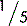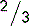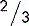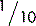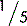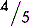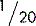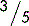APPENDIX D Answers
Answers to section 1 questions
Section 1
Prescription
Label
2. They can assist in prompt identification of different strengths of medicines in an emergency but may cause mistakes if the correct colour is misidentified.
3. It may be more economical for health authorities/retail outlets to purchase a product from overseas (parallel imports)
5.
Label 2
Metric system
3. Universal system of weights and measures which is used to express the strength of a medicine – the metric system
Answers to section 2 questions
4. The curve formed at the surface of a liquid in a container the centre of which is used as the precise volume
5. Do not administer the medicine; check your calculations again; try using another method; ask a reliable colleague to make an independent calculation; refer back to the prescriber