6 Treating ligaments and sprain injuries
Ligaments connect bone to bone in virtually every joint in the body. Ligamentous instability or laxity is caused by repetitive microtrauma or a one-time injury in which the ligament is stretched beyond its limits of integrity or torn (Dodds 2004). The persistent inflammation and immune system response in chronic inflammatory conditions such as rheumatoid arthritis disrupts the integrity of ligamentous structures and causes ligamentous laxity. Ligamentous laxity and joint hypermobility contribute to further joint degeneration and inflammation (Konttinen 1989).
Ligamentous laxity negatively affects the function and stability of every joint in which it occurs. Functional instability in the ankle following an acute ankle sprain is more due to ligamentous laxity and proprioceptive deficits in the joint than to any lack of muscle strength (Lentel 1995). Ligamentous laxity and apophyseal arthritis in the cervical spine leads to destruction of the disc, cartilage and the vertebral endplates due to inflammation and hypermobility of the spinal segments (Martel 1977).
Not all stresses at a joint cause ligamentous damage. Repetitive physiologic stresses at high strain such as those created while running or doing other repetitive load activities produce significant ligamentous laxity while a relatively few stresses at low strain rate such as those created doing squats do not (Steiner 1986).
In general, ligaments are slow to repair and healing requires a balance between stabilization sufficient to prevent re-injury and motion sufficient to provide adequate circulation, and forces sufficient to organize the repair tissue along functional lines. It is important to maintain proprioceptive input to the joint, the healing ligament and the surrounding muscle tissue during the healing process. Healing ligaments are weaker and more lax than normal ligaments and care must be taken to prevent re-injury during the repair process (Hart 1987).
It is outside the scope of this text to provide a comprehensive exploration of the pathology of ligamentous injury and repair. The clinician using FSM for pain management needs to know how to recognize ligamentous laxity and how to treat it successfully with FSM and adjunctive therapies.
Diagnosing ligamentous laxity
The index of suspicion for ligamentous injuries is created by the history and mechanism of injury. The definitive diagnosis for ligamentous laxity is made with a stress x-ray. A standard x-ray taken with the spine or extremity joint supported in a neutral position or with the patient supine is not useful in determining ligamentous stability. In the cervical spine and lumbar spine the “stress” for the stress x-ray is provided by gravity and positioning the body so the weight of the head or trunk stresses the spinal segments thought to be injured. The ribs and spinal architecture make ligamentous injuries in the thoracic spine extremely rare and their diagnosis and treatment is not addressed here.
Cervical ligamentous laxity
The lateral cervical flexion–extension x-ray is three views taken from the side first with the head in neutral, second with the head flexed forward as far as possible and third with the head extended back as far as possible. This series is used to discover increased translation between a vertebra and the one below it. If the line drawn along the anterior and posterior vertebral bodies is not smooth and linear there is some degree of ligamentous laxity.
Figure 6.1 • When the lateral cervical spine flexion x-ray shows the posterior and anterior margins of the vertebral body of C5 forward of the margins of C6 it indicates ligamentous injury to and laxity in the posterior ligaments that should stabilize the segment. If the segment slides posterior during extension it indicates that the anterior ligaments have also been injured. If total translation exceeds 3.5mm the segment is considered unstable and a surgical consult is advised.
The transverse ligament connects the lateral masses of C1 passing behind the odontoid process stabilizing it in the arch of C1. The transverse ligament is evaluated by judging the space between the dens and the arch of C1 in lateral view flexion extension x-rays.
The odontoid or dens is stabilized in the lateral plane by the alar ligaments. The alar ligaments attach the odontoid tip at lateral angles to the occiput at the foramen magnum – like a tent stays holding a post in neutral. The alar ligament is often injured when the head is rotated at the time of impact. Injuries to the alar ligaments allow excess translation between C0, C1 and C2 in lateral flexion and rotation creating symptoms of severe sub-occipital headache, pressure and pain in the distribution of the occipital nerve and referred pain from the C2 facet joint. The C2–3 facet joint becomes a pain generator when the ligamentous laxity at the dens allows abnormal movement of C1 on C2 placing abnormal biomechanical strain on the C2–C3 facet joint creating inflammation in the joint. The manifestations are severe myofascial trigger points in the sub-occipital muscles, hyperesthesia at C2 dermatome, joint pain and peri-orbital referred pain from the C2–C3 facet joint and a constant relentless headache.
This alar ligament injury can be diagnosed by identifying the mechanism of injury and symptom pattern and by appropriate imaging. The relationship between C0, C1 and C2 is assessed by the APOM (anterior to posterior open mouth) x-ray view. The APOM side bending view assesses ligamentous stability at this level. The APOM neutral view is compared to left and right APOM side bending views. The side bending APOM is collimated as for the APOM but the patient is asked to bend the head as far to the left and then to the right as possible. The patient should be warned that this important evaluation will increase symptoms and should be pre-medicated if necessary. The imaging is positive when the lateral mass C1 overhangs the vertebral body of C2 unevenly from side to side. Some slight translational movement at this level may be normal but it should be symmetrical. Any excessive or uneven translation of C1 on C2 combined with the characteristic symptoms and a history of trauma to the head or neck with the head in rotation is diagnostic. A thin slice high resolution MRI of the upper cervical spine (occiput to C3) can clearly visualize and demonstrate the ligamentous injury if performed and read properly.
Lumbar spinal ligaments are evaluated with six x-rays. Three views are taken from the front in an anterior–posterior (AP) view with the patient standing first with the low back in neutral, second with the patient side bending to the left and third with the patient side bending to the right. The lateral views show the low back vertebra first in neutral, then in flexion and last in extension. X-ray views are available to evaluate most joints with gravity or weights being used to assess the length of the ligament when it is stressed by weight. The reader is referred to their preferred radiology text to identify which views should be ordered to assess the joint in question.
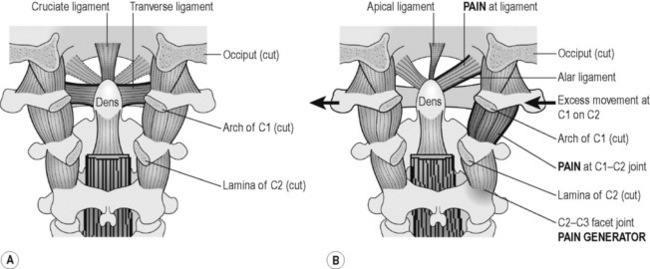
Figure 6.2 • A) C1 is held in place by a series of important ligaments. (1) The posterior view of the occiput, C1 and C2 – the occipital – atlanto – axial complex – shows the posterior osseous structures removed. The transverse ligament is the horizontal component of the cruciate ligament and is the most important stabilizer of C1 on C2 in flexion and extension. Injuries to this ligament are found on lateral view flexion – extension x-rays when the space between the anterior dens and the arch of C1 increases. The apical and alar ligaments stabilize C1 on C2 during rotation and lateral flexion. B) When the alar ligaments are injured the dens is not stable during lateral flexion and rotation. The excess movement creates inflammation and the ligaments, periosteum, nerves and facet joints become pain generators. Injuries to this ligament are discovered only in an APOM side bending x-ray.
Those skilled in manual therapy and diagnosis have been taught to evaluate the injured and the uninjured side to detect subtle excessive joint movement as part of their clinical training (Grieve 1981, White 1978).
The tools to diagnose a ligamentous injury are therefore easily available. The challenge for the clinician is in knowing when to look for and how to assess ligamentous injury. In general, if a patient is still in pain 12 weeks after an injury to the neck or any joint, then the injury involves something more than the soft tissues such as the muscles. The original physical diagnosis may have targeted the sprain strain injury to the muscles and ligaments but after 12 weeks these soft tissues should have healed.
In the cervical and lumbar spine, the primary suspects as cause of persistent pain are the facets and discs and ligaments that have been torn or stretched beyond their ability to heal. Injury to any or all three of these structures may contribute to neuropathic inflammation and activation which in turn creates biomechanical dysfunction, myofascial pain and trigger points. The lax ligaments allow excess motion at the injured spinal segment and the excess motion causes constant microtrauma and inflammation to the discs and facet joints and the nerves at that segment. Abnormal segmental motion stimulates proprioceptors that activate muscles to splint the joint protectively. Neuropathic pain secondary to inflammation from the disc and facet joint also creates pain and muscle splinting.
The splinted muscles constantly contract to stabilize the joint and may contain either latent or active trigger points making them taut and painful. These tissues are the most obvious pain generators and are likely to be the first treated because they are assumed to be the primary pain generators (Travell 1992). If treatment of the muscles with any therapy, including FSM, successfully reduces the pain and splinting, the patient will feel better for several hours but the joint will lose its stabilizing support and the patient will complain that the segmental pain and muscle tenderness is worse when it returns within hours of treatment. This reaction to treatment, while inconvenient is a valuable signal to evaluate the spine or extremity joint for ligamentous injury with a stress x-ray.
Flexion–extension x-rays or stress x-rays of any joint ordered and taken when the injury is less than 4 weeks old may not accurately represent the condition at the joint because the soft tissues around the injured ligaments swell for up to 4 weeks from the onset of an injury and create an artificial splint for the ligaments. The practitioner is advised to check the dates of injury and compare them to the dates of any previous imaging read as negative for excess movement and repeat the imaging if necessary.
If a spinal MRI shows several small disc bulges and one of them is located at the same level as the lax ligament demonstrated on x-ray by excess segmental translation, it suggests that this segment is the one contributing most to the symptoms. The reader is reminded that a disc does not have to have a frank herniation or fragment to be a pain generator. Small disc bulges are often read as normal by a radiologist because it is thought that a certain amount of bulging and degeneration is normal by a certain age in most people. End plate fractures and small tears in the disc annulus are invisible on imaging but sufficient to allow diffusion of the inflammatory material from the nucleus pulposus out into the spinal fluid and the lateral foramen where it can irritate both the spinal cord and the nerve (Olmarker 1993, 1995, Ozaktay 1995, 1998, Taylor 1993).
The findings on a flexion–extension spinal x-ray will show increased translation or sliding of the upper vertebra on the lower one moving forward or back or both when the head is flexed or extended. If the vertebra moves a total of 4mm in one direction, the joint may be medically unstable and a surgical consult is advisable especially in the cervical spine. Translation of the vertebra of more than 4mm can compromise the safety of the spinal cord especially if the patient is subject to additional trauma such as a fall or auto accident.
Ligamentous laxity in the peripheral joints such as the ankle, knee, elbow, wrist or shoulder has similar indicators. If the joint does not respond in 12 weeks to therapies that would be expected to repair the injury, then stress x-rays or a specific evaluation for joint ligament stability should be performed. In some joints suspected ligamentous tears require an arthrogram to determine the extent of the ligamentous and joint damage. Dye is injected into the joint in an arthrogram and if it leaks out this indicates that the capsular ligaments are ruptured.
History questions specific to ligamentous laxity
How were you injured?
If there is a specific mechanism of injury and the patient can identify a specific time when the pain started after the injury, then look for forces sufficient to damage the ligament in question. Auto accidents and falls commonly cause ligamentous injuries in the spine, especially the cervical spine. If there is no specific injury onset, then look for job or recreational activities that create chronic repetitive stress with the load focused on the ligament in an extremity joint or the spine such as running, repetitive lifting with the arms or turning the arm or shoulder or using the legs or feet to push. Participation in contact sports or impact sports such as tennis, handball or racquetball may be associated with ligamentous injuries. Look for systemic inflammatory conditions such as rheumatoid arthritis, lupus or inflammatory arthritis that may compromise tissue integrity by creating inflammation and joint degeneration.
Which way were you looking at the time of impact?
Was your head turned to one side or the other at the time of impact?
Did your head turn as you landed?
These three questions will tease out a mechanism of injury that would reasonably be expected to damage the alar ligaments and create ligamentous laxity and instability at C1–C2. The symptom picture of sub-occipital muscle tension and headaches, C2–C3 facet joint pain and periorbital referred pain and C2 hyperesthesia suggest this line of questioning.
What makes the pain worse?
Injured ligaments allow more segmental movement and any activity that stresses them will create excess movement at the injured joint and increase inflammation and pain. Look for increased pain with repetitive movement or activities that stress the joint in question. The pain may begin during the activity but it is more likely to increase 60 to 90 minutes after the activity.
Is neck pain worse when you read or use a computer?
Sometimes general questions are not adequate to prompt an accurate report and the questions need to be specific to the exact activity associated with increased pain. When ligaments in the neck are injured the pain will increase when bending the head or neck forward while typing or reading. Pain may increase during the activity or 1 to 2 hours after it.
Is the pain worse after you lift or do physical therapy exercises?
Cervical ligaments will be stressed and become painful after lifting the arms overhead or lifting a load out in front of the body during some work activities and during most exercises prescribed to strengthen muscles in the cervical spine. In general, if standard physical therapy exercises make the pain worse it indicates that the ligaments have been injured. Injured ligaments in the wrists or elbows become inflamed and painful after being stressed by lifting, pulling or rotating the wrist during exercise. The ligaments in the ankles and knees become inflamed and painful after walking or running or any activity that stresses them during exercise. The key here is that the pain may not start during the loading exercise but will start 1 to 2 hours after it.
Is the pain worse after massage or myofascial therapy?
If the tight muscles splinting the injured segment relax after massage or myofascial therapy then lax ligaments and the hypermobile segment will become more painful within 1 to 2 hours following the massage. This reaction is diagnostic and can be discovered in the history.
Is the pain worse in certain postures?
Certain postures put gravitational stresses on the ligaments and joints. Leaning even slightly forward while sitting to read or use a computer puts the head forward of the shoulders and stresses the vertebral ligaments of the cervical spine. Bending forward at the waist or leaning to the side stresses not only the lumbar discs but the ligaments as well.
Physical examination
Most practitioners reading this text have been trained to perform a physical examination to evaluate ligamentous laxity. This brief description is meant to be a reminder rather than a comprehensive or definitive instruction. The reader who desires more complete instruction is referred to any professional text on physical examination of the spine or extremities.
Gently palpate the area suspected of having injured ligaments. If the ligaments are lax there will be tenderness at the ligament–periosteum junction where the ligaments attach to the bone.
It is possible to detect subtle excessive joint movement by comparing the motion created by stressing the joints on both the injured and the uninjured side of the body if that has been part of your clinical training. Stabilize one side of the joint and gently press the other side of the joint away from the stabilized side. If the joint on the symptomatic side of the body moves more than the joint on the non-painful side this suggests a ligamentous injury. If this testing procedure has not been part of your clinical training or if you are not proficient in evaluating ligamentous injuries do not attempt to evaluate the joint by stressing it. It is beyond the scope of this text to provide instruction in physical examination and the reader is referred to any professional text of physical examination techniques.
Muscle involvement
Biomechanical abnormalities of a spinal segment or the extremity joint created by ligamentous injury contribute to muscle splinting and the formation of taut bands and myofascial trigger points in the muscles and fascia around the injured joint by both central and peripheral mechanisms. Neural feedback from the injured joint feeds back into the spinal cord and upregulates impulses going out to the joint from the spinal cord to increase muscle tension. The constant tension creates myofascial trigger points in the muscles. Trigger points cause the muscles to be short and taut and contribute to the compression and abnormal loading of the joints and ligaments creating further inflammation and biomechanical dysfunction. Myofascial trigger points sensitize pain nerves that feed back into the spine. This neural input from the muscles compounds the nociceptive sensitization of the nerves from the dorsal rami and amplifies the pain response in the area of the injured ligaments. Palpation of the muscles in the area around the injured ligament, whether in the spine or the extremity joints, will almost always reveal splinting, trigger points and tenderness in these muscles. As the ligaments are successfully treated and begin to stabilize the taut bands should soften and disappear as treatment progresses.
Movements that use trigger point laden muscles will create both local and referred pain from the myofascial trigger points. The same injuries that traumatize and damage the ligaments can also damage extremity joints, the facets and discs.
The practitioner treating ligamentous laxity may of necessity end up treating the extremity joint, or the disc, the nerve and the muscles associated with the injured ligament.
Treating ligamentous laxity
• The patient must be hydrated to benefit from microcurrent treatment.
• Hydrated means 1 to 2 quarts of water consumed in the 2 to 4 hours preceding treatment
• Athletes and patients with more muscle mass seem to need more water than the average patient.
• The elderly tend to be chronically dehydrated and may need to hydrate for several days prior to treatment in addition to the water consumed on the day of treatment
• DO NOT accept the statement, “I drink lots of water”
• ASK “How much water, and in what form, did you drink today before you came in?”
• Coffee, caffeinated tea, carbonated cola beverages do not count as water.
Channel A: condition frequencies
The frequencies listed are thought to remove or neutralize the condition for which they are listed except for 81, 49 / which are thought to increase secretions and vitality respectively
| 40 / | |
| 284 / | |
| 91 / | |
| 13 / | |
| 81 / | |
| 49 / | |
| 124 / | |
| 294 / | |
| 321 / | |
| 9 / |
Channel B: tissue frequencies
 The bursa is a gel filled sac that forms a cushion between adjacent tendons and between tendons and the periosteum.
The bursa is a gel filled sac that forms a cushion between adjacent tendons and between tendons and the periosteum. This frequency appears to influence the connective tissue that creates the matrix for the muscles, ligaments, tendons and fascia.
This frequency appears to influence the connective tissue that creates the matrix for the muscles, ligaments, tendons and fascia. The fascia is a layer of specialized connective tissue that surrounds every muscle and all of the viscera. The fascia secretes the ground substance that is required to repair itself and ligaments and tendons.
The fascia is a layer of specialized connective tissue that surrounds every muscle and all of the viscera. The fascia secretes the ground substance that is required to repair itself and ligaments and tendons. The ligaments are specialized connective tissue linking bone to bone at the periosteum often in the vicinity of the tendons, bursa in the extremity joints.
The ligaments are specialized connective tissue linking bone to bone at the periosteum often in the vicinity of the tendons, bursa in the extremity joints. Any dermatomal or peripheral nerve including proprioceptive and nociceptive nerves to the joints and musculoskeletal structures.
Any dermatomal or peripheral nerve including proprioceptive and nociceptive nerves to the joints and musculoskeletal structures.Treatment considerations
The conceptual framework for treatment of chronic ligamentous laxity with FSM is different than it is when treating any other chronic condition. The repair tissue laid down in the area of chronic ligamentous injuries is ineffective and disorganized due to chronic inflammation and constant aberrant motion. In most chronic injuries the first FSM strategy is to remove scar tissue but this is not done casually when treating chronic ligamentous injuries. Even though the pain and injury is chronic, the body needs to be encouraged to lay down scar tissue to repair the ligaments as if the injury was new. This is contrary to the treatment rationale for every other chronic condition treated with FSM.
The treatment rationale with ligamentous laxity is to treat the ligament to reduce or remove the impediments to healing. Frequencies are used to remove the pattern of being “torn or broken”, to reduce inflammation, chronic inflammation and calcium hardening and to increase the secretions of ground substance to enhance tissue repair and to increase vitality in the injured tissues. When the ligaments have been injured, the periosteum to which the ligaments attach, the tendons that connect muscle to the joint, and the bursas beneath the tendons in the extremity joints are usually collaterally inflamed or have been traumatized by the same mechanism of injury.
The desire on the part of the practitioner to soften the painful muscle tissue around the ligament by removing the scar tissue and disorganized ineffective repair tissue must be resisted. It will worsen the ligamentous pain and compromise joint stability. Treatment two or three times a week for 4 to 6 weeks while the patient is doing exercises designed specifically to strengthen the small inter-segmental muscles supporting the spinal joints or the stabilizing muscles in the peripheral joints will enhance the beneficial effects of exercise on ligamentous healing. This is not a simple or guaranteed outcome. Stabilizing injured ligaments is challenging and difficult. Use of FSM has been shown to make the process more effective.
Treatment protocol for chronic or acute ligamentous laxity
294, 321, 9, / 100
• Trauma, Paralysis, Allergy Reaction / ligament.
 These three frequencies – the basics – do not tend to change symptoms but they appear to be important in restoring normal function to injured tissue. If you think of a time when you have suffered a physical injury you may notice that it is possible to distinguish between the symptoms from the injury and the effect of the “fact” of the trauma, the shock of it to your system. 294 / is thought to address the “fact of the trauma” or the shock to the system created by the trauma.
These three frequencies – the basics – do not tend to change symptoms but they appear to be important in restoring normal function to injured tissue. If you think of a time when you have suffered a physical injury you may notice that it is possible to distinguish between the symptoms from the injury and the effect of the “fact” of the trauma, the shock of it to your system. 294 / is thought to address the “fact of the trauma” or the shock to the system created by the trauma. When a tissue is traumatized it sometimes behaves as if it has “lost” a line of instructions not unlike a computer when it freezes for the same reason. 321 / is thought to “reboot” the tissue and is described as being used to neutralize “paralysis” moving it past the lost instruction and on to the next step to facilitate return to function.
When a tissue is traumatized it sometimes behaves as if it has “lost” a line of instructions not unlike a computer when it freezes for the same reason. 321 / is thought to “reboot” the tissue and is described as being used to neutralize “paralysis” moving it past the lost instruction and on to the next step to facilitate return to function. When any tissue is traumatized the first response is the secretion of histamine to initiate the inflammatory response. When treating to arrest the immediate effects of a new injury removing the histamine from the injured area seems to stop the inflammatory progression.
When any tissue is traumatized the first response is the secretion of histamine to initiate the inflammatory response. When treating to arrest the immediate effects of a new injury removing the histamine from the injured area seems to stop the inflammatory progression.• Treatment time: Use for 1 to 2 minutes or if you are sensitive to the feel of tissue softening and time allows, use the frequency as long as the softening happens. Use these frequencies only on the first two to three treatments.
124 / 100, 191, 142
• Something torn or broken / ligament, tendon, fascia.
• 124Hz is thought to remove the “fact of being torn or broken” from the injured tissue. This is a conceptual shift to the medically trained mind. In an energetic model, conditions have a physical consequence in the tissue – the ligament is “torn or broken” – and at the same time there is also an energetic or vibrational pattern that impresses itself on the semiconductor field that is the injured tissue. It is as if the pattern of being “torn or broken” has impressed itself on the ligament’s field, interferes with the normal healing processes and prevents tissue repair. In an energetic model it is thought that removing this pattern of being “torn or broken” enables the normal repair processes to become effective. This frequency rarely if ever changes symptoms but it seems to enhance tissue repair of “torn or broken” tissues.
• Treatment time: Use for 1 to 4 minutes each or if you are sensitive to the feel of tissue softening and time allows, use the frequency as long as the softening happens. Use this frequency only on the first two to three treatments.
40, 284 / 100, 191, 142, 783, 77, 396
• Inflammation, chronic inflammation / Ligament, tendon, fascia, periosteum, connective tissue and nerve.
• Treatment time: Use these combinations for 2 to 4 minutes each. 40 / 116 reduced inflammation in the mouse model regardless of what tissue had been painted with arachidonic acid. 40 / 116 is the frequency to reduce general inflammation. Research has shown 40Hz to have a 4-minute time-dependent response in a mouse model of inflammation. 50% of the reduction in inflammation was present at 2 minutes. The full response was present at 4 minutes.
91 / 142, 396, 783
• Calcium ions, hardening / in fascia, nerves and periosteum.
• Calcium crystals flow into the tissues in response to inflammation and chronic inflammation.
• Treatment time: Use these combinations for 2 to 4 minutes each. The response and relief will vary from patient to patient in different tissues.
81, 49 / 142, 100, 191
• 81Hz is used for increasing secretions. / 142Hz is used for the fascia, 100 addresses the ligaments and 191 the tendons. The fascia secretes the ground substance necessary for repair of the fascia, ligaments and tendons. The additional tissue frequencies are included in the event that 81Hz is actually increasing collagen release from these tissues.
• Treatment time: Use for 2 to 4 minutes or if you are sensitive to the feel of tissue softening and time allows, use the frequency as long as the softening happens. This frequency combination appears to increase collagen deposition for enhanced ligamentous repair.
Treating ligamentous laxity in an extremity joint
Treating ligamentous laxity in an extremity joint adds the bursa to the list of tissues being treated.
40, 284 / 100, 191, 195, 783, 142, 77, 396
• Inflammation, chronic inflammation / ligament, tendon, bursa, periosteum, fascia, connective tissue and nerve.
• If the ligament being treated is in an extremity joint instead of the spine there will be a bursa associated. Ligamentous laxity in an extremity joint creates aberrant joint motion which usually inflames the bursa and associated joint structures.
81, 49 / 142, 100, 191
• 81Hz is used for increasing secretions. /142Hz is used for the fascia, 100 addresses the ligaments and tendons. The fascia secretes the ground substance necessary for repair of the fascia, ligaments and tendons. The additional tissue frequencies are included in the event that 81Hz is actually increasing collagen release from these tissues.
• Treatment time: Use for 2 to 4 minutes or if you are sensitive to the feel of tissue softening and time allows, use the frequency as long as the softening happens. This frequency combination appears to increase collagen deposition for enhanced ligamentous repair.

Treat the Muscles at the Spine and Extremity Joints
Note: 58 / 00, 02, 32 and 13 / 142, 62, 396 are missing. Treating myofascial tissue is different when treating an area with ligamentous laxity. Do not use the 58/’s or any frequency to remove scar tissue until a treatment trial has been done without them. Unless it is carefully done, removing scar tissue will destabilize the joint and cause an increase in pain.
If the disorganized and ineffective scar tissue must be removed in the process of treating the myofascial pain and adhesions between the nerves and the fascia, the injured joint and weakened lax ligaments must be artificially splinted for 1 to 2 days after the treatment by some means such as tape or a brace or a cervical collar. The muscles will eventually splint again because of biomechanical dysfunction and segmental inflammation at the joint but when they re-splint they should not have or develop myofascial trigger points for about 3 to 4 weeks. This window gives the patient time to progress in physical therapy strengthening the supporting muscles to improve joint stability.
The practitioner is advised to prepare the patient for this process and to be considerate of the patient’s schedule and priorities during the inevitable symptom increase during this project. It is prudent to inform all members of the treatment team about the treatment plan and to prepare them for the removal of scar tissue and the window of laxity that will follow. Physical therapists will need to adjust the exercise program to use lighter weights and more repetitions to increase vascularity and strengthen the supporting muscles without causing undue joint stress. Removing scar tissue or treating myofascial pain without these precautions can create significant exacerbations.
40, 284 / 100, 191, 195, 783, 142, 77, 396
• Inflammation, chronic inflammation / ligament, tendon, bursa, periosteum, fascia, connective tissue and nerve.
• These frequencies to reduce inflammation and chronic inflammation have already been used in the process of treating the ligamentous tissues and do not need to be repeated when treating the muscles.
91 / 142, 62, 396, 77, 191, 783
• Remove calcium ions and hardening from the fascia, the muscle belly elastic tissue, associated nerve fibers, the connective tissue, tendons and the periosteum where the tendon and ligaments attach.
• Treatment time: Use each frequency for 2 to 4 minutes each
• This frequency will produce softening in myofascial tissue. 91Hz may be used with each tissue frequency for 2 to 4 minutes each in a manual microcurrent unit (one that allows manual selection of a three-digit frequency) or in a unit that automatically changes frequencies after a set time interval of 2 minutes. If a manual microcurrent unit is being used and the practitioner is sensitive to the softening in the muscle, the frequency can be used for as long as it is producing changes in muscle texture. The tissue response may last for up to 5 or more minutes depending on the patient and the chronicity. Athletes with myofascial dysfunction respond very well to 91Hz and it may produce softening for up to 5 to 10 minutes with certain tissues.
• Narcotics: If patients are on high levels of narcotics or have had multiple injections with anesthetics it is sometimes necessary to run the frequencies to “remove” narcotics and anesthetics. It is not thought that these frequencies actually remove the narcotic or anesthetic. It is much more likely that they somehow influence the membrane in such a way as to make it more receptive to treatment.
• Use 43, 46, 19 / 396 if joint has been injected with anesthetics or if patient is on narcotics with contacts the same as for the rest of the treatment.
Note: Use of FSM with Prolo therapy
If the patient is planning to see a practitioner who uses prolo therapy to help stabilize the ligaments, FSM treatment should be suspended for 4 to 6 weeks after the prolo treatments while the desired scar tissue is being created. Prolo therapy involves the injection of an inflammatory sugar-based substance intended to create acute inflammation and stimulate healing and the formation of healthy scar tissue. If FSM treatment has not been effective in a 4 to 6 week treatment trial, prolo therapy is a reasonable next step and the two make a good combination.
If the pain is neuropathic pain instead of ligamentous pain, prolo therapy will increase nerve pain at least until the spinal ligaments that are contributing to the dysfunctional disc can be stabilized. FSM may be used to alleviate the neuropathic pain without compromising the result of the prolo therapy. Six weeks after the last prolo therapy injection when the ligament has been shown to be stable, FSM may be used to modify scar tissue and hardening in the fascia, muscle and nerve to normalize joint mechanics and function. The practitioner should avoid use of the frequencies for removing scar tissue (58/’s, 13, 3/) for more than 1 to 2 minutes each during the first few treatments after prolo therapy.
If there is dense scar tissue or bony stenosis of the nerve root or spinal cord or if a disc fragment is compressing the nerve root or cord at the involved level the patient’s pain may increase when polarized positive current is applied. If the patient is positioned comfortably, it is the only time the pain will increase during polarized positive treatment for nerve inflammation. It may increase in the dermatome or at the spine or both. Assess patient position to determine whether it is contributing to the pain increase.
Stop treating immediately if pain goes up during treatment. Move the patient to a seated position if possible. Move the contacts slightly up the spine superior to the nerve root being treated, reduce current levels and change the current from polarized positive to alternating. If this is going to reduce the reaction it will do so in 5 to 10 minutes. If the pain continues to increase, stop treating with current. The pain should go back down in a few hours although it may take up to 24 hours to reduce to base line.
This reaction is diagnostic. If physical examination findings of reduced sensation and deep tendon reflexes at the involved level or hyperactive deep tendon reflexes below the involved level are present this reaction suggests the need to x-ray or perform an MRI to confirm the presence of compression.
Treatment application for ligamentous laxity
• Current level: Use 100–300μamps for average patients. Use lower current levels, 40–100μamps, for very small or debilitated patients. Use higher current levels, 200–400μamps, for larger or very muscular patients. In general, higher current levels reduce pain more quickly. Do not use more than 500μamps as animal studies suggest that increases in ATP level off at current levels above 500μamps while current levels below 500μamps increase ATP up to 500%.
 ± Alternating or biphasic Current: Current is used in alternating mode for treatment of most tissues except nerves. “Alternating DC current” is actually DC (direct) current that alternates its polarity from positive to negative during the machine duty cycle.
± Alternating or biphasic Current: Current is used in alternating mode for treatment of most tissues except nerves. “Alternating DC current” is actually DC (direct) current that alternates its polarity from positive to negative during the machine duty cycle. + Polarized Positive Current: Current is polarized positive for most nerve treatments and may be beneficial in treating other tissues in some patients. When the current is polarized positive the DC wave form alternates from the zero line to positive in a square wave pattern. Some patients simply respond better to polarize positive current and some respond better to alternating or biphasic current. There is no explanation for this difference in response.
+ Polarized Positive Current: Current is polarized positive for most nerve treatments and may be beneficial in treating other tissues in some patients. When the current is polarized positive the DC wave form alternates from the zero line to positive in a square wave pattern. Some patients simply respond better to polarize positive current and some respond better to alternating or biphasic current. There is no explanation for this difference in response.• Waveslope: Moderate to Gentle
 The waveslope refers to the rate of increase of current in the wave as it rises in alternating mode from zero up to the treatment current level every 2.5 seconds on the Precision Microcurrent. Other microcurrent instruments may have slightly different duty cycles and the wave form may change more or less frequently. A sharp waveslope has a very steep leading edge on the wave shape indicating a very sharp increase in current. A gentle waveslope has a very gradual leading edge on the waveform indicating a gradual increase in current.
The waveslope refers to the rate of increase of current in the wave as it rises in alternating mode from zero up to the treatment current level every 2.5 seconds on the Precision Microcurrent. Other microcurrent instruments may have slightly different duty cycles and the wave form may change more or less frequently. A sharp waveslope has a very steep leading edge on the wave shape indicating a very sharp increase in current. A gentle waveslope has a very gradual leading edge on the waveform indicating a gradual increase in current. Use a gentle waveslope for acute pain or new injuries. A sharp waveslope is irritating in new injuries.
Use a gentle waveslope for acute pain or new injuries. A sharp waveslope is irritating in new injuries. Spinal Ligaments: FSM typically uses graphite gloves to conduct the current. The graphite gloves need to be kept moist so they conduct the current comfortably. The graphite gloves can be placed in a small warm wet towel or fabric sleeve, or alligator clips connected to the leads can be clipped to the wet contact.
Spinal Ligaments: FSM typically uses graphite gloves to conduct the current. The graphite gloves need to be kept moist so they conduct the current comfortably. The graphite gloves can be placed in a small warm wet towel or fabric sleeve, or alligator clips connected to the leads can be clipped to the wet contact. Place the positive leads in wet fabric to ensure a broad contact on the posterior spine at the involved spinal segment where the ligaments are painful in the cervical or lumbar spine.
Place the positive leads in wet fabric to ensure a broad contact on the posterior spine at the involved spinal segment where the ligaments are painful in the cervical or lumbar spine. Place the negative leads in wet fabric placed on the body anterior to the spinal contact.
Place the negative leads in wet fabric placed on the body anterior to the spinal contact.
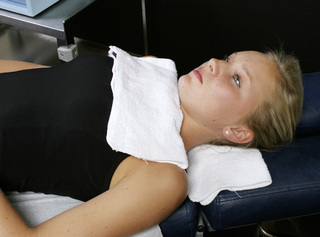
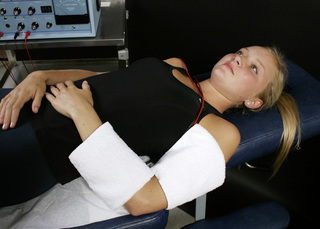
Figure 6.3 • Graphite gloves are placed in warm wet fabric contacts (face cloths). The graphite glove with the two positive leads in it is placed behind the neck and the graphite glove with the two negative leads in it is placed on the chest to allow current and frequencies to flow through the injured ligaments. This placement is used if there is no significant dermatomal nerve pain.
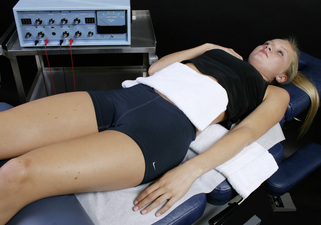
Figure 6.4 • Place the graphite gloves in separate warm wet fabric contacts (hand towel or face cloths). If there is no dermatomal nerve pain the lumbar ligaments can be treated by placing the positive leads glove fabric contact behind the back and the negative leads contact on the abdomen.
If two machines are being used to treat the ligaments and discs and the nerve and muscle simultaneously place the positive leads from both machines in the contact behind the back. Place the negative leads glove from the machine running the disc and ligament protocol on the abdomen and place the negative leads glove from the machine running the nerve and muscle protocol at the distal end of the affected nerve.
Treating spinal ligament combined with nerve pain
• Wrap the positive leads contact around the spine to treat the ligaments of the cervical or lumbar spine.
• Wrap the negative leads around the limb at the end of the dermatomal nerve if there is nerve pain that must be treated at the same time.
• Adhesive Electrode Pads may be used although the gloves seem to be more effective for reasons not understood. The electrode pads must be placed carefully so that the current and frequencies for the two channels cross in an interferential pattern through the area to be treated.
• Place the positive electrode from channel A on the skin over the injured spinal ligament. The negative electrode from channel A must be placed so that the current flows down an imaginary line diagonally through the body from the channel A positive electrode.
• Place the positive electrode from channel B on the skin over the contralateral spinal ligament or
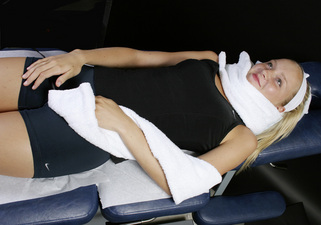
Figure 6.5 • If the nerve is to be treated at the same time as the cervical ligaments, wrap the positive leads glove in a warm wet fabric contact (hand towel) and wrap the contact around the neck so the current flows through the nerve from proximal to distal. Wrap the negative leads glove in a warm wet fabric contact (hand towel) and wrap the contact around the nerve root to be treated. The C5, C6, C7, C8, T1 and T2 nerve roots are being treating in this photograph. To treat the C3 or C4 nerve roots the negative contact would be placed up near the shoulder at those dermatomes.
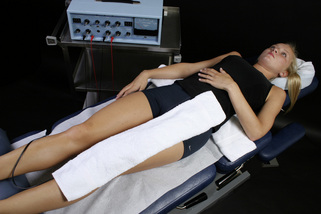
Figure 6.6 • If the nerve is to be treated at the same time as the ligaments the contact with the negative leads glove wrapped in it needs to be placed at the end of the nerve being treated in this case L3, L2, and L1. If all five nerve roots require treatment the towels can be connected to make one longer contact which is wrapped around the foot to treat L4, L5 and S1. The graphite glove with the positive leads is wrapped in the warm wet fabric contact (hand towel) and placed at the spine.
adjacent to the first contact over an extremity ligament. The negative electrode from channel B must be placed so that the current flows down an imaginary line diagonally through the body from the channel B positive electrode. The current and the frequencies must pass through the area to be treated as an “X” in three dimensions. The negative electrodes may be placed directly anterior or anterior and slightly inferior to the spinal contacts. The negative electrodes may be placed at the ends of the nerve root affected by the injured disc. A diagram for the placement would look like this:
| Positive Electrode Channel A | Positive Electrode Channel B |
| Area to be Treated | |
| Negative Electrode Channel B | Negative Electrode Channel A |
• Extremity Joint Ligaments: As a matter of habit the practitioner should wrap the contact containing the positive leads around the limb proximal to the joint in the event that the current may need to be polarized positive. The contact containing the negative leads should be wrapped around the limb and placed distal to the joint. If the practitioner is certain that the nerve is not involved and the current will not need to be polarized positive the contacts may be placed on opposite sides of the joint being treated.
• If wet towels are used, care should be taken to see that the towels do not touch each other. Current
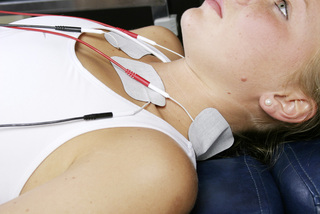
Figure 6.7 • Place the positive leads from channel A on the right side of the neck and the negative lead from channel A on the left upper chest just below the clavicle. Place the positive leads from channel B on the left side of the neck and the negative leads from channel B on the right upper chest just below the clavicle. The current and frequencies form an interferential pattern, crossing in three dimensions through the area to be treated.
will follow the path of least resistance and if the towels are less resistant than the patient the current will flow in the towels and avoid the tissue that needs treating. See the placement as shown in the photographs.
Adjunctive therapies
Ligaments are slow to heal and the muscles supporting the injured joint must be strengthened carefully to avoid overloading the injured area and exacerbating the instability. Comprehensive discussion of spinal and extremity joint stabilization exercises are beyond the scope of this text but the practitioner should be aware that such exercise protocols exist. In general, the exercises consist of small range, almost isometric muscle contractions using little or no weight. The muscle contractions increase vascularity and promote healing at the segment in addition to strengthening the muscles so they can assist in stabilization. The benefits of appropriate therapeutic exercises to train the muscles to compensate for the injured ligaments and stabilize the joint cannot be overestimated.
• Nutrition: Nutritional supplements to provide the building blocks for collagen repair are also helpful. Essential fatty acids, EPA/DHA, cold water fish oils at a dosage of one gram per day reduce inflammation and help ensure that repair tissues
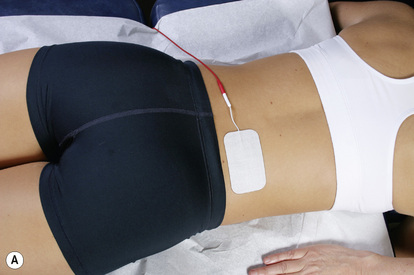
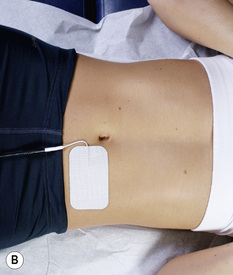
Figure 6.8 • To treat a lumbar ligament with no nerve involvement, place the positive lead from channel A on the right side of the low back. Place the negative lead from channel A on the left side of the abdomen at or below the same level as the disc being treated. Place the positive lead from channel B on the left side of the low back and the negative leads from channel B on the right side of the abdomen.
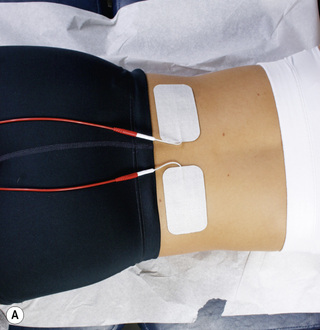
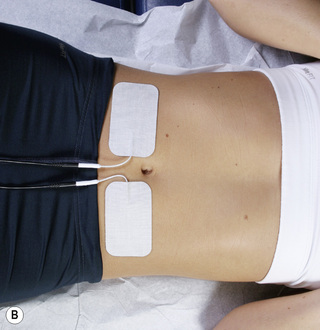
Figure 6.9 • Pads from both channel A and channel B in place on the lumbar spine and abdomen for treatment of lumbar ligaments without nerve involvement.
are flexible. Vitamin C provides one of the constituents of collagen and supplementation of one gram per day may help reduce inflammation and enhance collagen formation. The patient should have a source of high quality protein appropriate to individual nutritional requirements.
• Skin Taping: In some cases it may be useful to place adhesive, athletic tape on the skin overlying the injured joint. The tape on the skin inhibits
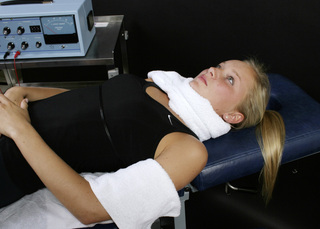
Figure 6.10 • When shoulder ligaments are injured and there is a component of C4 or C5 neuropathic pain, the graphite glove with the positive leads can be placed in a warm wet fabric contact wrapped around the neck and the graphite glove with the negative leads can be placed in a warm wet contact wrapped around the upper arm. The distal contact allows the current to flow through the ligaments and along the nerve so that both can be treated with the same set up.
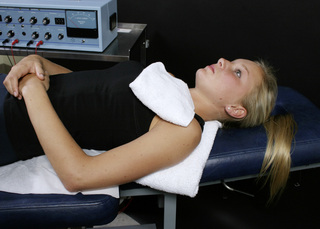
Figure 6.11 • If there is no nerve involvement, place the positive leads glove in a warm wet fabric contact behind the shoulder with the patient supine. Place the negative leads glove in a warm wet fabric contact (face cloth) on the anterior shoulder. The hands can be used to palpate and follow tissue changes under and around the towels.
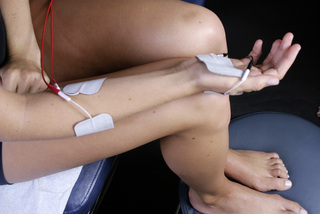
Figure 6.13 • Place the positive lead channel A adhesive electrode pad on the anterior surface of the forearm and the negative lead channel A pad on the posterior surface of the hand. Place the positive lead channel B pad on the posterior surface of the forearm and the negative channel B pad on the anterior surface of the hand. The current and the frequencies from channel A and channel B cross in an interferential pattern at the wrist. The same process is to be followed for using adhesive electrode pads on any extremity joint. Adhesive electrode pads are not usually comfortable at current levels above 100μamps. The current can be used polarized positive or alternating DC current and the proximal contact should always contain the positive lead.
muscle firing and serves to stabilize the joint below. Comprehensive description of skin taping is beyond the scope of this text but the practitioner treating ligamentous laxity should be aware that it is useful and pursue further training or refer the patient to someone with this skill until the ligament is repaired.
• Avoid: The patient should avoid any activity or therapy that stresses the joint or disrupts connective tissue, including yoga, lifting heavy
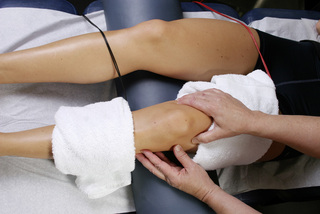
Figure 6.14 • Wrap the glove with the positive leads in a warm wet fabric contact (hand towel) and wrap the contact around the thigh just above the knee. Wrap the negative leads glove in a warm wet fabric contact (hand towel) and wrap that contact just below the knee or at the knee. The current can be used polarized positive or alternating DC current and the proximal contact should always contain the positive lead. Palpate the changes in the tender ligament being treated or mobilize the myofascial tissue by placing the hand under the towel during treatment.
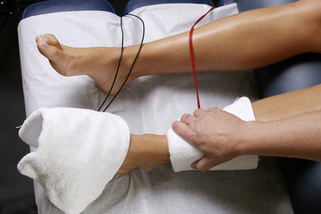
Figure 6.15 • Wrap the glove with the positive leads in a warm wet fabric contact (hand towel) and wrap the contact around the calf just above the ankle. Place the contacts higher on the calf if the gastrocnemius and soleus are involved in the ankle injury. Wrap the negative leads glove in a warm wet fabric contact (hand towel) and wrap that contact just below the ankle or at the ankle. The current can be used polarized positive or alternating DC current and the proximal contact should always contain the positive lead. Palpate the changes in the tender ligament being treated or mobilize the myofascial tissue by placing the hand under the towel during treatment.
weights, prolonged static postures that weight the lax joint, any ballistic movement that might tear fragile repair tissue, any movement that reproduces the mechanism of injury and any “deep” soft tissue therapy that might disrupt newly forming repair tissue. This caution applies to the use of FSM for myofascial therapy.
• Expected outcomes: The patient with a ligamentous injury should be warned that the process of repair will require 4 to 6 months of attention and effort even with the use of FSM as an adjunct. FSM can reduce inflammation, encourage enhanced tissue repair and speed up the repair process, reduce the number and severity of exacerbations and shorten the time required to achieve joint stability. The use of prolo therapy after a 4-month trial of FSM treatment may be helpful if healing is not proceeding as quickly as desired. In the author’s opinion, “prolo” therapy should not be used until there has been a period of stabilization exercises to prepare the area.
A successful outcome will depend on the extent of the original injury, the severity of the ligamentous injury, patient compliance with exercise, the patient’s overall health and nutritional status and, to some extent, good luck.
Case report
The patient was the 62-year-old female driver of a vehicle parked in icy conditions at the bottom of a steep driveway in front of a home that sat below the level of the roadway by 15 feet. Her vehicle was struck by a pickup truck that left the road way after losing control on the ice and was air born at the time of impact with the front quarter panel and door pillar on the driver side. She presented for treatment 4 months after the accident having been to a physical therapist, a massage therapist and a chiropractor for treatment previously. Her pain was worse after physical therapy, after massage and much worse after spinal manipulation so she discontinued both therapies after a short time. Her medical physician referred her for treatment with FSM.
The severity of the impact, the fact that she was still in moderate pain 4 months after the accident and the fact that her symptoms worsened after physical therapy and after massage and after spinal manipulation all suggested that her symptoms were due to ligamentous laxity. A full spinal series of x-rays including flexion–extension x-rays were taken. They demonstrated mild spinal degeneration consistent with her age and documented a total of 4mm translation of C4 on C5 and 3mm of translation at C5 on C6 in flexion and extension. She was placed in a cervical collar to be worn during any physical activity and referred to a spinal surgeon who saw no need for surgical treatment.
She was treated with FSM twice a week for 12 weeks and sent to physical therapy for spinal stabilization (STEP) exercises. At the end of 3 months the ligamentous laxity at C5 on C6 was 1mm and the ligamentous laxity of C4 on C5 was reduced to 1mm.
Dodds S.D., Panjabi M.M., Daigneault J.P. Radiofrequency probe treatment for subfailure ligament injury: a biomechanical study of rabbit ACL. Clin. Biomech.. 2004;19:175-183.
Grieve G.P. Common vertebral joint problems. Edinburgh: Churchill Livingstone, 1981.
Hart D.P., Dahners L.E. Healing of the medial collateral ligament in rats. The effects of repair, motion, and secondary stabilizing ligaments. J. Bone Joint Surg. Am.. 1987;69:1194-1199.
Konttinen Y.T., Santavirta S., Kauppit M., Isomaki H., Slati S. Atlantoaxial laxity in rheumatoid arthritis. Acta Orthopaedica Scandinavica. 1989;60(4):379-382.
Lentell G., Baas B., Lopez D., McGuire L., Sarrels M., Snyder P. The contributions of proprioceptive deficits, muscle function and anatomic laxity to functional instability of the ankle. J. Orthop. Sports Phys. Ther.. 1995;21:206-215.
Martel W. Pathogenesis of cervical discovertebral destruction in rheumatoid arthritis. Arthritis Rheum.. 1977;20:1217-1225.
Meyers T. Anatomy trains. Edinburgh: Churchill Livingstone, 2001;9-49.
Olmarker K., Rydevik B., Nordberg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425-1432.
Olmarker K., Blomquist J., Stromberg J., et al. Inflammatogenic properties of nucleus pulposus. Spine. 1995;20:665-669.
Ozaktay A.C., Cavanaugh J.M., Blagoev D.C. Phospholipase A2-induced electrophysiologic and histologic changes in rabbit dorsal lumbar spine tissues. Spine. 1995;20:2659-2668.
Ozaktay A.C., Kallakuri S., Cavanaugh J.M. Phospholipase A2 sensitivity of the dorsal root and dorsal root ganglion. Spine. 1998;23:1297-1306.
Steiner M.E., Grana W.A., Chilag K., Schelberg-Karnes E. The effect of exercise on anterior posterior knee laxity. Am. J. Sports Med.. 1986;14:24-29.
Taylor J.R., Twomey L.T. Acute injuries to cervical joints, An autopsy study of neck pain. Spine. 1993;18:1115-1122.
Travel J.G., Simons D.G. Myofascial pain and dysfunction. The trigger point manual. vol. 2. Baltimore: Williams & Wilkins; 1992.
White A.A., Panjabi M.H. Clinical biomechanics of the spine. Philadelphia: JB Lippincott, 1978.