CHAPTER 48 Host Modulation
Introduction
Host modulation is a relatively new term that has been incorporated into our dental jargon, but it has not been well defined. Host can be defined as “the organism from which a parasite obtains its nourishment,” or in the transplantation of tissue, “the individual who receives the graft.” Modulation is defined as “the alteration of function or status of something in response to a stimulus or an altered chemical or physical environment” (Taber’s Medical Dictionary, 2004). In diseases of the periodontium that are initiated by bacteria, the “host” clearly is the individual who harbors these pathogens; however, it was not clear for many years whether it was possible to modulate the host response to these pathogens and other stimuli leading to the breakdown of the attachment apparatus. Host modulation with chemotherapeutic therapy or drugs is the latest adjunctive therapeutic option for the management of periodontal diseases.
The concept of host modulation is fairly new to the field of dentistry but is universally understood by most physicians who routinely apply the principles of host modulation to the management of a number of chronic progressive disorders such as arthritis and osteoporosis. The concept of host modulation was first introduced to dentistry by Williams95 and Golub et al32 and then expanded on by many other scholars in the dental profession. In 1990, Williams concluded “there are compelling data from studies in animals and human trials indicating that pharmacologic agents that modulate the host responses believed to be involved in the pathogenesis of periodontal destruction may be efficacious in slowing the progression of periodontitis.”95 In 1992, Golub and colleagues discussed “host modulation with tetracyclines and their chemically modified analogues.”32 The future that these authors described has arrived, and to better understand this new era in disease management, we must first look to the theories of the pathogenesis of periodontitis.
Many clinicians previously believed that periodontal disease was an inevitable consequence of aging and was uniformly distributed in the population. They thought that disease severity was directly correlated with plaque levels (i.e., the worse the oral hygiene, the worse the periodontal disease) and that disease progression occurred in a continuous, linear manner throughout life. Now, as a result of better epidemiologic data, there has been a paradigm shift in how clinicians and scientists view the prevalence and progression of this common disease. It has been well established that periodontal disease is not a natural consequence of aging and disease severity is not correlated with plaque levels. Theories about the pathogenesis of periodontitis have evolved from a purely plaque-associated disease to the more recent hypotheses that place considerable emphasis on the host’s response to the bacteria.10 The first Surgeon General’s report on “Oral Health in America,” published in 2000, recognized the importance of dental health in the overall general health and well-being of a patient.87 Recent research findings indicate possible associations between chronic oral infections, such as periodontitis, and systemic disorders, such as diabetes, cardiovascular and lung diseases, stroke, osteoporosis, and rheumatoid arthritis. The Surgeon General’s report assesses these emerging associations and explores factors that may underlie oral-systemic disease connections. Along with these findings and the emergence of the discipline of periodontal medicine, there have been many developments in therapeutic approaches to the management of periodontitis. The development of the chemotherapeutic approach known as “host modulation” required a thorough understanding of the host response and the impact of a variety of risk factors.
Anecdotally, any dentist will be able to identify patients with abundant plaque and calculus deposits with widespread gingivitis and shallow pocketing but with minimal deep pocketing. By contrast, other patients, despite maintaining a high standard of plaque control, succumb to aggressive forms of periodontitis, with deep pocketing, tooth mobility, and early tooth loss. The former group of patients is periodontal disease resistant, whereas the latter group is periodontal disease susceptible. Clearly, the response of the periodontal tissues to plaque is different in these two types of patients, and certain patients undergo advanced periodontal breakdown even though they achieve a high standard of oral hygiene. The previous observations led researchers to realize that the host response to the bacterial challenge presented by subgingival plaque is perhaps the most important determinant of disease severity, progression, and even response to therapy. Although plaque bacteria are capable of causing direct damage to the periodontal tissues (e.g., by release of H2S, butyric acid, and other enzymes and mediators), it is now recognized that the great majority of the destructive events occurring in the periodontal tissues result from activation of destructive processes that occur as part of the host immune-inflammatory response to plaque bacteria. The host response is essentially protective by intent but paradoxically can also result in significant tissue damage, including breakdown of connective tissue fibers in the periodontal ligament and resorption of alveolar bone.
In 1985, research began to focus on bacterial-host interactions.53 It had been recognized that although bacterial pathogens initiate the periodontal inflammation, the host response to these pathogens is equally, if not more, important in mediating connective tissue breakdown, including bone loss. It has become clear that the host-derived enzymes known as the matrix metalloproteinases (MMPs), as well as changes in osteoclast activity driven by cytokines and prostanoids, cause most of the tissue destruction in the periodontium.63 This shift in paradigms, with a focus on the host response, led to the development of host modulatory therapies to improve therapeutic outcomes, slow the progression of disease, and allow for more predictable management of patients with periodontitis. This therapeutic strategy not only may be relevant to individuals at greater risk for periodontal disease but also may be important in the management of those at risk for a number of related systemic diseases. To better understand the host factors that clinicians are attempting to modulate, we need a more detailed assessment of the role of the host response in periodontal pathogenesis (Figure 48-1). After the accumulation of subgingival plaque bacteria, a variety of microbial substances, including chemotactic factors such as lipopolysaccharide (LPS), microbial peptides, and other bacterial antigens, diffuse across the junctional epithelium into the gingival connective tissues. The periodontium is anatomically unique in that the junctional epithelium ends on the tooth surface, which is nonliving tissue; there is no other such discontinuous lining over the entire surface of the body. The dentogingival junction indicates a priori vulnerability to bacterial attack. Epithelial and connective tissue cells are thus stimulated to produce inflammatory mediators that result in an inflammatory response in the tissues. The gingival vasculature dilates (vasodilation) and becomes increasingly permeable to fluid and cells. Fluid accumulates in the tissues, and defense cells migrate from the circulation toward the source of the chemotactic stimulus (bacteria and their products) in the gingival crevice. Neutrophils, or polymorphonuclear leukocytes (PMNs), predominate in the early stages of gingival inflammation to phagocytose and kill plaque bacteria. Bacterial killing by PMNs involves both intracellular mechanisms (after phagocytosis of bacteria within membrane-bound structures inside the cell) and extracellular mechanisms (by release of PMN enzymes and oxygen radicals outside the cell). As bacterial products enter the circulation, committed lymphocytes return to the site of infection, and B lymphocytes are transformed to plasma cells, which produce antibodies against specific bacterial antigens. Antibodies are released in the gingival tissues and, in the presence of complement, facilitate and enhance PMN phagocytosis and bacterial killing.
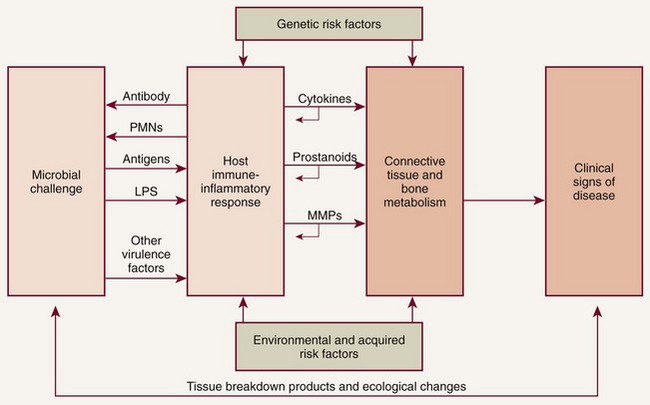
Figure 48-1 Schematic illustration of the pathogenesis of periodontitis. The microbial challenge presented by subgingival plaque bacteria results in an upregulated host immune-inflammatory response in the periodontal tissues that is characterized by the excessive production of inflammatory cytokines (e.g., interleukins, tumor necrosis factor), prostanoids (e.g., prostaglandin E2) and enzymes, including the matrix metalloproteinases (MMPs). These proinflammatory mediators are responsible for the majority of periodontal breakdown that occurs, leading to the clinical signs and symptoms of periodontitis. The process is modified by environmental (e.g., tobacco use) and acquired risk factors (e.g., systemic diseases) and genetic susceptibility. PMNs, Polymorphonuclear leukocytes; LPS, lipopolysaccharide.
(Modified from Kornman KS: Clin Infect Dis 28:520, 1999.)
Thus a host immune-inflammatory response is established in the gingival tissues, and the clinical signs of gingivitis develop. This response is essentially protective in intent, to combat the bacterial infection and prevent ingress of bacteria into the tissues. In persons who are not susceptible to periodontitis (disease resistant), these primary defense mechanisms control the infection, and chronic inflammation (i.e., chronic gingivitis) may persist indefinitely. In disease-susceptible individuals, however, inflammatory events extend apically and laterally to involve deeper connective tissues and alveolar bone. There is proliferation of the junctional epithelium, which becomes increasingly permeable and ulcerated, thus accelerating the ingress of bacterial products, and the inflammation worsens. Further defense cells are recruited to the area, including macrophages and lymphocytes. Large numbers of PMNs migrate into the tissues, secreting excessive quantities of destructive enzymes and inflammatory mediators. These enzymes include the MMPs, such as collagenase and gelatinase, which break down collagen fibers in the gingival and periodontal tissues. The infiltrating inflammatory and immune cells are accommodated by the breakdown of structural components of the periodontium. MMPs are a primary target for host modulation. Pharmacologic agents or chemotherapeutics can be administered to suppress excessive levels of MMPs.
Macrophages are recruited to the area and are activated (by binding to LPS) to produce prostaglandins (e.g., prostaglandin E2 [PGE2]), interleukins (e.g., IL-1α, IL-1β, IL-6), tumor necrosis factor alpha (TNF-α), and MMPs. The cytokines (interleukins and TNF-α) and prostanoids are additional targets for host modulatory therapeutics. Interleukins and TNF-α bind to fibroblasts, which are stimulated to produce additional quantities of PGE2, interleukins, TNF-α, and MMPs in positive-feedback cycles. The concentration of these enzymes and inflammatory mediators becomes pathologically high in the periodontal tissues. Host modulatory therapy (HMT) can be used to interrupt these positive-feedback loops and ultimately reduce the excessive levels of cytokines, prostanoids, and enzymes resulting in tissue destruction. MMPs break down collagen fibers, disrupting the normal anatomy of the gingival tissues and resulting in destruction of the periodontal ligament. The inflammation extends apically, and osteoclasts are stimulated to resorb alveolar bone by the high levels of prostaglandins, interleukins, and TNF-α in the tissues. The osteoclasts themselves are targets for host modulation. Drugs can be administered to downregulate osteoclastic activity and ultimately to inhibit bone resorption by these cells.
The elevations in the proinflammatory or destructive mediators in response to bacterial challenge are counterbalanced by elevations in antiinflammatory or protective mediators such as the cytokines IL-4 and IL-10, as well as other mediators, such as IL-1ra (receptor antagonist), and tissue inhibitors of metalloproteinases (TIMPs) (Figure 48-2). Under conditions of health, the antiinflammatory or protective mediators serve to control tissue destruction. If there are adequate levels of these antiinflammatory or protective mediators to keep the host response to the bacterial challenge in check, the individual will be disease resistant. If an imbalance occurs, with excessive levels of the proinflammatory or destructive mediators present in the host tissues, tissue destruction will ensue in the susceptible host. Another potential target for host modulation could entail the use of pharmacologic agents, which either mimic or result in elevations of endogenous antiinflammatory or protective mediators.
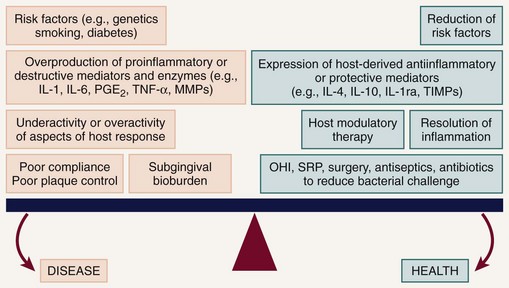
Figure 48-2 The periodontal balance. The balance between periodontal breakdown (“disease”) and periodontal stability (“health”) is tipped toward disease by risk factors, excessive production of inflammatory cytokines and enzymes (e.g., IL-1 and IL-6, interleukin-1 and -6; PGE2, prostaglandin E2; TNF-α, tumor necrosis factor alpha; MMPs, matrix metalloproteinases), underactivity or overactivity of aspects of the immune-inflammatory host response, poor compliance, and a pathogenic microflora. The balance can be tipped toward health by risk factor modification, upregulation, and restoration of balance between naturally occurring inhibitors of inflammation (e.g., IL-4 and IL-10, interleukins-4 and -10; IL-1ra, interleukin-1 receptor antagonist; TIMPs, tissue inhibitors of metalloproteinases), HMT (host modulatory therapy), and antibacterial treatments such as OHI (oral hygiene instructions), SRP (scaling and root planing), surgery, antiseptics, and antibiotics.
Thus plaque bacteria initiate the disease, and bacterial antigens that cross the junctional epithelium drive the inflammatory process. Therefore bacteria are essential for periodontitis to occur, but they are insufficient by themselves to cause disease. For periodontitis to develop, a susceptible host is also required. The majority of periodontal breakdown (bone loss, attachment loss) is caused by host-derived destructive enzymes (MMPs) and inflammatory mediators (prostaglandins, interleukins) that are released during the cascade of destructive events that occur as part of the inflammatory response66 (see Figure 48-1). Paradoxically, the inflammatory response, which is essentially protective in design, is responsible for much of the breakdown of the soft and hard periodontal tissues. Periodontal disease is characterized by high concentrations of MMPs, cytokines, and prostanoids in the periodontal tissues, whereas periodontal health is characterized by the opposite.65 The purpose of HMT is to restore the balance of proinflammatory or destructive mediators and antiinflammatory or protective mediators to that seen in healthy individuals. Pocket formation occurs as coronal junctional epithelium is broken down and restored at a more apical location. Plaque bacteria then migrate apically along the root surface deeper into the pocket, where the physical conditions favor the proliferation of gram-negative anaerobic species. Bacterial products continue to challenge the host, and the host continues its frustrated response against these bacteria and their products. Inflammation extends further and further apically, more bone is resorbed, and periodontal ligament (PDL) is broken down. The pocket deepens, and the associated attachment and bone loss result in clinical and radiographic signs of periodontitis. Intervention is required to prevent eventual tooth loss and other sequelae of the disease.
The nature of the host response to the presence of plaque is modified by genetic factors (helping to explain why aggressive periodontitis tends to have a familial aggregation) and systemic and environmental factors (e.g., smoking, diabetes, stress).
These risk factors may lead to the imbalance between the proinflammatory and antiinflammatory mediators seen in susceptible individuals (see Figure 48-2). Risk factors can affect onset, rate of progression, and severity of periodontal disease, as well as response to therapy22,38,80 (Box 48-1). Risk assessment is extremely important as we now recognize that some of these risk factors can be modified to reduce a patient’s susceptibility to periodontitis. Risk reduction strategies may include smoking cessation, improved control of diabetes, nutritional supplementation, improved oral hygiene, changes in medication, stress management, weight loss, and more frequent dental visits (Box 48-2). The use of chemotherapeutic agents or drugs specifically designed to treat periodontal diseases is emerging to aid as a risk reduction strategy.
BOX 48-1 Risk Factors for Periodontal Disease
PST, Periodontal screening test; HIV, human immunodeficiency virus.
Intervention in periodontal disease can now include host modulatory therapy (HMT) as one of the available adjunctive treatment options. The term adjunctive is meant to imply “in addition to conventional therapies” or “in addition to other established therapies.” For the management of periodontal diseases, conventional approaches were initially mechanical in nature, that is, surgery, as well as scaling and root planing (SRP). Initially, adjunctive therapies were solely antimicrobial such as the use of antiseptics and antibiotics (local and/or systemic). New adjunctive approaches involve modulation of the host response. Researchers are also investigating HMTs, which aim to modify or reduce destructive aspects of the host response so that the immune-inflammatory response to plaque is less damaging to the periodontal tissues. Removal of plaque by SRP targets one aspect of the pathogenic process by reducing the bacterial burden and therefore the antigenic challenge that drives the inflammatory response in the host tissues. However, the bacterial challenge is never completely eliminated after SRP, and recolonization by bacterial species occurs. HMTs offer the potential for downregulating destructive aspects and upregulating protective aspects of the host response so that, in combination with conventional treatments to reduce the bacterial burden, the balance between health (resolution of inflammation and wound healing) and disease progression (continued proinflammatory events) is tipped in the direction of a healing response.
HMT is a means of treating the host side of the host-bacteria interaction. The host response is responsible for most of the tissue breakdown that occurs, leading to the clinical signs of periodontitis. HMTs offer the opportunity for modulating or reducing this destruction by treating aspects of the chronic inflammatory response. HMTs do not “switch off” normal defense mechanisms or inflammation; instead, they ameliorate excessive or pathologically elevated inflammatory processes to enhance the opportunities for wound healing and periodontal stability.
HMT can be used to reduce excessive levels of enzymes, cytokines, and prostanoids and should not reduce levels below constitutive levels. HMTs can also modulate osteoclast and osteoblast function (Figure 48-3) but should not impact normal tissue turnover. HMT is key to addressing many of the risk factors that have adverse effects on the host response, which are either not easily managed (e.g., smoking, diabetes) or cannot be changed (e.g., genetic susceptibility). In addition, host modulatory agents might be used to increase the levels of a person’s own protective or antiinflammatory mediators. Use of systemic HMTs for treatment of a patient’s periodontal condition may also provide benefits for other inflammatory disorders such as arthritis, cardiovascular disease, dermatologic conditions, diabetes, rheumatoid arthritis, and osteoporosis. Also, patients who are currently taking host modulatory agents, such as nonsteroidal antiinflammatory drugs (NSAIDs), bisphosphonates, or tetracyclines, as well as newer agents targeting specific cytokines for the management of medical conditions, may be experiencing periodontal benefits from these systemic medications prescribed for the management of other chronic inflammatory conditions.
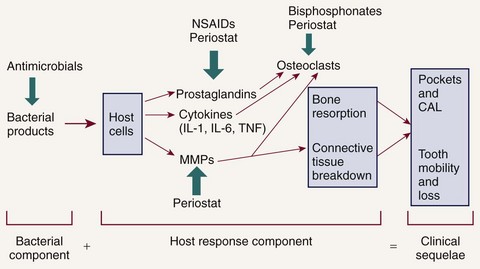
Figure 48-3 Potential adjunctive therapeutic approaches. Possible adjunctive therapies and points of intervention in the treatment of periodontitis are presented related to the pathologic cascade of events. CAL, Clinical attachment loss.
A variety of different drug classes have been evaluated as host modulation agents, including the NSAIDs, bisphosphonates, tetracyclines, enamel matrix proteins, growth factors, and bone morphogenetic proteins. This section discusses chemotherapeutics developed as HMTs researched as adjuncts to treat periodontitis to date, use of HMTs in clinical practice, and what the future might hold for HMTs.
Systemically Administered Agents
Nonsteroidal Antiinflammatory Drugs
NSAIDs inhibit the formation of prostaglandins, including PGE2, which is produced by neutrophils, macrophages, fibroblasts, and gingival epithelial cells in response to the presence of LPS, a component of the cell wall of gram-negative bacteria. PGE2 has been extensively studied in periodontal disease because it upregulates bone resorption by osteoclasts.33,43,64 Levels of PGE2 have been shown to be elevated in patients with periodontal disease compared with healthy patients.35,64 PGE2 also inhibits fibroblast function and has inhibitory and modulatory effects on the immune response.36
NSAIDs inhibit prostaglandins and therefore reduce tissue inflammation. They are used to treat pain, acute inflammation, and a variety of chronic inflammatory conditions. NSAIDs include the salicylates (e.g., aspirin), indomethacin, and the propionic acid derivatives (e.g., ibuprofen, flurbiprofen, and naproxen). The ability of NSAIDs to block PGE2 production, thereby reducing inflammation and inhibiting osteoclast activity in the periodontal tissues, has been investigated in patients with periodontitis. Studies have shown that systemic NSAIDs, such as indomethacin,97 flurbiprofen,96 and naproxen42 administered daily for up to 3 years, significantly slowed the rate of alveolar bone loss compared with placebo. However, NSAIDs have some serious disadvantages when considered for use as a HMT for periodontitis. Daily administration for extended periods is necessary for periodontal benefits to become apparent, and NSAIDs are associated with significant side effects, including gastrointestinal problems, hemorrhage (from decreased platelet aggregation), and renal and hepatic impairment. Furthermore, research shows that the periodontal benefits of taking long-term NSAIDs are lost when patients stop taking the drugs, with a return to or even an acceleration of the rate of bone loss seen before NSAID therapy, often referred to as a “rebound effect.”98 For these reasons, the long-term use of NSAIDs as an adjunctive treatment for periodontitis has never really developed beyond research studies.
It was previously anticipated that the selective cyclooxygenase-2 (COX-2) inhibitors may offer promise as adjunctive treatments in the management of periodontitis. The enzyme cyclooxygenase, which converts arachidonic acid to prostaglandins, exists in two functionally distinct isoforms, COX-1 and COX-2. COX-1 is constitutively expressed and has antithrombogenic and cytoprotective functions. Therefore, inhibition of COX-1 by nonselective NSAIDs causes side effects such as gastrointestinal ulceration and impaired hemostasis. COX-2 is induced after stimulation by various cytokines, growth factors, and LPS and results in the production of elevated quantities of prostaglandins. Inhibition of COX-2 by selective COX-2 inhibitors results in reduction of inflammation. Researchers considered that the use of selective COX-2 inhibitors offered the prospect for reducing periodontal inflammation without the side effects typically observed after long-term (nonselective) NSAID therapy, and preliminary studies identified that selective COX-2 inhibitors slowed alveolar bone loss in animal models5,41 and modified prostaglandin production in human periodontal tissues.89 However, the selective COX-2 inhibitors were later identified to be associated with significant and life-threatening adverse effects, resulting in some drugs being withdrawn from the market. In summary, NSAIDs (including the selective COX-2 specific inhibitors) are presently not indicated as adjunctive HMTs in the treatment of periodontal disease.
Bisphosphonates
The bisphosphonates are bone-seeking agents that inhibit bone resorption by disrupting osteoclast activity. Their precise mechanism of action is unclear, but research has shown that bisphosphonates interfere with osteoblast metabolism and secretion of lysosomal enzymes.94 More recent evidence has suggested that bisphosphonates also possess anticollagenase properties.59 The ability of bisphosphonates to modulate osteoclast activity clearly may be useful in the treatment of periodontitis. Research has demonstrated that in naturally occurring periodontitis in beagles, treatment with the bisphosphonate alendronate significantly increased bone density compared with placebo.74 In animal models of experimentally induced periodontitis, bisphosphonates reduced alveolar bone resorption.82,94 In human studies, these agents resulted in enhanced alveolar bone status and density.16,76
Some bisphosphonates have the unwanted effects of inhibiting bone calcification and inducing changes in white blood cell counts. Also, there have been recent reports of avascular necrosis of the jaws following bisphosphonate therapy, with the resultant risk of bone necrosis following dental extractions.11 The recent reports of bisphosphonate-related osteonecrosis of the jaw, although primarily associated with intravenous administration of bisphosphonates rather than oral administration, has impeded the development of bisphosphonates as an HMT to manage periodontitis. As with NSAIDs, at present there are no bisphosphonate drugs that are approved and indicated for treatment of periodontal diseases.
Sub-Antimicrobial-Dose Doxycycline
Sub-antimicrobial-dose doxycycline (SDD) is a 20-mg dose of doxycycline (Periostat) that is approved and indicated as an adjunct to SRP in the treatment of chronic periodontitis. It is taken twice daily for 3 months, up to a maximum of 9 months of continuous dosing. The 20-mg dose exerts its therapeutic effect by enzyme, cytokine, and osteoclast inhibition rather than by any antibiotic effect. Research studies have found no detectable antimicrobial effect on the oral flora or the bacterial flora in other regions of the body and have identified clinical benefit when used as an adjunct to SRP. At present, SDD (Periostat) is the only systemically administered HMT specifically indicated for the treatment of chronic periodontitis that is approved by the US Food and Drug Administration (FDA) and accepted by the American Dental Association (ADA). In addition, a modified-release SDD was recently approved by the FDA (Oracea) for the treatment of the common skin disorder rosacea and is routinely prescribed within the dermatology community. Studies conducted by Preshaw et al,73 utilizing this same modified-release SDD versus placebo in 266 subjects with periodontitis as an adjunct to SRP resulted in significantly greater clinical benefits than SRP alone in the treatment of periodontitis. It will be interesting to see what the long-term benefits to oral health will be in rosacea patients being prescribed this new drug. There has also been considerable off-label use of this new modified-release SDD for the treatment of periodontal diseases based on the understanding that once-a-day administration can increase the level of compliance as compared to twice-a-day oral administration.
Nonsteroidal Antiinflammatory Drugs
Topical NSAIDs have shown benefit in the treatment of periodontitis. One study of 55 patients with chronic periodontitis who received topical ketorolac mouthrinse reported that gingival crevicular fluid (GCF) levels of PGE2 were reduced by approximately half over 6 months and that bone loss was halted.45 In addition, locally administered ketoprofen has been investigated. To date, topically administered NSAIDs have not been approved as local HMTs for the management of periodontitis.
Enamel Matrix Proteins, Growth Factors, and Bone Morphogenetic Proteins
A number of local HMTs have been investigated for potential use as adjuncts to surgical procedures, not only to improve wound healing but also to stimulate regeneration of lost bone, periodontal ligament, and cementum, restoring the complete periodontal attachment apparatus. These have included enamel matrix proteins bone morphogenetic proteins (BMP-2, BMP-7), growth factors (platelet-derived growth factor, insulinlike growth factor), and tetracyclines. The locally applied HMTs currently approved by the FDA for adjunctive use during surgery are enamel matrix proteins (Emdogain), recombinant human platelet-derived growth factor-BB (GEM 21S), and BMP-2 (INFUSE), which are covered in much greater detail in other chapters. The initial local host modulatory agent approved by the FDA for adjunctive use during surgery to assist with clinical attachment gain and wound healing was Emdogain; this has been followed by platelet-derived growth factor combined with a resorbable synthetic bone matrix (GEM 21S) to assist in regenerative procedures approved recently by the FDA, as well as rhBMP-2 (INFUSE) soaked on to an absorbable collagen sponge to assist with ridge and sinus augmentation. The technology behind GEM 21 has already been marketed for use in wound healing, particularly in patients with diabetes, and INFUSE has been used for quite some time for the healing of fractures by the orthopedic community.
The remainder of this chapter focuses on the clinical utility of host modulation for nonsurgical procedures in clinical practice and the use of SDD (Periostat) in clinical practice.
Host Modulation and Comprehensive Periodontal Management
The term periodontal management suggests a much broader concept of periodontal care than the term periodontal treatment. This concept is extremely important considering the chronic nature of the disease. Management includes thorough medical and dental history and examination (clinical charting and radiographs), assessment of risk factors, diagnosis, development of a treatment strategy, initial and definitive treatment planning, review of treatment outcomes and reevaluation, long-term supportive periodontal therapy (maintenance care), and assessment of prognosis. In addition, as new data continue to emerge regarding biochemical assessments of disease activity (measuring levels of proinflammatory mediators, bone and connective tissue breakdown products in the GCF, saliva, and tissues of the oral cavity), new diagnostic and prognostic tests may become part of our established protocols for comprehensive periodontal disease management in the future. However, controlling the bacteria that cause periodontal infections remains a central focus of effective periodontal treatment. Understanding the importance of the host response and the impact of risk factors now allows clinicians to provide complementary treatment strategies simultaneously for their patients (Figure 48-4).
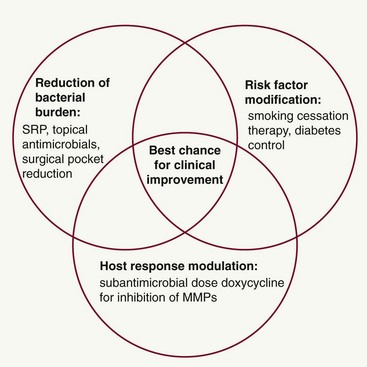
Figure 48-4 Complementary treatment strategies in periodontitis. The best chance for clinical improvement may come from implementing complementary treatment strategies that target different aspects of the periodontal balance. Reduction of the bacterial burden by scaling and root planing (SRP) is the cornerstone of treatment and can be augmented by the use of topical antimicrobials and surgical pocket therapy. In addition to this antibacterial treatment approach, the host response can be treated by the use of host modulatory therapy, such as sub-antimicrobial-dose doxycycline, for the inhibition of matrix metalloproteinases (MMPs). Risk factor assessment and modification must form a key part of any periodontal treatment strategy, including smoking cessation counseling. These different but complementary treatment strategies can be used together as part of a comprehensive management approach.
Those patients most likely requiring the use of HMT include those with risk factors that are either nonmodifiable or not easily modified. If the decision is made to use HMT, this must be discussed with the patient and the rationale for treatment thoroughly explained. This takes time at the chairside, but it is time well spent; patients become increasingly interested in their periodontal status and are more likely to develop ownership of their management, thereby enhancing compliance with all aspects of care, including plaque control, risk reduction, and treatment protocols. Compliance with HMT is greatly facilitated if the rationale for prescribing is clearly explained. The need for compliance with the prescribed drug regimen is important because with SDD, for example, a tablet must be taken twice daily (once in the morning and once in the evening) and should not be taken with calcium supplements. Compliance may be improved by administration of a modified-release SDD capsule taken only once a day. It should be emphasized to the patient that the use of HMT is not a substitute for excellent plaque control (just as it is not a substitute for excellent debridement and root surface instrumentation by the treating clinician). To achieve the best results, patients must be interested and well informed about their condition so that compliance is maximized. Furthermore, patients must also be convinced that comprehensive and frequent recall appointments are absolutely necessary in the maintenance phase of this chronic and often progressive disease, which can be very well controlled with adequate follow-up.
In addition to patient motivation, oral hygiene instruction, and SRP to reduce the bacterial challenge, a key treatment strategy when managing periodontitis patients is risk factor modification. The harmful effects of smoking on the periodontal tissues are well documented,46 and successful smoking cessation therapy will likely be a major benefit to patients with periodontitis. Smoking cessation counseling can be undertaken in the dental office (if staff are appropriately trained) or through collaboration with the patient’s physician or specialized clinics. Given the evidence that smokers have worse periodontal disease than nonsmokers83,99 and that the magnitude and predictability of clinical improvements after treatment are significantly reduced in smokers,1,69 smoking cessation counseling should form a major part of treatment for smokers with periodontitis. Patients with poorly controlled diabetes are also at increased risk for periodontitis,54 and periodontal therapy may have an impact on diabetic control.36 Collaboration with medical colleagues when treating diabetic patients with periodontitis is warranted to ascertain the degree of diabetic control.21 Other possible risks for periodontitis include nonmodifiable factors such as genetics, gender, and race. As the relevance of different risk factors is established through epidemiologic research, clinicians must remain aware of their responsibilities for informing and changing patients’ behaviors in relation to modifiable risks.
The management of patients with periodontitis can therefore involve the following complementary treatment strategies:
It is the responsibility of the dentist to customize the treatment plan for each individual patient by selecting and providing appropriate treatments, following discussion and informed decision making by the patient. Good communication and showing an interest in the patient’s condition are essential to maximize compliance and modify risk factors. The best chance for clinical improvement may come from a combination of targeted treatment approaches for each patient (see Figure 48-4). In fact, Novak et al published very striking improvements in probing depth reductions and clinical attachment level gains in a 6-month, randomized, multicenter, examiner-blinded, placebo-controlled study, which showed that combination therapy of HMT (SDD) plus a locally delivered antimicrobial (doxycycline hyclate gel) and SRP provided optimal improvements in clinical parameters when compared to SRP alone in the treatment of moderate-to-severe periodontitis.61
Sub-Antimicrobial-Dose Doxycycline (SDD)
As previously discussed, SDD is currently the only FDA-approved, systemically administered HMT indicated specifically in the treatment of periodontitis. SDD is used as an adjunct to SRP and must not be used as a stand-alone therapy (monotherapy). Because SDD, previously called “low-dose doxycycline” (LDD) and currently marketed as Periostat, is based on a sub-antimicrobial dosage of doxycycline, a member of the tetracycline family of compounds, the use of tetracyclines for the management of periodontal diseases must be put in perspective.
In regard to incorporation of a medical pharmacologic approach into the management of a disease in the dental practice setting, no class of drugs has made more of an impact on periodontal therapy than the tetracyclines. They have been used in conjunction with SRP, the “gold standard” of nonsurgical therapy, as well as with both resective and regenerative surgical procedures. The tetracyclines have been used locally and systemically as antimicrobial agents and more recently, systemically as a host modulation agent (SDD). The tetracyclines have been prescribed not only to address chronic periodontitis but also to manage specific and often more aggressive types of periodontitis. Most recently, the tetracyclines have been advocated for the management of patients with systemic diseases such as diabetes and rosacea (Oracea); doxycycline has led to improvements in both the periodontal health of compromised diabetic patients and long-term markers of glycemic control (e.g., glycated hemoglobin).37 As an adjunct to mechanical therapies, the goal of tetracycline therapy has been to enhance reattachment or even to stimulate new attachment of the supporting apparatus and osseous formation.
This section concentrates on the use of these pleiotropic compounds for modulation of the host response in the treatment of periodontitis.
Mechanisms of Action
In addition to its antibiotic properties, doxycycline (as well as the other members of the tetracycline family) has the ability to downregulate MMPs, a family of zinc-dependent enzymes that are capable of degrading extracellular matrix molecules, including collagen.6,78 MMPs are secreted by the major cell types in the periodontal tissues (fibroblasts, keratinocytes, macrophages, PMNs, endothelial cells) and play a key role in periodontitis. Excessive quantities of MMPs are released in inflamed periodontal tissues, resulting in breakdown of the connective tissue matrix. The predominant MMPs in periodontitis, particularly MMP-8 and MMP-9, derive from PMNs30 and are extremely effective in degrading type I collagen, the most abundant collagen type in gingiva and periodontal ligament.52 Levels of PMN-type MMPs have been shown to increase with severity of periodontal disease and decrease after therapy.24,30 The release of large quantities of MMPs in the periodontium leads to significant anatomic disruption and breakdown of the connective tissues, contributing to the clinical signs of periodontitis.
The rationale for using SDD as a HMT in the treatment of periodontitis is that doxycycline downregulates the activity of MMPs by a variety of synergistic mechanisms, including reductions in cytokine levels, and stimulates osteoblastic activity and new bone formation by upregulating collagen production (Figure 48-5).

Figure 48-5 Schematic of periodontal pocket indicating the pleiotropic mechanisms by which doxycycline inhibits connective tissue breakdown. Downregulation of destructive events occurring in the periodontal tissues by doxycycline results from modulation of a variety of different proinflammatory pathways.
(From Golub LM, Lee HM, Ryan ME, et al: Adv Dent Res 12:12, 1998.)
Clinical Research Data On Distinct Patient Populations
Tetracyclines work well as host modulation agents because of their pleiotropic effects on multiple components of the host response (see Figure 48-3). The only enzyme (MMP) inhibitors that have been approved for clinical use and tested for the treatment of periodontitis are members of the tetracycline family of compounds. In early studies investigating the use of different commercially available tetracyclines, Golub et al31 reported that the semisynthetic compound (e.g., doxycycline) was more effective than the parent compound tetracycline in reducing excessive collagenase activity in the GCF of chronic periodontitis patients. Because doxycycline was found to be a more effective inhibitor of collagenase than either minocycline or tetracycline9,25 and because of its safety profile, pharmacokinetic properties, and ready systemic absorption, recent clinical trials have focused on this compound. In an effort to eliminate the side effects of long-term tetracycline therapy, especially the emergence of tetracycline-resistant organisms, SDD capsules were prepared and tested.30 Each capsule contained 20 mg of doxycycline versus the commercially available 50 mg and 100 mg, antimicrobially effective, capsules or tablets. Multiple clinical studies using sub-antimicrobial doses of doxycycline have shown no difference in the composition or resistance level of the oral flora.85,93 More recent studies also demonstrate no appreciable differences in either fecal or vaginal microflora samples.91 In addition, these studies have shown no overgrowth of opportunistic pathogens, such as Candida, in the oral cavity, gastrointestinal system, or genitourinary system.
With regard to MMP inhibition, Golub et al24 reported that a 2-week regimen of SDD reduced collagenase in GCF and in the adjacent gingival tissues surgically excised for therapeutic purposes. Subsequent studies using SDD therapy adjunctive to routine scaling and prophylaxis indicated continued reductions in the excessive levels of collagenase in the GCF after 1 month of treatment. After cessation of SDD administration, however, there was a rapid rebound of collagenase activity to placebo levels, suggesting that a 1-month treatment regimen with this host modulation agent was insufficient to produce a long-term benefit.3 In contrast, during the same study, a 3-month regimen produced a prolonged drug effect without a rebound in collagenase levels to baseline during the no-treatment phase of the study. The mean levels of GCF collagenase were significantly reduced (47.3% from baseline levels) in the SDD group versus the placebo group, who received scaling and prophylaxis alone (29.1% reduction from baseline levels). Accompanying these reductions in collagenase levels were gains in the relative attachment levels in the SDD group.3,29 Continuous drug therapy over several months appears to be necessary for maintaining collagenase levels near normal over prolonged periods. However, it is reasonable to speculate that levels of these MMPs will eventually increase again in the more susceptible patients, and those individuals having the most risk factors and the greatest microbial challenge will require more frequent HMT than other patients.
General Patient Populations
Data from clinical trials of SDD are summarized in Table 48-1. In addition, a series of double-blind, placebo-controlled studies of 3, 6, and 9 months’ duration all showed clinical efficacy based on reductions in probing depth and gains in clinical attachment as well as biochemical efficacy, based on the inhibition of collagenase activity and protection of serum α1-antitrypsin (a naturally occurring protective mediator) from collagenase attack in the periodontal pocket.14,25,47 Golub et al27 showed that a 2-month regimen of SDD significantly decreased both the level of bone-type collagen breakdown products (ICTP; carboxy terminal peptide, a pyridinoline-containing cross-linked peptide of type I collagen) and MMP-8 and MMP-13 enzyme levels (neutrophil and bone-type collagenase) in chronic periodontitis subjects (Figure 48-6).
TABLE 48-1 Summary of Data Reported in Clinical Trials of Sub-Antimicrobial-Dose Doxycycline (SDD)72
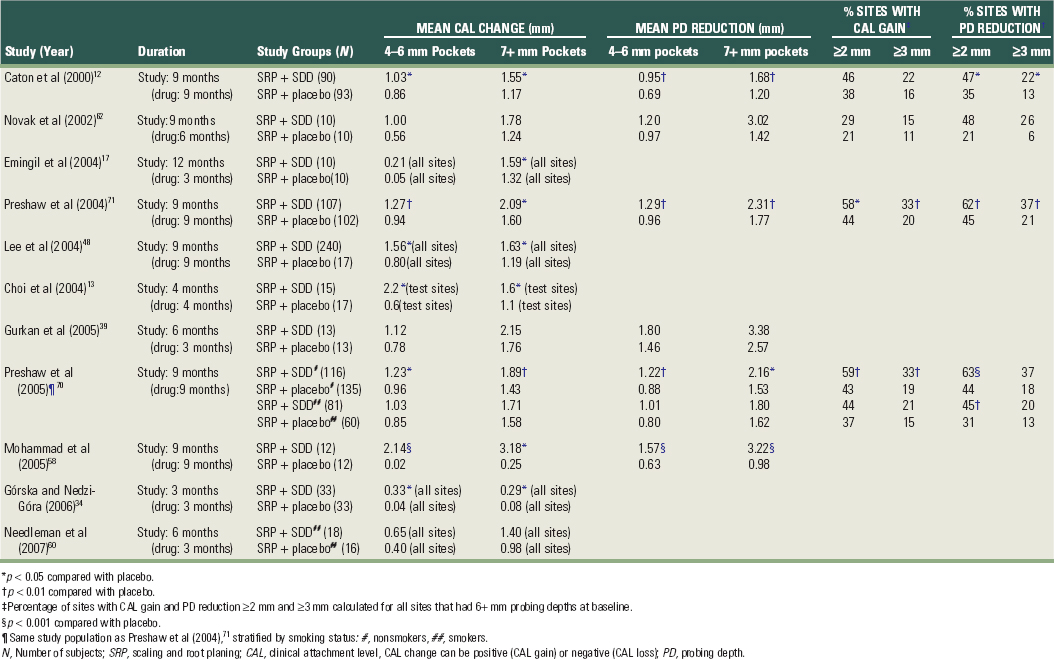
Figure 48-6 Effect of sub-antimicrobial-dose doxycycline (SDD) on gingival crevicular fluid (GCF) collagenase (MMP-8, MMP-13) and ICTP. A 2-month regimen of SDD significantly decreased levels of matrix metalloproteinases (MMP-8 and MMP-13, neutrophil and bone-type collagenases, respectively) and ICTP compared with placebo in GCF samples of adult periodontitis patients. Decreased levels of GCF bone-type collagen breakdown products (ICTP, pyridinoline-containing cross-linked peptide of type I collagen) in the SDD group versus the placebo group provides biochemical evidence of a reduction in bone resorption. RIA, Radioimmunoassay.
(From Golub LM, Lee HM, Greenwald RA, et al: Inflamm Res 46:310, 1997.)
The Phase III, 9-month, randomized, double-blind, placebo-controlled trial conducted at five dental centers demonstrated clinical efficacy and safety of SDD versus placebo as an adjunct to SRP. The benefits of HMT as an adjunct to mechanical therapy were again seen, with statistically significant reductions in probing depths and gains in clinical attachment levels, as well as the prevention of disease progression.12 When SDD administration was discontinued after 9 months of continuous therapy, the incremental improvements demonstrated in the SDD group were maintained for at least 3 months. There was no rebound effect in either the pocket depth reductions or the clinical attachment level gains; in fact, there appeared to be slight continued improvement in both these clinical parameters, presumably because of the enhanced clinical status of the patients who had benefited from adjunctive SDD, and possibly also because of the known persistence or substantivity of doxycycline in the bone and soft tissue of the periodontium. The clinical relevance of such findings confirms the utility of an MMP inhibitor in the management of chronic periodontitis.
High-Risk Patients: Smokers
The harmful effects of cigarette smoking and the reduced response to periodontal treatment in smokers compared with nonsmokers are well established.46 A recent metaanalysis of randomized clinical trials of SDD used as an adjunct to SRP revealed a benefit when using SDD in smokers with periodontitis60,70 (see Table 48-1). A hierarchic treatment response was observed such that nonsmokers who received SDD demonstrated the best clinical improvements and smokers who received placebo had the poorest treatment response. The responses of the smokers who received SDD and the nonsmokers who received placebo were intermediate to the two extremes and were broadly identical. This suggests that even patients traditionally considered resistant to periodontal treatment (i.e., smokers) can benefit from SDD, with a treatment response similar to that expected when treating a nonsmoker by scaling and root planing alone.
Special Patient Populations
More recent Phase IV (i.e., post-licensing) clinical studies have revealed success using SDD in particular populations of susceptible individuals. Much interest has focused on genetic susceptibility to periodontal disease, particularly whether a specific variation in the genes that regulate the cytokine IL-1 confers increased susceptibility to disease. This polymorphism is known as the periodontitis-associated genotype (PAG), the presence of which can be characterized using a commercially available screening test, the PST genetic susceptibility test. Investigation of patients who possess this gene polymorphism has been driven by the assumption that local phenotypic differences exist in chronic periodontitis associated with this genotype (e.g., that PAG-positive patients produce more IL-1 cytokines for a given bacterial challenge, resulting in increased tissue damage and more extensive periodontal disease). However, few studies exist of the impact of cytokine gene polymorphism on tissue IL-1 cytokine levels in periodontal disease to support this, although IL-1β levels in shallow periodontal pockets have been reported to be higher in patients with the genotype than those without.19 The studies that have investigated associations between PAG and periodontal disease status have thus far generated conflicting data (as reviewed by Taylor et al).84 A reasonable assumption currently is that there are genetic associations between polymorphisms in the IL-1 gene cluster and periodontal disease but that unambiguous results are not yet apparent because of the heterogeneity of the disease and/or the variable design of the reported studies. Cullinan et al concluded that IL-1 genotype is a contributory but nonessential risk factor for periodontal disease progression.15
A 5-month preliminary investigation by Ryan et al79 was designed to evaluate the impact of treatment on IL-1 and MMP levels in PST-positive patients who presented with elevated levels of these biochemical markers in their GCF. These patients were initially treated with SRP, resulting in no change in the levels of these biochemical markers after 1 month. Al-Shammari et al2 reported similar findings, with no changes in GCF levels of IL-1β and ICTP before and after SRP in patients who had not been genotyped. When the genotype-positive patients received SDD and these biochemical markers were monitored at 2 and 4 months, a significant decrease (50% to 61%) in the IL-1β and MMP-9 levels was noted after treatment with SDD. Correspondingly, gains in clinical attachment and reduced probing depths were also observed. The study concluded that a sub-antimicrobial dose of doxycycline may provide PST-positive patients with a therapeutic strategy that specifically addresses their exaggerated host response.
Another recent study was conducted in susceptible patients with severe generalized periodontitis using host modulation (SDD) as an adjunct to repeated subgingival debridement.62 Seventy percent of the patients who completed this 9-month double-blind, placebo-controlled study were smokers. SDD as an adjunct to mechanical therapy versus mechanical therapy alone resulted in significantly greater mean probing depth reductions in pockets of 7 mm or greater at baseline as early as 1 month after therapy (2.52 mm versus 1.25 mm, respectively). These improvements in the SDD group compared with the group receiving mechanical therapy only were maintained during the 5.25 months of therapy (2.85 mm versus 1.48 mm, respectively) and even at 3 months after stopping drug therapy (3.02 mm versus 1.41 mm), demonstrating that no rebound effect occurred. Because of the beneficial effects of HMT in susceptible patients, multicenter studies are using SDD in other susceptible populations, including diabetic, osteoporotic, and institutionalized geriatric patients, as well as smokers.
Since periodontitis is associated with many systemic diseases (e.g., osteoporosis, diabetes, and cardiovascular disease [CVD]), researchers have investigated the effect of SDD on these systemic conditions. Payne et al67 recently conducted a 2-year randomized, double-blind, placebo-controlled trial in osteopenic women at two dental centers demonstrating that SDD can effectively reduce the levels of localized and systemic inflammatory mediators in osteopenic patients in addition to improving on the clinical measurements of periodontitis28; SDD significantly reduced the progression of periodontal attachment loss75 and the severity of gingival inflammation and alveolar bone loss in postmenopausal osteopenic women.67 Golub et al28 reported that collagenase activity in the GCF of these osteopenic women was significantly reduced by SDD treatment. ICTP showed a similar pattern of change during SDD treatment with GCF collagenase activity and ICTP levels positively correlated at all time periods (p < 0.001). MMP-8 accounted for approximately 80% of total collagenase in the GCF, with much lower levels of MMP-1 and -13. SDD dramatically reduced the elevated MMP-8 levels by 60%. This 2-year study also demonstrated that long-term continuous use of SDD did not produce antibiotic side effects,92 confirming what had been reported previously in studies of continuous use of up to 12 months. Other studies have shown that SDD reduces systemic inflammatory biomarkers in CVD patients8 and SDD decreases glycosylated hemoglobin assay (HbA1c) in patients who are taking normally prescribed hypoglycemic agents.18 The impact of SDD therapy on periodontitis may be amplified by the concurrent use of other HMTS used to manage other inflammatory diseases. Additional studies are being conducted to investigate the impact of combined HMT use not only on periodontal disease but on other chronic inflammatory conditions.
Suggested Uses and Other Considerations
Until relatively recently, treatment options for periodontal disease have focused solely on reducing the bacterial challenge by nonsurgical therapy, surgery, and systemic or local antimicrobial therapy. The development of SDD as an HMT, driven by research into the pathogenesis of periodontal disease, is a great example of how translational research can lead to new treatments. By better understanding the biochemical processes that are important in periodontal disease, a pharmacologic principle (doxycycline downregulates MMP activity) has been used in the development of a new drug treatment. Data presented from research studies show the clinical benefits of adjunctive SDD, and the science behind SDD has been transferred into clinical practice. In other words, dentists now have the opportunity to use SDD for patient care, with the aim being to enhance the treatment response to conventional therapy.
Candidate Patients
When deciding whether to use SDD as an adjunct to SRP, first consider the patient’s motivation toward periodontal care, the medical history, and the patient’s willingness to take a systemic drug treatment. SDD is contraindicated in any patient with a history of allergy or hypersensitivity to tetracyclines. It should not be given to pregnant or lactating women or children less than 12 years old (because of the potential for discoloration of the developing dentition). Doxycycline may reduce the efficacy of oral contraceptives, and therefore alternative forms of birth control should be discussed, if necessary. There is a risk of increased sensitivity to sunlight (manifested by an exaggerated sunburn) seen with higher doses of doxycycline, although this has not been reported in the clinical trials using the sub-antimicrobial dose.
The rationale for using SDD must be clearly explained to the patient. By discussing the etiology of periodontal disease, the available treatment options, and the anticipated outcomes, patients become more interested in their periodontal management, are more likely to comply with treatment, and take more responsibility for managing their disease. Therefore the anticipated compliance and likely commitment to treatment must also be gauged when considering SDD therapy. Patients who show little enthusiasm for complying with the treatment plan or with oral hygiene practices are less likely to be good candidates for systemic drug therapy.
Treatable Periodontal Conditions
SDD is indicated in the management of chronic periodontitis, and studies to date have focused on chronic and aggressive forms of periodontitis.12,17,62,71 SDD should not be used in conditions such as gingivitis and periodontal abscess or when an antibiotic is indicated. SDD can be used in patients with aggressive periodontitis who are being treated nonsurgically. Furthermore, emerging studies have supported efficacy of SDD as an adjunct to periodontal surgery.20 SDD may also be of benefit in cases that are refractory to treatment, as well as in patients with risk factors such as smoking, diabetes, osteoporosis/osteopenia, genetic susceptibility, and in whom the treatment response might be limited.
Side Effects
Doxycycline at antibiotic doses (≥100 mg) is associated with adverse effects, including photosensitivity, hypersensitivity reactions, nausea, vomiting, and esophageal irritation. However, in the clinical trials of SDD (20-mg dose), it was reported that the drug was well tolerated, and the profile of unwanted effects was virtually identical in the SDD and placebo groups.12,17,62,71 The types of adverse events did not differ significantly between treatment groups, and the typical side effects of the tetracycline class were not observed indicating that the appearance of adverse events is dose related.12,71 Furthermore, there was no evidence of adverse events that could be attributed to antimicrobial effects of treatment and no evidence of developing antibiotic resistance of the microflora after 2 years of continuous use.12,85,86,92,93 Therefore the drug appears to be well tolerated, with a very low incidence of adverse effects.
Sequencing Prescription with Periodontal Treatment
SDD is indicated as an adjunct to mechanical periodontal therapy and should not be used as a stand-alone or monotherapy. SDD should be prescribed to coincide with the first round of SRP and is prescribed for 3 months, up to a maximum of 9 to 24 months of continuous dosing depending on the patient risk. Modification of any risk factors, such as smoking, nutrition, stress, contributing medications, faulty restorations, poor oral hygiene, and poor diabetic control, should also be addressed at this time. A patient’s refusal or inability to modify contributing risk factors is an important consideration for treatment planning and evaluation of therapeutic responses.
After initial periodontal treatment, the patient is enrolled into an intensive periodontal maintenance program. This involves regular monitoring of probing depths, reinforcement of oral hygiene, and re-motivation of the patient, with further SRP to disrupt the plaque biofilm and remove re-forming calculus deposits. The 3-month prescription of SDD fits in well with the typical maintenance recall interval of 3 months, which in turn is based on the duration reported for recolonization of treated periodontal pockets.50
Thus SDD therapy is commenced at the start of initial periodontal therapy and continues for 3 months until the first reevaluation or maintenance appointment. At maintenance appointments, the need for further prescription of SDD can be assessed. For patients demonstrating a good treatment response with significant reductions in probing depths, further SDD may not be necessary. Periodontal maintenance care must continue, with an emphasis on plaque control, monitoring, and prophylaxis. In other patients, the treatment response after completion of initial therapy may be less favorable. Sites with persisting or progressing pockets may require additional instrumentation, and the prescription of SDD may be extended for an additional 3 months. SDD may be combined with the use of locally applied antimicrobials and surgical procedures. Remember that periodontitis is a chronic disease, and the treatment (whether SDD is used or not) is long term. The patient must be regularly reevaluated to determine disease stability or progression and the need for additional active therapy.
Therefore patients may cycle between phases of “active” treatment (SRP + SDD) and long-term periodontal maintenance. The success of the maintenance phase of treatment will be affected by many factors, including the following:
Maintenance therapy is more likely to be successful in patients with good compliance, good oral hygiene, minimal or no systemic risk factors, and minimal residual deep pocketing. The patient who enters the maintenance program may have periodontal stability for months or years. However, plaque control may deteriorate, the patient may develop or acquire new risk factors, and disease progression (i.e., further loss of attachment) may become apparent, indicating that a further course of treatment is required. A further course of SRP will then be undertaken together with adjunctive SDD to restore periodontal stability.
Combining with Periodontal Surgery or Local Delivery Systems
Most clinical research to date has focused on using SDD as an adjunct to nonsurgical periodontal treatment. However, emerging data in which SDD was used as an adjunct to access flap surgery in 24 patients revealed better probing depth reductions in surgically treated sites greater than 6 mm compared with surgically treated sites in patients given placebo.20 Furthermore, the SDD group demonstrated greater reductions in ICTP (carboxy-terminal peptide, a breakdown product of collagen) than the placebo group, indicating that collagenolytic activity was reduced in the patients taking SDD.
SDD treatment can also be combined with the local delivery of antibiotics into the periodontal pocket through sustained-delivery systems. The two treatment approaches target different aspects of the pathogenic process: local delivery systems deliver antimicrobial concentrations of an antibacterial agent directly into the site of the pocket, whereas SDD is a systemic host response modulator. Thus combining these two complementary treatment strategies is another example of how antibacterial therapy (SRP + local antibiotics) can be combined with HMT (SDD) to maximize the clinical benefit for patients. Preliminary results from a 6-month, 180-patient clinical trial designed to evaluate the safety and efficacy of SDD combined with a locally applied antimicrobial (Atridox) and SRP versus SRP alone demonstrated that patients receiving the combination of treatments experienced more than a 2-mm improvement in mean attachment gains and probing depth reductions (p < 0.0001) compared with SRP alone.61
Monitoring Benefits of Therapy
To improve the ability of dentists to make appropriate treatment decisions for patients undergoing periodontal therapy, it would be extremely useful if they had access to the types of diagnostic tests available to their medical colleagues. Such tests might be used, for example, to distinguish between active and inactive lesions. Studies have shown that SRP alone, although effective for improving clinical parameters such as probing depths, may not be sufficient to reduce excessive levels of many underlying destructive mediators, particularly in more susceptible patients. It would be valuable therefore if it were possible to monitor the levels of such inflammatory mediators as treatment progresses. SDD results in downregulation of MMP activity in inflamed periodontal tissues.24,27 Therefore, in theory, MMP levels could be monitored before, during, and after SRP plus SDD treatment. Published data support a concomitant reduction in MMP levels in GCF13,17 and improvements in clinical parameters when combining SDD and SRP.17 Although chairside tests for MMPs have been developed,51 they are not in widespread use because of concerns about their specificity or sensitivity.
In the absence of new chairside tests or a centralized diagnostic facility for monitoring the inflammatory status of the tissues, dentists must rely on clinical periodontal monitoring to assess the outcomes of treatment. In addition to the reductions in probing depths and gains in attachment that may be observed after SRP plus SDD, the quality of the periodontal tissues also tends to improve after treatment with SDD. More sensitive radiographic techniques, assessments of bone density, and bone height changes used solely in clinical trials in the past may be possible in clinical practice in the future. Until such diagnostic techniques are made widely available, however, clinicians must rely on clinical judgment to determine the most appropriate course of therapy.
Emerging Host Modulatory Therapies
In the future, a variety of HMTs will likely be developed as adjunctive treatments for periodontitis. One of the most promising groups of potential HMTs is the chemically modified tetracyclines (CMTs). These nonantibiotic tetracycline analogs are tetracycline molecules that have been modified to remove all antibiotic properties, but which retain host modulatory, anticollagenolytic effects. The CMTs are also designed to be more potent inhibitors of proinflammatory mediators and can increase levels of antiinflammatory mediators such as IL-10. Because they have no antimicrobial properties, the clinician would be able to increase the dose for more susceptible patients with more risk factors and who might be more difficult to manage without getting side effects seen with antibiotics. CMTs, such as CMT-3 and CMT-8 (both of which lack antibiotic activity but retain anti-MMP activity), have been shown to inhibit osteoclastic bone resorption and promote bone formation,81 enhance wound healing,68 and inhibit proteinases produced by periodontal pathogens.35 CMTs also are being studied for other effects, such as inhibition of tumor cell invasion49 and attenuation of intimal thickening after arterial injury.44 CMTs will likely emerge as drugs that have beneficial effects in a variety of disease states because of their host modulation capabilities.
Other potential HMTs include the novel anticytokine drugs developed for the management of rheumatoid arthritis, a disease with a pathobiology similar to that of periodontitis.57 Cytokines such as TNF-α have been targeted by TNF-α antagonists (e.g., Infliximab, Etanercept), which have been shown to be effective in treating rheumatoid arthritis.90 As yet, such drugs have not been evaluated in the treatment of periodontal disease, but they could offer potential benefits given the importance of inflammatory cytokines, such as TNF-α, in periodontal pathogenesis. In addition, drugs designed to increase the levels of antiinflammatory or protective mediators, such as IL-1ra, can perform similar functions. In addition, evidence is emerging to support the benefits of combining HMTs that target different aspects of the disease processes. For example, the combination of SDD with bisphosphonates has also showed synergy in animal models of bone loss.
Recent research has indicated that there is an active biochemical resolution phase involved in inflammation. Failure of this resolution pathway may play a role in the pathogenesis of periodontal diseases.88 Restoration of the resolution pathway by the introduction of resolving molecules may provide new potential therapeutic methods for the management of inflammatory periodontitis.7 In rabbit animal models of periodontitis, topical application of Resolvin E1 has been shown to protect bone loss mediated by osteoclasts.40 In the future, a combination of antiinflammatory drugs with resolving agents tested in human clinical trials may provide evidence to support a brand new host modulation therapy for the management of periodontal diseases.
Host Modulation Factors in Systemic Disorders
In susceptible patients demonstrating an excessive local inflammatory response to the bacterial stimuli leading to periodontal disease, another consideration involves the loss of epithelial integrity in the periodontal pocket. This tissue response allows for bacterial penetration into the inflamed tissues and eventual entry of the bacteria into the systemic circulation. Patients with untreated periodontitis have an increased risk for transient bacteremias. Bacteremia and associated endotoxemia may incite the overproduction of destructive proinflammatory mediators at distant sites in the periodontitis patient. Therefore patients with periodontitis may be at greater risk for developing a number of systemic conditions associated with a similar overactive host response to external stimuli such as cardiovascular disease and diabetic complications. Elevated levels of cytokines, prostanoids, and enzymes are evident in all these conditions.
In the era of periodontal medicine, systemic host modulatory approaches need to be considered. As mentioned, host modulators used to manage periodontal disease, such as an MMP, cytokine, and prostanoid inhibitors, may have additional beneficial effects on systemic diseases that have been linked to periodontal disease, such as CVD and diabetes. In CVD, preliminary studies have indicated that individuals with periodontal disease are almost twice as likely to have a fatal heart attack and three times as likely to have a stroke.4 MMPs and cytokines have been found to play a major role in weakening the plaques formed with CVD, leading to rupture and eventual thrombosis and infarction.23 In fact, Golub et al26 suggested that tetracyclines could reduce the incidence of acute myocardial infarction56 by blocking collagenase and stabilizing the collagen cap on the atheroscleromatous arterial plaques. In diabetes, the same MMPs and cytokines involved in the development of periodontitis as the sixth long-term complication of diabetes55 also play a role in the development of other well-known complications of diabetes such as nephropathy, angiopathy, retinopathy, and wound-healing problems.77 Modulation of these proinflammatory mediators in diabetic patients may impede the development of multiple long-term complications. An inhibitor of MMPs, cytokines, and prostanoids used in the treatment of periodontitis may have an indirect effect on these disease processes if a patient’s risk for developing these disorders is increased by the presence of untreated periodontitis. HMT may also directly aid in the treatment and prevention of CVD and diabetic complications.
Summary
Periodontal pathogens and destructive host responses are involved in the initiation and progression of periodontitis. Therefore the successful long-term management of this disease may require a treatment strategy that integrates therapies that address both etiologic components. Evidence for the role of MMPs, cytokines, and other mediators in the pathogenesis of periodontal disease distinguishes them as viable targets for a chemotherapeutic approach. The introduction of novel, adjunctive therapies such as host modulation to enhance the efficacy of existing mechanical procedures can contribute favorably to an integrated approach for the long-term, clinical management of periodontitis.
HMTs are an emerging treatment concept in the management of periodontitis. The use of HMT as an adjunct may be particularly useful in susceptible, high-risk patients in whom a prolonged and excessive host response to the presence of bacteria promotes the activity of MMPs and osteoclasts. SDD is the only systemically administered HMT currently approved and indicated as an adjunct to SRP for treating periodontitis. Clinical trials have demonstrated a clear treatment benefit when using SDD versus SRP alone. SDD should be used as part of a comprehensive treatment strategy that includes antibacterial treatments (SRP, plaque control, oral hygiene instruction, local antimicrobials, and periodontal surgery), host response modulation (SDD), and assessment and management of periodontal risk factors. In the future, a range of HMTs targeting different aspects of the destructive cascade of breakdown events in the periodontal tissues are likely to be developed as adjunctive treatments for periodontitis. The further development of these agents will permit dentists to treat specific aspects of the underlying biochemical basis for periodontal disease. The goal is to maximize the treatment response by reducing inflammation and inhibiting destructive processes in the tissues, which will result in enhanced periodontal stability after conventional periodontal treatments such as SRP and surgery. The dentist is now in the exciting position to be able to combine established treatment strategies with new systemic and local drug treatments for this common, chronic disease.
The findings discussed with regard to the use of HMT to better manage chronic periodontal disease may have applications to other chronic systemic diseases such as arthritis, diabetes, osteoporosis, and cardiovascular disease. In addition, studies utilizing locally applied antimicrobials as part of an intensive periodontal therapy (IPT) regimen have shown very promising results. Future studies may demonstrate that in addition to our current standard therapies, IPT with adjunctive antibiotics and/or host modulation for the management of periodontal disease may have profound positive effects on the overall health status of high risk patients. The proper management of local infection and inflammation (periodontitis) will have a significant impact on general overall health of the population.
![]() Science Transfer
Science Transfer
At the present time, the only FDA-approved agent for host modulation treatment of periodontal disease is sub-antimicrobial dose doxycycline (SDD) 20 mg per day. This can be used in conjunction with initial therapy and needs to be taken over a prolonged period of up to 9 months. Patients with a history of allergy to the tetracyclines and doxycycline are not able to use this therapy. Also, it is contraindicated in pregnant and lactating women and children younger than 12 years of age. Patients taking oral contraceptives may have reduced protection from pregnancy with this therapy. Most patients will have a small (i.e., less than 1 mm) additive effect on pocket depth reduction when this systemic approach is added to scaling and root planing.
The downregulation of the destructive elements of the host immune response has the potential to add to the spectrum of treatment choices in the future; however, other agents, such as systemic nonsteroidal antiinflammatory drugs (NSAIDs) such as ibuprofen, can cause significant side effects with long-term use and are not advised. Antiosteoporotic agents, such as the bisphosphonates, have minimal effect on periodontal bone loss and carry other risks such as localized bone necrosis.
Modulation of host response may be valuable in enhancing the effects of locally released antimicrobial agents, such as doxycycline and minocycline, and in the nonsurgical treatment of periodontitis in smokers.
1 Ah MK, Johnson GK, Kaldahl WB, et al. The effect of smoking on the response to periodontal therapy. J Clin Periodontol. 1994;21:91.
2 Al-Shammari KF, Giannobile WV, Aldredge WA, et al. Effect of non-surgical periodontal therapy on C-telopeptide pyridinoline cross-links (ICTP) and interleukin-1 levels. J Periodontol. 2001;72:1045.
3 Ashley RA. Clinical trials of a matrix metalloproteinase inhibitor in human periodontal disease. SDD Clinical Research Team. Ann NY Acad Sci. 1999;878:335.
4 Beck JD, Offenbacher S, Williams R, et al. Periodontitis: a risk factor for coronary heart disease? Ann Periodonto. 1998;3:127.
5 Bezerra MM, de Lima V, Alencar VB, et al. Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2000;71:1009.
6 Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474.
7 Bhatavadekar NB, Williams RC. New directions in host modulation for the management of periodontal disease. J Clin Periodontol. 2009;36(2):124.
8 Brown DL, Desai KK, Vakili BA, et al. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24(4):733.
9 Burns FR, Stack MS, Gray RD, Paterson CA. Inhibition of purified collagenase from alkali-burned rabbit corneas. Invest Ophthalmol Vis Sci. 1989;30:1569.
10 Carranza FA, editor. Clinical periodontology, ed 8, Philadelphia: Saunders, 1996.
11 Carter G, Goss AN, Doecke C. Bisphosphonates and avascular necrosis of the jaw: a possible association. Med J Aust. 2005;182:8. 413
12 Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521.
13 Choi DH, Moon IS, Choi BK, et al. Effects of sub-antimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitis. J Periodontal Res. 2004;39:20.
14 Crout RJ, Lee HM, Schroeder K, et al. The “cyclic” regimen of low-dose doxycycline for adult periodontitis: a preliminary study. J Periodontol. 1996;67:506.
15 Cullinan MP, Westerman B, Hamlet SM, et al. A longitudinal study of interleukin-1 gene polymorphisms and periodontal disease in a general adult population. J Clin Periodontol. 2001;28:1137.
16 El-Shinnawi UM, El-Tantawy SI. The effect of alendronate sodium on alveolar bone loss in periodontitis (clinical trial). J Int Acad Periodontol. 2003;5:5.
17 Emingil G, Atilla G, Sorsa T, et al. The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. J Periodontol. 2004;75:106.
18 Engebretson SP, Hey-Hadavi J, Celenti R, et al. Low-dose doxycycline treatment reduces glycosylated hemoglobin in patients with type 2 diabetes: a randomized controlled trial. J Dent Res. 82(Spec. issue), 2003. abstract #1445
19 Engebretson SP, Lamster IB, Herrera-Abreu M, et al. The influence of interleukin gene polymorphism on expression of interleukin-1beta and tumor necrosis factor-alpha in periodontal tissue and gingival crevicular fluid. J Periodontol. 1999;70:567.
20 Gapski R, Barr JL, Sarment DP, et al. Effect of systemic matrix metalloproteinase inhibition on periodontal wound repair: a proof of concept trial. J Periodontol. 2004;75:441.
21 Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041.
22 Genco RJ. Host responses in periodontal diseases: current concepts. J Periodontol. 1992;63:338.
23 Genco RJ, Offenbacher S, Beck J, Rees T. Cardiovascular disease and oral infections. In: Rose LF, editor. Periodontal medicine. Ontario: BC Decker, 1999.
24 Golub LM, Ciancio S, Ramamurthy NS, et al. Low-dose doxycycline therapy: effect on gingival and crevicular fluid collagenase activity in humans. J Periodontal Res. 1990;25:321.
25 Golub LM, Evans RT, McNamara TF, et al. A non-antimicrobial tetracycline inhibits gingival matrix metalloproteinases and bone loss in Porphyromonas gingivalis-induced periodontitis in rats. Ann NY Acad Sci. 1994;732:96.
26 Golub LM, Greenwald RA, Thompson RW. Antibiotic use and risk of mycoardial infarction. JAMA. 1999;282:1997.
27 Golub LM, Lee HM, Greenwald RA, et al. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46:310.
28 Golub LM, Lee HM, Stoner JA, et al. Subantimicrobial-dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J Periodontol. 2008;79(8):1409.
29 Golub LM, McNamara TF, Ryan ME, et al. Adjunctive treatment with subantimicrobial doses of doxycycline: effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J Clin Periodontol. 2001;28:146.
30 Golub LM, Sorsa T, Lee HM, et al. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J Clin Periodontol. 1995;22:100.
31 Golub LM, Wolff M, Lee HM, et al. Further evidence that tetracyclines inhibit collagenase activity in human crevicular fluid and from other mammalian sources. J Periodont Res. 1985;20:12.
32 Golub LM, Suomalainen K, Sorsa T. Host modulation with tetracyclines and their chemically modified analogues. Curr Opin Dent. 1992;2:80.
33 Goodson JM, Dewhirst FE, Brunetti A. Prostaglandin E2 levels and human periodontal disease. Prostaglandins. 1974;6:81.
34 Górska R, Nedzi-Góra M. The effects of the initial treatment phase and of adjunctive low-dose doxycycline therapy on clinical parameters and MMP-8, MMP-9, and TIMP-1 levels in the saliva and peripheral blood of patients with chronic periodontitis. Arch Immunol Ther Exp (Warsz). 2006;54(6):419.
35 Grenier D, Plamondon P, Sorsa T, et al. Inhibition of proteolytic, serpinolytic, and progelatinase-b activation activities of periodontopathogens by doxycycline and the non-antimicrobial chemically modified tetracycline derivatives. J Periodontol. 2002;73:79.
36 Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:51.
37 Grossi SG, Skrepcinski FB, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713.
38 Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260.
39 Gürkan A, Cinarcik S, Hüseyinov A. Adjunctive subantimicrobial dose doxycycline: effect on clinical parameters and gingival crevicular fluid transforming growth factor-beta levels in severe, generalized chronic periodontitis. J Clin Periodontol. 2005;32(3):244.
40 Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179(10):7021.
41 Holzhausen M, Rossa CJr, Marcantonio EJr, et al. Effect of selective cyclooxygenase-2 inhibition on the development of ligature-induced periodontitis in rats. J Periodontol. 2002;73:1030.
42 Howell TH, Jeffcoat MK, Goldhaber P, et al. Inhibition of alveolar bone loss in beagles with the NSAID naproxen. J Periodontal Res. 1991;26:498.
43 Howell TH, Williams RC. Non-steroidal anti-inflammatory drugs as inhibitors of periodontal disease progression. Crit Rev Oral Biol Med. 1993;4:177.
44 Islam MM, Franco CD, Courtman DW, Bendeck MP. A nonantibiotic chemically modified tetracycline (CMT-3) inhibits intimal thickening. Am J Pathol. 2003;163:1557.
45 Jeffcoat MK, Reddy MS, Haigh S, et al. A comparison of topical ketorolac, systemic flurbiprofen, and placebo for the inhibition of bone loss in adult periodontitis. J Periodontol. 1995;66:329.
46 Kinane DF, Chestnutt IG. Smoking and periodontal disease. Crit Rev Oral Biol Med. 2000;11:356.
47 Lee HM, Golub LM, Chan D, et al. α1-Proteinase inhibitor in gingival crevicular fluid of humans with adult periodontitis: serpinolytic inhibition by doxycycline. J Periodontal Res. 1997;32:9.
48 Lee JY, Lee YM, Shin SY, et al. Effect of subantimicrobial dose doxycycline as an effective adjunct to scaling and root planning. J Periodontol. 2004;75(11):1500.
49 Lokeshwar BL, Escatel E, Zhu B. Cytotoxic activity and inhibition of tumor cell invasion by derivatives of a chemically modified tetracycline CMT-3 (COL-3). Curr Med Chem. 2001;8:271.
50 Magnusson I, Lindhe J, Yoneyama T, Liljenberg B. Recolon-ization of a subgingival microbiota following scaling in deep pockets. J Clin Periodontol. 1984;11:193.
51 Mantyla P, Stenman M, Kinane DF, et al. Gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis. J Periodontal Res. 2003;38:436.
52 Mariotti A. The extracellular matrix of the periodontium: dynamic and interactive tissues. Periodontol 2000. 1993;3:39.
53 Maynard J. Eras in periodontics. Periodontal Disease Management: a conference for the dental team. Boston: American Academy of Periodontology; 1993.
54 Mealey B. Diabetes and periodontal diseases. J Periodontol. 2000;71:664.
55 Mealy B. Diabetes mellitus. In: Rose LF, editor. Periodontal medicine. Ontario: BC Decker, 1999.
56 Meier CR, Derby LE, Jick SS, et al. Antibiotics and risk of subsequent first-time acute myocardial infarction. JAMA. 1999;281:427.
57 Mercado FB, Marshall RI, Bartold PM. Inter-relationships between rheumatoid arthritis and periodontal disease: a review. J Clin Periodontol. 2003;30:761.
58 Mohammad AR, Preshaw PM, Bradshaw MH, et al. Adjunctive subantimicrobial dose doxycycline in the management of institutionalised geriatric patients with chronic periodontitis. Gerodontology. 2005;22(1):37.
59 Nakaya H, Osawa G, Iwasaki N, et al. Effects of bisphosphonate on matrix metalloproteinase enzymes in human periodontal ligament cells. J Periodontol. 2000;71:1158.
60 Needleman I, Suvan J, Gilthorpe MS, et al. A randomized-controlled trial of low-dose doxycycline for periodontitis in smokers. J Clin Periodontol. 2007;34(4):325.
61 Novak MJ, Dawson DR3rd, Magnusson I, et al. Combining host modulation and topical antimicrobial therapy in the management of moderate to severe periodontitis: a randomized multicenter trial. J Periodontol. 2008;79(1):33.
62 Novak MJ, Johns LP, Miller RC, Bradshaw MH. Adjunctive benefits of subantimicrobial dose doxycycline in the management of severe, generalized, chronic periodontitis. J Periodontol. 2002;73:762.
63 Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821.
64 Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64:432.
65 Page RC. Milestones in periodontal research and the remaining critical issues. J Periodontal Res. 1999;34:331.
66 Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9.
67 Payne JB, Stoner JA, Nummikoski PV, et al. Subantimicrobial dose doxycycline effects on alveolar bone loss in post-menopausal women. J Clin Periodontol. 2007;34(9):776.
68 Pirila E, Ramamurthy N, Maisi P, et al. Wound healing in ovariectomized rats: effects of chemically modified tetracycline (CMT-8) and estrogen on matrix metalloproteinases -8, -13 and type I collagen expression. Curr Med Chem. 2001;8:281.
69 Preber H, Bergstrom J. Effect of cigarette smoking on periodontal healing following surgical therapy. J Clin Periodontol. 1990;17:324.
70 Preshaw PM, Hefti AF, Bradshaw MH. Adjunctive subantimicrobial dose doxycycline in smokers and non-smokers with chronic periodontitis. J Clin Periodontol. 2005;32:610.
71 Preshaw PM, Hefti AF, Novak MJ, et al. Subantimicrobial dose doxycycline enhances the efficacy of scaling and root planing in chronic periodontitis: a multi-center trial. J Periodontol. 2004;75:1068.
72 Preshaw PM. Host response modulation in periodontics. Periodontol 2000. 2008;48:92.
73 Preshaw PM, Novak MJ, Mellonig J, et al. Modified-release subantimicrobial dose doxycycline enhances scaling and root planing in subjects with periodontal disease. J Periodontol. 2008;79(3):440.
74 Reddy MS, Weatherford TW, Smith CA, et al. Alendronate treatment of naturally-occurring periodontitis in beagle dogs. J Periodontol. 1995;66:211.
75 Reinhardt RA, Stoner JA, Golub LM, et al. Efficacy of sub-antimicrobial dose doxycycline in post-menopausal women: clinical outcomes. J Clin Periodontol. 2007;34(9):768.
76 Rocha M, Nava LE, Vazquez de la Torre C, et al. Clinical and radiological improvement of periodontal disease in patients with type 2 diabetes mellitus treated with alendronate: a randomized, placebo-controlled trial. J Periodontol. 2001;72:204.
77 Ryan ME. Host response in diabetes-associated periodontitis: effects of tetracycline analogues. Stony Brook: State University of New York (PhD thesis); 1998.
78 Ryan ME, Ramamurthy NS, Golub LM. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr Opin Periodontol. 1996;3:85.
79 Ryan ME, Lee HM, Bookbinder MK, et al. Treatment of genetically susceptible patients with a subantimicrobial dose of doxycycline. J Dent Res. 2000;79:608. (abstract 3719)
80 Salvi GE, Lawrence HP, Offenbacher S, et al. Influence of risk factors on the pathogenesis of periodontitis. Periodontol 2000. 1997;14:173.
81 Sasaki T, Ohyori N, Debari K, et al. Effects of chemically modified tetracycline, CMT-8, on bone loss and osteoclast structure and function in osteoporotic states. Ann NY Acad Sci. 1999;878:347.
82 Shoji K, Horiuchi H, Shinoda H. Inhibitory effects of a bisphosphonate (risedronate) on experimental periodontitis in rats. J Periodont Res. 1995;30:277.
83 Stoltenberg JL, Osborn JB, Pihlstrom BL, et al. Association between cigarette smoking, bacterial pathogens and periodontal status. J Periodontol. 1993;64:1225.
84 Taylor JJ, Preshaw PM, Donaldson PT. Cytokine gene polymorphism and immunoregulation in periodontal disease. Periodontol 2000. 2004;35:158.
85 Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472.
86 Thomas JG, Metheny RJ, Karakiozis JM, et al. Long-term sub-antimicrobial doxycycline (Periostat) as adjunctive management in adult periodontitis: effects on subgingival bacterial population dynamics. Adv Dent Res. 1998;12:32.
87 US Department of Health and Human Services (DHHS). Oral health in America: a Report of the Surgeon General, Executive Summary. Rockville, MD: DHHS, NIDCR, National Institutes of Health; 2000.
88 Van Dyke TE. The management of inflammation in periodontal disease. J Periodontol. 2008;79(8 Suppl):1601.
89 Vardar S, Baylas H, Huseyinov A. Effects of selective cyclooxygenase-2 inhibition on gingival tissue levels of prostaglandin E2 and prostaglandin F2a and clinical parameters of chronic periodontitis. J Periodontol. 2003;74:57.
90 Vilcek J, Feldmann M. Historical review: cytokines as therapeutics and targets of therapeutics. Trends Pharmacol Sci. 2004;25:201.
91 Walker C, Preshaw PM, Novak J, et al. Long-term treatment with subantimicrobial dose doxycycline has no antibacterial effect on intestinal flora. J Clin Periodontol. 2005;32:1163.
92 Walker C, Puumala S, Golub LM, et al. Subantimicrobial dose doxycycline effects on osteopenic bone loss: microbiologic results. J Periodontol. 2007;78(8):1590.
93 Walker C, Thomas J, Nango S, et al. Long-term treatment with subantimicrobial dose doxycycline exerts no antibacterial effect on the subgingival microflora associated with adult periodontitis. J Periodontol. 2000;71:1465.
94 Weinreb M, Quartuccio H, Seedor JG, et al. Histomorphometrical analysis of the effects of the bisphosphonate alendronate on bone loss caused by experimental periodontitis in monkeys. J Periodontal Res. 1994;29:35.
95 Williams RC. Periodontal disease. N Engl J Med. 1990;322(6):373.
96 Williams RC, Jeffcoat MK, Howell TH, et al. Altering the progression of human alveolar bone loss with the non-steroidal anti-inflammatory drug flurbiprofen. J Periodontol. 1989;60:485.
97 Williams RC, Jeffcoat MK, Howell TH, et al. Indomethacin or flurbiprofen treatment of periodontitis in beagles: comparison of effect on bone loss. J Periodontal Res. 1987;22:403.
98 Williams RC, Jeffcoat MK, Howell TH, et al. Three year trial of flurbiprofen treatment in humans: post-treatment period. J Dent Res. 1991;70:468. (abstract 1617)
99 Wouters FR, Salonen LE, Frithiof L, Hellden LB. Significance of some variables on interproximal alveolar bone height based on cross-sectional epidemiological data. J Clin Periodontol. 1993;20:199.