12 Lymphoid (Immune) System
The lymphoid system protects against foreign invasions, such as macromolecules and microorganisms, and against virally altered cells. This system is composed of collections of nonencapsulated cells, known as the diffuse lymphoid system, and encapsulated collections of cells, lymph nodes, tonsils, thymus, and spleen.
Overview of the Lymphoid System
There are three lines of defense that the body has: the epithelium, which isolates the body from the external environment; the epidermis; and the various mucosae. These form physical obstacles that usually prevent foreign pathogens from gaining access to the sterile body compartments. These relatively thin barriers can be damaged by trauma, and some pathogens are able to penetrate them even if intact. Two additional lines of defense are innate (nonspecific) and adaptive (acquired) immune systems. In most cases, these systems can protect the body when these barriers have been violated.
Innate Immune System
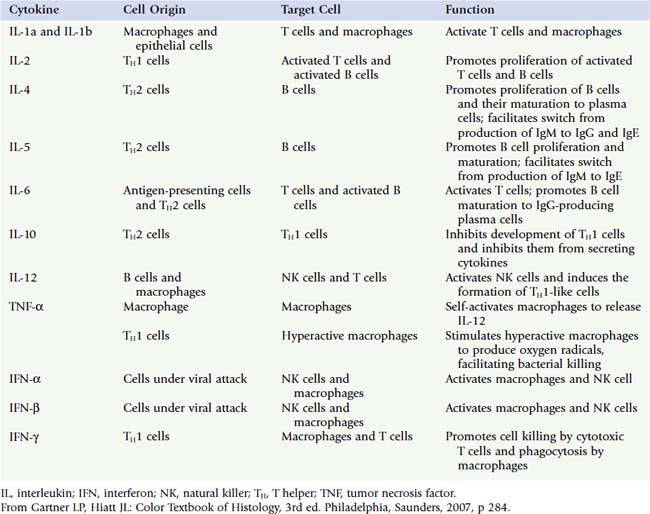
The more primitive and evolutionarily older but faster-responding innate (natural) immune system consists of complement, antimicrobial peptides, cytokines, macrophages, neutrophils, natural killer (NK) cells, and Toll-like receptors. This system is nonspecific and does not establish an immunologic memory of the agent that elicited its attack. Table 12.1 lists acronyms used in this chapter.
Table 12.1 ACRONYMS AND ABBREVIATIONS
| Acronym/Abbreviation | Definition |
|---|---|
| ADDC | Antibody-dependent cellular cytotoxicity |
| APC | Antigen-presenting cell |
| BALT | Bronchus-associated lymphoid tissue |
| B lymphocyte | Bursa-derived lymphocyte (bone marrow–derived lymphocyte) |
| C3b | Complement 3b |
| CD | Cluster of differentiation molecule (followed by an Arabic numeral) |
| CLIP | Class II associated invariant protein |
| CSF | Colony-stimulating factor |
| CTL | Cytotoxic T lymphocyte (T killer cell) |
| Fab | Antigen-binding fragment of an antibody |
| Fc | Crystallized fragment (constant fragment of an antibody) |
| GALT | Gut-associated lymphoid tissue |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HEV | High endothelial venule |
| IFN-γ | Interferon-γ |
| IL | Interleukin (followed by an Arabic numeral) |
| M cell | Microfold cell |
| MAC | Membrane attack complex |
| MALT | Mucosa-associated lymphoid tissue |
| MHC I and MHC II | Major histocompatibility class I molecules and class II molecules |
| MIIC vesicle | MHC class II–enriched compartment |
| NK cell | Natural killer cell |
| PALS | Periarterial lymphatic sheath |
| SIGs | Surface immunoglobulins |
| TAP | Transporter protein (1 and 2) |
| TCM | Central memory T cell |
| TCR | T cell receptor |
| TEM | Effector T memory cell |
| TH cell | T helper cell (followed by an Arabic numeral) |
| TLRs | Toll-like receptors |
| T lymphocyte | Thymus-derived lymphocyte |
| TNF-α | Tumor necrosis factor-α |
| T reg cell | Regulatory T cell |
| TSH | Thyroid-stimulating hormone |
From Gartner LP, Hiatt JL: Color Textbook of Histology, 3rd ed. Philadelphia, Saunders, 2007, p 274.
Table 12.2 TOLL-LIKE RECEPTORS AND THEIR PUTATIVE FUNCTIONS
| Domains | Receptor Pair | Function |
|---|---|---|
| Intracellular and extracellular (on cell membrane) | TLR1–TLR2 | Binds to bacterial lipoprotein; binds to certain proteins of parasites |
| TLR2–TLR6 | Binds to lipoteichoic acid of gram-positive bacterial wall and to zymosan | |
| TLR4–TLR4 | Binds to LPS of gram-negative bacteria | |
| TLR5–?* | Binds to flagellin of bacterial flagella | |
| TLR11–?* | Host recognition of Toxoplasmosis gondii | |
| Intracellular only | TLR3–?* | Binds to double-stranded viral RNA |
| TLR7–?* | Binds to single-stranded viral RNA | |
| TLR8–?* | Binds to single-stranded viral RNA | |
| TLR9–?* | Binds to bacterial and viral DNA | |
| TLR10–?* | Unknown | |
| TLR12–?* | Unknown |
LPS, lipopolysaccharide.
* Currently, TLR partner is unknown.
From Gartner LP, Hiatt JL: Color Textbook of Histology, 3rd ed. Philadelphia, Saunders, 2007, p 275.
Adaptive Immune System
The adaptive (acquired) immune system is specific and composed of T and B lymphocytes (T and B cells) and antigen-presenting cells (APCs), although they also use the components of the innate immune system to perform their task of protecting the body. These cells not only release cytokines to communicate with each other, but also contact one another, and by recognizing particular membrane bound molecules, they induce specific responses in the other cells to combat foreign substances known as antigens. By definition:
The cells of the adaptive immune system release cytokines, recruiting cells of the innate immune system to assist in the response against the invading antigens. The adaptive immune system is typified by the following four characteristics: specificity, diversity, memory, and ability to distinguish between self and nonself. There are two types of immune reactions mounted by the adaptive immune system:
The cells of the adaptive immune system develop in the bone marrow where B cells mature and develop into immunocompetent cells. T cells have to leave the bone marrow and enter the thymic cortex, however, to develop into immunocompetent cells. Immunocompetent B and T cells leave their primary lymphoid organs (bone marrow and thymus) to enter diffuse lymphoid tissue, lymph nodes, and the spleen—collectively known as secondary lymphoid organs. Here they search out and contact antigens.
Clonal Selection and Expansion
To be able to recognize and eliminate all the possible antigens and pathogens that one may contact in a lifetime, during embryogenesis about 1015 lymphocytes are established. Each lymphocyte has the property of recognizing a particular foreign antigen, and each proliferates to form a cluster of identical cells, where each cluster is known as a clone. The members of each clone possess the same membrane-bound antibodies (surface immunoglobulins [sIgs]) or the same T cell receptor (TCR) for B cells and T cells, respectively. If the sIg or the TCR is against the macromolecules of the self, that clone is either eliminated during embryonic development (clonal deletion) or inactivated so that it cannot initiate an immune response (clonal anergy), protecting the individual from autoimmunity.
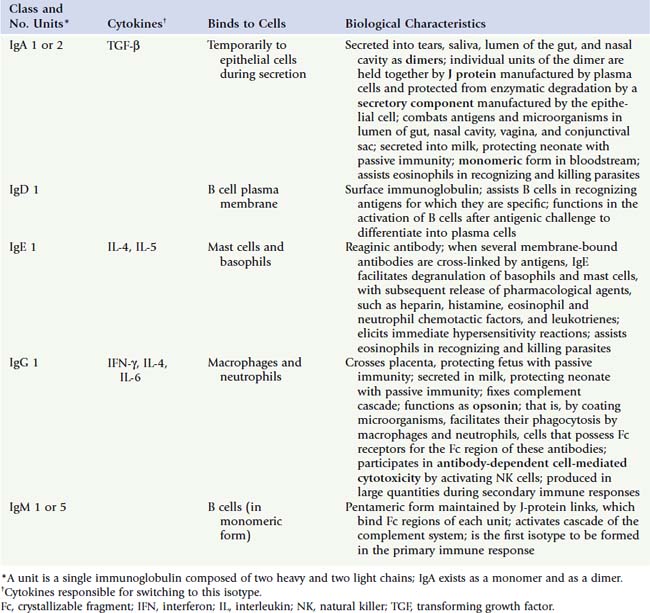
Immunoglobulins (Antibodies)
A special family of glycoproteins, known as antibodies (immunoglobulins), is manufactured in enormous numbers by plasma cells and in small quantities by B cells (that place them on their cell membranes as sIgs, B cell receptors). A representative antibody (IgG) resembles the letter Y and is composed of four polypeptide chains (Fig. 12.1).
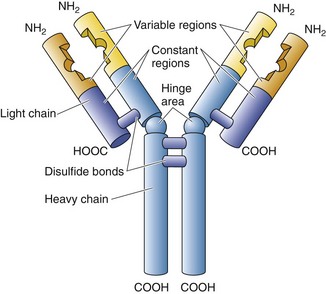
Figure 12.1 Drawing of a typical IgG.
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 3rd ed. Philadelphia, Saunders, 2007, p 278.)
Enzymatic cleavage of an antibody by papain occurs at the hinge region and forms an Fc fragment, the stem, whose amino acid sequence is constant, and two Fab fragments (antigen binding), each composed of a light chain and part of a heavy chain, whose distal portions are specific in their ability to bind only one particular epitope (the antigenic determinant region of an antigen). There are five different classes of immunoglobulins depending on various characteristic differences (Table 12.3).
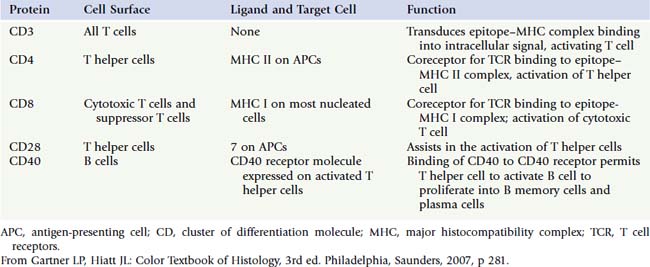
Cells of the Adaptive and Innate Immune Systems
The adaptive and innate immune systems rely on the following cells: B cells, T cells, macrophages and their subtype APCs, and NK cells.
B Lymphocytes (B Cells)
B cells develop and become immunocompetent in the bone marrow. These cells manufacture IgM and IgD antibodies and insert their Fc end into their plasmalemma (sIgs) so that the Fab end projects into the external milieu. The Fc portion is affixed to the cell membrane by two transmembrane proteins, Igβ and Igα, that, when the sIg contacts an epitope, transduce that information intracellularly, starting a sequence of steps whose consequence is:
Certain polysaccharides, such as peptidoglycans of bacterial membranes, are thymic-independent antigens because they can initiate a humoral immune response without T cell intermediaries. Only IgM antibodies are produced, however, and B memory cells are not formed.
T Lymphocytes (T Cells)
T cells develop in the bone marrow but have to enter the cortex of the thymus to express the necessary plasmalemma-bound molecules to become immunocompetent (see later in the section on the thymus). In contrast to B lymphocytes, T cells:
T cells can become activated only if they recognize not only the epitope but also the MHC molecule. If the T cell does not recognize the MHC molecule, it cannot mount an immune response; therefore, T cells are said to be MHC-restricted. T lymphocytes are classified into three broad categories:
Naïve T cells are immunologically competent and have CD45RA molecules on their plasmalemma, but have not as yet been challenged immunologically. When they are challenged, they proliferate to form memory and effector T lymphocytes.
Memory T cells possess CD45R0 molecules on their plasmalemma and are of two types: central memory T cells (TCMs), whose cell membrane sports CR7+ molecules, and effector memory T cells (CR7− cells, TEMs), which do not have CR7 molecules on their surface. These cells establish the immunologic memory of the immune system. TCMs reside in the paracortex of lymph nodes where they bind to APCs, inducing the APCs to release IL-12. This cytokine causes TCMs to proliferate and form TEMs. The newly formed TEMs travel to the site of inflammation, differentiate into effector T cells, and respond to the antigenic challenge.
CLINICAL CONSIDERATIONS
IgM is the first antibody to be formed by B cells until TH cells instruct them to switch to IgG synthesis. Individuals who have defective CD40 ligands are unable to switch isotypes and have excess blood levels of IgM, a condition known as hyper-IgM syndrome, resulting in humoral immunodeficiency–induced chronic infections.
All nucleated cells possess MHC I molecules, and these have to be recognized by CTLs to mount an immune response. Many tumor cells and virally altered cells stem the synthesis of MHC I molecules to avoid being recognized and destroyed by CTLs. NK cells are able to destroy these cells, however, because they do not need to recognize MHC I molecules.
Effector T Cells
Effector memory T cells give rise to effector T cells, three different groups of immunocompetent cells that have the ability to mount an immune response. The three categories are TH cells, CTLs and T killer cells, and T reg cells.
All TH cells display CD4 molecules on their plasmalemma and have the ability to work with cells that belong to the innate and the adaptive immune systems. TH cells also function in activating CTLs to kill foreign and virally altered cells and in activating B cells to differentiate into plasma cells to form antibodies. There are four subcategories of TH cells (a fifth one was placed into the T reg cell category), and they all secrete various cytokines (Table 12.6):
CLINICAL CONSIDERATIONS
Occasionally, the immune system develops a dysfunction, as in Graves’ disease, in which the thyroid follicular cells’ receptors for thyroid- stimulating hormone are no longer recognized as part of the self. Instead, these receptors become viewed as if they were antigens. Conditions where the self is viewed as if it were foreign are known as autoimmune diseases. Antibodies bind to the TSH receptors, causing the follicular cells to secrete an overabundance of thyroid hormone. Patients with Graves’ disease present with an enlarged thyroid gland and exophthalmos (protruding eyeballs).
Major Histocompatibility Complex Molecules
MHCs, located on the surface of APCs, including virally attacked and virally altered cells, function in holding short peptides cleaved from antigens, known as epitopes, that are presented to T cells. MHC molecules of every individual differ from MHC molecules of other individuals; T cells can recognize the self. There are two types of MHC molecules:
Loading Major Histocompatibility Complex I Molecules
Proteasomes cleave endogenous proteins into epitopes 8 to 12 amino acids in length. The epitopes, transferred into the rough endoplasmic reticulum by transporter proteins, TAP1 and TAP2, are bound to MHC I, and the complex is transferred to the Golgi apparatus for packaging and transport. The MHC I–epitope complex is transported to the plasma membrane of the cell to be presented to CTLs, which determine whether or not the cell has to be destroyed. If the cell is producing viral protein, it is driven into apoptosis; if the cell is producing self proteins, the cell is allowed to live.
Loading Major Histocompatibility Complex II Molecules
Antigen-Presenting Cells
APCs phagocytose and process antigens, load the epitopes on MHC II molecules, place the complex on their plasma membrane, and present the complex to T cells. APCs release cytokines such as IL-1, IL-6, IL-12, and TNF-α, which affect the immune response and a host of other signaling molecules that function outside the immune system.
Interaction Among Lymphoid Cells
To mount an immune response, lymphoid cells interact with one another and examine each other’s surface molecules. If the molecules of the presenter cell are not recognized, the lymphocyte to which they are presented is driven into apoptosis. If the molecules are recognized, the lymphocyte that recognizes them becomes activated—that is, it proliferates and differentiates. For activation to occur:
TH2 Cell–Mediated Humoral Immune Response
For all thymus-dependent antigens, B cells internalize and disassemble their antigen-sIg complex, load the MHC II, and place the MHC II–epitope complex on its plasmalemma to present it to a TH2 cell (Fig.12.2).
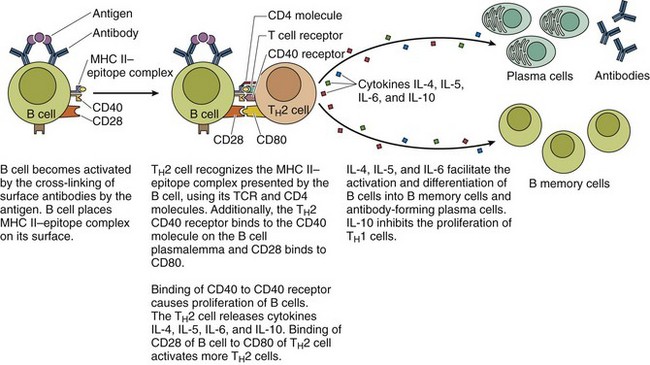
Figure 12.2 Activation of B cells by TH2 cells to produce B memory cells and antibody-forming plasma cells. The humoral response to thymus-independent antigens and the interaction with TH2 cells are not required.
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 3rd ed. Philadelphia, Saunders, 2007, p 285.)
CLINICAL CONSIDERATIONS
Acquired immunodeficiency syndrome (AIDS) is caused by human immunodeficiency virus (HIV), which has the ability to bind to the CD4 molecules of TH cells. After binding to the CD4 molecules, the virus introduces its core into the TH cell, debilitating it. As the virus increases in number and infects additional TH cells, the number of TH cells diminishes, and the patient is unable to mount an immune response and succumbs to opportunistic infections.
TH1 Cell–Mediated Killing of Virally Transformed Cells
The ability of a CTL to kill a virally transformed cell depends on two conditions:
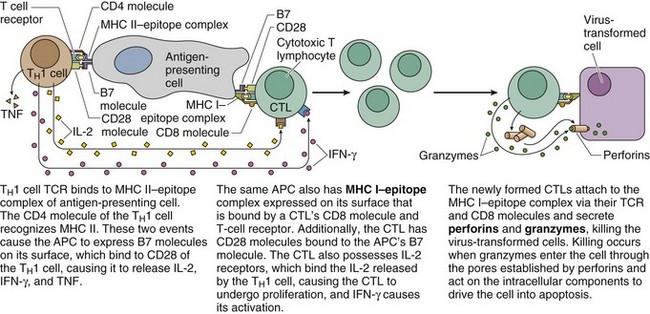
Figure 12.3 Activation of CTLs by TH1 cells. The TH1 cell and the CTL must be complexed to the same APC.
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 3rd ed. Philadelphia, Saunders, 2007, p 286.)
The activated CTLs bind, via TCR and CD8, to the epitope–MHC I complex of the virally transformed cells and kill the transformed cells by:
TH1 Cells Assist Macrophages in Killing Phagocytosed Bacteria
Macrophages have to be activated by TH1 cells before they can destroy bacteria that they phagocytosed. This process requires that first the TH1 cell become activated; the activated TH1 cell then instructs the macrophage to destroy the bacteria in its phagosomes (Fig. 12.4). The activation of the TH1 cell requires two steps:
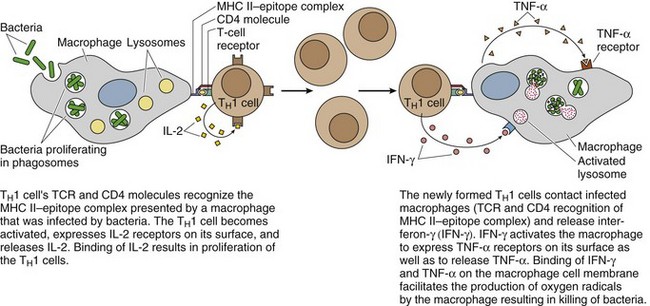
Figure 12.4 Activation of macrophages by TH1 cells.
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 3rd ed. Philadelphia, Saunders, 2007, p 287.)
The newly formed, activated TH1 cells bind to the macrophages with bacteria in their phagosomes.
Lymphoid Organs
Lymphoid organs are of two types:
Thymus
The thymus, a small endodermally derived organ located in the superior mediastinum, is divided into two lobes by its connective tissue capsule and functions in educating T cells to become immunocompetent. Although around the time of puberty the thymus begins to involute (degenerate) and becomes infiltrated by adipocytes, it is still functional in adults. Each lobe of the thymus is subdivided into incomplete lobules so that each lobule has its individual cortex, but shares the medulla with other lobules (Fig. 12.5).
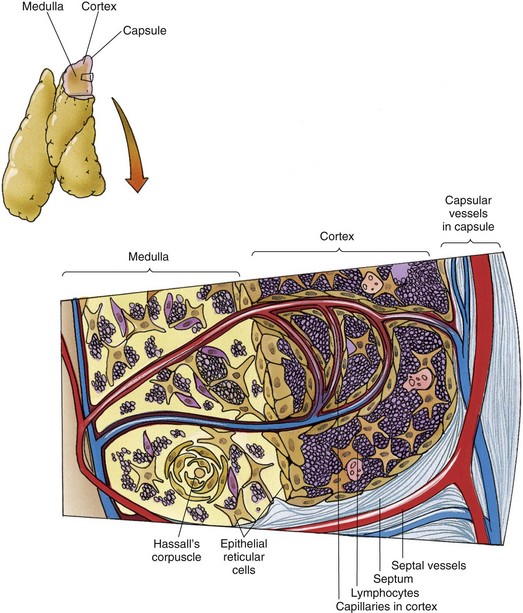
Figure 12.5 Diagram of the thymus depicting its histology and vascular supply.
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 3rd ed. Philadelphia, Saunders, 2007, p 288.)
The thymic cortex is occupied by numerous lymphocytes whose large nuclei and scant cytoplasm impart a dark, basophilic image in histologic sections. Immunoincompetent T cell precursors from the bone marrow enter the cortex of the thymus to proliferate and become immunocompetent T cells. To do this, they must contact various epithelial reticular cells of the cortex and develop some and eliminate other surface markers.
Epithelial Reticular Cells
There are six types of epithelial reticular cells, three in the cortex and three in the medulla:
CLINICAL CONSIDERATIONS
The blood supply of the thymus first gains entry into the medulla and forms a capillary bed at the junction of the cortex and the medulla. Branches of these capillaries enter the cortex and immediately become surrounded by a sheath of type I epithelial reticular cells that are held to one another by fasciae occludentes. These epithelial reticular cells form the blood thymus barrier in the thymic cortex, which ensures that macromolecules carried in the bloodstream cannot enter the cortex and interfere with the immunologic development of T cells. The endothelial cells of the cortical capillaries and the type I epithelial reticular cells possess their own basal lamina, which adds support to the barrier. The space between the epithelial sheath and the endothelium is patrolled by macrophages that destroy macromolecules that manage to escape from the capillaries. The cortex of the thymus drains into the venous network of the medulla.
Some individuals who are born without a thymus, a condition known as DiGeorge’s syndrome, are unable to generate T cells and are incapable of mounting a cell-mediated immune response. Because TH cells are required in the initiation of most humorally mediated immune responses, these patients are mostly immunoincompetent. As long as patients with DiGeorge’s syndrome are protected from infection, they can survive; however, most die of infections, or because many of these patients are also born without parathyroid glands, they die of calcium tetani (severe hypocalcemia).
Lymph Nodes
Lymph nodes are usually small, bean-shaped structures (≤3 cm in diameter) with a convex surface and a concave surface (hilum) invested by a connective tissue capsule (Fig. 12.6) that is usually embedded in adipose tissue. Deep to the capsule, the parenchyma is subdivided into:
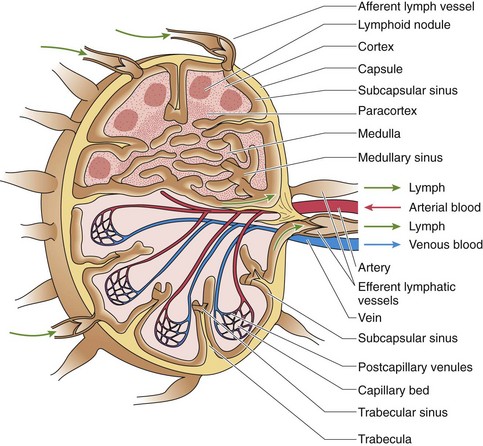
Figure 12.6 Diagram of a typical lymph node.
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 3rd ed. Philadelphia, Saunders, 2007, p 291.)
The capsule on the convex aspect sends trabeculae into the cortex, subdividing it into incomplete compartments; as the trabeculae continue into the paracortex and the medulla, they become more tortuous and less definite (see Fig. 12.6). Lymph nodes house T cells, B cells, dendritic cells, macrophages, and APCs, and function in clearing lymph and initiating immunologic reactions against foreign antigens. Lymph enters the lymph node via afferent lymph vessels that pierce the convex surface and whose valves prevent the lymph from flowing out of the node. The lymph percolates through the node and exits, via efferent lymph vessels, which also have valves to prevent the lymph from reentering the node at the hilum. Arteries enter and veins leave the lymph node at the hilum; these vessels use trabeculae to penetrate the parenchyma of the node. In the paracortex, the veins form high endothelial venules (HEVs).
The incomplete compartments of the cortex of a lymph node are bounded superiorly by the connective tissue capsule and laterally by trabeculae derived from the capsule (see Fig. 12.6). As the afferent lymph vessels pierce the capsule, they deliver their lymph into the subcapsular sinus, from which the lymph travels into paratrabecular sinuses that follow the trabeculae and deliver their lymph into the very tortuous medullary sinuses that are drained by efferent lymph vessels. These lymphatic sinuses are lined by simple squamous endothelial cells, and their lumina are spanned by an interdigitating complex of stellate reticular cells that not only slow the flow of lymph but also are used as scaffoldings by macrophages that phagocytose antigenic particulate matter.
The cortical compartments display dark, spherical secondary or primary lymphoid nodules.
The paracortex (see Fig. 12.6) is the T cell–rich region of the lymph node. Here HEVs permit the entry of B and T cells into the lymph node. B cells migrate to the cortex, and T cells remain in the paracortex.
The medulla (see Fig. 12.6) is composed of medullary sinusoids, trabeculae, and medullary cords, structures formed by reticular fibers, reticular cells, and macrophages, and B cells and plasma cells that were formed in secondary lymphoid follicles.
CLINICAL CONSIDERATIONS
In a healthy individual, lymph nodes are too soft to be able to be palpated. If the patient has a regional infection, however, the lymphocytes of the node draining that particular area proliferate; the node swells, becomes hard and painful, and may be palpated with ease. Each area of the body is drained by a series of lymph nodes that are connected to one another by lymph vessels. This formation of chains of lymph nodes is frequently responsible for the spread of infections or the metastasis of malignancy from one part of the body to another. As lymph percolates throughout the sinusoids of the lymph node, macrophages remove approximately 99% of foreign or undesirable particulate matter by phagocytosing it.
APCs that contacted antigens make their way to the lymph node nearest to their location, present the MHC-epitope complex to T helper cells, and initiate an immune response. When in the lymph node, these APCs are known as migrating dendritic cells.
Antigens that enter the lymph node via the afferent lymph vessels are picked up by follicular dendritic cells, which present the epitope to resident lymphocytes. When the antigen is recognized, a B cell becomes activated at the interface of the paracortex and cortex, it migrates into a primary lymphoid nodule, and begins to undergo rapid mitosis, forming a germinal center, transforming the primary into a secondary lymphoid nodule. If the activated B cells express improper sIgs, they are driven into apoptosis by the follicular dendritic cells; if they present proper sIgs, they are permitted to continue to differentiate into B memory cells and plasma cells. The newly differentiated cells migrate into the medulla of the lymph node and form medullary cords. Approximately 90% of the plasma cells leave the lymph node via the efferent lymph vessels and migrate to the bone marrow, where they manufacture and release antibodies until they die. The remaining 10% of plasma cells stay in the medullary cord and manufacture antibodies until they also die. Most B memory cells also leave their lymph node of origin to seed other secondary lymphatic organs, where they set up small clones in case the same antigen invades the body again. A few B memory cells remain in their lymph node of origin and establish a small clone there.
Spleen
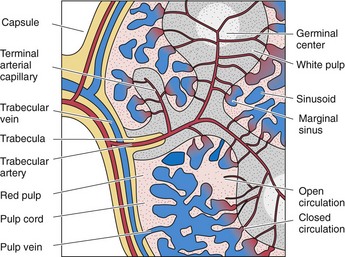
The spleen has a dense, irregular, and collagenous connective tissue capsule that is covered by the peritoneum, a simple squamous epithelium. The largest lymphoid organ, the spleen has a convex surface and a concave area, the hilum, where the capsule sends connective tissue trabeculae, bearing blood vessels and nerve fibers into the substance of the spleen. Attached to the capsule and the trabeculae is a three-dimensional complex of type III collagen fibers with their associated reticular cells that form the physical framework of the spleen. In contrast to lymph nodes, the spleen is not divided into a cortex, paracortex, and medulla; instead, it comprises white pulp, the marginal zone, and red pulp (sporting an abundance of tortuous sinusoids) that are intermingled (Fig. 12.7) to serve the functions of the spleen:
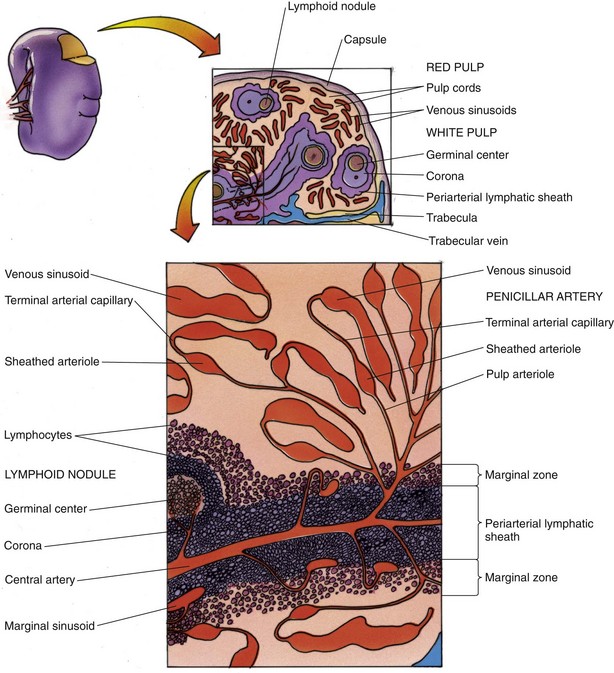
Figure 12.7 Diagram of the spleen.
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 3rd ed. Philadelphia, Saunders, 2007, p 294.)
Vascular Supply of the Spleen
The large artery supplying the spleen, the splenic artery, forms several branches before it enters the substance of the spleen at its hilum (Figs. 12.7 and 12.8).
White Pulp, Marginal Zone, and Red Pulp
The three components of the spleen are white pulp, marginal zone, and red pulp.
 mm wide, is the interface between the white pulp and red pulp (see Fig. 12.7). The cells of the marginal zone are interdigitating dendritic cells (APCs), macrophages, plasma cells, T cells, and B cells. Additionally, small sinusoids, marginal sinuses, abound in this region. Capillaries, derived from the central artery, enter the red pulp for a short distance, recur, and empty into the marginal sinuses.
mm wide, is the interface between the white pulp and red pulp (see Fig. 12.7). The cells of the marginal zone are interdigitating dendritic cells (APCs), macrophages, plasma cells, T cells, and B cells. Additionally, small sinusoids, marginal sinuses, abound in this region. Capillaries, derived from the central artery, enter the red pulp for a short distance, recur, and empty into the marginal sinuses.The functions of the spleen are intimately interconnected with the design of its vascular supply.
Mucosa-Associated Lymphoid Tissue
The mucosae of the respiratory, digestive, and urinary tracts display nonencapsulated clusters of lymphoid nodules and lymphocyte infiltrations known as MALT; examples are gut-associated lymphoid tissue (GALT), bronchus-associated lymphoid tissue (BALT), and tonsils.
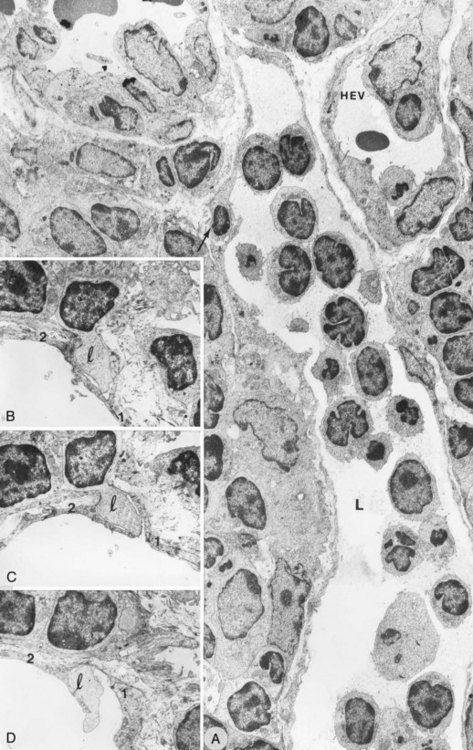
Figure 12.9 Transmission electron micrographs. A, ALPA vessel (L) of the interfollicular area full of lymphocytes that has an intraendothelial channel that includes lymphocytes (arrow) in the endothelial wall (×3000). B–D, Ultrathin serial sections that document various stages through an intraendothelial channel composed of one (1) and two (2) endothelial cells (×9000). l, lymphocyte.
(From Azzali G, Arcari MA: Ultrastructural and three-dimensional aspects of the lymphatic vessels of the absorbing peripheral lymphatic apparatus in Peyer’s patches of the rabbit. Anat Rec 258:76, 2000.)
Tonsils
Tonsils, a collection of partially encapsulated lymphoid nodules (palatine, pharyngeal, lingual, and numerous very small tonsils), are located at the entrance of the oral pharynx, protecting it from inhaled antigens. In the presence of an antigenic challenge, lymphocytes become activated and proliferate, enlarging the affected tonsil.