Skeletal System
Physiology of the Skeletal System
Congenital/Hereditary Diseases of Bone
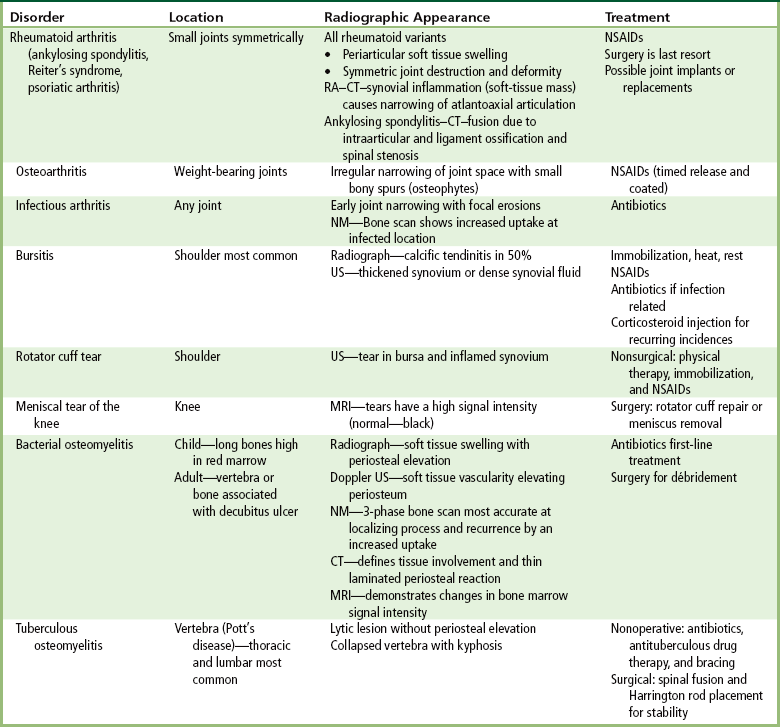
Inflammatory and Infectious Disorders
Rheumatoid Variants: Ankylosing Spondylitis, Reiter’s Syndrome, and Psoriatic Arthritis
Osteoarthritis (Degenerative Joint Disease)
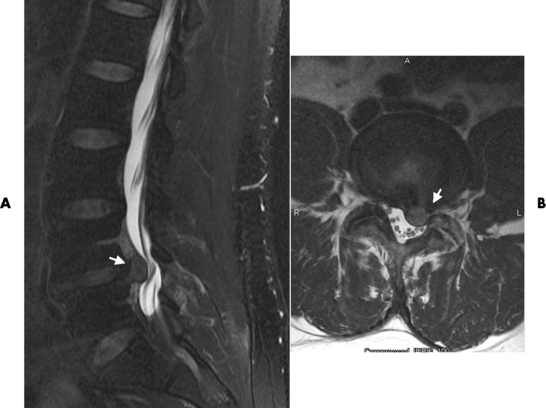
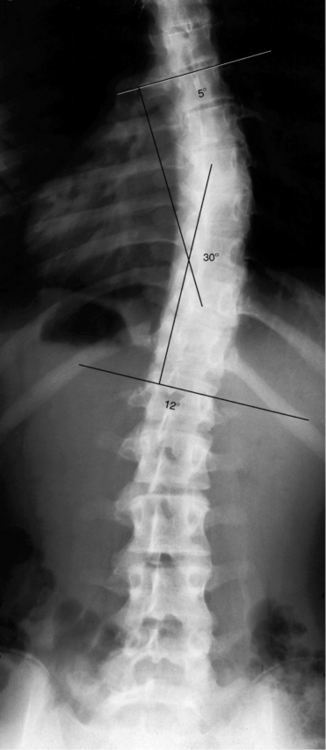
Fractures and Dislocations of the Spine
After reading this chapter, the reader will be able to:
1 Classify the more common diseases in terms of their attenuation of x-rays
2 Explain the changes in technical factors required for obtaining optimal-quality images in patients’ with various underlying pathologic conditions
3 Define and describe all bold-faced terms in this chapter
4 Describe the physiology of the skeletal system
5 Identify anatomic structures on both diagrams and radiographs of the skeletal system
6 Differentiate the various pathologic conditions affecting the skeletal system and their radiographic manifestations
Physiology of the skeletal system
The skeletal system is composed primarily of two highly specialized connective tissues: bone and cartilage. Bone consists of an organic matrix in which inorganic salts (primarily calcium and phosphate) are deposited. A fibrous membrane termed the periosteum covers the outer surfaces of bone, except at joint surfaces, where articular cartilage covers the bone and acts as a protective cushion. The periosteum contains a network of blood vessels from which nutrient arteries penetrate into the underlying bone. The main shaftlike portion is termed the diaphysis, and the ends of the bone are called epiphyses. The hollow, tubelike structure within the diaphysis, known as the medullary cavity, or marrow, is lined by an inner membrane termed the endosteum (Figure 4-1A).
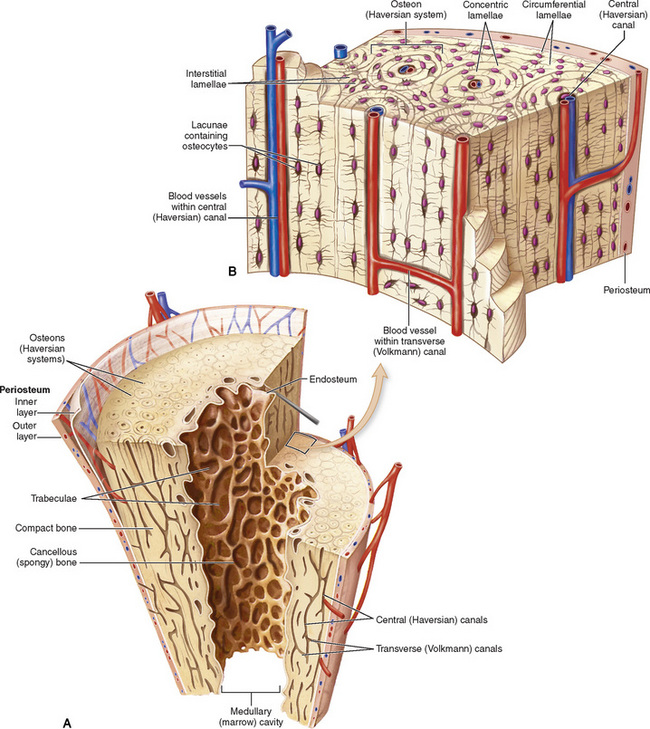
Figure 4-1 Long bone. A, Partial frontal section of a long bone (tibia) showing cancellous and compact bone. B, Frontal section of a long bone.
There are two major types of bone. The outer layer consists of compact bone, which to the naked eye appears dense and structureless. Under the microscope, the matrix of compact bone consists of complex structural units called “haversian systems.” Cancellous (spongy) bone is composed of a weblike arrangement of marrow-filled spaces separated by thin processes of bone, called trabeculae, that are visible to the naked eye (Figure 4-1B). The relative amount of each type of bone depends on the strength required and thus varies from bone to bone and in different portions of the same bone. For example, the shafts of long bones, such as the femur and tibia, have a thick outer layer of compact bone, whereas the layer of compact bone is relatively thin in irregular bones, such as vertebral bodies and facial bones, and in short bones, such as the carpal and tarsal bones.
Most bones form from models composed of hyaline cartilage (enchondral ossification). In a typical long bone, a primary ossification center appears in the center of the cartilage precursor in about the eighth week of intrauterine life, and bone formation extends so that the entire shaft is usually ossified before birth. Just before or after birth, secondary ossification centers appear in the epiphyses, the ends of developing long bones. Until the linear growth of bone is complete, the epiphysis remains separated from the diaphysis by a cartilaginous plate called the epiphyseal cartilage. The epiphyseal cartilage persists until the growth of the bone is complete. At that time, the epiphyseal plate ossifies, and the epiphysis and diaphysis fuse (at various ages depending on the specific bones). Where the diaphysis meets the epiphyseal growth plate is a slight flaring, known as the metaphysis.
The increase in length of a developing long bone occurs by growth of the epiphyseal cartilage followed by ossification. Bones grow in diameter by the combined action of two special types of cells called osteoblasts and osteoclasts. Osteoclasts enlarge the diameter of the medullary cavity by removing bone from the diaphysis walls. At the same time, osteoblasts from the periosteum produce new bone around the outer circumference. Osteoblasts and osteoclasts thus continuously resorb old bone and produce new bone. This constant process of remodeling occurs until the bone assumes its adult size and shape.
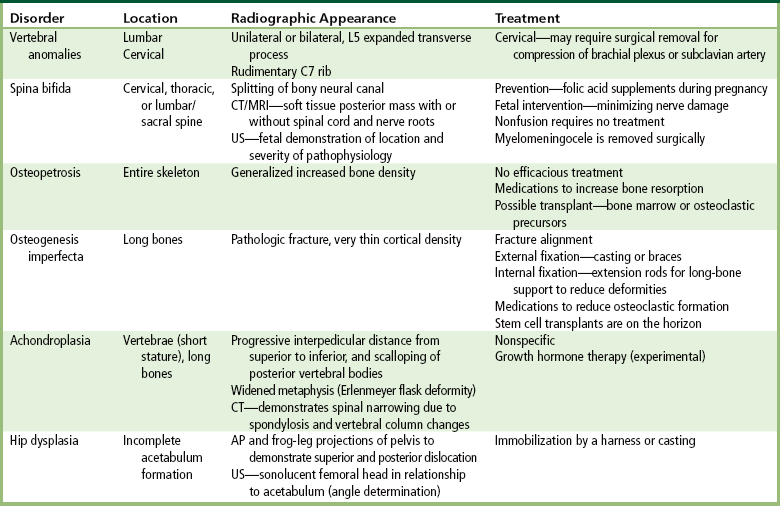
The radiographic determination of bone age is useful for evaluating physiologic age and growth potential, and for predicting adult stature. The best known and most widely accepted method of determining skeletal bone age (skeletal maturation) is that of Greulich and Pyle in their radiographic atlas compiled from thousands of examinations of American children at different ages. This atlas contains standard radiographs for age and sex that permit an assessment of bone age based on the presence or absence of ossification centers and their configuration, and the fusion of epiphyses in various portions of the hand and wrist.
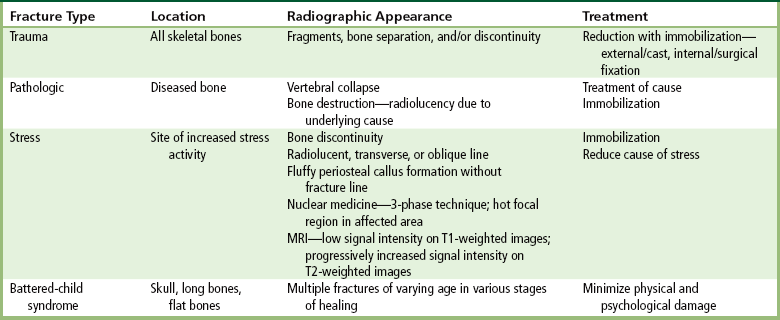
Throughout life, bone formation (ossification) and bone destruction (resorption) continue to occur. They are in balance during the early and middle years of adulthood. After about age 40 years, however, bone loss at the inner or endosteal surface exceeds bone gain at the outer margins. Thus in long bones, the thickness of compact bone in the diaphyses decreases, and the diameter of the medullary cavity increases. The bone eventually resembles a hollow shell and is less able to resist compressive and bending forces. This process may lead to collapse and loss of height of vertebral bodies as well as to fractures of long bones after relatively mild injury.
Bones can also develop within a connective tissue membrane (intramembranous ossification). The clavicles and flat bones of the skull have no cartilaginous stage and begin to take shape when groups of primitive cells differentiate into osteoblasts, which secrete matrix material and collagenous fibrils. Deposition of complex calcium salts in the organic bone matrix produces rodlike trabeculae that join in a network of interconnecting spicules to form spongy or cancellous bone. Eventually, plates of compact or dense bone cover the core layer of spongy bone. Flat bones grow in size by the addition of osseous tissue to their outer surfaces (appositional growth). They cannot grow by expansion, as enchondral bone does. Bones perform five basic functions (Box 4-1).
Congenital/hereditary diseases of bone
A transitional vertebra is one that has characteristics of vertebrae on both sides of a major division of the spine.
Radiographic Appearance
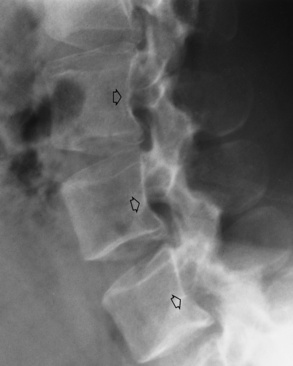
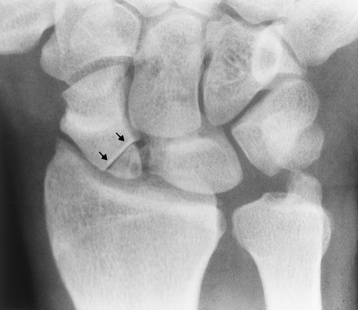
Transitional vertebrae most frequently occur at the lumbosacral junction and contain expanded transverse processes, which may form actual unilateral or bilateral joints with the sacrum (Figure 4-2). When unilateral, this process often leads to degenerative change involving the opposite hip and the intervertebral disk space above it. At the thoracolumbar junction, the first lumbar vertebra may have rudimentary ribs articulating with the transverse processes. The seventh cervical vertebra may also have a rudimentary rib (Figure 4-3).
Treatment
Most vertebral anomalies remain unnoticed and are incidental findings on radiographs. A cervical rib may compress the brachial nerve plexus (causing pain or numbness in the upper extremity) or the subclavian artery (decreasing blood flow to the arm) and therefore require surgical removal.
Spina Bifida
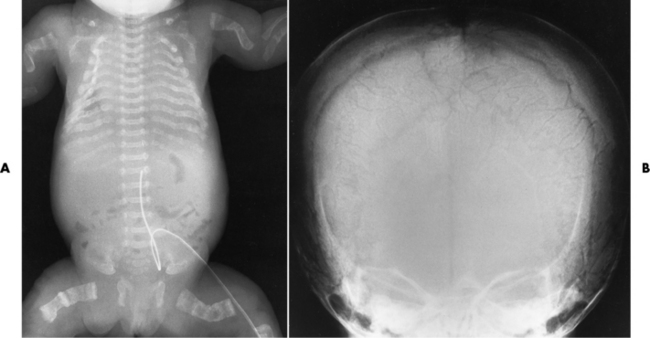
Spina bifida refers to a posterior defect of the spinal canal, resulting from failure of the posterior elements to fuse properly. A mild, insignificant form is spina bifida occulta, in which there is a splitting of the bony neural canal at the L5 or S1 level (Figure 4-4). Large defects are associated with spinal cord abnormalities and may lead to a variety of muscular abnormalities and lack of bladder or bowel control. In many cases a slight dimpling of the skin or a tuft of hair over the vertebral defect indicates the site of the lesion.
Large defects in the lumbar or cervical spine may be accompanied by herniation of the meninges (meningocele), or of the meninges and a portion of the spinal cord or nerve roots (myelomeningocele). A patient with a meningocele may be asymptomatic. Other malformations associated with a meningocele are clubfoot, gait disturbances, and bladder incontinence. The myelomeningocele has associated neurologic deficits at and below the site of protrusion. Almost all patients with myelomeningocele have the Chiari II malformation, with caudal displacement of posterior fossa structures into the cervical canal. Hydrocephalus is a frequent complication.
Radiographic Appearance
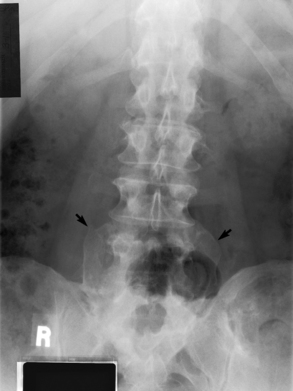
Spina bifida lesions are associated with large bony defects, absence of the laminae, and increased interpedicular distance (Figure 4-5). The herniated spinal contents are seen as a soft tissue mass posterior to the spine. Myelography, computed tomography (CT), or magnetic resonance imaging (MRI) can demonstrate the presence of spinal cord or nerve roots within the herniated sac. Prenatal real-time ultrasound can now demonstrate this serious malformation in utero on sagittal spine fetal images that determine the location and severity of the deformity.
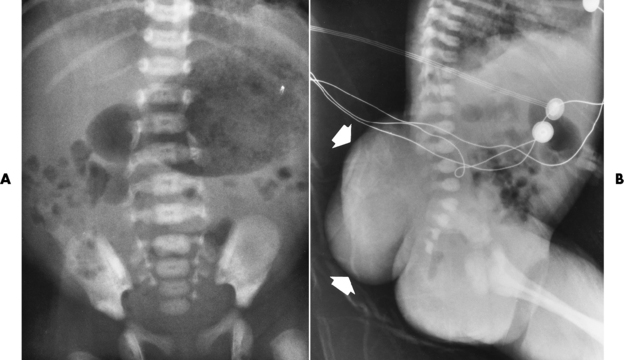
Figure 4-5 Meningomyelocele. A, Frontal projection of the abdomen shows greatly increased interpedicular distance of the lumbar vertebrae. B, In another patient, lateral projection demonstrates a large soft tissue mass (arrows) situated posterior to the spine. Notice the absence of the posterior elements in the lower lumbar and sacral regions.
Treatment
Prenatal intervention involves the daily supplement of folic acid, which has reduced the number of incidences of spina bifida. The ability to diagnose the fetus allows for fetal intervention, experimental surgery that helps minimize nerve deficits. Spina bifida occulta and meningoceles usually require no treatment. Sometimes a meningocele requires surgical repair, depending on the size and location of the protrusion. A myelomeningocele generally requires surgical repair. When this repair is being completed, a shunt is placed to prevent hydrocephalus.
Osteopetrosis
Osteopetrosis (marble bones) is a rare hereditary bone dysplasia in which failure of the resorptive mechanism of calcified cartilage interferes with the normal replacement by mature bone. It prevents the bone marrow from forming, so that the bones become very brittle and stress fractures occur often. The patient may also become anemic as a result of the lack of blood-producing bone marrow. Osteopetrosis varies in severity and age at clinical presentation, from a fulminant, often fatal condition involving the entire skeleton at birth or in utero to an essentially asymptomatic form that is an incidental radiographic finding.
Radiographic Appearance
Osteopetrosis results in a symmetric, generalized increase in bone density (Figure 4-6). To produce a diagnostic image, the radiographer must increase the exposure factors (milliampere seconds [mAs] and kilovolts peak [kVp]) to compensate for the increase in bone formation (increased attenuation factor). The image may appear blurred because of the structural changes; in some cases, a good image may be difficult to produce.
Treatment
Currently, no effective treatment exists for osteopetrosis. Medications designed to increase bone resorption and blood cell production may help control the disease. For the most severe cases bone marrow transplantation is the only way to improve the prognosis of this pathologic disorder. For less severe cases, bone marrow transplants increase the production of blood cells to manage the anemia. Transplantation of osteoclastic precursors may help control the balance of bone formation and resorption.
Osteogenesis Imperfecta
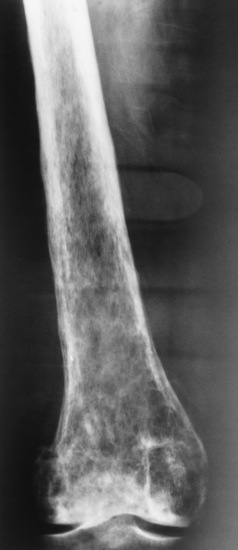
Osteogenesis imperfecta (brittle bones) is an inherited generalized disorder of connective tissue characterized by multiple fractures and an unusual blue color of the normally white sclera of the eye. Due to imperfectly formed or inadequate bone collagen, the adult patient with osteogenesis imperfecta is generally wheelchair bound because the skeletal structure does not support the body weight (Figure 4-7).
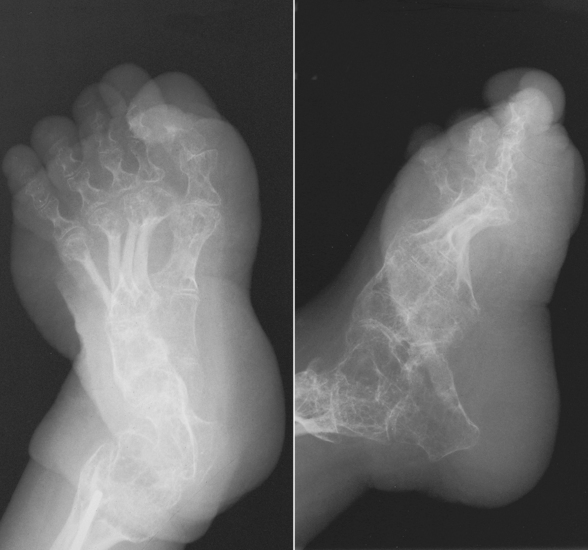
Figure 4-7 Osteogenesis imperfecta of the adult foot. Demineralization and the lack of bony cortices are demonstrated on anteroposterior and lateral projections.
Radiographic Appearance
Patients with this condition suffer repeated fractures caused by the severe osteoporosis and the thin, defective cortices (Figure 4-8A). The fractures often heal with exuberant callus formation (often so extensive as to simulate a malignant tumor), sometimes causing bizarre deformities. Because of the severe cortical bone loss in advanced stages of disease, producing a good radiographic image may require lowering the kilovoltage to compensate for the loss of bone quality. Ossification of the skull progresses slowly, leaving wide sutures and multiple juxtasutural accessory bones within a suture (wormian bones) that produce a mosaic appearance (Figure 4-8B). “Child abuse” may be confused with osteogenesis imperfecta because of the presentation of multiple fractures in different stages of the healing process.
Treatment
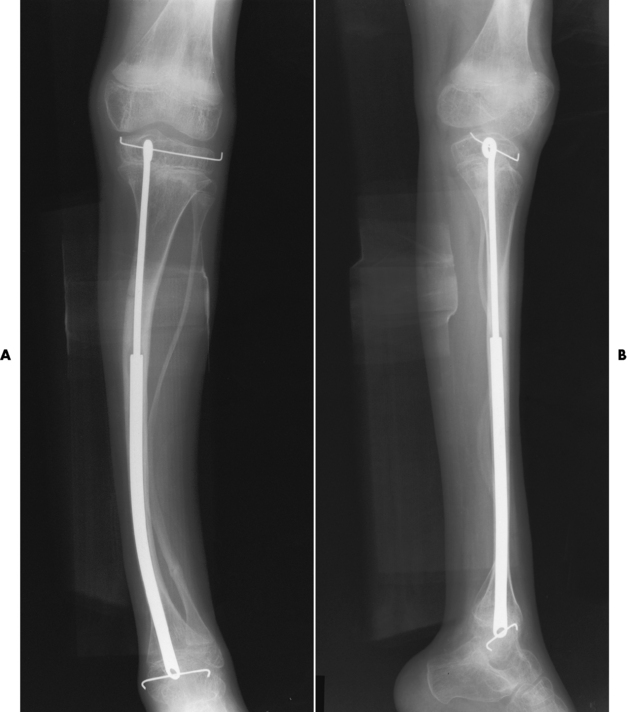
The aim of treatment is to reduce fractures by using proper safety measures. Because of defective cortices in severe cases, the long bones become less supportive. Therefore in some instances, extendable rods are surgically placed to provide more support, help prevent new fractures from occurring, and prevent long bone bowing (Figure 4-9). Currently, drugs are prescribed to regulate the osteoclastic formation, thus keeping the bone density more normal. As research continues, stem cell transplants may become a viable cure.
Achondroplasia
Achondroplasia is the most common form of dwarfism; it results from diminished proliferation of cartilage in the growth plate (decreased enchondral bone formation). This autosomal dominant condition does not affect membranous bone formation. Therefore the individual has short limbs, which contrast with the nearly normal length of the trunk (axial skeleton). Other characteristic physical features are a large head with frontal bulging, saddle nose, a prognathous (jutting) jaw, and prominent buttocks that give the false impression of lumbar lordosis.
Radiographic Appearance
Typical radiographic findings include progressive narrowing of the interpedicular distances from above downward, which is the opposite of normal, and scalloping of the posterior margins of the lumbar vertebral bodies (Figure 4-10). The decreased enchondral bone formation may make the long bones appear short and thick with a widened metaphysis (Erlenmeyer flask deformity) (Figure 4-11). CT is beneficial in demonstrating the degree of spinal narrowing caused by spondylosis and the changes in the vertebral column.
Treatment
Achondroplasia has no cure, and treatments address complications or deformities associated with the disease process. Patients afflicted with it live normal independent and productive lives. With today’s technology, the long bones can be surgically lengthened. Growth hormone treatment for achondroplasia is still considered experimental; however, preliminary reports indicate positive results in increasing bone growth.
Congenital Hip Dysplasia (Dislocation)
Congenital hip dysplasia, also known as developmental hip dysplasia, results from incomplete acetabulum formation caused by physiologic and mechanical factors. Physiologically, the fetus is exposed to increased hormone levels during delivery. Mechanically, as the fetus grows and occupies more space, the amount of amniotic fluid decreases, placing gentle pressure on the infant. Hip dysplasia is more common in females. During pediatric assessment of the hip, when the leg is flexed and abducted, the hip may “pop” out of joint and a “click” is felt or heard. The tendons and ligaments responsible for proper femoral head alignment are affected.
Radiographic Appearance
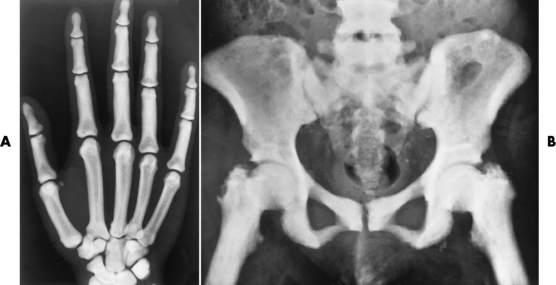
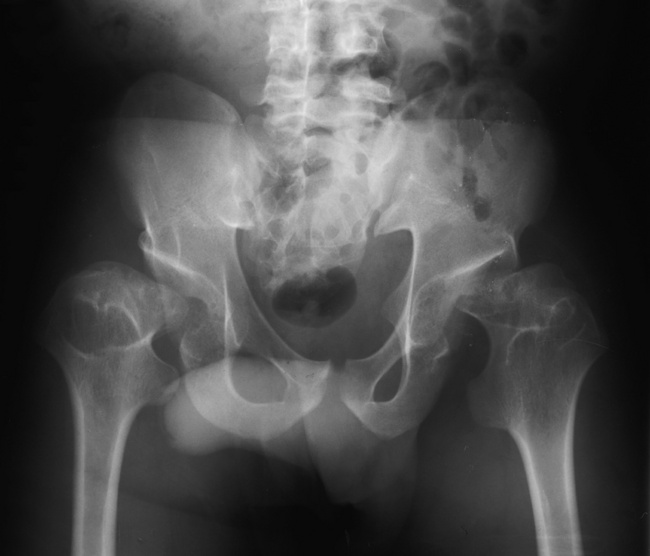
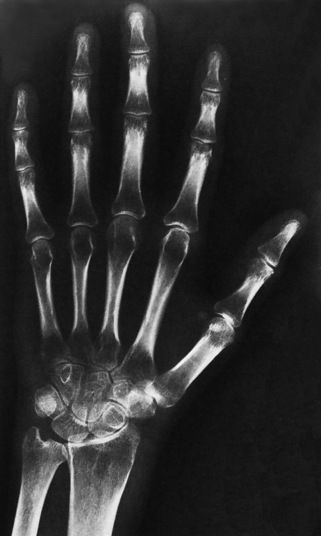
Anteroposterior (AP) pelvis and bilateral frog-leg (Cleaves) views are required to make a diagnosis (Figure 4-12). In many cases the AP image appears almost normal with only a slightly larger joint space. On the bilateral frog-leg view, the hip is usually dislocated superiorly and posteriorly. Because children with this disorder will undergo a multitude of follow-up images to recheck development, it is very important to shield the gonadal anatomy.
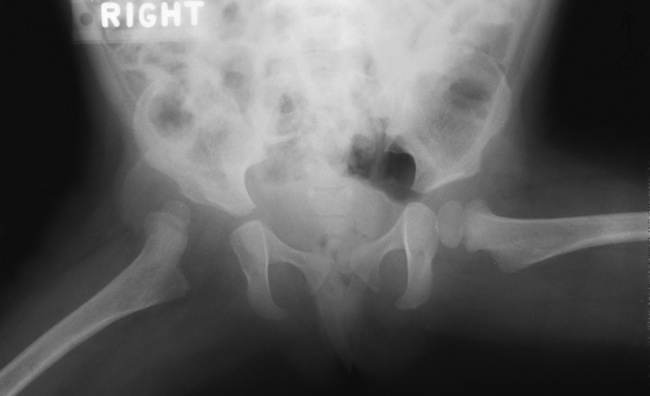
Figure 4-12 Hip dysplasia of the right hip in a 2-year-old child. Cleaves (frog-leg) view of the pelvis demonstrates superior and posterior displacement.
Ultrasound imaging (ultrasonography) now provides an alternative imaging method. The sonolucent femoral head can be viewed in relationship to the acetabulum to demonstrate the femoral angles. If sonography is available, its use allows the ionizing radiation dose to the child to be reduced, because previously, clinicians relied on two different x-ray projections for each assessment.
Treatment
Treatment depends on the type of femoral head movement: subluxation or dislocation. Children not diagnosed and treated before walking may appear to “waddle like a duck.” Immobilization of the femoral head is the most common treatment. To accomplish this, a harness or pelvic cast is used. Such immobilization allows the acetabulum to continue to form correctly before the infant begins to walk.
Inflammatory and infectious disorders
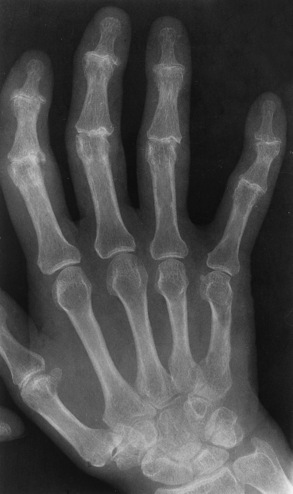
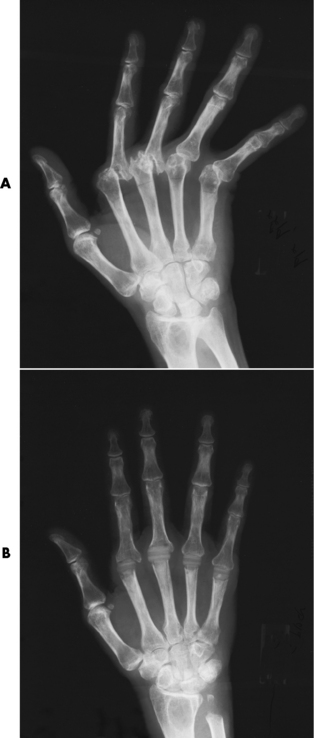
Rheumatoid arthritis is a chronic systemic disease of unknown cause that appears primarily as a nonsuppurative (noninfectious) inflammatory arthritis of the small joints of the hands and feet. Women are affected about three times more frequently than men, and the average age at onset in adults is 40 years. Rheumatoid arthritis usually has an insidious origin and may either run a protracted and progressive course, leading to a crippling deformity of affected joints, or undergo spontaneous remissions of variable length. There is usually symmetric involvement of multiple joints, and the disease often progresses proximally toward the trunk until practically every joint in the body is involved.
Rheumatoid arthritis begins as an inflammation of the synovial membrane (synovitis) that lines the joints. The excessive exudate, a result of the inflammation, causes proliferation of the synovium. The resulting mass of thickened granulation tissue (pannus, meaning “covers like a sheet”) causes erosion of the articular cartilage and underlying bony cortex, fibrous scarring, and even the development of ankylosis (bony fusion across a joint). The erosion occurs because the inflammatory cells produce lytic enzymes. A combination of the fusion of joint surfaces and an inflammatory laxity of ligaments leads to the development of crippling deformities in the end stage of the disease (Figure 4-13).
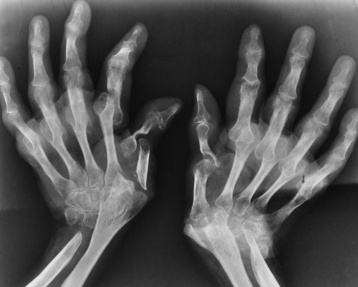
Figure 4-13 Mutilating rheumatoid arthritis. Severe, bilaterally symmetric destructive changes of the hands and wrists with striking subluxations.
Radiographic Appearance
The earliest radiographic evidence of rheumatoid arthritis is fusiform periarticular soft tissue swelling caused by joint effusion and hyperplastic synovial inflammation. Disuse and local hyperemia (increased blood flow) lead to periarticular osteoporosis that initially is confined to the portion of bone adjacent to the joint but may extend to involve the entire bone (Figure 4-14). Extension of the pannus from the synovial reflections onto the bone causes characteristic small foci of destruction at the edges of the joint, where articular cartilage is absent. These typical marginal erosions have poorly defined edges without a sclerotic rim and sometimes may be seen only on oblique or magnification projections. Destruction of articular cartilage causes narrowing of the joint space. The laying down of bony trabeculae across a narrow joint space may completely obliterate the joint cavity and produce solid bony ankylosis, which most frequently involves the bones of the wrist (see Figure 4-13).
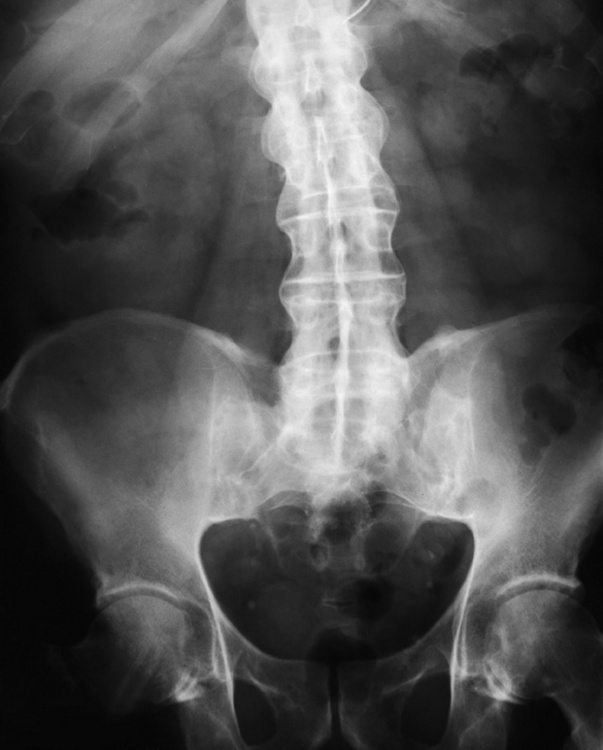
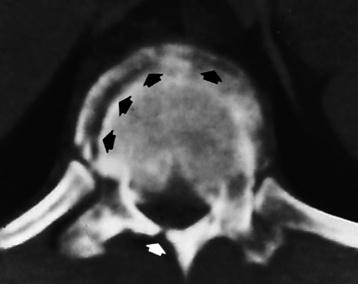
Ligamentous involvement produces a variety of contractures and subluxations causing the common ulnar deviation of the hands (see Figure 4-13). In the cervical spine, rheumatoid arthritis characteristically produces atlantoaxial subluxation (Figure 4-15), an increased distance between the anterior border of the odontoid and the superior border of the anterior arch of the atlas (normally less than 2.5 mm), which results from weakening of the transverse ligaments from synovial inflammation. The synovial inflammation appears as a soft tissue mass that causes a narrowing of the atlantoaxial articulation on CT images. CT also demonstrates associated erosion of the odontoid (in about two thirds of patients).
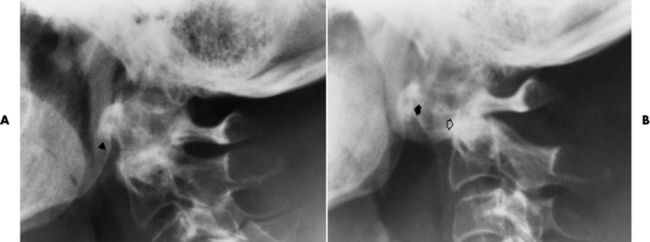
Figure 4-15 Subluxation of the atlantoaxial joint in rheumatoid arthritis. A, Routine lateral image of the cervical spine shows a normal relationship between the anterior border of the odontoid process and the superior portion of the anterior arch of the atlas (arrowhead). B, With flexion, there is wide separation between the anterior arch of atlas (solid arrow) and the odontoid (open arrow).
Rheumatoid nodules are soft tissue masses that usually appear over the extensor surfaces on the ulnar aspect of the wrist or the olecranon but occasionally are seen over other body prominences, tendons, or pressure points. These characteristic nodules, which develop in about 20% of patients with rheumatoid arthritis and do not occur in other diseases, aid in making the diagnosis.
Rheumatoid Variants: Ankylosing Spondylitis, Reiter’s Syndrome, and Psoriatic Arthritis
Radiographic Appearance of Ankylosing Spondylitis
Ankylosing spondylitis almost always begins in the sacroiliac joints, causing bilateral and usually symmetric involvement. Blurring of the articular margins and patchy sclerosis generally progress to narrowing of the joint space and may lead to complete fibrous and bony ankylosis (Figure 4-16). The disease typically progresses from the lumbar spine upward.
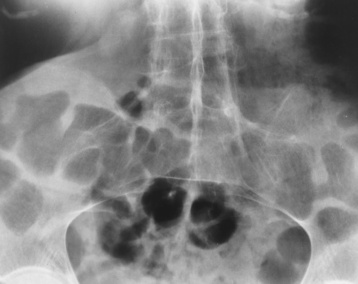
Figure 4-16 Ankylosing spondylitis. Bilateral, symmetric obliteration of the sacroiliac joints and “bamboo spine.”
Ossification in the paravertebral tissues and longitudinal spinal ligaments (poker spine) combines with extensive lateral bony bridges (syndesmophytes) between vertebral bodies to produce the characteristic “bamboo spine” of advanced disease (Figure 4-17; see Figure 4-16). Limitation of activity leads to generalized skeletal osteoporosis and a tendency to fracture in response to the stress of minor trauma (Figure 4-18). CT demonstrates both the fusion due to intraarticular and ligamentous ossification and any spinal stenosis caused by the displacement of the fractures.
Radiographic Appearance of Reiter’s Syndrome
Reiter’s syndrome (reactive arthritis) is characterized by arthritis, urethritis, and conjunctivitis. It primarily affects young adult men and appears to occur after certain types of venereal or gastrointestinal infections. Reiter’s syndrome most frequently involves the sacroiliac joints, heel, and toes (Figure 4-19). Unlike in ankylosing spondylitis, the sacroiliac involvement here is usually bilateral but asymmetric, and Reiter’s syndrome tends to cause only minimal changes in the spine.
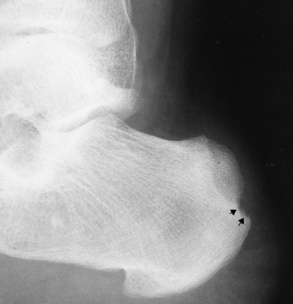
Figure 4-19 Reiter’s syndrome. Striking bony erosion (arrows) at insertion of the Achilles tendon on the posterosuperior margin of calcaneus.
Although the radiographic changes in peripheral joints often mimic rheumatoid arthritis, Reiter’s syndrome tends to be asymmetric and primarily involves the feet rather than the hands (Figure 4-20).
Radiographic Appearance of Psoriatic Arthritis
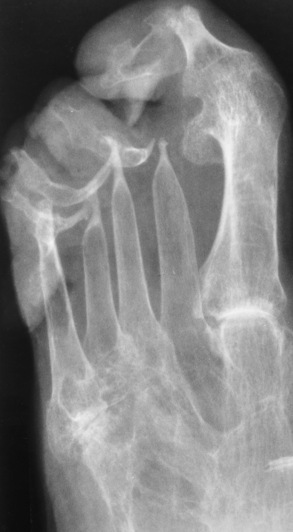
Psoriatic arthritis refers to a rheumatoid arthritis–like destructive process involving peripheral joints that develops in patients with typical skin changes of psoriasis (Figure 4-21). Unlike rheumatoid arthritis, psoriatic arthritis predominantly involves the distal rather than the proximal interphalangeal joints of the hands and feet, produces asymmetric rather than symmetric destruction, and causes little or no periarticular osteoporosis.
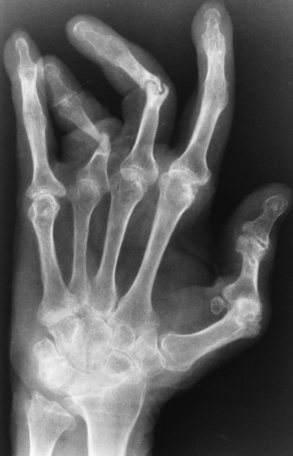
Figure 4-21 Psoriatic arthritis. Bizarre pattern of asymmetric bone destruction, subluxation, and ankylosis. Notice the particularly severe pencil-in-cup deformity of the third proximal interphalangeal joint, and the bony ankylosis involving the wrist and the phalanges of the second and fifth digits.
Characteristic findings include bony ankylosis of the interphalangeal joints of the hands and feet and resorption of the terminal tufts of the distal phalanges (Figure 4-22). Characteristics common to both Reiter’s syndrome and psoriatic arthritis include erosions and hypertrophic changes occurring in the origin and insertion of the tendons and ligaments.
Osteoarthritis (Degenerative Joint Disease)
Osteoarthritis is an extremely common generalized disorder characterized pathologically by loss of joint cartilage and reactive new bone formation. Part of the wear and tear of the aging process, degenerative joint disease tends to affect predominantly the weight-bearing joints (spine, hip, knee, ankle) and the interphalangeal joints of the fingers. A secondary form of degenerative joint disease may develop in a joint that has been repeatedly traumatized or subjected to abnormal stresses because of orthopedic deformities, or it may be a result of a septic or inflammatory arthritis that destroys cartilage.
Radiographic Appearance
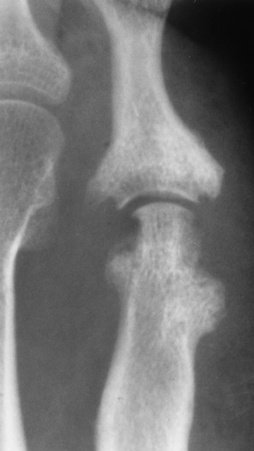
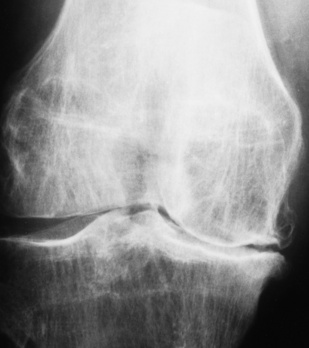
The earliest radiographic findings in degenerative joint disease are narrowing of the joint space, caused by thinning of the articular cartilage, and development of small bony spurs (osteophytes) along the margins of the articular edges of the bones. In contrast to the smooth, even narrowing of the joint space in rheumatoid arthritis, the joint space narrowing in degenerative joint disease is irregular and more pronounced in that part of the joint where weight-bearing stress is greatest and where degeneration of the articular cartilage is most noticeable. The articular ends of the bones become increasingly dense (periarticular sclerosis). Erosion of the articular cortex may produce typical irregular, cystlike lesions with sclerotic margins in the subchondral bone near the joint. Calcific or ossified loose bodies may develop, especially at the knee. With advanced disease, relaxation of the joint capsule and other ligamentous structures may lead to subluxation. Local osteoporosis does not occur unless pain causes prolonged disuse of the joint.
In the fingers, degenerative joint disease involves primarily the distal interphalangeal joints (Figure 4-23). Marginal spurs produce well-defined bony protuberances that appear clinically as the palpable and visible knobby thickening of Heberden’s nodes. In the hip, the most prominent finding is asymmetric narrowing of the joint space that involves predominantly the superior and lateral aspects of the joint, where the greatest stress of weight bearing exists (Figure 4-24). Joint space narrowing is also asymmetric in the knee, where it predominantly involves the medial femorotibial compartment (Figure 4-25).
Infectious Arthritis
Pyogenic (pus-forming) organisms may gain entry into a joint by the hematogenous route, by direct extension from an adjacent focus of osteomyelitis, or from trauma to the joint (e.g., after surgery). The onset of bacterial arthritis usually occurs abruptly with a high fever, shaking chills, and one or a few severely tender and swollen joints. The most common type today is a migratory arthritis from Lyme disease.
Radiographic Appearance
Soft tissue swelling is the first radiographic sign of acute bacterial arthritis. In children, fluid distention of the joint capsule may cause widening of the joint space and actual subluxation, especially about the hip and shoulder. Periarticular edema displaces or obliterates adjacent tissue fat planes (Figure 4-26A). Rapid destruction of articular cartilage causes joint space narrowing early in the course of the disease. The earliest bone changes, which tend to appear 8 to 10 days after the onset of symptoms, are small focal erosions in the articular cortex. Because of the delay in bone changes, detection of the characteristic soft tissue abnormalities is essential for early diagnosis. Severe, untreated infections cause extensive destruction and a loss of the entire cortical outline (see Figure 4-26). With healing, sclerotic bone reaction results in an irregular articular surface. If the articular cartilage has been completely destroyed, bony ankylosis usually follows.
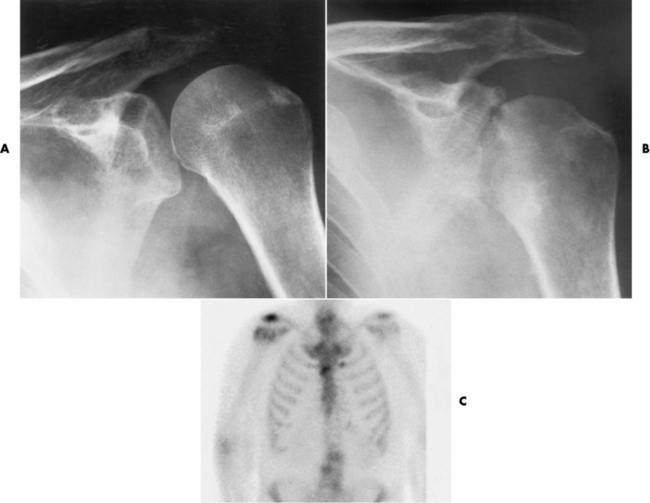
Figure 4-26 Acute staphylococcal arthritis. A, Several days after instrumentation of the shoulder for joint pain, there is separation of the humeral head from the glenoid fossa caused by fluid in the joint space. B, Six weeks later, pronounced cartilage and bone destruction are evident, with sclerosis on both sides of the glenohumeral joint. C, Septic arthritis. On a radionuclide bone scan, a focal area of increased activity in the right shoulder correlates with the clinical history of septic arthritis.
Radiographic Appearance of Tuberculous Arthritis
Tuberculous arthritis is a chronic, indolent (organic) infection that has an insidious (gradual) onset and a slowly progressive course (Figure 4-27). It usually involves only one joint, and it affects primarily the spine, hips, and knees. Most patients have a focus of tuberculosis elsewhere in the body, most commonly in the lungs.
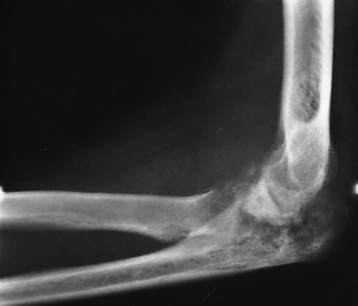
Figure 4-27 Tuberculous arthritis of the elbow with complete destruction of the joint space. A large chronic granulomatous mass can be seen in the antecubital region.
A distinctive early radiographic feature of tuberculous arthritis is the extensive juxtaarticular (near a joint) osteoporosis that precedes bone destruction—in contrast to bacterial arthritis, in which osteoporosis is a relatively late finding. Joint effusion leads to a nonspecific periarticular soft tissue swelling. Cartilage and bone destruction occur relatively late in the course of tuberculous arthritis and tend to initially involve the periphery of the joint, sparing the maximal weight-bearing surfaces that are destroyed in pyogenic arthritis. Therefore, joint space narrowing occurs late in tuberculous arthritis, in contrast to the early narrowing seen with bacterial infections. As in pyogenic arthritis, the earliest evidence of bone destruction is usually erosion at the margins of the articular ends of bone. With progressive disease, there is ragged destruction of the articular cartilage and subchondral cortex and disorganization of the joint, often with preservation of necrotic fragments of bone (sequestra) that may involve opposing surfaces (“kissing sequestra”).
Treatment of Arthritis
Arthritis therapy should protect affected joints, maintain mobility, and strengthen muscles. For this to occur, lifestyle changes, use of support devices, drugs, and surgery may be necessary. For those with rheumatoid arthritis and osteoarthritis, which make up 90% of all cases diagnosed, rest and exercise are recommended to minimize inflammation and preserve the range of motion. The first-line medications are nonsteroidal antiinflammatory drugs (NSAIDs), which decrease the inflammatory response but do not affect the disease process. Prostaglandin inhibitors, such as aspirin and ibuprofen (salicylates), fall into this category of drugs that reduce the triggering of inflammation. A more aggressive group of drugs, disease-modifying antirheumatic drugs, are used in advanced stages to reduce symptoms. The antimetabolite methotrexate, a cytotoxic drug, tempers cell division in the synovial joint. Invasive surgery involves replacing joints with new artificial joints (Figure 4-28) to increase joint mobility. For infectious arthritis, antibiotics usually eradicate the infection and cure the arthritis. Aspiration may be needed if fluid accumulates in the bursa.
Bursitis
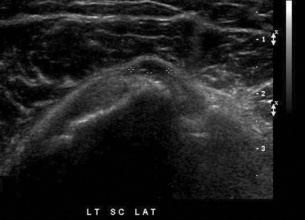
Bursitis refers to an inflammation of the bursae, small fluid-filled sacs located near the joints that reduce the friction caused by movement. Repeated physical activity commonly causes bursitis, but trauma, rheumatoid arthritis, gout, or infection also can cause this inflammation. Bursitis or tenosynovitis is usually not visualized on plain radiographs, but disorders of the bursa and synovium can be seen on ultrasound images (Figure 4-29). Plain radiographic images may exclude other disorders that cause similar symptoms.
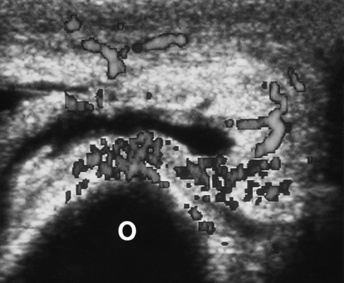
Figure 4-29 Bursitis. Transverse scan of the posterior aspect of the elbow shows the thick-walled, fluid-containing olecranon bursa. Power color Doppler imaging shows the bursa’s activity.
Radiographic Appearance
The major radiographic manifestation of bursitis is the deposition of calcification in adjacent tendons, which is a common cause of pain, limitation of motion (frozen joints), and disability about a joint. Calcific tendinitis most commonly involves the shoulder, and calcification may be demonstrated radiographically in about half the patients with persistent pain and disability in the shoulder region (Figure 4-30). However, calcification may also be detected in asymptomatic persons, and severe clinical symptoms may occur without evidence of calcification. Calcific tendinitis appears as amorphous calcium deposits that most frequently occur about the shoulder in the supraspinatus tendon, where they are seen directly above the greater tuberosity of the humerus. The deposits vary greatly in size and shape, from thin curvilinear densities to large calcific masses.
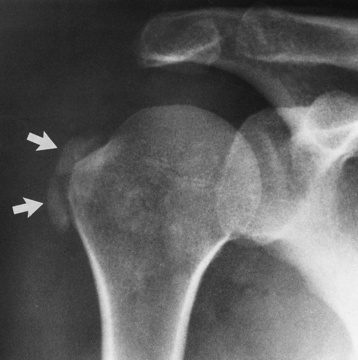
Figure 4-30 Calcific tendinitis. Frontal projection of the shoulder demonstrates amorphous calcium deposits (arrows) in the supraspinatus tendon.
In the acute early stages of bursitis, ultrasonography demonstrates the bursa filled with synovial fluid and having ill-defined margins. During the acute phase of true tendinitis, the thickened tendon has ill-defined margins. Both bursitis and tendinitis may demonstrate increased vascularity on Doppler ultrasound.
Treatment
First-line treatments for bursitis include application of heat, rest, and immobilization. Nonsteroidal antiinflammatory drugs are taken to reduce inflammation and relieve pain, as necessary. If the inflammation results from an infection, a regimen of antibiotics is appropriate. In recurring or severe bursitis, corticosteroid injections into the affected bursa may reduce the inflammation.
Rotator Cuff Tears
The rotator cuff of the shoulder is a musculotendinous structure composed of the teres minor, infraspinatus, supraspinatus, and subscapularis muscles. Rupture of the rotator cuff produces a communication between the shoulder joint and the subacromial bursa that can be demonstrated by arthrography (the injection of contrast material directly into the shoulder joint) (Figure 4-31). Currently, MRI is considered the modality of choice for demonstrating a rotator cuff disorder. The normal rotator cuff appears as a black structure, but tears cause it to have high signal intensity (Figure 4-32). However, ultrasound may be the preferred initial modality to demonstrate the tear of the tendon (Figure 4-33) because of its wider availability and lower cost.
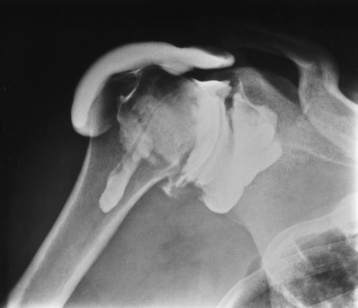
Figure 4-31 Shoulder arthrography of a rotator cuff tear. Opacification of the subacromial and subdeltoid bursae indicates abnormal communication between them and the glenohumeral joint cavity, thus confirming the diagnosis.
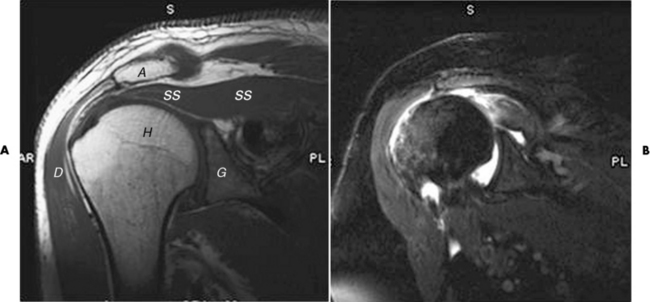
Figure 4-32 MRI of rotator cuff tear. A, Normal shoulder. Notice the low signal intensity of the supraspinatus tendon (ss). A, Acromion; C, clavicle; D, deltoid muscle; G, glenoid fossa; H, humeral head. B, T2 coronal with fat saturation with intra-articular contrast. Supraspinatus tendon is torn (white curved arrow) and retracted.
Tears of the Menisci of the Knee
Meniscal tears are a common cause of knee pain. Although occasionally the result of acute trauma, meniscal tears more frequently reflect a degenerative process caused by the chronic trauma inherent in human knee function. In the past, meniscal tears were best demonstrated by arthrography, which is invasive; now, MRI is clearly the imaging modality of choice, as it is noninvasive and has an accuracy of 90% to 98%.
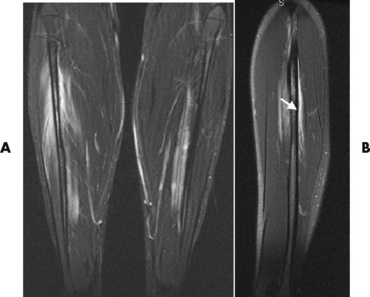
MRI demonstrates a tear as a sharply marginated line of high signal intensity (Figure 4-34) that crosses the normally dark, triangular meniscus. In addition, this modality can show the often-associated tears of the anterior and posterior cruciate ligaments and changes in the underlying bone. Ultrasound may demonstrate tenosynovitis of related tendons, which appears as a thickened synovial sheath (Figure 4-35).
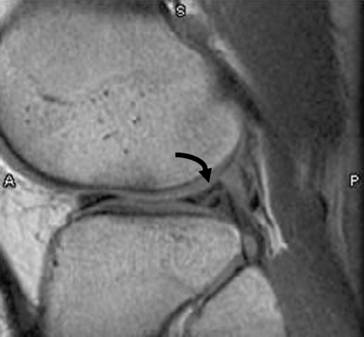
Figure 4-34 Meniscal tear. Sagittal proton density MR scan of knee shows bucket handle tear of the lateral meniscus (curved arrow).
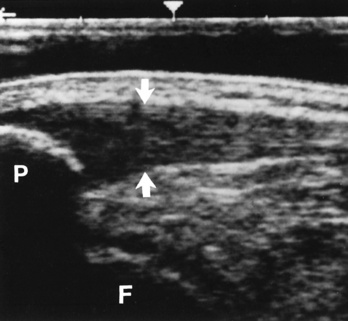
Figure 4-35 Acute patellar tendinitis. Longitudinal ultrasound scan shows thickening and decreased echogenicity of the upper two thirds of the tendon (arrows). F, Femoral condyle; P, patella.
Treatment for Intraarticular Components
Antiinflammatory medications and immobilization of the joint are the first line of treatment if the damage will repair itself. When the component is not completely destroyed, this treatment may result in cure (30% to 90% of cases respond to nonsurgical treatment). When irreparable joint damage has occurred, the orthopedist performs arthroscopic surgery to remove a complete or nonhealing tear or to attempt suture repair.
Bacterial Osteomyelitis
Bacterial osteomyelitis is an inflammation of the bone (osteitis) and bone marrow (myelitis) caused by a broad spectrum of infectious (most often gram-positive) organisms that reach bone by hematogenous spread, by extension from an adjacent site of infection, or by direct introduction of organisms (after trauma or surgery). Acute hematogenous osteomyelitis tends to involve bones with rich red marrow. In infants and children, the metaphyses of long bones, especially the femur and tibia, are most often affected; staphylococci and streptococci are the most common organisms. Patients with acute osteomyelitis experience fever and localized warmth, swelling, and tenderness. In adults, acute hematogenous osteomyelitis primarily occurs in the vertebrae, causing localized back pain and muscle spasm, and it rarely involves the long bones. Although the incidence and severity of osteomyelitis have decreased since the advent of antibiotics, this disease has now become more prevalent as a complication of intravenous drug abuse (in which case, gram-negative organisms are found). In diabetic patients and patients with other types of vascular insufficiency, a soft tissue infection may spread from a skin abscess or a decubitus ulcer, usually in the foot, to cause cellulitis and eventually osteomyelitis in adjacent bones.
Osteomyelitis begins as an abscess of the bone. Pus produced by the acute inflammation spreads down the medullary cavity and outward to the surface. Once the infectious process has reached the outer margin of the bone, it raises the periosteum from the bone and may spread along the surface for a considerable distance.
Because the earliest changes of osteomyelitis are usually not evident on plain radiographic images until about 10 days after the onset of symptoms, radionuclide (technetium Tc-99m) bone scanning is the most valuable imaging modality for the early diagnosis of osteomyelitis. Increased nuclide uptake, reflecting the inflammatory process and increased blood flow, is evident within hours of the onset of symptoms (Figure 4-36).
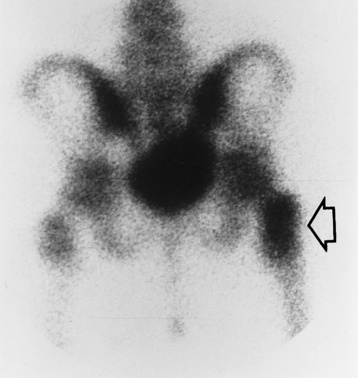
Figure 4-36 Osteomyelitis. Radionuclide bone scan (posterior projection) demonstrates increased uptake of radionuclide in the trochanteric portion of the right femur (arrow). Plain x-ray of the pelvis and hips obtained at the same time showed no detectable abnormality.
Radiographic Appearance
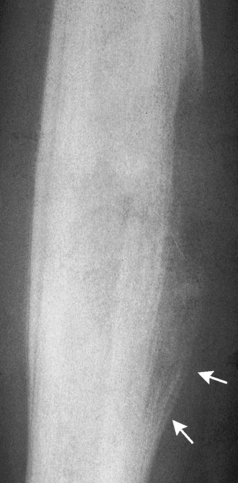
On plain radiographs, the earliest evidence of osteomyelitis in a long bone is a localized, deep soft tissue swelling adjacent to the metaphysis. The inflammation causes displacement or obliteration of the normal fat planes adjacent to and between the deep muscle bundles, unlike in skin infections, in which the initial swelling is superficial. The initial bony change appears as subtle areas of metaphyseal lucency reflecting resorption of necrotic bone. Soon, bone destruction becomes more prominent, producing a ragged, moth-eaten appearance (Figures 4-37 and 4-38). The more virulent the organism, the larger the area of destruction. Subperiosteal spread of inflammation elevates the periosteum and stimulates the deposition of layers of new bone parallel to the shaft. This results in a layered periosteal reaction that is characteristic of benign diseases, especially infection. Eventually, a large amount of new bone surrounds the cortex in a thick, irregular bony sleeve (involucrum). Disruption of cortical blood supply leads to bone necrosis. Segments of avascular dead bone (sequestra) remain as dense as normal bone and are clearly differentiated from the demineralized bone, infected granulation tissue, and pus around them (Figure 4-39). Power Doppler ultrasound demonstrates increased vascularity in the inflammation elevating the periosteum.
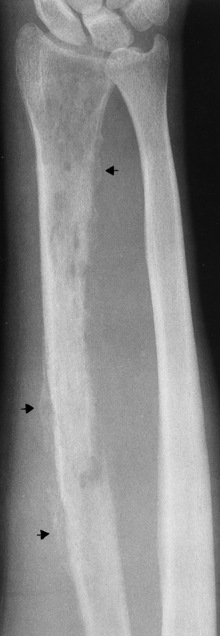
Figure 4-37 Osteomyelitis. Patchy pattern of bone destruction involves much of the shaft of the radius. Notice the early periosteal new bone formation (arrows).
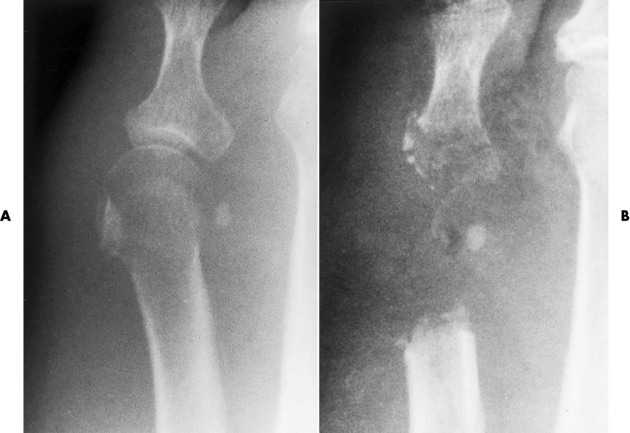
Figure 4-38 Staphylococcal osteomyelitis. A, Initial image of the first metatarsophalangeal joint shows soft tissue swelling and periarticular demineralization caused by increased blood flow to the region. B, Several weeks later, severe bony destruction about the metatarsophalangeal joint can be seen.
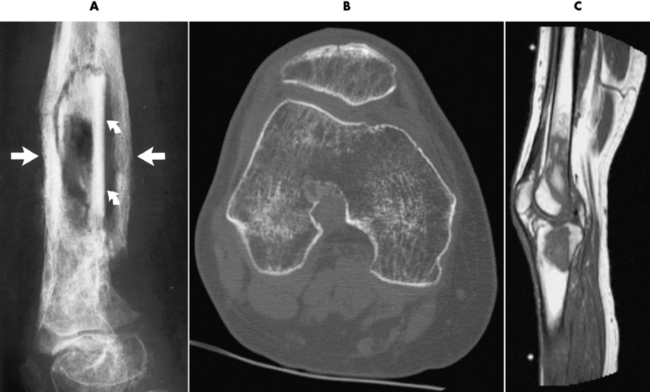
Figure 4-39 Chronic osteomyelitis. A, Involucrum (straight arrows) surrounds sequestra (curved arrows). B, After knee surgery, multiple foci can be seen to be lacking trabeculation in both femoral condyles in this 33-year-old woman. C, Sagittal MR image of the patient in B shows decreased signal intensity from the femoral condyle extending into the femoral shaft.
After the acute infection has subsided, a pattern of chronic osteomyelitis develops. The bone appears thickened and sclerotic with an irregular outer margin. The cortex may become so dense that the medullary cavity is difficult to demonstrate. Reactivation of infection may appear as the recurrence of deep soft tissue swelling or periosteal calcification, or the development of lytic abscess cavities within the bone. However, plain radiographs are often inadequate to determine whether an active infection is present. A three-phase radionuclide scan demonstrates increased uptake on all phases and is much more sensitive and accurate for establishing recurrence.
The earliest sign of vertebral osteomyelitis is subtle erosion of the subchondral bony plate with loss of the sharp cortical outline. This may progress to total destruction of the vertebral body associated with a paravertebral soft tissue abscess (Figure 4-40). Unlike neoplastic processes, osteomyelitis usually affects the intervertebral disk space and often involves adjacent vertebrae. Depending on the site of disease, anterior extension of osteomyelitis may cause retropharyngeal abscess, mediastinitis, empyema, pericarditis, subdiaphragmatic abscess, psoas muscle abscess, or peritonitis; posterior extension of inflammatory tissue can compress the spinal cord or produce meningitis if the infection penetrates the dura to enter the subarachnoid space.
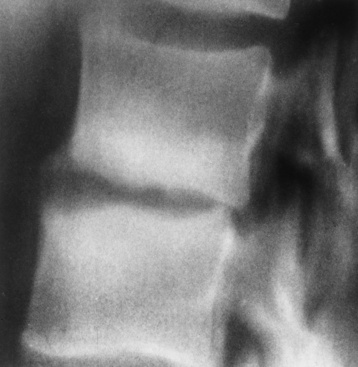
Figure 4-40 Bacterial vertebral osteomyelitis. Narrowing of the intervertebral disk space with irregularity of the end plates and reactive sclerosis.
CT can be of value in the diagnosis of osteomyelitis, especially that involving the spine. This modality can precisely define the size of the surrounding soft tissue mass, its relation to nearby vital structures (aorta, spinal cord), and the presence of abscess cavities requiring surgical drainage (see Figure 4-39B). In acute osteomyelitis of long bones, the bone destruction and periosteal reaction produce a thin laminated periosteal reaction.
MRI can assist in determining whether an abscess has formed and in excluding cellulitis. Bone marrow changes caused by inflammatory exudate result in decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images (see Figure 4-39C).
Treatment
Antibiotics to eradicate gram-negative or gram-positive organisms should be given for 4 to 8 weeks to prevent recurrence. Surgery may be required for débridement of necrotic tissue or for placement of a drain into an abscess. Bone grafts, usually of bone marrow, are placed to fill bony defects or to help bones heal. After surgery, the patient must continue to take antibiotics for approximately 3 weeks.
Tuberculous Osteomyelitis
Tuberculous osteomyelitis (which is rare today) most commonly involves the thoracic and lumbar spine. Pott’s disease (tuberculosis of the spine) occurs in the midthoracic spine and thoracolumbar region. Irregular, poorly marginated bone destruction within the vertebral body is often associated with a characteristic paravertebral abscess, an accumulation of purulent material that produces a fusiform soft tissue mass about the vertebra. The spread of tuberculous osteomyelitis causes narrowing of the adjacent intervertebral disk and the extension of infection and bone destruction across the disk to involve the adjacent vertebral body. Unlike bacterial infection, tuberculous osteomyelitis is rarely associated with periosteal reaction or bone sclerosis.
Radiographic Appearance
The infection tends to begin in the anterior part of the vertebral body adjacent to the intervertebral disk (Figure 4-41A). Caseous necrosis of the vertebral marrow produces a slow resorption of bony trabeculae, with bone destruction appearing 2 to 5 months after onset of infection. In the untreated patient, progressive vertebral collapse and anterior wedging lead to a characteristic sharp kyphotic angulation (gibbous deformity) (Figure 4-41B). A radionuclide bone scan may demonstrate early activity but cannot distinguish tumors or fractures. The gallium bone scan can help to define the total tissue involvement and determine response to therapy in tuberculous osteomyelitis. CT also may delineate the extent of soft tissue involvement and illustrate osseous destruction. Soft tissue calcifications identified on CT aid in distinguishing tuberculous osteomyelitis from other conditions. MRI demonstrates changes in the bone marrow, but these findings are no more specific than those of radionuclide bone scans.
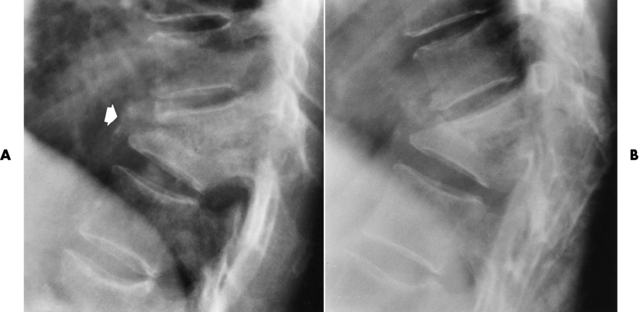
Figure 4-41 Tuberculous osteomyelitis. A, Initial image demonstrates vertebral collapse and anterior wedging of adjacent midthoracic vertebrae (arrow). Residual intervertebral disk space can barely be seen. B, In a image obtained several months later, there is virtual fusion of the collapsed vertebral bodies, producing the characteristic sharp kyphotic angulation (gibbous deformity).
Tuberculosis can, rarely, involve a low-grade chronic infection of the long bones that appears radiographically as a generally destructive lytic process with minimal or no periosteal reaction. The spectrum of radiographic appearances is wide, varying from localized, well-circumscribed, expansile lesions to diffuse, uniform, honeycomb-like areas of destruction that are often associated with pathologic fractures. Chronic draining sinuses may develop, especially in children.
Treatment
Nonoperative treatments for tuberculous osteomyelitis include a regimen of antibiotics, antituberculous drug therapy, and possible bracing. Antibiotics help prevent abscess development, antituberculous drug therapy eradicates the tuberculosis bacilli, and bracing provides support. With this treatment combination, most patients do not require surgery. If the pain, neurologic deficits, and deformities progress, surgical intervention may be necessary. Spinal fusion or Harrington rod placement for stability may be performed.
Metabolic bone disease
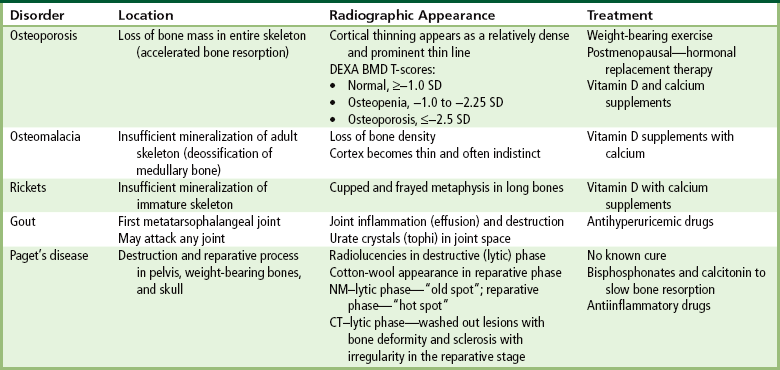
Osteoporosis is a generalized or localized deficiency of bone matrix in which the mass of bone per unit volume is decreased in amount but normal in composition. Bone is a living, constantly changing tissue, and normally a balance exists between the amount of old bone being removed (an osteoclastic process) and the amount of new bone replacement (an osteoblastic process). Osteoporosis is usually caused by accelerated resorption of bone, although decreased bone formation may lead to osteoporosis in some situations—for example, in Cushing’s syndrome, after prolonged steroid administration, and after prolonged disuse or immobilization as in a casted extremity (Figure 4-42).
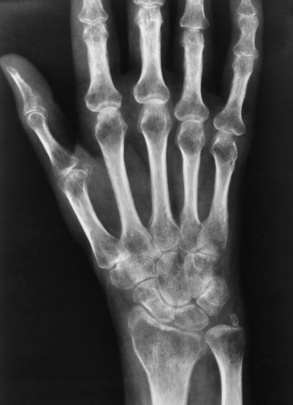
Figure 4-42 Disuse osteoporosis. Severe periarticular demineralization after prolonged immobilization of the hand.
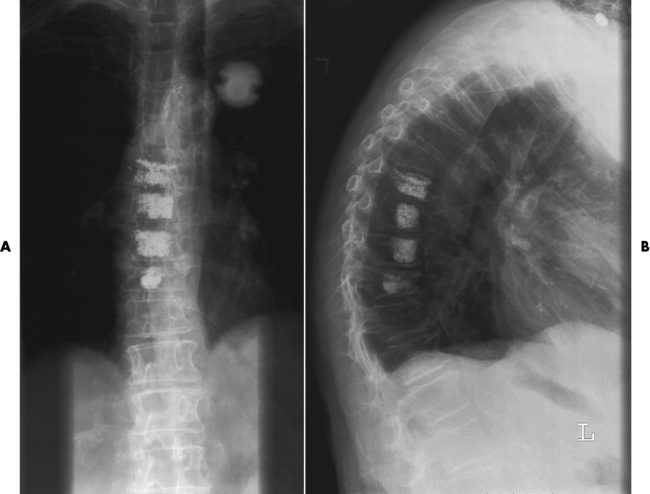
Loss of mineral salts causes osteoporotic bone to become more lucent than normal. This may be difficult to detect, because about 50% to 70% of the bone density must be lost before it can be demonstrated as a lucent area on routine radiographs. For patients with osteoporosis, it is essential to use the lowest practical kVp. This technique provides the extremely short scale of contrast necessary to visualize the demineralized osteoporotic bones. Today, quantitative CT (QCT), single-photon absorptiometry, and dual-energy x-ray absorptiometry (DEXA bone densitometry–qualitative) provide data that measure bone mineral content. A quantitative CT scan accurately demonstrates levels of bone mass and determines the rate of bone decay, which represents bone quantity. Three-dimensional quantitative CT is considered more sensitive and accurate than the other methods. Quantitative CT measurements depend on the trabecular bone, which demonstrates the earliest signs of osteoporosis. CT scans, because of their high radiation dose, are not done as frequently as bone densitometry. Dual-energy x-ray absorptiometry (DEXA) bone densitometry is used to image the hip and spine, assisting in the determination of bone quality. The patient’s history is entered into the computer system and two images, usually an AP lumbar spine and an AP hip, are taken (Figure 4-43). The image data are compared with those of other individuals of the same race, age, and weight to determine a bone-quality value. This value indicates whether the patient has normal bone (T-score greater than −1.0 standard deviation [SD]), osteopenia (T-score between −1.0 and −2.5 SD), or osteoporosis (T-score less than −2.5 SD).
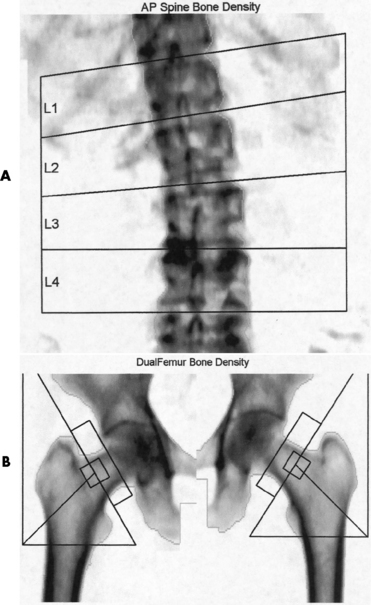
Figure 4-43 Osteoporosis. Results of DEXA bone mineral density (BMD) scan of the spine. A, A T-score average of 3.3 demonstrates osteoporosis. B, The dual femur of this 65-year-old woman has a T-score average of 2.3, indicating osteopenia.
The major causes of generalized osteoporosis are aging and postmenopausal hormonal changes. With increasing age, bones lose density and become more brittle, fracturing more easily and healing more slowly. Two factors contribute to this process: Elderly persons may become less active and they may have poor diets, often deficient in protein. In postmenopausal women, there is a deficiency in the gonadal hormonal levels that stimulate bone formation. This decreased bone formation plus an increase in bone resorption by a factor of three to five times results in a loss of density.
Radiographic Appearance
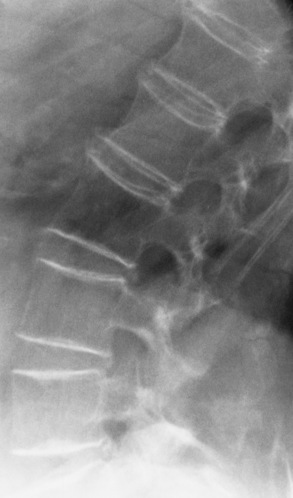
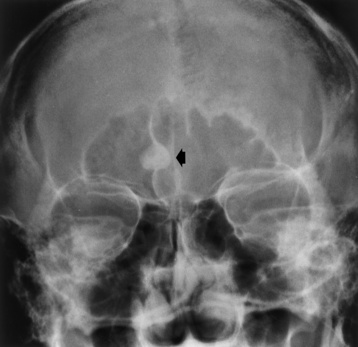
Regardless of the cause, the radiographic appearance is somewhat similar in all conditions producing osteoporosis. The most striking change is cortical thinning, with irregularity and resorption of the endosteal (inner) surfaces (Figure 4-44). These findings are most evident in the spine and pelvis. As the bone density of a vertebral body decreases, the cortex appears as a relatively dense and prominent thin line producing the typical picture-frame pattern. Because of the severe loss of bone density, anterior wedging or compression fractures of one or more vertebral bodies may result, most commonly in the middle and lower thoracic and upper lumbar areas. The intervertebral disk may expand into the weakened vertebral bodies and produce characteristic concave contours of the superior and inferior disk surfaces. In the skull, the calvaria may show a spotty loss of density, and deossification (bone loss) of the floor of the sella turcica and dorsum sellae is commonly seen.
Treatment
To help prevent osteoporosis, weight-bearing exercises help build bone density and muscle mass. For postmenopausal osteoporosis, hormonal replacement therapy and dietary supplements of calcium and vitamin D may reduce development of the disease process. In severe cases, vertebroplasty (kyphoplasty) is performed to prevent vertebral collapse (Figure 4-45).
Osteomalacia
Osteomalacia refers to insufficient mineralization of the adult skeleton. The lack of a balance between osteoid formation and mineralization influencing bone quality results in either excessive osteoid formation or, more frequently, insufficient mineralization. Proper calcification of osteoid requires that adequate amounts of calcium and phosphorus be available at the mineralization sites. In osteomalacia, failure of calcium and phosphorus deposition in bone matrix may be attributable to an inadequate intake of, or to a failure of absorption of, calcium, phosphorus, or vitamin D. Vitamin D is necessary for intestinal absorption of calcium and phosphorus and may have a direct effect on bone. At times, the level of vitamin D is sufficient but is not used because of resistance to the action of the vitamin at end organs, such as the kidneys. Other, nonnutritional causes of osteomalacia include chronic kidney failure and certain renal diseases in which calcium is lost to the urine and then bone breaks down as the body attempts to maintain a normal calcium level in the blood.
Radiographic Appearance
Regardless of the cause, osteomalacia appears radiographically as a loss of bone density because of the presence of nonmineralized osteoid. Although the cortex is thinned, it may stand out more prominently than normal because of the uniform deossification of medullary bone (Figure 4-46). In contrast to the situation in osteoporosis, the cortical borders in osteomalacia often appear indistinct. Fine-detail radiographs of the hands in patients with osteomalacia often demonstrate intracortical lines caused by local resorption or lack of mineralization, a finding not seen in osteoporosis.
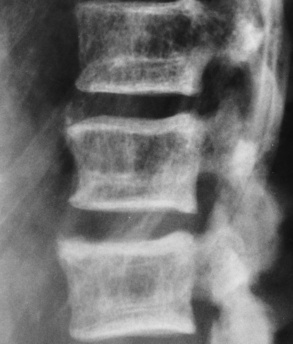
Figure 4-46 Osteomalacia. Striking prominence of the cortices of the vertebral bodies with increased trabeculation of spongy bone.
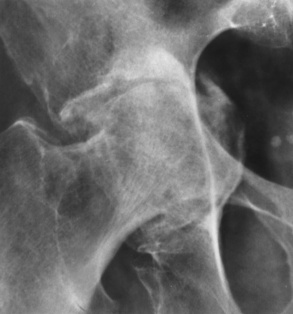
Bones that are softened by osteomalacia may bend or give way as a result of weight bearing. Bowing deformities primarily involve the pelvis, vertebral column, thorax, and proximal extremities. In the pelvis, there may be a characteristic inward bending of the sidewalls with deepening of the acetabular cavities (protrusio acetabuli) (Figure 4-47).
Rickets
Rickets is a systemic disease of infancy and childhood that is the equivalent of osteomalacia in the mature skeleton. In this condition, calcification of growing skeletal elements is defective because of a deficiency of vitamin D in the diet or a lack of exposure to ultraviolet radiation (sunshine), which converts sterols in the skin into vitamin D. Most common in premature infants, rickets usually develops between ages 6 months and 1 year.
Radiographic Appearance
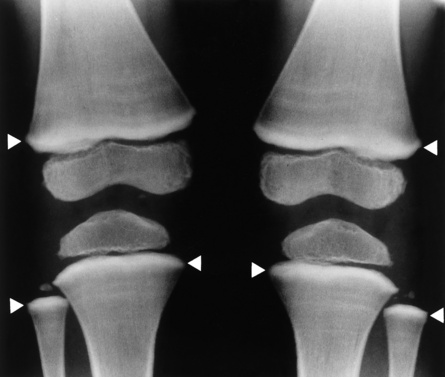
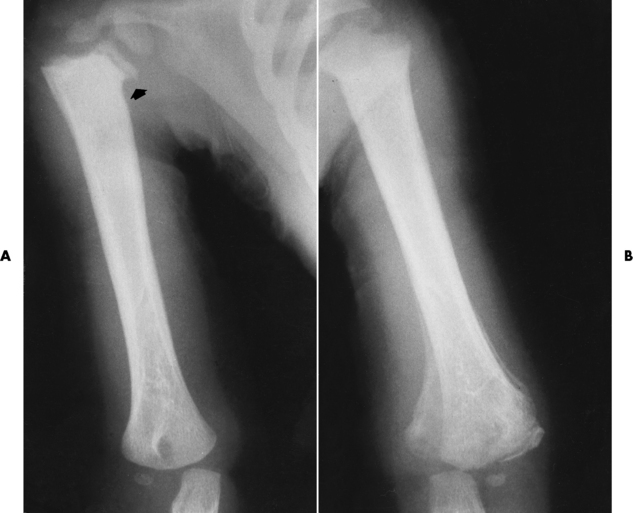
The early radiographic changes in rickets are best seen in the fastest-growing portions of bone, such as the sternal ends of the ribs, the proximal ends of the tibia and humerus, and the distal ends of the radius and ulna. An overgrowth of noncalcified osteoid tissue appears radiographically as a characteristic increase in distance between the ossified portion of the epiphysis and the end of the shaft. In response to the pull of muscular and ligamentous attachments, the metaphyseal ends of the bone become cupped and frayed, and the normally sharp metaphyseal lines disappear (Figure 4-48). Lack of calcification leads to a delayed appearance of the epiphyseal ossification centers. Because of poor mineralization, bowing of weight-bearing bones (especially the tibia) develops once the infant begins to stand or walk. Extensive osteoid tissue in the sternal ends of the ribs produces a characteristic beading (rachitic rosary).
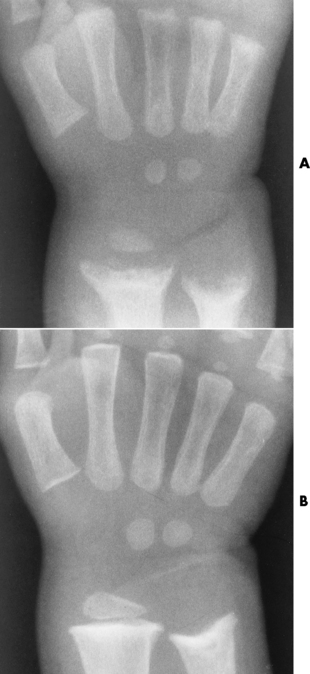
Figure 4-48 Rickets. A, Initial image of this wrist shows characteristic cupping and fraying of the metaphyseal ends of the radius and ulna, with disappearance of normally sharp metaphyseal lines. B, On a image obtained after therapy with vitamin D, there is remineralization of the metaphyseal ends of the radius and ulna, increased sharpness of metaphyseal lines, and a return of the epiphyseal centers to normal density and sharpness of outline.
Softening of the vertebral bodies leads to the development of kyphosis. In females, narrowing of the pelvic inlet may make normal delivery impossible in later life.
Treatment for Osteomalacia and Rickets
Treatment consists of doses of vitamin D with calcium supplements. Other treatments are required if the calcium loss has a nonnutritional cause.
Gout
Gout is a disorder in the metabolism of purine (a component of nucleic acids) in which an increase in the blood level of uric acid leads to the deposition of uric acid crystals in the joints, cartilage, and kidney. Several inherited enzyme defects can cause overproduction of uric acid (primary gout). In secondary gout, hyperuricemia can be caused by an overproduction of uric acid, which in turn may be caused by increased turnover of nucleic acids (e.g., as in metastatic carcinoma, myeloma, and hemolytic anemia), drugs (e.g., chemotherapy and thiazides used to treat hypertension), or a decrease in the excretion of uric acid resulting from kidney failure.
Radiographic Appearance
Acute gout manifests primarily as an exquisitely painful arthritis that initially attacks a single joint, usually the first metatarsophalangeal joint (Figure 4-49). Radiographic changes develop late in the disease and only after repeated attacks. Therefore negative radiographic findings do not exclude gout and thus do not aid in early diagnostic evaluation.
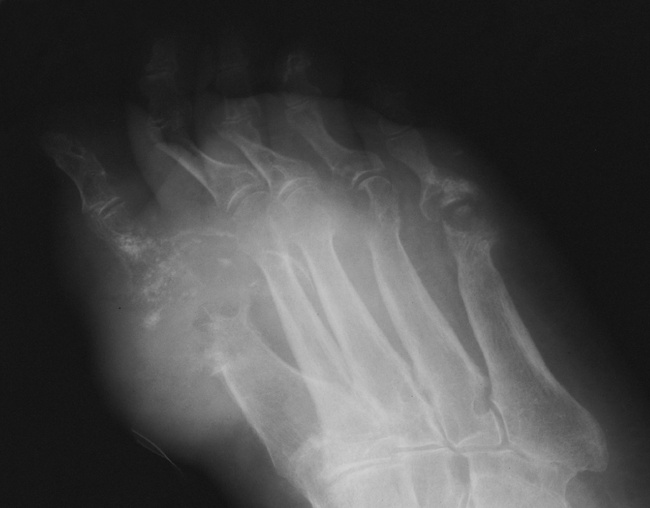
Figure 4-49 Gout. Effusion with tophi deposits at the first, fourth, and fifth metatarsophalangeal joints.
Deposition of nonopaque urate crystals in the joint synovial membrane and on the surface of articular cartilage incites an inflammatory reaction that produces the earliest radiographic signs of joint effusion and periarticular swelling (Figure 4-50). Clumps of urate crystals (tophi) form along the margins of the articular cortex and erode the underlying bone, leading to small, sharply marginated, punched-out defects at the joint margins of the small bones of the hand and foot. These erosions often have the appearance of cystlike lesions with thin sclerotic margins and characteristic overhanging edges (“rat bite” erosions) (Figure 4-51). In advanced disease, severe destructive lesions are associated with joint space narrowing and even fibrous ankylosis. Because patients are relatively free of symptoms between acute exacerbations of the disease, the bone density remains relatively normal, and diffuse osteoporosis is not part of the radiographic appearance.
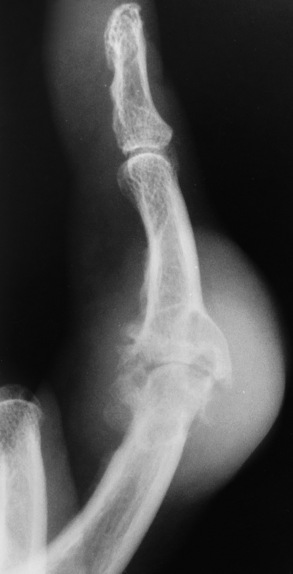
Figure 4-50 Gout. Severe joint effusion and periarticular swelling of the proximal interphalangeal joint of the finger. Note the associated erosion of the articular cartilage.
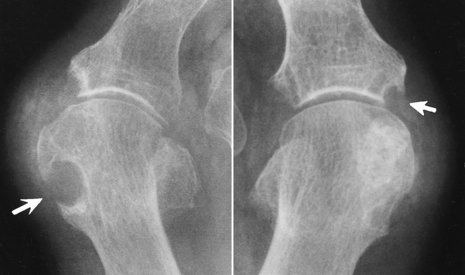
Figure 4-51 Two examples of typical “rat bite” erosions about the first metatarsophalangeal joint (arrows). The cystlike lesions have thin sclerotic margins and characteristic overhanging edges.
Continued deposition of urate crystals in the periarticular tissues causes the development of the characteristic large, lumpy soft tissue swellings representing gouty tophi. In addition to the first metatarsophalangeal joints, other common sites of tophi are the ear, the olecranon bursa, and the insertion of the Achilles tendon. Tophi consisting of only sodium urate appear as soft tissue densities. Deposition of calcium in these urate collections causes the tophaceous periarticular masses to become radiopaque (Figure 4-52).
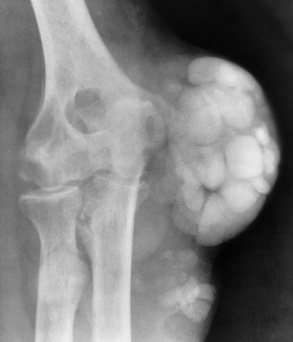
Figure 4-52 Gout. Massive deposition of calcium in a long-standing tophaceous lesion about the elbow.
Although some renal dysfunction occurs in almost all patients with gouty arthritis, no radiographic abnormalities in the urinary tract can be demonstrated unless uric acid stones form. Nonradiopaque pure uric acid stones can be demonstrated only on excretory urograms, where they appear as filling defects in the pelvicalyceal system or ureter. Stones containing the calcium salts of uric acid can be detected on plain abdominal radiographs, on which they appear radiopaque.
MRI of gout produces various patterns, although regions of persistent low signal intensity are characteristic.
Treatment
Patients receiving doses of antihyperuricemic drugs rarely experience tophi. Untreated gout may lead to decreased renal excretion because of renal damage resulting from crystal deposits in the interstitial tissue. Eventually, this process causes obstruction of the renal tubules and renal failure.
Paget’s Disease
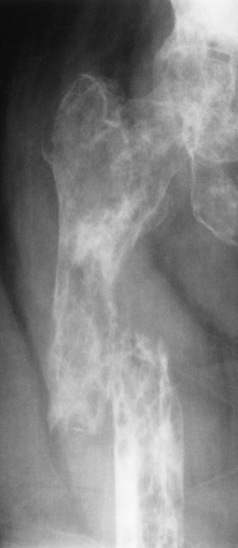
Paget’s disease (osteitis deformans) is one of the most common chronic metabolic diseases of the skeleton. Destruction of bone, followed by a reparative process, results in weakened, deformed, and thickened bony structures that tend to fracture easily. The disease, seen most commonly during middle life, affects men twice as often as women and has been reported to occur in about 3% of all persons older than 40 years. Although the destructive phase often predominates initially, there is more frequently a combination of destruction and repair in the pelvis and weight-bearing bones of the lower extremities. The reparative process may begin early and may be the prominent feature, often involving multiple bones. Paget’s disease affects particularly the pelvis, femurs, skull, tibias, vertebrae, clavicles, and ribs. The radionuclide bone scan is the most efficient method to visualize multicentric lesions (Figure 4-53A).
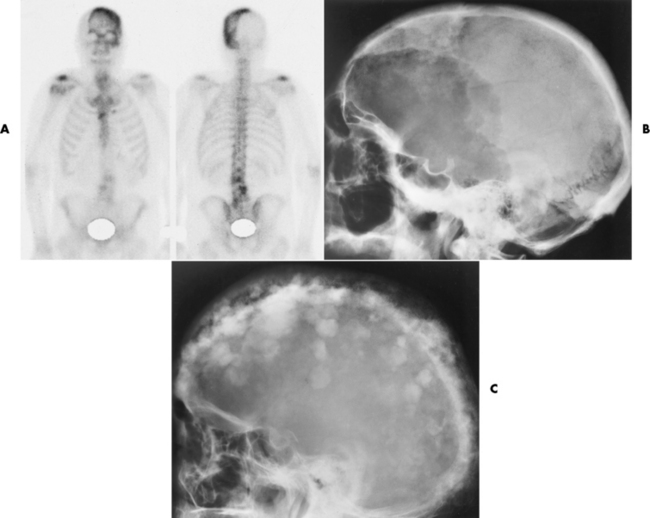
Figure 4-53 Paget’s disease of the skull. A, Nuclear medicine bone scan demonstrating a “cold spot” within a “hot spot” in the skull. The cold spot represents the initial destructive phase, whereas the hot spot is a result of the reparative process. B, Radiograph obtained during the destructive phase of the disease demonstrates an area of sharply demarcated radiolucency (osteoporosis circumscripta) that involves primarily the frontal portion of the calvaria. C, During the reparative phase in another patient, irregular areas of sclerosis produce the characteristic cotton-wool appearance of the skull.
Radiographic Appearance
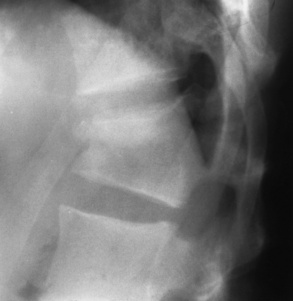
In the skull, Paget’s disease begins as an area of sharply demarcated radiolucency (osteoporosis circumscripta) that represents the destructive phase of the disease (Figure 4-53B). During the reparative process, the development of irregular islands of sclerosis and cortical thickening results in a mottled, cotton-wool appearance (Figure 4-53C). A bone scan demonstrates “cold spots” representing the initial destructive phase and “hot spots” as a result of the reparative process (see Figure 4-53A). In the spine, Paget’s disease characteristically causes enlargement of the vertebral body. Increased trabeculation, which is most prominent at the periphery of the bone, produces a rim of thickened cortex and a picture-frame appearance. Uniform dense sclerosis of one or more vertebral bodies (ivory vertebrae) may occur.
The pelvis is the most common and often the initial site of Paget’s disease. A distinctive early sign is coarsening of the trabeculae along the iliac margins, which causes thickening of the pelvic brim (Figure 4-54).
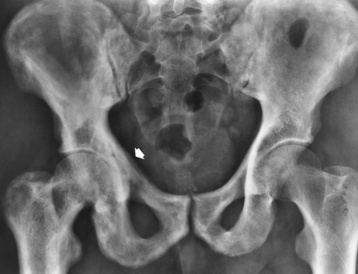
Figure 4-54 Paget’s disease of the pelvis. Diffuse sclerosis with cortical thickening involves the right femur and both iliac bones. Note the characteristic thickening and coarsening of the iliopectineal line (arrow) on the involved right side.
In the long bones, the destructive phase almost invariably begins at one end of the bone and extends along the shaft for a variable distance before ending in a typical, sharply demarcated, V-shaped configuration (blade of grass appearance). In the reparative stage, the bone is enlarged with an irregularly widened cortex and coarse, thickened trabeculae (Figure 4-55). Although dense, the bones are soft and weakened, and deformities are common. On CT, the lytic stage produces a washed-out appearance with cortical bone deformity. During the reparative process, the sclerosis appears as increased irregular cortical thickening and enlargement.
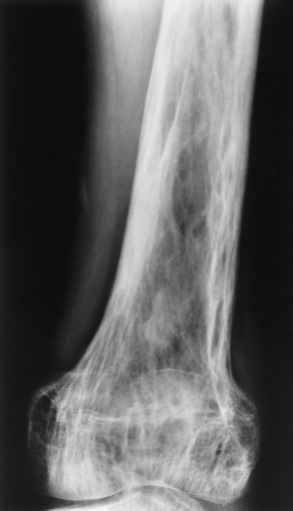
Figure 4-55 Paget’s disease of the knee. Note the characteristic cortical thickening, destruction of fine trabeculae, and accentuation of secondary trabeculae.
Paget’s disease may have severe clinical complications. The downward thrust of the heavy head on the softened bone of the skull base may compress the brainstem and cause numerous cranial nerve deficits. Expansion and distortion of softened vertebral bodies, sometimes with pathologic fractures, may compress the spinal cord and produce nerve root deficits. Multiple microscopic arteriovenous malformations in pagetoid bone may result in high-output cardiac failure. The most serious complication of Paget’s disease, the development of osteosarcoma, fortunately occurs in less than 1% of patients with this condition. MRI and CT provide the most accurate imaging if osteosarcoma is suspected.
Treatment
No known cure exists for Paget’s disease; however, the process may be slowed by the administration of bisphosphonates, which bind to the bone to minimize the resorption, and calcitonin, which inhibits the activity of the osteoclasts (resorption cells). Sometimes antiinflammatory drugs reduce the inflammation caused by cell breakdown.
Lead Poisoning
Lead poisoning results from the ingestion of lead-containing materials (especially paint) or from the occupational inhalation of lead fumes. Environmental exposure occurs in the drinking of water (leaded pipes) or the eating of food that is processed, preserved, or stored in containers made with lead. Currently, lead is
the number one environmental pollutant worldwide; however, the incidence in the United States has decreased as a result of public education and governmental restrictions of its use. In the past decade, the United States has experienced concern about imported toys that contain lead. The chronic form of lead poisoning may cause mental retardation, seizures, behavioral disorders, or delayed development. Ingested lead affects all systems of the body. Children are more susceptible to lower doses, and the effect on their central nervous system is more severe, causing interference in the cognitive thought process.
Radiographic Appearance
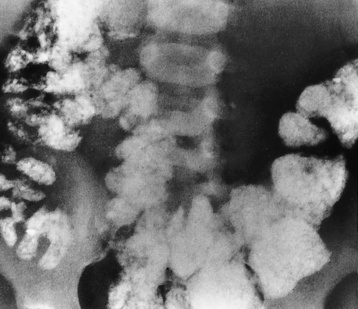
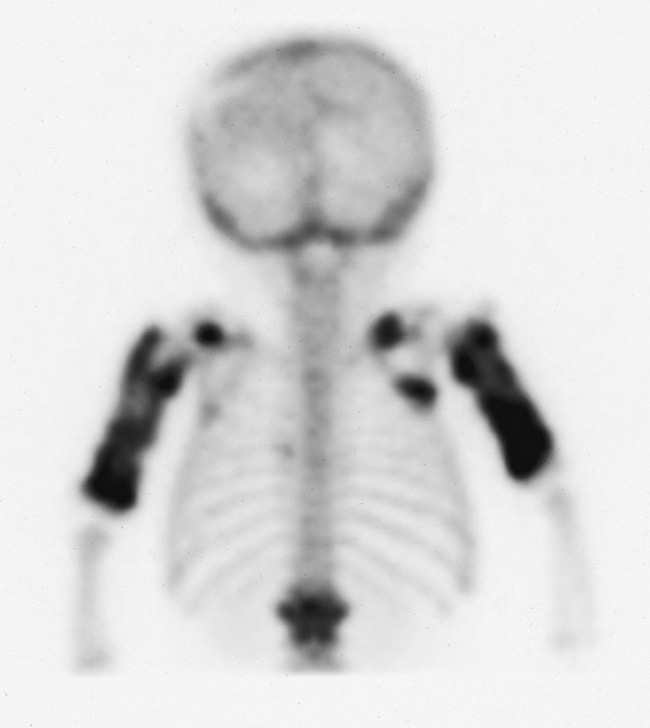
In children, because lead and calcium are used interchangeably by bone, high concentrations of lead are deposited in the most rapidly growing portions of the skeleton, especially the metaphyses at the distal ends of the femur. This results in classic lead lines, dense transverse bands extending across the metaphyses of the long bones (Figure 4-56) and along the margins of flat bones, such as the iliac crest. In young children who eat lead-containing paint (pica), plain abdominal radiographs may show extensive mottled opacities, which are suggestive of intestinal barium, even though no contrast material has been administered (Figure 4-57). CT is the modality of choice for demonstrating cerebral edema and structural changes.
Treatment
First, the source must be eliminated. If the drop in the body’s lead content is not quick enough, chelation therapy to aid in the urinary excretion of lead is prescribed for those who do not have kidney or liver disease. The objective is to keep the blood lead level lower than 60 µg/dL to prevent potential encephalopathy.
Fibrous dysplasia
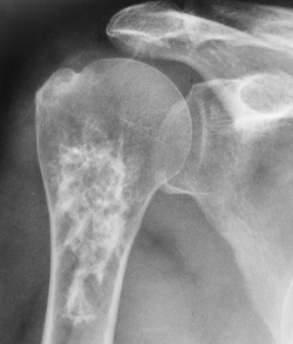
Fibrous dysplasia, a disorder that usually begins during childhood, is characterized by the proliferation of fibrous tissue within the medullary cavity. This proliferation causes loss of trabecular markings and widening of the bone. The disease may be confined to a single bone (monostotic) or the bones of one extremity, which is the most common form when it occurs in adults. The other form, in which the disease is widely distributed throughout the skeleton (polyostotic—among patients with this form, 25% have involvement of half the skeleton with more than 25% involvement of the skull), is present in children and occurs most often in girls. Fibrous dysplasia involves primarily the long bones (especially the femur and tibia), ribs, and facial bones.
Radiographic Appearance
Fibrous replacement of the medullary cavity typically produces a well-defined radiolucent area, which may vary from completely radiolucent to a homogeneous ground-glass density, depending on the amount of fibrous or osseous tissue deposited in the medullary cavity (Figure 4-58). Irregular bands of sclerosis may cross the cystlike lesion, giving it a multilocular appearance (Figure 4-59). The bone is often locally expanded (suggesting a balloon), and the cortex may be eroded from within, predisposing to pathologic fractures. In severe and long-standing disease, affected bones may become bowed or deformed. CT images have a similar appearance to that of diagnostic x-rays, a cross-sectional perspective demonstrating sclerotic formation with radiolucent lesions. CT better demonstrates the location of the lesion, the cause (tissue type—lucent or sclerotic), and the degree of expansion. For the most definitive diagnosis, CT complements radiographic findings.
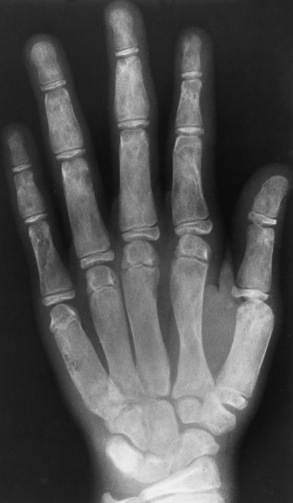
Figure 4-58 Fibrous dysplasia. Note the smudgy, ground-glass appearance of the medullary cavities with failure of normal modeling.
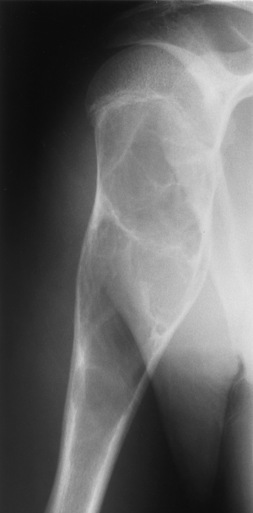
Figure 4-59 Fibrous dysplasia. An expansile lesion of the humerus contains irregular bands of sclerosis, giving it a multilocular appearance.
Fibrous dysplasia is the most common cause of an expansile focal rib lesion, which usually has a ground-glass or soap-bubble appearance.
Ischemic necrosis of bone
Ischemic necrosis of bone is caused by loss of the blood supply, which in turn can result from such varied conditions as thrombosis, vasculitis, disease of surrounding bone, and single or repeated episodes of trauma. Among the many conditions associated with ischemic necrosis are acute trauma (fracture or dislocation), steroid therapy and Cushing’s disease, hemolytic anemia (especially sickle cell disease), chronic alcoholism and chronic pancreatitis, Gaucher’s disease, radiation therapy, and caisson disease (a complication of underwater diving, the so-called “bends”).
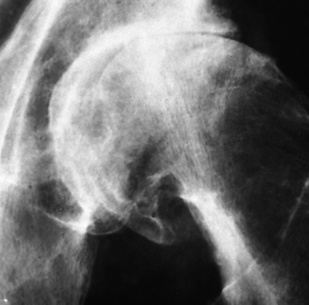
Radiographic Appearance
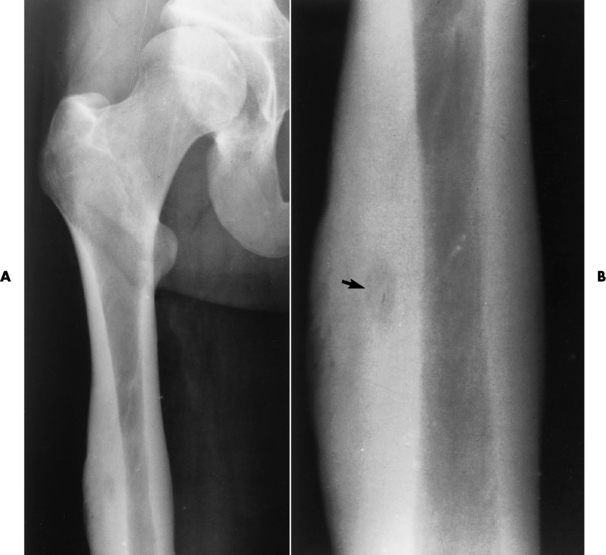
The femoral head is the most common site of ischemic necrosis. Initially, the ischemic bone may appear denser than adjacent viable bone. The first sign of structural failure is the development of a radiolucent subcortical band (the crescent sign) representing a fracture line (Figure 4-60A). As the disorder progresses, fragmentation, compression, and resorption of dead bone, along with proliferation of granulation tissue, revascularization, and production of new bone, produce a pattern of lytic and sclerotic areas with flattening of the femoral head and periosteal new bone formation (Figure 4-60B). Mechanical distortion about the hip leads to uneven weight bearing and accelerated secondary osteoarthritis.
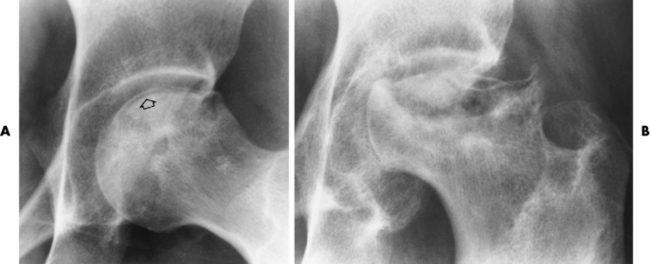
Figure 4-60 Ischemic necrosis of the femoral head. A, An arclike radiolucent cortical band (the crescent sign) (arrow) in the femoral head represents the fracture line. B, Eventually there is combination of lytic and sclerotic areas with severe flattening of the femoral head.
It is often necessary to obtain two radiographs in patients with ischemic necrosis. The first is taken with normal density, whereas the second is made with an increased kVp to allow for adequate penetration of the more opaque sclerotic ischemic bone.
Radionuclide bone scanning is more sensitive than plain radiography for detecting changes of ischemic necrosis. In the initial stages of infarction, radionuclide activity is absent in the area of involvement. As the disease progresses, this area of decreased activity may become rimmed by a zone of increased activity as a result of the reparative process.
MRI may be even more sensitive than radionuclide bone scanning for detecting ischemic necrosis. Infarction at the end of a bone causes changes in the fatty marrow, sharply reducing the normally intense T1-weighted signal from the marrow so that the infarcted area becomes gray or black (Figure 4-61).
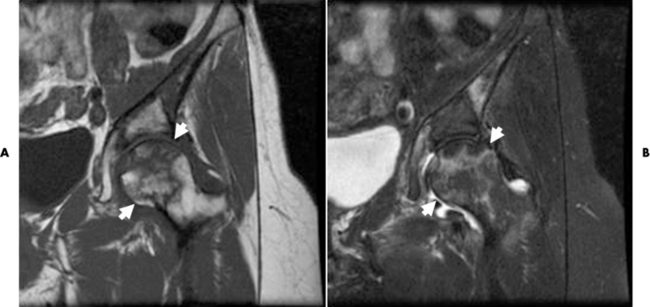
Figure 4-61 Ischemic necrosis. On this T1-weighted image, (A) the ischemic right femoral head has lost its normal bright homogenous signal intensity and appears dark and mottled (arrow), and an (B) the T2-weighted image of the affected area appears as a bright signal intensity, representing edema.
In addition to ischemic changes in the subchondral areas of bone, infarction may involve the shaft of a long bone. In many cases, this type of bone infarction is asymptomatic and is detected only on radiographs obtained for another purpose. A mature infarct in the shaft of a bone appears as a densely calcified
area in the medullary cavity (Figure 4-62). It may be sharply limited by a dense sclerotic zone or associated with dense streaks extending from the central region. Bone infarction must be differentiated from a calcified enchondroma, which contains amorphous, spotty calcific densities; is not surrounded by a sclerotic rim; and may expand the bone.
Benign bone tumors
Benign bone neoplasms generally displace soft tissue, whereas malignant bone tumors produce true soft tissue swelling. When there is bone expansion, an intact cortex with a sclerotic margin usually indicates a benign lesion. Benign bone neoplasms occur much less often than bone metastases.
Radiographic Appearance
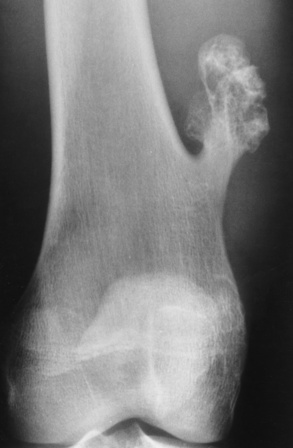
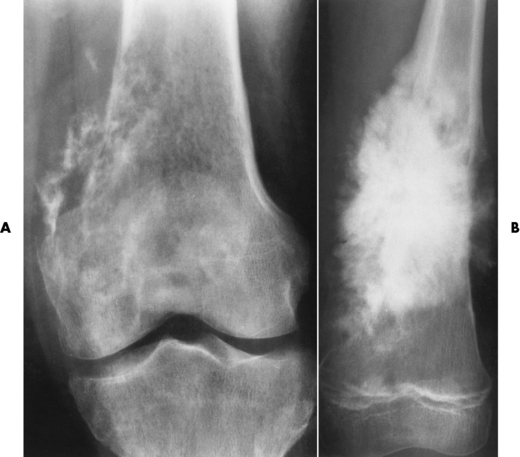
Osteochondroma (exostosis) is a benign projection of bone with a cartilaginous cap that arises in childhood or the teen years, especially about the knee. The exostosis occurs in the epiphyseal plate (the ring of Ranvier) and grows laterally from the epiphysis. A true osteochondroma must exhibit the cortex and medullary portion (spongiosa) as continuous bone growth. The cartilaginous cap of the lesion may convert to a malignancy if the cartilage cap becomes thicker and contains disorganized calcifications (chondrosarcoma or osteosarcoma). Ultrasound is a safe, quick, and inexpensive method to evaluate the thickness of the cartilaginous cap. A hereditary form of osteochondroma (autosomal dominant) produces multiple lesions and has an increased risk of malignancy.
A radiograph is the preferred method to demonstrate that the cortex of an osteochondroma blends with that of normal bone. The long axis of the tumor characteristically runs parallel to the parent bone and points away from the nearest joint (Figure 4-63). The best modality to demonstrate the thickness of the cartilaginous cap and thus rule out malignant conversion is MRI with long TR pulses (increased signal). On T2-weighted spin-echo images, the cartilaginous cap produces a high signal intensity.
Enchondromas
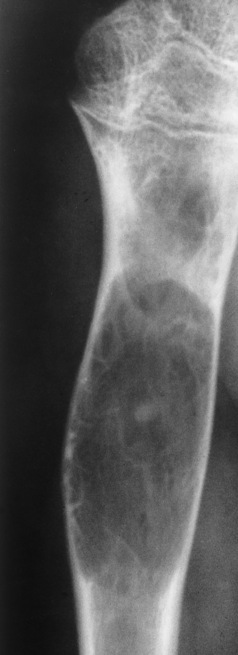
Enchondroma begins as a slow-growing benign cartilaginous tumors arising in the medullary canal (ectopic cartilaginous growth). This tumor destroys normal bone by erupting as a mixture of calcified and uncalcified hyaline cartilage. Enchondromas are most frequently found in children and young adults, and they involve primarily the small bones of the hands and feet. These tumors are often multiple.
As the well-demarcated tumor grows, it expands bone locally, causing thinning and endosteal scalloping of the cortex. This process often leads to a pathologic fracture with only minimal trauma (Figure 4-64). A characteristic finding in enchondroma is stippled, speckled, and ringlike or arclike calcifications within the lucent matrix because of the tumor composition. This radiographic appearance is also seen on CT images.
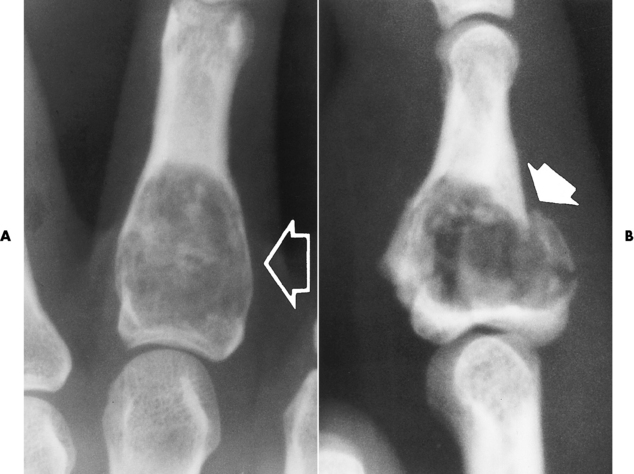
Figure 4-64 Enchondroma. A, A well-demarcated tumor (arrow) expands the bone and thins the cortex. B, Pathologic fracture (arrow).
T2-weighted MR images demonstrate a lesion having lobulated borders from the endosteal scalloping and containing focal areas of high signal intensity. On T1-weighted images, enchondromas have low to intermediate signal intensity.
Nuclear medicine bone scan findings are usually negative in cases of enchondromas, ruling out the possibility of malignancy.
Giant Cell Tumors
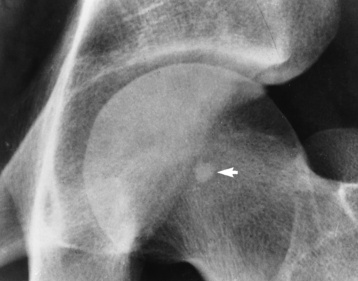
Giant cell tumor (osteoclastoma) typically arises at the end of the distal femur or proximal tibia of a young adult after epiphyseal closure (20 to 40 years old). It begins as an eccentric lucent lesion in the metaphysis, and it characteristically extends to the immediate subarticular cortex of the bone but does not involve the joint (Figure 4-65). MRI is used to determine intraarticular extension, soft tissue involvement, and bone marrow changes. When these results are combined with radiographic images, the accuracy is especially high. A few giant cell tumors may be premalignant or actually malignant.
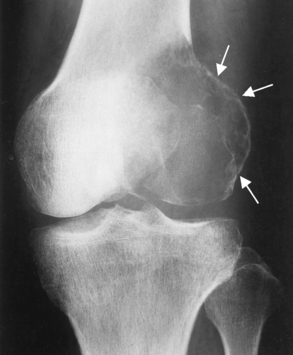
Figure 4-65 Giant cell tumor. Typical eccentric lucent lesion (arrows) in distal femoral metaphysis extends to the immediate subarticular cortex. Surrounding cortex, although thinned, remains intact.
As the tumor expands toward the shaft, it produces the characteristic radiographic appearance of multiple large bubbles separated by thin strips of bone.
Osteomas
Osteomas most often arise in the outer table of the skull, the paranasal sinuses (especially frontal and ethmoid), and the mandible. Detection of these tumors may be incidental on radiographs taken because of the pain produced by bone expansion.
Osteomas appear radiographically as well-circumscribed, extremely dense, round lesions that are rarely more than 2 cm in diameter (Figure 4-66).
Osteoid Osteomas
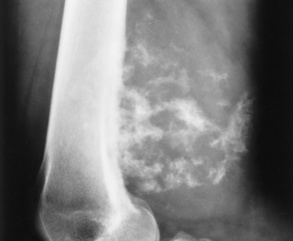
Osteoid osteoma usually develops in teenagers or young adults and produces the classic clinical symptom of local pain that is worse at night and is dramatically relieved by aspirin. Most osteoid osteomas occur in the femur and tibia, originate from osteoblastic cells, and are less than 1.5 cm in diameter. A radionuclide bone scan can help differentiate osteoid osteoma from osteomyelitis; osteoid osteoma has a double density, whereas osteomyelitis demonstrates only an outline because of the avascularity of the inner structure. Preoperatively, nuclear medicine studies localize the tumor for complete excision.
The tumor is typically seen as a small, round or oval, lucent center (the nidus), less than 1 cm in diameter, that is surrounded by a large, dense sclerotic zone of cortical thickening (Figure 4-67). The nidus may not be visualized, depending on the bone structure involved (cortical versus cancellous). Because this dense reaction may obscure the nidus on conventional radiographs, overpenetrated images or tomography (conventional or computed) may be necessary to demonstrate it. CT is commonly used if there is the need to demonstrate the central radiolucent nidus for percutaneous ablation. The nidus enhances following administration of contrast material. T1-weighted MR images demonstrate the nidus as isoechoic to muscle (same intensity as muscle). CT is considered the definitive diagnostic modality.
Simple Bone Cysts
A simple bone cyst (unicameral) is a true fluid-filled cyst with a wall of fibrous tissue that most often occurs in the proximal humerus or femur at the metaphysis. Although not a true neoplasm, a simple bone cyst may resemble one radiographically and clinically. Solitary bone cysts are asymptomatic and often discovered either incidentally or after pathologic fracture.
The simple bone cyst appears as an expansile lucent lesion that is sharply demarcated from adjacent normal bone and may have a thin rim of sclerosis around it. It has an oval configuration, with its long axis parallel to that of the host bone, and may cause cortical bone thinning (Figure 4-68). Its appearance on CT is very similar to that on radiographs, so CT is usually unnecessary. Simple bone cysts produce low signal intensity on T1-weighted MR images (high signal intensity on T2-weighted images). To aid in determining proper treatment, MRI determines whether septations exist that may not be visible on the radiograph.
Aneurysmal Bone Cysts
An aneurysmal bone cyst, rather than being a true neoplasm or cyst, consists of numerous blood-filled arteriovenous communications thought to be caused by trauma.
In long bones, an aneurysmal bone cyst is an expansile, eccentric, cystlike lesion that causes pronounced ballooning of the thinned cortex (Figure 4-69). In some cases, the cystlike lesion may have internal separations. At times, the cortex may be so thin that it is invisible on plain radiographs, and thus this benign lesion may be mistaken for a malignant bone tumor. A CT scan may assist in defining the well-lobulated lesion, but MRI better shows the internal loculations and fluid levels that produce low signal intensity on T2-weighted images. On T1-weighted images, the cyst has a low to intermediate signal intensity but contains high signal intensity if there has been acute hemorrhage.
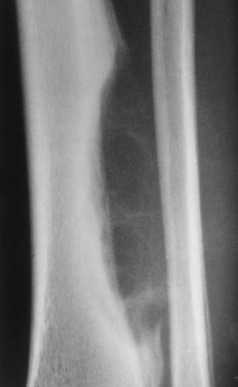
Figure 4-69 Aneurysmal bone cyst. This expansile, eccentric, cystic lesion of the tibia has multiple fine internal septa. Because severely thinned cortex is difficult to detect, the tumor resembles a malignant process.
Bone islands are solitary, sharply demarcated areas of dense compact bone that occur most commonly in the pelvis and upper femur. They appear in every bone except the skull (Figure 4-70). Although almost half of the bone islands enlarge over a period of years and many show activity on radionuclide scans, bone islands are asymptomatic and completely benign and must be distinguished from osteoblastic metastases.
Treatment of Benign Bone Tumors
An osteochondroma requires treatment only when mechanical friction (joint mobility) impinges on nerves, blood vessels, or muscles. For an enchondroma, curettage of the lesion is the common treatment. The treatment for giant cell tumor includes curettage of the bone and local resection. About 50% of giant cell tumors recur. Surgical excision of the nidus of an osteoid osteoma is essential for cure; it is not necessary to remove the reactive
calcification, although it may form the major part of the lesion. CT-guided percutaneous radiofrequency ablation (destruction) may provide the best treatment. Simple bone cyst treatment depends on the size and location of the cyst. If the cyst continues to develop, surgical curettage and implantation of bone chips is performed. Aneurysmal bone cysts require curettage to prevent further destruction from occurring. Bone islands require no treatment.
Malignant bone tumors
Malignant bone neoplasms generally cause soft tissue swelling and cortical bone erosion with a margin that is poorly defined or absent. The neoplasm extends into the soft tissue through spiculations (finger-like projections). Plain radiographs may identify a single lesion. A radionuclide bone scan or (PET) scan can detect silent lesions when minimal cellular destruction has occurred. Discovering additional lesions determines the treatment and prognosis. CT and MRI can precisely define the location of a malignant bone tumor and its extension into the medullary cavity and spread to surrounding structures (Figures 4-71 and 4-72).
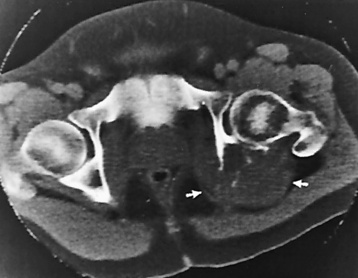
Figure 4-71 Osteogenic sarcoma. CT scan through the level of the femoral head shows destruction of the left ischium, which was seen on plain radiographs. In addition, the CT scan demonstrates a large soft tissue mass (arrows) in the area covered by gluteus maximus muscle and separated from rectum. The mass was not clinically palpable.
Figure 4-72 MRI of osteogenic sarcoma. A coronal image shows the intramedullary and soft tissue extent of the mass.
Radiographic Appearance
Osteogenic sarcoma generally occurs in the end of a long bone in the metaphysis (especially about the knee). This tumor consists of osteoblasts, which produce osteoid and spicules of calcified bone. Most osteogenic sarcomas arise in persons between 10 and 25 years old, although a smaller peak incidence is seen in older persons who have a preexisting bone disorder, particularly Paget’s disease. The usual initial complaints are local pain and swelling, sometimes followed by fever, weight loss, and secondary anemia. Pulmonary metastases develop early, and a plain chest radiograph should be obtained to exclude this unfavorable prognostic sign. If no metastases to the lung are detected by this modality, CT or PET should be performed.
The typical radiographic appearance of osteogenic sarcoma is a mixed destructive and sclerotic lesion associated with a soft tissue mass, irregular periosteal reaction, and reactive new bone formation (Figure 4-73A). In the classic sunburst pattern, horizontal bony spicules extend in radiating fashion into the soft tissue mass (Figure 4-73B). A characteristic finding is elevation of the periosteum at the periphery of a lesion, with subsequent new bone formation (Codman’s triangle) (Figure 4-74). CT scans are beneficial in demonstrating this finding in flat bones, where periosteal change is more difficult to visualize. MRI of the entire length of the bone is important to rule out skip lesions, and this modality is more accurate to visualize the tissue extension and medullary involvement. To determine the longitudinal involvement, specifically in the medullary cavity, T1-weighted images using a spin-echo sequence are best. The short-tau inversion recovery (STIR) sequence produces high-intensity signals. Both signal sequences are required because the T1-weighted images are slightly more specific, whereas the STIR sequence produces images that are slightly more sensitive.
Chondrosarcoma
Chondrosarcoma is a malignant tumor of cartilaginous origin that may originate anew or within a preexisting cartilaginous lesion (e.g., osteochondroma and enchondroma). Tumor grading of this particular neoplasm depends on the maturity and differentiation of the cells. Chondrosarcomas commonly occur in long bones, but often originate in a rib, scapula, or vertebra. The tumor is about half as common as osteogenic sarcoma; it develops at a later age (peak incidence in 35- to 60-year-olds), grows more slowly, and metastasizes later.
In addition to the bone destruction seen with all malignant tumors, chondrosarcoma often contains punctate or amorphous calcification within its cartilaginous matrix (Figure 4-75). On CT, as on radiographs, chondrosarcomas demonstrate endosteal scalloping and cortical destruction. To evaluate for possible pulmonary metastases, CT is the modality of choice. On T2-weighted MR images, the lobulated lesions have high signal intensity, whereas the septations appear as low signal intensity.
Ewing’s Sarcoma
Ewing’s sarcoma is a primary malignant tumor arising in the bone marrow of long bones. A tumor of children and young adults, Ewing’s sarcoma has a peak incidence in the midteens and is rare in persons over 30 years of age. The major clinical complaint is local pain, often of several months’ duration, that persistently increases in severity and may be associated with a tender soft tissue mass. Patients with this tumor characteristically have malaise and appear sick, often with fever and leukocytosis, suggestive of osteomyelitis.
The classic radiographic appearance of Ewing’s sarcoma is an ill-defined permeative area of bone destruction that involves a large central portion of the shaft of a long bone (underlying medullary destruction) and is associated with a fusiform layered periosteal reaction (onionskin appearance) parallel to the shaft (Figure 4-76). Tumor cells invade the cortical bone and spread into the soft tissue. For staging and follow-up, MRI is the modality of choice. MRI best demonstrates the soft tissue involvement on T1-weighted images, where the tumor has a low signal intensity when compared with normal bone marrow. On T2-wighted imaging, the tumor has a higher signal intensity than muscle, so fat-saturation sequences are best (STIR sequences provide an alternative when fat saturation is unavailable).
Multiple Myeloma
Multiple myeloma is a disseminated (widespread) malignancy of plasma cells that may be associated with bone destruction, bone marrow failure, hypercalcemia, renal failure, and recurrent infections. The disease affects primarily persons between 40 and 70 years of age. This frequently occurring primary bone tumor attacks the intramedullary canal of the diaphysis. Typical laboratory findings include an abnormal spike of monoclonal immunoglobulin and the presence of Bence Jones protein in the urine.
The classic radiographic appearance of multiple myeloma is multiple punched out osteolytic lesions scattered throughout the skeletal system and best seen on lateral views of the skull (Figures 4-77 and 4-78). Because the bone destruction is attributable to the proliferation of plasma cells distributed throughout the bone marrow, the flat bones containing red marrow (vertebrae, skull, ribs, pelvis) are primarily affected. The appearance may be indistinguishable from that of a metastatic carcinoma, although the lytic defects in multiple myeloma tend to be more discrete and uniform in size. Extensive plasma cell proliferation in the bone marrow with no tendency to form discrete tumor masses may produce generalized skeletal deossifications simulating postmenopausal osteoporosis. In the spine, decreased bone density and destructive changes in multiple myeloma are usually limited to the vertebral bodies, sparing the pedicles (which lack red marrow and are frequently destroyed by metastatic disease). The severe loss of bone substance in the spine often results in multiple vertebral compression fractures (Figure 4-79).
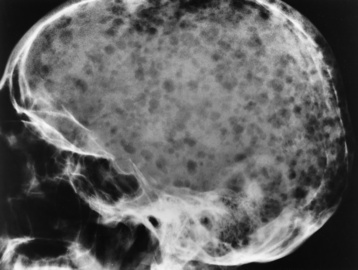
Figure 4-77 Multiple myeloma. Diffuse punched-out osteolytic lesions scattered throughout the skull.
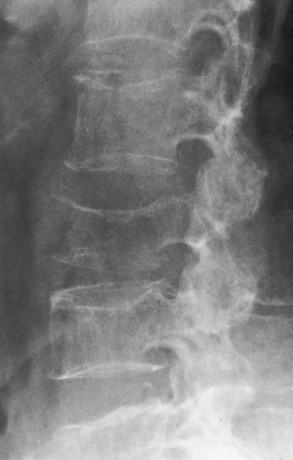
Figure 4-79 Multiple myeloma. Diffuse myelomatous infiltration has caused generalized demineralization of the vertebral bodies and compression fracture of L2.
Because multiple myeloma causes little or no stimulation of new bone formation, radionuclide bone scans may be normal even with extensive skeletal infiltration. On T1-weighted MR images, low-intensity tumor tissue replaces the normal high-intensity fatty marrow.
Treatment of Malignant Bone Tumors
The treatment of osteogenic sarcoma includes surgical removal with or without chemotherapy and/or radiation therapy. Although osteogenic sarcoma is usually fatal, about 30% of patients receiving chemotherapy are cured. For chondrosarcoma lesions, the treatment usually includes surgical excision. Because of poor response, chemotherapy and radiation therapy are not suggested. The more differentiated the involved cells, the higher the survival rate. Chondrosarcoma tumors are not sensitive to chemotherapy. Ewing’s sarcoma and multiple myeloma are treated with radiation therapy and chemotherapy. Chemotherapy for Ewing’s sarcoma increases the survival rate to about 30%. Multiple myeloma patients have a grim prognosis, most dying within 3 to 4 years. PET imaging helps to determine therapy effectiveness by demonstrating active tumor metastases in multiple myeloma (Figure 4-80).
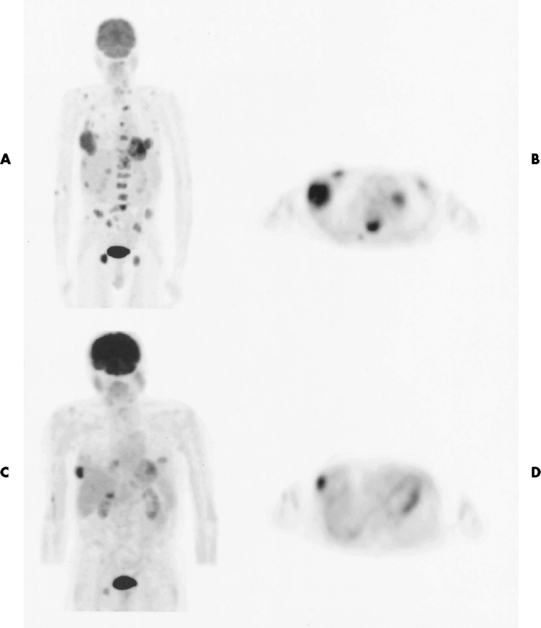
Figure 4-80 Multiple myeloma. The PET oncologic survey findings are positive, with a pattern of extensive metabolically active tumor metastases demonstrated throughout the axial skeleton and in both lower lung fields on coronal (A) and transverse (B) images. After 4 months, when the patient is in his last cycle of chemotherapy, the survey images (C and D) still demonstrate persistent, viable, metabolically active neoplasm. However, a dramatic improvement in the overall pattern in comparison with the earlier scan is seen.
Bone metastases
Metastases are the most common malignant bone tumors, spreading by means of the bloodstream or lymphatic vessels or by direct extension. The most common primary tumors are carcinomas of the breast, lung, prostate, kidney, and thyroid. Favorite sites of metastatic spread are bones containing red marrow, such as
the spine, pelvis, ribs, skull, and the upper ends of the humerus and femur. Metastases distal to the knees and elbows are infrequent but do occur, especially with bronchogenic (lung) tumors.
The detection of skeletal metastases is critical in the management of patients with known or suspected neoplastic disease, both at the time of initial staging and during the period of continuing follow-up care. The presence of metastases may exclude some patients from the radical “curative” therapy that is offered to those without disseminated disease, thus sparing them from fruitless, high-morbidity procedures for a nonremediable condition.
The best screening examination for the detection of asymptomatic skeletal metastases is the radionuclide bone scan (Figure 4-81) or the PET scan (Figure 4-82), which is unquestionably more sensitive than the radiographic skeletal survey. Because almost half the mineral content of a bone must be lost before the loss is detectable on plain radiographs, the skeletal survey should be abandoned as a general screening examination for the detection of asymptomatic skeletal metastases. False-negative findings on bone scans occur only with aggressively osteolytic lesions, especially in patients with multiple myeloma. The role of plain radiographs in screening for metastases is to further evaluate focal abnormalities detected on radionuclide scanning because a variety of lesions (e.g., infections, benign tumors, fibrous dysplasia, and bone islands) can also show positive findings on bone scans. Because the presence of multiple focal abnormalities is typical of metastatic disease, it is necessary to radiographically examine only a single lesion or area to confirm the diagnosis. In addition, because of the occasional false-negative results on a bone scan, it is imperative that all symptomatic sites in patients with neoplastic disease be examined by plain radiographs unless the radionuclide bone scan unequivocally demonstrates diffuse metastatic disease.
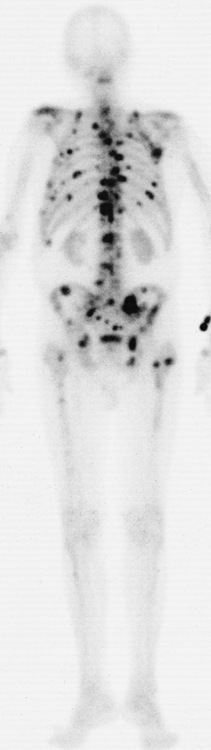
Figure 4-81 Screening radionuclide bone scan. Multiple focal areas of radionuclide uptake in the axial skeleton, representing metastases from prostate carcinoma.
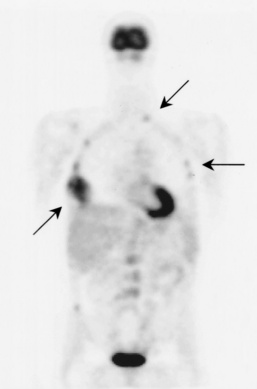
Figure 4-82 Fluorodeoxyglucose-PET scan showing metastases. Arrows point to metabolic activity in the skeletal system (ribs).
Radiographic Appearance
Metastatic disease may produce a broad spectrum of radiographic appearances: osteolytic, sclerotic, or mixed. Osteolytic metastases cause destruction without accompanying bone proliferation. They develop from tumor embolic deposits in the medullary canal and eventually extend to destroy cortical bone. The margins of the lucent lesions are irregular and poorly defined, rarely sharp and smooth. The most common primary lesions causing osteolytic metastases are carcinomas of the breast, kidney, and thyroid. Metastases from carcinomas of the kidney and thyroid typically produce a single large metastatic focus that may appear as an expansile trabeculated lesion (a “blowout”) (Figure 4-83). Metastases from breast carcinoma are most often multiple when first detected.
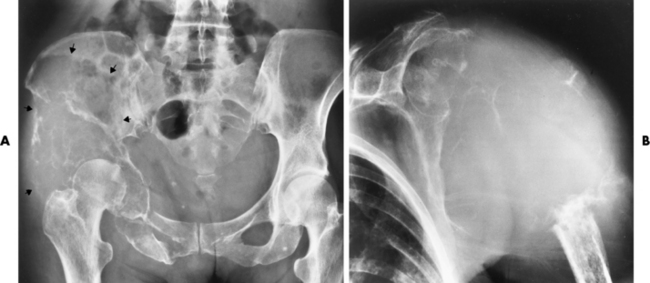
Figure 4-83 Blowout metastases. A, Lytic expansile destruction of the left ilium (arrows) in a patient with metastatic thyroid carcinoma. B, Osteolytic metastasis to the humerus from carcinoma of the kidney.
In the spine, osteolytic metastases tend to involve not only the vertebral bodies but also the pedicles and posterior arches. Destruction of one or more pedicles may be the earliest sign of metastatic disease and may help in differentiating this process from multiple myeloma, in which the pedicles are much less often involved. Because cartilage is resistant to invasion by metastases, preservation of the intervertebral disk space may help to distinguish metastases from an inflammatory process. Pathologic collapse of vertebral bodies frequently occurs in advanced disease.
Osteoblastic metastases are generally considered evidence of slow growth in a neoplasm that has allowed time for a proliferation of reactive bone. In men, osteoblastic metastases are usually a result of carcinoma of the prostate gland; carcinoma of the breast is the most common primary site of osteoblastic metastases in women. These lesions initially appear as ill-defined areas of increased density that may progress to complete loss of normal bony architecture. They may vary from small, isolated round foci of sclerotic density to a diffuse sclerosis involving most or all of a bone (Figure 4-84). In the spine, this may produce the characteristic uniform density of an “ivory” vertebral body (Figure 4-85).
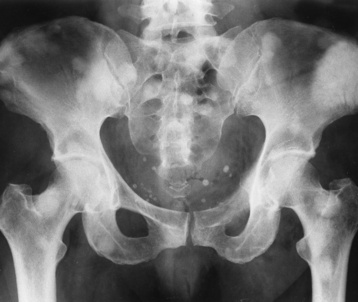
Figure 4-84 Osteoblastic metastases. Multiple areas of increased density involving the pelvis and proximal femurs, representing metastases from carcinoma of the urinary bladder.
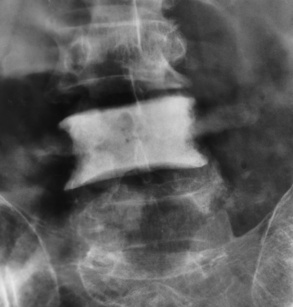
Figure 4-85 Ivory vertebra. Diffuse sclerosis of the L4 vertebral body from metastatic carcinoma of the prostate.
The combination of destruction and sclerosis in the mixed type of metastasis causes the affected bone to have a mottled appearance, with intermixed areas of lucency and increased density.
Bone destruction by metastases and associated extension of tumor in the adjacent soft tissues can be well demonstrated by CT and MRI (Figure 4-86). A PET scan can quickly determine if the patient has a solitary metastatic lesion or multiple lesions. New fusion technology (superimposition of two imaging modality scans, such as PET and MRI) can further define the number, size, and location of metastatic lesions, influencing the type of treatment best suited for the patient.
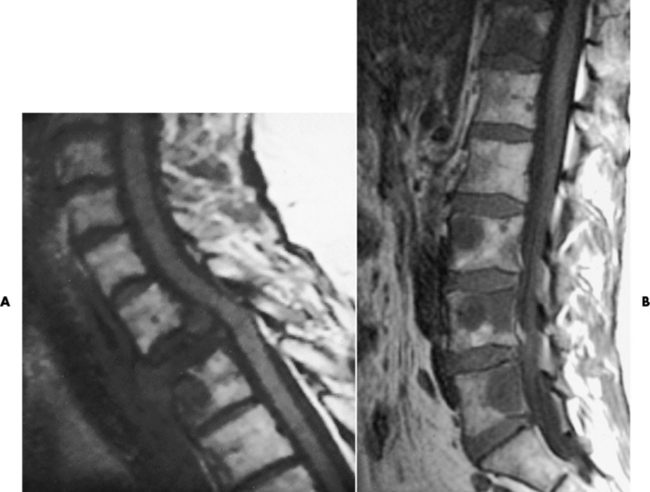
Figure 4-86 A, MR image of metastases. Sagittal image shows gross metastatic destruction and replacement of the body of T2, which has collapsed. The metastasis has a lower signal intensity than that of vertebral marrow or the spinal cord. The spinal cord is focally indented and displaced posteriorly by the tumor. Also, a low-signal-intensity lytic metastasis involves the anterior portion of T3. B, Vertebral metastasis from breast carcinoma. Many lytic lesions are evident on this MR image of the lumbar region.
Fractures
Fractures are the most common skeletal abnormality seen in a general radiology practice. A fracture is defined as a disruption of bone caused by mechanical forces applied either directly to the bone or transmitted along the shaft of a bone. Although often obvious, some fractures are subtle and difficult to detect. A fracture typically appears as a radiolucent line crossing the bone and disrupting the cortical margins. However, the fracture line may be thin and easily overlooked, whereas overlap of fragments may produce a radiopaque line. Secondary signs of an underlying fracture include joint effusion, soft tissue swelling, and interruption of the normal pattern of bony trabeculae.
Types of Fractures
Fractures are described and classified by their extent, direction, and position; the number of fracture lines; and the integrity of the overlying skin (Figure 4-87). A fracture that results in discontinuity between two or more fragments is a complete fracture; an incomplete fracture causes only partial discontinuity, with a portion of the cortex remaining intact. In closed fractures, the overlying skin is intact; if the overlying skin is disrupted, the fracture is open, or compound. Although it is a clinical distinction, the radiographic demonstration of bone clearly protruding through the skin and the presence of air in soft tissues about the fracture site on radiographs obtained immediately after the injury are highly suggestive of an open fracture.
The direction of a fracture is determined by its relationship to the long axis of long and short bones and to the longest axis of irregular bones (e.g., the talus or carpal navicular). A transverse fracture runs at a right angle to the long axis of a bone and most commonly results from a direct blow or is a fracture within pathologic bone. An oblique fracture runs a course of approximately 45 degrees to the long axis of the bone and is caused by angulation or by both angulation and compression forces. A spiral fracture encircles the shaft, is generally longer than an oblique fracture, and is caused by torsional forces. Avulsion fractures are generally small fragments torn from bony prominences; they are usually the result of indirectly applied tension forces within attached ligaments and tendons rather than direct blows.
A comminuted fracture is composed of more than two fragments. A butterfly fragment is an elongated triangular fragment of cortical bone generally detached from two other larger fragments of bone. A segmental fracture consists of a segment of the shaft isolated by proximal and distal lines of fracture.
A compression fracture results from a compression force that causes compaction of bone trabeculae and results in decreased length or width of a portion of a bone. Compression fractures most commonly occur in the vertebral body as a result of flexion of the spine; they may also be seen as impacted fractures of the humeral or femoral heads. A depressed fracture occurs in the skull or tibial plateau. In the skull, a small object with great force can produce a comminuted fracture, with portions of the fracture fragments driven inward. In the knee, the relatively hard lateral femoral condyle may impact on the relatively soft lateral tibial plateau with sufficient force to push the cortical surface of the tibia into the underlying cancellous bone.
A stress, or fatigue, fracture is the response of bone to repeated stresses, none of which is sufficient to cause a fracture. The earliest pathologic process in a stress fracture is osteoclastic resorption, followed by the development of periosteal callus in an attempt to repair and strengthen the bone. A pathologic fracture occurs in bone at an area of weakness caused by a process such as tumor, infection, or metabolic bone disease.
A greenstick fracture is an incomplete fracture with the opposite cortex intact. Greenstick fractures are found almost exclusively in infants and children because of the softness of their cancellous bone. A torus (buckle) fracture is one in which one cortex is intact with buckling or compaction of the opposite cortex. A bowing fracture is a plastic deformation caused by a stress that is too great to permit a complete recovery of normal shape but is less than the stress required to produce a fracture.
An undisplaced fracture occurs when a plane of cleavage exists in the bone without angulation or separation. Displacement refers to separation of bone fragments; the direction of displacement describes the relationship of the distal fragment with respect to the proximal fragment and is usually measured in terms of the thickness of the shaft. Angulation indicates an angular deformity between the axes of the major fragments and also describes the position of the distal fragment with respect to the proximal one. Dislocation refers to the displacement of a bone that is no longer in contact with its normal articulation. If there is only partial loss of continuity of the joint surfaces, the displacement is called a subluxation.
Radiographic Appearance
Radiographs are essential in the diagnosis and management of fractures. Initially, a radiograph documents the clinically suspected fracture and determines whether the underlying bone is normal or whether the fracture is pathologic and has occurred in abnormal bone. After the orthopedic reduction of a fracture, a second set of radiographs determines whether the fracture fragments are in anatomic position. Over the next several weeks or months, additional radiographs are obtained to assess fracture healing and to exclude possible complications.
In all cases of trauma, it is essential to have at least two projections of the injured part, preferably taken at 90 degrees to each other, to determine fracture continuity or displacement in the anterior or posterior, medial or lateral, and superior or inferior direction. It is also important to demonstrate the joint above and below the fracture to search for a dislocation or a second fracture that may have resulted from transmission of the mechanical force. An example of this mechanism is the fracture or dislocation of the head of the fibula that frequently occurs with a fracture of the distal part of the tibia at the ankle.
Treatment
The overall goal of fracture treatment is to restore function and stability with an acceptable cosmetic result and a minimum of residual deformity. In external, or closed, reduction, the fracture is treated by manipulation of the affected body part without surgical incision. Open reduction is a surgical procedure using direct or indirect manipulation of the fracture fragments and usually involving the application or insertion of some type of appliance or device to achieve and maintain the reduction (Figure 4-88). External fixation is accomplished with the use of splints, external reduction devices, or casts (Figure 4-89); internal fixation uses metal plates and screws, wires, rods, and nails, either alone or in combination, to maintain the reduction (Figure 4-90).
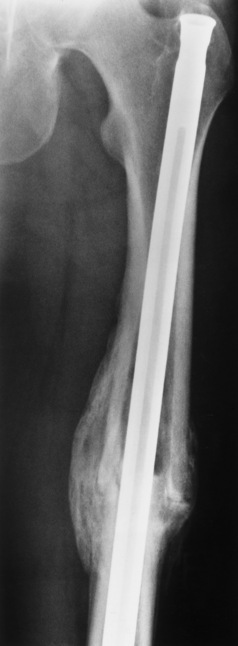
Figure 4-88 Open reduction with internal fixation. An intramedullary rod has been placed across a fracture of the femoral shaft. Note the extensive callus formation about the fracture site.
Most reduced fractures are immobilized or protected by an overlying cast. Fiberglass casting material causes less attenuation than plaster and thus produces less artifact. The radiopaque cast causes some obscuration of fine bony detail and, in severely osteoporotic bone, may make it difficult to visualize the fracture site. Therefore, if there is a question of healing that requires the demonstration of early callus formation or if there is a possibility of osteomyelitis, it is essential that the cast be removed by the physician before radiographs are obtained so that there is sufficient visibility of bone detail to resolve the questions. The radiographer must increase exposure factors to compensate for the use of plaster or fiberglass casting and whether the cast is wet or dry. Depending on the resource, some authorities recommend an increase in mAs, whereas others indicate an increase in kVp; it is recommended that one follow department protocol to produce the best images possible.
Fracture Healing
The radiographic evidence of fracture healing is a continuous external bridge of callus (calcium deposition) (Figure 4-91) that extends across the line of fracture and unites the fracture fragments (Figures 4-92 and 4-93). The callus uniformly ossifies and approaches the density of normal bone. It is essential that at least two views be taken (preferably 90 degrees to each other) to ensure that there is callus about the fracture line in all directions. Proper exposure of the radiograph is required because underexposed images may produce the illusion of obliteration of the fracture line by bony trabeculae, whereas a properly exposed image would demonstrate the continued presence of the fracture line and a lack of healing. If the findings are equivocal, either CT or conventional tomography may be required to determine the degree of union. “Stress” images, a series of radiographs obtained with the injured part in the neutral position and during the application of stress by a physician or designated assistant on the distal fragment or part in the plane of suspected motion, may demonstrate a change in the alignment of the fragment, which indicates a lack of union.
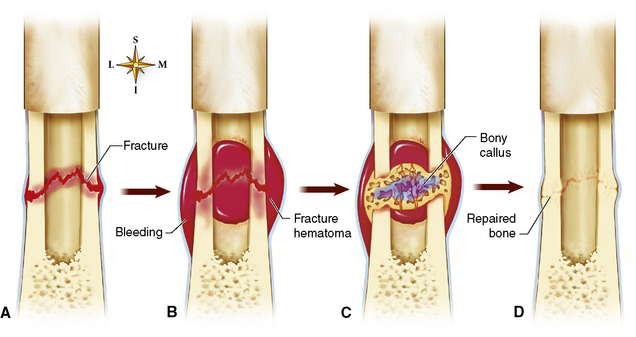
Figure 4-91 Bone fracture healing. A, Fracture of the femur B, Formation of a fracture hematoma. C, Formation of internal and external bony callus. D, Bone remodeling complete.
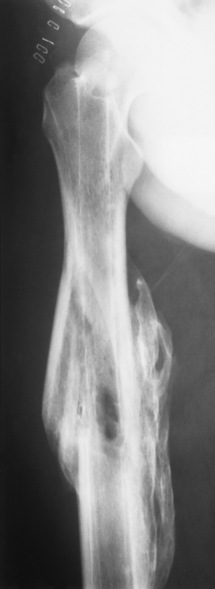
Figure 4-92 Normal union of a fracture. There is dense callus formation bridging the previous fracture of the midshaft of the femur. The original fracture line is completely obliterated.
Malunion is the healing of fracture fragments in a faulty position. It leads to impairment of normal function or a cosmetic appearance that may require surgical correction (Figure 4-94).
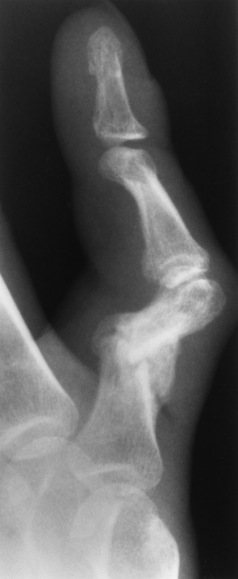
Figure 4-94 Malunion. Healing of proximal phalangeal fracture in poor position led to impairment of normal function.
Delayed union is an ill-defined term arbitrarily applied to any fracture that takes longer to heal than the average fracture at that anatomic location. Delayed union may result from infection, from inadequate immobilization, from limited blood supply, or from loss of bone at the fracture site.
Nonunion refers to a condition in which the fracture healing process has completely stopped and the fragments remain ununited even with prolonged immobilization. Radiographically, nonunion characteristically appears as smooth, well-defined sclerosis about the fracture margins with occlusion of the medullary canal by sclerotic bone (Figure 4-95). A persistent defect, consisting of fibrous tissue and cartilage, appears between the fragments. Nonunion occurs predominantly in adults, is rare in children, and requires operative intervention to reinitiate the healing process.
Pathologic Fractures
Pathologic fractures are those occurring in bone that has been weakened by a preexisting condition. The most common underlying process is metastatic malignancy or multiple myeloma. In children, developmental diseases, such as osteogenesis imperfecta and osteopetrosis, nutritional deficiencies (rickets, scurvy) may result in pathologic fractures. Pathologic fractures also may occur when there is a benign cause of weakened bone, such as simple bone cyst, enchondroma, aneurysmal bone cyst, or fibrous dysplasia. Metabolic disorders causing a diffuse loss of bone substance (osteoporosis, osteomalacia, hyperparathyroidism) also make the skeleton more susceptible to injury.
Radiographic Appearance
Clinically, pathologic fractures arise from minor trauma that would not affect normal bone. Radiographically, the fracture crosses an area of abnormal thinning, expansion, or bone destruction (Figure 4-96). The most common sites of pathologic fractures are the spine, femur, and humerus, areas in which metastatic disease is most common. In the spine, a pathologic fracture results in collapse of the vertebral body; indeed, a compressed vertebra in a patient older than 40 years should indicate underlying myeloma or metastatic disease.
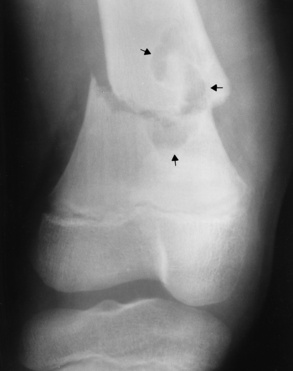
Figure 4-96 Pathologic fracture. A transverse fracture crosses the large benign tumor (arrows) of the distal femur.
CT or MRI may detect a subtle change in the abnormal bone that is obscured by an abnormal lytic area or by sclerotic changes on plain radiographs.
The radiographer must remember that patients with suspected pathologic fractures must be handled with extreme care to avoid causing either further injury to the bone in question or an additional pathologic fracture in another area.
Treatment
Treatment of a pathologic fracture varies depending on the cause. Patients with malignant causes of fracture receive palliative therapy with possible surgery to place an orthotic fixation device, joint replacement, or amputation. For benign lytic lesions (cysts), surgical bone grafts or implantation of bone chips may be required to increase bone strength and structure.
Stress Fractures
Stress (fatigue) fractures are the result of repeated stresses to a bone that would not be injured by isolated forces of the same magnitude. The type of stress fracture and the site where it occurs vary with the activity. Regardless of location, the activities resulting in stress fractures are usually strenuous, often new or different, and repeated with frequency before producing pain. Stress fracture frequently occurs in soldiers during basic training (“march” fracture). The most common sites are the shafts of the second and third metatarsals, the calcaneus, the proximal and distal shafts of the tibia and fibula, the shaft and neck of the femur, and the ischial and pubic rami.
Radiographic Appearance
Initially, findings on plain radiographs of the symptomatic area are within normal limits (Figure 4-97). The stress fracture is first visualized 10 to 20 days after the onset of symptoms as either a thin line of transverse or occasionally oblique radiolucency or as fluffy periosteal callus formation without evidence of a fracture line. When this radiographic appearance is detected at a site common to stress fractures, a history of athletic or other unusual activity should be elicited as the underlying cause.
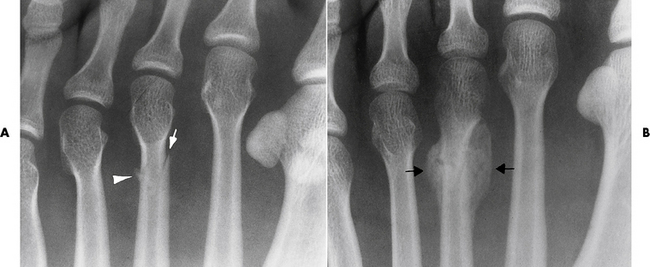
Figure 4-97 Stress fracture of the third metatarsal. Initial radiographic findings were within normal limits. A, Radiograph obtained 14 days after onset of symptoms demonstrates thin oblique lucency (arrow) interrupting one cortex, and a small amount of fluffy periosteal callus formation (arrowhead) along the opposite cortex. There is no evidence of complete fracture line. B, Second radiograph obtained 3 weeks later shows dense callus formation (arrows) about the fracture site.
Radionuclide bone scans using a triple-phase technique to maximize specificity can demonstrate a stress fracture before it can be detected on plain radiographs. MRI, used only for cases with indeterminate radiographic findings, is highly sensitive for detecting stress fractures (Figure 4-98). In some cases, MRI has higher specificity than radionuclide bone scans. Early stress fractures produce low signal intensity on T1-weighted images; there is a progressive increase in signal intensity with increased T2 weighting. A fat-saturation (fat-suppressing) technique may demonstrate associated medullary edema or hemorrhage, which produces a high signal compared with the fat-suppressed background.
Battered-Child Syndrome
Battered-child syndrome refers to multiple, repeated, physically induced injuries in young children caused by parents or guardians. The facility treating the suspected battered child, also known as suspected non-accidental trauma (SNAT), has a legal responsibility to report suspicious cases to child protective services. While the child is being evaluated, diagnosed, and treated, the environment must be protective for the child.
Shaken-baby syndrome, Munchausen syndrome by proxy, and sudden infant death syndrome are all situations investigated to prove or disprove abusive injury. A skeletal radiographic series is performed in such instances and includes an AP view of each extremity and the pelvis. AP and lateral projections of the chest and skull are also required.
Radiographic Appearance
The radiographic findings in this syndrome include multiple fractures of varying age in various stages of healing, fractures of the corners of metaphyses with or without associated epiphyseal displacement, and exuberant subperiosteal new bone formation along the shafts of long bones (Figure 4-99). Skull fractures or widening of the cranial sutures are commonly associated. Another highly suggestive finding is one or more fractures at otherwise unusual sites (usually fractured only by direct blows), such as the ribs, scapula, sternum, spine, or lateral ends of the clavicles (Figure 4-100).
Common Fractures and Dislocations
Colles’ fracture is a transverse fracture through the distal radius with dorsal (posterior) angulation and often overriding of the distal fracture fragment (Figure 4-101). In more than half the cases, there is an associated avulsion fracture of the ulnar styloid process. Colles’ fracture is usually caused by a fall on the outstretched hand and is the most common fracture of the wrist.
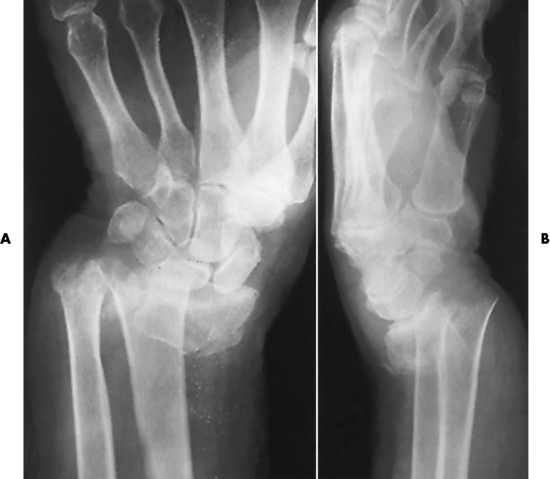
Figure 4-101 Colles’ fracture. Frontal (A) and lateral (B) projections of the wrist show overriding and dorsal displacement of distal fragment. There is also a displaced fracture of the distal ulna.
Navicular (scaphoid) fractures are the most common fractures involving the carpal bones. They are usually transverse and occur through the central part (the waist) of the bone. Although most navicular fractures can be identified on routine frontal projections of the wrist, subtle fractures may require specific oblique and angulated projections or images made using magnification techniques. In some cases, a navicular fracture cannot be detected on the initial examination despite strong clinical suspicion (Figure 4-102A). In this situation, the wrist should be placed in a cast or plaster splint and reexamined (without the cast or splint) in 7 to 10 days. At this time, resorption of bone at the margins of the fracture widens the fracture line and makes it more apparent radiographically (Figure 4-102B).
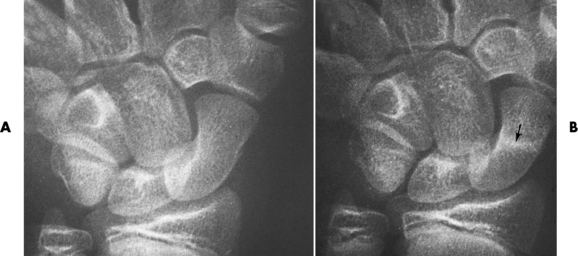
Figure 4-102 Navicular fracture. A, On a radiograph obtained immediately after injury, fracture cannot be detected. B, On a second radiograph obtained 3 weeks later, fracture is clearly signified by a sclerotic band (arrow) of opaque internal callus.
Nonunion is a serious complication of navicular fractures (see Figure 4-95). Its incidence increases with motion or displacement of the fracture fragments resulting from either poor immobilization or neglect. Because the blood supply to the navicular bone comes primarily from the distal portion, the proximal fragment may become avascular and undergo ischemic necrosis. This condition is seen as an increase in bone density associated with collapse of bone or loss of volume in the affected fragment (Figure 4-103).
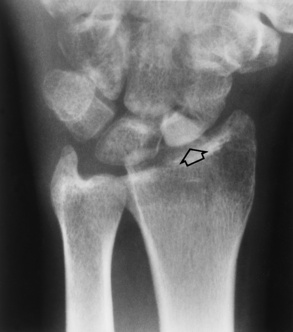
Figure 4-103 Avascular necrosis of a navicular fracture. Increased bone density is seen in the avascular proximal fragment (arrow).
A boxer’s fracture is a transverse fracture of the neck of the fifth metacarpal with volar (palmar) angulation of the distal fragment (Figure 4-104). This injury is typically the result of a blow struck with the fist.
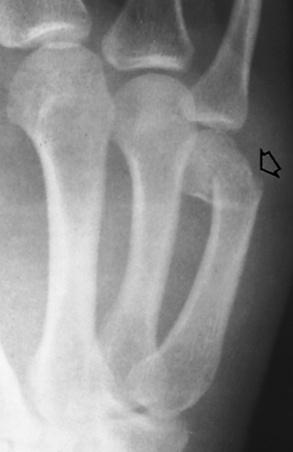
Figure 4-104 Boxer’s fracture. There is a fracture at the neck of the fifth metacarpal (arrow) with volar angulation of the distal fragment.
In the detection of fractures about the elbow, a valuable clue is displacement of the normal elbow fat pads (the fat pad sign). On lateral projections of the elbow, the anterior fat pad normally appears as a radiolucency closely applied to the anterior surface of the distal end of the humerus. The posterior fat pad, normally hidden in the depths of the olecranon fossa, should not be visible on standard lateral projections of the elbow. Any process producing synovial or hemorrhagic effusion within the elbow joint displaces the fat pads. The normally hidden posterior fat pad, when posteriorly displaced, becomes visible as a crescentic lucency behind the lower end of the humerus (Figure 4-105). The anterior fat pad becomes more rounded and further separated from the underlying bone. The posterior fat pad is by far the more sensitive indicator of an elbow joint effusion. Its presence on the lateral projection of the patient with elbow trauma strongly suggests an underlying fracture, especially of the radial head, and indicates the need for oblique projections if no fracture is seen on standard projections. If no fracture is identified, a second radiograph obtained 2 weeks or more after appropriate immobilization often shows a fracture by demonstrating a fracture line or callus formation indicating healing.
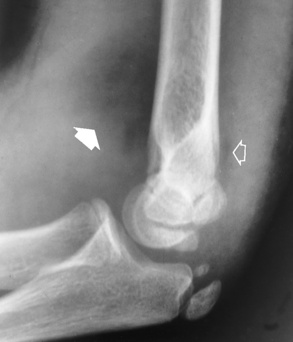
Figure 4-105 Fat pad sign in the elbow. The anterior fat pad (solid arrow) is clearly lifted from its fossa as a result of a large joint effusion in this child with a supracondylar fracture of the distal humerus. The normally hidden posterior fat pad is posteriorly displaced by effusion (open arrow).
Most fractures of the forearm involve both the radius and the ulna. If only one bone fractures, it is essential to examine both the elbow and the wrist to exclude the possibility of proximal or distal joint dislocation. A Monteggia fracture (Figure 4-106) is an isolated fracture of the shaft of the ulna associated with anterior dislocation of the radius at the elbow. A Galeazzi fracture is the combination of a fracture of the shaft of the radius and a dorsal (posterior) dislocation of the ulna at the wrist.
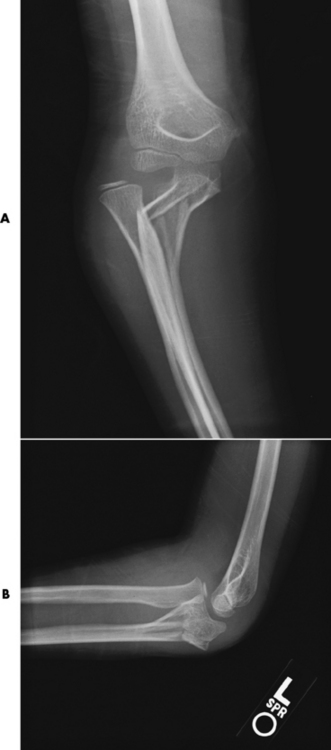
Figure 4-106 Monteggia fracture. A, The anterioposterior projection demonstrates lateral radial head dislocation with a proximal ulna fracture. B, The lateral projection demonstrates a displaced fracture of the proximal ulna with associated anterior dislocation of radial head.
Pott’s fracture involves both malleoli (i.e., of tibia and fibula) with dislocation of the ankle joint. A bimalleolar fracture is one involving both the medial and the lateral malleoli (Figure 4-107). Because of the mechanism of injury, the fracture on one side is transverse, whereas the fracture on the other side is oblique or spiral. Trimalleolar fractures involve the posterior lip of the tibia in addition to the medial and lateral malleoli and usually represent fracture-dislocations.
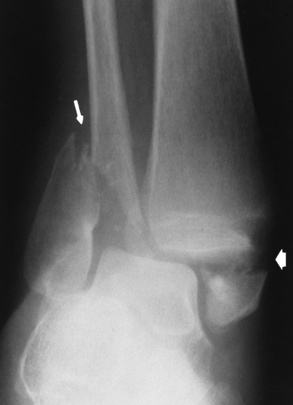
Figure 4-107 Bimalleolar fracture of the ankle. A transverse fracture of the medial malleolus (wide arrow) is associated with a low oblique fracture of the distal fibula (thin arrow).
One of the most frequent injuries to the foot is a transverse fracture at the base of the fifth metatarsal (a Jones fracture) (Figure 4-108). This fracture represents an avulsion injury that results from plantar flexion and inversion of the foot, as occurs when stepping off a curb or falling while walking on stairs. It is important to distinguish this fracture from the longitudinally oriented apophysis that is normally found in children at the lateral margin of the base of the fifth metatarsal.
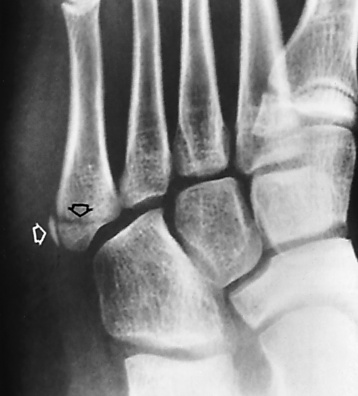
Figure 4-108 Jones fracture of the base of the fifth metatarsal. Note that the fracture line is transverse (black arrow) on the base of the fifth metatarsal, whereas the normal apophysis in this child has vertical orientation (white arrow).
The shoulder is by far the most commonly dislocated joint in the body (Figure 4-109). About 95% of shoulder dislocations are anterior, resulting from external rotation and abduction of the arm. As the anterior displacement occurs, the posterolateral surface of the humeral head impacts against the anterior or anteroinferior surface of the glenoid fossa, possibly resulting in a compression fracture of the humeral head, a fracture of the glenoid rim, or both. In most cases, the humeral head is displaced medially and anteriorly and comes to rest beneath the coracoid process.
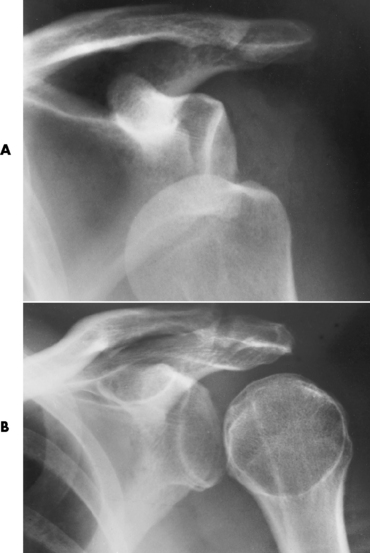
Figure 4-109 Dislocation of the shoulder. A, Anterior dislocation seen with inferior and medial displacement of the humeral head. B, Posterior dislocation demonstrated by the increased distance between the anterior glenoid rim and the humeral head.
Dislocations of the hip, with or without associated fracture of the acetabulum, are caused by severe injuries, such as automobile collisions, pedestrian accidents, or falls from a great height. Unlike in the shoulder, posterior dislocations of the hip are far more common than anterior dislocations, accounting for 85% to 90% of the cases (Figure 4-110).
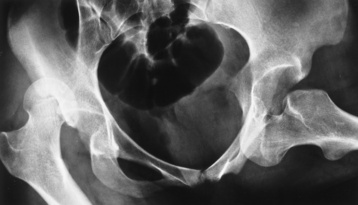
Figure 4-110 Dislocations of the hip. Frontal radiograph of teen-aged girl injured in motor vehicle collision demonstrates right posterior dislocation and left anterior dislocation of the hip. Right posterior dislocation is characterized by typical superolateral displacement of the femoral head, fixed adduction, and internal rotation (lesser trochanter superimposed on the femoral shaft). Left anterior dislocation is manifested by the characteristic inferomedial displacement of the femoral head, which has come to overlie the obturator foramen; fixed abduction; and external rotation (lesser trochanter depicted in profile).
Fractures and dislocations of the spine
Fractures and dislocations of the spine may be the result of direct trauma, hyperextension-flexion injuries (whiplash), or normal stresses in abnormal bone (osteoporosis, metastatic destruction). In the patient with spinal injury, the major goal of the radiographic evaluation is to determine whether a fracture or dislocation is present and whether the injury is stable or unstable. The spine can be regarded as consisting of two major columns. The anterior column consists of the vertebral bodies, intervertebral disks, and anterior and posterior longitudinal ligaments. The facets, apophyseal joints, pedicles, laminae, spinous processes, and all the intervening ligaments form the posterior column. If one of the two columns remains intact, the injury is considered stable. If both columns are disrupted, the injury is considered unstable.
If there is a strong suspicion of injury to the cervical spine, the initial radiograph should be a horizontal-beam lateral projection with the patient supine. This cross-table lateral radiograph must be checked by the physician before any other projections are obtained (Figure 4-111). It is essential to include all seven cervical vertebrae on the image, lest the relatively common injuries of C6 and C7 be overlooked (Figure 4-112). This requirement may require supine oblique projections or images made in the swimmer’s position, in which one arm of the patient is extended over the head while the other arm remains by the side so as to slightly oblique the upper torso and permit visualization of the cervicothoracic junction. A frontal projection of the spine and an open-mouth projection of the atlas and axis (C1 and C2) should be obtained next. In an acutely injured patient, oblique or flexion and extension projections should be performed only under the direct supervision of the attending physician. CT or even conventional tomography may be used to confirm the presence or absence of a fracture (especially of the posterior elements) and to visualize otherwise obscured areas of the spine, such as the craniovertebral and cervicothoracic junctions. CT can precisely localize fracture fragments in relation to the spinal canal and detect otherwise obscure fractures of the posterior elements. Myelography may be performed in patients with a spinal cord injury in the absence of an obvious fracture or dislocation to identify a condition amenable to surgical removal or repair (e.g., a herniated disk fragment or an epidural hematoma of the spinal cord).
Figure 4-111 A, Cross-table C-spine radiograph of a 23-month-old demonstrating internal decapitation B, Volume-rendered CT scan better delineates the separation of C5-C6.
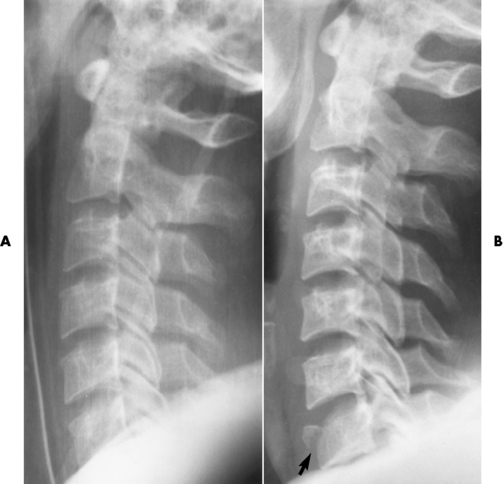
Figure 4-112 Fracture of the C7 vertebral body. A, On the initial lateral radiograph, only the upper six cervical vertebrae can be seen. The patient’s shoulders overlie the seventh cervical vertebra. B, With shoulders pulled down to expose the seventh cervical vertebra, the anterosuperior fracture (arrow) is clearly identified.
Several types of fractures are peculiar to the cervical spine. A Jefferson fracture, a comminuted fracture of the ring of the atlas, involves both the anterior and posterior arches and causes displacement of the fragments. The characteristic appearance on frontal radiographs or tomograms is a bilateral offset or spreading of the lateral articular masses of C1 in relation to the opposing articular surfaces of C2 (Figure 4-113).
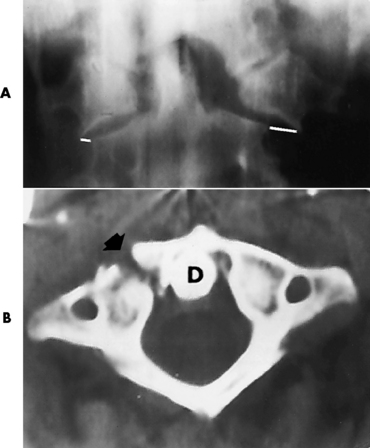
Figure 4-113 Jefferson fracture. A, On frontal projection, there is lateral displacement of the lateral masses of C1 bilaterally (white lines). B, CT scan in another patient shows a unilateral break in the arch of C1 (arrow). D, Dens.
Fractures of the odontoid process are usually transverse and located at the base of the dens at its junction with the body (Figure 4-114). On an open-mouth view, a lucency between the upper central incisor teeth often overlaps the dens; this must be differentiated from a rare vertical fracture of the dens.
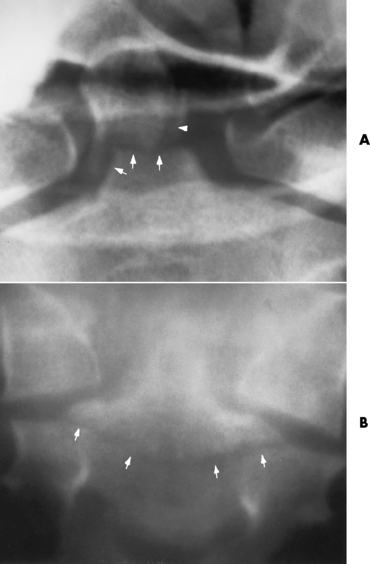
Figure 4-114 Fracture of the odontoid process. A, Open-mouth frontal projection shows combined oblique and transverse fracture at the base of the dens (arrows). There is also a separate cortical fragment on left (arrowhead), which most likely remains attached to the alar ligament. B, In another patient, a frontal tomogram shows a low fracture (arrows) through the body of C2.
The hangman’s fracture is the result of acute hyperextension of the head on the neck. It appears as a fracture of the arch of C2 anterior to the inferior facet and is usually associated with anterior subluxation of C2 on C3 (Figure 4-115). Although originally described in patients who had been hanged, this injury is now far more commonly the result of motor vehicle collisions.
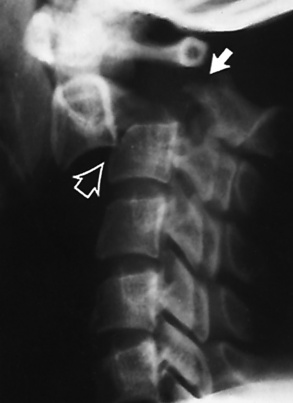
Figure 4-115 Hangman’s fracture. Neural arch fracture (solid arrow) associated with complete C2-C3 subluxation (open arrow).
Clay shoveler’s fracture is an avulsion fracture of a spinous process in the lower cervical or upper thoracic spine. The fracture is difficult to demonstrate on emergency cross-table lateral radiographs because the shoulders frequently obscure the lower cervical region. The diagnosis can be made from the frontal view by noting the double shadow of the spinous processes caused by the caudal displacement of the avulsed fragment (Figure 4-116). This double-spinous-process sign must be differentiated from a bifid spinous process, which usually lies at a higher level and on a more horizontal plane.
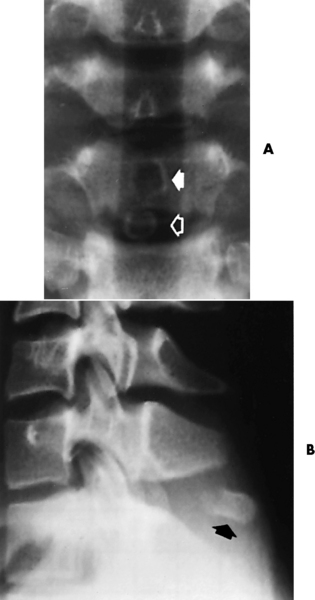
Figure 4-116 Clay shoveler’s fracture. A, Frontal projection of the cervical spine shows the characteristic double-spinous-process sign resulting from caudal displacement of an avulsed fragment (open arrow) with respect to the normal position of the major portion of the spinous process (solid arrow). B, Lateral projection clearly shows the avulsed fragment (arrow).
Most fractures of the thoracolumbar spine are attributable to compressive forces that cause anterior wedging or depression of the superior end plate of a vertebral body (Figure 4-117). The seat belt fracture is a transverse fracture of a lumbar vertebra that is often associated with significant visceral injuries (Figure 4-118). In this condition, a horizontal fracture of the vertebral body extends to involve some or all of the posterior elements.
Herniation of intervertebral disks
The intervertebral disks act as shock absorbers between the vertebrae, cushioning the movements of the spine. Each disk consists of a fibrous outer cartilage (annulus) surrounding a central nucleus pulposus, which is the essential part of the disk. The nucleus pulposus is a highly elastic, semifluid mass compressed like a spring between the vertebral surfaces. In youth, it contains a large amount of fluid to cushion the motion of the spine. With increasing age, the fluid and elasticity gradually diminish, leading to degenerative changes and back pain. Protrusion, or herniation, of a lumbar intervertebral disk is the major cause of severe acute, chronic, or recurring low back and leg pain. It most frequently involves the L4-L5 and L5-S1 levels in the lumbar region, where it often causes sciatica, pain that radiates down the sciatic nerve to the back of the thigh and lower leg. Other major sites are the C5-6 and C6-C7 levels in the neck and the T9-T12 levels in the thoracic region.
Radiographic Appearance
Although plain radiographs show characteristic narrowing of the intervertebral disk spaces with hypertrophic spur formation, bony sclerosis, spurs impinging on the neural foramina, and the vacuum phenomenon (lucent collections overlying the intervertebral disks), these findings are nonspecific and frequently occur in patients with minimal symptoms (Figure 4-119). The diagnosis of herniation of an intervertebral disk requires CT, MRI, or myelography to demonstrate impression of the disk on the spinal cord or individual nerve roots (Figures 4-120 to 4-125).
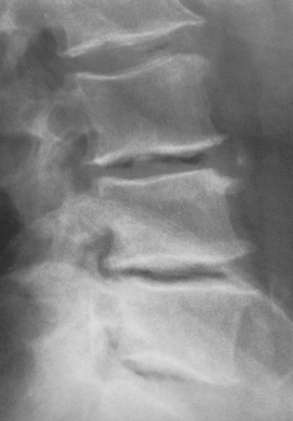
Figure 4-119 Degenerative disk disease. Hypertrophic spurring, intervertebral disk space narrowing, and reactive sclerosis. Note the linear lucent collections (vacuum phenomenon) overlying several intervertebral disks.
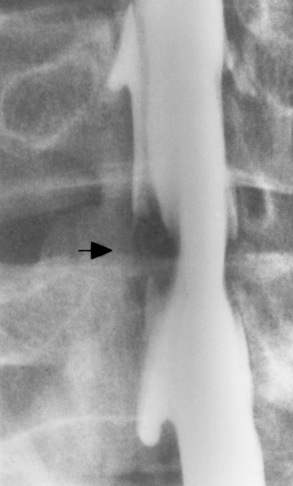
Figure 4-120 Lumbar disk herniation. Myelogram shows an extradural lesion (arrow) at the level of the intervertebral disk space. Note the amputation of the nerve root by disk compression.
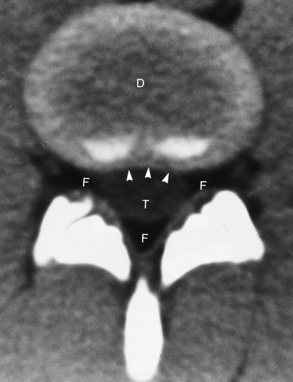
Figure 4-121 CT of a normal lumbar disk (D). The normal lumbar intervertebral disk has a concave posterior border (arrowheads). Note the normal epidural fat (F) surrounding the thecal sac (T).
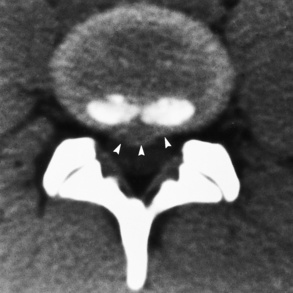
Figure 4-122 Central bulging of an intervertebral disk. CT scan shows convex posterior border of the disk (arrowheads). Note the preservation of epidural fat.
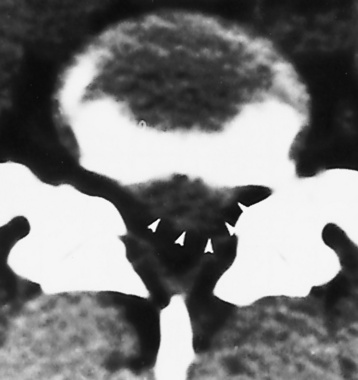
Figure 4-123 Disk herniation at the L5-S1 level. CT shows herniation of the disk (arrowheads) to the left with obliteration of epidural fat.
Treatment
Patients with symptoms suggestive of disk herniation are initially treated conservatively with bed rest, muscle relaxants, and analgesics before being subjected to radiographic studies. If conservative treatment is unsuccessful, surgery may be necessary to remove the cause of impingement and alleviate the pain and symptoms.
Scoliosis
Scoliosis is a twisting and curvature in the lateral perspective, somewhat like an S curve. The most common types of scoliosis can be classified as idiopathic, functional, neuromuscular, and degenerative. Idiopathic. the most common in young females. can be divided into infantile, juvenile. and adolescent. Adolescent scoliosis occurs in people 10 years and older with an abnormal curvature from an unknown cause. Functional scoliosis occurs because of a problem outside of the spine, such as one leg being shorter than the other for which the body compensates naturally. Neuromuscular scoliosis occurs when the vertebra fails to form completely or fails to separate appropriately. Adult-type scoliosis is degenerative in form and is usually caused by arthritis.
Radiographic Appearance
Full spine images should be acquired in the upright position with the patient standing using normal stance and weight distribution. The technologist must include the iliac crest while shielding the gonad region and the breast tissue; some even suggest removing the shoes for the most accurate images. The spinal curvature will be measured each clinic visit to determine whether the curvature is progressing or remaining constant (Figure 4-126). This evaluation is important in helping to determine the treatment necessary. Curvatures that are considered normal are less than 20 degrees, whereas curvatures of 40 to 50 degrees and greater need the most extensive treatment. To obtain more extensive information of the bony development, a CT scan demonstrates the vertebral structure. In some cases, an MRI is used to ensure that the spinal cord and spinal canal are within normal limits in preparation for surgical treatments.
Treatment
For curvatures of 25 to 40 degrees, bracing technology is the treatment of choice. The brace is custom fitted and is most effective if worn more than 12 hours a day. For the more severe cases, curvatures 40 degrees or greater, surgery is most often recommended and has a high success rate. Surgery possibly includes a spinal fusion and rods to provide as much correction as possible.
Spondylolysis and spondylolisthesis
Spondylolysis refers to a cleft in the pars interarticularis that is situated between the superior and inferior articular processes of a vertebra. Occurring in about 5% of the population, such clefts are usually bilateral, most commonly involve the fifth lumbar vertebra, and predispose to the forward displacement of one vertebra on the other that may cause chronic back pain. Spondylolysis is the term for a defect in the pars interarticularis without displacement; if displacement occurs, the condition is called spondylolisthesis.
Radiographic Appearance
A plain lateral radiograph of the lower lumbar spine clearly shows any spondylolisthesis and may demonstrate the lucent cleft in the pars interarticularis even if no displacement has occurred (Figure 4-127). In the grading of spondylolisthesis of the lumbosacral junction on the lateral projection, the superior surface of the sacrum is divided into four equal parts. A forward displacement of the fifth lumbar vertebra up to one fourth the thickness of the sacrum is called a first-degree spondylolisthesis; half the thickness, a second-degree spondylolisthesis; and so on.
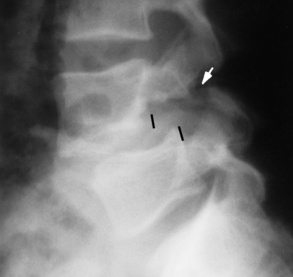
Figure 4-127 Spondylolisthesis. Lateral view of the lower lumbar spine shows a break in the pars interarticularis (arrow), with resultant anterior slippage of L4 with respect to L5. Vertical black lines indicate the posterior margins of the vertebral bodies.
The diagnosis of spondylolysis without displacement may require an oblique projection of the lumbar spine, in which the appearance of the posterior elements has been likened to that of a Scotty dog (Figure 4-128). The pedicle and transverse process
Figure 4-128 Spondylolysis. A, Diagram of the Scotty dog sign. B, Oblique projection of the lumbar spine demonstrates a defect in the pars interarticularis, which appears as a fracture through the neck of the Scotty dog (arrow).
form the eye and nose; the superior and inferior articular processes form the ear and leg; and the pars interarticularis forms the neck, which is “fractured” in a patient with spondylolysis.
Treatment
Passive treatment for spondylosis or spondylolisthesis includes bracing, physical therapy, restricted activity, and nonnarcotic analgesics to control pain to a tolerable level. Conservative therapy works for the majority of patients. Surgical treatment is necessary only when passive treatment is unsuccessful for controlling pain. A complete laminectomy, usually with spinal fusion, is performed to free the peripheral nerves and stabilize spinal movement.