CHAPTER 15 Microbiology and Treatment of Endodontic Infections
Apical periodontitis is essentially an inflammatory disease of microbial etiology primarily caused by infection of the root canal system.233 Although chemical and physical factors can induce periradicular inflammation, a large body of scientific evidence indicates that endodontic infection is essential to the progression and perpetuation of the different forms of apical periodontitis.23,110,160,287 Endodontic infection develops in root canals devoid of host defenses, either as a consequence of pulp necrosis (as a sequel to caries, trauma, periodontal disease, or iatrogenic operative procedures) or pulp removal for treatment.
Although fungi and most recently archaea and viruses have been found in association with endodontic infections,217,264,273,305 bacteria are the major microorganisms implicated in the pathogenesis of apical periodontitis. In advanced stages of the endodontic infectious process, bacterial organizations resembling biofilms can be observed adhered to the canal walls.161,167,257 Therefore, there is a current trend to include apical periodontitis in the roll of biofilm-induced oral diseases. Bacteria colonizing the root canal system enter in contact with the periradicular tissues via apical/lateral foramens or root perforations. As a consequence of the encounter between bacteria and host defenses, inflammatory changes take place in the periradicular tissues and give rise to the development of apical periodontitis. Depending on several bacterial and host-related factors, endodontic infections can lead to acute or chronic apical periodontitis.
The ultimate goal of the endodontic treatment is either to prevent the development of apical periodontitis or, in cases where the disease is already present, to create adequate conditions for periradicular tissue healing. Because apical periodontitis is an infectious disease, the rationale for endodontic treatment is unarguably to eradicate the occurring infection and/or prevent microorganisms from infecting or reinfecting the root canal or the periradicular tissues. The cardinal principle of any healthcare profession is the thorough understanding of disease etiology and pathogenesis, which provides a framework for effective treatment. In this context, understanding the microbiologic aspects of apical periodontitis is the basis for endodontic practice of high quality and founded on solid scientific insight. This chapter focuses on diverse aspects of endodontic microbiology, including pathogenetic, taxonomic, morphologic, and ecological issues. Special emphasis is also placed on the underlying principles for sound antimicrobial treatment of endodontic infections.
Apical Periodontitis as an Infectious Disease
The first recorded observation of bacteria in the root canal dates back to the 17th century and the Dutch amateur microscope builder Antony van Leeuwenhoek (1632-1723). He reported that the root canals of a decayed tooth “were stuffed with a soft matter” and that “the whole stuff” seemed to him to be alive,54 but the role of Leeuwenhoek’s “animalcules” in disease causation was unsuspected at that time. It took almost 200 years before his observation was confirmed and a cause-and-effect relationship between bacteria and apical periodontitis was suggested.
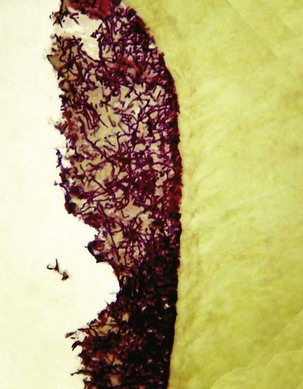
This occurred specifically in 1894, when Willoughby Dayton Miller, an American dentist working at the laboratory of Robert Koch in Berlin, Germany, published a milestone study reporting on the association between bacteria and apical periodontitis after the analysis of samples collected from root canals.155 By means of bacterioscopy of the canal samples, he found bacterial cells in the three basic morphologies known at the time: cocci, bacilli, and spirilla (or spirochetes) (Fig. 15-1). Morphologically, the endodontic microbiota was clearly different in the coronal, middle, and apical parts of the root canal. Spirochetes were found in high frequencies in abscessed cases, and an etiologic role was suspected for these bacteria. Most of the bacteria Miller had seen under light microscopy could not be cultivated using the technology available at his time. Those bacteria were conceivably anaerobic bacteria, which were only successfully cultivated about 50 to 100 years later with the advent of anaerobic culture techniques. However, it is now widely recognized that a large number of bacterial species living in diverse environments still remain to be cultivated by current technology,6,200 and the root canal is no exception (discussed later in this chapter). Based on his findings, Miller raised the hypothesis that bacteria were the causative agents of apical periodontitis.
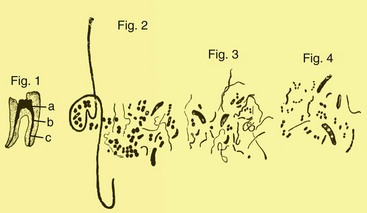
FIG. 15-1 Drawings from Miller’s classic paper showing different bacterial forms in a root canal sample observed by microscopy.
Approximately 70 years after Miller’s classic study, his assumptions were confirmed by an elegant study from Kakehashi et al.110 These authors investigated the response of the dental pulps of conventional and germ-free rats after exposure to the oral cavities. Histologic evaluation was performed and revealed that whereas pulp necrosis and apical periodontitis lesions developed in all conventional rats, the pulps of germ-free rats not only remained vital but also repaired themselves by hard-tissue formation. Dentinlike tissue sealed the exposure area and isolated the pulps again from the oral cavity.
The important role of bacteria in the etiology of apical periodontitis was further confirmed by Sundqvist’s classic study.287 This author applied advanced anaerobic culturing techniques to the evaluation of bacteria occurring in the root canals of teeth whose pulps became necrotic after trauma. Bacteria were found only in the root canals of teeth exhibiting radiographic evidence of apical periodontitis, confirming the infectious etiology of this disease. Anaerobic bacteria accounted for more than 90% of the isolates. Findings from Sundqvist’s study also served to demonstrate that in the absence of infection, the necrotic pulp tissue itself and stagnant tissue fluid in the root canal cannot induce and perpetuate apical periodontitis lesions.
Möller et al.160 also provided strong evidence about the microbial causation of apical periodontitis. Their study using monkeys’ teeth demonstrated that only devitalized pulps that were infected induced apical periodontitis lesions, whereas devitalized and noninfected pulps showed an absence of significant pathologic changes in the periradicular tissues. In addition to corroborating the importance of microorganisms for the development of apical periodontitis, this study also confirmed that necrotic pulp tissue per se is unable to induce and maintain an apical periodontitis lesion.
Routes of Root Canal Infection
Under normal conditions, the pulpodentin complex is sterile and isolated from oral microbiota by overlying enamel and cementum. In the event that the integrity of these natural layers is breached (e.g., as a result of caries, trauma-induced fractures and cracks, restorative procedures, scaling and root planning, attrition, abrasion) or naturally absent (e.g., because of gaps in the cemental coating at the cervical root surface), the pulpodentin complex is exposed to the oral environment and then challenged by microorganisms present in caries lesions, in saliva bathing the exposed area, or in dental plaque formed onto the exposed area. Microorganisms from subgingival biofilms associated with periodontal disease may also have access to the pulp via dentinal tubules at the cervical region of the tooth and lateral and apical foramens. Microorganisms may also have access to the root canal any time during or after professional endodontic intervention.
Whenever dentin is exposed, the pulp is put at risk of infection as a consequence of the permeability of normal dentin dictated by its tubular structure (Fig. 15-2). Dentinal tubules traverse the entire width of the dentin and have a conical conformation, with the largest diameter located near the pulp (mean, 2.5 µm) and the smallest diameter in the periphery, near the enamel or cementum (mean, 0.9 µm).75 The smallest tubule diameter is entirely compatible with the cell diameter of most oral bacterial species, which usually ranges from 0.2 to 0.7 µm. One might well assume that once exposed, dentin offers an unimpeded access pathway for bacteria to reach the pulp via tubules. This is not necessarily the case. It has been demonstrated that bacterial invasion of dentinal tubules occurs more rapidly in nonvital teeth than in vital ones.164 In vital teeth, outward movement of dentinal fluid and the tubular contents (including odontoblast processes, collagen fibrils, and the sheathlike lamina limitans that lines the tubules) influence dentinal permeability and can conceivably delay intratubular invasion by bacteria. Because of the presence of tubular contents, the functional or physiologic diameter of the tubules is only 5% to 10% of the anatomic diameter seen by microscopy.152 Other factors such as dentinal sclerosis beneath a carious lesion, tertiary dentin, smear layer, and intratubular deposition of fibrinogen also reduce dentin permeability and thereby limit or even impede bacterial progression to the pulp via dentinal tubules.183 Host defense molecules, such as antibodies and components of the complement system, may also be present in the dentinal fluid of vital teeth and can assist in the protection against deep bacterial invasion of dentin.3,172,173 So long as the pulp is vital, dentinal exposure does not represent a significant route of pulpal infection, except when dentin thickness is considerably reduced and dentin permeability is significantly increased.
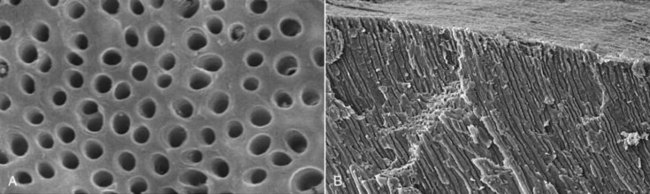
FIG. 15-2 Scanning electron micrographs of dentin showing tubules in cross-sectional (A), and longitudinal (B), views (A-B, ×850 and ×130, respectively).
Most of the bacteria in the carious process are nonmotile; they invade dentin by repeated cell division which pushes cells into tubules. Bacterial cells may also be forced into tubules by hydrostatic pressures developed on dentin during mastication.153 Bacteria inside tubules under a deep carious lesion can reach the pulp even before frank pulpal exposure.101 As mentioned, it has been assumed that the pulp will not be infected if it is still vital, and the few bacteria that reach the pulp can be of no importance, since the vital pulp can eliminate such a transient infection and rapidly clear or remove bacterial products. This efficient clearance mechanism tends to prevent injurious agents from reaching a high enough concentration to induce significant inflammatory reactions.182 On the other hand, if the vitality of the pulp is compromised and the defense mechanisms are impaired, even very few bacteria may initiate infection.
Bacteria have been isolated from traumatized teeth with necrotic pulps and apparently intact crowns.287,318 It has been suggested that bacteria from the gingival crevice or periodontal pockets might reach the root canals of teeth whose pulps became necrotic after trauma through severed blood vessels of the periodontium—a phenomenon called anachoresis.86 However, this theory has never been supported by scientific evidence. In fact, trauma can induce exposure of dentin by fracturing the crown or inducing the formation of enamel cracks. Macro- and microcracks in enamel can be present in most teeth (not only traumatized ones) and do not necessarily end at the enamel-dentin junction but extend deep into the dentin.142 A large number of dentinal tubules can be exposed to the oral environment by a single crack. These cracks can be clogged with dental plaque and provide portals of entry for bacteria. If the pulp remains vital after trauma, bacterial penetration into tubules is counteracted by the dentinal fluid and tubular contents, as discussed earlier, and pulpal health is not usually jeopardized. But if the pulp becomes necrotic as a consequence of trauma, it loses the ability to protect itself against bacterial invasion, and regardless of dentin thickness, dentinal tubules then will become true avenues through which bacteria can reach and colonize the necrotic pulp.
Direct exposure of the dental pulp to the oral cavity is the most obvious route of endodontic infection. Caries is the most common cause of pulp exposure, but bacteria may also reach the pulp via direct pulp exposure as a result of iatrogenic restorative procedures or trauma. The exposed pulp tissue enters in direct contact with oral bacteria from caries lesions, saliva, and/or plaque accumulated onto the exposed surface. Almost invariably, exposed pulps will undergo inflammation and necrosis and become infected. The time elapsed between pulp exposure and infection of the entire canal is unpredictable, but it is usually a slow process.45
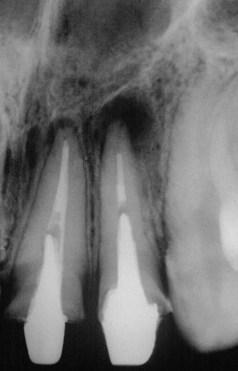
The egress of microorganisms and their products from infected root canals through apical, lateral, or furcation foramina, dentinal tubules, and iatrogenic root perforations can directly affect the surrounding periodontal tissues and give rise to pathologic changes in these tissues. However, there is no consensus as to whether the opposite is true, that is, whether subgingival biofilms associated with periodontal disease can directly cause pulpal disease. Conceptually, microorganisms in subgingival plaque biofilms associated with periodontal disease could reach the pulp by the same pathways intracanal microorganisms reach the periodontium and could thereby exert harmful effects on the pulp. However, it has been demonstrated that although degenerative and inflammatory changes of different degrees may occur in the pulp of teeth with associated marginal periodontitis, pulpal necrosis as a consequence of periodontal disease only develops if the periodontal pocket reaches the apical foramen, leading to irreversible damage to the main blood vessels that penetrate through this foramen to irrigate the pulp127 (Fig. 15-3). After the pulp becomes necrotic, periodontal bacteria can reach the root canal system via exposed dentinal tubules at the cervical area of the root or via lateral and apical foramina to establish an endodontic infectious process.
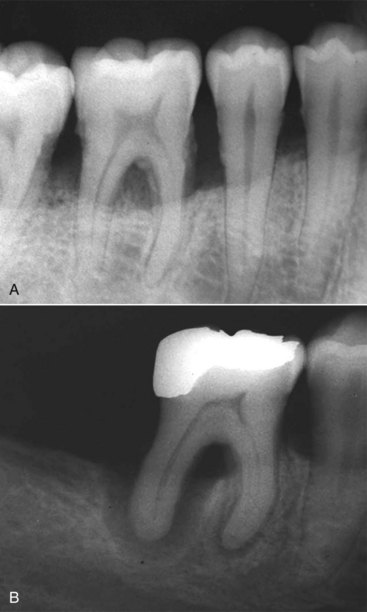
FIG. 15-3 Periodontal disease (A) mostly affects the pulp vitality when the subgingival biofilm reaches the apical foramen (B).
It has been claimed that microorganisms can reach the pulp by anachoresis. Theoretically, microorganisms are transported in the blood or lymph to an area of tissue damage, where they leave the vessel, enter the damaged tissue, and establish an infection77,203; there is no clear evidence showing that this process can represent a route for root canal infection. It has been revealed that bacteria could not be recovered from unfilled root canals when the bloodstream was experimentally infected, unless the root canals were overinstrumented during the period of bacteremia, with resulting injury to periodontal blood vessels and blood seepage into the canal.51 Another argument against anachoresis as a route of pulpal infection comes from the study by Möller et al.,160 who induced pulpal necrosis in monkeys’ teeth and reported that all cases of aseptic necrosis remained bacteria-free after 6 to 7 months of observation. Although anachoresis has been suggested to be the mechanism through which traumatized teeth with seemingly intact crowns become infected,86 current evidence indicates the main pathway of pulpal infection in these cases is dentinal exposure due to enamel cracks.141,142
Whatever the route of bacterial access to the root canal, necrosis of pulp tissue is a prerequisite for the establishment of primary endodontic infections. To reiterate: if the pulp is vital, it can protect itself against bacterial invasion and colonization. If the pulp becomes necrotic due to caries, trauma, operative procedures, or periodontal disease, it can be very easily infected. This is because host defenses do not function in the necrotic pulp tissue, and those in the periradicular tissues do not reach deep into the root canal space.
Another situation in which the root canal system is devoid of host defenses relates to cases where the pulp was removed for treatment. Microbial penetration in the canal can occur during treatment, between appointments, or even after root canal filling. The main causes of microbial introduction into the canal during treatment include remnants of dental biofilm, calculus or caries on the tooth crown; leaking rubber dam; contamination of endodontic instruments (e.g., after touch with the fingers); and contamination of irrigant solutions or other solutions of intracanal use (e.g., saline solution, distilled water, citric acid). Microorganisms can also enter the root canal system between appointments by leakage through the temporary restorative material; breakdown, fracture, or loss of the temporary restoration; fracture of the tooth structure; and in teeth left open for drainage. Microorganisms can penetrate the root canal system even after completion of the root canal filling by leakage through the temporary or permanent restorative material; breakdown, fracture, or loss of the temporary/permanent restoration; fracture of the tooth structure; recurrent decay, exposing the root canal filling material; or delay in placement of permanent restorations.242
Mechanisms of Microbial Pathogenicity and Virulence Factors
The ability of a microorganism to cause disease is regarded as pathogenicity. Virulence denotes the degree of pathogenicity of a microorganism, and virulence factors are the microbial products, structural components, or strategies that contribute to pathogenicity. Bacterial virulence factors comprise structural cellular components and released products. Bacterial strategies that contribute to pathogenicity include the ability to coaggregate and form biofilms, which confers protection against host defenses and antimicrobial agents. Some microorganisms routinely cause disease in a given host and are called primary pathogens. Other microorganisms cause disease only when host defenses are impaired and are called opportunistic pathogens. Bacteria that make up the normal microbiota are present as harmless commensals and live in balance with the host. One of the greatest beneficial effects of human microbiota is probably the tendency to protect the host from exogenous infections by excluding other microorganisms. Nevertheless, in certain situations, the balance may be disturbed by a decrease in the normal level of resistance, and then the commensal bacteria are usually the first to take advantage. Most bacteria involved with endodontic infections are normal inhabitants of the oral microbiota that exploit changes in the balance of the host/bacteria relationship, becoming opportunistic pathogens and causing endogenous infections.
Bacteria involved with the pathogenesis of primary apical periodontitis may have participated in the early stages of pulp inflammation and necrosis, or they may have gained entry into the root canal space any time after pulpal necrosis. In the former situation, involved bacteria are usually those present in the advanced front of caries lesions and from saliva bathing the affected area. Bacteria in caries lesions form authentic biofilms adhered to dentin (Fig. 15-4). Diffusion of bacterial products through dentinal tubules induces pulpal inflammation long before the tissue is exposed. After pulp exposure, the surface of the tissue can also be colonized and covered by bacteria present in the caries biofilm. The exposed pulp tissue is in direct contact with bacteria and their products and responds with severe inflammation. Some tissue invasion by bacteria may also occur. Bacteria in the battlefront have to survive the attack from the host defenses and at the same time acquire nutrients to keep themselves alive. In this bacteria/pulp clash, the latter invariably is “defeated” and becomes necrotic, so bacteria move forward and “occupy the territory,” that is, colonize the necrotic tissue. These events advance through tissue compartments, coalesce, and move towards the apical part of the canal until virtually the entire root canal is necrotic and infected (Fig. 15-5). At this stage, involved bacteria can be regarded as the early root canal colonizers or pioneer species.

FIG. 15-4 Scanning electron micrograph showing a bacterial biofilm covering dentin in a deep carious lesion. Note the presence of different bacterial morphotypes (×3500).
(From Torabinejad M, Walton RE: Endodontics: principles and practice, ed 4, Saunders/Elsevier, St. Louis, 2009.)
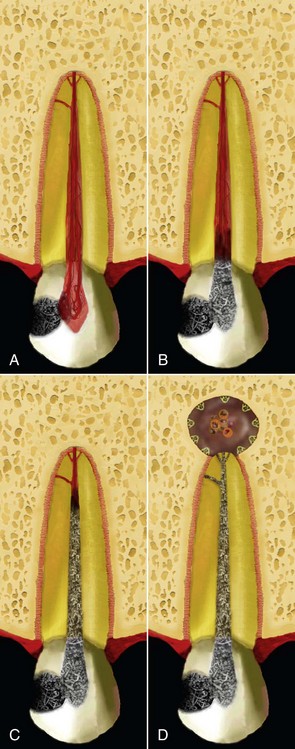
FIG. 15-5 Dynamics of pulp inflammation, necrosis, and infection from caries exposure (A) to apical periodontitis formation (D).
Bacteria colonizing the necrotic root canal induce damage to the periradicular tissues and give rise to inflammatory changes. In fact, periradicular inflammation can be observed even before the entire root canal is necrotic.7,161,279,320 Therefore, the early colonizers play an important role in the initiation of the apical periodontitis disease process. The environmental conditions in the root canal are modified by pioneer species and the disease process and may then be conducive to the establishment of bacterial groups different from the early colonizers. Once the pulp is necrotic, species other than those that participated in the initial infectious process may also have access to the canal via coronal exposure or exposed dentinal tubules, and a shift in the microbiota is observed. Rearrangement in the proportions of the pioneer species and latecomers occurs, and as the environment changes, some early colonizers are expected to no longer participate in the consortium in advanced disease. With the passage of time, the endodontic microbiota becomes more and more structurally and spatially organized.
Some virulence attributes required for pathogens to thrive in other sites may be of no value for bacteria that reach the root canal after necrosis—for instance, the ability to evade host defenses. This is because latecomers face no significant opposition from host defenses, which are no longer active in the canal after necrosis. Although colonization may appear an easy task for late colonizers, other environmental factors (e.g., interaction with pioneer species, oxygen tension, nutrient availability) will determine whether new species entering the canal will succeed in establishing themselves and join the early colonizers to make up a dynamic mixed community in the root canal. Ultimately, the root canals of teeth evincing radiographically detectable apical periodontitis lesions harbor both early colonizers that managed to stay in the canals and late colonizers that managed to adapt to the new but propitious environmental conditions.248
Bacteria exert their pathogenicity by wreaking havoc on the host tissues through direct and/or indirect mechanisms. Bacterial virulence factors that cause direct tissue harm include those that damage host cells and/or the intercellular matrix of the connective tissue. These factors usually involve secreted products, including enzymes, exotoxins, heat-shock proteins and metabolic end products.231 Furthermore, bacterial structural components, including lipopolysaccharide (LPS), peptidoglycan, lipoteichoic acid, fimbriae, flagella, outer membrane proteins and vesicles, lipoproteins, DNA, and exopolysaccharides can act as modulins by stimulating the development of host immune reactions capable not only of defending the host against infection but also of causing severe tissue destruction98,248,301 (Fig. 15-6). For instance, inflammatory and noninflammatory host cells can be stimulated by bacterial components to release chemical mediators such as cytokines and prostaglandins, which are involved in the induction of bone resorption characteristically observed in chronic apical periodontitis lesions.278 Another example of indirect damage caused by bacteria is the formation of purulent exudate in acute apical abscesses. Host defense mechanisms against bacteria emanating from the root canal appear to be the most important factor involved in formation of purulent exudate associated with abscesses. Formation of oxygen-derived free radicals, such as superoxide and hydrogen peroxide, alongside the release of lysosomal enzymes by polymorphonuclear leukocytes, gives rise to destruction of the connective extracellular matrix, leading to pus formation.300 Although bacterial products may certainly be involved in the pathogenesis of diseases related to endodontic infections, bacterial indirect destructive effects seem to be more significant in the tissue damage associated with acute and chronic apical periodontitis lesions.
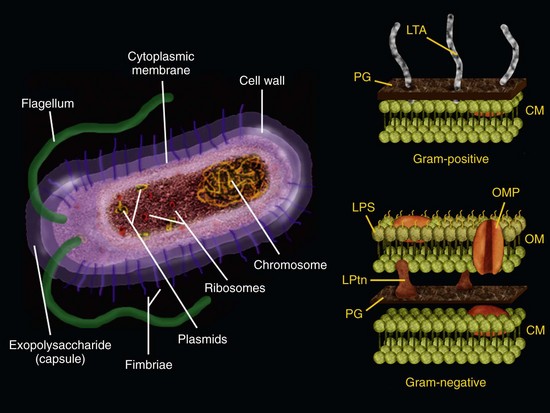
FIG. 15-6 Bacterial cell and its structural components that can act as virulence factors. Right, a detailed scheme of the bacterial cell walls from gram-positive and gram-negative bacteria. CM, Cytoplasmic membrane; LPS, lipopolysaccharide (endotoxin); LPtns, lipoproteins; LTA, lipoteichoic acid; OM, outer membrane; OMP, outer membrane protein; PG, peptidoglycan.
Depending on several factors, apical periodontitis can be chronic or acute. A chronic disease is usually associated with low virulence of the bacterial consortium involved, but this represents a persistent source of aggression to the tissues. Persistence in chronic infections is usually related to community organization in biofilms and the inaccessibility to host defenses because of the anatomic location of the infection. In chronic infections, the juxtaposition of bacterial biofilms to susceptible tissues that are not adapted to their presence triggers harmful inflammatory responses.42 Thus, bacteria in the necrotic root canal cause chronic infection by forming biofilms on the canal walls and maintaining a close contact with the apical periodontal ligament, which reacts in a damaging way because periodontal ligament and bone cells are never adapted to the presence of bacteria. An acute infection in turn is usually caused by a high virulent bacterial consortium. High virulence may be a result of synergism between species. Acute infections are usually related to bacterial cells in a planktonic state, at high numbers, with tissue invasion ability, counteracted by a diminished host resistance. It has been demonstrated for some pathogens that genes coding for many virulence factors are much more expressed in planktonic cells than in sessile (biofilm) cells, suggesting that planktonic cells are more likely to participate in acute infections.73
Apical periodontitis is a multifactorial disease that is resultant of the interplay of many host and bacterial factors. Few if any of the putative endodontic pathogens are individually capable of inducing all of the events involved in the pathogenesis of the different forms of apical periodontitis. Probably, the process requires an integrated and orchestrated interaction of the selected members of the mixed endodontic microbiota and their respective virulence attributes. Although LPS is undoubtedly the most studied and quoted virulence factor, it sounds simplistic to ascribe to this molecule all responsibility for apical periodontitis causation. This statement is further reinforced by the fact that some cases of primary infections and many cases of secondary/persistent infections harbor exclusively gram-positive bacteria. Therefore, the involvement of other factors must not be overlooked. In fact, the pathogenesis of different forms of apical periodontitis and even the same form in different individuals is unlikely to follow a stereotyped course with regard to the bacterial mediators involved.248
Biofilm and Community-Based Microbial Pathogenesis
Individual microorganisms proliferating in a habitat give rise to populations. Such populations often occur as microcolonies in the environment. Populations interact with one another to form a community. Thus, community refers to a unified assemblage of populations that coexist and interact at a given habitat. The community and habitat are part of a larger system called an ecosystem, which can be defined as a functional self-supporting system that includes the microbial community and its environment. To sum up, the following hierarchy becomes apparent: ecosystem, community, population, and the single individual (cell).
Populations perform functions that contribute to the overall community and maintain the ecological balance of the ecosystem. Each population occupies a functional role (niche) within the community. There are a limited number of niches within the community for which populations must compete. More competent populations occupy the niches and displace those less competent. As discussed later, highly structured and spatially organized microbial communities may exhibit properties that are greater than the sum of the component populations. In reality, complex microbial communities have been shown to be endowed with the ability to confront and withstand the challenges imposed by the environment by creating a mosaic of microenvironments that enable the survival and growth of the community members.146
Historically, microbiologists have faced periods of “reductionism” and “holism.”121 Reductionism is based on the idea that the whole can be understood by examining smaller and smaller pieces of it, that is, all complex systems can be completely understood in terms of their individual components. Through reductionist approaches, individual species are isolated from complex mixed communities and metabolically and genetically studied so as to allow understanding of the community by examining every single constituent. However, it has become quite apparent for the microbiota associated with many human infectious diseases that the whole is very often greater than the simple sum of its parts. This concept has prompted microbiologists to adopt a holistic approach to understand the community behavior associated with pathogenesis of many infectious diseases known to have a polymicrobial etiology. Holism holds that any component cannot be thoroughly understood except in their relation to the whole. The holistic theory has been largely employed in ecology: the interplay of the different parts composing the ecosystem will ultimately determine its properties.
Traditionally, the study of the etiology of the main human diseases (including oral diseases) has been based on reductionism. More recently, it has been recognized that the biofilm (dental plaque) associated with caries and periodontal diseases represents a sophisticated community that exert functions essential for the biofilm architecture and physiology, with consequent pathogenetic implications. Recent evidence indicates that apical periodontitis can also be due to collaborative activities of a biofilm community.
Community profiling studies143,209,261 revealed that bacterial composition of the endodontic microbiota differs consistently between individuals. This indicates that apical periodontitis has a heterogeneous etiology, where multiple bacterial combinations can play a role in disease causation. Moreover, community structure differs significantly between different disease forms (e.g., chronic apical periodontitis versus acute apical abscess),219,261 suggesting existence of a pattern associated with each form. Differences are still observed between individuals suffering from the same disease, disclosing an interindividual variability even more pronounced when different geographic locations are studied. There is seemingly a geography-related pattern, where individuals from a given location tend to cluster together based on similarities of the microbial community profiles.
The high interindividual variability observed for samples from the same clinical disease indicates that different compositions of bacterial communities can result in similar disease outcomes. Identification of community members can reveal the presence of some species or groups of species that may be important for the causation of some forms of disease. It is reasonable to realize that different magnitudes of disease, based on intensity of signs and symptoms, may be related to the species composition of the community. Indeed, it has been demonstrated that, despite individual uniqueness, bacterial communities associated with acute abscesses (severe pain and swelling) are more similar among them in comparison to bacterial communities associated with chronic apical periodontitis (no pain).219,261 Although there may be no specific species related to some forms of disease, specificity seems to be related to community level, and certain communities (species composition) are specifically associated with some forms of apical periodontitis. From the perspective of the single-pathogen concept, apical periodontitis can be considered as having no specific microbial etiology. However, based on the community-as-a-pathogen concept, it is possible to infer that some communities are more related to certain forms of the disease.219,261
Biofilm and Bacterial Interactions
The community-forming ability can be regarded as essential for microbial survival in virtually all environments. Indeed, the vast majority of microorganisms in nature invariably grow and function as members of metabolically integrated communities, or biofilms.42,147 Biofilm can be defined as a sessile multicellular microbial community characterized by cells that are firmly attached to a surface and enmeshed in a self-produced matrix of extracellular polymeric substance (EPS), usually polysaccharide42,55 (Fig. 15-7). The ability to form biofilms has been regarded as a virulence factor,90 and biofilm infections account for an estimated 65% to 80% of bacterial infections that affect humans in the developed world.41 Given its importance in varied aspects, there has been a high level of interest in the study of biofilm properties, not only in medical microbiology but also in different sectors of industrial and environmental microbiology.
Biofilms are not merely passive assemblages of bacterial cells that are stuck to surfaces; they are structurally and dynamically organized complex biologic systems. Bacterial cells in biofilms form microcolonies (±15% by volume) that are embedded and nonrandomly distributed in the EPS matrix (±85% by volume) and separated by water channels.44,55,276,281 Microcolonies are usually shaped as “towers” or “mushrooms.” Dental biofilms can reach up to 300 or more cell layers in thickness.276 Individual microcolonies may consist of a single bacterial species but more frequently are composed of several different species in a mixed community.
As the biofilm matures on the surface, extracellular polysaccharides are continually synthesized to form an extracellular matrix that eventually may constitute as much as 85% of the volume of the biofilm.41 Although the matrix is primarily composed of polysaccharides, it can also contain proteins and nucleic acids.90 The matrix is not only important physically as part of the scaffold that determines the biofilm structure, but it is also biologically active and can retain nutrients, water, and essential enzymes within the biofilm.5 The matrix can also protect the biofilm community from exogenous threats and may participate in adherence to the surface.
Community members form distinct populations or microcolonies separated by open water channels that traverse the biofilm matrix and create primitive circulatory systems.43 Fluid in these channels carries substrate, end products of bacterial metabolism and signal molecules involved in bacterial interactions.27 Thus, vital nutrients and communication molecules can diffuse, and wastes can be washed out through these channels.
Microcolonies that form in the biofilm arise from surface colonization by planktonic (unattached) bacterial cells. During the early stages of biofilm formation, bacteria bind to many host proteins and coaggregate with other bacteria. These interactions lead to changes in growth rate, gene expression, and protein production. It has been demonstrated by proteomic techniques or DNA arrays that genes expressed by cells in biofilms differed by 20% to 70% from those expressed by the same cells growing in planktonic culture.20,174,222 Thus, bacteria in biofilms adopt a radically different phenotype compared to their planktonic counterparts. Within biofilms, sophisticated systems of cell-cell communication (quorum sensing) are also used by some bacteria to coordinate gene expression. Phenotypic heterogeneity in biofilms is also observed as a result of exposure of microcolonies to a variety of gradients (e.g., oxygen tension, pH, osmolarity, type and amounts of nutrients, cell density), which contribute to form diverse microenvironments throughout the biofilm structure.
Biofilm Community Lifestyle
As a community of bacteria, biofilms have a collective physiology, responding in concert to environmental challenges. Many naturally occurring biofilms have a highly diverse microbiota. The component species form populations (microcolonies) that are not randomly distributed but are spatially and functionally organized throughout the mixed community.
The properties displayed by a mixed biofilm community are mostly dictated by the interactions between populations, which create novel physiologic functions that cannot be observed with individual components. Populations are strategically positioned for optimal metabolic interaction, and the resultant architecture favors the ecological role of the community in the ecosystem. The biofilm community lifestyle affords a number of advantages to colonizing bacteria, including those discussed in the sections that follow.148
A Broader Habitat Range for Growth
The metabolism of early colonizers alters the local environment, setting the stage for attachment and growth of latecomers (including more fastidious species). This may include increased availability of some types of nutrients, removal of harmful metabolic end products, and the development of appropriate physicochemical conditions (e.g., reduced oxygen tension and redox potential). As a consequence, the diversity of the microbiota increases over time because of bacterial succession.
Increased Metabolic Diversity and Efficiency
Nutrients are limited in most ecosystems, so the communities that can fix and process their resources most efficiently will thrive and be perpetuated. Bacteria living in mixed communities take part in a number of nutritional interrelationships, and food webs develop. For instance, products of the metabolism of one species may become the main source of nutrients for other species. Also in food webs, the concerted action of interacting species is required for the sequential breakdown of complex host-derived substrates to simpler products, which can favor less competent species. Many complex host macromolecules, especially glycoproteins, can only be degraded efficiently by mixed consortia of oral bacteria. Byproducts of the degradation of complex nutrients are trapped in the biofilm matrix and shared with other community members.
Protection From Competing Microorganisms, Host Defenses, Antimicrobial Agents, and Environmental Stress
Neighboring cells of a different species can produce enzymes such as beta-lactamases, catalase, and proteinases that are retained in the biofilm matrix and protect other bacteria against antimicrobials and host defenses. The same is true for metabolites and bacteriocins, which can inhibit competing species. The biofilm matrix also confers physical protection against phagocytosis.
Genetic Exchanges
Cell-cell associations and the biofilm architecture facilitate horizontal gene transfer in the community. Potential mechanisms mediating genetic exchange in biofilms include conjugation, transformation, and transduction. The main consequences of genetic exchanges among community members are related to the acquisition and dissemination of virulence and antibiotic-resistance genes.
Enhanced Pathogenicity
To cause disease, bacteria must adhere to host surfaces, obtain nutrients from the host, and multiply, invade tissues, overcome or evade the host defenses, and induce tissue damage. A diverse range of virulence traits are required for these particular stages of the disease process, and it is highly likely that each will require the concerted action of bacteria in a community. Similarly, it is possible that certain species can have more than one role in disease, and different species can perform similar functions. This helps explain why communities with different bacterial composition can be found in different individuals with similar disease. In mixed communities, a broad spectrum of relationships may arise between the component species, ranging from no effect or reduced pathogenicity to additive or synergistic pathogenic effects. Endodontic abscesses are examples of polymicrobial infections whereby bacterial species that individually have low virulence and are unable to cause disease can do so when in association with others as part of a mixed consortium (pathogenic synergism).30
Resistance to Antimicrobial Agents
From a clinical standpoint, biofilm increased resistance to antimicrobial agents is of special concern. Bacteria arranged in biofilms are more resistant to antibiotics than the same cells grown in planktonic state. The antibiotic concentration required to kill bacteria in the biofilm is about 100 to 1000 times higher than that needed to kill the same species in planktonic state.145 There are several possible mechanisms involved with biofilm resistance to antimicrobials.
Biofilm Structure May Restrict Penetration of Antimicrobial Agents
The agent may adsorb to and inhibit the bacteria at the biofilm surface, leaving cells deeply located in the biofilm relatively unaffected. The matrix in biofilms can also bind and retain neutralizing enzymes at concentrations that could inactivate the antimicrobial agent.280
Altered Growth Rate of Biofilm Bacteria
Many antibiotics can freely penetrate the biofilm matrix, but cells are often still protected. The occurrence of starved bacteria entering the stationary phase in biofilms seems to be a significant factor in the resistance of biofilm populations to antimicrobials. Bacteria grow slowly under conditions of low availability of nutrients in an established biofilm and as a consequence are much less susceptible than faster-dividing cells. Most antibiotics require at least some degree of cellular activity to be effective. Therefore, bacterial cells in stationary phase might represent a general mechanism of antibiotic resistance in the biofilm.90
Presence of “Persister” Bacteria
Increased tolerance of some biofilms to antibiotics may be largely due to the presence of a subpopulation of specialized survivor cells known as persisters.113 It remains unclear whether these bacteria actually represent a distinct phenotype or are simply the most resistant cells within a population.90
Quorum Sensing—Bacterial Intercommunication
Quorum sensing is a term currently used for systems based on cell-cell communication that regulate gene expression in a cell density–dependent manner.180 Quorum-sensing systems capacitate bacteria to behave collectively as a group. This phenomenon has been described in both gram-positive and gram-negative bacterial species.10,57,317 Quorum sensing involves the production, release, and subsequent detection of diffusible signaling molecules called autoinducers. Two predominant types of autoinducers, N-acyl-l-homoserine lactones (AHLs) and posttranslationally modified peptides, are used by gram-negative and gram-positive bacteria, respectively. For example, the human pathogens Pseudomonas aeruginosa and Staphylococcus aureus employ AHLs and peptides, respectively, to control the expression of multiple virulence genes in concert with cell population density. Several other small-molecule quorum-sensing autoinducers have recently been discovered, including Pseudomonas quinolone signal (PQS) and AI-2.35
As members of a bacterial population producing and releasing autoinducers multiply, the extracellular concentration of these signaling molecules also increases. When autoinducers reach a crucial threshold level, the group responds with a population-wide alteration in gene expression, activating or repressing target genes.181 Since alterations in gene expression are linked to the presence of autoinducers, bacteria can perform specific functions only when living in groups. Such behavior provides some advantages to a bacterial population, affording adaptability to and protection against threatening environments.
Quorum-sensing systems are known to regulate virulence, competence for DNA uptake, entry into stationary phase, and biofilm formation.35,46,184 With regard to virulence, quorum sensing allows the community to regulate expression of virulence genes in accordance with cell density. Thus, some opportunistic pathogens express virulence factors in response to sensing their own cell density. Using quorum sensing, bacteria can amass a high cell density before virulence factors are expressed, and in so doing, the bacteria are able to make a consolidated attack to overcome the host defenses and establish infection.116 Teleologically, it appears bacteria were able to strategically wait for a critical number of cells to be reached and then start inducing damage, not giving the host sufficient time to mount an effective defense response. In reality, with regard to energy yield, it would be difficult for bacteria to produce some virulence factors before a critical community density is reached. For instance, production of proteinases by a few cells are not expected to have significant impact on the host environment, representing “wasted energy” for the growing population.180
The process of biofilm formation, including surface attachment, proliferation, matrix production, and detachment, is also partially controlled by quorum sensing.74 Moreover, bacteria can monitor crowding as a measure of competition for nutrients. At high cell density, there may be too much competition to utilize available nutrients for growth, and starvation is anticipated. If bacteria are already starved and crowded, as they often are when growing as a biofilm, it may be advantageous to enter stationary phase. Entry into stationary phase dramatically alters patterns of gene expression to allow extended cell survival in the absence of nutrients.128 Control of cell density in biofilms is also important to prevent water circulatory channels, essential for biofilm physiology, from being clogged and then inactivated by cells under excessive growth.
Several oral bacteria have been demonstrated to produce quorum-sensing signaling molecules,71,194,321 indicating that bacteria in endodontic biofilms may also communicate with one another through this system.
Methods for Microbial Identification
The endodontic microbiota has been traditionally investigated by microbiologic culture methods. Culture is the process of propagating microorganisms in the laboratory by providing them with the required nutrients and proper physicochemical conditions, including temperature, moisture, atmosphere, salt concentration, and pH.272 Essentially, culture analyses involve the following steps: sample collection and transport, dispersion, dilution, cultivation, isolation, and identification. Endodontic samples are collected and transported to the laboratory in a viability-preserving, nonsupportive, anaerobic medium. They are then dispersed by sonication or vortex mixing, diluted, distributed onto various types of agar media, and cultivated under aerobic or anaerobic conditions. After a suitable period of incubation, individual colonies are subcultivated and identified on the basis of multiple phenotype-based aspects, including colony and cellular morphology, gram-staining pattern, oxygen tolerance, comprehensive biochemical characterization, and metabolic end-product analysis by gas-liquid chromatography. The outer cellular membrane protein profile as examined by gel electrophoresis, fluorescence under ultraviolet light, and susceptibility tests to selected antibiotics can be needed for identification of some species.61 Marketed packaged kits that test for preformed enzymes have also been used for rapid identification of several species.
Culture analyses of endodontic infections have provided a substantial body of information about the etiology of apical periodontitis, composition of the endodontic microbiota in different clinical conditions, effects of treatment procedures in microbial elimination, susceptibilities of endodontic microorganisms to antibiotics, and so on. Advantages and limitations of culture methods are listed in Box 15-1. As one can tell, however, some important limitations of culture methods make a comprehensive analysis of the endodontic microbiota difficult to achieve.
The difficulties in culturing or identifying many microbial species are of special concern. Unfortunately, not all microorganisms can be cultivated under artificial conditions, and this is simply because the nutritional and physiologic needs of most microorganisms are still unknown. Investigations of many aquatic and terrestrial environments using culture-independent methods have revealed that the cultivable members of these systems represent less than 1% of the total extant population.6,314 Furthermore, 50% to 80% of bacterial species composing the microbiota associated with diverse human sites, including the oral cavity, represent unknown and still uncultivated bacteria.2,58,120,185,282
That a given species has not been cultivated does not imply that this species will remain indefinitely impossible to cultivate. A myriad of obligate anaerobic bacteria were uncultivable 100 years ago, but further developments in anaerobic culturing techniques have to a large extent helped to solve this problem. It must be assumed that no single method or culture medium is suitable for isolating the vast diversity of microorganisms present in most environments.83 While we remain relatively unaware of the requirements for many bacteria to grow, identification methods not based on cultivability are required.
In some situations, even the successful cultivation of a given microorganism does not necessarily mean that this microorganism can be successfully identified. Culture-dependent identification is based on phenotypic traits observed in reference strains, with predictable biochemical and physical properties under optimal growth conditions. However, many phenotype-related factors can lead to difficulties in identification and even to misidentification.19,25,295 As a consequence of all these factors, phenotype-based identification does not always allow an unequivocal identification.
To sidestep the limitations of culture, tools and procedures based on molecular biology have become available and have been substantially improved to achieve a more realistic description of the microbial world without the need for cultivation (Fig. 15-8). Molecular biology techniques have been widely used to directly identify microorganisms in samples without the need for cultivation. In addition, molecular technology has also been applied to reliably identify cultivated bacteria, including strains with ambiguous or aberrant phenotypic behavior, rare isolates, poorly described or uncharacterized bacteria, and newly named species.*
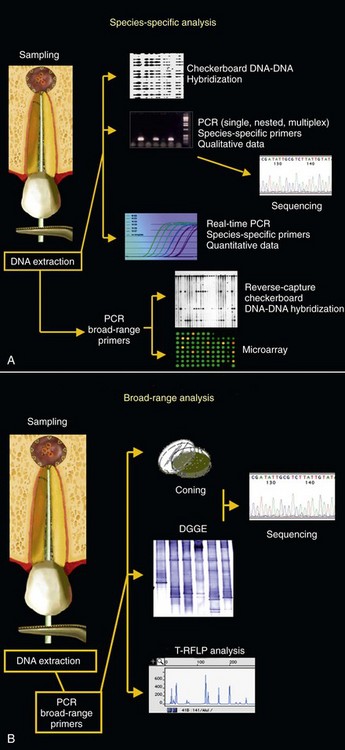
FIG. 15-8 Molecular biology methods used (or with potential to be used) in the study of endodontic infections. The choice for a particular technique will depend on the type of analysis to be performed. Some methods can be used for detection of target microbial species or groups (A) and others for a broader analysis of the microbiota (B).
Molecular approaches for microbial identification rely on certain genes that contain revealing information about the microbial identity. Of the several genes that have been chosen as targets for bacterial identification, the 16S rRNA gene (or 16S rDNA) has been the most widely used because it is universally distributed among bacteria, is long enough to be highly informative and short enough to be easily sequenced, possesses conserved and variable regions, and affords reliability for inferring phylogenetic relationships.319 Similarly, the 18S rRNA gene of fungi and other eukaryotes has also been extensively used for identification of these organisms.
Data from 16S rRNA gene sequences can be used for accurate and rapid identification of known and unknown bacterial species, using techniques that do not require cultivation. The 16S RNA gene of virtually all bacterial species in a given environment, including still uncultivated and uncharacterized bacteria, can be amplified by polymerase chain reaction (PCR) using broad-range (or universal) primers that are complementary to conserved regions of this gene. Sequencing of the variable regions flanked by the broad-range primers will provide information for accurate bacterial identification. Primers or probes that are complementary to variable regions can also be designed to detect specific target species directly in clinical samples (see Fig. 15-4).
Many molecular methods for the study of microorganisms exist; the choice of a particular approach depends on the questions being addressed (see Fig. 15-5). Broad-range PCR followed by cloning and sequencing can be used to unravel the breadth of microbial diversity in a given environment. Microbial community structures can be analyzed via fingerprinting techniques, such as denaturing gradient gel electrophoresis (DGGE) and terminal restriction fragment length polymorphism (T-RFLP). Fluorescence in situ hybridization (FISH) can measure abundance of target species and provide information on their spatial distribution in tissues. Among other applications, DNA-DNA hybridization arrays (checkerboard techniques, DNA microarrays), species-specific single PCR, nested PCR, multiplex PCR, and quantitative real-time PCR can be used to survey large numbers of clinical samples for the presence of target species. Variations in PCR technology can also be used to type microbial strains.
As with any other method, molecular methods have their own advantages and limitations (Box 15-2).
BOX 15-2 Advantages and Limitations of Molecular Biology Methods
Impact of Molecular Methods in Endodontic Microbiology
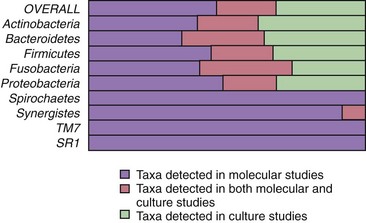
Traditionally, endodontic infections have been studied by means of culture approaches. Such studies identified a set of species thought to play an important role in the pathogenesis of apical periodontitis.288 More recently, not only have findings from culture-based methods been confirmed, but they have also been significantly supplemented with those from culture-independent molecular biology techniques.236 Molecular methods have confirmed and strengthened the association of many cultivable bacterial species with apical periodontitis and have also revealed new suspected endodontic pathogens.250 The list of candidate pathogens has expanded to include culture-difficult species or as-yet-uncultivated bacteria that had never been found in endodontic infections by culturing approaches. The results from molecular studies impact remarkably on the knowledge of bacterial diversity in endodontic infections. More than 400 different bacterial species have already been detected in different types of endodontic infections. Of these, about 45% were exclusively reported by molecular biology studies, compared to 32% detected by culture studies alone. Twenty-three percent of the total bacterial species richness has been detected by application of both culture and molecular studies (Fig. 15-9). As a consequence, it becomes quite apparent that the endodontic microbiota has been refined and redefined by molecular methods.250
Types of Endodontic Infections
Endodontic infections can be classified according to anatomic location (intraradicular or extraradicular infection). Intraradicular infection is caused by microorganisms colonizing the root canal system and can be subdivided into three categories according to the time microorganisms entered the root canal system: primary infection, caused by microorganisms that initially invade and colonize necrotic pulp tissue (initial or “virgin” infection); secondary infection, caused by microorganisms not present in the primary infection but introduced in the root canal at some time after professional intervention (i.e., secondary to intervention); and persistent infection, caused by microorganisms that were members of a primary or secondary infection and in some way resisted intracanal antimicrobial procedures and were able to endure periods of nutrient deprivation in treated canals. Both persistent and secondary infections are for the most part clinically indistinguishable, except for cases where signs and/or symptoms of infection arise in a previously noninfected tooth—a typical example of secondary infection. Extraradicular infection is characterized by microbial invasion of the inflamed periradicular tissues and is a sequel to the intraradicular infection. Admittedly, extraradicular infections can be dependent on or independent of the intraradicular infection.
Diversity of the Endodontic Microbiota
Microbiota is a collective term for microorganisms and should replace terms such as flora and microflora, which perpetuate an outdated classification of microorganisms as plants.52 Diversity refers to the number of different species present (richness) and their relative abundance (evenness) in a given ecosystem.105
The oral cavity harbors one of the highest accumulations of microorganisms in the body. Even though viruses, archaea, fungi, and protozoa can be found as constituents of the oral microbiota, bacteria are by far the most dominant inhabitants of the oral cavity. There are an estimated 10 billion bacterial cells in the oral cavity,156 and culture-independent studies (microscopy and molecular biology technologies) have shown that over 50% to 60% of the oral microbiota still remains to be cultivated and fully characterized.2,275 A high diversity of bacterial species has been revealed in the oral cavity by culturing approaches, but application of molecular biology methods to the analysis of the bacterial diversity has revealed a still broader and more diverse spectrum of extant oral bacteria. Over 700 bacterial taxa have been found in the human oral cavity, and phylogenetic studies have revealed that these taxa fall into 13 separate phyla: Firmicutes, Bacteroidetes, Actinobacteria, Fusobacteria, Proteobacteria, Spirochaetes, Synergistes, TM7, Sulphur River 1 (SR1), Chloroflexi, Cyanobacteria, Deinococcus, and Acidobacteria.1,112,133,185,186
Modern anaerobic culture and sophisticated molecular biology techniques have disclosed important aspects of the endodontic microbiota in the different types of infection. Of particular interest, they have allowed the assessment of the microbial diversity in endodontic infections, collectively revealing that more than 400 different microbial species/phylotypes have been found in infected root canals, usually in combinations involving many species in primary infections and a few ones in secondary/persistent infections. Endodontic bacteria fall into 9 of the 13 phyla that have oral representatives* (Fig. 15-10). Fungi and archaea have been only occasionally found in endodontic infections.
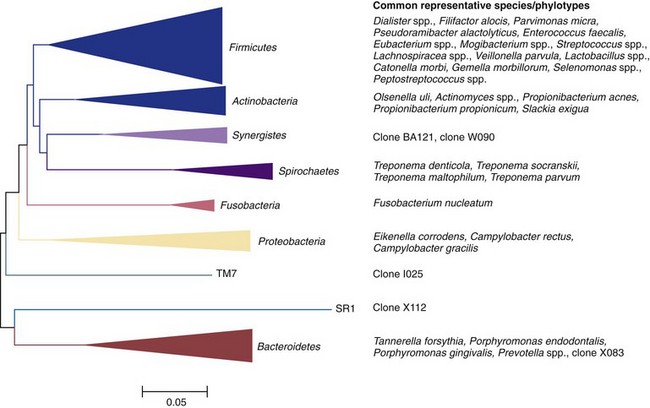
FIG. 15-10 Bacterial phyla with their respective endodontic representatives. Right, example species or phylotypes for each phylum.
Endodontic infections develop in a previously sterile place that does not contain a normal microbiota. Any species found has the potential to be an endodontic pathogen or at least play a role in the ecology of the endodontic microbial community. Culture and molecular studies reveal only prevalence of species; consequently, only association can be inferred. Causation is usually surmised on the basis of both frequency of detection and potential pathogenicity (in animal models or association with other human diseases), and several species have emerged as candidate or putative endodontic pathogens. The following sections discuss specific aspects of each type of endodontic infection.
Primary Intraradicular Infection
Microbial Composition and Diversity
Primary intraradicular infection is infection of the necrotic pulp tissue (Fig. 15-11). Participating microorganisms can have been involved in earlier stages of pulp invasion (usually via caries) that culminated in inflammation and further necrosis, or they can be latecomers that took advantage of the environmental conditions in the root canal after pulp necrosis.
FIG. 15-11 Apical periodontitis due to primary intraradicular infection. Pulp is necrotic and lesion size is usually directly proportional to complexity of the microbiota involved.
Primary infections are characterized by a mixed community conspicuously dominated by anaerobic bacteria. The number of bacterial cells may vary from 103 to 108 per root canal.220,265,287,306 Molecular studies have disclosed a mean of 10 to 20 species/phylotypes per infected canal.162,208,250,261 Canals of teeth with sinus tracts exhibit a mean number of 17 species.208 The size of the apical periodontitis lesion has been shown to be proportional to the number of bacterial species and cells in the root canal.208,260,287 A molecular study208 demonstrated that the number of taxa per canal was clearly in direct proportion to the lesion size: small lesions (<5 mm) harbored about 12 taxa, lesions from 5 to less than 10 mm harbored 16 taxa, and lesions over 10 mm harbored about 20 species. Some canals associated with large lesions may harbor even more than 40 taxa208; the larger the lesion, the higher the bacterial diversity and density in the canal.
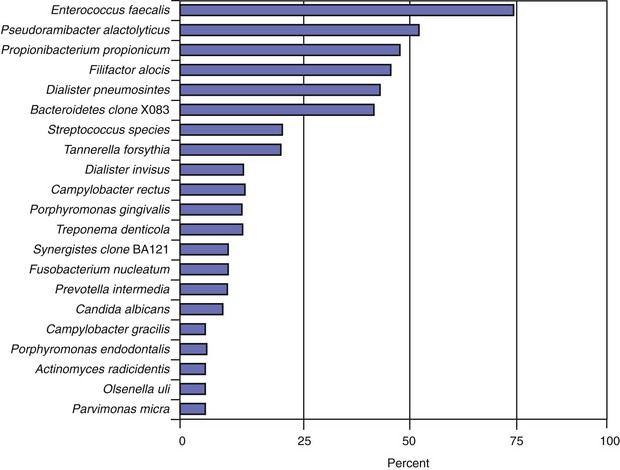
The most prevalent named bacterial species detected in primary infections, including abscessed cases, belong to diverse genera of gram-negative (Fusobacterium, Dialister, Porphyromonas, Prevotella, Tannerella, Treponema, Campylobacter, and Veillonella) and gram-positive (Parvimonas, Filifactor, Pseudoramibacter, Olsenella, Actinomyces, Peptostreptococcus, Streptococcus, Propionibacterium, and Eubacterium) bacteria.* Bacterial prevalence in primary infections may vary from study to study as a function of several factors, such as sensitivity and specificity of the detection and identification methods, sampling technique, geographic location, and accuracy or divergence in clinical diagnosis and disease classification. Even so, the species most frequently detected are expected to be the same in most well-conducted studies. Figs. 15-12 to 15-14 display the most frequently detected species associated with chronic apical periodontitis, acute apical periodontitis, and acute apical abscesses, respectively, as revealed by studies from the authors’ group using a highly sensitive molecular biology technique.

FIG. 15-12 Prevalence of bacteria detected in primary infections of teeth with chronic apical periodontitis. Data from the authors’ studies using a taxon-specific nested-polymerase chain reaction protocol.
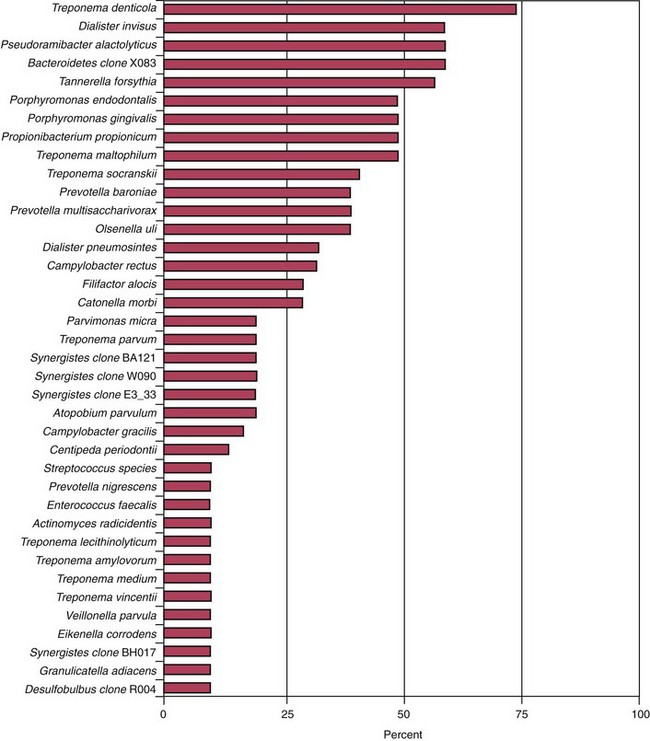
FIG. 15-13 Prevalence of bacteria detected in primary infections of teeth with acute apical periodontitis. Data from the authors’ studies using a taxon-specific nested-polymerase chain reaction protocol.
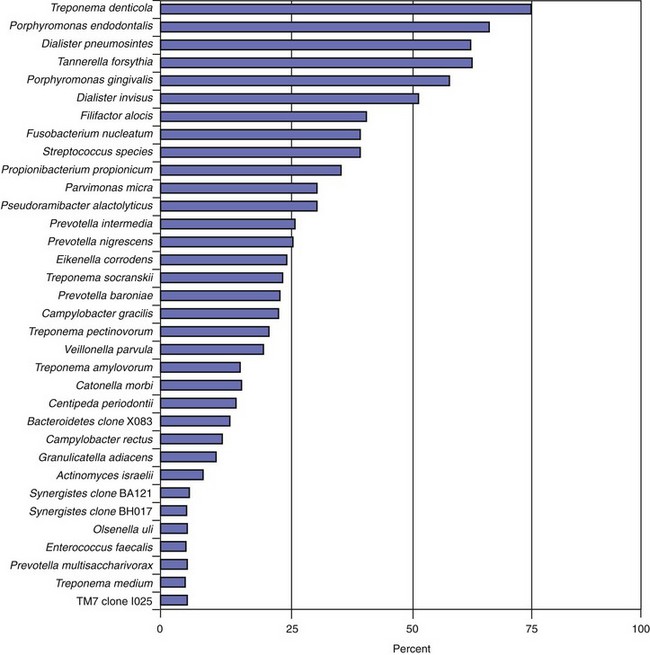
FIG. 15-14 Prevalence of bacteria detected in primary infections of teeth with acute apical abscesses. Data from the authors’ studies using a taxon-specific nested-polymerase chain reaction protocol.
About 40% to 55% of the endodontic microbiota in primary infections is composed of species still uncultivated.162,219 A molecular study revealed that 55% and 40% of the taxa detected in association with chronic apical periodontitis and acute apical abscesses, respectively, were uncultivated phylotypes.219 As for their abundance in these infections, as-yet-uncultivated phylotypes corresponded to 38% and 30% of the clones sequenced from samples of chronic apical periodontitis and abscesses, respectively.219 Molecular studies investigating the breadth of bacterial diversity in infected root canals have disclosed the occurrence of uncultivated phylotypes belonging to several genera, including Synergistes, Dialister, Prevotella, Solobacterium, Olsenella, Fusobacterium, Eubacterium, Megasphaera, Veillonella, and Selenomonas, as well as phylotypes related to the family Lachnospiraceae or the TM7 phylum.† Some uncultivated phylotypes can even be among the most prevalent bacteria in primary intraradicular infections, and others may be associated with pain.219 Two of the most prevalent such phylotypes found in endodontic infections include Synergistes clone BA121 and Bacteroidetes clone X083.207,208,251,253,255 Detection of these phylotypes in samples from endodontic infections suggests that they can be previously unrecognized bacteria that play a role in the pathogenesis of different forms of apical periodontitis. The fact that they have not yet been cultivated and phenotypically characterized does not mean that they are not important.
Symptomatic Infections
Acute apical periodontitis and acute apical abscesses are typical examples of symptomatic endodontic infections. In these cases, the infection is located in the canal, but it has also reached the periradicular tissues and, in abscessed cases, can spread to other anatomic spaces. Acute apical abscesses are caused by bacteria that egress the infected root canal and invade the periradicular tissues to establish an extraradicular infection and evoke purulent inflammation. Clinically, the disease leads to pain and/or swelling and has the potential to diffuse to sinuses and other facial spaces of the head and neck to form a cellulitis or other complications (Fig. 15-15). The microbiota involved in endodontic abscesses is mixed and dominated by anaerobic bacteria47,115,122,219,261 (see Fig. 15-14). Direct comparisons using molecular technology reveal an average of 12 to 18 taxa per abscess case, compared to 7 to 12 taxa present in root canals from chronic cases.219,261 Uncultivated phylotypes constitute approximately 40% of the taxa found in abscesses and collectively represent more than 30% of the 16S rRNA gene sequences retrieved in clone libraries.219
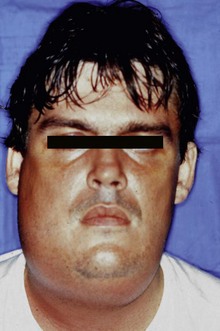
FIG. 15-15 Acute apical abscess. The infection has spread to other anatomic spaces to form cellulitis.
Whereas microbial causation of apical periodontitis is well established, there is no strong evidence disclosing specific involvement of a single species with any particular sign or symptom of apical periodontitis. Some gram-negative anaerobic bacteria have been suggested to be involved with symptomatic lesions,* but the same species may also be present in somewhat similar frequencies in asymptomatic cases,† so factors other than the mere presence of a given putative pathogenic species may play a role in the etiology of symptomatic endodontic infections.232,237 These factors could include differences in virulence ability among strains of the same species; bacterial interactions resulting in additive or synergistic effects among species in mixed infections; number of bacterial cells (load); environmental cues regulating expression of virulence factors; host resistance; and concomitant herpesvirus infection. Association of some or all of these factors (instead of an isolated event) is likely to determine the occurrence and intensity of symptoms.232,237
†References 15, 69, 89, 109, 262, and 263.
Cross-sectional studies suggest that bacterial succession occurs before symptoms arise.261 At a given moment during the endodontic infectious process, the microbiota may reach a certain degree of pathogenicity that elicits acute inflammation at the periradicular tissues, with consequent development of pain and sometimes swelling. Molecular studies using DGGE or T-RFLP analysis revealed that the structure of the endodontic bacterial communities in symptomatic teeth is significantly different from that of asymptomatic teeth.261 Differences are represented by different dominant species in the communities and larger numbers of species in symptomatic cases. A shift in the structure of the microbial community is likely to occur before appearance of symptoms. Such a shift is probably due to the arrival of new pathogenic species or variations and rearrangements in the number of members of the bacterial community. Differences in the type and load of dominant species and the resulting bacterial interactions may affect virulence of the whole bacterial community. This is in agreement with the community-as-a-pathogen concept discussed earlier.
Geographic Influence
Findings from laboratories in different countries are often quite different regarding the prevalence of the species involved in endodontic infections. Although these differences may be attributed to variations in identification methodologies, a geographic influence in the composition of the root canal microbiota has been suspected. Studies using molecular biology techniques directly compared the endodontic microbiota of patients residing in different geographic locations and suggested that significant differences in the prevalence of some important species can actually exist.14,204,241 In a more holistic approach, analysis of the bacterial community profiles of the microbiota associated with acute apical abscesses from the United States and Brazilian patients also revealed a geography-related pattern, with several species being exclusive for each location and others shared by the two locations, showing great differences in prevalence.143 The factors that can lead to differences in the composition of the endodontic microbiota and the impact of these differences on therapy, particularly in abscessed cases requiring systemic antibiotic therapy, remain elusive.
Spatial Distribution of the Microbiota—Anatomy of Infection
Knowledge of microbial location and organization within the root canal system assumes special importance to understanding the disease process and establishing effective antimicrobial therapeutic strategies. Most of the knowledge of the structure of the endodontic microbiota comes from morphologic studies,161,167,227,257 but these do not usually provide information about bacterial identity and numbers. Consequently, it is not possible to delineate the role of the visualized bacteria in the disease process. Because every bacterial cell observed in the root canal system might be an endodontic pathogen, findings from morphologic studies should be used to understand the topography of the root canal infection and establish therapeutic measures in an attempt to completely eradicate the infection or at least reduce the bacterial load to thresholds compatible with periradicular tissue healing.
Again, mounting evidence suggests that apical periodontitis, like caries and periodontal diseases, is also a biofilm-induced disease. Morphologic studies have shown that the root canal microbiota in primary infections is dominated by bacterial morphotypes that include cocci, rods, filaments, and spirilla (spirochetes) (Fig. 15-16), and fungal cells are sporadically found227,257 (Fig. 15-17). Bacteria colonizing the root canal system usually grow in sessile biofilms adhered to the dentinal walls and also as coaggregates (cells with distinct morphologies), aggregates (cells with the same morphology), and planktonic cells suspended in the fluid phase of the main canal161,167,257 (Fig. 15-18). Lateral canals and isthmuses connecting main canals may be clogged with bacterial cells primarily organized in biofilms166,202 (Figs. 15-19 and 15-20).
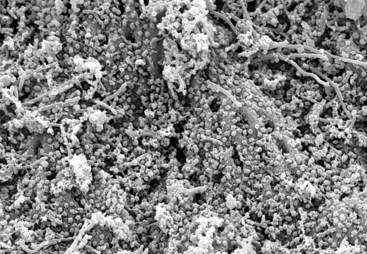
FIG. 15-16 Mixed bacterial population colonizing the root canal wall. Cocci are the predominant forms, but rods, filaments, and spirochetes are also observed. In some areas, coccoid cells are relatively apart from each other (×2200).
(From Siqueira JF Jr, Rôças IN, Lopes HP: Patterns of microbial colonization in primary root canal infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:174, 2002.)
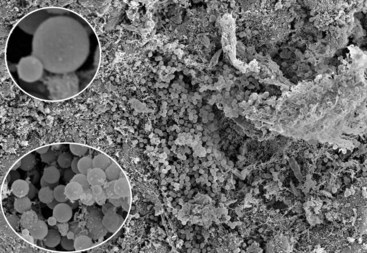
FIG. 15-17 Heavy colonization of yeast cells in the root canal of an extracted tooth with primary infection associated with apical periodontitis (×300). Note that some cells are in the stage of budding. A daughter cell is growing on the surface of the mother cell (insets) (insets: bottom ×2700, top ×3500).
(From Siqueira JF Jr, Sen BH: Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:632, 2004.)
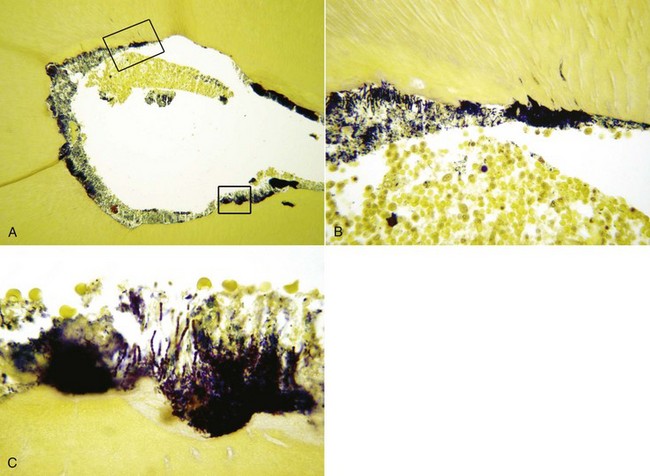
FIG. 15-18 Biofilm on the walls of a mesial root canal from a mandibular first molar (×100). The tooth was symptomatic, and an apical periodontitis lesion was present. Sections in B and C correspond to higher magnifications of the larger and smaller insets, respectively. Note the accumulation of polymorphonuclear neutrophils in the canal near the biofilm. (B, ×400; C, ×1000). Section stained with a Taylor-modified Brown and Brenn technique.
(Courtesy Dr. Domenico Ricucci.)
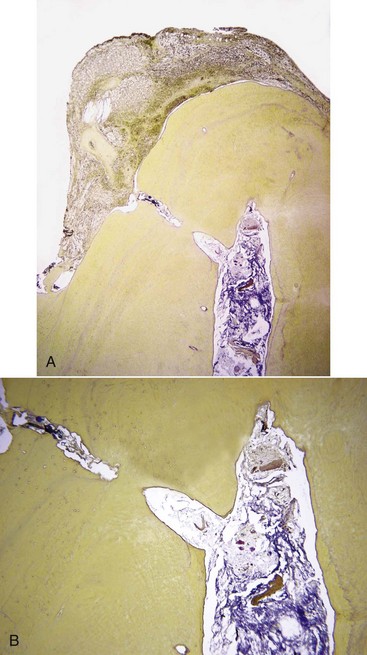
FIG. 15-19 A, Bacterial biofilm in the necrotic canal and in an apical ramification contiguous to inflamed periradicular tissues (×25). B, Higher magnification of A (×100). Sections stained with a Taylor-modified Brown and Brenn technique.
(Courtesy Dr. Domenico Ricucci.)
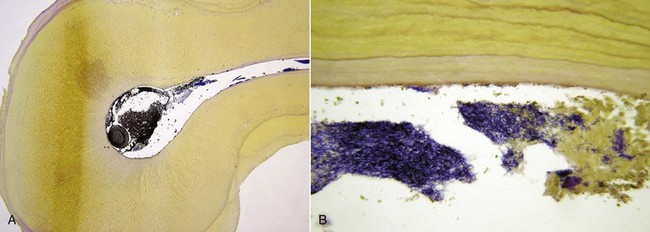
FIG. 15-20 Transverse sections of a maxillary second molar with fused mesial and palatal roots. A, Heavy bacterial infection of the canal, spreading to an isthmus (×25). B, Higher magnification of the isthmus clogged with bacteria (×400). Section stained with a Taylor-modified Brown and Brenn technique.
(Courtesy Dr. Domenico Ricucci.)
Bacterial cells from endodontic biofilms are very often seen penetrating the dentinal tubules (Fig. 15-21). The diameter of dentinal tubules is large enough to permit penetration of most oral bacteria. It has been reported that dentinal tubule infection can occur in about 70% to 80% of the teeth evincing apical periodontitis lesions.149,193 A shallow penetration is more common; bacterial cells can be observed reaching approximately 300 µm in some teeth257 (Fig. 15-22). Dividing cells can be frequently observed within tubules in in situ investigations257 (see Fig. 15-22), indicating that bacteria can derive nutrients within tubules, probably from degrading odontoblastic processes, denatured collagen, bacterial cells that die during the course of infection, and intracanal fluids that enter the tubules by capillarity.

FIG. 15-21 Heavy infection of the root canal walls, mainly by cocci, but some small rods are also seen. Cocci are penetrating into dentinal tubules (×3500).
(From Siqueira JF Jr, Rôças IN, Lopes HP: Patterns of microbial colonization in primary root canal infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:174, 2002.)
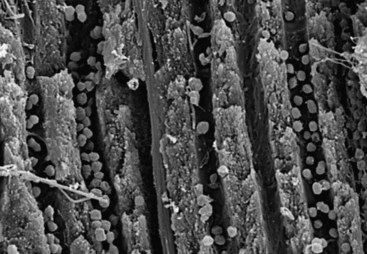
FIG. 15-22 Cocci in dentinal tubules approximately 300 µm from the main root canal (×5000).
(From Siqueira JF Jr, Rôças IN, Lopes HP: Patterns of microbial colonization in primary root canal infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:174, 2002.)
Several putative endodontic pathogens have been shown to be capable of penetrating dentinal tubules in vitro, including Porphyromonas endodontalis, Porphyromonas gingivalis, Fusobacterium nucleatum, Actinomyces israelii, Propionibacterium acnes, Enterococcus faecalis, Candida albicans, and streptococci.142,191,239,258,310 In their clinical study, Peters et al.193 isolated and identified bacteria present in root dentin at different depths, and the most common isolates belonged to the genera Prevotella, Porphyromonas, Fusobacterium, Veillonella, Peptostreptococcus, Eubacterium, Actinomyces, lactobacilli, and streptococci. Using immunohistologic analysis, Matsuo et al.149 observed the intratubular occurrence of F. nucleatum, Pseudoramibacter alactolyticus, Eubacterium nodatum, Lactobacillus casei, and Parvimonas micra inside dentinal tubules from the canal walls of extracted infected teeth with apical periodontitis.
Whereas bacteria present as planktonic cells in the main root canal may be easily accessed and eliminated by instruments and substances used during treatment, those organized in biofilms attached to the canal walls or located into isthmuses, lateral canals, and dentinal tubules are definitely more difficult to reach and may require special therapeutic strategies to be eradicated.
Microbial Ecology and the Root Canal Ecosystem
A root canal with necrotic pulp provides a space for bacterial colonization and affords bacteria a moist, warm, nutritious, and anaerobic environment, which is by and large protected from the host defenses because of lack of active blood circulation in the necrotic pulp tissue. Also, the root canal walls are nonshedding surfaces conducive to persistent colonization and formation of complex communities. The necrotic root canal might be considered a fertile environment for bacterial growth, and colonization not a difficult task for virtually all oral bacterial species. Although a large number of bacterial species (about 100 to 200) can be found in the oral cavity of a particular individual,187 only a limited assortment of these species (about 10 to 40) is consistently selected out for growth and survival within a root canal containing necrotic pulp tissue from the same individual. This indicates that ecological determinants operate in the necrotic canal and dictate which species will succeed in colonizing this previously sterile environment. The major ecological factors that determine the composition of the root canal microbiota include oxygen tension, type and amount of available nutrients, and bacterial interactions. Other factors such as temperature, pH, and receptors for adhesins may also be involved.
The root canal infection is a dynamic process, and different bacterial species apparently dominate at different stages. Shifts in the composition of the microbiota are largely due to changes in environmental conditions, particularly in regard to oxygen tension and nutrient availability. In the very initial phases of the pulpal infectious process, facultative bacteria predominate.63 After a few days or weeks, oxygen is depleted within the root canal as a result of pulp necrosis and consumption by facultative bacteria. Further oxygen supply is interrupted with loss of blood circulation in the necrotic pulp. An anaerobic milieu develops and is highly conducive to survival and growth of obligate anaerobic bacteria. With the passage of time, anaerobic conditions become even more pronounced, particularly in the apical third of the root canal; as a consequence, anaerobes will dominate the microbiota, outnumbering facultative bacteria (Fig. 15-23).
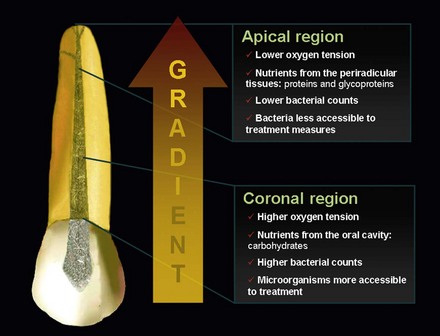
FIG. 15-23 Ecological conditions in different areas of the root canal. A gradient of oxygen tension and nutrients (type and availability) is formed. Consequently, the microbiota residing in different parts can also differ in diversity, density, and accessibility to treatment procedures.
The main sources of nutrients for bacteria colonizing the root canal system include: (1) the necrotic pulp tissue; (2) proteins and glycoproteins from tissue fluids and exudate that seep into the root canal system via apical and lateral foramens; (3) components of saliva that may coronally penetrate into the root canal; and (4) products of the metabolism of other bacteria. Because the largest amount of nutrients is available in the main canal, the most voluminous part of the root canal system, most of the infecting microbiota (particularly fastidious anaerobic species) is expected to be located in this region. Bacterial species that can best utilize and compete for nutrients in the root canal system will succeed in colonization.
In addition to being influenced by variations in oxygen levels, shifts in the microbiota colonizing the root canal system can also be dependent upon the dynamics of nutrient utilization. Saccharolytic species dominate the very early stages of the infectious process but are soon outnumbered by asaccharolytic species, which will dominate later stages.289 Even though the necrotic pulp tissue can be regarded as a finite source of nutrients to bacteria, given the small volume of tissue that is gradually degraded, induction of periradicular inflammation guarantees a sustainable source of nutrients, particularly in the form of proteins and glycoproteins present in the exudate that seep into the canal. At this stage of the infectious process, bacteria that have a proteolytic capacity or establish a cooperative interaction with those that can utilize this substrate in the metabolism start to dominate. Therefore, as the infectious process reaches the stage of induction of periradicular inflammation, proteins become the principal nutrient source, particularly in the apical part of the canal, favoring the establishment of anaerobic species that utilize peptides and/or amino acids in their metabolism (see Fig. 15-23).
Because primary endodontic infections are usually characterized by mixed communities, different bacterial species are in close proximity with one another, and interactions become inevitable. Thus, establishment of certain species in the root canal is also influenced by interactions with other species. In this regard, early colonizers play an important role in dictating which species will live along with them in the community. Bacterial interactions can be positive or negative. Positive interactions enhance the survival capacity of the interacting bacteria and enable different species to coexist in habitats where neither could exist alone. For instance, interbacterial nutritional interactions are important ecological determinants that result in higher metabolic efficiency of the whole community. Nutritional interactions are mainly represented by food webs, including utilization of metabolic end products from one species by another and bacterial cooperation for the breakdown of complex host-derived substrates. Fig. 15-24 displays a complex array of interbacterial nutritional interactions that can take place in infected root canals, where the growth of some species can be dependent upon products of metabolism of other species. Moreover, one species can provide growth conditions favorable to another—for example, by reducing oxygen tension in the environment and favoring the establishment of anaerobes, or by releasing some proteinases that can provide protection from host defenses. Negative interactions in turn act as feedback mechanisms that limit population densities. Examples include competition (for nutrients and space) and amensalism (when one species produces a substance that inhibits another species).
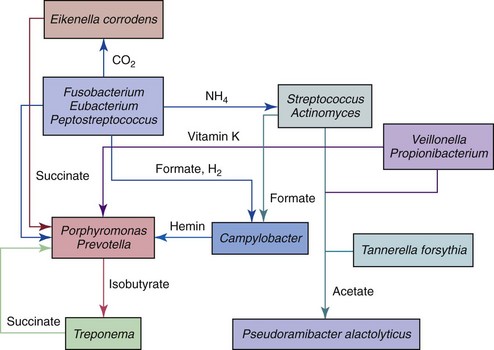
FIG. 15-24 Interbacterial nutritional interactions that can take place in infected root canals where growth of some species can be dependent upon products of metabolism of other species.
Many species adhere directly to host surfaces, other species adhere to bacteria already attached to the surface. The latter is called coaggregation and is a highly specific phenomenon with regard to the partners involved.117 Coaggregations differ from aggregations and agglutinations in that the latter two interactions occur between genetically identical cells.118 A given pair of species can attach to each other by means of specific receptor-adhesin interactions, which are usually lectinlike interactions (attachment of a specific protein on the surface of one species to a specific carbohydrate on the surface of the other). Coaggregation can favor colonization of host surfaces and also facilitate metabolic interactions between the partners. Coaggregation has been demonstrated for several pairs of bacterial taxa found in endodontic infections.114
Other Microorganisms in Endodontic Infections
Fungi
Fungi are eukaryotic microorganisms that may colonize the oral cavity, especially Candida species, but they have been only occasionally detected in primary intraradicular infections.60,126,159,259 One molecular study16 has reported the occurrence of C. albicans in 21% of samples from primarily infected canals.
Archaea
Archaea comprise a highly diverse group of prokaryotes distinct from bacteria. Members of this domain have been traditionally recognized as extremophiles, but some of these microorganisms have also been found to flourish in nonextreme environments, including the human body.59 To date, no member of the Archaea domain has been described as a human pathogen. Although one study failed to detect archaea in samples from primary endodontic infections,254 other studies detected a Methanobrevibacter oralis–like phylotype in some primarily infected canals.305,307
Viruses
Viruses are not cells but inanimate particles composed of a nucleic acid molecule (DNA or RNA) and a protein coat. On their own, they have no metabolism and need to infect living cells to use the cell’s machinery to replicate the viral genome. Because viruses require viable host cells to infect and replicate themselves, they cannot thrive in the root canal with necrotic pulp. Viruses have been reported to occur in the root canal only in noninflamed vital pulps of patients infected with the human immunodeficiency virus.78 Human cytomegalovirus (HCMV) and Epstein-Barr virus (EBV) have been detected in apical periodontitis lesions,215 where living host cells are present. It has been hypothesized that HCMV and EBV may be implicated in the pathogenesis of apical periodontitis as a direct result of virus infection and replication or as a result of virally induced impairment of local host defenses, which might give rise to overgrowth of pathogenic bacteria in the very apical part of the root canal.273 Bacterial challenge emanating from the canals may cause an influx of virus-infected cells into the periradicular tissues. Reactivation of HCMV and/or EBV by tissue injury induced by bacteria may evoke impairment of host immune response in the periradicular microenvironment, changing the potential of local defense cells to mount an adequate response against infectious agents. Herpesvirus-infected inflammatory cells are stimulated to release proinflammatory cytokines,157,313 and evidence of HCMV and/or EBV infection has been more frequently observed in symptomatic apical periodontitis lesions,214,216 large lesions,215,216 and lesions from HIV-positive patients.217 However, the role of herpesviruses in the pathogenesis of apical periodontitis has still to be elucidated.