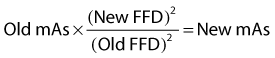Chapter 15 Radiography and Radiology
Radiography is the acquisition of radiographic images; radiology is the study and interpretation of those images. Radiography is an important part of the diagnostic armamentarium in the evaluation of lameness. Its most important role is to give information about bones and joints. However, it also can provide information about soft tissues, most particularly tendon, ligament, and joint capsule insertions. If radiography is to be used properly, then the area under investigation must be evaluated comprehensively and appropriately. A sufficient number of views, all which have been appropriately centered and exposed, should be obtained.
Obtaining high-quality radiographic images requires attention to detail. The horse must be correctly positioned and adequately restrained or sedated. For most weight-bearing examinations the horse should stand with the cannon bone of the limb to be examined in a vertical position. The horse should be standing on all four limbs, not resting a limb. The area under investigation should be cleaned to remove any surface dirt. For examinations of the foot, the shoe should be removed (if possible) to facilitate proper paring of the sole and frog, to ensure it is adequately clean, and to avoid superimposition of a radiodense shoe over the distal phalanx and navicular bone. The tail should be tied to facilitate correct positioning of the cassette or imaging plate when the stifle and hock regions are examined.
Radiographic Detail
The aim is to obtain as highly detailed radiographic images as possible. The detail that can be obtained is influenced by a number of factors, including those listed in Box 15-1.
BOX 15-1 Factors That Influence Radiographic Images
The use of a grid requires a higher exposure factor. If a focused grid is used, the x-ray beam must be perpendicular to the grid and centered on it, and the correct FFD should be used. Parallel grids have slightly more latitude when an FFD of more than 120 cm is used. The higher the grid ratio and lines per centimeter, the more effective the grid is in reducing scattered radiation, but the higher the grid factor. The grid factor denotes how much an exposure must be increased from nongrid values for comparable opacity. For example, if the grid factor is 2, then the milliampere-seconds (mAs) should be doubled.
Image Resolution
Image resolution is the objective measurement of how much detail can be provided by a film-screen combination and is measured in line pairs per millimeter. Resolution indicates the size of the smallest object that the system will record (i.e., the smallest distance that must exist between two objects before they can be distinguished as two separate entities). Image definition cannot be quantified but is the subjective impression of the amount of detail that can be seen on a radiograph.
Image Contrast
Contrast is the degree of definition on a radiographic image between adjacent structures of differing radiopacities. Opacity or radiopacity is the degree of whiteness of the object being radiographed. The denser the physical structure, the greater the degree to which the tissue absorbs x-rays, and the more opaque it appears on a radiograph.
Exposure Factors
Exposure factors affect the opacity and contrast of the radiographic image. The kilovoltage governs the quality and intensity of x-rays and affects both contrast and opacity. The quantity of x-rays reaching the x-ray film or imaging plate is the product of milliamperage and time and is also influenced by the focus-film distance (FFD), according to the inverse square law. The following equation is used to calculate the exposure of a change in distance:
The milliampere-seconds and FFD affect the opacity of the image, not the contrast.
Exposure Latitude
Exposure latitude is the degree of overexposure or underexposure that can be tolerated in a correctly developed film and still produce an image of acceptable radiographic quality. A low kilovoltage yields high contrast with low latitude, whereas a high kilovoltage results in low contrast but has wide latitude. For good bone detail the kilovoltage should be less than 70 kV. Attenuation of the x-ray beam depends heavily on the atomic number of the tissues, and it is desirable that photoelectric absorption predominate. Increasing the kilovoltage also results in more forward scatter. The use of computed or digital radiography potentially results in greater latitude for a single exposure.
To obtain a radiographic image with the same opacity as the original but with decreased contrast, the milliampere-seconds are halved and the kilovoltage is increased by 15% (approximately 10 kV). To increase the contrast but maintain the opacity, the milliampere-seconds are doubled and the kilovoltage is reduced by approximately 15%.
Image Sharpness and Resolution
Lack of image sharpness can be caused by a number of factors, including movement of the horse. Short exposure times help to reduce the risk of movement blur. Reducing the FFD can increase the amount of x-rays reaching the horse, and therefore the exposure time could be reduced. However, reduction of FFD results in an increase in geometric indistinctness or penumbra. Image blur also may be the result of poor screen-film contact. Contact can be tested by placing a wire mesh on top of the cassette and making an exposure using a large FFD. Any loss of image sharpness is the result of poor film-screen contact.
Image resolution also can be influenced by the focal spot size of the x-ray machine. High-output x-ray machines usually have different size focal spots. A small focal spot (e.g., 0.6 mm) usually results in better image resolution, but an increased exposure time is required to achieve the same milliamperes. When movement is likely to be a problem (e.g., proximal limb examinations), a larger focal spot (1.5 to 2.0 mm) is preferable to reduce exposure times.
Film and Screen Factors
Relative speed ratings of screens and distance factors must be considered when exposure factors are being selected. Speed classification of film-screen combinations allows comparison of systems from different manufacturers. Some manufacturers use 100, 200, 400, and so on, and others use 2, 4, 8, and so forth, but the interrelationship is the same. Speed 8 screens require half the exposure (milliampere-seconds) needed for speed 4 screens; speed 200 requires twice the exposure (milliampere-seconds) of speed 400. Although the same exposures are required to provide the same image opacity using similar film-screen speeds, the detail and resolution may vary. Generally when only one screen from a pair is used (when using single-emulsion film), the speed of the system will halve. Thus if one screen from a pair rated 400 is used, the speed will be 200.
Radiation Safety
Radiation safety (i.e., ensuring that personnel around the horse do not receive doses of radiation) is essential. Different codes of practice apply in various countries, but the basic principles are summarized in Box 15-2.
BOX 15-2 Basic Principles of Radiation Safety
Response of Bone to Stimuli: Wolff’s Law
Correct interpretation of radiographic images requires knowledge of the ways in which bone responds to various stimuli. Bone models according to Wolff’s law: it models according to the stresses placed on it so that it can be functionally competent with the minimum amount of bone tissue. The use of the terms modeling and remodeling creates considerable confusion because usage differs in histology and radiology. Histologically, bone remodeling refers to resorption and formation of bone that is coupled and occurs in basic multicellular units. This regulates the microstructure of bone without altering its shape and is a continuous process, replacing damaged bone with new bone. Thus remodeling cannot be appreciated radiologically. Radiologically, the term has been used to describe reshaping of bone to match form and function (e.g., after fracture repair). Strictly speaking, the term modeling should be used to describe the change in the shape of a bone as it adapts to the stresses applied to it.
Bone is a dynamic tissue, constantly reacting to the stimuli that it receives both internally and externally. However, it takes time to respond, and a 40% change in bone density must occur before changes are evident radiologically. Therefore radiographic images, although anatomically accurate, are relatively insensitive in the early stages of a disease process. This is known as the radiographic latent period. It is critical to appreciate these limitations when interpreting radiographic images. Bone can be undergoing abnormal modeling without identifiable structural change. Once radiological abnormalities have developed, some will persist over the long term without necessarily being associated with ongoing pain. Thus in effect these changes remain as scars reflecting previous injury. Aging of such lesions is impossible, and assessing clinical significance must be evaluated in the light of the clinical signs.
Bone can react to stimuli in only a limited number of ways. Bone can produce new bone, such as periosteal new bone, endosteal new bone, cortical thickening, increased thickness of trabeculae, callus formation, osteophyte and enthesophyte formation, and the palisading periosteal new bone typical of hypertrophic osteopathy. New bone often results in what has been described radiologically as sclerosis: increased opacity of the bone, caused by either new bone being laid down within the bone or superimposition of new bone on the surface of the bone. More than one radiographic image usually is required to determine why a structure appears to have increased opacity (i.e., is sclerotic). Strictly speaking, however, sclerosis is a localized increase in opacity of the bone caused by increased bone mass within the bone. Unless this can be determined with certainty it is preferable to use the term increased radiopacity.
Osteolysis is resorption of bone resulting in a radiolucency. Again, a lag period, usually of at least 10 days, occurs between the onset of osteolysis and its radiological detection. Osteolysis occurs for a variety of reasons, including pressure, infection, as part of early fracture repair, and as part of the disease process in osteoarthritis, osteochondrosis, osseous cystlike lesions, and subchondral bone cysts. Bone destruction and resorption usually are seen more easily in cortical bone rather than cancellous bone because of the greater contrast.
Generalized demineralization, or osteopenia, of bone throughout the body rarely occurs in the horse. Localized demineralization in a single limb usually is the result of disuse and is characterized by thinning of the cortices and a more obvious trabecular pattern. The proximal sesamoid bones are particularly sensitive indicators of disuse osteopenia in the horse.
Focal demineralization and loss of bone may be caused by pressure—for example, as seen in chronic proliferative synovitis in the fetlock—resulting in erosion of the dorsoproximal aspect of the sagittal ridge of the third metacarpal bone (McIII). It may be the result of infection, invasion by fibrous tissue, or a neoplasm.
Cortical thickness changes (models) according to Wolff’s law as an immature athlete develops into a mature, trained athlete. The dorsal cortex of the McIII and the third metatarsal bone becomes thicker. If a horse has marked conformational abnormalities, such as offset or bench knees, the bones model accordingly, with the lateral cortex of the distal limb bones becoming thicker.
Periosteal New Bone
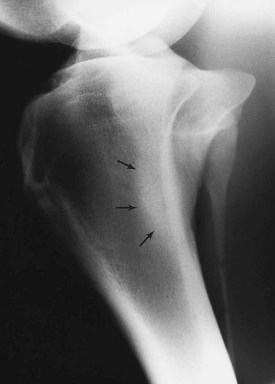
Blunt trauma to bone can lead to subperiosteal hemorrhage, resulting in lifting of the periosteum away from the bone. This process may stimulate the production of periosteal new bone (Figure 15-1). Some bones, such as the second and fourth metacarpal and metatarsal bones, seem particularly prone to such reactions. There is individual variation among horses in susceptibility to such reactions. Usually a lag period of at least 14 days occurs between trauma and the radiological detection of periosteal new bone. Such bone usually is much less dense than the parent bone; therefore soft exposures (or low kilovoltages) are essential for detection of this bone, which initially has a rather irregular outline. As the bone gradually consolidates and then models, it becomes more opaque and more smoothly outlined. Curiously, although a well-established splint exostosis would be expected to become inactive, many have increased radiopharmaceutical uptake (IRU) compared with the parent bone if examined scintigraphically.
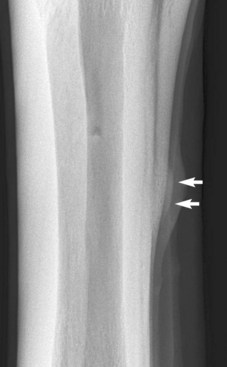
Fig. 15-1 Dorsomedial-palmarolateral oblique radiographic image of the metacarpal region. Soft tissue swelling overlies periosteal new bone (arrows) on the middiaphyseal region of the second metacarpal bone.
Periosteal new bone can also develop as a result of fracture, infection, inflammation, and neoplasia. Inflammation of the interosseous ligamentous attachment between the second metacarpal bone and the McIII or the fourth metacarpal bone and the McIII caused by movement and loading can result in periosteal new bone formation and a splint exostosis. It is curious that some of these formations develop rapidly without associated pain, whereas others can cause persistent pain and lameness for many weeks, despite a similar radiological appearance. The bony protuberances that develop on the proximolateral aspect of the metatarsal regions, often bilaterally, are even more enigmatic. They are rarely associated with clinical signs, although they often have IRU despite having been present for several years.
Endosteal New Bone
Endosteal new bone may develop as a result of trauma (e.g., a cortical or subcortical fracture or trauma at an enthesis) or inflammation, infection, or, less commonly, a tumor (Figure 15-2). Stress fractures of the dorsal cortex of the McIII are accompanied by the development of endosteal new bone, which may be more readily detected than the fracture itself.
Sclerosis
Sclerosis is the localized formation of new bone within bone and results in increased bone mass. It is most easily identified in trabecular bone (Figure 15-3) and occurs in response to several stimuli, including the following:
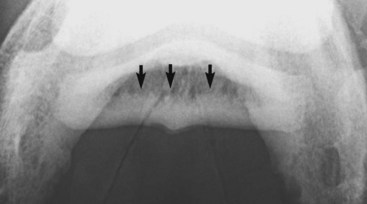
Fig. 15-3 Palmaroproximal-palmarodistal oblique radiographic image of a navicular bone. The flexor cortex is thickened, and there is extensive endosteal new bone (arrows) resulting in increased radiopacity of the spongiosa.
Enostosis-like lesions are the development of bone within the medullary cavity or on the endosteum, resulting in a relatively opaque (sclerotic) area of variable size. They frequently occur adjacent to the nutrient foramen, and the origin is unclear. They may develop as a focal or multifocal lesion. They vary in size and generally are seen in the diaphyseal regions of long bones in the horse. These lesions have been seen most frequently in the humerus, the radius, the tibia, the McIII, the third metatarsal bones, and the femur. When these lesions develop, they have focal intense IRU and may be associated with pain and lameness. However, they also are seen as incidental findings.
Small focal opacities in the proximal metaphyseal region of the tibia have been seen. The origin and clinical significance are not known. Care must be taken in the fetlock region not to misinterpret the radiopacity caused by the ergot as an opaque lesion within the proximal phalanx.
Osteophyte Formation
An osteophyte is a spur of bone on a joint margin that develops as a result of a variety of stimuli, including joint instability, or in association with intraarticular disease, particularly osteoarthritis. Not all periarticular modeling changes at the junction of the articular cartilage and periarticular bone are associated with ongoing joint disease, but radiological differentiation between a subclinical osteophyte and a clinically significant one is difficult. Small spurs frequently are seen on the dorsoproximal aspect of the third metatarsal bone close to the tarsometatarsal joint. Some are quiescent, unassociated with articular pathological findings, whereas others are progressive. Small spurs on the dorsoproximal aspect of the middle phalanx are frequent incidental findings in Warmblood breeds. Mature horses with offset- or bench-knee conformation frequently have spurs on the lateral aspect of the antebrachiocarpal joint without associated clinical signs.
The time of development of an osteophyte after stimulus varies depending on the inciting cause and individual variation. Two weeks to several months may pass before an osteophyte may be identified radiologically. A smoothly marginated osteophyte of uniform opacity is more likely to be long-standing, whereas a poorly marginated osteophyte with a lucent tip is likely to be active.
Some joints seem to have a greater propensity than others for the development of periarticular osteophyte formation. The reason for this tendency is unknown and may in part reflect the ease with which osteophytes can be detected radiologically. Even within what is currently considered a single disease process, osteoarthritis of the distal hock joints (bone spavin), some horses develop predominantly periarticular osteophytes (Figure 15-4), whereas others have narrowing of the joint space and subchondral sclerosis. A third group develops extensive radiolucent areas (Figure 15-5).
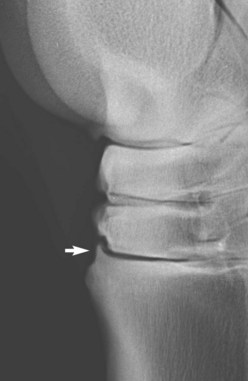
Fig. 15-4 Lateromedial radiographic image of a hock. There is a moderately sized periarticular osteophyte on the dorsoproximal aspect of the third metatarsal bone, close to and traversing the tarsometatarsal joint (arrow).
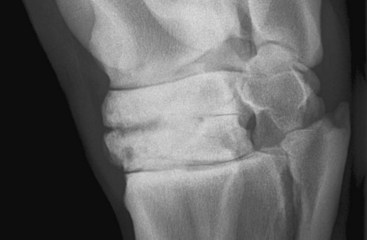
Fig. 15-5 Dorsolateral-plantaromedial oblique radiographic image of a hock. There is narrowing of the centrodistal joint space. Radiolucent regions are seen in the subchondral bone of the central and third tarsal bones dorsomedially. There is loss of trabecular architecture because of medullary increased radiopacity in the central and third tarsal bones. Note also the osseous cystlike lesion in the distal dorsomedial aspect of the third tarsal bone.
Enthesophyte Formation
Enthesophyte formation is new bone at the site of attachment of a tendon, ligament, or joint capsule to bone. Entheseous new bone reflects the bone’s response to stress applied through these structures, such as ligamentous tearing or capsular traction (Figure 15-6). Like osteophytes, enthesophyte formations take several weeks to months to develop and may or may not be associated with clinical signs. Knowledge of ligament, tendon, and capsular insertions is essential to determine which soft tissue structure may have been damaged. In some locations, such as the hock, differentiation between enthesophyte and osteophyte formation is not easy. The attachments of the cranialis tibialis, fibularis tertius, and dorsal tarsal ligament are close to the joint margins of the tarsometatarsal joint, and differentiation between entheseous new bone at these attachments and periarticular osteophyte formation may be difficult. Entheseous new bone may take the form of spur formation, such as at the site of attachment of the common digital extensor tendon on the distal phalanx or the site of attachment of the oblique sesamoidean ligament on the palmar aspect of the proximal phalanx. At other sites, such as the origin of the suspensory ligament (SL) on the plantar aspect of the third metatarsal bone, new bone formation may be more diffuse. Diffuse new bone formation on the caudal aspect of the occiput reflects tearing of the attachment of the nuchal ligament. New bone on the dorsal aspect of the radial, intermediate, ulnar, or third carpal bones may reflect entheseous new bone at the site of attachment of intercarpal ligaments or the joint capsule. Such new bone does not necessarily reflect osteoarthritis, but it may reflect slight joint instability, which itself may predispose to the development of osteoarthritis. Similarly, joint trauma resulting in sprain of periarticular ligaments and subsequent entheseous new bone may ultimately also result in osteoarthritis.
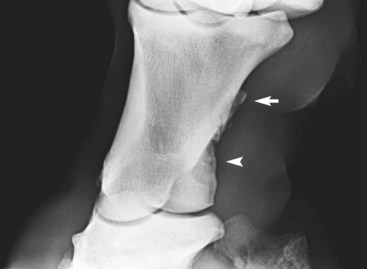
Fig. 15-6 Dorsolateral-palmaromedial oblique radiographic image of a pastern. There is entheseous new bone (arrow) on the middiaphyseal region of the proximal phalanx at the site of insertion of the oblique sesamoidean ligament. There is entheseous new bone further distally (arrowhead) at the origin of the ligament between the proximal phalanx and the cartilage of the foot, the chondrocompedal ligament.
Fracture of an enthesophyte and mineralization within the tendon or ligament attachment also may occur. A relatively common site is the radial tuberosity at the attachment of biceps brachii. Small linear opacities may be seen dorsal to the summits of the spinous processes in the thoracic region, which are associated with tearing of the attachment of the supraspinous ligament.
Spondylosis
Ossifying spondylosis occurs in the caudal half of the thoracic region in the horse. Spondyles (osteophytes) arise from the ventral aspect of the vertebral bodies near the intercentral articulations. The osteophytes extend across the intercentral articulation toward similar osteophytes on adjacent vertebrae. Usually a lucent line persists between the two spondyles, although sometimes complete bridging does occur. Spondylosis may be progressive in cranial and caudal directions, although in some horses it remains static.
New Bone of Unknown Origin
New bone sometimes develops on the dorsal aspect of the diaphysis and distal metaphyseal region of the middle phalanx. The cause of this is unknown. It may be asymptomatic.
Hypertrophic Osteopathy
Hypertrophic osteopathy (Marie’s disease) is typified by palisading periosteal new bone, which appears to be perpendicular to the cortices and irregular in outline in the acute stage. It affects principally the diaphyseal and metaphyseal regions of long bones and spares the joints. In the early stages, very soft exposures must be used to avoid overexposing this relatively radiolucent bone. Later the margins of the new bone become more opaque and smoother, and the distinction between the original cortex and the new bone becomes less obvious. These bony lesions develop secondary to a tumor, abscess, or other lesion in the thorax or abdomen, or in association with diffuse granulomatous disease. The bony lesions may regress and model if the primary lesion can be identified and successfully treated.
Osteitis
Osteitis is inflammation of bone. It may be noninfectious or infectious. Noninfectious osteitis usually is the result of trauma or inflammation of the adjacent soft tissues. It is characterized by new bone formation and, less commonly, bone resorption.
Infectious Osteitis and Osteomyelitis
Infectious osteitis is inflammation of bone as a result of infection. In bones with a myeloid cavity the term osteomyelitis is used if the myeloid cavity is affected. Infection results in soft tissue swelling, new bone formation, and bone resorption. In the distal phalanx and the distal sesamoid (navicular) bone, bone lysis predominates with little new bone formation. In other bones a combination of loss of bone and new bone formation usually occurs. A piece of dead radiopaque bone, or a sequestrum, may develop, surrounded by an area of lucent granulation tissue, the involucrum (Figure 15-7). An area of more radiopaque bone may be laid down surrounding the infected area to wall off infection. A radiolucent tract, a sinus, may develop between the infected tissues and the skin. In the early stages, diagnostic ultrasonography may be more sensitive than radiography for detecting infection.
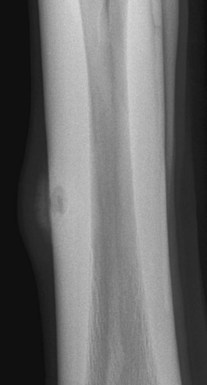
Fig. 15-7 Dorsomedial-palmarolateral radiographic image of a metacarpal region of a 5-year-old Warmblood that had been kicked 2 months earlier. There is soft tissue swelling overlying ill-defined periosteal new bone. There is a radiolucent defect in the lateral cortex of the third metacarpal bone with an acentric, more radiolucent area. These abnormalities are consistent with infectious osteitis.
Osseous Cystlike Lesions and Subchondral Bone Cysts
Osseous cystlike lesions are solitary, circular, or semicircular lucent areas in a bone; they usually develop in the subchondral bone. Sometimes a neck can be identified connecting the cyst to the joint cavity. Cystlike lesions may be unicameral (single chambered) or, less commonly, multicameral. A sclerotic rim frequently surrounds osseous cystlike lesions. Those that develop before skeletal maturity often appear to migrate away from the joint surface as normal endochondral ossification occurs. These single osseous cystlike lesions, which are not associated with any pathological changes in the articular cartilage, should be differentiated from the more poorly defined lucent zones that develop in the subchondral bone in osteochondrosis.
The cause of osseous cystlike lesions is probably multifactorial. Some are true subchondral bone cysts and have a fibrous cystic lining. There is increasing evidence that some of these lesions develop as a response to trauma to the articular cartilage and subchondral bone. They tend to occur most commonly in the medial condyle of the femur and in the center of the glenoid cavity of the scapula. Other osseous cystlike lesions may have a different histological appearance, despite similar radiological appearance. Osseous cystlike lesions in the proximal medial aspect of the radius tend to be associated with periosteal new bone in the region of insertion of the medial collateral ligament. Other common locations include the distal aspect of the McIII and the proximal, distal, and middle phalanges.
Care should be taken when evaluating some bones, especially the phalanges, not to confuse the myeloid cavity with an osseous cystlike lesion.
The development of some osseous cystlike lesions can be followed radiologically. Some start as a small, elliptical depression in the articular surface that progressively enlarges into the subchondral bone. A sclerotic margin develops.
Osseous cystlike lesions may be associated with lameness. Lesions deep in the bone, such as those seen in the first, second, and ulnar carpal bones, are rarely associated with lameness. Cystlike lesions that are close to an articular surface are more likely to be associated with lameness. However, the lameness may be remarkable in its variable nature and severity. Lameness may resolve spontaneously or after surgical intervention, despite persistence of a lesion, radiologically.
The incidence of the development of osseous cystlike lesions is higher in young horses than older horses. Some horses develop a subchondral bone cyst early in life that remains clinically quiescent, only to cause clinical signs later in life. However, subchondral bone cysts and osseous cystlike lesions can develop in skeletally mature horses.
It can be difficult to determine the likely clinical significance of a subchondral bone cyst or an osseous cystlike lesion based purely on radiological appearance. Scintigraphic evaluation may be helpful, because many have IRU, but it can also be confusing because a long-established cystlike lesion that has been clinically quiescent can suddenly become a source of pain without evidence of active bone modeling. It is always important to evaluate radiologically the joint in its entirety, because evidence of secondary osteoarthritis warrants a more guarded prognosis.
Osteochondrosis
Osteochondrosis is considered a disorder of endochondral ossification, although there is increasing evidence that primary subchondral bone lesions also may occur. Osteochondrosis can cause osseous cystlike lesions and osteochondritis dissecans. Osteochondritis dissecans may be generalized although it is evident clinically and radiologically only in certain joints. The femoropatellar, tarsocrural, fetlock, and scapulohumeral joints are most commonly affected. Radiological abnormalities vary depending on the joint involved and include the following:
Radiological changes are not always clinically significant but must be interpreted in the light of clinical signs. Flattening of contour of the lateral trochlear ridge of the femur, with sclerosis of the subchondral bone, may be seen in the absence of lameness in mature horses. However, identical changes may be present in horses with poor hindlimb action referable to pain associated with the femoropatellar joints (Figure 15-8). Flattening of contour or the presence of elliptical depressions in the trochleas of the talus with normal subchondral bone opacity is rarely associated with clinical signs. The more change in the subchondral bone, the more likely that clinical signs will be present. Small osteochondral fragments may become lodged in the synovial membrane and become progressively larger. Trauma may result in such lesions becoming dislodged and mobile and result in clinical signs.
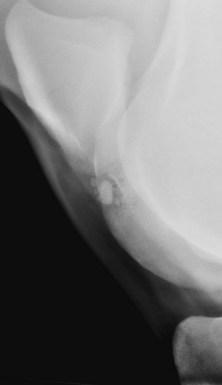
Fig. 15-8 Caudolateral-craniomedial oblique radiographic image of a stifle of an 8-year-old Warmblood show jumper with no history of lameness, undergoing a prepurchase examination. There is a concave depression in the middle of the lateral trochlea of the femur, with mild subchondral increased radiopacity and ill-defined lucent areas. In addition, there are one large and several small radiopaque bodies visible in the concave depression. This is osteochondrosis.
Fracture
A fracture is a discontinuity of bone that may be seen radiologically as one or more lucent lines. Mach lines, or bands, should not be confused with a fracture. A Mach line or band is a radiolucent line created by edge enhancement when one bone edge is superimposed over another bone. Superimposition of the second and fourth metacarpal bones on the McIII commonly results in Mach lines. In a caudocranial view of the proximal tibia, a Mach line is created by superimposition of the tibial crest on the tibia.
Large exostoses involving the second or fourth metacarpal bone or fourth metacarpal or fourth metatarsal bone often result in confusing lucent lines, apparently within the bone, which must not be interpreted as fractures. Likewise, some bony exostoses in these locations incorporate some fibrous tissue, resulting in a persistent lucent line that should not confused with a fracture. A nutrient foramen that traverses a bone should also not be interpreted as fracture. Most nutrient foramina are broader than a recent fracture would be expected to be. A notable exception is the vertically orientated nutrient foramen in the second metacarpal or second metatarsal bone and the fourth metacarpal or fourth metatarsal bone. It is important not to misinterpret lucent lines in the foot created by the frog clefts as fracture lines. Despite careful packing of the frog clefts and sulcus (with, for example, Play-Doh), complete packing is impossible if these are very deep and will result in narrow lucent lines. Care should be taken to see if such a lucent line (e.g., apparently in the navicular bone) extends beyond the bone margins. If the angle of projection is altered slightly, does the position of the line change relative to the medial and lateral borders of the bone? Can the same lucent line be seen in a dorsopalmar image? Care must also be taken not to confuse the lucent bands between separate centers of ossification with fracture lines. The cartilages of the foot and the fibula frequently develop as separate centers of ossification, and bony union may never develop between the different ossification centers, which results in lucent gaps within each bone that persist over the long term. A long, thick hair coat that is wet sometimes can result in the appearance of a lucent line or lines superimposed over bone, which must not be interpreted as a fracture.
Some stress or fatigue fractures are never detectable radiologically. Some can be seen as one or more lucent lines, or, if fractures are chronic, there may be endosteal new bone or increased medullary opacity. If a chronic stress fracture traverses a cortex, there may be periosteal callus, although the fracture line itself may not be detectable.
Ideally, the postulated radiolucent fracture line should be detectable on more than one radiographic image to confirm the presence of a fracture. However, this is not always possible, especially if the fracture is incomplete and nondisplaced. This is particularly true for fractures of the distal phalanx and incomplete dorsal cortical fractures of the proximal phalanx. For best results, the x-ray beam must be perpendicular to the plane of the fracture line. Many different views that differ by only 5 degrees in angle of projection may be required for detection of a fissure fracture. With a complete fracture, many different views may be required for determination of the precise configuration of the fracture.
Some fractures are not detectable in the standard radiographic images of the area under examination. A fracture of the lateral palmar process of the distal phalanx may not be detectable in lateromedial, dorsopalmar, and dorsoproximal-palmarodistal oblique images. Dorsolateral-palmaromedial oblique or sometimes a palmaroproximal-palmarodistal oblique image, or a dorsal 60° proximal 45° lateral-palmarodistal medial oblique view, is required. Sagittal and some slab fractures of the third carpal bone are visible only in dorsoproximal-dorsodistal oblique images of the flexed carpus (the skyline projection). Similarly, fractures of the medial pole of the patella are only detectable in cranioproximal-craniodistal oblique images of the flexed stifle.
The normal healing process of a fracture involves osteoclasis along the fracture line within 5 to 10 days, resulting in initial broadening of the fracture line. Therefore if a fracture is suspected but cannot be seen in the acute stage, radiography should be repeated after 5 to 10 days. Care should be taken not to underexpose the radiograph, because a faint lucent line may be missed. Paradoxically, early callus formation, which develops within 14 to 21 days, will be missed if the radiograph is overexposed or not viewed over high-intensity illumination or if a computer screen is viewed in excessively bright light.
A fracture should be evaluated to determine whether it is complete or incomplete and simple or comminuted, whether fracture fragments are displaced, whether articular involvement is present, and whether any other concurrent pathological condition is present that may influence the prognosis. An apical proximal sesamoid bone fracture may be accompanied by severe desmitis of the branch of the SL, which prognostically is the more severe injury. Biaxial transverse fractures of the proximal sesamoid bones result in disruption of the suspensory apparatus and severe risk of compromising the blood supply to the distal part of the limb. An avulsion fracture of the proximal phalanx at the attachment of the lateral collateral ligament of the metacarpophalangeal joint will result in loss of joint stability.
It is important to be aware that with a single, complete fracture, two lucent lines representing the fracture traversing, for example, the dorsal and palmar cortices, may be seen. These lines should not be confused with two fractures. However, care must always be taken to make sure that there is not more than a single fracture line. For example, a common fracture in steeplechasers that fall is a vertical fracture through the body of the accessory carpal bone. These horses have a good prognosis with conservative management. Rarely, one or more chip fractures involving the articular margins of the bone occur; such injuries warrant a much more guarded prognosis. An event horse that hits a fixed fence during cross-country jumping may fracture the medial trochlear ridge of the femur. Fracture fragments may readily be seen in a lateromedial image of the stifle. Such fractures also can be accompanied by a fracture of the medial pole of the patella, which can be seen only in a skyline image. Therefore it is essential to obtain a complete radiographic series and interpret each radiograph in its entirety.
Intraarticular fractures occur when a break in the articular surface occurs. Unless some degree of displacement is present, damage to the articular cartilage may not be seen but is assumed to exist. A small degree of displacement is indicated by the presence of a slight step in the two sides of the articular portion of the fracture line. Comminution at the joint surface sometimes occurs. This may not be evident in standard radiographic images. For example, lateral condylar fractures of the McIII may be accompanied by a Y fragment at the articular margin that is detectable only in dorsodistal-palmaroproximal oblique images.
Fractures on an articular margin are called chip fractures. In some locations, such as the antebrachiocarpal and middle carpal joints, it is important to assess the radiographs carefully for evidence of preexisting osteoarthritis, which would have predisposed to the fracture and may influence the prognosis. The veterinarian should bear in mind that more than one chip may exist.
Chip fractures of the articular margins should be differentiated where possible from separate centers of ossification, avulsion fractures that occurred in the neonatal period, and ectopic mineralization. The position of the mineralized body relative to the articular margin, the size and shape of the body, and the contour of the articular margin should all be assessed carefully. A recent chip fracture may have a sharp edge, and a detectable fracture bed from which it originated may be seen. Separate centers of ossification usually are well rounded and uniformly opaque with no discernible fracture bed. It is important to recognize that such pieces may be clinically silent, but some are associated with lameness. The clinical significance must be interpreted in the light of clinical signs and the response to diagnostic analgesia. Ectopic mineralization may be present within the joint capsule.
Osteochondral fragments, the result of osteochondritis dissecans, should not be confused with a fracture. Osseous fragments may be seen, for instance, distal to the trochleas of the talus at the entrance to the talocalcaneal-centroquartal (proximal intertarsal) joint. These fragments may have originated from the distal (cranial) aspect of the intermediate ridge of the tibia and usually are of no clinical significance. Well-rounded, smoothly marginated osseous opacities may be seen on the proximoplantar aspect of the proximal phalanx. These may be the result of avulsion fractures of the short, deep cruciate sesamoidean ligaments that were sustained early in life; they are not always clinically significant but may compromise performance at maximum levels. The variably present first and fifth carpal bones should not be mistaken for fractures.
In some joints, it may not be possible to determine radiologically the origin of a fracture fragment. For example, fragments in the femorotibial joint frequently are not associated with any detectable disruption in bone outlines, and only arthroscopic evaluation of the joint allows determination of the origin. It also may not be possible to determine whether an accompanying soft tissue pathological condition exists. A fracture of the medial intercondylar eminence of the tibia may occur alone. The prognosis is good with surgical removal. Sometimes, however, concurrent cranial cruciate ligament damage exists at its insertion cranial to the eminence, resulting in a much more guarded prognosis.
A slab fracture is a fracture that extends from one joint surface to another (e.g., from the proximal to the distal aspect of the third carpal bone or central or third tarsal bones). These fractures can be extremely difficult to detect in the acute stage if not displaced.
In some locations, determination of the origin of a fracture fragment is facilitated by ultrasonographic examination. For example, a fragment on the palmar aspect of the fetlock may be an avulsion fracture of the insertion of the palmar annular ligament on the proximal sesamoid bone, and this is readily confirmed ultrasonographically.
Fracture healing depends on many factors, including the age of the horse, its nutritional and metabolic status, the site and stability of the fracture, the presence or absence of periosteum or infection, and the blood supply to the bone. After initial mineral resorption along the fracture line and formation of fibrous callus, calcified periosteal and endosteal callus develop. The amount and quality of callus that develops depends on the degree of stability at the fracture site and the presence or absence of infection. If a fracture is stable, either because it is incomplete or because it has been stabilized by internal fixation, healing is predominantly by primary union and endosteal reaction with minimal periosteal callus. Instability results in the development of periosteal callus.
The fracture may become stable long before the fracture line disappears radiologically. Healing may be complete within 6 to 12 weeks, with progressive narrowing and ultimate disappearance of the fracture line. However, healing of some fractures takes considerably longer. A horse may be sound and able to withstand full work despite the persistence of a radiolucent fracture line. In some locations (e.g., lateral condylar fractures of the McIII) a persistent lucent line may be associated with recurrent and persistent pain.
Some bones tend to heal by fibrous union, resulting in a persistent lucent line. These include the accessory carpal bone, the proximal and distal sesamoid bones, and the distal phalanx. Unless a fracture of the navicular bone is stabilized by internal fixation, lucent areas tend to develop in the adjacent bone along the fracture line. These indicate a chronic fracture of the navicular bone of at least 6 to 8 weeks’ duration.
If a fracture line persists beyond 6 months, it can be considered to be a delayed union. Sclerosis of the bone adjacent to the fracture line may be present, and the ends of the bone may be slightly flared. Delayed union is not uncommon, but nonunion is unusual in the horse except in those bones that tend to heal by fibrous union.
Aging of a fracture is not easy to determine radiologically with any accuracy. The presence of periosteal callus (Figure 15-9) indicates a fracture of at least 14 days’ duration and often substantially longer. An acute fracture has very clearly defined margins, which become less distinct as resorption occurs along the fracture line during early healing.
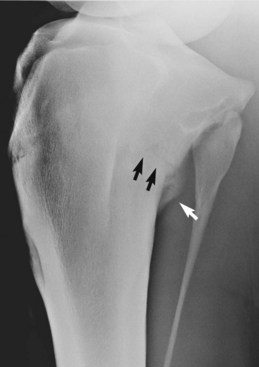
Fig. 15-9 Craniolateral-caudomedial oblique radiographic image of the proximal aspect of the tibia of a 3-year-old Thoroughbred filly. Periosteal callus formation (arrows) on the caudoproximal aspect of the tibia is associated with a stress fracture. The oblique lucent line in the proximal aspect of the tibia is a nutrient foramen.
Degenerative Joint Disease: Osteoarthrosis or Osteoarthritis
Arthritis means inflammation of a joint. The term osteoarthritis or osteoarthrosis indicates that bone has become involved and that a soft tissue component may (-itis) or may not (-osis) be present. The term secondary joint disease sometimes is used to denote that the degenerative changes are secondary to a known condition, such as infection or osteochondrosis. Any condition that damages cartilage, causes joint instability, or places abnormal forces on the joint may result in osteoarthritis. It may develop as a result of abnormal conformation or be the result of wear and tear on an athletic horse. However, advanced osteoarthritis sometimes is seen in relatively young horses with no identifiable predisposing cause.
Radiological abnormalities associated with osteoarthritis include the following:
However, it is important to recognize that in some joints advanced osteoarthritis may be present without any detectable radiological change. Periarticular osteophytes are not necessarily synonymous with clinically significant osteoarthritis. It is relatively unusual to see subchondral lucent zones in high-motion joints. These occur more commonly in the low-motion joints, such as the distal hock and proximal interphalangeal joints.
A relatively poor correlation exists between the degree of radiological change associated with osteoarthritis and the degree of pain and thus lameness. Advanced radiological change may be present when lameness is first recognized, which clearly must have predated the onset of recognizable clinical signs. In contrast, obvious lameness associated with joint pain may be present without detectable radiological change. Widespread wear lines may be present on the articular cartilage without associated radiological change.
Therefore dating the likely onset of clinical signs based on the radiological appearance of a joint can be difficult. It also can be difficult to predict the likely progression of minor radiological changes, such as small periarticular osteophytes, which may be present without clinical signs at, for example, a prepurchase examination.
Luxation and Subluxation
Luxation is the complete loss of contact between articular surfaces. Subluxation is partial loss of contact between joint surfaces and may be intermittent. Both are usually the result of trauma, although congenital luxation of the patella occurs occasionally. Subluxation of the proximal interphalangeal joint may occur without any obvious cause, especially in hindlimbs. Subluxation is easy to identify radiologically, but the radiographs must be evaluated carefully to identify any concurrent fracture that will adversely influence prognosis.
Subluxation may not be obvious radiologically, and stress radiographs obtained with the limb not bearing weight, with pressure applied in a mediolateral or dorsopalmar direction, may be necessary to determine if the bones can be moved relative to each other.
Dystrophic and Metastatic Mineralization
Soft tissue mineralization is classified as metastatic or dystrophic. Metastatic mineralization is the deposition of mineral in normal tissues and is associated with hypercalcemia, hyperphosphatemia, or hypercalciuria and is unusual in the horse. Dystrophic mineralization is the deposition of mineral in injured, degenerating, or necrotic tissue and occurs quite commonly in the horse in, for example, a damaged SL. It can occur secondary to any injury to soft tissue subsequent to infarction, hemorrhage, or inflammation. Dystrophic mineralization may ultimately become ossified.
Radiographic Examination
For each joint or region to be examined there is a standard radiographic technique that includes a basic minimum number of views for routine evaluation of the region. This usually includes a minimum of four images for joints distal to the elbow and stifle. However, radiographic technique must be flexible. For example, if a fracture is suspected, various images differing only slightly in angle of projection may be required. Some fractures are visible only on special projections. For example, a fracture of the medial pole of the patella may be detected only in a cranioproximal-craniodistal oblique image. Exposure factors need to be altered depending on the size of the horse and the area being evaluated. Exposure factors ideal for assessing trabecular structure within medullary bone result in overexposure of immature periosteal new bone. Underexposure may result in a fracture line or alterations in corticomedullary demarcation and trabecular structure being missed.
It is also important to realize how position sensitive some radiological abnormalities are and how easily artifacts can be created. For example, the accurate evaluation of corticomedullary demarcation and trabecular structure within the navicular bone in a palmaroproximal-palmarodistal oblique image depends highly on the position of the limb and the angle of the x-ray beam. The optimum angle of the x-ray beam depends on the shape of the foot. Inappropriate positioning of the foot or use of an x-ray beam that is not tangential to the flexor surface of the navicular bone will result in artifacts.
The x-ray beam should be coned down on the area of interest as much as possible and centered on the area of interest. Lesions will be missed if an attempt is made to evaluate too much in a single image (e.g., the fetlock, pastern, and foot). It frequently is helpful to obtain comparative images of the contralateral limb. These should be obtained with identical exposure factors and similar positioning of the limb and angulation of the x-ray beam for accurate evaluation. Comparison of radiographs with a known normal example of similar age can also be helpful. It is thus useful to compile an image library of normal examples.
Interpreting Radiographs
Radiographs should be evaluated in a systematic way. First, the quality of the radiographs should be assessed. Is the horse positioned appropriately? Are the radiographs of adequate quality? Are there any artifacts? The films should be viewed on a proper viewing box, both close to and from far away. The veterinarian should view the films under both normal and high-intensity illuminations, should interpret the entire film, and should follow all the bone margins and then evaluate the internal architecture. For computed or digital radiography the computer screen should be of high resolution and should be viewed in low-intensity light. The image should be windowed if necessary (i.e., alteration of brightness and contrast). The veterinarian should avoid lesion or disease spotting but aim to describe the radiological abnormalities and then deduce potential causes of the abnormality.
If a lesion is suspected but further information is required, the clinician should consider coning down, altering the exposure factors, slightly changing the angle of projection, or using special views. The radiographs should be compared with normal bone specimens, with an awareness that not all radiological abnormalities are necessarily of clinical significance. Knowledge of normal anatomy and normal variations is essential, together with knowledge of the sites of ligament, tendon, and joint capsule insertions.1,2 Recognition of breed differences also is important. Radiological abnormalities must be interpreted in the light of clinical signs. It is also important to recognize that there are discipline differences in the potential clinical significance of some lesions. For example, mild osteoarthritis in the antebrachiocarpal or middle carpal joint may compromise the performance and career of a flat racehorse but be of little clinical significance for a show horse or low-level show jumper. It must also be recognized that it is often not possible to predict the future development of lesions. A horse with a small osteophyte on the dorsoproximal aspect of the third metatarsal bone may look identical in a later year, but a different horse may have developed extensive periarticular osteophytes involving both the centrodistal and tarsometatarsal joints.
Determining the Age of a Lesion
It is often not possible to accurately determine the age of a lesion that is identified radiologically. Periosteal new bone usually takes at least 14 days to be visible radiologically after trauma. Once new bone is well consolidated and smoothly marginated, it is impossible to determine how long it might have been present. A nondisplaced fracture may take up to 10 days to become evident radiologically; loss of clarity of the fracture margins may indicate that it has been present longer. Radiological changes compatible with osteoarthritis can precede the onset of clinical signs and may be relatively advanced when lameness is first recognized. It is not possible to determine when the changes first developed.
Radiographic Technique
Standard radiographic projections are outlined in Table 15-1. For detailed descriptions of radiographic technique for all regions of the musculoskeletal system, readers are referred to Clinical Radiology of the Horse.3 Throughout this text, radiographic images are described using the technique advocated by the American College of Veterinary Radiology.4
TABLE 15-1 Standard Radiographic Projections and Suggested Extra Images
Cd, Caudal; Cr, cranial; D, dorsal; Di, distal; Pr, proximal; L, lateral; M, medial; O, oblique; Pa, palmar; Pl, plantar; Pr, proximal; V, ventral.
All radiographs should be permanently labeled photographically on the film, by use of special tape attached to the cassette when the film is exposed, a labeling light box system in the darkroom, or a computer. Labels should include at least the identity of the horse, the date, the limb examined, and medial or lateral when appropriate. The following facts also are preferable on the label: the name of the owner or agent of the horse, the name of the veterinary practice, and the identity of the view (e.g., PaL-DMO). For ease of identification, it can be helpful if the horse’s label is always positioned on the lateral aspect of the cassette or imaging plate; if a medial marker were omitted, it would then still be possible to differentiate between the medial and lateral sides.
Radiographs are part of the horse’s permanent medical and legal record and should always be retained by the veterinary practice for future reference unless the owner of the horse gives permission for the radiographs to be transferred to another veterinary practice. Electronic or copy radiographs are easily produced if a client wishes to have his or her own copy.
Digital radiography generally uses imaging plates with a larger exposure latitude compared with traditional film-screen combinations. Therefore it is potentially easier to obtain good-quality images with a single exposure. With a computerized system the images can be manipulated to enhance contrast and magnify areas of interest; therefore lesions that might previously have been missed may be more readily identified. With an appropriate archiving system, all images can be stored digitally rather than printed. It is crucial that an adequate backup system be in place to protect against data loss. Digital radiography is not a substitute for good radiographic technique but offers the advantage of providing images that may be of superior quality and can be stored and transmitted electronically.