Chapter 73Clinical Use of Stem Cells, Marrow Components, and Other Growth Factors
Scar tissue that forms after tendon and ligament injuries is architecturally and biomechanically inferior to normal tissues, rendering the structure susceptible to reinjury.1 The goals of regenerative therapies, such as stem cells and growth factors, are to restore normal structure and function to tissues to enhance healing, restore pain-free physical activity, and avoid reinjury. Successful regenerative healing of any tissue is intended to closely mimic events of development in which there are spatial and temporal interactions between scaffold, growth factors, and cell populations. An understanding of the molecular and mechanical processes involved in the development of tendon and ligamentous injuries and between acute and repetitive overload pathologies will help guide development of target therapies for each specific disorder.
For many of the regenerative therapies presently available, technology and marketing are ahead of laboratory and clinical research. Most of the products being used to manage equine tendon and ligament injuries have been evaluated experimentally to some extent, but long-term clinical safety and efficacy data are limited. Ideally, the efficacy data would be obtained using prospective, blinded, and controlled clinical trials, although these studies are exceedingly difficult and expensive to perform. At a minimum, results of new treatments should be compared retrospectively with carefully selected historical case-matched studies. As part of a comprehensive plan, benefits from adjunctive surgical manipulations should also be evaluated. Desmotomy of the accessory ligament (superior [proximal] check ligament) of the superficial digital flexor tendon (SDFT) should be considered for horses with superficial digital flexor (SDF) tendonitis. In areas where a tendon or ligament is anatomically confined by surrounding structures and therefore susceptible to compression, such as the fetlock canal and carpal canal for horses with tendon injuries, as well as the proximal metacarpal/metatarsal regions in horses with enlargement of the proximal aspect of the suspensory ligament, surgical release of the restricting fascia should be implemented as part of a multitargeted approach to tendon/ligament healing. Critical to a successful outcome of all treatment regimens is a carefully implemented rehabilitation program. Well documented in studies of regenerative healing is the importance of mechanical stimulation and the type and quantity of the scaffold, growth factor(s), and/or stem cells.2-6
Stem Cell Therapies
The therapeutic role of stem cells in regenerative medicine is not fully understood. It is unclear whether stem cells ultimately function as a tissue-specific cell, such as a tenocyte, or whether they primarily improve tissue repair through secretion of immunomodulatory and trophic bioactive factors.7 These questions are not purely pedantic in nature because if stem cells are truly immunomodulatory, then allogeneic transplantations should be possible. If allogeneic stem cells were efficacious and could be used safely, then “off-the-shelf” stem cell products could be developed to increase availability and rapid implementation of stem cell therapies.
The stem cell field continues to rapidly evolve experimentally and clinically. At a basic level, stem cells are broadly defined as undifferentiated cells that possess the ability to divide for indefinite periods in culture and may give rise to highly specialized cells of each tissue type (i.e., mesoderm, ectoderm, and endoderm). There are two broad categories of stem cells: embryonic and adult derived (see Hipp and Atala6 and Tweedell8 for recent reviews). Embryonic stem (ES) cells used to be defined as those derived from embryos, more specifically from day 8, preimplantation blastocyts. Using recent advances, ES cells can be generated from adult fibroblasts using many of the same technologies that were used to clone Dolly the sheep. These cells are known as induced pluripotent stem (iPS) cells. Adult-derived mesenchymal stem cells (MSCs) can be obtained from bone marrow, fat, umbilical cord blood, muscle, and many other tissues, including cartilage, trabecular bone, and tendon.6 Hematopoietic stem cells (HSCs) are those cells in bone marrow, which are the basis of bone marrow transplantation, and are capable of forming all types of blood cells. Arguments can be made about the optimal source of stem cells for regenerative therapy; importantly, studies are needed to define the need for stem cells in such endeavors. Presently, stem cell therapies for tendon or ligament regeneration in horses are not regulated by the Food and Drug Administration in the United States or elsewhere.9
Commonly Used Stem Cell Products
Presently, there are three approaches to using stem cell therapy in horses, and all use MSCs, although equine ES, iPS, and umbilical cord blood–derived cells are beginning to be investigated. First, MSCs in a mixed cell population can be harvested from bone marrow aspirates; second, MSCs from bone marrow can be cultured; and third, a mixed cell population of MSCs can be harvested from adipose tissue. Each technique has its strengths and weaknesses.
Bone Marrow–Derived MSCs (BM-MSCs)
BM-MSCs can be easily and noninvasively obtained and compared with other MSCs, and they have a greater capacity to differentiate into other tissue types.10-12 BM-MSCs have received the most scientific attention and hence are the best characterized. The process is straightforward. In standing sedated horses, bone marrow is collected from the sternum or the tuber coxae using local analgesia. Fresh bone marrow is either injected directly into the injured tissue, or the nucleated adherent cell population (containing the BM-MSCs) is isolated and expanded in the laboratory before injection.
Bone Marrow Aspirate and Injection of Fresh Bone Marrow
Bone marrow is aspirated from the sternum at a site where the cranial third of the girth would sit (Figure 73-1). This site is relatively safe and has large marrow spaces compared with the marrow spaces cranial and caudal to the girth.However, it must be borne in mind that several horses have died as a result of inadvertent puncture of the heart during bone marrow collection from the sternum. The horse has seven sternal marrow spaces, and they become progressively smaller toward the manubrium and xiphoid processes (Figure 73-2). Ultrasonography is used by some to locate the sternebrae to avoid inadvertent penetration of the intersternebral space and puncture of the heart. The site is clipped, surgically prepared, and a bleb of local anesthetic solution is injected subcutaneously over the aspiration site(s). Bone penetration and aspiration are facilitated by use of a bone marrow (Jamshidi) biopsy needle with a double-diamond stylet and interlocking T-handle, with a large inner diameter cannula (e.g., 11 gauge × 100 mm). Ideally, the site of injection in the tendon/ligament is aseptically prepared before obtaining the bone marrow aspirate, so that the bone marrow can be injected under ultrasonographic guidance immediately to avoid clotting in the syringe. If performed efficiently, anticoagulant is not necessary, which further simplifies the direct aspiration-injection technique. Advantages of this approach are the simplicity of the technique, ability to perform the procedure at the time of diagnosis, and the relatively low cost. The primary disadvantage is the low number of stem cells that are contained in raw bone marrow aspirates. In people and cats, the number of stem cells in raw bone marrow is reported to be 0.001% to 0.01% of the mononuclear cell population.13,14 Using the direct aspiration-injection technique, Herthel15 reported improved clinical outcome in horses affected with suspensory desmitis.

Fig. 73-1 To obtain a sternal bone marrow aspirate, the bone marrow biopsy needle is inserted at the cranial extent of where a girth would cross the ventral aspect of the chest. Using this location, the needle would most likely enter the third sternal bone marrow space (see Figure 73-2).
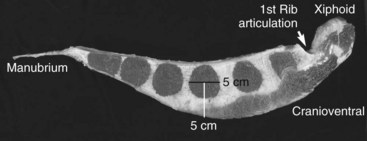
Fig. 73-2 Sagittal section of a sternum from a 450-kg adult horse (cranial is to the right) showing the typical six marrow spaces of the equine sternum. The needle shown in Figure 73-1 most likely enters the third marrow space. This marrow space is approximately 5 cm in diameter, and there it is approximately 5 cm from the ventral aspect of the sternum to the center of the sternal space. This picture demonstrates the diminishing amount of sternal cartilage surrounding the caudal sternal spaces. Although less ventral cartilage might facilitate entry into the marrow space, there is also less room for error dorsally and thereby increased risk of inadvertent cardiac puncture if the bone marrow aspirate needle is inserted too deeply. A generally safe guideline is to insert the bone marrow aspirate needle 2 to 3 cm after initial contact with the ventral aspect of the sternal cartilage. If no bone marrow aspirate is obtained, it is safest to move 2 to 3 cm cranial or caudal on the sternum and reattempt bone marrow aspiration.
Culture-Expanded BM-MSCs
There is experimental evidence to support the use of isolated and culture-expanded BM-MSCs from investigations in laboratory animals, in which MSCs were implanted into surgically created lesions. Improvement in tissue organization and composition and mechanics compared with controls was seen after injection of MSCs into tendons and ligaments.16-18 To obtain 10 × 106 cells, the recommended number for injection, approximately 3 weeks of culture is required to expand the selected cells. Implantation is performed as described using ultrasonographic guidance. After culture, the MSCs are suspended in the horse’s own bone marrow supernatant or plasma to avoid injection of allogeneic antigens and to gain potential beneficial effects of rich growth factors that could be present in the supernatant. Laboratory research has demonstrated significant anabolic effects of bone marrow supernatant on tendon- and ligament-derived cells.19-22
In a small case-control study (n = 11), BM-MSCs resulted in less reinjury of the SDFT in racehorses compared with a control population.23 Recently, in more than 1000 horses treated with cultured BM-MSCs, Smith24 found a significant decrease in reinjury rate for racehorses and other sports horses of all disciplines combined compared with conventionally treated historical controls. Ectopic bone formation, a potential problem when reimplanting BM-MSCs, was not observed in either study. Interestingly, Smith24 reported that early (<44 days from injury) implantation of cells was associated with significantly less reinjury compared with those horses in which a longer interval between injury and implantation was used. These results suggest that implantation of cells before fibrous tissue formation enhances healing.
There are several commercial and private laboratories providing services for isolation and expansion of BM-MSCs. Each laboratory has distinct directions for aspiration and shipping of bone marrow aspirate to the facility and for implantation of the resultant stem cell product. Therefore the chosen laboratory should be contacted directly for specific instructions.
Adipose-Derived MSCs (A-MSCs)
The currently available technique uses a mixed population of cells derived from adipose tissue surgically excised from the tail head region. The A-MSCs are not cultured but are simply isolated and reinjected into the horse. Compared with techniques using cultured BM-MSCs, this technique supplies a greater number of nucleated cells (not necessarily stem cells) in a short period (48 hours). The cells are reimplanted under ultrasonographic guidance. There are currently no published results for the application of A-MSCs in equine tendonitis, although the results of an experimental pilot study demonstrated significant improvement in histologic score in the A-MSC–treated tendons over phosphate-buffered saline–treated control tendons.25 Like culture-expanded MSCs, the laboratory chosen to provide the service should be contacted for detailed instructions.
The Future of Stem Cell Therapy
There are several avenues of stem cell therapy for tendon or ligament injuries presently under investigation. Equine ES cells, umbilical cord blood–derived stem cells, and tendon-derived stem cells are in use for treatment of tendonitis or desmitis. Equine iPS cells are being investigated. iPS cells could have greater differentiation capacity than other adult-derived stem cells, and iPS cells are “horse specific,” which will alleviate concerns of immune rejection. Finally, genetically modified stem cells have been investigated in vitro and in vivo and show tremendous promise for enhancing organized repair of tendons and other musculoskeletal tissues.26
Growth Factor–Based Biologics
Several medical treatments for equine tendonitis and desmitis have centered on delivery of single or multiple growth factors to the site of injury. Growth factors are protein signaling molecules that regulate cellular metabolism. They are temporally and spatially expressed during tendon and ligament development and repair.27-29 Growth factors stimulate cell proliferation, increase extracellular matrix synthesis, and promote vascular in-growth. In addition to the anabolic effects, growth factors down-regulate catabolic, matrix-degrading cytokines such as interleukins and matrix metalloproteinases.
The growth factors most widely studied in tendon and ligament healing include insulin-like growth factor–1 (IGF-1), platelet-derived growth factor (PDGF), bone morphogenetic protein–12, transforming growth factor–β (TGF-β), vascular endothelial growth factor (VEGF), growth/differentiation factor, and basic fibroblast growth factor.17,30-35 Growth factors are available individually as recombinant, purified proteins or can be injected within a less-defined milieu of a bone marrow aspirate or as platelet-rich plasma (PRP). Growth factors are delivered to a tendon or ligament using a series of intralesional injections. In the future, gene therapy techniques could be used to deliver more sustained levels of growth factor expression.7 There are numerous animal studies demonstrating the advantages of using growth factors for enhanced healing of tendonitis, desmitis, and tendon lacerations, but despite these experimental studies, there are few long-term, multicentered, human or equine clinical data available for any of the growth factors. For example, experimentally, intralesional administration of IGF-1 enhanced return of tendon fiber pattern and improved mechanical characteristics in a collagenase-induced SDF tendonitis model.34 Clinically, results after IGF-1 injection only appear comparable with those previously reported for flat-track racehorses treated with conservative therapy alone.
Platelet-Rich Plasma (PRP)
PRP is a fraction of venous blood with a concentrated platelet count, generally greater than two to four times normal baseline. During wound healing, platelet aggregation results in release of bioactive substances that promote tissue repair, regulate inflammation, and stimulate recruitment of stem cells.36 Use of PRP in tissue healing is aimed at enhancing the natural biological process induced after platelet aggregation and degranulation. Platelets contain high concentrations of several growth factors, including PDGF, TGF-β, and VEGF, all of which have been shown using in vitro and in vivo animal models to enhance tendon and ligament healing.3,19,37-39 PRP is generated through a simple centrifugation or filtration process of venous blood to concentrate platelets. The advantages of using PRP include ease of use, administration of autologous peptides, delivery of a combination of growth factors, and low cost (relative to stem cells). After contact of platelets with exposed basement membrane in damaged soft tissues, PRP clots, resulting in the formation of a fibrin scaffold to allow for cellular migration into the injury, and as a mechanism to retain the growth factors at the site of injury. The primary disadvantages are the lack of stem cells within a preparation and the variability between the various products with respect to platelet concentration and residual leukocyte content.
Several companies are marketing specialized devices designed to create PRP. Not surprisingly, when investigated, platelet concentrations are highly and positively correlated to growth factor concentrations.38 In addition, platelet concentrations are highly and positively correlated to tendon and ligament matrix gene expression. In other words, when platelet concentrations are high, there are more growth factors, as well as more extracellular matrix expression, such as collagen type I and cartilage oligomeric matrix protein, in both tendon and suspensory ligament. In contrast, white blood cell concentration is highly and positively correlated with collagen type III, which represents scar tissue, and with matrix-degrading enzyme gene expression. Collectively, these data support the notion of selecting a device with high platelet and low leukocyte concentration to maximize tissue repair and minimize further matrix loss.
Clinical evidence for the benefit of injecting PRP in people is encouraging.40 In horses, a case-control study in Standardbred racehorses with midbody suspensory desmitis demonstrated that a single injection of PRP resulted in an excellent prognosis for return to racing (the Editors note that the prognosis associated with the return to racing of Standardbred racehorses with suspensory desmitis is considered favorable with rest alone).41 Typically, PRP is injected into the affected tissue 7 to 10 days after injury when the inflammatory phase of wound healing is receding. The optimal number of platelets and frequency of injection have not been determined. A typical treatment protocol presently includes a single injection under ultrasonographic guidance using enough PRP to fill the defect as observed ultrasonographically. Horses are reassessed every 30 days, and if substantial improvement in clinical and ultrasonographic findings is not observed, then a second injection should be considered. The majority of horses only require one injection. As with all other therapies, a controlled rehabilitation regimen is critical for successful repair, restoration of pain-free function, and prevention of reinjury.