Chapter 34 Laminitis
 Pathophysiology of Laminitis
Pathophysiology of Laminitis
A tough, flexible, connective tissue suspensory apparatus suspends the distal phalanx (DP) to the inside of the hoof wall. The surface of the inner hoof wall is folded into leaflike lamellae (laminae) to increase the surface area of attachment between the hoof and the DP. A horse has laminitis when this attachment apparatus is compromised. Without the DP properly attached to the inside of the hoof, the weight of the horse and the forces of locomotion drive the DP down and away from the hoof wall. Arteries, veins, and nerves are sheared and crushed, and secondary to the lamellar pathology, the corium of the coronet and sole is damaged. Eventually even the DP itself may suffer deterioration and lysis of its dorsal margin. Unrelenting pain in the feet (bilateral forelimb usually) and a characteristic lameness occur. In acute laminitis the lamellar tissue suspending the DP from the inner hoof wall is affected at the junction between the connective tissue of the dermis or corium (the bone side) and the basal cell layer of the epidermal lamellae (the hoof side). This junction, the basement membrane zone or dermal/epidermal interface, appears to be the weak link in an otherwise robust and reliable structure. Wholesale epidermal cell detachment from and lysis of the lamellar basement membrane may occur,1,2 leading to disruption of lamellar anatomy and ultimately compromise (to varying degrees) of the suspensory attachment between the DP and hoof. Associated with basement membrane lysis and dysadhesion are changes to lamellar epidermal cell morphology that result in lamellar stretching and attenuation. Thus whether by loss of basement membrane attachment or by lamellar stretching (or both), the DP draws away from the inner hoof wall and sinks downward. The degree of separation and sinking varies between horses affected with laminitis, ranging from barely detectable (mild) to catastrophically severe (“sinkers”). Ponies and small horses are usually less severely affected than their heavier cousins. A good correlation exists between the severity, as seen with the microscope (histopathology), and the degree of lameness (using the Obel grading system3) shown by the horse.1 When a horse first shows laminitic pain, the anatomy of the hoof wall lamellae is already compromised. The higher the lameness grade, the more severe the microscopic damage. Any activity that places stress on an already weakened lamellar attachment apparatus (such as forced exercise) exacerbates damage and is contraindicated. The use of nerve blocks to eliminate pain encourages locomotion and does more damage. Favorable outcomes are associated with effective preventive and early therapeutic strategies.
Pathophysiology
Pathological deformation of the lamellar attachment apparatus begins during the developmental phase of laminitis. Tightly controlled metabolic processes are targeted, causing lamellar-specific pathology. One mechanism behind lamellar deformation involves lamellar enzymes. Enzymes are normal constituents of lamellar cells and respond to the stresses and strains of equine life and to constant growth. Sufficient enzyme is manufactured locally to release epidermal cell-to-cell and cell-to-basement membrane attachment as required, maintaining the correct shape and orientation of the lamellae. From time to time injury to the basement membrane requires its lysis and reconstruction. The controlled release of matrix metalloproteinases (MMPs) and specific tissue inhibitors of MMP (TIMPs) keeps repair and modeling in equilibrium.4,5 Normally the hoof lamellae slowly migrate distally past the stationary basement membrane that is firmly attached to the connective tissue covering the dorsal surface of the DP. Distal movement occurs because the hoof wall6 continually proliferates at the coronet and moves downward past the stationary DP by a process of controlled enzymatic modeling of the lamellar epidermis and the basement membrane zone. Accumulating evidence suggests that during developmental laminitis, compromise of the basement membrane zone occurs when production of constituent lamellar enzymes is increased and they are activated out of control. The enzymes known to be involved are metalloproteinase-2 (MMP-2), metalloproteinase-9 (MMP-9), membrane-type metalloproteinase (MMP-14), and aggrecanase (ADAMTS-4), and singly or together they destroy key components of the lamellar suspensory apparatus.7-10 A second, newly discovered lamellar deformation mechanism is seen in hyperinsulinemic horses. Experimental hyperinsulinemia is profoundly laminitogenic in ponies and horses, inducing clinical signs in 40 to 72 hours.11,12 Limited basement membrane dysadhesion occurred in insulin-affected horses but not to the extent seen in experimental carbohydrate-induced laminitis and was virtually absent in ponies. Mitosis, normally infrequent among lamellar epidermal cells, was a feature of hyperinsulinemic lamellar histopathology possibly contributing to lamellar lengthening.13 In laminitis induced by dosing horses with extract of Black walnut (Juglans nigra) heartwood, there was no evidence of basement membrane breakdown.14,15
Histopathology of Acute Laminitis
Most descriptions of laminitis histopathology derive from laminitis induced experimentally with a single large dose of carbohydrate of either grain starch or oligofructose.3,16,17 The sequences of microscopic events that lead to clinical laminitis follow a consistent temporal pattern,18 and the stages of histological laminitis can be identified by the degree of severity of these changes. Making the lamellar basement membrane clearly visible is important and requires staining lamellar tissues with periodic acid–Schiff (PAS) stain or with immunohistochemical methods using basement membrane–specific antibodies.1,2
Normal lamellar anatomical characteristics, assessed before allocating a laminitis grade to a section of lamellar hoof tissue, are as follows (Figure 34-1):
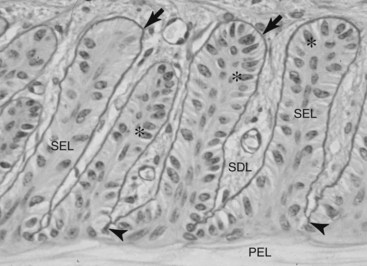
Fig. 34-1 Micrograph of normal hoof lamellae stained to highlight the basement membrane. The basement membrane (arrows) of each secondary epidermal lamella (SEL) is a magenta (black in the figure) line closely adherent to the SEL basal cells. At secondary dermal lamellae (SDL) tips, between the bases of each SEL, the basement membrane (BM) penetrates deeply (arrowheads) and is close to the anuclear, keratinized, primary epidermal lamella (PEL). The SEL tips are rounded (club-shaped). The basal cell nuclei are oval in shape (stars) and positioned away from the BM at the apex of each cell. The long axis of each basal cell nucleus is at right angles to the long axis of the SEL. The SDL are filled with connective tissue even at their very tips, between the SEL bases. These parameters of hoof lamellar anatomy form the basis of the histological grading system of laminitis histopathology. Stain, Periodic acid–Schiff; bar, 10 µm.
Grade 1 Histopathology
The earliest change attributable to laminitis is loss of shape and normal arrangement of the lamellar basal and parabasal cells. The basal cell nuclei become rounded instead of oval and take an abnormal position in the cytoplasm of the cell. The secondary epidermal lamellae become stretched, long, and thin, with tapering instead of club-shaped tips. These changes were present at 12 hours in serial lamellar biopsies taken after oligofructose dosing.18 First noticeable at the tips of the secondary epidermal lamellae, teat-shaped bubbles of loose basement membrane form. PAS staining shows this best (Figure 34-2).
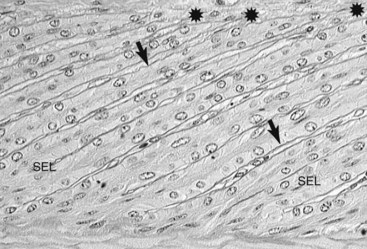
Fig. 34-2 Grade 1 histological laminitis (periodic acid–Schiff stain). Micrograph showing hoof lamellar tissues stained to highlight the basement membrane. The basement membrane (arrows) is stained dark magenta (black in the figure). At the now-tapered tips of the secondary epidermal lamellae (SELs), the basement membrane has lifted away (stars) from the underlying basal cells. Between the SEL bases the basement membrane (BM) is in its normal position, close to the primary epidermal lamellae; bar, 10 µm.
Examination of laminitis tissues with the electron microscope confirms lysis and separation of the lamellar basement membrane.19 Importantly, the greater magnification shows widespread loss of basal cell adhesion plaques (hemidesmosomes) and contraction of the basal cell cytoskeleton away from the inner cell surface. Electron microscopy shows why the basement membrane separates from the feet of the basal cells. The filaments that anchor hemidesmosomes to the lamina densa of the basement membrane no longer bridge the dermal/epidermal interface (Figure 34-3).13
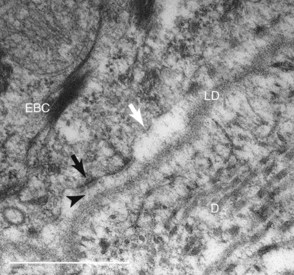
Fig. 34-3 Electron micrograph of hoof lamellar tip developing laminitis. The lamina densa (LD) of the basement membrane is separating from the plasmalemma of the lamellar basal cell (EBC). Some hemidesmosomes (black arrow) appear undamaged, but others (white arrow) have faded, are losing their anchoring filament attachments, and are drawing away from the basement membrane. Normally anchoring filaments (arrowhead) bridge the lamina densa firmly to the EBC. D, Dermis.
Grade 2 Histopathology
Because the basement membrane is no longer completely tethered to the basal cells, it slips farther away with each cycle of weight bearing by the horse. Portions of the lamellar basement membrane are lysed initially between the bases of the secondary epidermal lamellae (see Figure 34-2). The basement membrane retracts from the tips of secondary epidermal lamellae, taking the dermal connective tissue with it. The basement membrane–free epidermal cells appear not to be undergoing necrosis, at least initially, and clump together to form amorphous, basement membrane–free masses on either side of the lamellar axis.1
Grade 3 Histopathology
In laminitis the worst-case scenario is a rapid and near-total basement membrane separation from all the epidermal lamellae of the hoof toe, quarters, heel, and bars. Sheets of basement membrane peel away to form aggregations of loose, isolated basement membrane in the connective tissue adjoining the lamellae. The epidermal lamellar cells are left as isolated columns with little viable connection to the dermal connective tissue still attached to the DP. The hoof lamellar tips slide away from the basement membrane connective tissue attachments, at first microscopically, but as the degree of separation increases, the distance between hoof and DP becomes measurable in millimeters (Figure 34-4) and can be detected radiologically.20 This manifests clinically as a sinker. Because the basement membrane is the key structure bridging the epidermis of the hoof to the connective tissue of the DP, wholesale loss and disorganization of the lamellar basement membrane follow and inexorably lead to the pathology of hoof and bone that characterizes the chronic stage of laminitis.
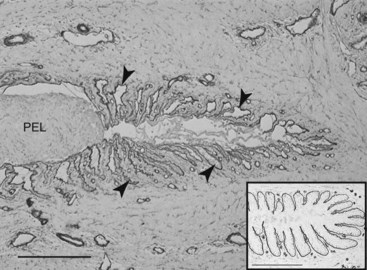
Fig. 34-4 Grade 3 histological laminitis (immunostain). The basement membrane of a lamellar tip is highlighted by type IV collagen immunostaining. The tip of the primary epidermal lamella (PEL) has completely detached from its basement membrane. The PEL basal cells are now an unattached, amorphous mass. Collapsed tubes of basement membrane, now empty of epidermal cells, are still attached to connective tissue (arrowheads). The PEL has already moved 0.03 mm from its dermal compartment, and soon the distance will be measured, using a tape measure, on a radiograph. The inset shows a normal lamellar tip immunostained the same way. Type IV collagen immunostain; bars, 10 µm.
The laminitis process also affects the lamellar capillaries. As the basement membrane and the connective tissue between the secondary epidermal lamellae disappear, so do the capillaries. They become obliterated, compressed against the edges of the primary dermal lamellae. Without a full complement of capillaries in the lamellar circulation, blood probably bypasses the capillary bed through dilated arteriovenous shunts,21 changing the nature of the foot circulation. A bounding pulse becomes detectable by finger palpation of the digital arteries. Furthermore, epidermal cell necrosis, intravascular coagulation, and edema are not universally present in sections made from tissues in the early stages of laminitis. The vessels in the primary dermal lamella, even the smallest, are predominantly open, without evidence of microvascular thrombi. The gross anatomical appearance of freshly dissected laminitis tissue is dryness. Sometimes the lamellae just peel apart.
Leukocytes, Inflammation, and Laminitis
It is rare to detect leukocytes in the lamellar tissues of normal horses.14 However, extravasation of leukocytes into the perivascular lamellar dermis occurs in carbohydrate-,1 Black walnut extract–,14,22 and hyperinsulinemia-induced13 forms of laminitis. Because leukocytic infiltration of tissues is associated with inflammation, the discovery that leukocytic infiltration is common to most, if not all, forms of laminitis has reemphasized an inflammatory pathway to laminitis development. There is molecular evidence that inflammatory mediators may activate many of the processes known to damage the lamellar interface.23,24 Polymorphonuclear leukocytes are rich in MMP-9, and their presence within lamellar epidermal compartments in grade 3 histopathology1 suggests that this basement membrane–degrading enzyme may have a pathological role in disease development. Also neutrophils produce reactive oxygen species and proinflammatory cytokines that probably contribute to cellular damage within the lamellar milieu.15 Lamellar damage and leukocyte infiltration are readily detected by calprotectin immunostaining,14,25 and because leukocyte infiltration precedes the expression of calprotectin in the lamellar epidermis, a role for leukocytes in initiating lamellar pathology has been suggested.22,23 However, carbohydrate-induced laminitis studied at the 12-hour postdosing time point shows basement membrane degradation occurring in advance of leukocyte infiltration, thus downplaying an initiating role for leukocytes in lesion development.25
Laminitis Theories
The enzymatic theory of laminitis, based on the triggering of lamellar MMP activity,26,27 challenged the alternative view that laminitis was caused by ischemia and ischemia/reperfusion injury damaging epidermal lamellae because blood flow was impeded.28 Evidence against ischemia/reperfusion involvement in Black walnut extract–induced laminitis came from studies measuring xanthine oxidase, a reactive oxygen intermediate that increases in tissues affected by ischemia and ischemia/reperfusion. Xanthine oxidase was absent in tissues with Black walnut extract–induced lamellar pathology, suggesting that a global lamellar hypoxic event had not occurred.29 Furthermore, there is evidence from three independent international laboratories that the foot circulation during the developmental phase of both carbohydrate- and hyperinsulinemia-induced laminitis is vasodilated.30-33 Laminitis did not occur if the foot was in a state of vasoconstriction during the developmental phase, suggesting that the trigger factors only cause laminitis if they reach the lamellar tissues at a high enough concentration and over a long enough time.30 In current laminitis therapy there is a consistent lack of efficacy of drugs addressing ischemia, suggesting other pathogenic processes, such as inflammation, may be more important.
The actual trigger factors of laminitis remain unidentified. Gram-negative bacterial endotoxin has been detected in the circulation of horses with carbohydrate-induced laminitis34 and is presumed to play a role in initiating lamellar pathology. Horses with colic, colitis, pleuropneumonia, and retained fetal membranes, the conditions often associated with laminitis development,35 develop pyrexia and appear “endotoxic,” but this is usually inferred from clinical signs rather than confirmed by endotoxin assay. Tumor necrosis factor and interleukin-6 (IL-6) along with other cytokines are expressed by mononuclear phagocytes within minutes of exposure to endotoxin.36-38 Decreases in digital blood flow and lamellar perfusion, secondary effects mediated by the platelet-derived vasoconstrictors 5-hydroxytryptamine and thromboxane, follow intravenous endotoxin administration.37 However, laminitis or even foot pain has never been elicited by the experimental administration of endotoxin into either the bloodstream or the peritoneal cavity.39,40 Gram-negative bacteria disappear early, before the onset of clinical signs, from hindgut samples taken from horses developing carbohydrate overload-induced laminitis, suggesting no role for endotoxin in laminitis pathogenesis.41 Instead a plethora of absorbed microbial toxins may affect lamellar compromise, including gram-positive exotoxins from the explosive proliferation of hindgut streptococci after carbohydrate overload.
Lamellar disintegration of laminitis occurs well before clinical signs. The molecular conformation of the lamellar basement membrane is altered 12 hours after dosing with oligofructose, and a major constituent of the basement membrane, collagen IV, begins to disappear. Previously, damage to the lamellar basement membrane was attributed to MMP release and activation, but new evidence places MMP activation many hours later than other molecular events.25 An enzyme capable of modifying the proteoglycan components of lamellar basement membrane is ADAMTS-4 (A Disintegrin And Metalloproteinase with ThromboSpondin motifs), the gene for which has the greatest fold increase of any thus far discovered in laminitis development.10,25,42 ADAMTS-4 gene expression occurs early in laminitis development, and the gene product may play a central role in the pathophysiology of the disease.
Metalloproteinase Inhibitors
The activity of tissue MMPs correlates strongly with the degree of malignancy and invasiveness of lethal human tumors, such as malignant melanoma, breast cancer, and colon cancer.43 Research in this field has generated a wide range of chemical agents capable of inhibiting MMP activity in vitro and in vivo. BB-94 (Batimastat, British Biotech, Oxford, England) blocks the activity of the laminitis MMPs in vitro and has the potential to be a useful tool in preventing and managing acute laminitis. Whether MMP inhibitors can prevent or ameliorate naturally occurring laminitis has yet to be established.
Natural Trigger Factors
Insulin
To test the hypothesis that hyperinsulinemia triggers laminitis, normal, lean ponies with no previous history of insulin resistance or laminitis were subjected to prolonged hyperinsulinemia and euglycemia.44 All the ponies developed laminitis within 72 hours of hyperinsulinemia, a finding supporting the importance of insulin in the pathogenesis of endocrinopathic laminitis. Standardbred horses subjected to the same hyperinsulinemia/euglycemia clamp protocol also developed clinical laminitis but sooner (<48 hours) and with histopathology notable for lamellar basement membrane separation.33 Hyperinsulinemia causes profound changes in the peripheral microcirculation,45 and a vascular (ischemic) pathogenesis for laminitis has long been sought.46 However, hoof wall surface temperature, measured continuously in the hyperinsulinemic and control horses, showed hyperinsulinemia induced early-onset, prolonged vasodilation. Lamellar insulin receptors are located within blood vessels (but not elsewhere) and in the presence of insulin activate vascular nitric oxide synthase to produce the potent vasodilator nitric oxide. Perhaps unregulated vasodilation results in excessive cellular uptake of glucose, lamellar glucotoxicity, and oxidative stress similar to that involved in the pathogenesis of atherosclerosis and the associated up-regulated MMP-9 activity in human diabetes.47
Trigger Factors of Bacterial Origin
Equine lamellae, cultured in vitro, have tested resistant to virtually all known cytokines, tissue factors, and prostaglandins. Gram-negative bacterial endotoxin, extract of Black walnut (J. nigra), and even anaerobic culture conditions fail to induce lamellar separation or significant MMP activation.48 Equine IL-6 added to cultured lamellar explants fails to activate lamellar MMPs or cause basement membrane disruption at the lamellar interface.25 Some notable exceptions occur, however. Factors present in the supernatant of cultures of Streptococcus bovis (S. bovis) isolated from the equine cecum activate equine hoof MMP-2 and cause lamellar separation.48 During carbohydrate overload, rapidly proliferating species of hindgut streptococci, predominantly S. bovis (now Streptococcus lutetiensis), ferment carbohydrate and produce large quantities of lactic acid.41,49,50 In the presence of virtually unlimited substrate, the population of S. bovis increases exponentially and then dies and lyses en masse. The liberated cellular components of lysed hindgut streptococci may cross the mucosal barrier of the damaged hindgut and reach the hoof lamellae hematogenously to initiate laminitis. Microbial and other factors probably associate with basement membranes throughout the body but probably only cause damage to lamellar basement membranes because of their uniquely equine involvement in weight bearing.
Equine Metabolic Syndrome
The term equine metabolic syndrome refers to horses with a history of laminitis, insulin resistance, cresty necks, and increased adipose tissue deposits in the withers, dorsal area of the back, and rump.51 Elevated serum insulin concentrations distinguish ponies that are susceptible to dietary pasture-associated laminitis.52-55 Furthermore, insulin concentrations are markedly elevated in ponies that develop laminitis after grazing high carbohydrate pasture, whereas glucose, free fatty acid, and cortisol concentrations remain normal.53,56 In contrast to people, insulin-resistant horses rarely develop pancreatic exhaustion and hyperglycemia and are capable of producing exceptionally high serum insulin concentrations.56,57 Insulin toxicity appears to be a key factor in triggering equine laminitis. The onset of laminitis is associated with plasma insulin that exceeds 100 µIU/mL (normal range = 8 to 30 µIU/mL).58
Horses and ponies at risk of laminitis should be blood tested for the early detection of hyperinsulinemia, and grain or other soluble carbohydrate should be withheld for 3 hours before testing. A single blood sample showing elevated insulin predicts that laminitis will occur or may become worse.58 Techniques should be used to lower insulin concentrations and restore insulin sensitivity. A weight-reducing diet with a low glycemic index and physical exercise reduce insulin resistance in horses.59 Insulin-sensitizing drugs of the type given to people with type 2 diabetes have been trialed in insulin-resistant horses. Metformin, at a higher dose than previously reported (15 mg/kg body weight per os [PO] every 12 hours), reversed insulin resistance during the first 6 to 14 days of treatment, but this effect diminished by 220 days.60 Pharmacokinetic and epidemiological studies and placebo-controlled trials may further define the potential applications of this drug in the treatment of insulin resistance and prevention of laminitis.
Equine Cushing’s Disease
Older ponies and horses sometimes develop a problem with their pituitary gland, which enlarges, becomes dysfunctional, and results in the development of equine Cushing’s disease (ECD). Pituitary enlargement is sometimes described as a tumor (pituitary adenoma), but most affected horses have simply pituitary hyperplasia (an increase in size for unexplainable reasons). The region of the pituitary involved is the pars intermedia, giving the condition its common medical name: pituitary pars intermedia dysfunction (PPID). The dysfunctional pituitary produces an excess of hormones and peptides that control other hormones. A sign that horses are affected by PPID is hirsutism; the hair coat grows unnaturally long and is not shed at the usual times.
The hormone imbalance is associated with hyperinsulinemia that disturbs hoof lamellar metabolism, promoting an insidious, relentlessly developing, chronic laminitis. Affected horses and ponies often have higher than normal concentrations of glucose, adrenocorticotropic hormone (ACTH), cortisone, and insulin in their blood. The levels of these substances vary throughout the day (diurnal or circadian rhythm), and care has to be taken with the interpretation of blood analysis. The clinical signs of ECD are pot belly and wasted top line, bulging supraorbital fat, polyuria and polydipsia, susceptibility to infections, and laminitis. Insulin status is a powerful prognostic indicator in horses with ECD, and insulin-resistant animals with a basal serum insulin concentration of greater than 188 µIU/mL are much more likely to develop laminitis and survive less than 2 years after diagnosis.56 Laminitis that develops in association with ECD is usually refractory to treatment. However, promising results have been obtained after the administration of pergolide mesylate (Permax, Valeant Pharmaceuticals International, Costa Mesa, California, United States), a drug registered for use in people. Doses in the range of 1 to 2 mg/horse/day have been recommended. The drug mechanism is to reduce production in the pituitary gland of the hormone (ACTH) that controls cortisol production in the adrenal gland. With cortisol under control, insulin responsiveness in hoof lamellae returns, and the laminitis stabilizes. Using pergolide mesylate, the ACTH concentration in horses with ECD decreases within 1 week.58
Exogenous Corticosteroids and Laminitis
Although not proven experimentally, an association was made between systemic or intraarticular administration of corticosteroids, including triamcinolone acetonide (TMC) and methylprednisolone acetate, and the development of laminitis in otherwise apparently healthy horses.61 A single dose of TMC (0.05 mg/kg) given intramuscularly was cleared slowly and induced hyperglycemia and hyperinsulinemia for at least 3 days.62 Larger intramuscular doses (0.2 mg/kg) prolonged this hyperinsulinemic effect, and serum insulin concentration of around 130 µIU/mL persisted for 6 days.62 However, none of the treated horses developed laminitis, although a “laminar” ring, coincident with the time of injection, grew down the hoof wall. In a retrospective clinical trial only 1 of 205 horses treated with TMC developed laminitis, and it had a previous episode of laminitis in its history.63 Nonetheless, clinicians must be aware of the risks associated with exogenously administered corticosteroids. As Bailey and Elliot61 warn, “Glucocorticoids might only increase the risk of developing laminitis when other causative factors are present, or when the lamellar tissues are somehow ‘primed’ to undergo changes leading to cell damage and dysfunction.” A risk factor may be the preexisting hyperinsulinemia associated with metabolic syndrome or ECD. Because hyperinsulinemia alone will precipitate clinical laminitis,11 injecting glucocorticoid into already insulin-resistant, hyperinsulinemic horses may elevate serum insulin concentrations into the laminitogenic range.
Supporting Limb Laminitis
Laminitis in the lamellae of a single hoof can occur whenever a horse’s limb is forced to bear weight unilaterally for prolonged periods. This can occur when an injury (bone or joint fracture) or disease process (infectious arthritis) in the contralateral limb is so painful that weight bearing is impossible. After days to weeks of unrelieved weight bearing, the supporting limb develops lamellar pathology, often to a severe degree. Presumably the immobile limb lacks adequate lamellar perfusion and glucose delivery that eventually trigger a lamellar pathology indistinguishable from that initiated by other causes. Development of this form of laminitis may be delayed or prevented by supporting the limb by a firmly applied elastic support bandage and shoeing with an effective support shoe. The horse should be provided with a deep bed of wood shavings or sand so that it can lie down comfortably and allow blood to circulate through its feet. Deep, compliant bedding also allows the horse to find a foot position that promotes foot circulation. The injured limb should be treated promptly and fitted with a cast or splint so that it can begin to take its share of weight bearing. Pain should be controlled with analgesics for the same reason. An exercise regimen of walking every 6 hours to encourage circulation of the contralateral foot has reduced the incidence of supporting limb laminitis in an equine hospital, but this practice may not be possible or advisable in all horses.64
 Diagnosis of Laminitis
Diagnosis of Laminitis
Laminitis is characterized by an acute-onset lameness of variable severity involving one or more feet. Most often both front feet are affected, with or without the hind feet, but unilateral laminitis does occur, usually caused by excessive load bearing because of severe contralateral limb lameness. Occasionally the hind feet are affected, without involvement of the front feet. The horse may be extremely reluctant to move and, if persuaded to move, tends to land heel first, with a short, pottery gait, with the hindlimbs placed unusually far underneath the body. There is a marked shortening of the caudal phase of the stride at the walk. Lameness may be accentuated as the horse turns. Lameness is worse on hard ground than on soft ground. Although many horses show severe lameness, in those with milder lameness the lameness may be less typical, although suggestive of foot pain. When standing still the horse may position the hindlimbs unusually far underneath the body and may constantly shift weight between the limbs. A horse with severe primary lameness that has developed laminitis in the contralateral limb may start to load the originally lame limb, shifting weight between the two limbs.
Usually, but not invariably, a clinically significant increase in digital pulse amplitude occurs; however, in a thick-skinned cob-type horse this may not be palpable. In the acute phase the affected feet may be hot. Pressure or percussion applied to the feet, especially in the toe region, usually causes pain, but if the horn is excessively hard, the horse may not react. Careful palpation around the coronary band may reveal an unusual depression associated with sinking of the distal phalanx (DP). An area of unusual softness may herald infection tracking proximally in association with laminitis complicated by submural abscessation.
Clinical signs are usually diagnostic, except in less severely affected horses or those with involvement of only the hind feet, when the characteristics of the lameness may suggest foot pain, but not necessarily pathognomonic for laminitis. The response to perineural analgesia varies and is not associated necessarily with the degree of pain and lameness. Apparent desensitization of the foot with palmar (abaxial sesamoid) nerve blocks may have absolutely no effect on the lameness in some horses, although some improvement may occur in others.
Because laminitis frequently develops secondarily to a primary disease process, it is critical to evaluate the entire horse and to identify any predisposing factors that require treatment, such as endotoxemia, septic metritis, equine metabolic syndrome, or equine Cushing’s disease. If equine metabolic syndrome is suspected, serum insulin concentrations and the response to an intravenous glucose tolerance test should be assessed. It has been suggested that the presence of three or more of the following may increase the risk of laminitis development tenfold: insulin resistance, compensatory β-cell response, hypertriglyceridemia, and obesity (body condition score, >6/9).1 Recent administration of corticosteroids may also be a risk factor; in a survey of 36 European sports horse practitioners, 12 had experienced the development of severe laminitis within 7 to 10 days of administration of corticosteroids in 22 horses.2 Corticosteroids incriminated included dexamethasone, triamcinolone, and betamethasone administered either systemically or intraarticularly for treatment of recurrent airway obstruction, joint disease, or back pain, at what would be considered to be normal dose rates. The majority, but not all, of the horses were considered overweight. Approximately 50% of affected horses were humanely destroyed because of uncontrollable sinking of the DPs.
Radiographic examination is critical for establishing a treatment protocol and prognosis. Although rotation of the DP often can be managed successfully, sinking warrants an extremely guarded prognosis. Lateromedial images help to determine whether the condition is acute or an exacerbation of a more chronic problem. Abnormal thickness of the dorsal hoof wall, with or without modeling of the toe of the DP, implies previous disease. Lateromedial images are also important to establish the baseline position of the DP within the hoof capsule. Dorsopalmar images may be useful for assessing mediolateral balance in horses with chronic, unstable laminitis and to delect radiolucent lines indicative of lamellar separation (Figure 34-6, B). If the foot is grossly misshapen, trimming it first is preferable; otherwise, a false impression of severe rotation of the DP, which merely reflects the abnormal hoof wall growth and the development of a lamellar wedge, may occur.
Standardizing the procedure (positioning, film-focus distance, and exposure factors) is essential to make meaningful comparisons between examinations. If using conventional radiography, use soft exposures or radiographic film with a large gray scale to enhance soft tissue detail. A grid is usually unnecessary. A horizontal x-ray beam should be perpendicular to the sagittal plane of the digit, centered between the toe and the heel, about 2 cm distal to the coronary band. Radiodense markers are placed on the dorsal aspect of the hoof wall and on the sole at the apex of the frog. The marker on the dorsal hoof wall should extend from the coronary band distally. This helps to establish the orientation of the DP and its position relative to the coronary band. Radiological abnormalities include rotation or sinking of the DP, increased thickness of the dorsal hoof wall, and radiolucent lines in the dorsal hoof wall, reflecting serum, necrotic tissue, or gas caused by infection or hoof wall separation.
 Medical Therapy of Laminitis
Medical Therapy of Laminitis
Laminitis often develops because disease is occurring in a body compartment other than the foot. Thus it is of paramount importance that the primary disease is treated urgently and effectively. If the duration and severity of the primary disease can be reduced by intensive therapy, a strong chance exists that the severity of lamellar pathology also may be reduced, thus improving the prognosis for the horse. Severe laminitis is sometimes the outcome despite the best of current therapy, but some horses can exhibit early, mild clinical signs of laminitis yet recover with no long-term ill effects.
Currently no drug regimen is able to arrest or block the triggering of laminitis, and the severity of the initial lamellar damage is what influences the outcome.1 An effective laminitis preventive may emerge when the mechanism behind the disintegration of the anatomy of the hoof wall lamellae is fully understood. The discovery that enzymes appear to be involved in the lamellar failure of laminitis2-5 offers hope that proteinase inhibitor therapy, specifically targeted at hoof wall metalloproteinases, may arrest laminitis development. Horses that were able to keep the feet cool during the laminitis developmental period did not develop laminitis,6 an observation that was the basis for developing distal limb cryotherapy as a useful preventive, first-aid measure. Bacteria are a source of laminitis trigger factors7; therefore effective antimicrobial therapy is a treatment priority.
Horses diagnosed with toxemia during enteritis, colitis, strangulating colic, pleuropneumonia, retained placenta, infectious metritis, and grain overload are at high risk of developing laminitis, and medical therapy and mechanical support for the distal phalanx (DP) ideally should be initiated before the clinical signs of foot pain appear. Addressing laminitis as soon as it appears in a sick horse should always be regarded as an emergency procedure. Even then, treatment may be too late. Antiendotoxin hyperimmune serum (Polymmune J, Veterinary Dynamics Inc., Templeton, California, United States) should be included in the intravenous fluid therapy for horses with, or at risk of developing, endotoxemia.
Nonsteroidal antiinflammatory drugs (NSAIDs) are given to reduce inflammation and foot pain. Flunixin meglumine (0.25 mg/kg thrice daily intravenously [IV] or 1.1 mg/kg twice daily IV) has a proven antiendotoxin effect by reducing prostaglandin production via cyclooxygenase inhibition8,9 and is valuable. Horses receiving flunixin meglumine that subsequently were given endotoxin had significantly lower blood prostaglandin and lactate concentrations and reduced clinical signs than control horses. However, the effectiveness of flunixin meglumine or any NSAID as an antilaminitis agent has never been tested. A proven cause-and-effect link between endotoxemia and laminitis has never been established.9
Phenylbutazone (4.4 mg/kg IV or PO every 12 hours) appears to be a potent NSAID for the control of foot pain and is popular with most clinicians. Phenylbutazone and flunixin meglumine at the lower dose rate can be used concurrently, the former to control severe foot pain and the latter to control the effects of endotoxemia. Intravenously administered ketoprofen (2.2 mg/kg twice daily) can be used interchangeably with flunixin meglumine. Horses with acute laminitis usually require NSAID therapy for at least 2 weeks, and because of its low cost, phenylbutazone (2.2 mg/kg) is the best choice for maintenance therapy. Care must be taken not to overdose small ponies.
However, NSAIDs were administered to horses during experimental induction of laminitis without altering the outcome; laminitis still occurred.10 When the laminitis process is triggered, virtually nothing by way of drug therapy stops its progress. The administration of phenylbutazone during the developmental and acute stages reduces foot pain and creates a more comfortable-looking horse, but the disease continues unabated. This creates an ethical dilemma: balancing the need to alleviate pain and suffering against the realization that most of what is administered is only palliative. When NSAIDs are in use, the horse should be confined to a stall with deep bedding. Exercise is contraindicated while the horse is under the influence of analgesics.
Cryotherapy
Distal limb cryotherapy to cool the feet, reduce lamellar tissue metabolism, and induce digital vasoconstriction is a proven preventive strategy when administered early in the developmental phase of laminitis. Cold-induced, digital vasoconstriction during the laminitis developmental phase may limit exposure to and impact of circulating trigger factors on lamellar anatomy. Cryotherapy may bestow additional protection by slowing the kinetics of lamellar enzyme activity below a threshold that causes damage. Nuclear scintigraphic studies showed cold therapy significantly decreased perfusion of the soft tissues of a horse’s foot within 30 minutes of the application of external cold.11 An ice water footbath was used successfully to treat a pony in the developmental stage of acute laminitis after experimental cecal catheterization.12
Two controlled studies testing the efficacy of cryotherapy for the prevention of laminitis were completed. In the first study,13 laminitis was induced in six horses using the oligofructose overload model. Each horse had one forelimb immersed in ice and water (mean temperature, 0.5 to 1.7°C) for a 48-hour experimental period, achieving a mean internal hoof temperature of 3.5 to 0.9°C. All horses developed clinical and histological laminitis in one or more of the untreated limbs. Laminitis did not develop in the treated limbs, and there was significantly reduced lamellar histological damage. In cooled limbs there was significantly less up-regulation of lamellar MMP mRNA compared with untreated limbs. Although cryotherapy markedly reduced the severity of laminitis, it did not completely prevent minor histological changes in four of the six horses. In the second study, cryotherapy was applied to all four limbs of six horses for 72 hours.14 Laminitis was induced as before, and the observation period was extended until 7 days after oligofructose dosing. The horses showed either no or very mild clinical signs of laminitis, and histological examination of lamellar tissues collected 7 days postinduction showed no evidence of laminitis. Control horses were lame at 7 days and had moderate to severe laminitis histopathologically.
Cryotherapy was instigated immediately after administration of the carbohydrate induction bolus in these studies. In a horse with grain overload or acute colitis such prompt initiation of cryotherapy may not be possible. It is unclear whether such a potent prophylactic effect would occur if cryotherapy was initiated later in the course of the disease when lameness was already present. However, the potential value of cryotherapy to prevent laminitis was demonstrated, and further clinical evaluation of the technique is justified.
Cryotherapy for laminitis requires maintaining the limbs from the proximal metacarpal region and distad in a slurry of crushed ice or a circulating cold water (1 to 4°C) apparatus continuously for 24 hours or even longer if the period of septic shock, pyrexia, and digital vasodilation persists. Cryotherapy is safe, well tolerated, and economical. Unlike people, horses do not find cold therapy noxious. We have kept the forelimbs of normal horses in circulating, very cold water continuously for 3 days, with no immediate or long-term ill effect. Initial experiments support the effectiveness of cryotherapy for halting laminitis onset.13,14 Keeping a horse with its feet in ice and water requires an extraordinary amount of time and dedication but can be done and, if laminitis is prevented, is worth the effort.
Digital Blood Flow Therapy
Vasodilatory therapy and hot water footbaths during the developmental phase of laminitis are contraindicated. Drugs with vasodilator action, such as isoxsuprine hydrochloride, acepromazine, and glyceryl trinitrate (applied as patches to the pastern), may be beneficial after lamellar damage has occurred, when healing is required, but should be administered with caution during the developmental phase of laminitis. However, neither orally administered isoxsuprine nor pentoxifylline produced significant improvement in digital or lamellar blood flow when tested under controlled conditions using ultrasound and laser Doppler flowmetry.15-17 Intravenously administered acepromazine maleate, long held to be effective at increasing lamellar microcirculatory blood flow, had no significant effect when tested by laser flowmetry.17 Glyceryl trinitrate tested in the same way, using a Black walnut extract laminitis induction model, had no effect on lamellar blood flow.16 The continued veterinary use of these drugs in the therapy of laminitis is questionable.
Exercise of an intensity that increases core temperature and local analgesia of the palmar or plantar nerves result in hoof wall heating (and by implication, vasodilation) and are contraindicated during the developmental stage of laminitis. Local analgesia of the foot to reduce foot pain and encourage the horse to walk may result in greater lamellar damage than in a rested, confined horse. Forced exercise of any horse with acute laminitis is strongly contraindicated.
Free Radical Scavengers
Dimethyl sulfoxide (DMSO) may be given intravenously for its free radical scavenging and antiinflammatory effects. DMSO (90% solution) mixed with polyionic solutions and 5% dextrose is best administered slowly at about 8 L/h. The concentration of DMSO must remain below 20% to avoid the risk of intravascular hemolysis. However, despite the potential value of DMSO, its promise as an effective laminitis therapy has not been fulfilled. Evidence exists that ischemia, reperfusion injury, and generation of free radicals are not involved in the pathogenesis of horses with laminitis induced with extract of Black walnut.18
Recommended Treatment Strategy
Cryotherapy is the frontline of defense against laminitis and should be instituted early, preferably before clinical signs of foot pain appear. Cryotherapy has a proven preventive effect when applied throughout the developmental period of carbohydrate-induced laminitis.13,14 Applied to all four limbs during the developmental phase of laminitis, distal limb cryotherapy is an effective means of ameliorating the disease and is recommended in horses at risk of developing acute laminitis. Clinically, distal limb cryotherapy has been applied for up to 5 days and successfully prevented clinical laminitis in horses affected by acute colitis.19
The list of pharmaceuticals that have been administered to horses with laminitis is long, and, apart from the NSAIDs, none have achieved particular prominence. Aggressive treatment of the primary disease using fluids and electrolytes, antibiotics and NSAIDs, and uterine lavage for horses with infectious metritis or retained placenta is recommended.
The administration of 4 L of mineral oil four times a day may be beneficial in horses with laminitis developing from grain overload. Mineral oil has a laxative effect and is said to block the absorption of toxins in the large intestine.20 Activated charcoal is an effective adsorbent of a range of toxins and may be useful in horses with grain overload if administered promptly. In Australia, doses of 1 to 5 g/kg/day have been used to treat plant toxicoses in large animals. The higher dose is indicated if a large quantity of grain has been consumed. However, activated charcoal has not been tested against alimentary laminitis; thus its true effectiveness is unknown. The application of cold therapy to all feet, strict confinement to a stall with a deep bedding of sand or shavings, and mechanical support for the DP are also recommended.
Laminitis developing secondary to equine Cushing’s disease (pituitary pars intermedia dysfunction) is usually refractory to treatment unless horses are given pergolide mesylate (1 to 2 mg/horse/day) to reduce adrenocorticotropic hormone (ACTH) production in the pituitary gland.21 Within 7 days of commencing pergolide treatment, plasma ACTH concentration and adrenal cortisol levels are significantly decreased and probably so is the associated hyperinsulinemia.22 Metformin (15 mg/kg body weight PO every 12 hours) reverses insulin resistance and decreases serum insulin concentration during the first 6 to 14 days of treatment, but the effect diminishes by 220 days.23 Responses to insulin reduction strategies may be disappointing if substantial lamellar pathology already exists. Preventive use of pergolide and metformin, before or shortly after the first laminitis episode, may be more efficacious.
Some horses that show the clinical signs of acute laminitis recover completely if treated promptly using a combination of rational medical therapy and mechanical support. However, horses recovering from even the mildest form of laminitis should be observed closely and allowed to rest. If no radiological evidence of palmar displacement of the DP within the hoof capsule is apparent, and the digital pulse amplitude is not palpably exaggerated 48 hours after treatment has ceased, the horse can be returned to its usual function with caution.
If radiographs show displacement of the DP, then the prognosis must be more guarded. Horses with a mild increase in the distance between the DP and the dorsal hoof wall, with or without rotation of the DP, often make an apparent recovery and remain sound indefinitely. However, horses with marginally greater displacement and rotation of the DP make only partial recoveries and often have a history of intermittent lameness, especially after exercise. Histopathological examination of the hoof lamellae of partially recovered horses showed a reduction in the number of secondary epidermal lamellae. Many of the secondary epidermal lamellae had distorted and abnormal shapes even several years after the initial episode of laminitis. Some secondary epidermal lamellae become isolated from the attachment to the primary epidermal lamellae and exist as isolated, unattached islands adrift in the lamellar connective tissue. If the surface area of the lamellae of the inner hoof wall is reduced after laminitis, the effectiveness of the lamellar-DP suspensory mechanism must also be reduced. Horses developing laminitis associated with substantial initial lamellar destruction, as manifest by radiological displacement of the DP, appear never to make a complete anatomical recovery and are prone to recrudescent episodes of laminitic foot pain.
Ultimately the prognosis is directly proportional to the severity and extent of lamellar pathology. Horses with more than 15 degrees of rotation, accompanied by distal displacement of the DP within the hoof capsule, within 4 to 6 weeks of the initial episode of laminitis have a poor prognosis. Prolapse of the DP through an already necrotic sole, accompanied by subsolar and sublamellar infection, usually occurs. Discharge of purulent exudate from the coronet and the heel is common. Osteitis and radiological evidence of lysis of the distal margin of the DP develop. Such horses require months of expensive supportive care and surgery, and although occasionally a horse does make a surprisingly good recovery, most suffer months of crippling foot pain and recumbency and eventually require euthanasia on humane grounds.
The road to recovery after a serious episode of laminitis is a rocky one. The extent of lamellar pathology lies hidden beneath the hoof wall, and we can only guess at what is really going on. Radiographs and the initial degree of pain expressed by the horse, often masked by analgesics such as phenylbutazone, give valuable clues. However, relentless sinking of the DP in the hoof capsule and involvement of all four feet make recovery unlikely.
 Chronic Laminitis
Chronic Laminitis
Christopher C. Pollitt and Simon N. Collins
Following the acute phase of laminitis a horse may recover fully, with no long-term adverse effects on foot function, or can progress into chronic laminitis characterized by displacement of the distal phalanx (DP) within the foot.1,2 This occurs because the damage to the lamellar interface is so extensive that the suspensory apparatus of the distal phalanx (SADP) can no longer maintain the normal anatomical relationship between the hoof capsule, dermis, and DP, resulting in lameness (Figure 34-5).2-7
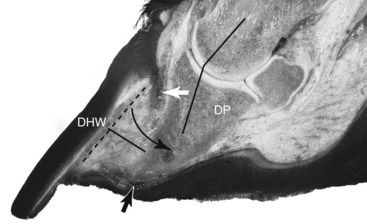
Fig. 34-5 Sagittal section of a foot with severe chronic laminitis showing presence of a large lamellar wedge. The attachment between the distal phalanx (DP) and the dorsal hoof wall (DHW) has failed, and hoof and bone are now widely separated. The dotted line shows the original position of the DP. The angled black line shows that the DP has rotated around the distal interphalangeal joint (in the direction of the curved black arrow) and is no longer in alignment with the pastern axis (longitudinal axis of the proximal and middle phalanges), that is, phalangeal rotation. The material now between the inner aspect of the hoof wall and the DP is abnormal and consists of hyperplastic epidermal tissue that forms a weak, pathological structure called the lamellar wedge (straight black line). The distal descent of the unsupported DP within the hoof capsule has affected the normal alignment of the dermal papillae, resulting in distorted growth of the proximal hoof wall tubules, and has caused the sole to become convex instead of concave (dropped sole). Two dark hemorrhagic zones (white and black arrows) show the sites of greatest pressure and trauma.
Continuous supportive foot management is necessary to minimize the debilitating effects.7 Affected horses are either chronic remissive laminitics (CRLs) or refractory exacerbative laminitics (RELs).8,9 Although CRLs are responsive to palliative treatment with nonsteroidal antiinflammatory drugs (NSAIDs), RELs are minimally responsive, and euthanasia is often the only option to relieve unrelenting pain. CRLs remain highly susceptible to recrudescent episodes of acute intense pain.7,9 Epidemiological estimates suggest that 50% of all acutely laminitic horses become chronic, and that 50% of these remain permanently lame.10
The anatomical dislocation of the DP, which can be detected radiologically as “rotation” or “sinking” (Figure 34-6), depends on the extent and localization of the lamellar pathology, the magnitude of the loads placed on the foot, the effect of treatment modalities used, and disease duration.2,7

Fig. 34-6 A, Lateromedial radiograph of a catastrophic “sinker” foot, showing a visible and palpable supracoronary depression (A), and compression and distortion of the coronary dermis between the hoof wall and the extensor process (B), following distal displacement of the distal phalanx (DP). A visible airline (C) provides evidence of failure of the suspensory apparatus of the DP and physical separation of the dermal and epidermal lamellae. The distal displacement of the DP has resulted in penetration of the apex of the bone into the solar dermis (D). B, Dorsopalmar radiograph of the sinker foot seen in A, showing a fine near-parallel array of radiolucent lines (A) indicating failure of the suspensory apparatus of the DP, as well as physical separation of the dermal and epidermal lamellae.
Disease progression and exacerbation are associated with the initiation of a number of changes, including lamellar wedge formation (see Figure 34-5), bone modeling and lysis (particularly at the apex of the DP), and abscessation within the solar dermis.2,5,7,11,12 DP dislocation results in the development of vascular deficits within the coronary and solar plexi, as well as within the sublamellar and lamellar dermis.14 There is also alteration to the normal pattern of hoof horn production from the coronary, lamellar, and solar regions of the foot, which leads to the development of a distorted hoof capsule.2,7
It is important to assess the nature and extent of DP dislocation because it relates directly to the degree of lameness,13,15 the likelihood of return to former performance levels,13,15,16 and survival outcome.13,16,17 The following anatomical variations in DP dislocation occur, relating to the site and extent of lamellar pathology2,7,18:
With sinking of the DP there may be a depression palpable at the coronary band,2,7 which is focal or extends around the circumference of the coronary band.
Radiological examination is essential to determine the position of the DP. Extreme changes are readily appraised by visual inspection alone. However, subtle and modest changes require objective evaluation, especially when distal displacement of the DP occurs. Two parameters are of diagnostic importance: the hoof–DP distance and the coronary band extensor process distance.
In a normal foot, the perpendicular distance between the middorsal aspect of the hoof wall and the dorsal aspect of the DP (hoof–DP distance) is up to 18 mm,19,20 and the vertical distance between the proximal aspect of the hoof capsule and the proximal aspect of the extensor process of the DP is approximately 4 mm.21 These measurements do not take into account breed variation in foot size, and a better determination of DP dislocation may be if the hoof–DP distance exceeds 25% of the length of the palmar cortex of the DP.20
Dislocation results in compression and shear within the solar dermis, as well as the development of foot pain detectable clinically as a positive response to the application of hoof testers.2,7 Recent work22 suggests that these events, combined with inflammatory responses, can induce neuropathic changes characterized by allodynia (pain from stimuli that are not normally painful) and hyperalgesia (lowering of the pain threshold). This results in a chronic pain state that is nonresponsive to traditional NSAID treatment.
DP dislocation may also be associated with vascular trauma occurring within the solar dermis, which subsequently becomes evident as hemorrhage within the sole horn directly beneath the solar margin of the DP.2,7 Lamellar pathology can result in the development of a “seroma” between the apical tips of the primary dermal lamellae and the basal region of the primary epidermal lamellae (adjacent to the inner hoof wall).2 This seroma can be discerned as a mild linear reduction in radiopacity along the dermoepidermal junction on lateromedial radiographic images optimized for evaluation of soft tissue (see Figure 34-6, A).
Progressive lamellar separation and inspissation of a seroma results in the appearance of a radiolucent airline seen in a lateromedial radiographic image7,11,23 or as a series of fine, near-parallel radiolucent lines in a dorsopalmar radiographic image (see Figure 34-6, B). The appearance of the airline indicates that extensive lamellar separation has occurred, and supportive foot management is required to stabilize the DP and protect the surviving lamellae. This may prevent further mechanical failure of the SADP and progressive dislocation of the DP. With continued hoof growth, the airline moves distally and eventually becomes evident at the weight-bearing border of the foot, providing a potential portal for secondary, opportunistic bacterial and fungal infection.
DP dislocation initiates a number of distinct secondary changes that further compromise foot function and alter the normal pattern of hoof horn production.2,7,11 Most notable is the development of a lamellar wedge (see Figure 34-5). The precise etiopathophysiological mechanisms that lead to its formation are not fully understood, and considerable variation in the histological appearance of this structure is reported.* This presumably reflects the progressive nature of the pathological processes that contribute to its formation over time, and the severity of the underlying lamellar pathology.
In mild laminitis, where lamellar separation is minimal, the lamellar wedge is characterized by elongation and attenuation of the normal lamellar architecture.2,5 More extensive separation results in lamellar fragmentation and a permanent reduction in the SADP surface area of attachment.2,7 Hyperplastic epidermal proliferation occurs, and the resultant tissue has been described as “ectopic” white line13 because its histological appearance, a mixture of tubular and lamellar horn, resembles that of the white line.
The dislocation of the DP within the hoof capsule triggers a series of events that alter the physical appearance of the hoof capsule.2,7,8,22 These changes include a flattening of the sole in direct response both to the anatomical dislocation of the DP and from excessive movement of the DP occurring within the hoof capsule during weight bearing. This can cause vascular trauma of the solar dermis and lead to abscessation and the production of a false sole (Figure 34-7). In severe laminitis, the sole becomes convex, and the DP may eventually prolapse through it, which results in severe unrelenting pain and prolonged recumbency. Solar prolapse of the DP often results in the development of proliferative granulation tissue and associated subsolar abscessation at the site of the prolapse.
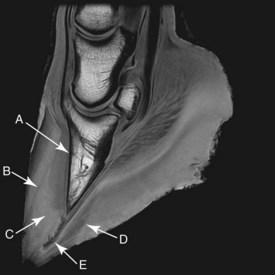
Fig. 34-7 High-resolution sagittal three-dimensional T1-weighted spoiled gradient-echo magnetic resonance image of a chronic laminitic foot, showing angular deviation between the dorsal surface of the hoof wall (viewed proximally) and the dorsal aspect of the distal phalanx (A); a linear hypointense area at the margin of the lamellar interface, indicating lamellar separation and/or seroma formation (B); ectopic white line of the lamellar wedge (high signal intensity within the lamellar interface) and the widened sublamellar dermis proximally (intermediate signal intensity) (C); double (false) sole (D); and track of solar abscessation passing through the widened white line at the hoof wall–sole junction (E).
Elongation, attenuation, and separation of the lamellar interface become apparent on the solar surface of the hoof as a progressive “widening” of the white line,2,7,8 which may be accompanied by hemorrhage. Dislocation also leads to pathological alterations in the coronary band. A combination of compression, vascular deficits, and reorientation of the dermal papillae alters the normal pattern of hoof horn production. There is reorientation of the dermal papillae of the coronary band,7,28 which results in a realignment of the direction of horn growth at the midline of the hoof and leads to differential rates of proximodistal hoof horn growth around the circumference of the hoof capsule. As a result, the growth rings diverge circumferentially from the dorsal aspect of the hoof wall to the heel. If left unattended this leads to the development of a distorted hoof capsule, characterized by both an excessive buildup of heel horn and dorsal concavity of the hoof wall. With advanced lesions there is a physical separation, or shear lesion, of the hoof capsule from the underlying coronary dermis,2 and if extreme this results in complete avulsion of the hoof capsule.2,7
DP dislocation is often accompanied by progressive demineralization of the distal margin of the DP.2,7,11 Radiological abnormalities vary from low-grade demineralization and slight modeling of the apex of the DP (a “ski tip”) to extensive radiolucent defects on the distal, dorsal, and palmar cortices, as well as DP solar margin fractures. Extensive new bone formation may occur along the middorsal aspect of the DP, interpreted as a reactive response to increased levels of tensile stress occurring within the SADP.
There are also specific hidden dangers associated with DP dislocation that are only now being fully understood.7 The solar dermis may become distorted around the distal margin of the DP, causing a reorientation of the dermal papillae of the sole dermis in this region. This distortion changes the direction of sole horn production and leads to the development of an in-growing mass of sole horn that progressively impinges on the distal dorsal aspect of the DP and is often associated with pronounced bone lysis. In addition, a similar change in orientation can occur in the coronary papillae, and similarly leads to an in-growing mass of hoof horn, which further compresses the coronary dermis. Foot treatments should be instigated to reduce the risk posed by altered in-growing horn production. Timely resection of the proximal and distal dorsal hoof wall overlying the in-growing zones promotes reorientation of the dermal papillae, leading to normal hoof horn growth. Treatment shoes and sole inserts should support the palmar/plantar aspect of the feet and spare (relieve) any sole pressure beneath the dorsal margin of the dislocated DP. Shoe breakover should align with the DP dorsal margin to minimize strain within the lamellar interface.
DP dislocation can also adversely alter the normal pattern of blood circulation within the foot2,7 and thus affect foot metabolism, and is therefore of prognostic importance.
 Venography
Venography
Christopher C. Pollitt, Simon N. Collins, and Greg Baldwin
Venography is an additional method of assessing the damaged anatomical interrelationships within a chronic laminitic foot and the associated alteration to the digital circulation. The lack of valves in the digital veins distal to the midpastern region enables near-complete retrograde filling of foot veins with 20 to 25 mL of radiodense contrast media (Figure 34-8). The technique for venography is described elsewhere.1,2 Serial venography has diagnostic and prognostic value because it may detect the progressive soft tissue pathology associated with DP rotation, and it provides objective monitoring of the response to remedial foot management.
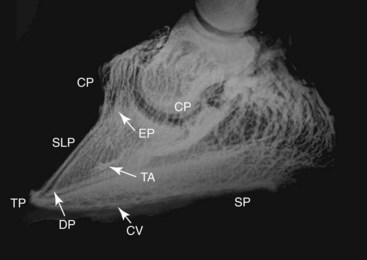
Fig. 34-8 Lateromedial venogram of a normal foot. Most of the vessels are large veins. Veins in the solar and terminal papillae are visible but not the lamellar veins and capillaries. The dorsal margin of the distal phalanx is proximal to the circumflex vein. CP, Coronary venous plexus; EP, extensor process of the distal phalanx; SLP, sublamellar plexus; TA, terminal arch vessels (arrow); DP, distal phalanx (distal margin arrow); TP, terminal papillae; CV, circumflex vein (arrow); SP, sole papillae.
(Venogram from Homestead Equine Hospital, Pacific, Missouri, United States.)
In venograms acquired before onset of laminitis the mean perpendicular distance between the dorsal aspect of the hoof wall and the dorsal aspect of the distal phalanx (DP) (hoof–DP distance) is approximately 18 mm, and the width of the sublamellar venous plexus is 3.0 mm with a linear distance between the apex of the DP and the circumflex vessels of 8.0 mm. The apex of the DP is proximal to the level of the circumflex plexus.3
Chronic laminitis is associated with alteration of the venographic appearance that correlates to disease severity and progression (Figures 34-9 through 34-11). Within 7 days of the onset of experimentally induced laminitis, there is an increase in the hoof–DP distance of less than 2 mm. This is associated with a corresponding increase in the width of the sublamellar vascular plexus, with evidence of contrast media penetration into the sublamellar dermis. The apex of the dislocated DP is level with the circumflex plexus. There is reduced contrast medium filling within the coronary plexus as a result of compression of the dermal tissues between the extensor process of the DP and the proximal aspect of the hoof wall.
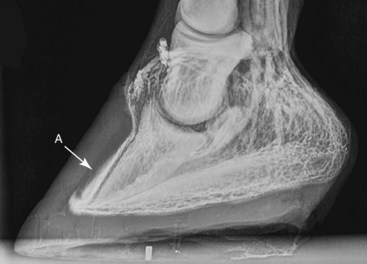
Fig. 34-9 Lateromedial venogram of a chronic laminitic foot, showing the presence of diffuse contrast agent (feathering) within the sublamellar dermis distally (A). Note the apex of the distal phalanx is at the level of the circumflex vessels dorsally.
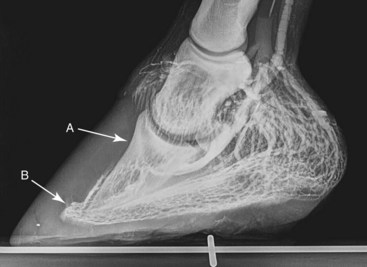
Fig. 34-10 Lateromedial venogram of a foot with recent-onset laminitis and mild rotation of the distal phalanx, showing complete venous deficit within the coronary plexus dorsally and the proximal region of the dorsal sublamellar plexus (A). The circumflex vessels dorsally have been distorted substantially around the apex of the distal phalanx (B), which is indicative of the hidden danger of an inward-growing solar horn.
(Venogram from Davide Zani, University of Milan, Italy.)
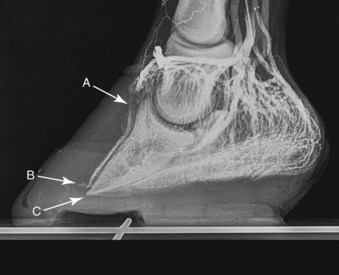
Fig. 34-11 Lateromedial venogram of the same laminitic foot as shown in Figure 34-10 following supportive farriery intervention. Foot trimming and remedial shoeing have resulted in the partial restoration of venous filling within the coronary plexus dorsally and the proximal region of the dorsal sublamellar plexus (A). However, a venous deficit has developed in the circumflex vessels dorsally (B), and the apex of the distal phalanx (C) has descended below the level of the circumflex vessels, resulting in a pronounced venous deficit in the solar plexus dorsally.
(Venogram from Davide Zani, University of Milan, Italy.)
There may also be distortion of the circumflex plexus as pathological abnormalities of the solar dermis around the apex of the DP develop. Inward-growing lamellae (lamellar wedge) and distorted inward-growing tubular horn of the proximal hoof wall and dorsal sole further alter patterns of contrast media distribution.
With more severe DP dislocation, the increase in hoof–DP distance is greater than 4 mm, and the magnitude of the filling deficit in the coronary plexus increases. The apex of the DP is distal to the circumflex plexus (see Figure 34-11). These events are associated with widening and distortion of the sublamellar and circumflex vascular beds. The sublamellar vascular bed increases in width with contrast medium penetration throughout the sublamellar dermis. The distance between the tip of the DP and the circumflex vessels also increases. Vascular filling deficits may also be evident within the distal, dorsal sublamellar plexus, evidence of an encroaching lamellar wedge and a prelude to DP lysis in this region.
Treatment of a chronic laminitic horse is aimed at stabilizing the foot, preventing further DP dislocation, and crucially restoring the circulation (Figures 34-11 and 34-12). Various treatment approaches have been suggested. These include rasping of the distal portion of the dorsal hoof wall and the dorsal aspect of the sole to relieve pressure on the circumflex vessels of the toe and to allow realignment of the solar plexus at the apex of the DP. The concurrent application of beveled, rocker, or reversed shoes may also help by reducing weight-bearing pressure in this region of the foot. Shoe breakover should be aligned with the dorsal distal margin of the DP to reduce strain within the lamellar interface. Complete or partial hoof wall resection, as well as paring of the lamellar wedge, is advocated to aid reinstatement of the sublamellar circulation (see Figure 34-12). Finally, coronary band hoof wall peels or deep coronary band grooving4 may be beneficial to relieve compression of the coronary dermis, and hence reestablish the coronary plexus circulation. In addition, this treatment option aids realignment of the dermal papillae and restoration of normal patterns of hoof wall growth.
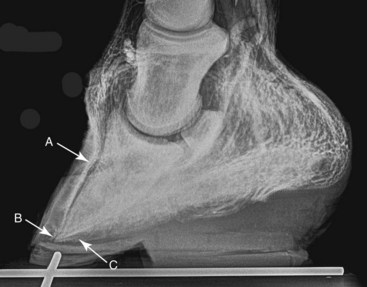
Fig. 34-12 Lateromedial venogram of the same laminitic foot as shown in Figures 34-10 and 34-11 following coronary band peeling and removal of the dorsal aspect of the hoof wall. This has resulted in restoration of venous filling within the coronary and sublamellar plexus (A). In addition, there has been realignment of the circumflex vessels at the apex of the distal phalanx (B) and a modest improvement of venous filling of the solar plexus (C).
(Venogram from Davide Zani, University of Milan, Italy.)
Serial venography gives important information on the progression of chronic laminitis pathology that is not detectable by standard radiology. The venographic changes, detectable early in the chronic phase, are a reference point for assessing the success of supportive therapy or subsequent clinical deterioration (see Figures 34-10 through 34-12). If no contrast medium filling deficits develop and the width of the sublamellar plexus remains stable, a guarded prognosis is justified. Deficits in venous filling with contrast media reflect focal dermal compression by distorted, aberrant lamellar and tubular hoof growth. This can potentially be corrected with judicious, timely hoof capsule resections. Filling deficits may reverse if hoof capsule modification is successful.
 Hoof Care of a Laminitic Horse
Hoof Care of a Laminitic Horse
Hoof care in all phases of laminitis is extremely important. The goal is to reduce stress to the damaged lamellae by minimizing the distracting forces affecting the displacement of the distal phalanx (DP) (rotation or sinking). These distracting forces are the weight of the horse, the constant pull of the deep digital flexor tendon (DDFT), and the leverage on the toe of the hoof capsule. Nothing can be done about the horse’s weight, but absolute stall rest can reduce the increased stress associated with movement. Some of the weight of the horse can be partially redirected away from the hoof wall in the resting horse by removing the shoes. Recruiting other parts of the ground surface of the hoof to bear weight can also reduce stress on the hoof wall with the use of particular bedding or sole pads. Elevating the heel reduces the stress on the DDFT. In horses with rapid rotation or penetration of the corium through the sole, a tenotomy of the DDFT1,3,4 was recommended to eliminate any pull on the DP. Finally, beveling (unweighting) the toe with a rasp helps increase ease of breakover, thereby decreasing the stress created by the leverage of the toe. Baseline radiographic examination is important to establish the relationship between the DP and the hoof capsule. I place both feet on two 8 × 13 × 18-cm wooden blocks that provide comfort to the horse, evenly weight the digits, and eliminate radiological distortion. There are linear radiodense markers embedded in the surface of the blocks. I also place small spherical radiodense markers at the dorsal aspect of the coronary band and at the apex of the frog and a linear marker on the dorsal aspect of the hoof capsule (Figure 34-13).
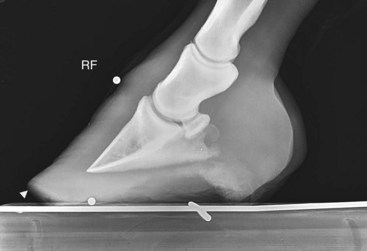
Fig. 34-13 Lateromedial radiographic image showing small, spherical radiodense markers at the apex of the frog and dorsal aspect of the hoof. There also is a wire imbedded on the surface of the wood block on which the foot is resting. Note that the toe of the hoof capsule has been unweighted (white arrowhead).
In the developmental phase of laminitis the goal is to recognize the possibility of disease and be prepared if the disease occurs. The goal is to prevent and/or minimize the distal displacement of the DP. This can be partially accomplished by transferring weight from the wall to the solar surface of the foot and by reducing the pull of the DDFT. If the horse is shod, the shoes should be removed carefully by rasping off each nail clinch, then removing each nail separately with a creased nail puller. This helps to reduce mechanical trauma, pain, and discomfort associated with shoe removal. The only hoof care necessary in this phase is to bevel (unweight) the toe with a rasp back to a point 2.5 to 4 cm in front of the true apex of the frog, depending on the size of the hoof (see Figure 34-13). In a normal horse, the frog can serve as a reference point for the tip of the DP, which is located approximately 1.9 to 2.5 cm dorsal to the apex of the frog (see Figure 34-13). Soft bedding materials such as wet sand or damp peat provide good support and relief, but they may be difficult and expensive to maintain. Alternatively, frog or sole pads can be used. Frog support using Lily pads (Nanric, Inc., Lawrenceburg, Kentucky, United States) or roll gauze taped to the frog was commonly used in the past but is being replaced by pads that adapt to the entire solar surface, providing more support and comfort to the entire foot. High-density foam is one of the most practical and economical methods for providing solar support. Sheets of 5-cm extruded foam, used for house insulation (Styrofoam Scoreboard, Dow Chemical, Midland, Michigan, United States), can be purchased at home centers or building supply companies. For foam application, stand the horse on small precut rectangular blocks of the foam insulation, and then cut and shape the block to the foot using a serrated bread knife, leaving 1.3 to 2.5 cm extending in front of the toe to prevent it from being pushed back as the horse walks. Attach the shaped pad to the foot with duct tape (Figure 34-14). The 5-cm extruded foam may be too thick for some horses or ponies with small feet, and sheets of 2.5- and 3.8-cm foam are more appropriate. In the developmental phase only one layer is usually necessary. Closed cell foam is also useful and is commercially available. These pads can be attached with duct or elastic tape (Elastikon, Johnson & Johnson Consumer Companies, Inc., Skillman, New Jersey, United States).
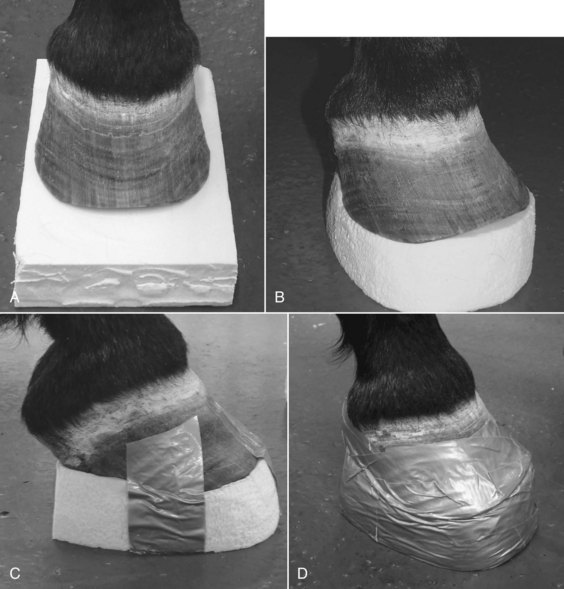
Fig. 34-14 These four pictures show a horse standing on a precut block of foam (A), the foam pad trimmed (B), duct tape applied to the bottom of the pad (C), and the pad attached to the foot with duct tape (D).
In the acute phase of laminitis the goal is to recognize the disease early and take appropriate actions to relieve the pain and provide support and comfort to minimize the effects of the disease. If the shoes have not been previously removed it is important to remove the shoes as atraumatically as possible, as outlined above, to provide solar support. In some horses it may not be possible to remove the shoes or it may aggravate the condition. Solar support can be accomplished with the shoe left in place in these situations. The only hoof care necessary is to unweight the toe if possible. Soft bedding materials such as wet sand or damp peat provide support and relief, especially for those horses from which the shoes cannot be removed.
In the acute phase of laminitis two layers of foam are used to provide support and elevate the heel. One layer is applied and is then removed after 24 hours when it becomes compressed. Ideally, this layer will compress evenly to approximately 2 cm. Remove the dorsal portion (from the apex of frog to the toe) of this pad to reduce the pressure over the most painful area of the sole. This trimmed pad and another foam layer are then attached to the foot with duct tape to raise the heel and provide more cushion to an area of the foot that can tolerate weight bearing (Figure 34-15). The foam pads should be replaced every 4 to 5 days or as needed. A commercial therapeutic boot system, Soft-Ride (Soft-Ride, Inc., Vermilion, Ohio, United States), which has a variety of orthotic inserts, provides support, relief from pain, and is relatively easy to apply. A commercial wedge pad system (Redden Ultimate, Nanric, Inc.) using rubber impression material (Advanced Cushion Support, Nanric, Inc.) is available and works very well in horses not getting adequate support from the foam insulation or other types of solar support (Figure 34-16). These commercial pads are taped or glued to the foot. The system combines a cuff with two attached, 5-degree wedge pads with a built-in dorsal to palmar breakover. The bottom wedge also has beveled edges for ease of medial to lateral breakover; this bottom wedge can be removed separately.
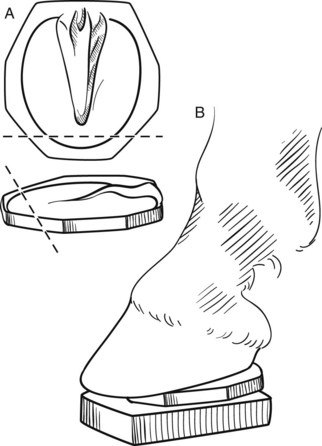
Fig. 34-15 The drawing illustrates the trimming (dashed line) of a compressed foam pad (A), which is then added to new layer of foam (B) before attaching the two to the hoof with duct tape.
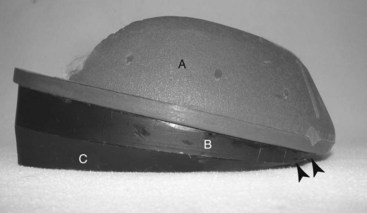
Fig. 34-16 Redden Ultimate (Nanric, Inc., Lawrenceburg, Kentucky, United States) is a wedge system that can be attached to the hoof by a cuff (A) with special glue or adhesive tape. There are two 5-degree wedge pads (B and C) attached to each other with screws. The bottom pad (C) has round edges for ease of lateral to medial breakover. The arrowheads show how the system eases the dorsal to palmar breakover.
The goal of hoof care in the chronic phase of laminitis is to minimize further rotation of the DP. The following principles should be used when preparing the hoof and selecting a therapeutic horseshoe: (1) the palmar aspect of the hoof should be supported using all structures (sole, bars, and frog, including the central and lateral sulci); (2) the sole should be protected by not removing any portion of the sole dorsal to the apex of the frog; (3) the hoof wall should be trimmed from the quarters to the heel only to realign the solar surface of the DP and the weight-bearing surface of the hoof; (4) the dorsal aspect of the hoof capsule should be protected by bringing the point of breakover back to a point 2.5 and 3.8 cm (depending on the size of the hoof) in front of the true apex of the frog; and (5) the heels should be raised to reduce the tension of the DDFT. Two of the goals of hoof care—realignment of the solar surface of the DP and raising the heel to reduce tension of the DDFT—seem counterintuitive. In a chronic unstable laminitic horse these two principles are necessary to reduce the tension of the tendon and provide comfort. In a chronic stable laminitic horse with only mild misalignment of the solar surface of the DP, raising the heel after trimming is not necessary. Specific hoof care management in the chronic phase depends on whether the condition is stable or unstable. In horses maintaining a stable condition, trimming alone may be all that is necessary, carefully following the stipulated principles. Generally, the traditional therapeutic horse shoes (heart bar shoe, reverse shoe with a wedge, reverse shoe with frog support, and the egg bar shoe with a treatment plate) advocated for chronic laminitis may be beneficial only if the condition is reasonably stable. Horses with instability of the DP require horse shoes that are adaptable and more compatible with the goals of hoof care. There are a number of commercially made, aluminum rail shoes (EDSS, Penrose, Colorado, United States; Aluminum 4 Point Rail Shoe, Nanric, Inc.) (Figure 34-17) that provide the additional requirements for support. The new design of these shoes automatically pulls the breakover back, raises the heel, and provides palmar support. Ideally, the breakover should be close to the tip of the DP. A rubber impression material (Advanced Cushion Support, Nanric, Inc.) is used between the palmar structures of the sole and the shoes to provide support of the entire foot.
Fig. 34-17 The Ovnicek aluminum shoe (EDSS, Penrose, Colorado, United States) has interchangeable plastic rails (A). The shoe is specially machined to pull the point of breakover (small arrows) back. It is designed to set back from the toe of the hoof (B). Sole support is provided with rubber impression material (C).
Horses with severe rotation and/or penetration of the sole usually have insufficient heel to realign the DP and the weight-bearing surface of the hoof by removing the heel alone. I have used a special shoeing technique to reestablish this relationship. A rubber impression material (Advanced Cushion Support, Nanric, Inc.) is used to provide the sole support and acts as a spacer between the weight-bearing surface of the hoof wall and the therapeutic shoe (Figure 34-18). These shoes cannot be attached to the hoof conventionally but rather are fastened to the hoof using an acrylic adhesive and fiberglass cloth (Equilox and Equilox Composite Cloth, Equilox International, Pine Island, Minnesota, United States). Routine clinical and radiological evaluations provide useful information to determine the horse’s progress every 5 to 6 weeks when the shoes are reset. This is a difficult technique to perform and to keep the foot balanced without placing undue stress to the hoof capsule. An alternative to this technique is to apply synthetic polymer (Steward Clogs, EDSS) (Figure 34-19) or wooden fabricated shoes3 (Figure 34-20). The wooden shoe can be easily adapted to complement the realignment of the DP. The same principles for treating a chronic laminitic horse are applicable for the wooden shoes.
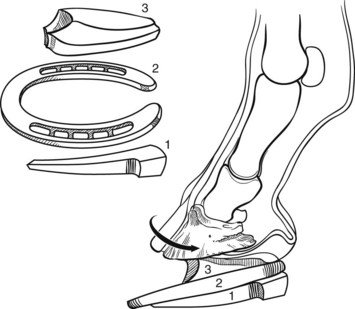
Fig. 34-18 This drawing illustrates the special shoeing technique for realigning the foot when there is penetration of the sole by the corium. The components of this technique are plastic rails (1), the EDSS shoe (2), and cushion elastomer (3). The cushion elastomer is used as a spacer to keep the contact surface of the shoe parallel to the solar surface of the distal phalanx. The shoe is attached with a hoof acrylic and fiberglass cloth because it cannot be attached to the hoof conventionally (not shown).

Fig. 34-20 An example of a wooden fabricated shoe. The front of the shoe is labeled A. The black arrowheads show how the system eases the dorsal to palmar breakover. The sides of the wooden shoe are beveled approximately the same as the dorsal aspect.
In horses with an unstable foot or feet exhibiting progressive rotation or penetration of the corium through the sole, a deep digital flexor tenotomy1,3,4 is recommended to reduce the active forces influencing separation of the lamellae and DP on the sole in the next section. This procedure relieves pain, prevents or minimizes rotation, and allows reestablishment of the normal relationship between the solar surface of the DP and the weight-bearing surface of hoof. Severing the DDFT causes partial luxation and hyperextension of the distal interphalangeal (DIP) joint, as well as overloading of the superficial digital flexor tendon (SDFT). This abnormal joint position may cause palmar foot pain and usually results in osteoarthritis of the DIP joint. Severe overloading of the SDFT may result in damage to the tendon, ultimately resulting in a flexural deformity of the metacarpophalangeal joint. Postoperative management of these horses is important to prevent or minimize these complications. Shoeing is crucial for protecting the DIP joint and reducing the load on the tendons. The horse is shod with a slight flexion of the DIP joint with a shoe in which the heels are raised and extend palmarly about 1 to 1.5 cm. The treatment of these horses with complicated laminitis may take months or years to resolve, takes perseverance and a team commitment, and can be an emotional roller-coaster for the owners. Owners should be made aware of these realities.
 Deep Digital Flexor Tenotomy for Managing Laminitis
Deep Digital Flexor Tenotomy for Managing Laminitis
Transection of the deep digital flexor tendon (DDFT) has been advocated in treatment of horses with chronic refractory laminitis.1-4 The procedure initially appeared to have merit when used in horses with acute laminitis, but the long-term results were poor because of the severe and unstable nature of the laminitis.2 The procedure attenuated pain, but most horses had ongoing displacement of the distal phalanx (DP) and suffered recurrent abscessation, and ultimately humane destruction was necessary.
The rationale for surgery is based on the biomechanical forces in the foot, which include the attachments of the dermal lamellae between the DP and the hoof wall, the downward vertical load through the bony column of the limb, the proximal palmar traction of the DDFT on the DP, the proximal pull of the common digital extensor tendon, and the digital cushion. The balance of these forces maintains a functional unit.
During a non–weight-bearing state, minimal force is imposed on the digit by the DDFT. When the limb is placed under load, the DDFT serves as a tension band and places shearing forces on the dorsal lamellae of the digit from its proximal palmar pull. The predominant force on the digit is the vertical load produced by the weight of the horse. Neither of these forces is necessarily detrimental under normal conditions; however, with compromise of the dorsal lamellae during laminitis, these forces result in distraction of the DP from the hoof capsule. The type and extent of displacement of the DP are determined by the degree of laminar damage and the load placed on the foot.
The rationale for tenotomy is to reduce the proximal palmar pull of the DDFT on the DP and therefore to decrease the shearing forces on the lamellae of the dorsal aspect of the foot. Transecting the tendon also permits lowering the heel if indicated to allow more normal alignment of the DP. The procedure generally is considered a salvage procedure for breeding horses or horses not intended for athletic endeavors, although some horses have returned to successful athletic careers. The degree of damage associated with the laminitis, rather than the surgery, limits future soundness.
No clear-cut guidelines dictate when and if surgery should be performed. In general, surgery is reserved for horses with chronic recurring laminitis that suffer from periodic hoof abscesses and show distal phalangeal rotation and contraction of the hoof wall, minimal to no sinking (distal displacement), and can produce a reasonable dorsal hoof wall. Horses with acute laminitis with instability of the DP–hoof wall unit are not good surgical candidates. If tenotomy is performed, additional measures, such as transfixation casting, must be provided.
Tenotomy may be repeated with recurrence of clinical signs or with the development of a metacarpophalangeal joint flexural deformity subsequent to a midpastern tenotomy. Performing repeat tenotomy is easier after a midmetacarpal tenotomy, although repeat tenotomy is also possible to perform following a midpastern tenotomy. I have performed repeat tenotomy of the DDFT numerous times in horses with chronic recurring pain associated with laminitis. Clinical improvement was seen after each procedure, although subjectively the effects were not as beneficial or prolonged as in the previous tenotomy. This may have been associated with progressive deterioration of the digit associated with laminitis or from adhesions of the DDFT preventing tension relief after transection.
Deep digital flexor tenotomy is performed in the midmetacarpal or midpastern region (Figures 34-21 and 34-22). When performed in the midmetacarpal region, the tenotomy may be done with the horse standing, under local analgesia, or under general anesthesia. An audible pop often occurs once transection is complete. A 1- to 2-cm gap in the transected tendon ends is immediately palpable. The stab incisions do not require closure, but a firm pressure bandage is applied.
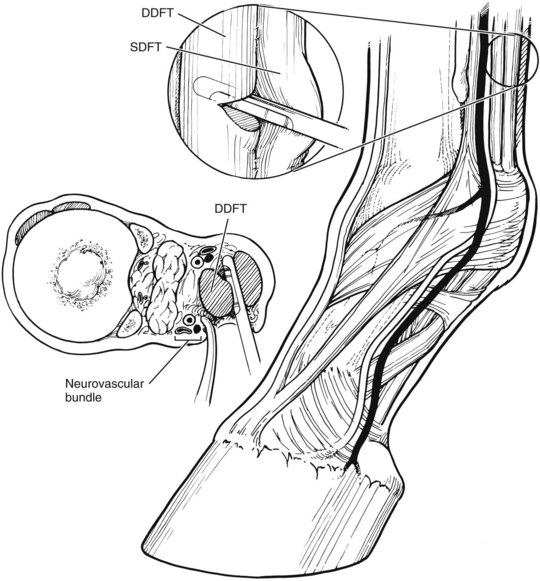
Fig. 34-21 Deep digital flexor tenotomy can be performed in the metacarpal region in a standing horse or with the horse under general anesthesia. DDFT, Deep digital flexor tendon; SDFT, superficial digital flexor tendon.
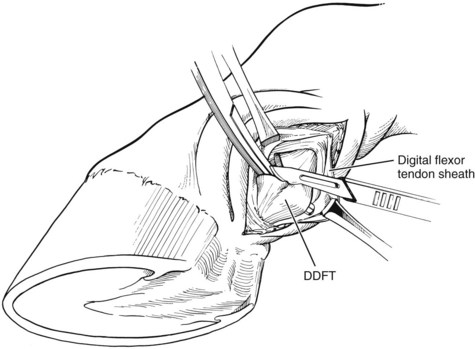
Fig. 34-22 Deep digital flexor tenotomy can be performed in the midpastern region with the horse under general anesthesia. DDFT, Deep digital flexor tendon.
A modification of this technique involves the use of self-fashioned “butter knife” malleable retractors. A 3-cm skin incision is made over the DDFT on the lateral aspect of the metacarpal region. Using blunt dissection along the dorsal and palmar borders of the DDFT, two planes are created for insertion of the butter knife retractors. Care is taken to exclude the neurovascular bundle from potential transection. The DDFT is sharply transected using a No. 10 scalpel, and the skin is closed in routine fashion.
Midpastern tenotomy is almost always performed with the horse recumbent, with the limb flexed to relax the DDFT (see Figure 34-22). Performing the procedure in a standing horse is possible if general anesthesia is prohibited, such as in a late-term pregnant mare. However, in a standing horse there is a higher risk of contamination of the surgical site, with possible involvement of the digital flexor tendon sheath (DFTS); therefore extreme caution should be taken.
It is mandatory to apply an extended and optimally elevated heel shoe in horses with midpastern tenotomy before or during surgery; an exception may be for horses with severe contraction of the hoof and surrounding soft tissues. The shoe should be worn for a minimum of 8 to 10 weeks to prevent luxation of the DIP joint. Although this shoe is not mandatory for all horses with midmetacarpal tenotomy, if clinical evidence exists of DIP joint luxation, an extended and elevated heel shoe should be applied. After tenotomy a horse should be confined to a restricted stall-size area. Bandages should be changed every 2 to 4 days for 8 to 10 weeks to minimize swelling.
Depending on the solar angle of the DP, the heel may be lowered to facilitate alignment, but in feet with extreme contraction, changes should be made gradually. Relative “unloading” of the dorsal aspect of the foot may be accomplished by shifting the breakover in a palmar direction.
The amount of tension release is greater after midpastern tenotomy because of the anatomical approximation to the insertion of the DDFT on the DP and unrestricted separation within the DFTS. The tendon may separate 6 to 10 cm after transection in this region. In contrast, separation after midmetacarpal tenotomy is limited by peritendon attachment to the subcutaneous tissue.
Clinical improvement is generally seen within 2 to 3 days of surgery. In general, clinical effects after tenotomy appear to be beneficial for several months. A flexural deformity of the metacarpophalangeal joint may develop from chronic pain, resulting in unweighting of the limb and contracture of scar tissue at the tenotomy site. Chronic pain may result from overloading the superficial digital flexor tendon before healing, osteoarthritis of the DIP joint, or chronic infections of the digit. Chronic infection may be a major complication, especially if infectious osteitis of the DP develops. Systemic antibiotics, local debridement, topical antimicrobial drugs, and bandaging are important in resolving infection. Treatment of such complicated conditions may take many months to resolve at great emotional and financial cost, and everybody involved must be aware of this.
 Other Management Aspects of Laminitis
Other Management Aspects of Laminitis
Medical management of laminitis (see page 372) and some aspects of hoof care focused on a horse with progressive rotation of the distal phalanx (DP) (see page 379) are discussed elsewhere. This section discusses some additional aspects of management, routine care, monitoring of the horse, and prevention of recurrence. It must be acknowledged that in equine practice, ideal management is not always possible, and compromises must sometimes be made to adapt to the prevailing circumstances.
In a horse with acute laminitis, some clinicians favor removal of the shoes, assuming that the horse can stand on soft bedding. However, in our experience, removal of the shoes often results in increased discomfort, and in the acute stages of laminitis, we prefer to leave the shoes in place. Alternatively, the normal shoes may be replaced with glue-on heart bar shoes, foam pads, frog pads, or custom-made boots,1 which seem to enhance comfort. Not all affected horses develop rotation or sinking of the DP; however, even if baseline radiographs reveal no evidence of rotation, each horse should be monitored carefully. If there is any evidence of increased discomfort, the feet should be reexamined radiologically. The client must be instructed carefully about observing the horse’s stance and the frequency of shifting weight between limbs, mobility, and the amount of time spent lying down, as well as advised that veterinary advice should be sought if there is any evidence of deterioration.
Ideally a horse with acute laminitis should be restricted to stable rest, but this is not always possible, especially for a child’s pony that lives outdoors throughout the year. In these circumstances, a small area of the field should be fenced off using electric fencing. This area should be tightly mown, and the grass clippings should be removed. Spreading a thick layer of wood shavings over the entire surface may be beneficial.
Many horses and ponies that develop laminitis are grossly overweight, and reduction of body weight is crucial for successful management. Owners are often reluctant to admit that the horse is overweight and find it difficult to restrict the diet adequately. It is helpful to weigh the horse or make an estimate of body weight using a height-specific weight tape2 and then set a target for the expected weight loss with monitoring at intervals. Strict instructions concerning dietary management should be made, stipulating the amounts of food, preferably by measured weights, that should be fed. Horses should receive a maximum of 7 MJ/kg dry matter equivalent to poor quality hay. Hay should be soaked for a minimum of 12 hours to reduce soluble carbohydrates. Concentrate foods and other glycemic foods, such as apples and carrots, should be eliminated; however, supplementation with vitamins C (5 mg/kg) and E (4 IU/kg) is potentially beneficial for their antioxidant effects. Low-energy chaff or small quantities of nonmolassed sugar beet pulp is acceptable. If a pony lives outdoors and cannot be stabled, then a strip grazing system should be established; each strip should be finely mown before the pony has access to it. A grazing muzzle may also be useful. If a horse has clinical evidence suggestive of equine metabolic syndrome, combined with increased serum insulin concentrations and an abnormal response to an intravenous glucose tolerance test, then oral administration of metformin (15 to 17.5 mg/kg PO twice daily), an insulin-sensitizing drug, is recommended, although clinical efficacy is not yet proven, and there are questions concerning bioavailability.3 Serum insulin levels should be measured episodically.
In horses with chronic laminitis, even if rotation of the DP occurred previously, the position of the DP may actually be stable. The heel tends to grow more quickly than the toe, and regular trimming of the heel and shortening the toe are imperative to try to reestablish correct alignment of the DP within the hoof capsule and relative to the phalangeal axis. It will take weeks to months to reestablish these alignments, and it is critical that this is a slow, deliberate process without using aggressive angle changes. The heart bar shoe recruits part of the frog to share load bearing, and if correctly fitted, it shifts weight-bearing load away from the toe, thus sparing the dorsal structures of the foot. This approach, combined with removal of the lamellar wedge, often results in improved comfort and alignment of the hoof wall and DP. Correct positioning of the frog support plate is critical so that even, mild pressure is applied over the length of the frog. Reverse shoes or W shoes (open toe shoe with a heart bar) nailed on with one or two nails both medially and laterally, or glued or taped in place, may provide comfort in some horses. Often what works in one horse may not work in another, and a different strategy must be used. It may be necessary to obtain follow-up radiographs at 2-week intervals to determine whether additional trimming is necessary.
The term derotation of the DP has become popular; however, we believe that this is an inappropriate term. Although lowering the heel changes the angle of the DP with respect to the middle phalanx, it cannot acutely change the position of the DP relative to the hoof wall. The position of the DP within the hoof capsule often appears improved after removal of excessive toe.
It is critical for the farrier and veterinarian to work together closely. Although in general a slow deliberate approach to trimming is used, if the veterinarian believes that the farrier is not trimming the foot sufficiently aggressively, obtaining lateromedial radiographs may be helpful to demonstrate excess toe, heel, or both, as well as the position of the DP relative to the dorsal hoof wall and the phalangeal axis. Lateromedial radiographs are also useful for demonstrating excessive separation of the dorsal hoof wall or accumulation of fluid, as well as for determining when limited dorsal hoof wall resection may be indicated. If sinking of one or more of the DPs is evident radiologically, the owner must be advised that the prognosis for recovery is extremely guarded.
If pain is not adequately controlled by systemic analgesics, daily monitoring of the horse, preferably by a veterinarian, is crucial to identify signs of impending penetration of the sole, such as increased softening of the sole dorsal to the apex of the frog and red discoloration. The coronary bands of all feet should be assessed carefully; depression at the coronary band may herald sinking of the DP. Increased lameness may be associated with a subsolar abscess that requires establishment of drainage. A horse that has developed or is at risk of development of mechanically induced laminitis because of a painful condition in the contralateral limb must be monitored particularly vigilantly. In these horses, subtle evidence of mechanical laminitis is easily missed and usually occurs between 4 and 6 weeks after injury to the contralateral limb. However, in some horses, usually racehorses, mechanical laminitis occurs early, even within the first few days, and is inexplicable. Often the first sign of contralateral mechanical laminitis is increased weight bearing on the injured limb, or treading. Increased weight bearing on the injured limb may be a sign of improved comfort, but the clinician should investigate the contralateral limb carefully for the development of mechanical laminitis. Chronic laminitis may be associated with neuropathic pain, which responds poorly to nonsteroidal antiinflammatory drugs.4 Treatment with gabapentin (2.5 to 5 mg/kg PO twice daily) may improve comfort5,6; however, the horse is likely to appear constantly drowsy, and to date there are no long-term data that document any long-term efficacy of this treatment.
Careful assessment of digital pulse amplitudes and foot temperature may be useful for detecting the development of laminitis or deterioration of preexisting laminitis. In older horses in which laminitis cannot be stabilized, consideration should always be given to the existence of primary equine Cushing’s disease, which must be treated appropriately.
It is critical that clients be kept well informed of the likely outcome and are well appraised of the long convalescent periods that often are required after substantial rotation of the DP occurs. They must be warned that unexpected relapses may occur despite steady progress. Clients must be aware that the prognosis is hopeless in some horses, and euthanasia may be required. Humane decisions for euthanasia can be difficult. Nonetheless, it is important to recognize that with early aggressive treatment some horses can make a complete recovery to athletic function despite marked rotation of one or more DPs. The prognosis is generally more favorable in ponies than in horses. Clients must be advised that continued long-term dietary management may be crucial to prevention of recurrent laminitis.
The prognosis in heavy breeds of horses with sinking of the DPs is generally hopeless. These horses seem particularly prone to the development of laminitis secondary to retention of the placenta, and although with aggressive management they can be salvaged until the foal is weaned, longer-term treatment is invariably unsuccessful.
