5 Orthodontic tooth movement
If a force is applied to a tooth it will elicit a response within the periodontium, resulting in remodelling of the periodontal ligament and alveolar bone, and ultimately tooth movement. The physical and biological principles that underlie this process form the basis of orthodontic practice and are discussed in this chapter.
Biological basis of tooth movement
It was first noted in the nineteenth century that a mechanical stimulus applied to bone could lead to remodelling and this is the basic principle, which facilitates orthodontic tooth movement.
Physiology of bone
Bone is a hard tissue composed of a collagen matrix impregnated with mineral salts. As well as providing the foundation of the musculoskeletal system in most vertebrates, it serves as a storage site for many important elements, especially calcium. Bone consists of three principle components:
There is close intercellular communication between osteoblasts and osteocytes, the main function of this osteoblast–osteocyte complex being to maintain integrity of the bone matrix.
Bone remodelling
Bone is a dynamic tissue, with resorption and deposition continually occurring and being closely linked and regulated. This process produces remodelling of the skeleton, in simple terms by osteoblastic deposition and osteoclastic resorption. However, the situation is complex and in addition to their direct role in bony deposition, several osteoblastic responses have been identified that indirectly facilitate osteoclastic resorption:
Regulation of bony deposition and resorption is important for normal maintenance of the skeleton and when these mechanisms break down, pathological change can occur. Numerous systemic and local factors have been implicated in bone remodelling (Table 5.1).
Table 5.1 Factors affecting bone remodelling and maintenance of the periodontal space
| Systemic factors | Local factors |
|---|---|
| Parathyroid hormone | Cytokines and growth factors |
| Vitamin D metabolites | Prostaglandins |
| Calcitonin | Leukotrienes |
Biomechanics of tooth movement
Early research into tooth movement investigated the histological response of tissues using animal models, whilst more recent work has focused on cellular activity following mechanical stimulation (Box 5.1).
Box 5.1 How has orthodontic tooth movement been investigated?
Early investigations into orthodontic tooth movement examined the histological effect within the periodontal ligament and alveolar bone of loading a tooth. Later experimental models were developed, both in vitro and in vivo, to study the effect that these forces had in greater detail. Numerous animal models have been used, including rats, cats and primates. Usually an orthodontic appliance is attached to the teeth and force applied over a given period of time, samples of cervicular fluid are collected for assay during the experimental period and then the animal is sacrificed for histological examination. Organ culture systems have also been used. As the periodontal ligament is similar to sutural joints, in both anatomy and function, one model involves placing mechanical stress across cranial sutures taken from newborn rabbits. Animal models tell us much about the histological and biochemical changes that occur during mechanical stress; however, the major drawback of such experiments is the difficulty in determining the individual cellular response. To examine this, a single cell type is cultured on a substrate that is mechanically deformed. Petri dishes with flexible bases are available, and these can be deformed by placing them over a convex template or applying a vacuum. Different cell types can be examined in this way and the size and periods of the deformation can be varied. Samples are taken periodically from the cell culture medium in which the cells are immersed for subsequent biochemical assay.
Pressure–tension theory
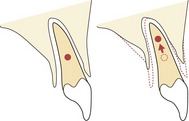
Histological studies carried out independently by Carl Sandstedt and Albin Oppenheim at the turn of the past century provided the foundation for current understanding of orthodontic tooth movement. When a force is placed on a tooth, bone is laid down on the tension side of the periodontal ligament and resorbed on the pressure side (Fig. 5.1). On the pressure side, when the force is light, multinucleate cells resorb bone directly. However, if the forces are higher and exceed capillary blood pressure, cell death can occur and a cell-free area forms. This is described as hyalinization, due to the glass-like appearance of these regions when viewed with light microscopy resembling hyaline cartilage. Resorption of these areas proceeds at a much-reduced rate. This process is described as undermining resorption and will result in slower tooth movement and greater pain and discomfort for the patient. Later work showed that even forces as light as 30g will produce some areas of hyalinization, and this tends to occur more with tipping than bodily movement of teeth, presumably because the force is dissipated more evenly through the periodontal ligament during bodily movement (Reitan, 1964).
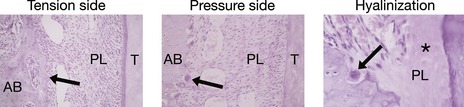
Figure 5.1 Histological appearance of the periodontium during orthodontic tooth movement.
Osteoblastic bone deposition (arrowed) on the tension side. Osteoclastic frontal resorption (arrowed) on the pressure side. Hyalinization occurs in areas of excess pressure and is characterized by a glass-like appearance in regions of the periodontal ligament (*) and areas of undermining resorption (arrowed). AB, alveolar bone; PL, periodontal ligament; T, tooth.
Courtesy of Professor Jonathan Sandy.
From histological and clinical studies there appears to be a range of force effective for tooth movement (Storey & Smith, 1952) although the optimum force magnitude for orthodontic tooth movement has yet to be described (Ren et al, 2003). Light continuous forces are thought to be more effective than heavy forces as these will increase the risk of hyalinization, with no increase in the desired tooth movement but with greater potential anchorage loss (Fig. 5.2). However, large variation does exist between individuals and more important than the absolute force is the stress generated in the periodontal ligament. Stress is force per unit area and depending on the type of tooth movement, stress distribution within the periodontium will vary. Therefore, different force levels are recommended for different types of tooth movement (Table 5.2). Tipping teeth requires less force than bodily movement, whilst intrusive forces need to be light as these are dissipated through the apices of the teeth, increasing the risk of root resorption.
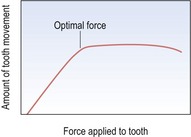
Figure 5.2 Graph of force applied to the periodontal ligament and subsequent tooth movement.
Up to a certain threshold, tooth movement increases with force. However, once this force exceeds a certain level, no additional movement is obtained. Indeed, extreme forces can even cause a reduction in movement.
Table 5.2 Range of forces for different tooth movements
| Tipping | 30–60 g |
| Bodily movements | 100–150 g |
| Rotational movements | 50–75 g |
| Extrusion | 50–75 g |
| Intrusion | 15–25 g |
Bone deflection and piezoelectricity
Orthodontic forces are transmitted within the periodontal ligament of the tooth and traditionally this has been thought to be via principle fibres of the periodontal ligament itself. However, when cross-linking of collagen is disrupted, the histological response of bone to orthodontic tooth movement appears normal (Heller & Nanda, 1979). Therefore, it has been proposed that the periodontal ligament represents a continuous hydrostatic system that distributes forces equally to all its regions (Baumrind, 1969). However, this would mean differential forces could not be distributed within the ligament, which is clearly not the case.
Teeth will displace a greater amount than the width of the periodontal ligament when force is initially applied to them, which implies that some deflection of alveolar bone also occurs. This might explain why differential forces can develop at the bone surfaces of the periodontal ligament. Bone deflection also produces stress-generated electrical potentials at the bone surface, which at one time were thought to be involved in bone remodelling. However, these are short-lived and very small and as such, unlikely to play an active role (McDonald, 1993).
Cellular shape change and signal transduction
When an external force is applied to a tooth, this stimulus generates an intracellular response, which ultimately leads to a change in function of affected and adjacent cells by production of local bone remodelling mediators.
A relationship appears to exist between cell shape and metabolic activity. Cell shape is controlled by the cytoskeleton, which terminates in specialized sites at the cell membrane, which form junctional complexes with the extracellular matrix. At these focal adhesion integrins, a family of proteins that span the cell membrane link the cytoskeleton to the extracellular matrix. They act as intracellular signalling receptors and are involved in numerous signalling pathways. Mechanical stress on cells within the periodontal ligament initiates both the cyclic AMP and phosphoinositide pathways, which results in an increase in production of secondary messengers. This produces an increase in intracellular calcium levels, which mediates further cellular events, including a stimulation of DNA synthesis (Harell et al, 1977).
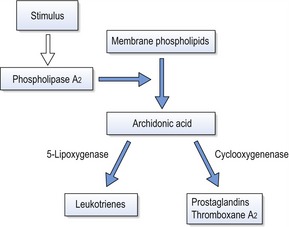
Production of arachidonic acid metabolites
Arachidonic acid is an unsaturated fatty acid produced from membrane phospholipids, which is metabolized into prostaglandins, leukotrienes and thromboxanes (Fig. 5.3). These molecules are potent mediators of inflammation and numerous in vitro and in vivo experiments have shown a relationship between mechanical stimulation and prostaglandin production in bone. Based on this work, prostaglandins have been used clinically via local administration in the gingivae to increase the efficiency of orthodontic tooth movement (Yamasaki et al, 1984). The other principle products of arachidonic acid metabolism, leukotrienes, have also been shown to increase around teeth moved orthodontically. This may explain the observation that on administration of a non-steroidal anti-inflammatory drug in an animal model, there is a decrease in osteoclast numbers, but not tooth movement (Sandy & Harris, 1984). This suggests some overlap between the pathways and a degree of redundancy within the system. Inhibition of leukotriene production itself results in inhibition of tooth movement (Mohammed et al, 1989).
Production of cytokines
Cytokines are low-molecular-weight proteins that regulate or modify the action of cells. These protein groups are diverse and complex, and include some potent stimulators of bone resorption. It appears that osteoclast activity is dependent on soluble factors produced by osteoblasts and stromal cells in the periodontal ligament, so-called osteoclast-stimulating factors. On mechanical stimulation, the initial response of bone is to inhibit the production of cytokines involved in osteoclast stimulation and hence promote osteogenesis.
Numerous cytokines are produced in the periodontal ligament when force is applied to teeth, including interleukins (ILs), tumour necrosis factors (TNFs) and epidermal growth factors (EGFs). Local production of these molecules by mechanically activated cells within the ligament may play an important role in mediating both the resorptive and formative phase of connective tissue remodeling.
Alteration in cellular function and remodelling
The ultimate effect of mechanical stimulation on cells within the periodontal ligament is an alteration in gene expression and cellular function; which allows remodelling to take place and teeth to be moved through alveolar bone. Initially there appears to be a reduction in DNA synthesis, followed by a gradual increase. In addition there is an increase in production of collagen and proteins associated with its breakdown, and that of components in the extracellular matrix, including metalloproteinases, collagenases and gelatinases. It appears that matrix degradation in the periodontal ligament is a prerequisite for cell proliferation, creating room to accommodate an increase in the cell population. On the compression side it is likely that enzymes produced by osteoblasts degrade the non-mineralized osteoid surface of the bone, while periodontal cells degrade the extracellular matrix of the periodontal ligament. There is an increase in recruitment and production of osteoclasts, mediated via factors released from cells in the periodontal ligament. Osteoclasts finally access the bone surface and degrade the mineralized matrix.
Mechanical basis of tooth movement
Orthodontic tooth movement is dictated by the force system delivered to the teeth and mediated through the orthodontic appliance and the biological response it evokes. The following are important concepts and definitions pertaining to orthodontic tooth movement and are relevant to its understanding:
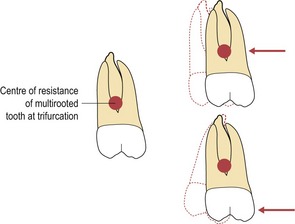
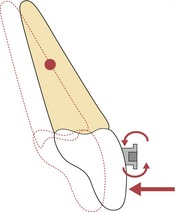
Figure 5.5 For bodily movement the force needs to pass through the centre of resistance of the tooth.
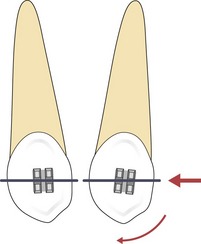
If the force vector does not pass through the centre of resistance, a moment is created and rotation will occur.
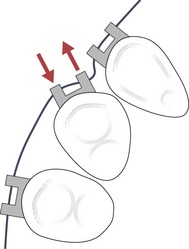
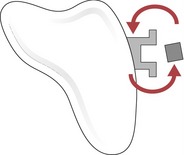
Figure 5.6 A couple is generated when a light archwire is engaged into the bracket slot of a rotated tooth.
Tipping and bodily tooth movement
A significant problem with orthodontic tooth movement is that the centre of resistance of a tooth is not directly accessible to force application. Force must be applied to the tooth crown, which is at a distance from the centre of resistance and therefore, a moment and some rotational force is always produced.
Tipping movements are relatively easy to generate by point contact on the crown of a tooth and this is how the active components on a removable appliance work (Fig. 5.7).
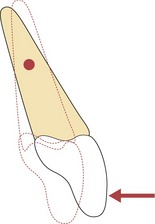
Figure 5.7 Application of a single force to the crown of a tooth will result in tipping as a moment is created.
Bodily movement is more difficult to produce and requires the combination of a force and a couple to control the rotational effect. This is essentially how the edgewise slot of a fixed appliance works. By placing a rectangular wire into the slot, a couple is created within the bracket slot, which will control root position and allow the tooth to move bodily in the direction of applied force (Fig. 5.8). The couple acts over the depth of the bracket slot and this will need to counter the moment created by a force applied at some distance from the centre of resistance of the tooth. Therefore, although the force applied to the tooth may be small, a large couple will need to be developed within the bracket to maintain bodily movement of the tooth. A small amount of space or ‘slop’ always exists between the archwire and bracket, so some tipping does occur. This can be countered by placing more torsion in the wire, or into the bracket base (Fig. 5.9). In reality, pure bodily movement or translation is an idealized impossibility. What happens is that the tooth or group of teeth will tip and then upright as they move along the archwire, giving the impression of bodily movement.
Force systems
In certain clinical situations the forces acting on the dentition and the resulting moments and couples can be readily determined. For example, with a cantilever spring acting on one tooth, the force system will consist of one force vector and the resulting couple and moment (Fig. 5.10). The movement of the tooth and the reactive forces are therefore fairly predictable. However, when using fixed appliances with continuous archwires numerous force vectors can be created and it becomes impossible to work out all the possible interactions. This is especially true in the initial alignment stage of treatment when numerous displaced teeth are often engaged with a flexible wire. Even so, all systems will follow the same basic physical laws, most notably Newton’s third law of motion: ‘For every action there is an equal and opposite reaction,’ the sum of the forces and the sum of the moments for any appliance system equaling zero. Therefore care must be taken to elicit the planned tooth movements while limiting the unwanted ones, as this can result in undesired treatment outcomes such as loss of anchorage.
Friction
Friction is the force that will resist the motion of two objects in contact with each other and acts tangentially to the two surfaces in contact. Friction affects tooth movement associated with all fixed appliances, but is particularly relevant for edgewise mechanics, which often rely upon sliding teeth along an archwire. Specifically, two types of friction need to be overcome:
Frictional resistance to sliding is proportional to the applied load and independent of the sliding surface area. In orthodontics, friction can result in binding between the archwire and bracket, leading to a reduction or failure of tooth movement, distortion of the archwires and loss of anchorage. Friction must be overcome before teeth will move and whilst frictional force can be measured in a laboratory, clinically the level is difficult to determine. In clinical practice with fixed appliances, friction is affected by a number of factors:
Anchorage
Anchorage is the resistance to unwanted tooth movement, or those sites that provide resistance to the reactive forces generated on activation of any orthodontic appliance. Anchorage needs to be carefully planned at the start of treatment to ensure the desired tooth movements are achieved. It maybe that equal movement of both the active and reactive units is desirable, such as expansion or closing a midline diastema, when anchorage is described as reciprocal. However, it is usual that to achieve the aims of treatment greater movement of the active than the reactive or anchorage unit is required. Depending how far the anchorage unit can move the anchorage requirements can be described as minimum or moderate. If no movement of the anchorage unit is permissible the requirement is described as maximum.
Sources of anchorage
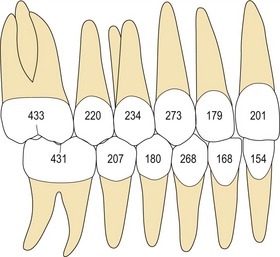
Figure 5.13 Root surface area (mm2) of the permanent dentition, giving an indication of the relative anchorage value of each tooth (Jepsen, 1963).
Headgear
Three factors should be considered when using headgear:
Direction of force
The force direction can be defined as:
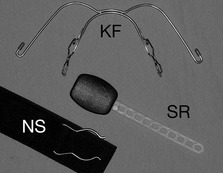
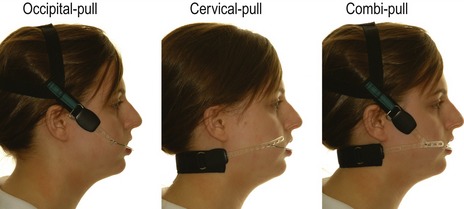
Generally, high-pull headgear is used in cases with increased vertical proportions, which include high maxillary–mandibular plane angles and vertical maxillary excess, because it has an intrusive effect on the posterior dentition. Headgear force in this direction can help to avoid further bite opening and restrain vertical maxillary development during orthodontic treatment. Similarly, low-pull headgear is used in cases with reduced vertical proportion and will extrude the posterior dentition. In a growing individual this can be helpful in reducing a deep overbite. Straight or combi-pull headgear is generally used in the presence of normal vertical proportions and is useful for molar distalization (Fig. 5.18).
Duration of force
How long the patient is instructed to wear the headgear will depend on the aims of treatment. Not only is headgear effective at supporting anchorage it can also be used to distalize the buccal dentition and even restrict growth of the maxilla. To achieve these latter two objectives requires a greater duration of wear and a higher level of force magnitude. In general the duration of wear required is:
Level of force
The chosen force level will also depend upon the aims of treatment. A higher level of force is required for distalization and orthopaedic change:
Headgear and safety
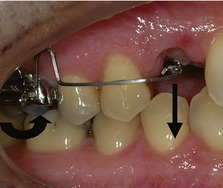
The application of a removable extraoral force to an orthodontic appliance with headgear has led to injuries being reported in a small number of patients (Fig. 5.19). These are predominantly of two types (Samuels, 1996):
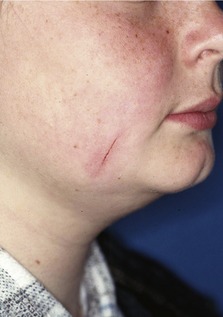
Figure 5.19 Facial laceration following accidental disengagement of a headgear facebow.
Courtesy of Ricarda Kane.
The most serious reported consequence of headgear injury is ocular damage, which can result in partial or total blindness in one or both eyes. Penetrating injuries of the eye can be relatively asymptomatic in the initial stages; however, oral microorganisms transmitted by the facebow can rapidly infect the eye because it acts as an excellent culture medium. These infections can be very difficult to treat and a sympathetic ophthalmitis can result, with the unaffected eye also becoming involved. Although the risk of this is small, because of the potentially devastating consequences, two independent safety devices are recommended when using headgear:
Anchorage loss
Anchorage loss is associated with undesirable tooth movement during orthodontic treatment. A common example is during overjet reduction, where teeth in the buccal segments move forward rather than those in the labial segments being retracted. If severe, too much space is lost and a residual overjet results. A number of factors can contribute to anchorage loss.
Heavy forces
Forces should be light enough to exceed the threshold for tooth movement where planned but below the threshold for movement of the anchorage unit. Heavy forces lead to unfavourable reactions in the periodontal ligament and tooth movement will plateau above a certain threshold. With greater force there is no increase in tooth movement. Assuming there are a greater number of teeth in the anchorage unit; by increasing forces, although there is no greater amount of tooth movement where it is wanted, greater force is applied to the teeth in the anchorage unit and they are more likely to move (Fig. 5.21). Hence anchorage is lost with heavier forces. This is the differential force theory proposed by Begg and utilised in his treatment philosophy by pitching tipping of teeth, requiring very light forces against bodily movement of the teeth in the anchorage unit.
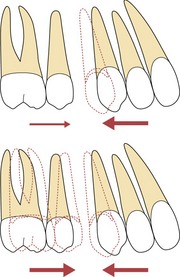
Figure 5.21 Forces should be kept light to reduce anchorage loss.
All orthodontic force will produce some reciprocal force on the anchorage unit. If this is small, there will be minimal movement of these teeth. With heavier force there is a risk that the anchorage unit will move, with no additional desired tooth movement; in this case retraction of the maxillary canine.
Maxillary arch
The maxillary arch is particularly susceptible to anchorage loss. This is probably due to a combination of factors, particularly the density of bone, width of the alveolus and more extensive tooth movement often required in the maxilla. Fortunately it is easier to support anchorage in the maxillary arch, primarily with the use of headgear.
Lindauer SJ. The basics of orthodontic mechanics. Sem Orthod. 2001;7:2-15.
Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod. 2006;28:221-240.
Quinn RS, Yoshikawa DK. A reassessment of force magnitude in orthodontics. Am J Orthod. 1985;88:252-260.
Baumrind S. A reconsideration of the propriety of the ‘pressure-tension’ hypothesis. Am J Orthod. 1969;55:12-22.
Harell A, Dekel S, Binderman I. Biochemical effect of mechanical stress on cultured bone cells. Calcif Tissue Res. 1977;22(Suppl):202-207.
Heller IJ, Nanda R. Effect of metabolic alteration of periodontal fibers on orthodontic tooth movement. An experimental study. Am J Orthod. 1979;75:239-258.
Jepsen A. Root surface measurement and a method for X-ray determination of root surface area. Acta Odontol Scand. 1963;21:35-46.
Kapila S, Angolkar PV, Duncanson MGJr, et al. Evaluation of friction between edgewise stainless steel brackets and orthodontic wires of four alloys. Am J Orthod Dentofacial Orthop. 1990;98:117-126.
McDonald F. Electrical effects at the bone surface. Eur J Orthod. 1993;15:175-183.
Mohammed AH, Tatakis DN, Dziak R. Leukotrienes in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1989;95:231-237.
Reitan K. Effects of force magnitude and direction of tooth movement on different alveolar types. Angle Orthod. 1964;34:244-255.
Ren Y, Maltha JC, Kuijpers-Jagtman AM. Optimum force magnitude for orthodontic tooth movement: a systematic literature review. Angle Orthod. 2003;73:86-92.
Samuels RH. A review of orthodontic face-bow injuries and safety equipment. Am J Orthod Dentofacial Orthop. 1996;110:269-272.
Sandy JR, Harris M. Prostaglandins and tooth movement. Eur J Orthod. 1984;6:175-182.
Storey E, Smith R. Force in orthodontics and its relation to tooth movement. Aust J Dent. 1952;56:11-18.
Yamasaki K, Shibata Y, Imai S, et al. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod. 1984;85:508-518.