CHAPTER 51 SUPPLEMENT A Diagnosis and Management of Endodontic-Periodontic Lesions
Factors Initiating Pulpal and Periradicular Diseases
Pulpal and periradicular diseases are initiated by numerous external factors that may include microorganisms, trauma, excessive heat, restorative procedures, restorative agents, and malocclusion. These insults lead to inflammatory changes in the pulp, starting from a reversible or irreversible pulpitis and ultimately progressing to pulpal necrosis and subsequent breakdown of the periodontium. Dental caries is a prominent cause of pulpal disease, and bacterial infection is the primary form of microbial insult to the pulp. A systematic review of literature from 1966 to 2000 showed causative effects of mutans streptococci and the lactobacilli for human dental caries, whereas others like sanguinis streptococci, S. salivarius, or enterococci could not be associated with the disease.145 Recent understanding in the caries process has adopted the importance of homeostatic balance in biofilm, which describes the microbial ecosystem on tooth surfaces, rather than the virulence of individual bacterial species.141 Regardless, pulpal infection is polymicrobial that often starts from incipient caries causing localized pulpal inflammation, or pulpitis.
Local invasion of the cariogenic bacteria or a shift in the bacterial content of biofilm can lead to inflammatory changes in the dental pulp, which frequently happens in the absence of caries extension into the pulp chamber. Bacterial by-products relevant to pulpitis include lactic acid, ammonia, urea, lipopolysaccharide (LPS), and lipoteichoic acid (LTA). It is notable that the dental pulp is capable of managing the microbial insults because of its extensive intrapulpal lymphatic system. However, an overwhelming pulpal inflammatory response may be induced through various mechanisms by various microbial challenges. LPS and LTA bind toll-like receptors (TLRs), present on the surface of some immune cells in the pulp, and induce the release of inflammatory mediators such as prostaglandins, cytokines, and chemokines.64 In particular, tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-8, IL-12, and chemokines CCL2 and CXCL2 are well described for their role in pulpitis.52 IL-1 is known to be released from macrophages after stimulation with LPS and responsible for bone resorption leading to periradicular periodontitis.59 During acute pulpitis, the inflammatory mediators trigger vasodilation, transient increase of pulpal blood flow (PBF), inflammatory cell infiltration, increased intrapulpal pressure, and finally ischemic necrosis of the pulp (Supplement A Figure 51-1).
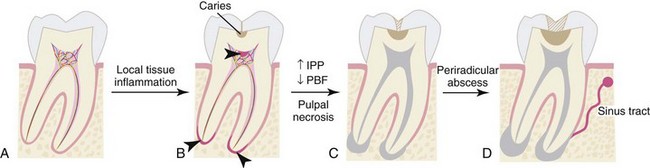
Supplement A Figure 51-1 Progression of the pulpal and periradicular pathosis. A, Normal tooth without any pulpal pathosis is richly vascularized and innervated. B, With microbial challenges, such as caries, local tissue inflammation can occur in the pulp adjacent to the site of carious lesions as well as in the apical regions (arrowheads). C, Pulpal inflammation can lead to reduction in pulpal blood flow (PBF) caused by an increase in intrapulpal pressure (IPP), causing pulpal necrosis (shown in gray). D, Pulpal necrosis, if left untreated, can lead to the chronic inflammation of periradicular tissues and abscess formation, leading to a draining sinus tract.
In an acute periradicular abscess, anaerobic bacteria are predominant over aerobic strains and anaerobes, and microaerophiles were predominant in 82% of the cases studied.72 The most prevalent isolated bacteria are Fusobacterium nucleatum, Parvimonas micra, and Porphyromonas endodontalis.133 Depending on the virulence of the organisms and host resistance, a lesion that has been chronic may exacerbate and become an acute periradicular abscess. The presence of spirochetes is also well documented in periradicular, as well as periodontal abscesses and has been studied by various identification techniques.27 Spirochetes most often isolated in root canal infections are Treponema denticola and Treponema maltophilium.66,123 When comparing chronic and acute periradicular abscesses, Baumgartner found that there was a significantly higher incidence of spirochetes in acute abscesses or cellulitis than in asymptomatic infected root canals. Treponema socranskii was the most frequently encountered species.10
Even in the absence of microbial infection, thermomechanical irritants can induce altered pulpal circulation and pulp tissue damage. Earlier studies demonstrated that thermal changes caused by dental procedures, such as tooth preparation, led to marked diminution of PBF and plasma extravasation, resulting in an inflammatory response.74,120 Extreme heat caused by dry tooth preparation is known to cause tooth “blushing,” representing vascular stasis and hemorrhage in the subodontoblastic vascular plexus.105 It was noted that even a small increase in pulpal temperature (5 to 6° C) is capable of inducing necrotic changes in the pulp.171 Pulpal necrosis will eventually lead to periradicular periodontitis. Likewise, chemical irritants impose measurable changes in the pulp status. A recent study showed cytotoxicity of dental resin material (2-hydroxyethyl methacrylate [HEMA]) for the pulp stromal cells through induction of apoptotic cell death.116 Currently, bonding agents are being considered as pulp capping materials as opposed to calcium hydroxide, but some investigators have raised concerns as to the adverse effects of these agents on the status of the pulp. Etching the dentin surface with high phosphoric acid content yields deleterious effects on dental pulp.129 Also, bonding resins as pulp capping material led to acute pulpitis and varying degrees of necrosis in human teeth.2 Root canal overfills with gutta percha and sealers invariably cause severe inflammatory reactions in the periradicular tissues, even though patients may be completely asymptomatic.122 Thus dental materials often contain chemical irritants that affect the pulpal and periodontal tissues, and one must be cognizant of the potential iatrogenic endodontic pathosis associated with their misuse.
Classification of Pulpal Diseases
The classification of diseases of the dental pulp depends on the extent of pulpal injury and its ability to repair. Many factors influence whether a tooth will be classified as normal, develop a reversible pulpitis, an irreversible pulpitis, or will become totally necrotic.
Previous studies have found little correlation between the histology of pulpal disease and the symptoms that the patient experienced before treatment.84,154 More recently, a review of the diagnosis of pulpal pain by Bender found that 80% of patients who give a previous history of pain manifested histopathologic evidence of chronic partial pulpitis with partial necrosis. This symptomatology would indicate that either endodontic therapy or extraction would be the indicated treatment choices. Bender concluded that a clinician is able to determine the degree of pulp histopathosis by asking the patient about their previous pain history and symptoms related to the involved tooth.12 There still remains controversy, however, as to the degree of correlation between pulpal symptoms and the histopathology of the pulpal tissues and additional studies may help to confirm the correlation between the two.
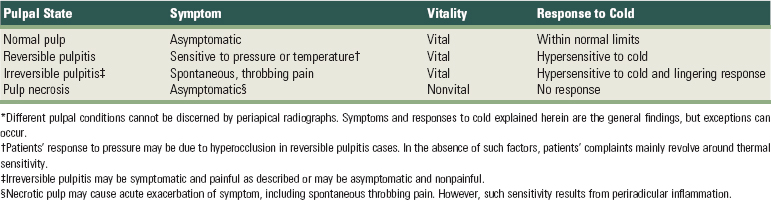
A thorough discussion of the classification of pulpal disease may be found in numerous endodontic textbooks. Additionally, an abbreviated classification of pulpal disease may be found in Supplement A Table 51-1.
Classification of Periradicular Diseases
The American Board of Endodontics has recently revised the classification of periapical diseases by referring to all “periapical” diseases as “periradicular” to indicate that pathogens residing in the root canal system may exit the tooth at numerous sites along the root surface. Bone loss, therefore, may occur along almost any portion of the root.
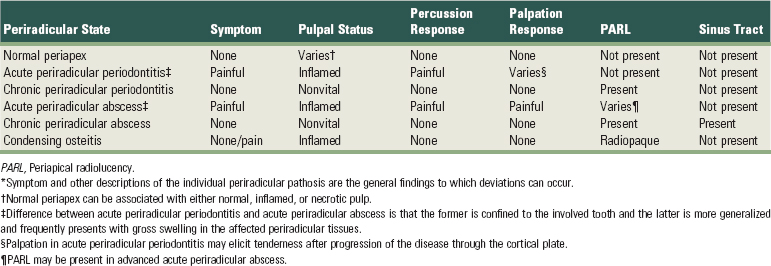
Periradicular diseases of endodontic origin are inflammatory processes occurring in the tissues that surround the tooth. They result from various infectious agents that originate in the root canal system and create a series of both inflammatory and immunologic responses. These agents exit through the apical foramen, lateral canals, or dentinal tubules.76,117 It is an infectious process caused by a large number of microbial species unlike classic infectious diseases occurring elsewhere in the body that may consist of only one or two specific organisms. These species reside in ecologically balanced communities referred to as biofilms.114 The classification of periradicular diseases and the characteristics of each are found in Supplement A Table 51-2.
Anatomic Considerations of the Pulpal and Periodontal Continuum
Persistent infection in the pulp tissue leads to secondary infection and breakdown of tissues in the periodontium. Conversely, severe periodontal disease may initiate or exacerbate inflammatory changes in the pulp tissue. This mutuality of infection between pulp and periodontium is mediated through physical routes, allowing for communication between the two structures. The main and obvious route of communication is the apical foramina. Advanced pulpitis will lead to pulp necrosis, which often is accompanied by inflammatory bone resorption at the root apex, as found in cases of chronic periradicular periodontitis (CPP) or abscess (Supplement A Figure 51-2). This is also known as retrograde periodontitis because it represents the periodontal tissue breakdown from an apical to a cervical direction and is the opposite of orthograde periodontitis that results from a sulcular infection. This is typically identified as periradicular radiolucency or PARL (Supplement A Figure 51-3). By far, the retrograde periodontitis is the most common example of pulpal diseases leading to secondary periodontal breakdown. Apical foramina can also lead to pulpal inflammatory changes secondary to severe periodontitis in cases in which the periodontal defect reaches the apical foramina.
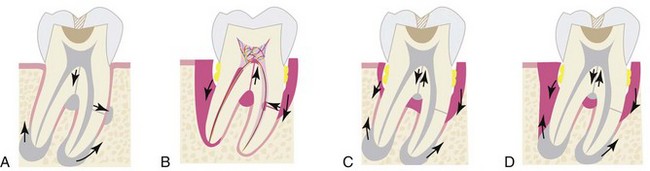
Supplement A Figure 51-2 Classification of endodontic-periodontic lesions. A, Primary pulpal infection can lead to chronic periradicular periodontitis by which a periapical radiolucency (PARL) can develop and migrate cervically. Mandibular molars can also have accessory canals in lateral orientation or in the furcation area. These accessory canals can allow migration of the primary pulpal infection and cause secondary breakdown of the periodontium at their respected loci. B, Primary periodontal infection can lead to extensive breakdown of alveolar crest bone that migrates from the cervical area to the apex. In these lesions, one would find generalized bone loss around a single tooth or often might involve multiple adjacent teeth. Because of the pulpal-periodontal continuum through main root canal foramina or through accessory canals, extensive periodontal infection can cause irritation in the pulp tissues. C, Both primary pulpal and primary periodontal infection can occur simultaneously in an “independent” endodontic-periodontic lesion, exhibiting the characteristics of both. D, Primary pulpal and primary periodontal infections can occur extensively in this “combined” endodontic-periodontic lesion.
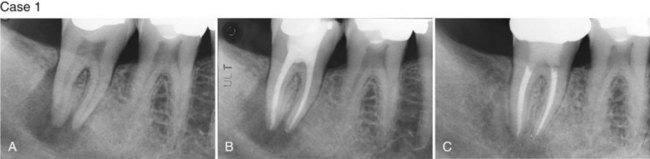
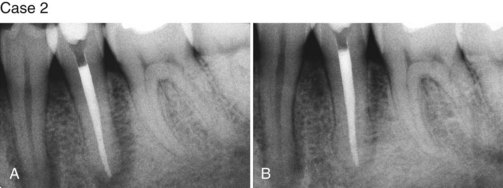
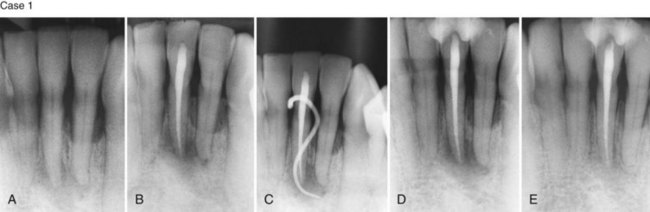
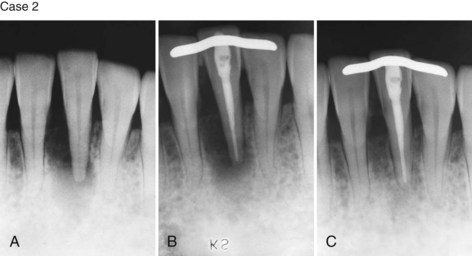
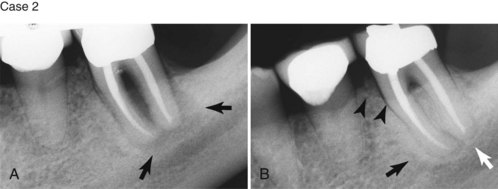
Supplement A Figure 51-3 Retrograde periodontitis. Case 1. A, Large periapical lesion extending around the periapex of tooth #31. No visible fractures were detected on the mesial or distal marginal ridges. The tooth tested nonvital. A sinus tract was visible on the buccal gingiva. B, Endodontic therapy was completed in two visits, and the canals were obturated. C, Healing of the periradicular bone is evident at 6 months, and a crown providing complete coverage has been placed. Case 2. A, Radiographic lesion extends from the periapex of tooth #20 after completion of the endodontic therapy with gutta percha. B, A 6-month recall radiograph reveals complete healing of the bony lesion. There was no vertical fracture present in the tooth.
(Case 1 courtesy Dr. Thomas Rauth.)
Alternatively, lateral or accessory canals may be the route of such communications. Prevalence of accessory root canals in various human teeth and their contribution to the complexity of the root canal system have been well established. Accessory canals are found along the length of the root canals, albeit to varying frequencies depending on their location. Earlier studies, using the “clearing technique” for transparent root canal visualization, showed that 59.5% of maxillary second premolars possess lateral canals; 78.2% of those are found in the apical regions of the root canals.156 Notably, accessory canals were also found in midroot and cervical regions, albeit with reduced frequencies at 16.2% and 4.0%, respectively. A subsequent study showed that 28.4% of permanent molars exhibit patent accessory canals in furcation regions,51 suggesting that these accessory canals allow the pulpal and periodontal continuum to exist. Root canal therapies fail frequently in maxillary molars because of unidentified second mesial canals, which are found in a surprisingly high percentage (80.8%) of teeth.67 Clearly, accessory canals can lead to CPP resulting from chronic pulpal diseases. This can be readily detected in periapical radiographs (Supplement A Figure 51-4), and the periodontal lesions heal after successful completion of endodontic therapy. Questions arise as to whether pulpal disease can develop from periodontal infections through accessory root canals. Kirkham76 reported that out of 100 permanent human teeth extracted as the result of severe periodontal disease, only 2 teeth possessed accessory canals within the periodontal pockets. Thus, the likelihood that primary periodontal infection reaching the dental pulp through accessory canals is rare.
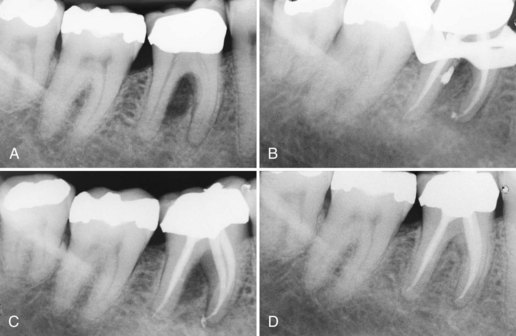
Supplement A Figure 51-4 Lateral canal-led periodontal defect from a primary endodontic infection. A, Bone loss is present in the furcation with sinus tract present on the buccal mucosa. Tooth #30 tested nonvital. B, During condensation, a large amount of sealer was expressed through a large lateral canal in the distal root. C, Sealer was removed after obturation by curettage of the furcation and irrigation with anesthetic solution through the sinus tract. D, Healing at 12 months demonstrates complete repair of periradicular bone.
(Courtesy Dr. Thomas Rauth.)
The third route is dentinal tubules. Dentinal tubules maintain a tapered structure along the length from the pulpodentinal complex (PDC) to the dentinoenamel junction (DEJ) with the diameter of 2.5 µm at PDC and 0.9 µm at the DEJ.149 It is therefore conceivable that dentin is a permeable structure, and that the permeability changes at different locations along the root according to the size and density of the dentinal tubules. Clearly, the tubular diameter is large enough to allow bacterial penetration and colonization inside the dentin tubule. Because of the tapered structure, permeability would increase when the carious lesion extends deeper into the dentin. Bacterial colonization in the tubules from infected root canals has been well documented.132 Also, bacterial invasion into dentinal tubules from the periodontal pocket has been demonstrated,50 suggesting that dentinal tubules may allow pulpal irritation from chronic periodontal infections.
Dentin permeability through dentinal tubules is a clinically important issue. The permeability may be measured through hydraulic conductance described earlier.121 Subsequently, investigators have studied the effects of various agents and stresses on dentin permeability. Root planing, as part of routine periodontal therapy, for example, is shown to decrease dentin permeability as the result of formation of smear layer, which is acid-labile.38 However, dentin permeability may increase on removal of the smear layer, resulting in tubular penetration of oral pathogens and pulpal irritation. Further study is necessary to delineate the role of dentinal tubules in causing the secondary infection in pulpal or periodontal tissues. However, clinicians need to be cognizant of the fact that patent dentinal tubules are effective conduits of irritants between these two otherwise distinct tissues.
Biologic Effects of Pulpal Infection on Periodontal Tissues
The effects of the pulpal disease on the surrounding periodontal tissues is widely accepted by both clinicians and researchers. Pulpal pathosis as a cause of periodontal disease has been studied by numerous researchers for more than 50 years. Early inflammatory changes in the pulp exert very little effect on the periodontium. Even a pulp that is significantly inflamed may have little or no effect on the surrounding periodontal tissues. It is believed that this initial pulpal inflammatory response is an attempt by the body to prevent the spread of infection to the periapical tissues.
When the pulp becomes necrotic, however, it produces a significant inflammatory response. This response can traverse the apical foramen, the furcation, lateral canals, dentinal tubules, or areas of trapped necrotic tissue along the surface of the root that extend past the periodontal ligament (PDL) and into the surrounding periradicular tissues.130 This initial inflammatory response of the pulp and subsequent necrosis that permeates through the periradicular spaces of the canal system includes various bacterial strains, fungi, yeasts, and viruses.107 The nature and extent of the periodontal destruction that follows depends on the virulence of the pathogens in the canal system, the duration of the disease, and the defense mechanisms of the host.20
Bacteria play a critical role in both endodontic and periodontal disease. In a classic study by Kakehashi et al,68 the infected pulps of germ-free rats remained vital, whereas the infected pulps of normal rats that were left open to the oral environment developed pulpal necrosis with subsequent inflammation and formation of periapical lesions. Other investigators reported similar results using other animal models.102
Most bacteria grow in biofilms. The biofilm is composed of a 15% cellular component and a matrix material that comprises the remaining 85%. The formation of biofilm communities is under the control of complex chemical signals that both regulate and guide the formation of the slime-enclosed colonies and water channels.24 Proteolytic bacteria predominate in early root canal infections and then change over time to a flora that contains a greater number of anerobes.34,138 Fungi and yeasts are also present in pulpal infections.158 Studies by various investigators report that the incidence of cultured samples of fungi and yeasts from untreated root canals vary from 0.5% to 26%,125 whereas the percentage in teeth that have been previously treated showed an increase of these organisms. Candida albicans was the most prevalent isolated species.159
Current evidence suggests the important role that viruses may play in the pathogenesis of both periodontal and endodontic disease. Viruses have been isolated from both patients with periodontal disease and from the dental pulp.22,45 A study by Contreras et al23 demonstrated that gingival herpes viruses were associated with the increased growth of pathogenic periodontal bacteria. Even with the numerous studies that have been published, additional research is needed to demonstrate the causal relationship between viral infections in both periodontal and pulpal disease.125
Several nonliving pathogens have also been implicated in the inflammatory process as well. These include foreign bodies, epithelial rests, cholesterol crystals, Russell bodies, Rushton hyaline bodies, and Charcot-Leyden crystals. These nonliving pathogens have not only been implicated in the inflammatory process but may also be responsible for the lack of healing of periradicular lesions in teeth that have received appropriate endodontic treatment.125 If the growth of the epithelial cells is stimulated by any of these living or nonliving pathogens, then the integrity of the periodontal tissues may be affected as well.
As the degree of pulpal inflammation becomes more extensive, a greater amount of destruction of the periodontal tissues ensues. Extension of the infection through the PDL space, tooth socket, and surrounding bone occurs, and the patient begins to experience a localized or diffuse swelling that may result in cellulitis that invades the various facial spaces. Most often, however, the infection erupts through the labial, buccal, or lingual mucosa and results in a draining sinus tract. In cases in which the path of least resistance for the infectious process is along the attached gingiva, the infection may dissect the PDL space and result in the formation of a deep but narrow periodontal pocket. This pocket usually extends to the main site of the infection when probed or traced with a gutta percha point. Some investigators have referred to this as a retrograde periodontitis to differentiate it from marginal periodontitis in which the spread of the disease originates in the periodontium and extends from the crestal gingival tissues to the apex of the root. Confusion often results among both general dentists and specialists as to whether the probable defect is the result of an endodontic or periodontal problem. A narrow probing defect combined with a nonvital pulpal response indicates that the problem is usually endodontic rather than periodontal.
In a few situations, adjacent teeth, their root surfaces, or furcation areas may also probe deeply. Care must be taken to thoroughly test all maxillary and mandibular teeth to correctly assess whether the problem is endodontic or periodontal. Once the correct diagnosis is made, only then should the treatment plan be formulated and discussed with the patient. When endodontic therapy is required and is the main cause of the swelling or breakdown of the periodontium, successful endodontic treatment usually results in healing of both the periapical and periodontal tissues. There are times, however, when trauma to the tooth, severe loss of adjacent periodontal tissues, continued tooth mobility, and occlusal trauma do not provide an environment that allows for periradicular healing to occur. In these cases, splinting is sometimes necessary to help stabilize the tooth and allow for potential repair of the periradicular tissues (Supplement A Figure 51-5).
Supplement A Figure 51-5 Case 1. A, Previous trauma of tooth #25 with complaints of pain to biting and chewing. The tooth tested nonvital and tooth #26 probed 6 mm on the lingual. B, Postoperative radiograph after obturation of the canal. Treatment was completed in two appointments with the interappointment placement of calcium hydroxide. C, 4 months later the tooth was mobile, and there was a sinus tract present. D, The occlusion was adjusted, and composite resin was bonded to the mesial and distal surfaces to stabilize both tooth #25 and 26. E, Healing of the periradicular lesion is apparent after 13 months and tooth #26 probed only 4 mm. Case 2. A, Previously traumatized tooth #25. The tooth was Class III mobile and tested nonvital to both CO2 and electric pulp testing. B, After obturation of the tooth with gutta percha, a cast gold splint was bonded to the lingual surface to stabilize the tooth. C, 13-month recall demonstrates repair of the periradicular bone and no mobility as a result of the placement of stabilization and splint.
(Case 1 Courtesy Dr. Thomas Rauth.)
When the endodontic infection is left untreated, it becomes one of the risk factors for the progression of periodontal disease. Untreated and unresolved infections of endodontic origin can sustain the growth of various endodontic pathogens that may lead to additional periodontal pocket formation, increased bone loss, calculus deposition, osteoclastic activity, and subsequent bone and tooth resorption. They may additionally impair wound healing and aggravate the development and progression of the periodontal disease state.31
The ability of the periodontium to regenerate and heal the lost attachment apparatus has been controversial. This is especially true when the teeth have been endodontically treated and the cement layer is no longer present.71 A study by Sanders et al127 demonstrated a 60% osseous regeneration rate in teeth that had not undergone endodontic treatment compared with a regeneration rate of only 33% in teeth that had endodontic treatment completed. Another study that compared the loss of attached gingival tissue found that there was a 0.2 mm greater loss of attached tissue in the presence of teeth with a root canal infection and a periapical radiolucency present.62 These same investigators in a later study found a three times greater loss of marginal proximal bone utilizing radiographic measurements in teeth with endodontic infections compared to those without endodontic infections or subsiding endodontic involvement.61 Other investigators, however, have reported that all periodontal tissues have the ability to regenerate, regardless of whether the tooth is vital, partially treated and medicated, partially filled, or endodontic treatment has been successfully completed.30 Additional research needs to be completed to better understand the relationship between the presence of endodontic infection and the increased loss of marginal bone and attached tissue in patients prone to periodontal disease.
It is clearly apparent that the endodontium and periodontium are closely related and that microorganisms and nonliving pathogens play an important role in both infections. Diseases in one area can lead to a secondary disease in the other. Diagnosis therefore is critically important and will dictate the appropriate course of treatment.
Biologic Effects of Periodontal Infection on the Dental Pulp
The effects of periodontal disease on the dental pulp appear to be more controversial compared to the effects of pulpal disease on the periodontium.11,131 Not all researchers agree about the effect of periodontal disease on the pulp. Even though inflammation and localized pulpal necrosis have been observed next to lateral canals exposed by periodontal disease,126,130,131 other research studies have not confirmed a correlation between periodontal disease and changes within the pulp.25,97,148 Langeland et al84 indicated that when pathologic changes do occur in the pulp of a tooth as a result of advanced periodontal disease, the pulp does not usually undergo degenerative changes as long as the main canal has not been involved. If the vasculature of the pulp remains vital, no inflammatory reaction occurs and there are no symptoms of pulpal pathosis. One animal study conducted by Bergenholtz14 found that 70% of animal specimens showed no pathologic changes even when 30% to 40% of the periodontal attachment had been lost. The remainder showed only minor inflammatory changes, formation of reparative dentin, or resorptive defects where the root had been exposed.14
Researchers and clinicians, however, have observed the spread of advanced periodontal lesions that extend to the apical foramen and result in pulpal necrosis. This retrograde infection may proliferate through large accessory canals on the periradicular surfaces of the tooth, canals positioned closer to the apical foramen, and the area in which the main canal exits the tooth apex.126 Kobayashi et al81 compared the microflora from root canals and periodontal pockets of caries-free teeth that were necrotic and tested nonvital with an electric pulp tester. The aerobic/anaerobic ratio in the periodontal pocket was 0.23 compared to 0.0022 in the root canal. Although there were far fewer bacteria in the root canal, both areas demonstrated similar bacterial strains. The authors concluded that the similarity of strains in both areas suggested that the periodontal pocket may be the source of bacteria found in infections within the root canal system.79
Protection and preservation of the cementum and dentin surrounding the tooth also play important roles in preserving the health of the pulp and preventing the ingress of periodontal pathogens. The presence of an intact layer of cementum is important in protecting the pulp from the plaque and other periodontal pathogens that migrate along the root surface during the development of advanced periodontal disease. Excessive root planing and curettage that remove the cementum and dentin of the root surface, encourages narrowing of the pulp canals. This process is thought to be reparative rather than inflammatory.11,85 Several studies also suggest that periodontal disease is degenerative to pulpal tissues resulting in continued calcification, fibrosis, collagen resorption and inflammation.84,94 Dentin thickness also contributes to the protection of the pulp. Stanley137 stated that if a 2-mm thickness of dentin remains between the pulp and irritating stimulus, there is little chance of pulpal damage.137 Weine164 summarized the precautions that can be taken during the course of periodontal therapy as (1) avoid using irritating chemicals on the root surface, (2) minimize the use of ultrasonic scalers when there is less than 2 mm of remaining dentin, and (3) allow minor pulpal irritations to subside before completing additional procedures.164 When these precautions are not followed and the microvasculature of the pulp is damaged during periodontal procedures that involve deep curettage or periodontal surgical efforts to save the tooth, necrosis may result.165
The healing success and failure rates after endodontic microsurgery were studied in teeth that had lesions of only endodontic origin compared to those teeth that had lesions of a combined endodontic-periodontal origin. Teeth that required periradicular surgery were chosen. Those teeth that had lesions only of endodontic origin had a success outcome of 95.2%, whereas those teeth that had combined lesions demonstrated a successful outcome of only 77.5%. This suggests that bone and tissue healing are negatively affected after endodontic surgery when the lesions are of combined origin.73
With all the available research, it appears that both the pulp and periodontal compartments influence the other. Periodontal disease, however, seems to have less of an influence on the pulpal tissues compared to the influence of pulpal disease on the periodontium. Clearly, advanced periodontal disease has some effect on the pulpal state (Supplement A Figure 51-6). Unless the microvasculature of the pulp is compromised during aggressive periodontal procedures, most periodontal interventions result in only a localized pulpal response and dentin hypersensitivity.155
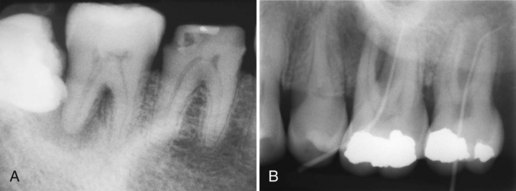
Supplement A Figure 51-6 Primary periodontal defects causing periradicular bony lesions and pulpal irritation. A, Primary periodontal lesion is evident on the distal of tooth #31. The defect was probed to a depth of 7 mm, and the tooth tested vital to both thermal and electrical pulp testing. The defect was most likely the result of the impacted third molar and formation of a chronic periodontal abscess. B, Primary periodontal lesions both probing 12 mm into the furcation. Teeth #14 and 15 tested vital to thermal and electrical testing. The patient’s chief complaint was discomfort to cold, thus exemplifying pulpitis secondary to the primary periodontal infection.
(B Courtesy Dr. Gregory Kolber.)
Differential Diagnosis of Pulpal and Periodontal Infection
Acute infection of both the periodontium and the pulp must be differentiated from one another for clinicians to be able to establish a correct diagnosis with reasonable certainty and to initiate appropriate therapy. A thorough understanding of both disease processes and the correct interpretation of clinical and radiographic findings will aid the dentist in establishing a diagnosis that results in the rapid relief of the acute and painful condition.
When the pulpal and periodontal abscesses are separate from one another, most clinicians feel that the diagnosis is usually easier. There are instances, however, when each primary disease may have similar clinical characteristics and make the diagnosis increasingly difficult. In other situations, there may be no demarcation between the two areas of pathosis that both clinically and radiographically appear as one large and continuous lesion with extreme pain and swelling. When this occurs, the clinician must avoid classifying these continuous lesions as true combined lesions. One must rely on all available clinical testing methods to help clarify the correct clinical diagnosis prior to beginning treatment.71
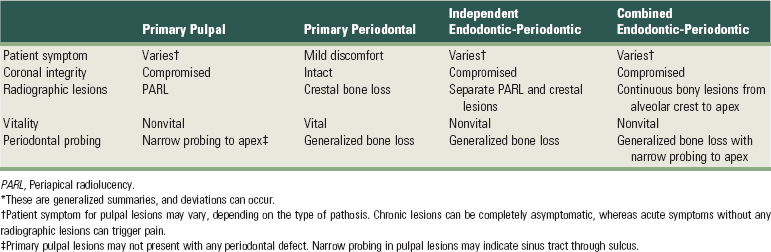
Making the accurate differentiation between pulpal and periodontal lesions can be challenging. If the lesion originates from a pulpal infection and yet was treated by extensive periodontal therapy, it will not resolve. Conversely, performing endodontic therapy on a tooth that has an extensive periodontal defect and a vital pulp will result in the persistence of the periodontal infection. Thus, identifying the primary cause of infection is a critical determinant of treatment outcome. Perhaps the most important consideration when making such a distinction is to base the diagnosis on multiple findings. Those include patients’ symptoms, coronal integrity, shape and size of the radiographic lesions, periodontal probing, and tooth vitality. It is possible that one or more of these findings suggest a pulpal or periodontal infection, whereas others point to the contrary. For instance, a tooth may exhibit extensive failing restorations, recurring decay, and radiographic lesions, suggesting probable pulpal involvement. However, the tooth may test completely vital and lack any evidence of an irreversible pulpitis upon thermal testing. Under these circumstances, one would rule out the primary pulpal infection and examine the patient for periodontal involvement. Thus, making the distinction between pulpal or periodontal infection requires collectively dissecting the multiple findings and synthesizing the most probable diagnosis (Supplement A Table 51-3).
Patients’ Subjective Symptoms
Patients suffering from the acute phase of pulpal infection presents with the symptoms that are generally absent in chronic periodontal infection. During the initial stage of pulpitis, patients may complain of sensitivity and pain exacerbated by certain stimuli, including temperature changes, pressure, and/or biting. If the symptoms result from a reversible pulpitis, they will usually resolve spontaneously with time as the result of various mechanisms such as closure of dentinal tubules, clearance of microbial irritants and toxins, and reparative dentin formation. Persistent inflammation leads to irreversible pulpitis, which is often associated with sharp and untriggered pain, although an asymptomatic irreversible pulpitis may also occur as described previously. The acute pain to thermal stimuli may subside after several days as the pulp becomes necrotic, and the bacteria and their by-products migrate down the complex canal system. As the infection extends to and then past the apical foramen or a lateral periradicular canal, the tooth becomes particularly sensitive to bite pressure and percussion. After several days, the necrotic tooth may develop either a periradicular abscess which results in elevation of the tooth from the dentoalveolar complex. Patients frequently report that the tooth feels “high” on occlusion. However, patients with irreversible pulpitis or chronic pulpal infection (i.e., necrosis) may be completely asymptomatic. Thus, the diagnosis of primary pulpal infection must be made with objective findings, such as the patients’ responses to percussion, palpation, biting, periodontal probing, and vitality testing as well as thorough evaluation of the patients’ subjective symptoms. Use of transillumination is also extremely important to rule out the possibility of root fracture.
In periradicular and periodontal abscesses, the extent of pain may vary. Generally, an acute periradicular abscess causes extreme pain to pressure, bite, percussion and, at times, to palpation if the infection has penetrated the cortical bony plate. Periodontal abscesses are thought to cause less pain because there is little or no elevation of the periosteum. Edema and swelling are characteristics that may be shared by both conditions. The swelling and edema with a periodontal abscess is generally confined to the cervical portion of the tooth. A periradicular abscess is usually more sensitive to palpation around the tooth apex if the infection has penetrated through the bony cortical plate. Redness and a smooth appearance of the marginal gingival tissues is more common with abscesses of periodontal origin, whereas redness can be detected more apically if a pulpal abscess has started to swell and elevate surrounding tissues. Objective findings in periodontal abscesses include bleeding on probing, suppuration, increased pocket depth, increased tooth mobility, and occasionally, lymphadenopathy.57 Abscesses of endodontic origin usually probe normally but may also display increased mobility, depending on the amount of bone loss. Patients may describe the tooth as feeling longer or higher than the adjacent teeth.48
Suppuration and drainage from periradicular and periodontal abscesses may also differ. Periodontal abscesses are associated with severe periodontal destruction. In a study investigating the incidence of anaerobes in periodontal abscesses, approximately 66% were determined to have a suppurative exudate that was evident during probing. In the same study, 100% of the patients had bleeding during probing, more than 75% had severe edema, redness and swelling, and 78% of the patients had some degree of mobility. Only a few of the patients suffered from lymphadenopathy. Over 60% of the patients had not received previous periodontal therapy and nearly 70% of the teeth were molars. The average measured pocket depth was 7.28 mm.57 In another study that cultured facultative anaerobic bacteria, the teeth most affected were maxillary and mandibular anteriors and mandibular molars. One of the typical features in this study, as in the previous one, was the fact that most of the teeth had been previously untreated.96 Other aspects of periodontal therapy that correlate highly with the development of acute periodontal abscess formation are (1) patients undergoing current periodontal treatment,135 (2) the incomplete removal of calculus,29 (3) a history of a previous abscess at the same site,99 and (4) the previous use of antibiotics for dental or nondental reasons.56 Drainage from periradicular abscesses usually comes from one of two sites. The most prevalent area of drainage is a sinus tract that develops when the area of swelling breaks through the mucoperiosteum and exits the mucosal tissue either near or at some distance from the site of the infection. Additionally, the path of least resistance may be along the PDL, and the infection may dissect the ligament along the surface of the root and exit the tooth at the height of the epithelial attachment. This results in a periodontal defect that probes along a narrow path to the apex of the root as previously discussed. Both the sinus tract and narrow sulcular lesions can usually be traced to the infected tooth or offending root using a gutta percha point.
Coronal Integrity
Periodontal infection without pulpal involvement may present with intact crown structure and absence of coronal defects (see Supplement A Figure 51-6, A). On the other hand, endodontic infection is almost always associated with loss of coronal integrity such as occurs with caries, failing restorations, extensive restorations and the existence of cracks or fractures that extend to the pulpal tissues (see Supplement A Figures 51-3 and 51-4). However, this does not mean that all periodontal infections are devoid of coronal defects nor that all endodontic lesions exhibit loss of coronal integrity. When pulp tissue is severed by trauma, for example, one might expect to find a necrotic pulp in the absence of coronal defects. If left untreated, such lesions originating from primary pulpal infection lead to the breakdown of the periodontium as in CPP or CPA. Of course, primary periodontal lesions can develop in teeth with coronal defects. Furthermore, true combined lesions (so-called “endo-perio” lesions) would present with a periodontal infection and with extensive coronal destruction. However, careful examination of the coronal status either during intraoral or radiographic examination can provide supportive information in deciding whether the lesion originates from an endodontic or periodontal infection.
Radiographic Appearance
Periapical radiographs can provide distinguishing information whether the lesion is of pulpal or periodontal origin. Although radiographic findings are objective data, interpretation of radiographs can be highly subjective, depending on who is reading the radiograph. Thus, it is important to understand to focus on specific entities on a radiograph, including the coronal status, crestal bone height and shape, presence of periapical or lateral radiolucency, bony trabeculation, integrity of lamina dura, and careful evaluation of the obturation of the root canal if present. Coronal status as revealed on a radiograph can also help in the differential diagnosis as described previously. Also, periradicular lesions originating from primary pulpal infection lead to a retrograde periodontitis that migrates from the root apex in a cervical direction (see Supplement A Figure 51-3). On the contrary, periodontal infections will lead to the loss of crestal bone from the cervical area of the tooth in an apical direction (Supplement A Figure 51-7). Thus, radiographic lesions appear different for endodontically treated teeth compared to periodontal lesions, and the shape of the bony lesions can help distinguish between the two. For example, radiographic lesions representing orthograde severe periodontitis will appear wider at the cervical end than the apical portion of the lesion. Periapical or lateral radiolucencies may also result from differences in trabeculation patterns not associated with pulpal infection. For this reason, it is critical to consider the integrity of the lamina dura, which almost always is violated in the periapical or lateral radiolucency, representing a chronic or acute pulpal infection. If the tooth had been endodontically treated, assessment of the previous obturation qualities (i.e., voids, short fills or overfills, missed canals, etc.) is also important. These radiographic features will provide very useful and often distinguishing information in making the differential diagnosis between pulpal and periodontal lesions more accurate.
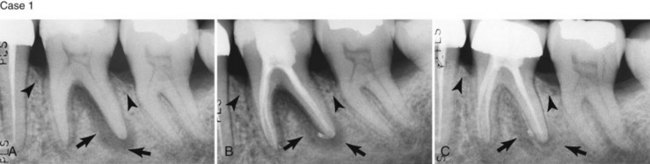
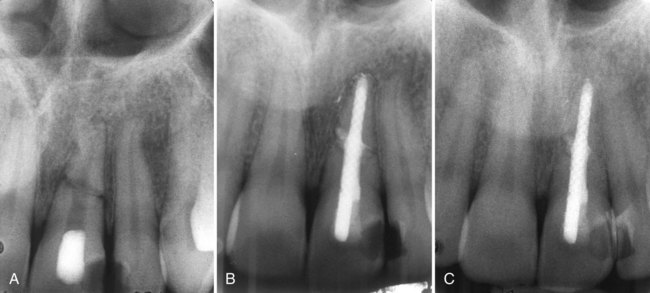
Supplement A Figure 51-7 Case 1. “Independent” endodontic-periodontic lesion. A, “Truly separate” endodontic-periodontic lesions. Vertical periodontal bone loss can be seen at the mesial surface of both teeth #18 and 19. A large endodontic lesion extends around the apex and into the bifurcation. An apical and furcal defect is evident radiographically. B, Final condensation film 2 weeks after initiating treatment. C, Nearly complete healing of the endodontic lesion at 6 months and restoration of the tooth with a PFM crown. The areas of vertical bone loss from the periodontal lesions are still present. Case 2. “Combined” endodontic-periodontic lesion. A, Nonvital tooth with a narrow 9-mm probing defect in the bifurcation of tooth #19 and a large periapical lesion. Normal probing depths were found on the remaining tooth surfaces. B, 9-month healing demonstrates significant bone repair in the furcation and periapical areas. A small amount of bone loss in the furcation still remains because of a persistent periodontal defect.
(Case 1 Courtesy Dr. Thomas Rauth.)
Vitality
The testing of tooth vitality often becomes one of the most important tests that can differentiate a periodontal and a periradicular infection. Teeth with a periodontal infection usually test vital to thermal testing unless the acute condition is a true combined lesion in which both endodontic and periodontal compartments have become diseased. Teeth with both a periradicular infection and a periodontal abscess usually test nonvital. Exceptions to this are either extremely calcified canals, extensively restored teeth, or multirooted teeth where some canals may be necrotic as the result of either pulpal or periodontal disease. Other canals may still retain vital tissue that responds to thermal or electric pulp testing. Thermal testing is usually the most reliable way of determining pulpal health or disease. Patients with an irreversible pulpitis often report a lingering painful response to a thermal stimulus. In later stages of pulpitis, heat exacerbates the symptom more than the cold, and the application of cold may even cause short-term pain relief.70 Although thermal testing can be informative as to the status of the pulp, a patient’s response to thermal stimuli may be confused with hypersensitivity resulting from exposed dentin and patent dentinal tubules without pulpitis.3 Therefore, thermal testing must be combined with other diagnostic criteria as discussed previously to distinguish between the lesions originating from pulpal or periodontal infection.
Treatment Considerations of Endodontic-Periodontic Lesions
In managing the lesions of pulpal or periodontal origin, making an accurate diagnosis as to the source of infection is a critical determinant of the treatment outcome. Primary pulpal lesions combined with secondary periodontal defects would completely resolve by conventional root canal therapy (RCT) alone. Supplement A Figure 51-4 shows a chronic pulpal infection associated with pulp necrosis that led to the periodontal defect via the endodontic-periodontic continuum through the lateral canal. This is a classic example of primary pulpal infection causing a secondary periodontal defect. In this case, RCT alone led to complete resolution of the periradicular periodontitis involving the furcal defect. In fact, this type of lesion and the healing pattern are observed in almost all periodontal defects resulting from primary pulpal infection and rapid bone loss in the periodontal tissues.
Lesions originating from pulpal infections require endodontic therapy, which would not resolve lesions resulting from primary periodontal infections. This is exemplified in Supplement A Figure 51-7. In Case 1, a preoperative radiograph of “independent” endodontic-periodontic lesion shows a periapical radiolucency spanning the entire length of the distal root of tooth #19 and moderate bone loss at the mesial aspect. On completion of endodontic therapy, the periodontal breakdown around the distal root completely resolved, whereas the mesial bony defect remained unchanged. Case 2 shows a “combined” endodontic-periodontic lesion involving extensive periradicular defects around the apices of tooth #19 and a periodontal defect in the bifurcation. After successful endodontic therapy alone, the periradicular lesions and the furcation defect have resolved, but incomplete healing is noted in the coronal aspect of the furcation. This finding presumes that the unhealed defect originates from a primary periodontal infection.
Therefore, endodontic-periodontic lesions require both endodontic and periodontal therapies for complete healing to occur. This is true whether the endodontic-periodontic lesions are independent or combined. One important consideration is the sequence of therapies: Which of the two should be performed first? As discussed earlier, endodontic lesions are often associated with more pronounced symptoms than periodontal lesions.
More importantly, in combined endodontic-periodontic lesions, some periodontal defects will resolve on completion of the endodontic treatment, whereas the opposite would not be the case. After resolution of the secondary periodontal defect stemming from a primary pulpal infection, the residual periodontal disease may be more accurately and predictably managed. These considerations indicate that combined endodontic-periodontic lesions are best treated by first performing the necessary endodontic care followed by periodontal therapy.
When patients present with an abscess, the periodontal and periradicular abscesses are managed differently. Previous studies have suggested that the treatment of the patient with an acute periodontal abscess should be performed in two stages. In the first stage, the management of the acute lesion is performed. In the second stage, a more comprehensive treatment of the original and any residual lesions are accomplished. The management of the acute periodontal abscess involves establishing drainage via the periodontal pocket and subgingival scaling and root planning.
Curettage of the epithelium lining, the pocket, and surrounding connective tissue is then accomplished followed by compression of the pocket wall. If the swelling is large and fluctuant, flap surgery or incision and drainage may be necessary to relieve the pressure. In cases in which the bone loss is too extensive and the prognosis for the tooth is hopeless, extraction may be required.153
Rationale use of antibiotics should be considered in acute periodontal abscesses.63 The use of systemic antibiotics may be indicated when patients have elevated temperatures, cellulitis, or systemic disease and are immunocompromised.153 In a study by Jaramillo,63 some periodontal pathogens showed resistance to tetracycline, metronidazole, and amoxicillin but not azithromycin. In the management of acute periradicular abscesses, the abscess should first be drained by performing a pulpectomy or incision and drainage. The choice of which technique to use may be based on time constraints. The incision and drainage procedure requires less time to accomplish and yet relieves the pressure that has accumulated under the subperiosteal tissues that results in a fluctuant, swollen, and painful lesion. The pulpectomy removes the infected tissues and microorganisms from within the canal system and calcium hydroxide is placed into each of the canals. Calcium hydroxide provides both biocompatibility and helps to block the canals from coronal leakage. Its use has proved to be a suitable intracanal medicament because of its stability and bactercidal effect in a limited space.69
If adequate drainage is achieved via incision and drainage and debridement and medication of the canal system, antibiotics generally are of no additional benefit. In the event of systemic complications, such as fever, lymphadenopathy, cellulitis, or in an immunocompromised patient, antibiotics may be prescribed in addition to drainage of the tooth.95
Penicillin V or amoxicillin are still the antibiotics of choice against the majority of bacteria isolated from acute endodontic infections. If penicillin V therapy is ineffective, the combination of penicillin V with metronidazole or amoxicillin–clavulanate potassium is recommended. The use of clindamycin is another excellent alternative.72 The management of pain associated with acute apical periodontitis is well controlled by prescribing systemic nonsteroidal antiinflammatory drugs (NSAIDs) with or without the additional use of Tylenol. Narcotics are usually unnecessary unless an acute flare-up occurs.139 The correct diagnosis of periodontal and periradicular abscesses is the most critical step in the resolution of the disease process. Failure to properly diagnose and treat these often encountered conditions result in the progression of the disease, continued loss of bone and periodontal attachment apparatus, and a poor prognosis and possible loss of the tooth.
Endodontic Procedural Complications and the Effects on the Periodontium
Numerous complications may arise during and following endodontic therapy. Some of these complications may be iatrogenic such as perforations, root fractures, sodium hypochlorite accidents, or the improper use of ultrasonic devices. The remainder may be the result of excessive masticatory forces, resorptive defects on either or both the internal or external periradicular surfaces, or dental malformations that occur during tooth development.
Many complications are difficult to avoid because of the complex morphology of the tooth and calcification of the root canal system. All of these complications result in the increased risk of treatment failure and the destruction of periodontal tissues surrounding the tooth. Lin et al89 remind us, however, that errors or complications encountered during endodontic therapy are not the direct cause of treatment failure. Instead, it is the inability to completely remove the pathogens during treatment of these complications that have a negative effect on surrounding periodontal tissues.89 Therefore, appropriate treatment is required to minimize the loss of these periodontal supporting structures and maximize the prognosis for tooth retention.
Perforations
Perforations may occur due to extensive caries or resorptive defects that have perforated through the dentin and into the periradicular tissues. Iatrogenic perforations may also occur during the preparation of the access cavity, instrumentation of the canal space, preparation of the tooth for a post, and extensive gouging of a tooth with rotary instruments during periodontal surgical procedures.
Perforations occurring during access preparation may be located either on the external surface of the tooth or perforate the furcation of multirooted teeth. Errors in instrumentation are usually the result of either overinstrumentation, stripping of the internal root curvature or “danger zone,”1 or canal transportation sometimes resulting in a perforation defect that extends into the periradicular tissues.
After endodontic therapy, a final restoration is placed to protect the tooth from further breakdown or fracture. Because most of the coronal tooth structure may have been previously destroyed, a post and core is often required to provide retention for the placement of a crown. Improper post space preparation may needlessly result in a perforation or stripping defect of the root and result in a guarded-to-hopeless prognosis for the tooth and exposure of the periradicular tissues to necrotic debris and oral pathogens. This will result in the additional loss of bone at the perforation site and future endodontic and periodontal complications.
The most important consideration in repair of a perforation defect is to minimize the time from the occurrence of the perforation until it is successfully sealed and to control any infection present. Keeping this time as short as possible ensures a more predictable result and minimizes the amount of periodontal destruction adjacent to the perforative defect.39,118 This may be done nonsurgically, surgically, or a combination of the two techniques, depending on the size of the defect, the amount of bone loss, and the location of the perforation. Surgical procedures that involve bicuspidation, hemisection, root amputation, reimplantation, and guided tissue regeneration are more complex, have a more guarded prognosis, and demand that the patient becomes diligent in their oral hygiene.5,9 In some cases, the placement of an implant may be a more predictable choice and better preserve the surrounding bone structure.
Numerous materials have been previously used for the repair of these perforations. Cavit, intermediate restorative material (IRM), super-EBA, amalgam, composites, glass ionomer cements, and iridium foil have been used with varying degrees of success.4 Most of the recent studies, however, have demonstrated the wide use of mineral trioxide aggregate (MTA) as the material of choice because of its biocompatibility, ability to be sealed, and its high degree of success.93,112,169
The location of the perforation, its size, and the condition of the pulpal and periradicular tissues has a profound effect on the prognosis of the tooth. Teeth that are nonvital with periradicular lesions demonstrate a less favorable prognosis compared to those that are vital and have intact PDLs with no apical pathosis present.104,113 Additionally, furcal perforations, perforations slightly below crestal bone, stripping perforations in the coronal third of the root, or older perforations with increased loss of bone demonstrate a poorer prognosis than those perforations that are small and situated more apically or above crestal bone.113
Prevention of root perforations depends on a thorough clinical examination. The examination should include the knowledge of tooth morphology and the angulation of the tooth being treated. Multiple-angled radiographs can help determine the position of the perforation defect, and the use of a fiberoptic light can aid in the proper diagnosis and the possible presence of any preexisting fractures. Magnifying loupes or an operating microscope aid the operator in observing small details that may otherwise go unnoticed and better allow microsurgical procedures to be completed more quickly and accurately. This usually results in more rapid healing and a repair procedure that has a higher degree of success.
Tooth Fractures
Whenever trauma occurs, the patient will usually be examined first by their general dentist and then possibly referred to either a periodontist or endodontist for further evaluation and treatment. It is extremely important for the generalist or specialist to determine if the tooth has received a simple concussion, luxation, or fracture of the crown or root. Multiple radiographs are helpful in distinguishing between these various injury types. It is suggested that a steep occlusal and two conventional periapical bisecting angled films are taken from both mesial and distal directions.7 If a tooth fracture is detected, a thorough diagnosis and treatment plan needs to be developed to render the appropriate treatment for the patient.
Teeth can fracture for a variety of reasons, and the fracture site and severity may be situated along any area of the tooth from the crown to the apical portion of the root. The treatment and prognosis of each of these fracture types vary greatly. The discussion of tooth fracture is divided into three separate categories that affect both the endodontic and periodontal treatment. The causes, clinical observations and diagnosis, treatment, and prognosis are discussed for each type of fracture modality.
Crown-Root Fractures
Crown-root fractures are of endodontic and periodontal concern when the fracture extends slightly below the marginal gingiva or crestal bone. These fractures are usually the result of a traumatic incident and are usually horizontal or oblique because of the direction and the intensity of force sustained during the trauma. Anterior teeth are most frequently involved, but premolars and molars may also be fractured, depending on the type of injury sustained. Teeth that are badly shattered in both the coronal and root areas and have sustained multiple fractures cannot be successfully treated endodontically or periodontally. They are most often extracted and restored utilizing a fixed bridge or an implant. Most of the time, however, a chisel-type fracture will occur that splits the crown and the root in an oblique direction and extends 2 to 3 mm below the marginal gingival and crestal bone generally results in the exposure of the pulp and often presents a challenge to the periodontist, endodontist, and restorative dentist to successfully retain the tooth.
The depth to which the fracture extends determines the restorability of the tooth and the type of restorative treatment employed. In cases in which the tooth is immature and development of the root is incomplete, the fractured segments may be temporarily bonded to allow for future root development and apical closure. Sound clinical judgment, the length of time from the occurrence of the fracture, and pulpal status must be taken into account to arrive at a proper course of treatment.35-37
If the root has completely developed, the fractured segment should be removed to determine the depth of the fracture defect. Questions that need to be answered during treatment planning are as follows:
These cases usually necessitate a multidisciplinary approach utilizing the talents of four to five different specialty areas.
Horizontal Root Fractures
Horizontal fractures most often occur in the region of the anterior maxilla. Many of these fractures go undiagnosed and heal without any treatment.26 Usually it is not until these patients visit a dental office years later that a radiograph reveals the presence of the previous trauma and subsequent healing of the fractured tooth segments. In a study by Andreasen et al,7 400 horizontal root–fractured teeth were studied in young individuals from 7 to 17 years of age. Their findings demonstrated that 30% of the teeth healed by hard tissue fusion, 20% healed with the interposition of the PDL and bone, 43% healed with the interposition of PDL alone, and only 22% became necrotic and exhibited inflammatory changes between the fragments. Variables that correlated positively with healing were (1) younger age, (2) immature root formation, (3) vital pulp, and (4) no tooth mobility or displacement of the coronal segment. Healing of the segments was also progressively worse as the separation between the fragments increased.7 Horizontal root fractures are usually categorized as coronal, midroot, or apical. The diagnosis of horizontal fractures is mostly based on information gained during the clinical and radiographic examinations.
Horizontal Fractures in the Coronal Area
Coronal fractures may require only endodontic treatment if the fracture is at the level of the gingival tissue. This is necessary only when the coronal portion of the tooth is missing and endodontics is necessary to allow for the placement of a final restoration. If the position of the fracture is below crestal bone and the crown is intact, the mobility of the coronal segment dictates whether to splint the teeth and wait to evaluate the traumatized tooth at a later appointment or remove the coronal segment, complete endodontic treatment, and then proceed with either periodontal crown lengthening or extrusion of the apical segment.54 Cvek26 reported that when comparing transverse fractures in the coronal third of the root to oblique fractures, the frequency of healing did not differ. Only 16% of the teeth in this study became necrotic with radiolucent areas adjacent to the fracture site.
When the crown has been lost and the coronal segment of the root is submerged below the marginal gingiva, periodontal treatment is necessary and the tissues may be apically repositioned around the submerged root segment. In complex crown fractures in younger patients, a multidisciplined approach is often needed to surgically treat the tissues around the fractured segment using guided bone regeneration followed by endodontic treatment and the placement an esthetic post to increase retention of the crown and more equally distribute the stresses of mastication.6 If the root has fully developed and has adequate root length, another common approach is to orthodontically extrude the root to preserve both the height of bone and contour of the surrounding tissue. Both these tissues migrate coronally with the root during the extrusion process. Following extrusion, the coronal fibers of the PDL are surgically released and the root is stabilized to prevent intrusion or relapse. After stabilization has occurred, the final restoration is fabricated and permanently cemented.54
Horizontal Fractures in the Midroot Area
Midroot fractures usually require no periodontal intervention with the possible exception of the patient who requires emergency repositioning and splinting of the teeth by a periodontist rather than a general dentist or endodontist. Midroot fractures that are stable usually require no endodontic intervention or splinting. As with midroot fractures in the coronal area, luxated teeth should be repositioned and passively splinted for 1 to 4 weeks. The exact time of stabilization varies between different investigators.7,110 Supplement A Figure 51-8 illustrates the successful use of a large Thermafil carrier to stabilize both the coronal and apical segments of a tooth with a horizontal midroot fracture. Delays in splinting of several days did not result in reduced healing.7 Frequent pulp testing and radiographic evaluation for resorptive defects should be done with all fractured teeth.
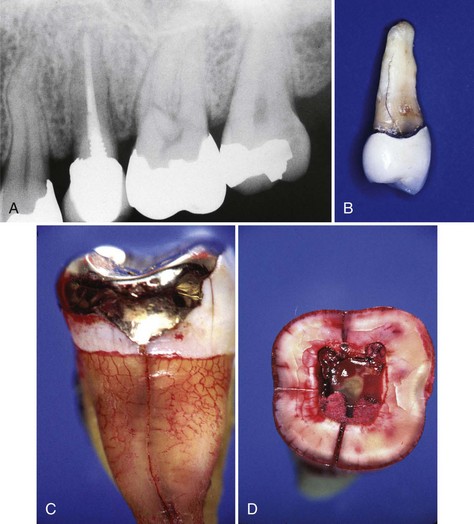
Supplement A Figure 51-8 Horizontal root fracture. A, Radiograph of tooth #9, 4 weeks after instrumentation. The radiograph shows a midroot intraalveolar horizontal root fracture with separation of the coronal and apical segments. Resorptive defects are evident at the fracture site and slight periapical bone loss is present at the periapex and mesial root surfaces. The tooth displayed slight mobility with normal probing depths. B, The segments were stabilized using a size #140 Thermafil obturator with a metal carrier. The tooth was restored with an acid-etched composite restoration. C, 12-year recall radiograph shows excellent long-term treatment success and a normal periradicular appearance.
(Courtesy Dr. Nadia Chugal.)
Necrosis of the coronal potion of the fracture necessitates endodontic therapy of usually just the coronal segment. Gutta percha or MTA are used to seal the coronal portion of the root, depending on the apical diameter and the presence, severity, and type of resorption. The apical segment usually remains vital.168 If both segments become nonvital, fixation using various post types and designs may be employed80 (see Supplement A Figure 51-8).
Horizontal Fractures in the Apical Area
Fractures occurring in the apical portion of the root are evaluated and treated similar to coronal and midroot fractures. The mobility of the coronal segment may be less due to the more apical position of the fracture site. This depends on the direction and severity of the trauma and the presence and degree of tooth luxation. Factors previously discussed regarding splinting and frequent evaluation of vitality and root resorption apply to apical fractures as well. Most coronal and apical segments remain vital and do not require either endodontic or periodontal intervention.7 If the apical segment does become necrotic, it is usually removed surgically and the necrotic coronal portion of the root is endodontically treated in the same manner as horizontal fractures occurring in the midroot area of the tooth.
Vertical Root Fractures
The early detection and diagnosis of vertical root fractures presents a challenge to both general dentists and specialists. Failure to properly diagnose this type of fracture usually results in prolonged patient suffering and frustration for both the patient and treating dentist. An accurate diagnosis can often be established by listening to the patients chief complaint, careful examination of both periapical and bitewing radiographs, and a thorough clinical examination.21 These steps help in accurately locating the involved tooth, correctly identifying the source of the problem, and decreasing the rapid breakdown of bone surrounding the tooth. Taking these necessary steps will have a profound effect on the success of the placement of a subsequent implant.151
Definition
Dentin is anisotropic and fractures usually extend perpendicular to the orientation of the dentinal tubules. Vertical root fractures result from internal and external stresses that exceed the elastic modulus of dentin. These stresses ultimately result in structural failure (Supplement A Figure 51-9).
Supplement A Figure 51-9 Vertical root fracture. A, Patient whose chief complaint is pain from bite pressure irritation, bleeding, and redness on the lingual marginal tissues. The radiograph reveals a slight loss of bone on the distal surface of tooth #13 and a short screw post. A lingual periodontal pocket probing 6 mm was present. A diagnosis of vertical root fracture was made. B, After extraction of the tooth, a vertical root fracture extending two-thirds of the root is evident. C, Lower molar previously diagnosed with an incomplete fracture tested vital and was referred back to the dentist for restoration with a full crown. The tooth was restored with an onlay, and an endodontic procedure was needed less than a year later because of pulpal necrosis. Initial healing was observed at 6 months, but the tooth required extraction 18 months later. This photograph shows the extension of the vertical fracture apically. D, A cross-sectional view at the cementoenamel junction demonstrates the presence of both a mesiodistal, as well as a lingual, fracture.
Incidence
The incidence of vertical root fractures in endodontically treated teeth has been reported to vary from 3.69% to as high as 10.9%.41,103,172 Fuss et al40 feel that this large difference may be due to the difficulty in making an accurate diagnosis before sending patients to have the tooth extracted. This concept may be valid because one study found that general dentists correctly diagnosed vertically fractured teeth only 33% of the time.142 The remaining 67% were referred for either periodontal therapy or endodontic retreatment.142
There are many factors that predispose teeth to vertical root fractures. These etiologic factors include previous endodontic treatment, extensive restorative procedures, morphologic tooth differences, age, sex, ethnicity, cultural differences, resorptive defects, and heavy masticatory forces. Most vertically fractured teeth have undergone previous endodontic treatment. In fact, the most common etiology associated with vertical root fractures is previous endodontic treatment and post placement. Vertical root fractures in teeth that have not been treated endodontically are uncommon. The only reported studies are investigations of Chinese and Thai populations. These studies point to differences in dietary habits, consumption of abrasive foods, and chewing betel nut. The incidence of fractures in males was 70% to 100% greater compared with females.18,19,42
The Chinese and Thai studies reported that vertical fractures occurred 78% more frequently in the mesial or mesiobuccal roots of first molars and in patients between the ages of 40 and 69 years. One study found that 40% of all vertical fractures occurred in teeth that have not been treated endodontically. The majority of the patients studied had relatively intact dentitions. Fractured teeth frequently had no previous restorative procedures and showed excessive wear on the occlusal surfaces.18,19,42
Both endodontic and restorative procedures remove valuable tooth structure that is critical to the stability of the tooth and its ability to resist constant masticatory loading. Stresses generated during these procedures may not appear for many years until the vertical fracture becomes apparent both clinically and radiographically.40 An investigation of instrumented compared to noninstrumented teeth demonstrated that instrumented mandibular premolars showed a higher risk to fracture than those that were not instrumented. This further emphasizes the role that structural integrity plays in reducing the possibility of tooth fracture.167
As with nonendodontically treated teeth, endodontically treated teeth with narrow mesiodistal diameters have a greater tendency to vertically fracture. This includes maxillary premolars, the mesial or mesiobuccal roots of molars and lower anterior teeth.142 Tamse reported that the incidence of vertical fractures was greatest in maxillary second premolars (27%) and the mesial roots of mandibular molars (24%).142 Other investigations have included lower premolars and distal roots of maxillary molars as well.77,150 Overpreparation of the canal system111,128 and the cementation of posts of improper design, length, and diameter increase stresses within the tooth and the propensity for the root to vertically fracture as well.101,124 All fractures extend from the internal root surface to at least one external surface. but occasionally some fractures extend to both surfaces. Most often, fractures will be oriented in a facial or buccolingual direction in anterior and premolar teeth and mesiodistally oriented in molars. When both the buccal and lingual surfaces of a tooth have deep and narrow probing defects, it is generally pathognomonic of a vertical fracture. Vertical forces applied to an endodontic spreader during lateral condensation cause stresses to be generated throughout the prepared canal. If the spreader load is too great, the spreader is too large or the canal has been overinstrumented, both in vitro and photoelastic investigations have demonstrated that these internal stresses can result in vertical root fracture.58,65,106,166
Clinical Findings
The subjective findings associated with vertical fractures are usually minimal. The patient often presents with reported discomfort to bite and chewing pressure and signs of mild inflammation. Generally, there is minimal swelling unless a flare-up has occurred. Flare-ups are usually the primary reason for the patient seeking dental care. When this happens, the tooth will appear to have all the signs and symptoms of a periodontal abscess. This is the most probable reason that so many vertical fractures are referred first for periodontal or endodontic treatment.
The fracture space contains various irritants that result in the inflammation of the surrounding periodontal tissues. Bacteria may be present either in the dentinal tubules or enter the fracture site from the oral environment. Sealer may be found in the fracture or adjacent tissues, especially when the fracture resulted from excessive condensation forces or forceful tapping of a post during the cementation procedure.160
Objective findings include periodontal probing patterns that are usually very deep and narrow at the fracture site.91,142 This is probably one the most common findings when a vertical root fracture is diagnosed. Periodontal defects tend to probe more widely because of the broader and more extensive loss of bone. Often, it is possible to retract the tissues, transilluminate the root, and successfully identify the fracture. At other times, the tooth may probe normally, but a sinus tract may be evident close to the gingival margin rather than at the apical area. Tamse et al142 found that a solitary, narrow buccal pocket existed in 67% of the vertical fractures examined and a sinus tract was evident 34% of the time. If a fracture is suspected but cannot be seen, a surgical flap may be necessary to positively identify its presence or absence.119
Fractures in maxillary premolars, mesial and mesiobuccal roots of molars, and lower anteriors usually extend in a faciolingual direction.91,142,144 Mandibular molar teeth with large intracoronal restorations may be transilluminated to diagnose the presence a fracture. These fractures may extend varying distances both pulpally, as well as down both mesial and distal tooth/root surfaces. Teeth with shallow but wide restorations that test necrotic and are painful to percussion generally have vertical fractures that have extended into the pulpal space and may not be salvageable and will subsequently require extraction.
Vertical root fractures extend varying lengths from the coronal or apical portions of the root. The degree of extension depends on both the amount and location of stress concentration within the root. Most of these fractures probably begin on the internal root surface caused by the stresses generated during restoration, endodontic instrumentation and condensation, or the placement of a post. Probing depths may vary, depending on the length of time the fracture has been present, although some vertically fractured teeth have probing depths that are normal.
Radiographic bone loss is significantly greater in teeth with periodontal or combined endodontic-periodontal lesions compared to teeth that are vertically fractured.108 In vertically fractured teeth, radiographic findings in maxillary and mandibular premolars and mesial and mesiobuccal roots of molars typically display one of three typical patterns that depend on the time the fracture has been present and the amount of bone loss that has occurred. Early patterns may appear as just a slight widening of the PDL space on the lateral tooth surface. As bone loss becomes more progressive, a more angular loss of crestal bone, or “halo” or “J-shaped” radiographic appearance, may become evident. Only a few teeth display the actual separation of tooth segments. Radiolucencies in the bifurcation area are more typical of vertical root fractures in mandibular molars.143,151 The use of computed tomography (CT) scans and the newer cone beam CT (CBCT) scans have been shown to be superior to periapical radiographs in the identification and diagnosis of vertical root fractures.53,170
Prevention of vertical root fractures relies on two basic concepts: (1) the preservation of radicular dentin and (2) the reduction of dentinal stresses resulting from forces encountered during instrumentation, condensation, and restorative procedures. Overinstrumentation of the canal space and the use of progressively larger hand or rotary instruments result in the loss of valuable tooth structure. Preservation of this tooth structure is necessary to prevent tooth fracture after the restoration of the tooth and years of masticatory function.83 Instrumentation and, in particular, underinstrumention exposes the tooth to greater forces during condensation with inflexible and larger spreader and plugger designs.65,106 More research is needed to arrive at an ideal instrumentation and filling technique based on the dimensions of the root, its curvature, and the stresses transmitted during endodontic treatment of the tooth.
Research of various post designs demonstrated that posts should be utilized only when there is too little tooth structure left to support the final restoration. Posts that transfer stresses throughout the entire root surface have a vented surface to allow for even cementation pressures, do not weaken root structure caused by excessive post width, and do not result in a wedging effect during cementation should be utilized. Post width should be no greater than one-third the width of the root. Post designs that are least likely to result in excessive stress production are currently prefabricated, passively cemented parallel designs of either metal or the newer more flexible carbon-fiber design.124,134,136 Cast posts and cores with minimal taper may be needed in certain cases as the result of canal morphology and the necessity for a protective ferrule to further prevent the possibility of root fracture.144
Treatment of vertical root fractures involved the use of composite resin bonding techniques in an attempt to repair the fractured root surface, continuous wave carbon dioxide (CO2) lasers to fuse bioactive glass paste in an attempt to fuse the fractured surfaces and the use of various filling materials in an attempt to seal the fractured segments.161 Most of these techniques have had varying success and still need to be further evaluated before their acceptance as a viable way to retain a vertically fractured tooth.
The prognosis for the vertically fractured tooth is virtually hopeless, and attempts to bond the fractured segments together, utilize CO2 lasers to fuse the segments, and use of other filling materials have met with met with varying success.161 The usual solution in single-rooted teeth is to remove the tooth and replace it with a fixed or removable appliance or a dental implant. In multirooted teeth, either an amputation or hemisection of the involved root is surgically performed.82 An alternate procedure is removal of the tooth, maintenance of the bony architecture, and placement of an implant.
Sodium Hypochlorite Accident
Sodium hypochlorite is the most commonly used irrigant in endodontic treatment. Concentrations that have been recommended range from 0.5% to 6%, but 2.5% is the most commonly used concentration.173 Extrusion of sodium hypochlorite into the periradicular tissues results in a rapid diffuse swelling and extreme pain. Tightly wedging the irrigating needle into the canal and forcing the irrigant into the surrounding periradicular tissues is the most common cause of extrusion. Other mishaps that result in the extrusion of sodium hypochlorite are caused by overinstrumentation of the canal, iatrogenic perforations that occur during access preparation, or the inadvertent injection of sodium hypochlorite into the tissues during patient anesthesia.100 In a recent survey of diplomats of the American Board of Endodontics, approximately one-third reported experiencing a sodium hypochlorite accident at some time during their years of practice. In the survey, findings indicated that accidents were more positively correlated with women, maxillary compared to mandibular teeth, posterior compared to anterior teeth, and necrotic compared to teeth with no necrosis or periapical lesions present. Sodium hypochlorite accidents may involve both the endodontist and periodontist if the general dentist refers the patient for further consultation. The endodontist may monitor the case for several weeks and subsequently obturate the canal when the swelling subsides. The periodontist may be asked to monitor scar tissue formation that often forms and causes continued discomfort and surgically intervene if necessary.
Avoidance of sodium hypochlorite accidents is best accomplished by the use of side-vented needles and loose insertion of the irrigation needle into the canal. Using light pressure to deliver the sodium hypochlorite and remove the debris created during canal instrumentation is also useful. If an accident does occur, the patient should be checked frequently, the tooth should be closely monitored and left open for drainage to occur, and referral to an endodontist is advised. The occurrence of a sodium hypochlorite accident by itself does not adversely affect the endodontic prognosis, and most signs and symptoms from the accident usually resolve within a month.78
Resorptive Defects
Resorption of tooth structure occurs as pathologic events leading to destruction of root canal and periradicular structural integrity. However, it is important to recognize that the resorptive process itself is a normal, physiologic event. In bone, for example, there is constant osteoclast-mediated bone resorption coupled with new bone formation as part of routine bone remodeling process.146 Even in dental tissues, physiologic root resorption is found in primary teeth without any inflammatory events. Thus resorption of mineralized tissues, whether bone, dentin, or cementum, is not pathologic at all. What is surprising is that such remodeling of hard tissue is not meant to occur in the permanent dentition under normal conditions. The mechanisms leading to resorption are essentially identical in pathologic root resorption as that for bone that involve the activation of odontoclast cells expressing tartrate-resistant acid phosphatase.75 In normal conditions, root resorption does not occur as the result of resistance of dental tissues to the physiologic bone remodeling process. The resistance may be explained by the fact that organic matrix of demineralized dentin or unmineralized predentin contain inhibitors of root resorption.163 This may be associated with the fact that dentin matrix possesses significantly reduced amount of osteopontin (OPN) compared with bone matrix,17 and OPN plays a critical role in odontoclast maturation during root resorption.152 Furthermore, predentin and cementoid covering the most inner and outer layers of the root, respectively, provide protection against the physiologic bone remodeling and resorptive activities.90,162 Therefore pathologic root resorption would entail disruption of these protective layers as can be evinced in cases of trauma, excessive heat, chemical injuries, and infection. Trauma, such as luxation, avulsion, or excessive pressure applied by orthodontic appliances, can lead to cemental tears, exposing deeper cementum and/or dentin. Minor injuries of this kind can be repaired without gross structural alteration by formation of a new cemental layer covering the injured sites and prevent further progression of the resorption. However, traumatic injuries causing larger denudation of the protective cementoid or predentin layers would allow progressive external or internal root resorption, respectively. External root resorption is also known as replacement resorption, osseous replacement, or ankylosis, which can be detected in routine radiographs as radicular defects replaced with bony trabeculation. On the contrary, internal root resorption frequently appears as a circular radiolucent lesion centered within the root canal in the absence of osseous replacement. After trauma, external root resorption may also be aggressive in nature and lead to the rapid destruction of dental tissues.55 These lesions are known as invasive cervical resorption because of the typical location that involves the cervical region of the root by fibrovascular tissues causing the resorptive defect. The etiology of these lesions may be linked with a history of trauma. Cervical root resorption is also associated with damage in the cementoid layer located at the cervical attachment apparatus.47 Other causes of root resorption include chemical injuries. Intracoronal bleaching may cause external cervical root resorption by cemental damage caused by the release of hydrogen peroxide or carbamide peroxide through the dentinal tubules.46 Excessive heat generated by ultrasonic instrumentation during RCT may also cause external root resorption due to the necrotic changes in the cemental layer and PDL cell damage. As described in greater detail elsewhere in this chapter, ultrasonic devices are frequently used for post removal during orthograde root canal retreatments, and the device setting and the contact time with the intracanal post system may influence the risk of external resorption. Perhaps the most frequent cause of root resorption is the presence of chronic inflammation of the pulp and periradicular tissues. This is known as inflammatory root resorption, can be internal or external, and is located mostly at the root apex associated with apical periodontitis.87 The presence of apical inflammatory root resorption may drastically alter the course of the endodontic therapy as it may shorten the root length and compromise the apical constriction (Supplement A Figure 51-10, A).
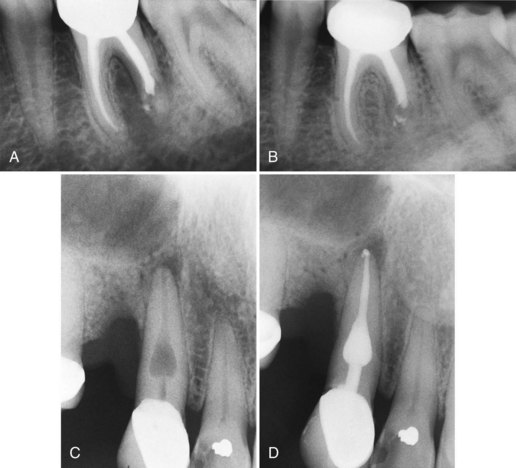
Supplement A Figure 51-10 Root resorption. A, Radiograph of tooth #19 after obturation of the canals. The shortened distal root is the result of apical inflammatory root resorption. A large radiolucent lesion extending into the bifurcation is evident. B, A 1-year recall radiograph shows complete repair of periradicular bone and some additional resorption of extruded filling material at the root apex. C, An internal resorptive lesion of relatively large size. The expansion of the canal space is diagnostic of a resorptive defect inside the tooth. However, it is not possible to diagnose the possibility of a possible external perforation from a two-dimensional radiograph. D, The final obturation film seems to indicate that the lesion and filling material appears to be confined to the canal space.
(Courtesy Dr. Thomas Rauth.)
Root resorptions have been treated by various means. If the cause of the resorption is chronic inflammation originating from pulpal infection, conventional RCT may lead to complete resolution of the resorptive process, although the defect cannot be restored (see Supplement A Figure 51-10, B). This is also the case for internal root resorption, which occurs with multinucleated giant cells and the granulation tissues in the radicular pulp. Internal resorptive lesions with perforating defects or external root resorption not resulting from pulpal infection have limited treatment options and their outcome is highly variable. RCT combined with perforation repair using MTA has been effective in some cases.13,113 Recent studies also assessed the potential use of bisphosphonate, which inhibits bone resorption by acting directly on osteoclasts.115 Preclinical studies evaluating the bisphosphonate’s ability to inhibit root resorption has resulted in partial success and demonstrated that the topical application of bisphosphonate reduced the extent of root resorption but not ankylosis.81,92 On the contrary, intracanal application of bisphosphonate did not have any effect on replacement resorption.147 Further research is necessary to elucidate the potential benefit of this novel approach in preventing root resorption.
Dental Anomalies
Dental malformations that occur during tooth development can result in the loss of periradicular bone and the possible loss of the tooth. Malformations include dens invaginatus (dens in dente), dens evaginatus, and lingual dental grooves.
Dens Invaginatus
Dens invaginatus is one of the more common developmental defects that occurs when the inner enamel epithelium invaginates into the dental papilla before calcification.49 Its etiology is unknown, but it may be genetic in origin. The lateral incisor is the most commonly affected tooth, and bilateral occurrence is not uncommon. It may often be detected by observance of an increased labiolingual or mesiodistal diameter, labial notching, a peg or conical morphology, and the presence of an exaggerated or “talon cusp.”15 Its presence increases the risk of pulpal disease and severe periodontal destruction.
The severity of dens invaginatus varies, and the classification by Oehlers is generally the most widely accepted.109 The classification increases in morphologic severity from Class I to Class III with Class III being the most anatomically complex and the most difficult to treat endodontically. If pulpal necrosis and periapical disease result from the exposure of the defect to the oral environment, either orthograde endodontic treatment or a combination of orthograde and surgical treatment may be necessary. Both gutta percha and MTA have been utilized to render orthograde treatment of complex defects16 (Supplement A Figure 51-11).
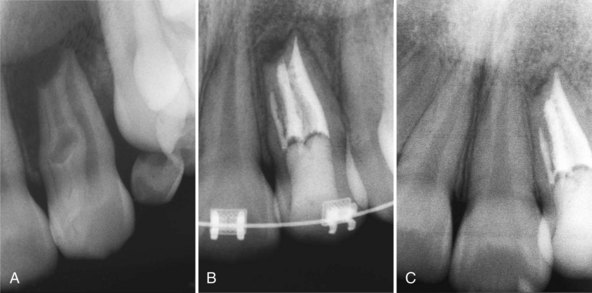
Supplement A Figure 51-11 Case of dens invaginatus causing primary endodontic infection. A, Preoperative radiograph of tooth #10 demonstrating a complex dens invaginatus with extensive apical bone loss. B, Significant bone healing is apparent and orthodontic therapy has been initiated C, A 4-year follow-up demonstrated continued bone repair.
(Courtesy Dr. Nadia Chugal.)
Dens Evaginatus
Dens evaginatus is another dental anomaly that has been well documented since 1925.88 The defect manifests itself as a tubercle or protrusion of enamel from the occlusal tooth surface that has a core of dentin usually containing a thin extension of pulpal tissue. It occurs in at least 2% of patients of Asian or Native American descent and is located mostly on mandibular premolar teeth but may also be found on molars and anteriors.98 The tubercles are susceptible to pulpal exposure caused by fracture, wear, orthodontic movement, or occlusal equilibration. Many of these teeth become necrotic before the root of the tooth has developed and require apexification procedures to allow for apical closure.
Prevention or prophylactic treatment before pulpal involvement is of paramount importance in the treatment of dens invaginatus. If the pulp is not exposed after removal of the tubercle, an alloy or a composite resin restoration may be utilized. If the pulp is exposed during removal of the tubercle, pulp capping may be accomplished by using either calcium hydroxide or MTA. Therefore, early recognition and timely treatment can prevent pulpal necrosis, extensive loss of periodontal supporting tissues, and the possible loss of the tooth.
Lingual Groove
Sometimes referred to as palatogingival grooves, lingual grooves are found most often on the distolingual surface of the maxillary lateral incisor, although other incisor teeth can be involved as well. The groove is a developmental anomaly where there is an infolding of the inner enamel epithelium and Hertwig’s epithelial root sheath. The groove may extend varying distances from the cingulum of the incisor in a cervicoapical direction toward the apex of the root. Microscopic investigation of teeth with lingual grooves demonstrates that more than 75% show deformation of the pulp chamber adjacent to the groove, diminished enamel and dentin thickness, and extension of the groove to the apical third of the root. Direct communication between the between the pulp and periodontium is rarely observed.86
Lingual grooves are associated with both periodontal and pulpal pathosis. A study43 using scanning electron microscopy traced the communication between the lingual groove and the pulp cavity. Accessory foramina were located in both areas. These accessory foramina could provide a pathway for infectious materials to migrate from the lingual groove to the pulp cavity resulting in pulpal necrosis.43 The presence of a lingual groove frequently results in a deep narrow periodontal defect. There are occasional pulpal communications that result in both periodontal and pulpal pathosis. Bonded restorative materials in combination with guided tissue regeneration have been used with variable results. The prognosis of these teeth is usually guarded to poor. Many are lost to extraction with the subsequent placement of an implant or bridge. Supplement A Figure 51-12 demonstrates a lateral incisor with two roots and a deep lingual groove that was successfully treated.
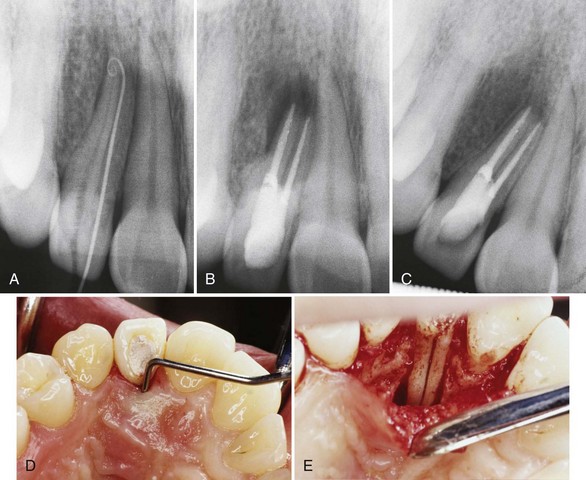
Supplement A Figure 51-12 Case of developmental lingual groove causing primary endodontic infection. A, Preoperative radiograph of tooth #7. A gutta percha marker extends to the apex. The radiograph is suggestive of a deep lingual groove or two separate roots. No caries or other abnormalities were present. B, A large lingual and periradicular defect developed after gutta percha obturation of both canals (see photo in D). C, 1-year recall after surgery showed radiographic healing, no symptoms, and a 3 mm probing depth. D, A 12 mm probing depth is demonstrated before surgical exploration. E, A large area of bone loss on the palatal and a deep lingual groove are evident during reflection of the flap. The groove was eliminated and Geristore resin was bonded to the lingual surface to repair the defect.
(Courtesy Dr. Nadia Chugal.)
Ultrasonic Devices
The retreatment of teeth that have been restored with various post designs has attracted researchers to study how to best remove these posts without weakening, fracturing, or perforating the surrounding tooth structure. Today, the use of ultrasonic devices to remove obstructions, cements, and metallic posts from within the canal space is used frequently when retreatment becomes necessary or a crown and post have fractured leaving the root and remaining post exposed and in need of further treatment.
Early studies suggested that the removal of posts without water spray could produce heat that could seriously effect the mineralized tissues that surround the tooth if temperatures in the periradicular tissues exceeded 50° C.32 Temperatures exceeding 10 degrees above body temperature are usually cited as a threshold beyond which vital tissues become irreversibly damaged. Resorptive defects resulting from the excessive heat generated from the ultrasonic tip to the metal post and the damage to both the cemental layer and PDL have been previously discussed.
More recent observations and case reports have clearly shown that if the intensity of the device and the contact time with the post is too long, significant heat conduction occurs through the dentin and into the surrounding PDL, bone, and soft tissue; it results in the necrosis of both the periodontal tissues surrounding the tooth and may cause the loss of the tooth and permanent damage to the periodontium.33,157 Most of these studies have concluded that there is a significant increase in root temperature that can reach values of more that 50° C if the contact time exceeds 15 to 30 seconds and there is no coolant used during the removal of the post.44,60 It therefore seems prudent to utilize a water coolant and intermittent contact times between the ultrasonic tip and the post of no more than 30 seconds. This should ensure that damage to the surrounding periodontal tissues is minimized.
Not utilizing a local anesthetic when removing a post from a tooth is often the best way to evaluate when the patient is beginning to experience discomfort. The ultrasonic tip can be removed as soon as the patient indicates any painful sensation and replaced once it has disappeared. Using this technique can avoid problems or damage to the surrounding periodontal structures.
To demonstrate the amount of heat transferred to the surrounding tissues, various in vitro studies have further investigated the effects of ultrasonic contact time with the post, the intensity setting of the device, the use of air and water coolants, and the amount of remaining tooth structure.33 Most of these studies have concluded that there is a significant increase in root temperature that can reach values of more that 50° C if the contact time exceeds 15 to 30 seconds and there is no coolant used during the removal of the post.44,60 It therefore seems prudent to utilize a water coolant and intermittent contact times between the ultrasonic tip and the post of no more than 30 seconds. This should ensure that damage to the surrounding periodontal tissues is minimized.
Minimizing or avoiding these various procedural complications will benefit the endodontist, periodontist, patient, and generalist in maintaining teeth that would have a guarded to poor prognosis and might be lost because of tooth-related defects such as resorption or operator-related errors resulting in the extraction of the tooth and loss of the supporting periodontal structures.
1 Abou-Rass M, Frank AL, Glick DH. The anticurvature filing method to prepare the curved root canal. J Am Dent Assoc. 1980;101:792-794.
2 Accorinte Mde L, Loguercio AD, Reis A, et al. Adverse effects of human pulps after direct pulp capping with the different components from a total-etch, three-step adhesive system. Dent Mater. 2005;21:599-607.
3 Addy M. Tooth brushing, tooth wear and dentine hypersensitivity—are they associated? Int Dent J. 2005;4(Suppl. 1):261-267.
4 Aguirre R, El Deeb ME, El Deeb ME. Evaluation of the repair of mechanical furcation perforations using amalgam, gutta-percha, or indium foil. J Endod. 1986;12:249-256.
5 Alhadainy HA. Root perforations. A review of literature. Oral Surg Oral Med Oral Pathol. 1994;78:368-374.
6 Altun C, Tozum TF, Guven G. Multidisciplinary approach to the rehabilitation of a crown fracture with glass-fibre-reinforced composite: a case report. J Can Dent Assoc. 2008;74:363-366.
7 Andreasen JO, Andreasen FM, Mejare I, et al. Healing of 400 intra-alveolar root fractures. Effect of treatment factors such as treatment delay, repositioning, splinting type and period and antibiotics. Dent Traumatol. 2004;20:203-211.
8 Bakland LK, Flores MT. Management of traumatic dental injuries. In: Torabinejad M, Walton RE, editors. Endodontics: Principles and Practice. ed 4. St. Louis, MO: Elsevier Saunders; 2009:163-184.
9 Barkhordar RA, Javid B. Treatment of endodontic perforations by guided tissue regeneration. Gen Dent. 2000;48:422-426.
10 Baumgartner JC, Khemaleelakul SU, Xia T. Identification of spirochetes (treponemes) in endodontic infections. J Endod. 2003;29:794-797.
11 Bender IB, Seltzer S. The effect of periodontal disease on the pulp. Oral Surg Oral Med Oral Pathol. 1972;33:458-474.
12 Bender IB. Pulp pain diagnosis—a review. J Endod. 2000;26:175-179.
13 Benenati FW. Treatment of a mandibular molar with perforating internal resorption. J Endod. 2001;27:474-475.
14 Bergenholtz G, Lindhe J. Effect of experimentally induced marginal periodontitis and periodontal scaling on the dental pulp. J Clin Periodontol. 1978;5:59-73.
15 Bishop K, Alani A. Dens invaginatus. Part 2: clinical, radiographic features and management options. Int Endod J. 2008;41:1137-1154.
16 Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod. 2009;35:777-790.
17 Butler WT, Brunn JC, Qin C. Dentin extracellular matrix (ECM) proteins: comparison to bone ECM and contribution to dynamics of dentinogenesis. Connect Tissue Res. 2003;44:171-178.
18 Chan CP, Lin CP, Tseng SC, et al. Vertical root fracture in endodontically versus nonendodontically treated teeth: a survey of 315 cases in Chinese patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:504-507.
19 Chan CP, Tseng SC, Lin CP, et al. Vertical root fracture in nonendodontically treated teeth—a clinical report of 64 cases in Chinese patients. J Endod. 1998;24:678-681.
20 Chen SY, Wang HL, Glickman GN. The influence of endodontic treatment upon periodontal wound healing. J Clin Periodontol. 1997;24:449-456.
21 Cohen S, Blanco L, Berman L. Vertical root fractures: Clinical and radiographic diagnosis. J Am Dent Assoc. 2003;134:434-441.
22 Contreras A, Slots J. Typing of herpes simplex virus from human periodontium. Oral Microbiol Immunol. 2001;16:63-64.
23 Contreras A, Umeda M, Chen C, et al. Relationship between herpesviruses and adult periodontitis and periodontopathic bacteria. J Periodontol. 1999;70:478-484.
24 Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318-1322.
25 Czarnecki RT, Schilder H. A histological evaluation of the human pulp in teeth with varying degrees of periodontal disease. J Endod. 1979;5:242-253.
26 Cvek M, Mejare I, Andreasen JO. Healing and prognosis of teeth with intra-alveolar fractures involving the cervical part of the root. Dent Traumatol. 2002;18:57-65.
27 Dahle UR, Tronstad L, Olsen I. Characterization of new periodontal and endodontic isolates of spirochetes. Eur J Oral Sci. 1996;104:41-47.
28 Dang DA, Walton RE. Vertical root fracture and root distortion: effect of spreader design. J Endod. 1989;15:294-301.
29 Dello Russo NM. The post-prophylaxis periodontal abscess: etiology and treatment. Int J Periodontics Restorative Dent. 1985;5:28-37.
30 Diem CR, Bowers GM, Ferrigno PD, et al. Regeneration of the attachment apparatus on pulpless teeth denuded of cementum in the rhesus monkey. J Periodontol. 1974;45:18-22.
31 Ehnevid H, Jansson L, Lindskog S, et al. Endodontic pathogens: propagation of infection through patent dentinal tubules in traumatized monkey teeth. Endod Dent Traumatol. 1995;11:229-234.
32 Eriksson AR, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent. 1983;50:101-107.
33 Ettrich CA, Labossiere PE, Pitts DL, et al. An investigation of the heat induced during ultrasonic post removal. J Endod. 2007;33:1222-1226.
34 Fabricius L, Dahlén G, Holm SE, Möller AJ. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res. 1982;90:200-206.
35 Flores MT, Andersson L, Andreasen JO, et al. Guidelines for the management of traumatic dental injuries. I. Fractures and luxations of permanent teeth. Dent Traumatol. 2007;23:66-71.
36 Flores MT, Andersson L, Andreasen JO, et al. Guidelines for the management of traumatic dental injuries. II. Avulsion of permanent teeth. Dent Traumatol. 2007;23:130-136.
37 Flores MT, Malmgren B, Andersson L, et al. Guidelines for the management of traumatic dental injuries. III. Primary teeth. Dent Traumatol. 2007;23:196-202.
38 Fogel HM, Pashley DH. Effect of periodontal root planing on dentin permeability. J Clin Periodontol. 1993;20:673-677.
39 Fuss Z, Trope M. Root perforations: classification and treatment choices based on prognostic factors. Endod Dent Traumatol. 1996;12:255-264.
40 Fuss Z, Lustig J, Katz A, et al. An evaluation of endodontically treated vertical root fractured teeth: impact of operative procedures. J Endod. 2001;27:46-48.
41 Fuss Z, Lustig J, Tamse A. Prevalence of vertical root fractures in extracted endodontically treated teeth. Int Endod J. 1999;32:283-286.
42 Gao YJ, Yin XM, Wu HJ. Relationship between vertical root fracture and the habits of chewing betel nut. Hunan Yi Ke Da Xue Xue Bao. 2001;26:161-162.
43 Gao ZR, Shi JN, Wang Y, Gu FY. Scanning electron microscopic investigation of maxillary lateral incisors with a radicular lingual groove. Oral Surg Oral Med Oral Pathol. 1989;68:462-466.
44 Garrido AD, Fonseca TS, Silva-Sousa YT, et al. Evaluation of root external temperature during the application of ultrasound in removal of intraradicular posts. Gen Dent. 2007;55:121-124.
45 Glick M, Trope M, Pliskin ME. Detection of HIV in the dental pulp of a patient with AIDS. J Am Dent Assoc. 1989;119:649-650.
46 Gokay O, Ziraman F, Cali Asal A, et al. Radicular peroxide penetration from carbamide peroxide gels during intracoronal bleaching. Int Endod J. 2008;41:556-560.
47 Gonzales JR, Rodekirchen H. Endodontic and periodontal treatment of an external cervical resorption. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e70-e77.
48 González-Moles MA, González NM. Bacterial infections of pulp and periodontal origin. Med Oral Patol Oral Cir Bucal. 2004;9(Suppl):34-36. 32-34
49 Gound TG. Dens invaginatus—a pathway to pulpal pathology: a literature review. Pract periodontics Aesthet Dent. 1997;9:585-594.
50 Guiliana G, Ammatuna P, Pizzo G, et al. Occurrence of invading bacteria in radicular dentin of periodontally diseased teeth: microbiological findings. J Clin Periodontol. 1997;24:478-485.
51 Gutman JL. Prevalence, location, and patency of accessory canals in the furcation region of permanent molars. J Periodontol. 1978;49:21-26.
52 Hahn CL, Liewehr FR. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. J Endod. 2007;33:213-219.
53 Hassan B, Metska ME, Ozok AR, et al. Detection of vertical root fractures in endodontically treated teeth by a cone beam computed tomography scan. J Endod. 2009;35:719-722.
54 Heithersay GS. Combined endodontic-orthodontic treatment of transverse root fractures in the region of the alveolar crest. Oral Surg Oral Med Oral Pathol. 1973;36:404-415.
55 Heithersay GS. Invasive cervical resorption following trauma. Aust Endod J. 1999;25:79-85.
56 Helovuo H, Hakkarainen K, Paunio K. Changes in the prevalence of subgingival enteric rods, staphylococci and yeasts after treatment with penicillin and erythromycin. Oral Microbiol Immunol. 1993;8:75-79.
57 Herrera D, Roldán S, González I, et al. The periodontal abscess (I). Clinical and microbiological findings. J Clin Periodontol. 2000;27:387-394.
58 Holcomb JQ, Pitts DL, Nicholls JI. Further investigation of spreader loads required to cause vertical root fracture during lateral condensation. J Endod. 1987;13:277-284.
59 Hong CY, Lin SK, Kok SH, et al. The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J Oral Pathol Med. 2004;33:162-169.
60 Huttula AS, Tordik PA, Imamura G, et al. The effect of ultrasonic post instrumentation on root surface temperature. J Endod. 2006;32:1085-1087.
61 Jansson LE, Ehnevid H, Lindskog S, et al. The influence of endodontic infection on progression of marginal bone loss in periodontitis. J Clin Periodontol. 1995;22:729-734.
62 Jansson LE, Ehnevid H, Lindskog SF, et al. Radiographic attachment in periodontitis-prone teeth with endodontic infection. J Periodontol. 1993;64:947-953.
63 Jaramillo A, Arce RM, Herrera D, et al. Clinical and microbiological characterization of periodontal abscesses. J Clin Periodontol. 2005;32:1213-1218.
64 Jiang HW, Zhang W, Ren BP, et al. Expression of toll like receptor 4 in normal human ododntoblasts and dental pulp tissue. J Endod. 2006;32:747-751.
65 Joyce AP, Loushine RJ, West LA, et al. Photoelastic comparison of stress induced by using stainless-steel versus nickel-titanium spreaders in vitro. J Endod. 1998;24:714-715.
66 Jung IY, Choi B, Kum KY, et al. Identification of oral spirochetes at the species level and their association with other bacteria in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:329-334.
67 Jung IY, Seo MA, Fouad AF, et al. Apical anatomy in mesial and mesiobuccal roots of permanent first molars. J Endod. 2005;31:364-368.
68 Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340-349.
69 Kawashima N, Wadachi R, Suda H, et al. Root canal medicaments. Int Dent J. 2009;59:5-11.
70 Keenan JV, Farman AG, Fedorowicz Z, et al: Antibiotic use for irreversible pulpitis, Cochrane Database Syst Rev 18:CD004969, 2005.
71 Kerns DG, Glickman GN. Endodontic and periodontal interrelationships. In: Cohen S, Hargreaves K, editors. Pathways of the Pulp. ed 9. St. Louis, MO: Elsevier Mosby; 2006:650-667.
72 Khemaleelakul S, Baumgartner JC, Pruksakorn S. Identification of bacteria in acute endodontic infections and their antimicrobial susceptibility. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:746-755.
73 Kim E, Song JS, Jung IY, et al. Prospective clinical study evaluating endodontic microsurgery outcomes for cases with lesions of endodontic origin compared with cases with lesions of combined periodontal-endodontic origin. J Endod. 2008;34:546-551.
74 Kim S, Dorscher-Kim JE, Liu M, et al. Functional alterations in pulap microcirculation in response to various dental procedures and materials. Proc Finn Dent Soc. 1992;88(Suppl. 1):65-71.
75 Kimura R, Anan H, Matsumoto A, et al. Dental root resorption and repair: histology and histometry during physiological drift of rat molars. J Periodontal Res. 2003;38:525-532.
76 Kirkham DB. The location and incidence of accessory pulpal canals in periodontal pockets. J Am Dent Assoc. 1975;91:353-356.
77 Kishen A. Mechanisms and risk factors for fracture predilection in endodontically treated teeth. Endod Topics. 2006;13:57.
78 Kleier DJ, Averbach RE, Mehdipour O. The sodium hypochlorite accident: experience of diplomates of the American Board of Endodontics. J Endod. 2008;34:1346-1350.
79 Kobayashi T, Hayashi A, Yoshikawa R, et al. The microbial flora from root canals and periodontal pockets of non-vital teeth associated with advanced periodontitis. Int Endod J. 1990;23:100-106.
80 Kocak S, Cinar S, Kocak MM, et al. Intraradicular splinting with endodontic instrument of horizontal root fracture—case report. Dent Traumatol. 2008;24:578-580.
81 Komatsu K, Shimada A, Shibata T, et al. Long-term effects of local pretreatment with alendronate on healing of replanted rat teeth. J Periodontal Res. 2008;43:194-200.
82 Kurtzman GM, Silverstein LH, Shatz PC. Hemisection as an alternative treatment for vertically fractured mandibular molars. Compend Contin Educ Dent. 2006;27:126-129.
83 Lam PP, Palamara JE, Messer HH. Fracture strength of tooth roots following canal preparation by hand and rotary instrumentation. J Endod. 2005;31:529-532.
84 Langeland K, Rodrigues H, Dowden W. Periodontal disease, bacteria, and Pulpal histopathology. Oral Surg Oral Med Oral Pathol. 1974;37:257-270.
85 Lantelme RL, Handelman SL, Herbison RJ. Dentin formation in periodontally diseased teeth. J Dent Res. 1976;55:48-51.
86 Lara VS, Consolaro A, Bruce RS. Macroscopic and microscopic analysis of the palato-gingival groove. J Endod. 2000;26:345-350.
87 Laux M, Abbott PV, Pajarola G, et al. Apical inflammatory root resorption: a correlative radiographic and histologic assessment. Int Endod J. 2000;33:483-493.
88 Leigh R. Dental pathology of the Eskimo. Dent Cosmos. 1925;67:884-898.
89 Lin LM, Rosenberg PA, Lin J. Do procedural errors cause endodontic treatment failure? J Am Dent Assoc. 2005;136:187-193.
90 Loe H, Waerhaug J. Experimental replantation of teeth in dogs and monkeys. Arch Oral Biol. 1961;3:176-184.
91 Lustig JP, Tamse A, Fuss Z. Pattern of bone resorption in vertically fractured, endodontically treated teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:224-227.
92 Lustosa-Pereira A, Garcia RB, de Moraes IG, et al. Evaluation of the topical effect of alendronate on the root surface of extracted and replanted teeth. Microscopic analysis on rats’ teeth. Dent Traumatol. 2006;22:30-35.
93 Main C, Mirzayan N, Shabahang S, et al. Repair of root perforations using mineral trioxide aggregate: a long term study. J Endod. 2004;30:80.
94 Mandi FA. Histological study of the pulp changes caused by periodontal disease. J Br Endod Soc. 1972;6:80-82.
95 Matthews DC, Sutherland S, Basrani B. Emergency management of acute apical abscesses in the permanent dentition: a systematic review of the literature. J Can Dent Assoc. 2003;69:660.
96 Matthews DC, Tabesh M. Detection of localized tooth-related factors that predispose to periodontal infections. Periodontol 2000. 2004;34:136-150.
97 Mazur B, Massler M. Influence of periodontal disease of the dental pulp. Oral Surg Oral Med Oral Pathol. 1964;17:592-603.
98 McCulloch KJ, Mills CM, Greenfeld RS, Coil JM. Dens evaginatus: review of the literature and report of several clinical cases. J Can Dent Assoc. 1998;64:104-106. 110-113
99 McLeod DE, Lainson PA, Spivey JD. Tooth loss due to periodontal abscess: a retrospective study. J Periodontol. 1997;68:963-966.
100 Mehdipour O, Kleier DJ, Averbach RE. Anatomy of sodium hypochlorite accidents. Compend Contin Educ Dent. 2007;28:544-546. 548, 550
101 Meister FJr, Lommel TJ, Gerstein H. Diagnosis and possible causes of vertical root fractures. Oral Surg Oral Med Oral Pathol. 1980;49:243-253.
102 Möller AJ, Fabricius L, Dahlén G, Ohman AE, Heyden G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89:475-484.
103 Morfis AS. Vertical root fractures. Oral Surg Oral Med Oral Pathol. 1990;69:631-635.
104 Motamedi MH. Root perforations following endodontics: a case for surgical management. Gen Dent. 2007;55:19-21.
105 Mullaney TP, Laswell HR. Iatrogenic blushing of dentin following full crown preparation. J Prosthet Dent. 1969;22:354-359.
106 Murgel CA, Walton RE. Vertical root fracture and dentin deformation in curved roots: the influence of spreader design. Endod Dent Traumatol. 1990;6:273-278.
107 Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348-381.
108 Nicopoulou-Karayianni K, Bragger U, Lang NP. Patterns of periodontal destruction associated with incomplete root fractures. Dentomaxillofac Radiol. 1997;26:321-326.
109 Oehlers FA. Dens invaginatus. II. Associated posterior corwn forms and pathogenesis. Oral Surg Oral med Oral Pathol. 1957;10:1302-1316.
110 Oikarinen K. Tooth splinting: a review of the literature and consideration of the versatility of a wire-composite splint. Endod Dent Traumatol. 1990;6:237-250.
111 Okitsu M, Takahashi H, Yoshioka T, et al. Effective factors including periodontal ligament on vertical root fractures. Dent Mater J. 2005;24:66-69.
112 Oliveira TM, Sakai VT, Silva TC, et al. Repair of furcal perforation treated with mineral trioxide aggregate in a primary molar tooth: 20-month follow-up. J Dent Child (Chic). 2008;75:188-191.
113 Pace R, Giuliani V, Pagavino G. Mineral trioxide aggregate as repair material for furcal perforation: case series. J Endod. 2008;34:1130-1133.
114 Palmer R. Oral bacterial biofilms - history in progress. Microbiology. 2009. Epub ahead of print
115 Papapoulos SE. Bisphosphonates: how do they work? Best Pract Res Clin Endocrinol Metab. 2008;22:831-847.
116 Paranjpe A, Cacalano NA, Hume WR, et al. N-acetylcysteine protects dental pulp stromal cells from HEMA-induced apoptosis by inducing differentiation of the cells. Free Radic Biol Med. 2007;43:1394-1408.
117 Paul BF, Hutter JW. The endodontic-periodontal continuum revisited: new insights into etiology, diagnosis and treatment. J Am Dent Assoc. 1997;128:1541-1548.
118 Pitt Ford TR, Torabinejad M, McKendry DJ, et al. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:756-763.
119 Pitts DL, Natkin E. Diagnosis and treatment of vertical root fractures. J Endod. 1983;9:338-346.
120 Raab WH. Temperature related changes in pulpal microcirculation. Proc Finn Dent Soc. 1992;88:469-479.
121 Reeder OWJr, Walton RE, Livingston MJ, et al. Dentin permeability: determinants of hydraulic conductance. J Dent Res. 1978;57:187-193.
122 Ricucci D, Langeland K. Apical limit of root canal instrumentation and obturation, part 2. A histological study. Int Endod J. 1998;31:394-409.
123 Rôças IN, Siqueira JFJr, Santos KR, et al. “Red complex” (Bacteriodes forsythus, Porphyromonas gingivalis, and Treponema denticola) in endodontic infections: a molecular approach. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:468-471.
124 Ross RS, Nicholls JI, Harrington GW. A comparison of strains generated during placement of five endodontic posts. J Endod. 1991;17:450-456.
125 Rothstein I, Simon JH. The endo-perio lesion: a critical appraisal of the disease condition. Endod Topics. 2006;13:34-56.
126 Rubach WC, Mitchell DF. Periodontal disease, accessory canals and pulp pathosis. J Periodontol. 1965;36:34-38.
127 Sanders JJ, Sepe WW, Bowers GM, et al. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects. Part III. Composite freeze-dried bone allografts with and without autogenous bone grafts. J Periodontol. 1983;54:1-8.
128 Sathorn C, Palamara JE, Palamara D, et al. Effect of root canal size and external root surface morphology on fracture susceptibility and pattern: a finite element analysis. J Endod. 2005;31:288-292.
129 Schuurs AH, Gruythuysen RJ, Wesselink PR. Pulp capping with adhesive resin-based composite vs. calcium hydroxide: a review. Endod Dent Traumatol. 2000;16:240-250.
130 Seltzer S, Bender IB, Nazimov H, Sinai I. Pulpitis-induced interradicular periodontal changes in experimental animals. J Periodontol. 1967;38:124-129.
131 Seltzer S, Bender IB, Ziontz M. The interrelationship of pulp and periodontal disease. Oral Surg Oral Med Oral Pathol. 1963;16:1474-1490.
132 Shovelton DH. The presence and distribution of microorganisms within non-vital teeth. Br Dent J. 1964;117:101-107.
133 Siqueira JFJr, Rôças IN. The microbiota of acute apical abscesses. J Dent Res. 2009;88:61-65.
134 Sirimai S, Riis DN, Morgano SM. An in vitro study of the fracture resistance and the incidence of vertical root fracture of pulpless teeth restored with six post-and-core systems. J Prosthet Dent. 1999;81:262-269.
135 Smith RG, Davies RM. Acute lateral periodontal abscesses. Br Dent J. 1986;161:176-178.
136 Standlee JP, Caputo AA, Collard EW, et al. Analysis of stress distribution by endodontic posts. Oral Surg Oral Med Oral Pathol. 1972;33:952-960.
137 Stanley HR. Design for a human pulp study. II. Oral Surg Oral Med Oral Pathol. 1968;25:756-764.
138 Sundqvist G. Ecology of the root canal flora. J Endod. 1992;18:427-430.
139 Sutherland S, Matthews DC. Emergency management of acute apical periodontitis in the permanent dentition: a systematic review of the literature. J Can Dent Assoc. 2003;69:160.
140 Tan PL, Aquilino SA, Gratton DG, et al. In vitro fracture resistance of endodontically treated central incisors with varying ferrule heights and configurations. J Prosthet Dent. 2005;93:331-336.
141 Takahashi N, Nyvad B. Caries ecology revisited: Microbial dynamics and the caries process. Caries Res. 2008;42:409-418.
142 Tamse A, Fuss Z, Lustig J, et al. An evaluation of endodontically treated vertically fractured teeth. J Endod. 1999;25:506-508.
143 Tamse A, Fuss Z, Lustig J, et al. Radiographic features of vertically fractured, endodontically treated maxillary premolars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:348-352.
144 Tamse A. Vertical root fractures in endodontically treated teeth: diagnostic signs and clinical management. Endod Topics. 2006;13:84.
145 Tanzer JM, Livingston J, Thompson A. The microbiology of primary dental caries in humans. J Dent Ed. 2001;65:1028-1037.
146 Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504-1508.
147 Thong YL, Messer HH, Zain RB, et al. Intracanal bisphosphonate does not inhibit replacement resorption associated with delayed replantation of monkey incisors. Dent Traumatol. 2009. Epub ahead of print
148 Torabinejad M, Kiger RD. A histologic evaluation of dental pulp tissue of a patient with periodontal disease. Oral Surg Oral Med Oral Pathol. 1985;59:198-200.
149 Torneck CD. Dentin-pulp complex. In: Ten Cate R, editor. Oral Histology: Development, Structure, and Function. St. Louis: Mosby, 1998.
150 Trope M, Ray HLJr. Resistance to fracture of endodontically treated roots. Oral Surg Oral Med Oral Pathol. 1992;73:99-102.
151 Tsesis I, Tamse A, Lustig J, et al. Vertical root fractures in endodontically treated teeth part I: clinical and radiographic diagnosis. Refuat Hapeh Vehashinayim. 2006;23:13-17.
152 Tyrovola JB, Spyropoulos MN, Makou M, et al. Root resorption and the OPG/RANKL/RANK system: a mini review. J Oral Sci. 2008;50:367-376.
153 Valgi P, Gorzo I. Periodontal Abscess: Etiology, diagnosis and treatment. Fogorv. 2004;97:151-155.
154 Van Hassel HJ. Physiology of the human dental pulp. Oral Surg Oral Med Oral Pathol. 1971;32:126-134.
155 Vandenwijngaert S, Vanlerberghe K. Effects of periodontal disease and its treatment on the condition of the pulp. Rev Belge Med Dent. 2000;55:313-320.
156 Vertucci F, Seelig A, Gillis R. Root canal morphology of the human maxillary second premolar. Oral Surg Oral Med Oral Pathol. 1974;38:456-464.
157 Walters JD, Rawal SY. Severe periodontal damage by an ultrasonic endodontic device: a case report. Dent Traumatol. 2007;23:123-127.
158 Waltimo TM, Sen BH, Meurman JH, et al. Yeasts in apical periodontitis. Crit Rev Oral Biol Med. 2003;14:128-137.
159 Waltimo TM, Sirén EK, Torkko HL, et al. Fungi in therapy-resistant apical periodontitis. Int Endod J. 1997;30:96-101.
160 Walton RE, Michelich RJ, Smith GN. The histopathogenesis of vertical root fractures. J Endod. 1984;10:48-56.
161 Wang YL, Lee BS, Tseng CL, et al. In vitro study of root fracture treated by CO2 laser and DP—bioactive glass paste. J Formos Med Assoc. 2008;107:46-53.
162 Wedenberg C, Lindskog S. Macrophage colonization of infected and noninfected dental tissues in vitro. Scan J Dent Res. 1986;94:311-319.
163 Wedenberg C, Yumita S. Evidence for an inhibitor of osteoclast attachment in dentinal matrix. Endod Dent Traumatol. 1990;6:255-259.
164 Weine FS. Endodontic-Periodontal Problems. In: Weine FS, editor. Endodontic Therapy. ed 5. St. Louis, MO: Mosby; 1995:640-673.
165 Whyman RA. Endodontic-periodontic lesions. Part II: Management. N Z Dent J. 1988;84:109-111.
166 Wilcox LR, Roskelley C, Sutton T. The relationship of root canal enlargement to finger-spreader induced vertical root fracture. J Endod. 1997;23:533-534.
167 Wu MK, van der Sluis LW, Wesselink PR. Comparison of mandibular premolars and canines with respect to their resistance to vertical root fracture. J Dent. 2004;32:265-268.
168 Yates JA. Root fractures in permanent teeth: a clinical review. Int Endod J. 1992;25:150-157.
169 Yildirim G, Dalci K. Treatment of lateral root perforation with mineral trioxide aggregate: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e55-e58.
170 Youssefzadeh S, Gahleitner A, Dorffner R, et al. Dental vertical root fractures: value of CT in detection. Radiol. 1999;210:545-549.
171 Zach L, Cohen G. Pulp response to externally applied heat. Oral Surg Oral Med Oral Pathol. 1962;19:515-530.
172 Zadik Y, Sandler V, Bechor R, et al. Analysis of factors related to extraction of endodontically treated teeth. Oral Surg Oral Med Oral Pathol Endod. 2008;106:e31-e35.