Gastrointestinal System
After reading this chapter, the reader will be able to:
1 Classify the more common diseases in terms of their attenuation of x-rays
2 Explain the changes in technical factors required for obtaining optimal quality radiographs in patients with various underlying pathologic conditions
3 Define and describe all bold-faced terms in this chapter
4 Describe the physiology of the gastrointestinal system
5 Identify anatomic structures on both diagrams and radiographs of the gastrointestinal system
6 Differentiate the various pathologic conditions affecting the gastrointestinal system and their radiographic manifestations
As in other body systems, certain pathologic conditions in the gastrointestinal (GI) system require alterations in the technical factors chosen for imaging. In patients with ascites, a common complication of advanced cirrhosis, an increased kilovolt peak (kVp) is required to penetrate the additional fluid content of the abdomen. On the other hand, a decreased kVp is needed in patients with suspected large or small bowel obstruction because of the excessive amount of gas in the abdominal cavity. When using a computed radiography or a direct digital imaging system, the radiographer should still consider the pathology condition in relationship to its attenuation factor.
The radiographer is usually called on to assist the radiologist during fluoroscopic examinations of the GI tract. Indeed, it is generally the radiographer’s task to coerce the patient into drinking (and not vomiting) the rather unpleasant-tasting contrast material and to urge the patient to turn around several times to provide adequate mucosal coating for the double-contrast upper GI series. Similarly, the radiographer may have to persuade the patient to retain barium and air during the often uncomfortable barium enema examinations. It is frequently time well spent for the radiographer to explain fully to the patient both the mechanics of the procedure and the extreme importance of patient cooperation.
Physiology of the digestive system
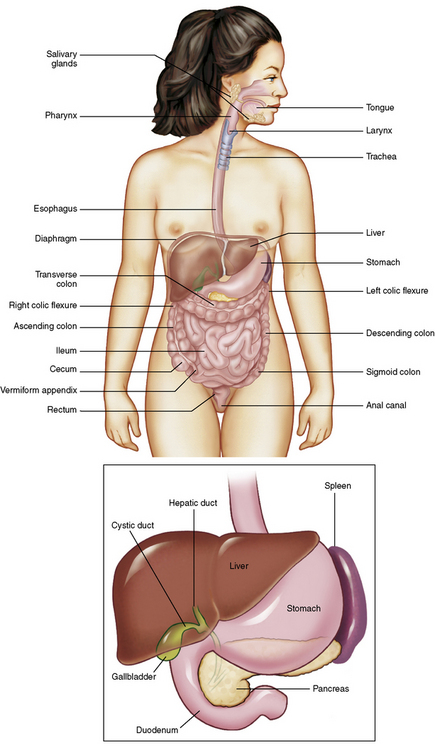
The basic function of the digestive system is to alter the chemical and physical composition of food so that it can be absorbed and used by body cells. This process depends on secretions of the endocrine and exocrine glands and on the controlled movement of ingested food through the tract so that absorption can occur (Figure 5-1).
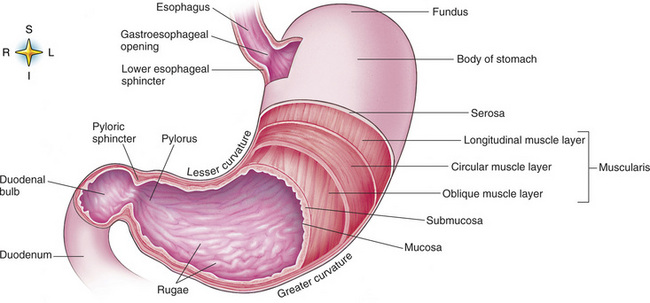
Digestion begins in the mouth with chewing (mastication), the mechanical breakdown of food. The secretion of saliva moistens the food in preparation for swallowing. Swallowing (deglutition) is a complex process that requires coordination of many muscles in the head and neck and the precise opening and closing of esophageal sphincters. Digestion continues in the stomach with the churning movement of gastric contents that have become mixed with hydrochloric acid and the proteolytic enzyme pepsin (Figure 5-2). The resulting milky white chyme is propelled through the pyloric sphincter into the duodenum by rhythmic smooth muscle contractions called peristalsis.
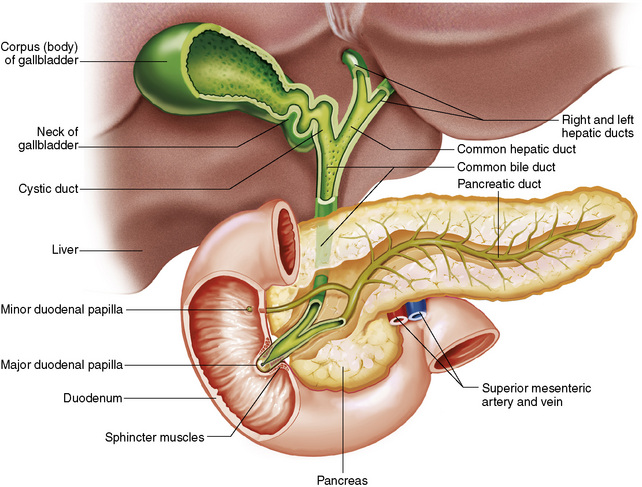
The greatest amount of digestion occurs in the duodenum, the first part of the small bowel. In addition to intestinal secretions containing mucus and enzymes, secretions of the pancreas and liver enhance digestion in this region. The pancreas secretes enzymes for the digestion of proteins (trypsin and chymotrypsin), fat (lipase), and carbohydrates (amylase). It also secretes an alkaline solution to neutralize the acid carried into the small intestine from the stomach. Bile is secreted by the liver, is stored in the gallbladder, and enters the duodenum through the common bile duct. Bile is an emulsifier, a substance that acts like soap by dispersing the fat into very small droplets that permit it to mix with water.
When digestion is complete, the nutrients are absorbed through the intestinal mucosa into blood capillaries and lymph vessels of the wall of the small bowel. The inner surface area of the small bowel is increased by the formation of numerous finger-like projections (villi), which provide the largest amount of surface area possible for digestion and absorption.
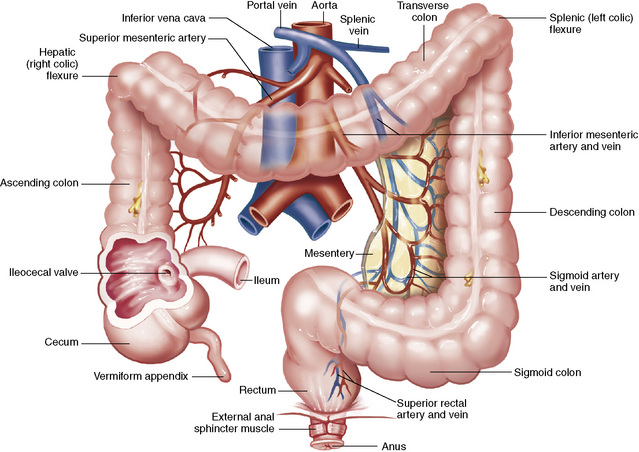
Material that has not been digested passes into the colon, where water and minerals are absorbed, and the remaining matter is excreted as feces (Figure 5-3). If the contents of the lower colon and rectum move at a rate that is slower than normal, extra water is absorbed from the fecal mass to produce a hardened stool and constipation. Diarrhea results from increased motility of the small bowel, which floods the colon with an excessive amount of water that cannot be completely absorbed.
The vermiform (worm-shaped) appendix arises from the inferomedial aspect of the cecum about 3 cm below the ileocecal valve. Although the appendix has no functional importance in digestion, it is often classified as an accessory digestive organ merely because of its location.
The liver is the largest gland in the body and is responsible for several vital functions. Liver cells detoxify (make harmless) a variety of poisonous substances that enter the blood from the intestines. Toxic chemicals that are changed to nontoxic compounds in the liver include ammonia (converted to urea and excreted by the kidneys), alcohol, and barbiturates. Liver cells secrete about 1 pint of bile each day. As mentioned, bile is an emulsifier; it is essential for the digestion and absorption of dietary fat and the fat-soluble vitamins A, D, E, and K. Bile is a greenish liquid consisting of water, bile salts, cholesterol, and bilirubin (a breakdown product of hemoglobin).
Liver cells play a vital role in the metabolism of proteins, fats, and carbohydrates. The liver is the major site of synthesis of the enzymes necessary for various cellular activities throughout the body. Liver cells also synthesize blood proteins, such as albumin, which maintains the correct amount of fluid within blood vessels, and the essential proteins required for blood clotting (fibrinogen and prothrombin). Therefore, liver damage may result in edema (excess water in the soft tissues) and a serious bleeding tendency. The liver plays an important role in maintaining the proper level of glucose in the blood by taking up excess glucose absorbed by the small intestine and storing it as glycogen. When the level of circulating glucose falls below normal, the liver breaks down glycogen and releases glucose into the bloodstream. Liver cells also store iron and vitamins A, B12, and D.
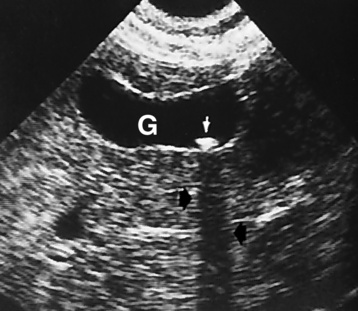
The gallbladder is a pear-shaped sac that lies on the undersurface of the liver (Figure 5-4). Its function is to store bile that enters by way of the hepatic and cystic ducts and to concentrate the bile by absorbing water. In response to the presence of dietary fat in the small bowel, the gallbladder contracts and ejects the concentrated bile into the duodenum.
The pancreas controls the level of circulating blood glucose by secreting insulin and glucagon in the islets of Langerhans. An increased concentration of glucose in the blood stimulates the beta cells to increase secretion of insulin, which decreases the blood glucose level probably by accelerating the transport of glucose into cells. A blood glucose concentration less than normal triggers the alpha cells to secrete glucagon, which accelerates the breakdown of glycogen into glucose by the liver.
As discussed, pancreatic secretions are vital for digestion. Pancreatic enzymes that pass through the pancreatic duct into the duodenum are necessary for the breakdown of proteins, carbohydrates, and fats.
Esophagus
Congenital Type
Congenital tracheoesophageal (TE) fistulas result from the failure of a satisfactory esophageal lumen to develop completely separate from the trachea. The lack of the development of the esophageal lumen resulting in a blind pouch describes congenital esophageal atresia. Esophageal atresia and TE fistulas are often associated with other congenital malformations involving the skeleton, cardiovascular system, and GI tract.
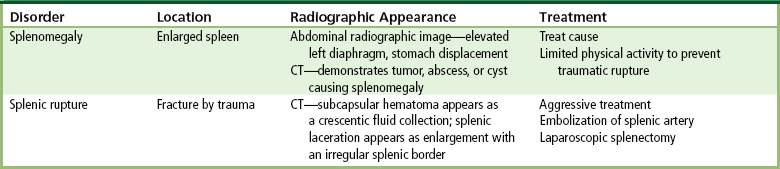
Radiographic Appearance: In the second most common type of esophageal anomaly, type I, both the upper and lower segments of the esophagus are blind pouches. This anomaly can be differentiated from the type III lesion (the most common type) only by plain abdominal radiographs, which demonstrate the absence of air below the diaphragm in the type I lesion and the presence of air below the stomach in the type III lesion.
In the type II form of TE fistula, the upper esophageal segment communicates with the trachea, whereas the lower segment ends in a blind pouch. Because there is no connection between the trachea and the stomach, there is no radiographic evidence of gas within the abdomen. Oral administration of contrast material in this condition immediately outlines the tracheobronchial tree.
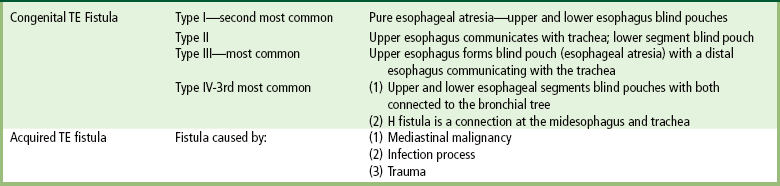
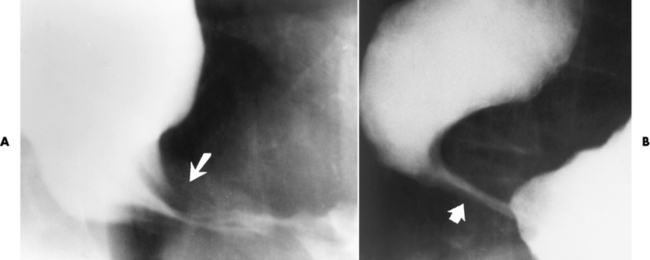
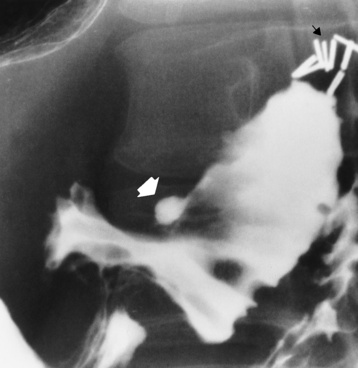
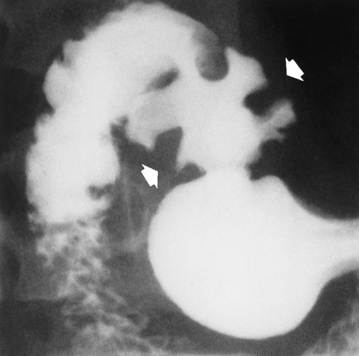
The type III TE fistula (seen in 85% to 90% of cases) consists of an upper segment that ends in a blind pouch at the level of the bifurcation of the trachea or slightly above it, and a lower segment attached to the trachea by a short fistulous tract. Radiographic demonstration of the looping of a small esophageal feeding tube indicates that the proximal esophagus ends in a blind pouch (Figure 5-5A). Plain radiographs of the abdomen demonstrate the presence of air in the bowel that has freely entered the stomach through the fistulous connection between the trachea and the distal esophagus.
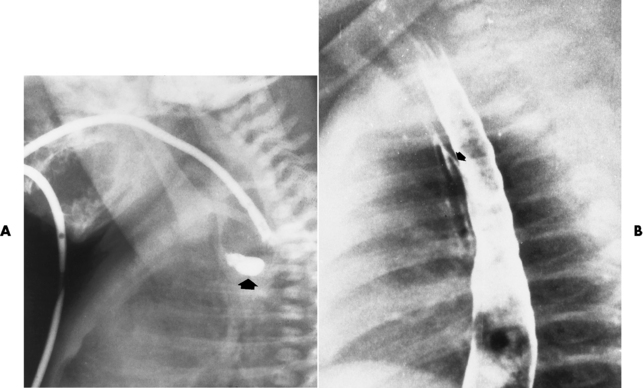
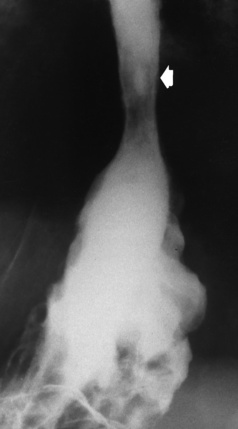
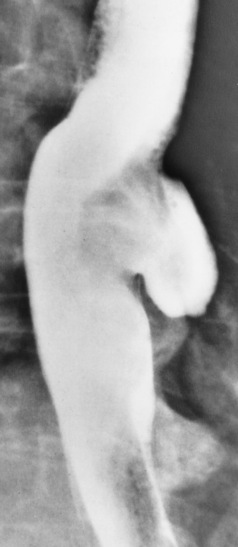
Figure 5-5 Congenital tracheoesophageal fistula. A, Type III fistula (arrow), in which contrast material (injected through a feeding tube) demonstrates occlusion of proximal esophageal pouch. B, Type IV, or H, fistula (arrow).
There are two forms of type IV TE fistula. In one, the upper and lower esophageal segments end in blind pouches, both of which are connected to the tracheobronchial tree. In this form, gas is seen in the stomach, and oral contrast material outlines both fistulas and the bronchial tree. In the other form of type IV TE fistula (called an H fistula), both the trachea and the esophagus are intact. These two structures are connected by a single fistulous tract that can be found at any level from the cricoid cartilage of the trachea to the tracheal bifurcation (Figure 5-5B). Unlike the other forms of TE fistula, the H fistula may not be identified in infancy and, if it is small and only occasionally causes emptying of material into the lungs, can permit survival into adulthood.
Computed tomography (CT) esophagography without use of a contrast agent that is performed using a multidetector CT scanner with three-dimensional reconstructions demonstrates TE fistulas. CT is less invasive than contrast radiography and provides clinicians with critical surgical planning information.
Acquired Type
About 50% of acquired fistulas between the trachea and esophagus are caused by malignancy in the mediastinum. Almost all the rest result from infectious processes or trauma.
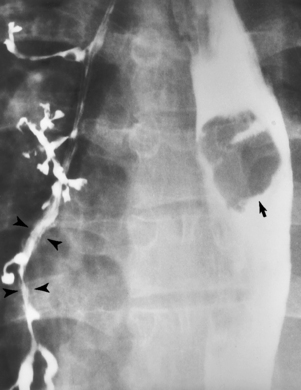
Fistulization between the esophagus and the respiratory tract is a major late complication of esophageal carcinoma and is often a terminal event (Figure 5-6). A fistula can also be a complication of erosion into the esophagus either by carcinoma of the lung arising near or metastasizing to the middle mediastinum or by mediastinal metastases from other primary sites. Regardless of therapy, the overall prognosis of malignant TE fistulas is dismal, and more than 80% of patients with this complication die within 3 months from uncontrollable hemorrhage or from pulmonary infection caused by repeated episodes of aspiration pneumonia.
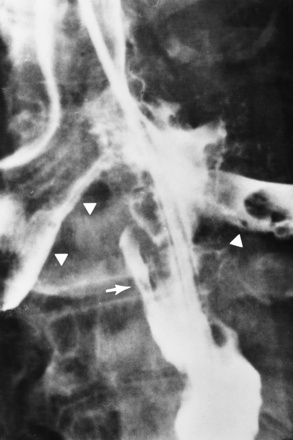
Figure 5-6 Esophagorespiratory fistula between esophagus (arrow) and bronchial tree (arrowheads), seen as complication of esophageal carcinoma.
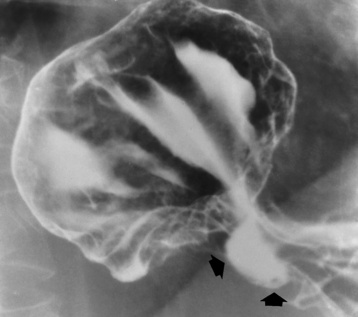
Fistulous communications between the esophagus and the tracheobronchial tree can be the result of esophageal instrumentation and perforation (Figure 5-7). It is most common after esophagoscopy but may also occur after instrumental dilation of strictures by bougienage, pneumatic dilation of the esophagus for the treatment of achalasia, or even the insertion of a nasogastric tube. Blunt or penetrating trauma to the chest, especially after crush injury, can result in esophageal perforation and fistulization.
Figure 5-7 Acquired tracheoesophageal fistula. A 23-year-old female aspirated her dentures, causing an erosion through the esophagus. The esophagram demonstrates the fistula (arrow) between the esophagus and trachea.
Radiographic Appearance: After traumatic perforation of the thoracic esophagus, chest radiographs may demonstrate air dissecting within the mediastinum and soft tissue, often with pleural effusion or hydropneumothorax. The introduction of an oral contrast agent may demonstrate the site of perforation and the extent of fistulization.
Esophagitis
Reflux (Gastroesophageal Reflux Disease)
Although the reflux of gastric acid contents is the most common cause of acute esophagitis, infectious and granulomatous disorders, physical injury (caustic agents, radiation injury), and medication may produce a similar
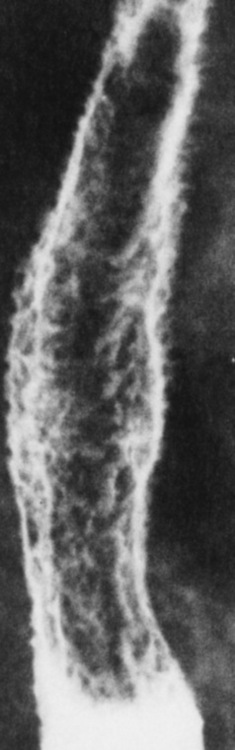
inflammatory response. Gastroesophageal reflux disease (GERD) describes any symptomatic condition or structural changes caused by reflux of the stomach contents into the esophagus. Alcohol, chocolate, caffeine, and fatty foods tend to decrease the pressure of the esophageal sphincter, allowing reflux to occur. Regardless of the cause, acute esophagitis produces burning chest pain that may simulate the pain of heart disease. Superficial ulcerations are most typical of reflux. The esophagus is often dilated, with a loss of effective peristalsis. Nonpropulsive peristaltic waves, ranging from mild tertiary contractions to severe segmental spasms, are an early finding.
Reflux esophagitis develops when the lower esophageal sphincter fails to act as an effective barrier to the entry of gastric acid contents into the distal esophagus. Although there is a higher-than-normal likelihood of gastroesophageal reflux in patients with sliding hiatal hernias, reflux esophagitis can be endoscopically demonstrated in only about one fourth of these patients. On the other hand, esophagitis is often encountered in patients in whom no hiatal hernia can be demonstrated.
Several radiographic approaches have been suggested for the demonstration of gastroesophageal reflux. One procedure is to increase intraabdominal pressure by straight-leg raising or manual pressure on the abdomen, often with Valsalva maneuver (forced expiration with the glottis closed). Having the patient turn from prone to supine or vice versa may demonstrate reflux of barium from the stomach into the esophagus. It must be remembered, however, that the failure to demonstrate reflux radiographically does not exclude the possibility that a patient’s esophagitis is related to reflux. As long as typical radiographic findings of reflux esophagitis are noted, there is little reason to persist in strenuous efforts to actually demonstrate retrograde flow of barium from the stomach into the esophagus.
Radiographic Appearance: The earliest radiographic findings in reflux esophagitis are detectable on double-contrast studies. They consist of superficial ulcerations or erosions that appear as streaks or dots of barium superimposed on the flat mucosa of the distal esophagus. In single-contrast studies of patients with esophagitis, the outer borders of the barium-filled esophagus are not sharply seen but rather have a hazy, serrated appearance with shallow, irregular protrusions indicating erosions of varying length and depth. Widening and coarsening of edematous longitudinal folds can simulate filling defects. In addition to diffuse erosion, reflux esophagitis can result in large, discrete, penetrating ulcers in the distal esophagus (Figure 5-8) or in a hiatal hernia sac (Figure 5-9). Fibrotic healing of diffuse reflux esophagitis or a localized penetrating ulcer may cause narrowing of the distal esophagus. Strictures resulting from reflux esophagitis tend to be smooth and tapering with no demonstrable mucosal pattern (Figure 5-10).
Barrett’s Esophagus
Barrett’s esophagus is a condition related to severe reflux esophagitis in which the normal squamous lining of the lower esophagus is destroyed and replaced by columnar epithelium similar to that of the stomach. Ulceration in Barrett’s esophagus typically occurs at the squamocolumnar junction (Z-line). In addition to exhibiting postinflammatory stricture, Barrett’s esophagus has an unusually high propensity for development of malignancy in the columnar cell–lined portion. These tumors are almost always adenocarcinomas, which are otherwise very rare in the esophagus (accounting for about 5% of esophageal cancers).
Radiographic Appearance: Although a hiatal hernia with gastroesophageal reflux is commonly demonstrated, Barrett’s ulcer is usually separated from the hiatal hernia by a variable length of normal-appearing esophagus (Figure 5-11), in contrast to reflux esophagitis, in which the distal esophagus is abnormal down to the level of the hernia. As in reflux esophagitis, fibrotic healing of the ulceration in Barrett’s esophagus often leads to a smooth, tapered stricture (Figure 5-12).
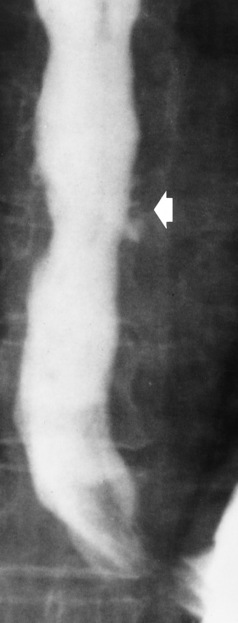
Figure 5-11 Barrett’s esophagus. Ulcerations (arrow) have developed at a distance from esophagogastric junction.
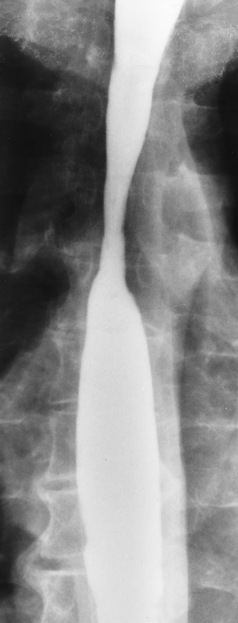
Figure 5-12 Barrett’s esophagus. Note the smooth, tapered stricture in the upper thoracic esophagus.
Because the distal esophagus consists of a gastric type of mucosa in Barrett’s esophagus, it actively takes up the intravenously injected radionuclide pertechnetate. The demonstration of a continuous concentration of the isotope from the stomach into the distal esophagus to a level that corresponds approximately to that of the ulcer or stricture is indicative of Barrett’s esophagus.
Candida and Herpesvirus
Candida (fungal) and herpesvirus are the organisms most often responsible for infectious esophagitis, which usually occurs in patients with widespread malignancy who are receiving radiation therapy, chemotherapy, corticosteroids, or other immunosuppressive agents. It also can develop in patients with acquired immunodeficiency syndrome (AIDS) and even in otherwise healthy adults who have received antibiotics (especially tetracycline) for upper respiratory infection.
Radiographic Appearance: The classic radiographic appearance of infectious esophagitis is an irregular cobblestone pattern with a shaggy marginal contour of the esophagus caused by deep ulcerations and sloughing of the mucosa (Figure 5-13). Candida infection manifests as plaques and nodules resulting from a superficial collection of fungi. Characteristics of herpetic esophagitis include small mucosal ulcers or plaques.
Treatment for Esophagitis
Modifications to lifestyle, including weight loss, changes in diet to prevent decreased esophageal sphincter control, and medications to reduce acidity, are the first line of treatment. One technique, called proton pump inhibitor therapy (using esomeprazole), can help control progression in reflux disease and Barrett’s esophagus. Other techniques used are photodynamic therapy and thermal ablation to destroy the Barrett’s tissue. If these therapies do not work, surgical fundoplication (tucks in the stomach fundus and distal esophagus) is an option. Infectious esophagitis caused by Candida or herpesvirus may require a regimen of antifungal or antiviral drugs to eradicate the cause.
Ingestion of Corrosive Agents
The ingestion of alkaline or acidic corrosive agents produces acute inflammatory changes in the esophagus. Superficial penetration of the toxic agent results in only minimal ulceration. Deeper penetration of the submucosa and muscular layers causes sloughing of destroyed tissue and deep ulceration. Ingestion of strong alkaline agents causes deeper lesions than ingestion of strong acids, and only half of those who ingest an acid suffer severe injury. Drug-induced esophagitis may occur in patients who have delayed esophageal transit time, which permits prolonged mucosal contact with the ingested substance. The most common drug causing esophageal ulceration is potassium chloride in tablet form. Other medications that can cause esophagitis are weak caustic agents that are harmless when they pass rapidly through the esophagus.
Radiographic Appearance: Healing of the intense mucosal and intramural inflammation of acute esophagitis may lead to pronounced fibrosis and stricture formation. These benign strictures tend to be long lesions with tapered margins and relatively smooth mucosal surfaces (Figure 5-14), in contrast to the irregular narrowing, mucosal destruction, and overhanging margins that are generally associated with malignant processes.
Treatment: The type of agent ingested determines the therapy. The local or state poison control service is usually called for specific treatments if the situation is not drug induced. Vomiting is generally not induced because this would cause a second exposure of the esophagus to the agent. Dilution by administration of milk or water is appropriate unless the corrosive agent is acidic (in this case, water should not be used as it would produce excessive heat).
Esophageal Cancer
Progressive difficulty in swallowing (dysphagia) in a person older than 40 years must be assumed to be caused by cancer until proven otherwise. Because the symptoms of esophageal carcinoma tend to appear late in the course of the disease, and because the lack of a limiting outer layer (serosa) commonly permits direct extension of the tumor by the time of the initial diagnosis, carcinoma of the esophagus has a dismal prognosis. Most carcinomas of the esophagus are of the squamous cell type and they occur most often at the esophagogastric junction. The incidence of carcinoma of the esophagus is far higher in men than in women. There is a strong correlation between excessive alcohol intake, smoking, and esophageal carcinoma.
Radiographic Appearance
The earliest radiographic evidence of infiltrating carcinoma of the esophagus appears on a double-contrast barium swallow image as a flat, plaquelike lesion, occasionally with central ulceration, that involves one wall of the esophagus (Figure 5-15). At this stage, there may be minimal reduction in the caliber of the lumen. Unless the patient is carefully examined in various positions, this earliest form of esophageal carcinoma can be missed. As the infiltrating cancer progresses, irregularity of the wall is seen, indicating mucosal destruction. Advanced lesions encircle the lumen completely, causing annular constrictions with overhanging margins and often some degree of obstruction. The lumen through the stenotic area is irregular, and mucosal folds are absent or severely ulcerated (Figure 5-16). Less commonly, carcinoma of the esophagus can appear as a localized polypoid mass, often with deep ulceration and a fungating appearance.
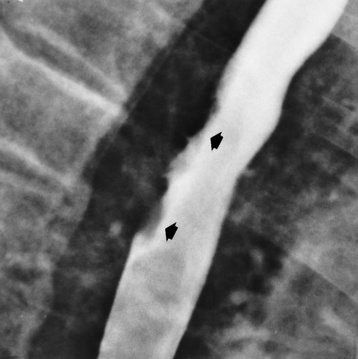
Figure 5-15 Early carcinoma of the esophagus. Flat, plaquelike lesion (arrows) involves the posterior wall of the esophagus.
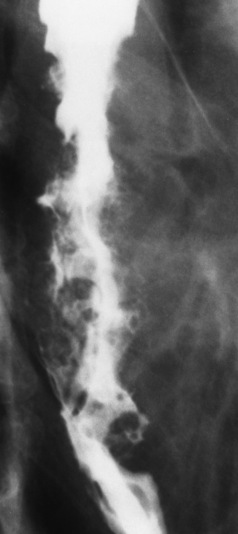
Figure 5-16 Carcinoma of the esophagus. Irregular narrowing with ulceration involves an extensive segment of the thoracic portion of the esophagus.
Luminal obstruction as a result of carcinoma causes proximal dilation of the esophagus and may result in aspiration pneumonia. Extension of the tumor to adjacent mediastinal structures may lead to fistula formation, especially between the esophagus and the respiratory tract (see Figure 5-20).
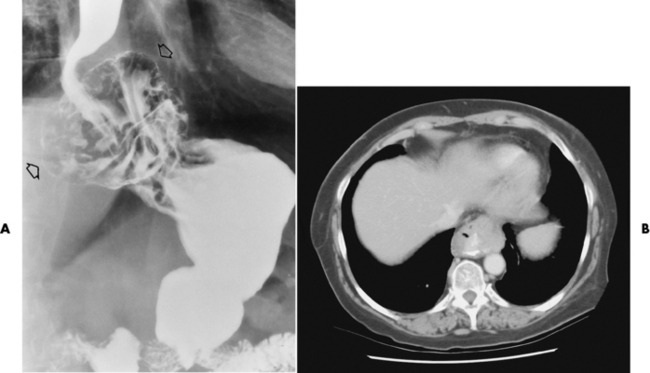
Wall thickening greater than 3 to 5 mm on a CT scan is suggestive of esophageal cancer. CT has become a major method of staging patients with esophageal carcinoma (with 90% accuracy), providing information on tumor size, extension, and resectability that was previously available only at thoracotomy (Figure 5-17). Evidence of tumor spread includes the obliteration of fat planes between the esophagus and adjacent structures (left atrium, aorta), the formation of a fistula to the tracheobronchial tree, and recognition of metastatic disease (e.g., low-density masses in the liver, enlargement of draining lymph nodes). Use of contrast enhancement improves the detail of tumor delineation.
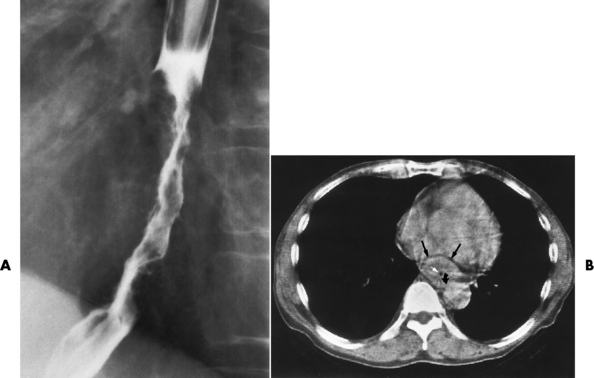
Figure 5-17 CT staging of esophageal carcinoma. A, Esophagram demonstrates infiltrating lesion causing irregular narrowing of the distal part of the esophagus. B, CT scan shows a mass of bulky carcinoma (black arrows) filling most of the lumen (white arrow). Obliteration of the fat plane adjacent to the aorta (curved arrow) indicates mediastinal invasion.
Treatment
If the cancerous lesion has not extended into surrounding tissue, surgical resection may result in cure. When the cancerous lesion involves surrounding tissue, treatment becomes palliative surgery together with radiation therapy or chemotherapy. If the esophagus is severely narrowed, a technique known as bougienage can be employed; this is the introduction of a long instrument to dilate and help maintain an adequate lumen. Laser therapy aids in treating the dysphagia in patients with unresectable lesions. A newer technique, photodynamic therapy using laser-activated chemicals, is a method of destroying tumor tissue. The prognosis of the patient who is diagnosed in the late stages of esophageal cancer is extremely poor.
Esophageal Diverticula
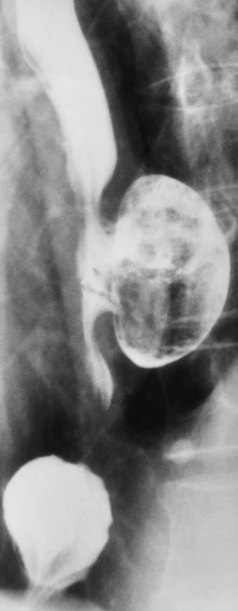
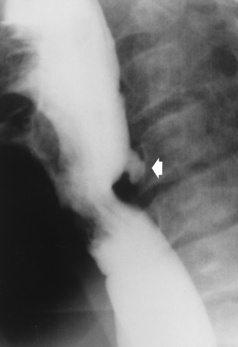
Esophageal diverticula (outpouchings) are common lesions that either contain all layers of the wall (traction or true diverticula) or are composed of only mucosa and submucosa herniating through the muscular layer (pulsion or false diverticula). Small diverticula do not retain food or secretions and are asymptomatic. When a diverticulum fills with food or secretions, aspiration pneumonia may result.
Radiographic Appearance
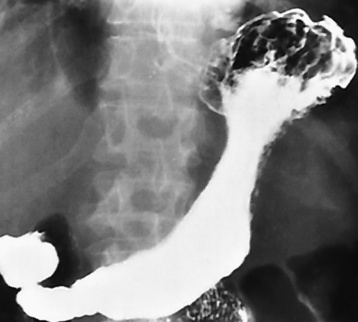
Zenker’s diverticula arise from the posterior wall of the upper (cervical) esophagus (Figure 5-18). Occasionally, they can become so large that they almost occlude the esophageal lumen. CT prominently demonstrates the cricopharyngeal muscle, which aids in locating the origin of Zenker’s diverticula at the pharyngoesophageal junction. Diverticula of the thoracic portion of the esophagus are primarily found opposite the bifurcation of the trachea, in the region of the hilum of the lung (Figure 5-19). These traction diverticula reflect motor function disturbance and develop in response to the pull of fibrous adhesions after infection of the mediastinal lymph nodes. Epiphrenic diverticula arise in the distal 10 cm of the esophagus (Figure 5-20). They are associated with incoordination of esophageal peristalsis and sphincter relaxation, which increases the intraluminal pressure in this segment.
Esophageal Varices
Esophageal varices are dilated veins in the wall of the esophagus that are most commonly the result of increased pressure in the portal venous system (portal hypertension), which is in turn usually a result of cirrhosis of the liver. In patients with portal hypertension, much of the portal blood cannot flow along its normal pathway through the liver to the inferior vena cava and then on to the heart. Instead, it must go by a circuitous collateral route, and increased blood flow through these dilated veins causes the development of esophageal (and gastric) varices. Esophageal varices are infrequently demonstrated in the absence of portal hypertension. “Downhill” varices are produced when venous blood from the head and neck cannot reach the heart because of an obstruction of the superior vena cava caused by tumors or inflammatory disease in the mediastinum. In this situation, blood flows “downhill” through the esophageal veins before eventually entering the portal vein, through which it flows to the inferior vena cava and the right atrium.
Radiographic Appearance
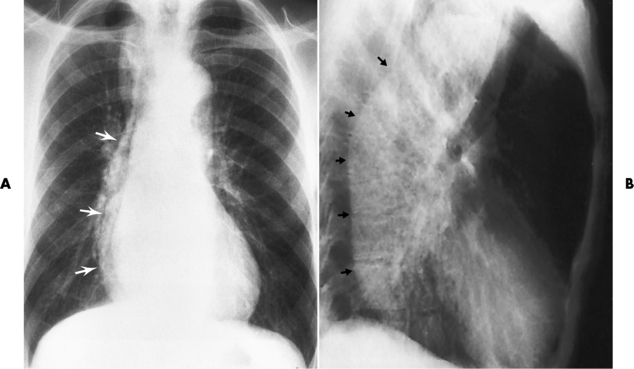
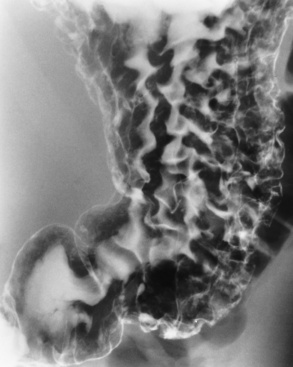
The characteristic radiographic appearance of esophageal varices is serpiginous (wavy border) thickening of folds, which appear as round or oval filling defects resembling the beads of a rosary (Figure 5-21). Precise technique is required to demonstrate esophageal varices. A double-contrast barium swallow study best demonstrates the serpiginous and wormlike filling defect. Complete filling of the esophagus with barium may obscure varices, and powerful contractions of the esophagus may squeeze blood out of the varices and make them impossible to detect. Upright and recumbent imaging may best demonstrate the varices dilated and empty, respectively.
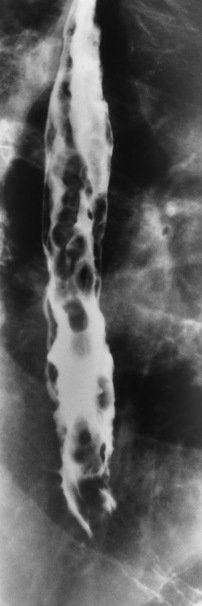
Figure 5-21 Esophageal varices. Note the diffuse round and oval filling defects, which resemble rosary beads.
Varices can be demonstrated with endoscopic ultrasound imaging (ultrasonography) as compressible hypoechoic or cystic masses in the GI tract from the outer to the submucosal layers.
The major complication of esophageal varices is bleeding. Their appearance in patients with cirrhotic liver disease implies significant portal venous hypertension and is an ominous sign, because up to 90% of the deaths from liver disease in patients with cirrhosis occur within 2 years of the diagnosis of varices.
Treatment
Vasoconstrictor drugs to constrict the dilated vessels are commonly used to treat esophageal varices. Active bleeding can be controlled by a technique called balloon tamponade, which creates pressure to stop the bleeding. If bleeding cannot be controlled, surgery is performed to tie off collateral vessels.
Hiatal Hernia
Hiatal hernia is the most common abnormality (occurring in 50% of the population) detected on upper GI examination. Its broad radiographic spectrum ranges from large esophagogastric hernias, in which much of the stomach lies within the thoracic cavity and there is a predisposition to volvulus (twisting), to small hernias that emerge above the diaphragm only under certain circumstances (related to changes in intraabdominal or intrathoracic pressure) and easily slide back into the abdomen through the hiatus (sliding hiatal hernia). The symptoms associated with hiatal hernia and its complications (esophagitis, esophageal ulcer, esophageal stenosis) are related to the presence of esophageal reflux rather than to the hiatal hernia itself. Most hiatal hernias do not produce symptoms and are clinically of no importance.
Radiographic Appearance
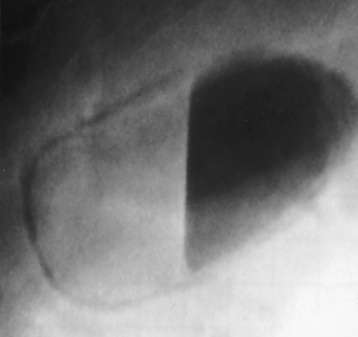
Although the diagnosis of hiatal hernia generally requires a barium study (Figure 5-22), at times a large hiatal hernia may appear on plain chest radiograph as a soft tissue mass in the posterior mediastinum, often containing a prominent air-fluid level (Figure 5-23). The esophagus and stomach are distinguished by their appearance; mucosal folds are linear and parallel in the esophagus, whereas in the stomach the folds appear numerous and thicker without a parallel orientation.
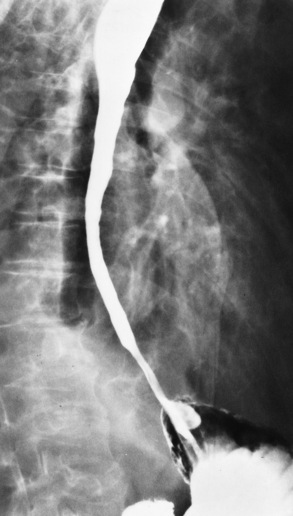
Achalasia
Achalasia is a functional obstruction of the distal section of the esophagus with proximal dilation caused by incomplete relaxation of the lower esophageal sphincter. It is related to a paucity or absence of ganglion cells in the myenteric neural plexuses of the distal esophageal wall.
Radiographic Appearance
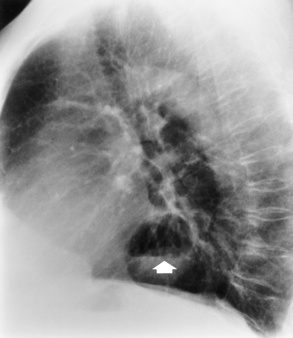
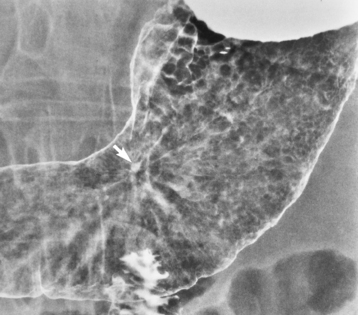
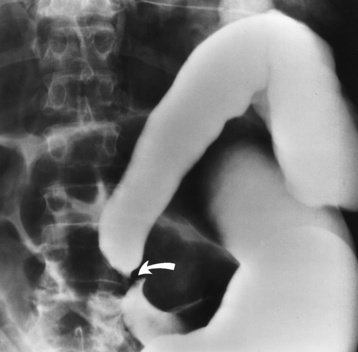
On plain chest radiographs, the dilated, tortuous esophagus may produce a widened mediastinum (often with an air-fluid level) on the right side adjacent to the cardiac shadow (Figure 5-24). The hallmark of achalasia, seen on barium studies, is a gradually tapered, smooth, conical, 1- to 3-cm narrowing of the distal esophageal segment (rat-tail or beak appearance) (Figure 5-25). On sequential radiographs, especially with the patient upright, only small spurts of barium are seen to pass through the narrowed distal segment to enter the stomach.
Foreign Bodies
A wide spectrum of foreign bodies can become impacted in the esophagus, usually in the cervical esophagus at or just above the level of the thoracic inlet (Figure 5-26). Symptomatically, the patient is unable to swallow without regurgitation. Most metallic objects, such as pins, coins, and small toys, are radiopaque and are easily visualized on radiographs or during fluoroscopy. Objects made of aluminum and some light alloys may be impossible to detect radiographically because the density of these metals is almost equal to that of soft tissue. It is essential that any suspected foreign body be evaluated on two projections to be certain that the object projected over the esophagus truly lies within it.
Radiographic Appearance
Nonopaque foreign bodies in the esophagus, especially pieces of poorly chewed meat (masticated food bolus), can be demonstrated only after the ingestion of barium (Figure 5-27). Such a foreign body usually becomes impacted in the distal esophagus just above the level of the diaphragm and is often associated with a distal stricture. The intraluminal filling defect usually has an irregular surface and may resemble a completely obstructing carcinoma.
Treatment
Medications are the first line of treatment to relax the esophagus and allow the foreign body to move naturally into the stomach. In some instances, especially for a sharper-pointed object, retrieval of the foreign body using endoscopy may be appropriate. Interventional approaches are attempted before the patient is taken to surgery to remove the obstruction. If the esophageal foreign body causes obstruction for more than 12 hours, there is an increased risk of perforation.
Perforation of the Esophagus
Perforation of the esophagus may be a complication of esophagitis, peptic ulcer, neoplasm, external trauma, or instrumentation. At times, perforation of a previously healthy esophagus can result from severe vomiting (the most common cause) or coughing, often from dietary or alcoholic indiscretion. Complete rupture of the wall of the esophagus may cause the sudden development of severe upper gastric pain simulating that of myocardial infarction. In the Mallory-Weiss syndrome, an increase in intraluminal and intramural pressures associated with vomiting (severe retching) after an alcoholic bout causes superficial mucosal laceration or fissures near the esophagogastric junction that produce severe hemorrhage. Endoscopy is required to best demonstrate lacerations, especially those close to the sphincter.
Radiographic Appearance
A perforation that extends throughout the entire esophageal wall can lead to free air in the mediastinum or periesophageal soft tissues. The administration of radiopaque contrast material may demonstrate extravasation through the perforation (Figure 5-28) or an intramural dissection channel separated by an intervening lucent line from the normal esophageal lumen. CT is the preferred modality to define the extent of the process.
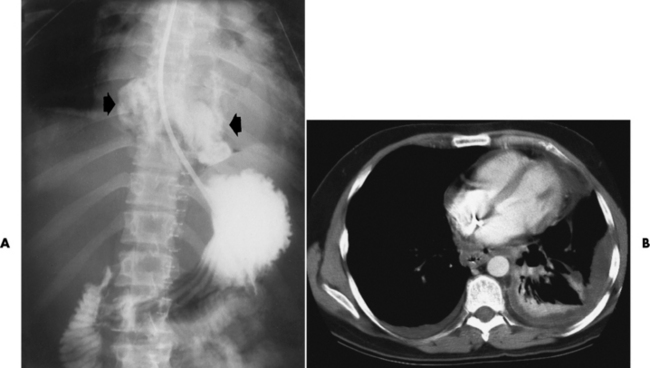
Figure 5-28 A, Esophageal perforation. Extravasation of contrast material (arrows) is seen in a previously healthy patient who experienced severe vomiting after excessive ingestion of alcohol. B, CT scan of the chest of a 41-year-old man illustrates perforation of the esophagus with drainage into the thoracic cavity.
Stomach
Inflammation of the stomach can be the result of a variety of irritants including alcohol, corrosive agents, and infection. Gastritis changes the normal surface pattern of the gastric mucosa. Helicobacter pylori can cause chronic gastritis that may lead to peptic ulcer disease.
Radiographic Appearance
Alcoholic gastritis may produce thickening of gastric folds (Figure 5-29), multiple superficial gastric erosions, or both. In corrosive gastritis, the acute inflammatory reaction heals by fibrosis and scarring, which result in severe narrowing of the antrum and may cause gastric outlet obstruction. In bacterial (phlegmonous) gastritis, inflammatory thickening of the gastric wall causes narrowing of the stomach that may mimic gastric cancer. The diagnosis of infectious gastritis can be made if there is evidence of gas bubbles (produced by the bacteria) in the stomach wall (Figure 5-30). These types of gastritis are known as erosive or acute gastritis.
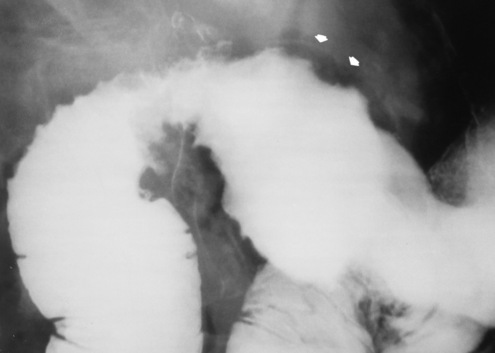
Figure 5-30 Phlegmonous emphysematous gastritis. Note the severe, irregular ulceration of the distal stomach, with air in the wall (arrows).
Chronic atrophic gastritis (nonerosive) refers to severe mucosal atrophy (wasting) that causes thinning and a relative absence of mucosal folds, with the fundus or entire stomach having a bald appearance. This is a nonspecific radiographic pattern that can be related to such factors as age, malnutrition, medication, and complications of alcoholism. Chronic atrophic gastritis also occurs in patients with pernicious anemia, who cannot absorb vitamin B12 because of an inability of the stomach to secrete intrinsic factor (or hydrochloric acid).
Treatment
The causative agent in erosive gastritis determines treatment. Eliminating the causative agent, such as alcohol, reduces the changes in the mucosal lining as well as the symptoms because production of the protecting mucus is not inhibited. If overproduction of stomach acid produces the changes, acid-reduction medications are used to help maintain the mucous defense barrier. Antibiotics are the appropriate medication for bacterial or infectious gastritis. Patients with H. pylori gastritis receive a regimen of multiple antibiotics because this infection may be antibiotic resistant. Use of a combination of antibiotics has resulted in an 80% to 90% cure rate.
Pyloric Stenosis
Pyloric stenosis, also known as infantile hypertrophic pyloric stenosis (IHPS), occurs when the two muscular layers of the pylorus become hyperplastic and hypertrophic. Environmental and hereditary factors are believed to cause this process in 2 to 4 per 1000 live births. The gastric antrum and the pyloric canal become lengthened, whereas the mucosa is usually edematous and thickened. This causes a complete or near-complete obstruction, preventing food from entering into the duodenum. The edematous and thickened pylorus may be palpated and is described as a mobile, hard “olive.”
Radiographic Appearance
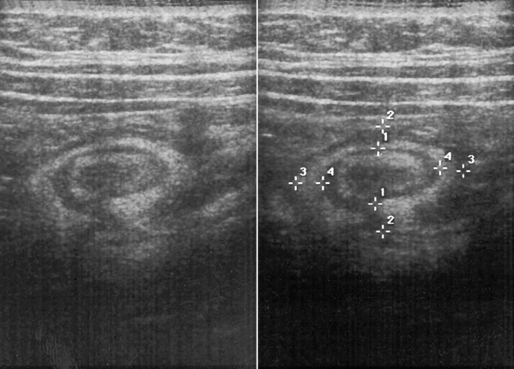
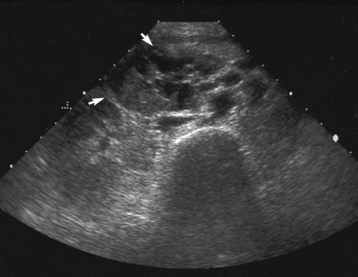
In today’s imaging arena, ultrasound is the modality of choice due to its high sensitivity and specificity, an accuracy approaching 100%. Pyloric stenosis appears as a thickened pyloric muscle (width greater than 3 mm) and an elongated pyloric canal (greater than 1.2 cm) on the longitudinal sonogram (Figure 5-31). The palpable olive appears as a “doughnut” or “target” sign in the cross-sectional image. When ultrasonographic findings are inconclusive, an upper GI series may aid in confirming the diagnosis by demonstrating the shouldering caused by a filling defect at the antrum as a result of the hypertrophic pyloric sphincter and delayed gastric emptying.
Peptic Ulcer Disease
Peptic ulcer disease is a group of inflammatory processes involving the stomach and duodenum. It is caused by the action of acid and the enzyme pepsin secreted by the stomach and occurs most frequently on the lesser curvature. The spectrum of peptic ulcer disease varies from small and shallow superficial erosions to huge ulcers that may perforate through the bowel wall.
The major complications of peptic ulcer disease are hemorrhage (20%), gastric outlet obstruction (5% to 10%), and perforation (less than 5%). Peptic ulcer disease is the most common cause of acute upper GI bleeding. Free perforation of a peptic ulcer located in the anterior wall of the stomach or duodenum is the most common cause of pneumoperitoneum with peritonitis (see later discussion “Pneumoperitoneum”). Narrowing of the lumen of the distal stomach or duodenal bulb caused by peptic ulcer disease is by far the most common cause of gastric outlet obstruction.
Duodenal Ulcer
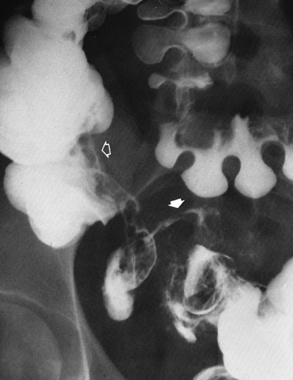
Duodenal ulcer is the most common manifestation of peptic ulcer disease. More than 95% of duodenal ulcers occur in the first portion of the duodenum (the duodenal bulb).
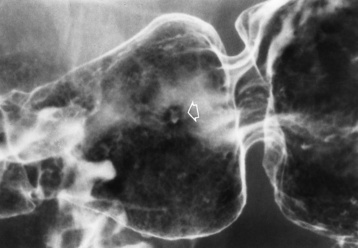
Radiographic Appearance: An unequivocal diagnosis of active duodenal ulcer requires the demonstration of an ulcer crater, which appears in profile as a small collection of barium projecting from the lumen. When seen en face (face on), the ulcer niche appears as a rounded or linear collection of contrast material surrounded by lucent folds that often radiate toward the crater (Figure 5-32). Secondary signs of duodenal ulcer disease include thickening of the mucosal folds and a deformity of the duodenal bulb. Acute ulcers incite muscular spasm, leading to deformity of the margins of the duodenal bulb that may be inconsistent and varied during the examination. With chronic ulceration, fibrosis and scarring cause a fixed deformity that persists even though the ulcer heals. Symmetrical narrowing of the duodenal bulb in its midportion may produce the typical cloverleaf deformity of chronic duodenal ulcer disease (Figure 5-33). CT demonstrates an irregularity or collection of contrast material in the gastric wall; however, as with barium studies, this appearance may be difficult to differentiate from that of malignancy.
Gastric Ulcer
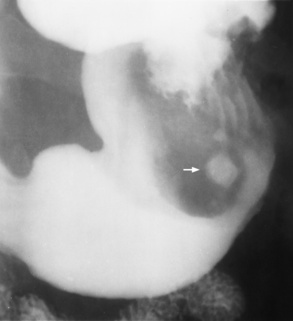
Gastric ulcers, another form of peptic ulcer disease, usually occur on the lesser curvature of the stomach. Unlike duodenal ulcers, which are virtually always benign, up to 5% of gastric ulcers are malignant.
Radiographic Appearance: Radiographic signs that indicate whether a gastric ulcer is more likely to be benign or malignant have been described. The classic sign of a benign gastric ulcer in profile is penetration, with clear projection of the ulcer outside the normal barium-filled gastric lumen because the ulcer represents an excavation in the wall of the stomach (Figure 5-34). A thin lucency at the base of the ulcer, reflecting mucosal edema caused by inflammatory exudate, is another sign of benignancy. When viewed en face, a gastric ulcer appears as a persistent collection of barium surrounded by a halo of edema (the ulcer collar) (Figure 5-35).
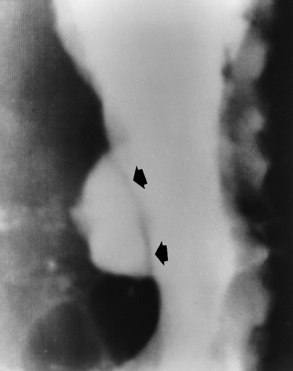
Figure 5-34 Benign gastric ulcer. Penetration of contrast material outside the normal, barium-filled gastric lumen associated with a thin, sharply demarcated, lucent line with parallel straight margins (arrows), representing edema at the base of the ulcer crater.
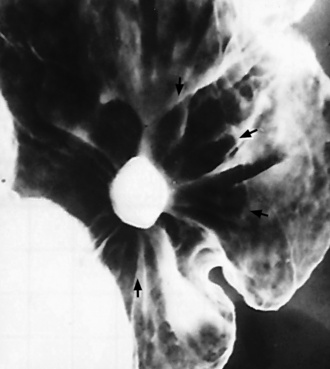
Figure 5-35 Benign gastric ulcer. On en face projection, prominent radiating folds extend directly to the ulcer. Lucency around the ulcer (arrows) represents inflammatory mass effect.
A hallmark of benign gastric ulcer is radiation of mucosal folds to the edge of the crater. However, because radiating folds can be identified in both malignant and benign ulcers, the character of the folds must be carefully assessed. If the folds are smooth and slender and appear to extend into the edge of the crater, the ulcer is most likely benign (Figure 5-36A). In contrast, irregular folds that merge into a mound of polypoid tissue around the crater are suggestive of malignancy (Figure 5-36B).
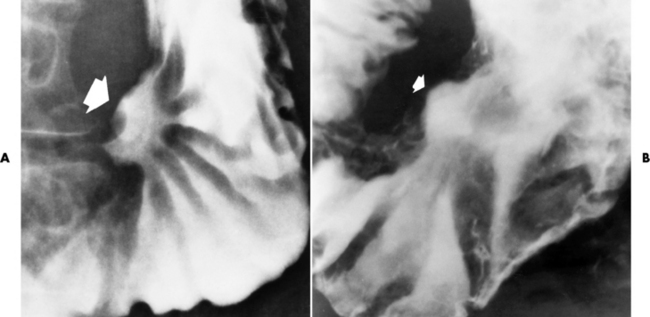
Figure 5-36 Radiating folds in gastric ulcers. A, Small, slender folds extending to the edge of the crater (arrow) indicate the benign nature of this ulcer. B, In this malignant gastric ulcer, thick folds radiate to an irregular mound of tissue around the ulcer (arrow).
Although the size, shape, number, and location of gastric ulcers have been suggested as criteria for distinguishing between benign and malignant lesions, these findings are of little practical value. One exception is ulcers in the gastric fundus above the level of the esophagogastric junction—essentially all of which are malignant.
An abrupt transition between the normal mucosa and the abnormal tissues surrounding a gastric ulcer is characteristic of a malignant lesion (Figure 5-37), in contrast to the diffuse and almost imperceptible transition between the normal gastric mucosa and the mound of edema surrounding a benign ulcer. Neoplastic tissue surrounding a malignant ulcer is usually nodular, unlike the smooth contour of the edematous mound around a benign ulcer. A malignant ulcer does not penetrate beyond the normal gastric lumen but remains within it because the ulcer merely represents a necrotic area within an intramural or intraluminal mass.
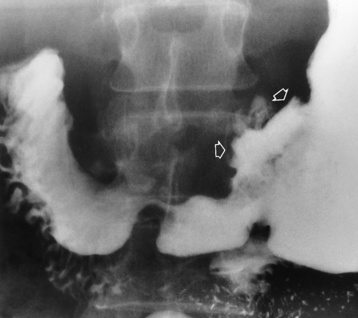
Figure 5-37 Malignant gastric ulcer. An abrupt transition occurs between normal mucosa and abnormal tissue surrounding an irregular gastric ulcer (arrows).
Most benign gastric ulcers heal completely with medical therapy (see “Treatment of Ulcers”) (Figure 5-38). Complete healing does not necessarily mean that the stomach returns to an absolutely normal radiographic appearance; bizarre deformities can result from fibrotic retraction and stiffening of the stomach wall. Although many malignant ulcers show significant healing, there is almost never complete disappearance of the ulcer crater.
The role of endoscopy in evaluating patients with gastric ulcers is controversial. At present, endoscopy is indicated only when the radiographic findings are not typical for a benign ulcer, when healing of the ulcer does not progress at the expected rate, or when the mucosa surrounding a healed ulcer crater has a nodular surface or any other feature suggestive of an underlying early gastric cancer.
Superficial Gastric Erosions
Superficial gastric erosions are ulcerations that are so small and shallow that they are rarely demonstrated on conventional single-contrast upper GI examinations. With the increasing use of double-contrast techniques, a superficial gastric erosion typically appears radiographically as a tiny fleck of barium, which represents the erosion, surrounded by a radiolucent halo, which represents a mound of edematous mucosa (Figure 5-39). Possible factors implicated in the production of superficial gastric erosions include alcohol, antiinflammatory drugs (aspirin, steroids), Crohn’s disease (see later discussion), and candidiasis (see earlier discussion “Candida and Herpesvirus”).
Treatment of Ulcers
Lifestyle modifications are the first line of treatment for ulcers. First, the patient should avoid foods that cause an increase in the acid secretions (i.e., alcohol and caffeine). Antacids aid in neutralizing stomach acid. If stress is the cause of the increase in acidic secretions, stress management is appropriate. When the ulceration is caused by an infection (H. pylori), antibiotics are given to kill the bacteria. If the acidic secretions cannot be controlled by these methods, histamine H2 antagonists help in reducing stomach acids and protecting the stomach lining. When more aggressive treatment is required, proton pump inhibitors may be used. Surgical treatment for management of complications may be necessary when ulcers do not respond to other treatments.
Cancer of the Stomach
Because pain is infrequently an early symptom, carcinoma of the stomach is rarely noted until the disease is far advanced and thus has a dismal prognosis (survival rate, 10%). The incidence of gastric cancer varies widely throughout the world. It is very high in Japan, Chile, and parts of Eastern Europe. It is low in the United States, where for reasons unknown it has been decreasing.
Several conditions appear to predispose persons to the development of carcinoma of the stomach. There is an increased risk of gastric cancer in patients with atrophic gastric mucosa, as in pernicious anemia, and in persons 10 to 20 years after a partial gastrectomy for peptic ulcer disease. A suggestive laboratory sign is achlorhydria, the absence of hydrochloric acid in gastric secretions obtained by means of a stomach tube. Most carcinomas occur in the distal stomach and are adenomatous in nature.
Radiographic Appearance
Gastric carcinoma can have a broad spectrum of radiographic appearances. Tumor infiltration of the gastric wall may stimulate intense fibrosis, which produces diffuse thickening, narrowing, and fixation of the stomach wall (a linitis plastica pattern) (Figure 5-40). The stomach is contracted into a tubular structure without normal pliability. This fibrotic process usually begins near the pylorus and progresses slowly upward; the fundus is the area least involved.
Another major form of gastric carcinoma is a large irregular polypoid mass (about one third of cancers). Irregularity and ulceration within the mass are suggestive of malignancy, whereas the presence of a stalk and normal-appearing gastric folds extending to the tumor are sign of benignancy. Ulceration can develop in any gastric carcinoma and occurs in approximately one third. It varies from shallow erosions in relatively superficial mucosal lesions to huge excavations within fungating polypoid masses (Figure 5-41).
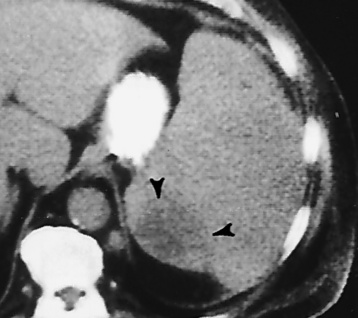
CT is of major value in the staging of gastric carcinoma, in planning its treatment, in assessing the response to therapy, and in detecting tumor recurrence (Figure 5-42). Carcinoma of the stomach may appear as thickening of the gastric wall or as an intraluminal mass. The earliest stage (stage I) demonstrates as an intraluminal mass without wall thickening. Stage II consists of wall thickening of greater than 1 cm without invasion of other tissue or organs. As the disease progresses, the stomach wall thickens and invades adjacent organs (stage III). Obliteration of the fat planes (the covering layers of fat) around the stomach is a reliable indicator of the extragastric spread of tumor (stage IV). CT can demonstrate direct tumor extension to intraabdominal organs and distant metastases, especially to the liver.
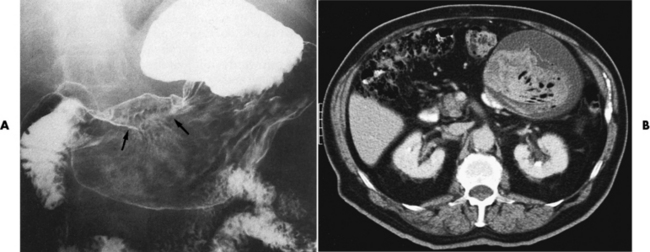
Figure 5-42 CT staging of gastric carcinoma. A, Double-contrast study demonstrates large lesser-curvature mass (arrows). B, CT scan shows a thickened gastric wall; the contrast agent demonstrates the lumen of the stomach.
Gastric carcinoma may also be demonstrated using endoscopic ultrasound. The gastric mucosa produces an increased echogenicity and demonstrates vertical invasion through the gastric wall. If diagnosed at a late stage, the lesion may extend into the perigastric lymph nodes.
Small bowel
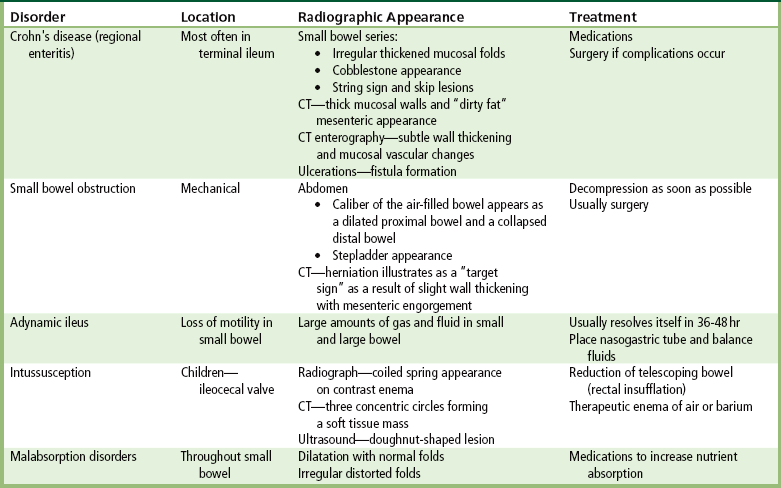
Crohn’s Disease (Regional Enteritis)
Crohn’s disease is a chronic inflammatory disorder of unknown cause that most often involves the terminal area of the ileum but can affect any part of the gastrointestinal tract. Although it can occur at any age, Crohn’s disease is most common in young adults. The underlying cause is unknown, although there appears to be some psychogenic element; stress or emotional upsets are frequently related to the onset or relapse of the disease.
The granulomatous inflammatory process in Crohn’s disease is frequently discontinuous, with diseased segments of bowel separated by apparently healthy portions (skip areas). Diffuse inflammation with edema involves all layers of the intestinal wall. Ulceration is common, and fistulas running in the bowel wall or extending to other organs are not infrequent.
The clinical spectrum of Crohn’s disease is broad, ranging from a relatively benign course with unpredictable acute attacks and remissions to severe diarrhea and an acute condition in the abdomen. Although acute Crohn’s disease may produce right lower quadrant pain simulating that of appendicitis, there is often blood in the stools that has come from the intensely
congested mucous membranes. Small bowel obstruction and fistula formation occur in up to half of patients. Rectal fissures and perirectal abscesses occur in about one third.
Radiographic Appearance
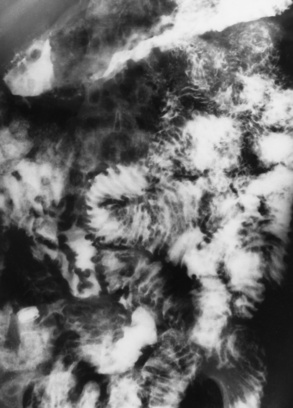
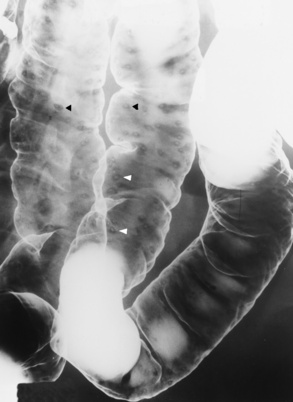
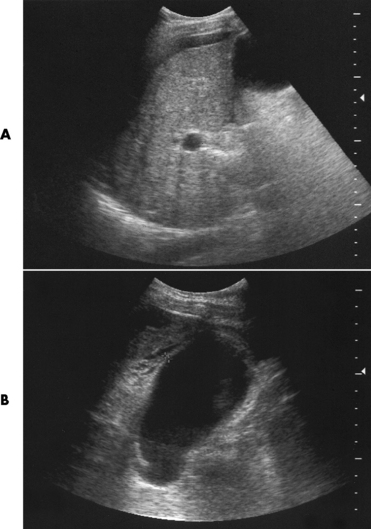
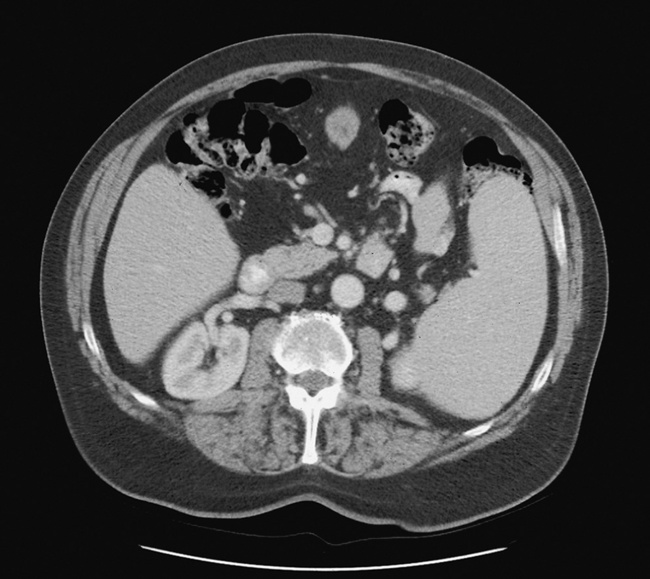
In the small bowel, the earliest radiographic changes of Crohn’s disease include irregular thickening and distortion of mucosal folds caused by submucosal inflammation and edema. Transverse and longitudinal ulcerations can separate islands of thickened mucosa and submucosa, leading to a characteristic rough cobblestone appearance (Figure 5-43). Rigid thickening of the entire bowel wall produces pipelike narrowing. Continued inflammation and fibrosis can result in a severely narrowed, rigid segment of small bowel in which the mucosal pattern is lost (string sign) (Figure 5-44). When several areas of small bowel are diseased, involved segments of varying length are often sharply separated from radiographically normal segments (skip lesions). On CT images, there is thickening of the wall of the small bowel, and the mesentery has an appearance that is described as “dirty fat.” The new technology of CT enterography demonstrates subtle findings, such as mild wall thickening and mucosal vascular changes. CT is recommended for a patient with active Crohn’s disease to determine the nature of the mass (Figure 5-45).
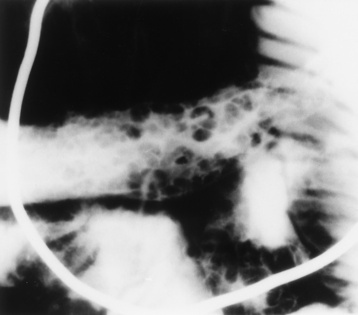
Figure 5-43 Crohn’s disease. Cobblestone appearance is produced by transverse and longitudinal ulcerations separating islands of thickened mucosa and submucosa.
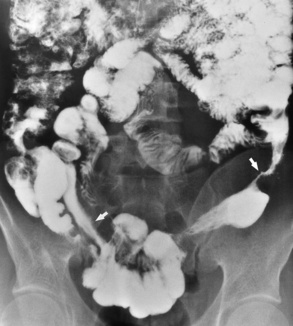
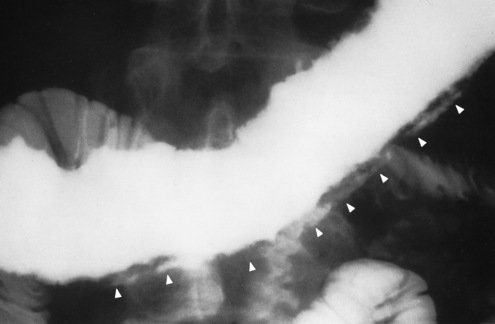
Figure 5-44 Crohn’s disease. Arrows point to widely separated areas of disease (skip lesions). The lesions are greatly narrowed segments of small bowel (string sign).
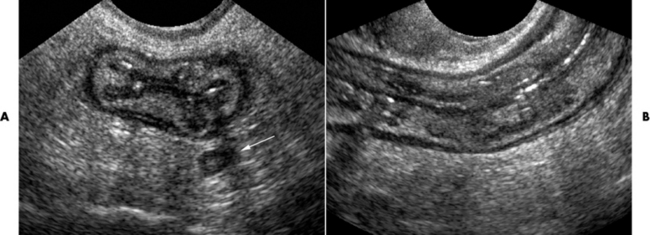
Figure 5-45 Crohn’s disease. Ultrasound. A, sagittal and B, demonstrates the ileum in long axis with a very thick wall and that wall layering is preserved.
Fistula formation, a hallmark of chronic Crohn’s disease, is found in at least half of all patients with this condition (Figure 5-46). The diffuse inflammation of the serosa and mesentery in Crohn’s disease causes involved loops of bowel to be firmly matted together by fibrous peritoneal and mesenteric bands. Fistulas apparently begin as ulcerations that burrow through the bowel wall into adjacent loops of small bowel and colon. In addition to fistulas between loops of bowel, a characteristic finding in Crohn’s disease is the appearance of fistulous tracts ending blindly in abscess cavities surrounded by dense inflammatory tissue. These abscess cavities can produce palpable masses, persistent fever, or pain. Although less common than bowel-to-bowel fistulas, internal fistulas extending from the bowel to the bladder or vagina can occur. A common complication is the development of external gastrointestinal fistulas, which usually extend to the perianal area and may be associated with fissures and perirectal abscesses.
Small Bowel Obstruction
Fibrous adhesions caused by previous surgery or peritonitis account for almost 75% of all small bowel obstructions. External hernias (inguinal, femoral, umbilical, incisional) are the second most common cause. Other general causes of mechanical small bowel obstruction include luminal occlusion (gallstone, intussusception) and intrinsic lesions of the bowel wall (neoplastic or inflammatory strictures, vascular insufficiency).
Radiographic Appearance
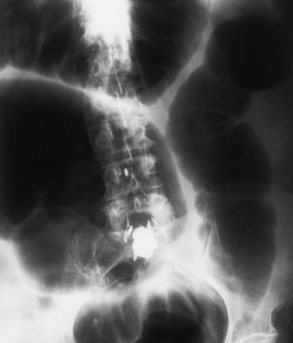
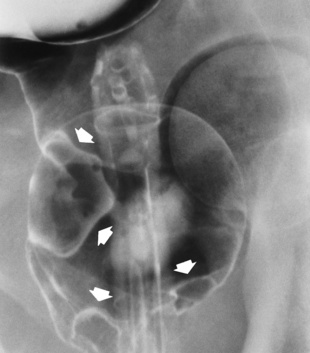
Distended loops of small bowel containing gas and fluid can usually be recognized radiographically within 3 to 5 hours of the onset of complete obstruction. Almost all gas proximal to a small bowel obstruction represents swallowed air. On upright or lateral decubitus projections, the interface between gas and fluid forms a straight horizontal margin (Figure 5-47). Although the presence of gas-fluid levels at different heights in the same loop has traditionally been considered evidence for mechanical obstruction, an identical pattern can also be demonstrated in some patients with adynamic ileus (see later discussion). The air-filled bowel appears as a dilated proximal bowel and a collapsed distal bowel. On upright images, a string-of-beads sign appears. In adynamic ileus, the bowel has no caliber change.
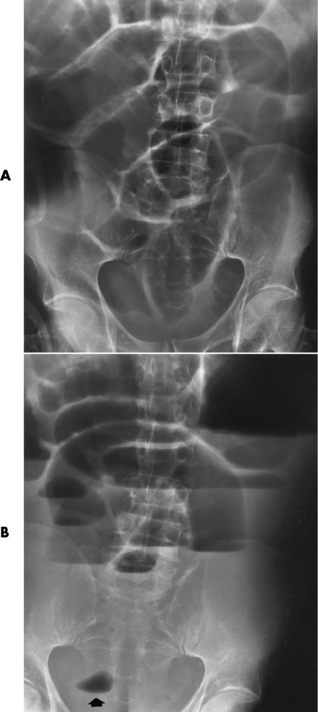
Figure 5-47 Small bowel obstruction. Supine (A) and upright (B) projections demonstrate large amounts of gas in dilated loops of small bowel. B, which was taken with the patient in the upright position and using a horizontal beam, demonstrates multiple prominent air-fluid levels. A single, small collection of gas (arrow) remains in colon.
As time passes, the small bowel may become so distended as to be almost indistinguishable from the colon. To make the critical differentiation between small and large bowel obstruction, it is essential to determine which loops of bowel contain abnormally large amounts of air. Small bowel loops generally occupy the more central portion of the abdomen, whereas colonic loops are positioned laterally around the periphery of the abdomen or inferiorly in the pelvis (Figure 5-48). Gas within the lumen of the small bowel outlines the thin valvulae conniventes, which completely encircle the bowel. In contrast, colonic haustral markings are thicker and farther apart and occupy only a portion of the transverse diameter of the bowel.
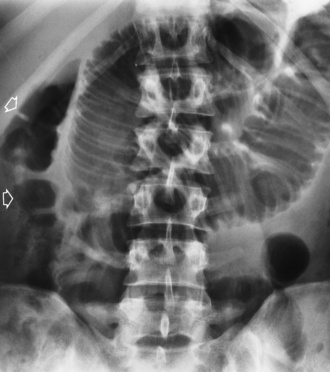
Figure 5-48 Small bowel obstruction. Dilated loops of small bowel occupy the central portion of the abdomen, with the nondilated cecum and ascending colon positioned laterally around the periphery of the abdomen (arrows).
The site of obstruction can usually be predicted with considerable accuracy if the number and position of dilated bowel loops are analyzed. The presence of a few dilated loops of small bowel located high in the abdomen (in the center or slightly to the left) indicates an obstruction in the distal duodenum or jejunum. The involvement of additional small bowel loops is suggestive of a lower obstruction. As more loops are affected, they appear to be placed one above the other upward and to the left, producing a characteristic stepladder appearance (Figure 5-49). The point of obstruction is always distal to the lowest loop of dilated bowel.
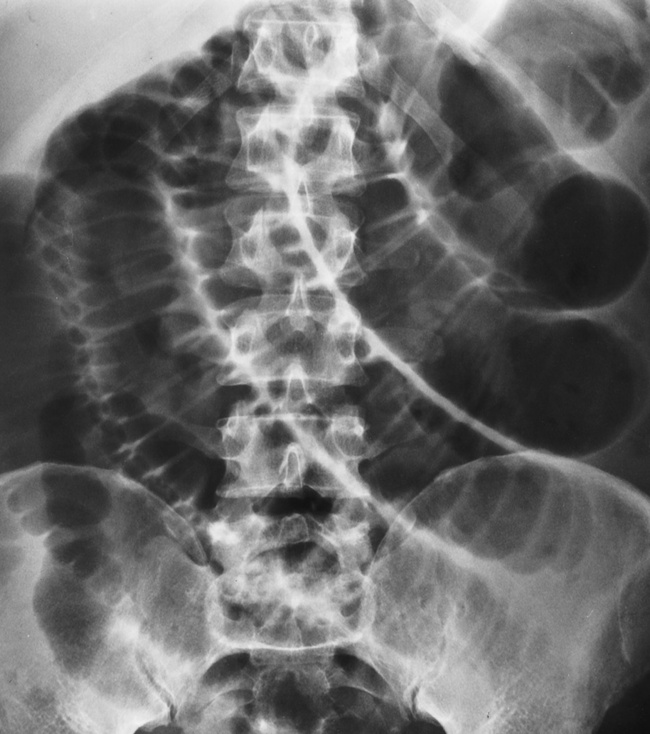
Figure 5-49 Low small bowel obstruction. Dilated loops of gas-filled bowel appear to be placed one above the other, upward and to the left, producing the characteristic stepladder appearance.
Patients with complete mechanical small bowel obstruction demonstrate little or no gas in the colon. This is a valuable point in the differentiation between mechanical obstruction and adynamic ileus, in which gas is seen within distended loops throughout the bowel. Although a small amount of gas or fecal accumulation may be present at an early stage of a small bowel obstruction (see Figure 5-48), the detection of a large amount of gas in the colon effectively eliminates this diagnosis.
The bowel proximal to an obstruction may contain no gas but be completely filled with fluid. This may produce a confusing picture of a normal-appearing abdomen or a large soft tissue abdominal mass.
Plain abdominal radiographs are occasionally insufficient for a distinction to be made between small and large bowel obstructions. In these instances, a carefully performed barium enema examination can document or eliminate the possibility of large bowel obstruction. If it is necessary to determine the precise site of small bowel obstruction, barium can be administered in either a retrograde (by means of an enema) or an antegrade (by way of the mouth) manner. Orally administered barium (not water-soluble agents) is the most effective contrast material for demonstrating the site of small bowel obstruction (Figure 5-50). The large amount of fluid proximal to a small bowel obstruction prevents any trapped barium from hardening or increasing the degree of obstruction. The density of barium permits excellent visualization far into the intestine; water-soluble agents, however, are lost to sight because of dilution and absorption. It must be emphasized that if plain radiographs clearly demonstrate a mechanical small bowel obstruction, a contrast examination is unnecessary.
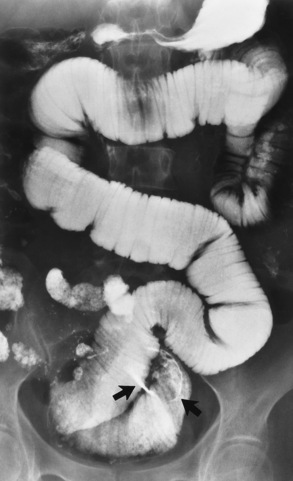
Figure 5-50 Barium upper gastrointestinal series demonstrates an impacted bezoar (arrows) to be the cause of a small bowel obstruction.
Strangulation of bowel caused by interference with the blood supply is a serious complication of small bowel obstruction. In a closed-loop obstruction (e.g., volvulus and incarcerated hernia) (Figure 5-51), the loops going both toward (afferent) and away from (efferent) the area of narrowing become obstructed. The involved segments usually fill with fluid and appear radiographically as a tumorlike soft tissue mass. A closed loop is a clinically dangerous form of obstruction, because the continuing outpouring of fluid into the enclosed space can raise intraluminal pressure and rapidly lead to occlusion of the blood supply to that segment of bowel. Because venous pressure is normally lower than arterial pressure, blockage of venous outflow from the strangulated segment occurs before obstruction of the mesenteric arterial supply. Ischemia can rapidly cause necrosis of the bowel with sepsis, peritonitis, and a potentially fatal outcome.
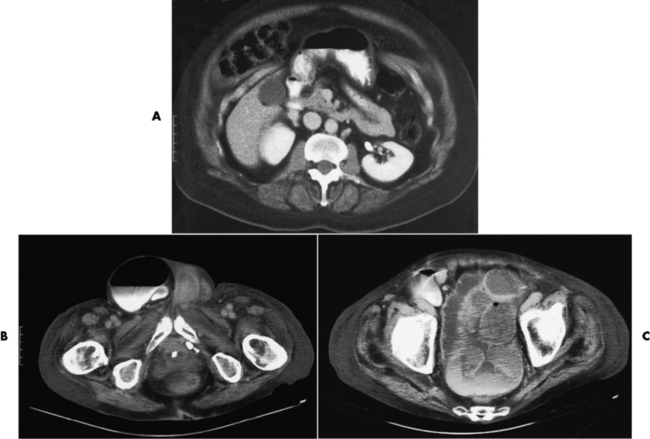
Figure 5-51 CT scans of bowel obstructions. A, Herniation of the small bowel and stomach through anterior abdominal wall. B, Scrotal herniation of the large bowel causing bowel obstruction. C, Dilated sigmoid colon caused by obstruction.
CT may aid in demonstrating small bowel obstruction when the plain abdominal radiographs are normal or nonspecific. In addition to showing the site, level, and cause of the obstruction, this modality may indicate whether there is strangulation of the involved loops of bowel. Specifically, herniation causes slight wall thickening that appears as a “target sign” as a result of engorgement of the superior and inferior mesenteric vessels. CT appears to be the most valuable modality for patients with a history of abdominal malignancy and for those who have signs of infection, bowel infarction, or a palpable abdominal mass (Figure 5-52).
Adynamic Ileus
Adynamic ileus is a common disorder of intestinal motor activity in which fluid and gas do not progress normally through a nonobstructed small and large bowel. A variety of neural, hormonal, and metabolic factors can precipitate reflexes that inhibit intestinal motility. Adynamic ileus occurs to some extent in almost every patient who undergoes abdominal surgery. Other causes of adynamic ileus are peritonitis, medications that decrease intestinal peristalsis (those with an atropine-like effect), electrolyte and metabolic disorders, and trauma. Adynamic ileus (or paralytic ileus) occurs more often than mechanical bowel obstruction. The clinical findings in patients with adynamic ileus vary from minimal symptoms to generalized abdominal distention with a sharp decrease in the frequency and intensity of bowel sounds.
Radiographic Appearance
The radiographic hallmark of adynamic ileus is the retention of large amounts of gas and fluid in dilated small and large bowel. The entire small and large bowel in adynamic ileus, unlike in mechanical small bowel obstruction, appears almost uniformly dilated with no demonstrable point of obstruction (Figure 5-53).
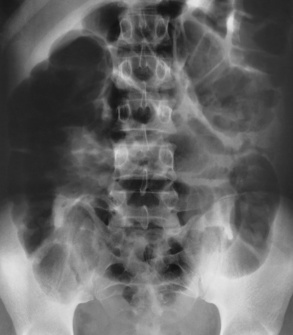
Figure 5-53 Adynamic ileus. Large amounts of gas and fluid are retained in loops of dilated small and large bowel. The entire bowel, small and large, appears almost uniformly dilated with no demonstrable point of obstruction.
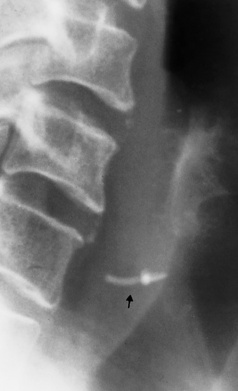
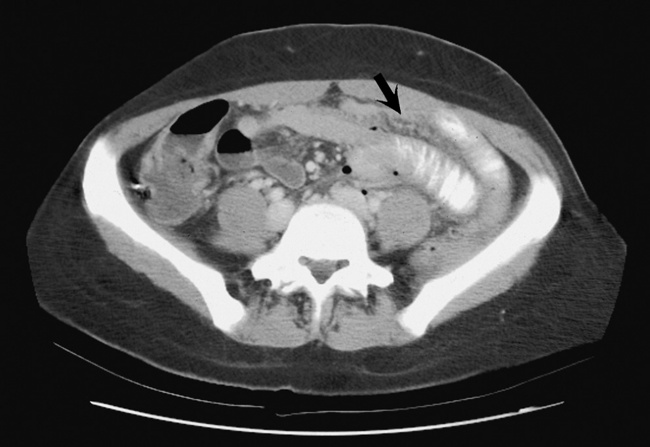
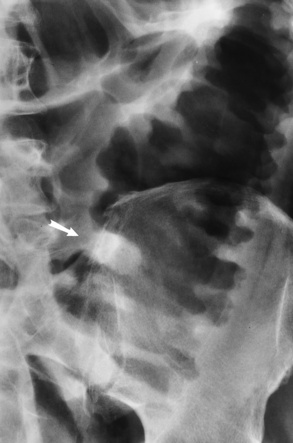
There are two major variants of adynamic ileus. Localized ileus refers to an isolated distended loop of small or large bowel (the sentinel loop), which is often associated with an adjacent acute inflammatory process. The portion of the involved bowel can offer a clue to the underlying disease. Localized segments of the jejunum or transverse colon are frequently dilated in patients with acute pancreatitis. Similarly the hepatic flexure of the colon can be distended in acute cholecystitis, the terminal ileum can be dilated in acute appendicitis, the descending colon can be distended in acute diverticulitis, and dilated loops can be seen along the course of the ureter in acute ureteral colic (Figure 5-54). Unfortunately, isolated segments of distended small bowel are commonly seen in patients with abdominal pain and thus the sentinel loop may be found “guarding” the wrong area.
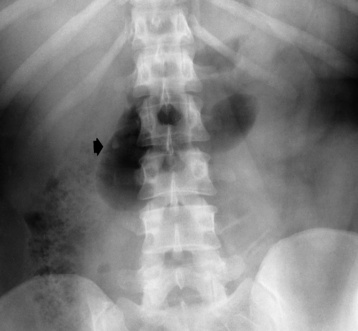
Figure 5-54 Localized ileus in patient with acute ureteral colic. Arrow points to impacted ureteral stone.
Colonic ileus refers to selective or disproportionate gaseous distention of the large bowel without an obstruction (Figure 5-55). Massive distention of the cecum, which is often horizontally oriented, characteristically dominates the radiographic appearance. Colonic ileus usually accompanies or follows an acute abdominal inflammatory process or abdominal surgery. The clinical presentation and the findings on plain abdominal radiographs simulate those in mechanical obstruction of the colon. A barium enema examination is usually necessary to exclude an obstructing lesion.
Treatment
Adynamic ileus caused by surgery usually resolves itself spontaneously in 36 to 48 hours if no complications are involved. Treatment involves insertion of a nasogastric tube to aspirate the stomach, decompress the bowel, and allow the intestine to rest. Electrolyte and fluid imbalances are corrected by intravenous (IV) injection.
Intussusception
Intussusception is a major cause of bowel obstruction in children; it is much less common in adults. Intussusception is the telescoping of one part of the intestinal tract into another because of peristalsis, which forces the proximal segment of bowel to move distally within the ensheathing outer portion. Once such a lead point has been established, it gradually progresses forward and causes increased obstruction. This process can compromise the vascular supply and produce ischemic necrosis of the intussuscepted bowel.
In children, intussusception is most common in the region of the ileocecal valve. The clinical onset tends to be abrupt, with severe abdominal pain, blood in the stool (“currant jelly” stool), and often a palpable right-sided mass. If the diagnosis is made early and therapy instituted promptly, the mortality of intussusception in children is less than 1%. However, if treatment is delayed more than 48 hours after the onset of symptoms, the mortality increases dramatically. In adults, intussusception is often chronic or subacute and is characterized by irregular recurrent episodes of colicky pain, nausea, and vomiting. A specific cause of intussusception often cannot be detected in children. In adults, however, the leading edge is frequently a polypoid tumor with a stalk (pedunculated) or an inflammatory mass.
Radiographic Appearance
Radiographically, an intussusception produces the classic coiled-spring appearance of barium trapped between the intussusceptum and the surrounding portions of bowel (Figure 5-56A). Reduction of a colonic intussusception can sometimes be accomplished by a barium enema examination (Figure 5-56B and C), although great care must be exercised to prevent excessive intraluminal pressure, which may lead to perforation of the colon. On CT images, intussusception appears as three concentric circles forming a soft tissue mass.
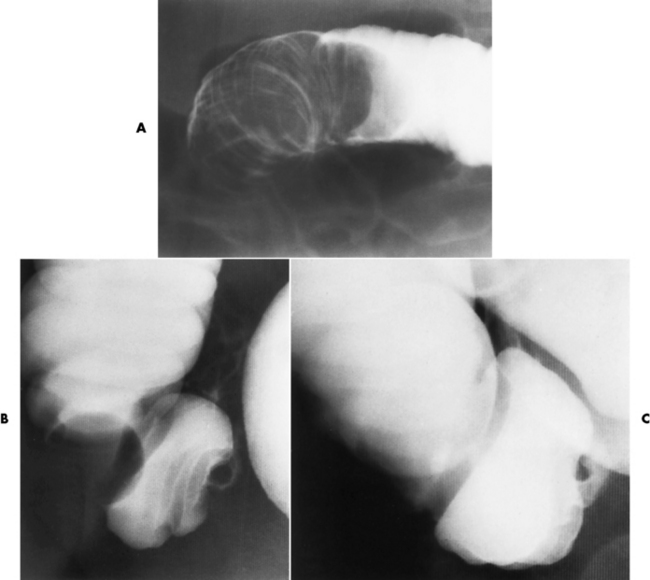
Figure 5-56 Intussusception. A, Obstruction of the colon at the hepatic flexure produces the characteristic coiled-spring appearance of intussuscepted bowel. Partial (B) and complete (C) reduction of intussusception by careful barium enema examination.
Ultrasound, used especially in children, demonstrates the obstructive mass as a doughnut-shaped lesion on the transverse scan and as a “pseudokidney” on the longitudinal scan (Figure 5-57).
Treatment
Reduction of intussusceptions by rectal insufflation of air (instead of barium) has been reported to be an effective technique in children. In older children and adults, a second barium enema examination after reduction is necessary to determine whether an underlying polyp or a tumor caused the intussusception.
Malabsorption Disorders
Malabsorption disorder refers to a multitude of conditions in which there is defective absorption of carbohydrates, proteins, and fats from the small bowel. Regardless of the cause, malabsorption results in steatorrhea—the passage of bulky, foul-smelling, high-fat-content stools that float.
Radiographic Appearance
Many of the diseases that cause malabsorption produce radiographic abnormalities in the small bowel, although malabsorption can exist without any detectable small bowel changes. The two major radiographic appearances are (1) small bowel dilatation with normal folds (Figure 5-58) and (2) a pattern of generalized, irregular, distorted small bowel folds (Figure 5-59).
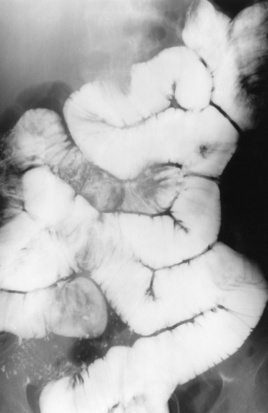
Figure 5-58 Diffuse dilatation of entire small bowel with excessive intraluminal fluid in a patient with malabsorption caused by sprue.
Treatment
Patients afflicted with malabsorption disorders take medications to assist in absorbing key nutrients to keep the body’s systems in good health. Probiotics are live microbial food supplements that aid in improving the intestinal microbial balance. These supplements enhance the bioavailability of nutrients to the body.
Colon
Acute appendicitis develops when the neck of the appendix becomes blocked by a fecalith or by postinflammatory scarring that creates a closed-loop obstruction within the organ. Because of inadequate drainage, fluid accumulates in the obstructed portion and serves as a breeding ground for bacteria. High intraluminal pressure causes distention and thinning of the appendix distal to the obstruction, which interferes with the circulation and may lead to gangrene and perforation. If the process evolves slowly, adjacent organs (terminal ileum, cecum, omentum) may wall off the appendiceal area so that a localized abscess develops; rapid vascular compromise may permit free perforation with the spilling of fecal material into the peritoneal cavity and the development of generalized peritonitis. Appendicitis occurs in all age groups but is more common in children and adolescents.
The clinical symptoms (and laboratory results) of acute appendicitis are usually so characteristic that there is no need for routine radiographs to make the correct diagnosis. The presence of severe right lower quadrant pain, low-grade fever, and slight leukocytosis, especially in younger adults, is presumed to be evidence of appendicitis. However, in some patients, especially the elderly, the clinical findings may be obscure or minimal. In addition, because the appendix is mobile and may be in an unusual location, the pain of acute appendicitis may mimic that of cholecystitis, diverticulitis, or pelvic inflammatory disease. When the symptoms are confusing, an imaging examination may be necessary for prompt diagnosis and surgical intervention before perforation occurs.
Radiographic Appearance
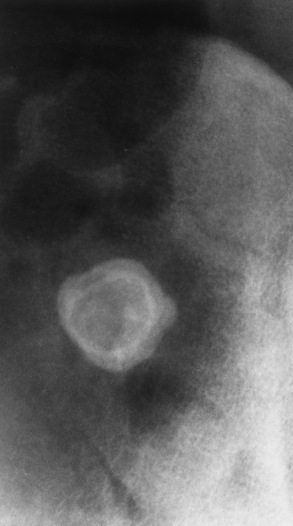
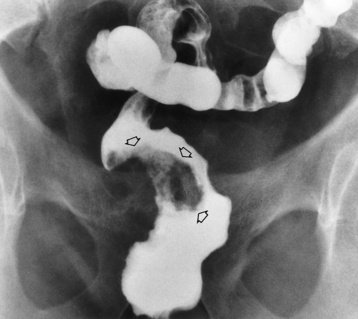
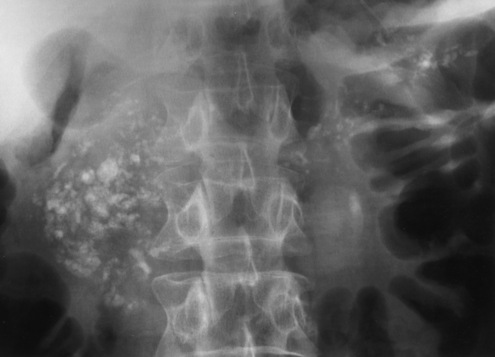
Plain abdominal radiographs demonstrate a round or oval, laminated calcified fecalith in the appendix (appendicolith) (Figure 5-60) in about one third of patients. Surgical experience indicatesthat the presence of an appendicolith in combination with symptoms of acute appendicitis usually implies that the appendix is gangrenous (necrotic) and likely to perforate. Most appendicoliths are located in the right lower quadrant overlying the iliac fossa. Depending on the length and position of the appendix, however, an appendicolith can also be seen in the pelvis or in the right upper quadrant (retrocecal appendix), where it can simulate a gallstone.
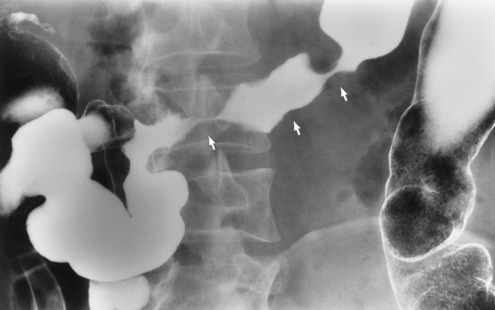
Because of the danger of perforation, barium enema examination is usually avoided in acute appendicitis. If it is performed, an irregular impression of the base of the cecum (caused by inflammatory edema), in association with failure of barium to enter the appendix, is a characteristic finding. Nevertheless, failure of barium to fill the appendix is not a reliable sign of appendicitis, because the normal appendix does not fill with barium in about 20% of cases. Partial filling of the appendix with distortion of its shape or caliber strongly suggests acute appendicitis (Figure 5-61), especially if there is a cecal impression. In contrast, a patent (open) appendiceal lumen effectively excludes the diagnosis of acute appendicitis, especially when barium extends to fill the rounded appendiceal tip.
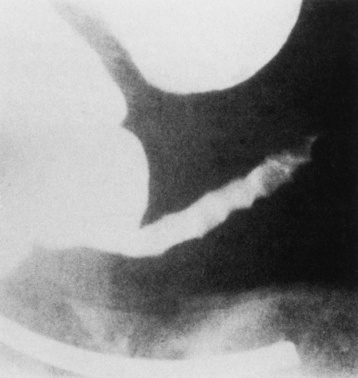
Figure 5-61 Acute appendicitis. Spot radiograph from barium enema examination shows incomplete filling of appendix.
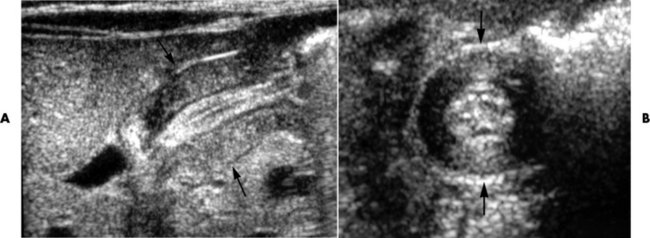
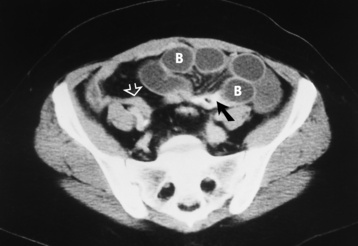
When the clinical presentation is unclear, high-resolution ultrasound with graded compression is the imaging modality of choice for diagnosing acute appendicitis, especially when use of ionizing radiation is contraindicated in the patient. A noncompressible appendix measuring 7 mm or more in maximal outer diameter is considered virtually pathognomonic of acute appendicitis (Figure 5-62).
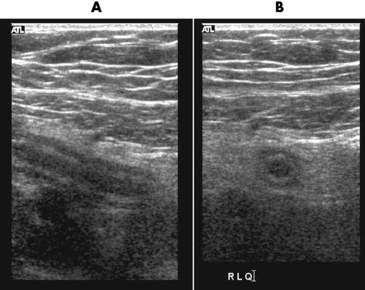
Figure 5-62 Ultrasound images of appendicitis. The Sagittal A, illustrates an inflamed appendix that is elongated and hypoechoic. The transverse scan, B, demonstrates the appendiceal lumen surrounded by hypoechoic hypoechoic inflamed tissue.
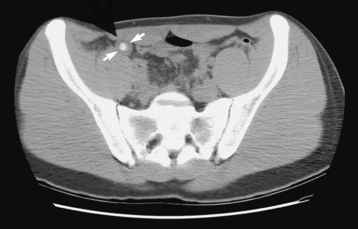
CT, the gold standard, shows an appendiceal abscess as a round or oval mass of soft tissue density that may contain gas. After administration of IV contrast material, the appendix appears as a dilated structure with a thickened, circumferentially enhancing wall (Figure 5-63). This modality provides a more precise evaluation of the nature, extent, and location of the pathologic process and can detect intraabdominal disease unrelated to appendicitis that may explain the patient’s clinical presentation.
Treatment
When a patient is diagnosed with appendicitis, an immediate appendectomy should be performed before perforation occurs to prevent complications (e.g., peritonitis, gangrene, and abscess formation). If perforation occurs, a regimen of antibiotics helps reduce the risk of peritonitis and sepsis.
Diverticulosis
Colonic diverticula are outpouchings that represent acquired herniations of mucosa and submucosa through the muscular layers at points of weakness in the bowel wall. The incidence of colonic diverticulosis increases with age. Rare in persons younger than 30 years diverticula can be demonstrated in up to half of persons older than 60 years. Diverticular disease is presumed to occur in individuals who frequently exert high pressures in the lumen while straining to pass a large bulk of stool. Those consuming low-fiber and low-bulk meals are more susceptible. Diverticula occur most commonly in the sigmoid colon and decrease in frequency in the proximal colon. Although most patients with diverticulosis have no symptoms, a substantial number have chronic or intermittent lower abdominal pain, frequently related to meals or emotional stress, and alternating bouts of diarrhea and constipation. Bleeding may be caused by inflammatory erosion of penetrating blood vessels at the base of the diverticulum. The leading cause of massive lower gastrointestinal bleeding in adults and guaiac-positive (indicating occult blood) stools in the elderly is diverticular disease.
Radiographic Appearance
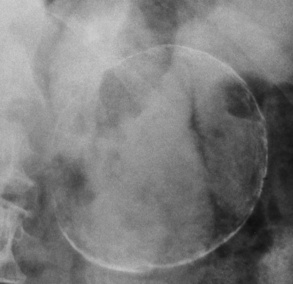
Colonic diverticula appear radiographically as round or oval outpouchings of barium projecting beyond the confines of the lumen. They vary in size from barely visible dimples to saclike structures 2 cm or more in diameter. Giant sigmoid diverticula up to 25 cm in diameter, which probably represent slowly progressing chronic diverticular abscesses, may appear as large, well-circumscribed, lucent cystic structures in the lower abdomen.
Diverticula are usually multiple and tend to occur in clusters, although a solitary diverticulum is occasionally found. When diverticula are multiple, deep crisscrossing ridges of thickened circular muscle (saw-toothed configuration) can produce a characteristic series of sacculations (Figure 5-64).
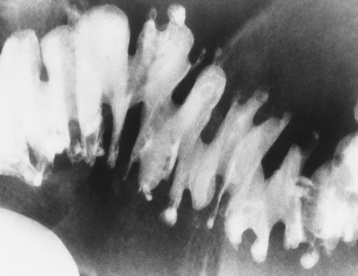
Figure 5-64 Diverticulosis. The typical saw-toothed configuration is produced by thickened circular muscle and is associated with multiple diverticula.
Diverticula also commonly occur in the esophagus and duodenum and infrequently may develop in the jejunum and ileum.
Diverticulitis
Diverticulitis is a complication of diverticular disease of the colon (necrosing inflammation in the diverticula), especially in the sigmoid region, in which perforation of a diverticulum leads to the development of a peridiverticular abscess. It is estimated that up to 20% of patients with diverticulosis eventually develop acute diverticulitis. Retained fecal material trapped in a diverticulum by the narrow opening of the diverticular neck causes inflammation of the mucosal lining, which then leads to perforation of the diverticulum. This usually results in a localized peridiverticular abscess that is walled off by fibrous adhesions. The inflammatory process may localize within the wall of the colon and produce an intramural mass, or it may dissect around the colon, causing segmental narrowing of the lumen. Extension of the inflammatory process along the colon wall can involve adjacent diverticula, resulting in a longitudinal sinus tract along the bowel wall. A common complication of diverticulitis is the development of fistulas to adjacent organs (bladder, vagina, ureter, small bowel).
Radiographic Appearance
The radiographic diagnosis of diverticulitis requires direct or indirect evidence of diverticular perforation. The most specific sign is extravasation, which can appear either as a tiny projection of contrast material from the tip of a diverticulum (Figure 5-65A) or as an obvious filling of a pericolic abscess (Figure 5-65B). A more common, although somewhat less specific, sign of diverticulitis is the demonstration of a pericolic soft tissue mass that is attributable to a localized abscess and represents a walled-off perforation. This extraluminal mass appears as a filling defect causing eccentric narrowing of the bowel lumen. The adjacent diverticula are spastic, irritable, and attenuated and frequently seem to drape over the mass. It is important to remember, however, that a peridiverticular abscess caused by diverticulitis can occur without radiographically detectable diverticula (Figure 5-66).
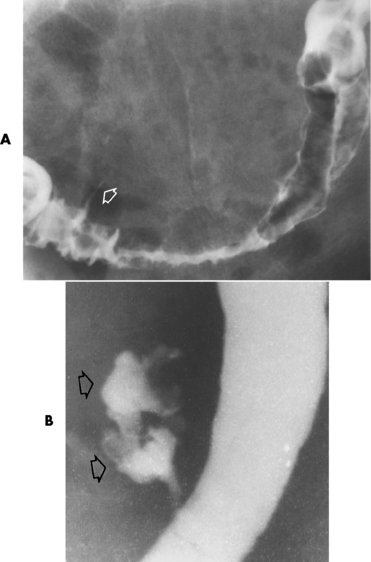
Figure 5-65 Diverticulitis. A, Thin projection of contrast material (arrow) implies extravasation from colonic lumen. Note severe spasm of the sigmoid colon caused by intense adjacent inflammation. B, The pericolic abscess is clearly filled with contrast material (arrows).
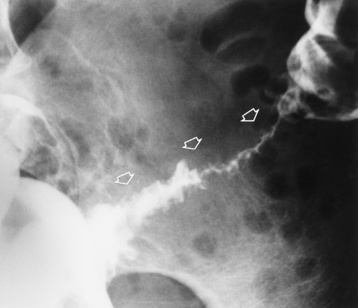
Figure 5-66 Sigmoid diverticulitis. Severe narrowing of the long involved portion of the sigmoid colon (arrows) in a patient with no radiographically detectable diverticula.
Severe spasm or fibrotic healing of diverticulitis can cause a rigidity and progressive narrowing of the colon that simulates annular carcinoma. Although radiographic distinction from carcinoma may be impossible, findings favoring the diagnosis of diverticulitis include the involvement of a relatively long segment, a gradual transition from diseased to normal colon, a relative preservation of mucosal detail, and fistulous tracts and intramural abscesses. At times, colonoscopy or surgery may be required to make a definitive diagnosis.
Acute diverticulitis on ultrasound appears as a hypoechoic projection that arises from the wall of the bowel and is surrounded by inflamed fat. CT demonstrates pericolic fluids or gas collections, usually with nonspecific colonic wall thickening that produces narrowing of the bowel lumen (Figure 5-67). Inflammatory stranding may appear in the adjacent fat.
Treatment of Diverticulosis and Diverticulitis
Noninvasive treatment is the first choice for diverticulosis, with use of dietary modifications (nothing with seeds, nuts, popcorn, etc.) and exercise (to increase peristalsis). If diverticulitis has developed, antibiotics and diet adjustments (liquids) are given until the bowel heals. Perforation requires surgical repair and a regimen of antibiotics.
Ulcerative Colitis
Ulcerative colitis is one of the two major inflammatory bowel diseases (the other is Crohn’s colitis; see later discussion). It primarily affects young adults and is highly variable in severity, clinical course, and ultimate prognosis. The cause is unknown, although an autoimmune cause has been suggested and a psychogenic factor may be involved because the condition is often aggravated by stress. The onset of the disease and subsequent exacerbations can be insidious or abrupt. The main symptoms are bloody diarrhea, abdominal pain, fever, and weight loss. A characteristic feature of ulcerative colitis is alternating periods of remission and relapse. Most patients have intermittent episodes of symptoms, with complete remission between attacks. In fewer than 15% of patients, ulcerative colitis is an acute severe process with a far higher incidence of serious complications, such as toxic megacolon (extreme dilatation of a segment of colon with systemic toxicity) and free perforation into the peritoneal cavity. Usually, ulcerative colitis involves only the mucosal layer of the colon.
Radiographic Appearance
In the radiographic evaluation of a patient with known or suspected ulcerative colitis, plain abdominal radiographs are essential. Large nodular protrusions of hyperplastic mucosa, deep ulcers outlined by intraluminal gas, or polypoid changes along with a loss of haustral markings (normal indentations in the wall of the colon) may indicate the diagnosis. Plain abdominal radiographs can also demonstrate evidence of toxic megacolon, a dramatic and ominous complication of ulcerative colitis that is characterized by extreme dilatation of a segment of colon (Figure 5-68), or evidence of an entire diseased colon, combined with systemic toxicity (abdominal pain and tenderness, tachycardia, fever, and leukocytosis). Toxic megacolon can lead to spontaneous perforation of the colon, which can be dramatic and sudden and can cause irreversible shock. Because there is such a high danger of spontaneous perforation, barium enema examination is absolutely contraindicated during a recognized attack of toxic megacolon.
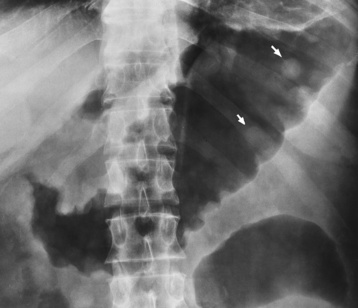
Figure 5-68 Toxic megacolon in ulcerative colitis. Note dilatation of the transverse colon with multiple pseudopolypoid projections extending into the lumen (arrows).
Ulcerative colitis has a strong tendency to begin in the rectosigmoid area. Although by radiographic criteria alone the rectum appears normal in about 20% of patients with ulcerative colitis, true rectal sparing is infrequent, and there is usually evidence of disease on sigmoidoscopy or rectal biopsy. Isolated right colon disease with a normal left colon does not occur, and ulcerative colitis may spread to involve the entire colon (pancolitis). The disease is almost always continuous, without evidence of the skip areas seen in Crohn’s disease. Except for “backwash ileitis” (minimal inflammatory changes involving a short segment of terminal ileum), ulcerative colitis does not involve the small bowel, a feature distinguishing it from Crohn’s disease, which may involve both the large and the small intestines.
On double-contrast studies, the earliest detectable radiographic abnormality in ulcerative colitis is fine granularity of the mucosa corresponding to the hyperemia and edema seen endoscopically (Figure 5-69). Once superficial ulcers develop, small flecks of adherent barium produce a stippled mucosal pattern. As the disease progresses, the ulcerations become deeper. Extension into the submucosa may produce broad-based ulcers with a collar-button appearance (Figure 5-70). Perirectal inflammation can cause widening of the soft tissue space between the anterior sacrum and the posterior rectum (retrorectal space).
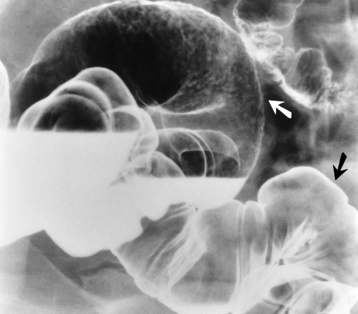
Figure 5-69 Ulcerative colitis involving primarily the rectosigmoid. Distal rectosigmoid mucosa (white arrow) is finely granular compared with the normal-appearing mucosa (black arrow) in the more proximal colon.
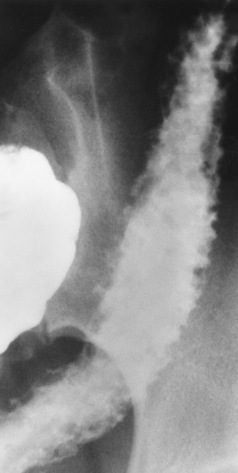
Figure 5-70 Ulcerative colitis. Progression of disease results in deep ulcerations (collar-button ulcers) extending into the submucosal layer.
When the disease is chronic, fibrosis and muscular spasm cause progressive shortening and rigidity of the colon (Figure 5-71). The haustral pattern is absent, and the bowel contour is relatively smooth because of ulcer healing and subsequent reepithelization. Eventually, the colon may appear as a symmetric, rigid, tubular structure (lead-pipe colon).
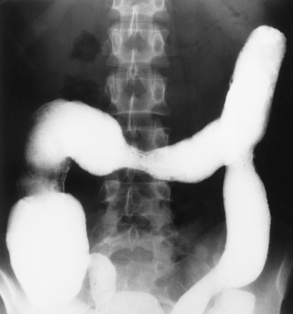
Figure 5-71 Chronic ulcerative colitis (lead-pipe colon). Muscular hypertrophy and spasm cause shortening and rigidity of the colon with loss of haustral markings.
Carcinoma of the colon is about 10 times more frequent in patients with ulcerative colitis than in the general population. During the first 10 years of disease, there is only a small risk of malignancy. Thereafter, it is estimated that there is a 20% chance per decade that carcinoma will develop in a patient with ulcerative colitis. Malignant lesions in patients with ulcerative colitis generally occur at a much younger age than they occur in the general population, and they tend to be extremely virulent. Carcinoma of the colon in patients with chronic ulcerative colitis often appears as a bizarre stricture rather than with the more characteristic polypoid or apple-core appearance of a primary colonic malignancy (Figure 5-72). The tumor typically produces a narrowed segment with an eccentric lumen, irregular contours, and margins that are rigid and tapered. Because it is frequently difficult to distinguish carcinoma from benign stricture in patients with ulcerative colitis, colonoscopy or surgery is often required for an unequivocal diagnosis.
Crohn’s Colitis
Crohn’s disease of the colon, the second major cause of inflammatory bowel disease, is identical to Crohn’s disease in the small bowel and must be distinguished from ulcerative colitis. The proximal portion of the colon is most frequently involved in Crohn’s disease; associated disease of the terminal ileum is seen in up to 80% of affected patients. Unlike in ulcerative colitis, in Crohn’s colitis the rectum is often spared, and isolated rectal disease very rarely occurs. Crohn’s disease usually has a patchy distribution, with involvement of multiple noncontiguous segments of colon (skip lesions), unlike the continuous colonic involvement in ulcerative colitis. Perirectal abnormalities (fissures, abscesses, fistulas) occur at some point during the course of disease in half of patients with Crohn’s colitis but are rare in those with ulcerative colitis. Crohn’s disease involves all layers of the gastrointestinal tract.
Radiographic Appearance
The earliest radiographic findings in Crohn’s disease of the colon are seen on double-contrast examinations. Isolated tiny, discrete erosions (aphthous ulcers) appear as punctate collections of barium with a thin halo of edema around them (Figure 5-73). Aphthous ulcers in Crohn’s disease have a patchy distribution against a background of normal mucosa, unlike the blanket of abnormal granular mucosa seen in ulcerative colitis.
As Crohn’s colitis progresses, the ulcers become deeper and more irregular, with a great variation in size, shape, and overall appearance. Deep linear, transverse, and longitudinal ulcers often separate intervening mounds of edematous but nonulcerating mucosa, producing a characteristic cobblestone appearance (see Figure 5-43). If the penetrating ulcers extend beyond the contour of the bowel, they can coalesce (grow together) to form long tracts running parallel to the long axis of the colon (Figure 5-74). The penetration of ulcers into adjacent loops of bowel or into the bladder, vagina, or abdominal wall causes fistulas, which can often be demonstrated radiographically. Inflammatory and fibrotic thickening of the bowel wall leads to narrowing of the lumen and stricture formation. Occasionally an eccentric stricture with a suggestion of overhanging edges can be difficult to distinguish from annular carcinoma (Figure 5-75). In most instances, however, characteristic features of Crohn’s disease elsewhere in the colon (deepulcerations, pseudopolyps, skip lesions, sinus tracts, fistulas) clearly indicate the correct diagnosis.
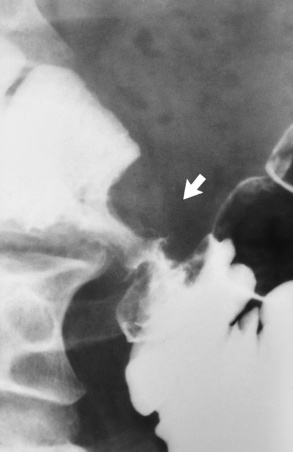
Figure 5-75 Chronic Crohn’s colitis. Benign stricture with overhanging edges in the transverse colon simulates carcinoma (arrow).
CT demonstrates the mesenteric and extraintestinal extent of the disease, and any abscess formation or colonic wall thickening. Magnetic resonance (MR) spectroscopy is currently under investigation as a way to help distinguish ulcerative colitis from Crohn’s colitis. Preliminary results indicate that this technique is able to differentiate between the two inflammatory bowel diseases in more than 90% of cases.
Patients with Crohn’s colitis appear to have a higher incidence of developing colon cancer than the general population, although this association is less striking than that between colon cancer and ulcerative colitis. Carcinoma complicating Crohn’s colitis is most common in the proximal portion of the colon and usually appears radiographically as a fungating mass with typical malignant features, unlike the mildly irregular stricture
characteristic of colon cancer in patients with ulcerative colitis. A small bowel examination may be necessary to demonstrate the terminal ileum.
Treatment of Ulcerative Colitis and Crohn’s Colitis
The initial treatments for ulcerative colitis and Crohn’s disease are identical, as both are inflammatory bowel diseases. First, nutritional supplements and dietary modifications (decrease bulk to reduce stool frequency) begin to diminish the inflammatory process. A regimen of antiinflammatory drugs interrupts the inflammatory cycle. Stress-related conditions require psychological support to control episodes. For ulcerative colitis, surgical resection may be necessary to prevent the spread of the condition. With proper treatment or spontaneous remission, many of the radiographic changes in this disease are reversible. In Crohn’s colitis, surgery is usually ineffective because the area adjacent to the anastomosis becomes involved as a result of the resection. For severe cases of the disorder, parenteral (IV) nutrition allows the intestine time to heal.
Ischemic Colitis
Ischemic colitis is characterized by the abrupt onset of lower abdominal pain and rectal bleeding. Diarrhea is common, as is abdominal tenderness on physical examination. Most patients are older than 50 years, and many have a history of prior cardiovascular disease.
Radiographic Appearance
The initial radiographic appearance of ischemic colitis is fine superficial ulceration caused by inflammatory edema of the mucosa. As the disease progresses, deep penetrating ulcers, pseudopolyps, and characteristic thumbprinting can be demonstrated. Thumbprinting refers to sharply defined, finger-like indentations along the margins of the colon wall (Figure 5-76). In most cases, the radiographic appearance of the colon returns to normal within 1 month if good collateral circulation is established. Extensive fibrosis during the healing phase can cause tubular narrowing and a smooth stricture. If blood flow is insufficient, acute bowel necrosis and perforation may result.
Treatment
In some cases, no intervention is needed, as the bowel heals itself. Patients taking vasoconstricting drugs are most susceptible, and when the diagnosis is ischemic colitis, these medications are discontinued and supportive care is given. Immediate surgical intervention is required for bowel infarctions and other complications.
Irritable Bowel Syndrome
Irritable bowel syndrome refers to several conditions that have an alteration in intestinal motility as the underlying pathophysiologic abnormality. Most consider these conditions to be functional disorders because there is a disruption of the food sequence breakdown in the stomach and intestines. Patients with irritable bowel syndrome may complain primarily of chronic abdominal pain and constipation (spastic colitis), chronic intermittent watery diarrhea, often without pain, or alternating bouts of constipation and diarrhea.
Radiographic Appearance
Although there are no specific radiographic findings in irritable bowel syndrome, patients with this condition usually undergo a barium enema examination to exclude another chronic disorder as the cause of the symptoms. When the patient is symptomatic, a barium enema may demonstrate areas of irritability and spasticity and accentuated haustration, although similar radiographic findings may be observed in normal asymptomatic persons, especially those who have received laxatives and enemas.
Treatment
No cure exists for irritable bowel syndrome. Because the precise cause of this functional disorder has not been determined, symptomatic relief is provided. It is necessary to identify trigger foods so that they can be avoided, thus decreasing the spasms and pain. Alternative therapies, relaxation, meditation, and physical exercise have value if the patient can use them to help ease some of the spasms and pain.
Cancer of the Colon
Carcinoma of the colon and rectum is the third leading cause of death from cancer in the United States. Even though colon and rectal cancer can be more easily diag nosed than most other malignant neoplasms it remains a leading cause of death. About half of colon carcinomas occur in the rectum and sigmoid, where they can be felt by rectal examination or seen with a sigmoidoscope. Carcinoma of the colon and rectum is primarily a disease of older persons, with a peak incidence in the 50- to 70-year range, and twice as common in men. Two diseases predispose to the development of cancer of the colon: long-standing ulcerative colitis and familial polyposis, a hereditary disease in which innumerable polyps develop in the colon and elsewhere in the intestinal tract.
Radiographic Appearance
Because cancer of the colon is curable if discovered early in its course, delay in diagnosis is the most significant factor in the poor prognosis. There is considerable evidence to indicate that many, if not most, carcinomas of the colon arise in preexisting polyps. Therefore, the early diagnosis of colonic cancer depends on polyp detection. Malignant polyps (Figure 5-77) tend to be sessile lesions (without stalks) with an irregular or lobulated surface, unlike benign polyps, which are usually smooth and often have a stalk (are pedunculated). Other radiographic criteria suggestive that a polyp is malignant include large size (especially if more than 2 cm in diameter), retraction or indentation (puckering) of the colon wall seen on profile view at the site of origin of a sessile polyp, and evidence of interval growth of a polyp on sequential examinations.
Annular carcinoma (described as apple-core or napkin-ring carcinoma) is one of the most typical forms of primary colonic malignancy (Figure 5-78). Annular carcinomas appear to arise from flat plaques of tumor (saddle lesions) that involve only a portion of the circumference of the colon wall. Unless meticulous care is taken to search for an area of minimal straightening or slight contour defects, the small and subtle, but lethal, saddle carcinoma can be easily overlooked. As the tumor grows, it characteristically infiltrates the bowel wall rather than forming a bulky intraluminal mass. This produces a classic bilateral contour defect with ulcerated mucosa, eccentric and irregular lumen, and overhanging margins. Progressive constriction of the bowel can cause complete colonic obstruction, most commonly in the sigmoid region.
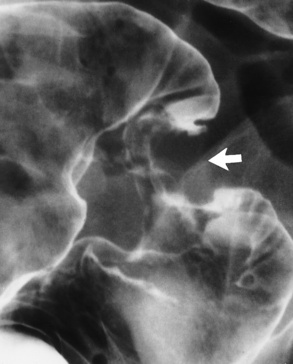
Figure 5-78 Annular carcinoma of the sigmoid colon. Note the sharply defined proximal and distal margins of this relatively short, apple-core lesion (arrow).
Ulceration is common in carcinoma of the colon. It can vary from an excavation within a large fungating mass to mucosal destruction within an annular apple-core tumor.
A patient with carcinoma of the colon has a 1% risk of having multiple synchronous (occurring simultaneously) colon cancers. Therefore, it is essential to carefully examine the rest of the colon once an obviously malignant lesion has been detected. In addition, such a patient has a 3% risk of developing additional metachronous cancers later.
CT virtual colonoscopy has become more frequently used as a screening tool for colon cancer. Today, two- or three-dimensional CT colonoscopy can demonstrate lesions of 8 to 10 mm with an accuracy similar to that obtained with traditional colonoscopy. CT virtual colonoscopy is an alternative for incomplete or equivocal results of traditional colonoscopy. CT is also the modality of choice for staging carcinoma of the colon and for assessing tumor recurrence (Figure 5-79). Carcinoma causes asymmetrical or circumferential thickening of the bowel wall with narrowing and deformity of the lumen. CT can demonstrate local extension of tumor to the pelvic organs as well as lymphadenopathy and metastases to the adrenal glands or liver (Figure 5-80).
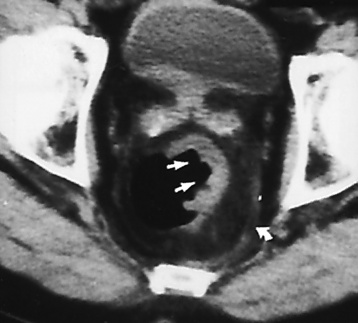
Figure 5-79 CT scan of a patient with rectal carcinoma shows a soft tissue mass on the lateral wall of the rectum containing a central ulceration (straight arrows). Thickening of the perirectal fascia (curved arrow), the presence of multiple lymph nodes (on the more cephalic images), and increased soft tissue density of perirectal fat were suggestive of tumor extension beyond the bowel wall, which was confirmed at surgery.
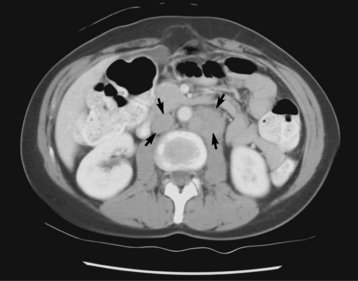
Figure 5-80 CT scan shows retroperitoneal and periaortic lymphadenopathy (arrows) in a 51-year-old woman with colon cancer.
Transrectal ultrasound produces the most accurate images for staging of local rectal cancer by demonstrating the depth of invasion within the bowel wall. Ultrasound also may determine the presence of a tumor in adjacent, normal-size lymph nodes.
Positron emission tomography (PET) with fluorodeoxyglucose F 18 (FDG-PET), although less specific than CT and ultrasound, has a higher accuracy for detecting distant nodular metastases. Fusion imaging, which superimposes the functional (PET) and anatomic (CT) studies, provides more specific detail to localize and evaluate the severity of the disease process.
Treatment
Surgical resection is required to remove a solitary carcinoma. Postoperative treatment may involve chemotherapy or radiation therapy. Following resection, FDG-PET in combination with a CT scan helps rule out recurrence. FDG-PET images can differentiate recurrence of the tumor from adhesions or scar tissue resulting from the resection. The presence of multiple lesions requires irradiation and chemotherapy.
Large Bowel Obstruction
About 70% of large bowel obstructions result from primary colonic carcinoma. Diverticulitis and volvulus account for most other cases. Colonic obstructions tend to be less acute than small bowel obstructions; the symptoms develop more slowly, and fewer fluid and electrolyte disturbances are produced.
Radiographic Appearance
The radiographic appearance of colonic obstruction depends on the competency of the ileocecal valve. If the ileocecal valve is competent, obstruction causes a large, dilated colon with a greatly distended, thin-walled cecum and little small bowel gas (Figure 5-81). The colon distal to the obstruction is usually collapsed and free of gas. If the ileocecal valve is incompetent, there is distention of gas-filled loops of both colon and small bowel, which may simulate an adynamic ileus.
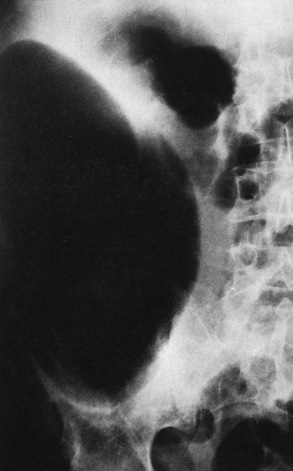
Figure 5-81 Large bowel obstruction. Huge dilatation of cecum (up to 13 cm in diameter) without perforation.
The major danger in colonic obstruction is perforation. If the ileocecal valve is competent, the colon behaves like a closed loop, and the increased pressure caused by the obstruction cannot be relieved. Because the cecum is spherical and has a large diameter, it is the most likely site for perforation. In acute colonic obstruction, perforation is very likely if the cecum distends to more than 10 cm; in intermittent or chronic obstruction, however, the cecal wall can become hypertrophied, and the diameter of the cecum can greatly exceed 10 cm without perforation (see Figure 5-81). In the patient with suspected large bowel obstruction, a low-pressure barium enema can be safely performed (Figure 5-82) and demonstrates the site and often the cause of the obstruction (Figure 5-83).
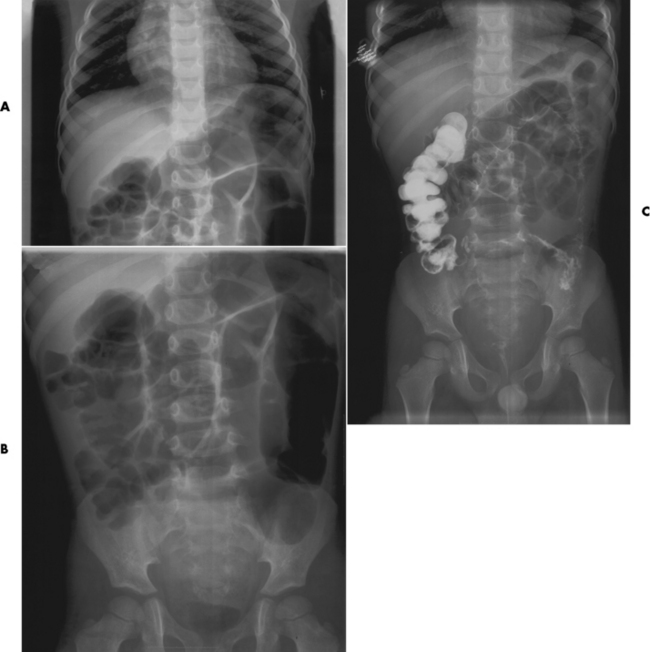
Figure 5-82 Upper (A) and lower (B) abdominal radiographs in a 4-year-old illustrate a gas pattern indicating a large bowel obstruction. A low-pressure barium enema was performed to relieve the obstruction, as seen on the post-evacuation view. C, As barium is seen in the cecum.
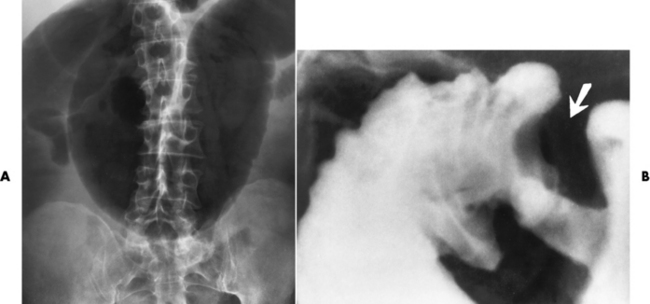
Figure 5-83 Large bowel obstruction caused by annular carcinoma of the sigmoid. A, Plain abdominal radiograph demonstrates pronounced dilatation of the gas-filled transverse and ascending colon. B, Barium enema demonstrates typical apple-core lesion (arrow) producing the colonic obstruction.
Some professionals recommend an abdominal/pelvic CT scan as an initial examination, especially for patients without a surgical history who have symptomatic signs suggesting infection, bowel infarction, or a palpable mass. CT can demonstrate causes of an obstruction, such as diverticulitis or appendicitis.
Volvulus of the Colon
Volvulus refers to a twisting of the bowel on itself that may lead to intestinal obstruction. Because twisting of the bowel usually requires a long, movable mesentery, volvulus of the large bowel most frequently involves the cecum and sigmoid colon. The transverse colon, which has a short mesentery, is rarely affected by volvulus. A sigmoid volvulus, more commonly found in the elderly, results from a low-fiber diet causing constipation.
Cecal Volvulus
The ascending colon and the cecum may have a long mesentery as a fault of rotation and fixation during the embryonic development of the gut. This situation predisposes to volvulus, with the cecum twisting on its long axis. It should be stressed, however, that cecal volvulus develops in only a few patients with an extremely mobile cecum.
Radiographic Appearance: In cecal volvulus, the distended cecum tends to be displaced upward and to the left, although it can be found anywhere within the abdomen. A pathognomonic sign of cecal volvulus is a kidney-shaped mass (representing the twisted cecum) with the twisted and thickening mesentery mimicking the renal pelvis (Figure 5-84A). A barium enema examination is usually required for definite confirmation of the diagnosis. This study demonstrates obstruction of the contrast column at the level of the stenosis, with the tapered edge of the column pointing toward the site of the twist (Figure 5-84B).
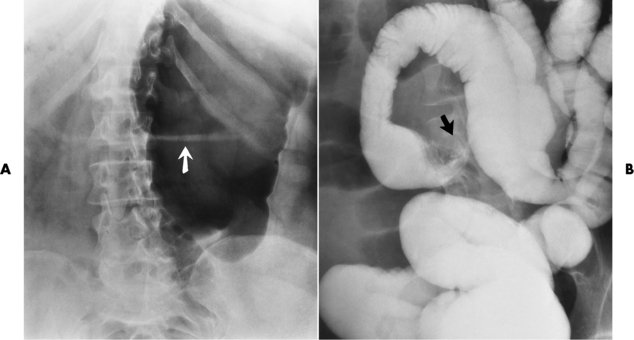
Figure 5-84 Cecal volvulus. A, The dilated, gas-filled cecum appears as a kidney-shaped mass with twisted and thickening mesentery (arrow) mimicking the renal pelvis. B, Barium enema examination demonstrates obstruction of the contrast column at the level of the stenosis (arrow); the tapered edge of the column points toward the torsion site.
Sigmoid Volvulus
A long, redundant loop of sigmoid colon can undergo a twist on its mesenteric axis and form a closed-loop obstruction. In sigmoid volvulus, the greatly inflated sigmoid loop appears as an inverted U-shaped shadow that rises out of the pelvis in a vertical or oblique direction and can even reach the level of the diaphragm. The affected loop appears devoid of haustral markings and has a sausage or balloon shape.
Radiographic Appearance: A barium enema examination demonstrates an obstruction to the flow of contrast material at the site of volvulus and considerable distention of the rectum. The lumen of the sigmoid tapers toward the site of stenosis, and a pathognomonic bird’s-beak appearance is produced (Figure 5-85).
Hemorrhoids
Hemorrhoids are varicose veins of the lower end of the rectum that cause pain, itching, and bleeding. Like varicose veins in the leg, hemorrhoids are caused by increased venous pressure. The most common cause of increased pressure is chronic constipation with resulting excessive muscular straining needed to empty the bowel. Increased venous pressure can also be produced by a pelvic tumor or a pregnant uterus.
Radiographic Appearance
On barium enema examinations, hemorrhoids occasionally can produce single or multiple rectal filling defects that simulate polyps (Figure 5-86). The proper diagnosis can easily be made by inspection, digital examination, or direct vision through the anoscope.
Treatment
Hemorrhoids may be treated nonsurgically and surgically. The nonsurgical approach consists of rubber band ligation. The surgical intervention consists of circumferential mucosectomy, which is a stapled hemorrhoidectomy, a safe and effective procedure for advanced stages (third- and fourth-degree hemorrhoidal prolapse).
Gallbladder
Gallstones consist of two major types: cholesterol stones and pigment stones. Cholesterol stones are predominant (75%) in the United States, whereas pigment stones occur more frequently in the tropics and Asian countries. Genetic predispositions associated with higher incidence include family history of the disorder, age more than 40 years, excess weight, and female sex. Gallstones can develop whenever bile contains insufficient bile salts and lecithin in proportion to cholesterol to maintain the cholesterol in solution. This situation can result from a decrease in the amount of bile salts present (because of decreased reabsorption in the terminal ileum as a result of inflammatory disease or surgical resection), or it can be caused by increased hepatic synthesis of cholesterol.
Radiographic Appearance
Because cholesterol is not radiopaque, most gallstones are radiolucent and visible only on contrast examinations or ultrasound. In up to 20% of patients, however, gallstones contain sufficient calcium to be detectable on plain abdominal radiographs (Figure 5-87). Gallstones can have a central nidus (or focus) of calcification, a laminated appearance (with alternating opaque and lucent rings), or calcification around the periphery. Occasionally, a nonopaque stone may contain gas-filled fissures that produce the Mercedes-Benz sign, a characteristic triradiate pattern similar to the German automobile trademark (Figure 5-88).
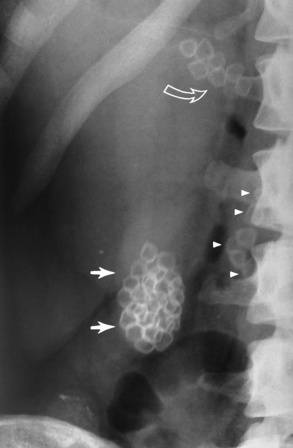
Figure 5-87 Calculi in the gallbladder (solid arrows) and bile ducts (open arrow). Calculi (arrowheads) lie in common bile duct, some of which overlie the spine and are difficult to detect.
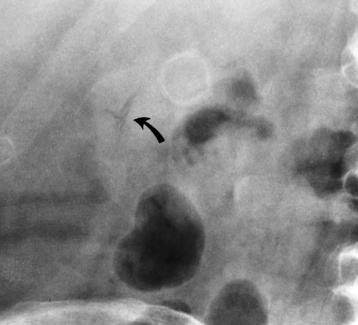
Figure 5-88 Mercedes-Benz sign of fissuring in a gallstone (arrow). Note the adjacent gallstone with a radiopaque rim.
Oral cholecystography (OCG) was the traditional technique for the diagnosis of gallstones. It is rarely used today because it has been replaced in most institutions by ultrasound (see later). Gallstones appear as freely movable filling defects in the opacified gallbladder. They fall by gravity to the dependent portion of the gallbladder and frequently layer out at a level that depends on the relationship of the specific gravity of the stone to that of the surrounding bile (Figure 5-89). Solitary gallstones are usually rounded; multiple stones are generally faceted. Large numbers of stones can have a sandlike or gravel-like consistency and be visible only when they layer out on radiographs obtained using a horizontal beam and with the patient in an erect or lateral decubitus position. Infrequently a gallstone is coated with tenacious mucus and adheres to the gallbladder wall.
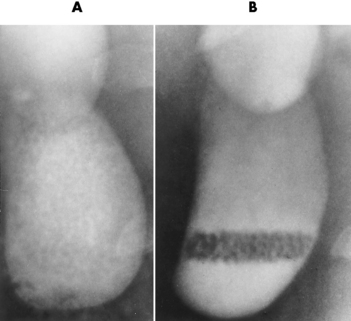
Figure 5-89 Oral cholecystogram showing multiple gallstones. A, With the patient supine, the stones are poorly defined and have a gravel-like consistency. B, On an erect image taken with a horizontal beam, the innumerable gallstones layer out and are easily seen.
Malabsorption of the radiopaque contrast material, hepatocellular dysfunction (serum bilirubin value ≥ 2 mg/dL), and intrinsic disease of the gallbladder (cystic duct obstruction, chronic cholecystitis) can lead to nonvisualization of the gallbladder on OCG. If these causes can be excluded, failure of the gallbladder to opacify after the administration of two doses of orally administered cholecystographic contrast material is highly reliable evidence of gallbladder disease.
Ultrasound is now the imaging modality of choice for demonstrating gallstones. This noninvasive technique is as accurate as OCG, is independent of hepatic function, and does not rely on patient compliance in taking oral contrast agents. In addition to imaging the gallbladder, ultrasound can provide important additional information by effectively demonstrating the biliary tree and hepatic parenchyma. Gallstones appear on ultrasound as foci of high-amplitude echoes associated with posterior acoustic shadowing (Figure 5-90). The mobility of free-floating gallstones may be demonstrated by performing the examination with the patient in various positions.
Acute Cholecystitis
Acute cholecystitis (inflammation of the gallbladder) usually (in 95% of cases) occurs after obstruction of the cystic duct by an impacted gallstone. Gallstones may injure the mucosal wall, allowing bacteria to enter.
Radiographic Appearance
Either ultrasound or radionuclide scanning can be used. The ultrasonographic diagnosis of acute cholecystitis requires the demonstration of a distended gallbladder containing gallstones. Important additional findings include edema of the gallbladder wall and focal tenderness elicited directly over the gallbladder. A normal gallbladder ultrasound image virtually excludes the diagnosis of acute cholecystitis. Because many disorders may mimic acute cholecystitis, ultrasound can also be used to evaluate the remainder of the right upper quadrant for any other acute abnormality.
When the results of a radionuclide cholescintigram are normal, they demonstrate the bile ducts, the gallbladder, and early excretion of radionuclide into the duodenum and proximal jejunum, within 30 minutes after the IV injection of the radionuclide (technetium Tc 99m) (Figure 5-91). Failure to accumulate radioactivity (after 4 hours) in the gallbladder is highly sensitive (98%) and specific for cystic duct obstruction. If associated with appropriate symptoms, this finding is virtually diagnostic of acute cholecystitis.
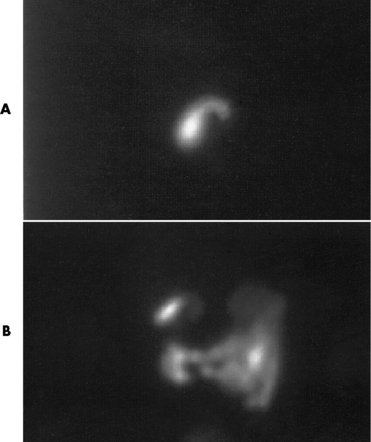
Figure 5-91 Acute cholecystitis. A, Radionuclide hepatobiliary study using a technetium Tc-99m disofenin in a 51-year-old woman with right upper quadrant pain demonstrates an engorged gallbladder at 1 hour after injection. B, At 28 minutes after injection of cholecystokinin (CCK), the gallbladder can be seen emptying into the small bowel with an ejection fraction of 83%, which is normal.
When ultrasound findings are inconclusive, MR cholangiopancreatography can demonstrate the cystic duct and any obstructing calculi located in the gallbladder neck. MR imaging (MRI) does not, however, evaluate the gallbladder wall thickness as well as ultrasound.
Emphysematous Cholecystitis
Emphysematous cholecystitis is a rare condition in which the growth of gas-forming organisms in the gallbladder is facilitated by stasis and ischemia caused by cystic duct obstruction (most often by stones). Emphysematous cholecystitis occurs most frequently in elderly men and in patients with poorly controlled diabetes mellitus.
Radiographic Appearance
Plain abdominal radiographs demonstrate gas in the gallbladder lumen that dissects into the wall or pericholecystic tissues. This produces the pathognomonic appearance of a rim of lucent bubbles or streaks of gas outside of and roughly parallel to the gallbladder lumen (Figure 5-92).
Treatment for Cholecystitis
No treatment is necessary for asymptomatic patients. For acute impaction and biliary colic, prompt treatment using an antispasmodic and an analgesic helps alleviate symptoms. Interventional treatments available today include extracorporeal shock wave therapy (lithotripsy), stone removal by endoscopic retrograde cholangiopancreatography (ERCP), and chemical dissolution. The most common surgical approach used currently is laparoscopic cholecystectomy. The radiographer may be requested to obtain images during operative cholangiography to determine ductal blockage and identify remaining stones. Imaging must be performed at the appropriate medium-to-low kVp (70 to 80) at the completion of contrast injection. The contrast agent should be free of air bubbles, which would otherwise simulate stones.
Porcelain Gallbladder
Porcelain gallbladder refers to extensive calcification in the wall of the gallbladder, which forms an oval density that corresponds to the size and shape of the organ (Figure 5-93). Because chronic cholecystitis produces a loss of wall function, the gallbladder becomes fibrotic and calcified. The term reflects the blue discoloration and brittle consistency of the gallbladder wall.
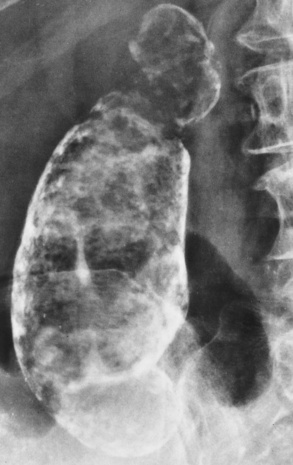
Figure 5-93 Porcelain gallbladder. Note the extensive mural calcification around the perimeter of the gallbladder.
Radiographic Appearance
The calcification in a porcelain gallbladder can appear as a broad continuous band in the muscular layers, or it may be multiple and punctate and occur in the glandular spaces of the mucosa.
The detection of extensive calcification in the wall of the gallbladder should indicate the possibility of carcinoma. Although a porcelain gallbladder is uncommon in cases of carcinoma of the gallbladder, there is a striking incidence of carcinoma in patients with porcelain gallbladder (up to 60% of the cases).
Liver
Hepatitis is the most prevalent inflammatory disease of the liver. The most common causes are viral infection and reactions to drugs and toxins. The viral types of hepatitis are hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), and hepatitis E virus (HEV).
Hepatitis A virus, previously known as infectious hepatitis, is transmitted in the digestive tract from oral or fecal contact. In most cases HAV is self-contained and has a favorable prognosis.
Hepatitis B virus, previously known as serum hepatitis, is contracted by exposure to contaminated blood or blood products, or through sexual contact. Healthcare workers are more susceptible to this virus and are usually required to have been vaccinated or to prove immunity. Ninety percent of patients with HBV recover without incident; however, because of the high incidence (200,000 to 300,000 new cases/year), the vaccine required as part of the childhood immunization process.
Hepatitis C virus, formally known as non-A, non-B hepatitis, is the common cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Because it is contracted by blood transfusion or sexual contact, some authorities believe that healthcare workers are more susceptible; however, in the general population, the source of 40% of cases is unknown. It is estimated that 50% of patients with HVC-related cirrhosis will develop cancer. In the United States and Western Europe, HCV cirrhosis is the principal cause of liver transplantation.
Hepatitis E virus is self-limited and is acquired by the ingestion of food or water that has been contaminated with fecal material.
Summary of Findings for the Gallbladder
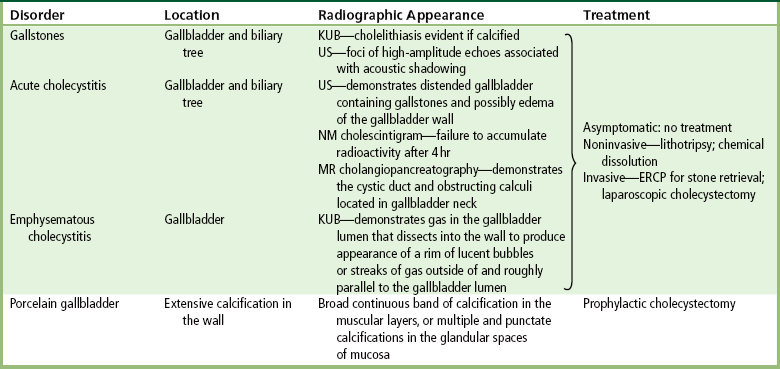
ERCP, endoscopic retrograde cholangiopancreatography; KUB, kidney-ureter-bladder radiograph; NM, nuclear medicine; US, ultrasound.
Radiographic Appearance
Viral hepatitis in the earliest stages is not visible on diagnostic images. As the disease progesses, complications of cirrhosis or hepatocellular carcinoma can be demonstrated on ultrasound, CT, and MRI. On ultrasound, cirrhosis due to viral hepatitis usually manifests as macronodules (Figure 5-94), which may vary in size from 3 mm to 5 cm. The liver has an internal coarse texture with increased echogenicity, and the surface may have a nodular appearance representing fibrosis (Figure 5-95). Portal hypertension, due to enlargement or blockage of the portal system, can also be detected. CT demonstrates hepatocellular necrosis with scattered inflammatory cells and evidence of a fatty liver. Normally the density of the liver is similar to that of the spleen. In fatty liver, infiltrates of fat appear as discrete or diffuse areas of low density within the liver. Contrast-enhanced CT in the portal venous phase is best for demonstrating a lobulated liver. Due to atrophy, the intrahepatic fissures appear more prominent. T1-weighted contrast MR images show the liver to have irregular diffuse enhancement with ill-defined margins; there may be rim enhancement in the venous phase. T2-weighted fat-suppressed MR images demonstrate a heterogeneous mass appearance with moderate elevation of the signal intensity. MRI enhancement with gadolinium is superior to that produced by iodinated contrast agents on CT for distinguishing abnormalities.
Cirrhosis of the Liver
Cirrhosis refers to the chronic destruction of liver cells and structure, with nodular regeneration of liver parenchyma and fibrosis; it is an end-stage liver disease. The major cause of cirrhosis is chronic alcoholism (i.e., 10 to 20 years of alcohol abuse), in which damage to the liver is related either to the toxic effect of alcohol or to the malnutrition that frequently accompanies chronic alcoholism. Other causes of liver destruction leading to cirrhosis are postnecrotic viral hepatitis, hepatotoxic drugs and chemicals that destroy liver cells, disease of the bile ducts (primary and secondary biliary cirrhosis), and excessive deposition of iron pigment within the liver (hemochromatosis).
Regardless of the cause, fibrous connective tissue replaces the destroyed liver cells with cells that have no liver cell function. Initially the liver enlarges because of regeneration, but it eventually becomes smaller as the fibrous connective tissue contracts, and the surface becomes bumpy and nodular.
Radiographic Appearance
Cirrhosis causes many physiologic changes that can be detected clinically or radiographically. In alcoholic cirrhosis, a large amount of fat accumulates within the liver. This fatty infiltration is beautifully demonstrated on CT. In normal individuals, the liver always appears brighter than the spleen, whereas in patients with cirrhosis the liver is much darker because of the large amount of fat (Figure 5-96). The portal veins appear as high-density structures surrounded by a background of low density caused by hepatic fat; this is the opposite of the normal pattern of portal veins, which are low-density channels on noncontrast scans. Ultrasound images of the liver demonstrate coarse echogenicity, which results from diffuse liver disease. Multiple hyperechoic micronodules (0.1 to 1 cm) or macronodules (varying up to 5 cm) cause a nodular surface pattern.
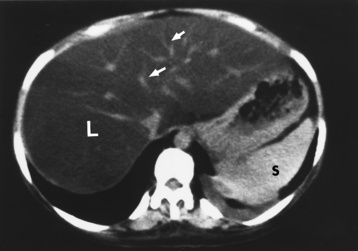
Figure 5-96 Fatty infiltration of the liver in cirrhosis. CT scan shows generalized decrease in the attenuation value of the liver (L), which is far less than that of the spleen (S). Portal veins appear as high-density structures (arrows) surrounded by a background of low-density hepatic fat.
Nodular regeneration of the liver combined with fibrosis causes obstruction of the portal vein, which drains blood from the gastrointestinal tract through the liver before emptying into the inferior vena cava near the heart. Increased pressure within this vessel causes pronounced enlargement of the spleen (splenomegaly). Because blood cannot flow through the obstructed portal vein, it must find an alternative route to bypass the liver. This leads to the development of collateral circulation, with large dilated veins becoming prominent on the abdominal wall in the area of the umbilicus. Another alternative route for blood to take in its return to the heart is the periesophageal veins, which dilate to become esophageal varices, which then can rupture and result in fatal hemorrhage (see Figure 5-21). Destruction of liver cells substantially decreases the ability of the organ to synthesize proteins, such as albumin, and several of the factors required for blood clotting. A deficiency of albumin (hypoalbuminemia) results in fluid leaking out of the circulation and the development of generalized edema, which is evidenced by swelling of the lower extremities. When edema involves the wall of the intestinal tract, it can produce regular, uniform thickening of small bowel folds.
One of the most characteristic symptoms of cirrhosis is the accumulation of fluid in the peritoneal cavity (ascites), which causes characteristic abdominal distention. The abdomen is tight and quite hard, and an increase in exposure factors is required if a large amount of fluid has accumulated. Ascites develops because of a combination of albumin deficiency and increased pressure within obstructed veins, which permits fluid to leak into the abdominal cavity. Large amounts of ascitic fluid are easily detectable on plain abdominal radiographs as a general abdominal haziness (ground-glass appearance) (Figure 5-97A). With the patient in a supine position, the peritoneal fluid tends to gravitate to dependent portions of the pelvis and accumulate within the pelvic peritoneal reflections, thus filling the recesses on both sides of the bladder and producing a symmetrical density resembling a dog’s ears. Smaller amounts of fluid (300 to 1000 mL) may widen the flank stripe and obliterate the right lateral inferior margin of the liver (the hepatic angle). Ascites is exquisitely shown on ultrasound as a mobile, echo-free fluid region shaped by adjacent structures (Figure 5-97 B) or on CT as an extravisceral collection of fluid with a low attenuation value (Figure 5-97C).
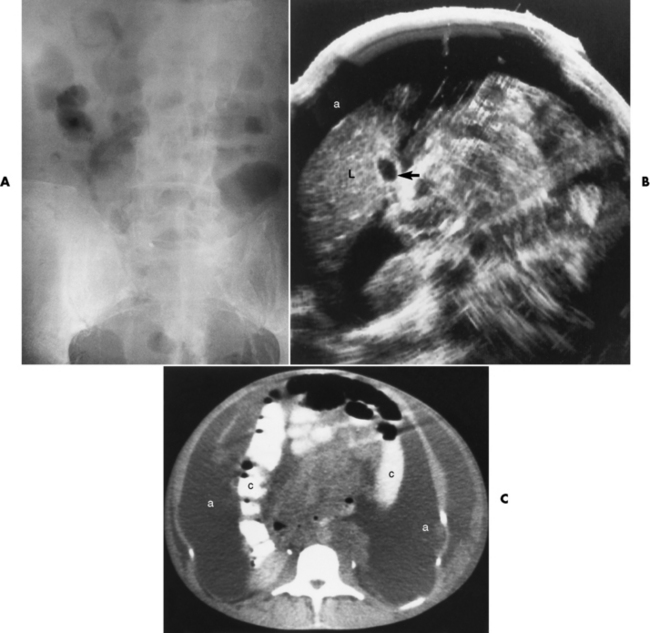
Figure 5-97 Ascites. A, Plain radiograph shows general abdominal haziness (ground-glass appearance). B, On an ultrasound image, a large amount of sonolucent ascitic fluid (a) separates the liver (L) and other soft tissue structures from the anterior abdominal wall. Note the relative thickness of the gallbladder wall (arrow). C, CT scan through the lower abdomen shows a huge amount of low-density ascitic fluid (a) with medial displacement of ascending and descending colon (c).
Cirrhosis may lead to the development of jaundice, either from destruction of liver cells or from obstruction of bile ducts. Because the liver cannot perform its usual task of inactivating the small amounts of female sex hormones secreted by the adrenal glands in both men and women, men with cirrhosis often have breast enlargement (gynecomastia). The inability of necrotic liver cells to detoxify harmful substances leads to an accumulation of ammonia and other poisonous material in the circulation. The patient becomes confused and disoriented, demonstrates a typical flapping tremor or shaking, becomes abnormally sleepy (exhibits somnolence), and may lapse into a potentially fatal hepatic coma. Ascites occurs in advance stages of cirrhosis; of those afflicted, only 20% survive 5 years.
Treatment
The damage caused by cirrhosis is irreversible and incurable except by liver transplantation. Most patients without a liver transplant die within 15 years of diagnosis. The patient may control the process by choosing a nutritional diet that decreases the metabolic load on the liver, stopping alcohol consumption, resting, and managing the complications of liver failure.
Hepatocellular Carcinoma
In the United States, primary liver cell carcinoma most commonly occurs in patients with underlying diffuse hepatocellular disease, especially alcoholic or postnecrotic cirrhosis. The clinical presentation varies from mild right upper quadrant discomfort and weight loss to hemorrhagic shock from massive intraperitoneal bleeding, which reflects rupture of the tumor into the peritoneal cavity. Invasion of the biliary tree may produce obstructive jaundice.
Radiographic Appearance
CT is the modality of choice in the diagnosis of hepatocellular carcinoma. The tumor appears as a large mass, with an attenuation value close to that of normal parenchyma, that tends to alter the contour of the liver by projecting beyond its outer margin (Figure 5-98). After the rapid administration of IV contrast material, there is usually dense, diffuse, and nonuniform enhancement of the tumor. Unlike metastases, hepatocellular carcinoma tends to be a solitary mass or to produce a small number of lesions (thus appearing multinodular). Hepatocellular carcinoma tends to invade the hepatic and portal venous systems, and tumor thrombi within these veins are well demonstrated on CT. Currently, a three-phase contrast helical CT study is performed to image the arterial phase, the portal circulation, and the contrast-staining tumor in the venous or excretory phase (Figure 5-99).
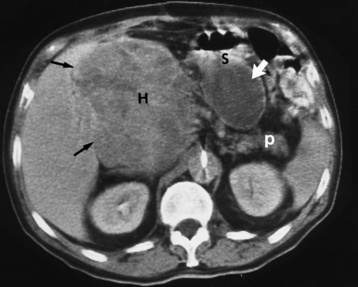
Figure 5-98 Hepatocellular carcinoma. CT scan shows huge mass (H) with an attenuation value slightly less than that of normal liver. Black arrows point to the interface between the tumor and normal liver. Incidentally shown is a pancreatic pseudocyst (white arrow) in the lesser sac between the stomach (S) and pancreas (p).
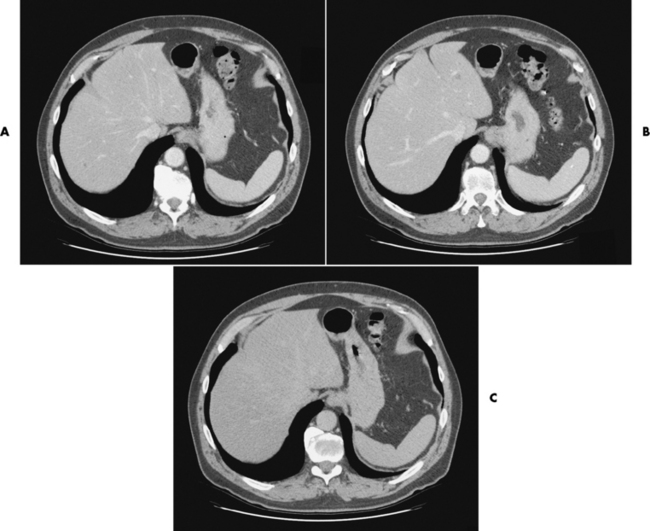
Figure 5-99 Three-phase CT scanning of the normal liver. A, Hepatic arteries. The bolus of contrast material is in the abdominal aorta. B, The inferior vena cava and the portal system are visible. C, The liver parenchyma is homogeneous, indicating normal study.
Ultrasonography is used to screen chronic hepatitis B virus carriers. With the use of microbubble contrast agents, Doppler ultrasound imaging demonstrates lesion vascularity through the use of phasing techniques similar to those used in CT. MRI may permit a specific diagnosis by demonstrating (during the arterial phase, after gadolinium injection) a characteristic capsule of compressed liver tissue, an accumulation of fat within the tumor, and the propensity of the tumor to spread into the hepatic and portal venous system.
Treatment
Although the overall prognosis for patients with hepatocellular carcinoma remains bleak, radiographic studies can determine whether the tumor can be successfully removed surgically. If the tumor is confined to one lobe of the liver and there is no evidence of metastases, arteriography is usually indicated to demonstrate the precise surgical anatomy and to detect small, staining tumor nodules that cannot be identified with noninvasive imaging techniques. Surgery and chemotherapy are the treatments of choice. Metastases occur late and are usually not the cause of death. A common cause of death is catastrophic bleeding into the peritoneal cavity from hepatic failure or esophageal varices.
Hepatic Metastases
Metastases are by far the most common malignant tumors involving the liver. Although some types of metastases (especially mucinous carcinoma of the colon or rectum) may produce diffuse, finely granular calcifications that can be seen on plain radiographs, the diagnosis of hepatic metastases usually requires CT, ultrasound, MRI, or radionuclide studies.
Radiographic Appearance
CT and MRI are probably the most sensitive techniques for detecting hepatic metastases. On CT, most metastases are relatively well marginated and appear less dense than normal liver parenchyma (Figure 5-100A). Although frequently detectable on noncontrast scans, most metastatic lesions are best seen as areas of increased density adjacent to normally enhancing hepatic parenchyma after the administration of IV contrast material. CT-guided fine-needle aspiration biopsy can be used to obtain cells that can be studied for a definitive diagnosis. MRI demonstrates liver metastases as areas of low signal intensity on T1-weighted sequences and bright lesions on T2-weighted sequences. This modality is especially important for patients who cannot receive IV iodinated contrast agents.
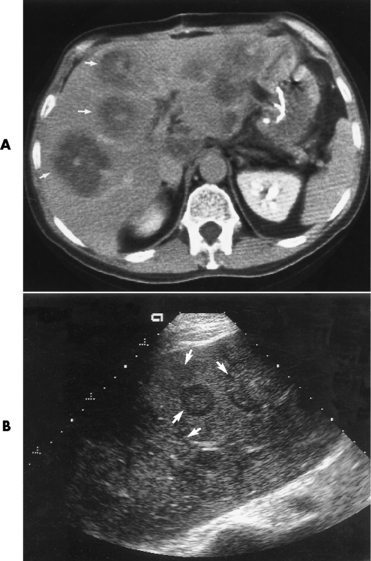
Figure 5-100 Hepatic metastases. A, CT scan shows multiple low-density metastases with high-density centers (arrows). B, Ultrasound image of the liver demonstrates a diffuse, patchy, nodular appearance (arrows).
Ultrasound (Figure 5-100B) and radionuclide scans can demonstrate hepatic metastases, but these modalities are slightly less sensitive than CT. CT has the additional advantage of being able to detect extrahepatic metastases, such as those to abdominal lymph nodes.
Pancreas
Acute pancreatitis is an inflammatory process in which protein- and lipid-digesting enzymes become activated within the pancreas and begin to digest the organ itself. Occasionally, this necrotic process extends into blood vessels, causing bleeding (acute hemorrhagic pancreatitis), which may be life threatening.
The most common cause of acute pancreatitis is excessive alcohol consumption. Less frequently, acute pancreatitis is related to gallstones, which may enter the common bile duct and obstruct the ampulla of Vater, forcing bile to reflux into the pancreas and causing an inflammatory reaction.
The first symptom of acute pancreatitis usually is the sudden onset of severe, steady abdominal pain that radiates to the back; this may indicate a perforated ulcer. Nausea and vomiting are common, and jaundice may develop if inflammatory edema of the head of the pancreas sufficiently obstructs the common bile duct. If a large area of the pancreas is affected, the absence of lipid enzymes from the pancreas prevents the proper absorption of fat, leading to the malabsorption syndrome. Blood tests and urinalysis typically show a high level of the pancreatic enzyme amylase, which confirms the diagnosis of acute pancreatitis.
Radiographic Appearance
The findings on plain abdominal radiographs are often normal in the patient with acute pancreatitis; even when abnormal, they are usually nonspecific and consistent with any intraabdominal inflammatory disease. The most common abnormalities include a localized adynamic ileus, usually involving the jejunum (the “sentinel loop”); generalized ileus with diffuse gas-fluid levels; isolated distention of the duodenal sweep (C-loop); and localized distention of the transverse colon to the level of the left colonic flexure (the colon cutoff sign). Pancreatic calcifications indicate that the patient has chronic pancreatitis, and moreover they may indicate an exacerbation of the inflammatory disease.
Ultrasound and CT are the imaging modalities that most precisely define the degree of pancreatic inflammation and the pathways of its spread throughout the abdomen. They are also of great clinical importance in the early diagnosis of complications of acute pancreatitis, such as abscess, hemorrhage, and pseudocyst formation.
CT in acute pancreatitis demonstrates diffuse or focal enlargement of the gland. The margins of a normal pancreas are sharply delineated by surrounding peripancreatic fat. Spread of inflammation and edema beyond the confines of the pancreas obscures the peripancreatic soft tissues and often thickens the surrounding fascial planes (Figure 5-100), making CT superior to ultrasound.
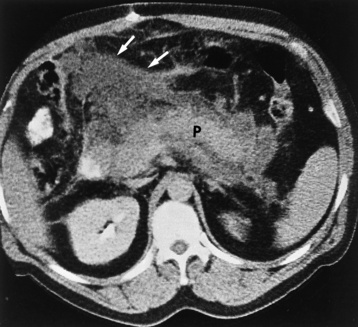
Figure 5-101 Acute pancreatitis. CT scan demonstrates diffuse enlargement of the pancreas (P) with obliteration of peripancreatic fat planes by an inflammatory process. Extension of the inflammatory reaction into the transverse mesocolon (arrows) is shown.
Acute pancreatitis may alter both the size and the parenchymal echogenicity of the gland on ultrasound examination. Although the pancreas usually enlarges symmetrically and retains its initial shape, nonspecific enlargement of the pancreatic head or tail can simulate focal pancreatic carcinoma. Gallstones causing pancreatitis can be demonstrated. The accompanying interstitial inflammatory edema causes the pancreas to appear relatively sonolucent in comparison with the adjacent liver (Figure 5-102). One limitation of ultrasound in patients with acute pancreatitis is the frequent occurrence of adynamic ileus with excessive intestinal gas, which may prevent adequate visualization of the gland.
Chronic Pancreatitis
Chronic pancreatitis results when frequent intermittent injury to the pancreas causes increasing damage that produces scar tissue. Recurring episodes usually result from chronic alcohol abuse, which may cause the gland to lose its ability to produce digestive enzymes, insulin, and glucagon. Three symptoms that help identify chronic pancreatitis are pain, malabsorption causing weight loss (exocrine failure–digestive enzymes), and diabetes (endocrine failure–insulin and glucagon).
Radiographic Appearance
Pancreatic calcifications are a pathognomonic finding in chronic pancreatitis, developing in about one third of patients with this disease (Figure 5-103). The small, irregular calcifications are seen most frequently in the head of the pancreas and can extend upward and to the left to involve the body and tail of the organ.
On ultrasound examination, the major feature of chronic pancreatitis is an alteration of the intrinsic echo pattern caused by calcification and fibrosis (Figure 5-104). The pancreas may be atrophic as a result of fibrous scarring or may appear significantly enlarged during recurrences of acute inflammation. Dilatation of the pancreatic duct as a result of gland atrophy and obstruction can be seen, although a similar pattern can be produced by the ductal obstruction in pancreatic cancer. CT can also demonstrate ductal dilatation, calcification, and atrophy of the gland in patients with chronic pancreatitis. However, because similar information can be obtained less expensively and without ionizing radiation with the use of ultrasound, CT is usually reserved for patients with chronic pancreatitis in whom technical factors make ultrasound suboptimal.
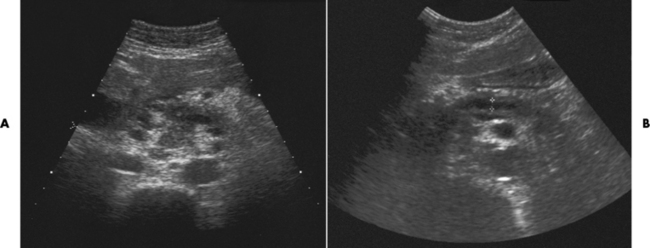
Figure 5-104 Chronic pancreatitis in a 40-year-old man with abdominal pain. He has a history of chronic alcoholism. A, An ultrasound image obtained in April shows the pancreas to be inhomogeneous with a patchy appearance. B, On the follow-up scan obtained in May, the pancreatic ducts are considerably dilated (cursors).
Enlargement of the pancreatic head can cause widening and pressure changes on the inner aspect of the duodenal sweep on barium studies. These changes produce narrowing of the lumen (the double-contour effect) and spiny protrusions of mucosal folds (spiculation) that can be indistinguishable from those of pancreatic carcinoma.
Treatment of Acute and Chronic Pancreatitis
Most cases of acute pancreatitis require supportive treatment only (e.g., IV fluids, pain and nausea medications) because the pancreas will heal itself. If acute pancreatitis is caused by stone blockage, procedures to remove the stone (e.g., ERCP) or possibly surgery to remove the gallbladder should be performed. In some cases, IV antibiotics are given to help reduce the inflammatory process and prevent infection.
In chronic pancreatitis, treatment is directed toward controlling pain and managing nutritional and metabolic problems. Consumption of alcohol must cease. Dietary changes are made to reduce fat and protein intake; supplements containing pancreatic enzymes are taken to aid in digestion. If the blood sugar becomes uncontrolled, insulin is prescribed. Most patients who follow their prescribed treatment do well.
Pancreatic Pseudocyst
Pancreatic pseudocysts are loculated (walled-off) fluid collections arising from inflammation, necrosis, or hemorrhage associated with acute pancreatitis or trauma (Figure 5-105). When the infected or traumatized pancreas continues to release enzymes, pseudocysts are commonly formed. The pseudocyst has a shaggy lining surrounded by dense white scar tissue and may or may not connect with the pancreatic duct.
Radiographic Appearance
On ultrasound examination, a pseudocyst typically appears as an echo-free cystic structure with a sharp posterior wall (Figure 5-106). Hemorrhage into the pseudocyst produces a complex fluid collection containing septations of echogenic areas. CT demonstrates pseudocysts as sharply marginated, fluid-filled collections that are often best delineated after the administration of IV contrast material (Figure 5-107).
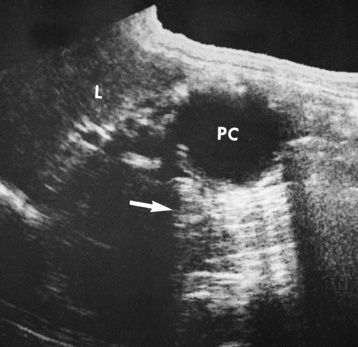
Figure 5-106 Ultrasound image of pancreatic pseudocyst. Longitudinal sonogram of the right upper quadrant demonstrates irregularly marginated pseudocyst (PC) with acoustic shadowing (arrow). L, Liver.
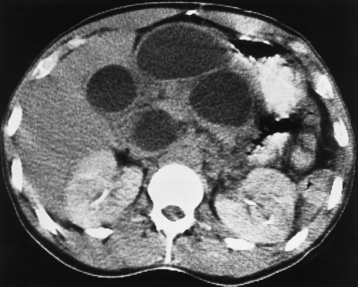
Figure 5-107 Multiple pancreatic pseudocysts. CT scan after IV administration of contrast material demonstrates four sharply marginated, fluid-filled collections.
Large pseudocysts are visible on plain radiographs of the abdomen when they displace the gas-filled stomach and bowel. Similarly, pseudocysts in the head of the pancreas can cause pressure defects and widening of the duodenal sweep, whereas those arising from the body or tail of the pancreas can displace and deform the stomach, proximal jejunum, or colon. However, because ultrasound is highly accurate in diagnosing pancreatic pseudocysts, it has completely replaced plain abdominal radiographs and barium studies, which display only indirect signs.
Treatment
Pseudocysts may undergo spontaneous resolution or persist as chronic collections that may require surgical intervention. Because ultrasound is relatively inexpensive and does not involve ionizing radiation, serial sonograms are usually used to monitor the progression of a pancreatic pseudocyst. Percutaneous drainage of a pseudocyst and endoscopic drainage of cysts extending into the stomach are interventional therapies used today.
Cancer of the Pancreas
The most common pancreatic malignancy is adenocarcinoma (90%), which often is far advanced and has metastasized before it is detected and thus has an extremely poor survival rate. Of these malignancies, 60% occur in the head of the pancreas. Less common pancreatic tumors are hormone-secreting neoplasms of the islet cells of the islets of Langerhans. Production of insulin by an insulinoma can lower the blood glucose level, leading to attacks of weakness, unconsciousness, and insulin shock. This tumor occurs most frequently in the tail and is usually benign. Ulcerogenic islet cell tumors (gastrinomas—usually malignant) produce the Zollinger-Ellison syndrome, which is characterized by intractable ulcer symptoms, hypersecretion of gastric acid, and diarrhea. Diarrheogenic islet cell tumors produce the WDHA syndrome—watery diarrhea, hypokalemia (low serum potassium), and achlorhydria (low serum chloride), which are major features of the clinical picture. Adrenocorticotropic hormone (ACTH) production by pancreatic islet cell tumors causes Cushing’s syndrome; the release of serotonin by pancreatic tumors may cause the carcinoid syndrome. Because these functional islet cell tumors usually become apparent through their hormonal effects rather than from the consequences of tumor bulk, they are often small and difficult to detect.
Radiographic Appearance
Because it is noninvasive and relatively inexpensive, ultrasound is often the initial screening modality for a patient with suspected pancreatic carcinoma. Ultrasound can demonstrate most tumors more than 2 cm in diameter that lie in the head of the pancreas, but lesions in the body and tail of the pancreas are more difficult to detect. Pancreatic carcinoma typically causes the gland to have an irregular contour and a semisolid pattern of intrinsic echoes (Figure 5-108).
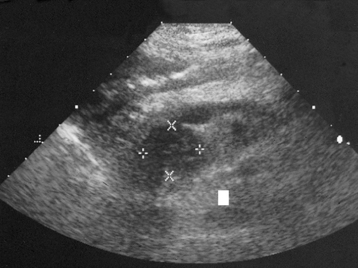
Figure 5-108 Pancreatic adenocarcinoma in a 66-year-old man with a 10-day history of painless jaundice and weight loss. Ultrasound image demonstrates a heterogeneous hypoechoic mass (dark area with measurement cursors) in the pancreatic head and uncinate process; the mass is obstructing the pancreatic and common bile ducts.
CT is the most effective modality for detecting pancreatic cancer in any portion of the gland and for defining its extent. CT can demonstrate the mass of the tumor, ductal dilatation, and invasion of neighboring structures. After the administration of IV contrast material, the relatively avascular tumor appears as an area of decreased attenuation in comparison with the normal pancreas (Figure 5-109). CT is the best procedure for staging pancreatic carcinoma and may prevent needless surgery in patients with nonresectable lesions. This technique may permit detection of hepatic metastases or involvement of regional vessels and adjacent retroperitoneal lymph nodes.
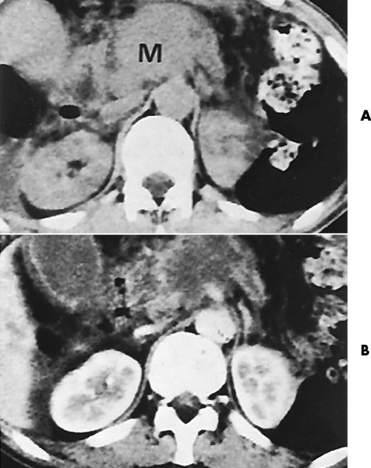
Figure 5-109 Carcinoma of the pancreas. A, Noncontrast CT scan demonstrates a homogeneous mass (M) in the body of the pancreas. B, After IV injection of a bolus of contrast material, there is enhancement of the normal pancreatic parenchyma and the surrounding vascular structures. The pancreatic carcinoma remains unchanged and thus appears as a low-density mass.
Cytologic examination of tissue obtained by percutaneous fine-needle aspiration under ultrasound or CT guidance can often provide the precise histologic diagnosis of a neoplastic mass, thus making surgical intervention unnecessary.
Carcinoma of the head of the pancreas often causes obstructive jaundice and the appearance of narrowing of the distal common bile duct on transhepatic cholangiography (Figure 5-110) or ERCP. Percutaneous biliary drainage may represent an alternative to surgical intervention for relieving biliary obstruction in patients with pancreatic carcinoma who cannot be cured. Although the transhepatic insertion of biliary drainage tubes does not alter the dismal prognosis, it can reduce patient morbidity and the need for hospitalization.
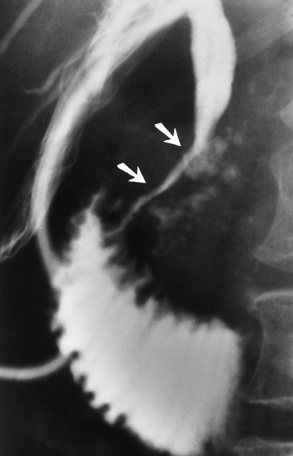
Figure 5-110 Carcinoma of the head of the pancreas. A transhepatic cholangiogram shows irregular narrowing of the common bile duct (arrows). Calcifications reflect the underlying chronic pancreatitis.
A barium upper GI series demonstrates distortion of the mucosal pattern and configuration of the duodenal sweep in about half of patients with carcinoma of the head of the pancreas. Early or small lesions, however, rarely produce detectable radiographic abnormalities; tumors of the body or tail of the pancreas must be quite large to be visible on barium examinations.
Treatment
Finding pancreatic cancer in the earliest stages can result in a cure (the 5-year survival rate is 2%). In most cases, the diagnosis of the disease occurs after metastasis, and most patients die within 12 to 24 months of diagnosis. Nevertheless, treatment can improve the quality of life. Surgical resection, radiation therapy, chemotherapy, and biologic therapy help alleviate the patient’s symptoms.
Surgical resection (total pancreatectomy) involves the removal of the entire pancreas, duodenum, common bile duct, gallbladder, spleen, and surrounding lymph nodes. The Whipple procedure consists of removing only the head of the pancreas, the duodenum, a portion of the stomach, and other nearby tissue. If a distal pancreatectomy is performed, only the body and tail of the pancreas are removed. In most cases, the purpose of the surgery is to alleviate biliary and small bowel obstruction. Surgery and chemotherapy are most effective for islet cell cancers.
Pneumoperitoneum
Free air in the peritoneal cavity associated with significant abdominal pain and tenderness is often caused by perforation of a gas-containing viscus and indicates a surgical emergency. Less frequently, pneumoperitoneum results from abdominal, gynecologic, intrathoracic, or iatrogenic causes and does not require operative intervention.
Radiographic Appearance
The radiographic demonstration of free air in the peritoneal cavity is a valuable sign in the diagnosis of perforation of the gastrointestinal tract. As little as 1 mL of free intraperitoneal gas can be identified. Free air is best demonstrated by examination of the patient in the upright position with a horizontal beam (Figure 5-111). Because the gas rises to the highest point in the peritoneal cavity, it accumulates beneath the domes of the diaphragm. Free intraperitoneal gas appears as a sickle-shaped lucency that is easiest to recognize on the right side between the diaphragm and the homogeneous density of the liver. On the left side, the normal gas and fluid shadows present in the fundus of the stomach can be confusing. The free air is shown to best advantage if the patient remains in an upright (or lateral decubitus) position for 10 minutes before a radiograph is obtained.
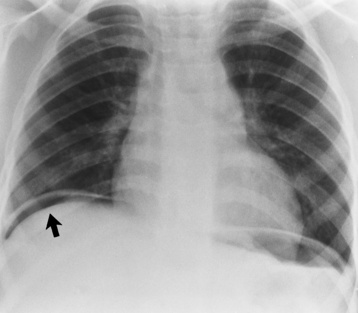
Figure 5-111 Pneumoperitoneum. Gas accumulating beneath the dome of the right hemidiaphragm (arrow) appears as a sickle-shaped lucency on this erect chest radiograph obtained with a horizontal beam.
If the patient is too ill to sit or stand, a lateral decubitus view (preferably with the patient on the left side) can be used. In this position, free air moves to the right side and collects between the lateral margin of the liver and the abdominal wall (Figure 5-112). Some gas also collects in the right iliac fossa and, when large amounts are involved, can be seen along the flank down to the pelvis.
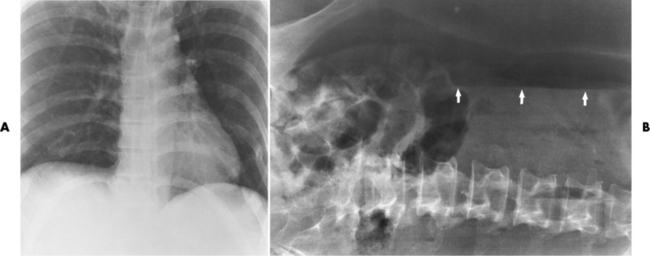
Figure 5-112 Pneumoperitoneum. A, Semierect projection obtained without a horizontal beam shows no evidence of free intraperitoneal gas beneath the domes of the diaphragm. B, On a lateral decubitus projection obtained with a horizontal beam in the same patient, free intraperitoneal gas is clearly shown collecting under the right side of the abdominal wall (arrows). Gas can even be seen extending down the flank to the region of the pelvis.
When the patient is in the supine position, free intraperitoneal gas accumulates between the intestinal loops and is much more difficult to demonstrate. However, a large quantity of gas can be diagnosed indirectly because the outer margins of the intestinal wall can be visualized (Figure 5-113). The demonstration of distinct inner and outer contours of the bowel wall is often the only sign of pneumoperitoneum in patients who are in such poor condition that they cannot be turned on their side or be examined while upright.
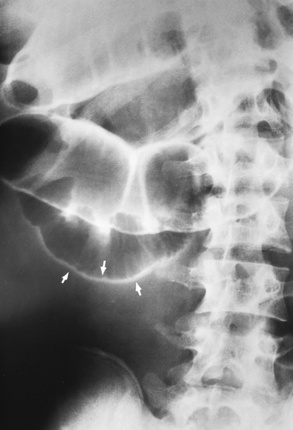
Figure 5-113 Double-wall sign of pneumoperitoneum demonstrated on supine projection. Large quantities of free intraperitoneal gas may be diagnosed indirectly because gas permits visualization of both inner and outer margins of the intestinal wall (arrows).
In children, pneumoperitoneum can manifest as a generalized greater-than-normal lucency of the entire abdomen. An important sign of pneumoperitoneum on the supine radiograph is demonstration of the falciform ligament. This almost vertical, curvilinear, water-density shadow in the upper abdomen to the right of the spine is outlined only when there is gas on both sides of it, as in pneumoperitoneum (Figure 5-114).
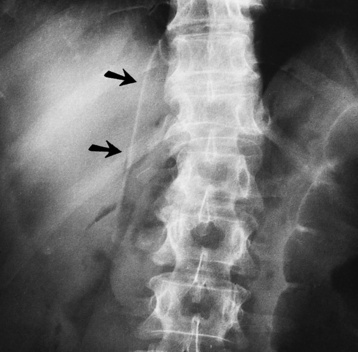
Figure 5-114 Falciform ligament sign of pneumoperitoneum. On a supine projection, the falciform ligament appears as a curvilinear water-density shadow (arrows) in the upper abdomen to the right of the spine. This finding implies that pneumoperitoneum with gas is on both sides of the ligament.
The most common cause of pneumoperitoneum with associated inflammation is perforation of a peptic ulcer, either gastric or duodenal. Colonic perforations, especially those involving the cecum, give the most abundant quantities of free intraperitoneal gas. Septic infection of the peritoneal cavity by gas-forming organisms can result in the production of a substantial amount of gas and the radiographic appearance of pneumoperitoneum. Pneumoperitoneum can also develop after penetrating injuries of the abdominal wall and after blunt trauma causing rupture of a hollow viscus.
Iatrogenic pneumoperitoneum is generally asymptomatic and usually follows abdominal surgery. Postoperative pneumoperitoneum can be radiographically detectable for up to 3 weeks after surgery, but usually it can no longer be demonstrated after the first postoperative week. Rarely, free air in the peritoneal cavity may be the result of perforation during an endoscopic procedure.
Spleen
Enlargement of the spleen (splenomegaly) is associated with numerous conditions, including infections (subacute bacterial endocarditis, tuberculosis, infectious mononucleosis, malaria), connective tissue disorders, neoplastic hematologic disorders (lymphoma, leukemia), hemolytic anemia and hemoglobinopathies, and portal hypertension (cirrhosis).
Radiographic Appearance
Plain abdominal radiographs can demonstrate the inferior border of the enlarged spleen well below the costal margin. An enlarged spleen can elevate the left hemidiaphragm, impress the greater curvature of the barium-filled stomach, and displace the entire stomach toward the midline. Splenomegaly can also cause downward displacement of the left kidney and the splenic flexure of the colon.
CT is of value when it is unclear whether a mass palpated in the left upper quadrant represents an enlarged spleen or a separate abdominal mass. When splenomegaly is present (Figure 5-115), CT findings may indicate the cause of the splenic enlargement by demonstrating a tumor (Figure 5-116), abscess, or cyst. Associated abdominal lymph node enlargement is suggestive of lymphoma; characteristic alterations in the size and shape of the liver and prominence of collateral venous structures in the splenic hilum indicate that splenomegaly may be a result of cirrhosis and portal hypertension.
Rupture
Rupture of the spleen is usually caused by trauma. Infrequently, it may be a complication of the palpation of a spleen enlarged by infection (especially infectious mononucleosis) or leukemia. In many patients suffering traumatic rupture of the spleen, the severity of clinical symptoms and the rapid loss of blood into the abdominal cavity require immediate surgery without radiographic investigation. However, in patients in whom bleeding stops temporarily or in whom there is slow bleeding over several days, radiographic studies may be of diagnostic value.
Radiographic Appearance
CT is the best imaging procedure for screening patients with blunt abdominal trauma for the presence of splenic injury. Indeed, its almost 100% sensitivity in detecting splenic injury has substantially decreased the need for abdominal arteriography and exploratory surgery. Subcapsular hematomas appear on CT as crescentic collections of fluid that flatten or indent the lateral margin of the spleen (Figure 5-117). Splenic lacerations, which may occur with or without an accompanying subcapsular hematoma, produce a CT appearance of splenic enlargement, an irregular cleft or defect in the splenic border, and free blood in the peritoneal cavity.
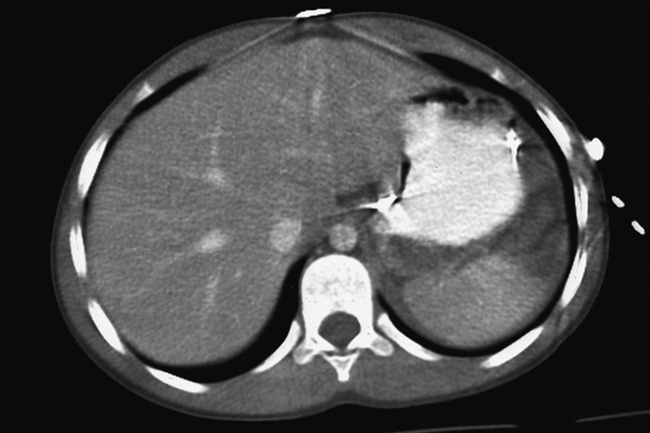
Figure 5-117 CT scan in a patient who was ejected from a motor vehicle. Traumatic splenic rupture and hematoma are illustrated by the decreased attenuation surrounding the spleen.
Before the advent of CT, splenic arteriography was the procedure of choice for demonstrating splenic rupture. Major positive findings include extravasation of contrast material into the splenic parenchyma, obstruction of a major splenic artery, and—in an enlarged spleen—vascular defects that indicate sites of rupture and hematoma.
Treatment of Splenic Disorders
Treatment depends on the causative factors. A very enlarged spleen has a higher incidence of rupture and thus the patient should limit physical activity to avoid trauma. In most cases, the patient with splenomegaly does well with conservative treatment. Aggressive treatment would include possible embolization of the splenic artery to stop the bleeding in a splenic rupture. Surgical procedures include laparoscopic splenectomy and distal pancreatectomy, if necessary.