CHAPTER 18 Endodontic and Periodontal Interrelationships
The interrelationships between pulpal and periodontal disease primarily occur by way of the intimate anatomic and vascular connections between the pulp and the periodontium; these interrelationships have been traditionally demonstrated using radiographic, histologic, and clinical criteria. Pulpal and periodontal problems are responsible for more than 50% of tooth mortality.16 Diagnosis is often challenging because these diseases have been primarily studied as separate entities, and each primary disease may mimic clinical characteristics of the other disease. Some studies suggest that these two diseases may have etiologic influences on the progression of the other disorder. Pulp tissue succumbs to degeneration by way of a multitude of insults such as caries, restorative procedures, chemical and thermal insults, trauma, and periodontal disease. When products from pulp degeneration reach the supporting periodontium, rapid inflammatory responses can ensue that are characterized by bone loss, tooth mobility, and sometimes sinus tract formation. If this occurs in the apical region, a periradicular lesion forms. If this occurs with crestal extension of the inflammation, a retrograde periodontitis or reverse pocket is formed. However, the lesion formed has little anatomic similarity to a periodontally induced defect.
Periodontal disease, by contrast, is a slowly progressing disease that may have a gradually atrophic effect on the dental pulp. A periodontal lesion is used to denote an inflammatory process in the periodontal tissues resulting from dental plaque accumulation on the external tooth surfaces. Research has shown that periodontitis is characterized by the presence of localized inflammation or tissue infarction, a decrease in cells, resorption, fibrosis, and coagulation necrosis.* Dystrophic calcification may cause some degeneration in the pulp and further influence periodontal disease. In addition, periodontal treatments such as deep root planing or curettage, use of localized medicaments, and gingival injury or wounding may accelerate further pulpal inflammation and provoke the interrelated disease process.70,76,77
In recent years, periodontal disease has been shown to be related to (and possibly even the cause of) pulpal disease, and pulpal disease may cause periodontal lesions that behave differently from chronic destructive periodontitis. The effects of periodontal disease on the pulp and the potential for healing of certain periodontal lesions after endodontic therapy have been documented extensively.† This chapter discusses the intercommunications between pulpal and periodontal tissues, the effects of pulpal disease on the periodontium, periodontal disease and its effects on the pulp, and the classification, differential diagnosis, and management and prognosis of endodontic and periodontal problems.
†References 7, 18, 45, 52, 67, and 77.
Intercommunication between Pulpal and Periodontal Tissue
Several possible channels between the pulp and periodontium that lead to the interaction of the disease process in both tissues have been suggested. These include neural (i.e., reflex) pathways, lateral canals, dentinal tubules, palatogingival grooves, periodontal ligament, alveolar bone, apical foramina, and common vasculolymphatic drainage pathways. The most interconnected and evidenced relationship between the two tissues is by way of the vascular system, as illustrated anatomically by the presence of the apical foramen, lateral (i.e., accessory) canals, and dentinal tubules.* These communications, when they exist, may serve as potential paths for inflammatory reciprocity.13,33,45,47,52,65
*References 7, 18, 45, 52, 67, and 77.
The apical foramen is the most direct route of communication to the periodontium, but by no means is it the only location where pulpal and periodontal tissues communicate with each other. Lateral and accessory canals, mainly in the apical area and in the furcation of molars, also connect the dental pulp with the periodontal ligament. Table 18-1 lists the incidence of furcation canals. These have been suggested as a direct pathway between pulp and periodontium and typically contain connective tissue and vessels that connect the circulatory system of the pulp with that of the periodontium. Research has demonstrated that inflammation in the interradicular periodontal tissues may develop following induction of pulpal inflammation.69,70 Serial sectioning of 74 teeth has revealed 45% of accessory canals are present primarily in the apical region.65 More significantly, lateral accessory canals in eight teeth were located more coronally on the roots. Among them, connection of the accessory canals with periodontal pockets was microscopically demonstrable in five of the specimens. One investigator introduced safranin dye into 102 molar teeth that were placed in a vacuum chamber and found 28% of the teeth had furcation canals, although only 10% of the total group exhibited canals on the lateral root surface.33
TABLE 18-1 Incidence of Furcation Canals
| Investigators | Techniques | Incidence |
|---|---|---|
| Rubach and Mitchell (1965)65 | Sectioned teeth | 45% |
| Lowman et al. (1973)52 | Dissecting microscope | Maxillary molars 59% |
| Mandibular molars 55% | ||
| Burch and Hulen (1974)13 | Radiopaque dye | Accessory furcal canals 76% |
| Vertucci and Williams (1974)81 | Hematoxylin dye | 46% |
| Kirkham (1975)43 | Radiopaque dye | 23% |
| Gutmann (1978)33 | Safranin dye | Maxillary molars 28.4% |
| Mandibular molars 27.4% |
In addition to the apical foramen and lateral accessory canals, dentinal tubules have also been suggested as another common pathway between periodontium and pulpal tissue. Dentinal tubules contain cytoplasmic extensions or odontoblastic processes that extend from the odontoblasts at the pulpodentin interface to the dentinoenamel junction (DEJ) or the cementodentinal junction (CDJ). It has been reported that the pulp chamber can communicate with the external root surface by way of dentinal tubules, especially when the cementum is denuded.33,85
Palatogingival grooves are developmental anomalies of the maxillary incisor teeth, with lateral incisors more often affected than central incisors (4.4% versus 0.28%, respectively).84 These usually begin in the central fossa, cross the cingulum, and extend apically with varying distances. Generally, the incidence of palatogingival grooves ranges from 1.9% to 8.5%.26,84 Investigators26 reported that 0.5% of the teeth examined had a palatogingival groove extension to the root apex, thus contributing to an endodontic pathologic condition. Bilateral buccal radicular grooves have also been reported on maxillary incisors.41
Perforation of the root creates a communication between the root canal system and the periodontal ligament. This may occur as a result of overinstrumentation during endodontic procedures, internal or external root resorption, or caries invading through the floor of the pulp chamber. The prognosis for teeth with root perforation is usually determined by the location of the perforation, the time left unsealed, the ability to seal the perforation, the chance of building new attachments, and the accessibility of the remaining root canals. Teeth that have perforations in the middle or apical third of the root have the greatest chance of healing. The closer the perforation is to the gingival sulcus, particularly into the coronal third of the root or the furcation region, the greater the likelihood of apical migration of the gingival epithelium in initiation of a periodontal lesion.4
A vertical root fracture can produce a “halo” effect around the tooth radiographically.63 Deep periodontal pocketing and localized destruction of alveolar bone are often related to longstanding root fractures. The fractured root can mimic a radiographic profile of occlusal trauma, with localized loss of lamina dura, altered trabecular pattern, and a widened periodontal ligament. The fracture site provides a portal of entry for irritants from the root canal system to the surrounding periodontal ligament. Vertical root fractures have contributed to the progression of periodontal destruction in the presence of apparently successful endodontic tooth therapy and overall periodontal site stability.64
Influence of Pulpal Pathologic Condition on the Periodontium
Pulpal pathosis as a cause of periodontal disease has received much attention during the last decade. Pulpal degeneration results in necrotic debris, bacterial byproducts, and other toxic irritants that can move toward the apical foramen, causing periodontal tissue destruction apically and potentially migrating toward the gingival margin. Investigators termed this retrograde periodontitis to differentiate the process from marginal periodontitis, in which the disease proceeds physically from the gingival margin toward the root apex. When pulpal disease progresses beyond the confines of the tooth, inflammation extends and affects the adjacent periodontal attachment apparatus.73 This inflammatory process often results in dysfunction of the periodontal ligament and resorption of alveolar bone, cementum, and even dentin. The endodontic infection has been regarded as a local modifying risk factor for periodontitis progression if left untreated.23 It is believed that an unresolved periapical infection could sustain endodontic pathogen growth, and infectious products would egress into the periodontium by way of the apex and lateral or accessory canals, as well as encourage osteoclastic activity. These may aggravate periodontal pocket formation and bone loss and impair wound healing to accelerate further periodontal disease development and progression. In addition, the medicaments (e.g., high concentrations of calcium hydroxide, corticosteroids, antibiotics) used for root canal therapy can irritate the periodontal attachment apparatus.10,11 The nature and extent of periodontal destruction depend on several factors, including virulence of the irritating stimuli present in the root canal system (e.g., microbiota, medications, foreign body reactions), duration of the disease, and host defense mechanisms.16 The rare prevalence of endodontic-periodontal lesions argues that periradicular pathoses in general have little effect on localized inflammatory bone resorption, given that few combined lesions are seen in cases of apical periodontitis.
The ability of the periodontium to regenerate lost attachment apparatus on pulpless teeth has been questioned, especially if these teeth contain a root canal filling and have been denuded of cementum. One investigator suggested that endodontically treated teeth may not respond as well as untreated teeth to periodontal procedures. He found 60% osseous regeneration of periodontal defects in teeth not treated endodontically, compared with 33% defect fill in endodontically treated teeth.66 However, in a monkey study, others reported that all tissue of the periodontium had a potential for regeneration after periodontal surgery, regardless of the status of the pulp (vital, filled, medicated, or open).20 Another study reported that pulpal status has little influence on initial cementogenesis and that substances leaching from certain root canal filling materials do not alter deposition of new cementum.61
Although endodontic infections have been highly correlated with deeper periodontal pockets and furcation involvement in mandibular molars, the causal relationship between the two pathoses has not yet been established.39 It has been suggested that endodontic treatment should occur before treatment of furcation lesions (i.e., bone regeneration) to ensure successful results. Extensive evidence is lacking to prove this hypothesis, but there is general agreement that with the proper endodontic treatment, periodontal disease of pulpal origin should heal. The question of whether endodontic infections play a significant role in affecting the health of the periodontium remains to be further elucidated.57
Influence of Periodontal Inflammation on the Pulp
Clinically, it is not uncommon to observe an advanced periodontitis spreading to the apical foramen, with associated pulp necrosis. It is also recognized that infection from a periodontal pocket may spread to the pulp through accessory canals, which occur most often in the furcation and closer to the apex of teeth. Investigators proved that pulpitis and pulp necrosis can occur as a result of periodontal inflammation involving accessory and apical canals.65 In addition, bacterial products and toxins may also gain access to the pulp by way of exposed dentinal tubules. The pulpal reaction is influenced not only by the stages of periodontal disease but also by the type of periodontal treatment, such as scaling, root planing, and administration of medication.32 Inflammatory lesions of varying severity and necrotic pulp tissue are usually found in teeth with large canals or in cases where periodontal breakdown has extended to the apex.32 One investigator stated that during periodontal therapy, the blood vessels supplying the pulp by way of accessory canals may be damaged.85 Another animal study found that 70% of root specimens examined showed no pathologic changes despite the fact that 30% to 40% of the periodontal attachment was lost.8 The remaining 30% of roots displayed only small inflammatory cell infiltrates or formation of reparative dentin or both in areas where pulp was adjacent to root exposed through periodontal destruction. These tissue changes were frequently associated with root surface resorption, suggesting that dentinal tubules must be uncovered before irritation can be transmitted.
These observations suggest that the presence of an intact cementum layer is important for the protection of the pulp from toxic elements produced by the plaque microbiota, so periodontal disease and periodontal treatments should be regarded as potential causes of pulpitis and pulpal necrosis. It has also been reported that the pulps of teeth with longstanding periodontal disease develop fibrosis and various forms of mineralization. Canals associated with periodontally involved teeth were reported to be narrower than canals of teeth that were not periodontally involved. This result is thought to be a reparative process rather than an inflammatory response.7,49
Although consensus supports the influence a degenerating or inflamed pulp can have on the periodontium, not all researchers agree about the effect of periodontal disease on the pulp. Specifically, inflammatory alterations and localized pulp necrosis have been observed adjacent to lateral canals in roots exposed by periodontal disease.65,69,70 Additional studies have failed to confirm a direct correlation between periodontal disease and pulp tissue changes.19,56,79 When pathologic changes occur in the pulp as a result of periodontal disease, the pulp usually does not degenerate so long as the main canal is not involved.48 It seems plausible to assume that periodontal disease rarely jeopardizes the vital function of the pulp. Generally, if the blood supply through the apical foramen remains intact, the pulp is usually capable of withstanding physiologic insults induced by periodontal disease.
Theoretic Pathways of Osseous Lesion Formation
For the clinician, the close relationship between pulpal and periodontal disease is reasonably established on clinical and radiographic levels. Because interpretations vary as to which came first (the proverbial “chicken or egg” controversy), clinical data gathering in endodontic and periodontal problems is often complex, requiring medical history review, pulp vitality testing, pocket and furcation probing, tooth mobility determinations, and critical examination of radiographs. When formulating a differential diagnosis, the clinician should first consider both the periodontal and pulpal status of the affected tooth. If an interrelationship in disease entities exists, appropriate treatment must be rendered to remove true causative factors and enhance the prognosis for tooth retention.72 Fig. 18-1 lists interrelationships between pulpal and periodontal disease.
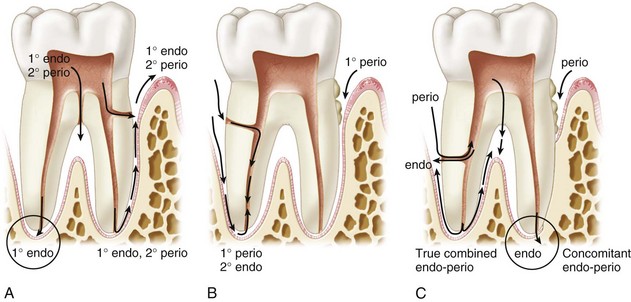
FIG. 18-1 Endodontic and periodontal pathways. A, Endodontic lesions. The pathway of inflammation is through the apical foramen, furcation canals, and lateral accessory canals to the periodontium. This results in a primary endodontic lesion, sometimes progressing toward secondary periodontal involvement. B, Periodontal lesions. This is the progression of periodontitis by way of lateral canal and apex to induce a secondary endodontic lesion. C, True combined endodontic and periodontal lesion and concomitant endodontic and periodontal lesions.
Primary Endodontic Lesions
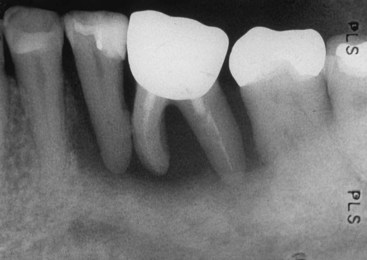
Disease processes of the dental pulp frequently involve inflammatory changes. Caries, restorative procedures, and traumatic injuries are the most common causes. Typically, endodontic lesions resorb bone apically and laterally and destroy the attachment apparatus adjacent to a nonvital tooth. Inflammatory processes in the periodontium occurring as a result of root canal infection not only may be localized at the apex but also may appear along the lateral aspects of the root (Fig. 18-2) and in furcation areas of two- and three-rooted teeth (Fig. 18-3).
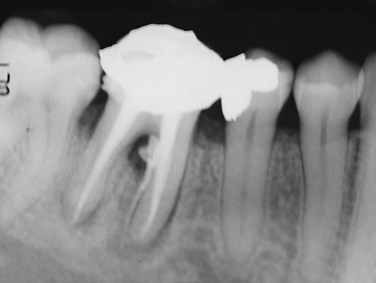
FIG. 18-2 Primary endodontic lesion. Mandibular molar showing endodontic filling material extending into the furcation and along the lateral root surface because of inadequate canal preparation. Furcation could be probed.
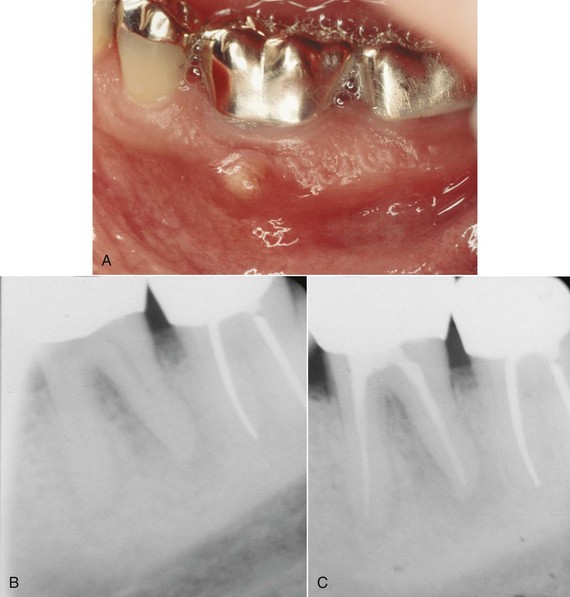
FIG. 18-3 Primary endodontic lesion on mandibular first molar. A, Abscess was noted on the buccal surface of the tooth. B, Apical radiolucency was evident on the distal root. C, Six months after root canal treatment, there is evidence of osseous healing at both the apex and furcation area.
The emergence of these processes may be associated with clinical signs of inflammation: pain, tenderness to pressure and percussion, increased tooth mobility, and swelling of the marginal gingiva, simulating a periodontal abscess. The suppurative process may cause a sinus tract along the periodontal ligament space or through patent channels (including the apical foramen and lateral accessory canals). This usually results in a narrow opening of the sinus tract into the gingival sulcus and pocket that can be easily traced with a gutta-percha cone or a periodontal probe. Such a tract can readily be probed down to the tooth apex, where no increased probing depth would otherwise exist around the tooth. In multirooted teeth, a periodontal ligament sinus tract can drain off into the furcation area and resemble a Grade III “through-and-through” furcation defect resulting from periodontal disease.
Clinically, endodontic testing procedures should reveal a necrotic pulp or, in multirooted teeth, at least an abnormal response, indicating the pulp is degenerating. Because the primary lesion is an endodontic problem that has merely manifested itself through the periodontal ligament, complete resolution is usually anticipated following nonsurgical endodontic therapy without any periodontal treatment.
Primary Endodontic Lesions With Secondary Periodontal Involvement
When a lesion of endodontic origin is not treated, usually pathosis will continue, leading to destruction of the periapical alveolar bone and progression into the interradicular area, causing breakdown of surrounding hard and soft tissues (Fig. 18-4). As drainage persists through the gingival sulcus, accumulation of plaque and calculus in the purulent pocket results in periodontal disease and further apical migration of the attachment. When this occurs, not only does the diagnosis become more difficult, but the prognosis and treatment may be altered. Diagnostically, these lesions have a necrotic root canal and plaque or calculus accumulation, demonstrable by a probe and radiograph. Radiographs may show generalized periodontal disease with angular defects at the initial site of the endodontic involvement.
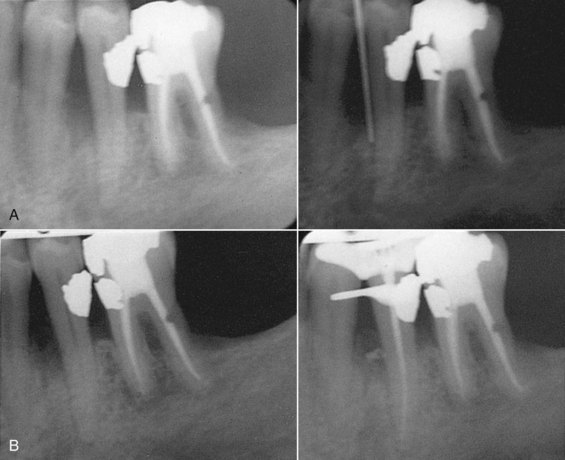
FIG. 18-4 Primary endodontic lesion with secondary periodontal involvement. A, Mesial aspect of mandibular second premolar had deep periodontal pocketing (periodontal probe in place demonstrated 6 mm probing depth) even after periodontal therapy. B, Pulp tests were performed on the premolar; the tooth was nonresponsive. After endodontic treatment, the periodontal pocket resolved (filling of a lateral accessory canal after obturation is demonstrated).
Resolution of the primary endodontic and secondary periodontal lesion relies on treatment of both conditions. When only endodontic therapy is provided, only part of the lesion can be expected to heal. If endodontic therapy is adequate, the prognosis depends on the severity of periodontal involvement and efficacy of periodontal therapy.
Primary Periodontal Lesions
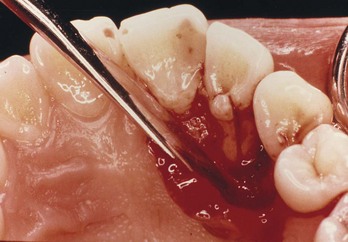
Periodontal disease has a progressive nature. It begins in the sulcus and migrates to the apex as deposits of plaque and calculus produce inflammation, causing loss of surrounding alveolar bone and supporting periodontal soft tissues. This leads to a loss of clinical attachment and formation of a periodontal abscess during the acute phase of destruction.71 The progression of periodontal disease to the formation of osseous defects and subsequent radiographic appearance along lateral aspects of roots and in furcation areas is well known. These defects may or may not be in association with trauma from occlusion, which can often be the cause of an isolated periodontal problem. Osseous lesions of periodontal origin are usually associated with tooth mobility, and the affected teeth respond positively to pulp testing. In addition, careful periodontal examination will usually reveal broad-based pocket formation and an accumulation of plaque and calculus. The bony lesion is usually more widespread and generalized than are lesions of endodontic origin (Fig. 18-5).

FIG. 18-5 Primary periodontal lesion. A, Mandibular cuspid exhibits extensive periodontal destruction; the tooth responded normally to pulp tests. B, Extracted tooth depicts extensive calculus accumulation and root concavity.
The prognosis for those teeth affected by periodontitis worsens as the disease process and periodontal destruction progress. Treatment depends on the extent of the periodontitis and on the patient’s ability to comply with potential long-term treatment and maintenance therapy. Because this is purely a periodontal problem, the prognosis depends exclusively on the outcome of periodontal therapy.
Primary Periodontal Lesions With Secondary Endodontic Involvement
As stated earlier, periodontal disease can have an effect on the pulp through dentinal tubules, lateral canals, or both. Primary periodontal lesions with secondary endodontic involvement differ from the primary endodontic lesion with secondary periodontal involvement only by the temporal sequence of the disease processes. The tooth with primary periodontal and secondary endodontic disease exhibits deep pocketing, with a history of extensive periodontal disease and, possibly, past treatment. When the pulp becomes involved, the patient often reports accentuated pain and clinical signs of pulpal disease. This situation exists when the apical progression of periodontal disease is sufficient to open and expose the pulp to the oral environment by way of lateral canals or dentinal tubules. On radiographs, these lesions may be indistinguishable from primary endodontic lesions with secondary periodontal involvement. The prognosis depends on continuing periodontal treatment subsequent to endodontic therapy.
True Combined Lesions
Pulpal and periodontal disease may occur independently or concomitantly in and around the same tooth. Once the endodontic and periodontal lesions coalesce, they may be clinically indistinguishable (Fig. 18-6). The prognosis of multi-rooted teeth with combined pulpal and periodontal lesions depends largely on the extent of the destruction caused by the periodontal disease component. A necrotic pulp or a failing endodontic treatment, plaque, calculus, and periodontitis will be present in varying degrees.
Concomitant Pulpal and Periodontal Lesions
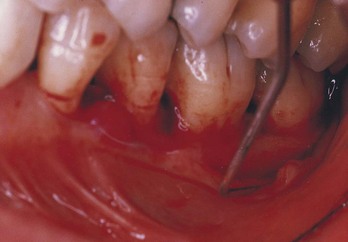
An additional classification has been proposed for lesions that may commonly be seen clinically and reflect the presence of two separate and distinct entities.6 This is referred to as the concomitant pulpal and periodontal lesion (Fig. 18-7). In essence, both disease states exist but with different causative factors and with no clinical evidence that either disease state has influenced the other. This situation often goes undiagnosed, and treatment is rendered to only one of the diseased tissues in the hope that the other will respond favorably. In actuality, both disease processes must be treated concomitantly, with the prognosis dependent on the removal of the individual etiologic factors and prevention of any further factors that may affect the respective disease processes.
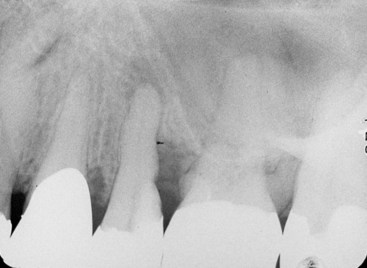
FIG. 18-7 Concomitant pulpal and periodontal lesion on the maxillary second premolar. An endodontic lesion was noted at the apex, with a noncommunicating periodontal pocket on the distal side.
With the application of research findings and clinical experience, pathways of osseous lesion formation may be theorized. These concepts are intended as a guide for the evaluation and understanding of the clinician’s successes and failures in treating teeth with osseous lesions. By understanding the nature of their formation, the clinician can better anticipate the healing potential after treatment.
Differential Diagnosis
During the course of treatment, clinicians are frequently presented with the dilemma of accurately assessing the contribution of endodontic and periodontal lesions. These lesions may be very separate from each other and present no extraordinary therapeutic consideration. In a few other situations, there is no obvious demarcation between the two lesions, which appear as one, both on radiographs and clinically. In the diagnosis of radiographic osseous lesions, one must resist the temptation to label everything a “combined lesion.” Table 18-2 summarizes the differential diagnosis between pulpal and periodontal lesions and highlights a number of common characteristics between these lesions.
TABLE 18-2 Differential Diagnosis Between Pulpal and Periodontal Disease
| Pulpal | Periodontal | |
|---|---|---|
| CLINICAL | ||
| Etiology | Pulp infection | Periodontal infection |
| Vitality | Nonvital | Vital |
| Restorative | Deep or extensive | Not related |
| Plaque/calculus | Not related | Primary cause |
| Inflammation | Acute | Chronic |
| Pockets | Single, narrow | Multiple, wide coronally |
| pH value | Often acid | Usually alkaline |
| Trauma | Primary or secondary | Contributing factor |
| Microbial | Few | Complex |
| RADIOGRAPHIC | ||
| Pattern | Localized | Generalized |
| Bone loss | Wider apically | Wider coronally |
| Periapical | Radiolucent | Not often related |
| Vertical bone loss | No | Yes |
| HISTOPATHOLOGY | ||
| Junctional epithelium | No apical migration | Apical migration |
| Granulation tissues | Apical (minimal) | Coronal (larger) |
| Gingival | Normal | Some recession |
| THERAPY | ||
| Treatment | Root canal therapy | Periodontal treatment |
Root fractures, especially vertical root fractures, present particular problems in diagnosis (Fig. 18-8). Symptoms and signs associated with vertical root fractures show a varying character and are frequently difficult to distinguish from those associated with periodontal and endodontic lesions. Breakdown can manifest itself on radiographs in a number of different ways. This may be anything from nothing detectable on radiographs to an area of rapid vertical bone loss, given that there is no way to predict when a patient may present for treatment. It is imperative for the clinician to take more than one radiograph at different angles, especially when no clear diagnosis emerges. In these cases, a minor alteration in the angulation may reveal a tooth fracture or periodontal furcation involvement. The diagnosis of vertical root fractures is often difficult because the fracture is usually not detectable by clinical inspection and radiographic examination unless there is a clear separation of the root fragments. Cemental tears or detachment of cementum from a root surface by means of trauma or aging has been reported14,51; the lesion often results in periodontal destruction and endodontic involvement.
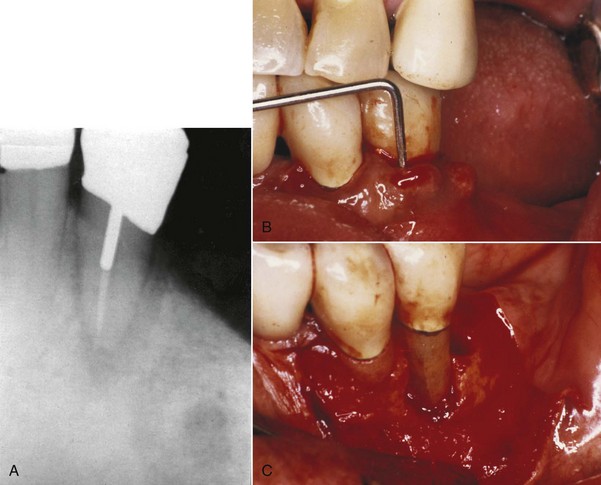
FIG. 18-8 Vertical root fracture. A, Radiograph revealed widened periodontal ligament with J-shaped radiolucency around the apex. B, Periodontal probe indicated more than 12 mm of probing depth. C, Exploratory surgery confirmed vertical root fracture.
Sometimes the definitive diagnosis of vertical root fractures has to be confirmed by exploratory surgical exposure of the root for direct visual examination.82 Vertical root fractures have been associated with root-filled teeth where excessive lateral forces were applied during compaction or possibly with stress induced by a post placement in root-filled teeth.58 Clinical survey of fractured teeth also reveals that fractures are more common in teeth with extensive restorations, in older patients, and in mandibular posterior teeth.30 Vertical root fractures that involve the gingival sulcus and periodontal pocket area usually have a hopeless prognosis because of continuous bacterial invasion of the fracture space from the oral environment. Single-rooted teeth are generally extracted. In multirooted teeth, a treatment alternative is hemisection or resection of the fractured root. See Chapter 1 for a further discussion of vertical root fractures.
Developmental grooves, primarily found in maxillary central and lateral incisors, are also capable of initiating localized periodontal destruction along the root surface.26,41,50 It is thought they may be a genetic attempt to form an accessory root, but once plaque and calculus invade the epithelial attachment, the groove becomes a pathway for microbes and their noxious allies (toxins), with ample substrate from food debris to create a self-supporting periodontal lesion. Bone demineralization follows the path of the groove. Palatogingival grooves are often associated with poor periodontal health because of patients’ inability to keep these areas clean, and a poor prognosis is usually assigned regardless of proper conventional therapy.30,84 It is easy to identify these grooves if one is aware of their existence. Clinically, these grooves may be asymptomatic, or symptomatic periodontal problems (either acute or chronic) may occur. It is thought that the pulp of these teeth may become secondarily involved and demonstrate symptoms of pulpal disease (Fig. 18-9). These lesions are often confused with the enamel projections in the furcation area of mandibular molars.55 The prevalence of cervical enamel projections (Fig. 18-10) ranges from 18% to 45%.36,55 Depending on the apical extent of these cervical grooves, some authors36,55 also find a high association with pathologic furcation involvement (up to 82.5%36).
Lateral Periodontal Cysts
The clinical presentation of a lateral periodontal cyst is often without symptoms (Fig. 18-11). It may be present as a gingival swelling on the facial aspect that may have pain and tenderness on palpation. Radiographic features are a well-circumscribed round or ovoid radiolucent area that usually has a sclerotic margin. Most lateral periodontal cysts are less than 1 cm in diameter and lie somewhere between the apex and cervical margin of a tooth. Three possible etiologies are reported in the literature: (1) reduced enamel epithelium, (2) remnants of dental lamina, or (3) cell rests of Malassez. Histologic evaluation reveals that these cysts are lined by epithelium that closely resembles reduced enamel epithelium lining. The lesion occurs predominantly in the fifth to seventh decades of life, with a predilection for males.3 The lesion is usually slow growing. Treatment consists of careful excision to help prevent recurrence. The most common location is the mandibular cuspid-bicuspid area, although numerous cases have been reported in the anterior maxilla. Investigators have reported finding unicystic and multicystic (including botryoid) varieties.3 They found that lateral periodontal cysts were lined predominantly or exclusively by thin, reduced enamel, epithelium-like tissue that contained many clear cells, and epithelial thickenings referred to as plaques. Glycogen was present in the epithelium of two thirds of the cases, although not exclusively in the clear cells, many of which were not positive for glycogen. Some of their botryoid variety differed histologically, being lined predominantly by nonkeratinizing stratified squamous epithelium with crowded and pyknotic nuclei and no clear cells. One case contained melanin, and another showed epithelial crypt formation and superficial palisaded low columnar cells, as seen in the glandular odontogenic cyst. This raised the question of whether the latter may form part of the clinicopathologic spectrum of a lateral periodontal cyst. The histogenesis of lateral periodontal cysts is uncertain, but they favor origin from reduced enamel epithelium. There are cases that either do not fit a characteristic endodontic or periodontal lesion or do not respond to treatment as expected. A biopsy and histologic analysis are often recommended. Systemic diseases such as scleroderma, metastatic carcinoma, and osteosarcoma can mimic endodontic and periodontal disease visible on a radiograph. The conscientious clinician must always be alert for lesions of nonendodontic or nonperiodontal origin and look for other causes.
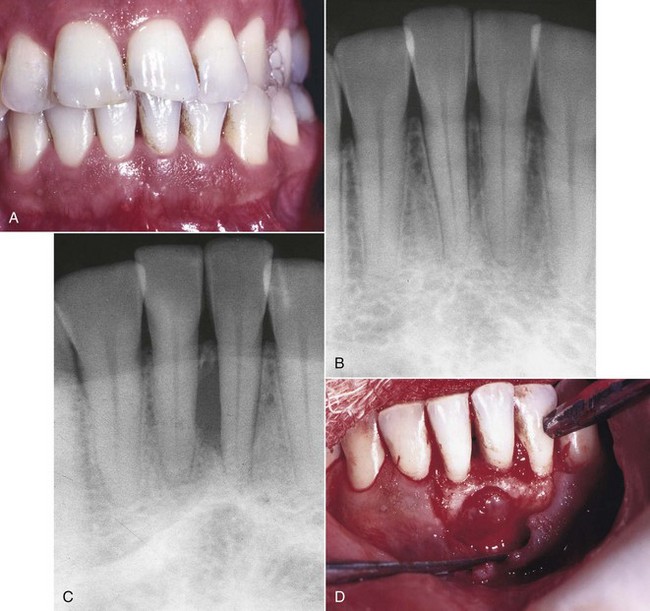
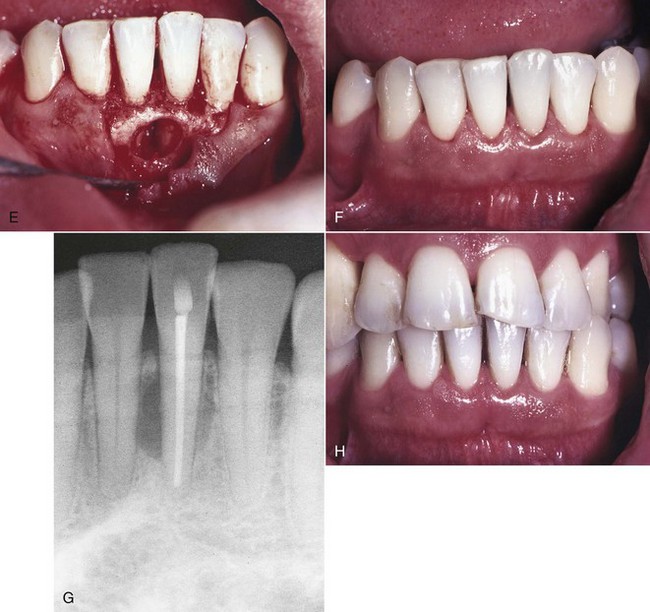
FIG. 18-11 Lateral periodontal cyst. A, Clinical photograph showing mild gingivitis around tooth #24; 5 mm probing defect on mesial aspect of tooth #24. B, Radiograph depicts beginning lesion 2 years earlier. C, Radiograph shows extent of lesion at time of referral to periodontist. D, Initial flap reflection—lesion present interproximal between teeth #24 and #25. E, Lesion was enucleated and submitted for histologic evaluation; diagnosis was lateral periodontal cyst. F, Three-month posttreatment clinical photograph; tooth #24 was nonvital and symptomatic and was referred for root canal treatment. G, One-year posttreatment radiograph showing completed root canal treatment and osseous defect still present; continuous recall recommended. H, One-year posttreatment clinical photograph; probing depths were within normal limits; continuous recall was recommended.
Treatment Alternatives
When traditional endodontic and periodontal treatments prove insufficient to stabilize an affected tooth, the clinician must consider treatment alternatives. Generally, a localized periodontal defect associated with an endodontically untreatable tooth or an iatrogenic tooth problem is reason to explore other treatment options. Alternate treatments often consist of resection or regenerative approaches. Resection techniques focus on eliminating the diseased roots or teeth; regenerative efforts are aimed at restoring lost biologic structures. Resection methods involve removal of affected roots or extraction of involved teeth. When a tooth requires extraction, one of the first options for restoring occlusal function should include placement of dental implants with hybrid prostheses. Bone replacement grafts using guided-tissue and bone-regeneration techniques are ways to reestablish biologic structures that were lost during this disease process. Fixed partial dentures are still a viable option for some patients and certainly should be considered if abutment teeth already have restorations or endodontic treatment.
Root resection is the removal of a root, with accompanying odontoplasty before or preferably after endodontic treatment.27 Formerly it was used when root canal therapy was considered too difficult, but now its indications are restricted to multirooted teeth in which one or more roots cannot be saved. The indications for root resection often include (but are not limited to) root fracture, perforation, root caries, dehiscence, fenestration, external root resorption involving one root, incomplete endodontic treatment of a particular root, severe periodontitis affecting only one root, and severe Grade II or III furcation involvement. Root resection is a technique-sensitive procedure (Fig. 18-12) requiring a careful diagnostic process for selection of those teeth that would likely be successful candidates, followed by meticulous interdisciplinary treatment. Factors such as occlusal forces, tooth restorability, and the value of the remaining roots must be examined before treatment. A carefully constructed treatment plan is crucial to the success of this resection procedure.31 Proper reshaping of the occlusal table and restoration of the clinical crown are essential, and the root surface must be recontoured to remove the root stump, thus preventing formation of a potential food trap.42

FIG. 18-12 Root resection to correct endodontic and periodontal defect. A, Pretreatment radiograph depicts severe periodontal involvement on the mesial. Endodontic treatment was performed before surgical removal of the mesial root. B, Five-year follow-up shows the remaining tooth well maintained.
The effectiveness of this approach remains controversial owing to the disparity of results reported in several long-term studies.* Retrospective longitudinal studies have observed the fate of sectioned teeth for time frames ranging from 3 to 12 years and have reported success rates ranging from 62% to 100%, with a low incidence (i.e., 10%) of periodontal breakdown. However, as most long-term studies will point out, the major cause of failure of resection procedures resides in failure of the endodontic and restorative components. Unique anatomic features, such as root length, curvature, shape, size, position of adjacent teeth, and bone density, may influence the end result. For example, root fusion makes resection all but impossible. The removal of roots purely to eliminate a resorptive or traumatic perforation defect, fractured root, or endodontically inoperable root usually results in definitive treatment. If, however, localized or generalized periodontal disease is present, conditions favorable for healing must be created, and concomitant periodontal therapeutic procedures can be implemented to restore the health of the periodontium.31 The final restoration of root-resected teeth will depend significantly on the nature of the resection, the amount of remaining tooth structure, the periodontal status, and the patient’s occlusion. The prosthetic aspects of tooth restoration must be carefully assessed and integrated into the anticipated surgical procedure to ensure proper positioning of tooth margins relative to the osseous crest and also to manage the anticipated changes in occlusal relationships and masticatory forces.83
Controversy has also existed regarding the benefits and need for endodontic therapy before root resection. Instances develop in which exploratory surgery is necessary; should the periodontal problem be more extensive than that determined presurgically, the removal of a root should be carried out at that time. In these instances, removal of the involved root without endodontic treatment would be acceptable, but root canal therapy should be performed as soon as possible after root removal.29,75,78 After resection of a vital root, the pulpal opening in the crown may be sealed and restored with a permanent amalgam restoration or sedative base material (e.g., Dycal) as a temporary solution. Investigators evaluated vital-root resection in maxillary molar teeth for 9 years.27 Amputated pulps were covered with Dycal base and amalgam. At 1 year, 38% of molar teeth remained vital, but at 5 years only 13% maintained vitality. These findings imply that the long-term prognosis for vital-root resection is poor, so endodontic therapy should be done before or immediately after resection. However, one investigator reported that vitality of resected teeth can be maintained even after 16 years.35 Nonetheless, it is generally agreed that whenever possible, endodontics should be performed in advance (before root resection). If this is not possible, the endodontic treatment should be performed as soon as possible after vital-root amputation. Otherwise, pulpal complications such as internal resorption, pulpal inflammation, and necrosis may occur.2,77
The concepts of guided tissue regeneration (GTR) or guided bone regeneration (GBR) have been used to promote bone healing after endodontic surgery.28,60 Fig. 18-13 illustrates an endodontic defect successfully treated with the GTR approach. Theoretically, the GTR barrier prevents contact of connective tissue with the osseous walls of the defect, protecting the underlying blood clot and stabilizing the wound.34 An investigator treated large periradicular lesions with GTR barrier membranes and demonstrated that periradicular healing occurred more rapidly at membrane sites than at control sites.59,60 The quality and quantity of the regenerated bone were superior with adjunctive use of the membrane than without such use. Similar findings were published in a case report with histologic examination of biopsy material obtained at barrier removal.62 In addition, during examination of clinical cases, the closer the lesion is to the gingival margin, the greater the fluid and bacterial contamination from the sulcus (and also a greater risk of mechanical trauma). When GTR is considered, the combined endodontic and periodontal lesion probably has the least favorable prognosis compared with cases having only a periodontal lesion.59
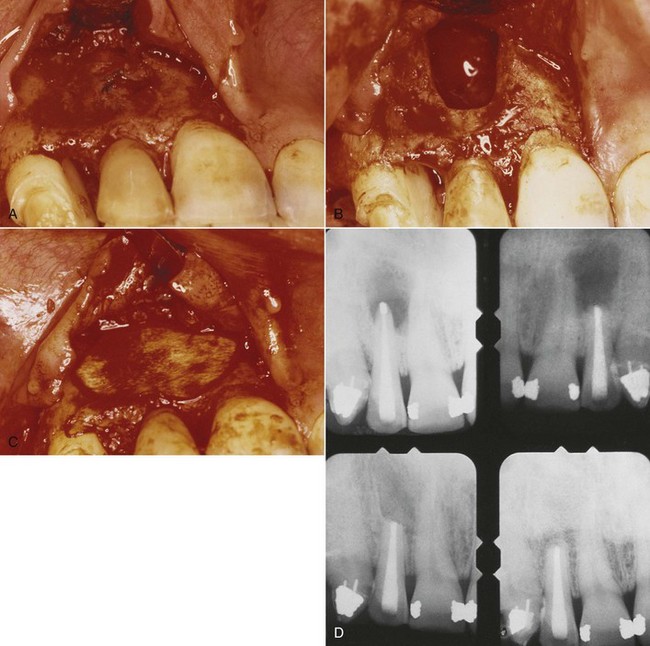
FIG. 18-13 Periradicular endodontic surgery using the combination of bone replacement grafts and collagen GTR (guided tissue regeneration) barrier membranes. A, Buccal flap was reflected (note extensive apical lesion). B, Osseous window was created with a bur. C, The defect was repaired with demineralized freeze-dried bone allograft (DFDBA) and covered with a collagen membrane. D, Radiographs show condition before surgery, at the time of surgery, and at 6 months and 2 years after surgery. At 2 years, radiograph reveals complete bone regeneration.
Many varieties of GTR membranes are available for clinical use, but it is logical to use bioresorbable collagen and polymer membranes in endodontic surgeries because there is often no need for a second surgery to retrieve the membrane. Studies reveal similar results achieved with nonresorbable and resorbable membranes.17 Long-term studies need to be completed to evaluate the success of this approach.
For more than 40 years, bone grafts have been used to treat osseous defects associated with periodontal disease.68 Because the “apicoectomy” (i.e., root-end resection) defect has a surrounding bony wall, the need for adjunctive bone grafting during this procedure is questionable (unless it is exceptionally large in diameter). With the introduction of the GTR concept, a combination of bone replacement graft and GTR membrane (Fig. 18-14) has shown promising results.1,21,40,62,80 Further studies are required to explore the true benefit of this combination treatment approach in root-end resection procedures.
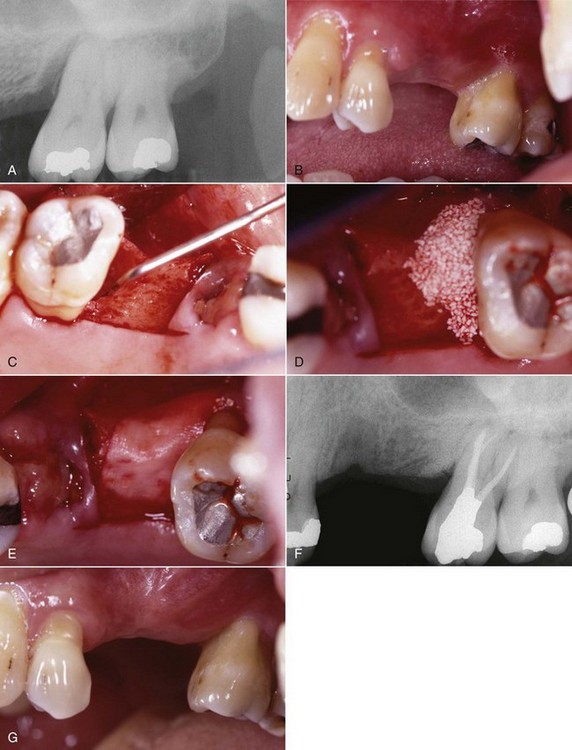
FIG. 18-14 Periodontal surgery using guided bone regeneration. A, Pretreatment radiograph demonstrates significant osseous defect on mesial aspect of tooth #14. B, Pretreatment clinical photograph of teeth #12 to #16. C, Full-thickness mucoperiosteal flap reflected with periodontal probe 7 mm in osseous defect. D, Osseous defect filled with BioOss (cancellous bovine bone); patient had root canal treatment started before surgical appointment; RCT was completed 6 weeks after osseous graft placement. E, Ossix membrane (collagen) placed over BioOss graft. F, One-year posttreatment radiograph with evidence of osseous repair. G, One-year posttreatment clinical photograph of area.
Forced Eruption or Extruson
Teeth that have fractured, undergone extensive decay, or experienced internal or external root resorption or lateral perforation may be candidates for a forced eruption procedure, especially straight, tapering, single-rooted teeth (Fig. 18-15). To obtain good access for endodontic or restorative procedures along with reduced probing depths, it may be necessary to perform extensive resection surgery to gain crown lengthening. Forced eruption may offer a better solution than surgical crown lengthening, which may produce poor aesthetics in some cases. A review of the literature reveals that most successfully treated cases are extrusions of less than 4 mm.5,9,46 The infrequently used treatment method of forced eruption serves as an alternative to the sacrifice of the natural root system. Forced eruption can preserve the natural root system and related periodontal architecture, resulting in years of additional service for the patient. It also can maintain adjacent tooth structure while retaining the option for future implant reconstruction if needed. Given the reported success of forced eruption, the technique should be employed more often by dentists.22,25 The increased use of implants in clinical treatments has stimulated clinicians’ interest in augmenting bone in patients who have deficient alveolar ridges that might preclude ideal implant placement.
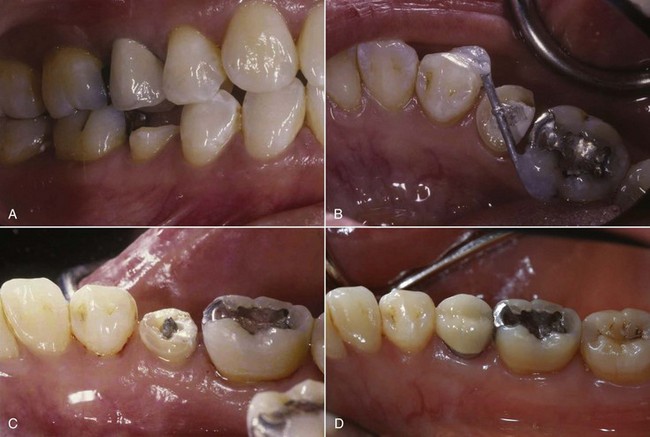
FIG. 18-15 Clinical case of forced eruption. A, Initial presentation of tooth #29 (buccal view). B, Bonded wire to help extrude #29. C, Lingual view of #29 after 3 weeks of extrusion. D, Lingual view of cemented porcelain crown on tooth #29; 4 months following extrusion and after 1 month of crown retention.
A nonsurgical technique for using the available bone for implant site development and fixture placement is orthodontic extrusion or forced eruption. The concept of extruding a tooth coronally by orthodontic forces and the clinical alterations in the soft-tissue architecture of the periodontium demonstrated during orthodontic extrusive movement of periodontally compromised teeth have demonstrated probing depth reduction in some cases.54 An investigator reported that tooth movement in the presence of inflammation might cause deepening of osseous defects. Often extrusions are accompanied by an immature-appearing tissue that is red compared with adjacent tissue. This results from the eversion of junctional epithelium, which is nonkeratinized in the sulcus; the vasculature is more readily visible until the newly exposed tissue has a chance to keratinize in about 28 days.37,38 ost extruded teeth should be retained in place twice as long as it took to force-erupt them in order to minimize relapse before attempting definitive restorative procedures.
Summary
Endodontic and periodontal lesions result from the close interrelationship of pulp tissue and the periodontium. The major pathways of communication between the two types of tissue are the apical foramina, lateral and accessory canals, and dentinal tubules. The differential diagnosis of endodontic and periodontal lesions is not always straightforward and requires clinical data accumulation from a number of diagnostic tests to obtain a correct diagnosis. When examining and treating the combined or individual lesion in endodontics and periodontics, the clinician must bear in mind that successful treatment depends on a correct diagnosis. Lesions with combined causes will require both endodontic and periodontal therapy, and endodontic therapy should usually be completed first. Root resection and regenerative techniques offer alternative approaches, enhancing the clinician’s ability to deal with these complex clinical problems.
1. Abramowitz PN, Rankow H, Trope M. Multidisciplinary approach to apical surgery in conjunction with the loss of buccal cortical plate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1994;77:502.
2. Allen AL, Gutmann JL. Internal root resorption after vital root resection. J Endod. 1977;3:438.
3. Altini M, Shear M. The lateral periodontal cyst: an update. Oral Pathol Med. 1992;21(6):245.
4. Basten CH-J, Ammons WFJr, Persson R. Long-term evaluation of root resected molars: a retrospective study. Int J Periodontics Restorative Dent. 1996;16:206.
5. Batenhorst K, Bowers G. Tissue changes resulting from facial tipping and extrusion of incisors in monkeys. J Periodontol. 1974;45:660.
6. Belk CE, Gutmann JL. Perspectives, controversies, and directives on pulpal-periodontal relationship. J Can Dent Assoc. 1990;56:1013.
7. Bender IB, Seltzer S. The effect of periodontal disease on the pulp. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1972;33:458.
8. Bergenholtz G, Lindhe J. Effect of experimentally induced marginal periodontitis and periodontal scaling on the dental pulp. J Clin Periodontol. 1978;5:59.
9. Berglundh T, Marinello C. Periodontal tissue reactions to orthodontic extrusion: an experimental study in the dog. J Clin Periodontol. 1991;18:330.
10. Blomlof L, Jansson L, Applegren R, Ehnevid H, Lindskog S. Prognosis and mortality of root-resected molars. Int J Periodontics Restorative Dent. 1997;17:190.
11. Blomlof L, Lengheden A, Linskog S. Endodontic infection and calcium hydroxide treatment effects on periodontal healing in mature and immature replanted monkey teeth. J Clin Periodontol. 1992;29:652.
12. Buhler H. Evaluation of root resected teeth: results after 10 years. J Periodontol. 1988;59:805.
13. Burch JG, Hulen S. A study of the presence of accessory foramina and the topography of molar furcations. Oral Surg Oral Med Oral Pathol. 1974;38:451.
14. Camargo PM, Pirih FQ, Wolinsky LE, Lefovic V, Kamrath H, White SN. Clinical repair of an osseous defect associated with a cemental tear: a case report. Int J Periodontics Restorative Dent. 2003;23(1):79.
15. Carnevale G, DiFebo G, Tonelli MP, Marin C, Fuzzi MA. Retrospective analysis of the periodontal-prosthetic treatment of molars and interradicular lesions. Int J Periodontics Restorative Dent. 1991;11:189.
16. Chen SY, Wang HL, Glickman GN. The influence of endodontic treatment upon periodontal wound healing. J Clin Periodontol. 1997;24:449.
17. Christgau M, Schmalz G, Reich E, Wenzel A. Clinical and radiographical split-mouth study on resorbable versus non-resorbable GTR-membranes. J Clin Periodontol. 1995;22:306.
18. Cutright DE, Bhaskar SN. Pulpal vasculature as demonstrated by a new method. Oral Surg Oral Med Oral Pathol. 1969;27:678.
19. Czarnecki RT, Schilder H. A histological evaluation of human pulp in teeth with varying degrees of periodontal disease. J Endod. 1979;5:242.
20. Diem CR, Bower GM, Ferrigno PD, Fedi PF Jr. Regeneration of the attachment apparatus on pulpless teeth denuded of cementum in Rhesus money. J Periodontol. 1974;45:18.
21. Duggins I, Clay J, Himel V, Dean J. A combined endodontic retrofill and periodontal guided tissue regeneration for the repair of molar endodontic furcation perforations: report of a case. Quintessence Int. 1994;25:109.
22. Durham TM, Goddard T, Morrison S. Rapid forced eruption: a case report and review of forced eruption techniques. Gen Dent. 2004;52(2):167.
23. Ehnevid H, Jansson L, Lindskog S, Blomlof L. Endodontic pathogens: propagation of infection through patent dentinal tubules in traumatized monkey teeth. Endod Dent Traumatol. 1995;11:229.
24. Erpenstein H. A three year study of hemisections molars. J Clin Periodontol. 1983;10:1.
25. Esposito S. Rapid forced eruption: a case report and review of forced eruption techniques. Gen Dent. 2003;51(1):58.
26. Everett FG, Kramer GM. The disto-lingual groove in the maxillary lateral incisor: a periodontal hazard. J Periodontol. 1972;443:352.
27. Filipowicz F, Umstott P, England M. Vital root resection in maxillary molar teeth: a longitudinal study. J Endod. 1984;10:264.
28. Garrett S. Periodontal regeneration around natural teeth. Ann Periodontol. 1996;1:621.
29. Gerstein K. The role of vital root resection in periodontics. J Periodontol. 1977;48:478.
30. Gher ME, Dunlap RM, Anderson MH, Kuhl LV. Clinical survey of fractured teeth. J Am Dent Assoc. 1987;114:174.
31. Green EN. Hemisection and root amputation. J Am Dent Assoc. 1986;112:511.
32. Guldener PH. The relationship between periodontal and pulpal disease. Int Endod J. 1985;18:41.
33. Gutmann JL. Prevalence, location and patency of accessory canals in the furcation region of permanent molars. J Periodontol. 1978;49:21.
34. Haney JM, Nilveus RE, McMillan PJ, Wikesjo UME. Periodontal repair in dogs: expanded polytetrafluoroethylene barrier membranes support would stabilization and enhance bone regeneration. J Periodontol. 1993;64:883.
35. Haskell EW. Vital root resection: a case report of long-term follow-up. Int J Periodontics Restorative Dent. 1984;4(6):56.
36. Hou GL, Tsai C. Relationship between periodontal furcation involvement and molar cervical enamel projections. J Periodontol. 1987;58:715.
37. Ingber JS. Forced eruption: alteration of soft tissue cosmetic deformities. Int J Periodontics Restorative Dent. 1989;9(6):416.
38. Ingber JS. Forced eruption. I. A method of treating isolated one and two wall infrabony osseous defects-rationale and case report. J Periodontol. 1974;45:199.
39. Jansson LE, Ehnevid H. The influence of endodontic infection on periodontal status in mandibular molars. J Periodontol. 1998;69:1392.
40. Kellert M, Chalfin H, Solomon C. Guided tissue regeneration: an adjunct to endodontic surgery. J Am Dent Assoc. 1994;125:1229.
41. Kerezoudis NP, Sisko GJ, Tsatsas V. Bilateral buccal radicular groove in maxillary incisors: case report. Int Endod J. 2003;36(12):898.
42. Kirchoff DA, Gerstein H. Presurgical occlusal contouring for root amputation procedures. Oral Surg. 1969;27:379.
43. Kirkham DB. The location and incidence of accessory pulpal canals in periodontal pockets. J Am Dent Assoc. 1975;91:353.
44. Klavan B. Clinical observation following root amputation in maxillary molar teeth. J Periodontol. 1975;46:1.
45. Koenigs JF, Brilliant JD, Foreman DW. Preliminary scanning electron microscope investigations of accessory foramina in the furcation areas of human molar teeth. Oral Surg Oral Med Oral Pathol. 1974;38:773.
46. Kozlovsky A, Tal H. Forced eruption combined with gingival fiberotomy: a technique for clinical crown lengthening. J Clin Periodontol. 1988;15:534.
47. Kramer IR. The vascular architecture of the human dental pulp. Arch Oral Biol. 1960;2:177.
48. Langeland K, Rodrigues H, Dowden W. Periodontal disease, bacteria and pulpal histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1974;37:257.
49. Lantelme RL, Handelman SL, Herbison RJ. Dentin formation in periodontally diseased teeth. J Dent Res. 1976;55:48.
50. Lee KW, Lee EC, Poon KY. Palato-gingival grooves in maxillary incisors. Br Dent J. 1968;124:14.
51. Leknes KN, Lie T, Selvig KA. Cemental tear: a risk factor in periodontal attachment loss. J Periodontol. 1996;67(6):583.
52. Lowman JV, Burke RS, Pelleu GB. Patent accessory canals: incidence in molar furcation region. Oral Surg Oral Med Oral Pathol. 1973;36:580.
53. Mandi FA. Histological study of the pulp changes caused by periodontal disease. J Br Endod Soc. 1972;6:80.
54. Mantzikos T, Shamus I. Forced eruption and implant site development: soft tissue response. Am J Orthod Dentofacial Orthop. 1997;112(6):596.
55. Masters DH, Hoskins SW. Projection of cervical enamel into molar furcations. J Periodontol. 1964;35:49.
56. Mazur B, Massler M. Influence of periodontal disease on the dental pulp. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1964;17:592.
57. Miyashita H, Bergenholtz G, Grondahl K, Wennstrom JL. Impact of endodontic conditions on marginal bone loss. J Periodontol. 1998;69:158.
58. Obermayr G, Walton RE, Leary JM, Krell KV. Vertical root fracture and relative deformation during obturation and post cementation. J Prosthet Dent. 1991;66:181.
59. Pecora G, Baek SH, Rethnam S, Kim S. Barrier membrane techniques in endodontic microsurgery. Dent Clin North Am. 1997;41:585.
60. Pecora G, Kim S, Celleti R, Davarpanah M. The guided tissue regeneration principle in endodontic surgery: one year postoperative results of large periapical lesions. Int Endod J. 1995;28(1):41.
61. Perlmutter S, Tagger M, Tagger E, Abram M. Effect of the endodontic status of the tooth on experimental periodontal reattachment in baboons: a preliminary investigation. Oral Surg Oral Med Oral Pathol. 1987;63:232.
62. Pinto VS, Zuolo ML, Mellonig JT. Guided bone regeneration in the treatment of a large periapical lesion: a case report. Pract Periodontics Aesthet Dent. 1995;7:76.
63. Pitts DL, Natkin E. Diagnosis and treatment of vertical root fractures. J Endod. 1983;9:338.
64. Polson AM. Periodontal destruction associated with vertical root fracture: report of four cases. J Periodontol. 1977;48:27.
65. Rubach WC, Mitchell DF. Periodontal disease, accessory canals and pulp pathosis. J Periodontol. 1965;36:34.
66. Sanders JJ, Sepe WW, Bowers GM, et al. Clinical evaluation of freeze-dried bone allograft in periodontal osseous defects. III. Composite freeze-dried bone allografts with and without autogenous bone grafts. J Periodontol. 1983;54:1.
67. Saunders RL. X-ray microscopy of the periodontal and dental pulp vessels in the monkey and in man. Oral Surg Oral Med Oral Pathol. 1966;22:503.
68. Schallhorn RG. Long-term evaluation of osseous grafts in periodontal therapy. Int Dent J. 1980;30:101.
69. Seltzer S, Bender IB, Nazimov H, Sinai I. Pulpitis induced interradicular periodontal change in experimental animals. J Periodontol. 1967;38:124.
70. Seltzer S, Bender IB, Ziontz M. The interrelationship of pulp and periodontal disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endodon. 1963;16:1474.
71. Silverstein L, Shatz PC, Amato AL, Kurtzman D. A guide to diagnosing and treating endodontic and periodontal lesions. Dent Today. 1998;17(4):112.
72. Simon JHS, Glick DH, Frank AL. The relationship of endodontic-periodontic lesions. J Periodontol. 1972;43:202.
73. Simring M, Goldberg M. The pulpal pocket approach: retrograde periodontitis. J Periodontol. 1964;35:22.
74. Sinai I, Soltanoff W. The transmission of pathologic changes between the pulp and the periodontal structures. Oral Surg Oral Med Oral Pathol. 1973;36:558.
75. Smukler H, Tagger M. Vital root amputation: a clinical and histologic study. J Periodontol. 1976;47:324.
76. Stahl SS. Pathogenesis of inflammatory lesions in pulp and periodontal tissues. Periodontics. 1966;4:190.
77. Stallard RE. Periodontic-endodontic relationships. Oral Surg Oral Med Oral Pathol. 1972;34:314.
78. Tagger M, Perlmutter S, Tagger E, Abrams M. Histological study of untreated pulps in hemisected teeth in baboons. J Endod. 1988;14:288.
79. Torabinejad M, Kiger RD. A histologic evaluation of dental pulp tissue of a patient with periodontal disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1985;59:198.
80. Tseng CC, Chen YH, Huang CC, Bowers GM. Correction of a large periradicular lesion and mucosal defect using combined endodontic and periodontal therapy: a case report. Int J Periodontics Restorative Dent. 1995;15:377.
81. Vertucci FJ, Williams RG. Furcation canals in the human mandibular first molar. Oral Surg Oral Med Oral Pathol. 1974;38:308.
82. Walton RE, Michelich RJ, Smith GN. The histopathogenesis of vertical root fractures. J Endod. 1984;10:48.
83. Ward HE. Preparation of furcally involved teeth. J Prosthet Dent. 1982;48:261.
84. Withers J, Brunsvold M, Killoy W, Rahe A. The relationship of palato-gingival grooves to localized periodontal disease. J Periodontol. 1981;52:41.
85. Whyman RA. Endodontic-periodontic lesion. I. Prevalence, etiology, and diagnosis. N Z Dent J. 1988;84:74.