CHAPTER 43 The Male and Female Reproductive Systems
The two most basic components of the reproductive system are the gonads and the reproductive tract. The gonads (testes and ovaries) perform an endocrine function, which is regulated within a hypothalamic-pituitary-gonadal axis. The gonads are distinct from other endocrine glands in that they also perform an exocrine function (gametogenesis). The reproductive tract is involved in several aspects of gamete development, function, and transport and, in women, allows fertilization, implantation, and gestation. Normal gametogenesis in the gonads and the development and physiology of the reproductive tract are absolutely dependent on the endocrine function of the gonads. The clinical ramifications of this hormonal dependence include infertility in the face of low sex hormone production, ambiguous genitalia in dysregulated hormone or receptor expression, and hormone-responsive cancers, especially uterine and breast cancer in women and prostate cancer in men.
THE MALE REPRODUCTIVE SYSTEM
The male reproductive system has evolved for continuous, life-long gametogenesis, coupled with occasional internal insemination with a high density of sperm (>60 × 106/mL in 3 to 5 mL of semen). In adult men the basic roles of gonadal hormones are (1) support of gametogenesis (spermatogenesis), (2) maintenance of the male reproductive tract and production of semen, and (3) maintenance of secondary sex characteristics and libido. There is no overall cyclicity of this activity in men.
THE TESTIS
Histophysiology
Unlike the ovaries, the testes reside outside the abdominal cavity in the scrotum (Fig. 43-1). This location maintains testicular temperature at about 2 degrees lower than body temperature, which is crucial for optimal sperm development. The human testis is covered by a connective tissue capsule and is divided into about 300 lobules by fibrous septa (Fig. 43-2). Within each lobule are two to four loops of seminiferous tubules. Each loop empties into an anastomosing network of tubules called the rete testis. The rete testis is continuous with small ducts, the efferent ductules, that lead the sperm out of the testis into the head of the epididymis on the superior pole of the testis (Fig. 43-2). Once in the epididymis, the sperm pass from the head, to the body, to the tail of the epididymis and then to the vas (ductus) deferens. Viable sperm can be stored in the tail of the epididymis and the vas deferens for several months.
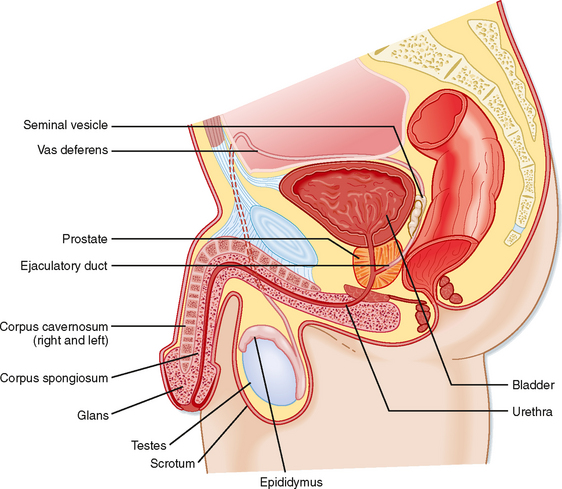
Figure 43-1 Anatomy of the male reproductive system.
(Modified from Drake RL et al: Gray’s Anatomy for Students. Philadelphia, Churchill Livingstone, 2005.)
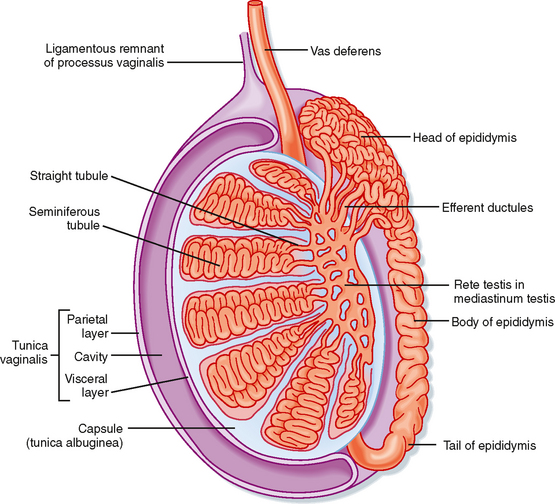
Figure 43-2 Anatomy and organization of the testis.
(Modified from Drake RL et al: Gray’s Anatomy for Students. Philadelphia, Churchill Livingstone, 2005.)
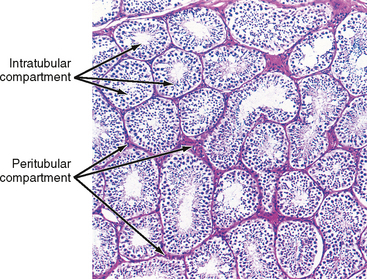
The presence of the seminiferous tubules creates two compartments within each lobule: an intratubular compartment, which is composed of the seminiferous epithelium of the seminiferous tubule, and a peritubular compartment, which is composed of neurovascular elements, connective tissue cells, immune cells, and the “interstitial cells of Leydig,” whose main function is to produce testosterone (Fig. 43-3).
The Intratubular Compartment
The seminiferous tubule is lined by a complex seminiferous epithelium composed of two cell types: sperm cells in various stages of spermatogenesis and the Sertoli cell, which is a “nurse cell” in intimate contact with all sperm cells (Fig. 43-4).
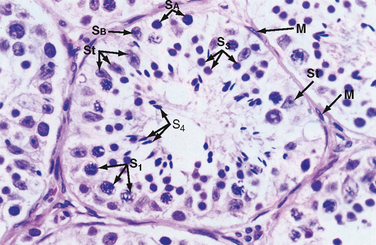
Figure 43-4 Histology of a seminiferous tubule. M, myoid cell just outside the basal lamina; S1, primary spermatocyte; S3, spermatid; S4, mature spermatid or spermatozoon; SB and SA, spermatogonia; St, Sertoli cell.
(From Young B et al: Wheater’s Functional Histology. A Text and Colour Atlas, 5th ed. London, Churchill Livingstone, 2006.)
Developing Sperm Cells
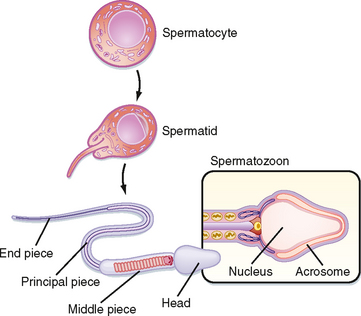
Spermatogenesis involves the processes of mitosis and meiosis. Stem cells, called spermatogonia, reside at the basal level of the seminiferous epithelium (Fig. 43-4). Spermatogonia divide mitotically to generate daughter spermatogonia (spermatocytogenesis). One or more spermatogonia remain within the stem cell population, firmly adherent to the basal lamina. However, the majority of these daughter spermatogonia enter meiotic division, which results in haploid spermatozoa on completion of meiosis. These divisions are accompanied by incomplete cytokinesis such that all daughter cells remain interconnected by a cytoplasmic bridge. This configuration contributes to the synchrony of development of a clonal population of sperm cells. Spermatogonia migrate apically away from the basal lamina as they enter the first meiotic prophase. At this time they are called primary spermatocytes (Fig 43-4). During the first meiotic prophase, the hallmark processes of sexual reproduction involving chromosomal reduplication, synapsis, crossing over, and homologous recombination take place. Completion of the first meiotic division gives rise to secondary spermatocytes, which quickly (i.e., within 20 minutes) completes the second meiotic division. The initial products of meiosis are haploid spermatids (Fig. 43-4). Spermatids are small, round cells that undergo a remarkable metamorphosis called spermiogenesis (Fig. 43-5). The products of spermiogenesis are the streamlined spermatozoa. As the spermatid matures into a spermatozoon, the size of the nucleus decreases and a prominent tail is formed. The tail contains microtubular structures that propel sperm, similar to a flagellum. The chromatin material in the sperm nucleus condenses, and most of the cytoplasm is lost. The acrosome is a membrane-enclosed structure on the head of the sperm that acts as a lysosome and contains hydrolytic enzymes that are important for fertilization. These enzymes remain inactive until the acrosomal reaction occurs (see later).
Spermatozoa (Fig. 43-4) are found at the luminal surface of the seminiferous tubule. Release of sperm, or spermiation, is controlled by Sertoli cells. The process of spermatogenesis takes about 72 days. A cohort of adjacent spermatogonia enter the process every 16 days so that the process is staggered at one point along a seminiferous tubule. In addition, the process is staggered along the length of a seminiferous tubule (i.e., not all spermatogonia enter the process of spermatogenesis at the same time along the entire length of the tubule or in synchrony with every other tubule; there are about 500 seminiferous tubules per testis; see later). Because the seminiferous tubules within one testis are about 400 m in length, spermatozoa are continually being generated at many sites within the testis at any given time.
The Sertoli Cell
Sertoli cells are the true epithelial cells of the seminiferous epithelium and extend from the basal lamina to the lumen (Fig. 43-4). Sertoli cells surround sperm cells and provide structural support within the epithelium, and they form adhering and gap junctions with all stages of sperm cells. Through the formation and breakdown of these junctions, Sertoli cells guide sperm cells toward the lumen as they advance to later stages of spermatogenesis. Spermiation requires the final breakdown of Sertoli–sperm cell junctions.
Another important structural feature of Sertoli cells is the formation of tight junctions between adjacent Sertoli cells (Fig. 43-6). These Sertoli-Sertoli cell occluding junctions divide the seminiferous epithelium into a basal compartment containing the spermatogonia and early-stage primary spermatocytes and an adluminal compartment containing later-stage primary spermatocytes and all subsequent stages of sperm cells. As early primary spermatocytes move apically from the basal to the adluminal compartment, the tight junctions need to be disassembled and reassembled. These tight junctions form the physical basis for the blood-testis barrier (Fig. 43-6), which creates a specialized, immunologically safe microenvironment for developing sperm. By blocking paracellular diffusion, the tight junctions restrict movement of substances between blood and the developing germ cells through a trans–Sertoli cell transport pathway and, in this manner, allow the Sertoli cell to control the availability of nutrients to germ cells.
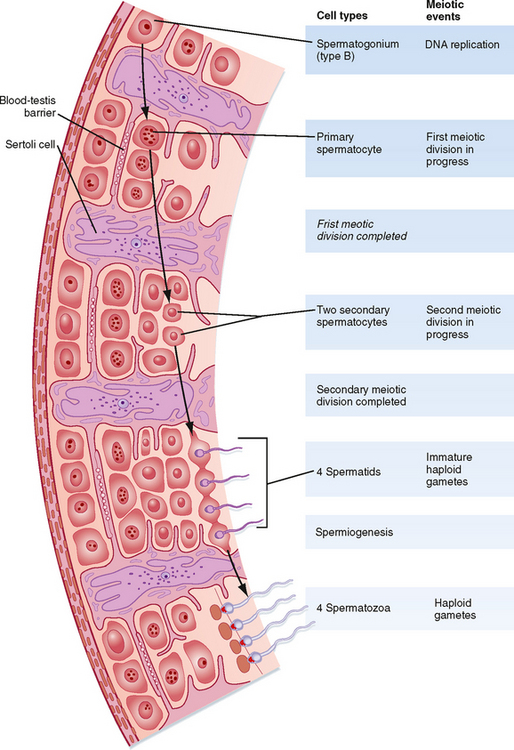
Figure 43-6 Interactions among the various cells of the testis in the hormonal regulation of spermatogenesis.
(From Carlson BM: Human Embryology and Developmental Biology. Philadelphia, Mosby, 2004.)
Healthy Sertoli cell function is essential for sperm cell viability and development. In addition, spermatogenesis is absolutely dependent on testosterone produced by peritubular Leydig cells (see later), yet it is the Sertoli cells that express the androgen receptor, not the developing sperm cells. Similarly, the pituitary hormone follicle-stimulating hormone (FSH) is also required for maximal sperm production, and again, it is the Sertoli cell that expresses the FSH receptor, not the developing sperm. Thus, these hormones support spermatogenesis indirectly through stimulation of Sertoli cell function.
Sertoli cells have multiple additional functions. They express the enzyme CYP19 (also called aromatase), which converts Leydig cell–derived testosterone to the potent estrogen estradiol-17β (see later). This local production of estrogen may enhance spermatogenesis in humans. Sertoli cells also produce androgen-binding protein (ABP), which maintains a high androgen level within the adluminal compartment, the lumens of the seminiferous tubules, and the proximal part of the male reproductive tract. Sertoli cells also produce a large amount of fluid. This fluid provides an appropriate bathing medium for the sperm and assists in moving the immotile spermatozoa from the seminiferous tubule into the epididymis. Sertoli cells perform an important phagocytic function by engulfing residual bodies, which represent cytoplasm shed by spermatozoa during spermiogenesis.
Finally, the Sertoli cell has an important endocrine role. During development, Sertoli cells produce antimüllerian hormone (AMH; also called müllerian inhibitory substance), which induces regression of the embryonic müllerian duct that is programmed to give rise to the female reproductive tract (see later). The Sertoli cells also produce the hormone inhibin. Inhibin is a heterodimer protein hormone related to the transforming growth factor-β family. FSH stimulates inhibin production, which then negatively feeds back on gonadotropes to inhibit FSH production. Thus, inhibin keeps FSH levels within a set point.
The Peritubular Compartment
The peritubular compartment contains the primary endocrine cell of the testis, the Leydig cell (Fig. 43-7). This compartment also contains the common cell types of loose connective tissue and an extremely rich peritubular capillary network that provides nutrients to the seminiferous tubules (by way of Sertoli cells) while conveying testosterone away from the testes to the peripheral circulation.
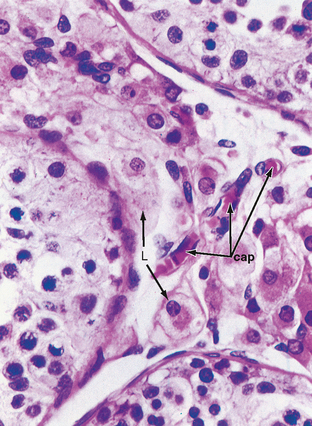
Figure 43-7 Histology of the peritubular space containing Leydig cells (L) and richly vascularized by peritubular capillaries (cap).
(Modified from Young B et al: Wheater’s Functional Histology. A Text and Colour Atlas, 5th ed. London, Churchill Livingstone, 2006.)
The Leydig Cell
Leydig cells are steroidogenic stromal cells. These cells synthesize cholesterol de novo, as well as acquire it through low-density lipoprotein (LDL) receptors and high-density lipoprotein (HDL) receptors (also called scavenger receptor BI [SR-BI]), and store cholesterol as cholesterol esters, as described for adrenocortical cells (see Chapter 42). Free cholesterol is generated by a cholesterol ester hydrolase and transferred to the outer mitochondrial membrane and then to the inner mitochondrial membrane in a steroidogenic acute regulatory (StAR) protein–dependent manner. As in all steroidogenic cells, cholesterol is converted to pregnenolone by CYP11A1. Pregnenolone is then processed to progesterone, 17-hydroxyprogesterone, and androstenedione by 3β-hydroxysteroid dehydrogenase (3β-HSD) and CYP17 (Fig. 43-8). Recall from Chapter 42 that CYP17 is a bifunctional enzyme with 17-hydroxylase activity and 17,20-lyase activity. CYP17 displays a robust level of both activities in the Leydig cell. In this respect the Leydig cell is similar to the zona reticularis cell, except that it expresses a higher level of 3β-HSD, so the Δ4 pathway is ultimately favored. Another major difference is that the Leydig cell expresses a Leydig cell–specific isoform of 17β-hydroxysteroid dehydrogenase (17β-HSD type 3), which converts androstenedione to testosterone (Fig. 43-8).
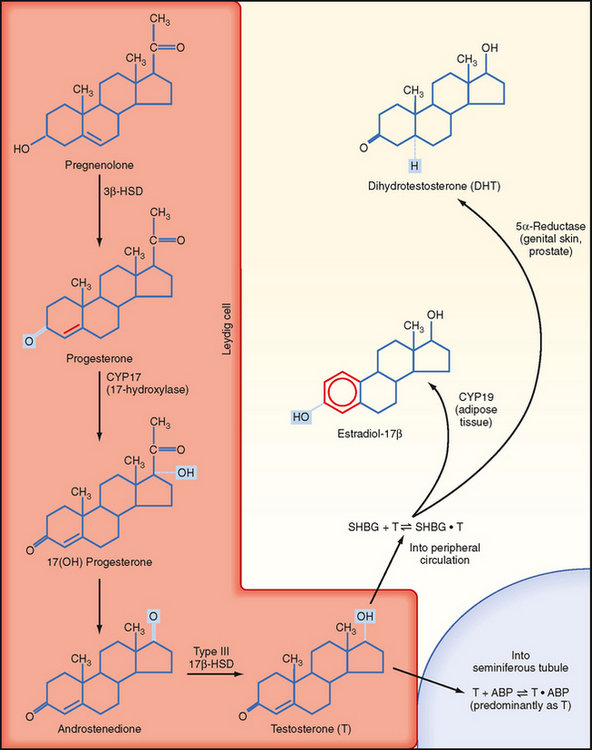
Figure 43-8 Steroidogenic pathway in Leydig cells (the first step of converting cholesterol to pregnenolone is omitted). Testosterone is sequestered by binding to androgen-binding protein (ABP) within the seminiferous tubules or circulates within the peripheral circulation bound to sex hormone–binding globulin (SHBG) and can be peripherally converted to dihydrotestosterone or estradiol-17β.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
FATES AND ACTIONS OF ANDROGENS
Intratesticular Androgen
The testosterone produced by Leydig cells has several fates and multiple actions. Because of the proximity of Leydig cells to the seminiferous tubules, significant amounts of testosterone diffuse into the seminiferous tubules and become concentrated within the adluminal compartment by ABP (Fig. 43-8). Testosterone levels within the seminiferous tubules that are greater than 100 times more concentrated than circulating testosterone levels are absolutely required for normal spermatogenesis. As mentioned earlier, Sertoli cells express the enzyme CYP19 (aromatase), which converts a small amount of testosterone into the highly potent estrogen estradiol-17β. Human sperm cells express at least one isoform of the estrogen receptor, and there is some evidence from aromatase-deficient men that this locally produced estrogen optimizes spermatogenesis in humans.
Peripheral Conversion to Estrogen
In several tissues (especially adipose tissue), testosterone is converted to estrogen (Fig. 43-8). Studies involving men with aromatase deficiency have shown that an inability to produce estrogen results in tall stature because of the lack of epiphyseal closure in long bones and osteoporosis. Thus, peripheral estrogen plays an important role in bone maturation and biology in men. These studies also implicated estrogen in promoting insulin sensitivity, improving lipoprotein profiles (i.e., increasing HDL, decreasing triglycerides and LDL), and exerting negative feedback on pituitary gonadotropins.
Peripheral Conversion to Dihydrotestosterone
Testosterone can also be converted into a potent, nonaromatizable androgen, 5α-dihydrotestosterone (DHT), by the enzyme 5α-reductase (Fig. 43-8). There are two isoforms of 5α-reductase, type 1 and type 2. Major sites of 5α-reductase 2 expression are the male urogenital tract, genital skin, hair follicles, and liver. 5α-Reductase 2 generates DHT, which is required for masculinization of the external genitalia in utero and for many of the changes associated with puberty, including growth and activity of the prostate gland (see later), growth of the penis, darkening and folding of the scrotum, growth of pubic and axillary hair, growth of facial and body hair, and increased muscle mass (Fig. 43-9). The onset of 5α-reductase 1 expression occurs at puberty. This isozyme is expressed primarily in the skin and contributes to sebaceous gland activity and the acne associated with puberty. Because DHT has strong growth-promoting (i.e., trophic) effects on its target organs, the development of selective 5α-reductase 2 inhibitors has benefited the treatment of prostatic hypertrophy and prostatic cancer.
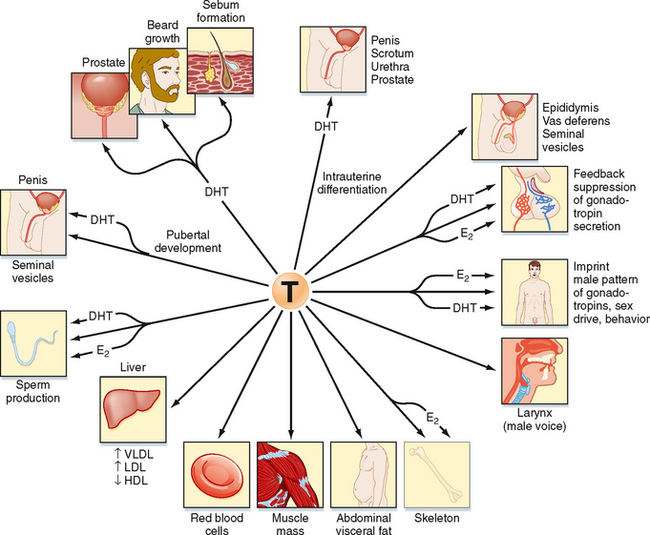
Figure 43-9 Spectrum of effects of testosterone (T). Note that some effects result from the action of testosterone itself, whereas others are mediated by dihydrotestosterone (DHT) and estradiol (E2) after they are produced from testosterone. VLDL, LDL, HDL, very-low-density, low-density, and high-density lipoproteins, respectively.
Peripheral Testosterone Actions
Testosterone has a direct action (i.e., without conversion to DHT) in several cell types (Fig. 43-9). As mentioned earlier, testosterone regulates Sertoli cell function. It induces development of the male tract from the mesonephric duct in the absence of 5αreductase. Testosterone has several metabolic effects, including increasing very low density lipoprotein (VLDL) and LDL while decreasing HDL, promoting the deposition of abdominal adipose tissue, increasing red blood cell production, promoting bone growth and health, and exerting a protein anabolic effect on muscle. Testosterone is sufficient to maintain erectile function and libido.
Mechanism of Androgen Action
Testosterone and DHT act through the same androgen receptor (AR). The AR resides in the cytoplasm bound to chaperone proteins in the absence of ligand. Testosterone-AR binding or DHT-AR binding causes dissociation of the chaperone proteins, followed by nuclear translocation of the androgen-AR complex, dimerization, binding to an androgen response element (ARE), and recruitment of coactivator proteins and general transcription factors to the vicinity of a specific gene’s promoter. It remains unclear how testosterone and DHT differ in their ability to activate the AR in the context of different cell types, although the presence of different coactivator proteins in different cell types is probably involved.
Transport and Metabolism of Androgens
As testosterone enters the peripheral circulation, it binds to and quickly reaches equilibrium with serum proteins. About 60% of circulating testosterone is bound to sex hormone–binding globulin (SHBG), 38% is bound to albumin, and about 2% remains as “free” hormone. Testosterone and its metabolites are primarily excreted in urine. Approximately 50% of excreted androgens are found as urinary 17-ketosteroids, with most of the remainder being conjugated androgens or diol or triol derivatives. Only about 30% of the 17ketosteroids in urine are from the testis; the rest are produced from adrenal androgens. Androgens are conjugated with glucuronate or sulfate in the liver, and these conjugated steroids are excreted in urine.
HYPOTHALAMIC-PITUITARYTESTICULAR AXIS
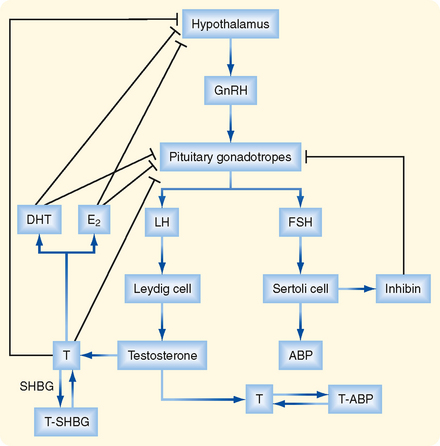
The testis is regulated by an endocrine axis (Fig. 43-10) involving parvicellular hypothalamic gonadotropin-releasing hormone (GnRH) neurons and pituitary gonadotropes that produce both luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
Regulation of Leydig Cell Function
The Leydig cell expresses the LH receptor, which acts on Leydig cells much like adrenocorticotropic hormone (ACTH) does on zona fasciculata cells in the adrenal cortex (see Chapter 42). Rapid effects include hydrolysis of cholesterol esters and new expression of StAR protein. Less acute effects include an increase in steroidogenic enzyme gene expression and expression of the LDL receptor and SR-BI (the HDL receptor). Over the long term, LH promotes Leydig cell growth and proliferation.
Testosterone negatively feeds back on LH production by the pituitary gonadotrope as testosterone and its metabolites, DHT, and estradiol-17β. All three steroid hormones inhibit the expression of LH-β and the GnRH receptor. These steroids also inhibit the release of GnRH by the hypothalamic neurons (Fig. 43-10).
Regulation of Sertoli Cell Function
The Sertoli cell is stimulated by both testosterone and FSH. In addition to stimulating the synthesis of proteins involved in the “nurse cell” aspect of Sertoli cell function (e.g., ABP), FSH stimulates synthesis of the dimeric protein inhibin. Inhibin is induced by FSH and negatively feeds back on the gonadotrope to selectively inhibit FSH production (Fig. 43-10).
THE MALE REPRODUCTIVE TRACT
Once spermatozoa emerge from the efferent ductules, they leave the gonad and enter the male reproductive tract (Fig. 43-1). The segments of the tract are as follows: the epididymis (head, body, and tail), the vas deferens, the ejaculatory duct, the prostatic urethra, the membranous urethra, and the penile urethra. Unlike the female tract, there is a contiguous lumen from the seminiferous tubule to the end of the male tract (i.e., the tip of the penile urethra), and the male reproductive tract connects to the distal urinary tract (i.e., male urethra). In addition to conveying sperm, the primary functions of the male reproductive tract are as follows:
There is an important “loophole” in the male reproductive axis that is based on the fact that intratesticular levels of testosterone need to be greater than 100-fold higher than circulating levels of the hormone to maintain normal rates of spermatogenesis; however, it is the circulating levels of testosterone that provide the negative feedback to the pituitary and hypothalamus. This means that exogenous administration of testosterone can raise circulating levels sufficient to inhibit LH but not sufficient to accumulate in the testis at the required concentration for normal spermatogenesis. However, the decreased LH levels will diminish intratesticular production of testosterone by Leydig cells, which results in reduced levels of spermatogenesis (Fig. 43-11). This “loophole” is currently being investigated as a possible strategy for developing a male oral contraceptive. It is also the basis for sterility in some cases of steroid abuse in men.
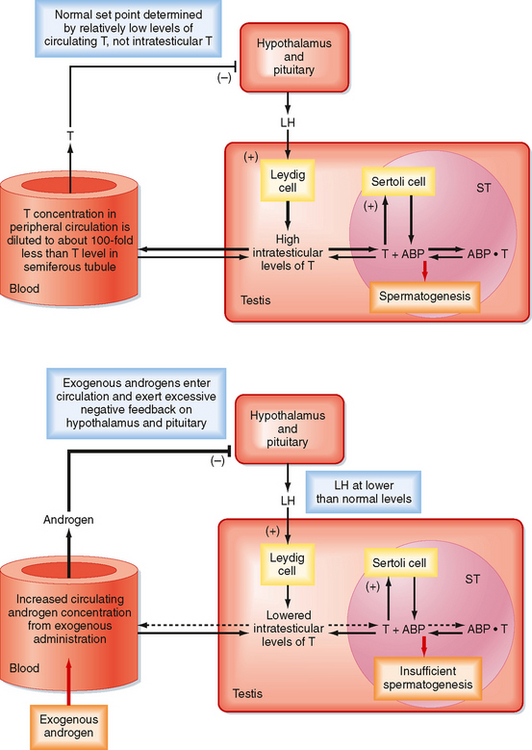
Figure 43-11 The difference in intratesticular testosterone versus circulating testosterone concentrations and its importance in the hypothalamic-pituitarytestis axis. Upper panel, Feedback loop in a normal adult man. Lower panel, Administration of testosterone (or an androgenic analogue) increases circulating testosterone (androgen) levels, which in turn increase negative feedback on release of LH. Decreased LH levels diminish Leydig cell activity and intratesticular production of androgen. Lowered intratesticular testosterone levels result in reduced sperm production and can cause infertility. Note that the inhibin feedback loop has been omitted from this diagram.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
Erection is a neurovascular event. The penis is composed of three erectile bodies: two corpora cavernosa and one corpus spongiosum (Fig. 43-12, A). The penile urethra runs through the corpus spongiosum. These three bodies are composed of erectile tissue—an anastomosing network of potential cavernous vascular spaces lined with continuous endothelia within a loose connective tissue support. During the flaccid state, blood flow to the cavernous spaces is minimal (Fig. 43-12, A). This is due to vasoconstriction of the vasculature (called the helicine arteries) and shunting of blood flow away from the cavernous spaces. In response to sexual arousal, the parasympathetic cavernous nerves innervating the vascular smooth muscle of the helicine arteries release nitric oxide (NO). NO activates guanylyl cyclase, thereby increasing cGMP, which decreases intracellular [Ca++] and causes muscular relaxation (Fig. 43-12, B). The vasodilation allows blood to flow into the cavernous spaces to induce engorgement and erection. It also presses on veins in the penis and reduces venous drainage (Fig. 43-12, B).
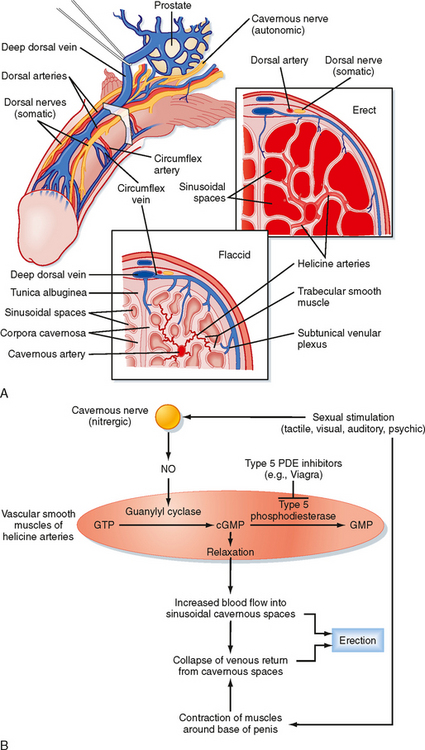
Figure 43-12 A, Arrangement of the vasculature and cavernous tissue within the penis. During the flaccid state, blood flow into the cavernous spaces is limited by contraction of the helicine arteries. B, Outline of neurovascular events leading to penile erection.
(From Bhasun S et al. In Larsen P et al [eds]: Williams Textbook of Endocrinology, 10th ed. Philadelphia, Saunders, 2003.)
An inability to achieve or maintain an erection is termed erectile dysfunction (ED) and is one cause of infertility. Multiple factors can lead to ED, including insufficient androgen production; neurovascular damage (e.g., from diabetes mellitus, spinal cord injury); structural damage to the penis, perineum, or pelvis; psychogenic factors (e.g., depression, performance anxiety); and prescribed medications and recreational drugs, including alcohol and tobacco. A major development in the treatment of some forms of erectile dysfunction is the use of selective cGMP phosphodiesterase inhibitors (e.g., Viagra), which assist in the maintenance of an erection (Fig. 43-12, B).
ANDROPAUSE
There is no distinct andropause in men. However, as men age, gonadal sensitivity to LH decreases and androgen production drops. As this occurs, serum LH and FSH levels rise. Although sperm production typically begins to decline after 50 years of age, many men can maintain reproductive function and spermatogenesis throughout life.
THE FEMALE REPRODUCTIVE SYSTEM
The female reproductive system is composed of the gonads, called ovaries, and the female reproductive tract, which includes the oviducts, uterus, cervix, vagina, and external genitalia.
THE OVARY
The ovary is located within a fold of peritoneum called the broad ligament, usually close to the lateral wall of the pelvic cavity (Fig. 43-13). Because the ovary extends into the peritoneal cavity, ovulated eggs briefly reside within the peritoneal cavity before they are captured by the oviducts.
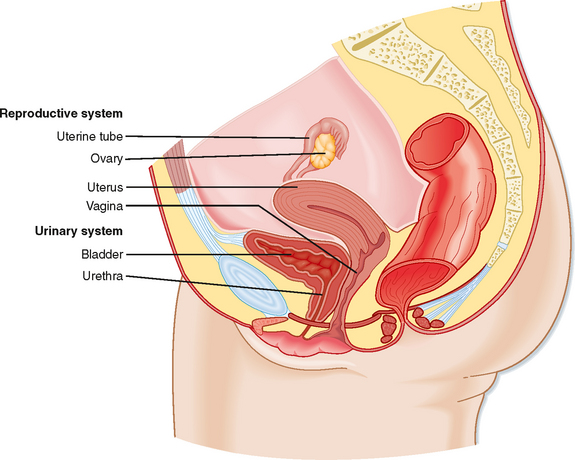
Figure 43-13 Anatomy of the female reproductive system.
(Modified from Drake RL et al: Gray’s Anatomy for Students. Philadelphia, Churchill Livingstone, 2005.)
The ovary is divided into an outer cortex and inner medulla (Fig. 43-14). Neurovascular elements innervate the medulla of the ovary. The cortex of the ovary is composed of a densely cellular stroma. Within this stroma reside the ovarian follicles (Fig. 43-14), which contain a primary oocyte surrounded by follicle cells. The cortex is covered by a connective tissue capsule, the tunica albuginea, and a layer of simple epithelium consisting of ovarian surface epithelial cells. There are no ducts emerging from the ovary to convey its gametes to the reproductive tract. Thus, the process of ovulation involves an inflammatory event that erodes the wall of the ovary. After ovulation, the ovarian surface epithelial cells rapidly divide to repair the wall.
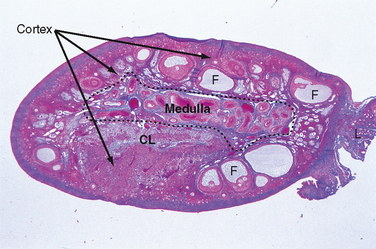
Figure 43-14 Histology of the ovary. CL, corpus luteum; F, follicle;
(Modified from Young B et al: Wheater’s Functional Histology. A Text and Colour Atlas, 5th ed. London, Churchill Livingstone, 2006.)
Growth, Development, and Function of the Ovarian Follicle
The ovarian follicle is the functional unit of the ovary, and it performs both gametogenic and endocrine functions. A histological section of the ovary from a premenopausal cycling woman contains follicular structures at many different stages of development. The life history of a follicle can be divided into the following stages:
Resting Primordial Follicle
Growth and Structure.
Resting primordial follicles (Fig. 43-15) represent the earliest and simplest follicular structure in the ovary. Primordial follicles appear during midgestation through the interaction of gametes and somatic cells. Primordial germ cells that have migrated to the gonad continue to divide mitotically as oogonia until the fifth month of gestation in humans. At this point the approximately 7 million oogonia enter the process of meiosis and become primary oocytes. During this time the primary oocytes become surrounded by a simple epithelium of somatic follicle cells, thereby creating the primordial follicles (Fig. 43-15). The follicle cells establish gap junctions with each other and the oocyte. The follicle cells themselves represent a true avascular epithelium surrounded by a basal lamina. Similar to Sertoli cell–sperm interactions, a subpopulation of granulosa cells remains intimately attached to the oocytes throughout their development. Granulosa cells provide nutrients such as amino acids, nucleic acids, and pyruvate to support oocyte maturation.
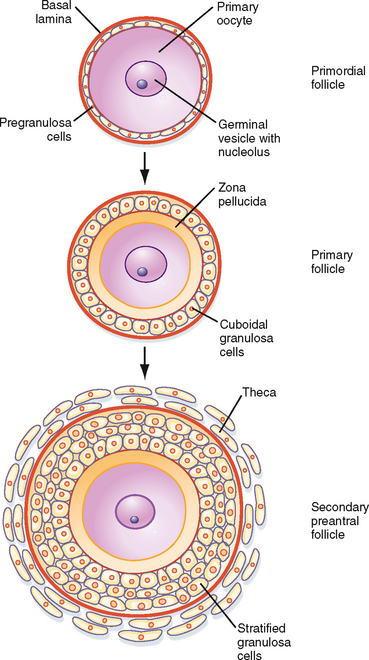
Figure 43-15 Development of a primordial follicle up to a secondary, preantral follicle.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
The primordial follicles represent the ovarian reserve of follicles (Fig. 43-16). This reserve is reduced from a starting number of about 7 million to less than 300,000 follicles at reproductive maturity. Of these, a woman will ovulate about 450 between menarche (first menstrual cycle) and menopause (cessation of menstrual cycles). At menopause, less than 1000 primordial follicles are left in the ovary. Primordial follicles are lost primarily from death as a result of follicular atresia. However, a small subset of primordial follicles will enter follicular growth in waves. Because the ovarian follicular reserve represents a fixed, finite number, the rate at which resting primordial follicles die or begin to develop (or both) will determine the reproductive life span of a woman. Age at the onset of menopause has a strong genetic component but is also influenced by environmental factors. For example, cigarette smoking significantly depletes the ovarian reserve. An overly rapid rate of atresia or development will deplete the reserve and give rise to premature ovarian failure.
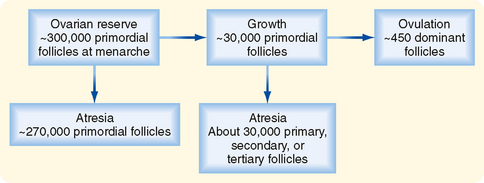
Figure 43-16 Fate of ovarian follicles.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
Pituitary gonadotropins maintain a normal ovarian reserve by promoting the general health of the ovary. However, the rate at which resting primordial follicles enter the growth process appears to be independent of pituitary gonadotropins. The decision of a resting follicle to enter the early growth phase is primarily dependent on intraovarian paracrine factors that are produced by both the follicle cells and oocytes.
The Gamete.
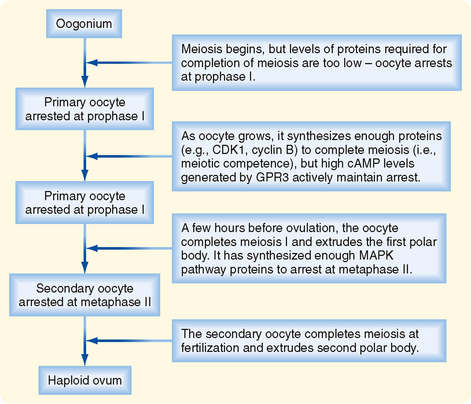
In primordial follicles, the gamete is derived from oogonia that have entered the first meiotic division; such oogonia are referred to as primary oocytes. Primary oocytes progress through most of prophase of the first meiotic division (termed prophase I) over a 2-week period and then arrest in the diplotene stage. This stage is characterized by the decondensation of chromatin, which supports the transcription needed for oocyte maturation. Meiotic arrest at this stage, which may last for up to 50 years, appears to be due to “maturational incompetence,” or lack of the cell cycle proteins needed to support the completion of meiosis. The nucleus of the oocyte, called the germinal vesicle, remains intact at this stage.
Growing Preantral Follicles
Growth and Structure.
The first stage of follicular growth is preantral, which refers to the development that occurs before the formation of a fluid-filled antral cavity. One of the first visible signs of follicle growth is the appearance of cuboidal granulosa cells. At this point the follicle is referred to as a primary follicle (Fig. 43-15). As granulosa cells proliferate, they form a multilayered (i.e., stratified) epithelium around the oocyte. At this stage the follicle is referred to as a secondary follicle (Fig. 43-15).
Once a secondary follicle acquires three to six layers of granulosa cells, it secretes paracrine factors that induce nearby stromal cells to differentiate into epithelioid thecal cells. Thecal cells form a flattened layer of cells around the follicle. Once a thecal layer forms, the follicle is referred to as a mature preantral follicle (Fig. 43-15). In humans it takes several months for a primary follicle to reach the mature preantral stage.
Follicular development is associated with an inward movement of the follicle from the outer cortex to the inner cortex, closer to the vasculature of the ovarian medulla. Follicles release angiogenic factors that induce the development of one to two arterioles that form a vascular wreath around the follicle.
The Gamete.
During the preantral stage, the oocyte begins to grow and produce cellular and secreted proteins. The oocyte initiates secretion of extracellular matrix glycoproteins, called ZP1, ZP2, and ZP3, that form the zona pellucida (Fig. 43-15). The zona pellucida increases in thickness and provides a species-specific binding site for sperm during fertilization (see later). Importantly, granulosa cells and the oocyte maintain gap junctional contact via cellular projections through the zona pellucida. The oocyte also continues to secrete paracrine factors that regulate follicle cell growth and differentiation.
Endocrine Function.
Granulosa cells express the FSH receptor during this period, but they are primarily dependent on factors from the oocyte to grow. They do not produce ovarian hormones at this early stage of follicular development. The newly acquired thecal cells are analogous to testicular Leydig cells in that they reside outside the epithelial “nurse” cells, express the LH receptor, and produce androgens. The main difference between Leydig cells and thecal cells is that thecal cells do not express high levels of 17β-HSD. Thus, the major product of theca cells is androstenedione, as opposed to testosterone. Androstenedione production at this stage is minimal.
Growing Antral Follicles
Growth and Structure.
Mature preantral follicles develop into early antral follicles (Fig. 43-17) over a period of about 25 days, during which they grow from a diameter of about 0.1 mm to a diameter of 0.2 mm. Once the granulosa epithelium increases to six to seven layers, fluid-filled spaces appear between cells and coalesce into the antrum. Over a period of about 45 days, this wave of small antral follicles will continue to grow to large, recruitable antral follicles that are 2 to 5 mm in diameter. This period of growth is characterized by about a 100-fold increase in granulosa cells (from about 10,000 to 1,000,000 cells). It is also characterized by swelling of the antral cavity, which increasingly divides the granulosa cells into two discrete populations (Fig. 43-17).
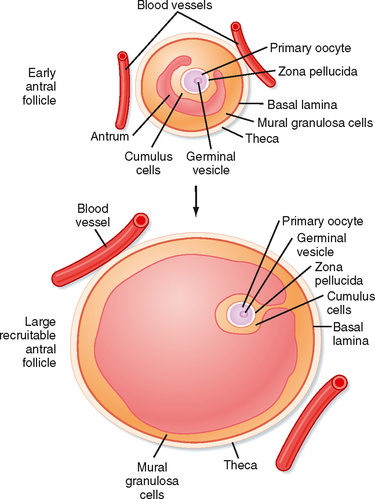
Figure 43-17 Development of an early antral follicle to a mature preovulatory follicle.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
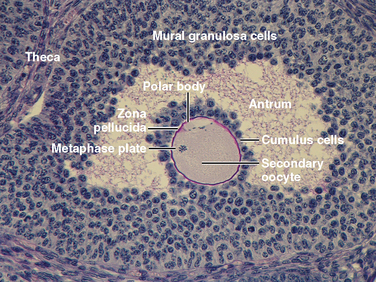
Mural granulosa cells (also called the stratum granulosum) form the outer wall of the follicle. The basal layer is adherent to the basal lamina and in close proximity to the outer-lying thecal layers. Mural granulosa cells become highly steroidogenic and remain in the ovary after ovulation to differentiate into the corpus luteum.
Cumulus cells are the inner cells that surround the oocyte (they are also referred to as the cumulus oophorus and corona radiata). The innermost layer of cumulus cells maintains gap and adhesion junctions with the oocyte. Cumulus cells are released with the oocyte (collectively referred to as the cumulus-oocyte complex) during the process of ovulation. Cumulus cells are crucial for the ability of the fimbriated end of the oviduct to “capture” and move the oocyte by a ciliary transport mechanism along the length of the oviduct to the site of fertilization (see later).
Early antral follicles are dependent on pituitary FSH for normal growth. Large antral follicles become highly dependent on pituitary FSH for their growth and sustained viability. As discussed later, 2- to 5-mm follicles are recruited to enter a rapid growth phase via the transient increase in FSH that occurs toward the end of the previous menstrual cycle.
The Gamete.
The oocyte grows rapidly in the early stages of antral follicles—growth then slows in larger follicles. During the antral stage, the oocyte synthesizes sufficient amounts of cell cycle components so that it becomes competent to complete meiosis I at ovulation (note that the human egg arrests after ovulation at a second point, metaphase II, until it is fertilized by sperm). Thus, in early primary and secondary follicles, the oocyte fails to complete meiosis I because of a dearth of specific meiosis-associated proteins. However, larger antral follicles gain meiotic competence but still maintain meiotic arrest until the midcycle LH surge. Meiotic arrest is achieved by maintenance of elevated cAMP levels in the mature oocyte (Figs. 43-18 and 43-19).
Endocrine Function.
The thecal cells of large antral follicles produce significant amounts of androstenedione and testosterone. Androgens are converted to estradiol-17β by the granulosa cells (see later). However, at this stage, FSH stimulates the proliferation of granulosa cells and induces the expression of CYP19 (aromatase) required for the synthesis of estrogen. Additionally, the mural granulosa cells of the large antral follicles produce increasing amounts of inhibin B during the early follicular phase. Low levels of estrogen and inhibin negatively feed back on FSH secretion, thereby contributing to selection of the follicle with the most FSH-responsive cells.
Dominant Follicle
Growth and Structure.
At the end of a previous menstrual cycle, a crop of large (2 to 5 mm) antral follicles (Fig. 43-17) are recruited to begin rapid, gonadotropin-dependent development. The total number of recruited follicles in both ovaries can be as high as 20 in a younger woman (<33 years old) but rapidly declines at older ages. The number of recruited follicles is reduced to the prolifera quota (one in humans) by the process of selection. As FSH levels decline, the rapidly growing follicles progressively undergo atresia until one follicle is left. Generally, the largest follicle with the most FSH receptors of the recruited crop becomes the dominant follicle. Selection occurs during the early follicular phase. By midcycle, the dominant follicle becomes a large preovulatory follicle that is 20 mm in diameter and contains about 50 million granulosa cells by the midcycle gonadotropin surge.
The Gamete.
The oocyte is competent to complete meiosis I but remains arrested in the dominant follicle until the LH surge. Growth of the oocyte continues, but at a slower rate, until the oocyte reaches a diameter of about 140 μm by ovulation.
Endocrine Function.
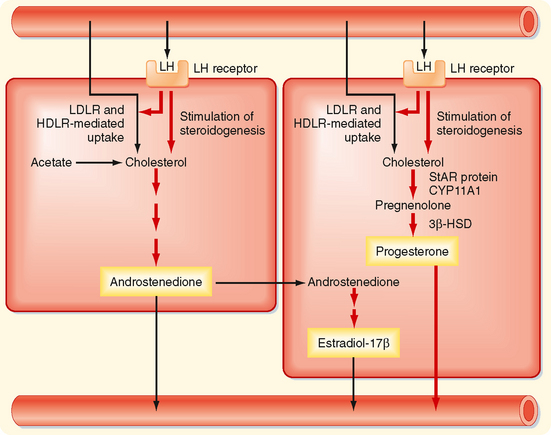
The newly selected follicle emerges for the first time during its development as a significant steroidogenic “gland.” Ovarian steroidogenesis requires both theca and granulosa cells (Fig. 43-20). As discussed earlier, thecal cells express LH receptors and produce androgens. Basal levels of LH stimulate the expression of steroidogenic enzymes, as well as the LDL receptor and HDL receptor (SR-B1), in the theca. Thecal cells show robust expression of CYP11A1 (side chain cleavage enzyme), 3β-HSD, and CYP17 with both 17-hydroxylase activity and 17,20-lyase activity. Androgens (primarily androstenedione but also some testosterone) released from the theca diffuse into the mural granulosa cells or enter the vasculature surrounding the follicle.

Figure 43-20 Two-cell model for steroidogenesis in the dominant follicle.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
The mural granulosa cells of the selected follicle have a high number of FSH receptors and are very sensitive to FSH, which up-regulates CYP19 (aromatase) gene expression and activity (Fig. 43-20). CYP19 converts androstenedione to the weak estrogen estrone and converts testosterone to the potent estrogen estradiol-17β. Granulosa cells express activating isoforms of 17β-HSD, which ultimately drives steroidogenesis toward the production of estradiol-17β. In addition, FSH induces the expression of inhibin B during the follicular phase.
Importantly, FSH also induces the expression of LH receptors in mural granulosa cells during the second half of the follicular phase (Fig. 43-20). Thus, mural granulosa cells become responsive to both gonadotropins, which allows these cells to maintain high levels of CYP19 in the face of declining FSH levels. Acquisition of LH receptors also ensures that mural granulosa cells respond to the LH surge.
The Dominant Follicle during the Periovulatory Period
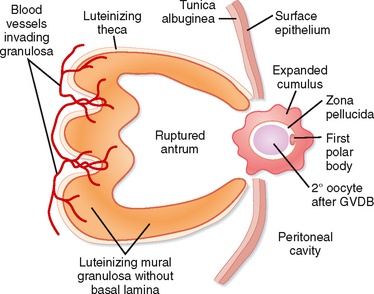
The periovulatory period is defined as the time from the onset of the LH surge to expulsion of the cumulus-oocyte complex out of the ovary (i.e., ovulation). This process lasts for 32 to 36 hours in women. Starting at the same time and superimposed on the process of ovulation is a change in the steroidogenic function of theca and mural granulosa cells. This process is called luteinization and culminates in the formation of a corpus luteum that is capable of producing high amounts of progesterone, along with estrogen, within a few days after ovulation. Thus, the LH surge induces the onset of complex processes during the periovulatory period that complete the gametogenic function of the ovary for a given month and switches the endocrine function to prepare the female reproductive tract for implantation and gestation.
Growth and Structure.
The LH surge induces dramatic structural changes in the dominant follicle that involves its rupture, ovulation of the cumulus-oocyte complex, and the biogenesis of a new structure called the corpus luteum from the remaining thecal and mural granulosa cells. Major structural changes occur during this transition:
The Gamete.
Before ovulation, the primary oocyte is competent to complete meiosis, but it is arrested in prophase I. The LH surge induces the oocyte to progress to metaphase II (Fig. 43-18). The oocyte subsequently arrests at metaphase II until fertilization. LH receptors are present on mural granulosa cells, not cumulus cells.
Endocrine Function.
Both theca and mural granulosa cells express LH receptors at the time of the LH surge. The LH surge induces differentiation of the granulosa cells—a process that continues for several days after ovulation. During the periovulatory period, the LH surge induces the following shifts in steroidogenic activity of the mural granulosa cells (Fig. 43-22):
The Corpus Luteum
Growth and Structure.
After ovulation, the remnant of the antral cavity fills with blood from damaged blood vessels in the vicinity of the stigma. This gives rise to a corpus hemorrhagicum (Fig. 43-23). Within a few days, red blood cells and debris are removed by macrophages, and fibroblasts fill in the antral cavity with a hyaline-like extracellular matrix. In the mature corpus luteum, the granulosa cells, now called granulosa lutein cells, enlarge and become filled with lipid (cholesterol esters). The enlarged granulosa lutein cells collapse into and partially fill in the old antral cavity. Proliferation of these cells is very limited. The theca, along with blood vessels, mast cells, macrophages, leukocytes, and other resident connective tissue cells, infiltrate the granulosa layer at multiple sites.
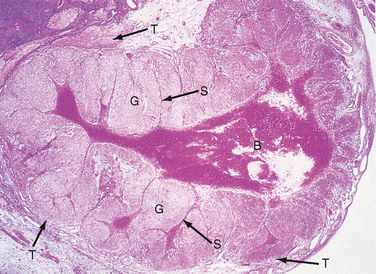
Figure 43-23 Histology of an early corpus luteum, called a corpus hemorrhagicum. B, blood clot in the former antral space; G, luteinized granulosa cells; S, septa of connective tissue cells and blood vessels within the granulosa layer; T, luteinized theca cells.
(From Young B et al: Wheater’s Functional Histology. A Text and Colour Atlas, 5th ed. London, Churchill Livingstone, 2006.)
The human corpus luteum is programmed to live for 14 days, plus or minus 2 days (corpus luteum of menstruation), unless “rescued” by the LH-like hormone human chorionic gonadotropin (hCG), which originates from an implanting embryo. If rescued, the corpus luteum of pregnancy will remain viable during the pregnancy (usually about 9 months). The mechanism by which the corpus luteum of menstruation regresses in 14 days is not fully understood. Regression appears to involve release of the prostaglandin PGF2α from both granulosa lutein cells and the uterus in response to declining levels of progesterone during the second week of the luteal phase. Several paracrine factors (endothelin, monocyte chemotactic protein-1) from immune and vascular cells are likely to play a role in the demise and removal of granulosa lutein cells. The corpus luteum is ultimately turned into a scar-like body called the corpus albicans, which sinks into the medulla of the ovary and is slowly absorbed.
The Gamete.
The LH surge induces two parallel events, ovulation and luteinization. If ovulation occurs normally, the corpus luteum is devoid of a gamete.
Endocrine Function.
Progesterone production by the corpus luteum (Fig. 43-22) increases steadily from the onset of the LH surge and peaks during the midluteal phase. The main purpose of this timing is to transform the uterine lining into an adhesive and supportive structure for implantation and early pregnancy. As discussed later, the midluteal phase is synchronized with early embryogenesis so that the uterus is optimally primed when a blastocyst tumbles into the uterus around day 22 of the menstrual cycle. Estrogen production transiently decreases in response to the LH surge but then rebounds and peaks at the midluteal phase.
Luteal hormonal output is absolutely dependent on basal LH levels (Fig. 43-22). In fact, progesterone output is closely correlated with the pulsatile pattern of LH release in women. Both FSH and LH are reduced to basal levels during the luteal phase by negative feedback from progesterone and estrogen. In addition, granulosa lutein cells secrete inhibin A, which selectively represses FSH secretion. The elevated estrogen levels at the midluteal phase may be responsible for the decrease in sensitivity of the corpus luteum to LH, so progesterone and estrogen levels decline during the second half of the luteal phase unless an increase in circulating LH-like activity (i.e., in the form of hCG) compensates for the decreased sensitivity to LH.
The corpus luteum must generate large amounts of progesterone to support implantation and early pregnancy. Accordingly, the life of the corpus luteum is very regular, and a shortened luteal phase typically leads to infertility. The quality of the corpus luteum is largely dependent on the size and health of the dominant follicle from which it developed, which in turn is dependent on normal hypothalamic and pituitary stimulation during the follicular phase. Numerous factors that perturb hypothalamic and pituitary output during the follicular phase, including heavy exercise, starvation, high prolactin levels, and abnormal thyroid function, can lead to luteal phase deficiency and infertility.
Atretic Follicles
Follicular atresia refers to the demise of an ovarian follicle. During atresia, the granulosa cells and oocytes undergo apoptosis. The thecal cells typically persist and repopulate the cellular stroma of the ovary. The thecal cells retain LH receptors and the ability to produce androgens and are collectively referred to as the “interstitial gland” of the ovary. Follicles can undergo atresia at any time during development.
Follicular Development with Respect to the Monthly Menstrual Cycle
The first half of the monthly menstrual cycle is referred to as the follicular phase of the ovary and is characterized by the recruitment and growth of 15 to 20 large antral follicles (2 to 5 mm in diameter), followed by selection of one of these follicles as the dominant follicle and growth of the dominant follicle until ovulation. The dominant follicle must contain a fully developed oocyte and somatic follicle cells that secrete high levels of estrogen. It takes several months for a primordial follicle to reach the size of a large antral follicle that can be recruited. Therefore, much of follicular development occurs independently of the monthly menstrual cycle. The second half of the monthly menstrual cycle is referred to as the luteal phase of the ovary and is dominated by hormonal secretions of the corpus luteum. Nevertheless, small follicles continue to develop within the ovarian stroma during the luteal phase.
Regulation of Late Follicular Development, Ovulation, and Luteinization: The Human Menstrual Cycle
As stated earlier, late follicular development and luteal function are absolutely dependent on normal hypothalamic and pituitary function. As in the male, hypothalamic neurons secrete GnRH in a pulsatile manner. GnRH, in turn, stimulates LH and FSH production by pituitary gonadotropes. A high frequency of GnRH pulses (1 pulse per 60 to 90 minutes) selectively promotes LH production, whereas a slow frequency promotes FSH production. A major difference between the male and female reproductive axes is the midcycle gonadotropin surge, which is dependent on a constant, high level of estrogen coming from the dominant follicle.
A highly dynamic “conversation” occurs among the ovary, pituitary, and hypothalamus in which the events of the menstrual cycle are orchestrated, beginning with the ovary at the end of the luteal phase of a previous, nonfertile cycle (Fig. 43-24):
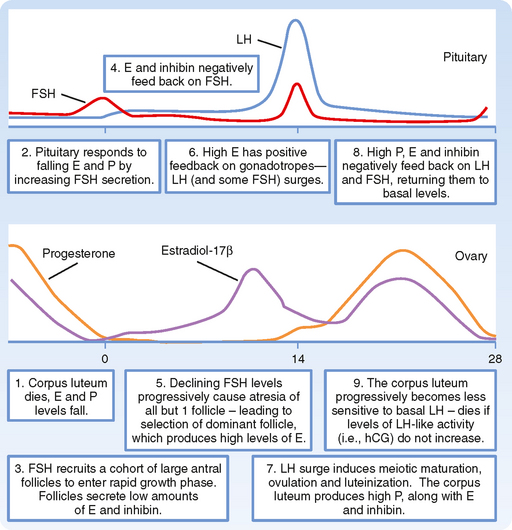
Figure 43-24 The human menstrual cycle, with emphasis on the “dialogue” between ovary and pituitary gonadotropes.
From this sequence of events it is evident that the ovary is the primary clock for the menstrual cycle. The timing of the two main pituitary-based events—the transient rise in FSH that recruits large antral follicles and the LH surge that induces ovulation—is determined by two ovarian events. These are respectively the highly regular life span of the corpus luteum and its demise after 14 days and growth of the dominant follicle to the point at which it can maintain a sustained high production of estrogen that induces a switch to positive feedback at the pituitary.
THE OVIDUCT
Structure and Function
The oviducts (also called the uterine tubes and the fallopian tubes) are muscular tubes with the distal ends close to the surface of each ovary and the proximal ends traversing the wall of the uterus. The oviducts are divided into four sections (going from distal to proximal): the infundibulum, or open end of the oviduct, which has finger-like projections called fimbriae that sweep over the surface of the ovary; the ampulla, which has a relatively wide lumen and extensive folding of the mucosa; the isthmus, which has a relatively narrow lumen and less mucosal folding; and the intramural or uterine segment, which extends through the uterine wall at the superior corners of the uterus (Fig. 43-25).
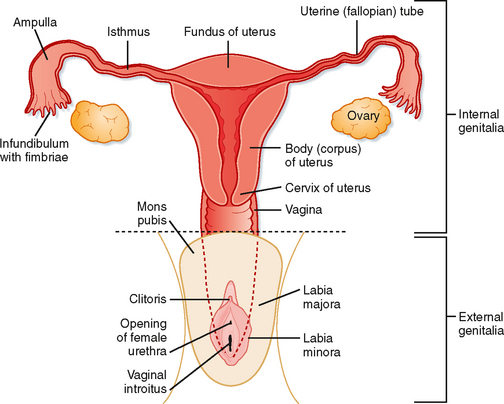
Figure 43-25 Schematic of the female reproductive system.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
The main functions of the oviducts are to
The timing of movement of the embryo into the uterus is critical because the uterus has an implantation window of about 3 days. The oviduct needs to hold the early embryo until it reaches the blastocyst stage (5 days after fertilization) and then allow it to pass into the uterine cavity.
The wall of the oviduct is composed of a mucosa (called the endosalpinx), a two-layered muscularis (called the myosalpinx), and an outer-lying connective tissue (the perisalpinx). The endosalpinx is thrown into many folds, almost to the extent that the lumen is obliterated, and is lined by a simple epithelium made up of two cell types: ciliated cells and secretory cells. The cilia are most numerous at the infundibular end and propel the cumulus-oocyte complex toward the uterus. The cilia on the fimbriae are the sole mechanism for transport of the ovulated cumulus-oocyte complex. Once the complex passes through the ostium of the oviduct and enters the ampulla, it is moved by both cilia and peristaltic contractions of the muscularis.
The secretory cells produce a protein-rich mucus that is conveyed along the oviduct to the uterus by the cilia. This ciliary-mucus escalator maintains a healthy epithelium, moves the cumulus-oocyte complex toward the uterus, and may provide directional cues for swimming sperm. Movement of the cumulus-oocyte complex slows at the ampullary-isthmus junction, where fertilization normally takes place. This appears to be due, in part, to thick mucus produced by the isthmus and increased tone of the muscularis of the isthmus. The composition of oviductal secretions is complex and includes growth factors, enzymes, and oviduct-specific glycoproteins. Note that the clinical process of in vitro fertilization has shown that secretions of the oviduct are not absolutely necessary for fertility. However, normal oviductal function is absolutely required for both fertilization and implantation after in vivo insemination. Normal oviductal function also minimizes the risk of ectopic implantation and pregnancy.
Hormonal Regulation during the Menstrual Cycle
In general, estrogen secreted during the follicular phase increases epithelial cell size and height in the endosalpinx. Estrogen increases blood flow to the lamina propria of the oviducts, promotes the production of oviduct-specific glycoproteins (whose functions are poorly understood), and increases ciliogenesis throughout the oviduct. Estrogen promotes the secretion of thick mucus in the isthmus and increases the tone of the muscularis of the isthmus, thereby keeping the cumulus-oocyte complex at the ampullary-isthmus junction for fertilization. High progesterone, along with estrogen, during the early luteal to midluteal phase decreases epithelial cell size and function. Progesterone promotes deciliation. It also decreases the secretion of thick mucus and relaxes the tone in the isthmus. In addition, it should be noted that oviductal epithelial cells express the LH receptor, which may synergize with estrogen to optimize oviductal function during the periovulatory period.
THE UTERUS
Structure and Function
The uterus is a single organ that sits in the midline of the pelvic cavity between the bladder and the rectum (Fig. 43-13). The mucosa of the uterus is called the endometrium; the three-layered, thick muscularis is called the myometrium; and the outer connective tissue and serosa are called the perimetrium. The parts of the uterus are (1) the fundus, which is the portion that rises superiorly from the entrance of the oviducts; (2) the body of the uterus, which makes up most of the uterus; (3) the isthmus, a short narrowed part of the body at its inferior end; and (4) the cervix, which extends into the vagina (Figs. 43-13 and 43-25). Because the cervical mucosa is distinct from the rest of the uterus and does not undergo the process of menstruation, it will be discussed separately later.
The established functions of the uterus are all related to fertilization and pregnancy (see later). The main functions of the uterus are to
To understand the function of the uterus and uterine changes during nonfertile menstrual cycles, the fine structure of the endometrium and the relationship of uterine blood supply to the endometrium will be reviewed (Fig. 43-26). The luminal surface of the endometrium is covered with a simple cuboidal/columnar epithelium. The epithelium is continuous with mucosal glands (called uterine glands) that extend deep into the endometrium. The mucosa is vascularized by spiral arteries, which are branches of the uterine artery that run through the myometrium. The terminal arterioles of the spiral arteries project just beneath the surface epithelium. These arterioles give rise to a subepithelial plexus of capillaries and venules that have ballooned, thin-walled segments called venous lakes or lacunae. The lamina propria itself is densely cellular. The stromal cells of the lamina propria play important roles during both pregnancy and menstruation.
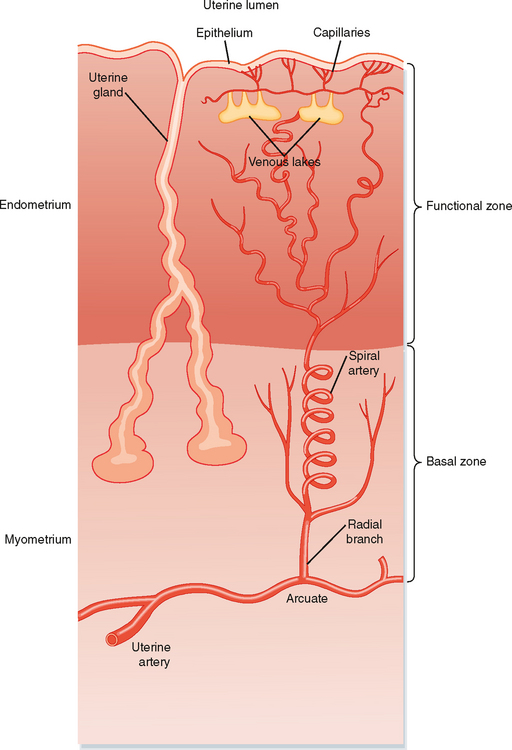
Figure 43-26 Diagram of the organization of glands and blood flow within the uterine endometrium.
(From Straus III. In Yen SSC et al [eds]: Reproductive Endocrinology, 4th ed. Philadelphia, Saunders, 1999.)
About two thirds of the luminal side of the endometrium is lost during menstruation and is called the functional zone (also called the stratum functionalis) (Fig. 43-26). The basal third of the endometrium that remains after menstruation is called the basal zone (also called the stratum basale). The basal zone is fed by straight arteries that are separate from the spiral arteries, and it contains all the cell types of the endometrium (i.e., epithelial cells from the remaining tips of glands, stromal cells, and endothelial cells).
HORMONAL REGULATION OF THE UTERINE ENDOMETRIUM DURING THE MENSTRUAL CYCLE
Proliferative Phase
Monthly oscillations in ovarian steroids induce the uterine endometrium to enter different stages. At the time of selection of the dominant follicle and its production of estrogen, the uterine endometrium is just ending menstruation. The stratum functionalis has been shed, and only the stratum basale remains (Fig. 43-27). The rising levels of estrogen during the mid to late follicular phase of the ovary induce the proliferative phase of the uterine endometrium. Estrogen induces all cell types in the stratum basale to grow and divide. In fact, the definition of an “estrogenic” compound has historically been one that is “uterotropic.” Estrogen increases cell proliferation directly through its cognate receptors (ER-α and ER-β), which regulate gene expression (Fig. 43-28). Estrogen also controls uterine growth indirectly through the local production of growth factors. In addition, estrogen induces the expression of progesterone receptors, thereby “priming” the uterine endometrium so that it can respond to progesterone during the luteal phase of the ovary.
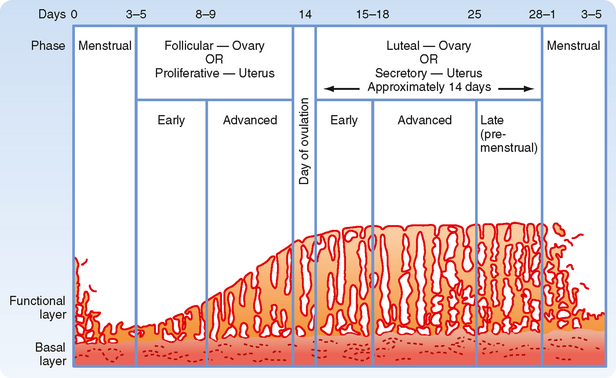
Figure 43-27 The menstrual cycle of the uterine endometrium.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
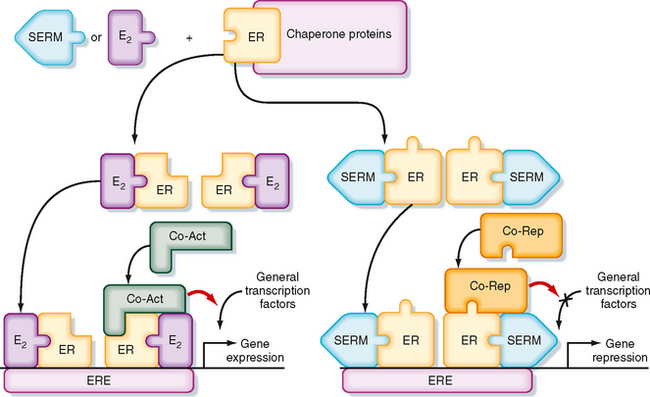
Figure 43-28 Molecular mechanism by which the estrogen receptor (ER) regulates gene expression. Left, Estradiol-17β binds to the ER and changes its conformation so that it binds as a dimer to estrogen-response element (ERE) and recruits coactivator proteins (Co-Act), which leads to stimulation of gene expression. Right, Selective estrogen receptor modulators (SERMs), such as tamoxifen in the breast, alter ER conformation so that it recruits corepressor proteins (Co-Rep), thereby inhibiting gene expression. In this case the SERM acts as an ER antagonist, but in some tissues, the same SERM can act as an ER agonist.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
Secretory Phase
By ovulation, the thickness of the stratum functionalis has been reestablished under the proliferative actions of estradiol-17β (Fig. 43-27). After ovulation, the corpus luteum produces high levels of progesterone, along with estradiol-17β. The luteal phase of the ovary switches the proliferative phase of the uterine endometrium to the secretory phase. In general, progesterone inhibits further endometrial growth and induces the differentiation of epithelial and stromal cells. Progesterone induces the uterine glands to secrete a nutrient-rich product that supports blastocyst viability. As the secretory phase proceeds, the mucosal uterine glands become corkscrewed and sacculated (Fig. 43-27). Progesterone also induces changes in adhesivity of the surface epithelium, thereby generating the “window of receptivity” for implantation of an embryo (see later). Additionally, progesterone promotes the differentiation of stromal cells into “predecidual cells,” which must be prepared to form the decidua of pregnancy or to orchestrate menstruation in the absence of pregnancy.
Progesterone opposes the proliferative actions of estradiol-17β and down-regulates the estrogen receptor (ER). Progesterone also induces inactivating isoforms of 17β-HSD, thereby converting the active estradiol-17β into the inactive estrone. This opposition of the mitogenic actions of estradiol-17β by progesterone is important to protect the uterine endometrium from estrogen-induced uterine cancer. In contrast, the administration of “unopposed estrogen” to women significantly increases the risk for uterine cancer.
Drugs called selective estrogen receptor modulators (SERMs) have been developed that inhibit ER function in a tissue-specific manner (Fig. 43-28). For example, the SERM tamoxifen is used as an ER antagonist for the treatment of breast cancer (whose early progression is promoted by estrogen). Binding of SERM to the ER induces conformational changes that allow corepressors to bind to the ER or promote degradation of the ER (or both; Fig. 43-28). Because tamoxifen has some uterotropic activity (i.e., makes uterine endometrial tissue grow), newer SERMs such as raloxifene have been developed to have ER antagonist activity on the breast, beneficial ER agonist activity on bone (see later), and no activity or ER antagonistic activity on the uterine endometrium.
Menstrual Phase
In a nonfertile cycle, death of the corpus luteum results in sudden withdrawal of progesterone, which leads to changes in the uterine endometrium that result in loss of the lamina functionalis (Fig. 43-27). Menstruation normally lasts for 4 to 5 days (called a period), and the volume of blood loss ranges from 25 to 35 mL. Menstruation coincides with the early follicular phase of the ovary.
Hormonal Regulation of the Myometrium
The smooth muscle cells of the myometrium are also responsive to changes in steroid hormones. Peristaltic contractions of the myometrium favor movement of the luminal contents from the cervix to the fundus at ovulation, and these contractions probably play a role in rapid, bulk transport of ejaculated sperm from the cervix to the oviducts. During menstruation, contractions propagate from the fundus to the cervix, thereby promoting expulsion of sloughed stratum functionalis. The size and number of smooth muscle cells are determined by estrogen and progesterone. Healthy, cycling women maintain a robust myometrium, whereas the myometrium progressively thins in postmenopausal women. The most drastic changes are seen during pregnancy, when the smooth muscle cells increase from 50 to 500 μm in length. The pregnant myometrium also has a greater number of smooth muscle cells and more extracellular matrix.
Disorders of menstruation are relatively common and include menorrhagia (heavy menstrual flow leading to loss of more than 80 mL of blood), metrorrhagia (irregular and sometimes prolonged menstrual flow between normal periods), and dysmenorrhea (painful periods). The existence of a few, irregular periods, called oligomenorrhea, and the absence of periods, called amenorrhea, are often due to dysfunction of the hypothalamic-pituitary-ovarian axis, as opposed to local pelvic pathophysiology.
Because endometrial tissue is naturally sloughed in fragments that contain viable cells, endometrial tissue occasionally gains access to other parts of the female tract (e.g., oviducts, ovary), as well as the lower part of the abdomen and associated structures (e.g., rectum, bladder). These implants give rise to endometriosis—a foci of hormonally responsive endometrial tissue outside the uterus. The spread of endometriosis may be due to reflux of menstrual tissue into the oviducts or movement of tissue through lymphatics, or both. Endometriosis frequently exhibits cyclic bleeding and is associated with infertility, pain on defecation, pain on urination, pain with sexual intercourse, or generalized pelvic pain.
THE CERVIX
Structure and Function
The cervix is the inferior extension of the uterus that projects into the vagina (Figs. 43-13 and 43-25). It has a mucosa that lines the endocervical canal, which has a highly elastic lamina propria and a muscularis that is continuous with the myometrium. The part of the cervix that extends into the vaginal vault is called the ectocervix, whereas the part surrounding the endocervical canal is called the endocervix. The openings of the endocervical canal at the uterus and vagina are called the internal cervical os and the external cervical os, respectively. The cervix acts as a gateway to the upper female tract—at midcycle, the endocervical canal facilitates sperm viability and entry. During the luteal phase, the endocervical canal impedes the passage of sperm and microbes, thereby inhibiting superimplantation of a second embryo or ascending infection into the placenta, fetal membranes, and fetus. The cervix physically supports the weight of the growing fetus. At term, cervical softening and dilation allow passage of the newborn and placenta from the uterus into the vagina.
Hormonal Regulation of Cervical Mucus during the Menstrual Cycle
The endocervical canal is lined by a simple columnar epithelium that secretes cervical mucus in a hormonally responsive manner. Estrogen stimulates the production of a copious quantity of thin, watery, slightly alkaline mucus that is an ideal environment for sperm. Progesterone stimulates the production of a scant, viscous, slightly acidic mucus that is hostile to sperm. During the normal menstrual cycle, the conditions of the cervical mucus are ideal for sperm penetration and viability at the time of ovulation.
THE VAGINA
Structure and Function
The vagina is one of the copulatory structures in women and acts as the birth canal (Figs. 43-13 and 43-25). Its mucosa is lined by a nonkeratinized, stratified squamous epithelium. The mucosa has a thick lamina propria enriched with elastic fibers and is well vascularized. There are no glands in the vagina, so lubrication during intercourse comes from (1) cervical mucus (especially with intercourse that occurs midcycle), (2) a transudate (i.e., ultrafiltrate) from the blood vessels of the lamina propria, and (3) the vestibular glands. The mucosa is surrounded by a relatively thin (i.e., relative to the uterus and cervix), two-layered muscularis and an outer connective tissue. The vaginal wall is innervated by branches of the pudendal nerve, which contribute to sexual pleasure and orgasm during intercourse.
Hormonal Regulation during the Menstrual Cycle
The superficial cells of the vaginal epithelium are continually desquamating, and the nature of these cells is influenced by the hormonal environment. Estrogen stimulates proliferation of the vaginal epithelium and increases its glycogen content (referred to as “cornification”—but in humans, true cornification or keratinization does not occur). The glycogen is metabolized to lactic acid by commensal lactobacilli, thereby maintaining an acidic environment. This inhibits infection by noncommensal bacteria and fungi. Progesterone increases the desquamation of epithelial cells.
THE EXTERNAL GENITALIA
Structure and Function
The female external genitalia are surrounded by the labia majora (homologues of the scrotum) laterally and the mons pubis anteriorly (Fig. 43-25). The vulva collectively refers to an area that includes the labia majora and the mons pubis, plus the labia minora, the clitoris, the vestibule of the vagina, the vestibular bulbs (glands), and the external urethral orifice. The vulva is also referred to as the pudendum by clinicians. The structures of the vulva serve the functions of sexual arousal and climax, directing the flow of urine, and partially covering the opening of the vagina, thereby inhibiting the entry of pathogens.
The clitoris is the embryological homologue of the penis and is composed of two corpora cavernosa, which attach the clitoris to the ischiopubic rami, and a glans. These structures are composed of erectile tissue and undergo the process of erection in essentially the same manner as the penis. Unlike the penis, clitoral tissue is completely separate from the urethra. Thus, the clitoris is involved in sexual arousal and climax at orgasm. The vagina is likewise involved in sexual satisfaction but also serves as the copulatory organ and birth canal.
Hormonal Regulation during the Menstrual Cycle
The structures of the vulva do not show marked changes during the menstrual cycle. However, the health and function of these structures are dependent on hormonal support. The external genitalia and vagina are responsive to androgens (testosterone and dihydrotestosterone) and estrogen. Androgens also act on the central nervous system (CNS) to increase libido in women.
BIOLOGY OF ESTRADIOL-17β AND PROGESTERONE
Biological Effects of Estrogen and Progesterone
Estradiol-17β and progesterone fluctuate during the menstrual cycle, and they have multiple effects that can be categorized according to whether they are directly related to the reproductive system or not. Both hormones have profound effects on the ovary, oviduct, uterus, cervix, vagina, and external genitalia and on the hypothalamus and pituitary. Estrogen and progesterone also have important effects on nonreproductive tissues, including the following:
Transport and Metabolism of Ovarian Steroids
Steroid hormones are slightly soluble in blood and are bound to plasma proteins. Approximately 60% of the estrogen is transported bound to sex hormone–binding globulin, 20% is bound to albumin, and 20% is in the free form. Progesterone binds primarily to cortisol-binding globulin (transcortin) and albumin. Because it has relatively low binding affinity for these proteins, its circulating half-life is about 5 minutes.
Although the ovary is the primary site of estrogen production, peripheral aromatization of androgens to estrogens can generate locally high levels of estradiol-17β in some tissues. Peripheral conversion of adrenal and ovarian androgens serves as an important source of estrogen after menopause (see later). The fact that CYP19 (aromatase) is expressed in the breast is the basis for the use of aromatase inhibitors in the treatment of estrogen-dependent breast cancer in postmenopausal women.
Estrogens and progestins are degraded in the liver to inactive metabolites, conjugated with sulfate or glucuronide, and excreted in urine. Major metabolites of estradiol include estrone, estriol, and catecholestrogens (2-hydroxyestrone and 2-methoxyestrone). The major metabolite of progesterone is pregnanediol, which is conjugated with glucuronide and excreted in urine.
ONTOGENY OF THE REPRODUCTIVE SYSTEMS
Unlike most other organ systems, the reproductive systems undergo significant changes in their activity during the life span of a man or woman (Fig. 43-29). Development of the reproductive systems occurs in utero and results in female or male fetuses. After birth and during infancy, the reproductive systems are largely quiescent. At puberty, the hypothalamic-pituitary-gonadal axes “wake up,” and the gonads begin producing sex steroids, which in turn induce the sexually dimorphic changes in appearance and behavior associated with men and women. The reproductive life span of women is set by their ovarian reserve and degree of follicular development (see earlier) and ends at menopause, usually in the fifth decade of life. The loss of estrogen production by the ovaries has a clear clinical impact on many postmenopausal women. Men continue to produce sperm throughout life but can experience a decline in androgen production (andropause), which is associated with its own clinical sequelae.
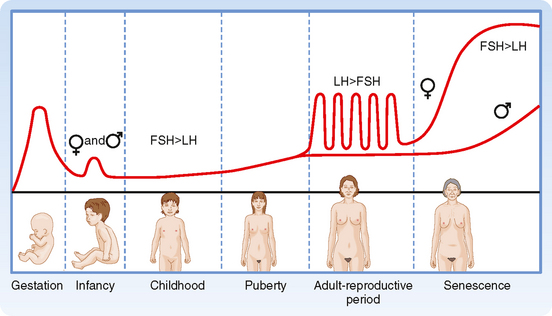
Figure 43-29 Pattern of gonadotropin secretion throughout life. Note the transient peaks during gestation and early infancy and the low levels thereafter in childhood. Women subsequently have monthly cyclic bursts, with luteinizing hormone (LH) exceeding follicle-stimulating hormone (FSH); men do not. Both genders show increased gonadotropin production after 50 years of age, with FSH exceeding LH.
PREGNANCY
The reproductive system of women undergoes dramatic changes during pregnancy. The production of gonadotropin and gonadal steroids is switched from the maternal hypothalamic-pituitary-ovarian axis, which is strongly repressed during pregnancy, to the fetal placenta. Indeed, it is the endocrine function of fetal placental tissue that (1) maintains a quiescent gravid uterus, (2) alters maternal physiology to ensure fetal nutrition in utero, (3) alters maternal pituitary function and mammary gland development to ensure ongoing fetal nutrition after birth, and (4) determines the time of labor and delivery (also called parturition). The placenta also plays an important role in fetal testosterone production and male differentiation of the reproductive system before the fetal hypothalamus and pituitary develop into a functional axis.
Fertilization, Early Embryogenesis, Implantation, and Placentation
Synchronization with Maternal Ovarian and Reproductive Tract Function
Fertilization, early embryogenesis, implantation, and early gestation are all synchronized with the human menstrual cycle (Fig. 43-30). Just before ovulation, the ovary is in the late follicular stage and produces high levels of estrogen. Estrogen promotes growth of the uterine endometrium and induces expression of the progesterone receptor. Estrogen ultimately induces the LH surge, which in turn induces meiotic maturation of the oocyte and ovulation of the cumulus-oocyte complex.
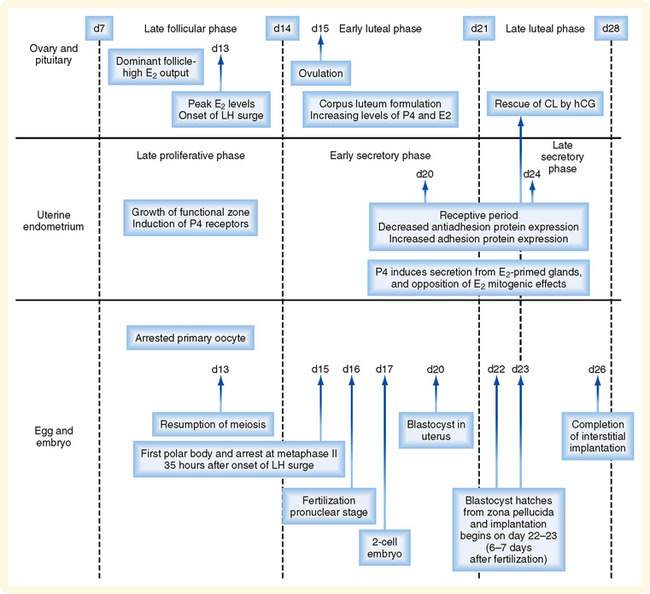
Figure 43-30 Synchronization of events of the menstrual cycle (ovary and endometrium) with fertilization, early development in the oviduct, and implantation in the uterus. E2, estradiol.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
The events between fertilization and implantation take about 6 days to complete, so implantation occurs at about day 22 of the menstrual cycle. At this time the ovary is in the midluteal phase and secreting large amounts of progesterone. Progesterone stimulates secretion from the uterine glands, which provide nutrients to the embryo. This is referred to as histiotropic nutrition and is an important mode of maternal-to-fetal transfer of nutrients for about the first trimester of pregnancy, after which it is replaced by hemotropic nutrition (see later). Progesterone inhibits myometrial contraction and prevents the release of paracrine factors (e.g., cytokines, prostaglandins, chemokines, and vasoconstrictors) that lead to menstruation. Progesterone induces the “window of receptivity” in the uterine endometrium, which exists from about day 20 to day 24 of the menstrual cycle. This receptive phase is associated with increased adhesivity of the endometrial epithelium and involves the formation of cellular extensions, called pinopodes, on the apical surface of endometrial epithelia, along with increased expression of adhesive proteins (e.g., integrins, cadherins) and decreased expression of antiadhesive proteins (e.g., mucins) in the apical cell membrane.
When a fertilized egg implants in the uterus, the uterine endometrium is at its full thickness, is actively secreting, and is capable of tightly adhering to the implanting embryo.
Fertilization
Fertilization accomplishes both recombination of genetic material to form a new, genetically distinct organism and initiation of events that begin embryonic development. Several steps must occur to achieve successful (unassisted) fertilization (Fig. 43-31), including the following:
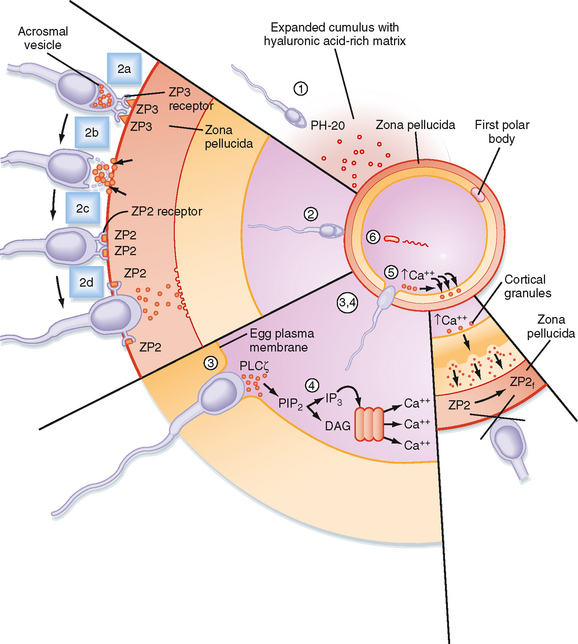
Figure 43-31 Events involved in fertilization (see text for details).
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
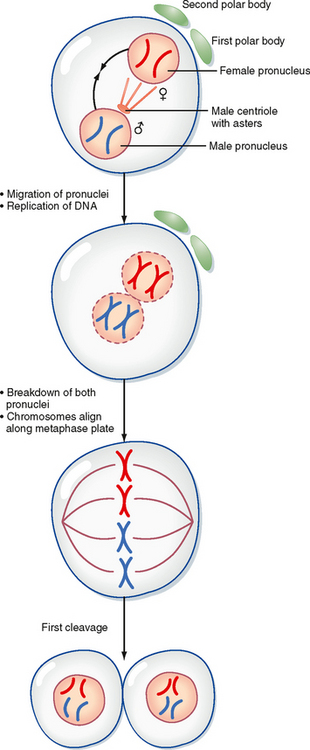
In mammalian eggs, a large initial release of Ca++ is followed by a series of subsequent, smaller Ca++ oscillations that can last for hours. A major consequence of this signaling pathway is that it “wakes up” the metabolically quiescent egg so that it can resume meiosis and begin embryonic development. This process is called egg activation.
The activated egg completes the second meiotic division as the sperm DNA decondenses and a pronucleus forms around it (Fig. 43-32). Once the egg has completed meiosis, a pronucleus forms around the female chromosomes as well. A centrosome, contributed by the sperm, becomes a microtubule organizing center from which microtubules extend until they contact the female pronucleus. The male and female DNA replicate as the two pronuclei are pulled together. Once the pronuclei contact each other, the nuclear membranes break down, the chromosomes align on a common metaphase plate, and the first cleavage occurs.
Early Embryogenesis and Implantation
Fertilization typically occurs on day 16 to 17 of the menstrual cycle, and implantation occurs about 6 days later. Thus, the first week of embryogenesis takes place within the lumens of the oviduct and uterus. For most of this time, the embryo remains encapsulated by the zona pellucida. The first two cleavages take about 2 days, and the embryo reaches a 16-cell morula by 3 days. The outer cells of the morula become tightly adhesive with each other and begin transporting fluid into the embryonic mass. During days 4 and 5, the transport of fluid generates a cavity, called the blastocyst cavity, and the embryo is now called a blastocyst (Fig. 43-33). The blastocyst is composed of two subpopulations of cells: an eccentric inner cell mass and an outer, epithelial-like layer of trophoblasts. The region of the trophoblast layer immediately adjacent to the inner cell mass is referred to as the embryonic pole, and it is this region that attaches to the uterine endometrium at implantation (Fig. 43-33).
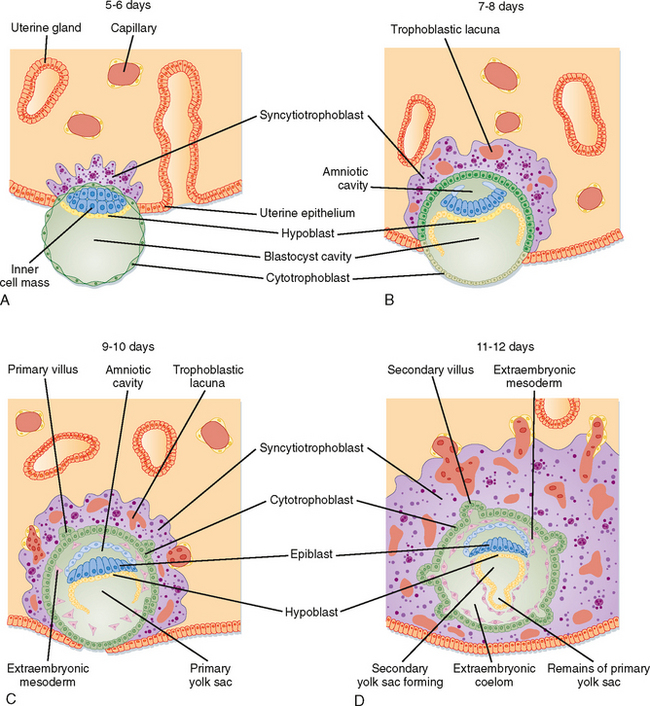
Figure 43-33 Events involved in early embryonic implantation.
(From Carlson BM: Human Embryology and Developmental Biology. Philadelphia, Mosby, 2004.)
The embryo resides within the oviduct during the first 3 days and then enters the uterus. By 5 to 6 days of development, the trophoblasts of the blastocyst secrete proteases that digest the outer-lying zona pellucida. At this point, corresponding to about day 22 of the menstrual cycle, the “hatched” blastocyst is able to adhere to and implant into the receptive uterine endometrium (Fig. 43-33).
At the time of attachment and implantation, the trophoblasts differentiate into two cell types: an inner layer of cytotrophoblasts and an outer layer of multinuclear/multicellular syncytiotrophoblasts (Fig. 43-33). The cytotrophoblasts initially provide a feeder layer of continuously dividing cells. Syncytiotrophoblasts initially perform three general types of function: adhesive, invasive, and endocrine. Syncytiotrophoblasts express adhesive surface proteins (i.e., cadherins and integrins) that bind to uterine surface epithelia and, as the embryo implants, to components of the uterine extracellular matrix. In humans, the embryo completely burrows into the superficial layer of the endometrium (Fig. 43-33). This mode of implantation, called interstitial implantation, is the most invasive among placental mammals. Invasive implantation involves adhesion-supported migration of syncytiotrophoblasts into the endometrium, along with the breakdown of extracellular matrix by the secretion of matrix metalloproteinases and other hydrolytic enzymes.
The endocrine function begins with the onset of implantation, when syncytiotrophoblasts start secreting the LH-like protein human chorionic gonadotropin, which maintains the viability of the corpus luteum and thus progesterone secretion. Syncytiotrophoblasts also become highly steroidogenic. By 10 weeks, the syncytiotrophoblasts acquire the ability to make progesterone at sufficient levels to maintain pregnancy independently of a corpus luteum. Syncytiotrophoblasts produce several other hormones, as well as enzymes that modify hormones.
As implantation and placentation progress, syncytiotrophoblasts take on the important functions of phagocytosis (during histiotropic nutrition) and bidirectional placental transfer of gases, nutrients, and wastes. Exchange across the syncytiotrophoblasts involves diffusion (e.g., gases), facilitated transport (e.g., GLUT1-mediated transfer of glucose), active transport (e.g., amino acids by specific transporters), and pinocytosis/transcytosis (e.g., of iron-transferrin complexes).
Placenta accreta is destruction of the endometrium and adherence of the placenta to the myometrium, a condition associated with potentially life-threatening postpartum hemorrhage. The decidual response occurs only in the uterus. Thus, the highly invasive nature of the human embryo poses considerable risk to the mother in the case of ectopic implantation. Ectopic implantation refers to the implantation of an embryo at a site other than the uterus, and an ectopic pregnancy refers to a developing embryo at a site of ectopic implantation. Most ectopic pregnancies (>90%) occur within the oviducts (called tubal pregnancies), but they can also occur in the ovary and abdominal cavity. Implantation in the oviducts is often associated with long-term infection and inflammation (called pelvic inflammatory disease) and obstruction of the tube. In a tubal pregnancy, the highly invasive nature of the human syncytiotrophoblast, which is normally moderated by the uterine decidual response, usually leads to burrowing of the implanted embryo through the wall of the oviduct. Although abdominal pregnancies can proceed to term, undetected oviductal pregnancies usually lead to rupture of the oviductal wall. The resulting internal hemorrhage can be catastrophic to the mother and requires immediate surgical intervention.
There is also a maternal response to implantation that involves transformation of the endometrial stroma. This response, called decidualization, involves an enlargement of stromal cells as they become lipid- and glycogen-filled decidual cells (at this time, the endometrium is referred to as the decidua). The decidua forms an epithelial-like sheet with adhesive junctions that inhibit migration of the implanting embryo. The decidua also secretes factors, such as tissue inhibitors of metalloproteinases (TIMPs), that moderate the activity of syncytiotrophoblast-derived hydrolytic enzymes in the endometrial matrix. Consequently, decidualization allows regulated invasion during implantation. Normally, the implanting embryo and placenta do not extend to and involve the myometrium.
Placental Endocrinology
Human Chorionic Gonadotropin.
The first hormone produced by syncytiotrophoblasts is hCG. hCG is structurally related to the pituitary glycoprotein hormones (see Chapter 40). As such, hCG is composed of a common α-glycoprotein subunit (α-GSU) and a hormone-specific β subunit (β-hCG). Antibodies used to detect hCG (i.e., in laboratory assays and over-the-counter pregnancy tests) are designed to specifically detect the β subunit. hCG is most similar to LH and binds with high affinity to the LH receptor. The β subunit of hCG is longer than that of LH and contains more sites for glycosylation, which greatly increases the half-life of hCG to 24 to 30 hours. The stability of hCG allows it to rapidly accumulate in the maternal circulation such that hCG is detectable within maternal serum within 24 hours of implantation. Serum hCG levels double every 2 days for the first 6 weeks and peak at about 10 weeks. Serum hCG then declines to a constant level at about 50% of the peak value (Fig. 43-34, A).
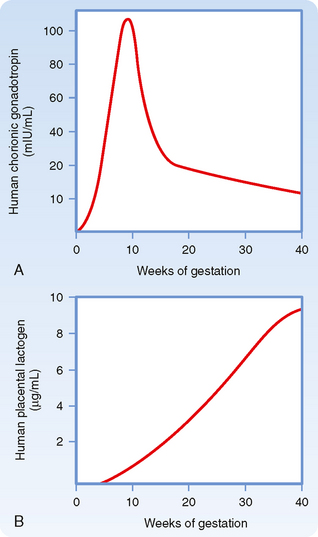
Figure 43-34 Circulating levels of human chorionic gonadotropin and human placental lactogen in maternal blood during pregnancy.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
The primary action of hCG is to stimulate LH receptors on the corpus luteum. This prevents luteolysis and maintains a high level of luteal-derived progesterone production during the first 10 weeks. The rapid increase in hCG is responsible for the nausea of “morning sickness” associated with early pregnancy. A small amount (i.e., 1% to 10%) of hCG enters the fetal circulation. hCG stimulates fetal Leydig cells to produce testosterone before the fetal gonadotropic axis is fully mature. hCG also stimulates the fetal adrenal cortex (see later) during the first trimester.
Progesterone.
The placenta produces a high amount of progesterone, which is absolutely required to maintain a quiescent myometrium and a pregnant uterus. Progesterone production by the placenta is largely unregulated—the placenta produces as much progesterone as the supply of cholesterol and the levels of CYP11A1 and 3β-HSD allow (Fig. 43-35). Notably, placental steroidogenesis differs from that in the adrenal cortex, ovaries, and testis in that cholesterol is transported into the placental mitochondria by a mechanism that is independent of StAR protein. Thus, this first step in steroidogenesis is not a regulated, rate-limiting step in the placenta as it is in other steroidogenic glands. This means that fetuses with an inactivating mutation in StAR protein will develop lipoid congenital adrenal hyperplasia (see Chapter 42) and hypogonadism but will have normal progesterone levels produced by their placenta. Progesterone production by the placenta does not require fetal tissue. Consequently, progesterone levels are largely independent of fetal health and cannot be used as a measure of fetal health. Maternal progesterone levels continue to increase throughout pregnancy.
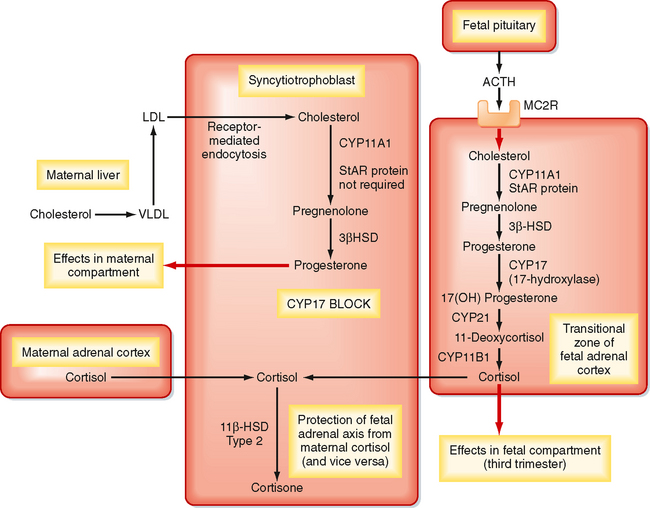
Figure 43-35 Production of progesterone and inactivation of cortisol by syncytiotrophoblast.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
Progesterone is released primarily into the maternal circulation and is required for implantation and maintenance of pregnancy. Progesterone also has several effects on maternal physiology and induces breast growth and differentiation. The switch from corpus luteum–derived progesterone to placental-derived progesterone (referred to as the lutealplacental shift) is complete at about the eighth week of pregnancy. Progesterone (and pregnenolone) are used by the transitional zone of the fetal cortex to make cortisol late in pregnancy.
Estrogen.
Estrogens are also produced by the syncytiotrophoblasts. Syncytiotrophoblasts are similar to ovarian granulosa cells in that they lack CYP17 and are dependent on another cell type to provide 19-carbon androgens for aromatization (Fig. 43-36). The ancillary, androgen-producing cell resides in the fetal adrenal cortex.
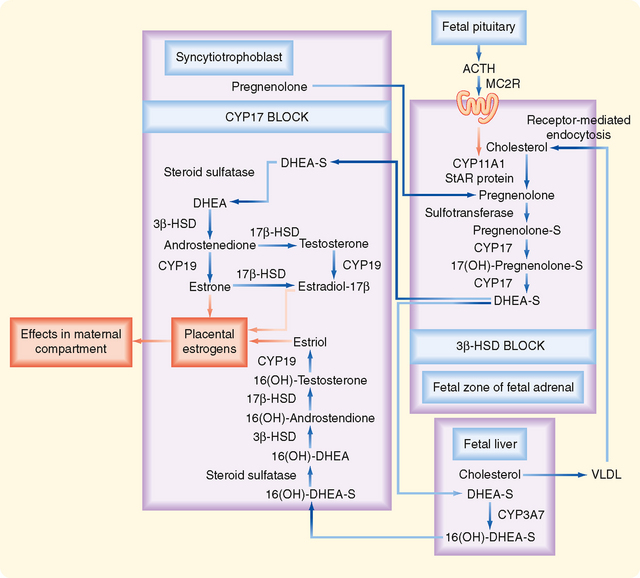
Figure 43-36 Estrogen biosynthesis by the fetoplacental unit. ACTH, adrenocorticotropic hormone; DHEA, dehydroepiandrostenedione; DHEA-S, dehydroepiandrostenedione sulfate; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; MC2R, melanocortin-2 receptor (ACTH receptor); 16(OH)-, 16-hydroxy-; StAR protein, steroidogenic acute regulatory protein; VLDL, very low density lipoproteins.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
The fetal adrenal cortex contains an outer definitive zone, a middle transitional zone, and an inner fetal zone. The definitive and transitional zones give rise to the zona glomerulosa and zona fasciculata, respectively. Aldosterone synthesis is initiated close to parturition. Synthesis of cortisol begins at about 6 months and increases during late gestation. The fetal zone is the predominant portion of the adrenal cortex in the fetus; it constitutes as much as 80% of the bulk of the large fetal adrenal and is the site of most fetal adrenal steroidogenesis. The fetal zone strongly resembles the zona reticularis in that it expresses little or no 3β-HSD (Fig. 43-36). The fetal zone primarily releases the sulfated form of the inactive androgen dehydroepiandrosterone sulfate (DHEAS) throughout most of gestation. Production of DHEAS from the fetal adrenal is absolutely dependent on fetal ACTH from the fetal pituitary by the end of the first trimester.
The DHEAS released from the fetal zone has two fates. First, DHEAS can go directly to the syncytiotrophoblast, where it is desulfated by a placental steroid sulfatase and used as a 19-carbon substrate for the synthesis of estradiol-17β and estrone (Fig. 43-36). The second fate of DHEAS is 16-hydroxylation in the fetal liver by the enzyme CYP3A7. 16-Hydroxyl-DHEAS is then converted by syncytiotrophoblasts to the major estrogen of pregnancy, called estriol (Fig. 43-36).
Maternal estrogen levels increase throughout pregnancy. Because estrogen production is dependent on a healthy fetus, estriol levels can be used to assess fetal health. The collective term used for the placental syncytiotrophoblasts and fetal organs in the context of estrogen production is the fetoplacental unit. Estrogens increase uteroplacental blood flow, enhance LDL receptor expression in syncytiotrophoblasts, and induce several components (e.g., prostaglandins, oxytocin receptors) involved in parturition. Estrogens increase breast growth directly and indirectly through the stimulation of maternal pituitary prolactin production. Estrogens also increase lactotrope size and number, thereby increasing overall pituitary mass by more than twofold by term. Estrogens also affect several aspects of maternal physiology.
Human Placental Lactogen.
Human placental lactogen (hPL), also called human chorionic somatomammotropin (hCS), is a 191–amino acid protein hormone produced in the syncytiotrophoblast that is structurally similar to growth hormone (GH) and prolactin (PRL). Its function overlaps those of both GH and PRL. It can be detected within the syncytiotrophoblast by 10 days after conception and in maternal serum by 3 weeks of gestation (Fig. 43-34). Maternal serum levels rise progressively throughout the remainder of the pregnancy. The quantity of hormone produced is directly related to the size of the placenta such that as the placenta grows during gestation, hPL secretion increases. As much as 1 g/day of hPL can be secreted late in gestation.
Like GH, hPL is protein anabolic and lipolytic. Its antagonistic action to insulin is the major basis for the diabetogenicity of pregnancy. Like PRL, it stimulates mammary gland growth and development. Mammary gland development in pregnancy results from the actions of hPL, PRL, estrogens, and progestins. hPL inhibits maternal glucose uptake and use, thereby increasing serum glucose levels. Glucose is a major energy substrate for the fetus, and hPL increases fetal glucose availability.
As with hCG, far less hPL is found in the fetal circulation than in the maternal circulation. This suggests that the hormones may play a more important role in the mother than in the fetus. hPL is not essential for the pregnancy.
Both hPL and PRL act as fetal growth hormones and stimulate production of the fetal growth-promoting hormones insulin-like growth factor I and II (IGF-I and IGF-II). Ironically, fetal GH does not appear to regulate growth, and anencephalic infants and GH-deficient children typically have normal birth weight.
Diabetogenicity of Pregnancy
Pregnancy represents an insulin-resistant state (Fig. 43-37). During the last half of pregnancy, when hPL levels are highest, maternal energy metabolism shifts from an anabolic state, in which nutrients are stored, to a catabolic state, sometimes described as accelerated starvation, in which maternal energy metabolism shifts toward fat utilization with sparing of glucose. As maternal use of glucose for energy decreases, lipolysis increases and fatty acids become major energy sources. The peripheral responsiveness to insulin decreases and pancreatic insulin secretion increases. Beta cell hyperplasia occurs in pregnancy. Although this does not usually lead to a clinical condition, pregnancy aggravates existing diabetes mellitus, and diabetes can develop for the first time in pregnancy. If the diabetes resolves spontaneously with delivery, the condition is referred to as gestational diabetes. Other hormones contributing to the diabetogenicity of pregnancy are estrogens and progestins because both these hormones decrease insulin sensitivity.
Parturition
Human pregnancy lasts an average of 40 weeks from the beginning of the last menstrual period (gestational age). This corresponds to an average fetal age of 38 weeks. Parturition is the process whereby uterine contractions lead to childbirth. Labor consists of three stages: strong uterine contractions that force the fetus against the cervix, with dilation and thinning of the cervix (several hours); delivery of the fetus (less than 1 hour); and delivery of the placenta, along with contractions of the myometrium to halt bleeding (less than 10 minutes).
Control of parturition in humans is complex, and the exact mechanisms underlying its control are not well understood.
Placental CRH and the Fetal Adrenal Axis
The placenta produces corticotropin-releasing hormone (CRH), which is identical to the 41–amino acid peptide produced by the hypothalamus. Placental CRH production and maternal serum CRH levels increase rapidly during late pregnancy and labor. Moreover, circulating CRH is either in the form of free CRH, which is bioactive, or complexed to a CRH-binding protein. Maternal levels of CRH-binding protein plummet during late pregnancy and labor, so free CRH levels increase. Placental CRH also accumulates in the fetal circulation and stimulates fetal ACTH secretion. ACTH stimulates both fetal adrenal cortisol production and fetoplacental estrogen production. In contrast to the inhibitory effect of cortisol on hypothalamic CRH production, cortisol stimulates placental CRH production. This establishes a self-amplifying positive feedback. CRH itself promotes myometrial contractions by sensitizing the uterus to prostaglandins and oxytocin (see later). Estrogens also directly and indirectly stimulate myometrial contractility. Moreover, this model correlates the onset of parturition with cortisol-induced maturation of fetal systems, including the lungs and gastrointestinal tract.
Estrogen and Progesterone Secretion
Although a rise in maternal serum estrogen and a drop in progesterone levels occur late in gestation in some species, no change in the ratio of the two hormones is seen in human serum. However, “functional” progesterone withdrawal involving changes in uterine progesterone receptor and progesterone metabolism has been proposed.
Oxytocin
Oxytocin is secreted from the pars nervosa of the pituitary gland (see Chapter 40). Oxytocin, which stimulates powerful uterine contractions, plays a major role in parturition. Oxytocin is released in response to stretch of the cervix through a neuroendocrine reflex, and it stimulates uterine contractions and thereby facilitates delivery. Oxytocin can be used to induce parturition, and uterine sensitivity to oxytocin increases before parturition. Because maternal serum oxytocin levels do not increase until after parturition has begun, oxytocin is not thought to initiate parturition. However, progesterone inhibits and estrogen stimulates the synthesis of oxytocin receptors, and although maternal serum progesterone levels do not decrease immediately before human parturition, estrogen levels rise and oxytocin receptor synthesis increases.
Prostaglandins
Prostaglandins and other cytokines increase uterine motility, and levels of these compounds increase during parturition, thereby facilitating delivery. Their exact role in the initiation of parturition is not known. Prostaglandin levels in amnionic fluid, fetal membranes, and uterine decidua increase before the onset of labor. The prostaglandins F2α and E2 increase uterine motility. Large doses of these compounds have been used to induce labor. Because estrogens stimulate prostaglandin synthesis in the uterus, amnion, and chorion, the rising estrogen levels late in gestation can increase uterine prostaglandin formation before parturition.
Uterine Size
Uterine size is thought to be a factor regulating parturition because stretch of smooth muscle, including the uterus, increases muscle contraction. In addition, uterine stretch stimulates uterine prostaglandin production. Multiple births generally occur prematurely. The tendency for early delivery can be a result of increased uterine size, increased fetal production of chemicals stimulating delivery, or both.
MAMMOGENESIS AND LACTATION
Structure of the Mammary Gland
The mammary gland is composed of about 20 lobes, each with an excretory lactiferous duct that opens at the nipple (Fig. 43-38). The lobes, in turn, are composed of several lobules that contain secretory structures called alveoli and the terminal portions of the ducts. The epithelium of the alveoli and ducts is a simple one, except for the presence of a myoepithelial cell layer on the basal side of the epithelium (but apical to the basal lamina). Myoepithelial cells are stellate, smooth muscle–like cells, and contraction of these cells in response to a stimulus (see later) expels milk from the lumens of the alveoli and ducts. Lobes and lobules are supported within a connective tissue matrix. The other major tissue component of the breast is adipose tissue. The lactiferous ducts empty at the nipple, which is a highly innervated, hairless protrusion of the breast designed for suckling by an infant. The nipple is surrounded by a pigmented, hairless areola that is lubricated by sebaceous glands. Protrusion of the nipple, called erection, is mediated by sympathetic stimulation of smooth muscle fibers in response to suckling and other mechanical stimulation, erotic stimulation, and cold.
Hormonal Regulation of Mammary Gland Development
At puberty, estrogen increases ductal growth and branching. With onset of the luteal phases of the ovary, progesterone and estrogen induce ductal growth and the formation of rudimentary alveoli. During nonpregnant cycles, the breasts develop somewhat and then regress. Estrogen also increases the deposition of adipose tissue, which makes a major contribution to the size and overall form of the breasts. Adipose tissue expresses CYP19, so accumulation of this tissue in the breast increases the local production of estrogens from circulating androgens.
Breast development is facilitated by pregnancy, during which extensive ductal growth and branching and lobuloalveolar development occur. The parenchymal growth of the breast during development occurs at the expense of stroma, which is degraded to make room for enlarging lobuloalveolar structures. Several placental hormones stimulate breast development, including estrogen, progesterone, placental lactogen, and a growth hormone variant (GH-V). Estrogen acts on the breast both directly and indirectly through increasing maternal pituitary PRL. Estrogen increases PRL secretion from pituitary lactotropes. Estrogen also stimulates lactotrope hypertrophy and proliferation, which accounts for the twofold increase in pituitary volume during pregnancy in humans. Although epithelial cells express genes encoding milk protein and enzymes involved in milk production, progesterone inhibits the onset of milk production and secretion (lactogenesis).
After parturition, the human breast produces colostrum, which is enriched with antimicrobial and antiinflammatory proteins. In the absence of placental progesterone, normal breast milk production occurs within a few days. The lobuloalveolar structures produce milk, which is subsequently modified by the ductal epithelium. Lactogenesis and maintenance of milk production (galactopoiesis) require stimulation by pituitary PRL in the presence of normal levels of other hormones, including insulin, cortisol, and thyroid hormone. Although placental estrogen stimulates PRL secretion during pregnancy, the stimulus for PRL secretion during the nursing period is suckling by the infant (Fig. 43-39). Levels of PRL are directly correlated with the frequency and duration of suckling at the nipple. The link between suckling at the nipple and PRL secretion involves a neuroendocrine reflex in which dopamine secretion at the median eminence is inhibited (the PRL release inhibitory factor; see Chapter 40). It is also possible that suckling increases the secretion of unidentified PRL-releasing hormones.

Figure 43-39 Neuroendocrine reflex caused by suckling at the nipple and leading to secretion of oxytocin and prolactin. In turn, these hormones induce continued milk production (galactopoiesis) and milk let-down. Prolactin also induces lactational amenorrhea.
(Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.)
PRL also inhibits release of GnRH, and consequently, nursing can be associated with lactational amenorrhea (Fig. 43-39). This effect of prolactin has been called “nature’s contraceptive,” and it may play a role in spacing out pregnancies. However, only regular nursing over a 24-hour period is sufficient to induce a PRL-induced anovulatory state in the mother. Thus, lactational amenorrhea is not an effective or reliable form of birth control for most women. The inhibition of GnRH by high levels of PRL is important clinically. A prolactinoma is the most common form of hormone-secreting pituitary tumor, and hyperprolactinemia is a significant cause of infertility in both sexes. Hyperprolactinemia can likewise be associated with galactorrhea, or the inappropriate flow of breast milk, in men and women.
Suckling at the nipple also stimulates the release of oxytocin from the pars nervosa (see Chapter 40) through a neuroendocrine reflex (Fig. 43-39). Contraction of myoepithelial cells induces milk let-down, or expulsion of milk from the alveolar and ductal lumens. Thus, the nursing infant does not gain milk by applying negative pressure to the breast from suckling. Rather, milk is actively ejected through a neuroendocrine reflex. Oxytocin release and milk let-down can be induced by psychogenic stimuli, such as the mother hearing a baby crying on television or thinking about her baby. Such psychogenic stimuli do not affect PRL release.
There are multiple behavioral methods of contraception. Total abstinence is the best way to avoid getting pregnant. Other methods include the rhythm method, which relies on abstinence from sexual intercourse during fertile periods around the time of ovulation. The fertile period extends from 3 to 4 days before the time of ovulation until 3 to 4 days afterward. A second method is withdrawal before ejaculation, coitus interruptus. Both these methods have higher failure rates (20% to 30%) than barrier methods (2% to 12%), intrauterine devices (IUDs) (<2%), and oral contraceptives (<1%) do. Barriers such as condoms or diaphragms are more effective when used with spermicidal jellies. Of all methods, only condoms provide effective protection from sexually transmitted diseases in sexually active individuals. IUDs are relatively effective. They prevent implantation by locally producing an inflammatory response in the endometrium. Some forms of IUDs contain copper, zinc, or progestins, which inhibit sperm transport or viability in the female reproductive tract.
Oral contraceptives have been marketed in the United States since the early 1960s. The doses of steroids used today are many-fold lower than those used 35 years ago. Properly used, oral contraceptives have a low failure rate. Many forms of oral contraceptives are marketed today. The trend over the years has been to decrease the dosage of steroids used because the side effects are dose dependent. All oral steroidal contraceptives contain either a combination of an estrogen and a progestin or a progestin alone. Oral contraceptives work through multiple mechanisms. Most block the LH surge that triggers ovulation. However, some pills, such as the progestin-only mini-pill, do not prevent LH surges. Fertility is also blocked by changing the nature of cervical mucus, by altering endometrial development, and by regulating fallopian tube motility. Because these contraceptives suppress FSH, they impair early follicular development.
Emergency contraception involves hormonal treatment designed to inhibit or delay ovulation, inhibit corpus luteum function, disrupt the function of the oviducts and uterus, or any combination of these mechanisms. For example, candidates for emergency contraception include women who are sexually assaulted or who experienced failure of a barrier method (e.g., ruptured condom). There are more than 20 types of commercially available “morning after” pills. The currently preferred medication is levonorgestrel (Plan B), which is a synthetic progestin-only pill. The efficacy of the pill is inversely correlated with the time that it is taken after intercourse. The exact mechanism of action is not known. Treatment has no effect if implantation has occurred.
Medical (hormonal) termination of pregnancy (abortion) can be achieved up to 49 days of gestation by the administration of mifepristone (RU-486), a progesterone receptor antagonist that induces collapse of the pregnant endometrium. Mifepristone is followed 48 hours later by the ingestion or vaginal insertion of a synthetic prostaglandin E (e.g., misoprostol), which induces myometrial contractions.
MENOPAUSE
Though related to the depletion of ovarian follicles, the causes and process of menopause are poorly understood. Age-related changes in the CNS, including critical patterns of GnRH secretion, precede follicular depletion and may play an important role in menopause. Because follicles do not develop in response to LH and FSH secretion, estrogen and progesterone levels drop. Loss of the negative-feedback inhibition of estrogen on GnRH and LH/FSH results in a marked rise in serum LH and FSH. FSH levels rise more than LH levels. This could result from loss of ovarian inhibin.
Menopause typically occurs between 45 and 55 years of age. It extends over a period of several years. Initially, the cycles become irregular and are periodically anovulatory. The cycles tend to shorten, primarily in the follicular phase. Eventually, the woman ceases to cycle altogether. Serum estradiol levels drop to about a sixth the mean levels for younger cycling women, and progesterone levels drop to about a third those in the follicular phase of younger women. Production of these hormones does not cease entirely, but the primary source of these hormones in postmenopausal women becomes the adrenal, although interstitial cells of the ovarian stroma continue to produce some steroids. Most circulating estrogens are now produced peripherally from androgens. Because estrone is the primary estrogen produced in adipose tissue, it becomes the predominant estrogen in postmenopausal women.
Most of the symptoms associated with menopause result from estrogen deficiency. The vaginal epithelium atrophies and becomes dry, and bone loss is accelerated and may lead to osteoporosis. The incidence of coronary artery disease increases markedly after menopause. Hot flashes result from periodic increases in core temperature, which produces peripheral vasodilation and sweating. Hot flashes are thought to be linked to increases in LH release and are probably associated not with the pulsatile rise in LH secretion but rather with central mechanisms controlling GnRH release. Hot flashes typically subside within 1 to 5 years of the onset of menopausal symptoms.