CHAPTER 27 The Cephalic, Oral, and Esophageal Phases of the Integrated Response to a Meal
In this chapter we will look at the processes that occur in the gastrointestinal (GI) tract in the early stages of the integrated response to a meal. Even before food is ingested there are changes in the physiology of the GI tract, and in this so-called cephalic phase, as well as in the oral phase (when ingested food is in the mouth), the responses of the GI tract to the presence of food are mainly associated with preparing the GI tract for digestion and absorption. We will also look at the transfer of food from the mouth to the esophagus, the esophageal phase of the meal.
CEPHALIC AND ORAL PHASES
The main feature of the cephalic phase is activation of the GI tract in readiness for the meal. The stimuli involved are cognitive and include anticipation or thinking about the consumption of food, olfactory input, visual input (seeing or smelling appetizing food when hungry), and auditory input. The latter may be an unexpected link but was clearly demonstrated in the classic conditioning experiments of Pavlov, in which he paired an auditory stimulus to the presentation of food to dogs; eventually, the auditory stimulus alone could stimulate secretion. A real-life analogy is presumably being told that dinner is ready. All these stimuli result in an increase in excitatory parasympathetic neural outflow to the gut. Sensory input, such as smell, stimulates sensory nerves that activate parasympathetic outflow from the brainstem. Higher brain sites are also involved (such as the limbic system, hypothalamus, and cortex) in the cognitive components of this response. The response can be both positive and negative; thus, anticipation of food and a person’s psychological status, such as anxiety, can alter the cognitive response to a meal. However, the final common pathway is activation of the dorsal motor nucleus in the brainstem, the region where the cell bodies of the vagal preganglionic neurons arise; activation of the nucleus leads to increased activity in efferent fibers passing to the GI tract in the vagus nerve. In turn, the efferent fibers activate the postganglionic motor neurons (referred to as motor because their activation results in change of function of an effector cell). Increased parasympathetic outflow enhances salivary secretion, gastric acid secretion, pancreatic enzyme secretion, gallbladder contraction, and relaxation of the sphincter of Oddi (the sphincter between the common bile duct and the duodenum). All these responses enhance the ability of the GI tract to receive and digest the incoming food. The salivary response is mediated via the ninth cranial nerve; the remaining responses are mediated via the vagus nerve.
Many of the features of the oral phase are indistinguishable from the cephalic phase. The only difference is that food is in contact with the surface of the GI tract. Thus, there are additional stimuli generated from the mouth, both mechanical and chemical (taste). However, many of the responses that are initiated by the presence of food in the oral cavity are identical to those initiated in the cephalic phase because the efferent pathway is the same. Here we will discuss the responses specifically initiated in the mouth, which consist mainly of the stimulation of salivary secretion.
The mouth is important for the mechanical disruption of food and for the initiation of digestion. Chewing subdivides and mixes the food with the enzymes salivary amylase and lingual lipase and with the glycoprotein mucin, which lubricates the food for chewing and swallowing. Minimal absorption occurs in the mouth, although alcohol and some drugs are absorbed from the oral cavity and this can be clinically important. However, as with the cephalic phase, it is important to realize that stimulation of the oral cavity initiates responses in the more distal GI tract, including increased gastric acid secretion, increased pancreatic enzyme secretion, gallbladder contraction, and relaxation of the sphincter of Oddi, mediated via the efferent vagal pathway.
Properties of Secretion
General Considerations
Secretions in the GI tract come from glands associated with the tract (the salivary glands, pancreas, and liver), from glands formed by the gut wall itself (e.g., Brunner’s glands in the duodenum), and from the intestinal mucosa itself. The exact nature of the secretory products can vary tremendously, depending on the function of that region of the GI tract. However, these secretions have several characteristics in common. Secretions from the GI tract and associated glands include water, electrolytes, protein, and humoral agents. Water is essential for generating an aqueous environment for the efficient action of enzymes. Secretion of electrolytes is important for the generation of osmotic gradients to drive the movement of water. Digestive enzymes in secreted fluid catalyze the breakdown of macronutrients in ingested food. Moreover, many additional proteins secreted along the GI tract have specialized functions, some of which are fairly well understood, such as those of mucin and immunoglobulins, and others that are only just beginning to be understood, such as those of trefoil peptides.
Secretion is initiated by multiple signals associated with the meal, including chemical, osmotic, and mechanical components. Secretion is elicited by the action of specific effector substances, called secretagogues, acting on secretory cells. Secretagogues work in one of the three ways that have already been described in the previous chapter—endocrine, paracrine, and neurocrine.
Constituents of Secretions
Inorganic secretory components are region or gland specific, depending on the particular conditions required in that part of the GI tract. The inorganic components are electrolytes, including H+ and HCO3−. Two examples of different secretions include acid (HCl) in the stomach, which is important to activate pepsin and to start protein digestion, and HCO3− in the duodenum, which neutralizes gastric acid and provides optimal conditions for the action of digestive enzymes in the small intestine.
Organic secretory components are also gland or organ specific and depend on the function of that region of the gut. The organic constituents are enzymes (for digestion), mucin (for lubrication and mucosal protection), and other factors such as growth factors, immunoglobulins, and absorptive factors.
Salivary Secretion
During the cephalic and the oral phase of the meal, considerable stimulation of salivary secretion takes place. Saliva has a variety of functions, including those important for the integrative responses to a meal and for other physiological processes (Table 27-1). The main functions of saliva in digestion include lubrication and moistening of food for swallowing, solubilization of material for taste, initiation of carbohydrate digestion, and clearance and neutralization of refluxed gastric secretions in the esophagus. Saliva also has antibacterial actions that are important for overall health of the oral cavity and teeth.
Table 27-1 Functions of Saliva and Chewing
Functional Anatomy of the Salivary Glands
There are three pairs of major salivary glands: parotid, submandibular, and sublingual. In addition, many smaller glands are found on the tongue, lips, and palate. These glands are the typical tubuloalveolar structures of glands located in the GI tract (Fig. 27-1). The acinar portion of the gland is classified according to its major secretion: serous (“watery”), mucous, or mixed. The parotid gland produces mainly serous secretion, the sublingual gland secretes mainly mucus, and the submandibular gland produces a mixed secretion.
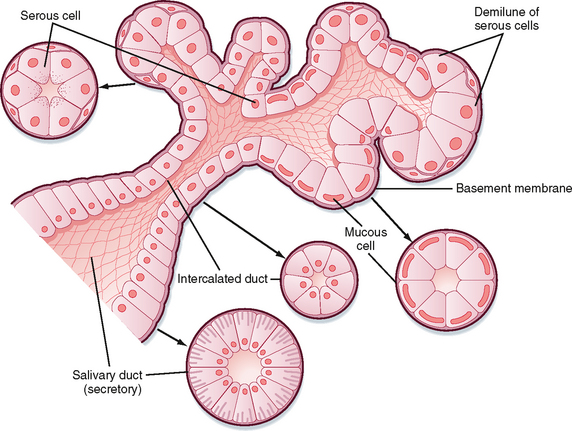
Figure 27-1 General structure of tubuloalveolar secretory glands associated with the digestive tract, for example, the salivary glands and the pancreas.
Cells in the secretory end pieces, or acini, are called acinar cells and are characterized by basally located nuclei, abundant rough endoplasmic reticulum, and apically located secretory granules that contain the enzyme amylase and other secreted proteins. There are also mucous cells in the acinus; the granules in these cells are larger and contain the specialized glycoprotein mucin. There are three kinds of ducts in the gland that transport secretions from the acinus to the opening in the mouth and also modify the secretion: intercalated ducts drain acinar fluid into larger ducts, the striated ducts, which then empty into even larger excretory ducts. A single large duct from each gland drains saliva to the mouth. The ductal cells lining the striated ducts, in particular, modify the ionic composition and osmolarity of saliva.
Composition of Saliva
The important properties of saliva are a large flow rate relative to the mass of gland, low osmolarity, high K+ concentration, and organic constituents, including enzymes (amylase, lipase), mucin, and growth factors. The later are not important in the integrated response to a meal but are essential for long-term maintenance of the lining of the GI tract.
The inorganic composition is entirely dependent on the stimulus and the rate of salivary flow. In humans, salivary secretion is always hypotonic. The major components are Na+, K+, HCO3−, Ca++, Mg++, and Cl−. Fluoride can be secreted in saliva, and fluoride secretion forms the basis of oral fluoride treatment for the prevention of dental caries. The concentration of ions varies with the rate of secretion, which is stimulated during the postprandial period.
The primary secretion is produced by acinar cells in the secretory end pieces (acini) and is modified by duct cells as saliva passes through the ducts. The primary secretion is isotonic, and the concentration of the major ions is similar to that in plasma. Secretion is driven predominantly by Ca++-dependent signaling, which opens apical Cl− channels in the acinar cells. Cl− therefore flows out into the duct lumen and establishes an osmotic and electrical gradient. Because the epithelium of the acinus is relatively leaky, Na+ and water then follow across the epithelium via the tight junctions (i.e., via paracellular transport). Transcellular water movement may also occur, mediated by aquaporin 5 water channels. The amylase content and rate of fluid secretion vary with the type and level of stimulus. The excretory duct cells and the striated duct cells modify the primary secretion to produce the secondary secretion. The duct cells reabsorb Na+ and Cl− and secrete K+ and HCO3− into the lumen. At rest, the final salivary secretion is hypotonic and slightly alkaline. Na+ is exchanged for protons, but some of the secreted protons are then reabsorbed in exchange for K+. HCO3−, on the other hand, is secreted only in exchange for Cl−, thereby providing excess base equivalents. The alkalinity of saliva is probably important in restricting microbial growth in the mouth, as well as in neutralizing refluxed gastric acid once the saliva is swallowed. When salivary secretion is stimulated, moreover, there is a decrease in K+ (but it always remains above plasma concentrations), Na+ increases toward plasma levels, Cl− and HCO3− increase, and thus the secretion becomes even more alkaline. Note that HCO3− secretion can be directly stimulated by the action of secretagogues on duct cells. The duct epithelium is relatively tight and lacks expression of aquaporin, and therefore water cannot follow the ions rapidly enough to maintain isotonicity at moderate or high flow rates during stimulated salivary secretion. Thus, with an increase in the secretion rate, there is less time for modification by the ducts, and the resulting saliva more closely resembles the primary secretion and therefore plasma. However, [HCO3−] remains high because it is secreted by duct and possibly acinar cells by the action of secretagogues (Fig. 27-2).
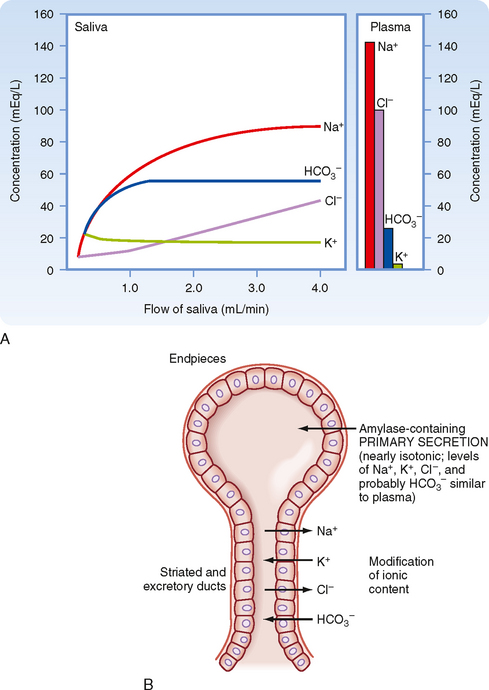
Figure 27-2 A, The composition of salivary secretion as a function of the salivary flow rate compared with the concentration of ions in plasma. Saliva is hypotonic to plasma at all flow rates. [HCO3−] in saliva exceeds that in plasma except at very low flow rates. B, Schematic representation of the two-stage model of salivary secretion. The primary secretion containing amylase and electrolytes is produced in the acinar cell. The concentration of electrolytes in plasma is similar to that in the primary secretion, but it is modified as it passes through ducts that absorb Na+ and Cl− and secrete K+ and HCO3−.
The organic constituents of saliva, proteins and glycoproteins, are synthesized, stored, and secreted by the acinar cells. The major products are amylase (an enzyme that initiates starch digestion), lipase (important for lipid digestion), glycoprotein (mucin, which forms mucus when hydrated), and lysozyme (attacks bacterial cell walls to limit colonization of bacteria in the mouth). Although salivary amylase begins the process of digestion of carbohydrates, it is not required in healthy adults because of the excess of pancreatic amylase. Similarly, the importance of lingual lipase is unclear.
Metabolism and Blood Flow of Salivary Glands
The salivary glands produce a prodigious flow of saliva. The maximal rate of saliva production in humans is about 1 mL/min/g of gland; thus, at this rate, the glands are producing their own weight in saliva each minute. Salivary glands have a high rate of metabolism and high blood flow; both are proportional to the rate of saliva formation. The blood flow to maximally secreting salivary glands is approximately 10 times that of an equal mass of actively contracting skeletal muscle. Stimulation of the parasympathetic nerves to salivary glands increases blood flow by dilating the vasculature of the glands. Vasoactive intestinal polypeptide (VIP) and acetylcholine are released from parasympathetic nerve terminals in the salivary glands and are vasodilatory during secretion.
Regulation of Salivary Secretion
Control of salivary secretion is exclusively neural. In contrast, control of most other GI secretions is primarily hormonal. Salivary secretion is stimulated by both the sympathetic and parasympathetic subdivisions of the autonomic nervous system. The primary physiological control of the salivary glands is by the parasympathetic nervous system. Excitation of either sympathetic or parasympathetic nerves to the salivary glands stimulates salivary secretion. If the parasympathetic supply is interrupted, salivation is severely impaired and the salivary glands atrophy.
Sympathetic fibers to the salivary glands stem from the superior cervical ganglion. Preganglionic parasympathetic fibers travel via branches of the facial and glossopharyngeal nerves (cranial nerves VII and IX, respectively). These fibers form synapses with postganglionic neurons in ganglia in or near the salivary glands. The acinar cells and ducts are supplied with parasympathetic nerve endings.
Parasympathetic stimulation increases the synthesis and secretion of salivary amylase and mucins, enhances the transport activities of the ductular epithelium, greatly increases blood flow to the glands, and stimulates glandular metabolism and growth.
Ionic Mechanisms of Salivary Secretion
Ion Transport in Acinar Cells.
Figure 27-3 shows a simplified view of the mechanisms of ion secretion by serous acinar cells. The basolateral membrane of the cell contains Na+,K+-ATPase and an Na+-K+-2Cl− symporter. The concentration gradient for Na+ across the basolateral membrane, which is dependent on Na+,K+-ATPase, provides the driving force for entry of Na+, K+, and Cl− into the cell. Cl− and HCO3− leave the acinar cell and enter the lumen via an anion channel located in the apical membrane of the acinar cell. This secretion of anions drives the entry of Na+ and thus water into the acinar lumen via the relatively leaky tight junctions.
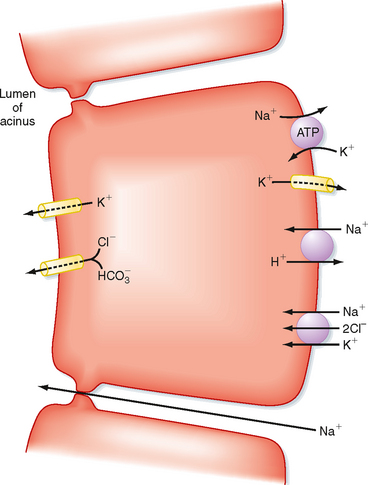
Figure 27-3 Ionic transport mechanism involved in the secretion of amylase and electrolytes in salivary acinar cells.
Acinar cell fluid secretion is strongly enhanced in response to elevations in intracellular [Ca++] as a result of activation of the muscarinic receptor for acetylcholine.
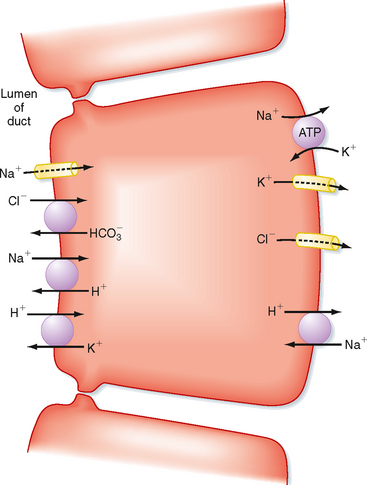
Ion Transport in Ductular Cells.
Figure 27-4 shows a simplified model of ion transport processes in epithelial cells of the excretory and striated ducts. Na+,K+-ATPase, located in the basolateral membrane, maintains the electrochemical gradients for Na+ and K+ that drive most of the other ionic transport processes of the cell. In the apical membrane, the parallel operation of Na+, H+, Cl−, HCO3−, and H+-K+ antiporters results in absorption of Na+ and Cl− from the lumen and secretion of K+ and HCO3− into the lumen. The relative impermeability of the ductular epithelium to water prevents the ducts from absorbing too much water by osmosis.
Swallowing
Swallowing can be initiated voluntarily, but thereafter it is almost entirely under reflex control. The swallowing reflex is a rigidly ordered sequence of events that propel food from the mouth to the pharynx and from there to the stomach. This reflex also inhibits respiration and prevents the entrance of food into the trachea during swallowing. The afferent limb of the swallowing reflex begins when touch receptors, most notably those near the opening of the pharynx, are stimulated. Sensory impulses from these receptors are transmitted to an area in the medulla and lower pons called the swallowing center. Motor impulses travel from the swallowing center to the musculature of the pharynx and upper esophagus via various cranial nerves and to the remainder of the esophagus by vagal motor neurons.
The acinar cells and duct cells of the salivary glands respond to both cholinergic and adrenergic agonists. Nerves stimulate the release of acetylcholine, norepinephrine, substance P, and VIP by salivary glands, and these hormones increase the secretion of amylase and the flow of saliva. These neurotransmitters act mainly by elevating the intracellular concentration of cAMP and by increasing the concentration of Ca++ in the cytosol. Acetylcholine and substance P, acting on muscarinic and tachykinin receptors, respectively, increase the cytosolic concentration of Ca++ in serous acinar cells. In contrast, norepinephrine, acting on β receptors, and VIP, acting at its receptor, elevate the cAMP concentration in acinar cells. Agonists that elevate the cAMP concentration in serous acinar cells elicit a secretion that is rich in amylase; agonists that mobilize Ca++ elicit a secretion that is more voluminous but has a lower concentration of amylase. Ca++-mobilizing agonists may also elevate the concentration of cGMP, which may mediate the trophic effects evoked by these agonists.
Xerostomia, or dry mouth, is caused by impaired salivary secretion. It can be congenital or develop as part of an autoimmune process. The decrease in secretion reduces pH in the oral cavity, which causes tooth decay and is associated with esophageal erosions. Reduced secretion also causes difficulty swallowing.
The ability to measure and monitor a wide range of molecular components that are indicative of overall health is useful in diagnosis and monitoring. Saliva is easy to access, and collection of it is noninvasive. It is used to identify individuals with disease (presence of biomarkers) and to monitor the progress of affected individuals under treatment. In endocrinology, levels of steroids can be measured in the free form rather than as the free and bound form as in plasma (e.g., the stress hormone cortisol and the sex hormones estradiol, progesterone, and testosterone). Viral infections such as human immunodeficiency virus (HIV), herpes, hepatitis C, and Epstein-Barr virus infection can be detected by polymerase chain reaction (PCR) techniques. Bacterial infections, such as Helicobacter pylori, can likewise be detected in saliva, and saliva is also used for monitoring of levels of drugs.
The timing of events in swallowing is shown in Figure 27-5. The voluntary phase of swallowing is initiated when the tip of the tongue separates a bolus of food from the mass of food in the mouth. First the tip of the tongue and later the more posterior portions of the tongue press against the hard palate. The action of the tongue moves the bolus upward and then backward into the mouth. The bolus is forced into the pharynx, where it stimulates the touch receptors that initiate the swallowing reflex. The pharyngeal phase of swallowing involves the following sequence of events, which occur in less than 1 second: (1) the soft palate is pulled upward and the palatopharyngeal folds move inward toward one another; these movements prevent reflux of food into the nasopharynx and open a narrow passage through which food moves into the pharynx; (2) the vocal cords are pulled together and the larynx is moved forward and upward against the epiglottis; these actions prevent food from entering the trachea and help open the upper esophageal sphincter (UES); (3) the UES relaxes to receive the bolus of food; and (4) the superior constrictor muscles of the pharynx then contract strongly to force the bolus deeply into the pharynx. A peristaltic wave is initiated with contraction of the pharyngeal superior constrictor muscles, and the wave moves toward the esophagus. This wave forces the bolus of food through the relaxed UES. During the pharyngeal stage of swallowing, respiration is also reflexively inhibited. After the bolus of food passes the UES, a reflex action causes the sphincter to constrict.
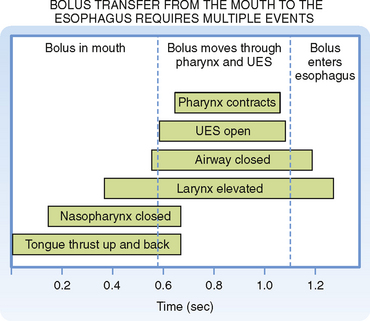
Figure 27-5 Timing of motor events in the pharynx and upper esophageal sphincter (UES) during a swallow.
Gastroesophageal reflux disease (GERD) is commonly referred to as heartburn or indigestion. It occurs when the lower esophageal sphincter allows the acidic contents of the stomach to reflux back into the distal part of the esophagus. This region of the esophagus, unlike the stomach, does not have a robust system to protect the mucosal lining. Thus, the acid will activate pain fibers and thereby result in discomfort and pain. This is not an uncommon phenomenon, even in healthy individuals. In the long term, continual reflux can result in damage to the esophageal mucosa. In this case, this condition is classed as GERD and can be treated by H2 receptor antagonists that reduce gastric acid secretion, such as ranitidine, or by proton pump inhibitors, such as omeprazole.
ESOPHAGEAL PHASE
The esophagus, the UES, and the lower esophageal sphincter (LES) serve two main functions (Fig. 27-6). First, they propel food from the mouth to the stomach. Second, the sphincters protect the airway during swallowing and protect the esophagus from acidic gastric secretions.
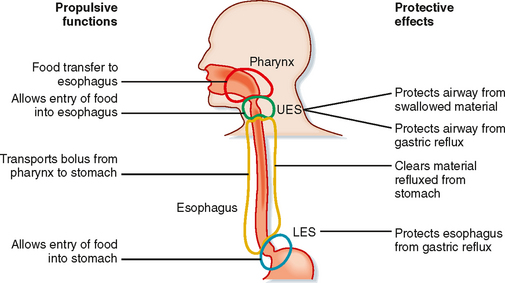
Figure 27-6 The esophagus and associated sphincters have multiple functions involved in movement of food from the mouth to the stomach and also in protection of the airway and esophagus.
The stimuli that initiate the changes in smooth muscle activity that result in these propulsive and protective functions are mechanical and consist of pharyngeal stimulation during swallowing and distention of the esophageal wall itself. The pathways are exclusively neural and involve both extrinsic and intrinsic reflexes. Mechanosensitive afferents in both the extrinsic (vagus) nerves and intrinsic neural pathways respond to esophageal distention. These pathways include activated reflex pathways via the brainstem (extrinsic, vagus) or solely intrinsic pathways. The striated muscle is regulated from the nucleus ambiguus in the brainstem, and the smooth muscle is regulated by parasympathetic outflow via the vagus nerve. The changes in function resulting from mechanosensitive stimuli and activation of reflex pathways are peristalsis of striated and smooth muscle, relaxation of the LES, and relaxation of the proximal portion of the stomach.
Functional Anatomy of the Esophagus and Associated Structures
The esophagus, like the rest of the GI tract, has two muscle layers—circular and longitudinal—but the esophagus is one of two places in the gut where striated muscle occurs, the other being the external anal sphincter. The type of muscle (striated or smooth) in the esophagus varies along its length. The UES and LES are formed by thickening of striated or circular smooth muscle, respectively.
Motor Activity during the Esophageal Phase
The UES, esophagus, and LES act in a coordinated manner to propel material from the pharynx to the stomach. At the end of a swallow, a bolus passes through the UES, and the presence of the bolus, via stimulation of mechanoreceptors and reflex pathways, initiates a peristaltic wave (alternating contraction and relaxation of the muscle) along the esophagus that is called primary peristalsis (Fig. 27-7). This wave moves down the esophagus slowly (3 to 5 cm/sec). Distention of the esophagus by the moving bolus initiates another wave called secondary peristalsis. Frequently, repetitive secondary peristalsis is required to clear the esophagus of the bolus. Stimulation of the pharynx by the swallowed bolus also produces reflex relaxation of the LES and the most proximal region of the stomach. Thus, when the bolus reaches the LES, it is already relaxed to allow passage of the bolus into the stomach. Similarly, the portion of the stomach that receives the bolus is relaxed. In addition, esophageal distention produces further receptive relaxation of the stomach. The proximal part of the stomach relaxes at the same time as the LES; this occurs with each swallow and its function is to allow the stomach to accommodate large volumes with a minimal rise in intragastric pressure. This process is called receptive relaxation (Fig. 27-8).
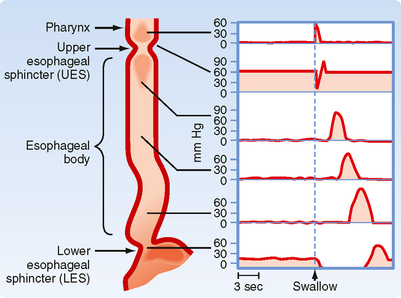
Figure 27-7 Changes in pressure in the different regions of the pharynx, esophagus, and associated sphincters initiated during a swallow. The pressure trace is a diagrammatic representation from that obtained during manometry in an awake human. Stimulation of the pharynx by the presence of a bolus initiates a decrease in pressure (= opening) of the UES and a peristaltic wave of contraction along the esophagus. Stimulation of the pharynx also relaxes the smooth muscle of the LES to prepare for entry of food.
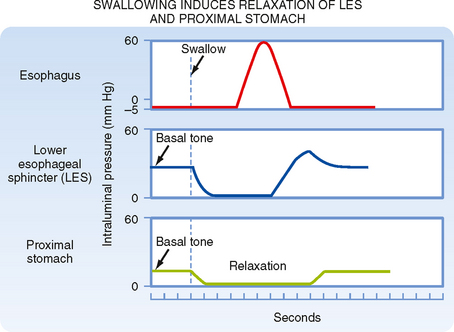
Figure 27-8 Swallowing in the form of pharyngeal stimulation induces neural reflex relaxation of the LES and the proximal part of the stomach to allow entry of food.
The LES also has important protective functions. It is involved in the prevention of acid reflux from the stomach back into the esophagus; an insufficient tonic contraction of the LES is associated with reflux disease, a gradual erosion of the esophageal mucosa, which is not as well protected as the gastric and duodenal mucosa. There is also some evidence that peristalsis in the absence of swallowing (secondary peristalsis) is important for clearing refluxed gastric contents.