CHAPTER 13 Early embryonic circulation
The early embryonic circulation is symmetrical (Fig. 13.1). It is modified throughout development to produce a functioning fetal circulation which is connected to the placenta, and changes rapidly at birth to accommodate disconnection from the placenta and the start of gaseous exchange in the lungs. Major restructuring of early vessels occurs as the embryo grows: anastomoses form and then disappear, capillaries fuse and give rise to arteries or veins, and the direction of blood flow may reverse several times before the final arrangement of vessels is completed.
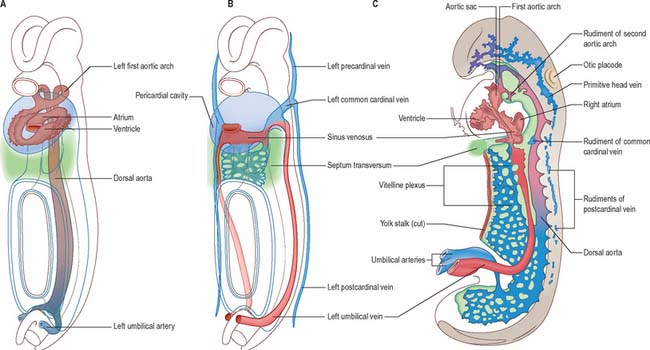
Fig. 13.1 The early, symmetrical blood vascular system. A, Ventrolateral view of the endothelial profile of the heart, the first aortic arch arteries and the dorsal aorta shown in relation to the major epithelial populations. B, Ventrolateral view of the main venous channels shown in relation to the major epithelial populations. C, Left lateral view of the blood vascular system of a stage 11 human embryo; only the endothelial lining of the heart tube is shown. At this stage the arteries and veins are in the process of development and no true circulation is yet established.
The earliest circulatory components develop by vasculogenesis in the extraembryonic tissues. The endothelial heart tubes, dorsal aortae, umbilical and early vitelline vessels arise by vasculogenesis within the embryo. Further vessel development occurs by a process of angiogenesis in which angioblasts, arising in splanchnic and somitic tissues, add endothelial sprouts and branches to earlier vessels. None of the main vessels of the adult arises as a single trunk in the embryo. A capillary network is first laid down along the course of each vessel; the larger arteries and veins are defined by selection and enlargement of definite paths in this network. Lymphatic vessels develop after the main arteries and veins are formed: they arise initially by angiogenesis from the cardinal veins and subsequently by proliferation of lymphangioblasts to form lymphatic capillaries.
Early blood vessels are initially surrounded by a fibronectin-rich matrix that is later incorporated into the endothelial basal lamina along with laminin, a particularly early constituent. Several layers of fibronectin-expressing cells are seen around larger vessels, such as the dorsal aortae. The endothelium does not synthesize a basal lamina in those regions where remodelling and angiogenesis is active, and the mesenchyme around such endothelium does not express α-actin or laminin until branching has stopped and differentiation of the tunica media begins (after a stable vascular pattern has formed). It is not known how differentiation of pericytes and smooth muscle cells is induced; the majority of arteries accumulate medial smooth muscle from the surrounding mesenchyme.
In early development, the arteries of the embryo are disproportionately large and their walls consist of little more than a single layer of endothelium. The cardiac orifices are also relatively large and the force of the cardiac contraction is weak, and consequently the circulation is sluggish, despite the rapid rate of contraction of the developing heart. However, the tissues are able to draw nourishment not only from the capillaries but also from the large arteries and the intraembryonic coelomic fluid.
It has been suggested that the rapidly expanding cardiovascular system is filled with plasma by the movement of fluid from the intraembryonic coelom to the veins. In general, the wall of the intraembryonic coelom is composed of proliferating cells which produce the splanchnopleuric and somatopleuric mesenchymal populations. However, the walls of a portion of the pericardioperitoneal canals are thinner, and possibly more permeable to coelomic fluid, at the time when the canals surround the hepatocardiac channels (veins). The latter lie between the hepatic plexus and the sinus venosus; the hepatocardiac channel on the right side is more developed and on the left it is more plexiform, with only a transitory connection to the sinus venosus. The differentiation of this specific coelomic region occurs just in advance of the expansion and filling of the right and left atria, at about stage 12.
As the heart muscle thickens, compacts and strengthens, the cardiac orifices become both relatively and absolutely reduced in size, the valves increase their efficiency, and the large arteries acquire their muscular walls and undergo a relative reduction in size. From this time onwards, the embryo is dependent for its nourishment on the expanding capillary beds, and the function of the larger arteries becomes restricted to that of controllable distribution channels to keep the embryonic tissues constantly and appropriately supplied.
The heart starts to beat early, before the development of the conduction system, and a circulation is established before a competent valvular mechanism has formed. Cardiac output increases in proportion with the weight of the embryo and cardiac rate increases with development. However, most of the increase in cardiac output results from a geometric increase in stroke volume. When dorsal aortic blood flow is matched to embryonic weight, blood flow remains constant over a more than 150-fold change in mass of the embryo.
After head folding, the embryo has bilateral primitive aortae, each consisting of ventral and dorsal parts that are continuous through the first embryonic aortic arches (see Ch. 35). The ventral aortae are fused and form a dilated aortic sac. The dorsal aortae run caudally, one on each side of the notochord. In the fourth week they fuse from about the level of the fourth thoracic to that of the fourth lumbar segment to form a single definitive descending aorta (Figs 13.1A,C, 13.2B). In general, more mature endothelial channels are seen in the rostral, more advanced regions of the embryo, whereas more caudally, a changing capillary plexus constantly remodels until it becomes confluent with the vascular channels of the connecting stalk. The dorsal continuation of the primitive dorsal aortae directs blood into an anastomosing network around the allantois which will form the umbilical arteries. Blood is channelled back to the developing heart from the allantois via umbilical veins, from anastomoses in the primitive yolk sac via the vitelline veins, and from the body via pre- and post-cardinal veins that join to form the common cardinal veins (Figs 13.1B,C, 13.2A).
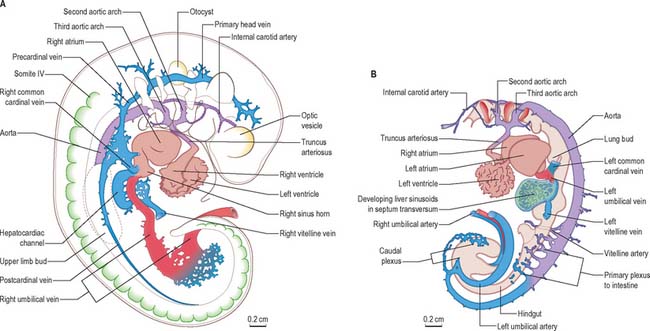
Fig. 13.2 Profile reconstructions of the blood vascular system of a stage 13 human embryo. The early circulation is now asymmetrical with the venous vessels enlarging on the right and diminishing on the left. A, Right side: the main venous channels to the heart can be seen. B, Left side: the aortic arch arteries, the main vessels arising from the dorsal aorta and the umbilical arteries are shown. Note: Only the endothelial lining of the heart chambers is shown and, because the muscular wall has been omitted, the pericardial cavity appears much larger than the contained heart.
EMBRYONIC ARTERIES
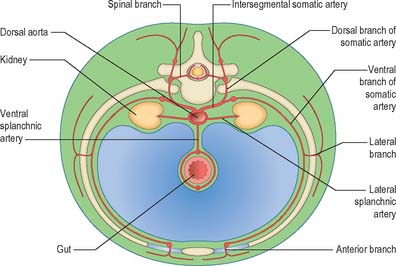
Initially the dorsal aortae are the only longitudinal vessels present. Their branches all run at right angles to the long axis of the embryo. Later these transverse arteries become connected by longitudinal anastomosing channels which persist in part, forming arteries such as the vertebral, internal thoracic, superior and inferior epigastric, and gastroepiploic. Each primitive dorsal aorta gives off somatic arteries (intersegmental branches to the body wall), a caudal continuation which passes into the body stalk (the umbilical arteries), lateral splanchnic arteries (paired segmental branches to the mesonephric ridge), and ventral splanchnic arteries (paired segmental branches to the digestive tube).
Somatic arteries
The somatic arteries are intersegmental in position. They persist, almost unchanged, in the thoracic and lumbar regions, as the posterior intercostal, subcostal and lumbar arteries. Each gives off a dorsal ramus, which passes backwards in the intersegmental interval and divides into medial and lateral branches to supply the muscles and superficial tissues of the back (Fig. 13.3). The dorsal ramus also gives off a spinal branch, which enters the vertebral canal and divides into a series of branches that supply the walls and joints of the osteoligamentous canal, and neural branches to the spinal cord and spinal nerve roots. After giving off its dorsal ramus, each intersegmental artery runs ventrally in the body wall, gives off a lateral branch and terminates in muscular and cutaneous rami.
Early umbilical arteries
Initially, the umbilical arteries are the direct caudal continuation of the primitive dorsal aortae. They are present in the body stalk before any vitelline or visceral branches emerge, indicating the dominance of the allantoic over the vitelline circulation in the human embryo. (On a comparative basis, the umbilical vessels are chorio-allantoic and therefore ‘somatovisceral’.) After the dorsal aortae fuse, the umbilical arteries arise from their ventrolateral aspects and pass medial to the primary excretory duct to the umbilicus. Later, the proximal part of each umbilical artery is joined by a new vessel that leaves the aorta at its termination and passes lateral to the primary excretory duct. This, possibly the fifth lumbar intersegmental artery, constitutes the dorsal root of the umbilical artery (the original stem, the ventral root). The dorsal root gives off the axial artery of the lower limb, branches to the pelvic viscera and, more proximally, the external iliac artery. The ventral root disappears entirely, and the umbilical artery now arises from that part of its dorsal root distal to the external iliac artery, i.e. the internal iliac artery.
Lateral splanchnic arteries
The lateral splanchnic arteries supply, on each side, the mesonephros, metanephros, testis or ovary and the suprarenal gland. All these structures develop, in whole or in part, from the intermediate mesenchyme, later termed the aorta-gonad-mesonephros region. One testicular or ovarian artery and three suprarenal arteries persist on each side. The phrenic artery branches from the most cranial suprarenal artery, and the renal artery arises from the most caudal. Additional renal arteries are frequently present and may be regarded as branches of persistent lateral splanchnic arteries.
Ventral splanchnic arteries
The ventral splanchnic arteries are originally paired vessels distributed to the capillary plexus in the wall of the yolk sac. After fusion of the dorsal aortae, they merge as unpaired trunks that are distributed to the increasingly defined and lengthening primitive digestive tube. Longitudinal anastomotic channels connect these branches along the dorsal and ventral aspects of the tube, forming dorsal and ventral splanchnic anastomoses (Fig. 13.3). These vessels obviate the need for so many ‘subdiaphragmatic’ ventral splanchnic arteries, and these are reduced to three, the coeliac trunk and the superior and inferior mesenteric arteries. As the viscera supplied descend into the abdomen, their origins migrate caudally by differential growth: the origin of the coeliac artery is transferred from the level of the seventh cervical segment to the level of the 12th thoracic; the superior mesenteric from the second thoracic to the first lumbar; and the inferior mesenteric from the twelfth thoracic to the third lumbar. However, above the diaphragm, a variable number of ventral splanchnic arteries persist, usually four or five, supplying the thoracic oesophagus. The dorsal splanchnic anastomosis persists in the gastroepiploic, pancreaticoduodenal and primary branches of the colic arteries, whereas the ventral splanchnic anastomosis forms the right and left gastric and the hepatic arteries.
EMBRYONIC VEINS
The early embryonic veins are often segregated into two groups, visceral and somatic, for convenience and apparent simplicity. The visceral group contains the derivatives of the vitelline and umbilical veins, and the somatic group includes all remaining veins. It should be noted that, with time, embryonic veins change the principal tissues they drain. They may receive radicles from obviously parietal tissues which become confluent with drainage channels that are clearly visceral, and so form a compound vessel. The arrangement of the early embryonic veins is initially symmetrical. The primitive tubular symmetric heart receives its venous return through the right and left sinual horns which are initially embedded in the mesenchyme of the septum transversum. Each horn receives, most medially, the termination of the principal vitelline vein, more laterally, the umbilical vein and, most laterally, having encircled the pleuroperitoneal canal, the common cardinal vein. This symmetric pattern changes as the heart and gut develop and the cardiac return is diverted to the right side of the heart.
Vitelline veins
The vitelline veins drain capillary plexuses developed in the splanchnopleuric mesenchyme of the secondary yolk sac. With head, tail and lateral fold formation, the upper recesses of the yolk sac are enclosed within the embryo as the splanchnopleuric gut tube, which extends from the stomodeal buccopharyngeal membrane to the proctodeal cloacal membrane. Derivatives from all these levels possess a venous drainage that is originally vitelline.
Early umbilical veins
The umbilical veins form by the convergence of venules that drain the splanchnopleure of the extraembryonic allantois. The human endodermal allantois is very small: it projects into the embryonic end of the connecting stalk, which is therefore regarded as precociously formed allantoic mesenchyme, whereas the umbilical vessels are considered to be allantoic. The peripheral venules drain the mesenchymal cores of the chorionic villous stems and terminal villi (extraembryonic somatopleuric structures). These are the radicles of the vena umbilicalis impar (usually single) which traverses the compacting mixed mesenchyme of the umbilical cord to reach the caudal rim of the umbilicus. Here, the single cordal vein divides into primitive right and left umbilical veins. Each curves rostrally in the somatopleuric lateral border of the umbilicus, i.e. where intraembryonic and extraembryonic or amniotic somatopleure are continuous, lying lateral to the communication between both the intraembryonic and extraembryonic coeloms. Rostrolateral to the umbilicus, the two umbilical veins reach, enter and traverse the junctional mesenchyme of the septum transversum and connect with septal capillary plexuses. They then continue, entering their corresponding cardiac sinual horns lateral to the terminations of the vitelline veins. This early symmetric disposition of the vitelline veins and anastomoses, umbilical and common cardinal veins, and the locus of the hepatic primordial complex are summarized in Figs 13.1B, 13.2A. For further development of the vitelline and umbilical veins see Chapter 73 (Figs 73.8, 73.9).
Cardinal veins and somatic venous complexes
The initial venous channels in the early embryo have traditionally been termed cardinal because of their importance at this stage. The cardinal venous complexes are first represented by two large vessels on each side, the precardinal portion being rostral and the postcardinal, caudal, to the heart. The two veins on each side unite to form a short common cardinal vein, which passes ventrally, lateral to the pleuropericardial canal, to open into the corresponding horn of the sinus venosus (Figs 13.1B, 13.2A, 13.4B). The precardinal veins undergo remodelling as the head develops. The postcardinal veins, which in the early embryo drain the body wall, are insufficient channels for venous return from the developing mesonephros and gonads and for the growing body wall. As the embryo increases in size, they are supplemented by a range of bilateral longitudinal channels that anastomose with the posterior cardinal system and with each other. These channels are the subcardinal, supracardinal, azygos line, subcentral and precostal veins (Fig. 13.4).
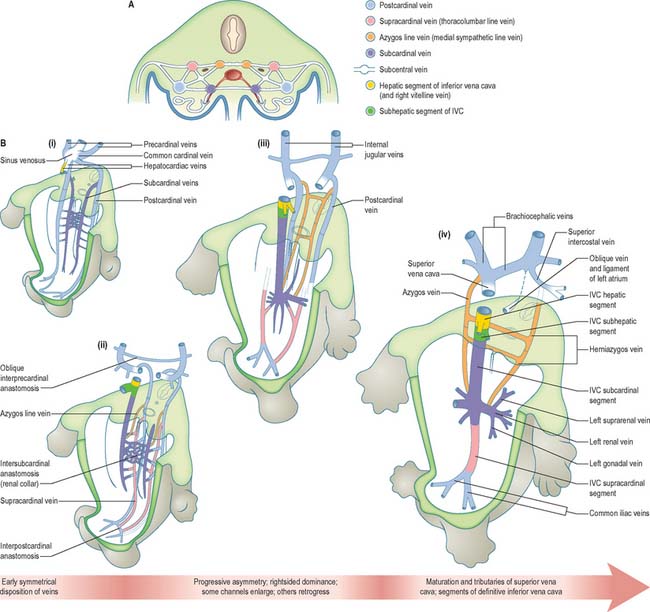
Fig. 13.4 Somatic venous development. A, Schematic section through the embryonic trunk. Principal longitudinal veins are colour-coded. Interconnections and intersegmental veins remain uncoloured. B, Plan of development of principal somatic veins from the early symmetric state, through states of increasing asymmetry, to the definitive arrangement.
Subcardinal veins
Subcardinal veins form in the ventromedial parts of the mesonephric ridges and become connected to the postcardinal veins by a number of vessels traversing the medial part of the ridges. The subcardinal veins assume the drainage of the mesonephros, and intercommunicate by a pre-aortic anastomotic plexus, which later constitutes the part of the left renal vein that crosses anterior to the abdominal aorta.
Supracardinal veins
Supracardinal veins form dorsolateral to the aorta and lateral to the sympathetic trunk and take over the intersegmental venous drainage from the posterior cardinal vein. The supracardinal veins are also referred to as the thoracolumbar line or lateral sympathetic line veins.
Azygos line veins
Azygos line veins form dorsolateral to the aorta and medial to the sympathetic trunk. These channels, also referred to as the medial sympathetic line veins, gradually take over the intersegmental venous drainage from the supracardinal veins. The intersegmental veins now reach their longitudinal channel by passing medial to the autonomic trunk, a relationship that the lumbar and intercostal veins subsequently maintain. Cranially, the azygos lines join the persistent cranial ends of the posterior cardinal veins.
Subcentral veins
Subcentral veins form directly dorsal to the aorta in the interval between the origins of the paired intersegmental arteries. These veins communicate freely with each other and with the azygos line veins; these connections ultimately form the retro-aortic parts of the left lumbar veins and of the hemiazygos veins.
Further development of the somatic veins
The supracardinal veins lie lateral to the aorta and the sympathetic trunks, which therefore intervene between them and the azygos lines (Fig. 13.4). They communicate caudally with the iliac veins and cranially with the subcardinal veins in the neighbourhood of the pre-aortic intersubcardinal anastomosis. The supracardinal veins also communicate freely with each other through the medium of the azygos lines and the subcentral veins. The most cranial of these connections, together with the supracardinal–subcardinal and the intersubcardinal anastomoses, complete a venous ring around the aorta below the origin of the superior mesenteric artery, termed the ‘renal collar’.
The ultimate arrangement of these embryonic abdominal and thoracic longitudinal cardinal veins may be summarized as follows. The terminal part of the left postcardinal vein forms the distal part of the left superior intercostal vein. On the right side, its cranial end persists as the terminal part of the azygos vein. The caudal part of the subcardinal vein is in part incorporated in the testicular or ovarian vein and partly disappears. The cranial end of the right subcardinal vein is incorporated into the inferior vena cava and also forms the right suprarenal vein. The left subcardinal vein, cranial to the intersubcardinal anastomosis, is incorporated into the left suprarenal vein. The renal and testicular or ovarian veins on both sides join the supracardinal–subcardinal anastomosis. On the left side, this is connected directly to the part of the inferior vena cava that is of subcardinal status via an intersubcardinal anastomosis. The right supracardinal vein forms much of the postrenal (caudal) segment of the inferior vena cava. The left supracardinal vein disappears entirely. The right azygos line persists in its thoracic part to form all but the terminal part of the azygos vein. Its lumbar part can usually be identified as a small vessel that leaves the vena azygos on the body of the 12th thoracic vertebra and descends on the vertebral column, deep to the right crus of the diaphragm, to join the posterior aspect of the inferior vena cava at the upper end of its postrenal segment. The left azygos line forms the hemiazygos veins. The subcentral veins give rise to the retro-aortic parts of the left lumbar veins and of the hemiazygos veins (Fig. 13.4).
EMBRYONIC LYMPHATIC VESSELS
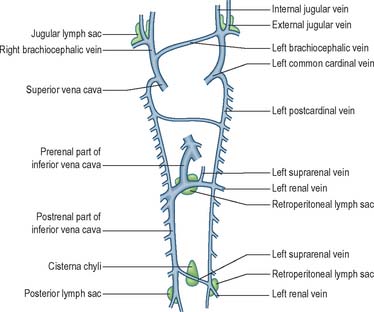
The earliest lymphatic vessels arise from budding of lymphatic endothelial cells from the cardinal veins to form lymph sacs (Eichman et al 2005). Six early lymph sacs can be identified, two are paired (the jugular and the posterior lymph sacs) and two are unpaired (the retroperitoneal sac and the cisterna chyli). In lower mammals an additional pair (subclavian) is present, but in the human embryo these are merely extensions of the jugular sacs.
The jugular lymph sac is the first to appear, at the junction of the subclavian vein with the precardinal, with later prolongations along the internal and external jugular veins; the posterior lymph sac encircles the left common iliac vein; the retroperitoneal sac appears in the root of the mesentery near the suprarenal glands; and the cisterna chyli appears opposite the third and fourth lumbar vertebrae (Fig. 13.5). The lymph vessels bud out from the lymph sacs along lines that correspond more or less closely with the course of embryonic blood vessels (most commonly veins); many also arise de novo in the mesenchyme and establish connections with existing vessels. In the body wall and the wall of the intestine, the deeper plexuses are the first to be developed; the vessels in the superficial layers are gradually formed by continued growth.
LYMPH NODES AND LYMPHOID TISSUES
Lymph vessels can be seen in the embryo in the cervical region from stage 16. Lymph nodes, which provide regional proliferative foci for lymphocytes, have been identified from week 9. Early lymph sacs become infiltrated by lymphoid cells, and the outer portion of each sac becomes the subcapsular sinus of the lymph node. Morphological differentiation of medullary and cortical compartments has not been observed until the end of week 10 (Tonar et al 2001). At the same time as these early lymph nodes are developing, the nasopharyngeal wall is infiltrated by lymphoid cells that are believed to herald the early development of the tubal and pharyngeal tonsils.
In the neonate, a considerable proportion of the total amount of lymphoid tissue is localized in lymph nodes: the subsequent increase in the amount of lymphoid tissues that occurs during childhood reflects the growth of these nodes. Definitive follicles with germinal centres are formed during the first postnatal year. The pharyngeal tonsil reaches its maximal development at 6 years and its subsequent involution is completed by puberty. Details of the development of gut-associated lymphoid tissue are given in Chapter 73.
Eichman A, Yuan Li, Moyon D, Lenoble F, Pardanaud L, Bréant C. Vascular development: from precursor cells to branched arterial and venous networks. Int J Dev Biol. 2005;49:259-267.
Sabin FR. On the origin of the abdominal lymphatics in mammals from the vena cava and the renal glands. Anat Rec. 1912;6:335-342.
Tonar Z, Kocova J, Liska V, Slipkja J. Early development of the jugular lymphatics. Sb Lek. 2001;102:217-225.