Psoriasis – Biologics
The biological response modifiers are breakthrough treatments for moderate-to-severe psoriasis and for other diseases such as rheumatoid arthritis and Crohn’s disease. Biologics are based on recombinant cytokines, fusion proteins or monoclonal antibodies (mouse or human) that bind to tumour necrosis factor-α (TNF-α), cytokine receptors or block T cell receptors to have their effect (Fig. 1).
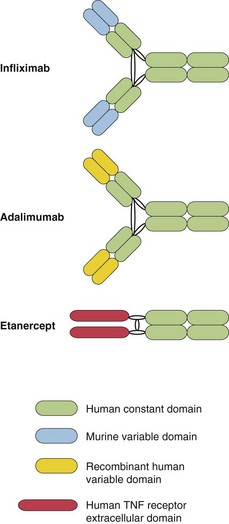
Fig. 1 Diagram of the structure of the biologics.
Infliximab and adalimumab are similar to normal human immunoglobulins. Etanercept contains the extracellular TNF receptor domain fused to parts of the human immunoglobulin heavy chain.
Scientific background
Research has confirmed psoriasis to be an immune-mediated inflammatory disease and has identified associated genes that are involved in immune regulation. These discoveries allow the pharmaceutical industry to design drugs that should work in psoriasis and in other diseases where the pathomechanisms are well understood (Fig. 2). The ‘biologics’ work by having a blocking effect on TNF-α, other cytokines (e.g. interleukin (IL)-12 and -23), dendritic cells and T lymphocytes involved in causing the disease. TNF-α is derived from Th1 cells and mediates inflammation by initiating a cascade of cytokines. IL-12 (derived from dendritic cells) and IL-23 are central to defining T helper cell types and T cell differentiation.
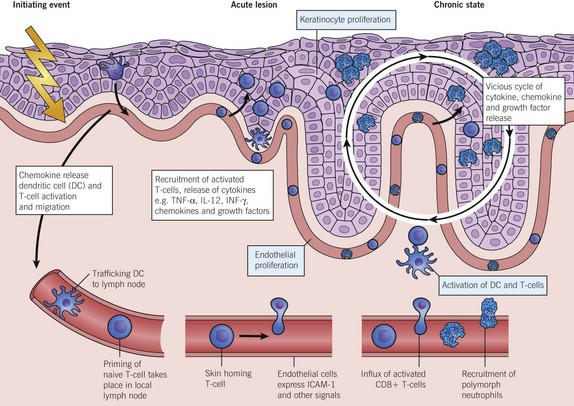
Fig. 2 A model of the immunopathogenesis of psoriasis
showing the molecular and cellular changes as they progress from the intitiating event through to the acute lesion and on to the chronic plaque. A vicious cycle of release of cytokines, chemokines and growth factors keeps the disease active. The biologics break the cycle by negating the inflammatory action of TNF-α, IL-12 and other factors.
Effects of activated chronic inflammatory cytokines
The chronic inflammatory cytokines that are active in severe psoriasis may be one reason why patients with the disease have higher rates of cardiovascular morbidity. In addition, some genes associated with psoriasis may predispose to cardiovascular disease and diabetes.
Associated joint disease
Psoriasis is associated with an arthropathy in 20–30% of cases. Some but not all biologics will improve psoriatic arthritis in addition to the skin disease.
Types of biologic available
The most commonly used biologics for psoriasis are the anti-TNF-α agents infliximab, etanercept and adalimumab, and the anti-IL-12/IL-23 drug ustekinumab. The biologics are very effective drugs, producing better responses than agents such as methotrexate. They are given by intravenous infusion or subcutaneous injection, at intervals that vary from twice weekly to once every 3 months. The most commonly used biologics will be discussed individually.
 Etanercept: a fusion protein that acts by binding to soluble and receptor-bound TNF-α. It was the first biologic to be approved by the National Institute for Health and Clinical Excellence (NICE) for use in patients whose psoriasis is moderate to severe and has failed to respond to other systemic drugs such as ciclosporin and methotrexate, or photochemotherapy (PUVA). In psoriasis, the severity of skin involvement is measured by the Psoriasis Area and Severity Index (PASI; see p. 19) and the impact on a patient’s life by the Dermatology Life Quality Index (DLQI; see p. 19). High scores on both of these parameters are required for the use and funding of this and other biologic drugs within the National Health Service in England, according to NICE. Etanercept is given by subcutaneous injection once or twice weekly (in a dose of 25 mg or 50 mg).
Etanercept: a fusion protein that acts by binding to soluble and receptor-bound TNF-α. It was the first biologic to be approved by the National Institute for Health and Clinical Excellence (NICE) for use in patients whose psoriasis is moderate to severe and has failed to respond to other systemic drugs such as ciclosporin and methotrexate, or photochemotherapy (PUVA). In psoriasis, the severity of skin involvement is measured by the Psoriasis Area and Severity Index (PASI; see p. 19) and the impact on a patient’s life by the Dermatology Life Quality Index (DLQI; see p. 19). High scores on both of these parameters are required for the use and funding of this and other biologic drugs within the National Health Service in England, according to NICE. Etanercept is given by subcutaneous injection once or twice weekly (in a dose of 25 mg or 50 mg).
 Adalimumab: a human monoclonal antibody directed against TNF-α approved by NICE and administered by subcutaneous injection (40 mg every 2 weeks).
Adalimumab: a human monoclonal antibody directed against TNF-α approved by NICE and administered by subcutaneous injection (40 mg every 2 weeks).
 Infliximab: a combined murine and human monoclonal antibody against TNF-α given by intravenous infusion at a dose of 5 mg/kg every 8 weeks. It is approved by NICE for severe psoriasis.
Infliximab: a combined murine and human monoclonal antibody against TNF-α given by intravenous infusion at a dose of 5 mg/kg every 8 weeks. It is approved by NICE for severe psoriasis.
 Ustekinumab: this human monoclonal biologic blocks the p40 subunit common to IL-12 and IL-23. Ustekinumab is approved by NICE and is given by subcutaneous injection (45 mg or 90 mg for patients weighing >100 kg) every 12 weeks after a loading regimen.
Ustekinumab: this human monoclonal biologic blocks the p40 subunit common to IL-12 and IL-23. Ustekinumab is approved by NICE and is given by subcutaneous injection (45 mg or 90 mg for patients weighing >100 kg) every 12 weeks after a loading regimen.
Pretreatment screening
Prior to initiating treatment, patients are counselled about potential side-effects of biologics (as shown below) and screened for other risk factors, as follows:
 Detailed history to exclude past or present cardiovascular or demyelinating disease and serious infections.
Detailed history to exclude past or present cardiovascular or demyelinating disease and serious infections.
 Note the presence of coexisting joint disease that may influence the selection of biologic.
Note the presence of coexisting joint disease that may influence the selection of biologic.
 Screen for infections including viral hepatitis, HIV and tuberculosis (the latter by chest X-ray and interferon-gamma release assay).
Screen for infections including viral hepatitis, HIV and tuberculosis (the latter by chest X-ray and interferon-gamma release assay).
Effectiveness of biologics
The biologics show varying degrees of effectiveness in treating psoriasis. Response to treatment can be compared by looking at the proportion of patients treated who achieve a 75% improvement in their PASI score (the ‘PASI 75’) after 10–12 weeks of biologic therapy. If there has been no response after 12–16 weeks, the drug is withdrawn. Etanercept, adalimumab and infliximab are also effective in psoriatic arthritis.
Therapeutic algorithm
Biologics are approved by NICE (in the UK) for use in patients with severe psoriasis who have a PASI >10 and a DLQI >10, and who have failed on (or are intolerant of) standard systemic treatments such as ciclosporin, methotrexate or PUVA. Etanercept, adalimumab and ustekinumab are all approved for use in this situation. If the psoriasis is very severe, inflixumab may be most appropriate as it seems to have a more rapid onset of action. Occasionally methotrexate is used in combination with etanercept or infliximab. Patients who have failed on one biologic may be tried on an alternative. Ustekinumab may be an appropriate alternative to etanercept or adalimumab due to its different mode of action.
Side-effects of biologics
The main problems with biologics are related to injection site reactions, serious infections (e.g. reactivation of latent tuberculosis), cancers, demyelination and cardiovascular events. Occasionally biologics actually cause a worsening of psoriasis. They are expensive in comparison to other treatments. Generally the biologics are well tolerated by patients.
Use of biologic agents in other skin diseases
These are detailed in Table 1.
Table 1 Use of biologics in other skin diseases
| Biologic | Type | Disease treated |
|---|---|---|
| Rituximab | IgG chimeric antibody directed against CD20 that potently depletes B cell numbers Given as an intravenous infusion |
Used successfully in B cell malignancies and in autoimmune diseases including systemic lupus erythematosus, dermatomyositis, systemic sclerosis and pemphigus |
| Infliximab | Combined murine and human monoclonal antibody against TNF-α | Beneficial off-licence use in hidradenitis suppurativa, pyoderma gangrenosum, sarcoidosis, Behçet’s disease and certain types of vasculitis |
| Omalizumab | Humanized monoclonal antibody that binds to IgE and thus inhibits the binding of IgE to high-affinity receptors on mast cells and basophils | Use is being considered in chronic urticaria and atopic dermatitis Preliminary results are said to be mixed |
Biologics
 Exciting new class of immunologically active drugs: biologics are selectively directed against the molecular pathways known to be involved in the pathogenesis of psoriasis.
Exciting new class of immunologically active drugs: biologics are selectively directed against the molecular pathways known to be involved in the pathogenesis of psoriasis.
 Properly controlled clinical trials: have demonstrated the efficacy of these agents in reducing the severity and extent of psoriasis and in improving quality of life.
Properly controlled clinical trials: have demonstrated the efficacy of these agents in reducing the severity and extent of psoriasis and in improving quality of life.
 NICE approval: in the UK, etanercept, adalimumab and ustekinumab can be used in patients with moderate-to-severe psoriasis who have appropriate PASI and DLQI scores.
NICE approval: in the UK, etanercept, adalimumab and ustekinumab can be used in patients with moderate-to-severe psoriasis who have appropriate PASI and DLQI scores.
 Infliximab infusion: is approved for use in more severe cases of resistant psoriasis.
Infliximab infusion: is approved for use in more severe cases of resistant psoriasis.
 Administration: biologics are given by subcutaneous injection or intravenous infusion. The frequency of dosing is very variable, ranging from twice weekly to once every 3 months.
Administration: biologics are given by subcutaneous injection or intravenous infusion. The frequency of dosing is very variable, ranging from twice weekly to once every 3 months.
 Infliximab in other diseases: off-licence reports indicate infliximab to be beneficial in severe cases of pyoderma gangrenosum, hidradenitis suppurativa and sarcoidosis.
Infliximab in other diseases: off-licence reports indicate infliximab to be beneficial in severe cases of pyoderma gangrenosum, hidradenitis suppurativa and sarcoidosis.
 Rituximab: a biologic that depletes B cells. It can be beneficial in pemphigus and some autoimmune diseases (off-licence use).
Rituximab: a biologic that depletes B cells. It can be beneficial in pemphigus and some autoimmune diseases (off-licence use).
 Atopic eczema: to date, there are no biologics approved for use in atopic eczema.
Atopic eczema: to date, there are no biologics approved for use in atopic eczema.