CHAPTER 9 Organization of Motor Function
Movements are the major way that we interact with the world. Most of our activities, whether running, reaching, eating, talking, writing, or reading, ultimately involve motor acts. Thus, motor control is a major task of the nervous system, and from an evolutionary perspective, it is probably the reason that nervous systems first arose. Not surprisingly, a large amount of the nervous system is devoted to motor control, which can be defined as the generation of signals to coordinate contraction of the musculature of the body and head either to maintain a posture or to make a movement (transition between two postures).
Given that large amounts of the nervous system are involved in motor control, it follows that damage or diseases of the nervous system often result in motor abnormalities. Conversely, particular motor symptoms help determine the location of the damaged or malfunctioning region, thus making assessment of motor function an important clinical tool for doctors.
In this chapter each major nervous system area involved in motor control will be described, starting with the spinal cord and then proceeding to the brainstem, cerebral cortex, cerebellum, and basal ganglia. Eye movement will be discussed at the end of the chapter because of the specialized circuits involved in their generation. Although each area will be described separately, it is important to keep in mind that they are highly interdependent and that most movements result from the coordinated action of multiple brain regions. For example, even spinal reflexes, which are mediated by local circuits in the cord, can be modified by descending motor commands, and virtually all voluntary movements are generated by activation of the spinal cord circuitry (or analogous brainstem nuclei for muscles in the head and face).
PRINCIPLES OF SPINAL CORD ORGANIZATION
The spinal cord has several levels of organization, including segmental organization, which will be our initial focus. Segmental organization refers to the fact that there are basic circuits and connections that take place at each level of the spinal cord and that are largely confined to a single or several neighboring segments. The basic spinal reflexes (i.e., the myotactic, inverse myotactic, and flexion reflexes) are mediated by such circuits. However, superimposed on this segmental organization is the propriospinal system, which is a series of neurons whose axons run up and down the spinal cord to connect the different levels of the cord to one another. This system allows the coordination of activity at different spinal levels, which is important for behavior involving forelimbs and hind limbs, such as locomotion. Finally, there are descending motor (and ascending sensory) tracts that interact with these spinal circuits. These motor pathways carry signals related to voluntary movement, but they are also important for the more automatically (or nonconsciously) controlled aspects of motor function, such as the setting of muscle tone (the resting resistance of muscles to changes in length).
Somatic Motor Neurons
Contractions of skeletal muscle fibers are responsible for movement of the body. Skeletal muscle fibers are innervated by large neurons, called αmotor neurons, in the ventral horn of the spinal cord or in cranial nerve nuclei. These neurons are large, multipolar neurons that range in size up to 70 μm in diameter (Fig. 4-10, A). Their axons leave the spinal cord through the ventral roots and from the brainstem via several cranial nerves. The motor axons are distributed to the appropriate skeletal muscles through peripheral nerves, and they terminate with synapses, called neuromuscular junctions or end plates, on skeletal muscle fibers.
A given skeletal muscle is supplied by a group of α motor neurons located in a motor nucleus. In the ventral horn, a motor nucleus is typically a sausage-shaped array of motor neurons that extend over several spinal cord segments.
A motor unit is an α motor neuron and all of the skeletal muscle fibers that its axon supplies. Each skeletal muscle fiber in mammals is supplied by just one α motor neuron. However, a given α motor neuron may innervate a variable number of skeletal muscle fibers; the number depends on how fine a control of the muscle is required. For highly regulated muscles, such as the eye muscles, an α motor neuron may supply only a few skeletal muscle fibers. However, in a proximal limb muscle, such as the quadriceps femoris, a single α motor neuron may innervate thousands of skeletal muscle fibers.
The motor unit can be regarded as the basic unit of movement. When an α motor neuron discharges under normal circumstances, all the muscle fibers of the motor unit contract. A given α motor neuron may participate in a variety of reflexes and in voluntary movement. Because decisions about whether the synaptic input from various sources will cause particular muscle fibers to contract are made at the level of the α motor neuron (in mammals), these motor neurons have been termed the final common pathway.
Another type of motor neuron is called the γ motor neuron. γ Motor neurons are smaller than α motor neurons; they have a soma diameter of about 35 μm. The γ motor neurons that project to a particular muscle are located in the same motor nucleus as the α motor neurons that supply that muscle. γ Motor neurons do not supply ordinary skeletal muscle fibers. Instead, they synapse on specialized striated muscle fibers, the intrafusal muscle fibers, that are found within muscle spindles (see later).
The skeletal muscle fibers that belong to a given motor unit are called a muscle unit. All the muscle fibers in a muscle unit are of the same histochemical type (i.e., they are all either slow twitch [type I] or fast twitch [type IIA or IIB]). For an in-depth presentation of muscle fiber types, see Chapter 12.
The first motor units to be activated, either by voluntary effort or during reflex action, are those with the smallest motor axons; these motor units generate the smallest contractile force and allow the initial contraction to be finely graded. As more motor units are recruited, motor neurons with progressively larger axons become involved, and they generate progressively larger amounts of tension. This orderly recruitment of motor units is called the size principle because the motor units are recruited in order of motor neuron axon size. The size principle depends on the fact that small motor neurons are activated more easily than large motor neurons. Recall that if an excitatory synapse is active, it will open channels in the postsynaptic membrane and cause an excitatory postsynaptic current (EPSC). The same size EPSC will generate a larger potential change at the axon hillock of a small motor neuron than it will at a larger motor neuron, simply as a consequence of Ohm’s law (V = IR) and the fact that smaller motor neurons have higher membrane resistance than larger motor neurons do. Thus, recalling that excitatory postsynaptic potentials (EPSPs) in the central nervous system (CNS) are small and need to summate to reach threshold for triggering spikes, it is easy to see that as the level of synaptic bombardment rises, the resulting depolarization will reach spiking threshold in smaller motor neurons first, assuming the same level of bombardment. As the size principle is usually obeyed, this assumption generally appears to hold; however, there can be exceptions, and in these cases one assumes that the descending motor pathways must provide differing levels of synaptic drive to the different sized motor neurons.
A clinically useful way to monitor the activity of motor units is electromyography. An electrode is placed within a skeletal muscle to record the summed action potentials of the skeletal muscle fibers of a muscle unit (see Fig. 12-7). If no spontaneous activity is noted, the patient is asked to contract the muscle voluntarily to increase the activity of motor units in the muscle. As the force of voluntary contraction increases, more motor units are recruited. In addition to the recruitment of more motor neurons, contractile strength increases with increases in the rate of discharge of the active α motor neurons. Electromyography is used for various purposes. For example, the conduction velocity of motor axons can be estimated by measuring the difference in latency of motor unit potentials when a peripheral nerve is stimulated at two sites separated by a known distance. Another use is to observe fibrillation potentials that occur when muscle fibers are denervated. Fibrillation potentials are spontaneously occurring action potentials in single muscle fibers. These spontaneous potentials contrast with motor unit potentials, which are larger and have a longer duration because they represent the action potentials in a set of muscle fibers that belong to a motor unit.
Autonomic motor neurons are discussed in Chapter 11.
Spinal Reflexes
A reflex is a relatively predictable, involuntary, and stereotyped response to an eliciting stimulus. Because of these properties, spinal reflexes have been used to identify and classify spinal cord neurons, determine their connectivity, and study their response properties. Thus, knowledge of spinal reflexes is essential for understanding spinal cord function.
The basic circuit that underlies a reflex is called a reflex arc. A reflex arc can be divided into three parts: an afferent limb (sensory receptors and axons) that carries information to the CNS, a central component (synapses and interneurons within the CNS), and an efferent limb (motor neurons) that causes the motor response. The knee jerk response to tapping on the patellar tendon with a reflex hammer by a doctor is a common example of a spinal reflex and illustrates the various components of the definition. The tap on the tendon actually causes brief stretching of the quadriceps muscle (eliciting stimulus) and thus activates sensory receptors (Ia fibers in muscle spindles). Activation of sensory receptors causes an excitatory signal to be sent to the spinal cord to activate motor neurons that go back to the quadriceps and cause it to contract, thereby resulting in a kick (stereotyped response). The person feels the kicking motion but has no sense that it was generated by himself or herself (involuntary). In this case, the afferent limb is represented by the Ia fibers and the efferent limb by the motor neurons. The central portion of this arc is minimal (a synapse from the Ia afferents onto the motor neurons), but in most reflexes it is more complex and can involve multiple types of interneurons.
It is the predictable linking of stimulus and response that makes reflexes a useful tool both for clinicians and for neuroscientists trying to understand spinal cord function. However, one danger to avoid is thinking that a particular neuron’s function is solely participation in a particular reflex because these same neurons are the targets of descending motor pathways and thus are involved in generating voluntary movement. Indeed, many of these neurons are active even when the afferent leg of their reflex arc is silent. One such example is the interneurons of the flexion reflex arc because they are also part of the central pattern generator for locomotion.
Later in this section we will discuss three well-known spinal reflexes because they illustrate important aspects of spinal cord circuitry and function and because of their behavioral and clinical importance. However, you should be aware that there are a number of additional reflexes that are mediated by spinal circuits (e.g., see micturition reflex, Fig. 11-3).
Sensory Receptors Responsible for Eliciting Spinal Reflexes
Each spinal reflex is elicited by the activation of one or more classes of sensory receptors. In the following section, two receptor types, muscle stretch receptors (muscle spindles) and Golgi tendon organs, are described in detail because these receptors are important both for spinal reflexes and as a source of the proprioceptive information that gives us an awareness of our limbs and helps guide voluntary movement.
The Muscle Spindle
Muscle spindles are found in almost all skeletal muscles and are particularly concentrated in muscles that exert fine motor control (e.g., the small muscles of the hand and eye).
Structure of the Muscle Spindle.
As its name implies, a muscle spindle is a spindle or fusiform-shaped organ composed of a bundle of specialized muscle fibers richly innervated both by sensory and by motor axons (Fig. 9-1). A muscle spindle is about 100 μm in diameter and up to 10 mm long. The innervated part of the muscle spindle is encased in a connective tissue capsule. Muscle spindles lie between regular muscle fibers and are typically located near the tendinous insertion of the muscle. The distal ends of the spindle are attached to the connective tissue within the muscle (endomysium). Thus, muscle spindles lie in parallel with the regular muscle fibers. This arrangement has important functional implications, as will be made clear later.
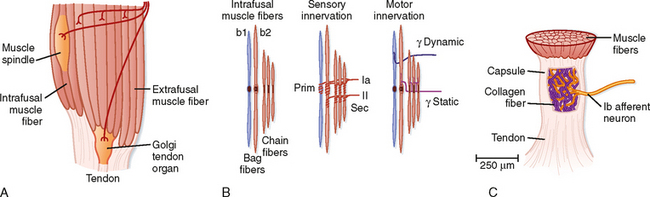
Figure 9-1 Muscle proprioceptors. Skeletal muscles contain sensory receptors embedded within the muscle (spindles) and within their tendons (Golgi tendon organs). A, Schematic of a muscle showing the arrangement of a spindle in parallel with extrafusal muscle fibers and a tendon organ in series with muscle fibers. B, Structure and motor and sensory innervation of a muscle spindle. C, Structure and innervation of a tendon organ.
The muscle fibers within the spindle are called intrafusal fibers to distinguish them from the regular or extrafusal fibers that make up the bulk of the muscle. Individual intrafusal fibers are much narrower than extrafusal fibers and do not run the length of the muscle. Thus, they are too weak to contribute significantly to muscle tension or to directly cause changes in the overall length of the muscle by their contraction.
Morphologically, two types of intrafusal muscle fibers are found within muscle spindles: nuclear bag and nuclear chain fibers (Fig. 9-1, B). These names are derived from the arrangement of nuclei in the fibers. Nuclear bag fibers are larger than nuclear chain fibers, and their nuclei are bunched together like a bag of oranges in the central, or equatorial, region of the fiber. In nuclear chain fibers, the nuclei are arranged in a row. Functionally, nuclear bag fibers are divided into two types: bag1 and bag2. As detailed later, bag2 fibers are functionally similar to chain fibers.
The neural innervation of an intrafusal fiber differs significantly from that of an extrafusal fiber, which is innervated by a single motor neuron. Intrafusal fibers are multiply innervated and receive both sensory and motor innervation. The sensory supply includes a single group Ia afferent and a variable number of group II afferent fibers (Fig. 9-1, B). Group Ia fibers belong to the largest-diameter class of sensory nerve fibers and conduct at 72 to 120 m/sec; group II fibers are intermediate in size and conduct at 36 to 72 m/sec. A group Ia afferent fiber forms a primary ending consisting of a spiral-shaped terminal composed of branches of the group Ia fiber on each of the intrafusal muscle fibers. Thus, terminals of primary endings are found on both types of nuclear bag fibers and on nuclear chain fibers. The group II afferent fiber forms a secondary ending, which is found on nuclear chain and bag2 fibers, but not on bag1 fibers. The primary and secondary endings have mechanosensitive channels that are sensitive to the level of tension on the intrafusal muscle fiber.
The motor supply to a muscle spindle consists of two types of γ motor axons (Fig. 9-1, B). Dynamic γ motor axons end on nuclear bag1 fibers, and static γ motor axons end on nuclear chain and bag2 fibers.
Muscle Spindles Detect Changes in Muscle Length.
Muscle spindles respond to changes in muscle length because they lie in parallel with the extrafusal fibers and therefore will also be stretched or shortened along with the extrafusal fibers. Because intrafusal fibers, like all muscle fibers, display spring-like properties, a change in their length will change the tension that they are under, and this change is sensed by mechanoreceptors of the Ia and II spindle afferents.
Figure 9-2 shows the changes in activity of the afferent fibers of a muscle spindle when the muscle is stretched. It is clear that Ia and II fibers respond differently to stretch. Group Ia fibers are sensitive both to the amount of muscle stretch and to its rate, whereas group II fibers respond chiefly to the amount of stretch. Thus, when a muscle is stretched to a new longer length, group II firing will increase in proportion to the amount of stretch (Fig. 9-2, left), and when the muscle is allowed to shorten, its firing rate will decrease proportionately (Fig. 9-2, right). Group Ia fibers show this same static-type response, and thus under steady-state conditions (i.e., constant muscle length), their firing rate will reflect the amount of muscle stretch, similar to that of group II fibers. However, while muscle length is changing, group Ia firing also reflects the rate of stretch or shortening that the muscle is undergoing. Its activity overshoots during muscle stretch and undershoots (and possibly ceases) during muscle shortening. These are called dynamic responses. This dynamic sensitivity also means that the activity of group Ia fibers is much more sensitive to transient stretches, such as shown in the middle diagrams of Figure 9-2. In particular, the tap profile is what occurs when a doctor uses a reflex hammer to hit the muscle tendon and thereby cause a brief stretching of the attached muscle. The change in muscle length is too brief for changes in group II firing to occur, but because the magnitude of the rate of change (slopes of the tap profile) is so high with this stimulus, large dynamic responses are elicited in the group Ia fibers. Thus, the functionality of reflex arcs involving Ia afferents is what is being assessed by using a reflex hammer to tap on tendons.
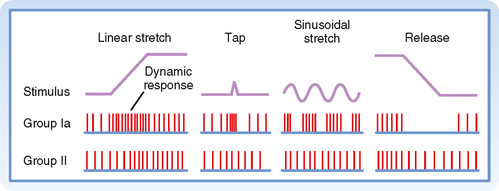
Figure 9-2 Responses of a primary ending (Ia) and a secondary ending (II) to changes in muscle length. Note the difference in dynamic and static responsiveness of these endings. The waveforms at the top represent the changes in muscle length. The middle and bottom rows show the discharges of a group Ia and II fiber, respectively, during the various changes in muscle length.
γ Motor Neurons Adjust the Sensitivity of the Spindle.
Up to this point we have described only how muscle spindles behave when there are no changes in γ motor neuron activity. The efferent innervation of muscle spindles is extremely important, however, because it determines the sensitivity of muscle spindles to stretch. For example, in Figure 9-3, A, the activity of a muscle spindle afferent is shown during a steady stretch. When the extrafusal portion of the muscle contracts (Fig. 9-3, B), the muscle spindle is unloaded by the resultant shortening of the muscle, and the muscle spindle afferent may stop discharging and thus become insensitive to further changes in muscle length. However, this unloading of the spindle can be counteracted if γ motor neurons are simultaneously stimulated. Such stimulation causes the intrafusal muscle fibers of the spindle to shorten along with the extrafusal muscle fibers (Fig. 9-3, C). Actually, only the two polar regions of the intrafusal muscle contract; the equatorial region, where the nuclei are located, does not contract because it has little contractile protein. As a result, when the polar regions contract, the equatorial region elongates and regains its sensitivity. Conversely, when a muscle relaxes and thus elongates, a concurrent decrease in γ motor neuron activity will allow the intrafusal fibers to relax as well and thereby prevent the tension on the central portion of the intrafusal fiber from reaching a level at which firing of the afferents is saturated. Thus, the γ motor neuron system allows the muscle spindle to operate over a wide range of muscle lengths while retaining high sensitivity to small changes in length.
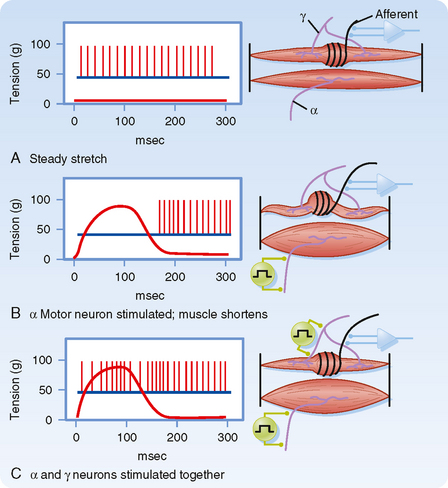
Figure 9-3 The activity of γ motor neurons can counteract the effects of unloading on the discharge of a muscle spindle afferent. A, The activity of a muscle spindle afferent is shown during steady stretch. B, α Motor neuron stimulation at time t = 0 msec causes contraction of the extrafusal fibers, which leads to muscle shortening and increased muscle tension, but unloading of the tension across the muscle spindle, which in turn induces the afferent to stop firing. Upon relaxation the muscle returns to its original length and tension is restored on the intrafusal fibers, causing the return of activity in the Ia afferent. C, Coactivation of α and γ motor neurons causes shortening of both extrafusal and intrafusal fibers. Thus, there is no unloading of the spindle, and the afferent maintains its spontaneous activity.
(Redrawn from Kuffler SW, Nicholls JG: From Neuron to Brain. Sunderland, MA, Sinauer, 1976.)
Descending motor commands from the brain typically activate α and γ motor neurons simultaneously and thus cause a synchronous contraction of extrafusal and intrafusal muscle fibers. This co-contraction means that as the muscle shortens from the contraction of extrafusal fibers, the polar regions of the intrafusal fibers also shorten, thereby maintaining relatively constant tension on the equatorial portion and thus the sensitivity of the spindle apparatus.
As mentioned earlier, there are two types of γ motor neurons—dynamic and static (Fig. 9-1). Dynamic γ motor axons end on nuclear bag1 fibers, and static γ motor axons synapse on nuclear chain and bag2 fibers. Thus, when a dynamic γ motor neuron is activated, the response of the group Ia afferent fiber is enhanced, but the activity of the group II afferents is unchanged; when a static γ motor neuron discharges, the responsiveness of the group II afferents and the static responsiveness of the group Ia afferents are increased. The effects of stimulating the static and dynamic fibers on a group Ia afferent’s response to stretch is illustrated in Figure 9-4. Descending pathways can preferentially influence dynamic or static γ motor neurons and thereby alter the nature of reflex activity in the spinal cord.
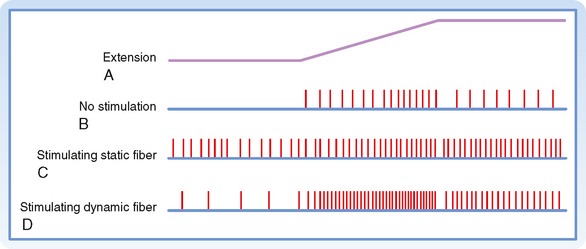
Figure 9-4 Effects of static and dynamic γ motor neurons on the responses of a primary ending to muscle stretch. The upper trace, A, is the time course of the stretch. B shows the discharge of group Ia fibers in the absence of γ motor neuron activity. In C, a static γ motor axon was stimulated, and in D, a dynamic γ motor axon was stimulated.
(Redrawn from Crowe A, Matthews PBC: J Physiol 174:109, 1964.)
Golgi Tendon Organ
A second type of mechanosensitive receptor associated with skeletal muscle is the Golgi tendon organ (Fig. 9-1). A Golgi tendon organ is innervated from the terminals of group Ib afferent fibers. The diameter of a Golgi tendon organ is about 100 μm and its length is about 1 mm. A group Ib fiber has a large diameter and conducts in the same velocity range as a group Ia fiber. The terminals of a Ib fiber are wrapped about bundles of collagen fibers in the tendon of a muscle (or in tendinous inscriptions within the muscle). Thus, the sensory ending is arranged in series with the muscle, in contrast to the parallel arrangement of the muscle spindle.
Because of their in-series relationship to the muscle, Golgi tendon organs can be activated either by muscle stretch or by contraction of the muscle. In both cases, however, the actual stimulus sensed by the Golgi tendon organ is the force that develops in the tendon to which it is linked. Thus, the response to stretch is the result of the spring-like nature of the muscle (i.e., by Hooke’s law, the force on a spring is proportional to how much it is stretched).
The distinction between the responsiveness of the muscle spindles and Golgi tendon organs can be made clear by comparing the firing patterns of Ia and Ib fibers when a muscle is stretched and then held at a longer length (Fig. 9-5). The Ia fiber’s firing rate will maintain its increase until the stretch is reversed. In contrast, the Ib fiber will show an initial large increase in firing, reflecting the increased tension on the muscle caused by the stretch, but will then show a gradual return toward its initial firing rate as the tension on the muscle is lowered because of cross-bridge recycling and the resultant lengthening of the sarcomeres. Therefore, Golgi tendon organs signal force, whereas spindles signal muscle length. Further evidence of this distinction is that Ib firing correlates with force level during isometric contraction even though muscle length, and therefore Ia activity, are unchanged.
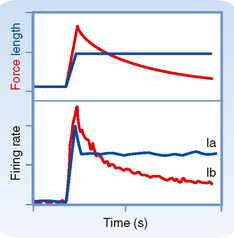
Figure 9-5 Changes in group Ia and Ib firing rates when muscle is stretched to a new length as indicated in the top graph (blue line). After a transient burst, the firing rate of the Ia fiber remains constant at a new higher level that is proportional to the increase in length (lower graph, blue line). In contrast, the Ib unit shows an initial rapid increase in firing followed by a slow decrease back toward its original level (bottom graph, red line) and has a firing profile that matches the tension level in the muscle caused by the stretch (top graph, red line).
The Myotatic or Stretch Reflex
The stretch reflex is key for the maintenance of posture and helps overcome unexpected impediments during a voluntary movement. Changes in the stretch reflex are involved in actions commanded by the brain, and pathological alterations in this reflex are important signs of neurological disease. The phasic stretch reflex occurs in response to rapid, transient stretches of the muscle, such as those elicited by a doctor using a reflex hammer or by an unexpected impediment to an ongoing movement. The tonic stretch reflex occurs in response to a slower or steady stretch applied to the muscle.
The Phasic (or Ia) Stretch Reflex.
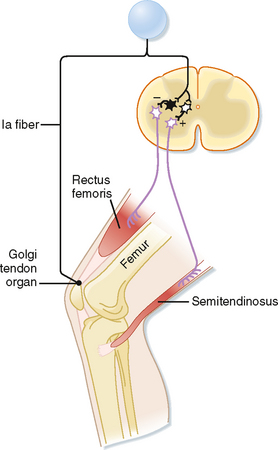
The phasic stretch reflex is elicited by the primary endings of the muscle spindles. The reflex arc responsible for the phasic stretch reflex is shown in Figure 9-6. A group Ia afferent fiber from a muscle spindle in the rectus femoris muscle is shown to branch as it enters the gray matter of the spinal cord. It will form excitatory synapses directly (monosynaptically) on virtually all α motor neurons that supply the same (also known as the homonymous) muscle and with many of its synergists, such as the vastus intermedius muscle in this case, which also acts to extend the leg at the knee. If the excitation is powerful enough, the motor neurons discharge and cause a contraction of the muscle. Note that the Ia fibers do not contact the γ motor neurons, possibly to avoid a positive-feedback loop situation. This selective targeting of α motor neurons is exceptional in that most other reflex and descending pathways will target both α and γ motor neurons.
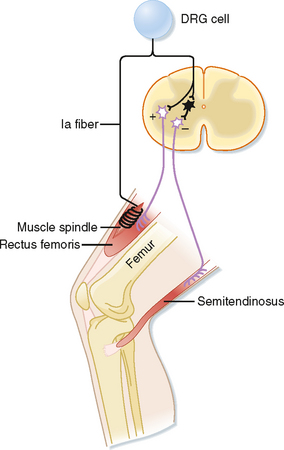
Figure 9-6 Reflex arc of the stretch reflex. The shortest pathway in this arc contains a single synapse within the CNS; hence, it is a monosynaptic reflex. The interneuron, shown in black, is a group Ia inhibitory interneuron.
Other branches of group Ia fibers end on a variety of interneurons; however, one type, the reciprocal Ia inhibitory interneuron (black cell in Fig. 9-6), is particularly important with regard to the stretch reflex. These interneurons are identifiable because they are the only inhibitory interneurons that receive input from both the Ia afferents and Renshaw cells (see Fig. 9-11). They end on α motor neurons that innervate the antagonist muscles, in this case the hamstring muscles, including the semitendinosus muscle, which act to flex the knee.
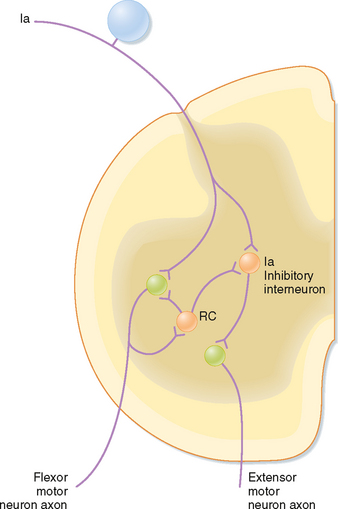
Figure 9-11 Renshaw cell (RC) connections with motor neurons and Ia inhibitory interneurons. The circuits shown mediate Ia reciprocal inhibition of antagonist muscles (in this case an extensor) and inhibition of this reciprocal inhibition by Renshaw cells. Note that there are equivalent Renshaw cells and Ia inhibitory interneurons associated with extensor motor neurons and Ia input from spindles in extensor muscles, but they are not shown for simplicity. Orange cells are inhibitory and blue and green ones are excitatory.
The organization of the stretch reflex arc guarantees that one set of α motor neurons is activated and the opposing set is inhibited. This arrangement is known as reciprocal innervation. Although many reflexes involve such reciprocal innervation, this type of innervation is not the only possible organization of a motor control system, and indeed, descending motor pathways can override such patterns.
The stretch reflex is quite powerful, in large part because of its monosynaptic nature. The power of this reflex also derives from the essentially maximal convergence and divergence that exist in this pathway, which is not apparent from the circuit diagrams, such as Figure 9-6, that are typically used to illustrate reflex pathways. That is, each Ia fiber will contact virtually all homonymous α motor neurons, and each such α motor neuron will receive input from every spindle in that muscle. Although its monosynaptic nature makes the Ia reflex rapid and powerful, it also means that there is relatively little opportunity for direct control of activity flow through its reflex arc. The CNS overcomes this problem by controlling muscle spindle sensitivity via the γ motor neuron system.
The Tonic Stretch Reflex.
The tonic stretch reflex can be elicited by passively bending a joint. This reflex circuit includes both group Ia and group II afferent fibers from muscle spindles. Group II fibers make monosynaptic excitatory connections with α motor neurons, but they also excite them through disynaptic and polysynaptic pathways. Normally, there is ongoing activity in the Ia and II afferents that helps maintain a baseline firing of α motor neurons; therefore, the tonic stretch reflex contributes to muscle tone. Its activity also contributes to our ability to maintain a posture. For example, if the knee of a soldier standing at attention begins to flex because of fatigue, the quadriceps muscle will be stretched, a tonic stretch reflex will be elicited, and the quadriceps will contract more, thereby opposing the flexion and restoring the posture.
Hyperactive stretch reflexes can lead to tremors and clonus. Although the negative-feedback action of the stretch reflex should help stabilize the limb, the conduction delay between the initiating stimulus (muscle stretch) and the response (muscle contraction) can cause it to be a source of instability resulting in rhythmic movements such as tremors and clonus. Clonus is elicited by a sustained stretch of a muscle in a person who has spinal cord damage. Normally, an imposed sustained stretch on a muscle will elicit an increase in Ia and II activity, which after a delay will cause a contraction in the muscle that opposes the stretch but does not completely return the muscle to its initial length because the gain of the stretch reflex is much less than 1.* This partial compensation, in turn, will lead to a decrease in Ia and II activity, which causes the limb to lengthen again, but not fully. This lengthening will once again increase Ia and II activity, and so on. The delay is key in setting up this oscillation because it leads to the feedback signal continuing even after the muscle has compensated and thus results in an overcompensation that leads to the next overcorrection. However, because the reflex gain is normally much less than 1, this oscillation dies out quickly (the overcompensation gets smaller and smaller), and the muscle comes to rest at an intermediate length. In contrast, when descending motor pathways are damaged, the resulting changes in spinal cord connectivity and increases in neuronal excitability result in a hyperactive reflex (which is equivalent to raising the gain close to 1). In this case, the successive overcompensations are much larger, and an overt but transient oscillation can be observed (clonus). If the gain equals 1, the clonus does not die out but rather persists for as long as the initial stretch stimulus is maintained.
* In general, gain of a system is defined as its output for a given input. Here, the input to the system is the imposed stretch, and the output is movement caused by the stretch reflex—evoked contraction.
The foregoing discussion suggests that stretch reflexes can act like a negative-feedback system to control muscle length. By following the stretch reflex arc, it is possible to see that changes in its activity will act to oppose changes in muscle length from a particular equilibrium point. For example, if the muscle’s length is increased, there will be an increase in Ia and II firing, which will excite homonymous α motor neurons and lead to contraction of the muscle and reversal of the stretch. Similarly, passive shortening of the muscle will unload the spindles and lead to a decrease in the excitatory drive to the motor neurons and thus relaxation of the muscle. So how are we able to rotate our joints? It is partly because the γ motor neurons are coactivated during a movement and thereby shift the equilibrium point of the spindle and partly because the gain or strength of the reflex is low enough that other input to the motor neuron can override the stretch reflex.
Inverse Myotatic or Ib Reflex
Just as the stretch reflex can be thought of as a feedback system to regulate muscle length, the inverse myotactic, or Ib, reflex can be thought of as a feedback system to help maintain force levels in a muscle. Using the upper part of the leg as an example, the Ib reflex arc is shown in Figure 9-7. In this example the receptor organs are Golgi tendon organs of the rectus femoris muscle. The afferent fibers branch as they enter the spinal cord and end on interneurons. There are no monosynaptic connections to α motor neurons. Rather, the Ib afferents synapse onto two classes of interneurons: interneurons that inhibit α motor neurons that supply the homonymous muscle, in this case the rectus femoris, and excitatory interneurons that activate α motor neurons to the antagonist (semitendinosus). Because there are two synapses in series in the CNS, this is a disynaptic reflex arc. Given these connections, Ib activity should have the opposite action of the Ia stretch reflex during passive stretch of the muscle, which explains this reflex’s other name, the inverse myotactic reflex. However, functionally, the two reflex arcs can act synergistically, as the following example shows. Recall that the Golgi tendon organs monitor force levels across the tendon that they supply. If during maintained posture, such as standing at attention, knee extensors, such as the rectus femoris muscle, begin to fatigue, the force in the patellar tendon will decline. The decline in force will reduce the activity of Golgi tendon organs in this tendon. Because the Ib reflex normally inhibits the α motor neurons to the rectus femoris muscle, reduced activity of the Golgi tendon organs will enhance the excitability of (i.e., disinhibit) the α motor neurons and thereby help reverse the decrease in force caused by the fatigue. Simultaneously, bending of the knee will stretch the knee extensors and activate the Ia fibers, which will then excite the same α motor neurons. Thus, coordinated action of both muscle spindle and Golgi tendon organ afferent fibers is needed to cause greater contraction of the rectus femoris muscle and maintenance of the posture.
Flexion Reflexes and Locomotion
The flexion reflex starts with activation of one or more of a variety of sensory receptors, including nociceptors, whose signals can be carried to the spinal cord via a variety of afferents, including group II and III fibers, collectively called the flexion reflex afferents (FRAs). In flexion reflexes, afferent volleys (1) cause excitatory interneurons to activate the α motor neurons that supply the flexor muscles in the ipsilateral limb and (2) cause inhibitory interneurons to inhibit the α motor neurons that supply the antagonistic extensor muscles (Fig. 9-8). This pattern of activity causes one or more joints in the stimulated limb to flex. In addition, commissural interneurons evoke the opposite pattern of activity in the contralateral side of the spinal cord (Fig. 9-8), which results in extension of the opposite limb, the crossed extension reflex. For our lower limbs (or in quadrupeds for both forelimbs and hind limbs), the crossed extension part of the reflex helps in maintaining balance by enabling the contralateral limb to be able to support the additional load that is transferred to it when the flexed limb is lifted.
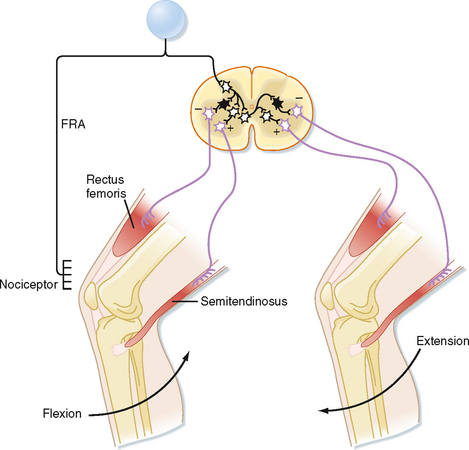
Figure 9-8 The reflex arc of the flexion reflex. Black interneurons are inhibitory and clear ones are excitatory. FRA, flexion reflex afferent.
After damage to the descending motor pathways, hyperactive stretch reflexes may result in spasticity, in which there is large resistance to passive rotation of the limbs. In this condition it may be possible to demonstrate what is called the clasp-knife reflex. When spasticity is present, attempts to rotate a limb about a joint will initially meet high resistance. However, if the applied force is increased, there will come a point at which the resistance suddenly dissipates and the limb rotates easily. This change in resistance is caused by reflex inhibition. The Ib reflex arc suggests that rising activity in this pathway could underlie the sudden release of resistance, and indeed, the clasp-knife reflex was once attributed to the activation of Golgi tendon organs when these receptors were thought to have a high threshold to muscle stretch. However, the tendon organs have since been shown to be activated at very low levels of force and are no longer thought to cause the clasp-knife reflex. It is now thought that this reflex is caused by the activation of other high-threshold muscle receptors that supply the fascia around the muscle. Signals from these receptors cause the activation of interneurons that lead to inhibition of the homonymous motor neurons.
Because flexion typically brings the affected limb in closer to the body and away from a painful stimulus, flexion reflexes are a type of withdrawal reflex. In Figure 9-8, the neural circuit of the flexion reflex is shown for neurons that affect only the knee joint. Actually, however, considerable divergence of the primary afferent and interneuronal pathways occurs in the flexion reflex. In fact, all the major joints of a limb (e.g., hip, knee, and ankle) may be involved in a strong flexor withdrawal reflex. Details of the flexor withdrawal reflex vary, depending on the nature and location of the stimulus. This variability in flexion reflex is called the local sign. Flexor withdrawal reflexes also occur in areas other than the limbs; for example, visceral disease may cause contractions of muscles in the chest wall or abdomen and thereby decrease the mobility of the trunk.
The interneurons subserving flexion reflexes also appear to be part of the central pattern generator (CPG) for generating locomotion and thus are an example of how the reflex circuits are used for multiple purposes. A CPG is a set of neurons and circuits capable of generating the rhythmic activity that underlies motor acts, even in the absence of sensory input. Using the FRA interneurons as an example, one can see that activation of the FRA interneurons leads to a pattern of flexor excitation and extensor inhibition on one side and the converse pattern on the opposite side and that if the FRA interneurons on each side of the spinal cord alternated in being active, a stepping pattern would emerge. That is, walking motion is the result of alternately activating flexors and extensors in each leg such that activation of the flexors (and extensors) in the two legs occurs out of phase with each other, exactly what would be produced by alternately activating the FRA interneurons on each side. Note that such a rhythmic activity pattern in the FRA circuits is not dependent on activity from the FRAs themselves (e.g., they could be activated by descending pathways from the brain).
To show that these circuits are actually involved in generating the locomotion rhythm, spinal cord preparations were made that showed spontaneous locomotion (i.e., if the brainstem is transected and weight is supported, the spinal cord circuits can generate activity that causes the limbs to generate a normal locomotion sequence.) In one such preparation, the electromyogram from the flexors and extensors of a limb were recorded and the FRAs then stimulated to see the effect on locomotion rhythm (Fig. 9-9). Before any stimulus, a spontaneous alternating pattern of flexor and extensor electromyographic (EMG) activity exists. If the FRAs were not involved in the locomotion circuit or at least were not a critical part of the circuits responsible for generating the rhythm (Fig. 9-9, B), we would expect the stimulus to produce only a transient response (i.e., a single EMG response of the flexors and brief inhibition of the extensors) and have no long-term effect on this pattern. Such a transient response is observed (Fig. 9-9, A; EMG records just after the stimulus). However, the stimulus also causes a permanent, approximately 180-degree phase shift in locomotor rhythm, as can be seen by comparing the times of contractions before and after the stimulus. The dashed vertical lines indicate the times at which a flexor EMG response would be expected if the stimulus had produced no phase shift from the EMG activity pattern; before the stimulus, each vertical line is aligned with the onset of a flexor EMG burst, whereas after the stimulus, each vertical line occurs at the end of the flexor burst. Therefore, we can conclude that the stimulus affected the locomotor CPG itself and that the FRA interneurons are a critical part of this CPG (Fig. 9-9, C).
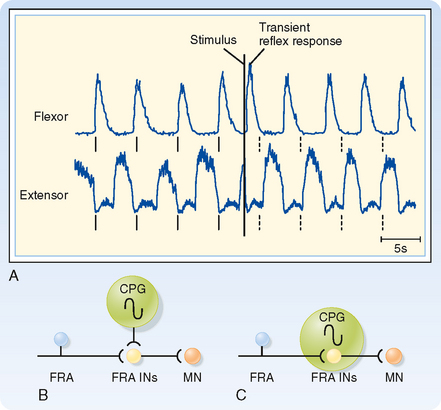
Figure 9-9 Phase reset of locomotion rhythm by FRA stimulation helps identify neuronal components of the underlying central pattern generator (CPG). A, EMG records from knee flexor and extensor muscles. Note the rhythmic alternating pattern before application of the stimulus. The solid vertical lines below each trace indicate the times at which flexor contraction is initiated. The dashed vertical lines indicate the times at which flexor contraction would have been initiated if the stimulus caused no lasting effect on the rhythmic pattern. B and C, Two possible models for the CPG underlying the locomotor rhythm seen in A. B does not include the FRA interneurons in the CPG, whereas C does. The data shown in A support the model shown in C.
(Data from Hultborn H et al: Ann N Y Acad Sci 860:70, 1998.)
A second important point illustrated by this experiment is that the locomotion CPG (and CPGs generally) can be influenced by strong afferent activity. The afferent influence ensures that the pattern generator adapts to changes in the terrain as locomotion proceeds. Such changes may occur rapidly during running, and locomotion must then be adjusted to ensure proper coordination.
Determining Spinal Cord Organization by Using Reflexes
As already discussed, divergence is an important aspect of reflex pathways. Convergence is another important organizational feature of reflex arcs. Convergence is defined as the termination of several neurons on one other neuron. For example, all group Ia afferent fibers from the muscle spindles of a particular hind limb muscle synapse onto any given α motor neuron to that muscle. Convergent input can be demonstrated by using the phenomenon of spatial facilitation, which is illustrated in Figure 9-10.
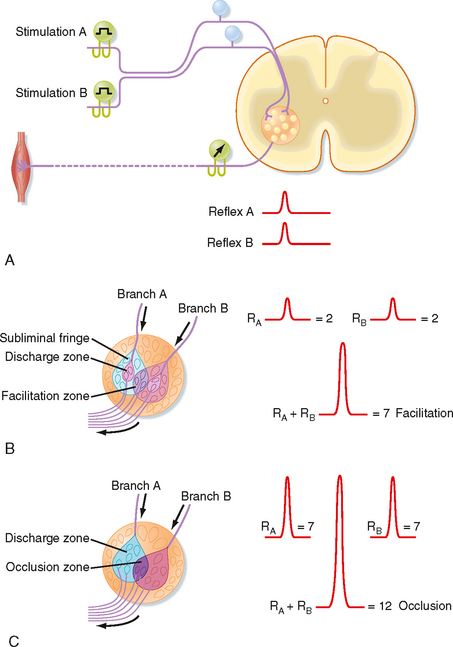
Figure 9-10 A, Arrangement for using electrically evoked afferent volleys and recordings from motor axons in a ventral root to study reflexes. B, Experiment in which combined stimulation of two muscle nerves resulted in spatial summation. In C, the combined volleys caused occlusion.
(Redrawn from Eyzaguirre C, Fidone SJ: Physiology of the Nervous System, 2nd ed. Chicago, Mosby—Year Book, 1975.)
In this example, a monosynaptic reflex is elicited by electrical stimulation of the group Ia fibers in each of two branches of a muscle nerve (Fig. 9-10, A). The reflex response is characterized by recording the discharges of α motor axons from the appropriate ventral root (as a compound action potential). When muscle nerve branch A is stimulated, a small compound action potential is recorded as reflex A. Similarly, when muscle nerve branch B is stimulated, reflex B is recorded. Figure 9-10, B, depicts the motor neurons contained within the motor nucleus. The discharge zones (pink colored areas) enclose α motor neurons that are activated above threshold when each muscle nerve branch is stimulated separately. Thus, two α motor neurons spike when each muscle nerve branch is stimulated alone (an additional seven motor neurons in the subliminal fringe are excited, but not sufficiently to trigger spikes). When the two nerves are stimulated at the same time, a much larger reflex discharge is recorded (see recordings at the right of Fig. 9-10, B). As the figure demonstrates, this reflex represents the discharge of seven α motor neurons: the four that spiked after the singular stimulation of each nerve (two per nerve) and three additional α motor neurons (located in the facilitation zone) that are made to discharge only when the two muscle nerves are stimulated simultaneously because they lie in the subliminal fringe for both nerves.
A similar effect could be elicited by repetitive stimulation of one of the muscle nerves, provided that the stimuli occur close enough together that some of the excitatory effect of the first volley still persists after the second volley arrives. This effect is called temporal summation. Both spatial summation and temporal summation depend on the properties of the EPSPs evoked in α motor neurons by the group Ia afferent fibers (see Fig. 6-8).
If a volley in one of the two muscle nerves in Figure 9-10 reaches the motor nucleus at a time when the motor neurons are highly excitable, the reflex discharge will be relatively large (see Fig. 9-10, C). A similar volley in the other muscle nerve might also produce a large reflex response. However, when the two muscle nerves are excited simultaneously, the reflex can be less than the sum of the two independently evoked reflexes if the cells reaching threshold to activation of either of the two nerves alone overlap significantly. In this case, each afferent nerve activates 7 α motor neurons, but the volleys in the two nerves together cause only 12 motor neurons to discharge. This phenomenon is called occlusion.
The phenomena of spatial and temporal summation and occlusion can also be used to demonstrate interactions between spinal cord neurons and the various reflex circuits. To start, a monosynaptic reflex discharge can be evoked by stimulating the group Ia afferent fibers in a muscle nerve. This tests the reflex excitability of a population of α motor neurons. The discharges of either extensor or flexor α motor neurons can be recorded by choosing the proper muscle nerve to be stimulated. Other kinds of afferent fibers are then stimulated along with the homonymous Ia afferent from the muscle to see whether the response to the Ia stimulation changes. For example, stimulation of group Ia afferent fibers in the nerve to the antagonist muscles produces inhibition of the response to the homonymous Ia stimulation (which is mediated by what is called the reciprocal Ia inhibitory interneuron). Alternatively, if the small afferent fibers of a cutaneous nerve are stimulated to evoke a flexion reflex, the responses to Ia stimulation of the α motor neurons that innervate the extensor muscles will be inhibited (and those of α motor neurons that innervate flexor muscles will be potentiated). As a final example, stimulation of a ventral root causes inhibition of Ia responses and inhibits the reciprocal Ia inhibition. Because the ventral root contains only motor neuron axons, this result implies the presence of axon collaterals that excite inhibitory interneurons that feed back onto the same motor neuron population (Fig. 9-11). These interneurons are named Renshaw cells. Because ventral root stimulation also inhibits the Ia inhibition of antagonist motor neurons, but no other classes of interneurons, the reciprocal Ia interneurons can be uniquely identified by their being inhibited by ventral root stimulation (and activated by Ia stimulation).
Topographic Organization of the Ventral Horn
Up to this point we have considered the functional organization of the spinal cord, largely without regard to its physical (i.e., anatomic) instantiation. We now turn to this aspect of spinal cord organization by discussing the organization of the ventral horn and in particular the topographic arrangement of the motor neurons contained therein. This topography has functional implications for how the descending motor tracts interact with the spinal cord machinery that we have been discussing.
Spinal cord motor neurons are organized topographically in rostrocaudally running columns in the ventral horn (Fig. 9-12). Motor neurons that supply the axial musculature form a column of cells that extends the length of the spinal cord. In the cervical and lumbosacral enlargements, these cells are located in the most medial part of the ventral horn. Motor neurons that supply the limb muscles form columns that extend for several segments in the lateral part of the ventral horn in the cervical and lumbosacral enlargements. Motor neurons to muscles of the distal part of the limb are located most laterally, whereas those that innervate more proximal muscles are located more medially. Motor neurons to flexors are dorsal to those that innervate extensors. Note that the α and γ motor neurons to a given muscle are found intermixed within the same motor neuron column.
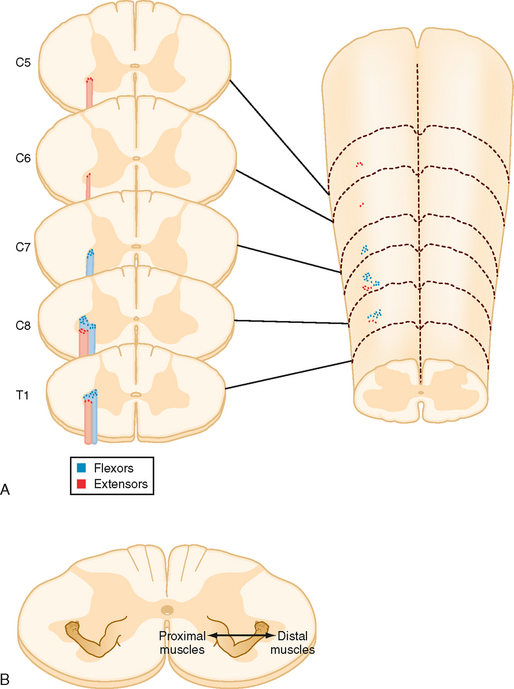
Figure 9-12 Musculotopic organization of motor neurons in the ventral horn of the spinal cord. A, Schematic of the cervicothoracic spinal cord and associated cross sections, showing the locations of motor neurons that innervate a flexor (blue dots) and an extensor (red dots). B, Spinal cord cross section with locations of different muscles represented by a drawing of the arm.
(Redrawn from Purves D et al [eds]: Neuroscience, 3rd ed. Sunderland, MA, Sinauer, 2004.)
The interneurons that connect with the motor neurons in the enlargements are also topographically organized. In general, interneurons that supply the limb muscles are located mainly in the lateral parts of the deep dorsal horn and the intermediate region that lies between the dorsal and ventral horns. Those that supply the axial muscles, however, are located in the medial part of the ventral horn. These interneurons receive synaptic connections from primary afferent fibers and from the axons of pathways that descend from the brain, and thus are both part of spinal reflex arcs and descending motor control pathways.
An important aspect of interneuronal systems is that the laterally placed interneurons project ipsilaterally to motor neurons that supply the distal or the proximal limb muscles, whereas the medial interneurons project bilaterally. This arrangement of the lateral interneurons allows the limbs to be controlled independently. In contrast, the bilateral arrangement of the medial interneurons allows bilateral control of motor neurons to the axial muscles to provide postural support to the trunk and neck.
DESCENDING MOTOR PATHWAYS
Classification of Descending Motor Pathways
Pyramidal versus Extrapyramidal Pathways
Descending motor pathways were traditionally subdivided into pyramidal tract and extrapyramidal pathways. This terminology reflects a clinical dichotomy between pyramidal tract disease and extrapyramidal disease. In pyramidal tract disease, the corticospinal, or pyramidal, tract is interrupted. The signs of this disease were originally attributed to the loss of function of the pyramidal tract (so named because the corticospinal tract passes through the medullary pyramid). However, in many cases of pyramidal tract disease, the functions of other pathways are also altered, and most pyramidal tract signs (see the later section Motor Deficits Caused by Lesions of Descending Motor Pathways) appear to not be caused by loss of the corticospinal tract or at least require damage to additional motor pathways. The term extrapyramidal is even more problematic. Thus, this classification system is not used in this book.
Lateral versus Medial Motor Systems
Another way of classifying the motor pathways is based on their sites of termination in the spinal cord and the consequent differences in their roles in the control of movement and posture. The lateral pathways terminate in the lateral portions of the spinal cord gray matter (Fig. 9-13). The lateral pathways can excite motor neurons directly, although interneurons are their main target. They influence reflex arcs that control fine movement of the distal ends of limbs, as well as those that activate supporting musculature in the proximal ends of limbs. The medial pathways end in the medial ventral horn on the medial group of interneurons (Fig. 9-13). These interneurons connect bilaterally with motor neurons that control the axial musculature and thereby contribute to balance and posture. They also contribute to the control of proximal limb muscles. In this book we use the lateral/medial terminology to classify the descending motor pathways. However, even this scheme is not perfect, partly because although motor neuron cell bodies form localized columns, motor neuron dendritic trees are rather large and typically span most of the ventral horn. Thus, any motor neuron can potentially receive input from so-called medial or lateral system pathways.
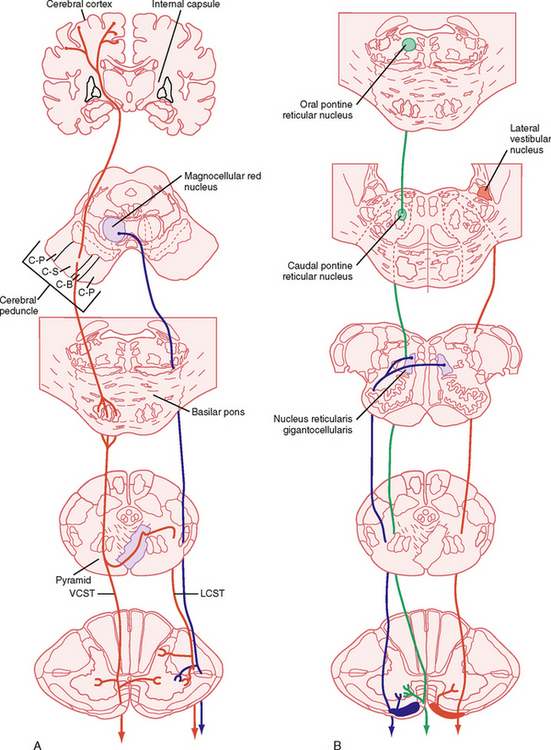
Figure 9-13 Descending motor pathways. Major pathways connecting the cortical and brainstem motor areas to the spinal cord are shown. A, Lateral system pathways, corticospinal (red) and rubrospinal (blue) pathways. Note that the ventral corticospinal pathway is part of the medial system, but is shown in A for simplicity. B, Medial system pathways, medullary (blue) and pontine (green) reticulospinal and lateral vestibulospinal (red) pathways. C-B, corticobulbar; C-P, corticopontine; C-S, corticospinal; LCST, lateral corticospinal tract; VCST, ventral corticospinal tract.
The Lateral System
Lateral Corticospinal and Corticobulbar Tracts
The corticospinal and corticobulbar tracts originate from a wide region of the cerebral cortex. This region includes the primary motor, premotor, supplementary, and cingulate motor areas of the frontal lobe and the somatosensory cortex of the parietal lobe. The cells of origin of these tracts include both large and small pyramidal cells of layer V of the cortex, including the giant pyramidal cells of Betz. Although Betz cells are a defining feature of the motor cortex, they represent a small minority (< 5%) of the cells that contribute to these tracts, in part because they are found only in the primary motor cortex, and even here they represent a minority of the cells contributing to the tract. These tracts leave the cortex and enter the internal capsule, then traverse the midbrain in the cerebral peduncle, pass through the basilar pons, and emerge as the pyramids on the ventral surface of the medulla (Fig. 9-13, A). The corticobulbar axons leave the tract as it descends the brainstem and terminate in the various cranial nerve motor nuclei. The corticospinal fibers continue caudally, and in the most caudal region of the medulla, about 90% of them cross to the opposite side. They then descend in the contralateral lateral funiculus as the lateral corticospinal tract. The lateral corticospinal axons terminate at all spinal cord levels, primarily on interneurons, but also on motor neurons. The remaining uncrossed axons continue caudally in the ventral funiculus on the same side as the ventral corticospinal tract, which belongs to the medial system. Many of these fibers ultimately decussate at the spinal cord level at which they terminate.
The lateral corticospinal tract is a relatively minor tract in lower mammals but becomes quantitatively and functionally very important in primates and in humans in particular, where it contains over 1 million axons. This number still represents a relatively small proportion of the outflow from the cortex because there are approximately 20 million axons in the cerebral peduncles. Nevertheless, the corticospinal pathway is critical for the fine independent control of finger movement inasmuch as isolated lesions of the corticospinal tract typically lead to a permanent loss of this ability, even though there is often recovery of other movement abilities with such lesions. Indeed, in primates, corticospinal synapses directly onto motor neurons are particularly prevalent for the motor neurons controlling finger muscles and are probably the basis of our ability to make independent, finely controlled finger movements.
The corticobulbar tract, which projects to the cranial nerve motor nuclei, has subdivisions that are comparable to the lateral and ventral corticospinal tracts. For example, part of the corticobulbar tract ends contralaterally in the portion of the facial nucleus that supplies muscles of the lower part of the face and in the hypoglossal nucleus. This component of the corticobulbar tract is organized like the lateral corticospinal tract. The remainder of the corticobulbar tract ends bilaterally.
Rubrospinal Tract
This tract originates in the magnocellular portion of the red nucleus, which is located in the midbrain tegmentum. These fibers decussate (cross) in the midbrain, descend through the pons and medulla, and then take up a position just ventral to the lateral corticospinal tract in the spinal cord. They preferentially affect motor neurons controlling distal musculature, similar to the corticospinal fibers. Red nucleus neurons receive input from the cerebellum and from the motor cortex, thus making this an area of integration of activity from these two motor systems.
The Medial System
The ventral corticospinal tract and much of the corticobulbar tract can be regarded as medial system pathways. These tracts end on the medial group of interneurons in the spinal cord and on equivalent neurons in the brainstem. The axial muscles are controlled by these pathways. These muscles often contract bilaterally to provide postural support or some other bilateral function, such as swallowing or wrinkling of the brow.
Other medial system pathways originate in the brainstem. These include the pontine and medullary reticulospinal tracts, the lateral and medial vestibulospinal tracts, and the tectospinal tract.
Pontine and Medullary Reticulospinal Tracts
The cells that give rise to the pontine reticulospinal tract are in the medial pontine reticular formation. The tract descends in the ventral funiculus, and it ends on the ipsilateral medial group of interneurons. Its function is to excite motor neurons to the proximal extensor muscles to support posture.
The medullary reticulospinal tracts arise from neurons of the medial medulla, in particular the nucleus gigantocellularis. The tracts descend bilaterally in the ventral lateral funiculus, and they end mainly on interneurons associated with medial motor neuron cell groups. The function of the pathway is mainly inhibitory.
Lateral and Medial Vestibulospinal Tracts
The lateral vestibulospinal tract originates in the lateral vestibular nucleus, also known as Deiter’s nucleus. This tract descends ipsilaterally through the ventral funiculus of the spinal cord and ends on interneurons associated with the medial motor neuron groups. The lateral vestibulospinal tract excites motor neurons that supply extensor muscles of the proximal part of the limb that are important for postural control. In addition, this pathway inhibits flexor motor neurons because it also excites the reciprocal Ia interneurons that receive Ia input from extensor muscles, which therefore inhibits flexor motor neurons. The excitatory input to the lateral vestibular nucleus is from both the semicircular canals and the otolith organs, whereas the inhibitory input is from the Purkinje cells of the anterior vermis region of the cerebellar cortex. An important function of the lateral vestibulospinal tract is to assist in postural adjustments after angular and linear accelerations of the head.
The medial vestibulospinal tract originates from the medial vestibular nucleus. This tract descends in the ventral funiculus of the spinal cord to the cervical and midthoracic levels, and it ends on the medial group of interneurons. Sensory input to the medial vestibular nucleus from the labyrinth is chiefly from the semicircular canals. This pathway thus mediates adjustments in head position in response to angular acceleration of the head.
The Tectospinal Tract
The tectospinal tract originates in the deep layers of the superior colliculus. The axons cross to the contralateral side, just below the periaqueductal gray matter. They then descend in the ventral funiculus of the spinal cord to terminate on the medial group of interneurons in the upper cervical spinal cord. The tectospinal tract regulates head movement in response to visual, auditory, and somatic stimuli.
Monoaminergic Pathways
In addition to the lateral and medial systems, less specifically organized systems descend from the brainstem to the spinal cord. These include several pathways that use monoamines as synaptic transmitters.
The locus coeruleus and the nucleus subcoeruleus are nuclei located in the rostral pons, and they are composed of norepinephrine-containing neurons. These nuclei project widely throughout the CNS and their projection to the spinal cord travels in the lateral funiculus. Their terminals are on interneurons and motor neurons. The dominant effect of the pathway is inhibitory.
The raphe nuclei of the medulla also project widely throughout the CNS and give rise to several raphe-spinal pathways. Many of the raphe-spinal cells contain serotonin. Terminals on dorsal horn interneurons are inhibitory, whereas terminals on motor neurons are excitatory. The dorsal horn projection may help reduce nociceptive transmission (Fig. 7-9), whereas the ventral horn projection may enhance motor activity.
In general, the monoaminergic pathways may alter the responsiveness of spinal cord circuits, including the reflex arcs. In this respect, they induce widespread changes in excitability rather than discrete movements or specific changes in behavior.
Motor Deficits Caused by Lesions of Descending Motor Pathways
A common cause of motor impairment in humans is interruption of the cerebral cortical efferent fibers in the internal capsule; such interruptions occur in capsular strokes. The resulting disorder is often termed a pyramidal tract syndrome, or upper motor neuron disease, although these names are misnomers. Motor changes characteristic of this disorder include (1) increased phasic and tonic stretch reflexes (spasticity); (2) weakness, usually of the distal muscles, especially the finger muscles; (3) pathological reflexes, including the sign of Babinski (dorsiflexion of the big toe and fanning of the other toes when the sole of the foot is stroked); and (4) a reduction in superficial reflexes, such as the abdominal and cremasteric reflexes. It is important to emphasize that if only the corticospinal tract is interrupted, as can occur with a lesion of the medullary pyramid, most of these signs are absent. In this situation, the most prominent deficits are weakness of the distal muscles, especially those of the fingers, and a Babinski sign. Spasticity does not occur, but instead muscle tone may actually decrease. Evidently, spasticity requires the disordered function of other pathways, such as the reticulospinal tracts, as would occur after loss of the descending cortical influence to the brainstem nuclei of origin of these tracts.
The effects of interruption of the medial system pathways are quite different from those produced by corticospinal tract lesions. The main deficits associated with medial system interruption are an initial reduction in the tone of postural muscles and loss of righting reflexes. Long-term effects include locomotor impairment and frequent falling. However, manual manipulation of objects is perfectly normal.
The Decerebrate Preparation
The decerebrate preparation has been useful for experimentally investigating how various descending pathways interact with the spinal cord circuitry. Surgical decerebration is achieved either by transecting the midbrain, often at an intercollicular level, or by occluding the blood vessels feeding this area. In the latter case, the anterior vermis of the cerebellum is also lesioned, an important distinction. With the intercollicular transection, some descending pathways, such as those originating in the cerebral cortex, are interrupted, whereas others, such as those originating in the brainstem, remain intact.
However, remember that the corticospinal tract is only a minor component of the cortical descending fibers. Many other cortical fibers project to locations throughout the brainstem, including the nuclei of origin for the medial descending pathways. Loss of these cortical control systems results in altered activity in the intact descending pathways. As a result, animals show hypertonia and suppression of some spinal reflexes, such as the flexion reflex, and exaggeration of others, such as the stretch reflex, a condition called decerebrate rigidity. Decerebrate animals maintain a posture that has been called exaggerated standing. Human patients with brainstem damage may also develop a decerebrate state that has many of the same reflex features as animal preparations. The prognosis in such patients is poor if signs of decerebration appear.
Loss of descending control on the reticular formation results in increased activity in the pontine reticulospinal pathway and decreased activity in the medullary reticulospinal pathway. This increase and decrease in activity, respectively, produce increased excitation and decreased inhibition (disinhibition) of the motor neurons, which explains the observed rigidity. Interestingly, this hypertonia can be relieved by cutting the dorsal roots, thus indicating that the reticulospinal tracts have a major effect on γ motor neurons, whose activity alters muscle stiffness only by increasing muscle spindle sensitivity and thereby causes increased activity in the Ia and II afferents that innervate the α motor neurons.
When vessel occlusion is used to generate the decerebrate state, the lateral vestibulospinal tract becomes hyperactive because of damage to Purkinje cells in the anterior vermis of the cerebellum, which provide the major inhibitory projection to the lateral vestibular nucleus. Interestingly, this hypertonia is not lost after transection of the dorsal roots, which implies that the lateral vestibulospinal tract is acting to a significant extent directly on the α motor neurons (either monosynaptically or via interneurons).
BRAINSTEM CONTROL OF POSTURE AND MOVEMENT
The importance of motor control pathways that originate in the brainstem is evident from observations of the extensor hypertonus and increased phasic stretch reflexes that occur in decerebrate animals. Particular brainstem systems have been identified that influence posture and locomotion. Brainstem circuits are also critically involved in the control of eye movement; these circuits are discussed in a separate section at the end of the chapter.
Postural Reflexes
Several reflex mechanisms are evoked when the head is moved or the neck is bent. There are three types of postural reflexes: vestibular reflexes, tonic neck reflexes, and righting reflexes. The sensory receptors responsible for these reflexes include the vestibular apparatus (see Chapter 8), which is stimulated by head movement, and stretch receptors in the neck.
The vestibular reflexes constitute one class of postural reflex. Rotation of the head activates sensory receptors of the semicircular canals (see Chapter 8). In addition to generating eye movement, the sensory input to the vestibular nuclei results in postural adjustments. Such adjustments are mediated by commands transmitted to the spinal cord through the lateral and medial vestibulospinal tracts and the reticulospinal tracts. The lateral vestibulospinal tract activates extensor muscles that support posture. For instance, if the head is rotated to the left, postural support is increased on the left side. This increased support prevents the subject from falling to the left as the head rotation continues. Any disease that eliminates labyrinthine function in the left ear will cause the person to tend to fall to the left. Conversely, a disease that irritates (stimulates) the left labyrinth will cause the person to tend to fall to the right. The medial vestibulospinal tract causes contractions of neck muscles that oppose the induced movement (vestibulocollic reflex).
Tilting the head also changes the linear acceleration on individual hair cells of the otolith organs of the vestibular apparatus. The resulting changes in hair cell activity can produce eye movement and postural adjustment. For example, tilting the head and body forward (without bending the neck and consequently without evoking the tonic neck reflexes) in a quadruped, such as a cat, results in extension of the forelimbs and flexion of the hind limbs. This vestibular action tends to restore the body toward its original orientation. Conversely, if the head and body are tilted backward (without bending the neck), the forelimbs flex and the hind limbs extend. Otolithic organs also contribute to the vestibular placing reaction. If an animal, such as a cat, is dropped, stimulation of the utricles leads to extension of the forelimbs in preparation for landing.
The tonic neck reflexes are another type of positional reflex. These reflexes are activated by the muscle spindles found in neck muscles. These muscles contain the largest concentration of muscle spindles of any muscle in the body. If the neck is bent (without tilting the head), the neck muscle spindles evoke tonic neck reflexes without interference from the vestibular system. When the neck is extended, the forelimbs extend and the hind limbs flex. The opposite effects occur when the neck is flexed. Note that these effects are opposite those evoked by the vestibular system. Furthermore, if the neck is bent to the left, the extensor muscles in the limbs on the left contract more, and the flexor muscles in the limbs on the right side relax.
The third class of postural reflex is the righting reflexes. These reflexes tend to restore an altered position of the head and body toward normal. The receptors responsible for righting reflexes include the vestibular apparatus, the neck stretch receptors, and mechanoreceptors of the body wall.
Brainstem Control of Locomotion
The spinal cord contains neural circuits that serve as central pattern generators for locomotion, as discussed earlier. These CPG circuits produce very regular rhythmic output that characterizes stereotyped behavior, such as walking. The irregularities of real-world environments, however, often require modification of this stereotyped output (e.g., if you are walking and see a hole in the floor where you are about to step, you can extend the forward swing of your leg past the hole onto solid ground beyond it).
Such modifications can be the result of sensory input to the spinal cord, as was shown in Figure 9-9, where stimulation of FRA fibers in a peripheral nerve caused a phase shift in the locomotor pattern. They can also be the result of descending commands along the motor pathways discussed earlier. In this case, sensory data (e.g., visual) can be used by the brain to make anticipatory modifications in CPG activity so that potential obstacles can be avoided. In addition, we can voluntarily control activation, or shutdown, of the CPG (i.e., deciding consciously when to start and stop walking). Such voluntary regulation of spinal CPGs originates in the cerebral cortex; however, much of the cortical influence on locomotion appears to be mediated via projections to brainstem regions known as locomotor regions. A locomotor region can be defined as a brain area that when stimulated, leads to sustained locomotion.
There are several such locomotor regions in the brainstem, and they are located at different levels ranging from the subthalamus to the medulla and are connected with each other. The best known is the midbrain locomotor region, which is thought to organize commands to initiate locomotion. It is located in the midbrain at the level of the inferior colliculus. Voluntary activity that originates in the motor cortex can trigger locomotion by the action of corticobulbar fibers projecting to the midbrain locomotor region. The commands are relayed through the reticular formation and then to the spinal cord via the reticulospinal tracts.
Motor Control by the Cerebral Cortex
Thus far in this chapter, emphasis has been placed on reflexes and relatively automatic types of movement. We will now discuss the neural basis for more complex, goal-directed voluntary movement. Such movement often varies when repeated and is frequently initiated as a result of cognitive processes rather than in direct response to an external stimulus. Thus, it requires the participation of motor areas of the cerebral cortex.
Let us first consider what is necessary to generate a voluntary movement. For example, to make a reaching movement with your arm, you must first identify the target (or goal) and locate it in external space. Next, a limb trajectory must be determined based on an internal representation of your arm and, in particular, your hand relative to the target. Finally, a set of forces necessary to generate the desired trajectory must be computed. This process is often thought of as a series of transformations between coordinate systems. For example, the location of a visually identified target is measured in a retinotopic space, but its location is perceived in an external or world space (i.e., the position of a nonmoving target is perceived as stable, even when the eye, and thus the target’s image on the retina, changes). Next, calculation of a trajectory would involve a body- or hand-centered system, and finally, forces must ultimately be computed in a muscle-based reference frame.
These steps form a linear sequence, and traditionally it was thought that a hierarchy of motor areas carried out the successive steps. For example, the target of the movement was thought to be identified by pooling sensory information in the posterior parietal cerebral cortex (Fig. 9-14, A). This information would then be transmitted to the supplementary motor and premotor areas, where a motor plan is developed and then forwarded to the primary motor cortex, whose activity would be related to the final execution stage (e.g., generation of appropriate force levels). The motor cortex would then transmit commands, via the descending pathways discussed earlier, to the spinal cord and brainstem motor nuclei.
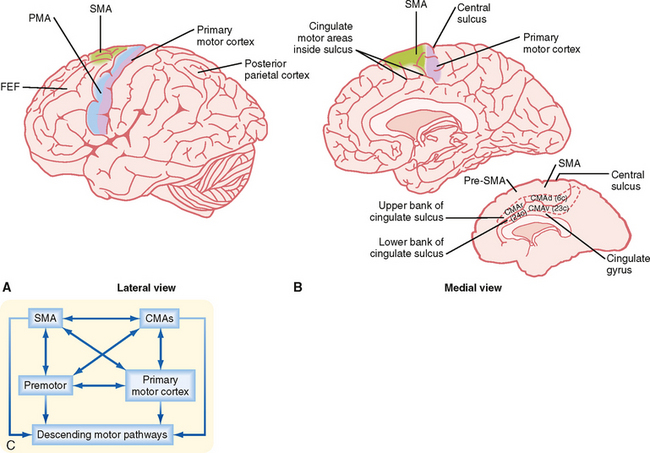
Figure 9-14 Motor areas of the frontal cortex. A and B, Lateral and medial views of a hemisphere showing the major cortical motor areas. FEF, frontal eye fields. The inset in B shows the walls of the cingulate sulcus, which contain the cingulate motor areas. Numbers in parentheses are Brodmann’s numbers for the CMAs. C, Diagram showing interconnections of the motor areas. PMA, premotor area; SMA, supplementary motor area; CMA, cingulate motor area.
Although there is significant evidence in support of this hierarchical view of the generation of voluntary movement by the cortical motor system, more recent results have suggested a different conception, namely, that the various motor areas should be thought of as forming a parallel distributed network rather than a strict hierarchy (Fig. 9-14, C). For example, each cortical motor area makes its own significant contribution to the descending motor pathways, with the primary motor cortex contributing only about half the fibers in the corticospinal tract that arise from the frontal lobe. Moreover, the various motor areas are all bidirectionally connected to each other, and the single-unit recording studies described later suggest that each of the areas plays a role in several of the stages of planning and executing a movement. This debate forms one of the themes of the following discussion because in its various guises, the distributed network versus hierarchical organization debate has been ongoing for decades and will probably continue for some time.
Cortical Motor Areas
The motor areas in the cerebral cortex were originally defined on the basis of experiments in which electrical stimuli applied to the cortex evoked discrete, contralateral movement. Movement, however, can also be evoked when other cortical areas are stimulated more intensely. Thus, motor areas are defined as those from which movement can be evoked by the lowest stimulus intensity. On the basis of these stimulation studies, the effects produced by lesions, anatomic experiments, electrophysiological recordings, and modern imaging studies in humans, several “motor” areas of the cerebral cortex have been recognized (Fig. 9-14), including the primary motor cortex in the precentral gyrus, the premotor area just rostral to the primary motor cortex, the supplementary motor cortex on the medial aspect of the hemisphere, and three cingulate motor areas located on the walls of the cingulate sulcus in the frontal lobe. There are also cortical regions scattered across all cortical lobes whose activity is related specifically to eye movement (see the section Eye Movement).
Somatotopic Organization of Cortical Motor Areas
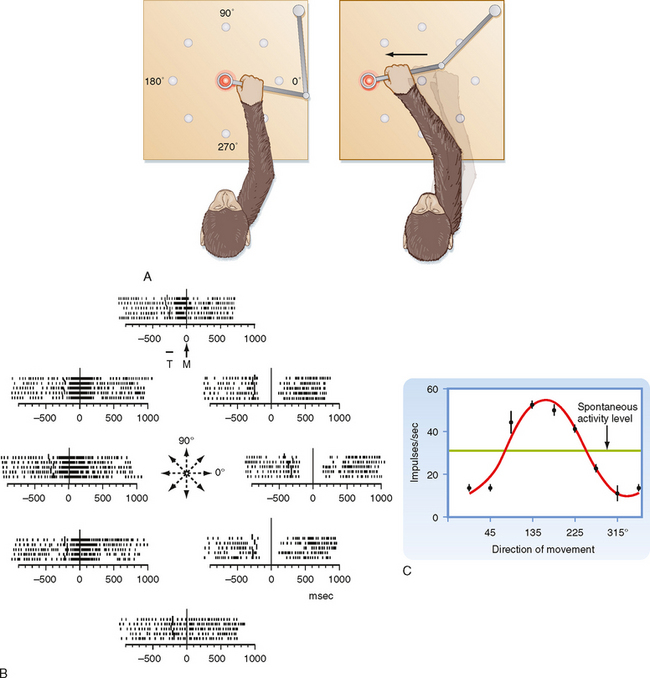
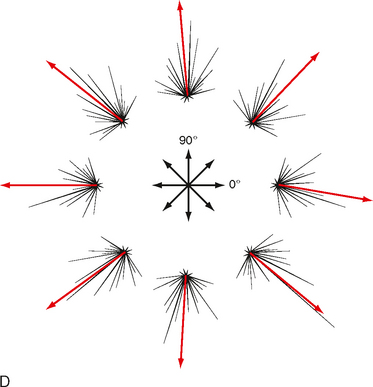
Primary Motor Cortex
The primary motor cortex (or just motor cortex) can be defined as the region of cortex from which movements are elicited with the least amount of electrical stimulation. It is essentially congruent with Brodmann’s cytoarchitectonic area 4 (Fig. 10-3). In humans it is located on the parts of the precentral gyrus that form the rostral wall of the central sulcus and the caudal half of the apex of the gyrus. Based on initial mapping studies, which were done with surface stimulation, the motor cortex was described as having a topographic organization that parallels that of the somatosensory cortex. The face, body, and upper limb were represented on the lateral surface with the face located inferiorly, near the lateral fissure, the torso most superiorly, and the lower extremity mostly on the medial aspect of the hemisphere. This somatotopic organization is often represented as a figurine or in a graphic form called a motor homunculus (Fig. 9-15, A). The distortion of the various body parts in the homunculus indicates approximately how much of the cortex is devoted to their motor control. This simple homunculus was likened to a piano keyboard and fit well with traditional conceptions of the motor cortex being the final cortical stage and acting as a relay for sending motor commands to the spinal cord.
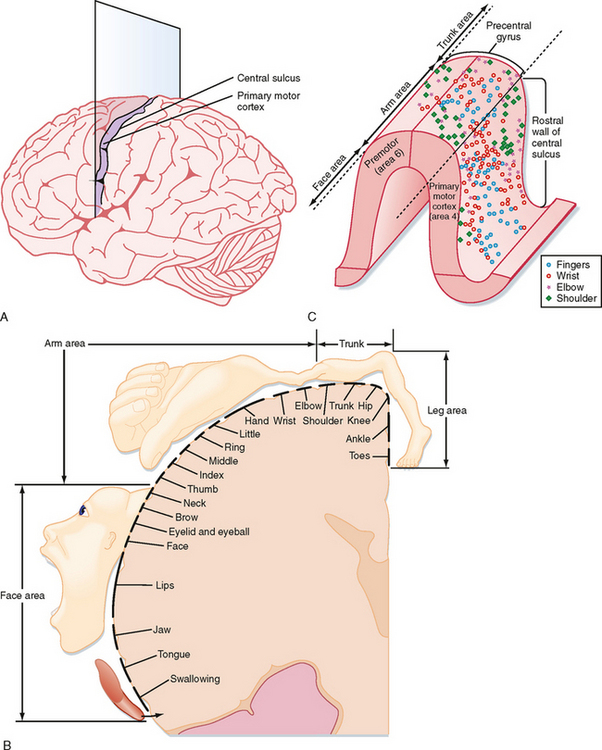
Figure 9-15 Traditional and modern views of motor cortex musculotopic organization. A, Lateral view of the cerebrum showing a plane of section through the precentral gyrus (primary motor cortex) to obtain the section shown in B. B, Classic view of motor cortex musculotopy. C, Modern view of motor cortex organization in which each body part is represented multiple times across several discrete regions.
Beginning in the 1960s and 1970s, mapping studies began using microelectrodes inserted to the deep, or output, layers of the cortex to apply stimuli. With this technique, called intracortical microstimulation (ICMS), much lower stimulus intensities could be used to evoke movements and thus allowed higher-resolution mapping of the motor cortex, which revealed a much more complex topography than was previously imagined (Fig. 9-15, C). Movement about each joint was found to be evoked by many noncontiguous columns throughout wide regions of the motor cortex. Thus, cell columns related to movement about a particular joint are actually interspersed among columns that control movement about many other joints. In sum, although the motor cortex may have large subdivisions corresponding to a limb or the head, within each such area there is a complex intermingling of cell columns that control the muscles within that body part.
Such mixing of cell columns makes functional sense because most movement requires the coordinated action of muscles throughout a limb and most connectivity in the cortex is localized (i.e., axon collaterals that connect different cell columns are primarily confined to a 1- to 3-mm region surrounding the column from which they originate). Thus, by having multiple cell columns that control movement about a joint and intermixing them with columns controlling movement about other joints, multijoint movement can be generated as a whole.
Although the somatotopy of the motor cortex is in part anatomically determined by the topography of the corticospinal pathway, it is also a dynamic map. Axon collaterals link the different cell columns, so activity in one column could potentially lead to movement about multiple joints. In fact, this can happen, but these intercolumnar connections are modulated by inhibitory GABAergic interneurons. This was shown by locally blocking GABA in one region of the motor cortex and then stimulating the neighboring region. Before the block, stimuli evoked contractions of one set of muscles, but once inhibition was blocked, contractions were also evoked in muscles controlled by the region that was no longer inhibited (Fig. 9-16). Functional connections between cell columns can be controlled on a millisecond time scale, and depending on their state, the somatotopic map can be radically changed. Longer-term plastic changes are also known to occur; for example, the use (or disuse) of a body part can affect the size of its representation.
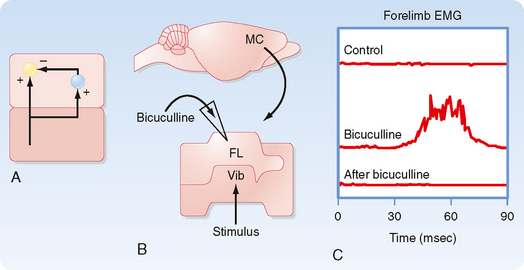
Figure 9-16 Dynamic nature of a motor cortex musculotopic map. Inhibitory GABAergic interneurons play an important role in shaping motor responses to stimulation of each region of the motor cortex. A, Schematic showing excitatory connections between two regions of primary motor cortex and local inhibitory neurons within a single region. B, Schematic of a rat brain indicating motor cortex regions where electrical stimuli were applied to evoke movements (Vib region) and bicuculline was applied to block GABAergic synapses (in the FL region). FL, forelimb; HL, hind limb; Vib, vibrissa. C, FL EMG records showing response to stimulation of the Vib region before, during the application of bicuculline, and after washing it out. Note that Vib stimulation evoked vibrissae movement in all conditions but evoked forelimb movement only when inhibitory interneurons were blocked.
(Data from Jacobs K, Donoghue J: Science 251:944, 1991.)
Supplementary Motor Area
The supplementary motor area (SMA) is located mainly on the medial surface of the hemisphere, just anterior to the primary motor cortex, and corresponds to the medial portion of Brodmann’s area 6 (Fig. 9-14). It is subdivided into two regions: the more posterior part is referred to as the SMA proper (or just SMA), and the anterior portion is called the pre-SMA. The SMA proper is similar to the other motor areas already listed: it contains a complete somatotopic map, it contributes to the corticospinal tract, and it is interconnected with the other motor areas. In contrast, the pre-SMA is not strongly connected with the other motor areas and spinal cord but rather is connected to the prefrontal cortex.
The results of stimulation studies show that as in the motor cortex, there is a complete somatotopic map in the SMA. Stimulation of the SMA can evoke isolated movement about single joints, similar to that after stimulation of the motor cortex, but higher-intensity and longer-duration stimulation is necessary; moreover, the evoked movements are often more complex than those evoked by stimulation of the motor cortex. However, longer-duration stimulation of the primary motor cortex can also evoke complex, apparently purposeful movement sequences, so the distinction is not absolute. In addition, stimulation of the SMA can produce vocalization or complex postural movements, but it can also have the opposite result, namely, a temporary arrest of movement or speech. Removal of the supplementary motor cortex retards movement of the opposite extremities and may result in forced grasping movements with the contralateral hand.
Premotor Area
This area lies rostral to the primary motor cortex and is contained in Brodmann’s area 6 on the lateral surface of the brain (Fig. 9-14). It can be distinguished from the primary motor cortex by the higher stimulus intensities needed to evoke movement. The premotor area has been divided into two functionally distinct subdivisions: dorsal and ventral. Like the motor cortex, both subdivisions are somatotopically organized and both contribute to the corticospinal tract. The dorsal division (PMd) contains a relatively complete map representing the leg, trunk, arm, and face. In contrast, the somatotopic map of the ventral division (PMv) is mostly limited to the arm and face, with only a small leg representation. Thus, the PMv appears to be specialized for control of upper limb and head movement. A second difference between the subdivisions is that PMd contains a large representation of the proximal muscles, whereas PMv has a large representation of the distal muscles.
Cingulate Motor Areas
These motor areas are located within the cingulate sulcus at approximately the same anterior-posterior level as the SMA. There are three cingulate motor areas (dorsal, ventral, and rostral) (Fig. 9-14, B). Each contains a somatotopic map and contributes to the corticospinal tract. Microstimulation in these areas evokes movement similar to that evoked by motor cortex stimulation, except that once again, higher stimulus intensities are needed. Single-cell recordings during movements have shown that the spontaneous activity of neurons in the cingulate motor areas is related to the preparation and execution of movements.
Connections of the Cortical Motor Areas
The motor areas of the cortex receive input from a number of sources, cortical and subcortical; however, the single largest source of synapses in an area is the area itself, specifically, the local intrinsic connections. Second, all the motor areas described earlier are bidirectionally connected to each other with high topographic specificity (Fig. 9-14, C). For example, the arm regions of the primary motor cortex and the cingulate motor areas project to each other. Sensory information comes from ascending pathways that relay in the thalamus. This information can reach the motor cortex directly from the thalamus or indirectly by way of the somatosensory cortex. Both somatosensory information and visual information are conveyed to the motor areas from the posterior parietal cortex. The motor areas of the cortex also receive information through circuits that interconnect them with the other major brain regions involved in motor control, namely, the cerebellum and basal ganglia. These two structures project to distinct parts of the thalamus (the ventral lateral [VL] and ventral anterior [VA] nuclei), which then project to the cortical motor areas.
The output of the cortical motor areas to the spinal cord and brainstem is conducted through several descending pathways. These pathways include not only direct projections through the corticospinal and corticobulbar tracts (to the cranial nerve nuclei) but also indirect projections to the red nucleus and to various nuclei in the reticular formation. Descending projections from these brainstem sites were reviewed earlier (see the section Descending Motor Pathways). Control of head and neck muscles is mediated by projections to the various cranial nerve nuclei. The motor regions also project to the cerebellum and basal ganglia, thus completing neuroanatomic loops with these structures. The major connection to the cerebellum is via the corticopontine projections to the basilar pontine nuclei, which in turn project to the cerebellum. In addition, the cortical motor areas project, mostly via disynaptic pathways that synapse in the midbrain, to the inferior olive, another important precerebellar nucleus. The cortical motor regions project directly to the striatum of the basal ganglia. Finally, there are major projections to the thalamus by which the cortex regulates the information that it receives.
Activity of Motor Cortex Cells
The role of individual motor cortex neurons in the control of movement has been extensively investigated in trained monkeys. In these experiments, discharges from a neuron in the primary motor cortex are recorded during the execution of a previously learned simple movement, such as wrist flexion, made immediately in response to a sensory cue (Fig. 9-17). Motor cortex neurons were found to change their firing rates before initiation of the movement, and the onset of this change was correlated with the reaction time (i.e., the time from the cue to onset of the movement). Moreover, in this task the change in firing of motor cortex neurons was often correlated to the contractile force of the muscle that generates the movement and to the rate of change in force rather than to the position of the joint. These findings suggest that these cells are involved in the final stages of planning and executing movements, consistent with the hierarchical view of the cortical motor areas.
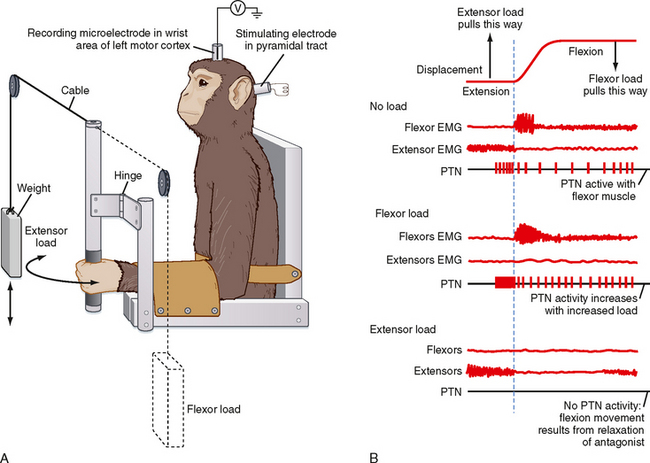
Figure 9-17 A, Experimental arrangement for recording from a corticospinal neuron while a monkey performs trained wrist movements. The stimulation electrode is used to elicit antidromic spikes that are used to identify the motor cortex neuron specifically as a pyramidal tract neuron. Stimuli are not applied while the monkey is performing movements. B, The pyramidal tract neuron (PTN) discharges before the onset of movement or EMG activity when flexors need to generate force (no load and flexor load conditions). Moreover, the firing rate is correlated with the level of flexor force that is needed. In the extensor load condition, flexors do not need to contract to generate movement, and thus there is no activity in this PTN. The top trace shows wrist movement, which is essentially identical for all three experimental conditions. Thus this cell’s activity encodes force magnitude and direction, but not displacement. Figure based on work of Evarts and colleagues.
However, even in these early experiments, the firing rates of some motor cortex cells appeared to relate to earlier planning stages. Moreover, even when a monkey was trained to withhold the movement for a certain period after the cue, the firing rates of motor cortex neurons still changed despite the absence of any movement. Such “set-related” activity has been amply confirmed in a variety of other tasks and suggests that motor cortex activity may be involved in the earlier planning stages along with activity in other motor areas of the cortex. It also suggests the possibility that other, perhaps subcortical, systems may be needed to generate a trigger signal for the initiation of movement.
Subsequent studies have used tasks in which animals were trained to move a manipulandum (a device with a handle to hold and a small circle on the end) to capture lighted targets on a surface in front of them (Fig. 9-18, A). These experiments demonstrated that cells in the arm region of the motor cortex showed changes in their firing rates to movement in many different directions and thus were described as broadly tuned (Fig. 9-18, B). That is, a cell that showed a maximal increase for movement in one particular direction, called its preferred direction, would also show somewhat smaller increases or even decreases for movement in other directions (Fig. 9-18, C). Moreover, the preferred directions of the different cells were uniformly distributed across all 360 degrees of possible movement directions. These results implied that a particular cell is probably involved in most arm movements, but they also raised the problem of how precise movements could be made with such broadly tuned cells. It was suggested that although changes in the activity of individual cells could not precisely predict or specify the direction of the upcoming movement, the net activity of the population could. To test this idea, models were made in which the activity of each cell is represented as a vector (Fig. 9-18, D). The direction of each cell vector is determined by the preferred direction of the cell, and the magnitude of the vector for a particular movement is proportional to the firing rate of the cell during the time preceding the movement. The individual cell vectors (Fig. 9-18, D, black lines) from hundreds of cells can then be vectorially summed to get a resultant or population vector (Fig. 9-18, D, red lines) that accurately predicts the upcoming movement.
Figure 9-18 A, Experimental setup in which a monkey holds onto the arm of the apparatus and captures light spots with the distal end of the arm. The monkey first captures the central light spot and then captures whichever of the surrounding targets that becomes illuminated. B, Raster plots showing the activity of one motor cortex cell during movement in eight different directions. T indicates the time at which the target light turns on, whereas M indicates the time at the onset of movement, which is at the center of each raster. Each mark on a raster represents a spike of a motor cortex cell, and each row of marks shows the cell’s activity during one trial. C, Cosine function was fit to the firing level as a function of the direction of movement. The horizontal bar indicates the average spontaneous firing rate in the absence of an upcoming movement. Note that for most directions, the activity in the periods just before and during movement changes significantly from baseline. D, Vector model of population activity in the motor cortex. Black lines are individual cell vectors. When all of them are summed for a particular direction of movement, the resulting population vector (red) points in essentially the direction of the upcoming movement.
One of the difficulties in assessing the relationship between firing of cortical cells and various movement parameters, such as force, velocity, displacement, and target location, is that these parameters are normally correlated with each other. Thus, variations of the tasks described earlier have been used to decorrelate these various parameters (e.g., using weights to vary the force needed to make a movement without changing the displacement as illustrated in Fig. 9-17, A, or rotating the starting position of the wrist so that different muscles are required to generate the same trajectory in external space). The results of these experiments showed that the activity of motor cortex cells may be related to each of the various motor planning stages. Furthermore, the activity of a single cell may correlate with one parameter initially and then switch as the time for onset of movement approaches.
Activity in Other Cortical Motor Areas
Activity in the premotor and supplementary motor areas is in many ways similar to that in the primary motor cortex. Cells in these areas show activity related to upcoming movement, and the activity is correlated with movement parameters, such as displacement, force, and target location, just as primary motor cortex activity can be, consistent with the distributed network view of the cortical motor areas. There do, however, appear to be some real differences between the areas as well, although these differences may be more quantitative than qualitative. For example, a higher percentage of cells in the premotor and supplementary motor areas show activity related to earlier motor planning stages than do cells in the primary motor cortex. In addition, the premotor and supplementary motor areas can be distinguished from each other by the apparently greater involvement of the premotor area in movements made to external cues (such as in the task shown in Fig. 9-18) and the greater involvement of the supplementary motor area in movements made in response to internal cues (i.e., self-initiated). Recent research has also revealed that each of these areas is functionally heterogeneous and can therefore be further subdivided; however, such details are beyond the present scope.
MOTOR CONTROL BY THE CEREBELLUM
Overview of the Role of the Cerebellum in Motor Control
More than 100 years ago, scientists showed that damage to the cerebellum led to deficits in motor coordination. That is, damage or loss of the cerebellum does not lead to paralysis, loss of sensation, or an inability to understand the nature of a task, but rather it leads to an inability to perform movements well. Yet it has been hard to define the cerebellum’s precise role or roles in generating movement despite, paradoxically, also having more detailed knowledge of its deceptively simple anatomic and physiological organization than any other CNS region. The cerebellum is proposed to play a critical role in the learning and execution of both voluntary and certain reflex movements. However, hypotheses about these roles face significant challenges that prevent their current acceptance. Here, the behavioral effects of damaging the cerebellum are presented, followed by a description of its connectivity, both intrinsic and with the rest of the CNS, and then finally its activity.
Behavioral Consequences of Cerebellar Damage
Damage to the cerebellum impairs motor function on the ipsilateral side of the body. This reflects a double crossing of most cerebellar-related output as it travels to the motor neurons. The first crossing typically occurs in the cerebellar efferent pathway, whereas the second crossing takes place in the descending motor pathways. For example, the cerebellum projects to the contralateral motor cortex, via the thalamus, and the corticospinal pathway recrosses the midline at the lower medulla.
The specific motor deficits that result from cerebellar lesions depend on which functional component of the cerebellum is most affected. If the flocculonodular lobe is damaged, the motor disorders resemble those produced by a lesion of the vestibular apparatus; such disorders include difficulty in balance and gait and often nystagmus. If the vermis is affected, the motor disturbance affects the trunk, and if the intermediate region or hemisphere is involved, motor disorders occur in the limbs. The part of the limbs affected depends on the site of damage; hemispheric lesions affect the distal muscles more than paravermal lesions do.
Types of motor dysfunction in cerebellar disease include disorders of coordination, equilibrium, and muscle tone. Incoordination is called ataxia and is often expressed as dysmetria, a condition in which errors in the direction and force of movement prevent a limb from being moved smoothly to a desired position. Ataxia may also be expressed as dysdiadochokinesia, in which rapid alternating supination and pronation of the arm is difficult to execute. When more complicated movement is attempted, decomposition of movement occurs, in which the movement is accomplished in a series of discrete steps rather than as a smooth sequence. An intention tremor appears when the subject is asked to touch a target; the affected hand (or foot) develops a tremor that increases in magnitude as the target is approached. When equilibrium is disturbed, impaired balance may be seen, and the individual tends to fall toward the affected side and may walk with a wide-based stance (gait ataxia). Speech may be slow and slurred, a defect called scanning speech. Muscle tone may be diminished (hypotonia), except for lesions of the anterior vermis (see earlier section on decerebrate rigidity); the diminished tone may be associated with a pendular knee jerk. This can be demonstrated by eliciting a phasic stretch reflex of the quadriceps muscle by striking the patellar tendon. The leg continues to swing back and forth because of the hypotonia, in contrast to the highly damped oscillation in a normal person.
These disorders reflect, in part, abnormal timing of muscle contractions. Normally, limb movements involve precisely timed EMG bursts in both agonist and antagonist muscles. There is an initial agonist burst followed by a burst in the antagonist and, finally, a second agonist burst. With cerebellar damage, the relative timing of these bursts is abnormal (Fig. 9-19).
Figure 9-19 Disruption of cerebellar activity alters the timing of EMG responses during movement. The cerebellar nuclei were cooled to block their functioning temporarily while monkeys performed movements about their elbow. Loss of cerebellar activity disrupts the relative timing of agonist and antagonist EMG bursts. This leads to abnormal acceleration of the limb and a movement trajectory that overshoots the target position (hypermetria).
(Data from Flament D, Hore J: J Neurophysiol 55:1221, 1986.)
Cerebellar Organization
The cerebellum (“little brain”) is located in the posterior fossa of the cranium, just below the occipital lobe, and is connected to the brainstem via three cerebellar peduncles (superior, middle, and inferior). From the outer surface, only the cortex is visible. Deep to the cortex is the white matter of the cerebellum, and buried within the white matter are the four cerebellar nuclei: proceeding medially to laterally, the fastigial, globose, emboliform, and dentate nuclei. The middle two nuclei are often grouped together and referred to as the interpositus nucleus. For the most part, cerebellar afferents to the cortex and nuclei enter the cerebellum via the inferior and middle peduncles, and efferents from the cerebellar nuclei leave via the superior peduncle.
The cerebellar cortex is subdivided into three rostrocaudally arranged lobes: the anterior lobe, the posterior lobe, and the flocculonodular lobe (Fig. 9-20, A). The cerebellar lobes are separated by two major fissures, the primary fissure and the posterolateral fissure, and each lobe is made up of one or more lobules. Each lobule of the cerebellar cortex is composed of a series of transverse folds called folia.
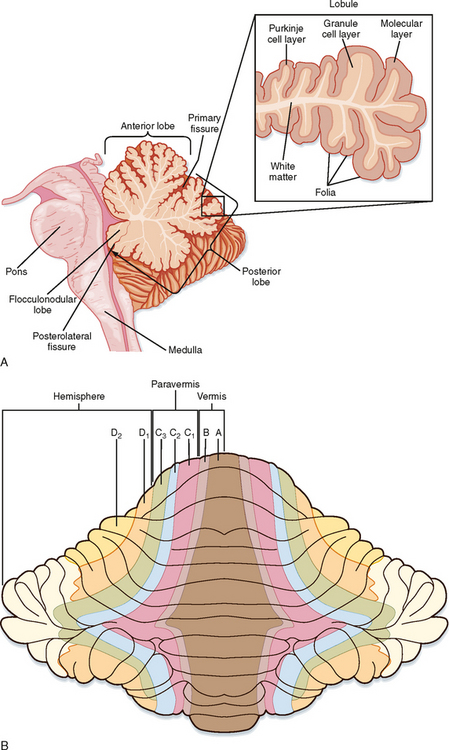
Figure 9-20 Anatomic divisions of the cerebellum. A, Midsagittal view showing folding of the cortex into lobe, lobules, and folia. B, Schematic view of an unfolded ferret cerebellar cortex to illustrate earlier compartmentation schemes for subdividing the cerebellar cortex into three (vermis, paravermis, and hemisphere) and then seven longitudinally running zones. Light yellow colored portion of each hemisphere indicates an area for which no data were available. C, Schematic of an unfolded rat cerebellum showing its subdivision into well over 20 compartments based on staining for molecular markers, in this case zebrin II (aldolase C). Letters and numbers on the right half of the cerebellum indicate the zebrin compartment number. Roman numerals down the center indicate cerebellar lobules. Names on left hemisphere indicate names of cerebellar lobules. CP, copula pyramis; Cr, crus; DPFL, dorsal paraflocculus; FL, flocculus; Par, paramedian; pf, primary fissure; Sim, simplex; VPFL, ventral paraflocculus.
(B, Modified from Voogd J. In Neurobiology of Cerebellar Evolution and Development. Chicago, American Medical Association, 1969; C, courtesy of Dr. Izumi Sugihara.)
The cerebellar cortex has also been divided into longitudinal compartments (Fig. 9-20, B and C). Initially, the cerebellar cortex was divided into three such compartments: the vermis, which lies on the midline; the paravermis, which lies adjacent to both sides of the vermis; and the lateral hemispheres. These regions have now been subdivided into many further compartments on the basis of myeloarchitectonics (patterns of axonal bundles in the white matter) and the expression patterns of specific molecules, such as aldolase C. Although the functional significance of these compartments is not fully known, the topography of cerebellar afferents, specifically the olivocerebellar system, is precisely aligned with them, and the receptive field properties of cerebellar Purkinje cells also tend to follow this organizational scheme.
Cerebellar Cortex
Afferent Systems
There are two major cerebellar afferent systems: mossy fibers and climbing fibers. Mossy fibers are named for their distinctive appearance in the cerebellar cortex: as a mossy fiber courses through the granule layer, on occasion it swells and sends out a bunch of short twisty branchlets. These entities are called rosettes and are points of synaptic contact between these fibers and neurons in the granule cell layer. Mossy fibers arise from many sources, including the spinal cord (the spinocerebellar pathways), dorsal column nuclei, trigeminal nucleus, nuclei in the reticular formation, primary vestibular afferents, vestibular nuclei, cerebellar nuclei, and the basilar pontine nuclei. The details of mossy fiber projection patterns are beyond the scope of the present chapter; however, several general points are worth noting:
In contrast to the diverse origins of mossy fibers, climbing fibers all originate from a single nucleus: the inferior olive, which is located in the rostral medulla, just dorsal and lateral to the pyramids. The olivary neurons are almost all projection cells whose axons leave the nucleus without giving off collaterals and then cross the brainstem to enter the cerebellum primarily via the inferior cerebellar peduncle. Like mossy fibers, olivocerebellar axons are excitatory and send collaterals to the cerebellar nuclei as they ascend through the cerebellar white matter to the cortex. In the cerebellar cortex, olivocerebellar axons may synapse with basket, stellate, and Golgi cells but form a special synaptic arrangement with Purkinje cells. Each Purkinje cell receives input from only a single climbing fiber, which “climbs” up its proximal dendrites and makes hundreds of excitatory synapses. Note, the terminal portion of the olivocerebellar axon is referred to as a climbing fiber. Conversely, each olivary axon will branch to form about 10 to 15 climbing fibers.
The inferior olive is a distinctive brain region for several reasons. As already noted, its neurons are virtually all projection cells, so there is little local chemical synaptic interaction between the cells. Instead, olivary neurons are electrically coupled to each other by gap junctions. In fact, the olive has the highest density of neuronal gap junctions in the CNS. This allows olivary neurons to have synchronized activity that gets transmitted to the cerebellum. Afferents to the olive may be divided into two main classes, excitatory input, which arises from many regions throughout the CNS, and inhibitory GABAergic input from the cerebellar nuclei and a few brainstem nuclei. Although these afferents can modulate the firing rates of olivary neurons (as is typical in most brain regions), the membrane conductance of olivary neurons limits this modulation to a range of a few hertz and endows these neurons with the potential to be intrinsic oscillators. Instead of just modulating firing rates, olivary afferent activity acts to modify the effectiveness of the electrical coupling between olivary neurons and thus changes the patterns of synchronous activity delivered to the cerebellum. Afferent activity may also modulate expression of the oscillatory potential of olivary neurons. Thus, the inferior olive appears to be organized to generate patterns of synchronous activity across the cerebellar cortex. The functional significance of these patterns remains controversial. One hypothesis is that they provide a gating signal for synchronizing motor commands to various muscle combinations.
Cellular Elements and Efferents of the Cortex
Despite its enormous expansion throughout vertebrate evolution, the basic anatomic organization of the cerebellar cortex has remained nearly invariant. The circuitry is also among the most regular and stereotyped of any brain region. The cerebellar cortex contains eight different neuronal types: Purkinje cells, Golgi cells, granule cells, Lugaro cells, basket cells, stellate cells, unipolar brush cells, and candelabrum cells. These cells are found in all regions of the cerebellar cortex, with the exception of unipolar brush cells, which are limited mainly to cerebellar areas receiving vestibular input (i.e., the flocculonodular lobe). These eight cell types are distributed among the three layers that make up the cerebellar cortex of higher vertebrates. The outer or superficial layer is the molecular layer (Fig. 9-20, A). Stellate and basket cells are found here. The deepest layer is the granule cell layer. This layer has the highest cellular density in the nervous system and contains granule, Golgi, and unipolar brush cells. Separating the molecular and granule cell layers is the Purkinje cell layer, formed by Purkinje cell somata, which are arranged as a one-cell-thick sheet of cells. Candelabrum cells are also located in this layer. Lugaro cells are situated slightly deeper at the upper border of the granule cell layer.
The sole efferent from the cortex is the Purkinje cell axon, which also has local collaterals and is GABAergic and inhibitory. Thus, the remaining seven cell types are local interneurons. Of these, the stellate, basket, Golgi, Lugaro, and candelabrum cells are also inhibitory GABAergic neurons, whereas the granule and unipolar brush cells are excitatory.
Microcircuitry of the Cortex
The dendrites, axons, and patterns of synaptic connections of most neurons within the cerebellar cortex are organized with respect to the transverse (short) and longitudinal (long) folial axes (Fig. 9-21). In the vermis, where the folia run perpendicular to the sagittal plane, these axes lie in the sagittal and coronal planes, respectively. In the hemispheres, where the folia are oriented at various angles with respect to the sagittal plane, this correspondence is lost, and the local folial axes must then serve as the reference axes.
Figure 9-21 Three-dimensional view of the cerebellar cortex showing some of the cerebellar neurons. The cut face at the left is along the long axis of the folium; the cut face at the right is at right angles to the long axis. BC, basket cell; CF, climbing fiber; CN, cerebellar nuclear cell; GC, Golgi cell; Glm, glomerulus; GrC, granule cell; MF, mossy fiber; PC, Purkinje cell; PF, parallel fiber; SC, stellate cell.
The Purkinje cell dendritic tree is the largest in the CNS. It extends from the Purkinje cell layer through the molecular layer to the surface of the cerebellar cortex and for several hundred microns along the transverse axis of the folium, but only 30 to 40 μm in the longitudinal direction. Thus, it is like a flat pancake that lies in a plane parallel to the transverse folial axis. Accordingly, a set of Purkinje cell dendritic trees can be thought of as a stack of pancakes, with the stack running along the longitudinal folial axis.
The dendritic trees of the molecular layer interneurons (stellate and basket cells) are oriented similar to the Purkinje cell dendritic tree, although they are much less extensive. The axons of stellate and basket cells run transversely across the folium and form synapses with Purkinje cells. Stellate and basket cells synapse onto Purkinje cell dendrites. In addition, basket cells make synapses on the Purkinje cell soma and form a basket-like structure around the base of the soma, which gives the basket cell its name.
Granule cells are small neurons with four to five short unbranched dendrites, each ending in a claw-like expansion that synapses with a mossy fiber rosette and with terminals from Golgi cell axons in a complex arrangement known as a glomerulus. The axons of granule cells ascend through the Purkinje cell layer to the molecular layer, where they bifurcate and form parallel fibers. The parallel fibers run parallel to the cerebellar surface along the longitudinal axis of the folium (perpendicular to the planes of the Purkinje, stellate, and basket cell dendritic trees) and form excitatory synapses with the dendrites of the Purkinje, Golgi, stellate, and basket cells.
The orthogonal relationship between the parallel fibers and the dendritic trees of the Purkinje cells and molecular layer interneurons (basket and stellate cells) has significant functional consequences. This arrangement allows maximal convergence and divergence to occur. A single parallel fiber, which can be up 6 mm long, will pass through more than 100 Purkinje cell dendritic trees (and also interneuron dendrites); however, it has the chance to make only one or two synapses with any particular cell because it crosses through the short dimension of the dendritic tree. Conversely, a given Purkinje cell receives synapses from about 200,000 parallel fibers. Thus, a beam of parallel fibers can be excited experimentally, which will excite a row of Purkinje cells and interneurons that are in line with this beam (Fig. 9-22). In addition, because the axons of the interneurons run perpendicular to the parallel fibers, this beam of excitation will be flanked by inhibition. Although this classic electrophysiological experiment clearly demonstrates the functional connectivity of the cerebellar cortex, whether such beams of excitation occur normally remains a controversial question.
Figure 9-22 Functional connectivity of the cerebellar cortex. The geometry of the cerebellar cortical circuits makes electrophysiological determination of the functional connectivity of the cellular elements possible. The figure shows a classic paradigm in which stimulation of the cerebellar cortex activates a beam of parallel fibers (brown). Recordings from the stellate and basket cells (green cells) and Purkinje cells (PCs) (orange cells) in line with this beam show that they are excited by the parallel fibers. In contrast, Purkinje cells located rostral or caudal to the beam receive only inhibition (purple areas) as a result of the perpendicular spatial relationship of the parallel fibers and the stellate and basket cell axons.
The Golgi cells are inhibitory interneurons in the granule cell layer. The geometry of their axonal and dendritic arbors is an exception to the orthogonal and planar organization of the cortex in that their dendrites and axons carve out roughly conical territories. One can think of it as two cones, tip to tip, where the soma is at the point at which the two cone tips meet. The dendritic tree forms the upper cone, which often extends into the molecular layer, and the axon forms the lower one. Golgi cells are excited by mossy and climbing fibers and by granule cell axons (parallel fibers) and inhibited by basket, stellate, and Purkinje cell axon collaterals. They in turn inhibit granule cells. Thus, they participate in both feedback (when excited by parallel fibers) and feedforward (when excited by mossy fibers) inhibitory loops that control activity in the mossy fiber—parallel fiber pathway to the Purkinje cell.
Lugaro cells have fusiform somata from which emerge two relatively unbranched dendrites, one from each side, that run along the transverse folial axis for several hundred microns, usually just under the Purkinje cell layer. Purkinje cell axon collaterals provide the main input to these neurons, with granule cell axons adding a minor input. The axon terminates mainly in the molecular layer on basket, stellate, and possibly Purkinje cells. Thus, these cells appear to sample the activity of Purkinje cells and provide both positive-feedback signals (they inhibit the interneurons that inhibit Purkinje cells) and negative-feedback signals (they directly inhibit the Purkinje cell).
Unipolar brush cells have only a single dendrite that ends as a tight bunch of branchlets that resemble a brush. These cells receive excitatory input from mossy fibers and inhibitory input from Golgi cells. It is thought that they synapse with granule and Golgi cells, which would make these cells an excitatory feedforward link in the mossy fiber—parallel fiber pathway.
Candelabrum cells are GABAergic cells located in the Purkinje layer. Their dendrites and axons terminate in the molecular layer, where the axonal arborization pattern resembles a candelabrum.
Cerebellar Nuclei
The cerebellar nuclei are the main targets of the cerebellar cortex. This projection is topographically organized such that each longitudinal strip of cortex targets a specific region of the cerebellar nuclei. The gross pattern is that the vermis projects to the fastigial and vestibular nuclei, the paravermal region projects to the interpositus, and the lateral hemisphere projects to the dentate nucleus.
The cerebellar nuclear neurons in turn provide the output from the cerebellum to the rest of the brain (with the primary exception of Purkinje cells that project to the vestibular nuclei). In discussing the output of the cerebellar nuclei, it is useful to group the nuclear cells according to whether they are GABAergic because the GABAergic cells project back to the inferior olive and form a negative-feedback loop to one of the cerebellum’s principal afferent sources. It is important to note that GABAergic cells project to the specific part of the inferior olive from which they receive input and from which their overlying longitudinal strip of cortex receives climbing fibers. Thus, the cerebellar cortex, cerebellar nuclei, and inferior olive are functionally organized as a series of closed loops. The non-GABAergic, excitatory nuclear cells project to a variety of targets from the spinal cord to the thalamus. In general, each nucleus gives rise to crossed ascending and descending projections that leave the cerebellum via the superior cerebellar peduncle. The fastigial nucleus also gives rise to significant uncrossed fibers, as well as a second crossed projection called the uncinate or hook bundle that leaves via the inferior cerebellar peduncle.
Although there are differences in the specific targets of each nucleus, in general, the ascending cerebellar projections target midbrain structures, such as the red nucleus and superior colliculus, and the VL nucleus of the thalamus, which connects to the primary motor cortex and thereby links the cerebellum to motor areas of the cerebrum. (The cerebral motor areas are likewise linked to the cerebellum by multiple pathways, including ones that relay in the basilar pons and inferior olive.) The descending fibers target mainly the basilar pontine nuclei, inferior olive, and several reticular nuclei. In addition, there is a small cerebellospinal pathway that arises principally from the fastigial nucleus. Finally, the fastigial nucleus has significant projections to the vestibular nuclei.
Activity of Purkinje Cells in the Cerebellar Cortex in the Context of Motor Coordination
Mossy fiber input to the cerebellar cortex, via their excitation of granule cells, causes a Purkinje cell to discharge single action potentials, referred to as simple spikes (Fig. 9-23). The spontaneous simple spike firing rate is typically around 20 to 50 Hz but can vary widely (from 0 to > 100 Hz), depending on the relative balance of excitation from parallel fiber input and inhibition from cerebellar cortex interneurons. Thus, this activity reflects the state of the cerebellar cortex.
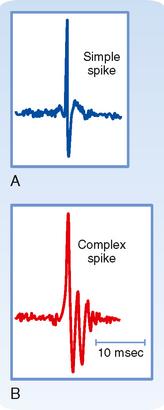
Figure 9-23 Responses of a Purkinje cell to excitatory input recorded extracellularly. A, Granule cells, via their ascending axons and parallel fibers, excite Purkinje cells and trigger simple spikes. B, Climbing fiber activity leads to high-frequency (≈500 Hz) bursts of two to four spikes known as complex spikes in Purkinje cells.
In contrast, a climbing fiber discharge causes a high-frequency burst of action potentials, called a complex spike (Fig. 9-23), in an all-or-none manner because of the massive excitation that is provided by the single climbing fiber to a Purkinje cell. This excitation is so powerful that there is essentially a one-to-one relationship between climbing fiber discharge and a complex spike. Thus, complex spikes essentially override what is happening at the cortex level and reflect the state of the inferior olive. The average firing rate of a spontaneous complex spike is only about 1 Hz.
Because the climbing fibers generate complex spikes at such a low frequency, they do not substantially change the average firing rates of Purkinje cells, and consequently, it is commonly argued that they have no direct role in shaping the output of the cerebellar cortex and are therefore not involved in ongoing motor control. Instead, it is commonly thought that their function is to alter the responsiveness of Purkinje cells to parallel fiber input. In particular, under certain circumstances, complex spike activity produces a prolonged depression in parallel fiber synaptic efficacy, termed LTD (long-term depression). This phenomenon is the proposed mechanism by which climbing fibers act in motor-learning hypotheses. Such hypotheses typically state that the parallel fiber system, and hence simple spikes, are involved in generating ongoing movement and, when there is a mismatch between the intended and actual movement, this error activates the inferior olive and complex spikes result, which then lead to LTD of the active parallel fiber synapses. This adjustment in synaptic weight will change the motor output in the future. If this change results in a properly executed movement, activation of the inferior olive will not occur and the motor program will be unchanged, but if there is still an error, the olivocerebellar system will trigger additional complex spikes that will cause further changes in synaptic efficacy, and so on. Major challenges to this view are that motor learning can occur when LTD is chemically blocked and that learned behavior can remain after removal of portions of the cerebellum where the memory is supposedly stored.
An alternative view is that the olivocerebellar system is directly involved in motor control (note that this does not preclude a role in motor learning as well) and, in particular, helps in the timing of motor commands. This view follows from the types of motor deficits observed in cerebellar damage and makes use of the special properties of the inferior olive mentioned earlier, namely, that it can generate rhythmic synchronous complex spike discharges across populations of Purkinje cells. These complex spikes would then produce synchronized inhibitory postsynaptic currents (IPSPs) on cerebellar nuclear neurons as a result of the convergence present in the Purkinje cell axon to the cerebellar nuclear projection. Because of the membrane properties of cerebellar nuclear neurons, these synchronized IPSPs could have a qualitatively different effect on nuclear cell firing than would the IPSPs caused by more numerous, but asynchronous simple spikes. Specifically, they could trigger rebound bursts in the nuclear cells that would then be transmitted to other motor systems as a gating signal. In fact, voluntary movements appear to be composed of a series of periodic accelerations that reflect a central oscillatory process. However, whether the olivocerebellar system helps time motor commands requires further evidence.
MOTOR CONTROL BY THE BASAL GANGLIA
The basal ganglia are the deep nuclei of the cerebrum. In association with other nuclei in the diencephalon and midbrain, the basal ganglia differ from the cerebellum in the way that they regulate motor activity. Unlike the cerebellum, the basal ganglia do not receive input from the spinal cord, but they do receive direct input from the cerebral cortex. The main action of basal ganglia is on the motor areas of the cortex by way of the thalamus. In addition to their role in motor control, the basal ganglia contribute to affective and cognitive functions. Lesions of the basal ganglia produce abnormal movement and posture.
Organization of the Basal Ganglia and Related Nuclei
The basal ganglia include the caudate nucleus, the putamen, and the globus pallidus (Fig. 9-24). The term striatum, derived from the striated appearance of these nuclei, refers only to the caudate nucleus and putamen. The striations are produced by the fiber bundles formed by the anterior limb of the internal capsule as it separates the caudate nucleus and putamen. The globus pallidus typically has two parts, an external segment and an internal segment. The combination of putamen and globus pallidus is often referred to as the lentiform nucleus.
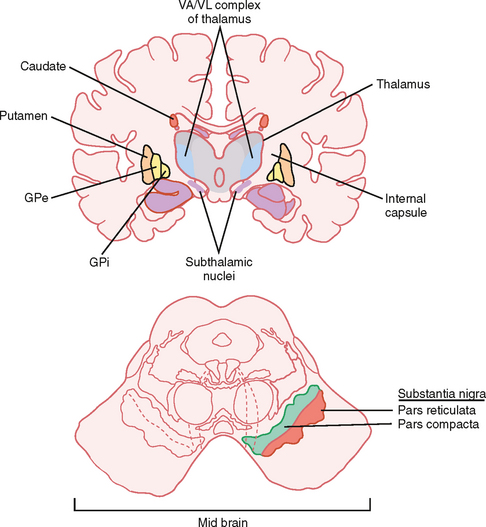
Figure 9-24 Components of basal ganglia and other closely associated brain regions. The main components of the basal ganglia are the caudate, putamen, globus pallidus, and substantia nigra pars reticulata. The motor loop of the basal ganglia connects with motor areas in the frontal cortex, the VA and VL thalamic nuclei, and the superior colliculus. Input from the substantia nigra pars compacta is critical for normal basal ganglia function. GPe, external segment of globus pallidus; GPi, internal segment of globus pallidus.
Associated with the basal ganglia are several thalamic nuclei. These include the ventral anterior (VA) and ventral lateral (VL) nuclei and several components of the intralaminar complex. Other associated nuclei are the subthalamic nucleus of the diencephalon and the substantia nigra of the midbrain (Fig. 9-24). The substantia nigra (“black substance”) derives its name from its content of melanin pigment. Many of the neurons in the pars compacta of this nucleus contain melanin, a byproduct of dopamine synthesis. The other subdivision of the substantia nigra is the pars reticulata. This structure can be regarded as an extension of the internal segment of the globus pallidus because these nuclei have an identical origin and similar connections.
Connections and Operation of the Basal Ganglia
Neurons of the striatum begin to discharge before movement occurs. This sequence suggests that these neurons help select the movement that is to be made. Activity in the putamen is related to the occurrence of movement of the body, whereas activity in the caudate nucleus is related to eye movement.
With the exception of the primary visual and auditory cortices, most regions of the cerebral cortex project topographically to the striatum. An important component of the cortical input to the striatum originates in the motor cortex. The corticostriatal projection arises from neurons in layer V of the cortex. The neurons appear to use glutamate as their excitatory neurotransmitter. The striatum then influences neurons in the VA and VL nuclei of the thalamus by two pathways, direct and indirect (Fig. 9-25, A). The thalamic neurons in turn excite neurons of the motor areas of the cerebral cortex.
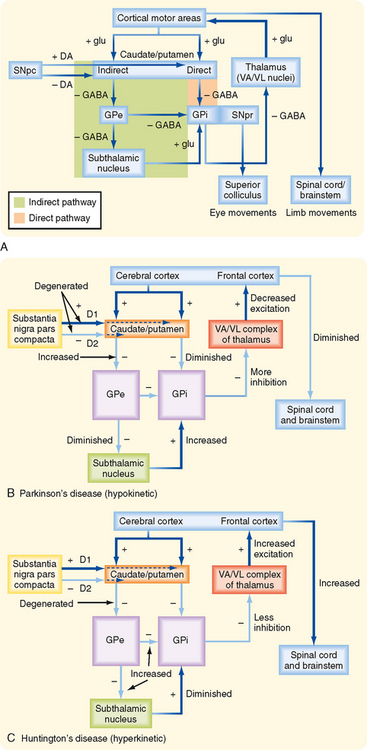
Figure 9-25 Functional connectivity of the basal ganglia for motor control. A, Connections between various basal ganglia components and other associated motor areas. The excitatory cortical input to the caudate and putamen influences output from the GPi and SNpr via a direct and an indirect pathway. Note that the two inhibitory steps in the indirect pathway mean that activity through this pathway has an effect on basal ganglia output to the thalamus and superior colliculus opposite that of the direct pathway. Note that DA is a neuromodulator that acts on D1 and D2 receptors on striatal neurons participating in the direct and indirect pathways, respectively. B, Changes in activity flow that occur in Parkinson’s disease in which the SNpc is degenerated. C, Changes in activity flow in Huntington’s disease in which inhibitory control of the GPe is lost. Plus (+) and minus (−) symbols, respectively, indicate the excitatory or inhibitory nature of a synaptic connection. DA, dopamine; glu, glutamate; GPe, GPi, globus pallidus external and internal; SNpc, SNpr, substantia nigra pars compacta and pars reticulata; VA/VL, ventral anterior and ventral lateral nuclei of the thalamus.
Direct Pathway
The overall action of the direct pathway through the basal ganglia to motor areas of the cortex is to enhance motor activity. In the direct pathway, the striatum projects to the internal segment of the globus pallidus (GPi). This projection is inhibitory, and the main transmitter is GABA. The GPi projects to the VA and VL nuclei of the thalamus. These connections also use GABA and are inhibitory. The VA and VL nuclei send excitatory connections to the prefrontal, premotor, and supplementary motor cortex. This input to the cortex influences motor planning, and it also affects the discharge of corticospinal and corticobulbar neurons.
The direct pathway appears to function as follows. Neurons in the striatum have little background activity, but during movement they are activated by their input from the cortex. In contrast, neurons in the GPi have a high level of background activity. When the striatum is activated, its inhibitory projections to the globus pallidus slow the activity of pallidal neurons. However, the pallidal neurons themselves are inhibitory, and they normally provide tonic inhibition of neurons in the VA and VL nuclei of the thalamus. Therefore, activation of the striatum causes disinhibition of neurons of the VA and VL nuclei. When disinhibited, the VA/VL neurons increase their firing rates, exciting their target neurons in the motor areas of the cerebral cortex. Because the motor areas evoke movement by activating α and γ motor neurons in the spinal cord and brainstem, the basal ganglia can regulate movement by enhancing the activity of neurons in the motor cortex.
Indirect Pathway
The overall effect of the indirect pathway is to reduce the activity of neurons in motor areas of the cerebral cortex. The indirect pathway involves inhibitory connections from the striatum to the external segment of the globus pallidus (GPe), which in turn sends an inhibitory projection to the subthalamic nucleus and to GPi. The subthalamic nucleus then sends an excitatory projection back to the GPi (Fig. 9-25, A).
In this pathway, pallidal neurons in the external segment are inhibited by the GABA released from striatal terminals in the globus pallidus. The GPe normally releases GABA in the subthalamic nucleus and thereby inhibits the subthalamic neurons. Therefore, striatal inhibition of the GPe results in the disinhibition of neurons of the subthalamic nucleus. The subthalamic neurons are normally active, and they excite neurons in the GPi by releasing glutamate. When the neurons of the subthalamic nucleus become more active because of disinhibition, they release more glutamate in the GPi. This transmitter excites neurons in the GPi and consequently activates inhibitory projections that affect the VA and VL thalamic nuclei. The activity of the thalamic neurons consequently decreases, as does the activity of the cortical neurons that they influence.
The direct and indirect pathways thus have opposing actions; an increase in the activity of either one of these pathways might lead to an imbalance in motor control. Such imbalances, which are typical of basal ganglion diseases, may alter the motor output of the cortex.
Actions of Neurons in the Pars Compacta of the Substantia Nigra on the Striatum
Dopamine is the neurotransmitter used by neurons of the pars compacta of the substantia nigra. In the nigrostriatal pathway, release of dopamine has an overall excitatory action on the direct pathway and an inhibitory action on the indirect pathway. This is, however, a modulatory type of effect. That is, dopamine is apparently causing its action not by generating postsynaptic potentials but rather by altering the striatal cells’ response to other transmitters. The different actions on the direct and indirect pathways result from different types of dopamine receptors being expressed by the spiny projection cells of the striatum that contribute to the direct and indirect pathways. D1 receptors are found on striatal cells that form the direct pathway by projecting to the GPi, whereas D2 receptors are found on striatal cells that participate in the indirect pathway and project to the GPe. The overall consequence of dopamine release in both cases is facilitation of activity in the motor areas of the cerebral cortex.
Subdivision of the Striatum into Striosomes and Matrix
On the basis of the associated neurotransmitters, the striatum has been subdivided into zones called striosomes and matrix. The cortical projections related to motor control end in the matrix area. The limbic system projects to the striosomes. Striosomes are thought to synapse in the pars compacta of the substantia nigra and to influence the dopaminergic nigrostriatal pathway.
Role of the Basal Ganglia in Motor Control
The basal ganglia mainly influence the motor cortex. Therefore, the basal ganglia have an important influence on the lateral system of motor pathways. Such an influence is consistent with some of the movement disorders observed in diseases of the basal ganglia. However, the basal ganglia must additionally regulate the medial motor pathways because diseases of the basal ganglia can also affect the posture and tone of proximal muscles.
The deficits seen in the various basal ganglia diseases include abnormal movement (dyskinesia), increased muscle tone (cogwheel rigidity), and slowness in initiating movement (bradykinesia). Abnormal movement includes tremor, athetosis, chorea, ballism, and dystonia. The tremor of basal ganglion disease is a “pill-rolling,” 3-Hz tremor that occurs when the limb is at rest. Athetosis consists of slow, writhing movement of the distal parts of the limbs, whereas chorea is characterized by rapid, flicking movement of the extremities and facial muscles. Ballism is associated with violent, flailing movement of the limbs (ballistic movement). Finally, dystonic movement is slow trunk movement that distorts body positions.
Parkinson’s disease is a common disorder characterized by tremor, rigidity, and bradykinesia. This disease is caused by loss of neurons in the pars compacta of the substantia nigra. Consequently, the striatum suffers a severe loss of dopamine. Neurons of the locus coeruleus and the raphe nuclei, as well as other monoaminergic nuclei, are also lost. The loss of dopamine diminishes the activity of the direct pathway and increases the activity of the indirect pathway (Fig. 9-25, B). The net effect is an increase in the activity of neurons in the internal segment of the globus pallidus. This results in greater inhibition of neurons in the VA and VL nuclei and less pronounced activation of the motor cortical areas. The consequence is slowed movement (bradykinesia).
Before the dopaminergic neurons are completely lost, administration of L-DOPA can relieve some of the motor deficits in Parkinson’s disease. L-DOPA is a precursor of dopamine, and it can cross the blood-brain barrier. Currently, the possibility of transplanting dopamine-synthesizing neurons into the striatum is being explored. Future research will no doubt focus on the potential for human embryonic stem cells to play such a therapeutic role.
Another basal ganglion disturbance is Huntington’s disease, which results from a genetic defect that involves an autosomal dominant gene. This defect leads to the preferential loss of striatal GABAergic and cholinergic neurons that project to the GPe as part of the indirect pathway (and also degeneration of the cerebral cortex, with resultant dementia). Loss of inhibition of the GPe presumably leads to diminished activity of neurons in the subthalamic nucleus (Fig. 9-25, C). Hence, the excitation of neurons of the GPi would be reduced. This will disinhibit neurons in the VA and VL nuclei. The resulting enhancement of activity in neurons in the motor areas of the cerebral cortex may help explain the choreiform movements of Huntington’s disease. The rigidity in Parkinson’s disease may in a sense be the opposite of chorea because overtreatment of patients with Parkinson’s disease with L-DOPA can result in chorea.
Hemiballism is caused by a lesion of the subthalamic nucleus on one side of the brain. In this disorder, involuntary, violent flailing movements of the limbs may occur on the side of the body contralateral to the lesion. Because the subthalamic nucleus excites neurons of the GPi, a lesion of the subthalamic nucleus would reduce the activity of these pallidal neurons. Therefore, neurons in the VA and VL nuclei of the thalamus would be less inhibited, and the activity of neurons in the motor cortex would be increased.
In all these basal ganglia disorders, the motor dysfunction is contralateral to the diseased component. This is understandable because the main final output of the basal ganglia to the body is mediated by the corticospinal tract.
Differences between the Basal Ganglia and Cerebellar Motor Loops
The organization of the motor loops that connect the basal ganglia and cerebellum with the motor regions of the cerebral cortex differs in several ways. The basal ganglia receive input from most areas of the cerebral cortex, whereas input to the cerebellum from the cerebral cortex is more restricted. Output from the basal ganglia is also more widespread and reaches the prefrontal cortex, as well as all the premotor areas. The cerebellar circuit influences only the premotor and motor cortex. Finally, the basal ganglia do not receive somatosensory information from ascending pathways in the spinal cord, and they have few connections with the brainstem. In contrast, the cerebellum is the target of several somatosensory pathways, and it has rich connections with brainstem nuclei.
EYE MOVEMENT
Eye movement has a number of features that distinguish it from other motor behavior. When compared with the movement that limbs, with their multiple joints and muscles, can perform, eye movement is relatively simple. For example, each eye is controlled by only three agonist-antagonist muscle pairs: the medial and lateral recti, the superior and inferior recti, and the superior and inferior obliques. These muscles allow the eye to rotate about three axes. Assuming that the head is in an upright position, the axes are the vertical axis, a horizontal axis that runs left to right, and the torsional axis (which is directed along the axis of sight). The medial and lateral recti control movement about the vertical axis; the other four muscles generate movement about the horizontal and torsional axes. Another simplifying feature is that there are no external loads to be compensated for. Furthermore, eye movement appears to be separable into a few distinct types, with each type being controlled by its own specialized circuitry. Thus, eye movement offers a number of advantages as a model system for studying motor control. Moreover, deficits in eye movement provide important clinical clues to the diagnosis of neurological problems. We first review the different eye movement types and then discuss the neural circuitry underlying their generation.
Types of Eye Movement
Vestibuloocular Reflex
Eye movement probably first evolved to hold the eye still, in contrast to limb movement, where one typically wants to generate movement with respect to the external world. The reason is that visual acuity degrades rapidly when there is eye movement relative to the external world (i.e., the visual scene slips across the retina). A major cause of such slippage is movement of the head. The vestibuloocular reflex (VOR) is one of the main mechanisms by which head movement is compensated in order to allow a stable visual scene to be maintained on the retina.
To maintain a stable visual scene on the retina, the VOR produces movement of the eyes that is equal and opposite the movement of the head. This reflex is initiated by stimulation of the receptors (hair cells) in the vestibular system (see Chapter 8). Recall that the vestibular organs are sensitive to head acceleration, not visual cues, and thus the VOR occurs in both the light and dark. Functionally, it is what is called an open loop system in that it generates an output (eye movement) in response to a stimulus (head acceleration), but its immediate behavior is not regulated by feedback about the success or failure of its output. It is worth noting, however, that in the light at least, any failure by the VOR to match eye and head rotation will result in what is called retinal slip (i.e., slip of the visual image across the retina), and this error signal can be fed back to the VOR circuits by other neuronal pathways and over time can lead to adjustments in the strength of the VOR to eliminate the error. This adaptation of the VOR is a major model for studying plasticity in the brain.
As stated, acceleration signals initiate the VOR. The output of the VOR, however, must be a change in eye position in the orbit. Thus, the problem to be solved by the nervous system is to translate the acceleration signals sensed by the vestibular organs into correct positional signals for the eyes. Mathematically, this can be thought of as a double integration. The first integration is done by the vestibular receptor apparatus because although the hair cells respond to head acceleration, the signals in the vestibular afferents are proportional to head velocity (at least for most stimuli that are encountered physiologically). The second integration, from velocity to position, occurs in the CNS in circuits described later.
The head can move in six different ways, often referred to as six degrees of freedom: three translational and three rotational. To compensate for these different types of movement, there are both translational and angular VORs, as well as separate subsystems for handling movement about different directions (e.g., rotation about a vertical or a horizontal axis).
Optokinetic Reflex
The optokinetic reflex (OKR) is a second mechanism by which the nervous system stabilizes the visual scene on the retina, and it often works in conjunction with the VOR. Whereas the VOR is activated only by head motion, the OKR is activated by movement of the visual scene, whether caused by motion of the scene itself or by head motion. That is, the sensory stimulus for this reflex is slip of the visual scene on the retina as detected by motion-sensitive retinal ganglion cells. An example of the former is when you are sitting in a train and a train on the adjacent track begins moving, your eyes rotate to keep the image of the neighboring car stable. This often leads to a sensation that you are moving (this is not entirely surprising because OKR circuits feed into the same circuits as used by the vestibular system).
The OKR can work in conjunction with the VOR to stabilize the visual image and is particularly important for maintaining a stable image when head movements are slow because the VOR works poorly in these conditions. In addition, as mentioned earlier, the VOR circuits by themselves act in an open loop mode and thus have no way to correct errors or calibrate their performance (i.e., detect a mismatch between head and eye rotation). The OKR allows for corrections and for calibration by triggering mechanisms to adjust the sensitivity of the VOR. Such mismatches occur as the head grows during childhood or when one puts on glasses.
Saccades
In animals whose eyes have a fovea, it becomes particularly advantageous to be able to move the eye with respect to the world (i.e., the main visual scene) so that objects of importance can be focused onto the fovea and scrutinized with this high-resolution part of the retina. Two classes of eye movement underlie this ability: saccadic and smooth pursuit. Movements that bring a particular region of the visual world onto the fovea are called saccades. For example, to read this sentence you are making a series of saccades to bring successive words onto your fovea to be read. However, even afoveate animals make saccades, and thus saccades may also be used to rapidly scan the visual environment.
Saccades are extremely rapid eye movements. In humans, eye velocity during a saccade can reach 800 degrees/sec, as compared with movement velocity of less than 10 degrees/sec generated in response to typical VOR and OKR stimuli (velocities of up to ≈120 degrees/sec can be produced by OKR stimuli in humans; however, they are still much slower than the maximal saccade velocities). Saccades can be made voluntarily or reflexively. Moreover, although they are usually made in response to visual targets, they can also be made toward auditory or other sensory cues, in the dark, or toward memorized targets.
Interestingly, visual processing appears to be suppressed just before and during saccades, particularly in the magnocellular visual pathway that is concerned with visual motion. This phenomenon is known as saccadic suppression and may function to prevent sensations of sudden, rapid movement of the visual world that would result during a saccade in the absence of such suppression. The mechanisms underlying saccadic suppression are not fully known, but in areas of the cortex related to visual processing, the responsiveness of the cells to visual stimuli is reduced and altered during saccades.
Smooth Pursuit
Once a saccade has brought a moving object of interest onto the fovea, the smooth pursuit system allows us to keep it stable on the fovea despite its continued motion. This ability appears to be limited to primates and allows prolonged continuous observation of a moving object. Note that in some respects, smooth pursuit might seem similar to the OKR, and in fact there may not be an absolute dividing line because as the target size grows, the distinction between target and background is lost; however, for small moving targets, smooth pursuit requires suppression of the OKR. You can see the effect of this suppression by moving your finger back and forth in front of this text while tracking it with your eyes. Your finger will be in focus while the words on this page will be part of the background scene and will become illegible as they slip along your retina.
Nystagmus
When there is a prolonged OKR or VOR stimulus (e.g., if you keep turning in one direction), these reflexes will initially counterrotate the eyes in an attempt to maintain a stable image on the retina, as described earlier. However, with a prolonged stimulus, the eyes will reach their mechanical limit, no further compensation will be possible, and the image will begin to slip on the retina. To avoid this situation, a fast saccade-like movement of the eyes occurs in the opposite direction, essentially resetting the eyes to begin viewing the visual scene again. Then the slow OKR- or VORinduced counterrotation will start anew. This alternation of slow and fast movement in opposite directions is nystagmus and can be displayed on a nystagmogram (Fig. 9-26). Thus, nystagmus can be defined as oscillatory or rhythmic movements of the eye in which there is a fast and a slow phase. The nystagmus is named according to the direction of the fast phase, because it is more easily observed.
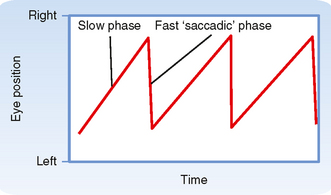
Figure 9-26 Nystagmogram showing eye movements that occur during nystagmus. The plot shows a left nystagmus because the fast phase is directed toward the left (downward on the graph).
In addition to being induced physiologically by VOR or OKR stimuli, nystagmus can result from damage to the vestibular circuits, either in the periphery (e.g., VIII nerve) or centrally (e.g., vestibular nuclei), and can be a useful diagnostic symptom.
Vergence
Conjugate eye movement is movement of both eyes in the same direction and in an equal amount. Such coordination allows a target to be maintained on both fovea during eye movement and is necessary to maintain binocular vision without experiencing diplopia (double vision). However, when objects are close (< 30 m), maintaining a target on both fovea requires eye movement that is no longer identical. Such disjunctive or vergence movements are also necessary for fixation of both eyes on objects that are approaching or receding. During convergence movements, accommodation of the lens for near vision and pupillary constriction also occur. In sum, the stimuli for vergence movements are diplopia and blurry images.
Neural Circuitry and Activity Underlying Eye Movement
Motor Neurons of the Extraocular Muscles
Three cranial nerve nuclei supply the extraocular muscles: oculomotor, trochlear, and abducens nuclei. Note that we will sometimes refer to these three nuclei collectively as the oculomotor nuclei; however, the context should make clear whether we mean the specific nucleus or all three. Motor neurons for the ipsilateral medial and inferior recti, ipsilateral inferior oblique, and contralateral superior rectus muscles reside in the oculomotor nucleus; those for the contralateral superior oblique muscle reside in the trochlear nucleus; and those for the ipsilateral lateral rectus muscle are located in the abducens nucleus. These motor neurons form some of the smallest motor units (1 : 10 nerve-to-muscle ratio), consistent with the very fine control needed for precise eye movement.
An important point regarding motor neurons to the extraocular muscles is that most have spontaneous activity when the eye is in the primary position (looking straight ahead) and their firing rate correlates with eye position and velocity. This spontaneous activity allows the antagonist muscle pairs to act in a push-pull fashion, which increases the responsiveness of the system. That is, as motor neurons innervating one muscle are activated and cause increased contraction, those to its antagonist are inhibited and lead to relaxation.
In addition to motor neurons, the abducens nuclei have internuclear neurons. These neurons project, via the medial longitudinal fasciculus, to medial rectus motor neurons in the contralateral oculomotor nucleus. As we will see, this projection facilitates the coordinated action of the medial and lateral recti that is needed for conjugate movements, such as occur in the VOR.
Circuits Underlying the Vestibuloocular Reflex
The VOR acts to counter head motion by causing rotation of the eyes in the opposite direction. There are separate circuits for rotational and translational movement of the head. The sensors for the former are the semicircular canal, and the sensors for the latter are the otoliths (the utricle and saccule). The circuits for the angular VOR are more straightforward (but still complex!), so we will focus on these pathways to illustrate how this reflex works; however, the basic scheme is the same: vestibular afferents go to vestibular nuclei, the vestibular nuclei in turn project to the various oculomotor nuclei, and motor neurons in the oculomotor nuclei give rise to axons that innervate the extraocular muscles. What varies are the specific vestibular and oculomotor nuclei that are involved.
Focusing on the angular VOR pathways, the pathway for generating horizontal eye movement originates in the horizontal canals, and the analogous one for vertical movement originates in the anterior and posterior canals. Figure 9-27, A, shows the basic circuit for the horizontal VOR. Note that only the major central circuits originating in the left horizontal canal and vestibular nuclei are shown; however, mirror image pathways arise from the right canal and vestibular nuclei. Vestibular afferents involved in the horizontal VOR pathway primarily synapse in the medial vestibular nucleus, which projects to the abducens nucleus bilaterally; inhibitory neurons project ipsilaterally and excitatory ones project contralaterally. Control of the medial rectus muscle is achieved by abducens internuclear neurons that project from the abducens to the part of the oculomotor nucleus controlling the medial rectus muscle. Note the double crossing of this pathway, which results in aligning of the responses of functional synergists (e.g., the left medial rectus with the right lateral rectus).
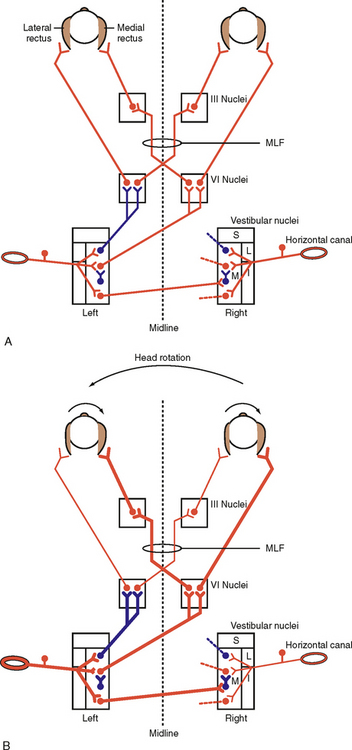
Figure 9-27 Circuits underlying the horizontal vestibuloocular reflex (VOR). A, The vestibular nuclei receive excitatory input from the horizontal canal afferents and project to the abducens (VI) nucleus. The VI nucleus innervates the lateral rectus and projects to the contralateral oculomotor (III) nucleus, which controls the medial rectus. Excitatory neurons are shown in red, inhibitory ones in blue. Note that only the major pathways originating in the left vestibular nuclei are shown. For clarity, only the beginnings of mirror image pathways from the right vestibular nuclei are shown (dotted lines). B, Flow of activity in the VOR circuitry induced by leftward head rotation. Increased soma size and axonal thickness indicate increased activity; thinner axons indicate decreased activity in comparison to levels at rest (A). Note that leftward rotation causes both an increase in activity of the left vestibular afferents and a decrease in activity of the right ones. MLF, medial longitudinal fasciculus. Vestibular nuclei: I, inferior; L, lateral; M, medial; S, superior.
The vertical VOR pathway primarily involves the superior vestibular nucleus, which has direct bilateral projections to the oculomotor nucleus.
Consider what happens in the horizontal canal pathway when there is head rotation to the left as shown in Figure 9-27, B. Leftward head rotation would cause the visual image to slip to the right. However, compensation by the VOR will be triggered by depolarization of the hair cells of the left canal in response to the angular acceleration (Fig. 8-27). The depolarized hair cells will cause increased activity in the left vestibular afferents and thereby excite neurons of the left medial vestibular nucleus. These include excitatory neurons that project to the contralateral abducens nucleus and synapse with both motor neurons and internuclear neurons. Excitation of the motor neurons will lead to contraction of the right lateral rectus and rotation of the right eye to the right, whereas excitation of the internuclear neurons of the right abducens nucleus will lead to excitation of the medial rectus motor neurons in the left oculomotor nucleus, thus causing the left eye to rotate to the right as well.
If we now follow the pathway starting with the inhibitory vestibular neurons that project from the left medial vestibular nucleus to the ipsilateral abducens nucleus, we can see that the activity of these cells leads to inhibition of motor neurons to the left lateral rectus and motor neurons to the right medial rectus (the latter via internuclear neurons to the right oculomotor nucleus). Consequently, these muscles will relax, thereby facilitating rotation of the eyes to the right. Thus, the eye is being pulled by the increased tension of one set of muscles and “pushed” by the release of tension in the antagonist set of muscles.
Note that the mirror image pathways originating from the right canal have been left out of Figure 9-27 for clarity, but the changes in activity through them with leftward head rotation would be exactly the opposite, and thus they would function synergistically with those that are shown. As an exercise the reader should work out the resulting changes in activity through these circuits. Remember that leftward head rotation hyperpolarizes the hair cells of the right canal, thereby leading to a decrease in right vestibular afferent activity and disfacilitation of the right vestibular nuclear neurons.
Now, consider the commissural fibers that connect the two medial vestibular nuclei. These fibers are excitatory but end on local inhibitory interneurons of the contralateral vestibular nucleus and thus inhibit the projection neurons of that nucleus. This pathway reinforces the actions of the contralateral vestibular afferents on their target vestibular nuclear neurons. In our example, commissural cells in the left vestibular nucleus will be activated and therefore cause active inhibition of the right medial vestibular nuclei projection neurons, which reinforces the disfacilitation caused by the decrease in right afferent activity. In fact, this commissural pathway is powerful enough to modulate the activity of the contralateral vestibular nuclei even after unilateral labyrinthectomy, which destroys the direct vestibular afferent input to these nuclei.
Finally, it is important to note that superimposed on the brainstem circuits is the cerebellum. Parts of the vermis and flocculonodular lobe receive primary vestibular afferents or secondary vestibular afferents (axons of the vestibular nuclear neurons), or both, and in turn project back to the vestibular nuclei directly and via a disynaptic pathway involving the fastigial nucleus. The exact role of these cerebellar circuits in generating the VOR is much debated, but they are critical inasmuch as damage to them leads to abnormal eye movement, such as spontaneous nystagmus, and other symptoms of vestibular dysfunction.
When a labyrinth is irritated in one ear, as in Ménière’s disease, or when a labyrinth is rendered nonfunctional, as may happen as a result of head trauma or disease of the labyrinth, the signals transmitted through the VOR pathways from the two sides become unbalanced. Vestibular nystagmus can then result. For example, irritation of the labyrinth of the left ear can increase the discharge of afferents that supply the left horizontal semicircular duct. The signal produced resembles that normally generated when the head is rotated to the left. Because the stimulus is ongoing, a left nystagmus results, with a slow phase to the right (caused by the VOR pathway) and a fast phase to the left. Destruction of the labyrinth in the right ear produces effects similar to those induced by irritation of the left labyrinth. Interestingly, the nystagmus is temporary, thus showing the ability of these circuits to adapt over time.
Circuits Underlying the Optokinetic Reflex
The stimulus eliciting the OKR is visual (retinal slip), so photoreceptors are the start of the reflex arc. Key brainstem centers for this reflex lie in the tegmentum and pretectal region of the rostral midbrain. They are the nucleus of the optic tract (NOT) and a group of nuclei collectively known as the accessory optic nuclei (AON). Direction-selective, motion-sensitive retinal ganglion cells are a major afferent source carrying visual information to these nuclei. In addition, input comes from primary and higher-order visual cortical areas in the occipital and temporal lobes. These latter afferent sources become particularly important in primates and humans. Cells of the NOT and AON have large receptive fields, and their responses are selective for the direction and speed of movement of the visual scene. Interestingly, the preferred directions of movement of the NOT/AON cells correspond closely to motion caused by rotation about axes perpendicular to the semicircular canals, thereby facilitating coordination of the VOR and OKR to produce stable retinal images. The efferent connections of these nuclei are numerous and complex and not fully understood. There are polysynaptic pathways to the oculomotor and abducens nuclei and monosynaptic input to the vestibular nuclei, which allows interaction with the VOR. There are projections to various precerebellar nuclei, including the inferior olive and basilar pontine nuclei. These pathways then loop through the flocculus and back to the vestibular nuclei. In sum, via several pathways operating in parallel, activity ultimately arrives at the various oculomotor nuclei whose motor neurons are activated, and proper counterrotation of the eyes results.
Clinical testing of labyrinthine function is done either by rotating the patient in a Bárány chair to activate the labyrinths in both ears or by introducing cold or warm water into the external auditory canal of one ear (caloric test). When a person is rotated in a Bárány chair, nystagmus develops during the rotation. The direction of the fast phase of the nystagmus is in the same direction as the rotation. When the rotation of the chair is halted, nystagmus develops in the opposite direction (postrotatory nystagmus) because stopping a rotation is equivalent to accelerating in the opposite direction.
The caloric test is more useful because it can distinguish between malfunction of the labyrinths on the two sides. The head is bent backward about 60 degrees so that the two horizontal canals are essentially vertical. If warm water is introduced into the left ear, the endolymph in the outer portion of the loop of the left semicircular canal tends to rise as the specific gravity of the endolymph decreases because of heating. This sets up a convection flow of endolymph, and as a result, the kinocilia of the left ampullary crest hair cells are deflected toward the utricle, the same as if there was head rotation to the left, the discharge of the afferents that supply this canal increases, and nystagmus occurs with the fast phase toward the left. The nystagmus produces a sense that the environment is spinning to the right, and the subject tends to fall to the right. The opposite effects are produced if cold water is placed in the ear. A mnemonic expression that can help in remembering the direction of the nystagmus in the caloric test is COWS (“cold opposite, warm same”). In other words, cold water results in a fast phase of nystagmus toward the opposite side, and warm water causes a fast phase toward the same side.
Circuits Underlying Saccades
Saccades are generated in response to activity in the superior colliculus or the cerebral cortex (frontal eye fields and posterior parietal areas). Activity in the superior colliculus is related to computation of the direction and amplitude of the saccade. Indeed, the deep layers of the superior colliculus contain a topographic motor map of saccade locations. From the superior colliculus, information is forwarded to distinct sites for control of horizontal and vertical saccades, referred to as the horizontal and vertical gaze centers, respectively. The horizontal gaze center consists of neurons in the paramedian pontine reticular formation (PPRF), in the vicinity of the abducens nucleus (Fig. 9-28, A). The vertical gaze center is located in the reticular formation of the midbrain, specifically, the rostral interstitial nucleus of the medial longitudinal fasciculus and the interstitial nucleus of Cajal. Because the circuitry and operation of the horizontal gaze center are better understood than those of the vertical gaze center, it is discussed here in detail. However, cells showing similar activity patterns have been described in the vertical gaze center.
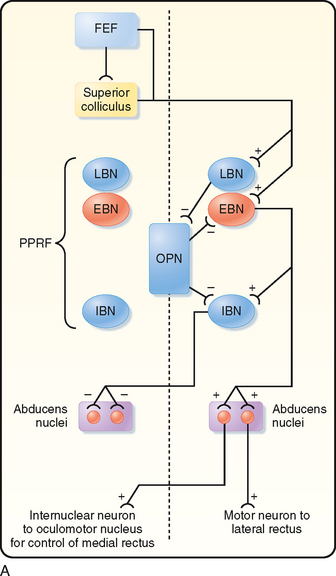
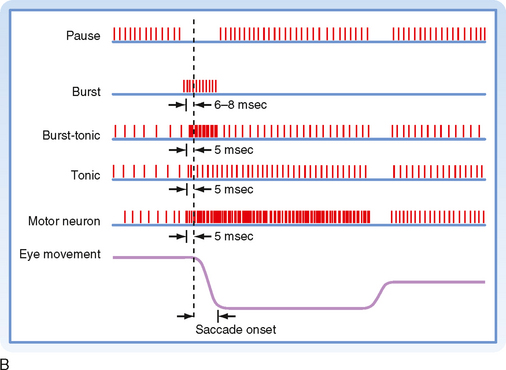
Figure 9-28 Horizontal saccade pathways. A, Circuit diagram of the major pathways. EBN, excitatory burst neuron; FEF, frontal eye field; IBN, inhibitory burst neuron; LBN, long lead burst neuron; OPN, omnipause neuron; PPRF, paramedian pontine reticular formation. B, Firing patterns of some of the neurons involved in making saccades. Excitation of burst neurons of the right horizontal gaze center causes abducens motor neurons on the right and medial rectus motor neurons on the left to be activated. The ascending pathway to the oculomotor nucleus is through the medial longitudinal fasciculus. The left horizontal gaze center is simultaneously inhibited.
Figure 9-28, A is an overview of the neural circuitry by which saccades are generated, and Figure 9-28, B shows the activity of certain types of neurons found in the gaze center that are responsible for horizontal saccades. Each horizontal gaze center has excitatory burst neurons that project to motor neurons in the ipsilateral abducens nucleus and to the internuclear neurons (which will excite medial rectus motor neurons in the contralateral oculomotor nucleus). It also has inhibitory burst neurons that inhibit the contralateral abducens. These burst neurons are capable of extremely high bursts of spikes (up to 1000 Hz). Moreover, the gaze center has neurons showing tonic activity and burst-tonic activity.
Normally, both inhibitory and excitatory burst neurons are inhibited by omnipause neurons located in the nucleus of the dorsal raphe. When a saccade is to be made, activity from the frontal eye fields or the superior colliculus, or both, leads to inhibition of the omnipause cells and excitation of the burst cells on the contralateral side. The resulting high-frequency bursts in the excitatory burst neurons provide a powerful drive to motor neurons of the ipsilateral lateral rectus and contralateral medial rectus (Fig. 9-28, A) while at the same time, inhibitory burst neurons permit relaxation of the antagonists. The initial bursts of these neurons allow strong contraction of the appropriate extraocular muscles, which overcomes the viscosity of the extraocular muscle, and permits rapid movement to occur.
Circuits Underlying Smooth Pursuit
Smooth pursuit involves tracking a moving target with one’s eyes (Fig. 9-29). Visual information about target velocity is processed in a series of cortical areas, including the visual cortex in the occipital lobe, several temporal lobe areas, and the frontal eye fields. It should be noted that in the past it was thought that the frontal eye fields were related only to control of saccades, but evidence has recently shown that there are distinct regions within the frontal eye fields dedicated to either saccade production or smooth pursuit. Indeed, there may be two distinct cortical networks, each specialized for one of these types of eye movement. Cortical activity from multiple cortical areas is fed to the cerebellum via parts of the pontine nuclei and nucleus reticularis tegmenti pontis. Specific areas in the cerebellum, namely, parts of the posterior lobe vermis, the flocculus, and the paraflocculus, receive this input and in turn project to the vestibular nuclei. From the vestibular nuclei, activity can then be forwarded to the oculomotor, abducens, and trochlear nuclei, as was described for the VOR earlier.
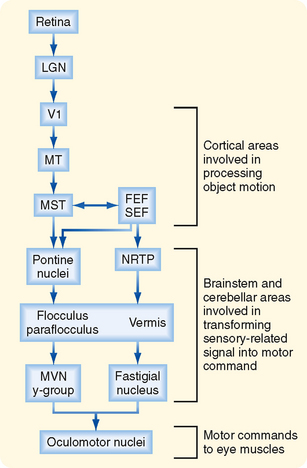
Figure 9-29 Smooth pursuit pathways. The stimulus for smooth pursuit eye movement is a moving visual target. This causes activity to flow through the circuitry diagramed in the figure and leads to maintenance of the fovea on the target. FEF, frontal eye field; LGN, lateral geniculate nucleus; MVN, medial vestibular nucleus; NRTP, nucleus reticularis tegmenti pontis; SEF, supplementary eye field; V1, primary visual cortex. MT and MST are higher-order visual association areas.
Circuits Underlying Vergence
The neural circuits underlying vergence movements are not well known. There are premotor neurons (neurons that feed onto motor neurons) located in the brainstem areas surrounding the various oculomotor nuclei. In some cortical visual areas and the frontal eye fields there are neurons whose activity is related to the disparity of the image on the two retinas or varies during vergence movements. How vergence signals in these cortical areas feed into the brainstem premotor neurons is not clear. The cerebellum also appears to play a role in vergence movements because cerebellar lesions impair this type of eye movement.