder-sphincteric function (Koff, 1997). For instance, it has been clearly shown that a significant proportion of normal infants exhibit prominent detrusor-sphincter discoordination and interrupted voiding during the first 1 to 2 years of life. This is manifested by a discoordinated and interrupted urine flow, which may even be brought to a complete stop for 1 to 2 minutes before restarting, producing a pattern of repeated small voidings in quick succession (Yeung et al, 1995a, 1995b, 1998). Urodynamic findings show association with high voiding pressures and interruption of flow but no impairment of overall bladder emptying. However, this type of dysfunction resolved with a period of successful toilet training and was only transitory or intermittent and did not persist. One must therefore be cautious in the assessment of young children with apparent voiding dysfunctions and resist the temptation to overinterpret intermittent or transient symptoms as pathologic and hence overinvestigate. However, if voiding dysfunctions are persistent well beyond the period of toilet training, especially if associated with urinary complications like recurrent urosepsis, then the possibility of underlying anatomic and neurologic causes must be considered and duly evaluated.
Key Points: Normal Bladder Function and Development in Infants and Children
Epidemiology and Classification of Non-Neuropathic Bladder-Sphincter Dysfunction in Children
Epidemiology and Prevalence
Non-neuropathic bladder-sphincter dysfunction in children is probably more common than what meets the eye, but many times it only presents to us when UTI, VUR, or urinary incontinence is manifested. It has been reported that 15% of 6-year-old children suffer this condition (Hoebeke, 2002).
Categorization of Non-Neuropathic Bladder-Sphincter Dysfunction in Children
Various conditions have been described under the category of non-neuropathic bladder-sphincter dysfunction, but it must be emphasized that these conditions should not be viewed rigidly as separate and distinct entities but rather as transitional phases of a complex sequence of events. For instance, a girl with dysfunctional voiding may start with having detrusor instability associated with sphincter and pelvic floor overactivity, then gradually evolve to develop fractionated voiding with increasing postmicturition residues, and finally develop bladder decompensation and the “lazy bladder” syndrome (van Gool et al, 1992). It must also be emphasized that use of the term “non-neuropathic” is based purely on the fact that no obvious and identifiable neurologic lesions can be identified. However, conditions like the urofacial syndrome complex (Ochoa syndrome) and the Hinman syndrome behave almost identically to the typical neuropathic bladder-sphincter dysfunctions. It is indeed conceivable that they do have an organic underlying neurologic cause, although the exact neuroanatomic lesion has not yet been identified. Hence the distinction between neuropathic and non-neuropathic bladder dysfunctions may not be as clear as traditionally thought.
In adults, lower urinary tract function has been well understood and standardization of terminology has been established by a committee working under the International Continence Society (ICS) since the early 1970s (Bates et al, 1976a, 1976b, 1979). In contrast, neural control over the bladder-sphincter unit in children is age dependent and is much more variable and complex. Definitions of normal versus abnormal lower urinary tract function have therefore been far less standardized. Various classifications for bladder dysfunctions in children have been described over the past few decades (Bellinger, 1996; Lapides, 1970; Wein, 1998). To avoid confusion, the classification proposed by the International Children’s Continence Society in 2007, with a standardized set of terminology and definitions for different lower urinary tract dysfunctions, was adopted (Nevéus et al, 2007).
Etiologic Classification of Bladder Dysfunction
Dysfunction of the lower urinary tract in children can be secondary to derangement of nervous control, disorders of detrusor and sphincteric muscle function, structural abnormalities, and other unclassified conditions (Nevéus et al, 2007).
Derangement of Nervous Control Conditions
Disorders of Detrusor and Sphincteric Muscle Function
Functional Classification of Bladder Dysfunction
This is based on the functional state of the bladder-sphincteric complex with respect to detrusor activity, bladder sensation, bladder compliance and capacity, and urethral function both during the filling and the voiding phase of cystometry (Fig. 127–5) (Nevéus et al, 2007).
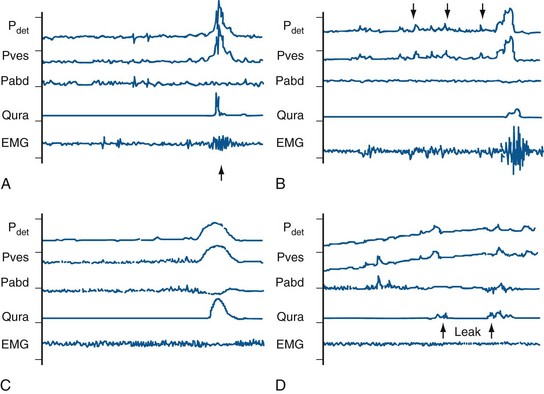
Figure 127–5 Various bladder functional patterns. A, Dyssynergic patterns with an increase in sphincteric electromyographic activities during micturition (arrow), leading to an interrupted urinary flow. B, Overactive bladder, characterized by frequent unstable detrusor contractions (arrows) during the filling phase. C, Normal female micturition pattern; note the pelvic floor relaxation as evidenced by a drop in abdominal pressure during micturition. D, Bladder with low (poor) compliance as evidenced by a rapid increase in intravesical pressure during filling, often associated with urinary leakage (arrows). EMG, electromyogram; Pabd, abdominal pressure; Pdet, detrusor pressure; Pves, intravesical pressure; Qura, uroflow.
(From Yeung CK, Barker GM, Läckgren G. Pathophysiology of bladder dysfunction. In: Gearhart JP, Rink RC, Mouriquand PDE, editors. Pediatric urology. 2nd ed. Philadelphia: Elsevier Saunders; 2010. p. 353–65.)
During the Filling (Storage) Phase
Detrusor Activity
Normal bladder volume increases during filling without a significant rise in detrusor pressure or involuntary phasic contractions despite provocation.
Overactive bladder is characterized by phasic involuntary detrusor contractions, which may occur either spontaneously or on provocation. The exact pathogenesis for detrusor overactivity has not been resolved. Various underlying causes have been proposed: (1) failure of maturation of CNS control mechanisms; (2) low detrusor levels of inhibitory vasoactive intestinal peptide; (3) abnormal enkephalin-mediated inhibitory neurotransmission; (4) denervation hypersensitivity; and (5) alterations in the properties of the detrusor myocytes (Wein and Barrett, 1992; Harrison and Abrams, 1994; Brading, 1997). Detrusor overactivity was frequently found in children with both diurnal and nocturnal enuresis (Bauer et al, 1980; Nevéus et al, 2007). However, the precise relationship between detrusor overactivity contraction exhibited on a cystometrogram and different types of urinary incontinence is still debated (Nevéus et al, 2007). Children with an unstable bladder typically present with a sudden urge to void that can be only be partially suppressed. Often a girl with an unstable bladder will squat down on the heel of one foot, demonstrating the pathognomonic sign of Vincent curtsy.
Detrusor overactivity may also be qualified when possible according to cause into neurogenic detrusor overactivity (formerly called detrusor hyperreflexia) when there is objective evidence of a relevant neurologic disorder.
Urethral Function
Normal positive urethral closure pressure is maintained during filling, even in the presence of increased abdominal pressure (guarding reflex), but it decreases immediately before micturition to allow flow.
Incompetent leakage of urine occurs in the absence of detrusor contraction despite urethral closure.
During the Voiding (Emptying) Phase
Detrusor Activity
Normal
Voiding is achieved by voluntarily initiated detrusor contraction that is sustained and cannot usually be suppressed voluntarily until at least after the age of 4 years. Complete bladder emptying is achieved in the absence of bladder outlet obstruction.
Underactive
Detrusor contraction is of inadequate magnitude and/or duration to effect bladder emptying with a normal time span. The term should be reserved for describing detrusor activity during micturition because it is not readily visible during the filling phase of the cystometrogram. It is often observed in the overdistended postobstructive bladder.
Urethral Function
This may be due to sphincteric overactivity or mechanical obstruction due to obstructive urethral lesions such as congenital obstructive posterior urethral membrane or syringoceles (Dhillon et al, 1993; Dewan and Goh, 1995).
Dysfunctional Elimination Syndrome
The association of constipation with urologic pathologies has been described in the literature since the 1950s, but it was only over the past decade that clinicians have paid more attention to this relationship and recognized its existence with the term dysfunctional elimination syndrome (DES). This term is used to reflect the broad spectrum of functional disturbances that may affect the urinary tract, including that of functional bowel disturbances, and can be classified as follows:
Koff and colleagues (1998) showed that the pattern of DES significantly influenced clinical outcome of ureteric reimplantation surgery for VUR in children. Children with constipation had the highest likelihood of developing a breakthrough UTI and thus required surgery. DES also had an adverse effect on the rate of spontaneous reflux resolution, with children requiring an average of 1.6 years longer to outgrow reflux than children without DES. For those requiring surgery, both the results and postoperative complications seemed to be negatively influenced by DES. Postoperative UTIs developed in 78% of children with DES. All children who had persistent reflux, recurrent reflux after 2 years, and contralateral reflux after unilateral reimplantation had DES.
Loening-Baucke (1997) looked at resolution of urinary incontinence and UTIs in children after treatment of their constipation. This study showed that after successful treatment of constipation, 89% of those with daytime incontinence and 63% with nighttime incontinence became dry. None of the children who had UTI had recurrence after treatment of constipation.
It therefore seems from these data that management of the underlying dysfunction should be given priority in the treatment protocol of children with conditions such as VUR, incontinence, and UTI because successful management may significantly improve outcome. Thus more attention should be paid to evaluate for the presence of functional bowel disturbances even in cases of urologic pathologies.
Bladder-Sphincter Dysfunction during Bladder Filling
Overactive Bladder, Urge Syndrome, and Urge Incontinence
Overactive bladder (OAB) is the most common term currently used in clinical medicine to describe a complex of lower urinary tract symptoms (LUTS). Traditionally, the infant bladder has been described as unstable (replaced with OAB now) or uninhibited, but recent studies using prolonged natural filling bladder monitoring techniques have shown that to the contrary, the bladder is normally quiescent and stable even in newborns (Yeung et al, 1995). Clinically, the condition of OAB is best exhibited by urge syndrome with or without urge incontinence. The syndrome, characterized by frequent attacks of sudden and imperative sensations of urge, due to detrusor overactivity during the filling phase, is more prevalent in girls. This burning sensation or urge to void occurs even with small bladder capacities. The unstable contractions are often counteracted by voluntary contraction of the pelvic floor muscles in an attempt to externally compress the urethra (hold maneuvers) exhibited as squatting (Vincent curtsy sign) in many cases (Vincent, 1966; van Gool and de Jonge, 1989). However, despite this move, urge incontinence can still occur with frequent urine leakage onto the underpants. Urge incontinence usually consists of small quantities of urine loss and occurs most often in the afternoon or when the child is preoccupied in play or other activities and is not alert enough to contract the pelvic floor in response to the urge sensation. Children with urge syndrome have small bladder capacities for age and often choose to drink extremely little in order to escape from the social embarrassment of frequent imperative urges to go to the toilet and from urinary incontinence, but otherwise they have complete relaxation of the pelvic floor musculature in most instances. In addition, habitual voluntary pelvic floor contraction to counteract every urge to void may often lead to inappropriate postponement of defecation, constipation, and fecal soiling (Vincent, 1966; O’Regan et al, 1986). This should not be confused with encopresis in children with behavioral problems.
Functional Urinary Incontinence
This can be defined as the involuntary loss of urine due to a failure of control of the bladder-sphincteric unit, which is frequent enough to constitute a social or hygienic problem, and in the absence of underlying anatomic causes (Nevéus et al, 2007). Apart from urge incontinence that is associated with detrusor overactivity and urge syndrome as described earlier, other forms of functional urinary incontinence exist as a result of failure of the bladder storage and/or sphincteric control mechanisms. Stress incontinence represents the involuntary leakage of urine occurring at times when the intravesical pressure exceeds the bladder outlet or urethral resistance, in the absence of measurable detrusor contraction. Unlike in adults and particularly in elderly women, true stress incontinence is extremely uncommon in neurologically normal children and is in general not associated with any demonstrable urodynamic abnormalities. It should therefore be differentiated from urge incontinence and detrusor overactivity. Insufficient bladder outlet or urethral resistance is usually not a main factor. Because the amount of urine leakage in most patients is usually small, the incontinence may at times be only scarcely discernible. It is possible therefore that the actual incidence of this condition may be underestimated.
Giggle Incontinence
Giggle incontinence is usually seen in girls and is characterized by involuntary and typically unpredictable wetting during giggling or laughter. In contrast to stress incontinence, it produces a much larger volume of urine leakage, often amounting to complete bladder emptying. Similar to stress incontinence, however, cystometry may be completely normal or occasionally demonstrate some detrusor overactivity. Treatment is notoriously difficult, but sometimes a course of anticholinergic drugs may help to ameliorate the symptoms.
Bladder-Sphincter Dysfunction during Bladder Emptying
Dysfunctional Voiding
Dysfunctional voiding is characterized by incomplete relaxation or overactivity of the pelvic floor muscles during micturition. It can manifest in different patterns depending on the degree of functional outflow obstruction caused, as well as the status of the detrusor activity. As outlined earlier, the underlying bladder-sphincter dysfunction may evolve progressively and change with time through a transitional phase of a complex sequence of events of bladder functional development.
Staccato and Fractionated Voiding
In staccato voiding the urinary stream is often delayed after the onset of detrusor contraction and is typically interrupted, resulting in a few small squirts of urine passed in quick succession. The interrupted voiding is caused by periodic bursts of pelvic floor muscle activities during micturition, resulting in the characteristic abrupt elevation or spikes of voiding pressures, coinciding with paradoxical cessation of urinary flow. The flow time is usually prolonged and bladder emptying is often incomplete, resulting in increased postmicturition residues and hence a predisposition to UTIs.
Fractionated voiding is characterized by infrequent and incomplete emptying secondary to detrusor inactivity. Micturition occurs in several small, discontinuous fractions due to poor and unsustained detrusor contractions, leaving significant postvoid residual urine volumes. Abdominal straining is usually evident as an effort to improve bladder emptying. However, this is often paradoxically counteracted by a reflex increase in activities of the pelvic floor muscles that is triggered by an increase in intravesical pressure. The bladder capacity is usually large for age and may gradually increase as the condition further progresses. Overflow incontinence may ultimately develop.
Infrequent Voiding and Underactive Bladder Syndrome (“Lazy Bladder”)
These two conditions are described together because they represent a spectrum of disease that more commonly occurs in girls. The old entity “lazy bladder” is now replaced by the term underactive bladder. At one end of the spectrum, the underactive bladder is generally regarded as the end point of long-standing dysfunctional voiding culminating in a fully decompensated system. As a result of the chronic functional bladder outflow obstruction, there is a gradual deterioration in detrusor contractility and emptying efficiency. Postmicturition residual urine and bladder capacity progressively increase, eventually developing into a large, floppy bladder with inefficient emptying. The child uses abdominal straining as the main driving force for bladder emptying, and detrusor contractions are small and unsustained with low pressures, at times being completely undetectable using conventional cystometry. Because urge sensation is either absent or diminished, voiding is infrequent and occasionally the child may not void for 8 to 10 hours or even longer if engaged in activities. The typical presentation of a child with infrequent voiding syndrome is where the mother always complains that the child never voids unless told to do so. The resultant large volume of postmicturition residual urines predispose to recurrent UTIs, which often constitute the primary complaint bringing the child to the pediatric urologist. Overflow incontinence is usually evident, which may be further associated with constipation and/or fecal soiling. Urodynamic studies typically reveal a large-capacity bladder with high compliance on filling. Detrusor contractions are noticeably absent, and voiding is associated with increased abdominal pressures, resulting in a fractionated flow pattern. Electromyography often shows increased activity in the pelvic floor muscle with each abdominal strain.
Hinman Syndrome and Occult Neuropathic Bladder
Hinman, Allen, and Dorfman first coined the terms non-neurogenic neurogenic bladder or subclinical neurogenic bladder, and later Hinman syndrome to describe a presumably acquired form of bladder-sphincter dysfunction in children that was characterized by a combination of bladder decompensation with incontinence, poor emptying, and recurrent urinary infections (Dorfman et al, 1969; Hinman and Baumann, 1973; Allen, 1977; Hinman, 1986). Most children also have significant bowel dysfunction including encopresis, constipation, and fecal impaction. In British literature the condition is often referred to as “occult neuropathic bladder” after the article by D. Innes Williams and colleagues (1975). The condition has all the clinical and urodynamic features typical of neuropathic bladder dysfunction, but no neurologic pathology can be demonstrated. Hinman and Baumann initially hypothesized that the condition could be ascribed to acquired psychologically abnormal behavior with voluntary discoordination between the detrusor muscle and the pelvic floor–external urethral sphincter complex during voiding (Hinman and Baumann, 1973). This notion, however, has not received strong support from later studies, and controversies still exist as to whether a subtle or occult spinal pathology can occur to account for an isolated bladder neuropathy in a child who does not exhibit other somatic neurologic deficits and has normal magnetic resonance imaging of the spinal cord. Urodynamic studies often revealed marked sphincteric overactivity and abrupt contractions of the pelvic floor as the child attempted to control incontinence from uninhibited bladder contractions. In its severe form, the bladder-sphincter dysfunction can cause full-blown bladder decompensation with day-and-night wetting, large postmicturition residual urine volumes, recurrent urinary infections, and significant damage to the upper urinary tracts (Dorfman et al, 1969; Hinman and Baumann, 1973; Williams et al, 1975; Allen, 1977; Hinman, 1986, 1997; Jayanthi et al, 1997). Imaging studies often reveal profound changes in the upper urinary tracts, with about two thirds of the children developing hydroureteronephrosis and over half having VUR. Nearly every patient has a grossly trabeculated, large-capacity bladder with a considerable volume of postmicturition residual urine. Treatment should therefore entail measures to control infection and to restore normal bladder storage and emptying function. Due to the great similarities to neuropathic bladder dysfunction, the management strategy in these children generally follows similar principles for the former condition.
Another piece of indirect evidence that a subtle or occult neurologic lesion may account for some cases of “non-neurogenic neurogenic” bladder-sphincteric dysfunction is the Ochoa (urofacial) syndrome. Children with this syndrome exhibit all the classical features of dysfunctional voiding including urinary incontinence, recurrent UTI, constipation, reflux and upper tract damage, but in addition have a peculiar painful or apparently crying facial expression during smiling (Ochoa and Gorlin, 1987; Ochoa, 1992). The condition has an autosomal recessive inheritance and the gene has been located on chromosome 10. Urodynamic studies characteristically showed a sustained contraction of the external sphincter during voiding. The resulting severe bladder-sphincter dysfunction is often associated with a dismal outcome. Of the 66 children reported by Ochoa, 33% had renal functional impairment, 26% had hypertension, and 24% had end-stage renal failure. Five patients received renal transplantation and 17 were dead (Ochoa, 1992). Because the neural ganglia controlling the facial muscles are situated close to the pontine micturition center, it is tempting to speculate that a small, genetically predetermined, congenital neurologic lesion in this area may be responsible for both the peculiar facial expression and the bladder dysfunction. Further studies in this direction may help to resolve some of the continuing dilemma.
Postvoid Dribbling
Postvoid dribbling refers to involuntary leakage of urine immediately after voiding. Typically this refers to post–toilet-trained girls who dribble soon after standing up following a void and are otherwise normal with no other associated urinary symptoms. In these circumstances it may be a result of vesicovaginal reflux whereby urine is trapped in the vagina during voiding, and once the child stands the urine begins to dribble out. When in doubt, it can be confirmed by performing a micturating cystourethrogram. The condition is harmless and tends to resolve with age, but the child may also be taught to empty her vagina by simply voiding with her thighs apart and leaning forward after voiding before getting up.
Dysfunctional Elimination Syndrome, Constipation, and Bladder Dysfunction
It has long been recognized that children with dysfunctional voiding and recurrent urinary infections often have associated bowel dysfunction including constipation, fecal impaction, and encopresis. However, only recently has the term dysfunctional elimination syndrome been given to recognize the existence of this relationship. Studies have been performed to explain how constipation and chronic rectal dilatation may interfere with the normal function of the lower urinary tract, resulting in bladder dysfunction, reflux, and UTIs (Yazbeck et al, 1987; O’Regan et al, 1997). The close proximity of the rectum to the posterior wall of the bladder makes it possible that any gross distension of the rectum by impacted feces can result in mechanical compression of the bladder and bladder neck leading to urinary obstruction (Shopfner, 1968; O’Regan et al, 1997). In addition, it has been observed that large fecal impaction may induce significant detrusor instability and other bladder dysfunctions, which in turn will result in urge syndrome, UTI, and reflux (Shopfner, 1968; Yazbeck et al, 1987; O’Regan et al, 1997). O’Regan and colleagues have reported a high incidence of enuresis in children with urinary infection and constipation. It has been noted that in constipated enuretic children who had detrusor instability, treatment of concomitant constipation resulted in cessation or improvement of enuresis (O’Regan et al, 1985, 1986). Animal studies have also revealed an increased predisposition to bacteriuria in rats with surgically induced fecal retention (Breda et al, 1975). The evidence available so far therefore strongly suggests that there is an important relationship among constipation, detrusor instability, reflux, UTI, and enuresis. Proper attention to associated bowel dysfunction should therefore form an integral part of the overall management in children with bladder dysfunctions.
Relationship among Bladder-Sphincter Dysfunction, Vesicoureteral Reflux, and Recurrent Urinary Tract Infections
Impairment in the functions of the lower urinary tract often coexists with recurrent UTIs and VUR, without any identified neurologic or other causal pathology (Koff and Murtag, 1983; Griffiths and Scholtmeijer, 1987; Sillen et al, 1992; Chandra et al, 1996; Yeung et al, 1998). Incidence of higher intravesical pressure, detrusor overactivity, detrusor-sphincter dyssynergia, or incomplete voiding have been reported in a significant proportion of children with VUR and conversely, micturating cystourethrograms (MCUs) performed in children with bladder dysfunctions often find concomitant VUR. The most common abnormalities of the lower urinary tract found to coexist with VUR are predominantly detrusor overactivity and uncoordinated detrusor-sphincter function during micturition. These abnormalities may occur secondary to urinary infection or can be acquired during the period when voluntary control of micturition is being established. Although the bladder instability can occur in most children with VUR, it is unlikely to have its role in initiating VUR (Koff and Murtag, 1984). However, reflux from incompetence of the ureterovesical junction (UVJ) may be worsened by detrusor instability (Stephens, 1979). The urethral sphincteric dysfunction or discoordination is characterized urodynamically by obstruction during voiding due to involuntary constriction of the urinary sphincter and is believed to be the normal response of the individual to an uninhibited bladder contraction while attempting not to void (Scholtmeijer and Griffifths, 1990; Koff, 1992; Homsy, 1994; Scholtmeijer and Nijman, 1994). It is considered as the most serious dysfunction because it has been reported that patients with this dysfunction often have bilateral high-grade reflux and injured upper renal tracts (Griffiths and Scholtmeijer, 1987; Orellana et al, 2004). The discoordinated bladder also regularly decompensates, and a significant number of children with moderate or severe VUR were reported to have overdistended bladder with weak contractions and high capacity (Hjalmas et al, 1994; Koff et al, 2002; Garat Barredo et al, 2004).
Previous studies showed that infants with UTI and VUR have a high prevalence of high voiding detrusor pressure (Fig. 127–6) (Sillen et al, 1992, 1996; Yeung et al, 1998). Male refluxers are characterized by considerably higher maximum voiding pressures associated with detrusor overactivity, which may represent a more pronounced form of detrusor-sphincter dyssynergia, as compared with age-matched female refluxers and normal nonrefluxing infants. In contrast, in the majority of female refluxing infants no detrusor hypercontractility was noted and instead they often displayed a urodynamic pattern with normal voiding pressures and relatively large bladder capacity with or without emptying inefficiency and postmicturition residues. The finding of higher voiding detrusor pressure in male infants compared with female infants may be attributed to higher urethral resistance of the longer male urethra and smaller urethral meatus and anatomic difference in the configuration of external sphincter. Studies have also shown that high intravesical pressure significantly contributes to the development and severity of VUR. It was shown that infants with bilateral high-grade VUR had significantly higher voiding detrusor pressures and higher prevalence of detrusor overactivity than those with lower-grade or unilateral VUR (Chandra and Maddix, 2000).
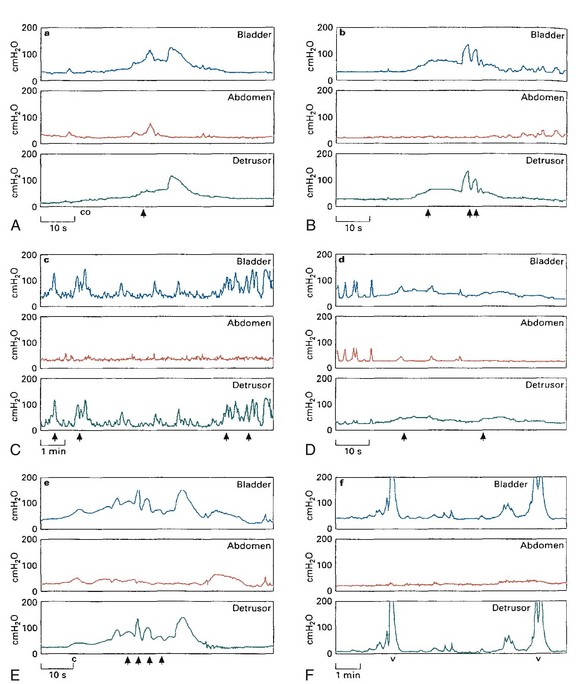
Figure 127–6 Urodynamic patterns in infants with vesicoureteral reflux. A, Normal: a period of low-pressure detrusor contraction (co) preceded a more formal contraction associated with the onset of urinary flow (arrow). B, Immature: urinary flow (arrows) was interrupted during periods of sudden elevation of detrusor pressure. C, Unstable: frequent high-pressure unstable bladder contractions associated with occasional leakage of urine (arrows). D, Inadequate: unsustained low-pressure detrusor contractions associated with the passage of small dribbles of urine (arrows) and a large PVR. E, Dyssynergic: significant interruption of urinary flow (arrows) associated with spikes of increase in detrusor pressure. F, Obstructive: note frequent voiding (v) with high detrusor pressures, greater than 20 cm H2O.
(From Yeung CK, Godley ML, Dhillon HK, et al. Urodynamic patterns in infants with normal lower urinary tracts or primary vesico-ureteric reflux. Br J Urol 1998;81:461–7.)
It has been further shown that the spontaneous resolution of VUR may be delayed in the presence of abnormal dynamics of the bladder (Godley et al, 2001; Sofia et al, 2004). Bladder dysfunction was shown to have a powerful relationship with the nonresolution of high-grade infant VUR. It is generally accepted that bladder dysfunction delays the spontaneous resolution of reflux in older children (Koff and Murtag, 1984; Taylor et al, 1982). Studies have also shown that there is a strong correlation between recurrent infections and nonresolution, which probably reflects the fact that most infections were seen in infants and children with bladder dysfunction. Therefore recurrent infections can be regarded as a marker of bladder dysfunction, indicating an investigation of bladder function (Sofia et al, 2004).
Moreover, successful treatment of the underlying bladder dysfunction resulted in marked increase in the rate of spontaneous resolution of reflux and significant reduction in recurrent UTIs. Therefore early detection and proper management of underlying lower urinary tract abnormalities are crucial in the treatment of VUR in infants and children. The first step in management in those children presenting postnatally with VUR or UTI should be a detailed evaluation of bladder function to choose an appropriate treatment modality and to prevent renal deterioration.
Why do children who have VUR have such a high incidence of bladder dysfunction? The correlation of this maturational lag in the bladder function and development of the CNS is the other untouched area of investigation. A similar association between gastroesophageal reflux and cerebral palsy is now well studied; understood and effective surgical treatment protocol has evolved over the past 2 decades.
Key Points: Epidemiology and Classification of Non-Neuropathic Bladder-Sphincter Dysfunction in Children
Evaluation of Non-Neuropathic Bladder-Sphincter Dysfunction
History
The majority of children with non-neuropathic bladder-sphincter dysfunction typically present after toilet training with symptoms of either nighttime or daytime urinary incontinence or both. Occasionally they can be recognized at an earlier age when the child presents for investigation of UTIs or VUR. In any case, it is important to obtain a detailed history from the child and guardian. This should include relevant questions to exclude neurologic and congenital abnormalities. Bowel dysfunction can coexist in the form of encopresis, constipation, and fecal impaction and should be noted during history-taking. The urinary history should focus on symptoms related to both the storage and evacuation of urine.
Voiding Diary
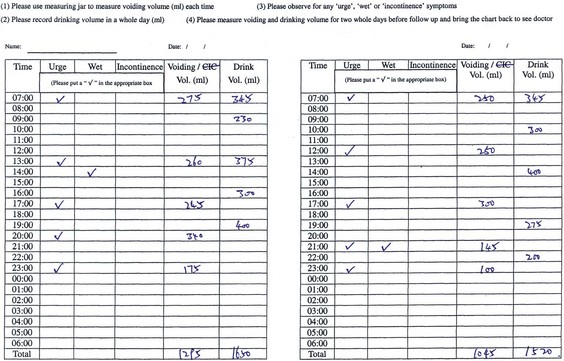
A voiding diary is used to record daily fluid intake and urine output at home under normal conditions. When properly filled in, it can be informative and give us clues to the underlying dysfunction. The number of voidings per day, the distribution of voids during the day, and each voided volume are recorded. It can also record any episodes of urgency and leakage. It is a useful tool to help identify those who may warrant further studies, as well as in follow-up of patients (Fig. 127–7).
Physical Examination
Physical examination is usually normal in children with non-neuropathic bladder-sphincter dysfunction, but careful examination is required to exclude those who may require further neurologic evaluation. Occasionally, in an otherwise normal child, one may find a palpable bladder on abdominal examination in cases of decompensated bladders. Abnormalities of the lower spine should be sought specifically to exclude the possibility of an occult spinal dysraphism. Lesions such as an asymmetric gluteal fold, hairy patch, dermovascular malformation, or lipomatous abnormality of the sacral region should prompt further imaging evaluation. The external genitalia may be examined to exclude any obvious anatomic problems that can explain the urinary symptoms in question. Rectal examination may reveal fecal impaction or distended rectum in those with chronic constipation. In the absence of any positive findings on examination, further investigations may be warranted.
Laboratory Investigations
Laboratory investigations are generally not routinely required unless the child presents with complications such as UTIs. However, to complement a full workup, urinalysis may be performed to rule out bacteriuria and glucosuria. Serum and urine osmolality may be looked at in cases of nocturnal enuresis to assess renal concentrating ability.
Ultrasonography
Ultrasonography (USG) is often the first-line investigation in children with non-neuropathic bladder-sphincter dysfunction because it is a simple, readily available, and noninvasive tool that is able to provide information both on anatomic and functional problems when performed by experienced pediatric radiologists.
Ultrasonography has been increasingly used in the study of the pelvic floor musculature. In boys the external sphincter, puborectalis, and bulbocavernosus have been observed to contract during a hold maneuver on USG. In girls, lengthening of the urethra and movement of the bladder neck in the direction of the symphysis are seen. However, in patients with non-neuropathic bladder-sphincter dysfunction, approximately one third of the children were unable to elicit movement of the pelvic floor or had paradoxical movement. The clinical significance of this remains unclear, but when introduced to a program of urotherapy, marked improvement of symptoms was reported. Similarly, the USG had been employed in the study of bladder neck mobility. Studies have shown that in a proportion of girls with hyperlaxity of joints, coughing or straining would result in a wide opening of the bladder neck and urethra. In this group of girls, urotherapy would prove difficult and occasionally some would go on to require surgery to the bladder neck (deJong, 2005).
More recently, the ultrasound has been used to measure bladder parameters used in calculating a “bladder volume and wall thickness index” (BVWI). This BVWI can be classified into normal, thick, or thin according to the measured parameters. Studies have shown that these classification corresponded closely to urodynamic findings of underlying bladder dysfunctions and can act as a reliable tool to guide for further invasive investigations (Yeung et al, 2004).
Other Imaging Studies
Radiologic examination of the spine may be necessary to rule out any neurogenic causes of bladder-sphincter dysfunction.
A micturating cystourethrogram may be performed in patients to rule out VUR. Information on the bladder emptying efficiency may be obtained, and the status of the urethra can be assessed to exclude any outflow obstruction.
Urodynamic Studies
Urodynamic studies are employed to describe the physiologic parameters involved in the bladder mechanics during filling and voiding.
Uroflowmetry
All urodynamics should be preceded by or combined with a uroflow study. When performing uroflowmetry, the child must experience normal desire to void for it to be representative and repeated recordings should be performed. Preferably, the voiding should be observed and correct sitting or standing position ascertained. In children, normal flow rates are different from adults and there is usually a poor correlation between maximal flow rate (Qmax) and outflow resistance as the detrusor is able to exert much stronger contractions to counteract any increased resistance. It is therefore important to study the pattern of the flow curve in addition to the flow rates in children. The precise shape of the flow curve is determined by detrusor contractility, the presence of any abdominal straining, and the bladder outlet. The normal pattern is that of a smooth bell shape. A tower-shaped curve produced by an explosive voiding contraction is seen in overactive bladders, and a low plateau-shaped curve is representative of an outlet obstruction. A staccato pattern is seen with sphincteric overactivity during voiding with peaks and troughs throughout voiding, and an interrupted flow is seen in acontractile or underactive bladders. When combined with cystometry and perineal EMG studies, further information on the different spectrum of functional bladder outflow obstructions can be analyzed (Nijman, 1997).
Conventional Fill Urodynamic Studies
This involves more sophisticated instruments and becomes more invasive to the patient, requiring a bladder catheter introduced transurethrally or suprapubically. The use of suprapubic catheterization has been suggested as a better alternative to transurethral catheterization. Although it may be considered more invasive, it is much more physiologic than to try to void with a catheter in the urethra, particularly in children. Not only is it obstructive to flow, but the trauma of placing the catheter just before the investigation may cause significant discomfort. For suprapubic catheterization, a 6-Fr double-lumen catheter is placed suprapubically under sedation and left in situ for 24 hours before commencement of the urodynamic study.
The suprapubic catheter is connected to a computer system and used to measure intravesical pressure. Another catheter is placed in the rectum to measure intra-abdominal pressure surrounding the bladder. By subtracting the latter from the intravesical pressure, one can calculate the detrusor pressure. Sphincteric activity can also be measured with simultaneous perineal EMG recordings. All the measured data are directly fed into a computer for analysis and display of graphical measurements. The urodynamic study looks at both the filling and voiding phases of the bladder. Bladder filling and storage are described according to bladder sensation, detrusor activity, bladder compliance, and bladder capacity. Pressure flow studies are carried out during the voiding phase.
Traditionally, the bladder is filled artificially with water/normal saline to speed up the procedure. The child is asked to indicate his or her desire to void (if old enough to do so) and then void into a specially designed seat with a uroflowmeter attached. The investigator observing the study should also make note of any events occurring during the study (e.g., any large movements of the patient, coughing, or in particular any urinary dribbling).
By combining cystometry with fluoroscopy, video-urodynamics can be performed with fluoroscopic images of the bladder, bladder neck, and urethra captured. Conventional fill urodynamics is performed in the usual manner with the child sitting in a specially designed chair with a uroflowmetry device in the fluoroscopy suite. Fluoroscopic images are taken during filling and voiding. This has the added advantage of being able to observe the shape of the bladder during filling and voiding, observing for signs of VUR, as well as the configuration of the urethra and pelvic floor.
Natural Fill Urodynamic Studies
More recently, studies have shown that the nonphysiologic filling of the bladder during conventional fill urodynamics, even at low filling rates, can lead to misrepresentations of true bladder activity during normal situations. Therefore natural fill urodynamic studies may be performed whereby the child is asked to drink to allow the bladder to fill up on its own rate. Urodynamic studies in children with urge syndrome usually reveal detrusor overactivity associated with a small bladder capacity but may occasionally be normal with incontinence only barely perceptible, particularly if conventional fill cystometry rather than natural filling cystometry is used. It appears that the artificial filling may inhibit the detrusor response and attenuate its maximum contractile potential, rendering detrusor instability much less pronounced and undetectable. Therefore natural fill cystometry is the preferred technique in children, or better still, the combined use of artificial and natural filling urodynamic studies is helpful to accurately delineate the underlying bladder dysfunction (Yeung et al, 1995).
Ambulatory Urodynamic Studies
The unfamiliar hospital and urodynamic laboratory environment, as well as the presence of a urodynamics investigator, can sometimes cause significant distress, particularly to young children. To overcome this, an ambulatory urodynamic study may be performed. This study is performed with ambulatory equipment strapped to the child and relies on infrared telemetry while the investigator sits behind a one-way mirror (Yeung, 1998).
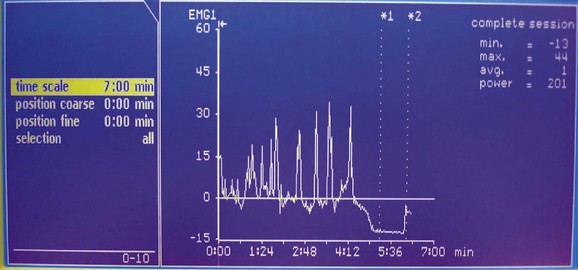
The system consists of an ambulatory urodynamic recorder with a specially designed built-in extension board that converts digital pressure signals to a modulated infrared wave. Two external infrared leads are connected to the output and fixed to the clothing of the patient (Fig. 127–8). The optimal emission from the recorder has a 10-m range and completes the circuit of the infrared on-line link by cable to the computer. Direct line of sight from the transmitter to the receiver is unnecessary because the infrared signals reflect from the walls of the room before reaching the receiver. For active and uncooperative patients, more than one receiver connected in parallel with the power unit and computer can be used at the same time to enhance signal collection. The first receiver to receive valid signals transmits the data to the computer and inhibits the other receivers simultaneously (Fig. 127–9).

Figure 127–8 The ambulatory urodynamic recorder with the external infrared leads mounted in a plastic holder that can be attached to the patient.
(From Yeung CK. Continuous real-time ambulatory urodynamic monitoring in infants and young children using infrared telemetry. Br J Urol 1998:81;76–80.)
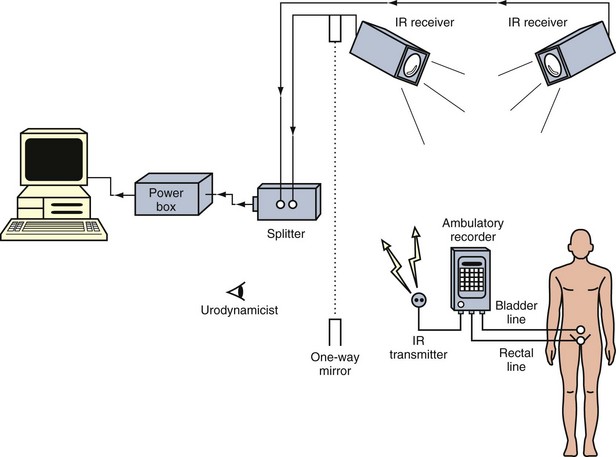
Figure 127–9 Schematic diagram to show the setup for continuous real-time ambulatory urodynamics using infrared telemetry.
(From Yeung CK. Continuous real-time ambulatory urodynamic monitoring in infants and young children using infrared telemetry. Br J Urol 1998:81;76–80.)
During the investigation the infant can conduct normal activities, be totally mobile, and be accompanied by one or both of the parents undisturbed in a private cubicle. From an adjoining room the hidden investigator observes and records the patient’s activities, including urinary dribbling and micturition, through a one-way mirror, while real-time on-line pressure signal displays are monitored continuously on the computer. Only natural fill studies are possible by this means. Overall, it allows for a continuous monitoring of bladder function under near-natural conditions for the child.
More recently, the Bluetooth system has been introduced. This works in much the same way as the infrared system but is much less bulky for the patient to carry around. It offers even more freedom of movement with a much longer transmission range and more reliable reception. If coupled with a camera recorder in the room the child is in, there is no need for the investigator to sit behind a one-way mirror throughout the procedure. With such advances in technology, it will not be surprising if urodynamics can soon be performed in the setting of one’s own home!
Key Points: Evaluation of Non-Neuropathic Bladder-Sphincter Dysfunction in Children
Management of Non-Neuropathic Bladder-Sphincter Dysfunction
Behavior Modification and Standard Urotherapy
Urotherapy is a nonpharmacologic and nonsurgical combination of cognitive, behavioral, and physical therapy with an aim to normalize micturition pattern and prevent further functional disturbances of the lower urinary tract. The children and their parents are educated on proper voiding mechanics, and their specific problem is explained to them to provide motivation for improvement. Specific instructions are then given as to how and when to void. Children are assessed and taught correct sitting or standing positions for voiding. They are taught how to relax the pelvic floor and avoid straining. Their drinking and voiding habits are studied and modified accordingly to include proper hydration with timed voiding. A proper assessment of their bowel function is imperative to successful management. To achieve good results, strong patient support with adequate motivation of both the child and the parents is essential.
Biofeedback and Pelvic Floor Rehabilitation
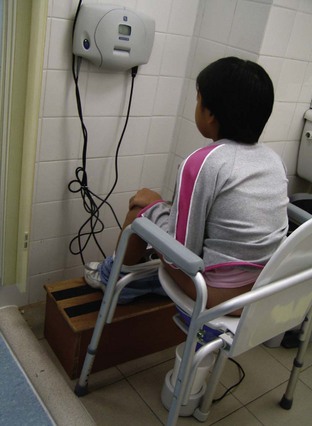
Biofeedback is based on the concept of building self-perception on detrusor contractions and pelvic floor relaxation in the patient. By combining uroflowmetry with real-time monitoring, the child is able to see how well he or she is voiding. It also helps the child understand what can be altered with voluntary control and with relaxation of the pelvic floor. Biofeedback as a treatment modality for children with dysfunctional voiding is based on this concept and has proven to be highly effective either on its own or in combination with standard urotherapy (Figs. 127-10 through 127-12).
Neuromodulation, Acupuncture, and Other Treatment Modalities
Recent studies reported that transcutaneous electrical nerve stimulation (TENS) is a simple, cost-effective, noninvasive treatment modality for the management of a wide variety of lower urinary tract disorders with few side effects. The mechanisms of action of the reported techniques remain unclear, but symptomatic improvements are common. Some reports indicate changes in urodynamic parameters during transcutaneous stimulation, suggesting that the TENS therapy was capable of inhibiting detrusor contractions. The beneficial effects of TENS over the sacral dermatomes (S2-S3) in patients with lower urinary tract symptoms associated with detrusor overactivity have been reported. The use of low-frequency electrical current to inhibit detrusor activity in adults is common and appears to modulate excitatory and inhibitory components of bladder control. Such studies provide the rationale for the application of TENS therapy to modulate detrusor function in patients with functional detrusor dysfunction.
Traditional acupuncture has also reported high efficacy in the treatment of nocturnal enuresis with low relapse rate. Reports have used acupoints innervated by sacral segments S2-S4, and stimulation involved both manual and electrostimulation. However, its use in the pediatric population remains limited by the intrinsic fear of needling in children with subsequent lack of cooperation (Bower et al, 2005).
Bowel Management
The principles of bowel management include rectal emptying of impacted stool and the maintenance of regular soft stools. A reliable bowel habit pattern can only be established when the child is able to achieve pain-free defecation of soft stool. An initial cleanout of the bowel can be achieved by oral laxatives and rectal enemas on a regular basis until there is complete disimpaction of the rectum. Occasionally, high-colonic washouts may be necessary to achieve a good result. Only when this has been achieved should the second stage of management commence. This involves prevention of further stool accumulation with continuation of regular oral laxatives and/or stool softeners, as well as dietary modification. Correct toileting posture and correct recruitment of abdominal muscles in the defecation process aids in the effectiveness of bowel emptying. However, success of a bowel management program can only be achieved with sound support from the family. Parental education is necessary in order to reinforce and encourage correct toileting habits at home.
Medications
Pharmacologic control of the lower urinary tract (LUT) can either act via the central nervous system (CNS) or peripherally. Several CNS regions have been identified to be involved with micturition control including the cortex, pontine tegmentum, medulla, and spinal structures. All these regions are modulated by several different neurotransmitters, which have become targets for drug treatment. However, many of the drugs developed with CNS action are not sufficiently selective to affect only the lower urinary tract, increasing the possibility of these drugs causing adverse drug reactions. Pharmacologic targets for treating bladder dysfunction may be aimed at the bladder, urethra, ganglia, or peripheral nerves. The main targets for drug therapy are receptors or ion channels known to be involved in bladder contraction (muscarinic receptors, L-type calcium channels). More recently, research has been focused more on alternative neurotransmission and modulation of afferent signaling from the LUT.
Antimuscarinics
Antimuscarinics are the gold standard in treatment of overactive bladders. Muscarinic receptors are found in the human detrusor muscle, and bladder contractions are initiated by stimulation of these receptors with the release of acetylcholine from cholinergic nerves. The main action of antimuscarinics is on the M1 and M3 receptor subtypes, which are thought to be responsible for the pathogenesis of detrusor overactivity. Antimuscarinics, such as oxybutynin, act by reducing the frequency and intensity of involuntary contractions, resulting in an increase in the functional bladder capacity. The clinical efficacy depends on various factors such as receptor affinity, pharmacokinetics, and the specificity for the bladder. Latest developments have focused on receptor specificity and efficacy-to-side-effect ratio. The nonselective pattern of activity and penetration of the blood-brain barrier are known to induce systemic and central side effects.
α-Adrenergic Blockers
The body of the bladder receives relatively sparse innervation by noradrenergic nerves. The density of noradrenergic nerves increases markedly toward the bladder neck, where the smooth muscle receives a dense noradrenergic nerve supply, particularly in males. The normal response to noradrenaline is relaxation of the detrusor and contraction of the bladder neck. α Blockers are therefore used in patients with evidence of bladder neck dysfunction for relaxation of the bladder neck.
Other Medications
Tricyclic antidepressants such as imipramine have been found to be effective for increasing urine storage by both decreasing the detrusor contractility and increasing outlet resistance. However, the precise mechanism of action is not well explained. Its possible effect on the bladder outlet has been described by inhibition of norepinephrine reuptake producing α-adrenergic stimulation. These agents are associated with a high incidence of side effects, and their use should be judicious.
β-Adrenergic agonists can cause significant increases in bladder capacity but can cause significant cardiovascular side effects.
Parasympathicomimetics, calcium antagonists, potassium channel openers, and prostaglandin inhibitors have all been described for use with effect on the bladder, but these are rarely used in children either due to their unfavorable side effects or due to a lack of proven efficacy.
Clean Intermittent Catheterization
Clean intermittent catheterization (CIC) may become necessary in children with decompensated bladders or lazy bladder syndrome in which bladder emptying efficiency is compromised and upper urinary tract dilatation may exist. Regular emptying of the bladder to achieve low-pressure emptying improves detrusor contractility and bladder emptying function. Therefore a regular CIC program can allow for bladder retraining. Some of these children may be able to eventually wean from use of CIC.
Surgery
When conservative management with nonpharmacologic and pharmacologic treatment fails, surgical approaches should be considered. Bladder augmentation may be performed to help produce a low-pressure system with increased bladder capacity. Augmentation may be performed using intestinal segments such as colon, ileum, or stomach. However, the complications that may ensue must be considered including mucus production, electrolyte imbalance, and even possible metaplasia/malignancy.
Surgical means have also been employed to reduce urethral/sphincteric pressure as an alternative to α blockers. Recent studies have focused on the use of balloon dilatation of the bladder neck and botulinum A toxin injection into the urethral urinary sphincter in children with non-neuropathic bladder-sphincter dysfunction with promising results. However, these methods seem to require repeated attempts, and long-term effects and efficacy have yet to be presented.
Key Points: Management of Non-Neuropathic Bladder-Sphincter Dysfunction in Children
Nocturnal Enuresis
Etiologic Factors for Nocturnal Enuresis
Primary nocturnal enuresis (PNE) is a common disorder among children. Despite extensive research there is still significant controversy regarding its etiology, and it is now generally accepted that multiple pathologic factors are probably involved. Among others, a common finding is that the nocturnal urine output in many enuretic children is in excess of the bladder reservoir capacity during sleep at night (Norgaard et al, 1985; Hjalmas, 1997, 1998 ). Nocturnal polyuria can be either absolute—which is usually associated with a derangement of the circadian rhythm of antidiuretic hormone (ADH) secretion—or relative, mainly due to a reduced functional bladder capacity during sleep at night. However, whether it is an increased urine production or a reduced bladder capacity that results in the mismatch between nocturnal bladder capacity and the amount of urine produced during sleep at night, there must be a simultaneous arousal failure in response to bladder fullness before bedwetting can occur.
Bladder-Sphincter Dysfunction in Nocturnal Enuresis
The exact role of bladder dysfunction in the pathogenesis of PNE has until recently remained largely controversial. Although some workers have observed markedly reduced functional bladder capacities among enuretic children compared with age-matched nonenuretic controls, others have previously reported that enuretic episodes were triggered by a full bladder and that bladder capacities in the enuretic children were completely normal (Starfield, 1967; Norgaard, 1989; Jarvelin et al, 1990). However, recent studies have convincingly proven that in a significant proportion of children with severe nocturnal enuresis, especially those who were refractory to conservative treatment and failed to respond to desmopressin therapy, there was a marked reduction in functional bladder capacities when compared with age-matched normal controls (Watanabe et al, 1994; Kirk et al, 1995; Rushton et al, 1996; Eller et al, 1998; Yeung et al, 1999). Furthermore, it has been shown that other types of bladder dysfunctions, notably dysfunctional voiding and marked detrusor overactivity with extremely high voiding pressures suggestive of an obstructive pattern, are not uncommon among enuretic children (Figs. 127-13 through 127-15) (Yeung et al, 1999).
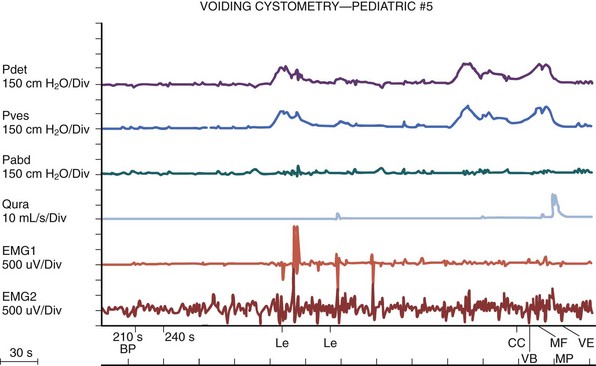
Figure 127–13 Urodynamic tracing showing primary bladder neck dysfunction characterized by a marked delay in initiation of flow after onset of detrusor contraction, often associated with high detrusor pressure but minimal pelvic electromyographic activities.
Sleep Arousal Disturbance and Brainstem Dysfunction in Nocturnal Enuresis
More recently, the involvement of the central nervous system in the etiology of childhood nocturnal enuresis has been assessed. Enuretic boys were shown to be more difficult to arouse than age-matched controls. The arousal inability in patients with nocturnal enuresis may relate to either elevated arousal thresholds or the presence of spontaneous uninhibited bladder contractions.
There is also accumulating evidence that a small functional bladder capacity and detrusor overactivity may play an important role in the pathophysiology of nocturnal enuresis and can further provide reliable predictive clues to the treatment response. Most interestingly, overnight studies in enuretic children with simultaneous sleep EEG and cystometry have revealed marked detrusor instability only after sleep at night but not during wakeful periods in the daytime (Watanabe, 1995; Yeung et al, 1999). This per se can result in a marked reduction in the nocturnal functional bladder capacity (Watanabe, 1995; Rushton et al, 1996; Eller et al, 1998; Yeung et al, 1999). Because this pattern has not been observed in normal nonenuretic subjects even during the newborn period, one may hypothesize that this could be due to a small neurologic lesion affecting a tiny area surrounding the vicinity of the pontine micturition center, the posterior hypothalamus (responsible for secretion of ADH), and the locus coeruleus (which is thought to play an important role in the initiation of cortical arousal and release of noradrenaline) (Yeung et al, 1995, 1998, 1999).
It should be noted that despite the high prevalence of underlying bladder dysfunction among patients with severe, refractory PNE, many have no identifiable daytime urinary symptoms including incontinence, frequency, and urgency. A detailed urodynamic study would, however, reveal detrusor instability in 30% to 90% of patients. The authors’ experience has shown that many enuretic children with reduced functional bladder capacity have learned, either consciously but more probably subconsciously, to restrain fluid intake during the daytime as an attempt to avoid the social inconvenience of urinary frequency and urge incontinence. This could often be easily spotted by a detailed home-recorded voiding diary by an extraordinarily low fluid intake and infrequent, small-volume voiding. By giving these patients a standard fluid intake and then asking them to repeat a home recording of micturition frequency and urinary volume, one could quite easily obtain a highly reliable estimation of the functional bladder capacity and hence identify those patients with reduced bladder capacities (Rittig et al, 1997; Yeung et al, 1999).
Relationship between Bladder and Brain Dysfunctions (Bladder-Brain Dialogue)
A deficiency of prepulse inhibition (PPI) of startle has been reported in enuretic children such that prestimulation 120 msec before the main stimulus resulted in significantly less inhibition of the blink reflex among enuretic children than healthy controls. Prepulse inhibition is based on an inhibition of the startle pathway by the pedunculopontine tegmental nucleus, which lies in close proximity to the pontine micturition center in the brainstem. It has been postulated that a deficient PPI and reduced ability to inhibit micturition during sleep might both originate from a common dysfunction in the pontine tegmentum. This suggests that a relationship may exist between nocturnal bladder function and brainstem functions in humans (Diao et al, 2005; Ornitz et al, 1999, 2000).
Furthermore, it has also been shown that following successful treatment of nocturnal enuresis in these children, both the sleep arousal threshold and the PPI of startle improved. This suggests that brainstem dysfunction may be a secondary effect to the underlying bladder dysfunction, further supporting the “bladder-brain dialogue” concept whereby there is a bidirectional communication between the bladder and the brain.
Key Points: Nocturnal Enuresis
Summary
Despite extensive clinical and animal research, it is perhaps embarrassing to admit that the exact neurologic mechanisms of the development of micturition control from newborn to infancy and later childhood, as well as the pathophysiologic pathways that are involved in various types of bladder dysfunction, have as yet remained largely unclear. It is, however, certain that the bladder and the brain talk with each other all the time as early as in the fetus, and there are extremely rapid and extensive changes and modulation of micturition reflexes during the early postnatal period, leading to copious potentials for developmental errors and voiding dysfunctions. Over the past decade experts have learned that both normal and abnormal bladder function are much more dynamic than previously believed. New investigative tools and research have allowed physicians to study in much greater details and precision the various components including neurologic, muscular, urodynamic, and hormonal mechanisms that may intricately interact to produce different patterns of bladder dysfunctions, as well as their relentless, dynamic changes.
Glassberg KI, Combs AJ. Nonneurogenic voiding disorders: what’s new? Curr Opin Urol. 2009;19:412-418.
Harrison SCW, Abrams P. Bladder function. In: Sant GR, editor. Pathophysiologic principles of urology. London: Blackwell Scientific Publications; 1994:93-121.
Hjalmas K, Arnold T, Bower W, et al. Nocturnal enuresis: an international evidence based management strategy [review article]. J Urol. 2004;171:2545-2561.
Hoebeke P, Bower W, Combs A, et al. Diagnostic evaluation of children with daytime incontinence. J Urol. 2010;183:699-703.
Mulders MM, Cobussen-Boekhorst H, de Gier RP, et al. Urotherapy in children: quantitative measurements of daytime urinary incontinence before and after treatment according to the new definitions of the International Children’s Continence Society. J Pediatr Urol. 2011;7(2):213-218.
Nevéus T, von Gontard A, Hoebeke P, et al. The standardization of lower urinary tract function in children and adolescents: report from the standardization committee of the International Children’s Continence Society. Neuro Urol. 2007;26:90-102.
Nørgaard JP, van Gool JD, Hjalmas K, et al. Standardization and definitions in lower urinary tract dysfunction in children. International Children’s Continence Society. Br J Urol. 1998;81(Suppl 3):1-16.
Palmer LS. Biofeedback in the management of urinary continence in children. Curr Urol Rep. 2010;11:122-127.
Sillen U. Bladder dysfunction and vesicoureteral reflux. Adv Urol. 2008;:815472.
Van Batavia JP, Combs AJ, Horowitz M, et al. Primary bladder neck dysfunction in children and adolescents III: results of long-term alpha-blocker therapy. J Urol. 2010;183:724-730.
Yeung CK. The normal infant bladder. Scand J Urol Nephrol Suppl. 1995;173:19-23.
Yeung CK, Godley ML, Ho CKW, et al. Some new insights into bladder function in infancy. Br J Urol. 1995;6:235-240.
Allen TD. The non-neurogenic neurogenic bladder. J Urol. 1977;117:232-238.
Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997;17:8402.
Bachelard M, Sillen U, Hansson S, et al. Urodynamic pattern in asymptomatic infants: siblings of children with vesicoureteric reflux. J Urol. 1999;162:1733-1737.
Bates P, Bradley WE, Glen E, et al. First report on the standardization of terminology of lower urinary tract function. Urinary incontinence. Procedures related to the evaluation of urine storage: cystometry, urethral closure pressure profile, units measurement. Br J Urol. 1976;48:39-42.
Bates P, Bradley WE, Glen E, et al. The standardization of terminology of lower urinary tract function. Eur Urol. 1976;2:274-276.
Bates P, Bradley WE, Glen E, et al. The standardization of terminology of lower urinary tract function. J Urol. 1979;121:551-554.
Bauer SB. Pediatric urodynamics: lower tract. O’Donnell B, Koff SA, editors. Pediatric urology. Oxford: Butterworth-Heineman, 1997, 125-151.
Bauer SB, Retik AB, Colodny AH, et al. The unstable bladder in childhood. Urol Clin North Am. 1980;7:321-336.
Bellinger MF. Myelomeningocele and neuropathic bladder. In: Gillenwater JY, Grayhack JT, Howards SS, Duckett JW, editors. Adult and pediatric surgery. Mosby-Year Book; 1996:2489-2528.
Blaivas JG. The neurophysiology of micturition: A clinical study of 550 patients. J Urol. 1982;127:958-963.
Bower YF, Diao M, Tang Jl, Yeung CK. Acupuncture for nocturnal enuresis in children: a systematic review and exploration of rationale. J Urol. 2005;174:1623-1627.
Bower YF, Yip SK, Yeung CK. Dysfunctional elimination symptoms in childhood and adulthood. J Urol. 2005;174:1623-1627.
Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57-67.
Bradley WE, Timm GW, Scott FB. Innervation of the detrusor muscle and urethra. Urol Clin North Am. 1974;1:3-27.
Breda G, Bianchi GP, Bonimi U, et al. Faecal stasis and bacteriuria: experimental research in rats. Urol Res. 1975;2:155-157.
Chandra M, Maddix H, McVicar M. Transient urodynamic dysfunction of infancy: relationship to urinary tract infections and vesicoureteral reflux. J Urol. 1996;155:673-677.
Chandra M, Maddix H. Urodynamic dysfunction in infants with vesicoureteral reflux. J Urol. 2000;136:754-759.
de Groat WC. Anatomy and physiology of the lower urinary tract. Urol Clin North Am. 1993;20:383-401.
de Groat WC, Araki I, Vizzard MA, et al. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92:127.
de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. Nervous control of the urogenital system, vol 3. London: Harwood Academic; 1993:227-290.
de Jong TP. Additional urodynamic information by ultrasound. Fourth course on pediatric urodynamics 2005;45–53.
DeLancey JOL. Structural aspects of urethrovesical function in the female. Neurourol Urodyn. 1988;7:509-519.
Dewan PA, Goh DG. Variable expression of the congenital obstructive posterior urethral membrane. Urology. 1995;45:507-509.
Dhillon HK, Yeung CK, Ransley PG, Duffy PG. Cowper’s gland cysts—a cause of transient intra-uterine bladder outflow obstruction? Fetal Diagn Ther. 1993;8:51-55.
Diao M, Yeung CK, Sihoe JDY, et al. Brainstem and bladder dysfunctions in nocturnal enuresis. Proceedings of the 2nd Joint Meeting of European Society for Paediatric Urology (ESPU) and American Academy of Pediatrics (AAP), Uppsala, Sweden, 2005.
Dorfman LE, Bailey J, Smith JP. Subclinical neurogenic bladder in children. J Urol. 1969;101:48-54.
Eller DA, Austin PF, Tanguay S, Homsy YL. Daytime functional bladder capacity as a predictor of response to desmopressin in monosymptomatic nocturnal enuresis. Eur Urol. 1998;33(Suppl. 3):25-29.
Fernandes ET, Reinberg Y, Vernier R, Gonzalez R. Neurogenic bladder dysfunction in children: review of pathophysiology and current management. J Pediatr. 1994;124:1-7.
Garat Barredo JM, Caffaratti Sfulcini J, De la Pena E. Treatment of bladder instability (non-neurogenic hyperactive bladder) in children, with tolterodine. Actas Urol Esp. 2004 Fe;28(2):122-128.
Godley ML, Desai D, Yeung CK, et al. The relationship between early renal status, and the resolution of vesico-ureteric reflux and bladder function at 16 months. Br J Urol. 2001;87:457-462.
Goellner MH, Ziegler EE, Fomon SJ. Urination during the first three years of life. Nephron. 1981;28:174-178.
Gosling JA. The structure of the female lower urinary tract and pelvic floor. Urol Clin North Am. 1985;12:207-214.
Gosling JA, Dixon JS, Critchley HOD, Thompson SA. A comparative study of the human external sphincter and periurethral levator ani muscle. Br J Urol. 1981;53:35-41.
Griffiths DJ, Scholtmeijer RJ. Vesicoureteral reflux and lower urinary tract dysfunction: evidence for two different reflux/dysfunction complexes. J Urol. 1987;137:240.
Harrison SCW, Abrams P. Bladder function. In: Sant GR, editor. Pathophysiologic principle of urology. Blackwell Scientific Publications; 1994:93-121.
Hinman F. Non-neurogenic neurogenic bladder (the Hinman syndrome)—15 years later. J Urol. 1986;136:769-777.
Hinman F. Non-neurogenic bladder (Hinman syndrome). In: O’Donnell B, Koff SA, editors. Pediatric urology. Butterworth-Heinemann; 1997:245-248.
Hinman F, Baumann FW. Vesical and ureteral damage from voiding dysfunction in boys without neurologic or obstructive disease. J Urol. 1973;109:727-732.
Hjalmas K. Urodynamics in normal infants and children. Scand J Urol Nephrol. 114(Suppl.), 1988. 30–27
Hjalmas K. Pathophysiology and impact of nocturnal enuresis. Acta Paediatrica. 1997;86:919-922.
Hjalmas K. Nocturnal enuresis: basics and new aspects. Eur Urol. 1998;33(Suppl. 3):53-57.
Hjalmas K, Hellstrom AL, Hanson E, Sillen U. Tamminen Mobius T for the International Reflux Study in children (1994) Infants and children with vesicoureteral reflux have big bladders. Proceedings of the 5th European Society for Pediatric Urology Annual Meeting, Gothenberg, Sweden, p. 13.
Hoebeke P. Voiding dysfunction, recurrent UTI, constipation and vesicoureteric reflux: A common disease complex. Dialogues in Pediatric Urology. 25(8), 2002.
Holmdahl G. Bladder dysfunction in boys with posterior urethral valves. Scan J Urol Nephrol. 1997;188(Suppl.):1-36.
Holmdahl G, Hanson E, Hanson M, et al. Four-hour voiding observation in healthy infants. J Urol. 1996;156:1809-1812.
Homsy YL, Nsouli I, Hamburger B, et al. Effects of oxybutynin on vesicoureteral reflux in children. J Urol. 1985;134:1168-1171.
Homsy YL. Dysfunctional voiding syndromes and vesicoureteral reflux. Pediatr Nephrol. 1994;8:116-121.
Jarvelin MR, Huttunen NP, Seppanen J, et al. Screening of urinary tract abnormalities among day and night-wetting children. Scand J Urol Nephrol. 1990;24:181-189.
Jayanthi VR, Khoury AE, McLorie GA, Agarwal SK. The non-neurogenic neurogenic bladder of early infancy. J Urol. 1997;158:1281-1285.
Kirk J, Rasmussen PV, Rittig S, Djurhuus JC. Micturition habits and bladder capacities in normal children and in patients with desmopressin-resistant enuresis. Scand J Urol Nephrol. 1995;Suppl. 173:49-50.
Koff SA. The relationship between dysfunctional voiding and reflux. J Urol. 1992;148:1703.
Koff SA. Non-neuropathic vesicourethral dysfunction in children. In: O’Donnell B, Koff SA, editors. Pediatric urology. Oxford (UK): Butterworth-Heinemann; 1997:217-228.
Koff SA, Murtagh DS. The uninhibited bladder in children: effect of treatment on recurrence of urinary infection and on vesicoureteral reflux resolution. J Urol. 1983;130:1138-1141.
Koff SA, Murtagh D. The uninhibited bladder in children: effect of treatment on vesicoureteric reflux resolution. Contrib Nephrol. 1984;39:211-220.
Koff SA, Mutabagani KH, Jayanthi VR. The valve syndrome pathophysiology and treatment with nocturnal bladder emptying. J Urol. 2002;167:291.
Koff SA, Wagner TT, Jayanthi VR. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J Urol. 1998;160(3-II):1019-1022.
Kokoua A, Homsy Y, Lavigne JF, et al. Maturation of the external urinary sphincter: a comparative histotopographic study in humans. J Urol. 1992;150:617-622.
Lapides J. Neuromuscular, vesical and urethral dysfunction. In: Campbell MF, Harrison H, editors. Urology. Philadelphia: WB Saunders; 1970:1343-1379.
Loening-Baucke V. Urinary incontinence and urinary tract infection and their resolution with treatment of chronic constipation of childhood. Pediatrics. 1997;100:228-232.
Maggi CA, Santicioli P, Meli A. Postnatal development of micturition in rats. Am J Physiol. 1986;250:R926-R931.
Mattiasson A. Bladder and urethral physiology and pathophysiology. In: Krane RJ, Siroky MB, Fitzpatrick JM, editors. Clinical urology. Philadelphia: JB Lippincott; 1994:536-557.
McLorie GA, Husmann DA. Incontinence and enuresis. Pediatr Clin North Am. 1987;34:1159-1174.
Nash DFE. The development of micturition control with special reference to enuresis. Ann R Coll Surg Engl. 1949;5:318-344.
Nevéus T, von Gontard A, Hoebeke P, et al. The standardization of lower urinary tract function in children and adolescents: Report from the standardization committee of the International Children’s Continence Society. Neuro Urol. 2007;26:90-102.
Nijman JM. The interpretation of urodynamic investigations in children. Second course on pediatric urodynamics. Utrecht (Netherlands): Utrecht University; 1997. p. 45–53
Norgaard JP. Urodynamics in enuresis I: reservoir function. Neurourol Urodyn. 1989;8:199-211.
Norgaard JP, Pedersen EB, Djurhuus JC. Diurnal anti-diuretic hormone levels in enuretics. J Urol. 1985;134:1029-1031.
Ochoa B. The urofacial (Ochoa) syndrome revisited. J Urol. 1992;148:580-583.
Ochoa B, Gorlin RJ. Urofacial (Ochoa) syndrome. Am J Med Genet. 1987;27:661-667.
Ohel G, Haddad S, Samueloff A. Fetal urine production and micturition and fetal behavioral state. Am J Perinatol. 1995;12:91-92.
O’Regan S, Yazbeck S, Hamberger B, Schick E. Constipation—a commonly unrecognized cause of enuresis. Am J Dis Child. 1986;140:260-261.
O’Regan S, Yazbeck S, Schick E. Constipation, bladder instability, urinary tract infection syndrome. Clin Nephrol. 1985;23:152-154.
O’Regan S, Yazbeck S, Schick E. Constipation and the urinary system. In: O’Donnell B, Koff SA, editors. Pediatric urology. Oxford (UK): Butterworth-Heinemann; 1997:245-248.
Orellana P, Baquedano P, Rangarajan V, et al. Relationship between acute pyelonephritis, renal scarring, and vesicoureteral reflux. Results of a coordinated research project. Pediatr Nephrol. 2004;19(10):1122-1126.
Ornitz EM, Russell AT, Hanna GL, et al. Prepulse inhibition of startle and the neurobiology of primary nocturnal enuresis. Biol Psychiatry. 1999;45(11):1455-1466.
Ornitz EM, Russell AT, Gabikian P, et al. Prepulse inhibition of startle, intelligence and familial primary nocturnal enuresis. Acta Paediatr. 2000;89(4):475-481.
Perez LM, Khoury J, Webster GD. The value of urodynamic studies in infants less than 1 year old with congenital spinal dysraphism. J Urol. 1992;148:584-587.
Rittig S, Schaumburg H, Schmidt F, et al. Long-term home studies of water balance in patients with nocturnal enuresis. Scand J Urol Nephrol. 1997;31(Suppl. 183):25-27.
Rushton HG, Belman AB, Zaontz M, et al. The influence of small bladder capacity and other predictors on the response to desmopressin in the management of monosymptomatic nocturnal enuresis. J Urol. 1996;156:651-655.
Scholtmeijer RY, Griffiths DJ. The role of videourodynamic studies in diagnosis and treatment of vesicoureteric reflux. J Ped Surg. 1990;25:669-671.
Scholtmeijer RJ, Nijman RJ. Vesicoureteric reflux and videourodynamic studies: results of a prospective study after three years of follow up. Urology. 1994;43:714-718.
Shopfner CE. Urinary tract pathology associated with constipation. Radiology. 1968;90:865-877.
Sillen U, Bachelard M, Hermanson G, Hjalmas K. Gross bilateral reflux in infants: gradual decrease of initial detrusor hypercontractility. J Urol. 1996;155:668-672.
Sillen U, Hjalmas K, Aili M, et al. Pronounced detrusor hypercontractility in infants with gross bilateral reflux. J Urol. 1992;148:598-599.
Sillen U, Solsnes E, Hellstrom AL, Sandberg K. The voiding pattern of healthy preterm neonates. J Urol. 2000;163:278-281.
Sofia M, Sillen U, Bachelard M, et al. Spontanious resolution of high grade infantile reflux. J Urol. 2004;172(2):694-699.
Starfield B. Functional bladder capacity in enuretic and non-enuretic children. J Pediatr. 1967;70:77-81.
Stephens FD. The vesicoureteral hiatus and paraureteral diverticula. J Urol. 1979;121:786-791.
Sugaya K, de Groat WC. Micturition reflexes in the in-vitro neonatal rat brainstem-spinal cord-urinary bladder preparation. Am J Physiol. 1994;266:R658.
Sugaya K, Roppolo JR, Yoshimura N, et al. The central neural pathways involved in the neonatal rat as revealed by the injection of pseudorabies virus into the bladder. Neurosci Lett. 1997;223:197.
Taylor CM, Corkery JJ, White RH. Micturition symptoms and unstable bladder activity in girls with primary vesicoureteral reflux. Br J Urol. 1982;54:494.
Thor KB, Blais DP, de Groat WC. Behavioral analysis of the postnatal development of micturition in kittens. Dev Brain Res. 1989;46:137-144.
van Gool JD, de Jonge GA. Urge syndrome and urge incontinence. Arch Dis Child. 1989;64:1629-1634.
van Gool JD, Hjalmas K, Tamminen-Mobius T, Olbing H. Historical clues to the complex of dysfunctional voiding, urinary tract infection and vesicoureteral reflux. The International Reflux Study in children. J Urol. 1992;148:1699-1702.
Vincent SA. Postural control of urinary incontinence—the Curtsey sign. Lancet. 1966;2:631-632.
Watanabe H. Sleep patterns in children with nocturnal enuresis. Scand J Urol Nephrol Suppl. 1995;173:55-56.
Watanabe H, Kawauchi A, Kitamori T, Azuma Y. Treatment system for nocturnal enuresis according to an original classification system. Eur Urol. 1994;25:43-50.
Wein AJ. Pathophysiology and categorization of voiding dysfunction. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell’s urology. Philadelphia: WB Saunders; 1998:917-926.
Wein AJ, Barrett DM. Physiology of micturition and urodynamics. In: Kelalis PP, editor. Clinical pediatric urology. Philadelphia: WB Saunders; 1992:187-217.
Wiegel JW. Neurogenic bladder and urinary diversion. In: Ashcraft KW, editor. Pediatric urology. Philadelphia: WB Saunders; 1990:175-210.
Williams DI, Hirst G, Doyle D. The occult neuropathic bladder. J Pediatr Surg. 1975;9:35-41.
Yazbeck S, Schick E, O’Regan S. Relevance of constipation to enuresis, urinary tract infection and reflux: a review. Eur Urol. 1987;13:318-321.
Yeates WK. Bladder function in normal micturition. In: Kolvin I, MacKeith RC, Meadow SR, editors. Bladder control and enuresis. London: W. Heinemann Medical Books Ltd; 1973:28-365.
Yeung CK. The normal infant bladder. Scand J Urol Nephrol Suppl. 1995;173:19-23.
Yeung CK. Continues real-time ambulatory urodynamic monitoring in infants and young children using infrared telemetry. Br J Urol. 1998;81:76-80.
Yeung CK, Barker GM, Läckgren G. Pathophysiology of bladder dysfunction. In: Gearhart JP, Rink RC, Mouriquand PDE, editors. Pediatric urology. 2nd ed. Philadelphia: Elsevier Saunders; 2010:353-365.
Yeung CK, Chiu HN, Sit FKY. Bladder dysfunction in children with refractory monosymptomatic primary nocturnal enuresis. J Urol. 1999;162:1049-1054.
Yeung CK, Godley ML, Dhillon HK, et al. Urodynamic patterns in infants with normal lower urinary tracts or primary vesico-ureteric reflux. Br J Urol. 1998;81:461-467.
Yeung CK, Godley ML, Duffy PG, Ransley PG. Natural filling cystometry in infants and children. Br J Urol. 1995;75:531-537.
Yeung CK, Godley ML, Ho CKW, et al. Some new insights into bladder function in infancy. Br J Urol. 1995;6:235-240.
Yeung CK, Sreedhar B, Leung VT, Metriweli C. Ultrasound bladder measurements in patients with primary nocturnal enuresis: a urodynamic and treatment outcome correlation. J Urol. 2004;171:2589-2594.
Zimmer EZ, Chao CR, Guy GP, et al. Vibroacoustic stimulation evokes human fetal micturition. Obstet Gynecol. 1993;81:178-180.
Zvara P, Carrier S, Kour NW, Tanagho EA. The detailed neuroanatomy of the human striated urethral sphincter. Br J Urol. 1994;74:182-187.