CHAPTER 25 Nonrespiratory Functions of the Lung
Although gas exchange is the primary function of the lung, the lung is also a major defense organ that protects the inside of the body from the outside world, and it is an important organ for metabolism. To cope with the inhalation of ubiquitous foreign substances, the respiratory system and, in particular, the conducting airways have developed unique structural features (e.g., the mucociliary clearance system), as well as specialized adaptive and innate immune response mechanisms. In addition, because the lung receives the total cardiac output, it is uniquely positioned to act as a metabolic regulator of venous blood before its entry into the systemic circulation. This chapter provides insight into mucociliary clearance and the immune defense systems of the lung, as well as describes the metabolic capabilities of the lung.
MUCOCILIARY CLEARANCE SYSTEM
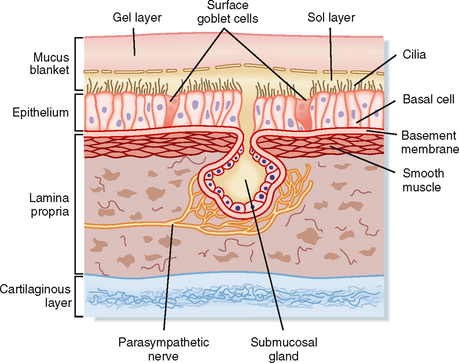
The mucociliary clearance system protects the lower respiratory system by trapping and removing inhaled pathogenic viruses and bacteria, in addition to nontoxic and toxic particulates (e.g., pollen, ash, mineral dust, mold spores, and organic particles), from the lungs. These particulates are inhaled with each breath and must be removed from the lungs. The three major components of the mucociliary clearance system are two fluid layers referred to as the sol (periciliary fluid) and gel (mucus layer) phases and the cilia, which are positioned on the surface of the airway epithelial cells (Fig. 25-1). The cilia are embedded in the periciliary fluid with only the tips of the cilia contacting the mucus. Inhaled material is trapped on the viscoelastic mucus, whereas the watery periciliary fluid allows the cilia to move freely. Effective clearance requires both ciliary activity and the appropriate balance of periciliary fluid and mucus.
PERICILIARY FLUID
The periciliary fluid layer is composed of nonviscous serous fluid, which is produced via active ion transport by the pseudostratified ciliated columnar epithelial cells that line the airways. Several mediators, under basal conditions and in response to inflammation, stimulate Cl− secretion by airway epithelial cells. The balance between Cl− secretion and Na+ absorption determines the volume and ionic composition of the periciliary fluid and maintains the depth of this fluid at about 5 to 6 μm (Fig. 25-1). When net NaCl transport into periciliary fluid is stimulated, diffusive entry of water (i.e., osmosis) into the periciliary fluid is enhanced because of the osmotic gradient that occurs transiently as a result of NaCl transport. Maintaining normal fluid depth and ionic composition in the periciliary fluid is important for rhythmic beating of the cilia and normal mucociliary clearance.
Cystic fibrosis (CF) is an autosomal recessive genetic disease that is characterized by thick, tenacious, and dehydrated airway secretions. In CF, mutations in CFTR, the cystic fibrosis transmembrane conductance regulator, which is a Cl− channel, result in a decreased ability to secrete Cl− and therefore enhance Na+ absorption. This reduces the volume of periciliary fluid and results in thick mucus that cannot be cleared from the lung by the mucociliary clearance system.
Mucus Layer
The mucus layer lies on top of the periciliary fluid layer and is composed of a complex mixture of macromolecules and electrolytes. Because the mucus layer is in direct contact with air, it entraps inhaled substances. The mucus layer is predominantly water (95% to 97%), 5 to 10 μm thick, and exists as a discontinuous blanket (i.e., islands of mucus). Mucus has low viscosity and high elastic properties and is composed of glycoproteins with groups of oligosaccharides attached to a protein backbone. Healthy individuals produce approximately 100 mL of mucus each day.
Cells That Produce Mucus
Four cell types contribute to the quantity and composition of the mucus layer: goblet cells, mucous cells, and serous cells within the submucosal tracheobronchial glands, as well as Clara cells. Goblet cells, also referred to as surface secretory cells, are present every five to six ciliated cells in the respiratory epithelium. They can be found up to the 5th tracheobronchial division and disappear beyond the 12th division. In many diseases, goblet cells appear further down the tracheobronchial tree, thus making the smaller airways more susceptible to obstruction by mucus plugging. Goblet cells secrete neutral and acidic glycoproteins rich in sialic acid in response to chemical stimuli. In the presence of infection or cigarette smoke or in patients with chronic bronchitis, goblet cells can increase in size and number, and they secrete copious amounts of mucus. Injury and infection change the properties of the mucus secreted by goblet cells by increasing its viscosity.
Submucosal tracheobronchial glands are present wherever there is cartilage in the upper regions of the conducting airways, and they secrete water, ions, and mucus into the airway lumen through a ciliated duct. The secretory cells of the submucosal gland include mucous cells located near the distal end of the duct and serous cells located at the most distal end of the duct. Although both cell types secrete mucus, their cellular morphology and mucus composition are distinctly different (Table 25-1). Mucous cells secrete acidic glycoproteins, whereas serous cells secrete neutral glycoproteins and bactericidal compounds, including lysozyme, lactoferrin, and antileukoprotease. Submucosal glands increase in number and size and can extend to the bronchioles in diseases such as chronic bronchitis (i.e., inflammation of the bronchi). This leads to increased mucus production, alterations in the chemical composition of the mucus (i.e., increased viscosity and decreased elasticity), and the formation of plugs that are manifested clinically as airway obstruction. Mucus secretion from submucosal tracheobronchial glands is under parasympathetic (cholinergic), sympathetic (adrenergic), and peptidergic (vasoactive intestinal polypeptide) neural control. Local inflammatory mediators such as histamine and arachidonic acid metabolites also stimulate mucus production.
Table 25-1 Properties of Submucosal Gland Cells
| Serous Cells | Mucous Cells | |
|---|---|---|
| Granules | Small, electron dense | Large, electron lucent |
| Glycoproteins | Acidic | |
| Hormones | α- > β-Adrenergic | β- > α-Adrenergic |
| Receptors | Muscarinic | Muscarinic |
| Degranulation |
Sputum is expectorated mucus. However, in addition to mucus, sputum contains serum proteins, lipids, electrolytes, Ca++, DNA from degenerated white cell nuclei (collectively known as bronchial secretions), and extrabronchial secretions, including nasal, oral, lingual, pharyngeal, and salivary secretions. The color of sputum correlates more closely with the amount of time that it has been present in the lower respiratory tract than with the presence of infection.
Clara cells, located in the epithelium of bronchioles, also contribute to the composition of mucus via secretion of a nonmucinous material containing carbohydrates and proteins. These cells play a role in bronchial regeneration after injury.
Cilia
There are approximately 250 cilia per airway epithelial cell, and each is 2 to 5 μm in length. Cilia are composed of nine microtubular doublets that surround two central microtubules held together by dynein arms, nexin links, and spokes. The central microtubule doublet contains an ATPase that is responsible for the contractile beat of the cilium. Cilia beat with a coordinated oscillation in a characteristic, biphasic, and wavelike rhythm called metachronism. They beat at approximately 1000 strokes/min, with a power forward stroke and a slow return or recovery stroke. During their power forward stroke, the tips of the cilia extend upward into the viscous mucus layer and thereby move it and the entrapped particles. On the reverse beat, the cilia release the mucus and withdraw completely into the sol layer. Cilia in the nasopharynx beat in the direction that propels the mucus into the pharynx, whereas cilia in the trachea propel mucus upward toward the pharynx, where it is swallowed.
Particle Deposition and Clearance
Deposition of particles in the lung depends on particle size and density, the distance over which the particle travels, and the relative humidity of the air. In general, particles larger than 10 μm are deposited by impaction in the nasal passages and do not penetrate into the lower respiratory tract. Particles 2 to 10 μm in size are deposited in the lower respiratory tract predominantly by inertial impaction at points of turbulent flow (i.e., nasopharynx, trachea, and bronchi) and at airway bifurcations because their inertia (i.e., tendency to move in a straight direction) prevents them from changing directions rapidly. The greater the mass and velocity of a particle, the greater its inertia and likelihood of impacting on a surface directly in front of it. In more distal areas, where airflow is slower, smaller particles (0.2 to 2 μm) are deposited on the surface by sedimentation secondary to gravity. Particle size and density, as well as airway diameter, are major factors that influence deposition of particles in the airway via sedimentation. For substances with elongated shapes (i.e., asbestos, silica), another important mechanism of deposition is interception. The elongated particle’s center of gravity is compatible with the flow of air; however, when the distal tip of the particulate comes in contact with a cell or mucus layer, deposition is facilitated. Particles less than 0.2 μm are deposited by diffusion via brownian motion in the smaller airways and alveoli. The particle’s diffusion coefficient is a major influence on the deposition of small particles. Unlike the deposition of larger particles in the upper airways, particle density does not influence diffusion. Diffusion deposition is enhanced with decreased particle size. These small particles come in contact with the alveolar epithelium in the terminal respiratory units where cilia and the mucociliary transport system do not exist. Thus, small particles can be cleared only by lymphatic drainage or phagocytosis by alveolar macrophages. Macrophages migrate through the alveoli and engulf foreign or effete autologous materials in the airway lumen. Clearance of material by alveolar macrophages is usually rapid (<24 hours).
In the conducting airways, the mucociliary clearance system transports deposited particles from the terminal bronchioles to the major airways, where they are coughed up and either expectorated or swallowed. Deposited particles can be removed in a matter of minutes to hours. In the trachea and main bronchi, the rate of particle clearance is 5 to 20 μm/min, but it is slower in the bronchioles (0.5 to 1 μm/min). In general, the longer inhaled material remains in the airways, the greater the probability that the material will cause lung damage because of slow clearance. The region from the terminal bronchioles to the alveoli is devoid of ciliated cells and is considered the “Achilles heel” in what is otherwise a highly effective system. In individuals with the occupational lung disease pneumoconiosis, the “black lung” disease of coal miners, the highest concentration of coal dust particles is usually seen just beyond the terminal bronchioles. The relatively slow rate of particle clearance in this area renders the terminal respiratory unit the most common location of airway damage for all types of occupational lung disease.
METABOLIC FUNCTIONS OF THE LUNG
The endothelial cells that line the capillaries of the lungs are exposed to the total cardiac output. Such exposure provides an ideal environment to metabolize substances and modify venous blood before its entry into the systemic circulation. The endothelial cells of the pulmonary capillary bed have developed a variety of metabolic processing mechanisms and cell surface receptors to carry out their unique role in metabolism. Endothelial cells within the pulmonary capillary bed metabolize many substances, including vasoactive amines, cytokines, lipid mediators, and proteins. Metabolism occurs through either intracellular or extracellular processing of substances as they pass through the capillaries or via direct synthesis and secretion by endothelial cells. For example, circulating inactivated angiotensin I is activated by extracellular enzymes on the surface of endothelial cells.
Serotonin, a vasoconstrictor, binds to a specific receptor on the surface of the endothelial cell and is internalized and metabolized by intracellular mechanisms. Approximately 80% of the serotonin entering the lung is metabolized in a single pass through the pulmonary capillary bed. Endothelial cells also have surface receptors for bradykinin, tumor necrosis factor (TNF), components of complement, immunoglobulin Fc fragments, and adhesion molecules. In addition, endothelial cells synthesize and secrete prostacyclin, endothelin, clotting factors, nitric oxide, prostaglandins, and cytokines. Vascular endothelial cells, however, lack 5-lipoxygenase and are not able to synthesize leukotrienes. Compounds not metabolized by the pulmonary capillary bed include epinephrine, dopamine, histamine, isoproterenol, angiotensin II, and substance P.
IMMUNE DEFENSE SYSTEM
To deal with inhaled viruses, bacteria, and noxious agents, the respiratory system has developed specialized defense mechanisms that form the basis of the mucosal immune system in the lung. To avoid a continuous inflammatory state, which can cause lung damage, the lung must discriminate between what is harmful and what is not. Although inflammation is a protective response to injury or to an invading pathogen, inflammation usually disrupts the normal physiology. Accordingly, the lung has evolved “first-line” defense mechanisms that are designed to handle the offending agent with minimal or no inflammation. If the first-line defense mechanisms fail, an inflammatory response is initiated. The mucosa of the lung contains specialized adaptive immune cells (e.g., T lymphocytes with limited antigen recognition abilities and plasma cells that synthesize a non–complement-binding antibody, IgA) and innate immune cells (e.g., alveolar macrophages, natural killer [NK] cells, and dendritic cells) (Table 25-2). These cells limit the immunological and inflammatory responses to foreign substances that enter the respiratory system.
Table 25-2 Innate and Adaptive Immune Cells in the Respiratory System
| Cell Type | Location | Function |
|---|---|---|
| TCRγδ lymphocytes | Intraepithelial | |
| TCRαβ lymphocytes | Lamina propria | |
| B lymphocytes | Submucosa | IgA antibody synthesis |
| Dendritic cells | Diffuse in the lung interstitium | |
| Alveolar macrophages | Alveoli and alveolar ducts | |
| NK cells | Diffuse in the lung interstitium | |
| NK/T cells | Diffuse in the lung interstitium | Immunoregulation (IL-4) |
Any process that interferes with normal ciliary beating will interfere with clearance of particles in the lung. Kartagener’s syndrome is associated with immotile cilia and comprises the triad of situs inversus with bronchiectasis and sinusitis, which causes a chronic infection.
Patients with asthma have increased mucus production and viscosity. This causes abnormalities in mucociliary clearance in the absence of infection.
Mucosa-Associated Lymphoid Tissue (MALT)
The respiratory, gastrointestinal, and urinary systems are part of the body’s mucosal immune system, which can function independently of the systemic immune system. In nonmucosal tissues (e.g., spleen, liver, kidney), the adaptive immune response is the body’s primary defense. However, the lung and other mucosal tissues are unique in that the adaptive immune response is initiated only after the insulting agent has bypassed the innate immune response.
An important feature that distinguishes lymph nodes in the systemic immune system from MALT is that true lymph nodes are encapsulated and have an afferent (entering) and efferent (leaving) pattern of lymphatic fluid drainage that is not present in MALT (Fig. 25-2). Once an antigen is processed through a lymph node, it can be assumed that systemic sensitization has or will soon occur. In contrast, although MALT is organized, it is not encapsulated and there is only afferent lymph drainage. It appears that there is direct communication between organs of MALT and that sensitization via one organ is transposed to all MALT tissues via a “lymphatic-like” drainage network. The systemic immune system and MALT may work independently of each other, and sensitization of one may not transpose to the other. This may serve as a defense mechanism in limiting sensitization only to mucosal tissue.
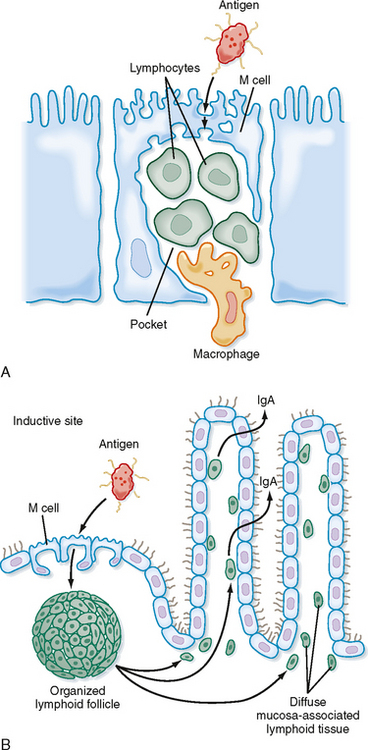
Figure 25-2 Representation of MALT, M cells, and IgA synthesis. A, M cells located in mucosal epithelium endocytose antigen in the lumen and transport it for processing to submucosal pockets of immune cells. B, Diagram of a mucous membrane showing secretion of IgA antibodies in response to antigen endocytosed by M cells at an inductive site. Activated B cells migrate from the lymphoid follicle to nearby MALT, where they differentiate into IgA-producing plasma cells.
The lymphatic system and the lymphoid tissue in the lungs filter fluids and particulates through lymph nodes and bronchus-associated lymphoid tissue known as BALT (e.g., lymph nodules, lymph aggregates). Solitary lymphocytes and dendritic cells are scattered throughout the respiratory tract in a diffuse submucosal network and play an important role in defense of the lung. Because inhaled particles are broadly dispersed throughout the respiratory tract, each type of lymphoid tissue plays an important and unique role in the overall defense of the lung.
Immunoglobulin A (IgA)
The lung also has several unique defense features that limit airway inflammation. One of the specialized features is a unique antibody system that uses specialized functional features of the IgA antibody. In submucosal areas, plasma cells synthesize and secrete IgA, which migrates to the submucosal surface of epithelial cells, where it binds to a surface protein receptor, poly-Ig (Fig. 25-3). The poly-Ig receptor aids in pinocytosis of IgA into the epithelial cell and eventual secretion (exocytosis) of IgA into the airway lumen. During exocytosis of the IgA complex, the poly-Ig is enzymatically cleaved, and a portion of it, the secretory piece, is still associated with the complex. The secretory piece remains attached to the IgA complex in the airway, and it helps protect the IgA complex from proteolytic cleavage in the lumen. The IgA-antibody system is very effective in binding particulates and viruses before they invade epithelial cells, and it aids in removal of these substances through the mucociliary clearance system. The IgA-antigen immune complex does not bind complement in the same classic manner as other immune complexes do; this limits its proinflammatory properties.
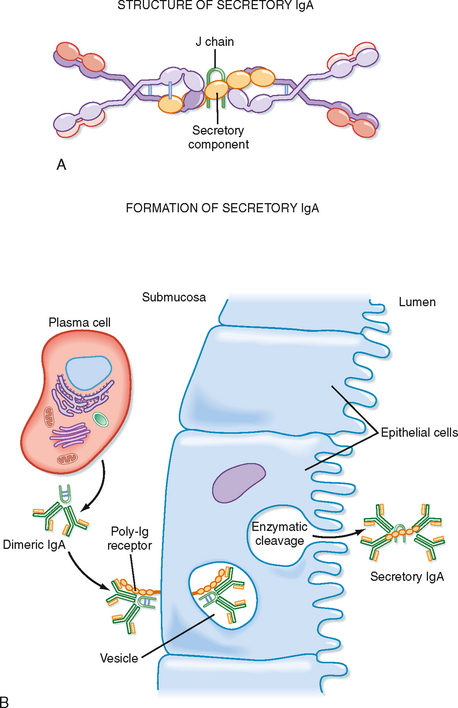
Figure 25-3 Structure and formation of secretory IgA. A, Secretory IgA consists of at least two IgA molecules that are covalently linked via a J chain and covalently associated with the secretory component. The secretory component contains five Ig-like domains and is linked to dimeric IgA between its fifth domain and one of the IgA heavy chains. B, Secretory IgA is formed during transport through epithelial cells.
In allergic diseases such as asthma, an antibody synthesis switchover occurs and IgE becomes the predominant antibody synthesized in response to the allergen. The IgE binds to tissue mast cells and in the presence of allergen leads to their degranulation and release of proinflammatory and bronchoconstricting mediators. Hypersensitivity lung diseases are associated with an altered immune response to nonpathological organisms. It is not a typical allergic response in that symptoms arise 4 to 6 hours after contact with the inciting agent and eosinophils are not a prominent component. The lung pathology is more of a granulomatous-like response with ensuing fibrosis. Goodpasture’s syndrome is an autoimmune response to the lung basement membrane that results in hemorrhagic disease. IgA deficiency is the most common inherited immunoglobulin deficiency and is frequently associated with chronic lung disease.
Adaptive and Innate Immune Cells
The vast majority of T lymphocytes are CD3+ cells with T cell receptors (TCRs) that are composed of α and β chains (TCRαβ cells). Another class of T lymphocytes with a TCR expressing γ and δ chains (TCRγδ cells) has been identified more recently. TCRαβ and TCRγδ cells can secrete similar mediators: interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-4, and IL-5. TCRγδ cells represent a minority of T cells in the peripheral blood and systemic lymphoid tissue; however, they preferentially localize to mucosal sites (i.e., skin, intestine, and lung). TCRγδ cells are the “first line of defense” of epithelial surfaces, and they prevent the development of inflammation mediated by antigen-specific T cells. These cells provide a bridge between adaptive and innate immunity. TCRγδ cells also suppress the IgE response to inhaled antigen.
Natural Killer Cells
Resident populations of functionally active NK cells are present in the lung interstitium. NK cells are a major component of the body’s innate immune defense system against invading pathogens such as herpes-viruses and various bacterial infections. NK cells are named for their ability to kill target cells without previous sensitization. The mechanism of killing is through the release of granular enzymes, perforins, and serine esterases. These enzymes create holes or pores within the target cell membranes that lead to cell death. In addition to their cytotoxic activity, they produce cytokines (i.e., IL-4, IL-5, IL-13, IFN-γ, and TNF-α) that are similar to those of lymphocytes. NK cells increase in number and cellular activity in humans with asthma.
Dendritic Cells and Alveolar Macrophages
Dendritic cells and alveolar macrophages are the first nonepithelial cells to respond to a foreign substance. If the foreign material stays within the air space in the lower respiratory system (alveolar ducts and alveoli), it will be phagocytized by alveolar macrophages and removed by the lymphatic system. However, if it penetrates and reaches the interstitial areas, it will come in contact with dendritic cells. Dendritic cells capture, process, and present antigen to T cells, as well as activate or suppress the T cell response.
Alveolar macrophages are found in the alveolus adjacent to the epithelium and, less frequently, in the terminal airways and interstitial space. They migrate freely throughout the alveolar spaces and serve as a first line of defense in the terminal air spaces. They phagocytize foreign particles and substances, as well as surfactant and cellular debris from dead cells. Once a particle is engulfed, the major mechanisms for destruction include the formation of O2 radicals, enzymatic activity, and halogen derivatives within lysosomes. The phagocytic activity of the alveolar macrophage inhibits the binding of particulates to the alveolar epithelium and their subsequent penetration into the interstitium. The alveolar macrophage transports engulfed particles to ciliated regions of the mucociliary transport system for elimination. Thus, the alveolar macrophage provides an important link between the alveolar spaces, the “Achilles heel” postterminal bronchiole region, and the mucociliary clearance system. In addition, the alveolar macrophage can suppress T cell activity by direct contact with the T cell or by the secretion of soluble factors such as nitric oxide, prostaglandin E2, and the immunosuppressive cytokines IL-10 and transforming growth factor β (TGF-β). The ability of the alveolar macrophage to dispose of foreign material rapidly and without mounting an inflammatory response enhances the lung defense system and is a major contributor to the overall defense system.
In certain circumstances, such as the inhalation of silica particles, alveolar macrophages phagocytize the particles but are unable to destroy them and the macrophages eventually die. The result is that alveolar macrophages now have localized and concentrated silica particles in the “Achilles heel” region of the lung. The silica particles are not removed from this region by the mucociliary transport system and thus accumulate there and enter the lung interstitium, which leads to a granulomatous-like inflammatory response, fibrosis, and restrictive lung disease. Silica is present in many work environments, including foundries, mining, and photography. There is concern that silicosis may become a leading problem in occupationally related lung disease.
Toll-like Receptors
Because most inhaled substances are nonpathogenic, the body has developed a recognition system to identify potentially harmful pathogenic substances. The system is based on the recognition of pathogen-associated molecular patterns (PAMPs) on the organism or substance, which are then recognized by a family of receptors on host cells called Toll-like receptors (TLRs). Activation of this system initiates inflammatory host defense mechanisms to fight off the pathogen. The TLRs are a family of transmembrane proteins with different specificities for various pathogens. TLR-4 is specific for the gram-negative bacterial product lipopolysaccharide, whereas TLR-2 is specific for lipoproteins associated with gram-positive bacteria. In the lung, bronchial epithelial cells and alveolar type II epithelial cells express TLR-2 and TLR-4. Macrophages and dendritic cells in the lung and other organs also express TLRs. Thus, in addition to classic phagocytic cells, bronchial and alveolar epithelial cells play active roles in host defense via the PAMP-TLR recognition system.
CLINICAL MANIFESTATIONS ASSOCIATED WITH ABNORMALITIES IN INNATE AND ADAPTIVE IMMUNITY
By far the most common pathological conditions associated with mucosal tissue are allergic responses (e.g., allergic asthma, allergic rhinitis, and food and skin allergies). As previously described, the predominant antibody response in MALT is IgA. However, in an allergic response, IgE is the predominant antibody synthesized. Sensitized CD4+ T cells and IL-4 are required for this to occur. IgE binds to the surface of tissue mast cells, and antigen stimulation leads to the degranulation of mast cells (Fig. 25-4). The released granules contain eosinophil chemotactic factors and leukotrienes that induce bronchoconstriction. Symptoms of wheezing, coughing, and shortness of breath occur within minutes as a result of the intense eosinophilia and airway edema. Resolution of the inflammatory response can occur spontaneously or in response to therapy (bronchodilator or antiinflammatory drugs). Low-grade inflammation may persist and result in a process called airway remodeling, manifested by permanent, nonreversible structural changes such as submucosal fibrosis and airway smooth muscle hypertrophy. The mechanisms responsible for airway remodeling in allergic diseases are not clearly understood, but chemokines and cytokines such as TGF-β, a potent profibrotic cytokine, play important roles.
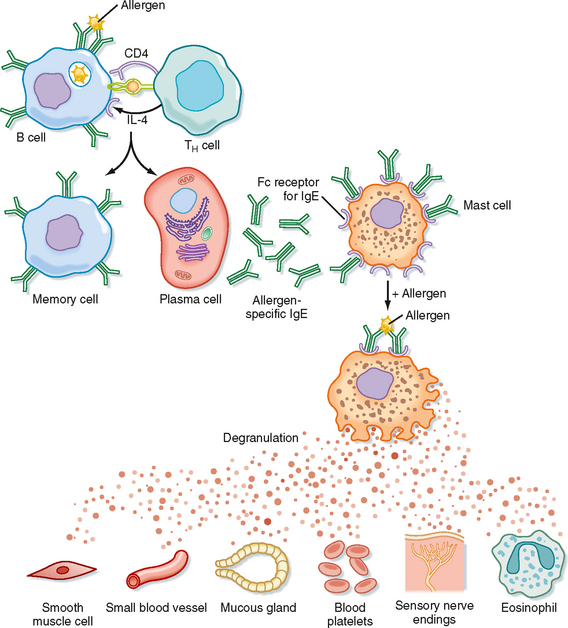
Figure 25-4 General mechanism underlying an allergic reaction. Exposure to an allergen activates B cells to form IgE-secreting plasma cells. The secreted IgE molecules bind to IgE-specific Fc receptors on mast cells and basophils. After a second exposure to the allergen, the bound IgE is cross-linked, which triggers the release of pharmacologically active mediators from mast cells and basophils. The mediators cause smooth muscle contraction, increased vascular permeability, and vasodilation.