CHAPTER 29 Oral Malodor
Semantics
Breath odor can be defined as the subjective perception after smelling someone’s breath. It can be pleasant, unpleasant or even disturbing, if not repulsive. If unpleasant, the terms breath malodor, halitosis, bad breath, or fetor ex ore can be applied. These terms, however, are not synonymous with oral malodor, which has its origin in the oral cavity. This is not always the case for all breath malodors. The term “oral malodor” is thus too restrictive.
Breath malodor should not be confused with the momentarily disturbing odor caused by food intake (e.g., garlic, onions, and certain spices), smoking, or medication (e.g., metronidazole) because these odors do not reveal a health problem. The same is true for “morning” bad breath, as habitually experienced on awakening. This malodor is caused by a decreased salivary flow and increased putrefaction during the night and spontaneously disappears after breakfast or oral hygiene measures. A persistent breath malodor, by definition, does reflect some pathology.
Epidemiology
Breath malodor is a common complaint among the general population. It has a significant socioeconomic impact but was neglected until recently by scientists and clinicians and is hardly covered in the medical curricula. Halitosis can lead to personal discomfort and social embarrassment and is still one of the biggest taboos in society. Almost $1 billion a year is spent in the United States on deodorant-type mouth (oral) rinses, mints, and related over-the-counter products to manage bad breath.56 It would be preferable to spend this money on a proper diagnosis and etiologic care instead of short-term and even inefficient masking attempts.
There are few studies that document the prevalence of oral malodor. Moreover, the studies that exist used different methodologies ranging from self-reported breath malodor to more objective assessments such as the measurement of the volatile sulfur compounds (VSCs). The incidence of halitosis remains therefore poorly documented in most countries. Several studies in industrialized countries reported incidences up to 50%, with a variable degree of intensity.5,61,63,94 A large-scale Japanese study of more than 2500 subjects (aged 18 to 64 years) reported that about one in four subjects exhibited VSC values higher than 75 parts per billion (ppb), which is considered to be the limit for social acceptance.63,132
From large-scale inventories in two multidisciplinary outpatient clinics for breath odor,19,20 no gender predominance seems to exist for bad breath, although other studies indicate a higher prevalence in women.65,100 It has also been observed that women seek treatment more often than men.63 Age can range from 5 years to over 80 years. No association was found between increased age and oral malodor.19,20 Most of the patients had been complaining about breath malodor for several years before seeking proper advice (recent report of the department involving 2000 patients).
Classification
There are three main categories of halitosis: genuine halitosis, pseudo-halitosis, and halitophobia. Genuine halitosis is the term that is used when the breath malodor really exists and can be diagnosed organoleptically or by measurement of the responsible compounds. When an obvious breath malodor cannot be perceived, but the patient is convinced that he or she suffers from it, this is called pseudo-halitosis. If the patient still believes that there is bad breath after treatment of genuine halitosis or diagnosis of pseudo-halitosis, one considers halitophobia, which is a recognized psychiatric condition.130
Etiology
In the vast majority, breath malodor originates from the oral cavity. Gingivitis, periodontitis, and especially tongue coating are the predominant causative factors.20,78,79,131,132
A recent large-scale study with 2000 halitosis patients has shown that for 76% an oral cause could be found (tongue coating [43%], gingivitis/periodontitis [11%], or a combination [18%] are most often encountered.84a Because a large part of the population has a tongue coating or gingivitis/periodontitis, there is a risk that an intraoral condition is too easily considered as the cause while more important pathologies are overlooked. Indeed, for a minority of patients (4% in the same recent study), extraoral causes can be identified, including ear-nose-throat (ENT) pathologies, systemic diseases (e.g., diabetes), metabolic or hormonal changes, hepatic or renal insufficiency, bronchial and pulmonary diseases, or gastroenterologic pathologies.20,69,83,123
In general, one can identify two pathways for bad breath. The first one involves an increase of certain metabolites in the blood circulation (e.g., due to a systemic disease), which will escape via the alveoli of the lungs during breathing (blood-gas exchange). The second pathway involves an increase of either the bacterial load or the amount of substrates for these bacteria at one of the lining surfaces of the oropharyngeal cavity, the respiratory tract, or the esophagus. All types of infections, ulcerations, or tumors at one of the previously mentioned areas can thus lead to bad breath. The most commonly involved bacteria are Porphyromonas gingivalis, Prevotella intermedia/nigrescens, Aggregatibacter actinomycetemcomitans (previously Actinobacillus actinomycetemcomitans), Campylobacter rectus, Fusobacterium nucleatum, Peptostreptococcus micros, Tannerella forsythia, Eubacterium spp, and spirochetes.
In a special patient category, subjects imagine they have breath malodor; this is called imaginary breath odor or halitophobia.75 The latter has been associated with obsessive-compulsive disorders and hypochondria.
Well-established personality disorder questionnaires (e.g., SCL-90) allow the clinician to assess the patient’s tendency for illusional breath malodor.21,23,24 The presence of a psychologist or psychiatrist at the malodor consultation can be especially helpful for such patients. Because of the complexity of this pathology, a malodor consultation is thus preferably multidisciplinary, combining the knowledge of a periodontologist or dentist, an ENT specialist, if necessary an internist, and a psychologist or psychiatrist. In the recent study of the department with 2000 patients, 16% were diagnosed with pseudo-halitosis or even halitophobia.84a
For oral malodor, the unpleasant smell of the breath mainly originates from VSCs, especially hydrogen sulfide (H2S), methylmercaptan (CH3SH), and less important dimethyl sulfide [(CH3)2S], as first discovered by Tonzetich.117 However, in certain conditions (e.g., when the saliva dries out on the mucosal surfaces), other compounds in mouth air may also play a role such as diamines (e.g., putrescine, cadaverine), indole, skatole, and volatile organic acids like butyric or propionic acid.33 Most of these compounds result from the proteolytic degradation by oral microorganisms of peptides present in saliva (sulfur-containing or non–sulfur-containing amino acids (Figure 29-1), shed epithelium, food debris, gingival crevicular fluid (GCF), interdental plaque, postnasal drip, and blood. In particular, gram-negative, anaerobic bacteria possess such proteolytic activity.
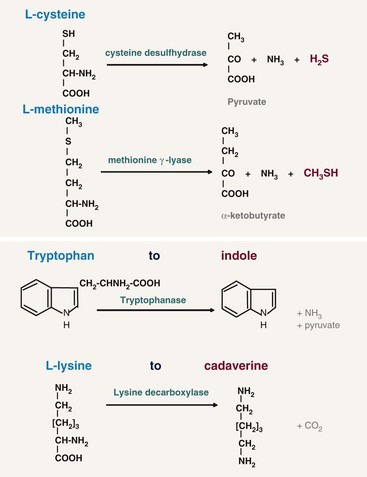
Figure 29-1 Proteolytic degradation by oral microorganisms of four amino acids (two containing sulfur and two not containing sulfur) to malodorous compounds.
For the extraoral causes of halitosis, other compounds besides the VSCs may be involved, which have not all been identified yet.50 Bad smelling metabolites can be formed/absorbed at any place in the body (e.g., the liver, the gut) and be transported by the bloodstream to the lungs. Exhalation of these volatiles in the alveolar air then causes halitosis, at least when the concentrations of the bad smelling metabolites are sufficiently high. The crevicular fluid reflects the circulating molecules in the blood and can thus also play a relevant role but due to the small amount probably not a very dominant one. The extraoral causes are much more difficult to detect, although they can sometimes be recognized by a typical odor. Uncontrolled diabetes mellitus can be associated with a sweet odor of ketones, liver disease can be revealed by a sulfur odor, and kidney failure can be characterized by a fishy odor because of the presence of dimethylamine and trimethylamine.83
Intraoral Causes
Tongue and Tongue Coating
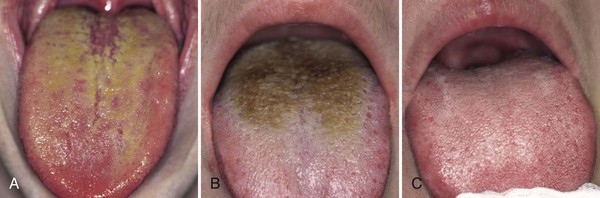
The dorsal tongue mucosa, with an area of 25 cm2, shows a very irregular surface topography.16,108 The posterior part exhibits a number of oval cryptolymphatic units, which roughen the surface of this area. The anterior part is even rougher because of the high number of papillae: the filiform papillae with a core of 0.5 mm in length, a central crater and uplifted borders; the fungiform papillae, 0.5 to 0.8 mm in length; the foliate papillae, located at the edge of the tongue, separated by deep folds; and the vallate papillae, 1 mm in height and 2 to 3 mm in diameter. These innumerable depressions in the tongue surface are ideal niches for bacterial adhesion and growth, sheltered from cleaning actions.18,131 Moreover, desquamated cells and food remnants also remain trapped in these retention sites and consequently can be putrefied by the bacteria.5 A fissurated tongue (deep fissures on dorsum, also called scrotal tongue or lingua plicata) and a hairy tongue (lingua villosa) have an even rougher surface (Figure 29-2).
The accumulation of food remnants intermingled with exfoliated cells and bacteria causes a coating on the tongue dorsum. The latter cannot be easily removed because of the retention offered by the irregular surface of the tongue dorsum (see Figure 29-2). As such, the two factors essential for putrefaction are united. Several investigators have identified the dorsal posterior surface of the tongue as the primary source of breath malodor.5,15,18,99 Indeed, high correlations have been reported between tongue coating and odor formation.5,18,63,131
Studies also suggest that oral malodor is associated with the total bacterial load of anaerobic bacteria in both saliva and tongue coating.89
Periodontal Infections
Several studies have shown a relationship between periodontitis and oral malodor. However, not all patients with gingivitis and/or periodontitis complain about bad breath, and there is some disagreement in the literature as to what extent oral malodor and periodontal disease are related.5,98,110 Bacteria associated with gingivitis and periodontitis are indeed able to produce VSCs.*
A study of Niles and Gaffar made clear that these gram-negative species in particular can cause an unpleasant smell by production of sulfur compounds.70 However, because of the large species diversity found in patients with halitosis, it can be suggested that breath malodor is the result of complex interactions between several bacterial species. A recent study indicates that some gram-positive microorganisms, such as Streptococcus salivarius, also contribute to oral malodor production by deglycosylating salivary glycoproteins, thus exposing their protein core to further degradation by gram-negative microorganisms.111 Recently, correlations have been found between Solobacterium moorei, a gram-positive bacillus, and oral malodor.38,45
Several studies have shown that the VSC levels in the mouth correlate positively with the depth of periodontal pockets (the deeper the pocket, the more bacteria, particularly anaerobic species) and that the amount of VSCs in breath increases with the number, depth, and bleeding tendency of the periodontal pockets.15,77,132 VSCs aggravate the periodontitis process by increasing the permeability of the pocket and mucosal epithelium and therefore exposing the underlying connective tissues of the periodontium to bacterial metabolites. Moreover, methylmercaptan enhances interstitial collagenase production, interleukin-1 (IL-1) production by mononuclear cells, and cathepsin B production, thus further mediating connective tissue breakdown.54,91 It was also shown that human gingival fibroblasts developed an affected cytoskeleton when exposed to methyl mercaptan.8,91 The same gas-altered cell proliferation and migration. VSCs are also known to impede wound healing. Thus, when periodontal surgery is planned, especially the insertion of implants, clinicians should recognize this pathologic role of VSCs.
Some studies, however, have shown that when the presence of tongue coating is taken into account, the correlation between periodontitis and oral malodor is much lower, indicating that tongue coating remains a key factor for halitosis. The prevalence of tongue coating is six times higher in patients with periodontitis, and the same bacterial species associated with periodontal disease can also be found in large numbers on the dorsum of the tongue, particularly when tongue coating is present.131 The reported association between periodontitis and oral malodor may thus primarily be due to the effects of periodontal disease on tongue coating. This theory is supported by a number of studies that have not found evidence for a strong direct association between periodontitis and risk of oral malodor.5,110
Other relevant malodorous pathologic manifestations of the periodontium are pericoronitis (the soft tissue “cap” being retentive for microorganisms and debris), major recurrent oral ulcerations, herpetic gingivitis, and necrotizing gingivitis/periodontitis. Microbiologic observations indicate that ulcers infected with gram-negative anaerobes (i.e., Prevotella and Porphyromonas species) are significantly more malodorous than noninfected ulcers.6
Dental Pathologies
Possible causes within the dentition are deep carious lesions with food impaction and putrefaction, extraction wounds filled with a blood clot, and purulent discharge leading to important putrefaction. Interdental food impaction in large interdental areas and crowding of teeth favor food entrapment and accumulation of debris. Acrylic dentures, especially when kept continuously in the mouth at night or not regularly cleaned, can also produce a typical smell. The denture surface facing the gingiva is porous and retentive for bacteria, yeasts, and debris, which are all factors that cause putrefaction.
Dry Mouth
Saliva has an important cleaning function in the oral cavity. Patients with xerostomia often present with large amounts of plaque on teeth and an extensive tongue coating. The increased microbial load and the escape of VSCs as gases when saliva is drying up explain the strong breath malodor.46 Several studies link stress with VSC levels, but it is not clear whether this can simply be explained by a reduction of salivary flow.52,84 Other causes of xerostomia are medication,64 alcohol abuse,28 Sjögren’s syndrome (a common autoimmune rheumatic disease),58 and diabetes.127
Extraoral Causes
Ear-Nose-Throat
During chronic or purulent tonsillitis, the deep crypts of the tonsils accumulate debris and bacteria, especially periopathogens, resulting in putrefaction. In the crypts, even calculus (e.g., subgingivally) can be formed (tonsilloliths or tonsil stones). Other examples include acute pharyngitis (viral or bacterial) and postnasal drip. The latter is a rather common condition, which is perceived by patients as a liquid flow in the throat, originating from the nasal cavity.99 It is often associated with chronic sinusitis or regurgitation esophagitis, in which the acidic content of the stomach reaches the nasopharynx and causes mucositis. Ozena (caused by Klebsiella ozaenae) is a rare atrophic condition of the nasal mucosa, with the appearance of crusts, that causes a very strong breath malodor. Finally, a foreign body in a nasal or sinus cavity can cause local irritation, ulceration, and subsequent putrefaction (e.g., children and mentally handicapped persons tend to put objects such as peas or wet paper in the nose).
Bronchi and Lungs
Pulmonary causes include chronic bronchitis, bronchiectasis (infection of standing mucus secretion in cystic dilations through walls of bronchioles), pneumonia, pulmonary abscesses, bronchial carcinoma, and carcinoma of the lung.57,59 The relevance of an early diagnosis is evident.
Gastrointestinal Tract
In contrast to the common public opinion, even among medical physicians, gastrointestinal pathologies are rarely responsible for bad breath.73 The following pathologies might be responsible for less than 1% of malodor cases:
There is some disagreement in the literature whether Helicobacter pylori infection is associated with halitosis.44,66 In a recent paper, H. pylori was shown to produce hydrogen sulfide and methylmercaptan, which suggests that this microorganism can contribute to the development of halitosis.55
Liver
Patients with various degrees of hepatocellular failure and/or portosystemic shunting of blood may acquire a sweet, musty, or even slightly fecal aroma of the breath, termed fetor hepaticus, which has been mainly attributed to the accumulation of dimethyl sulfide.10,116,122 Moreover, if the metabolizing function of the liver fails, the concentration of certain metabolites, normally processed in the liver, will increase and they will enter the systemic circulation again and will be exhaled.
Kidney
Kidney insufficiency, primarily caused by chronic glomerulonephritis, will lead to an increase of the amines dimethylamine and trimethylamine, which causes a typical fishy odor of the breath.109
Systemic Metabolic Disorders
Uncontrolled diabetes mellitus results in the accumulation of ketones, which have a sweet smell, like the odor of rotten apples. Insulin resistance leads to an increase of triglycerides and free fatty acids and ketones (like acetone, acetoacetate, and hydroxybutyrate) are formed during lipolysis.112
Trimethylaminuria
Trimethylaminuria is a hereditary metabolic disorder that leads to a typical fishy odor of the breath, urine, sweat, and other bodily secretions. Trimethylaminuria is an enzymatic defect that prevents the transformation of trimethylamine to trimethylaminoxide, resulting in abnormal amounts of this molecule. The prevalence is unknown but approaches 1% in the United Kingdom.62
Hormonal Causes
At certain moments during the menstrual cycle, a typical breath odor can develop; partners are often well aware of this odor. Evidence also indicates that VSC levels in the expired air are increased twofold to fourfold around the day of ovulation and in the perimenstrual period. Increases in VSCs are smaller in midfollicular phases.83
Physiology of Malodor Detection
The breath of a person contains up to 150 different molecules.81,120,121 The characteristics of the expired molecules determine whether we can smell them or not. Some gases can cause a striking odor at very low concentrations, whereas others need to be present in much higher quantities. The perception of the molecules depends on the following factors34:
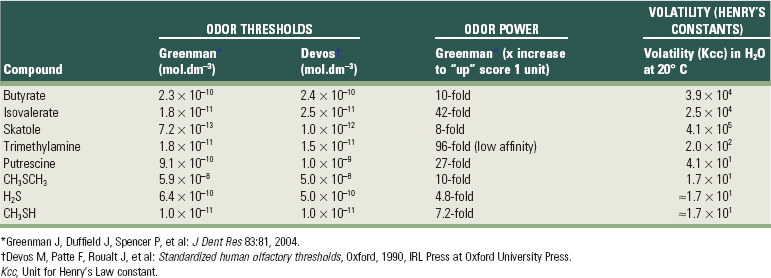
Table 29-1 provides an overview of these essential characteristics for the key malodorous compounds, clearly highlighting large interproduct variations. The odor power is the strongest for hydrogen sulfide and methylmercaptan; if the concentration of these products increases fivefold to tenfold, the odor will receive a higher organoleptic rating. For some other compounds, increases of 25 to 100 times are needed to reach a similar effect. Skatole and methyl mercaptan are detected at the lowest concentrations (low threshold). The three sulfur molecules have the lowest volatility (i.e., will escape the liquid phase first).34
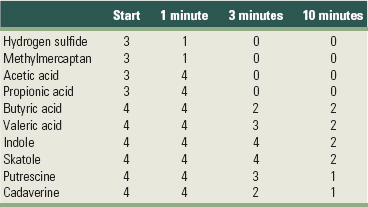
In a study of Kleinberg and Codipilly, aqueous solutions of oral odoriferous volatiles were placed on the skin of the back of the hand.46 Afterward, odor scores were given (organoleptic score, cfr. supra). The results are shown in Table 29-2. All metabolites caused an explicit odor, which decreased in intensity over time. Some molecules disappeared very fast (e.g., hydrogen sulfide and methylmercaptan), whereas others produced a bad smell for a longer period of time (e.g., indole and skatole, for 10 minutes and longer).
The olfactory response, rated by an organoleptic scale, follows an exponential curve when correlated with the concentration of different gases. In other words, when the concentrations of the molecules were compared to their organoleptic outcome, an optimal fit was only obtained when the concentrations of the gases were transformed to log values. The latter implies that all types of breath “measurements” (gas chromatography [GC], GC-mass spectrometer [MS], Halimeter, sulfur sensors, high-performance liquid chromatography [HPLC]) require log transformation to be comparable with organoleptic scores.34
Diagnosis of Malodor
Medical History
The proper diagnostic approach to a malodor patient starts with a thorough questioning about the medical history. Asking about all the relevant pathologies for breath malodor just discussed is not time-consuming; it may save time and expenses to achieve a proper differential diagnosis. As often repeated, “listen to the patient and the patient will tell you the diagnosis.” It should take place at the clinician’s desk in private and before any clinical examination (not in a dental chair). This will encourage the proper confidence needed for these patients. The patient’s history should be discretely and intermittently noted. The clinician should ask about the frequency (e.g., every month), the time of appearance during the day (e.g., after meals can indicate a stomach hernia), the time when the problem first appeared, whether others (nonconfidants) have identified the problem (to exclude imaginary breath odor), which medications are taken, and whether there are possible contributing factors such as mouth breathing, dry mouth, allergies, and nasal problems.
Clinical and Laboratory Examination
Self-Examination
It can be worthwhile to involve the patient in monitoring the results of therapy by self-examination, especially when an intraoral cause has been identified. For example, this can motivate the patient to continue the oral hygiene instructions. The following self-testing can be used:
Removing the odorous substances from the body allows a less emotional and thus more objective assessment. Smelling one’s own breath by expiring in the hands kept in front of the mouth is not relevant because the nose gets used to the odor and the smell of the skin and soaps used for handwashing may interfere. Moreover, studies have shown that self-assessment of oral malodor is notoriously unreliable and one should be careful with the information obtained from the patient.23
Oropharyngeal Examination
The oropharyngeal examination includes inspection of deep carious lesions, interdental food impaction, wounds, bleeding of the gums, periodontal pockets, tongue coating, dry mouth, and the tonsils and pharynx (for tonsillitis and pharyngitis).
Organoleptic Rating
Even though devices are available, the organoleptic assessment by a judge is still the “gold standard” in the examination of breath malodor. It is the easiest and most often used method because it gives a reflection of the everyday situation when halitosis is noticed. Moreover, the human nose can smell 10,000 different odors.39 In an organoleptic evaluation, a trained and preferably calibrated “judge” sniffs the expired air and assesses whether it is unpleasant by using an intensity rating, normally from 0 to 5, as proposed by Rosenberg and McCulloch.101 It is thus solely based on the olfactory organs of the clinician: 0 = no odor present, 1 = barely noticeable odor, 2 = slight but clearly noticeable odor, 3 = moderate odor, 4 = strong offensive odor, and 5 = extremely foul odor.
Before acting as a judge, persons must ensure they do not have anosmia (lost or impaired smelling capacity). A significant fraction of the adult population has partial loss of smelling acuity. After age 60, a decline of smelling acuity is common. Candidate odor judges should test their capacity to smell and recognize different odors (qualitative assessment), as well as their capacity to detect odors at low concentrations (quantitative assessment).22 The first aspect can be checked by using a commercially available test (Smell Identification test, Sensonic) that establishes a response curve based on the capacity to recognize and discriminate among smells. After scratching an odorous surface in a booklet, several options of smells are proposed. If a subject lacks the capacity to recognize certain odors, it will reveal a partial anosmia. The second aspect is tested by sniffing a series of dilutions of substances such as isovaleric acid, phenethyl alcohol, thiophene and pyridine, which are inexpensive organic components. These are presented to the candidate judge as dilutions, in one-log dilution steps, from 1 to 10−19. Concentrations of the odorous substance are presented in ascending order until the subject detects the substance, then in descending order until the person no longer detects it. This is the “psychophysical staircase method” for determination of the threshold level. The threshold corresponds to the average between ascending and descending levels. Clinicians can thus find out if their smelling threshold level is normal. Abnormalities in the capacity to judge or perceive odors can be caused by a viral infection of the nasal cavities, a concussion, and smoking. Whereas an infection can have transient effects on olfactory performance, a concussion and smoking have permanent effects.
To obtain a reliable result, both the judge and patient need to take a number of restrictions into account. Two days before the measurements they have to avoid the intake of spicy food, garlic, and onions. The use of any fragrance, shampoo, body lotion, tooth paste, or mouthrinse; smoking; and the consumption of alcohol or coffee are prohibited 12 hours before the organoleptic assessment is made. The patient should also not eat or drink on the morning of the organoleptic evaluation. The judge should not wear rubber gloves. Assessments should be repeated because breath odor can fluctuate from day to day. The patient should be encouraged to bring a confidant to the consultations who can identify whether the perceived odor is the one previously noticed.
The judge smells a series of different air samples (Figure 29-3), as follows:

Figure 29-3 A, Organoleptic assessment of the expired air. B, preferably, two calibrated experts will compare their scores at testing. C, Patient is asked to lick her wrist. D, After allowing the wrist to dry, the clinicians organoleptically assess it to evaluate the volatiles originating from the saliva and anterior part of the tongue. E, Inspection of the posterior part of the tongue and pharynx can reveal coating, ulcerations, or inflammation. F, A sample of the coating is taken from the back of the tongue, which is pulled with a gauze pad. G, Clinician smells the air expired by the nose.
Sometimes, the specific character of the odor can provide additional information such as the following:
Although the organoleptic assessment is still the gold standard for diagnosis of halitosis, the method also has some important drawbacks. The assessment can, for example, be influenced by several aspects like the position of the head, hunger, and the experience of the judge. Odor judges are also supposed to rest their noses for several minutes between the tests to avoid habituation. The most important disadvantage of the method, however, is that it clearly has a degree of subjectivity. Researchers are trying to improve the reliability and reproducibility of the organoleptic method.35 When using a panel of odor judges instead of one judge, the reliability is already considered to increase.130 Furthermore, the agreement among judges may be improved by standardization of the sense of smell using an odor solution kit for measuring the olfactory response.136 Training is also considered to reduce the odor judges’ errors.67
Portable Volatile Sulfur Monitor
The portable volatile sulfur monitor (Halimeter, Interscan, Chatsworth, CA) is an electronic device that analyzes the concentration of hydrogen sulfide and methyl mercaptan but without discriminating them (Figure 29-4). The mouth air is aspirated by inserting a drinking straw fixed on the flexible tube of the instrument. The straw is kept about 2 cm behind the lips, without touching any surface, while the subject keeps the mouth slightly open and breathes through the nose. The sulfur meter uses a voltametric sensor that generates a signal when exposed to sulfur-containing gases.

Figure 29-4 A, Portable sulfide monitor. B, Patient holds the aspirating tube in his mouth. Digital reading reveals the total amount of volatile sulfur compounds (VSCs) in parts per billion (ppb).
Absence of breath malodor leads to readings of 150 ppb or lower. Patients with elevated concentrations of VSCs easily reach 300 to 400 ppb. Using a recorder or specific software, a graphic presentation can be obtained, called a haligram, that gives the response in function of the time. The monitor needs regular calibration and replacement of the sensor biannually.
The Halimeter is easy to use as a chairside test and is relatively inexpensive. Patients are usually less embarrassed, and the absence of odor in case of halitophobia can be more convincingly proven than by organoleptic assessment. Several studies have shown good correlations between the organoleptic measurement and the Halimeter.103,104 The most important drawback of the device is that it detects only sulfur compounds and thus is used only for intraoral causes of halitosis. The absence of VSCs does not prove that no breath odor is present. The instrument has no specificity and can thus not discriminate among the different sulfur compounds. The sensitivity for methylmercaptan is five times lower than for hydrogen sulfide, and the device is almost insensitive to dimethyl sulfide. Moreover, ethanol and other compounds can disturb the measurements.
Gas Chromatography
A gas chromatography device can analyze air, saliva, or crevicular fluid (Figure 29-5). About 100 compounds have been isolated from the headspace of saliva and tongue coating, from ketones to alkanes and sulfur-containing compounds to phenyl compounds.14 In the expired air of a person, approximately 150 compounds can be found.81,120 The most important advantage of the technique (together with mass spectrometry) is that it can detect virtually any compound when using adequate materials and conditions. Moreover, it has a very high sensitivity and specificity.

Figure 29-5 Gas chromatography machinery, including thermal desorber (TD) to release molecules trapped in special collectors); gas chromatograph (GC) for separation of molecules; and mass spectrometer (MS) for identification of molecules.
Elaborate gas chromatography is only available in specialized centers but is especially useful for identifying nonoral causes.42,80,113,122 It is expensive and needs trained personnel.
Recently, a small, portable “gas chromatograph” (OralChroma, Abilit, Japan) has been introduced, which makes this technique available for periodontal clinics (Figure 29-6). It has the capacity to measure the concentration of the three key sulfur compounds (hydrogen sulfide, methylmercaptan, and dimethyl sulfide) separately. This can be helpful for a differential diagnosis. A high concentration of methylmercaptan compared to hydrogen sulfide indicates for example periodontitis.132 If only hydrogen sulfide is increased, there might be a problem with oral hygiene. Dimethyl sulfide can be present in case of some extraoral causes.116 Just like the Halimeter, the OralChroma cannot detect other than sulfur compounds and some intraoral and extraoral causes can thus be overlooked. The apparatus needs calibration and the sensor and column need to be replaced every 2 years.
Dark-Field or Phase-Contrast Microscopy
Gingivitis and periodontitis are typically associated with a higher incidence of motile organisms and spirochetes, so shifts in these proportions allow monitoring of therapeutic progress. Another advantage of direct microscopy is that the patient becomes aware of bacteria being present in plaque, tongue coating, and saliva. Too often, patients confuse plaque with food remnants.
Saliva Incubation Test
The analysis of the headspace above incubated saliva by gas chromatography reveals next to VSCs also other compounds like indole, skatole, lactic acid, methylamine, diphenylamine, cadaverine, putrescine, urea, ammonia, dodecanol, and tetradecanol. By adding some proteins, such as lysine or cysteine, the production of respectively cadaverine or hydrogen sulfide is dramatically increased. Organoleptic evaluation (or assessment of the VSCs) of the saliva headspace offers promising perspectives for monitoring treatment results.85 It is a less invasive test, especially for the patient, than smelling breath in front of the oral cavity.
Electronic Nose
Electronic noses identify the specific components of an odor and analyze its chemical makeup. They consist of a mechanism for chemical detection, such as an array of electronic sensors, and a mechanism for pattern recognition. They are smaller, less expensive, and easier to use than for example gas chromatography but can only be developed for specific applications if the important metabolites are already known. An artificial nose that has the same capacities as the human nose would be ideal. Currently, although significant improvements still need to be made, the first trials thus far have been promising.115
Treatment of Oral Malodor
The treatment of oral malodor (thus of intraoral origin) should preferably be cause related. Because oral malodor is caused by the metabolic degradation of available proteins to malodorous gases by certain oral microorganisms, the following general treatment strategies can be applied:
![]() Science Transfer
Science Transfer
The most common cause of oral malodor is bacterial metabolism in the oral cavity. Many of the foul-smelling molecules come from the anaerobic metabolism of proteins with subsequent increases in volatile sulfur-containing compounds (VSC) including hydrogen sulfide. The bacteria present in the biofilm attached to teeth, particularly those seen in the subgingival plaque of patients, play a major role in VSC production. Bacterial coating of the tongue are also important contributors to oral malodor.
Much of oral malodor can be controlled by periodontal therapy aimed at plaque removal and pocket reduction, coupled with an effective patient centered daily oral hygiene routine that includes toothbrushing and tongue scraping. The use of antimicrobal mouthwashes, such as those containing chlorhexidine, is also an important contributor to control of oral malodor.
Although electronic devices can detect some sulfur-containing compounds and gas chromotography can give an even wider and accurate measurement of up to 150 compounds, the use of the clinician’s nose to rate oral malodor on a scale of 0 to 5 is probably all that is needed to diagnose and treat oral malodor. Patients are generally unreliable in detecting oral malodor in their own mouths and so clinicans and friends and family can play a role in giving feedback on the status of bad breath.
Treatment should be centered on reducing the bacterial load/micronutrients by effective mechanical oral hygiene procedures, including tongue scraping. Periodontal disease should be treated and controlled, and as an auxiliary aid, oral rinses containing chlorhexidine and other ingredients may further reduce the oral malodor. If breath malodor persists after these approaches, other sources of the malodor, such as the tonsils, lung disease, gastrointestinal disease, and metabolic abnormalities (e.g., diabetes) should be investigated.
Issues related to oral malodor emphasize the clinician’s need for good diagnostic skills and stipulate an appreciation of chemistry. First, the etiology of breath malodor can be from a large variety of intraoral and extraoral causes, and much can be gained from a careful patient history before any oral or extraoral examination. Second, knowledge of the potential volatile substances and gases (e.g., VSCs) and their formation, sources, power, substantivity, and dilution capacity allows for treatment options and rational therapeutic interventions. Furthermore, this knowledge allows the dental clinician to help predict the outcome of therapeutic interventions, such as the short-term effectiveness of masking oral malodor and the longer-term effect of mechanically and chemically reducing the oral microbial load.
Mechanical Reduction of Intraoral Nutrients and Microorganisms
Because of the extensive accumulation of bacteria on the dorsum of the tongue, tongue cleaning should be emphasized.12,105,131 Previous investigations demonstrated that tongue cleaning reduces both the amount of coating (and thus bacterial nutrients), as well as the number of bacteria and thereby improves oral malodor effectively.18,31,32,37,90 Other reports indicated that the reduction of the microbial load on the tongue after cleaning is negligible and that the malodor reduction probably results from the reduction of the bacterial nutrients.60,89
Cleaning of the tongue can be carried out with a normal toothbrush, but preferably with a tongue scraper if a coating is established.74,76 Tongue cleaning using a tongue scraper reduced the halitosis levels with 75% after 1 week.76 This should be gentle cleaning to prevent soft tissue damage. It is best to clean as far backward as possible; the posterior portion of the tongue has the most coating.100 Tongue cleaning should be repeated until almost no coating material can be removed.13 Gagging reflexes often are elicited, especially when using brushes89; practice helps to prevent this.11 It can also be helpful to pull the tongue out with a gauze pad. Tongue cleaning has the additional benefit of improving taste sensation.89,129
Interdental cleaning and toothbrushing are essential mechanical means of dental plaque control. Both remove residual food particles and organisms that cause putrefaction. Clinical studies have shown that exclusively brushing the teeth has no appreciable influence on the concentration of VSCs.114 In a short-term study, a combination of tooth and tongue brushing or toothbrushing alone had a beneficial effect on bad breath for up to 1 hour (73% and 30% reduction in VSCs, respectively).118
Because periodontitis can cause chronic oral malodor, professional periodontal therapy is needed.5,15,77,132 A one-stage, full-mouth disinfection, combining scaling and root planing with the application of chlorhexidine, reduced the organoleptic malodor levels up to 90%.87 In a recent study of the same authors, initial periodontal therapy had only a weak impact on the VSC levels, except when combined with a mouthrinse containing chlorhexidine.85
Chewing gum may control bad breath temporarily because it can stimulate salivary flow.93 The salivary flow itself also has a mechanical cleaning capability. Not surprisingly, therefore, subjects with extremely low salivary flow rate have higher VSC ratings and tongue coating scores than those with normal saliva production.49 Waler128 showed that chewing of a gum without any active ingredient can reduce halitosis modestly.
On the other hand, clinicians can use bacterial nutrients to provoke malodor, to prove an intraoral origin of the bad breath for example, or to test the efficacy of different oral rinses. Rinsing with amino acids (e.g., cysteine challenge test) can result in a dramatic increase in hydrogen sulfide47 (see Figure 29-1).
Chemical Reduction of Oral Microbial Load
Mouth rinsing has become a common practice in patients with oral malodor.29 The active ingredients in oral rinses are usually antimicrobial agents such as chlorhexidine, cetylpyridinium chloride (CPC), essential oils, chlorine dioxide, hydrogen peroxide, and triclosan. All these agents have only a temporary reducing effect on the total number of microorganisms in the oral cavity.
Chlorhexidine
Chlorhexidine is considered the most effective antiplaque and antigingivitis agent.1-443 Its antibacterial action can be explained by disruption of the bacterial cell membrane by the chlorhexidine molecules, increasing its permeability and resulting in cell lysis and death.43,53 Because of its strong antibacterial effects and superior substantivity in the oral cavity, chlorhexidine rinsing provides significant reduction in VSC levels and organoleptic ratings.9,102,103,126,134
Rosenberg et al103 showed that a 0.2% chlorhexidine regimen produced a 43% reduction in VSC values and a greater than 50% reduction in organoleptic mouth odor ratings. De Boever and Loesche18 reported that a 1-week rinsing with 0.12% chlorhexidine gluconate, in combination with tooth and tongue brushing, significantly reduced VSC levels, mouth odor, and tongue odor by 73%, 69%, and 78%, respectively. Morning halitosis was reduced up to 90%.126 Halita (Dentaid, Spain), a new solution (0.05% chlorhexidine, 0.05% cetylpiridine chloride [CPC], 0.14% zinc lactate, no alcohol), has been even more efficient than chlorhexidine alone, suggesting that the other compounds are also important.95,97,126 This is explained by a synergistic effect between chlorhexidine and CPC on one hand and by the Zn++ on the other hand (see following discussion).
Unfortunately, as mentioned in some trials, chlorhexidine at concentrations ≥0.2% also has some disadvantages such as the increased tooth and tongue staining, bad taste, and some temporary reduction in taste sensation.25
Essential Oils
Previous studies evaluated the short-term effect (3 hours) of a Listerine rinse (which contains essential oils) compared with a placebo rinse.82 Listerine was found to be only moderately effective against oral malodor (±25% reduction versus 10% for placebo of VSCs after 30 minutes) and caused a sustained reduction in the levels of odorigenic bacteria. Similar VSC reductions were found after rinsing for 4 days.9
Chlorine Dioxide
Chlorine dioxide (ClO2) is a powerful oxidizing agent that can eliminate bad breath by oxidation of hydrogen sulfide, methylmercaptan, and the amino acids, methionine and cysteine. Studies demonstrated that single use of a ClO2–containing oral rinse slightly reduces mouth odor.26,27
Two-Phase Oil-Water Rinse
Rosenberg et al102 designed a two-phase oil-water rinse containing CPC. The efficacy of oil-water-CPC formulations is thought to result from the adhesion of a high proportion of oral microorganisms to the oil droplets, which is further enhanced by the CPC. A twice-daily rinse with this product (before bedtime and in the morning) showed reductions in both VSC levels and organoleptic ratings. These reductions were superior to Listerine and significantly superior to a placebo.51,102
Triclosan
Triclosan, a broad-spectrum antibacterial agent, has been found to be effective against most oral bacteria and has a good compatibility with other compounds used for oral home care. A pilot study demonstrated that an experimental mouth rinse containing 0.15% triclosan and 0.84% zinc produced a stronger and more prolonged reduction in mouth odor than a Listerine rinse.92 The anti-VSC effect of triclosan, however, seems strongly dependent on the solubilizing agents.133
Aminefluoride/Stannous Fluoride
The association of aminefluoride with stannous fluoride (AmF/SnF2) resulted in encouraging reductions of morning breath odor, even when oral hygiene is insufficient.88
Hydrogen Peroxide
Suarez et al114 reported that rinsing with 3% hydrogen peroxide (H2O2) produced impressive reductions (±90%) in sulfur gases that persisted for 8 hours.
Oxidizing Lozenges
Greenstein et al36 reported that sucking a lozenge with oxidizing properties reduces tongue dorsum malodor for 3 hours. This antimalodor effect may be caused by the activity of dehydroascorbic acid, which is generated by peroxide-mediated oxidation of ascorbate present in the lozenges.
Conversion of Volatile Sulfur Compounds
Metal Salt Solutions
Metal ions with affinity for sulfur are efficient in capturing the sulfur-containing gases. Zinc is an ion with two positive charges (Zn++), which will bind to the twice–negatively loaded sulfur radicals, and thus can reduce the expression of the VSCs. The same applies for other metal ions such as mercury and copper. Clinically, the VSC inhibitory effect was CuCl2 > SnF2 > ZnCl2. In vitro, the inhibitory effect was HgCl2 = CuCl2 = CdCl2 > ZnCl2 > SnF2 > SnCl2 > PbCl2.135
Compared with other metal ions, Zn++ is relatively nontoxic and noncumulative and gives no visible discoloration. Thus, Zn++ has been one of the most-studied ingredients for the control of oral malodor.128,135 Schmidt and Tarbet107 already reported that a rinse containing zinc chloride was remarkably more effective than a saline rinse (or no treatment) in reducing the levels of both VSCs (±80% reduction) and organoleptic scores (±40% reduction) for 3 hours.
As mentioned, Halita, a rinse containing 0.05% chlorhexidine, 0.05% CPC and 0.14% zinc lactate, has been even more efficient than a 0.2% chlorhexidine formulation in reducing the VSC levels and organoleptic ratings.86,126 The special effect of Halita may result from the VSC conversion ability of zinc, besides its antimicrobial action. The combination Zn++ and chlorhexidine seems to act synergistically.134
Toothpastes
Baking soda dentifrices have been shown to confer a significant odor-reducing benefit for time periods up to 3 hours.7,70 The mechanisms by which baking soda produces its inhibition of oral malodor might be related to its bactericidal effects and its transformation of VSCs to a nonvolatile state.
Gerlach et al30 compared the antimalodor efficacy of three different toothpastes and reported a slightly better outcome, especially for an SnF2-containing paste (±50% reduction), when compared to water (±35% reduction). In a study by Hoshi and van Steenberghe,40 a zinc citrate/triclosan toothpaste applied to the tongue dorsum appeared to control morning breath malodor for 4 hours. If the flavor oil was removed, however, the antimalodor efficacy of the active ingredients decreased. Another clinical study reported an up to 41% reduction in VSC levels after 7 days’ use of a dentifrice containing triclosan and a copolymer, but the benefit compared with a placebo was relatively small (17% reduction).71 Similar reductions were also found in two other more recent studies.41,72
Chewing Gum
Chewing gum can be formulated with antibacterial agents, such as fluoride or chlorhexidine, thus helping reduce oral malodor through both mechanical and chemical approaches. Tsunoda et al119 investigated the beneficial effect of chewing gum containing tea extracts for its deodorizing mechanism. Epigallocatechin (EGCg) is the main deodorizing agent among the tea catechins. The chemical reaction between EGCg and CH3SH results in a nonvolatile product. Waler128 compared different concentrations of zinc in a chewing gum and found that a 2-mg Zn++ acetate–containing chewing gum that remained in the mouth for 5 minutes resulted in an immediate reduction in the VSC levels of up to 45%, but the long-term effect was not mentioned.
Masking the Malodor
Treatments with rinses, mouth sprays, and lozenges containing volatiles with a pleasant odor have only a short-term effect.93,94 Typical examples are the mint-containing lozenges.
Another pathway is to increase the solubility of malodorous compounds in the saliva by increasing the secretion of saliva; a larger volume allows the retention of larger volumes of soluble VSCs.48 The latter can also be achieved by ensuring a proper liquid intake or by using a chewing gum; chewing triggers the periodontal-parotid reflex, at least when the lower (pre)molars are still present.
Summary
Breath malodor has important socioeconomic consequences and can reveal important diseases. A proper diagnosis and determination of the etiology allow initiation of the proper etiologic treatment. Although tongue coating and (less frequently) periodontitis and gingivitis are by far the most common causes of malodor, a clinician cannot take the risk of overlooking other, more challenging diseases. This can be done by a multidisciplinary consultation or if this is not feasible a trial therapy to deal quickly with intraoral causes (e.g., the full-mouth one-stage disinfection, including the use of the proper mouthrinses, tongue scrapers, and toothpastes). For more detailed information, the reader is encouraged to consult van Steenberghe124 and recent review papers.96,106,125
1 Addy M, Moran JM. Clinical indications for the use of chemical adjuncts to plaque control: chlorhexidine formulations. Periodontol 2000. 1997;15:52.
2 Addy M, Renton-Harper P. The role of antiseptics in secondary prevention. In: Lang NP, Karring T, editors. Proceedings of the second European Workshop in Periodontology. London: Quintessence, 1997.
3 Addy M, Moran J, Wade W. Chemical plaque control in the prevention of gingivitis and periodontitis. In: Lang NP, Karring T, editors. Proceedings of the 1st European Workshop in Periodontology. London: Quintessence, 1994.
4 Bollen CM, Quirynen M. Microbiological response to mechanical treatment in combination with adjunctive therapy: a review of the literature. J Periodontol. 1996;67:1143.
5 Bosy A, Kulkarni GV, Rosenberg M, et al. Relationship of oral malodor to periodontitis: evidence of independence in discrete subpopulations. J Periodontol. 1994;65:37.
6 Bowler PG, Davies BJ, Jones SA. Microbial involvement in chronic wound malodour. J Wound Care. 1999;8:216.
7 Brunette DM, Proskin HM, Nelson BJ. The effects of dentifrice systems on oral malodor. J Clin Dent. 1998;9:76.
8 Brunette DM, Ouyang Y, Glass-Brudzinski J, et al. Effects of methyl mercaptan on human gingival fibroblast shape, cytoskeleton and protein synthesis and the inhibition of its effect by Zn++. In: van Steenberghe D, Rosenberg M, editors. Bad breath: a multidisciplinary approach. Leuven, Belgium: Leuven University Press, 1996.
9 Carvalho MD, Tabchoury CM, Cury JA, et al. Impact of mouthrinses on morning bad breath in healthy subjects. J Clin Periodontol. 2004;31(2):85.
10 Chen S, Zieve L, Mahadevan V. Mercaptans and dimethyl sulfide in the breath of patients with cirrhosis of the liver: effect of feeding methionine. J Lab Clin Med. 1970;75:628.
11 Christensen GJ. Why clean your tongue? J Am Dent Assoc. 1998;129:1605.
12 Cicek Y, Orbak R, Tezel A, et al. Effect of tongue brushing on oral malodor in adolescents. Pediatr Int. 2003;45:719.
13 Clark GT, Nachnani S, Messadi DV. Detecting and treating oral and nonoral malodors. J Calif Dent Assoc. 1997;25:133.
14 Claus D, Geypens B, Rutgeers P, et al. Where gastroenterology and periodontology meet: determination of oral volatile organic compounds using closed-loop trapping and high-resolution gas chromatography-ion trap detection. In: van Steenberghe D, Rosenberg M, editors. Bad breath: a multidisciplinary approach. Leuven, Belgium: Leuven University Press, 1996.
15 Coil J, Tonzetich J. Characterization of volatile sulfur compounds production at individual gingival cervicular sites in humans. J Clin Dent. 1992;3:97.
16 Collins LM, Dawes C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J Dent Res. 1987;66:1300.
17 Crescenzo DG, Trastek VF, Allen MS, et al. Zenker’s diverticulum in the elderly: is operation justified? Ann Thorac Surg. 1998;66:347.
18 De Boever EH, Loesche WJ. Assessing the contribution of anaerobic microflora of the tongue to oral malodor. J Am Dent Assoc. 1995;126:1384.
19 Delanghe G, Ghyselen J, Bollen C, et al. An inventory of patients’ response to treatment at a multidisciplinary breath odor clinic. Quintessence Int. 1997;30(5):307.
20 Delanghe G, Ghyselen J, van Steenberghe D, et al. Multidisciplinary breath-odour clinic. Lancet. 1997;350:187.
21 Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull. 1973;9:13.
22 Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489.
23 Eli I, Baht R, Koriat H, et al. Self-perception of breath odor. J Am Dent Assoc. 2001;132:621.
24 Eli I, Baht R, Kozlovsky A, et al. The complaint of oral malodor: possible psychopathological aspects. Psychosom Med. 1996;58:156.
25 Fedorowicz Z, Aljufairi H, Nasser M, et al: Mouthrinses for the treatment of halitosis, Cochrane Database Syst Rev 8(4):CD006701, 2008.
26 Frascella J, Gilbert R, Fernandez P. Odor reduction potential of a chlorine dioxide mouthrinse. J Clin Dent. 1998;9:39.
27 Frascella J, Gilbert RD, Fernandez P, et al. Efficacy of a chlorine dioxide–containing mouthrinse in oral malodor. Compend Contin Educ Dent. 2000;21:241.
28 Friedlander AH, Marder SR, Pisegna JR, et al. Alcohol abuse and dependence: psychopathology, medical management and dental implications. J Am Dent Assoc. 2003;134:731.
29 Gagari E, Kabani S. Adverse effects of mouthwash use: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:432.
30 Gerlach RW, Hyde JD, Poore CL, et al. Breath effects of three marketed dentifrices: a comparative study evaluating single and cumulative use. J Clin Dent. 1998;9:83.
31 Gilmore EL, Bhaskar SN. Effect of tongue brushing on bacteria and plaque formed in vitro. J Periodontol. 1972;43:418.
32 Gilmore EL, Gross A, Whitley R. Effect of tongue brushing on plaque bacteria. Oral Surg Oral Med Oral Pathol. 1973;36:201.
33 Goldberg S, Kozlovsky A, Gordon D, et al. Cadaverine as a putative component of oral malodor. J Dent Res. 1994;73:1168.
34 Greenman J, Duffield J, Spencer P, et al. Study on the organoleptic intensity scale for measuring oral malodor. J Dent Res. 2004;83:81.
35 Greenman J, El Maaytah M, Duffield J, et al. Assessing the relationship between concentrations of malodor compounds and odor scores from judges. JADA. 2005;136:749.
36 Greenstein RB, Goldberg S, Marku-Cohen S, et al. Reduction of oral malodor by oxidizing lozenges. J Periodontol. 1997;68:1176.
37 Gross A, Barnes GP, Lyon TC. Effects of tongue brushing on tongue coating and dental plaque scores. J Dent Res. 1975;54:1236.
38 Haraszthy VI, Zambon JJ, Sreenivasan PK, et al. Identification of oral bacterial species associated with halitosis. J Am Dent Assoc. 2007;138:1113.
39 Hatt H. Molecular and cellular basis of human olfaction. Chem Biodivers. 2004;1:1857.
40 Hoshi K, van Steenberghe D. The effect of tongue brushing or toothpaste application on oral malodour reduction. In: van Steenberghe D, Rosenberg M, editors. Bad breath: a multidisciplinary approach. Leuven, Belgium: Leuven University Press, 1996.
41 Hu D, Zhang YP, Petrone M, et al. Clinical effectiveness of a triclosan/copolymer/sodium-fluoride dentifrice in controlling oral malodor: a three-week clinical trial. Compend Contin Educ Dent. 2003;24(9):34.
42 Hunter CM, Niles HP, Lenton PA, et al. Breath-odor evaluation by detection of volatile sulfur compounds: correlation with organoleptic odor ratings. Compend Contin Educ Dent. 2003;24:25.
43 Jones CG. Chlorhexidine: is it still the gold standard? Periodontol 2000. 1997;15:55.
44 Katsinelos P, Tziomalos K, Chatzimavroudis G, et al. Eradication therapy in Helicobacter pylori-positive patients with halitosis: long-term outcome. Med Princ Pract. 2007;16:119.
45 Kazor CE, Mitchell PM, Lee AM, et al. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J Clin Microbiol. 2003;41:558.
46 Kleinberg I, Codipilly M. The biological basis of oral malodor formation. In: Rosenberg M, editor. Bad breath: research perspectives. Tel Aviv: Ramot Publishing, 1995.
47 Kleinberg I, Codipilly M. Cysteine challenge testing: a powerful tool for examining oral malodour processes and treatments in vivo. Int Dent J. 2002;52:221.
48 Kleinberg I, Wolff MS, Codipilly DM. Role of saliva in oral dryness, oral feel and oral malodour. Int Dent J. 2002;52(suppl 3):236.
49 Koshimune S, Awano S, Gohara K, et al. Low salivary flow and volatile sulfur compounds in mouth air. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:38.
50 Kostelc JG, Preti G, Zelson PR, et al. Volatiles of exogenous origin from the human oral cavity. J Chromatogr. 1981;226:315.
51 Kozlovsky A, Goldberg S, Natour I, et al. Efficacy of a 2-phase oil: water mouthrinse in controlling oral malodor, gingivitis, and plaque. J Periodontol. 1996;67:577.
52 Kurihara E, Marcondes FK. Oral concentration of volatile sulphur compounds in stressed rats. Stress. 2002;5:295.
53 Kuyyakanond T, Quesnel LB. The mechanism of action of chlorhexidine. FEMS Microbiol Lett. 1992;79:211.
54 Lancero H, Niu J, Johnson PW. Exposure of periodontal ligament cells to methyl mercaptan reduces intracellular pH and inhibits cell migration. J Dent Res. 1996;75:1994.
55 Lee H, Kho HS, Chung JW, et al. Volatile sulfur compounds produced by Helicobacter pylori. J Clin Gastroenterol. 2006;40:421.
56 Loesche WJ. The effects of antimicrobial mouthrinses on oral malodor and their status relative to US Food and Drug Administration regulations. Quintessence Int. 1999;30:311.
57 Lorber B. “Bad breath”: presenting manifestation of anaerobic pulmonary infection. Am Rev Respir Dis. 1975;112:875.
58 Mathews SA, Kurien BT, Scofield RH. Oral Manifestations of Sjogren’s Syndrome. J Dent Res. 2007;87:308.
59 McGregor IA, Watson JD, Sweeney G, et al. Tinidazole in smelly oropharyngeal tumours. Lancet. 1982;1:110.
60 Menon MV, Coykendall AL. Effect of tongue scraping. J Dent Res. 1994;73:1492.
61 Meskin LH. A breath of fresh air. J Am Dent Assoc. 1996;127:1282.
62 Mitchell SC, Smith RL. Trimethylaminuria: the fish malodor syndrome. Drug Metab Dispos. 2001;29:517.
63 Miyazaki H, Sakao S, Katoh Y, et al. Correlation between volatile sulphur compounds and certain oral health measurements in the general population. J Periodontol. 1995;66:679.
64 Moore PA, Guggenheimer J. Medication-induced hyposalivation: etiology, diagnosis, and treatment. Compend Contin Educ Dent. 2008;29:50.
65 Morita M, Wang HL. Relationship between sulcular sulfide level and oral malodor in subjects with periodontal disease. J Periodontol. 2001;72:79.
66 Moshkowitz M, Horowitz N, Leshno M, et al. Halitosis and gastroesophageal reflux disease: a possible association. Oral Dis. 2007;13:581.
67 Nachnani S, Majerus G, Lenton P, et al. Effect of training on odor judges scoring intensity. Oral Dis. 2005;11:40.
68 Nakano Y, Yoshimura M, Koga T. Correlation between oral malodor and periodontal bacteria. Microbes Infect. 2002;4:679.
69 Newman MG. The role of periodontitis in oral malodor: clinical perspectives. In: van Steenberghe D, Rosenberg M, editors. Bad breath: a multidisciplinary approach. Leuven, Belgium: Leuven University Press, 1996.
70 Niles HP, Gaffar A. Advances in mouth odor research. In: Rosenberg M, editor. Bad breath: research perspectives. Tel Aviv: Ramot Publishing, 1995.
71 Niles HP, Vazquez J, Rustogi KN, et al. The clinical effectiveness of a dentifrice containing triclosan and a copolymer for providing long-term control of breath odor measured chromatographically. J Clin Dent. 1999;10:135.
72 Niles HP, Hunter C, Vazquez J, et al. The clinical comparison of a triclosan/copolymer/fluoride dentifrice vs a breath-freshening dentifrice in reducing breath odor overnight: a crossover study. Oral Dis. 2005;11(1):54.
73 Norfleet RG. Helicobacter halitosis. J Clin Gastroenterol. 1993;16:274.
74 Outhouse TL, Al-Alawi R, Fedorowicz Z, at al: Tongue scraping for treating halitosis, Cochrane Database Syst Rev 19(2):CD005519, 2006.
75 Oxtoby A, Field EA. Delusional symptoms in dental patients: a report of four cases. Br Dent J. 1994;176:140.
76 Pedrazzi V, Sato S, de Mattos Mda G, et al. Tongue-cleaning methods: a comparative clinical trial employing a toothbrush and a tongue scraper. J Periodontol. 2006;75(7):1009.
77 Persson S. Hydrogen sulfide and methylmercaptan in periodontal pockets. Oral Microbiol Immunol. 1992;7:378.
78 Persson S, Claesson R, Carlsson J. The capacity of subgingival microbiotas to produce volatile sulfur compounds in human serum. Oral Microbiol Immunol. 1989;4:169.
79 Persson S, Edlund MB, Claesson R, et al. The formation of hydrogen sulfide and methylmercaptan by oral bacteria. Oral Microbiol Immunol. 1990;5:195.
80 Phillips M, Cataneo RN, Cheema T, et al. Increased breath biomarkers of oxidative stress in diabetes mellitus. Clin Chim Acta. 2004;344:189.
81 Phillips M, Herrera J, Krishnan S, et al. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl. 1999;729:75.
82 Pitts G, Brogdon C, Hu L, et al. Mechanism of action of an antiseptic, anti-odor mouthwash. J Dent Res. 1983;62:738.
83 Preti G, Lawley HJ, Hormann CA. Non-oral and oral aspects of oral malodor. In: Rosenberg M, editor. Bad breath: research perspectives. Tel Aviv: Ramot Publishing, 1995.
84 Queiroz CS, Hayacibara MF, Tabchoury CP, et al. Relationship between stressful situations, salivary flow rate and oral volatile sulfur-containing compounds. Eur J Oral Sci. 2002;110:337.
84a Quirynen M, Dadamio J, Van den Velde S, et al. Characteristics of 2000 patients who visited a halitosis clinic. J Clin Periodontol. 2009;36(11):970-973.
85 Quirynen M, Zhao H, Soers C, et al. The impact of periodontal therapy and the adjunctive effect of antiseptics on breath odor-related outcome variables: a double-blind randomized study. J Periodontol. 2005;76(5):705.
86 Quirynen M, Zhao H, van Steenberghe D. Review of the treat-ment strategies for oral malodour. Clin Oral Invest. 2002;6:1.
87 Quirynen M, Mongardini C, van Steenberghe D. The effect of a 1-stage full-mouth disinfection on oral malodor and microbial colonization of the tongue in periodontitis: a pilot study. J Periodontol. 1998;69:374.
88 Quirynen M, Avontroodt P, Soers C, et al. The efficacy of amine fluoride/stannous fluoride in the suppression of morning breath odour. J Clin Periodontol. 2002;29:944.
89 Quirynen M, Avontroodt P, Soers C, et al. Impact of tongue cleansers on microbial load and taste. J Clin Periodontol. 2004;31:506.
90 Ralph WJ. Oral hygiene—why neglect the tongue? Aust Dent J. 1988;33:224.
91 Ratkay LG, Tonzetich J, Waterfield JD. The effect of methyl mercaptan on the enzymatic and immunological activity leading to periodontal tissue destruction. In: van Steenberghe D, Rosenberg M, editors. Bad breath: a multidisciplinary approach. Leuven, Belgium: Leuven University Press, 1996.
92 Raven S, Matheson JR, Huntington E, et al. The efficacy of combined zinc and triclosan system in the prevention of oral malodour. In: van Steenberghe D, Rosenberg M, editors. Bad breath: a multidisciplinary approach. Leuven, Belgium: Leuven University Press, 1996.
93 Reingewirtz Y, Girault O, Reingewirtz N, et al. Mechanical effects and volatile sulfur compound–reducing effects of chewing gums: comparison between test and base gums and a control group. Quintessence Int. 1999;30:319.
94 Replogle WH, Beebe DK. Halitosis. Am Fam Physician. 1996;53:1215.
95 Roldan S, Herrera D, Sanz M. Clinical and microbiological efficiency efficacy of an antimicrobial mouthrinse on oral halitosis. J Clin Periodontol. 2000;27:24.
96 Roldan S, Herrera D, Sanz M. Biofilms and the tongue: therapeutical approaches for the control of halitosis. Clin Oral Invest. 2003;7:189.
97 Roldán S, Herrera D, Santa-Cruz I, et al. Comparative effects of different chlorhexidine mouth-rinse formulations on volatile sulphur compounds and salivary bacterial counts. J Clin Periodontol. 2004;31(12):1128.
98 Rosenberg M. Bad breath and periodontal disease: how related are they? J Clin Periodontol. 2006;33:29.
99 Rosenberg M. Clinical assessment of bad breath: current concepts. J Am Dent Assoc. 1996;127:475.
100 Rosenberg M, Leib E. Experiences of an Israeli malodor clinic. In: Rosenberg M, editor. Bad breath: research perspectives. Tel Aviv: Ramot Publishing, 1995.
101 Rosenberg M, McCulloch CA. Measurement of oral malodor: current methods and future prospects. J Periodontol. 1992;63:776.
102 Rosenberg M, Gelernter I, Barki M, et al. Day-long reduction of oral malodor by a two-phase oil:water mouthrinse as compared to chlorhexidine and placebo rinses. J Periodontol. 1992;63:39.
103 Rosenberg M, Kulkarni GV, Bosy A, et al. Reproducibility and sensitivity of oral malodor measurements with a portable sulphide monitor. J Dent Res. 1991;70:1436.
104 Rosenberg M, Septon I, Eli I, et al. Halitosis measurement by an industrial sulphide monitor. J Periodontol. 1991;62:487.
105 Rowley EJ, Schuchman LC, Tishk MN, et al. Tongue brushing versus tongue scraping. Clin Prev Dent. 1987;9:13.
106 Sanz M, Roldan S, Herrera D. Fundamentals of breath malodour. J Contemp Dent Pract. 2001;2:1.
107 Schmidt NF, Tarbet WJ. The effect of oral rinses on organoleptic mouth odor ratings and levels of volatile sulfur compounds. Oral Surg Oral Med Oral Pathol. 1978;45(6):876.
108 Schroeder HE. Oral mucosa. In: Schroeder HE, editor. Oral structural biology. New York: Thieme Medical Publishers, 1991.
109 Simenhoff ML, Burke JF, Saukkonen JJ, et al. Biochemical profile or uremic breath. N Engl J Med. 1977;297:132.
110 Stamou E, Kozlovsky A, Rosenberg M. Association between oral malodour and periodontal disease-related parameters in a population of 71 Israelis. Oral Dis. 2005;11:72.
111 Sterer N, Rosenberg M. Streptococcus salivarius promotes mucin putrefaction and malodor production by Porphyromonas gingivalis. J Dent Res. 2006;85:910.
112 Stewart RD, Boettner EA. Expired-air acetone in diabetes mellitus. New Eng J Med. 1964;270:1035.
113 Suarez F, Springfield J, Furne J, et al. Differentiation of mouth versus gut as site of origin of odoriferous breath gases after garlic ingestion. Am J Physiol. 1999;276:G425.
114 Suarez FL, Furne JK, Springfield J, et al. Morning breath odor: influence of treatments on sulfur gases. J Dent Res. 2000;79:1773.
115 Tanaka M, Anguri H, Nonaka A, et al. Clinical assessment of oral malodor by the electronic nose system. J Dent Res. 2004;83:317.
116 Tangerman A, Meuwesearends MT, Jansen JBMJ. Cause and composition of foetor hepaticus. Lancet. 1994;343:483.
117 Tonzetich J. Production and origin of oral malodor: a review of mechanisms and methods of analysis. J Periodontol. 1977;48:13.
118 Tonzetich J, Ng SK. Reduction of malodor by oral cleansing procedures. Oral Surg Oral Med Oral Pathol. 1976;42:172.
119 Tsunoda M, Yamada S, Yasuda H. Deodorizing mechanism of epigallocatechin and chewing gum containing tea extracts. In: van Steenberghe D, Rosenberg M, editors. Bad breath: a multidisciplinary approach. Leuven, Belgium: Leuven University Press, 1996.
120 Van den Velde S, Quirynen M, Van hee P, et al. Differences between alveolar air and mouth air. Anal Chem. 2007;79:3425.
121 Van den Velde S, Quirynen M, Van hee P, et al. Halitosis associated volatiles in breath of healthy subjects. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:54.
122 Van den Velde S, Nevens F, Van hee P, et al. GC-MS analysis of breath odor compounds in liver patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875(2):344.
123 van Steenberghe D. Breath malodor. Curr Opin Periodontol. 1997;4:137.
124 van Steenberghe D, editor. Breath malodor: a step-by-step approach. Copenhagen: Quintessence, 2004.
125 van Steenberghe D, Quirynen M. Breath malodor. In: Lindhe J, Karring T, Lang NP, editors. Clinical periodontology and implant dentistry. Oxford: Blackwell, 2003.
126 van Steenberghe D, Avontroodt P, Peeters W, et al. Effect of different mouthrinses on morning breath. J Periodontol. 2001;72:1183.
127 Vernillo AT. Dental considerations for the treatment of patients with diabetes mellitus. J Am Dent Assoc. 2003;134:24S.
128 Waler SM. The effect of zinc-containing chewing gum on volatile sulfur-containing compounds in the oral cavity. Acta Odontol Scand. 1997;55:198.
129 Winkler S, Garg AK, Mekayarajjananonth T, et al. Depressed taste and smell in geriatric patients. J Am Dent Assoc. 1999;130:1759.
130 Yaegaki K, Coil J. Examination classification and treatment of halitosis; clinical perspectives. J Can Dent Ass. 2000;66:257.
131 Yaegaki K, Sanada K. Biochemical and clinical factors influencing oral malodor in periodontal patients. J Periodontol. 1992;63:783.
132 Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res. 1992;27:233.
133 Young A, Jonski G, Rolla G. A study of triclosan and its solubilizers as inhibitors of oral malodour. J Clin Periodontol. 2002;12:1078.
134 Young A, Jonski G, Rolla G. Inhibition of orally produced volatile sulfur compounds by zinc, chlorhexidine or cetylpyridinium chloride: effect of concentration. Eur J Oral Sci. 2003;5:400.
135 Young A, Jonski G, Rolla G, et al. Effects of metal salts on the oral production of volatile sulfur-containing compounds (VSC). J Clin Periodontol. 2001;28:776.
136 Zusho H, Asaka H, Okamoto H. Diagnosis of olfactory disturbance. Auris Nasus Larynx. 1981;8:19.