Definitions
Dry eye occurs when there is inadequate tear volume or function resulting in an unstable tear film and ocular surface disease.
1
Keratoconjunctivitis sicca (KCS) refers to any eye with some degree of dryness.
2
Xerophthalmia describes a dry eye associated with vitamin A deficiency.
3
Xerosis refers to the extreme ocular dryness and keratinization that occurs in eyes with severe conjunctival cicatrization.
4
Sjögren syndrome is an autoimmune inflammatory disease of which dry eyes is a typical feature.
Physiology
Tear film constituents
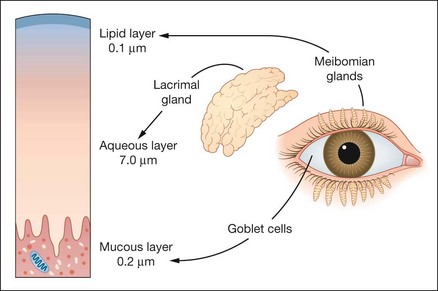
The tear film has three layers (Fig. 4.1):
1
Lipid layer secreted by the meibomian glands.
2
Aqueous layer secreted by the lacrimal glands.
3
Mucous layer secreted principally by conjunctival goblet cells.
Spread of the tear film
The tear film is mechanically spread over the ocular surface through a neuronally-controlled blinking mechanism. Three factors are required for effective resurfacing of the tear film:
•
Contact between the external ocular surface and the eyelids.
•
Normal corneal epithelium.
Outer lipid layer
1
Composition
•
The outer lipid layer is composed of a polar phase containing phospholipids adjacent to the aqueous-mucin phase and a non-polar phase containing waxes, cholesterol esters and triglycerides.
•
The polar lipids are bound to lipocalins within the aqueous layer. These are small secreted proteins that have the ability to bind hydrophobic molecules and may also contribute to tear viscosity.
•
Lid movement during blinking is important in releasing lipids from glands. The thickness of the layer can be increased by forced blinking, and conversely reduced by infrequent blinking.
2
Functions
•
To prevent evaporation of the aqueous layer and maintain tear film thickness.
•
To act as a surfactant allowing spread of the tear film.
•
Deficiency results in evaporative dry eye.
Middle aqueous layer
1
Secretion
•
The main lacrimal glands produce about 95% of the aqueous component of tears and the accessory lacrimal glands of Krause and Wolfring produce the remainder.
•
Secretion of tears has basic (resting) and much greater reflex components. The latter occurs in response to corneal and conjunctival sensory stimulation, tear break-up and ocular inflammation and is mediated via the 5th cranial nerve. It is reduced by topical anaesthesia and during sleep. Secretion can increase 500% in response to injury.
2
Composition
•
Water, electrolytes, dissolved mucins and proteins.
•
Growth factors derived from the lacrimal gland, the production of which increases in response to injury.
•
Pro-inflammatory interleukin cytokines which accumulate during sleep when tear production is reduced.
3
Functions
•
To provide atmospheric oxygen to the corneal epithelium.
•
Antibacterial activity due to proteins such as IgA, lysozyme and lactoferrin.
•
To wash away debris and noxious stimuli and allow the passage of leucocytes after injury.
•
To provide a smooth optical surface to the cornea by abolishing minute irregularities.
Inner mucous layer
1
Composition
•
Mucins are high molecular weight glycoproteins that may be transmembrane or secretory.
•
Secretory mucins are further classified as gel- forming or soluble. They are principally produced by the conjunctival goblet cells but also the lacrimal glands.
•
The superficial epithelial cells of the cornea and conjunctiva produce transmembrane mucins that form their glycocalyx (extracellular coating).
•
Epithelial staining with rose bengal indicates that the transmembrane and gel mucous layers are absent and the cell surface exposed. Damage to the epithelial cells will prevent normal tear film adherence.
2
Functions
•
To permit wetting by converting the corneal epithelium from a hydrophobic to a hydrophilic surface.
Deficiency of the mucous layer may be a feature of both aqueous deficiency and evaporative states. Goblet cell loss is associated with cicatrizing conjunctivitis, vitamin A deficiency, chemical burns and toxicity from medications.
Regulation of tear film components
1
Hormonal
•
Androgens are the prime hormones that regulate lipid production.
•
Oestrogens and progesterone receptors in the conjunctiva and the lacrimal glands are essential for normal function of these tissues.
2
Neural fibres adjacent to the lacrimal glands and goblet cells result in aqueous and mucus secretion.
Mechanism of disease
Inflammation in the conjunctiva and accessory glands is present in 80% of patients with KCS and may be the cause and consequence of dry eye, amplifying and perpetuating disease. The presence of inflammation is the rationale for steroid therapy. Hyperosmolarity of tears is also a key mechanism of disease and may be the major pathway for epithelial cell damage.
Classification
The following classification is usually applied although most individuals have considerable overlap between mechanisms (Table 4.1). The causes are shown in Tables 4.2 and 4.3.
Table 4.1 Classification of KCS
1 Aqueous layer deficiency
2 Evaporative
• Meibomian gland disease • Contact lens-associated |
Table 4.2 Causes of non-Sjögren KCS
1 Primary age-related hyposecretion is the most common 2 Lacrimal tissue destruction
• Inflammation (e.g. pseudotumour or sarcoidosis) 3 Absence or reduction of lacrimal gland tissue
4 Conjunctival scarring with obstruction of lacrimal gland ductules
• Stevens–Johnson syndrome 5 Neurological lesions with sensory or motor reflex loss
• Familial dysautonomia (Riley-Day syndrome) • Reduced sensation may also contribute to dry eye after refractive surgery and contact lens wear |
Table 4.3 Causes of evaporative KCS
1 Meibomian gland dysfunction
• Atopic keratoconjunctivitis • Congenital meibomian gland absence 2 Lagophthalmos
• Following blepharoplasty 3 Miscellaneous
• Environmental factors such as air conditioning |
Sjögren syndrome
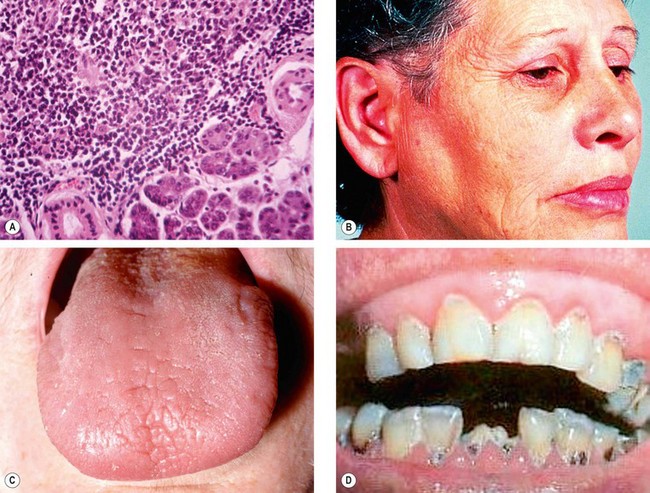
Sjögren syndrome is characterized by autoimmune inflammation and destruction of lacrimal and salivary glands (Fig. 4.2A). The condition is classified as primary when it exists in isolation, and secondary when associated with other diseases such as rheumatoid arthritis, SLE, systemic sclerosis, mixed connective tissue disease, primary biliary cirrhosis, chronic active hepatitis and myasthenia gravis. Primary Sjögren syndrome affects females more commonly than males and is characterized by the following:
1
Presentation is in adult life with grittiness of the eyes and dryness of the mouth.
2
Signs
•
Enlargement of salivary glands (
Fig. 4.2B) and occasionally lacrimal glands, with secondary diminished salivary flow rate and a dry fissured tongue (
Fig. 4.2C).
•
Dry nasal passages, diminished vaginal secretions and resultant dyspareunia.
•
Raynaud phenomenon and cutaneous vasculitis.
•
Arthralgia, myalgia and fatigue may be present.
3
Complications
•
Dental caries in severe untreated cases (
Fig. 4.2D).
•
Reflux oesophagitis and gastritis.
•
Malabsorption due to pancreatic failure.
•
Pulmonary disease, renal disease and polyneuropathy.
4
Diagnostic tests include serum autoantibodies, Schirmer test and biopsy of minor salivary glands.
5
Treatment options include a range of symptomatic treatments, salivary stimulants and immunosuppression with systemic steroids and cytotoxic agents.
Clinical features
Symptoms
The most common ocular symptoms are feelings of dryness, grittiness and burning that characteristically worsen during the day. Stringy discharge, transient blurring of vision, redness and crusting of the lids are also common.
Lack of emotional or reflex tearing is, however, uncommon. The symptoms of KCS are frequently exacerbated on exposure to conditions associated with increased tear evaporation (e.g. air-conditioning, wind and central heating) or prolonged reading, when blink frequency is reduced.
Signs
1
Posterior blepharitis and meibomian gland dysfunction may be present.
2
Conjunctiva may show mild keratinization and redness.
3
Tear film
•
In the normal eye, as the tear film breaks down, the mucin layer becomes contaminated with lipid but is washed away.
•
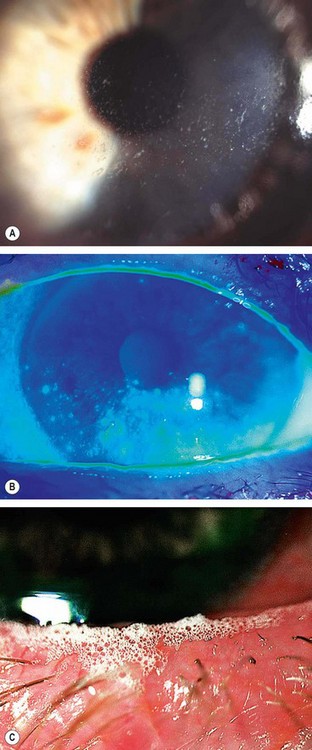
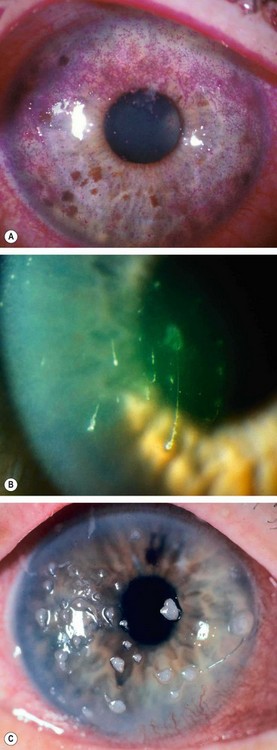
In the dry eye, the lipid-contaminated mucin accumulates in the tear film as particles and debris that move with each blink (
Fig. 4.3A).
•
The marginal tear meniscus is a crude measure of the volume of aqueous in the tear film. In the normal eye the meniscus is about 1 mm in height, while in dry eye it becomes thin (
Fig. 4.3B) or absent.
•
Froth in the tear film or along the eyelid margin occurs in meibomian gland dysfunction (
Fig. 4.3C).
4
Cornea
•
Punctate epithelial erosions that stain with fluorescein (
Fig. 4.4A).
•
Filaments consist of mucus strands lined with epithelium attached at one end to the corneal surface (
Fig. 4.4B) and stain well with rose bengal.
•
Mucous plaques consist of semi-transparent, white-to-grey, slightly elevated lesions of various sizes (
Fig. 4.4C). They are composed of mucus, epithelial cells and proteinaceous and lipoidal material and are usually seen in association with corneal filaments.
5
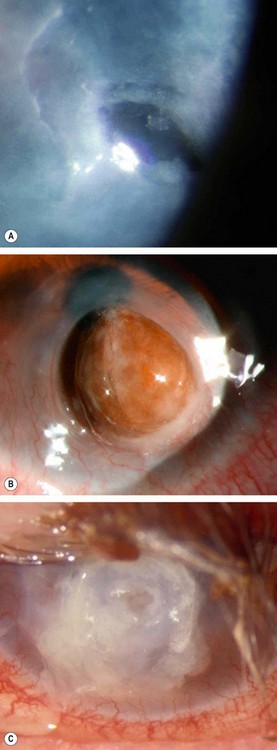
Complications which are rare but may develop in very severe cases include peripheral superficial corneal neovascularization, epithelial breakdown, melting (
Fig. 4.5A), perforation (
Fig. 4.5B) and bacterial keratitis (
Fig. 4.5C).
Special investigations
The aim of investigation is to confirm and quantify the diagnosis of dry eye. Unfortunately, although the repeatability of symptoms is good, that of clinical tests is poor, as is the correlation between symptoms and tests. The reliability of tests improves as the severity of dry eye increases. The tests measure the following parameters:
•
Stability of the tear film (break-up time, BUT).
•
Tear production (Schirmer, fluorescein clearance and tear osmolarity).
•
Ocular surface disease (corneal stains and impression cytology).
There is no clinical test to confirm the diagnosis of evaporative dry eye. It is therefore a presumptive diagnosis based on the presence of meibomian gland disease. Tarsal transillumination to visualize the meibomian glands can give an indication of gland drop-out. It is suggested the tests are performed in the following order because the Schirmer strip paper can damage the ocular surface and cause staining.
Tear film break-up time
The tear film BUT is abnormal in aqueous tear deficiency and meibomian gland disorders. It is measured as follows:
a
Fluorescein 2% or an impregnated fluorescein strip moistened with non-preserved saline is instilled into the lower fornix.
b
The patient is asked to blink several times.
c
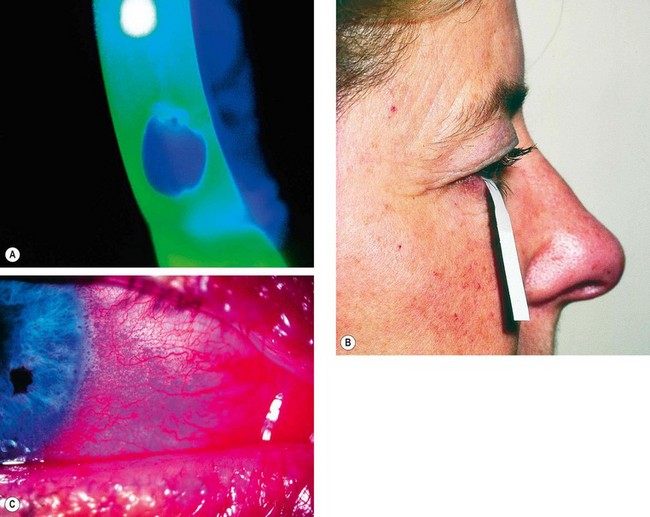
The tear film is examined at the slit-lamp with a broad beam using the cobalt blue filter. After an interval, black spots or lines appear in the fluorescein-stained film, indicating the formation of dry areas (
Fig. 4.6A).
d
The BUT is the interval between the last blink and the appearance of the first randomly distributed dry spot. A BUT of less than 10 seconds is abnormal.
The development of dry spots always in the same location may indicate a local corneal surface abnormality (e.g. epithelial basement membrane disease) rather than an intrinsic instability of the tear film.
Schirmer test
The Schirmer test is a useful assessment of aqueous tear production. The test involves measuring the amount of wetting of a special (no. 41 Whatman) filter paper, 5 mm wide and 35 mm long. The test can be performed with or without topical anaesthesia. In theory, when performed with an anaesthetic (Schirmer 2) it measures basic secretion whereas without anaesthetic (Schirmer 1) it measures maximum basic and reflex secretion. In practice, however, topical anaesthesia cannot abolish all sensory and psychological stimuli for reflex secretion. The test is performed as follows:
a
The eye is gently dried of excess tears. If topical anaesthesia is applied the excess should be removed from the inferior fornix with filter paper.
b
The filter paper is folded 5 mm from one end and inserted at the junction of the middle and outer third of the lower lid, taking care not to touch the cornea or lashes (
Fig. 4.6B).
c
The patient is asked to keep the eyes gently closed.
d
After 5 minutes the filter paper is removed and the amount of wetting from the fold measured.
e
Less than 10 mm of wetting after 5 minutes without anaesthesia and less than 6 mm with anaesthesia is considered abnormal.
Results can be variable and a single Schirmer test should not be used as the sole criterion for diagnosing dry eye, but repeatedly abnormal tests are highly supportive.
Ocular surface staining
1
Fluorescein stains corneal and conjunctival epithelium where there is sufficient damage to allow the dye to enter the tissues.
2
Rose bengal is a dye that has an affinity for dead or devitalized epithelial cells that have a lost or altered mucous layer. Corneal filaments and plaques are also shown up more clearly by the dye and the use of a red-free filter may help visualization. A 1% solution of rose bengal or a moistened impregnated strip can be used. Rose bengal may cause intense stinging that can last for up to a day, particularly in patients with severe KCS. To minimize irritation a very small drop should be used, immediately preceded by a drop of topical anaesthetic, and the excess washed out with saline.
3
The pattern of staining may aid diagnosis as follows:
•
Interpalpebral staining of the cornea and conjunctiva is common in aqueous tear deficiency (
Fig. 4.6D).
•
Superior conjunctival stain may indicate superior limbic keratoconjunctivitis.
•
Inferior corneal and conjunctival stain is often seen in patients with blepharitis or exposure.
Other tests
The following tests are rarely performed in clinical practice.
1
Fluorescein clearance test and the tear function index may be assessed by placing 5 µl of fluorescein on the ocular surface and measuring the residual dye in a Schirmer strip placed on the lower lateral lid margin at intervals of 1, 10, 20 and 30 minutes. The presence of fluorescein on each strip is examined under blue light and compared to a standard scale or measured using fluorophotometry. In normal eyes the value will have fallen to zero after 20 minutes. Delayed clearance is observed in all dry eye states.
2
Lactoferrin is the major protein secreted by the lacrimal gland. Tear lactoferrin is decreased in Sjögren syndrome and other lacrimal gland diseases. Commercially available immunoassay kits are available to measure lactoferrin in body fluids.
3
Phenol red thread test uses a thread impregnated with a pH sensitive dye. The end of the thread is placed over the lower lid and the length wetted (the dye changes from yellow to red in tears) is measured after 15 seconds. A value of 6 mm is abnormal. It is comparable to Schirmer test but takes less time.
4
Tear meniscometry is a technique to quantify the height and thus the volume of the lower lid meniscus.
5
Tear film osmolarity measurement techniques are available as research tools.
6
Impression cytology to determine goblet cell numbers.
Treatment
Dry eye is generally not curable and management is therefore structured around the control of symptoms and prevention of surface damage. The choice of treatment depends on the severity of the disease and involves one or more of the following measures alone or in combination.
Patient education
•
Establishment of a realistic expectation of outcome and emphasis on the importance of compliance.
•
Avoidance of toxic drugs or environmental factors and discontinuation of toxic topical medication if possible.
•
Review of work environment.
•
Emphasis on the importance of blinking whist reading or using a VDU.
•
Aids should be provided for patients with a loss of dexterity (e.g. rheumatoid arthritis). Plastic dropper bottles can be held in a nut-cracker as small unit dispensers may be very difficult to use.
•
Caution against laser refractive surgery.
•
Discussion of management of contact lens intolerance.
Tear substitutes
Tear substitutes have a relatively simple formulation that cannot approximate the complex number of components and structure of the normal tear film. Their delivery is also periodic rather than continuous. Almost all are based on replacement of the aqueous phase of the tear film. There are no mucus substitutes, and paraffin is only an approximation to the action of tear lipids.
1
Drops and gels
•
Cellulose derivatives (e.g. hypromellose) are appropriate for mild cases.
•
Carbomers (e.g. Viscotears
®, GelTears
®) adhere to the ocular surface and so are longer lasting.
•
Polyvinyl alcohol (e.g. Hypotears
®, Liquifilm
®)increases the persistence of the tear film and is useful in mucin deficiency.
•
Sodium hyaluronate (e.g. Vismed
®) may be useful in promoting conjunctival and corneal epithelial healing.
•
Autologous serum may be used in very severe cases.
•
Povidone and sodium chloride.
2
Ointments containing petrolatum mineral oil can be used at bedtime as daytime use is precluded by marked blurring.
Preservatives are a potential source of toxicity, especially after punctal occlusion. Non-preserved drops should therefore be used whenever possible.
Mucolytic agents
Acetylcysteine 5% drops (Ilube®) q.i.d. may be useful in patients with corneal filaments and mucous plaques. It may cause irritation following instillation. Acetylcysteine is also malodorous and has a limited bottle life so that it can only be used for up to 2 weeks. Debridement of filaments may also be useful.
Punctal occlusion
Punctal occlusion reduces drainage and thereby preserves natural tears and prolongs the effect of artificial tears. It is of greatest value in patients with moderate to severe KCS who have not responded to frequent use of topical treatment.
1
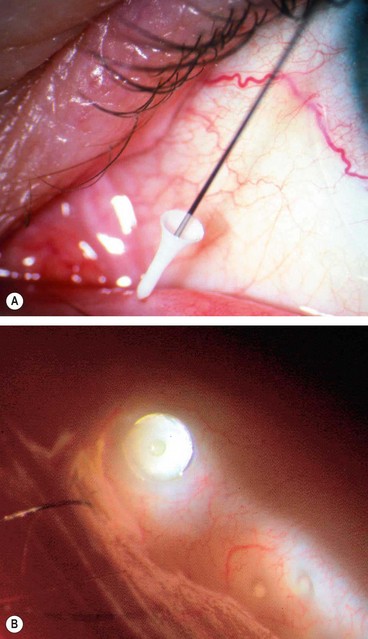
Temporary occlusion can be achieved by inserting collagen plugs into the canaliculi; these dissolve in 1–2 weeks. The main aim is to ensure that epiphora does not occur following permanent occlusion.
•
Initially the inferior puncta are occluded and the patient is reviewed after 1 or 2 weeks.
•
If the patient is now asymptomatic and without epiphora, the plugs can be removed and the inferior canaliculi permanently occluded (see below).
•
In severe KCS both the inferior and superior canaliculi can be plugged.
2
Reversible prolonged occlusion can be achieved with silicone (
Fig. 4.7) or long-acting (2–6 months) collagen plugs.
•
Problems include extrusion, granuloma formation and distal migration.
•
Plugs that pass into the horizontal portion of the canaliculus cannot be visualized and although they can usually be flushed out with saline, if they cause epiphora this is not always possible.
3
Permanent occlusion should be undertaken only in patients with severe dry eye with repeated Schirmer test values of 5 mm or less, and who have had a positive response to temporary plugs without epiphora. It should not be performed if possible in young patients who may have reversible pathology. All four puncta should not be occluded at the same time.
•
Permanent occlusion is performed following punctal dilatation by coagulating the proximal canaliculus with cautery; following successful occlusion, it is important to watch for signs of recanalization.
•
Diode laser cautery is less effective than thermal coagulation, with higher rates of recanalization.
Anti-inflammatory agents
1
Low dose topical steroids are effective supplementary treatment for acute exacerbations. The risks of long-term treatment must be balanced against the potential benefits of increased comfort.
2
Topical ciclosporin (0.05%, 0.1%) reduces T-cell mediated inflammation of lacrimal tissue, resulting in an increase in the number of goblet cells and reversal of squamous metaplasia of the conjunctiva.
3
Systemic tetracyclines may control associated blepharitis and reduce inflammatory mediators in the tears.
Contact lenses
Although long-term contact lens wear may increase tear film evaporation, reduce tear flow and increase the risk of infection, these effects can be outweighed by the reservoir effect of fluid trapped behind the lens.
1
Low water content HEMA lenses may be successfully fitted to moderately dry eyes.
2
Silicone rubber lenses that contain no water and readily transmit oxygen are effective in protecting the
cornea in extreme tear film deficiency, although deposition of debris on the surface of the lens can blur vision and be problematic. The continued availability of these lenses is in doubt.
3
Occlusive gas permeable scleral contact lenses provide a reservoir of saline over the cornea. They can be worn on an extremely dry eye with exposure.
Conservation of existing tears
1
Reduction of room temperature to minimize evaporation of tears.
2
Room humidifiers may be tried but are frequently disappointing because much apparatus is incapable of significantly increasing the relative humidity of an average-sized room. A temporary local increase in humidity can be achieved with moist chamber goggles or side shields to glasses but they may be cosmetically unacceptable.
Other options
1
Tarsorrhaphy diminishes surface evaporation by reducing the size of the palpebral aperture.
2
Botulinum toxin injection to the orbicularis muscle may help control the blepharospasm in severe dry eye. Injected at the medial canthus it reduces tear drainage, presumably by blocking lid movement.
3
Oral cholinergic agonists such as pilocarpine (5 mg q.i.d.) may reduce the symptoms of dry eye and dry mouth in patients with Sjögren syndrome. However, they may also cause blurred vision and intolerable sweating.
4
Zidovudine, an anti-retroviral agent, may be beneficial in primary Sjögren syndrome.
5
Submandibular gland transplantation for extreme dry eye requires extensive surgery and tends to produce unacceptable levels of mucus in the tear film.