CHAPTER 61 SUPPLEMENT A Reconstructive Periodontal Surgery
Recombinant Human Platelet-Derived Growth Factor-BB for Implant Site Development
Advances in tissue engineering are revolutionizing clinical medicine with the development of biological substitutes that augment or reconstitute organs, tissues, and tissue function.29 The potential for recombinant proteins to promote the regeneration of the periodontium and other oral tissues has demonstrated significant therapeutic promise in preclinical and clinical studies. Multiple controlled clinical trials have been completed evaluating the therapeutic effectiveness of recombinant growth factors for periodontal therapy,25,39 extraction socket ridge preservation,21 and sinus augmentation.8
Growth factors and other biological mediators have significant potential to improve wound healing and enhance the clinical benefits of bone replacement grafts.19,31,32,38,44 In 2005, the United States (US) Food and Drug Administration (FDA) approved for marketing a new dental bone filling device, GEM 21S (Osteohealth, Norristown, PA), which combines recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and beta-tricalcium phosphate (β-TCP) for the treatment of periodontal-related defects. GEM 21S is a completely synthetic grafting system for periodontal regeneration composed of highly purified rhPDGF (0.5 ml [0.3 mg/ml]) and synthetic β-TCP (0.5 cc Ca3 [PO4]). A large-scale, randomized controlled clinical trial demonstrated that rhPDGF-BB was safe and effective in the treatment of periodontal osseous defects when combined with β-TCP bone substitute.40 Since the commercial availability of GEM 21S, preclinical and clinical studies have focused on the effectiveness of rhPDGF-BB in combination with different scaffolds for periodontal and other regenerative indications, including dentoalveolar reconstruction as part of implant site development.
PDGF-BB exerts potent stimulatory effects as a chemoattractant and mitogen for osteoprogenitor cells, which are critical for new bone formation. The growth factor is also a key stimulatory factor and promoter of angiogenesis, complementing the actions of vascular endothelial growth factor (VEGF) in vessel formation.23,24 Cell culture studies highlight the potent biologic activity of PDGF-BB, with concentrations as low as 1 ng/ml of the growth factor significantly affecting the migration and proliferation periodontal ligament cell.35,45 In clinical applications, the potent biologic actions of PDGF-BB appear to occur early, amplifying a cascade of cellular events that continue over weeks at the surgical wound. Available evidence supports the premise that amplification of progenitor cell recruitment and differentiation promotes new vessel formation and regeneration during wound healing.23
The early modulation of signalling cascades in wound healing appears critical for periodontal regeneration. The physiologic time course of regulatory events stimulated by rhPDGF-BB has been evaluated after the single, local application of this growth factor combined with reconstructive surgery using β-TCP bone substitute in the treatment of periodontal intraosseous defects in humans.16,53 In these studies, wound and crevicular fluid samples were assayed for surrogate markers of wound healing (VEGF) and bone metabolism (pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen) over a 6-month period. The results revealed significant alterations in metabolic activity at the clinical site of application, consistent with the effects of rhPDGF-BB on clinical and radiographic periodontal regeneration.16,53 The latter findings paralleled differences in regenerative outcome. Collectively, these findings indicate that the local delivery of PDGF-BB exerts an early but sustained effect on periodontal bone metabolism.
Currently, rhPDGF-BB is approved by the FDA only for periodontal regeneration as part of the dental bone filling device GEM 21S, which is indicated in the treatment of intrabony periodontal defects, furcation periodontal defects, and gingival recession associated with periodontal defects (GEM 21S Package Insert). The effectiveness of GEM 21S has not been established in other regenerative indications such as alveolar bone defects and sinus augmentation. GEM 21S consists of 0.5 cc of β-TCP particles (0.25 to 1.0 mm) and 0.5 ml rhPDGF-BB (0.3 mg/ml). The clinical effectiveness of rhPDGF-BB and β-TCP in the treatment of periodontal intrabony defects has been rigorously tested in an 180 patient clinical trial,39 and “proof-of-principle” histologic evidence of regeneration has been shown.37,50 Although this device uses β-TCP as the scaffold/carrier, there has been considerable clinical interest in combining rhPDGF-BB with other bone replacement grafts, particularly bone allografts and xenografts.
Clinical case series reports also provide information on the clinical efficacy of combinatorial approaches using PDGF-BB with bone allografts. Camelo et al12 and Nevins et al39 reported human histologic evidence of periodontal regeneration in intraosseous defects and furcation defects treated using a combination of rhPDGF-BB and DFDBA (Supplement A Figure 61-1). Additional clinical reports have also demonstrated successful clinical cases utilizing an allograft scaffold for delivering rhPDGF-BB for periodontal therapy.34,39,41 In vitro data support the use of various scaffolds, including a mineral collagen bone substitute.58 This biomaterial has the benefit of combining a particulate bovine-derived anorganic bone matrix (90%) with collagen (10%) for biologic and biomechanical reasons. Supplement A Figure 61-2 demonstrates a presurgical radiograph of a mandibular right lateral incisor with 8-mm attachment loss and an angular intrabony defect that was treated with rhPDGF-BB and this mineral collagen bone substitute. The 18-month posttreatment evaluation revealed 5-mm attachment gain and radiographic suggestion of bone fill to the crest of the defect. The prognosis of the tooth improved from guarded to good, and the patient progressed to orthodontic therapy.
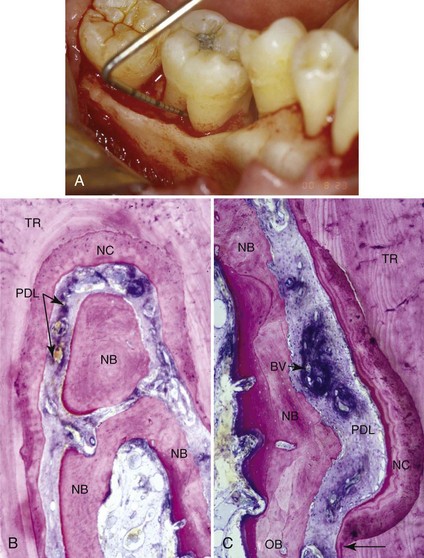
Supplement A Figure 61-1 A, Intraoperative photograph with probe in place showing a 5-mm furcation defect on the lingual of tooth #19. The furcation was treated with recombinant human platelet-derived growth factor-BB (rhPDGF-BB) mixed with demineralized freeze-dried bone allograft (DFDBA). After 9 months of healing, the tooth was resected and submitted for histologic analysis. B, Histologic section showing tooth root (TR), original bone (OB), new bone (NB), new cementum (NC), new periodontal ligament (PDL), and new blood vessel (BV) formation. New cementum is shown extending coronally from the notch placed at the apical extent of calculus during the treatment surgery. (Original magnification ×25.) C, Area of the fornix of the furcation shows new bone completely filling the original furcation defect, with restoration of a well-organized new PDL throughout the furcation. (Original magnification ×25.)
(A, from Nevins M, et al: Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol 74(9):1282-1292, 2003. B, and C, from Camelo M, et al: Periodontal regeneration in human class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft, Int J Periodontics Restorative Dent 23(3):213-225. 2003.)
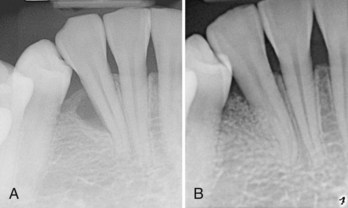
Supplement A Figure 61-2 A, Periapical radiograph of mandibular lateral incisor reveals evidence of vertical periodontal bone loss. The tooth treated with a regenerative periodontal surgical procedure grafting with mineral collagen bone substitute saturated with recombinant human platelet-derived growth factor-BB (rhPDGF-BB, 0.3 mg/ml). B, Periapical radiograph 18 months posttreatment reveals suggestion of bone fill consistent with the noted clinical improvement of 5 mm attachment gain.
(Courtesy of Dr. Marc Nevins. Boston.)
A recent report evaluated the healing of advanced buccal-wall extraction socket defects using a combination of rhPDGF-BB and particulate bovine-derived anorganic bone matrix (90%) with collagen (10%) at 4 and 6 months.42 Clinical examination and microscopic and computed tomography (CT) analysis of bone specimens demonstrated excellent bone fill at 4 months with robust new bone formation in and around the xenograft particles. This study is significant in that the histologic evaluation demonstrated multinucleated giant cells proximal to graft particles, together with evidence of reversal lines, suggestive of remodeling of the xenograft particles at 4 months posttreatment. Further research is needed to validate what appears to be a significant upregulation in bone metabolism.
Recent preclinical and clinical studies support earlier reports5,33 that rhPDGF-BB combined with other graft matrices can support improved bone formation and wound healing in alveolar ridge reconstruction and implant therapy. The pluripotent effects of PDGF on bone and soft tissues make it an important growth factor for complex esthetic regenerative procedures. Recent clinical reports suggest that rhPDGF can enhance the results of augmentation procedures, including combination hard and soft tissue grafting, and the treatment of defects at the time of implant placement.11,20
Simion et al55 reported the results of a canine study demonstrating the potential for a deproteinized cancellous bovine block, when infused with rhPDGF-BB, to regenerate significant amounts of new bone in severe mandibular vertical ridge defects without placement of a barrier membrane. The xenogenic block grafts were infused with rhPDGF-BB and stabilized in alveolar defects using two dental implants with or without collagen membranes. The alveolar ridge defects treated with the combination of rhPDGF-BB and xenograft without a collagen membrane demonstrated the greatest bone formation based on radiographic and histologic outcome measures. The histologic findings revealed robust osteogenesis throughout the block graft, with significant graft resorption and replacement (Supplement A Figure 61-3). In contrast, alveolar ridge defects treated with traditional guided bone regeneration (GBR) without the growth factor supported little or no bone formation. In two case reports, Simion reported on the use of rhPDGF-BB in combination with either a xenogeneic block bone graft or particulate graft to achieve advanced vertical and lateral alveolar ridge reconstruction.56,57 These case reports provide important human evidence on the use of this recombinant growth factor to successfully reconstruct significant alveolar ridge deformities in preparation for dental implant therapy.
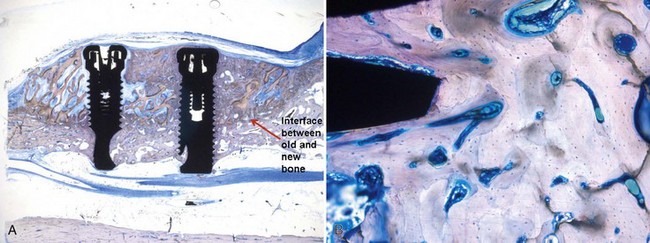
Supplement A Figure 61-3 A, Specimen (deproteinized bovine block + recombinant human platelet-derived growth factor-BB [rhPDGF-BB]). Note the formation of new bone around the implants. B, Specimen (deproteinized bovine block + rhPDGF-BB) reveals intense osteoblastic activity and remodeling with mature osteons (toluidine blue/pyronine G stain; magnification ×160).
(From Simion M, Rocchietta I, Kim D, et al: Int J Periodontics Restorative Dent 26:415, 2006.)
Collectively, available preclinical and clinical evidence documents the potential for rhPDGF-BB in combination with allogeneic and xenogeneic grafts to support bone formation and promote wound healing in alveolar ridge reconstruction. Nevertheless, the off-label use of rhPDGF-BB in combination with scaffolds other than β-TCP or for therapeutic indications other than periodontal defects must be based on firm scientific rationale and sound medical evidence. In the following clinical cases, the off-label use of rhPDGF-BB was determined to be consistent with good clinical practice and in the best interest of the patient after careful evaluation of clinical parameters and consideration of current and emerging evidence.
Clinical Case Reports
Ridge Preservation after Extraction
Tooth extraction results in resorption and remodeling of the alveolar process that can culminate over time in significant alterations in ridge morphology, especially in areas supporting removable dentures.3,4 Alveolar ridge preservation therefore is critical to ensure sufficient alveolar bone volume and favorable morphology to obtain ideal functional and esthetic prosthetic reconstruction supported prosthetic rehabilitation. In general, the facial alveolar process is at greater risk of undergoing remodeling than the lingual aspect following tooth extraction.*
Traditional approaches to site preservation have relied on a combination of bone replacement grafts and barrier membranes to compartmentalize extraction sites (GBR) in an effort to reduce the severity of crestal alveolar bone resorption. In this approach, a graft material is placed into the socket and covered by barrier membrane that extends past the crestal bone margin and under the marginal soft tissue. Mucoperiosteal flaps are typically elevated and advanced for the purpose of achieving primary closure. The application of graft plus barrier membrane for site preservation is most successful when the extraction socket walls are intact; the presence of osseous defects, such as dehiscences, often limits the effectiveness of this material-based approach. Further, although primary closure enhances bone healing, mobilization and advancement of the mucoperiosteal flap alters the natural location of the mucogingival junction, often significantly compromising vestibular anatomy and esthetics.
The therapeutic objective of combining a biologic or growth factor with a bone replacement graft in the clinical management of extraction sockets is to promote wound healing and bone regeneration, thereby minimizing alveolar ridge resorption, remodeling, and defect formation. The clinical goal is to achieve predictable ridge preservation after tooth extraction, even at sites requiring the correction of associated bony defects. Biologic agents offer the potential to change the surgical management of extraction sites, which includes the use of minimally invasive techniques, even with sites that have suffered extensive bone loss due to tooth associated pathology such as advanced periodontal disease, failed endodontic therapy, or a root fracture (Supplement A Figures 61-4 and 61-5).
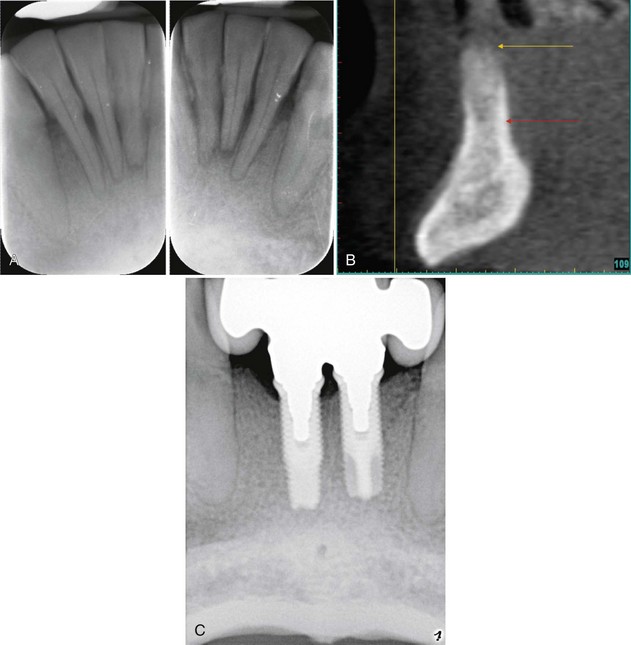
Supplement A Figure 61-4 A, Radiographs reveal advanced periodontal bone loss approaching the apical third of the mandibular incisors. B, Cone-beam computed tomographic (CBCT) image of anterior mandibular region after extraction of four incisors and ridge preservation using freeze-dried bone allograft (FDBA) and recombinant human platelet-derived growth factor-BB (rhPDGF-BB) reveals maintenance of the ridge height. C, Radiograph shows implant-supported prosthesis, which serves as an effective tooth replacement. Note the improved appearance of the crestal lamina dura of the adjacent canines.
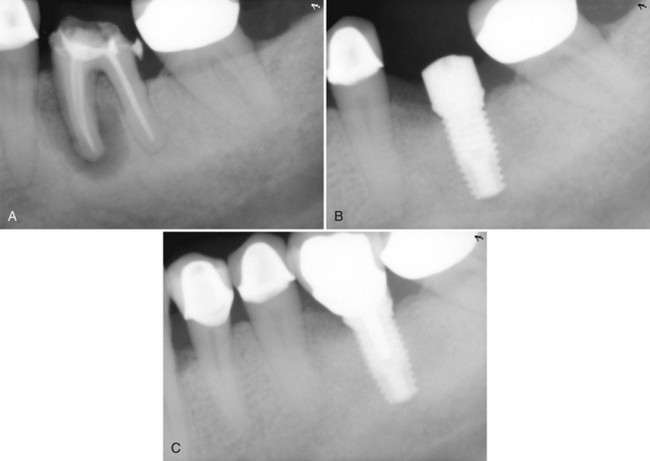
Supplement A Figure 61-5 A, Periapical radiograph of mandibular first molar with fractured mesial root reveals evidence of extensive periradicular bone loss. The tooth was extracted using a minimally invasive technique, and the socket was grafted with freeze-dried bone allograft (FDBA) saturated with recombinant human platelet-derived growth factor-BB (rhPDGF-BB, 0.3 mg/ml) without flap elevation and protected by a collagen membrane. B, Five months after extraction and ridge preservation the site was reentered surgically to place a dental implant that was allowed to heal for 3 months before restoration. C, Postoperative radiograph reveals implant supported crown restoration. Note the normal trabecular appearance of bone in region of the extracted molar with extensive alveolar bone loss.
Ridge Augmentation
Recent systematic reviews conclude that vertical and lateral ridge augmentation procedures are effective in correcting alveolar ridge deformities to support dental implant placement,18,51 with implant success rates variable but comparable to those placed in native bone.30 Autogenous grafts remain widely considered the gold standard for the correction of severe localized ridge deformities.36 Particulate autogenous grafts are often used in combination with barrier membranes and tenting screws to ensure space maintenance and graft containment.9,10,47 The clinical application of autogenous grafts, however, is often limited by constraints in available donor tissue volume and potential morbidity secondary to graft harvest. Although xenogeneic and allogeneic grafts obviate many of the donor site limitations of autografts, it is widely recognized that bone formation using these grafts is more variable and less robust than achieved following reconstruction with autogenous bone grafts.
Recent clinical interest has focused on the potential for growth factors to enhance the effectiveness of matrices in reconstructing alveolar ridge defects traditionally managed with block or particulate autogenous grafts. Currently, recombinant human bone morphogenetic protein-2 (rhBMP-2) is only marketed in combination with absorbable collagen sponge (Infuse Bone Graft; Medtronic Sofamor Danek, Inc, Memphis, TN) for augmentation of localized alveolar ridge defects associated with extraction sockets. Clinical cases illustrating the application of rhBMP-2 are presented elsewhere in this textbook.
The success of alveolar ridge augmentation procedures appears to depend highly on local vascularity, which is consistent with the pivotal role angiogenesis plays in skeletal development and bone fracture repair.26 The clinical rationale for using rhPDGF-BB in lateral ridge augmentation is to stimulate vascular in-growth and promote regeneration. Alveolar ridge deformities requiring significant vertical and/or horizontal augmentation present the greatest clinical challenge and therefore may benefit most from the use of a growth factor–enhanced matrix. The following case report illustrates the clinical application of rhPDGF-BB in the management of a severe anterior alveolar ridge defect.
A 33-year-old woman presented with localized severe chronic periodontitis involving maxillary central incisors, which had a history of endodontic and prosthetic treatment. (Supplement A Figure 61-6, A to C) The chief complaint was poor esthetics. Given the hopeless long-term prognosis, the central incisors were treatment planned for extraction. Severe vertical and horizontal bone loss was present after tooth extraction, and a combination approach was selected to reconstruct the ridge. Full-thickness mucoperiosteal flaps were elevated to expose and debride the ridge defect. A titanium tenting screw was next placed, approximating the desired level of vertical bone height (Supplement A Figure 61-6, D to E). Next, the ridge defect was grafted using particulate freeze-dried bone which had been wetted for 10 minutes with rhPDGF-BB (0.3 mg/ml). A small, expanded polytetrafluoroethylene (ePTFE) titanium-reinforced membrane (GORE-TEX, W. L. Gore & Associates, Inc, Flagstaff, AZ) was carefully adapted to contain the particulate bone graft. The flaps were mobilized and advanced for primary closure (Supplement A Figure 61-6, F to H).
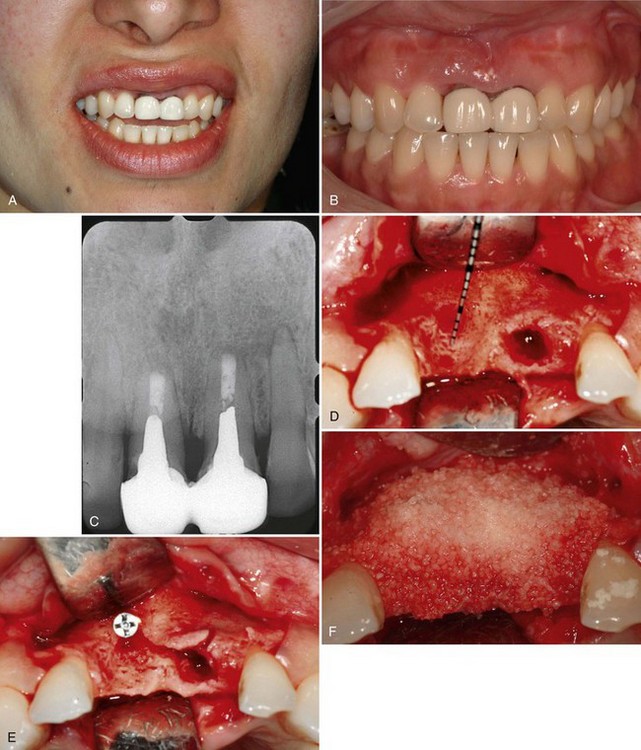
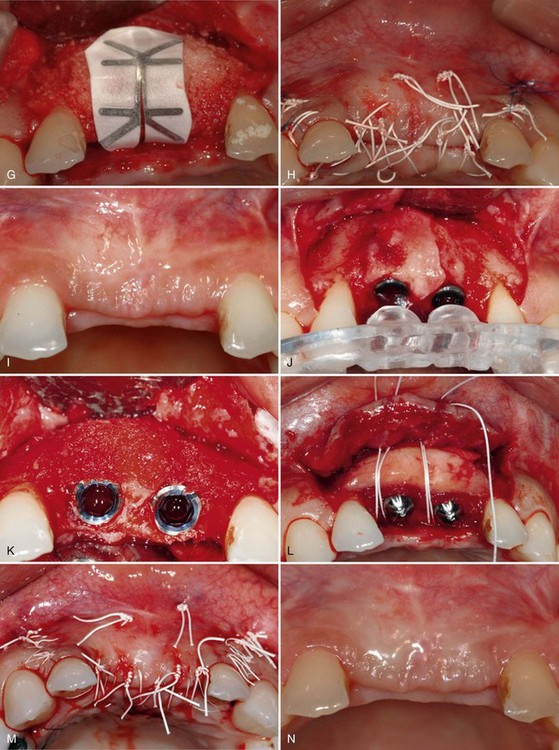
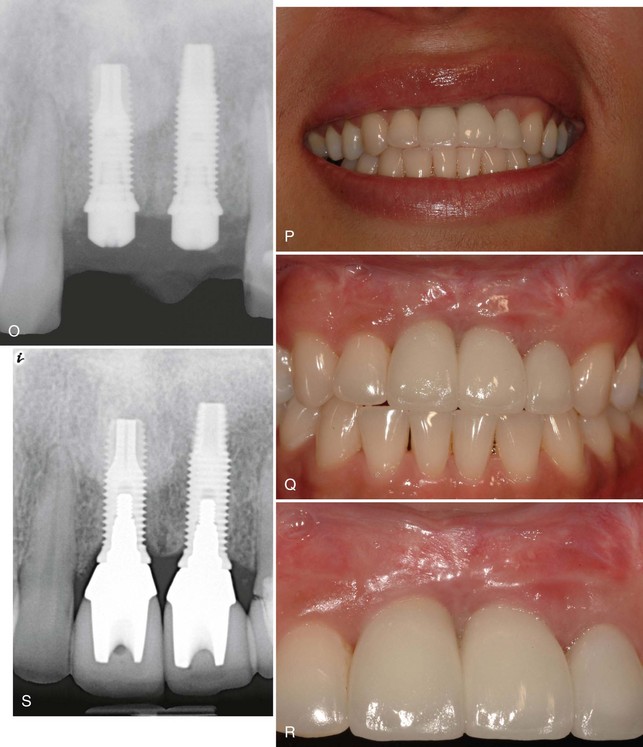
Supplement A Figure 61-6 A to C, A 32-year-old female patient presents with a history of trauma involving the maxillary central incisors, requiring conventional and surgical endodontic therapy. Note the mucosal scar tissue, advanced gingival recession, and bone loss. D, Surgical exposure 5 months postextraction reveals evidence of advanced horizontal alveolar bone loss and residual socket defect. Inadequate horizontal ridge dimension is available to support implant placement. E, Combination therapy was used to correct the ridge deformity with recombinant human platelet-derived growth factor-BB (rhPGDF), particulate bone allograft, and an expanded polytetrafluoroethylene (ePTFE) titanium-reinforced membrane. A titanium tenting screw was placed in site #8 to support ePTFE membrane. A ridge split was performed in site #9 to increase horizontal dimension. F, Particulate freeze-dried bone allograft (FDBA) was hydrated with rhPDGF-BB and grafted to the edentulous site. G, An e-PTFE titanium-reinforced membrane was adapted to facilitate graft containment. H, A combination of horizontal mattress and interrupted PTFE and nylon sutures were used to achieve tension-free primary closure. I, Healing progressed uneventfully with maintenance of flap closure. J, Significant horizontal ridge augmentation was confirmed at time of reentry for implant placement. Surgical guide is shown. K, Particulate demineralized FDBA (DFDBA) saturated with rhPDGF-BB was grafted over the buccal alveolar ridge to further develop ridge contour. L, A connective tissue graft harvested from the palate was placed for soft tissue augmentation. M, Primary closure was achieved after implant placement and grafting using PFTE sutures. N and O, Healing at 5 months demonstrated enhanced ridge form. Radiographic findings are consistent with excellent bone density and osteointegration. P to S, Postoperative clinical and radiographic views demonstrate that the clinical goals of treatment of an esthetic and functional result were achieved.
After 5 months of healing, reentry surgery was performed to evaluate the site for implant therapy. The reentry revealed increased ridge width sufficient for placement of two dental implants to replace the maxillary central incisors. Following implant placement, additional bone grafting of the facial ridge was performed with demineralized freeze-dried bone allograft (DFDBA) and rhPDGF-BB (0.3 mg/ml) to enhance the ridge dimension. Healing abutments were placed for space maintenance,52 and a connective tissue graft was placed to enhance the future soft tissue emergence. The site was allowed to heal for an additional 5 months before completing prosthetic treatment (Supplement A Figure 61-6, I to O). The final implant-supported crowns satisfied the patient’s esthetic demands, and the radiographic and clinical findings were consistent with a successful outcome (Supplement A Figure 61-6, P to S). This case illustrates the successful reconstruction of severe ridge deformity before implant placement using rhPDGF-BB in combination with allogeneic bone, averting the need to harvest autogenous bone from a secondary surgical site.
Sinus Augmentation
Grafting the floor of the maxillary sinus is the most common surgical procedure for increasing alveolar bone height to support placement of dental implants in the posterior maxilla.60 Multiple consensus conferences and systematic reviews conclude that sinus augmentation is a highly predictable technique.1,17,48,59,61 Recent interest in biologics and growth factors has been primarily focused on developing a substitute for bone grafts, particularly autografts, as well as increasing the rate of healing and quality of bone. Infuse bone graft, which combines rhBMP-2 with an absorbable collagen sponge, is also approved as an alternative to autogenous bone graft for sinus augmentation (Infuse Bone Graft, Medtronic Sofamor Danek, Inc, Memphis, TN) The effectiveness and safety of rhBMP-2 for sinus augmentation has been well documented in prospective studies.7,8
The clinical objective for combining rhPDGF-BB with a bone replacement graft in sinus augmentation is twofold—namely, to increase both the rate of bone formation and the density (or quality) of mineralized bone. The healing of allogeneic, xenogeneic, and composite grafts can take from 6 to 12 months for bone maturation.22,28 Although current and emerging evidence suggests that rhPDGF-BB has the potential to enhance new bone formation, controlled clinical trials remain necessary to establish the effects of this growth factor on regenerative outcome. The next case report illustrates the clinical and radiographic outcome after the use of rhPDGF-BB in combination with allogeneic bone for sinus augmentation.
A 59-year-old man presented with an edentulous right posterior maxilla seeking implant supported prosthetic treatment. The preoperative CT scan revealed insufficient bone volume caused by pneumatization of the maxillary sinus and crestal bone resorption (Supplement A Figure 61-7, A). Using a lateral window approach, the sinus was grafted with FDBA and DFDBA wetted with rhPDGF-BB (0.3 mg/ml). Following a 5-month healing period, a bone core was taken at the time of implant placement (Supplement A Figure 61-7, B). Histologic evaluation demonstrated significant vital bone and remodeling of the graft material, consistent with enhanced wound healing. Similar observations of more rapid graft remodeling and bone formation were made in a recent study evaluating rhPDGF-BB in combination with bovine-derived anorganic bone matrix for sinus augmentation.42
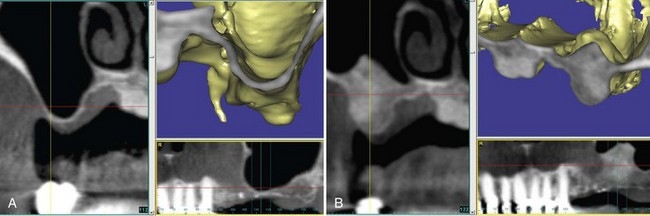
Supplement A Figure 61-7 A, Cone-beam computed tomography (CBCT) image reveals a maxillary sinus with extensive pneumatization and minimal bone at the floor. Alveolar bone deficiency prevents implant placement. B, Postoperative CT image of sinus 5 months after grafting with freeze-dried bone allograft (FDBA), demineralized FDBA (DFDBA), and recombinant human platelet-derived growth factor-BB (rhPDGF-BB). Radiographic evidence of significant bone augmentation bone was found, enabling implant placement.
Summary
The potent effects of rhPDGF-BB on wound healing may expand opportunities to provide implant therapy, as well as manage and improve implant survival and esthetic outcomes. The clinical goals of growth factor–enhanced therapy include less invasive surgical procedures with more robust and predictable treatment outcomes. Anatomic constraints limit the volume of available autogenous bone, and donor sites are often associated with significant postoperative morbidity and complications, regardless of whether the location is intraoral (e.g., ramus, chin, tuberosity, nasal septum) or extraoral (e.g., iliac crest, tibia, calvaria).15,27,43,49,62 In addition, the need for a second surgical procedure is often a deterrent to patients proceeding with implant therapy. The ability to achieve optimal and predictable implant site development without the use autogenous grafts therefore offers great advantage to clinician and patient.
The introduction of recombinant growth factors has increased options for combinatorial approaches to reconstructive surgery. Graft matrices that are space maintaining and osteoconductive aid in preventing soft tissue collapse and provide a scaffold for cellular migration and stabilization of the blood clot. Graft matrices, such as β-TCP and bone allograft, can also serve as delivery devices for drugs and biologics. The controlled delivery of growth factors may enable the successful application of minimally invasive protocols to maintain alveolar bone morphology and preserve soft tissue contours. Growth factors may also enable hard and soft tissue augmentation protocols to be accomplished in a surgical setting, thereby reducing the number of reconstructive surgical visits.
1 Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22(Suppl):49.
2 Amler MH, Johnson PL, Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960;61:32.
3 Atwood DA. Reduction of residual ridges: a major oral disease entity. J Prosthet Dent. 1971;26:266.
4 Atwood DA, Coy WA. Clinical, cephalometric, and densitometric study of reduction of residual ridges. J Prosthet Dent. 1971;26:280.
5 Becker W, Lynch SE, Lekholm U, et al. A comparison of ePTFE membranes alone or in combination with platelet-derived growth factors and insulin-like growth factor-I or demineralized freeze-dried bone in promoting bone formation around immediate extraction socket implants. J Periodontol. 1992;63:929.
6 Boyne PJ. Osseous repair of the postextraction alveolus in man. Oral Surg Oral Med Oral Pathol. 1966;21:805.
7 Boyne PJ, Marx RE, Nevins M, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997;17:11.
8 Boyne PJ, Lilly LC, Marx RE, et al. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005;63:1693.
9 Buser D, Dula K, Hirt HP, et al. Lateral ridge augmentation using autografts and barrier membranes: a clinical study with 40 partially edentulous patients. J Oral Maxillofac Surg. 1996;54:420.
10 Buser D, Dula K, Hess D, et al. Localized ridge augmentation with autografts and barrier membranes. Periodontol 2000. 1999;19:151.
11 Byun HY, Wang HL. Sandwich bone augmentation using recombinant human platelet-derived growth factor and beta-tricalcium phosphate alloplast: case report. Int J Periodontics Restorative Dent. 2008;28:83.
12 Camelo M, Nevins ML, Schenk RK, et al. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23:213.
13 Carlsson GE, Bergman B, Hedegard B. Changes in contour of the maxillary alveolar process under immediate dentures. A longitudinal clinical and x-ray cephalometric study covering 5 years. Acta Odontol Scand. 1967;25:45.
14 Carlsson GE, Persson G. Morphologic changes of the mandible after extraction and wearing of dentures. A longitudinal, clinical, and x-ray cephalometric study covering 5 years. Odontol Revy. 1967;18:27.
15 Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003;5:154.
16 Cooke JW, Sarment DP, Whitesman LA, et al. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12:1441.
17 Del Fabbro M, Testori T, Francetti L, et al. Systematic review of survival rates for implants placed in the grafted maxillary sinus. Int J Periodontics Restorative Dent. 2004;24:565.
18 Donos N, Mardas N, Chadha V. Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J Clin Periodontol. 2008;35:173.
19 Eingartner C, Coerper S, Fritz J, et al. Growth factors in distraction osteogenesis. Immuno-histological pattern of TGF-beta1 and IGF-I in human callus induced by distraction osteogenesis. Int Orthop. 1999;23:253.
20 Fagan MC, Miller RE, Lynch SE, et al. Simultaneous augmentation of hard and soft tissues for implant site preparation using recombinant human platelet-derived growth factor: a human case report. Int J Periodontics Restorative Dent. 2008;28:37.
21 Fiorellini JP, Howell TH, Cochran D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76:605.
22 Hanisch O, Lozada JL, Holmes RE, et al. Maxillary sinus augmentation prior to placement of endosseous implants: A histomorphometric analysis. Int J Oral Maxillofac Implants. 1999;14:329.
23 Hollinger JO, Hart CE, Hirsch SN, et al. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90(Suppl 1):48.
24 Homsi J, Daud AI. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control. 2007;14:285.
25 Howell TH, Fiorellini JP, Paquette DW, et al. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68:1186.
26 Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100.
27 Kurz LT, Garfin SR, Booth REJr. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324.
28 Landi L, Pretel RWJr, Hakimi NM, et al. Maxillary sinus floor elevation using a combination of DFDBA and bovine-derived porous hydroxyapatite: a preliminary histologic and histomorphometric report. Int J Periodontics Restorative Dent. 2000;20:574.
29 Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920.
30 Levin L, Nitzan D, Schwartz-Arad D. Success of dental implants placed in intraoral block bone grafts. J Periodontol. 2007;78:18.
31 Lind M, Deleuran B, Thestrup-Pedersen K, et al. Chemotaxis of human osteoblasts. Effects of osteotropic growth factors. APMIS. 1995;103:140.
32 Lind M, Deleuran B, Yssel H, et al. IL-4 and IL-13, but not IL-10, are chemotactic factors for human osteoblasts. Cytokine. 1995;7:78.
33 Lynch SE, Buser D, Hernandez RA, et al. Effects of the platelet-derived growth factor/insulin-like growth factor-I combination on bone regeneration around titanium dental implants. Results of a pilot study in beagle dogs. J Periodontol. 1991;62:710.
34 Lynch SE, Wisner-Lynch L, Nevins M, et al. A new era in periodontal and periimplant regeneration: use of growth-factor enhanced matrices incorporating rhPDGF. Compend Contin Educ Dent. 2006;27:672.
35 Matsuda N, Lin WL, Kumar NM, et al. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J Periodontol. 1992;63:515.
36 McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78:377.
37 Mellonig JT, Valderrama MP, Cochran DL. Histological and Clinical Evaluation of Recombinant Human Platelet-Derived Growth Factor Combined with Beta Tricalcium Phosphate for the Treatment of Human Class III Furcation Defects. Int J Periodontics Restorative Dent. 2009;29:169.
38 Mustoe TA, Pierce GF, Thomason A, et al. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237:1333.
39 Nevins M, Camelo M, Nevins ML, et al. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74:1282.
40 Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205.
41 Nevins M, Hanratty J, Lynch SE. Clinical results using recombinant human platelet-derived growth factor and mineralized freeze-dried bone allograft in periodontal defects. Int J Periodontics Restorative Dent. 2007;27:421.
42 Nevins ML, Camelo M, Schupbach P, et al. Human histologic evaluation of mineralized collagen bone substitute and recombinant platelet-derived growth factor-BB to create bone for implant placement in extraction socket defects at 4 and 6 months: a case series. Int J Periodontics Restorative Dent. 2009;29:129.
43 Nkenke E, Schultze-Mosgau S, Radespiel-Troger M, et al. Morbidity of harvesting of chin grafts: a prospective study. Clin Oral Implants Res. 2001;12:495.
44 Noda M, Camilliere JJ. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989;124:2991.
45 Oates TW, Rouse CA, Cochran DL. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J Periodontol. 1993;64:142.
46 Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967;17:21.
47 Pikos MA. Mandibular block autografts for alveolar ridge augmentation. Atlas Oral Maxillofac Surg Clin North Am. 2005;13:91.
48 Pjetursson BE, Tan WC, Zwahlen M, et al. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J Clin Periodontol. 2008;35:216.
49 Rawashdeh MA. Morbidity of iliac crest donor site following open bone harvesting in cleft lip and palate patients. Int J Oral Maxillofac Surg. 2008;37:223.
50 Ridgway HK, Mellonig JT, Cochran DL. Human histologic and clinical evaluation of recombinant human platelet-derived growth factor and beta-tricalcium phosphate for the treatment of periodontal intraosseous defects. Int J Periodontics Restorative Dent. 2008;28:171.
51 Rocchietta I, Fontana F, Simion M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: a systematic review. J Clin Periodontol. 2008;35:203.
52 Salama H, Garber DA, Salama MA, et al. Fifty years of interdisciplinary site development: lessons and guidelines from periodontal prosthesis. J Esthet Dent. 1998;10:149.
53 Sarment DP, Cooke JW, Miller SE, et al. Effect of rhPDGF-BB on bone turnover during periodontal repair. J Clin Periodontol. 2006;33:135.
54 Schropp L, Wenzel A, Kostopoulos L, et al. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23:313.
55 Simion M, Rocchietta I, Kim D, et al. Vertical ridge augmentation by means of deproteinized bovine bone block and recombinant human platelet-derived growth factor-BB: a histologic study in a dog model. Int J Periodontics Restorative Dent. 2006;26:415.
56 Simion M, Rocchietta I, Dellavia C. Three-dimensional ridge augmentation with xenograft and recombinant human platelet-derived growth factor-BB in humans: report of two cases. Int J Periodontics Restorative Dent. 2007;27:109.
57 Simion M, Rocchietta I, Monforte M, et al. Three-dimensional alveolar bone reconstruction with a combination of recombinant human platelet-derived growth factor BB and guided bone regeneration: a case report. Int J Periodontics Restorative Dent. 2008;28:239.
58 Stephan EB, Renjen R, Lynch SE, et al. Platelet-derived growth factor enhancement of a mineral-collagen bone substitute. J Periodontol. 2000;71:1887.
59 Tan WC, Lang NP, Zwahlen M, et al. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. Part II: transalveolar technique. J Clin Periodontol. 2008;35:241.
60 Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003;8:328.
61 Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003;8:328.
62 Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192.