CHAPTER 26 Smoking and Periodontal Disease
The Smoking Epidemic
Smoking is highly prevalent and can be considered an epidemic in both developed and developing nations. In the United States (US) in 1993, 25.4% of the population smoked, 27.0% of men and 24.0% of women.1 By 2007, this had decreased to 20.8% of the total population, 23.9% of men and 18.0% of women (http://www.cdc.gov/tobacco). Among dentate individuals during the period 1988 to 1994, 27.9% of adults were current smokers and 23.3% were former smokers.2 Smoking was higher in younger age groups (≤34 years) than in older age groups (≥55 years) and was more common in males (30.9%) compared to females (25.1%).2 In 2009, roughly 43.4 million Americans were smokers with 443,000 deaths in the US attributable to smoking each year (http://www.cdc.gov/tobacco). In the European Union, approximately 29.0% of the population are smokers, ranging from 16% in Sweden to 48% in Greece, and smoking accounts for 650,000 deaths each year (or 1 in 7 of all deaths) (http://www.ash.org.uk ). Furthermore, 79,000 nonsmoking Europeans die each year as a result of passive smoking. Worldwide, it is estimated that there are >1.3 billion smokers (around 1 billion men and 300 million women), with just over 80% living in low- and middle-income countries. Globally, smoking accounts for 1 in 5 deaths among men over 30 and 1 in 20 deaths among women over 30.
Smoking is harmful to almost every organ in the body and is associated with multiple diseases reducing the expectancy and quality of life. Diseases associated with smoking include lung cancer, heart disease, stroke, emphysema, bronchitis, and cancers of the oral cavity, bladder, kidney, stomach, liver, and cervix. Approximately half of long-term smokers will die early on account of smoking, and those who die before the age of 70 will lose on average around 20 years of life.3 Most deaths from smoking are due to lung cancer, chronic obstructive pulmonary disease, and coronary heart disease.
Tobacco smoke contains thousands of noxious chemicals, and comprises a gaseous phase and a solid (particulate) phase. The gas phase contains carbon monoxide, ammonia, formaldehyde, hydrogen cyanide, and many other toxic and irritant compounds, including more than 60 known carcinogens such as benzo(a)pyrene and dimethylnitrosamine. The particulate phase includes nicotine, “tar” (itself made up of many toxic chemicals), benzene, and benzo(a)pyrene. Tar is inhaled with the smoke and in its condensate form, is the sticky brown substance that stains fingers and teeth yellow/brown. Nicotine, an alkaloid, is found within the tobacco leaf and evaporates when the cigarette is lighted. It is quickly absorbed in the lung and reaches the brain within 10 to 19 seconds. Nicotine is highly addictive and causes a rise in blood pressure, increased heart rate and respiratory rate, and peripheral vasoconstriction.
All dental patients must be asked about their smoking status. Current smoking status is the minimal information that must be recorded (e.g., patient is currently smoking X cigarettes per day), but the importance of cumulative exposure to cigarette smoke mandates that it is more appropriate to record pack-years of smoking (Box 26-1). Biochemical tests can also be used to assess smoking status, including exhaled carbon monoxide (CO) and measurement of cotinine (the major metabolite of nicotine) in serum, saliva, or urine. Cotinine is measured in preference to nicotine as the half-life of nicotine is short (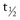 ≈ 1 to 2 hours),4 whereas that of cotinine is approximately 20 hours.5 Plasma and saliva cotinine concentration in smokers is approximately 300 ng/ml and the urine concentration is approximately 1500 ng/ml. Nonsmokers usually have plasma and saliva concentrations <2 ng/ml (unless they are passive smokers).
≈ 1 to 2 hours),4 whereas that of cotinine is approximately 20 hours.5 Plasma and saliva cotinine concentration in smokers is approximately 300 ng/ml and the urine concentration is approximately 1500 ng/ml. Nonsmokers usually have plasma and saliva concentrations <2 ng/ml (unless they are passive smokers).
BOX 26-1 The Challenge of Assessing Smoking Status
Current Smokers
Ask about current smoking and past smoking. Many smokers are trying to quit and therefore simply asking how many cigarettes they are smoking today may not give an accurate assessment of their lifetime exposure (e.g., a patient currently smoking 5 cigarettes per day may have been smoking 40 cigarettes per day until yesterday when they decided to cut down). To get around this problem, pack-years should be calculated as follows:
1 pack-year is the cumulative exposure that corresponds to smoking 1 pack of 20 cigarettes per day for 1 year. For example, a smoker who has smoked 20 cigarettes per day for 15 years has 15 pack-years of smoking.
Former Smokers
Ask the patient about past smoking. A patient with periodontitis may have a significant smoking history that has had an impact on their periodontal status, even though they may no longer smoke. Former smokers should always be congratulated on their achievement in quitting, but it is also very important to document the following:
Is the patient’s response accurate? Inaccurate or false reporting of smoking status is common (e.g., because the patient tells you what they think you want to hear or is embarrassed because they have not managed to cut down yet). Many patients report smoking 20 per day, because this is the number in a pack in most countries, so 20 may be a convenient response rather than an accurate response. Cultural factors may also influence responses.94
When is a smoker not a smoker?
It must be noted that many periodontal research studies have not used such definitions, however, and this can become particularly problematic in the context of what constitutes a former smoker. For example, from an exposure point of view, there is a big difference between someone who smoked 5 cigarettes per day for 10 years and quit 30 years ago compared to someone who smoked 40 cigarettes per day for 20 years and quit 6 months ago. It is always best in clinical practice to gather full information about the smoking history.
Smoking is the major risk factor for periodontitis, affecting the prevalence, extent, and severity of disease. In addition, smoking adversely impacts the clinical outcome of nonsurgical and surgical therapy, as well as the long-term success of implant placement. With 41.9% of periodontitis cases in the US attributable to smoking,2 it is essential to understand its impact on the initiation, progression, and management of the disease. This chapter discusses the effects of smoking on the prevalence, severity, etiology, and pathogenesis of periodontal disease, as well as the impact on treatment. The reader is referred to several excellent reviews on the topic for the detailed results of studies.6-13
Effects of Smoking on the Prevalence and Severity of Periodontal Diseases
Gingivitis
Controlled clinical studies have demonstrated that in human models of experimental gingivitis, the development of inflammation in response to plaque accumulation is reduced in smokers compared with nonsmokers (Table 26-1).14,15 In addition, cross-sectional studies have consistently demonstrated that smokers present with less gingival inflammation than nonsmokers.16-20 These data suggest that smokers have a decreased expression of clinical inflammation in the presence of plaque accumulation compared with nonsmokers. The microbiologic, immunologic, and physiologic factors that might account for this observation are discussed in detail later.
TABLE 26-1 Effects of Smoking on Prevalence and Severity of Periodontal Disease
| Periodontal Disease | Impact of Smoking |
|---|---|
| Gingivitis | ↓ Gingival inflammation and bleeding on probing |
| Periodontitis | ↑ Prevalence and severity of periodontal destruction |
| ↑ Pocket depth, attachment loss, and bone loss | |
| ↑ Rate of periodontal destruction | |
| ↑ Prevalence of severe periodontitis | |
| ↑ Tooth loss | |
| ↑ Prevalence with increased number of cigarettes smoked per day | |
| ↓ Prevalence and severity with smoking cessation |
↓, Decreased; ↑, increased.
Periodontitis
Although gingival inflammation in smokers appears to be reduced in response to plaque accumulation compared with nonsmokers, an overwhelming body of data points to smoking as a major risk factor for increasing the prevalence and severity of periodontal destruction. Multiple cross-sectional and longitudinal studies have demonstrated that pocket depth, attachment loss, and alveolar bone loss are more prevalent and severe in patients who smoke compared with nonsmokers.8,9,12,13 An assessment of the relationship between cigarette smoking and periodontitis was performed in more than 12,000 dentate individuals >18 years of age as part of the third National Health and Nutrition Examination Survey (NHANES III).2 Periodontitis was defined as one or more sites with clinical attachment loss ≥4 mm and pocket depth ≥4 mm. Smoking status was defined using criteria established by the Centers for Disease Control and Prevention (CDC) (see Box 26-1). Of >12,000 individuals studied, 9.2% had periodontitis. This represented approximately 15 million cases of periodontitis in the US. On average, smokers were four times as likely to have periodontitis as persons who had never smoked after adjusting for age, gender, race/ethnicity, education, and income/poverty ratio. Former smokers were 1.7 times more likely to have periodontitis than persons who had never smoked. This study also demonstrated a dose-response relationship between cigarettes smoked per day and the odds of having periodontitis. In subjects smoking nine or fewer cigarettes per day, the odds for having periodontitis were 2.8, whereas subjects smoking 31 or more cigarettes per day were almost six times more likely to have periodontitis. With former smokers, the odds of having periodontitis declined with the number of years since quitting. These data indicated that approximately 42% of periodontitis cases (6.4 million cases) in the US adult population were attributable to current smoking and approximately 11% (1.7 million cases) were attributable to former smoking. These data highlight the serious threat to dental public health posed by cigarette smoking and raise questions about the best methods for managing periodontitis in patients who smoke (Box 26-2).
BOX 26-2 Should We Change How We Manage Periodontal Disease?
FACT 1: Smoking is the major risk factor for periodontal disease.
FACT 2: According to the literature, smoking may be responsible for more than half of periodontitis cases among adults in the United States.2
FACT 3: Depending on the study, approximately 10%-15% of adults in most populations examined have advanced chronic periodontitis.
Questions
These questions are intended to be controversial! It is clear that smoking has a huge deleterious impact on periodontal status. Smoking cessation MUST be a core part of periodontal treatment protocols in patients with periodontitis who smoke. The answer to the first question is almost certainly a resounding “YES” but probably will never be able to be tested. The answer to the second question is more difficult. Certainly, smokers with periodontitis must be educated about the harm they are causing to their periodontal tissues and must be encouraged and helped to quit. Treatment outcomes are improved in smokers who quit compared to those who continue to smoke.92
Two Final (Contentious!) Points
These data are consistent with the findings of other cross-sectional studies performed in the United States and Europe. The odds ratio for periodontitis in current smokers has been estimated to range from as low as 1.5 to as high as 7.3, depending on the observed severity of periodontitis.11 A metaanalysis of data from six such studies involving 2361 subjects indicated that current smokers were almost three times more likely to have severe periodontitis than nonsmokers.10 The detrimental impact of long-term smoking on the periodontal and dentate status of older adults has been clearly demonstrated. Older adult smokers are approximately three times more likely to have severe periodontal disease,21,22 and the number of years of tobacco use is a significant factor in tooth loss, coronal root caries, and periodontal disease.23,24
Smoking also has been shown to affect periodontal disease severity in younger individuals. Cigarette smoking is associated with increased severity of generalized aggressive periodontitis in young adults,25 and those age 19 to 30 years who smoke are 3.8 times more likely to have periodontitis than nonsmokers.26 Longitudinal studies have demonstrated that young individuals smoking more than 15 cigarettes per day showed the highest risk for tooth loss.27 Also, smokers are more than six times as likely as nonsmokers to demonstrate continued attachment loss.28 Over a 10-year period, bone loss has been reported to be twice as rapid in smokers as in nonsmokers29 and proceeds more rapidly even in the presence of excellent plaque control.30 Less information is available on the effects of cigar and pipe smoking, but it appears that effects similar to cigarette smoking are observed with these forms of tobacco use.31-34 The prevalence of moderate and severe periodontitis and the percentage of teeth with ≥5 mm attachment loss was most severe in current cigarette smokers, but cigar and pipe smokers showed a severity of disease intermediate between the current cigarette smokers and nonsmokers.31 Tooth loss is also increased in cigar and pipe smokers compared with nonsmokers.34
Interestingly, former smokers have less risk for periodontitis than current smokers but more risk than nonsmokers, and the risk for periodontitis decreases with the increasing number of years since quitting smoking.2 This suggests that the negative effects of smoking on the host are reversible with smoking cessation and therefore that smoking cessation programs must be an integral component of periodontal education and therapy (Box 26-3). Several tobacco intervention approaches can be used in helping the patient deal with the nicotine withdrawal symptoms and psychologic factors associated with smoking cessation (Box 26-4).35,36
BOX 26-3 Helping Your Patients to Quit Smoking
Smoking cessation is a public health priority for governments around the world. Excellent online resources are available to provide information about the harmful effects of smoking and to help people to quit, as follows:
Smoking cessation must be an integral part of the management of dental patients who smoke, and it is the responsibility of all dental care professionals to address this issue with their patients. The dental team is well placed to provide this treatment, since they see patients on a regular basis over time as part of ongoing routine dental management. Furthermore, interventions to help patients quit smoking in dental practices are effective, with quit rates of 15%- 20% compared to around 5% in control groups.96 The whole dental team should therefore be involved in smoking cessation, but this is not always undertaken. Why? Some of the barriers to providing smoking cessation counseling in the dental practice are shown in Table 26-2.
TABLE 26-2 Barriers Against and Stimuli for Provision of Smoking Cessation Advice by the Dental Team in Dental Practice
| Barriers | Stimuli | |
| Professional characteristics | ||
| Practice organization | ||
| Health care system | Local availability of smoking cessation clinics |
Adapted from Rosseel JP, Jacobs JE, Hilberink SR, et al: Br Dent J 206:E13. 2009.
Various methods for helping patients to quit smoking in the dental environment have been described, and these are typically referred to as Brief Intervention Programs. One such program8 is the 5 As, as follows:
ASK Ask the patient about their smoking status (see Box 26-1).
This should be part of the medical history.
ADVISE Advise smokers of the associations between oral disease and smoking.
Be informative, honest, and helpful but not judgmental.
The patient’s response to this information will reveal their interest in quitting.
ASSESS Assess the patient’s interest and readiness to attempt smoking cessation.
Patients may not yet be in an action phase to quit smoking, which is why it is always important to make these assessments every time you see the patient.
ASSIST Assist the patient in their quit attempt.
If you are trained, there are many techniques that can be used (see Box 26-4).
Alternatively, assist the patient in seeking the help they need.
ARRANGE Arrange follow-up or referral to professional smoking cessation services.
The most important aspect of this is to keep in regular contact particularly around the quit date and in the immediate period after.
A simplified version that is particularly useful for the dental team is the AAR program, as follows:
ASK Ask the patient about their smoking status.
ADVISE Advise smokers of the associations between oral disease and smoking.
REFER Refer the patient to a professional smoking cessation program.
BOX 26-4 Methods for Smoking Cessation
Set a Quit Date
This is the very first step and is highly important. The patient must set their own quit date on which they will stop smoking and will remove all tobacco products from their environment.
Will Power Alone
Success rate at 12 months is 3%. This is the least effective method of smoking cessation, with only 3% of smokers managing to quit after 12 months.
Self-Help Materials
Success rate at 12 months is 4%. This includes a variety of literature and online resources that patients can access. The dental team can be helpful in providing literature and guiding patients towards resources they can access.
Brief Intervention Program in Primary Care
Success rate at 12 months is 5%–10%.36 Brief advice is very important and should always be given at every dental visit. Enhancing the role of specially trained dental hygienists and prevention auxiliaries in giving lifestyle advice is of major benefit. If the dental team gave even brief advice to most of their smoking patients and achieved a low cessation rate of 5% over time, a significant proportion of smokers would be assisted to quit each year.
Nicotine Replacement Therapy (NRT)
Success rate at 12 months is 10%–20%. NRT generally doubles the success rate of smoking cessation. For example, in primary care settings in which brief advice is given, 12-month success rates double from around 5% to around 10% if NRT is used. In an intensive setting such as a smokers clinic, success rates increase from around 10%-20%. NRT is not a magic cure but helps with craving and withdrawal when a person quits smoking, and although it does contain nicotine, it does not contain the toxic products, such as tar and carbon monoxide (CO), in cigarette smoke. NRT products include the following:
Bupropion
Success rate at 12 months is 20%–30%. This medication is used as an antidepressant at higher doses but is effective for smoking cessation at lower doses. It is usually prescribed starting 1–2 weeks before the quit date, initially at 150 mg once per day for 6 days then 150 mg twice daily for 7–9 weeks. There are serious potential drug interactions so this medication should be prescribed by the patient’s medical doctor.
Effects of Smoking on the Etiology and Pathogenesis of Periodontal Disease
The increased prevalence and severity of periodontal destruction associated with smoking suggests that the host-bacterial interactions normally seen in chronic periodontitis are altered, resulting in more extensive periodontal breakdown (Table 26-3). This imbalance between bacterial challenge and host response may be caused by changes in the composition of the subgingival plaque, with increases in the numbers and virulence of pathogenic organisms, changes in the host response to the bacterial challenge, or a combination of both. This section discusses recent evidence on the effects of smoking on the microbiology, immune-inflammatory response, and physiology of periodontitis.
TABLE 26-3 Effects of Smoking on the Etiology and Pathogenesis of Periodontal Disease
| Etiologic Factor | Impact of smoking |
|---|---|
| Microbiology | No effect on rate of plaque accumulation. |
| ↑ Colonization of shallow periodontal pockets by periodontal pathogens. | |
| ↑ Levels of periodontal pathogens in deep periodontal pockets. | |
| Immune-inflammatory response | Altered neutrophil chemotaxis, phagocytosis, and oxidative burst. |
| ↑ TNF-α and PGE2 in GCF. | |
| ↑ Neutrophil collagenase and elastase in GCF. | |
| ↑ Production of PGE2 by monocytes in response to LPS. | |
| Physiology | ↓ Gingival blood vessels with ↑ inflammation. |
| ↓ GCF flow and bleeding on probing with ↑ inflammation. | |
| ↓ Subgingival temperature. | |
| ↑ Time needed to recover from local anesthesia. |
↓, Decreased; ↑, increased; TNF-α, tumor necrosis factor alpha; GCF, gingival crevicular fluid; PGE2, prostaglandin E2; LPS, lipopolysaccharide.
Microbiology
Studies have failed to demonstrate a difference in the rate of plaque accumulation of smokers compared with nonsmokers, suggesting that if an alteration in the microbial challenge in smokers exists, it results from a qualitative rather than quantitative alteration in the plaque.17 Several studies have explored the possible changes in subgingival plaque caused by smoking, with conflicting and inconclusive results. In a study of 142 patients with chronic periodontitis, plaque samples from deep pockets (≥6 mm) showed no differences in the counts of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia.37 In a similar study of 615 patients using immunoassay, the prevalence of A. actinomycetemcomitans, P. gingivalis, P. intermedia, and Eikenella corrodens was not found to be significantly different between smokers and nonsmokers.38 In contrast, other studies have shown differences in the microbial composition of subgingival plaque between smokers and nonsmokers. In a study of 798 subjects with different smoking histories, it was found that smokers had significantly higher levels of Tannerella forsythia (previously called Bacteroides forsythus) and that smokers were 2.3 times more likely to harbor T. forsythia than nonsmokers and former smokers.39 Of particular interest was the observation that smokers do not respond to mechanical therapy as well as nonsmokers; this is associated with increased levels of T. forsythia, A. actinomycetemcomitans, and P. gingivalis remaining in the pockets after therapy in the smoking group when compared with nonsmokers.40-43
Many discrepancies between the findings of microbiologic studies are a function of the methodology involved, including bacterial counts versus proportions or prevalence of bacteria, number of sites sampled and the pocket depths selected, the sampling technique, the disease status of the subject, and the methods of bacterial enumeration and data analysis. In an attempt to overcome some of these problems, a recent study sampled subgingival plaque from all teeth with the exception of third molars in 272 adult subjects, including 50 current smokers, 98 past smokers, and 124 nonsmokers.44 Using checkerboard DNA-DNA hybridization technology to screen for 29 different subgingival species, it was found that members of the orange and red complexes, including Eikenella nodatum, Fusobacterium nucleatum ss vincentii, P. intermedia, Peptostreptococcus micros, Prevotella nigrescens, T. forsythia, P. gingivalis, and Treponema denticola, were significantly more prevalent in current smokers than in nonsmokers and former smokers. The increased prevalence of these periodontal pathogens was caused by an increased colonization of shallow sites (pocket depth ≤4 mm), with no differences among smokers, former smokers, and nonsmokers in pockets 4 mm or greater. In addition, these pathogenic bacteria were more prevalent in the maxilla than the mandible. These data suggest that smokers have a greater extent of colonization by periodontal pathogens than nonsmokers or former smokers and that this colonization may lead to an increased prevalence of periodontal breakdown.
Immune-Inflammatory Responses
The immune response of the host to plaque accumulation is essentially protective. In periodontal health and gingivitis, a balance exists between the bacterial challenge of plaque and the immune-inflammatory responses in the gingival tissues, with no resulting loss of periodontal support. In contrast, periodontitis is associated with an alteration in the host-bacterial balance that may be initiated by changes in the bacterial composition of subgingival plaque, changes in the host responses, or a combination of both.
Smoking exerts a major effect on the protective elements of the immune-inflammatory response, resulting in an increase in the extent and severity of periodontal destruction. The deleterious effects of smoking appear to result in part from a down-regulation of the immune response to bacterial challenge. The neutrophil is an important component of the host response to bacterial infection, and alterations in neutrophil number or function may result in localized and systemic infections. Critical functions of neutrophils include chemotaxis (directed locomotion from the bloodstream to the site of infection), phagocytosis (internalization of foreign particles such as bacteria), and killing using oxidative and nonoxidative mechanisms. Neutrophils obtained from the peripheral blood, oral cavity, or saliva of smokers or exposed in vitro to whole tobacco smoke or nicotine have been shown to demonstrate functional alterations in chemotaxis, phagocytosis, and the oxidative burst.45,46 In vitro studies of the effects of tobacco products on neutrophils have shown detrimental effects on cell movement, as well as the oxidative burst.47-51 In addition, levels of antibody to periodontal pathogens essential for phagocytosis and killing of bacteria, specifically immunoglobulin G2 (IgG2), have been reported to be reduced in smokers versus nonsmokers with periodontitis,42,52-54 suggesting that smokers may have reduced protection against periodontal infection. In contrast, elevated levels of tumor necrosis factor alpha (TNF-α) have been demonstrated in the gingival crevicular fluid (GCF) of smokers,55 as well as elevated levels of prostaglandin E2 (PGE2), neutrophil elastase, and matrix metalloproteinase-8 (MMP-8).56 In vitro studies also have shown that exposure to nicotine increases the secretion of PGE2 by monocytes in response to lipopolysaccharide (LPS).57
These data suggest that smoking may impair the response of neutrophils to plaque bacteria but may also increase the release of tissue-destructive enzymes. The exact changes in the immunologic mechanisms involved in the rapid tissue destruction seen in smokers are currently unclear. Further studies are needed to define the effects of tobacco use on the immune-inflammatory response and tissue destruction in periodontitis.
Physiology
Previous studies have shown that the clinical signs of inflammation are less pronounced in smokers than in nonsmokers.15,18 This may result from alterations in the inflammatory response in smokers, as outlined previously, or from alterations in the vascular response of the gingival tissues. Although no significant differences in the vascular density of healthy gingiva have been observed between smokers and nonsmokers,58 the response of the microcirculation to plaque accumulation appears to be altered in smokers compared with nonsmokers. With developing inflammation, increases in GCF flow, bleeding on probing,14 and gingival blood vessels59 are less in smokers than nonsmokers. In addition, the oxygen concentration in healthy gingival tissues appears to be less in smokers than nonsmokers, although this condition is reversed in the presence of moderate inflammation.60 Subgingival temperatures are lower in smokers than nonsmokers,61 and recovery from the vasoconstriction caused by local anaesthetic administration takes longer in smokers.62,63 These cumulative data suggest that significant alterations are present in the gingival microvasculature of smokers compared with nonsmokers and that these changes lead to decreased blood flow and decreased clinical signs of inflammation. This explains the long observed phenomenon of a transient increase in gingival bleeding when a smoker quits.
Effects of Smoking on Response to Periodontal Therapy
Nonsurgical Therapy
Numerous studies have indicated that current smokers do not respond as well to periodontal therapy as nonsmokers or former smokers (Table 26-4). The majority of clinical research supports the observation that pocket depth reduction is more effective in nonsmokers than in smokers after nonsurgical periodontal therapy, including oral hygiene instruction, scaling, and root planing.40-43, 64-66 In addition, gains in clinical attachment as a result of nonsurgical treatment are less pronounced in smokers than in nonsmokers. In a study of patients with previously untreated advanced periodontal disease, nonsurgical therapy resulted in significantly greater mean reductions in pocket depth and bleeding on probing in nonsmokers than in smokers when evaluated 6 months after completion of therapy.43 Average pocket reductions of 2.5 mm for nonsmokers and 1.9 mm for smokers were observed in pockets that averaged 7 mm before treatment. In another study, the nonsurgical management of pockets ≥5 mm showed that smokers had less pocket depth reduction than nonsmokers after 3 months (1.29 mm versus 1.76 mm), as well as less gain in clinical attachment levels.40 When a higher level of plaque control can be achieved as part of nonsurgical care, the differences in the resolution of 4 mm to 6 mm pockets between nonsmokers and smokers became clinically less significant.66 It can be concluded that smokers respond less well to nonsurgical therapy than nonsmokers. With excellent plaque control, however, these differences may be minimized (but the emphasis is on truly excellent plaque control). When comparing current smokers with former smokers and nonsmokers, the former and nonsmoking subjects appear to respond equally well to nonsurgical care,40 reinforcing the need for patients to be informed of the benefits of smoking cessation.
TABLE 26-4 Effects of Smoking on Response to Periodontal Therapy
| Therapy | Effects of Smoking |
|---|---|
| Nonsurgical | ↓ Clinical response to root surface debridement. |
| ↓ Reduction in pocket depth. | |
| ↓ Gain in clinical attachment levels. | |
| ↓ Negative impact of smoking with ↑ level of plaque control. | |
| Surgery and implants | ↓ Pocket depth reduction and ↓ gain in clinical attachment levels after access flap surgery. |
| ↑ Deterioration of furcations after surgery. | |
| ↓ Gain in clinical attachment levels, ↓ bone fill, ↑recession, and ↑ membrane exposure after GTR. | |
| ↓ Pocket depth reduction after bone graft procedures. | |
| ↑ Risk for implant failure and periimplantitis. | |
| Maintenance care | ↑ Pocket depth and attachment loss during maintenance therapy. |
| ↑ Disease recurrence in smokers. | |
| ↑ Need for re-treatment in smokers. | |
| ↑ Tooth loss in smokers after surgical therapy. |
↓, Decreased; ↑ increased; GTR, guided tissue regeneration.
Surgical Therapy and Implants
The less favorable response of the periodontal tissues to nonsurgical therapy that is observed in current smokers is also observed after surgical therapy. In a longitudinal comparative study of the effects of four different treatment modalities, including coronal scaling, root planing, modified Widman flap surgery, and osseous resection surgery, smokers (“heavy” defined as ≥20 cigarettes/day; “light” defined as ≤19 cigarettes/day) consistently showed less pocket reduction and less gain in clinical attachment than nonsmokers or former smokers.65 These differences were evident immediately after the completion of therapy and continued throughout 7 years of supportive periodontal therapy. During the 7 years, deterioration at furcation areas was greater in heavy and light smokers than in former smokers and nonsmokers. Smoking also has been shown to have a negative impact on the outcomes of guided tissue regeneration (GTR)67,68 and the treatment of infrabony defects by bone grafts.69 By 12 months after GTR therapy at deep infrabony defects, smokers demonstrated less than half the attachment gain as nonsmokers (2.1 mm versus 5.2 mm).67 In a second study, 73 smokers also showed less attachment gain than nonsmokers (1.2 mm versus 3.2 mm), more gingival recession, and less bone infill of the defect. Similarly, after the use of bone grafts for the treatment of infrabony defects, smokers showed less reduction in pocket depths than nonsmokers.69
Open flap access surgery without regenerative or grafting procedures is the most common surgical procedure used for accessing the root and bone surfaces. By 6 months after this procedure, smokers showed significantly less reduction of deep pockets (≥7 mm) than nonsmokers (3.0 mm for smokers versus 4.0 mm for nonsmokers) and significantly less gain in clinical attachment (1.8 versus 2.8 mm), even though all of the patients received supportive periodontal therapy every month for 6 months.70 Of clinical relevance was the observation that only 16% of deep pockets in smokers returned to 3 mm or less at 6 months after surgery, whereas 47% of deep pockets in nonsmokers were 3 mm or less after completion of therapy.
Three metaanalyses have investigated the influence of smoking on the short- and long-term outcomes of implant therapy and have identified that smoking increases the risk of implant failure.71-73 These studies used various definitions to define implant failure, including implant loss, implant bone loss, mobility, pain, and periimplantitis. Overall, the risk for implant failure in smokers appears to be approximately double the risk for failure in nonsmokers, and the risks appear to be higher in maxillary implants and when implants are placed in poor quality bone. Smoking has also been shown to be a risk factor for periimplantitis, with a majority of studies showing a significant increase in periimplant bone loss compared with nonsmokers.74 Collectively, these data indicate that implant failure is more common in smokers than nonsmokers, but since numerous factors can influence implant success (see Part 7), further controlled clinical trials are needed to address the role of smoking as an independent variable in implant failure. However, given the current evidence, patients should be informed and advised of the benefits of smoking cessation and the potential risks of smoking for implant failure.
Maintenance Therapy
The detrimental effect of smoking on treatment outcomes appears to be long-lasting and independent of the frequency of maintenance therapy. After four different modalities of therapy, including scaling, scaling and root planing, modified Widman flap surgery, and osseous surgery, maintenance therapy was performed by a hygienist every 3 months for 7 years.65 Smokers consistently had deeper pockets than nonsmokers and less gain in attachment when evaluated each year for the 7-year period. Even with more intensive maintenance therapy given every month for 6 months after flap surgery,70 smokers had deeper and more residual pockets than nonsmokers, even though no significant differences in plaque or bleeding on probing scores were found. These data suggest that the effects of smoking on the host response and the healing characteristics of the periodontal tissues may have a long-term effect on pocket resolution in smokers, possibly requiring more intensive management in the maintenance phase. Smokers also tend to experience more periodontal breakdown than nonsmokers after therapy.75,76 In studies of patients who failed to respond to conventional therapy, including different combinations of oral hygiene instruction, scaling and root planing, surgery, and antibiotics, approximately 90% of these poorly responding patients were smokers.75,77
It is clear from these studies that smokers (1) may present with periodontal disease at an early age, (2) may be difficult to treat effectively with conventional therapeutic strategies, and (3) may continue to have progressive or recurrent periodontitis. For this reason, smoking cessation counseling must be a cornerstone of periodontal therapy in smokers.
Effects of Smoking Cessation on Periodontal Treatment Outcomes
The effect of smoking cessation on periodontal status has been studied in a large number of cross-sectional and cohort observational studies, in which the periodontal status of smokers, former smokers and nonsmokers is compared.* Similarly, periodontal treatment outcomes have been assessed in smokers, former smokers, and nonsmokers.41,65,86-89 Collectively, these studies have demonstrated that smokers have significantly worse periodontal status (deeper probing depths, more attachment loss and bone loss) than either former smokers or nonsmokers and usually have poorer treatment outcomes. The periodontal status of former smokers is intermediate to that of current smokers or nonsmokers and appears to be usually closer to that of nonsmokers.
There are very few intervention studies on the effect of smoking cessation on periodontal treatment outcomes (i.e., studies in which smokers were helped to quit and the effect on periodontal status was then assessed). Two short-term studies have indicated that smoking has a negative impact on the gingival vasculature and that these changes are reversible on quitting smoking.90,91 One intervention study has assessed the impact of smoking cessation on outcomes after nonsurgical periodontal treatment.92 This study employed dental hygienists who were trained as smoking cessation advisors and achieved a 20% quit rate at 12 months in a population of smokers who also had periodontitis using a variety of strategies, including counseling, NRT, and bupropion. This confirms the effectiveness of dental health care professionals in providing smoking cessation counseling. All the patients in the study received nonsurgical therapy as treatment for their periodontitis, in addition to smoking cessation counseling. Those individuals who successfully quit smoking for the entire 12 months of the study had the best response to the periodontal treatment. The treatment responses in the nonquitters and the failed quitters (i.e., the “oscillators” who initially quit, but then resumed smoking) were significantly poorer than those seen in the quitters, and did not differ significantly from each other.
The benefit of smoking cessation on the periodontium is likely to be mediated through various pathways, such as a shift towards a less pathogenic microflora, recovery of the gingival microcirculation and improvements in aspects of the immune-inflammatory responses. In support of this, in the interventional study described previously,92 plaque samples were collected as the study progressed. It became clear that subgingival microbial profiles differed significantly between smokers and quitters at 6 and 12 months following smoking cessation.93 At 6 and 12 months posttreatment, the microbial community in smokers was similar to that observed at baseline (i.e., before periodontal treatment/smoking cessation counseling), whereas the quitters demonstrated significantly divergent profiles, and changes in bacterial levels contributed to this shift. These data suggest a critical role for smoking cessation in altering the subgingival biofilm.
To conclude, smoking is the major risk factor for periodontitis, and smoking cessation should be an integral part of periodontal therapy in patients who smoke and needs to be considered a priority for the management of periodontitis in smokers.
![]() Science Transfer
Science Transfer
Smoking results in a dramatic increased risk of periodontitis and increases the likelihood of poor responses to periodontal therapy. Nonsurgical therapy can be successful in smokers if excellent oral hygiene is achieved, but surgical treatments, including implant placement, all have decreased favorable outcomes in smokers. Smokers have less gingivitis and bleeding on probing, so it is more difficult to detect attachment loss and bone destruction; thus it is imperative that comprehensive pocket depth recording and full-mouth radiographs accompany their periodontal examination.
Dental clinicians have a unique and obligatory responsibility to inform smokers of their susceptibility to advanced periodontal destruction and poor response to surgical procedures. The demonstration of specific smoking-related periodontal problems is often the first salutary information that the patient accepts concerning the body’s deleterious reaction to smoking. This can trigger a desire for quitting smoking and the dental team has a special role in initiating smoking cessation. Nicotine replacement therapy and behavior modification approaches generally have a 20% success rate. The addition of systemic medication, such as bupropion and varenicline, may add another 10% of quitters at 1 year, but because of the occasional serious side effects (seizures, arrhythmias, tachycardia, depression, and suicidality), it is suggested that these agents may be best used under the direction of a physician.
Smoking cessation should precede periodontal surgical intervention, and if patients continue to smoke, clinicians may consider limiting periodontal treatment to nonsurgical approaches accompanied by rigorous short interval (2 to 3 months) maintenance visits until smoking ceases.
References
1 Garfinkel L. Trends in cigarette smoking in the United States. Prev Med. 1997;26:447-450.
2 Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71:743-751.
3 Doll R, Peto R, Wheatley K, et al. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309:901-911.
4 Pilotti A. Biosynthesis and mammalian metabolism of nicotine. Acta Physiologica Scandinavica. 1980;479:13-17.
5 Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78:696-698.
6 Heasman L, Stacey F, Preshaw PM, et al. The effect of smoking on periodontal treatment response: a review of clinical evidence. J Clin Periodontol. 2006;33:241-253.
7 AAP: Position paper. Tobacco use and the periodontal patient. J Periodontol. 1996;67:51-56.
8 Johnson GK, Hill M. Cigarette smoking and the periodontal patient. J Periodontol. 2004;75:196-209.
9 Johnson GK, Guthmiller JM. The impact of cigarette smoking on periodontal disease and treatment. Periodontol 2000. 2007;44:178-194.
10 Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol. 1996;1:1-36.
11 Papapanou PN. Risk assessments in the diagnosis and treatment of periodontal diseases. J Dent Educ. 1998;62:822-839.
12 Ramseier CA. Potential impact of subject-based risk factor control on periodontitis. J Clin Periodontol. 2005;32:283-290.
13 Tonetti MS. Cigarette smoking and periodontal diseases: etiology and management of disease. Ann Periodontol. 1998;3:88-101.
14 Bergstrom J, Preber H. The influence of cigarette smoking on the development of experimental gingivitis. J Periodontal Res. 1986;21:668-676.
15 Danielsen B, Manji F, Nagelkerke N, et al. Effect of cigarette smoking on the transition dynamics in experimental gingivitis. J Clin Periodontol. 1990;17:159-164.
16 Bergstrom J, Floderus-Myrhed B. Co-twin control study of the relationship between smoking and some periodontal disease factors. Community Dentistry and Oral Epidemiology. 1983;11:113-116.
17 Bergstrom J. Cigarette smoking as risk factor in chronic periodontal disease. Community Dentistry and Oral Epidemiology. 1989;17:245-247.
18 Bergstrom J. Oral hygiene compliance and gingivitis expression in cigarette smokers. Scand J Dent Res. 1990;98:497-503.
19 Preber H, Bergstrom J. Occurrence of gingival bleeding in smoker and non-smoker patients. Acta Odontol Scand. 1985;43:315-320.
20 Preber H, Bergstrèom J. Cigarette smoking in patients referred for periodontal treatment. Scand J Dent Res. 1986;94:102-108.
21 Beck JD, Koch GG, Rozier RG, Tudor GE. Prevalence and risk indicators for periodontal attachment loss in a population of older community-dwelling blacks and whites. J Periodontol. 1990;61:521-528.
22 Locker D, Leake JL. Risk indicators and risk markers for periodontal disease experience in older adults living independently in Ontario, Canada. J Dent Res. 1993;72:9-17.
23 Jette AM, Feldman HA, Tennstedt SL. Tobacco use: a modifiable risk factor for dental disease among the elderly. Am J Public Health. 1993;83:1271-1276.
24 Jette AM, Feldman HA, Douglass C. Oral disease and physical disability in community-dwelling older persons. J Am Geriatr Soc. 1993;41:1102-1108.
25 Schenkein HA, Gunsolley JC, Koertge TE, et al. Smoking and its effects on early-onset periodontitis. J Am Dent Assoc. 1995;126:1107-1113.
26 Haber J, Wattles J, Crowley M, et al. Evidence for cigarette smoking as a major risk factor for periodontitis. J Periodontol. 1993;64:16-23.
27 Holm G. Smoking as an additional risk for tooth loss. J Periodontol. 1994;65:996-1001.
28 Ismail AI, Morrison EC, Burt BA, et al. Natural history of periodontal disease in adults: findings from the Tecumseh Periodontal Disease Study, 1959-87. J Dent Res. 1990;69:430-435.
29 Bolin A, Eklund G, Frithiof L, Lavstedt S. The effect of changed smoking habits on marginal alveolar bone loss. A longitudinal study. Swed Dent J. 1993;17:211-216.
30 Bergstrom J, Eliasson S, Dock J. A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol. 2000;71:1338-1347.
31 Albandar JM, Streckfus CF, Adesanya MR, Winn DM. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol. 2000;71:1874-1881.
32 Feldman RS, Bravacos JS, Rose CL. Association between smoking different tobacco products and periodontal disease indexes. J Periodontol. 1983;54:481-487.
33 Feldman RS, Alman JE, Chauncey HH. Periodontal disease indexes and tobacco smoking in healthy aging men. Gerodontics. 1987;3:43-46.
34 Krall EA, Garvey AJ, Garcia RI. Alveolar bone loss and tooth loss in male cigar and pipe smokers. J Am Dent Assoc. 1999;130:57-64.
35 Parrott S, Godfrey C, Raw M, et al. Guidance for commissioners on the cost effectiveness of smoking cessation interventions. Health Educational Authority. Thorax. 1998;53(Suppl 5 Pt)2):S1-38.
36 Richmond RL. Physicians can make a difference with smokers: evidence-based clinical approaches. Int J Tuberc Lung D. 1999;3:100-112.
37 Preber H, Bergstrom J, Linder L. Occurrence of periopathogens in smoker and non-smoker patients. J Clin Periodontol. 1992;19:667-671.
38 Stoltenberg JL, Osborn JB, Pihlstrom BL, et al. Association between cigarette smoking, bacterial pathogens and periodontal status. J Periodontol. 1993;64:1225-1230.
39 Zambon JJ, Grossi SG, Machtei EE, et al. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol. 1996;67:1050-1054.
40 Grossi SG, Skrepcinski FB, DeCaro T, et al. Response to periodontal therapy in diabetics and smokers. J Periodontol. 1996;67:1094-1102.
41 Grossi SG, Zambon J, Machtei EE, et al. Effects of smoking and smoking cessation on healing after mechanical periodontal therapy. J Am Dent Assoc. 1997;128:599-607.
42 Haffajee AD, Cugini MA, Dibart S, et al. Socransky SS: The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24:324-334.
43 Renvert S, Dahlen G, Wikstrom M. The clinical and microbiological effects of non-surgical periodontal therapy in smokers and non-smokers. J Clin Periodontol. 1998;25:153-157.
44 Haffajee AD, Socransky SS. Relationship of cigarette smoking to the subgingival microbiota. J Clin Periodontol. 2001;28:377-388.
45 Eichel B, Shahrik HA. Tobacco smoke toxicity: loss of human oral leukocyte function and fluid-cell metabolism. Science. 1969;166:1424-1428.
46 Kenney EB, Kraal JH, Saxe SR, Jones J. The effect of cigarette smoke on human oral polymorphonuclear leukocytes. J Periodontal Res. 1977;12:227-234.
47 Codd EE, Swim AT, Bridges RB. Tobacco smokers’ neutrophils are desensitized to chemotactic peptide-stimulated oxygen uptake. J Lab Clin Med. 1987;110:648-652.
48 Kalra J, Chaudhary AK, Prasad K. Increased production of oxygen free radicals in cigarette smokers. Int J Exp Pathol. 1991;72:1-7.
49 Lannan S, McLean A, Drost E, et al. Changes in neutrophil morphology and morphometry following exposure to cigarette smoke. Int J Exp Pathol. 1992;73:183-191.
50 Ryder MI, Fujitaki R, Johnson G, Hyun W. Alterations of neutrophil oxidative burst by in vitro smoke exposure: implications for oral and systemic diseases. Ann Periodontol. 1998;3:76-87.
51 Selby C, Drost E, Brown D, et al. Inhibition of neutrophil adherence and movement by acute cigarette smoke exposure. Exp Lung Res. 1992;18:813-827.
52 Califano JV, Schifferle RE, Gunsolley JC, et al. Antibody reactive with Porphyromonas gingivalis serotypes K1-6 in adult and generalized early-onset periodontitis. J Periodontol. 1999;70:730-735.
53 Gunsolley JC, Pandey JP, Quinn SM, et al. The effect of race, smoking and immunoglobulin allotypes on IgG subclass concentrations. J Periodontal Res. 1997;32:381-387.
54 Tangada SD, Califano JV, Nakashima K, et al. The effect of smoking on serum IgG2 reactive with Actinobacillus actinomycetemcomitans in early-onset periodontitis patients. J Periodontol. 1997;68:842-850.
55 Bostrom L, Linder LE, Bergstrom J. Clinical expression of TNF-alpha in smoking-associated periodontal disease. J Clin Periodontol. 1998;25:767-773.
56 Soder B. Neutrophil elastase activity, levels of prostaglandin E2, and matrix metalloproteinase-8 in refractory periodontitis sites in smokers and non-smokers. Acta Odontol Scand. 1999;57:77-82.
57 Payne JB, Johnson GK, Reinhardt RA, et al. Nicotine effects on PGE2 and IL-1b release by LPS-treated human monocytes. J Periodontal Res. 1996;31:99-104.
58 Persson L, Bergstrom J. Smoking and vascular density of healthy marginal gingiva. Eur J Oral Sci. 1998;106:953-957.
59 Bergstrom J, Persson L, Preber H. Influence of cigarette smoking on vascular reaction during experimental gingivitis. Scand J Dent Res. 1988;96:34-39.
60 Hanioka T, Tanaka M, Ojima M, et al. Oxygen sufficiency in the gingiva of smokers and non-smokers with periodontal disease. J Periodontol. 2000;71:1846-1851.
61 Dinsdale CR, Rawlinson A, Walsh TF. Subgingival temperature in smokers and non-smokers with periodontal disease. J Clin Periodontol. 1997;24:761-766.
62 Ketabi M, Hirsch RS. The effects of local anesthetic containing adrenaline on gingival blood flow in smokers and non-smokers. J Clin Periodontol. 1997;24:888-892.
63 Trikilis N, Rawlinson A, Walsh TF. Periodontal probing depth and subgingival temperature in smokers and non-smokers. J Clin Periodontol. 1999;26:38-43.
64 Ah MK, Johnson GK, Kaldahl WB, et al. The effect of smoking on the response to periodontal therapy. J Clin Periodontol. 1994;21:91-97.
65 Kaldahl WB, Johnson GK, Patil KD, Kalkwarf KL. Levels of cigarette consumption and response to periodontal therapy. J Periodontol. 1996;67:675-681.
66 Preber H, Bergstrom J. The effect of non-surgical treatment on periodontal pockets in smokers and non-smokers. J Clin Periodontol. 1986;13:319-323.
67 Tonetti MS, Pini Prato G, Cortellini P. Effect of cigarette smoking on periodontal healing following GTR in infrabony defects. A preliminary retrospective study. J Clin Periodontol. 1995;22:229-234.
68 Trombelli L, Kim CK, Zimmerman GJ, Wikesjo UM. Retrospective analysis of factors related to clinical outcome of guided tissue regeneration procedures in intrabony defects. J Clin Periodontol. 1997;24:366-371.
69 Rosen PS, Marks MH, Reynolds MA. Influence of smoking on long-term clinical results of intrabony defects treated with regenerative therapy. J Periodontol. 1996;67:1159-1163.
70 Scabbia A, Cho KS, Sigurdsson TJ, et al. Cigarette smoking negatively affects healing response following flap debridement surgery. J Periodontol. 2001;72:43-49.
71 Hinode D, Tanabe S, Yokoyama M, et al. Influence of smoking on osseointegrated implant failure: a meta-analysis. Clin Oral Implants Res. 2006;17:473-478.
72 Klokkevold PR, Han TJ. How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? Int J Oral Max Impl. 2007;22(Suppl):173-202.
73 Strietzel FP, Reichart PA, Kale A, et al. Smoking interferes with the prognosis of dental implant treatment: a systematic review and meta-analysis. J Clin Periodontol. 2007;34:523-544.
74 Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35:292-304.
75 MacFarlane GD, Herzberg ML, Wolff LF, Hardie NA. Refractory periodontitis associated with abnormal polymorphonuclear leukocyte phagocytosis and cigarette smoking. J Periodontol. 1992;63:908-913.
76 Magnusson I, Walker CB. Refractory periodontitis or recurrence of disease. J Clin Periodontol. 1996;23:289-292.
77 Magnusson I, Low SB, McArthur WP, et al. Treatment of subjects with refractory periodontal disease. J Clin Periodontol. 1994;21:628-637.
78 Baljoon M, Natto S, Bergstrom J. The association of smoking with vertical periodontal bone loss. J Periodontol. 2004;75:844-851.
79 Baljoon M, Natto S, Bergstrom J. Long-term effect of smoking on vertical periodontal bone loss. J Clin Periodontol. 2005;32:789-797.
80 Bergstrom J, Eliasson S, Preber H. Cigarette smoking and periodontal bone loss. J Periodontol. 1991;62:242-246.
81 Bergstrom J, Eliasson S, Dock J. Exposure to tobacco smoking and periodontal health. J Clin Periodontol. 2000;27:61-68.
82 Do LG, Slade GD, Roberts-Thomson KF, Sanders AE. Smoking-attributable periodontal disease in the Australian adult population. J Clin Periodontol. 2008;35:398-404.
83 Haffajee AD, Socransky SS. Relationship of cigarette smoking to attachment level profiles. J Clin Periodontol. 2001;28:283-295.
84 Paulander J, Wennstrom JL, Axelsson P, Lindhe J. Some risk factors for periodontal bone loss in 50-year-old individuals. A 10-year cohort study. J Clin Periodontol. 2004;31:489-496.
85 Thomson WM, Broadbent JM, Welch D, et al. Cigarette smoking and periodontal disease among 32-year-olds: a prospective study of a representative birth cohort. J Clin Periodontol. 2007;34:828-834.
86 Hughes FJ, Syed M, Koshy B, et al. Prognostic factors in the treatment of generalized aggressive periodontitis: II: Effects of smoking on initial outcome. J Clin Periodontol. 2006;33:671-676.
87 Meinberg TA, Canarsky-Handley AM, McClenahan AK, et al. Outcomes associated with supportive periodontal therapy in smokers and nonsmokers. J Dent Hyg. 2001;75:15-19.
88 Preshaw PM, Lauffart B, Zak E, et al. Progression and treatment of chronic adult periodontitis. J Periodontol. 1999;70:1209-1220.
89 Ryder MI, Pons B, Adams D, et al. Effects of smoking on local delivery of controlled-release doxycycline as compared to scaling and root planing. J Clin Periodontol. 1999;26:683-691.
90 Morozumi T, Kubota T, Sato T, et al. Smoking cessation increases gingival blood flow and gingival crevicular fluid. J Clin Periodontol. 2004;31:267-272.
91 Nair P, Sutherland G, Palmer RM, et al. Gingival bleeding on probing increases after quitting smoking. J Clin Periodontol. 2003;30:435-437.
92 Preshaw PM, Heasman L, Stacey F, et al. The effect of quitting smoking on chronic periodontitis. J Clin Periodontol. 2005;32:869-879.
93 Fullmer SC, Preshaw PM, Heasman PA, Kumar PS. Smoking cessation alters subgingival microbial recolonization. J Dent Res. 2009;88:524-528.
94 Scott DA, Palmer RM, Stapleton JA. Validation of smoking status in clinical research into inflammatory periodontal disease. J Clin Periodontol. 2001;28:715-722.
95 Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390-395.
96 Rosseel JP, Jacobs JE, Hilberink SR, et al. What determines the provision of smoking cessation advice and counselling by dental care teams? Br Dent J. 2009;206:E13.