CHAPTER 57 Pleura, lungs, trachea and bronchi
The lungs are the essential organs of respiration and are responsible for the uptake of oxygen into the blood and the removal of carbon dioxide. The functional design of the thorax facilitates this complex process. The muscles of respiration and the diaphragm, acting together, increase the intrathoracic volume, creating a negative pressure within the pleural space which surrounds the lung and causing expansion of the lung (see Ch. 58). The resultant reduction in intra-alveolar pressure prompts the conduction of air through the upper respiratory tract into the trachea and airways and thence into the alveoli, where gaseous exchange occurs. The process of breathing exposes the lung to noxious agents, including gases, dust particles, bacteria and viruses; the mucous barrier, mucociliary escalator, branching pattern of the airways and the cough reflex are all anatomical defences against these insults. Anatomical defects may compromise respiratory function; for example, chest wall abnormalities may cause restrictive lung disease. Similarly, ultrastructural abnormalities such as ciliary dysfunction (as seen in Kartagener’s syndrome) lead to recurrent respiratory infections and airway damage.
PLEURA
Each lung is covered by pleura, a serous membrane arranged as a closed invaginated sac. The visceral or pulmonary pleura adheres closely to the pulmonary surface and its interlobar fissures. Its continuation, the parietal pleura, lines the corresponding half of the thoracic wall and covers much of the diaphragm and structures occupying the middle region of the thorax. The visceral and parietal pleurae are continuous with each other around the hilar structures, and they remain in close, though sliding, contact at all phases of respiration. The potential space between them is the pleural cavity, which is maintained at a negative pressure by the inward elastic recoil of the lung and the outward pull of the chest wall.
The right and left pleural sacs form separate compartments and touch only behind the upper half of the sternal body (see Fig. 53.6A,B), although they are also close to each other behind the oesophagus at the midthoracic level. The region between them is the mediastinum (interpleural space). The left pleural cavity is the smaller of the two, because the heart extends further to the left. The upper and lower limits of the pleurae are about the same on the two sides, but the left sometimes descends lower in the midaxillary line.
The interlobar fissures and posterior azygo-oesophageal and retrosternal pleural reflections are the only aspects of the normal pleura that can be visualized on a chest radiograph or CT scan (Fig. 57.1A–D; see Figs 55.16, 55.17). Demonstration of significant pleural shadowing in any other regions usually implies pathological abnormalities of the pleura. Thoracoscopy allows the direct inspection of both the parietal and visceral surfaces. The parietal pleura is translucent and at thoracoscopy the underlying muscles and blood vessels are visible. The visceral pleura is also translucent and has a grey variegated appearance due to the underlying lung and the vascular network in the subpleural layer.
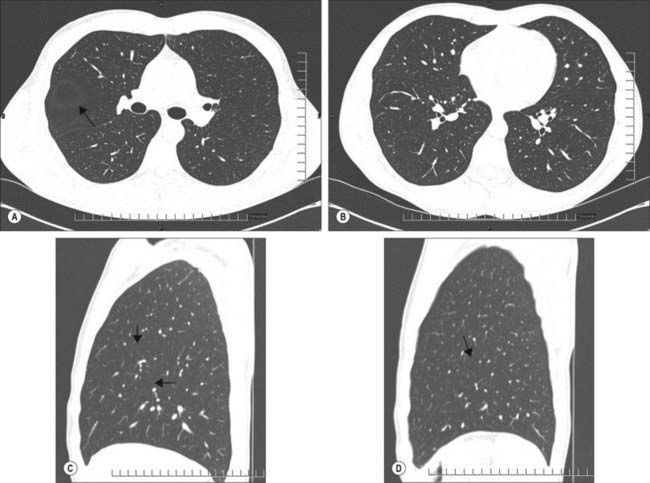
Fig. 57.1 A, Axial section through the lungs below the carina demonstrates the left and right major fissures. The circular avascular area anterior to the right major fissure and surrounded by a halo of white (arrow) is due to the horizontal (minor) fissure. In this subject the fissure is slightly domed and passing through the plane of the section. B, Axial section through the lungs at the mid left atrial level demonstrating the left and right major fissures as they pass more anteriorly and separate the lower lobes from the lingula and middle lobes. C, Sagittal section through the right lung demonstrating the major and minor fissures (arrows). D, Sagittal section through the left lung demonstrating the major fissure (arrow).
PARIETAL PLEURA
Different regions of parietal pleura are customarily distinguished by name, thus the costovertebral pleura lines the internal surface of the thoracic wall and the vertebral bodies, the diaphragmatic pleura lies on the thoracic surface of the diaphragm, the cervical pleura lies over the pulmonary apices (and is therefore also called the dome of the pleura), and the mediastinal pleura is applied to the structures between the lungs.
Costovertebral pleura
Costovertebral pleura lines the sternum, ribs, transversus thoracis and intercostal muscles and the sides of the vertebral bodies; normally it is easily separated from these structures. External to the pleura is a thin layer of loose connective tissue, the endothoracic fascia, which corresponds to the transversalis fascia of the abdominal wall. Anteriorly, the costal pleura begins behind the sternum, where it is continuous with the mediastinal pleura along a junction extending from behind the sternoclavicular joint down and medially to the midline behind the sternal angle. From here, the right and left costal pleurae descend in contact with each other to the level of the fourth costal cartilages and then diverge. On the right side, the line descends to the back of the xiphisternal joint, while on the left the line diverges laterally and descends at a distance of 2–2.5 mm from the sternal margin to the sixth costal cartilage, forming the cardiac notch. On each side, the costal pleura sweeps laterally, lining the internal surfaces of the costal cartilages, ribs, transversus thoracis and intercostal muscles. Posteriorly, it passes over the sympathetic trunk and its branches to reach the sides of the vertebral bodies, where it is again continuous with the mediastinal pleura. The costovertebral pleura is continuous with the cervical pleura at the inner margin of the first rib and below it becomes continuous with the diaphragmatic pleura along a line which differs slightly on the two sides. On the right, this line of costodiaphragmatic reflection begins behind the xiphoid process, passes behind the seventh costal cartilage to reach the eighth rib in the midclavicular line, the tenth rib in the midaxillary line, and then ascends slightly to cross the twelfth rib level with the upper border of the twelfth thoracic spine (see Fig. 53.6A,B). On the left, the line initially follows the ascending part of the sixth costal cartilage, but then follows a course similar to that on the right, although it may be slightly lower.
Diaphragmatic pleura
The diaphragmatic pleura is a thin, tightly adherent layer which covers most of the upper surface of the diaphragm. It is continuous with the costal pleura and on its medial aspect is continuous with the mediastinal pleura along the line of attachment of the pericardium to the diaphragm.
Cervical pleura
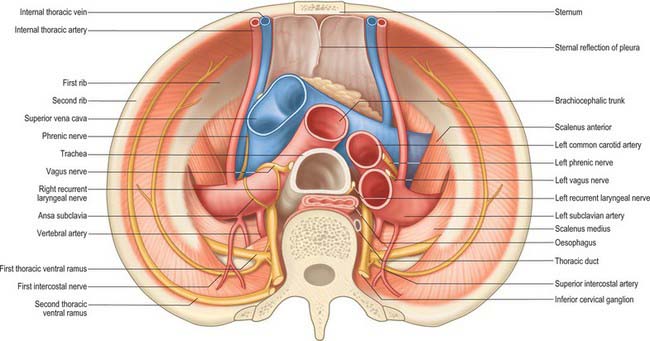
The cervical pleura is a continuation of the costovertebral pleura over the pulmonary apex (Fig. 57.2). It ascends medially from the internal border of the first rib to the apex of the lung, as high as the lower edge of the neck of the first rib, and then descends lateral to the trachea to become the mediastinal pleura. As a result of the obliquity of the first rib, the cervical pleura extends 3–4 cm above the first costal cartilage, but not above the neck of the first rib. The cervical pleura is strengthened by a fascial suprapleural membrane, which is attached in front to the internal border of the first rib, and behind to the anterior border of the transverse process of the seventh cervical vertebra. It contains a few muscular fibres, which spread from the scaleni. Scalenus minimus extends from the anterior border of the transverse process of the seventh cervical vertebra to the inner border of the first rib behind its subclavian groove, and also spreads into the pleural dome, which it therefore tenses: it has been suggested that the suprapleural membrane is the tendon of scalenus medius. The cervical pleura (like the pulmonary apex) reaches the level of the seventh cervical spine approximately 2.5 cm from the midline. Its projection is a curved line from the sternoclavicular joint to the junction of the medial and middle thirds of the clavicle, its summit being 2.5 cm above it. The subclavian artery ascends laterally in a furrow below the summit of the cervical pleura (Fig. 57.2).
Mediastinal pleura
The mediastinal pleura is the lateral boundary of the mediastinum and forms a continuous surface above the hilum of the lung from sternum to vertebral column. On the right it covers the right brachiocephalic vein, the upper part of the superior vena cava, the terminal part of the azygos vein, the right phrenic and vagus nerves, the trachea and oesophagus. On the left it covers the aortic arch, left phrenic and vagus nerves, left brachiocephalic and superior intercostal veins, left common carotid and subclavian arteries, thoracic duct and oesophagus. At the hilum of the lung it turns laterally to form a tube that encloses the hilar structures and is continuous with the pulmonary pleura.
VISCERAL PLEURA
The pulmonary pleura is inseparably adherent to the lung over all its surfaces, including those in the fissures, except at the root or hilum of the lung and along a line descending from this, which marks the attachment of the pulmonary ligament (Figs 57.3, 57.4).
INFERIOR PULMONARY LIGAMENTS
Below the hilum the mediastinal pleura extends as a double layer, the pulmonary ligament, from the lateral surface of the oesophagus to the mediastinal surface of the lung, where it is continuous with the parietal pleura (Figs 57.3, 57.4). It is continuous above with the pleura around the hilar structures and below it ends in a free sickle-shaped border.
PLEURAL RECESSES
The pleura extends considerably beyond the inferior border of the lung, but not as far as the attachment of the diaphragm, which means that the diaphragm is in contact with the costal cartilages and intercostal muscles below the line of pleural reflection from the thoracic wall to the diaphragm. In quiet inspiration the inferior margin of the lung does not reach this reflection, and the costal and diaphragmatic pleurae are separated merely by a narrow slit, the costodiaphragmatic recess. In quiet inspiration the lower limit of the lung is normally 5 cm above the lower pleural limit. A similar costomediastinal recess exists behind the sternum and the costal cartilages, where the thin anterior margin of the lung falls short of the line of pleural reflection. The extent of this recess, the anterior costomediastinal line of pleural reflection, and the position of the anterior margin of the lung all exhibit individual variation.
The inferior border of the right costodiaphragmatic recess is an important consideration in the surgical posterior approach to the kidney. Usually the pleura crosses the twelfth rib at the lateral border of erector spinae, so that the medial region of the kidney is above the pleural reflection (see Fig. 53.6B). However, if the twelfth rib does not project beyond the muscle, the eleventh rib may be mistaken for the twelfth in palpation, and an incision prolonged to this level will damage the pleura. Whether the lowest palpable rib is the eleventh or twelfth can be ascertained by counting from the second rib (identified at its junction with the sternal angle).
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
The parietal and visceral pleurae are developed from somatopleural and splanchnopleural layers of the lateral plate mesoderm respectively, which means that the parietal pleura is supplied by arteries from somatic sources. The costovertebral pleura is supplied by branches of intercostal and internal thoracic arteries; the mediastinal pleura is supplied by branches from bronchial, upper diaphragmatic, internal thoracic and mediastinal arteries; the cervical pleura is supplied by branches from the subclavian artery; and the diaphragmatic pleura is supplied by the superficial part of the microcirculation of the diaphragmatic muscle. The veins join systemic veins in the thoracic wall which drain into the superior vena cava. Lymph from the costovertebral parietal pleura drains into the internal thoracic chain anteriorly and intercostal chains posteriorly, while that from the diaphragmatic pleura drains into the mediastinal, retrosternal and coeliac axis nodes.
The visceral pleura forms an integral part of the lung and accordingly its arterial supply and venous drainage are provided by the bronchial vessels. The bronchial arteries at the hilum form an anulus that surrounds the main bronchus, and pleural branches from this anulus supply the visceral pleura facing the mediastinum, interlobar surfaces, apical surface and part of the diaphragmatic surface. The visceral pleura is drained by pulmonary veins, apart from an area around the hilum that drains into bronchial veins. The lymphatic drainage of the visceral pleura is to the deep pulmonary plexus within the interlobar and peribronchial spaces.
INNERVATION
The costal and peripheral diaphragmatic parietal pleurae are innervated by intercostal nerves, and the mediastinal and central diaphragmatic parietal pleurae are innervated by the phrenic nerve. Irritation of the former results in pain that is referred along the intercostal nerves to the appropriate part of the thoracic or abdominal wall, whereas irritation of the central diaphragmatic pleura causes pain that is referred to the lower neck and shoulder tip, i.e. to the C3, 4 dermatomes. The visceral pleura is innervated by visceral afferents that reach it by travelling along the bronchial vessels with the autonomic fibres.
PNEUMOTHORAX
Any breach of the chest wall and parietal pleura or visceral pleura leads to the accumulation of air within the pleural cavity (pneumothorax). Fluid (hydrothorax), blood (haemothorax) and rarely lymph (chylothorax) can also accumulate in this space. Pneumothoraces may occur spontaneously or following trauma (e.g. rib fractures, penetrating injuries from sharp instruments, iatrogenic injury). Significant air in the pleural space is visible on a chest radiograph: there is separation of the parietal and visceral pleurae and an absence of pulmonary vascular markings in the corresponding area. Occasionally, a ball valve-like effect occurs, so that air enters the pleural space during inspiration but cannot escape in expiration. This tension pneumothorax can be life-threatening and should be suspected whenever there are unilateral decreased breath sounds and hyperresonance on percussion, hypotension, jugular venous distension and contralateral tracheal deviation. A tension pneumothorax requires immediate decompression by the insertion of an intercostal drain or wide bore catheter. Fluid collection in the pleural space may be due to congestive cardiac failure, hypoalbuminaemia, inflammatory, infective or neoplastic conditions. A pleural effusion causes obliteration of the costophrenic angle and the diaphragm, and a lateral meniscus is visible on a frontal chest radiograph. Drainage of the fluid and subsequent analysis is required for diagnostic purposes. Where there is a collection of pus (empyema) or blood, pleural drainage is essential for therapeutic purposes. Ultrasonography is useful in assessing the size and characteristics of an effusion, such as the presence of loculation and debris. It may even demonstrate underlying consolidated lung. Computed tomography is utilized to assess the underlying hidden lung parenchyma and mediastinal glands. For further review of the radiology of the pleurae and lungs, see Armstrong (2000).
MICROSTRUCTURE
The pleural surface is smooth and moistened by serous fluid. It consists of a single layer of flat mesothelial cells separated by a basal lamina from an underlying lamina propria of loose connective tissue. Ultrastructurally, pleural mesothelial cells are like those of the peritoneum in that they have highly folded basal plasma membranes, their adjacent cell surfaces interdigitate and are joined by desmosomes, and their luminal surfaces bear numerous microvilli and some cilia. Pinocytotic vesicles are common in their cytoplasm.
The deeper layers of the lamina propria are continuous with the tissue surrounding the pulmonary lobules. Blood and lymph vessels and nerves all travel within the pleura.
Serosa
The serosa is formed by the visceral pleura, which is a transparent single sheet of mesothelium covering a thin lamina propria of connective tissue, and is closely attached to the various structures of the lung, except at the hilum. Beneath the serosa, loose connective tissue covers the entire pulmonary surface: it extends from the hilum along the conducting tubes and blood vessels within the substance of the lung. The connective tissue partitions the lung into numerous lobules, small polyhedral tissue masses which each receives a lobular bronchiole and the terminal branches of arterioles, venules, lymphatics and nerves. Lobules vary in size, the superficial ones are the largest, and are visible as polygonal areas 5–15 mm across.
LUNGS
The lungs are the essential organs of respiration. They are situated on either side of the heart and other mediastinal contents (Figs 57.3, 57.4). Each lung is free in its pleural cavity, except for its attachment to the heart and trachea at the hilum and pulmonary ligament respectively. When removed from the thorax, a fresh lung is spongy, can float in water, and crepitates when handled, because of the air within its alveoli. It is also highly elastic and so it retracts on removal from the thorax. Its surface is smooth and shiny and is separated by fine, dark lines into numerous small polyhedral domains, each crossed by numerous finer lines, indicating the areas of contact between its most peripheral lobules and the pleural surface.
At birth the lungs are pink, but in adults they are dark grey and patchily mottled. As age advances, this maculation becomes black, as granules of inhaled carbonaceous material are deposited in the loose connective tissue near the lung surface. Darkening is often more marked in men than women, in those who have dwelt in industrial areas and in smokers. The posterior pulmonary border is usually darker than the anterior. In the upper, less movable parts of the lung, this surface pigmentation tends to be concentrated opposite the intercostal spaces. Lungs from fetuses or stillborn infants who have not respired differ from those of infants who have taken a breath in that they are firm, non-crepitant and do not float in water.
The adult right lung usually weighs 625 g, and the left 565 g, but the range of wet weights is considerable, not least because it reflects the amount of blood or serous fluid contained within the lungs when weighed. In proportion to body stature, the lungs are heavier in men than in women.
PULMONARY SURFACE FEATURES
Each lung has an apex, base, three borders and two surfaces (Figs 57.3, 57.4). In shape, each lung approximates to half a cone.
Apex
The apex, the rounded upper extremity, protrudes above the thoracic inlet where it contacts the cervical pleura, and is covered in turn by the suprapleural membrane. As a consequence of the obliquity of the inlet, the apex rises 3–4 cm above the level of the first costal cartilage; it is level posteriorly with the neck of the first rib. Its summit is 2.5 cm above the medial third of the clavicle. The apex is therefore in the root of the neck (see Fig. 35.5A). It has been claimed that, because the apex does not rise above the neck of the first rib, it is really intrathoracic, and that it is the anterior surface that ascends highest in inspiration. The subclavian artery arches up and laterally over the suprapleural membrane, grooves the anterior surface of the apex near its summit and separates it from scalenus anterior. The cervicothoracic (stellate) sympathetic ganglion, the ventral ramus of the first thoracic spinal nerve and the superior intercostal artery all lie posterior to the apex. Scalenus medius is lateral, the brachiocephalic artery, right brachiocephalic vein and trachea are adjacent to the right medial surface of the lung, and the left subclavian artery and left brachiocephalic vein are adjacent to the left medial surface of the apex of the lung.
Base
The basal surface is semilunar and concave, and rests upon the superior surface of the diaphragm, which separates the right lung from the right lobe of the liver and the left lung from the left lobe of the liver, the gastric fundus and spleen. Since the diaphragm extends higher on the right than on the left, the concavity is deeper on the base of the right lung. Posterolaterally, the base has a sharp margin that projects a little into the costodiaphragmatic recess.
Costal surface
The costal surface of the lung is smooth and convex, and its shape is adapted to that of the thoracic wall, which is vertically deeper posteriorly. It is in contact with the costal pleura; in specimens that have been preserved in situ, this surface exhibits grooves that correspond with the overlying ribs.
Medial surface
The medial surface has a posterior vertebral and anterior mediastinal part. The vertebral part lies in contact with the sides of the thoracic vertebrae and intervertebral discs, the posterior intercostal vessels and the splanchnic nerves. The mediastinal area is deeply concave, because it is adapted to the heart at the cardiac impression, which is much larger and deeper on the left lung where the heart projects more to the left of the median plane. Posterosuperior to this concavity is the somewhat triangular hilum, where various structures enter or leave the lung, collectively surrounded by a sleeve of pleura that also extends below the hilum and behind the cardiac impression as the pulmonary ligament.
Other impressions on the lung surface
In addition to these pulmonary features, cadaveric lungs that have been preserved in situ can show a number of other impressions that indicate their relations with surrounding structures (Figs 57.3, 57.4). On the right lung the cardiac impression is related to the anterior surface of the right auricle, the anterolateral surface of the right atrium and partially to the anterior surface of the right ventricle. The impression ascends anterior to the hilum as a wide groove for the superior vena cava and the terminal portion of the right brachiocephalic vein. Posteriorly this groove is joined by a deep sulcus which arches forwards above the hilum and is occupied by the azygos vein. The right side of the oesophagus makes a shallow vertical groove behind the hilum and the pulmonary ligament. Towards the diaphragm it inclines left and leaves the right lung, and therefore does not reach the lower limit of this surface. Posteroinferiorly the cardiac impression is confluent with a short wide groove adapted to the inferior vena cava. Between the apex and the groove for the azygos, the trachea and right vagus are close to the lung, but do not mark it.
On the left lung (Fig. 57.4) the cardiac impression is related to the anterior and lateral surfaces of the left ventricle and auricle. The anterior infundibular surface and adjoining part of the right ventricle is also related to the lung as it ascends in front of the hilum to accommodate the pulmonary trunk. A large groove arches over the hilum, and descends behind it and the pulmonary ligament, corresponding to the aortic arch and descending aorta. From its summit a narrower groove ascends to the apex for the left subclavian artery. Behind this, above the aortic groove, the lung is in contact with the thoracic duct and oesophagus. In front of the subclavian groove there is a faint linear depression for the left brachiocephalic vein. Inferiorly, the oesophagus may mould the surface in front of the lower end of the pulmonary ligament.
Pulmonary borders
The inferior border is thin and sharp where it separates the base from the costal surface and extends into the costodiaphragmatic recess, and is more rounded medially where it divides the base from the mediastinal surface. It corresponds, in quiet respiration, to a line drawn from the lowest point of the anterior border which passes to the sixth rib at about the midclavicular line, then to the eighth rib in the midaxillary line (usually 10 cm above the costal margin), and then continues posteriorly, medially and slightly upwards to a point 2 cm lateral to the tenth thoracic spine (see Fig. 53.5A,B). The diaphragm rises higher on the right to accommodate the liver, and so the right lung is vertically shorter (by approximately 2.5 cm) than the left. However, cardiac asymmetry means that the right lung is broader, and has a greater capacity and weight than the left. The posterior border separates the costal surface from the mediastinal surface, and corresponds to the heads of the ribs. It has no recognizable markings and is really a rounded junction of costal and vertebral (medial) surfaces. The thin, sharp, anterior border overlaps the pericardium. On the right it corresponds closely to the costomediastinal line of pleural reflection, and is almost vertical. On the left it approaches the same line above the fourth costal cartilage; below this point it shows a variable cardiac notch, the edge of which passes laterally for 3.5 cm before curving down and medially to the sixth costal cartilage 4 cm from the midline. It therefore does not reach the line of pleural reflection here (see Fig. 53.5A) and so the pericardium is covered only by a double layer of pleura (area of superficial cardiac dullness). However, surgical experience suggests that the line of pleural reflection, the anterior pulmonary margin and the costomediastinal pleural recess are all variable.
PULMONARY FISSURES AND LOBES
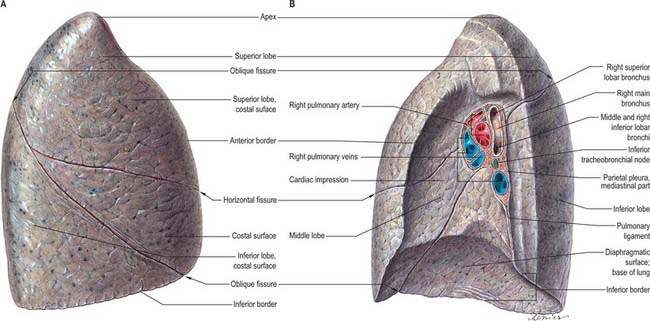
Right lung
The right lung is divided into superior, middle and inferior lobes by an oblique and a horizontal fissure (Fig. 57.3). The upper, oblique fissure separates the inferior from the middle and upper lobes, and corresponds closely to the left oblique fissure, although it is less vertical, and crosses the inferior border of the lung approximately 7.5 cm behind its anterior end. On the posterior border it is either level with the spine of the fourth thoracic vertebra or slightly lower. It descends across the fifth intercostal space and follows the sixth rib to the sixth costochondral junction. The short horizontal fissure separates the superior and middle lobes. It passes from the oblique fissure, near the midaxillary line, horizontally forwards to the anterior border of the lung, level with the sternal end of the fourth costal cartilage, then passes backwards to the hilum on the mediastinal surface. The horizontal fissure is usually visible on a frontal chest radiograph. The oblique fissure is usually visible on a lateral radiograph and on a high resolution CT scan as a curvilinear band from the lateral aspect to the hilum (Fig. 57.1A–D). The small middle lobe is cuneiform and includes some of the costal surface, the lower part of the anterior border and the anterior part of the base of the lung. Sometimes the medial part of the upper lobe is partially separated by a fissure of variable depth which contains the terminal part of the azygos vein, enclosed in the free margin of a mesentery derived from the mediastinal pleura, and forming the ‘lobe of the azygos vein’. This varies in size, and sometimes includes the apex of the lung. It is always supplied by one or more branches of the apical bronchus. Radiographically, a pleural effusion may be limited to the azygos fissure. Less common variations are the presence of an inferior accessory fissure, which separates the medial basal segment from the remainder of the lower lobe, and a superior accessory fissure, which separates the apical segment of the lower lobe from the basal segments. The identification of the completeness of the fissures is important prior to lobectomy, because individuals with incomplete fissures are more prone to develop postoperative air leaks, and may require further procedures such as stapling and pericardial sleeves (see Venuta et al 1998).
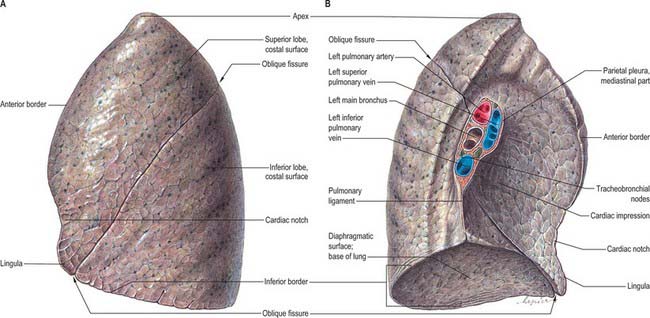
Left lung
The left lung is divided into a superior and an inferior lobe by an oblique fissure (Fig. 57.4) which extends from the costal to the medial surfaces of the lung both above and below the hilum. Superficially this fissure begins on the medial surface at the posterosuperior part of the hilum. It ascends obliquely backwards to cross the posterior border of the lung 6 cm below the apex, then descends forwards across the costal surface, to reach the lower border almost at its anterior end. It finally ascends on the medial surface to the lower part of the hilum. At the posterior border of the lung the fissure usually lies opposite a surface point 2 cm lateral to the midline between the spines of the third and fourth thoracic vertebrae, but it may be above or below this level. Traced around the chest, the fissure reaches the fifth intercostal space (at or near the midaxillary line) and follows this to intersect the inferior border of the lung close to, or just below, the sixth costochondral junction (7.5 cm from the midline). The left oblique fissure is usually more vertical than the right, and is indicated approximately by the medial border of the scapula when the arm is fully abducted above the shoulder. A left horizontal fissure is a normal variant found occasionally.
The superior lobe, which lies anterosuperior to the oblique fissure, includes the apex, anterior border, much of the costal and most of the medial surfaces of the lung. At the lower end of the cardiac notch a small process, the lingula, is usually present. The larger inferior lobe lies behind and below the fissure, and contributes almost the whole of the base, much of the costal surface and most of the posterior border of the lung.
Bronchopulmonary segments
Each of the principal bronchi divides into lobar bronchi. Primary branches of the right and left lobar bronchi are termed segmental bronchi because each ramifies in a structurally separate, functionally independent, unit of lung tissue called a bronchopulmonary segment (Figs 57.5-57.7).
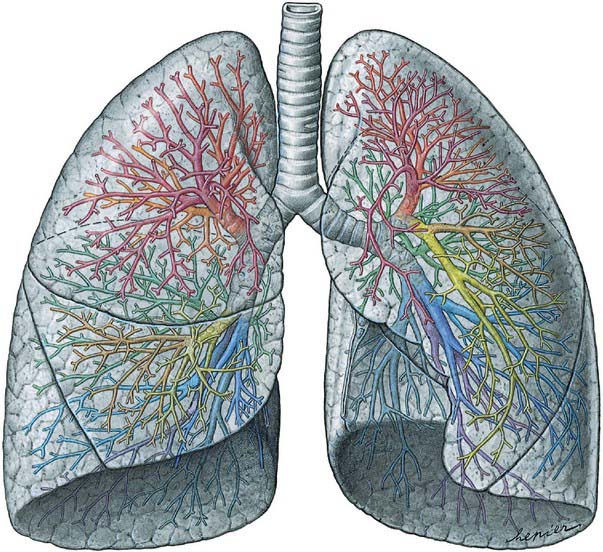
Fig. 57.5 Lungs and bronchi, ventral aspect. The lobar and segmental bronchi are projected onto the lung in different colours (compare with Fig. 57.6).
(From Sobotta 2006.)
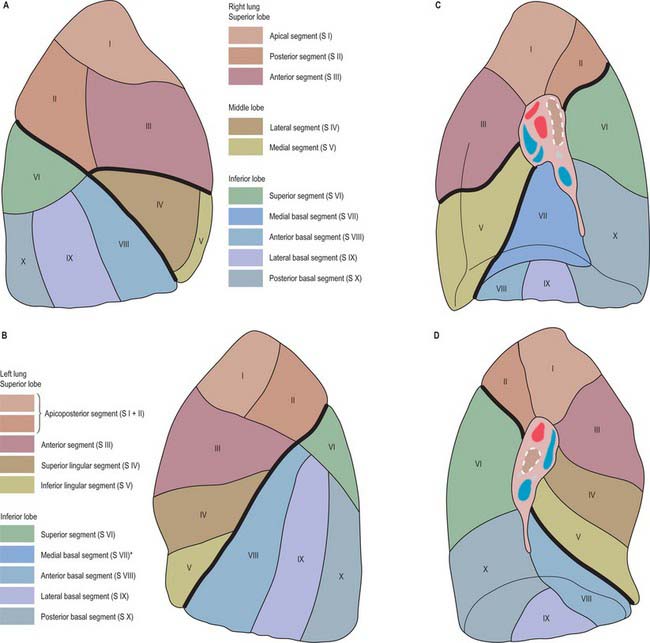
Fig. 57.6 Bronchopulmonary segments of the right and left lungs, coloured to indicate the different territories. A, Right lung, lateral aspect. B, Left lung, lateral aspect. C, Right lung, medial aspect. D, Left lung, medial aspect. *Generally, this segment is not a separate unit, but is fused with the anterior basal segment.
(From Sobotta 2006.)
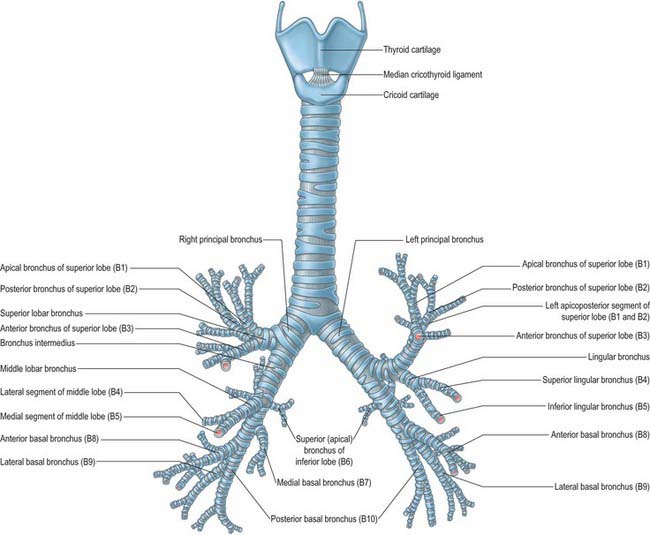
Fig. 57.7 The cartilages of the larynx, trachea and bronchi: anterior aspect. The bronchopulmonary segments are shown in brackets.
(Redrawn from an original figure drawn from a metal cast made by the late Lord Russell Brock, GKT School of Medicine, London.)
The main segments are named and numbered as follows:
| Right lung | |
| Superior lobe: | I, apical; II, posterior; III, anterior |
| Middle lobe: | IV, lateral; V, medial |
| Inferior lobe: | VI, superior (apical); VII, medial basal; VIII, anterior basal; IX, lateral basal; X, posterior basal |
| Left lung | |
| Superior lobe: | I, apical; II, posterior; III, anterior; IV, superior lingular; V, inferior lingular |
| Inferior lobe: | VI, superior (apical); VIII, anterior basal; IX, lateral basal; X, posterior basal |
Each segment is surrounded by connective tissue that is continuous with the visceral pleura, and is a separate respiratory unit. The vascular and lymphatic arrangements of the segments are described on pages 996–998.
PULMONARY HILA
The pulmonary root connects the medial surface of the lung to the heart and trachea and is formed by a group of structures which enter or leave the hilum (Figs 57.3, 57.4; see also Fig. 55.4B). These are the principal bronchus, pulmonary artery, two pulmonary veins, bronchial vessels, a pulmonary autonomic plexus, lymph vessels, bronchopulmonary lymph nodes and loose connective tissue, all of which are enveloped by a sleeve of pleura. The pulmonary roots, or pedicles, lie opposite the bodies of the fifth to seventh thoracic vertebrae. The phrenic nerve, pericardiacophrenic artery and vein, and anterior pulmonary plexus are common anterior relations of both hila, and the vagus nerve and posterior pulmonary plexus are common posterior relations. The pulmonary ligament is a common inferior relation. The major structures in both roots are similarly arranged, so that the upper of the two pulmonary veins is anterior, the pulmonary artery and principal bronchus are more posterior, and the bronchial vessels are most posterior. The arrangement of bronchopulmonary segments and the pulmonary hila permit the resection of abscesses and localized lesions caused by lung cancer.
Right hilum
The right root is situated behind the superior vena cava and right atrium and below the terminal part of the azygos vein. The usual sequence of hilar structures from above downwards is: superior lobar bronchus, pulmonary artery, principal bronchus, and lower pulmonary vein (Fig. 57.3).
Left hilum
The left root lies below the aortic arch and in front of the descending thoracic aorta. The usual vertical sequence of structures at the left hilum is pulmonary artery, principal bronchus, and lower pulmonary vein (Fig. 57.4). The pulmonary artery is longer on the left side, and each of its branches from the hilum to the oblique fissure must be identified in pulmonary resections.
SECONDARY PULMONARY LOBULES
Each segmental bronchus supplies a bronchopulmonary segment. Progressive subdivisions of the bronchus occur within each segment and the bronchi become increasingly narrow. All intrapulmonary bronchi are kept patent by cartilaginous plates, which decline in size and number and finally disappear when the tubes are less than 1 mm in diameter (bronchioles). The terminal bronchiole is the most peripheral bronchiole not to have alveoli in its wall. Distal to each terminal bronchiole is an acinus, which consists of three to four orders of respiratory bronchioles, leading to three to eight orders of alveolar ducts. The walls of these ducts consist of alveolar sacs or the mouths of alveoli. The primary lobule is the lung distal to the respiratory bronchiole. The secondary lobule is the smallest subsection of the peripheral lung bounded by connective tissue septa and consists of approximately six terminal bronchioles. The connective tissue septa are uneven in both size and shape.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
The lungs have two functionally distinct circulatory pathways. The pulmonary vessels convey deoxygenated blood to the alveolar walls and drain oxygenated blood back to the left side of the heart, and the much smaller bronchial vessels, which are derived from the systemic circulation, provide oxygenated blood to lung tissues that do not have close access to atmospheric oxygen, i.e. those of the bronchi and larger bronchioles.
The pulmonary artery bifurcates into right and left pulmonary arteries which pass to the hila of the lungs. On entering the lung tissue, both arteries divide into branches that accompany segmental and subsegmental bronchi and lie mostly dorsolateral to them. The pulmonary capillaries form plexuses immediately outside the epithelium in the walls and septa of alveoli and alveolar sacs. The plexus forms a single layer in the interalveolar septa, with meshes smaller than the capillaries, and exceedingly thin walls. Pulmonary veins, two from each lung, drain the pulmonary capillaries. Their radicles coalesce into larger branches which traverse the lung independently of the pulmonary arteries and bronchi. Communicating freely, they form large vessels that ultimately accompany the arteries and bronchial tubes to the pulmonary hilum, where the bronchi often separate the dorsolateral artery and the ventromedial vein. The pulmonary veins open into the left atrium and convey oxygenated blood for systemic distribution by the left ventricle.
At the hilum, the pulmonary vessels accompany the main bronchial divisions. This is not the case in the bronchopulmonary segments, where a segmental bronchus, its branches and associated arteries occupy a central position in each segment, but the many tributaries of the pulmonary veins run between segments, serving adjacent segments (which therefore drain into more than one vein). Some veins also lie beneath the visceral pleura, including the pleura in the interlobar fissures. It follows from this that a bronchopulmonary segment is not a complete vascular unit with an individual bronchus, artery and vein. During resection of segments, it is obvious that the planes between them are not avascular but are crossed by pulmonary veins and sometimes by branches of arteries. This pattern of bronchi, arteries and veins exhibits considerable variation: veins are the most variable, and arteries are more variable than bronchi.
Pulmonary arteries
The right pulmonary artery divides into two large branches as it emerges behind the superior vena cava (Fig. 57.8). A lymph node usually occupies the bifurcation. The superior branch, which is the smaller of the two, goes to the superior lobe and usually divides into two further branches, which supply the majority of that lobe. The inferior branch descends anterior to the intermediate bronchus and immediately posterior to the superior pulmonary vein. It provides a small recurrent branch to the superior lobe, then at the point where the horizontal fissure meets the oblique fissure, it gives off the branch to the middle lobe anteriorly, and the branch to the superior segment of the inferior lobe posteriorly. It then continues a short distance before dividing to supply the rest of the inferior lobe segments.
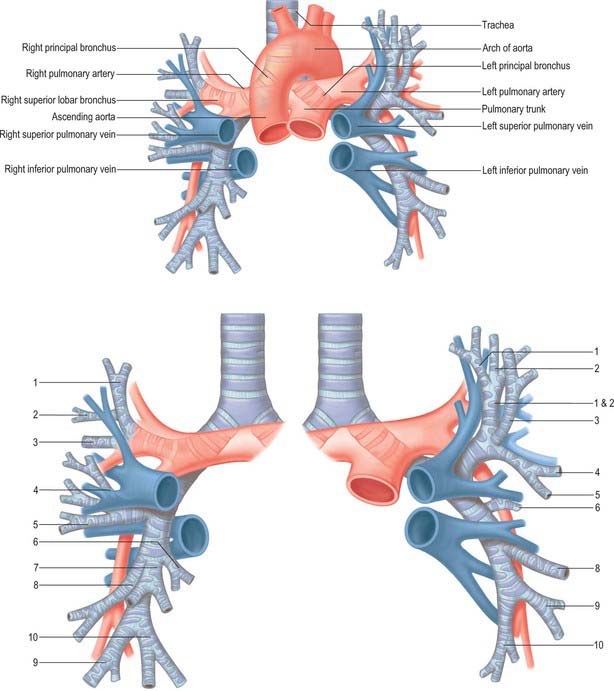
Fig. 57.8 The relationship between the central airways and the pulmonary vessels. The numbers in the lower figure denote the numbers of the bronchopulmonary segments.
The left pulmonary artery emerges from within the concavity of the aortic arch and descends anterior to the descending aorta to enter the oblique fissure (Fig. 57.8). The branches of the left pulmonary artery are extremely variable. The first and largest branch is usually given off to the anterior segment of the left superior lobe. Before reaching the oblique fissure, the artery gives off a variable number of other branches to the superior lobe, and as it enters the fissure it usually supplies a large branch to the superior segment of the inferior lobe. Lingular branches arise within the fissure, and the rest of the lower lobe is supplied by many varied branching patterns: it was a surgical aphorism of the late Lord Brock that when performing a left upper lobectomy, … ‘there was always one more branch of the pulmonary artery than you thought!’.
Pulmonary sequestration
When a portion of lung exists without the appropriate bronchovascular connections, it is usually supplied by the systemic vasculature. Extralobar pulmonary sequestration segments are covered by visceral pleura and usually found below the left lower lobe, whereas intralobar abnormalities are usually embedded in normal lung, classically the posterior basal segment of the left lower lobe. Extralobar pulmonary sequestration per se is asymptomatic, but is often associated with other congenital abnormalities.
Pulmonary arteriography and embolization
A thrombus that forms in the legs or pelvic veins may embolize and travel in the right side of the circulation through the right atrium and right ventricle and then lodge in the pulmonary vasculature. The clinical abnormalities observed depend on the size of the embolus. Large emboli may lodge in the main pulmonary artery branches and cause right ventricular dysfunction and hypoxia. Smaller emboli may lodge in segmental pulmonary arteries and cause pleuritic chest pain, shortness of breath and haemoptysis. Pulmonary emboli cause a ventilation/perfusion mismatch that can have serious physiological implications leading to a significant reduction in the oxygenation of blood. Ventilation/perfusion scans with radiolabelled xenon and technetium usually demonstrate segmental abnormalities in perfusion with normal ventilation in the corresponding regions. Pulmonary emboli may also be evaluated by contrast-enhanced spiral CT or pulmonary angiograms and appear as filling defects.
Pulmonary veins
There are usually four pulmonary veins, two from each lung. They originate from capillary networks in the alveolar walls and return oxygenated blood to the left atrium. All the main tributaries of the pulmonary veins receive smaller tributaries, both intra- and inter-segmental; by repeated junctions, tributary veins finally form a single trunk in each lobe, i.e. three in the right lung, and two in the left. The right middle and superior lobar veins usually join and so two veins, superior and inferior, leave each lung.
In the pulmonary hilum, the usual distribution of veins is as follows. The superior pulmonary vein is anteroinferior to the pulmonary artery, and the inferior is the most inferior hilar structure and also slightly posterior. On the right, the union of apical, anterior and posterior veins (draining the upper lobe) with a middle lobar vein (formed by lateral and medial tributaries; Fig. 57.8) forms the superior right pulmonary vein. The inferior right pulmonary vein is formed by the hilar union of superior (apical) and common basal veins from the lower lobe. The union of superior and inferior basal tributaries forms the common basal vein. Occasionally the three right lobar veins remain separate. The right superior pulmonary vein passes posterior to the superior vena cava, the inferior behind the right atrium. On the left, the superior left pulmonary vein, which drains the upper lobe, is formed by the union of apicoposterior (draining the apical and posterior segments), anterior and lingular veins (Fig. 57.8). The inferior left pulmonary vein, which drains the lower lobe, is formed by the hilar union of two veins, superior (apical) and common basal, the latter formed by the union of a superior and an inferior basal vein. Both superior and inferior left pulmonary veins pass anterior to the descending thoracic aorta. Sometimes the two left pulmonary veins form a single trunk, or they may be augmented by an accessory lobar vein from each lobe, which unite to form a third left pulmonary vein.
The right and left pulmonary veins perforate the fibrous pericardium and open separately in the posterosuperior aspect of the left atrium (see Figs 56.1, 56.2B). Their terminations are separated centrally by the oblique pericardial sinus, and laterally by smaller and variable pulmonary venous pericardial recesses that are directed medially and upwards. The pulmonary veins are devoid of valves.
Lymphatics
Pulmonary lymphatic vessels originate in a superficial subpleural plexus. A deep plexus accompanies the branches of the pulmonary vessels and bronchi. Superficial efferents turn round lung borders and the margins of fissures to converge in the bronchopulmonary nodes. There is little anastomosis between the superficial and deep lymphatics, except in the hilar regions. In peripheral parts of the lungs, small channels connect superficial and deep lymphatic vessels, and are capable of dilatation to direct lymph from the deep to the superficial channels when outflow from deep vessels is obstructed by pulmonary disease. There is a tendency for vessels from the upper lobes to pass to the superior tracheobronchial nodes, and those from lower lobes to the inferior tracheobronchial group; however, lymphatic vessels of adjoining lobes connect deep in the fissures and so these connections are not exclusive. At the level of pulmonary lobation, the arrangement of lymphatic vessels follows the central artery of a lobule and its peripheral veins. Lymphoid aggregations, non-follicular in appearance, occur in peribronchial sites and in ‘placoid’ formations adjoining pulmonary pleura.
INNERVATION
The autonomic nervous system controls many aspects of airway function, including regulation of airway smooth muscle tone, mucus secretion from submucosal glands and surface epithelial goblet cells, vascular permeability and blood flow (see Belvisi 2002).
Pulmonary plexuses
The pulmonary plexuses lie anterior and posterior to the other hilar structures of the lungs (see Fig. 56.20). The anterior plexus is small and is formed by rami from vagal and sympathetic cervical cardiac nerves via connections with the superficial cardiac plexus. The posterior pulmonary plexus is formed by the rami of vagal and sympathetic cardiac branches from the second to fifth or sixth thoracic sympathetic ganglia. The left plexus also receives branches from the left recurrent laryngeal nerve. Further details of the pulmonary plexuses are given in the description of the cardiac plexuses in Chapter 56.
MICROSTRUCTURE OF RESPIRATORY SURFACES
Thin-walled respiratory surfaces (alveoli) are distributed as isolated patches within the walls of respiratory bronchioles, and as tube-like alveolar ducts and balloon-like alveolar sacs which contain groups of adjacent alveoli.
Alveolar area
Normal adult human alveoli have a mean total surface area of 143 m2: an adult respiratory system contains of the order of 300 million alveoli. Their inflated diameter varies with lung position, and is greater in the upper regions than in the lower, because of the increased gravitational pressure at the lung base. These values vary considerably between normal young individuals; the differences become even more marked with age as a consequence of degenerative changes.
Alveolar structure
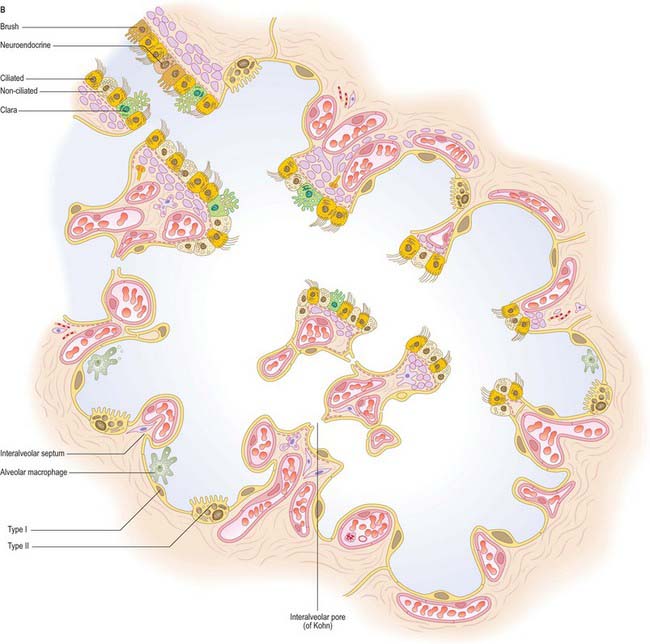
The alveoli are thin-walled pouches which provide the respiratory surface for gaseous exchange (Figs 57.9-57.11). Their walls contain two types of epithelial cell (pneumocytes) and cover a delicate connective tissue within which a network of capillaries ramifies. Since the walls are extremely thin, they present a minimal barrier to gaseous exchange between the atmosphere and the blood in the capillaries. Adjacent alveoli are frequently in close contact and then the intervening connective tissue forms the central part of an interalveolar septum. Alveolar macrophages are present within the alveolar lumen, and migrate over the epithelial surface.
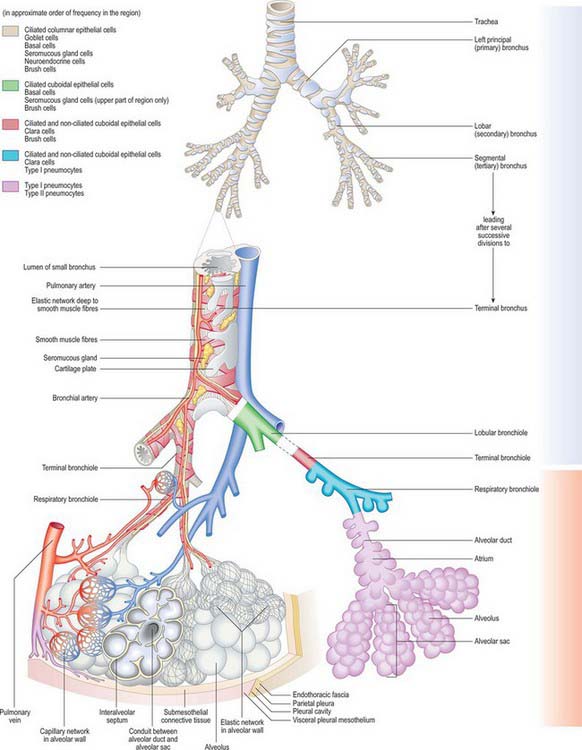
Fig. 57.9 The respiratory tree and its blood supply and drainage, lymphatic drainage and nerve supply. The distribution of the different epithelial cells is shown. Blue = vessels which contain deoxygenated blood; red = vessels which contain oxygenated blood.
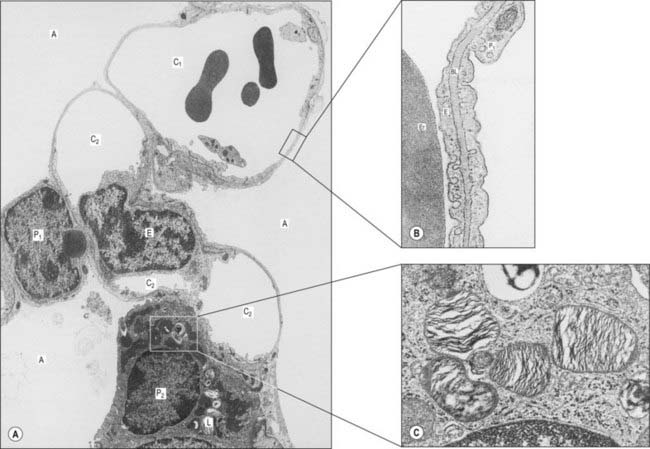
Fig. 57.11 A, Electron micrograph showing the septum between three adjacent alveoli (A). The septum contains two capillaries (C1 and C2) in section; the lower one is cut obliquely through the nucleus of an endothelial cell (E). Type I pneumocytes (P1) line the alveolar airspaces except where a type II pneumocyte (P2), with cytoplasmic lamellar bodies (L), is located. B, The thin portion of an interalveolar septum. Part of an erythrocyte (Er) is shown in the capillary lumen, lined by endothelium (E). The alveolar airspace (right) is lined by a type I pneumocyte (P1). Between these two cells is a shared basal lamina (BL). C, Cytoplasmic lamellar bodies in a type II pneumocyte are composed mainly of phospholipids which contribute to the surfactant secreted by these pneumocytes and Clara cells of the respiratory bronchioles.
(By permission from Young B, Heath JW 2000 Wheater’s Functional Histology. Edinburgh: Churchill Livingstone.)
Interalveolar septum
The alveolar lining epithelium varies in thickness, but extensive areas of it are as little as 0.05 μm thick (see Fig. 57.11). The epithelium lies on a basal lamina, which, in the thin portions of a septum, is fused with the basal lamina surrounding the adjacent capillaries. The total barrier to diffusion between air and blood in these thin portions may be as little as 0.2 μm. The thick portions of a septum contain connective tissue elements, including elastic fibres, collagen type III fibres, resident and migratory cells (Fig. 57.10).
Alveolar epithelial cells (pneumocytes)
The alveolar epithelium is a mosaic of types I and II pneumocytes. Type I pneumocytes are simple squamous epithelial cells and form over 90% of the alveolar area. Their basal laminae fuse with the basal laminae of the adjacent capillary endothelium to form the thin portions of interalveolar septa. Their cytoplasm is thin (0.05–0.2 μm) which facilitates gaseous diffusion between the lumen of the alveolus and its capillaries. The edges of adjacent cells overlap; they are joined by tight junctions, creating a strict diffusion barrier between the alveolar surface and underlying tissues. Together with a similar endothelial barrier, this arrangement limits the movement of fluid from blood and interstitial spaces into the alveolar lumen (the blood–air barrier). If damaged, type I cells, which do not divide, are replaced by type II cells, which proliferate and differentiate into type I pneumocytes.
The smaller type II cells are often more numerous than type I cells, but they contribute less than 10% of the alveolar surface area. They are rounded cells and protrude from the alveolar surface, particularly at the angles between alveolar profiles. In the human lung they are often associated with interalveolar pores of Kohn. Their cytoplasm contains numerous characteristic secretory lamellar bodies consisting of concentric whorls of phospholipid-rich membrane, the precursors of alveolar surfactant (Fig. 57.11C), which they can recycle.
Interalveolar pores (of Kohn)
These are small pores lined by epithelium (usually type II alveolar cells), which cross interalveolar septa to link adjacent alveolar airspaces. Humans have up to seven pores per alveolus, ranging in size from 2 to 13 μm. These small passages may sustain the flow of air in the event of blockage of one of the alveolar ducts and also provide routes of migration for alveolar macrophages.
Alveolar macrophages
Alveolar macrophages are derived from circulating monocyte precursors. They originate in haemopoietic tissue in the bone marrow, migrate into the alveolar lumen from adjacent blood vessels and connective tissue, and wander about on the epithelial surfaces. They clear the respiratory spaces of inhaled particles which are small enough to reach the alveoli (hence their alternative name of ‘dust cells’). Most of them migrate with their phagocytosed load to the bronchioles, where they are swept into the mucociliary rejection current and removed from the lung. Others migrate through the epithelium of the alveoli into the lymphatics which drain the connective tissue of the lung, and so pass into lymphoid tissue around the pulmonary lobules. Under normal conditions alveolar macrophages have a granular cytoplasm because they contain phagocytosed particles: in smokers the particles have a characteristic appearance, and are called tar bodies. When actively phagocytic, macrophages release proteases: if antiproteases (e.g. α1antitrypsin) that are normally present in the alveolar lining are deficient, macrophage activity may damage the lung. Alveolar macrophages are involved in the turnover of surfactant.
Alveolar macrophages can be recovered from sputum, and are of diagnostic importance if they appear abnormal. For instance, whenever erythrocytes leak from pulmonary capillaries, the macrophages that engulf the extravasated cells become brick red, and are detectable in ‘rusty’ sputum. They are typical of congestive heart failure, and often termed heart-failure cells. Macrophages that migrate back into the connective tissue of the lung settle in patches that are visible beneath the visceral pleura, e.g. carbon-filled cells give the lungs a mottled appearance. However, if the inhaled particles are abrasive or chemically active, they may elude macrophage removal and damage the respiratory surface, producing fibrosis and a concomitant reduction in the respiratory area. This occurs in many industrial diseases, e.g. pneumoconiosis, caused by coal dust, or asbestosis, where the long thin fibres of asbestos can cause considerable damage and may trigger fatal mesothelioma in the pleural lining.
Alveolar surfactant
The alveolar surface is normally covered by a film of pulmonary surfactant, which is a complex mixture, mainly of phospholipids (particularly dipalmitoylphosphatidylcholine and phosphatidylglycerol), with some protein and neutral lipid (Devendra & Spragg 2002). Surfactant is stored in lamellar bodies and secreted in the form of tubular myelin (unrelated to myelin of the nervous system) by type II pneumocytes. It is recycled by type II pneumocytes, or cleared (i.e. phagocytosed) by alveolar macrophages. Clara cells of the bronchiolar epithelium are believed to secrete surfactant of a different composition.
Surface tension at the alveolar surface is very high, because the alveoli are minute. This opposes expansion during inspiration, and tends to collapse the alveoli in expiration. The detergent-like properties of pulmonary surfactant greatly reduce the surface tension, and make ventilation of the alveoli much more efficient.
TRACHEA AND BRONCHI
The trachea is a tube formed of cartilage and fibromuscular membrane, lined internally by mucosa. The anterolateral portion is made up of incomplete rings of cartilage, and the posterior aspect by a flat muscular wall. It is 10–11 cm long, and descends from the larynx (Fig. 57.7) from the level of the sixth cervical vertebra to the upper border of the fifth thoracic vertebra, where it divides into right and left principal (pulmonary) bronchi. It lies approximately in the sagittal plane, but its point of bifurcation is usually a little to the right. The trachea is mobile and can rapidly alter in length; during deep inspiration, the bifurcation may descend to the level of the sixth thoracic vertebra. Its external transverse diameter is typically 2 cm in adult males, and 1.5 cm in adult females. In children it is smaller, more deeply placed and more mobile. The lumen in live adults has an average transverse diameter of 12 mm, although this increases after death because the smooth muscle making up its posterior aspect relaxes. In the first postnatal year, the tracheal diameter does not exceed 4 mm, while during later childhood its diameter in millimetres is approximately equal to age in years. The transverse shape of the lumen is variable, especially in the later decades of life, and may be round, lunate or flattened. At bronchoscopy the posterior wall of the trachea bulges into the lumen and this is exaggerated in expiration and coughing. The distal end of the trachea is visible as a concave spur. A tracheal bronchus may occasionally arise from the lateral wall of the trachea, more frequently from the right side: it may be supernumerary or it may represent a displaced upper lobe airway.
TRACHEAL RELATIONS
Cervical part of the trachea
Anterior relations
The cervical trachea is crossed anteriorly by skin and by the superficial and deep cervical fasciae. It is also crossed by the jugular arch and overlapped by sternohyoid and sternothyroid. The second to fourth tracheal cartilages are crossed by the isthmus of the thyroid gland, above which an anastomotic artery connects the bilateral superior thyroid arteries; below this and in front are the pretracheal fascia, inferior thyroid veins, thymic remnants and the thyroid ima artery (when it exists). In children the brachiocephalic artery crosses obliquely in front of the trachea at, or a little above, the upper border of the manubrium; the left brachiocephalic vein may also rise a little above this level.
Posterior relations
The oesophagus lies behind the cervical trachea, separating it from the vertebral column and the prevertebral fascia.
Lateral relations
The paired lobes of the thyroid gland, which descend to the fifth or sixth tracheal cartilage, and the common carotid and inferior thyroid arteries are lateral relations. The recurrent laryngeal nerves ascend on each side, in or near the grooves between the sides of the trachea and the oesophagus.
Thoracic part of the trachea
Anterior relations
As it descends through the superior mediastinum, the thoracic trachea lies behind the manubrium sterni, the attachments of sternohyoid and sternothyroid, the thymic remnants and the inferior thyroid vein. The brachiocephalic and left common carotid arteries come to lie on the right and left respectively of the trachea as they diverge upwards into the neck. At a lower level, the aortic arch, the brachiocephalic and left common carotid arteries, left brachiocephalic veins, deep cardiac plexus and some lymph nodes are all anterior to the trachea.
Posterior relations
The oesophagus is posterior to the trachea and separates it from the vertebral column.
Lateral relations
Laterally and on the right are the right lung and pleura, right brachiocephalic vein, superior vena cava, right vagus nerve and azygos vein. On the left are the arch of the aorta, and the left common carotid and left subclavian arteries. The left recurrent laryngeal nerve is at first situated between the trachea and aortic arch, and then lies in, or just in front of, the groove between the trachea and the oesophagus.
RIGHT MAIN BRONCHUS
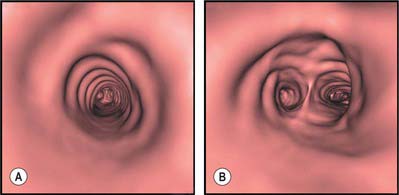
The right principal bronchus is approximately 2.5 cm long, and is wider, shorter and more vertical than the left (Figs 57.7, 57.8, 57.12). These differences explain why inhaled foreign bodies enter the right principal bronchus more often than the left (these events are more common in children and they may present with breathlessness, unilateral wheeze or recurrent aspirations: a chest radiograph may show air trapping in the affected lobe). The right main bronchus gives rise to its first branch, the superior lobar bronchus, then enters the right lung opposite the fifth thoracic vertebra. The azygos vein arches over it, and the right pulmonary artery lies at first inferior, then anterior, to it. After giving off the superior lobar bronchus, which arises posterosuperior to the right pulmonary artery, the right main bronchus crosses the posterior aspect of the artery, enters the pulmonary hilum posteroinferior to it, and divides into a middle and an inferior lobar bronchus. Normal variants in the bronchial anatomy are occasionally seen and consist of either displaced or supernumerary airways (see Ghaye et al 2001). Abnormalities include a common origin of the right upper and middle lobe bronchi; an accessory cardiac bronchus; and a right lower lobe bronchus that may arise from the left main stem bronchus. These anatomical variants are largely asymptomatic, but occasionally may cause haemoptysis, recurrent infection and development of bronchiectasis of the airway.
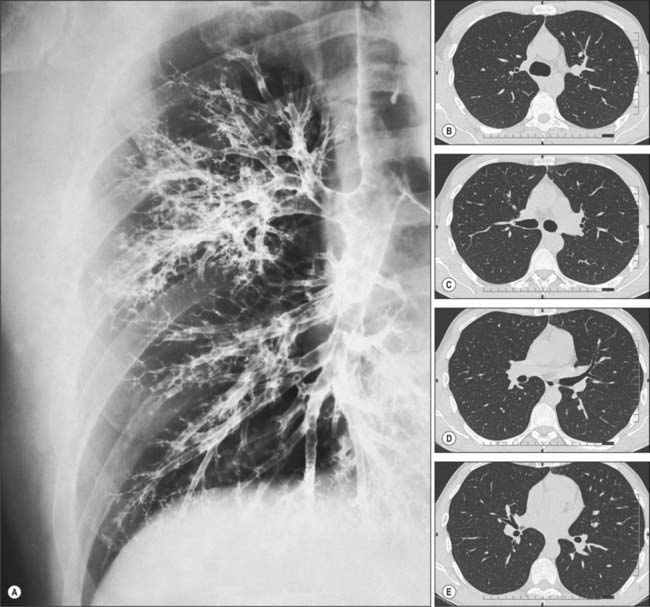
Fig. 57.12 A, Bronchogram showing the branching pattern of the trachea and bronchi of the right lung, in a slightly oblique anteroposterior view. In this procedure, a radiopaque contrast medium has been introduced into the respiratory tract to coat the walls of the respiratory passages. For identification of the major branches (compare with Fig. 57.5. B–E and Fig. 57.6) CT images demonstrating lobar and segmental bronchial branching. (B–E correspond to the 1st, 2nd, 3rd and 4th intercostal spaces, respectively.)
Right superior lobar bronchus
The right superior lobar bronchus arises from the lateral aspect of the parent bronchus and runs superolaterally to enter the hilum; 1 cm from its origin it divides into three segmental bronchi.
Segmental anatomy
The apical segmental bronchus continues superolaterally towards the apex of the lung, which it supplies, and divides near its origin into apical and anterior branches. The posterior segmental bronchus serves the posteroinferior part of the superior lobe, passes posterolaterally and slightly superiorly, and soon divides into a lateral and a posterior branch. The anterior segmental bronchus runs anteroinferiorly to supply the rest of the superior lobe, and divides near its origin into a lateral and an anterior branch of equal size (Figs 57.5, 57.6).
Right middle lobar bronchus
The right middle lobar bronchus normally starts 2 cm below the superior lobar bronchus, from the front of right bronchus intermedius, and descends anterolaterally.
Right inferior lobar bronchus
The right inferior lobar bronchus is the continuation of the principal bronchus beyond the origin of the middle lobar bronchus.
Segmental anatomy
At or a little below its origin from the principal bronchus, the right inferior lobar bronchus gives off a large superior (apical) segmental bronchus posteriorly. This runs posteriorly to the upper part of the inferior lobe, and then divides into medial, superior and lateral branches; the first two usually arise from a common stem. After giving off the superior segmental branch, the right inferior lobar bronchus descends posterolaterally. The medial basal segmental bronchus branches from its anteromedial aspect, and runs inferomedially to serve a small region below the hilum. The inferior lobar bronchus continues downwards and then divides into an anterior basal segmental bronchus, which descends anteriorly, and a trunk that soon divides into a lateral basal segmental bronchus, which descends laterally, and a posterior basal segmental bronchus, which descends posteriorly. In more than half of all right lungs a subsuperior (subapical) segmental bronchus arises posteriorly from the right inferior lobar bronchus 1–3 cm below the superior segmental bronchus, and is distributed to the region of lung between the superior and posterior basal segments (Figs 57.5, 57.6).
LEFT MAIN BRONCHUS
The left principal bronchus, which is narrower and less vertical than the right, is 5 cm long. Passing to the left inferior to the aortic arch, it crosses anterior to the oesophagus, thoracic duct and descending aorta; the left pulmonary artery is at first anterior and then superior to it. It enters the hilum of the left lung at the level of the sixth thoracic vertebra and divides into a superior and an inferior lobar bronchus.
Left superior lobar bronchus
The left superior lobar bronchus arises from the anterolateral aspect of its parent stem, curves laterally, and soon divides into two bronchi which correspond to the branches of the right principal bronchus as it supplies the right superior and middle lobes, except that on the left side both are distributed to the left superior lobe because there is no separate middle lobe.
Segmental anatomy
The superior division of the left superior lobar bronchus ascends 1 cm, gives off an anterior segmental bronchus, continues a further 1 cm as the apicoposterior segmental bronchus, and then divides into apical and posterior branches. The apical, posterior and anterior segmental bronchi are largely distributed as they are in the right superior lobe. The inferior division descends anterolaterally to the anteroinferior part of the left superior lobe (the lingula) and forms the lingular bronchus, which divides into superior and inferior lingular segmental bronchi. This is different from the pattern in the right middle lobe, where the corresponding distribution is lateral and medial (Figs 57.5, 57.6).
Left inferior lobar bronchus
The left inferior bronchus descends posterolaterally and divides to supply territories of the lung that are distributed in essentially the same manner as they are in the right lung.
Segmental anatomy
The superior (apical) segmental bronchus arises from the inferior lobar bronchus posteriorly 1 cm from its origin. After a further 1–2 cm, the inferior lobar bronchus divides into an anteromedial and a posterolateral stem. The latter divides into lateral and posterior basal segmental bronchi. The anterior basal segmental bronchus is an independent branch of the inferior lobar bronchus occasionally. A subsuperior (subapical) segmental bronchus arises posteriorly from the left inferior lobar bronchus sometimes (Figs 57.5, 57.6).
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Trachea
The trachea is supplied with blood mainly by branches of the inferior thyroid arteries. The thoracic portion of the trachea is also supplied by branches of the bronchial arteries that ascend to anastomose with the tracheal branches of the inferior thyroid arteries. Veins draining the trachea end in the inferior thyroid venous plexus. The lymph vessels pass to the pretracheal and paratracheal lymph nodes.
Bronchi
Bronchial arteries
The bronchial arteries supply oxygenated blood to maintain the pulmonary tissues. They are derived from the descending thoracic aorta either directly or indirectly (Baile 1996). The right bronchial artery is usually a branch of the third posterior intercostal artery. There are normally two left bronchial arteries (upper and lower) that branch separately from the thoracic aorta. The bronchial arteries accompany the bronchial tree and supply bronchial glands, the walls of the bronchial tubes and larger pulmonary vessels. The bronchial branches form a capillary plexus in the muscular tunic of the air passages which supports a second, mucosal plexus that communicates with branches of the pulmonary artery and drains into the pulmonary veins. Other arterial branches ramify in interlobular loose connective tissue and most end in either deep or superficial bronchial veins; some also ramify on the surface of the lung, forming subpleural capillary plexuses. Bronchial arteries supply the bronchial wall as far as the respiratory bronchioles, and anastomose with branches of the pulmonary arteries in the walls of the smaller bronchi and in the visceral pleura. These bronchopulmonary anastomoses may be more numerous in the newborn and are subsequently obliterated to a marked degree. In addition to the main bronchial arteries, smaller bronchial branches arise from the descending thoracic aorta: one of these may lie in the pulmonary ligament and may cause bleeding during inferior lobectomy.
Bronchial veins
Usually two on each side, the bronchial veins drain blood from larger bronchi and from hilar structures. They form two distinct systems. Deep bronchial veins commence as intrapulmonary bronchiolar plexuses that communicate freely with the pulmonary veins and eventually join a single trunk that ends in a main pulmonary vein or in the left atrium. Superficial bronchial veins drain extrapulmonary bronchi, visceral pleura and the hilar lymph nodes. They also communicate with the pulmonary veins and end in the azygos vein on the right and in the left superior intercostal or the accessory hemiazygos veins on the left. Bronchial veins do not receive all the blood conveyed by bronchial arteries, some enters the pulmonary veins (see pp. 996–997). The main bronchial arteries and veins run on the dorsal aspect of the extrapulmonary bronchi.
Lymphatic drainage
The deep lymphatic plexus reaches the hilum by travelling along the pulmonary vessels and bronchi. In larger bronchi the deep plexus has submucosal and peribronchial parts, but in smaller bronchi there is only a single plexus that extends to the bronchioles. The walls of the alveoli have no lymphatic vessels.
INNERVATION
The anterior and posterior pulmonary plexuses innervate the trachea and the bronchi. The two plexuses are interconnected. The trachea is innervated by branches of the vagi, recurrent laryngeal nerves and sympathetic trunks. The nerves enter the lung as networks that travel along branches of the bronchi and pulmonary and bronchial vessels as far as the visceral pleura. Efferent vagal preganglionic axons synapse on small ganglia within the walls of the tracheobronchial tree: these may act as sites of integration and/or modulation of the input from extrinsic nerves, or permit some local control of aspects of airway function by local reflex mechanisms. The parasympathetic nervous system is the dominant neuronal pathway in the control of airway smooth muscle tone. Stimulation of cholinergic nerves causes bronchoconstriction, mucus secretion and bronchial vasodilation. Sympathetic nerves may control tracheobronchial blood vessels, but innervation of human airway smooth muscle by sympathetic-adrenergic fibres has yet to be convincingly demonstrated.
MICROSTRUCTURE OF THE CONDUCTING AIRWAYS
The conducting airways are lined internally by a mucosa, and the epithelium lies on a thin connective tissue lamina propria. External to this is a submucosa, also composed of connective tissue, in which are embedded airway smooth muscle, glands, cartilage plates (depending on the level in the respiratory tree), vessels, lymphoid tissue and nerves. Cartilage is present from the trachea to the smallest bronchi, but is absent (by definition) from bronchioles.
Epithelium
The epithelia of the trachea, bronchi and bronchioles are, in general, similar to each other, with graded variations in the numbers of different cell types. The extrapulmonary and larger intrapulmonary passages are lined with respiratory epithelium, which is pseudostratified, predominantly ciliated, and contains interspersed mucus-secreting goblet cells. There are fewer cilia in terminal and respiratory bronchioles, and the cells are reduced in height to low columnar or cuboidal. The epithelium of smaller bronchi and bronchioles is folded into conspicuous longitudinal ridges, which allow for changes in luminal diameter (Fig. 57.9). The epithelium in the respiratory bronchioles progressively reduces in height towards the alveoli, and is eventually composed of cuboidal, non-ciliated cells. Respiratory bronchioles have lateral pouches in their walls, which are lined with squamous cells, so providing an accessory respiratory surface.
Six distinct types of epithelial cell have been described in the conducting airways, namely, ciliated columnar, goblet, Clara, basal, brush and neuroendocrine. Lymphocytes and mast cells migrate into the epithelium from the underlying connective tissue.
Ciliated columnar cells
Ciliated columnar cells are the driving force of the mucociliary rejection current (escalator) in the bronchial tree. They vary from low to tall columnar, and each has up to 300 cilia projecting from the apical surface. The cilia extend into a watery fluid secreted by serous cells of the submucosal glands, but their tips are in contact with a more superficial layer of thicker mucus secreted by surface goblet cells and mucous cells in the submucosal glands. The rate of ciliary beating is usually 12–16 per second, although mechanical stimulation of the epithelial surface, and inflammatory mediators, increase the rate. In addition to tight junctions which seal the apical intercellular space from the airway lumen, the ciliated cells are coupled by gap junctions which allow a change in rate of beating to spread from stimulated cells to their neighbours (probably via calcium signalling) so that their metachronal coordination remains intact.
Goblet cells
Goblet cells are present from the trachea (6000–7000 per mm2) down to the smaller bronchi, but are normally absent from bronchioles. They contain an apical region full of large secretory vacuoles filled with mucinogen. When the epithelium is irritated, e.g. by tobacco smoke, there is an overall increase in the number of goblet cells, and they also extend into the bronchioles.
Clara cells
Clara cells are cuboidal non-ciliated cells with apices which bulge into the lumen. They contain numerous electron-dense secretory granules and many lysosomes. Clara cells produce surfactant lipoprotein and thus share functional similarities with the type II alveolar cell of pulmonary alveoli, although their secretory granules differ in structure and composition. They may also regulate ion transport.
Basal cells
Basal cells are small rounded cells that occur in parts of the airway lined by pseudostratified respiratory epithelium; they are mitotic stem cells for other epithelial cell types. Basal cells contact the basal lamina and are most frequent in the larger conducting passages.
Brush cells
Brush cells are slender, non-ciliated cells with characteristically long, stiff apical microvilli from which they derive their name. Although infrequent, they are present throughout all parts of the conducting airway passages, including the respiratory epithelium of the nasopharynx. They are in contact with afferent nerve fibres basally and are presumed to have a sensory receptor function.
Neuroendocrine cells
Neuroendocrine cells, also termed dense-core or small granule (Kulchitsky) cells, are found mainly in the basal part of the epithelium. They have a rounded shape and the cytoplasm basal to their nuclei contains numerous small dense-cored vesicles, approximately 150 nm diameter. They form part of the dispersed neuroendocrine system of amine precursor uptake and decarboxylation (APUD) cells. Neuroendocrine cells are most numerous in fetal lungs and their number decreases dramatically after birth. There appears to be little further change in their frequency during adult life, although they may proliferate in certain pulmonary diseases.
Lymphocytes
Small lymphocytes, mainly T cells derived from mucosa-associated lymphoid tissue in the walls of the passages, occur within the epithelium throughout the conducting airway tissues, particularly in the extrapulmonary portion: they are concerned with the immune surveillance of the epithelium. Clusters of lymphocytes sometimes lie beneath non-ciliated epithelial cells of the microfold (M-cell) type.
Submucosal glands
Tubuloacinar, seromucous glands are present in the submucosa of the trachea and bronchi and, to a lesser extent, in the larger bronchioles. They contain separate mucous and serous cells, and are an important source of the mucous layer at the surface of the ciliated respiratory epithelium. Their secretions include mucins; the bacteriostatic substances lysozyme and lactoferrin; secretory antibodies (IgA) produced by plasma cells in the submucosal connective tissue; and protease inhibitors (e.g. α1-antitrypsin) important for neutralizing leukocyte-derived proteases, particularly elastase, in the respiratory tract. The secretory acini and tubules are surrounded by myoepithelial cells, which are innervated by autonomic fibres.
Connective tissue and muscle
Broad, longitudinal bands of elastin (Fig. 57.13) within the submucosa follow the course of the respiratory tree and connect with the elastin networks of the interalveolar septa (see Fig. 57.9). This elastic framework is a vital mechanical element of the lung, and is responsible for elastic recoil during expiration.
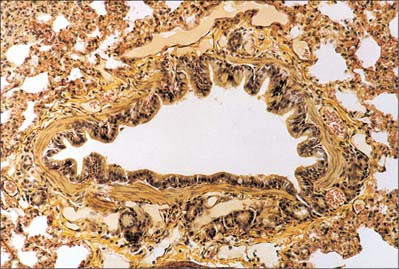
Fig. 57.13 Large bronchiole stained for elastin, showing smooth muscle in the bronchiolar wall and a few small seromucous glands (below).
In the trachea and extrapulmonary bronchi, the smooth muscle is mainly confined to the posterior, non-cartilaginous part of the tracheal tube (Fig. 57.14). Along the entire intrapulmonary bronchial tree, smooth muscle forms two opposed helical tracts which become thinner and finally disappear at the level of the alveoli. The tone of these muscle fibres is under nervous and hormonal control: groups of muscle cells are coupled by gap junctions to spread excitation within fascicles.
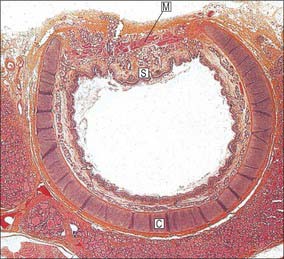
Fig. 57.14 Micrograph of the trachea of a child, sectioned transversely, showing an incomplete cartilage ring (C), joined posteriorly by muscular tissue (M). Seromucous submucosal glands (S) are concentrated in this region and between adjacent cartilages. Note thyroid tissue (bottom).
(By permission from Stevens A, Lowe JS 1996 Human Histology, 2nd edn. London: Mosby.)
Muscle cell contraction narrows the airway, while relaxation permits bronchodilation. Some tone normally exists in the muscular bands, which relax slightly during inspiration and contract during expiration, thereby assisting the tidal flow of air. Abnormal contraction may be caused by circulating smooth muscle stimulants or by local release of excitants such as serotonin, histamine and leukotrienes, which produces bronchospasm. Numerous mast cells are present in the connective tissue of the respiratory tree, especially towards the bronchioles.
Cartilaginous support
The trachea and extrapulmonary bronchi contain a framework of incomplete rings of hyaline cartilage which are united by fibrous tissue and smooth muscle (Fig. 57.14). Intrapulmonary bronchi contain discontinuous plates or islands of hyaline cartilage in their walls.
Tracheal cartilages
Tracheal cartilages vary from 16 to 20 in number. Anteriorly, each cartilage takes the form of an incomplete ring which surrounds approximately two-thirds of the tracheal circumference. Posteriorly, where the cartilages are deficient, the tube is flat and is completed by fibroelastic tissue and smooth muscle. The cartilages are horizontally stacked and separated by narrow intervals. They are typically 4 mm vertically, and 1 mm thick. Their external surfaces are vertically flat, and their internal surfaces are convex. Two or more cartilages often unite, partially or completely, and sometimes bifurcate at their ends. They may become calcified in the elderly. The cartilages are shorter, narrower and less regular in extrapulmonary bronchi, but they are generally similar in shape and arrangement.
The first and last tracheal cartilages differ from the rest (Fig. 57.7). The first cartilage is the broadest. It often bifurcates at one end and is connected by the cricotracheal ligament to the inferior border of the cricoid cartilage, with which it may blend. It may also blend with the second cartilage. The last cartilage is centrally thick and broad and its lower border, the carina, is a triangular hook-shaped process which curves down and backwards between the bronchi (Fig. 57.9). It forms an incomplete ring on each side, and encloses the start of a principal bronchus.
Bronchial cartilages
The irregularity of the cartilaginous plates in the extrapulmonary bronchi increases as they are traced distally, and as the major bronchi approach their lungs and lobes, the plates surround their dorsal aspects. In intrapulmonary bronchi, discontinuous plates of cartilage progressively form less and less of the bronchial wall, and they are not present in the bronchioles.
PULMONARY DEFENSIVE MECHANISMS
The respiratory tract presents a huge surface area that is vulnerable to desiccation, microbial invasion and the mechanical and chemical effects of inhaled particles. Inhaled air is humidified chiefly in the upper respiratory tract where it passes, with some turbulence, over the nasal and buccopharyngeal mucosae. The secretions of the various glands of the bronchial tree also help to prevent desiccation. Goblet cells secrete sulphated acid mucosubstances; cells in mucous glands beneath the epithelial surface contain mainly carboxylated mucosubstances, particularly those associated with sialic acid, although sulphated groups also occur; cells of serous glands contain neutral mucosubstances. Goblet cells respond mainly to local irritation, while tubular glands, both mucous and serous, are mainly under neural and hormonal control. Excessive or altered secretions may obstruct the flow of air. In addition to mucosubstances secreted by bronchial glands, antibacterial and antiviral substances, e.g. lysozyme, antibodies of the IgA type and possibly interferon, also appear in the secreted fluid.
Inhaled particles may be removed via the mucociliary rejection current. Cilia sweep the fluid overlying the surfaces of bronchioles, bronchi and trachea upwards at 1 cm/min, and much of the inhaled matter trapped in the viscous fluid may be removed in this way. Particles small enough to reach the alveoli may be removed by alveolar phagocytes (see p. 998). Alveolar epithelium has limited powers of regeneration but normally is continually replaced. The lifespan of alveolar squamous cells is approximately 3 weeks, and that of alveolar phagocytes is 4 days. Inhaled particles may also be cleared by the cough reflex.
Numerous lymphoid nodules occur in the bronchial lining. They provide foci for the production of lymphocytes and give local immunological protection against infection both by cell-mediated (T-cell) activities, and by the production of immunoglobulins (mainly IgA) from B cells, which are passed on to gland cells for secretion to the epithelial surface.
Anatomy of coughing
Coughing involves an initial deep inspiration, followed by forceful contraction of the expiratory muscles and diaphragm against a closed glottis. This leads to an abrupt rise in pleural pressure (6.5 to 13 kPa) and intra-alveolar pressure. Subsequent glottal opening causes a rapid peak expiratory flow of air, followed by some collapse of the trachea and central airways, which is responsible for the post-peak plateau in flow.
Coughing is initiated when mechanically and chemically sensitive vagal afferents innervating the airways are activated. Data from experimental studies suggest that the receptors innervated by these afferents may be broadly divided into three groups: slowly (SAR) and rapidly (RAR) adapting stretch receptors and bronchopulmonary C fibres. The vagal afferents terminate centrally in largely nonoverlapping regions of the caudal half of the nucleus of the solitary tract; second order neurones from this nucleus terminate in respiratory-related regions of the medulla, pons and spinal cord. Afferent nerves innervating other viscera, and somatosensory nerves innervating the chest wall, diaphragm and anterior abdominal musculature are also likely be involved in regulating cough.
The identity of the afferent terminals in the airways is uncertain; receptors similar to taste buds are considered to be responsible at the laryngeal aditus, and some of the ‘brush cells’ of the respiratory epithelium appear to have neural contacts, and may therefore represent sensory cells with basal synaptic outputs (Widdicombe 2002). Other than the larynx, the carina and branching points of the tracheobronchial tree appear to be the most sensitive areas of the epithelium lining the airways.
For further details, see Kubin et al (2006) and Canning (2006).
BRONCHOSCOPY
Bronchoscopy allows the direct visualization of the vocal cords, trachea and major airways as far as the first division of the subsegmental airway. Occasionally bronchoscopy can also provide some information about structures adjacent to the airways, for example, significant subcarinal lymphadenopathy may cause splaying or widening of the carina. Bronchoscopy enables the acquisition of samples (e.g. bronchial lavage, bronchial brushings, bronchial and transbronchial biopsies), provides information about staging in lung cancer, and facilitates therapeutic procedures (e.g. foreign body removal, ablation of tumours and insertion of airway stents). The information obtained by continuous (spiral) volume CT scanning can be reconstructed to provide three-dimensional images which can be used to create endobronchial views (virtual bronchoscopy, Fig 57.15) or to demonstrate the pulmonary arteries.
Armstrong P. The normal chest. In: Armstrong P, Wilson AG, Dee P, Hansell DM. Images of the Diseases of the Chest. London: Mosby; 2000:21-62.
Baile EM. The anatomy and physiology of the bronchial circulation. J Aerosol Med. 1996;9:1-6.
Belvisi MG. Overview of the innervation of the lung. Curr Opin Pharmacol. 2002;2:211-215.
Canning BJ. Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:33S-47S.
Devendra G, Spragg RG. Lung surfactant in subacute pulmonary disease. Respir Res. 2002;3:19-22.
Ghaye B, Szapiro D, Fanchamps JM, Dondelinger RF. Congenital bronchial abnormalities revisited. Radiographics. 2001;21:105-119.
Jeffery PK. Microscopic structure of the lung. In: Gibson GJ, Geddes DM, Costabel U, Sterk PJ, Corrin B. Respiratory Medicine. 3rd edn. London: Elsevier Science; 2003:34-50.
Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618-627.
Venuta F, Rendina EA, De Giacoma T, et al. Technique to reduce air leaks after pulmonary lobectomy. Eur J Cardiothorac Surg. 1998;13:361-364.
Widdicombe J. Neuroregulation of cough: implications for drug therapy. Curr Opin Pharmacol. 2002;2:256-263.