Immunology of the skin
The immunological components of skin can be separated into structures, cells and immunogenetics.
Structures
The epidermal barrier is an important example of innate immunity, as most micro-organisms that have contact with the skin do not penetrate it. Equally, the generous blood and lymphatic supplies to the dermis are important channels through which immune cells can pass to or from their sites of action.
Cells
Professional antigen presenting cells
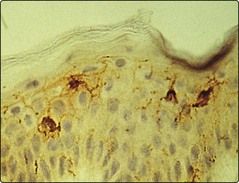
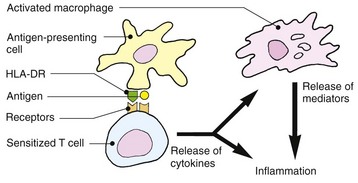
The Langerhans cells (epidermis) and dermal dendritic cells are the outermost sentinels of the cellular immune system (Fig. 1). They are dendritic, bone marrow-derived cells. Langerhans cells are characterized ultrastructurally by a unique cytoplasmic organelle known as the Birbeck granule. Recent work has shown the important role ultraviolet radiation plays in inducing photoimmunosuppression, which is mediated by effects on the skin dendritic cell population.
T lymphocytes
T cells are defined by expression of the T cell receptor (TCR), the structure of which determines the foreign antigens the T cell will recognize. Consequently, TCR specificity is closely regulated to prevent circulation of T cells strongly recognizing self-proteins. The TCR recognizes the antigen as presented by the major histocompatibility complex (MHC) and this interaction is stabilized by CD8+ (MHC class I) or CD4+ (MHC class II) molecules.
T lymphocyte circulation through normal skin for immunosurveillance is regulated by lymphocyte surface molecules that promote ‘skin homing’, including cutaneous leucocyte antigen (CLA), CCR4, CCR6 and CCR10. Epithelial danger signals induced by infection and inflammation increase cutaneous and endothelial expression of skin homing receptor ligands thereby enhancing the influx of lymphocytes into the cutaneous compartment.
Different types of T cell with differing functions are recognized in the skin, for example:
 CD4+ function is classified by cytokine production (Fig. 2), which also determines how they regulate class-switching of B cells to IgG (Th1) or IgE (Th2) production.
CD4+ function is classified by cytokine production (Fig. 2), which also determines how they regulate class-switching of B cells to IgG (Th1) or IgE (Th2) production.
 CD8+ cells are capable of cytokine production (Tc1 and Tc2, etc.) and target cell killing mediated by granzyme B and perforin production.
CD8+ cells are capable of cytokine production (Tc1 and Tc2, etc.) and target cell killing mediated by granzyme B and perforin production.
 NKT (CD4+ or CD8+ or double or nil expressing) cells express a T cell receptor and NK cell surface markers. NKT cells are capable of high levels of cytokine production.
NKT (CD4+ or CD8+ or double or nil expressing) cells express a T cell receptor and NK cell surface markers. NKT cells are capable of high levels of cytokine production.
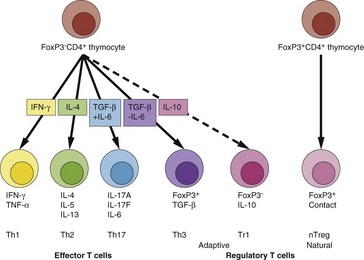
The cytokine/function polarization of CD4+ T cells, which was until recently limited to the Th1/Th2 paradigm, has been expanded. Cytokines that are critical to lineage development are indicated in the arrow boxes. Below the cells are the effector or regulatory cytokine repertoires of each cell type (nTreg are contact dependent) and their abbreviated names.
Mast cells
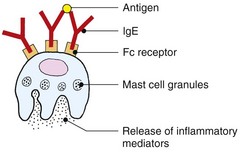
Mast cells are principally known for their ability to degranulate and release histamine and other vasoactive molecules. This is very rapid because the granules are preformed. Degranulation arises in response to cross-linking of the high-affinity IgE receptor. Cross-linking arises when IgE molecules on the surface of the receptors bind the same protein antigen. Mast cells also synthesize a wide range of cytokines, and experimental models support the concept that mast cells play an important role in skin immune responses. Mast cells are normal residents of the dermis, and their circulating counterparts are basophils. Mast cell numbers increase during inflammatory reactions.
Keratinocytes
Keratinocytes synthesize antimicrobial peptides, produce proinflammatory cytokines (especially IL-1) and express immune reactive molecules such as major histocompatibility complex (MHC) class I and II molecules on their surface. They signal to cutaneous dendritic cells and have been shown to be able to induce specific dendritic cell: T cell functional outcomes. For example, keratinocyte production of thymic stromal lymphopoietin (TSLP) induces dendritic cells to drive T cells towards an inflammatory Th2 phenotype.
Immunogenetics
The tissue-type antigens of an individual are found in the MHC, located in humans on the human leucocyte antigen (HLA) gene cluster on chromosome 6. The classical HLA genes are HLA-A, B and C (MHC class I) and DP, DQ and DR (MHC class II). The MHC class I complexes (CD8+ restricted) are ubiquitously expressed but MHC class II (CD4+ restricted) is confined to professional antigen presenting cells (including B lymphocytes, Langerhans cells, dermal dendritic cells and macrophages). During inflammation, other cell types such as endothelial cells and keratinocytes can express MHC class II. There are specific HLA genes associated with an increased likelihood of certain diseases, some of which are ‘autoimmune’ in nature (Table 1).
Table 1 Skin disease associations of HLA antigens
| Disease | HLA antigen | Relative risk |
|---|---|---|
| Behçet’s disease | B5 | 10 |
| Dermatitis herpetiformis | B8DRw3 | 15>15 |
| Pemphigus | DRw4 | 10 |
| Psoriasis | B13Dw7Cw6 | 41012 |
| Psoriatic arthropathy | B27 | 10 |
| Bw38 | 9 | |
| Reiter’s disease | B27 | 35 |
Hypersensitivity reactions and the skin
Hypersensitivity is the term applied when an adaptive immune response is inappropriate or exaggerated to the degree that tissue damage results. The skin can exhibit all the main types of hypersensitivity response.
Type I (immediate)
Allergen-specific immunoglobulin (Ig) E bound to the surface of mast cells causes degranulation on antigen exposure (as discussed above). The result in the skin is urticaria, although massive histamine release can cause anaphylaxis. The response occurs within minutes, although a delayed component is recognized. Factors other than IgE can cause mast cell degranulation.
Type II (antibody-dependent cytotoxicity)
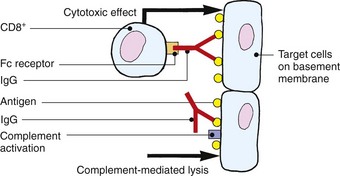
IgG antibodies directed against an antigen on target skin cells or structures induce cytotoxicity by killer T cells or by complement activation. For example, IgG pemphigus antibodies directed against desmoglein on the keratinocyte surface result in activation of complement, attraction of effector cells and lysis of the keratinocytes. Intraepidermal blisters result.
Type III (immune complex disease)
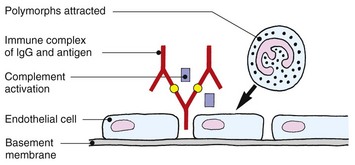
Immune complexes formed by the combination of antigen and IgG or IgM antibodies in the blood are deposited in the walls of small vessels, often those of the skin. Complement activation, platelet aggregation and the release of lysosomal enzymes from polymorphs cause vascular damage. This leucocytoclastic vasculitis is seen, for example, with systemic lupus erythematosus and dermatomyositis, but also occurs with microbial infections such as infective endocarditis.
Type IV (cell mediated or delayed)
Lymphocytes sensitized by cutaneous dendritic cells in the draining lymph node proliferate and undertake immunosurveillance of the tissues. On re-encounter with their cognate antigen–MHC complex, they become activated and induce inflammation and/or cell killing. From antigen exposure to sensitization takes 7–14 days. However, long-lived memory cells are able to undertake rapid expansion at a subsequent exposure and provide lasting immunity. Allergic contact dermatitis (see p. 34) and the tuberculin reaction to intradermally administered antigen are both forms of type IV reaction. The responses to skin infections such as leprosy or tuberculosis are granulomatous variants of the reaction.
Immunology
 Skin provides a physical barrier to infection and possesses antimicrobial peptides.
Skin provides a physical barrier to infection and possesses antimicrobial peptides.
 Dendritic cells in the skin, including epidermal Langerhans cells, form outposts of the cellular immune system and can present antigens to immunocompetent cells, e.g. T lymphocytes.
Dendritic cells in the skin, including epidermal Langerhans cells, form outposts of the cellular immune system and can present antigens to immunocompetent cells, e.g. T lymphocytes.
 T cells circulate through normal skin and form part of the skin-associated lymphoid tissue. They are localized by adhesion molecules.
T cells circulate through normal skin and form part of the skin-associated lymphoid tissue. They are localized by adhesion molecules.
 Keratinocytes can be immunologically active cells.
Keratinocytes can be immunologically active cells.
 All four types of hypersensitivity reaction occur in the skin.
All four types of hypersensitivity reaction occur in the skin.
 Genetic factors modulate immunological responses. Certain HLA antigens are associated with increased risk of skin disease, e.g. HLA-DRw4 with pemphigus.
Genetic factors modulate immunological responses. Certain HLA antigens are associated with increased risk of skin disease, e.g. HLA-DRw4 with pemphigus.