Chapter 63Gene Therapy
It was well stated by Anderson in Nature in 1998: “Despite our present lack of knowledge, gene therapy will almost certainly revolutionize the practice of medicine over the next 25 years.”1 Gene therapy has the potential to revolutionize intraarticular therapy, both for the prevention of osteoarthritis (OA) and its management.
There are two approaches to gene therapy: (1) identifying a genetic disease (e.g., OA) and then replacing the defective gene, or (2) using gene therapy to increase levels of selected therapeutic proteins, as with interleukin-1 receptor antagonist (IL-1ra). Until gene defects are clearly identified, gene therapy for OA is likely to use the second approach, promoting production of disease-modifying agents such as catabolic antagonists or anabolic promoters.
Review of Components of Gene Therapy
A gene, which is a functional unit of DNA, consists of a DNA sequence that produces a single polypeptide. This gene sequence codes for a specific messenger RNA that goes from nucleus to cytoplasm to translate the amino acid sequence (protein).
Gene therapy is targeted on the production of a selected therapeutic protein to alter a disease process. This protein may enhance (e.g., insulin-like growth factor–1 [IGF-1]) or repress (e.g., IL-1ra) a specific cellular process. The essential components of gene therapy include (1) isolation of a gene (called cloning), (2) manipulation of the gene (engineering), and (3) transfer of the gene into the host cells (transfection/transduction). The gene sequence is initially isolated and characterized and is then engineered by the addition of regulatory elements (promoters) that allow control of protein production.
The essential component of gene transfer is the vector. Gene delivery vectors facilitate the transfer of the therapeutic gene into the nucleus of the target cell. Once in the nucleus, the gene is decoded (expressed) to produce a protein. Viruses, the most commonly used vectors, have the ability to transfer genes into a host cell in an efficient, logical, and easy manner. Viruses used as vectors have been rendered incapable of replicative spread by removal of viral genes and insertion of the therapeutic gene(s). Viral vectors are more efficient than nonviral vectors.
There are two potential methods of gene transfer. Ex vivo gene transfer is an indirect technique where the cells are collected from the joint and grown in the laboratory (e.g., synovial cells). The gene is then transferred (transduced) into cultured cells using a viral vector, and the transduced cells are reimplanted after testing for protein production. Ex vivo transfer is safer but less convenient than the second technique, in vivo transfer. In vivo transfer involves direct transfer of a vector to the target tissues. For example, synovial cells could be transfected by direct transmission of an IL-1ra gene using an adenovirus vector injected into a joint (Figure 63-1). Adenoviruses are the most common vectors proposed for use in human patients with clinical disease2 and were used in our equine studies.3-9 Adenoviral vectors can transduce dividing and nondividing cells and are amenable to in vivo transfer. The adenoviral vector takes up an episomal location (extrachromosomal) in the host nucleus. Second-generation vectors give protein expression for 21 to 40 days.
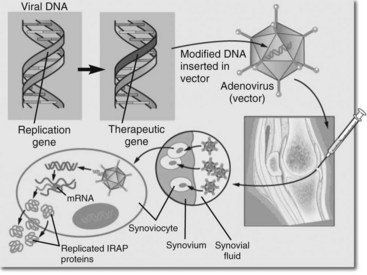
Fig. 63-1 Schematic drawing representing in vivo gene transfer to the synovium. This is the way in which gene therapy with interleukin-1 receptor antagonist was performed.
(Reproduced with permission from McIlwraith CW: Milne lecture: from arthroscopy to gene therapy—30 years of looking in joints, Proc Am Assoc Equine Pract 51:83, 2005.)
Gene Therapy and Joint Disease
Our investigation of the potential value of gene therapy in the treatment of OA is partially based on limitations of traditional therapy. Currently, most therapeutic agents need to be directly administered intraarticularly, and the half-life of most commonly used agents is short.10,11 Gene transfer provides an excellent alternative to conventional therapy because a single intraarticular injection results in the production of the specific therapeutic protein within diseased joint(s) for a prolonged period.12 The potential for the use of gene therapy is best illustrated by our work demonstrating that experimental OA can be suppressed by the use of gene therapy. Cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF) modulate the synthesis of metalloproteinases (MMPs) by synovial cells and are largely responsible for the mediation of joint disease.13-15 The mechanism by which IL-1 causes up-regulation of deleterious mediators is illustrated in Figure 63-2.
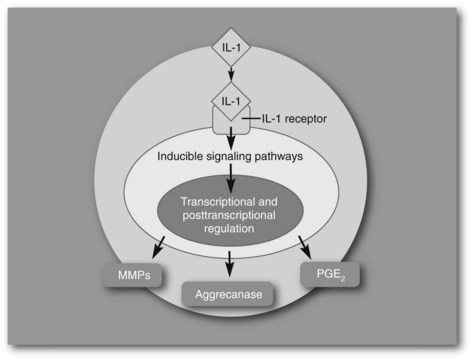
Fig. 63-2 Diagram of interleukin-1 (IL-1) activation of matrix metalloproteinase, aggrecanase, and prostaglandin E2 release acting through IL-1 receptors on the cell membranes.
(Reproduced with permission from McIlwraith CW: Milne lecture: From arthroscopy to gene therapy—30 years of looking in joints, Proc Am Assoc Equine Pract 51:73, 2005.)
Any of the articular tissues, including synovium, articular cartilage, and meniscus, are possible targets for gene transfer. However, the synovium is the best because of its large surface area and contact with the joint space, and has been used with IL-1ra. Synovium is also a convenient target because the gene vector can be administered intraarticularly. Extracellular matrix would limit vector penetration in cartilage cells.
Use of Gene Therapy to Prevent Articular Cartilage Degradation in Osteoarthritis
It is now well accepted that the IL-l “system” is important in the pathogenesis of OA and articular cartilage degradation. There are two agonists, IL-lα and IL-lβ, with IL-1–converting enzyme responsible for activation of both cytokines. There is also an IL-1ra. Both the agonists and the antagonists function through type I and type II receptors on the cell membrane of the target cell (this can be synovium or a chondrocyte) (see Figure 63-2). Up-regulators of IL-l gene transcription include IL-l itself, TNF, and IL-2. IL-1 in turn up-regulates MMP activity, prostaglandin E2, and IL-6, which are important in articular cartilage matrix degradation. The type I IL-l receptor mediates biological responses to IL-6. The type II receptor does not deliver biological signals but tracks IL-1 and inhibits its action. Type II soluble receptors are also potential therapeutic agents. Following intraarticular administration of IL-1ra protein in the Pond-Nuki model of OA, there was a dose-dependent reduction in the incidence of osteophytes, size and grade of tibial cartilage erosion, cartilage histological changes, and MMP-1 expression.16 However, multiple and regular dosing of the protein is essential.
The first report of gene transfer of IL-1ra was in 1993.17 A collaborative effort between the University of Pittsburgh School of Medicine Department of Orthopedic Surgery and Molecular Genetics and Colorado State University (CSU) Orthopaedic Research Center was successful in treating experimental equine OA using gene therapy with equine IL-1ra using an adenoviral vector. Our collaborators Evans and Robbins had previously done work in experimental animals using an adenoviral vector to transport the equine IL-1ra gene, and it was this adenoviral vector containing the equine gene that was used in our studies. The first step was the identification and sequencing of the gene and coding equine IL-1ra, previously done in our laboratory in 1998.18
Proof-of-principle experiments began. First, in vitro expression of an active equine IL-1ra protein after gene transfer of the equine IL-1ra gene sequence to cultured equine synoviocytes using an adenoviral vector was validated.19 Synoviocytes were transfected with Ad-EqIL-1ra at 0, 1, 10, and 100 multiplicity of infection. We assayed the media for and found sufficient active protein (Quantikine human IL-1ra immunoassay; R&D Systems, Minneapolis, Minnesota, United States). Then in an in vivo dose titration study we found that our Ad-EqIL-1ra vector led to a dose-dependent increase in the concentration of IL-1ra in synovial fluid. The maximum duration of IL-1ra production (35 days) was with 10 or 20 × 1010 particles per joint. However, a higher dose of 50 × 1010 produced an undesired synovial leukocytosis.
Next we tested this regimen using our previously described equine OA model20,21 using eight treated and eight control horses.4 Fourteen days after surgery, horses received 20 × 1010 Ad-EqIL-1ra viral particles per joint and continued in a standardized exercise protocol. Relevant levels of IL-1ra protein were measured in the synovial fluid. At 8 weeks, treated horses showed marked reduction in full-thickness cartilage erosions in the induced OA joints and lower inflammatory scores in the synovium compared with control horses. Safranin O fast green staining was depleted in the control joints but was close to normal in the treated joints. There was increased proteoglycan synthesis in the articular cartilage of treated joints. From these data we concluded that transduction of cells with Ad-EqIL-1ra led to synthesis of biologically active equine IL-1ra and excellent suppression of experimentally induced equine OA.
To put the therapeutic benefit of this gene therapy into perspective compared with conventionally used medication, gene therapy was better at reducing the progression of experimentally created OA than were triamcinolone acetonide (Vetalog; Fort Dodge Animal Health, Overland Park, Kansas, United States)20 and methylprednisolone acetate (Depo-Medrol; Pfizer, New York, New York, United States),21 both of which were tested in similar fashion. This was the first study to demonstrate both clinical improvement as a result of gene therapy and significant improvements in gross and histological changes. We concluded that we may have discovered a practical and potentially effective therapy for equine OA. However, subsequent studies revealed that repeated administration of the viral vector induced substantial reactive synovitis, and now our focus has been in trying to achieve a better, nonreactive vector. Work is centered on attempts at developing an adeno-associated viral (aaV) vector, which has been difficult and frustrating. However, recent work in our laboratory has shown good green fluorescent protein expression with an equine aaV vector, and we are optimistic that we have found a better method of gene therapy with IL-1ra in the horse.22,23
Gene Therapy and Promotion of Cartilage Healing
There is potential value in using anabolic genes, such as IGF-1, to heal existing cartilage defects. Adenoviral-mediated IGF-1 gene transduction of equine synovium caused persistent elevations of synovial fluid IGF-1 ligand.24 Enhancement of cartilage healing in an equine model was demonstrated with genetic modification of chondrocytes with IGF-1.25
Following our positive results with IL-1ra gene transfer in experimentally induced OA, the possibility of gene therapy with both IL-1ra and IGF-1 was explored. We reasoned that promotion of anabolism in combination with inhibition of catabolism could provide an appropriate environment for articular cartilage healing. In another collaborative effort among our laboratory, Cornell University, University of Pittsburgh, and Harvard, we have evaluated the value of the combined gene therapy protocol using IL-1ra to decrease the effect of IL-1 on cartilage repair and IGF-1, which had previously been shown to enhance cartilage healing and to reduce the deleterious effects of IL-1.26-28 Using an osteoarthritic IL-1 coculture (synovium and articular cartilage), gene transduction with IGF-1 and IL-1ra proteins was demonstrated using an adenoviral vector with the reduction of proteoglycan loss from the cartilage.26 There was also restoration of cartilage matrix without IL-1 being detected using the same in vitro system.27 Combination gene therapy was then evaluated in vivo using full-thickness articular chondral defects treated with microfracture in the horse.28 Gene therapy enhanced the quality of the repair tissue in full-thickness equine chondral defects compared with microfracture alone, resulting in increased concentration of type II collagen and aggrecan in the repair tissue.
Gene Therapy and Bone Healing
The delivery of growth factors using gene therapy to enhance bone healing was reviewed.29 Initial work at CSU was done evaluating an adenoviral-bone morphogenetic protein–2 (BMP-2) gene therapy protocol in an infected nonunion fracture model.30 Beneficial effects were obtained, and more recently the use of adeno-BMP-2 gene therapy is being evaluated in a splint gap osteotomy model in the horse.