3 Sterilization, Disinfection, and Antisepsis
An important aspect of the control of infections is an understanding of the principles of sterilization, disinfection, and antisepsis (Box 3-1).
Box 3-1
Definitions
Antisepsis: Use of chemical agents on skin or other living tissue to inhibit or eliminate microbes; no sporicidal action is implied
Disinfection: Use of physical procedures or chemical agents to destroy most microbial forms; bacterial spores and other relatively resistant organisms (e.g., mycobacteria, viruses, fungi) may remain viable; disinfectants are subdivided into high-, intermediate-, and low-level agents
Germicide: Chemical agent capable of killing microbes; spores may survive
High-level disinfectant: A germicide that kills all microbial pathogens except large numbers of bacterial spores
Intermediate-level disinfectant: A germicide that kills all microbial pathogens except bacterial endospores
Low-level disinfectant: A germicide that kills most vegetative bacteria and lipid-enveloped or medium-size viruses
Sporicide: Germicide capable of killing bacterial spores
Sterilization: Use of physical procedures or chemical agents to destroy all microbial forms, including bacterial spores
Sterilization
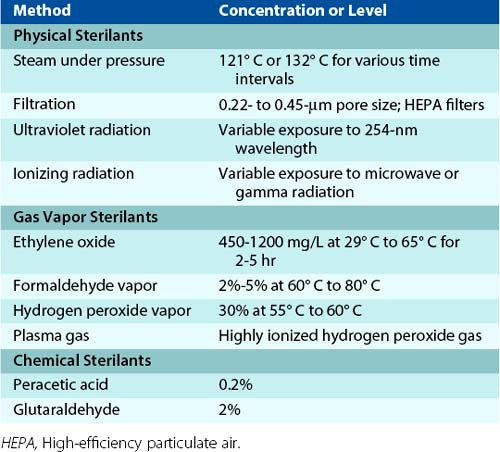
Sterilization is the total destruction of all microbes, including the more resilient forms such as bacterial spores, mycobacteria, nonenveloped (nonlipid) viruses, and fungi. This can be accomplished using physical, gas vapor, or chemical sterilants (Table 3-1).
Table 3-1 Methods of Sterilization
| Method | Concentration or Level |
|---|---|
| Physical Sterilants | |
| Steam under pressure | 121° C or 132° C for various time intervals |
| Filtration | 0.22- to 0.45-µm pore size; HEPA filters |
| Ultraviolet radiation | Variable exposure to 254-nm wavelength |
| Ionizing radiation | Variable exposure to microwave or gamma radiation |
| Gas Vapor Sterilants | |
| Ethylene oxide | 450-1200 mg/L at 29° C to 65° C for 2-5 hr |
| Formaldehyde vapor | 2%-5% at 60° C to 80° C |
| Hydrogen peroxide vapor | 30% at 55° C to 60° C |
| Plasma gas | Highly ionized hydrogen peroxide gas |
| Chemical Sterilants | |
| Peracetic acid | 0.2% |
| Glutaraldehyde | 2% |
HEPA, High-efficiency particulate air.
Physical sterilants, such as moist and dry heat, are the most common sterilizing methods used in hospitals and are indicated for most materials, except those that are heat sensitive or consist of toxic or volatile chemicals. Filtration is useful for removing bacteria and fungi from air (with high-efficiency particulate air [HEPA] filters) or from solutions. However, these filters are unable to remove viruses and some small bacteria. Sterilization by ultraviolet or ionizing radiation (e.g., microwave or gamma rays) is also commonly used. The limitation of ultraviolet radiation is that direct exposure is required.
Ethylene oxide is a commonly used gas vapor sterilant. Although it is highly efficient, strict regulations limit its use, because ethylene oxide is flammable, explosive, and carcinogenic to laboratory animals. Sterilization with formaldehyde gas is also limited, because the chemical is carcinogenic. Its use is restricted primarily to sterilization of HEPA filters. Hydrogen peroxide vapors are effective sterilants because of the oxidizing nature of the gas. This sterilant is used for the sterilization of instruments. A variation is plasma gas sterilization, in which hydrogen peroxide is vaporized, and then reactive free radicals are produced with either microwave-frequency or radio-frequency energy. Because this is an efficient sterilizing method that does not produce toxic byproducts, plasma gas sterilization has replaced many of the applications for ethylene oxide. However, it cannot be used with materials that absorb hydrogen peroxide or react with it.
Two chemical sterilants have also been used: peracetic acid and glutaraldehyde. Peracetic acid, an oxidizing agent, has excellent activity, and the end products (i.e., acetic acid and oxygen) are nontoxic. In contrast, safety is a concern with glutaraldehyde, and care must be used when handling this chemical.
Disinfection
Microbes are also destroyed by disinfection procedures, although more resilient organisms can survive. Unfortunately, the terms disinfection and sterilization are casually interchanged and can result in some confusion. This occurs because disinfection processes have been categorized as high level, intermediate level, and low level. High-level disinfection can generally approach sterilization in effectiveness, whereas spore forms can survive intermediate-level disinfection, and many microbes can remain viable when exposed to low-level disinfection.
Even the classification of disinfectants (Table 3-2) by their level of activity is misleading. The effectiveness of these procedures is influenced by the nature of the item to be disinfected, number and resilience of the contaminating organisms, amount of organic material present (which can inactivate the disinfectant), type and concentration of disinfectant, and duration and temperature of exposure.
Table 3-2 Methods of Disinfection
| Method | Concentration (Level of Activity) |
|---|---|
| Heat | |
| Moist heat | 75° C to 100° C for 30 min (high) |
| Liquid | |
| Glutaraldehyde | 2%-3.5% (high) |
| Hydrogen peroxide | 3%-25% (high) |
| Formaldehyde | 3%-8% (high/intermediate) |
| Chlorine dioxide | Variable (high) |
| Peracetic acid | Variable (high) |
| Chlorine compounds | 100-1000 ppm of free chlorine (high) |
| Alcohol (ethyl, isopropyl) | 70%-95% (intermediate) |
| Phenolic compounds | 0.4%-5.0% (intermediate/low) |
| Iodophor compounds | 30-50 ppm of free iodine/L (intermediate) |
| Quaternary ammonium compounds | 0.4%-1.6% (low) |
High-level disinfectants are used for items involved with invasive procedures that cannot withstand sterilization procedures (e.g., certain types of endoscopes and surgical instruments with plastic or other components that cannot be autoclaved). Disinfection of these and other items is most effective if cleaning the surface to remove organic matter precedes treatment. Examples of high-level disinfectants include treatment with moist heat and use of liquids such as glutaraldehyde, hydrogen peroxide, peracetic acid, and chlorine compounds.
Intermediate-level disinfectants (i.e., alcohols, iodophor compounds, phenolic compounds) are used to clean surfaces or instruments where contamination with bacterial spores and other highly resilient organisms is unlikely. These have been referred to as semicritical instruments and devices and include flexible fiberoptic endoscopes, laryngoscopes, vaginal specula, anesthesia breathing circuits, and other items.
Low-level disinfectants (i.e., quaternary ammonium compounds) are used to treat noncritical instruments and devices, such as blood pressure cuffs, electrocardiogram electrodes, and stethoscopes. Although these items come into contact with patients, they do not penetrate through mucosal surfaces or into sterile tissues.
The level of disinfectants used for environmental surfaces is determined by the relative risk these surfaces pose as a reservoir for pathogenic organisms. For example, a higher level of disinfectant should be used to clean the surface of instruments contaminated with blood than that used to clean surfaces that are “dirty,” such as floors, sinks, and countertops. The exception to this rule is if a particular surface has been implicated in a nosocomial infection, such as a bathroom contaminated with Clostridium difficile (spore-forming anaerobic bacterium) or a sink contaminated with Pseudomonas aeruginosa. In these cases, a disinfectant with appropriate activity against the implicated pathogen should be selected.
Antisepsis
Antiseptic agents (Table 3-3) are used to reduce the number of microbes on skin surfaces. These compounds are selected for their safety and efficacy. A summary of their germicidal properties is presented in Table 3-4. Alcohols have excellent activity against all groups of organisms, except spores, and are nontoxic, although they tend to dry the skin surface because they remove lipids. They also do not have residual activity and are inactivated by organic matter. Thus the surface of the skin should be cleaned before alcohol is applied. Iodophors are also excellent skin antiseptic agents, having a range of activity similar to that of alcohols. They are slightly more toxic to the skin than is alcohol, have limited residual activity, and are inactivated by organic matter. Iodophors and iodine preparations are frequently used with alcohols for disinfecting the skin surface. Chlorhexidine has broad antimicrobial activity, although it kills organisms at a much slower rate than alcohol. Its activity persists, although organic material and high pH levels decrease its effectiveness. The activity of parachlorometaxylenol (PCMX) is limited primarily to gram-positive bacteria. Because it is nontoxic and has residual activity, it has been used in handwashing products. Triclosan is active against bacteria but not against many other organisms. It is a common antiseptic agent in deodorant soaps and some toothpaste products.
| Antiseptic Agent | Concentration |
|---|---|
| Alcohol (ethyl, isopropyl) | 70%-90% |
| Iodophors | 1-2 mg of free iodine/L; 1%-2% available iodine |
| Chlorhexidine | 0.5%-4.0% |
| Parachlorometaxylenol | 0.50%-3.75% |
| Triclosan | 0.3%-2.0% |
Mechanisms of Action
The following section briefly reviews the mechanisms by which the most common sterilants, disinfectants, and antiseptics work.
Moist Heat
Attempts to sterilize items using boiling water are inefficient, because only a relatively low temperature (100° C) can be maintained. Indeed, spore formation by a bacterium is commonly demonstrated by boiling a solution of organisms and then subculturing the solution. Boiling vegetative organisms kills them, but the spores remain viable. In contrast, steam under pressure in an autoclave is a very effective form of sterilization; the higher temperature causes denaturation of microbial proteins. The rate of killing organisms during the autoclave process is rapid but is influenced by the temperature and duration of autoclaving, size of the autoclave, flow rate of the steam, density and size of the load, and placement of the load in the chamber. Care must be taken to avoid creating air pockets, which inhibit penetration of the steam into the load. In general, most autoclaves are operated at 121° C to 132° C for 15 minutes or longer. Including commercial preparations of Bacillus stearothermophilus spores can help monitor the effectiveness of sterilization. An ampule of these spores is placed in the center of the load, removed at the end of the autoclave process, and incubated at 37° C. If the sterilization process is successful, the spores are killed and the organisms fail to grow.
Ethylene Oxide
Ethylene oxide is a colorless gas (soluble in water and common organic solvents) that is used to sterilize heat-sensitive items. The sterilization process is relatively slow and is influenced by the concentration of gas, relative humidity and moisture content of the item to be sterilized, exposure time, and temperature. The exposure time is reduced by 50% for each doubling of ethylene oxide concentration. Likewise, the activity of ethylene oxide approximately doubles with each temperature increase of 10° C. Sterilization with ethylene oxide is optimal in a relative humidity of approximately 30%, with decreased activity at higher or lower humidity. This is particularly problematic if the contaminated organisms are dried onto a surface or lyophilized. Ethylene oxide exerts its sporicidal activity through the alkylation of terminal hydroxyl, carboxyl, amino, and sulfhydryl groups. This process blocks the reactive groups required for many essential metabolic processes. Examples of other strong alkylating gases used as sterilants are formaldehyde and β-propiolactone. Because ethylene oxide can damage viable tissues, the gas must be dissipated before the item can be used. This aeration period is generally 16 hours or longer. The effectiveness of sterilization is monitored with the Bacillus subtilis spore test.
Aldehydes
As with ethylene oxide, aldehydes exert their effect through alkylation. The two best-known aldehydes are formaldehyde and glutaraldehyde, both of which can be used as sterilants or high-level disinfectants. Formaldehyde gas can be dissolved in water (creating a solution called formalin) at a final concentration of 37%. Stabilizers, such as methanol, are added to formalin. Low concentrations of formalin are bacteriostatic (i.e., they inhibit but do not kill organisms), whereas higher concentrations (e.g., 20%) can kill all organisms. Combining formaldehyde with alcohol (e.g., 20% formalin in 70% alcohol) can enhance this microbicidal activity. Exposure of skin or mucous membranes to formaldehyde can be toxic. Glutaraldehyde is less toxic for viable tissues, but it can still cause burns on the skin or mucous membranes. Glutaraldehyde is more active at alkaline pH levels (“activated” by sodium hydroxide) but is less stable. Glutaraldehyde is also inactivated by organic material; so items to be treated must first be cleaned.
Oxidizing Agents
Examples of oxidants include ozone, peracetic acid, and hydrogen peroxide, with the last used most commonly. Hydrogen peroxide effectively kills most bacteria at a concentration of 3% to 6% and kills all organisms, including spores, at higher concentrations (10% to 25%). The active oxidant form is not hydrogen peroxide but rather the free hydroxyl radical formed by the decomposition of hydrogen peroxide. Hydrogen peroxide is used to disinfect plastic implants, contact lenses, and surgical prostheses.
Halogens
Halogens, such as compounds containing iodine or chlorine, are used extensively as disinfectants. Iodine compounds are the most effective halogens available for disinfection. Iodine is a highly reactive element that precipitates proteins and oxidizes essential enzymes. It is microbicidal against virtually all organisms, including spore-forming bacteria and mycobacteria. Neither the concentration nor the pH of the iodine solution affects the microbicidal activity, although the efficiency of iodine solutions is increased in acid solutions because more free iodine is liberated. Iodine acts more rapidly than do other halogen compounds or quaternary ammonium compounds. However, the activity of iodine can be reduced in the presence of some organic and inorganic compounds, including serum, feces, ascitic fluid, sputum, urine, sodium thiosulfate, and ammonia. Elemental iodine can be dissolved in aqueous potassium iodide or alcohol, or it can be complexed with a carrier. The latter compound is referred to as an iodophor (iodo, “iodine”; phor, “carrier”). Povidone iodine (iodine complexed with polyvinylpyrrolidone) is used most commonly and is relatively stable and nontoxic to tissues and metal surfaces, but it is expensive compared with other iodine solutions.
Chlorine compounds are also used extensively as disinfectants. Aqueous solutions of chlorine are rapidly bactericidal, although their mechanisms of action are not defined. Three forms of chlorine may be present in water: elemental chlorine (Cl2), which is a very strong oxidizing agent; hypochlorous acid (HOCl); and hypochlorite ion (OCl2). Chlorine also combines with ammonia and other nitrogenous compounds to form chloramines, or N-chloro compounds. Chlorine can exert its effect by the irreversible oxidation of sulfhydryl (SH) groups of essential enzymes. Hypochlorites are believed to interact with cytoplasmic components to form toxic N-chloro compounds, which interfere with cellular metabolism. The efficacy of chlorine is inversely proportional to the pH, with greater activity observed at acid pH levels. This is consistent with greater activity associated with hypochlorous acid rather than with hypochlorite ion concentration. The activity of chlorine compounds also increases with concentration (e.g., a twofold increase in concentration results in a 30% decrease in time required for killing) and temperature (e.g., a 50% to 65% reduction in killing time with a 10° C increase in temperature). Organic matter and alkaline detergents can reduce the effectiveness of chlorine compounds. These compounds demonstrate good germicidal activity, although spore-forming organisms are 10- to 1000-fold more resistant to chlorine than are vegetative bacteria.
Phenolic Compounds
Phenolic compounds (germicides) are rarely used as disinfectants. However, they are of historical interest, because they were used as a comparative standard for assessing the activity of other germicidal compounds. The ratio of germicidal activity by a test compound to that by a specified concentration of phenol yielded the phenol coefficient. A value of 1 indicated equivalent activity, greater than 1 indicated activity less than phenol, and less than 1 indicated activity greater than phenol. These tests are limited, because phenol is not sporicidal at room temperature (but is sporicidal at temperatures approaching 100° C), and it has poor activity against non–lipid-containing viruses. This is understandable, because phenol is believed to act by disrupting lipid-containing membranes, resulting in leakage of cellular contents. Phenolic compounds are active against the normally resilient mycobacteria, because the cell wall of these organisms has a very high concentration of lipids. Exposure of phenolics to alkaline compounds significantly reduces their activity, whereas halogenation of the phenolics enhances their activity. The introduction of aliphatic or aromatic groups into the nucleus of halogen phenols also increases their activity. Bis-phenols are two phenol compounds linked together. The activity of these compounds can also be potentiated by halogenation. One example of a halogenated bis-phenol is hexachlorophene, an antiseptic with activity against gram-positive bacteria.
Quaternary Ammonium Compounds
Quaternary ammonium compounds consist of four organic groups covalently linked to nitrogen. The germicidal activity of these cationic compounds is determined by the nature of the organic groups, with the greatest activity observed with compounds having 8- to 18-carbon long groups. Examples of quaternary ammonium compounds include benzalkonium chloride and cetylpyridinium chloride. These compounds act by denaturing cell membranes to release the intracellular components. Quaternary ammonium compounds are bacteriostatic at low concentrations and bactericidal at high concentrations; however, organisms such as Pseudomonas, Mycobacterium, and the fungus Trichophyton are resistant to these compounds. Indeed, some Pseudomonas strains can grow in quaternary ammonium solutions. Many viruses and all bacterial spores are also resistant. Ionic detergents, organic matter, and dilution neutralize quaternary ammonium compounds.
Alcohols
The germicidal activity of alcohols increases with increasing chain length (maximum of five to eight carbons). The two most commonly used alcohols are ethanol and isopropanol. These alcohols are rapidly bactericidal against vegetative bacteria, mycobacteria, some fungi, and lipid-containing viruses. Unfortunately, alcohols have no activity against bacterial spores and have poor activity against some fungi and non–lipid-containing viruses. Activity is greater in the presence of water. Thus 70% alcohol is more active than 95% alcohol. Alcohol is a common disinfectant for skin surfaces and, when followed by treatment with an iodophor, is extremely effective for this purpose. Alcohols are also used to disinfect items such as thermometers.
1. Define the following terms and give three examples of each: sterilization, disinfection, and antisepsis.
2. Define the three levels of disinfection and give examples of each. When would each type of disinfectant be used?
3. What factors influence the effectiveness of sterilization with moist heat, dry heat, and ethylene oxide?
4. Give examples of each of the following disinfectants and their mode of action: iodine compounds, chlorine compounds, phenolic compounds, and quaternary ammonium compounds.
1. There is not a uniform definition of sterilization and disinfection. In general, sterilization represents the total destruction of all microbes, including the more resilient forms such as bacterial spores, mycobacteria, nonenveloped viruses, and fungi. Examples of agents used for sterilization are ethylene oxide, formaldehyde gas, hydrogen peroxide, peracetic acid, and glutaraldehyde. Disinfection results in the destruction of most organisms, although the more resilient microbes can survive some disinfection procedures. Examples of disinfectants include moist heat, hydrogen peroxide, and phenolic compounds. Antisepsis is used to reduce the number of microbes on the skin surfaces. Examples of antiseptic agents include alcohols, iodophors, chlorhexidine, parachlorometaxylenol, and triclosan.
2. Disinfection is subdivided into high-level, intermediate-level, and low-level. High-level disinfectants include moist heat, glutaraldehyde, hydrogen peroxide, peracetic acid, and chlorine compounds. Intermediate-level disinfection includes alcohols, iodophor compounds, and phenolic compounds. Low-level disinfectants include quaternary ammonium compounds. Although some agents are used both for sterilization and disinfection, the difference is the concentration of the agent and duration of treatment. The types of disinfectants that are used are determined by the nature of the material to be disinfected and how it will be used. If the material will be used for an invasive procedure but cannot withstand sterilization procedures (e.g., endoscopes, surgical instruments that cannot be autoclaved), then a high level disinfectant would be used. Intermediate-level disinfectants are used to clean surfaces and instruments where contamination with highly resilient organisms is unlikely. Low-level disinfectants are used to clean noncritical instruments and devices (e.g., blood pressure cuffs, electrodes, stethoscopes).
3. The effectiveness of moist heat is greatest when applied under pressure. This allows the temperature to be elevated. Other factors that determine the effectiveness of moist heat are the duration of exposure and penetration of the steam into the contaminated material (determined by load size and flow rate of steam). Dry heat is effective if applied at a high temperature for a long duration. Ethylene oxide sterilization is a slow process that is influenced by the concentration of the gas, relative humidity, exposure time, and temperature. The effectiveness improves with a higher concentration of ethylene oxide, elevated temperatures, and a relative humidity of 30%.
4. Iodine compounds precipitate proteins and oxidize essential enzymes. Examples include tincture of iodine and povidone iodine (iodine complexed with polyvinylpyrrolidone). Chlorine compounds are strong oxidizing agents, although the precise mechanism of action is not well defined. Examples include elemental chlorine, hypochlorous acid, and hypochlorite ion. The most common commercial chlorine compound is bleach. Phenolic compounds act by disrupting lipid-containing membranes, resulting in a leakage of cellular contents. Examples include phenol (carbolic acid), o-phenylphenol, o-benzyl-p-chlorophenol, and p-tert-amyl-phenol. Quaternary ammonium compounds also denature cell membranes and include benzalkonium chloride and cetylpyridinium chloride.